Abstract
Betulinic acid (BA) inhibits the migration, invasion, and cytoskeletal reorganization of fibroblast-like synoviocytes (RA-FLS) in patients with rheumatoid arthritis. Here, to further explore the mechanism of action of BA in collagen-induced arthritis (CIA) rats, we investigated the pharmacodynamic effects of BA on synovial inflammation in a rat model of type II CIA. After inducing hind paw swelling, the rats were divided into four groups: healthy controls (normal), and rats that underwent CIA and received methotrexate treatment (MTX), BA treatment (BA), or no treatment (CIA). Body weight and hind paw swelling were determined regularly, and arthritis scores were calculated weekly. On day 35, rats were sacrificed and their hind ankle joints sectioned and stained with hematoxylin and eosin for histopathological evaluation. BA significantly reduced CIA-induced hind paw swelling, synovial tissue proliferation, cartilage destruction, and vasospasm. BA treatment also decreased serum interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) levels in rats with CIA. The CCK-8 assay was used to detect the proliferation of isolated vimentin+CD68− RA-FLS; RA-FLS were stimulated with TNF-α in vitro. BA significantly inhibited TNF-α-stimulated RA-FLS proliferation, as well as IL-1β and IL-6 secretion. BA also downregulated the transcription of vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β) and decreased the expression of the NF-кB pathway proteins (NF-kB-P65, IkBα, and IKKα/β) in the TNF-α-stimulated RA-FLS. These results indicate that BA alleviated the symptoms of CIA by inhibiting synoviocyte proliferation, modifying TNF-α- and NF-кB-related inflammatory pathways, and downregulating inflammatory mediators and growth factors including IL-1β, IL-6, VEGF, and TGF-β.
Keywords: betulinic acid, NF-кB pathway, rheumatoid arthritis, type II collagen, vimentin+CD68− fibroblast-like synoviocytes
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease, and is the most common inflammatory joint disease affecting 1% of the world’s population.1 If RA is not actively treated at an early stage, it gradually progresses and leads to the destruction of joints and their loss of function. This reduces the patient’s quality of life and can even result in disability. Chronic inflammation, synovial tissue hyperplasia, vasculitis, and osteoarticular cartilage destruction are prominent features of RA.2 Although the mechanism of RA is unclear, evidence from genetic, biological, and histopathological analyses both in in vivo models and in clinical research indicate that chronic inflammation and joint destruction are associated with stromal tissue dysregulation and immune-mediated etiologies.2 Reorganization of fibroblast-like synoviocytes (RA-FLS) are synovial mesenchymal cells located on the synovial membranes that play crucial roles in the pathogenesis of RA.3 When activated, RA-FLS exhibit abnormal biological behavior and properties similar to those of cancerous cells, such as anchorage-independent growth, migration, invasion, and overproduction of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), which are essential for RA progression.4–6 TNF-α secretion induces a large number of inflammatory cytokines, chemokines, and matrix metalloproteinases, which ultimately leads to synovial inflammation and joint destruction.2 Therefore, inhibiting RA-FLS proliferation and migration or downregulating proinflammatory cytokines are potential therapeutic strategies for RA. Furthermore, the current nonsteroidal, immunosuppressive, and hormone-based drugs produce many side effects in patients, leading to poor compliance. Thus, new drugs for the treatment of RA with a low incidence of side effects and high efficacy are urgently needed.
We previously discovered that the ethanol extract of Eucommia ulmoides significantly inhibits the in vitro proliferation of RA-FLS.7 In animal models, the extract significantly decreases serum interleukin (IL)-17 and IL-23 levels as well as the number of Th17 cells in the spleen.8 Furthermore, the extract markedly improves the imbalance in the receptor activator of nuclear factor kappa B ligand/osteoprotegerin (RANKL/OPG) ratio and reduces toe swelling during collagen-induced arthritis (CIA) in rats. We demonstrated that betulinic acid (BA), which was present in E. ulmoides, is responsible for inhibiting RA-FLS proliferation in vitro.8,9 BA, a natural pentacyclic triterpene compound, is widely present in Betula pubescens, Ziziphus mauritiana, and plants of Apocynaceae. BA can also be synthesized from betulin.10 BA has multiple activities, including antitumor, antioxidant, anti-inflammatory, and immunoregulatory properties.11–13 BA has been shown to alleviate the inflammatory response of osteoarthritic chondrocytes stimulated by IL-1β, and to act synergistically with fluvastatin against atherosclerosis in type II collagen-stimulated arthritis that is mediated via Toll-like receptor-4.14 BA treatment may be clinically beneficial to patients with RA, although its mechanism of action may be varied and it is unclear whether its efficacy would be affected by metabolism and other biological factors.15 Therefore, we established a rat CIA model to explore the pharmacodynamic effects of BA in vivo. Synoviocytes were also extracted from rats with CIA for in vitro experiments. Our study results can provide a reference for the mechanism of BA treatment of RA.
Materials and methods
Animals
To better meet the statistical requirements, 32 male Wistar rats (each weighing 200 ± 10 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Animal Certificate of Conformity: SCXK (Shanghai), No. 2016-0002) and fed for 3 days in the Laboratory Animal Center of the Shanghai University of Traditional Chinese Medicine (SHUTCM). All procedures involving animals were performed in accordance with the ethical standards for the care and use of laboratory animals and related ethical regulations of SHUTCM (ethical committee approval number: PZSHUTCM19011101).
Establishment of the CIA model and treatment groups
Bovine type II collagen (30 mg; Sigma-Aldrich, USA) was mixed with 15 mL of complete Freund’s adjuvant (Sigma-Aldrich) and 0.1 mol/L of acetic acid solution to produce a 2 mg/mL collagen emulsion. Sample size calculation was used to compare the averages of multiple samples, according to the mean (Xi) and SD (Si) of the hind paw swelling of rats in the study of Wang Jian-Ying et al.,7 where it was calculated using the following formula n = Ψ2(ΣSi2/g)/(Σ(Xi − X)2/(g − 1))16 that six rats were needed per group. However, to further eliminate statistical differences, we decided to use eight rats per group, with 32 rats in the four groups. Each rat was intradermally administered 100 µL of the emulsion (i.e. 200 µg type II collagen) on day 1 (primary injection) and on day 7 (booster injection) at the base of the tail. After 8 days, the hind paws were slightly swollen with more evident swelling on the 14th day, indicating the successful establishment of the CIA model. Subsequently, 24 rats were randomly assigned into three groups of eight rats per group to receive either no treatment (the CIA group), BA treatment (the BA group), or methotrexate treatment (MTX) treatment (the MTX group). BA (90 mg/kg/day; Shanghai, China), and MTX (1.5 mg/kg/day; Shanghai, China) were intragastrically administered daily for three weeks. Untreated rats and rats in the CIA group that received no treatment received intragastric administration of distilled water (4 mL/kg/day for 3 weeks).
Hind paw swelling measurements and arthritis score
Hind paw swelling and rat body weights were monitored once a week between days 14 and 35 (measurements were also taken before treatment and on day 0). Paw volume (indicated by paw swelling) was measured using a paw volume meter (Shandong Academy of Medical Sciences, China) and rats were assigned arthritis scores on a scale of 0–4, with a higher score indicating more serious joint symptoms. The scoring system was as follows: 0, no joint inflammation (no redness); 1, slight swelling in the red spot; 2, moderate swelling of joints; 3, severe swelling of the joints; 4, rigid or even deformed joints, indicating severe dysfunction.7
Serum and tissue collection
One hour after treatment administration on day 35, rats were euthanized with an overdose of 20% uratin (EINECS No. 200-123-1, Hubei Yuancheng Pharmaceutical Co., Ltd., China) injection. The abdominal aorta was bled and centrifuged at 4°C at 3000 r/min for 5 min. The serum obtained was stored at −80°C prior to testing. The leg joint of the hind limb was quickly removed and fixed in 10% neutral formaldehyde solution.
Histopathological staining
The ankle joint was placed in 10% formic acid formaldehyde solution for decalcification, embedded in paraffin, and sectioned at a 6-µm thickness. Sections were then subjected to hematoxylin and eosin (H&E) and immunohistochemical staining, followed by the microscopic observation of pathological changes.
Isolation of RA-synoviocytes from rats with CIA
Six Wistar rats with CIA were used as donors for synoviocytes isolation. The rats were euthanized and their knee joints were removed, then soaked in 75% alcohol for 2 min. Muscle tissues were removed and the synovial layer tissues were separated in a sterile environment. The separated synovial tissues were soaked in phosphate-buffered saline (PBS) for 5 min, rinsed with PBS, and placed in a Petri dish. Tissues were minced and digested by adding type II collagenase (2 mg·mL−1) was suspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS; Cat. #10099-141, Gibco, Life Technologies Corporation, Beijing, China). This cell suspension was filtered through a 100-mesh screen and centrifuged at 1000 r/min for 5 min. The supernatant was discarded, and the cell pellet was resuspended in 4 mL of DMEM. The obtained cell suspension was transferred to a clean culture flask and cultured at 37°C. After incubation for 24 h, any unattached cells were removed and the remaining cells returned to culture. The cell culture medium was changed every 2–3 days, and cell growth was observed under an inverted microscope. Finally, adherent synovial fibroblasts were obtained.
Immunostaining for fluorescent activated cell sorting
Synoviocytes were trypsinized at 37°C, washed twice with PBS, and cell numbers counted by flow cytometry (Becton Dickinson, USA). The cell concentration was adjusted to 1 × 107 cells/mL and stained with antivimentin (Cat. #sc-58899, Santa Cruz Biotechnology, USA) and anti-CD68 (1 μg/106 cells; Cat. #ab31630, Abcam, UK) directly conjugated fluorescent antibodies. Cells were then centrifuged at 1000 r/min at 4°C for 5 min, rinsed twice with 2 mL of PBS supplemented with 2% FBS, and resuspended after centrifugation. Vimentin+CD68− synovial cells were sorted by flow cytometry.
Cell viability assay
Cell viability was measured by the 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid benzene)-2 H-tetrazole monosodium salt (CCK-8) assay. Briefly, RA-FLS (3000 cells per well) were plated onto 96-well plates for 2 h and incubated with different concentrations of BA (0, 1, 5,10, 20, 50, and 100 μg/mL) for 24 h or treated with different concentrations of BA (0, 5, 10, and 20 μg/mL) for 24, 48, or 72 h at 37°C before 20 μL of 5 mg/mL CCK-8 (Sigma-Aldrich) in PBS was added to each well. After a further 0.5 h incubation with CCK-8, the supernatant was removed and the optical density values at 450 nm (OD450) were measured in dimethyl sulfoxide (Sigma-Aldrich). The proliferation rate was calculated as: 1 − (1 − OD490/blank OD450) × 100%.
Determination of IL-1β and IL-6 levels in TNF-α-stimulated RA-FLS
Vimentin+CD68− RA-FLS from passage numbers 3–5 were suspended at 2 × 108 cells/mL and plated into 96-well plates (at 2000 cells/well). After further incubation for 6 h, supernatants were removed and standard growth medium with 20 ng/mL TNF-α (eBioscience, USA) was added to each well except wells designated as negative control wells. After 24 h incubation under standard growth conditions, the medium was removed, and replaced with solutions of BA (5 and 10 μg/mL) and 20 μg/mL of recombinant human TNF receptor Fc fusion protein (rhTNFR:Fc., Qilu Pharmaceutical Co., Ltd., China) in a serum-free medium. After another 24 h, supernatants were collected to determine the concentrations of IL-1β and IL-6 using enzyme-linked immunosorbent assay kits (eBioscience) according to the manufacturer’s instructions.
Determination of VEGF and TGF-β the mRNA levels in TNF-α-stimulated RA-FLS
Vimentin+CD68− RA-FLS obtained after FACS were cultured in standard growth media in 24-well plates under standard culture conditions at a cell density of 1 × 109 cells/mL. After 24 h of incubation, the growth medium was removed and cells in all wells, except the negative control, were exposed to 100 ng/mL TNF-α prepared in DMEM supplemented with 10% FBS. After 1 h of TNF-α stimulation, BA (5, 10, and 20 μg/mL) and 20 μg/mL rhTNFR:FC, both dissolved in serum-free media, were added and cells were further incubated for 4 h. Subsequently, the treatment medium was removed and RNA was extracted and purified using an RNA Mini Kit (Ambion, USA). RNA was then reverse transcribed into cDNA using the SuperScript III First-Strand Synthesis SuperMix (Invitrogen, USA). Quantitative reverse-transcription PCR was performed using a relative quantification approach, with the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene as a reference and SYBR Green as the fluorescent dye. Reactions were performed using the following amplification conditions: predenaturation at 50°C for 2 min and polymerase activation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, and annealing and extension at 60°C for 60 s. The primer sequences used for PCR were as follows: in the 5′3′ direction, VEGF forward primer: CACGACAGAAGGGGAGCAG, VEGF reverse primer: TACACGTCTGCGGATC-TTGG; TGFβ forward primer: TGGCGTTACCTTGGTAACC, TGFβ reverse primer: GGTGTTGAGCCCTTTCCA. The relative expression of each target gene compared with β-actin was calculated using the 2−ΔΔCt method.
Evaluation of NF-kB pathway activation in TNF-α-stimulated RA-FLS by western blot analysis
Vimentin+CD68− RA-FLS were cultured and stimulated with TNF-α as described in the section “Determination of the mRNA levels of VEGF and TGF-β in TNF-α-stimulated RA-FLS” above. Subsequently, the cells were treated with BA (10 μg/mL) and 20 μg/mL rhTNFR:FC. Cells were then trypsinized, centrifuged, and lysed in a buffer containing 50 mm of Tris-HCl (pH 7.4), 150 mM of NaCl, 1% Triton X 100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM of Na3VO4, 1 mM of NaF, 1 mM of phenylmethane sulfonyl fluoride, and 1 mM of protease inhibitor cocktail. The cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% gel, and transferred to polyvinylidene difluoride membranes prior to incubation with specific primary antibodies (phospho-NF-κB p65 (Ser536) rabbit mAb #3033, phospho-IκBα (Ser32) rabbit mAb #2859, and phospho-IKKα/β (Ser176/180) rabbit mAb #2697 (all from Cell Signaling Technology, Danvers, MA, USA)) at 4°C after blocking with 5% nonfat milk. Subsequently, the membranes were washed three times with PBS containing 0.05% Tween® 20 (PBS-T) and incubated with a horseradish peroxidase-conjugated antirabbit secondary antibody (Cell Signaling Technology, USA) for 1 h at room temperature. Membranes were washed again with PBS-T three times, and pNF-kB-P65, pIkBα, and pIKKα/β signals were visualized using enhanced chemiluminescence (Cat. #32106, Pierce, USA) according to the manufacturer’s instructions. The relative band intensity was quantified using ImageJ v. 1.47.
Statistical analyses
Statistical analyses were performed using the IBM SPSS Statistics 22.0 software. The arthritis scores were analyzed by Fisher’s exact test and the comparisons among groups were analyzed by one-way ANOVA. Differences were considered statistically significant at P < 0.05, P < 0.01, and P < 0.001. Plots were generated in GraphPad Prism 5.00.288 (GraphPad Software, Inc., USA).
Results
BA alleviated disease symptoms in rats with CIA
To explore whether BA can alleviate the symptoms of rheumatoid arthritis in CIA rats, the weight, toe volume, and joint swelling of rats in different groups were observed or scored, as shown in Figure 1. Rats immunized with type II collagen but not healthy controls showed symptoms of arthritic disease from day 14. These included weight gain, different degrees of joint swelling in their hind paws, joint malformations, and impaired or slower movement. After treatment by BA or MTX, significant recovery was observed, as indicated by reduction in hind paw joint redness (Figure 1(a)). The body weights of rats treated with BA or MTX groups were significantly higher than those of rats that did not receive treatment (the CIA group) (Figure 1(b)). The body weights of BA-treated rats gradually increased after treatment, although their weights were still less than healthy control rats. On the 7th day after collagen immunization, the hind paw volumes of rats significantly increased compared to that of healthy controls (Figure 1(c)). From day 14, the hind paw volume started to decrease with BA and MTX treatment (P < 0.01) (Figure 1(c)). From days 14 to 21, the arthritis scores (Figure 1(d)) of BA- and MTX-treated rats were lower than those of rats with CIA that received no treatment, although the differences were not significant (P > 0.05). The reduction in arthritis scores reached significance after 28 days (P < 0.01) and 35 days (P < 0.001).
Figure 1.
Improvement in symptoms after BA or MTX treatment in rats with CIA. Rats were intragastrically administered BA (90 mg/kg/day) and MTX (1.5 mg/kg/day) daily for 3 weeks; rats in the CIA group and healthy rats received distilled water (0.4 mL/100 g/day) over the same period. (a) Images showing hind paw swelling in rats from each group. (b) Effect of BA on the change in body weight, (c) hind paw volume, and (d) arthritis scores in rats. *P < 0.01 (Fisher’s exact test) compared with the normal group; #P < 0.001 compared with the CIA group. All values represent the mean ± SD from eight independent experiments.
BA treatment improved histopathological features in the joints of rats with CIA
To further evaluate the effect of BA on the joints of CIA rats, the joints of different groups of rats were sliced and immunohistochemically tested. As shown in Figure 2, histopathological analysis showed that the cartilage of the ankle joint surface of rats with CIA was severely damaged and that the synovial lining layer had marked fibrous synovial hyperplasia and inflammatory cell infiltration. Cartilage destruction occurred in vasospasms, irregular joint spaces, and joint cavity adhesions. BA treatment significantly reduced synovial hyperplasia, inflammatory cell infiltration, and vasospasm formation and improved pathological conditions such as joint cavity adhesion when compared to rats that did not receive treatment.
Figure 2.
H&E stained (200×) sections of ankle joints from rats in all groups: (a) synovial hyperplasia, (b) cell infiltration, (c) cartilage destruction, and (d) joint cavity.
BA treatment inhibited secretion of inflammatory cytokines in rats with CIA
BA significantly reduces serum levels of IL-1β, IL-6, and TNF-α, as shown in Figure 3. High levels of IL-1β, IL-6, and TNF-α were observed in the sera of rats with the CIA group, whereas BA and MTX treatment significantly decreased the levels of these cytokines (P < 0.01 and P < 0.05, respectively, compared to the CIA group). Rats that received BA treatment had the lowest levels of these cytokines (P < 0.01 compared to other groups).
Figure. 3.

BA reduces the levels serum IL-1β, IL-6, and TNF-α in rats with CIA. All values are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the normal group and #P < 0.01 compared with the CIA group.
Isolation and identification of vimentin+CD68− RA-FLS
A large number of rat synovial fibroblasts were obtained after the culture of the synovial tissue from rats with CIA (Figure 4(a)). The cells appeared as clear fusiform dendrites under the microscope and had distinctive fibroblast-like features. Vimentin+CD68− synovial fibroblasts accounted for approximately 30.5% of the entire cell population. After sorting, the purity of cells was increased to ⩾93.4% (Figure 4(b)).
Figure 4.
Identification of vimentin+CD68− synovial fibroblasts by flow cytometry: (a) CIA synovial fibroblast-like cells and (b) flow cytogram of identification of vimentin+CD68− synovial fibroblast cells.
BA significantly inhibited the proliferation of RA-synoviocytes from CIA rats
As shown in Figure 5(a), as the concentration of BA increases, the number of cells decreases; however, we know that BA is not cytotoxic when it is below the 20 µg/mL administration concentration (Figure 5(b)). Thus, we used the CCK-8 assay to detect the effect of BA on the cell proliferation of RA-synoviocytes from CIA rats. BA treatment effectively inhibited the proliferation of synovial fibroblasts from rats with CIA, and the comparison among groups showed that the inhibitory effect was more pronounced at a BA dose of 20 μg/mL, but this probably also show certain toxicity (Figure 5(c); P < 0.001).
Figure 5.
BA inhibits the proliferation of RA-FLS from rats with CIA. All data are mean ± SD of three independent experiments. (a) RA-FLS at a density of 3 × 104 cells per well were treated with different concentrations of BA (0, 5, 10, and 20 µg/mL) for 24 h. (b) Cell viability determined using the CCK-8 assay. The 0 μg/mL value represents data from the healthy controls. *P < 0.01 compared with the healthy control group; #P < 0.001 compared with the 0, 1, 5, and 10 μg/mL BA treatment groups. (c) RA-FLS at a density of 3 × 104 cells per well were treated with different concentrations of BA (5, 10, and 20 µg/mL) for 48, 72, or 96 h. Cell proliferation was measured using the CCK-8 assay. *P < 0.01, **P < 0.001, compared with the 0 μg/mL BA treatment groups.
BA significantly decreased IL-1β and IL-6 production by RA-FLS stimulated with TNF-α in vitro
In vitro TNF-α-stimulated vimentin+CD68− RA-FLS secreted large amounts of the inflammatory factors, IL-1β, and IL-6 (Figure 6), which were significantly reduced after BA treatment or after neutralization of TNF-α by rhTNFR:Fc. The secretion of IL-1β was decreased in a dose-dependent manner after BA treatment, as there were significant differences between the 0 and 10 μg/mL BA treatment groups (P < 0.001). Similarly, treatment with BA significantly decreased IL-6 secretion compared to the control group (P < 0.001). Interestingly, BA treatment reduced TNF-α-stimulated IL-6 secretion to a larger extent than that achieved by TNF-α neutralization (P < 0.001).
Figure 6.
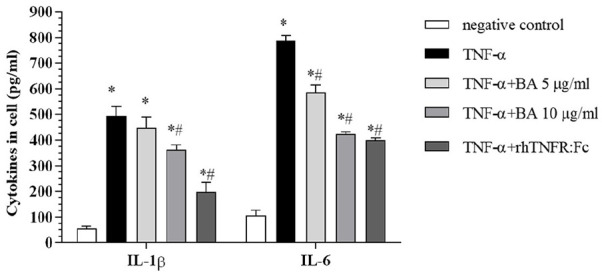
Effect of BA on the secretion of IL-1β and IL-6 in TNF-α-stimulated RA-FLS. DMSO was used as a blank and TNF-α-stimulated cells were used as the control group. All values were calculated from three independent experiments and are represented as means ± SD. *P < 0.001 compared with the blank group; #P < 0.001 compared with the control group.
BA significantly inhibited VEGF and TGF-β transcription by TNF-α-stimulated RA-FLS
TNF-α-stimulated vimentin+CD68− RA-FLS showed a high expression of VEGF and TGF-β mRNA transcripts (Figure 7). BA treatment led to a significant dose-dependent decrease in VEGF mRNA levels, and all tested doses resulted significantly lower VEGF expression compared to the control group (P < 0.01). The levels of TGF-β mRNA also decreased with increasing BA concentrations, and were significantly lower compared to the control group (P < 0.01). Furthermore, the levels of TGF-β mRNA decreased to normal levels after BA treatment, and the difference among different treatment groups was not significant (P > 0.05).
Figure 7.

Effect of BA on TNF-α-stimulated expression of VEGF and TGF-β in RA-FLS. DMSO was used as a blank. TNF-α-stimulated cells were used as a control group. All values are the mean ± SD of three independent experiments. **P < 0.01 compared with the blank group; #P < 0.05 and ##P < 0.01 compared with the control group.
BA inhibited TNF-α-stimulated activation of the NF-κB pathway in RA-FLS
To elucidate the mechanism of the anti-inflammatory effects of BA, the activation of IKK and NF-kB, as indicated by their phosphorylation, was determined using western blotting analysis. As shown in Figure 8, the levels of phosphorylated NF-kB-P65, IkBα, and IKKα/β proteins in TNF-α-stimulated RA-FLS, as determined by western blotting, were significantly higher than those in the negative control group (P < 0.001). TNF-α-stimulated synoviocytes in the control group showed high levels of phosphorylated NF-kB-P65, IkBα, and IKKα/β expression, as indicated by thick and dark bands (Figure 8(a)), which were significantly reduced after BA treatment (Figure 8(b)). The levels of phosphorylated NF-kB-P65, IkBα, and IKKα/β proteins in the BA treatment group cells were significantly lower than those in the TNF-α-stimulated control group cells (P < 0.001). Notably, BA treatment and TNF-α neutralization produced the same inhibitory effects on the NF-kB pathway, and no significant differences in the levels of all three proteins were seen between both treatment groups (P > 0.05).
Figure 8.
BA inhibits the production of IKK, Iκ-B, and P65 proteins in RA-FLS cells stimulated by TNF-α. (a) Western blot analysis of NF-κB, IκBα, and IKK protein levels. RA-FLS were treated with BA (10 µg/mL) or DMSO for 24 h followed by TNF-α stimulation for 10 min. Cells that received no drugs were designated as the negative control group, whereas TNF-α administration was designated as the TNF-α group. (b) Quantification of IKK, Iκ-B, and P65 protein levels. GAPDH was used as the internal control. *P < 0.001 compared with the negative control, #P < 0.001 compared with the TNF-α group. All values are the mean ± SD of three independent experiments.
Discussion
The pathogenesis of RA is complex. RA predominantly affects synovial joints and can progress rapidly.17,18 In the present study, we used a classical CIA rat model to simulate RA and observed the effects of BA treatment on disease progression. The beneficial effects of BA treatment included restoration of body weight loss, reduction of hind paw swelling, and improvement in arthritis scores, indicating BA can reverse disease pathology caused by CIA and delay disease progression.
Several findings from clinical studies have shown that inflammation occurs during the pathogenesis of RA.19 Inflammatory cytokines, including IL-1β, IL-6, and TNF-α, can promote the occurrence and progression of inflammation in RA.20 In this study, BA significantly inhibited the secretion of inflammatory cytokines, which was detected systemically, and this is a likely molecular mechanism of BA-mediated alleviation of arthritis. In patients with RA, synovial cells reach the articular surface to destroy cartilage, and stimulate the formation of osteoclasts, thereby increasing bone erosion and destruction.21,22 Recent studies have suggested that BA can inhibit synovitis cell migration and invasion in patients with RA.23 In addition, the proliferation rate of synovial fluid endothelial cells in patients with RA is higher than that in healthy controls.24 Herein, we extracted, sorted, and cultured rat synovitis cells to investigate the effects of BA and showed that BA significantly inhibited the proliferation of synoviocytes in a dose-dependent manner.
TNF-α is a key proinflammatory cytokine that plays an important role in immunoregulation. It induces and stimulates the secretion of other inflammatory cytokines such as IL-1β and IL-6. The upregulation of TNF-α induces cartilage destruction and collagen breakdown, and promotes platelet aggregation and inflammatory cell infiltration.25 BA significantly reduced the secretion of IL-1β and IL-6 induced by TNF-α stimulation compared to the control group. Inflammatory cytokines and hypoxia lead to the upregulation of vascular endothelial growth factor (VEGF) in patients with RA.26 Studies have shown that high serum VEGF levels are associated with joint damage,27 especially during early stages of RA. Elevated levels of VEGF are associated with devastating lesions.28 Therefore, antiangiogenic therapy could be a potential strategy for RA and the use of the anti-VEGF monoclonal antibody (bevacizumab) is a potential treatment strategy.29 In this study, we used TNF-α monoclonal antibody treatment, which resulted in decreased serum VEGF levels and was consistent with the results of previous studies.30 Transforming growth factor (TGF)-β is a multifunctional growth factor that regulates various biological processes including cell differentiation, proliferation, inflammatory responses, and tissue remodeling and formation.31 TGF-β can also promote the secretion of VEGF.32 Collectively, both VEGF and TGF-β can regulate inflammation.33 Therefore, we also studied TGF-β expression in cells and found that BA significantly decreased the transcription of both VEGF and TGF-β in TNF-α-stimulated RA-FLS. No significant difference was observed between the downregulation of TGF-β after treatment with BA or after TNF-α neutralization. This suggested that the BA-induced downregulation of VEGF and TGF-β may be an effective mechanism to alleviate CIA-simulated RA symptoms.
NF-κB is a transcription factor involved in the regulation of a variety of inflammatory cytokines.34 The NF-κB pathway can be activated by inflammatory factors such as IL-1β and TNF-α, and this pathway is important for controlling synovial inflammation and joint destruction.23 In this study, we used western blot analysis to detect the levels of phosphorylation of NF-κB pathway proteins and discovered that both BA treatment and TNF-α neutralization significantly reduced the TNF-α-stimulated protein production of P65, IKK, and IК-B to a similar extent. This could also be an effective mechanism for the therapeutic effect of BA on synovitis cells in CIA rats.
Although our study involves both in vivo and in vitro experiments and, to some extent, explains the effect of BA on synovial inflammation in CIA rats, there are still many deficiencies in elucidating the mechanism of BA in CIA rats. For example, we did not discuss whether BA has any effect on the migration and invasion of RA-FLSs; In addition, due to various reasons, our western blot analysis did not set different drug concentration gradients, so it did not show dose-dependent results. Our research did not have time to explore JNK and ERK pathways. If these problems can be better solved, it is very important to further clarify the significance that the mechanism of BA effect in CIA rats. We hope that we can improve these problems in the follow-up research; of course, we also welcome other researchers to discuss together.
Conclusion
In summary, BA inhibited the proliferation of synovitis cells and downregulated the expression of IL-1β, IL-6, TNF-α, and other inflammatory factors in serum and synoviocytes. Moreover, BA decreased the serum levels of VEGF and TGF-β to inhibit TNF-α-stimulated inflammatory effects and NF-κB signaling. Thus, BA relieved synovial inflammation in rats with CIA. The results of our study explain the mechanism of action of BA and indicate there is potential developing BA as clinical treatment for RA.
Acknowledgments
Many thanks to the National Natural Science Foundation of China and other project funds for their strong support for this research. Many thanks to Associate Professor Yuan Ying, the corresponding author, and other co-authors for their dedicated contributions.
Footnotes
Author contributions: J.-Y.W. designed and performed the main experiments; K.-L. performed experiments, compiled the data, and drafted and wrote the manuscript; Y.Y. and Y.-Y.P. supervised the study and revised the manuscript; and X.-Y.C. and L.Z. performed some experiments.
Animal welfare: The present study followed international, national, and Shanghai University of Traditional Chinese Medicine (SHUTCM) guidelines for humane animal treatment and complied with relevant legislation.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Experimental Animal Welfare and Ethics Committee of Shanghai University of Traditional Chinese Medicine (approval number: PZSHUTCM19011101).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Natural Science Fund (grant nos 81773922 and 81973730), Shanghai Natural Science Fund (grant no. 19ZR1452000), Shanghai Municipality Further Accelerates the Three-Year Action Plan for the Development of Traditional Chinese Medicine (2018–2020), and “Internet+TCM Health Service” Research and Transformation Platform Construction Fund (grant no. ZY (2018-2020)-CCCX-2001-01).
ORCID iD: Ying Yuan  https://orcid.org/0000-0002-5274-6221
https://orcid.org/0000-0002-5274-6221
References
- 1. Meyer PW, Ally MM, Tikly M, et al. (2019) Tobacco-derived lipopolysaccharide, not microbial translocation, as a potential contributor to the pathogenesis of Rheumatoid Arthritis. Mediators of inflammation 870: 4693870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G. (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews Immunology 7: 429–442. [DOI] [PubMed] [Google Scholar]
- 3. Bartok B, Firestein GS. (2010) Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunological Reviews 233(1): 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fu D, Yang Y, Xiao Y, et al. (2012) Role of p21-activated kinase 1 in regulating the migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Rheumatology (Oxford) 51(7): 1170–1180. [DOI] [PubMed] [Google Scholar]
- 5. Huang M, Wang L, Zeng S, et al. (2017) Indirubin inhibits the migration, invasion, and activation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Inflammation Research 66(5): 433–440. [DOI] [PubMed] [Google Scholar]
- 6. Li F, Li X, Kou L, et al. (2014) SUMO-conjugating enzyme UBC9 promotes proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation 37(4): 1134–1141. [DOI] [PubMed] [Google Scholar]
- 7. Jian-Ying W, Yuan Y, Xiao-Jun C, et al. (2016) Extract from Eucommia ulmoides Oliv. ameliorates arthritis via regulation of inflammation, synoviocyte proliferation and osteoclastogenesis in vitro and in vivo. Journal of Ethnopharmacology 194: 609–616. [DOI] [PubMed] [Google Scholar]
- 8. Jian-Ying W, Xiao-Jun C, Lei Z, et al. (2019) Anti-inflammatory effects of Eucommia ulmoides Oliv. male flower extract on lipopolysaccharide-induced inflammation. Chinese Medical Journal 132: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jian-Ying W, Xiao-Jun C, Lei Z, et al. (2018) Comparative studies of different extracts from Eucommia ulmoides Oliv. against rheumatoid arthritis in CIA rats. Evidence-Based Complementary and Alternative Medicine 2018: 7379893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xing S, Juan R, Yan Z, et al. (2019) Research progress in preparation and pharmacological effects of betulinic acid and its derivatives. China Pharmacy 30: 570–576. [Google Scholar]
- 11. Chintharlapalli S, Papineni S, Ramaiah SK, et al. (2007) Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Research 67: 2816–2823. [DOI] [PubMed] [Google Scholar]
- 12. Lingaraju MC, Pathak NN, Begum J, et al. (2015) Betulinic acid attenuates renal oxidative stress and inflammation in experimental model of murine polymicrobial sepsis. European Journal of Pharmaceutical Sciences 70: 12–21. [DOI] [PubMed] [Google Scholar]
- 13. Yun Y, Han S, Park E, et al. (2003) Immunomodulatory activity of betulinic acid by producing pro-inflammatory cytokines and activation of macrophages. Archives of Pharmacal Research 26(12): 1087–1095. [DOI] [PubMed] [Google Scholar]
- 14. Jingbo W, Aimin C, Qi W, et al. (2015) Betulinic acid inhibits IL-1beta-induced inflammation by activating PPAR-gamma in human osteoarthritis chondrocytes. International Immunopharmacology 29: 687–692. [DOI] [PubMed] [Google Scholar]
- 15. Mathew LE, Rajagopal V, Helen A. (2017) Betulinic acid and fluvastatin exhibits synergistic effect on toll-like receptor-4 mediated anti-atherogenic mechanism in type II collagen induced arthritis. Biomedicine & Pharmacotherapy 93: 681–694. [DOI] [PubMed] [Google Scholar]
- 16. Zhen Qiu S, Yong Yong X. (2019) Medical Statistics, 4th edn. Beijing, China: People’s Medical Publishing House, p. 574. [Google Scholar]
- 17. Tokoroyama T, Ando M, Setoguchi K, et al. (2016) Prevalence, incidence and prognosis of chronic kidney disease classified according to current guidelines: A large retrospective cohort study of rheumatoid arthritis patients. Nephrology Dialysis Transplantation 32: 2035–2042. [DOI] [PubMed] [Google Scholar]
- 18. Wangyang Y, Yi L, Wang T, et al. (2018) MiR-199a-3p inhibits proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes via suppressing retinoblastoma 1. Bioscience Reports 38: BSR20180982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siebert S, Tsoukas A, Robertson J, et al. 2015. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacological Reviews 67(2): 280–309. [DOI] [PubMed] [Google Scholar]
- 20. Liou LB. (2003) Different monocyte reaction patterns in newly diagnosed, untreated rheumatoid arthritis and lupus patients probably confer disparate C-reactive protein levels. Clinical and Experimental Rheumatology 21(4): 437–444. [PubMed] [Google Scholar]
- 21. Müller-Ladner U, Pap T, Gay RE, et al. (2005) Mechanisms of disease: The molecular and cellular basis of joint destruction in rheumatoid arthritis. Nature Clinical Practice Rheumatology 1(2): 102–110. [DOI] [PubMed] [Google Scholar]
- 22. Pap T, Meinecke I, Muller-Ladner U, et al. (2005) Are fibroblasts involved in joint destruction? Annals of the Rheumatic Diseases 64(Suppl. 4): iv52–iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li N, Gong Z, Li X, et al. (2019) Betulinic acid inhibits the migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. International Immunopharmacology 67: 186–193. [DOI] [PubMed] [Google Scholar]
- 24. Walsh DA, Wade M, Mapp PI, et al. (1998) Focally regulated endothelial proliferation and cell death in human synovium. American Journal of Pathology 152(3): 691–702. [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Chen R, Cai G. (2005) Targeting NF-kappaBeta and TNF-alpha activation by electroacupuncture to suppress collagen-induced rheumatoid arthritis in model rats. Alternative Therapies in Health and Medicine 21: 26–34. [PubMed] [Google Scholar]
- 26. Taylor PC. (2005) Serum vascular markers and vascular imaging in assessment of rheumatoid arthritis disease activity and response to therapy. Rheumatology (Oxford) 44(6): 721–728. [DOI] [PubMed] [Google Scholar]
- 27. Paleolog EM. (2002) Angiogenesis in rheumatoid arthritis. Arthritis Research 4: S81–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ballara S, Taylor PC, Reusch P, et al. (2001) Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis and Rheumatism 44(9): 2055–2064. [DOI] [PubMed] [Google Scholar]
- 29. Lainer DT, Brahn E. (2005) New antiangiogenic strategies for the treatment of proliferative synovitis. Expert Opinion on Investigational Drugs 14(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 30. Strunk J, Bundke E, Lange U. (2006) Anti-TNF-alpha antibody Infliximab and glucocorticoids reduce serum vascular endothelial growth factor levels in patients with rheumatoid arthritis: A pilot study. Rheumatology International 26(3): 252–256. [DOI] [PubMed] [Google Scholar]
- 31. Yuan Y, Shen C, Zhao SL, et al. (2019) MicroRNA -126 affects cell apoptosis, proliferation, cell cycle and modulates VEGF/TGF-beta levels in pulmonary artery endothelial cells. European Review for Medical and Pharmacological Sciences 23: 3058–3069. [DOI] [PubMed] [Google Scholar]
- 32. White RA, Malkoski SP, Wang XJ. (2010) TGFbeta signaling in head and neck squamous cell carcinoma. Oncogene 29: 5437–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dzhalilova DS, Diatroptov ME, Tsvetkov IS, et al. (2018) Expression of HIF-1alpha, NF-kappab, and VEGF genes in the liver and blood serum levels of HIF-1alpha, erythropoietin, VEGF, TGF-beta, 8-isoprostane, and corticosterone in Wistar rats with high and low resistance to hypoxia. Bulletin of Experimental Biology and Medicine 165: 781–785. [DOI] [PubMed] [Google Scholar]
- 34. Sweeney SE, Firestein GS. (2004) Signal transduction in rheumatoid arthritis. Current Opinion in Rheumatology 16: 231–237. [DOI] [PubMed] [Google Scholar]







