Abstract
Recently many therapeutic classes have emerged in advanced hormone receptor-positive breast cancer, which is the leading cause of cancer death in women. In absence of visceral crisis, treatment relies on endocrine therapy combined with cyclin dependent kinase 4 and 6 inhibitor. Many mechanisms lead to resistance to endocrine therapy, including the activation of intracellular signaling pathways critical for cell survival. Approximately 70% of breast tumors harbor an alteration in the phosphoinositide 3 kinase (PI3K)/Akt pathway, leading to its hyper activation. This pathway is involved in the regulation of growth, proliferation and cell survival as well as in angiogenesis and is consequently a major target in the oncogenesis. An aberrant PIK3CA mutation is a common phenomenon in breast cancer and found in approximately 40% of patients with advanced hormone receptor-positive breast cancer. For the moment, the only positive trials showing a progression free survival benefit in this population are BOLERO-2 (2012), SOLAR-1 (2019), which tested everolimus, a mammalian target of rapamycin inhibitor, and alpelisib, a PI3K inhibitor, and led to their marketing authorization. However, many other inhibitors of this pathway are promising; nevertheless their development is actually limited by toxicity, mainly cutaneous (rash), digestive (diarrhea) and endocrine (diabetes).
Keywords: endocrine resistance, HR positive advanced breast cancer, PIK3 mutations, PI3K/Akt/mTOR inhibitor, PI3K/Akt/mTOR pathway
Introduction
Breast cancer is the most common cancer in women (2.4 million cases) and the leading cause of cancer deaths (520,000 deaths, of which more than 40,000 per year in the US).1,2 In the metastatic setting, the median overall survival (OS) is around 3 years, regardless of hormonal status or HER2 and the 5-year survival rate is only 25.9%.3 Metastatic disease remains incurable despite the latest therapeutic advances and recent data suggesting an improvement in OS.4 The challenge of treatment is to prolong survival and control the symptoms of the disease while respecting the quality of life.
Around 70% of breast cancers are luminal estrogen receptor-positive, HER2-negative (ER+ HER2−) subtype.5 For this subtype, endocrine therapy (ET) is the core treatment unless there is a visceral crisis or a proof of endocrine resistance. For postmenopausal women, ET includes Selective Estrogen Receptor Modulator (tamoxifen or toremifene), Selective Estrogen Receptor Down-regulator (fulvestrant), non-steroidal aromatase inhibitor (anastrozole and letrozole) and steroidal aromatase inhibitor (exemestane). For pre-menopausal patients, Ovarian Function Suppression (gonadotropin-releasing hormone agonists such as goserelin) or ovarian ablation (with radiation or surgery)6 is combined with ET of postmenopausal women.
Endocrine resistance and tumor progression eventually occurs after exposure to first line ET, with a median time of exposure of 1 year.7 In addition, the efficacy of ET drops with each subsequent line: clinical benefit rate of fulvestrant or aromatase inhibitor (AI) is 70% versus 30% as frontline and second line or above respectively. Endocrine resistance encompasses different situations: primary resistance (progression of disease within the first 6 months of first-line) or secondary resistance (progression disease after 6 months of exposure).
Several mechanisms involved in endocrine resistance have been discovered.8 Dysregulation of activating signal transduction pathways such as that of the Epidermal Growth Factor Receptor or the Insulin Growth Factor Receptor. Dysregulation of the phosphoinositide 3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is particularly involved in the case of secondary endocrine resistance like dysregulation of the cell cycle involving the cyclin D/cyclin dependent kinase 4 and 6 (CDK4/6)/Rb pathway.9
This led to the strategy of combination therapies associating ET and targeted therapies like PI3K pathway inhibitors or CDK4/6 inhibitors. Palbociclib, a CDK4/6 inhibitor (CDK4/6i), is approved in combination with letrozole or fulvestrant for the treatment of postmenopausal women with advanced HR+ HER2– breast cancer. Pre/peri-menopausal patients received also a luteinizing hormone releasing hormone agonist.
First in class, the PALOMA-1/TRIO-18 trial showed that the addition of palbociclib to letrozole significantly prolonged progression-free survival (PFS) as compared with letrozole alone as first line therapy (median PFS 20.2 months versus 10.2 months [hazard ratio, 0.49; 95% confidence interval (CI): 0.32–0.75; p = 0.0004]10 with no OS benefit in the whole population but a trend towards it in the endocrine sensitive one.11 Second, MONARCH 2 and MONALEESA 3 demonstrated similar PFS benefit with abemaciclib and ribociclib with a benefit in OS as the first or second line of treatment of postmenopausal patients. In addition, an OS benefit has been shown in premenopausal women with frontline ribociclib in the MONALEESA 7 trial: median OS 40.9 months in the placebo arm versus not reached in the ribociclib arm (hazard ratio, 0.71; 95% CI: 0.53–0.84).12 Based on these studies, international guidelines now recommend a combination of endocrine therapy and a CDK4/6i (palbociclib, ribociclib or abemaciclib) for HR+, HER 2– advanced breast cancer with no sign of visceral crisis from the first line or later if the patient had already had ET for their metastatic disease.12
However, resistance to CDK4/6 inhibitors is unavoidable in most patients, prompting the exploration of resistance pathways to these treatments. Among the various possible causes of resistance to CDK4/6is, pathological activation of the PI3K/Akt/mTOR pathway has been demonstrated.13–15
The PI3K/Akt/mTOR pathway in estrogen-receptor positive breast cancer
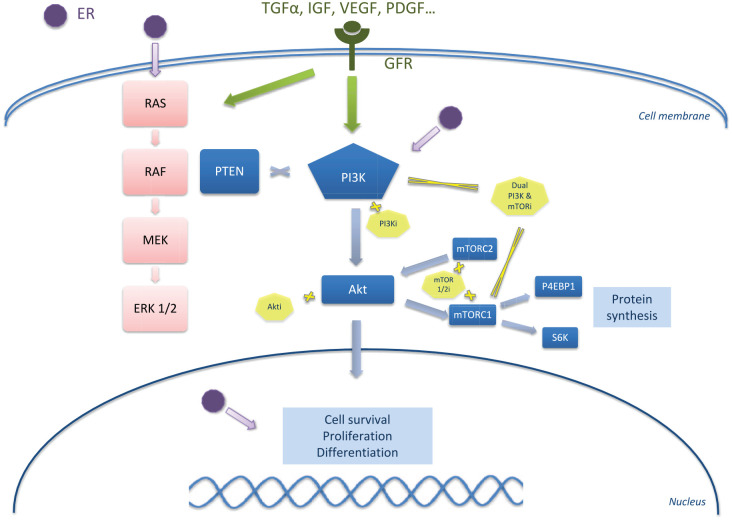
PI3K/Akt/mTOR is one of the major intracellular signaling pathways. The PI3K signaling regulates diverse cellular functions, including cell proliferation, survival, translational regulation of protein synthesis, glucose metabolism, cell migration and angiogenesis.16 Resistance to different therapeutic classes, including chemotherapy, ET and anti-HER2 therapies, are linked to the constitutive activation of the PI3K pathway.17 The PI3K/Akt/PTEN/mTOR pathway is activated in approximately >70% of HR+ breast cancers through AKT1 mutation, loss of PTEN or PI3K activator mutation.18 Discovered in the 1980s, PI3Ks are a family of lipid kinases that phosphorylate the 3′- hydroxyl group of phosphatidylinositols at the level of the plasma membrane.19 PI3K can be divided into three classes of enzyme isoforms (I–III) according to the coding genes, to their substrate preference and structure.20 Class I PI3Ks is composed of class IA and class IB. Class IA PI3Ks are heterodimers consisting of two subunits: a catalytic subunit (p110α, p110β, p110δ) is stabilized by dimerization with a regulatory subunit (p85α, p55α, p50α, p85β, p55γ), forming complexes that are activated downstream of receptor tyrosine kinases. The different isoforms of PI3K have various tissue distributions that inform the expected activity and toxicity profile. The α and β isoforms are ubiquitously expressed and regulate a wide range of physiological processes. The γ and δ isoforms, on the other hand, are preferentially expressed in leukocytes and control different aspects of immune responses (in particular in autoimmune toxicities), explaining the interest of their combination with immune checkpoint inhibitors. The central role in this pathway is played by class IA PI3Ks, which phosphorylates phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) to generate phosphatidylinositol-3 (PIP3),4,5. This subtype is the type most clearly involved in the development of human cancer.21 PIP3, which subsequently leads to the phosphorylation of AKT, a serine/threonine kinase with three isoforms (AKT1, AKT2 and AKT3). AKT is a downstream target of the PI3K pathway and plays a major role in the survival, growth, proliferation and metabolism of glucose in cells.22 Activated AKT induces the activation of the mTOR pathway. mTOR is an atypical serine/threonine protein kinase composed by two distinct protein complexes named mTOR complex 1 (mTORC1) and 2 (mTORC2).23 mTORC1 is highly sensitive to rapamycin and promotes cell growth and cell cycle progression by inducing anabolic processes and inhibiting catabolic processes, respectively. mTORC2 responds to growth factors and regulates metabolism and cell survival, as well as the cytoskeleton. These two complexes mTORC1 and mTORC2 are downstream and upstream of Akt, respectively.24 Activation of the PI3K/Akt/mTOR pathway, which promotes cell proliferation and induces inhibition of pro-apoptotic proteins, is an essential element in the control of cell growth and survival.25 The signal is turned off by PTEN (Phosphatase and TENsin homolog, deleted on chromosome 10), which is a tumor suppressor gene, by dephosphorylating PIP3 to PI-4,5-P2 inhibiting activation of AKT. The PI3K/Akt/mTOR and estrogen-receptor pathways crosstalk by direct or indirect interaction (Figure 1): signaling through the first activates estrogen independent ER transcriptional activity that promotes cell multiplication. Next to it, activation of the estrogen pathway triggers the synthesis of many components of the PI3K/Akt/mTOR pathway.13,26
Figure 1.
Targeting the PI3K/Akt/mTOR pathway.
Akti, capivasertib, ipatasertib; Dual PI3K & mTORi, dactolisib, samotolisib; mTOR1/2i, everolimus, temsirolimus, ridafarolimus; PI3Ki: alpelisib, buparlisib, pictilisib, tazelisib, pilarlisib.
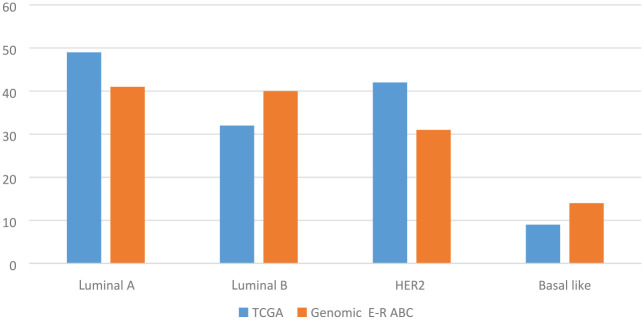
Hyperactivation of the PI3K pathway can occur through several mechanisms: mutation of PI3K (catalytic domain, or helical), loss of PTEN function (deletion or loss of expression, epigenetics), AKT mutation or by the regulatory function of proteins TSC1/TSC2 (tuberous sclerosis complex). The most frequent mutations observed in PIK3CA are clustered in hotspots affecting the helical (exon 9) and kinase (exon 20) domains of the protein.27 PIK3CA mutation frequency varied by subtype of breast cancer: 30–50% of advanced ER+ HER2− breast cancers have an activating PIK3CA mutation; however, PIK3CA is mutated less frequently in ER-negative breast cancers, except for androgen receptor-positive, triple-negative breast cancers (Figure 2).28,29 The clinical development of pan-Class I PI3K inhibitors including buparlisib (BKM120),30 pilaralisib (XL147)31 and pictilisib (GDC-0941)32 has been limited by major toxicities and modest clinical efficacy. The clinical development of these agents has been stopped.33 Isoform-specific inhibitors have allowed these treatments to be given at higher active doses with fewer side effects.34 Thus far, the most successful PI3K inhibitor clinically is alpelisib, which selectively inhibits p110α at least 50 times more than other isoforms.35
Figure 2.
PIK3CA mutation frequency by molecular subtype of breast cancer.
Genomic E-R ABC, Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers; TCGA: The Cancer Genome Atlas
Pivotal clinical trials targeting the PI3K/Akt/mTOR pathway in estrogen-receptor positive breast cancer
Targeting the PI3K/Akt/mTOR pathway involves mTOR inhibitors, PI3K inhibitors, AKT inhibitors or dual mTOR/PI3K inhibitors (Table 1).36–52 Here we detail the phase I to III trials targeting each class with their clinical outcomes and toxicity profile.
Table 1.
Summary of phases I–II–III trials with PI3K_AKT inhibitors in HR positive advanced breast cancer.
| Class | SMI | Study | NCT | Phase and trial design | Population number | Patient population | Treatment | PFS (months) | OS (months) | ORR | Toxicity (Grade 3 and Grade 4 AEs) in experimental arm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PI3K p110α isoform |
Alpelisib
(BYL719) |
SOLAR-136 | NCT02437318 | III randomized, double-blind placebo-controlled, multicenter |
N = 572 Cohort PIK3CA-mut: n = 341 Cohort PIK3CA-WT n = 231 |
Postmenopausal ER+, HER2− ABC progressing after prior ET | Alpelisib (300 mg/d) or placebo + fulvestrant* | Alpelisib: 11 Control: 5.7 HaR = 0.65, p < 0.0001 |
N/A | Alpelisib+fulvestrant: 26.6% Placebo+fulvestrant: 12.8% |
Hyperglycemia 36.6% Rash 9.9% Diarrhea 6.7% |
| Mayer et al.37 | NCT01791478 | Ib open-label study |
N = 26 Cohort PIK3CA-mut: n = 16 Cohort PIK3CA-WT n = 10 |
Postmenopausal ER+, HER2− ABC progressing on/after prior ET | Alpelisib (300 mg/d) + letrozole (2,5 mg/d) | N/A | N/A |
PIK3CA-mut.
25% PIK3CA-WT 10% |
Diarrhea 10% Hyperglycemia 10% AST/ALT elevation 5% |
||
| Juric et al.38 | NCT01219699 | Ib open-label study |
N = 87 Cohort PIK3CA-mut: n = 49 Cohort PIK3CA-WT n = 32 UK status n = 6 |
Postmenopausal ER+, HER2− ABC progressing on/after prior ET | Alpelisib (starting at 300 mg/d) + fulvestrant* |
PIK3CA-mut.,
9.1 PIK3CA-WT, 4.7 |
N/A |
PIK3CA-mut.
29% PIK3CA-WT 0% |
Hyperglycemia 22% Maculopapular rash 13% Rash 8% |
||
| Rugo et al.39 | NCT02437318 | II open-label non-comparative study |
N = 100 Fulvestrant cohort n = 64 Letrozole cohort n = 36 |
Men and women with PIK3CA-mut. ER+, HER2– ABC progressing on/after CDKi + ET | Alpelisib (300 mg/d) + fulvestrant* or Alpelisib + letrozole (2.5 mg/d) |
N/A | N/A | 20% fulvestrant and 18% letrozole | Hyperglycemia 38.1% (fulvestrant) and 27.8% (letrozole)
Rash 4.8% (fulvestrant) and 27.8% (letrozole) |
||
| Sharma et al.40 | NCT02379247 | I/II |
N = 43 Cohort PIK3CA-mut.: n = 19 Cohort PIK3CA-WT: n = 23 |
HER2– ABC, after one line of CT, ER+ or not | Alpelisib (250 mg, 300 mg, 350 mg/d) + nab-paclitaxel (100 mg/m2 d 1, 8, 15 every 28 d) |
PIK3CA-mut.,
13 PIK3CA-WT, 7 HaR = 0.39, p = 0.03 |
N/A | 57% | Neutropenia 31% Hyperglycemia 29% Anemia 12% Diarrhea 7% |
||
| Juric et al.41 | NCT01872260 | Ib/II three-arm study |
N = 98 Arm LEE+LET: n = 41 Arm BYL+LET: n = 21 Arm LEE+BYL+LET: n = 36 |
Postmenopausal women with ER+, HER2– ABC | Letrozole (2.5 mg/d) + escalating doses of LEE qd [(300–500 mg) 3-wks-on/1-wk-off)] or BYL (200–250 mg) | N/A | N/A | 16/27 evaluable patients | Nausea 6% Hyperglycemia 17% Neutropenia 22% Fatigue 11%) |
||
|
Pan-PI3K
inhibitors |
Buparlisib
(BKM120) |
BELLE-243 | NCT01610284 | III randomized, double-blind, placebo-controlled, multicenter study |
N = 1147 Buparlisib+fulvestrant: n = 576 Placebo + fulvestrant: n = 571 |
HER2– ER+ ABC progressing on/after prior ET and after up to one line of CT | Fulvestrant*± buparlisib (100 mg/d) | Buparlisib: 6·9 Placebo: 5 HaR = 0·78, p = 0·00021 |
N/A | Buparlisib: 11.8% Placebo: 7.7% |
Increased ALT 25% Increased AST 18% Hyperglycemia 15% Rash 8%) |
| BELLE-342 | NCT01633060 | III randomized, double-blind, placebo-controlled, multicenter study |
N = 432 Buparlisib: n = 289 Placebo: n = 143 |
HER2– ER+ ABC progressing on/after prior ET and mTOR inhibitors | Fulvestrant (500 mg) ± buparlisib (100 mg/d) | Buparlisib: 3.9 Placebo: 1.8 HaR = 0·67, p = 0.003 |
N/A | Buparlisib: 8% Placebo: 2% |
Increased ALT 22% Increased AST 18% Hyperglycemia 12% Hypertension 16% Fatigue 3% |
||
| BELLE-444 | NCT01572727 | II/III randomized, double-blind, placebo-controlled, multicenter study |
N = 416 Buparlisib: n = 209 Placebo: n = 207 |
HER2– ABC, ER+ (73%) or not, no prior CT for ABC; prior ET allowed | Paclitaxel (80 mg/m2 per wk) in 28-day ± buparlisib (100 mg/d) | Buparlisib + paclitaxel: 8.0 Placebo + paclitaxel: 9.2 HaR = 1·18 |
N/A | Buparlisib + paclitaxel: 22.6% Placebo + paclitaxel: 27.1% |
Neutropenia 14.9% Hyperglycemia 8.9% Rash 7.9% Increased ALT 6.9% Fatigue 5.9% Diarrhea 5.4% Alopecia 5.0% |
||
| PI3K p110α isoform |
Taselisib
(GDC-0032) |
SANDPIPER45 | NCT02340221 | III randomized, double-blind, placebo-controlled, multicenter study |
N = 516 Taselisib: n = 340 Placebo: n = 176 |
Postmenopausal women with ER+, HER2– ABC progressing on/after prior ET | Fulvestrant*± taselisib (4 mg/qd) | Taselisib: 7.4 Placebo: 5.4 HaR = 0·7, p = 0.0037 |
N/A | Taselisib: 28% Placebo: 11.9% |
Diarrhea 12% Hyperglycemia 10% Colitis 3% Stomatitis 2% |
| Dickler et al.46 | NCT01296555 | II open-label |
N = 60 Cohort PIK3CA-mutation: n = 20 Cohort PIK3CA-WT: n = 27 UK status: n = 13 |
Postmenopausal women with ER+, HER2– ABC, progressing after ⩾1 ET line | Fulvestrant*+ taselisib (6 mg/d) |
IK3CA-mut.,
7.6 PIK3CA-WT, 5.4 UK status, 5.3 |
PIK3CA-mut.,
19.2 PIK3CA-WT, 27 UK status, N/A |
PIK3CA-mut.:
38.5% PIK3CA-WT: 14.3% UK status: 20% |
Colitis 13.3% Diarrhea 11.7% Hyperglycemia 6.7% |
||
| Saura et al.47 | NCT01296555 | Ib dose escalation study | N = 28 | ER+, ABC, postmenopausal women progressing after ⩾1 ET line |
Letrozole (2.5 mg) + taselisib (6–9 mg/d) | N/A | N/A |
PIK3CA-mut.:
38% PIK3CA-WT: 9% |
Diarrhea 14% Hyperglycemia 7% Mucosal inflammation 7% |
||
| PIPA48 | NCT02389842 | Ib/II three-arm study | N = 24 | ER+, HER2– ABC, with PIK3CA-mut., progressing after ⩾1 ET line | Taselisib (2 mg/d) + fulvestrant* + palbociclib (125 mg/d on a 3/1 scheme) | 7.9 | N/A | 33% | Neutropenia 57% Rash 11% |
||
|
Pan- PI3K
inhibitors |
Pictilisib
(GDC-0941) |
PEGGY49 | NCT01740336 | II randomized, placebo-controlled |
N = 183 Pictilisib: n = 91 Placebo: n = 92 |
ER+, HER2– ABC, first-/second-line CT | Paclitaxel (90 mg/m2 weekly for 3 weeks in every 28 d) + 260 mg pictilisib or placebo (daily on days 1–5 every week) | Pictilisib: 8.2 Placebo: 7.8 HaR = 0·95, p = 0.83 |
N/A | Pictilisib: 22% Placebo: 19.6% |
67% Neutropenia> peripheral neuropathy> anemia> diarrhea |
| FERGI50 | NCT01437566 | II randomized, double-blind, placebo-controlled, multicenter study | Part 1: n = 168 Part 2: n = 61 |
HER2– ER+ ABC progressing after ET and only PIK3CA-mut. for Part 2 | Fulvestrant* ± pictilisib (340 mg/d in Part 1 or 260 mg/d in Part 2) | Part 1: Pictilisib: 6.6 Placebo: 5.1 HaR = 0·74, p = 0.096 Part 2: Pictilisib: 5.4 Placebo: 10 HaR = 1·07, p = 0.84 |
N/A | Part 1: Pictilisib: 7.9% Placebo: 6.3% Part 2: Pictilisib: 7.3% Placebo: 5% HaR = 1·07, p = 0.84 |
Part 1/Part 2 Maculopapular rash 9% Diarrhea 8% increased ALT 5% Fatigue 8% |
||
| Pan-Akt inhibitors |
Capivasertib
(AZD5363) |
FAKTION51 | NCT01992952 | II randomized, double-blind, placebo-controlled, multicenter study |
N = 140 Capivasertib: n = 69 Placebo: n = 71 |
Postmenopausal ER+, HER2− ABC progressing on/after prior ET | Fulvestrant*± capivasertib [(400 mg t/d) 4-d-on/3-d-off] | Capivasertib: 10.3 Placebo: 4.8 HaR = 0·58, p = 0·0018 |
Capivasertib: 26.0 Placebo: 20.0 HaR = 0·59, p = 0·071 |
Capivasertib: 29% Placebo: 8% |
Hypertension 32% Diarrhea 14% Rash 20% Infection 6%) |
| BEECH52 | NCT01625286 | Ib (Part A) open label; II (Part B) randomized, placebo-controlled, double-blind |
N = 148 Part A: n = 38 Part B: n = 110 |
ER+ HER2– ABC, no prior CT, PIK3CA-mut. or not | Part A: paclitaxel 90 mg/m2 (d 1, 8 and 15 of a
28-d cycle) + escalating doses of capivasertib Part B: paclitaxel ± capivasertib (400 mg b.i.d. 4 d on/3 d off) |
Capivasertib: 10.9 Placebo: 8.4 HaR = 0·80, p = 0·308 |
N/A | Part A: 10.5% Part B: Placebo: 57% Capivasertib: 59% |
Diarrhea 10% Hyperglycemia 10% AST/ALT elevation 5% |
Fulvestrant 500 mg by IM injection at cycle 1, days 1 and 15, and then on day 1 of each subsequent 28-day cycle.
ABC, advanced breast cancer; AI, aromatase inhibitor; CT, chemotherapy; d, day; ER+, estrogen receptor positive; ET, endocrine therapy; HER2−, HER2 negative; HaR, hazard ratio; LET, letrozole; N/A, not applicable; NCT, Clinical Trials.gov identifier; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PIK3CA-mut., mutation in the PIK3CA gene; SMI, small molecule inhibitor; wk, week; WT, wild-type.
mTOR inhibition
The HORIZON study was designed to study the benefit of the addition of temsirolimus, a selective mTORC1 inhibitor, to letrozole in postmenopausal women with HR-positive locally advanced or metastatic breast cancer with no prior exposure to AIs for unresectable or metastatic disease.53 Patients were eligible if their disease did not relapse during the first year following the completion of adjuvant ET. PFS was comparable in both groups (hazard ratio, 0.90; 95% CI: 0.76–1.07; p = 0.25) with no improvement in the temsirolimus group. The same results were observed for OS. In addition, PFS was similar in patients with or without prior adjuvant endocrine therapy (hazard ratio, 0.84; 95% CI: 0.66–1.08; hazard ratio, 0.87; 95% CI: 0.69–1.11, respectively). The authors report also a slight but significant benefit of the combination in patients younger than 65 years (median PFS, 9.0 versus 5.6 months; hazard ratio, 0.75; 95% CI: 0.60–0.93; p = 0.009). This drug led to a significant increase in grade 3 and 4 adverse events with temsirolimus (37% versus 24%), including hyperglycemia, diarrhea, mucositis/stomatitis and hyperlipidemia.
The clinical benefit of everolimus, a rapamycin derivative that inhibits specifically mTORC1, was proven in the randomized phase III, placebo controlled BOLERO-2 trial.54 A total of 724 patients with HR-positive locally advanced or metastatic breast cancer that relapsed or progressed while receiving previous therapy with a non-steroidal AI in the adjuvant setting or to treat advanced disease (or both) were included. Primary endpoint was PFS with a significant benefit for the everolimus arm: hazard ratio 0.43; 95% CI: 0.35–0.54; p < 0.001. Median PFS survival was 10.6 versus 4.1 months, according to central assessment (hazard ratio 0.36; 95% CI: 0.27–0.47; p < 0.001). OS was similar in both groups: 31.0 (everolimus group) versus 26.6 months (placebo group) (hazard ratio, 0.89; 95% CI: 0.73–1.10; p = 0.14).55
PI3K inhibition
The hypothesis of the BELLE-3 trial was that resistance to mTOR inhibitors is potentially due to a feedback activation of the PI3K/Akt/mTOR pathway. Buparlisib is a pan-Class I PI3K inhibitor. The trial included patients who progressed on or after ET combined with mTOR inhibitors. Median PFS was low in both groups but significantly longer in the buparlisib plus fulvestrant versus placebo plus fulvestrant group: 3.9 versus 1.8 months (hazard ratio 0.67, 95% CI: 0.53–0.84, one-sided p = 0.00030), respectively.42 However, it was decided by the trial sponsor that development of the safety profile of buparlisib was inadequate with further investigations and the sponsor stopped its development, in particular due to psychiatric side effects of depression and anxiety. Subgroup analyses showed a greater benefit in the population with PIK3CA mutation in the ctDNA: hazard ratio 0.46 (95% CI: 0.29–0.73); p = 0.00031 versus hazard ratio 0.73 (95% CI: 0.53–1.00); p = 0.026. These results support future trials testing α-selective PI3K inhibitors in combination with ET in patients with PIK3CA mutations.
In the SOLAR-1 trial all patients had received AI during adjuvant therapy or advanced disease and were considered endocrine-resistant as they relapsed during ET or within the 12 months following its completion. Patients were included in two cohorts based on PIK3CA mutation and were randomized to receive fulvestrant plus alpelisib or placebo. Primary end-point was the PFS in the PIK3CA-mutated patient group. In total, 341 of the 572 patients (59%) with HR-positive HER2 negative advanced breast cancer included in the SOLAR-1 trial had confirmed PIK3CA mutation.36 After a median follow-up of 20 months, median PFS was almost doubled in the alpelisib plus fulvestrant group: 11.0 versus 5.7 months (hazard ratio 0.65; 95% CI: 0.50–0.85; p < 0.001). Overall response (26.6% versus 12.8%) and clinical response (61.5% versus 45.3%) rates were also greater in the combination group. In the cohort of patients without PIK3CA-mutated cancer at the final efficacy analysis the median PFS was 7.4 months in the alpelisib–fulvestrant group and 5.6 months in the placebo–fulvestrant group (hazard ratio 0.85; 95% CI: 0.58–1.25), confirming a lack of benefit in patients without a tumor harboring PIK3CA mutation.
AKT inhibition
Similarly, the addition of the investigational AKT 1-3 isoform inhibitor capivasertib to fulvestrant significantly extended PFS for endocrine resistant HR-positive HER2-negative advanced breast cancer patients in the FAKTION phase II study: PFS was 10.3 months for capivasertib compared with 4.8 months for placebo (hazard ratio, 0.57; 95% CI: 0.39–0.84; one-sided p = 0.0017; two-sided 0.0035).51 However, this benefit of capivasertib over placebo was not consistent with the BEECH trial.52 In this last, patients were also considered resistant to ET and received capivasertib or placebo, in combination with weekly paclitaxel and no ET. Capivasertib was well tolerated. Median PFS in the overall population was 10.9 months with capivasertib versus 8.4 months with placebo (hazard ratio 0.80; 80% CI 0.60–1.06; p = 0.308). The result was not better in the subgroup of PIK3CA mutated patients.
Ipatasertib, another AKT inhibitor, was tested with paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer in the LOTUS trial: a multicenter, randomized, double-blind, placebo-controlled, phase II trial.56 PFS was longer in patients who received ipatasertib than in those who received placebo: 6.2 versus 4.9 months (hazard ratio 0.60, 95% CI: 0.37–0.98; p = 0.037), supporting the combination of targeted therapy with chemotherapy. A similar combination will be tested in the IPATunity130 trial: a pivotal randomized phase III trial evaluating ipatasertib + paclitaxel for PIK3CA/AKT1/PTEN-altered advanced breast cancer with both triple-negative and hormone receptor-positive HER2-negative (HR+/HER2–).57
Profile of tolerance and management of side effects
These new treatments have frequent but reversible side effects including hyperglycemia, rash, stomatitis, diarrhea, nausea and fatigue. In the different studies, targeting selectively the PI3Kα isoform decreased the side effects compared with pan-Class I inhibitors. Therefore, in the SOLAR-1 trial evaluating alpelisib (BYL719), an α-specific PI3K inhibitor combined with fulvestrant, the most common grade 3 or 4 adverse events of special interest were hyperglycemia (high blood sugar), rash and diarrhea.58 This toxic profile is similar to other PI3K inhibitors.
Hyperglycemia is a known effect of PI3K pathway inhibitors and is considered an on-target effect. It results partially from the induction of a fasting metabolic state characterized by reduced glucose utilization in favor of fatty acids for energy production. In addition, glucose transport capacity, glycolysis and glycogen synthesis are decreased.59 High blood sugar occurs early around the 15th day in 63.7% of the patients with alpelisib–fulvestrant, leading to an early stopping of the drug in 6.3% of patients. Grade 3 [fasting plasma glucose (FPG) >250–500 mg/dL] and Grade 4 (FPG >500 mg/dL) hyperglycemia were reported in 33% and 3.9% of patients, respectively. Metformin is usually given to manage hyperglycemia in people taking alpelisib–fulvestrant. Hyperosmolar and ketoacidotic states are rare but can occur in patients with pre-existing diabetes.60
Skin toxicity (including (including rash, follicular rash, generalized rash and maculopapular rash) also occurs mostly after 2 weeks of exposure to alpelisib, in around 53.9% of patients (Grade 3/4 in 20.1%). This toxicity was mostly treated with local and/or systemic corticosteroids. Use of anti-rash drugs (antihistamines) prior to the onset of the skin toxicity was associated with a decreased frequency of skin damage (26.7% versus 53.9%). Regarding diarrhea, it occurs later, with a median onset time of 139 days (about 5 months). It was reported in 57.7% (Grade 3/4 in 6.7%). The most commonly used treatments were antipropulsives. Gastrointestinal adverse effects such as diarrhea are regular with metformin. However, in people taking alpelisib with fulvestrant, prescribing metformin did not increase diarrhea. In the case of adverse events requiring a reduction in dose, the dose of alpelisib should be reduced first to 250 mg once daily and then to 200 mg. If dose reduction below 200 mg/day is required, alpelisib should be discontinued.61 The most common side effects that led to stopping taking alpelisib and fulvestrant were hyperglycemia (6.3% of patients) and rash (3.2% of patients).36
Conclusion
To date, the frontline reference treatment for advanced or metastatic HR+ HER2 negative breast cancer is a combination of ET with a CDK4/6i. At progression, targeting the PI3K/Akt/mTOR pathway seems with alpelisib and ET has proven a significant PFS benefit. Management of toxicity, including diarrhea and hyperglycemia, is critical as it may lead to the early cessation of the drug in the case of insufficient management. This will probably limit explorations combining hormone therapy, CDK4/6i and PI3K/Akt/mTOR inhibitor.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jean Sebastien Frenel  https://orcid.org/0000-0002-5273-0561
https://orcid.org/0000-0002-5273-0561
Contributor Information
Pauline du Rusquec, Department of Medical Oncology, Institut Curie, PSL Research University, Paris, France.
Cyriac Blonz, Department of Medical Oncology, Institut de cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
Jean Sebastien Frenel, Department of Medical Oncology, Institut de cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
Mario Campone, Department of Medical Oncology, Institut de cancerologie de l’ouest site René Gauducheau, Saint Herblain, France.
References
- 1. Fitzmaurice C, Allen C, Barber RM, et al. ; Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol 2017; 3: 524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cancer of the Breast (Female) - Cancer Stat Facts. SEER, https://seer.cancer.gov/statfacts/html/breast.html (accessed 9 May 2020).
- 3. Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005-2015). Breast Edinb Scotl 2018; 39: 131–138. [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi K, Ito Y, Matsuura M, et al. Impact of immunohistological subtypes on the long-term prognosis of patients with metastatic breast cancer. Surg Today 2016; 46: 821–826. [DOI] [PubMed] [Google Scholar]
- 5. Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardoso F, Senkus E, Costa A, et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol 2018; 29: 1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 2001; 19: 2596–2606. [DOI] [PubMed] [Google Scholar]
- 8. Ma CX, Reinert T, Chmielewska I, et al. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 2015; 15: 261–275. [DOI] [PubMed] [Google Scholar]
- 9. Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 2011; 18: 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 11. Turner NC, Slamon DJ, Ro J, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 2018; 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 12. Tripathy D, Im S-A, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 13. Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 2011; 29: 4452–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa C, Wang Y, Ly A, et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov 2020; 10: 72–85. [DOI] [PubMed] [Google Scholar]
- 15. O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018; 8: 1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katso R, Okkenhaug K, Ahmadi K, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2001; 17: 615–675. [DOI] [PubMed] [Google Scholar]
- 17. McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006; 46: 249–279. [DOI] [PubMed] [Google Scholar]
- 18. Miller TW, Rexer BN, Garrett JT, et al. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res BCR 2011; 13: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Santis MC, Gulluni F, Campa CC, et al. Targeting PI3K signaling in cancer: challenges and advances. Biochim Biophys Acta Rev Cancer 2019; 1871: 361–366. [DOI] [PubMed] [Google Scholar]
- 20. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 2006; 7: 606–619. [DOI] [PubMed] [Google Scholar]
- 21. Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27: 5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009; 9: 550–562. [DOI] [PubMed] [Google Scholar]
- 23. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med 2016; 67: 11–28. [DOI] [PubMed] [Google Scholar]
- 25. Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene 2008; 27: 5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol 2019; 30: x3–x11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle 2004; 3: 1221–1224. [DOI] [PubMed] [Google Scholar]
- 28. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lehmann BD, Bauer JA, Schafer JM, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 2014; 16: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodon J, Braña I, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs 2014; 32: 670–681. [DOI] [PubMed] [Google Scholar]
- 31. Shapiro GI, Rodon J, Bedell C, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res 2014; 20: 233–245. [DOI] [PubMed] [Google Scholar]
- 32. Sarker D, Ang JE, Baird R, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res 2015; 21: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018; 15: 273–291. [DOI] [PubMed] [Google Scholar]
- 34. Dienstmann R, Rodon J, Serra V, et al. Picking the Point of Inhibition: A Comparative Review of PI3K/AKT/mTOR Pathway Inhibitors. Mol Cancer Ther 2014; 13: 1021–1031. [DOI] [PubMed] [Google Scholar]
- 35. Fritsch C, Huang A, Chatenay-Rivauday C, et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 2014; 13: 1117–1129. [DOI] [PubMed] [Google Scholar]
- 36. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019; 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 37. Mayer IA, Abramson VG, Formisano L, et al. A phase Ib study of Alpelisib (BYL719), a PI3Kα-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res 2017; 23: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Juric D, Janku F, Rodón J, et al. Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol 2018; e184475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rugo HS, Ruiz Borrego M, Chia SKL, et al. Alpelisib (ALP) + endocrine therapy (ET) in patients (pts) with PIK3CA-mutated hormone receptor-positive (HR+), human epidermal growth factor-2-negative (HER2-) advanced breast cancer (ABC): first interim BYLieve study results. J Clin Oncol 2019; 37: 1040–1040. [Google Scholar]
- 40. Sharma P, Abramson VG, O’Dea A, et al. Clinical and biomarker results from phase I/II study of PI3K inhibitor BYL 719 (alpelisib) plus nab-paclitaxel in HER2-negative metastatic breast cancer. J Clin Oncol 2018; 36: 1018–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Juric D, Ismail-Khan R, Campone M, et al. Abstract P3-14-01: phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy and molecular analysis. Cancer Res 2016; 76: P3-14. [Google Scholar]
- 42. Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3). Lancet Oncol 2018; 19: 87–100. [DOI] [PubMed] [Google Scholar]
- 43. Baselga J, Im S-A, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martín M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2– advanced breast cancer (BELLE-4). Ann Oncol 2017; 28: 313–320. [DOI] [PubMed] [Google Scholar]
- 45. Baselga J, Dent SF, Cortés J, et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): primary analysis from SANDPIPER. J Clin Oncol 2018; 36: LBA1006–LBA1006. [Google Scholar]
- 46. Dickler MN, Saura C, Richards DA, et al. Phase II study of taselisib (GDC-0032) in combination with fulvestrant in patients with her2-negative, hormone receptor–positive advanced breast cancer. Clin Cancer Res 2018; 24: 4380–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saura C, Sachdev J, Patel MR, et al. Abstract PD5-2: Ph1b study of the PI3K inhibitor taselisib (GDC-0032) in combination with letrozole in patients with hormone receptor-positive advanced breast cancer. Cancer Res 2015; 75: PD5-2-PD5-2. [Google Scholar]
- 48. Pascual J, MacPherson IR, Armstrong AC, et al. PIPA: a phase Ib study of β-isoform sparing phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib (T) plus palbociclib (P) and fulvestrant (FUL) in PIK3CA-mutant (mt) ER-positive and taselisib (T) plus palbociclib (P) in PIK3CA-mutant (mt) ER-negative advanced breast cancer. J Clin Oncol 2019; 37: 1051–1051.30817251 [Google Scholar]
- 49. Vuylsteke P, Huizing M, Petrakova K, et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann Oncol Off J Eur Soc Med Oncol 2016; 27: 2059–2066. [DOI] [PubMed] [Google Scholar]
- 50. Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016; 17: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2020; 21: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turner NC, Alarcón E, Armstrong AC, et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol 2019; 30: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2013; 31: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med 2012; 366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 2014; 25: 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017; 18: 1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dent R, Kim S-B, Oliveira M, et al. IPATunity130: A pivotal randomized phase III trial evaluating ipatasertib (IPAT) + paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered advanced triple-negative (TN) or hormone receptor-positive HER2-negative (HR+/HER2–) breast cancer (BC). J Clin Oncol 2018; 36: TPS1117–TPS1117. [Google Scholar]
- 58. Rugo HS, André F, Yamashita T, et al. 324PAlpelisib (ALP) + fulvestrant (FUL) for patients with hormone receptor–positive (HR+), HER2− advanced breast cancer (ABC): management and time course of key adverse events of special interest (AESIs) in SOLAR-1. Ann Oncol; 30. Epub ahead of print 1 October 2019. DOI: 10.1093/annonc/mdz242.019. [DOI] [Google Scholar]
- 59. Busaidy NL, Farooki A, Dowlati A, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol Off J Am Soc Clin Oncol 2012; 30: 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nunnery SE, Mayer IA. Management of toxicity to isoform α-specific PI3K inhibitors. Ann Oncol Off J Eur Soc Med Oncol. Epub ahead of print 18 October 2019. DOI: 10.1093/annonc/mdz440. [DOI] [Google Scholar]
- 61. PIQRAY® (alpelisib) Dosing & Administration | HCP, https://www.hcp.novartis.com/products/piqray/metastatic-breast-cancer/dosing-and-administration/ (accessed 27 October 2019).