Abstract
Background:
Advances in radiation treatment (RT), specifically volumetric planning with detailed dose and volumetric data for specific brain structures, have provided new opportunities to study neurobehavioral outcomes of RT in children treated for brain tumor. The present study examined the relationship between biophysical and physical dose metrics and neurocognitive ability, namely learning and memory, 2 years post-RT in pediatric brain tumor patients.
Procedure:
The sample consisted of 26 pediatric patients with brain tumor, 14 of whom completed neuropsychological evaluations on average 24 months post-RT. Prescribed dose and dose–volume metrics for specific brain regions were calculated including physical metrics (i.e., mean dose and maximum dose) and biophysical metrics (i.e., integral biological effective dose and generalized equivalent uniform dose). We examined the associations between dose–volume metrics (whole brain, right and left hippocampus), and performance on measures of learning and memory (Children’s Memory Scale).
Results:
Biophysical dose metrics were highly correlated with the physical metric of mean dose but not with prescribed dose. Biophysical metrics and mean dose, but not prescribed dose, correlated with measures of learning and memory.
Conclusions:
These preliminary findings call into question the value of prescribed dose for characterizing treatment intensity; they also suggest that biophysical dose has only a limited advantage compared to physical dose when calculated for specific regions of the brain. We discuss the implications of the findings for evaluating and understanding the relation between RT and neurocognitive functioning.
Keywords: dosimetry, late effects, learning and memory, pediatric brain tumor, radiation
Brain and central nervous system tumors are among the most common childhood cancers.1 Treatment advances have resulted in increased survival rates among pediatric brain tumor (PBT) patients; however, radiation therapy (RT) is associated with suboptimal cognitive, behavioral, and emotional outcomes.2–5 Many survivors of PBT experience long-term impairments in cognitive abilities and decreased academic functioning, reducing the likelihood of vocational success. Therefore, research aimed at limiting cognitive morbidity is of paramount importance.
1 |. NEUROPSYCHOLOGICAL LATE EFFECTS
The adverse effects of RT on the developing brain are well-documented. A mild decline in IQ among PBT patients is reported as early as 1 year and as late as 10 years post-treatment.6,7 This decline in IQ reflects the failure of pediatric patients to make expected gains in their cognitive abilities relative to peers, rather than a loss of previously acquired skills.8 Deficits in learning and memory are among the most common sequelae of RT, particularly among children with third ventricle tumors9 and medulloblastoma.10,11 Children with a brain tumor perform more poorly than comparative groups9,12 and normative means10 on tests of verbal and/or visual learning and memory.9,10,13
The hippocampus is a critical structure for memory.14 Despite the established link between RT and hippocampal damage in animal models,15,16 evidence is limited in humans but remains indicative of damage. Among children treated for infratentorial tumors, there is evidence for early decline in hippocampal volume with return to positive growth 2 years post-diagnosis,17 however, hippocampal volume may remain reduced relative to healthy peers several years post-diagnosis.18 Notably, memory performance of adult survivors of PBT is related to hippocampal volume but not whole brain or putamen volumes.19 Findings from a recent study examining associations between hippocampal subfield volumes (cornus ammonis [CA] 1, CA2–3, dentate gyrus [DG]-CA4, stratum radiatum–lacunosum–moleculare [SRLM], and subiculum) and verbal short-term memory revealed smaller DG-CA4, CA1, CA2–3, and SRLM volumes in PBT survivors compared to healthy children; and positive correlations between verbal memory and DG-CA4, CA1, and SRLM volumes.20
2 |. DOSIMETRY AND NEUROPSYCHOLOGICAL LATE EFFECTS
Although considerable evidence exists for a relationship between cognitive abilities and RT, previous research has relied primarily on prescribed dose (i.e., total dose to the target volume) or contrasts based on the volume of brain irradiated (i.e., whole brain vs. restricted field) to assess radiation exposure.21,22 However, these broad-based metrics do not account for variability in RT procedures from one patient to the next, including variations in dose, volume, and beam orientation, which are integral to minimizing damage to normal brain tissue and sparing critical brain structures. For example, prescribed dose does not account for dose–volume heterogeneity, commonly related to tissue late effects. Manipulation of these RT variables in treatment planning has made the use of prescribed dose an imprecise measure of RT.
Three dimensional (3D) conformal therapy and methods of dosimetry now allow for analysis using differential and cumulative dose–volume histograms (DVHs), derived from dosimetry calculated on a fine spatial scale. These analyses map the heterogeneous levels of dose throughout the brain, so that the dose delivered to the whole brain and specific structures can be assessed. In an attempt to better account for variability in the distribution of dose over treatment volume, Merchant and colleagues partitioned RT dose–volume data into three ranges (low, intermediate, and high) to represent the fractional volumes that received each dose in the total brain, supratentorial brain, and left and right temporal lobes.23 They showed that IQ after RT was significantly correlated with dosimetry of the total and supratentorial brain volumes.
For further precision, information derived from 3D conformal therapy and dosimetry can be used to calculate radiobiologic indices, such as generalized equivalent uniform dose (gEUD) and integral biological effective dose (IBED). In addition to the physical RT dose, these indices model the actual biological effect resulting from dose. Radiobiological approaches to understanding long-term side effects of RT have shown that biological effects depend, for any given tissue, on the dose, volume, fractionation sizes, and alpha/beta ratio, where alpha and beta are the radiobiological cell survival parameters for the tissue within the treatment volume. gEUD refers to the absorbed dose that, when homogeneously delivered to a tumor, causes the same expected number of clonogens to survive as the actual nonhomogeneous absorbed dose distribution.24 The underlying assumption is that homogeneous irradiation of a tumor with absorbed dose D, and any nonhomogeneous irradiations with EUD equal to D are equivalent in a biological sense. IBED is calculated using the linear quadratic model of cell survival.25 Ultimately, the biological effect of RT, not the physical dose, is most relevant to late-effects research. Calculation of gEUD or IBED yields a single value and makes it possible to compare patients treated with the same dose, but with different dose volumes.
IBED has been the focus of limited research on neurobehavioral outcomes following RT (see Zureick26 for limited use of gEUD). Reimers and colleagues reported a significant relationship between IBED to the isocenter of the treatment field and verbal IQ.27 Ris and colleagues used IBED as a method for more precise modeling of neurobehavioral late effects in a sample of five children with PBT.28 Decline in digit span performance from year 1 to year 2 post-RT significantly correlated with whole brain IBED but not prescribed dose. To our knowledge, no other studies have examined the relation between IBED and neuropsychological late effects. Although several studies have shown a relationship between prescribed dose and neurocognitive late effects, very few studies have employed more refined metrics to determine if any advantage is conferred in the study of late effects. General indices of dose may be satisfactory predictors of global cognitive abilities such as IQ, but more precise metrics may be better predictors of specific cognitive abilities such as learning and memory.
3 |. THE PRESENT STUDY
The primary aims of the current study are twofold. First, this study extends Ris et al. by contrasting the values of prescribed dose and physical and biophysical metrics used to capture dose heterogeneity in brain tissue.28 Second, this study directly assesses the associations among RT dose metrics and verbal and visual–spatial learning and memory. Because biophysical metrics of IBED and gEUD take into consideration individualized treatment variables, not accounted for by prescribed dose, we predicted that biophysical metrics would be more strongly associated with learning and memory performance than prescribed dose.
4 |. METHODS
4.1 |. Participants
This study is part of a longitudinal project on cognitive, behavioral, and social–emotional outcomes following PBT treated with or without RT. Participants were children and adolescents aged 3–17 years who recently underwent surgical resection of a brain tumor. They were recruited from neuro-oncology clinics at four urban medical centers. Detailed eligibility criteria are reviewed elsewhere.4,29 Briefly, exclusion criteria include severe preexisting conditions or ineligible tumor types (e.g., glioblastoma multiforme), severe postsurgical complications, or history of neurofibromatosis type 1. Ultimately, 69 participants were enrolled in the study; 2 died prior to data collection and 4 dropped out, yielding 63 participants, 30 of whom were treated with RT. Only those receiving RT and for whom detailed dosimetric data were available are included in this study, yielding a sample of 26 patients diagnosed between the ages of 5 and 16 years. A summary of demographic and tumor-/treatment-related variables is presented in Table 1. The sample is predominantly male, with the majority of parents completing at least some college. The majority of participants had infratentorial tumors, were treated with craniospinal RT with a boost to the tumor bed, and received chemotherapy. Fourteen participants had neurocognitive data available at follow-up approximately 2 years postsurgery, so associations between dose metrics and memory are described for this subsample. Specifically, four participants dropped out, six were lost to follow-up, one died in the interim between baseline and follow-up evaluations, and one was not administered the memory measures. Participants with neuropsychological data did not differ from those without on demographic and tumor-related variables, including gender, parental education, age at surgery, tumor location, and prescribed dose (all P-values > 0.09).
TABLE 1.
Demographic and tumor-related variables
| Variable | |
|---|---|
| Gender (Male; n) | 21 |
| Age at surgery [Mean (SD)] | 10.66 (3.87) |
| Age at follow-up (T2) [Mean (SD)] | 12.60 (3.45) |
| Number of months post-surgery at follow-up [Mean (SD)] | 24.83 (10.92) |
| Education of primary caregiver (n) | |
| Some high school | 2 |
| High school graduate | 4 |
| Some college | 6 |
| College graduate | 7 |
| Graduate degree | 4 |
| Type of tumor (n) | |
| Medulloblastoma/PNET | 12 |
| Astrocytoma | 3 |
| Germ cell | 3 |
| Ependymoma | 2 |
| Optic nerve glioma | 2 |
| Other (e.g., atypical teratoid-rhabdoid, | |
| bithalamic, and brainstem glioma) | 4 |
| Location of tumor (n) | |
| Supratentorial | 10 |
| Optic chiasm | 3 |
| Extrafrontal | 2 |
| Fronto-parietal/temporal | 2 |
| Other (pineal, sella) | 3 |
| Infratentorial | 16 |
| Posterior fossa | 13 |
| Other (multifocal, | |
| fourth ventricle) | 3 |
| Shunt placement | |
| Yes(n) | 2 |
| Chemotherapy | |
| Yes(n) | 16 |
| Type of radiation (n) | |
| Focal | 9 |
| Nonfocal | 1 |
| Craniospinal + Boost | 16 |
| Prescribed Dose (cGy) [Mean (SD)] | 5210.77 (603.52) |
Note: Missing data–chemotherapy (0); shunt placement (3); education of primary caregiver (3); follow-Up (12).
4.2 |. Dose reconstruction
Calculation of RT dose was performed throughout the brain volume using the results of the respective treatment planning systems. RT doses were calculated using the collapsed cone convolution super-position algorithm (Pinnacle, Tomotherapy Planning Station) or pencil beam algorithm (Brainscan, iPlanDose). The former provides excellent agreement between computed and measured doses under a wide variety of conditions, with almost all points within 3% of agreement,30,31 and the latter provides equivalent agreement in homogenous media such as the brain.
4.3 |. Contouring
Neuroanatomical structures including the right hippocampus, left hippocampus, and whole brain were delineated manually by a single author (M.L.) in all RT patients on 1.5T T1 magnetic resonance (MR) images (see Figure 1). As MR images were acquired over a period of time and locations, the scan parameters varied. Most images were standard post-contrast T1-weighted 2D sequences (2000 ms TR, 3 ms TE, 384 × 512), 5 mm slice thickness, acquired from base of skull to top of head; others were post-contrast T1-weighted FLAIR images (2000 ms TR, 27 ms TE, 512 × 512) or 3D T1-weighted SPGR sequences (4.2 ms TR, 1.9 ms TE, 256 × 256, 1.3–1.5 mm slice thickness).
FIGURE 1.
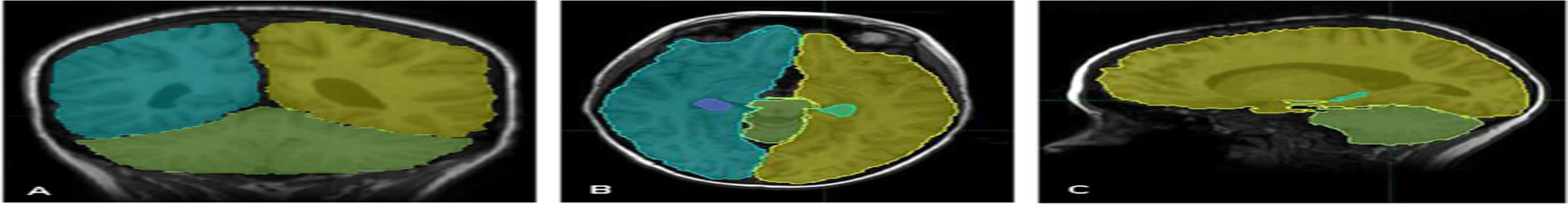
Examples of slice contours oriented within the A) coronal B) axial, and C) sagittal planes. Each cerebral hemisphere is traced along with the hippocampi and cerebellum
Contouring was performed in MimVista (Mim Software Inc, Cleveland, OH) using the 2D brush tool and standard neuroanatomical atlases within MimVista. The hippocampi were generated following the methodology described by Gondi and colleagues.32 Inter-rater reliability was assessed by comparing volumes of two contourers repeating contours 10 times on one patient on separate days. Mean volume differences were found to be 3.3%, with a maximum difference of 3.8%.
4.4 |. Dose Metrics
4.4.1 |. Prescribed dose
Refers to the total dose prescribed to the target volume. For patients receiving craniospinal RT with a boost to the tumor bed, the prescribed dose is the sum of these two doses.
4.4.2 |. Physical dose metrics
Mean dose.
For a given structure, the mean dose is the average dose to that structure: Dose to a subvolume × volume of that subvolume / sum of all subvolumes.
Maximum dose.
This refers to the maximum dose of any subvolume in that structure for a given structure.
4.4.3 |. Biophysical dose metrics
Generalized equivalent uniform dose (gEUD).
Equivalent uniform dose was first proposed to provide a metric for nonuniform tumor dose33 and then extended to the more general form for nonuniform normal tissue dose.24 It provides a single metric that attempts to reflect the biological effects to a structure resulting from a heterogeneous dose. It is calculated as
Mathematically, gEUD is the quantity sum of all subvolume ʋi times the dose to that subvolume Di to an exponent a, then taken to the inverse of a after summing. The value of a is generally negative for tumors and positive for normal tissues. A value of zero results in gEUD equaling the mean dose.
Integral biological effective dose (IBED)
The calculation of IBED is a two-step process. In the first step, the heterogeneous dose distribution of a treatment volume is condensed to a dose–volume relation using a differential dose DVH. This step breaks the volume into many small subvolumes, sums the volumes in the dose increments, and presents the results in a DVH. In the second step, IBED is calculated from the DVH data.
Normal tissue response to RT can be modeled using linear (α) and quadratic (β) terms. Using the terms of the linear quadratic model of cell survival, IBED30,31 is calculated as
where n is the number of fractions delivered, di is the dose delivered to the ith element of the volume from the DVH, α / β is a measure of the early and late response for a particular tissue, Δv is the volume of the ith element, and V is the total volume of the treated volume. The α/β ratio was assumed to be constant for normal brain tissue with a value of 2.9(i).
4.5 |. Measures
Subtests from the Children’s Memory Scale (CMS)34 assessed verbal and visual–spatial learning and memory. Subtest raw scores were converted to scaled scores.
Stories.
Children listened to two stories and after each they recalled the story verbatim immediately and following a 30 min delay.
Word Pairs.
Participants listened to a list of word pairs, and after each of the three learning trials, they repeated the list (i.e., immediate memory) as well as after a 30 min delay.
Faces.
Participants studied a series of faces; then another series of faces was presented immediately thereafter and 30 min later, and participants responded “yes” if the face was presented earlier and “no” if it was not.
Dot Locations.
Participants studied the location of blue dots inside a large grid, and then replicated this pattern using chips on a blank grid. Participants had three trials to learn the locations, before being asked to recall them after a 30 min delay.
4.6 |. Procedure
Participants were recruited during neuro-oncology follow-up visits, and parents provided informed consent in accordance with institutional review boards. Patient charts and RT treatment plans were reviewed by a radiation oncology physicist blind to the results of memory testing (M.L.) to record treatment data. Total doses to the tumor site and craniospinal axis were recorded. For volumes other than craniospinal axis treatment, the planning data were reviewed and the planning target volumes and doses were recorded. The memory tests included in the study were collected during follow-up evaluations that occurred approximately 24 months following surgical resection.
4.7 |. Statistical analyses
Due to the nonnormal distributions of the dosimetric variables, Spearman’s rho rather than Pearson’s r examined the strength and direction of the relationship between dosimetry variables for the whole brain and right and left hippocampi. Spearman’s rho examined relationships between dose metrics for brain regions and memory performance. One-sample t-tests compared the sample mean on memory measures to the normative mean scaled score of 10.
5 |. RESULTS
5.1 |. Correlations among dose metrics
Table 2 presents the correlation matrix for RT dose metrics. Prescribed dose was not significantly correlated with other metrics, with the exception of maximum dose delivered to the whole brain and right hippocampus. For whole brain and hippocampal metrics, mean dose, IBED, and gEUD were highly correlated; maximum dose also correlated with other dose metrics in the hippocampi alone.
TABLE 2.
Correlations among radiation metrics (N = 26)
| Prescribed dose | IBED | gEUD | Mean | Max | |
|---|---|---|---|---|---|
| Whole brain | |||||
| IBED | −0.076 | 1.00 | 0.812** | 0.973** | −0.281 |
| gEUD | 0.382 | - | 1.00 | 0.835** | 0.109 |
| Mean | −0.036 | - | - | 1.00 | −0.238 |
| Max | 0.795** | - | - | - | 1.00 |
| Right hippocampus | |||||
| IBED | 0.151 | 1.00 | 0.984** | 0.991** | 0.580** |
| gEUD | 0.231 | - | 1.00 | 0.994** | 0.670** |
| Mean | 0.207 | - | - | 1.00 | 0.631** |
| Max | 0.493* | - | - | - | 1.00 |
| Left hippocampus | |||||
| IBED | −0.035 | 1.00 | 0.984** | 0.990** | 0.666** |
| gEUD | 0.058 | - | 1.00 | 0.996** | 0.733** |
| Mean | 0.028 | - | - | 1.00 | 0.709** |
| Max | 0.319 | - | - | - | 1.00 |
Note: Nonparametric (Spearman’s rho) correlations are reported.
=P < 0.05;
=P < 0.01.
5.2 |. Memory performance among PBT patients
Table 3 presents descriptive statistics for the memory tests. Mean performance for the study sample was significantly lower than the standardization mean on delayed recall of Faces (t = −3.29, P < 0.01; but not immediate recall, t = −2.05, P < 0.07) and Word Pair learning (t = −3.12, P < 0.01; but not delayed recall, t < 1). Significant differences were not observed for Dot Locations learning or delayed recall (t’s < 1); and immediate and delayed recall for Stories (t < 1, t = 1.37, P < 0.20, respectively).
TABLE 3.
Performance on memory subtests (n = 14)
| M | SD | Range | |
|---|---|---|---|
| Dot Locations | |||
| Learning | 9.43 | 3.61 | 3–16 |
| Delay | 10.42 | 3.13 | 3–14 |
| Faces | |||
| Immediate | 8.07 | 3.52 | 1–13 |
| Delay* | 7.79 | 2.52 | 3–12 |
| Stories | |||
| Immediate | 10.36 | 2.65 | 6–15 |
| Delay | 10.86 | 2.35 | 7–15 |
| Word Pair | |||
| Learning* | 8.29 | 2.05 | 5–12 |
| Delay | 9.36 | 3.00 | 4–16 |
Note
=P < 0.01 for one-sample t-test (normative mean of 10).
5.3 |. Correlations among dose metrics and memory performance
Table 4 presents the correlation matrix relating dose metrics to memory performance. Memory measures were not correlated with prescribed dose. Conversely, Word Pair delayed recall was significantly associated with whole brain and right hippocampus mean dose, IBED, and gEUD; and left hippocampus gEUD, though correlations with IBED and mean dose were large (r > −0.50) and marginally significant. Additionally, Dot Locations learning and recall was associated with whole brain IBED, gEUD, and mean dose (r < −0.56).
TABLE 4.
Correlations among radiation metrics and memory subtests (time 2 scores; n = 14)
| Stories | Faces | Word Pair | Dot Loc | |||||
|---|---|---|---|---|---|---|---|---|
| Imm. | Delay | Imm. | Delay | Learn. | Delay | Learn. | Delay | |
| Prescribed dose | ||||||||
| 0.159 | 0.142 | −0.061 | −0.235 | 0.227 | −0.409 | −0.140 | −0.243 | |
| Whole brain | ||||||||
| IBED | −0.292 | −0.328 | −0.095 | −0.461 | −0.161 | −0.606* | −0.561* | −0.582* |
| gEUD | −0.292 | −0.323 | −0.071 | −0.476 | −0.099 | −0.648* | −0.632* | −0.636* |
| Mean | −0.268 | −0.289 | −0.007 | −0.408 | −0.167 | −0.575* | −0.603* | −0.580* |
| Max | 0.087 | 0.100 | 0.115 | −0.028 | −0.002 | −0.391 | −0.177 | −0.218 |
| Right hippocampus | ||||||||
| IBED | −0.190 | −0.104 | −0.032 | −0.278 | −0.018 | −0.571* | −0.327 | −0.286 |
| gEUD | −0.125 | −0.069 | −0.027 | −0.259 | 0.000 | −0.551* | −0.382 | −0.373 |
| Mean | −0.181 | −0.124 | −0.020 | −0.248 | −0.023 | −0.590* | −0.375 | −0.389 |
| Max | 0.130 | 0.143 | −0.011 | −0.093 | 0.030 | −0.417 | −0.036 | −0.237 |
| Left hippocampus | ||||||||
| IBED | −0.145 | −0.172 | −0.135 | −0.525 | −0.101 | −0.528 | −0.279 | −0.242 |
| gEUD | −0.125 | −0.181 | −0.124 | −0.505 | −0.105 | −0.566* | −0.340 | −0.373 |
| Mean | −0.116 | −0.154 | −0.097 | −0.498 | −0.066 | −0.519 | −0.326 | −0.304 |
| Max | 0.292 | 0.205 | −0.127 | −0.336 | −0.076 | −0.411 | 0.050 | −0.189 |
Note: Nonparametric (Spearman’s rho) correlations are reported.
=P < 0.05.
6 |. DISCUSSION
The current study examined the relationship between physical and biophysical RT dose metrics and their relationship to learning and memory outcomes 2 years post-RT in a sample of PBT patients. This study addressed a number of unique aspects of dosimetry and its relationship to neurocognitive outcome including: (1) the relative value of prescribed dose in characterizing RT exposure in pediatric patients and its association with neurocognitive outcome; (2) the relationship between different measures of physical and biophysical dose; and (3) the relative value of using physical dose versus biophysical dose to predict neurocognitive outcome.
Prescribed dose is the most commonly employed metric in neurocognitive late-effects research examining treatment intensity as a predictor of cognitive decline. In our sample, prescribed dose was not significantly correlated with more refined physical or biophysical dose metrics. Moreover, prescribed dose was not significantly correlated with memory performance, thereby replicating and extending the findings of Ris and colleagues, which focused specifically on brief attention and working memory.28 Taken together, prescribed dose in pediatric samples with heterogeneous tumor types may be less meaningful as a marker than biophysical metrics, potentially because it does not reflect the volume of brain irradiated.
Biophysical metrics and the physical metric of mean dose were highly correlated. To the extent that dose is homogeneous, biophysical metrics and mean dose behave similarly. Homogeneity of dose is more likely in smaller brain structures, such as the hippocampus, relative to larger structures. In calculating biophysical metrics, lower dosage volumes are minimized and higher volumes are maximized given that higher doses often produce significant biological effects and lower doses result in no or subclinical effects. Our findings reveal near perfect correlations between biophysical metrics and mean dose in the hippocampus, with somewhat decreased association between whole brain metrics. Thus, biophysical metrics do not appear to offer any unique information over and above that accounted for by physical metrics in this sample, particularly for smaller brain structures. Our findings likely reflect the fact that more than half of the sample treated with RT received craniospinal RT plus a posterior fossa boost, resulting in increased homogeneity of dose to brain structures.
Given that biophysical metrics and mean dose were highly correlated, it is not surprising that both were predictive of learning and memory; and considerably more so than prescribed and maximum doses. As a group, our sample performed in the average range on measures of verbal and visual memory, with the exception of Word Pair learning and delayed recall of Faces, which fell in the low average to average range but significantly below the normative mean. Learning and memory outcomes following RT are mixed, with PBT patients demonstrating significantly worse verbal learning and memory than controls,28,35 stable or improved verbal and visual memory over time36; or decline in verbal memory but not visual memory26 or vice versa.37
Both verbal and visual memory measures were sensitive to the effects of RT. Word Pair delayed recall, which is commonly associated with hippocampal functioning, was similarly correlated with whole brain and hippocampal dose metrics. Although we observed more statistically significant correlations with the right than left hippocampus, the magnitude of the correlations were similar. Functional imaging studies from the adult literature indicate that successful verbal associative encoding requires activation of the left hippocampus as well as the left inferior frontal gyrus.38 While the left hippocampus appears to be necessary for verbal associative learning, it may not be sufficient, as there is evidence for bilateral recruitment.39,40 Significant correlations with the right hippocampus may suggest the presence of a compen-satory mechanism supporting average range performance, as is often the case when involvement of the adjacent hemispheric homologue is implicated.41–43
Dot Locations learning and recall were significantly correlated with whole brain dose metrics, though not specifically to the right or left hippocampus. These findings may reflect the notion that memory is a distributed process, particularly visual memory which is thought to be less lateralized than verbal memory, likely reflecting the broader distribution of the visual–perceptual system. Although the hippocampus is susceptible to damage in PBT patients, white matter is also at considerable risk.11,44 In patients with TBI, visual memory performance was associated with wide spread reductions in grey and white matter volume of several cortical and subcortical structures, implicating the alteration of multiple systems subserving attentional and memory processing.45
Our findings must be considered in the light of the methodological limitations. First, the sample is small, which limits the generalizability of the findings, statistical power to detect significant effects, and robustness of observed relationships. Second, a number of participants received craniospinal RT with a boost to the tumor bed, thereby reducing dose heterogeneity and variation between biophysical and physical metrics. Third, this sample is notable for diagnostic heterogeneity. Although memory problems are commonly reported in children with infratentorial and midline tumors, the small sample precludes accounting for tumor location. Fourth, the study employed a variety of memory measures, with the exception of the commonly used list learning task.
With advances in radiation oncology, we are able to better explore relationships between RT and neurocognitive outcome. The current study suggests that prescribed dose in a heterogeneous sample does not correlate with physical and biophysical metrics or predict memory outcomes. In contrast, measures of dosimetry derived from physical and biophysical properties correlate in a predictable way and are predictive of memory, although biophysical metrics hold no clear advantage over mean dose. Understanding the effects of RT dosimetry on cognitive function is essential to develop new ways to target and administer RT in children with brain tumors. In fact, although mean dose does not account for the multidimensionality of individualized RT protocols, it is highly correlated with more precise metrics that take into account a number of individualized variables. Given the small sample size, replication of these findings is needed in a larger, more homogeneous sample.
Abbreviations:
- CA
cornus ammonis
- DG
dentate gyrus
- DVH
dose-volume histogram
- gEUD
generalized equivalent uniform dose
- IBED
integral biological effective dose
- MR
magnetic resonance
- PBT
pediatric brain tumor
- RT
radiation treatment
- SRLM
stratum radiatum–lacunosum–moleculare
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology. 2015;17(Suppl 4):iv1–iv62. 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulhern RK, Hancock J, Fairclough D, et al. Neuropsychological status of children treated for brain tumors: a critical review and integrative analysis. Med Pediatr Oncol. 1992;20:181–191. [DOI] [PubMed] [Google Scholar]
- 3.Patel SK, Mullins WA, O’Neil SH, Wilson K. Neuropsychological differences 415 between survivors of supratentorial and infratentorial brain tumours. J Intellect Disabil Res. 2011;55:30–40. [DOI] [PubMed] [Google Scholar]
- 4.Raghubar KP, Mahone EM, Yeates KO, Cecil KM, Makola M, Ris MD. Working memory and attention in pediatric brain tumor patients treated with and without radiation therapy. Child Neuropsychol 2017;23:642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–3476. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe-Hirsch E, Renier D, Lellouch-Tubiana A, et al. Medulloblastoma in childhood: progressive intellectual deterioration. Childs Nerv Syst. 1990;6:60–65. [DOI] [PubMed] [Google Scholar]
- 7.Ris MD, Noll RB. Long-term neurobehavioral outcome in pediatric brain-tumor patients: review and methodological critique. J Clin Exp Neuropsychol. 1994;16:21–42. [DOI] [PubMed] [Google Scholar]
- 8.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–2306. [DOI] [PubMed] [Google Scholar]
- 9.King TZ, Fennell EB, Williams L, et al. Verbal memory abilities of children with brain tumors. Child Neuropsychol. 2004;10:76–88. [DOI] [PubMed] [Google Scholar]
- 10.George AP, Kuehn SM, Vassilyadi M, et al. Cognitive sequelae in children with posterior fossa tumors. Pediatr Neurol. 2003;28:42–47. [DOI] [PubMed] [Google Scholar]
- 11.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. [DOI] [PubMed] [Google Scholar]
- 12.Nagel BJ, Delis DC, Palmer SL, Reeves C, Gajjar A, Mulhern RK. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology. 2006;20:105–112. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DL, McCabe MA, Nicholson HS, et al. Quality of long-term survival in young children with medulloblastoma. J Neurosurg. 1994;80:1004–1010. [DOI] [PubMed] [Google Scholar]
- 14.Eichenbaum H What H.M. taught us. J Cogn Neurosci. 2013;25:14–21. [DOI] [PubMed] [Google Scholar]
- 15.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. [DOI] [PubMed] [Google Scholar]
- 16.Raber J, Fan Y, Matsumori Y, et al. Irradiation attenuates neurogenesisand exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–389. [DOI] [PubMed] [Google Scholar]
- 17.Nagel BJ, Palmer SL, Reddick WE, et al. Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. AJNR Am J of Neuroradiol. 2004;25:1575–1582. [PMC free article] [PubMed] [Google Scholar]
- 18.Riggs L, Bouffet E, Laughlin S, et al. Changes to memory structures in children treated for posterior fossa tumors. J Int Neuropsychol Soc. 2014;20:168–180. [DOI] [PubMed] [Google Scholar]
- 19.Jayakar R, King TZ, Morris R, Na S. Hippocampal volume and auditory attention on a verbal memory task with adult survivors of pediatric brain tumor. Neuropsychology. 2015;29:303–319. [DOI] [PubMed] [Google Scholar]
- 20.Decker AL, Szulc KU, Bouffet E, et al. Smaller hippocampal subfield volumes predict verbal associative memory in pediatric brain tumor survivors. Hippocampus. 2017;27:1140–1154. [DOI] [PubMed] [Google Scholar]
- 21.Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Dev Med Child Neurol. 2000;42:741–745. [DOI] [PubMed] [Google Scholar]
- 22.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose,boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32:1760–1768. [DOI] [PubMed] [Google Scholar]
- 23.Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry 465 predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–1554. [DOI] [PubMed] [Google Scholar]
- 24.Niemierko A A generalized concept of equivalent uniform dose (EUD). Med Phys. 1999;26:1100. [Google Scholar]
- 25.Fowler JF. Brief summary of radiobiological principles in franctionated radiotherapy. Semin Radiat Oncol. 1992;2:16–21. [Google Scholar]
- 26.Zureick AH, Evans CL, Niemierko A, et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer. 2018. 10.1002/cncr.31143 [DOI] [PubMed] [Google Scholar]
- 27.Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003;40:26–34. [DOI] [PubMed] [Google Scholar]
- 28.Ris MD, Ryan PM, Lamba M, et al. An improved methodology for modeling neurobehavioral late-effects of radiotherapy in pediatric brain tumors. Pediatr Blood Cancer. 2005;44:487–493. [DOI] [PubMed] [Google Scholar]
- 29.Robinson KE, Wolfe KR, Yeates KO, Mahone EM, Cecil KM, Ris MD. Predictors of adaptive functioning and psychosocial adjustment in children with pediatric brain tumor: a report from the brain radiation investigative study consortium. Pediatr Blood Cancer. 2015;62:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahnesjo A Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Med Phys. 1989;16:577–592. [DOI] [PubMed] [Google Scholar]
- 31.Mohan R, Chui C, Lidofsky L. Differential pencil beam dose computation model for photons. Med Phys. 1986;40:64–73. [DOI] [PubMed] [Google Scholar]
- 32.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reporting Niemierko A. and analyzing dose distributions: a concept of 489 equivalent uniform dose. Med Phys. 1997;24:103–110. [DOI] [PubMed] [Google Scholar]
- 34.Cohen MJ. Children’s Memory Scale Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 35.Margelisch K, Studer M, Ritter BC, Steinlin M, Leibundgut K, Heinks T. Cognitive dysfunction in children with brain tumors at diagnosis. Pedi atr Blood Cancer. 2015;62:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Pinto M, Conklin HM, Li C, Merchant TE. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2012;84:e363–e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. [DOI] [PubMed] [Google Scholar]
- 38.Kim H Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. [DOI] [PubMed] [Google Scholar]
- 39.Herting MM, Nagel BJ. Differences in brain activity during a verbal associative memory encoding task in high and low-fit adolescents. J Cogn Neurosci. 2013;25:595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler SM, McLelland VC, Sheard E, McAndrews MP, Rovet JF. Hippocampal functioning and verbal associative memory in adolescents with congenital hypothyroidism. Front Endocrinol. 2015:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D,Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. [DOI] [PubMed] [Google Scholar]
- 42.Jensen EJ, Hargreaves I, Bass A, Pexman P, Goodyear BG, Federico P. Cortical reorganization and reduced efficiency of visual word recognition in right temporal lobe epilepsy: a functional MRI study. Epilepsy Research. 2011;93:155–163. [DOI] [PubMed] [Google Scholar]
- 43.Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J. Functional MRI of global and local processing in children. Neuroimage. 2002;16:415–424. [DOI] [PubMed] [Google Scholar]
- 44.Reddick WE, Taghipour DJ, Glass JO, et al. Prognostic factors that increase the risk for reduced white matter volumes and deficits in attention and learning for survivors of childhood cancers. Pediatr Blood Cancer. 2014;61:1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauer J, Moreno-López L, Manktelow A, et al. Neural correlates of visual memory in patients with diffuse axonal injury. Brain Injury. 2017;31:1513–1520. [DOI] [PubMed] [Google Scholar]


