Abstract
Background
Several systematic reviews compared recombinant gonadotrophin with urinary gonadotrophins (HMG, purified FSH, highly purified FSH) for ovarian hyperstimulation in IVF and ICSI cycles and these reported conflicting results. Each of these reviews used different inclusion and exclusion criteria for trials. Our aim in producing this review was to bring together all randomised studies in this field under common inclusion criteria with consistent and valid statistical methods.
Objectives
To compare the effectiveness of recombinant gonadotrophin (rFSH) with the three main types of urinary gonadotrophins (i.e. HMG, FSH‐P and FSH‐HP) for ovarian stimulation in women undergoing IVF or ICSI treatment cycles.
Search methods
An extended search was done according to Cochrane guidelines including the Menstrual Disorders & Subfertility Group's Specialised Register of controlled trials (up to May 2010), The Cochrane Central Register of Controlled Trials (up to May 2010), MEDLINE (1966 to May 2010), EMBASE (1980 to May 2010), CINAHL (1982 to May 2010), National Research Register, and Current Controlled Trials (up to May 2010).
Selection criteria
All randomised controlled trials reporting data comparing clinical outcomes for women undergoing IVF/ICSI cycles and using recombinant FSH in comparison with HMG or highly purified HMG, purified urinary FSH (FSH‐P), and highly purified urinary FSH (FSH‐HP) for ovarian hyperstimulation in IVF or ICSI cycles were included.
Data collection and analysis
Data selected by three reviewers (MvW, IK, and AV). Data extraction and risk assessment done by four reviewers (MvW, IK, AB and AV). Primary outcome measure was live birth rate and OHSS per randomised woman. Binary outcomes were analysed using odds ratios and also reported in absolute terms. Grouped analyses were carried out for all outcomes to explore whether relative effects differed due to key features of the trials.
Main results
We included 42 trials with a total of 9606 couples. Comparing rFSH to all other gonadotrophins combined, irrespective of the down‐regulation protocol used, did not result in any evidence of a statistically significant difference in live birth rate (28 trials, 7339 couples, odds ratio (OR) 0.97, 95% CI 0.87 to 1.08). This suggests that for a group with a 25% live birth rate using urinary gonadotrophins the rate would be between 22.5% and 26.5% using rFSH. There was also no evidence of a difference in the OHSS rate (32 trials, 7740 couples, OR 1.18, 95% CI 0.86 to 1.61). This means that for a group with 2% risk of OHSS using urinary gonadotrophins, the risk would be between 1.7% and 3.2% using rFSH.
When different urinary gonadotrophins were considered separately, there were significantly fewer live births after rFSH than HMG (11 trials, N=3197, OR 0.84, 95% CI 0.72 to 0.99). This means that for a live birth rate of 25% using HMG, use of rFSH instead would be expected to result in a rate between 19% and 25%. There was no evidence of a difference in live births when rFSH was compared with FSH‐P (5 trials, N=1430, OR 1.26, 95% CI 0.96 to 1.64) or when rFSH was compared with FSH‐HP (13 trials, N=2712; OR 1.03, 95% CI 0.86 to 1.22).
Authors' conclusions
Clinical choice of gonadotrophin should depend on availability, convenience and costs. Differences between urinary gonadotrophins were considered unlikely to be clinically significant. Further research on these comparisons is unlikely to identify substantive differences in effectiveness or safety.
Keywords: Female; Humans; Pregnancy; Birth Rate; Fertilization in Vitro; Fertilization in Vitro/methods; Follicle Stimulating Hormone; Follicle Stimulating Hormone/therapeutic use; Gonadotropins; Gonadotropins/therapeutic use; Gonadotropins/urine; Live Birth; Live Birth/epidemiology; Ovulation Induction; Ovulation Induction/methods; Recombinant Proteins; Recombinant Proteins/therapeutic use; Sperm Injections, Intracytoplasmic; Sperm Injections, Intracytoplasmic/methods
Plain language summary
Recombinant FSH versus urinary gonadotrophins (HMG, purified FSH, highly purified FSH) for ovarian hyperstimulation in IVF and ICSI cycles
Several systematic reviews compared recombinant FSH with urinary gonadotrophins (HMG, purified FSH, highly purified FSH) for ovarian hyperstimulation in IVF and ICSI cycles and these reported conflicting results. We included 42 trials with in total 9606 couples. Comparing rFSH with urinary gonadotrophins overall did not result in any difference in live birth rate, OHSS or any of the other outcomes. Comparing rFSH with HMG/HP‐HMG resulted in a significantly lower live birth rate in the rFSH group though differences were small. There was no proof of a difference in live birth when comparing rFSH with FSH‐P or with FSH‐HP. We may conclude that all these gonadotrophins are equally effective and safe, and that further trials are unwarranted.
Summary of findings
Summary of findings for the main comparison. rFSH versus urinary gonadotrophins: primary analyses for ovarian stimulation in assisted reproductive technology cycles.
| rFSH versus urinary gonadotrophins: primary analyses for ovarian stimulation in assisted reproductive technology cycles | ||||||
| Patient or population: patients with ovarian stimulation in assisted reproductive technology cycles Settings: Intervention: rFSH versus urinary gonadotrophins: primary analyses | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RFSH versus urinary gonadotrophins: primary analyses | |||||
| Live birth (or ongoing pregnancy) by urinary gonadotrophin | Study population | OR 0.97 (0.87 to 1.08) | 7339 (28 studies) | ⊕⊕⊕⊕ high | ||
| 245 per 1000 | 239 per 1000 (220 to 260) | |||||
| Medium risk population | ||||||
| 237 per 1000 | 232 per 1000 (213 to 251) | |||||
| Live birth (or ongoing pregnancy) by urinary gonadotrophin ‐ rFSH versus HMG/HMG‐HP | Study population | OR 0.84 (0.72 to 0.99) | 3197 (11 studies) | ⊕⊕⊕⊕ high | ||
| 255 per 1000 | 223 per 1000 (198 to 253) | |||||
| Medium risk population | ||||||
| 237 per 1000 | 207 per 1000 (183 to 235) | |||||
| Live birth (or ongoing pregnancy) by urinary gonadotrophin ‐ rFSH versus FSH‐P | Study population | OR 1.26 (0.96 to 1.64) | 1430 (5 studies) | ⊕⊕⊕⊕ high | ||
| 170 per 1000 | 205 per 1000 (164 to 251) | |||||
| Medium risk population | ||||||
| 154 per 1000 | 187 per 1000 (149 to 230) | |||||
| Live birth (or ongoing pregnancy) by urinary gonadotrophin ‐ rFSH versus FSH‐HP | Study population | OR 1.03 (0.86 to 1.22) | 2712 (13 studies) | ⊕⊕⊕⊕ high | ||
| 267 per 1000 | 273 per 1000 (239 to 308) | |||||
| Medium risk population | ||||||
| 267 per 1000 | 273 per 1000 (239 to 308) | |||||
| OHSS by urinary gonadotrophin | Study population | OR 1.18 (0.86 to 1.61) | 7740 (32 studies) | ⊕⊕⊕⊕ high | ||
| 19 per 1000 | 22 per 1000 (16 to 30) | |||||
| Medium risk population | ||||||
| 17 per 1000 | 20 per 1000 (15 to 27) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
The first phase of vitro fertilisation (IVF) and intra‐cytoplasmic sperm injection (ICSI) consists of ovarian hyperstimulation to produce multiple follicles for follicle aspiration. The strategy of stimulating the ovaries with gonadotrophins is well established. This review addresses the use of gonadotrophins for ovarian induction in IVF and ICSI cycles. Couples that have an indication for IVF or ICSI after ovarian stimulation with gonadotropins are couples that have a low chance to conceive naturally. The most prevalent indications for IVF or ICSI are a tubal factor, a male factor and or unexplained subfertility.
Description of the intervention
The first generation of gonadotrophins, used in the 1970's, was human menopausal gonadotrophin, produced from the urine of menopausal women (HMG, a combination of follicle stimulating hormone (FSH) and luteinizing hormone (LH) in a 1:1 ratio). Since the 1980's, a variety of urinary gonadotrophins have been produced, such as purified FSH (FSH‐P) which contains less than one international unit (IU) of LH per 75 IU of FSH. The third generation of urinary gonadotrophins was highly purified FSH (FSH‐HP) with less than 0.1 IU of LH per 75 IU of FSH. An early systematic review (Daya 1995) reported a higher clinical pregnancy rate per cycle with FSH‐P and FSH‐HP when compared with HMG, but a later review (Agrawal 2000) reported no difference between these urinary gonadotrophin products.
The fourth generation of gonadotrophins was produced using recombinant DNA technology (recombinant FSH, rFSH), which is free from LH activity. The production of rFSH is independent of urine collection, thus guaranteeing a high availability of a bio chemically pure FSH preparation that is free from urinary protein contaminants. The production process also yields FSH with minimal batch‐to‐batch discrepancy (Bergh 1999) and low immunogenicity which allows subcutaneous administration.
How the intervention might work
In the follicular phase of a normal menstrual cycle a cohort of 10 to 20 ovarian antral follicles develops. Of this cohort only one follicle obtains dominance over the others and shows continued growth until ovulation takes place. The aim in standard IVF or ICSI is to achieve the maturation of a much larger part of the ovarian antral follicle cohort. This is accomplished by ovarian stimulation with FSH containing gonadotrophins.
Why it is important to do this review
Several systematic reviews and one international Health Technology Assessment report compared rFSH with urinary gonadotrophins (HMG, FSH‐P, FSH‐HP) Daya 1998; Larizgoitia 2000;Daya 2002;Van Wely 2003;NCC‐WCH 2004;Al‐Inany 2003; Al‐Inany 2008;Coomarisamy 2008).
These reviews addressed several comparisons. Two reviews compared rFSH to urinary FSH and found higher pregnancy rates per cycle started for rFSH (Daya 2002, updated from Daya 1998).
Three reviews compared rFSH versus urinary gonadotrophins (HMG, FSH‐P, FSH‐HP together) and found no evidence of a difference between these two groups (Larizgoitia 2000;Al‐Inany 2003;NCC‐WCH 2004).
Three reviews compared rFSH with HMG and reported evidence of a difference in live birth and clinical pregnancy rate per cycle between rFSH and HMG (Van Wely 2002;Al‐Inany 2008;Coomarisamy 2008).
Apart from the different comparisons, three aspects in particular deserve attention.
Firstly, gonadotrophin‐releasing hormone (GnRH) agonists and GnRH antagonist are often used in conjunction with gonadotrophins to facilitate cycle control and achieve pituitary down‐regulation in ovarian stimulation during assisted reproductive treatment cycles. There is evidence of effectiveness in increased clinical pregnancy rate with the use of GnRHa when compared with no GnRHa in ovarian stimulation for IVF (Hughes 1992). Long pituitary‐down GnRHa protocols were found to increase clinical pregnancy rates when compared with short or ultrashort GnRHa protocols (Daya 1998). Nowadays, GnRH antagonists are also often used for down‐regulation.
Secondly many trials have been performed by pharmaceutical companies and the conflict of interest may have introduced bias.
Thirdly, it is now customary to freeze supernumerary embryos and to transfer frozen/thawed embryos if transfer of fresh embryos has failed. Hence, we will include all studies which involved fresh or frozen cycles embryo transfer, then explore any influence different settings have on the treatment differences.
The systematic reviews mentioned above reported conflicting results. Each of these reviews used different inclusion and exclusion criteria for trials (Daya 1995; Agrawal 2000; Daya 2002; Al‐Inany 2003; Larizgoitia 2000;Van Wely 2003;NCC‐WCH 2004;Al‐Inany 2008; Coomarisamy 2008). Some based their conclusion on rates per cycle that had been invalidly analysed as if each woman contributed a single cycle. Legitimate but different choices of meta‐analytic model (fixed or random effects, odds ratio or risk ratio) complicate comparison further. Our aim in producing this review is to bring together all randomised studies in this field under common inclusion criteria with consistent and valid statistical methods.
Objectives
To compare the effectiveness of recombinant gonadotrophin (rFSH) with the three main types of urinary gonadotrophins (i.e. HMG, FSH‐P and FSH‐HP) for ovarian stimulation in women undergoing IVF or ICSI treatment cycles.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials only. Quasi‐randomised controlled trials, in which allocation was, for example, by alternation or reference to case record number or to dates of birth, were excluded. Crossover trials were excluded since the design was not appropriate in this context (Vail 2003).
Trials in which fresh and frozen embryos are transferred were included.
Types of participants
Normogonadotrophic (defined as having normal serum concentration of FSH and LH) women undergoing fresh and/or frozen‐thawed IVF or ICSI treatment cycles.
Types of interventions
Ovarian stimulation with recombinant gonadotrophin (rFSH) versus HMG, FSH‐P or FSH‐HP, with or without the use of a GnRH down‐regulation protocol in women undergoing IVF and ICSI cycles.
Types of outcome measures
Primary outcomes
Effectiveness: One or more live birth(s) per woman or, if not reported, one or more pregnancy ongoing beyond 20 weeks per woman
Adverse: Rate of ovarian hyperstimulation syndrome per woman
Secondary outcomes
Effectiveness: Cumulative live birth/ongoing pregnancy per woman including the result of frozen‐thawed embryo transfers
Clinical pregnancy rate per woman (as confirmed by the presence of foetal heart rate) Patient acceptability/satisfaction
Number of oocytes produced per cycle Adverse: Multiple pregnancy rate per woman and per pregnancy Miscarriage rate per woman
Process outcomes: Amount of gonadotrophins used per woman per cycle ( total dose in IU [international units]) Duration of ovarian stimulation per woman per cycle
Search methods for identification of studies
We searched the Menstrual Disorders & Subfertility Group's Specialised Register of controlled trials (until May 2010), The Cochrane Central Register of Controlled Trials (CENTRAL) on the latest issue of the Cochrane Library (until May 2010), MEDLINE (1966 to May 2010), EMBASE (1980 to May 2010), CINAHL (1982 to May 2010), National Research Register, and web‐based trials databases such as Current Controlled Trials. There was no language restriction. Additionally all references in the published reviews, identified trials and background papers were checked and authors were contacted if necessary. The search strategy was developed by Marian Showell, the trial search coordinator of the Cochrane Menstrual Disorders & Subfertility Group (MDSG). The search strategy is shown in Appendix 1. Furthermore, trial registries were searched for additional (ongoing) trials. We also contacted pharmaceutical companies for any published, unpublished or ongoing studies not identified with our search strategy.
Data collection and analysis
Selection of studies
Three reviewers (MvW, IK, and AV) independently examined the electronic search results for reports of possibly relevant trials and these reports were retrieved in full. All reviewers applied the selection criteria independently to the trial reports, rechecking trial eligibility and resolving disagreements by discussion with the other reviewers (HI, AB, FV and JT).
Data extraction and management
Four reviewers (MvW, IK, AB and AV) independently extracted the outcomes data and information on funding, location, clinical and design details and participants. Any differences were resolved by discussion among reviewers. Details of the studies were entered into the Table of Included Studies. Studies that appeared to meet the inclusion criteria but were excluded from the review are presented in the Table of Excluded Studies, briefly stating the reason for exclusion but giving no further information.
Assessment of risk of bias in included studies
Four reviewers (MvW, IK, AB and AV) extracted information regarding the risk of bias (threats to internal validity) under six domains (also see :Table 2).
1. Methodological quality of trials.
| Methodology | Adequate | Unclear | Inadequate |
| Randomisation | Computer‐generated, random number table, lots, coin toss, etc | 'random' stated without further explanation | [Study excluded] |
| Concealment | Third party, sequentially numbered coded drugs containers or envelopes | Missing or inadequate detail e.g. "sealed envelopes" | Open, not able to validate e.g. lots, coin toss, shuffle |
| Blinding | Double blinded, i.e. both the patient and the doctor | Unclear whether blinding was used | Not double blinded, not blinded |
| Incomplete outcome data addressed | No losses to follow‐up or evidence that any losses to follow‐up were low and comparable between groups (i.e. all data presented as ITT) | Unclear whether all data is presented according to ITT | Not all data is presented according to ITT and losses to follow‐up are considerable |
| Selective reporting | Major outcomes had been reported in sufficient detail to allow analysis, independently of their apparent statistical significance. | Unclear whether major outcomes were reported in sufficient details (often in abstracts) | Major outcomes had not been reported in sufficient detail to allow analysis |
| Other bias | No evidence of miscellaneous errors or circumstances that might influence internal validity of trial results | It is unknown whether there are miscellaneous errors or circumstances that might influence internal validity of trial results | Evidence of miscellaneous errors or circumstances that might influence internal validity of trial results |
1. Sequence generation. Evidence that an unpredictable random process was used.
2. Allocation concealment. Evidence that the allocation list was not available to anyone involved in the recruitment process.
3. Blinding of participants, clinicians and outcome assessors. Evidence that knowledge of allocation was not available to those involved in subsequent treatment decisions or follow‐up efforts.
4. Completeness of outcome data. Evidence that any losses to follow‐up were low and comparable between groups.
5. Selective outcome reporting. Evidence that major outcomes had been reported in sufficient detail to allow analysis, independently of their apparent statistical significance.
6. Other potential sources. Evidence of miscellaneous errors or circumstances that might influence internal validity of trial results.
Missing details were sought from the authors. All details are presented in the Risk of bias table following each included study. Any differences were resolved by discussion.
Measures of treatment effect
All binary outcomes were summarised using the odds ratio (OR) with 95% confidence intervals (CI).
Ordinal scales used in patient acceptability, amount of gonadotrophin used and duration of ovarian stimulation were treated as continuous outcomes. Means and standard deviations were abstracted, calculated or requested.
Unit of analysis issues
All outcomes were expressed per woman randomised. Where only data 'per cycle' were available, and participants had contributed multiple cycles, data were omitted from meta‐analysis.
The secondary outcome multiple pregnancy, was also expressed per clinical pregnancy.
Dealing with missing data
Where there was insufficient information in the published report, we attempted to contact the authors for clarification. If missing data became available, they were included in the analysis. It was anticipated that trials conducted over 10 years ago might not have data on live birth rates of the study participants. Data extracted from the trials were analysed on an intention to treat basis. Where randomised cases were missing from outcome assessment, we first contacted the authors for additional data. If further data were not available, we assumed that missing participants had failed to achieve pregnancy and had not suffered reported adverse events.
Assessment of heterogeneity
Presence of statistical heterogeneity of treatment effect among trials was determined using the I2 statistic. We adopted the following broad interpretation: 0% to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; 75% to 100%, considerable heterogeneity present (Higgins 2008).
Assessment of reporting biases
We assessed the methods section for relevant determined outcomes. When data was measured but not reported in the paper this was considered internal reporting bias.
To evaluate external reporting bias funnel plots for primary outcomes and for clinical pregnancy rate are presented. When there was evidence of small‐study effects, publication bias was considered as only one of a number of possible explanations. We also informally compared results for clinical pregnancy rates between those reporting ongoing pregnancy or live birth and those that did not.
Data synthesis
Review Manager software was used to perform the meta‐analyses using a fixed effect model. For binary outcomes, we used the Peto approach. For reporting purposes, primary outcomes were translated to absolute risks. Results for continuous outcomes were combined using mean difference. If studies had reported acceptability scores using different scales, the standardised mean difference would have been used.
Prospectively it was planned to undertake four different stratifications of the primary outcomes:
different urinary gonadotrophins (HMG, FSH‐P and FSH‐HP)
different GnRH protocols (antagonist, long GnRHa, short/ultrashort GnRHa protocol, and no GnRHa)
use of fresh or frozen‐thawed embryos
different sponsors (commercial, non‐commercial)(Lexchin 2003)
Subgroup analysis and investigation of heterogeneity
If excessive heterogeneity existed within strata it would have been explored informally, using the clinical and design details recorded in the Table of included studies. Heterogeneity between strata was anticipated, and possible reasons discussed.
Sensitivity analysis
We assessed the influence of excluding data from reports that pooled multiple cycles per woman.
We assessed the influence of risk of bias on effect size by removing trials deemed to be at high risk.
Analyses were repeated using a random effects model to explore whether different conclusions would be reached.
All sensitivity analyses were reported for live birth, OHSS and clinical pregnancy only.
Results
Description of studies
For details on the studies please see: Characteristics of included studies;Characteristics of excluded studies, Characteristics of studies awaiting classification.
Results of the search
The search strings identified a total of 121 references, handsearching identified another 11 papers. Most references identified by the search were excluded at the first screening step as they were clearly irrelevant. The most frequent reasons for exclusion at this level were: article was a review or a commentary or case study; the treatment was not IVF or ICSI, or study was clearly a non‐randomised design. A total 53 studies, 40 full‐text papers and 13 abstracts from congress proceedings, were then formally assessed.
Included studies
Fourty‐two trials described in 43 publications (including 8 abstracts from congress proceedings) met all selection criteria and were included in the review. The total number of study participants was 9606 and the total number of cycles was 9644.
PATIENTS
The studies included in this review used different inclusion criteria such that the individual studies differ in indication for treatment, female age and number of previous cycles. Detail can be found in the Characteristics of included studies table.
INTERVENTIONS
Twelve of the 42 included trials compared rFSH with HMG or HP‐HMG (n=3775). The size of these trials varied from 40 to 721. In these 12 trials the following down regulation protocols were used: antagonist by one trial (Bosch 2008), long GnRH agonist by eight trials (Andersen 2006;Balasch 2003; EISG 2002; Gordon 2001; Hompes 2008; Kilani 2003;Ng 2001;Westergaard 2001), short GnRH agonist by two trials (Rashidi 2005; Strehler 2001) and no down regulation by one trial (Jansen 1998).
Seven of the 42 included trials compared rFSH with FSH‐P (n=1560). The size of these trials varied from 40 to 721. In these 7 trials the following down regulation protocols were used: long GnRH agonist by six trials (Alvino 1995;Drakakis 2002;Gordon 2001;Hedon 1995; Out 1995; RHFSHG 1995), and no down regulation by one trial (Meden‐Vrtovec 2003).
Twenty‐two of the 42 included trials compared rFSH with FSH‐HP (n=4147). In these 23 trials the following down regulation protocols were used: long GnRH agonist by 18 trials (Abate 2009; Antoine 2007; Baker 2009; Berger 1999; Bergh 1997; Dickey 2002; Dickey 2003; Franco 2000; Frydman 2000; Gallego 2003a; Ghosh 1999; Hoomans 1999;Lenton 2000; Mohamed 2006;Nardo 2000Schats 2000; Selman 2002; O´Dea 1993), short GnRH agonist by two trials ( Cheon 2004; Hugues 2001) and no down regulation by three trials (Ferraretti 1999; Germond 2000; Machado 1999).
One study compared HMG, FSH‐P, FSH‐HP and rFSH in 124 women (137 cycles) (Kornilov 1999). In this study a long GnRH agonist was used for down‐regulation.
The studies included in this review varied in initial gonadotrophin dose given, primary outcome measurements and methodological quality.
Three trials studied the results of transfer of frozen‐thawed embryos in addition to transfer of fresh embryos (Out 1995; Hompes 2008; Andersen 2006). In one trial, the frozen cycles resulted in an additional 22 ongoing pregnancies in the rFSH group and seven ongoing pregnancies in the FSH‐P group (Out 1995). In the FIRM trial there were an extra four deliveries in the rFSH group and an extra five deliveries in the HP‐HMG group following frozen‐thawed cycles (Hompes 2008). The cryo‐cycle results of the Merit trial (Andersen 2006) could be extracted from another publication on that trial (Ziebe 2007).
Another heterogeneity between the trials was the used fertilisation method, in 20 of the 42 trials only IVF was done, in six of the trials only ICSI was done and in 16 trials both IVF and ICSI cycles were done.
OUTCOMES
Of the included trials we were able to retrieve intention to treat data from 28 trials on live birth, 31 trials had data on OHSS and from 41 trials full data on clinical pregnancy was available.
Excluded studies
Fifteen studies were excluded from the analysis. Of these ten studies did not meet the selection criteria (see Characteristics of excluded studies). Six studies were not truly randomised (Duijkers 1997;Manassiev 1997;Serhal 2000;Ruvolo 2009; Requena 2010). One study compared two down regulation protocols besides rFSH and HMG (GnRH agonist plus HMG versus GnRH antagonist plus rFSH) in oocyte donors (Martinez 2008). One study was a duplicate of Dickey 2003a study (Dickey 2003b). One study compared rFSH with a combination of uFSH and rFSH (Pacchiarotti 2007). One study compared rFSH plus HMG versus FSH‐HP plus HMG (Raga 1999).
Five studies are awaiting classification. For two studies we were not able to obtain the abstract (Kahn 1999; Strowitzki 2007). For one study the abstract contained no usable data and author nor sponsor were able to provide more data (Olivennes F 1999). The other two studies are probably double publications (Chakravarty 2000; Righini 1998)
Risk of bias in included studies
The risk of bias per included trial was judged in the table Characteristics of included studies. Also see Figure 1 and Figure 2.
1.
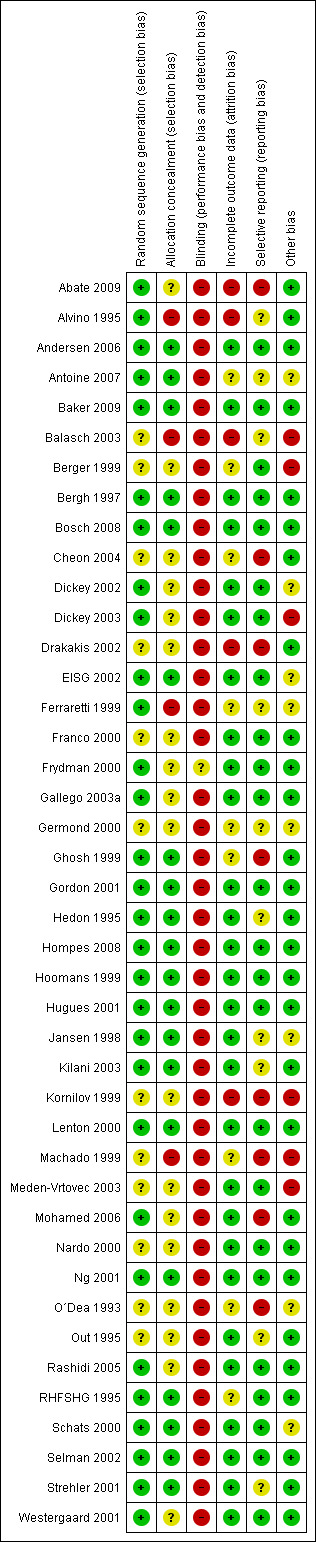
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
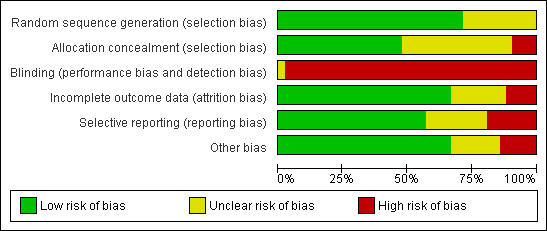
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
We received additional data on randomisation, concealment of allocation, blinding, sponsoring and/or data relevant for effect size calculation from the authors of 24 studies (Abate 2009; Antoine 2007; Baker 2009; Balasch 2003; Bergh 1997; Bosch 2008; Cheon 2004; Dickey 2002; Dickey 2003; EISG 2002; Ferraretti 1999; Gordon 2001; Hompes 2008; Hoomans 1999; Jansen 1998; Kilani 2003; Meden‐Vrtovec 2003; Mohamed 2006; Ng 2001;Out 1995;Schats 2000; Selman 2002; Strehler 2001; Westergaard 2001). In 7 trials, additional information was not needed (Alvino 1995; Andersen 2006; Franco 2000; Frydman 2000; Hedon 1995;Hugues 2001; Lenton 2000 , and for 11 trials we tried but failed to contact study authors (Berger 1999;Kornilov 1999; Drakakis 2002; Gallego 2003a; Ghosh 1999; O´Dea 1993; Germond 2000; Machado 1999Nardo 2000; Rashidi 2005; RHFSHG 1995).
Allocation
All 42 included studies were randomised controlled trials but for five studies the method of randomisation remained unclear (O´Dea 1993;Berger 1999;Kornilov 1999;Nardo 2000;Drakakis 2002;Cheon 2004 ). Allocation to the intervention or control group was adequately concealed in 28 of 42 trials (Andersen 2006; Antoine 2007; Baker 2009Bergh 1997; Bosch 2008; Dickey 2002; Dickey 2003;EISG 2002; Frydman 2000; Germond 2000; Ghosh 1999; Gordon 2001; Hedon 1995;Hompes 2008;Hoomans 1999; Hugues 2001; Jansen 1998; Kilani 2003; Lenton 2000; Meden‐Vrtovec 2003; Mohamed 2006;Ng 2001; RHFSHG 1995; Schats 2000; Selman 2002; Strehler 2001; Westergaard 2001). The allocation concealment was qualified as inadequate in six trials (Alvino 1995; Balasch 2003; Franco 2000; Ferraretti 1999; Machado 1999;Kornilov 1999) and unclear in eight trials (Abate 2009; Berger 1999; Cheon 2004; Drakakis 2002; Gallego 2003a; Nardo 2000; O´Dea 1993Rashidi 2005). Also see Figure 1.
Blinding
Partially blinded outcome assessment was reported in 13 of the 42 RCTs. However, we considered only double blinded trials to be adequately blinded. Therefore none of the included trials had adequate blinding.
Incomplete outcome data
For the pregnancy outcomes, data were presented according to intention to treat (ITT) or otherwise the ITT data could usually be retraced from extra information on the respective trials. For abstracts however, it was not always known whether data were ITT or not. The data from the following RCTs may not be presented according to ITT (Berger 1999, Gallego 2003a, Ghosh 1999, Machado 1999, Kornilov 1999;O´Dea 1993). Therefore sensitivity analyses were performed excluding these trials. Pregnancy data from one of the trials that was planned to be included in the primary analysis only could not be extracted as data were presented as a percentage per embryo transfer only and absolute pregnancy numbers could not be retrieved (Kornilov 1999).
In three trials some women underwent multiple cycles: 165 cycles in 148 couples (Berger 1999), 79 cycles in 71 women (Machado 1999), 137 cycles in 124 women (Kornilov 1999) and 241 women undergoing 254 cycles (Cheon 2004). In all other trials only one fresh IVF or ICSI cycle was offered to each couple randomised into the trial. The results of these four trials with multiple cycles were presented per cycle and not per woman randomised. Therefore sensitivity analyses were performed excluding these trials.
The sensitivity analyses were done for the outcomes live birth, OHSS and clinical pregnancy and are presented for the general outcome and grouped according to the types of urinary gonadotrophins, type of down‐regulation, use of fresh/frozen protocol and sponsor.
Oocytes were usually presented per woman with oocytes or per oocyte pick‐up. The number of oocytes per couple randomised could therefore not be determined. As the pooled result would be biased we did not pool the data, hence results are presented per study only. In theory this practice of not including failures in the outcome can produce massive bias, for instance in the case that one protocol would result in more cancellations. We can therefore not exclude that even the per trial results are biased.
Amount of gonadotrophin used and duration of ovarian stimulation was usually presented only for those couples that actually received gonadotrophins thus not including cancelled cycles. As the pooled result would be biased we did not pool the data, hence results are presented per study only. Amount of gonadotrophin used and duration of ovarian stimulation had more problems. Several older trials compared different starting doses of rFSH and urinary FSH, generally lower rFSH starting doses were used. Furthermore in some trials fixed dosages were used. This will have impact on the outcome amount of gonadotrophin used and duration of treatment. Thus besides the bias due to not presenting the results per woman randomised, a difference in these outcomes may also be due to the trial design. With these outcomes even the results per trial may well present biased outcomes.
Due to these serious problems with oocytes retrieved, amount of gonadotrophins used and duration of ovarian stimulation we have removed these outcomes from the analyses table. The data can be found under additional tables (Table 3, Table 4; Table 5) for oocytes retrieved, amount of gonadotrophins used and duration of ovarian stimulation respectively.
2. Number of oocytes retrieved.
| Study | recombinant FSH | Urinary gonadotrophins | SMD [95% CI) | ||||
| mean | SD | N | mean | SD | N | ||
| Out 1995 | 10.84 | 5.7 | 585 | 8.95 | 5.7 | 396 | 1.89 [1.16, 2.62] |
| RHFSHG 1995 | 9.3 | 5.0 | 55 | 10.7 | 5.3 | 59 | ‐1.40 [‐3.29, 0.49] |
| Hedon 1995 | 9.7 | 5.8 | 57 | 8.9 | 5.8 | 33 | 0.80 [‐1.69, 3.29] |
| Bergh 1997 | 12.2 | 5.5 | 119 | 7.6 | 4.4 | 102 | 4.60 [3.29, 5.91] |
| Jansen 1998 | 11.2 | 6.8 | 54 | 8.3 | 6.2 | 35 | 2.90 [0.16, 5.64] |
| Ghosh 1999 | 12.2 | 4.7 | 22 | 14.4 | 4.3 | 25 | ‐2.20 [‐4.79, 0.39] |
| Berger 1999 | 9.1 | 5.1 | 47 | 11.9 | 7.3 | 53 | ‐2.80 [‐5.25, ‐0.35] |
| Hoomans 1999 | 8.84 | 5.0 | 83 | 9.79 | 5.0 | 82 | ‐0.95 [‐2.48, 0.58] |
| Kornilov 1999 | 14.4 | 6.0 | 28 | 13.8 | 5.7 | 109 | 0.60 [‐1.87, 3.07] |
| Ferraretti 1999 | 4.1 | 2.4 | 66 | 3.7 | 2.3 | 75 | 0.40 [‐0.38, 1.18] |
| Lenton 2000 | 10.2 | 6.0 | 68 | 10.8 | 6.1 | 69 | ‐0.60 [‐2.63, 1.43] |
| Nardo 2000 | 3.8 | 6.0 | 75 | 3.8 | 6.0 | 35 | 0.00 [‐2.41, 2.41] |
| Schats 2000 | 13.1 | 7.7 | 232 | 11.4 | 7.6 | 231 | 1.70 [0.31, 3.09] |
| Frydman 2000 | 11.0 | 5.9 | 130 | 8.8 | 4.8 | 116 | 2.20 [0.86, 3.54] |
| Franco 2000 | 10.7 | 6.8 | 60 | 10.5 | 5.7 | 60 | 0.20 [‐2.05, 2.45] |
| Ng 2001 | 12.6 | 8.9 | 17 | 9.6 | 8.1 | 16 | 3.00 [‐2.80, 8.80] |
| Gordon 2001 | 12.0 | 6.0 | 34 | 10.0 | 7.0 | 49 | 2.00 [‐0.81, 4.81] |
| Westergaard 2001 | 12.9 | 6.8 | 188 | 12.9 | 6.7 | 186 | 0.00 [‐1.37, 1.37] |
| Hugues 2001 | 7.8 | 4.9 | 56 | 8.8 | 5.7 | 30 | ‐1.00 [‐3.41, 1.41] |
| Strehler 2001 | 12.29 | 7.8 | 296 | 9.67 | 5.92 | 282 | 2.62 [1.49, 3.75] |
| Drakakis 2002 | 10.8 | 5.2 | 36 | 12.0 | 7.5 | 29 | ‐1.20 [‐4.42, 2.02] |
| Selman 2002 | 8.9 | 4.7 | 133 | 8.7 | 3.4 | 131 | 0.20 [‐0.79, 1.19] |
| Dickey 2002 | 13.6 | 6.9 | 56 | 13.7 | 6.3 | 111 | ‐0.10 [‐2.25, 2.05] |
| EISG 2002 | 14.0 | 8.5 | 339 | 12.8 | 8.5 | 361 | 1.20 [‐0.06, 2.46] |
| Dickey 2003 | 11.9 | 6.9 | 60 | 11.8 | 6.3 | 60 | 0.10 [‐2.26, 2.46] |
| Kilani 2003 | 6.8 | 3.9 | 43 | 7.9 | 4.6 | 44 | ‐1.10 [‐2.89, 0.69] |
| Balasch 2003 | 9.1 | 4.35 | 25 | 11.79 | 4.55 | 25 | ‐2.69 [‐5.16, ‐0.22] |
| Meden‐Vrtovec 2003 | 7.1 | 5.3 | 70 | 6.1 | 4.2 | 61 | 1.00 [‐0.63, 2.63] |
| Gallego 2003a | 10.4 | 5.48 | 43 | 10.49 | 7.79 | 45 | ‐0.09 [‐2.89, 2.71] |
| Cheon 2004 | 14.6 | 9.2 | 131 | 15.4 | 6.9 | 123 | ‐0.80 [‐2.79, 1.19] |
| Rashidi 2005 | 8.7 | 8.5 | 30 | 9.0 | 6.2 | 30 | ‐0.30 [‐4.06, 3.46] |
| Andersen 2006 | 11.8 | 5.7 | 368 | 10.0 | 5.4 | 363 | 1.80 [1.00, 2.60] |
| Mohamed 2006 | 6.8 | 3.2 | 121 | 6.2 | 2.8 | 120 | 0.60 [‐0.16, 1.36] |
| Hompes 2008 | 10.77 | 6.64 | 247 | 7.86 | 4.54 | 247 | 2.91 [1.91, 3.91] |
| Antoine 2007 | 11.9 | 5.7 | 72 | 10.9 | 4.9 | 73 | 1.00 [‐0.73, 2.73] |
| Baker 2009 | 17.1 | 9.4 | 70 | 16.3 | 9.2 | 70 | 0.80 [‐2.28, 3.88] |
| Bosch 2008 | 14.4 | 8.1 | 126 | 11.3 | 6.0 | 122 | 3.10 [1.33, 4.87] |
| Abate 2009 | 5.0 | 2.6 | 186 | 6.0 | 2.8 | 215 | ‐1.00 [‐1.53, ‐0.47] |
3. Amount of gonadotrophin used (IU).
| Study | recombinant FSH | urinary gonadotrophins | MD [95% CI) | ||||
| mean | SD | N | mean | SD | N | ||
| Hedon 1995 | 2265.0 | 743.0 | 57 | 2213.0 | 743.0 | 33 | 52.00 [‐266.54, 370.54] |
| Out 1995 | 2138.0 | 715.0 | 585 | 2385.0 | 715.0 | 396 | ‐247.00 [‐338.19, ‐155.81] |
| RHFSHG 1995 | 2270.0 | 714.0 | 60 | 2095.0 | 591.0 | 63 | 175.00 [‐57.24, 407.24] |
| Alvino 1995 | 2400.0 | 487.0 | 30 | 2250.0 | 731.0 | 30 | 150.00 [‐164.31, 464.31] |
| Bergh 1997 | 1643.0 | 383.0 | 119 | 2393.0 | 1005.0 | 102 | ‐750.00 [‐956.82, ‐543.18] |
| Jansen 1998 | 1410.0 | 228.0 | 54 | 1365.0 | 228.0 | 35 | 45.00 [‐51.97, 141.97] |
| Berger 1999 | 2475.0 | 488.0 | 89 | 2445.0 | 405.0 | 76 | 30.00 [‐106.27, 166.27] |
| Kornilov 1999 | 1590.0 | 709.0 | 28 | 2027.0 | 704.0 | 109 | ‐437.00 [‐730.99, ‐143.01] |
| Hoomans 1999 | 1479.0 | 285.0 | 83 | 2139.0 | 285.0 | 82 | ‐660.00 [‐746.97, ‐573.03] |
| Schats 2000 | 1695.0 | 375.0 | 232 | 1823.0 | 383.0 | 231 | ‐128.00 [‐197.05, ‐58.95] |
| Lenton 2000 | 1673.0 | 488.0 | 68 | 1823.0 | 488.0 | 69 | ‐150.00 [‐313.44, 13.44] |
| Franco 2000 | 1913.0 | 975.0 | 60 | 1898.0 | 810.0 | 60 | 15.00 [‐305.73, 335.73] |
| Nardo 2000 | 2486.0 | 800.0 | 75 | 2780.0 | 800.0 | 35 | ‐294.00 [‐614.97, 26.97] |
| Frydman 2000 | 2070.0 | 765.0 | 130 | 3053.0 | 1020.0 | 116 | ‐983.00 [‐1210.48, ‐755.52] |
| Westergaard 2001 | 2242.0 | 375.0 | 190 | 2280.0 | 435.0 | 189 | ‐38.00 [‐119.79, 43.79] |
| Gordon 2001 | 2025.0 | 350.0 | 39 | 1981.0 | 570.0 | 59 | 44.00 [‐138.26, 226.26] |
| Strehler 2001 | 2150.0 | 797.0 | 296 | 1516.0 | 545.0 | 282 | 634.00 [523.14, 744.86] |
| Hugues 2001 | 1353.0 | 679.0 | 52 | 1981.0 | 972.0 | 32 | ‐628.00 [‐1012.03, ‐243.97] |
| Ng 2001 | 1800.0 | 270.0 | 20 | 1650.0 | 270.0 | 20 | 150.00 [‐17.34, 317.34] |
| Selman 2002 | 4538.0 | 1575.0 | 134 | 3878.0 | 1125.0 | 133 | 660.00 [331.87, 988.13] |
| Dickey 2002 | 2169.0 | 685.0 | 58 | 2444.0 | 836.0 | 111 | ‐275.00 [‐510.08, ‐39.92] |
| EISG 2002 | 2775.0 | 810.0 | 354 | 2768.0 | 817.0 | 373 | 7.00 [‐111.30, 125.30] |
| Drakakis 2002 | 2664.0 | 832.0 | 36 | 3251.0 | 937.0 | 29 | ‐587.00 [‐1023.08, ‐150.92] |
| Kilani 2003 | 2025.0 | 795.0 | 50 | 1680.0 | 530.0 | 50 | 345.00 [80.16, 609.84] |
| Dickey 2003 | 2354.0 | 779.0 | 59 | 2314.0 | 847.0 | 57 | 40.00 [‐256.41, 336.41] |
| Balasch 2003 | 2449.0 | 885.0 | 25 | 1922.0 | 379.0 | 25 | 527.00 [149.61, 904.39] |
| Gallego 2003a | 1666.0 | 685.0 | 43 | 2262.0 | 685.0 | 45 | ‐596.00 [‐882.31, ‐309.69] |
| Meden‐Vrtovec 2003 | 1253.0 | 173.0 | 70 | 1283.0 | 270.0 | 61 | ‐30.00 [‐108.95, 48.95] |
| Cheon 2004 | 1322.0 | 526.0 | 131 | 2124.0 | 882.0 | 123 | ‐802.00 [‐982.02, ‐621.98] |
| Rashidi 2005 | 2138.0 | 800.0 | 30 | 2250.0 | 800.0 | 30 | ‐112.00 [‐516.85, 292.85] |
| Mohamed 2006 | 5533.0 | 2398.0 | 121 | 3213.0 | 1527.0 | 120 | 2320.00 [1812.85, 2827.15] |
| Andersen 2006 | 2385.0 | 622.0 | 368 | 2508.0 | 729.0 | 363 | ‐123.00 [‐221.30, ‐24.70] |
| Antoine 2007 | 2349.0 | 779.0 | 72 | 2526.0 | 802.0 | 73 | ‐177.00 [‐434.34, 80.34] |
| Hompes 2008 | 1781.0 | 468.0 | 256 | 1932.0 | 628.0 | 250 | ‐151.00 [‐247.68, ‐54.32] |
| Baker 2009 | 2715.0 | 905.0 | 76 | 2641.0 | 841.0 | 76 | 74.00 [‐203.76, 351.76] |
| Bosch 2008 | 2624.0 | 801.0 | 140 | 2481.0 | 994.0 | 140 | 143.00 [‐68.46, 354.46] |
| Abate 2009 | 3536.0 | 1099.0 | 186 | 2106.0 | 719.0 | 215 | 1430.00 [1245.12, 1614.88] |
4. Duration of ovarian stimulation.
| Study | recombinant FSH | Urinary gonadotrophins | MD [95% CI) | ||||
| mean | SD | N | mean | SD | N | ||
| Hedon 1995 | 10.2 | 2.1 | 57 | 10.3 | 2.1 | 33 | ‐0.10 [‐1.00, 0.80] |
| Out 1995 | 10.7 | 2.0 | 595 | 11.3 | 2.0 | 396 | ‐0.60 [‐0.85, ‐0.35] |
| RHFSHG 1995 | 9.9 | 2.3 | 60 | 9.4 | 1.8 | 63 | 0.50 [‐0.23, 1.23] |
| Bergh 1997 | 11.0 | 1.6 | 119 | 13.5 | 3.7 | 102 | ‐2.50 [‐3.27, ‐1.73] |
| Jansen 1998 | 6.2 | 0.97 | 54 | 6.0 | 0.97 | 35 | 0.20 [‐0.21, 0.61] |
| Hoomans 1999 | 9.9 | 1.5 | 83 | 9.6 | 1.5 | 82 | 0.30 [‐0.16, 0.76] |
| Kornilov 1999 | 8.1 | 1.7 | 28 | 8.9 | 18.0 | 109 | ‐0.80 [‐4.24, 2.64] |
| Franco 2000 | 10.1 | 1.8 | 60 | 10.3 | 1.9 | 60 | ‐0.20 [‐0.86, 0.46] |
| Lenton 2000 | 10.2 | 2.1 | 68 | 10.7 | 1.7 | 69 | ‐0.50 [‐1.14, 0.14] |
| Nardo 2000 | 10.4 | 1.6 | 75 | 10.9 | 2.1 | 35 | ‐0.50 [‐1.28, 0.28] |
| Schats 2000 | 11.6 | 1.9 | 232 | 12.4 | 2.7 | 231 | ‐0.80 [‐1.23, ‐0.37] |
| Frydman 2000 | 11.7 | 1.9 | 130 | 14.5 | 3.3 | 116 | ‐2.80 [‐3.48, ‐2.12] |
| Strehler 2001 | 9.5 | 3.2 | 259 | 9.1 | 2.1 | 248 | 0.40 [‐0.07, 0.87] |
| Ng 2001 | 9.0 | 3.7 | 20 | 10.0 | 3.0 | 20 | ‐1.00 [‐3.09, 1.09] |
| Westergaard 2001 | 9.9 | 1.5 | 190 | 10.0 | 1.5 | 189 | ‐0.10 [‐0.40, 0.20] |
| Gordon 2001 | 10.0 | 1.5 | 39 | 9.3 | 1.5 | 59 | 0.70 [0.09, 1.31] |
| Hugues 2001 | 12.9 | 2.0 | 52 | 12.6 | 2.0 | 32 | 0.30 [‐0.58, 1.18] |
| Selman 2002 | 13.7 | 1.4 | 134 | 13.4 | 1.5 | 133 | 0.30 [‐0.05, 0.65] |
| Drakakis 2002 | 9.8 | 1.3 | 36 | 9.2 | 1.2 | 29 | 0.60 [‐0.01, 1.21] |
| EISG 2002 | 11.0 | 2.7 | 354 | 11.2 | 2.6 | 373 | ‐0.20 [‐0.59, 0.19] |
| Dickey 2002 | 9.0 | 1.4 | 58 | 9.5 | 1.6 | 111 | ‐0.50 [‐0.97, ‐0.03] |
| Dickey 2003 | 9.3 | 1.7 | 59 | 9.0 | 1.6 | 57 | 0.30 [‐0.30, 0.90] |
| Balasch 2003 | 14.7 | 3.15 | 25 | 12.67 | 2.2 | 25 | 2.03 [0.52, 3.54] |
| Meden‐Vrtovec 2003 | 7.0 | 1.2 | 70 | 6.9 | 1.3 | 61 | 0.10 [‐0.33, 0.53] |
| Gallego 2003a | 10.47 | 1.3 | 43 | 9.89 | 1.15 | 45 | 0.58 [0.07, 1.09] |
| Kilani 2003 | 12.9 | 3.5 | 50 | 11.0 | 2.8 | 50 | 1.90 [0.66, 3.14] |
| Cheon 2004 | 9.2 | 1.8 | 131 | 9.5 | 1.8 | 123 | ‐0.30 [‐0.74, 0.14] |
| Rashidi 2005 | 8.0 | 3.7 | 30 | 8.0 | 4.5 | 30 | 0.00 [‐2.08, 2.08] |
| Andersen 2006 | 10.1 | 1.7 | 368 | 10.4 | 1.9 | 363 | ‐0.30 [‐0.56, ‐0.04] |
| Hompes 2008 | 11.88 | 2.57 | 256 | 12.6 | 3.13 | 250 | ‐0.72 [‐1.22, ‐0.22] |
| Bosch 2008 | 10.0 | 1.9 | 140 | 9.9 | 1.8 | 140 | 0.10 [‐0.33, 0.53] |
| Abate 2009 | 13.3 | 1.2 | 186 | 12.3 | 1.0 | 215 | 1.00 [0.78, 1.22] |
Preference of patients for rFSH or urinary gonadotrophins was a planned secondary outcome. However, there were no RCTs that compared the preference of the patients.
Selective reporting
Selective reporting can never be completely excluded, but funnel plots for live birth rate (Figure 3), OHSS (Figure 4) and clinical pregnancy (Figure 5) did not suggest presence of selective reporting.
3.
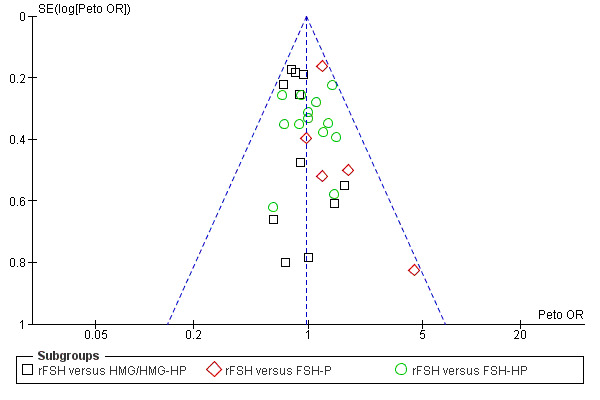
Funnel plot of comparison: 1 rFSH versus urinary gonadotrophins, outcome: 1.1 Live birth (or pregnancy ongoing beyond 20 weeks).
4.
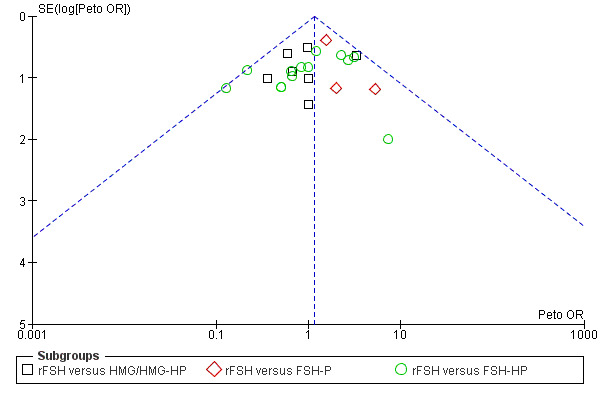
Funnel plot of comparison: 1 rFSH versus urinary gonadotrophins, outcome: 1.5 Ovarian Hyperstimulation Syndrome (OHSS).
5.
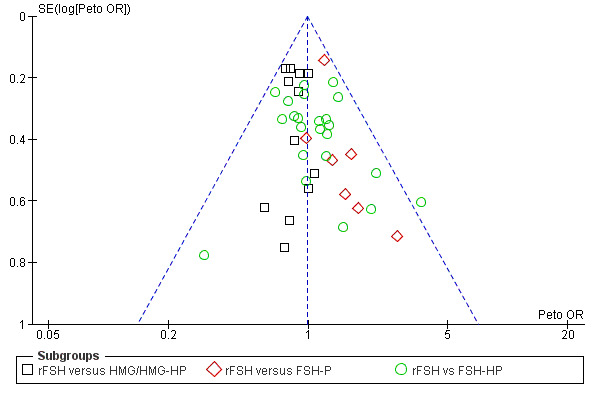
Funnel plot of comparison: 1 rFSH versus urinary gonadotrophins, outcome: 1.9 Clinical pregnancy.
Other potential sources of bias
Of the 42 studies 22 were industry sponsored. This is an important issue since there could be a conflict of interest. Ten studies were sponsored by Serono (Alvino 1995, Bergh 1997, Franco 2000, Frydman 2000, Hedon 1995, Lenton 2000, Machado 1999, Schats 2000, O´Dea 1993; RHFSHG 1995
Three RCTs were sponsored by Organon (Hoomans 1999, Jansen 1998, Out 1995)
Seven RCTs were sponsored by Ferring (Kornilov 1999, Andersen 2006, Dickey 2002, Dickey 2003, EISG 2002, Hompes 2008, Westergaard 2001)
Two RCTs were sponsored by IBSA ( Antoine 2007, Baker 2009).
For six RCTs the funding was unclear: (Berger 1999, Drakakis 2002, Gallego 2003a, Germond 2000, Ghosh 1999, Nardo 2000)
A further 14 trials reported having no funding or governmental funding (Abate 2009, Balasch 2003, Bosch 2008,Cheon 2004, Ferraretti 1999, Gordon 2001, Hugues 2001,Kilani 2003, Meden‐Vrtovec 2003,Mohamed 2006, Ng 2001, Rashidi 2005, Selman 2002, Strehler 2001).
Miscarriages were in some studies presented per biochemical pregnancy (very early miscarriages), in some per clinical pregnancy and in others per ongoing pregnancy (late miscarriages). Similarly multiple pregnancy data was mostly presented for clinical multiple pregnancies and sometimes only for ongoing multiple pregnancies.
Effects of interventions
See: Table 1
Primary analysis for rFSH versus urinary gonadotrophins
Data are presented in "Data and analyses" under "1 rFSH versus urinary gonadotrophins". The outcomes live birth, OHSS and clinical pregnancy data are presented by grouping according to types of urinary gonadotrophins, type of down‐regulation, fresh/frozen policy, and pharmaceutical sponsor. Also see the Summary of findings table 1.
Primary outcomes
Primary efficacy outcome: Live birth
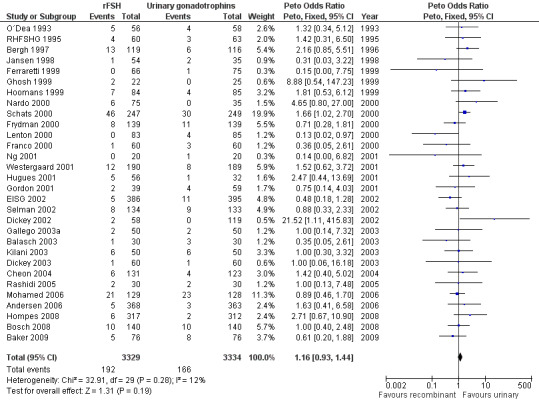
There was no evidence of a statistically significant difference in the primary outcome live births or pregnancies ongoing beyond 20 weeks (28 trials, N=7339; Analysis 1.1; OR 0.97, 95% CI 0.87 to 1.08) for rFSH versus urinary gonadotrophins. This means that of 25% live births using urinary gonadotrophins, use of rFSH instead would be expected to result in a live birth rate between 22.5% and 26.5%. There was no indication of statistical heterogeneity. Visual inspection of the forest plot showed that the OR and 95%CI of the individual trials overlapped and the I2 was 0%.
1.1. Analysis.
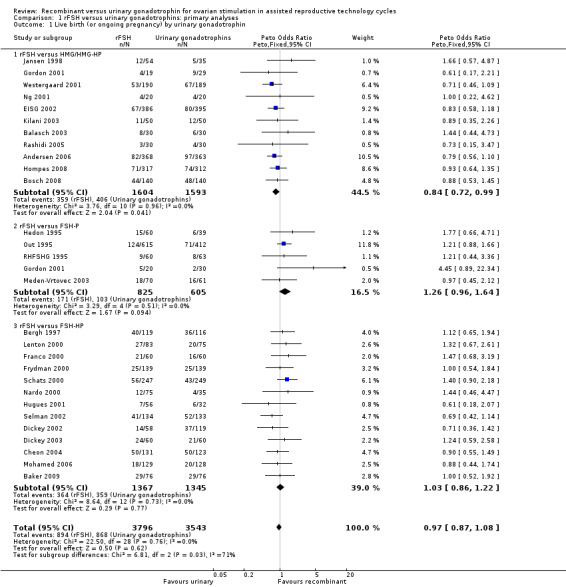
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 1 Live birth (or ongoing pregnancy) by urinary gonadotrophin.
1.1 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by the different urinary gonadotrophins
See Analysis 1.1.
Of the 28 trials with data on live births 11 trials compared rFSH versus HMG/HP‐HMG, 5 trials compared rFSH with FSH‐P and 13 trials compared rFSH with FSH‐HP (for the corresponding forest plot see Figure 6 .
6.
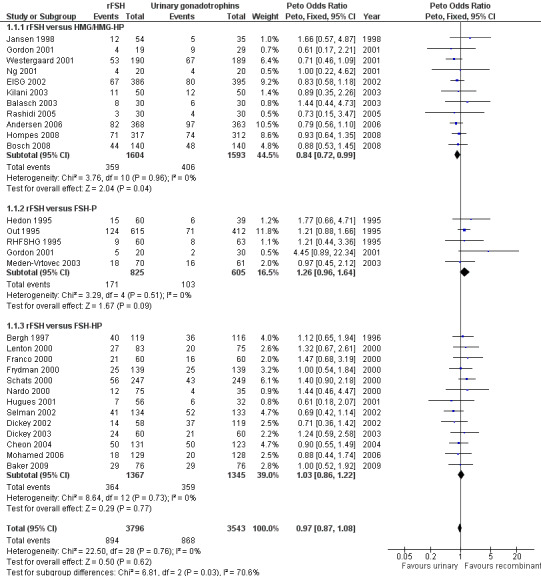
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.1 Live birth (or ongoing pregnancy) by urinary gonadotrophin.
There were significantly fewer live births after rFSH as compared to HMG (OR 0.84, 95% CI 0.72 to 0.99; 11 trials, N=3197; Analysis 1.1 ). This means that for a live birth rate of 25%, use of rFSH instead would be expected to result in a live birth rate between 19% and 25%. There was no indication of statistical heterogeneity. Visual inspection of the forest plot showed that the OR and 95% CI of the individual trials largely overlapped and the I2 was 0%. Pooling using a random effects model resulted in the same OR.
There was no evidence of a statistically significant difference in live birth between rFSH and FSH‐P (5 trials, N=1430; Analysis 1.1. OR 1.26, 95% CI 0.96 to 1.64; I2 of 0%;) and between rFSH and FSH‐HP (13 trials, N=2712; Analysis 1.1. OR 1.03, 95% CI 0.86 to 1.22; I2 of 0%).
1.2 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by down regulation protocol
See Analysis 1.2
1.2. Analysis.
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 2 Live birth (or ongoing pregnancy) by down regulation protocol.
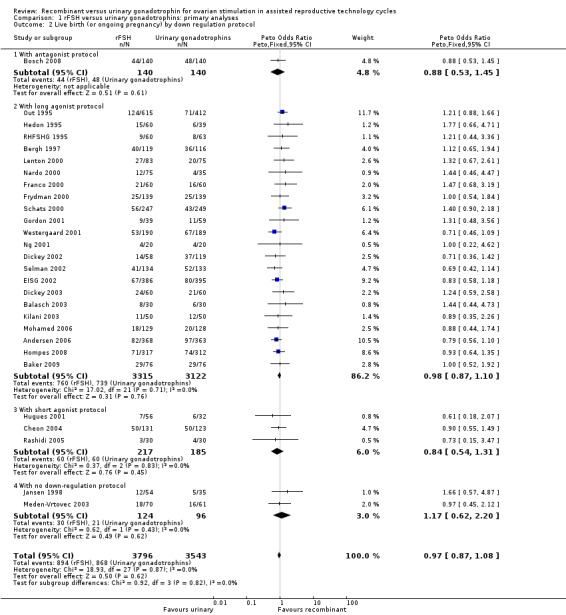
Of the 28 trials with data on live births 1 trial used an antagonist protocol, 22 trials used a long GnRH agonist protocol, three used a short GnRH agonist protocol, and two did not use down‐regulation (for the corresponding forest plot see Figure 7).
7.
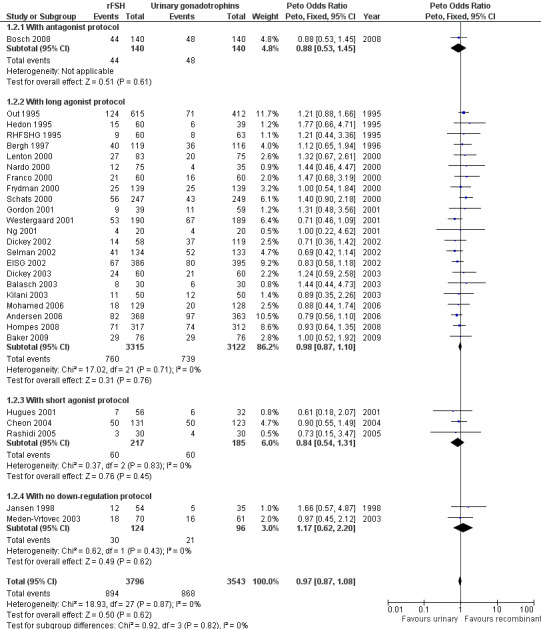
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.2 Live birth (or ongoing pregnancy) by down regulation protocol.
There was no evidence of a statistically significant difference in live birth between rFSH and urinary gonadotrophins for any of the down regulation protocols (antagonist protocol, 1 trial, N=280; Analysis 1.2; OR 0.88, 95% CI 0.53 to 1.45), (long GnRHa protocol, 22 trials, N=6437; OR 0.98, 95% CI 0.87 to 1.10; Analysis 1.2), (short GnRHa protocol,3 trials, N=402; Analysis 1.2; OR 0.84, 95% CI 0.54 to 1.31), (no down regulation, 2 trials, N=220; Analysis 1.2; OR 1.17, 95% CI 0.62 to 2.20 ). There was no indication of statistical heterogeneity ( I2 of 0%).
1.3 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by fresh/frozen policy
See Analysis 1.3
1.3. Analysis.
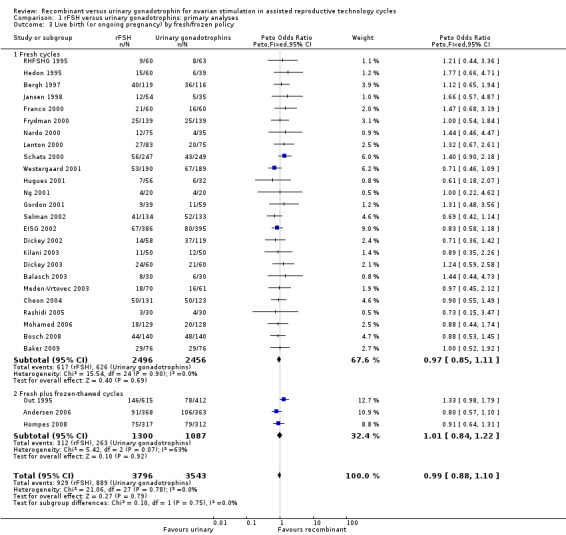
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 3 Live birth (or ongoing pregnancy) by fresh/frozen policy.
Of the 28 trials with data on live births the outcome of frozen‐thawed cycles was known for only three trials (for the corresponding forest plot see Figure 8). There was no evidence of a statistically significant difference between rFSH and urinary gonadotrophins for live births after fresh cycles (25 trials, N=4952; Analysis 1.3; OR 0.97, 95% CI 0.85 to 1.11) and for cumulative live birth rate after fresh and frozen‐thawed cycles (3 trials, N=2387; Analysis 1.3; OR 1.01, 95% CI 0.84 to 1.22). There was no indication of statistical heterogeneity ( I2 of 0%).
8.
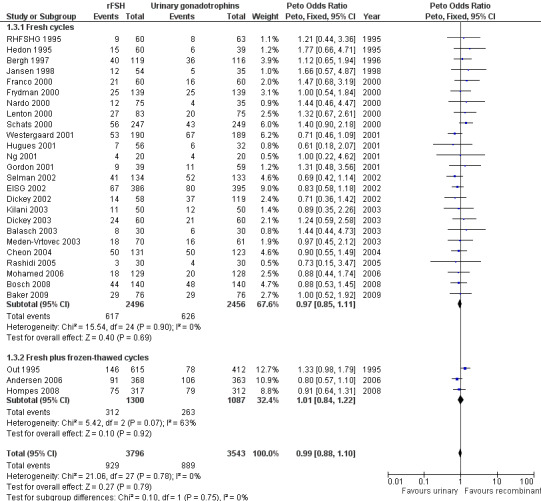
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.3 Live birth (or ongoing pregnancy) by fresh/frozen policy.
1.4 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by pharmaceutical sponsor
See Analysis 1.4
1.4. Analysis.
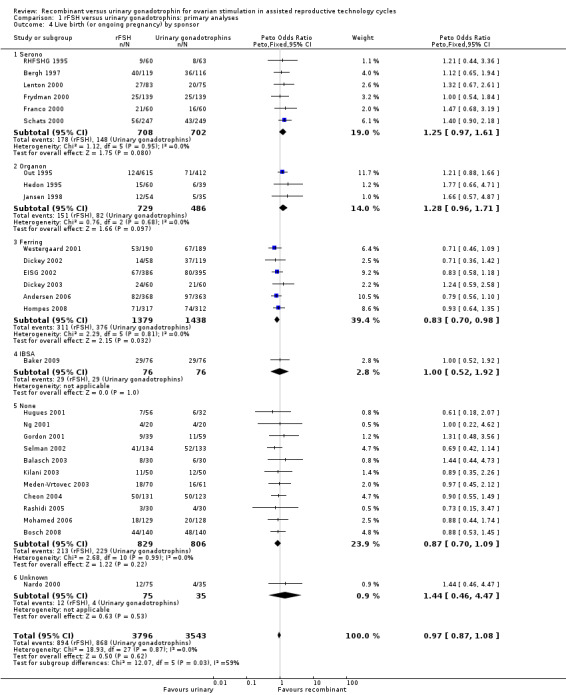
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 4 Live birth (or ongoing pregnancy) by sponsor.
Of the 28 trials with data on live births six trials were sponsored by Serono, three trials were sponsored by Organon ‐ now MSD, six trials were sponsored by Ferring, one trials was sponsored by IBSA, 11 trials were not sponsored by a pharmaceutical company and for one trial sponsoring was unknown (for the corresponding forest plot see Figure 9).
9.
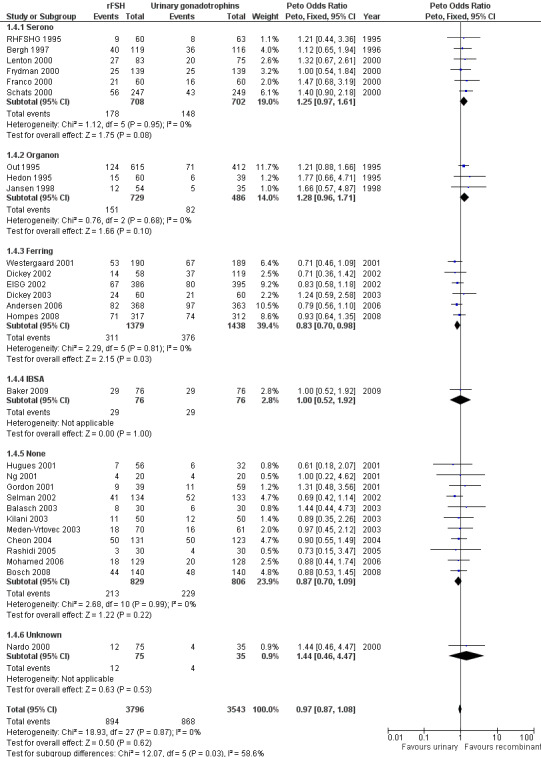
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.4 Live birth (or ongoing pregnancy) by sponsor.
There was no evidence of a statistically significant difference in live births between rFSH and urinary gonadotrophins for the trials sponsored by Serono (6 trials, N=1410; Analysis 1.4; OR 1.25, 95% CI 0.97 to 1.61), the trials sponsored by Organon (3 trials, N=1215; Analysis 1.4; OR 1.28, 95% CI 0.96 to 1.71), the trial sponsored by IBSA (1 trials N=152; Analysis 1.4; OR 1.00, 95% CI 0.52 to 1.92), and the non‐sponsored trials (11 trials, N=1635; Analysis 1.4; OR 0.87, 95% CI 0.70 to 1.09). However, there were significantly fewer live births after rFSH as compared to urinary gonadotrophins for the trials sponsored by Ferring (6 trials, N=2817; Analysis 1.4; OR 0.83, 95% CI 0.69 to 0.98). To evaluate whether this is a pharmaceutical effect or really a result from the interventions done an additional unplanned sub analysis was performed (see last results section Extra unplanned analysis). There was no indication of statistical heterogeneity for any of these grouped comparisons (I2 was 0%).
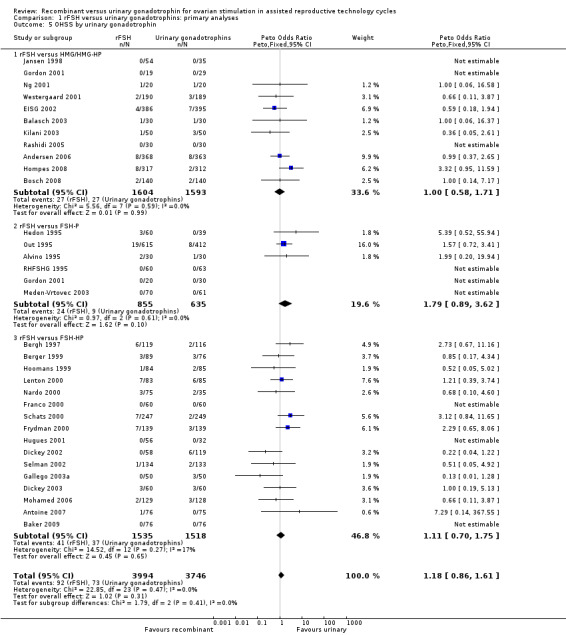
Primary safety outcome: OHSS
There was no evidence of a statistically significant difference in the primary safety outcome OHSS for rFSH versus urinary gonadotrophins (32 trials, N=7740; Analysis 1.5; OR 1.18, 95% CI 0.86 to 1.61; I2 of 0%). This means that for a typical rate of 2% OHSS using urinary gonadotrophins, use of rFSH instead would be expected to result in an OHSS rate between 1.7% and 3.2% OHSS. There was no indication for statistical heterogeneity. Visual inspection of the forest plot showed that the OR and 95%CI of the individual trials overlapped and the I2 was 0%.
1.5. Analysis.
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 5 OHSS by urinary gonadotrophin.
1.5 OHSS grouped by the different urinary gonadotrophins
See Analysis 1.5
Of the 32 trials with data on OHSS 11 trials compared rFSH versus HMG/HP‐HMG, 6 trials compared rFSH with FSH‐P and 16 trials compared rFSH with FSH‐HP (for the corresponding forest plot see Figure 10).
10.
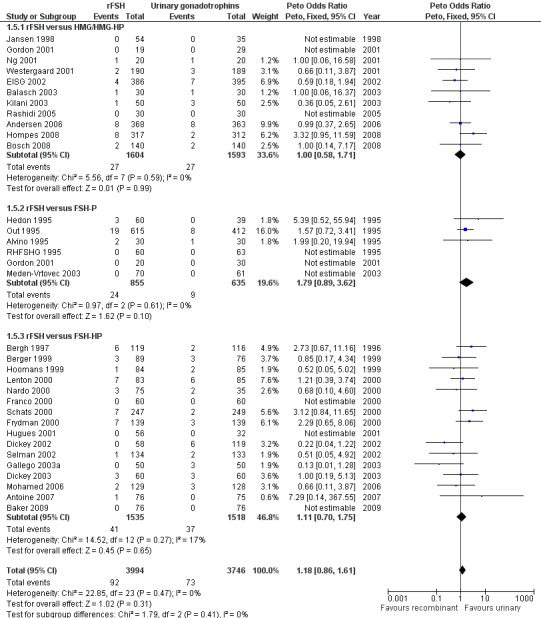
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.5 OHSS by urinary gonadotrophin.
There was no evidence of a statistically significant difference in OHSS for rFSH versus HMG (11 trials, N=3197; Analysis 1.5; OR 1.00, 95% CI 0.58 to 1.71), also not for rFSH versus FSH‐P (6 trials, N=1490; Analysis 1.5; OR 1.79, 95% CI 0.89 to 3.62), and for rFSH versus FSH‐HP (16 trials, N=3053; Analysis 1.5; OR 1.11, 95% CI 0.70 to 1.75; I2 was 0%). There was no indication for statistical heterogeneity for any of these grouped comparisons ( I2 was 0%).
1.6 OHSS grouped by down regulation protocol
See Analysis 1.6
1.6. Analysis.
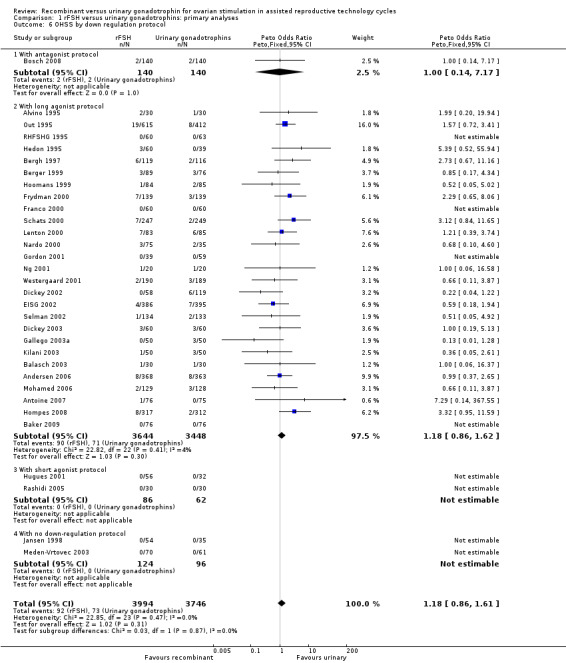
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 6 OHSS by down regulation protocol.
Of the 32 trials with data on live birth 1 trial used an antagonist protocol, 27 trials used a long GnRH agonist protocol, two used a short GnRH agonist protocol, and two did not use down‐regulation (for the corresponding forest plot see Figure 11).
11.
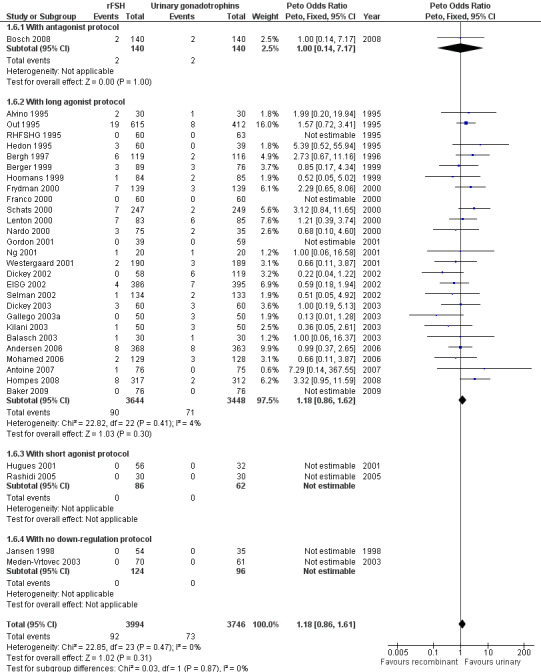
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.6 OHSS by down regulation protocol.
There was no evidence of a statistically significant difference in OHSS between rFSH and urinary gonadotrophins for any of the down regulation protocols (antagonist protocol, N=280; Analysis 1.6; OR 1.00, 95% CI 0.14 to 7.17), (long GnRHa protocol, N=7092; Analysis 1.6; OR 1.18, 95% CI 0.86 to 1.62), (short GnRHa protocol, N=148; Analysis 1.6; OR not estimable due to lack of OHSS cases), (no down regulation, N=220; OR not estimable due to lack of OHSS cases). There was no indication for statistical heterogeneity for any of these grouped comparisons ( I2 of 0%).
1.8 OHSS grouped by sponsor
See Analysis 1.7
1.7. Analysis.
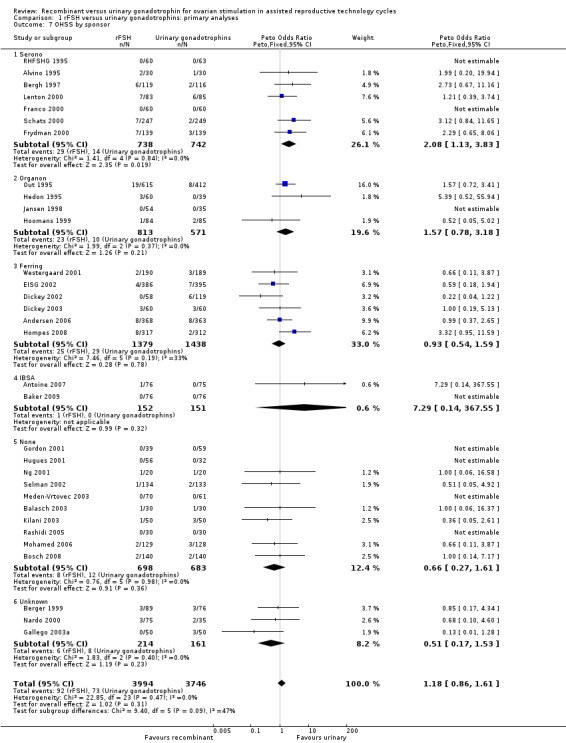
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 7 OHSS by sponsor.
Of the 32 trials with data on OHSS seven trials were sponsored by Serono, four trials were sponsored by Organon ‐ now MSD, six trials were sponsored by Ferring, two trials were sponsored by IBSA, 10 trials were not sponsored by a pharmaceutical company and for three trials sponsoring was unknown (for the corresponding forest plot see Figure 12).
12.
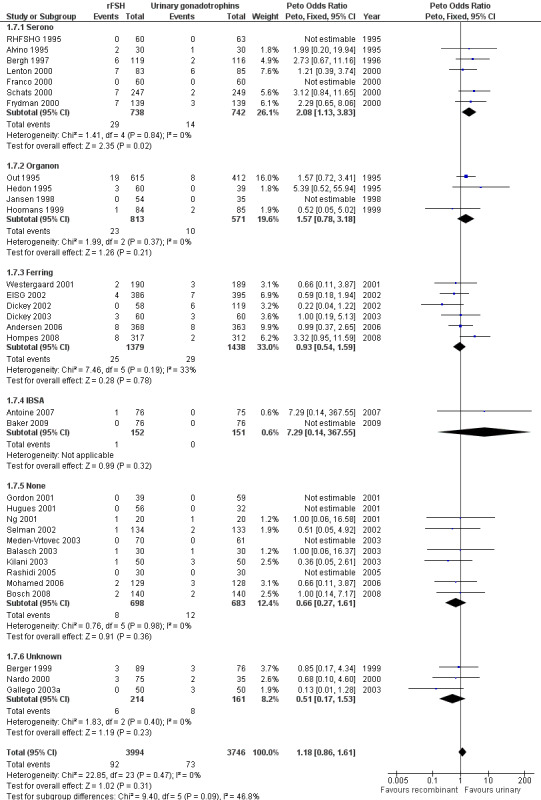
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.8 OHSS by sponsor.
There was more OHSS in the rFSH group as compared to urinary gonadotrophins in studies sponsored by Serono (7 trials, N=1480; Analysis 1.7; OR 2.08, 95% CI 1.13 to 3.83). There was no evidence of a statistically significant difference in OHSS between rFSH and urinary gonadotrophins for the trials sponsored by Organon (4 trials, N=1387; Analysis 1.7; OR 1.57, 95% CI 0.78 to 3.18), the trials sponsored by Ferring (6 trials, N=2817; Analysis 1.7; OR 0.93, 95% CI 0.54 to 1.59), the trials sponsored by IBSA (2 trials N=303; Analysis 1.7; OR 7.29, 95% CI 0.14 to 368), the sponsoring unknown trials (3 trials N=375; Analysis 1.7; OR 0.51, 95% CI 0.17 to 1.53) and the non‐sponsored trials (10 trials, N=1381; Analysis 1.7; OR 0.66, 95% CI 0.27 to 1.61). There was no indication for statistical heterogeneity for any of these grouped comparisons (I2 was 0%).
Secondary outcomes
Clinical pregnancy rate
There was no evidence of a statistically significant difference in the clinical pregnancy rate (41 trials, N=9482; Analysis 1.8; OR 0.99, 95% CI 0.91 to 1.09) for rFSH versus urinary gonadotrophins. This means that for a typical clinical pregnancy rate of 28% using urinary gonadotrophins, use of rFSH instead would be expected to result in a clinical pregnancy rate between 26% and 30%. There was no indication of statistical heterogeneity. Visual inspection of the forest plot showed that the OR and 95%CI of the individual trials overlapped and the I2 was 0%.
1.8. Analysis.
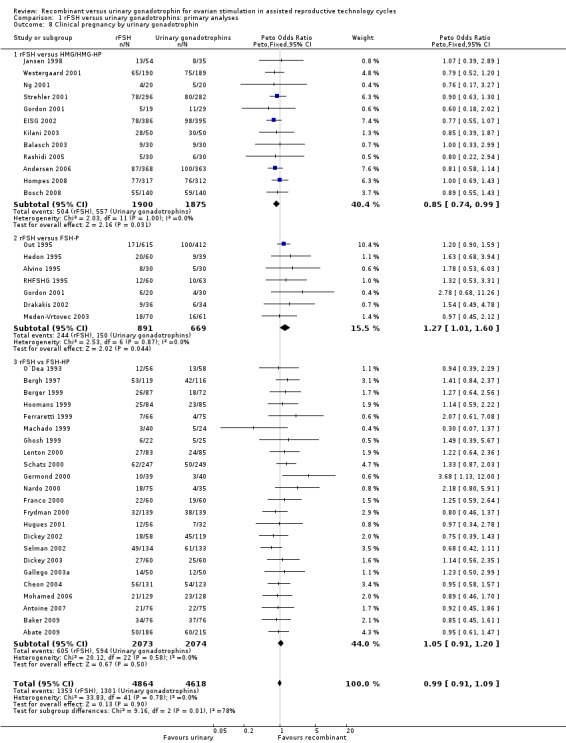
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 8 Clinical pregnancy by urinary gonadotrophin.
1.9 Clinical pregnancy rate grouped by the different urinary gonadotrophins
See Analysis 1.8
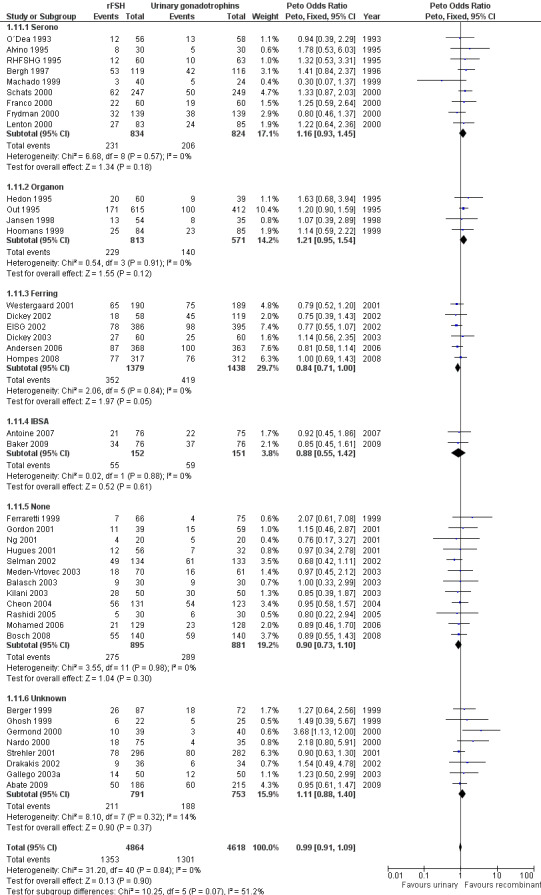
Of the 41 trials with data on clinical pregnancies 12 trials compared rFSH versus HMG/HP‐HMG, seven trials compared rFSH with FSH‐P and 23 trials compared rFSH with FSH‐HP (for the corresponding forest plot see Figure 13).
13.
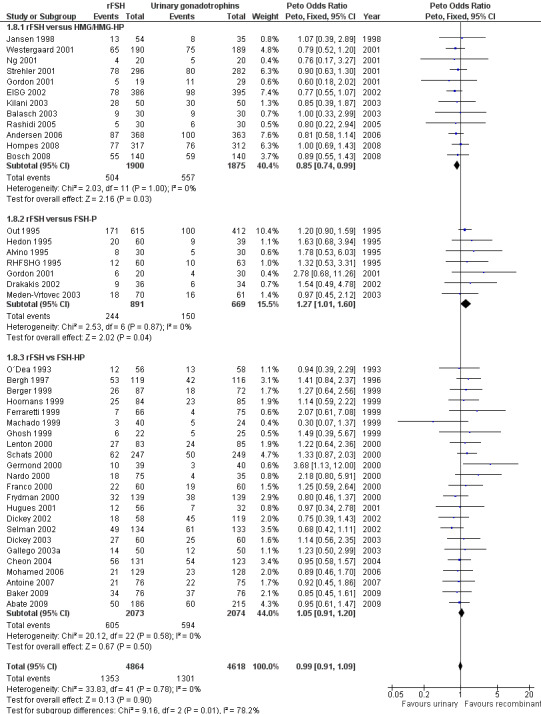
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.9 Clinical pregnancy by urinary gonadotrophin.
There were significantly fewer clinical pregnancies after rFSH as compared to HMG/HP‐HMG (OR 0.85, 95% CI 0.74 to 0.99; I2 of 0%; 12 trials, N=3775; Analysis 1.8). This means that for a typical clinical pregnancy rate of 28% using HMG/HP‐HMG, use of rFSH instead would be expected to result in a clinical pregnancy rate between 23% and 28%.
There were significantly more clinical pregnancies after rFSH as compared to FSH‐P (OR 1.27, 95% CI 1.01 to 1.60; I2 of 0%; 7 trials, N=1560; Analysis 1.8 ). This means that for a typical clinical pregnancy rate of 28% using FSH‐P, use of rFSH instead would be expected to result in a clinical pregnancy rate between 28% and 40%.There was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and FSH‐P (23 trials, N=4147; Analysis 1.8; OR 1.05, 95% CI 0.91 to 1.20; I2 of 0%).
1.10 Clinical pregnancy rate grouped by down regulation protocol
See Analysis 1.9
1.9. Analysis.
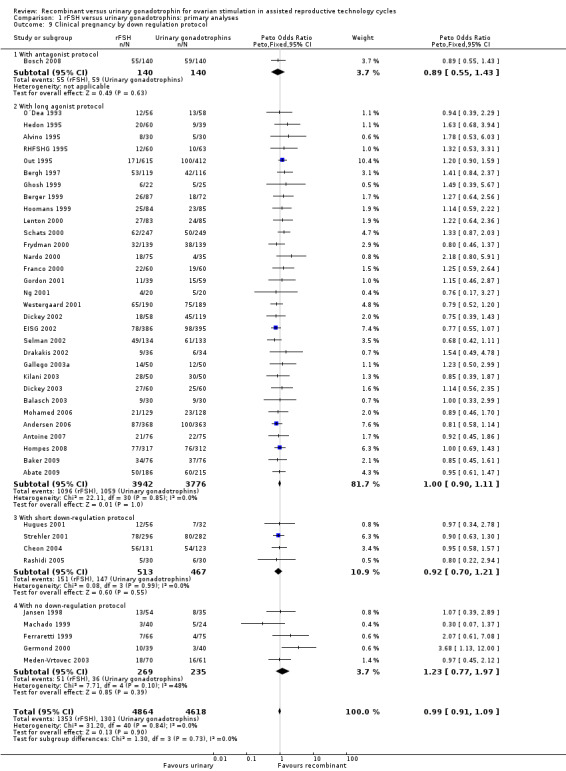
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 9 Clinical pregnancy by down regulation protocol.
Of the 41 trials with data on clinical pregnancies one trial used an antagonist protocol, 31 trials used a long GnRH agonist protocol, four used a short GnRH agonist protocol, and five did not use down‐regulation (for the corresponding forest plot see Figure 14).
14.
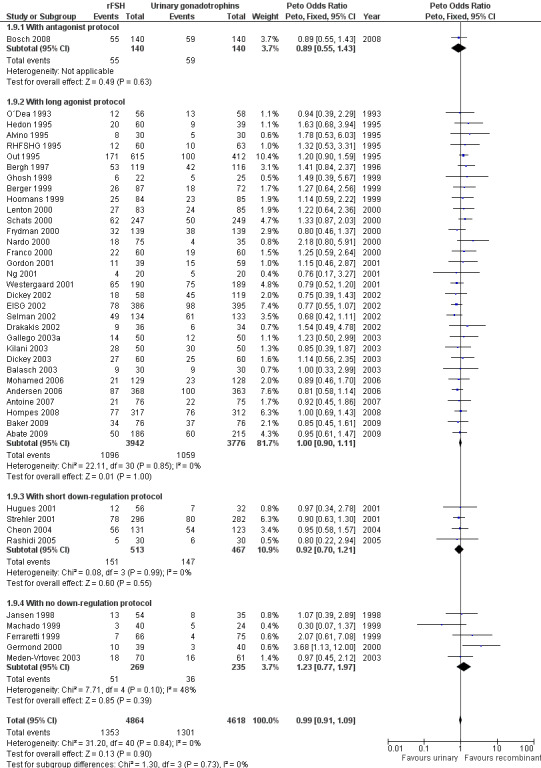
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.10 Clinical pregnancy by down regulation protocol.
There was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and urinary gonadotrophins for any of the down regulation protocols (antagonist protocol, N=280; Analysis 1.9; OR 0.89, 95% CI 0.55 to 1.43), (long GnRHa protocol, N=7718; Analysis 1.9; OR 1.00, 95% CI 0.90 to 1.11), (short GnRHa protocol, N=980; Analysis 1.9; OR 0.92, 95% CI 0.70 to 1.21), (no down regulation, N=504; Analysis 1.9; OR 1.23, 95% CI 0.77 to 1.97). There was no indication of any statistical heterogeneity of these grouped comparisons (I2 was 0%).
1.11 Clinical pregnancy rate grouped by fresh/frozen policy
See Analysis 1.10
1.10. Analysis.
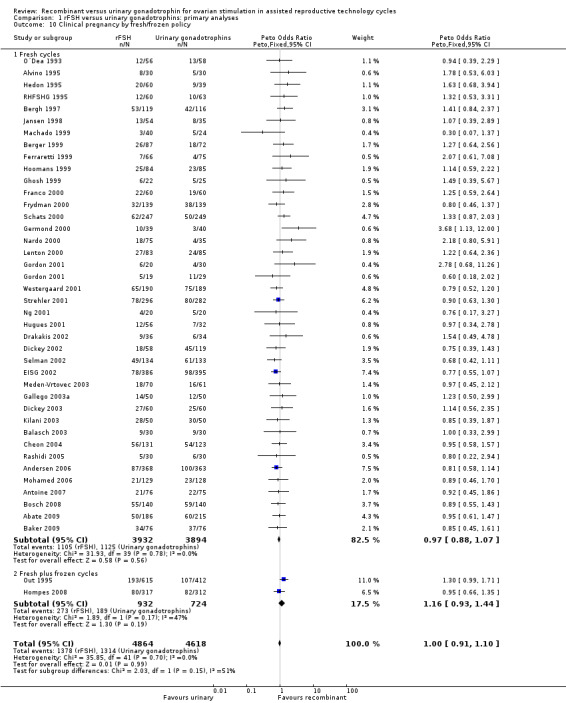
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 10 Clinical pregnancy by fresh/frozen policy.
Of the 41 trials with data on clinical pregnancies the outcome of frozen‐thawed cycles was known for only two trials (for the corresponding forest plot see Figure 15). There was no evidence of a statistically significant difference between rFSH and urinary gonadotrophins for clinical pregnancy rate after fresh cycles (39 trials, N=8744; Analysis 1.10; OR 0.97, 95% CI 0.88 to 1.07) and for cumulative clinical pregnancy rate after fresh and frozen‐thawed cycles (2 trials, N=1656; Analysis 1.10; OR 1.16, 95% CI 0.93 to 1.44). There was no indication of statistical heterogeneity ( I2 of 0%).
15.
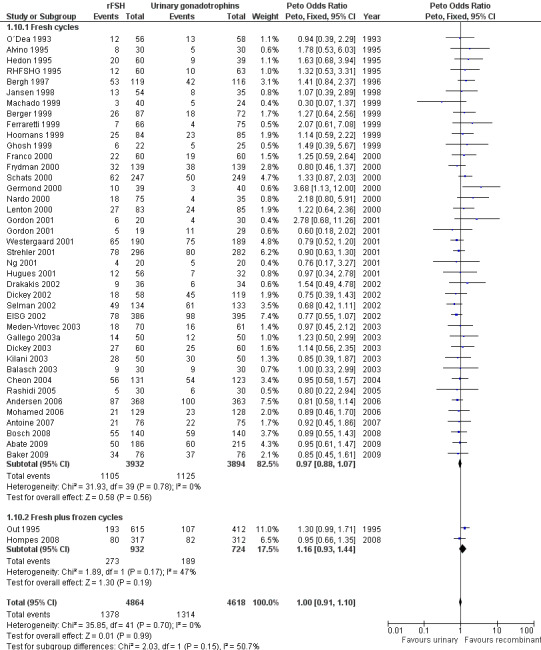
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.11 Clinical pregnancy by fresh/frozen policy.
1.12 Clinical pregnancy rate grouped by pharmaceutical sponsor
See Analysis 1.11
1.11. Analysis.
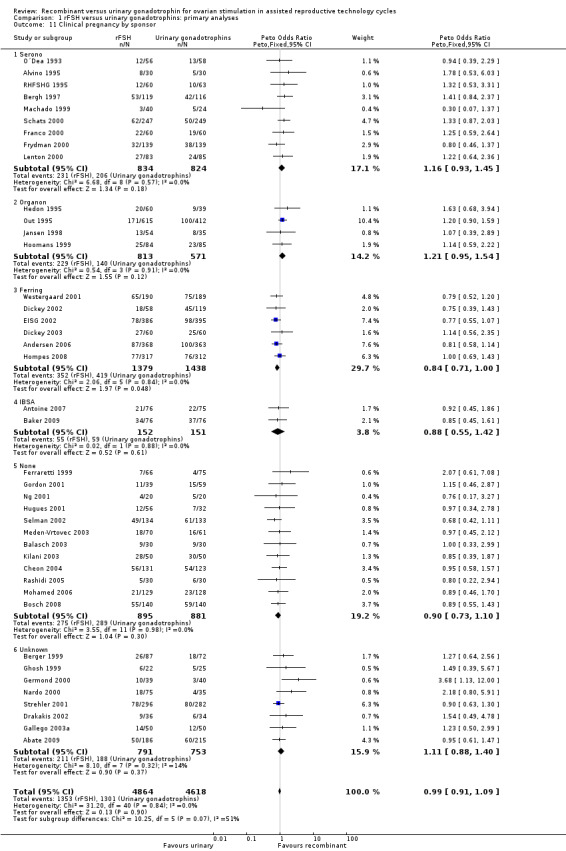
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 11 Clinical pregnancy by sponsor.
Of the 41 trials with data on clinical pregnancies nine trials were sponsored by Serono, four trials were sponsored by Organon ‐ now MSD, six trials were sponsored by Ferring, two trials were sponsored by IBSA, 12 trials were not sponsored by a pharmaceutical company and for eight trials sponsoring was unknown (for the corresponding forest plot see Figure 16).
16.
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.12 Clinical pregnancy by sponsor.
There was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and urinary gonadotrophins for the trials sponsored by Sereno (9 trials, N=1658; Analysis 1.11; OR 1.16, 95% CI 0.93 to 1.45), the trials sponsored by Organon (4 trials, N=1384; OR 1.21, 95% CI 0.95 to 1.54), the trials sponsored by IBSA (2 trials, N=303; Analysis 1.11; OR 0.88, 95% CI 0.55 to 1.42), and the non‐sponsored trials (12 trials, N=1776; Analysis 1.11; OR 0.90, 95% CI 0.73 to 1.10). However, there were borderline significantly fewer live births after rFSH as compared to urinary gonadotrophins for the trials sponsored by Ferring (6 trials, N=2817; Analysis 1.11; OR 0.84, 95% CI 0.71 to 1.00). To evaluate whether this is a pharmaceutical effect or really a result from the interventions done an additional unplanned sub analysis was performed (see last results section Extra unplanned analysis) There was no indication of statistical heterogeneity for any of these grouped comparisons (I2 was 0%).
Further secondary outcomes
There were no data available on patient acceptability or satisfaction for trials that compared rFSH and urinary gonadotrophins.
There was no evidence of a statistically significant difference in multiple pregnancy rate per woman (25 trials, N=6329; Analysis 1.12; OR 0.91, 95% CI 0.76 to1.09) for rFSH versus urinary gonadotrophins (for the corresponding forest plot see Figure 17), nor for multiple pregnancy rate as expressed per clinical pregnancy (25 trials, N=6329; Analysis 1.13; OR 0.96, 95% CI 0.78 to 1.18) (for the corresponding forest plot see Figure 18). For both multiple pregnancy outcomes the I2 was 0%.
1.12. Analysis.
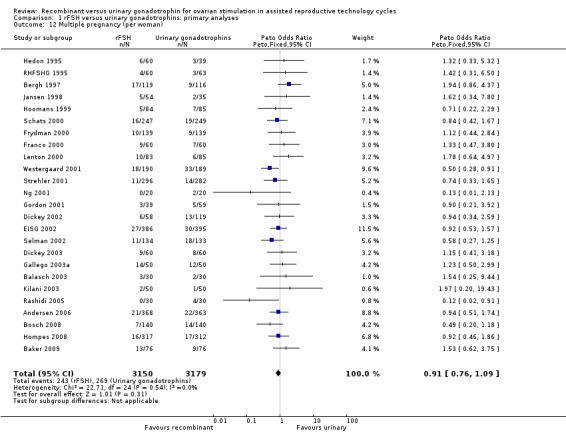
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 12 Multiple pregnancy (per woman).
17.
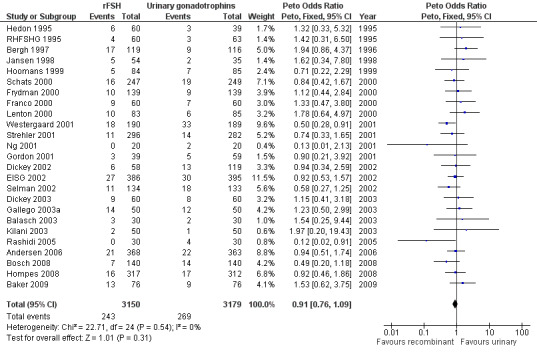
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.15 Multiple pregnancy (per woman).
1.13. Analysis.
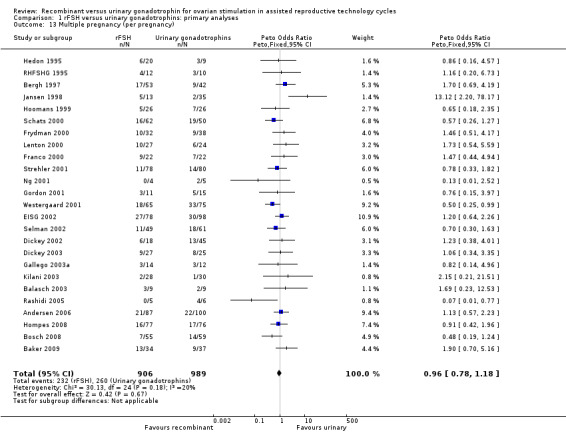
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 13 Multiple pregnancy (per pregnancy).
18.
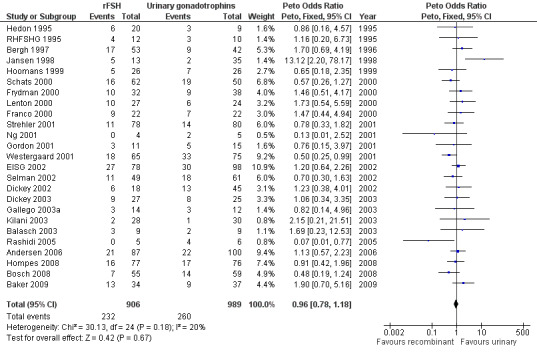
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.16 Multiple pregnancy (per pregnancy).
There was no evidence of a statistically significant difference in miscarriage rate (30 trials, N=6663; Analysis 1.14; OR 1.16, 95% CI 0.95 to 1.47) with an I2 of 0 (for the corresponding forest plot see Figure 19 ).
1.14. Analysis.
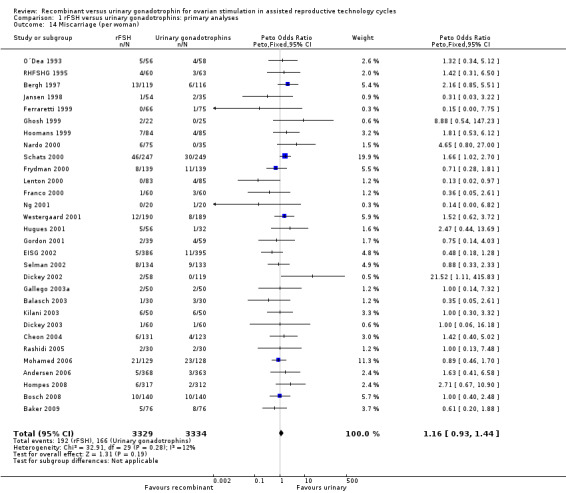
Comparison 1 rFSH versus urinary gonadotrophins: primary analyses, Outcome 14 Miscarriage (per woman).
19.
Forest plot of comparison: 1 rFSH versus urinary gonadotrophins: primary analyses, outcome: 1.17 Miscarriage (per woman).
For the oocytes retrieved there was data from 37 trials, entailing 8564 couples. However, as oocytes could not be retrieved per woman randomised and because there was very large statistical heterogeneity between the trials data was not presented in a forest plot and a mean difference (MD) was not calculated. Data are presented under additional tables (Table 3).
Data differed largely between the individual trials for amount of gonadotrophins used (IU) and for duration of ovarian stimulation (days) (I2 of 96% and 90% respectively). Data was not presented in a forest plot and was not pooled. Data are presented under additional tables (Table 4 and Table 5).
2 Sensitivity analyses for rFSH versus urinary gonadotrophins excluding lower quality trials
Data are presented in "Data and analyses" under "2 Sensitivity analyses excluding lower quality trials". These sensitivity analyses were done for the outcomes live birth, OHSS and clinical pregnancy only and excluded trials that were not ITT and/or that did perform multiple cycles. Live birth, OHSS and clinical pregnancy data are presented grouped for the types of urinary gonadotrophins and grouped for type of down‐regulation.
Primary outcomes
Primary efficacy outcome: Live birth
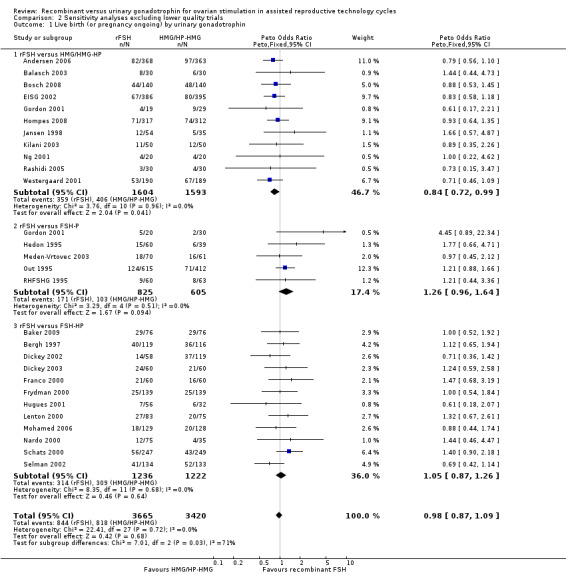
One trial was excluded in the sensitivity analyses as this trial performed multiple cycles and results were presented per cycle and not per woman (Cheon 2004). In the primary analysis there was no evidence of a statistically significant difference between rFSH and urinary gonadotrophins in live births or pregnancy ongoing beyond 20 weeks (28 trials, N=7339; Analysis 1.5; OR 0.97, 95% CI 0.87 to 1.08; I2 of 0%) and exclusion of this trial did not affect the result (27 trials, N=7085; Analysis 2.1; OR 0.98, 95% CI 0.87 to 1.09; I2 of 0%) .
2.1. Analysis.
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 1 Live birth (or pregnancy ongoing) by urinary gonadotrophin.
2.1 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by the different urinary gonadotrophins
See Analysis 2.1
The one trial with data on live births that was excluded in the sensitivity analysis compared rFSH with FSH‐HP. Exclusion of this trial from the subgroup rFSH versus FSH‐HP did not affect the outcome of the primary analyses as presented in Analysis 1.1 .
Of the 27 trials with data on live births 11 trials compared rFSH versus HMG/HP‐HMG, 5 trials compared rFSH with FSH‐P and 13 trials compared rFSH with FSH‐HP.
There were significantly fewer live births after rFSH as compared to HMG ( OR 0.84, 95% CI 0.72 to 0.99; 11 trials, N=3197; Analysis 2.1 ). There was no indication of statistical heterogeneity. Visual inspection of the forest plot showed that the OR and 95%CI of the individual trials largely overlapped and the I2 was 0%. Pooling using a random effects model resulted in the same OR.
There was no evidence of a statistically significant difference in live births between rFSH and FSH‐P (5 trials, N=1430; Analysis 2.1; OR 1.26, 95% CI 0.96 to 1.64; I2 of 0%) and between rFSH and FSH‐HP (12 trials, N=2458; Analysis 2.1; OR 1.05, 95% CI 0.87 to 1.26; I2 of 0%)
2.2 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by down regulation protocol
See Analysis 2.2
2.2. Analysis.
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 2 Live birth (or pregnancy ongoing) rFSH vs FSH‐P by down‐regulation.
The one trial with data on live births that was excluded in the sensitivity analysis used a short GnRHa protocol. Exclusion of this trial from the subgroup long agonist protocol did not affect the outcome of the primary analyses presented in Analysis 1.2.
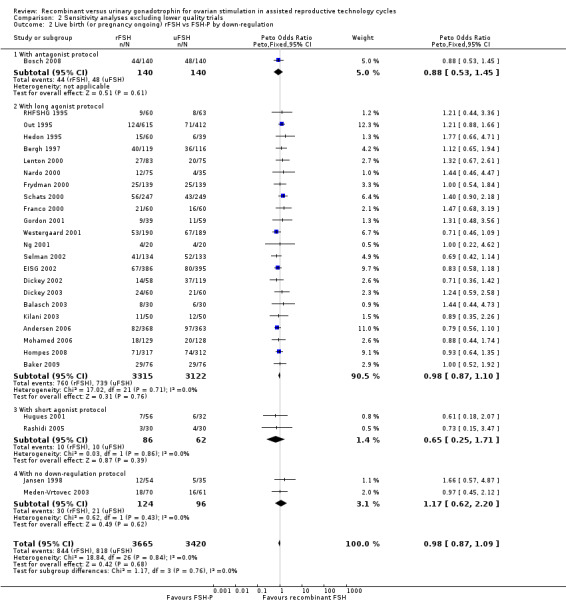
Of the 27 trials with data on live births 1 trial used an antagonist protocol, 22 trials used a long GnRH agonist protocol, three used a short GnRH agonist protocol, and two did not use down‐regulation.
There was no evidence of a statistically significant difference in live births between rFSH and urinary gonadotrophins for any of the down regulation protocols (antagonist protocol, 1 trial, N=280; Analysis 2.2; OR 0.88, 95% CI 0.53 to 1.45), (long GnRHa protocol, 22 trials, N=6437; Analysis 2.2; OR 0.98, 95% CI 0.87 to 1.10), (short GnRHa protocol, 1 trial, N=148; Analysis 2.2; OR 0.65, 95% CI 0.25 to 1.71), (no down regulation, 2 trials, N=220; Analysis 2.2; OR 1.17, 95% CI 0.62 to 2.20). There was no indication of statistical heterogeneity ( I2 of 0%).
2.3 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by fresh/frozen policy
See Analysis 2.3
2.3. Analysis.
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 3 Live birth (or ongoing pregnancy) by fresh/frozen policy.
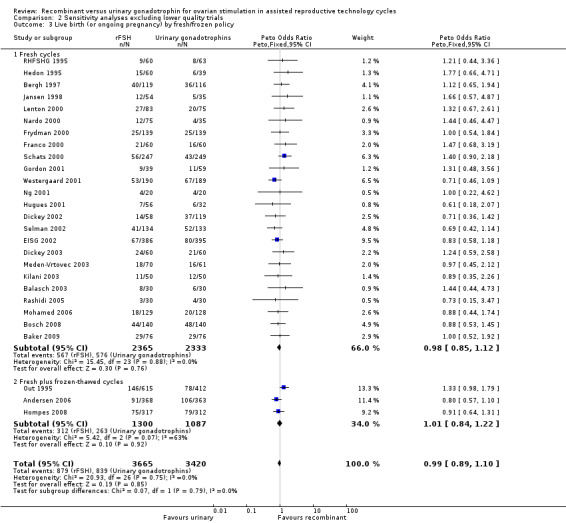
The one trial with data on live births that was excluded in the sensitivity analysis performed only fresh cycles. Exclusion of this trial from the subgroup fresh cycles did not affect the outcome of the primary analyses in Analysis 1.3.
Of the 27 trials with data on live births the outcome of frozen‐thawed cycles was known for only three trials. There was no evidence of a statistically significant difference between rFSH and urinary gonadotrophins for live births after fresh cycles (24 trials, N=4698; Analysis 2.3; OR 0.98, 95% CI 0.85 to 1.12) and for cumulative live birth rate after fresh and frozen‐thawed cycles (3 trials, N=2387; Analysis 2.3; OR 1.01, 95% CI 0.84 to 1.22). There was no indication of statistical heterogeneity ( I2 of 0%).
2.4 Live birth (or pregnancy ongoing beyond 20 wk's) grouped by pharmaceutical sponsor
See Analysis 2.4
2.4. Analysis.
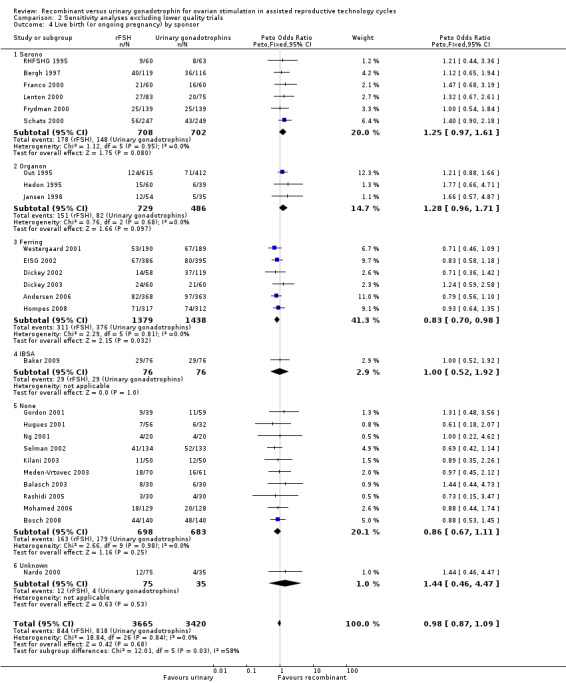
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 4 Live birth (or ongoing pregnancy) by sponsor.
The one trial with data on live births that was excluded in the sensitivity analysis was not a commercially sponsored trial. Exclusion of this trial from the subgroup none did not affect the outcome of the primary analyses as presented in Analysis 1.3 .
Of the 27 trials with data on live births six trials were sponsored by Serono, three trials were sponsored by Organon ‐ now MSD, six trials were sponsored by Ferring, one trials was sponsored by IBSA, 10 trials were not sponsored by a pharmaceutical company and for one trials sponsoring was unknown.
There was no evidence of a statistically significant difference in live births between rFSH and urinary gonadotrophins for the trials sponsored by Sereno (6 trials, N=1410; Analysis 2.4; OR 1.25, 95% CI 0.97 to 1.61), the trials sponsored by Organon (3 trials, N=1215; Analysis 2.4; OR 1.28, 95% CI 0.96 to 1.71), the trial sponsored by IBSA (1 trials N=152; Analysis 2.4; OR 1.00, 95% CI 0.52 to 1.92), and the non‐sponsored trials (10 trials, N=1381; Analysis 2.4; OR 0.86, 95% CI 0.67 to 1.11). However, there were significantly fewer live births after rFSH as compared to urinary gonadotrophins for the trials sponsored by Ferring (6 trials, N=2817; Analysis 2.4; OR 0.83, 95% CI 0.69 to 0.98). There was no indication of statistical heterogeneity for any of these grouped comparisons (I2 was 0%).
Primary safety outcome: OHSS
Two trials with data on OHSS were excluded in the sensitivity analyses as it was not certain whether data were according to ITT (Berger 1999, Gallego 2003a). Furthermore, one of the trials use multiple cycles and presented data (Berger 1999). In the primary analysis there was no evidence of a statistically significant difference between rFSH and urinary gonadotrophins in live birth or pregnancy ongoing beyond 20 weeks (32 trials, N=7740; Analysis 1.5; OR 1.18, 95% CI 0.86 to 1.61; I2 of 0%) for rFSH versus urinary gonadotrophins and exclusion of the two trials did not change that finding (30 trials, N=7475; Analysis 2.5; OR 1.24, 95% CI 0.90 to 1.7).
2.5. Analysis.
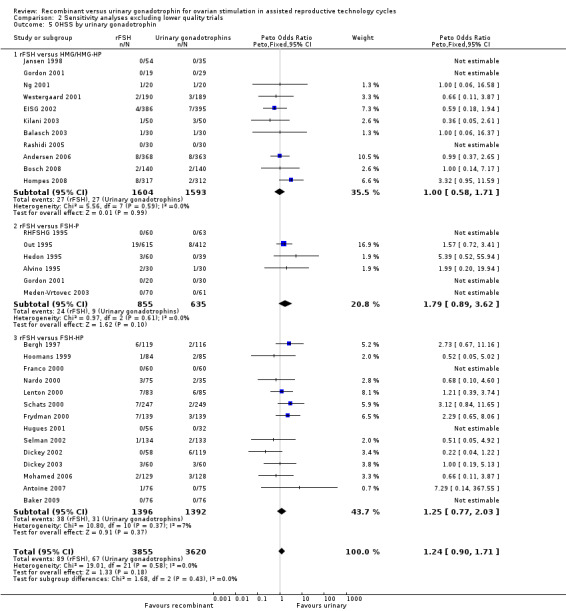
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 5 OHSS by urinary gonadotrophin.
2.5 OHSS grouped by the different urinary gonadotrophins
See Analysis 2.5
The two trials with data on OHSS that were excluded in the sensitivity analyses compared rFSH with FSH‐HP. Exclusion of these trials from the subgroup rFSH versus FSH‐HP did not affect the outcome of the primary analyses as presented in Analysis 1.5.
Of the 30 trials with data on OHSS, 11 trials compared rFSH versus HMG/HP‐HMG, 6 trials compared rFSH with FSH‐P and 14 trials compared rFSH with FSH‐HP.
There was no evidence of a statistically significant difference in OHSS for rFSH versus HMG (11 trials, N=3197; Analysis 2.5; OR 1.00, 95% CI 0.58 to 1.71), also not for rFSH versus FSH‐P (6 trials, N=1490; Analysis 2.5; OR 1.79, 95% CI 0.89 to 3.62), and for rFSH versus FSH‐HP (14 trials, N=2788; Analysis 2.5; OR 1.25, 95% CI 0.77 to 2.03; I2 was 0%).
2.6 OHSS grouped by down regulation protocol
See Analysis 2.6
2.6. Analysis.
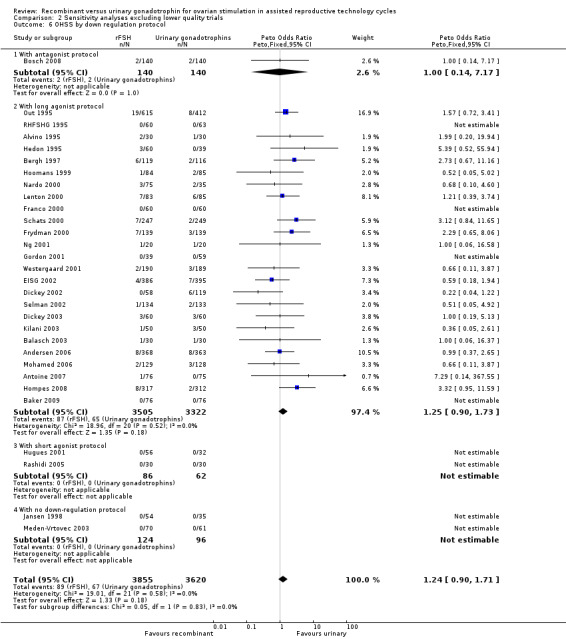
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 6 OHSS by down regulation protocol.
The two trials with data on OHSS that were excluded in the sensitivity analyses used a long GnRHa protocol. Exclusion of these trials from the subgroup long agonist protocol did not affect the outcome of the primary analyses as presented in Analysis 1.6.
Of the 30 trials with data on live birth 1 trial used an antagonist protocol, 27 trials used a long GnRH agonist protocol, two used a short GnRH agonist protocol, and two did not use down‐regulation.
There was no evidence of a statistically significant difference in OHSS between rFSH and urinary gonadotrophins for any of the down regulation protocols (antagonist protocol, N=280; Analysis 2.6; OR 1.00, 95% CI 0.14 to 7.17), (long GnRHa protocol, N=6827; Analysis 2.6; OR 1.25, 95% CI 0.90 to 1.73; OR 1.18, 95% CI 0.86 to 1.62), (short GnRHa protocol, N=148; Analysis 2.6; OR not estimable due to lack of OHSS cases), (no down regulation, N=220; OR not estimable due to lack of OHSS cases). There was no indication of statistical heterogeneity for any of these grouped comparisons ( I2 of 0%).
2.7 OHSS grouped by sponsor
See Analysis 2.7
2.7. Analysis.
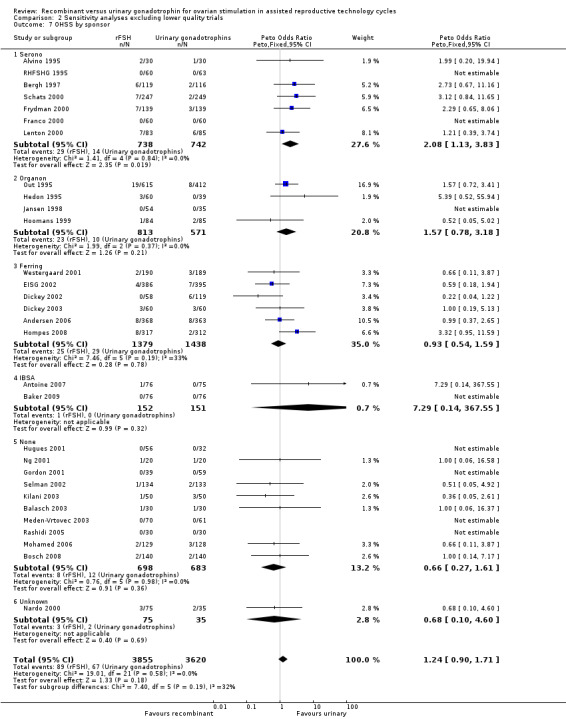
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 7 OHSS by sponsor.
For the two trials with data on OHSS that were excluded in the sensitivity analyses the sponsoring was unknown. Exclusion of these trials from the subgroup sponsoring unknown did not affect the outcome of the primary analyses as presented in Analysis 1.7.
Of the 30 trials with data on OHSS seven trials were sponsored by Serono, four trials were sponsored by Organon ‐ now MSD, six trials were sponsored by Ferring, two trials were sponsored by IBSA, 10 trials were not sponsored by a pharmaceutical company and for one trials sponsoring was unknown.
There was more OHSS in the rFSH group as compared to urinary gonadotrophins in studies sponsored by Serono (7 trials, N=1480; OR 2.08, 95% CI 1.13 to 3.83).
There was no evidence of a statistically significant difference in OHSS between rFSH and urinary gonadotrophins for the trials sponsored by Organon (4 trials, N=1387; Analysis 2.7; OR 1.57, 95% CI 0.78 to 3.18), the trials sponsored by Ferring (6 trials, N=2817; Analysis 2.7; OR 0.93, 95% CI 0.54 to 1.59), the trials sponsored by IBSA (2 trials, N=303; Analysis 2.7; OR 7.29, 95% CI 0.14 to 368), and the non‐sponsored trials (10 trials, N=1381; Analysis 2.7; OR 0.66, 95% CI 0.27 to 1.61). There was no indication of statistical heterogeneity for any of these grouped comparisons (I2 was 0%).
Secondary outcomes
Clinical pregnancy rate
Six trials with data on clinical pregnancies were excluded in the sensitivity analyses as it was not certain whether data were presented according to ITT and/or because multiple cycles were done and data were presented per cycle and not per woman (O´Dea 1993; Berger 1999; Ghosh 1999 ;Machado 1999Gallego 2003a; Cheon 2004). In the primary analysis there was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and urinary gonadotrophins (41 trials, N=9482; OR 0.99, 95% CI 0.91 to 1.09; I2 of 0%) and exclusion of the six trials did not change that result (35 trials, N=8744; OR 0.99, 95% CI 0.90 to 1.09; I2 of 0%).
2.8 Clinical pregnancy rate grouped by the different urinary gonadotrophins
See Analysis 2.8
2.8. Analysis.
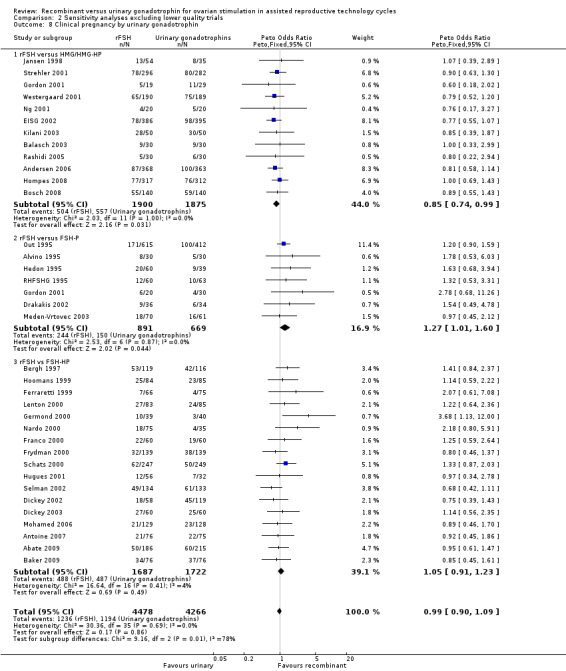
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 8 Clinical pregnancy by urinary gonadotrophin.
The six excluded trials all compared rFSH with FSH‐HP. Exclusion of these six trials from the subgroup rFSH versus FSH‐HP did not affect the outcome of the primary analyses as presented Analysis 1.8.
Of the 35 trials with data on clinical pregnancies, 12 trials compared rFSH versus HMG/HP‐HMG, seven trials compared rFSH with FSH‐P and 17 trials compared rFSH with FSH‐HP.
There were significantly fewer clinical pregnancies after rFSH as compared to HMG (OR 0.85, 95% CI 0.74 to 0.99; I2 of 0%; 12 trials, N=3775; Analysis 2.8). There were significantly more clinical pregnancies after rFSH as compared to FSH‐P (OR 1.27, 95% CI 1.01 to 1.60; I2 of 0%; 7 trials, N=1560; Analysis 2.8). There was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and FSH‐HP (17 trials, N=3409; Analysis 2.8; OR 1.05, 95% CI 0.91 to 1.23; I2 of 0%).
2.9 Clinical pregnancy rate grouped by down regulation protocol
See Analysis 2.9
2.9. Analysis.
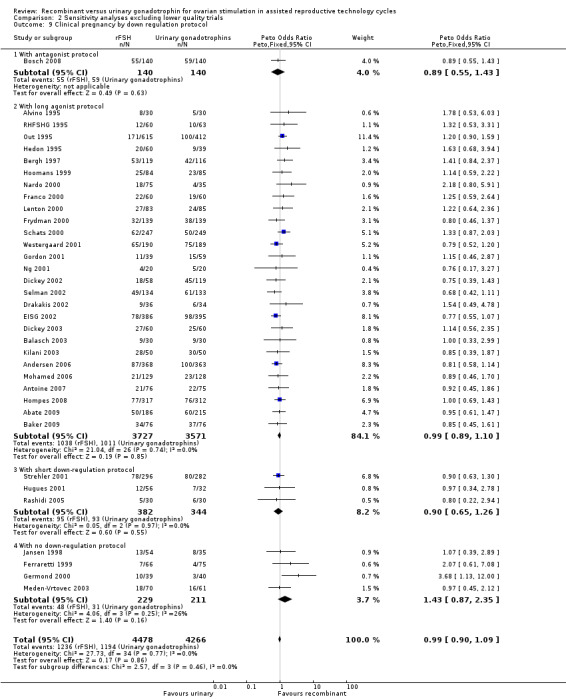
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 9 Clinical pregnancy by down regulation protocol.
Of the six excluded trials with data on clinical pregnancies four used a long agonist protocol and one used a short agonist protocol and one used no down regulation. Exclusion of these six trials from the respective down regulation subgroups did not affect the outcome of the primary analyses as presented Analysis 1.9.
Of the 35 trials with data on clinical pregnancies that were included in the sensitivity analysis, one trial used an antagonist protocol, 27 trials used a long GnRH agonist protocol, three used a short GnRH agonist protocol, and five did not use down‐regulation.
There was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and urinary gonadotrophins for any of the down regulation protocols (antagonist protocol, N=280; Analysis 2.9; OR 0.89, 95% CI 0.55 to 1.43), (long GnRHa protocol, N=7298; Analysis 2.9; OR 0.99, 95% CI 0.89 to 1.10), (short GnRHa protocol, N=726; Analysis 2.9; OR 0.90, 95% CI 0.65 to 1.26), (no down regulation, N=440; Analysis 2.9; OR 1.43, 95% CI 0.87 to 2.35). There was no indication of statistical heterogeneity for these grouped comparisons (I2 was 0%).
2.11 Clinical pregnancy rate grouped by fresh/frozen policy
See Analysis 2.10
2.10. Analysis.
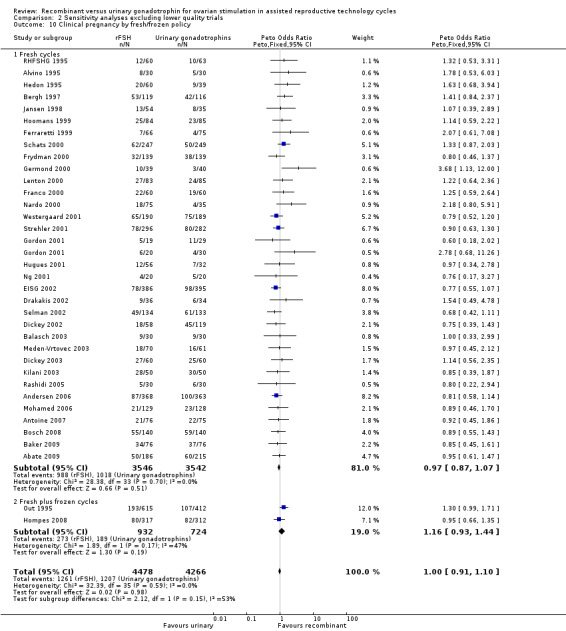
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 10 Clinical pregnancy by fresh/frozen policy.
Of the 35 trials with data on clinical pregnancies the outcome of frozen‐thawed cycles was known for only two trials. There was no evidence of a statistically significant difference between rFSH and urinary gonadotrophins for clinical pregnancy rate after fresh cycles (33 trials, N=8744; Analysis 2.10; OR 1.00, 95% CI 0.91 to 1.10) and for cumulative clinical pregnancy rate after fresh and frozen‐thawed cycles (2 trials, N=1656; Analysis 2.10; OR 1.16, 95% CI 0.93 to 1.44). There was no indication of statistical heterogeneity ( I2 of 0%).
2.12 Clinical pregnancy rate grouped by pharmaceutical sponsor
See Analysis 2.11
2.11. Analysis.
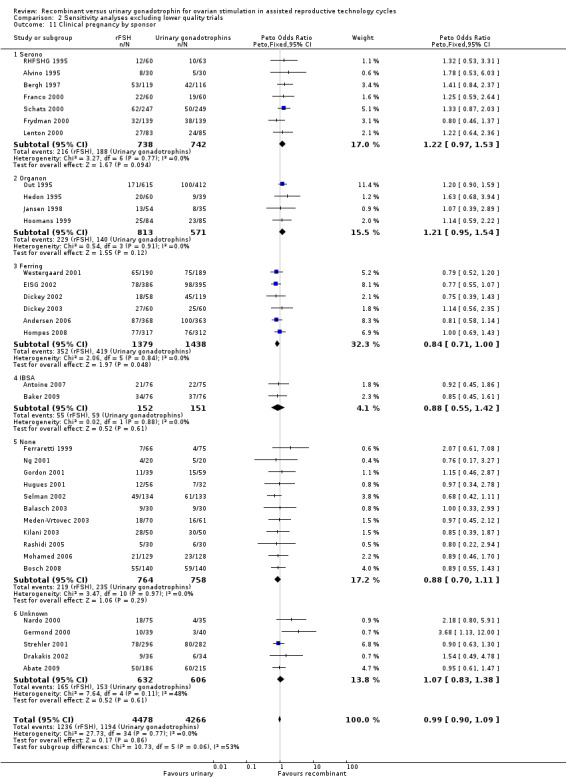
Comparison 2 Sensitivity analyses excluding lower quality trials, Outcome 11 Clinical pregnancy by sponsor.
Of the six excluded trials with data on clinical pregnancies all used fresh cycles. Exclusion of these six trials from the respective fresh and frozen subgroups did not affect the outcome of the primary analyses as presented under 1.11.
Of the 35 trials with data on clinical pregnancies that were included in the sensitivity analysis, seven trials were sponsored by Serono, four trials were sponsored by Organon ‐ now MSD, six trials were sponsored by Ferring, two trials were sponsored by IBSA, 11 trials were not sponsored by a pharmaceutical company and for five trials sponsoring was unknown.
There was no evidence of a statistically significant difference in clinical pregnancy rate between rFSH and urinary gonadotrophins for the trials sponsored by Sereno (7 trials, N=1480; Analysis 2.11; OR 1.22, 95% CI 0.97 to 1.53), the trials sponsored by Organon (4 trials, N=1384; OR 1.21, 95% CI 0.95 to 1.54), the trials sponsored by IBSA (2 trials N=303; Analysis 2.11; OR 0.88, 95% CI 0.55 to 1.42), and the non‐sponsored trials (11 trials, N=1522; Analysis 2.11; OR 0.88, 95% CI 0.70 to 1.11). However, there were borderline significantly fewer live births after rFSH as compared to urinary gonadotrophins for the trials sponsored by Ferring (6 trials, N=2817; Analysis 2.11; OR 0.84, 95% CI 0.71 to 1.00). There was no indication of statistical heterogeneity for any of these grouped comparisons (I2 was 0%).
Extra unplanned analysis
For live birth rate and clinical pregnancy rate statistically significant differences were found only for the subgroup of trials that compared rFSH versus HMG/HP‐HMG (Figure 6) and for the subgroup of trials that were sponsored by Ferring (Figure 9). Of the six trials that were sponsored by the pharmaceutical company Ferring four were trials that compared rFSH and HMG/HP‐HMG. To evaluate whether the differences in live birth rate and clinical pregnancy rate are (partly) due to a sponsor effect we also pooled the data of five mostly small non‐sponsored trials (N=548) that compared rFSH and HMG/HP‐HMG (Gordon 2001; Ng 2001; Kilani 2003; Balasch 2003; Bosch 2008) and we pooled the data of the four large Ferring‐sponsored trials (N=2520) that compared rFSH and HMG/HP‐HMG (Westergaard 2001;EISG 2002; Andersen 2006; Hompes 2008).
The pooled OR for clinical pregnancy rate of the five non‐sponsored trials was 0.84 (95% CI 0.58 to 1.21). The pooled OR for clinical pregnancy rate of the four trials that had been sponsored by Ferring was 0.84 (95% CI 0.70 to 1.00).
The pooled OR for live birth rate of the five non‐sponsored trials was 0.90 (95% CI 0.62 to 1.31). The pooled OR for live birth rate of the four trials that had been sponsored by Ferring was 0.81 (95% CI 0.68 to 0.98).
Discussion
Summary of main results
This review compared the effectiveness of recombinant gonadotrophin (rFSH) with the three main types of urinary gonadotrophins (i.e. HMG, FSH‐P and FSH‐HP) for ovarian stimulation in women undergoing IVF or ICSI treatment cycles. Overall, there was no evidence of a difference in pregnancy outcomes when rFSH was compared to urinary gonadotrophins as a whole. Comparing rFSH with HMG/HP‐HMG resulted in a lower live birth rate in the rFSH group though differences were small and considered unlikely to be clinically significant. There was no proof of a difference in live births when comparing rFSH with FSH‐P or with FSH‐HP and there was no evidence of a difference observed in OHSS for any of the comparisons.
There was no evidence of differences in live births, OHSS or clinical pregnancy when rFSH was compared to urinary gonadotrophins as a whole in any of the down regulation groups. There was also no evidence of a difference in cumulative live birth and clinical pregnancy rates following frozen‐thawed cycles for rFSH versus urinary gonadotropins. Stratification for sponsoring did only result in a difference in the trials that were sponsored by Ferring. The live birth and clinical pregnancy rate were lower for rFSH compared to urinary FSH in the Ferring sponsored trials. This will be discussed further under the sub header Potential biases in the review process.
Overall completeness and applicability of evidence
Of the 42 trials that were included in the primary analyses all 42 trials had data on clinical pregnancy but only 28 trials had data on live birth and 32 trials had data on OHSS. In the sensitivity analysis excluding the trials of lower quality 35 trials had data on clinical pregnancy, 27 trials had data on live birth and 30 trials had data on OHSS. For the trials that compared rFSH and HMG the data were most complete ‐ these trials were all published after 2001. The data of trials that compared rFSH and uFSH‐P and uFSH‐HP were more incomplete as these included older trials that had been published before 2000 in a time when clinical pregnancy was still an accepted endpoint.
The evidence is broadly applicable for standard IVF and ICSI cycles.
The data on oocyte retrieval, gonadotrophin dose used and duration of stimulation was generally not presented per woman randomised. Therefore, these outcomes are likely to be biased and no conclusions on the basis of these comparisons should be drawn.
The preference of patients for a particular type of gonadotrophin was not evaluated in any trial. As all gonadotrophins are now given subcutaneously and with so few clinical differences a clear preference profile for a trial is not easy to make. Differences in preference are likely to be influenced by the way of presenting the drug (using a pen, needing only a single administration). This was however not the aim of the present study.
Quality of the evidence
Most included studies used computer generated randomisation with a proper method of allocation concealment. The quality of the trials varied from low to moderate and appeared to be high in some of the larger sponsored trials.
Potential biases in the review process
The majority of the included trials were industry sponsored and this may have introduced a bias in favour of the gonadotrophin produced by the sponsor. The subgroup analyses in which we grouped the primary outcomes for pharmaceutical sponsoring suggest that live birth and clinical pregnancy rate were lower for rFSH compared to urinary FSH in the Ferring sponsored trials. However, further analysis of the Ferring sponsored trials and the non‐sponsored trials that compared HMG/HP‐HMG with rFSH showed comparable summary OR and confidence intervals that overlapped. Hence, though we cannot rule out that a sponsor effect is involved in this finding the potential effect of this sponsor bias appears to be limited.
We only did grouped analyses for down‐regulation method, use of fresh or fresh and frozen cycles and sponsoring. There were more differences between trials that we did not explore further in sub analyses. For instance, the fertilisation method may affect the effectiveness of gonadotrophins (Al‐Inany 2009) and a sub optimal dose may also have impact on the results.
Agreements and disagreements with other studies or reviews
Our results are in agreement with all the previous reviews (Daya 1998; Larizgoitia 2000;Daya 2002;Van Wely 2003NCC‐WCH 2004; Al‐Inany 2008; Al‐Inany 2009; Coomarisamy 2008), although there appears to be a difference with two older reviews that found rFSH to result in more clinical pregnancies than FSH‐P and FSH‐HP (Daya 1998; Daya 2002). This review also found evidence of a higher clinical pregnancy rate for rFSH in comparison to FSH‐P but no evidence of a difference between rFSH and FSH‐HP. The difference between the Daya reviews and the present review can however be explained. If we were to have combined only the trials that were performed before 2001 the clinical pregnancy rate would indeed have been in significantly in favour of rFSH. In other words, this difference in clinical pregnancy rate between rFSH and FSH‐HP was only found in the older trials performed before 2000 and not in the trials performed after 2000, which explains why this difference was no longer detectable.
Authors' conclusions
Implications for practice.
It appears that all available gonadotrophins are equally effective and safe. The choice of one or the other product will depend upon the availability of the product, the convenience of its use, and the associated costs.
Implications for research.
We included 42 trials, entailing 9606 couples. Any specific differences are likely to be too small to justify further research.
Feedback
Comments from Dr Masoud Afnan
Summary
The summary states that there are no statistically significant differences between rec FSH and all urinary gonadotrophins. However the detailed analysis of rec FSH and hMG (analysis 1.1.1) does show a statistically significant difference OR 0.84 (0.72‐0.99). Heterogeniety is 0%. I believe that this difference is clinically important, and biologically plausible. Masoud Afnan
Reply
Thank you for your feedback. We agree that the text in the Results section of the Abstract did not make it sufficiently clear that it referred to comparison between recombinant gonadotrophin versus urinary gonadotrophins overall. We have edited the text to clarify this. We have also added to the abstract the live birth data for recombinant gonadotrophin versus individual urinary gonadotrophins. However, we have noted that we not consider the difference for HMG to be clinically important.
Contributors
Dr Masoud Afnan, Director of Obstetrics and Gynaecology, Beijing United Family Hospital
Review authors: Madelon van Wely, Irene Kwan, Anna Burt, Jane Thomas, Andy Vail, Fulco Van der Veen, Hesham Al‐Inany
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2012 | Feedback has been incorporated | Abstract edited. Results for individual urinary gonadotropins included in abstract. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 15 March 2011 | Amended | Minor edit. Added to the Plain language summary the word significantly: Comparing rFSH with HMG/HP‐HMG resulted in a "significantly" lower live birth rate in the rFSH group though differences were small. Added the grading HIGH for the primary adverse outcome OHSS to the summary of findings table. |
| 15 March 2011 | Amended | Minor edit. Added to the abstract how many people selected studies and extracted data. Added dates to the searches in the abstract |
| 21 February 2011 | Amended | Minor edit made EISG study was reported in the table as having been conducted with Puregon whereas it should be Gonal F‐ this is now corrected |
| 3 February 2011 | Amended | Minor edit to with the addition of the correct DOI to the reference Van Wely 2002 |
| 19 November 2007 | New citation required and conclusions have changed | Substantive amendment of protocol |
Notes
Three previously published Cochrane reviews have been superseded by this review . These three reviews are now withdrawn Van Wely 2002; Daya 2003 and Daya 2000.
Acknowledgements
We would like to thank authors of papers and representatives of pharmaceutical companies for providing extra information.
Appendices
Appendix 1. Search strategy
MDSG 1.04.09
Keywords CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in vitro fertilization" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "*Ovulation Induction" or "ovulation" or "COH" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "ovarian stimulation" or Title CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in vitro fertilization" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "*Ovulation Induction" or "ovulation" or "COH" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "ovarian stimulation"
AND
Keywords CONTAINS "recombinant" or "recombinant FSH" or "recombinant hFSH" or "rFHS" or "rFSH" or "rh‐FSH" or Title CONTAINS "recombinant" or "recombinant FSH" or "recombinant hFSH" or "rFHS" or "rFSH" or "rh‐FSH"
AND
Keywords CONTAINS "urinary FSH" or "HMG" or "human menopausal gonadotrophin" or "FSH HMG" or "uFSH" or Title CONTAINS "urinary FSH" or "HMG" or "human menopausal gonadotrophin" or "FSH HMG" or "uFSH"
EMBASE
Database: EMBASE <1980 to 200 Week 20> Search Strategy:
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/
2 ((in vitro adj5 fertilization).tw. or in vitro.mp.) adj5 fertilisation.tw. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
3 intracytoplasmic sperm injection$.tw.
4 (ivf or icsi).tw.
5 exp ovulation induction/ or exp superovulation/
6 ovulat$ induc$.tw.
7 superovulation.tw.
8 (ovar$ adj5 stimulat$).tw.
9 (ovar$ adj1 induc$).tw.
10 or/1‐9
11 recombinant follicle stimulating hormone.tw.
12 recombinant FSH.tw.
13 rFSH.tw.
14 (recombinant adj5 gonadotropin$).tw.
15 (recombinant adj5 gonadotrophin$).tw.
16 or/11‐15
17 HMG.tw.
18 FSH‐P.tw.
19 FSH‐HP.tw.
20 (urin$ adj5 gonadotropin$).tw.
21 (urin$ adj5 gonadotrophin$).tw.
22 uFSH$.tw.
23 (urin$ adj5 FSH).tw.
24 (urin$ adj5 follicle$ stimulat$).tw.
25 human menopausal gonadotrop#in$.tw.
26 or/17‐25
27 26 and 16 and 10
28 Controlled study/ or randomized controlled trial/
29 double blind procedure/
30 single blind procedure/
31 crossover procedure/
32 drug comparison/
33 placebo/
34 random$.ti,ab,hw,tn,mf.
35 latin square.ti,ab,hw,tn,mf.
36 crossover.ti,ab,hw,tn,mf.
37 cross‐over.ti,ab,hw,tn,mf.
38 placebo$.ti,ab,hw,tn,mf.
39 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf.
40 (comparative adj5 trial$).ti,ab,hw,tn,mf.
41 (clinical adj5 trial$).ti,ab,hw,tn,mf.
42 or/28‐41
43 nonhuman/
44 animal/ not (human/ and animal/)
45 or/43‐44
46 42 not 45
47 27 and 46
MEDLINE
Database: Ovid MEDLINE(R) <1950 to May Week 4 2010> Search Strategy: 1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ 2 ((in vitro adj5 fertilization).tw. or in vitro.mp.) adj5 fertilisation.tw. [mp=title, original title, abstract, name of substance word, subject heading word] 3 intracytoplasmic sperm injection$.tw. 4 (ivf or icsi).tw. 5 exp ovulation induction/ or exp superovulation/ 6 ovulat$ induc$.tw. 7 superovulation.tw. 8 (ovar$ adj5 stimulat$).tw. 9 (ovar$ adj1 induc$).tw. 10 or/1‐9 11 recombinant follicle stimulating hormone.tw. 12 recombinant FSH.tw. 13 rFSH.tw. 14 (recombinant adj5 gonadotropin$).tw. 15 (recombinant adj5 gonadotrophin$).tw. 16 or/11‐15 17 HMG.tw. 18 FSH‐P.tw. 19 FSH‐HP.tw. 20 (urin$ adj5 gonadotropin$).tw. 21 (urin$ adj5 gonadotrophin$).tw. 22 uFSH$.tw. 23 (urin$ adj5 FSH).tw. 24 (urin$ adj5 follicle$ stimulat$).tw. 25 human menopausal gonadotrop#in$.tw. 26 or/17‐25 27 26 and 16 and 10 28 randomized controlled trial.pt. 29 controlled clinical trial.pt. 30 randomized.ab. 31 placebo.ab. 32 cross‐over studies/ 33 (crossover or cross‐over or cross over).tw. 34 clinical trials as topic.sh. 35 randomly.ab. 36 trial.ti. 37 or/28‐36 38 humans.sh. 39 37 and 38 40 27 and 39
CINAHL
Database: CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature <1982 to May Week 3 2010> Search Strategy: 1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ 2 ((in vitro adj5 fertilization).tw. or in vitro.mp.) adj5 fertilisation.tw. [mp=title, subject heading word, abstract, instrumentation] 3 intracytoplasmic sperm injection$.tw. 4 (ivf or icsi).tw. 5 exp ovulation induction/ or exp superovulation/ 6 ovulat$ induc$.tw. 7 superovulation.tw. 8 (ovar$ adj5 stimulat$).tw. 9 (ovar$ adj1 induc$).tw. 10 or/1‐9 11 recombinant follicle stimulating hormone.tw. 12 recombinant FSH.tw. 13 rFSH.tw. 14 (recombinant adj5 gonadotropin$).tw. 15 (recombinant adj5 gonadotrophin$).tw. 16 or/11‐15 17 HMG.tw. 18 FSH‐P.tw. 19 FSH‐HP.tw. 20 (urin$ adj5 gonadotropin$).tw. 21 (urin$ adj5 gonadotrophin$).tw. 22 uFSH$.tw. 23 (urin$ adj5 FSH).tw. 24 (urin$ adj5 follicle$ stimulat$).tw. 25 human menopausal gonadotrop#in$.tw. 26 or/17‐25 27 26 and 16 and 10 28 exp clinical trials/ 29 Clinical trial.pt. 30 (clinic$ adj trial$1).tw. 31 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw. 32 Randomi?ed control$ trial$.tw. 33 Random assignment/ 34 Random$ allocat$.tw. 35 Placebo$.tw. 36 Placebos/ 37 Quantitative studies/ 38 Allocat$ random$.tw. 39 or/28‐38 40 27 and 39
CENTRAL
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <2rd Quarter 2010> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ 2 ((in vitro adj5 fertilization).tw. or in vitro.mp.) adj5 fertilisation.tw. [mp=title, original title, abstract, mesh headings, heading words, keyword] 3 intracytoplasmic sperm injection$.tw. 4 (ivf or icsi).tw. 5 exp ovulation induction/ or exp superovulation/ 6 ovulat$ induc$.tw. 7 superovulation.tw. 8 (ovar$ adj5 stimulat$).tw. 9 (ovar$ adj1 induc$).tw. 10 or/1‐9 11 recombinant follicle stimulating hormone.tw. 12 recombinant FSH.tw. 13 rFSH.tw. 14 (recombinant adj5 gonadotropin$).tw. 15 (recombinant adj5 gonadotrophin$).tw. 16 or/11‐15 17 HMG.tw. 18 FSH‐P.tw. 19 FSH‐HP.tw. 20 (urin$ adj5 gonadotropin$).tw. 21 (urin$ adj5 gonadotrophin$).tw. 22 uFSH$.tw. 23 (urin$ adj5 FSH).tw. 24 (urin$ adj5 follicle$ stimulat$).tw. 25 human menopausal gonadotrop#in$.tw. 26 or/17‐25 27 26 and 16 and 10
Data and analyses
Comparison 1. rFSH versus urinary gonadotrophins: primary analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth (or ongoing pregnancy) by urinary gonadotrophin | 28 | 7339 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 1.1 rFSH versus HMG/HMG‐HP | 11 | 3197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 1.2 rFSH versus FSH‐P | 5 | 1430 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.96, 1.64] |
| 1.3 rFSH versus FSH‐HP | 13 | 2712 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.86, 1.22] |
| 2 Live birth (or ongoing pregnancy) by down regulation protocol | 28 | 7339 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 2.1 With antagonist protocol | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.53, 1.45] |
| 2.2 With long agonist protocol | 22 | 6437 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.87, 1.10] |
| 2.3 With short agonist protocol | 3 | 402 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.54, 1.31] |
| 2.4 With no down‐regulation protocol | 2 | 220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.62, 2.20] |
| 3 Live birth (or ongoing pregnancy) by fresh/frozen policy | 28 | 7339 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.88, 1.10] |
| 3.1 Fresh cycles | 25 | 4952 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 3.2 Fresh plus frozen‐thawed cycles | 3 | 2387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
| 4 Live birth (or ongoing pregnancy) by sponsor | 28 | 7339 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 4.1 Serono | 6 | 1410 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.97, 1.61] |
| 4.2 Organon | 3 | 1215 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.96, 1.71] |
| 4.3 Ferring | 6 | 2817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 4.4 IBSA | 1 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.52, 1.92] |
| 4.5 None | 11 | 1635 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.70, 1.09] |
| 4.6 Unknown | 1 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.46, 4.47] |
| 5 OHSS by urinary gonadotrophin | 32 | 7740 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.86, 1.61] |
| 5.1 rFSH versus HMG/HMG‐HP | 11 | 3197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.58, 1.71] |
| 5.2 rFSH versus FSH‐P | 6 | 1490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [0.89, 3.62] |
| 5.3 rFSH versus FSH‐HP | 16 | 3053 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.70, 1.75] |
| 6 OHSS by down regulation protocol | 32 | 7740 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.86, 1.61] |
| 6.1 With antagonist protocol | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.17] |
| 6.2 With long agonist protocol | 27 | 7092 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.86, 1.62] |
| 6.3 With short agonist protocol | 2 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 With no down‐regulation protocol | 2 | 220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 OHSS by sponsor | 32 | 7740 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.86, 1.61] |
| 7.1 Serono | 7 | 1480 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.13, 3.83] |
| 7.2 Organon | 4 | 1384 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.78, 3.18] |
| 7.3 Ferring | 6 | 2817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.54, 1.59] |
| 7.4 IBSA | 2 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.29 [0.14, 367.55] |
| 7.5 None | 10 | 1381 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.27, 1.61] |
| 7.6 Unknown | 3 | 375 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.17, 1.53] |
| 8 Clinical pregnancy by urinary gonadotrophin | 41 | 9482 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 8.1 rFSH versus HMG/HMG‐HP | 12 | 3775 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.74, 0.99] |
| 8.2 rFSH versus FSH‐P | 7 | 1560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [1.01, 1.60] |
| 8.3 rFSH vs FSH‐HP | 23 | 4147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.91, 1.20] |
| 9 Clinical pregnancy by down regulation protocol | 41 | 9482 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 9.1 With antagonist protocol | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.55, 1.43] |
| 9.2 With long agonist protocol | 31 | 7718 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.90, 1.11] |
| 9.3 With short down‐regulation protocol | 4 | 980 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 9.4 With no down‐regulation protocol | 5 | 504 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.77, 1.97] |
| 10 Clinical pregnancy by fresh/frozen policy | 41 | 9482 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 10.1 Fresh cycles | 39 | 7826 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 10.2 Fresh plus frozen cycles | 2 | 1656 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.93, 1.44] |
| 11 Clinical pregnancy by sponsor | 41 | 9482 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 11.1 Serono | 9 | 1658 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.93, 1.45] |
| 11.2 Organon | 4 | 1384 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| 11.3 Ferring | 6 | 2817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.71, 1.00] |
| 11.4 IBSA | 2 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.55, 1.42] |
| 11.5 None | 12 | 1776 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.73, 1.10] |
| 11.6 Unknown | 8 | 1544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.88, 1.40] |
| 12 Multiple pregnancy (per woman) | 25 | 6329 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.76, 1.09] |
| 13 Multiple pregnancy (per pregnancy) | 25 | 1895 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.78, 1.18] |
| 14 Miscarriage (per woman) | 30 | 6663 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.93, 1.44] |
Comparison 2. Sensitivity analyses excluding lower quality trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth (or pregnancy ongoing) by urinary gonadotrophin | 27 | 7085 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 1.1 rFSH versus HMG/HMG‐HP | 11 | 3197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 1.2 rFSH versus FSH‐P | 5 | 1430 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.96, 1.64] |
| 1.3 rFSH versus FSH‐HP | 12 | 2458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.87, 1.26] |
| 2 Live birth (or pregnancy ongoing) rFSH vs FSH‐P by down‐regulation | 27 | 7085 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 2.1 With antagonist protocol | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.53, 1.45] |
| 2.2 With long agonist protocol | 22 | 6437 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.87, 1.10] |
| 2.3 With short agonist protocol | 2 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.25, 1.71] |
| 2.4 With no down‐regulation protocol | 2 | 220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.62, 2.20] |
| 3 Live birth (or ongoing pregnancy) by fresh/frozen policy | 27 | 7085 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.89, 1.10] |
| 3.1 Fresh cycles | 24 | 4698 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.85, 1.12] |
| 3.2 Fresh plus frozen‐thawed cycles | 3 | 2387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.84, 1.22] |
| 4 Live birth (or ongoing pregnancy) by sponsor | 27 | 7085 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 4.1 Serono | 6 | 1410 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.97, 1.61] |
| 4.2 Organon | 3 | 1215 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.96, 1.71] |
| 4.3 Ferring | 6 | 2817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 4.4 IBSA | 1 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.52, 1.92] |
| 4.5 None | 10 | 1381 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.67, 1.11] |
| 4.6 Unknown | 1 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.46, 4.47] |
| 5 OHSS by urinary gonadotrophin | 30 | 7475 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 5.1 rFSH versus HMG/HMG‐HP | 11 | 3197 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.58, 1.71] |
| 5.2 rFSH versus FSH‐P | 6 | 1490 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [0.89, 3.62] |
| 5.3 rFSH versus FSH‐HP | 14 | 2788 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.77, 2.03] |
| 6 OHSS by down regulation protocol | 30 | 7475 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 6.1 With antagonist protocol | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.17] |
| 6.2 With long agonist protocol | 25 | 6827 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.90, 1.73] |
| 6.3 With short agonist protocol | 2 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 With no down‐regulation protocol | 2 | 220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 OHSS by sponsor | 30 | 7475 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 7.1 Serono | 7 | 1480 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.13, 3.83] |
| 7.2 Organon | 4 | 1384 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.78, 3.18] |
| 7.3 Ferring | 6 | 2817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.54, 1.59] |
| 7.4 IBSA | 2 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.29 [0.14, 367.55] |
| 7.5 None | 10 | 1381 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.27, 1.61] |
| 7.6 Unknown | 1 | 110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.10, 4.60] |
| 8 Clinical pregnancy by urinary gonadotrophin | 35 | 8744 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 8.1 rFSH versus HMG/HMG‐HP | 12 | 3775 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.74, 0.99] |
| 8.2 rFSH versus FSH‐P | 7 | 1560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [1.01, 1.60] |
| 8.3 rFSH vs FSH‐HP | 17 | 3409 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.91, 1.23] |
| 9 Clinical pregnancy by down regulation protocol | 35 | 8744 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 9.1 With antagonist protocol | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.55, 1.43] |
| 9.2 With long agonist protocol | 27 | 7298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.89, 1.10] |
| 9.3 With short down‐regulation protocol | 3 | 726 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 9.4 With no down‐regulation protocol | 4 | 440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.87, 2.35] |
| 10 Clinical pregnancy by fresh/frozen policy | 35 | 8744 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 10.1 Fresh cycles | 33 | 7088 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.87, 1.07] |
| 10.2 Fresh plus frozen cycles | 2 | 1656 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.93, 1.44] |
| 11 Clinical pregnancy by sponsor | 35 | 8744 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 11.1 Serono | 7 | 1480 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.97, 1.53] |
| 11.2 Organon | 4 | 1384 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| 11.3 Ferring | 6 | 2817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.71, 1.00] |
| 11.4 IBSA | 2 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.55, 1.42] |
| 11.5 None | 11 | 1522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 11.6 Unknown | 5 | 1238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.83, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abate 2009.
| Methods | Open randomised controlled trial in three centre's in Italy. Based on a computer‐generated randomisation list. Randomisation after adequate down‐regulation, stratified by centre. Allocation concealment unclear Timing March 2007 to October 2008. Power calculation not stated. |
|
| Participants | 401 women with indication for IVF or ICSI, <3 prior oocyte retrievals, age 26‐38 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: FSH‐HP (Fostimon) Starting dose 225 IU. Down regulation with GnRHa (triptorelin acetate 0.1 mg/day s.c.) long protocol. Transfer of 1to3 embryos IVF or ICSI. |
|
| Outcomes | Ongoing pregnancy rate Clinical pregnancy rate Number of pregnancy loss OHSS? Number of oocytes retrieved Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only. Funding is unclear. Additional information requested but not retrieved |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | No reference, and no acknowledgement of support from independent trials unit or industry |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not formally blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Summary outcome data tabled make no mention of exclusions, but clear from percentages given that not all participants included in all analyses. |
| Selective reporting (reporting bias) | High risk | OHSS was an outcome according to the methods section but was not reported on, the pregnancy rates were presented as percentages |
| Other bias | Low risk | |
Alvino 1995.
| Methods | Open randomised controlled trial Method of randomisation: a blocked randomisation code, inadequate concealment. Power calculation not reported Not blinded |
|
| Participants | 60 women undergoing IVF, <4 previous cycles, age 23to36 yrs | |
| Interventions | Intervention: rFSH (Gonal F)
Control: FSH‐P (Metrodin). Both 225 IU daily for 5 days. Long down regulation with GnRHa (Leuprolide 0.5mg daily) IVF in all cycles Transfer of 2to3 embryos |
|
| Outcomes | Clinical pregnancy rate Number of embryo transferred OHSS rate Dose of gonadotrophin used |
|
| Notes | One treatment cycle only Funded by Serono |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | a computer generated blocked randomization code |
| Allocation concealment (selection bias) | High risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study described as "open" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 withdrawals noted, but not clear how many analysed in each group |
| Selective reporting (reporting bias) | Unclear risk | This cannot be clarified from the abstract |
| Other bias | Low risk | |
Andersen 2006.
| Methods | Randomised, open‐label, assessor‐blind, parallel‐group RCT Based on a computer‐generated randomisation list by an independent statistician not involved in the study Sequentially numbered sealed envelopes used to conceal treatment allocation Blinded; all investigators, embryologists, laboratory personnel and sponsor staff, including the statistician responsible for analysis All handling of study medication was done by nurses and precaution taken to ensure treatment assignments were not available to the investigators Patients instructed not to discuss their drug assignment with the investigator All information regarding treatment allocation kept in locked cupboard not accessible by the investigator All randomisation envelopes inspected and accounted for before breaking of the blind Power calculation carried out |
|
| Participants | 731 women with indication for IVF, <4 previous cycles, age 21 to 37 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: HP‐HMG (Menopur) Starting dose 225 IU. Down regulation with GnRHa (triptorelin acetate 0.1 mg/day s.c., long protocol. Transfer of 1to2 embryos All cycles IVF, no ICSI. |
|
| Outcomes | Live birth rate Ongoing pregnancy Clinical pregnancy rate Number of pregnancy loss Multiple pregnancy rate OHSS rate Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only. Frozen embryos derived from the study are followed‐up for 3 yrs. Data not yet available. Funded by Ferring. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated randomisation list by an independent statistician not involved in the study |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes used to conceal treatment allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study, but attempted blinding of all investigators, embryologists, laboratory personnel and sponsor staff, including the statistician responsible for analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All pregnancy data were presented according to ITT principles. However, number of oocytes retrieved was presented per patient with oocytes. Dose of gonadotrophin used and treatment duration were presented per patient that received gonadotrophins. The actual number of women with oocyte retrieval and the number of women that received at least one dosage of gonadotrophins however were reported. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Antoine 2007.
| Methods | Randomized, controlled, investigator‐blind multi‐center trial (abstract) Based on a computer‐generated randomisation list by an independent statistician not involved in the study Sequentially numbered sealed opaque envelopes used to conceal treatment allocation Blinded; all physicians and embryologists. Power calculation carried out (no of oocytes) |
|
| Participants | One hundred fifty IVF patients, <3 previous cycles, age 18to39 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: HP‐FSH (Fostimon) Starting dose of 225 IU. Down regulation long protocol GnRHa. Both IVF and ICSI. Transfer of 1to3 embryos |
|
| Outcomes | Clinical pregnancy rate OHSS rate Number of oocytes retrieved Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only Funded by Institut Biochimique SA (IBSA), Switzerland. Additional information requested and retrieved from dr Cometti from IBSA Switserland. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated randomisation list by an independent statistician not involved in the study |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes used to conceal treatment allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | All physicians and embryologists but not double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This can not be determined from the extended abstract |
| Selective reporting (reporting bias) | Unclear risk | This can not be determined from the extended abstract |
| Other bias | Unclear risk | This can not be determined from the extended abstract |
Baker 2009.
| Methods | Randomized, controlled, investigator‐blind multi‐center trial. Based on a computer‐generated randomisation list by an independent statistician not involved in the study Sequentially numbered sealed opaque envelopes used to conceal treatment allocation Blinded; all physicians and embryologists. All handling of study medication was done by the study coordinators and precaution taken to ensure treatment assignments were not available to the physician investigators. Study coordinators and patients instructed not to discuss their drug assignment with their physician. All information regarding treatment allocation kept in locked cupboard not accessible by the treating physicians. Power calculation carried out (no of oocytes) |
|
| Participants | One hundred fifty‐two IVF patients, <3 previous cycles, age 18to39 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: HP‐FSH (Fostimon) Starting dose of 300 IU. Down regulation after oral contraceptive pills with daily leuprolide acetate (long protocol). Both IVF and ICSI. Transfer of 1to3 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate OHSS rate Number of oocytes retrieved Number of pregnancy loss Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only Funded by Institut Biochimique SA (IBSA), Switzerland. Additional information requested and retrieved from dr Valerie Baker. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated randomisation list by an independent statistician not involved in the study |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Physicians and embryologists blinded, but not participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Main analyses based on numbers randomised |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Balasch 2003.
| Methods | Randomised, open‐label, single‐centre study. Allocation by using computer generated table on‐site. Embryologist blinded. Ratio HMG versus rFSH was 1:1. Timing of trial unclear, 60 patients were randomised. Power calculation was not done. |
|
| Participants | Sixty couples undergoing ICSI and having unexplained or male‐related primary infertility. No previous IVF/ICSI cycles and age 26to37 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: HMG (Pergonal) im Starting dose 150 IU. Long luteal GnRHa protocol with a single depot triptorelin injection. ICSI only. Transfer of 2to3 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate OHSS rate Number of oocytes retrieved Number of pregnancy loss Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only No Funding. Additional information requested and retrieved from dr Balasch. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation by using computer generated table on‐site. Pairs of individuals matched prior to randomisation. Not clear from description whether pairs were allocated concurrently or sequentially. |
| Allocation concealment (selection bias) | High risk | Inadequate due to use of table on‐site |
| Blinding (performance bias and detection bias) All outcomes | High risk | Embryologist was blinded but as the allocation was inadequate the effect of blinding cannot be considered adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 pairs of women allocated, only 50 individuals analysed and unclear whether these were 25 of the original pairs: "25 pairs of patients ... were finally considered" but also describes cancellation rate as "13.3% (4 patients) and 20% (6 patients)" in the two groups, implying that loss was not 5 pairs. |
| Selective reporting (reporting bias) | Unclear risk | Key results not reported in valid format as unpaired analyses presented. |
| Other bias | High risk | Inappropriate analysis methods applied |
Berger 1999.
| Methods | Randomised, open‐label, multi‐centre study (Abstract). Randomisation and allocation not described. Ratio rFSH vs FSH‐HP was 1:1. No party was blinded. Timing of trial unclear. Power calculation was not done. |
|
| Participants | 165 cycles in 148 couples undergoing IVF or ICSI. Number of previous cycles not known, female age <40. | |
| Interventions | Intervention: rFSH (Puregon)
highly purified FSH (Metrodin‐HP) Starting dose 150 IU for rFSH and 225 for FSH‐HP. Long luteal GnRHa protocol with a single depot triptorelin injection. ICSI only. Transfer of 2 embryos |
|
| Outcomes | Clinical pregnancy rate OHSS rate Number of oocytes retrieved Dose of gonadotrophin used |
|
| Notes | some couples had more than one cycle Funding unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but no further detail |
| Allocation concealment (selection bias) | Unclear risk | No reference in this conference abstract |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reference in this conference abstract |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | High risk | 148 participants but 165 cycles counted as if statistically independent |
Bergh 1997.
| Methods | RCT Computer‐generated randomisation list. Only authorised access to randomisation list. Allocation using sealed numbered opaque envelopes. Blinded: administering physician and assessor Power calculation carried out |
|
| Participants | 235 women undergoing assisted reproductive techniques, <4 previous ART cycles, female age 18to38. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: highly purified FSH (Metrodin‐HP) Starting dose Long down regulation with GnRHa (buserelin 300 uG) 3 times/day intranasally IVF and ICSI Transfer of 2to3 embryos |
|
| Outcomes | Dose of gonadotrophin used Clinical pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate Miscarriage rate OHSS rate Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Duration of treatment |
|
| Notes | One treatment cycle only Funded by Serono Extra information from dr Bergh on randomisation procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Only authorised access to randomisation list, allocation using sealed numbered opaque envelopes (determined by correspondence with author). |
| Blinding (performance bias and detection bias) All outcomes | High risk | Administering physician and assessor but not participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four post‐randomisation exclusions described: one withdrew consent and three logistical errors. 12 excluded from urinary arm "primarily because of poor ovarian response", but able to re‐create ITT analysis for key outcomes. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Bosch 2008.
| Methods | Randomized, controlled, single‐center trial. Randomisation based on a computer‐generated randomisation list. The randomisation list was centralised at the institution’s call centre, and the centre was contacted to allocate each randomised patient into a group. Timing clear. Power calculation carried out (ongoing pregnancy was primary outcome) |
|
| Participants | Two hundred and eighty patients undergoing their first IVF/ICSI treatment. Female age 18to37 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: highly purified HP‐HMG (Menopur) The starting dose for HMG and rFSH was 225 IU/day s.c. for the first two days of stimulation. Then, a serum E2 determination was performed for individual adjustments. On stimulation Day 6, 0.25 mg of the GnRH antagonist Cetrorelix (Cetrotide®, Serono) was started daily, and continued until the end of stimulation. Both IVF and ICSI cycles. Transfer of 1to3 embryos |
|
| Outcomes | Live birth rate Ongoing pregnancy Clinical pregnancy rate Number of pregnancy loss Multiple pregnancy rate OHSS rate Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only No pharmaceutical funding Additional information requested and retrieved from dr Bosch |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | The randomisation list was centralised at the institution’s call centre, and the centre was contacted to allocate each randomised patient into a group. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open label". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up described in CONSORT diagram. Possible to recreate ITT analyses |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Cheon 2004.
| Methods | RCT Methods of randomisation and allocation concealment not reported 'Double‐blind' study: both the clinicians and the embryologists were blinded. Power calculation not reported |
|
| Participants | 241 women undergoing 254 cycles of COH for IVF and IVF‐ET, < 4 previous cycles, female age 25 to 40 yrs. | |
| Interventions | Intervention: rFSH
Control: highly purified FSH (Metrodin‐HP) Short down regulation with GnRHa (Suprefact) IVF only Transfer of 2to4 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Cancellation rate (?? due to OHSS) No.of oocytes retrieved |
|
| Notes | No funding Some women underwent more than one cycle. Extra information requested and retrieved from dr Cheon on live birth rates, allocation remained unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised" but no further detail |
| Allocation concealment (selection bias) | Unclear risk | No reference |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "double‐blind" but does not refer to clinicians or participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcmoes analysable for stated numbers of participants, but not clear whether number analysed was same as number randomised. |
| Selective reporting (reporting bias) | High risk | Absence of adverse event reporting. Miscarriages only calculable by subtraction of live births from clinical pregnancies. |
| Other bias | Low risk | |
Dickey 2002.
| Methods | RCT, multicentric. Methods of randomisation: blocks‐of‐three design: SAS ProcPlan. Randomised into three groups. Computer‐generated central allocation. Power calculation based on oocytes retrieved. |
|
| Participants | 177 women who met down‐regulation criteria and planning to undergo IVF‐ET ). Female age 18‐39 yrs, number of previous cycles not an inclusion criterion (see notes). | |
| Interventions | Intervention: rFSH (Follistim)
Control: highly purified FSH (Bravelle) SC Control: highly purified FSH (Bravelle) IM Long down regulation with GnRHa (leuprolide acetate 0.5 mg) daily sc. IVF only Transfer of maximally 4 embryos |
|
| Outcomes | Dose of gonadotrophin used Clinical pregnancy rate Ongoing pregnancy rate Live birth rate Miscarriage rate OHSS rate Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Duration of treatment |
|
| Notes | One treatment cycle only. Funded by Ferring. Additional information was obtained from Ferring USA (via dr Marshall). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated but in blocks of 3 so that every third participant predictable. |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 post randomisation exclusions described. Possible to re‐create ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Unclear risk | Suspicion of publication bias. See comments for Dickey 2003. |
Dickey 2003.
| Methods | RCT, multicentric. Methods of randomisation: blocks‐of‐six design: SAS ProcPlan. Computer‐generated central allocation. Power calculation based on oocytes retrieved. |
|
| Participants | 120 women who met down‐regulation criteria and planning to undergo IVF‐ET (no ICSI). Female age 18to39 yrs, number of previous cycles not an inclusion criterion. | |
| Interventions | Intervention: rFSH (Follistim)
Control: highly purified FSH (Bravelle) Long down regulation with GnRHa (leuprolide acetate 0.5 mg) daily sc. IVF only Transfer of maximally 4 embryos |
|
| Outcomes | Dose of gonadotrophin used Clinical pregnancy rate Ongoing pregnancy rate Live birth rate Miscarriage rate OHSS rate Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Duration of treatment |
|
| Notes | One treatment cycle only. Funded by Ferring. Additional information was obtained from Ferring USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods of randomisation: blocks‐of‐six design: SAS ProcPlan. |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "nearly identical in design to Dickey 2002 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Possible to re‐create ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | High risk | Strong suggestion of publication bias in selection and method of presentation: "This report presents the pooled data from two independent trials...The present data form part of the clinical development program...". Includes data from two arms of Dickey 2002 as if part of single trial. |
Drakakis 2002.
| Methods | RCT Methods of randomisation and allocation concealment not reported Power calculation not reported Not blinded |
|
| Participants | 70 women undergoing IVF‐ET or ICSI‐ET | |
| Interventions | Intervention: rFSH Control: urinary FSH | |
| Outcomes | Clinical pregnancy rate No.of oocytes retrieved |
|
| Notes | Long down regulation with GnRHa (buserelin acetate intranasally) Funding not stated It was not stated what brand of rFSH or urinary FSH was used, however the urinary FSH contained 75% FSH and 25% LH. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into two groups" without further detail. |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference to blinding and groups treated differently to determine suitable dose. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clear from denominator that reported pregnancy rate includes fewer participants than randomised, but not clear for other reported outcomes |
| Selective reporting (reporting bias) | High risk | Absence of adverse event reporting and no follow‐up beyond undefined "pregnancy rate". |
| Other bias | Low risk | |
EISG 2002.
| Methods | RCT Randomisation achieved by computer‐generated allocation sequence and copies of randomised list distributed in sealed, opaque numbered envelopes stratified for IVF and ICSI. Size of randomisation blocks was four Power calculation carried out Not blinded. |
|
| Participants | 781 women undergoing IVF/ICSI. Female age 23/36 yrs and less than 4 previous ART cycles. | |
| Interventions | Intervention: rFSH (Gonal F)
Control: HMG‐HP (Menopur) Starting dose 225 IU. Long luteal GnRHa protocol with triptorelin, buserelin or leuprolid or depot injections with triptorelin or goserelin IVF and ICSI Transfer of 1to3 embryos |
|
| Outcomes | Clinical pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate Miscarriage rate OHSS rate Dose of gonadotrophin used Duration of stimulation Both IVF and ICSI. |
|
| Notes | Long down regulation with GnRHa Funded by Ferring |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open label". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 post‐randomisation exclusions before therapy suggests poor timing of randomisation, but specified by group. Further 34 excluded for "protocol violation" but reasons and allocated group given for all. Possible to re‐create ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Unclear risk | Unclear reporting and selection of different subsets of patients for particular endpoints. |
Ferraretti 1999.
| Methods | Randomised trial. Allocation by drawing from a random table. 4‐arm RCT, 2 arms used (rFSH vs FSH‐HP) Not blinded Power calculation not carried out |
|
| Participants | 141 women undergoing assisted reproductive techniques with no ovarian response in a previous cycle. Female age 29to45 yrs. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: highly purified uFSH (Metrodin‐HP) Starting dose 300 IU No down regulation Transfer of maximally 3 embryos |
|
| Outcomes | Clinical pregnancy rate Miscarriage rate Number of oocytes retrieved |
|
| Notes | One treatment cycle only No funding Extra information retrieved from dr Ferraretti on funding and randomisation procedure. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random table was used |
| Allocation concealment (selection bias) | High risk | No, allocation done by drawing |
| Blinding (performance bias and detection bias) All outcomes | High risk | Appears unlikely given treatments compared |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Hard to say on basis of available abstract |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting |
| Other bias | Unclear risk | Unclear reporting |
Franco 2000.
| Methods | RCT Randomisation by drawing lots, using a randomisation table No allocation concealment Power calculation not reported Not blinded |
|
| Participants | 120 women undergoing ICSI | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: highly purified FSH (Metrodin‐HP) Starting dose 150 IU rFSH and 225 IU FSH‐HP Long GnRHa protocol with leuprolide acetate 0.5 mg daily ICSI only Transfer of maximally 4 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate No of oocytes retrieved OHSS rate |
|
| Notes | Funded by Serono | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Apparently self‐contradictory information: "by drawing lots, using a randomisation table previously elaborated for the study". |
| Allocation concealment (selection bias) | Unclear risk | No reference. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference and appears unlikely given treatments compared |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers randomised and analysed were equal |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Frydman 2000.
| Methods | Double‐blind RCT Methods of randomisation was sealed numbered envelopes 'Double‐blind' study: unclear which party was blind Power calculation carried out |
|
| Participants | 278 women undergoing IVF. Female age 18 to 38 yrs and less than 4 previous ART cycles. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: highly purified FSH (Metrodin‐HP) Starting dose 150 IU daily Downregulation long GnRHa protocol either depot, sc or intranasal. IVF only Transfer of 2to5 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate OHSS rate Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Long down regulation with GnRHa (Leuprorelin or buserelin) One cycle only Funded by serono Righini (reference at Studies awaiting classification) possible double publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list prepared using a computer program. |
| Allocation concealment (selection bias) | Unclear risk | No reference |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double blind" but no description of which parties. Other studies in review describe double‐blind as investigator and embryologist rather than participant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Possible to trace drop‐out at each stage of treatment |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Gallego 2003a.
| Methods | RCT Randomisation using a table of random numbers Methods of allocation concealment not clear No blinding Power calculation not reported |
|
| Participants | 100 women undergoing IVF, aged below 40 yrs, no previous IVF cycles. | |
| Interventions | Intervention: rFSH (Puregon)
Control: FSH (Neofertinorm, FSH‐HP) Starting dose 150 IU for rFSH and 225 IU for FSH‐HP daily Downregulation long GnRHa protocol (leuprorelin 0,5 mg/day). IVF only Transfer of maximally 3 embryos |
|
| Outcomes | Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate OHSS rate No. of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Funding not reported Tried but failed to contact dr Gallego for more information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Possible to trace drop‐out at each stage of treatment |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Germond 2000.
| Methods | Randomised parallel trial in two centre's (abstract). Trial recruited between jantoJuly 1999 Not blinded Power calculation not reported |
|
| Participants | 79 women undergoing assisted reproductive techniques, above 35 years of age. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: highly purified FSH (Metrodin‐HP) Starting dose 225 IU No down regulation Transfer of 2to3 embryos |
|
| Outcomes | Clinical pregnancy rate | |
| Notes | One treatment cycle only Funding unknown |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail in abstract |
| Allocation concealment (selection bias) | Unclear risk | No detail in abstract |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference, and appears unlikely given treatments compared |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail in abstract |
| Selective reporting (reporting bias) | Unclear risk | No detail in abstract |
| Other bias | Unclear risk | No detail in abstract |
Ghosh 1999.
| Methods | Randomised trial (abstract). Allocation using opaque sequentially numbered sealed envelopes. Not blinded Power calculation not carried out IVF only |
|
| Participants | 47 women undergoing IVF, female age <37 yrs, < 4 previous IVF cycles. | |
| Interventions | Intervention: rFSH (Puregon)
Control: highly purified FSH (Metrodin‐HP) Starting dose 150 IU (rFSH) and 225 IU (FSH‐HP) resp. Long luteal GnRHa protocol with buserelin s.c. 500 µg daily, reduced to 200 µg IVF only Transfer of maximally 3 embryos |
|
| Outcomes | Clinical pregnancy rate Number of oocytes retrieved |
|
| Notes | One treatment cycle only Funding unknown Extra information requested but no response Chakravarty (reference at Studies awaiting classification) possible double publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list |
| Allocation concealment (selection bias) | Low risk | Allocation using opaque sequentially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that randomised and analysed numbers the same for cumulative pregnancy rate, but not necessarily for other outcomes. |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported in sufficient detail, and some key outcomes missing. |
| Other bias | Low risk | |
Gordon 2001.
| Methods | Randomised clinical single centre trial Assessor blind Randomisation by computer allocation by the hospital pharmacist. A total of 128 patients were studied of whom 29 received HMG, 39 received rFSH and 60 received urinary FSH. Power calculation not done |
|
| Participants | 128 women undergoing IVF, age 18to38 yrs, no previous IVF cycles. | |
| Interventions | Intervention: rFSH
Control: FSH and HMG (humegon) Starting dose 225 IU. Long luteal GnRHa protocol with buserelin acetate 0.15 mg nasal spray IVF, no ICSI. Transfer of 1to3 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Miscarriage rate No. of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Long down regulation with GnRHa (Buserelin) No pharmaceutical funding Extra information requested and retrieved from dr Uma Gordon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized computer‐generated code". |
| Allocation concealment (selection bias) | Low risk | Third‐party allocation by the hospital pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "preparations differed in appearance", but administered by "nursing staff independent of clinician's activities". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers randomised and analysed the same. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Hedon 1995.
| Methods | RCT Assessor‐blind Randomised in blocks in a 3:2 ratio. Randomisation using a randomisation list. Central allocation of a subject number in order of entrance, which corresponded to medication boxes with the medication power calculation not reported |
|
| Participants | 99 women undergoing IVF, female age 18to39, <4 previous ART cycles. | |
| Interventions | Intervention: rFSH (Puregon)
Control: FSH‐P (Metrodin) Starting dose 150 IU (rFSH) and 225 IU (FSH‐P) resp. Long luteal GnRHa protocol with triptorelin 0.1 mg s.c. daily IVF only Transfer of maximally 3 embryos |
|
| Outcomes | Clinical pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate No of oocytes retrieved OHSS rate Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Funded by Organon 1 cycle only, no cryo cycles included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a randomisation list on a 3:2 ratio.. |
| Allocation concealment (selection bias) | Low risk | Central allocation of a subject number in order of entrance, which corresponded to medication boxes with the medication (size of blocks not known though) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assessor‐blind only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 participants excluded post‐randomisation who did not reach stage of receiving FSH treatment. Possible to re‐create ITT analyses for review. |
| Selective reporting (reporting bias) | Unclear risk | Further outcome details (including live birth) given in subsequent publication, raising concern of potential publication bias. |
| Other bias | Low risk | |
Hompes 2008.
| Methods | Multicentre RCT in the Netherlands. Allocation by central computer randomisation using an interactive voice response system. Randomisation in permuted blocks of random size stratified on centre. Not blinded. Ratio HMG vs rFSH was 1:1. Trial took place between and , 629 patients were randomised. Power calculation based on ongoing pregnancy rate per cycle (1 first cycle only). Supported by Ferring Pharmaceuticals. | |
| Participants | Couples with an indication for IVF or ICSI undergoing their first IVF‐ICSI treatment due to male factor, tubal factor, unexplained, combination or other factors. Women were healthy, aged 18to39 (mean 32), no PCOS, no hypogonadotropic hypogonadism, and FSH day above 12 IU‐L. BMI was not an exclusion criterion (mean BMI 24). | |
| Interventions | Long GnRHa protocol with triptorelin acetate 0.1 mg/day sc. Ovarian hyperstimulation with HP‐HMG (Menopur) sc daily vs rFSH (Follitropin Alpha, Gonal‐F), 225 IU sc daily for 5 days, then adjusted. 250 uG sc rHCG when >=3 follicles of >=17 mm. IVF performed in all cycles, 1to2 embryos transferred on day 3. Luteal support with vaginal progesterone gel from day of ET until conformation of clinical pregnancy or neg betaHCG test. | |
| Outcomes | Primary endpoint: ongoing pregnancy. Secondary endpoints: live birth, clinical pregnancy, early pregnancy loss, multiple pregnancy, oocytes retrieved, fertilisation rate, embryo quality, OHSS, cancellation rate, total dose of gonadotropin, days of stimulation. | |
| Notes | Additional information was obtained from the authors and Ferring Pharmaceuticals in The Netherlands. Clinical pregnancy defined as at least 1 IUG with fetal heartbeat 5to6 weeks after ET. Ongoing pregnancy was defined as at least 1 viable IUG at 10 weeks after ET. Supported by Ferring Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by computer randomisation. |
| Allocation concealment (selection bias) | Low risk | Central procedure using an interactive voice response system. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open‐label". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Possible to trace participants dropping out at each stage of treatment and re‐create ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Hoomans 1999.
| Methods | RCT Randomisation using a random number table. Authorised access to randomised list Allocation using sealed envelopes, opaque and numbered 'Open' trial, not blinded Power calculation carried out |
|
| Participants | 169 women undergoing IVF, female age 18to39 yrs, < 3 previous cycles. | |
| Interventions | Intervention: rFSH (Puregon)
Control: highly purified FSH (Metrodin‐HP) Starting dose 150 IU (rFSH) and 225 IU (FSH‐HP) resp. Long luteal GnRHa protocol with buserelin s.c. daily IVF only Transfer of maximally 3 embryos |
|
| Outcomes | Clinical pregnancy rate Miscariage rate No of oocytes retrieved OHSS rate Dose of gonadotrophin used Duration of stimulation |
|
| Notes | One cycle only Funded by Organon Extra information from dr Hoomans on randomisation procedure and outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed envelopes, opaque and numbered. Only authorised access to randomised list. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants post‐randomisation failed to reach FSH treatment. Sufficient detail given by group on drop outs at all stages to recreate ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Hugues 2001.
| Methods | RCT Randomisation by random number table Allocation in concealed envelopes Not blinded Power calculation not reported |
|
| Participants | 88 women undergoing IVF, female age 18to38 yrs, | |
| Interventions | Intervention: rFSH (Follitropin a and Follitropin b)
Control: highly purified FSH (Metrodin‐HP) Startin dose 100 IU rFSH vs 150 IU FSH‐HP Short down regulation with 25 uG sc daily GnRHa (decapeptyl) IVF only Transfer of maximally 3 embryos |
|
| Outcomes | Pregnancy rate Ongoing pregnancy rate Ectopic pregnancy rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Not funded by a pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random number table |
| Allocation concealment (selection bias) | Low risk | Allocation in concealed numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "not blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Jansen 1998.
| Methods | RCT Randomisation by subject number from a randomisation list corresponding with patient boxes in which medication was kept Allocation at a ratio of 3:2 (rFSH/HMG) Assessor blind Power calculation carried out |
|
| Participants | 109 women undergoing IVF, female age <40 yrs, <3 previous ART cycles | |
| Interventions | Intervention: rFSH (Puregon)
Control: HMG (Humegon) Starting dose: 150 IU rFSH vs 225 IU HMG IVF only No down regulation Transfer of 1to3 embryos |
|
| Outcomes | Clinical pregnancy rate Ongoing pregnancy rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | One cycle only Funded by Organon Additional information was obtained from dr Jansen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Centralised scheme. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assessor blind only. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants failed to receive gonadotrophin treatment, and further 23 did not reach embryo transfer. sufficient detail given to re‐create ITT analyses. |
| Selective reporting (reporting bias) | Unclear risk | Further outcome details (including live birth) given in subsequent publication, raising concern of potential publication bias. |
| Other bias | Unclear risk | "the study had to be truncated prematurely due to non‐medical factors outside our control". |
Kilani 2003.
| Methods | RCT Random number table made by a computer Randomisation sequence and sealed envelops containing treatment assignments prepared at separate location and conducted by separate personnel Power calculation carried out Blind to ultrasound and laboratory (performing hormone assay) personnel |
|
| Participants | 100 women undergoing IVF. Female age 18to37, none taken more than 3 IVF/ICSI cycles in the past. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: HMG (HMG HP Menopur) Starting dose 150 IU. Long down regulation with GnRHa (Triptorelin) Both IVF and ICSI cycles. transfer of 1to2 embryos |
|
| Outcomes | Live birth rate Term pregnancy rate Multiple pregnancy rate Miscarriage rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | No pharmaceutical funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table made by a computer. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes prepared independently |
| Blinding (performance bias and detection bias) All outcomes | High risk | Partial only: ultrasound and laboratory personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers randomised and analysed the same. |
| Selective reporting (reporting bias) | Unclear risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Kornilov 1999.
| Methods | RCT, four groups (rFSH, FSH‐HP, FSH‐P, HMG) Randomisation procedure not known No power calculation Not blinded |
|
| Participants | 124 women underwent 137 cycles if IVF‐ET | |
| Interventions | Intervention: rFSH (Gonal‐F) 28 cycles
Control: FSH‐HP (Metrodin HP) 35 cycles, FSH‐P (Metrodin) 34 cycles, HMG (Humegon) 40 cycles Starting dose 150 IU ‐ 300 IU. Long down regulation with GnRHa (Triptorelin) IVF cycles. transfer of 2to3 embryos |
|
| Outcomes | Clinical pregnancy rate per embryo transfer Multiple pregnancy rate per embryo transfer Miscarriage rate per embryo transfer No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Pregnancy rate was not presented per randomised woman, treated groups did not match in number (40 vs 28 cycles) and there was a significant difference in age distribution between groups. More information was requested at several instances but the authors did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference to blinding ‐ unlikely given treatments compared. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear on numbers of participants randomised. Percentages rather than numbers given for various outcomes and failure to distinguish 'participants' from 'cycles' prevents assured calculation of drop‐out. |
| Selective reporting (reporting bias) | High risk | Absence of OHSS data. Incomplete reporting of other outcomes. |
| Other bias | High risk | Data described as cycles rather than participants ‐ may include multiple cycles for individual women. Marked difference in average age between groups. |
Lenton 2000.
| Methods | RCT (open) Computer‐generated randomisation (in blocks of 4), stratified by centre, using sealed envelopes containing the name of the drug to be used. Not blinded Power calculation carried out |
|
| Participants | 168 women undergoing IVF with female age 18to38 and no previous ART cycle. | |
| Interventions | Intervention: rFSH (Follitropin alpha, Gonal‐F)
Control: highly purified FSH (Metrodin‐HP) Starting dose: 150 IU daily Downregulation with long GnRHa protocol with buserelin (900 uG intra nasal daily) IVF or ICSI Transfer of 1to2 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Long down regulation with GnRHa (Buserelin) Funded by Serono |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Machado 1999.
| Methods | Randomised trial (abstract). Allocation by allocation by drawing straws 4 arm study, 2 arms with CC not included here Not blinded Power calculation not carried out |
|
| Participants | 79 cycles in 71 women undergoing assisted reproductive techniques. Female age 26to43 | |
| Interventions | Intervention: rFSH (Puregon)
Control: highly purified FSH (Metrodin‐HP) Starting dose 300 IU No down regulation Transfer of maximally 4 embryos |
|
| Outcomes | Clinical pregnancy rate | |
| Notes | Some women had more than one cycle Requested extra information but no info yet. No response authors Funded by Serono |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation by allocation by drawing straws allowing no verification of process. |
| Allocation concealment (selection bias) | High risk | Allocation by allocation by drawing straws |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not formally blinded and not well concealed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This could not be determined from the abstract |
| Selective reporting (reporting bias) | High risk | Complete data was only available for clinical pregnancy |
| Other bias | High risk | Some women had more than one cycle.and not possible to distinguish repeat cycles from report. |
Meden‐Vrtovec 2003.
| Methods | RCT Randomisation by tossing coins Allocation status in sequentially number envelopes Authorised access to randomised list Not blinded No power calculation |
|
| Participants | 131 women undergoing IVF | |
| Interventions | Intervention: rFSH Control: FSH‐P | |
| Outcomes | Pregnancy rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | No down regulation Funded by the Ministry of Science Extra information from dr Meden‐Vrtovec on randomisation procedure and on live births. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by tossing coins, allowing no verification of process. |
| Allocation concealment (selection bias) | Unclear risk | Allocation status reported in correspondence to use sequentially numbered envelopes. Unclear how this is compatible with coin toss method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Reported in correspondence. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that numbers randomised and analysed were equal. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | High risk | Reported in correspondence to be part of a larger trial, raising concern of publication bias. |
Mohamed 2006.
| Methods | Randomised, open‐label, parallel‐group RCT Allocation by computer on site with randomisation based on a computer‐generated randomisation list. Not blinded Power calculation carried out |
|
| Participants | 257 women with indication for IVF, age above 39 yrs, number of previous cycles not an inclusion criterion but women nulliparous. | |
| Interventions | Intervention: rFSH (Gonal‐F)
Control: HP‐HMG (Fostimon) Starting dose 300 IU. Down regulation with GnRHa (buserelin 0.4 mg/day s.c., long protocol. Transfer of maximally 3 embryos All cycles IVF, no ICSI. |
|
| Outcomes | Clinical pregnancy rate Number of pregnancy loss Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Treatment duration |
|
| Notes | One treatment cycle only. Not funded Additional information on randomisation, allocation concealment, funding and on ongoing pregnancies was obtained from dr Sbracia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No actual blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | High risk | Multiple pregnancies not reported although these must have been known from the fetal heart beat monitoring at 4 weeks after transfer. Outcome definitions not clear. |
| Other bias | Low risk | |
Nardo 2000.
| Methods | RCT Methods of randomisation and allocation concealment not reported Assessor‐blind Power calculation not reported |
|
| Participants | 110 women undergoing IVF | |
| Interventions | Intervention: rFSH (Gonal F, Puregon)
Control: highly purified FSH (Metrodin‐HP) Starting dose: Long down regulation with GnRHa (Leuprorelin) |
|
| Outcomes | Take home baby rate Pregnancy rate OHSS rate Miscarriage rate No of oocytes retrieved Duration of stimulation |
|
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assessor‐blind only. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers randomised and analysed appear to be equal. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Ng 2001.
| Methods | RCT Randomisation using computer generated random numbers, in sealed sequential envelopes opened by nurses not involved in the study Assessor‐blind Power calculation carried out |
|
| Participants | 40 women undergoing ICSI, female age <39, no details on number of previous ART cycles. | |
| Interventions | Intervention: rFSH (Gonal F)
Control: HMG (Pergonal) Starting dose 300 IU for 2 days, then 150 IU. Long down regulation with GnRHa (Buserelin) 0.15 mg nasal spray 4 times daily. ICSI in all cycles. Transfer of 1to3 embryos. |
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Ectopic pregnancy rate Miscarriage rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | No pharmaceutical funding. Extra information obtained on randomisation procedure, funding and pregnancy data from dr Ng. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed sequential envelopes opened by nurses not involved in the study. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assessor‐blind only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers randomised and analysed were equal |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Out 1995.
| Methods | RCT multicentric (18 centre's) Randomisation using a randomisation list. Central allocation of a subject number in order of entrance, which corresponded to medication boxes with the medication Assessor blind Power calculation carried out Randomised at a ratio of 3:2 (rFSH:FSH‐P) |
|
| Participants | 1027 women undergoing IVF, female age 18to39 and <4 previous IVF cycles. | |
| Interventions | Intervention: rFSH (Puregon)
Control: FSH‐P (Metrodin) Starting dose: 150 IU rFSH vs 225 IU FSH‐P Long down regulation with GnRHa (Buserelin 150 uG 4 time daily) IVF only Transfer of maximally 3 embryos |
|
| Outcomes | Clinical pregnancy rate Ongoing pregnancy rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Funded by Organon (all authors were from Organon NL) Extra information on randomisation procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Description of sequentially numbered medication boxes, but no reference to concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Assessor‐blind only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | Unclear risk | Further outcome details (including live birth) given in subsequent publication, raising concern of potential publication bias. |
| Other bias | Low risk | |
O´Dea 1993.
| Methods | Randomised trial (abstract). Allocation method unknown. Not blinded Power calculation not carried out IVF only |
|
| Participants | 47 women undergoing IVF | |
| Interventions | Intervention: rFSH (Puregon)
Control: highly purified FSH (Metrodin‐HP) Starting dose 150 IU (rFSH) and 225 IU (FSH‐HP) resp. Long luteal GnRHa protocol with leuprolide acetate 0.5 mg s.c. daily Unclear how many embryos were transferred |
|
| Outcomes | Clinical pregnancy rate Miscarriage rate |
|
| Notes | One treatment cycle only Funded by Serono More information asked ‐ study too old |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No reference to method. |
| Allocation concealment (selection bias) | Unclear risk | No reference to concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This could not be determined from the abstract |
| Selective reporting (reporting bias) | High risk | Only usable data on clinical pregnancy and miscarriage rate. |
| Other bias | Unclear risk | This could not be determined from the abstract |
Rashidi 2005.
| Methods | Randomised controlled single centre trial. Randomisation by a computer‐generated list of random numbers, which were placed in sealed envelopes. Embryologists were blinded to treatment. Power calculation carried out (based on metaphase II oocyte number). |
|
| Participants | Sixty women undergoing ovarian stimulation for ICSI. Female age <=35 yrs. | |
| Interventions | Intervention: rFSH
Control: HMG Starting dose 150 IU Down regulation in a long protocol with decapeptyl 3.75 mg IM. Transfer of maximally 4 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Number of pregnancy loss Multiple pregnancy rate OHSS rate Number of oocytes retrieved Number of embryo transferred Dose of gonadotrophin used Treatment duration |
|
| Notes | No pharmaceutical funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No detail beyond "sealed envelopes". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Embryologist only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numers randomised and analysed were equal. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
RHFSHG 1995.
| Methods | RCT Randomisation using computer‐generated random number sequence in sealed opaque numbered envelopes No power calculation reported No blinding |
|
| Participants | 127 women undergoing IVF, female age 18to38 yrs and < 4 previous IVF cycles. | |
| Interventions | Intervention: rFSH
Control: FSH‐P Starting dose 225 IU daily Long down regulation with GnRHa (Buserelin 200 uG s.c. daily) IVF in all cycles. Transfer of maximally 5 embryos. |
|
| Outcomes | Live birth rate Ongoing pregnancy rate Clinical pregnancy rate Multiple pregnancy rate Ectopic pregnancy rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Funded by Serono | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 post‐randomisation exclusions ‐ 3 neither treatment and 1 both treatments received ‐ not reported intended group allocation. Otherwise possible to trace participants reaching each stage. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Schats 2000.
| Methods | RCT (Data pooled from 2 studies) Randomised using computer‐generated list. Allocation list held in Pharmacy. Sequentially numbered sealed envelopes used. Assessor blind Power calculation carried out |
|
| Participants | 496 women undergoing IVF/ICSI with female age 18to38 and <3 previous ART cycles. | |
| Interventions | rFSH (Gonal F)
Control: FSH (Metrodin HP) Starting dose: 150 IU daily Long down regulation with GnRHa (Buserelin or Leuprolide) IVF or ICSI Transfer of maximally 3 embryos. |
|
| Outcomes | Delivery rate Clinical pregnancy rate Miscarriage rate Multiple pregnancy rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | One cycle only Funded by Serono Extra information from dr Schats on the allocation procedure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Held in Pharmacy. Sequentially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Partial: assessor‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Possible to trace participants reaching each stage |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Unclear risk | Not clear how and when the decision was taken to combine the two studies ('Feronia' and 'Apis') "to make the study more powerful" (communication from author). |
Selman 2002.
| Methods | RCT Randomisation by using computer‐generated list. Allocation using sequentially numbered sealed envelopes. No power calculation No blinding |
|
| Participants | 267 women undergoing a first IVF/ICSI cycle with female age between 18to38 yrs. | |
| Interventions | Intervention: rFSH (Gonal F)
Control: highly purified FSH (Metrodin‐HP, Fostimon) Starting dose: 225 IU daily Long down regulation with GnRHa (Triptorelin) Transfer of maximally 3 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate Miscarriage rate OHSS rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | One cycle only No funding dr Selman provided extra information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by using computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Allocation using sequentially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as "open". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
Strehler 2001.
| Methods | RCT, open label, single centre Randomisation based on a computer‐generated randomisation list. Allocation by computer. Timing January 1998 and June 1999 Power calculation not reported |
|
| Participants | 578 women undergoing IVF of ICSI, female age <40 and <5 previous ART cycles. | |
| Interventions | Intervention: rFSH (Gonal F)
Control: HMG (Menogon) Starting dose 150to450 IU Short down regulation with GnRHa (Nefarelin acetate) IVF and ICSI Transfer of 1to3 embryos |
|
| Outcomes | Clinical pregnancy rate Multiple pregnancy rate No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Funding not stated Extra information from dr Strehler. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation by computer. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference to blinding and seems unlikely given treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | Unclear risk | Key outcomes ‐ live birth and OHSS rates ‐ not reported. Live birth may not have been recorded, but OHSS or its absence should have been apparent in the study timeframe. |
| Other bias | Low risk | |
Westergaard 2001.
| Methods | RCT, single centre Randomisation based on a computer‐generated randomisation list. Computerised allocation on day 14 after adequate down‐regulation. Trial took place between October 1998 and January 2000 Not blinded No power calculation performed |
|
| Participants | 379 women undergoing IVF, female age 21to39, first IVF attempt 71% | |
| Interventions | Intervention: rFSH (Gonal F)
Control: HMG (Menogon) Starting dose: 225 IU Long luteal GnRHa protocol with buserelin acetate 0.15 mg nasal spray 4 times daily or 0.5 mg SC for 14 days IVF and ICSI Transfer of 1to2 embryos |
|
| Outcomes | Live birth rate Clinical pregnancy rate Multiple pregnancy rate OHSS No of oocytes retrieved Dose of gonadotrophin used Duration of stimulation |
|
| Notes | Unconditionally supported by Ferring dr Westergaard provided extra information on funding, randomisation procedure and drop‐outs (there where no drop‐outs) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | On‐site computer program. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No reference to blinding and seems unlikely given treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described in sufficient detail to allow re‐creation of ITT analyses. |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported in sufficient detail for inclusion. |
| Other bias | Low risk | |
rFSH ‐ recombinant follicle stimulating hormone uFSH ‐ urinary follicle stimulating hormone
uFSH‐HP highly purified follicle stimulating hormone
HMG human menopausal gonadotrophin HP‐HMG ‐ highly purified menotropin GnRHa ‐ gonadotrophin‐releasing hormone agonist OHSS ‐ ovarian hyperstimulation syndrome s.c. ‐ subcutaneously
IM ‐ intra muscular COH ‐ controlled ovarian hyperstimulation IVF ‐ in vitro fertilisation ICSI ‐ intracytoplasmic sperm injection ET ‐ embryo transfer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dickey 2003b | Abstract. Duplicate of Dickey 2003 study. |
| Duijkers 1997 | Quasi randomised. Used alternating day allocation. Included 20 couples. Published in a Thesis. |
| Manassiev 1997 | Not truly randomised, allocation by area of residence. |
| Martinez 2008 | Compared two down regulations methods besides HMG vs rFSH in oocyte donors (leuprorelin + human menopausal gonadotropins (HMG) versus ganirelix + recombinant follicle‐stimulating hormone (rFSH)). |
| Pacchiarotti 2007 | Compared rFSH with a combination of uFSH and rFSH in a RCT |
| Raga 1999 | The study was randomised as rFSH versus uFSH‐HP in poor responders. However, in both study arms HMG (150 IU daily) was added during the first four days of stimulation. |
| Requena 2010 | Not truly randomised, allocation by order of inclusion. |
| Ruvolo 2009 | No randomisation. The study prospectively compared rFSH and HMG (in 19 and 10 women) but did allocate the treatments by randomisation. |
| Serhal 2000 | Not truly randomised, allocation by alternating weeks. |
Characteristics of studies awaiting assessment [ordered by study ID]
Chakravarty 2000.
| Methods | RCT |
| Participants | Couples undergoing IVF or ICSI |
| Interventions | rFSH vs uFSH‐HP in agonist cycles |
| Outcomes | Clinical pregnancy rate |
| Notes | Double publication of Gosh? |
Kahn 1999.
| Methods | Prospective, randomised study over multiple cycles including cryo cycles. |
| Participants | not known yet |
| Interventions | rFSH versus urinary FSH |
| Outcomes | Live birth rate |
| Notes | Apparant long‐term treatment of couples that were initially randomised in the Out et al trial (1995) |
Olivennes F 1999.
| Methods | Randomised small study with 30 couples |
| Participants | Couples undergoing IVF |
| Interventions | rFSH vs HMG in antagonist cycles |
| Outcomes | Not clear, according to Professors Engel and Olivennes there was no difference in clinical outcomes |
| Notes | ESHRE abstract Tours |
Reyftmann 1997.
| Methods | not known yet |
| Participants | not known yet |
| Interventions | not known yet |
| Outcomes | Not clear |
| Notes | Not able to get abstract or more data on trial |
Righini 1998.
| Methods | RCT |
| Participants | Couples undergoing IVF |
| Interventions | rFSH versus uFSH‐HP |
| Outcomes | Not clear |
| Notes | IFFS abstract |
Strowitzki 2007.
| Methods | RCT |
| Participants | Couples undergoing IVF or ICSI |
| Interventions | Fixed antagonist protocol + HP‐HMG or rFSH |
| Outcomes | Clinical and ongoing pregnancy? |
| Notes | Not able to get abstract or more data on trial |
Differences between protocol and review
The protocol and review was written by different authors. The protocol was written by the following authors: Irene Kwan, Hesham G Al‐Inany, Anna L Burt, Jane Thomas, Andy Vail. The review was written by the following authors: Madelon van Wely, Irene Kwan, Anna L Burt, Jane Thomas, Andy Vail, Fulco Van der Veen, Hesham G Al‐Inany.
An extra unplanned sub analysis was done to evaluate whether a sponsor effects was due to the sponsoring or due to the treatment given in these sponsored studies. Indeed this last option appeared more likely.
Contributions of authors
MvW searched the literature, contacted authors of studies, entered the data, did the analyses, wrote the review, and responded to reviewer comments.
IK developed and wrote the draft of the protocol, developed the title and the intended methods of the review, entered the protocol into RevMan; and responded to peer reviewers' comments on the protocol, searched the literature, entered part of the data and contacted authors of studies.
AV developed the intended methods of the review, provided statistical expertise, checked the data and the risk of bias tables.
JT helped to develop the protocol and the intended methods of the review.
AB helped to develop the protocol and the intended methods of the review, searched the literature and entered part of the data.
FV helped to write the review and was consultant on clinical issues.
AI helped to develop the protocol, the title and the intended methods of the review, responded to peer reviewers' comments and was consultant on clinical issues.
Sources of support
Internal sources
None, Not specified.
-
Academic Medical Center, Netherlands.
Review was partly written during working hours, i.e. partly paid by salary
External sources
None, Not specified.
No external support, no grants, Not specified.
Declarations of interest
None known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Abate 2009 {published and unpublished data}
- Abate A, Nazarro A, Salerno A, Marzano F, Cossut P, Perino M. Efficacy of recombinant versus human derived follicle stimulating hormone on the oocyte and embryo quality in IVF‐ICSI cycles: randomized, controlled, multi‐centre trial. Gynecological Endocrinology 2009;25(8):479‐84. [DOI] [PubMed] [Google Scholar]
Alvino 1995 {published data only}
- Alvino, H, Norman, R.J, Matthews, C.D. Recombinant human follicle stimulating hormone (Gonal‐F,Serono) compared to urinary follicle stimulating hormone (Metrodin) in IVF cycles: a randomised control study. Fertility Society of Australia/ Australasian Gynaecological endoscopy Society Conference, Melbourne, Australia. 19‐25 November. 1995.
Andersen 2006 {published data only}
- Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified HMG or recombinant FSH in patients undergoing IVF: a randomized assessor‐blind controlled trial. Human Reproduction 2006;21(12)::3217‐27. [DOI] [PubMed] [Google Scholar]
- Ziebe S, Lundin K, Janssens R, Helmgaard L, Arce JC. Influence of ovarian stimulation with HP‐HMG or recombinant FSH on embryo quality parameters in patients undergoing IVF.. Human Reproduction 2007;22:2404–13. [DOI] [PubMed] [Google Scholar]
Antoine 2007 {published and unpublished data}
- Antoine J.M, Mouzon J, Nicollet B, Salle B, Urbancsek J, Grudzinskas J.G. Effectiveness and tolerability of hFSH compared to rFSH in ICSI:the European study. IBSA Satelite Symposium abstract, ESHRE, Lyon. 2007; Vol. (http://www.ibsa.ch/it/eshre_2007_lyon_abstracts‐3.pdf).
Baker 2009 {published and unpublished data}
- Baker VL, Fujimoto VY, Kettel LM, Adamson GD, Hoehler F, Jones CE, Soules MR. Clinical efficacy of highly purified urinary FSH versus recombinant FSH in volunteers undergoing controlled ovarian stimulation for in vitro fertilization: a randomized, multicenter, investigator‐blind trial.. Fertility and Sterility 2009;91:1005‐11. [DOI] [PubMed] [Google Scholar]
Balasch 2003 {published and unpublished data}
- Balasch J, Penarrubia J, Fabregues F, Vidal E, Casamitjana R, Manau D, Carmona F, Creus M, Vanrell JA. Ovarian responses to recombinant FSH or HMG in normogonadotrophic women following pituitary desensitization by a depot GnRH agonist for assisted reproduction. Reprod Biomed Online 2003;7(1):35‐42. [DOI] [PubMed] [Google Scholar]
Berger 1999 {published data only}
- Berger E, Chabloz P, Quay N, Sann A, Walton S, Germond M, Birkhauser M. An open, randomized, group‐comparative bi‐centre study comparing recombinant FSH Follitropinum beta 150 IU and highly purified urinary FSH 225 IU as a fixed dose regimen in IVF/ICSI treatment. Human Reproduction. 1999; Vol. 14 suppl 1:61‐62.
Bergh 1997 {published data only}
- Bergh C, Howles CM, Borg K, Hamberger L, Josefsson B, Nilsson L, Wikland M. Recombinant human follicle stimulating hormone (r‐hFSH; Gonal‐F) versus highly purified urinary FSH (Metrodin HP): results of a randomized comparative study in women undergoing assisted reproductive techniques. Human Reproduction 1997;12(10):2133‐9. [DOI] [PubMed] [Google Scholar]
Bosch 2008 {published and unpublished data}
- Bosch E, Vidal C, Labarta E, Remohi J, Pellicer A. Highly purified HMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonist ‐ a randomised study.. Human reproduction 2008;23:2346‐51. [DOI] [PubMed] [Google Scholar]
Cheon 2004 {published data only}
- Cheon KW, Byun HK, Yang KM, Song IO, Choi KH, Yoo KJ. Efficacy of recombinant human follicle‐stimulating hormone in improving oocyte quality in assisted reproductive techniques. Journal of Reproductive Medicine 2004;49(9):733‐8. [PubMed] [Google Scholar]
Dickey 2002 {published and unpublished data}
- Dickey RP, Thornton M, Nichols J, Marshall DC, Fein SH, Nardi RV, Bravelle IVF Study Group. Comparison of the efficacy and safety of a highly purified human follicle‐stimulating hormone (Bravelle) and recombinant follitropin‐beta for in vitro fertilization: a prospective, randomized study. Fertility Sterility 2002;77(6):1202‐8. [DOI] [PubMed] [Google Scholar]
Dickey 2003 {published and unpublished data}
- Dickey RP, Nichols JE, Steinkampf MP, Gocial B, Thornton M, Webster BW, Bello SM, Crain J, Marshall DC, Bravelle IVF Study Group. Highly purified human‐derived follicle‐stimulating hormone (Bravelle) has equivalent efficacy to follitropin‐beta (Follistim) in infertile women undergoing in vitro fertilization. Reproductive Biology and Endocrinolology 2003;1:63 (http://www.RBEj.com/content/1/1/63). [DOI] [PMC free article] [PubMed] [Google Scholar]
Drakakis 2002 {published data only}
- Drakakis P, Loutradis D, Kallianidis K, Milingos S, Dionyssiou‐Asteriou A, Michalas S. The clinical efficacy of recombinant FSH (r‐FSH) as compared to highly purified urinary gonadotrophin (HMG‐FD) and the use of a low starting dose of r‐FSH in IVF or ICSI. A randomized prospective study. Italian Journal of Gynaecology Obstetrics 2002;14(3):64‐8. [Google Scholar]
EISG 2002 {published and unpublished data}
- European and Israeli Study Group on Highly Purified Menotropin versus Recombinant Follicle‐Stimulating Hormone (EISG). Efficacy and safety of highly purified menotropin versus recombinant follicle‐stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trial. Fertility Sterility 2002;78(3):520‐8. [DOI] [PubMed] [Google Scholar]
Ferraretti 1999 {published and unpublished data}
- Ferraretti A.P, Gianaroli, L, Magli, M.C, Bafaro, G, Colacuri N. Female poor responders. Molecular and Cellular Endocrinology 2000;161:59‐66. [DOI] [PubMed] [Google Scholar]
- Ferraretti, A.P, Gianaroli, L, Magli, M.C, Feliciani, E, Gergolet, M, Fortini. Recombinant FSH versus urinary FSH in non‐down regulated poorly responding patients. Abstract book, 11th World Congress of In vitro Fertilization and Human Reproductive Genetics. 1999; Vol. 263, Abstract P196.
Franco 2000 {published data only}
- Franco JG Jr, Baruffi RL, Coelho J, Mauri AL, Petersen CG, Garbellini E. A prospective and randomized study of ovarian stimulation for ICSI with recombinant FSH versus highly purified urinary FSH. Gynecological Endocrinology 2000;14(1):5‐10.. [DOI] [PubMed] [Google Scholar]
Frydman 2000 {published data only}
- Frydman R, Howles CM, Truong F for the French Multicentre Trialists. A double‐blind, randomized study to compare recombinant human follicle stimulating hormone (FSH; Gonal‐F) with highly purified urinary FSH (Metrodin) HP) in women undergoing assisted reproductive techniques including intracytoplasmic sperm injection. Human Reproduction 2000;15(3):520‐5. [DOI] [PubMed] [Google Scholar]
Gallego 2003a {published data only}
- Gallego Pastor E, Fernandez‐Shaw S, Mayoral M, Rodriguez L, Grande C, Pons I, Martinez V, Garcia del Real. The treatment with recombinant FSH improvement the embryo quality in IVF cycles: A prospective randomiced study. Revista Iberoamericana de Fertilidad y Reproduccion Humana 2003;20(1):43‐50. [Google Scholar]
Germond 2000 {published and unpublished data}
- Germond, M, De Palma, R, Senn, A, Inaudi, P, Dessole, S, De Grandi. P. Recombinant versus highly purified urinary FSH to induce ovulation induction and pregnancies in women over 35 years in an IVF/ICSI programme. Human Reproduction 2000;15(Special Issue):Abstract O‐118. [Google Scholar]
Ghosh 1999 {published data only}
- Chakravarty B, Chattopadhyay R, Ghosh S, Goswami S K, Kabir S N. Randomized comparative study of recombinant versus highly purified urinary follicle stimulating hormone in controlled ovarian stimulation: our experience.. International Journal of Obstetrics and Gynecology. 2000; Vol. 70:suppl 4:40 (FC4 11.03).
- Ghosh S, Chattopadhyay R, Goswami S, Chakravarty BN. Recombinant FSH versus highly purified urinary FSH ‐ our experience. In Abstract book, 11th World Congress of In vitro Fertilization and Human Reproductive Genetics. 1999; Vol. 264, issue Abstract P‐197.
Gordon 2001 {published and unpublished data}
- Gordon UD, Harrison RF, Fawzy M, Hennelly B, Gordon AC. A randomized prospective assessor‐blind evaluation of luteinizing hormone dosage and in vitro fertilization outcome. Fertility Sterility 2001;75(2):324‐31. [DOI] [PubMed] [Google Scholar]
Hedon 1995 {published data only}
- Hedon B, Out HJ, Hugues JN, Camier B, Cohen J, Lopes P, Zorn JR, Heijden B, Coelingh Bennink HJ. Efficacy and safety of recombinant follicle stimulating hormone (Puregon) in infertile women pituitary‐suppressed with triptorelin undergoing in‐vitro fertilization: a prospective, randomized, assessor‐blind, multicentre trial. Human Reproduction 1995;10(12):3102‐6. [DOI] [PubMed] [Google Scholar]
Hompes 2008 {published and unpublished data}
- Hompes PG, Broekmans FJ, Hoozemans DA, Schats R, for the FIRM group. Effectiveness of highly purified human menopausal gonadotropin vs. recombinant follicle‐stimulating hormone in first‐cycle in vitro fertilization‐intracytoplasmic sperm injection patients. Effectiveness of highly purified human menopausal gonadotropin vs. recombinant follicle‐stimulating hormone in first‐cycle in vitro fertilization‐intracytoplasmic sperm injection patients. Effectiveness of highly purified human menopausal gonadotropin vs. recombinant follicle‐stimulating hormone in first‐cycle in vitro fertilization‐intracytoplasmic sperm injection patients.. Fertil Steril 2007;89:1685‐93. [DOI] [PubMed] [Google Scholar]
Hoomans 1999 {published data only}
- Hoomans EH, Andersen AN, Loft A, Leerentveld RA, Kamp AA, Zech H. A prospective, randomized clinical trial comparing 150 IU recombinant follicle stimulating hormone (Puregon) and 225 IU highly purified urinary follicle stimulating hormone (Metrodin‐HP) in a fixed‐dose regimen in women undergoing ovarian stimulation. Human Reproduction 1999;14(10):2442‐7. [DOI] [PubMed] [Google Scholar]
Hugues 2001 {published data only}
- Hugues JN, Bry‐Gauillard H, Bstandig B, Uzan M, Cedrin‐Durnerin I. Comparison of recombinant and urinary follicle‐stimulating hormone preparations in short‐term gonadotropin releasing hormone agonist protocol for in vitro fertilization‐embryo transfer. Journal of Assisted Reproductrion and Genetics 2001;18(4):191‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jansen 1998 {published data only}
- Jansen CA, Os HC, Out HJ, Coelingh Bennink HJ. A prospective randomized clinical trial comparing recombinant follicle stimulating hormone (Puregon) and human menopausal gonadotrophins (Humegon) in non‐down‐regulated in‐vitro fertilization patients. Human Reproduction 1998;13(11):2995‐9. [DOI] [PubMed] [Google Scholar]
Kilani 2003 {published data only}
- Kilani Z, Dakkak A, Ghunaim S, Cognigni GE, Tabarelli C, Parmegiani L, Filicori M. A prospective, randomized, controlled trial comparing highly purified HMG with recombinant FSH in women undergoing ICSI: ovarian response and clinical outcomes. Human Reproduction 2003;18(6):1194‐9. [DOI] [PubMed] [Google Scholar]
Kornilov 1999 {published data only}
- Kornilov N V, Shlykova S A, Loginova J A, Tomas C, Ashorn R G. Comparison of four different gonadotropins for ovarian stimulation in IVF treatment. In Vitro Fertilization and Human Reproductive Genetics, 11th World Congress, Bologona, Italy. 1999:Abstract book: 379‐83.
Lenton 2000 {published data only}
- Lenton E, Soltan A, Hewitt J, Thomson A, Davies W, Ashraf N, Sharma V, Jenner L, Ledger W, McVeigh E. Induction of ovulation in women undergoing assisted reproductive techniques: recombinant human FSH (follitropin alpha) versus highly purified urinary FSH (urofollitropin HP). Human Reproduction 2000;15(5):1021‐7. [DOI] [PubMed] [Google Scholar]
Machado 1999 {published and unpublished data}
- Machado, M.G, Borges de Souza, M.C, Oliveira, J.B.A, Henriques, C.A, Mancebo. A.C.A. Highly purified gonadotropin and recombinant gonadotropin: study in IVF cycles. Gynecol. Endocrinol 1999;13, supplement 3:37, Abstract FC‐51. [Google Scholar]
Meden‐Vrtovec 2003 {published data only}
- Meden‐Vrtovec H, Mocnik‐Roznik S, Tomazevic T, Virant‐Klun I. Recombinant FSH vs. urinary FSH for ovarian stimulation in in vitro fertilization. Journal of Reproductive Medicine 2003;48(10):799‐803. [PubMed] [Google Scholar]
Mohamed 2006 {published and unpublished data}
- Mohamed MA, Sbracia M, Pacchiarotti A, Micara G, Linari A, Tranquilli D, Espinola SM, Aragona C. Urinary follicle‐stimulating hormone (FSH) is more effective than recombinant FSH in older women in a controlled randomized study. Fertility and Sterility 2006;85(5):1398‐403. [DOI] [PubMed] [Google Scholar]
Nardo 2000 {published data only}
- Nardo, L.G, Bellanca, S.A, Messina, K, Nardo, F. Efficacy of recombinant follicle stimulating hormone versus urinary follicle stimulating hormone in in‐vitro fertilization: A prospective, randomized, assessor‐blind study. Italian Journal of Gynaecology and Obstetrics 2000;12(2):53. [Google Scholar]
Ng 2001 {published and unpublished data}
- Ng EH, Lau EY, Yeung WS, Ho PC. HMG is as good as recombinant human FSH in terms of oocyte and embryo quality: a prospective randomized trial. Human Reproduction 2001;16(2):319‐25. [DOI] [PubMed] [Google Scholar]
O´Dea 1993 {published and unpublished data}
- O’Dea, L, Loumaye, E, Liu. H. A randomized, comparative, multicenter clinical trial of recombinant and urinary human FSH in in vitro fertilization and embryo transfer (IVFET). The American Fertility Society and The Canadian Fertility and Andrology Society 1993 Annual Meeting, Program Supplement. 1993:S50‐S51, abstract O‐106.
Out 1995 {published data only}
- Out HJ, Mannaerts BM, Driessen SG, Bennink HJ. A prospective, randomized, assessor‐blind, multicentre study comparing recombinant and urinary follicle stimulating hormone (Puregon versus Metrodin) in in‐vitro fertilization. Human Reproduction 1995;10(10):2534‐40. [DOI] [PubMed] [Google Scholar]
Rashidi 2005 {published data only}
- Rashidi BH, Sarvi F, Tehrani ES, Zayeri F, Movahedin M, Khanafshar N. The effect of HMG and recombinant human FSH on oocyte quality: a randomized single‐blind clinical trial. Eur J Obstet Gynecol Reprod Biol 2005;120 (2):190‐4. [DOI] [PubMed] [Google Scholar]
RHFSHG 1995 {published data only}
- Recombinant human FSH Study Group (RHFSHG). Clinical assessment of recombinant human follicle‐stimulating hormone in stimulating ovarian follicular development before in vitro fertilization.. Fertility Sterility 1995;63(1):77‐86. [PubMed] [Google Scholar]
Schats 2000 {published data only}
- Schats R, Sutter PD, Bassil S, Kremer JA, Tournaye H, Donnez J. Ovarian stimulation during assisted reproduction treatment: a comparison of recombinant and highly purified urinary human FSH. On behalf of The Feronia and Apis study group. Human Reproduction 2000;15(8):1691‐7. [DOI] [PubMed] [Google Scholar]
Selman 2002 {published data only}
- Selman HA, Santo M, Sterzik K, Coccia E, El‐Danasouri I. Effect of highly purified urinary follicle‐stimulating hormone on oocyte and embryo quality. Fertility Sterility 2002;78(5):1061‐7. [DOI] [PubMed] [Google Scholar]
Strehler 2001 {published data only}
- Strehler E, Abt M, El‐Danasouri I, Santo M, Sterzik K. Impact of recombinant follicle‐stimulating hormone and human menopausal gonadotropins on in vitro fertilization outcome. Fertility Sterility 2001;75(2):332‐6. [DOI] [PubMed] [Google Scholar]
Westergaard 2001 {published data only}
- Westergaard LG, Erb K, Laursen SB, Rex S, Rasmussen PE. Human menopausal gonadotropin versus recombinant follicle‐stimulating hormone in normogonadotropic women down‐regulated with a gonadotropin‐releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized study. Fertility Sterility 2001;76(3):543‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dickey 2003b {published data only}
- Dickey RP, Nichols JE, Steinkampf MP, Gocial B, Crain JL, Webster BW, Scobey MJ, Marshall DC. Bravelle (highly‐purified hFSH) vs. Follistim® (rFSH) in IVF: pooled analysis from two prospective, randomized clinical trials. Fertility and Sterility. 2003; Vol. Vol. 79, Supplement 2:17.
Duijkers 1997 {published data only}
- Duijkers IJ, Willemsen WN, Hollanders HM, Hamilton CJ, Thomas CM, Vemer HM. Follicular fluid hormone concentrations after ovarian stimulation using gonadotropin preparations with different FSH/LH ratios. II. Comparison of HMG and recombinant FSH. Int J Fertil Womens Med 1997;42:431‐5. [PubMed] [Google Scholar]
Manassiev 1997 {published data only}
- Manassiev NA, Davies WAR, Leonard T, Pavlovich B, Philips A, Tenekedjiev K. Initial results from the comparison of recombinant FSH and urinary FSH in an IVF programme. Human Reproduction 1997;12 (S1):265. [Google Scholar]
Martinez 2008 {published data only}
- Martínez F, Clua E, Parera N, Rodríguez I, Boada M, Coroleu B. Prospective, randomized, comparative study of leuprorelin + human menopausal gonadotropins versus ganirelix + recombinant follicle‐stimulating hormone in oocyte donors and pregnancy rates among the corresponding recipients.. Gynecol Endocrinol 2008;24(4):188‐93. [DOI] [PubMed] [Google Scholar]
Pacchiarotti 2007 {published data only}
- Pacchiarotti A, Aragona C, Gaglione R, Selman H. Efficacy of a combined protocol of urinary and recombinant follicle‐stimulating hormone used for ovarian stimulation of patients undergoing ICSI cycle. J Assist Reprod Genet 2007;24(9):400‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raga 1999 {published data only}
- Raga F, Bonilla‐Musoles F, Casan EM, Bonilla F. Recombinant follicle stimulating hormone stimulation in poor responders with normal basal concentrations of follicle stimulating hormone and oestradiol: improved reproductive outcome. Human Reproduction 1999;14(6):1431‐4. [DOI] [PubMed] [Google Scholar]
Requena 2010 {published data only}
- Requena A, Landeras JL, Martínez‐Navarro L, Calatayud C, Sánchez F, Maldonado V, Muñoz M, Fernández M, González A, López S, López R, Pacheco A, Calderón G, Martínez V. Could the addition of hp‐hMG and GnRH antagonists modulate the response in IVF‐ICSI cycles?Read More: http://informahealthcare.com/doi/abs/10.3109/14647270903586356. Hum Fertil 2010;13(1):41‐49. [DOI] [PubMed] [Google Scholar]
Ruvolo 2009 {published data only}
- Ruvolo G, Bosco L, Cittadini E. Ovarian stimulation protocol influences the apoptotic rate of human cumulus cells: a comparative study between recombinant and urinary human follicle‐stimulating hormone (FSH). Fertility and Sterility 2009;92(3):Supplement. [Google Scholar]
Serhal 2000 {published data only}
- Serhal P, Phophong P, Ranieri D.M. Comparison between HMG and recombinant‐FSH for ovarian stimulation in patients undergoing IVF. Human Reproduction 2000;15:143:(Abstract P‐112). [Google Scholar]
References to studies awaiting assessment
Chakravarty 2000 {published data only}
- Chakravarty B, Chattopadhyay R, Ghosh S, Goswami S K, Kabir S N. Randomized comparative study of recombinant versus highly purified urinary follicle stimulating hormone in controlled ovarian stimulation: our experience. International Journal of Obstetrics and Gynecology. 2000; Vol. 70:suppl 4:40 (FC4 11.03).
Kahn 1999 {published data only}
- Kahn J, Sunde A, During V, Out H. A prospective randomized comparative cohort study of either recombinant FSH (Puregon) or urinary FSH (Metrodin) in in‐vitro fertilization treatment. Middle East Fertility Society Journal 1999;4:206–14. [Google Scholar]
Olivennes F 1999 {published data only}
- Olivennes F, Belaich‐Allart J, Alvarez S, Bouchard P, Frydman R. A prospective randomized study comparing the use of HMG versus rec‐FSH with the single dose GnRH antagonist (Cetrorelix) protocol in IVF‐embryo transfer. Abstract of the 15the Annual meeting of the ESHRE, Tours, France Human Reproduction. 1999; Vol. 14:O111.
Reyftmann 1997 {published data only (unpublished sought but not used)}
- Reyftmann, L, Déchaud, H, Arnal, F, et al. Comparaison de lefficacité de Gonal F versus Humegon sure les résultats de la stimulation ovarienne pour FIV. FFER, 2eme Journées de la Fédération Francaise d'Etude de la Reproduction. 1997.
Righini 1998 {published data only}
- Righini C, Avril C, Camier B, Carles F, Cornet D, Moreau L, Ragage J P, Sage J C, Thebault A, Troung F, Howles C M. Recombinant human follicle‐stimulating hormone (r‐hFSH; GonalF) versus highly purified urinary hFSH (u‐hFSH‐HP; Metrodin HP) for the induction of superovulation in women undergoing assisted reproducitve techniques. Fertility Sterility 1998;IFFS Abstracts (S131):P‐019. [Google Scholar]
Strowitzki 2007 {published data only}
- Strowitzki T. By treatment protocols: differences in treatmentoutcomes after antagonist downregulation. 5thWorld Congresson Ovulation Induction.. 5thWorld Congresson Ovulation Induction. Rome, Italy, September 13–15.. 2007.
Additional references
Agrawal 2000
- Agrawal R, Holmes J, Jacobs HS. Follicle‐stimulating hormone or human menopausal gonadotropin for ovarian stimulation in in vitro fertilization cycles: a meta‐analysis. Fertility and Sterility 2000;73(2):338‐43. [DOI] [PubMed] [Google Scholar]
Al‐Inany 2003
- Al‐Inany H, Aboulghar M, Mansour R, Serour G. Meta‐analysis of recombinant versus urinary‐derived FSH: an update. Human Reproduction 2003;18(2):305‐13. [DOI] [PubMed] [Google Scholar]
Al‐Inany 2008
- Al‐Inany HG, Abou‐Setta AM, Aboulghar MA, Mansour RT, Serour GI. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta‐analysis. Reprod Biomed Online 2008;16:81‐88. [DOI] [PubMed] [Google Scholar]
Al‐Inany 2009
- Al‐Inany HG, Abou‐Setta AM, Aboulghar MA, Mansour RT, Serour GI. Highly purified HMG achieves better pregnancy rates in IVF cycles but not ICSI cycles compared with recombinant FSH: a meta‐analysis. Gynecol Endocrinol 2009;25:372‐378. [DOI] [PubMed] [Google Scholar]
Bergh 1999
- Bergh C. What are the clinical benefits of recombinant gonadotrophins? Recombinant follicle stimulating hormone. Human Reproduction 1999;14(6):1418‐20. [DOI] [PubMed] [Google Scholar]
Coomarisamy 2008
- Coomarasamy A, Afnan M, Cheema D, Veen F, Bossuyt PM, Wely M. Urinary HMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down‐regulation protocol in IVF or ICSI treatment: a systematic review and meta‐analysis. Hum Reprod 2008;23:310‐315. [DOI] [PubMed] [Google Scholar]
Daya 1995
- Daya S, Gunby J, Hughes EG, Collins JA, Sagle MA. Follicle‐stimulating hormone versus human menopausal gonadotropin for in vitro fertilization cycles: a meta‐analysis. Fertility and Sterility 1995;64(2):347‐54. [PubMed] [Google Scholar]
Daya 1998
- Daya S. Gonadotrophin‐releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daya 2000
- Daya S, Gunby J. Recombinant versus urinary follicle stimulating hormone for ovarian stimulation in assisted reproduction cycles. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
Daya 2002
- Daya S. Updated meta‐analysis of recombinant follicle‐stimulating hormone (FSH) versus urinary FSH for ovarian stimulation in assisted reproduction. Fertility and Sterility 2002;77(4):711‐4. [DOI] [PubMed] [Google Scholar]
Daya 2003
- Daya S. Follicle‐stimulating hormone and human menopausal gonadotropin for ovarian stimulation in assisted reproduction cycles. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1992
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Koppel P, Collins JA. The routine use of gonadotropin‐releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta‐analysis of randomized controlled trials. Fertility and Sterility 1992;58:888‐96. [DOI] [PubMed] [Google Scholar]
Larizgoitia 2000
- Larizgoitia I, Estrada M D, Garcia‐Altes A. Recombinant FSH as adjuvant in assisted reproduction: some data on the efficacy and efficiency of recombinant FSH urinary FSH. Barcelona, Spain: Catalan Agency for Health Technology Assessment and Research (CAHTA). 2000:1‐16.
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCC‐WCH 2004
- National Collaborating Centre for Women's and Children's Health. Fertility: assessment and management for people with fertility problems. Clinical guideline. London,UK: RCOG Press, 2004. [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials.. Human Reproduction 2003;18:1000‐4. [DOI] [PubMed] [Google Scholar]
Van Wely 2002
- Wely M, Westergaard LG, Bossuyt PMM, Veen F. Human menopausal gonadotropin versus recombinant follicle stimulation hormone for ovarian stimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003973] [DOI] [PubMed] [Google Scholar]
Van Wely 2003
- Wely M, Westergaard LG, Bossuyt PM, Veen F. Effectiveness of human menopausal gonadotropin versus recombinant follicle‐stimulating hormone for controlled ovarian hyperstimulation in assisted reproductive cycles: a meta‐analysis. Fertility and Sterility 2003;80(5):1086‐93. [DOI] [PubMed] [Google Scholar]
Ziebe 2007
- Ziebe S, Lundin K, Janssens R, Helmgaard L, Arce JC, MERIT (Menotrophin vs Recombinant FSH in vitro Fertilisation Trial) Group. Influence of ovarian stimulation with HP‐hMG or recombinant FSH on embryo quality parameters in patients undergoing IVF. Human Reproduction 2007;22:2404‐2413. [DOI] [PubMed] [Google Scholar]


