Abstract
Background:
While air pollution has been associated with depression and anxiety in adults, its impact on childhood mental health is understudied.
Objective:
We examined lifetime exposure to traffic-related air pollution (TRAP) and symptoms of depression and anxiety at age 12 years in the Cincinnati Childhood Allergy and Air Pollution Study cohort.
Methods:
We estimated exposure to elemental carbon attributable to traffic (ECAT), a surrogate of diesel exhaust, at birth, age 12 years, and average exposure throughout childhood, using a validated land use regression model. We assessed depression and anxiety at age 12 years by parent report with the Behavior Assessment System for Children-2, and by child report with the Child Depression Inventory-2 (CDI-2) and the Spence Children’s Anxiety Scale (SCAS). Associations between TRAP at birth, age 12 years, and childhood average and mental health outcomes were estimated using linear regression models adjusting for covariates including parent depression, secondhand smoke exposure, race, household income, and others.
Results:
Exposure to ECAT was not significantly associated with parent-reported depression or anxiety. However, exposure to ECAT at birth was associated with increased child-reported depression and anxiety. Each 0.25 μg/m3 increase in ECAT was associated with a 3.5 point increase (95% CI 1.6–5.5) in CDI-2 scores and 2.3 point increase (95% CI 0.8–3.9) in SCAS total anxiety scores. We observed similar associations between average childhood ECAT exposures but not for concurrent exposures at age 12.
Conclusions:
TRAP exposure during early life and across childhood was significantly associated with self-reported depression and anxiety symptoms in children. The negative impact of air pollution on mental health previously reported among adults may also be present during childhood.
Keywords: Air pollution, Anxiety, Child mental health, Depression, Exposure
1. Introduction
Approximately one-half of all Americans will meet diagnostic criteria for a mental health disorder during their lifetime with initial onset frequently occurring during childhood or adolescence (Kessler et al., 2005; Paus et al., 2008). Adolescence is a particularly important developmental period in which major morphological and functional changes in the brain, combined with hormonal influences, contribute to emotional turmoil (Arain et al., 2013), and these changes are linked with increased incidence of mental health problems (Paus et al., 2008). Depression and anxiety are of particular concern, as these are the most prevalent mental health disorders affecting 14% and 30% of U.S. adolescents, respectively (Caspi et al., 1996; Kessler et al., 2005; Kim-Cohen et al., 2003; Merikangas et al., 2010; Paus et al., 2008; Pollack et al., 1996). Children with mental health problems experience more academic difficulties, are more likely to be engaged in the justice and welfare systems, and are at elevated risk for suicide, the second leading cause of death among adolescents and young adults (Heron, 2016; Liu et al., 2011). The impact of mental health disorders extends beyond childhood as these are associated with lifelong implications including school drop-out, substance use, suicide risk, and recurrent unemployment (Fergusson and Woodward, 2002; Weissman et al., 1999). Recent trends indicate a near doubling in the rate of hospitalization for mental health concerns among children over the last decade demonstrating a significant rise in the prevalence of these disorders during childhood (Plemmons et al., 2018) spurring a need for more research about the etiology and possible prevention of these disorders.
While genetics, family history, socioeconomic status, and medical conditions play an important role in mental health disorders, environmental factors may also influence their development through oxidative stress and neuroinflammatory pathways (Ng et al., 2008; Vogelzangs et al., 2013). Air pollution is a common environmental exposure known to induce systemic inflammation and oxidative stress with adverse health consequences including respiratory and cardiovascular disease (Allen et al., 2017a; Block and Calderón-Garcidueñas, 2009; Block et al., 2012; Campbell et al., 2005; Chen et al., 2008; Chen and Schwartz, 2009; Costa et al., 2014, 2017; Dockery et al., 1993; Dockery, 2009; Dominici et al., 2006; Gauderman et al., 2007; Health Effects Institute, 2010; Lelieveld et al., 2015; Newman et al., 2013; Pope III and Dockery, 2006; Weuve et al., 2012). Recent toxicological studies demonstrate that some air pollutants, including particulate matter and elemental carbon, may affect the central nervous system through multiple mechanisms including neuroinflammation, microglial activation, and altered synaptic plasticity (Block and Calderón-Garcidueñas, 2009; Costa et al., 2014, 2017). Air pollution may also act through oxidative stress mechanisms to induce dopaminergic neurotoxitcity (Block and Calderón-Garcidueñas, 2009). Accumulating epidemiologic evidence supports the neurotoxic effects of air pollution with studies reporting associated cognitive deficits and externalizing behaviors in children, and accelerated cognitive decline in adults (Allen et al., 2017a; Block et al., 2012; Campbell et al., 2005; Chen and Schwartz, 2009; Costa et al., 2017; Newman et al., 2013; Weuve et al., 2012).
Less well studied, however, is the impact of air pollution on mental health symptoms, including depression and anxiety. Among adults, reports of air pollution and adverse mental health outcomes, including psychiatric emergency room visits, first appeared in the 1980s (Briere et al., 1983; Bullinger, 1989). Recent studies in the U.S., China, and South Korea, have reported that short-term increases in air pollution are linked to elevated risk for suicide, symptoms of depression and anxiety, and emergency department visits for mental health disorders in adults (Bakian et al., 2015; Chen et al., 2018; Lim et al., 2012; Pun et al., 2016; Szyszkowicz et al., 2009). In European cohorts, associations with long-term exposure to air pollution and depression have been inconsistent but are suggestive of a potentially harmful relationship (Vert et al., 2017; Zijlema et al., 2016).
Research among children and adolescents is sparse, though exposure to various components of air pollution has been associated with increased symptoms of depression and likelihood of depression diagnosis (Roberts et al., 2019) as well as treatment for a mental health disorder (Oudin et al., 2016). In addition, prenatal exposure to polycyclic aromatic hydrocarbons (PAHs) due to the combustion of fossil fuels, including air pollution and other organic materials has been associated with increased depression and anxiety in children at age 6–7 years (Perera et al., 2012).
Given the rising incidence of childhood mental health concerns and the scarcity of research specifically with children, the objective of our study was to examine the association between exposure to traffic-related air pollution (TRAP) across childhood and symptoms of depression and anxiety among children at age 12 years.
2. Methods
2.1. Study population
Study participants were drawn from the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a prospective cohort study of children and their parents residing in the Greater Cincinnati, Ohio region (LeMasters et al., 2006; Ryan et al., 2005). Birth records were obtained from the Ohio and Kentucky Departments of Health from 2001 to 2003. Study eligibility required having a birth record address located less than 400 m (defined as having an average daily truck count > 1000) or more than 1500 m from a major roadway. Eligibility also required having at least one biological parent with atopy confirmed by skin prick testing (LeMasters et al., 2006). Enrolled children and their parents completed clinical evaluations at ages 1, 2, 3, 4, 7, and 12 years that included physical assessments and surveys regarding the participants’ health and general wellbeing, housing characteristics, residential history, and secondhand smoke (SHS) exposure. The focus of the current study is on mental health outcomes, involving assessments of depression and anxiety collected at age 12 years. The study was approved by the Institutional Review Boards of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. Participants and parents provided informed assent and consent, respectively.
2.2. Traffic-related air pollution (TRAP) exposure
We estimated participants’ residential exposure to elemental carbon attributable to traffic sources (ECAT) using a previously developed and validated land-use regression (LUR) model (Ryan et al., 2007, 2008). Briefly, PM2.5, elemental carbon (EC), and elemental components of PM2.5 were measured at 27 sampling sites operated in the study region from 2001 to 2006 (Ryan et al., 2007). Using chemical mass balance and UNMIX models, the average fraction of elemental carbon in PM2.5 from traffic sources (ECAT) was determined for each sampling site and serves as our surrogate marker of the traffic-related air pollution mixture (Hu et al., 2006). We subsequently developed, validated, and applied a LUR model to estimate annual average concentrations of ECAT at participants’ homes from birth through 12 years (Ryan et al., 2007, 2008). For this analysis, we estimated: 1) prenatal/early life exposure based on LUR-derived ECAT concentrations at the birth address, 2) current exposure based on the home address at age 12 years, and 3) average childhood exposure based on all reported home addresses from birth through 12 years.
2.3. Outcome measures
Trained staff collected assessments of depression and anxiety symptoms at age 12 years. Parents completed the Behavior Assessment System for Children-2 (BASC-2) (Reynolds and Kamphaus, 2004) Parent Rating Scales that yields composite scores for Externalizing Problems, Internalizing Problems, Behavioral Symptoms, and Adaptive Skills, and subscale scores of which the Anxiety, Depression, Somatization, and Withdrawal scales were examined. Children completed the short form of the Children’s Depression Inventory-2 (CDI-2) (Kovacs, 1992) that assesses symptoms of depression within the past two weeks and yields a total depression score. Children also completed the Spence Children’s Anxiety Scale (SCAS) (Spence, 1998) that assesses six domains of anxiety including panic/agoraphobia, social phobia, separation anxiety, obsessive compulsive disorder, physical injury fears, and generalized anxiety, as well as a total anxiety score. All three scales yield age-standardized T-scores with means of 50 and standard deviations of 10.
BASC-2 forms were completed by the parents and were immediately reviewed for completeness. A trained examiner provided standard instructions to the children for completion of the CDI-2 and SCAS and allowed the children to complete the forms on their own. If the children had trouble reading the items or completing the forms, the examiner offered to read the items aloud and assisted with form completion. We used publisher-supplied software to score all instruments. Examiners addressed elevated depression scores, with both children and parents, and offered resources and assistance with referrals for further evaluation as needed.
2.4. Covariates
Parents completed the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996) to assess attitudes and symptoms specific to signs of depression; this assessment was considered in all models examining child depression outcomes. Parents also completed the Parenting Relationship Questionnaire (PRQ) (Kamphaus and Reynolds, 2008) to describe the parent-child relationship from the parent’s perspective. The PRQ yields T-scores for Attachment, Communication, Discipline Practices, Involvement, Parenting Confidence, Satisfaction with School, and Relational Frustration; this measure was considered in all models.
Exposure to lead and tobacco smoke have been linked with internalizing symptoms in children (Ashford et al., 2008; Bandiera et al., 2010; Bouchard et al., 2009; Yolton et al., 2008) and were considered in our models. Blood samples collected at age 12 years were analyzed for lead concentrations obtained by ICP-MS at the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health’s Wadsworth Center. Hair samples were collected at age 2 and 4 year study visits and analyzed for cotinine by radioimmunoassay at the Hospital for Sick Children (Toronto, Canada). The mean of 2-and 4-year cotinine concentrations was used to estimate early childhood SHS exposure.
2.5. Statistical analysis
Descriptive statistics and graphical plots were used to examine the distribution of all variables, examine potential outliers, and describe the population, exposures, and outcome measures. Means and standard deviations or medians with 25th and 75th percentiles are reported, as appropriate, for the continuous variables; frequencies are reported for categorical variables. Concentrations of hair cotinine below the analytic limit of detection (LOD) were replaced by the LOD divided by the square root of 2 (Hornung and Reed, 1990). Missing hair cotinine concentrations (n = 58) were imputed and replaced based on parent report of number of cigarettes smoked by household members (0, 1–10, 11–20, > 20) and available hair cotinine concentrations within each category.
We developed separate unadjusted linear regression models to assess associations between early, current, and average childhood TRAP exposures and each outcome. Adjusted linear regression models were subsequently developed for models with significant associations between TRAP exposure and mental health outcomes. Covariates considered for inclusion in adjusted models were selected based on prior literature or their potential roles as confounders of the relationship between TRAP exposure and mental health. These included maternal age at delivery, maternal education, parent marital status and depression (BDI-II); average household income from birth to 12 years, an index of community deprivation based on census-tract level SES data, PRQ scores; child age at study visit, sex, race; early childhood hair cotinine levels, and blood lead measured at 12 years. For each outcome, covariates were included in the adjusted models if significantly (p < 0.05) associated with the outcome or if inclusion resulted in a > 10% change in the ECAT parameter estimate. We assessed potential effect modification of ECAT exposure by child sex by including an interaction term in the adjusted models. Any covariate or effect modifier retained in one of the models for the mental health outcomes was included in all final models with the exception of the BDI-II which was only included in analyses of child depression. Given that the range of estimated ECAT exposures is approximately 0.2–0.9 μg/m3, effect estimates for ECAT exposure are presented for a 0.25 μg/m3 change in ECAT exposure. Statistical analyses were performed using SAS® version 9.4 (SAS Institute).
3. Results
3.1. Participant characteristics
A total of 344 children and their parents completed the study visit and were included in the analysis. Five children did not complete the self-report measures (CDI-2 and SCAS) because their visits were completed by mail; these instruments were skipped for mailed visits because we did not have the ability to provide necessary support if scores were elevated. Children were, on average, age 12.2 years, and 44.5% (n = 153) were female (Table 1). The majority (75.9%, n = 261) of participants were white, reflecting the racial distribution of the greater Cincinnati region. Mothers of participants were, on average, age 30.7 years at the time of the participants’ birth, and 78.4% completed high school prior to the age 12 study visit. There were no significant differences between participants who completed the age 12 study visit and those who did not (n = 419) with respect to sex, race, maternal education at enrollment, household income at enrollment, or exposure to ECAT at the birth record address (Supplementary Table 1). Average [SD] exposure to ECAT among the participants at the birth record address (0.39 [0.14] ug/m3) was similar to average exposure at the time of the age 12 study visit (0.37 [0.12] μg/m3), and average ECAT from birth through the age 12 study visit (0.38 [0.10] μg/m3) (Table 1). However, individual participant’s exposure to ECAT varied throughout childhood due to changes in residential location; the correlation between ECAT exposure at the birth record address and age 1 year was 0.96 while the correlations between ECAT exposure at birth and age 12 years and the average childhood exposure were 0.44 and 0.80, respectively.
Table 1.
Child, Maternal, and Household Characteristics of CCAAPS Participants (n = 344).
| Characteristic | Mean/n | SD/% |
|---|---|---|
| Child characteristics | ||
| Age at 12 y Study Visit (y) | 12.2 | 0.8 |
| Female | 153 | 44.5% |
| Race/Ethnicity | ||
| White | 261 | 75.9% |
| Black/More than One Race | 83 | 24.1% |
| Maternal characteristics | ||
| IQ (Wechsler Abbreviated Scale of Intelligence (WASI-2) | 105.2 | 13.3 |
| Depression (BDI-II) | 6.4 | 4.0 |
| Age at Study Enrollment (years) | 30.7 | 5.9 |
| Maternal Education at 12 y Study Visit | ||
| ≤ High School | 72 | 21.6% |
| Some College or Trade School | 94 | 28.1% |
| ≥ College Degree | 168 | 50.3% |
| Household/Parenting characteristics | ||
| Household Income at enrollment | ||
| < $20,000 | 58 | 17.4% |
| $20,000 to < $ 40,000 | 54 | 16.2% |
| $40,000 to < $70,000 | 96 | 28.8% |
| $70,000 to < $90,000 | 89 | 26.7% |
| > $90,000 | 36 | 10.8% |
| Parent Relationship Questionnaire (PRQ) T-scores | ||
| Attachment | 52.2 | 9.6 |
| Communication | 50.7 | 10.1 |
| Discipline Practices | 48.3 | 10.4 |
| Involvement | 52.3 | 11.3 |
| Parenting Confidence | 51.4 | 9.5 |
| Relational Frustration | 48.2 | 9.5 |
| ECAT exposure measures | ||
| Early Life ECAT at Birth Record Address (μg/m3) | 0.39 | 0.14 |
| Current ECAT at 12 y Study Visit (μg/m3) | 0.37 | 0.12 |
| Average Childhood ECAT; Birth to 12 y Study Visit (μg/m3) | 0.38 | 0.10 |
3.2. Mental health findings
Mental health outcomes ascertained from the CDI-2, SCAS, and BASC-2 are provided in Table 2. The prevalence of elevated depression scores (T ≥ 65) is 9.7% in this cohort. The prevalence of elevated SCAS scores (T ≥ 65) is less than 3% for all anxiety subscales. The prevalence of ‘at-risk’ BASC-2 scores (T ≥ 60) range from 8.1% to 14.2%. Correlations between child reported depression and anxiety, assessed by the CDI-2 and SCAS, respectively, and parent-reported child depression and anxiety, assessed by the BASC-2, were low (r = 0.24 and r = 0.35, respectively).
Table 2.
Summary of Child and Parent Reported Measures of Depression and Anxiety.
| Responder | Assessment | Index | na | Mean (SD) | n (%) > 65 |
|---|---|---|---|---|---|
| Child Reported | CDI-2 | Total Depression | 339 | 52.7 (10.2) | 33 (9.7) |
| SCAS | Total Anxiety | 339 | 44.2 (8.2) | 3 (0.9) | |
| Generalized Anxiety | 339 | 46.4 (6.6) | 1 (0.3) | ||
| Obsessive Compulsive Disorder | 339 | 46.0 (7.5) | 9 (2.6) | ||
| Panic/Agoraphobia | 339 | 46.9 (7.4) | 7 (2.1) | ||
| Fear of Physical Injury | 339 | 49.5 (8.2) | 9 (2.6) | ||
| Separation Anxiety | 339 | 45.4 (6.5) | 4 (1.2) | ||
| Social Phobia | 339 | 46.7 (8.0) | 5 (1.5) | ||
| Parent Reported | BASC-2 | Internalizing Composite | 344 | 50.6 (11.1) | 31 (9.0) |
| Depression Subscale | 344 | 49.9 (10.2) | 28 (8.1) | ||
| Anxiety Subscale | 344 | 52.1 (12.0) | 49 (14.2) | ||
| Somatization Subscale | 344 | 49.4 (11.4) | 30 (8.7) | ||
| Withdrawal Subscale | 344 | 50.0 (11.0) | 34 (9.9) |
Number of participants with completed assessments
3.3. TRAP and child-reported internalizing symptoms
Unadjusted analyses of prenatal/early life, current, and average childhood ECAT exposures identified significant associations with child-reported depression and anxiety outcomes (Supplementary Table 3). Prenatal/early life exposure to ECAT was univariately associated with elevated CDI-2 scores for total depression as well as SCAS scores for total anxiety, generalized anxiety, obsessive-compulsive disorders, and panic/agoraphobia (Supplementary Table 3). In addition, a positive association was observed between average childhood ECAT exposure and CDI-2 and SCAS generalized anxiety scores (Supplementary Table 3).
After adjustment for covariates (Fig. 1), prenatal/early life ECAT was significantly associated with increased CDI-2 T-scores for depression (β = 3.01, 95% CI 1.03, 4.99 per 0.25 μg/m3 increase in ECAT). Although not statistically significant in adjusted models, CDI-2 T-scores were elevated with increasing average childhood ECAT (β = 2.46, 95% CI −0.28, 5.20, p = 0.08).
Fig. 1.
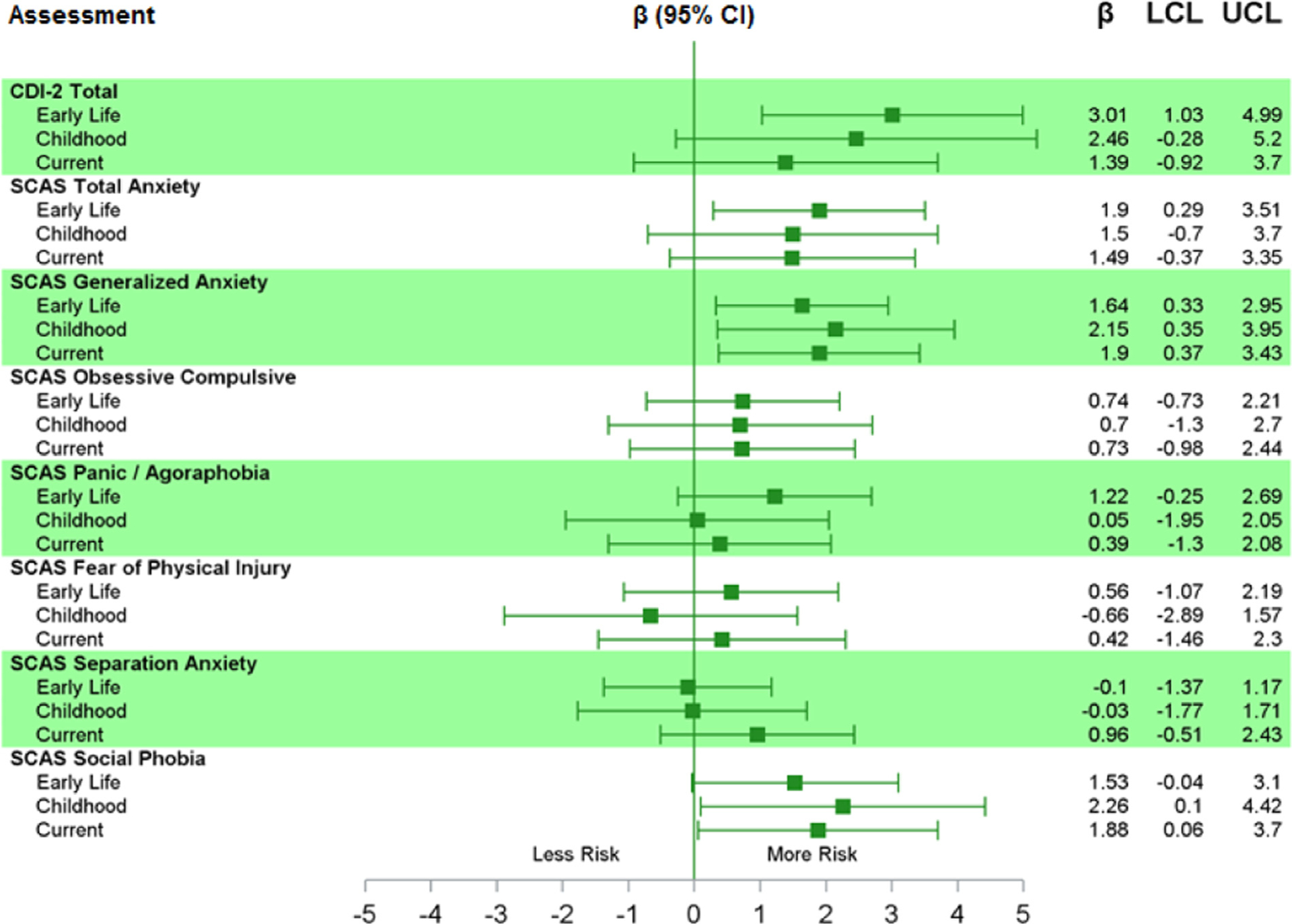
Adjusted* Parameter Estimates (per 0.25 μg/m3 increase) for the Association between Early, Current, and Average Childhood ECAT Exposure and Child-Reported Symptoms of Depression and Anxiety. *Adjusted for maternal age at delivery, average household income birth to 12 y study visit, maternal BDI-II scores, PRQ Relational Frustration T-score, child race, average hair cotinine from samples collected at ages 2 and 4 years.
In the final adjusted models, a 0.25 μg/m3 increase in ECAT exposure in prenatal/early life was significantly associated with a 1.90 point increase (95% CI 0.29, 3.51) in SCAS total anxiety T-score and a 1.64 point increase (95% CI 0.33, 2.95) in generalized anxiety T-score (Fig. 1). Current ECAT exposure was not associated with total anxiety, obsessive compulsive, panic/agoraphobia, fear of physical injury or separation anxiety T-scores, but was associated with increased generalized anxiety (β = 1.90, 95% CI 0.37, 3.43) and social phobia (β = 1.88, 95% CI 0.06, 3.70). A similar pattern was observed with average childhood exposure to ECAT with an estimated 2.15 point increase (95% CI 0.35, 3.95) in generalized anxiety T scores per 0.25 μg/ m3 increase in average childhood ECAT exposure and an estimated 2.26 point increase (95% CI 0.10, 4.42) in social phobia T scores per 0.25 μg/m3 increase in average childhood ECAT exposure.
3.4. TRAP and parent-reported internalizing behaviors
No significant associations were observed between ECAT exposures during the three time periods and the BASC-2 internalizing composite score or the depression, anxiety, somatization, or withdrawal subscales (Supplementary Table 2). Therefore, we did not conduct additional analyses of parent-reported (BASC-2) behaviors.
4. Discussion
In this longitudinal exposure study, TRAP, assessed through a measure of ECAT, was associated with increased self-reported symptoms of depression and anxiety at age 12 years. Though prior studies have found exposure to air pollution to be associated with cognitive deficits and increased behavior problems in children, and prior studies link air pollution to mental health symptoms in adults, to our knowledge this is the first epidemiologic study to identify associations between childhood exposure to TRAP and elevated depression and anxiety at age 12. The availability of residential addresses throughout childhood allowed us to examine exposures at varying time points, and we observed exposures occurring during early childhood to have the greatest association with depression at age 12. Similarly, exposure to TRAP during early childhood was the only period significantly associated with total anxiety, though nonsignificant trends were evident for TRAP exposure at age 12, as well as average childhood exposure, with total anxiety scores. Notably, increased generalized anxiety was consistently associated with TRAP exposure at all childhood time periods, and for social phobia, associations were positive and significant for current and average childhood exposure.
Our results support the hypothesis that air pollution exposure is associated with adverse mental health outcomes in children and are consistent with previous epidemiologic studies in adult populations (Briere et al., 1983; Bullinger, 1989; Lim et al., 2012; Power et al.,2015; Pun et al., 2016; Szyszkowicz et al., 2009). The majority of studies in adults, however, examined short-term exposure to air pollution and acute episodes of depression. Szyszkowicz et al. reported a 2–3% increased risk for emergency department visits for depression in the week following elevated ozone exposure across multiple cities in Canada (Szyszkowicz and Kousha, 2016). Similarly, an analysis of daily hospital admissions for mental health disorders in Shanghai, China reported a 1.27% and 1.88% increase in admissions per 10 μg/m3 increase in 2-day moving average PM10 and NO2 exposure, respectively (Chen et al., 2018). In Salt Lake City, UT the odds of suicide were significantly increased following elevated NO2 and PM2.5 1–3 days prior (Bakian et al., 2015).
Studies in adults have also reported associations between air pollution and mental health measured through surveys. In Seoul, South Korea, increased symptoms of depression were associated with elevated 3-day moving average exposures to PM10, NO2, and ozone (Lim et al., 2012). In a nationwide U.S. cohort of elderly adults, increased PM2.5 was significantly associated with both anxiety and depression, with 180 and 30-day moving averages being the most significant exposure windows (Pun et al., 2016). Three studies have examined longer-term exposure to air pollution and mental health outcomes. In the Nurses’ Health Study, significantly increased odds of anxiety symptoms were associated with higher exposure to PM2.5, with exposures occurring less than 1 year before symptoms being more significant than exposures 15 years before symptoms (Power et al., 2015). Long-term exposure to ozone and PM2.5 was also associated with the onset of depression in this study (Kioumourtzoglou et al., 2017). In contrast, a study of elderly adults in Boston, MA did not observe significant associations between long-term air pollution exposure and depression symptoms (Wang et al., 2014). These conflicting results may be due to differences in study populations, exposure assessment, and outcome measures.
Research among children and adolescents is meager but suggests exposure to air pollution is associated with increased symptoms of depression, depression diagnosis (Roberts et al., 2019), and treatment for mental health disorders (Oudin et al., 2016). Perera also reported that prenatal exposure to PAHs, that may include TRAP, is associated with increased depression and anxiety in children at age 6–7 years (Perera et al., 2012).
Prior studies have reported links between TRAP and cognition and executive function (Freire et al., 2009; Porta et al., 2016; Sunyer et al., 2015). Other studies, including CCAAPS, have also reported prenatal and childhood TRAP exposure to be significantly associated with behavior problems assessed at school-age including symptoms of attention-deficit hyperactivity disorder (Harris et al., 2016; Min and Min, 2017; Newman et al., 2013). In addition, studies have reported increased odds of autism spectrum disorders with higher prenatal exposures to traffic-related pollutants, PM2.5, and proximal distance to major roadways (Volk et al., 2011, 2013).
Multiple mechanisms by which air pollution may adversely affect the central nervous system and neurobehavior are biologically plausible including the induction of proinflammatory cytokines leading to neuroinflammation in the brain, endothelial dysfunction, disruption of the blood-brain barrier, oxidative stress, and neuronal damage (Block and Calderón-Garcidueñas, 2009; Brockmeyer and D’Angiulli, 2016; Calderón-Garcidueñas et al., 2008, 2016). With respect to depression and anxiety, air pollution may also induce dopaminergic and/or glutamatergic neurotoxicity. In mice, air pollution provokes behaviors that represent depression and anxiety (Bolton et al., 2013; Davis et al., 2013; Fonken et al., 2011).
While air pollution is a complex mixture of toxic compounds including gases, trace metals, and particulate matter of varying sizes, ultrafine particles (UFPs, particles < 100 nm in diameter) are of particular interest due to their dominant particle concentrations in ambient air, ability to bind toxic compounds to their large surface area, capability to translocate to other organ systems, and potential to directly impact the brain via the olfactory epithelium (Allen et al., 2017a, 2017b; Health Effects Institute, 2010). Experimental evidence in mice also supports the neurotoxicty of UFPs as recent evidence demonstrates UFP exposures lead to inflammation, microglial activation, disruption to white matter development, elevated glutamate, and other pathology (Allen et al., 2017b). The primary contributors to UFPs in ambient air are tailpipe emissions from mobile sources, particularly diesel fuel, and epidemiologic studies examining traffic-specific pollutants including NO2, black carbon, and ultrafine particles (UFP) are most consistent in their observed associations with adverse cognitive development.
We observed significant associations when examining child self-reported symptoms, but not parent-reported child behaviors. Incongruence between parent and child ratings of psychiatric symptoms have been previously reported in which children and adolescents reported more severe symptoms than their parents, possibly due to parents’ unawareness of symptom severity (Moretti et al., 1985; Stanger and Lewis, 1993). Angold and Nauta (Angold et al., 1987; Nauta et al., 2004) reported a discordance in anxiety measures to be greater among children in a non-clinical group than among children with a diagnosed anxiety disorder. By definition, internalizing symptoms are more diffi-cult for others to detect because they project inward. In contrast, externalizing symptoms, including hyperactivity and aggressive behaviors, project outward and are more evident to parents, teachers, and others. The observed low correlation between parent and child report of symptoms of depression and anxiety lends additional support to the possibility that parents may be unaware of their children’s experience of these internalizing symptoms.
Our study has some important strengths including its longitudinal design and collection of complete childhood address histories allowing us to estimate exposures from birth through age 12 years. Our findings suggest early life exposure, as estimated at the birth record address, represents the most relevant period of exposure with respect to depression outcomes, and was also significantly associated with anxiety symptoms. Though we did not capture residential mobility during the entire pregnancy, due to temporal proximity it is likely that TRAP exposure estimated at the birth record address is highly correlated with TRAP exposures during the third trimester, a previously identified period of enhanced vulnerability to the neurotoxic effects of air pollution (Allen et al., 2017b). The comprehensive assessment of multiple covariates, including SHS exposure, maternal depression, and the parent-child relationship, is another strength of the study.
Our assessment of depression and anxiety at age 12 years is also an important component to our design given that adolescence is a developmental period during which the incidence of mental health problems increases (Paus et al., 2008). The prevalence of depression among adolescents has been estimated at rates of 5–14% (Birmaher et al., 1996; Jellinek and Snyder, 1998; Merikangas et al., 2010), and anxiety has been estimated at 32% (Merikangas et al., 2010). These rates appear to be increasing from generation to generation, with earlier onset ages (Gershon et al., 1987; Gotlib and Hammen, 2014). While multiple factors influence an individuals’ risk for adverse mental health outcomes, environmental factors contributing to depression and anxiety in children are poorly understood. Identifying and intervening on modifiable environmental exposures, including air pollution, associated with childhood depression and anxiety is a significant public health challenge given that childhood mental health disorders often persist into adulthood, leading to continued depressive and anxiety disorders, suicidal behavior and psychiatric hospitalization as well as academic failure, recurrent unemployment, and relationship difficulties. Like adults, children with psychiatric disorders are often undertreated; 70–80% of depressed children receive no treatment (Cicchetti and Toth, 1998). Identification of air pollution as a contributor to the increased prevalence of these disorders during childhood may provide additional leverage to inform policy around air quality.
It is important to note that the observed increase in reported symptoms in our cohort of typically developing children is relatively small, 3-points and 2-points, for depression and total anxiety, respectively. These are equivalent to one-third to one-fourth of a standard deviation in the T-scores, respectively, and are not likely to result in a clinical diagnosis of a mental health disorder in a low-risk sample like ours. However, even small effects of exposure may have a substantial impact at the population level by shifting the population distribution and resulting in increased risk of poor outcomes (Bellinger, 2012).
The study is not without limitations. We acknowledge attrition of the cohort over time, which is common in longitudinal research, but we did not find significant differences between those who completed the study at 12 and those who did not. We estimated TRAP exposure at the homes of study participants, and therefore there may be some exposure misclassification due to time spent away from the home. However, we expect this exposure misclassification to be non-differential, and therefore bias is toward the null. Future studies may also consider underlying genetic susceptibility or modifying factors that may increase susceptibility to air pollution including stress and APOE4 gene variants (Calderón-Garcidueñas et al., 2018; Cooney, 2011). Additional limitations include the potential for residual confounding due to unmeasured covariates that may affect neurobehavior and the use of a single pollutant (ECAT) as our air pollution exposure metric. However, previous studies support the hypothesis that air pollutants emitted from traffic, including elemental and black carbon, NO2, and UFPs are most consistently linked to adverse neurobehavioral outcomes.
5. Conclusions
Our results demonstrate that early life exposure to TRAP is associated with self-reported symptoms of depression and anxiety at age 12 years when controlling for relevant covariates. In addition, current exposure to TRAP at age 12 years, and average exposure across childhood, is associated with self-reported symptoms of generalized anxiety and social phobia when controlling for relevant covariates. To our knowledge, these findings are the first to demonstrate that childhood exposure to TRAP is associated with mental health symptoms during adolescence and adds to the growing body of epidemiologic evidence for the role of air pollution in neurobehavioral and mental health disorders.
Supplementary Material
Acknowledgements
We thank the CCAAPS participants for their time and contribution to this research.
Funding
This work was supported by the National Institutes of Environmental Health Sciences (NIEHS R01 ES019890, R01 ES11170, R01 ES019890, P30 ES006069, and T32 ES10957).
Abbreviations:
- BASC-2
Behavior Assessment System for Children-2
- BDI-II
Beck Depression Inventory-II
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- CDI-2
Child Depression Inventory-2
- ECAT
elemental carbon attributable to traffic
- LOD
limit of detection
- LUR
land-use regression
- NO2
nitrogen dioxide
- PM
particulate matter
- PRQ
Parenting Relationship Questionnaire
- SCAS
Spence Children’s Anxiety Scale
- SD
standard deviation
- SHS
secondhand smoke
- TRAP
Traffic-related air pollution
- UFP
ultrafine particle
Footnotes
Declarations of interest
None.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2019.03.005.
References
- Allen J, Klocke C, Morris-Schaffer K, Conrad K, Sobolewski M, Cory-Slechta D, 2017a. Cognitive effects of air pollution exposures and potential mechanistic underpinnings. Curr. Environ. Health Rep 4, 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, et al. , 2017b. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 59, 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Weissman MM, John K, Merikancas KR, Prusoff BA, Wickramaratne P, et al. , 1987. Parent and child reports of depressive symptoms in children at low and high risk of depression. J. Child Psychol. Psychiatry 28, 901–915. [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. , 2013. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat 9, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford J, Van Lier PA, Timmermans M, Cuijpers P, Koot HM, 2008. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J. Am. Acad. Child Adolesc. Psychiatry 47, 779–787. [DOI] [PubMed] [Google Scholar]
- Bakian AV, Huber RS, Coon H, Gray D, Wilson P, McMahon WM, et al. , 2015. Acute air pollution exposure and risk of suicide completion. Am. J. Epidemiol 181, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera FC, Arheart KL, Caban-Martinez AJ, Fleming LE, McCollister K, Dietz NA, et al. , 2010. Secondhand smoke exposure and depressive symptoms. Psychosom. Med 72, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck Depression Inventory -II The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bellinger DC, 2012. A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environ. Health Perspect 120, 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, 1996. Childhood and adolescent depression: a review of the past 10 years. Part II. J. Am. Acad. Child Adolesc. Psychiatry 35, 1575–1583. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L, 2009. Air pollution: mechanisms of neuroinflammation and cns disease. Trends Neurosci. 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen J-C, et al. , 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, et al. , 2013. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ. Health Perspect 121, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, et al. , 2009. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in us young adults. Arch. General. Psychiatry 66, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J, Downes A, Spensley J, 1983. Summer in the city: urban weather conditions and psychiatric emergency-room visits. J. Abnorm. Psychol 92, 77. [DOI] [PubMed] [Google Scholar]
- Brockmeyer S, D‘Angiulli A, 2016. How air pollution alters brain development: the role of neuroinflammation. Transl. Neurosci 7, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger M, 1989. Psychological effects of air pollution on healthy residents – a time-series approach. J. Environ. Psychol 9, 103–118. [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, et al. , 2008. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol Pathol. 36, 289–310. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Leray E, Heydarpour P, Torres-Jardón R, Reis J, 2016. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: the clinical impact on children and beyond. Rev. Neurol 172, 69–80. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Gónzalez-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Mukherjee PS, Kulesza RJ, et al. , 2018. Hallmarks of alzheimer disease are evolving relentlessly in metropolitan mexico city infants, children and young adults. Apoe4 carriers have higher suicide risk and higher odds of reaching nft stage v at ≤ 40 years of age. Environ. Res 164, 475–487. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy S, Meacher D, Sioutas C, et al. , 2005. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 26, 133–140. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA, 1996. Behavioral observations at age 3 years predict adult psychiatric disorders: Longitudinal evidence from a birth cohort. Arch. General. Psychiatry 53, 1033. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu C, Chen R, Wang W, Li W, Kan H, et al. , 2018. Ambient air pollution and daily hospital admissions for mental disorders in shanghai, china. Sci. Total Environ 613, 324–330. [DOI] [PubMed] [Google Scholar]
- Chen H, Goldberg M, Villeneuve PJ, 2008. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev. Environ. Health 23, 243–298. [DOI] [PubMed] [Google Scholar]
- Chen J-C, Schwartz J, 2009. Neurobehavioral effects of ambient air pollution on cognitive performance in us adults. Neurotoxicology 30, 231–239. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, 1998. The development of depression in children and adolescents. Am. Psychol 53, 221–241. [DOI] [PubMed] [Google Scholar]
- Cooney CM, 2011. Stress–Pollution Interactions: An Emerging Issue in Children’s Health Research National Institute of Environmental Health Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang Y-C, Dao K, Roque P, 2014. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed. Res. Int 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang Y-C, Dao K, Roqué PJ, 2017. Neurotoxicity of traffic-related air pollution. Neurotoxicology 59, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, et al. , 2013. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. PloS One 8, e64128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. , 1993. An association between air pollution and mortality in six us cities. New Engl. J. Med 329, 1753–1759. [DOI] [PubMed] [Google Scholar]
- Dockery DW, 2009. Health effects of particulate air pollution. Ann. Epidemiol 19, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. , 2006. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama 295, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, 2002. Mental health, educational, and social role outcomes of adolescents with depression. Arch. General. Psychiatry 59, 225–231. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. , 2011. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippo-campal cytokine expression and morphology. Mol. Psychiatry 16, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Ramos R, Puertas R, Lopez-Espinosa M-J, Julvez J, Aguilera I, et al. , 2009. Association of traffic-related air pollution with cognitive development in children. J. Epidemiol. Community Health 2008, 084574. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. , 2007. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 369, 571–577. [DOI] [PubMed] [Google Scholar]
- Gershon ES, Hamovit JH, Guroff JJ, Nurnberger JI, 1987. Birth-cohort changes in manic and depressive disorders in relatives of bipolar and schizoaffective patients. Arch. General. Psychiatry 44, 314–319. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hammen CL, 2014. Handbook of Depression, Third ed. Guilford Press, New York, NY. [Google Scholar]
- Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. , 2016. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicology Teratol. 57, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Effects Institute, 2010. Traffic-Related Air Pollution: a Critical Review of the Literature on Emissions, Exposure, and Health Effects. Health Effects Institute [Google Scholar]
- Heron M, 2016. Deaths: Leading Causes for 2014 (National Vital Statistics Report). National Cetner for Health Statistics, Hyattsville, MD. [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Hu S, McDonald R, Martuzevicius D, Biswas P, Grinshpun SA, Kelley A, et al. , 2006. Unmix modeling of ambient PM2.5 near an interstate highway in cincinnati, OH, USA. Atmos. Environ 40, 378–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinek MS, Snyder JB, 1998. Depression and suicide in children and adolescents. Pediatr. Rev./Am. Acad. Pediatr 19, 255–264. [DOI] [PubMed] [Google Scholar]
- Kamphaus RW, Reynolds CK, 2008. Parenting Relationship Questionnaire Pearson Clinical Assessment, SanAntonio, TX. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch. General. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R, 2003. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch. General. Psychiatry 60, 709–717. [DOI] [PubMed] [Google Scholar]
- Kioumourtzoglou M-A, Power MC, Hart JE, Okereke OI, Coull BA, Laden F, et al. , 2017. The association between air pollution and onset of depression among middle-aged and older women. Am. J. Epidemiol 185, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, 1992. The Children’s Depression Inventory Pearson Assessments, Eagan, MN. [Google Scholar]
- Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A, 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525, 367. [DOI] [PubMed] [Google Scholar]
- LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. , 2006. High prevalence of aeroallergen sensitization among infants of atopic parents. J. Pediatr 149, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y-H, Kim H, Kim JH, Bae S, Park HY, Hong Y-C, 2012. Air pollution and symptoms of depression in elderly adults. Environ. Health Perspect 120, 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen X, Lewis G, 2011. Childhood internalizing behaviour: analysis and implications. J. Psychiatr. Ment. Health Nurs 18, 884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J-p, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. , 2010. Lifetime prevalence of mental disorders in us adolescents: results from the national comorbidity survey replication–adolescent supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J. y., Min K. b., 2017. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int 99, 221–227. [DOI] [PubMed] [Google Scholar]
- Moretti M, Fine S, Haley G, Marriage K, 1985. Childhood and adolescent depression: child-report versus parent-report information. J. Am. Acad. Child Psychiatry 24, 298–302. [DOI] [PubMed] [Google Scholar]
- Nauta MH, Scholing A, Rapee RM, Abbott M, Spence SH, Waters A, 2004. A parent-report measure of children’s anxiety: psychometric properties and comparison with child-report in a clinic and normal sample. Behav. Res. Ther 42, 813–839. [DOI] [PubMed] [Google Scholar]
- Newman NC, Ryan P, LeMasters G, Levin L, Bernstein D, Hershey GKK, et al. , 2013. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ. Health Perspect 121, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI, 2008. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol 11, 851–876. [DOI] [PubMed] [Google Scholar]
- Oudin A, Bråbäck L, Åström DO, Strömgren M, Forsberg B, 2016. Association between neighbourhood air pollution concentrations and dispensed medication for psychiatric disorders in a large longitudinal cohort of swedish children and adolescents. BMJ Open 6, e010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN, 2008. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, et al. , 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ. Health Perspect 120, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemmons G, Hall M, Doupnik S, Gay J, Brown C, Browning W, et al. , 2018. Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics e20172426. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, et al. , 1996. Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am. J. Psychiatry 153, 376–381. [DOI] [PubMed] [Google Scholar]
- C.A. Pope III, D.W. Dockery, 2006. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manag. Assoc 56, 709–742. [DOI] [PubMed] [Google Scholar]
- Porta D, Narduzzi S, Badaloni C, Bucci S, Cesaroni G, Colelli V, et al. , 2016. Air pollution and cognitive development at age 7 in a prospective italian birth cohort. Epidemiology 27, 228–236. [DOI] [PubMed] [Google Scholar]
- Power MC, Kioumourtzoglou M-A, Hart JE, Okereke OI, Laden F, Weisskopf MG, 2015. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. bmj 350, h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Manjourides J, Suh H, 2016. Association of ambient air pollution with depressive and anxiety symptoms in older adults: Results from the nshap study. Environ. Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW, 2004. Behavior Assessment System for Children, second ed. American Guidance Services Publishing, Circle Pines, MN. [Google Scholar]
- Roberts S, Arseneault L, Barratt B, Beevers S, Danese A, Odgers CL, et al. , 2019. Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in london-based children from a uk longitudinal cohort study. Psychiatry Res. 272, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, LeMasters G, Biagini J, Bernstein D, Grinshpun S, Shukla R, et al. , 2005. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J. Allergy Clin. Immunol 116, 279–284. [DOI] [PubMed] [Google Scholar]
- Ryan P, LeMasters G, Biswas P, Levin L, Hu S, Lindsey M, et al. , 2007. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ. Health Perspect 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PH, LeMasters GK, Levin L, Burkle J, Biswas P, Hu S, et al. , 2008. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci. Total Environ 404, 139–147. [DOI] [PubMed] [Google Scholar]
- SAS Institute I. Statistical analysis software, version 9.4 Cary, NC. [Google Scholar]
- Spence SH, 1998. A measure of anxiety symptoms among children. Behav. Res. Ther 36, 545–566. [DOI] [PubMed] [Google Scholar]
- Stanger C, Lewis M, 1993. Agreement among parents, teachers, and children on internalizing and externalizing behavior problems. J. Clin. Child Psychol 22, 107–116. [Google Scholar]
- Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, López-Vicente M, et al. , 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 12, e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz M, Rowe B, Colman I, 2009. Air pollution and daily emergency department visits for depression. Int. J. Occup. Med. Environ. Health 22, 355–362. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M, Kousha T, 2016. Air pollution and emergency department visits for headache and migraine. Health Scope. [Google Scholar]
- Vert C, Sánchez-Benavides G, Martínez D, Gotsens X, Gramunt N, Cirach M, et al. , 2017. Effect of long-term exposure to air pollution on anxiety and depression in adults: a cross-sectional study. Int. J. Hyg. Environ. Health 220, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman A, De Jonge P, Penninx B, 2013. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiatry 3, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R, 2011. Residential proximity to freeways and autism in the charge study. Environ. Health Perspect 119, 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R, 2013. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Eliot MN, Koutrakis P, Gryparis A, Schwartz JD, Coull BA, et al. , 2014. Ambient air pollution and depressive symptoms in older adults: results from the mobilize boston study. Environ. Health Perspect 122, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, et al. , 1999. Depressed adolescents grown up. Jama 281, 1707–1713. [DOI] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F, 2012. Exposure to particulate air pollution and cognitive decline in older women. Arch. Intern. Med 172, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Khoury J, Hornung R, Dietrich K, Succop P, Lanphear B, 2008. Environmental tobacco smoke exposure and child behaviors. J. Dev. Behav. Pediatr 29, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlema W, Wolf K, Emeny R, Ladwig K, Peters A, Kongsgård H, et al. , 2016. The association of air pollution and depressed mood in 70,928 individuals from four european cohorts. Int. J. Hyg. Environ. Health 219, 212–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


