Abstract
Background:
Diabetic (DM) inactivation of small conductance calcium-activated potassium (SK) channels contributes to coronary endothelial dysfunction. However, the mechanisms responsible for this down-regulation of endothelial SK channels are poorly understood. Thus, we hypothesized that the altered metabolic signaling in diabetes regulates endothelial SK channels and human coronary microvascular function.
Methods:
Human atrial tissue, coronary arterioles and coronary artery endothelial cells (HCAECs) obtained from DM and non-diabetic (ND) patients (n = 12/group) undergoing cardiac surgery were used to analyze metabolic alterations and endothelial SK channel function, coronary microvascular reactivity and SK gene/protein expression/localization.
Results:
The relaxation response of DM coronary arterioles to the selective SK channel activator SKA-31 and calcium ionophore A23187 was significantly decreased compared to that of ND arterioles (p<0.05). Diabetes increases the level of NADH and the NADH/NAD+ ratio in human myocardium and HCAECs p<0.05). Increase in intracellular NADH (100 μM) in the HCAECs caused a significant decrease in SK channel currents in HCAECs (p<0.05), whereas, intracellular application of NAD+ (500 μM) increased the endothelial SK channel currents (p<0.05). Mitochondrial reactive oxygen species (mROS) of HCAECs and NADPH oxidase (NOX) and PKC protein expression in the human myocardium and coronary microvasculature were increased respectively (p<0.05).
Conclusions:
Diabetes is associated with metabolic changes in the human myocardium, coronary microvasculature and HCAECs. Endothelial SK channel function is regulated by the metabolite pyridine nucleotides, NADH and NAD+, suggesting that metabolic regulation of endothelial SK channels may contribute to coronary endothelial dysfunction in the patients with diabetes.
Keywords: Metabolic syndrome, Diabetes, SK channels, Coronary microcirculation, Endothelial function, Endothelium-dependent hyperpolarization
Graphical abstract

INTRODUCTION
Diabetes mellitus (DM) affects more than 20% of the United States population and is increasing dramatically in prevalence.1 Patients with DM have a significantly higher risk for coronary macrovascular and microvascular diseases than patients without.2–4 DM is associated with impairment of microvascular endothelial function2 and dysregulation of coronary blood flow, which in turn could underlie decreased cardiac function and result in increased cardiovascular morbidity and mortality.1, 5–8 Regulation of electrical signaling in the coronary endothelial cells plays a key role in endothelial function.8–11 This electrical signaling is mediated by small conductance calcium-activated-potassium channels (SK), which are largely responsible for coronary arteriolar relaxation mediated by endothelium-dependent hyperpolarizing factors (EDHF). 8–12 Previous studies reported that DM reduced endothelial SK channel currents, and accordingly endothelial dependent hyperpolarization and peripheral vascular relaxation in rodent models.13 Inactivation of endothelial SK channels in this patient population contributes to human coronary arteriolar endothelial dysfunction and impaired microvascular relaxation.14, 15 Inhibition of SK channels also contributes to coronary/peripheral microvascular dysfunction after cardioplegic ischemia and reperfusion (CP-I/R) in animals and humans, whereas, SK inhibition is more pronounced in DM patients. 14–17
However, the precise mechanisms responsible for DM dysregulation of SK channels and coronary endothelial function are still undefined. Recent study indicates that metabolites,18 such as, pyridine nucleotides can regulate ion channels, such as cardiac sodium channels (INa) 19–22 voltage-gated potassium channels (Kv) of smooth muscle cells23, 24 and large conductance calcium-activated potassium (BK) channels of pulmonary arterial smooth muscle cells.25 However, little is known on the role of pyridine nucleotides on DM dysregulation of coronary endothelial SK channels. Thus, we hypothesized that the altered metabolic signaling in DM dysregulates endothelial SK channels and contributes to coronary microvascular endothelial dysfunction.
The goal of this study is to elucidate the role of metabolic signaling during diabetes on coronary endothelial SK channel function and coronary microvascular relaxation. Specifically, using human atrial tissue samples, in-vitro coronary arterioles and human coronary artery endothelial cells (HCAECs), we examined the metabolic changes in human myocardium and HCAECs, measured the effect of metabolic molecules on SK currents in HCAECs, and evaluated the relaxation response of human coronary arterioles to selective SK activators.
MATERIALS and METHODS
Human Subjects and Tissue Harvesting
The discarded right-atrial-tissue samples were harvested from patients (n = 24) undergoing cardiac surgery during right atrial cannulation and before exposure of the heart to CP-I/R. 14–16 All procedures were approved by the Institutional Review Board (IRB) of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients.
Cell Culture
Human coronary artery endothelial cells (HCAECs, passage 3) harvested from donors (patients) with and without diabetes (Lonza, Walkersville, MD) were cultured and grown in the EGMTM-2 Bullet Kit medium (Lonza) in a humidified incubator with 5%CO2 at 37°C according to the manufacturer’s protocols. 14, 15
Microvessel Reactivity
The methods for measurement of in-vitro microvessel reactivity have been described previously.14–16 Coronary arterioles (80 to150 μm internal diameters) were dissected from harvested right atrial appendage-tissue-samples during atrial cannulation before the onset of CP-I/R. After a 60-minute stabilization period in the organ chamber, the microvessels were pre-constricted with endothelin-1 (10−8-10−7M) to 30–50% of the baseline diameter. After achievement of this constricted steady state, dose-dependent relaxation was measured in response to the application of the following vasodilators: the selective SK channel activator SKA-31 (10−9-10−5M), or the endothelium-dependent, receptor-independent vasodilator A23187 (10−9-10−5M). One or 3 interventions were performed on each vessel. The order of drug administration was random. In some cases, endothelial denudation was carried out by the intraluminal injection of air bubbles and all vessels denuded of endothelium showed complete relaxation to sodium nitroprusside.
Real-Time PCR Quantification
Total RNA from flash-frozen human atrial tissue samples was isolated using the RNeasy Mini plus Kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction and was reverse transcribed into complementary DNA (cDNA) using SuperScript® VILO™ Master Mix (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s protocol. Quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) was carried out using gene-specific primers, Fast SYBR® Green Master Mix (Thermo Fisher Scientific, Waltham, MA) and 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Forward primers: AAGCGACTGAGTGACTATGCTC (hSK3); GCTGGCAGGAACTGGCATTG (hSK4); TCGTGGAAGGACTCATGACCA(hGAPDH). Reverse primers: TCACGAAGAGCTGGACTTCAC(hSK3); AAGGCCACGATGAGGCAGAG(hSK4); CGCCAGTAGAGGCAGGGATG (hGAPDH); The qRT-PCR reaction was activated with an initial denaturation step at 95°C for 20 s, followed by cycles of denaturation at 95°C for 3 s, and annealing and extension at 60°C for 30 s. Samples were run in triplicate and averaged. Gene expression levels were normalized to the level of GAPDH.
Immunoblot
Atrial tissues samples and HCAECs were solubilized in SDS-PAGE buffer. Total protein (40μg) was fractionated on an 8% to 16% SDS-PAGE and then transferred to a polyvinylidene diflouride membrane (Immobilon-P; Millipore Corporation, Bedford, Mass) as previously described.11–12 Membranes were incubated for 1 hour at room temperature with 1:200 dilutions of individual rabbit polyclonal primary antibodies to SK-3, SK-4(IK-1) (Alomone Labs Ltd, Jerusalem, Israel). The membranes were then incubated for 1 hour with horseradish peroxidase-conjugated secondary anti-Ig, and washed 3 times in Tris saline buffer. Peroxidase activity was visualized with enhanced chemiluminescence (Thermo Scientific) and the images were captured with a digital camera system (G Box, Syngene, Cambridge, UK). The western blot bands were quantified with densitometry using ImageJ software (National Institute of Health, Bethesda, MD). Specificities of the anti-SK-3, and anti-SK-4 (IK) antibodies were demonstrated in previous studies, respectively.14–16
Liquid Chromatography-tandem to Mass Spectrometry (LC/MS-MS)
Polar metabolites were extracted from 100 mg flash-frozen atrial tissue samples with 1ml of ice-cold 80% (v/v) methanol and 0.6 ml acetonitrile and analyzed using a 5500 QTRAP hybrid triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC HPLC system (Shimadzu) with SRM.26 Peak areas from the total ion current for each metabolite SRM transition were integrated using MultiQuant v2.0 software (AB/SCIEX). LC/MS-MS was run independently for samples from n=4/group. Data analysis was performed using Metabo Analyst 3.027
Two-dimensional Thin Layer Chromatography (2D TLC)
Human atrial tissue samples (n = 4/group) were extracted with ice-cold 80% (v/v) methanol and 0.6 ml acetonitrile and the supernatant was separated by 2D TLC on a silica gel-coated aluminum plate with a fluorescent indicator 254 nm (Sigma-Aldrich). The first separation was conducted in the solvent system Isopropanol, NH4OH, and water, in volume proportions (6:3:1); followed by the second dimension in the solvent system N-butanol, acetone, acetic acid, 5% ammonium hydroxide, and water, in volume proportions (9:3:2:2:4). Individual spots were identified in UV light based on the migration of the standard molecule alone.
Measurements of NADH and NAD+ in HCAECs
Human myocardial and HCAECs levels of NADH and NAD+ were also, quantitatively measured from ND and DM atrial tissue and HCAECs homogenates using the EnzyChrom NAD+/NADH Assay Kit, according to the manufacturer’s protocol (Bioassay Systems). NADH and NAD+ concentrations in the sample were determined by colorimetric measurement at 565 nm and the NAD+ standard curves.19, 28
Patch-Clamp Recording of Endothelial K+ Currents
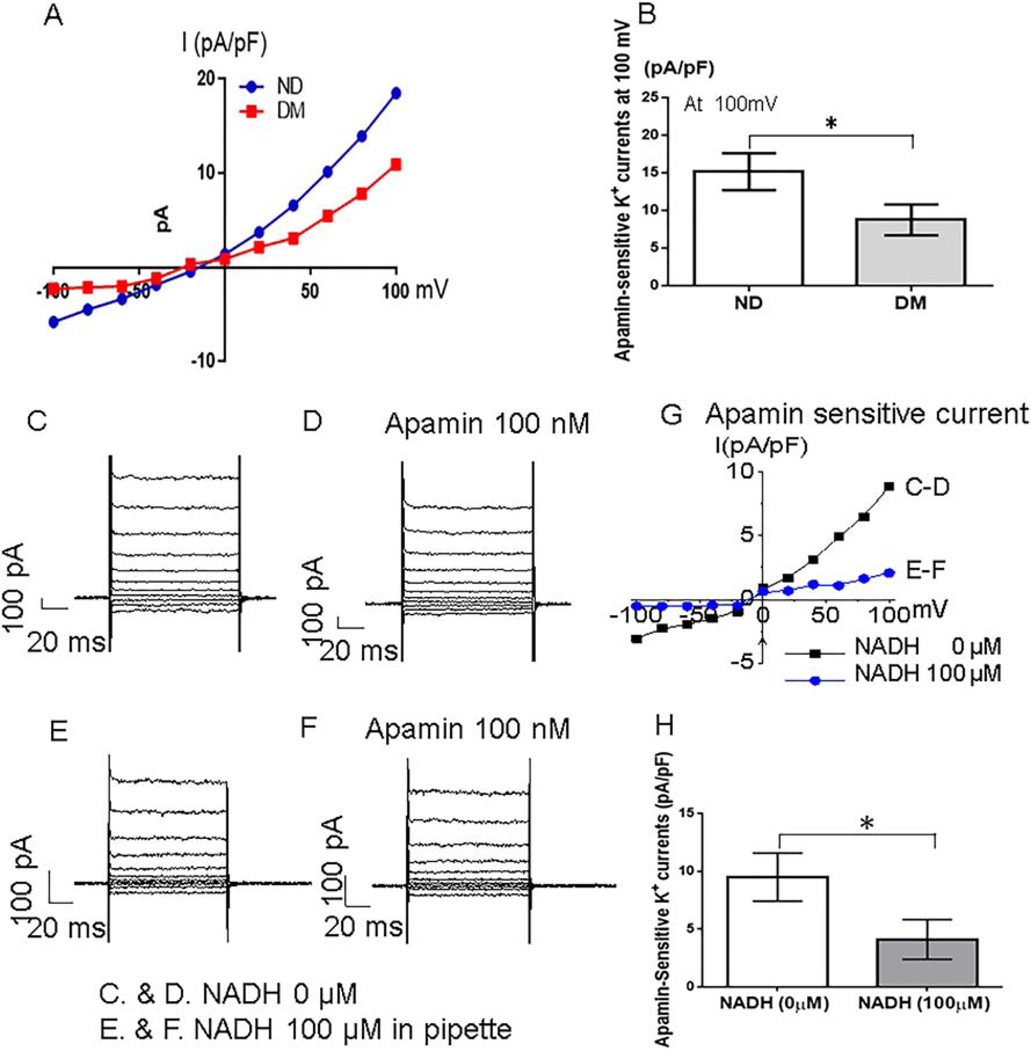
The primarily cultured HCAECs (passage 3) were washed twice with Ca2+− free DMEM, incubated with 0.05% trypsin and 0.02% EDTA for 1–2 mi.14 An Axopatch-200B amplifier, digidata 1440a A/D converter, pClamp 10 software (Molecular Devices, Foster City, USA) were used to record K+ currents of HCAECs in the whole-cell configuration in the voltage-clamp mode. The bath solution contained (in mM): 140 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, 30 glucose (pH 7.4; 22°C). The patch pipette resistance was 1–3 MΩ and filled with the pipette solution contained (in mM): 110 K-Aspartate, 20 KCl, 1 MgCl2, 8.5 CaCl2, 10 HEPES, 8 NaCl, 0.01 Niflumic acid and 10 BAPTA (pH 7.2, with calculated free Ca2+ 400 nmol/L). The cells were depolarized every 5s from a holding potential of −50 mV by 150 ms test pulses in the range between −100 to +100 mV in 20mV increments. Low-pass filter frequency was 2 kHz and sampling rate was 10 kHz. The selective SK blocker apamin was used to identify SK channels current in HCAECs. The effects of intracellular NADH (100μM) or NAD+ (500μM) on K+ currents were examined in the presence or absence apamin (100 nM).14 Experiments shown in Figure 3. were conducted at room temperature (20°−22°C), while the experiments shown in Figure 4 were conducted at 36°C.
Figure 3.
Primary-cultured human coronary arterial endothelial cells (HCAECs) of diabetics (DM) has less SK channels current than of non-diabetics (ND). A, An examples of basal whole-cell I-V relationships in HCAECs of ND and DM at room temperature. Currents were elicited by 20 mV step pulses between −100 and +100 mV from a holding potential of −50 mV with 400nM free calcium pipette solution. B, Bar graph shows apamin-sensitive component of potassium current at +100 mV in DM and ND HCAECs, *p<0.05 vs. ND, Mean ± SD, n = 5/group. NADH inhibits apamin-sensitive current in HCAECs; C and D, Representative whole cell current traces of HCAECs in control solution at room temperature (C), and in the presence of apamin (100 nM) (D). E, The traces in the presence of 100 μM intracellular NADH and no apamin. F, The same as (C), but with apamin in the bath solution. G, I-V curves obtained from data shown in A-D. H, Cumulative bar graph shows apamin-sensitive potassium currents at +100 mV with or without intracellular NADH. (N = 4/group. Mean ± SD, *p<0.05).
Figure 4.
Intracellular NAD+ significantly increases apamin-sensitive potassium current in DM HCAECs. A, Representative traces of the whole cell currents of HCAECs at holding potential of −50 mV and test potentials from −100 to +100 mV in 20 mV increments in control solution at 36°C. B and C, show representative traces in the presence of 500 μM intracellular NAD+ in the absence (B) or presence (C) of 100 nM extracellular apamin. D, Cumulative I-V curves in the absence of the drugs (black), and with intracellular NAD+ (red and blue), and with extracellular apamin (blue). E, At +100 mV, the effect of intracellular NAD+ is significant (* p<0.05). However, the increase in the outward potassium current is completely blocked by extracellular apamin (* p<0.05).
Measurement of mROS in the HCAECs
HCAECs were loaded with 5 μmol/L MitoSox Red and 100 nmol/L MitoTracker Green FM (Invitrogen) for 10 min at 37 °C. Images were taken on a Zeiss LSM710 confocal microscope (Carl Zeiss GmbH, Germany) using an argon laser excitation (514 nm) with emission collection through a 560 nm long pass filter. The mean values of the whole cell fluorescence of MitoSOX™ Red were obtained with ImageJ software.29
Chemicals
A23187, Endothelin-1 and SKA-31 were obtained from Sigma-Aldrich and dissolved in ultrapure distilled water on the day of the study.
Data Analysis
Data are presented as the standard deviation (SD) or mean and standard error of the mean (SEM). Microvessel responses are expressed as percent relaxation of the pre-constricted diameter. Microvascular reactivity was analyzed using 2 way repeated-measures ANOVA with a post hoc Bonferroni test. Clinical, Western blot and Oxyblot data were analyzed by Student t-test or χ2 test (GraphPad Software, Inc, San Diego, CA). P values < 0.05 were considered significant.
RESULTS
Patient Characteristics
The patient characteristics are listed in table 1. All patients with preoperative hypertension were on anti-hypertensive medication (β-blocker, aspirin, calcium channel blocker, or angiotensin-converting enzyme inhibitor). The pre-operative blood hemoglobin A1c (Hgb A1c) levels were 8.2 ± 0.84 in the DM patients, and 5.3 ± 0.33 in the ND patients ( n =12/group).
Table 1:
Patient Characteristics
| Patient Characteristics | ND | DM | P values |
|---|---|---|---|
| Age (y)* | 73 ± 10 | 69 ± 9.0 | 0.40 |
| Number of cases | 12 | 12 | 1.00 |
| Male n(%) | 10 (%) | 9 (%) | 1.00 |
| HbA1c (%)* | 5.3 ± 0.33 | 8.2 ± 0.84 | 0.0001 |
| Blood Glucose (mg/dL)* | 112 ± 14.0 | 156 ± 20.2 | 0.0005 |
| Obesity (BMI>30) n(%) | 3 (25.0%) | 7 (58.3%) | 0.21 |
| Hypertension n(%) | 3 (25.0%) | 9 (75%) | 0.04 |
| Atrial fibrillation n(%) | 1 (8.3%) | 1 (8.3%) | 1.00 |
| Heart failure n(%) | 1 (8.3%) | 2 (16.7%) | 1.00 |
| Hypercholesterolemia n(%) | 3 (25.0%) | 9 (75%) | 0.04 |
| Valve replacement n(%) | 9 (75%) | 1 (8.3%) | 0.003 |
| CABG only n(%) | 0 (%) | 9 (75%) | 0.0003 |
| CABG + Valve replacement n(%) | 2 (16.6%) | 2 (16.7%) | 1.00 |
| Pre-operative aspirin n(%) | 3 (25.0%) | 8 (66.7%) | 0.09 |
| Pre-operative β-blocker n(%) | 1 (8.3%) | 7 (58.3%) | 0.03 |
| Pre-operative CaCB n(%) | 1 (8.3%) | 5 (41.6%) | 0.16 |
| Pre-operative ACEI n(%) | 2 (16.7%) | 6 (50.0%) | 0.19 |
| Pre-operative statin n(%) | 3 (25.0%) | 9 (75.0%) | 0.04 |
ND: non-diabetes; DM: diabetes; BMI: body mass index; CABG: coronary artery bypass grafting; CaCB: calcium channel blocker; ACEI: angiotensin converting enzyme inhibitor
Data expressed as mean ± SD.
Microvascular Reactivity
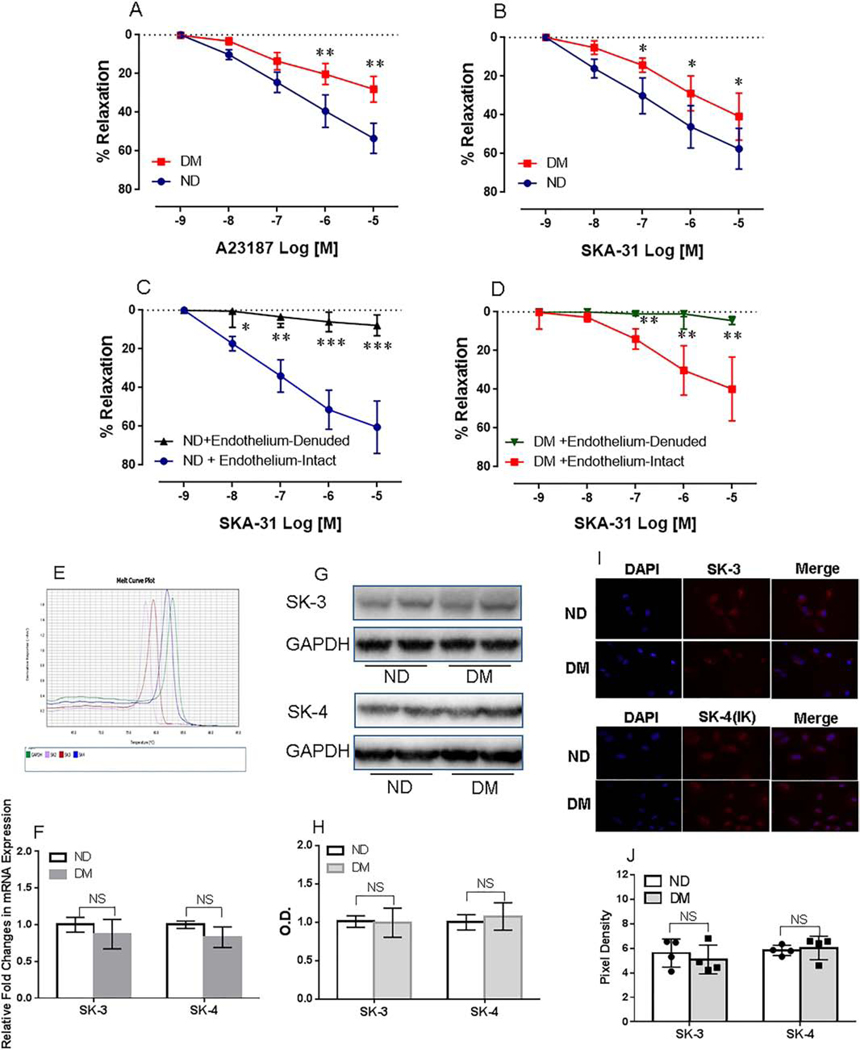
There were no significant differences in the basal microvessel diameters between ND (120 ± 23) and DM (125 + 21) groups (p =0.21). The degree of pre-contraction induced by endothelin-1 (36 ± 6% in the ND group, and 31 ± 5 % in the DM group, respectively) were similar. Both of the calcium ionophore A23187 (10−9-10−5M, Figure 1A) and the selective SK channel activator SKA-31 (10−9-10−5M, Figure 1B) caused dose-dependent relaxation responses. However, the relaxation responses of coronary arterioles to A23187 (Figure 1A) and SKA-31 (Figure 1B) were more pronounced in the ND group than that of DM group respectively. In addition, the relaxation responses of diabetic and non-diabetic arterioles to SKA-31 were abolished by endothelium denudation (Figure 1C and Figure D).
Figure 1.
Effects of SK channels modulators on human coronary microvascular vasodilation. A, Dose-dependent vasodilation of human coronary microvessels in response to the endothelium-dependent, receptor independent vasodilator A23187 (10−9−10−5M), n =12/group, **p<0.001; B, Dose-dependent vasodilation of human coronary arterioles in response to the selective SK channel activator SKA-31 (10−9-10−5M) from patients with or without DM, n =12/group; *p<0.05. C, Coronary microvascular vasodilation in response to the selective SK channel activator SKA-31 (10−9-10−5M) in the presence or absence of endothelium denudation in the non-diabetic (ND) vessels, n= 5/group, *p<0.05 or **p<0.001 or ****p<0.0001 vs. Endothelium-intact; D, Coronary microvascular vasodilation in response to the selective SK channel activator SKA-31 (10−9-10−5M) in the presence or absence of endothelium in the diabetic (DM) vessels, n = 5/group, **p<0.001 vs. Endothelium-intact, Mean ± SD; E, Traces showing the primers of SK-3 and SK-4 specificity; F, Bar graph showing the fold changes in SK-3 and SK-4 mRNA expression of atrial tissue samples collected from diabetic and non-diabetic patients, n = 4/group, mean ± SD; G, Representative immunoblots of human right atrial tissue lysates for SK-3 and SK-4 (IK-1); H, Densitometric analysis of signal intensity (fold changes) in the SK-3 and SK-4 protein expression of atrial tissue samples obtained from diabetic (DM) and non-diabetic patients (ND), n =6/group; I, Immunofluorescence staining of SK-3 and SK-4 (IK-1) in the cultured human coronary arterial endothelial cells (HCAECs) from diabetic (DM) and non-diabetic (ND) patients. J, Densitometric analysis of optical density, mean ± SD, n = 4/group; NS = no significances.
SK Channels Gene/Protein Expression and Localization
There were no significant differences in SK-3 and SK-4 (IK) mRNA (Figure 1E, F) and protein expression (Figure 1G,H) of atrial tissues between ND and DM. There were no significant differences in SK-3 and SK-4 (IK) distributions in the HCAECs between ND and DM groups (Figure 1I,J).
Metabolic Changes in the Human Atrial Myocardium
2D TLC indicates for altered trends in content of ATP, ADP, NAD and NADH under DM conditions (Figure 2A). Quantitative LC/MS-MS analysis revealed a significant decrease of ATP in the DM myocardium, but not ADP compared to non-diabetic group (Figure 2B, C). In the atrial myocardium (Figure 2D) and HCAECs (Figure 2E), diabetes slightly and insignificantly enhanced NAD+, but significantly increased NADH and ratio of NADH/NAD+ compared to ND tissue and cells (p<0.05, Figure 2D, and Figure 2E).
Figure 2.
NADH/NAD+ ratio is increased on DM relatively to ND group. A, Representative 2D TLC (two-dimensional thin layer chromatography) separation of ATP, ADP, NADH, and NAD+ in DM and ND human right-atrial tissue samples. The migration positions of the metabolites is shown with 1, 2, at the right of the TLC; B and C, LC-MS-MS identified altered ATP (B) and ADP (C) levels in atrial myocardium from diabetic (DM) and non-diabetic (ND) patients; The bar diagrams of LC-MS-MS showing the metabolite content based on the average spot intensity (pixels) after background correction. The bars represent the average value of 6 independent extractions with 50 mg of tissue, n=4/group, Values are mean ± SD; *p = 0.02; D, Atrial tissue levels of NAD+, NADH and the ratio of NADH/NAD+ from diabetic (DM) and non-diabetic (ND) patients by using the EnzyChrom NAD+/NADH Assay Kit; n = 12/group, *p<0.05 vs. ND; E, Intracellular levels of NADH, NAD+ and the ratio of NADH/NAD+ diabetic (DM) and non-diabetic (ND) HCAECs measured by using the EnzyChrom NAD+/NADH Assay Kit, n = 4/group, Mean ± SD, *p<0.05 vs. ND.
Metabolic Regulation of Endothelial SK Channel Function
The apamin-sensitive component of the HCAECs’ currents was significantly decreased in the DM HCAECs compared to ND HCAECs (Figure 3A and Figure 3B). Interestingly, either extracellular application of highly selective SK channel blocker apamin (100nM, Figure 3D,G) alone or administration of intracellular NADH+ (100 μM, Figure 3E,H) caused a significant decrease in endothelial K+ currents. However, when HCAECs were pre-incubated with apamin (100nM), NADH failed to induce additional inhibition of the currents (Figure 3F,G), indicating that NADH inhibition of endothelial K+ currents was via inhibition of SK channels. At 100mV, NADH significantly decreased the apamin-sensitive K+ currents (*p<0.05, Figure 3H).
In contrast, intracellular NAD+ (500μM) significantly increased the whole cell currents in the HCAECs by about 63% (Figure 4, *p<0.05). However, this increase was blocked in the presence of extracellular apamin (100 nM) (*p<0.05), implying that NAD+ activates SK channels. Thus, the increase in NADH/NAD+ ratio in the diabetic myocardium and diabetic endothelial cells relatively to ND (Figure 2D and Figure 2E) correlates with NADH inhibition (Figure 3C–H) and NAD+ activation (Figure 4) of SK channel currents, and is in agreement with the differences in DM and ND HCAECs.
DISCUSSION
We have previously observed that DM impairs coronary microvascular function through a decreased response to the endothelium-dependent vasodilator ADP, substance P and bradykinin.2, 3, 14, 15 In the present study, we observed that DM significantly reduced the relaxation response to the endothelium-dependent, receptor-independent vasodilator A23187 of human coronary arterioles as compared to response observed in patients without DM. We have previously reported the selective SK channel activator NS309 induced dose- and endothelium-dependent relaxation response of human coronary arterioles along with a diminished effect in vessels from patients with DM.14–16 In the current study, we found that another potent, selective SK channel activator, SKA-31, also significantly induced a dose- and endothelium-dependent relaxation response of human atrial coronary arterioles. Similarly, this effect was also reduced in patients with DM. These findings were consistent with previous studies, whereby endothelial SK currents were significantly reduced in diabetic HCAECs and therefore confirm that diabetic inhibition of endothelial SK channels contributes to the decreased relaxation response of coronary arterioles to the highly selective SK channel activator SKA-31.
The role of metabolic changes on coronary arterial function as well as vascular ion channels has been well studied by other investigators.7, 18, 30–36 It has been reported that metabolic changes can either downregulate or upregulate smooth muscle Kv (voltage dependent potassium channel) and BK (large conductance calcium-activated potassium channel) channel activities.7 For example, exposure of vessels to moderate metabolic stress was found to enhance smooth muscle Kv and BK activity/expression.32, 33, 35 In contrast, exposure to severe metabolic stress decreases smooth muscle Kv and BK activity.18, 30, 31, 36 It can be postulated, therefore, that DM may downregulate endothelial SK channels and upregulate smooth muscle Kv and BK channels in a compensative manner. As such, the role of metabolic changes on regulation of vascular and ion channel function needs clarification with the context of DM. Indeed, we have found that coronary arteriolar function is better preserved in patients with well-controlled DM when compared with patients with poorly controlled DM. To elucidate the cause of this phenomenon, we recruited patients with poorly controlled diabetes (HgbA1c ≥ 8.0) to investigate DM dysregulation of endothelial SK channel and coronary arteriolar function.
There are 4 types of SK channels, SK-1, SK-2, SK-3 and SK-4 (IK). We and others have found that SK-2, SK-3 and SK-4 (IK) are predominately present in the endothelial cells.8, 14, 16 In particular SK-2 is found to be located in the nuclei of endothelial cells, while SK-3 and SK-4 are predominately in the sarcolemmal membrane.8 We have previously demonstrated that there were no significant changes in the SK-3 and SK-4 protein expression in the atrial myocardium and HCAECs between ND and DM patients.14, 15 In this study, we further measured SK-3 and SK-4 mRNA levels in the human atrial tissues by RT-PCR. We did not find significant changes in SK-3 and SK-4 mRNA expression in the patients with DM compared to ND patients. Consistent with our previous studies, we did not observe significant changes in SK protein expression/localization in the human myocardium and HCAECs. These findings support the notion that the DM inhibition/inactivation of SK channels is via post-translational modification.
It has been well recognized that DM is associated with altered metabolic signaling, such as pyridine nucleotide redox in the myocardium and vasculature.37, 38 However, direct measures of NAD+ or NADH in the DM heart are very limited and the results are controversial. The myocardial NAD+ pool is reduced in streptozotocin-induced DM,39 heart failure19, 22, 40 and myocardial ischemia and reperfusion,28 whereas, NADH/NAD+ redox ratio are unchanged in rat DM models. 39, 41 In this study, we found that DM increased levels of NADH and the ratio of NADH/NAD+ in human atrial myocardium and HCAECs, as supported by two other critical observations in the DM heart mitochondria: (A) NAD+-dependent sirtuins are decreased leading to increased acetylation of lysine residues in mitochondrial proteins,42 and (B) interventions to normalize NADH/NAD+ ratio (NAD supplementation or caloric restrictions) have been shown to be beneficial to the DM heart.43, 44 Obviously, there are limitations in currently available methods to accurately measure NADH/NAD+ ratio within cellular compartments.
Previous studies indicate that pyridine nucleotide redox plays an important role in the regulation of ion channels.19–21, 45 For example, an increase in the cytosolic NADH/NAD+ ratio decreased in cardiac INa currents, whereas, increased intracellular NAD enhanced cardiac INa.19, 20 Interestingly, increased intracellular NADH downregulated BK, whereas, increased intracellular NAD+ enhanced BK currents in the pulmonary arterial smooth muscle cells of rabbits in the setting of hypoxia.25 In addition, application of NAD(H)P reduced Kv currents in the smooth muscle cells, whereas, NAD(P)+ or NAD+ enhanced Kv,21, 23, 46 and KATP currents.34, 47 In order to further investigate the impact of metabolites of NADH and NAD+ on endothelial SK channels, we applied NADH and NAD+ to the HCAECs by using similar concentrations of NADH and NAD+ as previously reported. With this, we observed that NADH inhibited, whereas, NAD+ enhanced endothelial SK currents. This is the first report demonstrating that pyridine nucleotides NADH/NAD+ also regulate SK channels in human endothelial cells.
Recent evidence suggests that Nox and dysfunctional mitochondria mutually stimulate to enhance ROS production and PKC expression/activation which play a pivotal role in endothelial dysfunction during diabetes. Specifically, PKC-α, -β and δ has been shown to be involved in hyperglycemia-induced abnormalities of endothelium-dependent vasodilatation in humans and clinical treatment with a PKC-β inhibitor improves endothelial function in DM patients.48, 49 In the present study, we also found that Nox, protein oxidation, mROS and PKC were significantly increased in the DM myocardium, coronary microvesels and HCAECs (Figure S1 and Figure S2, see in supplementary data), which may contribute to diabetes-related coronary arteriolar endothelial and smooth muscle dysfunction.2, 3 Additionally, recent studies indicate that NADH inhibited cardiac INa channels via mROS and PKC signaling pathways.19, 20, 22 Thus, it can be speculated that chronic over-production/activation of NADH, Nox, mROS and PKC during DM may negatively regulate SK channel function. Whether NADH directly or indirectly mediates endothelial SK channel suppression via PKC during DM requires further investigation.20
There are a number of limitation in the current study. For example, the number of cases recruited in the current study was relative small (n=12/group). In addition, the microvessels obtained from harvested right atrial tissue samples may not necessarily represent the coronary microvascular function of left ventricles. However, it is inapplicable to harvest tissue samples from human left ventricle for coronary microvessel study. Obviously, Investigation into the impact of metabolic signaling in SK activity and endothelial function in the human coronary arterioles and endothelial cells of diabetic patients with translational research models is a crucial step linking bench to bedside.
Conclusion
Diabetes is associated with metabolic changes in the human myocardium and HCAECs. Endothelial SK channel function is regulated by the metabolite pyridine nucleotides, NADH and NAD+, suggesting that metabolic regulation of endothelial SK channels may contribute to coronary endothelial dysfunction in the patients with DM.
Supplementary Material
HIGHLIGHTS.
Diminished relaxation response of human coronary arterioles to the selective SK channel activator SKA-31 in patients with diabetes
Increased the pyridine nucleotides NADH and the ratio of NADH/NAD+ in human diabetic myocardium and HCAECs
Inhibited SK channels of HCAECs by intracellular application of NADH Increased endothelial SK channels by administration of NAD+
ACKNOWLEDGEMENT
We would like to thank all nurses, physician assistants, perfusionists at cardiac-surgery-operation rooms in Lifespan Hospitals for collecting tissue samples and the data of patient characteristics. We would like to thank nurses and physician assistants at Division of Cardiac Surgery, Lifespan Hospitals for collecting patient consent forms.
SOURCES OF FUNDING
This research project was supported by the NIH1R01HL127072-01A1, 1R01 HL136347-01, NIGMS/NIH grant (pilot project) 1P20GM103652 and AHA-Grant-in-Aid-15GRNT25710105 to J.F.; R01-HL-46716 and U54GM115677 to F.W.S, and RO1HL128831, to F.W.S & A.U.
Funding: This research project was mainly supported by the National Institute of Health (NIH) 1R01HL127072-01A1, 1R01 HL136347-01, National Institute of General Medical Science (NIGMS) of the NIH [5P20-GM103652 (Pilot Project and CORE)] and AHA-Grant-in-Aid (#15GRNT25710105) to J.F. This work was supported in part by R01-HL46716 to F.W.S., RO1HL128831 to F.W.S and A.U.
Footnotes
Conflict of interests
No any potential conflicts of interest exist for all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 2.Feng J, Liu Y, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, Clements RT, Bianchi C, Sellke FW. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Anderson K, Singh AK, Ehsan A, Mitchell H, Liu Y, Sellke FW. Diabetes upregulation of cyclooxygenase 2 contributes to altered coronary reactivity after cardiac surgery. Ann Thorac Surg. 2017;104:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9:434–449 [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Liu Y, Dobrilovic N, Chu LM, Bianchi C, Singh AK, Sellke FW. Altered apoptosis-related signaling after cardioplegic arrest in patients with uncontrolled type 2 diabetes mellitus. Circulation. 2013;128:S144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: Regulation of flow and beyond. Circ Res. 2016;118:157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Kohler R. Genetic deficit of sk3 and ik1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332 [DOI] [PubMed] [Google Scholar]
- 9.Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance ca2+-activated k+ channel. Circ Res. 2006;99:537–544 [DOI] [PubMed] [Google Scholar]
- 10.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda). 2006;21:69–78 [DOI] [PubMed] [Google Scholar]
- 11.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272 [DOI] [PubMed] [Google Scholar]
- 12.Feletou M, Vanhoutte PM. Edhf: An update. Clin Sci (Lond). 2009;117:139–155 [DOI] [PubMed] [Google Scholar]
- 13.Weston AH, Absi M, Harno E, Geraghty AR, Ward DT, Ruat M, Dodd RH, Dauban P, Edwards G. The expression and function of ca(2+)-sensing receptors in rat mesenteric artery; comparative studies using a model of type ii diabetes. Br J Pharmacol. 2008;154:652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Xie A, Singh AK, Ehsan A, Choudhary G, Dudley S, Sellke FW, Feng J. Inactivation of endothelial small/intermediate conductance of calcium-activated potassium channels contributes to coronary arteriolar dysfunction in diabetic patients . J Am Heart Assoc. 2015;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Cole V, Lawandy I, Ehsan A, Sellke FW, Feng J. Decreased coronary arteriolar response to kca channel opener after cardioplegic arrest in diabetic patients. Mol Cell Biochem. 2018;445:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Liu Y, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Nishimura KK, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation. 2008;118:S46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Sellke EW, Feng J, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human skeletal muscle microvascular endothelial dysfunction related to cardiopulmonary bypass. Surgery. 2008;144:239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Terata K, Chai Q, Li H, Kleinman LH, Gutterman DD. Peroxynitrite inhibits ca2+-activated k+ channel activity in smooth muscle of human coronary arterioles. Circ Res. 2002;91:1070–1076 [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Sanyal S, Gao G, Gurung IS, Zhu X, Gaconnet G, Kerchner LJ, Shang LL, Huang CL, Grace A, London B, Dudley SC, Jr. Cardiac na+ current regulation by pyridine nucleotides. Circ Res. 2009;105:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Shi G, Yang KC, Gu L, Kanthasamy AG, Anantharam V, Dudley SC Jr., Role of protein kinase c in metabolic regulation of the cardiac na(+) channel. Heart Rhythm. 2017;14:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilfoil PJ, Tipparaju SM, Barski OA, Bhatnagar A. Regulation of ion channels by pyridine nucleotides. Circ Res. 2013;112:721–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Gu L, Sulkin MS, Liu H, Jeong EM, Greener I, Xie A, Efimov IR, Dudley SC Jr., Mitochondrial dysfunction causing cardiac sodium channel downregulation in cardiomyopathy. J Mol Cell Cardiol. 2013;54:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tur J, Chapalamadugu KC, Katnik C, Cuevas J, Bhatnagar A, Tipparaju SM. Kvbeta1.1 (akr6a8) senses pyridine nucleotide changes in the mouse heart and modulates cardiac electrical activity. Am J Physiol Heart Circ Physiol. 2017;312:H571–H583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dwenger MM, Ohanyan V, Navedo MF, Nystoriak MA. Coronary microvascular kv1 channels as regulatory sensors of intracellular pyridine nucleotide redox potential. Microcirculation. 2018;25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Park M, So I, Earm YE. Nadh and nad modulates ca(2+)-activated k+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflugers Arch. 1994;427:378–380 [DOI] [PubMed] [Google Scholar]
- 26.Brown KK, Spinelli JB, Asara JM, Toker A. Adaptive reprogramming of de novo pyrimidine synthesis is a metabolic vulnerability in triple-negative breast cancer. Cancer Discov. 2017;7:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia J, Wishart DS. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics. 2016;55:14 10 11–14 10 91 [DOI] [PubMed] [Google Scholar]
- 28.Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, Sadoshima J, Tian R. Elimination of nadph oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J Am Heart Assoc. 2014;3:e000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafique E, Torina A, Reichert K, Colantuono B, Nur N, Zeeshan K, Ravichandran V, Liu Y, Feng J, Benjamin LE, Irani K, Harrington EO, Sellke FW, Abid MR. Mitochondrial redox plays a critical role in the paradoxical effects of napdh oxidase-derived ros on coronary endothelium. Cardiovasc Res. 2017;113:234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated k(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152 [DOI] [PubMed] [Google Scholar]
- 31.Lu T, He T, Katusic ZS, Lee HC. Molecular mechanisms mediating inhibition of human large conductance ca2+-activated k+ channels by high glucose. Circ Res. 2006;99:607–616 [DOI] [PubMed] [Google Scholar]
- 32.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite role of kv1.5 channels in coronary metabolic dilation. Circ Res. 2015;117:612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive kv currents through s-glutathionylation. Pflugers Arch. 2015;467:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical atp-binding cassette protein, and its regulation of the katp channel. Circ Res. 2008;102:164–176 [DOI] [PubMed] [Google Scholar]
- 35.Svoboda LK, Reddie KG, Zhang L, Vesely ED, Williams ES, Schumacher SM, O’Connell RP, Shaw R, Day SM, Anumonwo JM, Carroll KS, Martens JR. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel kv1.5. Circ Res. 2012;111:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs camp-mediated dilation by reducing kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–1219 [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Yang X, Zhang Z, Song J, Guan YF, Zou DJ, Miao CY. Depletion of nad pool contributes to impairment of endothelial progenitor cell mobilization in diabetes. Metabolism. 2016;65:852–862 [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Li WL, Liu JM, Miao CY. Nampt and nampt-controlled nad metabolism in vascular repair. J Cardiovasc Pharmacol. 2016;67:474–481 [DOI] [PubMed] [Google Scholar]
- 39.Khanra R, Dewanjee S, T KD, Sahu R, Gangopadhyay M, De Feo V, Zia-Ul-Haq M. Abroma augusta l. (malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med. 2015;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC Jr., Margulies KB, Coon JJ, Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouzu H, Miki T, Tanno M, Kuno A, Yano T, Itoh T, Sato T, Sunaga D, Murase H, Tobisawa T, Ogasawara M, Ishikawa S, Miura T. Excessive degradation of adenine nucleotides by up-regulated amp deaminase underlies afterload-induced diastolic dysfunction in the type 2 diabetic heart. J Mol Cell Cardiol. 2015;80:136–145 [DOI] [PubMed] [Google Scholar]
- 42.Berthiaume JM, Hsiung CH, Austin AB, McBrayer SP, Depuydt MM, Chandler MP, Miyagi M, Rosca MG. Methylene blue decreases mitochondrial lysine acetylation in the diabetic heart. Mol Cell Biochem. 2017;432:7–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatt NM, Aon MA, Tocchetti CG, Shen X, Dey S, Ramirez-Correa G, O’Rourke B, Gao WD, Cortassa S. Restoring redox balance enhances contractility in heart trabeculae from type 2 diabetic rats exposed to high glucose. Am J Physiol Heart Circ Physiol. 2015;308:H291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthiaume JM, Kurdys JG, Muntean DM, Rosca MG. Mitochondrial nad(+)/nadh redox state and diabetic cardiomyopathy. Antioxid Redox Signal. 2019;30:375–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M, Yang KC, Dudley SC Jr., Cardiac sodium channel mutations: Why so many phenotypes? Nat Rev Cardiol. 2014;11:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tipparaju SM, Liu SQ, Barski OA, Bhatnagar A. Nadph binding to beta-subunit regulates inactivation of voltage-gated k(+) channels. Biochem Biophys Res Commun. 2007;359:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dabrowski M, Trapp S, Ashcroft FM. Pyridine nucleotide regulation of the katp channel kir6.2/sur1 expressed in xenopus oocytes. J Physiol. 2003;550:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–111 [DOI] [PubMed] [Google Scholar]
- 49.Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ 3rd, Wolka AM, Vinik AI. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase c-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30:896–902 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.