Abstract
Background.
The leaves of Bersama abyssinica are used for the treatment of diabetes mellitus in folk medicine system of Ethiopia. The present study was done based on the traditional claim of B abyssinica for the treatment of diabetes mellitus.
Methods.
The α-amylase inhibition and antioxidant activities of B abyssinica extracts were evaluated by using 3,5-dinitrosalicylic acid method and diphenyl-2-picrylhydrazyl assay model, respectively. Blood glucose lowering activity of the extracts was studied in 4 animal models; normoglycemic, oral glucose loaded, and streptozotocin-induced diabetic mice models.
Results.
Among the extracts, the crude extract showed the highest α-amylase enzyme inhibition activity with an IC50 of 6.57 μg/mL. The water fraction showed the strongest antioxidant activity with an IC50 of 3.43 μg/mL. The crude extract at doses of 200, and 400 mg/kg showed significant (P < .05) hypoglycemic activity in normoglycemic mice. All doses of the crude extract significantly (P < .05) reduced blood glucose levels of oral glucose-loaded mice. In streptozotocin-induced diabetic mice models, both the crude and solvent fractions showed a significant (P < .05) blood glucose lowering effect as compared with the negative control group post 8 hour treatment.
Conclusion.
The results demonstrated the beneficial biochemical effects of B abyssinica extract by inhibiting α-amylase and scavenging the free radicals. The crude extract and solvent fractions of B abyssinica had significant blood glucose lowering effect in all animal models.
Keywords: antidiabetic, diabetes mellitus, antioxidant, α-amylase, Bersama abyssinica, streptozotocin
Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 It is an important public health problem, 1 of 4 priority noncommunicable diseases targeted for action by world leaders.2 The epidemiology of diabetic mellitus estimated that there were 451 million (age 18-99 years) people with diabetes worldwide. These figures will be expected to increase to 693 million by 2045. In 2017, approximately 5 million deaths worldwide were attributable to diabetes in the 20- to 99-year age range.3 In Ethiopia, the number of people aged 20 to 79 years living with diabetes was estimated to be 1.3 million adults with diabetes, and the prevalence was 2.9%. This figure is projected to reach 1.8 million by 2030.4
Alternative systems of medicine based on plant extracts have thrived through the ages and are still practiced by a large population for the management of diabetes.5 Globally, medicinal plants have been used as a source of medicine and 80% to 85% of populations rely on these medicinal plants using the extracts or their active components as a traditional medicine to meet their primary health care needs.6 Currently, more than 1000 plants have been described as efficacious in the treatment of diabetes mellitus. Many of the currently available drugs have been directly or indirectly derived from plants. The popular oral antidiabetic drug glucophage (metformin) is derived from Galega officinalis.7,8 During the past few years, some of the new bioactive drugs isolated from hypoglycemic plants showed antidiabetic activity with more efficacy than oral hypoglycemic agents used in clinical therapy.9,10 Several plant species have been used for prevention or management of diabetes. Some of herbal drugs with proven antidiabetic and related beneficial effects used in treatment of diabetes are Allium sativum, Aloe vera, Coccinia indica, Eugenia jambolana, Ficus bengalensis, Momordica charantia, Ocimum sanctum syn., Trigonella foenum graecum, Vernonia amygdalina,11,12 Falcaria vulgaris,13 and Stevia rebaudiana.14
The antidiabetic activity of medicinal plants depends on a variety of mechanisms. Generally, the mechanisms of action could be grouped as pancreatic beta cell potassium channel blocking; cyclic adenosine monophosphate (cAMP; secondary messenger) stimulation; providing certain necessary elements like calcium, zinc, magnesium, manganese, and copper for the beta-cells; Inhibition of β-galactocidase and α-glucocidase; preventing oxidative stress that is possibly involved in pancreatic β-cell dysfunction found in diabetes15; stimulation of glycogenesis, glycolysis, and citric acid cycle and hexose monophosphate shunt; inhibition of gluconeogenesis and glycogenolysis16; improvement in digestion along with reduction in blood sugar and urea; protection of destruction and promotion of regeneration of the β-cells, initiate insulin release; reduction in insulin resistance and/or inhibition in renal glucose reabsorption.17
Free radical, which cause damage and mutation of cells, are harmful to human health. Antioxidants play a significant role against them, and recently consumption of natural materials, including cereals, pulses, nuts, fruits, and vegetables that have antioxidant activity, has increased.18 Natural products play a major role in the discovery of new therapeutic agents and have received much attention as sources of bioactive substances, including antioxidants.19–21
Antioxidant action has beneficial effects on preservation of β cell function in diabetes.13,22,23 Induction of oxidative stress is a key process in the pathogenesis of different illnesses, including diabetes mellitus and its complications, and the role of antioxidants in treating diabetes and its complications through prevention of oxidative stress has been explained in different studies.20,24–26 Interestingly, the current study revealed that Bersama abyssinica leaves have strong in vitro antioxidant activities indicating that the plant could possess antidiabetic activity.
Bersama abyssinica (Melianthaceae) belongs to the genus Bersama, which comprises 4 species. Other species include B engleriana, B swynnertonii, B swinnyi, and B yangambiensis.27 The antidiabetic activity of medicinal plants is due to the presence of phenolic compounds (anthraquinones, C-glycosylated anthrones, 2-hydroxy-3-methyl-anthraquinone, physcion, etc), flavonoids, terpenoids, alkaloids, glycosides, steroid, peptides, lipids, and other constituents.28–30 Phytochemical analysis carried out in Ethiopia showed that the leaf extract of B abyssinica contains alkaloids, glycosides, flavonoids, steroids, phenols, tannins, triterpenene, anthraquinones, polysterols, and coumarins.31 Consequently, this phytoconstituents may contribute for antidiabetic activity.
Plant extracts with cytotoxic actions are also therapeutically useful for diabetic treatment.32,33 In line with these, B abyssinica has been used for its cytotoxic effect.31,34–36 More important, this study has shown that the leaf crude extract and solvent fractions of B abyssinica possess in vitro α-amylase enzyme inhibitory activity, suggesting that the plant could be a potential candidate for diabetes mellitus. The practice of using plants for management of diabetes is also documented in Ethiopia just like other ailments. The leaves of B abyssinica have been used in the treatment of diabetes mellitus in Ethiopian folk medicine without any scientific proof for safety and efficacy.37,38 Accordingly, this study has been taken up to investigate the in vitro and in vivo antidiabetic activity of leaves crude extract and solvent fractions of B abyssinica.
Methods
Plant Materials
The fresh leaves of B abyssinica were collected from Tara Gedam, South Gondar zone, Amhara region, in December 2018. The botanical identification and authentication of the plant material was performed by Mr Abiyu Enyew (botanist) and the voucher specimen was deposited in the herbarium of Biology Department, Faculty of Natural and Computational Science, University of Gondar.
Experimental Animals
Healthy Male Swiss albino mice (weighing 20-28 g and age of 6–10 weeks) were purchased from the Ethiopian Public Health Institute (EPHI). Female rodents were excluded for greater compatibility nature of males for the models,39 except for oral toxicity study. The animals were kept in polypropylene cages, maintained under standard condition (12 hours light and 12 hours dark cycle), and were allowed for free access to a pellet diet and water ad libitum. After a randomized grouping and before initiation of the experiment, the animals were acclimatized to the laboratory conditions. The mice were maintained and cared according to the international guidelines given by Organization for Economic Cooperation and Development (OECD). In all animal models, an inhalational anesthetic, halothane, was used to alleviate animal pain, and they were euthanized by cervical dislocation after completing the experiments.
Preparation of Plant Crude Extract
The leaves of the plant were thoroughly washed with distilled water to remove dirt and then dried under shade at room temperature (25 to 27 °C). The dried plant materials were ground into coarse powder by the electrical mill. Then, the coarse powdered plant materials were macerated separately in 80% methanol for 72 hours and then the extracts were filtered by using Whatman filter paper no. 1. The marc was remacerated 2 times with fresh solvent, each for 72 hours, and the filtrates obtained from the successive maceration were concentrated under reduced pressure using rotary evaporator (Hamato) followed by hot air oven (Medit-MedizinTechnik) set at 40 °C. The semidried residues were frozen in the refrigerator overnight and then, dried using a lyophilizer (Labfreez) to completely remove the solvent residue. Then, the dried leaves extracts were kept separately in a desiccator until used for the experiment.40,41
Fractionation of Crude Extract
Solvent fractionation of leaf crude extract was carried out using water, ethyl acetate, and chloroform. Briefly, the leaf crude extract was dissolved in 400 mL of distilled water and this solution was transferred into a separating funnel. An equal volume of chloroform was added to it and was shaken vigorously. The mixture was separated into 2 layers, and after waiting for a while, the chloroform fraction was removed. The partition with chloroform was repeated 2 times. The chloroform layer was combined and subjected to evaporation using hot air oven set at 40 °C to get the chloroform fraction. To the separating funnel containing aqueous layer, 400 mL of ethyl acetate was added. The mixture was separated into 2 layers, and then the ethyl acetate was separated, and the procedure was repeated 2 times. The ethyl acetate layer was pooled and concentrated using hot air oven set at 40 °C to obtain the ethyl acetate fraction. The remaining aqueous layer was concentrated using hot air oven set at 40 °C and frozen in the refrigerator overnight and then concentrated in a lyophilizer to remove the water. After drying, the solvent fractions were stored in a desiccator until used for the experiment.42,43
Induction of Experimental Diabetes
Diabetes was induced by using streptozotocin (STZ), which was dissolved in 0.1 M cold citrate buffer (pH = 4.5). A 150 mg/kg dose of the freshly prepared STZ solution was administered intraperitonially to overnight fasted mice. After 30 minutes’ administration of STZ, food and water were allowed freely to the animals. Following 6 hours’ administration of STZ, 5% glucose solution in a volume of 1 mL/kg was given to the animals for the next 24 hours to prevent death secondary to hypoglycemic shock. Animals were screened for diabetes after 3 days of STZ injection and those with fasting blood glucose level >200 mg/dL were included in the study as diabetic mice.44
Acute Toxicity Study
Based on the limit test standard of the OECD No. 425 Guideline,45 acute oral toxicity test was carried out for leaf crude extract of B abyssinica. One female Swiss albino mouse was fasted for 4 hours on the first day of the test and then 2 g/kg of the extracts was given orally by oral gavage and was observed strictly for physical or behavioral changes like changes in skin, urination, lacrimation, reduction in feeding activity, excitation, paw licking, increased respiratory rate, decreased motor activity, diarrhea, weight loss, and paralysis for 1 day and given special attention during the first 4 hours. Based on the results from the first mouse, the other 4 female mice were recruited and fasted for 4 hours and then 2 g/kg single dose of the crude extract was given and were observed strictly in the same manner. The observation was continued for a total of 2 weeks for any signs of toxicity.45
Grouping and Dosing of Animals
For all mice models (normoglycemic, oral glucose loaded, and STZ-induced diabetic model) male mice were used since male mice are more sensitive to STZ and insulin than female mice (female mice are insensitive to STZ and insulin).46–48 In all cases, mice were assigned randomly into 5 groups of 6 mice each (n = 6).
In the normoglycemic and oral glucose loaded animal models, there was a negative control group (groups I), which received distilled water (DW); a positive control group (group II), which received glibenclamide 5 mg/kg (GLC 5 mg/kg); test groups (groups III-V) received 3 different doses of the B abyssinica crude extract (BAC).
In the single dose–treated diabetic animal model, there was a negative control group (groups I), which received the vehicle (distilled water = DW); a positive control group (group II), which received the standard drug (GLC 5 mg/kg); 12 test groups (group III-XIV) received different doses of the B abyssinica crude extract (BAC); chloroform fraction (CFF); ethyl acetate fraction (EAF); and aqueous fraction (AQF).
As per the OECD guideline, the extract doses to be administered were determined based on the acute toxicity study and the volume of administration was 1 mL/100 g of body weight of the mouse.45 The middle dose was one-tenth of the limit dose (200 mg/kg), the higher dose was twice the middle dose (400 mg/kg), and the lower dose was calculated as half of the middle dose (100 mg/kg). Glibenclamide was selected as a standard drug for the study based on earlier studies.13,49 The study was conducted using the oral route of administration because the plant materials are traditionally used by people via the oral route.37
Determination of α-Amylase Inhibition Activity
The α-amylase inhibition assay was performed using the 3,5-dinitrosalicylic acid (DNSA) method.50 The crude and solvent fractions of B abyssinica were dissolved in buffer ((Na2HPO4/NaH2PO4 (0.02 M), NaCl (0.006 M) at pH 6.9) to give concentrations ranging from 50 to 1000 μg/mL. A volume of 200 µL of α-amylase solution (Molychem) (2 units/mL) was mixed with 200 µL of the extract and was incubated for 10 minutes at 30 °C. Thereafter, 200 µL of the starch solution (1% in water w/v) was added to each tube and incubated for 3 minutes. The reaction was terminated by the addition of 200 µL DNSA reagent (12 g of sodium potassium tartrate tetrahydrate in 8.0 mL of 2 M NaOH and 20 mL of 96 mM 3,5-DNSA solution) and was boiled for 10 minutes in a water bath at 85 °C. The mixture was cooled to ambient temperature and was diluted with 5 mL of distilled water, and the absorbance was measured at 540 nm using a UV-visible spectrophotometer (Agilent Technologies). The blank with 100% enzyme activity was prepared by replacing the plant extract with 200 µL of the buffer. A blank reaction was similarly prepared using the plant extract at each concentration in the absence of the enzyme solution. A positive control sample was prepared using acarbose (Bayer) and the reaction was performed similarly to the reaction with plant extract as mentioned above.
The inhibition of α-amylase was expressed as percentage of inhibition and was calculated by the following equation: Inhibition (%) = [(Ac − Acb) − (As − Asb) / (Ac − Acb)] × 100, where Ac is the absorbance of control; Acb is the absorbance of control blank; As is the absorbance of sample; and Asb is the absorbance of sample blank. The % α-amylase inhibition was plotted against the extract concentration and the IC50 values were obtained from the graph.
In Vitro Antioxidant Activity in DPPH Assay Model
The free radical scavenging activity of the plant crude, solvent fractions, and ascorbic acid were determined in vitro by diphenyl-2-picrylhydrazyl (DPPH; Sigma Aldrich) assays according to the method described earlier.51,52 Aliquots of 100 µL of a methanolic solution containing different concentrations ranging from 50 to 1000 μg/mL were added to 3.9 mL of a 0.004% methanolic solution of DPPH. Absorbance at 517 nm was determined after 30 minutes, and the percent inhibition activity was calculated. IC50 values denote the concentration of the sample required to scavenge 50% DPPH free radicals. The percentage of the scavenging of the DPPH free radical was calculated by the formula: (A0 − A1)/A0 × 100, where A0 is the absorbance of the control and A1 is the absorbance of the extract/standard.
Effect of Plant Extract on Blood Glucose Level of Normoglycemic Mice
Healthy normal mice were fasted overnight for 14 hours, but water was allowed ad libitum, and then randomly divided into 5 different groups (6 mice in each). Using aseptic conditions, blood samples were collected from tips of the tail of each mouse to determine blood glucose level (BGL) just before treatment (at 0 hours) as a baseline, and then at 1, 2, 4, and 6 hours posttreatment.53
Effect of Plant Extract on Blood Glucose Level After Oral Glucose Tolerance Test
Mice were used for the oral glucose tolerance test (OGTT) after fasting overnight for 14 hours, as there is increased insulin sensitivity (insulin-stimulated glucose utilization) specifically in mice.54,55 Mice were grouped and treated as described above and the baseline blood glucose level was determined. Then 2 g/kg of glucose solution was administered orally to each mouse after 30 minutes of extract administration. Blood sugar level was measured for each animal just before treatment (at 0 minute) as a baseline, and then at 30, 60, and 120 minutes following glucose administration.56
Antihyperglycemic Activity of a Single Dose of the Extracts on STZ-Induced Diabetic Mice
In this model, STZ-induced diabetic mice were assigned randomly into 5 groups (n = 6) after fasting for 14 hours. Then, mice were treated with distilled water, glibenclamide, and 80% methanolic crude extracts and solvent fractions according to their respective grouping as described above. Blood glucose level was measured just before treatment (at 0 hour) as a baseline, and then at 2, 4, 6, and 8 hours posttreatment.53,57
Statistical Analysis
All the results were expressed as the mean ± standard error of means (SEM) for 6 mice in each group. Statistical analysis was performed by using the Statistical Package for Social Sciences (SPSS) version 24 software. Between- and within-group analysis were carried out using 1-way analysis of variance, followed by Tukey’s multiple comparison tests. The result was considered significant when P < .05.
Results
The Percentage Yield of Plant Material Extraction
From 129 g (12% yield) of 80% leaves crude extract, the yields of ethyl acetate chloroform, and aqueous fractions of leaves B abyssinica were 9%, 11.44%, and 70.62%, respectively.
Acute Toxicity Test
The acute toxicity study indicated that the crude extract caused no mortality in limit dose of 2000 mg/kg within the first 24 hours as well as for the following 14 follow-up days. Physical and behavioral observations of the experimental mice also revealed no visible signs of overt toxicity. This indicates that median lethal dose (LD50) of the extract is greater than 2000 mg/kg.
In Vitro α-Amylase Inhibition Activity of Crude Extract and Solvent Fractions
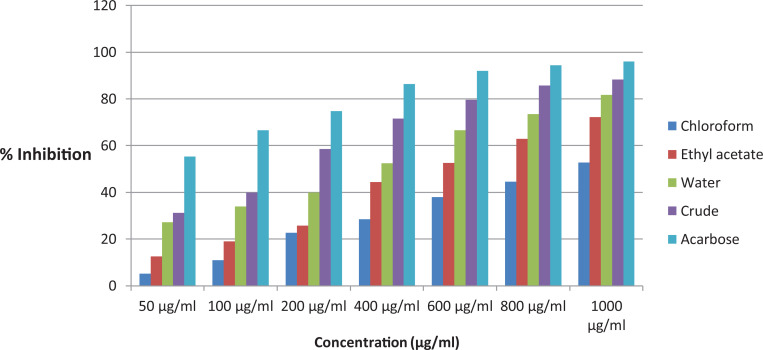
In vitro α-amylase inhibitory study evaluating the percent of α-amylase inhibition as a function of extract concentrations and the IC50 values were calculated (Figure 1). Concentration-dependent inhibitions were observed for various concentrations of the tested extracts and the standard. Among the extracts, the crude extract exhibited the lowest IC50 of 6.57 ± 0.74 μg/mL and the IC50 values of water fraction, ethyl acetate fraction, and the chloroform fraction were 13.33 ± 0.57, 20.34 ± 0.67, and 30.97 ± 0.84 μg/mL, respectively. The standard positive control acarbose showed an IC50 of 2.26 ± 0.53 μg/mL (Table 1).
Figure 1.
α-Amylase inhibitory activity of the leaves crude extract and solvent fractions of Bersama abyssinica.
Table 1.
α-Amylase Inhibitory Activities of the Crude Extract and Solvent Fractions.a
| Concentration (μg/mL) | Percentage inhibition | ||||
|---|---|---|---|---|---|
| Chloroform fraction | Ethyl acetate fraction | Aqueous fraction | Crude extract | Acarbose | |
| 50 | 5.21 ± 0.36 | 12.54 ± 0.67 | 27.24 ± 1.34 | 31.24 ± 0.46 | 55.31 ± 0.66 |
| 100 | 10.94 ± 0.84 | 18.95 ± 0.49 | 33.92 ± 0.94 | 39.93 ± 1.27 | 66.57 ± 0.38 |
| 200 | 22.71 ± 0.55 | 25.73 ± 1.08 | 39.94 ± 0.67 | 58.48 ± 0.92 | 74.8 ± 1.07 |
| 400 | 28.47 ± 0.94 | 44.31 ± 0.66 | 52.48 ± 0.57 | 71.52 ± 0.77 | 86.35 ± 0.64 |
| 600 | 37.90 ± 0.27 | 52.64 ± 0.87 | 66.57 ± 0.38 | 79.65 ± 0.64 | 91.93 ± 0.55 |
| 800 | 44.63 ± 0.49 | 62.92 ± 0.37 | 73.49 ± 0.77 | 85.64 ± 0.61 | 94.37 ± 0.48 |
| 1000 | 52.82 ± 0.67 | 72.19 ± 0.73 | 81.67 ± 0.64 | 88.34 ± 0.81 | 95.94 ± 0.64 |
| IC50 | 30.97 ± 0.84 | 20.34 ± 0.67 | 13.33 ± 0.57 | 6.57 ± 0.74 | 2.26 ± 0.53 |
Abbreviation: IC50, half maximal inhibitory concentration.
a Each value of percentage inhibition of α-amylase is presented as means ± standard error of the mean (SEM), n = 3.
Antioxidant Activity of Crude Extract and Solvent Fractions
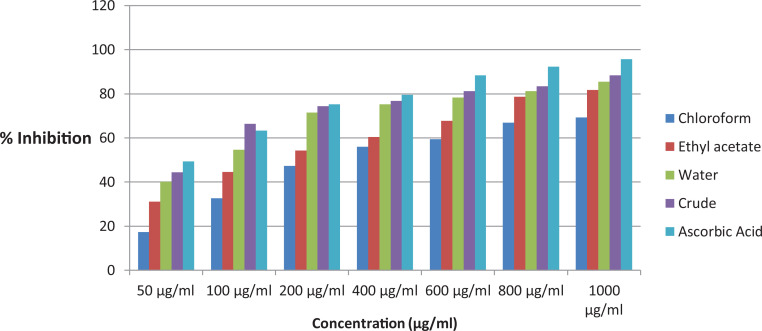
The leaf crude extract and solvent fractions of B abyssinica were tested for antioxidant activity using DPPH free radical. The extracts showed antioxidant activity as shown in Figure 2. The DPPH free radical scavenging activities of the B abyssinica extracts were concentration dependent with an IC50 value of 3.43 ± 0.57, 5.35 ± 0.67, 8.54 ± 0.92, and 14.64 ± 0.49 μg/mL for the water fraction, crude extract, ethyl acetate fraction, and the chloroform fraction, respectively (Table 2). The highest antioxidant activity was observed in the water fraction with an IC50 value of 3.43 ± 0.57 μg/mL, and IC50 of ascorbic acid was found to be 2.65 ± 0.84 μg/mL.
Figure 2.
Free radical scavenging activity of the leaves crude extract and solvent fractions of Bersama abyssinica.
Table 2.
Antioxidant Activities of the Crude Extract and Solvent Fractions.
| Concentration (μg/mL) | % inhibition of DPPH | ||||
|---|---|---|---|---|---|
| Chloroform fraction | Ethyl acetate fraction | Crude extract | Aqueous fraction | Ascorbic acid | |
| 50 | 17.24 ± 1.61 | 31.07 ± 0.87 | 40.19 ± 0.85 | 44.37 ± 0.66 | 49.37 ± 1.24 |
| 100 | 32.64 ± 0.95 | 44.54 ± 1.08 | 54.67 ± 1.07 | 66.40 ± 0.57 | 63.24 ± 0.99 |
| 200 | 47.37 ± 0.88 | 54.22 ± 0.67 | 71.51 ± 0.38 | 74.37 ± 1.01 | 75.19 ± 0.84 |
| 400 | 55.94 ± 0.17 | 60.37 ± 0.52 | 75.27 ± 0.44 | 76.79 ± 0.67 | 79.55 ± 0.37 |
| 600 | 59.37 ± 1.03 | 67.79 ± 0.38 | 78.27 ± 0.67 | 81.27 ± 0.59 | 88.34 ± 0.84 |
| 800 | 66.84 ± 0.66 | 78.64 ± 0.47 | 81.27 ± 0.82 | 83.49 ± 0.64 | 92.27 ± 0.55 |
| 1000 | 69.32 ± 0.37 | 81.71 ± 1.28 | 85.44 ± 1.34 | 88.37 ± 0.55 | 95.74 ± 0.16 |
| IC50 μg/mL | 14.64 ± 0.49 | 8.54 ± 0.92 | 5.35 ± 0.67 | 3.43 ± 0.57 | 2.65 ± 0.84 |
Abbreviations: DPPH, 2,2-diphenyl-1-picrylhydrazinel IC50, half maximal inhibitory concentration.
a Each value of percentage inhibition of DPPH free radical is presented as means ± standard error of the mean (SEM), n = 3.
Hypoglycemic Activity on Normoglycemic Mice
The activity of the leaf crude extract of B abyssinica on normoglycemic mice was as shown in Table 3. Within-group analysis showed that DW treated negative control and BAC100 mg/kg treated groups did not reduce blood glucose level significantly (P > .05) as compared with baseline blood glucose level at all points of time. However, BAC 200 mg/kg, BAC 400 mg/kg, and GLC 5 mg/kg treated groups lowered their baseline blood glucose level significantly. The percentage of reduction of baseline blood glucose level were 25.59%, 42.60%, and 49.42% for BAC 200 mg/kg, BAC 400 mg/kg, and GLC 5mg/kg, respectively.
Table 3.
Hypoglycemic Activity of the Leaf Crude Extract on Normoglycemic Mice.*
| Group | Blood glucose level (mg/dL) | ||||
|---|---|---|---|---|---|
| 0 hour | 1 hour | 2 hours | 4 hours | 6 hours | |
| DW10 | 84.67 ± 3.04 | 83.83 ± 3.53 | 80.17 ± 2.74 | 78.83 ± 2.47 | 74.83 ± 4.26 |
| GLC5 | 86.00 ± 3.19 | 73.00 ± 2.43β2 | 57.67 ± 1.02a3,β3 | 53.83 ± 1.85a3,β3 | 43.50 ± 1.57a3,β3 |
| BAC100 | 80.33 ± 3.54 | 81.17 ± 2.44 | 74.33 ± 4.84n2 | 73.33 ± 3.67n3 | 69.17 ± 2.64n3 |
| BAC200 | 77.50 ± 2.54 | 74.33 ± 4.19 | 66.50 ± 2.89a1 | 61.83 ± 3.04a2,β2 | 57.67 ± 1.81a2,β3,n1 |
| BAC400 | 91.17 ± 4.83 | 82.17 ± 4.67 | 63.33 ± 3.03a2,β3 | 56.83 ± 2.81a3,β3 | 52.33 ± 3.05a3,β3 |
Abbreviations: BAC, Bersama abyssinica leaf crude extract; DW, distilled water; GLC, glibenclamide.
* Each value represents mean ± SEM; n = 6 for each treatment. aCompared with the negative control, bcompared with BAC100, ccompared with BAC200, dcompared with BAC400, and βcompared with baseline blood glucose level, ncompared with GLC5. 1 P < .05, 2 P < .01, 3 P < .001.
Between-groups analysis showed that there was no significant difference in blood glucose level of all groups at baseline. During subsequent measurement of blood glucose level, it was found that GLC 5 mg/kg treated group lowered blood glucose level significantly as compared with negative control (DW) treated group. There was also significant difference in blood glucose level of GLC 5 mg/kg treated group and extract treated groups. The middle and the higher doses of crude extract treated groups (200 and 400 mg) also reduced blood glucose level significantly as compared to negative control. However, the lower doses were failed to decrease blood glucose level significantly as compared to negative control.
Antihyperglycemic Activity on Oral Glucose Loaded Mice
The activity of crude extract of B abyssinica on oral glucose tolerance test in oral glucose loaded mice is shown in Table 4. Oral administration of 2000 mg/kg of glucose to normoglycemic mice 30 minutes after treatment with distilled water, test extracts, and glibenclamide showed peak blood glucose level in 30 minutes. At first there was no significant difference in blood glucose level of mice.
Table 4.
Effect of the Leaf Crude Extract on Oral Glucose Loaded Mice.*
| Group | Blood glucose level (mg/dL) | |||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | 120 min | |
| DW10 | 85.83 ± 2.23 | 186.33 ± 3.64 | 141.33 ± 4.36 | 114.33 ± 3.33 |
| GLC5 | 89.17 ± 3.28 | 156.33 ± 3.95a3,β3 | 77.00 ± 6.23a3,μ3 | 73.33 ± 5.78a3,μ3 |
| BAC100 | 84.17 ± 2.01 | 178.17 ± 1.07 β3,n2 | 137.17 ± 7.54 β3,n3,μ2 | 87.67 ± 8.53a1,μ3 |
| BAC200 | 82.83 ± 2.6 | 169.50 ± 3.67a1,β3 | 113.33 ± 8.55a1,n2,β2,μ3 | 83.50 ± 2.69a2,μ3 |
| BAC400 | 87.50 ± 4.01 | 160.83 ± 2.45a3,β3 | 91.17 ± 4.67a3,μ3 | 75.83 ± 3.99a3,μ3 |
Abbreviations: BAC, Bersama abyssinica leaf crude extract; DW, distilled water; GLC, glibenclamide.
* Each value represents mean ± SEM; n = 6 for each treatment. aCompared with the negative control, bcompared with BAC100, ccompared with BAC200, dcompared with BAC400, μcompared with the blood glucose level at 30 minutes and βcompared with baseline blood glucose level, ncompared with GLC5. 1 P < .05, 2 P < .01, 3 P < .001.
There was a significant blood glucose level variation between 30 and 60 minutes as compared with baseline blood glucose level (P < .001 and P < .001, respectively) for BAC100 mg/kg treated group; and (P < .001 and P < .01, respectively) for BAC200 mg/kg treated group. The higher dose and glibenclamide treated group showed significant (P < .001) blood glucose level variation between 30 minutes as compared with baseline blood glucose level; while there was no significant (P > .05) difference between post 2-hour glucose loading as compared with baseline blood glucose level for BAC 400 mg/kg and GLC 5 mg/kg treated groups. Within-group analysis revealed that oral glucose loading caused a statistically significant reduction in BGL at 60 and 120 minutes in all groups as compared with the respective BGL at 30 minutes after glucose administration.
Between-group analysis showed that significant (P < .05, P < .001, P < .001) blood glucose reductions were started after 30 minutes of glucose loading for BAC 200 mg/kg, BAC 400 mg/kg, and GLC 5 mg/kg treated groups, respectively, as compared with negative control. After 1 hour of glucose loading, both BAC400 mg/kg and GLC 5 mg /kg treated groups reduced blood glucose level significantly (P < .001) as compared with negative control. Similarly, BAC 200 mg/kg treated groups reduced blood glucose level significantly (P < .05) as compared with negative control.
Following 2 hours of glucose loading, the BAC 100 mg/kg, BAC 200 mg/kg, and BAC 400 mg/kg treatment groups significantly (P < .05, P < .01, P < .001, respectively) reduced peak blood glucose level as compared with negative control. Likewise, GLC 5 mg /kg significantly (P < .001) reduced peak blood glucose level as compared with negative control.
Antihyperglycemic Activity of Plant Extract on Streptozotocin-Induced Diabetic Mice
The effects of B abyssinica crude extract and fractions on STZ-induced diabetes are shown in Table 5. Between- and within-group analyses were performed to see BGL differences across the various groups and time points, respectively. The between-group analysis indicated that there was no significant difference in baseline fasting BGL across all groups. Similarly, there was no significant difference in BGL at all time points when groups treated with plant extract as compared with each other.
Table 5.
Antihyperglycemic Activity of a Single Dose of Leaf Crude Extract and Solvent Fractions on Diabetic Mice.*
| Group | Blood glucose level (mg/dL) | ||||
|---|---|---|---|---|---|
| 0 hour | 2 hours | 4 hours | 6 hours | 8 hours | |
| DW10 | 342.83 ± 6.98 | 356.67 ± 7.96 | 334.67 ± 11.65 | 330.33 ± 9.89 | 324.83 ± 7.67n3 |
| GLC5 | 338.33 ± 12.06 | 283.33 ± 11.14β2,n3 | 216.33 ± 9.67β3,n3 | 172.17 ± 8.99 β3,n3 | 160.33 ± 5.17β3,n3 |
| BAC100 | 328.67 ± 20.05 | 309.00 ± 17.87 | 284.67 ± 18.67 | 244.83 ± 19.55n3 | 219.00 ± 10.67β2,n3 |
| BAC200 | 329.67 ± 17.31 | 316.17 ± 17.35 | 293.83 ± 15.08 | 233.83 ± 17.54β3,n3 | 208.50 ± 6.44β3,n3 |
| BAC400 | 352.67 ± 11.62 | 325.67 ± 10.78 | 260.33 ± 17.67β3,n3 | 214.67 ± 12.36β3,n3 | 182.00 ± 11.35β3,n3 |
| CFF100 | 347.50 ± 8.81 | 352.00 ± 8.45 | 341.67 ± 10.58 | 333.67 ± 11.26 | 321.00 ± 13.05 |
| CFF200 | 334.00 ± 6.77 | 326.83 ± 9.09 | 314.67 ± 14.67 | 294.33 ± 11.04β1 | 268.33 ± 8.58β3 |
| CFF400 | 355.83 ± 6.52 | 335.83 ± 6.77 | 320.17 ± 16.32β1 | 290.00 ± 8.27β3 | 264.33 ± 9.79β3,n1 |
| EAF100 | 350.67 ± 7.92 | 340.00 ± 7.79 | 322.00 ± 12.37 | 305.00 ± 9.52β1 | 273.17 ± 13.40β3 |
| EAF200 | 365.50 ± 5.58 | 347.17 ± 4.71 | 321.00 ± 20.24β3 | 285.83 ± 15.64β3 | 275.50 ± 7.08β3 |
| EAF400 | 344.67 ± 9.88 | 318.00 ± 9.27 | 291.17 ± 19.35β3 | 241.50 ± 13.68β3,n3 | 230.00 ± 14.43β3,n3 |
| AQF100 | 347.50 ± 9.02 | 332.83 ± 6.78 | 310.00 ± 11.87 | 292.50 ± 12.23β2 | 252.50 ± 11.74β3,n2 |
| AQF200 | 339.50 ± 6.67 | 315.83 ± 10.23 | 286.17 ± 15.27β2 | 265.50 ± 10.07β3,n2 | 247.50 ± 9.48β3,n2 |
| AQF400 | 362.33 ± 7.13 | 332.67 ± 6.85β1 | 293.83 ± 14.42β3 | 239.33 ± 17.52β3,n3 | 214.83 ± 7.98β3,n3 |
Abbreviations: BAC, Bersama abyssinica leaf crude extract; DW, distilled water; CFF, chloroform fraction; EAF, ethyl acetate fraction; AQF, aqueous fraction; GLC, glibenclamide.
* Each value represents mean ± SEM; n = 6 for each treatment. nCompared with the negative control, βcompared with baseline blood glucose level. 1 P < .05, 2 P < .01, 3 P < .001,
Within-group comparison showed that there was a significant BGL reduction observed in all doses of crude extract and solvent fractions treated groups at different time points as compared with the baseline fasting BGL. However, maximum percent reduction in BGL was recorded as 25.71% in CHF400, 33.27% in EAF400, 40.71% in AQF400, 48.39% in BAC400, and 52.61% in GLC5 treated group at the eighth hour as compared with the respective baseline fasting level.
The B abyssinica crude extract and fractions treated groups produced a significant BGL reduction; for BAC 400 mg/kg at 4 hours (P < .001); for all doses of BAC (P < .001), EAF 400 mg/kg (P < .001), AQF 200 mg/kg (P < .01), and AQF 400 mg/kg (P < .001) at 6 hours; for all doses of BAC (P < .001), AQF 400 mg/kg (P < .001), EAF 400 mg/kg (P < .001), AQF 100 mg/kg (P < .01), AQF 200 mg/kg (P < .01), and CHF 400 mg/kg (P < .05) at 8 hours as compared with the negative control. Similarly, the standard drug (GLC 5 mg/kg) produced a significant BGL reduction at 2 hours (P < .01), 4 hours (P < .001), 6 hours (P < .001), and 8 hours (P < .001) as compared with the negative control.
Discussion
Diabetes mellitus is one of the most common chronic diseases and is associated with hyperlipidemia and comorbidities such as obesity and hypertension.58 Many new bioactive phytochemicals isolated from the plants having hypoglycemic and antihyperglycemic effects show the same antidiabetic activity and sometimes even more potent than already known oral hypoglycemic agents.59,60 The present study investigated the in vitro and in vivo antidiabetic and antioxidant activity of the 80% methanolic crude extract and solvent fractions of B abyssinica on normal and diabetic mice.
The acute oral toxicity profile of the hydromethanolic leaf extract of B abyssinica was determined based on OECD guidelines 2008:425.45 At a dose of 2000 mg/kg, mortality and delayed toxicity were not observed in the 14 days after the treatment period. In this study, the LD50 value of the hydromethanolic root extract of B abyssinica was found to be >2000 mg/kg. This indicates that the extract is better tolerated and safe on oral administration.
N-methylnitrocarbamoyl-d-glucosamine popularly referred to as streptozotocin (STZ) is a well-known and established diabetic agent used as a screening model for evaluating the antidiabetic potential or capacity of compounds in a wide range of animals.61,62 The compound (STZ) is a potent methylating agent for DNA and acts as nitric oxide donor in pancreatic cells. Pancreatic cells are particularly sensitive to damage by nitric oxide (via inhibition of aconitase activity) and free radicals because of their low levels of free radical scavenging enzymes.63 In accordance, findings from the current investigation showed that STZ administration (intraperitoneally) at 150 mg/kg body weight effectively (71.6%) induced diabetes mellitus in physiologically normal mice as reflected by hyperglycemia.
It is well known that inhibition of intestinal α-glucosidase and pancreatic α-amylase activity results in delaying carbohydrate digestion of absorbable monosaccharides, causing reduction of postprandial hyperglycemia.64 The plant crude extracts and solvent fractions showed pancreatic α-amylase inhibitory activity. The search for a new group of agents from natural resources especially from plant medicines become an attractive approach for the treatment of postprandial hyperglycemia. The finding in Table 2 showed the percentage inhibition of crude extracts, and solvent fractions of B abyssinica against α-amylase. All of them demonstrated a dose-dependent reduction in α-amylase inhibition activity. The highest inhibition appeared in the crude extract (88.34%); while the chloroform fraction showed the weakest effect (52.82%). The α-amylase inhibitory activity in crude extract might be due to higher abundance of the compounds and is worth investigating further and isolating pure active compounds. Flavonoids, tannins, and phenolic acids are a major group of polyphenolic compounds that have been reported to possess inhibitory activity against α-amylase.65 In a previous study, the phytochemical analysis revealed that the extracts were rich in polyphenolic components, which suggests that the bioactive exerting the inhibitory effect against α-amylase may be present in all plant extracts at different concentration.
The maximum antioxidant activity (88.37%) was observed in the aqueous fraction, while the lowest antioxidant activity was observed in the chloroform fraction (69.32%). Ascorbic acid was used as a standard and the percentage of inhibition was found to be (95.74%). The detailed results of both fractions are shown in Table 3. The interesting fact of our study was that the crude extract contained more antioxidant compounds as compared with leaf crude extract and leaf solvent fractions. The overall results showed that the aqueous fraction has better antioxidant potential than the crude and other solvent fractions. The finding of the antioxidant activity of B abyssinica extract showed a dose-dependent antioxidant activity.
The hypoglycemic activity of B abyssinica leaf crude extract on normoglycemic mice, the negative control, and the lower dose (BAC 100 mg/kg) treated groups did not show significant reduction of blood glucose level as compared to their baseline blood glucose level. However, the higher doses (BAC 200 mg/kg and BAC 400 mg/kg) and glibenclamide-treated groups showed significant reduction of blood glucose level as compared with their base line blood glucose level. Glibenclamide is oral antidiabetic drug that reduce blood glucose level by increasing insulin secretion due to closure of potassium-ATP channels, membrane depolarization and stimulation of calcium ion influx and also improve glucose utilization.66 B abyssinica leaf crude extract showed similar glucose lowering effect with glibenclamide in dose-dependent manner. The mechanisms of glucose lowering effect of the extract might be comparable to glibenclamide or other unknown mechanisms. It might be due to the counter-regulatory effect of pancreatic cells in mice.
In general, in OGTT the crude extract showed a significant reduction in blood glucose level from 30 minutes compared with negative control. Mice treated with crude extract have better glucose utilization capacity. Postprandial glucose lowering ability of the extract may be attributed to inhibition of glucose absorption, stimulation of peripheral glucose utilization, decrease in glycogenolysis, and gluconeogenesis.67 This suggests that the extract is endowed with the ability to enhance regulatory mechanisms, indicating a potential advantage of the extract in minimizing hyperglycemia-related complications of diabetes. Although there was an increase in blood glucose level after glucose loading from the base line glucose level, all doses (100 mg/kg, 200 mg/kg, and 400 mg/kg) and the standard drug reduced significantly from peak level to baseline contained by 2 hours as compared with negative control. Previous studies showed that glucose lowering of plant extract may be due to a decrease in glucose absorption, increased peripheral glucose utilization and glycolysis, and decrease in glycogenolysis and gluconeogenesis.56
Among the 3 solvent fractions, namely ethyl acetate, chloroform, and aqueous, the aqueous fraction showed by far the best and anticipated antihyperglycemic activity in STZ-induced diabetic mice in single dose fractions treated groups compared with the base line and diabetic control groups. This might be because of the polar character of secondary metabolites such as flavonoids68,69 sterols/triterpenoids,70 alkaloids and phenolics.71 This result was comparable to a previous study, which reported that the aqueous fraction had superior glucose lowering activity among the fraction used.72 In the antihyperglycemic activity of single dose of solvent fractions on STZ-induced diabetic mice, both the crude and solvent fractions showed significant glucose lowering activity as compared with negative control. This may be due to the fact that the plant extracts may contain the most common phytochemical constituents and secondary metabolites, which have potent hypoglycemic, antihyperglycemic, and glucose suppressive effects. The selective and noninteraction of compounds in the hydromethanolic extract of leaf of B abyssinica may be responsible for its potent antihyperglycemic activity. The leaf crude extract and solvent fractions showed a comparatively delayed onset of blood glucose lowering effect than glibenclamide. This might be due to the presence of compounds with higher glycemic index that could give rise to free glucose after digestion and tend to raise blood glucose level following absorption. The presence of such effect may delay the action of the plant extract.49
Plants with hypoglycemic and antihyperglycemic activities may contain one or more chemical constituents. In this study, the preliminary phytochemical screening of the 80% methanolic leaf crude extract of B abyssinica, contains alkaloids, glycosides, flavonoids, steroids, phenols, tannins, triterpenene, anthraquinones, polysterols, and coumarins, but there is absence of saponins.31 Similarly, the gas chromatography–mass spectrometry (GC-MS) analysis of the methanolic fraction revealed the presence of various phytocompounds in leaves, such as terpenes, vitamin, carotenoid (rhodopin), flavonoids, and steroids.73 These secondary metabolites include flavonoids,68,69 sterols/triterpenoids,70 alkaloids, and phenolics.71 Effects might be achieved by facilitating insulin release from pancreatic β-cells, inhibiting glucose absorption in the gut, stimulating glycogenesis in the liver and/ or increasing glucose utilization by the body.74 Apart from lowering blood glucose effect, these phytochemicals are known to regenerate the damaged β-cells and stopping oxidative stress on beta cells in experimental diabetic rats.75 In line with this finding, any of the secondary metabolites that are found in B abyssinica crude extracts and solvent fractions may, therefore, be responsible for the observed glucose suppressive and antihyperglycemic activity of the extract and some of the bioactive constituents in this study could act synergistically or independently enhancing the activity of glycolytic and glyconeogenic enzymes. However, there is a need of bioactivity guided investigation to isolate the lead compound responsible for antidiabetic activity and to establish the possible mechanism(s) of action.
Conclusions
The leaf extract of B abyssinica has shown significant glucose lowering activity both in normal and STZ-induced diabetic mice. This traditional medicinal plant extracts also showed high α-amylase inhibitory and antioxidant activity, indicating that the polyphenols present in the extracts have potential to reduce α-amylase activity and scavenging DPPH. This study therefore supports the use of B abyssinica herb for management of diabetes mellitus by traditional healer. Further studies are required to identify the lead compound(s) present in B abyssinica with its molecular mechanism of action on peroxisome proliferator-activated receptors, insulin sensitization, histological analysis, and other insulin targets based on the pathophysiology of diabetes mellitus.
Acknowledgments
The authors would like to acknowledge University of Gondar for material support and for allowing to use the laboratory facility.
Footnotes
Author Contributions: Zemene Demelash Kifle conceived the idea, drafted the proposal, collected the plant materials, carried out all experiments, and prepared the final manuscript for publication. Both authors were involved in the design, write up and preparing the manuscript to be submitted. Both authors have read and agreed the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zemene Demelash Kifle  https://orcid.org/0000-0001-7030-2782
https://orcid.org/0000-0001-7030-2782
Ethical Approval: Ethical clearance was obtained from the Research and Ethics Committee, Department of Pharmacology, University of Gondar with a reference number of SOP 4/116/11 to conduct the experiment.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Global report on diabetes, author. 2016 https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1 Accessed June 5, 2020.
- 3. Cho N, Shaw J, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. [DOI] [PubMed] [Google Scholar]
- 4. Meresa A, Gemechu W, Basha H, et al. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents. Am J Adv Drug Deliv. 2017;5:040–058. [Google Scholar]
- 5. Rizvi SI, Matteucci E, Atukeren P. Traditional medicine in management of type 2 diabetes mellitus. J Diabetes Res. 2013;2013:580823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elujoba AA, Odeleye O, Ogunyemi C. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr J Tradit Complement Altern Med. 2005;2:46–61. doi:10.4314/ajtcam.v2i1.31103 [Google Scholar]
- 7. Eddouks M, Chattopadhyay D, Zeggwagh NA. Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evid Based Complement Alternat Med. 2012;2012:142087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mentreddy SR, Mohamed AI, Rimando AM. Medicinal plants with hypoglycemic/anti-hyperglycemic properties: a review. Accessed June 5, 2020 https://naldc.nal.usda.gov/download/45708/PDF
- 9. Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity—a review of ten years of herbal medicine research (1990-2000). Int J Diabetes Metab. 2006;14:1–25. [Google Scholar]
- 10. Koski RR. Practical review of oral antihyperglycemic agents for type 2 diabetes mellitus. Diabetes Educ. 2006;32:869–876. [DOI] [PubMed] [Google Scholar]
- 11. Mamun-or-Rashid A, Hossain MS, Hassan N, Dash BK, Sapon MA, Sen MK. A review on medicinal plants with antidiabetic activity. J Pharmacogn Phytochem. 2014;3:149–159. [Google Scholar]
- 12. Shukia R, Sharma SB, Puri D, Prabhu KM, Murthy PS. Medicinal plants for treatment of diabetes mellitus. Indian J Clin Biochem. 2000;15(suppl 1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R. Antidiabetic, hematoprotective and nephroprotective effects of the aqueous extract of Falcaria vulgaris in diabetic male mice. Arch Biol Sci. 2018;70:655–664. [Google Scholar]
- 14. Hagh-Nazari L, Goodarzi N, Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R. Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comparative Clin Pathol. 2017;26:455–463. [Google Scholar]
- 15. Jarald E, Joshi SB, Jain DC. Diabetes Vs herbal medicines. Iran J Pharmacol Ther. 2008;7:97–106. [Google Scholar]
- 16. Thomson I, Khanavi M, Taheri T, et al. Stimulation of hepatic glycogenolysis and inhibition of gluconeogenesis are the mechanisms of antidiabetic effect of Centaurea bruguierana ssp. Belangerana. Asian J Animal Vet Adv. 2012;7:1166–1174. [Google Scholar]
- 17. Narayan DS, Patra VJ, Dinda SC. Diabetes and Indian traditional medicines an overview. Int J Pharm Pharm Sci. 2012;4:45–53. [Google Scholar]
- 18. Hamelian M, Zangeneh MM, Amisama A, Varmira K, Veisi H. Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl Organomet Chem. 2018;32:e4458. [Google Scholar]
- 19. Zangeneh MM, Zangeneh A, Pirabbasi E, Moradi R, Almasi M. Falcaria vulgaris leaf aqueous extract mediated synthesis of iron nanoparticles and their therapeutic potentials under in vitro and in vivo condition. Appl Organomet Chem. 2019;33:e5246. [Google Scholar]
- 20. Zangeneh MM, Saneei S, Zangeneh A, et al. Preparation, characterization, and evaluation of cytotoxicity, antioxidant, cutaneous wound healing, antibacterial, and antifungal effects of gold nanoparticles using the aqueous extract of Falcaria vulgaris leaves. Appl Organomet Chem. 2019;33:e5216. [Google Scholar]
- 21. Zhaleh M, Zangeneh A, Goorani S, et al. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl Organomet Chem. 2019;33:e5015. [Google Scholar]
- 22. Dutta A, Lahkar M, Handique C. Evaluation of antidiabetic activity of Oxalis corniculata in streptozotocin induced diabetic rats. Int J Basic Clin Pharmacol. 2016;5:2178–2183. [Google Scholar]
- 23. Bhaskaran M, Mruthunjaya K, Manjula S, Rajan D. Evaluation of anti-diabetic activity of leaves of Actinodaphne hookeri Meissn. Int J Pharm Sci Res. 2019;10:83–96. [Google Scholar]
- 24. Zangeneh MM, Ghaneialvar H, Akbaribazm M, et al. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J Photochem Photobiol B. 2019;197:111556. [DOI] [PubMed] [Google Scholar]
- 25. Zangeneh MM, Joshani Z, Zangeneh A, Miri E. Green synthesis of silver nanoparticles using aqueous extract of Stachys lavandulifolia flower, and their cytotoxicity, antioxidant, antibacterial and cutaneous wound-healing properties. Appl Organomet Chem. 2019;33:e5016. [Google Scholar]
- 26. Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J. 2016;24:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heywood VH. Flowering Plants of the World. 2nd ed Oxford University Press; 1993. [Google Scholar]
- 28. Demissew S, Friis I, Awas TA, et al. Four new species of Aloe (Aloaceae) from Ethiopia, with notes on the ethics of describing new taxa from foreign countries. Kew Bulletin. 2011;66:111–121. [Google Scholar]
- 29. Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. [DOI] [PubMed] [Google Scholar]
- 30. Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis. 2005;15:149–160. [DOI] [PubMed] [Google Scholar]
- 31. Anza FWM, Libsu S, Mamo F, Endale M. Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica . J Adv Botany Zoology. 2015;3. [Google Scholar]
- 32. Chai TT, Yeoh LY, Ismail NIM, Ong HC, Manan FA, Wong FC. Evaluation of glucosidase inhibitory and cytotoxic potential of five selected edible and medicinal ferns. Trop J Pharm Res. 2015;14:449–454. [Google Scholar]
- 33. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. [DOI] [PubMed] [Google Scholar]
- 34. Esubalew ST, Belete A, Lulekal E, Gabriel T, Engidawork E, Asres K. Review of ethnobotanical and ethnopharmacological evidences of some Ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiop J Health Dev. 2017;31:161–187. [Google Scholar]
- 35. Tuasha N, Petros B, Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J Ethnobiol Ethnomed. 2018;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abebe W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. Eur J Med Plants. 2016;14(4). doi:10.9734/EJMP/2016/25670 [Google Scholar]
- 37. Schmelzer GH, Gurib-Fakim A. Plant resources of tropical Africa 11 (1). Medicinal plants 1 Accessed June 5, 2020 https://library.wur.nl/WebQuery/wurpubs/fulltext/417238
- 38. Lather A, Gupta V, Tyagi V, Kumar V, Garg S. Phytochemistry and pharmacological activities of Bersama engleriana Guerke—an overview. Int J Pharm. 2010;1:89–94. [Google Scholar]
- 39. Etuk E. Animals models for studying diabetes mellitus. Agric Biol J N Am. 2010;1:130–134. [Google Scholar]
- 40. Sarker SD, Latif Z, Gray AI. Natural Products Isolation. 2nd ed. Biotechnology™ Humana Press Inc; 2006. [Google Scholar]
- 41. Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Front Pharmacol. 2016;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Molla M, Gemeda N, Abay SM. Investigating potential modes of actions of Mimusops kummel fruit extract and solvent fractions for their antidiarrheal activities in mice. Evid Based Complement Alternat Med. 2017;2017:4103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kashimawo AJ, Kolawole JA, Ahmadu AA. Bioassay guided fractionation and α-amylase inhibitory activity of lupeol from the stem bark of Faidherbia albida Del. Mimosaceae: Accessed June 5, 2020. http://www.ijpsi.org/Papers/Vol6(6)/D06062932.pdf [Google Scholar]
- 44. Baquer NZ, Kumar P, Taha A, Kale RK, Cowsik SM, McLean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36:383–396. [DOI] [PubMed] [Google Scholar]
- 45. Organisation for Economic Co-operation and Development. OECD Guideline for the testing of chemicals. Acute oral toxicity: up and down procedure (UDP). Accessed June 5, 2020 https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf
- 46. Deeds M, Anderson J, Armstrong A, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5.47.1-5.20. [DOI] [PubMed] [Google Scholar]
- 48. Vital P, Larrieta E, Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol. 2006;190:425–432. [DOI] [PubMed] [Google Scholar]
- 49. Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement Alternat Med. 2012;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wickramaratne MN, Punchihewa JC, Wickramaratne DBM. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina . BMC Complement Alternat Med. 2016;16:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 52. Jalalvand AR, Zhaleh M, Goorani S, et al. Chemical characterization and antioxidant, cytotoxic, antibacterial, and antifungal properties of ethanolic extract of Allium saralicum RM Fritsch leaves rich in linolenic acid, methyl ester. J Photochem Photobiol B. 2019;192:103–112. [DOI] [PubMed] [Google Scholar]
- 53. Birru EM, Abdelwuhab M, Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complement Alternat Med. 2015;15:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222:G13–G25. [DOI] [PubMed] [Google Scholar]
- 55. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7:110–113. [Google Scholar]
- 57. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Alternat Med. 2015;15:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. [DOI] [PubMed] [Google Scholar]
- 59. Rai PK, Srivastava AK, Sharma B, Dhar P, Mishra AK, Watal G. Use of laser-induced breakdown spectroscopy for the detection of glycemic elements in Indian medicinal plants. Evid Based Complement Alternat Med. 2013;2013:406365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watal G, Dhar P, Srivastava SK, Sharma B. Herbal medicine as an alternative medicine for treating diabetes: the global burden. Evid Based Complement Alternat Med. 2014;2014:596017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dimo T, Rakotonirina SV, Tan PV, et al. Effect of Sclerocarya birrea (Anacardiaceae) stem bark methylene chloride/methanol extract on streptozotocin-diabetic rats. J Ethnopharmacol. 2007;110:434–438. [DOI] [PubMed] [Google Scholar]
- 62. Oliveira HC, dos Santos MP, Grigulo R, et al. Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol. 2008;115:515–519. [DOI] [PubMed] [Google Scholar]
- 63. Spinas GA. The dual role of nitric oxide in islet beta-cells. News Physiol Sci. 1999;14:49–54. [DOI] [PubMed] [Google Scholar]
- 64. Aloulou A, Hamden K, Elloumi D, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Alternat Med. 2012;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim JS, Kwon CS, SoN KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64:2458–2461. [DOI] [PubMed] [Google Scholar]
- 66. Hegde K, Arathi A, Mathew A. Evaluation of antidiabetic activity of hydro alcoholic extract of Chrysophyllum cainito fruits. Int J Pharm Sci Res. 2016;7:4422–4428. [Google Scholar]
- 67. Porchezhian E, Ansari SH, Shreedharan NK. Antihyperglycemic activity of Euphrasia officinale leaves. Fitoterapia. 2000;71:522–526. [DOI] [PubMed] [Google Scholar]
- 68. Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127:457–462. [DOI] [PubMed] [Google Scholar]
- 69. Oliver-Bever B. Medicinal plants in tropical West Africa. Cambridge University Press; 1986. [Google Scholar]
- 70. Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–275. [DOI] [PubMed] [Google Scholar]
- 71. Kameswara Rao B, Giri R, Kesavulu MM, Appa RC. Herbal medicine: in the management of diabetes mellitus. Manphar Vaidhya Patrika. 1997;1:33–35. [Google Scholar]
- 72. Nardos A, Makonnen E, Debella A. Effects of crude extracts and fractions of Moringa stenopetala (Baker f) Cufodontis leaves in normoglycemic and alloxan-induced diabetic mice. Afr J Pharm Pharmacol. 2011;5:2220–2225. [Google Scholar]
- 73. Zekeya N, Chacha M, Shahada F, Kidukuli A. Analysis of phytochemical composition of Bersama abyssinica by gas chromatography-mass spectrometry. J Pharmacogn Phytochem. 2014;3:246–252. [Google Scholar]
- 74. Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76:1223–1238. [DOI] [PubMed] [Google Scholar]
- 75. Alexandru V, Balan M, Gaspar A, Coroiu V. Antioxidant activity, phenolics and flavonoid content of some selected Romanian medicinal plants. Planta Medica. 2007;73:261. [Google Scholar]