Abstract
Objective:
Late-life depression (LLD) is a chronic and heterogeneous disorder. Recent studies have implicated non-normative age-related processes in its pathogenesis. This investigation examined both cross-sectional and longitudinal associations between skeletal muscle mitochondrial function and LLD.
Methods:
Data from 603 men and women from the Baltimore Longitudinal Study on Aging were analyzed, of whom 167 provided data from a follow-up visit. Muscle bioenergetics was measured by postexercise recovery rate of phosphocreatine (PCr) using phosphorus magnetic resonance spectroscopy. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression (CES-D) Scale.
Results:
There was no cross-sectional association between baseline depression status and either the PCr recovery rate constant (kPCr; t= −0.553, df=542; p = 0.580) or mitochondrial capacity largely independent of exercise intensity (adenosine triphosphate maximum [ATPmax]; t = 0.804, df=553; p = 0.422). Covariate-adjusted Firth logistic regression models however showed that greater decreases in skeletal muscle mitochondrial function from baseline to follow-up were associated with higher odds of clinically significant depressive symptoms (CES-D 16) at follow-up (ΔATPmax: odds ratio = 2.63, χ2 = 5.62, df =1; p = 0.018; ΔkPCr: odds ratio = 2.32, χ2 =5.79, df =1; p = 0.016).
Conclusion:
Findings suggest that declining skeletal muscle mitochondrial function in older adults is associated with clinically significant depressive symptoms at follow-up, thereby providing preliminary support for the hypothesis that mitochondrial dysfunction may be a potential key pathophysiological mechanism in adults with LLD. (Am J Geriatr Psychiatry 2019; 27:963–971)
Keywords: Mitochondrial function, aging, depression, fatigue, longitudinal
INTRODUCTION
Depression in later life (LLD) is a severe public health problem owing to its high prevalence, chronicity, and association with mortality.1 Treatment of LLD has been hampered by failure to understand its etiologic heterogeneity and by using diagnostic criteria and pathophysiological models based primarily on studies of younger adults. Biological and clinical changes associated with increased aging are associated with incident LLD and interact with LLD to create poorer health trajectories. For instance, older biological age as measured by a multibiomarker algorithm is associated with incident LLD, independent of the association of chronological age.2 Increased biological age is associated with the biological syndrome of frailty,3 which has been shown to interact with LLD to increase morbidity and mortality rates in later life. A better understanding of age-associated biological pathways that are associated with LLD, including their characteristic clinical presentations, is critical for the development of acute treatment strategies for this disorder.
A component of biological aging associated with frailty characteristics such as mobility deficits and fatigue in later life is mitochondrial dysfunction.4,5 Prior studies have established mitochondrial dysfunction as a driver of mammalian aging.6 Recent studies have shown, using phosphorus magnetic resonance spectroscopy (31P-MRS), that lower mitochondrial capacity is associated with lower activity levels, decreased aerobic capacity and mobility,7 and greater fatigability in later life.4 Mitochondrial dysfunction has also been identified in numerous neuroimmune and neurodegenerative diseases including depression.8–11 Decreased adenosine triphosphate (ATP) production rates have been observed in younger patients with depression,9 and most recently, adults with depression showed significantly impaired mitochondrial respiration compared with healthy controls.10
However, to date, no study has examined the association between skeletal muscle bioenergetics and depressive symptoms in later life. Therefore, the primary goal of this investigation is to test the hypothesis that greater deficits in mitochondrial function are associated cross-sectionally and longitudinally with clinically significant depressive symptoms. To test these hypotheses, data from the Baltimore Longitudinal Study on Aging (BLSA) was used.
METHODS
Study Participants
The BLSA conducted by the Intramural Research Program of the National Institute on Aging is America’s longest-running scientific study of human aging, with its goals of describing longitudinal physical and cognitive changes that define aging, and to identify genetic, physical, behavioral, and environmental factors that affect the rate of change in these traits. A secondary data analytic plan was approved by the BLSA in August 2016, and data were transferred in February 2017. At this data release, 603 participants had data from at least one qualifying visit on 31P-MRS measurements of postexercise phosphocreatine (PCr) recovery rate, demographic (age, sex) and medical comorbidity variables, and depression assessments. Of these, 167 had complete data at an additional follow-up time point (mean follow-up 1.65 years ± 0.56). Trained technicians administered all assessments following standardized protocols in the BLSA.
Assessment: 31P-MRS
Details of the 31P-MRS methods can be found in previous publications.4,7,12,13 In brief, in vivo 31P-MRS measurements of phosphorus-containing metabolites were obtained from the quadriceps muscles using a 3T Philips Achieva MR scanner (Philips, Best, the Netherlands). Participants were positioned supine on the bed of the scanner, with slight flexion in their knees, and their legs secured with straps to minimize movement during exercise. Participants performed rapid, intense ballistic knee extension exercises, and a series of 31P-MRS acquisitions were obtained before, during, and after exercise, using a 10-cm 31P-tuned, flat surface coil (PulseTeq, Surrey, United Kingdom) secured over the vastus lateralis muscle of the left thigh. A total of 75 spectra were obtained over a total acquisition time of 7 minutes 30 seconds. The duration of exercise was controlled to achieve depletion in PCr signal amplitude to a value within 33%–67% of the resting amplitude. Spectra were processed using jMRUI (version 5.0; MRUI Consortium) and quantified using a nonlinear least squares algorithm (AMARES).14–16 Skeletal muscle oxidative adenosine triphosphate (ATP) resynthesis rate was determined by fitting the postexercise recovery of PCr to the following mono-exponential function:
where PCr0 is the PCr peak area after exercise was performed, ΔPCr is the depletion of PCr peak area from baseline to exercise conclusion, and τPCr is the PCr exponential recovery time constant.12 Accurate kPCr quantification was ensured by examining the quality of all PCr recovery plots. Participants who showed poor fit were excluded from the dataset, as well as those who did not show full PCr recovery postexercise. The 75 dynamic scans, however, allowed for full PCr recovery in the majority of participants, minimizing excluded data. Two measures derived from the 31P-MRS were used in this investigation: kPCr, which is the PCr recovery rate constant, and ATP maximum (ATPmax), which is a measure of mitochondrial capacity largely independent of exercise intensity.17 ATPmax was calculated using the equation:
Although kPCr was quantified using the series of 75 dynamic scans, PCr0 for the calculation of ATPmax was collected from a single resting scan that was acquired prior to the start of the exercise.
Clinical Assessments
Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression (CES-D) Scale.18 Scores range from 0–60; a score of 16 or more is strongly correlated with a major depressive episode.19 Fatigability was assessed using the Pittsburgh Fatigability Scale (PFS).20 The PFS is a 20-item self-report measure of fatigability, associated with both performance and perceived exertion on standard walking tasks,20,21 with 10 items each assessing physical fatigability and mental fatigability. For this investigation, only 9 of 10 items for each domain were assessed, and thus, total PFS scores range from 0–90. Medical comorbidities were assessed as the sum of 24 dichotomous (yes/no) variables in which participants were asked whether a doctor had told them they had cardiac (e.g., heart attack, congestive heart failure), pulmonary (e.g., asthma), cerebrovascular (e.g., hypertension, stroke, diabetes, hypercholesterolemia), cancer, or musculoskeletal diseases (e.g., osteoarthritis, osteoporosis). Physical ability was assessed by the Short Physical Performance Battery (SPPB).22 Cognitive ability was assessed by the Folstein Mini-Mental State Exam.23
Statistical Analysis
To test for differences between subjects with only one assessment (termed baseline sample, although it should be noted that subjects have had previous assessments as part of the BLSA) and those with a follow-up assessment (termed longitudinal sample), two-sample t-tests were used for continuous measures and Chi-squared exact tests were used for categorical variables. To test for differences between subjects who were depressed at follow-up and those who were not depressed, among those with a follow-up assessment, the Mann-Whitney U tests were used for continuous measures (because the number of depressed subjects was small) and the Fisher’s exact tests were used for categorical variables.
To assess the cross-sectional association between depression status and skeletal muscle mitochondrial function, we fit unadjusted and adjusted linear regression models with either kPCr or ATPmax as the predictor and depression status as the response. In the adjusted models, we included age, sex, number of medical comorbidities as covariates, and percentage of PCr depletion. The latter is accounted for because those who do not show at least a 25% depletion of 25% PCr show inaccurate recovery curves given the small dynamic range.
To examine the association between change in depressive symptom severity and skeletal muscle mitochondrial function, we considered four multiple linear regression models. For each model, the response was change in CES-D from a subject’s baseline assessment to the first follow-up assessment, calculated as the difference in CES-D values (follow-up – baseline) divided by the number of years between visits. A different primary predictor was used in each model: (1) baseline ATPmax; (2) baseline kPCr; (3) change in ATPmax from baseline to follow-up; or (4) change in kPCr from baseline to follow-up. Change scores for kPCr and ATPmax in (3) and (4) were calculated in the same way as the change scores for CES-D. In all four models, we adjusted for baseline CES-D, sex, age, number of medical comorbidities, and baseline PCr depletion percentage (for models that examined change in skeletal muscle mitochondrial function, both baseline and follow-up PCr depletion percentage were tested; the addition of the follow-up PCr depletion percentage did not change the results, so only models including baseline PCr depletion percentage as a covariate were reported). All predictors were centered and scaled prior to entering the models, using the sample mean and standard deviation from the longitudinal sample. Unadjusted models were also fit for comparison.
To assess the association between depression status at follow-up and skeletal muscle mitochondrial function, we considered four multiple logistic regression models similar to the models described earlier. For each of these models, the response was depression status at follow-up (CES-D ≥ 16 versus CES-D <16). The predictors for these models were the same as those described earlier, with an additional covariate indicating the time between baseline and follow-up also included in each model. Considering that the number of events (subjects with CES-D ≥ 16 at follow-up) was rare, we used Firth logistic regression to estimate logistic regression parameters. This method for estimating logistic regression model parameters has been shown to reduce bias in estimates when the sample size is small or the event of interest rare.24 Unadjusted models were fit for comparison. All predictors were standardized before entering the models to allow for meaningful comparisons between coefficient estimates and to show effects for observable differences in the predictors. Coefficients from this analysis correspond to the mean change in CES-D (in linear models) or change in log odds (in the logistic models) for every one standard deviation change in each predictor. All analyses were conducted in R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). Firth logistic regression was conducted using the logistf package (Heinze et al; https://cran.r-project.org/package=logistf).
RESULTS
Cross-Sectional Association Between Mitochondrial Function and Depression
Table 1 displays the characteristics of the full sample (n= 603), which averaged age 69.7 ± 13.7 years, 54% women, and 66.5% Caucasian. The sample is physically (usual walking speed: 1.20 m/s ± 0.23; SPPB: 11.64 ± 1.13) and cognitively intact (Mini-Mental State Exam: 28.4 ± 1.49), with on average 2.98 ± 1.94 medical comorbidities and low depressive symptoms (mean CES-D: 5.00 ± 5.09, with 4.8% reporting significant depressive symptoms; CES-D ≥16).
TABLE 1.
Baseline Characteristics for the Sample From the Baltimore Longitudinal Study on Aginga
| Variable | Total Sample (n = 603) |
Baseline Data (n = 436) |
Longitudinal Data (n = 167) |
Statisticsb | CES-D ≥16 at Follow-Up (n = 9) |
CES-D <16 at Follow-Up (n = 158) |
Statisticsc |
|---|---|---|---|---|---|---|---|
| Demographic | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age, years | 69.70 (13.69) | 66.56(14.06) | 77.89 (8.21) | t = −12.24, df= 505; p < 0.001 | 80.78 (10.44) | 77.73 (8.08) | χ2 = 2.48, df = 1; p = 0.115 |
| Educational level | 17.01 (2.69) | 17.03 (2.63) | 16.95 (2.82) | t = 0.32, df= 576; p = 0.747 | 17.67 (2.65) | 16.91 (2.83) | χ2 = 0.78, df = 1; p = 0.376 |
| Sex, no. M/F (% F) | 278/325 (53.9) | 201/235 (53.9) | 77/90 (53.9) | χ2 < 0.01, df = 1; p = 0.999 | 2/7 (77.8) | 75/83 (52.5) | p = 0.180 |
| Race, no. Caucasian/black (% black) | 385/149 (25.7) | 261/116(28.2) | 124/33(19.8) | χ2 = 5.24, df = 1; p = 0.022 | 8/1 (111) | 116/32 (20.3) | p = 0.686 |
| Medical comorbidities | 2.98 (1.94) | 2.71 (1.86) | 3.66(1.96) | t = −5.48, df= 573; p <0.001 | 4.44 (1.42) | 4.59 (1.89) | χ2 = 0.03, df = 1; p = 0.865 |
| MMSE | 28.38(1.49) | 28.35 (1.54) | 28.42 (1.40) | t = −0.48, df= 499; p = 0.635 | 28.00 (1.58) | 28.45 (1.39) | χ2 = 0.75, df = 1; p = 0.387 |
| Physical abilities | |||||||
| Gait speed (m/s) | 1.20(0.23) | 1.22 (0.22) | 1.15(0.25) | t = 2.76, df= 271; p = 0.006 | 1.20 (0.48) | 1.15 (0.23) | χ2 = 0.13, df = 1; p = 0.722 |
| PFS (total) | 18.35 (12.80) | 18.26(12.71) | 18.57(13.07) | t = −0.26, df = 537; p = 0.799 | 26.89 (17.84) | 18.06 (12.61) | χ2 = 2.22, df = 1; p = 0.136 |
| Physical fatigability | 11.70 (6.92) | 11.58(6.86) | 12.03 (7.08) | t = −0.68, df = 550; p = 0.496 | 16.11 (9.84) | 11.77 (6.84) | χ2 = 2.51, df = 1; p = 0.113 |
| Mental fatigability | 6.69 (6.79) | 6.75 (6.73) | 6.56 (6.93) | t = 0.29, df= 543; p = 0.774 | 10.78 (8.20) | 6.30 (6.80) | χ2 = 2.59, df = 1; p = 0.107 |
| SPPB | 11.64(1.13) | 11.71 (0.93) | 11.46(1.51) | t = 1.93, df= 213; p = 0.055 | 10.44 (3.61) | 11.52 (1.29) | χ2 = 0.96, df = 1; p = 0.328 |
| Depression scores | |||||||
| CES-D | 5.00 (5.09) | 5.36 (5.33) | 4.14(4.36) | t = 2.82, df= 378; p = 0.005 | 12.11 (7.56) | 3.69 (3.65) | χ2= 11.69, df = 1; p = 0.001 |
| Mitochondrial markers | |||||||
| ATPmax | 0.41 (0.14) | 0.42 (0.15) | 0.38(0.13) | t = 3.50, df= 342; p = 0.001 | 0.45 (0.21) | 0.37 (0.12) | χ2 = 0.49, df = 1; p = 0.483 |
| kPCr | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.00) | t = 4.81, df= 362; p <0.001 | 0.02 (0.01) | 0.02 (0.00) | χ2 = 0.92, df = 1; p = 0.339 |
Notes. ATPmax: a measure of mitochondrial capacity largely independent of exercise intensity; kPCr: phosphocreatine recovery rate constant; MMSE: Mini-Mental Status Exam; PFS: Pittsburgh Fatigability Scale, with Physical Fatigability and Mental Fatigability representing subdomains of the PFS; SD: standard deviation.
Data presented as mean (SD) unless otherwise indicated.
For comparing subjects with only baseline data to those with longitudinal data, two-sample t tests were used for continuous variables, and the χ2 tests were used for categorical variables.
Within the longitudinal sample, the Mann-Whitney U tests (using the χ2 approximation) were used for continuous variables, and the Fisher’s exact tests were used for categorical variables for the comparisons of baseline values between those with and without elevated depressive symptoms at visit 2.
Cross-sectionally, there was no bivariate association between depression status and either kPCr (t = 0.50, df = 561; p = 0.615) or ATPmax, (t = −0.67, df = 549; p = 0.503), or after adjustment for age, sex, baseline PCr depletion percentage, and medical comorbidities (t=−0.553, df = 542; p = 0.580 and t = 0.804, df = 553; p = 0.422, respectively). This was true for the total baseline sample as well as the longitudinal sample (data for the latter are not shown).
Prior Mitochondrial Function as a Predictor of Depression
Table 1 compares the characteristics of the baseline (n = 436) and longitudinal (n = 167) samples. Participants in the longitudinal sample were older (aged 77.9 years ± 8.2) and less diverse (74% versus 63% Caucasian), with slower gait (usual walking speed 1.15 m/s ± 0.25 versus 1.22 m/s ± 0.22), poorer physical performance (SPPB: 11.46 ± 1.51 versus 11.71 ± 0.93) and greater comorbidity burden (3.66 ± 1.96 versus 2.71 ± 1.86) than subjects in the baseline sample. The longitudinal sample had more initial impairment in mitochondrial function than the baseline sample.
Nine of the 167 participants had a CES-D of 16 or more at follow-up; two of these participants had a CES-D of 16 or more at baseline. These participants did not significantly differ at baseline from the nondepressed individuals on any of the variables of interest with the exception of CES-D (mean CES-D 12.11 ± 7.56 versus 3.69 ± 3.65; Table 1). Linear and Firth logistic regression models (Table 2) were fit to test whether baseline mitochondrial functioning predicted changes in or elevated levels of CES-D, adjusting for age, sex, medical comorbidities, baseline PCr depletion percentage, and CES-D. No association was observed (Table 2, models 1a, 2a, 3a, 4a).
TABLE 2.
Skeletal Muscle Mitochondrial Function as a Predictor of Depression in Later Life
| Outcome | Primary Predictor | Model | N | Coefficient Estimate | Statistics | Adjusted R2 With Predictor | Adjusted R2 Without Predictor | |
|---|---|---|---|---|---|---|---|---|
| Linear models | ΔCES-D | ATPmax | 1a | 164 | 0.501 | t = 1.87, df = 157; p = 0.063 | 0.058 | 0.043 |
| ΔATPmax | 1b | 161 | −0.407 | t = −1.69, df = 154; p = 0.093 | 0.050 | 0.039 | ||
| kPCr | 2a | 167 | 0.251 | t = 1.01, df= 160; p = 0.316 | 0.042 | 0.042 | ||
| ΔkPCr | 2b | 167 | −0.241 | t = −1.02, df = 160; p = 0.309 | 0.042 | 0.042 | ||
| AIC With Predictor | AIC Without Predictor | |||||||
| Firth logistic Models | CES-D ≥ 16 | ATPmax | 3a | 164 | 0.661 (OR= 1.94) | χ2 = 2.53, df = 1;p = 0.112 | −12.769 | −12.571 |
| ΔATPmax | 3b | 161 | −0.980 (OR = 0.38) | χ2 = 5.62, df = 1;p = 0.018 | −15.634 | −12.442 | ||
| kPCr | 4a | 167 | 0.454 (OR= 1.58) | χ2 = 1.86, df = 1; p = 0.173 | −17.019 | −17.351 | ||
| ΔkPCr | 4b | 167 | −0.852 (OR = 0.43) | χ2 = 5.79, df = 1;p = 0.016 | −20.757 | −17.351 |
Notes. Δ refers to change in that variable between visits 1 and 2. Each row corresponds to a different model. The top four rows (models 1a and b and 2a and b) show standardized effect estimates for the primary predictors of ΔCES-D (i.e., slope of CES-D from visit 1 to visit 2), with each model adjusted for PCr depletion percentage and CES-D at visit 1, sex, age, and comorbid score. The bottom four rows (models 3a and b and 4a and b) show standardized effect estimates (i.e., expected change in the outcome for an increase of one standard deviation in the predictor) for the primary predictors of depression status at visit 2 using CES-D ≥ 16 to indicate depression. Each of these four Firth logistic regression models is adjusted for PCr depletion percentage and CES-D at visit 1, sex, age, comorbid score, and time from visit 1 to visit 2. For the logistic models, smaller AIC is better. AIC: XXXX; ATPmax: adenosine triphosphate maximum, a measure of mitochondrial capacity largely independent of exercise intensity; kPCr: phosphocreatine recovery rate constant; OR: odds ratio.
Declining Mitochondrial Function as a Predictor of Depression
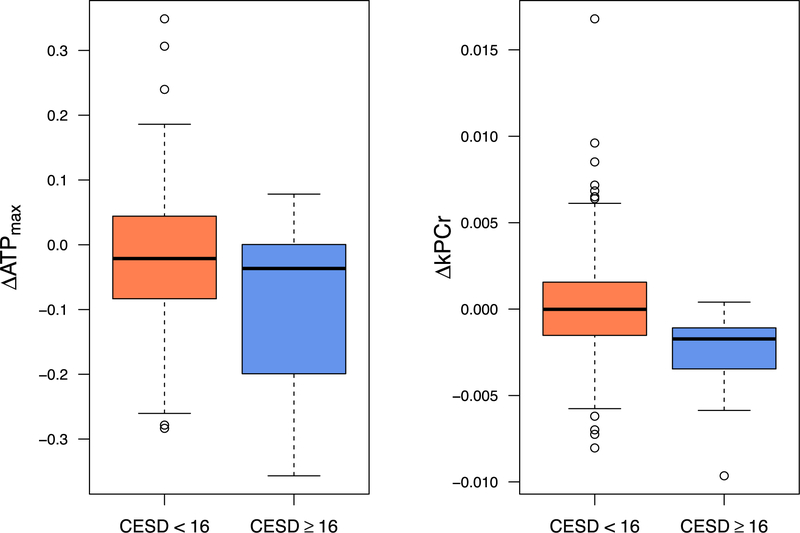
Linear regression and Firth logistic models with the mitochondrial change scores as the primary predictors were fit. Although linear models continued to show no significant association between change in skeletal muscle bioenergetics and change in CES-D scores from baseline to follow-up (Table 2, models 1b and 2b), separate covariate adjusted Firth logistic regression models with depression status at followup as the dichotomous outcome revealed that greater decreases in both ATPmax (, z = −2.371; p = 0.018; model 3b) and kPCr (, z = −2.406; p = 0.016; model 4b) from baseline to follow-up corresponded to higher odds of being depressed at followup, showing a main effect of decline in mitochondrial function predicting LLD at follow-up (Fig. 1).
FIGURE 1.
Box plots examining change in skeletal muscle mitochondrial function and incident depression at follow-up. Box plots graphically depict mean change in skeletal muscle mitochondrial function as a function of depression status at follow-up (CES-D ≥16 versus CES-D <16). These box plots offer complementary information to the results from the logistic regression models.
DISCUSSION
In this study, preliminary evidence was observed that declining skeletal muscle mitochondrial functioning in older adults is associated with clinically significant depressive symptoms at follow-up. No crosssectional association between mitochondrial function and either symptoms of depression or depression status was observed, nor was declining skeletal muscle mitochondrial function associated with depressive symptom increases from baseline to follow-up.
These preliminary findings from the longitudinal analyses serve as further support that non-normative aging processes may play a role in the pathophysiology of LLD.2, 25–27 The mitochondrion is critical for energy production, which drives numerous systems including neurotransmitter release and reuptake, and muscle contraction.28,29 Mitochondrial dysfunction is associated with dysfunction across a myriad of physiological systems associated with LLD including muscle breakdown4,7,30,31 chronically elevated inflammation, endothelial vascular dysfunction28,32 and greater neurodegeneration including Alzheimer-associated beta amyloid accumulation.33 Additionally, LLD at follow-up in this study was preceded by greater fatigability, although differences did not reach significance because of the rarity of LLD at follow-up in the BLSA. Fatigue is a key component of the syndrome of frailty and is associated with greater dysfunction in skeletal muscle bioenergetics. Given these findings, the production of energy via mitochondrial functioning may be a key pathophysiologic mechanism of LLD and, in particular the frail-depressed subtype of LLD, given that skeletal muscle mitochondrial dysfunction is associated with frailty characteristics such as slow gait, low physical activity levels, and grater fatigability.26
Although the findings from this study are preliminary and in need of replication given the small number of individuals with clinically significant depressive symptoms, they do lead to questions about the nature of the relationship between mitochondrial dysfunction and LLD. For instance, does the association between non-normative components of aging such as skeletal muscle mitochondrial dysfunction and LLD extend to dysfunction in the central nervous system? This would mean that peripheral deficits observed in the skeletal muscle would represent systemic bioenergetic deficits that might be manifest in the central nervous system in the form of neuroinflammation, cytochrome c-oxidase dysfunction, or cellular senescence.34–36 However, to date, no study has measured bioenergetics in the brain, the skeletal muscle, and the plasma simultaneously to answer this question. Another question is whether the association between skeletal muscle bioenergetics and LLD is mediated by psychosocial deficits that result from mitochondrial dysfunction in the periphery. Under this hypothesized relationship, age-related decrements in energy production result in behavioral decline and a sedentary and disengaged life style, thereby leading to greater loneliness and isolation and risk for LLD. These are important research questions to consider in light of these findings. However, to date, no study has examined the relationship between central and peripheral mitochondrial functioning and their clinical correlates, nor assessed the potential mediating role that psychosocial domains such as loneliness may play in the mitochondria-LLD relationship. Future studies should attempt to replicate the preliminary findings that skeletal muscle mitochondrial function is associated with increased risk of LLD. If so, new studies should be designed to establish the role that mitochondrial dysfunction plays in the development of LLD.
The results described earlier should be considered in light of limitations, including the fact that the CES-D is a screening instrument, not a diagnostic tool. Additionally, the rate of depression (CES-D ≥16) observed at follow-up in this study (5%), although consistent with epidemiologic rates of LLD, was a statistically rare event. Furthermore, only 167 of 603 participants had available longitudinal data, and this subsample was older, sicker, and less diverse than the baseline sample. The use of change scores in this study can make interpretation somewhat difficult, as we cannot identify which of the original four variables (change in mitochondrial function or change in depression) is producing the effect. We hope to further explore in larger datasets the relationship between measures of mitochondrial function and changes in depression score or incident depression using cross-lagged panel models to improve interpretability.
Offsetting these limitations are considerable strengths. The BLSA is a healthy sample with low rates of LLD. Therefore, the ability to observe an association between declining mitochondrial function and LLD is a stronger test of the hypothesis. Furthermore, similar to a previous study of LLD,25 the use of longitudinal data identified an association between declining mitochondrial function and LLD not observed using baseline values to predict outcome. Consequently, a more nuanced understanding of the LLD-mitochondria relationship may have been observed.
CONCLUSIONS
Deterioration of skeletal muscle mitochondrial functioning is associated with clinically significant depressive symptoms at follow-up in later life. The production of energy via mitochondrial functioning may be a key pathophysiologic mechanism of LLD and in particular the frail-depressed. Targeting mitochondrial dysfunction in adults with LLD may decrease depression and improve long-term health trajectories in this high-risk clinical population.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute on Aging and by funding from the National Institute for Mental Health (K23-MH099097; K01-MH113850).
Drs. Brown, Ciarleglio, Roose, Rutherford and Ms. Chen had full access to the data and take responsibility for its integrity and the accuracy of the analysis.
References
- 1.Lavretsky H, Lesser IM, Wohl M, et al. : Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry 1999; 7:309–316 [PubMed] [Google Scholar]
- 2.Brown PJ, Wall MM, Chen C, et al. : Biological age, not chronological age, is associated with late life depression. J Gerontol A Biol Sci Med Sci 2018; 73:1370–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. : Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 4.Santanasto AJ, Glynn NW, Jubrias SA, et al. : Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci 2015; 70:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell DJ, Bharadwaj MS, Van Horn CG, et al. : Respirometric profiling of muscle mitochondria and blood cells are associated with differences in gait speed among community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2015; 70:1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Freire M, de Cabo R, Bernier M, et al. : Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci 2015; 70:1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coen PM, Jubrias SA, Distefano G, et al. : Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci 2013; 68:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amorini AM, Nociti V, Petzold A, et al. : Serum lactate as a novel potential biomarker in multiple sclerosis. Biochim Biophys Acta 2014; 1842:1137–1143 [DOI] [PubMed] [Google Scholar]
- 9.Gardner A, Johansson A, Wibom R, et al. : Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord 2003; 76:55–68 [DOI] [PubMed] [Google Scholar]
- 10.Karabatsiakis A, Bock C, Salinas-Manrique J, et al. : Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl Psychiatry 2014; 4:e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rango M, Bonifati C, Bresolin N: Parkinson’s disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab 2006; 26:283–290 [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Reiter DA, Shardell M, et al. : 31P Magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2016; 71:1638–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santanasto AJ, Coen PM, Glynn NW, et al. : The relationship between mitochondrial function and walking performance in older adults with a wide range of physical function. Exp Gerontol 2016; 81:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naressi A, Couturier C, Castang I, et al. : Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 2001; 31:269–286 [DOI] [PubMed] [Google Scholar]
- 15.Naressi A, Couturier C, Devos JM, et al. : Java-based graphical user interface for the MRUI quantitation package. MAGMA 2001; 12:141–152 [DOI] [PubMed] [Google Scholar]
- 16.Vanhamme L, Van Huffel S, Van Hecke P, et al. : Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson 1999; 140:120–130 [DOI] [PubMed] [Google Scholar]
- 17.McCully KK, Fielding RA, Evans WJ, et al. : Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 1993; 75:813–819 [DOI] [PubMed] [Google Scholar]
- 18.Weissman MM, Sholomskas D, Pottenger M, et al. : Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977; 106:203–214 [DOI] [PubMed] [Google Scholar]
- 19.Beekman AT, Deeg DJ, Van Limbeek J, et al. : Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in the Netherlands. Psychol Med 1997; 27:231–235 [DOI] [PubMed] [Google Scholar]
- 20.Glynn NW, Santanasto AJ, Simonsick EM, et al. : The Pittsburgh Fatigability scale for older adults: development and validation. J Am Geriatr Soc 2015; 63:130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eldadah BA: Fatigue and fatigability in older adults. PM R 2010; 2:406–413 [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. : A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [DOI] [PubMed] [Google Scholar]
- 24.Firth D: Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27–38 [Google Scholar]
- 25.Brown PJ, Roose SP, Zhang J, et al. : Inflammation, depression, and slow gait: a high mortality phenotype in later life. J Gerontol A Biol Sci Med Sci 2016; 71:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown PJ, Rutherford BR, Yaffe K, et al. : The depressed frail phenotype: the clinical manifestation of increased biological aging. Am J Geriatr Psychiatry 2016; 24:1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutherford BR, Taylor WD, Brown PJ, et al. : Biological aging and the future of geriatric psychiatry. J Gerontol A Biol Sci Med Sci 2017; 72:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard M, McManus MJ, Gray JD, et al. : Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A 2015; 112:E6614–E6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei L, Wallace DC: Mitochondrial etiology of neuropsychiatric disorders. Biol Psychiatry 2018; 83:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menshikova EV, Ritov VB, Fairfull L, et al. : Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 2006; 61:534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taivassalo T, Gardner JL, Taylor RW, et al. : Endurance training and detraining in mitochondrial myopathies due to single large-scale mtDNA deletions. Brain 2006; 129:3391–3401 [DOI] [PubMed] [Google Scholar]
- 32.Voloboueva LA, Giffard RG: Inflammation, mitochondria, and the inhibition of adult neurogenesis. J Neurosci Res 2011; 89: 1989–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva DF, Selfridge JE, Lu J, et al. : Mitochondrial abnormalities in Alzheimer’s disease: possible targets for therapeutic intervention. Adv Pharmacol 2012; 64:83–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brites D, Fernandes A: Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 2015; 9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diniz BS: The molecular intersection between senescence and major depression in the elderly. Am J Geriatr Psychiatry 2018; 26:1097–1105 [DOI] [PubMed] [Google Scholar]
- 36.Holper L, Mann JJ: Test-retest reliability of brain mitochondrial cytochrome-c-oxidase assessed by functional near-infrared spectroscopy. J Biomed Opt 2018; 23:1–9 [DOI] [PubMed] [Google Scholar]