Abstract
Two experiments were conducted to determine the available P (aP) release of Smizyme TS G5 2,500 (Origination, LLC., Maplewood, MN) phytase. Pigs were weaned at approximately 21-d of age, randomly allotted to pens based on initial body weight (BW) and fed a common diet. On d 21 post-weaning, pens were blocked by BW and randomly allotted to 1 of 8 (experiment 1) or 7 (experiment 2) dietary treatments with five pigs per pen and eight pens per treatment. Treatments were formulated to include increasing aP from either inorganic P (0.12%, 0.18%, or 0.24% in experiment 1 and 0.11%, 0.19%, or 0.27% in experiment 2 from monocalcium P) or increasing phytase (150, 250, 500, 750, or 1,000 FTU/kg in experiment 1 and 250, 500, 1,000, or 1,500 FTU/kg in experiment 2). Prior to beginning the 21-d studies, all pigs were fed the lowest inorganic P diet for a 3-d period. At the conclusion of each experiment, the pig closest to the pen mean BW was euthanized and fibulas were collected to determine bone ash weight and percentage bone ash. Fibulas were processed using defatted bone mineral procedures. In both experiments, pigs fed increasing aP from inorganic P had increased (linear, P < 0.01) average daily gain (ADG), G:F, and final BW. Additionally, pigs fed diets with increasing phytase had increased (experiment 1 linear, P < 0.01; experiment 2 linear and quadratic, P < 0.05) performance across all growth response criteria. For bone composition, pigs fed increasing aP from inorganic P had increased bone ash weights (linear, P < 0.01) and percentage bone ash (experiment 1 quadratic, P < 0.01; experiment 2 linear, P < 0.01). Similarly, pigs fed increasing phytase had increased bone ash weights (linear, P < 0.01) and percentage bone ash (experiment 1 linear, P < 0.01; experiment 2 linear and quadratic, P < 0.05). The percentage aP released from Smizyme TS G5 2,500 for both experiments varied depending on the response criteria used. As the amount of phytase in the diet increased, the calculated aP release increased when ADG (experiment 1 linear, P < 0.01; experiment 2 linear and quadratic, P < 0.01), G:F (linear, P < 0.01), or percentage bone ash (experiment 1 linear and quadratic, P < 0.05; experiment 2 linear, P < 0.01) were used the predictor variable. When combining the data from experiment 1 and 2, the aP release prediction equations for Smizyme TS G5 2,500 are aP = (0.197 × FTU)/(584.956 + FTU), aP = (0.175 × FTU)/(248.348 + FTU), and aP = (0.165 × FTU)/(178.146 + FTU) when using ADG, G:F, and percentage bone ash, respectively as the predictor variable.
Keywords: bone ash, nursery pig, phosphorus, phytase
INTRODUCTION
Phytase is an enzyme commonly added to swine diets to improve the digestibility of phytate-bound-phosphorus. Phytate or phytic acid is a sixfold dihydrogen phosphate ester of inositol that is the major storage form (60–82%) of phosphorus (P) found in feedstuffs of plant origin (Ravindran et al., 1994). Pigs and other monogastric animals do not synthesize adequate levels of endogenous phytase to effectively cleave the phosphates from the phytate (Selle and Ravindran, 2008). Therefore, the P found in corn–soybean meal-based diets has limited availability, requiring nutritionists to add P from inorganic sources, such as monocalcium phosphate, in order to meet P requirements. Alternatively, adding phytase from an exogenous source has been shown to improve the hydrolysis of phytic acid, making P more available for absorption (Simons et al., 1990; Cromwell et al., 1993). This allows nutritionists to reduce dietary inorganic P without compromising growth, while subsequently improving diet costs and decreasing the amount of P excreted in swine waste (Simons et al., 1990; Selle and Ravindran, 2008; Lei et al., 2013).
Phytase was first commercialized in 1991 and nearly 20 years later it was coined one of the most significant discoveries in animal nutrition (Cromwell, 2009; Lei et al., 2013). While many commercially available phytase sources have already undergone evaluation to determine their available P (aP) release, new phytase sources must be analyzed to determine their efficacy (Jones et al., 2010; Goncalves et al., 2016; Gourley et al., 2018). Smizyme TS G5 2,500 (Origination, LLC., Maplewood, MN) is a newly available bacterial derived 6-phytase produced through the fermentation of Escherichia coli. Little research has been conducted to demonstrate its efficacy for use in the U.S swine industry. Therefore, the objective of these studies was to evaluate the effects of Smizyme TS G5 2,500 phytase (Origination, LLC., Maplewood, MN) on the growth performance and bone ash of 10- to 21-kg nursery pigs to develop an aP release curve.
MATERIALS AND METHODS
The Kansas State University Institutional Animal Care and Use Committee approved the protocol (4036) used in these experiments. Ingredients containing Ca and P were analyzed (Ward Laboratories, Inc., Kearney, NE) in duplicate prior to the manufacturing of experimental diets to determine nutrient loading values used for formulation (Table 1). Additionally, phytase was acquired for each experiment and the analyzed (Eurofins Scientific Inc., Des Moines, IA) activity was 2,630,000 FTU/kg (experiment 1) and 2,314,000 FTU/kg (experiment 2). Diets were corn–soybean meal-based and contained 1.24% standardized ileal digestible (SID) Lys with other amino acids set to meet or exceed NRC (2012) requirement estimates. All diets were formulated to contain a Ca:P ratio of 1.10:1 with no allowance for the release of Ca by phytase. All dietary treatments were manufactured at the Kansas State University O.H. Kruse Feed Technology Innovation Center in Manhattan, KS.
Table 1.
Analyzed ingredient composition (as-fed basis)a
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| Ingredient | Ca, % | P, % | Ca, % | P, % |
| Corn | 0.07 | 0.24 | 0.06 | 0.20 |
| Soybean meal | 0.56 | 0.64 | 0.60 | 0.64 |
| Limestone | 40.24 | 0.03 | 40.28 | 0.06 |
| Monocalcium P | 17.90 | 20.23 | 17.42 | 20.66 |
aIngredient samples were taken from the Kansas State University O.H. Kruse Feed Technology Innovation Center in Manhattan, KS, and submitted for analysis (Ward Laboratories, Inc., Kearney, NE).
Diet Manufacturing
At the time of feed manufacturing, eight (experiment 1) or seven (experiment 2) identical 907 kg batches of basal diet were produced and packaged in 22.7 kg bags (Table 2). For each experimental diet, a subset of bags from the basal diet was added to the mixer along with treatment specific ingredients to achieve the final experimental diets (Tables 3 and 4). Complete diet samples were taken during bagging of experimental diets with a subsample collected from every fourth bag and pooled into one homogenized sample per dietary treatment. After homogenization, each diet sample was ground to reduce particle size and then divided into three separate sub-samples per dietary treatment. Samples were stored at −20 °C until they were submitted for phytase and nutrient analysis.
Table 2.
Composition of the basal mix (as-fed basis)a
| Item | Experiment 1 and 2 |
|---|---|
| Ingredient, % | |
| Corn | 64.39 |
| Soybean meal | 34.05 |
| Sodium chloride | 0.61 |
| l-Lysine-HCl | 0.30 |
| dl-Methionine | 0.12 |
| l-Threonine | 0.12 |
| l-Valine | 0.01 |
| Trace mineral premix | 0.15 |
| Vitamin premix | 0.25 |
| Calculated analysis | |
| SID amino acids | |
| Lysine, % | 1.24 |
| Isoleucine:lysine | 64 |
| Leucine:lysine | 131 |
| Methionine:lysine | 33 |
| Methionine and cystine:lysine | 57 |
| Threonine:lysine | 64 |
| Tryptophan:lysine | 19 |
| Valine:lysine | 70 |
| Histidine:lysine | 42 |
| Total lysine, % | 1.41 |
| Metabolizable energy, kcal/kg | 3,324 |
| Net energy (NE), kcal/kg | 2,444 |
| SID lysine:NE, g/Mcal | 5.06 |
| Crude protein, % | 22.0 |
| Calcium, %b | – |
| Phosphorus, %c | – |
| Available phosphorus, % | 0.07 |
| STTD P, %d | 0.16 |
aThe basal batch was used as the major ingredient in each experimental diet.
bThe calculated analysis of Ca in experiments 1 and 2 was 0.31% and 0.32%, respectively.
cThe calculated analysis of P in experiment 1 and 2 was 0.37% and 0.35%, respectively.
dSTTD P = Standardized total tract digestible phosphorus.
Table 3.
Composition of experimental diets (as-fed basis), experiment 1a
| Inorganic P | Phytaseb | |||||||
|---|---|---|---|---|---|---|---|---|
| Ingredient, % | 0.12% | 0.18% | 0.24% | 150 | 250 | 500 | 750 | 1,000 |
| Basal mix | 98.75 | 98.75 | 98.75 | 98.75 | 98.75 | 98.75 | 98.75 | 98.75 |
| Limestone | 0.30 | 0.32 | 0.35 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Monocalcium P | 0.25 | 0.53 | 0.85 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Sandc | 0.71 | 0.41 | 0.05 | 0.70 | 0.70 | 0.69 | 0.68 | 0.67 |
| Phytased | – | – | – | 0.006 | 0.009 | 0.019 | 0.029 | 0.038 |
| Calculated analysis | ||||||||
| Crude protein, % | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 |
| Ca, % | 0.46 | 0.52 | 0.59 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 |
| P, % | 0.42 | 0.48 | 0.54 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 |
| Phytase, FTU/kg | – | – | – | 150 | 250 | 500 | 750 | 1,000 |
| Ca:P ratio | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| Analyzed compositione | ||||||||
| Crude protein, % | 21.9 | 22.0 | 21.8 | 21.5 | 21.8 | 21.9 | 21.0 | 21.6 |
| Ca, % | 0.44 | 0.43 | 0.54 | 0.38 | 0.43 | 0.40 | 0.46 | 0.41 |
| P, % | 0.45 | 0.48 | 0.56 | 0.43 | 0.41 | 0.43 | 0.44 | 0.44 |
| Phytase, FTU/kgf,g | – | – | – | 190 | 310 | 500 | 790 | 850 |
| Ca:P ratio | 0.98 | 0.90 | 0.96 | 0.88 | 1.05 | 0.93 | 1.05 | 0.93 |
aDiets were fed for 21 d from approximately 10–23 kg.
bSmizyme TS G5 2,500 (Origination, LLC., Maplewood, MN).
cSand was used to equalize the addition of the sum of limestone, monocalcium P, and phytase across experimental diets.
dPhytase premix was analyzed for phytase level and contained 2,630,000 FTU/kg (Eurofins Scientific Inc., Des Moines, IA).
eComplete diet samples were taken during bagging of experimental diets with a subsample collected from every fourth bag and pooled into one homogenized sample per dietary treatment. After homogenization, each diet was placed in a grinder to reduce particle size and then divided into three separate samples per dietary treatment. Samples were stored at −20 °C until they were submitted for duplicate analysis of crude protein, Ca, and P (Ward Laboratories, Inc., Kearney, NE).
fOne sample of each diet containing phytase was submitted to Eurofins Scientific Inc. (Des Moines, IA) for complete phytase analysis.
g AOAC (2000).
Table 4.
Composition of experimental diets (as-fed basis), experiment 2a
| Inorganic P | Phytaseb | ||||||
|---|---|---|---|---|---|---|---|
| Ingredient, % | 0.11% | 0.19% | 0.27% | 250 | 500 | 1,000 | 1,500 |
| Basal mix | 98.77 | 98.77 | 98.77 | 98.77 | 98.77 | 98.77 | 98.77 |
| Limestone | 0.18 | 0.23 | 0.28 | 0.18 | 0.18 | 0.18 | 0.18 |
| Monocalcium P | 0.20 | 0.55 | 0.95 | 0.20 | 0.20 | 0.20 | 0.20 |
| Sandc | 0.85 | 0.45 | 0.00 | 0.84 | 0.83 | 0.81 | 0.78 |
| Phytased | – | – | – | 0.011 | 0.022 | 0.043 | 0.065 |
| Calculated analysis | |||||||
| Crude protein, % | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 | 21.7 |
| Ca, % | 0.42 | 0.50 | 0.59 | 0.42 | 0.42 | 0.42 | 0.42 |
| P, % | 0.38 | 0.46 | 0.54 | 0.38 | 0.38 | 0.38 | 0.38 |
| Phytase, FTU/kg | – | – | – | 250 | 500 | 1,000 | 1,500 |
| Ca:P ratio | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| Analyzed compositione | |||||||
| Crude protein, % | 21.2 | 20.7 | 20.9 | 20.9 | 21.0 | 21.5 | 21.1 |
| Ca, % | 0.38 | 0.49 | 0.59 | 0.41 | 0.37 | 0.38 | 0.37 |
| P, % | 0.38 | 0.44 | 0.53 | 0.38 | 0.37 | 0.39 | 0.37 |
| Phytase, FTU/kgf,g | – | – | – | 265 | 470 | 1,000 | 1,450 |
| Ca:P ratio | 0.99 | 1.10 | 1.12 | 1.07 | 1.00 | 0.97 | 0.98 |
aDiets were fed for 21 d from approximately 10–22 kg.
bSmizyme TS G5 2,500 (Origination, LLC., Maplewood, MN).
cSand was used to equalize the addition of the sum of limestone, monocalcium P, and phytase across experimental diets.
dPhytase premix was analyzed for phytase level and contained 2,314,000 FTU/kg (Eurofins Scientific Inc., Des Moines, IA).
eComplete diet samples were taken during bagging of experimental diets with a subsample collected from every fourth bag and pooled into one homogenized sample per dietary treatment. After homogenization, each diet was placed in a grinder to reduce particle size and then divided into three separate samples per dietary treatment. Samples were stored at −20 °C until they were submitted for duplicate analysis of crude protein, Ca, and P (Ward Laboratories, Inc., Kearney, NE).
fOne sample of each diet containing phytase was submitted to Eurofins Scientific Inc. (Des Moines, IA) for complete phytase analysis.
g AOAC (2000).
Animals and Housing
Experiments were conducted at the Kansas State University Swine Teaching and Research Center in Manhattan, KS. The nursery barn was environmentally controlled, and each pen contained a four-hole, dry self-feeder, and nipple waterer for ad libitum access to feed and water.
A total of 320 (experiment 1) or 280 (experiment 2) pigs (DNA 241 × 600, initially 10 kg body weight [BW]) were used in 21-d growth trials. Pigs were weaned at approximately 21 d of age, randomly allotted to pens based on initial BW, and fed a common starter diet. On d 21 post-weaning, considered d 0 of the studies, pens were blocked by BW and randomly allotted to one of eight (experiment 1) or seven (experiment 2) dietary treatments with five pigs per pen (two barrows and three gilts or three barrows and two gilts) and eight pens per treatment. In experiment 1, treatments consisted of three diets with increasing (0.12%, 0.18%, or 0.24%) inorganic P from monocalcium P, or five diets with increasing (150, 250, 500, 750, or 1,000 FTU/kg) phytase added to the diet containing 0.12% aP. In experiment 2, treatments consisted of three diets with increasing (0.11%, 0.19%, or 0.27%) inorganic P from monocalcium P, or four diets with increasing (250, 500, 1,000, or 1,500 FTU/kg) phytase added to the diet containing 0.11% aP. In both trials, the source of phytase was Smizyme TS G5 2,500 (Origination, LLC., Maplewood, MN), a microbial phytase derived from the fermentation of E. coli. Prior to beginning the 21-d studies, all pigs were fed the lowest inorganic P diet for a 3-d period (d 18 to 21 post-weaning).
During the experiments, pigs and feeders were weighed every 7 d to determine average daily gain (ADG), average daily feed intake (ADFI), and G:F. At the conclusion of each study, the pig closest to the pen mean BW was euthanized via penetrating captive bolt and fibulas were collected to determine bone ash weight and percentage bone ash (32 barrows and 32 gilts [experiment 1] or 28 barrows and 28 gilts [experiment 2]). After collection, bones were individually placed in plastic bags with permanent identification and stored at −20 °C until processing. On the day of processing, bones were autoclaved for 1 h at 121 °C. After cooling, any leftover extraneous soft tissue including cartilage caps was cleaned from the fibulas. A total of 64 (experiment 1) or 56 (experiment 2) fibulas (one from each pig) were placed in Soxhlet extractors containing petroleum ether for 7 d as a means of removing water and fat. They were then dried at 105 °C for 24 h and ashed at 600 °C for 24 h.
Chemical Analysis
Three samples per dietary treatment from the pooled feed samples were sent to a commercial laboratory (Ward Laboratories, Inc., Kearney, NE) for duplicate analysis of crude protein (AOAC 990.03, 2006), Ca (AOAC 985.01, 2006), and P (AOAC 985.01, 2006). Additionally, one sample of each diet containing phytase was submitted for complete phytase analysis (Eurofins Scientific Inc., Des Moines, IA) using the AOAC official method 2000.12.
Statistical Analysis
Studentized residuals were evaluated for pen means or individual bone ash measurements to ensure data met the assumption of normal distribution. Data were analyzed as a randomized complete block design with a pen as the experimental unit. An initial base model was evaluated using the MIXED procedure of SAS version 9.4 (SAS Institute, Inc., Cary, NC). Treatment was considered a fixed effect and weight block a random effect. Linear and quadratic contrasts were evaluated within increasing inorganic P or phytase treatments. Contrast coefficients were adjusted to account for unequal spacing in phytase doses.
For pens of pigs fed the inorganic P diets, the marginal intake of aP per day was calculated for each pen using the equation: dietary aP% minus 0.12% (aP in the basal diet, experiment 1) or 0.11% (aP in the basal diet, experiment 2) multiplied by ADFI. A standard curve was then developed for each response criteria using the marginal aP release as the predictor variable. The equation for the standard curve was used to calculate aP release from each pen fed the different phytase dosages based on the observed value for each response criteria. This value was then converted to a marginal aP% using the pen ADFI.
A mixed-model ANOVA with weight block as a random effect was performed to evaluate aP release as a function of phytase dosage, assuming an intercept of no aP release for the control diet without phytase. All release values were calculated using formulated P and phytase levels. The GLIMMIX procedure of SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for the analysis.
Release values were combined from the two experiments and were used in a non-linear regression to fit a model predicting aP release curves dependant on phytase dosage as a continuous variable using the individual pen data. Separate aP release curves were developed using aP release derived from ADG, G:F, and percentage bone ash data. Model parameters were estimated using the nls function from the stats package in R (version 3.5.1 [2018-07-02]). Results were considered to be significant with P-values ≤ 0.05 and were considered marginally significant with P-values of > 0.05 and ≤ 0.10.
RESULTS
Chemical Analysis
Analysis of manufactured diets resulted in crude protein, Ca and P values that were reasonably consistent with formulation (Tables 3 and 4). Phytase analysis of complete diets showed a stepwise increase in phytase concentration.
Experiment 1
From d 0 to 21, pigs fed increasing aP from inorganic P had improved (linear, P < 0.001; Table 5) ADG, G:F, and final BW, with a tendency for increased ADFI (linear, P = 0.080). Additionally, pigs fed increasing aP from phytase had improved (linear, P < 0.010) performance across all growth response criteria measured.
Table 5.
Effects of increasing aP from inorganic P or Smizyme TS G5 2,500 phytase on nursery pig growth performance and bone ash values, experiment 1a,b
| Inorganic P, % aPc | Phytase, FTU/kgd | Inorganic P | Phytase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0.12% | 0.18% | 0.24% | 150 | 250 | 500 | 750 | 1,000 | SEMe | Linear | Quadratic | Linear | Quadratic |
| BW, kg | |||||||||||||
| d 0 | 10.4 | 10.5 | 10.4 | 10.3 | 10.4 | 10.4 | 10.4 | 10.4 | 0.17 | 0.548 | 0.284 | 0.693 | 0.671 |
| d 21 | 21.4 | 22.7 | 23.1 | 21.9 | 22.2 | 22.9 | 23.2 | 23.6 | 0.41 | <0.001 | 0.162 | <0.001 | 0.259 |
| d 0–21 | |||||||||||||
| ADG, g | 523 | 585 | 605 | 551 | 563 | 593 | 608 | 629 | 14.6 | <0.001 | 0.198 | <0.001 | 0.273 |
| ADFI, g | 882 | 944 | 937 | 868 | 910 | 923 | 931 | 947 | 24.8 | 0.080 | 0.204 | 0.007 | 0.725 |
| G:F, g/kg | 592 | 624 | 647 | 633 | 619 | 643 | 655 | 665 | 9.9 | <0.001 | 0.732 | <0.001 | 0.190 |
| Bone ash, gf | 0.58 | 0.66 | 0.81 | 0.68 | 0.71 | 0.75 | 0.74 | 0.92 | 0.031 | <0.001 | 0.344 | <0.001 | 0.683 |
| Bone ash, %f | 45.1 | 44.6 | 51.8 | 48.7 | 49.2 | 50.0 | 51.5 | 52.2 | 0.890 | <0.001 | 0.001 | <0.001 | 0.066 |
aA total of 320 nursery pigs (DNA 241 × 600, initially 10.4 kg BW) were used in a 21-day growth study with 5 pigs per pen and 8 pens per treatment.
bADG, average daily gain; ADFI, average daily feed intake; G:F, gain-to-feed ratio.
cInorganic P was added to the diet by increasing monocalcium P.
dSmizyme TS G5 2,500, Origination, LLC., Maplewood, MN.
eSEM, standard error of the mean.
fOne pig per pen (eight pens per treatment) was euthanized and fibulas were collected to determine bone ash weight and percentage bone ash. A total of 64 fibulas (one from each animal) were autoclaved for 1 h and then placed in Soxhlet extractors containing petroleum ether for 7 d as a means of removing water and fat. They were then dried at 105 °C for 24 h, and ashed at 600 °C for 24 h.
For bone composition, pigs fed increasing aP from inorganic P had increased (linear, P < 0.001) bone ash weights, resulting in increased (quadratic, P < 0.001) percentage bone ash, while those fed increasing phytase had increased bone ash weights (linear, P < 0.001) and percentage bone ash (linear, P < 0.001 and quadratic, P = 0.066).
The percentage aP released from Smizyme TS G5 2,500 varied depending on the response criteria used (Table 6). As the amount of phytase in the diet increased, the calculated aP release increased linearly (P < 0.001) when using ADG, G:F, or bone ash weight as the indicator of release. When using percentage bone ash as the indicator of release, aP increased in a linear and quadratic fashion (linear, P < 0.001 and quadratic, P = 0.028).
Table 6.
Calculated aP release values based on different response criteria, experiment 1a,b
| Phytase, FTU/kgc | Probability, P < | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 150 | 250 | 500 | 750 | 1,000 | SEMd | Linear | Quadratic |
| ADG | 0.031 | 0.052 | 0.094 | 0.109 | 0.139 | 0.019 | <0.001 | 0.184 |
| G:F | 0.098 | 0.058 | 0.117 | 0.148 | 0.166 | 0.022 | <0.001 | 0.096 |
| Bone ash weighte | 0.066 | 0.078 | 0.104 | 0.096 | 0.191 | 0.017 | <0.001 | 0.847 |
| Percentage bone ashe | 0.095 | 0.102 | 0.114 | 0.142 | 0.149 | 0.014 | <0.001 | 0.028 |
aThe marginal intake of available P (aP) per day was calculated for each pen using the equation: dietary aP% minus 0.12% (the aP in the basal diet) multiplied by ADFI. A standard curve was then developed for each response criteria using the marginal aP release as the predictor variable. The equation for the standard curve was used to calculate aP release from each pen fed the different phytase dosages based on the observed value for each response criteria.
bADG, average daily gain; G:F, gain-to-feed ratio.
cSmizyme TS G5 2,500 (Origination, LLC., Maplewood, MN).
dSEM, standard error of the mean.
eOne pig per pen (eight pens per treatment) was euthanized and fibulas were collected to determine bone ash weight and percentage bone ash. A total of 64 fibulas (one from each animal) were autoclaved for 1 h and then placed in Soxhlet extractors containing petroleum ether for 7 d as a means of removing water and fat. They were then dried at 105 °C for 24 h, and ashed at 600 °C for 24 h.
Experiment 2
From d 0 to 21, pigs fed increasing aP from either inorganic P (linear, P < 0.001; Table 7) or phytase (linear, P < 0.001 and quadratic, P < 0.05) had improved performance across all growth response criteria measured.
Table 7.
Effects of increasing aP from inorganic P or Smizyme TS G5 2,500 phytase on nursery pig growth performance and bone ash values, experiment 2a,b
| Inorganic P, % aPc | Phytase, FTU/kgd | Inorganic P | Phytase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0.11% | 0.19% | 0.27% | 250 | 500 | 1,000 | 1,500 | SEMe | Linear | Quadratic | Linear | Quadratic |
| BW, kg | ||||||||||||
| d 0 | 10.4 | 10.3 | 10.4 | 10.3 | 10.3 | 10.4 | 10.3 | 0.15 | 0.952 | 0.252 | 0.963 | 0.768 |
| d 21 | 20.6 | 21.9 | 23.4 | 21.5 | 22.4 | 22.6 | 22.9 | 0.34 | <0.001 | 0.881 | <0.001 | <0.001 |
| d 0–21 | ||||||||||||
| ADG, g | 485 | 553 | 620 | 532 | 574 | 581 | 600 | 10.8 | <0.001 | 0.957 | <0.001 | <0.001 |
| ADFI, g | 810 | 874 | 937 | 843 | 888 | 910 | 914 | 20.0 | <0.001 | 0.979 | <0.001 | 0.031 |
| G:F, g/kg | 600 | 633 | 664 | 631 | 646 | 639 | 658 | 7.2 | <0.001 | 0.853 | <0.001 | 0.045 |
| Bone ash, gf | 0.60 | 0.70 | 0.87 | 0.69 | 0.74 | 0.85 | 0.87 | 0.032 | <0.001 | 0.449 | <0.001 | 0.061 |
| Bone ash, %f | 47.3 | 50.1 | 53.5 | 50.4 | 50.5 | 52.5 | 52.9 | 0.694 | <0.001 | 0.696 | <0.001 | 0.035 |
aA total of 280 nursery pigs (DNA 241 × 600, initially 10.3 kg BW) were used with 5 pigs per pen and 8 pens per treatment.
bADG, average daily gain; ADFI, average daily feed intake; G:F, gain-to-feed ratio.
cInorganic P was added to the diet by increasing monocalcium P.
dSmizyme TS G5 2,500, Origination, LLC., Maplewood, MN.
eSEM, standard error of the mean.
fOne pig per pen (eight pens per treatment) was euthanized and fibulas were collected to determine bone ash weight and percentage bone ash. A total of 56 fibulas (one from each animal) were autoclaved for 1 h and then placed in Soxhlet extractors containing petroleum ether for 7 d as a means of removing water and fat. They were then dried at 105 °C for 24 h, and ashed at 600 °C for 24 h.
For bone composition, pigs fed increasing aP from inorganic P had increased (linear, P < 0.001) bone ash weights and percentage bone ash, while those fed increasing phytase had increased bone ash weights (linear, P < 0.001 and quadratic, P = 0.061) and percentage bone ash (linear, P < 0.001 and quadratic, P = 0.035).
Similar to experiment 1, the percentage aP released from Smizyme TS G5 2,500 in experiment 2 varied depending on the response criteria used (Table 8). As the amount of phytase in the diet increased, the calculated aP release increased when using ADG (linear and quadratic, P < 0.001), G:F (linear, P < 0.001 and quadratic, P = 0.066), or bone ash weight (linear, P < 0.001 and quadratic, P = 0.059) as the indicator of release. Likewise, when using percentage bone ash, the calculated aP release increased linearly (P < 0.001) with a quadratic tendency (P = 0.055).
Table 8.
Calculated aP release values based on different response criteria, experiment 2a,b
| Phytase, FTU/kgc | Probability, P < | ||||||
|---|---|---|---|---|---|---|---|
| Item | 250 | 500 | 1,000 | 1,500 | SEMd | Linear | Quadratic |
| ADG | 0.057 | 0.107 | 0.112 | 0.136 | 0.013 | <0.001 | <0.001 |
| G:F | 0.083 | 0.123 | 0.100 | 0.154 | 0.022 | <0.001 | 0.066 |
| Bone ash weighte | 0.060 | 0.094 | 0.165 | 0.173 | 0.023 | <0.001 | 0.059 |
| Percentage bone ashe | 0.088 | 0.091 | 0.143 | 0.152 | 0.024 | <0.001 | 0.055 |
aThe marginal intake of available P (aP) per day was calculated for each pen using the equation: dietary aP% minus 0.11% (the aP in the basal diet) multiplied by ADFI. A standard curve was then developed for each response criteria using the marginal aP release as the predictor variable. The equation for the standard curve was used to calculate aP release from each pen fed the different phytase dosages based on the observed value for each response criteria.
bADG, average daily gain; G:F, gain-to-feed ratio.
cSmizyme TS G5 2,500 (Origination, LLC., Maplewood, MN).
dSEM, standard error of the mean.
eOne pig per pen (eight pens per treatment) was euthanized and fibulas were collected to determine bone ash weight and percentage bone ash. A total of 56 fibulas (one from each animal) were autoclaved for 1 h and then placed in Soxhlet extractors containing petroleum ether for 7 d as a means of removing water and fat. They were then dried at 105 °C for 24 h, and ashed at 600 °C for 24 h.
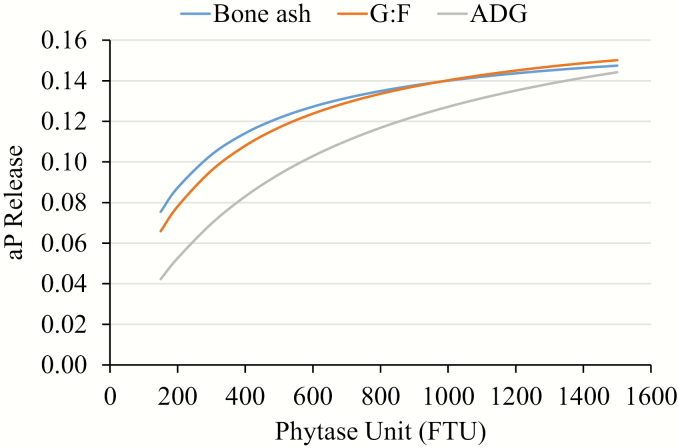
Combining the release values for both experiments, the release equations for Smizyme TS G5 2,500 are aP = (0.197 × FTU)/(584.956 + FTU), aP = (0.175 × FTU)/(248.348 + FTU), and aP = (0.165 × FTU)/(178.146 + FTU) when ADG, G:F, and percentage bone ash, respectively, where used as the predictor variable (Figure 1).
Figure 1.
Available P release curve for Smizyme TS G5 2,500 phytase as predicted by ADG, G:F, and percentage bone ash.
DISCUSSION
Smizyme TS G5 used in the present studies is a bacterial derived 6-phytase produced through the fermentation of E. coli in a Pichia Pastoris yeast medium. The efficacy of different phytase sources to release P is affected by the solubility of phytate bound P in feedstuffs, which influences its susceptibility to hydrolysis (Létourneau-Montminy et al., 2012). Additionally, the position of hydrolysis onset, preferred pH conditions, and temperature stability of the phytase further impact its efficacy (Humer et al., 2015). In vitro studies have shown that the optimal pH for E. coli derived phytase activity is between 2 and 4.5 (Adeola et al., 2004). A model conducted by Létourneau-Montminy et al. (2011) indicated that microbial phytases are largely active in the upper portion of the digestive tract, or stomach of the pig, where more acidic conditions are found. Because P solubility, hydrolysis, and absorption are affected by the physicochemical conditions along the digestive tract, microbial phytase activity is observed to significantly decrease in the small intestine due to increasing pH (Létourneau-Montminy et al., 2011). Rather, the majority of free P achieved from phytate hydrolysis in the stomach is absorbed in the small intestine. Any remaining concentrations of phytate bound P cleaved in the hindgut are of little physiological importance because P released in the hindgut is not absorbed (Rutherfurd et al., 2014; Humer et al., 2015).
Phosphorus is involved in many biochemical pathways, including soft tissue growth and skeletal integrity (Crenshaw, 2001). Jendza et al. (2006) evaluated the efficacy of an E. coli-derived phytase to determine its equivalency relative to P from monosodium phosphate. They determined that the equivalency requirement to maximize bone mineralization is more sensitive than the requirement needed to maximize growth performance. Several other studies have confirmed that lean growth and structural integrity are often independent of one another (Augspurger et al., 2003; Veum et al., 2006), therefore providing different aP release predictions based on response criteria (Jones et al., 2010; Gourley et al., 2018). In our studies, we also observed that the aP release with incrementally increasing phytase was different depending on the response criteria measured. We chose to use 10 kg nursery pigs to determine the phosphorus release of Smizyme phytase based on previous research (Gourley et al., 2018). Young growing pigs have a higher P requirement than finishing pigs, therefore diets with a wide range of P levels can be fed and still be below the pig’s P requirement. It was also observed that as the inclusion of inorganic P or phytase in the diet increased, percentage bone ash increased. This confirms that changes in nutritional status have an impact on bone ash percent (Crenshaw, 2001).
Multiple experiments have been conducted to determine the efficacy of microbial phytases on mineral digestibility, growth performance, or bone characteristics (Adeola et al., 2004; Jendza et al., 2006; Veum et al., 2006; Jones et al., 2010). By adding graded levels of inorganic P or exogenous phytase to P deficient diets and evaluating percentage bone ash, phytase activity and aP release can be determined. Due to differences in phytase characteristics and stability, phytase sources often differ in the amount of P released per FTU included in the diet (Goncalves et al., 2016). One FTU can be defined as the amount of enzyme activity that liberates 1 µmol inorganic orthophosphate per minute from 0.0051 mol L−1 sodium phosphate at a pH of 5.5 and 37 °C (AOAC, 2006). Numerous analytical methods have been developed to determine phytase activity, including the commonly used AOAC Official Method 2000.12 (Jones et al., 2010). However, due to the intrinsic differences between phytase sources, it is important when new or enhanced phytases enter the marketplace to evaluate their unique aP release.
Currently, there is limited research regarding the use of Smizyme phytase in swine diets. Arredondo et al. (2019) conducted a 12-d study to evaluate the apparent total tract digestibility (ATTD) of nutrients in growing pigs fed 250–2,500 FTU/kg Smizyme TS G5 in P-deficient diets. Results indicated that as phytase dose increased, the ATTD of Ca and P, and the standardized total tract digestibility of P increased in a linear and quadratic fashion (P < 0.01). In a broken line analysis, 1,041 and 1,107 FTU were needed to maximize the ATTD of Ca and P, respectively. Similarly, in the present studies when evaluating growth performance and bone ash, increasing phytase inclusion from 150 to 1,500 FTU resulted in linear and quadratic improvements in ADG, G:F, and percentage bone ash. When increasing phytase concentration up to 1,500 FTU a quadratic response was observed, indicating diminishing marginal improvements as phytase dose increases beyond 1,000 FTU. Zhang et al. (2000) also reported that growth criteria and bone ash percent response to phytase are better represented by a non-linear equation.
These studies have provided a range of aP release for Smizyme TS G5 2,500 phytase in nursery pigs weighing 10–21 kg when fed at levels between 150 and 1,500 FTU/kg. In summary, the magnitude of aP release at different FTU inclusion rates depends on the response criteria measured. When comparing the amount of phytase needed to reach a particular aP release value in the diet (Goncalves et al., 2016), Smizyme TS G5 2,500 appears to have a similar aP release to other commercially available phytase sources based on manufacture recommendation.
ACKNOWLEDGMENTS
Contribution no. 20-183-J of the Kansas Agricultural Experiment Station, Manhattan, 66506-0201. Appreciation is expressed to Origination, LLC. (Maplewood, MN) for partial financial support; and to Hilda I. Calderón for her statistical assistance.
Conflict of interest statement. The authors declare no conflict of interest; however, J.M.F. works for the company that provided partial financial support and markets the phytase used in these studies. B.L.G. is also involved with the manufacturing and marketing of the phytase.
LITERATURE CITED
- Adeola O., Sands J. S., Simmins P. H., and Schulze H.. . 2004. The efficacy of an Escherichia coli-derived phytase preparation. J. Anim. Sci. 82:2657–2666. doi: 10.2527/2004.8292657x [DOI] [PubMed] [Google Scholar]
- AOAC 2000. Official methods of analysis of AOAC international. 17th ed. Gaithersburg, (MD): Association of Official Analytical Chemists. [Google Scholar]
- AOAC. 2006. Official methods of analysis AOAC international. 18th ed. Arlington (VA): Association of Official Analytical Chemists. [Google Scholar]
- Arredondo M. A., Casas G. A., and Stein H. H.. . 2019. Increasing levels of microbial phytase increases the digestibility of energy and minerals in diets fed to pigs. Anim. Feed Sci. Technol. 248:27–36. doi: 10.1016/j.anifeedsci.2019.01.001 [DOI] [Google Scholar]
- Augspurger N. I., Webel D. M., Lei X. G., and Baker D. H.. . 2003. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci. 81:474–483. doi: 10.2527/2003.812474x [DOI] [PubMed] [Google Scholar]
- Crenshaw T. D. 2001. Calcium, phosphorus, vitamin D, and vitamin K in swine nutrition. In: Lewis A. and Southern L. L., editors. Swine nutrition. 2nd ed. Boca Raton (FL): CRC Press; p. 196–221. [Google Scholar]
- Cromwell G. L. 2009. ASAS centennial paper: landmark discoveries in swine nutrition in the past century. J. Anim. Sci. 87:778–792. doi: 10.2527/jas.2008-1463 [DOI] [PubMed] [Google Scholar]
- Cromwell G. L., Stahly T. S., Coffey R. D., Monegue H. J., and Randolph J. H.. . 1993. Efficacy of phytase in improving the bioavailability of phosphorus in soybean meal and corn-soybean meal diets for pigs. J. Anim. Sci. 71:1831–1840. doi: 10.2527/1993.7171831x [DOI] [PubMed] [Google Scholar]
- Goncalves M. A. D., Dritz S. S., Tokach M. D., DeRouchey J. M., Woodworth J. C., and Goodband R. D.. . 2016. Fact sheets—comparing phytase sources for pigs and effects of superdosing phytase on growth performance of nursery and finishing pigs. J. Swine Health Prod. 24:97–101. [Google Scholar]
- Gourley K. M., Woodworth J. C., DeRouchey J. M., Dritz S. S., Tokach M. D., and Goodband R. D.. . 2018. Determining the available phosphorus release of Natuphos E 5,000 G phytase for nursery pigs. J. Anim. Sci. 96:1101–1107. doi: 10.1093/jas/sky006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humer E., Schwarz C., and Schedle K.. . 2015. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 99:605–625. doi: 10.1111/jpn.12258 [DOI] [PubMed] [Google Scholar]
- Jendza J. A., Dilger R. N., Sands J. S., and Adeola O.. . 2006. Efficacy and equivalency of an Escherichia coli-derived phytase for replacing inorganic phosphorus in the diets of broiler chickens and young pigs. J. Anim. Sci. 84:3364–3374. doi: 10.2527/jas.2006-212 [DOI] [PubMed] [Google Scholar]
- Jones C. K., Tokach M. D., Dritz S. S., Ratliff B. W., Horn N. L., Goodband R. D., DeRouchey J. M., Sulabo R. C., and Nelssen J. L.. . 2010. Efficacy of different commercial phytase enzymes and development of an available phosphorus release curve for Escherichia coli-derived phytases in nursery pigs. J. Anim. Sci. 88:3631–3644. doi: 10.2527/jas.2010-2936 [DOI] [PubMed] [Google Scholar]
- Lei X. G., Weaver J. D., Mullaney E., Ullah A. H., and Azain M. J.. . 2013. Phytase, a new life for an “old” enzyme. Annu. Rev. Anim. Biosci. 1:283–309. doi: 10.1146/annurev-animal-031412-103717 [DOI] [PubMed] [Google Scholar]
- Létourneau-Montminy M. P., Jondreville C., Sauvant D., and Narcy A.. . 2012. Meta-analysis of phosphorus utilization by growing pigs: effect of dietary phosphorus, calcium and exogenous phytase. Animal 6:1590–1600. doi: 10.1017/S1751731112000560 [DOI] [PubMed] [Google Scholar]
- Létourneau-Montminy M. P., Narcy A., Lescoat P., Magnin M., Bernier J. F., Sauvant D., Jondreville C., and Pomar C.. . 2011. Modeling the fate of dietary phosphorus in the digestive tract of growing pigs. J. Anim. Sci. 89:3596–3611. doi: 10.2527/jas.2010-3397 [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington (DC): National Academy Press. [Google Scholar]
- Ravindran V., Ravindran G., and Sivalogan S.. . 1994. Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem. 50:133–136. doi: 10.1016/0308-8146(94)90109-0. [DOI] [Google Scholar]
- Rutherfurd S. M., Chung T. K., and Moughan P. J.. . 2014. Effect of microbial phytase on phytate P degradation and apparent digestibility of total P and Ca throughout the gastrointestinal tract of the growing pig. J. Anim. Sci. 92:189–197. doi: 10.2527/jas.2013-6923. [DOI] [PubMed] [Google Scholar]
- Selle P. H., and Ravindran V.. . 2008. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 113:99–122. doi: 10.1016/j.livsci.2007.05.014 [DOI] [Google Scholar]
- Simons P. C. M., and Versteegh H. A. J.. . 1990. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br. J. Nutr. 64:525–540. doi: 10.1079/bjn19900052 [DOI] [PubMed] [Google Scholar]
- Veum T. L., Bollinger D. W., Buff C. E., and Bedford M. R.. . 2006. A genetically engineered Escherichia coli phytase improves nutrient utilization, growth performance, and bone strength of young swine fed diets deficient in available phosphorus. J. Anim. Sci. 84:1147–1158. doi: 10.2527/2006.8451147x [DOI] [PubMed] [Google Scholar]
- Zhang Z. B., Kornegay E. T., Radcliffe J. S., Wilson J. H., and Veit H. P.. . 2000. Comparison of phytase from genetically engineered Aspergillus and canola in weanling pig diets. J. Anim. Sci. 78:2868–2878. doi: 10.2527/2000.78112868x [DOI] [PubMed] [Google Scholar]