Abstract
Background:
Osteochondral lesions of the talus (OLTs) with large subchondral cysts are challenging to treat.
Purpose:
To determine the safety and efficacy of autologous chondral grafting and malleolus osteotomy for treating OLTs associated with large subchondral cysts.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 19 patients underwent autologous chondral grafting and malleolus osteotomy. We obtained the visual analog scale (VAS), American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot, and magnetic resonance observation of cartilage repair tissue (MOCART) scores at 1 and 2 years postoperatively. The International Cartilage Repair Society (ICRS) score was collected 2 years postoperatively during second-look arthroscopic surgery.
Results:
In all patients, the osteotomy site healed without nonunion or malunion. Only 1 patient developed joint space narrowing. No donor site complications occurred. The mean AOFAS score significantly improved at 1 year (from 72.8 ± 4.8 preoperatively to 93.7 ± 4.6; t = –13.708; P < .0001). The 1- and 2-year AOFAS scores were similar (t = –0.755; P = .455), indicating stable improvement. The mean VAS score significantly decreased at 1 year (from 4.68 ± 0.67 preoperatively to 0.47 ± 0.69; t = 18.974; P < .0001). The 1- and 2-year VAS scores were similar (t = –0.705; P = .455), as were the 1- and 2-year MOCART scores (64.2 ± 7.5 vs 67.4 ± 7.3, respectively; t = –1.312; P = .198). The ICRS scores were as follows: 7 points (abnormal) in 1 (5.2%) patient, 8 to 11 points (nearly normal) in 9 (47.4%) patients, and 12 points (normal) in 9 (47.4%) patients.
Conclusion:
Osteotomy combined with autologous osteochondral transplantation provided good functional outcomes in patients with OLTs and large subchondral cysts. Second-look arthroscopic surgery showed healthy cartilage healing.
Keywords: autologous chondral graft, malleolus osteotomy, osteochondral lesion, cyst, talus
Osteochondral lesions of the talus (OLTs) are common and may cause pain and dysfunction of the ankle.5,7,8,27 OLTs can be a consequence of ankle sprain or trauma. Symptomatic OLTs that do not respond to nonoperative management, consisting of immobilization, protected weightbearing, nonsteroidal anti-inflammatory drugs, and/or physical therapy, need to be surgically treated.7,12 For superficial OLTs or those measuring less than 1.5 cm2, bone marrow stimulation (BMS) is a possible treatment option. BMS using the microfracture technique has been used to stimulate cartilage regeneration, with good results.6,18 However, in patients with full-thickness cartilage lesions and large subchondral cysts, the optimal treatment remains to be determined.7,16,24 The large cavity of the debrided cyst may compromise the structural support of the articulation, and the subchondral bone defect may hinder cartilage regeneration. Recent studies7,18,24,28 have shown that in patients with OLTs associated with large subchondral cysts, structural bone grafting, such as osteochondral autografts/allografts and autologous chondrocyte implantation, may provide good results. However, the potential donor site morbidity and long-term outcomes, including arthroscopic and radiological outcomes, remain to be investigated.
The purpose of this study was to analyze both the initial clinical and magnetic resonance imaging (MRI) findings as well as the results of second-look arthroscopic surgery performed at least 2 years after autologous chondral grafting and malleolus osteotomy in patients with OLTs and large subchondral cysts. To the best of our knowledge, this study is the first to describe the correlations among MRI findings, second-look arthroscopic results, and clinical and functional outcomes over a relatively long follow-up period. We also evaluated knee function and the osteotomy site to assess potential donor site complications.
Methods
Patient Selection
This study was a retrospective, nonrandomized sequential review of all patients with symptomatic OLTs associated with large subchondral cysts (diameter, >10 mm) who underwent ankle osteotomy and osteochondral graft implantation in our hospital between October 2014 and January 2017. The inclusion criteria were as follows: (1) symptomatic pain or dysfunction of the ankle joint, (2) talar cartilage lesion with a diameter of >10 mm with both cartilage surface involvement and subchondral bone cyst formation, (3) failure of nonoperative treatment for at least 3 months, and (4) no significant limitation of range of motion of the involved ankle (<10° in difference from the contralateral ankle). The exclusion criteria were active infections of the ankle and bony deformities of the ankle (varus or valgus of >10°) or subtalar joint (varus or valgus of >10°). All study participants provided signed informed consent. This study was approved by the ethics committee of our hospital.
Preoperative Planning
We performed radiography, computed tomography, and MRI in every patient with a talar cartilage injury before surgery. Patients with stage V OLTs with subchondral cysts according to the Hepple classification10 were considered to be eligible for autologous chondral grafting. The length, width, and depth of the cyst were measured on coronal, sagittal, and axial MRI scans. The diameter and depth of the cyst in each plane were measured, and the largest values were used to determine the required graft size. If the subchondral cyst was larger than the lesion on the cartilage surface, the osteochondral graft size was determined according to the area of the surface lesion, not according to the area of the cystic lesion.
Surgical Procedure
Surgery was performed under spinal anesthesia with the patient in the supine position and wearing a tourniquet. Standard anterolateral and anteromedial portals were routinely used to inspect the articular surface of the talar dome. Arthroscopic grading of the OLTs was performed. Autologous osteochondral transplantation was performed via either anterior malleolar osteotomy (for lesions located in zone 1) or medial malleolar osteotomy (for lesions located in zone 4 or 7), as described by Varner and Kolstad.25 The talar lesion was debrided and refreshed, and the necrotic sequestrum and sclerotic tissues were removed and curetted until bleeding was observed from the surrounding bone. The depth and diameter of the lesion were measured to determine the size of the graft required.
Autologous bone was harvested from the nonweightbearing distal articular surface of the femoral bone. A harvester tube (Smith & Nephew) was used to obtain an osteochondral cylinder of the correct size. The harvester tube set that we used has only 4 sizes (4.5, 6.5, 8.5, and 10.5 mm). As the cystic lesions measured more than 10 mm in diameter in our patients, 2 osteochondral cylinders were used in each patient. All cylinder grafts were implanted perpendicularly using the press-fit technique to avoid causing further injuries to the defect. If the bottom of the cyst was larger than the surface osteochondral lesion, cancellous bone from the calcaneus or femoral condyle was harvested to fill the defect. No additional fixation of the graft was needed. Medial malleolar osteotomy was performed with 2 or 3 cannulated screws (Tianjin ZhengTian Medical Instrument), while anterior malleolar osteotomy was performed with a 2.7-mm mini-locking plate (Tianjin ZhengTian Medical Instrument).
Postoperative Care and Rehabilitation
Antibiotics were applied to prevent infections. A removable splint in a neutral position was used for about 1 week to protect the ankle and prevent dorsiflexion contracture. The sutures were removed after 2 weeks. Patients were instructed to avoid weightbearing on the operated leg for 6 weeks and to perform full range of motion passive ankle exercises at least 800 times a day. At 6 weeks after surgery, a radiographic examination was performed to confirm bone healing. If good healing was observed, the patient was instructed to use weightbearing boots for walk training for 2 to 3 weeks. After removal of the boots, the patients were told to wear sports shoes to walk normally. Generally, jogging was permitted 3 months after surgery, and participation in competitive sports was gradually allowed from 6 months postoperatively.
Follow-up Assessments
Patients were evaluated at 3 months, 6 months, 1 year, and 2 years after surgery and once a year thereafter. During the follow-up, ankle function was evaluated using a physical examination, the visual analog scale (VAS), and the American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot scale. The Hospital for Special Surgery (HSS) scores of both the donor knee and healthy knee were evaluated to detect any complications at the donor site. Radiological follow-up was performed using computed tomography and MRI. The patients’ radiographs were graded using the magnetic resonance observation of cartilage repair tissue (MOCART) score to identify the condition of the cartilage.19 The radiographs were graded independently by the authors (L.B., S.L.), and the highest numerical score for each patient was included in the results.
About 2 years after surgery, the patients underwent second-look arthroscopic surgery at the time of hardware removal. International Cartilage Repair Society (ICRS) scores were obtained during second-look surgery.
Statistical Analysis
Statistical analysis was conducted using PASW v18.0 software (IBM). Data were reported as mean ± standard deviation. The independent-samples t test was used to compare differences in mean values between groups. A P value of <.05 was considered statistically significant.
Results
A total of 25 patients with symptomatic OLTs and large subchondral cysts underwent ankle osteotomy and osteochondral graft implantation during the study period. Of these, 3 patients were lost to follow-up and were excluded from the study (2 of these patients had moved, and 1 patient suffered an accidental fracture of the same ankle 8 months after surgery). Another 3 patients were excluded because of bony malalignment. The remaining 19 patients, consisting of 5 women and 14 men, were available for follow-up. The mean age of the patients was 35.1 ± 9.5 years, and the mean follow-up duration was 32.5 ± 6.2 months. The patient and lesion characteristics are shown in Table 1, and the surgical details and overall outcomes are shown in Table 2.
Table 1.
Patient and Lesion Characteristicsa
| Patient No. | Sex | BMI, kg/m2 | Age, y | Associated Lesions | Zone | Length of Cyst, mm | Width of Cyst, mm | Depth of Cyst, mm | Lesion Area, mm2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 24.0 | 31 | Bony impingement | 4, 7 | 13.6 | 11.3 | 10.2 | 153.6 |
| 2 | M | 25.7 | 31 | Bony impingement, kissing lesion | 7, 8 | 11.5 | 9.8 | 7.3 | 112.7 |
| 3 | M | 22.9 | 37 | ATFL injury | 4, 7 | 15.2 | 11.3 | 8.2 | 171.8 |
| 4 | F | 23.1 | 33 | Kissing lesion | 4, 5, 7 | 23.5 | 14.5 | 16.7 | 340.7 |
| 5 | F | 22.2 | 52 | None | 2, 4, 5 | 22.6 | 12.5 | 9.6 | 282.5 |
| 6 | M | 26.2 | 39 | ATFL injury | 7, 8 | 12.0 | 7.8 | 8.1 | 758.2 |
| 7 | M | 23.8 | 28 | None | 4, 7 | 13.3 | 7.8 | 7.4 | 103.7 |
| 8 | F | 20.5 | 29 | Malunion of medial malleolus | 1, 4 | 12.6 | 9.7 | 7.2 | 122.2 |
| 9 | F | 20.4 | 27 | None | 4, 7 | 10.8 | 7.9 | 10.1 | 85.3 |
| 10 | M | 25.8 | 32 | Kissing lesion | 4, 7 | 13.6 | 10.2 | 9.1 | 138.7 |
| 11 | M | 23.6 | 31 | None | 4, 7 | 14.1 | 12.7 | 8.3 | 179.1 |
| 12 | M | 27.5 | 33 | Bony impingement, kissing lesion | 4, 7 | 12.9 | 8.6 | 7.4 | 110.9 |
| 13 | F | 22.8 | 26 | None | 4, 5, 7 | 11.2 | 8.4 | 6.9 | 94.1 |
| 14 | M | 23.1 | 27 | Gout | 1-4 | 11.7 | 9.3 | 7.8 | 108.8 |
| 15 | M | 24.2 | 33 | Bony impingement, ATFL injury | 4, 5, 7 | 10.9 | 8.9 | 8.1 | 97.0 |
| 16 | M | 26.3 | 39 | ATFL injury | 4, 5, 7 | 10.6 | 9.4 | 7.9 | 99.7 |
| 17 | M | 25.3 | 31 | Bony impingement, kissing lesion | 1, 2, 4 | 10.2 | 8.5 | 7.2 | 87.6 |
| 18 | M | 27.2 | 26 | ATFL injury | 4, 5, 7 | 11.1 | 8.5 | 7.7 | 94.4 |
| 19 | M | 26.5 | 33 | Bony impingement | 3, 5, 6 | 10.8 | 9.5 | 8.4 | 102.6 |
aATFL, anterior tibiofibular ligament; BMI, body mass index; F, female; M, male.
Table 2.
Surgical Details and MOCART Scores of All Patientsa
| Patient No. | Follow-up, mo | Graft (Diameter), mm | Osteotomy | ICRS Score Before Arthroscopic Surgery | ICRS Score After Arthroscopic Surgery | MOCART Score |
|---|---|---|---|---|---|---|
| 1 | 27 | 2 FC (6.5 + 6.5) | MMO | 4 | 11 | 70 |
| 2 | 35 | 1 FC (10.5) | MMO | 5 | 11 | 60 |
| 3 | 48 | 2 FC (9.5 + 6.5) | MMO | 5 | 12 | 65 |
| 4 | 42 | 2 FC (10.5 + 10.5) + C | AMO (C) | 4 | 8 | 55 |
| 5 | 36 | 2 FC (10.5 + 10.5) | AMO (C) | 3 | 10 | 55 |
| 6 | 40 | 1 FC (10.5) + C | MMO | 6 | 10 | 65 |
| 7 | 33 | 2 FC (6.5 + 6.5) | MMO | 6 | 12 | 75 |
| 8 | 35 | 1 FC (10.5) | MMO | 4 | 12 | 70 |
| 9 | 28 | 1 FC (10.5) | MMO | 5 | 10 | 75 |
| 10 | 27 | 1 FC (10.5) | MMO | 4 | 8 | 70 |
| 11 | 31 | 2 FC (8.5 + 6.5) + C | MMO | 3 | 9 | 70 |
| 12 | 30 | 1 FC (10.5) + C | AMO (M) | 3 | 8 | 60 |
| 13 | 35 | 1 FC (10.5) | MMO | 4 | 9 | 75 |
| 14 | 29 | 1 FC (10.5) | AMO (M) | 3 | 10 | 65 |
| 15 | 26 | 1 FC (10.5) | MMO | 5 | 10 | 70 |
| 16 | 25 | 1 FC (8.5) + C | MMO | 6 | 11 | 70 |
| 17 | 31 | 1 FC (8.5) | AMO | 3 | 8 | 60 |
| 18 | 28 | 1 FC (10.5) | MMO | 4 | 12 | 80 |
| 19 | 25 | 1 FC (10.5) | AMO (L) | 3 | 12 | 75 |
aAMO, anterior malleolar osteotomy; C, cancellous bone from calcaneus; FC, femoral column; ICRS, International Cartilage Repair Society; L, lateral side; M, medial side; MMO, medial malleolar osteotomy; MOCART, magnetic resonance observation of cartilage repair tissue.
Outcomes and Complications
In all 19 study patients, the osteotomy site healed with no instances of nonunion or malunion (Figure 1). Joint space narrowing occurred in only 1 patient during the follow-up period. A patient developed skin necrosis at the anterior malleolar osteotomy site, which healed with dressing. Another patient was found to have narrowing of the joint line after 4 years of follow-up. No donor site complications were found, and no osteoarthritis changes were detected at the osteotomy site. HSS scores did not differ between the donor knee and the healthy knee (99.5 ± 1.1 vs 99.4 ± 1.5, respectively; t = 0.245; P = .808).
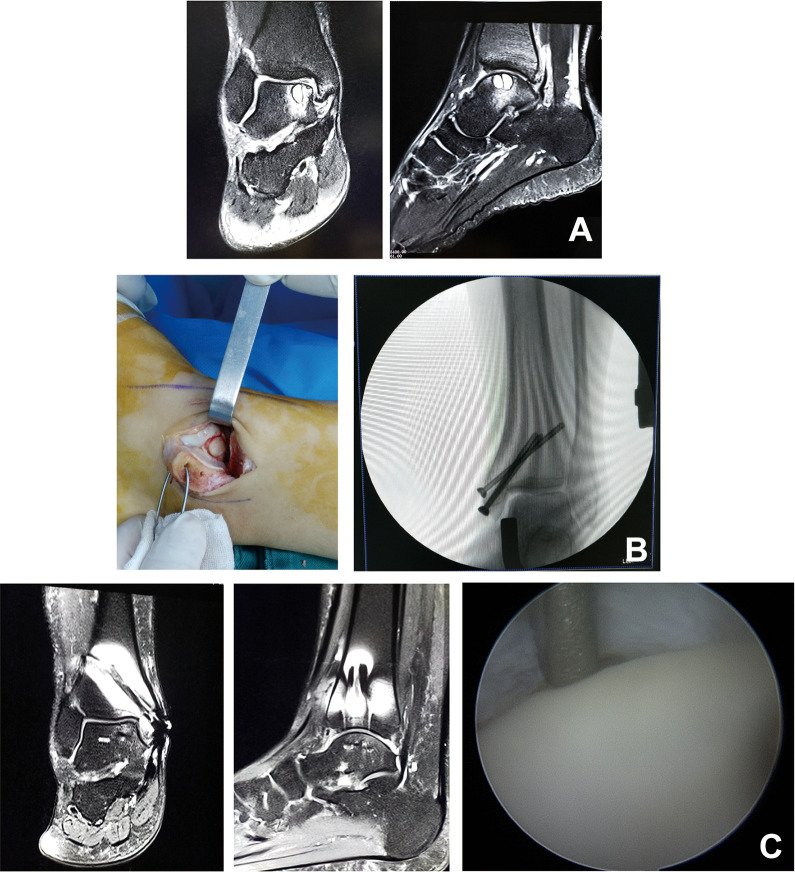
Figure 1.
A 33-year-old male patient. (A) Magnetic resonance imaging (MRI) showed a large talar cyst. (B) Medial malleolar osteotomy was performed for osteochondral grafting and fixed with 3 cannulated screws. (C) At 2 years after surgery, second-look arthroscopic surgery and MRI showed nearly normal cartilage.
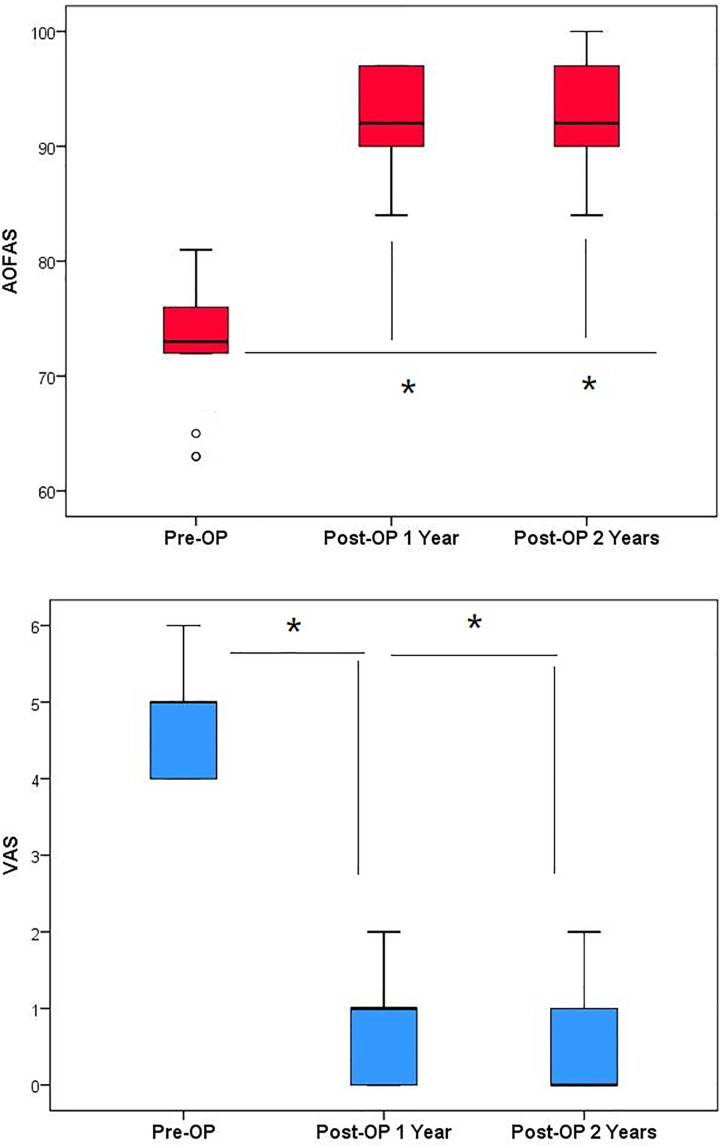
The mean AOFAS score significantly improved from 72.8 ± 4.8 (range, 63-81) preoperatively to 93.7 ± 4.6 (range, 84-100) at 1-year follow-up (t = –13.708; P < .0001). A comparison of the 1- and 2-year follow-up data showed that this outcome was stable, with no significant changes in the AOFAS score (t = –0.755; P = .455) (Figure 2). Pain was also relieved after surgery, as indicated by a significant decrease in the mean VAS score from 4.68 ± 0.67 preoperatively to 0.47 ± 0.69 at 1 year (t = 18.974; P < .0001). Furthermore, the VAS score at 2 years after surgery did not differ from that at 1 year after surgery (t = –0.705; P = .455); thus, pain subsided postoperatively and did not intensify during the follow-up period. The MOCART score at 1 year of follow-up (64.2 ± 7.5 [range, 45-70]) showed no significant difference from that at 2 years (67.4 ± 7.3 [range, 45-75]) (t = –1.312; P = .198).
Figure 2.
American Orthopaedic Foot and Ankle Society (AOFAS) ankle-hindfoot and visual analog scale (VAS) scores preoperatively and at 1 and 2 years postoperatively. Open circles indicate outliers.
*Statistically significant difference compared with preoperatively (P < .05).
There were 5 patients with kissing lesions on the tibial side. We compared the data of these 4 patients with those of the other 15 patients without kissing lesions. At 1-year follow-up, the AOFAS scores did not differ between the patients with and without kissing lesions (90.2 ± 5.3 vs 93.3 ± 3.5, respectively; t = –1.382; P = .185). VAS pain scores also did not differ between patients with and without kissing lesions (0.75 ± 0.95 vs 0.60 ± 0.63, respectively; t = –0.380; P = .708). However, the MOCART score was significantly lower among patients with kissing lesions versus without (57.5 ± 8.7 vs 66.0 ± 6.3, respectively; t = –2.223; P = .040). At the 2-year follow-up, there were no significant differences in these 3 parameters between the patients with and without kissing lesions: AOFAS score, 90.2 ± 5.3 versus 94.6 ± 4.1 (t = –1.781; P = .193); VAS score, 0.50 ± 1.00 versus 0.47 ± 0.64 (t = 0.083; P = .935); and MOCART score, 61.3 ± 6.3 versus 69.0 ± 6.9 (t = –2.035; P = .058), respectively.
Second-Look Evaluation
Second-look arthroscopic surgery records were presented in a random order and assessed by 2 independent observers to reduce the risk of bias. In all 19 patients, the mean ICRS score was 10.3 ± 1.5 (range, 7-12). Only 1 (5.3%) patient had a score of 7, which was defined as abnormal. A total of 9 (47.4%) patients had scores of 8 to 11, which were defined as nearly normal: 2 (10.5%) patients had 8 points, 1 (5.3%) patient had 9 points, 4 (21.1%) patients had 10 points, and 2 (10.5%) patients had 11 points. Nearly half of the patients (9/19; 47.4%) had full scores of 12 points, which were defined as normal cartilage.
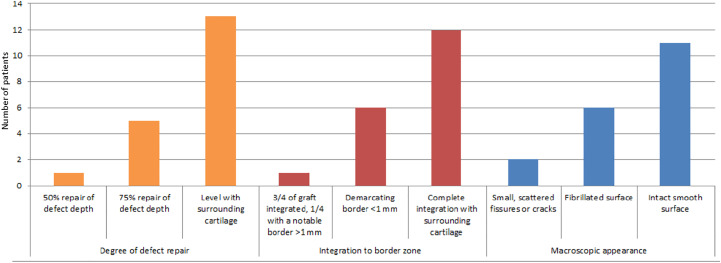
The results for the “degree of defect repair” parameter of the ICRS score were as follows: 50% repair of defect depth in 1 (5.3%) patient, 75% repair of defect depth in 5 (26.3%) patients, and level with surrounding cartilage in 13 (68.4%) patients. The results for the “integration to border zone” parameter were as follows: three-fourths of graft integrated and one-fourth with a notable border in 1 (5.3%) patient, demarcating border less than 1 mm in 6 (31.6%) patients, and complete integration with surrounding cartilage in 12 (63.2%) patients. The macroscopic appearance of cartilage was graded as follows: small scattered fissures or cracks in 2 (10.5%) patients, fibrillated surface in 6 (31.6%) patients, and intact smooth surface in 11 patients (57.9%) (Figure 3).
Figure 3.
Findings of second-look arthroscopic surgery according to the International Cartilage Repair Society score.
Discussion
Because of the poor regenerative ability of cartilage, the treatment of cartilage injuries still relies on the BMS technique or the transplantation of cartilage substitutes.7 For superficial lesions (Hepple stages I-III) with intact subchondral bone and a surface area of less than 1.5 cm2, the microfracture technique is often used.5,7 Additionally, the fibrocartilage regenerated by BMS exhibits good biological potency.6,26 However, the treatment of Hepple stage V OLTs remains a challenge for surgeons. This study showed that the use of malleolar osteotomy to expose the articular surface, combined with autologous cartilage grafting, yielded positive outcomes in patients with large OLTs and subchondral cysts. After more than 2 years of clinical follow-up, good functional outcomes and pain relief were obtained. Joint function was stable after surgery, and no recurrences were observed. At the second-look arthroscopic examination, most joints received an ICRS grade of “normal” or “nearly normal.” No further symptomatic improvement or loss of function occurred during the follow-up period.
Kwak et al17 conducted a long-term follow-up (2-10 years) of patients who had undergone autologous chondrocyte implantation; the authors reported that the mean AOFAS, Tegner activity, and Finsen scores improved, with good long-term results. Zhu and Xu28 used autologous osteochondral grafts combined with cancellous bone grafts to treat OLTs with large cysts in 12 patients, of whom 5 underwent a second-look examination; after 25.4 months of follow-up, good clinical results were obtained. Compared to BMS, the autologous graft technique seems to have better long-term effects. Kim et al15 evaluated 70 patients with OLTs treated using the arthroscopic microfracture technique; the authors reported that symptomatic improvement occurred early after the arthroscopic procedure and was maintained for up to 3 years after surgery. In our experience, in patients with full-thickness cartilage injuries and large cysts eroding the subchondral cancellous bone, the microfracture technique does not result in enough new fibrocartilage formation to fill the defect and provide effective mechanical support. Hence, in such patients, an autologous cartilage graft needs to be transplanted to fill the defect.
Although cartilage graft harvesting from the knee has been shown to have little effect on the donor site,1 many authors have focused on other grafts to avoid knee complications. Chen et al4 used tibial column transplantation with the periosteum instead of knee cartilage grafts; the authors reported that the VAS and AOFAS scores were significantly improved postoperatively, and the MOCART score was more than 64. Overall, the clinical results were excellent after an average follow-up period of 44.8 months. Arauz et al2 used absorbable cartilage screws to fix fresh-frozen talus allografts in 8 patients and obtained good results after an average follow-up period of 46.8 months. However, 2 of the patients showed partial resorption, and 1 patient showed perigraft lysis (<30%). There were no cases of collapse. Those authors concluded that this technique was suitable for patients with irregular lesions with a diameter greater than 20 mm. Sawa et al22 filled defects with cancellous bone harvested from the distal tibial metaphysis and sutured the cartilage pieces in situ. Postoperative MRI showed good continuity and curvature of the cartilage surface. Hu et al11 used osteoperiosteal cylinder grafts from the iliac crest, which also resulted in good clinical outcomes. Shimozono et al23 retrospectively reviewed the use of autologous grafts in 25 patients and the use of allogeneic bone in 16 patients. Over an average follow-up of 2 years, the autografts provided better clinical and MRI outcomes than the allografts. The rate of chondral wear on MRI was higher with the allografts than with the autografts, and the allograft-treated patients had a higher rate of clinical failure. In the current study, we did not find any donor site complications at the knee or any osteoarthritis changes at the osteotomy site. Although cartilage harvesting from the knee may be avoided with other types of grafts and graft techniques, autologous cartilage implantation is a safe and effective method to treat OLTs combined with large subchondral cysts.
Ankle osteotomy can provide good visualization and a large surgical field for lesion debridement and bone grafting in patients with OLTs. However, osteotomy also increases the risk of traumatic arthritis. Some authors have therefore used new graft matrixes to easily fill the defect via arthroscopic surgery or arthrotomy. Osteotomy is avoided because with these new matrixes, there is no need to insert the cartilage cylinder vertically into the lesion. Galla et al9 used autologous matrix-induced chondrogenesis with autologous spongiosa grafting to fill defects via arthrotomy. A total of 23 patients, evaluated for a mean duration of 33.5 ± 10.4 months (range, 24-52.9 months), obtained a significant improvement in ankle function and pain relief. Baumfeld et al3 used an all-arthroscopic procedure with autologous matrix-induced chondrogenesis combined with the microfracture technique to treat talar osteochondral defects. During their short-term follow-up (8-20 months), the AOFAS score significantly improved from 46.4 to 89.5. Kanatli et al14 treated 23 patients with a cell-free polymer-based scaffold under arthroscopic surgery and obtained satisfactory clinical and radiological outcomes. For arthroscopically reachable lesions, these collagen or scaffold matrixes combined with cancellous bone grafts may be a solution to avoid osteotomy. However, a longer follow-up is needed to assess the long-term outcomes of these materials.
In our study, we evaluated the osteotomy site using plain radiography to determine whether the joint space was narrowed. We found that only 1 patient had joint space narrowing. Furthermore, during the follow-up period, there were no instances of nonunion at the osteotomy site or local osteoarthritis. The safety of osteotomy and bone grafting was further confirmed by second-look arthroscopic surgery. Osteotomy combined with autologous cartilage implantation is still the ideal choice in the absence of advanced materials and cellular collagen matrixes.
A kissing lesion of talar cartilage is relatively rare in the literature. In this case series, only 5 (26.3%) patients had kissing lesions. Irwin et al13 reported that 31% (26/83) of their patients had kissing lesions on both the tibial and talar sides. We comparatively analyzed the data of patients with and without kissing lesions. Patients without kissing lesions had significantly better MOCART scores than patients with kissing lesions at 1 year after surgery. However, at 2 years, this difference seemed to have decreased. Furthermore, no significant differences were observed in the AOFAS score after 1 year of follow-up. This trend did not change after 2 years for both the AOFAS score and VAS score. Thus, it appears that kissing lesions did not affect the clinical outcomes of patients with OLTs, even those combined with large subchondral cysts. Ross et al20 reviewed the data of 31 patients with cartilage injuries of the tibial plafond, including 14 patients with kissing lesions on the talar side with a mean size of 38 mm2 (range, 7.1-113 mm2). All patients were treated using the microfracture technique. The authors found no significant differences in functional outcomes between patients with kissing lesions and those with isolated tibial lesions. Intact subchondral bone healed well after treatment with the microfracture technique. In our study, although patients with kissing lesions initially had lower MOCART scores, no differences were observed at the 2-year follow-up. Furthermore, the AOFAS scores were comparable in patients with and without kissing lesions. However, our study could not provide definitive evidence on the optimal treatment for kissing lesions. In addition, hemorrhaging is more frequent at the osteotomy site than in the case of conventional microfracture surgery, and this may provide more growth factors that promote healing of the tibial lesion.21 Thus, the effectiveness of BMS may be enhanced by osteotomy.
The present study has certain limitations. First, during second-look arthroscopic surgery, we did not collect cartilage samples for a histological examination to avoid causing iatrogenic injuries. However, the second-look examination confirmed that the graft had healed well in nearly all patients. Second, this study was a retrospective analysis without a control group. In the future, we intend to perform a randomized controlled trial to compare autologous chondral grafts with biological scaffolds for the treatment of OLTs with large subchondral cysts.
Conclusion
In the current study, osteotomy combined with autologous osteochondral transplantation provided good midterm functional results in patients with OLTs and large subchondral cysts. All patients achieved satisfactory functional and MRI outcomes. In addition, second-look arthroscopic surgery showed healthy cartilage healing and a good geometric shape of the graft. However, in patients with kissing lesions on the tibial side, functional outcomes may be affected.
Footnotes
Final revision submitted February 15, 2020; accepted March 12, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by grants from the Health and Family Planning Commission of Shenzhen Municipality (SZXJ2018085), Sanming Project of Medicine of Shenzhen (SZSM201612078), Medical Scientific Research Foundation of Guangdong Province (A2017202), and Natural Science Foundation of Guangdong Province (2017A030310616). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Peking University Shenzhen Hospital.
References
- 1. Andrade R, Vasta S, Pereira R, et al. Knee donor-site morbidity after mosaicplasty: a systematic review. J Exp Orthop. 2016;3(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arauz JMY, Vecchio JJD, Bilbao F, Raimondi N. Osteochondral lesions of the talus treatment with fresh frozen allograft. Foot Ankle Surg. 2017;23(4):296–301. [DOI] [PubMed] [Google Scholar]
- 3. Baumfeld T, Baumfeld D, Prado M, Nery C. All-arthroscopic AMIC® (AT-AMIC) for the treatment of talar osteochondral defects: a short follow-up case series. Foot (Edinb). 2018;37:23–27. [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Tang K, Yuan C, Zhou Y, Tao X. Intermediate results of large cystic medial osteochondral lesions of the talus treated with osteoperiosteal cylinder autografts from the medial tibia. Arthroscopy. 2015;31(8):1557–1564. [DOI] [PubMed] [Google Scholar]
- 5. Choi WJ, Jo J, Lee JW. Osteochondral lesion of the talus. Am J Sports Med. 2012;40(1):419–424. [DOI] [PubMed] [Google Scholar]
- 6. Choi WJ, Takao M, Jin WL. Microfracture treatment of osteochondral lesions of the talus. Oper Tech Orthop. 2014;24(3):157–162. [Google Scholar]
- 7. Dekker TJ, Dekker PK, Tainter DM, Easley ME, Adams SB. Treatment of osteochondral lesions of the talus: a critical analysis review. JBJS Rev. 2017;5(3):01874474-201703000-00001. [DOI] [PubMed] [Google Scholar]
- 8. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28(2):154–161. [DOI] [PubMed] [Google Scholar]
- 9. Galla M, Duensing I, Kahn TL, Barg A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2789–2795. [DOI] [PubMed] [Google Scholar]
- 10. Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. 1999;20(12):789–793. [DOI] [PubMed] [Google Scholar]
- 11. Hu Y, Guo Q, Jiao C, et al. Treatment of large cystic medial osteochondral lesions of the talus with autologous osteoperiosteal cylinder grafts. Arthroscopy. 2013;29(8):1372–1379. [DOI] [PubMed] [Google Scholar]
- 12. Ibáñez M, Calvo AB, Alvarez V, Lépore S. Osteochondral lesions of the talus: clinical and functional assessment of conservative vs scope treatment. J Clin Periodontol. 2014;2(4 Suppl):420–428. [Google Scholar]
- 13. Irwin RM, Shimozono Y, Yasui Y, Megill R, Deyer TW, Kennedy JG. Incidence of coexisting talar and tibial osteochondral lesions correlates with patient age and lesion location. Orthop J Sports Med. 2018;6(8):23259 67118790965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanatli U, Eren A, Eren TK, Vural A, Geylan DE, Oner AY. Single-step arthroscopic repair with cell-free polymer-based scaffold in osteochondral lesions of the talus: clinical and radiological results. Arthroscopy. 2017;33(9):1718–1726. [DOI] [PubMed] [Google Scholar]
- 15. Kim TY, Song SH, Baek JH, Hwang YG, Jeong BO. Analysis of the changes in the clinical outcomes according to time after arthroscopic microfracture of osteochondral lesions of the talus. Foot Ankle Int. 2019;40(1):74–79. [DOI] [PubMed] [Google Scholar]
- 16. Kraeutler MJ, Chahla J, Dean CS, et al. Current concepts review update: osteochondral lesions of the talus. Foot Ankle Int. 2017;38(3):331–342. [DOI] [PubMed] [Google Scholar]
- 17. Kwak SK, Kern BS, Ferkel RD, Chan KW, Sina K, Applegate GR. Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med. 2014;42(9):2156–2164. [DOI] [PubMed] [Google Scholar]
- 18. Looze CA, Capo J, Ryan MK, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. 2017;8(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. [DOI] [PubMed] [Google Scholar]
- 20. Ross KA, Hannon CP, Deyer TW, et al. Functional and MRI outcomes after arthroscopic microfracture for treatment of osteochondral lesions of the distal tibial plafond. J Bone Joint Surg Am. 2014;96(20):1708–1715. [DOI] [PubMed] [Google Scholar]
- 21. Salamanna F, Contartese D, Nicoli Aldini N, et al. Bone marrow aspirate clot: a technical complication or a smart approach for musculoskeletal tissue regeneration? J Cell Physiol. 2018;233(4):2723–2732. [DOI] [PubMed] [Google Scholar]
- 22. Sawa M, Nakasa T, Ikuta Y, et al. Outcome of autologous bone grafting with preservation of articular cartilage to treat osteochondral lesions of the talus with large associated subchondral cysts. Bone Joint J. 2018;100(5):590–595. [DOI] [PubMed] [Google Scholar]
- 23. Shimozono Y, Hurley ET, Nguyen JT, Deyer TW, Kennedy JG. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2018;100(21):1838–1844. [DOI] [PubMed] [Google Scholar]
- 24. Vantienderen RJ, Dunn JC, Kusnezov N, Orr JD. Osteochondral allograft transfer for treatment of osteochondral lesions of the talus: a systematic review. Arthroscopy. 2017;33(1):217–222. [DOI] [PubMed] [Google Scholar]
- 25. Varner KE, Kolstad K. Autologous osteochondral transplantation for medial talar dome osteochondral lesions. Tech Foot Ankle Surg. 2006;5(3):175–183. [Google Scholar]
- 26. Yoshimura I, Kanazawa K, Takeyama A, et al. Arthroscopic bone marrow stimulation techniques for osteochondral lesions of the talus: prognostic factors for small lesions. Am J Sports Med. 2013;41(3):528–534. [DOI] [PubMed] [Google Scholar]
- 27. Zengerink M, Struijs PAA, Tol JL, Dijk CNV. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Y, Xu X. Osteochondral autograft transfer combined with cancellous allografts for large cystic osteochondral defect of the talus. Foot Ankle Int. 2016;37(10):1113–1118. [DOI] [PubMed] [Google Scholar]