Version Changes
Revised. Amendments from Version 1
All authors reviewed the reviewer comments and contributed to the revision of Version 1. We have made minor clarifications in the text. In response to the reviewer comments, the title was clarified to indicate that this manuscript broadens G6PD testing beyond P. vivax malaria. We have also given more thought to how point of care G6PD testing could potentially be delivered at primary level facilities, and the costs of testing and P. vivax infection. An important correction in Version 2 is that G6PD deficiency has an X-linked inheritance pattern and is not autosomal dominant.
Abstract
Safe access to the most effective treatment options for Plasmodium vivax malaria are limited by the absence of accurate point-of-care testing to detect glucose-6-phosphate dehydrogenase (G6PD) deficiency, the most common human genetic disorder. G6PD-deficient patients are at risk of life-threatening hemolysis when exposed to 8-aminoquinolines, the only class of drugs efficacious against P. vivax hypnozoites. Until recently, only qualitative tests were available in most settings. These can identify patients with severe G6PD deficiency (mostly male) but not patients with intermediate G6PD deficiency (always female). This has led to and reinforced a gap in awareness in clinical practice of the risks and implications of G6PD deficiency in females—who, unlike males, can have a heterozygous genotype for G6PD. Increasing recognition of the need for radical cure of P. vivax, first for patients’ health and then for malaria elimination, is driving the development of new point-of-care tests for G6PD deficiency and their accessibility to populations in low-resource settings. The availability of user-friendly, affordable, and accurate quantitative point-of-care diagnostics for the precise classification of the three G6PD phenotypes can reduce sex-linked disparities by ensuring safe and effective malaria treatment, providing opportunities to develop supportive counseling to enhance understanding of genetic test results, and improving the detection of all G6PD deficiency phenotypes in newborns and their family members.
Keywords: G6PD deficiency, Plasmodium vivax, neonatal hyperbilirubinaemia, gender, sex, disparity, G6PD testing, primaquine, tafenoquine, genetic counselling, haemolysis, G6PD heterozygous females
Box 1. Learning points.
-
1.
Deficiency of the essential enzyme glucose-6-phosphate dehydrogenase (G6PD) in humans is caused by mutations in the G6PD gene located on the X-chromosome (Xq28). As such, males are either G6PD deficient or normal while females can be deficient, intermediate, or normal. The standard qualitative tests typically used to diagnose G6PD deficiency can identify deficient subjects but cannot reliably differentiate intermediate G6PD activity. Consequently, accurate assessment of G6PD status is more difficult in females, especially at the point of care where it is needed to inform Plasmodium vivax malaria case management.
-
2.
Evidence for haemolysis associated with anti-malarials in G6PD-intermediate females has been documented, albeit infrequently, since 1958. Differentiating females with intermediate activity from those with normal activity has not been prioritised due to technical complexity of the testing. As a result, front-line health care providers are often unaware of the sex-related, oxidative drug–associated risk for female patients.
-
3.
New quantitative point-of-care G6PD tests that provide accurate results for both males and females present important opportunities to address the sex-linked disparities related to safe and efficacious malaria treatment in women and girls. These tests can also be used to address other G6PD deficiency–associated medical indications, including improved management of neonatal hyperbilirubinemia in low-resource settings.
-
4.
Realisation of these opportunities requires community engagement and improved counseling to enhance understanding of genetic test results and the implications for offspring and other family members.
-
5.
Using G6PD testing beyond malaria treatment can further enhance the cost-effectiveness of the test, an important consideration for low-resource and malaria-endemic settings.
Introduction
A new, single-dose radical cure for Plasmodium vivax, tafenoquine has received registration in Australia (Kozenis®), the United States (Krintafel®), and more recently in Brazil and Thailand. This combined with the drive to eliminate malaria, is encouraging malaria-endemic countries to increase access to testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency. G6PD deficiency is the most common sex-linked genetic abnormality in humans, affecting more than 400 million people worldwide 1, 2, and it is associated with increased haemolytic risk during treatment with oxidative drugs, including radical curative treatments against Plasmodium vivax malaria. The World Health Organisation (WHO) estimates that there were 7.5 million cases of P. vivax in 2017 alone 3, a large proportion of which occurred in populations with high G6PD deficiency prevalence 4. For most countries outside sub-Saharan Africa approaching malaria elimination, P. vivax is now the main contributor to malaria disease burden, and there is recognition of the need for broader access to radical cure with either the current, standard, 7- to 14-day primaquine course or the new, single-dose tafenoquine, both of which are contraindicated in people with G6PD deficiency 5– 7. For G6PD-deficient patients with P. vivax malaria, WHO recommends an 8-week course of primaquine (0.75mg/kg weekly), which is qualified in the WHO malaria treatment guideline as a conditional recommendation with very low quality evidence. Where G6PD testing is not available, WHO recommends “all females should be considered as potentially having intermediate G6PD activity and given the 14-day regimen of primaquine, with counselling on how to recognise symptoms and signs of haemolytic anaemia” 8. Tafenoquine is the first drug to be contraindicated additionally in females with intermediate G6PD activity 9– 13.
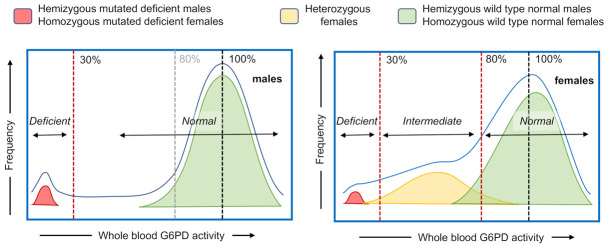
The G6PD gene is located on the X chromosome, so males have only one gene that expresses the G6PD enzyme and are either deficient in G6PD enzyme activity or normal, whereas females have two genes (but only one is expressed in each cell), and can have deficient, intermediate, or normal levels of G6PD activity ( Figure 1) 2, 14.
Figure 1. Schematic of population histograms demonstrating the relationship between phenotype and genotype in G6PD deficiency in males (left panel) and females (right panel).
The G6PD gene is located on the X chromosome, such that females have two genes and males have only one. Males with a mutated G6PD allele (in red, G6PD DEF) that expresses a compromised (deficient) G6PD enzyme protein typically have a blood G6PD value of less than 30% of normal. Females with two mutated G6PD-deficient alleles (in red, G6PD DEF1, DEF2) also typically have a blood G6PD value of less than 30% of normal. Males with a wild type G6PD allele (in green, G6PD WT) that expresses a fully functional enzyme have G6PD activity in an approximate normal distribution around the 100% median, as do females with two wild type G6PD alleles (in green, G6PD WT1, WT2). Heterozygous females with both wild type and mutated G6PD alleles (in yellow, G6PD WT, DEF1) can express a spectrum of whole blood G6PD activity, ranging from severely deficient (<30%) to beyond the World Health Organization definition of normal for females (>80%), with the majority in the intermediate (30% to 80%) activity range. The colored zones indicate the distribution of enzymatic activities associated with the genotypes as described above; the blue line represents the cumulative G6PD activity-based histogram.
Historically, it has been challenging to accurately identify and diagnose females with intermediate G6PD deficiency because existing quantitative diagnostic tests are complex and require good laboratory infrastructure 15– 17. While qualitative rapid tests and other near-patient methods, such as the fluorescent spot test, may be sufficient to differentiate gross deficiencies, they do not reliably assess intermediate status. Instead, the tests misclassify females with intermediate enzyme activity as normal. As a result, males and females who are severely G6PD deficient can be identified correctly and treated with weekly primaquine (or no treatment in the case of other oxidative drugs), whereas females with an intermediate G6PD status remain under-diagnosed and thus inadvertently exposed to oxidative treatments.
As countries prepare to increase access to P. vivax radical cure with the curative regimens of primaquine 18, 19 and tafenoquine (Kozenis® and Krintafel®) 11, 12, respectively, there are several opportunities that should be considered within broader national health systems for expanding the acceptability, utility, and concurrently, the cost-effectiveness of G6PD testing beyond malaria case management 15. In this article, we discuss an important opportunity to reduce sex-related health disparities in an era of new tools and initiatives that improve the diagnosis of G6PD deficiency. This is in alignment with the need to recognise sex as a key determinant of health and its importance in health research to understand the impact on health outcomes 20. We also propose a research agenda to investigate this opportunity ( Table 1).
Table 1. Research questions to investigate the implications of a new drive to increase access to safe radical cure of Plasmodium vivax malaria, including diagnosis of intermediate G6PD deficiency in females and availability of new point-of-care tests for G6PD deficiency.
| Research topics/agenda | Considerations |
|---|---|
| Studies to assess the feasibility of introducing point-of-
care quantitative tests into health clinics in low-resource settings, including ethnographic/qualitative studies on a barriers/facilitators model. |
A quantitative G6PD test may need to be integrated into dynamic and
contextually specific malaria case management strategies, newborn screening policies, and other complex health services. Feasibility studies will help to ensure that this integration can be successfully scaled up across areas where G6PD deficiency is prevalent. |
| Ethnographic research to inform appropriate messaging
and frontline staff training with respect to G6PD deficiency genetic counseling content and tools for target populations in low-resource, high disease burden settings. |
Current practices vary widely and scant evidence exists regarding G6PD
deficiency counseling best-practices. Ample lessons from other genetic conditions can be leveraged to design and validate such tools. |
| Studies comparing the costs of implementing current
G6PD tests, including costs of inaccurate diagnosis of intermediate G6PD, and new point-of-care tests. |
As new point-of-care tests become available, stakeholders within the
health system will need clear guidance on the costs of various products. These costing studies will need to consider factors such as training, distribution, and product specifications such as shelf life. |
| Cost-effectiveness studies that take into consideration
broader clinical benefits to the individual than those of Plasmodium vivax cure, in different G6PD prevalence settings. |
Improve the value proposition of G6PD testing by considering other
clinical benefits (not only as part of malaria case management) such as averting exposure to other oxidative agents, improved management of neonatal hyperbilirubinaemia in offspring and averting kernicterus. |
| Clinical studies to better define risk of clinical haemolysis in
intermediate females given different anti-relapse regimens. |
Clinical data focused on intermediate females will help inform downstream
decisions regarding appropriate anti-relapse regimens. Currently, these decisions are often made based on a clinician’s individual risk-benefit assessments. The need for this type of medical expertise can restrict 8-aminoquinoline usage to the highest tiers of the health system. |
G6PD deficiency in females
In a given population with a mutated G6PD allele frequency of 10%, this same number will indicate the proportion of affected males. The proportion of females with the homozygous mutated genotype will be around 1%, while a large proportion of females (20%) will be heterozygous with a variable phenotype due to X-chromosome inactivation 15, 18, 21. Of the heterozygous females, around 60% will have an intermediate phenotype 22. Thus while males represent the highest proportion of individuals with severe G6PD phenotypes, there is also a comparable proportion of females with intermediate G6PD deficiency. While this is based on well-established knowledge, the characterisation of G6PD phenotypes in heterozygous women has received little attention at the patient level, possibly because of the more labor-intense laboratory techniques needed to characterise it ( Table 2). In clinical settings worldwide, the most commonly performed tests are qualitative tests (e.g., fluorescent spot test), which result in a systematic underestimation of the number of females at risk (as intermediates are classified as normal) and a perception among health workers that G6PD deficiency only concerns males 23.
Table 2. Scientific findings and technical advancement for the characterisation of women with intermediate G6PD activity.
| Reference | Year
published |
Main findings |
|---|---|---|
| Beutler et al. | 1955 | Development of the GSH test for sensitivity to primaquine |
| Beutler et al. | 1955 | Development of the Heinz Bodies test for sensitivity to
primaquine |
| Alving, et al. | 1958 | Primaquine associated haemoglobin reduction observed
in individuals with intermediate activity |
| Childs, et al. | 1958 | |
| Gross, et al. | 1958 | |
| Tarlov, et al. | 1962 | |
| Brewer, et al. | 1960 * | Development of the methaemoglobin reduction test (MRT)
for sensitivity to primaquine |
| Stamatoyannopoulos, et al. | 1967 | Description of the enzymatic phenotypes in small samples
of known G6PD heterozygous females |
| Panizon, et al. | 1970 | |
| Rinaldi, et al. | 1976 | |
| Beutler et al. | 1977 | Gold standard spectrophotometric assay |
| Van Noorden, et al. | 1985 | Description of new or improved cytochemical techniques
for detection of G6PD in erythrocytes |
| Vives-Corrons, et al. | 1986 | |
| Vogels, et al. | 1986 | |
| Fanello, et al. | 2008 | Dapsone associated haemolysis in G6PD heterozygous
females (no phenotype) |
| Premji, et al. | 2009 | |
| Tiono, et al. | 2009 | |
| Pamba, et al. | 2012 | |
| Shah, et al. | 2012 | Development of the cytofluorometric reading of MRT |
| Chu, et al. | 2017 | Primaquine and tafenoquine associated haemolysis in
G6PD heterozygous females with intermediate activity |
| Rueangweerayut, et al. | 2017 |
* For ease of visualization, this article is listed out of chronological order
Other than the malaria radical curative indication, quantitative screening for G6PD deficiency can be expanded also geographically to clinical scenarios to improve patient care in females with intermediate G6PD status (where evidence is available) with an additional benefit of cost sharing:
-
(i)
Newborn screening to identify risk for pathologic hyperbilirubinaemia. Evidence indicates that qualitative G6PD tests do not detect all clinically relevant cases of G6PD deficiency, particularly in female neonates 24– 28. This results in comparatively less emphasis on the clinical management of female neonates who have a risk of developing pathologic levels of serum bilirubin.
-
(ii)
The use of dapsone through the antimalarial drug chloroguanil-dapsone-artesunate (not used in malaria treatment anymore but is used for other medical indications). Individual study analysis of phase 3 clinical trials with chloroguanil-dapsone-artesunate did not identify risk of severe haemolysis in G6PD heterozygous females 29, 30. However, a complete analysis of the data, including 200 heterozygous females, demonstrated that heterozygous females showed a wide range of reactions, from large to imperceptible haemoglobin drops 31. One published case report describes a Greek female who tested G6PD normal by a screening test then experienced severe haemolysis to dapsone; quantitative testing was recommended 32.
-
(iii)
Guidelines for rasburicase therapy in the context of genotyping. The Clinical Pharmacogenetics Implementation Consortium published guidelines that recognise the limitations of G6PD genotype results to inform the use of rasburicase therapy in females for management of tumor lysis syndrome as G6PD genotyping does not correlate with the three phenotypes in heterozygous females 33, 34.
With the introduction of new quantitative point-of-care G6PD diagnostics, the three G6PD phenotypes can be detected accurately and characterised precisely for the first time without the need for expensive, complicated assays that require good laboratory infrastructure. Immediate results can be obtained at the patient level in the clinic. Quantitative G6PD testing could be used prior to prescription of other haemolytic agents (such as co-trimoxazole or nitrofurantoin) and new data could contribute to the literature on their effects in heterozygous females with intermediate G6PD activity. Where G6PD testing is driven by malaria case management, often rural and lower socioeconomic populations will benefit, as these populations often experience higher rates of malaria infection. Recent renewed attention to the characterisation at a cellular level of heterozygous G6PD deficiency may help to raise awareness of the clinical implications of intermediate G6PD activity in females 35– 37.
Plasmodium vivax case management
A significant barrier to safe radical cure of P. vivax malaria with 8-aminoquinolines is the limited availability of G6PD tests. Until recently, primaquine has been administered either with no G6PD testing or with the use of qualitative tests 38. Females in the lower range of intermediate G6PD activity are at risk of potentially clinically significant haemolysis from high dose 14-day primaquine (0.5mg/kg daily) and tafenoquine (300mg single dose) (Kozenis® and Krintafel®) 9, 13, 19, 39, 40. In a systematic review comparing treatment with chloroquine versus chloroquine and primaquine, G6PD “normal” (classified using qualitative tests) females taking primaquine had significantly greater haemoglobin reductions than males 41. Recent studies exploring the efficacy of a high dose short course (7-day) primaquine regimen (1mg/kg) have shown consistently that females classified as normal by the fluorescent spot test are at risk for clinically significant primaquine-induced haemolysis 19, 40. One of the studies was carried out in an area with a high prevalence (15–18%) of G6PD deficiency; heterozygous females showed a significantly greater drop in haematocrit as compared to wild type homozygous females given the same treatments, and 2/16 females receiving dihydroartemisinin-piperaquine with the 7-day primaquine regimen needed a blood transfusion 18, 40. In the second study, 2 females from the site in Hanura, Indonesia had clinically significant haemolysis (a 31–40% haemoglobin reduction from pre-treatment) where 229 total participants were treated with the 7-day primaquine regimen 19. Prevalence of G6PD deficiency in the area was unknown but previous reports in West Timor region gave a prevalence of 3.2% among males 42. In clinical studies using tafenoquine 300mg (equivalent to the 14-day low dose primaquine regimen) where females with intermediate G6PD activity were excluded, no haemolysis-related adverse events were observed 11, 12. The inability to diagnose intermediate G6PD activity can negatively impact the safe radical cure of P. vivax malaria with high dose 14 or 7-day primaquine regimens and tafenoquine 300mg single dose in girls and women. The risk of haemolysis in malaria-endemic locations is concerning because of low access to medical supervision and health facilities where haemolytic events can be detected with haemoglobin testing and managed with blood transfusion.
From a policy and clinical practice perspective if national malaria programs decide to support radical cure with primaquine in the absence of G6PD testing, important ethical considerations will be raised and difficult tradeoffs between ensuring patient safety and expanding access to critical treatments must be made. In places where no testing is done, more males are at risk of severe haemolysis simply because there are more G6PD deficient males than females. In places where only qualitative testing is done, malaria programs may not be comfortable treating women given the limitations of that platform, as explained above. As such, some policies may indicate that radical cure be given only to males after qualitative G6PD testing at the point of care and all females referred to a health facility. In the absence of testing, practitioners may also assume all females (normal, intermediate and deficient) are G6PD deficient and they will be given the 8-week high dose primaquine regimen recommended for G6PD deficient individuals. These policies and practices generally are not standardized and can change quickly based on new information and varying perceptions of risk among key decision-makers. Decisions to include qualitative G6PD testing in clinical guidelines or use them as a matter of policy do provide broader access to G6PD testing and improve health disparities in malaria treatment. However, the benefit of this expanded access is mostly in males who have the most severe G6PD deficiency and are at highest risk of severe haemolysis. Alternatively, quantitative G6PD testing allows equal access to an accurate G6PD diagnosis, safe treatment and convenient health care delivery.
Anticipation of mandatory quantitative G6PD testing to support anti-relapse treatment of P. vivax with tafenoquine has spurred the development of new quantitative point-of-care G6PD diagnostic tests, which only recently have become commercially available. These tests demonstrate greater accuracy in identifying G6PD deficiency in females, including those with the intermediate phenotype 43, 44. A diagnosis of G6PD intermediate status presents an important opportunity to address disparities in appropriate treatment and care 17, such as providers’ low understanding and recognition of haemolytic responses, patients’ low awareness of adverse symptoms and the need for prompt follow-up, and appropriate genetic counseling.
Increased newborn screening for G6PD deficiency
The diagnostic gap for G6PD testing extends to newborn screening globally. Newborn screening for G6PD deficiency has been recommended by the World Health Organization among populations where 3–5% of males are affected 45. It has been recognised that even if the majority of G6PD deficient patients are asymptomatic as children and adults, they have an increased risk of kernicterus resulting from significant neonatal hyperbilirubinaemia 46. Screening for G6PD deficiency is recommended in newborns with jaundice, especially when family history or background suggest the likelihood of G6PD deficiency, or when the response to phototherapy is poor 45, 47– 50. Nonetheless, there is a high heterogeneity in practice among different countries and within countries between rural and urban areas, with urban and peri-urban areas having greater access to G6PD screening programs. The same heterogeneity applies to screening methods, with G6PD deficiency screening performed in some settings via high accuracy, gold-standard quantitative spectrophotometric methods, while in low-resource settings it is more commonly done using low-accuracy, qualitative diagnostic tests.
The diagnostic limitations of qualitative tests restrict the potential for downstream public health interventions to improve clinical care for all infants, particularly female infants. For example, a robust G6PD newborn screening program paired with health education programs implemented in Sassari District, Sardinia, Italy, resulted in a 75% reduction in G6PD deficiency–related complications, showing that individual diagnosis helped prevent haemolytic triggers in the at-risk population of young male children. The benefit was observed disproportionally in boys, suggesting the intervention had been less effective in girls, in part because girls with low-intermediate G6PD activity were misclassified as normal and not “at risk.” 51.
Most point-of-care tests for G6PD deficiency currently are not indicated for use with neonates and clinical studies to support this indication should be performed. Quantitative point-of-care G6PD tests should enable diagnosis of female newborns with the intermediate phenotype. This could improve management of neonatal hyperbilirubinaemia, including closer clinical follow-up with targeted early bilirubin testing, avoidance of haemolytic triggers, and focused parental support on signs and symptoms of hyperbilirubinaemia to prevent kernicterus 26– 28, 52. Additional studies using new quantitative point-of-care tests for G6PD deficiency in the first year of life will show whether it is possible to use the results obtained at birth to provide a definitive diagnosis of the phenotype at least as G6PD deficient, or normal with intermediate perhaps requiring further follow up. This, coupled with the capacity to retain data throughout a patient’s life via personal or health system records, may enable once-per-lifetime testing of G6PD status.
As with adult management of malaria, newborn screening with only qualitative G6PD tests will identify males who are most frequently at the highest risk of developing G6PD related complications in the early neonatal period but will miss G6PD intermediate females. This means that largely avoidable G6PD related complications in female infants may be managed insufficiently, again introducing health related gender disparities from birth 26– 28, 52. Using quantitative G6PD testing for newborn screening would eliminate this health disparity.
Counseling with G6PD testing
While G6PD deficiency testing has been conducted systematically in certain settings to support malaria treatment, it is not usually treated as a test requiring genetic counseling. Little guidance currently exists to help health care workers relay results to patients, explain the hereditary nature and X-linked inheritance pattern of the condition, and describe the clinical implications. While genetic counseling is still emerging in its application in low-resource settings 53– 55, the malaria field could draw lessons from genetic counseling in other domains, such as sickle cell disease, thalassaemia, other haemoglobinopathies, prenatal testing, and cancer genetics 56– 61. In many regions, where malaria is highly prevalent, inherited blood disorders overlap in prevalence, such that investment in genetic counseling capacity-building in these areas may be leveraged 22. This could be achieved by increasing the number of genetics professionals being trained annually and building such opportunities into genomics research projects, or by equipping other healthcare staff, including nurses and community healthcare workers to interpret genetic knowledge 62.
While an individual can potentially go through life unaware of his or her G6PD deficiency, there are still lifelong benefits to being informed. Particularly for women, awareness of G6PD status and understanding of inheritance bring a direct clinical advantage, with the added benefit of prompting testing in their newborns and other family members. In low-resource settings, screening for haemoglobinopathies often occurs too late to intervene, either during a pregnancy or after the birth of the severely sick child, bringing social stigma to the woman who “caused” the disease in the child 63. In contrast, for G6PD deficiency, an opportunity exists to develop genetic counseling specifically designed to minimise stigma and maximise the importance of knowing one’s G6PD status in order to prevent haemolytic triggers actively and inform other decisions throughout an individual’s lifespan, such as food and environmental factors to avoid. Counseling may be particularly important to clarify potential gender biases given the X-linked inheritance and variable penetrance amongst male and female individuals, as well as population biases due to increased screening of certain populations 64.
Community engagement and sensitisation
Community engagement is essential for creating awareness and minimising stigma 63. Extensive literature indicates the importance of community engagement and community leadership for the success of programs involving screening for genetic disorders 56, 59, 61, 65, 66, including some targeting G6PD deficiency in Sardinia, Italy 51. Lessons from studies performed at the community level for neglected tropical diseases suggest that coverage rates, adherence rates, and general acceptability of new interventions improve when communities and community leadership are involved 67, 68. For example, in a recent evaluation of rapid tests for onchocerciasis, participants reported that the manner in which new tests were introduced and results were provided influenced community perceptions of the acceptability of the tests and confidence in the test results 69.
These same strategies can be adapted and applied to the expansion of G6PD diagnostics. Women who learn their G6PD status—and who understand the broader consequences of their condition through enhanced genetic counseling—may encourage their family members to get tested. This could have an important ripple effect within families and communities in malaria-endemic settings, whereby more individuals seek testing (possibly independent of a malaria episode), learn their G6PD status, and use this information for decisions beyond malaria treatment.
Cost-effectiveness of G6PD testing
The health and economic advantages of introducing quantitative, point-of-care G6PD testing in malaria case management will be optimised if the clinical benefits of testing can be extended beyond the current primary indication, which is for malaria treatment. At a minimum, retention of the G6PD test result by the patient or the health system may eliminate the need to test again the next time a patient requires radical cure for P. vivax or develops a haemolytic crisis, during which testing would not be reliable 70. At a higher level, knowing one’s G6PD status at subsequent clinical visits would allow the patient and clinician to prevent a severe haemolytic crisis by avoiding oxidative medications, or if such a prescription is unavoidable, to closely monitor for signs of haemolysis so interventions could be performed earlier. This benefit could expand even further if, through appropriate community sensitisation and genetic counseling, a woman with intermediate G6PD activity seeks G6PD testing for her newborn. This could prompt closer follow-up care and avert hyperbilirubinaemia-related complications. For example, in Singapore, the implementation of universal screening for G6PD deficiency in the context of the Kernicterus Surveillance Programme has led to elimination of kernicterus in the country 71, 72, which is cost effective at the household and national level.
Clearly, when G6PD status can be retained by the individual or medical facility, repeated testing is not necessary, which in turn generates cost savings. However, when G6PD status cannot be reliably retained (e.g., in migratory populations) or requires confirmatory testing (i.e., initial test during an acute illness), repeated G6PD testing will reduce the cost-effectiveness of any health-related program, such as malaria elimination.
Current rough estimates indicate that a quantitative reader machine for G6PD (i.e., biosensor) will be in the range of 50–300USD, with test kits in the range of 3–5USD per unit; this will likely be less expensive than gold-standard spectrophotometric assay in terms of equipment (reader vs. temperature-controlled spectrophotometer), reagents (temperature-stable and ready-to-use vs. refrigerated), and operational time and time to result (clinical staff vs. specialised laboratory), but more expensive than the widely used qualitative fluorescent spot test or RDT. If only a G6PD qualitative RDT (1.75 USD per test) is used, the estimated cost per person over one year per episode is 39.10 USD in Thailand (Devine et al., 2017). However, the household and provider cost of one P. vivax malaria episode to an individual in the same country is estimated to be 150 USD (Devine et al., 2019).
Point-of-care quantitative tests could be used in secondary-level health care facilities. Introduction in selected primary-level facilities would depend on the ease of use by health care providers who deliver care at this level and is a considerable limitation to the implementation of quantitative G6PD testing in field settings. Depending on country settings, health seeking behaviors and healthcare access, a significant proportion of malaria patients would still not have access to safe radical cure unless radical cure can be administered safely by village health workers. Alternative service delivery models would need to be investigated to extend the reach of 8-aminoquinolines while ensuring safety. A combination of screening males with the more affordable qualitative test and females with the quantitative test may reduce commodity costs, but operational feasibility and costs of maintaining two products and training on their appropriate would need to be investigated.
With increased utilisation of and experience in quantitative G6PD testing, data will need to be captured to estimate precisely the associated costs and benefits across all performance domains. This will better inform future test development. It will also impact country-level decision-making in terms of cost trade-offs within limited health care budgets and acceptable costs for incorporating G6PD testing into health systems. The cost-effectiveness will be weighted in part by the prevalence of G6PD deficiency in a population. For example, if the prevalence is low or negligible, the benefits of testing for G6PD deficiency may be limited to the clinical benefit for which it was indicated, most likely radical cure of P. vivax malaria 73– 75. Because efficacy of radical cure is highly related to completion of primaquine regimens, ensuring dose adherence will greatly enhance the cost effectiveness; this is less of a challenge with the single day tafenoquine regimen. Deliberations over cost-benefit trade-offs at the country and district level should also include careful consideration of the ethical and equity considerations discussed above.
Conclusion
The sex-linked differences in G6PD deficiency and limitations of current G6PD diagnostic tools have led to a disparity in accurate G6PD diagnosis in females. The interaction of P. vivax malaria, G6PD status, 8-aminoquinolines, and treatment restrictions linked to pregnancy and the post-partum period results in a sex related inequity. Females are at higher risk for misclassification of a sex-linked disorder in women, low awareness of the risk for iatrogenic haemolysis of the G6PD intermediate phenotype, and no P. vivax anti-relapse vivax treatment for a large part of their reproductive life.
Standard high-dose primaquine and new anti-relapse treatment regimens against P. vivax malaria require G6PD testing. New, affordable, point-of-care quantitative G6PD diagnostics have been developed to support access to these drugs in malaria-endemic populations. In addition to the benefits at multiple levels of the health care system, these new diagnostic tools can help bring awareness to the front-line practitioner of the nuances of G6PD deficiency in females and the potential implications beyond malaria.
Furthermore, integration of G6PD test results across multiple clinical indications, such as hyperbilirubinaemia in neonates, will likely require improved genetic counseling, health systems strengthening, and improved record keeping and data management. In settings in which G6PD deficiency is prevalent, these efforts may result in greater cost-benefits beyond the use of G6PD tests for malaria treatment alone, and lead to more equitable malaria treatment.
Data availability
Underlying data
No data are associated with this article
Acknowledgments
The authors would like to acknowledge Athena Anderle and Ingela Ziemek for editorial support in the production of the manuscript.
Funding Statement
This work was supported by the Wellcome Trust through a Wellcome Trust Strategic Award [096527] and a Research Enrichment - Public Engagement Award to MK [200344]. CSC and GB are part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Unit, funded by the Wellcome Trust (United Kingdom) [A150007]. This work was funded by the United Kingdom’s Department for International Development (DFID) [204139], and the Bill & Melinda Gates Foundation [OPP1107113]. The findings and conclusions contained within are those of the authors and do not necessarily reflect the positions of DFID or the Bill & Melinda Gates Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Howes RE, Battle KE, Satyagraha AW, et al. : G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol. 2013;81:133–201. 10.1016/B978-0-12-407826-0.00004-7 [DOI] [PubMed] [Google Scholar]
- 2. Luzzatto L, Nannelli C, Notaro R: Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am. 2016;30(2):373–93. 10.1016/j.hoc.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 3. (WHO), W.H.O: World Malaria Report 2018.2018. Reference Source [Google Scholar]
- 4. Howes RE, Piel FB, Patil AP, et al. : G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9(11):e1001339. 10.1371/journal.pmed.1001339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howes RE, Battle KE, Mendis KN, et al. : Global Epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95(6 Suppl):15–34. 10.4269/ajtmh.16-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization: Control and elimination of Plasmodium vivax malaria—a technical brief.2015. Reference Source [Google Scholar]
- 7. Robinson LJ, Wampfler R, Betuela I, et al. : Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12(10):e1001891. 10.1371/journal.pmed.1001891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization: Guidelines for the treatment of malaria. Third edition.2015. Reference Source [Google Scholar]
- 9. Chu CS, Bancone G, Moore KA, et al. : Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Plasmodium vivax Malaria: A Nested Cohort in a Trial of Radical Curative Regimens. PLoS Med. 2017;14(2):e1002224. 10.1371/journal.pmed.1002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu CS, Freedman DO: Tafenoquine and G6PD: a primer for clinicians. J Travel Med. 2019;26(4): pii: taz023. 10.1093/jtm/taz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lacerda MVG, Llanos-Cuentas A, Krudsood S, et al. : Single-Dose Tafenoquine to Prevent Relapse of Plasmodium vivax Malaria. N Engl J Med. 2019;380(3):215–228. 10.1056/NEJMoa1710775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Llanos-Cuentas A, Lacerda MVG, Hien TT, et al. : Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria. N Engl J Med. 2019;380(3):229–241. 10.1056/NEJMoa1802537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rueangweerayut R, Bancone G, Harrell EJ, et al. : Hemolytic Potential of Tafenoquine in Female Volunteers Heterozygous for Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency ( G6PD Mahidol Variant) versus G6PD-Normal Volunteers. Am J Trop Med Hyg. 2017;97(3):702–711. 10.4269/ajtmh.16-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luzzatto L: G6PD deficiency: a polymorphism balanced by heterozygote advantage against malaria. Lancet Haematol. 2015;2(10):e400–1. 10.1016/S2352-3026(15)00191-X [DOI] [PubMed] [Google Scholar]
- 15. Domingo GJ, Advani N, Satyagraha AW, et al. : Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: challenges and opportunities. Int Health. 2019;11(1):7–14. 10.1093/inthealth/ihy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Domingo GJ, Satyagraha AW, Anvikar A, et al. : G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12: 391. 10.1186/1475-2875-12-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Seidlein L, Auburn S, Espino F, et al. : Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J. 2013;12: 112. 10.1186/1475-2875-12-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu CS, Bancone G, Nosten F, et al. : Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar J. 2018;17(1): 101. 10.1186/s12936-018-2248-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor WRJ, Thriemer K, von Seidlein L, et al. : Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet. 2019;394(10202):929–938. 10.1016/S0140-6736(19)31285-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sex and Gender Sensitive Research Call to Action Group: Sex and gender in health research: updating policy to reflect evidence. Med J Aust. 2019. 10.5694/mja2.50426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crow JF: Hardy, Weinberg and language impediments. Genetics. 1999;152(3):821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bancone G, Gilder ME, Chowwiwat N, et al. : Prevalences of inherited red blood cell disorders in pregnant women of different ethnicities living along the Thailand-Myanmar border [version 2; peer review: 2 approved]. Wellcome Open Res. 2017;2:72. 10.12688/wellcomeopenres.12338.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Broek L, Heylen E, van den Akker M: Glucose-6-phosphate dehydrogenase deficiency: not exclusively in males. Clin Case Rep. 2016;4(12):1135–1137. 10.1002/ccr3.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderle A, Bancone G, Domingo GJ, et al. : Point-of-Care Testing for G6PD Deficiency: Opportunities for Screening. Int J Neonatal Screen. 2018;4(4):34. 10.3390/ijns4040034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan M, Beutler E, Vreman HJ, et al. : Neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. Pediatrics. 1999;104(1 Pt 1):68–74. 10.1542/peds.104.1.68 [DOI] [PubMed] [Google Scholar]
- 26. Kaplan M, Hammerman C, Vreman HJ, et al. : Acute hemolysis and severe neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. J Pediatr. 2001;139(1):137–40. 10.1067/mpd.2001.115312 [DOI] [PubMed] [Google Scholar]
- 27. Riskin A, Gery N, Kugelman A, et al. : Glucose-6-phosphate dehydrogenase deficiency and borderline deficiency: association with neonatal hyperbilirubinemia. J Pediatr. 2012;161(2):191–6.e1. 10.1016/j.jpeds.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 28. Wang FL, Boo NY, Ainoon O, et al. : Comparison of detection of glucose-6-phosphate dehydrogenase deficiency using fluorescent spot test, enzyme assay and molecular method for prediction of severe neonatal hyperbilirubinaemia. Singapore Med J. 2009;50(1):62–7. [PubMed] [Google Scholar]
- 29. Premji Z, Umeh RE, Owusu-Agyei S, et al. : Chlorproguanil-dapsone-artesunate versus artemether-lumefantrine: a randomized, double-blind phase III trial in African children and adolescents with uncomplicated Plasmodium falciparum malaria. PLoS One. 2009;4(8):e6682. 10.1371/journal.pone.0006682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tiono AB, Dicko A, Ndububa DA, et al. : Chlorproguanil-dapsone-artesunate versus chlorproguanil-dapsone: a randomized, double-blind, phase III trial in African children, adolescents, and adults with uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2009;81(6):969–78. 10.4269/ajtmh.2009.09-0351 [DOI] [PubMed] [Google Scholar]
- 31. Pamba A, Richardson ND, Carter N, et al. : Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120(20):4123–33. 10.1182/blood-2012-03-416032 [DOI] [PubMed] [Google Scholar]
- 32. Todd P, Samaratunga IR, Pembroke A: Screening for glucose-6-phosphate dehydrogenase deficiency prior to dapsone therapy. Clin Exp Dermatol. 1994;19(3): 217–8. 10.1111/j.1365-2230.1994.tb01168.x [DOI] [PubMed] [Google Scholar]
- 33. Relling MV, McDonagh EM, Chang T, et al. : Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clin Pharmacol Ther. 2014;96(2):169–74. 10.1038/clpt.2014.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson KM, Yang W, Haidar CE, et al. : Concordance between glucose-6-phosphate dehydrogenase (G6PD) genotype and phenotype and rasburicase use in patients with hematologic malignancies. Pharmacogenomics J. 2019;19(3):305–314. 10.1038/s41397-018-0043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bancone G, Kalnoky M, Chu CS, et al. : The G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjects. Sci Rep. 2017;7(1):9822. 10.1038/s41598-017-10045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalnoky M, Bancone G, Kahn M, et al. : Cytochemical flow analysis of intracellular G6PD and aggregate analysis of mosaic G6PD expression. Eur J Haematol. 2018;100(3):294–303. 10.1111/ejh.13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters AL, Van Noorden CJ: Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem. 2009;57(11):1003–11. 10.1369/jhc.2009.953828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Recht J, Ashley EA, White NJ: Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis. 2018;12(4):e0006230. 10.1371/journal.pntd.0006230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brito-Sousa JD, Santos TC, Avalos S, et al. : Clinical Spectrum of Primaquine-induced Hemolysis in Glucose-6-Phosphate Dehydrogenase Deficiency: A 9-Year Hospitalization-based Study From the Brazilian Amazon. Clin Infect Dis. 2019;69(8):1440–1442. 10.1093/cid/ciz122 [DOI] [PubMed] [Google Scholar]
- 40. Chu CS, Phyo AP, Turner C, et al. : Chloroquine Versus Dihydroartemisinin-Piperaquine With Standard High-dose Primaquine Given Either for 7 Days or 14 Days in Plasmodium vivax Malaria. Clin Infect Dis. 2019;68(8):1311–1319. 10.1093/cid/ciy735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Commons RJ, Simpson JA, Thriemer K, et al. : The haematological consequences of Plasmodium vivax malaria after chloroquine treatment with and without primaquine: a WorldWide Antimalarial Resistance Network systematic review and individual patient data meta-analysis. BMC Med. 2019;17(1):151. 10.1186/s12916-019-1386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tantular IS, Matsuoka H, Kasahara Y, et al. : Incidence and mutation analysis of glucose-6-phosphate dehydrogenase deficiency in eastern Indonesian populations. Acta Med Okayama. 2010;64(6):367–73. 10.18926/AMO/41322 [DOI] [PubMed] [Google Scholar]
- 43. Alam MS, Kibria MG, Jahan N, et al. : Field evaluation of quantitative point of care diagnostics to measure glucose-6-phosphate dehydrogenase activity. PLoS One. 2018;13(11):e0206331. 10.1371/journal.pone.0206331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pal S, Bansil P, Bancone G, et al. : Evaluation of a Novel Quantitative Test for Glucose-6-Phosphate Dehydrogenase Deficiency: Bringing Quantitative Testing for Glucose-6-Phosphate Dehydrogenase Deficiency Closer to the Patient. Am J Trop Med Hyg. 2019;100(1):213–221. 10.4269/ajtmh.18-0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67(6):601–11. [PMC free article] [PubMed] [Google Scholar]
- 46. Bhutani VK, Maisels MJ, Stark AR, et al. : Management of jaundice and prevention of severe neonatal hyperbilirubinemia in infants >or=35 weeks gestation. Neonatology. 2008;94(1):63–7. 10.1159/000113463 [DOI] [PubMed] [Google Scholar]
- 47. Cunningham AD, Hwang S, Mochly-Rosen D: Glucose-6-Phosphate Dehydrogenase Deficiency and the Need for a Novel Treatment to Prevent Kernicterus. Clin Perinatol. 2016;43(2):341–54. 10.1016/j.clp.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaplan M, Hammerman C: Glucose-6-phosphate dehydrogenase deficiency and severe neonatal hyperbilirubinemia: a complexity of interactions between genes and environment. Semin Fetal Neonatal Med. 2010;15(3):148–56. 10.1016/j.siny.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 49. Olusanya BO, Emokpae AA, Zamora TG, et al. : Addressing the burden of neonatal hyperbilirubinaemia in countries with significant glucose-6-phosphate dehydrogenase deficiency. Acta Paediatr. 2014;103(11):1102–9. 10.1111/apa.12735 [DOI] [PubMed] [Google Scholar]
- 50. Olusanya BO, Ogunlesi TA, Kumar P, et al. : Management of late-preterm and term infants with hyperbilirubinaemia in resource-constrained settings. BMC Pediatr. 2015;15:39. 10.1186/s12887-015-0358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meloni T, Forteleoni G, Meloni GF: Marked decline of favism after neonatal glucose-6-phosphate dehydrogenase screening and health education: the northern Sardinian experience. Acta Haematol. 1992;87(1–2):29–31. 10.1159/000204709 [DOI] [PubMed] [Google Scholar]
- 52. Fu C, Luo S, Li Q, et al. : Newborn screening of glucose-6-phosphate dehydrogenase deficiency in Guangxi, China: determination of optimal cutoff value to identify heterozygous female neonates. Sci Rep. 2018;8(1):833. 10.1038/s41598-017-17667-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abacan M, Alsubaie L, Barlow-Stewart K, et al. : The Global State of the Genetic Counseling Profession. Eur J Hum Genet. 2019;27(2):183–197. 10.1038/s41431-018-0252-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marsh V, Kombe F, Fitzpatrick R, et al. : Consulting communities on feedback of genetic findings in international health research: sharing sickle cell disease and carrier information in coastal Kenya. BMC Med Ethics. 2013;14:41. 10.1186/1472-6939-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhong A, Darren B, Loiseau B, et al. : Ethical, social, and cultural issues related to clinical genetic testing and counseling in low- and middle-income countries: a systematic review. Genet Med. 2018. 10.1038/s41436-018-0090-9 [DOI] [PubMed] [Google Scholar]
- 56. Anie KA, Treadwell MJ, Grant AM, et al. : Community engagement to inform the development of a sickle cell counselor training and certification program in Ghana. J Community Genet. 2016;7(3):195–202. 10.1007/s12687-016-0267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Culhane-Pera KA, Moua M, Vue P, et al. : Leaves imitate trees: Minnesota Hmong concepts of heredity and applications to genomics research. J Community Genet. 2017;8(1):23–34. 10.1007/s12687-016-0284-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Culhane-Pera KA, Straka RJ, Moua M, et al. : Engaging Hmong adults in genomic and pharmacogenomic research: Toward reducing health disparities in genomic knowledge using a community-based participatory research approach. J Community Genet. 2017;8(2):117–125. 10.1007/s12687-017-0292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Green NS, Mathur S, Kiguli S, et al. : Family, Community, and Health System Considerations for Reducing the Burden of Pediatric Sickle Cell Disease in Uganda Through Newborn Screening. Glob Pediatr Health. 2016;3:2333794x16637767. 10.1177/2333794X16637767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. He LQ, Njambi L, Nyamori JM, et al. : Developing clinical cancer genetics services in resource-limited countries: the case of retinoblastoma in Kenya. Public Health Genomics. 2014;17(4):221–7. 10.1159/000363645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Housten AJ, Abel RA, Lindsey T, et al. : Feasibility of a Community-Based Sickle Cell Trait Testing and Counseling Program. J Health Dispar Res Pract. 2016;9(3): pii: 1. [PMC free article] [PubMed] [Google Scholar]
- 62. Wonkam A, de Vries J: Returning incidental findings in African genomics research. Nat Genet. 2020;52(1):17–20. 10.1038/s41588-019-0542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marsh VM, Kamuya DM, Molyneux SS: 'All her children are born that way': gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethn Health. 2011;16(4–5):343–59. 10.1080/13557858.2010.541903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ley B, Luter N, Espino FE, et al. : The challenges of introducing routine G6PD testing into radical cure: a workshop report. Malar J. 2015;14:377. 10.1186/s12936-015-0896-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cao A, Congiu R, Sollaino MC, et al. : Thalassaemia and glucose-6-phosphate dehydrogenase screening in 13- to 14-year-old students of the Sardinian population: preliminary findings. Community Genet. 2008;11(3):121–8. 10.1159/000113873 [DOI] [PubMed] [Google Scholar]
- 66. Hsu LL, Green NS, Donnell Ivy E, et al. : Community Health Workers as Support for Sickle Cell Care. Am J Prev Med. 2016;51(1 Suppl 1):S87–98. 10.1016/j.amepre.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Amazigo U, Crump A, Godal T: Recognising the role of community-directed treatment and of women in the fight against NTDs. Lancet Glob Health. 2017;5(6):e569–e570. 10.1016/S2214-109X(17)30171-7 [DOI] [PubMed] [Google Scholar]
- 68. Tchounkeu YF, Onyeneho NG, Wanji S, et al. : Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106(6):340–347. 10.1016/j.trstmh.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 69. Dieye Y, Storey HL, Barrett KL, et al. : Feasibility of utilizing the SD BIOLINE Onchocerciasis IgG4 rapid test in onchocerciasis surveillance in Senegal. PLoS Negl Trop Dis. 2017;11(10):e0005884. 10.1371/journal.pntd.0005884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chu CS, Bancone G, Soe NL, et al. : The impact of using primaquine without prior G6PD testing: a case series describing the obstacles to the medical management of haemolysis. Wellcome Open Res. 2019;4:25. 10.12688/wellcomeopenres.15100.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ho NK: Neonatal jaundice. A second 4-year experience in Toa Payoh Hospital (1986-1989). J Singapore Paediatr Soc. 1991;33(3–4):149–55. [PubMed] [Google Scholar]
- 72. Shah VA, Yeo CL: Identifying risk of neonatal hyperbilirubinaemia and early discharge for glucose-6-phosphate dehydrogenase deficient newborns in Singapore. Ann Acad Med Singapore. 2007;36(12):1003–9. [PubMed] [Google Scholar]
- 73. Devine A, Parmiter M, Chu CS, et al. : Using G6PD tests to enable the safe treatment of Plasmodium vivax infections with primaquine on the Thailand-Myanmar border: A cost-effectiveness analysis. PLoS Negl Trop Dis. 2017;11(5):e0005602. 10.1371/journal.pntd.0005602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peixoto HM, Brito MA, Romero GA, et al. : Cost-effectiveness analysis of rapid diagnostic tests for G6PD deficiency in patients with Plasmodium vivax malaria in the Brazilian Amazon. Malar J. 2016;15:82 10.1186/s12936-016-1140-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu JZ, Francis RO, Lerebours Nadal LE, et al. : G6PD Deficiency in an HIV Clinic Setting in the Dominican Republic. Am J Trop Med Hyg. 2015;93(4):722–9. 10.4269/ajtmh.14-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]