Abstract
Previous studies have identified ~50 genes that contribute to non-syndromic autosomal dominant sensorineural deafness (DFNA). However, in numerous families with hearing loss, the specific gene mutation remains to be identified. In the present study, the clinical characteristics and gene mutations were analyzed in a Chinese pedigree with hereditary hearing loss. The clinical characteristics of the family members were assessed and a detailed audiology function examination was performed. Whole-exome sequencing (WES) was performed to identify the gene mutation responsible for the hearing loss. Sanger sequencing was used to verify the candidate mutation detected in the family. The family consisted of 31 members, seven of whom were diagnosed with sensorineural deafness of varying degrees. No mutation was identified by the general deafness gene chip. However, a novel heterozygous mutation in exon 3 (c.152C>T; Pro51Leu) of the gene crystallin µ (CRYM) was identified by WES. This result was further verified by Sanger sequencing. Co-segregation of genotypes and phenotypes suggested that this novel mutation was instrumental for the hearing loss/DFNA. In conclusion, the present study identified a novel pathogenic mutation, NM_001888.5(CRYM): c.152C>T(Pro51Leu), associated with DFNA. This mutation has not been reported previously and further functional studies are warranted.
Keywords: sensorineural deafness, CRYM, mutation, sequencing
Introduction
Deafness is the most common sensory deficit disorder, accounting for a high incidence of disability (1). According to a survey from the World Health Organization (WHO) in 2013, there are ~360 million people with varying degrees of deafness worldwide (2). According to epidemiological data, ~1 in 1,000 newborns is diagnosed with congenital deafness (3). Deafness directly affects cognition, thinking and memory, resulting in a decline in the quality of life, thereby contributing to the burden of families and society (4). The progression and development of modern medicine have gradually reduced the proportion of deafness cases caused by environmental factors (5). However, the proportion of patients with hearing impairment caused by genetic factors has largely remained to be determined (6). Based on deafness combined with the presence or absence of malformations of the external ear, hereditary deafness may be classified into syndromic deafness and non-syndromic deafness (7). Nearly 70% of cases of hereditary deafness may be attributed to non-syndromic hearing loss (NSHL) and 50% of cases of NSHL have Mendelian disease (8). At present, ~145 chromosomal loci are known to be associated with non-syndromic deafness (9). The coagulation factor C homology (COCH) gene was the first gene to be identified to cause non-syndromic deafness (Online Mendelian Inheritance in Man ID, 603196) (10). Patients with a mutation in this gene may present with a series of symptoms caused by cochlear and vestibular dysfunction (11). At present, detection of the COCH mutation is a subject of intense research (12). Screening for gene mutations using traditional Sanger sequencing is time-consuming and expensive (13).
Recent developments in whole-exome sequencing (WES) technology have shifted this paradigm and currently, rapid sequencing of exomes, transcriptomes and genomes may be completed at a relatively low cost (14). Of note, the application of this technology to catalog the mutational landscapes of genetic disorders has revealed a novel approach to explore single-gene diseases (15,16). The use of WES technology in patients with hereditary deafness and targeted next-generation sequencing of genes associated with deafness in hearing-impaired individuals has enabled the identification of informative mutations (17). WES is not only able to provide an efficient diagnosis for known deafness genes but also unravel novel gene mutations that may help us understand the molecular mechanism underlying deafness or hearing loss (18).
The present study aimed to identify genetic factors associated with hearing loss in a Chinese pedigree with hearing loss. WES was employed to identify a novel gene mutation in the gene crystallin µ (CRYM) that was accountable for the disease.
Materials and methods
Subjects
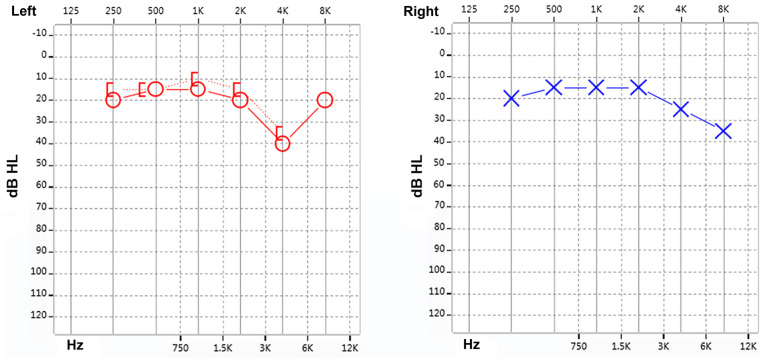
The present study reported on familial non-syndromic autosomal dominant sensorineural deafness in four generations of a Chinese pedigree. The genealogical proband was a 35-year-old female, recruited from Xinqiao Hospital (Chongqing, China) of the Army Medical University in September 2015. This proband was subjected to pure-tone audiometry, Distortion Product Otoacoustic Emission (DPOAE) and Auditory Brainstem Response (ABR) evaluation. The test results were indicative of sensorineural deafness in the right ear and a high-frequency hearing loss in the left ear (Fig. 1). The results presented a progressive aggravation of hearing loss with increasing frequency illustrated by a downsloping of the hearing curve. The patient was diagnosed with sensorineural deafness according to the guidelines for clinical evaluation and etiologic diagnosis of hearing loss by the American College of Medical Genetics and Genomics (19). The patient and her family members were enrolled in the present study. The study protocol was approved by the Ethics Committee of Xinqiao Hospital (Chongqing, China) and the study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the proband and the remaining family members. Furthermore, all of the participants agreed to the publication of their results on clinical characteristics and genetic data with protection of their privacy. Subsequently, the detailed medical history was collected, including family history of deafness and consanguineous marriages, year of onset of deafness, age, progression, and history of ototoxic drugs and noise exposure.
Figure 1.
Results of the audiogram function test for the proband. dB, decibels; HL, hearing level.
DNA extraction
Whole blood samples of all subjects were collected and subjected to genomic DNA extraction using the RelaxGene Blood DNA System (Tiangen Biotech) as per the manufacturer's instructions. The extracted DNA was stored at -20˚C until further analysis.
Audiology function examination
An audiology function examination was performed using pure tone audiometry, DPOAE and ABR on all family members using standard procedures as described previously (20).
Gene chip detection analysis
A total of 15 mutations of four deafness-associated genes were detected, including those in gap junction protein beta 2 (GJB2; 35delG, 176del16, 235delC and 299delAT), GJB3 (538C>T), solute carrier family 26 member 4 (2168A>G, IVS7-2A>G, 1174A>T, 122G>A, 1229C>T, 1975G>C, 2027T>A and IVS15+5 G>A) and mitochondrial 12S ribosomal RNA (1494C>T and 1555A>G) using the Heredity Hearing Loss Array Detection Kit purchased from Capital Bio Corp. The chip was imaged using a LuxScan™ 10 KB Microarray Scanner (Capital Bio Corp.) (21). Data evaluation was performed using SPSS 19.0 (IBM Corp.).
WES analysis
Specific primer sequences were designed by Primer Premier 5.0 (Premier Biosoft International). The Ion PI™ Hi-Q™ Sequencing 200 Kit (Thermo Fisher Scientific, Inc.) was employed for target gene mutation detection by computational mapping analysis. Genomic DNA samples were sheared by sonication. The sheared genomic DNA was then hybridized with the NimbleGen 2.0 probe sequence capture array from Roche (http://www.nimblegen.com/products/seqcap/ez/v2/index.html) to enrich the exonic DNA (Joy Orient). The libraries were first tested for exon-enrichment by quantitative PCR and for size distribution and concentration using the Agilent Bioanalyzer 2100 (Agilent Technologies, Inc.). The samples were then sequenced on an Illumina Hiseq 2500 platform (Illumina, Inc.). Each sample was tested in two parallel reactions.
Results
Family investigation and clinical phenotype
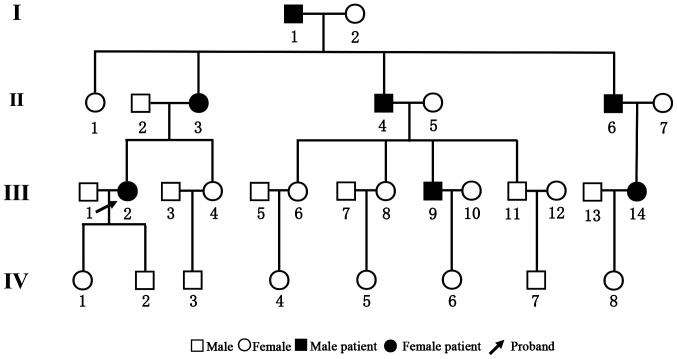
The pedigree chart is presented in Fig. 2. The family consisted of 31 members belonging to four generations. There were 14 males and 17 females. All family members underwent pure-tone audiometry examinations and the results are listed in Table I. A total of 7 patients, aged 31-79 years, were diagnosed with sensorineural deafness of varying degrees. None of the patients had a history of ototoxic drug exposure. Among them, cases I1, II3 and II4 were diagnosed with severe deafness on both ears. Cases III2, III9 and III14 demonstrated mild sensorineural deafness. Case II6 had mixed deafness (conductive deafness and sensory deafness) on the right ear and sensorineural deafness on the left ear. The results of the hearing test for the remaining family members are listed in Supplementary Table SI. There was no predilection for sex and a significant phenomenon of continuous transmission was noted. At least one parent of affected subjects was diagnosed with deafness, suggesting that the disease had an autosomal dominant pattern of inheritance.
Figure 2.
Pedigree chart of the family with the c.152C>T mutation in CRYM.
Table I.
Clinical characteristics of the family members.
| Subject | Age (years) | Sex | Age at onset (years) | Pure tone test | Degree of hearing loss | DPOAE | Binaural ABR threshold (dBnHL) |
|---|---|---|---|---|---|---|---|
| I1 | 78 | Male | 47 | Binaural full frequency sensorineural deafness | High severity on both sides | Binaural full frequency extraction in abnormal range | 110 |
| II2 | 56 | Male | 54 | Binaural sensorineural deafness | Moderate on both sides | Binaural full frequency extraction in abnormal range | 50 |
| II3 | 61 | Female | 52 | Binaural mixed deafness | Severe on both sides | Binaural full frequency extraction in abnormal range | 70 |
| II4 | 56 | Male | 43 | Binaural sensorineural deafness | Severe on both sides | Binaural full frequency extraction in abnormal range | 100 |
| II6 | 56 | Male | 50 | Binaural mixed deafness | Severe on both sides | Binaural full frequency extraction in abnormal range | 70 |
| III2 | 35 | Female | 32 | Sensorineural deafness in right ear, high-frequency hearing loss in left ear | Normal hearing threshold | Binaural partial frequency extraction in abnormal range | 30 |
| III9 | 31 | Male | 31 | Binaural sensorineural deafness with high frequency | Normal hearing threshold | Binaural partial frequency extraction in abnormal range | 30 |
| III14 | 32 | Female | 31 | Binaural sensorineural deafness with high frequency | Normal hearing threshold | Binaural partial frequency extraction in abnormal range | 40 |
DPOAE, Distortion Product Otoacoustic Emission; ABR, Auditory Brainstem Response; dBnHL, decibel normal hearing level.
Gene chip identification of deafness-associated genes
The routine diagnosis of deafness genes was performed by microarray in order to identify hot mutations in the study subjects. The gene chip contained 4 genes and 15 loci. The details of the gene chip and the mutations it is able to detect are listed in Table II. However, none of these mutations was identified by the gene chip in any of the participants.
Table II.
General deafness gene chip for target capture.
| Gene | Variant |
|---|---|
| GJB2 | NM_004004.6:c.35delG |
| GJB2 | 176del16 |
| GJB2 | 235delC |
| GJB2 | 299delAT |
| GJB3 | NM_024009.3:c.538C>T |
| SLC26A4 | 2168A>G |
| SLC26A4 | NM_000441.2:c.919-2A>G |
| SLC26A4 | 1174A>T |
| SLC26A4 | 1226G>A |
| SLC26A4 | 1229C>T |
| SLC26A4 | 1975G>C |
| SLC26A4 | 2027T>A |
| SLC26A4 | NM_000441.2:c.1707+5G>A |
| MT-RNR1 | 1494C>T |
| MT-RNR1 | 1555A>G |
GJB2, gap junction protein β 2; SLC26A4, solute carrier family 26 member 4; MT-RNR1, mitochondrially encoded 12S ribosomal RNA.
Mutation identified by WES
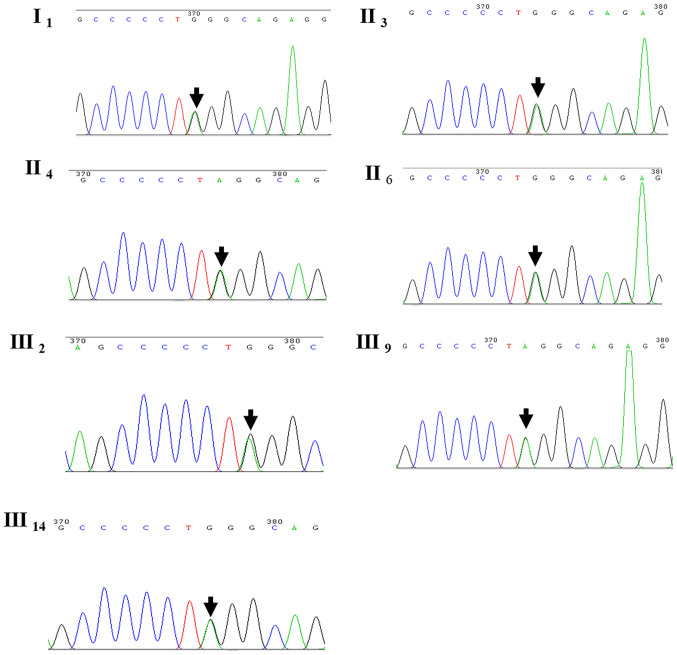
WES was performed in four family members, namely cases III2, III14, IV2 and IV8. A heterozygous mutation of CRYM was detected in cases III2 and III14; however, it was absent in subjects IV2 and IV8. The heterozygous mutation detected was c.152C>T(Pro51Leu). However, no mutations in other known genes associated with deafness were observed. Whether the mutation was pathogenic and accounted for a familial trait remain to be verified. Sanger sequencing was used to verify the results obtained by WES in the remaining family members participating in the study. Of note, the CRYM mutation was identified in all other seven family members with deafness, as in the proband III2 (Fig. 3).
Figure 3.
Results of the direct Sanger sequencing of the crystallin µ gene in seven patients with dominant sensorineural deafness. The mutation site is indicated by black arrows.
Bioinformatics analysis
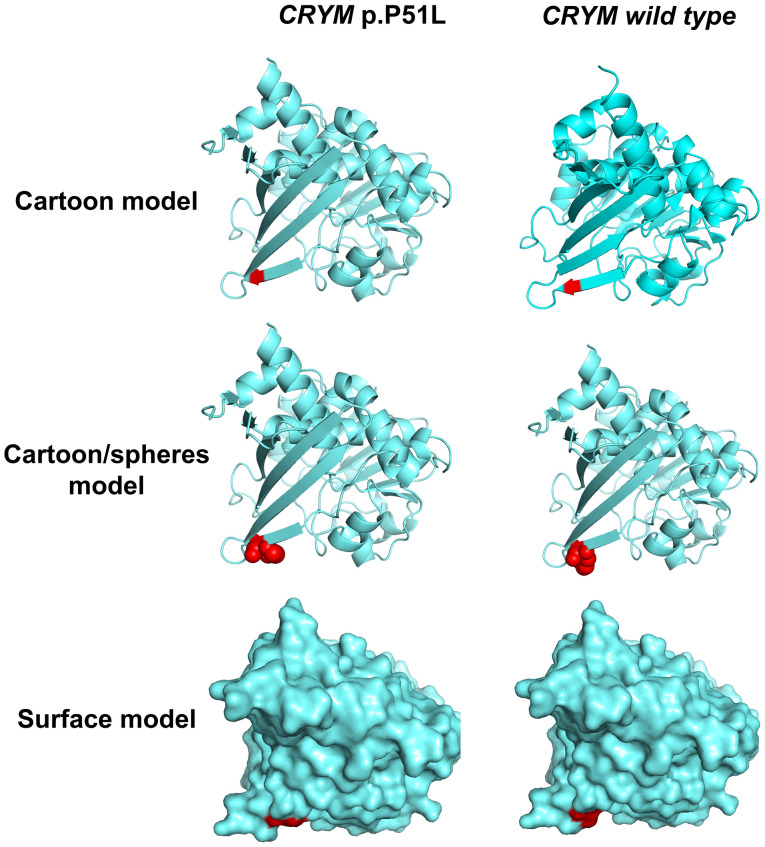
PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) was used to predict the possible impact of an amino acid substitution on the structure and function of the mutation, which indicated that the CRYM c.152C>T(Pro51Leu) mutation is probably damaging, with a score of 1.000 (sensitivity, 0.00; specificity, 1.00), and the Protein Variation Effect Analyzer (http://provean.jcvi.org/index.php) indicated a deleterious effect with a score of -5.395 (score below -2.5 means deleterious effect). Collectively, these results strongly indicated that the p.P51L mutation was likely to be deleterious to the protein. The result of a multiple sequence alignment suggested that the proline at position 51 of CRYM is highly conserved among various species (Fig. 4). In addition, the 3-dimensional protein structures were predicted and displayed with a cartoon, a cartoon/spheres and a surface model, which were generated using PyMOL software (version 2.3; Schrödinger LLC; Fig. 5). The protein structure parameters indicated that The Inertia Axis Aligned Bounding Box (IABB) dimensions were (43.63, 47.33, 63.49) and (43.32, 47.22, 63.49) and the IABB volume was 131,101.14 and 129,861.34 for the wild-type and muted type, respectively. This indicates that this substitution is able to destabilize the protein conformation.
Figure 4.
Conservation analysis of the crystallin µ gene. The mutation site is indicated by a black arrow. WT, wild-type; Mu, mutant.
Figure 5.
Display of the predicted 3-dimensional protein structure of CRYM. The red colour indicated the amino acid mutation site. CRYM, crystallin µ.
Follow-up
The only follow-up of the proband was by a phone call after 4 years. The subjective sensation of her hearing was not significantly changed according to the telephone survey and answered a subjective questionnaire with the help of her family member. No further hearing examinations were completed on the other family members and no family members were prescribed hearing aids.
Discussion
In the present study, a Chinese pedigree with hereditary sensorineural hearing loss of unknown genetic etiology was analyzed for a causative mutation using WES. A novel mutation, NM_00188.5(CRYM): c.152C>T(Pro51Leu), was detected. This mutation, not being a common variant, has a high likelihood of being pathogenic in patients with sensorineural hearing loss.
Deafness is a hereditary disorder with a high degree of genetic heterogeneity. DFNA is primarily the result of a single gene mutation (22). To date, a total of 105 genes have been reported to be associated with NSHL (23). Among the genes linked to this condition, COCH was the first gene to be reported to be associated with vestibular function (24). Clinical symptoms of COCH mutation are autosomal dominant, non-syndromic and characterized by progressive sensorineural hearing loss (10). At onset, the disease involves high-frequency hearing impairment. It progresses with age to severe hearing loss affecting low and high frequencies (25).
Recently, with the advent of comprehensive genetic testing for hereditary hearing loss using massively parallel sequencing (26), further gene mutations accounting for NSHL have been identified, thereby highlighting its genetic heterogeneity (27). The application of a deafness diagnostic screening panel based on a deafness mutations/gene database has gained popularity (28). A gene diagnostic chip for hereditary deafness has been used previously to detect gene mutations associated with hearing impairment among high-risk pregnant females (29) and newborns (30), and for pre-natal diagnosis of deafness to avoid the birth of children with congenital deafness (31).
At present, gene chip is used for rapid genetic diagnosis and in epidemiological surveys of hearing loss. However, it was originally devised for hot-spot mutations that have already been reported. Therefore, it cannot be used to detect other mutations in these genes. With the rapid development of WES technology, the scientific community has begun to widely adopt second-generation sequencing technology to solve biological problems. This novel generation of sequencers is able to rapidly sequence whole genomes and zoom in to deeply sequence the target regions of interest (32). Numerous genetic variants of unknown significance may be detected in patients by WES. In the present study, a regular gene chip was used to screen mutations for hearing loss, although no positive results were obtained. However, with the application of NGS, a novel mutation of CRYM, c.152C>T(Pro51Leu), responsible for hearing impairment was identified.
CRYM, which is also termed Thyroid Hormone Binding Protein (THBP) or Deafness, autosomal dominant, 40 (DFNA40), is located on chromosome 16. Its transcript length is 1,482 bp with eight exons encoding 314 amino acids. CRYM is mainly expressed in the heart and brain. It is particularly abundant in the inner ear (33). A previous study has demonstrated that CRYM is expressed in the human cochlea (34). CRYM has a critical role in the physiological regulation of the activity of thyroid hormone (35). An experimental study has indicated that CRYM may have a role in the development of cortical and hippocampal pyramidal cells in the early postnatal period (36). Furthermore, CRYM expression is possibly upregulated through the activator protein-1 (AP-1) site in the promoter (37). Another important CRYM function has also been identified: When combined with ketimine reductase, it may act as a classical imine reductase (38). In addition, CRYM has a critical role and is regarded as a novel androgen-regulated gene whose expression is elevated in prostate cancer (39). It has been demonstrated in an animal model that CRYM mutations cause auditory dysfunction through thyroid hormone-binding effects on the cochlea (40). It is well known that thyroid hormone is crucial for normal development, as well as maintenance of hearing function (41). CRYM is divided into two classes: Taxon-specific and ubiquitous. This gene encodes a taxon-specific crystallin protein that binds NADPH and has a sequence similarity to bacterial ornithine cyclodeaminases, which is particularly abundant in kangaroo lenses (42).
To date, CRYM-null patients have not been reported (35). Abe et al (43) were the first to demonstrate that CRYM mutation is associated with hearing loss. In their study, they indicated that one missense and one stop-lost variant in CRYM was a pathogenic mutation for hearing loss in two different families of Japanese origin. A search for the CRYM mutation in Clinvar was performed (https://www.ncbi.nlm.nih.gov/clinvar/?term=CRYM%5Bgene%5D). Most of the identified mutations in other loci of CRYM were benign, of uncertain significance or had conflicting interpretations of pathogenicity. The details of the mutations identified are listed in Table III. The previously unreported novel CRYM mutation identified in the present study is likely to be pathogenic.
Table III.
Summary of mutations in the crystallin µ gene and the clinical significance of pedigrees with associated dominant sensorineural deafness.
| Nucleic acid mutation | Amino acid variation | Molecular consequence | Clinical significance |
|---|---|---|---|
| c.945A>T | p.Ter315Tyr | Single nucleotide variant | Pathogenic |
| c.941A>C | p.Lys314Thr | Missense | Pathogenic |
| c.907G>A | p.Ala303Thr | Missense | Uncertain significance |
| c.864C>G | p.Thr288= | Single nucleotide variant | Benign |
| c.807T>C | p.Phe269= | Single nucleotide variant | Benign/likely benign |
| c.761C>T | p.Ala254Val | Missense | Uncertain significance |
| c.741C>T | p.Tyr247= | Single nucleotide variant | Benign |
| c.662C>T | p.Ala221Val | Missense | Likely benign |
| c.580G>A | p.Ala194Thr | Missense | Uncertain significance |
| c.523_524delinsTT | p.Glu175Leu | Missense | Conflicting interpretations of pathogenicity |
| c.490-12C>T | Single nucleotide variant | Benign | |
| c.489+9A>G | Single nucleotide variant | Likely benign | |
| c.480C>T | p.Ser160= | Single nucleotide variant | Likely benign |
| c.479C>T | p.Ser160Phe | Missense | Uncertain significance |
| c.474G>A | p.Gln158= | Single nucleotide variant | Uncertain significance |
| c.343A>G | p.Ile115Val | Missense | Uncertain significance |
| c.325-12T>C | Single nucleotide variant | Benign | |
| c.279G>A | p.Gln93= | Single nucleotide variant | Likely benign |
| c.135C>A | p.Pro45= | Single nucleotide variant | Likely benign |
| c.108C>A | p.Ser36Arg | Missense | Conflicting interpretations of pathogenicity |
| c.8G>T | p.Arg3Leu | Missense | Uncertain significance |
In conclusion, the present study identified a novel mutation, NM_00188.5(CRYM): c.152C>T(Pro51Leu), which further strengthened the association between CRYM mutation and NSHL. Further functional studies regarding mutations in this clinical condition may assist in clarifying the pathogenic mechanism underlying familial sensorineural deafness.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AD designed the study; QL performed all of the experiments; XZ and JY carried out the audiology function examination; MW collected the clinical data, analyzed the data, and prepared the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Xinqiao Hospital (Chongqing, China) and the study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the proband and the remaining family members.
Patient consent for publication
All of the participants agreed to the publication of their results on clinical characteristics and genetic data with protection of their privacy.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sheffield AM, Smith RJH. The epidemiology of deafness. Cold Spring Harb Perspect Med. 2019;9(a033258) doi: 10.1101/cshperspect.a033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadha S, Cieza A. World Health Organization and its initiative for ear and hearing care. Otolaryngol Clin North Am. 2018;51:535–542. doi: 10.1016/j.otc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Yao GD, Li SX, Chen DL, Feng HQ, Zhao SB, Liu YJ, Guo LL, Yang ZM, Zhang XF, Sun CX, et al. Combination of hearing screening and genetic screening for deafness-susceptibility genes in newborns. Exp Ther Med. 2014;7:218–222. doi: 10.3892/etm.2013.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasak JM, Allen P, McVay T, Lewis D. Hearing loss: Diagnosis and management. Primary Care. 2014;41:19–31. doi: 10.1016/j.pop.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Momi SK, Wolber LE, Fabiane SM, MacGregor AJ, Williams FM. Genetic and environmental factors in age-related hearing impairment. Twin Res Hum Genet. 2015;18:383–392. doi: 10.1017/thg.2015.35. [DOI] [PubMed] [Google Scholar]
- 7.Kremer H. Hereditary hearing loss; about the known and the unknown. Hear Res. 2019;376:58–68. doi: 10.1016/j.heares.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Vona B, Müller M, Dofek S, Holderried M, Löwenheim H, Tropitzsch A. A big data perspective on the genomics of hearing loss. Laryngorhinootologie. 2019;98 (Suppl 1):S32–S81. doi: 10.1055/a-0803-6149. (In English, German) [DOI] [PubMed] [Google Scholar]
- 9.Petersen M, Willems P. Non-syndromic, autosomal-recessive deafness. Clin Genet. 2006;69:371–392. doi: 10.1111/j.1399-0004.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 10.Robertson NG, Lu L, Heller S, Merchant SN, Eavey RD, McKenna M, Nadol JB Jr, Miyamoto RT, Linthicum FH Jr, Lubianca Neto JF, et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat Genet. 1998;20:299–303. doi: 10.1038/3118. [DOI] [PubMed] [Google Scholar]
- 11.Khetarpal U. DFNA9 is a progressive audiovestibular dysfunction with a microfibrillar deposit in the inner ear. Laryngoscope. 2000;110:1379–1384. doi: 10.1097/00005537-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 12.JanssensdeVarebeke S, Topsakal V, Van Camp G, Van Rompaey V. A systematic review of hearing and vestibular function in carriers of the Pro51Ser mutation in the COCH gene. Eur Arch Otorhinolaryngol. 2019;276:1251–1262. doi: 10.1007/s00405-019-05322-x. [DOI] [PubMed] [Google Scholar]
- 13.Ku C, Cooper DN, Iacopetta B, Roukos DH. Integrating next-generation sequencing into the diagnostic testing of inherited cancer predisposition. Clin Genet. 2013;83:2–6. doi: 10.1111/cge.12028. [DOI] [PubMed] [Google Scholar]
- 14.Levy SE, Myers RM. Advancements in next-generation sequencing. Annu Rev Genomics Hum Genet. 2016;17:95–115. doi: 10.1146/annurev-genom-083115-022413. [DOI] [PubMed] [Google Scholar]
- 15.Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: Discovery to translation. Nat Rev Genet. 2013;14:681–691. doi: 10.1038/nrg3555. [DOI] [PubMed] [Google Scholar]
- 16.Mardis ER. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008;24:133–141. doi: 10.1016/j.tig.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Vona B, Müller T, Nanda I, Neuner C, Hofrichter MA, Schröder J, Bartsch O, Läßig A, Keilmann A, Schraven S, et al. Targeted next-generation sequencing of deafness genes in hearing-impaired individuals uncovers informative mutations. Genet Med. 2014;16:945–953. doi: 10.1038/gim.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T, Wei X, Chai Y, Li L, Wu H. Genetic etiology study of the non-syndromic deafness in Chinese Hans by targeted next-generation sequencing. Orphanet J Rare Dis. 2013;8(85) doi: 10.1186/1750-1172-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alford RL, Arnos KS, Fox M, Lin JW, Palmer CG, Pandya A, Rehm HL, Robin NH, Scott DA, Yoshinaga-Itano C, et al. American College of Medical Genetics and Genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet Med. 2014;16:347–355. doi: 10.1038/gim.2014.2. [DOI] [PubMed] [Google Scholar]
- 20.Mehraei G, Gallardo AP, Shinn-Cunningham BG, Dau T. Auditory brainstem response latency in forward masking, a marker of sensory deficits in listeners with normal hearing thresholds. Hear Res. 2017;346:34–44. doi: 10.1016/j.heares.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan D, Xiang G, Chai X, Qing J, Shang H, Zou B, Mittal R, Shen J, Smith RJ, Fan YS, et al. Screening of deafness-causing DNA variants that are common in patients of European ancestry using a microarray-based approach. PLoS One. 2017;12(e0169219) doi: 10.1371/journal.pone.0169219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nance WE. The genetics of deafness. Ment Retard Dev Disabil Res Rev. 2003;9:109–119. doi: 10.1002/mrdd.10067. [DOI] [PubMed] [Google Scholar]
- 23.DiStefano MT, Hemphill SE, Oza AM, Siegert RK, Grant AR, Hughes MY, Cushman BJ, Azaiez H, Booth KT, Chapin A, et al. ClinGen expert clinical validity curation of 164 hearing loss gene-disease pairs. Genet Med. 2019;21:2239–2247. doi: 10.1038/s41436-019-0487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kok YJ, Bom SJ, Brunt TM, Kemperman MH, van Beusekom E, van der Velde-Visser SD, Robertson NG, Morton CC, Huygen PL, Verhagen WI, et al. A Pro51Ser mutation in the COCH gene is associated with late onset autosomal dominant progressive sensorineural hearing loss with vestibular defects. Hum Mol Genet. 1999;8:361–366. doi: 10.1093/hmg/8.2.361. [DOI] [PubMed] [Google Scholar]
- 25.Kemperman MH, Bom SJ, Lemaire FX, Verhagen WI, Huygen PL, Cremers CW. DFNA9/COCH and its phenotype. Nat Genet. 2002;61:66–72. doi: 10.1159/000066806. [DOI] [PubMed] [Google Scholar]
- 26.Shearer AE, DeLuca AP, Hildebrand MS, Taylor KR, Gurrola J II, Scherer S, Scheetz TE, Smith RJ. Comprehensive genetic testing for hereditary hearing loss using massively parallel sequencing. Proc Natl Acad Sci USA. 2010;107:21104–21109. doi: 10.1073/pnas.1012989107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgert N, Smith RJ, Camp GV. Function and expression pattern of nonsyndromic deafness genes. Curr Mol Med. 2009;9:546–564. doi: 10.2174/156652409788488775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe S, Yamaguchi T, Usami SI. Application of deafness diagnostic screening panel based on deafness mutation/gene database using invader assay. Genet Test. 2007;11:333–340. doi: 10.1089/gte.2007.0002. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y, Gu MS, Suo F, Wang CX, Liu XH, Liu FM. Application of gene detection technique in the antenatal diagnosis of hereditary hearing loss. Eur Rev Med Pharmacol Sci. 2017;21:1452–1455. [PubMed] [Google Scholar]
- 30.He X, Li X, Guo Y, Zhao Y, Dong H, Dong J, Zhong L, Shi Z, Zhang Y, Soliman M, et al. Newborn screening of genetic mutations in common deafness genes with bloodspot-based gene chip array. Am J Audiol. 2018;27:57–66. doi: 10.1044/2017_AJA-17-0042. [DOI] [PubMed] [Google Scholar]
- 31.Atik T, Bademci G, Diaz-Horta O, Blanton SH, Tekin M. Whole-exome sequencing and its impact in hereditary hearing loss. Genet Res (Camb) 2015;97(e4) doi: 10.1017/S001667231500004X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrauwen I, Hasin-Brumshtein Y, Corneveaux JJ, Ohmen J, White C, Allen AN, Lusis AJ, Van Camp G, Huentelman MJ, Friedman RA. A comprehensive catalogue of the coding and non-coding transcripts of the human inner ear. Hear Res. 2016;333:266–274. doi: 10.1016/j.heares.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usami S, Takumi Y, Suzuki N, Oguchi T, Oshima A, Suzuki H, Kitoh R, Abe S, Sasaki A, Matsubara A. The localization of proteins encoded by CRYM, KIAA1199, UBA52, COL9A3, and COL9A1, genes highly expressed in the cochlea. Neuroscience. 2008;154:22–28. doi: 10.1016/j.neuroscience.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Mori JI, Hashizume K. Mu-crystallin, a NADPH-dependent T(3)-binding protein in cytosol. Trends Endocrinol Metab. 2007;18:286–289. doi: 10.1016/j.tem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Hommyo R, Suzuki SO, Abolhassani N, Hamasaki H, Shijo M, Maeda N, Honda H, Nakabeppu Y, Iwaki T. Expression of CRYM in different rat organs during development and its decreased expression in degenerating pyramidal tracts in amyotrophic lateral sclerosis. Neuropathology. 2018;38:247–259. doi: 10.1111/neup.12466. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Nishio SI, Ishii H, Sekido T, Takeshige K, Ohkubo Y, Hiwatashi D, Takeda T, Komatsu M. Possible roles of the AP-1 site in the cytosolic T3 binding protein promoter and insights into its physiological significance. Horm Metab Res. 2013;45:501–506. doi: 10.1055/s-0033-1337933. [DOI] [PubMed] [Google Scholar]
- 38.Hallen A, Cooper AJ, Smith JR, Jamie JF, Karuso P. Ketimine reductase/CRYM catalyzes reductive alkylamination of α-keto acids, confirming its function as an imine reductase. Amino Acids. 2015;47:2457–2461. doi: 10.1007/s00726-015-2044-8. [DOI] [PubMed] [Google Scholar]
- 39.Malinowska K, Cavarretta IT, Susani M, Wrulich OA, Uberall F, Kenner L, Culig Z. Identification of mu-crystallin as an androgen-regulated gene in human prostate cancer. Prostate. 2009;69:1109–1118. doi: 10.1002/pros.20956. [DOI] [PubMed] [Google Scholar]
- 40.Oshima A, Suzuki S, Takumi Y, Hashizume K, Abe S, Usami S. CRYM mutations cause deafness through thyroid hormone binding properties in the fibrocytes of the cochlea. J Med Genet. 2006;43(e25) doi: 10.1136/jmg.2005.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffith AJ, Szymko YM, Kaneshige M, Quiñónez RE, Kaneshige K, Heintz KA, Mastroianni MA, Kelley MW, Cheng SY. Knock-in mouse model for resistance to thyroid hormone (RTH): An RTH mutation in the thyroid hormone receptor beta gene disrupts cochlear morphogenesis. J Assoc Res Otolaryngol. 2002;3:279–288. doi: 10.1007/s101620010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wistow G. Lens crystallins: Gene recruitment and evolutionary dynamism. Trends Biochem Sci. 1993;18:301–306. doi: 10.1016/0968-0004(93)90041-k. [DOI] [PubMed] [Google Scholar]
- 43.Abe S, Katagiri T, Saito-Hisaminato A, Usami S, Inoue Y, Tsunoda T, Nakamura Y. Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet. 2003;72:73–82. doi: 10.1086/345398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.