Abstract
High fat diets have detrimental effects on cognitive performance, and can increase oxidative stress and inflammation in the brain. The aging brain provides a vulnerable environment to which a high fat diet could cause more damage. We investigated the effects of a high fat/high cholesterol (HFHC) diet on cognitive performance, neuroinflammation markers, and phosphorylated Tau (p-Tau) pathological markers in the hippocampus of Young (4-month old) versus Aged (14-month old) male rats. Young and Aged male Fisher 344 rats were fed a HFHC diet or a normal control diet for 6 months. All animals underwent cognitive testing for 12 days in a water radial arm maze to assess spatial and working reference memory. Hippocampal tissue was analyzed by immunohistochemistry for structural changes and inflammation, and Western blot analysis. Young and Aged rats fed the HFHC diet exhibited worse performance on a spatial working memory task. They also exhibited significant reduction of NeuN and calbindin-D28k immunoreactivity as well as an increased activation of microglial cells in the hippocampal formation. Western blot analysis of the hippocampus showed higher levels of p-Tau S202/T205 and T231 in Aged HFHC rats, suggesting abnormal phosphorylation of Tau protein following the HFHC diet exposure. This work demonstrates HFHC diet-induced cognitive impairment with aging and a link between high fat diet consumption and pathological markers of Alzheimer’s disease.
Keywords: Aging, Spatial memory, Cognition, Tau phosphorylation, Hippocampus
1. INTRODUCTION
The incidence of obesity is growing and now includes at least one-third of the adult population in the United States, with another third of the population characterized as overweight [1]. One ofthe greatest factors contributing to the incidence of obesity isalteration in the diet, including both content and amount of fatintake. Obesity is considered a significant risk factor for other conditions such as cardiovascular disease, type 2 diabetes, hypertension, osteoarthritis, various forms of cancer, and depression [2,3]. Studies have shown that type 2 diabetes and obesity increase the risk of cognitive dysfunction and dementia [4–6]. Indeed, reduced cognitive performance following consumption of a high fat (HF)diet has been observed in humans [7–10] and animals [11–14]. Mechanisms likely involved in diet-induced damage to the brain include impaired glucoregulation [15], increased oxidative stress, and increased inflammation in brain tissue [16–19]. Previous work from our laboratory provided evidence for impairment of cognitive function in rats fed a high fat high cholesterol (HFHC) diet, and suggested that alterations in the blood-brain barrier (BBB) and in microglial activation may contribute to the reduced spatial and working memory observed [12,20,21]. However, in depth analysis of biological mechanisms for observed neuroinflammation and memory loss with the HFHC diets has not been undertaken so far.
Human epidemiological studies suggest that elevated intake of saturated fat in middle age may increase the risk to develop Alzheimer’s disease (AD) [22] as well as age-related milder forms of cognitive impairment [23–26]. A randomized controlled trial demonstrated that a 4-week consumption of a high saturated fat/high glycemic index meal significantly increased the levels of A42 in the cerebrospinal fluid (CSF) [27]. Animal studies utilizing transgenic mouse models of AD have shown that a diet rich in saturated fat and cholesterol can increase AD-pathology hallmarks, such as amyloid levels, Tau phosphorylation, and behavioral deficits [28–30]. Furthermore, diet-induced obesity has been shown to potentiate Tau-pathology in mice [31,32], but it is not known whether these effects are exacerbated with aging, nor is it known whether the Tau phosphorylation cascade is involved in HFHC-diet induced cognitive impairment. Therefore, the aims of this study were to investigate the consequences of long-term consumption of a HFHC diet on cognitive performance, hippocampal morphology, and expression of phosphorylated Tau in young versus aged rats.
2. Materials and methods
2.1. Animals and diets
Male Fischer 344 rats (Harlan, Indianapolis, IN) were given one week to acclimate to the vivarium, housed two to a cage and kept on a controlled 12 h light/12 h dark cycle and ad libitum access to food and water. Young and Aged rats were randomly assigned to the dietary groups: Young Control (4-month old, n = 8), Young HFHC (4-month old, n = 11), Aged Control (14-month old, n = 7), and Aged HFHC (14-month old, n = 8). The control diet consisted of standard rat chow (Harlan Teklad 8656, Indianapolis, IN) and provided 34% protein, 13% fat, and 53% carbohydrate (in kcal). The treatment diet (HFHC) consisted of (by weight): 10% hydrogenated coconut oil and 2% cholesterol (“Custom Diet D2-AIN93 without choline bitartrate and with 2% cholesterol”, MP Biomedicals, Solon, OH) and provided (in kcal) 15% protein, 36% fat and 49% carbohydrate. Rats were fed the control or HFHC diet for six months. Young rats were 10 months old and aged rats were 20 months old at the end of the study. Animal protocols were approved by the Medical University of South Carolina Institutional Care and Use Committee and carried out according to guidelines from the National Institutes of Health. Body weights (measured in grams) were evaluated every other week throughout the study in order to monitor effects of the diet. Food consumption was measured weekly per cage (two rats per cage) throughout the study.
2.2. Cognitive assessment
The 8-arm water radial arm maze (WRAM) was used in order to assess working and reference spatial memory according to previously published protocols from our laboratory [12,33,34]. Four escape platforms were placed in the maze; the four baited arms were assigned randomly and kept consistent over the 12 days of testing. Every day, four trials were administered (maximum of 3 min each). After each trial, the platform found was removed, allowing for a win-shift paradigm of this task [35,36]. Three types of errors were quantified: Working Memory Correct (WMC), Reference Memory (RM), and Working Memory Incorrect (WMI) [35]. WMC errors were first and repeat entries into an arm that previously contained a platform. RM errors were first entries into any arm that never contained a platform. WMI errors were repeat entries into an arm that never contained a platform, i.e. repeat entries into a reference memory arm [12,33,37]. Data were blocked into two phases: an initial learning phase (days 2–6), and a latter phase, or asymptotic (days 7–12). The first day of testing was considered a training day and was not included in the analyses. For WMC errors, trial 1 was not included in the analysis because it is not possible to make a WMC error on the first trial of each day.
2.3. Immunohistological evaluation of the hippocampus
Rats were anesthetized deeply with isoflurane gas (Novaplus) and the brain was rapidly removed. The right hemisphere was fixed in 4% paraformaldehyde for 48 h and transferred to 30% sucrose in phosphate buffered saline at 4 ◦C. Sections of hippocampus (40 m) were processed for immunohistochemistry. Tissue from the left hemisphere was dissected, frozen and processed for biochemical analysis as described previously [38,39]. Immunohistochemistry was performed according to our previously published protocols [12,20], using the following antibodies: NeuN (Millipore, 1:1000), Calbindin-D28k (Millipore, 1:1000), MAP2(microtubule-associated protein, Chemicon, 1:1000), and OX-6(major histocompatibility complex, MHC, Class II marker, Serotec, 1:1000). Free-floating sections were incubated with primary antibodies for 48 h at 4 ◦C, washed, and then incubated with secondary antibodies directed against the appropriate species for 1 h at room temperature. Sections were washed and placed in the Elite ABC reagent (Vector, Burlingame, CA), washed and incubated with diaminobenzidine (DAB; 0.02%) with nickel ammonium sulfate (NAS) (Fisher Scientific, Pittsburgh, PA; 25 mM). Sections were washed, mounted on subbed slides, air dried overnight, dehydrated, coverslipped with Permount (Fisher Scientific, Pittsburgh, PA, USA) and examined with a light microscope (Nikon Optiphot). Sections from all groups were incubated in the same bath to avoid group inter-variability in staining.
2.4. Densitometry
Semi-quantification of hippocampal NeuN and calbindin D-28k immunostaining density was performed using a Nikon Optiphot light microscope and Nikon Elements Image Software (NIS Elements, Melville, NY). Analysis was performed blinded of at least 3 sections per animal with a periodicity of every 12th section. Staining density, measured with a gray scale ranging from 0 to 256, was obtained when background staining was subtracted from mean staining intensities for each section. Mean intensity values were averaged per animal and then analyzed for statistical significance using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA) as described previously [12,40,41].
Semi-quantification of the number of activated microglia was also performed with the NIS Elements software (Nikon). Cells outside of the gray matter of the hippocampus were not counted (i.e. fimbria/fornix region). Because stereological cell counting requires a sample size of at least 100 profiles per brain [42], we opted to hand-count the cells due to the low number of OX-6immunoreactive cells per brain (<50 microglial cells observed per section). Since the counting method utilized herein did not account for shrinkage of brain tissue due to fixation, the cell counts for OX-6 immunoreactive cells are expressed as percent of the Young control group.
2.5. Western blot for phosphorylated Tau
Frozen hippocampal tissues were homogenized as described previously [43]. Total amounts of 40 g protein per sample were separated on 10% sodium dodecylsulfate-polyacrylamide gels, and transferred to nitrocellulose membranes (BioRad Laboratories). After blocking, membranes were incubated overnight at 4 ◦C with primary antibodies to phosphorylated Tau (p-Tau) S202/T205 (clone AT-8; 1:500, Fisher Scientific) and p-Tau pT231 (clone AT180; 1:1000, Innogenetics). After a washing step, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare) for 2 h at room temperature. Blots were developed with electrochemiluminescence (ECL Prime, GE Healthcare) and visualized using a CCD camera (LAS-3000, Fuji Film). Density of bands was analyzed with Multi Gauge (version 3.0) software (Fuji Film). All samples were run in one blot for each marker. -actin was used as a loading standard, and protein levels were normalized to -actin levels in the respective blots.
2.6. Statistical analysis
Changes in body weight, food consumption, densitometry measurements, and Western blot results were analyzed with two-way (Age × Diet) ANOVA followed by Tukey’s post-hoc test. Significance was set at p < 0.05. For all behavioral error analyses, two-way ANOVAs were employed with a primary focus on diet and age effects during Trial 4, which is the most demanding trial in a session. Post-hoc analyses used the Tukey’s multiple comparisons test. Pearson correlation analyses were performed to determine if there were relationships between hippocampal staining density, p-Tau expression and memory errors. GraphPad Prism version 6 (GraphPad Software, La Jolla, CA) was used for all statistical analyses.
3. Results
3.1. Food consumption and change in body weight
Analysis of food consumption per cage (Fig. 1A) showed a significant interaction between age and diet (F1,13 = 5.27, p = 0.039) and a significant effect of age (F1,13 = 9.37, p = 0.009). Tukey’s post-hoc analysis showed that Aged Control rats had lower food consumption, compared to Young Control rats (p = 0.008). The weight gain was significantly affected by the age of the rats (F1,30 = 53.96, p < 0.0001) and by the diet (F1,30 = 8.31, p = 0.007), with Young rats being the most affected by the diet (Fig. 1B). Post-hoc analysis revealed that Young HFHC gained a significantly greater amount of weight compared to Young Control rats (p = 0.030), whereas no significant difference was observed for Aged HFHC rats compared to Aged Control rats. As expected, post-hoc analyses revealed that the young rats gained a significantly greater amount of weight than the aged animals (Young Control > Aged Control; p = 0.0006 and Young HFHC > Aged HFHC; p < 0.0001). In fact, the Aged Control rats gained only a few grams after the 6 months dietary treatment, which is consistent with their lower food consumption throughout the study.
Fig. 1.
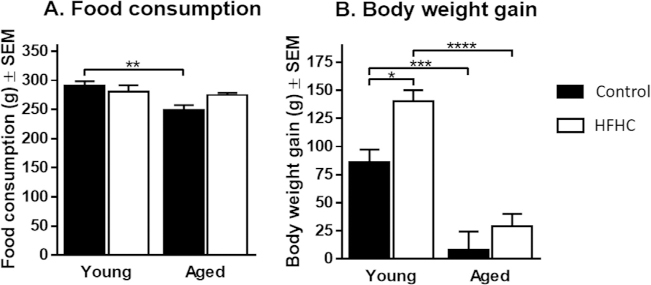
Food consumption and changes in body weight upon high fat/high cholesterol (HFHC) diet. (A) Food consumption per week with 2 animals per cage (mean ± SEM)for the Young Control, Young HFHC, Aged Control and Aged HFHC groups. Significant differences were tested with a two-way ANOVA followed by Tukey’s post-hoc test.Difference was statistically significant between Young Control and Aged Control groups with p < 0.01 (**). (B) Change in body weight at the end of the study (mean ± SEM)for the Young Control, Young HFHC, Aged Control and Aged HFHC groups. Significant differences were tested with a two-way ANOVA followed by Tukey’s post-hoc test.Differences were statistically significant when p < 0.0001 (****), p < 0.001 (***), and p < 0.05 (*).
3.2. WRAM behavior
The WRAM test was administered during the last two weeks of dietary treatment. A two-way ANOVA did not show any significant effects of diet on total acquisition time over the 12 days of testing (Fig. 2A), thus suggesting that the body weight gain of HFHC rats did not affect their ability to swim.
Fig. 2.
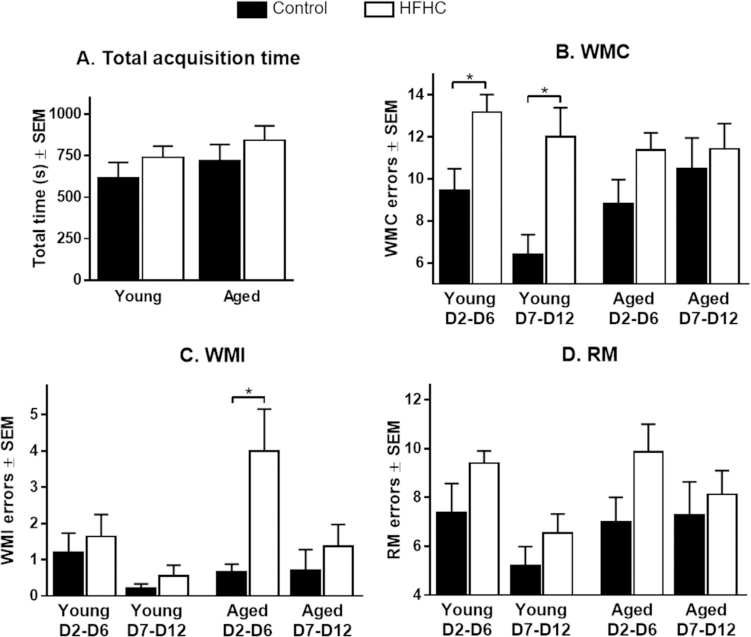
Water Radial Arm Maze (WRAM) performance. (A) Total acquisition time over the 12 days of testing (mean ± SEM), (B) Working Memory Correct (WMC) errors, (C)Working Memory Incorrect (WMI) errors, and (D) Reference Memory (RM) errors committed in Trial 4 by the four groups during the learning phase (D2-D6) and the asymptoticphase (D7-D12). Data are represented as mean errors ± SEM. Significant differences were tested with two-way ANOVA followed by Tukey’s post-hoc test. Differences werestatistically different when p < 0.05(*).
Testing days were divided into two blocks: the learning phase (days 2–6) and the asymptotic phase (days 7–12). Evaluation ofWMC errors committed in Trial 4, which represents the highest working memory load, throughout the 12 days of testing revealed a significant main effect of the HFHC diet during both the learning phase (F1,30 = 10.30, p = 0.003) and the asymptotic phase (F1,30 = 6.36, p = 0.017), with Young HFHC rats exhibiting a significantly greater number of WMC errors compared to Young Control rats in both phases (p = 0.027 and p = 0.009, respectively) (Fig. 2B). During the asymptotic phase, Young Control rats committed less WMC errors than all three other groups. Both Young Control and Young HFHC rats exhibited a learning curve (although less pronounced for the Young HFHC), committing fewer errors during the asymptotic phase compared to the learning phase (behavioral data are summarized in Table 1 and Fig. 2). On the contrary, Aged Control and Aged HFHC rats did not exhibit a learning curve and even made slightly more errors during the asymptotic phase (Table 1).
Table 1.
Mean number of the sum of errors ± SEM made by each group for the learning and asymptotic phases during Trial 4. Slopes of the linear regression are also presented. WMC: Working Memory Correct, WMI: Working Memory Incorrect and RM: Reference Memory.
| Measure n= |
Young Control 8 |
Young HFHC 11 |
Aged Control 7 |
Aged HFHC 8 |
|
|---|---|---|---|---|---|
| WMC | Learning | 9.4 ± 1.0 | 13.2 ± 0.8 | 8.8± 1.1 | 11.4 ± 0.8 |
| Asymptotic | 6.4 ± 0.9 | 12.0 ± 1.4 | 10.5 ± 1.5 | 11.4 ± 1.2 | |
| Slope | −0.50 ± 0.23 | −0.19 ± 0.27 | 0.28 ± 0.31 | 0.01 ± 0.23 | |
| WMI | Learning | 1.2 ± 0.5 | 1.6 ± 0.6 | 0.7 ± 0.2 | 4.0 ± 1.2 |
| Asymptotic | 0.2 ± 0.1 | 0.6 ± 0.3 | 0.7 ± 0.6 | 1.4 ± 0.6 | |
| Slope | −0.17 ± 0.09 | −0.18 ± 0.12 | 0.01 ± 0.11 | −0.36 ± 0.17 | |
| RM | Learning | 7.4 ± 1.2 | 9.4 ± 0.5 | 7.0 ± 1.0 | 9.9 ± 1.1 |
| Asymptotic | 5.2 ± 0.8 | 6.6 ± 0.8 | 7.3 ± 1.4 | 8.1 ± 1.0 | |
| Slope | −0.36 ± 0.23 | −0.47 ± 0.14 | 0.05 ± 0.28 | −0.29 ± 0.25 |
A significant overall effect of diet (F1,30 = 6.90, p = 0.014) was observed for WMI errors performed in Trial 4 (Fig. 2C). Tukey’s post-hoc test revealed that Aged HFHC rats performed significantly more WMI errors compared to Aged Control rats during the learning phase (p = 0.029). Even though Aged HFHC rats exhibited a learning curve, they performed worse than any other group in terms of WMI errors (Table 1). Young Control and HFHC rats committed very few WMI errors with no significant effects of the diet. Both previous work from our group and work by others have shown behavioral deficits from HFHC diet. However, a number of factors can affect the results in the current study (including age, rat strain, time of exposure, gender, diet composition) and because we utilized a slightly different diet composition and a different age when animals were exposed to the diet, it is not surprising that we did not see major diet effect in the WMI of Young rats.
A diet effect was observed on RM errors performed during Trial 4 (F1,30 = 6.57, p = 0.016, Fig. 2D). Tukey’s multiple comparisons test did not reveal any significant (p < 0.05) difference between the groups. However, we observed a trend for significance (p = 0.076) between Aged Control and Aged HFHC rats during the learning phase. Since the two-way ANOVA did not show any effect of age on the RM errors, we decided to collapse Young and Aged rats in each diet group to see if the diet would have an effect. A two-way ANOVA showed a significant effect of the diet (F1,62 = 8.99, p = 0.004), suggesting that the diet affected the learning ability of the rats. A significant difference was found between Control rats and HFHC rats during the learning phase of the task (p = 0.028). This further supports our findings about a possible diet effect. Young Control and Young HFHC rats exhibited a learning curve and made less than 7 errors during the asymptotic phase (Table 1), while neither Aged Control nor Aged HFHC rats reduced the number of RM errors and committed more than 7 errors on average during the asymptotic phase (Table 1), suggesting that both HFHC diet and aging affected the capability of learning this complex task.
3.3. Effects of HFHC diet and age on neuronal morphology
NeuN immunohistochemistry was performed in order to determine overall neuronal morphological changes. Densely packed pyramidal neurons in the CA1 and CA3 regions and granule cells in the dentate gyrus were observed in all groups (Fig. 3A–L). However, Young Control rats revealed the most robust immunoreactivity in all regions, whereas the aged HFHC diet rats exhibited a significantly reduced NeuN immunoreactivity in the CA1, CA3, and dentate gyrus compared to all other groups (Fig. 3). Analysis of NeuN in Aged HFHC rats suggested a weaker staining within neu-rons both in the pyramidal cell layers (Fig. 3D and H), and in the granule cell layer of the dentate gyrus (Fig. 3L). A two-way ANOVA for NeuN densitometry results (Fig. 3M–O) confirmed a significant interaction of age and diet for CA1 (F1,30 = 7.99, p = 0.008) as well as an effect of diet for CA1 (F1,30 = 5.89, p = 0.022), CA3 (F1,30 = 11.84, p = 0.002), and dentate gyrus (F1,31 = 8.30, p = 0.007). In the CA1, Aged HFHC rats exhibited a lower relative density compared to Young HFHC (p = 0.018). Moreover, Aged HFHC rats had a significantly lower relative density compared to Aged Control rats in the CA1 (p = 0.007), CA3 (p = 0.011) and dentate gyrus (p = 0.016) (Fig. 3N and O).
Fig. 3.
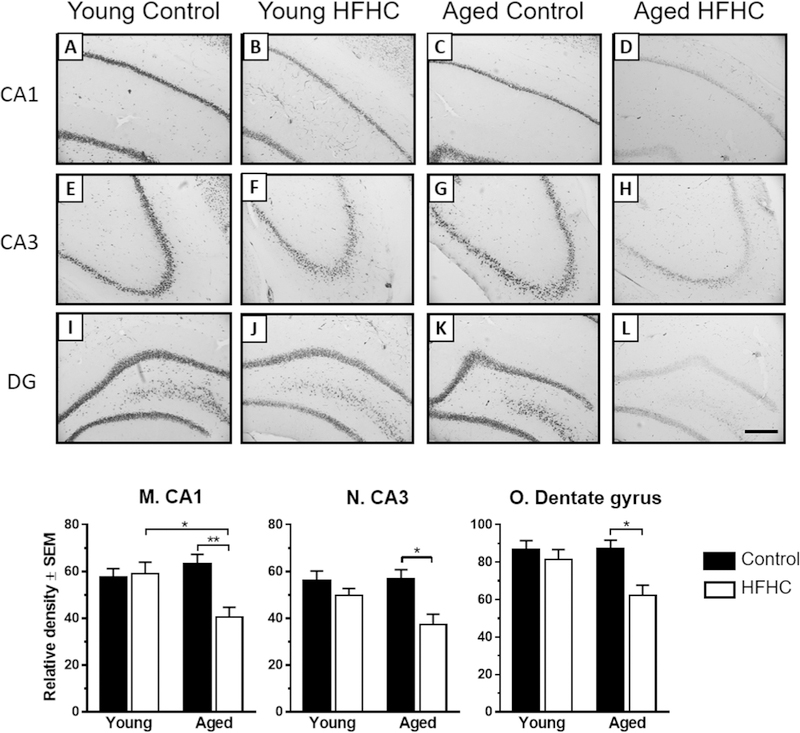
Neuronal nuclei (NeuN) immunoreactivity. (A–L) Representative photomicrographs of NeuN-immunostained sections showing the CA1, CA3 and dentate gyrus (DG)regions of the hippocampus area obtained from Young Control, Young HFHC, Aged Control and Aged HFHC rats. Scale bar in (L) represents 100 _m (for A–L). NeuN densitometry(M-O): Mean density measures of immunostained sections for NeuN in CA1, CA3 and dentate gyrus. Two-way ANOVA followed by Tukey’s post-hoc test revealed significantdifferences between groups at the level of p < 0.01 (**) and p < 0.05 (*).
3.4. Alterations in calbindin-D28k and MAP2 immunoreactivity
Calbindin-D28k is one of the major calcium-binding proteins in the brain, and plays a role in neuronal survival as well as synaptic plasticity [44,45]. Calbindin-D28k undergoes reduced expression in the rat hippocampus with aging [46,47]. Therefore, we investigated the influence of HFHC diet on expression of calbindin-D28k in hippocampus of young and aged rats. Hippocampal expression of calbindin-D28k was mainly found in the CA1 stratum pyramidale, CA3 stratum lucidum, and the dentate gyrus stratum granulosum and stratum moleculare (Fig. 4A–H). Aged HFHC rats had a reduced calbindin-D28k immunoreactivity overall in this brain region compared to all other groups. The young rats (Control and HFHC) as well as the Aged Control group exhibited abundant calbindin-D28k immunoreactivity throughout the pyramidal cell layer (Fig. 4A–C, E–G), while a partial and regional loss of calbindin-D28k immunoreactivity was observed in the Aged HFHC group (Fig. 4D and H). Even though clear morphological distribution differences were observed in discrete hippocampal regions and neuronal populations between the groups, an overall densitometry measurement did not reveal significant density effects. Thus, a twoway ANOVA for calbindin-D28k densitometry in the CA1 showed a significant effect of age (F1,20 = 4.60, p = 0.044) but no effect of the diet (p > 0.05). Even though the densitometry measurements (Fig. 4I) seem to point out a diet effect, the high variability observed in the Young HFHC group is likely preventing any significant difference between the groups.
Fig. 4.
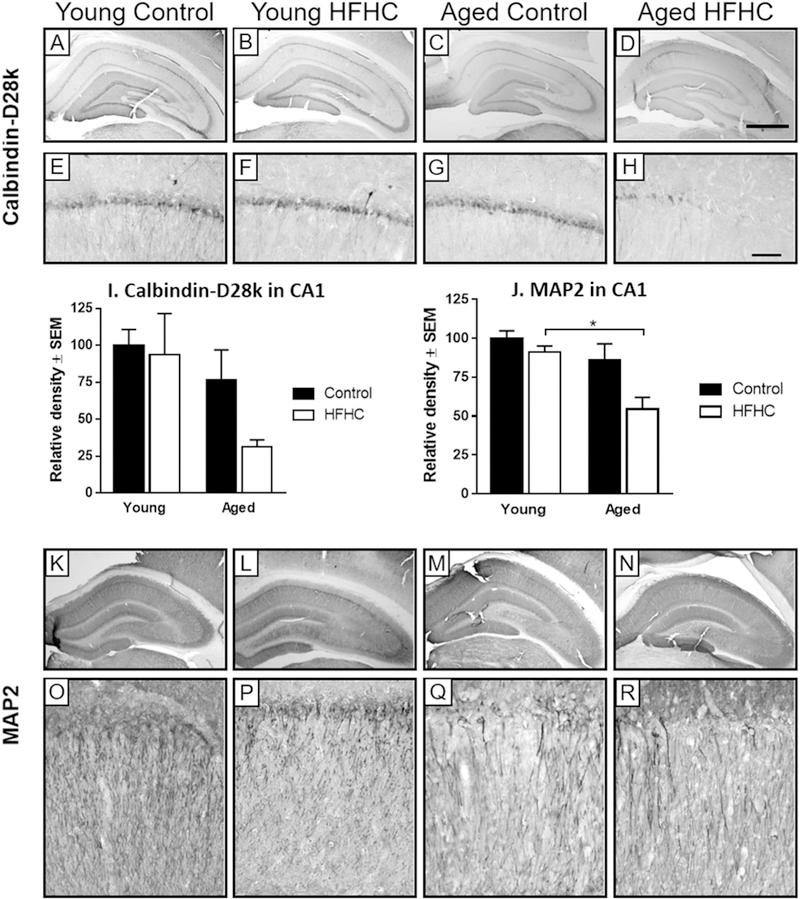
Calbindin-D28k and MAP2 immunoreactivity. Representative photomicrographs of calbindin-D28k (A–H) and MAP2 (K–R) -immunostained sections with differentmagnifications of the hippocampus area, obtained from Young Control, Young HFHC, Aged Control and Aged HFHC rats. The graph (I) represents the relative density forcalbindin-D28k in the CA1 area (mean ± SEM) for each group. A two-way ANOVA for calbindin-D28k densitometry in the CA1 showed a significant effect of age (F1,20= 4.60,p = 0.044) but no effect of the diet (p > 0.05). The graph (J) represents the relative density (mean ± SEM) for MAP2 in the CA1 area for each group. A two-way ANOVA for MAP2in the CA1 revealed a significant effect of age (F1,29= 10.25, p = 0.003) and diet (F1,29= 6.61, p = 0.016) but no interaction between age and diet. The magnified images (E–H andO–R) show the CA1 region. Scale bar in (D) represents 1000 _m (for A–D, K–N), in (H) it represents 100 _m (for E–H, O–R).
MAP2 is related to changes in structural stability of dendrites, and has been shown to be decreased in some models of neurodegeneration [48,49]. MAP2 immunoreactivity was expressed in the cell bodies and dendrites of CA1 and CA3 pyramidal neurons (Fig. 4K–R). The Young Control group revealed the most robust MAP2 immunoreactivity, both in cell bodies and dendrites. The Young HFHC rats had slightly reduced MAP2 immunoreactivity in the dendrites in comparison with the Young Control group. In comparison to that reduction, both Aged Control and Aged HFHC rats displayed a more extensive reduction of MAP2 immunoreactivity in cell bodies and dendrites, especially in the CA1 region. Both Aged groups displayed focal areas of lost immunoreactivity (Fig. 4Q and R). Densitometry measurements of MAP2 in the CA1 revealed an overall effect of age (F1,29 = 10.25, p = 0.003) and diet (F1,29 = 6.61, p = 0.016). Aged HFHC rats exhibited a significantly lower MAP2 immunoreactivity than the Young HFHC (p = 0.042) and the Young Control group (p = 0.006, Fig. 4J), confirming morphological observations demonstrated in Fig. 4.
3.5. Neuroinflammatory markers
In order to examine neuroinflammation as a possible mechanism for the observed reduction in NeuN, calbindin-D28k and MAP2 immunoreactivity in the CA1, we utilized antibodies directed against the MHC Class II marker OX-6 (Fig. 5), which labels activated microglial cells [50,51]. The majority of activated microglia were found in the CA3 region of the hippocampus (Fig. 5). The CA3 region is shown in the larger micrographs in order to visualize distribution of OX-6 positive cells. Both Young Control and Young HFHC rats displayed a few scattered OX-6 positive cells in the CA3 region of the hippocampus (Fig. 5A–D). On average, only a few OX-6 immunoreactive cells were observed per section in the two young groups. The Aged Control and the Aged HFHC groups displayed 2.5 and 4 times more OX-6 positive cells per section, respectively (Fig. 5E–H). A two-way ANOVA revealed an overall significant effect of diet on activated microglial cell numbers (F1,30 = 21.88, p < 0.0001) but no effect of age (Fig. 5I). Aged HFHC rats exhibited a significantly higher percent of OX-6 positive cells compared to Young HFHC rats (p = 0.0002). While the OX-6 antibody was used as a marker for activated microglia, differences in the morphological signs of the level of activation were observed. Both the Young HFHC and Aged HFHC rats revealed microglial cells with larger cell bodies, more processes, and increased staining in both cell bodies and processes (Fig. 5E–H), compared to the Young Control rats. These morphological features indicate greater activation and possibly a greater degree of inflammation [52].
Fig. 5.
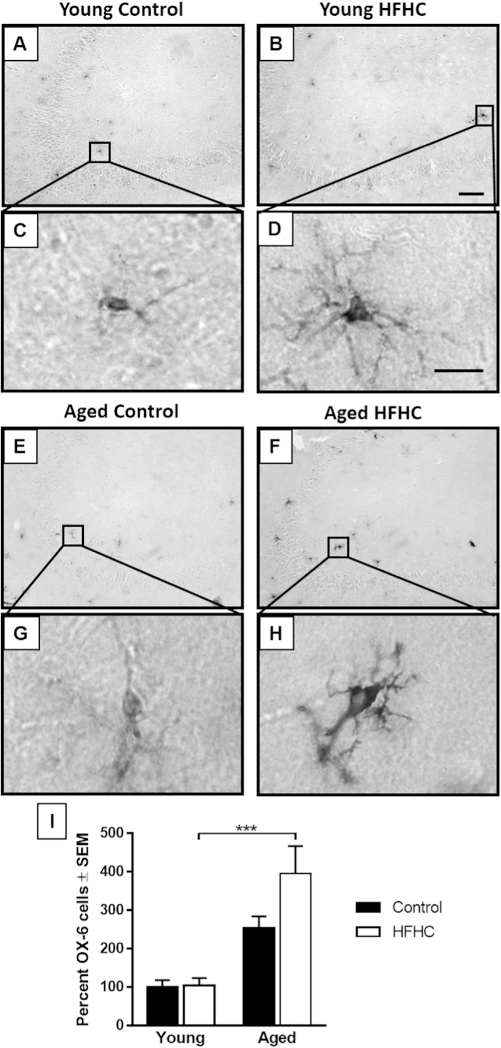
OX-6 immunoreactivity. (A–H) Representative photomicrographs of OX-6immunostained sections showing the CA3 region of the hippocampus area, obtainedfrom Young Control (A and C), Young HFHC (B and D), Aged Control (E and G) andAged HFHC (F and H) rats. Scale bar in (B) represents 100 _m (for A, B, E, F) and in(D) 10 _m for (C, D, G, H). (I) The graph represents the average percent of positiveOX-6 cells (mean ± SEM) for each group. A two-way ANOVA revealed a significanteffect of age, with Tukey’s post-hoc comparisons showing significant differenceswith p < 0.001 (***).
3.6. Expression of p-Tau in hippocampus
The levels of p-Tau at S202/T205 and T231 have previously been used as a reliable marker for the severity of AD, including early and late stages of neurofibrillary tangle development [53]. We investigated effects of age and diet on protein levels of p-Tau in hippocampus extracts by Western blot, using antibodies specific for the S202/T205 and T231 phosphorylation loci, respectively. In aged rats, the HFHC diet increased expression of both p-Tau S202/T205 and T231 (Fig. 6A and B). Both p-Tau S202/T205 and T231 expression levels were affected by the diet (F1,30 = 8.19, p = 0.008 and F1,30 = 13.13, p = 0.001, respectively) and by the age of the rats (F1,30 = 14.52, p = 0.0006 and F1,30 = 4.235, p = 0.048, respectively). Post-hoc tests revealed that Aged HFHC rats had a significantly higher expression of p-Tau S202/T205 when compared to Aged Control (p = 0.027) and to Young HFHC rats (p = 0.004, Fig. 6A). Aged HFHC rats exhibited a significantly higher expression of p-Tau T231 compared to Aged Control (p = 0.007) and to Young HFHC rats (p = 0.034, Fig. 6B). Interestingly, significant positive correlations were found between the expression of both p-Tau S202/T205 and T231 levels with the WMC errors during the asymptotic phase for Young HFHC rats (r = 0.83 with p = 0.005 and r = 0.74 with p = 0.023, respectively), supporting the notion that an increased expression of p-Tau is related to poor memory performance in HFHC animals. Since no significant difference was observed between Young Control and Young HFHC rats for either of the p-Tau levels, the analysis was focused on a possible diet effect by collapsing the Young and Aged groups of rats. A Student’s t-test reveals that the HFHC diet has a significant effect on both p-Tau S202/T205 and T231 levels (p = 0.028 and p = 0.003, respectively), supporting the idea that an increased expression of p-Tau in HFHC rats could be related to poor memory performance. Although this relationship was not found for the Aged groups, this may suggest that the overall increase in pTau levels in the Aged HFHC diet group may mask any correlation between WMC errors and p-Tau in this group. P-Tau S202/T205 levels and the high number of RM errors during the asymptotic phase showed a positive correlation (r = 0.68, p = 0.044), further supporting the idea that p-Tau is linked to memory impairment.
Fig. 6.
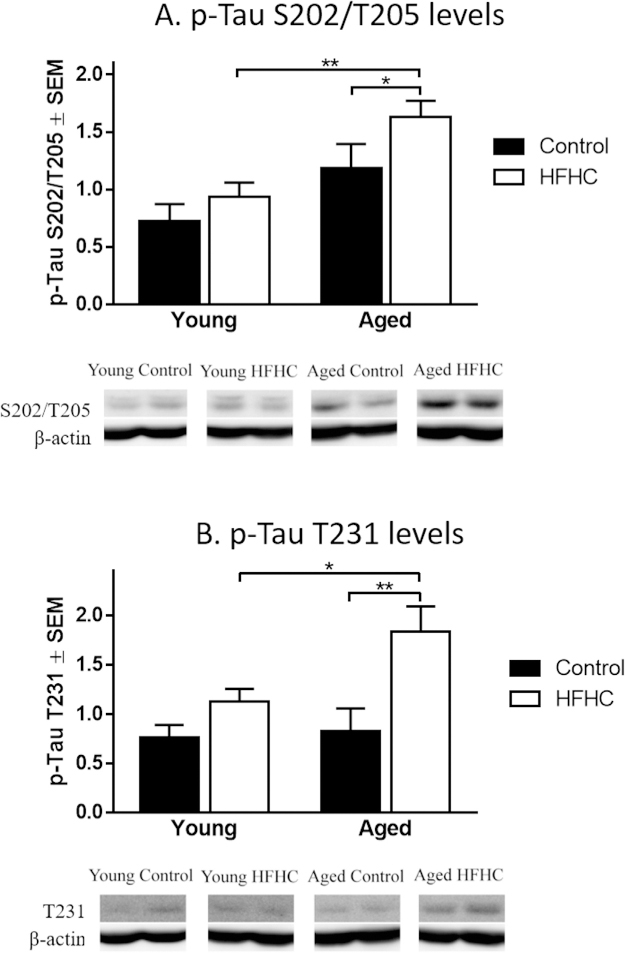
Changes in p-Tau expression levels by Western blot analysis. Mean relative changes (±SEM) in p-Tau at the phosphorylation sites S202/T205 and T231 (using AT-8 andAT-180 antibodies, respectively) obtained by Western blot analysis of the hippocampus area from Young Control, Young HFHC, Aged Control and Aged HFHC rats. Significantdifferences were tested with a two-way ANOVA followed by Tukey’s post-hoc test. Differences were statistically significant when p < 0.01 (**) and p < 0.05 (*).
4. Discussion
In the current study, we examined long-term effects of a HFHC diet in young and aged Fischer 344 rats to determine whether the effects of this diet were exacerbated in aged subjects. The findings herein demonstrated a significantly reduced performance in the WRAM with aging, as shown by us and others previously [54–56]. In addition, aged rats treated with the HFHC diet exhibited pronounced memory impairment and altered hippocampal morphology, including significant reduction in NeuN and calbindin-D28k immunoreactivities, increased activation of microglial cells, and increased expression of p-Tau, beyond changes observed in aged rats on a control diet, providing a possible biological link for HFHC-related effects on memory.
The WRAM test employed herein is a complex task that not only assesses hippocampus-dependent reference memory changes but also spatial working memory and working memory load [see e.g. [54]]. Both the Young Control and Young HFHC rats learned the task, exhibiting a learning curve and a decreasing number of errors during the asymptotic phase of testing (days 7–12). On the other hand, neither the Aged Control nor the Aged HFHC group exhibited a significant learning curve and performed on average more errors in the asymptotic phase during Trial 4 than both groups of Young rats. Thus, an effect of age but not diet was observed on reference memory, with the Aged HFHC group having the highest average errors in this assessment. Normal aging has been shown to affect spatial memory abilities in rats [56,57]. Other studies have shown significant effects of HF diet on spatial reference memory in young rats [58], but it is possible that the lack of significant effect of diet on this aspect of the task in the Aged group is due to the fact that Aged Control rats already had a lack of learning of this measure in the task, displaying a ceiling effect. Nonetheless, it was clear that the Aged rats on HFHC diet constituted the group exhibiting the worst performance on both the WMI and RM aspects of this task, confirming data from clinical studies, that HFHC diets predispose individuals to cognitive impairment with aging.
Indeed, there were diet-related effects on WMI and WMC errors. During the learning phase, the Aged HFHC-treated rats performed a significantly larger number of WMI errors compared to Aged Control rats, especially in Trial 4, i.e. when the working memory load is the largest. Overall, Young HFHC-treated rats committed a larger number of WMC errors compared to the control rats, thereby revealing a diet effect. These data are in line with previous investigations in adult or juvenile rodents, showing a significant reduction in hippocampus-dependent behaviors following prolonged treatment with a HFHC diet [12,58,59], as well as in frontal cortex-related working memory [60]. Our findings demonstrate exacerbated cognitive impairment in aged rodents with this type of diet, and indicate that the aging process gives rise to a higher vulnerability to diet-induced alterations in hippocampal function. A parallel vulnerability to HFHC diet has been suggested in rats that have sustained injury, e.g. upon traumatic brain injury [61], and in rats subjected to intermittent hypoxia [62]. Epidemiological studies in humans further support this notion, since lifetime dietary patterns can predict cognitive performance in older adults [63], and may even predict incidence of AD [64].
In order to investigate neuronal and glial correlates for the age-and diet-related changes found in the WRAM testing, hippocampal morphology was analyzed. Significant reduction in NeuN immunoreactivity was found in the Aged HFHC treated group, compared to aged and young control rats. Alterations included a thinner pyramidal cell layer, as well as reduced cellular immunoreactivity, with the greatest NeuN loss of immunoreactivity observed in the Aged HFHC rats. No age-related difference was observed, as expected from previous studies [65]. However, an interaction of age and diet was found for NeuN immunoreactivity in the CA1, making the significant differences observed between groups difficult to interpret. The significant loss of NeuN immunoreactivity observed in the CA1 of Aged HFHC rats could reflect a depletion of NeuN protein or loss of antigenicity [66].
One of the hypotheses that have been proposed in order to understand cognitive aging and alterations to the hippocampus is the “calcium hypothesis” in which calcium homeostasis is shifted, causing altered action potentials and synaptic plasticity [67–69], particularly in the hippocampal CA1 region [70]. In order to evaluate these effects, we analyzed the levels of calbindin-D28k, a major calcium-binding protein in the brain. The Aged HFHC rats revealed a reduction in calbindin-D28k immunoreactivity compared to all other groups, with focal areas showing a complete loss of calbindin-D28k immunoreactivity. This phenomenon was observed to a greater extent in aged vs. young rats. Although no previous studies have been conducted related to calcium-regulating proteins and hippocampal function with HFHC diet in aging, a previous study [71] demonstrated that maternal intake of HFHC diet during pregnancy in mice gave rise to reduced neuronal progenitors in the dentate gyrus and a reduced number of calretinin-expressing neurons in the hippocampus of the fetuses, providing an interesting parallel with the findings obtained here.
We observed a diet-and age-dependent reduction in MAP2 immunoreactivity in the CA1 region of the hippocampus, similar to findings with the HFHC diet in middle-aged rats previously published by our group [12,20]. MAP2 is a microtubule-associated protein shown to be decreased in some models of neurodegeneration and AD [48,49]. In a previous study, a reduction of hippocampal MAP2 density staining was observed in rats fed for only 7 days with a HF diet (containing 10% lard, and 20% high fructose corn syrup added to the water), demonstrating the damaging short-term effect of saturated fat and simple sugars on hippocampal morphology [72]. Our results demonstrate a decrease in dendritic integrity in Aged HFHC rats compared to Young HFHC and Control rats, suggesting that a combination of HFHC diet and aging can have detrimental effects on the dendritic integrity. Mechanisms responsible for a reduction in MAP2 from an HFHC diet may include alterations in the c-Jun NH2-terminal protein kinase (JNK) subgroup of MAP kinases, since previous studies have shown that MAP2 polypeptides are hypophosphorylated in Jnk1(−/−) brains, resulting in compromised ability to bind microtubules and promote their assembly [73]. These results suggest that JNK1 is required for maintaining the cytoskeletal integrity of neuronal cells and is a critical regulator of MAP activity and microtubule assembly. The JNK stress signaling pathway was implicated in the metabolic response to the consumption of a HF diet [74], including the development of obesity and insulin resistance, and would represent a future target for drug interventions in obesity associated with aging.
Neuroinflammation is involved in age-related cognitive impairment [75], and has been implicated in HFHC-induced cognitive impairment [12]. Activated microglia was observed in the hippocampus of both Young HFHC and Aged HFHC groups, as well as in the Aged Control group. These features suggest greater activation and possibly a greater degree of inflammation [76]. Taken together, the Young HFHC and Aged HFHC rats both revealed a greater level of activation compared to their control counterparts, however, the Aged HFHC rats revealed the highest number and highest level of activation compared to all other groups. In previous work, we have demonstrated that long-term administration of HFHC diets in rats give rise to a significant elevation of microglial activation in the hippocampus [12,20]. A study from our group even suggested a central role for pro-inflammatory cytokines in HFHC diet-induced effects on the hippocampus, demonstrating that the IL-1 receptor inhibitor Kineret blocked microglial activation induced by HFHC diets in hippocampal transplants [77]. Findings from others also imply that neuroinflammation plays a central role for obesity and HFHC dietinduced cognitive impairment [78] and therefore presents another strong candidate for intervention.
Lastly, we observed an effect of diet on hippocampal levels of pTau using antibodies specific to phosphorylation at Ser202/Thr205 and Thr231. The levels of p-Tau at these sites have previously been used as a marker for AD severity [53]. Our findings are in agreement with recent studies showing that diet-induced obesity (DIO) increased Tau phosphorylation and decreased learning abilities in the THY-Tau22 transgenic mouse model of AD [31], and in wild-type mice [32]. A recent study in Zucker rats also found that aging significantly increased phosphorylation of Tau at Ser396, and both aging and obesity increased phosphorylation of Tau at Thr231 [79]. Takalo et al. demonstrated that Tau induction following HFHC diet exposure in mice occurred independently of the peripheral metabolic status [80], providing an interesting target for future in depth molecular studies of this pathway.
5. Conclusion
Long-term exposure to a HFHC diet caused cognitive impairment that was exacerbated by aging. The reduced performance in the WRAM was coupled with markers for decreased neuronal viability and dendritic integrity while activation of microglial cells in the same brain region was increased. Aged rats with HFHC diet also had increased levels of p-Tau, providing a potential biological link between aging and HFHC diet-induced memory impairment.
HIGHLIGHTS.
Young and Aged rats were exposed for 6 months to a high fat high cholesterol diet (HFHC).
Cognitive performance, neuroinflammation markers and phosphorylated Tau were examined.
Young and Aged rats on HFHC diet exhibited worse performance on spatial memory task.
Aged HFHC rats showed higher levels of p-Tau compared to Aged control and Young HFHC rats.
This work demonstrates HFHC diet-induced cognitive impairment with aging.
Acknowledgements
This work was made possible by a grant from National Institute on Aging (AG044920), and the Swedish Alzheimer Foundation. XW was recipient of a scholarship from Chinese Scholarship Council, PR China. LRF was supported by Grant Number K12HD055885 from the National Institute of Child Health and Human Development (NICHD) and the Office of Research on Women’s Health (ORWH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. The authors would like to thank Claudia Umphlet and Laura Columbo for excellent technical support.
Footnotes
Disclosure statement
The authors have no conflict of interest to disclose.
References
- [1].Flegal KM, Carroll MD, Kit BK, Ogden CL, Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010, JAMA 307 (2012) 491–497. [DOI] [PubMed] [Google Scholar]
- [2].Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH, The disease burden associated with overweight and obesity, JAMA 282 (1999) 1523–1529. [DOI] [PubMed] [Google Scholar]
- [3].Guh DP, Zhang W, Bansback N, Amasi Z, Birmingham CL, Anis AH, The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis, BMC Public Health 9 (2009) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kivipelto M, Ngandu T, Fratiglioni L, Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease, Arch. Neurol 62 (2005) 1556–1560. [DOI] [PubMed] [Google Scholar]
- [5].Umegaki H, Neurodegeneration in diabetes mellitus, Adv. Exp. Med. Biol 724 (2012) 258–265. [DOI] [PubMed] [Google Scholar]
- [6].Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K, Central obesity and increased risk of dementia more than three decades later, Neurology 71 (2008) 1057–1064. [DOI] [PubMed] [Google Scholar]
- [7].Craft S, The role of metabolic disorders in Alzheimer disease and vascular dementia, Arch. Neurol 66 (2009) 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Craft S, Watson GS, Insulin and neurodegenerative disease: shared and specified mechanisms, Lancet Neurol 3 (2004) 169–178. [DOI] [PubMed] [Google Scholar]
- [9].Parrott MD, Greenwood CE, Dietary influences on cognitive function with aging: from high-fat diets to healthful eating, Ann. N. Y. Acad. Sci 1114 (2007) 389–397. [DOI] [PubMed] [Google Scholar]
- [10].Morris MC, Evans DA, Bienas JL, Tangney CC, Wilson RS, Dietary fat intake and 6-year cognitive change in an older biracial community population, Neurology 62 (2004) 1573–1579. [DOI] [PubMed] [Google Scholar]
- [11].Baran S, Campbell A, Kleen J, Foltz C, Wright R, Diamond D, Conrad CD, Combination of high fat diet and chronic stress retracts hippocampal dendrites, Neuroreport 16 (2005) 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Granholm AC, Bimonte-Nelson HA, Moore A, Nelson M, Freeman LR, Sambamurti K, Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat, J. Alzheimers Dis 14 (2008) 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Winocur G, Greenwood CE, The effects of high fat diets and environmental influences on cognitive performance in rats, Behav. Brain Res 101 (1999) 153–161. [DOI] [PubMed] [Google Scholar]
- [14].Winocur G, Greenwood CE, Studies of the effects of high fat diets on cognitive function in a rat model, Neurobiol. Aging 26 (Suppl. 1) (2005) 46–49. [DOI] [PubMed] [Google Scholar]
- [15].Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C, Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice, Behav. Brain Res 175 (2006) 374–382. [DOI] [PubMed] [Google Scholar]
- [16].Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC, Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms, Nutr. Neurosci 17 (2014) 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN, High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling, J.Neurochem 114 (2010) 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ, Cognitive impairment following high fat diet consumption is associated with brain inflammation, J. Neuroimmunol 219 (2010) 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang X, Dong F, Ren J, Driscoll M, Culver B, High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex, Exp. Neurol 191 (2005) 318–325. [DOI] [PubMed] [Google Scholar]
- [20].Freeman LR, Haley-Zitlin V, Stevens C, Granholm AC, Diet-induced effects on neuronal and glial elements in the middle-aged rat hippocampus, Nutr. Neurosci 14 (2011) 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Freeman LR, Granholm AC, Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet, J. Cereb. Blood Flow Metab 32 (2012) 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morris MC, Tangney CC, Dietary fat composition and dementia risk, Neurobiol. Aging 35 (Suppl. 2) (2014) S59–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kalmijn S, Launer L, Ott A, Witteman J, Hofman A, Breteler M, Dietary fat intake and the risk of incident dementia in the Rotterdam study, Ann. Neurol 42 (1997) 776–782. [DOI] [PubMed] [Google Scholar]
- [24].Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS, Dietary fats and the risk of incident Alzheimer disease, Arch. Neurol 60 (2003) 194–200. [DOI] [PubMed] [Google Scholar]
- [25].Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, Kivipelto M, Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study, Int. J. Geriatr. Psychiatry 23 (2008) 741–747. [DOI] [PubMed] [Google Scholar]
- [26].Okereke OI, Rosner BA, Kim DH, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Grodstein F, Dietary fat types and 4-year cognitive change in community-dwelling older women, Ann. Neurol 72 (2012) 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S, Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment, Arch. Neurol 68 (2011) 743–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Levin-Allerhand J, Lominska C, Smith J, Increased amyloid-levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet, J. Nutr. Health Aging 6 (2002) 315–319. [PubMed] [Google Scholar]
- [29].Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lütjohann D, Heerschap A, Kiliaan AJ, DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice, Neurobiol. Dis 33 (2009) 482–498. [DOI] [PubMed] [Google Scholar]
- [30].Zhang L, Dasuri K, Fernandez-Kim SO, Bruce-Keller AJ, Freeman LR, Pepping JK, Beckett TL, Murphy MP, Keller JN, Prolonged diet induced obesity has minimal effects towards brain pathology in mouse model of cerebral amyloid angiopathy: implications for studying obesity-brain interactions in mice, Biochim. Biophys. Acta 1832 (2013) 1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Leboucher A, Laurent C, Fernandez-Gomez F-J, Burnouf S, Troquier L, Eddarkaoui S, Demeyer D, Caillierez R, Zommer N, Vallez E, Bantubungi K, Breton C, Pigny P, Buée-Scherrer V, Staels B, Hamdane M, Tailleux A, Buée L, Blum D, Detrimental effects of diet-Induced obesity on t pathology are independent of insulin resistance in t transgenic mice, Diabetes 2 (2013) 1681–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Koga S, Kojima A, Kuwabara S, Yoshiyama Y, Immunohistochemical analysis of tau phosphorylation and astroglial activation with enhanced leptin receptor expression in diet-induced obesity mouse hippocampus, Neurosci.Lett 571 (2014) 11–16. [DOI] [PubMed] [Google Scholar]
- [33].Bimonte-Nelson H, Singleton R, Hunter C, Price K, Moore A, Granholm AC, Ovarian hormones and cognition in the aged female rat: I. Long-term but not short term, ovariectomy enhances spatial performance, Behav. Neurosci 117 (2003) 1395–1406. [DOI] [PubMed] [Google Scholar]
- [34].French K, Bimonte-Nelson HA, Granholm AC, Galantamine effects on memory, spatial cue utilization, and neurotrophic factors in gaed female rats, Cell Transplant 16 (2007) 197–205. [DOI] [PubMed] [Google Scholar]
- [35].Jarrard LE, Okaichi H, Steward O, Goldschmidt RB, On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus, Behav. Neurosci 98 (1984) 946–954. [DOI] [PubMed] [Google Scholar]
- [36].M’Harzi M, Jarrard L, Strategy selection in a task with spatial and nonspatial components: effects of fimbria-fornix lesions in rats, Behav. Neural Biol 58 (1992) 171–179. [DOI] [PubMed] [Google Scholar]
- [37].Hunter C, Bimonte HA, Granholm AC, Behavioral comparison of 4 and 6-month old Ts65Dn mice: age-related impairments in working and reference memory, Behav. Brain Res 138 (2003) 121–131. [DOI] [PubMed] [Google Scholar]
- [38].Backman C, Rose G, Hoffer B, Henry M, Bartus R, Friden P, Granholm AC, Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy, J.Neurosci 16 (1996) 5437–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Granholm AC, Sanders L, Seo H, Lin L, Ford K, Isacson O, Estrogen alters amyloid precursor protein as well as dendritic and cholinergic markers in a mouse model of Down syndrome, Hippocampus 13 (2003) 905–914. [DOI] [PubMed] [Google Scholar]
- [40].Lockrow J, Prakasam A, Huang P, Bimonte-Nelson HA, Sambamurti K, Granholm AC, Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model, Exp. Neurol 216 (2008) 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Willis L, Small BJ, Bickford PC, Umphlet C, Moore A, Granholm AC, Dietary blueberry supplementation affects growth but not vascularization of neural transplants, J. Cereb. Blood Flow Metab 28 (2008) 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gundersen H, Bagger P, Bendtsen T, Evans S, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. , The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis, APMIS 96 (1988) 857–881. [DOI] [PubMed] [Google Scholar]
- [43].Sarlus H, Hoglund C, Karshikoff B, Wang X, Lekander M, Schultzberg M, Oprica M, Allergy influences the inflammatory status of the brain and enhances tau-phosphorylation, J. Cell. Mol. Med 16 (2012) 2401–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chard PS, Jordan J, Marcuccilli C, Miller R, Leiden J, Roos R, Ghadge GD, Regulation of excitatory transmission at hippocampal synapses by cabindin D28k, PNAS 92 (1995) 5144–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Westerink RH, Beekwilder JP, Wadman WJ, Differential alterations of synaptic plasticity in dentate gyrus and CA1 hippocampal area of Calbindin-D28K knockout mice, Brain Res 1450 (2012) 1–10. [DOI] [PubMed] [Google Scholar]
- [46].Krzywkowski P, Potier B, Billard J, Dutar P, Lamour Y, Synaptic mechanisms and calcium binding proteins in the aged rat brain, Life Sci 59 (1996) 421–428. [DOI] [PubMed] [Google Scholar]
- [47].Villa A, Podini P, Panzeri M, Racchetti G, Meldolesi J, Cytosolic Ca2+ binding proteins during rat brain ageing: loss of calbindin and calretinin in the hippocampus with no change in the cerebellum, Eur. J. Neurosci 6 (1994) 1491–1499. [DOI] [PubMed] [Google Scholar]
- [48].Fifre A, Sponne I, Koziel V, Kriem B, Yen Potin FT, Bihain BE, Olivier JL, Oster T, Pillot T, Microtubule-associated protein MAP1A MAP1B, and MAP2 proteolysis during soluble amyloid beta-peptide-induced neuronal apoptosis. Synergistic involvement of calpain and caspase-3, J. Biol. Chem 281 (2006) 229–240. [DOI] [PubMed] [Google Scholar]
- [49].Takahashi RH, Capetillo-Zarate E, Lin MT, Milner TA, Gouras GK, Accumulation of intraneuronal beta-amyloid 42 peptides is associated with early changes in microtubule-associated protein 2 in neurites and synapses, PLoS One 8 (2013) e51965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Salimi K, Humpel C, Down-regulation of complement receptor 3 and major histocompatibility complex I and II antigen-like immonureactivity accompanies ramification in isolated rat microglia, Brain Res 946 (2002) 283–289. [DOI] [PubMed] [Google Scholar]
- [51].Sheffield L, Berman N, Microglial expression of MHC class II increases in normal aging of nonhuman priamtes, Neurobiol. Aging 19 (1998) 47–55. [DOI] [PubMed] [Google Scholar]
- [52].Luo X-G, Ding J-Q, Chen S-D, Microglia in the aging brain: relevance to neurodegeneration, Mol Neurodegeneration 5 (2010) 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Augustinack JC, Schneider A, Mandelkow E-M, Hyman BT, Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease, Acta Neuropathol. (Berl.) 103 (2001) 26–35. [DOI] [PubMed] [Google Scholar]
- [54].Bimonte HA, Nelson ME, Granholm AC, Age-related deficits as working memory load increases: relationships with growth factors, Neurobiol. Aging 24 (2003) 37–48. [DOI] [PubMed] [Google Scholar]
- [55].Sharma S, Rakoczy S, Brown-Borg H, Assessment of spatial memory in mice, Life Sci 87 (2010) 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Veng L, Granholm AC, Rose GM, Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats, Physiol. Behav 80 (2003) 27–36. [DOI] [PubMed] [Google Scholar]
- [57].Shukitt-Hale B, McEwen JJ, Szprengiel A, Joseph JA, Effect of age on the radial arm water maze – a test of spatial learning and memory, Neurobiol.Aging 25 (2004) 223–229. [DOI] [PubMed] [Google Scholar]
- [58].Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, Ferreira G, Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats, Brain Behav. Immun 40 (2014) 9–17. [DOI] [PubMed] [Google Scholar]
- [59].Kanoski SE, Davidson TL, Different patterns of memory impairments accompany short-and longer-term maintenance on a high-energy diet, J. Exp.Psychol. Anim. Behav. Process 36 (2010) 313–319. [DOI] [PubMed] [Google Scholar]
- [60].Camer D, Yu Y, Szabo A, Fernandez F, Dinh CH, Huang XF, Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory, Prog. Neuropsychopharmacol. Biol. Psychiatry 59 (2015) 68–75. [DOI] [PubMed] [Google Scholar]
- [61].Hoane MR, Swan AA, Heck SE, The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat, Behav. Brain Res 223 (2011) 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Goldbart AD, Row BW, Kheirandish-Gozal L, Cheng Y, Brittian KR, Gozal D, High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia, Brain Res 1090 (2006) 190–196. [DOI] [PubMed] [Google Scholar]
- [63].Hosking DE, Nettelbeck T, Wilson C, Danthiir V, Retrospective lifetime dietary patterns predict cognitive performance in community-dwelling older Australians, Br. J. Nutr 112 (2014) 228–237. [DOI] [PubMed] [Google Scholar]
- [64].Gu Y, Scarmeas N, Dietary patterns in Alzheimer’s disease and cognitive aging, Curr. Alzheimer Res 8 (2011) 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM, The neurobiology of aging, Epilepsy Res 68 (2006) S5–S20. [DOI] [PubMed] [Google Scholar]
- [66].Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T, Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note, Brain Res 1015 (2004) 169–174. [DOI] [PubMed] [Google Scholar]
- [67].Disterhoft JF, Moyer JR, Thompson LT, The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging, Ann. N. Y. Acad. Sci 747 (1994) 382–406. [DOI] [PubMed] [Google Scholar]
- [68].Khachaturian ZS, Calcium, membranes, aging, and Alzheimer’s disease. Introduction and overview, Ann. N. Y. Acad. Sci 568 (1989) 1–4. [DOI] [PubMed] [Google Scholar]
- [69].Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW, Calcium dysregulation in neuronal aging and Alzheimer’s disease: history and new directions, Cell Calcium 24 (1998) 417–433. [DOI] [PubMed] [Google Scholar]
- [70].Hayakawa N, Kato H, Araki T, Age-related changes of astorocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector, Mech.Ageing Dev 128 (2007) 311–316. [DOI] [PubMed] [Google Scholar]
- [71].Niculescu MD, Lupu DS, High fat diet-induced maternal obesity alters fetal hippocampal development, Int. J. Dev. Neurosci 27 (2009) 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Calvo-Ochoa E, Hernandez-Ortega K, Ferrera P, Morimoto S, Arias C, Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus, J. Cereb. Blood Flow Metab 34 (2014) 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M, JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins, Dev. Cell 4 (2003) 521–533. [DOI] [PubMed] [Google Scholar]
- [74].Vernia S, Cavanagh-Kyros J, Garcia-Haro L, Sabio G, Barrett T, Jung DY, Kim JK, Xu J, Shulha HP, Garber M, Gao G, Davis RJ, The PPAR-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway, Cell Metab 20 (2014) 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Johnson RW, Feeding the beast: can microglia in the senescent brain be regulated by diet? Brain Behav. Immun 43 (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kettenmann H, Hanisch UK, Noda M, Verkhratsky A, Physiology of microglia, Physiol. Rev 91 (2011) 461–553. [DOI] [PubMed] [Google Scholar]
- [77].Freeman LR, Small BJ, Bickford PC, Umphlet C, Granholm AC, A high-fat/high-cholesterol diet inhibits growth of fetal hippocampal transplants via increased inflammation, Cell Transplant 20 (2011) 1499–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nguyen JC, Killcross AS, Jenkins TA, Obesity and cognitive decline: role of inflammation and vascular changes, Front. Neurosci 8 (2014) 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Spolcova A, Mikulaskova B, Krskova K, Gajdosechova L, Zorad S, Olszanecki R, Suski M, Bujak-Gizycka B, Zelezna B, Maletinska L, Deficient hippocampal insulin signaling and augmented Tau phosphorylation is related to obesity-and age-induced peripheral insulin resistance: a study in Zucker rats, BMC Neurosci 15 (2014) 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, Mäkinen P, Leinonen V, Pihlajamäki J, Soininen H, Laakso M, Tanila H, Hiltunen M, High-fat diet increases Tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status, J. Nutr. Biochem 25 (2014) 634–641. [DOI] [PubMed] [Google Scholar]


