Abstract
Background
Heavy menstrual bleeding (HMB) impacts the quality of life of otherwise healthy women. The perception of HMB is subjective and management depends upon, among other factors, the severity of the symptoms, a woman's age, her wish to get pregnant, and the presence of other pathologies. Heavy menstrual bleeding was classically defined as greater than or equal to 80 mL of blood loss per menstrual cycle. Currently the definition is based on the woman's perception of excessive bleeding which is affecting her quality of life.
The intrauterine device was originally developed as a contraceptive but the addition of progestogens to these devices resulted in a large reduction in menstrual blood loss: users of the levonorgestrel‐releasing intrauterine system (LNG‐IUS) reported reductions of up to 90%. Insertion may, however, be regarded as invasive by some women, which affects its acceptability.
Objectives
To determine the effectiveness, acceptability and safety of progestogen‐releasing intrauterine devices in reducing heavy menstrual bleeding.
Search methods
We searched the Cochrane Gynaecology and Fertility Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO and CINAHL (from inception to June 2019); and we searched grey literature and for unpublished trials in trial registers.
Selection criteria
We included randomised controlled trials (RCTs) in women of reproductive age treated with LNG‐IUS devices versus no treatment, placebo, or other medical or surgical therapy for heavy menstrual bleeding.
Data collection and analysis
Two authors independently extracted data, assessed risk of bias and conducted GRADE assessments of the certainty of evidence.
Main results
We included 25 RCTs (2511 women). Limitations in the evidence included risk of attrition bias and low numbers of participants.
The studies compared the following interventions.
LNG‐IUS versus other medical therapy
The other medical therapies were norethisterone acetate, medroxyprogesterone acetate, oral contraceptive pill, mefenamic acid, tranexamic acid or usual medical treatment (where participants could choose the oral treatment that was most suitable).
The LNG‐IUS may improve HMB, lowering menstrual blood loss according to the alkaline haematin method (mean difference (MD) 66.91 mL, 95% confidence interval (CI) 42.61 to 91.20; 2 studies, 170 women; low‐certainty evidence); and the Pictorial Bleeding Assessment Chart (MD 55.05, 95% CI 27.83 to 82.28; 3 studies, 335 women; low‐certainty evidence).
We are uncertain whether the LNG‐IUS may have any effect on women's satisfaction up to one year (RR 1.28, 95% CI 1.01 to 1.63; 3 studies, 141 women; I² = 0%, very low‐certainty evidence). The LNG‐IUS probably leads to slightly higher quality of life measured with the SF‐36 compared with other medical therapy (MD 2.90, 95% CI 0.06 to 5.74; 1 study: 571 women; moderate‐certainty evidence) or with the Menorrhagia Multi‐Attribute Scale (MD 13.40, 95% CI 9.89 to 16.91; 1 trial, 571 women; moderate‐certainty evidence).
The LNG‐IUS and other medical therapies probably give rise to similar numbers of women with serious adverse events (RR 0.91, 95% CI 0.63 to 1.30; 1 study, 571 women; moderate‐certainty evidence). Women using other medical therapy are probably more likely to withdraw from treatment for any reason (RR 0.49, 95% CI 0.39 to 0.60; 1 study, 571 women, moderate‐certainty evidence) and to experience treatment failure than women with LNG‐IUS (RR 0.34, 95% CI 0.26 to 0.44; 6 studies, 535 women; moderate‐certainty evidence).
LNG‐IUS versus endometrial resection or ablation (EA)
Bleeding outcome results are inconsistent. We are uncertain of the effect of the LNG‐IUS compared to EA on rates of amenorrhoea (RR 1.21, 95% CI 0.85 to 1.72; 8 studies, 431 women; I² = 21%; low‐certainty evidence) and hypomenorrhoea (RR 0.98, 95% CI 0.73 to 1.33; 4 studies, 200 women; low‐certainty evidence) and eumenorrhoea (RR 0.55, 95% CI 0.30 to 1.00; 3 studies, 160 women; very low‐certainty evidence). We are uncertain whether both treatments may have similar rates of satisfaction with treatment at 12 months (RR 0.95, 95% CI 0.85 to 1.07; 5 studies, 317 women; low‐certainty evidence).
We are uncertain if the LNG‐IUS compared to EA has any effect on quality of life, measured with SF‐36 (MD −14.40, 95% CI −22.63 to ‐6.17; 1 study, 33 women; very low‐certainty evidence). Women with the LNG‐IUS compared with EA are probably more likely to have any adverse event (RR 2.06, 95% CI 1.44 to 2.94; 3 studies, 201 women; moderate‐certainty evidence). Women with the LNG‐IUS may experience more treatment failure compared to EA at one year follow up (persistent HMB or requirement of additional treatment) (RR 1.78, 95% CI 1.09 to 2.90; 5 studies, 320 women; low‐certainty evidence); or requirement of hysterectomy may be higher at one year follow up (RR 2.56, 95% CI 1.48 to 4.42; 3 studies, 400 women; low‐certainty evidence).
LNG‐IUS versus hysterectomy
We are uncertain whether the LNG‐IUS has any effect on HMB compared with hysterectomy (RR for amenorrhoea 0.52, 95% CI 0.39 to 0.70; 1 study, 75 women; very low‐certainty evidence).
We are uncertain whether there is difference between LNG‐IUS and hysterectomy in satisfaction at five years (RR 1.01, 95% CI 0.94 to 1.08; 1 study, 232 women; low‐certainty evidence) and quality of life (SF‐36 MD 2.20, 95% CI −2.93 to 7.33; 1 study, 221 women; low‐certainty evidence).
Women in the LNG‐IUS group may be more likely to have treatment failure requiring hysterectomy for HMB at 1‐year follow‐up compared to the hysterectomy group (RR 48.18, 95% CI 2.96 to 783.22; 1 study, 236 women; low‐certainty evidence).
None of the studies reported cost data suitable for meta‐analysis.
Authors' conclusions
The LNG‐IUS may improve HMB and quality of life compared to other medical therapy; the LNG‐IUS is probably similar for HMB compared to endometrial destruction techniques; and we are uncertain if it is better or worse than hysterectomy.
The LNG‐IUS probably has similar serious adverse events to other medical therapy and it is more likely to have any adverse events than EA.
Keywords: Female; Humans; Antifibrinolytic Agents; Antifibrinolytic Agents/administration & dosage; Antifibrinolytic Agents/therapeutic use; Contraceptives, Oral; Contraceptives, Oral/administration & dosage; Contraceptives, Oral/therapeutic use; Endometrium; Endometrium/surgery; Hysterectomy; Intrauterine Devices, Medicated; Levonorgestrel; Levonorgestrel/administration & dosage; Levonorgestrel/therapeutic use; Mefenamic Acid; Mefenamic Acid/administration & dosage; Mefenamic Acid/therapeutic use; Menorrhagia; Menorrhagia/drug therapy; Menorrhagia/surgery; Norethindrone; Norethindrone/administration & dosage; Norethindrone/therapeutic use; Progesterone; Progesterone/administration & dosage; Progesterone/therapeutic use; Quality of Life; Randomized Controlled Trials as Topic; Tranexamic Acid; Tranexamic Acid/administration & dosage; Tranexamic Acid/therapeutic use; Treatment Outcome
Plain language summary
Use of progestogen‐releasing intrauterine systems for heavy menstrual bleeding
Review question
Cochrane authors assessed the effectiveness, acceptability and safety of the levonorgestrel‐releasing intrauterine system (LNG‐IUS) for treating heavy menstrual bleeding.
Background
Heavy or excessive menstrual bleeding is a common problem in women of reproductive age (between the first period and menopause). Women who feel that their menstrual bleeding is excessive will have reduced quality of life and are likely to seek medical help. A wide variety of medical treatments, of variable effectiveness, are available for women with heavy bleeding. These include oral tablets, such as non‐steroidal anti‐inflammatory drugs (NSAIDs), anti‐fibrinolytic drugs, the contraceptive pill, drugs containing progestogen and a progestogen‐releasing intrauterine system, a device placed inside the womb which regularly delivers small amounts of progestogen; it can also be used for contraception. Surgery, either hysterectomy (removal of the womb) or endometrial ablation (removal of the inner lining of the womb), is also commonly used, often when drug treatments are ineffective.
Study characteristics
This review contains 25 RCTs conducted up to June 2019 that included 2511 participants with heavy menstrual bleeding.
Key results
All the studies we included assessed the effects of one progestogen‐releasing intrauterine system (releasing 20 micrograms of levonorgestrel daily) (LNG‐IUS) and our conclusions refer only to this device. The LNG‐IUS may be more effective in reducing heavy menstrual bleeding and improving quality of life than other medical treatments.
We are uncertain if there is any difference between the LNG‐IUS and the techniques to remove the inner lining of the womb in reducing heavy menstrual bleeding, and improving quality of life. The effect on satisfaction may also be similar. Women using LNG‐IUS are probably more likely to have any adverse event, but this did not seem to cause women to stop taking their treatment.
We are uncertain if LNG‐IUS is as effective as hysterectomy in reducing menstrual blood loss but satisfaction and improvements in quality of life may be similar. Although a proportion of women trying the LNG‐IUS eventually went on to have a hysterectomy for their heavy menstrual bleeding, the LNG‐IUS appeared to have lower overall costs than either endometrial ablation or hysterectomy.
Certainty of the evidence
Many of the trials in this review were small (< 100 participants) and some were at high risk of bias. Ratings for the overall certainty of the evidence for each comparison ranged from very low to moderate. Limitations in the evidence included inadequate reporting of study methods and inconsistency. One large trial compared the LNG‐IUS with hysterectomy over a 10‐year period and a number of other trials made assessments two years after starting treatment, so we have some information on the long‐term effects of treatments.
Summary of findings
Summary of findings 1. LNG‐IUS compared to any other medical treatment for heavy menstrual bleeding.
| IUS compared to any other medical treatment for heavy menstrual bleeding | |||||||
| Patient or population: heavy menstrual bleeding Setting: any Intervention: IUS Comparison: any other medical treatment | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with any other medical treatment | Risk with IUS | ||||||
| Bleeding | Percentage reduction in blood loss at end of study (from baseline) ‒ Alkaline haematin method | The mean percentage reduction in blood loss at end of study (from baseline) ‒ Alkaline haematin method was 13 to 35 | MD 66.91 higher (42.61 higher to 91.20 higher) | ‐ | 170 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 5 | |
| Percentage reduction in blood loss at end of study (from baseline) ‒ PBAC score | The mean percentage reduction in blood loss at end of study (from baseline) ‒ PBAC score was 2 to 53 | MD 55.05 higher (27.83 higher to 82.28 higher) | ‐ | 335 (3 RCTs) | ⊕⊕⊝⊝ LOW 3 5 | ||
| Satisfaction | Proportion of women satisfied with treatment up to 1‐year follow‐up | 603 per 1000 | 771 per 1000 (609 to 982) | RR 1.28 (1.01 to 1.63) | 141 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | |
| Quality of life | Overall MMAS (difference between group in MMAS at 2 years) | The mean quality of life score (between group difference in MMAS over 2 years) (scores range 0 to 100, with lower scores indicating greater severity) |
MD 13.40 higher (9.89 higher to 16.91 higher) |
571 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | Overall MMAS score and mean quality of life with any other medical treatment cannot be reported, as the results were reported as mean difference | |
| Perception of general health (difference between group in SF‐36 at 2 years) | The mean quality of life score (between group difference in SF‐36 over 2 years) ‒ Perception of general health (score 0 to 100, with higher scores indication better perception of general health) | MD 2.90 higher (0.06 higher to 5.74 higher) |
571 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | |||
| Adverse events | Proportion of women with serious adverse events | 178 per 1000 | 162 per 1000 (112 to 232) |
RR 0.91 (0.63‐1.30) | 571 (1 RCT) |
⊕⊕⊕⊝ MODERATE 3 | There were no serious adverse reactions attributable to study treatments. There was no clear evidence of difference between the two groups in the frequency of unrelated serious adverse events. |
| Withdrawal from treatment for any reason |
570 per 1000 | 279 per 1000 (222 to 342) | RR 0.49 (0.39 to 0.60) | 571 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | ||
| Treatment failure | 568 per 1000 | 193 per 1000 (148 to 250) | RR 0.34 (0.26 to 0.44) | 535 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | ||
| Cost | No numeric data available for this outcome | ||||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded 1 level for risk of bias (attrition)
2 Downgraded 1 level for considerable heterogeneity
3 downgraded 1 level for risk of bias (performance)
4 Downgraded 1 level for imprecision (small studies)
5 Downgraded 1 level for risk of bias (selection)
Summary of findings 2. LNG‐IUS compared to endometrial ablation for heavy menstrual bleeding.
| IUS compared to endometrial ablation for heavy menstrual bleeding | |||||||
| Patient or population: heavy menstrual bleeding Setting: any Intervention: LNG‐IUS Comparison: endometrial ablation | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with endometrial ablation | Risk with IUS | ||||||
| Bleeding | PBAC score at 12 months | ‐ | see comment | not estimable | 122 (2 studies) | ⊕⊝⊝⊝ VERY LOW123 | Substantial heterogeneity so trials not combined. The trial at high risk of bias reported no difference between treatments and the trial with a lower risk of bias reported that PBAC score was lower with endometrial ablation |
| Improvement in HMB within 12 months: amenorrhoea | 186 per 1000 | 224 per 1000 (158 to 319) | RR 1.21 (0.85 to 1.72) | 431 (8 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| Improvement in HMB within 12 months: hypomenorrhoea | 462 per 1000 | 452 per 1000 (337 to 614) | RR 0.98 (0.73 to 1.33) | 200 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| Improvement in HMB within 12 months: eumenorrhoea | 269 per 1000 | 148 per 1000 (81 to 269) | RR 0.55 (0.30 to 1.00) | 160 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | ||
| Satisfaction with treatment | Within 1‐year follow‐up | 811 per 1000 | 770 per 1000 (689 to 868) | RR 0.95 (0.85 to 1.07) | 317 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Quality of life | Within 12 months' follow‐up ‒ General health (SF‐36) | The mean quality of life (SF‐36) within 12 months' follow‐up ‒ General health was 54.9 | MD 14.4 lower (22.63 lower to 6.17 lower) | ‐ | 33 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 4 | |
| Adverse events | Total proportion of women with any adverse events | 277 per 1000 | 571 per 1000 (399 to 815) | RR 2.06 (1.44 to 2.94) | 201 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Treatment failure | Discontinuation of initial treatment, adjunct medical therapy or persistent HMB at 12 months | 131 per 1000 | 234 per 1000 (143 to 381) | RR 1.78 (1.09 to 2.90) |
320 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Requirement for surgery (hysterectomy) at 12 months | 78 per 1000 | 200 per 1000 (116 to 345) | RR 2.56 (1.48 to 4.42) |
400 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| Cost | According to 1 study, the cost per woman of the LNG‐IUS is about 50% of the endometrial ablation. | ||||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded 1 level for high risk of bias (performance and detection bias)
2 Downgraded 1 level for risk of bias (attrition)
3 Downgraded 1 level for substantial heterogeneity
4 Downgraded 1 level for imprecision
Summary of findings 3. LNG‐IUS compared to hysterectomy for heavy menstrual bleeding.
| IUS compared to hysterectomy for heavy menstrual bleeding | |||||||
| Patient or population: heavy menstrual bleeding Setting: any Intervention: IUS Comparison: hysterectomy | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with hysterectomy | Risk with IUS | ||||||
| Bleeding | Amenorrhea at 12 months | 1000 per 1000 | 520 per 1000 (390 to 700) | RR 0.52 (0.39 to 0.70) | 75 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| Satisfaction | At 5 years' follow‐up | 930 per 1000 | 940 per 1000 (875 to 1000) | RR 1.01 (0.94 to 1.08) | 232 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | |
| Quality of life | Scores at end of study (change values) ‒ SF‐36: General health | The mean change in quality of life scores at end of study (change values) ‒ SF‐36: General health was −4.5 | MD 2.20 higher (2.93 lower to 7.33 higher) | ‐ | 221 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | |
| Adverse events | Only individual adverse events were reported | ||||||
| Treatment failure | Requirement for hysterectomy for HMB at 12 months' follow‐up | 0 per 1000 | 0 per 1000 (0 to 0) | RR 48.18 (2.96 to 783.22) | 236 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | |
| Cost | According to one study, the cost per woman is lower with LNG‐IUS at 12 months and at ten years. | ||||||
| Withdrawal of treatment no data available for these outcomes | |||||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | |||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded 1 level for risk of bias (performance and detection bias)
2 Downgraded 1 level for risk of bias (attrition)
3 Downgraded 1 level for imprecision
Background
Description of the condition
Heavy menstrual bleeding (HMB) is a common problem in women of reproductive age (Shapley 2004); it has a measurable effect on their quality of life (NICE 2018). It can also lead to iron deficiency anaemia (NICE 2018), which can be life threatening in the most severe cases (Moragianni 2007). The prevalence of HMB typically ranges from 9% to 14% in studies that assessed menstrual loss objectively or from 20% to 52% in studies based on subjective assessment (Fraser 2009; NICE 2018).
HMB has been classically defined as menstrual blood loss equal to or greater than 80 mL per menstrual cycle, which can be objectively measured by the alkaline haematin test (Cole 1971; Hallberg 1966); this measurement of menstrual loss is, however, impractical in routine practice. Another (more indirect) method of measuring menstrual loss is the pictorial blood loss assessment chart (PBAC) which was first reported in 1990 (Higham 1990). This method is highly variable, with sensitivity ranging from 58% to 97% and specificity ranging from 52% to 89% (NICE 2018); it is undertaken by the patient herself and has been more commonly used in research in the last decade than the objective alkaline haematin method. Currently, the woman's perception of heavy menstrual loss that impairs quality of life is the key determinant for referral and subsequent treatment (Munro 2012; NICE 2018)
Description of the intervention
The primary objectives of treating HMB are to reduce the amount of menstrual blood loss and to improve quality of life. Hysterectomy was traditionally considered the definitive treatment for HMB and it was one of the most commonly performed operations in women, with HMB being a leading indication (Farquhar 2002). Although total hysterectomy is invariably 100% successful in treating HMB and is associated with high success rates, it is major surgery. There are different routes for hysterectomy, all with different incidence of short‐term complications such as bleeding, infection, and wound healing problems, and a lengthy postoperative recovery period. There have been indications that the use of hysterectomy for HMB is declining from previous levels: in an analysis of inpatient hysterectomy rates in the USA between 1998 and 2010, overall 247,973 (36.4%) fewer hysterectomies were performed in 2010 compared with 2002, with a decline of 29% of hysterectomies performed for abnormal bleeding (Wright 2013). Given that HMB is a benign condition, many women prefer a less invasive surgical option that conserves the uterus. Endometrial resection and ablation procedures involve the destruction of the endometrium (inner lining of the womb) and the underlying basal glands by various means. These methods are safer than hysterectomy but also can cause complications, and there is a relatively high rate of further surgical treatment long term (Cooper 2011; Fergusson 2013). Thus, alternative medical therapy with the avoidance of possibly unnecessary surgery is an attractive alternative. A wide variety of medications is available to reduce HMB (Heikinheimo 2017). Many of these treatments, both hormonal and non‐hormonal, are usually first line options, with surgery only being used when medical therapy is ineffective or unsuccessful or inappropriate.
The intrauterine device was originally used primarily as a method of contraception. Progestogen‐releasing intrauterine systems were initially introduced in an effort to reduce intrauterine device expulsion. It became apparent that prolonged contraceptive use of these systems was associated with a profound reduction in menstrual blood loss (Andersson 1994; Berqvist 1983).
Progestasert was the first hormonally impregnated device, releasing 65 µg of progesterone per day; it required re‐insertion approximately yearly but was discontinued in 2001.
The levonorgestrel‐releasing intrauterine system (LNG‐IUS) (Mirena®,Bayer) has been available to manage HMB in the USA since 2009 and even earlier than this in Europe. It is available in 130 countries worldwide and is used by more than 15 million women around the world (Bayer S.A. 2019). It is a T‐shaped device, which releases levonorgestrel (LNG), a potent 19‐testosterone‐derived progestin, directly into the uterine cavity at a rate of 20 µg/day over a 5‐ to 7‐year time period and is associated with a profound reduction in menstrual blood loss (McNicholas 2015; Rowe 2016). New levonorgestrel‐releasing devices are currently available for contraception, one releasing 17.5 µg daily for five years; and the other two lasting three years, one releasing 18.6 µg daily and the second releasing 14 µg daily. There are no available studies for their use for HMB (Goldstuck 2017).
The LNG‐IUS insertion is an invasive procedure which may not be acceptable to some women. A disadvantage of the device is frequent and variable intermenstrual bleeding and spotting during the first few months of use (Zigler 2017). It is also an expensive intervention should its use be discontinued earlier than the five‐year lifespan for which it is licensed as an effective contraceptive. Discontinuation may be because of pelvic discomfort or dissatisfaction with the side effects.
How the intervention might work
Local hormone delivery results in high levonorgestrel levels in the endometrial tissue but systemic circulation levels are low. These effects can appear as early as one month after insertion, making it an effective method of non‐surgical management of HMB (Nilsson 1978). Locally released hormone leads to endometrial thinning, glandular atrophy (decreased size of glands), and inflammation. It leads to a reduction in HMB of more than 80% over 3‐ to 6‐months' treatment (Reid 2005b); and perceived subjective reduction in HMB is similar to that achieved after endometrial ablative treatments (Kaunitz 2009).
The LNG‐IUS has been compared favourably to other medical treatments for heavy cyclical blood loss (Kaunitz 2009; Milsom 1991). It improves dysmenorrhoea and may reduce the incidence of pelvic inflammatory disease, particularly in those under the age of 25 years, by thickening the utero‐cervical mucus. Twenty per cent of the women using the LNG‐IUS were amenorrhoeic during the first year of use (Sergison 2019). It also appears to have reduced the number of women undergoing hysterectomy (Reid 2005b).
Why it is important to do this review
HMB or menorrhagia is a common gynaecological condition and has an enormous effect on quality of life of affected women, and on the healthcare system. In the USA, the direct and indirect cost of management of HMB is approximately USD 1 billion and USD 12 billion, respectively (Liu 2007). The last update of this review, in 2015, suggested that the LNG‐IUS was more effective than oral medication as a treatment for HMB, was better at improving quality of life and appeared to be more acceptable long term. When compared to endometrial ablation, it was not clear whether the LNG‐IUS offers any benefits with regard to reduced HMB; and satisfaction rates and quality of life measures were similar. Since the previous publication of this review in 2015, a number of studies have been conducted to compare the progestogen‐releasing intrauterine system with other treatment modalities. Therefore, it was important to review these new studies with the aim of improving clinical practice.
Objectives
To determine the effectiveness, acceptability and safety of progestogen‐releasing intrauterine devices in reducing heavy menstrual bleeding.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of progestogen‐releasing intrauterine devices, specifically levonorgestrel‐releasing intrauterine system (LNG‐IUS) devices versus no treatment, placebo, or other medical or surgical therapies used to reduce heavy menstrual bleeding (HMB). We excluded quasi‐randomised trials.
Types of participants
Inclusion criteria
Women of reproductive years with regular heavy periods measured either objectively (by the alkaline haematin method), semi‐objectively (by PBAC score) or subjectively (patient perception)
Exclusion criteria
Postmenopausal bleeding (more than 1 year from the last menstrual period)
Irregular menses (periods either less than 21 days or more than 35 days apart) and intermenstrual bleeding (bleeding between periods) at presentation
Pathological causes of HMB
Primary use of progesterone‐releasing intrauterine system for any reason other than HMB, e.g. contraception or relief of climacteric symptoms
Conditions where the use of the LNG‐IUS is contraindicated
Source of recruitment
Community, primary care, family planning or specialist clinics
Types of interventions
Progestogen‐releasing intrauterine devices versus no treatment, placebo or any other medical or surgical treatment for the reduction of HMB.
Types of outcome measures
Primary outcomes
1) Menstrual bleeding
-
Objective assessment of menstrual blood loss (mL; measured by the alkaline haematin method) (Hallberg 1966) or semi‐objective assessment by the pictorial bleeding assessment chart score (PBAC) (Higham 1990)
measurement of menstrual blood loss at end of study compared between groups
measurement of change from baseline compared between group
prevalence of amenorrhoea, hypomenorrhoea or eumenorrhoea after treatment
-
Subjective assessment of menstrual blood loss
women's perception of improvement recorded in a reproducible format.
2) Satisfaction with treatment
Secondary outcomes
1) Quality of life: participant's perceived change in quality of life provided this was recorded in a reproducible and validated format (for example SF‐12 or SF‐36), or subjectively by participant questionnaires.
2) Adverse events
proportion of women with adverse events of any type
proportion of women with specific individual adverse events
3) Withdrawal from treatment because of adverse events or any reason
4) Treatment failure
5) Requirement of surgery for the treatment of HMB, either hysterectomy or any surgery (endometrial ablation or hysterectomy)
6) Resource cost
Search methods for identification of studies
Electronic searches
For the latest search (12 June 2019), we searched the following electronic databases, trial registers and websites.
Electronic databases (performed by Marian Showell Information Specialist CGFG).
Cochrane Gynaecology and Fertility Group specialised register; Procite platform, searched 12 June 2019 (Appendix 1)
CENTRAL; Ovid platform (Issue May 2019), searched 12 June 2019 (Appendix 2)
MEDLINE; Ovid platform, searched from 1946 to 12 June 2019 (Appendix 3)
Embase; Ovid platform searched from 1980 to 12 June 2019 (Appendix 4)
PsycINFO; Ovid platform searched from 1806 to 12 June 2019 (Appendix 5)
CINAHL; EBSCO platform searched from 1961 to 12 June 2019 (Appendix 6)
Trial registers and websites (performed by Magdalena Bofill).
ClinicalTrials.gov
World Health Organization (WHO) International Clinical Trials Registry Portal (ICTRP)
Searching other resources
We also searched the reference lists of eligible studies and relevant reviews and contacted the pharmaceutical company that supplies LNG‐IUS to identify further eligible studies for inclusion.
Data collection and analysis
Selection of studies
For the 2020 update of this review, two review authors (AL and MB) conducted an initial screen of titles and abstracts retrieved by the search and obtained the full text of studies that appeared eligible for the review, according to the inclusion criteria. The same two review authors independently examined the full‐text articles and selected studies that were eligible for inclusion.
We resolved disagreements by discussion. AL or MB corresponded with study investigators, as required, to clarify study eligibility.
For earlier versions of the review, the same selection process was undertaken by at least two review authors (AL and either IC, MR or MH; please see Contributions of authors below).
Data extraction and management
For the 2020 update of the review, two authors (AL and MB) independently extracted data from the eligible studies. We resolved disagreements by discussion until agreement was reached.
Data extracted included study characteristics and effect estimates. Where studies had multiple publications, we used the main trial report as the reference and derived additional details from the secondary papers, as required. AL corresponded with study investigators for additional data on the methods and results, but did not always receive replies. Where data were missing, we attempted to either impute values from similar studies or calculate values from formulas given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
For the 2020 update of the review, two review authors (AL and MB) independently assessed the new included studies for risk of bias, using the Cochrane 'Risk of bias' tool (Higgins 2011). Studies were assessed for allocation (random sequence generation and allocation concealment), blinding (of participants and personnel and separately of assessors), completeness of outcome data, selective reporting and other bias (such as comparability of groups at baseline or other potential source of bias). We graded each domain as low risk of bias, unclear risk of bias or high risk of bias. We noted source of funding for each study in the Characteristics of included studies table, although this is not a part of the Cochrane 'Risk of bias' tool. We resolved disagreements by discussion until consensus was reached.
For previous versions of the review, at least two review authors (AL and IC) independently assessed the included studies for method of randomisation, allocation concealment, blinding, methods of dealing with incomplete data and presence of intention‐to‐treat analysis and power calculations, and source of funding.
We include 'Risk of bias' assessments for each study in the Characteristics of included studies table and for each outcome the overall risk of bias (for each study) for all studies included in the comparison contribute to the overall certainty of evidence for the outcome. In addition, we have incorporated differences in the risk of bias into the interpretation of review findings by means of sensitivity analyses.
Measures of treatment effect
For dichotomous data (e.g. treatment failure or amenorrhoea rates), we used the number of events in the two groups to calculate Mantel‐Haenszel risk ratios (RRs), together with their 95% confidence intervals (CIs). Where there was a statistical difference between the two groups, we calculated number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) — these are estimates of the number of women who would need to receive treatment in order for one woman to receive the benefit or harm.
For continuous data (e.g. PBAC bleeding score), we calculated mean differences (MD) with 95% CIs between treatment groups. We treated ordinal data (e.g. quality of life scores) as continuous data. We only included continuous data in the meta‐analysis if the underlying distribution of the measurement appeared normal. We checked data roughly for skewness by calculating the ratio of the mean to its standard deviation; where this value was less than 1, we reported the data in tables as descriptive data. We also used tables when authors reported their results as a median plus range or when trial results were incomplete (e.g. we could not extract measures of variance).
In some trials, change scores (from baseline) were reported in preference to, or as well as, final values after treatment. We also included these data in the meta‐analysis. Where data were not reported in numbers in the text of the publications, we estimated data from figures or graphs.
Unit of analysis issues
We planned to include only first phase data from cross‐over trials, but found no cross‐over trials to include in the review.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis when possible, and made attempts to obtain missing data from the authors of the included studies when necessary. When these data were unobtainable and imputation or calculation were not feasible, we analysed only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. When we pooled studies in meta‐analysis, we examined heterogeneity (variation) between the results of different studies by inspecting the scatter in the data points and their overlap and, more formally, by checking the results of the I² value (Higgins 2011). This quantity describes the percentage of total variation across studies that is due to heterogeneity rather than chance. Interpretation of a given degree of heterogeneity will differ according to whether the estimates show the same direction of effect.
A rough guide to interpretation of the I² value is as follows (Higgins 2011).
0% to 40% might not be important
30% to 60% may represent moderate heterogeneity
50% to 90% may represent substantial heterogeneity
75% to 100% may represent considerable heterogeneity
When we identified considerable heterogeneity (I² > 90%) from the analyses, we did not pool the data but displayed the individual summary effect estimates in forest plots without totals.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of trials. As there were fewer than 10 studies contributing to each outcome, it was not possible for us to use of a funnel plot to further explore the potential for reporting bias and small‐study effects.
Data synthesis
If the included studies were sufficiently similar, we pooled their results in meta‐analysis using both fixed‐effect and random‐effects models. We presented results in the review using a fixed‐effect model where there was no evidence of substantial heterogeneity; otherwise we presented a random‐effects model. We made the following comparisons.
LNG‐IUS versus placebo/no treatment
LNG‐IUS versus any other medical treatment
LNG‐IUS versus endometrial ablation
LNG‐IUS versus hysterectomy
We stratified these comparisons by follow‐up interval, where necessary; we assessed outcomes up to 12 months after initiation of treatment or more than 12 months for outcomes such as satisfaction with treatment and treatment failure. Otherwise, we reported end‐of‐study data.
An increase in the risk of the LNG‐IUS intervention of a particular outcome which may be beneficial (e.g. satisfaction with treatment) is displayed graphically in the forest plots to the right of the centre line; otherwise, a decrease in the risk (e.g. adverse events) is displayed in the forest plots to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analysis to determine the separate evidence for the following outcomes.
Individual adverse events (e.g. nausea, vaginitis)
Method of assessing menstrual blood loss (alkaline haematin or PBAC scores)
Quality of life domain scores (e.g. physical functioning, general health)
Menstrual bleeding assessments (amenorrhoea, hypomenorrhoea, eumenorrhoea or subjectively assessed perception of improvement)
When we detected substantial heterogeneity (I² > 50%), we explored possible explanations by checking the data, examining clinical and methodological differences between the studies and conducting post hoc sensitivity analyses. We considered any substantial heterogeneity that we identified, especially when there was a variation in the direction of the effect, in our interpretation of the results.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high or unclear overall risk of bias;
eligibility was restricted to studies of participants with no evidence of fibroids;
analysis was stratified according to the type of medical treatment, type of endometrial ablation and type of hysterectomy in the control group;
eligibility was restricted to the LNG‐IUS.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro (GRADEpro GDT). This table evaluated the overall certainty of the body of evidence for the primary review outcomes of menstrual bleeding and satisfaction with treatment; and for the secondary outcomes of quality of life, adverse events, treatment withdrawal, treatment failure and cost, using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We have justified, documented, and incorporated into reporting of results our judgements about evidence certainty (high, moderate or low) for each outcome.
Results
Description of studies
Results of the search
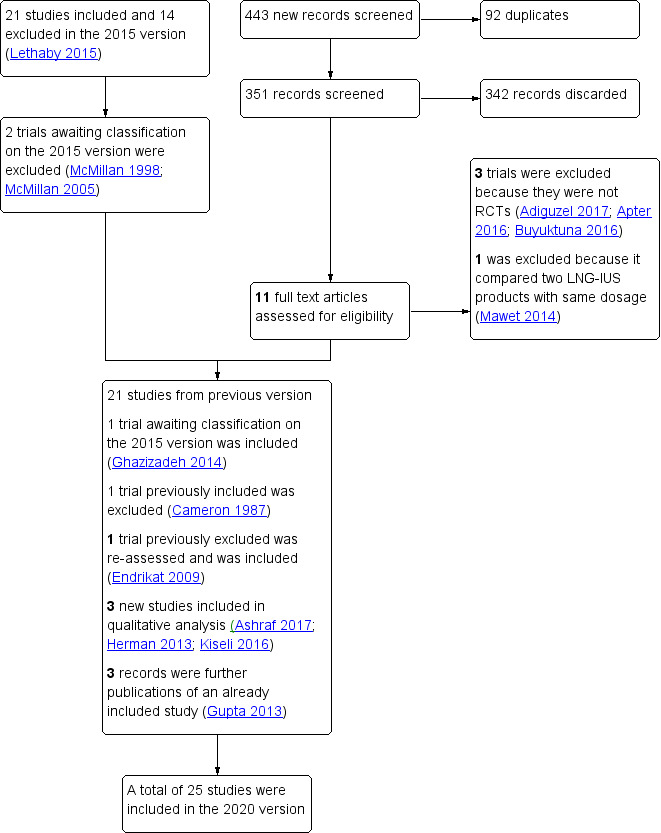
For the 2020 update, we identified a further nine potentially relevant studies from electronic databases and two studies from searches for closer inspection; we included three new studies in qualitative analysis and one new publication from a previously included study. For detailed search results, see Figure 1.
1.
Study flow diagram.
Included studies
Prior to the 2020 update, 21 randomised controlled trials (RCTs) met the criteria for inclusion in the review.
In the 2020 version, we considered two studies that were ongoing in the 2015 version. One study has not yet reported results (SHiPP 2013) — we contacted the authors and data will probably be available for the next review update; and the second (Herman 2013) has recently published a short report. Of the three studies awaiting classification in 2015, we have included Ghazizadeh 2014; and we have excluded two as no data have been published and we received no response from the authors (McMillan 1998; McMillan 2005).
We have excluded one study included prior to 2020, Cameron 1987, as the intervention is no longer available. It compared an intrauterine device (Progestasert) releasing 65 µg of progesterone daily but the device has been unavailable since 2001.
We reassessed one previously excluded study: originally we excluded it as a quasi‐randomized trial, but the researchers used a computer‐generated randomised list and women would take the next number on the list, so we decided to include it in this version (Endrikat 2009).
Twenty‐five studies with a total of 2511 randomly assigned women met the criteria for inclusion in the review, although not all participants contributed to the assessment of every outcome.
Details of the included studies and those awaiting classification or ongoing are displayed in Characteristics of included studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Participants
Participants were mostly recruited either from gynaecology clinics or by referral from general practitioners but some women referred themselves by responding to advertisements. In a few trials, women had been scheduled for hysterectomy; and in seven trials, women had failed first line medical therapy (Barrington 2003; de Souza 2010; Ghazizadeh 2014; Hurskainen 2001; Malak 2006; Sesti 2012; Tam 2006). A majority of trials excluded women with fibroids of any kind or either those greater than a certain diameter or those large enough to distort the uterine cavity. One research group investigated the effects of treatments separately in women with fibroids (but excluding submucous fibroids of any size distorting the uterine cavity or intramural or subserous fibroids greater than 5 cm in diameter) and women without any evidence of fibroids (in two separate publications). Many studies required women to have completed their families. Menstrual blood loss was usually confirmed by the alkaline haematin method or Pictorial Bleeding Assessment Chart (PBAC) scores prior to the initiation of treatment in consecutive menstrual cycles but in two trials, women were eligible if they considered their menstrual blood flow excessive. In one trial, participants complaining of HMB were only included if they had confirmed adenomyosis, but in two other trials adenomyosis was an exclusion criterion. One trial investigated the effects of treatments for HMB in women taking anticoagulant medication after cardiac valve replacement.
Interventions
The following interventions and comparisons were undertaken.
1. Comparisons with no treatment (1 trial)
One trial compared the levonorgestrel‐releasing intrauterine system (LNG‐IUS) with no treatment in women with anticoagulant therapy for cardiac valve replacement (Kilic 2009).
2. Comparisons with other medical treatments (9 trials)
Two trials compared the LNG‐IUS with norethisterone (long cycle) (Ashraf 2017; Irvine 1998)
One trial compared the LNG‐IUS with medroxyprogesterone acetate (MPA) (10 days) (Kaunitz 2010)
Three trials compared the LNG‐IUS with the combined oral contraceptive pill (COC) (Endrikat 2009; Sayed 2011; Shabaan 2011)
One trial compared the LNG‐IUS with mefenamic acid (Reid 2005a)
One trial compared the LNG‐IUS with a control group that was given a variety of medical treatments (tranexamic acid, mefenamic acid, combined oestrogen‐progestogen or progesterone alone) (Gupta 2013)
One trial compared the LNG‐IUS with tranexamic acid and norethisterone long cycle (Kiseli 2016)
3. Comparisons with surgical treatments: endometrial ablation (12 trials)
Three trials compared the LNG‐IUS with transcervical resection of the endometrium (Crosignani 1997; Kittelsen 1998; Malak 2006)
Seven trials compared the LNG‐IUS with thermal ablation (Barrington 2003; Herman 2013; de Souza 2010; Shaw 2007; Soysal 2002; TALIS 2006; Tam 2006)
One trial compared the LNG‐IUS with rollerball ablation (Ergun 2012)
One trial compared the LNG‐IUS with bipolar ablation and transcervical endometrial resection (Ghazizadeh 2014)
4. Comparisons with surgical treatments: hysterectomy (3 trials)
Three trials compared the LNG‐IUS with hysterectomy (Hurskainen 2001; Ozdegirmenci 2011; Sesti 2012)
All of the trials used the LNG‐IUS that releases 20 µg/day of levonorgestrel.
Outcomes
The effectiveness of LNG‐IUS in reducing HMB was measured either by PBAC scores or the alkaline haematin method. No trials were identified that measured women's own perception of improvement in HMB. Bleeding outcomes can be summarised as follows.
Alkaline haematin measurements (mL) at 3 months (Irvine 1998), 6 months (Kaunitz 2010; Reid 2005a), 12 months (Hurskainen 2001; Sayed 2011; Shabaan 2011) or at 5 and 10 years (Hurskainen 2001).
PBAC scores were measured at 6 months (Ashraf 2017; Barrington 2003; Crosignani 1997; Kilic 2009; Reid 2005a), 12 months (Crosignani 1997; de Souza 2010; Ergun 2012; Kittelsen 1998; Malak 2006; Sayed 2011; Sesti 2012; Shabaan 2011; Shaw 2007; Soysal 2002; TALIS 2006), 24 months (Herman 2013; Kittelsen 1998; Sesti 2012; Shaw 2007; TALIS 2006) and 5 years (de Souza 2010).
Categorisation of bleeding patterns as either amenorrhoea, hypomenorrhoea, spotting or normal was based on PBAC scores in 13 trials (Ashraf 2017; Herman 2013; Barrington 2003; Crosignani 1997; de Souza 2010; Endrikat 2009; Ergun 2012; Irvine 1998; Kiseli 2016; Malak 2006; Ozdegirmenci 2011; TALIS 2006; Tam 2006).
One trial measured total menstrual fluid loss (Reid 2005a).
Satisfaction with treatment was measured by 12 trials mostly at 12 months after the initiation of treatment, but also at longer time points (Herman 2013; Crosignani 1997; de Souza 2010; Endrikat 2009; Ergun 2012; Hurskainen 2001; Irvine 1998; Kiseli 2016; Malak 2006; Shaw 2007; Soysal 2002; TALIS 2006). Satisfaction was typically measured on a 5‐point scale, from very unsatisfied to very satisfied. Satisfaction rates in this review were scored when participants answered in the top two categories: very satisfied or somewhat/moderately satisfied.
Treatment failure was measured by 10 trials (Ashraf 2017; Endrikat 2009; Ergun 2012; Kaunitz 2010; Kittelsen 1998; Malak 2006; Sayed 2011; Shabaan 2011; TALIS 2006; Shaw 2007). Treatment failure was defined in various ways. In the trials where the LNG‐IUS was compared with either COC or medroxyprogesterone acetate (MPA) (other medical treatments), treatment failure was defined as either menstrual blood loss of 80 mL or more (alkaline haematin) and 50% or more reduction from baseline; or by the removal or expulsion of the LNG‐IUS or initiation of different treatment (either medical or surgical). In trials where the LNG‐IUS was compared with surgery (balloon, rollerball or transcervical resection of the endometrium (TCRE)), treatment failure was defined as an increase of HMB or no improvement in haemoglobin levels, major change in treatment (either expulsion or removal of the LNG‐IUS or initiation of alternative treatment or PBAC score ≥ 75 and re‐surgery in the surgical group or removal of the LNG‐IUS).
When the LNG‐IUS was compared to endometrial ablation and hysterectomy the requirement for further surgery for the treatment of HMB was reported by 10 studies. Six reported the requirement of further hysterectomy at different time frames (Herman 2013; de Souza 2010; Ergun 2012; Shaw 2007; Soysal 2002; TALIS 2006); and four reported the requirement of either endometrial ablation or hysterectomy at different time frames (Ergun 2012; Kittelsen 1998; Malak 2006; TALIS 2006).
Withdrawal from treatment for any reason was measured in one trial (Gupta 2013). The reasons given for withdrawal included adverse events, lack of efficacy, lack of tolerability, menopause or personal reasons.
Quality of life was measured by 14 trials (Crosignani 1997; de Souza 2010; Endrikat 2009; Gupta 2013; Hurskainen 2001; Kiseli 2016; Malak 2006; Ozdegirmenci 2011; Sayed 2011; Sesti 2012; Shabaan 2011; Soysal 2002; TALIS 2006; Tam 2006). The scales used included Medical Outcomes Study Short Form 36 Survey questionnaire (SF‐36), Psychological General Well‐Being Index (PGWBI), Menorrhagia Multi‐Attribute Scale (MMAS), EuroQol Group 5‐Dimension (EQ‐5D) questionnaire and visual analogue scale, RAND‐36 item Health Survey, World Health Organization Quality of Life Short Form (Turkish version) (WHOQOL‐BREF‐TR), and Health‐Related Quality of Life‐4 (HRQoL‐4). Other quality of life instruments in the included studies that measured specific aspects of quality of life, such as sexual functioning and anxiety, were not eligible for the review.
Adverse events were measured in 12 trials (Crosignani 1997; Endrikat 2009; Gupta 2013; Hurskainen 2001; Irvine 1998; Kittelsen 1998; Malak 2006; Ozdegirmenci 2011; Reid 2005a; Soysal 2002; TALIS 2006; Tam 2006). These were mostly measured incidentally and were secondary outcomes in the trials. Two trials also measured discontinuation from the study because of adverse events (Irvine 1998; Kittelsen 1998). Some adverse events were not directly compared because they were associated specifically with the mode of treatment, for example bowel perforation in hysterectomy or expulsion rate of the LNG‐IUS.
Costs were compared between groups in two trials (Hurskainen 2001; TALIS 2006); one compared costs of the LNG‐IUS with hysterectomy and the other with thermal balloon ablation.
Duration of follow‐up varied between the included studies. The single placebo/no treatment controlled study had minimal follow‐up of three months. Trials comparing the LNG‐IUS with various types of medical treatment ranged from two months' to two years' follow‐up. This latter trial, ELIPSE, is planning to monitor participants for five and 10 years. Over half of the trials comparing the LNG‐IUS with endometrial ablation had 12 months' follow‐up, one had six months' follow‐up and the remaining three trials had two, three and five years' follow‐up. Two of the trials comparing hysterectomy with the LNG‐IUS had 12 months' follow‐up; in the remaining trial, conducted in Finland, participants were monitored for 10 years. Outcomes from trials with minimal follow‐up should be considered with caution: from the case series studies, it is known that menstrual irregularity may be problematic in the first months after insertion of the LNG‐IUS (Suvisaari 1996), thus assessment of this method after two or three months may give a misleadingly poor outcome.
We could pool some of the outcomes from the studies in the meta‐analysis. We could not pool other outcomes because the data were heavily skewed or measures of variation were not reported and individual participant data were not available for transformation.
Excluded studies
We excluded four trials from this 2020 update. We excluded one that was previously included because the comparison was an IUS releasing progesterone that was withdrawn from the market in 2001 (Cameron 1987). We excluded another because participants were seeking contraception and not HMB treatment (Apter 2016); and two others because they were not RCTs (Adiguzel 2017; Buyuktuna 2016).
Prior to the 2015 update, five studies were excluded from those considered potentially eligible: two because there was no indication that they were randomised (Karacaoglu 2001; Romer 2000); one because it was not randomised (the first half received the device and the second half received medical treatment) (Milsom 1991); one because only 22% of the participants had HMB (Janssen 1999); and one because it experienced difficulties in recruitment so the trial was terminated (Rogerson 1999). A further study that had been included in previous versions of the review was excluded in the 2015 update because it no longer measured relevant outcomes (Lahteenmaki 1998).
Of 27 potentially relevant studies retrieved in the 2015 update, a further eight were excluded: one because it was an observational cohort study; four because the randomisation methods were not adequate (participants could choose treatment or allocation was by order of arrival or predefined application order); two because participants had endometrial hyperplasia; and one because of a substantial imbalance in the dropout rates between groups. We present details of all the excluded studies in Characteristics of excluded studies.
Risk of bias in included studies
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Random sequence generation
All included studies were randomised controlled trials, and adequate methods of randomisation (such as computer‐generated randomisation, permuted blocks or drawing from a hat) were reported in 18 trials (Ashraf 2017; Crosignani 1997; de Souza 2010; Endrikat 2009; Gupta 2013; Irvine 1998; Kaunitz 2010; Kiseli 2016; Kittelsen 1998; Ozdegirmenci 2011; Reid 2005a; Sayed 2011; Sesti 2012; Shabaan 2011; Shaw 2007; Soysal 2002; TALIS 2006; Tam 2006); and one trial protocol (Herman 2013). The method of randomisation was not reported in the remaining six trials and we considered these studies at unclear risk of bias (Barrington 2003; Ergun 2012; Ghazizadeh 2014; Hurskainen 2001; Kilic 2009; Malak 2006).
Allocation concealment
Adequate methods of allocation concealment were undertaken in 14 trials (Herman 2013; Crosignani 1997; Endrikat 2009; Gupta 2013; Hurskainen 2001; Irvine 1998; Kaunitz 2010; Reid 2005a; Sayed 2011; Sesti 2012; Shaw 2007; Soysal 2002; TALIS 2006; Tam 2006). In the remaining 11 trials, measures to conceal allocation were not reported and we scored these studies at unclear risk of bias (Ashraf 2017; Barrington 2003; de Souza 2010; Ergun 2012; Ghazizadeh 2014; Kilic 2009; Kiseli 2016; Kittelsen 1998; Malak 2006; Ozdegirmenci 2011; Shabaan 2011).
Blinding
We considered all trials at high risk of bias for blinding for the outcomes that were likely to be influenced by the lack of blinding. Mostly it was not feasible to blind participants to the differing nature of the interventions. As a majority of the primary outcomes were self‐reported by the participants, this means that assessments also were generally unblinded. We considered these studies at high risk of bias for these domains because knowledge of treatment could have influenced the responses made. One trial attempted to blind participants until randomised allocation was completed (Sesti 2012). Surgeons and assessors were blinded to allocation but the participants scored the PBAC instrument, quality of life and postoperative pain and the knowledge of treatment may have influenced their responses.
We considered 13 trials at low risk of bias for blinding (performance bias and detection bias) as they reported haematin alkaline or hematocrit, which are unlikely to be influenced by the lack of blinding (de Souza 2010; Endrikat 2009; Ergun 2012; Ghazizadeh 2014; Irvine 1998; Kaunitz 2010; Kilic 2009; Ozdegirmenci 2011; Sayed 2011; Shabaan 2011; Shaw 2007; Soysal 2002; Tam 2006).
Incomplete outcome data
We assessed 12 trials at low risk of bias because there were either no, or minimal, dropouts (dropouts were included in the analyses; or sensitivity analysis was performed to assess the impact of imputation for missing data) (Ashraf 2017; Herman 2013; Crosignani 1997; Endrikat 2009; Gupta 2013; Hurskainen 2001; Kaunitz 2010; Kilic 2009; Reid 2005a; Sesti 2012; Soysal 2002; Tam 2006). We considered five trials at unclear risk of bias because dropouts were minimal but differed by randomised group or increased with long‐term follow‐up (Barrington 2003; de Souza 2010; Ghazizadeh 2014; Malak 2006; TALIS 2006). We considered eight studies at high risk of bias because there was substantial dropout or a large imbalance in the dropout per group or reasons were not provided for dropouts (Ergun 2012; Kiseli 2016; Kittelsen 1998; Ozdegirmenci 2011; Sayed 2011; Shabaan 2011; Shaw 2007; Tam 2006).
Selective reporting
We considered 18 trials at low risk of bias because there was no clear evidence of selective reporting (Ashraf 2017; Crosignani 1997; Endrikat 2009; Gupta 2013; Hurskainen 2001; Irvine 1998; Kaunitz 2010; Kiseli 2016; Kittelsen 1998; Malak 2006; Ozdegirmenci 2011; Reid 2005a; Sayed 2011; Sesti 2012; Shabaan 2011; Shaw 2007; Soysal 2002; TALIS 2006); all prespecified outcomes were clearly reported in the results sections of the papers. We considered five studies at unclear risk of bias because adverse events were not reported or the outcomes were not clearly specified (Barrington 2003; de Souza 2010; Ergun 2012; Kilic 2009; Tam 2006). We considered two studies at high risk of reporting bias, because not all previously specified outcomes from the protocol were reported in one (Herman 2013); and the other provided no details about how the bleeding and satisfaction were assessed (Ghazizadeh 2014).
Other potential sources of bias
We considered 18 trials at low risk of bias because groups appeared to be comparable at baseline and there was no evidence of any other sources of bias (Ashraf 2017; Crosignani 1997; de Souza 2010; Gupta 2013; Hurskainen 2001; Irvine 1998; Kaunitz 2010; Kilic 2009; Kiseli 2016; Kittelsen 1998; Ozdegirmenci 2011; Sayed 2011; Sesti 2012; Shabaan 2011; Shaw 2007; Soysal 2002; TALIS 2006; Tam 2006). We considered four studies at unclear risk of bias because the authors did not report on participants' characteristics by group, or there were unequal numbers in the randomised groups with no explanation given, or it was not clear whether the imbalance in one participant's characteristics at baseline between groups could have resulted in bias (Herman 2013; Ergun 2012; Malak 2006; Reid 2005a). We considered three trials at high risk of other bias, two because the primary outcome differed substantially at baseline between groups and analyses were performed without adjustment (Barrington 2003; Ghazizadeh 2014); and one because three of the authors (including the principal author) were employees of a pharmaceutical company (which also funded the study) (Endrikat 2009).
A summary of the quality of the included studies is provided in Figure 2 and Figure 3.
Effects of interventions
See: Table 1; Table 2; Table 3
1 Progestogen‐releasing intrauterine system versus placebo/no treatment
One small study (40 women) compared the levonorgestrel‐releasing intrauterine device (LNG‐IUS) with no treatment in women taking anticoagulant medication after cardiac valve replacement (where women were at increased risk of heavy menstrual bleeding (HMB)) (Kilic 2009). At six months' follow‐up, women had lower PBAC scores after treatment with the LNG‐IUS compared with no treatment (mean difference (MD) −99.50, 95% confidence interval (CI) −115.75 to −83.25; 1 study, 40 participants; Analysis 1.1).
1.1. Analysis.

Comparison 1: LNG‐IUS versus placebo or no treatment, Outcome 1: Mean PBAC score at 6 months follow‐up
This study reported no other outcomes.
2 Progestogen‐releasing intrauterine system versus any other medical therapy
Nine trials compared a progestogen‐releasing intrauterine device with other medical treatment (Ashraf 2017; Endrikat 2009; Gupta 2013; Irvine 1998; Kaunitz 2010; Kiseli 2016; Reid 2005a; Sayed 2011; Shabaan 2011). The LNG‐IUS was compared with long‐cycle norethisterone in two trials (Ashraf 2017; Irvine 1998), to a 10‐day dose of medroxyprogesterone acetate (MPA) (Kaunitz 2010), to the oral contraceptive pill in women without and women with fibroids (Endrikat 2009; Sayed 2011; Shabaan 2011), to mefenamic acid (Reid 2005a), and to a variety of medical treatments (chosen by the patient and physician according to preference), including mefenamic acid, tranexamic acid, norethindrone, a combined oestrogen‐progestogen or progesterone‐only oral contraceptive pill or medroxyprogesterone acetate (MPA) injection (Gupta 2013). One trial compared the LNG‐IUS with norethisterone long cycle and tranexamic acid (Kiseli 2016).
Primary outcomes
2.1 Objective and semi‐objective measurements of menstrual blood loss
Compared with medical treatment, the LNG‐IUS was associated with reduced menstrual bleeding in most trials measuring this outcome.
Where summary effect measures could be calculated, treatment with the LNG‐IUS was associated with a greater percentage reduction from baseline by the alkaline haematin method when compared to the oral contraceptive pill (MD 66.91 mL, 95% CI 42.61 to 91.20; 2 studies, 170 women; I² = 81%; low‐certainty evidence), or by PBAC scores (MD 55.05, 95% CI 27.83 to 82.28; 3 studies, 335 women; I² = 79%; low‐certainty evidence; Analysis 2.2). Two of the trials in this pooled analysis also reported reduced menstrual blood loss at the end of study with the LNG‐IUS compared to the combined oral contraceptive, although substantial heterogeneity meant we could not pool the data (Analysis 2.1) (Sayed 2011; Shabaan 2011).
2.2. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 2: Percentage reduction in blood loss at end of study (from baseline)
2.1. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 1: Mean menstrual blood loss at end of study
Four other studies where we could not pool the data confirmed the benefits found with the LNG‐IUS (three studies with alkaline haematin measurement (Irvine 1998; Kaunitz 2010; Reid 2005a); and one with PBAC score measurement (Reid 2005a); one study found that menstrual fluid loss was reduced with the LNG‐IUS (Reid 2005a). These analyses considered any comparison of the LNG‐IUS with any medical therapy as a whole, given that women often are given choices of the most appropriate medical treatment, according to their personal circumstances/preference: mefenamic acid or oral progestogens. With regard to data on the delivery method of progestogens (intrauterine device versus oral treatment), two trials compared the LNG‐IUS with a long‐course oral progestogen,either norethisterone acetate (NET) or 10‐day MPA; in the LNG‐IUS versus NET comparison, there was no clear evidence of difference in reduction of HMB but the LNG‐IUS was more successful at reducing HMB than MPA (administered for 10 days).
One small study did not report clear evidence of difference in the proportion of women with amenorrhoea for longer than three months (RR 11.05, 95% CI 0.67 to 182.23; 35 women; Analysis 2.6) (Irvine 1998).
2.6. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 6: Improvement in HMB
We identified no trials that measured women's own perception of improvement in HMB.
2.2 Satisfaction
We are uncertain whether the LNG‐IUS compared to other medical treatment improves satisfaction with treatment for women with HMB (RR 1.28, 95% CI 1.01 to 1.63; 3 studies, 141 women; I² = 0%; very low‐certainty evidence; Analysis 2.8) (Endrikat 2009; Irvine 1998; Kiseli 2016).
2.8. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 8: Proportion of women satisfied with treatment up to one year follow up
Secondary outcomes
2.3 Quality of life
Quality of life outcomes were measured by three trials (Gupta 2013; Sayed 2011; Shabaan 2011).
We are uncertain whether the LNG‐IUS improves the quality of life of women compared to other medical treatment when it is self‐rated as good or excellent (RR 1.20, 95% CI 0.72 to 2.00; I² = 0%; 2 studies, 170 participants; very low) (Analysis 2.9) (Sayed 2011; Shabaan 2011).
2.9. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 9: Quality of life (good or excellent)
Results varied for different aspects of quality of life. There was clear evidence of difference favouring the LNG‐IUS in terms of unhealthy days in the past month physical (MD −1.40, 95% CI −2.17 to −0.63; I² = 70%; 2 studies, 170 participants) (Sayed 2011; Shabaan 2011) and in activity limitation (lost days in the past month) (MD −5.07, 95% CI −5.79 to −4.35; I² = 0%; 2 studies, 170 participants) (Sayed 2011; Shabaan 2011). The unhealthy days in the past month (mental) reported evidence of difference favouring other medical therapy (MD 1.44, 95% CI 0.61 to 2.27; 2 studies, 170 participants; I² = 94%). However, this result should be interpreted cautiously, as it has a very heterogeneity ( Analysis 2.10).
2.10. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 10: Quality of life (unhealthy days and lost days)
According to moderate‐certainty evidence the LNG‐IUS probably improves the quality of life of women with HMB compared to other medical treatments at 2‐year follow‐up using MMAS (Menorrhagia Multi‐Attribute Scale) summary score (MD 13.40, 95% CI 9.89 to 16.91; 1 study, 571 participants; moderate‐certainty evidence; Analysis 2.11) (Gupta 2013). However, there was no clear evidence of difference using MMAS between groups at five years follow‐up in the same trial (MD 3.90, 95% CI ‐0.60 to 8.40; 424; Analysis 2.12) (Gupta 2013). One trial reported quality of life using the SF36 and EQ‐5D at two and five years follow‐up (Gupta 2013). At two years follow‐up, there was difference favouring the LNG‐IUS compared to other medical treatments in physical role (MD 5.90, 95% CI 2.65 to 9.15), emotional role (MD 4.60, 95% CI 1.25 to 7.95), social functioning (MD 5.10, 95% CI 2.04 to 8.16), and energy and vitality (MD 5.30, 95% CI 2.46 to 8.14), perception of general health (MD 2.90, 95% CI 0.06 to 5.74; moderate‐certainty evidence) and pain (MD 7.80, 95% CI 4.55 to 11.05); but not in physical functioning (MD 2.70, 95% CI −0.00 to 5.40), mental health (MD 1.50, 95% CI −0.95 to 3.95), the EQ‐5D descriptive (MD 0.01, 95% CI −0.02 to 0.04) or visual analogue scale (MD 2.00, 95% CI −0.55 to 4.55). The evidence still favoured the LNG‐IUS at five years‐follow up for general health perception (MD 4.70, 95% CI 0.60 to 8.80); However, the remaining domains, such as physical functioning (MD 1.60, 95% CI ‐2.70 to 5.90), physical role (MD 2.70, 95% CI ‐2.10 to 7.50), emotional role (MD ‐2.00, 95% CI ‐6.80 to 2.80), social functioning (MD 2.20, 95% CI ‐2.50 to 6.90), mental health (MD ‐1.60, 95% CI ‐5.20 to 2.00), energy and vitality (MD 2.80, 95% CI ‐1.20 to 6.80), pain (MD 3.70, 95% CI ‐1.30 to 8.70), the EQ‐5D descriptive (MD ‐0.02, 95% CI ‐0.06 to 0.02) or visual analogue scale (MD 0.60, 95% CI ‐3.20 to 4.40) did not report clear evidence of difference at five years follow‐up.
2.11. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 11: Quality of life scores (between group difference in SF36 and EQ5D over 2 years)
2.12. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 12: Quality of life scores (between group difference in SF36 and EQ5D over 5 years)
Two trials reported no evidence of difference between the LNG‐IUS and other medical treatment in quality of life. One used the menorrhagia severity score (Analysis 2.13) (Endrikat 2009). The second, with 64 participants using the WHO QoL BrefTR, reported no differences in any domain: physical (MD −0.38, 95% CI −6.04 to 5.28), psychological (MD 0.56, 95% CI −5.18 to 6.30), social (MD −0.02, 95% CI −7.10 to 7.06), environmental (MD −0.43, 95% CI −4.93 to 4.07) and environmental TR (MD −0.32, 95% CI −4.92 to 4.28) (Analysis 2.14) (Kiseli 2016).
2.13. Analysis.
Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 13: Quality of life (menorrhagia severity score)
| Quality of life (menorrhagia severity score) | ||||
| Study | Comparison | N | Results | Conclusion/comment |
| Endrikat 2009 | COCP (20 ug ethinyl oestradiol + 1 mg norethindrone acetate) versus LNG IUS | Total: N = 42 FAS (full analysis set): N = 39 (19 in COCP group and 20 in LNG IUS group) |
Mean adjusted severity score (%) at end of study COCP: 16.24 LNG IUS: 12.02 No measure of variation reported |
Authors concluded that there was no difference between treatment groups (lower values were considered more beneficial) |
2.14. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 14: Quality of Life (WHO QoL‐Bref TR)
2.4 Adverse events
One large study reported no clear evidence of difference in the rate of serious side effects between groups (RR 0.91, 95% CI 0.63 to 1.30; 1 study, 571 women; moderate‐certainty evidence)(Analysis 2.15) (Gupta 2013).
2.15. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 15: Proportion of women with serious adverse events
There was also no clear evidence of difference in the prevalence of individual side effects but breast tenderness (RR 2.85, 95% CI 1.29 to 6.29; I² = 0%; 3 studies, 244 women) (Irvine 1998; Kaunitz 2010; Reid 2005a), and ovarian cysts (RR 3.28, 95% CI 1.31 to 8.21; I² = 0%; 3 studies, 784 women; Analysis 2.16) favour other medical treatments (Gupta 2013; Kaunitz 2010; Reid 2005a).
2.16. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 16: Individual adverse events
Individual side effects reported with no difference were as follows.
Pelvic pain (RR 2.22, 95% CI 0.94 to 5.23; 4 studies, 823 women; I² = 0%) (Endrikat 2009; Gupta 2013; Kaunitz 2010; Reid 2005a)
Mood swings (RR 1.08, 95% CI 0.60 to 1.95; 1 study, 31 women) (Irvine 1998)
Intermenstrual bleeding and menstrual irregularity (RR 0.98, 95% CI 0.53 to 1.82; 2 studies, 70 women; I² = 82%) (Endrikat 2009; Irvine 1998)
Nausea (RR 0.52, 95% CI 0.10 to 2.59; 1 study, 51 women) (Reid 2005a)
Diarrhoea (RR 0.26, 95% CI 0.03 to 2.17; 1 study, 51 women) (Reid 2005a)
Upper respiratory infection (RR 1.73, 95% CI 0.67 to 4.44; 2 studies, 213 women; I² = 45%) (Kaunitz 2010; Reid 2005a)
Headache (RR 1.20, 95% CI 0.75 to 1.93; 4 studies, 823 women; I² = 0%) (Endrikat 2009; Gupta 2013; Kaunitz 2010; Reid 2005a)
Vaginitis (RR 3.08, 95% CI 0.86 to 10.95; 1 study, 162 women) (Kaunitz 2010)
Acne (RR 1.02, 95% CI 0.31 to 3.41; 1 study, 162 women) (Kaunitz 2010)
Hypertension (RR 5.13, 95% CI 0.61 to 42.90; 1 study, 162 women) (Kaunitz 2010)
Sinusitis (RR 1.71, 95% CI 0.42 to 6.91; 1 study, 162 women) (Kaunitz 2010)
Fatigue (RR 2.05, 95% CI 0.39 to 10.88; 1 study, 162 women) (Kaunitz 2010)
Urinary tract infection (RR 2.05, 95% CI 0.53 to 7.92; 1 study, 162 women) (Kaunitz 2010)
Increased weight (RR 0.82, 95% CI 0.23 to 2.94; 1 study, 162 women) (Kaunitz 2010)
Lower abdominal pain (RR 0.42, 95% CI 0.13 to 1.44; 2 studies, 201 women; I² = 0%) (Endrikat 2009; Kaunitz 2010)
Any adverse event (RR 1.13, 95% CI 0.85 to 1.48; 2 studies, 101 women; I² = 0%) (Endrikat 2009; Kiseli 2016)
2.5 Withdrawal from treatment
2.5.1 For adverse events
There was no clear evidence of difference between groups in the withdrawal from treatment because of side effects (RR 1.07, 95% CI 0.74 to 1.54; 4 studies, 819 women; I² = 40%; Analysis 2.17) (Endrikat 2009; Gupta 2013; Irvine 1998; Kaunitz 2010).
2.17. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 17: Withdrawal from treatment
2.5.2 For any reason
One large study reported on the proportions of women who were still on treatment at 2‐year follow‐up (Gupta 2013). The reasons for discontinuation included adverse effects, lack of efficacy (treatment failure) and personal reasons (such as lack of tolerability, wish to become pregnant, pregnancy or menopause, among others). Women with the LNG‐IUS were less likely to withdraw from treatment within two years than those allocated to medical treatment (RR 0.49, 95% CI 0.39 to 0.60; 1 study, 571 women; Analysis 2.17).
2.6 Failure of treatment
Failure of treatment was reported in six trials in this comparison (Ashraf 2017; Endrikat 2009; Kaunitz 2010; Kiseli 2016; Sayed 2011; Shabaan 2011) (Analysis 2.18). The definition for treatment failure used in these trials was either PBAC score over 100 at the end of treatment or requirement for alternative treatment. Failure of treatment was less likely with the LNG‐IUS compared to medical treatment (RR 0.34, 95% CI 0.26 to 0.44; 6 studies, 535 women; I² = 62%; moderate‐certainty evidence) (Ashraf 2017; Endrikat 2009; Kaunitz 2010; Kiseli 2016; Sayed 2011; Shabaan 2011) (Analysis 2.18).
2.18. Analysis.

Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 18: Treatment failure (PBAC > 100 at end of treatment or requirement for alternative treatment )
Exploration of heterogeneity and sensitivity analyses
We identified substantial heterogeneity when studies were pooled for assessment of reduction in HMB. Two trials at high overall risk of bias measured menstrual blood loss at the end of the study in two different groups of participants: those with no fibroids; and those with fibroid‐related HMB by two different methods, alkaline haematin and PBAC scores. Although the summary estimates varied for HMB outcomes, the direction of effect always favoured the LNG‐IUS. The comparator was mostly the oral contraceptive pill but one study also compared the LNG‐IUS with MPA, an oral progestogen. The benefits we found with LNG‐IUS in the forest plots were confirmed by studies at low risk of bias, which we were not able to pool. There were generally too few studies to perform many sensitivity analyses. Adverse events were mainly recorded by studies at low risk of bias. For one outcome, withdrawal from treatment because of adverse events, the removal of a study at unclear overall risk of bias did not affect the findings. Thus, sensitivity analyses suggested that the findings were not influenced by trial quality, or women's fibroid status. We planned a sensitivity analysis restricting inclusion to LNG‐IUS studies only, but since only studies of the LNG‐IUS were considered in this update we could not perform it.
3 Progestogen‐releasing intrauterine system versus endometrial ablation
Three trials compared the LNG‐IUS with transcervical resection of the endometrium (TCRE) (Crosignani 1997; Kittelsen 1998; Malak 2006); one trial compared the LNG‐IUS with rollerball ablation (Ergun 2012); one trial compared LNG‐IUS with bipolar (Herman 2013); and six trials compared the LNG‐IUS with thermal balloon ablation (Barrington 2003; de Souza 2010; Shaw 2007; Soysal 2002; TALIS 2006; Tam 2006).
Primary outcomes
3.1 Objective and semi‐objective measurements of HMB
Findings were mixed with regard to bleeding outcomes. PBAC scores were lower after treatment with ablation in two studies (Crosignani 1997; Soysal 2002); they were lower in two other studies (both using balloon as control) in the LNG‐IUS groups (Shaw 2007; TALIS 2006); and there was no clear evidence of difference between groups in the remaining trials measuring this outcome (Barrington 2003; Ergun 2012; Kittelsen 1998; Malak 2006). When menstrual bleeding was categorised in terms of its flow (amenorrhoea, hypomenorrhoea etc.), there was no evidence of a difference in amenorrhoea (RR 1.21, 95% CI 0.85 to 1.72; participants = 431; studies = 8; I2 = 21%; low‐certainty evidence), hypomenorrhoea (RR 0.98, 95% CI 0.73 to 1.33; participants = 200; studies = 4; I2 = 0%; low‐certainty evidence) or eumenorrhoea rates (RR 0.55, 95% CI 0.30 to 1.00; I² = 61%; 3 studies, 160 women; low‐certainty evidence); and improvement in bleeding pattern was more likely in women with the LNG‐IUS (RR 1.20, 95% CI 1.02 to 1.41; I² = 40%; 3 studies, 172 women; Analysis 3.2).
3.2. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 2: Improvement in HMB
One trial reported data in a format that we could not include in the analysis (Herman 2013), and showed no clear evidence of difference at 24 months: mean PBAC scores were 64.8 in the LNG‐ IUS group and 14.2 in the EA group (difference 50.5, 95% CI −2.70 to 197.50).
We identified no trials that measured women's own perception of improvement in HMB.
3.2 Satisfaction with treatment
Satisfaction with treatment varied over time. There was no clear evidence of difference between treatments at 12 months' follow‐up (RR 0.95, 95% CI 0.85 to 1.07; 5 studies, 317 women; I² = 0%; low‐certainty evidence) (Crosignani 1997; Ergun 2012; Shaw 2007; Soysal 2002; TALIS 2006). At 24 months there was evidence of difference favouring the endometrial ablation group (RR 0.89, 95% CI 0.80 to 1.00; 2 studies, 319 women; I² = 0%) (Herman 2013; Shaw 2007); and at five years' follow up there was evidence of difference favouring the LNG‐IUS (RR 1.25, 95% CI 1.01 to 1.53; 1 study, 52 women; Analysis 3.4) (de Souza 2010).
3.4. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 4: Proportion of women satisfied with treatment
Secondary outcomes
3.3 Quality of life
Quality of life was measured by five trials, only two of which could be displayed in forest plots (TALIS 2006; Tam 2006).
In one trial there was no clear evidence of difference in the following measures.
Physical functioning (MD −3.10, 95% CI −11.10 to 4.90; 1 trial, 33 women) (Tam 2006)
Role limitation (physical) (MD −2.50, 95% CI −9.32 to 4.32; 1 trial, 33 women) (Tam 2006)
Bodily pain (MD 0.40, 95% CI −6.43 to 7.23; 1 trial, 33 women) (Tam 2006)
Vitality (MD −5.20, 95% CI −11.46 to 1.06; 1 trial, 33 women) (Tam 2006)
Overall SF‐36 score (MD 2.60, 95% CI −5.98 to 11.18; 1 trial, 79 women) (TALIS 2006)
One trial reported evidence of difference favouring the endometrial ablation group compared to the LNG‐IUS using the SF‐36 (Tam 2006).
Improved general health (MD −14.40, 95% CI −22.63 to −6.17; 1 trial, 33 women; very low‐certainty evidence) (Tam 2006)
Social functioning (MD −6.70, 95% CI ‐12.82 to −0.58; 1 trial, 33 women) (Tam 2006)
Emotional role limitation (MD −10.10, 95% CI −17.03 to −3.17; 1 trial, 33 women) (Tam 2006)
Mental health (MD −11.20, 95% CI −17.08 to −5.32; 1 trial, 33 women) in participants having endometrial ablation when compared to the LNG‐IUS (Tam 2006)
In three other trials also measuring SF‐36, we identified no clear evidence of differences in domains between groups, except for role limitations due to physical functioning; this was improved in the ablation group when compared to the LNG‐IUS group in one trial (descriptive results; Analysis 3.6). Another trial assessed outcomes at five years (de Souza 2010). It found that physical and emotional well‐being, assessed by participants as responses to a questionnaire, were improved in those having the LNG‐IUS compared to endometrial ablation but no differences were found in psychological well‐being, as assessed by the Psychological General Well‐being Index.
3.6. Analysis.
Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 6: Quality of life (QOL) scores at 12 months (SF36) ‐ descriptive results
| Quality of life (QOL) scores at 12 months (SF36) ‐ descriptive results | |||||
| Study | SF36 domains | LNG IUS group | Ablation group | Statistical test | Notes |
| Crosignani 1997 | Physical functioning: Social functioning: Role limitation (physical): Role limitation (emotional): Bodily pain: General health perception: Vitality: Mental health: | Median 85.0, IQR 62.8‐95.0 Median 75.0, IQR 50.0‐87.5 Median 100.0 IQR 50.0‐100.0 Median 66.7, IQR 33.3‐100.0 Median 41, IQR 41.0‐84.0 Median 65.0, IQR 51.0‐79.5 Median 55.0, IQR 47.5‐65.0 Median 60.0, IQR 46.0‐68.0 | Median 90.0, IQR 71.9‐94.7 Median 75.0, IQR 56.2‐87.5 Median 100.0 IQR 50.0‐100.0 Median 100.0 IQR 66.7‐100.0 Median 72.0, IQR 55.0‐92.0 Median 72.5, IQR 64.5‐77.0 Median 55.0, IQR 40.0‐70.0 Median 64.0, IQR 46.7‐68.0 | Mann Whitney U test No statistically significant differences between groups for any domain | |
| Soysal 2002 | Physical functioning: Social functioning: Role limitation (physical): Role limitation (emotional) Pain: General health perception Vitality: Mental health: |
Median: 72.5, IQR 53.7 to 91.2 Median 50, IQR 3.7 to 96.8 Median 25, IQR ‐25 to 75 Median 33.3, IQR ‐58.3 to 124.9 Median 51, IQR 30 to 72 Median 52, IQR 25.5 to 78.5 Median 45, IQR 26.2 to 63.7 Median 52, IQR 25 to 79 |
Median: 75, IQR 42.5 to 40 Median 50, IQR 125 to 87.5 Median 50, IQR ‐25 to 125 Median 33.3, IQR ‐33.3 to 99.9 Median 51, IQR 20 to 82 Median 47, IQR 19.5 to 74.5 Median 45, IQR 10 to 80 Median 52, IQR 22 to 82 |
Mann Whitney U test No significant differences between groups for any domain, except for role limitations due to physical functioning: Mean difference 20.22 (1.98 to 38.45) |
|
3.4 Adverse events
The proportion of women with adverse events reported clear evidence of difference favouring the endometrial ablation/resection group compared to the LNG‐IUS (RR 2.06, 95% CI 1.44 to 2.94; 3 studies, 201 women; I² = 0%; moderate‐certainty evidence)(Analysis 3.9) (Crosignani 1997; Malak 2006; Soysal 2002).
3.9. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 9: Total proportion of women with adverse events
Most of individual side effects did not report clear evidence of difference between groups.
Endometritis (RR 0.70, 95% CI 0.21 to 2.35; 2 studies, 120 women; I² = 79%) (Kittelsen 1998; TALIS 2006)
Pelvic pain or PID (RR 1.18, 95% CI 0.46 to 3.01; 3 studies, 180 women; I² = 0%) (Kittelsen 1998; Malak 2006; TALIS 2006)
Myometritis (RR 0.38, 95% CI 0.02 to 8.89; 1 study, 41 women) (Kittelsen 1998)
Adenomyosis (RR 0.38, 95% CI 0.02 to 8.89; 1 study, 41 women) (Kittelsen 1998)
Abnormal Pap (RR 0.16, 95% CI 0.01 to 2.99; 1 study, 41 women) (Kittelsen 1998)
Oedema (RR 3.45, 95% CI 0.15 to 80.03; 1 study, 41 women) (Kittelsen 1998)
Mood swings (RR 2.33, 95% CI 0.36 to 15.32; 2 studies, 132 women; I² = 0%) ( Malak 2006; Soysal 2002)
Nausea (RR 9.00, 95% CI 0.50 to 161.29; 1 study, 72 women) (Soysal 2002)
Headache (RR 2.04, 95% CI 0.64 to 6.50; 3 studies, 201 women; I² = 0%) (Crosignani 1997; Malak 2006; Soysal 2002)
Leg pain (RR 3.04, 95% CI 0.32 to 28.57; 2 studies, 141 women; I² = 0%) (Crosignani 1997; Soysal 2002)
Dysmenorrhoea (RR 0.25, 95% CI 0.03 to 2.17; 2 studies, 151 women; I² = 0%) (Soysal 2002; TALIS 2006)
Lower abdominal pain (RR 1.65, 95% CI 0.55 to 4.97; 4 studies, 242 women; I² = 0%) (Crosignani 1997; Kittelsen 1998; Malak 2006; Soysal 2002)
Actinomycoses (RR 2.93, 95% CI 0.12 to 69.74; 1 study, 79 women) (TALIS 2006)
Decreased libido (RR 1.03, 95% CI 0.15 to 6.90; 1 study, 69 women) (Crosignani 1997)
Hair loss (RR 5.14, 95% CI 0.26 to 103.35; 1 study, 69 women) (Crosignani 1997)
Anxiety or depression (RR 2.06, 95% CI 0.20 to 21.67; 1 study, 69 women) (Crosignani 1997)
Hypertension (RR 3.09, 95% CI 0.13 to 73.21; 1 study, 69 women) (Crosignani 1997)
Endometriosis (RR 0.38, 95% CI 0.02 to 8.89; 1 study, 41 women)(Kittelsen 1998)
Bleeding or spotting (RR 1.39, 95% CI 0.74 to 2.58; 4 studies, 241 women; I² = 67%) (Malak 2006; Soysal 2002; TALIS 2006; Tam 2006)
Hematometra (RR 0.33, 95% CI 0.01 to 7.87; 1 study, 60 women) (Malak 2006)
Vaginitis (RR 2.00, 95% CI 0.40 to 10.11; 1 study, 60 women) (Malak 2006)
Genital ulceration (RR 0.33, 95% CI 0.01 to 7.87; 1 study, 60 women) (Malak 2006)
There was clear evidence of difference in the following side effects favouring the endometrial ablation/resection group (Analysis 3.10).
3.10. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 10: Individual adverse events
Breast pain (RR 7.57, 95% CI 1.78 to 32.23; 3 studies, 201 women; I² = 0%) (Crosignani 1997; Malak 2006; Soysal 2002)
Weight gain (RR 2.60, 95% CI 1.16 to 5.84; 2 studies, 141 women; I² = 0%) (Crosignani 1997; Soysal 2002)
Bloating (RR 4.57, 95% CI 1.63 to 12.82; 3 studies, 141 women; I² = 0%) (Crosignani 1997; Malak 2006; Soysal 2002)
Acne or greasy skin (RR 8.40, 95% CI 1.57 to 44.76; 3 studies, 201 women; I² = 0%) (Crosignani 1997; Malak 2006; Soysal 2002)
3.5 Failure of treatment
Failure of treatment was defined as discontinuation of initial treatment, requirement of adjunct medical therapy or the persistence of menorrhagia.
Within the first year after treatment, treatment failure appeared to be more likely in the LNG‐IUS group than in the endometrial ablation group (RR 1.78, 95% CI 1.09 to 2.90; 5 studies, 320 women; I² = 0%; low‐certainty evidence) (Ergun 2012; Kittelsen 1998; Malak 2006; Shaw 2007; TALIS 2006), but there was no evidence of clear difference between groups at longer follow‐ups: at 24 months (RR 1.51, 95% CI 0.94 to 2.42; 3 studies, 199 women; I² = 0%) (Kittelsen 1998; Shaw 2007; TALIS 2006); and at 36 months (RR 1.50, 95% CI 0.61 to 3.69; 1 study, 60 women; Analysis 3.11) (Kittelsen 1998).
3.11. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 11: Treatment failure: discontinuation of initial treatment, adjunct medical therapy or persistent HMB)
For the comparisons where the comparator was a surgical procedure (endometrial ablation/resection or hysterectomy), studies reported the requirement for further surgery for the treatment of HMB.
The requirement for surgery (endometrial ablation or hysterectomy) for the treatment of HMB did not report clear evidence of difference between the LNG‐IUS and the endometrial resection/ablation group up to 36 months (Analysis 3.12).
3.12. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 12: Treatment failure: requirement for surgery for the treatment of HMB (ablation or hysterectomy)
At 12 months, RR was 1.47 (95% CI 0.67 to 3.23; 3 studies, 174 women; I² = 0%) (Ergun 2012; Kittelsen 1998; Malak 2006)
At 24 months, RR was 0.77 (95% CI 0.31 to 1.91; 2 studies, 139 women; I² = 65%) (Kittelsen 1998; TALIS 2006)
At 36 months, RR was 0.11 (95% CI 0.01 to 1.98; 1 study, 60 women) (Kittelsen 1998).
The requirement of hysterectomy for the treatment of HMB reported clear evidence of difference at 12 months' follow‐up in favour of the endometrial ablation/resection group (RR 2.56, 95% CI 1.48 to 4.42; 3 studies, 400 women; I² = 0%; low‐certainty evidence) (Herman 2013; Ergun 2012; Soysal 2002). There was no evidence of clear difference at two years' follow‐up (RR 1.14, 95% CI 0.50 to 2.60; 2 studies, 142 women; I² = 38%) (Shaw 2007; TALIS 2006); and five years' follow‐up (RR 0.16, 95% CI 0.02 to 1.21; 1 study, 58 women; Analysis 3.13) (de Souza 2010).
3.13. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 13: Treatment failure: requirement for hysterectomy
3.6 Resource cost
One trial published in 2006 found that the expected cost of treatment with the LNG‐IUS was NZD 1241 compared to NZD 2418 for balloon ablation. This finding was robust to sensitivity analysis which included a 25% decrease in the price of primary cost drivers and to variations in the rates of failed treatment.
Exploration of heterogeneity and sensitivity analyses
There were insufficient studies contributing to each outcome to undertake many sensitivity analyses. We noted some differences where we could perform these. In the forest plot measuring PBAC score at 12 months, substantial heterogeneity was demonstrated in the two studies measuring this outcome, one at low risk and the other at unclear risk of bias. When we excluded the study at unclear risk of bias, the PBAC score was improved in women undergoing ablation when compared with the LNG‐IUS. This finding was supported by the findings from the other study at low risk of bias, which we could not pool. The reported findings were not influenced by the type of endometrial ablation that was undertaken, or by whether the women had fibroids. We also identified substantial heterogeneity in satisfaction rates in the subgroup, from one year to five years' follow‐up. Both studies were at high overall risk of bias and the comparator was thermal balloon ablation. One of these studies measured satisfaction five years after treatment was initiated, however, while the other used different questioning after two years' follow‐up. Differences in the timing of the outcome and the questions asked may explain the discrepant findings between the two trials. As the included studies only assessed the LNG‐IUS, it was not possible to undertake our planned sensitivity analysis restricted to only studies of the LNG‐IUS.
4 Progestogen‐releasing intrauterine system versus hysterectomy
Three trials compared the LNG‐IUS with hysterectomy: one where abdominal, vaginal or laparoscopic hysterectomy was performed; another where only laparoscopic supracervical hysterectomy was performed; and for the third, the type of hysterectomy was not described (Hurskainen 2001; Ozdegirmenci 2011; Sesti 2012).
Primary outcomes
4.1 Objective and semi‐objective measurements of HMB
One trial (comparing the LNG‐IUS and supracervical laparoscopic hysterectomy) reported that the PBAC score difference between groups varied over time, showing no evidence of difference at 12 months (MD −0.20, 95% CI −5.52 to 5.12; 72 women) and clear evidence of difference favouring hysterectomy at 24 months, as the PBAC score increased with the LNG‐IUS (MD 52.66, 95% CI 28.86 to 76.46; 72 women; Analysis 4.2) (Sesti 2012). Amenorrhoea was reported in one trial (comparing the LNG‐IUS and abdominal hysterectomy): there was clear evidence of difference between groups at 12 months' follow‐up favouring hysterectomy (RR 0.52, 95% CI 0.39 to 0.70; 1 study, 75 women; very low‐certainty evidence)(Analysis 4.3) (Ozdegirmenci 2011). No trials were identified that measured women's own perception of improvement in HMB.
4.2. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 2: PBAC score
4.3. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 3: Amenorrhea at 12 months
4.2 Satisfaction with treatment
We are uncertain whether there was a difference in satisfaction with treatment at five years' follow‐up in one trial (RR 1.01, 95% CI 0.94 to 1.08; 1 study, 232 women; low‐certainty evidence; Analysis 4.4) (Hurskainen 2001).
4.4. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 4: Satisfaction with treatment (5 years follow‐up)
Secondary outcomes
4.3 Quality of life
One study at high risk of bias reported no clear evidence of differences in quality of life scales (physical, psychological, social and environmental domains) between groups (descriptive data) (Analysis 4.5) (Ozdegirmenci 2011).
4.5. Analysis.
Comparison 4: LNG‐IUS versus hysterectomy, Outcome 5: Quality of life scores at 12 months follow‐up (descriptive data)
| Quality of life scores at 12 months follow‐up (descriptive data) | |||||
| Study | Outcome | LNG IUS | Hysterectomy | Statistical test, results | Notes |
| Ozdegirmenci 2011 | WHOQOL‐BREF TR: Physical Psychological Social Environmental |
n = 43 Median = 68, IQR 59 to 77 Median = 58, IQR 51 to 66 Median = 67, IQR 59 to 75 Mean = 62, SD = 15 |
n = 32 Median = 72, IQR 57 to 84 Median = 62, IQR 50 to 75 Median = 67, IQR 55 to 78 Mean = 68, SD = 13 |
Mann Whitney U test, no difference between groups Student's T test, no difference between groups |
|
One study with 72 women, at moderate risk of bias, found improved emotional role and mental health scores (SF‐36) in women with the LNG‐IUS compared with hysterectomy (MD 16.10, 95% CI 8.88 to 23.32 and MD 36.80, 95% CI 30.37 to 43.23, respectively); and lower pain scores (SF‐36) in those having hysterectomy compared to the LNG‐IUS (MD −14.80, 95% CI −23.31 to −6.29; Analysis 4.6) (Sesti 2012). The same study reported no difference on the following measures using SF‐36.
4.6. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 6: Quality of life scores at end of study (final values)
General health (MD −0.80, 95% CI −6.03 to 4.43: 1 study, 72 women; low‐certainty evidence)
Physical (MD −5.00, 95% CI −10.77 to 0.77; 1 study; 72 women)
Physical role (MD −2.00, 95% CI −7.21 to 3.21; 1 study 72 women)
Social function (MD 1.80, 95% CI −5.00 to 8.60; 1 study; 72 women)
Vitality (MD 5.60, 95% CI −0.78 to 11.98; 1 study; 72 women)
One study, with 221 women, of unclear risk of bias also did not find evidence of a clear difference between groups at 10 years' follow‐up (Analysis 4.7) (Hurskainen 2001).
4.7. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 7: Quality of life scores at end of study (change values)
EQ‐5D (MD −1.09, 95% CI −1.15 to −1.03; 1 study, 221 women)
General health SF‐36 (MD 2.20, 95% CI −2.93 to 7.33; 1 study, 221 women)
Physical functioning SF‐36 (MD 0.40, 95% CI −5.51 to 6.31; 1 study, 221 women)
Emotional well‐being SF‐36 (MD 2.50, 95% CI −3.35 to 8.35; 1 study, 221 women)
Social functioning SF‐36 (MD 6.10, 95% CI −1.47 to 13.67; 1 study, 221 women)
Energy/vitality SF‐36 (MD 0.70, 95% CI −5.67 to 7.07; 1 study, 221 women)
Pain SF‐36 (MD 0.40; 95% CI ‐7.32 to 8.12; 1 study, 221 women)
Role ‒ physical SF‐36 (MD 5.00, 95% CI −7.49 to 17.49; 1 study, 221 women)
Role ‒ emotional SF‐36 (MD 4.20, 95% CI −10.03 to 18.43; 1 study, 221 women)
General Health ‒ visual analogue score (VAS) (MD 3.00, 95% CI −2.74 to 8.74; 1 study, 221 women)
4.4 Adverse events
A long list of immediate and long‐term adverse events was reported; the vast majority did not report clear evidence of difference between groups.
Early adverse events
Headache (RR 11.00, 95% CI 0.63 to 192.99; 1 study, 86 women) (Ozdegirmenci 2011)
Breast tenderness (RR 7.00, 95% CI 0.37 to 131.56; 1 study, 86 women) (Ozdegirmenci 2011)
Acne (RR 5.00, 95% CI 0.25 to 101.18; 1 study, 86 women) (Ozdegirmenci 2011)
Depressive episode (RR 3.96, 95% CI 0.45 to 35.06; 2 studies, 318 participants; I² = 0%) (Hurskainen 2001; Ozdegirmenci 2011)
Infected pelvis haematoma (RR 1.47, 95% CI 0.54 to 4.01; 1 study, 232 women) (Hurskainen 2001)
Postoperative fever (RR 0.49, 95% CI 0.05 to 5.35; 1 study, 232 women) (Hurskainen 2001)
Urinary retention (RR 0.25, 95% CI 0.03 to 2.17; 1 study, 232 women) (Hurskainen 2001)
Bladder perforation (RR 0.14, 95% CI 0.01 to 2.69; 1 study, 232 women) (Hurskainen 2001)
Bowel perforation (RR 0.33, 95% CI 0.01 to 7.96; 1 study, 232 women) (Hurskainen 2001)
Wound rupture (RR 0.20, 95% CI 0.01 to 4.05; 1 study, 232 women) (Hurskainen 2001)
Peritonitis (RR 0.33, 95% CI 0.01 to 7.96; 1 study, 232 women) (Hurskainen 2001)
Ileus (RR 0.20, 95% CI 0.01 to 4.05; 1 study, 232 women) (Hurskainen 2001)
Severe abdominal pain (RR 1.97, 95% CI 0.50 to 7.67; 1 study, 232 women) (Hurskainen 2001)
Vesicovaginal fistula (RR 0.33, 95% CI 0.01 to 7.96; 1 study, 232 women) (Hurskainen 2001)
Postoperative bleeding (RR 0.49, 95% CI 0.05 to 5.35; 1 study, 232 women) (Hurskainen 2001)
Intestinal occlusion (RR 2.95, 95% CI 0.12 to 71.65; 1 study, 232 women) (Hurskainen 2001)
Ureter lesion (RR 0.33, 95% CI 0.01 to 7.96; 1 study, 232 women) (Hurskainen 2001)
Thromboembolic disease (RR 2.95, 95% CI 0.12 to 71.65; 1 study, 232 women) (Hurskainen 2001)
Long‐term adverse events were reported in different publications at different follow‐up times in one study (Hurskainen 2001).
Abdominal pain (increase from baseline) (RR 0.60, 95% CI 0.30 to 1.22; 232 women)
Urge incontinence (RR 0.73, 95% CI 0.39 to 1.35; 221 women)
Stress incontinence (RR 0.70, 95% CI 0.48 to 1.01; 221 women)
Urinary tract infections (RR 0.45, 95% CI 0.20 to 1.03; 221 women)
There was clear evidence of difference between groups in one early adverse event: wound infection was more common in the hysterectomy group (RR 0.17, 95% CI 0.05 to 0.66; 2 studies, 307 women) (Hurskainen 2001; Ozdegirmenci 2011).
There was clear evidence of difference in two long‐term adverse events.
Increase from baseline in back pain favoured the LNG‐IUS compared to hysterectomy (RR 0.58, 95% CI 0.42 to 0.80; 1 study, 232 women) (Hurskainen 2001)
The prevalence of ovarian cysts was greater in women with the LNG‐IUS than those having hysterectomy (RR 2.72, 95% CI 1.24 to 5.97; 1 study, 180 women) (Hurskainen 2001) (Analysis 4.9)
4.9. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 9: Long term adverse events
4.5 Requirement of further surgery.
There was clear evidence of difference on the requirement of further surgery fir the treatment of HMB favouring hysterectomy (RR 48.18, 95% CI 2.96 to 783.22; 236women; one study; low‐certainty evidence; low‐certainty evidence) (Hurskainen 2001) (Analysis 4.10)
4.10. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 10: Treatment failure: requirement for surgery for HMB at 12 months follow up (hysterectomy)
4.6 Resource cost
Total healthcare costs and product losses per woman were lower in women with the LNG‐IUS than those having hysterectomy at 12 months' follow‐up (USD 1530, 95% CI 1203 to 1858 vs USD 4222, 95% CI 3808 to 4636) in one study published in 2001 (TALIS 2006). Women in this trial were monitored for a further nine years and at 10‐year follow‐up, 46% who were initially treated with the LNG‐IUS had a hysterectomy. Costs were still substantially lower in the LNG‐IUS group (discounted rate USD 3423) than in the hysterectomy group (USD 4937).
Exploration of heterogeneity and sensitivity analyses
We identified no heterogeneity. Sensitivity analyses suggested that the findings were not influenced by trial quality, women's fibroid status or type of hysterectomy. We planned a sensitivity analysis restricting inclusion to LNG‐IUS studies only but since we only considered studies of the LNG‐IUS in this update we did not perform the planned sensitivity analyses.
Discussion
Summary of main results
This review has assessed the effectiveness and safety of the levonorgestrel‐releasing intrauterine device (LNG‐IUS) in 25 studies with 2511 participants in total. The LNG‐IUS was compared with no treatment in one trial, with medical treatments in nine trials, with endometrial ablation in 12 trials and with hysterectomy in three trials.
The LNG‐IUS was compared with no treatment in women who had had a cardiac valve replacement and whose heavy menstrual bleeding (HMB) was considered potentially a side effect of their use of anticoagulant medications. The LNG‐IUS was associated with reduced menstrual blood loss after six months of approximately 100 Pictorial Bleeding Assessment Chart (PBAC) points, although the mean score (155.6) after treatment was still considered within the range of heavy menstruation. No other outcomes were reported for this comparison. Quality of life and satisfaction with treatment were not measured, so it is not possible to determine whether women found the treatment beneficial.
We have low confidence in the evidence of the effect of the LNG‐IUS compared to other medical treatments on menstrual bleeding outcomes, as the level of certainty was low. The evidence was, however, consistent across the different ways of measuring menstrual blood loss: these were percentage reduction in blood loss at end of study from baseline using either alkaline haematin method or PBAC, or as treatment success (PBAC < 100 at end of treatment or no requirement for alternative treatment). Two studies by the same authors confirmed that the benefits in reduction of HMB with the LNG‐IUS were found in both fibroid‐related and idiopathic HMB when compared with the oral contraceptive pill but both of these studies were at high overall risk of bias. It is important to note that participants with fibroid‐related menorrhagia were excluded if they had submucous fibroids of any size distorting the uterine cavity or intramural or subserous fibroids greater than 5 cm in diameter.
We have very little confidence that the rate of satisfaction is higher amongst LNG‐IUS users compared to other medical therapies (Figure 4). The improvement in quality of life is probably higher on the LNG‐IUS compared to other medical treatments. Probably specific side effects (such as breast tenderness and ovarian cysts) are more common with the LNG‐IUS, but there is no clear difference for serious adverse events. The difference on the withdrawal from treatment because of adverse events is probably the same for both groups, but withdrawal for any reason is probably lower with the LNG‐IUS. Treatment failure is also probably lower with the LNG‐IUS compared to other medical treatment.
4.

Forest plot of comparison: 2 LNG‐IUS versus any other medical treatment, outcome: 2.8 Proportion of women satisfied with treatment up to one year follow up.
When the LNG‐IUS was compared with endometrial ablative methods, evidence was mixed and inconsistent and mostly of low or very low overall certainty. We are uncertain whether there is a difference in bleeding outcomes, satisfaction with treatment (Figure 5), quality of life or rate of failure between treatments. The LNG‐IUS probably leads to a higher proportion of women with side effects (all adverse progestogenic effects). Endometrial resection/ablation may reduce the requirement for hysterectomy up to 1‐year follow‐up, but there is no difference in longer follow‐up. One trial conducted in New Zealand suggested that the LNG‐IUS was more cost effective than thermal balloon ablation.
5.

Forest plot of comparison: 3 IUS versus endometrial ablation, outcome: 3.4 Proportion of women satisfied with treatment.
The evidence from trials comparing the LNG‐IUS with hysterectomy was of low and very low certainty for the primary outcomes. Women having a hysterectomy had higher amenorrhoea rates when compared to those using the LNG‐IUS at one year. Quality of life and satisfaction with treatment were increased regardless of randomised treatment (Figure 6). One of the three studies included women with adenomyosis; in this study, quality of life was improved with both hysterectomy and the LNG‐IUS. Adverse event profiles differed: hysterectomy may be associated with more wound infection and back pain and the LNG‐IUS probably increases the incidence of ovarian cysts. Hysterectomy was less cost effective when compared to using the LNG‐IUS, in spite of the high rate of hysterectomy in the LNG‐IUS group at long‐term follow‐up (46% in 10 years in one trial)
6.

Forest plot of comparison: 4 IUS versus hysterectomy, outcome: 4.4 Satisfaction with treatment (5 years follow‐up).
Overall completeness and applicability of evidence
The review included 25 studies that compared the progestogen‐releasing intrauterine system with placebo/no treatment, medical treatment, endometrial ablation or hysterectomy in women with HMB. Some of the women in the trials had fibroid‐related bleeding (excluding large intramural/subserous fibroids and submucous fibroids causing distortion) and in others the HMB was associated with conditions such as adenomyosis or anticoagulant therapy. The effectiveness of the LNG‐IUS on bleeding outcomes was assessed in various ways: objectively by the alkaline haematin method, semi‐objectively by scores on the PBAC or according to definitions of menorrhagia or hypomenorrhoea. The findings with respect to HMB seemed consistent regardless of the method of measurement.
Reduction of HMB should correlate with improved quality of life. In the UK, NICE has provided a working definition of HMB based on quality of life, rather than measured blood loss (NICE 2018). It defines HMB as "excessive menstrual blood loss which interferes with a woman's physical, emotional, social and material quality of life, and which can occur alone or in combination with other symptoms" and emphasises that treatment should aim at improving quality of life measurements. Treatment with the LNG‐IUS generally improved health‐related quality of life measurements, irrespective of the quality‐of‐life instrument used and this treatment improved quality of life when compared to medical treatment and surgery. The rates of satisfaction were at least as good as medical treatment and surgery (either endometrial ablation or hysterectomy).
The LNG‐IUS was associated with a number of progestogen‐related adverse events in the included studies but there was no evidence that these effects resulted in increased withdrawal from treatment. Incidence of ovarian cysts increased but these were generally symptomless and had a high rate of resolution (Hurskainen 2001).
Only two studies measured comparative costs: one compared the LNG‐IUS with thermal balloon ablation (TALIS 2006); and the other compared the LNG‐IUS with hysterectomy for up to 10 years' follow‐up (Hurskainen 2001). The LNG‐IUS had a lower cost when compared to surgery. In the latter trial, at 10 years' follow‐up 46% of the women allocated to the LNG‐IUS eventually underwent hysterectomy but the discounted direct and indirect costs remained substantially lower than in the hysterectomy group.
Many of the trials in this review were small (< 100 participants) and some were at high risk of bias which means findings are sometimes inconsistent. One large trial compared the LNG‐IUS with hysterectomy over a 10‐year period and a number of other trials made assessments two years after starting treatment, so we have some information on the long‐term effects of treatments. Future research needs to measure satisfaction with treatment.
Quality of the evidence
Ratings for the overall quality of the evidence for each comparison ranged from very low to high (Table 1; Table 2; Table 3). Limitations in the evidence included inadequate reporting of study methods and heterogeneity.
Overall, the risk of bias in the included trials varied. All of the trials' participants were unblinded, mostly because of the nature of the interventions. Although the primary bleeding outcome was sometimes measured objectively, women were still required to estimate their bleeding by filling in a pictorial chart in some trials and it was not possible to exclude the likelihood that knowledge of their treatment influenced their responses. Over half of the included studies reported adequate allocation procedures and allocation concealment and less than half either had minimal missing data or used measures to prevent bias from attrition and loss to follow‐up. We report further details on the quality of the evidence in Figure 2 and Figure 3.
Many of the trials in this review were small (< 100 participants). One large trial compared the LNG‐IUS with hysterectomy over a 10‐year period and a number of other trials made assessments two years after starting treatment, so we have some information on the long‐term effects of treatments. Future research needs to measure satisfaction.
Potential biases in the review process
We made great effort to retrieve all eligible studies by implementing a comprehensive search strategy which included searches of grey literature, but it is not possible to exclude the chance that we missed some unpublished studies. We followed procedures to reduce other potential bias in the review process, such as duplicate selection of studies, data extraction and quality assessment.
Agreements and disagreements with other studies or reviews
Most studies comparing medical treatments for HMB have concluded that the LNG‐IUS is the most effective option, with the additional advantages that it offers contraception (NICE 2018), and the progestogen component of menopausal hormone therapy (Baldwin 2013; Somboonporn 2011). Reviews have generally concluded that the LNG‐IUS offers a considerable advantage over other medical treatments in reducing HMB (Kaunitz 2012; Matteson 2013; NICE 2018), with median percentage reductions often exceeding 90%, certainly in the short term, but these reviews have not been able to determine whether the benefits persist long term and whether these translate into reduced adverse events. The long‐term results of the ECLIPSE trial (Gupta 2013), included in this review, may be able to provide answers. This large trial found that the LNG‐IUS improved quality of life when compared to usual medical treatment for up to two years; and a large observational study (Xu 2014), undertaken in several countries in the Asia Pacific region, also found that both satisfaction with treatment and quality of life measures were greater in women using the LNG‐IUS when compared to oral medical treatments (antifibrinolytics, oral progestins or the contraceptive pill), although both groups achieved benefits over time. Another review reported that the LNG‐IUS was 20% less costly than oral medical treatment (mainly because of the high proportion of women in this latter group requiring additional surgical treatment) and also more effective (You 2006).
With respect to minimal surgery, two systematic reviews have suggested that the LNG‐IUS appears to be at least as effective as endometrial ablation (Middleton 2010; Kaunitz 2009 — although the former acknowledges that evidence is limited) with similar failure rates and quality of life.
Agreement is more mixed with regards to comparisons with hysterectomy which generally focus on quality of life and costs; these studies are strongly influenced by the perspective of the health system in the country of origin. One cost‐effectiveness review with a UK perspective concluded that hysterectomy was the preferred strategy for the first treatment of HMB when three options were compared: hysterectomy; endometrial ablation; and the LNG‐IUS (Roberts 2011). Although hysterectomy was more expensive, it produced more quality‐adjusted life years (QALYs), with the incremental cost‐effectiveness ratio for hysterectomy when compared with the LNG‐IUS being GBP 1440 per additional QALY. The results were highly sensitive to the utility values used in the analysis. Another review, using a healthcare Hong Kong perspective, concluded that hysterectomy was the most effective option (comparison of hysterectomy, endometrial ablation, LNG‐IUS and medical therapy) with the highest cost (You 2006). The incremental cost per additional QALY gained by hysterectomy was USD 23,500. The hysterectomy group gained a higher number of QALYs than the LNG‐IUS, oral medical treatment and endometrial ablation groups 99%, 99% and 98% of the time respectively and was more costly than the other three groups over 85% of the time. Another UK cost‐utility study compared the 5‐yearly cost of the LNG‐IUS followed by hysterectomy; the LNG‐IUS followed by endometrial ablation; immediate endometrial ablation by either thermal balloon or microwave; and hysterectomy (Clegg 2007). The LNG‐IUS followed by endometrial ablation dominated all the alternative treatments. By contrast, a review using a US payer perspective concluded that the LNG‐IUS resulted in the lowest treatment costs and the fewest number of hysterectomies performed over five years compared with all other initial strategies and resulted in the most QALYs gained among non‐surgical options (Ganz 2013). Initial treatment with the LNG‐IUS was the least costly and most effective option for women desiring to preserve their fertility. The discrepancy between the findings of these reviews is likely to relate to differences in the methods used and the cost input. However, assuming a USD 50,000 per QALY, LNG‐IUS treatment was either the dominant or a cost‐effective strategy and its overall associated costs were lower than those of other treatments for HMB.
Authors' conclusions
Implications for practice.
The levonorgestrel‐releasing intrauterine system (LNG‐IUS) results in a larger reduction in menstrual blood loss from baseline in women with HMB compared to other medical treatment or placebo, including selected women with fibroids. It appears to be more effective than oral medical therapies and results in better quality of life, higher satisfaction with treatment and lower withdrawal from treatment at two years.
There is very limited and low‐quality evidence that the LNG‐IUS appeared to have similar effectiveness to endometrial ablation methods; and quality of life outcomes were similar. The LNG‐IUS is associated with adverse events such as breast or pelvic pain and bloating when compared with other treatments, which are not directly comparable to the adverse events encountered with surgery. Both the LNG‐IUS and hysterectomy improved health‐related quality of life, which was most apparent within the five years after treatment. Although many women treated with the LNG‐IUS eventually had hysterectomy (up to 46% within 10 years), the LNG‐IUS remained cost effective.
Implications for research.
Further research is required to compare either specific types of endometrial destruction techniques and specific hysterectomy techniques with the LNG‐IUS. This is especially important on the comparison with hysterectomy as different routes can have different results and it can be very valuable to compare them.
Trials should plan for long‐term follow‐up and should focus on measuring satisfaction with treatment, acceptability of the treatment and quality of life of women with HMB.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2020 | Amended | Correction of typo |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 15 May 2020 | New citation required but conclusions have not changed | The addition of 5 new trials did not lead to a change in the conclusions of the review. |
| 15 May 2020 | New search has been performed | Updated in 2019/2020. 5 new trials included (Ashraf 2017; Herman 2013; Endrikat 2009 (excluded at the last published version); Ghazizadeh 2014 (awaiting classification at the last version); Kiseli 2016); 1 study previously included has been excluded (Cameron 1987). One new publication from a previously included study (Gupta 2013). |
| 20 January 2015 | New citation required and conclusions have changed | Twelve new trials added to the review. Conclusions changed. |
| 20 January 2015 | New search has been performed | Review updated in 2015. Twelve new trials added to the review (de Souza 2010; Ergun 2012; Gupta 2013; Kaunitz 2010; Kilic 2009; Malak 2006; Ozdegirmenci 2011; Sayed 2011; Sesti 2012; Shabaan 2011; Shaw 2007; Tam 2006). |
| 23 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
2020 update: we would like to thank Helen Nagels, Managing Editor, and Marian Showell, Information Specialist for their expert assistance with the review. We acknowledge and thank also Sarah Lensen, Jan Bosteels and Rik van Eekelen for providing peer review comment; Fiona Steward for her comments and feedback and Jason Elliot‐Smith for copy‐editing the review.
Prior to the 2020 update: the authors acknowledge the helpful comments of those who refereed previous versions of this review. Special thanks are also due to Mrs Helen Nagels, Ms Sarah Hetrick, Mrs Michele Proctor and Ms Shauna Sylvester, Review Group Managers and Managing Editors, for their tireless professionalism and help with the inevitable problems that arise; to Mrs Marian Showell, Mrs Sue Furness and Mrs Lisa McComb‐Williams, Trials Search Co‐ordinators and Information Specialists, for their assistance with identifying trials; and to Mrs Sue Hall, previous Secretary of the Review Group, for her secretarial help.
The authors also acknowledge the contribution of previous authors of the review: Inez Cooke, Munawar Hussain, Josephine Rishworth and Margaret Rees.
Appendices
Appendix 1. Cochrane Gynecology and Fertility Group (CGFG)
Searched 12 June 2019
Procite platform
Keywords CONTAINS "menorrhagia" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional bleeding" or "dysfunctional uterine bleeding" or "dysfunctional uterine bleeding" or "dysfunctional uterine bleeding" or "abnormal uterine bleeding" or "abnormal vaginal bleeding" or "excessive menstrual bleeding" or "excessive menstrual loss" or Title CONTAINS "menorrhagia" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional bleeding" or "dysfunctional uterine bleeding" or "abnormal uterine bleeding" or "abnormal vaginal bleeding" or "excessive menstrual bleeding" or "excessive menstrual loss"
AND
Keywords CONTAINS "progestagen" or "Progesterone" or "progestin" or "progestins" or "progestogen" or "progestogens" or "Levonorgestrel" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "IUD" or "LNG‐IUS" or "Mirena" or "Gestagen" or Title CONTAINS "progestagen" or "Progesterone" or "progestin" or "progestins" or "progestogen" or "progestogens" or "Levonorgestrel" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "IUD" or "LNG‐IUS" or "Mirena" or "Gestagen"
165 records
Appendix 2. CENTRAL search strategy
Searched 12 June 2019 (Issue May 2019)
Ovid platform
1 exp Menorrhagia/ (345) 2 Menorrhagia.tw. (545) 3 heavy menstrua$.tw. (338) 4 abnormal uterine bleeding.tw. (324) 5 (dysfunctional adj3 bleeding).tw. (175) 6 hypermenorr$.tw. (31) 7 excessive menstrua$.tw. (35) 8 DUB.tw. (53) 9 heavy period$.tw. (15) 10 ablation.tw. (6984) 11 hysterectom$.tw. (5976) 12 endometrial resection.tw. (94) 13 or/1‐12 (13781) 14 progest$.tw. (7789) 15 exp progestins/ or exp progesterone/ (3675) 16 exp Levonorgestrel/ (811) 17 Levonorgestrel.tw. (1483) 18 IUS.tw. (373) 19 LNG.tw. (669) 20 mirena.tw. (135) 21 IUD.tw. (1278) 22 medicated intrauterine device$.tw. (12) 23 intrauterine contraceptive device$.tw. (176) 24 exp intrauterine devices, medicated/ or exp intrauterine devices, copper/ (380) 25 intrauterine device$.tw. (1389) 26 or/14‐25 (12211) 27 26 and 13 (776)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 12 June 2019
Ovid platform 1 exp Menorrhagia/ (4148) 2 Menorrhagia.tw. (3155) 3 heavy menstrua$.tw. (882) 4 abnormal uterine bleeding.tw. (1957) 5 (dysfunctional adj3 bleeding).tw. (960) 6 hypermenorr$.tw. (293) 7 excessive menstrua$.tw. (182) 8 DUB.tw. (979) 9 heavy period$.tw. (97) 10 ablation.tw. (84538) 11 hysterectom$.tw. (34525) 12 endometrial resection.tw. (305) 13 or/1‐12 (125495) 14 progest$.tw. (94897) 15 exp progestins/ or exp progesterone/ (77878) 16 exp Levonorgestrel/ (4114) 17 Levonorgestrel.tw. (4466) 18 IUS.tw. (1096) 19 LNG.tw. (1694) 20 mirena.tw. (275) 21 IUD.tw. (6865) 22 medicated intrauterine device$.tw. (29) 23 intrauterine contraceptive device$.tw. (1702) 24 exp intrauterine devices, medicated/ or exp intrauterine devices, copper/ (3247) 25 intrauterine device$.tw. (5203) 26 or/14‐25 (137446) 27 26 and 13 (3838) 28 randomised controlled trial.pt. (483466) 29 controlled clinical trial.pt. (93106) 30 randomized.ab. (445540) 31 randomised.ab. (88852) 32 placebo.tw. (203762) 33 clinical trials as topic.sh. (187249) 34 randomly.ab. (312445) 35 trial.ti. (199928) 36 (crossover or cross‐over or cross over).tw. (80559) 37 or/28‐36 (1281431) 38 exp animals/ not humans.sh. (4587805) 39 37 not 38 (1178728) 40 27 and 39 (563)
Appendix 4. Embase search strategy
Searched from 1980 to 12 June 2019
Ovid platform
1 exp Menorrhagia/ (9105) 2 Menorrhagia.tw. (5084) 3 heavy menstrua$.tw. (1606) 4 abnormal uterine bleeding.tw. (3230) 5 (dysfunctional adj3 bleeding).tw. (1198) 6 hypermenorr$.tw. (412) 7 excessive menstrua$.tw. (244) 8 DUB.tw. (1348) 9 heavy period$.tw. (169) 10 ablation.tw. (126123) 11 hysterectom$.tw. (51103) 12 endometrial resection.tw. (439) 13 or/1‐12 (187121) 14 progest$.tw. (102750) 15 Levonorgestrel.tw. (5715) 16 IUS.tw. (1985) 17 LNG.tw. (2628) 18 mirena.tw. (1558) 19 IUD.tw. (6322) 20 medicated intrauterine device$.tw. (32) 21 intrauterine contraceptive device$.tw. (1426) 22 intrauterine device$.tw. (5983) 23 exp gestagen/ or exp levonorgestrel/ or exp progesterone/ (143023) 24 exp intrauterine contraceptive device/ or exp copper intrauterine device/ (15299) 25 or/14‐24 (191571) 26 13 and 25 (6960) 27 Clinical Trial/ (948276) 28 Randomized Controlled Trial/ (548503) 29 exp randomization/ (82653) 30 Single Blind Procedure/ (35282) 31 Double Blind Procedure/ (158186) 32 Crossover Procedure/ (59278) 33 Placebo/ (321204) 34 Randomi?ed controlled trial$.tw. (203351) 35 Rct.tw. (32522) 36 random allocation.tw. (1875) 37 randomly allocated.tw. (32455) 38 allocated randomly.tw. (2435) 39 (allocated adj2 random).tw. (803) 40 Single blind$.tw. (22703) 41 Double blind$.tw. (191160) 42 ((treble or triple) adj blind$).tw. (941) 43 placebo$.tw. (284098) 44 prospective study/ (523079) 45 or/27‐44 (2027296) 46 case study/ (61537) 47 case report.tw. (372039) 48 abstract report/ or letter/ (1055499) 49 or/46‐48 (1479435) 50 45 not 49 (1976729) 51 26 and 50 (1560)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 12 June 2019
Ovid platform
1 exp Menstrual Disorders/ (1208) 2 Menorrhagia.tw. (82) 3 heavy menstrua$.tw. (22) 4 abnormal uterine bleeding.tw. (24) 5 (dysfunctional adj3 bleeding).tw. (31) 6 hypermenorr$.tw. (2) 7 excessive menstrua$.tw. (6) 8 DUB.tw. (131) 9 heavy period$.tw. (11) 10 ablation.tw. (4028) 11 hysterectom$.tw. (798) 12 endometrial resection.tw. (4) 13 or/1‐12 (6233) 14 progest$.tw. (4708) 15 exp Progestational Hormones/ (2335) 16 exp Intrauterine Devices/ (128) 17 Levonorgestrel.tw. (103) 18 IUS.tw. (137) 19 LNG.tw. (57) 20 mirena.tw. (10) 21 IUD.tw. (213) 22 intrauterine contraceptive device$.tw. (13) 23 intrauterine device$.tw. (267) 24 or/14‐23 (5360) 25 13 and 24 (149) 26 random.tw. (55362) 27 control.tw. (425404) 28 double‐blind.tw. (22136) 29 clinical trials/ (11340) 30 placebo/ (5258) 31 exp Treatment/ (1004154) 32 or/26‐31 (1384561) 33 25 and 32 (84)
Appendix 6. CINAHL search strategy
Searched from 1961 to 12 June 2019
Ebsco platform
| # | Query | Results |
| S38 | S23 AND S37 | 125 |
| S37 | S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 | 1,188,882 |
| S36 | TX allocat* random* | 10,322 |
| S35 | (MH "Quantitative Studies") | 22,647 |
| S34 | (MH "Placebos") | 11,328 |
| S33 | TX placebo* | 57,156 |
| S32 | TX random* allocat* | 10,322 |
| S31 | (MH "Random Assignment") | 55,019 |
| S30 | TX randomi* control* trial* | 169,987 |
| S29 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,018,439 |
| S28 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 233 |
| S27 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 233 |
| S26 | TX clinic* n1 trial* | 245,100 |
| S25 | PT Clinical trial | 86,725 |
| S24 | (MH "Clinical Trials+") | 261,135 |
| S23 | S12 AND S22 | 541 |
| S22 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 | 9,042 |
| S21 | TX intrauterine contraceptive device | 425 |
| S20 | (MM "Intrauterine Devices") OR "intrauterine devices" | 2,863 |
| S19 | TX medicated intrauterine device* | 7 |
| S18 | TX IUD | 1,328 |
| S17 | TX mirena | 117 |
| S16 | TX LNG | 433 |
| S15 | TX Levonorgestrel | 1,840 |
| S14 | (MM "Levonorgestrel") OR "Levonorgestrel" | 1,819 |
| S13 | (MH "Progestational Hormones+") | 4,799 |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 | 34,939 |
| S11 | TX endometrial resection | 110 |
| S10 | TX hysterectom* | 9,908 |
| S9 | TX ablation | 23,660 |
| S8 | TX heavy period* | 258 |
| S7 | TX excessive menstrua* | 54 |
| S6 | TX hypermenorr* | 24 |
| S5 | TX (dysfunctional uterine bleeding) | 129 |
| S4 | TX abnormal uterine bleeding | 555 |
| S3 | TX heavy menstrua* | 484 |
| S2 | TX Menorrhagia | 1,319 |
| S1 | (MM "Menorrhagia") | 711 |
Data and analyses
Comparison 1. LNG‐IUS versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean PBAC score at 6 months follow‐up | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐99.50 [‐115.75, ‐83.25] |
Comparison 2. LNG‐IUS versus any other medical treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean menstrual blood loss at end of study | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.1 Alkaline haematin method (mL) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.2 PBAC score | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Percentage reduction in blood loss at end of study (from baseline) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Alkaline haematin method | 2 | 170 | Mean Difference (IV, Random, 95% CI) | 66.91 [42.61, 91.20] |
| 2.2.2 PBAC score | 3 | 335 | Mean Difference (IV, Random, 95% CI) | 55.05 [27.83, 82.28] |
| 2.3 Menstrual blood loss by alkaline haematin ‐ descriptive results | 3 | Other data | No numeric data | |
| 2.4 PBAC scores for menstrual blood loss ‐ descriptive results | 1 | Other data | No numeric data | |
| 2.5 Percentage change in MBL (from baseline to end of study) | 2 | Other data | No numeric data | |
| 2.6 Improvement in HMB | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.05 [0.67, 182.23] |
| 2.6.1 Amenorrhoea greater than 3 months | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.05 [0.67, 182.23] |
| 2.7 Total menstrual fluid loss ‐ descriptive results | 1 | Other data | No numeric data | |
| 2.8 Proportion of women satisfied with treatment up to one year follow up | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.01, 1.63] |
| 2.9 Quality of life (good or excellent) | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.72, 2.00] |
| 2.10 Quality of life (unhealthy days and lost days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.10.1 Unhealthy days in past month (physical) | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.17, ‐0.63] |
| 2.10.2 Unhealthy days in past month (mental) | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | 1.44 [0.61, 2.27] |
| 2.10.3 Activity limitation in past month (lost days) | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐5.07 [‐5.79, ‐4.35] |
| 2.11 Quality of life scores (between group difference in SF36 and EQ5D over 2 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.11.1 Overall MMAS | 1 | Mean Difference (IV, Fixed, 95% CI) | 13.40 [9.89, 16.91] | |
| 2.11.2 SF36: physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐0.00, 5.40] | |
| 2.11.3 SF36: physical role | 1 | Mean Difference (IV, Fixed, 95% CI) | 5.90 [2.65, 9.15] | |
| 2.11.4 SF 36: Emotional role | 1 | Mean Difference (IV, Fixed, 95% CI) | 4.60 [1.25, 7.95] | |
| 2.11.5 SF 36: Social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 5.10 [2.04, 8.16] | |
| 2.11.6 SF 36: Mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐0.95, 3.95] | |
| 2.11.7 SF 36: Energy and vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [2.46, 8.14] | |
| 2.11.8 SF 36: Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 7.80 [4.55, 11.05] | |
| 2.11.9 SF 36: Perception of general health | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [0.06, 5.74] | |
| 2.11.10 EQ5D descriptive | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] | |
| 2.11.11 EQ5D visual analogue scale | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐0.55, 4.55] | |
| 2.11.12 over 2 years | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable | |
| 2.12 Quality of life scores (between group difference in SF36 and EQ5D over 5 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.12.1 Overall MMAS | 1 | Mean Difference (IV, Fixed, 95% CI) | 3.90 [‐0.60, 8.40] | |
| 2.12.2 SF 36 Physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐2.70, 5.90] | |
| 2.12.3 SF36: Physical role | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.10, 7.50] | |
| 2.12.4 SF 36: Emotional role | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐6.80, 2.80] | |
| 2.12.5 SF36 Social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐2.50, 6.90] | |
| 2.12.6 SF 36 Mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐5.20, 2.00] | |
| 2.12.7 SF 36 Energy/vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐1.20, 6.80] | |
| 2.12.8 SF 36 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐1.30, 8.70] | |
| 2.12.9 SF 36 General health perception | 1 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [0.60, 8.80] | |
| 2.12.10 EQ‐5D Descriptive system | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] | |
| 2.12.11 EQ‐5D VAS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐3.20, 4.40] | |
| 2.13 Quality of life (menorrhagia severity score) | 1 | Other data | No numeric data | |
| 2.14 Quality of Life (WHO QoL‐Bref TR) | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐2.55, 2.19] |
| 2.14.1 Physical domain | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐6.04, 5.28] |
| 2.14.2 Psychological domain | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐5.18, 6.30] |
| 2.14.3 Social domain | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐7.10, 7.06] |
| 2.14.4 Environmental | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐4.93, 4.07] |
| 2.14.5 Environmental‐TR | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐4.92, 4.28] |
| 2.15 Proportion of women with serious adverse events | 1 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.30] |
| 2.16 Individual adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.16.1 Pelvic pain | 4 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.94, 5.23] |
| 2.16.2 Mood swings | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.60, 1.95] |
| 2.16.3 Intermenstrual bleeding and menstrual irregularity | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.82] |
| 2.16.4 Breast tenderness | 3 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.29, 6.29] |
| 2.16.5 Nausea | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.59] |
| 2.16.6 Diarrhoea | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.17] |
| 2.16.7 Upper respiratory infection | 2 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.67, 4.44] |
| 2.16.8 Ovarian cysts | 3 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [1.31, 8.21] |
| 2.16.9 Headache | 4 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.75, 1.93] |
| 2.16.10 Vaginitis | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.86, 10.95] |
| 2.16.11 Acne | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.31, 3.41] |
| 2.16.12 Hypertension | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.12 [0.61, 42.90] |
| 2.16.13 Sinusitis | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.42, 6.91] |
| 2.16.14 Fatigue | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.39, 10.88] |
| 2.16.15 Urinary tract infection | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.53, 7.92] |
| 2.16.16 Increased weight | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.23, 2.94] |
| 2.16.17 Lower abdominal pain | 2 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.13, 1.44] |
| 2.16.18 Any adverse events | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.48] |
| 2.17 Withdrawal from treatment | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.17.1 For adverse events | 4 | 819 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.54] |
| 2.17.2 For any reason | 1 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.39, 0.60] |
| 2.18 Treatment failure (PBAC > 100 at end of treatment or requirement for alternative treatment ) | 6 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.26, 0.44] |
2.3. Analysis.
Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 3: Menstrual blood loss by alkaline haematin ‐ descriptive results
| Menstrual blood loss by alkaline haematin ‐ descriptive results | |||||
| Study | Prog IUS | Medical treatment | Follow‐up | Statistical test | Notes |
| Irvine 1998 | Median = 6 mL, range = 0 to 284 mL n = 22 | Norethisterone group: Median=20mls, range=4‐137mls n=22 | 3 months | Wilcoxon rank‐sum test: t = 315.5, P value = 0.033 | |
| Kaunitz 2010 | Absolute change from baseline: Median = ‐128.8 mL, range ‐393.6 to 1242.2 mL n = 80 |
Absolute change from baseline: Medroxyprogesterone group: Median = ‐17.8 mL, range ‐271.5 to 78.6 mL n = 82 |
6 months | Wilcoxon rank‐sum test, P < 0.001 | |
| Reid 2005a | Median = 5 mL, range = 0 to 45 mL n = 25 | Mefenamic acid group: Median = 100 mL, range = 46 to 168 mL n = 26 | 6 months | Wilcoxon rank‐sum test: P value < 0.001 (for MBL at 6 months follow‐up between groups, summary figures not supplied) | |
2.4. Analysis.
Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 4: PBAC scores for menstrual blood loss ‐ descriptive results
| PBAC scores for menstrual blood loss ‐ descriptive results | |||||
| Study | IUS group | Control group | Follow‐up | Statistical test | Notes |
| Reid 2005a | Median = 25, range = 0 to 402 n = 25 | Mefenamic acid group: Median = 159, range = 50 to 307 n = 26 | Follow‐up = 6 months | Wilcoxon rank‐sum test: P value < 0.001 (for MBL at 6 months follow‐up between groups, summary figures not supplied) | |
2.5. Analysis.
Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 5: Percentage change in MBL (from baseline to end of study)
| Percentage change in MBL (from baseline to end of study) | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
| Endrikat 2009 | COCP (20 ug ethinyl oestradiol + 1 mg norethindrone acetate) versus LNG IUS | Total: N = 42 FAS (full analysis set): N = 39 (19 in COCP group and 20 in LNG IUS group) |
Mean percentage change COCP: ‐68% LNG IUS: ‐83% No measure of variation reported |
Authors concluded that LNG IUS was associated with a significantly greater percentage change from baseline than COCP (P = 0.0024). Estimate for median difference between the 2 interventions: ‐62 (95% CI ‐89 to ‐18). |
|
| Shabaan 2011 | COCP (30 mcg ethinyl oestradiol + 150 mcg levonorgestrel) versus LNG IUS | Total: N = 112 At 12 months, only N = 64 had alkaline haematin assessment At 12 months, only N = 64 had PBAC assessment |
Mean (SD) percentage change from baseline: Alkaline haematin: COCP: 35.0 (77.0) LNG IUS: 87.4 (11.3) PBAC: COCP: 2.5 (93.2) LNG IUS: 86.6 (17.0) |
Alkaline haematin: P = 0.013 PBAC: P < 0.001 |
|
2.7. Analysis.
Comparison 2: LNG‐IUS versus any other medical treatment, Outcome 7: Total menstrual fluid loss ‐ descriptive results
| Total menstrual fluid loss ‐ descriptive results | |||||
| Study | IUS group | Control group | Follow‐up | Statistical test | Notes |
| Reid 2005a | Median = 27, range = 0 to 156 n = 25 | Median = 157, range = 76 to 319 n = 25 | 6 months | Wilcoxon rank‐sum test: P value < 0.001 (for values at 6 months follow‐up between groups, summary figures not supplied) | The authors claim that this is the first study to measure total fluid menstrual loss as opposed to menstrual blood loss |
Comparison 3. LNG‐IUS versus endometrial ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean menstrual blood loss at end of study | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Mean PBAC score at 12 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Improvement in HMB | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Amenorrhoea within 12 months | 8 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.85, 1.72] |
| 3.2.2 Hypomenorrhoea within 12 months | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.73, 1.33] |
| 3.2.3 Eumenorrhoea within 12 months | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.00] |
| 3.2.4 Improvement in bleeding pattern within 12 months | 3 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.02, 1.41] |
| 3.3 PBAC score after treatment (descriptive results) | 6 | Other data | No numeric data | |
| 3.4 Proportion of women satisfied with treatment | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Within one year follow‐up | 5 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 3.4.2 At 2 years follow up | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 3.4.3 At 5 years follow‐up | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.01, 1.53] |
| 3.5 Quality of life (SF36) within 12 months follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.5.1 Physical functioning | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐11.10, 4.90] |
| 3.5.2 Role limitation (physical) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐9.32, 4.32] |
| 3.5.3 Bodily pain | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐6.43, 7.23] |
| 3.5.4 General health | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐14.40 [‐22.63, ‐6.17] |
| 3.5.5 Vitality | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐11.46, 1.06] |
| 3.5.6 Social functioning | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐12.82, ‐0.58] |
| 3.5.7 Role limitation (emotional) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐10.10 [‐17.03, ‐3.17] |
| 3.5.8 Mental health | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐11.20 [‐17.08, ‐5.32] |
| 3.5.9 Overall SF36 score | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐5.98, 11.18] |
| 3.6 Quality of life (QOL) scores at 12 months (SF36) ‐ descriptive results | 2 | Other data | No numeric data | |
| 3.7 Quality of life within 5 years follow‐up (proportion with improved wellbeing) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 Physical | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.11, 1.92] |
| 3.7.2 Emotional | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.09, 2.30] |
| 3.8 Quality of life within 5 years ‐ psychological wellbeing (continuous) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 10.30 [‐6.18, 26.78] |
| 3.9 Total proportion of women with adverse events | 3 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.44, 2.94] |
| 3.10 Individual adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.10.1 Endometritis | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.21, 2.35] |
| 3.10.2 Pelvic pain or PID | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.46, 3.01] |
| 3.10.3 Myometritis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.89] |
| 3.10.4 Adenomyosis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.89] |
| 3.10.5 Abnormal PAP | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.99] |
| 3.10.6 Oedema | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [0.15, 80.03] |
| 3.10.7 Breast pain | 3 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.57 [1.78, 32.23] |
| 3.10.8 Weight gain | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.16, 5.84] |
| 3.10.9 Mood swings | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.36, 15.32] |
| 3.10.10 Bloating | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.57 [1.63, 12.82] |
| 3.10.11 Acne or greasy skin | 3 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.40 [1.57, 44.76] |
| 3.10.12 Nausea | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.00 [0.50, 161.29] |
| 3.10.13 Headache | 3 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.64, 6.50] |
| 3.10.14 Leg pain | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.32, 28.57] |
| 3.10.15 Dysmenorrhoea | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.17] |
| 3.10.16 Lower abdominal pain | 4 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.55, 4.97] |
| 3.10.17 Actinomycoses | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 69.74] |
| 3.10.18 Decreased libido | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 6.90] |
| 3.10.19 Hair loss | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.26, 103.35] |
| 3.10.20 Anxiety or depression | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.20, 21.67] |
| 3.10.21 Hypertension | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 73.21] |
| 3.10.22 Endometriosis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.89] |
| 3.10.23 Bleeding or spotting | 4 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.74, 2.58] |
| 3.10.24 Hematometra | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 3.10.25 Vaginitis | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.40, 10.11] |
| 3.10.26 Genital ulceration | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 3.11 Treatment failure: discontinuation of initial treatment, adjunct medical therapy or persistent HMB) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.11.1 at 12 months | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.09, 2.90] |
| 3.11.2 at 24 months | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.94, 2.42] |
| 3.11.3 at 36 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.61, 3.69] |
| 3.12 Treatment failure: requirement for surgery for the treatment of HMB (ablation or hysterectomy) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.12.1 at 12 months | 3 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.67, 3.23] |
| 3.12.2 at 24 months | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.31, 1.91] |
| 3.12.3 at 36 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.98] |
| 3.13 Treatment failure: requirement for hysterectomy | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.13.1 at 12 months | 3 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.48, 4.42] |
| 3.13.2 at 24 months | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.50, 2.60] |
| 3.13.3 at 5 years | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.21] |
| 3.14 Total cost per woman | 1 | Other data | No numeric data |
3.1. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 1: Mean menstrual blood loss at end of study
3.3. Analysis.
Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 3: PBAC score after treatment (descriptive results)
| PBAC score after treatment (descriptive results) | |||||
| Study | LNG IUS group | Ablation group | Follow‐up | Statistical test | Notes |
| Barrington 2003 | Median = 19, range 0 to 100 n = 21 | Median = 27, range 0 to 424 n = 23 | 6 months | Mann‐Whitney test P value 0.69 (no significant difference between groups) | Pre‐operative menstrual PBAC scores differed between groups, P value = 0.02 |
| Crosignani 1997 | Mean = 38.8, SD = 37.1 n = 30 | Mean = 23.5, SD = 32.6 n = 30 | 12 months | Mann‐Whitney U test P value 0.015 favouring ablation | |
| Ergun 2012 | Mean = 70, no measure of variation reported n = 18 |
Mean = 55, no measure of variation reported n = 24 |
12 months | Mann Whitney U test No significant difference between groups |
|
| Kittelsen 1998 | Median = 7.0 Range = 0 to 101 n = 19 | Median = 4.0 Range = 0 to 182 n = 22 | 36 months | Wilcoxon rank sum test (difference from baseline between groups) P value = 0.86 | |
| Shaw 2007 | Median = 26, range 0 to 68 n = 21 |
Median = 62, range 0 to 142 n = 20 |
12 months | Mann Whitney test, P < 0.001, favouring LNG IUS | |
| TALIS 2006 | Mean = 20.6 SD = 28.8 n = 37 | Mean = 75.4 SD = 91.1 n= 31 | 24 months | Wilcoxon test, P value = 0.002, favouring LNG IUS | |
3.5. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 5: Quality of life (SF36) within 12 months follow‐up
3.7. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 7: Quality of life within 5 years follow‐up (proportion with improved wellbeing)
3.8. Analysis.

Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 8: Quality of life within 5 years ‐ psychological wellbeing (continuous)
3.14. Analysis.
Comparison 3: LNG‐IUS versus endometrial ablation, Outcome 14: Total cost per woman
| Total cost per woman | ||||
| Study | Details of cost | LNG IUS: Mean cost | Ablation: Mean cost | Comments |
| TALIS 2006 | Expected cost | Mean = NZD 1241 | Mean = NZD 2418 | Economic modelling examined the expected cost and outcome for women entering treatment (costs of procedure, recovery, medications, equipment, GP, lost income and failed treatment) |
Comparison 4. LNG‐IUS versus hysterectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 PBAC score | 1 | Other data | No numeric data | |
| 4.1.1 At 12 months follow‐up | 1 | Other data | No numeric data | |
| 4.1.2 At 24 months follow‐up | 1 | Other data | No numeric data | |
| 4.2 PBAC score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 at 3 months | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐15.90 [‐25.63, ‐6.17] |
| 4.2.2 at 6 months | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 30.70 [24.79, 36.61] |
| 4.2.3 at 12 months | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐5.52, 5.12] |
| 4.2.4 at 24 months | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 52.66 [28.86, 76.46] |
| 4.3 Amenorrhea at 12 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.39, 0.70] |
| 4.4 Satisfaction with treatment (5 years follow‐up) | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 4.5 Quality of life scores at 12 months follow‐up (descriptive data) | 1 | Other data | No numeric data | |
| 4.6 Quality of life scores at end of study (final values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.6.1 SF36: General health | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐6.03, 4.43] |
| 4.6.2 SF36: Physical function | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐10.77, 0.77] |
| 4.6.3 SF36: Role (physical) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐7.21, 3.21] |
| 4.6.4 SF36: Role (emotional) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 16.10 [8.88, 23.32] |
| 4.6.5 SF36: Mental health | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 36.80 [30.37, 43.23] |
| 4.6.6 SF36: Social function | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐5.00, 8.60] |
| 4.6.7 SF36: Vitality | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐0.78, 11.98] |
| 4.6.8 SF36: Pain | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐14.80 [‐23.31, ‐6.29] |
| 4.7 Quality of life scores at end of study (change values) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.7.1 EQ‐5D | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.15, ‐1.03] |
| 4.7.2 SF36: General health | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐2.93, 7.33] |
| 4.7.3 SF36: Physical functioning | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.51, 6.31] |
| 4.7.4 SF36: Emotional wellbeing | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐3.35, 8.35] |
| 4.7.5 SF36: Social functioning | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [‐1.47, 13.67] |
| 4.7.6 SF36: Energy/vitality | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐5.67, 7.07] |
| 4.7.7 SF36: Pain | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐7.32, 8.12] |
| 4.7.8 SF36: Role ‐ physical | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐7.49, 17.49] |
| 4.7.9 SF36: Role ‐ emotional | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐10.03, 18.43] |
| 4.7.10 General Health ‐ VAS | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐2.74, 8.74] |
| 4.8 Immediate adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.8.1 Headache | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.00 [0.63, 192.99] |
| 4.8.2 Breast tenderness | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.37, 131.56] |
| 4.8.3 Acne | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.25, 101.18] |
| 4.8.4 Depressive episode | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [0.45, 35.06] |
| 4.8.5 Wound infection | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.66] |
| 4.8.6 Infected pelvic haematoma | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.54, 4.01] |
| 4.8.7 Postoperative fever | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.35] |
| 4.8.8 Urinary retention | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.17] |
| 4.8.9 Bladder perforation | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.69] |
| 4.8.10 Bowel perforation | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 4.8.11 Wound rupture | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.05] |
| 4.8.12 Peritonitis | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 4.8.13 Ileus | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.05] |
| 4.8.14 Severe abdominal pain | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.50, 7.67] |
| 4.8.15 Vesicovaginal fistula | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 4.8.16 Postoperative bleeding | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.35] |
| 4.8.17 Intestinal occlusion | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.65] |
| 4.8.18 Ureter lesion | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 4.8.19 Thromboembolic disease | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.65] |
| 4.9 Long term adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.9.1 Back pain (increase from baseline) | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.80] |
| 4.9.2 Abdominal pain (increase from baseline) | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.30, 1.22] |
| 4.9.3 Urge incontinence | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.35] |
| 4.9.4 Stress incontinence | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.01] |
| 4.9.5 Urinary tract infections | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.20, 1.03] |
| 4.9.6 Incidence of ovarian cysts | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.24, 5.97] |
| 4.10 Treatment failure: requirement for surgery for HMB at 12 months follow up (hysterectomy) | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 48.18 [2.96, 783.22] |
| 4.11 Total cost per woman | 1 | Other data | No numeric data | |
| 4.11.1 At 12 months follow‐up | 1 | Other data | No numeric data | |
| 4.11.2 At 5 years follow‐up | 1 | Other data | No numeric data | |
| 4.11.3 At 10 years follow‐up | 1 | Other data | No numeric data |
4.1. Analysis.
Comparison 4: LNG‐IUS versus hysterectomy, Outcome 1: PBAC score
| PBAC score | ||||
| Study | LNG IUS group | Laparoscopic supracervical hysterectomy group | Statistical test, results | Notes |
| At 12 months follow‐up | ||||
| Sesti 2012 | Mean = 3.5, SD = 16.0, n=36 | Mean = 3.7, SD = 3.0, n=36 | Fisher's exact test, no significant difference between groups | |
| At 24 months follow‐up | ||||
| Sesti 2012 | Mean = 56.4, SD = 72.8, n=36 | Mean = 3.74, SD = 3.05, n=36 | Fisher's exact test, P < 0.001 | |
4.8. Analysis.

Comparison 4: LNG‐IUS versus hysterectomy, Outcome 8: Immediate adverse events
4.11. Analysis.
Comparison 4: LNG‐IUS versus hysterectomy, Outcome 11: Total cost per woman
| Total cost per woman | ||||||
| Study | Details of cost | LNG IUS: Mean cost | LNG IUS: 95% CI | Hyst: Mean cost | Hyst: 95% CI | P value |
| At 12 months follow‐up | ||||||
| Hurskainen 2001 | Total health care costs + product losses/woman | USD 1530 | 1203 to 1858 | USD 4222 | 3808 to 4636 | LNG IUS significantly lower than hysterectomy (figures not reported) |
| At 5 years follow‐up | ||||||
| Hurskainen 2001 | Total cost per participant Discounted total costs (by 3%) (direct and indirect)/woman |
USD 2966 USD 2817 |
2362 to 3679 2222 to 3530 |
USD 4718 USD 4660 |
4072 to 5238 4014 to 5180 |
LNG IUS significantly lower than hysterectomy (figures not reported) |
| At 10 years follow‐up | ||||||
| Hurskainen 2001 | Total cost per participant Discounted total costs per participant |
USD 3780 USD 3423 |
not reported | US$5089 US$4937 |
not reported | LNG IUS significantly lower than hysterectomy (figures not reported) |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashraf 2017.
| Study characteristics | ||
| Methods | Parallel, prospective, randomized multicentre clinical study | |
| Participants | Setting: conducted at the gynaecology units of Bahawal Victoria Hospital (BVH), Jubilee Female Hospital, Civil Hospital and private clinics of consultant gynaecologists in Bahawalpur, Pakistan. Inclusion criteria
Exclusion criteria
Follow‐up: 6 months |
|
| Interventions | A. norethisterone containing tablet was given orally at a dose of 5 mg ×3/day for 5 to 26 days of cycle over consecutive cycles: 40 B. LNG‐IUS (Mirena®): 40 |
|
| Outcomes | PBAC at baseline, 3 and 6 months | |
| Notes | time frame: March to August 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated by lottery method into two equal groups: A and B |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only outcome was PBAC (scored by participants) which could be influenced by participants' knowledge of treatment |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin and haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. Very unlikely for the difference of the treatments (one oral and one IUS) |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin and haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent dropouts after participants withdrawn for hysterectomy (2 in each arm) |
| Selective reporting (reporting bias) | Low risk | All the outcomes previously stated were reported. Objective of authors was only to measure PBAC, however they acknowledged that oral NET is associated with adverse events . |
| Other bias | Low risk | Similar at baseline Disclaimer: none Conflict of Interest: none Source of funding: the study was funded by the departmental grant of the Post Graduate Medical College (PGMC), Islamia University of Bahawalpur |
Barrington 2003.
| Study characteristics | ||
| Methods | Parallel group study in single centre
No. of women randomised: 50 Dropouts: at 6 months 4/25 (16%) in LNG‐IUS group and 2/25 (8%) in ablation group No. of women analysed: 44 No power calculation or ITT analysis Source of funding not stated |
|
| Participants | Country: UK Women with menorrhagia refractory to medical treatment referred by GPs to gynaecology clinic in district hospital Exclusion criteria
|
|
| Interventions | (1) Levonorgestrel‐releasing intrauterine system (LNG‐IUS, Mirena)
(2) Thermal balloon ablation after pre‐operative endometrial thinning with gosarelin 1 month prior Duration: 6 months |
|
| Outcomes |
|
|
| Notes | Preoperative menstrual bleeding was higher in the thermal balloon group compared to the LNG‐IUS group (P value 0.02). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding highly unlikely, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Haematin alkaline and haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding highly unlikely, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Haematin alkaline and haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All women received their allocated treatment. 44/50 analysed for primary outcome at 6 months. Reasons for withdrawal/dropout given; however because women in LNG‐IUS group were able to request removal of the device for inefficacy, this could have caused bias (women having hysterectomy not able to "withdraw" for this reason) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not clearly specified |
| Other bias | High risk | Preoperative menstrual bleeding was significantly higher in the thermal balloon group compared to the LNG‐IUS group. Bias is likely as menstrual bleeding was measured postoperatively without adjustment for higher scores |
Crosignani 1997.
| Study characteristics | ||
| Methods | Parallel group, single centre RCT. No. of women randomised: 70 No. of women analysed: 69 Exclusions post randomisation: 0. Losses to follow‐up: 6 months = 0, 12 months = 1 Power calculation for sample size was performed and analysis was by ITT Funding was partially supported by the Italian National Research Council and Leiras Pharmaceuticals provided the intrauterine devices |
|
| Participants | Country: Italy
Aged 38 to 53 years, all referred for a hysterectomy because of heavy menstrual bleeding Inclusion criteria
Exclusion criteria
|
|
| Interventions | (1) Levonorgestrel‐releasing (20 ug/day) intrauterine contraceptive system inserted within 7 days of menstruation (2) Endometrial resection in the early proliferative phase using a rollerball and a 90° loop. All the resections were performed by the same surgeon Duration: 12 months. Follow‐up assessments at 6 and 12 months. |
|
| Outcomes |
|
|
| Notes | The Academic Department undertaking the study was specifically interested in hysteroscopic surgery and hence the endometrial resection results may be better than those applicable to the general population of clinicians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For primary outcome of menstrual bleeding, 1/70 not included in the analysis. For analysis of PBAC scores, 10/70 not included (5 from each group) and for analysis of quality of life, 8/70 not included (4 in each group). Reasons for attrition were given |
| Selective reporting (reporting bias) | Low risk | Outcomes clearly specified and reported |
| Other bias | Low risk | No evidence of an imbalance between groups at baseline |
de Souza 2010.
| Study characteristics | ||
| Methods | Parallel group RCT, single centre No. of women randomised: 58 No. of women analysed: At 12 months, 55 (1 woman in Mirena group and 2 women in balloon group required hysterectomy). At 5 years, 52 (1 woman required hysterectomy and 3 were lost to follow‐up in Mirena group but 27/30 were analysed; 6 women required hysterectomy and 3 were lost to follow‐up in balloon group but 25/28 were analysed) Power calculation for sample size: difference of > 40% between proportions, but this calculation did not allow for dropouts ITT analysis but did not take dropouts into account Funding: Bayer provided the materials used in the study (both interventions) |
|
| Participants | Country: Brazil Women recruited between January 2005 and March 2007, with mean age 42 and 44 years and baseline PBAC 542 and 420. Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions | (1) Levonorgestrel‐releasing IUS (Mirena) (2) Thermal balloon ablation (Thermachoice) under general anaesthesia Both procedures initiated during the first 15 days of a menstrual cycle |
|
| Outcomes |
Assessed at 1, 6 and 12 months after the procedures and additionally at 5 years |
|
| Notes | 2 publications: 1 assessed outcomes at 12 months and the other at 5 years after treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 12‐month assessment, minimal dropouts but at 5 years' assessment only 17/30 in Mirena group and 11/28 in balloon group were still premenopausal and evaluated for Hb, quality of life scores and bleeding pattern |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not prespecified or reported |
| Other bias | Low risk | Groups appeared comparable at baseline |
Endrikat 2009.
| Study characteristics | ||
| Methods | A multicentre (9 centres in Canada) parallel‐group open‐label RCT | |
| Participants | Inclusion criteria
Exclusion criteria
Mean (SD) age: 41.8 (4.3) in LNG‐IUS group; 42.2 (4.4) in combined oral contraceptives group |
|
| Interventions |
Duration: 12 months |
|
| Outcomes | Primary: MBL (assessed by PBAC) Secondary: Rx success (MBL score < 100 at 12 months); Hb; quality of life (menorrhagia severity score); adverse events |
|
| Notes | Three of the authors (including the principal author) were employees of a pharmaceutical company (which also funded the study) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participating subjects were randomised in order of arrival at the treatment centre" according to computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Centralisation of randomisation sequence; and (quote): "a randomised subject could not be replaced by another subject" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible; outcomes likely to be influenced |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Blinding not possible; outcome unlikely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible; outcomes likely to be influenced |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Blinding not possible; outcome unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes assessed in the full analysis set (FAS) population and compared with per protocol analyses |
| Selective reporting (reporting bias) | Low risk | Measures of variation in the estimates not reported in the publication but the authors supplied a copy of the full report |
| Other bias | High risk | Three of the authors (including the principal author) were employees of a pharmaceutical company (which also funded the study), groups similar at baseline |
Ergun 2012.
| Study characteristics | ||
| Methods | Single centre parallel group RCT No. of women randomised: 58 No. of women analysed: 42 (reasons for dropouts not given) No ITT analysis or power calculation for sample size Funding: not reported |
|
| Participants | Country: Turkey Women with abnormal uterine bleeding which had not responded to medical treatment Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Duration: 12 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'random' but no method described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial dropout and no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not clearly prespecified |
| Other bias | Unclear risk | Characteristics of randomised participants not reported, so not clear if groups were comparable at baseline. Unequal numbers in randomised groups |
Ghazizadeh 2014.
| Study characteristics | ||
| Methods | Parallel randomised controlled trial | |
| Participants | Department of Obstetrics and Gynecology and Maternal‐Fetal and Neonatal Research Center and Breastfeeding Research Center, Tehran University of Medical Sciences Inclusion criteria
Exclusion criteria
Setting: Tehran, Iran Time frame: October 2009 to November 2010 |
|
| Interventions | Bipolar endometrial ablation (Novasure) (n = 30) Hysteroscopic endometrial resection (HER) (n = 32) Mirena (lg‐IUS) (n = 48) |
|
| Outcomes | Decreased menstrual blood loss Interaction between bleeding and normal activity Anaemia (estimated 6.8 mg/dL as cut‐off for anaemia) Patients’ satisfaction (checklist 6 months' follow‐up; some up to 12) |
|
| Notes | Funding: not specified; includes the statement "No competing financial interest" Outcomes do not match correctly on the report. Study authors contacted for more details, no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The title says RCT, but no data provided on the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label, lack of blinding likely to influence outcome. |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified, lack of blinding likely to influence outcome. |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Not blinded, lack of blinding unlikely to influence the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers in tables do not match numbers in text; study authors have been contacted for confirmation |
| Selective reporting (reporting bias) | High risk | No quantification of bleeding; not specified at what time satisfaction was measured |
| Other bias | High risk | Past medical history was positive in 12% Mirena, 13.3% Novasure, and 53.1% of HER (P < 0.0001) Ultrasonography was performed in 35.4% of patients in the Mirena group, 66.7% in the Nova‐Sure group, and 96.8% in the hysteroscopic endometrial resection group (P < 0.0001) |
Gupta 2013.
| Study characteristics | ||
| Methods | Parallel group RCT, multicentre (n = 63 in UK) No. of women randomised: 571 No. of women analysed: At 2 years, 231 in medical treatment group and 247 in LNG‐IUS group but sensitivity analysis with imputation of missing data was undertaken Power calculation for sample size: 90% power to detect small to moderate (0.3 SD) differences in primary outcome at any 1 time point ‒ allowed for 20% dropout ITT analysis Funding: NIHR Health Technology Assessment Programme |
|
| Participants | Country: UK Mean age: 42 years Inclusion criteria
Exclusion criteria
|
|
| Interventions | (1) Levonorgestrel‐releasing IUS (2) Usual medical treatment (mefenamic acid, tranexamic acid, norethindrone, combined oestrogen‐progestogen or progesterone‐only oral contraceptive pill, medroxyprogesterone acetate injection, chosen by the physician and patient according to contraceptive needs and desire to avoid hormone therapy) Women are permitted to change treatments, as well as between groups or could discontinue treatment ‒ to replicate usual practice. Duration: 6 months, 2, 5 and 10 years |
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerised minimised randomisation procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "assigned by telephone or web based central randomisation service" at clinical trials unit in University of Birmingham |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Haematin alkaline and haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Haematin alkaline or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear explanations given for missing data and sensitivity analyses performed where values were imputed for missing data |
| Selective reporting (reporting bias) | Low risk | Clear and comprehensive protocol |
| Other bias | Low risk | Groups comparable at baseline |
Herman 2013.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants |
|
|
| Interventions | Bipolar endometrial ablation (Novasure®) = 138 LNG‐IUS (Mirena®) = 132 |
|
| Outcomes |
|
|
| Notes | Short communication of results; full trial awaiting publication. Authors contacted for further details. This study is funded by The Netherlands Organisation for Health Research and Development (ZonMW) grant. The authors declare that they have no competing interests Between July 2012 and December 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation will be performed by accessing a web‐based randomisation programme; patients will be randomised into two groups in a ratio of 1:1, using permuted block randomizations with a variable block size". (protocol) |
| Allocation concealment (selection bias) | Low risk | "Participants will be given a computer generated numeric code. Data handling will be done anonymously, with the patient code only available to the local investigator and the research nurse working in the local centre" (protocol) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding highly unlikely, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin and haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding highly unlikely, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin and haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 91% completed 24 months' follow‐up |
| Selective reporting (reporting bias) | High risk | Some of the outcomes on the protocol are not included on the trial report (complications, sick leave and costs) |
| Other bias | Unclear risk | No information about baseline characteristics, conflict of interest or funding |
Hurskainen 2001.
| Study characteristics | ||
| Methods | Multicentre (n = 5), parallel group study.
No. of women randomised: 236 No. of women analysed: 228 at 12 months, 232 at 5 years, 221 at 10 years Dropouts: LNG‐IUS group: 1.6% at 6 months' and 2.5% at 12 months' follow‐up; hysterectomy group: 9.4% at 6 months and 4.3% at 12 months' follow‐up. Power calculation for sample size and ITT analysis. Source of funding: Academy of Finland, STAKES and research funds of the University Hospitals in Finland. Mirena was provided free of charge by Leiras. |
|
| Participants | Country: Finland
Women, aged 35 to 49 (mean age 43) referred by GPs or gynaecologists to 5 university hospitals
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | Results analysed at 1, 5 and 10 years' follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by centre in clusters by drawing from a hat |
| Allocation concealment (selection bias) | Low risk | Quote: "numbered opaque sealed envelopes" ‐ physicians and other study personnel did not participate in allocation execution |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Haematin alkaline or haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Haematin alkaline or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropouts at 12 months (3/119 (2.5%) in LNG group and 5/117 (4.3%) in hysterectomy group). At 5‐year follow‐up, 2/119 (1.7%) lost to follow‐up in LNG group and 2/117 (1.7%) lost to follow‐up in hysterectomy group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared similar at baseline |
Irvine 1998.
| Study characteristics | ||
| Methods | Parallel group, single centre RCT No. of women randomised: 44 No. of women analysed: 36 Exclusions post randomisation: 0. Withdrawal from treatment: 3 months ‒ 2 from LNG group and 6 from Norethisterone group Power calculation for sample size was performed Both ITT (for primary outcome) and per protocol analysis performed Source of funding: not stated |
|
| Participants | Country: UK Women aged 18 to 45 years all referred to specialist clinic complaining of regular heavy menstrual bleeding 151 women were screened but 197 were excluded from eligibility (41 measured menstrual blood loss < 80 mL; 62 declined to do menstrual blood loss measurements; 4 declined to participate) Inclusion criteria
Exclusion criteria
|
|
| Interventions | (1) Levonorgestrel‐releasing (20 µg/day) intrauterine contraceptive system inserted within 7 days of menstruation (2) Norethisterone 5 mg ×3 daily taken on Day 5 to 26 of the menstrual cycle for 3 cycles Duration: 3 months |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Outcomes assessed at 3 months, which is relatively short period to assess the effectiveness of the LNG‐IUS Power calculation performed prior to commencement of trial to assess group size and ITT analysis of data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque consecutively numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors stated that both ITT and per protocol analyses were performed but it appears that this was only for menstrual blood loss and satisfaction. Per protocol analyses were undertaken for all other outcomes. Completers of the trial at 3 months were 20/22 (90.9%) in LNG group and 16/22 (72.7%) in NET group. Side effects were collected in only 12/22 (54.5%) of NET group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared similar at baseline |
Kaunitz 2010.
| Study characteristics | ||
| Methods | Multicentre (n = 55), parallel group RCT No. of women randomised: 165 No. of women analysed: 165 for absolute change and 160 for treatment success ITT analysis and use of 'last observation carried forward' for dropouts Power calculation for sample size: allowed for 20% dropouts, 69 women per treatment group to detect a significant difference in HMB between groups (83% reduction with LNG‐IUS and 50% reduction with MPA) (90% power) and 40% difference in proportions with successful treatment (99% power). Funding: Bayer Schering Pharma AG |
|
| Participants | Country: USA, Canada and Brazil Women with mean age 38 or 39 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
Follow‐up at 3 and 6 months |
|
| Notes | Screening phase for 2 to 3 menstrual cycles to assess baseline HMB (alkaline haematin method). Two publications: one reported HMB outcomes assessed at 6 months; and one reported Hb and ferritin levels at 6 months. At the end of the study, women assigned to MPA were able to choose to use a LNG‐IUS and those allocated to LNG‐IUS were allowed to continue its use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "centralised interactive voice system" with "random permuted block lengths of 4 to attain balance within the strata and by country" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using a centralized interactive voice response system (ClinPhone, East Windsor, NJ)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropouts and analysis by ITT and last observation carried forward |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared similar at baseline |
Kilic 2009.
| Study characteristics | ||
| Methods | Single centre, parallel group RCT No. of women randomised: 40 No. of women analysed: 40 Power calculation for sample size: 9 per group for 80% power to detect a 20% decrease in the PBAC score with SD 14 ITT analysis Funding: not stated |
|
| Participants | Country: Turkey Women taking anticoagulant therapy after cardiac valve replacement, with median age 36 years. Inclusion criteria
Exclusion criteria: not reported |
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | HMB is a potential side effect of treatment with anticoagulant therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "simple randomisation method" but this method was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "closed envelopes" ‒ insufficient information to know whether allocation was properly concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all participants completed the study" |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not assessed |
| Other bias | Low risk | Groups appeared comparable at baseline ‒ no other potential bias |
Kiseli 2016.
| Study characteristics | ||
| Methods | Single‐centre RCT Central computer‐generated randomization schedule used for allocation Participants unable to be blinded, given the differences between the regimens Duration of trial: 2 control cycles pre‐treatment; 6 cycles on treatment |
|
| Participants | Country: Turkey Mean age: 42.1 years Number of participants: 84 women were randomised and 62 completed the trial Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Tranexamic acid 1 g ×4 daily from day 1 for 4 days Norethindrone 5 mg ×3/day from day 14 to 23 of the cycle Levonorgestrel IUS (20 μg/day) inserted during the first few days of menses Treatment for 6 cycles |
|
| Outcomes | PBAC score and associated percentage reduction in blood loss (end scores) World Health Organization QoL‐Short Form (Turkish version), in which women report limitations in physical health, psychological status, social support, and "relating to their environment" |
|
| Notes | The authors deny receiving any funding Authors contacted: TIME FRAME: May 2005 to December 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: 'Neither patients nor researchers were blinded to treatment'' Different dosage schedules make blinding impossible Potential knowledge of treatment may have influenced the primary outcome of MBL which was measured by PBAC |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: ''Neither patients nor researchers were blinded to treatment'' Different dosage schedules make blinding impossible Potential knowledge of treatment may have influenced the primary outcome of MBL which was measured by PBAC |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant loss to follow‐up, but similar numbers for each group 62/84 randomised women (74%) included in analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared comparable at baseline and no other potential bias was identified |
Kittelsen 1998.
| Study characteristics | ||
| Methods | Single centre, parallel group design No. of women randomised: n = 60 No. of withdrawals: n = 7 (6 in the LNG‐IUS group because of unwanted adverse events and 1 in the TCRE group because of dislike of treatment option after randomisation) Power analysis for sample size was performed Analysis was not by ITT Source of funding: Leiras Finland. |
|
| Participants | Country: Norway Inclusion criteria
Exclusion criteria
|
|
| Interventions | (1) Levonorgestrel‐releasing intrauterine system (LNG‐IUS) (Mirena) inserted within 7 days of the start of menstruation (2) Transcervical resection of the endometrium (TCRE) performed regardless of day of menstrual cycle Duration: 20 months, 3 years |
|
| Outcomes |
|
|
| Notes | Study has been extended to 36 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random permuted blocks" using a computer code |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sealed envelopes" opened at entry, unclear if sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/30 (36.7%) in LNG group had discontinued treatment by 36 months. 7/29 (24.1%) in TCRE group discontinued (4 because of treatment failure) in the study by 36 months |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups comparable at baseline |
Malak 2006.
| Study characteristics | ||
| Methods | Single centre parallel group RCT No. of women randomised: 60 No. of women analysed: 56 (4 patients in the LNG‐IUS group discontinued treatment) Power calculation for sample size: 30 women per group with 90% power to find a success rate of 85% in LNG‐IUS group and 70% in endometrial resection group No ITT analysis Funding: not stated |
|
| Participants | Country: Egypt Women scheduled to undergo hysterectomy for treatment of excessive uterine bleeding with or without dysmenorrhoea, with mean age 46 and 47 years Inclusion criteria
Exclusion criteria
|
|
| Interventions | Screening period of 2 months prior to enrolment. PBAC score > 100 considered excessive bleeding
|
|
| Outcomes | Primary
Other outcomes
Follow‐up at 6 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation table" |
| Allocation concealment (selection bias) | Unclear risk | Quote:"sealed envelopes" but unclear if sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal dropout (4/30 in LNG‐IUS group) but none in the ER group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | There was a significant difference in parity status between the 2 randomised groups |
Ozdegirmenci 2011.
| Study characteristics | ||
| Methods | Single centre parallel group study No. of women randomised: 86 No. of women analysed: 75 (11 lost to follow‐up from hysterectomy group) Power calculation for sample size: total of 72 participants for 90% power and d = 0.70 effect size. 20% more patients enrolled to allow for loss to follow‐up Analysis not by ITT Funding: not stated |
|
| Participants | Country: Turkey Women with clinical suspicion of adenomyosis complaining of menorrhagia and/or dysmenorrhoea and with confirmed adenomyosis, with mean age 44 and 46 years Inclusion criteria
Exclusion criteria
All women had menorrhagia |
|
| Interventions |
|
|
| Outcomes | Primary
Other
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated codes" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial losS to follow‐up from the hysterectomy group (26%) and none from the LNG‐IUS group |
| Selective reporting (reporting bias) | Low risk | Outcomes were clearly specified and reported |
| Other bias | Low risk | Groups appeared comparable at baseline and no other potential bias |
Reid 2005a.
| Study characteristics | ||
| Methods | Single centre, parallel group study design. Potential for bias because main author was aware of the allocation and measured the outcomes No. of women randomised and analysed: 51 No. of women who discontinued treatment: 4 in LNG group and 5 in MFA group Power calculation for sample size performed and ITT analysis Source of funding: Schering. |
|
| Participants | Country: UK Women were either referred by GPs or self referred after ads in the local press Inclusion criteria
Exclusion criteria
|
|
| Interventions | (1) Levonorgestrel‐releasing intrauterine system (2) Mefenamic acid 500 mg 3×/day for first 4 days of cycle Duration: 3 cycles and 6 cycles | |
| Outcomes | Primary
|
|
| Notes | The principal author reported a conflict of interest: he had received travel support from Schering. This is the first study to measure TMFL as opposed to menstrual blood loss. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered opaque sealed envelopes" prepared off site |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients lost to follow‐up or dropouts included in final analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | The authors did not present a table of baseline characteristics of the participants |
Sayed 2011.
| Study characteristics | ||
| Methods | Single centre parallel group RCT No. of women randomised: 58 No. of women analysed: 44 were still in the study at 12 months' follow‐up but bleeding outcomes measured in substantially fewer because of treatment failure Power calculation for sample size ‒ 29 participants per group (with 15% attrition) for 90% power ITT analysis claimed by authors but missing data not included in analyses Funding: Bayer Schering Pharma (LNG‐IUS supply), Proctor & Gamble (sanitary pads), Assiut University Egypt (lab work) |
|
| Participants | Country: Egypt Participants recruited from outpatient gynaecology clinics of Assiut University, mean age 37 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Follow‐up at 3, 6, 9 and 12 months |
|
| Outcomes | Primary
Other
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated table of random numbers" |
| Allocation concealment (selection bias) | Low risk | The randomization was conducted with allocation concealment. The assignments provided by a computer‐generated table of random numbers were written on pieces of paper. The pieces of paper were then inserted into envelopes that were immediately sealed. When a participant was enrolled, the first envelope on the pile was opened and her allocation was made |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial loss to follow‐up and treatment failure ‒ bleeding outcomes only measured in 20/58 (PBAC) and 22/58 (alkaline haematin) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared comparable at baseline and no other potential bias |
Sesti 2012.
| Study characteristics | ||
| Methods | Single centre parallel group RCT No. of women randomised: 72 No. of women analysed: 72 Power calculation for sample size ITT analysis Funding: not stated |
|
| Participants | Country: Italy Participants were women with HMB unresponsive to medical treatment with mean age 47 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
(Both performed by the same surgeons using the same technique) |
|
| Outcomes | Primary
Secondary
Follow‐up at 3, 6, 12 and 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated list" |
| Allocation concealment (selection bias) | Low risk | Quote: "serially numbered opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of patients until interventions were assigned, surgeons performing the procedures blinded |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors blinded, but some outcomes based on patient self‐report, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or exclusions |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared balanced at baseline and no other potential bias |
Shabaan 2011.
| Study characteristics | ||
| Methods | Single centre parallel group RCT No. of women randomised: 112 No. of women analysed: 95 (completed 12 months' follow‐up) Power calculation for sample size ‒ 90% power, 15% attrition required 112 participants Authors claimed ITT analysis but no methods used to account for missing data Funding: Bayer Schering Pharma (LNG‐IUS), Proctor & Gamble (sanitary pads) and Assiut University (laboratory work). |
|
| Participants | Country: Egypt Women recruited from gynaecology outpatient clinics of Assiut University Hospital, with mean age 39 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Follow‐up at 6 and 12 months. |
|
| Outcomes | Primary
Secondary
Follow‐up at 6 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted using a computer generated table of random numbers with allocation concealment" |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | No blinding, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial loss to follow‐up and bleeding outcomes measured in only 64/112 at 12 months (because of treatment failure) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appear comparable at baseline and no other potential bias |
Shaw 2007.
| Study characteristics | ||
| Methods | Single centre, parallel group RCT No. of women randomised: 66 No. of women analysed: 50 (completers at 12 months) – fewer women analysed because treatment failures not counted Power calculation for sample size: 30 women per group to have 80% power to detect a 50‐point difference in PBAC scores between treatments Analysis was not by ITT (no method to account for missing data) Funding: ATOS Medical provided balloons and partly funded research nurse sessions |
|
| Participants | Country: UK Women with idiopathic menorrhagia in whom prior medical oral treatment had failed: mean age 42 or 43 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
Follow‐up 3, 6, 9, 12 and 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated balanced random number blocks" |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially sealed opaque envelope" opened only when patient had signed the consent form |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence the outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence the outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial attrition from trial by 12 months |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared balanced at baseline and no other potential bias |
Soysal 2002.
| Study characteristics | ||
| Methods | Single centre study with parallel groups No. of women randomised: 72 No. of women analysed: 66 (for primary outcome) No women lost to follow‐up. Different denominators for other outcomes Power calculation for sample size – 30 patients per group to have 80% chance of detecting a mean difference of 15 mL in bleeding No ITT analysis (authors did not have a plan to deal with missing data) Source of funding not stated |
|
| Participants | Country: Turkey Patients with mean age 44 years recruited from university medical centre Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Follow‐up at 3, 6 and 12 months |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Study powered on menstrual blood loss, not quality of life. A much larger study would be required to adequately assess quality of life. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "numbered opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropouts |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared similar at baseline |
TALIS 2006.
| Study characteristics | ||
| Methods | Single centre, parallel groups No. of women randomised: 83 (2 excluded post randomisation in each group leaving a total of 79) No. of women analysed (for bleeding scores): 30 and 26 in LNG‐IUS group at 12 and 24 months, 29 and 20 in TBA group at 12 and 24 months (excluding failures) Lost to follow‐up: 1 (12 months) and 3 (24 months) in LNG‐IUS group; 2 (12 months) and 8 (24 months) in TBA group. Power calculation for sample size based on 50‐point PBAC difference ‒ 30 women per group No ITT analysis Funding was provided for sanitary pads and tampons but name of company was not reported |
|
| Participants | Country: New Zealand Patients with a complaint of heavy menstrual bleeding (mean age 41 to 43 years) recruited from hospital outpatient clinic Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomisation" in blocks |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Unclear risk | Alkaline haematin or haemoglobin were not measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal exclusions after randomisation (n = 4). These women were excluded because they did not fulfil inclusion criteria. Loss to follow‐up was mostly minimal but > 20% were lost by 24 months in the TBA group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared similar at baseline |
Tam 2006.
| Study characteristics | ||
| Methods | Single centre, parallel group RCT No. of women randomised: 44 No. of women analysed: 33 (11 patients withdrew prior to receiving treatment) No power calculation for sample size No ITT analysis Funding: not stated |
|
| Participants | Country: Hong Kong Women with excessive menstrual bleeding recruited from outpatient gynaecology clinic, with mean age 44 and 45 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random number series" |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes (personal correspondence with author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome |
| Blinding of participants and personnel (performance bias) Haematin alkaline, Haemoglobin All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, lack of blinding likely to influence outcome. |
| Blinding of outcome assessment (detection bias) (Haematine alkaline and haemoglobin) All outcomes | Low risk | Not blinded, lack of blinding unlikely to influence outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial exclusions post randomisation and prior to treatment ‒ greater in TBA group |
| Selective reporting (reporting bias) | Unclear risk | Authors stated that side effects of treatment were assessed but these were not reported clearly |
| Other bias | Low risk | Groups appear comparable at baseline and no other potential bias |
COC: combined oral contraceptive DVT: deep vein thrombosis EQ‐5D: Euroqol Group 5‐Dimension Self Report Questionnaire Fe: iron FSH: follicle stimulating hormone GnRH: gonadotropin‐releasing hormone HADS: Hospital Anxiety and Depression Scale Hb: haemoglobin HMB: heavy menstrual bleeding HT: hormone therapy ITT: intention‐to‐treat IUCD: intrauterine contraceptive device LNG‐IUS: levonorgestrel‐releasing intrauterine system MPA: medroxyprogesterone acetate MRI: magnetic resonance imaging NET: norethisterone acetate NSAID: non‐steroidal anti‐inflammatory drug OC: oral contraceptive PBAC: pictorial blood loss assessment chart PID: Pelvic inflammatory disease RCT: randomised controlled trial SD: standard deviation SF‐36: Medical Outcomes Study 36‐Item Short‐Form Health Survey TBA: thermal balloon ablation TCRE: transcervical resection of the endometrium TMFL: total menstrual fluid loss TVUS: transvaginal ultrasound VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu Hashim 2013 | Participants had pathology ‒ endometrial hyperplasia |
| Adiguzel 2017 | This is not an RCT. Women were offered the different treatments and were free to choose their option |
| Apter 2016 | The comparison was relevant LNG‐IUS versus Etonorgestrel implant, participants needed contraception and did not have heavy menstrual bleeding |
| Buyuktuna 2016 | This is not an RCT |
| Cameron 1987 | The intrauterine system used in this trial has been off the market since 2001 |
| Ghazizadeh 2011 | Although the study was described as random, the authors stated that participants were allowed to choose their treatment |
| Gupta 2006 | Relevant comparison but participants were able to choose their treatment and were not randomised |
| Janssen 1999 | This randomised double‐blind trial compared the effects of a multiload intrauterine device releasing 0.0 (control group), 1.5, 3.0 and 6.0 µg of 3‐ketodesogestrel daily on menstrual blood loss but 22% of the participants did not have heavy menstrual bleeding |
| Karacaoglu 2001 | This study was written in Turkish and translated by Dr Ahmet Metin Gulmezoglu. There is no indication that it was randomised |
| Karimi‐Zarchi 2013 | Participants had pathology ‒ endometrial hyperplasia |
| Kucuk 2008 | Quasi‐randomised trial ‒ "by a predefined application order" |
| Lahteenmaki 1998 | This study did not measure any of the review's outcomes |
| Lee 2013 | Observational cohort study ‒ participants were not randomised |
| Mawet 2014 | The comparison is between two different brands of the same product (LNG‐IUS 20 µg daily) |
| McMillan 1998 | Unknown characteristics of study |
| McMillan 2005 | Unknown characteristics of study |
| Milsom 1991 | This study compared flurbiprofen (an NSAID), tranexamic acid and LNG‐IUS in 35 women with menorrhagia. The first 20 consecutive women were treated with LNG‐IUS and the remaining 15 women were randomised to either flurbiprofen or tranexamic acid in a cross‐over design. The reduction in menstrual blood loss was compared between all treatments. There was no randomization |
| Rogerson 1999 | This trial experienced difficulties in recruiting patients and was stopped |
| Romer 2000 | This study was a controlled comparative study with equal numbers of patients in each group, endometrial ablation and LNG‐IUS. There is no indication that it was randomised |
| Tosun 2014 | Substantially biased study with large imbalance in dropouts, so final results not informative |
LNG‐IUS: levonorgestrel‐releasing intrauterine system NSAID: non‐steroidal anti‐inflammatory drug
Characteristics of ongoing studies [ordered by study ID]
SHiPP 2013.
| Study name | SHiPP (Stopping Heavy Periods Project), Identifier NCT02002260 |
| Methods | Parallel group RCT, open label. Randomisation by permuted blocks with a variable block size. |
| Participants | Women presenting for gynaecologic care with a self‐report of heavy menstrual bleeding secondary to ovulatory disorders or endometrial haemostatic disorders. Age 18 to 51 years. Plan to recruit 212 women. |
| Interventions | LNG‐IUS compared with combined oral contraceptives (any combined oral contraceptive chosen by primary care provider provided it contains 30 mg to 35 mg of ethinyl estradiol) |
| Outcomes | Primary: menstrual bleeding questionnaire (quality of life) at randomisation, 6 weeks, 3, 6 and 12 months Secondary: treatment failure |
| Starting date | February 2013 ‒ estimated completion date January 2017 |
| Contact information | Kristen Matteson Assistant Professor of Obstetrics and Gynecology Women and Infants Hospital of Rhode Island USA |
| Notes | Conducted in primary care centres. Author contacted. Doing final follow‐up August 2019, submitting for publication 2020. |
LNG‐IUS: levonorgestrel‐releasing intrauterine system PBAC: pictorial blood loss assessment chart RCT: randomised controlled trial
Differences between protocol and review
At the 2020 update:
We changed the name of the review to 'Progestogen‐releasing intrauterine systems for heavy menstrual bleeding' as it is a wider terminology and the progesterone‐releasing intrauterine system has been off the market for more than 15 years. One study that was included in the review prior to the 2020 update was excluded for the latter reason (Cameron 1987).
We added requirement for further surgery as a secondary outcome, and amended our primary outcome by adding eumenorrhoea after treatment to the list of methods of objective assessment, as this is an outcome of interest to women reading our review.
We also added the following outcomes to the 'Summary of findings' tables: adverse events; treatment failure; withdrawal from treatment; and requirement for surgery.
We deleted the following outcomes specified in the protocol: 'Duration of MBL in days'; 'Number of sanitary pads per cycle'; 'Acceptability of treatment'; and 'Mortality'. We excluded 'Duration of menstrual blood loss' and 'Number of sanitary pads per cycle' as there is no evidence of a correlation between the extent of blood loss and these outcomes (Chimbira 1980). We considered the outcome 'Acceptability of treatment' to be too similar to 'Satisfaction with treatment'. We considered 'Mortality' a rare event that was unlikely to be measured in studies.
One study that had been included prior to the 2015 update was excluded in 2015 because it did not measure any of the amended outcomes (Lahteenmaki 1998).
Contributions of authors
2020 update
Anne Lethaby and Magdalena Bofill screened potential studies for eligibility, and performed data extraction and risk of bias assessment of one new trial. Two new included trials had been included in other Cochrane Reviews. MBR did the data extraction and risk of bias assessment and double‐checked it with the previously extracted data, entered data, modified the review to incorporate the results of the additional studies and prepared the draft of the final review. AL commented on the draft and final version of the review. VJ resolved differences by providing a third‐party ruling and commented on the final version of the review.
2015 update
Munawar Hassain reviewed potential studies for eligibility and performed data extraction of the included studies. He also wrote the Background section and commented on the draft text of the final review. Josephine Rishworth assessed the quality of the included studies. Anne Lethaby reviewed potential studies for eligibility, assessed the quality of the included studies, performed data extraction, entered data, modified the review to incorporate the results of the additional studies and prepared the draft of the final review.
Margaret Rees assessed the quality of the included studies and commented on the final draft.
Prior to the 2015 update
Inez Cooke registered the title, reviewed potential studies for eligibility, assessed the quality of the included studies, performed data extraction, submitted the protocol in 1996 and prepared a draft of the review. For the 2003 update of the review, Inez reviewed potential studies for eligibility, assessed quality, performed data extraction, edited and commented on the text of the final review and wrote the discussion section.
Margaret Rees reviewed potential studies for eligibility, assessed the quality of the included studies, performed data extraction and reviewed and edited the completed draft of the review for the 1999 publication.
Anne Lethaby conducted additional searches in 1999, reviewed potential studies for eligibility, assessed the quality of the included studies, performed data extraction, entered data and prepared the draft of the final review with the inclusion of additional studies. For the 2003 update of the review, Anne performed additional searches, reviewed potential studies for eligibility, assessed the quality of the included studies, performed data extraction, entered data and modified the review to incorporate the results of the additional studies. A final search was performed in July 2005 just prior to publication of the update.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, National Womens Hospital and University of Auckland, Auckland, New Zealand
External sources
NHS Executive Anglia and Oxford Region R & D Programme, UK
Health Research Council, Auckland, New Zealand
Declarations of interest
AL, MB and VJ have no conflict of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Ashraf 2017 {published data only}
- *.Ashraf M, Habib-Ur-Rehman, A, Shehzad, Z, AlSharari S, Murtaza G. Clinical efficacy of levonorgestrel and norethisterone for the treatment of chronic abnormal uterine bleeding. Journal of the Pakistan Medical Association 2017;67(9):1331-8. [28924270] [PubMed] [Google Scholar]
- Ashraf M, Murtaza G. Levonorgestrel intrauterine system and norethisterone for the treatment of chronic abnormal uterine bleeding: A randomized clinical comparison in the patients of Bahawalpur, Pakistan. Value in Health 2016;19(7):A577. [ 613235950] [Google Scholar]
Barrington 2003 {published data only}
- Barrington JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;108(1):72-4. [DOI] [PubMed] [Google Scholar]
Crosignani 1997 {published data only}
- Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in treatment of dysfunctional uterine bleeding. Obstetrics and Gynecology Clinics of North America 1997;90(2):257-63. [DOI] [PubMed] [Google Scholar]
de Souza 2010 {published data only}
- Silva-Filho AL, Pereira FAN, Souza SS, Loures LF, Rocha APC, Valadares CN, et al. Five-year follow-up of levonorgestrel-releasing intrauterine system versus thermal balloon ablation for the treatment of heavy menstrual bleeding: a randomised controlled trial. Contraception 2013;87(4):409-15. [DOI] [PubMed] [Google Scholar]
- *.Souza SS, Camargos AF, Rezende CP, Pereira FAN, Araujo CAA, Filho ALS. A randomised prospective trial comparing the levonorgestrel-releasing intrauterine system with thermal balloon ablation for the treatment of heavy menstrual bleeding. Contraception 2010;81(3):226-31. [DOI] [PubMed] [Google Scholar]
Endrikat 2009 {published data only}
- Endrikat J, Shapiro H, Lukkari-Lax E, Kunz M, Schmidt W, Fortier M. A Canadian, multicentre study comparing efficacy of a levonorgestrel-releasing intrauterine system to an oral contraceptive in women with idiopathic menorrhagia. Journal d'Obstetrique et Gynecologie du Canada : JOGC [Journal of Obstetrics and Gynaecology Canada : JOGC] 2009;31(4):340-7. [DOI] [PubMed] [Google Scholar]
Ergun 2012 {published data only}
- Ergun B, Bastu E, Kuru O, Sen S, Kilic Y, Dural O. Comparison of rollerball endometrial ablation and levonorgestrel releasing intrauterine system in the management of abnormal uterine bleeding. International Journal of Gynaecology and Obstetrics 2012;119(S3):S672. [Google Scholar]
- *.Ergun B, Kuru O, Sen S, Kilic Y, Bastu E. Rollerball endometrial ablation versus levonorgestrel releasing intrauterine system in the management of abnormal uterine bleeding. Ginecologia.ro 2012;8(30):199-201. [Google Scholar]
Ghazizadeh 2014 {published data only}
- Ghazizadeh S, Panahi Z, Ghanbari Z, Menshadi AT, Farahmandian T, Javadian P. Comparative efficacy of Novasure, the levonorgestrel-releasing intrauterine system and hysteroscopic endometrial resection in the treatment of menorrhagia: a randomised clinical trial. Journal of Gynecologic Surgery 2014;30(4):215-8. [Google Scholar]
Gupta 2013 {published data only}
- *.Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. New England Journal of Medicine 2013;368(2):128-37. [DOI] [PubMed] [Google Scholar]
- Gupta JK, Daniels JP, Middleton LJ, Pattison HM, Prileszky G, Roberts TE, et cols. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of the levonorgestrel-releasing intrauterine system in primary care against standard treatment for menorrhagia: The ECLIPSE trial. Health technology Assessment 2015;19(88):1-118. [DOI: 10.3310/hta19880] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai J, Middleton L, Daniels J, Tryposkiadis K, Pattison H, Gupta J. Usual medical treatments or levonorgestrel-IUS for women with heavy menstrual bleeding: long-term randomised pragmatic trial in primary care. British Journal of General Practice 2016;66(653):e861-e870. [DOI: 10.3399/bjgp16X687577] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera S, Roberts T, Barton P, Daniels J, Middleton L, Gennard L, et al. Cost effectiveness of levonorgestrel intrauterine system for menorrhagia. BJOG 2013;120:390-1. [Google Scholar]
- Sanghera S, Roberts TE, Barton P, Frew E, Daniels J, Middleton L, et al. Levonorgestrel-releasing intrauterine system vs usual medical treatment for menorrhagia: an economic evaluation alongside a randomised controlled trial. PLOS One 2014;9(3):e91891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 2013 {published data only}
- Beelen P, Van Den Brink MJ, Geomini P, Dekker J, Herman M, Duijnhoven R, et al. RCT comparing the LNG-IUS versus endometrial ablation in women with heavy menstrual bleeding (MIRA). International Journal of Gynaecology and Obstetrics 2018;143(3):318-9. [10.1002/ijgo.12582] [Google Scholar]
- *.Herman MC, den Brink MJ, Geomini PM, Meurs HS, Huirne JA, Eising HP, et al. Levonorgestrel releasing intrauterine system (Mirena) versus endometrial ablation (Novasure) in women with heavy menstrual bleeding: a multicentre randomised controlled trial. BMC Women's Health 2013;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurskainen 2001 {published data only}
- Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, Kujansuu E, et al. The effect of hysterectomy or levonorgestrel-releasing intrauterine system on sexual functioning among women with menorrhagia: a 5-year randomised controlled trial. British Journal of Obstetrics and Gynaecology 2007;114(5):563-8. [DOI] [PubMed] [Google Scholar]
- Halmesmaki K, Hurskainen R, Tiitinen A, Teperi J, Grenman S, Kivela A, et al. A randomized controlled trial of hysterectomy or levonorgestrel-releasing intrauterine system in the treatment of menorrhagia - effect on FSH levels and menopausal symptoms. Human Reproduction (Oxford, England) 2004;19(2):378-82. [DOI] [PubMed] [Google Scholar]
- Halmesmaki KH, Hurskainen RA, Paavonen JA, Tuppurainen MT. Randomised controlled trial of the effect of hysterectomy or LNG-IUS use on bone mineral density: a five-year follow up. Therapy 2006;3(4):509-15. [Google Scholar]
- Heliovaara-Peippo S, Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel-releasing intrauterine system on lower abdominal pain and back pain among women treated for menorrhagia: a five-year randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2009;88(12):1389-96. [DOI] [PubMed] [Google Scholar]
- Heliovaara-Peippo S, Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel-releasing intrauterine system on lower urinary tract symptoms: a 10-year follow-up study of a randomised trial. British Journal of Obstetrics and Gynaecology 2010;117(5):602-9. [DOI] [PubMed] [Google Scholar]
- Heliovaara-Peippo S, Hurskainen R, Teperi J, Aalto A-M, Grenman S, Halmesmaki K, et al. Quality of life and costs of levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of menorrhagia: a 10 year randomised controlled trial. Obstetrical & Gynecological Survey 2014;69(4):204-5. [DOI] [PubMed] [Google Scholar]
- Heliovaara-Peippo S, Hurskainen R, Teperi J, Aalto A-M, Grenman S, Halmesmaki K, et al. Quality of life and costs of levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of menorrhagia: a 10-year randomised controlled trial. American Journal of Obstetrics and Gynecology 2013;209(6):535.e1-14. [DOI] [PubMed] [Google Scholar]
- Heliovaara-Peippo S, Oksjoki R, Halmesmaki K, Kaaja R, Teperi J, Grenman S, et al. The effect of hysterectomy or levonorgestrel-releasing intrauterine system on cardiovascular disease risk factors in menorrhagia patients: a 10-year follow-up of a randomised trial. Maturitas 2011;69(4):354-8. [DOI] [PubMed] [Google Scholar]
- Hurskainen R, Teperi J, Aalto A-M, Grenman S, Kivela A, Kujansuu E, et al. Levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of essential menorrhagia: predictors of outcome. Acta Obstetrica et Gynecologica Scandinavica 2004;83(4):401-3. [DOI] [PubMed] [Google Scholar]
- Hurskainen R, Teperi J, Rissanen P, Aalto A-M, Grenman S, Kivela A, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia. JAMA 2004;291(12):1456-63. [DOI] [PubMed] [Google Scholar]
- *.Hurskainen R, Teperi J, Rissanen P, Aalto A-M, Grenman S, Kivela A, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet 2001;357(9252):273-7. [DOI] [PubMed] [Google Scholar]
- Inki P, Hurskainen R, Palo P, Ekholm E, Grenman S, Kivela A, et al. Comparison of ovarian cyst formation in women using the levonorgestrel-releasing intrauterine system versus hysterectomy. Ultrasound in Obstetrics & Gynecology 2002;20(4):381-5. [DOI] [PubMed] [Google Scholar]
- Leminen H, Heliovaara-Peippo S, Halmesmaki K, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel-releasing intrauterine system on premenstrual symptoms in women treated for menorrhagia: secondary analysis of a randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2012;91(3):318-25. [DOI] [PubMed] [Google Scholar]
Irvine 1998 {published data only}
- Irvine GA, Campbell-Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for the treatment of idiopathic menorrhagia. British Journal of Obstetrics and Gynaecology 1998;105(6):592-8. [DOI] [PubMed] [Google Scholar]
Kaunitz 2010 {published data only}
- Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, DeSanctis Y, Jensen J. Levonorgestrel-releasing intrauterine system for heavy menstrual bleeding improves hemoglobin and ferritin levels. Contraception 2012;86(5):452-7. [DOI] [PubMed] [Google Scholar]
- *.Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding. Obstetrics and Gynecology 2010;116(3):625-32. [DOI] [PubMed] [Google Scholar]
Kilic 2009 {published data only}
- Kilic S, Yuksel B, Doganay M, Bardakci H, Akinsu F, Uzunlar O, et al. The effect of levonorgestrel-releasing intrauterine device on menorrhagia in women taking anticoagulant medication after cardiac valve replacement. Contraception 2009;80(2):152-7. [DOI] [PubMed] [Google Scholar]
Kiseli 2016 {published data only}
- Kiseli M, Kayikcioglu F, Evliyaoglu O, Haberal A. Comparison of therapeutic efficacies of norethisterone, tranexamic acid and levonorgestrel-releasing intrauterine system for the treatment of heavy menstrual bleeding: a randomized controlled study. Gynecologic and Obstetric Investigation 2016;81(5):447-53. [CRSSTD: 7929734] [DOI] [PubMed] [Google Scholar]
Kittelsen 1998 {published data only}
- Istre O, Trolle B. Treatment of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Fertility and Sterility 2001;76(2):304-9. [DOI] [PubMed] [Google Scholar]
- *.Kittelsen N, Istre O. A randomized study comparing levonorgestrel intrauterine system (LNG IUS) and transcervical resection of the endometrium (TCRE) in the treatment of menorrhagia: preliminary results. Gynaecological Endoscopy 1998;7(2):61-5. [Google Scholar]
- Rauramo I, Elo I, Istre O. Long-term treatment of menorrhagia with levonorgestrel intrauterine system versus endometrial resection. Obstetrics and Gynecology 2004;104(6):1314-21. [DOI] [PubMed] [Google Scholar]
Malak 2006 {published data only}
- Malak KA, Shawki O. Management of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Gynecological Surgery 2006;3(4):275-80. [Google Scholar]
Ozdegirmenci 2011 {published data only}
- Ozdegirmenci O, Kayikcioglu F, Akgul MA, Kaplan M, Karcaaltincaba M, Haberal A, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertility and Sterility 2011;95(2):497-502. [DOI] [PubMed] [Google Scholar]
Reid 2005a {published data only}
- Reid PC, Virtanen-Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss measurements. British Journal of Obstetrics and Gynaecology 2005;112(8):1121-5. [DOI] [PubMed] [Google Scholar]
Sayed 2011 {published data only}
- Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomised clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. International Journal of Gynaecology and Obstetrics 2011;112(2):126-30. [DOI] [PubMed] [Google Scholar]
Sesti 2012 {published data only}
- Sesti F, Piancatelli R, Pietropoli A, Ruggeri V, Piccione E. Levonorgestrel-releasing intrauterine system versus laparoscopic supracervical hysterectomy for the treatment of heavy menstrual bleeding: a randomised study. Journal of Women's Health 2012;21(8):851-7. [DOI] [PubMed] [Google Scholar]
Shabaan 2011 {published data only}
- Shabaan MM, Zakherah MS, El-Nashar SA, Sayed GH. Levonorgestrel-releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomised clinical trial. Contraception 2011;83(1):48-54. [DOI] [PubMed] [Google Scholar]
Shaw 2007 {published data only}
- *.Shaw RW, Symonds IM, Tamizian O, Chaplain J, Mukhopadhyay S. Randomised comparative trial of thermal balloon ablation and levonorgestrel intrauterine system in patients with idiopathic menorrhagia. Australian & New Zealand Journal of Obstetrics & Gynaecology 2007;47(4):335-40. [DOI] [PubMed] [Google Scholar]
- Shaw RW, Symonds IM, Tamizian O. Randomised comparative trial of thermal balloon ablation (TBA) v levonorgestrel intrauterine system (LNG IUS) in idiopathic menorrhagia. In: XVIII FIGO World Congress of Gynecology and Obstetrics. Vol. 4. 2006:119. [DOI] [PubMed]
Soysal 2002 {published data only}
- Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralblatt fur Gynakologie 2002;124(4):213-9. [DOI] [PubMed] [Google Scholar]
TALIS 2006 {published data only}
- Brown PM, Farquhar CM, Lethaby A, Sadler LC, Johnson NP. Cost-effectiveness analysis of levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG 2006;113(7):797-803. [DOI] [PubMed] [Google Scholar]
- *.Busfield RA, Farquhar CM, Sowter MC, Lethaby A, Sprecher M, Yu Y, et al. A randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG 2006;113(3):257-63. [DOI] [PubMed] [Google Scholar]
Tam 2006 {published data only}
- Tam WH, Yuen PM, Ng DPS, Leung PL, Lok IH, Rogers MS. Health status function after treatment with thermal balloon ablation and levonorgestrel intrauterine system for idiopathic menorrhagia: a randomised study. Gynecologic and Obstetric Investigation 2006;62(2):84-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu Hashim 2013 {published data only}
- Abu Hashim H, Zayed A, Ghayaty E, El Rakhawy M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomised controlled trial. Journal of Gynecologic Oncology 2013;24(2):128-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adiguzel 2017 {published data only}
- *.Adiguzel C, Seyfettinoglu S, Aka Satar D, Arlier S, Eskimez E, Kaya F, Nazik H. Evaluation of quality of life and cost-effectiveness of definitive surgery and the levonorgestrel intrauterine system as treatment options for heavy menstrual bleeding. Turkish Journal of Medical Sciences 2017;47(3):789-94. [DOI: 10.3906/sag-1512-115] [DOI] [PubMed] [Google Scholar]
Apter 2016 {published data only (unpublished sought but not used)}
- *.Apter D, Briggs P, Tuppurainen M, Grunert J, Lukkari-Lax E, Rybowski S, Gemzell-Danielsson K. A 12-month multicenter, randomized study comparing the levonorgestrel intrauterine system with the etonogestrel subdermal implant. Fertility and Sterility 2016;106(1):151-7.e5. [DOI: 10.1016/j.fertnstert.2016.02.036] [DOI] [PubMed] [Google Scholar]
Buyuktuna 2016 {published data only}
- *.Buyuktuna N, Sumer F. Comparison of mirena intrauterine device with oral progesterone treatment and surgical treatment in idiopathic menorrhagia patients: Results of a cost-minimisation analysis. Value in Health 2016;19(7):A698. [613234471] [Google Scholar]
Cameron 1987 {published data only}
- *.Cameron IT, Leask R, Kelly RW, Baird DT. The effects of danazol, mefenamic acid, norethisterone and a progesterone-impregnated coil on endometrial prostaglandin concentrations in women with menorrhagia. Prostaglandins 1987;34(1):99-110. [DOI] [PubMed] [Google Scholar]
- Cameron IT. Dysfunctional uterine bleeding. Bailliere's Clinical Obstetrics and Gynaecology 1989;3(2):315-27. [DOI] [PubMed] [Google Scholar]
Ghazizadeh 2011 {published data only}
- Ghazizadeh S, Bakhtiari F, Rahmanpour H, Davari-Tanha F, Ramezanzadeh F. A randomised clinical trial to compare levonorgestrel-releasing intrauterine system (Mirena) vs transcervical endometrial resection for treatment of menorrhagia. International Journal of Women's Health 2011;3:207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2006 {published data only}
- Gupta B, Mittal S, Misra R, Deka D, Dadhwal V. Levonorgestrel-releasing intrauterine system vs transcervical endometrial resection for dysfunctional uterine bleeding. International Journal of Gynaecology and Obstetrics 2006;95:261-6. [DOI] [PubMed] [Google Scholar]
Janssen 1999 {published data only}
- Janssen CAH. Menorrhagia and the 3-keto-desogestrel-copper medicated intrauterine device. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1999;85:135-6. [DOI] [PubMed] [Google Scholar]
Karacaoglu 2001 {published data only}
- Karacaoglu U, Cebi Z, Savan K, Yasar L, Cankaya A, Kupelioglu L, et al. Comparing hysteroscopic ablation with levonorgestrel-releasing intrauterine device in abnormal uterine bleeding cases. Jinekoloji Ve Obstetrik Dergisi 2001;15(3):172-5. [Google Scholar]
Karimi‐Zarchi 2013 {published data only}
- Karimi-Zarchi M, Dehghani-Firoozabadi R, Tabatabaie A, Dehghani-Firoozabadi Z, Teimoori S, Chiti Z, et al. A comparison of the effect of levonorgestrel IUD with oral medroxyprogesterone acetate on abnormal uterine bleeding with simple endometrial hyperplasia and fertility preservation. Clinical and Experimental Obstetrics & Gynecology 2013;40(3):421-4. [PubMed] [Google Scholar]
Kucuk 2008 {published data only}
- Kucuk U, Ertan K. Continuous oral or intramuscular medroxyprogesterone acetate versus the levonorgestrel releasing intrauterine system in the treatment of perimenopausal menorrhagia: a randomised prospective controlled trial in female smokers. Clinical and Experimental Obstetrics & Gynecology 2008;35(1):57-60. [PubMed] [Google Scholar]
Lahteenmaki 1998 {published data only}
- Lahteenmaki P, Haukkamaa M, Puolakka J, Riikonen U, Sainio S, Suvisaari J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ 1998;316:1122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- *.Lee BS, Ling X, Asif S, Kraemer P, Hanisch JU, Inki P. Levonorgestrel-releasing intrauterine system versus conventional medical therapy for heavy menstrual bleeding in the Asia-Pacific region. International Journal of Gynaecology and Obstetrics 2013;121:24-30. [DOI] [PubMed] [Google Scholar]
- Xu L, Lee BS, Asif S, Kraemer P, Inki P. Satisfaction and health-related quality of life in women with heavy menstrual bleeding; results from a non interventional trial of the levonorgestrel-releasing intrauterine system or conventional medical therapy. International Journal of Women's Health 2014;6:547-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mawet 2014 {published data only}
- Mawet M, Nollevaux F, Nizet D, Wijzen F, Gordenne V, Tasev N, et al. Impact of a new levonorgestrel intrauterine system, Levosert(), on heavy menstrual bleeding: results of a one-year randomised controlled trial. European Journal of Contraception & Reproductive Health Care 2014;19(3):169-79. [10.3109/13625187.2014.894184] [DOI] [PMC free article] [PubMed] [Google Scholar]
McMillan 1998 {unpublished data only}
- McMillan L. Randomised controlled study comparing the efficacy of Levonorgestrel intrauterine device with Nd:YAG laser endometrial ablation for the treatment of menorrhagia and premenstrual syndrome. National Research Register, id N0080056736 .
McMillan 2005 {published data only}
- McMillan L. Randomised controlled study comparing the efficacy of levonorgestrel intrauterine device with surgical treatment of menorrhagia by total endometrial ablation. National Research Register, id N0080056730 .
Milsom 1991 {published data only}
- Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. American Journal of Obstetrics and Gynecology 1991;164(3):879-83. [DOI] [PubMed] [Google Scholar]
Rogerson 1999 {unpublished data only}
- Rogerson L, Crocombe W, Duffy S. SMART - Satisfaction with Mirena and ablation: a randomised trial (abstract). In: British Society of Gynaecological Endocrinology. 1999.
Romer 2000 {published data only}
- Romer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2000;90(1):27-9. [DOI] [PubMed] [Google Scholar]
Tosun 2014 {published data only}
- Tosun AK, Tosun I, Suer N. Comparison of levonorgestrel-releasing intrauterine device with oral progestins in heavy menstrual bleeding (HMB) cases with uterine leiomyoma (LNG-IUD and oral progestin usage in myoma uteri). Pakistan Journal of Medical Sciences Quarterly 2014;30(4):834-9. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
SHiPP 2013 {unpublished data only}
- SHiPP (Stopping Heavy Periods Project), Identifier NCT02002260. Ongoing study. February 2013 ‒ estimated completion date January 2017. Contact author for more information.
Additional references
Andersson 1994
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994;49(1):56-72. [DOI] [PubMed] [Google Scholar]
Baldwin 2013
- Baldwin MK, Jensen JT. Contraception during the perimenopause. Maturitas 2013;76(3):235-42. [DOI] [PubMed] [Google Scholar]
Bayer S.A. 2019
- Bayer SA. Number of countries around the world where Mirena is available. email to: Magdalena Bofill 2019.
Berqvist 1983
- Berqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. British Journal of Obstetrics and Gynaecology 1983;90(3):255-8. [DOI] [PubMed] [Google Scholar]
Chimbira 1980
- Chimbira TH, Anderson ABM, Turnbull AC. Relation between measured menstrual blood loss and patient's subjective assessment of loss, duration of bleeding, number of sanitary towels used, uterine weight and endometrial surface area. British Journal of Obstetrics and Gynaecology 1980;87(7):603-9. [DOI] [PubMed] [Google Scholar]
Clegg 2007
- Clegg JP, Guest JF, Hurskainen R. Cost-utility of levonorgestrel intrauterine system compared with hysterectomy and second generation endometrial ablative techniques in managing patients with menorrhagia in the UK. Current Medical Research and Opinions 2007;23(7):1637-48. [DOI] [PubMed] [Google Scholar]
Cole 1971
- Cole SK, Billewicz WZ, Thomson AM. Sources of variation in menstrual blood loss. Journal of Obstetrics and Gynaecology of the British Commonwealth 1971;78(10):933-9. [DOI] [PubMed] [Google Scholar]
Cooper 2011
- Cooper K, Lee AJ, Chien P, Raja EA, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. British Journal of Obstetrics and Gynaecology 2011;118(10):1171-9. [DOI: 10.1111/j.1471-0528.2011.03011.x] [DOI] [PubMed] [Google Scholar]
Farquhar 2002
- Farquhar C, Steiner C. Hysterectomy rates in the United States 1990-1997. Obstetrics and Gynecology 2002;99(2):229-34. [DOI] [PubMed] [Google Scholar]
Fergusson 2013
- Fergusson RJ, Lethaby A, Shepperd S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD000329.pub2] [DOI] [PubMed] [Google Scholar]
Fraser 2009
- Fraser IS, Langham S, Uhl-Hochgraeber K. Health-related quality of life and economic burden of abnormal uterine bleeding. Expert Review of Obstetrics and Gynecology 2009;4(2):179-89. [Google Scholar]
Ganz 2013
- Ganz ML, Shah D, Gidwani R, Filonenko A, Su W, Pocoski J, et al. The cost effectiveness of the levonorgestrel-releasing intrauterine system for the treatment of idiopathic heavy menstrual bleeding in the United States. Value in Health 2013;16(2):325-33. [DOI] [PubMed] [Google Scholar]
Goldstuck 2017
- Goldstuck N. Clarification of the role of the Jaydess(Skyla) LNG- IUS 13.5mg and Kyleena LNG-IUS 19.5mg as intrauterine contraceptive systems. Expert Review of Medical Devices 2017;14(8):593-9. [DOI: 10.1080/17434440.2017.1350169] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro Guideline Development Tool. GRADEpro, Version accessed 4 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org. [ gradepro.org.]
Hallberg 1966
- Hallberg L, Hogahl AM, Nilsson L, Rybo G. Menstrual blood loss - a population study. Acta Obstetricia et Gynecologica Scandinavica 1966;45(3):320-51. [DOI] [PubMed] [Google Scholar]
Heikinheimo 2017
- Heikinheimo O, Fraser I. The current status of hormonal therapies for heavy menstrual bleeding. Best Practice & Research. Clinical Obstetrics & Gynaecology 2017;40:111-20. [DOI: 10.1016/j.bpobgyn.2017.01.001] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011] edition. The Cochrane Collaboration, 2011. [Google Scholar]
Higham 1990
- Higham JM, O'Brien PMS, Shaw RW. Assessment of menstrual blood using a pictorial chart. British Journal of Obstetrics and Gynaecology 1990;97(8):734-9. [DOI] [PubMed] [Google Scholar]
Kaunitz 2009
- Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstetrics and Gynecology 2009;113(5):1104-16. [DOI] [PubMed] [Google Scholar]
Kaunitz 2012
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding. Drugs 2012;72(2):193-215. [DOI] [PubMed] [Google Scholar]
Liu 2007
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilisation in abnormal uterine bleeding. Value Health 2007;10(3):183-94. [DOI] [PubMed] [Google Scholar]
Matteson 2013
- Matteson KA, Rahn DD, Wheeler TL, Casiano E, Siddiqui NY, Harvie HS, et al. Nonsurgical management of heavy menstrual bleeding: a systematic review. Obstetrics and Gynecology 2013;121(3):632-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicholas 2015
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert J. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration–approved duration. Obstetrics and Gynecology 2015;125(3):599-604. [DOI: 10.1097/AOG.0000000000000690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2010
- Middleton LJ, Champaneria R, Daniels JP, Bhattacharya S, Cooper KG, Hilken NH, et al. Hysterectomy, endometrial destruction and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ 2010;341:c3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moragianni 2007
- Moragianni VA, Somkuti SG. Profound hypothyroidism-induced acute menorrhagia resulting in life-threatening anemia. Obstetrics and Gynecology 2007;110(2 Pt 2):515-7. [DOI] [PubMed] [Google Scholar]
Munro 2012
- Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them? American Journal of Obstetrics and Gynecology 2012;207(4):259-65. [DOI: 10.1016/j.ajog.2012.01.046] [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Clinical Excellence. Heavy menstrual bleeding. pubmed.ncbi.nlm.nih.gov/21938862.
Nilsson 1978
- Nilsson CG, Luukkainen T, Arko H. Endometrial morphology of women using a d-norgestrel-releasing intrauterine device. Fertility and Sterility 1978;29(4):397-401. [DOI] [PubMed] [Google Scholar]
Reid 2005b
- Reid PC, Mukri F. Trends in number of hysterectomies performed in England for menorrhagia: examination of health episode statistics 1989 to 2002-3. BMJ 2005;330(7497):938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2011
- Roberts TE, Tsourapas A, Middleton LJ, Champaneria R, Daniels JP, Cooper KG, et al. Hysterectomy, endometrial ablation and levonorgestrel releasing intrauterine system (Mirean) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ 2011;342:d2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2016
- Rowe P, Farley T, Peregoudov A, Piaggio G, Boccard S, Landoulsi S, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception 2016;93(6):498-506. [DOI: 10.1016/j.contraception.2016.02.024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sergison 2019
- Sergison J, Maldonado L, Gao X, Hubacher D. Levonorgestrel intrauterine system associated amenorrhea: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2019;220(5):440-8. [DOI: 10.1016/j.ajog.2018.12.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapley 2004
- Shapley M, Jordan K, Croft PR. An epidemiological survey of symptoms of menstrual loss in the community. British Journal of General Practice 2004;54(502):359-64. [PMC free article] [PubMed] [Google Scholar]
Somboonporn 2011
- Somboonporn W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: systematic review and meta-analysis. Menopause 2011;18(10):1060-6. [DOI] [PubMed] [Google Scholar]
Suvisaari 1996
- Suvisaari J, Lahteenmaki P. Detailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel-releasing intrauterine system. Contraception 1996;54(4):201-8. [DOI] [PubMed] [Google Scholar]
Wright 2013
- Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SM, Lu Y-S, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstetrics and Gynecology 2013;122(2):233-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2014
- Xu L, Lee BS, Asif S, Kraemer P, Inki P. Satisfaction and health-related quality of life in women with heavy menstrual bleeding; results from a non interventional trial of the levonorgestrel-releasing intrauterine system or conventional medical therapy. International Journal of Women's Health 2014;6:547-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
You 2006
- You JH, Sahota DS, MoYuen P. A cost utility analysis of hysterectomy, endometriation resection and ablation and medical therapy for menorrhagia. Human Reproduction 2006;21(7):1878-83. [DOI] [PubMed] [Google Scholar]
Zigler 2017
- Zigler RE, McNicholas C. Unscheduled vaginal bleeding with progestin-only contraceptive use. American Journal of Obstetrics and Gynecology 2017;216(5):443-50. [DOI: 10.1016/j.ajog.2016.12.008] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lethaby 1999
- Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD002126] [DOI] [PubMed] [Google Scholar]
Lethaby 2005
- Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002126.pub2] [DOI] [PubMed] [Google Scholar]
Lethaby 2015
- Lethaby A, Hussain M, Rishworth J, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD002126.pub3] [DOI] [PubMed] [Google Scholar]


