Abstract
Background:
Patients with severe influenza-related acute respiratory distress syndrome (ARDS) have high morbidity and mortality. Moreover, nosocomial lower respiratory tract infection (NLRTI) complicates their clinical management and possibly worsens their outcomes. This study aimed to explore the clinical features and impact of NLRTI in patients with severe influenza-related ARDS.
Methods:
This was an institutional review board approved, retrospective, observational study conducted in eight medical centers in Taiwan. From January 1 to March 31 in 2016, subjects were enrolled from intensive care units (ICUs) with virology-proven influenza pneumonia, while all of those patients with ARDS requiring invasive mechanical ventilation and without bacterial community-acquired pneumonia (CAP) were analyzed. Baseline characteristics, critical-illness data and clinical outcomes were recorded.
Results:
Among the 316 screened patients with severe influenza pneumonia, 250 with acute respiratory failure requiring intubation met the criteria of ARDS, without having bacterial CAP. Among them, 72 patients developed NLRTI. The independent risk factors for NLRTI included immunosuppressant use before influenza infection [odds ratio (OR), 5.669; 95% confidence interval (CI), 1.770–18.154], extracorporeal membrane oxygenation (ECMO) use after ARDS (OR, 2.440; 95% CI, 1.214–4.904) and larger corticosteroid dosage after ARDS (OR, 1.209; 95% CI, 1.038–1.407). Patients with NLRTI had higher in-hospital mortality and longer ICU stay, hospitalization and duration on mechanical ventilation.
Conclusion:
We found that immunosuppressant use before influenza infection, ECMO use, and larger steroid dosage after ARDS independently predict NLRTI in influenza-related ARDS. Moreover, NLRTI results in poorer outcomes in patients with severe influenza.
The reviews of this paper are available via the supplemental material section.
Keywords: acute respiratory distress syndrome, influenza, mortality, nosocomial lower respiratory tract infection
Introduction
Bacterial co-infection worsens the clinical course and outcome in patients with influenza. Postmortem analysis of histopathological samples collected during the 1918 Spanish pandemic influenza revealed typical features of acute bacterial pneumonia, and 95.8% of the lung cultures tested positive for bacteria.1 In the 2009 global pandemic H1N1 influenza A virus infection, 18–34% of patients admitted to intensive care units (ICUs) developed bacterial coinfection,2–5 and their in-hospital mortality rate was as high as 30.9%.5
Hospital-acquired pneumonia and ventilator-associated pneumonia (VAP) cause a significant increase in health-care resource utilization and prolong hospitalization. A meta-analysis showed that VAP increased both ICU stay and hospital stay by 8.7 and 11.5 days, respectively.6 In patients with acute respiratory failure developing subsequent VAP, 47% of the isolated pathogens were Gram-negative bacilli (GNB), such as Pseudomonas aeruginosa, Acinetobacter baumannii and Stenotrophomonas maltophilia.7 Among patients with influenza pneumonia and acute respiratory failure, 26% later developed VAP, and the most commonly identified pathogens were GNB, including A. baumannii and P. aeruginosa.2
To date, however, limited data are available on patients with severe influenza infection and nosocomial lower respiratory tract infection (NLRTI), as well as their clinical impact. The present study aimed to describe the clinical characteristics, risk factors and outcomes of patients with severe influenza-related acute respiratory distress syndrome (ARDS) having NLRTI.
Methods
Study design, setting and participants
This multicenter retrospective observational study was conducted in Taiwan. Eight medical centers, including four hospitals in northern Taiwan, two hospitals in central Taiwan and two hospitals in southern Taiwan, participated in the clinical investigation. The data collection period was from 1 January 2016 to 31 March 2016 in all ICUs. All patients were screened for eligibility if the diagnosis of virology-proven influenza was confirmed by the Taiwan Centers for Disease Control on the basis of a rapid influenza diagnostic test, reverse transcription-polymerase chain reaction, or viral culture. We enrolled patients with influenza-related ARDS requiring intubation and mechanical ventilation. The severity of ARDS was graded according to the Berlin definition,8 and ARDS was defined by the acute onset of respiratory distress within 1 week, diffuse opacities in both lungs confirmed using imaging modalities, no evidence of cardiogenic pulmonary edema, and hypoxemia with an arterial partial pressure of oxygen/fraction of inspired oxygen ratio <300 with positive end-expiratory pressure ⩾5 cm H2O.
The study protocol was approved by the Institutional Review Boards of all eight hospitals (Taipei Veterans General Hospital, 2016-05-020CC; Taichung Veterans General Hospital, CE16093A; Tri-Service General Hospital, 1-105-05-086; National Taiwan University Hospital, 201605036RIND; Chang Gung Memorial Hospital, 201600988B0; China Medical University Hospital, 105-REC2-053(FR); Kaohsiung Medical University Hospital, KUMHIRB-E(I)-20170097; and Kaohsiung Chang Gung Memorial Hospital, 201600988B0). The requirement for written informed consent was waived because all patient information was anonymized and de-identified during data recording.
Data collection and measurements
Demographic data including age, sex, body mass index and comorbidities along with critical illness data, such as laboratory results, severity scores and ARDS severity upon initial presentation of influenza-related ARDS, were recorded. Important interventions performed in the ICU, including extracorporeal membrane oxygenation (ECMO), prone position, renal replacement therapy and use of vasopressors, sedatives, neuromuscular blockers and glucocorticoids within 14 days after influenza-related ARDS, were analyzed. The outcome measures encompassed development of NLRTI, in-hospital mortality, ICU stay, hospital stay and duration on mechanical ventilation. Length of hospital stay was calculated from onset of influenza-related ARDS to discharge or death. We recorded the Acute Physiology and Chronic Health Evaluation II (APACHE II) scores9 and Sequential Organ Failure Assessment (SOFA) scores10 on the day of ICU admission and followed up the SOFA scores up to 1 week after hospitalization.
Immunosuppressant use before influenza infection was defined as an oral prednisolone equivalent dose >5 mg/day or >150 mg cumulative dose or other immunosuppressant usage within 1 month before influenza infection.
Patients with bacterial community-acquired pneumonia (CAP) at the onset of ARDS were excluded. The diagnosis of CAP was in accordance with the guideline developed by the American Thoracic Society and Infectious Disease Society of North America.11,12 Antibiotics prescribed upon diagnosis of influenza-related ARDS and lasting for at least 5 consecutive days was also defined as the presence of CAP.
NLRTI was suspected in patients meeting the criteria of ventilator-associated events defined by the Centers for Disease Control and Prevention.13 Evidence of infection was confirmed by two intensivists (KCK and CCS) in agreement with the NLRTI after a detailed chart review of symptoms and vital signs, laboratory data and chest images. Newly developed or progressive respiratory symptoms, unstable vitals, elevated white blood cell counts or left shift, C-reactive protein or procalcitonin level, and new infiltrates on chest radiographs were suggestive of NLRTI. The presence of pathogenic bacteria in the respiratory specimen obtained 48 h after hospitalization and the administration of appropriate antibiotic treatment for at least 7 days were required for confirming a clinical diagnosis of NLRTI. The respiratory specimen was obtained from tracheal aspirates, bronchoalveolar lavage fluids, protected sheath brushing samples or biopsied tissues.
The primary outcome was development of NLRTI. Other outcome measurements were in-hospital mortality, ICU days, hospital days, and duration of mechanical ventilation.
Statistical analysis
The results were presented as mean ± standard deviation, median with interquartile range, or number (%) whenever appropriate. We used the Kolmogorov–Smirnov and Shapiro–Wilk tests to examine the normality of continuous variables. Independent t tests were used to compare normally distributed continuous variables, and the Mann–Whitney U test was used to compare non-normally distributed continuous variables. We used the Pearson χ2 test or Fisher’s exact test to compare categorical variables. Variables showing significant differences between groups were entered into univariate and multivariate logistic regression analyses by using the enter method to determine factors that independently predict NLRTI. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows/Macintosh, Version 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc for Windows, Version 17.9.4 (MedCalc Software bvba, Mariakerke, Belgium).
Results
During the study period, we enrolled 316 patients having complete medical records with virology-proven complicated influenza. Patients were excluded if they had not received invasive mechanical ventilation, did not have a diagnosis of ARDS or confirmed bacterial CAP. Finally, 250 patients were eligible for further analysis (Figure 1).
Figure 1.
Flow chart of the study.
aVirology-proven methods include the rapid influenza diagnostic test, reverse transcription-polymerase chain reaction and virus culture.
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; NLRTI, nosocomial lower respiratory tract infection.
In Table 1, the patients’ characteristics and clinical data are summarized. Most respiratory specimens of NLRTI (81.9%) were collected from tracheal aspirates. In patients with NLRTI, more immunosuppressant use before influenza infection, greater need of extracorporeal membrane oxygenation (ECMO) support, more use of vasopressors and larger steroid dosage were observed.
Table 1.
Characteristics of the 250 subjects with influenza-related ARDS categorized according to NLRTI.
| NLRTI n = 72 |
Without NLRTI n = 178 |
p value | |
|---|---|---|---|
| Baseline data | |||
| Age (years) | 61.0 ± 13.6 | 58.8 ± 14.8 | 0.282 |
| Male | 47 (65.3%) | 110 (61.8%) | 0.606 |
| Body mass index (kg/m2) | 25.1 ± 4.9 | 25.3 ± 5.7 | 0.789 |
| Malignancy | 11 (15.3%) | 21 (11.8%) | 0.456 |
| Type II diabetes mellitus | 22 (30.6%) | 51 (28.7%) | 0.764 |
| Liver cirrhosis | 8 (11.1%) | 24 (13.5%) | 0.611 |
| Cerebrovascular disease | 5 (6.9%) | 15 (8.4%) | 0.696 |
| End-stage renal disease | 6 (8.3%) | 9 (5.1%) | 0.379 |
| Congestive heart failure | 5 (6.9%) | 19 (10.7%) | 0.365 |
| Immunosuppressant use before influenza infectiona | 9 (12.5%) | 5 (2.8%) | 0.005 |
| Subtypes of influenza | |||
| Type A | 57 (79.2%) | 141 (79.2%) | 0.989 |
| Type B | 6 (8.3%) | 14 (7.9%) | |
| Positive, unknown subtype | 9 (12.5%) | 23 (12.9%) | |
| Laboratory data | |||
| Albumin (mg/dL) | 2.8 ± 0.5 | 2.8 ± 0.6 | 0.770 |
| Serum C-reactive protein (mg/dL) | 15.4 ± 9.4 | 15.3 ± 10.6 | 0.959 |
| Serum lactate level (mg/dL) | 24.8 ± 24.3 | 31.9 ± 41.5 | 0.200 |
| Severity scores | |||
| APACHE II | 23.7 ± 8.5 | 23.6 ± 8.5 | 0.914 |
| SOFA score | |||
| Day 1 | 11.7 ± 3.9 | 10.9 ± 2.9 | 0.494 |
| Day 3 | 10.4 ± 5.0 | 10.9 ± 3.2 | 0.733 |
| Day 7 | 9.0 ± 3.9 | 8.8 ± 2.9 | 0.878 |
| PaO2/FiO2 | 102.0 ± 66.3 | 107.9 ± 61.1 | 0.510 |
| ARDSb | 0.920 | ||
| Mild | 8 (11.1%) | 19 (10.7%) | |
| Moderate to severe | 64 (88.9%) | 159 (89.3%) | |
| Management | |||
| ECMOc | 21 (29.2%) | 26 (14.6%) | 0.008 |
| Prone positionc | 17 (23.6%) | 41 (23.0%) | 0.922 |
| Renal replacement therapyc,d | 5 (6.9%) | 25 (14.0%) | 0.118 |
| Vasopressorc | 46 (63.9%) | 88 (49.4%) | 0.038 |
| Sedationc | 57 (79.2%) | 128 (71.9%) | 0.236 |
| Neuromuscular blockadec | 46 (63.9%) | 109 (61.2%) | 0.696 |
| Steroid usec | 43 (59.7%) | 116 (65.2%) | 0.418 |
| Mean steroid dosagec (mg/kg per day)e | 1.6 ± 2.9 | 1.0 ± 1.4 | 0.017 |
Data are presented as mean ± standard deviation and number (%).
Oral prednisolone equivalent dosage > 5 mg/day or > 150 mg cumulative dose within 1 month before influenza infection; or regular treatment using other immunosuppressants within 1 month before influenza infection.
In accordance with the Berlin definition.
In our cohorts, 20 (95.2%) out of 21 patients with NLRTI received ECMO before development of NLRTI. Other managements, including prone position, renal replacement therapy, vasopressors, sedatives, neuromuscular blockade and steroid, were all initiated before NLRTI.
Excluding those with end-stage renal disease receiving regular hemodialysis.
In NLRTI group, mean steroid dosage was calculated only before NLRTI. In patients without NLRTI, mean steroid dosage was calculated from steroid cumulative dosage for 14 days via prednisolone equivalent dose (mg) after ARDS.
APACHE II, Acute Physiology and Chronic Health Evaluation; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; NLRTI, nosocomial lower respiratory tract infection; PaO2, arterial partial pressure of oxygen; SOFA, Sequential Organ Failure Assessment.
The patients with NLRTI also had worse outcomes, including more in-hospital mortality, prolonged hospital and ICU stays and longer duration on mechanical ventilation (Table 2). The median time of acquiring NLRTI was 11 days, and the most commonly isolated pathogenic bacterium was A. baumannii, which was resistant to carbapenem in 96.6% of cases (see Supplemental Material Table 1 online).
Table 2.
Outcomes of the 250 patients with influenza-related acute respiratory distress syndrome categorized according to NLRTI.
| NLRTI n = 72 |
Without NLRTI n = 178 |
p value | |
|---|---|---|---|
| In-hospital mortality | 34 (47.2%) | 51 (28.7%) | <0.001 |
| ICU stay (days) | 30.4 ± 21.5 | 15.1 ± 13.0 | <0.001 |
| Hospital stay (days) | 47.9 ± 36.7 | 25.6 ± 18.6 | <0.001 |
| Ventilation duration (days) | 26.8 ± 18.3 | 13.6 ± 11.0 | <0.001 |
Data are presented as mean ± standard deviation and number (%).
ICU, intensive care unit; NLRTI, nosocomial lower respiratory tract infection.
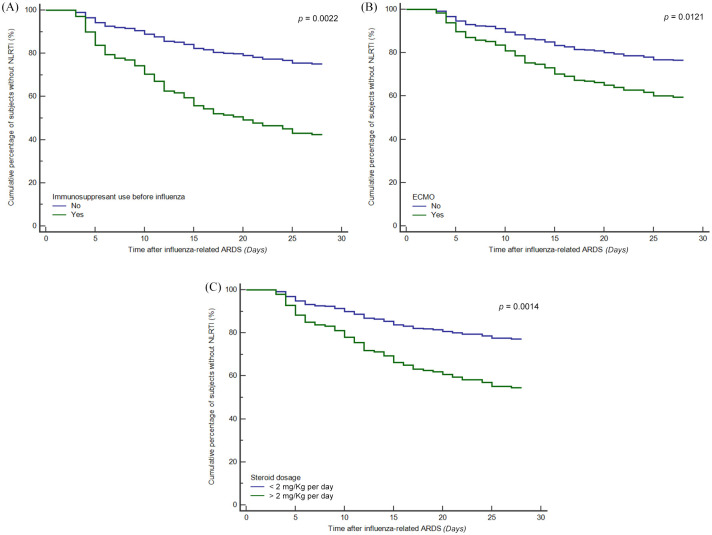
To further clarify the clinical risk factors for NLRTI among patients with influenza-related ARDS, we used univariate and multivariate logistic regression analyses (Table 3). Immunosuppressant use before influenza infection (OR, 5.669; 95% CI, 1.770–18.154), ECMO use (OR, 2.440; 95% CI, 1.214–4.904) and mean steroid dosage after influenza-related ARDS (OR, 1.209; 95% CI, 1.038–1.407) were significant variables. The Hosmer–Lemeshow test in our multivariate logistic regression analysis model showed it was a good fit (p = 0.189). In the Cox proportional hazards model, we also found that patients with immunosuppressant use before influenza infection, ECMO use after ARDS, and larger steroid dosage after ARDS had a higher probability of developing NLRTI during their clinical course [Figure 2(A) to (C)].
Table 3.
Risk factors for nosocomial lower respiratory tract infection according to univariate and multivariate logistic regression analyses.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p value | Odds ratio | 95% confidence interval | p value | |
| Immunosuppressant use before influenza infectiona | 4.943 | 1.596–15.311 | 0.006 | 5.669 | 1.770–18.154 | 0.003 |
| ECMO use | 2.407 | 1.248–4.642 | 0.009 | 2.440 | 1.214–4.904 | 0.012 |
| Vasopressor user | 1.809 | 1.030–3.179 | 0.039 | 1.567 | 0.853–2.879 | 0.148 |
| Mean steroid dosage after ARDS (kg/mg per day) | 1.171 | 1.019–1.347 | 0.026 | 1.209 | 1.038–1.407 | 0.015 |
Oral prednisolone equivalent dosage >5 mg/day or >150 mg cumulative dose within 1 month before influenza infection; using other immunosuppressants for more than 1 month.
ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation.
Figure 2.
Cox proportional hazard model for survival estimates by clinical risk factors. (A) Survival estimates for development of nosocomial lower respiratory tract infection (NLRTI) in influenza-related acute respiratory distress syndrome (ARDS) stratified by immunosuppressant use before influenza. Immunosuppressant use before influenza had significantly more NLRTI (p = 0.0022). (B) Survival estimates for development of NLRTI in influenza-related ARDS stratified by extracorporeal membrane oxygenation (ECMO) use. ECMO group had significantly more NLRTI (p = 0.0121). (C) Survival estimates for development of NLRTI in influenza-related ARDS stratified by mean steroid dosage after ARDS. Mean prednisolone equivalent dosage more than 2 mg/kg per day after ARDS had significantly more NLRTI (p = 0.0014).
In our cohort, liver cirrhosis, more severe clinical condition at presentation and during hospitalization, ECMO use, and development of NLRTI were associated with in-hospital mortality (Supplemental Tables 2 and 3).
Discussion
The aim of this study was to explore the clinical features of NLRTI in severe influenza-related ARDS. Multivariate regression analysis showed that immunosuppressant use before influenza infection, ECMO use after ARDS, and larger steroid dosage after influenza-related ARDS were predictors for NLRTI. We also found that patients with NLRTI had poorer outcomes, including higher mortality, longer ICU and hospital stays and longer duration on mechanical ventilation. To our knowledge, this is the first study to report the risk factors associated with NLRTI in severe influenza-related ARDS.
To date, few studies have investigated the incidence of nosocomial infections in patients with severe influenza. A report on the 2009 H1N1 pandemic in Argentina showed that 25% of patients had pneumonia on admission and 26% of patients developed VAP. The mortality rate in the VAP group was 45.2%.2 Similarly, our multicenter data showed that 28.8% of patients with influenza-related ARDS had NLRTI, and the in-hospital mortality rate was 47.2%.
Bacterial co-infection in patients with influenza resulted in the utilization of more medical resources. During the 2009 Spanish H1N1 influenza pandemic, patients in the co-infection group needed more invasive mechanical ventilation and vasopressor use.4 Our data also showed that the NLRTI group required more ECMO support and vasopressor use.
Several risk factors for bacterial co-infection in patients with influenza have been identified. Elderly patients aged more than 65 years old, preschoolers and pregnant women, as well as patients with obesity, chronic pulmonary diseases, cardiovascular disorders, hepatic dysfunction, renal insufficiency, diabetes and an immunocompromised status had a higher risk of bacterial coinfection.14 During the 2009 H1N1 influenza pandemic in Spain, older patients with higher APACHE II and SOFA scores had a higher risk of bacterial coinfection.4 In our cohort, we also noted that immunosuppressant use before influenza infection increased the risk of NLRTI.
Corticosteroid use is associated with various adverse events, including glaucoma, hyperglycemia, cardiovascular diseases, osteoporosis and avascular necrosis of the bone.15–19 In addition, infection is of the utmost concern and is well documented.20 Several observational studies utilizing big databases found that even under a prednisone equivalent dose as low as 5 mg/day, patients with rheumatoid arthritis had an increased risk of bacterial infection with a hazard ratio ranging from 1.32 to 1.78.21,22 Wolf et al. reported an increased risk for pneumonia requiring hospitalization in patients with rheumatoid arthritis on a prednisone dose of 5 mg/day.23 Our result is also consistent with these findings.
The incidence of VAP in patients receiving ECMO ranged from 15.8% to 74%.24–26 ECMO itself was an independent risk factor for both pneumonia and other infections in patients receiving heart transplantation.27 A possible mechanism underlying ECMO-related infection is the decreased monocyte response to pathogens induced by extracorporeal support.28 The impacts of VAP on patients receiving ECMO included longer ECMO duration and increased mortality.29 Our study was the first to show the association between ECMO and NLRTI in patients with severe influenza-related ARDS.
A meta-analysis showed that adjunctive therapy with glucocorticoids was harmful to patients with influenza. Steroid use was associated with an increased risk of mortality (OR, 3.06; 95% CI, 1.58–5.92) in patients with influenza.30 Although no randomized trials have as yet assessed the effect of glucocorticoids on influenza-related ARDS, experiences from treating H1N1 influenza-related ARDS demonstrated that early corticosteroid use is strongly associated with mortality. Patients receiving steroids had more frequent nosocomial pneumonia and showed a trend towards a longer duration of mechanical ventilation. In our study, we also found that larger steroid dosage after influenza-related ARDS increased the risk of NLRTI in such patients.
The pathogens involved in community-acquired bacterial co-infection and nosocomial infection are different. Streptococcus pneumoniae, P. aeruginosa and Staphylococcus aureus were the most commonly encountered bacteria in the community setting.4,5 In contrast, in patients with severe influenza developing subsequent VAP, A. baumannii and P. aeruginosa were the most frequent causative organisms. A. baumannii was isolated from 29 respiratory specimens collected from our NLRTI group, and it was also the most commonly found microorganism in our patients. Notably, nearly all of the isolated A. baumannii bacteria in our series were resistant to carbapenem. This poses a great challenge to our clinicians when prescribing appropriate empiric antibiotics to patients in the NLRTI group.
This study has several limitations. First, this was a retrospective cohort study; therefore, some medical data might be missing. For example, the relative low percentage of sedatives use in our cohort reflected lack of complete medications records. Some patients might be treated with pain control or antipsychotics. Second, the true incidence of nosocomial pneumonia remains uncertain. Owing to the retrospective nature of this study, confirming hospital-acquired pneumonia was difficult. Quantitative cultures from respiratory specimens were not fully available in our cohort. However, NLRTI could serve as a surrogate for clinicians to understand the disease course of severe influenza and to help identify patients at a high risk of bacterial infection. Third, although our study focused on bacterial NLRTI, emerging opportunistic infections, such as those caused by Aspergillus sp. or Candida sp., should also be considered and preemptive diagnosis and treatment should be mandatory. Fourth, although some patients received steroid treatment during hospitalization, whether it was used for ARDS or other indications could not be analyzed. Fifth, the management strategy of influenza-related ARDS might not be standardized between different study sites. Finally, our study concentrated on patients with influenza-related ARDS requiring invasive mechanical ventilation; therefore, whether the results can be applied to patients with less severe influenza remains unknown. To further understand nosocomial bacterial infections, a well-designed prospective clinical study is needed.
Conclusion
This study found that immunosuppressant use before influenza infection, ECMO use, and larger steroid dosage after influenza-related ARDS independently predict NLRTI in influenza-related ARDS. Moreover, NLRTI results in poorer outcomes in patients with severe influenza.
Supplemental Material
Supplemental material, Author_Response for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_Tables for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Acknowledgments
We thank Professor Meng-Chih Lin, the President of the Taiwan Society of Pulmonary and Critical Care Medicine, who organized and coached the TSIRC team.
Footnotes
Author contribution(s): Wei-Chih Chen: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Software; Visualization; Writing-original draft; Writing-review & editing.
Kuo-Chin Kao: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing-review & editing.
Chau-Chyun Sheu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Ming-Cheng Chan: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing-review & editing.
Yu-Mu Chen: Data curation; Investigation; Project administration; Writing-review & editing.
Ying-Chun Chien: Data curation; Formal analysis; Investigation; Writing-review & editing.
Chung-Kan Peng: Conceptualization; Data curation; Investigation; Methodology; Writing-review & editing.
Shinn-Jye Liang: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing-review & editing.
Han-Chung Hu: Data curation; Formal analysis; Investigation; Project administration; Writing-original draft preparation.
Ming-Ju Tsai: Data curation; Formal analysis; Investigation; Writing-review & editing.
Wen-Feng Fang: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing-review & editing.
Wann-Cherng Perng: Conceptualization; Methodology; Project administration; Supervision; Validation; Writing-review & editing.
Hao-Chien Wang: Conceptualization; Methodology; Project administration; Supervision; Validation; Writing-review & editing.
Chieh-Liang Wu: Conceptualization; Methodology; Resources; Supervision; Validation; Writing-review & editing.
Kuang-Yao Yang: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Supervision; Validation; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: The study protocol was approved by the Institutional Review Boards of all eight hospitals (Taipei Veterans General Hospital, 2016-05-020CC; Taichung Veterans General Hospital, CE16093A; Tri-Service General Hospital, 1-105-05-086; National Taiwan University Hospital, 201605036RIND; Chang Gung Memorial Hospital, 201600988B0; China Medical University Hospital, 105-REC2-053(FR); Kaohsiung Medical University Hospital, KUMHIRB-E(I)-20170097; and Kaohsiung Chang Gung Memorial Hospital, 201600988B0.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by research grants from Taipei Veterans General Hospital (V108C-057), and Taipei Veterans General Hospital-National Yang-Ming University-Excellent Physician Scientists Cultivation Program (106-V-B-015). In addition, this work was financially supported by the ‘Cancer Progression Research Center, National Yang-Ming University’ from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Informed consent: The requirement for written informed consent was waived because all patient information was anonymized and de-identified during data recording.
ORCID iDs: Han-Chung Hu  https://orcid.org/0000-0003-1603-868X
https://orcid.org/0000-0003-1603-868X
Kuang-Yao Yang  https://orcid.org/0000-0002-2803-980X
https://orcid.org/0000-0002-2803-980X
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Wei-Chih Chen, Department of Chest Medicine, Taipei Veterans General Hospital, Taipei; Institute of Emergency and Critical Care Medicine and Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei.
Kuo-Chin Kao, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Taoyuan; Department of Respiratory Therapy, Chang Gung University College of Medicine, Taoyuan.
Chau-Chyun Sheu, Division of Pulmonary and Critical Care Medicine, Kaohsiung Medical University Hospital, Kaohsiung; School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung.
Ming-Cheng Chan, Division of Chest Medicine, Department of Internal Medicine, and Section of Critical Care and Respiratory Therapy, Taichung Veterans General Hospital, Taichung; Central Taiwan University of Science and Technology, Taichung.
Yu-Mu Chen, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung.
Ying-Chun Chien, Division of Chest Medicine, Department of Internal Medicine, National Taiwan University Hospital, Taipei.
Chung-Kan Peng, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei.
Shinn-Jye Liang, Division of Pulmonary and Critical Care, Department of Internal Medicine, China Medical University Hospital, Taichung.
Han-Chung Hu, Department of Thoracic Medicine, Chang Gung Memorial Hospital, Taoyuan; Department of Respiratory Therapy, Chang Gung University College of Medicine, Taoyuan.
Ming-Ju Tsai, Division of Pulmonary and Critical Care Medicine, Kaohsiung Medical University Hospital, Kaohsiung; School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung.
Wen-Feng Fang, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung; Department of Respiratory Care, Chang Gung University of Science and Technology, Chiayi.
Wann-Cherng Perng, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei.
Hao-Chien Wang, Division of Chest Medicine, Department of Internal Medicine, National Taiwan University Hospital, Taipei.
Chieh-Liang Wu, Center for Quality Management, Taichung Veterans General Hospital, Taichung; Office of Medical Administration, Taichung Veterans General Hospital, Taichung.
Kuang-Yao Yang, Department of Chest Medicine, Taipei Veterans General Hospital, No. 201, Sec. 2, Shih-Pai Road, Taipei, 11217; Institute of Emergency and Critical Care Medicine and Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei; Cancer Progression Research Center, National Yang-Ming University, Taipei.
References
- 1. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Estenssoro E, Rios FG, Apezteguia C, et al. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med 2010; 182: 41–48. [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302: 1872–1879. [DOI] [PubMed] [Google Scholar]
- 4. Martin-Loeches I, Sanchez-Corral A, Diaz E, et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest 2011; 139: 555–562. [DOI] [PubMed] [Google Scholar]
- 5. Rice TW, Rubinson L, Uyeki TM, et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 2012; 40: 1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis 2010; 51(Suppl. 10): S120–S125. [DOI] [PubMed] [Google Scholar]
- 7. Markowicz P, Wolff M, Djedaini K, et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med 2000; 161: 1942–1948. [DOI] [PubMed] [Google Scholar]
- 8. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 9. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 10. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl. 2): S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events. Crit Care Med 2013; 41: 2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recomm Rep 2018; 67: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (Lond) 2006; 20: 407–416. [DOI] [PubMed] [Google Scholar]
- 16. Agudo-Tabuenca A, Gimeno-Orna JA, Saenz-Abad D. Assessment of the efficacy and safety of a protocol to manage glucocorticoid-induced hyperglycemia in diabetic patients during hospital stay. Endocrinol Diabetes Nutr. Epub ahead of print 23 March 2019. DOI: 10.1016/j.endinu.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 17. Buttgereit F, Burmester GR, Lipworth BJ. Inflammation, glucocorticoids and risk of cardiovascular disease. Nat Clin Pract Rheumatol 2009; 5: 18–19. [DOI] [PubMed] [Google Scholar]
- 18. Sosa M, Gomez de Tejada MJ. Glucocorticoid-induced osteoporosis. N Engl J Med 2019; 380: 1378–1379. [DOI] [PubMed] [Google Scholar]
- 19. Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am 2012; 41: 595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am 2016; 42: 157–176, ix–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011; 306: 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dixon WG, Abrahamowicz M, Beauchamp ME, et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis 2012; 71: 1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 2006; 54: 628–634. [DOI] [PubMed] [Google Scholar]
- 24. Pieri M, Agracheva N, Fumagalli L, et al. Infections occurring in adult patients receiving mechanical circulatory support: the two-year experience of an Italian national referral tertiary care center. Med Intensiva 2013; 37: 468–475. [DOI] [PubMed] [Google Scholar]
- 25. Aubron C, Cheng AC, Pilcher D, et al. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol 2013; 34: 24–30. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt M, Brechot N, Hariri S, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis 2012; 55: 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pons S, Sonneville R, Bouadma L, et al. Infectious complications following heart transplantation in the era of high-priority allocation and extracorporeal membrane oxygenation. Ann Intensive Care 2019; 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hadley JS, Wang JE, Michaels LC, et al. Alterations in inflammatory capacity and TLR expression on monocytes and neutrophils after cardiopulmonary bypass. Shock 2007; 27: 466–473. [DOI] [PubMed] [Google Scholar]
- 29. Bougle A, Bombled C, Margetis D, et al. Ventilator-associated pneumonia in patients assisted by veno-arterial extracorporeal membrane oxygenation support: epidemiology and risk factors of treatment failure. PLoS One 2018; 13: e0194976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, et al. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2016; 3: CD010406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_3_v.1 for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplementary_Tables for Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome by Wei-Chih Chen, Kuo-Chin Kao, Chau-Chyun Sheu, Ming-Cheng Chan, Yu-Mu Chen, Ying-Chun Chien, Chung-Kan Peng, Shinn-Jye Liang, Han-Chung Hu, Ming-Ju Tsai, Wen-Feng Fang, Wann-Cherng Perng, Hao-Chien Wang, Chieh-Liang Wu and Kuang-Yao Yang in Therapeutic Advances in Respiratory Disease