Abstract
Background
The underlying mechanism of micro (mi)RNA-211 in bone cell apoptosis after fracture remains unclear. This study aimed to determine the effect and function of miRNA-211 in bone cell apoptosis in fracture patients.
Methods
Serum samples were collected from patients with fractures and healthy controls. Serum miR-211 expression was detected by quantitative PCR. MC3T3-E1 cells were transfected with a transforming growth factor (TGF)-β inhibitor and phosphoinositide 3-kinase (PI3K) inhibitor. The viability of MC3T3-E1 cells was detected by the MTT assay, and apoptosis was detected by flow cytometry. Caspase-3/9 activity and the protein expression of TGF-β, PI3K, and p-Akt were detected by western blot and immunoprecipitation.
Results
In the fracture group, miRNA-211 expression was significantly up-regulated compared with controls. We used miRNA-211 mimics to up-regulate miRNA-211 expression, and observed inhibited cell viability and induced apoptosis and lactate dehydrogenase (LDH) activity. miRNA-211 up-regulation also suppressed the expression of TGF-β, PI3K, and p-Akt proteins. Conversely, miRNA-211 down-regulation increased cell viability and reduced apoptosis and LDH activity, as well as inducing the expression of TGF-β, PI3K, and p-Akt. Inhibiting TGF-β decreased the effect of anti-miRNA-211 on osteocyte apoptosis.
Conclusion
Our data indicate that miRNA-211 functions via the TGF-β/PI3K/Akt signaling pathway in patients with fractures.
Keywords: MicroRNA-211, fracture, transforming growth factor-β, phosphoinositide 3-kinase/Akt, bone cell apoptosis, osteocytes, osteoporosis
Introduction
Osteoporosis is a chronic inflammatory bone disease characterized by trabecula structural damage and reduced bone mineral density, whose course is regulated by immunoregulation.1 Estrogen deficiency-induced fracture is the most common fracture type and is frequently seen in middle-aged and older individuals, especially women. The jaw is an active part of human bone reconstruction which is also subject to fracture.2 Jaw fractures may affect oral implant treatments and delay the healing of jaw injury.3 Additionally, they can lead to the loss of jaw bone mass, accelerate alveolar bone atrophy, and reduce the height of alveolar bone, thereby affecting the outcome of prosthodontic therapy.3
Micro (mi)RNAs are a class of short, single-stranded non-coding RNAs that are extensively distributed in animals and plants.4 They function in post-transcriptional regulation by binding specifically with the 3′ untranslated region of target mRNAs, thereby regulating the expression of multiple genes involved in several biological processes including cell growth, differentiation, apoptosis, and tumorigenesis.5 miRNA expression in cells can be changed under the disease environment, which can induce a series of corresponding biological functional changes through alterations in post-transcriptional regulation.4
The phosphoinositide 3-kinase (PI3K)/Akt pathway is an important downstream signaling pathway of transforming growth factor (TGF)-β family members.6 The activated PI3K/Akt signaling pathway regulates transcription of the target gene in the nucleus.7 The TGF-β response is cell type-specific, and is regulated by a variety of components in the PI3K/Akt pathway as well as other signal transduction pathways.6 TGF signals can mediate the PI3K/Akt signaling pathway to activate the nuclear translocation of PI3K/Akt, thus activating or inhibiting the transcription of target genes that they regulate together.8 TGF-β plays important roles in bone metabolism and can directly act on osteoblasts and osteoclasts.9 As a TGF-β-specific intracellular signal transduction pathway, PI3K/Akt transmits its signals from the cytoplasm to the cell nucleus, thus regulating expression of the target gene.9
The healing process of osteoporotic fractures is more complicated than that of ordinary fractures, involving numerous cytokines in the bone microenvironment.10 Of these cytokines, TGF-β is an important growth factor. TGF-β has multiple functions including controlling the proliferation and differentiation of multiple bone cells.10 In animal experiments, TGF-β has been identified at the site of fractures at various stages of healing.11 The TGF-β content in the trabecula of patients with osteoporosis is significantly lower than in healthy individuals, resulting in increased bone turnover and loss of bone mass.11 However, the administration of a small dose of TGF-β promotes osteoblast proliferation, thereby reducing bone turnover and slowing the loss of bone mass. The increased in vivo expression of TGF-β induced by drug treatment may promote osteoblast differentiation, resulting in increased trabecula formation and accelerated bone formation, thus preventing loss of bone mass.12 The aims of the present study were to determine the effects and function of miRNA-211 on cell apoptosis after fracture.
Materials and methods
Osteoporosis patients
Forty-one patients with osteoporosis (22 male and 19 female) were recruited in this study. The mean age was 44 years (range, 28 to 52 years). Patients complicated with diseases such as malignant tumors, diabetes mellitus, and hypertension were excluded from this study. Written informed consent was obtained from all participants. Osteoporosis patients were recruited from the Department of Orthopedics, Dongying People’s Hospital. Patients with fractures were identified by computed tomography (CT). Serum samples of osteoporosis patients and 40 healthy volunteers were collected and stored at –80°C.
Quantitative reverse transcription (qRT)-PCR
Total RNA was isolated from serum using TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA). cDNA was synthesized from 1 µg total RNA using a Reverse Transcription kit (Invitrogen Corp.). qPCR was performed using an ABI 7900 RealTime PCR System (Applied Biosystems Life Technologies, Foster City, CA, USA). The fold changes of miRNA were calculated using the 2-ΔΔCT method.
Cell culture, differentiation, and transfection
MC3T3-E1 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Cell Culture, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco Cell Culture) in a humidified atmosphere of 5% CO2 at 37°C. Cells were transfected with miRNA-211, small interfering RNA-211, and negative mimics, purchased from Genechem (Shanghai, China), using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. MC3T3-E1 cells were transfected with the TGF-β inhibitor LY2157299 and PI3K inhibitor LY294002 which were both purchased from Genechem.
Western blot analysis
MC3T3-E1 cells were washed twice with phosphate-buffered saline (PBS) and lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer. The protein content was quantified with the bicinchoninic acid assay kit according to the manufacturer’s instructions (Beyotime Institute of Biotechnology, Shanghai, China). A total of 50 µg of protein was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% non-fat milk solution for 1 hour then incubated with primary antibodies against TGF-β (Santa Cruz Biotechnology, Santa Cruz, CA, USA), PI3K (Santa Cruz Biotechnology), p-Akt (Santa Cruz Biotechnology), or GAPDH (Santa Cruz Biotechnology) at 4°C overnight. Membranes were then washed with Tris-buffered saline with Tween and incubated with a goat anti-rabbit IgG secondary antibody conjugated with horseradish peroxidase at room temperature for 1 hour. Immunolabelling was visualized by application of the ECL Plus detection system (Bio-Rad, USA) and analyzed using Image Lab 3.0 software (Bio-Rad, Hercules, CA, USA).
Cell proliferation assay (MTT assay)
Cells were inoculated into 96-well plates at a density of 1 × 104 cells/ml/well and cultured with 20 µL MTT for 4 hours at 37°C. Next, 150 µl of DMEM medium was added to each well and the samples were agitated at 50 oscillations/min for 30 minutes at 37°C. The optical density was measured at 490 nm by a Universal Microplate Reader (BioTek, Winooski, VT, USA).
Apoptosis rate
Cells were added to 1 mL pre-cooled 70% ethanol at 4°C for 2 hours, stained with 5 µL of Annexin V-FITC and 5 µL of propidium iodide, and incubated for 15 minutes. The apoptosis rate was detected using flow cytometry (BD Biosciences, San Jose, CA, USA).
Luciferase reporter assay
The 3′ untranslated region sequence of TGF-β containing the putative miRNA-211 binding site was inserted into the pMIR-REPORT Luciferase vector (Ambion Inc., Austin, TX, USA). Cells were co-transfected with miRNA-211 mimics and TGF-β-pMIR-REPORT using Lipofectamine 2000. After 48 hours, luciferase activity was measured using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) and normalized to respective controls.
Immunoprecipitation
Cells were cultured in 6-mm diameter Petri dishes containing slides (1 × 106 cells/plate), washed with PBS and fixed with 4% paraformaldehyde for 15 minutes at room temperature. They were then permeablilized with 0.1% Triton X-100 at room temperature for 20 minutes, and blocked with 5% donkey serum in PBS for 1 hour at room temperature. Slides were then incubated overnight at 4°C with a primary antibody against TGF-β (Santa Cruz Biotechnology). Following three washes with PBS, they were incubated with an Alexa Fluor 555-conjugated secondary antibody (1: 300 dilution; Invitrogen) for 1 hour and stained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 minutes in the dark. The slides were then examined under a fluorescence microscope (Q500MC Leica image analysis system; Leica, Solms, Germany). Lactate dehydrogenase (LDH) activity levels and caspase-3/9 activity were detected by the LDH kit (Abcam Technology, Cambridge, UK) and caspase-3/9 activity kit (Beijing Shengboyuan Biotechnology Co., Ltd., Beijing, China), respectively, according to the manufacturers’ protocols.
Statistical analysis
Data are presented as the mean ± standard deviation. P < 0.05 was considered to indicate a statistically significant difference. The Student’s t-test or one-way analysis of variance and Tukey’s post-hoc test were used to detect significance.
Ethics
The research protocol was approved by the Care and Use Committee of Dongying People’s Hospital. Participants have provided their written informed consent to participate in this study.
Results
The expression of miRNA-211 in osteoporosis patients
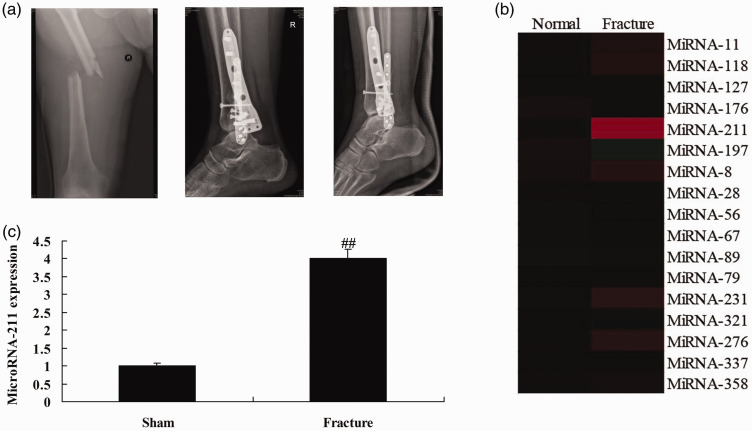
To determine the role of miRNAs in healing after fracture in osteoporosis patients, qRT-PCR was used to measure the expression of miRNAs in patients with osteoporosis. Figure 1b,c shows that miRNA-211 expression was significantly up-regulated in the osteoporosis group compared with healthy controls (P<0.01).
Figure 1.
The expression of miRNA-211 in patients with fractures.
(a) CT of patients. Gene chip (b) and qRT-PCR (c) analysis of the expression of miRNA-211 in patients with fractures. Normal, healthy control; Fracture, patient with fracture. ##p<0.01 versus control group.
miRNA-211 regulates bone cell apoptosis
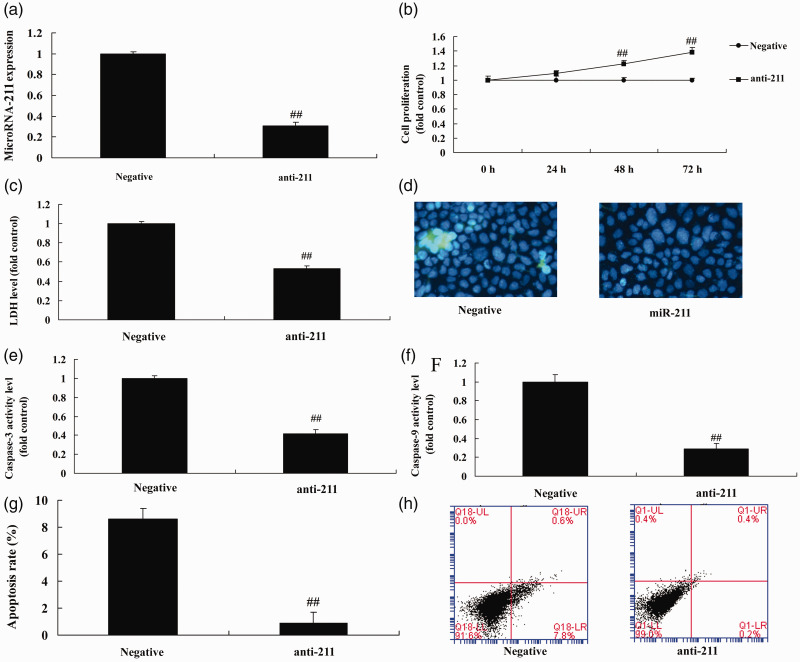
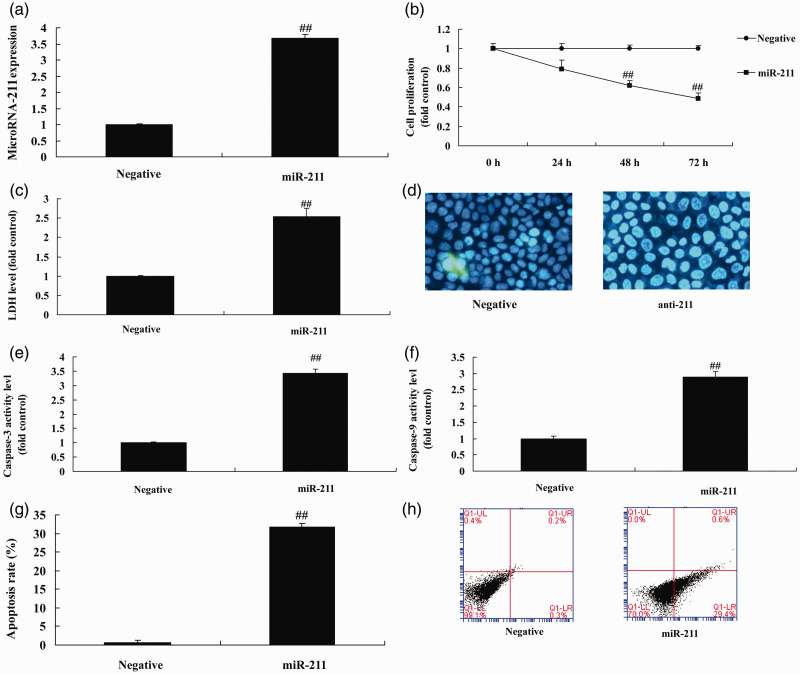
To determine the effect of miRNA-211 in healing after fracture in osteoporosis patients, we used anti-miRNA-211 to inhibit the expression of miRNA-211 in vitro. As shown in Figure 2a, significant inhibition of miRNA-211 was detected compared with the negative mimics group (P<0.01). This down-regulation of miRNA-211 expression significantly promoted cell growth, and significantly reduced LDH activity levels, caspase-3/9 activity, and apoptosis compared with the negative mimics group (Figure 2b–h) (all P<0.01). Transfection of miRNA-211 mimics significantly up-regulated the expression of miRNA-211 in vitro compared with the negative mimics (Figure 3a) (P<0.01), and over-expression of miRNA-211 significantly reduced cell growth and significantly promoted LDH activity levels, caspase-3/9 activity, and apoptosis compared with the negative mimics group (Figure 3b–h) (all P<0.01).
Figure 2.
Down-regulation of miRNA-211 regulates bone cell apoptosis.
qRT-PCR analysis of miRNA-211 expression. (b) Cell growth. (c) LDH activity. (d) DAPI staining. (e, f) Caspase-3/9 activity levels. (g, h). Apoptosis rate. Negative, negative mimics group; anti-211, down-regulation of miRNA-211 group. ##p<0.01 versus negative mimics group.
Figure 3.
Over-expression of miRNA-211 regulates bone cell apoptosis.
(a) qRT-PCR analysis of miRNA-211 expression. (b) Cell growth. (c) LDH activity. (d) DAPI staining. (e, f) Caspase-3/9 activity levels. (g, h). Apoptosis rate. Negative, negative mimics group; miR-211, over-expression of miRNA-211 group. ##p<0.01 versus negative mimics group.
miRNA-211 regulates bone cell apoptosis via the TGF-β/PI3K/Akt signaling pathway
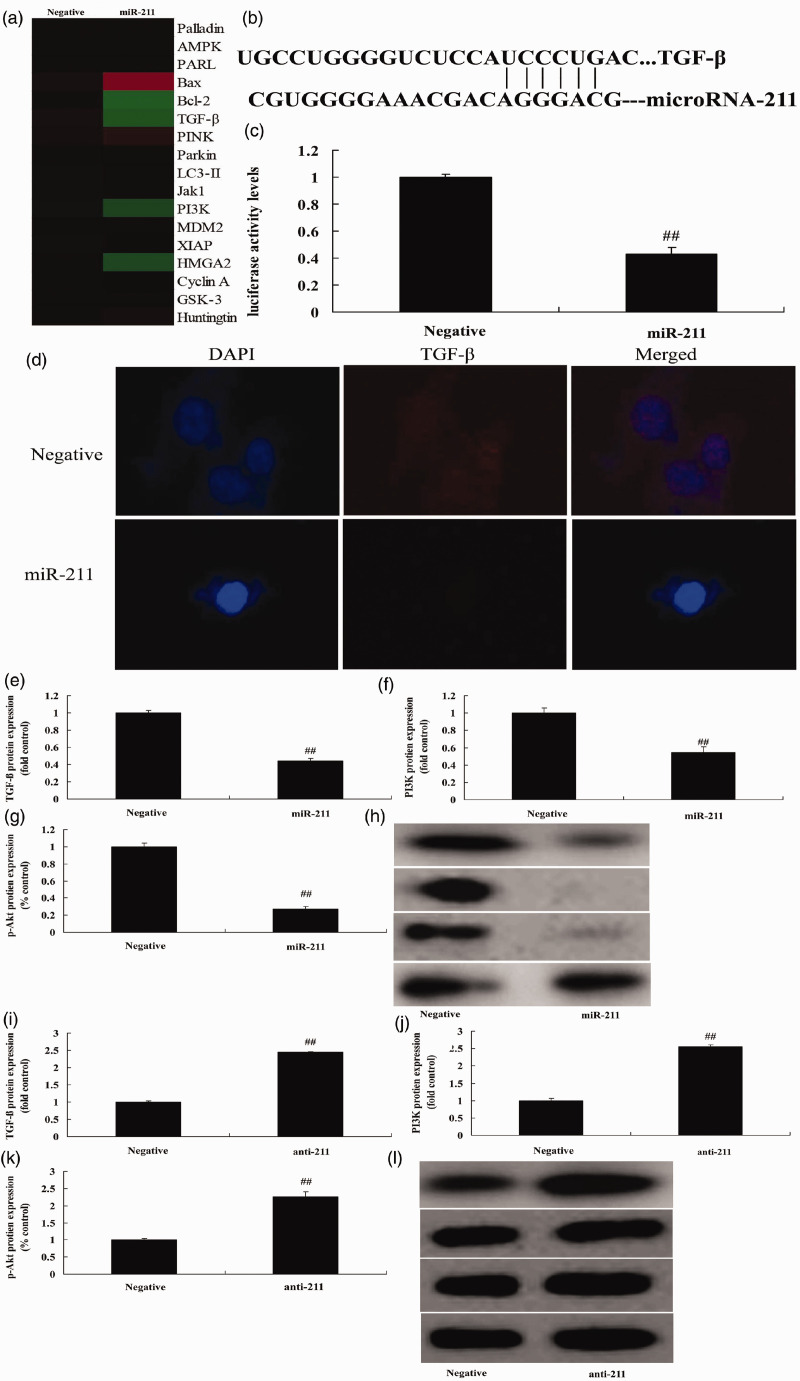
To explore the mechanism of miRNA-211 in healing after fracture in osteoporosis patients, the gene chip was used to measure changes in PI3K/Akt expression. Up-regulation of miRNA-211 following transfection significantly suppressed TGF-β and PI3K expression compared with the negative mimics group (Figure 4a) (P<0.01). Moreover, the putative miRNA-211-binding sequence of TGF-β RNA and up-regulation of miRNA-211 significantly reduced luciferase activity levels compared with the negative mimics group (Figure 4b,c) (P<0.01). Up-regulation of miRNA-211 significantly suppressed TGF-β, PI3K, and p-Akt expression compared with the negative mimics group (Figure 4d–h ) (P<0.01), while miRNA-211 down-regulation significantly induced TGF-β, PI3K, and p-Akt expression compared with the negative mimics group (Figure 4i–l) (P<0.01).
Figure 4.
miRNA-211 regulates bone cell apoptosis via the TGF-β/PI3K/Akt signaling pathway.
(a) Gene chip analysis. (b) Putative miRNA-211-binding sequence of TGF-β RNA. (c) Luciferase activity levels. (d) Immunofluorescence analysis of TGF-β expression. (e–g) TGF-β, PI3K, and p-Akt protein expression by statistical analysis and western blotting (h) following the over-expression and down-regulation of miRNA-211. Negative, negative mimics group; miR-211, over-expression of miRNA-211 group; anti-211, down-regulation of miRNA-211 group. ##p<0.01 versus negative mimics group.
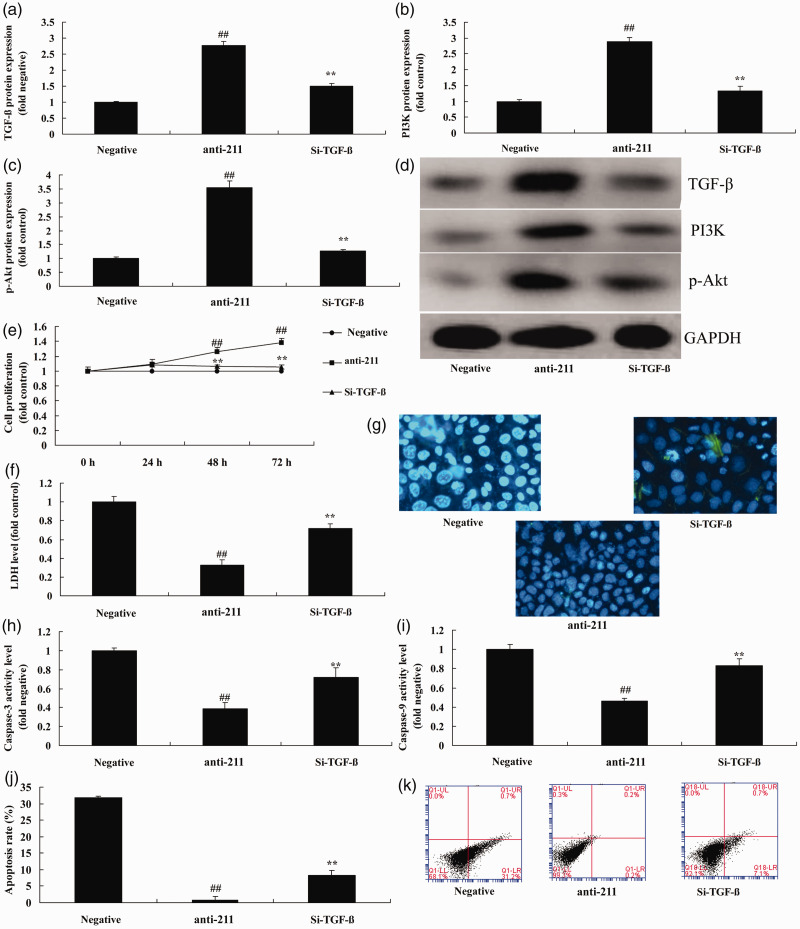
TGF-β inhibitor decreases the effect of anti-miRNA-211 up-regulation on bone cell apoptosis
Next, we investigated the role of TGF-β in the function of miRNA-211 in bone cell apoptosis following fracture. As shown in Figure 5a–d, treatment with 20 nM of LY2157299, a TGF-β inhibitor, significantly suppressed TGF-β, PI3K, and p-Akt expression following anti-miRNA-211 up-regulation (P<0.01). LY2157299 significantly decreased the effect of anti-miRNA-211 up-regulation on bone cell growth, LDH activity levels, caspase-3/9 activity, and apoptosis following anti-miRNA-211 up-regulation (Figure 5e–k) (all P<0.01).
Figure 5.
TGF-β inhibitor decreases the effect of anti-miRNA-211 up-regulation on bone cell apoptosis.
(a–c) TGF-β, PI3K, and p-Akt protein expression by statistical analysis and western blotting (d). (e). Cell growth. (f). LDH activity. (g). DAPI staining. (h, i) Caspase-3/9 activity levels. (j, k) Apoptosis rate. Negative, negative mimics group; anti-211, down-regulation of miRNA-211 group; Si- TGF-β, down-regulation of miRNA-211 and si-TGF-β group ##p<0.01 versus negative mimics group.
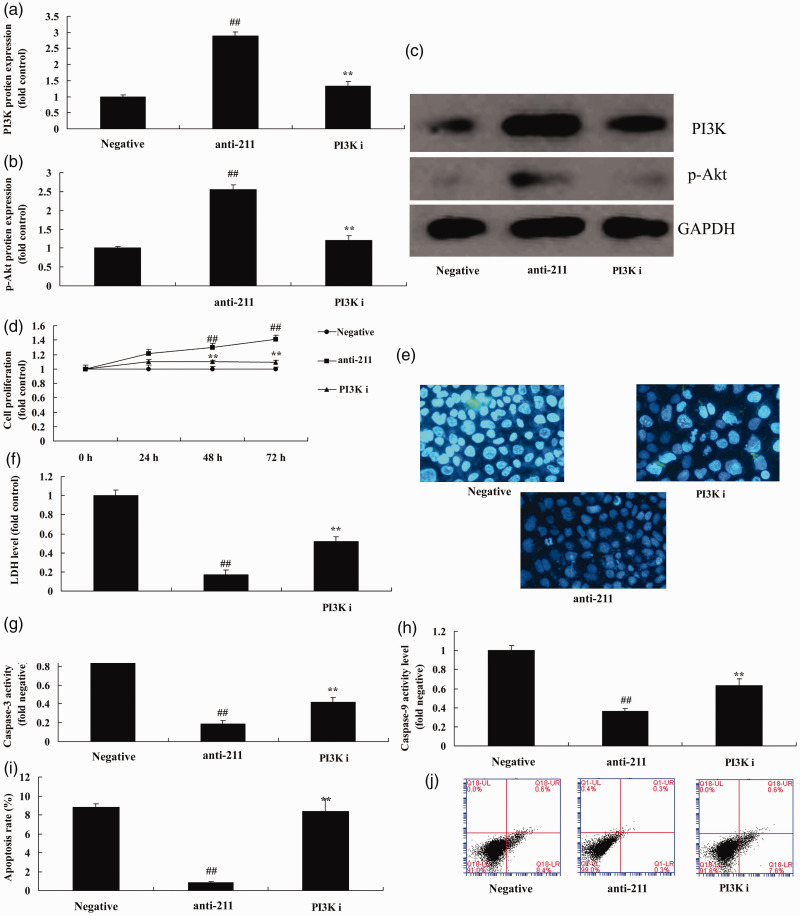
PI3K inhibitor decreases the effect of anti-miRNA-211 up-regulation on bone cell apoptosis
Similarly, to study the role of PI3K in the effect of anti-miRNA-211 up-regulation in bone cell apoptosis following fracture, 200 nM of the PI3K inhibitor LY294002 was used. This significantly reduced the expression of PI3K and p-Akt following anti-miRNA-211 up-regulation (Figure 6a–c) (P<0.01). LY294002 also significantly inhibited the effect of anti-miRNA-211 up-regulation on bone cell growth, LDH activity levels, caspase-3/9 activity, and apoptosis (Figure 6d–j) (all P<0.01).
Figure 6.
PI3K inhibitor decreases the effect of anti-miRNA-211 up-regulation on bone cell apoptosis.
(a, b) PI3K and p-Akt protein expression by statistical analysis and western blotting (c). (d). Cell growth. (e). LDH activity. (f). DAPI staining. (g, h) Caspase-3/9 activity levels. (i, j) Apoptosis rate. Negative, negative mimics group; anti-211, down-regulation of miRNA-211 group; PI3K i, down-regulation of miRNA-211 and PI3K inhibitor group ##p<0.01 versus negative mimics group.
Discussion
Osteoporosis is a systemic disease characterized by reduced bone formation and increased bone absorption, which is associated with enhanced bone fragility and susceptibility to fracture.13 Its clinical manifestations and major signs include pain, reduction in body height, a hump back, and fragility fractures.14 A decrease in bone mineral density is one criterion currently used to diagnose osteoporosis, which develops from a combination of genetic and environmental factors.13,15 miRNA expression is both tissue- and period-specific, and plays an important regulatory role in the growth and differentiation of tissues and cells.16 We found that miRNA-211 expression was significantly up-regulated in the fracture group compared with healthy controls. We also showed that the up-regulation of miRNA-211 reduced cell growth and promoted LDH and caspase-3/9 activity and apoptosis in the fracture group, while the opposite was observed following miRNA-211 down-regulation.
Calcium is a critical element of the human body, particularly of bone.17 Free calcium ions are an important second messenger in cells, involved in regulating a variety of pathophysiological processes including cell proliferation, differentiation, movement, and apoptosis.18 A number of studies have verified that increased calcium ions in extracellular fluid promotes multiple osteogenic capabilities, such as osteoblast proliferation and differentiation and extracellular matrix calcification.18
Osteoclasts absorb old bone while osteoblasts form new bone to repair the remaining lacuna after bone absorption. Estrogen slows down the apoptosis of osteoblasts and accelerates the apoptosis of osteoclasts.19 The sudden decrease in estrogen after menopause therefore leads to increased osteoclast activity, significantly increased osteoclast numbers, and reduced osteoblast number, giving rise to an imbalance between bone reconstruction and bone absorption and thus inducing post-menopausal fractures.19,20
TGF-β is a multi-functional regulatory growth factor for cell growth and differentiation, which plays a vital role in the balance between bone formation and bone absorption.21–23 TGF-β1 is a member of the TGF-β family and promotes autocrine or paracrine signaling in multiple types of immune cells, boosts the differentiation of bone marrow mesenchymal stem cells (BMSCs) into osteoblasts, stimulates osteoblast proliferation, and suppresses the differentiation of BMSCs into osteoclasts.12,21 It also stimulates the synthesis of bone matrix proteins and blocks their degradation. Its role in bone reconstruction has previously been studied,24 and our data suggested that the up-regulation of miRNA-211 suppressed TGF-β, PI3K, and p-Akt expression. The roles of TGF-β1 and PI3K/Akt in osteoporotic bone have been investigated,7,25 while abnormalities in the TGF-β/PI3K/Akt signal transduction pathway in ovariectomized rats may explain the genesis of primary fractures. Our study found that TGF-β and PI3K inhibitors weakened the effect of miRNA-211 down-regulation on bone cell apoptosis in fracture patients.
Conclusions
The present study demonstrated that miRNA-211 expression is up-regulated in patients with fractures. Furthermore, miRNA-211 appeared to function as a regulator of bone cell apoptosis by directly targeting TGF-β/PI3K/Akt. These findings illustrate a potential crucial function of miRNA-211 in the development of fractures, and suggest a potential therapeutic target against bone cell apoptosis.
Authors’ contributions
TS and DY carried out the studies, participated in collecting data, and drafted the manuscript. YW and QS performed the statistical analysis and participated in its design. TS, DY, YW, and QS helped to draft the manuscript. All authors read and approved the final manuscript.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Tongxin Sun https://orcid.org/0000-0002-3913-9160
References
- 1.Mak JC, Mason RS, Klein L, et al. An initial loading-dose vitamin D versus placebo after hip fracture surgery: randomized trial. BMC Musculoskelet Disord 2016; 17: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo-Cardiel G, Lopez-Echaury AC, Saucedo-Ortiz JA, et al. Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells, a randomized clinical trial. Dent Traumatol 2017; 33: 38–44. [DOI] [PubMed] [Google Scholar]
- 3.Ondari JN, Masika MM, Ombachi RB, et al. Unblinded randomized control trial on prophylactic antibiotic use in gustilo II open tibia fractures at Kenyatta National Hospital, Kenya. Injury 2016; 47: 2288–2293. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Su H, Wang X, et al. MiR-148a regulates bone marrow mesenchymal stem cells-mediated fracture healing by targeting insulin-like growth factor 1. J Cell Biochem 2018; 120: 1–12. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Xu X. MicroRNA-137 dysregulation predisposes to osteoporotic fracture by impeding ALP activity and expression via suppression of leucine-rich repeat-containing G-protein-coupled receptor 4 expression. Int J Mol Med 2018; 42: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 6.Choi JH, Hwang YP, Kim HG, et al. Saponins from the roots of Platycodon grandiflorum suppresses TGFbeta1-induced epithelial-mesenchymal transition via repression of PI3K/Akt, ERK1/2 and Smad2/3 pathway in human lung carcinoma A549 cells. Nutr Cancer 2014; 66: 140–151. [DOI] [PubMed] [Google Scholar]
- 7.Bai L, Liang R, Yang Y, et al. MicroRNA-21 regulates PI3K/Akt/mTOR signaling by targeting TGFbetaI during skeletal muscle development in pigs. PLoS One 2015; 10: e0119396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanlon V, Walia B, Yu J, et al. Loss of Cbl-PI3K interaction modulates the periosteal response to fracture by enhancing osteogenic commitment and differentiation. Bone 2017; 95: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsubaki M, Kato C, Manno M, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem 2007; 304: 53–60. [DOI] [PubMed] [Google Scholar]
- 10.Fischer C, Doll J, Tanner M, et al. Quantification of TGF-ss1, PDGF and IGF-1 cytokine expression after fracture treatment vs. non-union therapy via masquelet. Injury 2016; 47: 342–349. [DOI] [PubMed] [Google Scholar]
- 11.Xu MT, Sun S, Zhang L, et al. Diabetes mellitus affects the biomechanical function of the callus and the expression of TGF-beta1 and BMP2 in an early stage of fracture healing. Braz J Med Biol Res 2016; 49: e4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buijs JT, Stayrook KR, Guise TA. TGF-beta in the bone microenvironment: role in breast cancer metastases. Cancer Microenviron 2011; 4: 261–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qvist AH, Vaesel MT, Jensen CM, et al. Plate fixation compared with nonoperative treatment of displaced midshaft clavicular fractures: a randomized clinical trial. Bone Joint J 2018; 100-B: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 14.Gitajn IL, Titus AJ, Tosteson AN, et al. Deficits in preference-based health-related quality of life after complications associated with tibial fracture. Bone Joint J 2018; 100-B: 1227–1233. [DOI] [PubMed] [Google Scholar]
- 15.Barenius B, Inngul C, Alagic Z, et al. A randomized controlled trial of cemented versus cementless arthroplasty in patients with a displaced femoral neck fracture. Bone Joint J 2018; 100-B: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 16.Seeliger C, Karpinski K, Haug AT, et al. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res 2014; 29: 1718–1728. [DOI] [PubMed] [Google Scholar]
- 17.Yin ZY, Yin J, Huo YF, et al. Rapamycin facilitates fracture healing through inducing cell autophagy and suppressing cell apoptosis in bone tissues. Eur Rev Med Pharmacol Sci 2017; 21: 4989–4998. [PubMed] [Google Scholar]
- 18.Huang YZ, Zhao L, Wang CL, et al. RBM5-AS1 participates in fracture healing and inhibits apoptosis of bone cells through the up-regulation of beta-catenin. Eur Rev Med Pharmacol Sci 2018; 22: 5091–5097. [DOI] [PubMed] [Google Scholar]
- 19.Vi L, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2015; 30: 1090–1102. [DOI] [PubMed] [Google Scholar]
- 20.Pan X, Jiang L, Zhong L, et al. Arsenic induces apoptosis by the lysosomal-mitochondrial pathway in INS-1 cells. Environ Toxicol 2016; 31: 133–141. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee A, Larson EA, Carlos AS, et al. Congenic mice provide in vivo evidence for a genetic locus that modulates intrinsic transforming growth factor beta1-mediated signaling and bone acquisition. J Bone Miner Res 2012; 27: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Li HY, Wang D, et al. Delphinidin protects beta2m-/Thy1+ bone marrow-derived hepatocyte stem cells against TGF-beta1-induced oxidative stress and apoptosis through the PI3K/Akt pathway in vitro. Chem Biol Interact 2019; 297: 109–118. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Zhang Y, Chi P. Pirfenidone suppresses TGFbeta1 induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol Med Rep 2018; 18: 3907–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estai MA, Suhaimi F, Das S, et al. Expression of TGF-beta1 in the blood during fracture repair in an estrogen-deficient rat model. Clinics (Sao Paulo) 2011; 66: 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li BS, Huang JY, Guan J, et al. Camptothecin inhibits the progression of NPC by regulating TGF-beta-induced activation of the PI3K/AKT signaling pathway. Oncol Lett 2018; 16: 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.