Abstract
Background
Primary percutaneous coronary intervention (PPCI) is the preferred treatment for ST‐segment elevation myocardial infarction. Although coronary flow is restored after PPCI, impaired myocardial perfusion (known as no‐reflow) related to poor clinical outcomes is frequently observed. To overcome this phenomenon, drugs, such as atorvastatin, abciximab and others, have been tried as adjunctive treatment to PPCI. Among these drugs, verapamil and adenosine are among the most promising. No other systematic reviews have examined use of these two drugs in people with acute myocardial infarction (AMI) undergoing PPCI. This is an update of the version previously published (2013, Issue 6), for which the people of interest in the review were those treated with PPCI ‐ not those given fibrinolytic therapy.
Objectives
To study the impact of adenosine and verapamil on no‐reflow during PPCI in people with AMI.
Search methods
We updated searches of the following databases in June 2014 without language restriction: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Web of Science and BIOSIS, China National Knowledge Infrastructure and clinical trials registers (ClinicalTrials.gov, Current Controlled Trials, Australian and New Zealand Clinical Trials Registry, the World Health Organization (WHO) International Clinical Trials Registry Platform). We also handsearched The American Journal of Cardiology.
Selection criteria
We selected randomised controlled trials (RCTs) in which adenosine or verapamil was the primary intervention. Participants were individuals diagnosed with AMI who were undergoing PPCI.
Data collection and analysis
Two review authors collected studies and extracted data. When necessary, we contacted trial authors to obtain relevant information. We calculated risk ratios (RRs), P values and 95% confidence intervals (CIs) of dichotomous data.
Main results
We included in our review 11 RCTs (one new study with 59 participants) involving 1027 participants. Ten RCTs were associated with adenosine and one with verapamil. We considered the overall risk of bias of included studies to be moderate. We found no evidence that adenosine reduced short‐term all‐cause mortality (RR 0.61, 95% CI 0.25 to 1.48, P value = 0.27), long‐term all‐cause mortality (RR 0.78, 95% CI 0.22 to 2.74, P value = 0.70), short‐term non‐fatal myocardial infarction (RR 1.32, 95% 0.33 to 5.29, P value = 0.69) or myocardial blush grade (MBG) 0 to 1 after PPCI (RR 0.96, 95% CI 0.76 to 1.22, P value = 0.75). The incidence of thrombolysis in myocardial infarction (TIMI) flow grade < 3 after PPCI (RR 0.62, 95% CI 0.42 to 0.91, P value = 0.01) was decreased. Conversely, adverse events with adenosine, such as bradycardia (RR 6.32, 95% CI 2.98 to 13.41, P value < 0.00001), hypotension (RR 11.43, 95% CI 2.75 to 47.57, P value = 0.0008) and atrioventricular (AV) block (RR 6.78, 95% CI 2.15 to 21.38, P value = 0.001), were significantly increased.
Meta‐analysis of verapamil as treatment for no‐reflow during PPCI was not performed because data were insufficient.
Authors' conclusions
It is difficult to draw conclusions because of the insufficient quality and quantity of current research studies. We considered the overall risk of bias of included studies to be moderate. Adenosine as treatment for no‐reflow during PPCI could reduce angiographic no‐reflow (TIMI flow grade < 3) but was found to increase adverse events. What's more, no evidence could be found to suggest that adenosine reduced all‐cause mortality, non‐fatal myocardial infarction or the incidence of myocardial blush grade 0 to 1. Additionally, the efficacy of verapamil for no‐reflow during PPCI could not be analysed because data were insufficient. Further clinical research into adenosine and verapamil is needed because of the limited numbers of available trials and participants.
Plain language summary
Drugs used as add‐on therapy to heart procedure following heart attack
Acute myocardial infarction (AMI), or heart attack, is one of the major causes of mortality worldwide. Approximately one‐third of people suffering heart attacks die before they reach the hospital. Primary percutaneous coronary intervention (PPCI) is a procedure whereby the coronary artery is widened without surgery, using a stent. Although this procedure restores blood flow through the coronary artery, perfusion through all areas of the heart may not occur. This is known as no‐reflow. To try to resolve this problem, healthcare workers have tried drugs such as adenosine and verapamil as add‐on treatment. We include 11 studies in this review with a total of 1027 participants. Ten studies compared adenosine or placebo as an addition to PPCI, and one compared verapamil or placebo.
No evidence suggests that adenosine reduced short‐term or long‐term all‐cause mortality or non‐fatal myocardial infarction to a greater extent than placebo treatment. However, we found evidence to show that adenosine can decrease angiographic no‐reflow after PPCI. Conversely, we also found evidence indicating that adverse events (bradycardia, hypotension, atrioventricular block) were increased with adenosine, although these effects were short‐lived. Included studies were few, with low numbers of participants and a relatively short period of follow‐up, and event rates of mortality and angiographic no‐reflow were low. Our meta‐analyses therefore had insufficient numbers to support a clear‐cut conclusion about the impact of adenosine in people with AMI undergoing PPCI.
Background
Description of the condition
Acute myocardial infarction (AMI), one of the major causes of mortality worldwide, can be defined from different perspectives related to clinical, electrocardiographic, cardiac biomarker and pathological characteristics. Different types of myocardial infarction have been identified: Type 1 is defined as spontaneous myocardial infarction related to ischaemia due to a primary coronary event such as plaque erosion and/or rupture, fissuring or dissection (Thygesen 2007). It is estimated that more than three million people have acute ST‐elevation myocardial infarction (STEMI) each year, and more than four million have non‐ST‐elevation myocardial infarction (White 2008). Approximately one‐third of people with myocardial infarction die before they reach the hospital and can receive effective treatment (Huikuri 2001). Rapid reperfusion of the infarcted coronary artery is required in STEMI and can be carried out by pharmacological or mechanical therapy; primary percutaneous coronary intervention (PPCI) is accepted as superior to fibrinolysis (Keeley 2003; Weaver 1997; Zijlstra 1993). People of interest for this review included those having PPCI ‐ not those given fibrinolytic therapy. PPCI is associated with normal epicardial flow in more than 90% of patients (Boersma 2006; Simes 1995). More than 50% of PPCI patients had door‐to‐balloon time (from time of arrival at the initial hospital to time of first balloon inflation at the PPCI hospital) of within 90 minutes (Rathore 2009), and less than 5% of interhospital‐transferred patients had undergone PPCI within 90 minutes after first medical contact (Boersma 2006). Although epicardial coronary flow can be restored after PPCI, impaired myocardial perfusion, known as the no‐reflow phenomenon, is frequently observed. The pathogenetic components of no‐reflow are very complicated and include a variable combination of distal atherothrombotic embolisation, ischaemic injury, reperfusion‐related injury and susceptibility of coronary microcirculation to injury (Niccoli 2009a). Diagnosis of this phenomenon can be confirmed by angiography, electrocardiography, myocardial contrast echocardiography and cardiac magnetic resonance (Niccoli 2009a). Prevalence ranges from 5% to 50% after PPCI, depending on the assessment methods used (Eeckhout 2001; Rezkalla 2008). In the present meta‐analysis, angiography was used to identify the no‐reflow phenomenon because data were available across all included studies. Angiographic no‐reflow can be defined as (1) a thrombolysis in myocardial infarction (TIMI) flow grade < 2 despite vessel patency and absence of dissection, spasm or distal macroembolus, or (2) a myocardial blush grade (MBG) of 0 to 1 (Niccoli 2009a). More important, AMI with no‐reflow is strongly associated with poor clinical outcomes (Henriques 2003; Morishima 1995).
Description of the intervention
To improve myocardial perfusion or to prevent no‐reflow after PPCI, many drugs, such as atorvastatin, tirofiban and others, have been tried clinically as adjunctive treatment to PPCI (Kunadian 2008). Both adenosine and verapamil have vasodilatory effects and have been commonly studied (Kunadian 2008).
Adenosine, an endogenous nucleoside, has vasodilatory actions on both arteries and arterioles, mediated through adenosine A2A and A2B receptors (Forman 2006). It reduces the release of vasoconstrictors by inhibiting activation of neutrophils and platelets, and it prevents endothelial damage and microvascular spasm, both of which contribute to the cardioprotective and antithrombotic effects of adenosine (Berne 1980; Minamino 1998). Adenosine can decrease free radical formation, thereby limiting the degree of myocardial injury (Forman 2006). Moreover, under some circumstances, it may act as an antiarrhythmic agent by blocking the atrioventricular (AV) nodes via adenosine A receptors, and it has inhibitory effects on the central nervous system (Mustafa 2009). Because of the extremely short half‐life of intravascular adenosine (one to two seconds in humans), its adverse effects are very short‐lived (Forman 2006). Adverse effects of intracoronary adenosine in people with AMI include hypotension (14.3%), bradycardia (20%), second‐degree AV block (18.5%) and bronchospasm (0.6%). Fortunately, these adverse effects last for only two to three minutes after adenosine is administered (Fokkema 2009).
Verapamil, a calcium channel blocker, can decrease oxygen demand by lowering heart rate and arterial pressure, and can improve myocardial perfusion by relieving microvascular spasm (Taniyama 1997), which may subsequently reduce infarct size. Furthermore, verapamil may have a direct effect on calcium flux across the sarcolemmal membrane or within intracellular compartments by enhancing calcium homeostasis, which could be protective of reversibly injured myocardial cells, in addition to its vasodilatory effect and its inhibitory effect on platelet aggregation during AMI (Ikeda 1981; Taniyama 1997). Verapamil has been used as an antiarrhythmic agent because of its action on AV nodes (Akhtar 1989; Singh 1987) and AV block. Side effects are more common with verapamil than with adenosine (Vijayalakshmi 2006).
How the intervention might work
Reperfusion‐related injury after PPCI has been associated with high activated neutrophil count, and with activation and aggregation of platelets, and can result in damage to the endothelium, plugging of capillaries, production of oxygen free radicals and release of potent vasoconstrictors such as thromboxane‐A, endothelin‐1 and other inflammatory mediators. These can subsequently contribute to sustained spasm of the coronary microcirculation (Niccoli 2009a). Also, calcium overload can occur in myocytes. To treat patients and prevent the no‐reflow phenomenon, adenosine and verapamil have been used as pharmacological strategies (Niccoli 2010), as they are effective with different mechanisms of no‐reflow by inducing vasodilation, antithrombotic effects and anti‐inflammatory effects, and by decreasing free radical production and calcium overload in myocytes. Adenosine is safe and feasible in people with AMI as an adjunct to PPCI, and it can decrease the incidence of no‐reflow (Marzilli 2000). Similarly, verapamil can reverse no‐reflow after PPCI (Werner 2002).
According to the European Society of Cardiology guidelines for AMI (Van de Werf 2008), adenosine and verapamil have been endorsed as treatments for no‐reflow with a class IIb recommendation at evidence levels of B and C, respectively.
Why it is important to do this review
First, AMI with no‐reflow after PPCI is difficult to treat and is strongly associated with poor clinical in‐hospital and long‐term outcomes (Niccoli 2009a; Niccoli 2010). Second, randomised controlled trials of adenosine and verapamil in people with AMI who are undergoing PPCI have been conducted for many years (Fokkema 2009; Grygier 2011; Marzilli 2000; Micari 2005; Petronio 2005; Taniyama 1997). However, their efficacy for this patient group has not been systematically reviewed to date. This review was undertaken to summarise available evidence and to provide important clinical guidance for management of no‐reflow with AMI after PPCI.
Objectives
To study the impact of adenosine and verapamil on no‐reflow during PPCI in people with AMI.
Methods
Criteria for considering studies for this review
Types of studies
We selected randomised controlled trials only.
Types of participants
We included in our review people diagnosed with AMI and undergoing PPCI, irrespective of age, sex and ethnic group. We also included individuals with suboptimal reperfusion after PPCI. As this review focuses on the impact of adenosine or verapamil on patients given PPCI, we excluded those with AMI receiving emergency thrombolytic therapy before PPCI. As asthma, renal impairment, malignant disease and haemodynamic instability may influence the outcomes of interest, we also excluded studies that included participants with these conditions.
Haemodynamic instability is defined as systolic blood pressure persistently < 90 mmHg or mean arterial pressure < 60 mmHg, or as the need to use inotropic drugs or an intra‐aortic balloon pump to keep systolic pressure at ≥ 90 mmHg.
Types of interventions
Comparison: adenosine or verapamil versus placebo.
We included trials in which adenosine or verapamil was compared with placebo, regardless of dosage, frequency, duration or route of administration (intravenous or intracoronary).
Types of outcome measures
As case‐specific event rates are not available at the same time point for every study, we defined short term as four weeks to six weeks, and defined long term as six months to eighteen months.
Primary outcomes
All‐cause mortality at short term and at long term.
Non‐fatal myocardial infarction at short term and at long term.
Secondary outcomes
Thrombolysis in myocardial infarction (TIMI) flow grade < 3 after PPCI and at short term or at discharge.
Myocardial blush grade (MBG) 0 to 1 after PPCI and at short term or at discharge.
Adverse events of specific interest, including hypotension, bradycardia and AV block (all types).
Economic outcome: treatment costs (US dollars (USD)).
Quality of life.
Search methods for identification of studies
Electronic searches
We searched for randomised controlled trials in the following electronic databases from the date of last research included in the previously published version of the review in Febuary 2012 to 25 June 2014 for the update.
Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 5).
MEDLINE Ovid (1948 to Week 3 June 2014).
EMBASE Classic and EMBASE Ovid (1947 to 25 June 2014).
Information for Scientific Information (ISI) Web of Science (1970 to 25 June 2014).
ISI BIOSIS Previews (1969 to 25 June 2014).
China National Knowledge Infrastructure (CNKI) (http://www.cnki.net/) (1980 to 25 June 2014).
We have applied the Cochrane RCT filter to MEDLINE (sensitivity‐maximising) and EMBASE, and an adaptation of it to Web of Science and BIOSIS (Lefebvre 2011).
Please see Appendix 1 for search strategies used in this update, and Appendix 2 for details of the original search conducted in 2012.
We applied no language restrictions, and we had non‐English language papers translated for full assessment.
Searching other resources
We searched the following clinical trial registers on 25 June 2014.
ClinicalTrials.gov (http://www.clinicaltrials.gov/).
Current Controlled Trials (http://www.controlled‐trials.com/isrctn/).
Australian and New Zealand Clinical Trials Registry (ANZCTR) (http://www.anzctr.org.au/).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch/).
We handsearched The American Journal of Cardiology from 2000 to 2014.
Data collection and analysis
Selection of studies
Two review authors (NTS and LL) independently screened titles and abstracts. When potentially relevant abstracts were identified, we retrieved the full articles to assess whether they met our inclusion criteria. Review authors resolved disagreements by discussion, or by consultation with a third review author (SQ), until we reached a consensus. Two review authors (LL and SQ) translated Chinese language papers.
Data extraction and management
Two review authors (NTS and SQ) independently extracted data from the trial reports, using a data extraction form. When we could not resolve disagreements by discussion, we consulted a third review author (LL) from our team, or we contacted study authors for clarification. We entered the following information onto the data extraction form.
General information: record number, trial identification number, date of publication.
Characteristics of the included trial: design, allocation concealment, duration.
Participants: diagnosis, numbers of people in experimental and control groups, age, sex, withdrawals/losses to follow‐up (reason/description).
Interventions: dose, route and timing of administration.
Outcomes: as defined above, with length of follow‐up.
For binary data, we extracted the total number of participants (denominator) and the number of events (numerator) in each group.
Assessment of risk of bias in included studies
Two review authors (NTS and SQ) independently assessed risk of bias of the included studies under the guidance of the Cochrane Heart Group, by using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook). We resolved disagreements by discussion, or we contacted study authors to obtain further information. We present risk of bias assessment tables for the following domains (Cochrane Handbook).
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
Each domain can be categorised as having low, unclear or high risk of bias.
Measures of treatment effect
We analysed extracted data using Review Manager 5 software. For dichotomous outcomes, we used the risk ratio (RR), as it has been shown that the risk ratio is more intuitive than the odds ratio, and that the odds ratio tends to be interpreted by clinicians as the risk ratio (Boissel 1999; Deeks 2000). This misinterpretation can lead to overestimation of the effect. We provide the 95% confidence interval (CI) and the P value for each point estimate. We did not use continuous data in this review.
Unit of analysis issues
We addressed special issues in the analysis of studies using non‐standard design, such as cross‐over trials, cluster‐randomised trials and studies with multiple treatment groups, if they met our inclusion criteria. Petronio 2005 was a multiple treatment group study, from which we extracted only relevant data.
Dealing with missing data
We tried to contact study authors to obtain missing data, and we received a reply from Desmet 2011. We used an imputation method to determine missing data, but our overall findings were not affected, so we have not used the imputation method result in this review (Analysis 1.2).
1.2. Analysis.
Comparison 1 All‐cause mortality, Outcome 2 All‐cause mortality at long term.
Assessment of heterogeneity
We assessed clinical heterogeneity by considering variability in participants, interventions and outcomes of studies. We assessed statistical heterogeneity using the Chi² test and the I² test; we interpreted I² values between 50% and 90% as detecting substantial heterogeneity (Cochrane Handbook; Higgins 2003). The importance of the value of I² depends on the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test, confidence interval for I²).
Assessment of reporting biases
We had planned to investigate potential publication bias of included studies by constructing a funnel plot (Egger 1997). However, although we include in this review a total of 11 trials, the maximum number included for any single outcome is nine trials, which is deemed insufficient to support a meaningful funnel plot (Cochrane Handbook).
Data synthesis
We combined trial results using the Mantel‐Haenszel risk ratio, fixed‐effect model, except when statistical heterogeneity (I² > 50%) was observed, in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
If clinical heterogeneity was suspected, we planned subgroup analyses based on the following.
Participant characteristics (age, sex).
For adenosine, early administration (within four hours of onset of myocardial infarction symptoms) versus late administration to AMI participants.
Route of administration.
Sensitivity analysis
We performed sensitivity analyses to assess the impact of variable risks of bias, eligibility criteria, heterogeneity or publication status of included studies. In addition, we compared the combined results of fixed‐effect and random‐effects models when appropriate.
Results
Description of studies
We have provided the details of each included study in the Characteristics of included studies section.
Results of the search
We identified 2177 studies from the search of electronic databases. We identified 105 records via other resources: 37 studies from Clinical Trials.gov; eight from Current Controlled Trials; 15 from ANZCTR; four from WHO ICTRP; 39 from CNKI; and two from handsearching of The American Journal of Cardiology. After de‐duplication, we screened 1448 abstracts, of which 1399 were excluded. We reviewed the remaining 49 articles in full‐text version. We excluded 38 studies, leaving 11 RCTs for inclusion in the review. We found three ongoing studies (Characteristics of ongoing studies). We excluded the four former studies (Huang 2009; Jing 2010; Micari 2004; Zhen‐Guo 2010) that awaited classification, as we could obtain no further information, although we had sent emails to the corresponding authors.
We present the Quality of Reporting Meta‐analysis (QUOROM) flow diagram in Figure 1.
1.
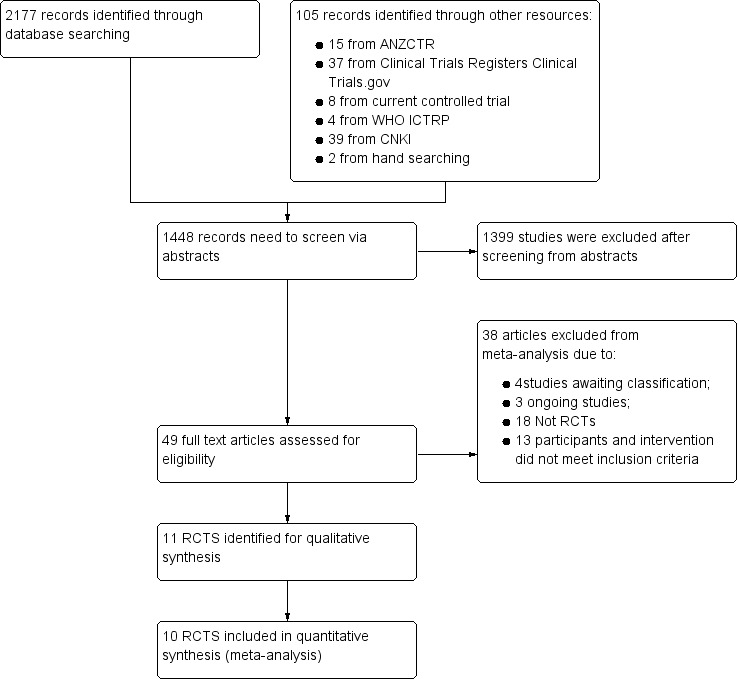
Study flow diagram: 2014 update search (one additional study included).
Included studies
All included studies were conducted with inpatients. Six were carried out in European countries, including the Netherlands (Fokkema 2009; Stoel 2008), Poland (Grygier 2011), Belgium (Desmet 2011) and Italy (Marzilli 2000; Petronio 2005). The remaining five were completed in China (Ji 2007; Wang 2008; Zhang 2012), Japan (Taniyama 1997) and the USA (Micari 2005).
The 11 included RCTs covered 1027 participants, of whom 76% were men. All participants were diagnosed with acute myocardial infarction (AMI) and required primary percutaneous coronary intervention (PPCI). Studies included from 28 to 448 participants.
Of the 11 included trials, 10 compared adenosine versus placebo, and one (Taniyama 1997) compared verapamil versus placebo, as an adjunct to PPCI. The route of administration in all included studies was intracoronary, except for Micari 2005, which administered treatment intravenously. The dose of adenosine ranged from 300 μg to 60 mg, given by infusion or by bolus. Normal saline was used as the placebo in all included trials.
All studies reported outcomes within one month, two trials (Petronio 2005; Zhang 2012) reported six‐month follow‐up and two trials (Desmet 2011; Stoel 2008) reported 12‐month follow‐up.
Excluded studies
Excluded studies are reported in the Characteristics of excluded studies section, along with reasons for exclusion.
Risk of bias in included studies
We have summarised risk of bias results in Figure 2 and Figure 3.
2.
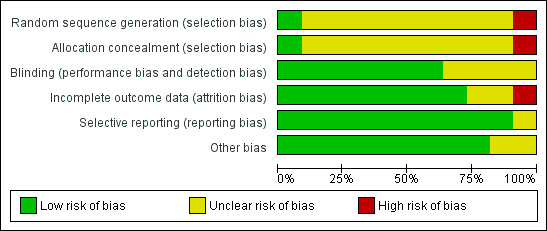
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
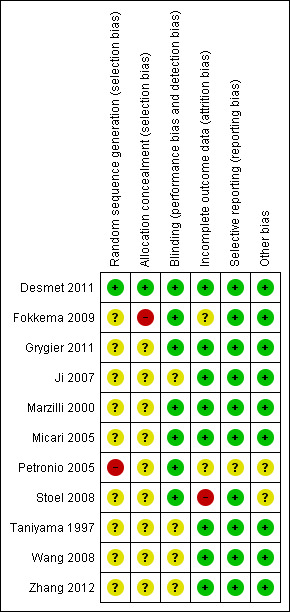
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although all included studies were described as randomised trials, investigators provided no clear description of randomisation and concealment of allocation, except for Desmet 2011, which reported adequate random sequence generation and adequate concealment of allocation.
Blinding
Details of random sequence generation, concealment and blinding are the most frequent poorly reported parameters in the included trials. In this review, two RCTs stated that they were double‐blind (Desmet 2011; Ji 2007), but only Desmet 2011 provided adequate sequence generation, allocation concealment and blinding. Because of perceived difficulties in blinding, we investigated evidence suggesting that those collecting and analysing outcome data were blinded to group allocation. Five studies reported blinding of this nature (Fokkema 2009; Grygier 2011; Marzilli 2000; Micari 2005; Petronio 2005).
Incomplete outcome data
Most studies did not report missing data and drop‐outs. Desmet 2011 and Fokkema 2009 reported missing data in some outcomes, and Stoel 2008 reported losses to follow‐up. We performed no intention‐to‐treat analyses to account for missing data.
Selective reporting
We have reported in the Results section all outcomes predefined in the Methods section of published trial reports.
Other potential sources of bias
We found no other obvious potential sources of bias.
Effects of interventions
All‐cause mortality
All‐cause mortality at short term (adenosine)
Seven included studies reported mortality at one‐month follow‐up for 830 participants (Desmet 2011; Fokkema 2009; Grygier 2011; Marzilli 2000; Micari 2005; Petronio 2005; Zhang 2012). None demonstrated a clinically or statistically significant difference between adenosine and control groups, with a fixed‐effect model risk ratio (RR) of 0.61 (95% confidence interval (CI) 0.25 to 1.48, P value = 0.27 and I² = 0%) (Analysis 1.1). We found no evidence of a reduction in all‐cause mortality with adenosine at one‐month follow‐up.
1.1. Analysis.
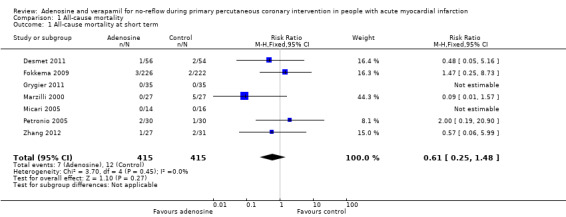
Comparison 1 All‐cause mortality, Outcome 1 All‐cause mortality at short term.
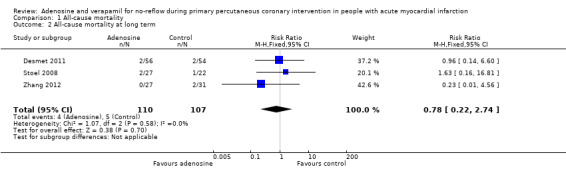
All‐cause mortality at long term (adenosine)
Three studies reported mortality at one‐year follow‐up for 217 participants (Desmet 2011; Stoel 2008; Zhang 2012). We found no evidence of a reduction in all‐cause mortality with adenosine at long‐term follow‐up (fixed‐effect model, RR 0.78, 95% CI 0.22 to 2.74, P value = 0.70, I² = 0%) (Analysis 1.2).
Non‐fatal myocardial infarction at short term (adenosine)
Five studies assessed non‐fatal myocardial infarction at one‐month follow‐up for 658 participants (Fokkema 2009; Grygier 2011; Marzilli 2000; Micari 2005; Zhang 2012). The fixed‐effect model RR was 1.32 (95% CI 0.33 to 5.29, P value = 0.69, I² = 0%) (Analysis 2.1). No evidence currently supports the use of adenosine in reducing non‐fatal myocardial infarction at short‐term follow‐up.
2.1. Analysis.
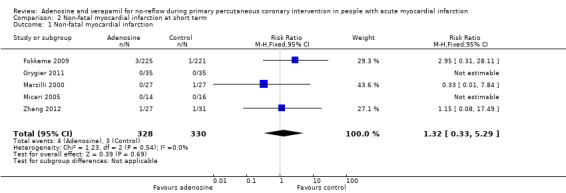
Comparison 2 Non‐fatal myocardial infarction at short term, Outcome 1 Non‐fatal myocardial infarction.
Thrombolysis in myocardial infarction (TIMI) flow grade < 3 after PPCI (adenosine)
Nine trials with 907 participants reported data on TIMI flow grade < 3 measured after PPCI (Desmet 2011; Fokkema 2009; Grygier 2011; Ji 2007; Marzilli 2000; Micari 2005; Petronio 2005; Wang 2008; Zhang 2012). The fixed‐effect model RR was 0.62 (95% CI 0.42 to 0.91, P value = 0.01, I² = 37%) (Analysis 3.1), indicating that adenosine can reduce the incidence of TIMI flow grade < 3 after PPCI.
3.1. Analysis.
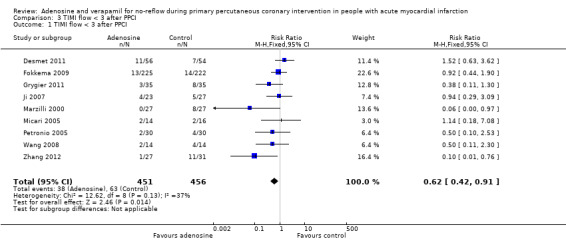
Comparison 3 TIMI flow < 3 after PPCI, Outcome 1 TIMI flow < 3 after PPCI.
Myocardial blush grade (MBG) 0 to 1 after PPCI (adenosine)
Three trials with 621 participants reported MBG 0 to 1 after PPCI (Desmet 2011; Fokkema 2009; Grygier 2011). The fixed‐effect model RR was 0.96 (95% CI 0.76 to 1.22, P value = 0.75, I² = 0%) (Analysis 4.1). Currently no evidence shows a reduction in the incidence of MBG 0 to 1 with adenosine.
4.1. Analysis.
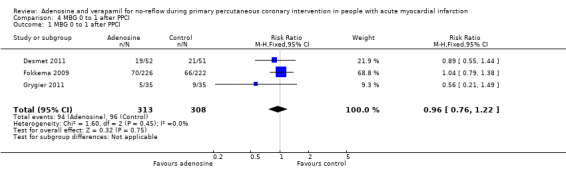
Comparison 4 MBG 0 to 1 after PPCI, Outcome 1 MBG 0 to 1 after PPCI.
Adverse events
Bradycardia (adenosine)
Five included RCTs with 518 people reported on bradycardia after PPCI (Fokkema 2009; Ji 2007; Marzilli 2000; Wang 2008; Zhang 2012). We observed no evidence of statistical heterogeneity between the two groups. The fixed‐effect model RR was 6.32 (95% CI 2.98 to 13.41, P value < 0.00001, I² = 0%) (Analysis 5.1), indicating a significantly greater likelihood of bradycardia in the adenosine group compared with the control group. Bradycardia was defined as heart rate less than 60 beats per minute.
5.1. Analysis.
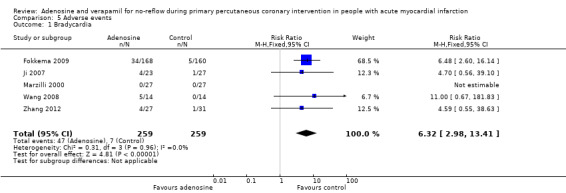
Comparison 5 Adverse events, Outcome 1 Bradycardia.
Hypotension (adenosine)
Three RCTs with 451 people reported on hypotension after PPCI (Fokkema 2009; Grygier 2011; Marzilli 2000). The fixed‐effect model RR was 11.43 (95% CI 2.75 to 47.57, P value = 0.0008) (Analysis 5.2). Evidence showed increased hypotension after PPCI with adenosine, compared with controls. Hypotension was defined as systolic blood pressure ≤ 90 mmHg.
5.2. Analysis.
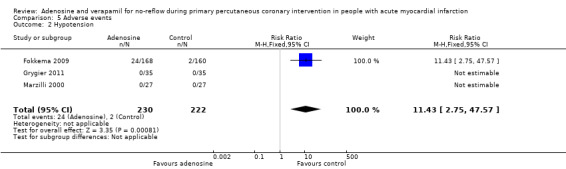
Comparison 5 Adverse events, Outcome 2 Hypotension.
Atrioventricular (AV) block (adenosine)
Atrioventricular block after PPCI was reported in six included studies with 639 participants (Desmet 2011; Fokkema 2009; Marzilli 2000; Micari 2005; Petronio 2005; Zhang 2012). As statistical heterogeneity between trials was significant (I² = 50%), we used the random‐effects model (RR 6.78, 95% CI 2.15 to 21.38, P value = 0.001) (Analysis 5.3). Evidence favoured adenosine over control in reducing the occurrence of AV block after PPCI. A sensitivity analysis comparing fixed‐effect and random‐effects models to control for heterogeneity did not substantively alter the results.
5.3. Analysis.
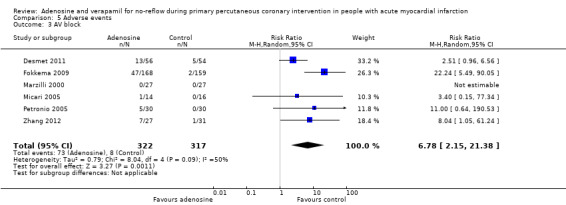
Comparison 5 Adverse events, Outcome 3 AV block.
Economic outcome
No data were available across all randomised controlled trials.
Quality of life
No data were available across all randomised controlled trials.
Discussion
Summary of main results
We have included 11 randomised controlled trials (RCTs) involving 1027 participants with acute myocardial infarction (AMI) for primary percutaneous coronary intervention (PPCI), in which the primary intention was to study the impact of adenosine and verapamil on no‐reflow during PPCI in people with AMI.
Only one of the 11 trials used verapamil (Taniyama 1997) and could not be included in the meta‐analyses. In the Taniyama 1997 study, after verapamil had been administered, no malignant ventricular arrhythmia or haemodynamic abnormalities were observed, and the number of participants with TIMI flow grade < 3 decreased to three from six. However, this study did not report outcomes such as all‐cause mortality, non‐fatal myocardial infarction or myocardial blush grade (MBG) 0 to 1.
We included 10 RCTs involving 987 participants for the study of adenosine. We found no evidence indicating that adenosine reduced short‐term (risk ratio (RR) 0.61, 95% confidence interval (CI) 0.25 to 1.48, P value = 0.27 ) or long‐term (RR 0.78, 95% CI 0.22 to 2.74, P value = 0.70) all‐cause mortality, or non‐fatal myocardial infarction (RR 1.32, 95% CI 0.33 to 5.29, P value = 0.69), to a greater extent than control. Evidence suggests that adenosine can reduce the incidence of TIMI flow grade < 3 after PPCI (RR 0.62, 95% CI 0.42 to 0.91, P value = 0.01), but MBG 0 to 1 is not statistically significant (RR 0.96, 95% CI 0.76 to 1.22, P value = 0.75). Adverse events reported with adenosine include bradycardia (five RCTs), hypotension (four RCTs) and all types of atrioventricular (AV) block (six RCTs). The incidence of adverse events (bradycardia: RR 6.32, 95% CI 2.98 to 13.41, P value < 0.00001; hypotension: RR 11.43, 95% CI 2.75 to 47.57, P value = 0.0008; AV block: RR 6.78, 95% CI 2.15 to 21.38, P value = 0.001)) after the procedure was significantly increased among groups treated with adenosine compared with placebo groups.
Results of the previous meta‐analysis show that adenosine could not significantly reduce the incidence of TIMI flow < 3 after PPCI (RR 0.72, 95% CI 0.49 to 1.07), but a trend toward reduction of TIMI was noted. Unlike the previous analysis, the present update analysis included a study from Zhang et al. Although this study included a small population, it had a large effect on the results of TIMI flow grade < 3 after PPCI. In contrast with the previous meta‐analysis, the present updated meta‐analysis suggests that adenosine is associated with a lower incidence of TIMI flow grade < 3 after PPCI. The present updated meta‐analysis includes one new, small study, which had unclear risk of bias across multiple domains and reported a large effect on the outcome of TIMI flow grade < 3 after PPCI. In fact, other than the study from Fokkema, all included studies were small studies. Risk of small study bias is high, and this has likely influenced the results (Nuesch 2010).
The present updated meta‐analysis had some limitations. First, most included studies had a relatively short period of follow‐up and included a limited number of participants; few studies reported long‐term follow‐up, and event rates of mortality and angiographic no‐reflow were low. The present meta‐analysis therefore had poor statistical power to support clear conclusions about the impact of adenosine in people with AMI undergoing PPCI. Second, compared with the outcome of TIMI flow grade < 3, the summative size of the effect of adenosine on short‐term all‐cause mortality (39% risk reduction) is clinically important, especially in the context of large numbers of individuals with AMI undergoing PPCI. Third, the included new study had unclear risk of bias across multiple domains and high risk of small study bias. Because of these potential clinical benefits, the conclusion should be interpreted cautiously. Large, high‐quality RCTs in this field are needed to explore the potential risks and benefits of these interventions.
Overall completeness and applicability of evidence
In designing this review, we hoped to determine the impact of adenosine or verapamil on AMI by searching for studies that met the following criteria.
Participants with AMI undergoing PPCI.
Adenosine or verapamil given as the primary intervention adjunctive to PPCI.
Key outcomes, including mortality, non‐fatal MI, TIMI flow grade and so forth, reported.
We believe that these parameters are important in helping us answer the clinical question: What is the impact of adenosine or verapamil as an adjunct to PPCI in people with AMI? Extracted and analysed data show that adenosine did not produce a significant reduction in primary outcomes (all‐cause mortality at short term and at long term, and non‐fatal MI at short term). However, we found no data on risks of non‐fatal MI at long term, so we could not investigate this outcome on the basis of available evidence.
According to the guidelines of the European Society of Cardiology, adenosine is recommended as a drug of choice for the treatment of patients with no‐reflow phenomenon, with a IIb class recommendation at evidence level B (Van de Werf 2008). Although no evidence in our previous review suggests that adenosine can reduce the incidence of TIMI flow grade < 3 after PPCI, in this updated review we show that adenosine reduces the incidence of angiographic no‐reflow, and the incidence of MBG 0 to 1 or mortality at short‐term or at long‐term follow‐up remains unchanged. In terms of the change in TIMI flow grade < 3 after PPCI, we found that before this new study (Zhang 2012) was considered, adenosine had already shown a trend towards improving coronary blood flow, but without statistical significance; after addition of the new study, which demonstrated that adenosine could significantly reduce the incidence of TIMI flow grade < 3 after PPCI, we noted the beneficial effects of adenosine in reducing the incidence of TIMI < 3 after PPCI. The change may be a result of the different means of administration and dosage of adenosine used in this new study, in which adenosine was given at 50 or 70 μg/kg/min for 3 hours intravenously. In the light of heterogeneity observed in each study and the small number of included participants, we believe that additional high‐quality RCTs are needed to confirm the efficacy of adenosine for no‐reflow in patients with AMI undergoing PPCI .
Our analyses clearly indicate that the incidence of adverse effects in the adenosine groups (bradycardia, hypotension, AV block) is greater than in the control groups. However, these adverse effects were shown to resolve spontaneously within a few minutes after the procedure.
Participant‐oriented outcomes, such as quality of life and costs of treatment, were seldom reported. Therefore, quality of life and economic outcomes were not included in the analysis. We suggest that future research should focus on participant outcomes.
Quality of the evidence
Although included studies did not describe methods of randomisation and allocation concealment, most studies did report details indicating whether outcomes were assessed by blinded researchers or whether the researchers were independent to the study. So, the overall risk of bias of trials included in this systematic review was judged to be moderate (please refer to Figure 2 and Figure 3).
Potential biases in the review process
We have strictly followed our published protocol in implementing the processes of searching for trials, extracting data and analysing data. However, the possibility of unidentified and/or unpublished papers is a potential source of bias for any systematic review. Another potential risk of bias may be performance bias due to imbalance of co‐interventions across intervention and control arms.
Agreements and disagreements with other studies or reviews
We know of no other systematic reviews of adenosine and verapamil given to individuals with AMI undergoing PPCI.
Authors' conclusions
Implications for practice.
Evidence favours adenosine as an adjunct to PPCI in reducing the incidence of angiographic no‐reflow (TIMI flow grade < 3), but no evidence shows reduced mortality. Although the incidence of adverse effects of adenosine (hypotension, bradycardia, AV block) is statistically significant, the effects are short‐lived.
In accordance with the current review, and in keeping with international guidelines (Steg 2012; Van de Werf 2008), we believe that adenosine as adjunctive therapy for patients with AMI undergoing PPCI should not be excluded from clinical practice.
Implications for research.
Although primary outcomes (all‐cause mortality and non‐fatal MI) showed no significant differences between adenosine and control groups, this evidence was based on relatively few included RCTs with few participants that were not powered to deliver a definitive judgement on adenosine in people with AMI. We hope that future RCTs can focus on both short‐term and long‐term clinical outcomes (all‐cause mortality and non‐fatal MI). Our finding is subject to small study bias, and conclusions should be interpreted cautiously.
It is worth noting that myocardial blush grade and thrombolysis in myocardial infarction flow grade are important outcomes for assessment of angiographic no‐reflow after PPCI. Although adenosine has been shown to be superior to placebo as treatment for angiographic no‐reflow after PPCI, the myocardial blush grade is currently limited. Larger trials of high quality are needed. Because no available data can be used, quality of life and health economic outcomes are not included in the analysis. Future trials should incorporate quality of life and health economic outcomes.
This review identified only one trial of verapamil for inclusion. Additional RCTs testing verapamil as an adjunct to PPCI in people with AMI are necessary, to explore its potential as adjunctive treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2014 | New search has been performed | This update includes 1 new study. |
| 25 June 2014 | New citation required and conclusions have changed | Results of TIMI flow grade < 3 after PPCI have changed after a new study was added (Zhang 2012). The rest of our conclusions remain the same. |
Acknowledgements
We would like to thank the editorial team of the Cochrane Heart Group for editorial suggestions provided.
We are very grateful to all previous contributors who authored the original review, which formed the template for this updated version. We thank Dr. Kyaw Aung Naing (KAN) for designing the review, drafting the protocol, extracting data from studies and drafting the original review.
Thanks also to Nicole Martin from the Cochrane Heart Group for developing the search strategy for this review, and to Joanne Abbott for providing search results and full‐text articles for the original review. We also thank Dr. Yuhan Sun for conducting and interpreting analyses for the update.
Appendices
Appendix 1. Search strategies 2014
CENTRAL
#1 MeSH descriptor Vasodilator Agents, this term only #2 (vasodilator*) #3 (vasorelaxant*) #4 ("vasoactive antagon*") #5 MeSH descriptor Adenosine, this term only #6 MeSH descriptor Nucleosides, this term only #7 (#6), from 1966 to 1971 #8 (adenosine) #9 (adenocard) #10 MeSH descriptor Verapamil, this term only #11 (verapamil) #12 finoptin #13 (iproveratril) #14 (i?optin*) #15 (lekoptin) #16 (dexverapamil) #17 (calan) #18 (falicard) #19 (cordilox) #20 (adenocor) #21 (vertab) #22 (securon) #23 (univer) #24 (verapress) #25 (adeno‐jec) #26 (adenoscan) #27 (apo‐verap) #28 (berkatens) #29 (chronovera) #30 (covera) #31 "ethimil mr" #32 (finoptin) #33 geangin #34 (hypaneze) #35 "ipoveratril hydrochloride" #36 (iproveratril) #37 (novo‐veramil) #38 (nu‐verap) #39 (verelan) #40 (#1 OR #2 OR #3 OR #4 OR #5 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39) #41 MeSH descriptor Angioplasty, Balloon, Coronary, this term only #42 (coronary near/5 angioplast*) #43 (pci) #44 "percutaneous coronary intervention*" #45 (ptca) #46 (balloon near/3 angioplast*) #47 (coronary near/5 balloon dilation*) #48 (coronary near/5 stent*) #49 (#41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48) #50 (#40 AND #49)
MEDLINE Ovid
1 Vasodilator Agents/ 2 vasodilator*.tw. 3 vasorelaxant*.tw. 4 (vasoactive adj antagon*).tw. 5 Adenosine/ 6 NUCLEOSIDES/ 7 limit 6 to yr="1966 ‐ 1971" 8 adenosine.tw. 9 adenocard.tw. 10 Verapamil/ 11 verapamil.tw. 12 finoptin.tw. 13 iproveratril.tw. 14 i?optin*.tw. 15 lekoptin.tw. 16 dexverapamil.tw. 17 calan.tw. 18 falicard.tw. 19 cordilox.tw. 20 adenocor.tw. 21 vertab.tw. 22 securon.tw. 23 univer.tw. 24 verapress.tw. 25 adeno‐jec.tw. 26 adenoscan.tw. 27 apo‐verap.tw. 28 berkatens.tw. 29 chronovera.tw. 30 ethimil mr.tw. 31 finoptin.tw. 32 geangin.tw. 33 hypaneze.tw. 34 ipoveratril hydrochloride.tw. 35 iproveratril.tw. 36 novo‐veramil.tw. 37 nu‐verap.tw. 38 Angioplasty, Balloon, Coronary/ 39 (coronary adj5 angioplast*).tw. 40 pci.tw. 41 percutaneous coronary intervention*.tw. 42 ptca.tw. 43 (balloon adj3 angioplast*).tw. 44 (coronary adj5 balloon dilation*).tw. 45 (coronary adj5 stent*).tw. 46 or/38‐45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomized.ab. 50 placebo.ab. 51 drug therapy.fs. 52 randomly.ab. 53 trial.ab. 54 groups.ab. 55 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 56 exp animals/ not humans.sh. 57 55 not 56 58 covera.tw. 59 verelan.tw. 60 1 or 2 or 3 or 4 or 5 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 58 or 59 61 46 and 57 and 60
EMBASE
1 Vasodilator Agents/ 2 vasodilator*.tw. 3 vasorelaxant*.tw. 4 (vasoactive adj antagon*).tw. 5 Adenosine/ 6 NUCLEOSIDES/ 7 limit 6 to yr="1966 ‐ 1971" 8 adenosine.tw. 9 adenocard.tw. 10 Verapamil/ 11 verapamil.tw. 12 finoptin.tw. 13 iproveratril.tw. 14 i?optin*.tw. 15 lekoptin.tw. 16 dexverapamil.tw. 17 calan.tw. 18 falicard.tw. 19 cordilox.tw. 20 adenocor.tw. 21 vertab.tw. 22 securon.tw. 23 univer.tw. 24 verapress.tw. 25 adeno‐jec.tw. 26 adenoscan.tw. 27 apo‐verap.tw. 28 berkatens.tw. 29 chronovera.tw. 30 ethimil mr.tw. 31 finoptin.tw. 32 geangin.tw. 33 hypaneze.tw. 34 ipoveratril hydrochloride.tw. 35 iproveratril.tw. 36 novo‐veramil.tw. 37 nu‐verap.tw. 38 Angioplasty, Balloon, Coronary/ 39 (coronary adj5 angioplast*).tw. 40 pci.tw. 41 percutaneous coronary intervention*.tw. 42 ptca.tw. 43 (balloon adj3 angioplast*).tw. 44 (coronary adj5 balloon dilation*).tw 45 (coronary adj5 stent*).tw. 46 or/38‐45 47 covera.tw. 48 verelan.tw. 49 1 or 2 or 3 or 4 or 5 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 47 or 48 50 random$.tw. 51 factorial$.tw. 52 crossover$.tw. 53 cross over$.tw. 54 cross‐over$.tw. 55 placebo$.tw. 56 (doubl$ adj blind$).tw. 57 (singl$ adj blind$).tw. 58 assign$.tw. 59 allocat$.tw. 60 volunteer$.tw. 61 crossover procedure/ 62 double blind procedure/ 63 randomized controlled trial/ 64 single blind procedure/ 65 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 66 (animal/ or nonhuman/) not human/ 67 65 not 66 68 46 and 49 and 67
Web of Science and BIOSIS
#21 #20 AND #19 #20 Topic=(((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))) ; #19 #18 AND #12 ; #18 #17 OR #16 OR #15 OR #14 OR #13 #17 Topic=((coronary near/5 stent*)) #16 Topic=(((coronary )near/5 ("balloon dilation*"))) #15 Topic=(("percutaneous coronary intervention*") or (ptca) or (balloon near/3 angioplast*)) ; #14 Topic=(pci) #13 Topic=((coronary near/5 angioplast*)) #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #11 Topic=((iproveratril) or (novo‐veramil) or (nu‐verap) or (verelan)) #10 Topic=((finoptin) or (geangin) or (hypaneze) or ("ipoveratril hydrochloride")) #9 Topic=((berkatens) or (chronovera) or (covera) or (ethimil)) #8 Topic=((verapress) or (adeno‐jec) or (adenoscan) or (apo‐verap)) #7 Topic=((adenocor) or (vertab) or (securon) or (univer)) #6 Topic=((calan) or (falicard) or (cordilox)) ; #5 Topic=((i?optin*) or (lekoptin) or (dexverapamil)) #4 Topic=((verapamil) or ( finoptin) or ( iproveratril)) #3 Topic=(("vasoactive antagon*") or (adenosine) or (adenocard)) #2 Topic=((vasorelaxant*)) #1 Topic=((vasodilator*))
CNKI
1. 腺苷 and 急性心肌梗死
2. 维拉帕米 and 急性心肌梗死
Clinical Trial Registers
1. adenosine and myocardial infarction 2. verapamil and myocardial infarction
Appendix 2. Search strategies 2012
CENTRAL
#1 MeSH descriptor Vasodilator Agents, this term only #2 (vasodilator*) #3 (vasorelaxant*) #4 ("vasoactive antagon*") #5 MeSH descriptor Adenosine, this term only #6 MeSH descriptor Nucleosides, this term only #7 (#6), from 1966 to 1971 #8 (adenosine) #9 (adenocard) #10 MeSH descriptor Verapamil, this term only #11 (verapamil) #12 finoptin #13 (iproveratril) #14 (i?optin*) #15 (lekoptin) #16 (dexverapamil) #17 (calan) #18 (falicard) #19 (cordilox) #20 (adenocor) #21 (vertab) #22 (securon) #23 (univer) #24 (verapress) #25 (adeno‐jec) #26 (adenoscan) #27 (apo‐verap) #28 (berkatens) #29 (chronovera) #30 (covera) #31 "ethimil mr" #32 (finoptin) #33 geangin #34 (hypaneze) #35 "ipoveratril hydrochloride" #36 (iproveratril) #37 (novo‐veramil) #38 (nu‐verap) #39 (verelan) #40 (#1 OR #2 OR #3 OR #4 OR #5 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39) #41 MeSH descriptor Angioplasty, Balloon, Coronary, this term only #42 (coronary near/5 angioplast*) #43 (pci) #44 "percutaneous coronary intervention*" #45 (ptca) #46 (balloon near/3 angioplast*) #47 (coronary near/5 balloon dilation*) #48 (coronary near/5 stent*) #49 (#41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48) #50 (#40 AND #49)
MEDLINE Ovid
1 Vasodilator Agents/ 2 vasodilator*.tw. 3 vasorelaxant*.tw. 4 (vasoactive adj antagon*).tw. 5 Adenosine/ 6 NUCLEOSIDES/ 7 limit 6 to yr="1966 ‐ 1971" 8 adenosine.tw. 9 adenocard.tw. 10 Verapamil/ 11 verapamil.tw. 12 finoptin.tw. 13 iproveratril.tw. 14 i?optin*.tw. 15 lekoptin.tw. 16 dexverapamil.tw. 17 calan.tw. 18 falicard.tw. 19 cordilox.tw. 20 adenocor.tw. 21 vertab.tw. 22 securon.tw. 23 univer.tw. 24 verapress.tw. 25 adeno‐jec.tw. 26 adenoscan.tw. 27 apo‐verap.tw. 28 berkatens.tw. 29 chronovera.tw. 30 ethimil mr.tw. 31 finoptin.tw. 32 geangin.tw. 33 hypaneze.tw. 34 ipoveratril hydrochloride.tw. 35 iproveratril.tw. 36 novo‐veramil.tw. 37 nu‐verap.tw. 38 Angioplasty, Balloon, Coronary/ 39 (coronary adj5 angioplast*).tw. 40 pci.tw. 41 percutaneous coronary intervention*.tw. 42 ptca.tw. 43 (balloon adj3 angioplast*).tw. 44 (coronary adj5 balloon dilation*).tw. 45 (coronary adj5 stent*).tw. 46 or/38‐45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomized.ab. 50 placebo.ab. 51 drug therapy.fs. 52 randomly.ab. 53 trial.ab. 54 groups.ab. 55 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 56 exp animals/ not humans.sh. 57 55 not 56 58 covera.tw. 59 verelan.tw. 60 1 or 2 or 3 or 4 or 5 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 58 or 59 61 46 and 57 and 60
EMBASE
1 Vasodilator Agents/ 2 vasodilator*.tw. 3 vasorelaxant*.tw. 4 (vasoactive adj antagon*).tw. 5 Adenosine/ 6 NUCLEOSIDES/ 7 limit 6 to yr="1966 ‐ 1971" 8 adenosine.tw. 9 adenocard.tw. 10 Verapamil/ 11 verapamil.tw. 12 finoptin.tw. 13 iproveratril.tw. 14 i?optin*.tw. 15 lekoptin.tw. 16 dexverapamil.tw. 17 calan.tw. 18 falicard.tw. 19 cordilox.tw. 20 adenocor.tw. 21 vertab.tw. 22 securon.tw. 23 univer.tw. 24 verapress.tw. 25 adeno‐jec.tw. 26 adenoscan.tw. 27 apo‐verap.tw. 28 berkatens.tw. 29 chronovera.tw. 30 ethimil mr.tw. 31 finoptin.tw. 32 geangin.tw. 33 hypaneze.tw. 34 ipoveratril hydrochloride.tw. 35 iproveratril.tw. 36 novo‐veramil.tw. 37 nu‐verap.tw. 38 Angioplasty, Balloon, Coronary/ 39 (coronary adj5 angioplast*).tw. 40 pci.tw. 41 percutaneous coronary intervention*.tw. 42 ptca.tw. 43 (balloon adj3 angioplast*).tw. 44 (coronary adj5 balloon dilation*).tw 45 (coronary adj5 stent*).tw. 46 or/38‐45 47 covera.tw. 48 verelan.tw. 49 1 or 2 or 3 or 4 or 5 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 47 or 48 50 random$.tw. 51 factorial$.tw. 52 crossover$.tw. 53 cross over$.tw. 54 cross‐over$.tw. 55 placebo$.tw. 56 (doubl$ adj blind$).tw. 57 (singl$ adj blind$).tw. 58 assign$.tw. 59 allocat$.tw. 60 volunteer$.tw. 61 crossover procedure/ 62 double blind procedure/ 63 randomized controlled trial/ 64 single blind procedure/ 65 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 66 (animal/ or nonhuman/) not human/ 67 65 not 66 68 46 and 49 and 67
Web of Science and BIOSIS
#21 #20 AND #19 #20 Topic=(((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))) ; #19 #18 AND #12 ; #18 #17 OR #16 OR #15 OR #14 OR #13 #17 Topic=((coronary near/5 stent*)) #16 Topic=(((coronary )near/5 ("balloon dilation*"))) #15 Topic=(("percutaneous coronary intervention*") or (ptca) or (balloon near/3 angioplast*)) ; #14 Topic=(pci) #13 Topic=((coronary near/5 angioplast*)) #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #11 Topic=((iproveratril) or (novo‐veramil) or (nu‐verap) or (verelan)) #10 Topic=((finoptin) or (geangin) or (hypaneze) or ("ipoveratril hydrochloride")) #9 Topic=((berkatens) or (chronovera) or (covera) or (ethimil)) #8 Topic=((verapress) or (adeno‐jec) or (adenoscan) or (apo‐verap)) #7 Topic=((adenocor) or (vertab) or (securon) or (univer)) #6 Topic=((calan) or (falicard) or (cordilox)) ; #5 Topic=((i?optin*) or (lekoptin) or (dexverapamil)) #4 Topic=((verapamil) or ( finoptin) or ( iproveratril)) #3 Topic=(("vasoactive antagon*") or (adenosine) or (adenocard)) #2 Topic=((vasorelaxant*)) #1 Topic=((vasodilator*))
CNKI
1. 腺苷 and 急性心肌梗死
2. 维拉帕米 and 急性心肌梗死
Clinical Trial Registers
1. adenosine and myocardial infarction 2. verapamil and myocardial infarction
Data and analyses
Comparison 1. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality at short term | 7 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.48] |
| 2 All‐cause mortality at long term | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.74] |
Comparison 2. Non‐fatal myocardial infarction at short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐fatal myocardial infarction | 5 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.33, 5.29] |
Comparison 3. TIMI flow < 3 after PPCI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TIMI flow < 3 after PPCI | 9 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.91] |
Comparison 4. MBG 0 to 1 after PPCI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MBG 0 to 1 after PPCI | 3 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
Comparison 5. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bradycardia | 5 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.32 [2.98, 13.41] |
| 2 Hypotension | 3 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.43 [2.75, 47.57] |
| 3 AV block | 6 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 6.78 [2.15, 21.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Desmet 2011.
| Methods | Date of publication: 2011 Design: prospective, single‐centre, double‐blind, placebo‐controlled clinical study Allocation: randomised Country: Belgium | |
| Participants | Diagnosis: acute STEMI for PPCI Number: 110 (adenosine 56; control 54) Age, years: adenosine (mean 61.4 ± 12.3); control (mean 60.6 ± 10.8) Sex: 82% men Inclusion: chest pain suggestive of myocardial ischaemia for at least 20 minutes; time from onset of symptoms of 12 hours; ECG showing ST‐segment elevation of 0.1 mV in 2 or more limb leads or 0.2 mV in 2 or more contiguous precordial leads, presumed new left bundle branch block Exclusion: contraindication to heparin, low‐molecular‐weight heparin or clopidogrel; anticipated difficult vascular access; cardiogenic shock; inability to give informed consent; high‐grade atrioventricular block; severe asthma; treatment with theophylline, glibenclamide or dipyridamole; prior coronary artery surgery; participation in any investigational drug or device study within the previous 30 days Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: 30 days and 1 year (collected from hospital records and telephone interviews) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study drug was constituted by the hospital pharmacy in numbered vials according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "This list was kept in a sealed envelope in the hospital pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded: "Vials containing adenosine and those containing placebo had an identical appearance. All study personnel were blinded to treatment allocation until the study had been completed and all analyses had been performed" "Coronary angiograms obtained before and after primary PCI were reviewed by two experienced observers blinded to treatment allocation and clinical data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We found no data on MBG and no clear description of it in the full text. However, communication with the study author confirmed that data for MBG were missing because of technical problems involved in determining MBG (e.g. when the image was focused on the coronary lesion, not including the myocardial region of interest needed to determine MBG, or when the coronary artery was not filmed in such a way that MBG could be assessed) |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Fokkema 2009.
| Methods | Date of publication: 2009 Design: not reported Allocation: randomised Country: Netherlands | |
| Participants | Diagnosis: acute STEMI for PPCI Number: 448 (adenosine 226; control 222) Age, years: adenosine (mean 62.4 ± 12.2); control (mean 62.5 ± 13.0) Sex: 75% men Inclusion: chest pain suggestive of myocardial ischaemia for at least 30 minutes; time from onset of symptoms of 12 hours before hospital admission; and ECG showing ST‐segment elevation of 0.1 mV in 2 or more leads Exclusion: cardiogenic shock; presence of a life‐threatening disease with a life expectancy of 6 months; receiving pharmacotherapy for chronic obstructive pulmonary disease; no informed consent Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: 30 days (collected from hospital records, written questionnaires and telephone interviews) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description |
| Allocation concealment (selection bias) | High risk | "a single‐center, prospective randomized open trial with blinded evaluation of end points" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Coronary angiograms were reviewed by 2 observers blinded for treatment allocation and clinical data" "QuBE was measured blinded to clinical data and treatment allocation by 2 observers" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals or drop‐outs were reported, but for some outcomes not all participant data were addressed, with no reason given. Missing outcome data were balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Grygier 2011.
| Methods | Date of publication: 2011 Design: not reported Allocation: randomised Country: Poland | |
| Participants | Diagnosis: acute STEMI for PPCI Number: 70 (adenosine 35; control 35) Age, years: adenosine (mean 65.3 ± 11), control (mean 64.5 ± 13) Sex: 63% men Inclusion: referred for PPCI within 6 hours of symptoms; culprit lesion was suitable for PCI and had presented with TIMI flow 0 to 2 Exclusion: chronic obstructive pulmonary disease or asthma; those who had received previous thrombolysis; patients presenting with TIMI flow 3 Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: 1 month |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the patient was included in the present study and randomized after informed consent had been given"; no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Angiograms were reanalyzed as a single group by 2 observers, who had not participated in the invasive procedure and were unaware of the treatment received" "MBG was assessed by 2 observers who were unaware of the others' findings and of the clinical data" "physicians in charge of the patients on the coronary care floor were informed of the angiographic results of the primary PCI but were unaware of the study treatment administered during the procedure" "The electrocardiograms were analysed in a blinded fashion by an experienced cardiologist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Ji 2007.
| Methods | Date of publication: 2007 Design: double‐blinded Allocation: randomised Country: China | |
| Participants | Diagnosis: acute STEMI for PPCI Number: 50 (adenosine 23; control 27) Age, years: adenosine (mean 59.35 ± 12.06), control (mean 60.59 ± 11.24) Sex: 82% men Inclusion: chest pain persisting at Ieast 30 minutes but no longer than 12 hours; ECG ST‐segment elevation (> 0.1 mV) of at Ieast 2 contiguous ECG leads; cuIprIt Iesion was suitabIe for PCI; informed consent Exclusion: cardiogenic shock or severe heart failure (Killip cIassification ≥ 3); received thromboIytics in the emergency room; history of bronchospasm or undergoing therapy with theophyline; presented with TIMI 3 flow; heart rate < 50 bpm; bIood pressure < 90/60 mmHg Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: 4 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, but no clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Marzilli 2000.
| Methods | Date of publication: 2000 Design: not reported Allocation: randomised Country: Italy | |
| Participants | Diagnosis: AMI for PPCI Number: 54 (adenosine 27; control 27) Age, years: adenosine (mean 58.15 ± 11), control (mean 61.9 ± 9) Sex: 80% men Inclusion: referred for PTCA within 3 hours from the onset of AMI; culprit lesion was suitable for PPCI; presented with TIMI flow from 0 to 2; informed consent Exclusion: TIMI 3 flow and having spontaneous reperfusion; history of bronchospasm and/or undergoing therapy with theophylline derivatives; had received thrombolytics in the emergency room Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Duration: during hospitalisation, no further follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the patient was included in the study and randomised after informed consent had been obtained"; no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Angiograms were reread as a single group by observers who had not participated in the invasive procedure and were blinded to the treatment received" "Physicians in charge of the patients in the intensive coronary care unit were informed of the angiographic results of the PTCA but were blinded to the intracoronary treatment administered during the procedure" "Images were recorded on S‐VHS tapes and analysed offline by 2 experienced observers blinded to the angiographic data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported in Results |
| Other bias | Low risk | Not obvious |
Micari 2005.
| Methods | Date of publication: 2005 Design: not reported Allocation: randomised Country: USA | |
| Participants | Diagnosis: acute STEMI for PPCI Number: 30 (adenosine 14; control 16) Age, years: adenosine (mean 57), control (mean 57) Sex: 67% men Inclusion: symptoms consistent with myocardial ischaemia for 30 minutes and 2‐mm ST‐segment elevation in 2 contiguous electrocardiographic leads; STEMI presenting within 6 hours from the onset of AMI Exclusion: history of AMI; wall motion abnormalities in 1 vascular territory; cardiomyopathy Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: 4 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients with ST‐elevation AMI referred for percutaneous coronary stenting were randomized to receive intravenous adenosine"; no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The thrombolysis In myocardial infarction flow grade after PCI was assessed by a reader blinded to the clinical and echocardiographic data" "Analysis of the myocardial contrast echocardiographic data was performed blinded to the clinical and angiographic data" "The analysis of wall motion index was performed blinded to all other data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data were reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Petronio 2005.
| Methods | Date of publication: 2005 Design: not reported Allocation: randomised Country: Italy | |
| Participants | Diagnosis: AMI for PPCI Number: 90 (adenosine 30; abciximab 30; control 30) Age, years: adenosine (mean 56 ± 14), abciximab (mean 60 ± 11), control (mean 61 ± 13) Sex: 83% men Inclusion: presentation > 6 hours from symptom onset; chest pain lasting 30 minutes and resistant to nitrates; 0.2‐mV ST‐segment elevation in 2 contiguous leads on a 12‐lead electrocardiogram; TIMI flow 0 to 1 in the infarct‐related artery at the diagnostic angiogram; absence of contraindications to abciximab and adenosine Exclusion: significant left main coronary disease; cardiogenic shock; AMI due to bypass graft occlusion; thrombolytic therapy before angioplasty Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: 1 month and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization occurred in a sequential alternating fashion based on the order of admission at our laboratory" |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The angiograms were reread as a single group, in chronological order, by experienced observers blinded to patient treatment" "Interpretation of the echocardiographic was blinded to patient treatment" "The ECGs were analysed in a blinded fashion by an experienced cardiologist" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in Methods are reported in Results |
| Other bias | Unclear risk | Not obvious |
Stoel 2008.
| Methods | Date of publication: 2008 Design: not reported Allocation: randomised Country: Netherlands | |
| Participants | Diagnosis: acute STEMI for PPCI Number: 49 (adenosine 27; control 22) Age, years: adenosine (mean 67.3 ± 15.6), control (mean 66.3 ± 11.7) Sex: 65% men Inclusion: suboptimal reperfusion (< 70% STRes with persistent ST‐elevation > 2 mV in at least 1 anterior lead and > 1 mV in a non‐anterior lead) more than 10 minutes after last balloon inflation Exclusion: haemodynamic instability; prior myocardial infarction; an ECG unsuitable for calculation of STRes (left bundle branch block, paced or severe disturbed rhythm); history of obstructive pulmonary disease Withdrawals or losses to follow‐up: 1 from adenosine group |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: hospital stay and 12 months (by telephone interview) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patient and operators were blinded for the study medication" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 lost to follow‐up in adenosine group, but no intention‐to‐treat analysis was reported and no reason was given |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Unclear risk | Not obvious |
Taniyama 1997.
| Methods | Date of publication: 1997 Design: not reported Allocation: randomised Country: Japan | |
| Participants | Diagnosis: acute AMI for PPCI Number: 40 (verapamil 20; control 20) Age, years: verapamil (mean 61 ± 11), control (mean 67 ± 14) Sex: 78% men Inclusion: diagnosis of a first AMI; single‐vessel disease; percutaneous transluminal coronary angioplasty of a totally occluded infarct‐related artery; TIMI flow grade 0 within 12 hours of symptom onset; residual diameter stenosis < 50%; no ischaemic event during follow‐up; adequate quality of echocardiogram Exclusion: severe congestive heart failure Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Verapamil
Control: do not give anything |
|
| Outcomes |
Follow‐up: mean of 25 days after AMI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No clear statement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Wang 2008.
| Methods | Date of publication: 2008 Design: not reported Allocation: randomised Country: China | |
| Participants | Diagnosis: AMI for PPCI Number: 28 (adenosine 14; control 14) Age, years: adenosine (mean 48.6 ± 13.7); control (mean 46.2 ± 13.1) Sex: 82% men Inclusion: chest pain for at least 30 minutes; time from onset of symptoms of 12 hours before hospital admission; an ECG showing ST‐segment elevation of 0.1 mV in 2 or more leads; cardiac marker increased to twice the upper limit; not received fibrinolysis at emergency department; HR ≥ 50/min, BP ≥ 90/60 mmHg Exclusion: cardiogenic shock; Killip class ≥ 3; history of asthma; HR < 50/min, TIMI flow 3 Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine
Control
|
|
| Outcomes |
Follow‐up: during hospitalisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Zhang 2012.
| Methods | Date of publication: 2012 Design: not reported Allocation: randomised Country: China |
|
| Participants | Diagnosis: AMI for PPCI Number: 59 (adenosine 32; control 27) Age, years: adenosine (mean 59.4 ± 10.2); control (mean 65.0 ± 12.2) Sex: 81% men Inclusion: had typical chest pain presenting within 12 hours of onset, with ST‐segment elevation in at least 2 contiguous leads of > 0.2 mV in precordial leads, > 0.1 mV in limb leads or new left bundle branch block (LBBB); candidates for primary PCI treatment Exclusion: thrombolytic treatment before PCI treatment; previous myocardial infarction; history of coronary artery bypass graft (CABG) or PCI; clinical evidence of significant reactive airway disease (e.g. asthma); advanced cancer, or end‐stage disease Withdrawals or losses to follow‐up: not reported |
|
| Interventions | Adenosine: 70 μg/kg/min for 3 hours
Control
|
|
| Outcomes |
Follow‐up: 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in Methods are reported in Results |
| Other bias | Low risk | Not obvious |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babbitt 1988 | Not an RCT |
| Dani 2009 | Not an RCT. Intervention groups did not meet inclusion criteria |
| De Luca 2012 | Participants received elective PCI, which did not meet inclusion criteria |
| Dillon 2001 | Not an RCT |
| Fischell 1998 | Not an RCT. Participants received saphenous vein graft percutaneous coronary intervention |
| Forman 2008 | Not an RCT |
| Garratt 1998 | Not an RCT. Intervention was a combination of adenosine plus lidocaine, with no control group |
| Granger 1997 | Not an RCT |
| Hang 2005 | Single‐centre, non‐randomised, prospective study with a retrospective control group |
| Harding 2006 | Not an RCT |
| Heidland 2000 | Participants were patients with stable angina |
| Huang 2009 | Abstract only, cannot contact study authors |
| Ibrahim 2002 | Not an RCT |
| Jing 2010 | Abstract only, cannot contact study authors |
| Kelly 2005 | Not an RCT |
| Kim 2009 | Participants had stable or unstable angina and received non‐urgent PCI, which did not meet inclusion criteria |
| Kloner 2006 | Participants received both PPCI and thrombolysis as reperfusion therapy |
| Kopecky 2003 | Intervention was adenosine agonist (AMP579), which did not meet inclusion criteria |
| Lee 2007 | Participants underwent non‐urgent PCI, which did not meet inclusion criteria |
| Lim 2004 | Intervention group got adenosine plus nicorandil; control group was given adenosine alone |
| Masaya 2004 | Retrospective study, not an RCT |
| Matsuo 2007 | Participants were not patients with AMI |
| Micari 2004 | Abstract only, cannot contact study authors |
| Michaels 2002 | Participants received saphenous vein graft percutaneous coronary intervention |
| Parikh 2004 | Not an RCT; intervention group did not meet inclusion criteria |
| Parikh 2007 | Not an RCT |
| Rezkalla 2010 | Not an RCT. Retrospective study |
| Ross 2005 | Participants received both PPCI and thrombolysis as reperfusion therapy |
| Sakuma 2010 | Study was a prospective, non‐randomised, open‐label trial |
| Tian 2008 | Participants underwent non‐urgent PCI, which did not meet inclusion criteria |
| Tsao 2009 | Complex coronary artery disease. Not an RCT |
| Vijayalakshmi 2006 | Participants were not only STEMI but also had non‐STEMI and unstable angina (acute coronary syndrome), so did not meet inclusion criteria |
| Werner 2002 | Not an RCT. AMI participants received not only direct PTCA but also rescue PTCA |
| Wilkinson 2010 | Not an RCT. Participants received saphenous vein graft percutaneous coronary intervention. Conference proceeding report |
| Zhen‐Guo 2010 | Abstract only, cannot contact study authors |
Characteristics of ongoing studies [ordered by study ID]
Garcia‐Dorado 2008.
| Trial name or title | Myocardial protection during reperfusion in patients with acute coronary syndrome with ST‐segment elevation submitted to primary angioplasty: effect of intracoronary adenosine on infarct size and ventricular remodeling |
| Methods | RCT |
| Participants | STEMI |
| Interventions | Intracoronary adenosine |
| Outcomes | Infarct size measured by MRI |
| Starting date | October 2008 |
| Contact information | |
| Notes | http://clinicaltrials.gov/show/NCT00781404 |
Gershlick 2011.
| Trial name or title | Randomized controlled trial comparing intracoronary administration of adenosine or sodium nitroprusside to control for attenuation of microvascular obstruction during primary percutaneous coronary intervention |
| Methods | RCT |
| Participants | STEMI undergoing primary percutaneous coronary intervention |
| Interventions | Intracoronary adenosine or sodium nitroprusside to control |
| Outcomes | Cardiac magnetic resonance measured infarct size, degree of ST resolution, incidence before and after angiographic procedure, true no‐reflow, overall major cardiac events |
| Starting date | 27 April 2011 |
| Contact information | |
| Notes | https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2010‐023211‐34 |
Niccoli 2009b.
| Trial name or title | Randomized evaluation of intracoronary nitroprusside vs adenosine after thrombus aspiration during primary percutaneous coronary intervention for the prevention of no‐reflow in acute myocardial infarction: the REOPEN‐AMI study protocol |
| Methods | RCT |
| Participants | AMI patients undergoing primary or rescue percutaneous coronary intervention and thrombus aspiration |
| Interventions | Receive intracoronary nitroprusside, adenosine or placebo |
| Outcomes | ST‐segment resolution, TIMI < 3, major cardiac events |
| Starting date | December 2007 |
| Contact information | |
| Notes | NCT00590070 |
Contributions of authors
Review authors contributed to the updated review by:
reviewing selected literature (LL, SQ);
appraising the papers (LL, NTS);
extracting the data (SQ, NTS);
entering data into Review Manager 5 (SQ, NTS);
conducting the analyses (SYH, SQ);
interpreting the analyses (SYH, LL); and
drafting the final updated review (LL, NTS, SQ).
Sources of support
Internal sources
Guangxi Medical University, China.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Desmet 2011 {published data only}
- Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, et al. High‐dose intracoronary adenosine for myocardial salvage in patients with acute ST‐segment elevation myocardial infarction. European Heart Journal 2011;32(7):867‐77. [PUBMED: 21196444] [DOI] [PubMed] [Google Scholar]
Fokkema 2009 {published data only}
- Fokkema ML, Vlaar PJ, Vogelzang M, Gu YL, Kampinga MA, Smet BJ, et al. Effect of high‐dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circulation. Cardiovascular Interventions 2009;2(4):323‐9. [PUBMED: 20031735] [DOI] [PubMed] [Google Scholar]
Grygier 2011 {published data only}
- Grygier M, Araszkiewicz A, Lesiak M, Janus M, Kowal J, Skorupski W, et al. New method of intracoronary adenosine injection to prevent microvascular reperfusion injury in patients with acute myocardial infarction undergoing percutaneous coronary intervention. The American Journal of Cardiology 2011;107(8):1131‐5. [PUBMED: 21310372] [DOI] [PubMed] [Google Scholar]
Ji 2007 {published data only}
- Ji ZG, Han JM, Liu G, Liu K. Effect of adenosine on ischemia‐reperfusion injury during percutaneous coronary intervention. Journal of Clinical Rehabilitative Tissue Engineering Research 2007; Vol. 11, issue 51:10399‐403.
Marzilli 2000 {published data only}
- Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 2000;101(18):2154‐9. [PUBMED: 10801755] [DOI] [PubMed] [Google Scholar]
Micari 2005 {published data only}
- Micari A, Belcik TA, Balcells EA, Powers E, Wei K, Kaul S, et al. Improvement in microvascular reflow and reduction of infarct size with adenosine in patients undergoing primary coronary stenting. The American Journal of Cardiology 2005;96(10):1410‐5. [PUBMED: 16275189] [DOI] [PubMed] [Google Scholar]
Petronio 2005 {published data only}
- Petronio AS, Carlo M, Ciabatti N, Amoroso G, Limbruno U, Palagi C, et al. Left ventricular remodeling after primary coronary angioplasty in patients treated with abciximab or intracoronary adenosine. American Heart Journal 2005;150(5):1015. [PUBMED: 16290987] [DOI] [PubMed] [Google Scholar]
Stoel 2008 {published data only}
- Stoel MG, Marques KM, Cock CC, Bronzwaer JG, Birgelen C, Zijlstra F. High dose adenosine for suboptimal myocardial reperfusion after primary PCI: a randomized placebo‐controlled pilot study. Catheterization and Cardiovascular Interventions 2008;71(3):283‐9. [DOI] [PubMed] [Google Scholar]
Taniyama 1997 {published data only}
- Taniyama Y, Ito H, Iwakura K, Masuyama T, Hori M, Takiuchi S, et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. Journal of the American College of Cardiology 1997;30(5):1193‐9. [PUBMED: 9350914] [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang X, Ding ZJ, Chen JZ. Journal of Clinical Medicine In Practice 2008; Vol. 12, issue 5.
Zhang 2012 {published data only}
- Zhang H, Tian NL, Hu ZY, Wang F, Chen L, Zhang YJ, et al. Three hours continuous injection of adenosine improved left ventricular function and infarct size in patients with ST‐segment elevation myocardial infarction. Chinese Medical Journal 2012;125(10):1713‐9. [PUBMED: 22800889] [PubMed] [Google Scholar]
References to studies excluded from this review
Babbitt 1988 {published data only}
- Babbitt DG, Perry JM, Forman MB. Intracoronary verapamil for reversal of refractory coronary vasospasm during percutaneous transluminal coronary angioplasty. Journal of the American College of Cardiology 1988;12:1377‐81. [DOI] [PubMed] [Google Scholar]
Dani 2009 {published data only}
- Dani S, Prajapati J, Jain S, Joshi H, Thanvi S, Sharma K, et al. Comparative data of intracoronary nicorandil and intracoronary adenosine in no (slow) reflow after primary percutaneous transluminal coronary angioplasty. American Journal of Cardiology 2009;103 (9):4B. [Google Scholar]
De Luca 2012 {published data only}
- Luca G, Iorio S, Venegoni L, Marino P. Evaluation of intracoronary adenosine to prevent periprocedural myonecrosis in elective percutaneous coronary intervention (from the PREVENT‐ICARUS trial). American Journal of Cardiology 2012;109:202‐7. [DOI] [PubMed] [Google Scholar]
Dillon 2001 {published data only}
- Dillon WC, Hadian D, Ritchie ME. Refractory no‐reflow successfully treated with local infusion of high‐dose adenosine and verapamil ‐ a case report. Angiology 2001;52:137‐41. [DOI] [PubMed] [Google Scholar]
Fischell 1998 {published data only}
- Fischell TA, Carter AJ, Foster MT, Hempsall K, DeVries J, Kim DH, et al. Reversal of "no reflow" during vein graft stenting using high velocity boluses of intracoronary adenosine. Catheterization and Cardiovascular Diagnosis 1998;45:360‐5. [DOI] [PubMed] [Google Scholar]
Forman 2008 {published data only}
- Forman MB, Hou DM, Jackson EK. Treating acute "no‐reflow" with intracoronary adenosine in 4 patients during percutaneous coronary intervention. Texas Heart Institute Journal 2008;35:439‐46. [PMC free article] [PubMed] [Google Scholar]
Garratt 1998 {published data only}
Granger 1997 {published data only}
- Granger CB. Adenosine for myocardial protection in acute myocardial infarction. American Journal of Cardiology 1997;79(12A):44‐8. [DOI] [PubMed] [Google Scholar]
Hang 2005 {published data only}
- Hang CL, Wang CP, Yip HK, Yang CH, Guo GB, Wu CJ, et al. Early administration of intracoronary verapamil improves myocardial perfusion during percutaneous coronary interventions for acute myocardial infarction. Chest 2005;128(4):2593‐8. [DOI] [PubMed] [Google Scholar]
Harding 2006 {published data only}
- Harding SA. The role of vasodilators in the prevention and treatment of no‐reflow following percutaneous coronary intervention. Heart 2006;92(9):1191‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heidland 2000 {published data only}
- Heidland UE, Heintzen MP, Michel CJ, Strauer BE. Effect of adjunctive intracoronary adenosine on myocardial ischemia, hemodynamic function and left ventricular performance during percutaneous transluminal coronary angioplasty: clinical access to ischemic preconditioning?. Coronary Artery Disease 2000, issue 5:421‐8. [DOI] [PubMed]
Huang 2009 {published data only}
- Huang D, Ma J, Zhang F, Wang X, Ge L, Qian J, et al. Effects of different vasodilators on no‐reflow phenomenon during percutaneous coronary intervention in patients with acute myocardial infarction. European Heart Journal 2009;30:248‐9. [Google Scholar]
Ibrahim 2002 {published data only}
- Ibrahim D, Huseyin Y, Cengiz E, Oktay S. Treatment of no‐reflow phenomenon with verapamil after primary stent deployment during myocardial infarction. Japanese Heart Journal 2002;43:573‐80. [DOI] [PubMed] [Google Scholar]
Jing 2010 {published data only}
- Jing W, Yun‐Dai C, Xiao Z, Guang Z. Adenosine improves myocardial perfusion in patients treated with percutaneous coronary intervention for first acute myocardial infarction. Circulation 2010;122(2):e162. [Google Scholar]
Kelly 2005 {published data only}
- Kelly RV, Cohen MG, Stouffer GA. Incidence and management of "no‐reflow" following percutaneous coronary interventions. American Journal of the Medical Sciences 2005;329(2):78‐85. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim SJ, Kim W, Park SH, Kim JT, Hwang SH, Gang DG, et al. Effect on myocardial protection of intracoronary adenosine and nicorandil injection in patients undergoing non urgent percutaneous coronary intervention. American Journal of Cardiology 2009;103(9):18B. [DOI] [PubMed] [Google Scholar]
Kloner 2006 {published data only}
- Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD‐2 trial. European Heart Journal 2006;27(20):2400‐5. [DOI] [PubMed] [Google Scholar]
Kopecky 2003 {published data only}
- Kopecky SL, Aviles RJ, Bell MR, Lobl JK, Tipping D, Frommell G, et al. A randomized, double‐blinded, placebo‐controlled, dose‐ranging study measuring the effect of an adenosine agonist on infarct size reduction in patients undergoing primary percutaneous,transluminal under coronary angioplasty: the ADMIRE (AmP579 Delivery for Myocardial Infarction REduction) study. American Heart Journal 2003;146(1):146‐52. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
Lim 2004 {published data only}
- Lim SY, Bae EH, Jeong MH, Kang DG, Lim YS, Kim KH, et al. Effect of combined intracoronary adenosine and nicorandil on no‐reflow phenomenon during percutaneous coronary intervention. Circulation Journal 2004;68(10):928‐32. [DOI] [PubMed] [Google Scholar]
Masaya 2004 {published data only}
- Masaya K, Keigo D, Shota S, Hiroaki T, Seiji H, Daiji H. Intracoronary verapamil rapidly terminates reperfusion tachyarrhythmias in acute myocardial infarction. Chest 2004;126(3):702‐8. [DOI] [PubMed] [Google Scholar]
Matsuo 2007 {published data only}
- Matsuo H, Watanabe S, Watanabe T, Warita S, Kojima T, Hirose T, et al. Prevention of no‐reflow/slow‐flow phenomenon during rotational atherectomy ‐ a prospective randomized study comparing intracoronary continuous infusion of verapamil and nicorandil. American Heart Journal 2007; Vol. 154, issue 5:994.e1‐6. [DOI] [PubMed]
Micari 2004 {published data only}
- Micari A, Belcik T, Balcells E, Kaul S, Wei K, Powers ER, et al. Intravenous adenosine improves microvascular reflow assessed by contrast echocardiography in patients with acute myocardial infarction. Circulation 2004;110:718. [Google Scholar]
Michaels 2002 {published data only}
- Michaels AD, Appleby M, Otten MH, Dauterman K, Ports TA, Chou TM, et al. Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: results of the randomized, controlled vasodilator prevention on no‐reflow (VAPOR) trial. Journal of Invasive Cardiology 2002;14(6):299‐302. [PubMed] [Google Scholar]
Parikh 2004 {published data only}
- Parikh K, Shah HD, Chag M, Shah U, Baxi H, Chandarana A, et al. Improvement in MACE by sequential administration of boluses or intracoronary adenosine and sodium nitroprusside during percutaneous coronary interventions. European Heart Journal 2004;25:518‐9. [Google Scholar]
Parikh 2007 {published data only}
- Parikh KH, Chag MC, Shah KJ, Shah UG, Baxi HA, Chandarana AH, et al. Intracoronary boluses of adenosine and sodium nitroprusside in combination reverse slow/no‐reflow during angioplasty: a clinical scenario of ischemic preconditioning. Canadian Journal of Physiology and Pharmacology 2007;85:476‐82. [DOI] [PubMed] [Google Scholar]
Rezkalla 2010 {published data only}
- Rezkalla SH, Dharmashankar KC, Abdalrahman IB, Kloner RA. No‐reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. Journal of Interventional Cardiology 2010;23(5):429‐36. [DOI] [PubMed] [Google Scholar]
Ross 2005 {published data only}
- Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, Investigators Amistad‐II. A randomized, double‐blinded, placebo‐controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD‐II). Journal of the American College of Cardiology 2005;45(11):1775‐80. [DOI] [PubMed] [Google Scholar]
Sakuma 2010 {published data only}
- Tadamichi S, Chikaaki M, Takehito T, Toshiharu O, Hiromichi T, Takenori O, et al. Exogenous adenosine triphosphate disodium administration during primary percutaneous coronary intervention reduces no‐reflow and preserves left ventricular function in patients with acute anterior myocardial infarction: a study using myocardial contrast echocardiography. International Journal of Cardiology 2010;140(2):200‐9. [DOI] [PubMed] [Google Scholar]
Tian 2008 {published data only}
- Tian F, Chen YD, Lü SZ, Song XT, Yuan F, Fang F, et al. Intracoronary adenosine improves myocardial perfusion in late reperfused myocardial infarction. Chinese Medical Journal 2008;121(3):195‐9. [PubMed] [Google Scholar]
Tsao 2009 {published data only}
- Tsao TP, Cheng SM, Cheng CC, Yang SP. Comparison of intracoronary adenosine and isosorbide dinitrate on no‐reflow/slow flow during rotational atherectomy. Acta Cardiologica 2009;64(2):225‐30. [DOI] [PubMed] [Google Scholar]
Vijayalakshmi 2006 {published data only}
- Vijayalakshmi K, Whittaker VJ, Kunadian B, Graham J, Wright RA, Hall JA, et al. Prospective, randomised, controlled trial to study the effect of intracoronary injection of verapamil and adenosine on coronary blood flow during percutaneous coronary intervention in patients with acute coronary syndromes. Heart (British Cardiac Society) 2006;92(9):1278‐84. [PUBMED: 16449518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 2002 {published data only}
Wilkinson 2010 {published data only}
- Wilkinson JR, Wragg A, Kapur A, Dymond DS. A single centre experience of pharmacological pre‐treatment of saphenous vein grafts to prevent no reflow post intervention. EuroIntervention Conference: EuroPCR. 2010.
Zhen‐Guo 2010 {published data only}
- Zhen‐Guo J, Jian‐Miao H, Gang L. Myocardial protective effect of adenosine during percutaneous coronary intervention. Circulation 2010;122(2):e247. [Google Scholar]
References to ongoing studies
Garcia‐Dorado 2008 {published data only}
- Garcia‐Dorado D. Myocardial protection during reperfusion in patients with acute coronary syndrome with ST segment elevation submitted to primary angioplasty: effect of intracoronary adenosine on infarct size and ventricular remodeling. ClinicalTrials.gov 2008. [http://clinicaltrials.gov/show/NCT00781404]
Gershlick 2011 {published data only}
- Gershlick AH. Randomized controlled trial comparing intracoronary administration of adenosine or sodium nitroprusside to control for attenuation of microvascular obstruction during primary percutaneous coronary intervention. ClinicalTrials.gov 2011. [https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2010‐023211‐34] [PubMed]
Niccoli 2009b {published data only}
- Niccoli G, D'amario D, Spaziani C, Cosentino N, Marino M, Rigattieri S, et al. Randomized evaluation of intracoronary nitroprusside vs. adenosine after thrombus aspiration during primary percutaneous coronary intervention for the prevention of no‐reflow in acute myocardial infarction: the REOPEN‐AMI study protocol. Journal of Cardiovascular Medicine 2009;10(7):585‐92. [PUBMED: 19384242] [DOI] [PubMed] [Google Scholar]
Additional references
Akhtar 1989
- Akhtar M, Tchou P, Jazayeri M. Use of calcium channel entry blockers in the treatment of cardiac arrhythmias. Circulation 1989;80(6 Suppl):IV31‐9. [PUBMED: 2688983] [PubMed] [Google Scholar]
Berne 1980
- Berne RM. The role of adenosine in the regulation of coronary blood flow. Circulation Research 1980;47(6):807‐13. [PUBMED: 6254686] [DOI] [PubMed] [Google Scholar]
Boersma 2006
- Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in‐hospital fibrinolysis in acute myocardial infarction patients. European Heart Journal 2006;27(7):779‐88. [PUBMED: 16513663] [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Deeks 2000
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analyses of binary data. Abstracts of 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. Cape Town: The Cochrane Collaboration, 2000.
Eeckhout 2001
- Eeckhout E, Kern MJ. The coronary no‐reflow phenomenon: a review of mechanisms and therapies. European Heart Journal 2001;22(9):729‐39. [PUBMED: 11350105] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forman 2006
- Forman MB, Stone GW, Jackson EK. Role of adenosine as adjunctive therapy in acute myocardial infarction. Cardiovascular Drug Reviews 2006;24(2):116‐47. [PUBMED: 16961725] [DOI] [PubMed] [Google Scholar]
Henriques 2003
- Henriques JP, Zijlstra F, 't Hof AW, Boer MJ, Dambrink JH, Gosselink M, et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 2003;107(16):2115‐9. [PUBMED: 12695301] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huikuri 2001
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. The New England Journal of Medicine 2001;345(20):1473‐82. [PUBMED: 11794197] [DOI] [PubMed] [Google Scholar]
Ikeda 1981
- Ikeda Y, Kikuchi M, Toyama K, Watanabe K, Ando Y. Inhibition of human platelet functions by verapamil. Thrombosis and Haemostasis 1981;45(2):158‐61. [PUBMED: 6789494] [PubMed] [Google Scholar]
Keeley 2003
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361(9351):13‐20. [PUBMED: 12517460] [DOI] [PubMed] [Google Scholar]
Kunadian 2008
- Kunadian V, Zorkun C, Williams SP, Biller LH, Palmer AM, Ogando KJ, et al. Intracoronary pharmacotherapy in the management of coronary microvascular dysfunction. Journal of Thrombosis and Thrombolysis 2008;26(3):234‐42. [PUBMED: 18818881] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Minamino 1998
- Minamino T, Kitakaze M, Asanuma H, Tomiyama Y, Shiraga M, Sato H, et al. Endogenous adenosine inhibits P‐selectin‐dependent formation of coronary thromboemboli during hypoperfusion in dogs. The Journal of Clinical Investigation 1998;101(8):1643‐53. [PUBMED: 9541494] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morishima 1995
- Morishima I, Sone T, Mokuno S, Taga S, Shimauchi A, Oki Y, et al. Clinical significance of no‐reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. American Heart Journal 1995;130(2):239‐43. [PUBMED: 7631601] [DOI] [PubMed] [Google Scholar]
Mustafa 2009
- Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handbook of Experimental Pharmacology 2009;193:161‐88. [PUBMED: 19639282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Niccoli 2009a
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no‐reflow in humans. Journal of the American College of Cardiology 2009;54(4):281‐92. [PUBMED: 19608025] [DOI] [PubMed] [Google Scholar]
Niccoli 2010
- Niccoli G, Marino M, Spaziani C, Crea F. Prevention and treatment of no‐reflow. Acute Cardiac Care 2010;12(3):81‐91. [PUBMED: 20698732] [DOI] [PubMed] [Google Scholar]
Nuesch 2010
- Nuesch E, Trelle S, Reichenbach S, Rutjes AWS, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [PUBMED: 20639294] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathore 2009
- Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, et al. Association of door‐to‐balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ (Clinical research ed.) 2009;338:b1807. [PUBMED: 19454739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rezkalla 2008
- Rezkalla SH, Kloner RA. Coronary no‐reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheterization and Cardiovascular Interventions 2008;72(7):950‐7. [PUBMED: 19021281] [DOI] [PubMed] [Google Scholar]
Simes 1995
- Simes RJ, Topol EJ, Holmes DR Jr, White HD, Rutsch WR, Vahanian A, et al. GUSTO‐I Investigators. Link between the angiographic substudy and mortality outcomes in a large randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion. Circulation 1995;91(7):1923‐8. [PUBMED: 7895348] [DOI] [PubMed] [Google Scholar]
Singh 1987
- Singh BN, Nademanee K. Use of calcium antagonists for cardiac arrhythmias. American Journal of Cardiology 1987;59(3):153B‐62B. [PUBMED: 2433930] [DOI] [PubMed] [Google Scholar]
Steg 2012
- Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. European Heart Journal 2012;33(20):2569‐619. [PUBMED: 22922416] [DOI] [PubMed] [Google Scholar]
Thygesen 2007
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. European Heart Journal 2007;28(20):2525‐38. [PUBMED: 17951287] [DOI] [PubMed] [Google Scholar]
Van de Werf 2008
- Werf F, Bax J, Betriu A, Blomstrom‐Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation: the Task Force on the Management of ST‐Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. European Heart Journal 2008;29(23):2909‐45. [PUBMED: 19004841] [DOI] [PubMed] [Google Scholar]
Weaver 1997
- Weaver WD, Simes RJ, Betriu A, Grines CL, Zijlstra F, Garcia E, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA 1997;278(23):2093‐8. [PUBMED: 9403425] [PubMed] [Google Scholar]
White 2008
- White HD, Chew DP. Acute myocardial infarction. Lancet 2008;372(9638):570‐84. [PUBMED: 18707987] [DOI] [PubMed] [Google Scholar]
Zhang 2012
- Zhang H, Tian NL, Hu ZY, Wang F, Chen L, Zhang YJ, et al. Three hours continuous injection of adenosine improved left ventricular function and infarct size in patients with ST‐segment elevation myocardial infarction. Chinese Medical Journal 2012;125(10):1713‐9. [PUBMED: 22800889] [PubMed] [Google Scholar]
Zijlstra 1993
- Zijlstra F, Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. New England Journal of Medicine 1993;328(10):680‐4. [PUBMED: 8433726] [DOI] [PubMed] [Google Scholar]


