Abstract
Spontaneous tumor lysis syndrome (SPTLS) is a rare phenomenon that can manifest in rapidly proliferating hematological malignancies and solid tumors prior to initiating cytotoxic therapy. We encountered a patient who originally presented with diffuse lymphadenopathy, abdominal distention, and dyspnea, who had laboratory abnormalities suggestive of SPTLS. His peripheral flow cytometry and lymph node biopsy revealed blastoid-variant mantle cell lymphoma. Prior to initiating chemotherapy, acute kidney injury (AKI) and uric acid had improved with intravenous fluids and the initiation of allopurinol. However, after beginning chemotherapy, the patient developed a second AKI concerning for tumor lysis syndrome (TLS). He went on to have renal recovery and did not require renal replacement therapy. With the exception of case reports, there is limited evidence to guide general medicine clinicians who encounter cases of SPTLS. Expert-based guidelines are available to guide use of rasburicase, an uricase enzyme, before initiation of chemotherapy for certain malignancies when risk for TLS is considered high. Despite these guidelines, the role of rasburicase in preventing AKI remains controversial after inconclusive results in a meta-analysis. The causative relationship between uric acid and AKI in TLS is based on a mechanism of tubular obstruction. There are also mechanisms by which uric acid may cause AKI without tubular obstruction related to acute hyperuricemic nephropathy. Further characterization of the role of uric acid in causing AKI in patients without tubular obstruction may identify new mechanisms of injury and offer insight into new treatment strategies.
Keywords: tumor lysis syndrome, uric acid nephropathy, rasburicase
Introduction
Tumor lysis syndrome (TLS) is an oncologic emergency that often develops after initiating chemotherapy for hematologic malignancies and lymphomas with a high proliferation rate and sensitivity to chemotherapy.1,2 The elevated levels of uric acid may lead to acute renal failure through multiple mechanisms including obstruction by precipitated uric acid crystals, cytokine release syndrome, and a decreased glomerular filtration rate.3-5 The Cairo-Bishop definition of TLS, which describes both laboratory and clinical TLS, was developed in 2004 to help clinicians identify the diagnosis and grade its severity.6 Laboratory TLS is defined by multiple electrolyte abnormalities including hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.7 Clinical TLS is determined by electrolyte changes plus a medical complication such as renal failure, seizure, or cardiac arrhythmia.
Spontaneous TLS (SPTLS) is a rare phenomenon that can develop in high-grade non-Hodgkin lymphomas and hematological malignancy due to rapid proliferation of malignant cells and auto-lysis.1 In this article, we describe a case that resulted in a diagnosis of blastoid-variant mantle cell lymphoma (MCL), with acute kidney injury (AKI) and a uric acid level of more than 20 mg/dL at the time of presentation.
Case Presentation
A 62-year-old male presented to the emergency department with a 2-week history of progressive shortness of breath with exertion. His medical history was relevant for β-thalassemia minor, hypertension, and a 40-year pack history of tobacco use. He initially had shortness of breath with a productive cough and was provided with a brief course of azithromycin by an outpatient physician. While he experienced relief from the cough, his exertional dyspnea continued to worsen, and he developed profound fatigue and night sweats. In the days preceding hospital admission, he reported a noticeable reduction in his urine output, which was associated with painless abdominal distention.
His initial blood counts showed leukocytosis to 21 000/mm3 with 10% lymphocytes and many atypical lymphocytes. He was also found to be thrombocytopenic to 42 000/mm3. His initial creatinine was 3.37 mg/dL with blood urea nitrogen (BUN) of 44 mg/dL and initial potassium level of 5.8 mEq/L and phosphorus of 6.4 mg/dL. The urinalysis was largely normal with a pH of 5.0, specific gravity of 1.015, and no protein or reported crystals. Computed tomography scans of the chest, abdomen, and pelvis were obtained and revealed extensive lymphadenopathy both above and below the diaphragm. There was no evidence of ureteral obstruction on imaging. The largest lymph node was 1.9 cm in diameter, and the spleen was enlarged to 23 cm. Uric acid and lactate dehydrogenase (LDH) levels were then obtained and revealed a uric acid level of 20 mg/dL and LDH of more than 1600 IU/L.
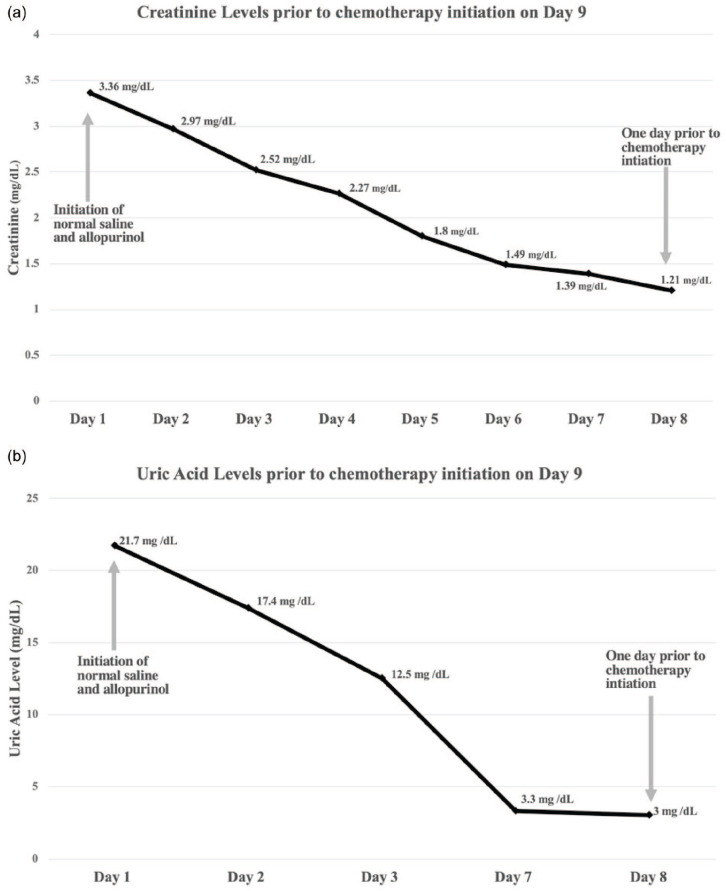
The AKI and acute presentation of dyspnea led to concern for sepsis at presentation. Broad spectrum antibiotics were started, but then discontinued when imaging ruled out consolidations and when there were no infection found in the urinary tract. The patient denied use of nonsteroidal anti-inflammatory drugs and was not exposed to contrast or other nephrotoxins prior to admission. The patient did report oliguria prior to admission and after fluid resuscitation, his renal function gradually improved to 1.2 mg/dL from 3.37mg/dL in 8 days (Figure 1a). During this period of time, his uric acid decreased to 3.0 mg/dL from 21.7 mg/dL (Figure 1b). He was then transferred to the inpatient oncology team while histology results from an axillary lymph node biopsy were pending.
Figure 1.
(a) Trend of creatinine during first 8 days of admission. (b) Trend in uric acid during first 8 days of admission.
Both the lymph node biopsy and peripheral flow cytometry revealed findings consistent with blastoid-variant MCL. The Ki67 of the specimen was greater than 90%. With concern that he would develop an AKI on initiation of chemotherapy, he was preemptively started on continuous normal saline 200 mL/h and allopurinol at a dose of 300 mg 3 times daily. Due to the improvement of his uric acid at the time, he did meet our hospital criteria to receive rasburicase prior to the initiation of chemotherapy. Chemotherapy with rituxumab and hyper-CVAD (cyclophosphamide, vincristine, adriamycin, and dexamethasone) was initiated. The patient remained on allopurinol while receiving chemotherapy.
Within 24 hours of beginning chemotherapy, the potassium, phosphate, and BUN levels rose significantly. The creatinine then began to increase with a peak of 2.69 mg/dL and a BUN of 105 mg/dL. During the 48 hours immediately after starting chemotherapy, the potassium level increased to more than 6.2 mEq/L and the phosphorus level to 12 mg/dL. The uric acid increased minimally from 3 to 4 mg/dL. The patient continued to produce urine and responded to medical therapy primarily targeting hyperkalemia. Dialysis was not required, and as the chemotherapy cycle concluded, renal function started to improve after 3 days and creatinine trended to a new baseline of 0.7 mg/dL.
Discussion
For patients with newly diagnosed lymphoma, risk for development of TLS is stratified from low to high based on the anticipated aggressiveness of the malignancy and its anticipated sensitivity to chemotherapy.1,8 As it pertains to the patient we have described, MCL of the blastoid variant subtype is considered high risk for TLS in cases where the LDH level is more than twice the upper limit of normal.9 On review, the presenting renal dysfunction, uric acid level of more than 20 mg/dL and hyperkalemia, with an ultimate diagnosis of an aggressive non-Hodgkin’s lymphoma, is clinically consistent with a diagnosis of spontaneous TLS. In our review of the literature, we did not identify other examples of SPTLS with blastoid-variant lymphoma. SPTLS is a rare, but accepted phenomenon that is thought to occur only in rapidly proliferating non-Hodgkin’s lymphomas and hematological malignancies.1 Since many cases of SPTLS can mimic other acute non-neoplastic diseases processes, the diagnosis is challenging to confirm without malignant pathology. Additionally, consensus guidelines are not available to guide the general medicine clinician on how to specifically manage the renal dysfunction associated with this condition.
For patients with SPTLS or standard TLS, uric acid elevation is thought to be the cause of AKI with most cases involving deposition of uric acid crystals in the nephron resulting in obstruction and increased tubular pressure.10,11 Increased tubular pressure directly decreases filtration at the glomerulus. Ultimately, the inability to excrete water and electrolytes may lead to uncontrollable electrolyte abnormalities and fluid overload, which can subsequently result in an urgent requirement for dialysis. In our case, we initially assumed uric acid had caused the patient’s AKI by the mechanism described above; however, both ultrasound and computed tomography imaging did not demonstrate evidence of nephrolithiasis. Furthermore, the gradual improvement of renal function and continued urine output also left us questioning this classical mechanism of kidney injury. This led to our review of alternative causes of AKI related to hyperuricemia.
During the workup of our patient, urine eosinophils were absent, and urine microscopic analysis did not reveal pathologic casts. Additionally, the patient had oliguria rather than anuria and did not require dialysis. For these reasons, the patient’s acute uric acid nephropathy and AKI were the result of elevated uric acid levels, but more likely due to crystal independent mechanisms.4,10,12 Using animal models, uric acid has been shown to contribute to AKI by impairing renal autoregulation, the bioavailability of nitric oxide, and proximal tubular proliferation.4,13,14 Hahn et al10 reviewed this topic and described how elevated uric acid levels are associated with elevated levels of inflammatory mediators systemically and in the nephron. The release of cytokines including monocyte chemoattractant protein-1, tumor necrosis factor-α, and intercellular adhesion molecule 1 have been implicated in the nephrotoxicity that uric acid potentiates. In a rat model, mild hyperuricemia has also been described as causing a 50% reduction in glomerular filtration rate and renal blood flow.10,13 Uric acid impairs the bioavailability of nitric oxide, which results in further renal tissue ischemia through vasoconstriction.14 Considering these models for renal injury, future therapies may be developed to treat crystal-independent renal dysfunction related to hyperuricemia.
The current standard for management of renal failure in TLS is based on minimizing tubular obstruction, diluting crystals, and preventing uric acid crystal formation by providing intravenous fluid and uric acid lowering therapy in the form of allopurinol or rasburicase. Use of allopurinol, as in our case, is beneficial in preventing uric acid production but does not reduce the level of uric acid if already elevated.15 Expert guidelines recommend use of rasburicase, a urate oxidase enzyme, for high-risk patients before initiating chemotherapy.11,16 For patients with spontaneous TLS, we did not identify any clear expert-based guidelines for clinicians. This may be due to the rarity of the condition or the possibility of confounding causes of AKI unrelated to uric acid elevation. Use of rasburicase to prevent AKI seems physiologically intuitive, but high-level clinical evidence to support this effect is not present to date. In a nonrandomized study of pediatric patients at high risk for developing TLS, one group trended changes in uric acid and creatinine while receiving either rasburicase or allopurinol, and the results favored improved renal outcomes with the use of rasburicase.17 There has been one clinical trial evaluating the use of rasburicase in adult patients at risk for TLS, and the results did not show a reduced risk of developing an AKI. The study did, however, show that rasburicase correlated with reduced laboratory TLS.18 There has been one meta-analysis performed in review of rasburicase based mostly on trials in pediatric patients. Despite 5 pediatric trials showing a benefit in favor of rasburicase, it was determined that evidence was insufficient to promote the use of rasburicase to prevent TLS or AKI due to the inconclusive findings in a single randomized clinical trial.19 For our case, there is no evidence to guide a decision on whether or not to use rasburicase. Our case adds to a growing need to conduct randomized trials evaluating the use of rasburicase in both SPTLS and TLS.
Summary
In summary, we have reported the first case of SPTLS in a patient with blastoid-variant MCL. We have reviewed the limitations, which many non-oncologist providers may encounter on realization of this condition. Development of consensus diagnostic criteria and management guidelines for spontaneous TLS would be of benefit for non-oncology, general medicine providers. More robust evidence is needed to clarify the role of rasburicase in patients at risk for TLS and in theory, for those with SPTLS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: At the University of Florida, institutional review board or ethic board approval is not required for publication of case reports that do not disclose identifiable patient information.
Informed Consent: Written informed consent and verbal consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Vishal Patel  https://orcid.org/0000-0003-3759-9523
https://orcid.org/0000-0003-3759-9523
References
- 1. Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. Am J Med. 1993;94:133-139. [DOI] [PubMed] [Google Scholar]
- 2. Montesinos P, Lorenzo I, Martín G, et al. Tumor lysis syndrome in patients with acute myeloid leukemia: identification of risk factors and development of a predictive model. Haematologica. 2008;93:67-74. [DOI] [PubMed] [Google Scholar]
- 3. Kjellstrand CM, Cambell DC, von Hartitzsch B, Buselmeier TJ. Hyperuricemic acute renal failure. Arch Intern Med 1974; 133:349-359. [PubMed] [Google Scholar]
- 4. Shimada M, Dass B, Ejaz AA. Paradigm shift in the role of uric acid in acute kidney injury. Semin Nephrol. 2011;31:453-458. [DOI] [PubMed] [Google Scholar]
- 5. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11. [DOI] [PubMed] [Google Scholar]
- 7. Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mato AR, Riccio BE, Qin L, et al. A predictive model for the detection of tumor lysis syndrome during AML induction therapy. Leuk Lymphoma. 2006;47:877-883. [DOI] [PubMed] [Google Scholar]
- 9. Cairo MS, Coiffier B, Reiter A, Younes A; TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586. [DOI] [PubMed] [Google Scholar]
- 10. Hahn K, Kanbay M, Lanaspa MA, Johnson RJ, Ejaz AA. Serum uric acid and acute kidney injury: a mini review. J Adv Res. 2017;8:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darmon M, Vincent F, Camous L, et al. ; Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique (GRRR-OH). Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique. Br J Haematol. 2013;162:489-497. [DOI] [PubMed] [Google Scholar]
- 12. Shimada M, Johnson RJ, May WS, Jr, et al. A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol Dial Transplant. 2009;24:2960-2964. [DOI] [PubMed] [Google Scholar]
- 13. Sánchez-Lozada LG, Tapia E, Santamaría J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237-247. [DOI] [PubMed] [Google Scholar]
- 14. Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553-3562. [DOI] [PubMed] [Google Scholar]
- 15. Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767-2778. [DOI] [PubMed] [Google Scholar]
- 17. Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998-3003. [DOI] [PubMed] [Google Scholar]
- 18. Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheuk DK, Chiang AK, Chan GC, Hay SY. Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2017;(3): CD006945. [DOI] [PMC free article] [PubMed] [Google Scholar]