Abstract
Background
Studies using 1 mg of colchicine to prevent postoperative atrial fibrillation (POAF) reported conflicting results. Moreover, colchicine was associated with significant gastrointestinal (GI) side effects. This study examined whether low-dose colchicine effectively prevents POAF and whether low-dose therapy is associated with lower rates of colchicine-induced GI side effects.
Methods
In this prospective, randomized, double-blind, placebo-controlled study, consecutive adult patients admitted for elective cardiac surgeries randomly received a 1-mg dose of colchicine (n = 81) or placebo (n = 71) orally 12 to 24 hours before surgery followed by a daily dose of 0.5 mg until hospital discharge. The primary efficacy endpoint was the development of at least one episode of POAF of ≥5 minutes. The primary safety endpoint was the development of adverse events, especially diarrhea.
Results
The in-hospital mortality rate was 3.9%. POAF occurred in 13 patients (16.1%) in the colchicine group and 13 patients (18.3%) in the placebo group (odds ratio 0.85 [95% Confidence Interval = 0.37−1.99]). Diarrhea occurred in two patients in each group and necessitated treatment discontinuation in one patient in each group.
Conclusion
Low-dose colchicine did not prevent POAF in patients undergoing cardiac surgery. These results should be interpreted cautiously because of the small sample size and early study termination.
ClinicalTrials.gov Unique Identifier number: NCT03015831
Keywords: Atrial fibrillation, colchicine, cardiac surgery, randomized controlled trial, gastrointestinal toxicity, arrhythmia
Introduction
Atrial fibrillation (AF) is the most common arrhythmia following coronary artery bypass graft (CABG) and other cardiac surgeries, with an estimated incidence of 30% (range, 10%–50%).1–5 Most patients (73%) typically develop AF by the third day after surgery, and the length of hospital stay is a mean of 3 days longer for such patients. Postoperative AF (POAF) increases morbidity and the risk of stroke, and this complication can lead to increased hospital expenditure because of prolonged hospital stay.6,7 POAF is also associated with increased risks of delirium and other neurocognitive disorders.8 Several preoperative, intraoperative, and postoperative factors are associated with the development of POAF, including advanced age, hypertension, diabetes mellitus, obesity, left atrial enlargement, diastolic dysfunction, left ventricular hypertrophy, surgical atrial injury, atrial ischemia, volume overload, and electrolyte imbalance.9
Although the exact mechanism of POAF is not well known, it is believed that inflammation and oxidative stress play vital roles in its pathogenesis. Chung et al. identified significantly higher C-reactive protein (CRP) levels in patients with confirmed AF than in controls. They hypothesized that the increase in the levels of CRP, a major inflammatory marker, reflects an inflammatory state that promotes the persistence of AF.9
Moreover, previous studies illustrated that anti-inflammatory therapy including steroids could potentially reduce the incidence of POAF.10–12 However, studies using colchicine, an anti-inflammatory agent, for the prevention of POAF in patients undergoing cardiac surgery yielded inconsistent results.13–15
In our previous END-AF trial, we demonstrated that treatment with colchicine in patients undergoing open-heart surgery (2 mg preoperatively followed by 1 mg daily) failed to significantly reduce the incidence of POAF. Moreover, diarrhea occurred in 24.6% of patients treated with colchicine, and more than half of such patients required treatment discontinuation.16
The present study, END-AF Low Dose, is an investigator-initiated trial that evaluated the effect of low-dose colchicine (1 mg administered 12–24 hours prior to cardiac surgery, followed by 0.5 mg daily until discharge) on the incidence of POAF and adverse events, particularly colchicine-related gastrointestinal (GI) side effects.
Methods
Design
This prospective, multicenter, double-blind, randomized placebo-controlled study was conducted at three major hospitals in Amman, Jordan between October 2017 and July 2019. The study was approved by the internal review board of each participating hospital. All participants provided written informed consent. The study was designed to enroll a total of 1200 patients to permit subgroup analysis and the assessment of secondary endpoints. Recognizing the slow recruitment in the early phase of the trial and assessing on the results of our previous trial, we revised the target enrollment to 362 patients as explained in the statistical analysis section.
Participants
All consecutive adult patients undergoing elective open-heart surgeries were screened for inclusion. The exclusion criteria were as follows: a) emergency cardiac surgery, b) hypersensitivity to or current treatment with colchicine, c) history of supraventricular arrhythmias, including AF, d) absence of sinus rhythm on hospital admission, e) serum creatinine level >2.5 mg/dL, f) severe hepatic disease or transaminase levels >1.5× the upper limit of normal, g) myopathy or elevated baseline creatine kinase levels, and h) pregnancy or lactation in women.
Intervention
Upon enrollment, patients were treated with 1 mg of colchicine or matching placebo 12 to 24 hours prior to surgery, followed by colchicine (0.5 mg) or placebo immediately after their surgery (via a nasogastric tube) and daily treatment at this dose until hospital discharge. Randomization was conducted using computer-generated random serial number sequences. None of the investigators was involved directly or indirectly with randomization. The colchicine and identical placebo tablets were provided in sealed envelopes that were number-coded.
Monitoring parameters
Electrocardiography (ECG) was performed upon patient admission. After surgery, patients were initially admitted to the intensive coronary care unit (ICCU), in which they were continuously monitored. Daily ECG was routinely ordered for all patients until they were discharged from the hospital. ECG was also performed when AF was suspected because of patients’ complaints or the presence of an irregular pulse. AF episodes were considered significant if they persisted for at least 5 minutes.
Patients were also monitored for adverse effects including but not limited to GI effects (mainly diarrhea), hepatotoxicity, myotoxicity, bone-marrow toxicity, alopecia, and anorexia
Endpoints
The primary efficacy endpoint was the development of at least one episode of AF (lasting 5 minutes or longer) occurring after cardiac surgery at any point during hospitalization. The primary safety endpoint was the development of adverse effects, especially diarrhea.
Statistical analysis
Based on data from our previous trial, we estimated that 21% of the patients in the placebo group would develop AF during hospitalization. At a power of 80% and a two-sided alpha level of 0.05, the sample size needed to detect an absolute difference of 10% in the rate of POAF between the two study groups was 181 in each group. The study was stopped early because of futility with respect to the primary endpoint, and this diminished the power of the study.
Data were analyzed using IBM SPSS version 20 (Armonk, NY, USA). Data were described using means and percentages. An independent-samples t-test was used to compare means, and the chi-squared test was used to compare percentages. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
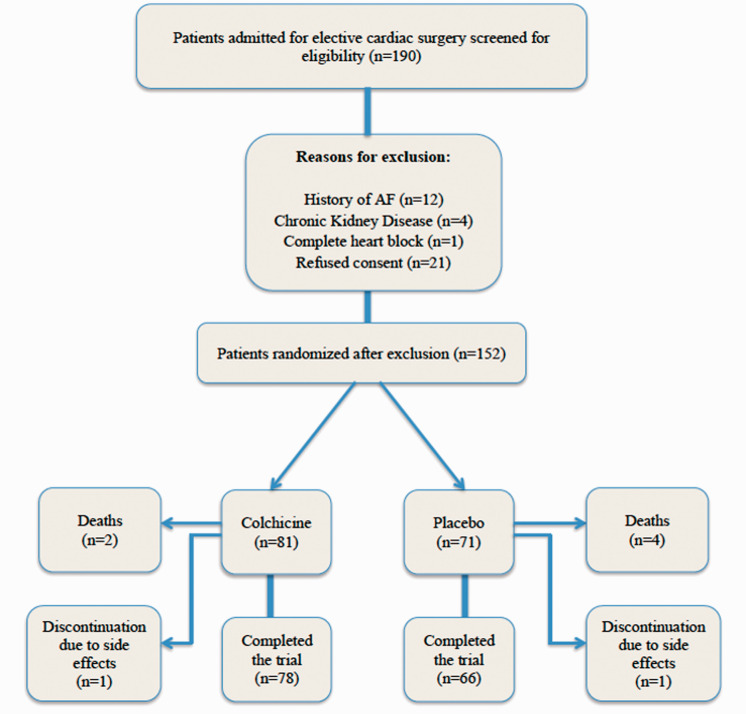
All patients (n = 190) who were admitted to the participating hospitals for elective cardiac surgery were screened for eligibility. Thirty-eight patients were excluded for reasons including a history of AF (n = 12), chronic renal disease (n = 4), complete heart block (n = 1), and refusal of consent (n = 21). The remaining 152 patients were randomized to receive colchicine (n = 81) or placebo (n = 71) (Figure 1).
Figure 1.
Flow diagram of study participation.
Baseline characteristics were similar between the groups. The mean age for all patients was 59.4 years. Females comprised 24% of the study cohort. The groups were comparable concerning most characteristics including body mass index, serum creatinine levels, estimated glomerular filtration rate, average heart rate, and left ventricular ejection fraction.
Hypertension, diabetes mellitus, hypercholesterolemia, and myocardial infarction were more prevalent in the placebo group, and the colchicine group included a higher number of active smokers. However, none of the differences was statistically significant (Table 1).
Table 1.
Baseline clinical and demographic characteristics.
| Colchicine (n = 81) | Placebo (n = 71) | P | |
|---|---|---|---|
| Age (years) | 59.0 ± 15.2 | 59.8 ± 13.1 | 0.730 |
| Women (%) | 28.4 | 18.3 | 0.204 |
| BMI (kg/m2) | 29.3 ± 5.4 | 29.3 ± 5.6 | 1.00 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.3 | 1.00 |
| eGFR (mL/minute/1.73 m2) | 101.4 ± 38.8 | 98.5 ± 34.6 | 0.629 |
| Heart rate (beats/minute) | 74.7 ± 13.9 | 72.5 ± 11.8 | 0.298 |
| Hypertension (%) | 55.6 | 62.0 | 0.526 |
| Diabetes mellitus (%) | 39.5 | 49.3 | 0.293 |
| Hypercholesterolemia (%) | 54.3 | 66.2 | 0.184 |
| Active smoking (%) | 29.6 | 19.7 | 0.223 |
| Prior MI (%) | 18.5 | 26.8 | 0.303 |
| LVEF (%) | 56.8 ± 10.9 | 56.6 ± 10.5 | 0.909 |
BMI = body mass index, eGFR = estimated glomerular filtration rate, MI = myocardial infarction, LVEF = left ventricular ejection fraction.
Of the 152 patients, 99 had CABG as their only surgery. Most of the remaining patients underwent valve replacement or combined surgeries (Table 2).
Table 2.
Types of cardiac surgery performed.
| Procedure | Number of patients (n = 152) |
|---|---|
| CABG | 99 |
| Aortic valve replacement | 10 |
| Atrial septal defect closure | 2 |
| Ventricular septal defect repair | 1 |
| Subaortic membrane excision | 2 |
| Mitral valve replacement | 8 |
| Bentall procedure | 1 |
| Composite aortic graft | 1 |
| Fontan procedure | 1 |
| Mitral valve repair | 1 |
| Combined surgeries | 26 |
CABG = coronary artery bypass grafting.
Main outcome
Twenty-six patients (17%) developed AF. The rate of AF was 16.0% (n = 13) in the colchicine group, versus 18.3% (n = 13) in the placebo group. Comparing the colchicine and placebo groups, the odds ratio was 0.85 (95% confidence interval [CI] = 0.37–1.99).
Of the 26 patients who developed AF, 14 (53.9%) had AF on the day of surgery or the first postoperative day, and 24 of 26 (92.3%) episodes of AF occurred within the first 3 postoperative days. The mean duration of AF episodes was 31 ± 41.3 hours (range, 1.5–144) in the colchicine group, versus 37 ± 71.7 hours (range, 0.75–264) in the placebo group.
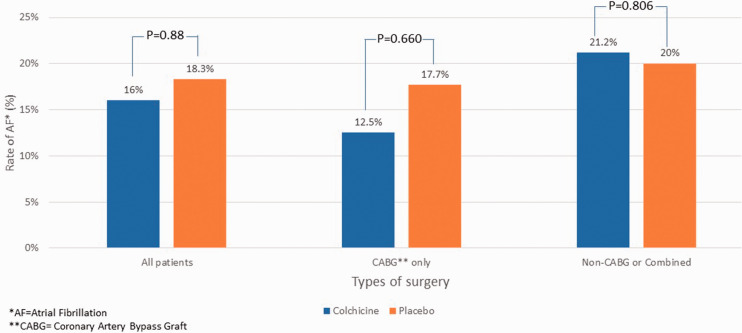
Of the 99 patients who had undergone only CABG, 12.5% of the 48 patients in the colchicine group and 17.7% of the 51 patients in the placebo group developed AF. Among the 53 patients who underwent non-CABG or combined surgeries, 33 patients (21.2%) in the colchicine group and 20 patients (20%) in the placebo group developed AF ( Figure 2).
Figure 2.
Rate of AF in different cardiac surgery groups.
The choice of surgical technique was left to the discretion of the treating physicians. The majority (77.8%) of CABG-only surgeries were performed off-pump. AF developed in 13 and 2 patients undergoing off-pump or on-pump surgeries, respectively (9.1% vs. 16.9%).
Safety and adverse effects
Four patients developed diarrhea, including two patients in each group. Diarrhea led to treatment discontinuation in one patient in each group. Anorexia was not observed during the trial. Two patients (one in each group) complained of excessive diaphoresis that spontaneously resolved. However, treatment was discontinued in both patients. No other major adverse effects were detected during the study.
Deaths
Six patients died during hospitalization because of surgical complications, including four patients in the placebo group and two patients in the colchicine group. All six patients were included in the analysis. Two of these patients had undergone only CABG, and four patients underwent valvular or combined surgeries. In addition, two procedures were repeat surgeries. Three deaths in patients who had AF at the time of death were unrelated to AF.
Discussion
Several studies have reported the role of colchicine in the prevention of POAF. All such studies treated patients at a dose of 1 mg. Although the efficacy of colchicine in reducing AF differed among the studies, all studies reported significant colchicine-induced GI side effects.
In the COPPS trial involving 360 patients who underwent cardiac surgery, colchicine reduced the incidence of POAF versus placebo by 44% (P = 0.021).13 In the COPPS-2 study, colchicine did not significantly reduce the frequency of POAF (33.9% vs. 41.2%). GI intolerance occurred in 12 patients (6.7%) in the placebo group and 26 patients (14.4%) in the colchicine group.14
In the open-label END-AF trial, we randomized patients who underwent open-heart surgery to receive 1 mg of colchicine vs. no colchicine. Colchicine failed to significantly reduce POAF and significantly increased the rate of GI side effects, especially diarrhea (44 of 179 patients, 25%), resulting in treatment discontinuation in half of these patients.16
In a meta-analysis reported by Lee et al., data from three randomized studies of 912 patients found that colchicine effectively prevented POAF (risk ratio [RR] = 0.65; 95% CI = 0.46–0.91; P < 0.01). The study concluded that although colchicine effectively prevented POAF, this treatment was linked to significant GI adverse effects, mainly diarrhea (RR = 2.20; 95% CI = 1.31–3.70; P = 0.003).17
Similarly, a systematic review and meta-analysis of five randomized controlled trials including 1412 patients found that colchicine significantly reduced the incidence of POAF by 30% compared with placebo or usual care (18% vs. 27%, P = 0.0002). The use of colchicine was associated with substantial increases in drug-related side effects, especially GI intolerance (21% vs. 8.2%, P < 0.0001).18
Colchicine was used at a dose of 1 mg in all previous trials. Considering these results, we decided to test whether low-dose colchicine (0.5 mg) could reduce the risk of POAF without increasing the risk of GI side effects.
Low-dose colchicine was previously tested in several settings for preventing different cardiovascular diseases. In a prospective randomized placebo-controlled study, patients with ST-segment–elevation myocardial infarction were randomized to receive weight-adjusted doses of colchicine or placebo. Patients weighing less than 60 kg received a daily dose of 0.5 mg. Colchicine treatment reduced infarct size, suggesting a potential benefit against ST-segment–elevation myocardial infarction.19
Nidorf et al. randomly assigned 532 patients with stable coronary artery disease to treatment with colchicine 0.5 mg/day or no colchicine and followed patients for a median of 3 years. Colchicine treatment reduced the risk of the primary composite outcome of acute coronary syndrome, out-of-hospital cardiac arrest, and ischemic stroke by 67%.20
Recently Tardif et al. conducted a randomized double-blind trial of 4745 patients who recently experienced myocardial infarction. Patients were randomized to treatment with low-dose (0.5 mg) colchicine or placebo. Treatment with colchicine reduced the risk of the primary composite endpoint of cardiovascular ischemic events by 23% (P = 0.02). Diarrhea occurred at equal rates in both groups.21
The results of these trials confirmed that low-dose colchicine was beneficial against various cardiovascular diseases. We therefore hypothesized that this low dose may also be effective in reducing the incidence of POAF while simultaneously averting GI intolerance and diarrhea.
Based on the sample calculation, our intention was to treat 362 patients in both arms. An interim data analysis by the Data Safety Monitoring Board indicated the futility of continuing with the trial because the rate of AF between the two groups did not differ and no difference was likely to occur with continued treatment. Among the 152 randomized patients, AF occurred in 26 patients (17%), including 16% of patients in the colchicine group and 18.3% of patients in the placebo group. The relatively low incidence of POAF was reassuring, and the finding was probably related to improvements in surgical techniques and other perioperative factors. Although we analyzed our results according to the type of surgery and the use of a perfusion pump, the sample size was too small to detect any difference in POAF within surgical subgroups.
Diarrhea, the major GI side effect of colchicine, occurred in only two patients receiving colchicine, including one patient who required treatment discontinuation. It is worth mentioning that two patients on placebo also complained of diarrhea. This low rate of GI intolerance is in line with the results of other trials using a similar dose
Our results indicate that a regimen of 1 mg of colchicine preoperatively followed by 0.5 mg daily postoperatively until hospital discharge did not reduce the risk of POAF despite being well tolerated.
A few limitations must be discussed. There was no continuous cardiac rhythm monitoring from the time patients were transferred from the ICCU until hospital discharge. Resting ECG was performed daily and whenever arrhythmia was suspected. Thus, asymptomatic episodes of AF may have been missed, leading to underestimation of the true incidence of POAF. However, missed episodes were likely to have occurred equally in both groups, and thus, the overall conclusion of the trial would not have been affected. Another limitation was the early termination of the trial because of futility regarding the primary endpoint. This decision diminished the power of the study to detect a difference in the rate of POAF between the two groups.
Conclusion
Although low-dose colchicine was well tolerated in patients undergoing cardiac surgery, this regimen did not prevent POAF. Thus, there is insufficient evidence supporting the administration of colchicine to reduce the incidence of AF after cardiac surgery.
Acknowledgements
We would like to express appreciation to the following physicians who participated in the study: Adnan Lahham, MD; Ahmad Harassis, MD; Aktham Hiari, MD; Asem Nammas, MD; Ashraf Abu Samen, MD; Hadi Abu Hantash, MD; Hussein Amarat, MD; Ibrahim Abu Ata, MD; Ibrahim Jarrad, MD; Islam Abu Sido, MD; Kamel Toukan, MD; Khalid Salaymeh, MD; Marwan Nimri, MD; Nayef Dibs, MD; Nidal Hamad, MD; Osama Baghal, MD; Qasem Shamayleh, MD; Suhail Hammoudeh, MD; Tareq Goussous, MD; Tayseer Qahwaji, MD; Walid Dehmes, MD; Zaher Kasih, MD; and Zakaria Khalil, MD.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Ramzi A. Tabbalat https://orcid.org/0000-0003-2628-9618
Yousef S. Khader https://orcid.org/0000-0002-7830-6857
Hassan Abu Khalaf https://orcid.org/0000-0003-2166-6445
References
- 1.Lauer M, Eagle K, Buckley M, et al. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis 1989; 31: 367–378. [DOI] [PubMed] [Google Scholar]
- 2.Filardo G, Damiano RJ, Jr, Ailawadi G, et al. Epidemiology of new-onset atrial fibrillation following coronary artery bypass graft surgery. Heart 2018; 104: 985–992. [DOI] [PubMed] [Google Scholar]
- 3.Polat A, Şahin İ, Yücel C, et al. Coronary vasculature and postoperative atrial fibrillation: a risk factor analysis. Turk Gogus Kalp Dama 2013; 21: 567–573.
- 4.Zaman A, Archbold R, Helft G, et al. Atrial Fibrillation After Coronary Artery Bypass Surgery. Circulation 2000; 101: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 5.Nair S. Atrial fibrillation after cardiac surgery. Ann Card Anaesth 2010; 13: 196–205. [DOI] [PubMed] [Google Scholar]
- 6.Tamis J, Steinberg J. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol 2000; 23: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazar H, Fitzgerald C, Gross S, et al. Determinants of Length of Stay After Coronary Artery Bypass Graft Surgery. Circulation 1995; 92: 20–24. [DOI] [PubMed] [Google Scholar]
- 8.Alqahtani A. Atrial fibrillation post cardiac surgery trends toward management. Heart Views 2010; 11: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung M, Martin D, Sprecher D, et al. C-Reactive Protein Elevation in Patients with Atrial Arrhythmias. Circulation 2001; 104: 2886–2891. [DOI] [PubMed] [Google Scholar]
- 10.Ishii Y, Schuessler R, Gaynor S, et al. Inflammation of Atrium After Cardiac Surgery Is Associated with Inhomogeneity of Atrial Conduction and Atrial Fibrillation. Circulation 2005; 111: 2881–2888. [DOI] [PubMed] [Google Scholar]
- 11.Ishii Y, Schuessler R, Gaynor S, et al. Postoperative atrial fibrillation: the role of the inflammatory response. J Thorac Cardiovasc Surg 2017; 153: 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho K, Tan J. Benefits and Risks of Corticosteroid Prophylaxis in Adult Cardiac Surgery. Circulation 2009; 119: 1853–1866. [DOI] [PubMed] [Google Scholar]
- 13.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Post pericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011; 124: 2290–2295. [DOI] [PubMed] [Google Scholar]
- 14.Imazio M, Brucato A, Ferrazzi P, et al . Colchicine for Prevention of Post pericardiotomy Syndrome and Postoperative Atrial Fibrillation. The COPPS-2 randomized clinical trial. JAMA 2014; 312: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 15.Vrachatis D, Kossyvakis C, Angelidis C, et al. Colchicine in Post-operative Atrial Fibrillation: a Review. Curr Pharm Des 2018; 24: 695–701. [DOI] [PubMed] [Google Scholar]
- 16.Tabbalat R, Hamad N, Alhaddad I, et al. Effect of ColchiciNe on the InciDence of Atrial Fibrillation in Open Heart Surgery Patients: END-AF Trial. Am Heart J 2016; 178: 102–107. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Singh N, Howe C, et al. Colchicine for Prevention of Post-Operative Atrial Fibrillation. JACC Clin Electrophysiol 2016; 2: 78–85. [DOI] [PubMed] [Google Scholar]
- 18.Lennerz C, Barman M, Tantawy M, et al. Colchicine for primary prevention of atrial fibrillation after open-heart surgery: systematic review and meta-analysis. Int J Cardiol 2017; 249: 127–137. [DOI] [PubMed] [Google Scholar]
- 19.Deftereos S, Giannopoulos G, Angelidis C, et al. Anti-Inflammatory Treatment with Colchicine in Acute Myocardial Infarction. Circulation 2015; 132: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 20.Nidorf S, Eikelboom J, Budgeon C, et al. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. J Am Coll Cardiol 2013; 61: 404–410. [DOI] [PubMed] [Google Scholar]
- 21.Tardif JC, Kouz S, Waters D, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019; 381: 2497–2505. [DOI] [PubMed] [Google Scholar]