Abstract
Atrial fibrillation is a common and morbid arrhythmia. Stroke is a major hazard of atrial fibrillation and may be preventable with oral anticoagulation. Yet since atrial fibrillation is often asymptomatic, many individuals with atrial fibrillation may be unaware and do not receive treatment that could prevent a stroke. Screening for atrial fibrillation has gained substantial attention in recent years as several studies have demonstrated that screening is feasible. Advances in technology have enabled a variety of approaches to facilitate screening for atrial fibrillation using both medical-prescribed devices as well as consumer electronic devices capable of detecting atrial fibrillation. Yet controversy about the utility of atrial fibrillation screening remains owing to concerns about potential harms resulting from screening in the absence of randomized data demonstrating effectiveness of screening on outcomes such as stroke and bleeding. In this review we summarize current literature, present technology, population-based screening considerations, and consensus guidelines addressing the role of atrial fibrillation screening in practice.
Keywords: atrial fibrillation, screening, stroke, ECG, wearable technology, atrial flutter
Why Screening?
The burden of atrial fibrillation
Atrial fibrillation (AF) is a common arrhythmia, estimated to affect at least 33.5 million individuals worldwide.1 The prevalence of AF is expected to rise to nearly 12 million people in the US by 2030 and 17.9 million people in the European Union by 2060, in part due to aging populations.2,3 The financial impact of AF on both US and European healthcare systems is substantial.4–6
Individuals with AF are at increased risk for morbidity and death, largely due to heart failure and ischemic stroke.7,8 There is also increasing recognition of an association between AF and cognitive decline, which may be related in part to both overt and silent strokes.9 Fortunately, oral anticoagulation (OAC) is highly effective for preventing strokes related to AF.10–12 However, AF is frequently asymptomatic, and up to 5% of individuals with a diagnosis of AF present with stroke as the first clinically-evident manifestation of their arrhythmia.13
Rationale for screening and current recommendations
Population-based screening for AF has several potential benefits, including identification of individuals with unrecognized AF who would benefit from OAC to prevent stroke, as well as an opportunity to implement more promptly interventions known to reduce AF burden and symptoms (Figure 1).14–16 As discussed below, however, randomized trials of AF screening have not demonstrated a reduction in stroke or other hard outcomes, although adequately powered studies have not yet reported. Furthermore, the potential benefits gained from early identification and treatment of screen-detected AF must be balanced against the potential harms and uncertainties posed by screening. First, even very specific screening tests (e.g., 12-lead ECG) applied at the population level will result in false positive diagnoses, potentially leading to excess bleeding associated with unnecessary OAC use.17–23 Second, prolonged application of continuous forms AF monitoring increase the likelihood of detecting short episodes of AF,24–26 which may be of uncertain clinical significance.27–29 Given emerging evidence that AF burden is related to stroke risk, it remains unclear whether very infrequent and short AF episodes detectable only through continuous screening increase the risk of stroke sufficiently to merit OAC.27–30 Third, not all forms of rhythm monitoring are sufficient to establish a diagnosis of AF. For example, some forms of rhythm detection may frequently lead to indeterminate results,31 while others require confirmatory, electrocardiographic testing,32 contributing to patient anxiety, increased costs, and further risk of false positive results.
Figure 1.
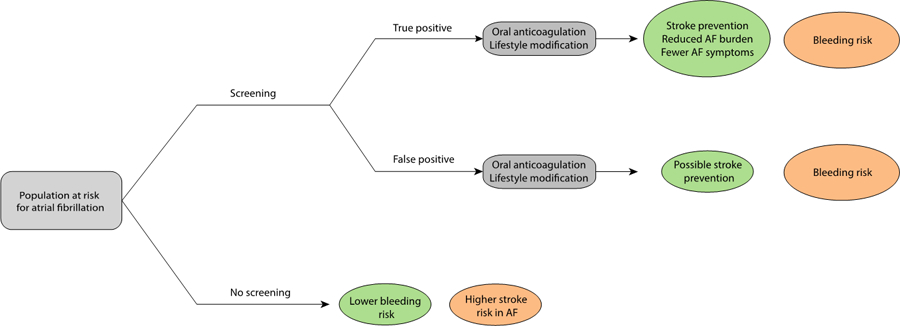
Rationale, risks, and benefits of atrial fibrillation screening.
The rationale for atrial fibrillation screening is depicted, as well as the relevant benefits (green) and risks (orange) expected with each approach. Individuals with undiagnosed incident AF are at risk for developing cardioembolic stroke prior to initiation of risk-based anticoagulation. Screening may lead to earlier diagnosis of AF, initiation of risk-based anticoagulation to prevent strokes, and an opportunity to institute risk factor modification strategies (e.g., weight loss, alcohol cessation, blood pressure control, sleep apnea management) to reduce AF symptoms and burden.14,15,62,63 For true positives (individuals with AF correctly identified as having AF using screening), the benefits may outweigh the risk of bleeding conferred by anticoagulation. For false positives (individuals without AF incorrectly identified as having AF using screening), the risk of bleeding likely outweighs any potential benefit of anticoagulation on non-AF related stroke. Without screening, fewer cases of otherwise undiagnosed AF will be identified, leading to lower overall bleeding risk from a lower rate of anticoagulation, but also more strokes resulting from unrecognized AF.
Consistent with uncertainty surrounding the balance of benefit and harm of population-based screening for AF, consensus guidelines offer varying endorsements (summarized in Table 1). For example, the European Society of Cardiology provides a class I recommendation for performing AF screening by “pulse taking or ECG rhythm strip” in individuals aged greater than 65 years.33 The National Heart Foundation of Australia and New Zealand provides a “strong” recommendation for “opportunistic point-of-care screening in the clinic or community” in individuals aged 65 or older by pulse palpation followed by ECG or an ECG rhythm strip (including a handheld ECG).34 The US Preventive Services Task Force indicates that evidence is insufficient to “assess the balance of benefits and harms” of AF screening using ECG at present.35
Table 1.
Summary of guideline recommendations and consensus statements pertaining to AF screening
| Society | Year | Recommendation | Class / strength of recommendation | Level / quality of evidence |
|---|---|---|---|---|
| European Society of Cardiology33 | 2016 | Opportunistic screening for AF is recommended by pulse taking or ECG rhythm strip in patients >65 years of age | I | B |
| Systematic ECG screening may be considered to detect AF in patients aged 75 years or older, or those at high stroke risk | IIb | B | ||
| National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand34 | 2018 | Opportunistic point-of-care screening in the clinic or community should be conducted in people aged 65 years or more | Strong | Moderate |
| United States Preventive Services Task Force35 | 2018 | The current evidence is insufficient to assess the balance of benefits and harms of screening for AF with electrocardiography | I | - |
| European Heart Rhythm Association (endorsed by Heart Rhythm Society, Asia Pacific Heart Rhythm Society, and Sociedad Latinoamericana de Estiulacion Cardiaca y Electrofisiologia)71 | 2017 | Opportunistic screening for AF is recommended by ESC guidelines in persons aged 65 years or older | Recommended | - |
| Systematic ECG screening may be considered to detect AF in patients aged 75 years or older, or those at high stroke risk | Recommended | - | ||
| ECG confirmation of AF is needed before considering the patient for anticoagulation therapy | Recommended | - | ||
| Repeated recordings can be considered to document AF in selected asymptomatic patients | May be used | - | ||
| When performed in high risk populations, screening for AF is advised because of its cost-effectiveness | May be used | - |
AF-SCREEN36, an international consensus group founded to promote discussion and research about AF screening as a strategy to reduce stroke and death, as well as provide advocacy for implementation of country-specific AF screening programs, has also issued several documents summarizing evidence relevant to AF screening and providing expert consensus recommendations. The recommendations of AF-SCREEN are generally similar to those of the European Society of Cardiology, and include single-timepoint screening for all individuals aged ≥ 65 years, as well as more intensive screening (e.g., 2 weeks of twice-daily intermittent monitoring) for individuals aged ≥ 75 years or at elevated risk for stroke.37 Additional emphases made by SCREEN-AF include the importance of linking AF screening to a treatment pathway, as well as a preference for screening using devices capable of generating an ECG waveform.37
Screening versus empiric treatment
As with any disease for which screening is being considered, the value of screening is diminished if the relevant treatment is sufficiently safe and effective that its administration to all at-risk individuals provides net benefit.38 To this end, there has been substantial interest in identifying whether it is possible to identify individuals at sufficiently high risk for AF and stroke that the empiric use of OAC may be justified, such as individuals with embolic stroke of undetermined source (ESUS). Subclinical AF is observed on continuous cardiac rhythm monitoring in approximately 30% of individuals within 3 years of an stroke of undetermined etiology, implicating occult cardioembolism as a likely stroke etiology.39 Yet two large randomized trials in patients with ESUS have raised concerns about this approach. Empiric use of OAC with rivaroxaban or dabigatran did not reduce the risk of recurrent stroke as compared to aspirin in the New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE-ESUS)40 or Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of the Oral Thrombin Inhibitor Dabigatran Etexilate versus Acetylsalicylic Acid in Patients with Embolic Stroke of Undetermined Source (RE-SPECT ESUS) trials, respectively.41 Moreover, the risk of major bleeding was increased approximately two-fold with OAC in NAVIGATE-ESUS. Apixaban for Treatment of Embolic Stroke of Undetermined Source (ATTICUS) and AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke (ARCADIA) are additional trials comparing apixaban to aspirin among patients with ESUS and are currently underway.42,43 In contrast to empiric use, OAC treatment in individuals confirmed to have subclinical AF using continuous monitoring following stroke of unclear etiology has been shown to reduce recurrent stroke.39 As a result, on the basis of available evidence, it appears that monitoring to document AF is likely to remain the clinical standard even in patients in whom a high suspicion for AF exists.
What has been done?
Overview of previous AF screening studies
Multiple studies have investigated the feasibility of population-based AF screening. A summary of the findings of non-randomized studies is listed in Table 2 and a summary of randomized trials is listed in Table 3. Screening has consistently been reported to lead to higher rates of AF detection. The yield of screening for identifying previously undiagnosed AF has generally ranged from about 0.1% to 5%, with a higher incidence among older individuals and with the use of continuous screening methods.44 The test characteristics of AF screening have been reported to vary substantially by screening modality, with sensitivities ranging from 86–100% and specificities ranging from 75–98% (Table 4). Although screening studies have not demonstrated a reduction in hard outcomes such as stroke or mortality, studies adequately powered to detect such an effect have not yet reported.
Table 2.
Summary of non-randomized studies of atrial fibrillation screening
| Author/study | Year | Country | Setting | Screened age (years) | N | Modality/approach | Screening period | New atrial fibrillation detection rate* |
|---|---|---|---|---|---|---|---|---|
| Wheeldon et al.72 | 1998 | United Kingdom | Primary care | ≥ 65 | 1,422 | 12-lead ECG | One-time | 5.4% |
| Engdahl et al.55 | 2013 | Sweden | Community invitation | 75–76 | 848 | 12-lead ECG, then 2-week single-lead handheld ECG twice daily | 2 weeks | 1.0% (initial assessment) 3.5% (2 weeks) |
| Lowres et al. (SEARCH-AF)58 | 2014 | Australia | Pharmacy | ≥ 65 | 1,000 | Single-lead handheld ECG | One-time | 1.5% |
| Kearley et al.53 | 2014 | United Kingdom | Primary care | ≥ 75 | 1,000 | Single-lead handheld ECG, blood pressure monitor | One-time | 1.4% |
| Svennberg et al. (STROKESTOP)54 | 2015 | Sweden | Community invitation | 75–76 | 7,173 | 12-lead ECG, then 2-week single-lead handheld ECG twice daily | 2 weeks | 0.5% (initial assessment) 3.0% (2 weeks) |
| Kaassenbrood et al.57 | 2016 | Netherlands | Influenza vaccination | ≥ 60 | 3,269 | Single-lead handheld ECG | One-time | 1.1% |
| Chan et al.73 | 2016 | Hong Kong | Community invitation | ≥ 18 | 13,122 | Single-lead handheld ECG | One-time | 0.8% |
| Sandhu et al. | 2016 | Canada | Pharmacy | ≥ 65 | 1,145 | Single-lead handheld ECG | One-time | 2. |
| Chan et al.32 | 2017 | Hong Kong | Primary care | ≥ 65 | 5,969 | Single-lead handheld ECG, blood pressure monitor | One-time | 1.2% |
| Quinn et al.52 | 2018 | Canada | Primary care | ≥ 65 | 2,171 | Pulse palpation, single-lead handheld ECG, blood pressure monitor | One-time | 0.6% |
| Perez et al.47 | 2019 | United States | Consumer volunteers | ≥ 22 | 419,297 | PPG-based smart-watch followed by ECG patch in subset | 9 months | 0.52%† 0.036% (confirmed AF) |
| Guo et al. (Huawei Heart Study)60 |
2019 | China | Consumer volunteers | ≥ 18 | 187,912 | PPG-based smart-watch or band followed by 12-lead ECG in subset | 2 weeks to 6 months | 0.23%† 0.12% (confirmed AF) |
at initial assessment unless otherwise specified
abnormal pulse notification only
Table 3.
Summary of randomized trials of atrial fibrillation screening
| Author/study | Year | Country | Setting | Screened age (years) | N | Modality/approach | Screening period | New atrial fibrillation detection rate |
|---|---|---|---|---|---|---|---|---|
| Morgan and Mant.22 | 2002 | United Kingdom | Primary care | All | Systematic (N=1,499) | Pulse assessment and lead II rhythm strip | 6 months | 0.8% |
| Opportunistic (N=1,502) | Clinical pulse assessment with discretionary 12-lead ECG if abnormal | 6 months | 0.5% | |||||
| Fitzmaurice et al. (SAFE)49 | 2007 | United Kingdom | Primary care | ≥ 65 | Systematic (N=4,933) | Pulse assessment and 12-lead ECG | One-time | 1.62% |
| Opportunistic (N=4,933) | Pulse assessment and 12-lead ECG if abnormal | One-time | 1.64% | |||||
| No screening (N=4,936) | - | - | 1.04% | |||||
| Halcox et al. (REHEARSE-AF)59 | 2017 | United Kingdom | Primary care or research visits | > 65 | Screening (N=501) | Single-lead handheld ECG twice weekly | 12 months | 3.7% |
| No screening (N=500) | - | - | 1.0% | |||||
| Steinhubl et al. (mSToPS)48 | 2018 | United States | Health plan enrollees | ≥ 75, ≥ 55 (male with 1 AF/stroke risk factor), ≥ 65 (female with 1 AF/stroke risk factor) | Immediate screening (N=1,366) | Single-lead patch monitor for up to 14 days (screened period) | 4 months | 3.9% |
| Delayed screening (N=1,293) | Single-lead patch monitor for up to 14 days (unscreened period) | 4 months | 0.9% |
Table 4.
Ongoing studies of AF screening
| Study | Country | Status | N | Modality | Approach | Endpoint |
|---|---|---|---|---|---|---|
| DANCAVAS74 | Denmark | Nearly complete | 35,000 | 3-lead ECG | One-time | All-cause mortality |
| STROKESTOP II66 | Sweden | Nearly complete | 8,000 | Zenicor single-lead ECG | Twice daily for 14 days | New-onset AF Initiation of OAC |
| SCREEN-AF75 | Canada | Recently completed | 856 | Zio patch monitor, WatchBP oscillometric device | 2 weeks at start and at 3 months | New-onset AF |
| AF-CATCH76 | China | Follow-up | 7,641 | AliveCor single-lead ECG | 1x/yr vs. 4x/yr vs. 1x/wk in 1st month | New-onset AF |
| MonDAFIS77 | Germany | Follow-up | 3,470 | Mobile telemetry | Post-stroke, up to 7 days | Oral anticoagulation |
| D2AF78 | Netherlands | Ongoing | 19,200 | MyDiagnostick single-lead ECG, WatchBP oscillometric device | One-time | New-onset AF |
| LOOP79 | Denmark | Ongoing | 6,000 | Implantable loop recorder | 4 years continuous | Stroke or systemic embolism |
| mSToPS48 | United States | Ongoing | 6,000 | Zio patch monitor | 2–4 weeks | Stroke, systemic embolism, myocardial infarction, death |
| VITAL-AF80 | United States | Ongoing | 35,000 | AliveCor single-lead ECG | At each encounter | New-onset AF |
| GUARD-AF56 | United States | Ongoing | 52,000 | Zio patch monitor | 2 weeks | Stroke Major bleeding |
| London Pharmacy Study81 | United Kingdom | Starting soon | 600 | Pulse palpation, AliveCor single-lead ECG | One-time | Test accuracy of screening modalities |
| SAFER82 | United Kingdom | Ongoing | 120,000 | Zenicor single-lead ECG | Twice daily for 14 days | Stroke |
| Fitbit Heart Study61 | United States | Ongoing | 100,000 | Wrist-based PPG | Wearable PPG-based algorithm followed by ECG patch | New-onset AF |
Overview of AF screening modalities
There are many available methods to screen for asymptomatic AF, ranging from less expensive, intermittent methods with lower sensitivity and/or specificity17,20,22,45 to more expensive, continuous methods which can record electrocardiographic data for weeks to years at a time (Figure 2).46–48 Traditional modalities have included manual pulse palpation and 12-lead ECG. Newer devices include a variety of handheld, wearable and implantable technologies, including consumer-facing smartphones and watches/bands. Non-invasive oscillometric (e.g. blood pressure monitor) devices capable of detecting an irregular pulse are practical and widespread. Handheld devices (including apps integrated into smart phones) detect AF by generating a single-lead ECG as the user applies two fingers from each hand. Smart watches/bands monitor for arrhythmia using photoplethysmography, although the latest iterations have recently incorporated the ability to produce a single-lead ECG. It is important to recognize that automated interpretation of single-lead ECGs generated by handheld or wearable devices is typically performed by proprietary algorithms with varying complexity and accuracy, although all devices able to output an ECG tracing have the advantage that such tracings can later be read manually by a provider. Nevertheless, handheld or wearable single-lead ECG tracings still typically require confirmation with a traditional 12-lead ECG to ensure a proper diagnosis of AF, and substantial delays between a preliminary diagnosis of AF and a confirmatory test may reduce the effectiveness of a screening intervention. Continuous ECG-based methods (e.g. ambulatory ECG monitors, patches, and implantable cardiac monitors) are expensive, but have the greatest sensitivity for detecting AF.
Figure 2.
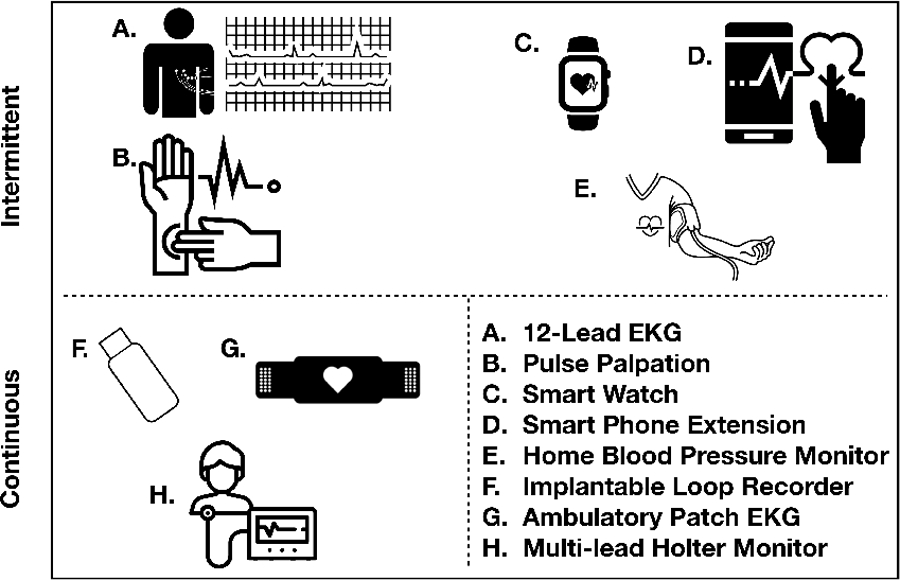
Overview of atrial fibrillation screening modalities Depicted is a summary of established modalities for atrial fibrillation screening.
Modalities in the top row (A-E) detect atrial fibrillation using intermittent assessment of cardiac rhythm, while modalities in the bottom row (F-H) detect atrial fibrillation through continuous monitoring.
While many devices have a role for the detection of AF, the most appropriate approach to deploy may ultimately depend on factors such as underlying AF and stroke risk, convenience, and costs. Clinicians, payers, and patients (who may also be consumers) are each likely to have different perspectives on optimal device selection, resulting in an array of different methods by which AF screening is likely to take place. Randomized trials and decision analytic models may help determine the most effective and efficient AF screening strategy to utilize in specific clinical contexts.
Review of previous studies by modality
Pulse palpation
Two randomized trials have investigated the efficacy of AF screening utilizing pulse palpation. Morgan and Mant22 randomized 3,001 individuals of all ages in four primary care practices in the UK to systematic screening implemented via trained nurses performing pulse palpation along with a lead II rhythm strip, versus “opportunistic screening”, defined as a reminder to document whether any pulses taken during routine clinical care were potentially suspicious for AF. In both cases, pulse palpation findings suspicious for AF were followed by a 12-lead ECG. At six months, 4.5% of individuals in the systematic screening arm had AF detected versus 1.3% of individuals in the opportunistic arm (mean difference 3.2%, 95% CI 2.0–4.4). Using any irregularity of the pulse as the positivity criterion, the sensitivity of pulse palpation was 91% and specificity was 74%. An important limitation of this study was that individuals with prevalent AF were not excluded, and 82% of individuals with screen-detected AF had a previous diagnosis of AF present in their medical record. This not only enriched the risk of AF beyond what might be expected in a screening population, but also introduced the potential for ascertainment bias.
The Screening for Atrial Fibrillation with Electrocardiography (SAFE) trial49 was a large, cluster-randomized clinical trial reported in 2007 investigating screening for AF using pulse palpation in 50 primary care centers in the United Kingdom. SAFE randomized 14,802 individuals aged ≥65 years to a) one-time screening with 12-lead ECG, b) one-time pulse palpation with a 12-lead ECG if the pulse felt irregular, and c) no screening. After 12 months, AF was detected in 1.63% of individuals in the intervention practices versus 1.04% in control practices (mean difference 0.59%, 95% CI 0.20–0.98). Rates of AF detection were nearly identical with 12-lead ECG screening (1.64%) versus pulse palpation with follow up 12-lead ECG (1.62%), despite the fact that 2,357 ECGs were performed in the former group versus only 238 in the latter. Economic analyses based on the results of SAFE have therefore suggested superior cost-effectiveness when pulse palpation is performed prior to 12-lead ECG.50 Of note, it is largely on the basis of the SAFE study that the European Society of Cardiology guidelines recommend screening with either 12-lead ECG or pulse palpation followed by 12-lead ECG if abnormal in individuals aged >65 years.51
Oscillometric devices
There have been several non-randomized studies investigating the efficacy of devices that leverage oscillometric technology (i.e., blood pressure cuff) to detect AF. In a Canadian study by Quinn et al.52, individuals aged ≥ 65 underwent AF screening using pulse palpation, single-lead ECG, or oscillometric device deployed within 22 primary care clinics. Individuals with a positive result on 1 or more tests underwent 12-lead ECG with or without 24-hour Holter monitor. Out of 2,052 participants who underwent all 3 screening tests, 56 had confirmed AF (prevalence of screen-detected AF 2.7%), of which 12 (0.6%) represented a new diagnosis of AF. Oscillometric screening demonstrated superior specificity when compared to pulse palpation, with a positive predictive value of 53.4% (95% CI 42.0–64.9) for AF. Notably, screening increased the proportion of AF patients receiving adequate anticoagulation (defined as taking a direct-acting OAC or warfarin with time in the therapeutic range > 65%) from 63% to 82%.
In a second study by Kearley et al. in the United Kingdom53, 1,000 ambulatory patients aged ≥ 75 in 6 general practices underwent AF screening with an oscillometric device or single-lead ECG with 12-lead ECG used as the reference standard. Of 1,000 individuals screened, 79 (7.9%) individuals had AF detected on 12-lead ECG, of which 11 (1.4% of the 889 individuals without known AF) represented a new AF diagnosis. The osillometric device had a sensitivity of 94.9% (95% CI 87.5–98.6) and a specificity of 89.7% (95% CI 87.5–91.6) for AF.
Chan et al. also assessed AF screening using an oscillometric device within a sample of 5,969 primary care patients in Hong Kong using single-lead ECG interpreted by cardiologists followed by confirmatory 12-lead ECG as the reference standard.32 Individuals enrolled were age ≥ 65 or had a diagnosis of hypertension or diabetes, and did not have prevalent AF. Of the 5,969 individuals screened, AF was diagnosed and confirmed by 12-lead ECG in 72 patients (1.21%). The oscillometric device demonstrated sensitivity of 80.6% (95% CI 69.5–88.9) and specificity of 98.7% (95% CI 98.3–98.9) for AF.
12-lead ECG
Several non-randomized studies have investigated the feasibility of traditional 12-lead ECG for AF screening. In the SAFE study described above, systematic deployment of 12-lead ECG led to a 0.60% absolute increase in AF diagnosis when compared to no screening.49 More recently, in the STROKESTOP study54, Svennberg et al. invited half of the population in two regions of Sweden aged 75–76 years to AF screening with 12-lead ECG followed by single-lead ECG monitoring for up to 14 days. The initial 12-lead ECG component of the screening intervention led to a new diagnosis of AF in 37 individuals (0.5% of the screened population). In a second Swedish study55, Engdahl et al. invited all inhabitants aged 75–76 years in a large municipality to a similar stepwise screening program with 12-lead ECG followed by intermittent single-lead ECG in selected individuals. The initial 12-lead component of the screening program resulted in a new diagnosis of AF in 10 individuals (1.2% of the screened population).
Patch Monitor
The mSToPS trial48 randomized 1,659 individuals to either immediate or delayed (after 4 months) AF screening with a single-lead patch monitor for up to 28 days. Eligibility for the study included age ≥ 75 years, male aged ≥ 55 years with at least 1 stroke risk factor, and female aged ≥ 65 years with at least 1 stroke risk factor. At 4 months, new AF was identified in 3.9% of the immediate screening group versus 0.9% in the delayed group (absolute difference 3.0%, 95% CI 1.8–4.1). At 1 year, new AF was identified at a rate of 6.7% per 100 person-years in individuals screened versus 2.6% per 100 person-years in matched controls who did not undergo any screening. Results of an observational comparison between the active and delayed screening arms with a matched control group on the incidence of stroke and other outcomes have not yet been reported.
The ongoing reducinG Stroke by Screening for UndiAgnosed atRial Fibrillation in Elderly inDividuals Study (GUARD-AF, ClinicalTrials.gov NCT4126486)56 is a randomized trial of AF screening using the Zio XT (iRhythm, San Francisco, CA) patch monitor deployed within primary care practices. GUARD-AF aims to enroll 52,000 individuals aged ≥ 70 years and is designed to detect a potential reduction in incident stroke rates with AF screening.
Single-lead handheld ECG
There has been substantial recent interest in screening for AF using handheld devices with the ability to produce single-lead ECG waveforms. In the same STROKESTOP study by Svennberg et al. using 12-lead ECGs54, the addition of up to 14 days of monitoring with a single-lead handheld ECG increased the rate of new AF detection from 0.5% to 3.0% (95% CI 2.7–3.5). In the Swedish stepwise screening study conducted by Engdahl et al.55, two weeks of intermittent single-lead ECG recording led to a new diagnosis of AF in 7.4% (95% CI 5.2–10.4). In the Netherlands, Kaasenbrood et al.57 performed pragmatic screening of individuals obtaining the influenza vaccine within 10 primary care practices. Using the MyDiagnostick handheld ECG device, 3,269 individuals (90.4% above age 60 due to Dutch influenza vaccine guidelines) were screened in a single day, with 37 (1.1%) of new AF cases detected. The majority of individuals diagnosed with new AF (78.4%) merited OAC based on an elevated CHA2DS2-VASc10 stroke risk score.
Several studies have investigated deployment of AF screening in the pharmacy setting. In the Screening Education and Recognition in Community pHarmacies of Atrial Fibrillation (SEARCH-AF) study58, iPhone-based single-lead ECG screening was deployed in 10 pharmacies in Sydney, Australia. Screening of 1,000 individuals resulted in newly identified AF in 15 (1.5%, 95% CI 0.8–2.5). Of these, 10 (1.0%) had no previous history of AF, and 9 were ultimately prescribed OAC for stroke prevention. Retrospective application of an automated AF detection algorithm to raw waveforms acquired from the single-lead ECG resulted in test sensitivity of 98.5% (95% CI 92–100) and specificity of 91.4% (95% CI 89–93) compared to cardiologist review. The Program for the Identification of “Actionable” Atrial Fibrillation in the Pharmacy Setting (PIAAF-Pharmacy) study screened individuals aged ≥ 65 years in 30 Canadian pharmacies with single-lead handheld ECG. Out of 1,145 individuals who underwent single time-point screening, a new diagnosis of AF was made in 24 individuals (2.4%, 95% CI 1.6–3.4).
Both SEARCH-AF and the STROKESTOP study performed basic cost-effectiveness analyses utilizing estimated efficacy and costs. In SEARCH-AF, screening was estimated to be cost-effective for men and women aged 65–84 years, with an incremental cost-effectiveness ratio of $4,066 per quality-adjusted life year saved. In STROKESTOP, deployment of the studied screening program was estimated to incur a cost of €4,614 ($5,039) per quality-adjusted life year saved. Although both of these estimates suggest that the cost-effectiveness of AF screening is favorable, both models depend on a reasonably high proportion of screen-positive patients receiving and persisting on OAC therapy.
Chan et al. have reported two non-randomized studies of AF screening in Chinese populations using handheld single-lead ECGs. In the first study, 13,122 individuals from Hong Kong were invited to participate in the screening intervention.45 The overall prevalence of detected AF was 1.8% (95% CI 1.6–2.0). Of these, 101 individuals (0.8% of the screened population) were found to have previously unknown AF. In a second study of 5,969 individuals in a primary care setting in Hong Kong, AF was diagnosed in 72 patients (1.21%) and confirmed with a 12-lead ECG.32
Recently, a large randomized trial of single-lead handheld ECG screening has been reported, REHEARSE-AF.59 Using the AliveCor Kardia monitor linked to a WiFi-enabled iPod, 1,001 individuals aged ≥65 years who were free of prevalent AF with a CHA2DS2-VASc score ≥2 were randomized to screening or routine care. Screened participants were instructed to acquire single-lead ECGs at home twice weekly over 12 months (with optional additional recordings for symptoms). At 12 months, twice-weekly ECGs were recorded by all participants in 39 of 52 study weeks, with at least one weekly ECG recorded by all participants in 48 of 52 weeks and 60,440 recordings taken in total. AF was diagnosed in 19 (3.8%) of individuals in the screening intervention arm versus 5 (1.0%) in the no screening arm (hazard ratio for AF diagnosis 3.9, 95% CI 1.4–10.4). There was no statistically significant difference in rates of ischemic stroke or transient ischemic attack in individuals randomized to screening versus no screening (4 vs. 8, hazard ratio 0.51, 95% CI 0.15–1.7), respectively. In a basic cost analysis, the overall cost of intervention was reported at $204,830, with an estimated screening-related cost per AF diagnosis of $10,780.
Photoplethysmography-based devices
The latest AF screening modality to be deployed are devices (e.g., smart-watch, band, smart phone) utilizing photoplethysmography (PPG) to detect peripheral pulse irregularity. One of the largest assessments of a wearable PPG-enabled device for AF screening, the Apple Heart Study, was recently reported.47 The Apple Heart Study was a single-arm non-randomized screening intervention using a PPG-enabled Apple Watch linked to an iPhone app. Consumer volunteers who owned an Apple Watch and a compatible iPhone were invited to participate and download the Heart Study app if they reported no history of AF or use of an anticoagulant prior to enrollment. Detection of 5 of 6 irregular PPG-derived tachograms within a 48-hour period triggered a notification via the Heart Study app, notifying participants to contact a telemedicine physician for a consultation. Eligible individuals were then mailed a 7-day ECG patch monitor for confirmation. Of 419,297 participants, a pulse notification was triggered in 2,161 (0.52%) over 9 months. Patch monitors were sent to 658 of these participants, of which 450 were worn, returned, and considered analyzable. New AF was confirmed in 153 (34%) of these 450 individuals, or 0.036% of the overall study sample. Among 86 participants who received a pulse notification while wearing the ECG patch, 72 had demonstrable AF simultaneously on the patch, corresponding to a positive predictive value of 84% for the pulse notification. The planned HEARTLINE study (NCT04276441) will assess the efficacy of the Apple Watch Series 4 (capable of generating a single-lead ECG) to diagnose AF, encourage adherence to OAC therapy, and potentially prevent AF-related stroke.
A second PPG-based screening intervention was recently reported in the Huawei Heart Study based in China.60 Consumers aged ≥18 years with an eligible Huawei phone running an Android operating system who owned a PPG-enabled device (smart watch or band) were invited to participate and download a mobile health app. Periodic PPG-based measurements were taken every 10 minutes, with a resulting 60 seconds of continuous data collection. Individuals could also trigger active measurements voluntarily. An alert for “suspected AF” would be initiated based on a proprietary automated PPG-based algorithm, after which individuals underwent confirmatory testing arranged either remotely or through networked hospitals. Over six months, 187,912 individuals underwent screening and 424 (0.23% of those screened) received an alert for “suspected AF.” Of these, 262 (61.8%) had follow-up evaluations with a medical history, physical exam, and either ECG or 24-hour Holter monitor. Of the 262 with full assessments, 227 (87.0%) were confirmed as having AF. With confirmed AF using ECG or 24-hour Holter monitor as the gold standard, the PPG-based algorithm was estimated to have a PPV of 91.6% (95% CI 91.5–91.8).
An additional study, the Fitbit Heart Study61, has recently launched and aims to validate a distinct PPG algorithm.
Future
AF screening will continue to grow in clinical use, as a result of current guidelines and endorsements based primarily on the ability of existing devices to accurately detect AF. The presence of affordable, consumer-facing devices coupled with a strong desire to avoid stroke will also continue to drive the uptake of AF screening, independent of the guideline process. The lack of benefit from empiric anticoagulation in several randomized trials in populations with a high prevalence of undiagnosed AF (e.g. post embolic stroke of undetermined source) leaves screening for AF as the current clinical approach.
Despite enthusiasm for AF screening, there are key unresolved issues that must be answered. These include which populations to screen, with what device, using what screening methodology, for how long to screen, and what duration or burden of AF is sufficient to justify initiation of OAC. Organizations such as the USPSTF are unlikely to endorse wide-spread, population-based AF screening among asymptomatic individuals until randomized trial data demonstrate a reduction in stroke with AF screening. Fortunately, there are now numerous randomized trials of AF screening which are either underway, or soon to start (Table 3) which are designed to be adequately powered to address many of these questions. The previously mentioned AF-SCREEN consensus group is also planning to perform individual-patient data meta-analysis of such trials. This will allow meta-regression, to help understand if there are any aspects of specific AF screening programs (e.g. setting, population, etc.) that lend themselves to a cost-effective reduction in stroke.
Another important consideration is the next steps following a new diagnosis of AF obtained via screening. As progressively more AF is detected using consumer devices, there may be an opportunity to leverage these very same devices to facilitate engagement in interventions known to reduce AF burden and symptoms, such as targeted weight loss14, alcohol cessation15, blood pressure control62, and sleep apnea management.63 Illustrating this principle, the mAFA II trial, which included a subset of individuals diagnosed with AF via screening with single-lead handheld ECG as part of the Huawei Heart Study, has recently reported a 4.1% absolute reduction in the primary endpoint of ischemic stroke, systemic thromboembolism, death, and rehospitalization (driven by a 3.3% reduction in rehospitalization) after randomization to a smartphone-based integrated care delivery app.16
There is also emerging interest in whether the efficiency of AF screening can be maximized by targeting individuals at highest risk for incident AF. Indeed, clinical risk factors,64,65 biomarkers,66 genetic predisposition,67 cardiac structural features,68,69 and electrocardiographic artificial intelligence algorithms70 have all been reported to serve as promising risk markers for AF which may facilitate screening. Many groups are also examining whether the combination of AF screening with other public health initiatives (e.g. blood pressure screening, vaccination, etc.) enhances screening acceptability to individuals and maximizes cost-effectiveness. The benefits of population-based AF screening will become much better understood over the next 5 years. It is plausible that multiple methods of screening for AF, rather than a single approach, will be considered acceptable in the future.
Table 5.
Summary of test characteristics of selected AF screening modalities
| Modality | Sensitivity (reported range) | Specificity (reported range) | References |
|---|---|---|---|
| Pulse palpation | 91.6 (16.0–100) | 78.8 (65.0–91.0) | 17,20,22,50 |
| 12-lead ECG | 92.7 (66.0–100) | 97.4 (85.0–98.0) | 18,50 |
| Blood pressure cuff | 95.5 (69.5–98.6) | 91.9 (86.7–98.9) | 32,50,53 |
| Single-lead ECG | 96.1 (36.8–98.0) | 94.0 (91.4–100) | 21,50,83 |
| Patch monitor | 100* | 96.6* | 84 |
| Photoplethysmography | 92.9 (77.0–100.0) | 97.7 (86.7–99.7) | 47,50,60,73 |
Uncertainty not reported
Sources of funding
Dr. Lubitz is supported by NIH grant 1R01HL139731 and American Heart Association 18SFRN34250007. Dr. Khurshid is supported by NIH grant T32HL007208.
Abbreviations
- AF
Atrial fibrillation
- OAC
oral anticoagulation
- ESUS
embolic stroke of undetermined source
- NAVIGATE-ESUS
New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus ASA to Prevent Embolism in Embolic Stroke of Undetermined Source
- RE-SPECT ESUS
Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of the Oral Thrombin Inhibitor Dabigatran Etexilate versus Acetylsalicylic Acid in Patients with Embolic Stroke of Undetermined Source
- ATTICUS
Apixaban for Treatment of Embolic Stroke of Undetermined Source
- ARCADIA
AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke
- SAFE
Screening for Atrial Fibrillation with Electrocardiography
- GUARD-AF
reducinG Stroke by Screening for UndiAgnosed atRial Fibrillation in Elderly inDividuals
- SEARCH-AF
Screening Education and Recognition in Community pHarmacies of Atrial Fibrillation
- PIAAF
Program for the Identification of “Actionable” Atrial Fibrillation
- PPG
photoplethysmography
Footnotes
Disclosures:
Dr. McIntyre has received speaking fees from Servier Laboratories and Bayer AG. Dr. Healey receives sponsored research support and speaking fees from Medtronic, Boston Scientific, Abbott Pharmaceuticals, Servier Laboratories, Bristol Myers Squibb / Pfizer, ARCA Biopharm, speaking fees from Cipher Pharma, and is the Senior Scientist and Stuart Connelly Chair in Cardiology Research. Dr. Lubitz receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer AG, Boehringer Ingelheim, and Fitbit, and has consulted for Bristol Myers Squibb / Pfizer and Bayer AG, and participates in a research collaboration with IBM.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JH, Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 3.Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman A, Witteman JCM, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. [DOI] [PubMed] [Google Scholar]
- 5.Kim MH, Johnston SS, Chu B-C, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 6.Wodchis WP, Bhatia RS, Leblanc K, Meshkat N, Morra D. A review of the cost of atrial fibrillation. Value Health. 2012;15:240–248. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987;147:1561–1564. [PubMed] [Google Scholar]
- 8.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG, AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 9.Diener H-C, Hart RG, Koudstaal PJ, Lane DA, Lip GYH. Atrial Fibrillation and Cognitive Function: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:612–619. [DOI] [PubMed] [Google Scholar]
- 10.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 11.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 12.Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991;84:527–539. [DOI] [PubMed] [Google Scholar]
- 13.Lubitz SA, Yin X, McManus DD, Weng L-C, Aparicio HJ, Walkey AJ, Rafael Romero J, Kase CS, Ellinor PT, Wolf PA, Seshadri S, Benjamin EJ. Stroke as the Initial Manifestation of Atrial Fibrillation: The Framingham Heart Study. Stroke. 2017;48:490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, McEvoy RD, Abhayaratna WP, Kalman JM, Lau DH, Sanders P. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018;20:1929–1935. [DOI] [PubMed] [Google Scholar]
- 15.Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, Wong G, Nalliah C, Sugumar H, Wong M, Kotschet E, Kaye D, Taylor AJ, Kistler PM. Alcohol Abstinence in Drinkers with Atrial Fibrillation. New England Journal of Medicine. 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH, Guo Y, Lip GYH, Lane DA, Chen Y, Wang L, Eckstein J, Thomas GN, Tong L, Mei F, Xuejun L, Xiaoming L, Zhaoliang S, Xiangming S, Wei Z, Yunli X, Jing W, Fan W, Sitong Y, Xiaoqing J, Bo Y, Xiaojuan B, Yuting J, Yangxia L, Yingying S, Zhongju T, Li Y, Tianzhu L, Chunfeng N, Lili Z, Shuyan L, Zulu W, Bing X, Liming L, Yuanzhe J, Yunlong X, Xiaohong C, Fang W, Lina Z, Yihong S, Shujie J, Jing L, Nan L, Shijun L, Huixia L, Rong L, Fan L, Qingfeng G, Tianyun G, Yuan W, Xin L, Yan R, Xiaoping C, Ronghua C, Yun S, Yulan Z, Haili S, Yujie Z, Quanchun W, Weidong S, Lin W, Chan E, Guangliang S, Chen Y, Wei Z, Dandi C, Xiang H, Anding X, Xiaohan F, Ziqiang Y, Xiang G, Fulin G. Mobile Health Technology to Improve Care for Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, Raftery J, Davies M, Lip G. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9:iii–iv, ix–x, 1–74. [DOI] [PubMed] [Google Scholar]
- 18.Taggar JS, Coleman T, Lewis S, Heneghan C, Jones M. Accuracy of methods for diagnosing atrial fibrillation using 12-lead ECG: A systematic review and meta-analysis. Int J Cardiol 2015;184:175–183. [DOI] [PubMed] [Google Scholar]
- 19.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke. 2012;43:2788–2790. [DOI] [PubMed] [Google Scholar]
- 20.Sudlow M, Rodgers H, Kenny RA, Thomson R. Identification of patients with atrial fibrillation in general practice: a study of screening methods. BMJ 1998;317:327–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165:193–194. [DOI] [PubMed] [Google Scholar]
- 22.Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract 2002;52:373–374, 377–380. [PMC free article] [PubMed] [Google Scholar]
- 23.Mandrola J, Foy A, Naccarelli G. Screening for Atrial Fibrillation Comes With Many Snags. JAMA Intern Med 2018;178:1296–1298. [DOI] [PubMed] [Google Scholar]
- 24.Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau C-P, Morillo CA, Hobbelt AH, Rienstra M, Connolly SJ. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 25.Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ, ASSERT-II Investigators. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH, Harrison TN, Liu TI, Solomon MD. Association of Burden of Atrial Fibrillation With Risk of Ischemic Stroke in Adults With Paroxysmal Atrial Fibrillation: The KP-RHYTHM Study. JAMA Cardiol 2018;3:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP, American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke Risk as a Function of Atrial Fibrillation Duration and CHA2DS2-VASc Score. Circulation. 2019;140:1639–1646. [DOI] [PubMed] [Google Scholar]
- 29.Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, Mairesse GH, Mabo P, Camm AJ, Healey JS. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre WF, Healey J. Stroke Prevention for Patients with Atrial Fibrillation: Beyond the Guidelines. J Atr Fibrillation 2017;9:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selder JL, Breukel L, Blok S, van Rossum AC, Tulevski II, Allaart CP. A mobile one-lead ECG device incorporated in a symptom-driven remote arrhythmia monitoring program. The first 5,982 Hartwacht ECGs. Neth Heart J 2019;27:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan P-H, Wong C-K, Pun L, Wong Y-F, Wong MM-Y, Chu DW-S, Siu C-W. Diagnostic performance of an automatic blood pressure measurement device, Microlife WatchBP Home A, for atrial fibrillation screening in a real-world primary care setting. BMJ Open. 2017;7:e013685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 34.NHFA CSANZ Atrial Fibrillation Guideline Working Group, Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, Hendriks J, Hespe C, Hung J, Kalman JM, Sanders P, Worthington J, Yan TD, Zwar N. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ 2018;27:1209–1266. [DOI] [PubMed] [Google Scholar]
- 35.US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng C-W, Wong JB. Screening for Atrial Fibrillation With Electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:478–484. [DOI] [PubMed] [Google Scholar]
- 36.Freedman B AF-SCREEN international collaboration. Eur Heart J 2016;37:3490–3491. [DOI] [PubMed] [Google Scholar]
- 37.Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao T-F, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VWY, Levin L-Å, Lip GYH, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu C-W, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP, AF-Screen Collaborators. Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration. Circulation. 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 38.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res 2019;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanna T, Diener H-C, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 40.Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, Swaminathan B, Lavados P, Wang Y, Wang Y, Davalos A, Shamalov N, Mikulik R, Cunha L, Lindgren A, Arauz A, Lang W, Czlonkowska A, Eckstein J, Gagliardi RJ, Amarenco P, Ameriso SF, Tatlisumak T, Veltkamp R, Hankey GJ, Toni D, Bereczki D, Uchiyama S, Ntaios G, Yoon B-W, Brouns R, Endres M, Muir KW, Bornstein N, Ozturk S, O’Donnell MJ, De Vries Basson MM, Pare G, Pater C, Kirsch B, Sheridan P, Peters G, Weitz JI, Peacock WF, Shoamanesh A, Benavente OR, Joyner C, Themeles E, Connolly SJ, NAVIGATE ESUS Investigators. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N Engl J Med 2018;378:2191–2201. [DOI] [PubMed] [Google Scholar]
- 41.Diener H-C, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, Kreuzer J, Cronin L, Cotton D, Grauer C, Brueckmann M, Chernyatina M, Donnan G, Ferro JM, Grond M, Kallmünzer B, Krupinski J, Lee B-C, Lemmens R, Masjuan J, Odinak M, Saver JL, Schellinger PD, Toni D, Toyoda K. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N Engl J Med 2019;380:1906–1917. [DOI] [PubMed] [Google Scholar]
- 42.Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nägele T, Brachmann J, Jung W, Gahn G, Schmid E, Bäezner H, Keller T, Petzold GC, Schrickel J-W, Liman J, Wachter R, Schön F, Schabet M, Lindner A, Ludolph AC, Kimmig H, Jander S, Schlegel U, Gawaz M, Ziemann U. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): Rationale and study design. Int J Stroke. 2017;12:985–990. [DOI] [PubMed] [Google Scholar]
- 43.Kamel H, Longstreth W, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, Di Tullio MR, Hod EA, Soliman EZ, Chaturvedi S, Moy CS, Janis S, Elkind MS. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: Rationale and methods. International Journal of Stroke. 2019;14:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowres N, Olivier J, Chao T-F, Chen S-A, Chen Y, Diederichsen A, Fitzmaurice DA, Gomez-Doblas JJ, Harbison J, Healey JS, Hobbs FDR, Kaasenbrood F, Keen W, Lee VW, Lindholt JS, Lip GYH, Mairesse GH, Mant J, Martin JW, Martín-Rioboó E, McManus DD, Muñiz J, Münzel T, Nakamya J, Neubeck L, Orchard JJ, Pérula de Torres LÁ, Proietti M, Quinn FR, Roalfe AK, Sandhu RK, Schnabel RB, Smyth B, Soni A, Tieleman R, Wang J, Wild PS, Yan BP, Freedman B. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med 2019;16:e1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan N-Y, Choy C-C. Screening for atrial fibrillation in 13 122 Hong Kong citizens with smartphone electrocardiogram. Heart. 2017;103:24–31. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH, MAFA II Investigators. Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 47.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP, Apple Heart Study Investigators. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, Topol EJ. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. JAMA 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzmaurice DA, Hobbs FDR, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GYH, Allan TF. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP, Okoli G, Sterne JA, Feder G, Eaton D, Hingorani A, Fawsitt C, Lobban T, Bryden P, Richards A, Sofat R. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- 51.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castellá M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Alexandru Popescu B, Schotten U, Van Putte B, Vardas P. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration With EACTS. Rev Esp Cardiol (Engl Ed) 2017;70:50. [DOI] [PubMed] [Google Scholar]
- 52.Quinn FR, Gladstone DJ, Ivers NM, Sandhu RK, Dolovich L, Ling A, Nakamya J, Ramasundarahettige C, Frydrych PA, Henein S, Ng K, Congdon V, Birtwhistle RV, Ward R, Healey JS. Diagnostic accuracy and yield of screening tests for atrial fibrillation in the family practice setting: a multicentre cohort study. cmajo 2018;6:E308–E315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kearley K, Selwood M, Van den Bruel A, Thompson M, Mant D, Hobbs FR, Fitzmaurice D, Heneghan C. Triage tests for identifying atrial fibrillation in primary care: a diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open. 2014;4:e004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 55.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–937. [DOI] [PubMed] [Google Scholar]
- 56.Bristol-Myers Squibb. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000. February 29 -. Identifier NCT02392754, A Study to Determine if Identification of Undiagnosed Atrial Fibrillation in People at Least 70 Years of Age Reduces the Risk of Stroke (GUARD-AF); 2019 Oct 15 [cited 2020 Mar 08]; Available from: https://clinicaltrials.gov/ct2/show/NCT04126486. [Google Scholar]
- 57.Kaasenbrood F, Hollander M, Rutten FH, Gerhards LJ, Hoes AW, Tieleman RG. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace. 2016;18:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 59.Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: The REHEARSE-AF Study. Circulation. 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH, MAFA II investigators. Mobile Health Technology for Atrial Fibrillation Screening Using Photoplethysmography-Based Smart Devices: The HUAWEI Heart study. J Am Coll Cardiol 2019; [DOI] [PubMed] [Google Scholar]
- 61.Fitbit, Inc. Fitbit Heart Study. https://healthsolutions.fitbit.com/heartstudy-info/.
- 62.Dzeshka MS, Shantsila A, Shantsila E, Lip GYH. Atrial Fibrillation and Hypertension. Hypertension. 2017;70:854–861. [DOI] [PubMed] [Google Scholar]
- 63.Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Lévy P, Kalman JM, Sanders P. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol 2018;3:532–540. [DOI] [PubMed] [Google Scholar]
- 64.Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of Atrial Fibrillation in a Racially Diverse Cohort: The Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hulme OL, Khurshid S, Weng L-C, Anderson CD, Wang EY, Ashburner JM, Ko D, McManus DD, Benjamin EJ, Ellinor PT, Trinquart L, Lubitz SA. Development and Validation of a Prediction Model for Atrial Fibrillation Using Electronic Health Records. JACC Clin Electrophysiol 2019;5:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, Al-Khalili F, Friberg L, Frykman V, Hijazi Z, Rosenqvist M, Engdahl J. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. EP Europace 2020;22:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weng L-C, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B, Trinquart L, McManus DD, Staerk L, Lin H, Lunetta KL, Ellinor PT, Benjamin EJ, Lubitz SA. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation. 2018;137:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner JD, Skelton WP, Khouzam RN. Is It Time to Incorporate the Left Atrial Size to the Current Stroke Risk Scoring Systems for Atrial Fibrillation?☆. Curr Probl Cardiol 2016;41:251–259. [DOI] [PubMed] [Google Scholar]
- 69.Bruun Pedersen K, Madsen C, Sandgaard NCF, Hey TM, Diederichsen ACP, Bak S, Brandes A. Left atrial volume index and left ventricular global longitudinal strain predict new-onset atrial fibrillation in patients with transient ischemic attack. Int J Cardiovasc Imaging. 2019;35:1277–1286. [DOI] [PubMed] [Google Scholar]
- 70.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S, Friedman PA. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 71.Mairesse GH, Moran P, Van Gelder IC, Elsner C, Rosenqvist M, Mant J, Banerjee A, Gorenek B, Brachmann J, Varma N, Glotz de Lima G, Kalman J, Claes N, Lobban T, Lane D, Lip GYH, Boriani G, ESC Scientific Document Group, Fauchier L, Jung W, Savelieva I, Freedman B, Chen SA, Isa R, Turakhia M, Sapp JL, Lip G, Gorenek B, Sticherling C, Fauchier L, Goette A, Jung W, Vos MA, Brignole M, Elsner C, Dan G-A, Marin F, Boriani G, Lane D, Blomstrom Lundqvist C, Savelieva I. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). EP Europace 2017;19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 72.Wheeldon NM, Tayler DI, Anagnostou E, Cook D, Wales C, Oakley GD. Screening for atrial fibrillation in primary care. Heart. 1998;79:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan P-H, Wong C-K, Poh YC, Pun L, Leung WW-C, Wong Y-F, Wong MM-Y, Poh M-Z, Chu DW-S, Siu C-W. Diagnostic Performance of a Smartphone-Based Photoplethysmographic Application for Atrial Fibrillation Screening in a Primary Care Setting. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diederichsen ACP, Rasmussen LM, Søgaard R, Lambrechtsen J, Steffensen FH, Frost L, Egstrup K, Urbonaviciene G, Busk M, Olsen MH, Mickley H, Hallas J, Lindholt JS. The Danish Cardiovascular Screening Trial (DANCAVAS): study protocol for a randomized controlled trial. Trials. 2015;16:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gladstone DJ. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000. February 29 -. Identifier NCT02392754, Home-Based Screening for Early Detection of Atrial Fibrillation in Primary Care Patients Aged 75 Years and Older (SCREEN-AF); 2015 Mar 19 [cited 2020 Mar 08]; Available from: https://clinicaltrials.gov/ct2/show/NCT02392754. [Google Scholar]
- 76.Chen Y, Wang J. GW28-e0871 Rationale and design of the randomized controlled trial of intensive versus usual ECG screening for atrial fibrillation in elderly Chinese by an automated ECG system in the community health center in Shanghai (AF-CATCH). Journal of the American College of Cardiology. 2017;70:C77–C78. [Google Scholar]
- 77.Haeusler KG, Kirchhof P, Heuschmann PU, Laufs U, Busse O, Kunze C, Thomalla G, Nabavi DG, Röther J, Veltkamp R, Endres M. Impact of standardized MONitoring for Detection of Atrial Fibrillation in Ischemic Stroke (MonDAFIS): Rationale and design of a prospective randomized multicenter study. Am Heart J 2016;172:19–25. [DOI] [PubMed] [Google Scholar]
- 78.Uittenbogaart SB, Verbiest-van Gurp N, Erkens PMG, Lucassen WAM, Knottnerus JA, Winkens B, van Weert HCPM, Stoffers HEJH. Detecting and Diagnosing Atrial Fibrillation (D2AF): study protocol for a cluster randomised controlled trial. Trials. 2015;16:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diederichsen SZ, Haugan KJ, Køber L, Højberg S, Brandes A, Kronborg C, Graff C, Holst AG, Nielsen JB, Krieger D, Svendsen JH. Atrial fibrillation detected by continuous electrocardiographic monitoring using implantable loop recorder to prevent stroke in individuals at risk (the LOOP study): Rationale and design of a large randomized controlled trial. Am Heart J 2017;187:122–132. [DOI] [PubMed] [Google Scholar]
- 80.Ashburner JM, Atlas SJ, McManus DD, Chang Y, Trisini Lipsanopoulos AT, Borowsky LH, Guan W, He W, Ellinor PT, Singer DE, Lubitz SA. Design and rationale of a pragmatic trial integrating routine screening for atrial fibrillation at primary care visits: The VITAL-AF trial. American Heart Journal. 2019;215:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veale EL, Stewart AJ, Mathie A, Lall SK, Rees-Roberts M, Savickas V, Bhamra SK, Corlett SA. Pharmacists detecting atrial fibrillation (PDAF) in primary care during the influenza vaccination season: a multisite, cross-sectional screening protocol. BMJ Open. 2018;8:e021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dymond A Screening for atrial fibrillation with ECG to reduce stroke [Internet]. [cited 2020 Feb 20]; Available from: http://www.isrctn.com/ISRCTN16939438
- 83.Giebel GD, Gissel C. Accuracy of mHealth Devices for Atrial Fibrillation Screening: Systematic Review. JMIR Mhealth Uhealth. 2019;7:e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mena LJ, Félix VG, Ochoa A, Ostos R, González E, Aspuru J, Velarde P, Maestre GE. Mobile Personal Health Monitoring for Automated Classification of Electrocardiogram Signals in Elderly. Comput Math Methods Med 2018;2018:9128054. [DOI] [PMC free article] [PubMed] [Google Scholar]


