Abstract
Glutamate-induced excitotoxicity has been reported to be involved in the pathophysiology of neurodegenerative disorders. It has been proposed that valproic acid (VPA), which is used in epileptic and bipolar disorders, may be protective against excitotoxic insult. The aim of the present study was to investigate the effects of VPA against the glutamate excitotoxicity in the SH-SY5Y human neuroblastoma cell line and determine its anti-oxidant capacity by measuring oxidative and anti-oxidant biochemical parameters. SH-SY5Y human neuroblastoma cells were pre-treated with 1, 5 or 10 mM VPA prior to exposure to 15 mM glutamate. The MTT assay was performed to determine cell viability. To detect oxidative insult in glutamate toxicity and the potential anti-oxidant effect of VPA, the cell catalase (CAT), superoxide dismutase (SOD), malondialdehyde and hydrogen peroxide (H2O2) activity was determined. A progressive decline in cell viability was observed with increasing glutamate concentrations (1-50 mM). Treatment with 1 mM VPA was revealed to be effective in increasing the viability of cells exposed to glutamate for 24 h. Oxidative damage, including an increase in H2O2 and MDA, was observed in SH-SY5Y cells treated with glutamate and was reduced by pre-treatment with VPA. CAT activity was decreased following glutamate exposure, but VPA did not prevent this decrease. SOD activity was increased by treatment with VPA alone and was not affected by glutamate exposure. Overall, the present results confirmed the critical role of oxidative stress in glutamate-induced excitotoxicity. They also suggested that VPA may exert an anti-oxidant effect against glutamate-induced excitotoxicity by decreasing oxidative parameters, including H2O2 and MDA, but only had a slight effect on CAT and SOD activity, which have an anti-oxidant capacity.
Keywords: valproic acid, glutamate excitotoxicity, SH-SY5Y, oxidative stress parameter
Introduction
The excitatory neurotransmitter glutamate is involved in the pathophysiology of certain neurological disorders, as well as in neuron loss, by increasing cell component damage, including mitochondrial dysfunction (1). Glutamate excitotoxicity is also considered a major mechanism underlying neuronal death, leading to neurodegeneration, as occurring in hypoxia, ischemia, traumas and chronic neurodegenerative disorders (2). It has been indicated that glutamate mediates excitotoxicity in primary cultured neurons by stimulating the N-methyl-d-aspartate receptor, which leads to increased calcium permeability and formation of reactive oxygen species (ROS), and the release of lysosomal enzymes (3,4). Oxidative stress is known to be involved in several human neuropathologies, including acute hypoxia-ischemia/reperfusion and chronic neurodegenerative disorders, e.g. Parkinson's and Alzheimer's diseases (5,6). Following acute glutamate administration, increased intracellular ROS accumulation, Ca+2 levels, production of peroxynitrite and depletion of glutathione were observed in the cerebral cortex of rats (7). ROS generation is involved in the pathophysiology of several neuropsychiatric disorders, since 20% of the total amount of oxygen in the body is metabolized by the brain, and the brain has a limited anti-oxidant capacity (8,9). High levels of extracellular glutamate, resulting in glutathione depletion and cell injury, in addition to the inhibition of glutamate toxicity by several anti-oxidants, including α-tocopherol and superoxide dismutase (SOD), indicate oxidative glutamate toxicity. Cultured cortical neurons from mice overexpressing the free radical-scavenging enzyme SOD were indicated to be resistant to glutamate toxicity and the involvement of glutathione in neuro-degeneration was demonstrated (10,11). Excitotoxic neuronal injury is linked to the generation of free radicals and glutathione is involved in neurodegeneration (10,11). It has also been speculated that the principal function of ascorbate and α-tocopherol as anti-oxidants may be based on a synergistic effect with glutathione in the central nervous system (10,11).
Valproic acid (VPA), widely used as an anti-convulsant agent and efficient mood stabilizer, has been reported to have a utility in the treatment of excitotoxicity in the hippocampus (12). The neuroprotective effect and anti-oxidant activity of VPA were studied in primary cultured rat cerebral cortical cells and chronic VPA treatment was suggested to inhibit glutamate-induced cell death, DNA fragmentation, intracellular free calcium imbalance, lipid peroxidation and protein oxidation (13). VPA directly inhibits histone deacetylase (HDAC), causing histone hyperacetylation and heat shock protein (HSP) induction, where HSP induction is correlated with damage resistance. VPA was indicated to exert a neuroprotective effect through these mechanisms in the cerebral ischemia model (14), it was also indicated that VPA has neuroprotective effects against brain ischemia due to its anti-inflammatory and anti-oxidant activities, as well as against HDAC and glycogen synthase kinase 3 (GSK3) inhibition (15,16).
In the present study, the neuroprotective effect of VPA against oxidative glutamate toxicity was studied in the SH-SY5Y cell line, which is frequently used as a model for the study of oxidative stress associated with neuronal death and its anti-oxidant capacity, by measuring oxidative and anti-oxidant biochemical parameters.
Materials and methods
Cell culture
The study was performed using SH-SY5Y human neuroblastoma cells originally obtained from the American Type Culture Collection and kindly supplied by Dr İbrahim Akalın (Department of Medical Genetics, İstanbul Medeniyet University, İstanbul, Turkey). Every effort was made for the cell lines to be kept pure and free from contamination in the laboratory. The preventions were taken to avoid contamination; the sampling area, incubator and water bath was kept clean, the laboratory enviroment was sanitized, the biological safety cabinet was used for all procedures, all cell culture equipment, including reagents and media were sterile, antibiotics were included and sterile water was used to prevent contamination.
The SH-SY5Y cells were grown in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), penicillin (100 unit/ml) and streptomycin (100 µg/ml). The cells were incubated at 37˚C with 5% CO2. SH-SY5Y cells were seeded into 96-well plates (1x104 cells/well). To investigate the effects of L-glutamate and VPA, SH-SY5Y cells were incubated in complete culture medium for 24 h prior to the addition of L-glutamate or VPA.
Drug concentrations
Cells were treated with 9 different concentrations of glutamate (1, 5, 10, 15, 20, 25, 30, 40 and 50 mM; L-glutamate; cat. no. G1251; Sigma-Aldrich; Merck KGaA) to determine the glutamate toxicity in the cultured SH-SY5Y cells. The glutamate concentrations that caused a significant reduction in cell viability were determined by drawing dose-cell viability curves. The glutamate concentration of 15 mM that caused a ~20% decrease in cell viability after 24 h was then used for subsequent experiments. The cells were challenged with this concentration calculated from the dose-response experiment for 2 different time intervals (3 and 24 h). Cell viability was determined by MTT assays, as described below.
SH-SY5Y cells were treated with 1, 5 and 10 mM VPA (Depakin 400 mg/4 ml; lyophilized powder; Sanofi S.A.) for 2 h prior to exposure to 15 mM glutamate. The effect of VPA treatment was tested following 2 different time intervals (3 and 24 h).
Cell viability assay
An MTT (Thermo Fisher Scientific, Inc.) assay was used to evaluate cell viability. After adding MTT solution (5 mg/ml) to each well, cells were incubated for 3 h with 5% CO2 at 37˚C. Following the removal of the culture medium, 200 µl dimethyl sulfoxide was used to dissolve the formazan product. Absorbance values were measured at 560 nm using a microplate reader (Multiskan™ GO microplate spectrophotometer; Thermo Fisher Scientific, Inc.). Cell viability was calculated by considering the controls as 100%.
Cell lysate preparation
SH-SY5Y cells were harvested by trypsin-EDTA 0.25% (Thermo Fisher Scientific, Inc.) and collected by centrifugation at 1,000-2,000 x g for 10 min at 4˚C. Cells were then harvested in ice-cold buffer (0.05 M potassium phosphate pH 7.0, 1 mM EDTA) and homogenized by sonication on ice. The solution was centrifuged at 12,000 x g at 4˚C for 20 min to remove cell debris. The supernatant was used for determining the quantity of total protein and for the enzyme activity assay. The protein concentration was determined using the bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc). All spectrophotometric measurements were made using an Epoch microplate spectrophotometer (BioTek Instruments, Inc.).
Catalase (CAT) activity assay
CAT activity in cell lysates was tested by spectrophotometrically monitoring the degradation of hydrogen peroxide (H2O2) for 3 min at 240 nm at 25˚C in the presence of 3% H2O2 and 0.1 mM EDTA in 0.05 M potassium phosphate buffer with a pH of 7.0.
SOD activity assay
The SOD assay was performed by quantifying the inhibition of nitro blue tetrazolium (NBT) at 560 nm. The assay mixture (200 µl) comprised 0.0033 mM riboflavin, 10 mM L-methionine, 0.033 mM NBT and 0.66 mM EDTA-Na2 in 0.05 M potassium phosphate buffer (pH 7.8). The 96-well plates containing the assay mixture were incubated for 20 min with 300 nmol/m2/sec at 560 nm excitation at 25˚C. One unit of SOD activity was defined as the amount of protein (in mg) causing 50% inhibition of photoreduction, following which specific enzyme activity was expressed as units/mg protein.
Malondialdehyde (MDA) activity assay
Lipid peroxidation products, including MDA, react with thiobarbituric acid to form a colored product with an absorption maximum of 532 nm. The results are expressed as the molar equivalent of MDA (calculated from the standard curve prepared with tetraethoxypropane) per mg of protein.
H2O2 activity assay
H2O2 in cells was quantified using a H2O2 assay kit (cat no. ab102500; Abcam). In brief, at 24 h after drug administration, cells were harvested, homogenized and centrifuged. The supernatant was used for the assay. The absorbance was detected at 570 nm using a microplate reader and the optical density was used for quantification of H2O2 levels. Distilled water was used as a negative control instead of cell lysate sample.
DAPI staining
At 24 h after glutamate exposure and/or pre-treatment with VPA, the cells were fixed with methanol/acetic acid at a ratio of 3:1 at room temperature for 10 min (17). Cells were then washed twice with PBS, stained with DAPI (BioShop Canada, Inc.) for 5 min and examined by fluorescence microscopy (Axio Vert.A1; Carl Zeiss AG). Apoptosis-associated changes in cellular and nuclear morphology were examined and a reduced nuclear size, chromatin condensation, nuclear fragmentation and intense fluorescence were considered to indicate apoptosis. Cells were imaged using Zen 2,6 Blue Edition software (Carl Zeiss, AG).
Statistical analysis
Values are expressed as the mean ± standard error of the mean and analyzed by one-way analysis of variance, followed by a Bonferroni's multiple-comparisons post-hoc test by Grahpad Prism 8 software (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Dose-response curve of glutamate and VPA
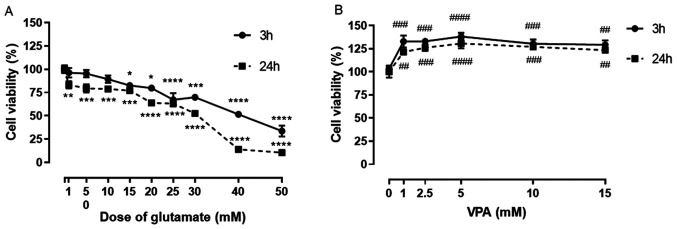
A dose-response curve of glutamate was plotted to determine the toxic concentration of glutamate in SH-SY5Y cells at 3 and at 24 h of exposure. The decrease in cell viability after exposure to glutamate for 3 h was not significant at the glutamate concentrations of 1, 5 and 10 mM. The significant decrease in cell viability started with 15 mM glutamate and decreased progressively with increasing glutamate concentrations. The progressive decline in cell viability was also observed at all concentrations of glutamate (1-50 mM) at 24 h of exposure (Fig. 1A). The glutamate concentration of 15 mM that caused a ~20% decrease in cell viability after 24 h was then used for the subsequent experiments.
Figure 1.
Effect of L-glutamate on SH-SY5Y cell viability. (A) Graph indicating the glutamate-induced dose-dependent decrease in cell viability in SH-SY5Y cells as % of control. Cells were treated with 9 different concentrations of glutamate (1, 5, 10, 15, 20, 25, 30, 40 and 50 mM). The cell viability determined after 3 and 24 h of treatment. (B) Graph of the effect of different VPA concentrations (1-15 mM) on cell viability at 3 and 24 h after treatment. Cell viability (% of control) is expressed as the mean value of four separate experiments (n=8 per condition). *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. control in A and ##P<0.01, ###P<0.001 and ####P<0.0001 vs. control in B. VPA, valproic acid.
VPA treatment alone at concentrations of 1, 2.5, 5, 10 and 15 mM significantly increased the cell viability of SH-SY5Y cells, when compared to the control, at 3 and 24 h. A significant increase was observed in cells treated with 1 mM VPA compared with the control (~130% of control; Fig. 1B), but no further increases could be observed with further increases in VPA concentration (Fig. 1B).
Effect of VPA pre-treatment on the viability of cells with glutamate-induced excitotoxicity
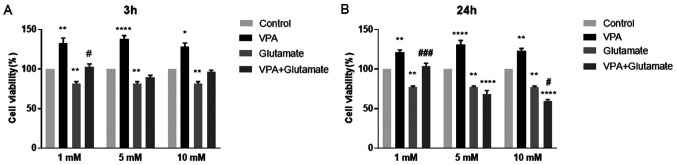
The effects of different concentrations of VPA (1, 5 and 10 mM) on cell viability, alone and prior to glutamate (15 mM) exposure for 3 and 24 h, are displayed in Fig. 2.
Figure 2.
Effect of VPA pre-treatment on the viability of SH-SY5Y cells with glutamate-induced excitotoxicity. Viability of SH-SY5Y cells pre-treated with or without VPA (1, 5, 10 mM) followed by exposure to 15 mM glutamate for (A) 3 h or (B) 24 h to induce excitotoxicity. *P<0.05, **P<0.01 and ****P<0.0001 vs. control group; #P<0.05 vs. glutamate group in A; **P<0.01 and ****P<0.0001 vs. control group; #P<0.05 and ###P<0.001 vs. glutamate group in B. Cell viability (% of control) is expressed as the mean value of four separate experiments (n=8 per experiment). VPA, valproic acid.
The viability of glutamate-treated cells was significantly increased in the group pre-treated with 1 mM VPA when compared with that of the cells treated with glutamate alone for 3 h. An increase in cell viability at 3 h was also observed after pre-treatment with 5 and 10 mM VPA; however, these changes were insignificant (Fig. 2A). Of note, pre-treatment with 1 mM VPA significantly increased the viability of cells exposed to glutamate for 24 h (Fig. 2B). However, 5 and 10 mM VPA concentrations were ineffective in reducing glutamate-induced excitotoxicity; conversely, they further decreased the viability of cells after glutamate exposure for 24 h. Thus, the concentration of 1 mM VPA was used in the further experiments to investigate the potential anti-oxidant effect of VPA in treating glutamate-injured neurons (Fig. 2).
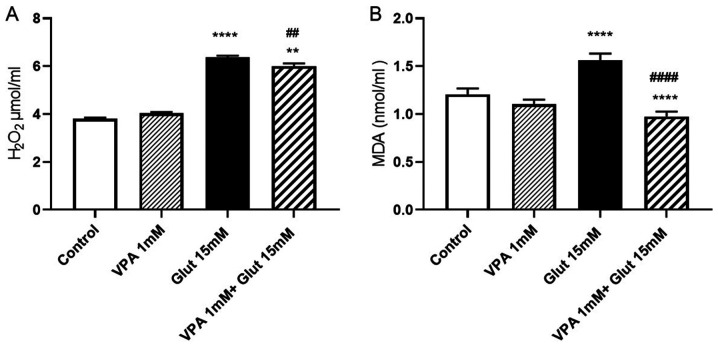
Effects of VPA pre-treatment on H2O2 and MDA contents in glutamate-induced excitotoxicity
At 24 h of incubation with 15 mM glutamate, H2O2 levels were increased as compared with those in the control group (P<0.0001). VPA treatment alone (1 mM) did not change the H2O2 levels as compared with those in the control. To explore whether VPA has protective effects against free radical-induced cell injury by scavenging free radicals, the effect of 1 mM VPA on H2O2 levels was evaluated. Treatment with VPA was indicated to decrease H2O2 levels in cells exposed to glutamate when compared to treatment with glutamate alone (P<0.01), but was not able to reduce them to the control levels (P<0.01 vs. control group; Fig. 3A).
Figure 3.
(A) H2O2 and (B) MDA levels in VPA-pre-treated SH-SY5Y cells exposed to glutamate to induce excitotoxicity. **P<0.01 and ****P<0.0001 vs. control group; ##P<0.01 vs. glutamate group in A. ****P<0.0001 vs. control group and ####P<0.0001 vs. glutamate group in B. Values are expressed as the mean value of four separate experiments (n=8 per experiment). H2O2, hydrogen peroxide; MDA, malondialdehyde; VPA, valproic acid; Glut, glutamate.
MDA levels were significantly increased after a 24-h incubation with glutamate (P<0.0001). Pre-treatment with VPA (1 mM) reversed the increase of MDA after glutamate exposure, when compared to the control and glutamate alone groups (P<0.0001 vs. control group; P<0.0001 vs. glutamate alone group; Fig. 3B).
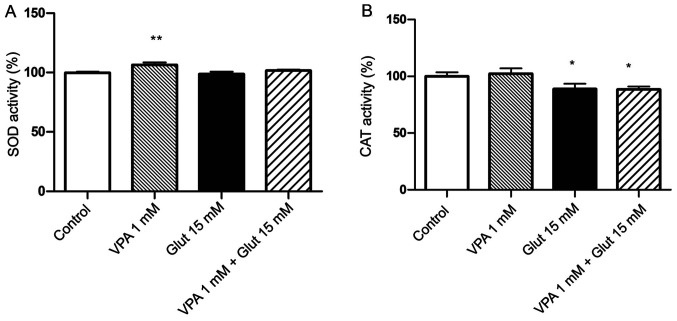
Effects of VPA pre-treatment on anti-oxidant enzyme activities in cells with glutamate-induced excitotoxicity
The SOD activity was increased in the 1 mM VPA alone group as compared with that in the control group (P<0.01; Fig. 4A). The SOD activity slightly decreased after glutamate exposure, as compared to the control group, but this change was not statistically significant. CAT activity was significantly decreased after exposure to 15 mM glutamate (Fig. 4B). VPA treatment alone slightly increased CAT activity, but the decrease was not significant; furthermore, pre-treatment with VPA had no obvious effect to inhibit the decrease of CAT in glutamate-exposed cells (P<0.05 vs. control; Fig. 4B).
Figure 4.
(A) SOD and (B) CAT activity in control cells, VPA-treated cells, cells exposed to glutamate, as well as VPA-pre-treated SH-SY5Y cells exposed to glutamate excitotoxicity. Cell viability was calculated by considering the controls as 100%. **P<0.01 vs. control in A; *P<0.05 vs. control in B. Values are expressed as the mean value of four separate experiments (n=8 in each experiment). SOD, superoxide dismutase; CAT, catalase; VPA, valproic acid; Glut, glutamate.
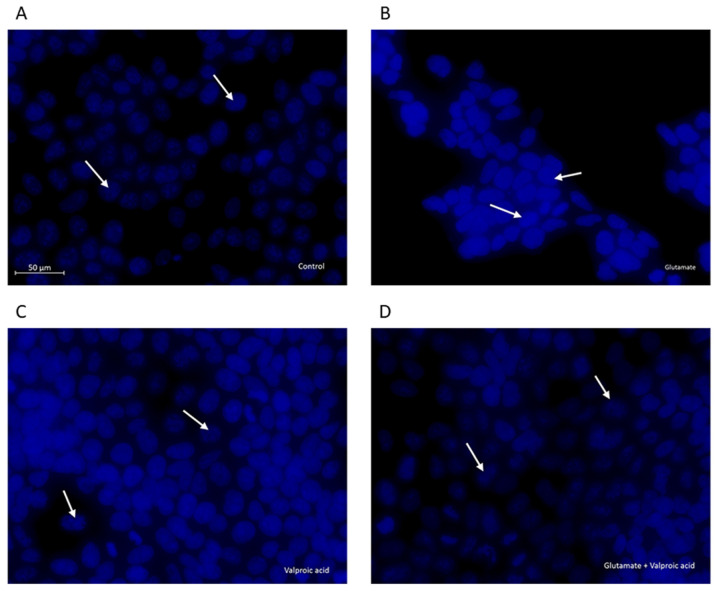
Effects of VPA pre-treatment on nuclear changes in cells with glutamate-induced excitotoxicity
Changes in cellular and nuclear morphology were examined in the control, glutamate-exposed, valproic acid-treated and valproic acid-pre-treated glutamate-exposed cells by DAPI staining (Fig. 5). The control cell nuclei were regular in shape. Morphological characteristics of apoptosis, including reduced nuclear size, chromatin condensation, nuclear fragmentation and intense fluorescence, were observed in glutamate-exposed cells. These changes in nuclear morphology were alleviated by pre-treatment of cells with VPA (1 mM) following exposure to 15 mM glutamate.
Figure 5.
Morphological assessment of the nuclei of SH-SY5Y cells by fluorescence microscopy. (A) Untreated cells were uniformly stained with DAPI. (B) Changes in the cellular and nuclear morphology of glutamate-exposed cells resulted in chromatin condensation and nuclear alterations. Effect of (C) valproic acid alone and (D) pre-treatment prior to glutamate exposure. The nuclei were stained with DAPI. Each photomicrograph was representative of three independent samples. Apoptotic cell nuclei are indicated by white arrows (scale bar, 50 µm).
Discussion
The present study focused on an acute excitotoxicity model of cell culture created by short-term exposure to glutamate for up to 24 h. Glutamate exposure for 24 h was also indicated to cause excitotoxicity in cortical neurons in primary culture (3). In the present study, the effect of VPA on the early stages of acute glutamate-induced neurotoxity, including intracellular ROS, were investigated in SH-SY5Y cells. Exposure to 15 mM glutamate was found to be the least effective compared with other higher doses of glutamate at causing toxicity in SH-SY5Y cells, as indicated by an MTT assay performed after glutamate treatment for 24 h. Among 3 concentrations of VPA (1, 5 and 10 mM), only pre-treatment with 1 mM VPA significantly inhibited glutamate-induced toxicity to increase the number of viable cells. Therefore, 15 mM glutamate and 1 mM VPA were used in the subsequent experiments to investigate the potential anti-oxidant effect of VPA to prevent glutamate-induced neuronal injury.
It was observed that acute exposure of glutamate to induce neurotoxicity significantly enhanced H2O2 levels, suggesting that glutamate-stimulated excitotoxicity in these cells is caused by oxidative damage. Major detrimental forms of ROS include superoxide, hydroxyl radicals and H2O2. H2O2 is one of the key targets for interventions among various ROS products. As H2O2 is neither a radical nor an ion, it is able to readily cross the cell membrane and affect cellular structures distant from its origin. Oxidative stress lead to protein dysfunction, DNA damage and lipid peroxidation, resulting in cell death in a manner that is dependent on the excessive production of ROS (18). A recent study revealed that 1-50 mM glutamate affected H2O2 synthesis by brain mitochondria, and this effect was associated with complex II, a source of superoxide formation in mitochondria, and is dependent on the mitochondrial potential (19). Ha et al (20) revealed that, following prolonged exposure to glutamate, extracellular H2O2 accumulated in a time- and concentration-dependent manner in HT22 cells. H2O2 formation due to mitochondrial superoxide leakage perpetuates oxidative stress in neuronal injury.
In the present study, increased levels of MDA were observed in cells exposed to glutamate. MDA, a product of the breakdown of polyunsaturated fatty acid, commonly known as a marker of oxidative stress, indicates that glutamate excitotoxicity may be associated with oxidative stress. MDA also serves as a convenient indicator of lipid peroxidation (21). In combination, the increased levels of H2O2 and MDA suggested that glutamate-induced neurotoxicity in SH-SY5Y cells is mediated by oxidative damage. This was consistent with the results of Sun et al (17), who indicated that glutamate exerted its toxicity through oxidative damage in SH-SY5Y cells.
The other result of the present study was that pre-treatment with 1 mM VPA decreased the glutamate-induced increase in H2O2 and MDA levels, revealing a neuroprotective effect of VPA by decreasing oxidative stress. Previous studies also confirmed that the protective effects of VPA are associated with a reduction of oxidative stress. Chronic treatment with VPA was reported to exert neuroprotective effects against excitotoxicity via inhibition of oxidative damage by decreasing glutamate-induced MDA levels (13). Frey et al (22) also demonstrated that valproate prevented amphetamine-induced lipid peroxidation in the hippocampus and in the prefrontal cortex, revealing the neuroprotective effects of VPA in response to oxidative stress. VPA has also been reported to inhibit the activation of the JNK pathway by decreasing ROS production in a model of spinal cord injury (23). It was reported that treatment with VPA following cerebral ischemia prevented ROS production via the inhibition of HDAC and induction of HSP (24). Silva et al (15) suggested that VPA exerted neuroprotective effects by attenuating the increased HDAC and GSK3 immunoreactivity, which are involved in inflammation and brain function in certain areas of the brain of ischemic animals. Inhibition of these enzymes was demonstrated to reduce ischemic cerebral damage by restoring failing mitochondrial bioenergetics and preventing ROS production (14,25).
The mechanisms through which VPA and other mood stabilizers decrease ROS generation remain to be fully elucidated, but it has been suggested that buffering overloaded intracellular calcium, stabilizing mitochondrial function and increased expression of endoplasmic reticulum stress protein may have a role in it (13,26,27). The inhibition of the GRP78 expression led to an increase in ROS and intracellular calcium levels following oxidative insult (28).
Oxidative stress occurs when cellular anti-oxidant defenses are inadequate to maintain the levels of ROS below the toxic threshold, due to excessive ROS production and/or loss of anti-oxidant defenses (29,30). CAT is one of the most common anti-oxidant enzymes in almost all living organisms that are exposed to oxygen; it catalyzes the reduction of H2O2 to water and removes organic hydroperoxides (31). SOD is a protective enzyme involved in catalyzing the dismutation of superoxide to less reactive H2O2 and molecular oxygen (32,33). These anti-oxidants may protect neuronal cells against oxidative damage by H2O2 (18,34,35). It has been reported that anti-oxidant systems in neurodegenerative disorders have coordinated effects induced by SOD and CAT (36).
In the present study, it was demonstrated that CAT activity is significantly decreased in cells exposed to glutamate. However, VPA did not exert any significant effect on CAT activity in cells cultured with glutamate. These results suggest that CAT activity has a role in glutamate-induced oxidative damage, but VPA does not appear to have a sufficient effect on CAT activity in cells exposed to glutamate. It has also been reported that CAT activity in the brain is significantly lower than that in other organs, including the kidney and liver (37). The low CAT activity may be reduced further by glutamate toxicity, an effect that VPA addition may not be sufficient to alleviate. VPA alone significantly increased the levels of this anti-oxidant enzyme, but exerted no significant effects on glutamate-exposed cells. It was reported that SOD activity may vary among different cell types, e.g. among adult rat brain cells; the specific activity of total SOD has been reported to be ~10-fold higher in glial cells than in neurons (38).
The results of the present study suggested that VPA exerts a protective effect on glutamate-induced excitotoxicity via decreasing ROS production without influencing anti-oxidant enzymes. Studies have reported controversial results regarding the effect of VPA on anti-oxidant enzymes. It has been indicated that the activity of glutathione S-transferase, glutathione reductase, glutathione peroxidase, SOD and CAT was significantly reduced in the cerebral cortex and cerebellum (39). A study on epileptic children also demonstrated a significant decrease in the anti-oxidant activity of SOD and CAT after VPA treatment (40). Glutathione reductase activity was indicated to be slightly lower in pediatric patients receiving VPA monotherapy and polytherapy, as compared with that in newly diagnosed pediatric patients (41). Other studies have revealed an increase in anti-oxidant enzymes by VPA treatment. It has been suggested that VPA treatment may alter the balance between oxidant and anti-oxidant systems (42). In primary cultured rat cerebral cortical cells, chronic treatment with lithium and valproate at therapeutic concentrations increased the activity of glutathione S-transferase (43).
However, the present study was not without its limitations. The potential contamination of the cells was not investigated, but the present results are in line with the literature and they will be confirmed in vivo or in primary cultured cells in future projects.
In conclusion, the present study demonstrated that pre-treatment with 1 mM VPA effectively prevented the decline in neuronal cell viability induced by glutamate exposure in SH-SY5Y cells via decreasing oxidative stress.
Acknowledgements
The authors would like to thank Miss Ilgın Akpınar from İstanbul University, Institution of Science, Department of Botany (Istanbul, Turkey) and Dr İbrahim Akalın from İstanbul Medeniyet University, Department of Medical Genetics (Istanbul, Turkey) for their technical contributions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BTB designed the study. BTB, EO and GA performed the experiments and analyzed and interpreted the data. All authors contributed to the writing of the manuscript and read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Wang R, Reddy PH. Role of Glutamate and NMDA receptors in Alzheimer's disease. J Alzheimers Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleich S, Römer K, Wiltfang J, Kornhuber J. Glutamate and the glutamate receptor system: A target for drug action. Int J Geriatr Psychiatry. 2003;18 (Suppl 1):S33–S40. doi: 10.1002/gps.933. [DOI] [PubMed] [Google Scholar]
- 3.Schubert D, Piasecki D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J Neurosci. 2001;21:7455–7462. doi: 10.1523/JNEUROSCI.21-19-07455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD. Researching glutamate-induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front Cell Neurosci. 2015;9(91) doi: 10.3389/fncel.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;10 (Supp l):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 6.Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Bharath MM, Shankar SK. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: Implications for Parkinson's disease. Neurochem Res. 2012;37:358–369. doi: 10.1007/s11064-011-0619-7. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Singh RL, Babu GN. Cell death mechanisms in the early stages of acute glutamate neurotoxicity. Neurosci Res. 2010;66:271–278. doi: 10.1016/j.neures.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB. Mitochondrial involvement in brain function and dysfunction: Relevance to aging, neurodegenerative disorders and longevity. Neurochem Res. 2001;26:739–764. doi: 10.1023/a:1010955807739. [DOI] [PubMed] [Google Scholar]
- 10.Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497:1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama K, Nakaki T. Inhibition of GTRAP3-18 may increase neuroprotective glutathione (GSH) synthesis. Int J Mol Sci. 2012;13:12017–12035. doi: 10.3390/ijms130912017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vajda FJ. Valproate and neuroprotection. J Clin Neurosci. 2002;9:508–514. doi: 10.1054/jocn.2002.1133. [DOI] [PubMed] [Google Scholar]
- 13.Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry. 2005;58:879–884. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Pang T, Wang YJ, Gao YX, Xu Y, Li Q, Zhou YB, Xu L, Huang ZJ, Liao H, Zhang LY, et al. A novel GSK-3β inhibitor YQ138 prevents neuronal injury induced by glutamate and brain ischemia through activation of the Nrf2 signaling pathway. Acta Pharmacol Sin. 2016;37:741–752. doi: 10.1038/aps.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva MR, Correia AO, Dos Santos GCA, Parente LLT, de Siqueira KP, Lima DGS, Moura JA, da Silva Ribeiro AE, Costa RO, Lucetti DL, et al. Neuroprotective effects of valproic acid on brain ischemia are related to its HDAC and GSK3 inhibitions. Pharmacol Biochem Behav. 2018;167:17–28. doi: 10.1016/j.pbb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Wu H, Klebe D, Hong Y, Zhang J. Valproic acid: A new candidate of therapeutic application for the acute central nervous system injuries. Neurochem Res. 2014;39:1621–1633. doi: 10.1007/s11064-014-1241-2. [DOI] [PubMed] [Google Scholar]
- 17.Sun ZW, Zhang L, Zhu SJ, Chen WC, Mei B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci Bull. 2010;26:8–16. doi: 10.1007/s12264-010-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobysheva NV, Selin AA, Vangeli IM, Byvshev IM, Yaguzhinsky LS, Nartsissov YR. Glutamate induces H2O2 synthesis in nonsynaptic brain mitochondria. Free Radic Biol Med. 2013;65:428–435. doi: 10.1016/j.freeradbiomed.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Ha JS, Lim HM, Park SS. Extracellular hydrogen peroxide contributes to oxidative glutamate toxicity. Brain Res. 2010;1359:291–297. doi: 10.1016/j.brainres.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 21.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: Markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord. 2010;125:287–294. doi: 10.1016/j.jad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci. 2006;31:326–332. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Maeng S, Kang SR, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. J Neurotrauma. 2014;31:582–594. doi: 10.1089/neu.2013.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xuan A, Long D, Li J, Ji W, Hong L, Zhang M, Zhang W. Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci. 2012;90:463–468. doi: 10.1016/j.lfs.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, Spano P, Carruba MO, De Simoni MG, Nisoli E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang JF, Azzam JE, Young LT. Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience. 2003;116:485–489. doi: 10.1016/s0306-4522(02)00655-3. [DOI] [PubMed] [Google Scholar]
- 27.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Bowes RC, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B. Free radicals and antioxidants-quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Ďuračková Z. Free radicals and antioxidants for Non-experts. In: Systems Biology of Free Radicals and Antioxidants. Laher I (ed). Springer, Berlin, Heidelberg, pp3-38, 2014. [Google Scholar]
- 31.Jornada LK, Valvassori SS, Steckert AV, Moretti M, Mina F, Ferreira CL, Arent CO, Dal-Pizzol F, Quevedo J. Lithium and valproate modulate antioxidant enzymes and prevent ouabain-induced oxidative damage in an animal model of mania. J Psychiatr Res. 2011;45:162–168. doi: 10.1016/j.jpsychires.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- 33.Yasui K, Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm Res. 2006;55:359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- 34.Chadwick W, Zhou Y, Park SS, Wang L, Mitchell N, Stone MD, Becker KG, Martin B, Maudsley S. Minimal peroxide exposure of neuronal cells induces multifaceted adaptive responses. PLoS One. 2010;5(e14352) doi: 10.1371/journal.pone.0014352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 36.Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant'Anna M, Cunha AB, Post RM. Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Br J Psychiatry. 2008;30:243–245. doi: 10.1590/s1516-44462008000300011. [DOI] [PubMed] [Google Scholar]
- 37.Wilson JX. Antioxidant defense of the brain: A role for astrocytes. Can J Physiol Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- 38.Savolainen H. Superoxide dismutase and glutathione peroxidase activities in rat brain. Res Commun Chem Pathol Pharmacol. 1978;21:173–176. [PubMed] [Google Scholar]
- 39.Chaudhary S, Parvez S. An in vitro approach to assess the neurotoxicity of valproic acid-induced oxidative stress in cerebellum and cerebral cortex of young rats. Neuroscience. 2012;225:258–268. doi: 10.1016/j.neuroscience.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Wu JY, Weng LH, Li XX, Yu LJ, Xu Y. Valproic acid protects against MPP+-mediated neurotoxicity in SH-SY5Y Cells through autophagy. Neurosci Lett. 2017;638:60–68. doi: 10.1016/j.neulet.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Sołowiej E, Sobaniec W. The effect of antiepileptic drug therapy on antioxidant enzyme activity and serum lipid peroxidation in young patients with epilepsy. Neurol Neurochir Pol. 2003;37:991–1003. (In Polish) [PubMed] [Google Scholar]
- 42.Yiş U, Seçkin E, Kurul SH, Kuralay F, Dirik E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009;84:232–237. doi: 10.1016/j.eplepsyres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Wang JF, Shao L, Sun X, Young LT. Glutathione S-transferase is a novel target for mood stabilizing drugs in primary cultured neurons. J Neurochem. 2004;88:1477–1484. doi: 10.1046/j.1471-4159.2003.02276.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.