Abstract
Objectives:
Peripheral artery disease is a major cardiovascular disease affecting more than 200 million people globally and up to 4 times more frequent in the diabetic population. It can lead to lower extremity amputations or revascularisation and is associated with an increased risk of myocardial infarction, stroke and early mortality. This novel cross-sectional study aimed to explore the feasibility and acceptability of incorporating diabetic foot screening at routine diabetic retinopathy screening appointments.
Methods:
Participants underwent foot screening during the interval between pupil dilatation and retinal photography as part of the eye screening procedure. Lower limb arterial assessment included ankle brachial index, pulse volume waveform and protective light touch sensation.
Results:
Of 364 participants invited, 88% (n = 321) met the inclusion criteria. About 26.4% (n = 86) had asymptomatic peripheral artery disease and 3% (n = 10) had peripheral sensory neuropathy. Binary logistical regression analysis identified age (p < 0.005), existing coronary heart disease (p < 0.005) and gender (p = 0.03) as predictors of peripheral artery disease.
Conclusion:
Incorporating foot examination during eye screening appointments is feasible and was well received by participants and staff alike. Undiagnosed early peripheral artery disease was evident in a third of the study population emphasising the benefit of introducing foot surveillance into eye screening appointments for the early identification of lower limb arterial disease and peripheral sensory neuropathy.
Keywords: Diabetes foot screening, peripheral arterial disease, diabetic peripheral neuropathy, diabetic retinopathy screening
Introduction
Cardiovascular disease (CVD) is the main cause of death in persons with diabetes worldwide.1,2 In 2010, peripheral arterial disease (PAD) was estimated to affect more than 200 million people globally, and approximately 20% over the age of 60 years in the United Kingdom.3,4 PAD is considered to be one of the most prevalent of morbid diseases5 with the associated disability and mortality increasing in the last two decades, more so in women than men, and no longer restricted to the elderly population and thereby representing a major public health challenge worldwide.6
Intermittent claudication is the commonest complaint in symptomatic PAD, but non-invasive vascular assessments such as ankle brachial index (ABI) reveal asymptomatic PAD to be several times more common.7,8 Delaying the diagnosis of PAD can potentially lead to the need for revascularisation and lower extremity amputation (LEA) associated with an increased risk of myocardial infarction (MI), stroke and early mortality.9 Diagnosis of PAD at the asymptomatic stage will therefore facilitate interventions to modify known risk factors.7
Diabetes is one of the main causes of non-traumatic LEA due to micro and macro-vascular complications such as peripheral neuropathy (DPN) and/or PAD. Co-existing microvascular disease (MVD) such as diabetes-related retinopathy and nephropathy increases the amputation risk, by more than 20 times10,11 and death following amputation.12
In 2019, there were an estimated 463 million people with diabetes globally which is anticipated to increase to 700 million by 2045.13,14 The International Diabetes Federation (IDF) has stated that every 30 seconds a lower limb or part of a lower limb is lost to amputation somewhere in the world as a consequence of diabetes which amounts to as many as 20 per day in the United Kingdom.13,15 Approximately 55% of those with diabetes, who have undergone an LEA, will require amputation of the contralateral limb within 2–3 years with an increased mortality rate of up to 77% within 5 years.16 A delayed diagnosis can lead to critical limb ischaemia (CLI) which has a very poor prognosis with the mortality rate at 15%–30% within 1 month increasing to 50% at 1 year and reaching 74% after 5 years17 which is higher than breast cancer, colon cancer, and prostate cancer.18 Good diabetes control, including improvement in diet, increased activity levels and eliminating unhealthy behaviours (e.g. excessive alcohol, smoking and unhealthy sleep patterns) along with medication adherence can however contribute to delaying the onset or progression of diabetes-related complications such as peripheral neuropathy and peripheral arterial disease.19,20
NICE recommends foot examination for persons with diabetes as part of their annual review.21 NICE and several other guidelines recommend ankle brachial pressure index (ABI) as the preferred non-invasive method for detecting PAD and the 10-g monofilament for detecting impaired peripheral sensory neuropathy, in primary and community care settings.22–24 However, inconsistencies in diabetic foot screening guidelines exist between regional, national and international recommendations which contributes to inequalities of care.25
The recommended use of ABI for PAD diagnosis is not however without its limitations as highlighted in a recent guideline update by NICE.21 The presence of distal peripheral neuropathy, medial arteriosclerosis and incompressible arteries particularly affect the performance of ABI.26–28 The recognised limitations of ABI in the diabetic population, therefore, suggests that other methods for detecting PAD should be explored.
Pulse volume waveform (PVW) is usually used to locate arterial lesions and is especially useful in the setting of calcified vessels as it does not require the vessel to be occluded, as needed to perform an ABI, and therefore results are not influenced by the presence of arteriosclerosis.29 PVW analysis carried out at ankle level provides a qualitative assessment of peripheral arterial blood flow.
In an attempt to combat the rising number of diabetes-related LEAs, integrating foot checks into established diabetic eye screening programmes could therefore be a viable option. Diabetic retinopathy screening (DRS) programmes have contributed to relegating diabetes-related retinopathy from being the leading cause of certifiable blindness among working age adults in England and Wales,30 and in a retrospective analysis of newly recorded certifications of visual impairment in Wales during 2007–2015, sight loss was reduced by almost 50%.31 It is well recognised that the success of any screening intervention is dependent on the early detection of complications and timely referral for treatment.32 In the context of PAD if not picked up early, disease will progress to non-healing ulcerations which often necessitate challenging distal revascularisation or angioplasty to improve distal blood flow.33
In the recent Fourth National Diabetes Foot-care Audit (2019), it was reported that 2.7% of people with diabetes presenting with severe foot ulceration underwent major amputation within 6 months, with 14% dying within 1 year.34
Methods
This cross-sectional feasibility study aimed to explore the acceptability of targeted screening for PAD and peripheral sensory neuropathy on the same occasion as diabetes retinopathy screening. This study was conducted over an 8-week period during 2017 at two hospital sites in Wales (University Hospital of Wales and Royal Glamorgan Hospital) where diabetic retinopathy screening was being carried out.
Ethical approval was granted by both the Chelsea Research Ethics Committee, London and Cardiff Metropolitan University Ethics Committee, Cardiff.
Persons with diabetes registered on the Diabetic Eye Screening Service Wales (DESW), Optimize database, due for eye screening appointments at the two designated hospital sites in Wales during the recruitment period were sent the study information and invitation letter offering foot screening at their forthcoming eye screening appointment. For inclusion into the study, participants had to be at least 18 years of age and able to give informed consent, be transferrable to a patient couch, lie supine for up to 10 min and have at least one lower limb.
The DESW Healthcare Assistants identified participants fulfilling the inclusion criteria. Following the assessment of visual acuity and the administration of Tropicamide eye drops, those agreeing to take part were then escorted by the Podiatry Assistant to an adjacent room for their foot checks. For the arterial assessment, measuring ABI and PVW, required the participant to lay supine with the dual chamber blood pressure cuffs from an automated device (Dopplex Ability, Huntleigh Healthcare), attached to all four limbs. The participant remained supine, motionless and not allowed to talk or smoke for the duration of the test of approximately 3–5 min. All mobile phones were required to be at least 1 metre away from the device. Systolic blood pressures and PVW of each limb were measured automatically and simultaneously, negating the need to rest the participant between measurements. The device automatically calculated the ABI (the highest ankle pressure divided by the highest brachial pressure for each lower limb) and visually displayed the PVW profile. ABI measurements were classified as normal (0.9–1.30), mild obstruction (0.7–0.89), moderate obstruction (0.4–0.69), severe obstruction (<0.4) or poorly compressible (>1.30).35
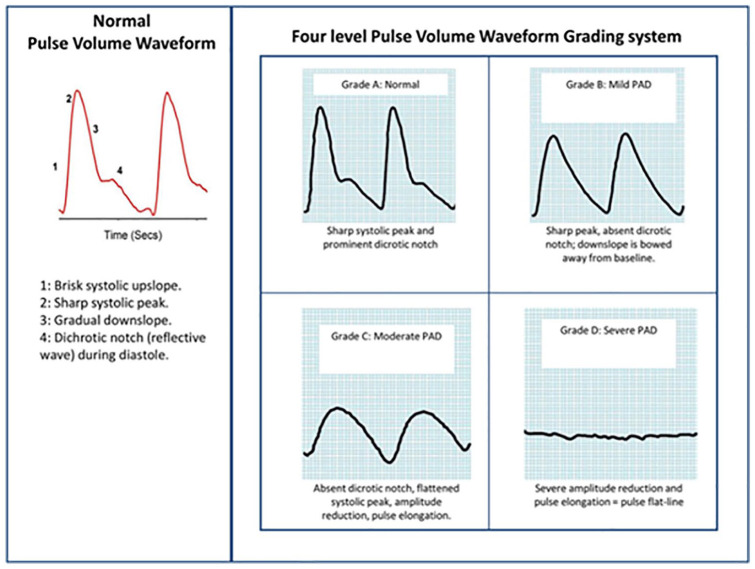
The Rumwell and McPharlin36 grading system was used to grade the PVW profiles as normal (Grade A), mild obstruction (Grade B), moderate obstruction (Grade C) and severe obstruction (Grade D) (Figure 1).
Figure 1.
Pulse volume waveform grading system (Rumwell and McPharlin).36
Light touch peripheral sensory neuropathy was assessed using the 10-g monofilament21 at five plantar load bearing sites on each foot. Following the foot screening, the participant was accompanied back to the original room where retinal photography was conducted. All results were stored on computer software for scrutiny and analysis.
The results of the retinal images were sent to the Diabetic Eye Screening Wales central grading centre with the foot screening results being sent to the chief investigator (JL). Onward referrals were according to the locally agreed pathways. GPs received copies of both eye and foot screening reports. Randomly selected participants (n = 100) took part in a short telephone questionnaire, exploring their combined screening experience.
Statistical analysis
Contingency tables were analysed using Pearson’s chi-square test and continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean values plus standard deviation (SD) and comparisons made with the independent-samples t-test. Data not normally distributed are presented as median values and interquartile ranges (IQR), and non-parametric analyses performed using the Mann–Whitney U test. All comparisons were two-sided and correlation analysis undertaken using Pearson’s method. Binary logistic regression was used to identify predictors of PAD, with variance inflation used to confirm the absence of multi-collinearity between individuals and this was confirmed by goodness of fit determined using Pearson’s and Hosmer–Lemeshow’s test. Statistical significance was deemed present with a p < 0.05. Statistical analyses were performed with the IBM SPSS Statistics software version 22.0 (IBM Corp., Armonk, NY and Minitab® version19).
Results
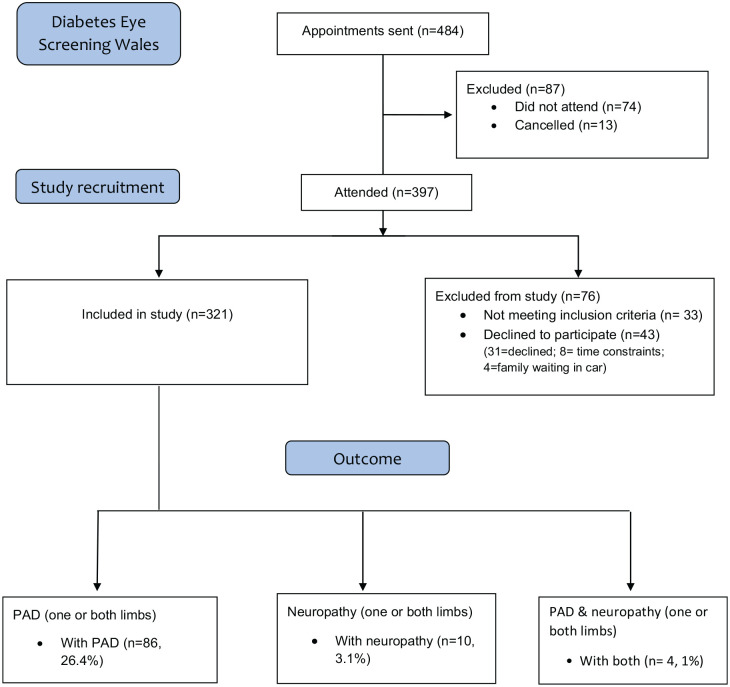
Of the 484 individuals invited for diabetic retinopathy screening, 13 cancelled their appointment and a further 74 did not attend. Of the 397 that did attend, 33 did not meet the study’s inclusion criteria and of 43 who did not take part, 31 declined, 8 had time constraints and 4 had family member awaiting in a car. Finally, of the 364 individuals invited, 321 (88%) participated in the combined screening service (Table 1).
Table 1.
Participant recruitment flow diagram.
|
PAD: peripheral artery disease.
The mean age of the study cohort was 64 years, 56% were males, 7% were current smokers with 53% considering themselves to be non-smokers and 40% were ex-smokers (Table 2).
Table 2.
Demographic characteristics of the PAD and non-PAD groups.
| Variables | Number of
individuals n = 321 (%) |
Number with PAD n = 86 (%) |
Number No PAD n = 235 (%) |
Significance between PAD and no PAD
groups (p value) |
|---|---|---|---|---|
| Age, years (mean/SD) | 63.88 / 14.05 | 71.1 / 11.5 | 61 / 14.2 | <0.005 |
| Gender, males/female | 180/141 (56/44) |
40/45 (22/32) |
140/96 (78/68) |
0.03 |
| Smoking | ||||
| Current | 23 (7) | 5 (23) | 18 (78) | |
| Previous | 128 () | 23 (18) | 105 (82) | NS |
| Non | 170 (53) | 57 (34) | 113 (66) | |
| Hypertension | 206 (64) | 70 (34) | 136 (66) | NS |
| Dyslipidaemia | 210 (7) | 69 (33) | 141 (67) | NS |
| Stroke | 31 (10) | 31 (100) | 0 (0) | NS |
| CHD | 61 (3) | 61 (100) | 0 (0) | < 0.005 |
| Previous PAD | 23 (7) | 3 (13) | 20 (87) | NS |
| Previous DVT | 9 (3) | 1 (11) | 8 (89) | NS |
| Neuropathy | 10 (3) | 3 (30) | 7 (70) | NS |
| Previous vascular intervention | 36 (11) | 2 (6) | 34 (94) | < 0.005 |
| BMI (mean/SD) | 31.61 / 6.14 | 31.44 / 6.36 | 31.74 / 6.12 | NS |
| Waist circumference (cm) (mean/SD) | 104.63 / 13.24 | 106.4 / 16.7 | 104.1 / 13.9 | NS |
PAD: peripheral artery disease; DVT: deep vein thrombosis; SD: standard deviation; NS: nonsignificant; CHD: coronary heart disease; BMI: body mass index.
Whereas 64% were known to have hypertension, other vascular complications, that is, coronary heart disease (CHD), stroke, and previous PAD were recorded in only 10% or less of the participants. There were 3% with impaired peripheral sensory neuropathy with two or more sites insensate using the 10 g monofilament, in one or both feet.
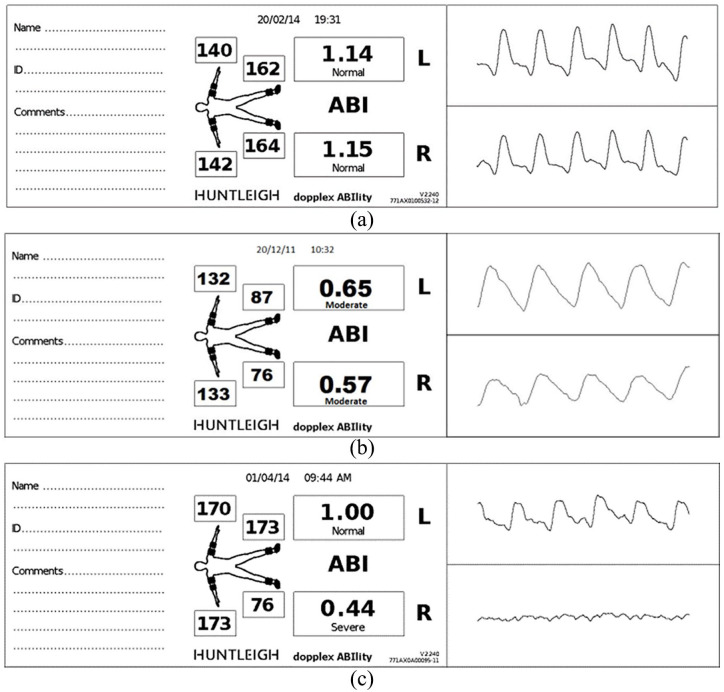
In this study, those with an ABI < 0.9 and/or PVW Grades B, C or D were considered as having PAD. About 26.4% (n = 86) were found to have previously undetected, asymptomatic PAD as self-reported by the participants (Figure 2(a)–(c)).
Figure 2.
Example of (a) both limbs normal ABI/Grade A PVW, (b) both limbs moderate ABPI/Grade B PVW and (c) left limb normal ABI/Grade A PVW, and right limb severe obstruction ABI/severe obstruction Grade D PVW.
Binary Logistical regression was used to explore for the potential risk factors for PAD. Of the variables investigated in this study, the following predictors were found to be significantly associated with the presence of PAD, age, coronary heart disease, gender and ABI (Table 3).
Table 3.
Binary logistic regression analysis to determine significant predictors of PAD.
| Predictors of PAD | p value | OR | 95% CI |
|---|---|---|---|
| Age | <0.005 | 1.0368 | (1.0095, 1.0649) |
| CHD | <0.005 | 2.8651 | (1.4390, 5.7048) |
| Gender | 0.03 | 2.0291 | (1.1017, 3.7372) |
| ABI | <0.005 | 0.0265 | (0.0033, 0.2128) |
| The adjusted R2 value = 17.2% |
PAD: peripheral artery disease; OR: odds ratio; CI: confidence interval; CHD: coronary heart disease; ABI: ankle brachial index.
The combined adjusted R2 value of 17.2% confirms that this is a predictive model of PAD. The model was also adjusted for the effect of age as it could be argued that the effect of all the variables was due to age. ABI, gender and CHD remained significant even when the age of the sample was adjusted for. The analysis of variance confirms that this is highly significant both combined (p < 0.001) and individually (Table 4).
Table 4.
The analysis of variance confirming significance both combined (p < 0.001) and individually.
| Source | DF | Wald test | |
|---|---|---|---|
| Chi-square | p value | ||
| Regression | 4 | 41.79 | <0.001 |
| Age | 1 | 7.05 | 0.008 |
| ABI | 1 | 11.67 | 0.001 |
| CHD 0 = No 1 = YES |
1 | 8.97 | 0.003 |
| Gender 1 = male 2 = female |
1 | 5.16 | 0.023 |
DF: degrees of freedom; ABI: ankle brachial index; CHD: coronary heart disease.
Interestingly, body mass index (BMI) was not found to be significantly related to PAD in this group, neither was smoking habits. When considering differences between those with PAD and those without, age, gender, CHD and previous vascular intervention were the only variables to show a statistically significant difference between the two groups.
Although specific data were not collected regarding diabetes complications due to lack of GP engagement, a large proportion of participants (between 70–80%) were ‘unaware’ of the impact that diabetes can have on the circulation to the lower limb and feet, and therefore, did not recognise the importance of regular foot checks, thereby increasing the risk of ulceration and potential amputation. After the completion of the study, a random selection of participants (n = 100) were contacted by telephone and asked about their combined screening experience. All of those contacted said they would welcome the addition of foot screening to the eye screening service in the future.
Discussion
Diabetic foot disease is one of the most devastating chronic complications of diabetes worldwide with widely different amputation rates, due to variations in foot care to include examination procedures and frequency and the inadequate detection and modulation of cardio-metabolic risk factors. Screening for PAD in studies involving the general population without cardiovascular risk has proven to be unyielding.37 However, in this novel, targeted feasibility study in people undergoing screening for diabetes-related retinopathy, determining both ABI and PVW, and assessing peripheral neuropathy we revealed that almost a third of the participants had previously undiagnosed PAD with a much small proportion (3%), having DPN. The mean age of those with PAD were a decade older than those without; however, similarly to Sampson,6 this study also highlighted that more females than males were found to have PAD.
Although the non-invasive assessment of ABI and pulse volume waveforms (PVW) are well recognised and recommended means of identifying asymptomatic PAD, they are predominantly utilised in secondary care. PVW has been shown to be more reliable than ABI in the diabetic population, being uninfluenced by the presence of arteriosclerosis38 and ABI is also considered less reliable in those with lower levels of disease.27,38 However, in this study, a device combining ABI with PVW was used allowing both results to be ascertained simultaneously. The use of PVW in this study also supports NICE recommendations for research into other modalities for identifying PAD, other than ABI, in this high-risk population.39
Those reporting CHD were also found to have asymptomatic PAD. Treating PAD in its asymptomatic stage is highly beneficial, particularly with respect to mitigating common risk factors of atherosclerotic disease in the different vascular beds7 with long-term health care cost benefits.40
Feedback from both participants and staff confirmed that they would welcome a combined foot and eye screening service, thereby limiting the number of outpatient sessions, a strategy which has also been suggested by others32 with the potential to also reduce cardiovascular disease and premature mortality in this vulnerable high-risk population.
It is not only important for all health professionals to be more astute in identifying PAD to ensure timely management of associated risk factors but there is also a real need to further increase awareness of PAD especially in people with diabetes. A public education programme by the Peripheral Arterial Disease Coalition for the early detection and prevention of PAD, but also to improve the quality of life of people with PAD,41 developed for both health care professionals and the public could be adopted and incorporated into diabetes education.
This feasibility study confirms the acceptability, to both the people with diabetes and health care professionals, of introducing a foot care assessment element on the same occasion as diabetic retinopathy screening.
Limitations
This feasibility study was limited in its scope, due to the absence of data from the diabetic screening service and primary care, to relate the foot examination findings with the retinopathy status and associated risk factors.
Health economic evaluation was not intended at this stage.
Conclusion
Screening for diabetes-related retinopathy and PAD has the ability to detect early disease which can facilitate timely interventions to reduce the risk of vision loss, lower limb and cardiovascular disease, premature death and reduce health care costs while also improving quality of life. This study demonstrated that combining foot and eye screening service is feasible and was well received by participants and staff alike, who expressed a wish to see such a service being routinely provided in the future. Combined screening for diabetes-related complications would empower the people with diabetes by increasing their knowledge and awareness of the existence and importance of co-morbidities such as diabetes-related retinopathy, cardiovascular disease, diabetic neuropathy and nephropathy.
Acknowledgments
The research team thanks the Diabetic Eye Screening Wales Team, the staff from Podiatry departments of both Cardiff & Vale and Cwm Taf University Health Boards in Wales, particularly Mrs Susan Dyananda, Mr Scott Cawley and Mrs Kathryn Watts from Cardiff & Vale UHB and Mrs Denise Jenkins, Miss Keri Hutchinson and Mrs Sandra Exell from Cwm Taf UHB who were instrumental in facilitating data collection.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from London-Chelsea Research Ethics Committee REC approval ID 15/LO/2208.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a Research Enterprise and Innovation Fund from Cardiff Metropolitan University.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: Jane EA Lewis  https://orcid.org/0000-0002-9837-6131
https://orcid.org/0000-0002-9837-6131
References
- 1. World Health Organization. Cardiovascular diseases (CVDs) fact sheet, 2019, https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed 14 March 2019).
- 2. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 3. Soyoye DO, Ikem RT, Kolawole BA, et al. Prevalence and correlates of peripheral arterial disease in Nigerians with type 2 diabetes. Adv Med 2016; 2016: 3529419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmad N, Thomas GN, Gill P, et al. Lower limb amputation in England: prevalence, regional variation and relationship with revascularisation, deprivation and risk factors. A retrospective review of hospital data. J R Soc Med 2014; 107(12): 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mueller T, Hinterreiter F, Luft C, et al. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg 2014; 59(5): 1291–1299. [DOI] [PubMed] [Google Scholar]
- 6. Sampson UK, Fowkes FG, McDermott MM, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart 2014; 9(1): 145–158.e21. [DOI] [PubMed] [Google Scholar]
- 7. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015; 116: 1509–1526. [DOI] [PubMed] [Google Scholar]
- 8. McDermott M, Guralnik J, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation 2008; 117: 2484–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340. [DOI] [PubMed] [Google Scholar]
- 10. Beckman JA, Duncan MD, Damrauer SM, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation 2019; 140: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohammedi K, Woodward M, Zoungas S, et al. Absence of peripheral pulses and risk of major vascular outcomes in patients with type 2 diabetes. Diabetes Care 2016; 39(12): 2270–2277. [DOI] [PubMed] [Google Scholar]
- 12. López-de-Andrés A, Jiménez-García R, Esteban-Vasallo MD, et al. Time trends in the incidence of long-term mortality in T2DM patients who have undergone a lower extremity amputation. Results of a descriptive and retrospective cohort study. J Clin Med 2019; 8(10): 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. IDF diabetes atlas. 9th ed., 2019, https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf
- 14. Hoffstad O, Mitra N, Walsh J, et al. Diabetes, lower-extremity amputation, and death. Diabetes Care 2015; 38: 1852–1857. [DOI] [PubMed] [Google Scholar]
- 15. Diabetes UK, www.diabetes.org.uk (accessed 20 July 2019).
- 16. Fortington LV, Geertzen JH, Van Netten JJ, et al. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg 2013; 46(1): 124–131. [DOI] [PubMed] [Google Scholar]
- 17. Schofield C, Libby G, Brennan GM, et al. Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care 2006; 29(10): 2252–2256. [DOI] [PubMed] [Google Scholar]
- 18. Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol 2016; 68(18): 2002–2015. [DOI] [PubMed] [Google Scholar]
- 19. Robbins JM, Strauss G, Aron D, et al. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc 2008; 98(6): 489–493. [DOI] [PubMed] [Google Scholar]
- 20. Reddy PH. Can diabetes be controlled by lifestyle activities? Curr Res Diabetes Obes J 2019; 1(4): 555568. [PMC free article] [PubMed] [Google Scholar]
- 21. NICE clinical guidelines (NG19), 2015, https://www.health-ni.gov.uk/publications/endorsed-nice-clinical-guidelines-20152016 (accessed 20 July 2019).
- 22. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. J Am Med Assoc 2001; 286: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 23. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease. Intern Angiol 2007; 26(2): 81–157. [PubMed] [Google Scholar]
- 24. NICE QoF indicators, 2018, http://www.nice.org.uk/standards-and-indicators/qofindicators (accessed 3 November 2019).
- 25. Formosa C, Gatt A, Chockalingam N. A critical evaluation of existing diabetic foot screening guidelines. Rev Diabet Stud 2016; 13(2–3): 158–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trevethan R. Subjecting the Ankle-Brachial Index to timely scrutiny: is it time to say goodbye to the ABI? Scand J Clin Lab Invest 2018; 78(1–2): 94–101. [DOI] [PubMed] [Google Scholar]
- 27. Nativel M, Potier L, Alexandre L, et al. Lower extremity arterial disease in patients with diabetes: a contemporary narrative review. Cardiovasc Diabetol 2018; 17: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care 2005; 28(9): 2206–2210. [DOI] [PubMed] [Google Scholar]
- 29. Soloman JA, Mondschein JI. Chapter 32: CT and MRI of the acute abdomen and pelvis. In: Torigian D, Ramchandani P. (eds) Radiology secrets plus. 3rd ed. Amsterdam: Elsevier, 2011, pp. 235–240. [Google Scholar]
- 30. Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014; 4: e004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas RL, Luzio SD, North RV, et al. Retrospective analysis of newly recorded certifications of visual impairment due to diabetic retinopathy in Wales during 2007–2015. BMJ Open 2017; 7: e015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lawrenson JG, Graham-Rowe E, Lorencatto F, et al. What works to increase attendance for diabetic retinopathy screening? an evidence synthesis and economic analysis. Health Technol Assess 2018; 22(29): 1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes Metab 2015; 17(5): 435–444. [DOI] [PubMed] [Google Scholar]
- 34. National diabetes foot care audit fourth annual report, https://files.digital.nhs.uk/F8/645631/NDFA%204AR%20-%20One-Page%20Summary%20v1.0.pdf (accessed 3 December 2019).
- 35. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. J Am Coll Cardiol 2017; 135: e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rumwell C, McPharlin M. Arterial evaluation: vascular technology. Davis, CA: University of California, Davis, 1998, pp. 60–69. [Google Scholar]
- 37. Davies JH, Richards J, Conway K, et al. Primary care pad screening: a worthwhile venture? a cross-sectional observational study. Brit J Gen Pract 2017; 67(655): e103–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lewis JEA, Williams P, Davies JH. Non-invasive assessment of peripheral arterial disease: automated ankle brachial index measurement and pulse volume analysis compared to duplex scan. SAGE Open Med 2016; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. NICE guidelines (CG147), 2018. (accessed 20 July 2019). [Google Scholar]
- 40. Vaidya A, Joore MA, Ten Cate-Hoek AJ, et al. Screen or not to screen for peripheral arterial disease: guidance from a decision model. BMC Public Health 2014; 14: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lovell M. The peripheral arterial disease coalition and peripheral arterial disease awareness campaign. J Vasc Nurs 2007; 25(4): 94–95. [DOI] [PubMed] [Google Scholar]