Abstract
Purpose: To determine the efficacy of acupuncture on the management of hormone therapy–related side effects in breast cancer patients. Methods: Randomized controlled trials of acupuncture versus a control or placebo in breast cancer patients that examined reductions in therapy-related side effects were retrieved from PubMed, EMBASE, Web of Science, and the Cochrane Library through April 2020. Data on patient symptoms (hot flashes, fatigue, pain, stiffness, and gastrointestinal symptoms), physical capacity, cytokines, and general psychosomatic well-being were analyzed. We evaluated and analyzed the quality of all included studies with the 5.2 Cochrane Handbook standards using Stata software (version 10.0) and Revman software (version 5.2), respectively. We assessed the risk of bias using the Cochrane Risk of Bias tool and evaluated the quality of evidence using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach. Results: The pooled results suggested that acupuncture led to moderate improvements in hot flashes, fatigue, and stiffness. No significant differences were observed in pain, gastrointestinal symptoms, Kupperman index scores, Overall quality of life, tumor necrosis factor levels, and interleukin levels. Conclusions: Evidence for outcome indicators of symptom management were downgraded by the GRADE system for inconsistency, indirectness, and imprecision in the included RCTs. Nonetheless, acupuncture is a moderately appropriate alternative therapy for hormone therapy–related side effects in breast cancer patients. However, it still lacks large-sample, multicenter, prospective RCTs. Future research should focus on standardizing comparison groups and treatment methods, be at least single-blinded, assess biologic mechanisms, have adequate statistical power, and involve multiple acupuncturists.
Keywords: breast cancer, acupuncture, meta-analysis, alternative therapy, GRADE
Introduction
Breast cancer is the most common cancer affecting women. Furthermore, it is the overall leading cause of death for women in the world, the second in Africa and Southeast Asia after cervical cancer, and the fifth in the West Pacific. Globally, breast cancer was the most common cancer type, representing 13.8% of all cancer cases. A study in China, India, and Russia, showed that breast cancer was the second cause of death among women after lung cancer in 2014.1
The side effects of breast cancer therapy represent a major public health concern.2 The prevalence of psychoneurological cluster symptoms in these patients varies across studies from 25% to 100%, depending on the type and stage of breast cancer and treatment modality.3-6 Furthermore, adverse effects have been associated with postoperative symptoms (eg, fatigue, pain, limb stiffness, and immunity reaction to psychological stress). Hormone therapy–related symptoms can be classified due to the therapy’s antiestrogenic actions (eg, hot flashes, vaginal bleeding, discharge, or dryness) or as more general effects (eg, nausea, vomiting, and wide range of physical and mental symptoms). Severe surgical trauma, hormone-associated treatment, and psychosomatic stress were the main causes of postoperative complications, especially breast cancer hormone–associated symptoms, which are reported in up to 50% of women.7
Acupuncture is widely accepted as beneficial for breast cancer survivors, both on and off treatment. There is growing evidence for complementary and integrative medicine for the treatment of psychoneurological cluster symptoms and overall quality of life (QOL) improvements.8-10 Alternative medicines have gained increasing attention for use in the management of adverse postoperative reactions and symptoms.11-14 Previous research in the United States has been unclear in determining whether acupuncture reduces postoperative symptoms in breast cancer patients.15-19
Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomized acupuncture interventions trials is common. Acupuncture recommendations, the criteria which randomized controlled trials (RCTs) consider, and the evidence used to reach their final conclusions are often equivocal. Systematic and transparent systems for decision making can help ensure that all important criteria are considered and that the best available evidence informs decisions. The results of previous studies were inconsistent and controversial.14,18 Therefore, assessing the effectiveness of acupuncture in the management of hormone therapy–related side effects in breast cancer by conducting a systematic review is valuable.
To explore this issue further, we used a Cochrane systematic review and the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to conduct a comprehensive evaluation and generate recommendations on the basis of the relevant evidence regarding the use of acupuncture in managing the hormone therapy–related side effects of breast cancer.
Methods
Search Strategy and Selection Criteria
We included reports of placebo or controlled RCTs on acupuncture for side effect management in postoperative hormone therapy–related breast cancer patients. Only English-language literature was searched; no publication date or status restrictions were imposed. Female participants were included if they were (1) aged 18 years or older; (2) patients diagnosed with breast cancer by pathology or cytology20; or (3) were actively undergoing breast cancer treatment and/or hormone therapy. All types, doses, and regimens of acupuncture and electroacupuncture were included, as were placebo and control groups. Primary outcome measures were breast cancer therapy–related side effects, and a secondary outcome measure was physical well-being. Participants were excluded if they (1) did not meet the diagnostic criteria of breast cancer; (2) had postoperative lymphatic drainage of breast cancer; (3) the body function could not tolerate acupuncture therapy; (4) had mental disorders or taking psychoactive drugs with a definite clinical diagnosis; (5) receiving blood transfusion or steroid treatment; (6) with a second solid tumor; (7) with contraindications of acupuncture therapy: those with bleeding tendency, severe allergic and infectious skin diseases, thyroid dysfunction, epilepsy, cerebral cortex damage, and a cardiac pacemaker installed.
Data Sources and Search Strategy
PubMed (1966 to April 2020), EMBASE (1974 to April 2020), the Cochrane Library (issue 4 through 2020), and the Web of Science (1974 to April 2020) were searched through April 2020 using the following terms: (“breast neoplasms”[MeSH Terms] OR “breast neoplasm”[Title/Abstract] OR “breast cancer”[Title/Abstract] OR “breast tumor”[Title/Abstract] OR “breast neoplasms”[Title/Abstract] OR “breast cancers”[Title/Abstract] OR “breast tumors”[Title/Abstract]) AND “electro-acupuncture” [MeSH Terms] OR “electro-acupuncture”[Title/Abstract] OR “Electroacupuncture” [MeSH Terms] OR “Electroacupuncture”[Title/Abstract] OR (“Acupunc-ture”[MeSH Terms] OR “Acupuncture”[Title/Abstract]) AND (random* OR “Clinical Trials as Topic”[Mesh] OR “Clinical Trial” [Publication Type]). Reference lists were reviewed to identify additional studies, and the final bibliography was distributed to experts to identify missing studies.
Data Abstraction and Assessment of Bias Risk
The titles and abstracts that matched the criteria of our meta-analysis were independently read by 2 professional appraisers (Tang Yong and Xiping Shen), and the full texts of the RCTs determined to have met the criteria were obtained. Final decisions on inclusion were made after examination of the full manuscripts. Data points from the included reports were individually extracted at least twice by 4 independent reviewers. Data included the number of participants, their characteristics, the intervention setting, placebo/control group, underlying participant disease status, study design, and acupuncture treatment characteristics.
The methodological quality of all studies was assessed using the criteria stated in the Cochrane Handbook version 5.2, with all results reported according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.21,22
All studies were assessed for risk of bias by at least 2 reviewers using the Cochrane Collaboration risk of bias tool on the basis of sequence generation, allocation concealment, blinding of the study participants and investigators, whether incomplete outcome data were addressed, selective outcome reporting, and other sources of bias. Final assessments were based on a selection consensus, data extraction, and the quality of inter-reviewer assessments.21
Data Analyses
For continuous outcomes, standardized mean differences (SMDs) or mean difference (MD) and 95% confidence intervals (CIs) were calculated. Binary outcomes were pooled using an odds ratio (OR) and 95% CI. A subgroup analysis was conducted according to the endpoint measurement methods and evaluated subjects. The meta-analysis was performed with Stata software (version 10.0, Stata Corp)23 and Review Manager Software (RevMan, version 5.2).24 A meta-regression and subgroup analysis approach was adopted to explore the possible sources of heterogeneity among the RCTs21; heterogeneities were estimated using Cochran’s Q test, with P < .05 indicating a statistically significant heterogeneity.
Grading of the Evidence
We used the GRADE approach to assess the certainty of our estimates and produce evidence profiles using GRADE proGDT (GRADE pro Guideline Development Tool software, McMaster University, Hamilton, Ontario, Canada, 2015). Evidence was graded as high, moderate, low, or very low quality. All controlled intervention studies that were included were graded as high-quality evidence by default and then downgraded on the basis of prespecified criteria, including risk of bias (assessed by the Cochrane risk of bias tool), inconsistency (substantial unexplained heterogeneity, I2 > 50%, P < .10), indirectness (presence of factors that limit result generalizability), imprecision (whether the 95% CI for pooled effect estimates and crossed a minimally important difference for benefit or harm), and publication bias (significant evidence of publication bias).25
Results
Description of Studies
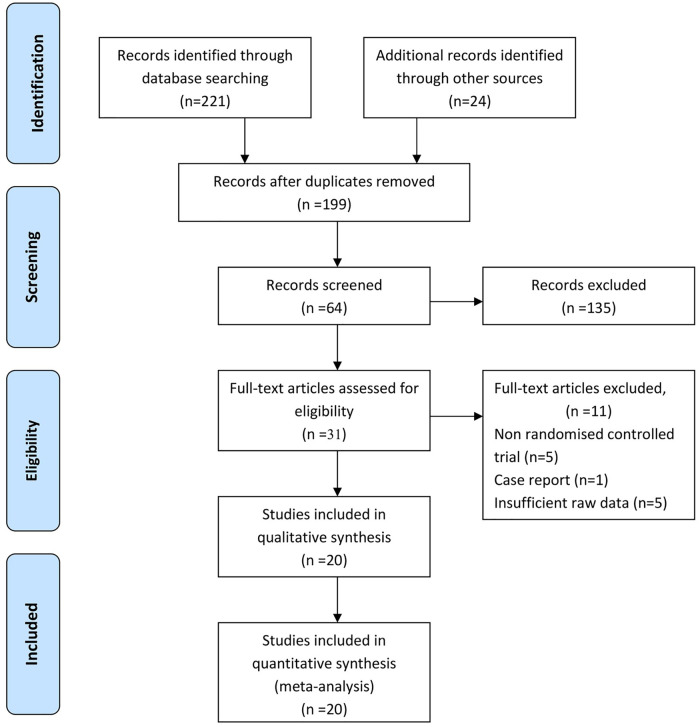
Twenty RCTs with a total of 2001 patients were included in the present review. In total, 12 studies from North America,16,18,19,25-32 9 from the European Union countries,17,33-39 and 1 each from Australia32 and Korea40 were included. All studies were from English databases (Figure 1).
Figure 1.
A flowchart of the results of the literature search.
Study Characteristics
All RCTs included patients with breast cancer (stages 0 to III). The mean age of participants ranged from 30 to 65 years, and all were female. Various controls were compared among the 20 RCTs examined, using 7 sham needles as controls,16,26,30,31,35,36,38 4 using no control treatment,19,33,35,38 and 2 using routine health education.17,18 One study used progressive relaxation programs32 and one used gabapentin41 as a comparison treatment.
Participants were treated with hormone therapy, postoperative radiation, and chemotherapy during the acupuncture intervention.16,17,28,29,34-36 Participants in 6 RCTs28,38,40,41 underwent postoperative radiation and chemotherapy with acupuncture. Participants in 3 RCTs19,26,39 were administered tamoxifen concurrent with acupuncture. Program intensities varied, ranging from twice weekly for 20 to 50 minutes each, with follow-up durations ranging from 6 weeks to 6 months. Acupuncture needling protocols, points, and meridian systems varied, with outcomes including pain-related functional interference, fatigue, gastrointestinal symptoms, postoperative upper limb stiffness, hot flashes, and sleep and mood disorder symptoms (Table 1).
Table 1.
Characteristics of the Included Studies.
| Author/year/country | No. of acupuncture group/control group | Mean age of acupuncture group (years) | Mean age of control group | Status of cancer (years) | Current treatment | Hormone therapy | Duration | Outcome measures/results |
|---|---|---|---|---|---|---|---|---|
| Bao et al26/2013/USA | 23/24 | 61 (44-82) | 61 (45-85) | 0-III | Hormone replacement therapy | Letrozole and/or anastrozole, and/or exemestane ≥1 month | 8 weeks | Significant improvements in physical well-being (HAQ-DI; P < .03) and pain (VAS), significant reduction of IL-17 (P < .00), no significant modulation in proinflammatory cytokine (P > .05) |
| Crew et al27/2007/USA | 9/9 | 47 ± 1.1 | 43 ± 1.5 | II-III | Medicated with tamoxifen, postoperative radiation and chemotherapy | Letrozole and/or anastrozole, and/or exemestane, 6 months | 6 weeks | Significant improvements in anxiety (HADS-A; P < .00), depression (HADS-D; PSS; P < .00) |
| Crew et al28/2010/USA | 20/18 | 58 (44-77) | 57 (37-77) | I-III | Medicated with tamoxifen, chemotherapy and radiotherapy | Letrozole and/or anastrozole, and/or exemestane, 6 months | 6 weeks | Significant improvement in pain and physical well-being (WOMAC; BPI; P < .01), quality of life (FACT-B; BPI; P < .01) |
| Deng et al16/2007/USA | 42/30 | 53.5 | 54 | Unclear | Medicated with tamoxifen, postoperative radiation and chemotherapy | Tamoxifen and/or aromatase inhibitors, within 3 weeks | 4 weeks | No significant improvement in hot flashes (P > .05) |
| Nedstrand et al33/2005/Sweden | 19/19 | 53 | Unclear | Unclear | Medicated with tamoxifen, postoperative radiation, chemotherapy, and radiotherapy | Tamoxifen treatments mentioned, no details | 6 months | Significant reduction in hot flashes (P < .00), KI (P < .00) |
| Frisk et al34/2008/Sweden | 36/36 | 56.5 | 53.4 | I-III | Medicated with tamoxifen, postoperative radiation, and chemotherapy | >2 years sequential estrogen/progestagen combination, >2 years after menopause, given combined estrogen/progestagen | 6 months | Significant reduction in hot flashes (P < .00), KI (P < .05) |
| Hervik and Mjaland35/2009/Norway | 30/29 | 53.6 ± 6.4 | 52.3 ± 6.9 | Unclear | Postoperative radiation and chemotherapy | Tamoxifen for at least 3 months, mentioned, no details | 6 weeks | Significant reduction in hot flashes (P < .00), KI (P < .00) |
| Hervik and Mjaland36/2014/Norway | 43/45 | 52.5 | 50.2 | Unclear | Postoperative, medicated with tamoxifen | Tamoxifen for 3 months | 10 weeks | Significant reduction in KI (P < .10) |
| Hershman et al29/2018/USA | 110/57 | 60.8 | 60.6 | I-III | Chemotherapy, hormone therapy | Anastrozole, letrozole, exemestane | 6 weeks | No significant improvement in pain (BPI-WP; P > .05), physical well-being, and endocrine symptoms (WOMAC; FACT-ES; P > .05) |
| Johnston et al37/2011/USA | 5/7 | 55 ± 6.40 | 53 ± 7.2 | Unclear | Medicated with hormone replacement therapy, postoperative radiation and chemotherapy | Hormone replacement therapy mentioned, no details | 8 weeks | Significant improvement in fatigue (BFI; P > .05) and cognitive dysfunction (FACT-B; P > .05) |
| Lesi et al38/2016/Italy | 105/85 | 49 (31-65) | 50 (27-63) | Unclear | Postoperative chemotherapy | Hormone replacement therapy mentioned, no details | Significant improvements in sleep disturbances (PSQI; P < .00), hot flashes (GCS; P < .00) | |
| Liljegren et al30/2012/Sweden | 38/36 | 58 ± 6.8 | 58 ± 9.3 | I | Medicated with tamoxifen, and chemotherapy | Tamoxifen treatments mentioned, at least 2 months | 6 weeks | Significant reduction in hot flashes (P < .00), significant improvement in physical well-being (WOMAC; P < .01) |
| Mao et al31/2014/USA | 19/21 | 57.5 ± 10.1 | 60.9 ± 6.5 | I-III | Postoperative, medicated with tamoxifen, and chemotherapy | Anastrozole, letrozole, exemestane | 12 weeks | Significant improvements in pain (BPI; P < .00), stiffness (WOMAC; P < .00), and no significant improvement in physical well-being (PPT; P > .05) |
| Mao et al19/2014/USA | 19/21 | 57.5 ± 10.1 | 60.9 ± 6.5 | I-III | Hormone therapy | Anastrozole, letrozole, exemestane | 12 weeks | Significant improvements in fatigue (BFI; P < .00), anxiety (HADS; P < .044), depression (HADS; P < .01), sleep quality (PSQI; P < .05) |
| Mao et al39/2015/USA | 30/32 | 52.9 ± 8.6 | 52 ± 8.9 | I-III | Hormone replacement therapy | Tamoxifen, aromatase inhibitor mentioned, no details | 8 weeks | Significant reduction in hot flash (HFCS; P < .00) |
| Molassiotis et al17/2013/UK | 56/49 | 46 | 53 | I-IIIa | Medicated with tamoxifen, postoperative radiation and chemotherapy | Hormone treatments mentioned, no details | 10 weeks | No significant improvement in fatigue (MFI; P > .05), emotional well-being (HADS; P > .05), and quality of life (FACT-B; P > .05) |
| Nedstrand et al32/2006/Sweden | 17/14 | 30-64 (53) | Unclear | Unclear | Postoperative radiation and chemotherapy | Tamoxifen treatments mentioned, at least 12 weeks | 6 months | Significant reduction in hot flashes (P < .00), KI (P < .00), pain (VAS; P < .00), psychological well-being (SCL; P < .00), mood well-being (MS; P < .00) |
| Smith et al18/2013/Australia | 10/10 | 55 ± 8.8 | 53 ± 12.5 | Unclear | Surgical treatment | Hormone treatments mentioned, no details | 6 weeks | No significant reduction in fatigue (BPI-SF; P > .05), significant improvement on quality of life (MYCaW; P = .00) |
| Garland et al40/2017/Canada | 30/28 | 52.9 ± 8.6 | 50.4 ± 8.4 | 0-III | Postoperative chemotherapy | Tamoxifen, normatase inhibitors | 8 weeks | Significant improvement in sleep disturbances (PSQI; P < .00), hot flashes (HFCS; P < .00) |
| Yao et al41/2016/Korea | 15/15 | 56.2 ± 5.82 | 55.8 ± 5.02 | I-III | Chemotherapy, radiation, and therapy | Not mentioned | 6 weeks | Significant improvement in lymphedema (P < .00), quality of life (QLQ-30; P < .05) |
Abbreviations: HAQ–DI, Health Assessment Questionnaire Disability Index; VAS, Visual Analogue Scale; IL 17, interleukin 17; HADS–A, Hospital Anxiety and Depression Scale–Anxiety; HADS–D, Hospital Anxiety and Depression Scale–Depression; PSS, Perceived Stress Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; BPI, Brief Pain Inventory; FACT–B, Functional Assessment of Cancer Therapy–breast Cancer; KI, Kupperman Index; FACT–ES, Functional Assessment of Cancer Therapy–endocrine Symptoms; PSQI, Pittsburgh Sleep Quality Index; GCS, Greene Climacteric Scale; PPT, Physical Performance Test; BFI, Brief Fatigue Inventory; HFCS, high fructose corn syrup; MFI, Multidimensional Fatigue Inventory; SCL, Symptom Checklist; MS, Mood Scale; BPI–SF, Brief Pain Inventory–Short Form; MYCaW, Measure Yourself Concerns and Wellbeing questionnaire.
Acupuncture Treatment Characteristics
One RCT28 used the National Acupuncture Detoxification Association protocol, 3 RCTs17,18,27 adopted the Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture, 1 RCT used28 traditional Chinese medicine textbooks, 2 RCTs19,30 manualized traditional Chinese medicine theory protocols, and the others utilized published literature. All RCTs used full-body or auricular needles, varying in size from 0.18 mm × 13 mm to 0.20 mm × 40 mm, and fixed-point selection.
In 7 RCTs,16,26,30,31,35,36,38 participants in the control group were administered sham acupuncture. Control group participants underwent progressive relaxation programs,32,39 no treatment, observation at baselinem27,28,34,37 normal practice including pharmacologic and nonpharmacologic treatments,32,40 health education,17 and waitlist controls.19 With the exception of 2 RCTs, which used electroacupuncture32 and thermoacupuncture,40 all patients in the treatment groups were treated with manual acupuncture.
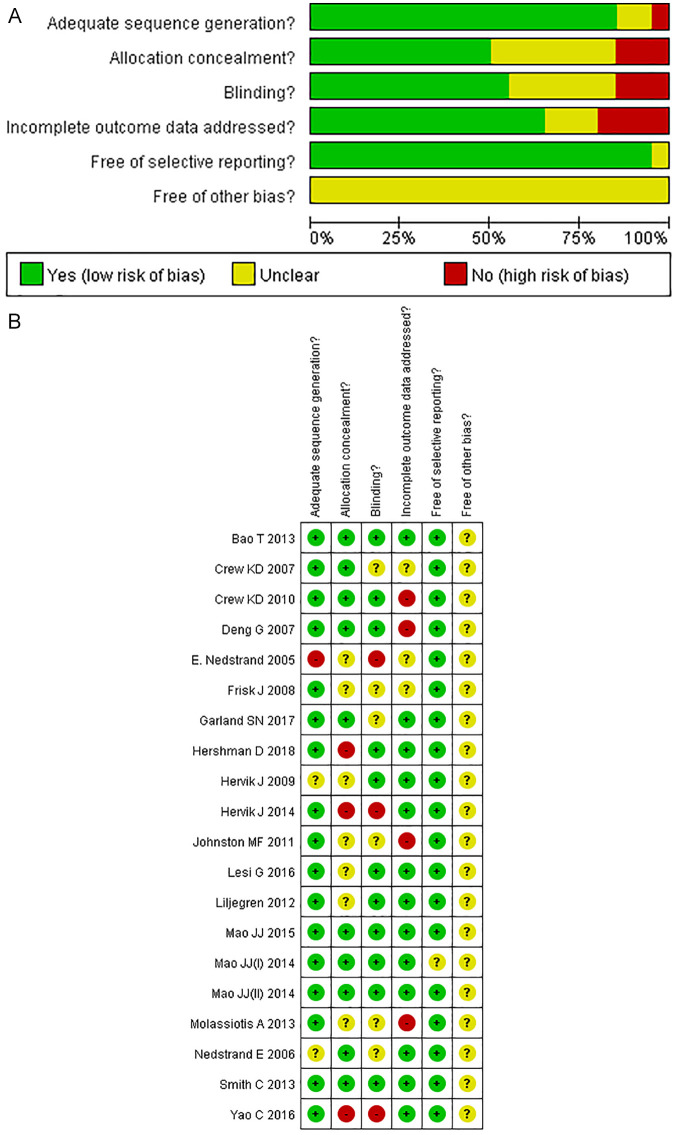
Study Methodological Quality
Of the 20 RCTs examined, 15 utilized randomization.16,18,19,26-29,31,32,34,37-40 The most common randomization method used was computer-generated random numbers. The other 5 did not clearly report a randomization process. In 8 RCTs, blocks were concealed and sequences were stored (in sealed, opaque, numbered envelopes) or otherwise concealed.17-19,28,30,33,35,38 Blinding protocols were described in 17 studies. Blinding was most often imposed by a statistician on instructors and evaluators (Tables 2 and 3).
Table 2.
Methodological Quality of Included Studiesa.
| Author/year/country | Randomization | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Bao et al26/2013/USA | Randomized using a computer-generated random numbers table | Mentioned | Yes (patients, oncologist, and statistician) | No | No | Unclear |
| Crew et al27/2007/USA | Randomized using a computer-generated random numbers table | Mentioned | No | Unclear | No | Unclear |
| Crew et al28/2010/USA | Randomized using a computer-generated random numbers table | Using opaque, numbered envelopes | Yes (patients) | Unclear | No | Unclear |
| Deng et al16/2007/USA | Randomized using a computer-generated random numbers table | Mentioned | Yes (subject, patients) | Yes | No | Unclear |
| Nedstrand et al33/2005/Sweden | Unclear | Using opaque, numbered envelopes | Unclear | Unclear | No | Unclear |
| Frisk et al34/2008/Sweden | Randomized using random number table | Unclear | Unclear | Unclear | No | Unclear |
| Hervik and Mjaland35/2009/Norway | Unclear | Using opaque, numbered envelopes | Yes (patients) | No | No | Unclear |
| Hervik and Mjaland36/2014/Norway | Mentioned | No | Yes (patients) | No | No | Unclear |
| Hershman et al29/2018/USA | Mentioned | No | No | Yes | Yes | Unclear |
| Johnston et al37/2011/USA | Randomized using a computer-generated random numbers table | Unclear | Unclear | Yes | No | Unclear |
| Lesi et al38/2016/Italy | Randomized using a computer-generated random numbers table | Unclear | Yes (subject and patients) | No | No | Unclear |
| Liljegren et al30/2012/Sweden | Randomized using a computer-generated random numbers table | Using opaque, numbered envelopes | Yes (study investigators, subject, statistician, patients) | No | Unclear | Unclear |
| Mao et al31/2014/USA (I) | Randomized using a computer-generated random numbers table | Using opaque, numbered envelopes | Yes (patients) | No | No | Unclear |
| Mao et al19/2014/USA (II) | Randomized using a computer-generated random numbers table | Using opaque, numbered envelopes | Yes (investigator, study staff, statistician | No | No | Unclear |
| Mao et al39/2015/USA | Randomized using random permuted blocks | Unclear | Unclear | Yes | No | Unclear |
| Molassiotis et al17/2013/UK | Unclear | Using opaque, numbered envelopes | Unclear | No | No | Unclear |
| Nedstrand et al32/2006/Sweden | Randomized using random number table | Mentioned | Yes (subject, patients) | No | No | Unclear |
| Smith et al18/2013/Australia | Randomized using random number table | Using opaque, numbered envelopes | Unclear | No | No | Unclear |
| Garland et al40/2017/Canada | Mentioned | No | No | No | No | Unclear |
| Yao et al41/2016/Korea | Randomized using random number table | Mentioned | Yes | No | No | Unclear |
Each item can be categorized based on the answer “yes,” “unclear,” or “no” depending on the appropriateness of the reported information of included randomized controlled trials.
Table 3.
Effect Sizes of Acupuncture Versus Control Interventions.
| Outcomes | No. of studies | No. of patients | Standardized mean difference (95% confidence interval) | Heterogeneity P | I 2 | Test for overall effect P |
|---|---|---|---|---|---|---|
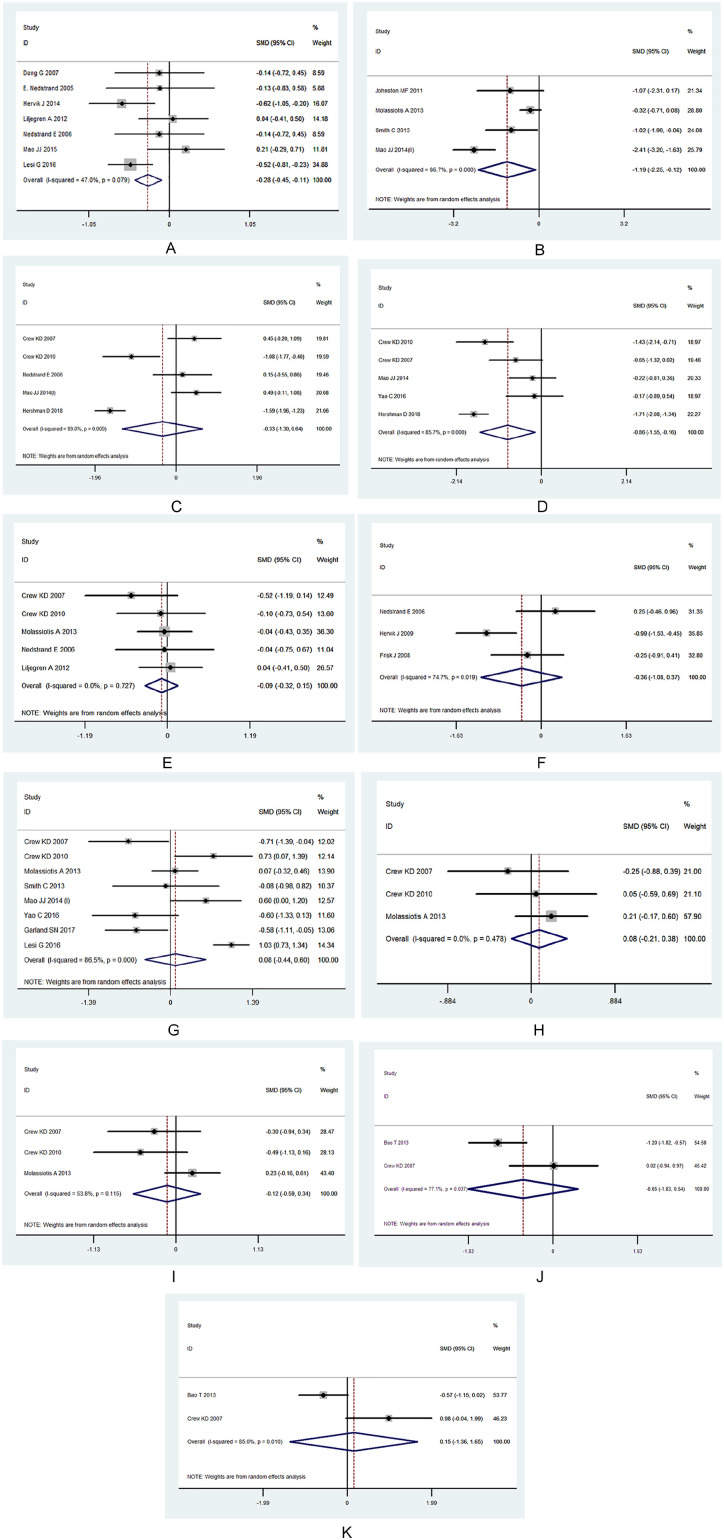
| Hot flashes | 7[14,32,33,36,38,30,39] | 578 | −0.28 (−0.45 to −0.11) | P = .07 | 47% | P = .00 |
| Fatigue | 4[17,18,37,31] | 177 | −1.19 (−2.25 to −0.12) | P = .00 | 86.7% | P = .02 |
| Pain | 5[18,27,28,31,32] | 319 | −0.33 (−1.31 to 0.64) | P = .00 | 93% | P = .50 |
| Stiffness | 5[16,27,28,29,31] | 316 | −0.86 (−1.56 to −0.16) | P = .00 | 85.7% | P = .02 |
| Gastrointestinal symptoms | 5[15,27,28,32,30] | 282 | −0.09 (−0.32 to 0.15) | P = .72 | 0% | P = .47 |
| Kupperman index | 3[34,32,35] | 157 | −0.36 (−1.08 to 0.37)k | P = .74 | 0.01% | P = .34 |
| Physical well-being | 8[17,18,27,28,38,31,40,41] | 576 | 0.08 (−0.44 to 0.60) | P = .00 | 86.5% | P = .76 |
| Social well-being | 3[17,27,28] | 176 | 0.08 (−0.21 to 0.38) | P = .47 | 0.00% | P = .58 |
| Emotional well-being | 176 | −0.12 (−0.59 to 0.34) | P = .11 | 53.8% | P = .61 | |
| TNF | 2[26,27] | 64 | −0.65 (−1.83 to 0.54) | P = .03 | 77.1% | P = .28 |
| IL | 2[26,27] | 64 | 0.15 (−1.36 to 1.65) | P = .01 | 85% | P = .85 |
Abbreviations: TNF, tumor necrosis factor; IL, interleukin,
The remaining RCTs did not mention whether other sources of bias were present. Overall, the Cochrane risk of bias generally revealed a high methodological quality among the RCTs included in this analysis (Figure 2A). The RCTs had an overall low risk of bias (Figure 2B).
Figure 2.
Risk of bias summary A. Risk of bias summary B.
Outcome Analysis
The pooled results suggested that acupuncture led to moderate improvements in hot flashes (SMD = −0.28; 95% CI = −0.45 to −0.11; P = .00), fatigue (SMD = −1.19; 95% CI = −2.25 to 0.12; P = .02), pain (SMD = −1.05; 95% CI = −1.89 to −0.21; P = .01), and stiffness (SMD = −0.06; 95% CI = −1.15 to −0.05; P = .03). No significant differences were observed in gastrointestinal symptoms, Kupperman index scores, physical well-being, social well-being, emotional well-being, tumor necrosis factor levels, or interleukin levels (Table 3; Figure 3A-K).
Figure 3.
(A) Hot flashes. (B) Fatigue. (C) Pain. (D) Stiffness. (E) Gastrointestinal symptoms. (F) Kupperman index. (G) Physical well-being. (H) Social well-being. (I) Emotional well-being. (J) TNF. (K) Interleukin-1.
Meta-Regression and Subgroup Analyses
Heterogeneity was present in the comparison of fatigue (I2 = 86.7%), pain (I2 = 82.7%), stiffness (I2 = 62.8%), physical well-being (I2 = 86.5%), emotional well-being (I2 = 53.8%), tumor necrosis factor (TNF) levels (I2 = 77.1%), and interleukin (IL) levels (I2 = 85%). A meta-regression revealed that the effect of the age, clinical stage, intervention, hormone therapy, acupuncture protocol, acupuncture points, control group, indications, and duration of acupuncture administration on physical well-being and gastrointestinal symptoms explained heterogeneity (Supplementary file table, available online).
Further subgroup analyses indicated that acupuncture >6 weeks had potential advantages for improvement in physical well-being (SMD = 0.66; 95% CI = 0.44 to 0.88; P = .00) when compared with <6 weeks of treatment (Figure 4).
Figure 4.
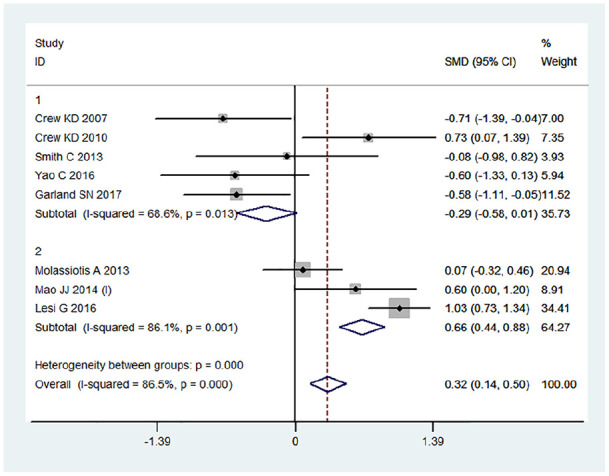
Subgroup forest plots.
GRADE Assessment
Table 4 shows a summary of the overall quality of evidence assessment for the effect of acupuncture on the relevant outcome measures. Certainty in the evidence was variable for hot flashes (low), fatigue (moderate), pain (low), stiffness (low), Kupperman index scores (low), physical well-being (low), TNF levels (low), and IL levels (low). Evidence for all outcomes was downgraded due to inconsistency and imprecision.
Table 4.
GRADE Quality of Evidence Assessment for Acupuncture on Symptom Management of Postoperative Side Effects of Breast Cancer.
| Quality
assessment |
Summary of finding
table |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients |
Effect |
||||||||||
| Outcome/no. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Acupuncture | Control | Relative (95% CI) absolute | ||
| Hot flashes (n = 6) | Randomized trials | Seriousa | Not seriousb | No serious indirectness | Seriousc | None | 299 | 269 | MD = 0.28 (0.45-0.11) | Low | Important |
| Fatigue (n = 4) | Randomized trials | No serious risk of bias | Not seriousb | No serious indirectness | No serious | None | 92 | 85 | MD = 0.06 (1.03-0.92) | Moderate | Important |
| Pain (n = 5) | Randomized trials | Serious | Not serious | No serious indirectness | Seriousc | None | 188 | 131 | MD = 0.98 (0.41-213) | Low | Important |
| Stiffness (n = 4) | Randomized trials | No serious risk of bias | Not serious | No serious indirectness | Seriousc | None | 170 | 116 | MD = 0.62 (0.54-0.76) | Low | Important |
| Kupperman index (n = 5) | Randomized trials | No serious risk of bias | Not serious | No serious indirectness | Seriousd | None | 83 | 74 | MD = 0.21 (0.83-0.4) | Low | Imporatant |
| Physical well-being (n = 7) | Randomized trials | No serious risk of bias | Seriousb | No serious indirectness | Seriousc | None | 305 | 271 | MD = 0.07 (0.4-0.24) | Low | Important |
| TNF (n = 2) | Randomized trials | No serious risk of bias | Not serious | No serious indirectness | Seriousc | None | 33 | 33 | MD = 0.64 (1.83-0.53) | Low | Important |
| IL-1 (n = 2) | Randomized trials | No serious risk of bias | No serious | No serious indirectness | Seriousc | None | 33 | 33 | MD = 0.15 (1.36-1.65) | Low | Important |
Abbreviations: GRADE, grading of recommendations assessment, development, and evaluation; CI, confidence interval; MD, mean differences; TNF, tumor necrosis factor; IL-1, interleukin-1; RCT, randomized controlled trial..
RCTs did not mention or not use the blinding method and randomized grouping.
Evidence of significant interstudy heterogeneity.
Confidence in estimates of effect is poor; RCTs do not calculate the number for optimal information size, and small sample size.
Similarity of point estimates, extent of overlap of CIs is poor.
Discussion
Previous studies have suggested that a substantial proportion of patients with breast cancer use acupuncture and that acupuncture may relieve cancer treatment-related side effects.42-44 The present meta-analysis and systematic review provides preliminary support for the feasibility and safety of acupuncture for breast cancer patients.
The pooled results in the present meta-analysis of 7 relevant RCTs suggest that acupuncture had a small effect on symptom (hot flashes, fatigue, and stiffness) frequency and severity in breast cancer survivors after 6 to 8 weeks of treatment. Subgroup effects of acupuncture on physical well-being over 6 weeks revealed a significant effect of acupuncture compared with a sham treatment. However, it did not find statistically significant differences in treatment-related physical functioning damage, pain, and inflammatory markers (TNF and IL) between the control and real acupuncture groups.
Pathology Parameters
The existing literature often lacks specifics concerning the precise acupuncture methods used. The specific effects of acupuncture are not well understood, and there is clearly a strong placebo component, especially in the management of hot flashes and upper limb stiffness among patients with cancer. Despite the consensus recommendations of the National Institutes of Health, implementation of acupuncture protocols has not been ideal and is still not accepted as a standard treatment. This is in part because acupuncture’s putative mechanisms (including the placebo response) are not well understood. Regardless of the specific molecular basis of these effects, acupuncture for hot flashes and upper limb stiffness is safe and inexpensive, relieves considerable suffering, and may be especially valuable for patients lacking pharmacological symptom control. Although further research is needed, the present review supports the use of acupuncture as an adjunctive treatment for psychosomatic symptoms in breast cancer.32,39
Hormone therapy (eg, letrozole, anastrozole, and exemestane) was particularly prominent across the RCTs included here and has become one of the most common methods for preoperative and postoperative breast cancer treatment. As estrogen deprivation affects tissues beyond the breast, including bone, uterine, and cardiovascular tissues, tamoxifen’s estrogen agonist activity may lead to harmful effects.43 Shah et al17 evaluated the estrogen level decline caused by decreased cerebral cortex endorphin levels, which causes secondary vasomotor and psychological symptoms, including increased endometrial carcinoma, irritability, hot flashes, vaginal dryness, loss of libido, menstrual disorders, fatigue, and musculoskeletal side effects.44
The present meta-analysis included 4 studies on the treatment of stiffness by acupuncture. To address this, 1 RCT used a percutaneous electroacupuncture stimulation treatment mode,28 whereas 3 studies utilized body acupuncture,33,35,36 including 24 points. Pseudo-acupuncture sites deviated from true points by 0.5 inches.
Due to some variability in application, the results of these studies are unclear, regarding the efficacy of acupuncture in the treatment of hot flashes in breast cancer patients taking hormone therapy. Whether the average treatment effect across these studies was clinically significant is complicated by the risk of syndrome differentiation and complex intervention bias, which require careful consideration. The forest plot depicts a wide 95% CI, indicating that the samples included in the present study were small and the follow-up times insufficient, yielding poor accuracy and test efficiency. These results should thus be interpreted with caution.
Physical Parameters
Emotional stress and declines in QOL measures are highly prevalent among postoperative breast cancer patients. The present meta-analysis does not allow for conclusions about how to improve the overall multidimensional physiological and the emotional and social functioning of these patients, however. For example, in 1 RCT,34 patients were administered antidepressants (serotonin reuptake inhibitors such as venlafaxine, paroxetine, sertraline, and fluoxetine) for their vasoconstrictive symptoms (eg, hot flashes). In another,35 gabapentin was used with acupuncture to treat anxiety attacks and sleep disturbances. While antidepressants reduced the frequency and severity of hot flashes, antidepressants and gabapentin relieved some symptoms of anxiety, depression, and pain. Given this, the present meta-analysis could not accurately determine the efficacy of consolidated test validity on the overall QOL measures in the patients assessed.
The variability of physical well-being was assessed based only on the postoperative treatment cycle. The difference in sensitivity between the primary outcome (eg, hot flashes, stiffness) and secondary outcome (general QOL) reports may contribute to the inconsistency between the body function and the overall QOL assessment.
The present meta-analysis included 5 studies14,17,27,29,34 in which all patients underwent different postoperative chemotherapy regimens (CMF regimens, anthracyclines + paclitaxel or CMF + anthracyclines + paclitaxel) within 1 to 6 months of their recruitment. Critically, cytotoxic reactions to chemotherapeutic agents and somatic immune damage may affect appetite and intestinal motility to varying degrees. These agents also cause nausea, bone marrow depression, and ovarian dysfunction. Within 2 to 4 weeks of each adjuvant chemotherapy course, there is also a mild to moderate decrease in the number of white blood cells per serology, which leads to changed levels of ILs and TNF, physical fatigue, and mental exhaustion, in addition to digestive tract changes. High levels of cytokines can increase cell migration, which is dependent on both signal transducers and activators of transcription and nuclear factor κ-light-chain-enhancer of activated B cells. Local in vivo inflammation caused by tumor growth results in elevated inflammatory cytokines such as IL-6 and TNF-α in the tumor microenvironment, which may lead to tumor cell migration and metastasis.45,46 The results of the present study revealed no significant changes in the levels of ILs, as shown previously.40,47
This systematic review has presented an updated view on the clinical applicability of acupuncture, and related therapies for symptom management, the small sample size effect of partial outcome (gastrointestinal symptoms, Kupperman index, physical well-being, social well-being, emotional well-being, TNF, and IL) exists due to the influence of the small sample size. Regardless, the pooled results and meta-analysis still needs to be objectively evaluated.
External and Internal Validity
The present study included a comprehensive and reproducible search of the literature examining the effect of acupuncture on the management of the postoperative side effects of breast cancer, as well as a transparent study selection process. Collation and synthesis of all the available evidence from a large body (20 RCTs, n = 2124) of controlled intervention RCTs was performed, providing for the maximum safeguards against bias. We also included an assessment of the overall quality of evidence using the GRADE assessment approach.
Despite our inclusion of many RCTs, a limited number of studies reported particular indications of acupuncture, and many analyses also had only 2 outcomes available for inclusion: social well-being, emotional well-being, TNF, and IL (2 RCTs). In spite of this, we elected to utilize the GRADE assessment for all outcomes.
Substantial unexplained heterogeneity was present in all substitution study analyses. Furthermore, we saw substantial heterogeneity among subtraction studies for fatigue, pain, gastrointestinal symptoms, Kupperman index scores, physical well-being, emotional well-being, TNF levels, and IL levels. Subgroup analyses did not explain this heterogeneity, so we downgraded these studies due to inconsistency. Inconsistency was not excluded entirely, however, as we were unable to test for heterogeneity with this small number of RCTs. Furthermore, bias was not excluded as we were unable to test for funnel plot asymmetry due to a lack of sufficient power (<10 studies included in the analysis).
Furthermore, no indirectness was present in the RCTs assessed here, as common postoperative treatment symptoms and adverse reactions and side effects were available in all analyses. However, small sample sizes (median = 21 participants) were another potential source of indirectness among the studies assessed here. Ultimately, we did not downgrade the evidence due to indirectness because of the large number of included studies, which represented a diverse range of study conditions and symptoms/phenotypes across participants. We also did not downgrade for indirectness because of the relatively short duration of follow-up (median = 4-12 weeks). We suggest that this follow-up was sufficient to assess the question of applicability and generalizability.
The effects of the duration of acupuncture practice were observed to be heterogeneous. Subgroup analysis showed that physical well-being improved significantly over the 10-week period of acupuncture practice and revealed a linear relationship between positive effects and duration of practice, suggesting that intervention over a relatively long time period (over 2 months) had a potential impact on psychosomatic function.
The major problems in the clinical application of this technique include factors such as the inconsistency of evidence of its effect, the lack of clinical recommendations because of physician attitudes, internal inconsistency of each acupuncture style, diversity of the population of breast cancer patients, and practical concerns from senior instructors. Diversity and biological characteristics of breast cancer with different acupuncture prescription phenomena have widely existed in published RCTs evaluating acupuncture for improving the overall well-being in postoperative breast cancer patients. This causes poor consistency between the systematic reviews of acupuncture interventions in the original studies; the measures of comparison were not uniform. To reduce potential clinical heterogeneity in acupuncture-treatment-related RCTs, the selected setting in acupuncture therapy should be specific, that is, it must identify the population intervention comparison outcome (PICO). In addition, based on GRADE, included RCTs did not calculate the number for optimal information size and the sample size of RCTs was insufficient. This inconsistency and imprecision are major reasons for the downgrade of the level of evidence and strength of recommendation for using acupuncture in breast cancer research and underlies insignificant clinical efficacy. Future RCTs should focus on an adequately large sample size to explore the standardized protocols and detect the interaction between the different biological and sociological characteristics of acupuncture intervention and breast cancer patients.
Finally, we revealed evidence for serious imprecision in all of the analyses included here. The 95% CIs for fatigue, pain, stiffness, gastrointestinal symptoms, Kupperman index scores, physical well-being, TNF levels, and IL levels crossed minimally important differences. Given this, these analyses were downgraded due to serious imprecision. Weighing the strengths and limitations of the RCTs included in the present study, we assessed the quality of the evidence using the GRADE guidelines as low for fatigue and stiffness, low for pain, gastrointestinal symptoms, Kupperman index scores, TNF, IL levels, and general psychosomatic well-being.
Two studies were excluded because of the use of anticonvulsants and opioids as treatment strategies.48,49 There were some concerns around serious methodological defects found in the retrieved original RCTs of acupuncture for therapy-related side effects management with breast cancer patients in the regional language database. Randomization was merely mentioned without any specific methodologies provided. These studies were therefore excluded because they did not meet our research inclusion criteria.
In summary, we systematically assessed current evidence regarding the use of acupuncture in patients suffering from hormone therapy–related side effects. The overall body of evidence was found to be of low-moderate quality. Our critical appraisal of the evidence using the GRADE approach resulted in the formulation of a weak recommendation regarding the use of acupuncture in the hormone therapy–related side effects in breast cancer patients. Nonetheless, the results of this meta-analysis revealed that acupuncture is a moderately appropriate complementary and alternative therapy for hormone therapy–related side effects in breast cancer patients. However, it still lacks large-sample, multicenter, prospective RCTs. Future research should focus on standardizing comparison groups and treatment methods, be at least single-blinded, assess biologic mechanisms, have adequate statistical power, and involve multiple acupuncturists.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We are indebted to the following education and research institutions for financial support. (1) Undergraduate teaching reform project of Guangxi higher education in 2020: exploration on the cultivation of undergraduates’ preclinical research reserve ability based on evidence-based medicine. (2) Guangxi science and technology plan project (Guangxi science and technology base and special talent program): an evidence-based evaluation study of the GRADE system in the evidence system of non-drug complementary alternative medicine.
ORCID iD: Pan Yuanqing  https://orcid.org/0000-0002-6823-9566
https://orcid.org/0000-0002-6823-9566
References
- 1. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20:2015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist. 2015;20:598-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kudach C, Dunwoody C, Wesmiller S. The relationship of age and postoperative pain in women after surgery for breast cancer. Pain Manag Nurs. 2018;19:348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamash KI, Umberger W, Aktas A, Walsh D, Cheruvu VK. The effect of the pain symptom cluster on performance in women diagnosed with advanced breast cancer: the mediating role of the psychoneurological symptom cluster. Pain Manag Nurs. 2018;19:627-636. [DOI] [PubMed] [Google Scholar]
- 5. Brunelle C, Swaroop M, Asdourian M, Skolny M, Sayegh H, Taghian AG. Precautionary behaviors and breast cancer-related lymphedema. Lymphat Res Biol. 2017;15:292-294. [DOI] [PubMed] [Google Scholar]
- 6. Tsaras K, Papathanasiou IV, Mitsi D, et al. Assessment of depression and anxiety in breast cancer patients: prevalence and associated factors. Asian Pac J Cancer Prev. 2018;19:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali MM, Moeller M, Rybicki L, Moore HCF. Prevalence and correlates of patient-reported symptoms and comorbidities in breast cancer survivors at a tertiary center. J Cancer Surviv. 2017;11:743-750. [DOI] [PubMed] [Google Scholar]
- 8. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arif F, Haroon SF, Balagamwala MA. Implementation of acupuncture therapy in the care of patients with breast cancer. J Acupunct Meridian Stud. 2017;10:155-156. [DOI] [PubMed] [Google Scholar]
- 10. Standish LJ, Dowd F, Sweet E, et al. Breast cancer integrative oncology care and its costs. Integr Cancer Ther. 2017;16:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu J, Zhang Z. Review and prospect of clinical application of ear-acupuncture treating post-operative syndromes of breast cancer [in Chinese]. Zhongguo Zhen Jiu. 2018;38:1249-1254. [DOI] [PubMed] [Google Scholar]
- 12. Bao T, Seidman AD, Piulson L, et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer. 2018;101:12-19. doi: 10.1016/j.ejca.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36:2647-2655. [DOI] [PubMed] [Google Scholar]
- 14. Tong T, Pei C, Chen J, Lv Q, Zhang F, Cheng Z. Efficacy of acupuncture therapy for chemotherapy-related cognitive impairment in breast cancer patients. Med Sci Monit. 2018;24:2919-2927. doi: 10.12659/MSM.909712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halsey EJ, Xing M, Stockley RC. Acupuncture for joint symptoms related to aromatase inhibitor therapy in postmenopausal women with early-stage breast cancer: a narrative review. Acupunct Med. 2015;33:188-195. [DOI] [PubMed] [Google Scholar]
- 16. Deng G, Vickers A, Yeung S, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25:5584-5590. [DOI] [PubMed] [Google Scholar]
- 17. Molassiotis A, Bardy J, Finnegan-John J, et al. A randomized, controlled trial of acupuncture self-needling as maintenance therapy for cancer-related fatigue after therapist-delivered acupuncture. Ann Oncol. 2013;24:1645-1652. [DOI] [PubMed] [Google Scholar]
- 18. Smith C, Carmady B, Thornton C, et al. The effect of acupuncture on post-cancer fatigue and well-being for women recovering from breast cancer: a pilot randomised controlled trial. Acupunct Med. 2013;31:9-15. [DOI] [PubMed] [Google Scholar]
- 19. Mao JJ, Xie SX, Farrar JT, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer. 2014;50:267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. Accessed June 23, 2020 https://handbook-5-1.cochrane.org/
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 23. Stata (version 10.0) [Computer program]. Stata Corp. Accessed January 11, 2008 http://www.sassy-software.com 2008
- 24. Cochrane Training. Cochrane RevMan. Accessed June 23, 2020 https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman
- 25. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383-394. [DOI] [PubMed] [Google Scholar]
- 26. Bao T, Cai L, Giles JT, et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat. 2013;138:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crew KD, Capodice JL, Greenlee H, et al. Pilot study of Acupuncture for the treatment of joint symptoms related to adjuvant aromatase inhibitor therapy in postmenopausal breast cancer patients. J Cancer Surviv. 2007;1:283-291. [DOI] [PubMed] [Google Scholar]
- 28. Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28:1154-1160. [DOI] [PubMed] [Google Scholar]
- 29. Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA. 2018;320:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liljegren A, Gunnarsson P, Landgren BM, Robeus N, Johansson H, Rotstein S. Reducing vasomotor symptoms with acupuncture in breast cancer patients treated with adjuvant tamoxifen: a randomized controlled trial. Breast Cancer Res Treat. 2012;35:791-798. [DOI] [PubMed] [Google Scholar]
- 31. Mao JJ, Farrar JT, Bruner D, et al. Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: a randomized trial. Cancer. 2014;120:3744-3751. doi: 10.1002/cncr.28917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nedstrand E, Wyon Y, Hammar M, Wijima K. Psychological well-being improves in women with breast cancer after treatment with applied relaxation or electro-acupuncture for vasomotor symptom. J Psychosom Obstet Gynaecol. 2006;27:193-199. [DOI] [PubMed] [Google Scholar]
- 33. Nedstrand E, Wijma K, Wyon Y, Hammar M. Vasomotor symptoms decrease in women with breast cancer randomized to treatment with applied relaxation or electro-acupuncture: a preliminary study. Climacteric. 2005;8:243-250. [DOI] [PubMed] [Google Scholar]
- 34. Frisk J, Carlhäll S, Källström AC, Lindh-Astrand L, Hammar M. Long-term follow-up of acupuncture and hormone therapy on hot flushes in women with breast cancer: a prospective, randomized, controlled multicenter trial. Climacteric. 2008;11:166-174. [DOI] [PubMed] [Google Scholar]
- 35. Hervik J, Mjåland O. Acupuncture for the treatment of hot flashes in breast cancer patients, a randomized, controlled trial. Breast Cancer Res Treat. 2009;116:311-316. [DOI] [PubMed] [Google Scholar]
- 36. Hervik J, Mjåland O. Long term follow up of breast cancer patients treated with acupuncture for hot flashes. Springerplus. 2014;3:141. doi: 10.1186/2193-1801-3-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnston MF, Hays RD, Subramanian SK, et al. Patient education integrated with acupuncture for relief of cancer-related fatigue randomized controlled feasibility study. BMC Complement Altern Med. 2011;11:49. doi: 10.1186/1472-6882-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lesi G, Razzini G, Musti MA, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: a prospective multicenter randomized controlled trial (AcCliMaT). J Clin Oncol. 2016;34:1795-1802. [DOI] [PubMed] [Google Scholar]
- 39. Mao JJ, Bowman MA, Xie SX, Bruner D, DeMichele A, Farrar JT. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J Clin Oncol. 2015;33:3615-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garland SN, Xie SX, Li Q, Seluzicki C, Basal C, Mao JJ. Comparative effectiveness of electro-acupuncture versus gabapentin for sleep disturbances in breast cancer survivors with hot flashes: a randomized trial. Menopause. 2017;24: 517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yao C, Xu Y, Chen L, et al. Effects of warm acupuncture on breast cancer-related chronic lymphedema: a randomized controlled trial. Curr Oncol. 2016;23:e27-e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simons DG, Travell J. Myofascial trigger points, a possible explanation. Pain. 1981;10:106-109. [DOI] [PubMed] [Google Scholar]
- 43. Vogel L. Hormone therapy study restokes debate over breast cancer risk. CMAJ. 2019;191:E1088-E1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shah JP, Thaker N, Heimur J, et al. Myofascial trigger points then and now: a historical and scientific perspective. PM R. 2015;7:746-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lai HC, Lin YW, Hsieh CL. Acupuncture-analgesia-mediated alleviation of central sensitization. Evid Based Complement Alternat Med. 2019;2019:6173412. doi: 10.1155/2019/6173412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. NIH Consensus Conference. Acupuncture. JAMA. 1998;280: 1518-1524. [PubMed] [Google Scholar]
- 47. Zhang H, Lü JJ, Huang QM, Liu L, Liu QG, Eric OA. Histopathological nature of myofascial trigger points at different stages of recovery from injury in a rat model. Acupunct Med. 2017;35:445-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu W, Giobbie-Hurder A, Freedman RA, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. 2020;25:310-318. doi: 10.1634/theoncologist.2019-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benson S, Hagen S, Hoffmann O, et al. Can a brief psychological expectancy intervention improve postoperative pain? A randomized, controlled trial in patients with breast cancer. Pain. 2019;160:1562-1571. [DOI] [PubMed] [Google Scholar]