Abstract
Typhuloid fungi are a very poorly known group of tiny clavarioid homobasidiomycetes. The phylogenetic position and family classification of the genera targeted here, Ceratellopsis, Macrotyphula, Pterula sensu lato and Typhula, are controversial and based on unresolved phylogenies. Our six-gene phylogeny with an expanded taxon sampling shows that typhuloid fungi evolved at least twice in the Agaricales (Pleurotineae, Clavariineae) and once in the Hymenochaetales. Macrotyphula, Pterulicium and Typhula are nested within the Pleurotineae. The type of Typhula (1818) and Sclerotium (1790), T. phacorrhiza and S. complanatum (synonym T. phacorrhiza), are encompassed in the Macrotyphula clade that is distantly related to a monophyletic group formed by species usually assigned to Typhula. Thus, the correct name for Macrotyphula (1972) and Typhula is Sclerotium and all Typhula species but those in the T. phacorrhiza group need to be transferred to Pistillaria (1821). To avoid undesirable nomenclatural changes, we suggest to conserve Typhula with T. incarnata as type. Clavariaceae is supported as a separate, early diverging lineage within Agaricales, with Hygrophoraceae as a successive sister taxon to the rest of the Agaricales. Ceratellopsis s. auct. is polyphyletic because C. acuminata nests in Clavariaceae and C. sagittiformis in the Hymenochaetales. Ceratellopsis is found to be an earlier name for Pterulicium, because the type, C. queletii, represents Pterulicium gracile (synonym Pterula gracilis), deeply nested in the Pterulicium clade. To avoid re-combining a large number of names in Ceratellopsis we suggest to conserve it with C. acuminata as type. The new genus Bryopistillaria is created to include C. sagittiformis. The families Sarcomyxaceae and Phyllotopsidaceae, and the suborder Clavariineae, are described as new. Six new combinations are proposed and 15 names typified.
Key words: Agaricomycetes, basidioma evolution, Clavariaceae, clavarioid fungi, Pleurotineae, Sclerotium, Typhulaceae
Taxonomic novelties: New suborder: Clavariineae Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen
New family: Phyllotopsidaceae Locquin ex Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen; Sarcomyxaceae Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen
New genus: Bryopistillaria Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen
New combinations: Bryopistillaria sagittiformis (Pat.) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen; Macrotyphula megasperma (Berthier) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen; Macrotyphula phacorrhiza (Reichard: Fr.) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen; Typhula podocarpi (Crous) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen
Typification: Lectotypification: Ceratella ferryi Quél. & Fautrey, Clavaria aculina Quél., Clavaria microscopica Malbr. & Sacc., Pistillaria aciculata Durieu & Lév. ex Sacc., Pistillaria aculeata Pat., Pistillaria acuminata Fuckel, Pistillaria attenuata Syd. & P. Syd., Pistillaria carestiae Ces. in Bres. & Sacc., Pistillaria equiseticola Boud., Pistillaria helenae Pat., Pistillaria juncicola Bourdot & Galzin, Pistillaria queletii Pat., Pistillaria sagittiformis Pat., Sclerotium complanatum Tode, Typhula brunaudii Quél.
Epitypification: Pistillaria acuminata Fuckel, Pistillaria queletii Pat., Pistillaria sagittiformis Pat., Sclerotium complanatum Tode
Introduction
Clavarioid fungi have club- or coral-shaped basidiomata with the hymenium fully exposed and include at least 540 species (Corner, 1950, Kirk et al., 2008). Phylogenetic studies have demonstrated that clavarioid basidiomata have arisen multiple times from ancestors with agaricoid or corticioid basidiomata in several lineages of Basidiomycota (Pine et al., 1999, Dentinger and McLaughlin, 2006), but molecular phylogenies of clavarioid fungi and allied taxa are far from being complete. Clavarioid lineages are known to have evolved in the Agaricales, Cantharellales, Gomphales, Hymenochaetales, Russulales, Thelephorales and Trechisporales among the Agaricomycetes (Pine et al., 1999, Hibbett and Binder, 2002, Hibbett, 2004, Dentinger and McLaughlin, 2006, Birkebak et al., 2013), but fully supported multigene phylogenies are lacking in most cases and details as to how transitions to a clavarioid basidioma type have occurred are vague. A more comprehensive taxon sampling and addition of more molecular markers is still needed in many groups, including clavarioid fungi, to better understand the evolution of basidioma configuration.
In this study, we target a group of clavarioid fungi with tiny basidiomata (Fig. 1A–K), here referred to as “typhuloid” following e.g. Corner, 1950, Petersen, 1974, Petersen et al., 2014, and Olariaga et al. (2016). Corner (1950: 145) characterised typhuloid fungi by their: i) small basidiomata and limited growth, ii) distinct stipe and fertile head, iii) simple hymenium, iv) epiphytic habitat on wood, stems or leaves, rather than being terricolous, v) smooth, white ellipsoid spores, vi) monomitic, generally clamped hyphae, and vii) agglutination of the hyphae on the surface of the stem. Three genera were regarded as typhuloid, Typhula, Pistillaria and Pistillina (Corner 1950), and were separated from pteruloid fungi (Deflexula, Pterula) with a dimitic hyphal system and from Ceratellopsis with highly reduced clavarioid basidiomata with generally sterile apices. Berthier (1976), in his monograph “Typhula and allied genera”, treated typhuloid fungi in a broader sense and considered Ceratellopsis, Macrotyphula, Pterula and Typhula to represent a natural group without proposing any family classification. Hirticlavula elegans, a member of Clavariaceae producing minute, hairy basidiomata, has also been considered somewhat typhuloid (Petersen et al. 2014), and limits between typhuloid fungi and other reduced clavarioid fungi are not always clear. Typhuloid fungi represent one of the most overlooked, poorly known and enigmatic groups of homobasidiomycetes. The family classification of these fungi is uncertain or based on weakly supported phylogenies with a very limited taxon sampling. Macrotyphula and Typhula were previously placed in Clavariadelphaceae (Corner, 1970, Hawksworth et al., 1995), but recent classifications place both genera in Typhulaceae together with Sclerotium (Kirk et al., 2008, Knudsen and Vesterholt, 2012). The family classification of Ceratellopsis is even more controversial. Initially included by Corner (1970) in Clavariadelphaceae, Jülich (1982) accommodated it in Typhulaceae. Hawksworth et al. (1995) and Begerow et al. (2018), probably following Corner, included it in Gomphaceae, although Ceratellopsis lacks the synapomorphic characters of this family, such as pistillarin, ampullate septa and cyanophilous, ornamented spores (Hosaka et al. 2006). As no molecular data of Ceratellopsis has been available, its phylogenetic relationships and classification have remained doubtful. Besides the above-mentioned genera treated by Berthier (1976), Mucronella and Hirticlavula, both producing tiny clavarioid basidiomata, have been assigned to the Clavariaceae based on molecular data (Birkebak et al., 2013, Petersen et al., 2014). Regardless of their phylogenetic origin, all typhuloid fungi share similar taxonomic problems. For many species of those genera only the type specimen or very few collections are known, species limits are unclear and distribution data are meagre. Molecular data of only a handful of species are available in public sequence repositories and the high morphological diversity of the group remains poorly sampled.
Fig. 1.
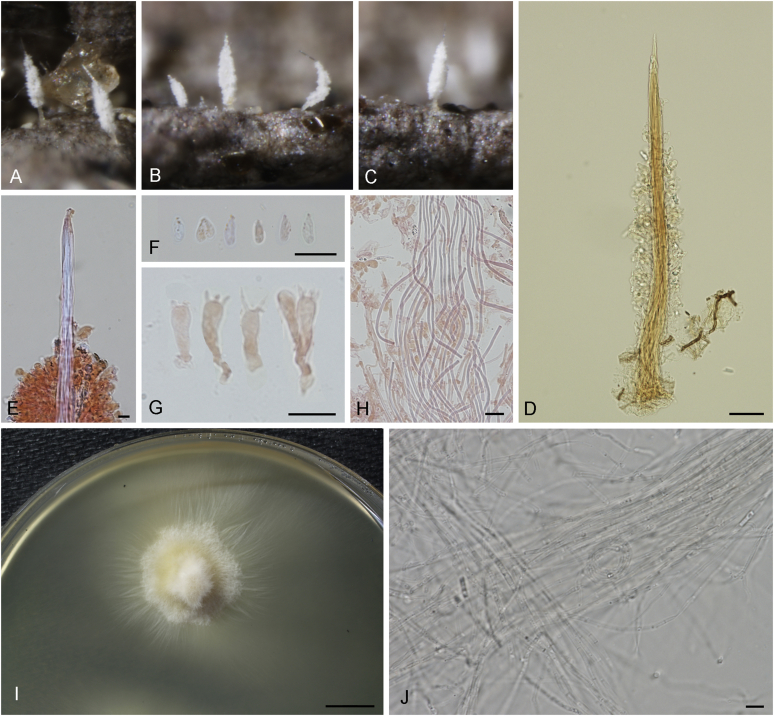
Diversity of typhuloid and pleurotoid fungi suggested to be closely related to Typhula. A.Macrotyphula fistulosa s.l. (IO.14.214, ARAN-Fungi). B.Macrotyphula juncea (IO.16.53, S). C.Typhula phacorrhiza, current type of Typhula (ARAN-Fungi 7446), here combined in Macrotyphula. D. Compressed sclerotium of T. phacorrhiza (ARAN-Fungi 7446). E.Typhula incarnata, showing sclerotia at the base (IO.14.92, S), proposed conserved type of Typhula. F.Typhula uncialis (IO.14.94, S, UPS), type of Gliocoryne. G.Typhula crassipes (IO.14.83, S, UPS). H.Typhula subhyalina (IO.15.06, S), type of Pistillina and Dacryopsella. I.Typhula erythropus (IO.16.83, ARAN-Fungi). J.Ceratellopsis aff. aculeata (ARAN-Fungi 11746). K.Pterulicium gracile (IO.14.142, S, UPS). L.Phyllotopsis nidulans (ARAN-Fungi). M.Pleurocybella porrigens (BIO-Fungi 13431). N.Sarcomyxa serotina (IO.14.130, S, UPS). Photographs I. Olariaga, except L by J.I. Iturrioz.
Typhula and segregated genera, Macrotyphula, and phylogenetic position of Typhulaceae
The phylogenetic position of Typhulaceae has been inferred from only two species, Typhula phacorrhiza and Macrotyphula fistulosa, type of Typhula and Macrotyphula, respectively. Through analyses of a 5-locus dataset, Matheny et al. (2006) recovered Typhulaceae in the hygrophoroid clade (Agaricales) with phylogenetic confidence, as sister to the Hygrophoraceae in a supported clade encompassing Pterulaceae and members of two pleurotoid agaric genera (Sarcomyxa serotina and Phyllotopsis spp.) (Fig. 1L–N). Binder et al. (2010), employing a broader taxon sampling of the Agaricomycetes, recovered also Typhulaceae as sister to Hygrophoraceae but without support, while Pterulaceae was supported as closely related to Stephanosporaceae instead (Binder et al. 2010). Other studies have not been able to confirm or reject the inclusion of Typhulaceae in the hygrophoroid clade, but recovered agaric or pleurotoid genera, such as Phyllotopsis, Pleurocybella, Tricholomopsis, as sister taxa of Typhulaceae with phylogenetic confidence (Dentinger and McLaughlin, 2006, Lodge et al., 2014). Dentinger et al. (2016) resolved for the first time several deep nodes of the Agaricales through a 208-locus dataset containing 35 taxa of Agaricales, and found that the hygrophoroid clade, as defined by Matheny et al. (2006), was paraphyletic. Also, Hygrocybe conica (Hygrophoraceae) was recovered as sister to the Clavariaceae, while Pterulaceae (Pterula multifida; recovered in the hygrophoroid clade by Matheny et al. (2006)), appeared in a branch with Pleurotus ostreatus. Thus, the results by Dentinger et al. (2016) question the inclusion of the Typhulaceae in the hygrophoroid clade. In addition to its uncertain phylogenetic position, the monophyly of Typhulaceae has not been tested appropriately.
Two genera, Typhula and Macrotyphula, are currently accepted in Typhulaceae (e.g. Berthier, 1976, Knudsen and Vesterholt, 2012). Macrotyphula differs from Typhula in having large, yellow-brown basidiomata (30–300 mm) that never arise from sclerotia and non-amyloid spores (Berthier 1976). In contrast, Typhula includes species with smaller basidiomata (generally under 10 mm long) that often arise from sclerotia and usually have amyloid spores. Some Typhula species are important plant pathogens that cause economic loss in cereal crops (e.g. Ekstrand 1955). These are popularly known as “snow moulds”, producing symptoms known as “Typhula blight” (Matsumoto et al., 2001, Hoshino et al., 2008). Several economically important species like T. incarnata and T. ishikariensis have been subjected to extensive research on their ecology, physiology and genetics (e.g. Matsumoto, 1992, Vergara et al., 2004, Blunt et al., 2015, Chang, 2015, Koch, 2016). Generic limits of Typhula are not fully delineated and lack a complete consensus. Probably due to the fact that its species show diverse basidioma morphologies, sclerotial anatomy and asexual morph states (Berthier 1976), a number of genera have been segregated from Typhula, such as Cnazonaria, Dacryopsella, Gliocoryne, Phacorhiza, Pistillaria, Pistillina, Scleromitra and Sphaerula. These genera have been used to a certain extent. Of these, Corner (1950) recognised Pistillaria (with Cnazonaria, Gliocoryne, Scleromitra and Sphaerula in synonymy), Pistillina and Typhula (with Phacorhiza in synonymy). Donk (1954) adopted also Pistillaria, Typhula and Pistillina, and further synonymised Dacryopsella under Pistillina. Pistillaria has been recognised generally based on a ceraceous consistency of the fresh fruitbodies, horny when dried, and the absence of sclerotia (Corner, 1950, Corner, 1970, Pilát, 1958), but generic limits between Pistillaria and Typhula have been long debated (Corner, 1950, Bourdot and Galzin, 1928, Donk, 1954, Berthier, 1976). Pistillina has been distinguished by basidiomata with a globose fertile part (Corner 1950). Berthier (1976), after examining extensive material and type specimens available, merged all these genera under Typhula (Sphaerula, Scleromitra and Dacryopsella were not treated in the monograph), but recognised Cnazonaria, Gliocoryne, Pistillaria and Pistillina as subgenera. After the publication of Berthier's monograph, a few authors have continued to use Pistillaria and Pistillina at the generic level (Shiryaev and Kotiranta, 2007, Kaygusuz and Çolak, 2017, Begerow et al., 2018, Petersen and Læssøe, 2019). Recently, the new monotypic genus Tygervalleyomyces was described in Typhulaceae based on analyses of the 28S region (Crous et al. 2017). The asexual morph of Tygervalleyomyces podocarpi, the only known morph, is similar to the asexual morph of Typhula crassipes (Berthier 1976, as Typhula corallina) in the cylindrical conidia with a truncate base, and in fact these two species have highly similar 28S sequences (98 %) and nested within a larger highly supported clade containing other Typhula species (Crous et al. 2017). In view of this, the status of Tygervalleyomyces needs to be re-evaluated in the light of a phylogeny with a broader sampling of Typhula species. Olariaga & Salcedo (2013) synonymised Typhula and Macrotyphula due to the fact that T. phacorrhiza formed a monophyletic group with Macrotyphula in previous analyses (Pine et al., 1999, Hibbett, 2007), as well as morphological similarities. The designation of T. phacorrhiza as lectotype of Typhula by Donk (1933) has been considered unfortunate (Berthier, 1976, Olariaga, 2009, Olariaga and Salcedo, 2013), because T. phacorrhiza, with long filiform basidiomata and unique compressed sclerotia, is an atypical species in Typhula (Remsberg, 1940, Corner, 1950, Berthier, 1976). In fact, T. phacorrhiza shares many features with M. fistulosa, i.e. the pale brown, large basidiomata, the stipe surface with thin hyphae and caulotrichomes, the basal tomentum formed by thick-walled, scarcely septate hyphae and the presence of a hyaline, striped encrustation on the medulla hyphae (Olariaga & Salcedo 2013). Molecular phylogenetic analyses show that these species are closely related and nested in the Agaricales (Binder et al. 2010). Nevertheless, taxon sampling in phylogenetic studies of Typhulaceae is extremely poor and the synonymy of Typhula and Macrotyphula needs to be further explored.
The type of Sclerotium is conspecific with the type of Typhula
The genus Sclerotium, also included in Typhulaceae (Kirk et al. 2008), is currently treated as an artificial genus that accommodates fungi producing sclerotia but not, or rarely, a sexual morph (Xu et al. 2010). Tode (1790) included originally eight species in Sclerotium, of which Fries (1821) treated S. complanatum in the first place and Clements & Shear (1931: 411) thus selected this species as the type of Sclerotium. A number of authors, especially during the XIXth century, described numerous species in Sclerotium, including ascomycetous and basidiomycetous fungi (e.g. Fries, 1822, Léveillé, 1843, Duby, 1830, Desmaziéres, 1848, Rostrup, 1866), and numerous plant pathogens (Xu et al. 2010). Until now, 464 names have been described or combined in Sclerotium (Index Fungorum, viewed on 11 June 2019) and it is evident that species assigned to Sclerotium have multiple evolutionary origins, but very few attempts to disassemble it have been made (Xu et al. 2010). With the end of the asexual-sexual morph dual nomenclature, many names in Sclerotium may turn out to have priority over species names in use. Several early authors observed that some Sclerotium species appeared in connection or directly attached to basidiomata of Typhula species (e.g. Berkeley, 1837, Léveillé, 1843 (as Clavaria), Rostrup 1866). Sclerotium complanatum, type of Sclerotium, is characterised by producing compressed sclerotia attached to the substrate by a small stalk (Tode 1790) which conform to those produced by T. phacorrhiza (Berkeley, 1837, Rostrup, 1866, Schröter, 1889). Thus, it is generally accepted that S. complanatum is a synonym of T. phacorrhiza (Xu et al., 2010, Kaygusuz and Çolak, 2017), although only Remsberg (1940) has proposed this synonymy according to our search. Other authors attributed S. complanatum to the sclerotial morph of T. gyrans (Fries, 1874, Corner, 1950, Donk, 1962), but this view appears to have been abandoned. In the meantime, the taxonomic identity of S. complanatum has not been reassessed and the name remains untypified. As currently asexual names compete with sexual names for priority, the possible synonymy of S. complanatum and T. phacorrhiza would make Sclerotium and Typhula taxonomic synonyms, and all Typhula names in use, including those being applied to economically important plant pathogens, would have to be transferred to the older and equally sanctioned genus Sclerotium. Examining in depth the taxonomic concept of S. complanatum and proposing a typification is thus of paramount importance to deal with a possible scenario of undesirable nomenclatural changes.
The poorly known genus Ceratellopsis, a possible earlier synonym of Pterulicium
Ceratellopsis differs from Typhula in having minute filiform basidiomata with a sterile apex and a non-corticate stipe (Corner, 1950, Berthier, 1976). Short basidia up to 20 μm have also been suggested to be a diagnostic character (Jülich 1982). Pterulicium gracile, called Pterula gracilis until very recently (Leal-Dutra et al. 2020), strongly resembles species of Ceratellopsis because of its minute white basidiomata with a sterile apex, at least at early stages of development (Corner, 1950, Berthier, 1976), but it differs microscopically in having skeletal hyphae, typical for Pterulaceae, 2-spored basidia and no stipe (Corner, 1950, Olariaga, 2009). Furthermore, we have made collections with very minute basidiomata with a clearly delimited stipe suggesting Ceratellopsis, but having skeletal hyphae as typical in Pterulaceae. Limits between Ceratellopsis and Pterulaceae, thus, are not always clear-cut.
As pointed out by Donk, 1954, Konrad and Maublanc, 1937 introduced Ceratellopsis based on the validly published but illegitimate Ceratella Pat. (Patouillard 1887; later homonym of Ceratella Hook. f. 1844) and explicitly indicated Ceratellopsis queletii as the type of Ceratellopsis. While Donk (1954) followed this typification, Corner (1950), noted that C. queletii might represent a rudimentary Pterula, and he selected instead Ceratellopsis aculeata as type so that Ceratellopsis could be used with certainty and not reduced to a synonym of Pterula. This choice, nevertheless, is not permissible (Turland et al. 2018; Art. 7.8) since the original type indication of Ceratellopsis by Konrad & Maublanc (1937) is unequivocal and irrevocable. Also, Olariaga (2009) proposed tentatively that C. queletii might be a synonym of Pterulicium gracile, but up to present, no consistent and stable interpretation of C. queletii has been provided and the taxonomic status of Ceratellopsis remains unresolved. Twenty-four names have been described or combined in Ceratellopsis, but several of them represent P. gracile (Berthier 1976) or Typhula species.
The confirmation that C. queletii, type of Ceratellopsis, is a synonym of P. gracile would have important consequences in the classification of Pterulaceae. The family, centered on the genus Pterula, has included several clavarioid genera with a dimitic hyphal system (Corner 1970). Based on molecular studies, four resupinate genera (Aphanobasidium, Coronicium, Merulicium, Radulomyces; Larsson, 2007, Larsson et al., 2004) and the polyporoid Radulotubus (Zhao et al. 2016) were later transferred to Pterulaceae. In the light of analyses of the ITS, 28S and RPB2 regions, employing a rich taxon sampling of the Pterulaceae, Leal-Dutra et al. (2020) elucidated generic limits in the family. This study discovered Pterula to be polyphyletic and splits its species into the new genus Myrmepterula, Phaeopterula and Pterulicium, leaving in Pterula sensu stricto a handful of species around Pterula plumosa. Deflexula was shown to be paraphyletic because some species nest in the Pterula clade, while the type D. fascicularis is in the Pterulicium clade. In total, 46 names earlier treated in Pterula and Deflexula were combined in Pterulicium, a genus up to then monospecific. Pterula gracilis was found to belong to the Pterulicium clade and accordingly combined as Pterulicium gracile. In this framework, would the synonymy between C. queletiti and P. gracile be confirmed, the name Ceratellopsis (1937) would have priority over Pterulicium (1950). Thus, the identity of C. queletii needs urgent clarification.
In the present study, we expand the taxon sampling of typhuloid fungi based on the multigene datasets used by Matheny et al. (2006) and Binder et al. (2010). With these data, our main goals were to: 1) provide a robust phylogenetic hypothesis for typhuloid fungi, especially for T. phacorrhiza (type of Typhula), types of genera segregated from Typhula, Sclerotium complanatum (type of Sclerotium) and Ceratellopsis species; 2) test the monophyly of Typhula; 3) assign typhuloid fungi to appropriate families; and 4) propose an updated nomenclature in the light of a robust multigene phylogeny.
Material and methods
Molecular techniques
DNA was extracted from fresh (stored in 1 % SDS extraction buffer) basidiomata, using a DNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. The following six gene regions were amplified: 1) nu5.8S rDNA, the 5′ end of the nuclear 28S rDNA (spanning domains D1–D2), part of the nuSSU rDNA (ca. 1 600 bp), RPB1 (900 bp; A–C), RPB2 (5–7 region, ca. 1 100 bp) and EF-1α (1 100 bp). The ITS (ITS1-5.8S-ITS2) and 28S regions were amplified in one piece using the primers ITS5-LR7, or otherwise as separate pieces: ITS using ITS5-ITS4, (White et al. 1990); and 28S using LR0R-LR5 (or LR3) and LR3R-LR7 (Vilgalys & Hester 1990). The same primers were used for sequencing. The ITS was sequenced using the primers ITS1-ITS4 and/or in a few instances ITS5, 5.8S and ITS3. The SSU region was amplified in one piece employing primers NS1-NS8 or in two pieces using NS1-NS4 and NS3-NS8 (White et al. 1990). PCR products of the RPB1 region were obtained using gRPB1-A (Stiller & Hall 1997) and fRPB1-C rev primers (Matheny et al. 2002). The sequence spanning RPB2 regions 5–7 was amplified in one piece, using fRPB2-5F and bRPB2-7R, or if required, in two pieces with primers fRPB2-5F-gRPB2-6R and bRPB2-6F-bRPB2-7R (Liu et al., 1999, Matheny, 2005). For samples that did not succesfully amplify or for sequencing, Typhula-specific primers were designed for the RPB2 region and used in different combinations (Table 1). The EF-1α region was PCR amplified and sequenced employing 983F and 2218R primers (Rehner & Buckley 2005). Typhula-specific primers of the EF-1α region were designed and used for PCR amplification and sequencing of problematic samples (Table 1). PCR amplifications were performed using Illustra™ Hot Start Mix RTG PCR beads (GE Healthcare, UK) in a 25 μL volume, containing 3 μL of genomic DNA, 10 μM of each primer and distilled water. PCRs were conducted in Applied Biosystems GeneAmp® PCR System 9700 and 2720 Thermal Cyclers. Amplifications were performed using the following program: initial denaturation at 95 °C for 5 min, followed by 35–40 cycles of 95 °C for 45–60 s, 52–58 °C for 50 s, 72 °C for 1 min, followed by a final extension at 72 °C for 10 min. PCR amplifications of protein-coding genes follow O’Donnell et al. (2011, RPB1) and Hansen et al. (2013, RPB2, EF-1α). PCR products were purified using the enzymatic method Exo-sap-IT (USB Corporation, Santa Clara, California, USA). When multiple bands were amplified in the RPB1 and RPB2 regions, PCR products were size-fractionated in a 1 % agarose gel, stained with GelRed™ (Biotium Inc.), visualised over a UV trans-illuminator, excised and purified using QIAquick spin columns (Qiagen). Purified PCR products were sequenced at Macrogen Europe service (www.macrogen.com).
Table 1.
Newly designed Typhula-specific primers for the RPB2 and EF-1α (5’–3’) regions.
| Locus | Primer | Sequence | PCR | Sequencing |
|---|---|---|---|---|
| EF-1α | 1007F-Typh | SCGAGGAYCGTTTCAACGAG | X | |
| EF-1α | 1447F-Typh | GCATGCCHTGGTWCAAGG | X | X |
| EF-1α | 1825F-Typh | GAACGTVTCCGTYAAGGAYA | X | |
| EF-1α | 2100R-Typh | ATKGGCTTGGARGGRACRA | X | |
| RPB2 | RPB2-5Fint-Typh | AARAARCGDYTNGAYYTSGC | X | |
| RPB2 | RPB2-6F-Typh | TGGGGAYTGGAGTCGTTGGA | X | X |
| RPB2 | RPB2-6R-Typh | TCCAACGACTCCARTCCCCA | X | X |
| RPB2 | RPB2-7Rint-Typh | TASGTGTTACGAGGRGACT | X |
Type specimens, taxon and molecular sampling
Type specimens of small typhuloid fungi available at E, FH, M, PC, S and UPS herbaria were examined: Ceratella ferryi, Ceratellopsis carestiae, C. rickii, C. acuminata, C. equiseticola, Clavaria aculina, C. microscopica, Pistillaria attenuata, P. juncicola, Pterulicium gracile, Typhula brunaudii, T. crassipes, T. sclerotioides, T. sphaeroidea, T. subhyalina and T. uncialis. Material deposited in G (customs blocked the loan), PAD (not available on loan) and SAPA (several contact attempts unsuccessful) could not be examined. The notation “!” indicates that type or other original material was examined by us. Cultures of the taxa collected and described in this study were deposited in the CBS-KNAW culture collection at the Westerdijk Fungal Biodiversity Institute.
For molecular analyses, types and other species of genera considered to be typhuloid or assigned to Typhulaceae were sampled, namely Ceratellopsis, Macrotyphula, Pterulicium gracile, Typhula s.l. and Sclerotium. A collection identified as C. acuminata, with skeletal hyphae, was included to test the limits between Ceratellopsis and Pterula. Nucleotide sequences were aligned in the six-gene dataset (nu5.8S rDNA, nu28S rDNA, nu18S rDNA, RPB1, RPB2 and EF-1α) assembled by Binder et al. (2010; TreeBASE no. S10185), in order to preliminarily explore their phylogenetic affinities. Nucleotide sequences were aligned in Aliview (Larsson 2014). This alignment was subjected to a maximum likelihood (ML) analysis using the “RAxML HPC2 on XSEDE” tool (Stamatakis 2014) in the CIPRES Science Gateway (Miller et al. 2010), starting from a random tree. A GTR-GAMMA model with four rate categories was selected for tree inference. For branch confidence, 1 000 ML bootstrap replicates were conducted with a GTRCAT model (ML-BP). Targeted typhuloid taxa nested in Agaricales (ML-BP 92 %), except for Ceratellopsis sagittiformis that was placed in Hymenochaetales. Based on this analysis, a first 6-locus (5.8S, 28S, 18S, RPB1, RPB2, EF-1α) dataset (the Agaricales matrix) was prepared to phylogenetically place typhuloid fungi among the Agaricales. Three taxa, Amylocorticium cebennense, Plicaturopsis crispa and Serpulomyces borealis, were included as outgroup for rooting purposes based on previous studies (Binder et al. 2010). A second dataset with the same molecular markers (the Pleurotineae matrix) included Typhulaceae and closely related families, with a more species-inclusive sampling than in the Agaricales matrix. Cantharocybe gruberi was used as an outgroup based on analyses of the Agaricales matrix. A third 28S alignment (the Clavariaceae matrix) was constructed based on Birkebak et al. (2013) to infer phylogenetic relationships of Ceratellopsis within the Clavariaceae, and employed Anomoporia bombycina, A. kamtschatica and Podoserpula pusio as outgroup taxa. A fourth 4-locus (5.8S, 28S, 18S, RPB2) dataset (the Hymenochaetales matrix) was assembled to further explore phylogenetic relationships of C. sagittiformis within the Hymenochaetales. Sequences of Calocera cornea were set as outgroup.
Sequence alignment and phylogenetic analyses
Sequences were edited and assembled using Sequencher v. 4.10.1 (Gene Codes Corp., Ann Arbor, MI) and deposited in GenBank (Table 2). Additional sequences were downloaded from GenBank and from the following genomes through the MycoCosm portal (Grigoriev et al. 2014): Agaricus bisporus, Coprinopsis cinerea (Muraguchi et al. 2015), Laccaria bicolor (Martin et al. 2008), Onnia scaura, Phellinus ferrugineofuscus, Plicaturopsis crispa (Kohler et al. 2015), Pterulicium gracile (Varga et al. 2019, deposited as Pterula gracilis), Radulomyces confluens and Trichaptum abietinum (Table 2). Nucleotide sequences were aligned manually using Aliview (Larsson 2014). Protein-coding genes were translated to amino acids to determine intron positions. In order to check gene-tree congruence, each individual gene-region was analysed using a ML approach, as explained above. Gene congruence was assessed manually by comparing supported clades among single-gene genealogies (Mason-Gamer & Kellogg 1996). Clades were considered in conflict if a supported clade (ML-BP >70 %) for one marker was contradicted with significant support by another one. Since no conflict was detected, markers were concatenated in the Agaricales, Typhulaceae and Hymenochaetales alignments. Introns were excluded and the third codon position was partitioned in the protein-coding genes (RPB1, RPB2 and EF-1α). Each matrix was subjected to ML and Bayesian analyses. ML analyses were conducted as explained above. Bayesian analyses were implemented in MrBayes v. 3.2.6 (Ronquist et al. 2012), using two parallel runs of eight Metropolis-coupled Markov chain Monte Carlo (MCMCMC) chains for 30 M generations, starting from a random tree, and sampling one tree every 1 000th generation from the posterior distribution. Substitution models were sampled across the GTR space during the MCMC simulation (Ronquist et al. 2012). Stationarity was assumed when average standard deviation of split frequencies fell below 0.01. A burn-in sample of 30 000 trees was discarded. To assess branch confidence, a 50 % majority rule consensus tree was computed with the remaining 30 002 trees using the SUMT command of MrBayes. Bayesian posterior probability (PP) values ≥0.95 were considered to be significant. The alignments and respective phylogenetic trees were deposited in TreeBASE, study number S25967.
Table 2.
Sequenced specimens used in this study, with GenBank accession numbers for 5.8S, 28S, 18S, RPB1, RPB2 and EF-1α regions. Numbers in parentheses following the species names indicate multiple collections of a species. The GenBank accessions of sequences generated in this study are in bold. Asterisks indicate sequences obtained from genome data through the JGI portal (https://jgi.doe.gov/). Abbreviations of datasets are: ag = Agaricales, cl = Clavariineae; hy = Hymenochaetales, pl = Pleurotineae.
| Original name | Updated name | Voucher specimen | Dataset | GenBank accession numbers |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 5.8S | 28S | 18S | RPB1 | RPB2 | EF-1α | ||||
| Agaricus bisporus∗ | — | H97 | ag | Genome | Genome | Genome | genome | Genome | genome |
| Alloclavaria purpurea | — | PBM 2731 (CUW) | hy | DQ486690 | DQ457657 | DQ437679 | — | — | — |
| Anomoporia bombycina | — | CFMR:L-6240 | cl | — | GU187564 | — | — | — | — |
| A. kamtschatica | — | GB/M Edman K426 | cl | — | DQ144615 | — | — | — | — |
| Anthracophyllum archeri | — | PBM 2201 (WTU) | ag | DQ444308 | AY745709 | DQ092915 | DQ435799 | DQ385877 | DQ028586 |
| Amanita brunnescens | — | PBM 2429 (CUW) | ag | AY789079 | AY631902 | AY707096 | AY788847 | AY780936 | AY881021 |
| Aphanobasidium pseudotsugae | — | HHB-822 (CFMR) | ag, pl | GU187509 | GU187567 | GU187620 | GU187695 | GU187781 | GU187695 |
| Armillaria mellea | — | PBM 2470 (CUW) | ag | AY789081 | AY700194 | AY787217 | AY788849 | AY780938 | AY881023 |
| Baeospora myosura | — | PBM 2748 (CUW) | ag | DQ484063 | DQ457648 | DQ435796 | DQ435801 | DQ470827 | GU187762 |
| Basidioradulum radula | — | GEL 2493 (KASSEL) | hy | DQ234537 | AY700184 | AY771611 | — | — | — |
| Blasiphalia pseudogrisella | — | P. Hoijer 4393 (H7031951)/ IO.14.231 (S) | hy | MF319048 | MF318899 | MF318990 | — | MT24239 | — |
| Bolbitius vitellinus | — | MTS 5020 (WTU) | ag | DQ200920 | AY691807 | AY705955 | DQ435802 | DQ385878 | DQ408148 |
| Camarophyllopsis schulzeri | — | S. Jacobsson 3453 (H) | cl | — | AM946415 | — | — | — | — |
| Cantharellopsis prescotii | — | I. Kytovuori 08-0808/ H6059300 | hy | MF319050 | MF318901 | MF318992 | — | MF288855 | — |
| Cantharocybe gruberi | — | PBM 510 (WTU) | ag, pl | DQ200927 | DQ234540 | DQ234546 | DQ435808 | DQ385879 | DQ059045 |
| Ceratellopsis acuminatea | — | CBS 146691 | ag, cl | MT232347 | MT232298 | MT232493 | MT24236 | MT24230 | MT242352 |
| C. aculeatea | — | ARAN-Fungi 13729 | cl | — | MT232300 | — | — | — | — |
| C. aff. acuminatea | — | ARAN-Fungi 11746 | cl | MT232348 | MT232299 | — | — | — | — |
| C. sagittiformis(1) | Bryopistillaria sagittiformis | IO.15.41 (S) | hy | — | MT232301 | — | — | MT24231 | — |
| C. sagittiformis(2) | B. sagittiformis | IO.15.85 (S) | hy | — | MT232302 | — | — | MT24232 | — |
| C. sagittiformis(3) | B. sagittiformis | IO.14.164 (S) | hy | MT232349 | MT232303 | — | — | MT24233 | — |
| Cheimonophyllum candidissimum | — | PBM 2411 (WTU) | ag | DQ486687 | DQ457654 | DQ435812 | DQ447888 | DQ470831 | GU187760 |
| Calocera cornea | — | GEL 5359 (KASSEL) | hy | AY789083 | AY701526 | AY771610 | — | AY536286 | — |
| Clavaria acuta(1) | — | RHP55840 (TENN043602) | cl | — | HQ877681 | — | — | — | — |
| C. acuta(2) | — | MTS4577 (WTU) | cl | — | HQ877679 | — | — | — | — |
| C. acuta(3) | — | JFA10440 (WTU) | cl | — | HQ877680 | — | — | — | — |
| C. alboglobospora | — | TENN042295 | cl | — | HQ877682 | — | — | — | — |
| C. argillacea | — | TFB10720 (TENN058804) | cl | — | HQ877683 | — | — | — | — |
| C. australiana | — | ADM1311 (TENN051311) | cl | — | HQ877685 | — | — | — | — |
| C. aff. fragilis | — | SAT98-349-01 (WTU) | cl | — | HQ877688 | — | — | — | — |
| C. fumosa | — | GG_151003 | cl | — | EF535268 | — | — | — | — |
| C. fuscata | — | RHP55840 (TENN043602) | cl | — | HQ877681 | — | — | — | — |
| C. inaequalis | — | MB 04-016 (WTU) | cl | — | AY745693 | — | — | — | — |
| C. pullei | — | KGN98 | cl | — | AY586646 | — | — | — | — |
| C. cf. rubicundula | — | TENN043695 | cl | — | HQ877697 | — | — | — | — |
| Clavaria sp.(1) | — | TFB11835 (TENN060720) | cl | — | HQ877692 | — | — | — | — |
| Clavaria sp.(2) | — | JMB10061001 (TENN065665) | cl | — | HQ877684 | — | — | — | — |
| C. stegasauroides | — | PBM3373 | cl | — | HQ877698 | — | — | — | — |
| C. zollingeri | — | JMB08040912 (TENN064095) | cl | — | HQ877700 | — | — | — | — |
| Clavicorona taxophila | — | 9186 | cl | — | AF115333 | — | — | — | — |
| Clavulinopsis amoena | — | PBM3381 | cl | — | HQ877702 | — | — | — | — |
| C. corallinorosacea | — | PBM3380 | cl | — | HQ877707 | — | — | — | — |
| C. fusiformis | — | MGW672 (TENN064110) | cl | — | HQ877717 | — | — | — | — |
| C. sulcata | — | PBM3379 | cl | — | HQ877709 | — | — | — | — |
| Clitocella mundula | — | TJB 7599 (CORT) | ag | DQ494694 | AY700182 | DQ089017 | DQ447937 | DQ474128 | — |
| C. candicans | — | WTU | ag | DQ202268 | AY645055 | AY771609 | DQ447891 | DQ385881 | DQ408149 |
| C. subditopoda | — | WTU | ag | DQ202269 | AY691889 | AY771608 | DQ447892 | AY780942 | DQ408150 |
| Coltricia perennis | — | P. Salo 11024 (H) | hy | MF319056 | MF318907 | MF318996 | — | MF288856 | — |
| Collybia tuberosa | — | TENN 53540 | ag | AY854072 | AY639884 | AY771606 | AY857982 | AY787219 | AY881025 |
| Conocybe lactea | — | PBM 2411 (WTU) | ag | DQ486693 | DQ457660 | DQ437683 | DQ447893 | DQ470834 | — |
| Coprinus comatus | — | ECV 3198 (UC) | ag | AY854066 | AY635772 | AY665772 | AY857983 | AY780934 | AY881026 |
| Coprinopsis cinerea∗ | — | AmutBmut #326 | ag | genome | Genome | genome | genome | genome | genome |
| Coronicium alboglaucum | — | NH4208 | pl | AY463400 | AY586650 | — | — | — | — |
| Cortinarius iodes | — | WTU | ag | AF389133 | AY702013 | AY771605 | AY857984 | AY536285 | AY881027 |
| Cotylidia sp. | — | WTU | hy | AY854079 | AY629317 | AY705958 | — | AY883422 | — |
| C. undulatea | — | IO.15.126 (S) | hy | MT232350 | MT232304 | — | — | MT24234 | — |
| Crepidotus cf. applanatus | — | PBM 717 (WTU) | ag | DQ202273 | AY380406 | AY705951 | AY333303 | AY333311 | DQ028581 |
| Cristinia sp. | — | FP-100305 (CFMR) | pl | GU187526 | GU187585 | GU187637 | GU187470 | GU187793 | GU187718 |
| Entoloma prunuloides | — | TJB 4765 (CORT) | ag | DQ206983 | AY700180 | AY665784 | DQ447898 | DQ385883 | DQ457633 |
| Globulicium hiemale | — | Hjm 19007 (GB) | hy | DQ873595 | DQ873595 | DQ873594 | — | — | — |
| Gymnopus contrarius | — | PBM 2711 (WTU) | ag | DQ486708 | DQ457670 | DQ440643 | DQ447902 | DQ472716 | GU187700 |
| Gyroflexus brevibasidiata | — | IO.14.230 (S) | hy | MT232351 | MT232305 | — | — | MT24235 | — |
| Fibricium rude | GEL 2121 (KASSEL) | hy | — | AY700202 | AY654888 | — | — | — | |
| Fistulina Antarctica | — | — (AFTOL-ID 1335) | ag | DQ486702 | AY293181 | AY293131 | DQ447899 | DQ472713 | GU187698 |
| Flammulina velutipes | — | TENN 52002 | ag | AY854073 | AY639883 | AY665781 | AY858966 | AY786055 | AY883423 |
| Hemimycena gracilis | — | PBM 2715 (WTU) | ag | DQ490623 | DQ457671 | DQ440644 | DQ447905 | DQ472719 | GU187709 |
| Hirticlavula elegans | — | JHP-13.364 (O) | cl | — | KJ939349 | — | — | — | — |
| Hodophilus aff. foetens | — | ECV4175 (TENN065670) | cl | — | HQ877678 | — | — | — | — |
| Hodophilus hymenocephalus(1) | — | WTU | cl | — | DQ457679 | — | — | — | — |
| H. hymenocephalus(2) | — | DJL98_081505 | cl | — | EF561628 | — | — | — | — |
| Hohenbuehelia tremula | — | PBM 2301 (WTU)/ DAOM 180808 | pl | DQ182504 | KU355405 | DQ440645 | — | KU355434 | KU355465 |
| Hydropus cf. scabripes | — | WTU | ag | DQ404389 | DQ411536 | DQ444855 | DQ447908 | DQ457634 | — |
| Hygrocybe coccinea | — | PBM 915 (WTU) | ag, cl | DQ490629 | DQ457676 | DQ444858 | DQ447910 | DQ472723 | GU187705 |
| H. aff. conica | — | CBS 300.56 | cl | — | DQ534589 | — | — | — | — |
| Hygrophorus pudorinus | — | PBM 2721 (WTU) | ag | DQ490631 | DQ457678 | DQ444861 | DQ447912 | DQ472725 | GU187710 |
| Hyphodontia alutaria | — | KHL 11889 (GB) | hy | DQ873603 | DQ873603 | DQ873602 | — | — | — |
| Hyphodontiella multiseptata | — | Ryberg 021022 (GB) | cl | — | EU118634 | — | — | — | — |
| Inocybe myriadophylla | — | JV 19652F (WTU) | ag | DQ221106 | AY700196 | AY657016 | DQ447916 | AY803751 | DQ435791 |
| Inonotus griseus | — | Dai 13436 | hy | KX674583 | KX364823 | — | — | KX364919 | — |
| Infundibulicybe gibba | — | JCS 0704B (WTU) | ag | DQ490635 | DQ457682 | DQ115780 | DQ447913 | DQ472727 | GU187759 |
| Kneiffiella subalutacea | — | KHL 11888 (GB) | hy | DQ873630 | DQ873631 | DQ873629 | — | — | — |
| Kuehneromyces rostratus | — | PBM 2703 (WTU) | ag | DQ490638 | DQ457684 | DQ457624 | DQ447918 | DQ472730 | GU187712 |
| Laccaria bicolor∗ | — | S238N | ag | genome | genome | genome | genome | genome | genome |
| Lachnella villosa | — | CBS 609.87 | ag | DQ097362 | DQ097347 | AY705959 | — | DQ472732 | GU187721 |
| Leifia flabelliradiata | — | KG Nilsson 36270 (GB) | hy | DQ873635 | DQ873635 | — | — | — | — |
| Lepista irina | — | PBM 2291 (WTU) | ag | DQ221109 | DQ234538 | AY705948 | DQ447919 | DQ385885 | DQ028591 |
| Lycoperdon pyriforme | — | DSH 96-054 (WTU) | ag | AY854075 | AF287873 | AF026619 | AY860523 | AY218495 | AY883426 |
| Macrolepiota dolichaula | — | HKAS | ag | DQ221111 | DQ411537 | AY771602 | DQ447920 | DQ385886 | DQ435785 |
| Macrotyphula fistulosa(1) | — | IO.14.219 (S)/ IO.15.123 (ARAN-Fungi, S)/ TUB 011469 | ag | — | DQ071735 | MT232494 | DQ068002 | MT24236 | MT242353 |
| M. fistulosa(2) | — | IO.14.214 (ARAN-Fungi, S) | ag | MT232352 | KY224088 | MT232495 | MT24237 | — | MT242354 |
| M. juncea s.l. | — | IO.14.177 (S) | ag | MT232353 | MT232306 | MT241267 | — | MT24237 | MT242355 |
| Megacollybia platyphylla | — | TENN 59432 | ag | DQ249275 | AY702016 | AY786053 | DQ447923 | DQ385887 | DQ435786 |
| Mucronella calva(1) | — | JS16142 | cl | — | AY586689 | — | — | — | — |
| M. calva(2) | — | GEL4458 | cl | — | AJ406588 | — | — | — | — |
| M. aff. calva | — | KHL10317 | cl | — | AY586690 | — | — | — | — |
| M. flava | — | IO.16.84 (S) | ag, cl | MT232354 | MT232307 | MT232496 | MT24238 | — | MT242356 |
| M. fusiformis | — | DJM 1309 | ag, cl | — | DQ284905 | — | — | — | — |
| M. pendula | — | PBM 3437 | ag, cl | — | HQ829921 | — | — | — | — |
| Muscinupta laevis | — | V. Haikonen 19745 (H6059292) | hy | MF319066 | MF318921 | MF319004 | — | MF288861 | — |
| Mycetinis alliaceus | — | TENN 55620 | ag | AY854076 | AY635776 | AY787214 | AY860526 | AY786060 | AY883431 |
| Odonticium romellii | — | KHL s. n. (GB) | hy | DQ873639 | DQ873639 | DQ873638 | — | — | — |
| Onnia scaura∗ | — | P53A | hy | genome | Genome | genome | — | genome | — |
| Oxyporus populinus | — | Dai 12793/ DSH 93-188 | hy | KF111019 | KF111021 | AF026616 | — | KT210380 | — |
| Peniophorella praetermissa | — | GEL 2182 (KASSEL) | hy | AY854081 | AY700185 | AY707094 | — | AY787221 | — |
| P. pubera | — | KHL 13154 (GB) | hy | DQ873599 | DQ873599 | DQ873598 | — | — | — |
| Pluteus romellii | — | ECV 3201 (UC) | ag | AY854065 | AY634279 | AY657014 | AY862187 | AY786063 | AY883433 |
| Phaeomarasmius proximans | — | PBM 1951 (WTU) | ag | DQ404381 | AY752970 | AY752970 | — | AY333314 | DQ028592 |
| Phellinidium ferrugineofuscum∗ | — | SpK3Phefer14 | genome | genome | genome | — | genome | — | |
| Phellinus tremulae | — | KCTC 6659/ NJB2011-PT2-F | hy | AY189703 | KU139202 | AY178026 | KU139277 | — | — |
| Phyllotopsis nidulans | — | IO.14.196 (S) | ag, pl | — | MT232308 | MT232497 | MT24239 | MT24238 | MT242357 |
| Phyllotopsis sp. | — | MB 35 (WTU) | ag, pl | DQ404382 | AY684161 | AY707090 | DQ447933 | AY786061 | DQ059047 |
| Porotheleum fimbriatum | — | CBS 788.86 | ag | DQ490626 | DQ457673 | DQ444854 | DQ447907 | DQ472721 | — |
| Pleurocybella porrigens | — | JFA 12544 (WTU)/TUB012154/ UPS F-611822 | ag, pl | MT232355 | MT232309 | GU187660 | DQ067994 | MT24239 | GU187740 |
| Pleurotus eryngii | — | X102 | ag, pl | KX977448 | — | FJ379286 | — | — | — |
| P. ostreatus | — | TENN 53662 | NG_027634 | AY645052 | AY657015 | AY862186 | AY786062 | AY883432 | |
| Plicaturopsis crispa∗ | — | FD-325 SS-3 | ag, pl | genome | genome | genome | genome | genome | genome |
| Podoserpula pusio | — | Hlepp-329 | ag | — | EF535271 | — | — | — | — |
| Porodaedalea pini | — | No-6170-T | hy | JX110037 | JX110081 | — | — | JX109951 | — |
| Pseudoclitocybe cyathiformis | — | JFA 12811 (WTU)/GLM 46020 (GB) | ag | GU187553 | EF551313 | GU187659 | DQ067939 | GU187815 | GU187742 |
| Pterulicium echo(1) | — | DJM 302S58 (MINN) | ag, pl | DQ494693 | AY458123 | DQ092911 | — | GU187805 | GU187743 |
| P. echo(2) | cf. Pterula | ZRL20151311 | pl | LT716065 | KY418881 | KY418947 | KY418979 | KY419026 | KY419076 |
| P. gracile∗(1) | — | CBS 309.79 | ag, pl | genome | genome | genome | genome | genome | genome |
| P. gracile(2) | — | IO.14.142 (S) | pl | MT232356 | MT232310 | MT232498 | — | — | MT242358 |
| Radulomyces confluens∗ | — | OMC1631 | ag, pl | genome | genome | genome | genome | genome | genome |
| R. molaris | — | ARAN-Fungi 2003 | ag, pl | — | MT232311 | MT232499 | MT24230 | MT24230 | MT242359 |
| Ramariopsis biformis | — | JMB10061006 (TENN065660) | cl | — | HQ877712 | — | — | — | — |
| R. crocea | — | JMB10071001 (TENN065661) | cl | — | HQ877715 | — | — | — | — |
| R. aff. kunzei | — | Marr5064 (WTU) | cl | — | HQ877719 | — | — | — | — |
| R. pseudosubtilis | — | RHP27722 (TENN027722) | cl | — | HQ877723 | — | — | — | — |
| R. tenuiramosa | — | GG_061104 | cl | — | EF535269 | — | — | — | — |
| Repetobasidium conicum | — | KHL 12338 (GB) | hy | DQ873647 | DQ873647 | DQ873646 | — | — | — |
| R. mirificum | — | FP-133558-sp | hy | — | AY293208 | AY293155 | — | — | — |
| Resinicium bicolor | — | GEL 2071 (KASSEL) | hy | DQ218310 | AY700183 | — | — | DQ457635 | — |
| Rickenella fibula | — | PBM 2503 (WTU) | hy | DQ241782 | AY700195 | AY771599 | — | DQ408115 | — |
| Rhodocollybia maculate | — | WTU | ag | DQ404383 | AY639880 | AY752966 | DQ447936 | AY787220 | DQ061279 |
| Sarcomyxa serotina | — | WTU/ DSH 93-218 | ag, pl | DQ494695 | AY691887 | AF026590 | DQ447938 | DQ859892 | GU187754 |
| Schizophyllum radiatum | — | FH | ag | AY571060 | AY571023 | AY705952 | DQ447939 | DQ484052 | — |
| Schizopora radula | — | Dai 12631 | hy | KT203307 | KT203328 | — | — | KT210382 | — |
| Sclerotium complanatum | Macrotyphula phacorrhiza | Microf. Exs. No. 49 (UPS) | pl | — | MT234400 | — | — | — | — |
| Sistotrema confluens | — | FCUG 298 | hy | DQ267125 | AY647214 | AY757260 | — | DQ381837 | — |
| Stephanospora caroticolor | — | TUB019072/ IOC 137-97/ R44008 | ag, pl | KM086827 | AF518652 | AF518591 | KF211335 | — | GU187747 |
| Trichaptum abietinum∗ | — | R44008 | hy | genome | genome | genome | — | genome | — |
| Tubulicrinis globisporus | — | KHL 12133 (GB) | hy | DQ873655 | DQ873655 | DQ873654 | — | — | — |
| T. inornatus | — | KHL 11763 (GB) | hy | DQ873659 | DQ873659 | DQ873658 | — | — | — |
| Tygervalleyomyces podocarpi | Typhula podocarpi | CPC 29979 | NR_156661 | NG_059851 | — | — | — | — | |
| Typhula capitata | — | IO.15.122 (S, UPS)/ CBS 143727 | ag, pl | MT232357 | MT232312 | MT232500 | MT24231 | MT24231 | MT242360 |
| T. crassipes | — | IO.14.83 (S, UPS) | ag, pl | MT232358 | KY224094 | — | — | MT24232 | MT242361 |
| T. erythropus | — | IO.14.123 (S, UPS)/ CBS 143797 | ag, pl | MT232359 | KY224096 | MT232501 | MT24232 | MT24233 | MT242362 |
| T. gyrans | — | IO.14.103 (S)/ CBS 143796 | ag, pl | MT232360 | KY224097 | MT232502 | MT24233 | MT24234 | MT242363 |
| T. micans | — | IO.14.165 (S) | ag, pl | MT232361 | KY224102 | MT232503 | MT24234 | MT24235 | MT242364 |
| T. incarnata | — | IO. 14. 92 (S)/ CBS 143742/ CBS 350.79 | ag, pl | MT232362 | MT232313 | MT232504 | MT24235 | MT24236 | MT242365 |
| T. phacorrhiza(1) | Macrotyphula phacorrhiza | IO.14.200 (S) | ag, pl | MT232363 | MT232314 | MT232505 | — | MT24237 | MT242366 |
| T. phacorrhiza(2) | M. phacorrhiza | IO.14.167 (S) | ag, pl | MT232364 | MT232315 | MT232506 | MT24236 | MT24238 | MT242367 |
| T. phacorrhiza(3) | M. juncea s.l. | DSH 96-059 | pl | — | AF393079 | AF026630 | — | AY218525 | — |
| T. phacorrhiza(4) | M. phacorrhiza | ARAN-Fungi 7446 | pl | — | MT232316 | — | — | — | MT242368 |
| T. sclerotioides | — | IO.14.22 (S) | ag, pl | MT232365 | MT232317 | MT232507 | MT24237 | MT24239 | MT242369 |
| T. subhyalina | — | IO.15.06 (S)/ CBS 143735 | ag, pl | MT232366 | MT232318 | MT232508 | — | MT24230 | MT242370 |
| T. uncialis | — | IO.14.74 (S) | ag, pl | MT232367 | MT232319 | MT232509 | MT24238 | MT24231 | MT242371 |
| Uncultured Basidiomycota(5) | Ceratellopsis sp. | Soil sample | cl | DQ341741 | DQ341741 | — | — | — | — |
| Uncultured Basidiomycota(1) | Ceratellopsis sp. | Soil sample | cl | HQ433218 | HQ433218 | — | — | — | — |
| Uncultured Basidiomycota(2) | Ceratellopsis sp. | Soil sample | cl | GQ159941 | GQ159941 | — | — | — | — |
| Uncultured Basidiomycota(3) | Ceratellopsis sp. | Soil sample | cl | EU691875 | EU691875 | — | — | — | — |
| Uncultured Basidiomycota(4) | Ceratellopsis sp. | Soil sample | cl | EU861817 | EU861817 | — | — | — | — |
| Uncultured Basidiomycota(5) | Ceratellopsis sp. | Soil sample | cl | DQ341741 | DQ341741 | — | — | — | — |
| Uncultured Basidiomycota(6) | Ceratellopsis sp. | Soil sample | cl | EF434117 | EF434117 | — | — | — | — |
| Uncultured Basidiomycota(7) | Ceratellopsis sp. | Soil sample | cl | GQ159939 | GQ159939 | — | — | — | — |
| Xerula radicata | — | TENN 59235 | ag | DQ241780 | AY645051 | AY654884 | DQ447946 | AY786067 | DQ029194 |
| Xylodon rimosissimus | — | CBS 105.045/ Ryberg 021031 (GB) | hy | DQ873627 | DQ873628 | DQ873626 | — | LN714662 | — |
Results
Origins of typhuloid fungi within Agaricales and Pleurotineae
A total of 118 (21 5.8S, 23 28S, 18 18S, 13 RPB1, 23 RPB2, 20 EF-1α) sequences were generated for this study (Table 2). The Agaricales matrix comprised 81 taxa and contained 6 292 unambiguously aligned nucleotide positions (161 nu5.8S, 1 480 nu28S, 1 745 nu18S, 861 RPB1, 1 056 RPB2 and 989 EF-1α), with all genes available for 86.4 % of taxa. The Pleurotineae matrix had 39 taxa and contained 6 215 unambiguously aligned nucleotide positions (159 nu5.8S, 1 418 nu28S, 1 732 nu18S, 863 RPB1, 1 056 RPB2 and 987 EF-1α), with all genes available for 72.2 % of taxa. The Bayesian analysis of the Agaricales and Pleurotineae datasets reached average standard deviations of split frequencies > 0.01 after 12 195 000 and 425 000 generations, respectively. The Bayesian majority rule consensus tree of the Agaricales was fully resolved and many deeper branches received high support by Bayesian PP (Fig. 2). The majority of these branches were, however, not supported by ML-BP (< 70 %). Typhuloid fungi do not form a monophyletic group. Specimens with skeletal hyphae nest in Pleurotineae (Pterulicium gracile) and in Clavariineae (C. acuminata). Sequences of the specimen of P. gracile collected by us (IO.14.142) were identical to those obtained from the available genome of P. gracile (CBS 309.79) (Fig. 3), employed also by Leal-Dutra et al. (2020). Clavariineae forms a strongly supported clade (PP 1, ML-BP 86) that is resolved as an early diverging lineage within Agaricales (PP 1). Ceratellopsis acuminata forms a highly supported clade with Ramariopsis kunzei (PP 1, ML-BP 100). The remaining Agaricales form a strongly supported monophyletic group (PP 1), within which Ampulloclitocybe clavipes, Cantharocybe gruberi, Hygrocybe coccinea, Hygrophorus pudorinus and Pseudoarmillariella ectypoides, corresponding to the Hygrophoraceae, constitute a strongly supported lineage in the Bayesian analysis (PP 0.98), as a sister group to the rest of the Agaricales (PP 1). The suborders Agaricineae (PP 0.96), the Tricholomatineae (PP 1), the Marasmiineae (PP 0.99) and the Pluteineae (PP 0.95) also received high support in the Bayesian analysis. Xeromphalina campanella, previously assigned to the hygrophoroid clade, is supported as an early diverging sister lineage to the Marasmiineae (PP 0.99). Species of Typhula and Macrotyphula form a well-supported clade together with other pleurotoid, clavarioid, corticioid and gasteroid species (PP 0.95) that is referred to the Pleurotineae. The Agaricales (ag) and Pleurotineae (pl) phylogenies (Fig. 2, Fig. 3) show similar supported nodes in the Pleurotineae, except that the Pleurotaceae formed a sister group to the rest of the Pleurotineae in both Bayesian and ML analyses of the Pleurotineae matrix (PP 1, ML-BP 99). In all analyses T. phacorrhiza is nested within a monophyletic Macrotyphula (ag PP 1, ML-BP 100; pl PP 1, ML-BP 100). A specimen identified as Sclerotium complanatum is nested within a clade of three T. phacorrhiza collections in the analyses of the Pleurotineae matrix. The Macrotyphula clade is encompassed in a larger supported clade together with Phyllotopsis and Pleurocybella porrigens (pl PP 1). Stephanospora caroticolor, Cristinia rhenana, Pterulaceae and Radulomycetaceae (Aphanobasidium, Radulomyces) form a strongly supported clade (pl PP 1, ML-BP 73) sister to the Macrotyphula clade, Phyllotopsis and Pleurocybella porrigens. All Typhula species but its type T. phacorrhiza, form a distinct separate lineage (pl PP 1, ML-BP 100). It includes the types of Cnazonaria, Dacryopsella, Gliocoryne, Phacorhiza, Pistillaria, Pistillina, Scleromitra, Sphaerula and Tygervalleyomyces. Typhula is a sistergroup to the clade formed by Macrotyphula, Phyllotopsis, Pleurocybella porrigens, Pterulaceae and Stephanosporaceae in the Pleurotineae phylogeny (Fig. 3), albeit without support (pl PP 0.94). Pleurotus and Hohenbuehelia tremula are supported as a sistergroup to the rest of the Pleurotineae in the Pleurotineae phylogeny (Fig. 3, PP 1, ML-BP 99).
Fig. 2.
Bayesian inference 50 % majority rule consensus phylogram of the Agaricales from 5.8S-18S-28S-RPB1-RPB2-EF-1α sequence data, with the placement of typhuloid fungi (in blue font). Bayesian posterior probabilities (PP) and Maximum Likelihood bootstrap values (ML-BP) are shown on branches, ordered as PP/ML-BP. Thickened branches received support at least in one analysis (ML-BP ≥ 70 % and/or PP ≥ 95 %). Suborder names recognised within the Agaricomycetes are indicated on the right side.
Fig. 3.
Bayesian inference 50 % majority rule consensus phylogram of the Pleurotineae from 5.8S-18S-28S-RPB1-RPB2-EF-1α sequence data. Bayesian posterior probabilities (PP) and Maximum Likelihood bootstrap values (ML-BP) are shown on branches, ordered as PP/ML-BP. Thickened branches received support at least in one of the analyses (ML-BP ≥ 70 % and/or PP ≥ 95 %). Family names recognised within the Pleurotineae are marked with colour boxes. Basidioma types are indicated with different colours for ingroup taxa.
The Clavariaceae phylogeny
The Clavariaceae matrix comprised 53 taxa and contained 1 505 unambiguously aligned nucleotide positions (28S rDNA). Bayesian and ML analyses produced very similar topologies (Fig. 4). Species of Mucronella form a highly supported sister group to the rest of the Clavariaceae (PP 1, ML-BP 100). Clavaria, Camarophyllopsis, Hodophilus and Hirticlavula elegans form a highly supported monophyletic group (PP 1, ML-BP 84), characterised by lacking clamp connections on context hyphae in all species but Clavicorona taxophila. Three species of Ceratellopsis form a distinct clade with several environmental sequences (uncultured Basidiomycota in GenBank). The relationships of the Ceratellopsis clade to the other genera with clamp connections, Ramariopsis and Clavulinopsis, are not resolved with support.
Fig. 4.
Bayesian inference 50 % majority rule consensus phylogram of the Clavariineae from 28S sequence data. Bayesian posterior probabilities (PP) and Maximum Likelihood bootstrap values (ML-BP) are shown on branches, ordered as PP/ML-BP. Thickened branches received support at least in one analysis (ML-BP ≥ 70 % and/or PP ≥ 95 %). Basidioma types are indicated with different colours for ingroup taxa.
The Hymenochaetales phylogeny
The Hymenochaetales matrix contained 37 taxa and 4 239 unambiguously aligned characters. The majority rule consensus tree of the Bayesian analysis is provided in Fig. 5. The Hymenochaetales is recovered as monophyletic with high support in the Bayesian analysis (PP 1, ML-BP 62). It comprises two larger clades: a) a clade containing Coltricia, Kneiffiella, Xylodon and Hymenochaetaceae, corresponding to clades C–F in Larsson et al. (2006), along with two species of Repetobasidium (PP 0.91, ML-BP 26); and b) a clade corresponding to the Rickenella clade (clade B in Larsson et al. 2006) (PP 1, ML-BP 57). Within the Rickenella clade, the three Ceratellopsis sagittiformis specimens are encompassed in a well-supported clade (PP 1, ML-BP 65) with species of agaricoid (Blasiphalia, Cantharellopsis, Gyroflexus, Rickenella), clavarioid (Alloclavaria), corticioid (Globulicium, Hyphodontia, Peniophorella, Resinicium), cyphelloid (Muscinupta) and thelephoroid (Cotylidia) basidiomata. The position of Hyphodontia alutaria and Resinicium bicolor is in conflict; both species form a supported monophyletic group with Rickenella fibula and C. sagittiformis in the Bayesian analysis (PP 99), as opposed to a monophyletic group with Cotylidia spp. in the ML analysis (ML-BP 75).
Fig. 5.
Bayesian inference 50 % majority rule consensus phylogram of the hymenochaetoid clade from 5.8S-18S-28S-RPB2 sequence data. Bayesian posterior probabilities (PP) and Maximum Likelihood bootstrap values (ML-BP) are shown on branches, ordered as PP/ML-BP. Thickened branches received support at least in one analysis (ML-BP ≥ 70 % and/or PP ≥ 95 %). Basidioma types are indicated with different colours for ingroup taxa.
Taxonomy
Clavariineae Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, subord. nov. MycoBank MB831365.
Basidiomata clavarioid, more rarely agaricoid with waxy decurrent gills, or corticioid. Hyphal system monomitic, or more rarely dimitic. Basidiospores hyaline, usually thin-walled, smooth or ornamented, usually with multiguttulate contents, sometimes with amyloid or dextrinoid reactions, usually with a cubic apiculus. Basidia claviform, with up to 4 sterigmata, chiastic, sometimes characteristically long (< 50 μm) or short (> 20 μm), occasionally sometimes with a loop-like basal clamp (Clavaria subgen. Holocoryne). Cystidia usually absent. Pileipellis either a hymeniderm or a trichoderm with rounded terminal elements in genera with agaricoid basidiomata. Basal tomentum composed of narrow, usually < 2 μm broad thick-walled hyphae in clavarioid genera (Ceratellopsis, Clavaria, Clavulinopsis, Ramariopsis), possibly also in other stipitate genera. Clamp connections present or absent, sometimes restricted to basidia. Saprotrophic on dead wood, herbaceous plants or leaves, or biotrophic with grasses and bryophytes. Presence of EF-1α intron 21 (numbering according to Matheny et al. 2007) in some genera (absent in Ceratellopsis).
Type family: Clavariaceae Chevall.
Notes: This suborder contains a single family. Similar isotopic ratios to those found in the Hygrophoraceae suggest that at least non-lignicolous members of Clavariaceae have some kind of biotrophic association with plants (Birkebak et al. 2013), whereas genera occurring on dead plant remnants are probably saprotrophic (Ceratellopsis, Mucronella, Hirticlavula). Very narrow and slightly thick-walled hyphae in the basal tomentum and mycelium are characteristic for many species of Clavariaceae, including species in Clavaria, Clavulinopsis, Ramariopsis (Olariaga 2009) and Ceratellopsis (Fig. 6) and it might be a synapomorphic character of the Clavariineae. The presence of EF-1α intron 21, absent in the rest of the Agaricales (Matheny et al. 2007) seems so far unique to some Clavariaceae (Clavaria, Clavulinopsis, Camarophyllopsis).
Fig. 6.
Ceratellopsis acuminata (epitype, Huhtinen 15/07, S). A–C. Dried basidiomata. D. Basidioma observed using a light microscope. E. Close-up of basidioma apex. F. Basidiospores. G. Basidia. H. Medullar hyphae resembling skeletal hyphae. I. One-year-old culture in MEA, kept at 5 °C (culture ex-epitype, CBS 146691). J. Hyphae from cultured mycelium (culture ex-epitype, CBS 146691). Mounting media were Melzer's reagent (D), Congo Red in ammonia (E–H) and water (I). Scale bars: D = 100 μm, E–H = 10 μm; I = 10mm. Photographs I. Olariaga.
Clavariaceae Chevall., Fl. Gen. Env. Paris 1: 102. 1826. [“Clavariae”; “ordre”; considered family according to Art. 18.2].
Type genus: Clavaria Vaill. ex L. : Fr.
Genera: Camarophyllopsis, Ceratellopsis, Clavaria, Clavulinopsis, Hirticlavula, Hodophilus, Hyphodontiella, Mucronella, Ramariopsis.
Ceratellopsis Konrad & Maubl., Icon. Select. Fung. 6: 502. 1937.
Basionym: Ceratella Pat., Hymenomyc. Eur.: 137. 1887. [nom. illeg. Art. 53.1, later homonym of Ceratella Hook f. 1844].
Presumed saprobic on bark, dead wood or culms. Basidiomata gregarious, clavarioid, 0.2–1(–2) mm high, lanceolate with a sterile pointed apex, white. Stipe usually present, short, glabrous or pubescent. Hyphal system monomitic or dimitic. Basidiospores without iodine reactions. Basidia claviform, short, 10–16 μm long. Generative hyphae present in the medulla, unidirectional, cylindrical, septate, thick-walled, 1–2.5 μm broad, sometimes dextrinoid. Skeletal hyphae present in the medulla of C. acuminata and further undescribed species known by us. Clamp connections scattered.
Type: Ceratellopsis acuminata (Fuckel) Corner, typ. cons. prop.
Notes: The background of the name Ceratellopsis requires, however, further clarification as its type has been a matter of controversy. According to our nomenclatural study, Ceratellopsis is a validly published replacement name based on Ceratella Pat. and typified by Pistillaria queletii. The final epithet Ceratella was first employed by Quélet (1886) for an unranked infrageneric name. Later, although Patouillard (1887) referred to Ceratella as “CERATELLA (Quél.)” he did not have in mind Clavaria [unranked] Ceratella as the basionym of a new combination. As explained in the introduction of the Hyménomycètes (Patouillard 1887: VII), authors of generic names were cited between round brackets only when Patouillard's circumscriptions were absolutely different from the original ones. Therefore, we consider Ceratella as the name of a new taxon to be cited as “Ceratella Pat.” (J. McNeill, pers. comm.) in agreement with Donk (1954) and the ING (Farr & Zijlstra 2020). Nevertheless, Ceratella Pat. (1887) is illegitimate as a later homonym of Ceratella Hook f. (1846). When Ceratellopsis was introduced, Konrad & Maublanc (1937) referred to it as a new name for “Ceratella (Quélet p.p.), Patouillard (1887)” and proposed Ceratellopsis queletii as the type, without providing a Latin description. Since Ceratellopsis was a replacement name for Ceratella Pat., and not a new taxon, Ceratellopsis is a validly published generic name even though it lacked a Latin description and was published later than 1935 (Art. 39.1), because such is not required for a replacement name. The type proposed for Ceratellopsis by Konrad & Maublanc is also in order, since Ceratella queletii was one of the three species listed under Ceratella Pat. (1887). A relevant fact that might have affected the typification of Ceratellopsis is whether the combination Ceratella queletii was validly published when Patouillard erected Ceratella Pat. Patouillard (1887) listed C. queletii as “C. Queletii” without explicitly citing its basionym Pistillaria queletii. However, we interpret that Patouillard (1887: VI) gave an indirect reference to the basionym that fulfils conditions for valid publication of C. queletii (Art. 41.3 and 38.14) by explicitly stating that Tabulae Analyticae Fungorum, the place of publication of Pistillaria queletii, basionym C. queletii, was one of the main works on which he based his Hyménomycètes (Art. 41.4, Ex. 9), and because Patouillard himself was author of the basionym.
For details on our choice to suggest C. acuminata as the conserved type for Ceratellopsis see notes under Pterulicium and the Discussion.
Ceratellopsis acuminata (Fuckel) Corner, Ann. Bot. Mem. 1: 202. 1950. Fig. 6.
Basionym: Pistillaria acuminata Fuckel, Fungi Rhen. Exs. (suppl.) 4: no 1888. 1867.
Synonym: Ceratella acuminata (Fuckel) Pat., Essai Tax. Hyménomyc.: 49. 1900.
Basidiomata gregarious, 0.2–0.4 mm high, simple, with a short stipe and a sterile apex. Fertile part cylindrical to oblong, sharply delimited from the stipe and the apex, white, 0.1–0.3 × 0.02–0.04 mm. Stipe short, cylindrical, glabrous, hyaline white, 0.04–0.12 × 0.01–0.02 mm. Apex pointed, acute, hyaline white, 0.04–0.1 mm long. Basidiospores ellipsoid to pip-shaped, sometimes in tetrads, hyaline, without iodine reactions, (3–)4–6 × (1.5–)2–3 μm. Basidia claviform, 2–4-spored, 10–16 × 3.5–4.5 μm, clamped. Generative hyphae cylindrical, hyaline, thin-walled, clampless, sometimes with scarce septa at the stipe base, 1–2.2 μm broad. Skeletal hyphae present in the medulla, cylindrical, refractive, slightly dextrinoid, 1.2–2.8 μm broad. Colonies on MEA 30–40 mm diam after 1 yr at 5 °C, superficial, effuse, convex, tomentose, hard-textured, with erect white tufts and strong smell reminiscent of Scleroderma. Reverse white. Margin regular and distinct. Vegetative hyphae cylindrical, closely septate, very slightly thick-walled, hyaline, 2.5−4 μm broad, with scattered clamp connections. Asexual morph not observed in culture.
Typus: Germany, Nassau, Johannisberger Schlosswald, ad pini sylv. folia putrida falae humus, Fuckel, Fungi Rhen. Exs. no 1888 (S-F128455 !, lectotype of Pistillaria acuminata designated here, MycoBank MBT387677). Isolectotypes: S-F128454 (!), S-F267533 (!), FH00608504 (!), K(M) 159801, M. Sweden, Härjedalen, Tänndalen, Hamrafjället Nature Protection Area, on dead leaves of Dryas octopetala, 4 Aug. 2015, S. Huhtinen 15/07 (S, epitype of Pistillaria acuminata designated here, MycoBank MBT389356). Culture ex-epitype: CBS 146691.
Known distribution: Denmark, Finland, France, Germany, Norway, Spain and Sweden.
Additional materials examined: Denmark, Sjælland, Bognæs skov, on leaves of Leymus on exposed beach, 26 Oct. 2019, T. Læssøe, DMS-10058526 (C). Finland, Perä-Pohjanmaa, Rovaniemi, Lovevaara Nature Protection area, brookside herb-rich forest, on leaf litter under alders, 7 Sep. 2012, S. Huhtinen 12/15 (TUR 197690). France, Val-d'Oise, Montmorency, ad cortices, 1889, Boudier herbarium (PC). Norway, Finnmark, Nord Varanger, Varanger Peninsula, Fosefjellet (ca. 3 km NW of Vadsö), on hare dung (Lepus tumidus) in moist chamber, 27 Jul. 1966, N. Lundqvist 4965g (UPS F-152857). Sweden, Gästrikland, Gävle, Lövudden, Salix viminali, folia dejecta, 25 Jun. 1953, J.A. Nannfeldt 12806 (UPS F-152650, as Ceratellopsis sp.); Lycksele Lappmark, Saxnäs, Satsfjället, on dead fern stems, 28 Jul. 2010, K. Hansen, K. Gillen & I. Olariaga, IO.10.01 (S); Västergötland, Håkantorp, Äspås hållplats, on Quercus robur leaves, 2 Oct. 1955, S. Kilander (UPS F-152830).
Notes: Ceratellopsis acuminata differs from C. aculeata in having skeletal hyphae in the basidioma core. Another collection identified as C. aff. acuminata by us (ARAN-Fungi 11746) possessed also skeletal hyphae, but had longer and shorter basidiomata and nested in a different clade than the epitype of C. acuminata (Fig. 4). This substantiates the idea that an additional species of Ceratellopsis exists and when more specimens become available the species limits should be studied further.
Corner (1950: 203) mentioned a type collection of Pistillaria acuminata (that Donk had examined in ms) without providing a collection number or a herbarium. Since we believe that Corner's type indication did not fulfil requirements for achieving a valid typification (Art. 7.11), we propose here a lectotypification of C. acuminata. The four syntypes examined are very meagre. Therefore, we select as epitype a recent collection from which a living culture and several gene regions have been obtained. We found C. acuminata to have a very broad host range and distribution, and feel justified in selecting Swedish material collected on Dryas leaves as epitype.
Ceratellopsis aculeata (Pat.) Corner, Ann. Bot. Mem. 1: 200. 1950.
Basionym: Pistillaria aculeata Pat., Tab. anal. Fung. 1: 26. 1883.
Synonyms: Ceratella aculeata Pat., Essai Tax. Hyménomyc.: 49. 1900.
(?) Pistillaria mucedinea Boud., Bull. Soc. bot. Fr. 24: 308. 1878. [1877].
(?) Ceratellopsis mucedinea (Boud.) Corner, Ann. Bot. Mem. 1: 204. 1950.
Typus: No type specimen in the Patouillard herbarium (FH, PC). Lectotype of Pistillaria aculeata designated here: Patouillard, Tab. Anal. Fung. 1: fig. 58. 1883. MycoBank MBT387467.
Specimens examined: Denmark, Lolland, Maribo Søndersø, on stems of Cladium mariscus, 9 Oct. 2000, T. Læssøe, DMS-376001 (C). Spain, Aragón, Huesca, on Pinus bark, 26 Feb. 2017, R. Blasco, ARAN-Fungi 13729; Basque Country, Gipuzkoa, Larraul, Usarrobi erreka, 9 Jun. 2012, I. Olariaga, ARAN-Fungi A3064020. Sweden, Öland, Norra Mosse, on damp, dead parts of Cladium, 2 Jul. 1988, S. Elborne, C-F-94548. UK, England, Wicken fen, on Cladium mariscus, 12 Aug. 1926, E.J.H. Corner (PC).
Notes: Medulla hyphae in Ceratellopsis aculeata are thick-walled and have scarce septa, as noted by Corner (1950). Originally described as occurring on fallen leaves, C. aculeata has been considered to typically occur on dead leaves of Cladium mariscus (Corner, 1950, Hansen and Knudsen, 1997). Specimens collected on bark or dead wood share a similar basidioma configuration, hyphae and spores.
As Corner (1950) suggested Pistillaria mucedinea is very close to C. aculeata. The small size of basidiomata (0.5–0.75 mm) and the 4-spored basidia described in the protologue support this view. Furthermore, our study of an authentic specimen kept at PC, collected on bark as described in the protologue, has scarcely septate thick-walled hyphae as observed in the material on Cladium mariscus. We agree with Corner (1950) and even suggest P. mucedinea might be conspecific with C. aculeata and list it as a possible earlier synonym. However, a better insight on species limits in Ceratellopsis needs to be acquired to further test this.
Names formerly placed in Ceratellopsis and imperfectly known, excluded here or illegitimate
Ceratellopsis aciculata (Durieu & Lév. ex Sacc.) Corner, Ann. Bot. Mem. 1: 200. 1950.
Basionym: Pistillaria aciculata Durieu & Lév. ex Sacc., Syll. Fung. 6: 759. 1887.
Typus: Lectotype designated here: Bory de Saint-Vincent & Durieu de Maisonneuve, Expl. Sci. Algérie 1(5): tab. 32, fig. 4. 1846. MycoBank MBT387461.
Notes: Pistillaria aciculata, published as a nomen nudum (Bory de Saint-Vincent & Durieu de Maisonneuve 1846), was invalid until Saccardo provided a description. The illustration provided by Bory de Saint-Vincent & Durieu de Maisonneuve (1846) shows brown, pointed acute structures that do not look like a fertile fungus but rather incipient basidiomata of a marasmioid fungus. This figure is, to our knowledge, the only original element and it is accordingly proposed as lectotype.
Ceratellopsis asphodeli (Pat.) Corner, Ann. Bot. Mem. 1: 203. 1950.
Basionym: Ceratella microscopica var. asphodeli Pat., Cat. Pl. Cell. Tunisie: 66. 1897.
Typus: No type specimen in the Patouillard herbarium (FH, PC). No original illustration.
Notes: The 2-spored basidia and the presence of cystidia described in Patouillard (1897) suggest that C. asphodeli is a synonym of Pterulicium gracile. The pink tones can be present in P. gracile (Olariaga 2009).
Ceratellopsis biformis Khurana in Berthier, Bull. Soc. Linn. Lyon. 45: 190. 1976 [nom. illeg., Art. 39, 40].
Notes: The description provided by Berthier (1976) based on Corner's notes of a fungus on Quercus leaves from India suggests that C. biformis may belong to Ceratellopsis as conceived here due to its narrow, 1.5–2 μm broad, medulla hyphae. Nevertheless, C. biformis was never validly published since neither a Latin diagnosis nor a type specimen were provided for it.
Ceratellopsis brondaei (Quél.) Corner, Ann. Bot. Mem. 1: 203. 1950.
Basionym: Clavaria brondaei Quél., Revue mycol. (Toulouse) 14(54): 65. 1892.
Typus: No type specimen in PC and TL.
Notes: Quélet (1892) described C. brondaei apparently based only on the Brondeau plate no. 165 (“Alb. 165”). The illustration provided in the protologue (plate 126, fig. 3), probably a reproduction of plate no. 165, shows a small white clavarioid fungus, said to grow in forest on soil among tiny mosses. The description, except the ecology, tallies with a species of Ceratellopsis as treated here, but in the absence of microscopic information and a type specimen, a reliable interpretation cannot be provided, as Corner (1950) stated.
Ceratellopsis caespitulosa (Sacc.) Corner, Ann. Bot. Mem. 1: 203. 1950.
Basionym: Pistillaria caespitulosa Sacc., Atti del Congr. bot. di Palermo. 1902.
Typus: [from Saccardo, Syll. Fung. 17: 202. 1905]: France, Côte d’Or, in cortice emortuo Loniceræ periclymeni [Lonicera periclymenum], PAD.
Notes: The denticulate “basidia” and the 1-septate biguttulate spores suggest that C. caespitulosa is an asexual morph fungus, probably conspecific with Isaria friesii (Leotiomycetes, Ascomycota).
Ceratellopsis carestiae (Ces.) Corner, Ann. Bot. Mem. 1: 203. 1950.
Basionym: Pistillaria carestiae Ces. in Bres. & Sacc., Malpighia 11: 255. 1897.
Typus: Italy, Piemonte, Alagna Valsesia, sur ramis secchiSyringa vulgaris, 13 Oct. 1857, Ab. Carestia no 27 (S-F15983 !, ex Bresadola herbarium; lectotype designated here, MycoBank MBT387464).
Notes: The material constitutes an asexual fungal state growing on bark, very probably conspecific with Isaria friesii. The spore content, described as divided in two (“plasma bipartito”) is due, in fact, to the 1-septate spores, as in C. caespitulosa (see above).
Ceratellopsis corneri Berthier, Bull. mens. Soc. linn. Lyon 43: 188. 1974.
Typus: France, Lyon, Soucieu-en-Jarrest, sur l´ecorce pourrissante d'un arbre abattu (Gymnosperme?), Bussy, 11 Apr. 1970 (holotype G).
Notes: Due to the 4–6 μm broad medulla hyphae and the amyloid spores, C. corneri does not conform to Ceratellopsis. We consider it that C. corneri should be examined and compared to Mucronella instead.
Ceratellopsis dryopteridis (S. Imai) Corner, Ann. Bot. Mem. 1: 203. 1950.
Basionym: Pistillaria dryopteridis S. Imai, Sapporo Trans. Sapporo nat. Hist. Soc. 13(4): 386. 1934.
Typus: Japan, Ishikari province, Nov. probably at SAPA.
Notes: The filiform 1–5 mm long basidiomata and 9–12.5 μm long spores suggest that C. dryopteridis should not be excluded from Ceratellopsis. The spores of C. dryopteridis are asperulate and therefore a relationship with Pterula is suggested here, but the type specimen, if it exists, should be examined to confirm this.
Ceratellopsis equiseticola (Boud.) Corner, Ann. Bot. Mem. 1: 204. 1950.
Basionym: Pistillaria equiseticola Boud., Bull. Trimestr. Soc. Mycol. France 33(1): 13. 1917.
Typus: France, Saône-et-Loire, Clovey (?), ad caules Equiseti limosi [Equisetum fluviatile], May 1915, Boudier herbarium (PC !, as P. equisetina; lectotype designated here, MycoBank MBT387465).
Notes: As earlier suggested by Berthier (1976), we conclude that C. equiseticola is a synonym of P. gracile after examining type material.
Ceratellopsis graminicola (Bourdot & Galzin) Corner, Ann. Bot. Mem. 1: 204. 1950.
Basionym: Pistillaria graminicola Bourdot & Galzin, Hymenomyc. France: 139. 1928.
Typus: No type specimen in the Bourdot & Galzin herbarium (PC). No original illustration.
Notes: The 12–18 μm long, 2–4-spored basidia, small spores (6–7 × 4 μm) and narrow, 1.5–2.5 μm broad hyphae given in the original description would indicate that C. graminicola should be retained in Ceratellopsis, rather than being conspecific with P. gracile. It might be conspecific with C. aculeata or C. acuminata, but details on its hyphal structure are necessary to provide a solid interpretation.
Ceratellopsis helenae (Pat.) Corner, Ann. Bot. Mem. 1: 204. 1950.
Basionym: Pistillaria helenae Pat., Tab. Anal. Fung. 1: 26. 1883.
Typus: No type specimen in the Patouillard herbarium (FH, PC). Lectotype designated here: Patouillard, Tab. Anal. Fung. 1: fig. no. 57. 1883. MycoBank MBT387466.
Notes: The forked or sparsely branched basidiomata, with a tendency to be caespitose, and the presence of a distinct stipe, suggest that C. helenae is a synonym of Typhula crassipes. Although basidiomata of T. crassipes are usually simple, we have observed branched basidiomata as those depicted in the lectotype figure. Also, 2-spored basidia and incarnate tones are sometimes present in T. crassipes (Olariaga 2009). Corner (1950) compared C. helenae to P. gracile, but the latter lacks a stipe.
Ceratellopsis kubickae Pilát, Česká Mykol. 12(4): 217. 1958.
Typus: Czech Republic, prope Třeboň, ad folium putridum Salicis auritae [Salix aurita], 15 May 1958, Kubíčka (PRM 655767).
Notes: Pilát (1958) described C. kubickae as monomitic and compared it with P. gracile. Berthier (1976) investigated the type material and proposed that C. kubickae is a synonym of P. gracile, and that Pilát (1958) overlooked skeletal hyphae. In our opinion, the 2-spored basidia and the absence of a stipe in C. kubickae support it is a synonym of P. gracile.
Ceratellopsis mucosa (Berk. & M.A. Curtis) Corner, Ann. Bot. Mem. 1: 205. 1950.
Basionym: Typhula mucosa Berk. & M.A. Curtis, Grevillea 2(14): 18. 1873.
Typus: USA, South Carolina, Society Hill, in herb. mort., 1852, Carolina inf. No. 3832 (syntypes FH 596847, K).
Notes: The original description is very meagre, and we are unable to propose a reliable interpretation without checking type material. Corner (1950) failed also to provide a specific interpretation and stated that C. mucosa “may be Ceratellopsis, Pterula, or a rudimentary Pistillaria”.
Ceratellopsis rickii (Oudem.) Corner, Ann. Bot. Mem. 1: 205. 1950.
Basionym: Mucronella rickii Oudem., Ned. kruidk. Archf., 3 sér. 2(3): 667. 1902.
Synonym: Cnazonaria rickii (Oudem.) Donk, Meded. Bot. Mus. Herb. Rijks Univ. Utrecht 9: 99. 1933.
Typus: The Netherlands, Limburg, Valkenburg, in caulibus herbarum praesertim Asparagi off. [Asparagus officinalis], May 1901, J. Rick, herb. Oudemans (holotype L). Isotype: Bourdot & Galzin herbarium (PC !).
Notes: Jülich (1980) reduced C. rickii to a synonym of P. gracile after examining type material. We confirm this synonymy based on characters seen on the cited isotype.
Ceratellopsis rosella (Fr.) Corner, Ann. Bot. Mem. 1: 206. 1950.
Basionym: Pistillaria rosella Fr., Epicr. syst. mycol.: 587. 1838. [1836–1838].
Typus: No type specimen in the Fries herbarium (UPS). No original illustration.
Notes: The pink colour described in the protologue is almost unique to T. micans among typhuloid fungi and we thus agree with Berthier (1976) in considering C. rosella a synonym of T. micans.
Ceratellopsis sydowii (Bres.) Corner, Ann. Bot. Mem. 1: 206. 1950.
Basionym: Clavaria sydowii Bres. in Sydow, Hedwigia 35: (61). 1896.
Typus: Germany, Saxony, Muskau, O.L. Bergpark, ad ramulos Robiniae pseudoacaciae, Jul. 1895, P. Sydow, Mycoth. March. 4405 (syntypes CHRB, MIN, NCU).
Notes: The caespitose growth habit on dicot. bark, pale pink colour (“dilute carnei”), long basidia (24–26 μm) and spore size mentioned in the protologue (9–10 × 5–5.5 μm) suggest that C. sydowii is a synonym of T. crassipes.
Ceratellopsis terrigena Berthier, Bull. Soc. Linn. Lyon 43(6): 188. 1974.
Typus: France, Rhône, Lyon, Izeron, sur terre nue d'un talus en sous-bois, 7 Aug. 1966, CL. 29 (holotype G).
Notes: Due to its 1.5–3.5 μm broad medulla hyphae C. terrigena does not conform to Ceratellopsis as here defined. Also, the presence of striking protruding cystidia is unknown for any other species of Ceratellopsis. The shape of cystidia and the absence of clamp connections may suggest C. terrigena to be allied with Alloclavaria in the Rickenella clade (Hymenochaetales).
Ceratellopsis thujicola (Kauffman) Corner, Ann. Bot. Mem. 1: 206. 1950.
Basionym: Pistillaria thujicola Kauffman, Pap. Mich. Acad. Sci. 9: 207. 1929 [1928].
Typus: USA, Michigan, Alger: Rock River, on inner side of Thuja occidentalis on loose bark, 8 Sep. 1927, C.H. Kauffman (holotype MICH11745).
Notes: The up to 10 mm long branched basidiomata do not conform clearly to Ceratellopsis as here defined, and we thus exclude it from this genus. The type material should be examined to propose a more precise interpretation.
Ceratellopsis tremula (Sacc.) Corner, Ann. Bot. Mem. 1: 207. 1950.
Basionym: Pistillaria ferryi subsp. tremula Sacc. in Sacc. & D. Sacc., Syll. Fung. 17: 202. 1905.
Typus: Italy, Padova, horto botanico Patavino, ad fructum putremTrichosanthis anguineae [Trichosanthes sanguinea], ubi Botrytis vulgaris et Acremoniella atra, Feb. 1904 (syntype PAD).
Notes: The 3–5 mm long basidiomata, 2-spored basidia and 8–11 μm long spores conform to P. gracile, but the type specimen needs to be examined to confirm the synonymy. Although this synonymy is listed in Index Fungorum (2019, viewed on 11 June 2019), it has not been otherwise proposed to our knowledge.
Pleurotineae Aime, Dentinger & Gaya, Biol. J. Linn. Soc.: 10.1111/bij.12553, 16. 2015.
Phyllotopsidaceae Locquin ex Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, fam. nov. MycoBank MB831374.
Basidiomata pleurotoid or clavarioid and sometimes arising from a sclerotium. Spore deposit white to salmon pink. Hyphal system monomitic. Basidiospores hyaline, cylindrical, allantoid or subglobose, smooth, without iodine reactions. Cheilocystidia sometimes present in pleurotoid genera. Clamp connections present, rarely absent. Saprotrophic.
Type genus: Phyllotopsis E.-J. Gilbert & Donk ex Singer
Representative genera: Macrotyphula, Phyllotopsis and Pleurocybella.
Notes: Macrotyphula, Phyllotopsis and Pleurocybella were suggested to be closely related by Dentinger & McLaughlin (2006) and our analyses confirm that they form a monophyletic group. Despite this, no obvious synapomorphic characters support the relationship between the typhuloid Macrotyphula and the pleurotoid Phyllotopsis and Pleurocybella (Moncalvo et al. 2002). All three genera contain saprotrophic species, mostly lignicolous, and possess clamp connections.
Macrotyphula R.H. Petersen, Mycologia 64: 140. 1972. nom. cons. prop.
Type: Clavaria fistulosa Holmsk.: Fr. (synonym: Macrotyphula fistulosa (Holmsk. : Fr.) R.H. Petersen).
Synonyms: Sclerotium Tode, Fung. mecklenb. sel. 1: 2. 1790 : Fr., Syst. Mycol. 2: 246. 1822. nom. rej. prop. Type: Sclerotium complanatum Tode : Fr. (synonym: Typhula phacorrhiza (Reichard : Fr.) Fr.).
Clavariadelphus subgen. Typhulopsis Corner, Ann. Bot. Mem. 1: 692. 1950 [nom. inval. Art. 40.3, two species were indicated as type].
Macrotyphula megasperma (Berthier) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, comb. nov. MycoBank MB831762.
Basionym: Typhula megasperma Berthier, Bull. Soc. Linn. Lyon 45: 78. 1976.
Macrotyphula phacorrhiza (Reichard : Fr.) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, comb. nov. MycoBank MB831761.
Basionym: Clavaria phacorrhiza Reichard, Schriften Berlin. Ges. Naturf. Freunde 1: 315. 1780. [“Clauaria phacorhiza”] : Fr., Syst. mycol. 1: 495. 1821.
Synonyms: Sclerotium complanatum Tode, Fung. Mecklenb. Sel. 1: 5. 1790.
Typhula phacorrhiza (Reichard : Fr.) Fr., Observ. Mycol. 2: 298. 1818. [“Typhla”].
Phacorhiza filiformis Grev., Scott. Crypt. Fl. 2: 93. 1824 [nom. nov. based on Clavaria phacorrhiza Reichard]
Typhula phacorrhiza var. complanata (Tode) Sacc., Syll. Fung. 6: 745. 1888. [∗ complanata].
Typus: Lectotype of Clavaria phacorrhiza: Sowerby, Col. Fig. Eng. Fung. 2: tab. 233. 1798, as “phacorhiza”, designated by Olariaga & Salcedo (2013: 42). Lectotype of Sclerotium complanatum designated here: Tode, Fung. Mecklenb. 1: tab. 1, fig. 9. 1790. MycoBank MBT387906. Germany, Bayern, Oberbayern, Landkreis Miesbach, valley Kleinthal near Miesbach, in a garden under Ribes nigrum and Ribes rubrum, on wet litter of various plants, mostly of Ribes, 2 Mar. 1992, F. Brand, Microf. Exs. No. 49 (UPS, as Sclerotium complanatum; epitype of Sclerotium complanatum designated here; Isoepitypes in B, BPI, CANB, DAOM, FH, GZU, H, HAL, HMAS, LE, M, MA, NMW, PRM, TNS). MycoBank MBT389352.
Specimens examined: Austria, prope Tullnerbach, in silva “Wiener Wald, ad petiolos Fraxini excelsioris L., C. de Keissler, Krypt. Exs. 1840 (PC, as Sclerotium complanatum). France, Pyrénées atlantiques, Borce, Le Gave d'Aspe, on the ground, among Rubus idaeus and Myrrhis odorata, 12 Oct. 2014, J.C. Zamora & I. Olariaga, IO.14.200 (S); without locality, dans les bois, parmi les tas de feuilles pourries, Desmazières, Pl. Crypt. N. France, Ed. 1 536 (PC, as Sclerotium complanatum). Spain, Navarre, Basaburua, Orokieta, Loiandi, on the ground among needles under Picea abies, 27 Oct. 2017, I. Olariaga, J. Martín, J. Teres & J.M. Riezu, ARAN-Fungi 7446. Sweden, Skåne, Eriksdal, Vitabäckskällan Nature Reserve, on wet ground under Alnus, 3 Oct. 2014, N.-O. Nilsson & I. Olariaga, IO.14.167 (S); Uppland, Uppsala-Näs, Vreta, in a compost-heap, 18 Oct. 1975, L. Jonsell, Fung. Exs. Suec. 3249 (PC).
Typhula phacorrhiza var. heterogenea Berthier, Bull. Soc. Linn. Lyon 45: 197. 1976.
Typus: USA, Idaho, Priest River, inter foliis Alni tenuifoliae [Alnus tenuifolia], 19 Oct. 1920, Weir 16943 (CGE, holotype).
Notes: This taxon, due to its gelatinised sclerotia with normal epidermoid layer (Berthier 1976), may also belong to Macrotyphula. The type material appears to have lost its basidiomata (Berthier 1976) and we find it premature to place it in Macrotyphula.
Pterulaceae Corner, Beih. Nova Hedwigia 33: 194. 1970.
Type genus: Pterula Fr. : Fr.
Representative genera: Allantula, Coronicium, Dimorphocystis, Merulicium, Pterula, Pterulicium.
Pterula Fr. : Fr., Syst. Orb. Veg. 1: 90. 1825.
Basionym: Anthina [unranked] Pterula (Fr.) Fr., Syst. Mycol. 3: 285. 1832: Fr., idem.
Type: Pterula plumosa (Schwein. : Fr.) Fr., selected by Donk (1954: 472).
Pterulicium Corner, Ann. Bot. Mem. 1: 699. 1950. Type: Pterulicium xylogenum Corner
Synonyms: Ceratellopsis Konrad & Maubl., Icon. Select. Fung. 6: 502. 1937. Type: Ceratellopsis queletii (Pat.) Konrad & Maubl. (synonym Pterulicium gracile (Desm. & Berk.) Leal-Dutra, Dentinger, G.W. Griff.), typ. rej. prop.
Deflexula Corner, Ann. Bot. Mem. 1: 695. 1950. Type: Deflexula fascicularis (Bres. & Pat.) Corner
Notes: Since the type of Ceratellopsis nests in Pterulicium (Fig. 3), the correct name for Pterulicium is Ceratellopsis under the current nomenclatural rules. A proposal to preserve the current usage of Ceratellopsis and Pterulicium is, however, in preparation (see Discussion).
Pterulicium gracile (Desm. & Berk.) Leal-Dutra, Dentinger & G.W. Griff. in Leal-Dutra, Griffith, Neves, McLaughlin, McLaughlin, Clasen & Dentinger, IMA Fungus 11(2): 15. 2020.
Basionym: Typhula gracilis Desm. & Berk. in Berk, Ann. Nat. Hist., Ser. 1, 1: 202. 1838. [“Typhula? gracilis”].
Synonyms: Clavaria gracilis (Desm. & Berk.) P. Karst., Bidrag Kännedom Finlands Natur Folk 37: 181. 1882. [“Cl? gracilis”; nom. illeg., later homonym of Clavaria gracilis Bolton and C. gracilis Pers. : Fr., Art. 53].
Pistillaria gracilis (Desm. & Berk.) Pat., Tab. Anal. Fung. 6: 30. 1887.
Ceratella gracilis (Desm. & Berk.) Pat., J. Bot. (Morot) 3: 36. 1889.
Hirsutella gracilis (Desm. & Berk.) Pat., Essai tax. Hyménomyc.: 50. 1900.
Pterula gracilis (Desm. & Berk.) Corner, Ann. Bot. Mem. 1: 514. 1950.
Clavaria microscopica Malbr. & Sacc., Michelia 2(6): 42. 1880.
Clavaria aculina Quél., Compt. Rend. Assoc. Franç. Avancem. Sci. 9: 670. 1881. [“1880”]
Pistillaria aculina (Quél.) Pat., Tab. Anal. Fung. 6: 29. 1887.
Ceratella aculina (Quél.) Pat., Hymenomyc. Eur.: 137. 1887.
Cnazonaria aculina (Quél.) Donk., Meded. Ned. Mycol. Ver. 22: 97. 1933.
Pistillaria queletii Pat., Tab. Anal. Fung. 1: 22. 1882.
Ceratella queletii (Pat.) Pat., Hymenomyc. Eur.: 137. 1887. [valid combination following Arts. 41.3 and 38.14].
Ceratellopsis queletii (Pat.) Konrad & Maubl., Icon. Sel. Fung. 6: 502. 1937.
Typhula brunaudii Quél., Compt. Rend. Assoc. Franç. Avancem. Sci 13: 283. 1885. [1884].
Clavaria brunaudii (Quél.) Sacc., Syll. fung. 6: 730. 1888.
Ceratella ferryi Quél. & Fautrey, Revue. Mycol. (Toulouse) 15(57): 15. 1893.
Pistillaria ferryi (Quél. & Fautrey) Sacc., Syll. Fung. 11: 141. 1895.
Ceratella microscopica var. asphodeli Pat., Cat. Pl. Cell. Tunisie: 66. 1897.
Ceratellopsis asphodeli (Pat.) Corner, Ann. Bot. Mem. 1: 203. 1950.
Pistillaria attenuata Syd. & P. Syd., Hedwigia 39: (1). 1900.
Pistillaria acicula Bourdot & Galzin, Hymenomyc. France: 139. 1928. [“1927”].
Pistillaria juncicola Bourdot & Galzin, Hymenomyc. France: 138. 1928. [“1927”].
Typus: UK, without locality, ex Desmazières herbarium (PC, not found, lectotype designated in Corner (1950: 515) by type indication). Isolectotype: UPS (!).
Additional materials examined: France, Charente-Maritime, Saintonge, maïs [Zea mays], P. Brunaud, Quélet herbarium (PC !, lectotype of Typhula brunaudii designated here, MycoBank MBT387470); Côte-d'Or, Noidan, sur tiges séches de Coix lacryma-jobi, Fautrey, Jun. 1892, Fungi Sel. Gall. Exs. no 6203 (PC!, lectotype of Ceratella ferryi designated here, isolectotypes: BR, ILL, MIN, UPS, MycoBank MBT387491); Rhône, Saint Priest, les Bouys, vers le Souey, “sur joncs [Juncus] purrisants”, H. Bourdot, 27 Sep. 1918, Bourdot 24978 (PC !, lectotype of Pistillaria juncicola designated here, MycoBank MBT387471); Seine-Maritime, environs de Rouen, sur jonc [Juncus], Apr. 1880, A. Le Breton, Quélet herbarium (PC !, lectotype of Clavaria aculina designated here, MycoBank MBT387469); Seine-Maritime, Rouen, sur les joncs [Juncus] morts et humides, forêt de Noumare (?), A. Malbranche, (PC !, lectotype of Clavaria microscopica designated here, MycoBank MBT387468). Germany, Brandenburg, Finkenkrug pr. Nauen, ad folia culmosque graminum, Calamagrostidis epigeii [Calamagrostis epigejos], Agrostidis albae [Agrostis alba] etc., Mycoth. Mar. no. 4803 (S F15411 !, lectotype of Pistillaria attenuata designated here, isolectotypes: FH00608505 (!), MICH, MycoBank MBT387678). Lectotype of Pistillaria queletii designated here: Patouillard, Tab. Anal. Fung. 1: fig. 45. 1883. MycoBank MBT389354. Sweden, Skåne, Kristianstads kommun, Balsberget Nature Reserve, on dead standing Juncus effusus stalks, at damp place, N.-O. Nilsson & I. Olariaga, 2 Oct. 2014, IO.14.142 (S, epitype of Pistillaria queletii designated here, MycoBank MBT387907).
Notes: In order to contribute to nomenclatural stability, an epitype specimen that represents P. gracile is proposed above for Pistillaria queletii (see Discussion). No good and recent specimen of P. gracile from France was available to epitypify C. queletii. Nevertheless, European material of P. gracile is morphologically and genetically homogeneous — ITS and 28S sequences available from France, Germany and Canada (CBS 309.79, CBS 325.58, CBS 554.85) are identical or nearly so to the sequences obtained by us from Sweden — and therefore we epitypify C. queletii using a specimen of P. gracile collected in Sweden.
Sarcomyxaceae Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, fam. nov. MycoBank MB831375.
Basidiomata pleurotoid, with gelatinous context in pileus. Gills slightly decurrent, crowded, forking. Stem lateral, floccose. Spore deposit white. Basidiospores cylindrical to allantoid, amyloid. Basidia claviform, (2–)4-spored, clamped. Cheilo- and pleurocystidia fusiform to clavate, more or less thick-walled. Pileipellis and part of trama gelatinised. Clamp connections present. Saprotrophic, lignicolous.
Type genus: Sarcomyxa P. Karst.
Only genus: Sarcomyxa.
Notes: The family contains a single genus with unique pleurotoid basidiomata, gelatinised pileipellis, fusiform to clavate cheilo- and pleurocystidia and amyloid spores (Knudsen & Vesterholt 2012). Due to its isolated position within the Pleurotineae (Fig. 2, Fig. 3), a new family is coined to accommodate Sarcomyxa.
Typhulaceae Jülich, Biblioth. Mycol. 85: 393. 1982 [“1981”]
Type genus: Typhula (Pers. : Fr.) Fr.
Typhula (Pers.) Fr., Obs. Mycol. 2: 296. 1818: Fr., Syst. Mycol. 1: 494. 1821, nom. cons. prop.
Basionym: Clavaria [unranked] Typhula Pers. : Fr., Syn. Meth. Fung. 1: XVIII. 1801
Type: Typhula incarnata Lasch, typ. cons. prop.
Synonyms: Pistillaria Fr., Syst. Mycol. 1: 496. 1821: Fr., idem. Type: Pistillaria micans (Pers. : Fr.) Fr., selected by Clem. & Shear, Gen. Fungi: 345. 1931 (synonym Typhula micans (Pers. : Fr.) Fr.).
Cnazonaria Corda in J. Sturm, Deutschl. Fl., Pilze 2: 55. 1829. Type: Clavaria setipes Grev. (synonym Typhula gyrans (Batsch : Fr.) Fr.).
Scleromitra Corda in Sturm, Deutschl. Fl., 3 Abt., 2: 59. 1829. Type: Scleromitra coccinea Corda (synonym T. micans (Pers. : Fr.) Fr.).
Phacorhiza Pers., Mycol. Eur. 1: 192. 1822 : Fr., Syst. Mycol. 3 (Index): 140. 1832. Type: Phacorhiza sclerotioides (Pers. : Fr.) Pers. (synonym Typhula sclerotioides (Pers. : Fr.) Fr.).
Pistillina Quél., Compt. Rend. Assoc. Franc. Avancem. Sci. 9: 671. 1881. [“1880”]. Type: Pistillina hyalina Quél. (synonym Typhula subhyalina Courtec.).
Sphaerula Pat., Tab. Anal. Fung. 1: 27. 1883. Type: Sphaerula capitata Pat. (synonym Typhula capitata (Pat.) Berthier)
Gliocoryne Maire, Bull. Soc. Bot. France 55: 121. 1909. Type: Clavaria uncialis Grev. (synonym Typhula uncialis (Grev.) Berthier)
Dacryopsella Höhn., Anz. Kaiserl. Akad. Wiss. Wien, Math.-Naturwiss. Kl., Abt. 1, 124: 50. 1915. Type: Dacryopsis typhae Höhn. (synonym T. subhyalina Courtec.).
Tygervalleyomyces Crous, Persoonia 39: 387. 2017. Type: Tygervalleyomyces podocarpi Crous.
New combinations in Typhula
Typhula podocarpi (Crous) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen comb. nov. MycoBank MB831376.
Basionym: Tygervalleyomyce podocarpi Crous, Persoonia 39: 387. 2017.
Notes: The asexual and only known morph of T. podocarpi, described from South Africa, conforms to that of Typhula crassipes, described from Germany (Berthier 1976). The ITS region of T. podocarpi differs in 23 positions from that of European specimens of T. crassipes and we thus consider T. podocarpi to be a separate species.
Rickenellaceae Vizzini, Micol. Veg. Medit. 25(2): 144. 2010.
Type genus: Rickenella Raithelh.
Representative genera: Alloclavaria, Atheloderma, Blasiphalia, Bryopistillaria, Cantharellopsis, Contumyces, Cotylidia, Ginnsia, Globulicium, Gyroflexus, Loreleia, Muscinupta, Odonticium, Peniophorella, Resinicium, Rickenella, Sidera, Skvortzovia and Tsugacorticium. Excluded from Rickenellaceae: Repetobasidium.
Notes: Genera in the Rickenella clade have been assigned to the Repetobasidiaceae (Zhang et al. 2018), often left without family assignation (Kirk et al., 2008, Knudsen and Vesterholt, 2012), or included in the family Rickenellaceae (Begerow et al. 2018). Our analyses suggest that Repetobasidium does not nest in the Rickenella clade, and Repetobasidiaceae cannot therefore be used to accommodate genera in the Rickenella clade. Consequently, we adopt Rickenellaceae as the correct placement for genera in this clade, excluding Repetobasidium so that Rickenellaceae is not superfluous as when erected by Vizzini (2010).
Bryopistillaria Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, gen. nov. MycoBank MB831377.
Etymology: From ancient Greek (“bryon”, moss), referring to its habitat on bryophytes, and from Pistillaria, referring to its similarity with several species placed in that genus.
Biotrophic on mosses, and maybe in addition saprobic on dead leaves and culms. Basidiomata gregarious or fasciculate (2–5 basidiomata), simple clavarioid, 0.6–1 mm high, initially lanceolate and with sterile apex, then cylindrical or claviform, with rounded fertile apex, white. Stipe short or absent, cylindrical, glabrous or pubescent. Hyphal system monomitic. Basidiospores without iodine reactions, smooth. Basidia claviform, 16–21 μm long. Medulla hyphae parallel-arranged, cylindrical, septate, thin-walled, 3–4 μm broad, without iodine reactions. Clamp connections absent.
Type: Bryopistillaria sagittiformis (Pat.) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen
Bryopistillaria sagittiformis (Pat.) Olariaga, Huhtinen, Læssøe, J.H. Petersen & K. Hansen, comb. nov. Fig. 7. MycoBank MB831378.
Fig. 7.
Bryopistillaria sagittiformis (epitype, IO.15.85, S). A. Basidioma growth habit. B. Close-up of basidiomata. C. Basidioma observed using a light microscope. D. Close-up of basidioma apex. E. Basidiospores. F. Basidia. G. Hymenium section showing a subhymenium with globose to sugblobose cells. H. Subhymenium cells. I. Thin-walled septate medullar hyphae. J. Caulinar hair. K. Crystals. Mounting media were Melzer's reagent (H, K) and Congo Red in ammonia (C–G, I–J). Scale bars: C = 100 μm; D = 50 μm; E–K = 10 μm. Photographs I. Olariaga, except B–C by J.H. Petersen.
Basionym: Pistillaria sagittiformis Pat., Tab. Anal. Fung. 1: 26. 1883 [“sagittæformis”]
Synonym: Ceratellopsis sagittiformis (Pat.) Corner, Ann. Bot. Mem. 1: 206. 1950. [“sagittæformis”].
Basidiomata gregarious or caespitose in groups of 2–5 basidiomata, 0.7–1.2 mm high, simple, with a short stipe. Fertile part narrowly claviform, sharply delimited from the stipe, white, 0.6–0.1 × 0.15–0.3 mm. Stipe short, cylindrical, glabrous or with sparse hairs, hyaline white, 0.1–0.4 × 0.1–0.2 mm. Subiculum spreading out on the substratum among basidiomata. Apex pointed and sterile in very young basidiomata, hyaline white, then obtuse and fertile. Basidiospores ellipsoid, sometimes in tetrads, hyaline, smooth, without iodine reactions, 4.5–6.5(–8) × 3–3.5(–4) μm. Basidia claviform, (1–2–)4-spored, 16–23 × 4.5–7 μm, clampless. Subhymenium composed of globose to subglobose hyphae, thin-walled, hyaline, 4–10(–12) μm broad. Generative hyphae cylindrical to fusiform, hyaline, thin-walled, clampless, 2.5–6(–9) μm broad, without iodine reactions. Hyphae on the stipe surface cylindrical, thin-walled, clampless, 3–3.5 μm broad. Caulinar hairs sparse, cylindrical, thin-walled, up to 50 × 3 μm. Subiculum formed by cylindrical hyphae, thin-walled, straight, branching at right angles, clampless, 3–4 μm broad. Skeletal hyphae absent. Crystals sometimes present among the medulla hyphae, bipyramidal or sphaeroid. Attempts to obtain cultures from shed spores on MEA unsuccessful.
Typus: Lectotype designated here: Patouillard, Tab. Anal. Fung. 1: fig. 56. 1883. MycoBank MBT389355. Estonia, Otepää, Karula National Park, Peräjärve forest trail, on living Pleurozium schreberi, 13 Sep. 2015, I. Olariaga, IO.15.85 (S, epitype designated here; isoepitype in UPS). MycoBank MBT387918.
Known distribution: Denmark, Estonia, Finland, France, Sweden.
Additional materials examined: Denmark, Lolland, Biowide plot 120, Hejrede Sø, 5 Nov. 2014, T. Læssøe & T. Smidth, DMS-695059 (C); Sjælland, Allindelille Fredskov, on dead stem of Alnus, 20 Oct. 1977, H. Knudsen, C-F-124736; Amager Strandpark, on moss, 17 Nov. 2014, T. Læssøe, C-F-113951; Asserbo Plantage, on damp Juniperus bark and living mosses, 28 Feb. 2019, O. Martin, DMS-10005573 (C); Vestskoven, on damp Juniperus bark and mosses, 14 Feb. 2018, T. Kehlet, DMS-9242450 (C); Biowide plot 079, Melby Hede, 6 Nov. 2014, T. Læssøe, DMS-695405 (C); Biowide Plot 070, Gjessøvej, on moss, 31 Aug. 2015, T. Borgen & T. Læssøe, C-F-114679; Biowide plot 120, Hejrede Sø, base of Poaceae plant, 5 Nov. 2014, T. Læssøe & T. Smidth, C-F-114417; Strødam Reservatet, on mosses, rotten leaves and bark, 8 Nov. 2003, T. Læssøe, DMS-398331(C); Jægersborg Dyrehave, on moss and algae, 20 Jun. 1998, T. Læssøe, DMS-384562 (C-F-38168); Møns Klinteskov, v. Nælderenden, on moss on branch in damp hole, 23 Oct. 1971, H. Knudsen, C-F-94572; Stabjerggård, on Tortula, 14 Nov. 1976, H. Knudsen, C-F-94573. Finland, Pohjois-Häme, Laukaa, Hallalähde, on water dripping hillside west of the spring, on stock covered with moss, abundant on Pleurozium schreberi, 14 Sep. 2004, T. Rämä (TUR 178089). France, Pyrénées atlantiques, Escot, Le Barescou, on living mosses, 8 Oct. 2016, I. Olariaga (ARAN-Fungi 4625). Sweden, Dalarna, Särna, ca. 1.5 km W from Kryptjärnen, on living Thuidium tamariscinum and Plagiomnium, spreading on a dead herbaceous culm, 31 Aug. 2015, I. Olariaga, IO.15.41 (S); Skåne, Eriksdal, Vitabäckskällan Nature Reserve, on living leaves of Scorpidium cossonii, 3 Oct. 2014, N.-O. Nilsson & I. Olariaga, IO.14.164 (S); Skåne, Tomelilla, Årupkärrets Nature Reserve, on living Scorpidium cossonii, in a fen, 3 Oct. 2014, N.-O. Nilsson & I. Olariaga, IO.14.163 (S); Uppland, Uppsala, the cemetery wall, southeast corner adjacent to Carolinaparken, on low mosses (Tortula ruralis), 12 Sep. 1932, S. Lundell (UPS F-152985, as Ceratellopsis cf. sagittiformis).
Notes: The original plate of B. sagittiformis shows a fungus with a fertile apex, 2-spored basidia and aseptate medulla hyphae reminiscent of skeletal hyphae. Corner (1950) suggested this plate might correspond to a Pterula species, but this view cannot be verified, as no type specimen appears to exist in the Patouillard herbarium (FH, PC). Instead, we interpret B. sagittiformis, as a species with obtuse fertile basidiomata at least when not extremely young, medulla hyphae 3–4 μm broad, lacking clamp connections and occurring usually, though apparently not strictly, on living mosses (Hansen & Knudsen 1997). It is possible that mosses are always present but not necessarily act directly as substrate for the basidiomata. Accordingly, we propose an epitype specimen to stabilise its current interpretation (Corner, 1950, Hansen and Knudsen, 1997).
Key to genera that contain species that can be considered typhuloid
-
1.
Lichenised; growing on a thallus containing green algaes……………………………………………………………………Multiclavula
-
1.
Non-lichenised; not associated with green algae…………………………………………………………………………………………2
-
2.
Basidia with transverse septa; on living bryophytes……………………………………………………………Eocronartium muscicola
-
2.
Basidia without septa; on various substrates incl. bryophytes ……………………………………………………………………………3
-
3.
Clamp connections verticillate on context hyphae…………………………………………………………………Lutypha sclerotiophila
-
3.
Clamp connections absent or simple when present………………………………………………………………………………………4
-
4.
With a sclerotium………………………………………………………………………………………………………………………………5
-
4.
Without a sclerotium..…………………………………………………………………………………………………………………………7
-
5.
With skeletal hyphae; smell phenolic when fresh…………………………………………unbranched forms of Pterula sclerotiicola
-
5.
Without skeletal hyphae; smell not phenolic (living basidiomata)………………………………………………………………………6
-
6.
Sclerotium compressed; sclerotial rind normal (i.e. with cells rooting in the medulla) with medulla gelatinised; basidiomata brown; fertile part cylindrical……………………………………………………………………Macrotyphula phacorrhiza and M. megasperma
-
6.
Sclerotium usually not compressed, sclerotial rind inverse (i.e. with cells not rooting in the medulla), if normal, with medulla rarely gelatinised; basidiomata brown or with other colours, fertile part cylindrical to convex……………………………………Typhula p.p.
-
7.
With skeletal hyphae.…………………………………………………………………………………………………………………………8
-
7.
Without skeletal hyphae…………………..…………………………………………………………………………………………………9
-
8.
Stipe absent; protruding hymenial cystidia producing conidia; basidia 2-spored; spores 9–16 × 3.5–7 μm; smell phenolic when fresh…………………………………………………………………………………………………………………………Pterulicium gracile
-
8.
Stipe short; hymenial cystidia absent; basidia 1–4-spored; spores smaller; smell not phenolic…………………Ceratellopsis p.p.
-
9.
Spores ornamented…………………………………………………………………………………………………………Ramariopsis p.p.
-
9.
Spores smooth.………………………………………………………………………………………………………………………………10
-
10.
With hymenial cystidia………………………………………………………………………………………………………………………11
-
10.
Without hymenial cystidia...…………………………………………………………………………………………………………………13
-
11.
Fertile part more or less cylindrical; terricolous; clamps absent; caulocystidia absent……………………“Ceratellopsis” terrigena
-
11.
Fertile part globose to spathulate; on dead plant remnants; clamp connections sometimes present; caulocystidia sometimes present..………………………………………………………………………………………………………………………………………12
-
12.
Spores ellipsoid, fusiform or sigmoid……………………………………………………………………………………………Physalacria
-
12.
Spores broadly ellipsoid to subglobose……………………………………………………………………Actiniceps (= Chaetotyphula)
-
13.
Basidiomata < 2 mm high; apex sterile and pointed at least in young basidiomata…………………………………………………14
-
13.
Basidiomata higher, if < 2 mm high then apex fertile even in young basidiomata……………………………………………………15
-
14.
Medulla hyphae < 2.5 μm broad; subhymenial hyphae not swollen, < 2.5 μm broad; on dead plant remnants; clamps (always?) present………………………………………………………………………………………………………………………Ceratellopsis p.p.
-
14.
Medulla hyphae 2.5–6(–9) μm broad; subhymenial hyphae globose to subglobose, 4–10(–12) μm broad; often on living mosses; clamps absent…………………………………………………………………………………………Bryopistillaria sagittiformis
-
15.
Basidiomata pale brown; usually > 10 mm high; spores inamyloid……………………………………………………Macrotyphula p.p.
-
15.
Basidiomata if > 10 mm high then colour not brown; spores amyloid or not…………………………………………………………16
-
16.
Clamps present and abundant on hyphae in the context………………………………………………………………………………17
-
16.
Clamps absent or restricted to basidia and loop-like……………………………………………………………………………………19
-
17.
Not positively geotropic; fertile part and stipe usually sharply delimited; growing usually on dead leaves or stems of plants………………………………………………………………………………………………………………………………Typhula p.p.
-
17.
Usually positively geotropic; fertile part and stipe not clearly differentiated; growing on highly decayed wood or polypores..…………………………………………………………………………………………………………………………………………18
-
18.
With gloeocystidia; spores subglobose………………………………………………………………………………………Dentipratulum
-
18.
Without gloeocystidia; spores ellipsoid……………………………………………………………………………………………Mucronella
-
19.
Basidiomata < 1.5 mm high; stipe with 150–250 μm long erect hairs with obtuse ends; on dead wood………………………………………………………………………………………………………………………Hirticlavula elegans
-
19.
Basidiomata larger, hairs absent or considerably shorter; terrestrial or on leaves and herbaceous remnants of plants.……………………………..……………………………………………………………………………………………………………20
-
20.
On plant remnants; spores usually amyloid……………………………………………………………………………………Typhula p.p.
-
20.
Terrestrial; spores non-amyloid………………………………………………..………………………………………………………Clavaria
Discussion
Evolution of typhuloid fungi and family delimitation in the Agaricales and the Pleurotineae
This study provides the most robust phylogenetic hypothesis for typhuloid fungi to date, and it resolves for the first-time relationships with other genera in the Agaricales. Terminal nodes recovered in our Agaricales analyses using ML and Bayesian approaches are consistent to a great extent with those obtained by Matheny et al. (2006). In addition, several more basal nodes received support when analysed using a Bayesian method (Fig. 2, Fig. 3), while ML bootstrap values were low for most of those. As RAxML does not allow for a free model-choice, the same dataset was analysed using IQ-TREE (Nguyen et al. 2015) and Garli (Zwickl 2006), but the supported topology was identical and bootstrap values were similar to those obtained in RAxML. It has long been known that Bayesian PP tend to be higher than ML bootstrap values (e.g. Susko 2009), and have been claimed to refer to different properties of phylogenetic confidence (García-Sandoval 2014). Although our dataset is the most comprehensive ever assembled for typhuloid fungi, it might still contain too little molecular characters to reconstruct the comparatively deep divergences among clades. In any case, it was highly expected that novel supported topologies would be recovered for the first time when more taxa were added to the analyses, even if many nodes so far only received support in the Bayesian analyses.
Typhulaceae, Pleurotaceae, Pterulaceae, Stephanosporaceae, Sarcomyxa, Pleurocybella and Phyllotopsis are resolved as a monophyletic group for the first time (Fig. 2, Fig. 3), corresponding to the Pleurotineae (Dentinger et al. 2016). Our results confirm that Sarcomyxa and the Typhulaceae belong to Pleurotineae as anticipated by Dentinger et al. (2016). The back-bone of the Pleurotineae phylogeny is fully resolved (Fig. 3) and suggest that Macrotyphula and Typhula, with a typhuloid basidioma configuration, might have evolved from a pleurotoid ancestor. The phylogenetic relationships of Phyllotopsis, Pleurocybella and Sarcomyxa, of debated family placement, are also resolved in our Bayesian Pleurotineae phylogeny (Fig. 3). These three pleurotoid genera have been placed, not always together, in families containing white-spored agarics, such as Marasmiaceae, Mycenaceae, Pleurotaceae and Tricholomataceae (e.g. Ju¨lich, 1982, Moncalvo et al., 2000, Kirk et al., 2008, Begerow et al., 2018), or even Pterulaceae (Begerow et al. 2018), but previous phylogenies did not support any of these family placements (e.g. Matheny et al., 2006, Binder et al., 2010). Based on our phylogenies and the high Bayesian PP (Fig. 2, Fig. 3), we propose the new family Sarcomyxaceae to encompass Sarcomyxa and validate the family Phyllotopsidaceae to accommodate Macrotyphula, Phyllotopsis and Pleurocybella. On the other hand, Pterulaceae and Radulomycetaceae, recently split sister families (Leal-Dutra et al. 2020, contains an assemblage of fungi with clavarioid, corticioid and polyporoid basidioma types (Zhao et al. 2016), monomitic or dimitic, defined as “morpho-anatomically a very diverse family with colourless spores” (Begerow et al. 2018), but well-supported in molecular phylogenies (Fig. 3; Matheny et al., 2006, Binder et al., 2010). All members of Pterulaceae and Radulomycetaceae studied when fresh (Aphanobasidium filicinum, Pterula subulata, Pterulicium gracile, P. sclerotiicola, Radulomyces confluens, R. molaris, and R. rickii had a distinct phenolic or naphthalene odour, as sometimes described for P. multifida (e.g. Petersen 1999). It is thus suggested here that such an odour, produced by a probably unidentified fungal metabolite, may be a synapomorphic character of Pterulaceae, but further species must be tested to draw a final conclusion.
According to our results, the hygrophoroid clade as recovered by Matheny et al. (2006), is not monophyletic, because the Hygrophoraceae, the Pleurotineae and Xeromphalina campanella, all previously assigned to the hygrophoroid clade (Matheny et al. 2006), are encompassed in three independent lineages within the Agaricales (Fig. 2). The position of the Hygrophoraceae inferred from our analyses is not consistent with the 208-locus phylogeny by Dentinger et al. (2016), which placed Hygrocybe conica (Hygrophoraceae) and Clavaria fumosa (Clavariaceae) in a monophyletic group described as the Hygrophorineae. The dataset employed by Dentinger et al. (2016) contained 36 taxa of the highly diverse Agaricales and only a single taxon of the Hygrophoraceae. Phylogenetic analyses of a matrix with few taxa, even when the number of overall characters is large, can be subject to strong systematic biases and can be susceptible to long-branch attraction (Heath et al. 2008). In our preliminary analyses, H. coccinea nested in Clavariaceae when no other Hygrophoraceae were included in the matrix. With the addition of a few taxa of the Hygrophoraceae (Fig. 2), Clavariaceae and Hygrophoraceae do not form a monophyletic group, but are suggested to be successive sister taxa to the rest of the Agaricales. Our data indicates that Dentinger et al. (2016) recovered H. conica and C. fumosa in a monophyletic clade due to a long-branch attraction artifact and we anticipate that this clade will no longer be resolved when more species of Hygrophoraceae are included in phylogenomic analyses of large multigene datasets. Following our phylogenetic hypothesis, we propose the new suborder Clavariineae to accommodate the Clavariaceae.
Typhula phacorrhiza is a synonym of Sclerotium complanatum that nests in Macrotyphula
Earlier hypotheses on the phylogenetic position of Typhula and Typhulaceae relied on a misidentified specimen of T. phacorrhiza (DSH96-059; Pine et al. 1999) that belongs to the Macrotyphula juncea species complex (Olariaga et al. in prep.). Therefore, this is the first time that T. phacorrhiza is included in a multigene phylogenetic study. As previously suggested, based on morphology (Olariaga & Salcedo 2013), T. phacorrhiza and Sclerotium complanatum belong to Macrotyphula. The bulk of species of Typhula form a distinct separate clade (referred to as the Typhula-core clade). Thus, our analyses confirm that the selection of T. phacorrhiza as type of Typhula is unfortunate (Berthier, 1976, Olariaga and Salcedo, 2013), also for the sake of nomenclatural stability. The examination of material deposited in herbaria under S. complanatum showed that this name is usually applied to sclerotia of T. phacorrhiza (e.g. Pl. Crypt. N. France, ed. 1, no 536; Microf. Exs. no 49; Krypt. Exs. no 1840; see material examined under T. phacorrhiza) and that S. complanatum is a synonym of T. phacorrhiza as proposed long ago (Remsberg 1940). Furthermore, the Typhula-core clade contains the types of all genera segregated from Typhula at some point. Based on analyses of a more inclusive species sampling of Typhula (Olariaga et al., in prep), we consider it more appropriate to merge all those genera, as done by some authors (Berthier, 1976, Knudsen and Vesterholt, 2012), rather than splitting Typhula into several genera that could be recognised only with great difficulty.
A strict application of nomenclatural rules in the light of our phylogenetic hypothesis (Fig. 2, Fig. 3) would result in a high number of undesirable name changes. The correct generic name for species of Macrotyphula and T. phacorrhiza would be Sclerotium. However, the adoption of Sclerotium for Macrotyphula species would be misleading, because nearly all Macrotyphula species lack sclerotia and Macrotyphula is a well-established name. At the same time, species in the Typhula-core clade would need to be transferred to Pistillaria, the oldest alternative genus name for the group. Pathogenic species of Typhula, such as T. idahoensis, T. incarnata or T. ishikariensis, on which extensive literature has been published, would undergo name changes. Also, the epithet of T. incarnata would need to be changed to avoid creating a later illegitimate homonym of Pistillaria incarnata Desm. In order to preserve nomenclatural stability, two proposals are in preparation to conserve: 1) Typhula with T. incarnata as conserved type, and 2) Macrotyphula against Sclerotium (Olariaga et al. unpubl.). In accordance with our proposals, we combine in Macrotyphula two closely related taxa treated by Berthier in Typhula subgen. Typhula: T. phacorrhiza and T. megasperma.
Ceratellopsiss. auct. is polyphyletic and misapplied
Despite our efforts to find good material, very few specimens of Ceratellopsis were available for this study. Besides those types examined by Berthier (1976), only a few more type specimens of Ceratellopsis could be located and examined. The type specimens of most names placed in Ceratellopsis appear to be lost and can only be interpreted through their protologues. Most non-type specimens of Ceratellopsis were characterised by having a central medulla of narrow, thick-walled hyphae, a poorly differentiated subhymenium of cylindrical < 2 μm broad hyphae, a sterile basidioma apex, a well-delimited stipe, basidia < 20 μm long and spores < 8 μm long. Some of these collections had skeletal hyphae in the medulla, as also observed in the type specimens of C. acuminata, while other specimens assigned to C. aculeata and C. mucedinea had scarcely septate hyphae with thinner walls. Specimens identified as C. sagittiformis differed in having broader (up 3–4(–8) μm), more often septate medulla hyphae, a well differentiated subhymenium with swollen, globose to subglobose hyphae (4–10(–12) μm) and a basidioma apex becoming fertile. Three type specimens (C. attenuata, C. equiseticola and C. rickii) and several other specimens filed under the names C. aculeata and C. acuminata represent P. gracile, because they possessed skeletal hyphae, 2-spored basidia, acuminate cystidia and lacked a stipe. As earlier suggested (Corner, 1950, Berthier, 1976), several names originally described or combined in Ceratellopsis represent in fact P. gracile.
This study provides the first molecular data of Ceratellopsis. It resolves the genus as polyphyletic, with species belonging to both the Clavariaceae and the Hymenochaetales. Among the specimens sampled for the molecular phylogenetic study, those with a sterile pointed apex and narrow thick-walled medulla hyphae nest in the Clavariaceae. This position is supported by the fact that tiny clavarioid basidiomata are also known in other Clavariaceae, such as in Hirticlavula (Petersen et al. 2014) and Mucronella (Birkebak et al. 2013). Narrow hyphae on the base of the stipe, typical in Clavariaceae (Olariaga 2009), have also been observed in species of Ceratellopsis. For the first time, we demonstrate that skeletal hyphae are present in some species of Ceratellopsis and that it is not a unique character for Pterulaceae among typhuloid fungi. The species of Ceratellopsis placed in Clavariaceae differ from P. gracile in having basidia < 20 μm long, a well-delimited stipe and in lacking protruding acuminate cystidia. This lineage of Ceratellopsis conforms to Corner’s (1950) concept of Ceratellopsis, i.e. “Ceratellopsis aculeata and the species which appear to resemble it”, except for the presence of skeletal hyphae in some species. The examination of a specimen identified as C. aculeata by Corner (PC), with very narrow, scarcely septate medulla hyphae, confirms this view. In accordance with this, we conclude that C. corneri and C. terrigena, having medullar hyphae 4–6 μm and 1.5–3.5 μm broad, respectively, do not belong to this lineage and must be placed elsewhere (see Taxonomy).
The inclusion of C. sagittiformis in the Rickenella clade of the hymenochaetoid clade is a novel and unexpected find although material identified as C. sagittiformis is often associated with living bryophytes, as several members of the clade are (Korotkin et al. 2018). The Rickenella clade encompasses fungi with a diverse basidioma configuration (agaricoid, clavarioid, cyphelloid, thelephoroid, corticioid, etc.) that often are associated with mosses (Larsson et al. 2006). Within the Rickenella clade, C. sagittiformis resembles Muscinupta laevis in having white basidiomata, in growing on living bryophytes and in lacking clamp connections (Eriksson & Ryvarden 1975, as Cyphellostereum laeve), but our phylogenetic analyses lack support to confirm a close relationship. Phylogenetic relationships within the Rickenella clade were previously explored using only ribosomal nuclear markers (5.8S and 28S; Larsson et al. 2006), and only recently using also the RPB2 region. Even with the addition of the 18S and the RPB2 regions and more taxa to the analyses of the hymenochaetoid clade, relationships within the Rickenella clade remain largely unresolved (Korotkin et al. 2018, Fig. 5). The three specimens of C. sagittiformis have identical sequences and formed a distinct lineage. Based on this and the fact that C. sagittiformis is the only bryophilous, reduced clavarioid species known to belong to the Rickenella clade, we propose the new genus Bryopistillaria to accommodate it (see Taxonomy).
Proposing a reliable and stable interpretation of C. queletii, type of Ceratellopsis, is necessary to be able to provide a solid interpretation of Ceratellopsis. The name Ceratellopsis queletii appears not to have been employed since its original description (Patouillard 1883). Regrettably, no type specimen exists in PC and FH (G. E. Tocci pers. comm.) and no original specimen is likely to be present in any other fungarium. The absence of a stipe, the sterile apex, the 2-spored basidia and the presence of protruding acuminate cystidia, clearly depicted in the original illustration of C. queletii selected here as lectotype, are characteristic of P. gracile (Corner, 1950, Berthier, 1976, Jülich, 1980). The spore size estimated by Corner from the original Patouillard plate of C. queletii is smaller (6 × 3 μm) than the range we have estimated (8–8.5 × 4.5–5 μm) and the size reported by Quélet (“8 μm?”, 1884) for C. queletii. Spores in P. gracile are generally reported as slightly larger than our measure estimates from the Patouillard plate, but those are almost within the ranges published for P. gracile: 9–16 × 4–7 μm (Corner 1950), 10.5–12.5 × 5.5–5.6 μm (Berthier 1976), 10–14 × 5.5–6.5 μm (Jülich 1980) and 8–11 × 5–7 μm (Daniëls & Moreno-Arroyo 2007). Based on all this, we consider C. queletii a synonym of P. gracile, as already Corner (1950) suggested.
Leal-Dutra et al. (2020), using one of the specimens of P. gracile employed in our analyses (CBS 309.79), showed that it belongs to the Pterulicium clade. This taxonomic conclusion reduces Pterulicium, as recently circumscribed, to a later synonym of Ceratellopsis and the fourty-six names combined in Pterulicium (Leal-Dutra et al. 2020), besides its type Pterulicium xylogenum, would have to be combined again in Ceratellopsis. In addition, a new genus name would be required for C. acuminata and C. aculeata. In order to avoid nomenclatural changes that may cause confusion, a proposal to conserve Ceratellopsis with C. acuminata as conserved type is in preparation. The acceptance of this proposal would preserve the current usage of Ceratellopsis and Pterulicium, typified by P. xylogenum, a presumed causal agent of culm rot disease of bamboo (Harsh et al. 2005) and possibly also of sugarcane (Corner 1952).
Conclusions
This study reveals that typhuloid fungi appeared several times among the Agaricomycetes and contributes to the understanding of fungal evolution and shifts of basidioma configuration. Novel phylogenetic hypotheses are provided for several groups of typhuloid fungi and pleurotoid agarics, and pertinent family and generic classifications are proposed. Future phylogenomic analyses will hopefully incorporate more taxa, including typhuloid fungi, and will serve to further test our phylogenetic hypotheses.
Acknowledgements
Bart Buyck's kind assistance to locate type specimens was indispensable during I.O.’s stay in PC herbarium. We thank also curator and assistants from C (Christian Lange), G (Philippe Clerk), FH (Genevieve E. Tocci), K (Angela Bond), M (Dagmar Triebel), PAD (Rosella Marcucci) and TL (Paul Semandi) herbaria for sending us material on loan or providing valuable information on types kept at their institutions. Mari Azpiroz, curator of ARAN herbarium is deeply thanked for efficiently arranging loans of specimens from herbaria. We wish to thank Rafael Blasco for providing us with material of Ceratellopsis and Nils-Otto Nilsson for good guidance and help collecting typhuloid fungi in Scania (Sweden). Juan Ignacio Iturrioz is thanked for lending us a photograph of Phyllotopsis nidulans. We express our gratitude to Francis Martin for kindly granting us permission to use partial sequences obtained from genomes of Agaricus bisporus, Onnia scaura, Phellinus ferrugineofuscus, Radulomyces confluens and Trichaptum abietinum. We are grateful to two anonymous reviewers for their insightful comments on the paper, as well as to L.A. Parra, J.C. Zamora and J. McNeill for advice on the nomenclatural status and the possibility to conserve the name Ceratellopsis. This study was funded by the Swedish Taxonomy Initiative (Svenska artprojektet) administered by the Swedish Species Information Center (ArtDatabanken), through grants 143/2013 and 22/2016 to I.O. A grant from the SYNTHESYS initiative funded a stay to examine collections kept in the Muséum national d'Histoire naturelle of Paris (PC).
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Begerow D., McTaggart A., Agerer R. In: Wolfgang F., editor. 2018. Basidiomycota and Entorhizomycota. A. Engler's Syllabus of Plant Families, part 1/3. Stuttgart, Germany. [Google Scholar]
- Berkeley M.A. Notices of British fungi. No. II. Magazine of Zoology and Botany. 1837;1:507–513. [Google Scholar]
- Berthier J. Monographie des Typhula Fr., Pistillaria Fr. et genres voisins. Bulletin mensuel de la Société linnéenne de Lyon. 1976 Special issue. [Google Scholar]
- Binder M., Larsson K.-H., Matheny P.B., et al. Amylocorticiales ord. nov. and Jaapiales ord. nov.: Early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia. 2010;102:865–880. doi: 10.3852/09-288. [DOI] [PubMed] [Google Scholar]
- Birkebak J.M., Mayor J.R., Ryberg K.M., et al. A systematic, morphological and ecological overview of the Clavariaceae (Agaricales) Mycologia. 2013;105:896–911. doi: 10.3852/12-070. [DOI] [PubMed] [Google Scholar]
- Blunt T.D., Brunk G., Koski T., et al. Typhula blight development in Poa annua and Poa pratensis as influenced by persistence of the fungicides chlorothalonil and fludioxonil under snow cover. Canadian Journal of Plant Pathology. 2015;37:165–178. [Google Scholar]
- Bory de Saint-Vincent M.M., Durieu de Maisonneuve M.C. Vol. 1. Livraison 6. Imprimerie Nationale; Paris: 1846. (Exploration scientifique de l'Algérie. Botanique). [Google Scholar]
- Bourdot H., Galzin A. Société Mycologique de France; Sceaux: 1928. Hyménomycètes de France. [Google Scholar]
- Chang S.-W. Genetic relationships among Typhula ishikariensis varieties from Wisconsin. Weed & Turfgrass Science. 2015;4:135–143. [Google Scholar]
- Clements F.E., Shear C.L. Carnegie Institution of Washington; New York: 1931. The genera of fungi. [Google Scholar]
- Corner E.J.H. A monograph of Clavaria and allied genera. Annals of Botany Memoirs. 1950;1:1–740. [Google Scholar]
- Corner E.J.H. Addenda Clavariacea II. Pterula and Pterulicium. Annals of Botany. 1952;16:531–569. [Google Scholar]
- Corner E.J.H. Supplement to “A monograph of Clavaria and allied genera”. Beihefte zur Nova Hedwigia. 1970;33:1–299. [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I., et al. Fungal Planet description sheets: 625–715. Persoonia. 2017;39:270–467. doi: 10.3767/persoonia.2017.39.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniëls P.P., Moreno-Arroyo B. Contribución al estudio de la diversidad fúngica andaluza II. Boletín de la Sociedad Micológica de Madrid. 2007;31:257–268. [Google Scholar]
- Dentinger B.T.M., Gaya E., O'Brien H., et al. Tales from the crypt: genome mining from fungarium specimens improves resolution of the mushroom tree of life. Biological Journal of the Linnean Society. 2016;117:11–32. [Google Scholar]
- Dentinger B.T.M., McLaughlin D.J. Reconstructing the Clavariaceae using nuclear large subunit rDNA sequences and a new genus segregated from Clavaria. Mycologia. 2006;98:746–762. doi: 10.3852/mycologia.98.5.746. [DOI] [PubMed] [Google Scholar]
- Desmaziéres J.B.H.J. Seizième notice sur les plantes cryptogames rècemment découvertes en France. Annales des Sciences Naturelles; Botanique, série 3. 1848;10:342–361. [Google Scholar]
- Donk M.A. Revision der Niederländischen Homobasidiomycetae-Aphyllophoraceae II. Mededeelingen Nederlandsche Mycologische Vereeniging. 1933;22:1–278. [Google Scholar]
- Donk M.A. The generic names proposed for Hymenomycetes-III. Reinwardtia. 1954;2:441–493. [Google Scholar]
- Donk M.A. The generic names proposed for Hymenomycetes. XII, Deuteromycetes. Taxon. 1962;11:75–104. [Google Scholar]
- Duby J.E. Editio secunda. Vol. 2. Va Desray; Paris: 1830. (Botanicon Gallicum seu Synopsis Plantarum in flora gallica descriptarum). [Google Scholar]
- Ekstrand H. Höstsädens och vallgräsens övervintering. Statens Växtskyddsanstalt Meddelande. 1955;67:1–125. [Google Scholar]
- Eriksson J., Ryvarden L. Fungiflora; Oslo: 1975. The Corticiaceae of North Europe. Vol. 3. Coronicium-Hyphoderma. [Google Scholar]
- Farr E.R., Zijlstra G. 2020. Index Nominum Genericorum (Plantarum)http://botany.si.edu/ing/ 1996+ (consulted on 27 March 2020) [Google Scholar]
- Fries E.M. 1821. Systema mycologicum. I. Lund. [Google Scholar]
- Fries E.M. 1822. Systema mycologicum II. Lund. [Google Scholar]
- Fries E.M. 1874. Hymenomycetes europaei. Uppsala, Sweden. [Google Scholar]
- García-Sandoval R. Why some clades have low bootstrap frequencies and high Bayesian posterior probabilities. Israel Journal of Ecology & Evolution. 2014;60:41–44. [Google Scholar]
- Grigoriev I.V., Nikitin R., Haridas S., et al. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Research. 2014;42:D699–704. doi: 10.1093/nar/gkt1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L., Knudsen H. 1997. Nordic Macromycetes. Vol. 3 Heterobasidioid, aphyllophoroid and gastromycetoid basidiomycetes. Copenhagen, Denmark. [Google Scholar]
- Hansen K., Perry B.A., Dranginis A.W., et al. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected lifehistory traits. Molecular Phylogenetics and Evolution. 2013;67:311–335. doi: 10.1016/j.ympev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Harsh S.N.K., Singh Y.P., Gupta H.K., et al. A new culm rot disease of bamboo in India and its management. Journal of Bamboo and Rattan. 2005;4:387–398. [Google Scholar]
- Hawksworth D.L., Kirk P.M., Sutton B.C., et al. 8th edn. CAB International. Cambridge University Press; 1995. Ainsworth & Bisby's Dictionary of Fungi. [Google Scholar]
- Heath T.A., Hedtke S.H., Hillis D.M. Taxon sampling and the accuracy of phylogenetic analyses. Journal of Systematics and Evolution. 2008;46:239–257. [Google Scholar]
- Hibbett D.S. Trends in Morphological Evolution in Homobasidiomycetes Inferred Using Maximum Likelihood: A Comparison of Binary and Multistate Approaches. Systematic Biology. 2004;53:889–903. doi: 10.1080/10635150490522610. [DOI] [PubMed] [Google Scholar]
- Hibbett D.S. After the gold rush, or before the flood? Evolutionary morphology of mushroom forming fungi (Agaricomycetes) in the early 21st century. Mycological Research. 2007;111:1001–1018. doi: 10.1016/j.mycres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Hibbett D.S., Binder M. Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proceedings of the Royal Society: Biology. 2002;269:1963–1969. doi: 10.1098/rspb.2002.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Bates S.T., Beever R.E., et al. Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders. Mycologia. 2006;98:949–959. doi: 10.3852/mycologia.98.6.949. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Tronsmo A.M., Yumoto I. Snow mold fungus, Typhula ishikariensis group III, in arctic Norway can grow at sub-lethal temperature after freezing stress and during flooding. Sommerfeltia. 2008;13:125–131. [Google Scholar]
- Jülich W. On Mucronella rickii and Pterula gracilis. Persoonia. 1980;10:535–543. [Google Scholar]
- Ju¨lich W. “1981”. Higher taxa of Basidiomycetes. Bibliotheca Mycologica. 1982;85:1–485. [Google Scholar]
- Kaygusuz O., Çolak Ö. Typhula spathulata – first record from Turkey. Czech Mycology. 2017;69:125–131. [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W., et al. 10th edn. CAB International; Oxon: 2008. Dictionary of the Fungi. [Google Scholar]
- Knudsen H., Vesterholt J. 2nd edn. 2012. Funga Nordica. Copenhagen, Denmark. [Google Scholar]
- Koch P. Optimal fungicide timing for suppression of Typhula blight under winter covers. Agronomy Journal. 2016;109:1771–1776. [Google Scholar]
- Kohler A., Kuo A., Nagy L.G., et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics. 2015;47:410–415. doi: 10.1038/ng.3223. [DOI] [PubMed] [Google Scholar]
- Konrad P., Maublanc A. Tome VI; Paris, France: 1937. “1924-1935” Icones selectae fungorum. [Google Scholar]
- Korotkin H.B., Swenie R.A., Miettinen O., Budke J.M., Chen K-H., Lutzoni F., Smith M.E., Matheny P.B. Stable isotopic analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. American Journal of Botany. 2018;105(11):1–9. doi: 10.1002/ajb2.1183. [DOI] [PubMed] [Google Scholar]
- Larsson K.-H. Re-thinking the classification of corticioid fungi. Mycological Research. 2007;111:1040–1063. doi: 10.1016/j.mycres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30:3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K.-H., Larsson E., Kõljalg U. High phylogenetic diversity among corticioid homobasidiomycetes. Mycological Research. 2004;108:983–1002. doi: 10.1017/s0953756204000851. [DOI] [PubMed] [Google Scholar]
- Larsson K.-H., Parmasto E., Fischer M., et al. Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia. 2006;98:926–936. doi: 10.3852/mycologia.98.6.926. [DOI] [PubMed] [Google Scholar]
- Leal-Dutra C.A., Griffith G.W., Neves M.A., et al. Reclassification of Pterulaceae Corner (Basidiomycota: Agaricales) introducing the ant-associated genus Myrmecopterula gen. nov., Phaeopterula Henn. and the corticioid Radulomycetaceae fam. nov. IMA Fungus. 2020;11:2. doi: 10.1186/s43008-019-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillé D.M. Mémoire sur le genre Sclerotium. Annales des Sciences Naturelles, série 2. 1843;20:218–248. [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Lodge D.J., Padamsee M., Matheny P.B., et al. Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales) Fungal Diversity. 2014;64:1–99. [Google Scholar]
- Martin F., Aerts A., Ahren D., et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature. 2008;452:88–92. doi: 10.1038/nature06556. [DOI] [PubMed] [Google Scholar]
- Mason-Gamer R.J., Kellogg E.A. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Graminaeae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- Matheny P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales) Molecular Phylogenetics and Evolution. 2005;35:1–20. doi: 10.1016/j.ympev.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Matheny P.B., Hofstetter V., Aime M.C., et al. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia. 2006;98:982–995. doi: 10.3852/mycologia.98.6.982. [DOI] [PubMed] [Google Scholar]
- Matheny P.B., Liu Y.L., Ammirati J.F., et al. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales) American Journal of Botany. 2002;89:688–698. doi: 10.3732/ajb.89.4.688. [DOI] [PubMed] [Google Scholar]
- Matheny P.B., Wang Z., Binder M., et al. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi) Molecular Phylogenetics and Evolution. 2007;43:430–451. doi: 10.1016/j.ympev.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Matsumoto N. Evolutionary ecology of the pathogenic species of Typhula. Transactions of the Mycological Society of Japan. 1992;33:269–285. [Google Scholar]
- Matsumoto N., Tkachenko O.B., Hoshino T. In: Low temperature plant microbe interactions under snow. Iriki N., Gaudet D.A., Tronsmo A.M., Matsumoto N., Yoshida M., Nishimune A., editors. 2001. The pathogenic species of Typhula; pp. 49–59. (Hokkaido National Agricultural Experimental Station, Japan). [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Proceedings of the Gateway Computing Environments Workshop. (GCE); 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; p. 18. [Google Scholar]
- Moncalvo J.-M., Lutzoni F.M., Rehner S.A., et al. Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Systematic Biology. 2000;49:278–305. doi: 10.1093/sysbio/49.2.278. [DOI] [PubMed] [Google Scholar]
- Moncalvo J.-M., Vilgalys R., Redhead S.A., et al. One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution. 2002;23:357–400. doi: 10.1016/S1055-7903(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Muraguchi H., Umezawa K., Niikura M., et al. Strand-Specific RNA-Seq Analyses of Fruiting Body Development in Coprinopsis cinerea. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., von Haeseler A., et al. IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Molecular Biology and Evolution. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K., Rooney A.P., Mills G.L., et al. Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic. Fungal Genetics and Biology. 2011;48:252–265. doi: 10.1016/j.fgb.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Olariaga I. University of the Basque Country; 2009. The order Cantharellales in the Iberian Peninsula and the Balearic Islands. Ph.D. Dissertation. [Google Scholar]
- Olariaga I., Corriol G., Salcedo I., et al. A new species of Typhula with sigmoid spores: Typhula suecica. Karstenia. 2016;56:27–38. [Google Scholar]
- Olariaga I., Salcedo I. “2012” New combinations and notes on clavarioid fungi. Mycotaxon. 2013;121:37–44. [Google Scholar]
- Patouillard N.T. 1883. Tabulae analyticae fungorum. Fasc. 1. Poligny, France. [Google Scholar]
- Patouillard N.T. 1887. Les Hyménomycètes d'Europe. Anatomie générale et classification des champignons supérieurs. Paris, France. [Google Scholar]
- Patouillard N.T. 1897. Catalogue raisonné des plantes cellulaires de la Tunisie. Paris, France. [Google Scholar]
- Petersen R.H. Notes on clavarioid fungi. XIV. Cultures of Lentaria byssiseda. Mycologia. 1974;66:530–532. [Google Scholar]
- Petersen J.H. 1999. Key to the genera of clavarioid fungi in Northern Europe.https://www.mycokey.com/MycokeyDK/DKkeysPDFs/ClavarioidGenusKeyPrint.pdf Mycokey webpage. [Google Scholar]
- Petersen J.H., Davey M.L., Læssøe T. Hirticlavula elegans, a new clavarioid fungus from Scandinavia. Karstenia. 2014;54:1–8. [Google Scholar]
- Petersen J.H., Læssøe T. 2019. Mycokey 4.1.http://www.mycokey.com/ [Google Scholar]
- Pilát A. Species nova generis Ceratellopsis Konr. et Maubl. in Bohemia: Ceratellopsis kubičkae sp. n. Česká Mykologie. 1958;12:213–217. [Google Scholar]
- Pine E.M., Hibbett D.S., Donoghue M.J. Phylogenetic relationships of cantharelloid and clavarioid Homobasidiomycetes based on mitochondrial and nuclear rDNA sequences. Mycologia. 1999;91:944–963. [Google Scholar]
- Quélet L. Quelques espèces critiques ou nouvelles de la Flore Mycologique de France. Compte Rendu de l'Association Francaise Pour l'Avancement des Sciences. 1884;12:498–512. [Google Scholar]
- Quélet L. 1886. Enchiridion Fungorum in Europa media et praesertim in Gallia vigentium. Paris, France. [Google Scholar]
- Quélet L. Description des champignons nouveaux. Les plus remarquables représentés dans les aquarelles de Louis de Brondeau, avec des observations sur les genres Gyrocephalus, Pers., et Ombrophila, Fr. Revue Mycologique (Toulouse) 1892;14:64–67. [Google Scholar]
- Rehner S., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Remsberg R.E. Studies in the genus Typhula. Mycologia. 1940;32:52–96. [Google Scholar]
- Ronquist F., Teslenko M., Mark P van der, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostrup E. Dyrkningsforsøg med sclerotier. Botanisk Tidsskrift. 1866;1:199–223. [Google Scholar]
- Schröter J. In: Krytogamen-Flora von Schlesien. Cohn F., editor. 1889. Pilze; pp. 1–814. Germany. [Google Scholar]
- Shiryaev A., Kotiranta H. The genera Typhula and Pistillaria (Typhulaceae, “Aphyllophorales”) in Finland. A check-list of the species. Karstenia. 2007;47:49–54. [Google Scholar]
- Stamatakis A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30 doi: 10.1093/bioinformatics/btu033. 1312–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller J.W., Hall B.D. The origin of red algae: implications for plastid evolution. Proceedings of the National Academy of Science of the United States of America. 1997;94:4520–4525. doi: 10.1073/pnas.94.9.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susko E. Bootstrap support is not first-order correct. Systematic Biology. 2009;58:211–223. doi: 10.1093/sysbio/syp016. [DOI] [PubMed] [Google Scholar]
- Tode H.J. Lüneburg; Germany: 1790. Fungi Mecklenburgensis selecti. [Google Scholar]
- Turland N.J., Wiersema J.H., Barrie F.R., et al. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile. 2018;159:1–254. [Google Scholar]
- Varga T., Krizsan K., Foldi C., et al. Megaphylogeny resolves global patterns of mushroom evolution. Nature, Ecology & Evolution. 2019;3:668–678. doi: 10.1038/s41559-019-0834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara G.V., Bughara S.S., Jung G. Genetic variability of grey snow mould (Typhula incarnata) Mycological Research. 2004;108:1283–1290. doi: 10.1017/s0953756204001078. [DOI] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzini A. Segnalazioni di Muscinupta laevis (Basidiomycota, Agaricomycetes) per il Nord Italia. Micologia e Vegetazione Mediterranea. 2010;25:141–148. [Google Scholar]
- White T.J., Bruns T.D., Lee S., et al. In: PCR protocols: a guide to methods and applications. Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. USA. [Google Scholar]
- Xu Z., Harrington T., Gleason M., et al. Phylogenetic placement of plant pathogenic Sclerotium species among teleomorph genera. Mycologia. 2010;102:337–346. doi: 10.3852/08-189. [DOI] [PubMed] [Google Scholar]
- Zhang M., Tai-Lui L., Chen F. Rickenella danxiashanensis, a new bryophilous agaric from China. Phytotaxa. 2018;350:283–290. [Google Scholar]
- Zhao C.-L., Chen H., He S.-H., et al. Radulotubus resupinatus gen. et sp. nov. with a poroid hymenophore in Pterulaceae (Agaricales, Basidiomycota) Nova Hedwigia. 2016;103:265–278. [Google Scholar]
- Zwickl D.J. The University of Texas at Austin; 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. Dissertation. [Google Scholar]