Abstract
Ovarian carcinomas have the poorest prognosis and the highest mortality among gynecological malignancies. Neoadjuvant chemotherapy (NACT) is considered as a novel therapeutic strategy and an alternative treatment for advanced epithelial ovarian cancer (AEOC). The aim of the present study was to identify the core genes related to platinum-based NACT resistance in AEOC and to allow screening at the molecular level for the most appropriate ovarian cancer patients for NACT. We obtained three drug-resistant microarrays GSE114206, GSE41499 and GSE33482 from the Gene Expression Omnibus (GEO) database as well as a microarray representing NACT, GSE109934. Bioinformatics analysis revealed the nature of the four potential candidate genes for using in functional enrichment analyses and interaction network construction. The potential associations and possible genetic alterations among the DEGs were summarized using the STRING database in Cytoscape and the cBioPortal visualization tool, respectively. A total of 63 genes were identified as DEGs from GSE109934 representing NACT. From the drug-resistant GSE114206 and GSE41499 datasets, 106 DEGs containing 36 upregulated genes and 70 downregulated genes were selected, and from the drug-resistant GSE114206 and GSE33482 datasets, 406 DEGs with 157 upregulated genes and 249 downregulated genes were selected. The 36 upregulated DEGs and the 70 downregulated genes were notably abundant in the different categories. In KEGG pathway analysis, the 157 upregulated genes and the 249 downregulated genes were concentrated in distinctive signaling pathways. Four potential genes associated with NACT and platinum-based chemoresistance were screened, including nuclear factor of activated T-cells, cytoplasmic 1 (NAFTc1), Kruppel-like factor 4 (KLF4), nuclear receptor subfamily 4 group A member 3 (NR4A3) and hepatocyte growth factor (HGF). Our study showed that the mRNA expression levels of NAFTc1, NR4A3 and HGF were increased in drug-resistant OC cell lines (all P<0.01), whereas the mRNA expression levels of KLF4 were notably lower in the SKOV3-CDDP and HeyA8-CDDP cell line (all P<0.01) but higher in the A2780-CBP cell line. The NAFTc1, KLF4, NR4A3 and HGF genes may be potential therapeutic targets for NACT and platinum-based chemoresistance factors as well as candidate biomarkers in AEOC. Determination of the expression levels of these four genes in tumor tissues before planning NACT treatment or initial surgery would be beneficial for AEOC patients.
Keywords: differentially expressed genes, neoadjuvant chemotherapy, potential therapeutic targets, platinum-based chemoresistance, epithelial ovarian carcinoma
Introduction
Ovarian carcinoma (OC) has the highest mortality rate among gynecological and reproductive malignant tumors in the United States. The early symptoms that patients exhibit are not obvious and are nonspecific due to the fact that the ovary lies deep within the pelvic cavity. In addition, OC is often initially diagnosed in an advanced stage due to the lack of effective detection measures. The 5-year survival rate of patients with OC is only 15–40% (1,2). The primary treatments for advanced epithelial ovarian cancer (AEOC) patients include primary cytoreductive surgery, chemotherapy, radiotherapy and endocrine therapy. In the 1970s, Griffiths and Fuller (3,4) proposed neoadjuvant chemotherapy (NACT) as an alternative treatment for AEOC patients. The aim of NACT is to improve the preoperative status of patients, reduce the tumor volume and post-surgical complications, create conditions that allow surgery, and improve the quality of life and prognosis of the patient. However, when eliminating tissue plane, NACT can promote inflammatory adhesion, which complicates surgery in a small number of AEOC patients. Moreover, NACT can induce resistance to platinum-based chemotherapies in tumor cells by exposing large tumor volumes to chemotherapy (5). To date, there is no uniform standard for accurately evaluating and screening OC patients to determine their suitability for NACT. Currently, the common evaluation methods used internationally by gynecological oncologists include cancer antigen 125 (CA125), human epididymis protein 4 (HE4), computed tomography (CT), MRI and laparoscopic exploration (6–8). However, any single method mentioned above is not ideal enough to screen for patients indicative for NACT (9). There is an urgent need for a novel biological predictor that can facilitate the selection of patients suitable for NACT. Consequently, to maximize the therapeutic effect of NACT and avoid tumor cell resistance caused by the overuse of NACT, biomarkers are needed to screen the most suitable ovarian cancer patients for NACT and to enable therapy targeted at the process of platinum-based chemoresistance at the molecular level. Simultaneously, it is important to elucidate the resistance mechanisms of tumor cells to chemotherapeutic drugs and to attempt to reverse their resistance.
The present study was based on NACT and drug-resistant microarrays from the GEO database. We identified four differentially expressed genes (DEGs) associated with NACT and chemoresistance in AEOC. Therefore, these core genes may be potential therapeutic targets for NACT and platinum-based chemoresistance factors as well as candidate biomarkers in AEOC.
Materials and methods
Microarray data
Three eligible profiles (GSE114206, GSE41499 and GSE33482) of drug resistance and one gene expression profiles (GSE109934) of NACT were obtained from the GEO databases (http://www.ncbi.nlm.nih.gov/geo/) (10,11).
Identification of DEGs
GEO2R is an interactive web tool that identifies differentially expressed genes (DEGs) by comparing two sets of samples that belong to the GEO database (12). Platinum-sensitive OC samples were compared with platinum-resistant samples by GEO2R. Platinum sensitivity was defined by a complete response during adjuvant chemotherapy and clinical remission for at least 6 months after the completion of chemotherapy. Platinum resistance was defined as progressive or persistent disease or progression within 6 months of completing platinum therapy. The pre-NACT OC samples and the post-NACT samples of the GSE109934 profile were divided into a platinum-sensitive group and platinum-resistant group. The pre-NACT OC samples were compared with the post-NACT samples using GEO2R. The defining values used to screen out statistically significant DEGs were |log FC| ≥1 and P<0.05. As a followed up, we visually displayed the DEGs in the form of heat maps using Morpheus (https://software.broadin stitute.org/morpheus/) online tools. To screen for DEGs that were more likely to be associated with neoadjuvant chemotherapy and platinum-based chemotherapy resistance, we used a Venn diagram to filter the results. In addition, the patient clinical information of the microarray profiles GSE114206, GSE41499, GSE33482, and GSE109934, including the demographic features, FIGO stage, and histological type as well as grade were listed.
GO and KEGG analyses of DEGs
To understand the nature of the DEGs, we determined their biomolecular functions via their Gene Ontology (GO) annotations. To better understand gene and protein functions, we used the Metascape (http://metascape.org/) online tool to analyze the DEGs. One of the hallmarks of the Kyoto Encyclopedia of Genes and Genome (KEGG) database (http://www.genome.jp/) is the ability to correlate fully sequenced gene catalogs with higher-level cellular and ecosystem functions. In addition, EasyChart (http://www.ehbio.com/ImageGP/) was used to highlight the different signaling pathways associated with gene enrichment. Using the The Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov/) online tool (13) for GO categories and KEGG pathway enrichment analysis, we were able to reveal the roles of these DEGs in the underlying mechanisms of neoadjuvant chemotherapy and platinum-based chemotherapy resistance.
PPI network construction and module analysis
STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) (http://string-db.org/) is a search tool for studying genetic interactions and assessing protein-protein interaction (PPI) through the retrieval of data on interacting genes/proteins (14). Exploring protein interactions allow us to more fully understand the potential regulatory mechanisms that govern biological processes. Therefore, the visualization analysis software Cytoscape (15) was used to construct a network and map the underlying relationships among the DEGs.
Protein/gene interaction network and biological process annotation analyses
GeneMANIA (http://www.genemania.org/) (16,17) is a vast database of genomics and proteomics that can be used to predict gene function and prioritize genes based on a protein/gene interaction network. Coremine Medical (http://www.coremine.com/medic al/) is an online tool that can be used to conduct homologous similarity analysis of gene sequence, and consult the biological processes affected via query genes based on ontology language, semantic networks, intelligent analysis and other technical support.
Variation of the 4 genes in EOC
The cBioPortal (https://www.cbioportal.org/) is a comprehensive genetic database that includes data on DNA mutations, gene amplification and methylation. The cBioPortal v1.11.3 visualization and analysis tools were used to summarize the possible genetic variation in 4 abnormally expressed genes in EOC and to further analyze the clinical value of this genetic variation.
Cell culture
The ovarian cancer cell lines SKOV3, HeyA8 and A2780 were obtained from the University of Texas M.D. Anderson Cancer Center (Houston, TX, USA) via ATCC. The cell lines were continuously subcultured in a moist incubator at 37°C under 5% carbon dioxide in 10% fetal bovine serum (FBS) in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.). We established cisplatin-resistant cell lines, SKOV3-CDDP and HeyA8-CDDP, and carboplatin-resistant cell line, A2780-CBP, by successively increasing the concentrations of cisplatin and carboplatin, respectively, as previously described (18,19).
Migration and invasion assays
To assess the migration and invasion potential of the cells in vitro, migratory and invasive tests were conducted in Transwell chambers with an aperture of 8 µm (Corning Costar, Inc.). Cells (5×104) were evenly mixed with 100 µl RPMI-1640 medium without serum and transferred to the upper Transwell chamber. After incubation for 24 h, the migratory cells adhering to the sub-membrane surface were fixed (4% paraformaldehyde) and stained for 10 min at room temperature (0.1% crystal violet). For the invasion assay, the bottom of the transfer hole in the upper chamber was covered with Matrigel (BD Bioscience). Each trial was repeated three times. The number of migrating and invading cells in 10 randomly selected areas were counted at ×100 magnification under a microscope (Olympus).
Quantitative RT-PCR analysis
Using the GoScript™ reverse transcription system (Promega), 1 µg total RNA in TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was reverse transcribed to cDNA based on the manufacturer's instructions. The oligonucleotide sequence information used to amplify the target mRNA is shown in Table I. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize the level of mRNA expression. The PCR conditions for the reaction were as follows: An initial denaturation step at 95°C for 5 min, followed by 40 cycles of 95°C for 12 sec and 60°C for 30 sec. GoTap qPCR Master Mix kit (Promega) was used to detect the quantity of each target mRNA, and relative expression was calculated using the 2−ΔΔCq method (20).
Table I.
Human primer sequences used for real-time quantitative PCR.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Annealing temperature (°C) |
|---|---|---|---|
| NFATc1 | 5′-CGATCCCGGGGTAGCAGCCT-3′ | 5′-CACCGCCATACTGGAGCCGC-3′ | 62.4 |
| KLF4 | 5′-TCGGACCACCTCGCCTTACA-3′ | 5′-CTGGGCTCCTTCCCTCATCG-3′ | 60.5 |
| NR4A3 | 5′-GGGAGCCGCTGGGCTTG-3′ | 5′-CAGTGGGCTTTGAGTGCTGTG-3′ | 61.0 |
| HGF | 5′-TAGGCACTGACTCCGAACA-3′ | 5′-AGGAGATGCAGGAGGACAT-3′ | 60.7 |
| GAPDH | 5′-CGTGGAAGGACTCATGACCA-3′ | 5′-GGCAGGGATGATGTTCTGGA-3′ | 62.0 |
NAFTc1, nuclear factor of activated T-cells, cytoplasmic 1; KLF4, Kruppel-like factor 4; NR4A3, nuclear receptor subfamily 4 group A member 3; HGF, hepatocyte growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
GraphPad version 6.0 statistical software (GraphPad Software, Inc.) was used to analyze the experimental data. Student's t-test was used to analyze comparisons between groups. Quantitative data are presented as the mean ±SD. All statistics were two-tailed, and P<0.05 was considered statistically significant.
Results
Gene expression profiles
Microarray data for the drug-resistant profiles, GSE114206, GSE41499 and GSE33482, and the gene expression profile, GSE109934 for NACT were obtained from GEO. The platforms for these datasets were GPL13497, GPL3921, GPL6480 and GPL19956, respectively. The GSE114206 profile contained 12 samples, including 6 platinum-resistant samples and 6 platinum-sensitive samples; the GSE41499 profile contained 8 samples, including 4 platinum-resistant samples and 4 platinum-sensitive samples, and the GSE33482 profile contained 12 samples, including 6 cisplatin-resistant samples and 6 cisplatin-sensitive samples. The GSE109934 profile consisted of 38 samples (a total of 19 patients; each patient provided a tissue sample before and after NACT), including 19 pre-NACT samples and 19 post-NACT samples, and finally each received 2–6 cycles of chemotherapy before surgery and 9 of these were classified as platinum resistant and 10 cases classified as platinum sensitive. A series of matrix files were downloaded as TXT files. Data preprocessing included the conversion and rejection of unqualified data, calibration, filing of missing data and standardization. The demographics and clinical characteristics of these samples of the microarray profiles GSE114206, GSE41499 and GSE109934, except for the GSE33482 profile are summarized in Tables SI and SII.
DEG screening
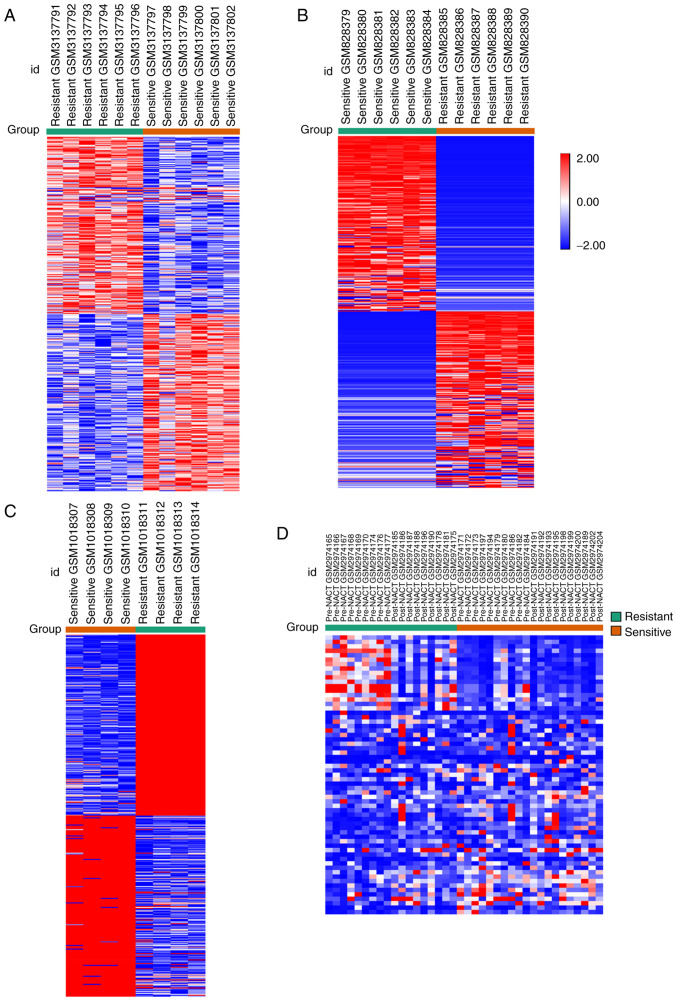
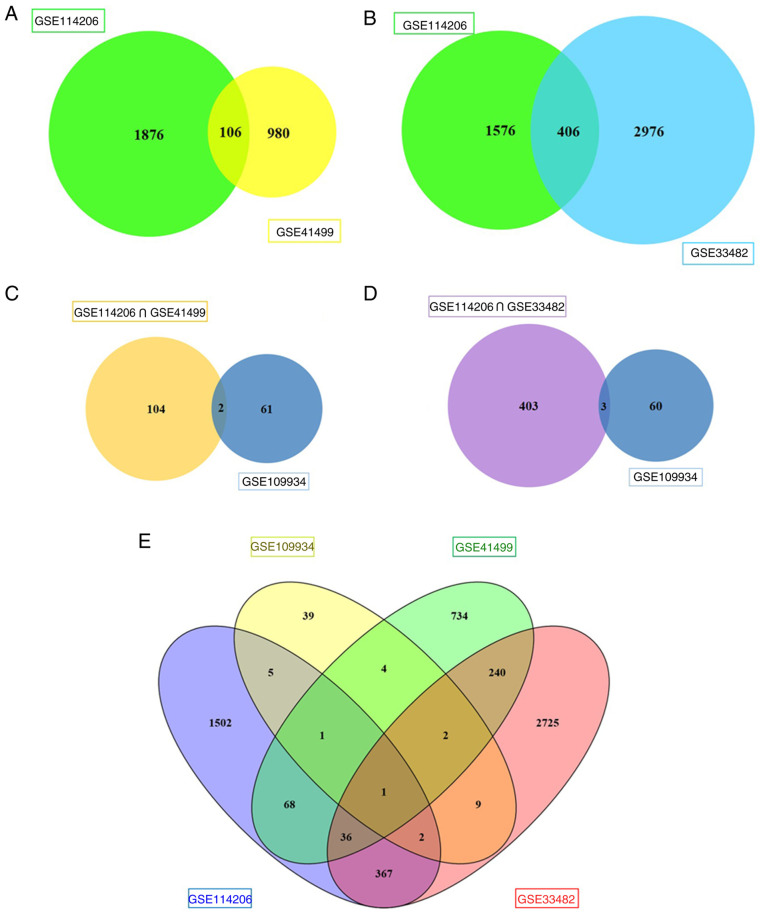
A comparison of the chemotherapy-sensitive samples and the chemotherapy-resistant samples identified the DEGs based on GEO2R analysis and the defined values of |log FC| ≥1 and P<0.05. A total of 1,976, 1,086 and 3,382 DEGs were identified from the GSE114206, GSE41499 and GSE33482 datasets, respectively (expression heat map of the top DEGs are shown in Fig. 1A-C from GSE114206, GSE33482 and GSE41499). A total of 63 genes were selected as DEGs from the GSE109934 profile by comparing the platinum-resistant group with the platinum-sensitive group, and the two groups both contained pre-NACT samples with post-NACT samples. The expression heat maps are shown in Fig. 1D. Of these DEGs, 1,040 upregulated and 936 downregulated genes were selected from GSE114206, and 536 upregulated and 550 downregulated genes were selected from GSE41499. Subsequently, 106 DEGs from the two datasets (GSE114206 and GSE41499) were selected, of which 36 genes were upregulated and 70 genes were downregulated (Fig. 2A). A total of 2,052 upregulated and 1,330 downregulated genes were identified in GSE33482, and 45 upregulated and 18 downregulated genes were identified in GSE109934. Subsequently, 406 DEGs with the same expression pattern as the DEGs of the GSE114206 and GSE33482 datasets were selected, which included 157 upregulated genes and 249 downregulated genes (Fig. 2B). To further screen genes related to drug resistance before and after neoadjuvant chemotherapy, 106 DEGs and 406 DEGs from the drug-resistant profiles were compared with GSE109934 (the NACT profile). The results indicated that two core genes, nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) and Kruppel-like factor 4 (KLF4), were potentially associated with neoadjuvant chemotherapy and platinum-based chemoresistance (Fig. 2C). Three key genes [NFATc1, nuclear receptor subfamily 4 group A member 3 (NR4A3) and hepatocyte growth factor (HGF)] were selected (Fig. 2D). The results of the overall screening are shown in the Venn diagram in Fig. 2E. Four genes potentially associated with NACT and platinum-based chemoresistance were screened, including NAFTc1, KLF4, NR4A3 and HGF.
Figure 1.
Heat map of the top 400 DEGs. (A-C) The top 200 upregulated DEGs and the top 200 downregulated DEGs in the resistant chemotherapy group compared with the sensitive chemotherapy group. (A) Expression heat map of the top DEGs in GSE114206. (B) Expression heat map of the top DEGs in GSE33482. (C) Expression heat map of the top DEGs in GSE41499. (D) A total of 63 differentially expressed genes were selected as DEGs from GSE109934 profile by comparing the platinum-resistant group with the platinum-sensitive group, and the two groups both contained pre-NACT samples with post-NACT samples, of which 45 upregulated and 18 downregulated genes are shown. Red, upregulated DEGs; blue, downregulated DEGs. DEGs, differentially expressed genes; NACT, neoadjuvant chemotherapy.
Figure 2.
Venn diagram. (A) A total of 106 DEGs in the two datasets (GSE114206 and GSE41499) were singled out, of which 36 genes were upregulated and 70 genes were downregulated. (B) A total of 406 DEGs in the two datasets (GSE114206 and GSE33482) were chosen, of which 157 genes were upregulated and 249 genes were downregulated. (C) Two core genes, NFATc1 and KLF4, were potentially associated with NACT and platinum-based chemoresistance. (D) Three key genes NFATc1, NR4A3 and HGF were selected. (E) The results of the overall screening of the Venn diagram are shown. DEGs, differentially expressed genes; NACT, neoadjuvant chemotherapy; NAFTc1, nuclear factor of activated T-cells, cytoplasmic 1; KLF4, Kruppel-like factor 4; NR4A3, nuclear receptor subfamily 4 group A member 3; HGF, hepatocyte growth factor.
GO and KEGG analyses
The results of the GO and KEGG analyses are presented in Tables II and III, respectively, based on P<0.05 in the DAVID tool. Of the 106 DEGs, those that were upregulated DEGs were most abundant in the BP categories ‘Negative regulation of signal transduction’ and ‘Negative regulation of cell communication’ as well as MF category ‘Transcription regulator activity’. The downregulated DEGs were primarily associated with ‘Positive regulation of defense response’ in the BP category, ‘Plasma membrane part’ in the CC category, and ‘Cytokine activity’ and ‘Growth factor activity’ in the MF category. Consistent with the results of the KEGG analysis, the downregulated DEGs were enriched in ‘Toll-like receptor signaling pathway’ development (Table II).
Table II.
Gene Ontology analysis and KEGG enrichment analyses of 106 differentially expressed genes correlated with platinum-based chemoresistance in ovarian carcinoma.
| Category | Term | Count | % | P-value |
|---|---|---|---|---|
| Upregulated | ||||
| GOTERM_BP_FAT | GO:0009968~negative regulation of signal transduction | 4 | 1.2 | 1.5E-2 |
| GOTERM_BP_FAT | GO:0010648~negative regulation of cell communication | 4 | 1.2 | 2.0E-2 |
| GOTERM_MF_FAT | GO:0030528~transcription regulator activity | 8 | 2.4 | 2.6E-2 |
| Downregulated | ||||
| GOTERM_BP_FAT | GO:0031349~positive regulation of defense response | 6 | 0.9 | 1.9E-5 |
| GOTERM_BP_FAT | GO:0008285~negative regulation of cell proliferation | 10 | 1.4 | 3.2E-5 |
| GOTERM_BP_FAT | GO:0042127~regulation of cell proliferation | 14 | 2.0 | 3.9E-5 |
| GOTERM_BP_FAT | GO:0006955~immune response | 12 | 1.7 | 2.3E-4 |
| GOTERM_BP_FAT | GO:0051174~regulation of phosphorus metabolic process | 10 | 1.4 | 3.0E-4 |
| GOTERM_CC_FAT | GO:0044459~plasma membrane part | 21 | 3.0 | 1.5E-3 |
| GOTERM_CC_FAT | GO:0005886~plasma membrane | 29 | 4.1 | 2.7E-3 |
| GOTERM_CC_FAT | GO:0005576~extracellular region | 19 | 2.7 | 3.2E-3 |
| GOTERM_CC_FAT | GO:0044421~extracellular region part | 11 | 1.6 | 1.2E-2 |
| GOTERM_CC_FAT | GO:0005615~extracellular space | 9 | 1.3 | 1.3E-2 |
| GOTERM_MF_FAT | GO:0005125~cytokine activity | 6 | 0.9 | 1.4E-3 |
| GOTERM_MF_FAT | GO:0008083~growth factor activity | 5 | 0.7 | 4.8E-3 |
| GOTERM_MF_FAT | GO:0005068~transmembrane receptor protein tyrosine kinase adaptor protein activity | 2 | 0.3 | 2.1E-2 |
| GOTERM_MF_FAT | GO:0005539~glycosaminoglycan binding | 4 | 0.6 | 2.1E-2 |
| GOTERM_MF_FAT | GO:0001871~pattern binding | 4 | 0.6 | 2.7E-2 |
| KEGG_PATHWAY | hsa04620: Toll-like receptor signaling pathway | 4 | 0.6 | 2.9E-2 |
GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; BP, biological process; CC, cell component; MF, molecular function.
Table III.
Gene ontology analysis and KEGG enrichment analyses of 406 differentially expressed genes correlated to platinum-based chemoresistance in ovarian carcinoma.
| Category | Term | Count | % | P-value |
|---|---|---|---|---|
| Upregulated | ||||
| GOTERM_BP_FAT | GO:0051240~positive regulation of multicellular organismal process | 10 | 0.8 | 2.7E-4 |
| GOTERM_BP_FAT | GO:0002474~antigen processing and presentation of peptide antigen via MHC class I | 4 | 0.3 | 3.9E-4 |
| GOTERM_BP_FAT | GO:0048002~antigen processing and presentation of peptide antigen | 4 | 0.3 | 1.8E-3 |
| GOTERM_BP_FAT | GO:0007267~cell-cell signaling | 14 | 1.1 | 2.0E-3 |
| GOTERM_BP_FAT | GO:0008283~cell proliferation | 11 | 0.8 | 4.5E-3 |
| GOTERM_CC_FAT | GO:0044421~extracellular region part | 26 | 2.0 | 1.7E-7 |
| GOTERM_CC_FAT | GO:0005576~extracellular region | 39 | 3.0 | 2.2E-7 |
| GOTERM_CC_FAT | GO:0044459~plasma membrane part | 39 | 3.0 | 2.4E-6 |
| GOTERM_CC_FAT | GO:0031226~intrinsic to plasma membrane | 25 | 1.9 | 3.7E-5 |
| GOTERM_CC_FAT | GO:0005887~integral to plasma membrane | 24 | 1.8 | 7.7E-5 |
| GOTERM_MF_FAT | GO:0005125~cytokine activity | 8 | 0.6 | 1.5E-3 |
| GOTERM_MF_FAT | GO:0030246~carbohydrate binding | 10 | 0.8 | 3.5E-3 |
| GOTERM_MF_FAT | GO:0032393~MHC class I receptor activity | 3 | 0.2 | 9.2E-3 |
| GOTERM_MF_FAT | GO:0004947~bradykinin receptor activity | 2 | 0.2 | 2.6E-2 |
| GOTERM_MF_FAT | GO:0008454~alpha-1,3-mannosylglycoprotein 4-beta-N-acetylglucosaminyltransferase activity | 2 | 0.2 | 2.6E-2 |
| KEGG_PATHWAY | hsa04060: Cytokine-cytokine receptor interaction | 12 | 0.9 | 7.3E-5 |
| KEGG_PATHWAY | hsa04940: Type I diabetes mellitus | 5 | 0.4 | 9.4E-4 |
| KEGG_PATHWAY | hsa05330: Allograft rejection | 4 | 0.3 | 6.3E-3 |
| KEGG_PATHWAY | hsa05332: Graft-versus-host disease | 4 | 0.3 | 7.9E-3 |
| KEGG_PATHWAY | hsa04630: Jak-STAT signaling pathway | 6 | 0.5 | 2.3E-2 |
| Downregulated | ||||
| GOTERM_BP_FAT | GO:0050830~defense response to Gram-positive bacterium | 5 | 0.2 | 1.2E-4 |
| GOTERM_BP_FAT | GO:0002449~lymphocyte mediated immunity | 7 | 0.3 | 3.1E-4 |
| GOTERM_BP_FAT | GO:0002460~adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains | 7 | 0.3 | 5.2E-4 |
| GOTERM_BP_FAT | GO:0002250~adaptive immune response | 7 | 0.3 | 5.2E-4 |
| GOTERM_BP_FAT | GO:0009968~negative regulation of signal transduction | 11 | 0.5 | 6.9E-4 |
| GOTERM_CC_FAT | GO:0044421~extracellular region part | 32 | 1.5 | 5.2E-6 |
| GOTERM_CC_FAT | GO:0005615~extracellular space | 23 | 1.1 | 1.5E-4 |
| GOTERM_CC_FAT | GO:0005576~extracellular region | 47 | 2.2 | 1.8E-4 |
| GOTERM_CC_FAT | GO:0044459~plasma membrane part | 46 | 2.2 | 2.6E-3 |
| GOTERM_CC_FAT | GO:0031012~extracellular matrix | 13 | 0.6 | 2.7E-3 |
| GOTERM_MF_FAT | GO:0008081~phosphoric diester hydrolase activity | 5 | 0.2 | 2.1E-2 |
| GOTERM_MF_FAT | GO:0005164~tumor necrosis factor receptor binding | 3 | 0.1 | 2.8E-2 |
| GOTERM_MF_FAT | GO:0005125~cytokine activity | 7 | 0.3 | 3.6E-2 |
| GOTERM_MF_FAT | GO:0005509~calcium ion binding | 19 | 0.9 | 4.0E-2 |
| GOTERM_MF_FAT | GO:0022890~inorganic cation transmembrane transporter activity | 6 | 0.3 | 4.2E-2 |
| KEGG_PATHWAY | hsa04514: Cell adhesion molecules (CAMs) | 8 | 0.4 | 6.6E-3 |
| KEGG_PATHWAY | hsa04060: Cytokine-cytokine receptor interaction | 11 | 0.5 | 1.2E-2 |
| KEGG_PATHWAY | hsa05330: Allograft rejection | 4 | 0.2 | 2.2E-2 |
| KEGG_PATHWAY | hsa04940: Type I diabetes mellitus | 4 | 0.2 | 3.3E-2 |
| KEGG_PATHWAY | hsa04672: Intestinal immune network for IgA production | 4 | 0.2 | 4.9E-2 |
GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; BP, biological process; CC, cell component; MF, molecular function.
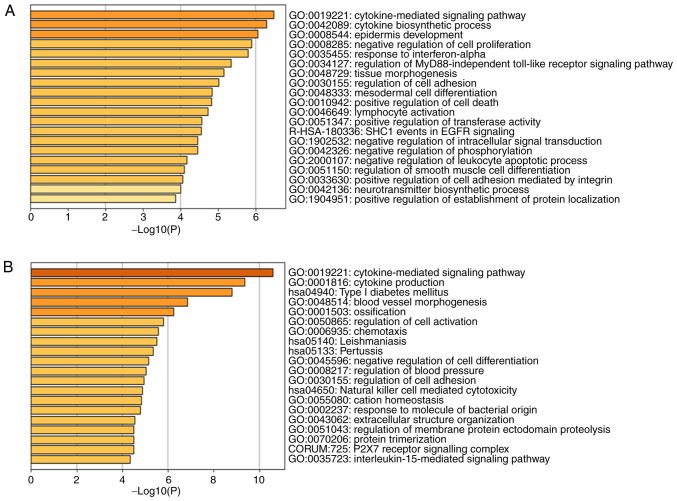
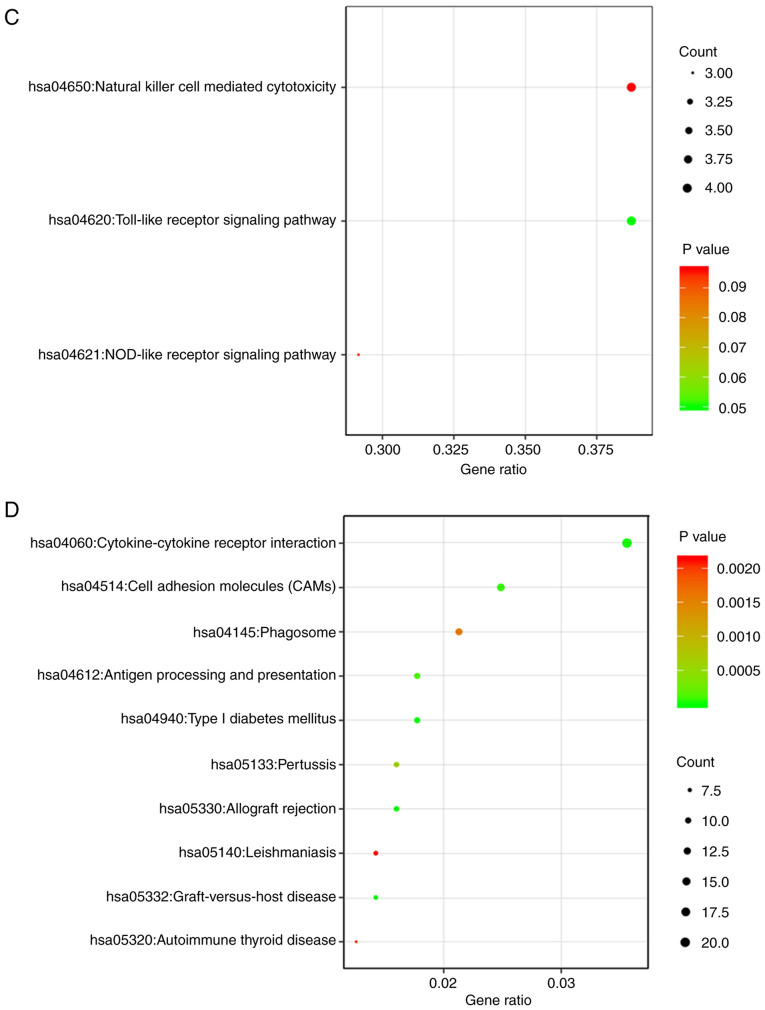
Of the 406 DEGs, the upregulated DEGs were principally concentrated in the BP category ‘Positive regulation of multicellular organismal process’, the CC category ‘Extracellular region part’, and the MF categories ‘Cytokine activity’ and ‘Carbohydrate binding’. The downregulated DEGs were primarily associated with ‘Defense response to a Gram-positive bacterium’ in the BP category, ‘Extracellular region part’ in the CC category, and ‘Phosphoric diester hydrolase activity’ in the MF category. The results also showed that the upregulated DEGs were generally associated with ‘Cytokine-cytokine receptor interaction’, ‘Type I diabetes mellitus’ and ‘JAK-STAT signaling pathway’, whereas the downregulated DEGs were associated with ‘Cell adhesion molecules (CAMs)’, ‘Cytokine-cytokine receptor interaction’ and ‘Allograft rejection’ according to the KEGG pathway analysis (Table III). The outcomes of the 106 DEGs and 406 DEGs are also presented as a bar chart and scatterplot diagram of the results of the GO and KEGG analyses, respectively. According to the results of a bar chart and scatterplot diagram in 106 DEGs, the significantly enriched GO terms were cytokine-mediated signaling pathway, cytokine biosynthetic process and epidermis development in Fig. 3A and the significantly enriched KEGG terms were Toll-like receptor signaling pathway in Fig. 3C. The results of a bar chart and scatterplot diagram in 406 DEGs showed that GO terms were mainly enriched in the cytokine-mediated signaling pathway, cytokine production and type I diabetes mellitus in Fig. 3B while KEGG terms were mainly enriched in cytokine-cytokine receptor interaction, cell adhesion molecules and phagosome in Fig. 3D. Only the top three terms are listed.
Figure 3.
GO and KEGG pathway analysis of the 106 DEGs and 406 DEGs. (A and B) Barplots were designed by Metascape tool. (C and D) Dotplots were conducted through Easychart tool. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes.
PPI network
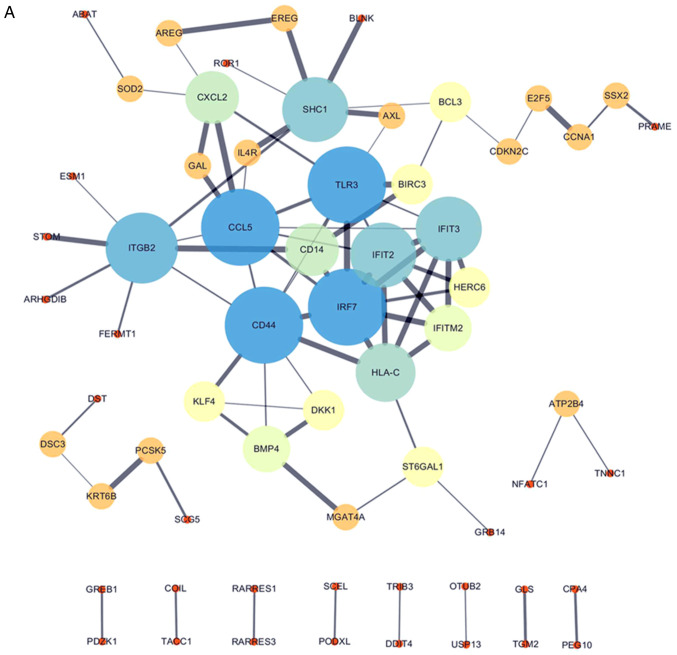
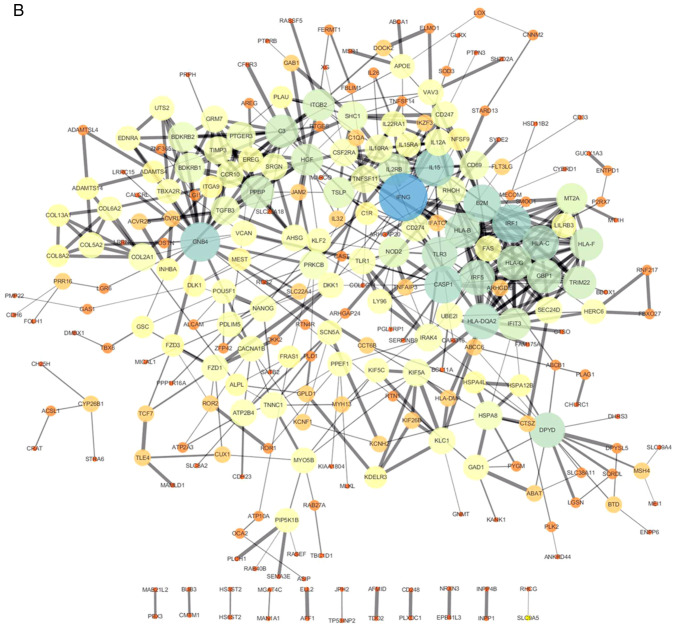
All 106 DEGs from the 2 datasets (GSE114206 and GSE41499) (Fig. 4A) and all 406 DEGs from the 2 datasets (GSE114206 and GSE33482) (Fig. 4B) were analyzed using the STRING database to further examine the characteristics and the potential interconnections of the DEGs. A PPI network of the DEGs was generated in Cytoscape (Fig. 4A and B).
Figure 4.
PPI network of the DEGs. (A) Gene coexpression network of 106 DEGs from the 2 cohort profile data sets (GSE114206 and GSE41499). (B) Gene coexpression network of 406 DEGs from the 2 cohort profile data sets (GSE114206 and GSE33482). PPI, protein-protein interaction; DEGs, differentially expressed genes.
Protein/gene interaction network and biological processes
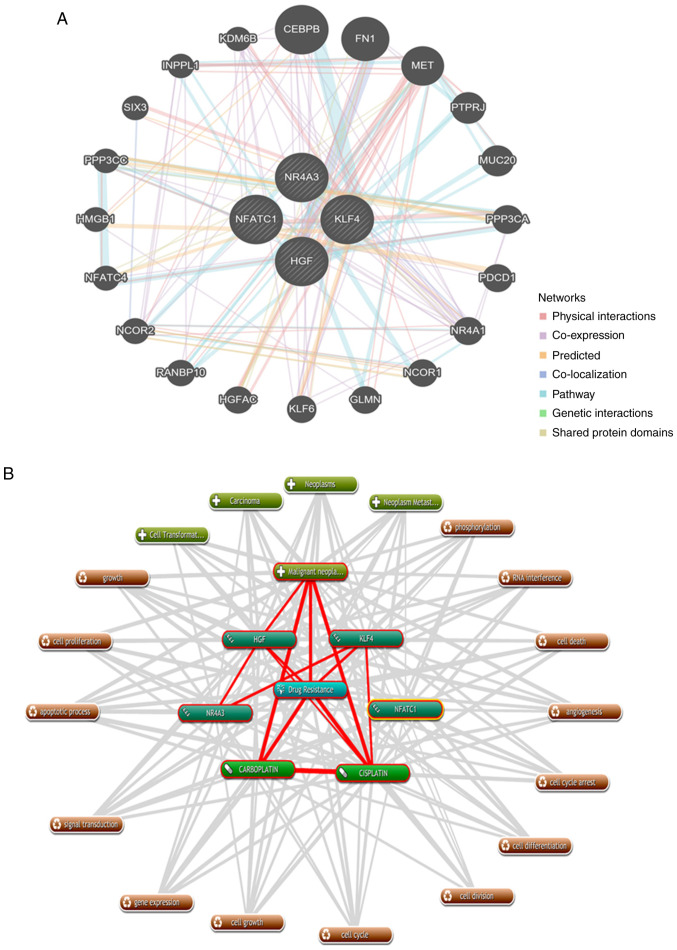
GeneMANIA was used to analyze and elucidate the functional roles of NFATc1, KLF4, NR4A3 and HGF in drug tolerance. More importantly, additional bioinformatics analysis exposed the interrelationships among the four genes that play a role in chemotherapeutic resistance in EOC. A protein/gene interaction network was formed for the four genes and 20 genes/proteins that corresponded to drug-tolerance, which included CEBPB, FN1, MET, PTPRJ, MUC20, PPP3CA, PDCD1, NR4A1, NCOR1, GLMN, KLF6, HGFAC, RANBP10, NCOR2, NFATC4, HMGB1, PPP3CC, SIX3, INPPL1 and KDM6B. As shown in Fig. 5A, NFATc1, KLF4, NR4A3 and HGF were associated with 20 drug-resistant proteins/genes.
Figure 5.
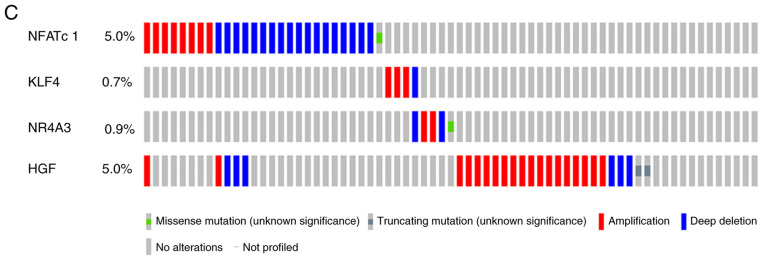
Protein/gene interaction network and biological process annotation of the differentially expressed genes. (A) Protein/gene-protein/gene interaction network of the four proteins/genes with 20 drug resistance-related proteins/genes in OC. (B) Four genes/proteins identified using Coremine Medical tools were associated with drug resistance and OC. Input terms were NFATc1, KLF4, NR4A3, HGF, drug resistance, cisplatin, carboplatin and OC. (C) Genetic alterations. Red indicates amplification and blue indicates deep deletion of genes in 57 of the 594 serous ovarian cancer patients (10%). OC, ovarian cancer; NAFTc1, nuclear factor of activated T-cells, cytoplasmic 1; KLF4, Kruppel-like factor 4; NR4A3, nuclear receptor subfamily 4 group A member 3; HGF, hepatocyte growth factor.
As shown in Fig. 5B, text mining analysis indicated that these four key genes directly or indirectly participate in 14 physiological processes that contribute to drug-resistance in OC, including cell proliferation, cell differentiation, apoptosis, cell division and phosphorylation (P<0.05). Additionally, the OncoPrint module in the cBioPortal was used to evaluate genetic conversions and demonstrated that 10% (57/594) of patients exhibited genetic metamorphosis (Fig. 5C).
RT-qPCR analysis
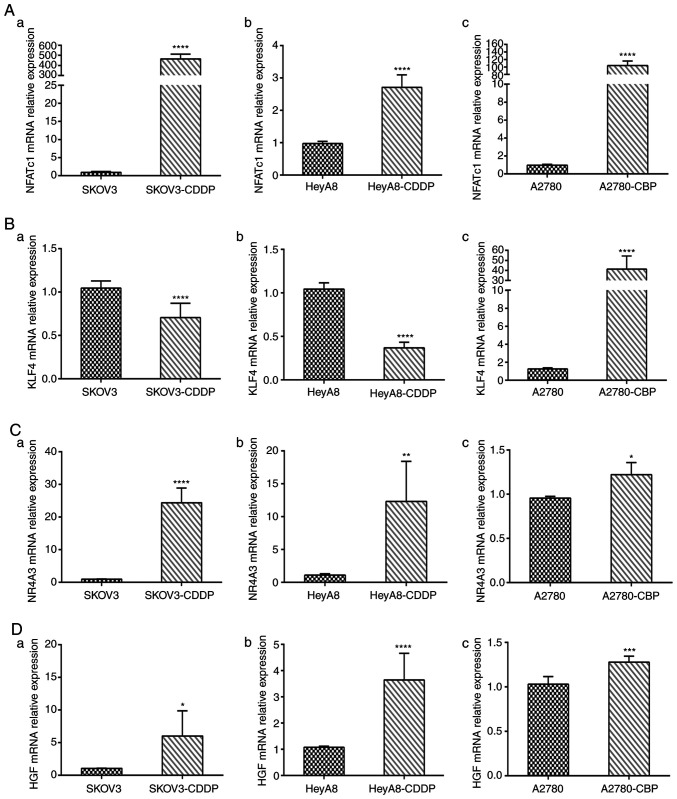
We quantified the mRNA levels of the four genes NFATc1, KLF4, NR4A3 and HGF in sensitive ovarian cells and cisplatin- and carboplatin-resistant cell lines. The mRNA expression levels of NFATc1 were significantly increased in cisplatin- and carboplatin-resistant cells compared with those in sensitive OC cells (all P-values <0.0001; Fig. 6A-a-c). In addition, mRNA expression of KLF4 was significantly lower in the SKOV3-CDDP and HeyA8-CDDP cell lines than that in the SKOV3 and HeyA8 cell lines (both P<0.0001; Fig. 6B-a and -b). However, mRNA expression of KLF4 was increased in the A2780-CBP cells (P<0.0001; Fig. 6B-c). Compared with sensitive cells, the expression of NR4A3 was increased in the SKOV3-CDDP, HeyA8-CDDP and A2780-CBP cells (P<0.0001, P<0.01, and P<0.05, respectively) (Fig. 6C-a-c). We also observed that the expression of HGF was significantly increased in SKOV3-CDDP, HeyA8-CDDP and A2780-CBP cells compared with that in the corresponding sensitive ovarian cancer cells (P<0.05, P<0.0001, and P<0.001, respectively) (Fig. 6D-a-c).
Figure 6.
Using RT-qPCR to detect gene mRNA expression in cisplatin- and carboplatin-resistant cells and sensitive ovarian cancer cells. (A) NFATc1 mRNA expression level in parental sensitive/resistant: (a) SKOV3/SKOV3-CDDP, (b) HeyA8/HeyA8-CDDP and (c) A2780/A2780-CBP cell lines. (B) KLF4 mRNA expression level in (a) SKOV3/SKOV3-CDDP, (b) HeyA8/HeyA8-CDDP and (c) A2780/A2780-CBP cell lines. (C) NR4A3 mRNA expression level in parental sensitive/resistant: (a) SKOV3/SKOV3-CDDP, (b) HeyA8/HeyA8-CDDP and (c) A2780/A2780-CBP cell lines. (D) KLF4 mRNA expression level in parental sensitive/resistant: (a) SKOV3/SKOV3-CDDP, (b) HeyA8/HeyA8-CDDP and (c) A2780/A2780-CBP cell lines. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, compared with the parental cell line. CDDP, cisplatin; CBP, carboplatin.
Cell migration and invasion assay analyses
The migratory and invasive potential of sensitive ovarian cells and cisplatin- and carboplatin-resistant cells in the Transwell migration assay is shown in Fig. S1A. Quantification revealed a significant 218.0±8.01% increase in migration in the SKOV3-CDDP cell line compared with that in sensitive SKOV3 cells (P<0.01; Fig. S1B-a), a 169.0±5.15% increase in migration in the HeyA8-CDDP cells compared with that in HeyA8 cells (P<0.01; Fig. S1B-b), and a significant 225.9±14.08% increase in migration in the A2780-CBP cell line compared with that in A2780 cells (P<0.01; Fig. S1B-c). Quantification revealed a significant 216.8±19.52% increase in invasion in the SKOV3-CDDP cell line compared with that in sensitive SKOV3 cells (P<0.01; Fig. S1C-a), a 271.9±22.81% increase in invasion in the HeyA8-CDDP cells compared with that in HeyA8 cells (P<0.01; Fig. S1C-b), and a significant 222.2±6.61% increase in invasion in the A2780-CBP cell line compared with that in A2780 cells (P<0.01; Fig. S1C-c).
Discussion
Ovarian cancer (OC) is the primary cause of death worldwide in female reproductive malignancies (21,22) OC patients have no apparent symptoms at early stages, and the majority of patients are diagnosed at late stages. The current standard treatment strategy for advanced epithelial ovarian cancer (AEOC) is primary cytoreductive surgery in combination with platinum-based first-line chemotherapy and radiotherapy or endocrine therapy (23,24). When adequate evaluation suggests that patients are less likely to receive a satisfactory outcome from tumor cell depletion surgery, neoadjuvant chemotherapy (NACT) followed by interval-based nodulation (NACT-IDS) after three platinum-based chemotherapy cycles has become an increasingly common treatment strategy (25–28). Overall, research has shown that the survival rate of patients with stage III or IV OC treated with NACT-IDS is not inferior to that of patients receiving primary cytoreductive surgery followed by chemotherapy (27). However, Rauh-Hain et al (5) suggested that compared with the primary surgery (PDS) group, patients in the NACT-IDS group had a higher rate of resistance to platinum after initial platinum chemotherapy. Therefore, there is an urgent clinical need to identify predictive markers for drug resistance and to accurately assess the risk before treatment to identify AEOC patients who are most likely to benefit from NACT and to optimize the role of NACT in OC treatment.
Therefore, the present study will aid gynecologic oncologists to better understand the mechanisms of OC resistance and avoid the occurrence of early chemotherapy resistance in their patients. Additionally, the core genes in this study may contribute to the identification of candidate biomarkers and the introduction of novel therapeutic targets for EOC.
In our research, the combined analyses identified 4 DEGs between pre-NACT and post-NACT cells that were also in common between sensitive and cisplatin- /carboplatin-resistant OC cells; these genes were ascertained as those most likely to be associated with neoadjuvant chemotherapy and platinum-based chemoresistance in EOC.
The nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) gene is a member of the NFAT gene family and encodes a component of the nuclear factor of activated T cells DNA-binding transcription complex and has been identified as a transcription factor in recent years.
The NFAT family
As required components for promoting and activating the expression of IL-2 and IL-2 receptors, members of the NFAT family are primarily described as being present in inactive T cells and functioning as a set of T cell-specific transcription factors (29–31). From the ongoing study of the NFAT family, we have found that NFAT is not only T cell-specific but is also expressed in a wide range of lymphoid cells and possibly in non-lymphocytes, such as natural killer (NK) cells and macrophages. More recently, five NFAT subfamily members, including the NFAT1, NFAT2, NFAT3, NFAT4 and NFAT5 families, have been discovered (32,33). Four members of the NFAT gene subfamily, NFAT1, NFAT2, NFAT3 and NFAT4, encode four proteins that are regulated by the calcium and calcineurin signaling pathway. The NFAT5 gene was found to encode a protein regulated by the cellular response to hyperosmotic stress (34,35).
Structure of the NFAT family
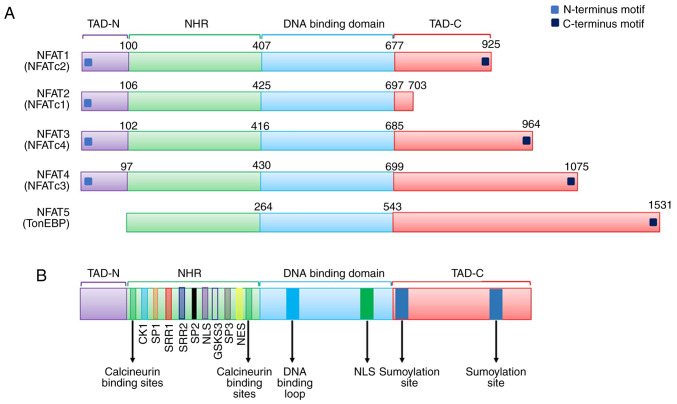
The general structure of the NFAT protein family is shown in Fig. 7A. The conventional NFAT protein (NFAT1-4) contains N- and C-terminal transactivation domains (TAD-N and TAD-C, respectively), which are highly variable in different NFAT proteins and isoforms. Additionally, except for the absence of a calcineurin-binding domain in the structure of NFAT5, all other conventional members have a Rel homology region (RHR), which consists of an NFAT homology region (NHR) and a DNA-binding domain (DBD). NFAT5 also lacks Fos/Jun residues, so it cannot bind to DNA through c-Fos and Jun. However, NFAT5 preserves the DNA contact residues of NFAT1 through NFAT4, such as the Rel homology domain, which can help bind DNA sequences (36). Deeper study of the NFAT family has shown that NHR contains several conserved regulatory motifs among NFAT family members, such as a nuclear localization sequence (NLS), a nuclear export signal (NES), serine-rich regions (SRRs), serine-proline repeat motifs (SPs), and a phosphorylation site. Moreover, NFAT family members contain a DNA-binding loop, binding sites for Fos and Jun in NFAT1-4, and a NES in the DNA-binding domain (DBD) (37). A schematic of the primary alignment of NFAT1-5 is displayed in Fig. 7B.
Figure 7.
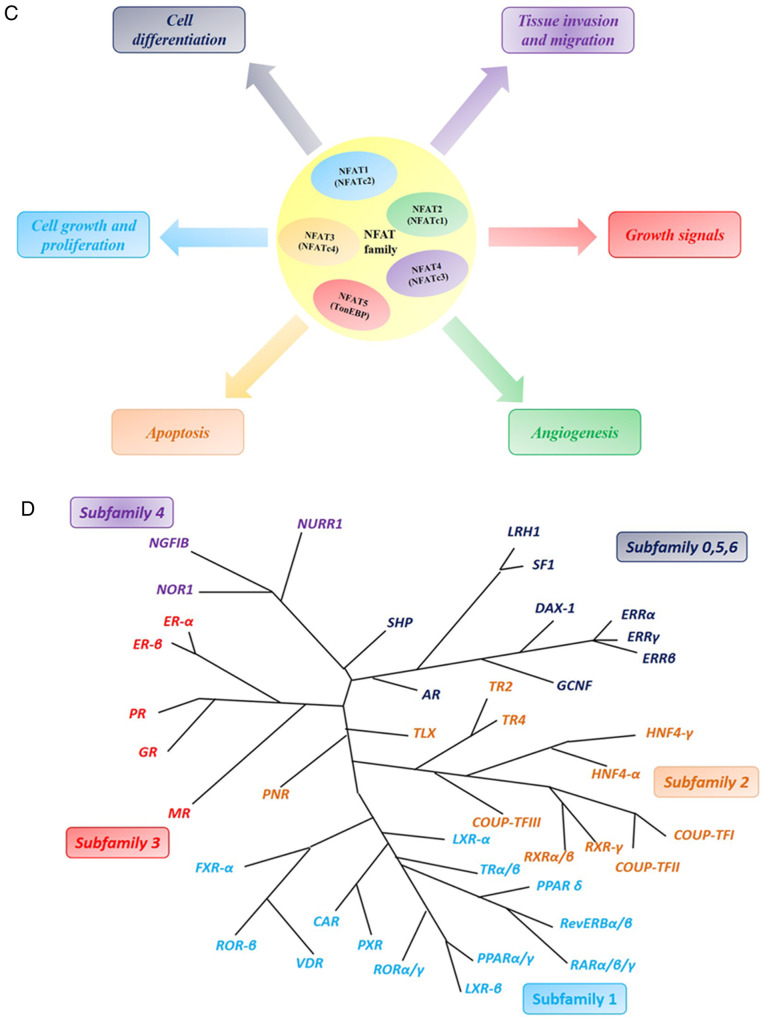
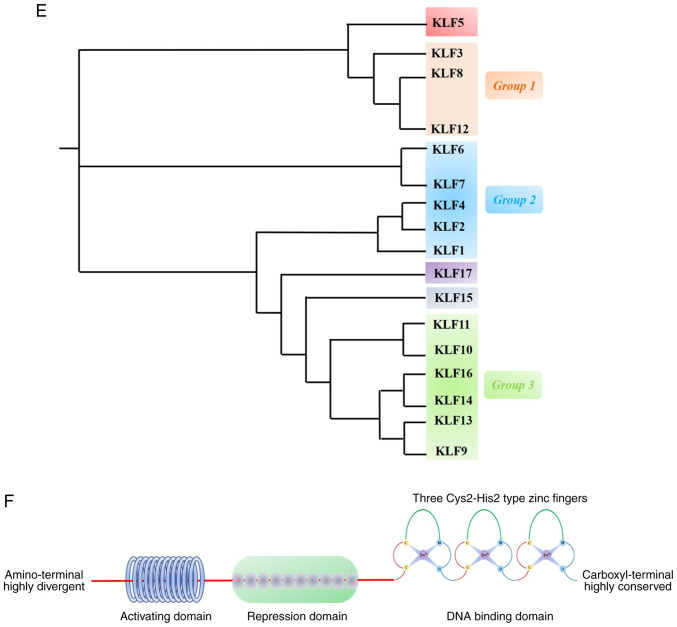
(A) General structure of the NFAT protein family is shown. NFAT protein consists of N- and C-terminus transactivation domains which are tremendously alterable among the different NFAT members and isoforms. Except that NFAT5 has no calcineurin-binding domain in its structure, all other conventional members have a structure named as REL homology region (RHR), which consisted of a NFAT homology region (NHR) and DNA-binding domain (DBD). (B) Schematic primary alignment of NFAT. The NHR contains a nuclear localization sequence (NLS), a nuclear export signal (NES) and two calcineurin binding sites. NHR also contains serine-rich regions (SRRs), serine-proline-repeat motifs (SPs), and a phosphorylation site, such as casein kinase 1 (CK1), GSK3 docking site. The DNA binding domain, contains DNA-binding loop sites that directly contacts DNA core sequence (A/T) GGAAA and a nuclear localization sequence NLS. C-terminus transactivation domains (TAD-C) consist of two sumoylation sites. (C) Tumor-related processes regulated by NFAT transcription factors. The nuclear factor of activated T-cells (NFAT) family includes five subfamilies, namely, the NFAT1 family, the NFAT2 family and the NFAT3, NFAT4 and NFAT5 family. NFAT proteins directly regulate the expression of genes related to cellular proliferation, apoptosis, angiogenesis and tissue invasion mechanisms among others. (D) NOR1 (NR4A3) belongs to one of the NR4A family. NR4A subfamily members are one of the subfamily of nuclear receptors (NRs). NRs are major drug targets for the therapy of reproductive abnormalities and cancer. We classified and present 48 NR members. (E) Phylogenetic classification of the human Krüppel-like factor (KLF) family. Human Genome Organization Gene Nomenclature Committee has numbered the Krüppel-like factor family in the order in which members of were discovered. Based on the structural similarity of the N-terminal, 17 human KLF proteins can be divided into three major subfamilies. Due to the presence of recognizable protein-protein interactions motifs, KLF5, 15 and 17 are not classified into any other family. KLF3, 8, and 12 (group 1) usually bind to the C-terminal binding proteins (CtBPs), which act as inhibitors of gene transcription. Members of the second group of KLF factors, including 1, 2, 4, 6, and 7, generally reckon as transcriptional activators because they all have acidic activation domains. KLF9, 10, 11, 13, 14 and 16 (group 3) share a Sin3a-interacting domain (SID), an α-helical motif that interworks with the repressor protein Sin3a. (F) Structure and function domains of KLF4, where the amino terminal is the transcriptional activation domain, the carboxyl terminal (triple Cys2-His2 zinc finger) is the DNA-binding domain, which are highly conserved among KLFs, and repression domain is the intermediate target of post-translational regulation.
Function of the NFAT family
The NFAT family was originally regarded as transcription factors that affect T cell activation. Therefore, in recent decades, members of this family have been major molecular targets for the development of immunosuppressive drugs, such as cyclosporine A, which modulates T cell immunity in autoimmune diseases (38,39). Accumulating evidence indicates that NFATs are indirectly involved in the regulation of the cell cycle and other processes, which suggests their broader role in normal physiological processes. Participation of NFAT in the processes of tumorigenicity and transformation has been reported (32). In addition, NFAT proteins can directly regulate the expression of genes related to cellular proliferation, angiogenesis and tissue invasion mechanisms in cancers (40,41) (Fig. 7C). Xu et al (42) demonstrated that NFATc1 is extremely overexpressed in ovarian tumor tissues compared with normal tissues. Additionally, c-myc was upregulated to accelerate cell proliferation through activation of the ERK1/2/p38/MAPK signaling pathway by NFATc1. Recent research has also found that NFATc1 modulates cell proliferation, migration, and invasion in SKOV3 cells (43). Additionally, NFATc1 siRNA can dramatically decrease SKOV3 cell growth (44). Furthermore, NFATc1 overexpression is linked to a poor prognosis in urothelial carcinoma (45). NFAT1 also plays a vital role in aspects of drug-resistant cancers. Murray et al (46) demonstrated that NFATc1 is a crucial factor in mediating drug-resistance and upgrades the tumor response to chemotherapeutic drugs in pancreatic cancer. Our research results also demonstrated that the mRNA expression levels of NFATc1 were significantly increased in cisplatin- and carboplatin-resistant cells compared with those in sensitive OC cells. Thus, our findings in the present study are consistent with the mentioned above studies.
Taken together, the results indicate that NFATc1 may be a drug resistance candidate and that better NFAT inhibitors may be promising targets for neoadjuvant chemotherapy and platinum-based chemoresistance in epithelial ovarian carcinoma. However, more research is needed to further detail the roles of NFATc1 in chemoresistance.
The nuclear receptor subfamily 4 group A member 3 (NR4A3) gene, also called the oxidored-nitro domain protein 1 (NOR1), is a member of the NR4A family. NR4A subfamily members are a subfamily of nuclear receptors (NRs).
NRs were identified in metazoans by Mangelsdorf et al (47) and represent the largest family of transcription factors. A number of small lipophilic ligands and cellular signaling pathways, including vitamins, fatty acids and cholesterol metabolites, can regulate a large conserved family of ligand-dependent transcription factors known as NRs (48). There is increasing evidence that NRs play important roles in a number of human physiological processes, including cell development, reproduction, circadian rhythms, and metabolism, and therefore, they have become primary drug targets for the treatment of cancer, cardiovascular disease and metabolic syndrome (49–51). NRs have also been among the most successful targets for drugs approved to treat many diseases, including cancer (52,53). The 48 members of the NR superfamily of the human genome are divided into seven subfamilies (54,55). A phylogenetic tree can be used to classify the subfamilies based on their sequences (56). The seven subfamilies of the 48 NRs are presented in Fig. 7D.
Subfamily 4 consists of neuron-derived orphan receptor-1 (NR4A3), nerve growth factor 1B (NR4A1) and nurr-related factor 1 (NR4A2). A previous study reported that compared with the control myometrium, the expression of NR4A subfamily members was significantly restrained in leiomyoma (57). In hematopoietic neoplasms, downregulated NRs, NR4A3 and NR4A1, which are believed to be tumor suppressors, were found to contribute to the formation of acute myeloid leukemia (58,59). In contrast, Haller et al (60) demonstrated that the overexpression of NR4A3 could stimulate cell proliferation in mouse salivary gland cancer. NOR1 may act as an antigen in tumor cells, and altered NOR1 expression can help clarify the function of the NOR1 protein in liver cancer (61). These results are consistent with our findings indicating that the NR4A3 protein is significantly increased in drug-resistant ovarian cancer. Currently, there are few studies on the NR4A3 gene and protein in epithelial ovarian tumors. There has been controversy in previous studies regarding the role of the NR4A3 gene in promoting cancer or tumor suppression in different types of tumors. It is speculated that the detection of altered NOR1 expression before surgery in patients with advanced EOC could help to identify whether tumor cells are resistant to platinum-based chemotherapy drugs. However, the exact function of the NR4A3 gene and protein has yet to be determined with a sufficient number of validated experimental trials.
KLF4, also known as gut-enriched Krüppel-like factor or GKLF, belongs to the Krüppel transcription factor family and has multiple functions, including cell differentiation, embryogenesis and pluripotency. Shields et al (62) first isolated and identified KLF4 from a NIH3T3 cDNA library. The Human Genome Organization Gene Nomenclature Committee numbered the members of the Krüppel-like factor family in the order in which they were discovered. Based on the structural similarity of their N-terminus, as shown in Fig. 7E, 17 human KLF proteins can be divided into three major subfamilies. Due to the presence of recognizable protein-protein interaction motifs, Krüppel-like factors 5, 15 and 17 are not classified into any other family. The KLF4 gene is affiliated with the second group of the Krüppel family of transcription factors. Furthermore, group 2 also includes Krüppel-like factors 1, 2, 6 and 7, which are characterized by three zinc fingers in the carboxyl terminus sequences and acidic activation domains and thus generally act as transcriptional activators (63). The general structure of Krüppel-like factor 4 protein is shown in Fig. 7F.
Recent research has found that KLF4-encoded proteins can induce G1-to-S transition of the cell cycle via the p53 gene after DNA damage (64). As a tumor suppressor, it has been documented that overexpression of KLF4 in the human colon cancer cell line RKO can reduce the ability of tumor cells to migrate and invade (65). By using RT-qPCR technique, we found that mRNA expression of KLF4 was significantly lower in the SKOV3-CDDP and HeyA8-CDDP cell lines than that noted in the SKOV3 and HeyA8 cell lines in our research. Furthermore, we also observed that the migration and invasion ability of drug-resistant OC cell lines SKOV3-CDDP and HeyA8-CDDP were higher than the SKOV3 and HeyA8 cell lines. Therefore, the results of our study were in line with the above previous experiments. In addition, KLF4 has also been observed to inhibit tumors in lung cancer (66), cervical cancer (67), and pancreatic cancer (68). It is still controversial whether KLF4 plays a role in carcinogenesis (69) or cancer suppression (70) in breast cancer. Additionally, it is not fully understood how the KLF4 gene promotes or suppresses cancer in different tumors. Additionally, KLF4 has a considerable effect on cancer drug resistance. A recent report noted that KLF4 expression was dramatically higher in normal ovarian tissue than in ovarian cancer tissue. However, the efficacy of chemotherapy drugs in OC cells can be significantly improved by small molecule inducers. Thus, the attempt to induce KLF4 expression by APTO-253 is a new therapeutic approach to treat OC and avoid the emergence of chemotherapy resistance (71). Future studies will be conducted to explore the mechanism of the KLF4 protein in carcinogenesis and chemotherapy resistance.
Mesenchymal cells typically produce a protein knowns as hepatocyte growth factor (HGF) that adheres to the hepatocyte growth factor receptor. This protein regulates cell growth and cell motility in a variety of cells and tissue types.
The HGF receptor and c-Met are highly expressed in some OC cell lines and in epithelial cells of ovarian tumors. Sowter et al (72) demonstrated that the primary cause of OC cell migration may be closely related to HGF in ovarian tumor fluid. Considerable evidence has indicated that HGF/Met is overexpressed in a consistent fraction of OCs (73,74). Rasola et al found that 100 ng/ml exogenous HGF enhanced CDDP- and PTX-induced apoptosis in OC cells via the caspase-dependent apoptosis pathways, and dose-dependent effects were observed (75). In a comparison of microRNA and gene expression of targets in patients treated with NACT and primary debulking surgery (PDS) followed by platinum/taxane chemotherapy, Mariani et al (76) reported that HGF and its receptor c-Met were notably increased in post-NACT patients. Similarly, the mRNA expression levels of HGF were dramatically increased in cisplatin- and carboplatin-resistant ovarian cancer cell lines. Taken together, these studies explain why the HGF/MET axis has become a candidate target of numerous therapeutic clinical trials in platinum-based chemotherapy resistance in patients with NACT.
PDS in combination with chemotherapy often leads to drug resistance and relapse in advanced OC, and there is a clear need to pursue the development of novel alternative therapies. In the present study, we used NACT as a basis to explore the mechanism underlying drug resistance in combination with sensitive and resistant ovarian cancer cells and to analyze the induction of chemoresistance in tumor cell clone chemotherapy. In conclusion, analysis of the altered expression of NFATc1, NR4A3, KLF3 and HGF as candidate biomarkers will contribute to the selection of patients suitable for this modality of treatment. Furthermore, inhibitors of these targets may improve the therapeutic efficacy of NACT and avoid platinum-based chemotherapy resistance. However, the present study had a limitation. More in-depth experimental studies are necessary to sufficiently confirm the role of these DEGs in drug resistance mechanism of OC.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation of China (grant nos. 81972448, 81602292, 81802617 and 81602293), the Postgraduate Innovation Fund of the 13th Five-Year Comprehensive Investment, Tianjin Medical University (grant no. YJSCX201812) and the Natural Science Foundation of Tianjin (grant no. 18JCQNJC81200).
Availability of data and materials
The datasets analyzed during the current study are available in the GEO repository (http://www.ncbi.nlm.nih.gov/geo/).
Authors' contributions
KZ drafted this manuscript. KZ, WW and LC conceived and designed the study. YL, JH, FG and WT collected the data and performed the analyses. YW and FX revised the manuscript, interpreted the data and approved the final version of manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Usach I, Blansit K, Chen LM, Ueda S, Brooks R, Kapp DS, Chan JK. Survival differences in women with serous tubal, ovarian, peritoneal, and uterine carcinomas. J Obstet Gynecol. 2015;212:188.e1–e6. doi: 10.1016/j.ajog.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Kyrgiou M, Salanti G, Pavlidis N, Paraskevaidis E, Ioannidis JP. Survival benefits with diverse chemotherapy regimens for ovarian cancer: Meta-analysis of multiple treatments. J Natl Cancer Inst. 2006;98:1655–1663. doi: 10.1093/jnci/djj443. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58:131–142. doi: 10.1016/S0039-6109(16)41440-4. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 5.Rauh-Hain JA, Nitschmann CC, Worley MJ, Bradford LS, Berkowitz RS, Schorge JO, Campos SM, Del CM, Horowitz NS. Platinum resistance after neoadjuvant chemotherapy compared to primary surgery in patients with advanced epithelial ovarian carcinoma. Gynecol Oncol. 2013;129:63–68. doi: 10.1016/j.ygyno.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Ghasemi N, Ghobadzadeh S, Zahraei M, Mohammadpour H, Bahrami S, Ganje MB, Rajabi S. HE4 combined with CA125: Favorable screening tool for ovarian cancer. Med Oncol. 2014;31:808. doi: 10.1007/s12032-013-0808-0. [DOI] [PubMed] [Google Scholar]
- 7.Qin L, Huang H, Chen M, Liang Y, Wang H. Clinical study of a CT evaluation model combined with serum CA125 in predicting the treatment of newly diagnosed advanced epithelial ovarian cancer. J Ovarian Res. 2018;11:49. doi: 10.1186/s13048-018-0422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Xiao Z, Liu Z, Lv F. MRI can be used to differentiate between primary fallopian tube carcinoma and epithelial ovarian cancer. Clin Radiol. 2020;75:457–465. doi: 10.1016/j.crad.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman-Rothe N, Curry E, Zeller C, Liber D, Stronach E, Gabra H, Ghaem-Maghami S, Brown R. Chromatin H3K27me3/H3K4me3 histone marks define gene sets in high-grade serous ovarian cancer that distinguish malignant, tumour-sustaining and chemo-resistant ovarian tumour cells. Oncogene. 2013;32:4586–4592. doi: 10.1038/onc.2012.477. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Meltzer PS. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 13.Huang Da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 14.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43((Database Issue)):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono K, Demchak B, Ideker T. Cytoscape tools for the web age: D3.js and Cytoscape.js exporters. F1000Res. 2014;3:143. doi: 10.12688/f1000research.4510.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan R, Wen XY, Wu J, Duan R, Cao H, Lam S, Hou D, Wang Y, Hu J, Chen Z. Knockdown of ZNF403 inhibits cell proliferation and induces G2/M arrest by modulating cell-cycle mediators. Mol Cell Biochem. 2012;365:211–222. doi: 10.1007/s11010-012-1262-6. [DOI] [PubMed] [Google Scholar]
- 17.Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q. GeneMANIA: A real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008;9(Suppl 1):S4. doi: 10.1186/gb-2008-9-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin F, Liu L, Liu X, Li G, Zheng L, Li D, Wang Q, Zhang W, Li L. Downregulation of tumor suppressor gene ribonuclease T2 and gametogenetin binding protein 2 is associated with drug resistance in ovarian cancer. Oncol Rep. 2014;32:362–372. doi: 10.3892/or.2014.3175. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Liu X, Li T, Wei L, Yang A, Lu Y, Zhang J, Li L, Wang S, Yin F. Cross-validation of genes potentially associated with overall survival and drug resistance in ovarian cancer. Oncol Rep. 2017;37:3084–3092. doi: 10.3892/or.2017.5534. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 23.Hoskins WJ. Epithelial ovarian carcinoma: Principles of primary surgery. Gynecol Oncol. 1994;55(Suppl):S91–S96. doi: 10.1006/gyno.1994.1346. [DOI] [PubMed] [Google Scholar]
- 24.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 25.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Neoadjuvant chemotherapy in the Medicare cohort with advanced ovarian cancer. Gynecol Oncol. 2011;123:461–466. doi: 10.1016/j.ygyno.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, Levine DA, Gardner GJ, Abu-Rustum NR, Barakat RR. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs. neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124:10–14. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 28.Nicklin JL, McGrath S, Tripcony L, Garrett A, Land R, Tang A, Perrin L, Chetty N, Jagasia N, Crandon AJ, et al. The shift toward neo-adjuvant chemotherapy and interval debulking surgery for management of advanced ovarian and related cancers in a population-based setting: Impact on clinical outcomes. Aust N Z J Obstet Gynaecol. 2017;57:651–658. doi: 10.1111/ajo.12665. [DOI] [PubMed] [Google Scholar]
- 29.Rincon M, Flavell RA. Transcription mediated by NFAT is highly inducible in effector CD4+ T helper 2 (Th2) cells but not in Th1 cells. Mol Cell Biol. 1997;17:1522–1534. doi: 10.1128/MCB.17.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Rao A, Harrison SC. Signal integration by transcription-factor assemblies: Interactions of NF-AT1 and AP-1 on the IL-2 promoter. Cold Spring Harb Symp Quant Biol. 1999;64:527–531. doi: 10.1101/sqb.1999.64.527. [DOI] [PubMed] [Google Scholar]
- 32.Viola JP, Carvalho LD, Fonseca BP, Teixeira LK. NFAT transcription factors: From cell cycle to tumor development. Braz J Med Biol Res. 2005;38:335–344. doi: 10.1590/S0100-879X2005000300003. [DOI] [PubMed] [Google Scholar]
- 33.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Rodríguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee N, Kim D, Kim WU. Role of NFAT5 in the immune system and pathogenesis of autoimmune diseases. Front Immunol. 2019;10:270. doi: 10.3389/fimmu.2019.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aramburu J, López-Rodríguez C. Regulation of Inflammatory Functions of Macrophages and T Lymphocytes by NFAT5. Front Immunol. 2019;10:535. doi: 10.3389/fimmu.2019.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mognol GP, Carneiro FR, Robbs BK, Faget DV, Viola JP. Cell cycle and apoptosis regulation by NFAT transcription factors: New roles for an old player. Cell Death Dis. 2016;7:e2199. doi: 10.1038/cddis.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JU, Kim LK, Choi JM. Revisiting the concept of targeting NFAT to control T cell immunity and autoimmune diseases. Front Immunol. 2018;9:2747. doi: 10.3389/fimmu.2018.02747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendickova K, Tidu F, Fric J. Calcineurin-NFAT signalling in myeloid leucocytes: New prospects and pitfalls in immunosuppressive therapy. EMBO Mol Med. 2017;9:990–999. doi: 10.15252/emmm.201707698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- 41.Kawahara T, Kashiwagi E, Ide H, Li Y, Zheng Y, Miyamoto Y, Netto GJ, Ishiguro H, Miyamoto H. Cyclosporine A and tacrolimus inhibit bladder cancer growth through down-regulation of NFATc1. Oncotarget. 2015;6:1582–1593. doi: 10.18632/oncotarget.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu W, Gu J, Ren Q, Shi Y, Xia Q, Wang J, Wang S, Wang Y, Wang J. NFATC1 promotes cell growth and tumorigenesis in ovarian cancer up-regulating c-Myc through ERK1/2/p38 MAPK signal pathway. Tumour Biol. 2016;37:4493–4500. doi: 10.1007/s13277-015-4245-x. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Duan Z, Yu J, Dang HX. NFATc1 regulates cell proliferation, migration, and invasion of ovarian cancer SKOV3 cells in vitro and in vivo. Oncol Rep. 2016;36:918–928. doi: 10.3892/or.2016.4904. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Yu J, Duan Z, Dang HX. The effect of NFATc1 on vascular generation and the possible underlying mechanism in epithelial ovarian carcinoma. Int J Oncol. 2016;48:1457–1466. doi: 10.3892/ijo.2016.3355. [DOI] [PubMed] [Google Scholar]
- 45.Kawahara T, Inoue S, Fujita K, Mizushima T, Ide H, Yamaguchi S, Fushimi H, Nonomura N, Miyamoto H. NFATc1 expression as a prognosticator in urothelial carcinoma of the upper urinary tract. Transl Oncol. 2017;10:318–323. doi: 10.1016/j.tranon.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray OT, Wong CC, Vrankova K, Rigas B. Phospho-sulindac inhibits pancreatic cancer growth: NFATc1 as a drug resistance candidate. Int J Oncol. 2014;44:521–529. doi: 10.3892/ijo.2013.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 49.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 50.Xie CQ, Jeong Y, Fu M, Bookout AL, Garcia-Barrio MT, Sun T, Kim BH, Xie Y, Root S, Zhang J, et al. Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol. 2009;23:724–733. doi: 10.1210/me.2008-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hegele RA. Retinoid X receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353:2088. doi: 10.1056/NEJM200511103531921. [DOI] [PubMed] [Google Scholar]
- 53.Jeong Y, Xie Y, Xiao G, Behrens C, Girard L, Wistuba II, Minna JD, Mangelsdorf DJ. Nuclear receptor expression defines a set of prognostic biomarkers for lung cancer. PLoS Med. 2010;7:e1000378. doi: 10.1371/journal.pmed.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McEwan IJ. The nuclear receptor superfamily at thirty. Methods Mol Biol. 2016;1443:3–9. doi: 10.1007/978-1-4939-3724-0_1. [DOI] [PubMed] [Google Scholar]
- 55.Weikum ER, Liu X, Ortlund EA. The nuclear receptor superfamily: A structural perspective. Protein Sci. 2018;27:1876–1892. doi: 10.1002/pro.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nuclear Receptors Nomenclature Committee, corp-author. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/S0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 57.Yin H, Lo JH, Kim JY, Marsh EE, Kim JJ, Ghosh AK, Bulun S, Chakravarti D. Expression profiling of nuclear receptors identifies key roles of NR4A subfamily in uterine fibroids. Mol Endocrinol. 2013;27:726–740. doi: 10.1210/me.2012-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730–735. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 59.Wenzl K, Troppan K, Neumeister P, Deutsch AJ. The nuclear orphan receptor NR4A1 and NR4A3 as tumor suppressors in hematologic neoplasms. Curr Drug Targets. 2015;16:38–46. doi: 10.2174/1389450115666141120112818. [DOI] [PubMed] [Google Scholar]
- 60.Haller F, Bieg M, Will R, Korner C, Weichenhan D, Bott A, Ishaque N, Lutsik P, Moskalev EA, Mueller SK, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019;10:368. doi: 10.1038/s41467-018-08069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang B, Wang W, Li W, Li X, Li X, Li G. Differential expression of oxidored nitro domain containing protein 1 (NOR1), in mouse tissues and in normal and cancerous human tissues. Gene. 2012;493:18–26. doi: 10.1016/j.gene.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 62.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/S1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, Sun R, Yang VW. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dang DT, Chen X, Feng J, Torbenson M, Dang LH, Yang VW. Overexpression of Krüppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. Oncogene. 2003;22:3424–3430. doi: 10.1038/sj.onc.1206413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Hofstetter WL, He Y, Hu W, Pataer A, Wang L, Wang J, Zhou Y, Yu L, Fang B, Swisher SG. KLF4 inhibition of lung cancer cell invasion by suppression of SPARC expression. Cancer Biol Ther. 2010;9:507–513. doi: 10.4161/cbt.9.7.11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang WT, Zheng PS. Krüppel-like factor 4 functions as a tumor suppressor in cervical carcinoma. Cancer. 2012;118:3691–3702. doi: 10.1002/cncr.26698. [DOI] [PubMed] [Google Scholar]
- 68.Zammarchi F, Morelli M, Menicagli M, Di Cristofano C, Zavaglia K, Paolucci A, Campani D, Aretini P, Boggi U, Mosca F, et al. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am J Pathol. 2011;178:361–372. doi: 10.1016/j.ajpath.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, Zheng H, Ai W. Krüppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–2172. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA, Schiemann WP, Keri RA. Krüppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia. 2011;13:601–610. doi: 10.1593/neo.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang B, Shen A, Ouyang X, Zhao G, Du Z, Huo W, Zhang T, Wang Y, Yang C, Dong P, et al. KLF4 expression enhances the efficacy of chemotherapy drugs in ovarian cancer cells. Biochem Biophys Res Commun. 2017;484:486–492. doi: 10.1016/j.bbrc.2017.01.062. [DOI] [PubMed] [Google Scholar]
- 72.Sowter HM, Corps AN, Smith SK. Hepatocyte growth factor (HGF) in ovarian epithelial tumour fluids stimulates the migration of ovarian carcinoma cells. Int J Cancer. 1999;83:476–480. doi: 10.1002/(SICI)1097-0215(19991112)83:4<476::AID-IJC7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 73.Di Renzo MF, Olivero M, Katsaros D, Crepaldi T, Gaglia P, Zola P, Sismondi P, Comoglio PM. Overexpression of the Met/HGF receptor in ovarian cancer. Int J Cancer. 1994;58:658–662. doi: 10.1002/ijc.2910580507. [DOI] [PubMed] [Google Scholar]
- 74.Li H, Zhang H, Zhao S, Shi Y, Yao J, Zhang Y, Guo H, Liu X. Overexpression of MACC1 and the association with hepatocyte growth factor/c-Met in epithelial ovarian cancer. Oncol Lett. 2015;9:1989–1996. doi: 10.3892/ol.2015.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rasola A, Anguissola S, Ferrero N, Gramaglia D, Maffe A, Maggiora P, Comoglio PM, Di Renzo MF. Hepatocyte growth factor sensitizes human ovarian carcinoma cell lines to paclitaxel and cisplatin. Cancer Res. 2004;64:1744–1750. doi: 10.1158/0008-5472.CAN-03-2383. [DOI] [PubMed] [Google Scholar]
- 76.Mariani M, McHugh M, Petrillo M, Sieber S, He S, Andreoli M, Wu Z, Fiedler P, Scambia G, Shahabi S, Ferlini C. HGF/c-Met axis drives cancer aggressiveness in the neo-adjuvant setting of ovarian cancer. Oncotarget. 2014;5:4855–4867. doi: 10.18632/oncotarget.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the GEO repository (http://www.ncbi.nlm.nih.gov/geo/).