Abstract
Background
Thoracic aortic aneurysm (TAA) is an uncommon disease with an incidence of 10.4 per 100,000 inhabitants. It occurs mainly in older individuals and is evenly distributed among both sexes. There are no signs or symptoms indicative of the presence of the disease. Progressive but unpredictable enlargement of the dilated aorta is the natural course of the disease and can lead to rupture. Open chest surgical repair using prosthetic graft interposition has been a conventional treatment for TAAs. Despite improvements in surgical procedures perioperative complications remain significant. The alternative option of thoracic endovascular aneurysm repair (TEVAR) is considered a less invasive and potentially safer technique, with lower morbidity and mortality compared with conventional treatment. Evidence is needed to support the use of TEVAR for these patients, rather than open surgery. This is an update of the review first published in 2009.
Objectives
This review aimed to assess the efficacy of TEVAR versus conventional open surgery in patients with thoracic aortic aneurysms.
Search methods
For this update the Cochrane Vascular Information Specialist searched the Specialised Register (last searched January 2016) and CENTRAL (2015, Issue 12).
Selection criteria
Randomised controlled trials in which patients with TAAs were randomly assigned to TEVAR or open surgical repair.
Data collection and analysis
Two review authors independently identified and evaluated potential trials for eligibility. Excluded studies were further checked by another author. We did not perform any statistical analyses as no randomised controlled trials were identified.
Main results
We did not find any published or unpublished randomised controlled trials comparing TEVAR with conventional open surgical repair for the treatment of thoracic aortic aneurysms.
Authors' conclusions
Stent grafting of the thoracic aorta is technically feasible and non‐randomised studies suggest reduction of early outcomes such as paraplegia, mortality and hospital stay. High quality randomised controlled trials assessing all clinically relevant outcomes including open‐conversion, aneurysm exclusion, endoleaks, and late mortality are needed.
Plain language summary
Thoracic endoscopic stent graft versus open surgery for thoracic aneurysm
Background
An aneurysm is a localised dilation or widening of an artery. Thoracic aneurysm is a relatively infrequent disease that affects both older men and women. The cause of thoracic aneurysm is unknown but the aneurysms generally do not cause symptoms. They are, however, likely to increase in size. Patients who do not receive surgical treatment at the time of diagnosis have a greater chance of dying from rupture of the aneurysm. Aneurysms greater than 5 cm carry a higher risk of bursting. Surgical repair of aneurysms requires general anaesthesia and opening of the chest wall to place an artificial graft in the area of the diseased vessel. This is associated with procedure‐related deaths and complications such as paraplegia, stroke, and renal failure and excludes some patients because of age and accompanying illnesses. Endovascular repair is a recently introduced, minimally invasive technique in which a stent is delivered through a blood vessel and fixed to the aneurysm. A seal forms between the stent and the vessel wall so that blood does not flow between the two. We searched for evidence of the effectiveness of endovascular repair compared with open surgical repair for thoracic aneurysms.
Key results
No randomised controlled trials were found in the medical literature (current until January 2016). Reports from non‐randomised studies suggest that endovascular repair is technically feasible and may reduce early negative outcomes including death and paraplegia. However, stent devices have late complications that are uncommon to open surgery (for example, development of leaks, graft migration, need for re‐intervention) and patients receiving stents may require frequent surveillance with computed tomography (CT) scans.
Quality of the evidence
In the absence of studies eligible for inclusion in the review it was not possible to assess the quality of the evidence.
Background
Description of the condition
Aneurysms are usually defined as a permanent localised dilation of an artery where the diameter is at least 50% greater than the expected normal size of the artery. The majority of aortic aneurysms are localised in the abdominal aorta, but the thoracic part of the aorta is not immune to vascular degeneration. Thoracic aortic aneurysms (TAAs) are usually associated with atherosclerosis. Due to the uncommon nature of the disease the data on natural history, prevalence, and mortality are limited and sometimes differ. A study published in 1982 reported an incidence of TAAs of 5.9 per 100,000 in a stable community over 30 years of observation (Bickerstaff 1982), while another survey documented an incidence of 10.4 per 100,000 from 1980 to 1994 (Clouse 1998). The two‐fold increase in the incidence of TAAs may be attributed to the advent of more sophisticated diagnostic instruments, such as computed tomography (CT), or the result of an aging population. However, according to some reports, TAAs occur mainly in older individuals and, contrary to abdominal aortic aneurysms which affect mainly males, the incidence of TAAs is evenly distributed among both sexes (Bickerstaff 1982; Clouse 1998).
Given the silent nature of TAAs it is difficult to identify signs and symptoms that warn of the presence of the disease. Most of the time such aneurysms are diagnosed by chance. When the diagnosis is made, identification of factors that might help us to recognise patients who are in most need of treatment, or who are at high risk of rupture, is necessary. While age, the presence of hypertension, chronic obstructive pulmonary disease, and renal failure have been identified as risk factors associated with aneurysmal disease and its rupture, the diameter of the aneurysm is the most important predictor to be considered when evaluating patients with TAAs (Griepp 1999). A number of retrospective studies have documented that the size of the aortic aneurysm is the major factor predicting rupture. Although small aneurysms may rupture, risk of rupture and mortality related to rupture become significant when the diameter of the affected aorta exceeds 5 cm (Cambria 1995; Coady 1997; Lobato 1998; Perko 1995). The five‐year mortality rate of aneurysms exceeding 6 cm varies from 38% to 64% (Coady 1997; Joyce 1964). In addition, the rate of increase in the size of aortic aneurysms is part of the natural course of the disease and is an another indicator that predicts rupture. Indeed, TAAs may have an expansion rate of between 0.2 cm and 0.4 cm per year. Growth rate is directly related to the initial size of the diseased aorta (Cambria 1995; Coady 1997; Hirose 1993). Some authors estimate that each 1 cm increase in the size of the thoracic aneurysm is translated into a further increase in the probability of rupture by a factor of 1.9, compared with a factor of 1.5 observed for abdominal aneurysms (Juvonen 1997).
Data from several retrospective surveys indicate that for patients who did not receive surgery at the time of diagnosis, rupture was the most common cause of death, with death rates ranging from 42% to 74% (Bickerstaff 1982; Crawford 1986; Perko 1995). The five‐year survival rate was 39% for patients with TAAs and 23% for those with thoracoabdominal aneurysm (Perko 1995). When rupture occurs, patients mostly die within six hours and, although 46% of patients arrive alive at the hospital, the overall mortality rate of ruptured TAAs reaches 97% (Johansson 1995).
Description of the intervention
The high mortality of aneurysmal disease has lead many to suggest surgical repair of the dilated aorta, even though the anatomical morphology of the thoracic aorta together with the characteristics of patients affected by TAAs pose a considerable challenge to surgeons. Open surgical repair using prosthetic graft interposition is the conventional treatment for TAAs mainly because of its feasibility and effectiveness in excluding the degenerated aorta from the systemic circulation. Open surgical repair of TAAs is associated with significant perioperative complications including 30‐day mortality and paraplegia, with rates of 4.8% and 4.6% respectively. Stroke and renal failure are also important complications to be considered (Coselli 2000; Deeb 1995; LeMaire 2003). Despite noteworthy improvements in surgical procedures with extracorporeal circulation for peripheral organ preservation, the development of different techniques for spinal cord protection, together with the refinement of prosthetic grafts for aortic repair, morbidity and mortality rates remain high (Hamerlijnck 1989; Huynh 2002; Thurnher 2002).
How the intervention might work
In view of these complexities, and the fact that many high risk patients were regularly excluded from open surgical repair, a less invasive and potentially safer technique became imperative. Thus an alternative for open surgical repair for aortic aneurysm has become the performance of endovascular repair using stent grafts. The aim of this technique is to reach the target site by performing an access through a remote vessel to deliver the stent, secure endograft fixation, and allow the formation of a haemostatic seal between the graft and the vessel wall. The endovascular repair (EVAR) technique has been successfully used in the treatment of abdominal aortic aneurysms and with respect to conventional open surgical repair has been shown to reduce early post‐operative complications and death (Greenhalgh 2004; Prinssen 2004). The success of the stent‐graft placement performed in abdominal aortic aneurysms prompted experts to translate the technique to thoracic aneurysm repair. Dake was the first to report the use of stent graft in 13 patients with TAAs with positive outcomes over 11 months of observation (Dake 1994).
Identification of the best vascular access through which to deliver the stent is important, as well as performing imaging studies that help assess the best site for the insertion of the stent‐graft delivery system. Non‐surgical repair may be an attractive therapeutic option for patients with TAAs. According to some authors the morbidity and mortality associated with EVAR of thoracic aneurysm (TEVAR) appear to be less than with conventional treatment. In addition, while mid‐term survival seems satisfactory in patients receiving endovascular treatment the long‐term outcomes for high risk patients are disappointing, probably due to co‐existing disease (Demers 2004; Ehrlich 1998; Greenberg 2000; Marcheix 2006; Mitchell 1999).
Why it is important to do this review
Thus, convincing evidence that TEVAR is better than open surgical treatment for TAAs is needed. Potential advantages of TEVAR include reduction in the rate of paraplegia; reduced time under anaesthesia; avoidance of arterial cross‐clamping, renal failure, and cardiovascular complications; reduced length of hospital stay, and particularly a reduction in the length of stay in an intensive care unit.
Objectives
This review aimed to assess the efficacy of thoracic endovascular aneurysm repair (TEVAR) versus conventional open surgery in patients with thoracic aortic aneurysms (TAA).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) evaluating TEVAR compared with conventional open surgical repair.
Types of participants
All asymptomatic patients in whom a thoracic aortic aneurysm was diagnosed by computed tomography (CT), magnetic resonance angiography (MRA), or conventional angiography. Only studies reporting the size of TAAs were eligible for consideration. Mycotic aneurysms (aneurysms caused by an infection of the arterial wall or when a pre‐existing aneurysm has become secondarily infected), acute or chronic dissections, and all patients with connective tissue disorders were excluded.
Types of interventions
All types of endovascular devices were considered when compared with conventional open surgical treatment.
Types of outcome measures
Primary outcomes
short‐term mortality rates (30 days, or in hospital, i.e. procedure related);
aneurysm exclusion (no flow in the aneurysmal sac or extravasations (discharge of blood from a vessel to the tissues) beyond the sac) on follow‐up imaging 30 days after the procedure;
major complications (haemorrhage, open conversion, myocardial infarction, stroke, renal failure, respiratory failure, spinal chord ischaemia, bowel ischaemia, lower limb ischaemia).
Secondary outcomes
minor complications;
long‐term complications and mortality (re‐intervention rates for problems related to the TAA or its treatment were sought where possible, as was cause of death with or without re‐intervention, i.e. device related);
quality of life (based on validated questionnaires).
Search methods for identification of studies
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (January 2016). In addition the CIS searched the Cochrane Register of Studies (CRS) http://www.metaxis.com/CRSWeb/Index.asp (CENTRAL (2015, Issue 12)). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in theCochrane Library (www.cochranelibrary.com).
Searching other resources
The reference lists of all relevant studies found were screened.
Data collection and analysis
Selection of studies
Two authors (IA and RC) assessed all studies identified from the literature search. Disagreements were resolved through discussion with the review team and the agreed arbitrator (AM).
Data extraction and management
Where eligible studies were identified, we planned for two review authors (IA and CR) to independently extract data. We planned to use a standard data extraction form to extract the following information: characteristics of the study (design, method of randomisation, blinding of outcome evaluators, and balance of prognostic factors (age, sex, size of the aneurysm)); participants; interventions; outcomes (types of outcome measures, adverse events). We planned to contact the authors of trials when these information were incomplete. We planned to resolve any disagreement by discussion.
Assessment of risk of bias in included studies
For the assessment of study quality, we planned to use the risk of bias approach for Cochrane reviews (Higgins 2011) based on the assessment of the following domains of each included study: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting bias, and other potential sources of bias (Higgins 2011).
We planned for two authors to independently evaluate the risk of bias of the included studies. Any disagreements were to be resolved by discussion or by a third review author.
Measures of treatment effect
We planned to carry‐out meta‐analyses of primary and secondary outcomes. For the analysis of dichotomous outcomes, we planned to use risk ratios with 95% confidence intervals (CI); for the analysis of continuous outcomes, we planned to use the mean difference (MD) or standardised mean difference (SMD) and 95% CI.
Unit of analysis issues
The unit of analysis was planned to be each participant recruited into the trials.
Dealing with missing data
In the event of missing data, we planned to contact the corresponding authors to try to obtain the relevant information.
We will not use any imputation methods. We preferred the intention‐to‐treat data where this approach was available. Where intention‐to‐treat data are not available, we planned to conduct analyses using data of available cases.
Assessment of heterogeneity
We planned to consider whether the clinical and methodological characteristics of the studies were sufficiently homogeneous to pool data in a meta‐analysis. We planned to quantify inconsistency among the pooled estimates using the I2 statistic.
We planned to consider a Chi2 test with a P value of 0.10 to be significant, and interpret the I2 statistic as: 0% to 40% unimportant heterogeneity; 30% to 60% moderate heterogeneity; 50% to 90% substantial heterogeneity; and 75% to 100% considerable heterogeneity.
Assessment of reporting biases
Where there are more than 10 studies that can be combined into a meta‐analysis, we planned to use a funnel plot to examine for small‐study effects, which may indicate publication bias.
Data synthesis
We planned to use the fixed‐effect model to pool the data for each outcome. Where important or substantial heterogeneity (for example I2 greater than 50%) was detected, we planned to meta‐analyse data of the treatment effect using a random‐effects model (with two or more studies).
Subgroup analysis and investigation of heterogeneity
Where sufficient data are available, we plan to perform subgroup analyses based on several participant characteristics such as sex, the size of the aneurysm, its anatomical localisation (ascending, descending, or thoracoabdominal) and condition of patients (high risk versus low risk), and characteristics of the intervention (such as fenestrated or branched endografts bifurcated stent graft).
Sensitivity analysis
Where a sufficient number of studies was available, we planned to perform sensitivity analyses to assess the consistency and robustness of the results of the meta‐analyses based on the following scenarios: including only studies with low risk of selection bias (sequence generation and allocation concealment) (Wood 2008); including only trials with low risk of detection bias (blinding of outcome assessor) (Savovic 2012); including only trials with low risk of attrition of bias; including only trials that did not deviate from intention‐to‐treat analysis (Abraha 2015).
Summary of findings
We planned to present the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the primary outcomes (Types of outcome measures) in a 'Summary of findings' table, according to the GRADE principles as described by Higgins 2011 and Atkins 2004. Since we planned to assess different comparisons of interventions, we planned to develop a 'Summary of findings' table for each comparison using the GRADEpro software (GRADEproGDT), to assist in the preparation of the 'Summary of findings' table.
Results
Description of studies
See Figure 1.
1.
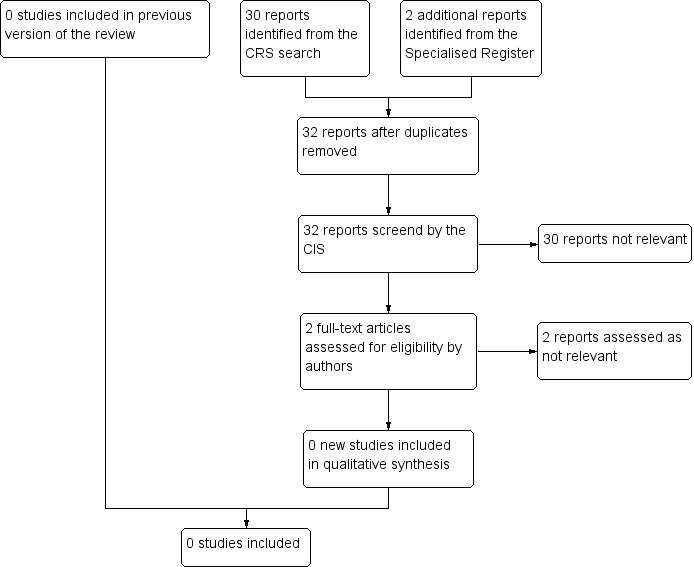
Study flow diagram.
Results of the search
No studies were identified that met any of the inclusion criteria. A number of case series, cohort studies, and controlled studies with historic controls were found. These were excluded because of lack of randomisation (seeCharacteristics of excluded studies).
Risk of bias in included studies
It was not possible to review methodological quality in the absence of studies eligible for inclusion in the review.
Effects of interventions
No published or unpublished randomised controlled trials were found comparing thoracic endovascular aneurysm repair (TEVAR) with conventional open surgical repair for the treatment of thoracic aortic aneurysms (TAAs).
Discussion
Summary of main results
Thoracic surgery for the treatment of aneurysms is performed in a complex setting and entails general anaesthesia, thoracotomy with extensive surgical resection, left lung collapse, and aortic cross‐clamping. As a result, important postoperative complications such as haemorrhage, spinal cord injury, stroke, and renal insufficiency must be considered. Distal aortic perfusion, cerebrospinal drainage, and intercostal artery implantation are some of the advances made to avoid complications associated with thoracic surgery but morbidity remains high. Moreover, factors such as the age of the patient and the presence of serious co‐morbidities such as coronary heart disease, chronic lung disease, diabetes, and hypertension may exclude a number of patients from receiving surgical treatment.
The advent of endovascular stent interposition, first described by Parodi (Parodi 1991) for abdominal aneurysm and later by Dake (Dake 1994) for the thoracic tract, has generated great interest among thoracic surgeons. This is mainly because an endovascular stent is considered a minimally invasive device and therefore, a good alternative for patients affected by thoracic aortic aneurysms (TAAs) who are not suitable for open surgical treatment.
This review documents that there are no published or pending randomised controlled trials that compare thoracic endovascular aneurysm repair (TEVAR) with open surgical treatment for patients with TAAs, assessing early and late mortality and major complications. Most of the current information relating to endovascular intervention comes from reports of case series, observational studies, or registry data with differing presentations.
Overall completeness and applicability of evidence
There is currently no evidence from randomised controlled trials.
Quality of the evidence
It was not possible to review methodological quality or the quality of the evidence in the absence of studies eligible for inclusion in the review.
Potential biases in the review process
Potential biases in the review process were minimised by performing extensive literature search.
Agreements and disagreements with other studies or reviews
Although no RCTs comparing TEVAR with open surgical treatment for patients with TAAs were identified, at least two controlled clinical trials can be mentioned. The first study, published in 2002, compared 19 patients who received endovascular stents with an historic cohort of 10 patients who received open surgical repair. Endovascular deployment was successful in all but one patient and early outcomes such as mean length of intensive care unit stay and hospital stay were better in the TEVAR group. The study, however, was not randomised and follow up was limited (Najibi 2002).
The second trial, published in 2005, started as a prospective non‐randomised multicenter phase II study. Promising results in terms of technical success and positive early outcomes prompted the FDA to approve the Gore TAG thoracic endoprosthesis (Makaroun 2005). At a later stage the study became a controlled trial in which the data of the 142 patients were compared with 94 controls (Bavaria 2007). According to the final analysis of the trial, mortality at 30 days was better in the endograft group, as well as other outcomes such as paraplegia and the length of hospital stay. However, the two‐year survival rate was similar in the two groups. The significant limitations of the trial were that it was not a randomised trial; at least half of the control group was historical, which may augment further the selection bias; and symptomatic aneurysms were significantly higher in the open surgical cohort (38% versus 21%). In addition, data on aortic characteristics were unavailable in many of the control patients and the follow up was limited to two years, within which time 17% of the patients had exclusion of their aneurysm of a size greater than 0.5 cm, and at least 17% of the patients were lost to follow up (Bavaria 2007).
Despite the reported early advantages, stent devices for thoracic aneurysm have late complications that are uncommon with open surgery. Such complications include endoleaks, graft migration, stent fractures, and aneurysm‐related death. Type I or attachment site endoleak is the most frequent complication and its incidence may reach 30% of cases (Neuhauser 2005). Usually Type I endoleaks require additional stent‐graft deployment for sealing or spot coiling, but sometimes such endoleaks may close spontaneously due to the formation of spontaneous thrombosis of the aortic false lumen and not require any further treatment. While resolution may occur within one week to eight months, endoleaks may progress with dilatation of the false lumen necessitating close follow up, supplementary procedures, or even open surgical treatment.
The available controlled clinical trials on TEVAR efficacy had promising results in terms of early mortality and reduction in postoperative complications but they failed to give generalisable conclusions, not only because of their internal low methodological quality but also in that high risk patients were not the target population. This reduces the original expectation that stent grafts could be an alternative for patients excluded from open surgical treatment because of their high risk status. The recent report of the Society of Thoracic Surgeons in fact states that the indication for stent grafting of a thoracic aortic aneurysm should be based on a predicted operative risk that is clearly lower than the risk of either conventional open repair or optimal medical management (Svensson 2008). To this it must be added that patients who receive TEVAR will be in need of frequent surveillance CT scans after the intervention is performed and at a later stage they will probably need surgical re‐intervention.
Although graft stenting for TAAs is technically feasible and may reduce the number and of severity of early outcomes, high quality randomised controlled trials assessing all clinically relevant outcomes, including early and late mortality, open conversion, aneurysm exclusion, endoleaks, are needed.
Authors' conclusions
Implications for practice.
Technically, endovascular repair of thoracic aneurysms may be a good alternative to open surgical repair. However, its benefit cannot be established as there are no published randomised controlled trials. The available information comes from non‐randomised studies, which show benefit in terms of early mortality and complications such as paraplegia. Although such evidence may suggest that endovascular repair can be appropriate in selected patients, high quality studies are needed to produce generalisable conclusions.
Implications for research.
High quality randomised controlled trials evaluating thoracic endovascular aneurysm repair (TEVAR) for thoracic aortic aneurysms are needed. These prospective trials should have adequate follow up, enough to evaluate the durability of endovascular treatment in terms of endoleak rate, re‐intervention rate, open‐conversion rate, and rupture‐free survival. In addition, clinically relevant outcomes including early and late mortality, major complications, and hospital and intensive care unit stay must be considered. However, it would probably be extremely difficult to perform a randomised trial given the current stage of surgical endovascular practice.
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2016 | New citation required but conclusions have not changed | Searches of CENTRAL and Specialised Register were rerun. No randomised controlled studies were found. Minor edits made. Conclusions not changed. |
| 19 January 2016 | New search has been performed | Searches of CENTRAL and Specialised Register were rerun. No randomised controlled studies were found. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 29 September 2008 | Amended | Converted to new review format. |
Appendices
Appendix 1. CRS search strategy
| Search run on Tue Jan 19 2016 | ||
| #1 | MESH DESCRIPTOR Aortic Aneurysm, Thoracic EXPLODE ALL TREES | 52 |
| #2 | MESH DESCRIPTOR Aorta, Thoracic | 139 |
| #3 | (thorac* near aneurysm):TI,AB,KY | 68 |
| #4 | (thoracic near3 (balloon* or dilat* or bulg*)):TI,AB,KY | 7 |
| #5 | TAA:TI,AB,KY | 134 |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 | 343 |
| #7 | MESH DESCRIPTOR Angioplasty EXPLODE ALL TREES | 4101 |
| #8 | (angioplas* or percutan* or PTA or venoplasty):TI,AB,KY | 12137 |
| #9 | (recanali* or revascular*):TI,AB,KY | 6550 |
| #10 | dilat*:TI,AB,KY | 6912 |
| #11 | (balloon or baloon):TI,AB,KY | 6481 |
| #12 | MESH DESCRIPTOR Endovascular Procedures | 168 |
| #13 | endovascular:TI,AB,KY | 1171 |
| #14 | MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES | 406 |
| #15 | MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES | 389 |
| #16 | MESH DESCRIPTOR Stents EXPLODE ALL TREES | 3123 |
| #17 | endoluminal:TI,AB,KY | 124 |
| #18 | (stent*):TI,AB,KY | 6755 |
| #19 | (endostent*):TI,AB,KY | 1 |
| #20 | (graft*):TI,AB,KY | 15917 |
| #21 | (endograft*):TI,AB,KY | 57 |
| #22 | (endoprosthe*):TI,AB,KY | 230 |
| #23 | (EVAR):TI,AB,KY | 98 |
| #24 | (EVRAR):TI,AB,KY | 0 |
| #25 | (TEVAR):TI,AB,KY | 24 |
| #26 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | 40372 |
| #27 | #6 AND #26 | 80 |
| #28 | * NOT SR‐PVD:CC AND 28/03/2013 TO 29/02/2016:DL | 240623 |
| #31 | #27 AND #28 | 30 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Appoo 2006 | Case series of 99 patients with descending thoracic aneurysms treated with first or second generation endografts (1999 ‐ 2005) |
| Azizzadeh 2003 | Case‐control study. Analysis of 18 patients with delayed neurologic deficit after open cardiovascular surgery for thoracoabdominal or thoracic aortic repair |
| Bavaria 2007 | Multicentre non‐randomised controlled trial of 140 consecutive patients with descending thoracic aneurysms treated with EVAR compared with 94 retrospective case series treated with open cardiovascular surgery for thoracoabdominal or thoracic aortic repair |
| Cambria 2002 | Retrospective data on 28 patients treated with EVAR for thoracoabdominal aneurysm |
| Dake 1998 | Prospective uncontrolled clinical trial of 103 patients treated with EVAR for thoracoabdominal aneurysms |
| Fattori 2006 | Register data of 457 consecutive patients (113 emergency and 334 elective cases) who received an endovascular Talent device |
| Hassoun 2005 | Ongoing, prospective non‐randomised phase II trial |
| Hassoun 2006 | Retrospective clinical trial involving 139 patients who underwent endovascular descending thoracic aortic repair |
| Ince 2003 | Retrospective analysis of six patients treated with EVAR for aortic aneurysm after previous coarctation surgery |
| Makaroun 2005 | Prospective non‐randomised uncontrolled trial of 139 patients treated with Gore‐TAG device |
| Mitchell 1999 | Prospective consecutive treatment of 103 patients with home‐made first‐generation device |
| Najibi 2002 | Non‐randomised controlled trial of 19 patients receiving endovascular treatment and 10 historic controls receiving open surgical repair |
| Wheatley 2006 | Non‐randomised multi‐institutional analysis of 158 consecutive patients treated with Gore‐TAG device |
Differences between protocol and review
Economic analysis was removed as a secondary outcome for this 2016 update as Cochrane Vascular does not have the expertise to carry out complete economic evaluations. Economic data available from studies identified for future updates will be included in the discussion section.
Contributions of authors
Dr Iosief Abraha: selected and assessed trials for possible inclusion; and wrote the majority of the text of this review Dr Carlo Romagnoli: prepared the abstract and approved the final version Dr Roberto Cirocchi: selected and assessed trials for possible inclusion; and approved the final version Dr Alessandro Montedori: resolved any differences in the assessment of trials, helped in writing the 'Description of studies', wrote the 'Plain language summary'; and approved the final version
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
Dr Iosief Abraha: none known Dr Carlo Romagnoli: none known Dr Roberto Cirocchi: none known Dr Alessandro Montedori: none known
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Appoo 2006 {published data only}
- Appoo JJ, Moser WG, Fairman RM, Cornelius KF, Pochettino A, Woo EY, et al. Thoracic aortic stent grafting: improving results with newer generation investigational devices. Journal of Thoracic and Cardiovascular Surgery 2006;131(5):1087‐94. [DOI] [PubMed] [Google Scholar]
Azizzadeh 2003 {published data only}
- Azizzadeh A, Huynh TT, Miller CC 3rd, Estrera AL, Porat EE, Sheinbaum R, et al. Postoperative risk factors for delayed neurologic deficit after thoracic and thoracoabdominal aortic aneurysm repair: a case‐control study. Journal of Vascular Surgery 2003;37(4):750‐4. [DOI] [PubMed] [Google Scholar]
Bavaria 2007 {published data only}
- Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low‐risk patients: a multicenter comparative trial. Journal of Thoracic and Cardiovascular Surgery 2007;133(2):369‐77. [DOI] [PubMed] [Google Scholar]
Cambria 2002 {published data only}
- Cambria RP, Brewster DC, Lauterbach SR, Kaufman JL, Geller S, Fan C‐M, et al. Evolving experience with thoracic aortic stent graft repair. Journal of Vascular Surgery 2002;35(6):1129‐36. [DOI] [PubMed] [Google Scholar]
Dake 1998 {published data only}
- Dake MD, Miller DC, Mitchell RS, Semba CP, Moore KA, Sakai T. The "first generation" of endovascular stent‐grafts for patients with aneurysms of the descending thoracic aorta. Journal of Thoracic and Cardiovascular Surgery 1998;116(5):689‐703; discussion ‐4. [DOI] [PubMed] [Google Scholar]
Fattori 2006 {published data only}
- Fattori R, Nienaber CA, Rousseau H, Beregi J‐P, Heijmen R, Grabenwoger M, et al. Results of endovascular repair of the thoracic aorta with the Talent Thoracic stent graft: the Talent Thoracic Retrospective Registry. Journal of Thoracic and Cardiovascular Surgery 2006;132(2):332‐9. [DOI] [PubMed] [Google Scholar]
Hassoun 2005 {published data only}
- Hassoun HT, Dake MD, Svensson LG, Greenberg RK, Cambria RP, Moore RD, et al. Multi‐institutional pivotal trial of the Zenith TX2 thoracic aortic stent‐graft for treatment of descending thoracic aortic aneurysms: clinical study design. Perspectives in Vascular Surgery and Endovascular Therapy 2005;17(3):255‐64. [DOI] [PubMed] [Google Scholar]
Hassoun 2006 {published data only}
- Hassoun HT, Mitchell RS, Makaroun MS, Whiting AJ, Cardeira KR, Matsumura JS. Aortic neck morphology after endovascular repair of descending thoracic aortic aneurysms. Journal of Vascular Surgery 2006;43(1):26‐31. [DOI] [PubMed] [Google Scholar]
Ince 2003 {published data only}
- Ince H, Petzsch M, Rehders T, Kische S, Korber T, Weber F, et al. Percutaneous endovascular repair of aneurysm after previous coarctation surgery [see comment]. Circulation 2003;108(24):2967‐70. [DOI] [PubMed] [Google Scholar]
Makaroun 2005 {published data only}
- Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. Journal of Vascular Surgery 2005;41(1):1‐9. [DOI] [PubMed] [Google Scholar]
Mitchell 1999 {published data only}
- Mitchell RS, Miller DC, Dake MD, Semba CP, Moore KA, Sakai T. Thoracic aortic aneurysm repair with an endovascular stent graft: the "first generation". Annals of Thoracic Surgery 1999;67(6):1971‐4; discussion 9‐80. [DOI] [PubMed] [Google Scholar]
Najibi 2002 {published data only}
- Najibi S, Terramani TT, Weiss VJ, Mac Donald MJ, Lin PH, Redd DC, et al. Endoluminal versus open treatment of descending thoracic aortic aneurysms. Journal of Vascular Surgery 2002;36(4):732‐7. [PubMed] [Google Scholar]
Wheatley 2006 {published data only}
- Wheatley GH 3rd, Gurbuz AT, Rodriguez‐Lopez JA, Ramaiah VG, Olsen D, Williams J, et al. Midterm outcome in 158 consecutive Gore TAG thoracic endoprostheses: single center experience. Annals of Thoracic Surgery 2006;81(5):1570‐7; discussion 7. [DOI] [PubMed] [Google Scholar]
Additional references
Abraha 2015
- Abraha I, Cherubini A, Cozzolino F, Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta‐epidemiological study. British Medical Journal (Clinical research ed.) 2015;350:h2445. [PUBMED: 26016488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bickerstaff 1982
- Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Peenen HJ, Cherry KJ, et al. Thoracic aortic aneurysms: a population‐based study. Surgery 1982;92(6):1103‐8. [PubMed] [Google Scholar]
Cambria 1995
- Cambria RA, Gloviczki P, Stanson AW, Cherry KJ Jr, Bower TC, Hallett JW Jr, et al. Outcome and expansion rate of 57 thoracoabdominal aortic aneurysms managed nonoperatively. American Journal of Surgery 1995;170(2):213‐7. [DOI] [PubMed] [Google Scholar]
Clouse 1998
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population‐based study. JAMA 1998;280(22):1926‐9. [DOI] [PubMed] [Google Scholar]
Coady 1997
- Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms?. Journal of Thoracic and Cardiovascular Surgery 1997;113(3):476‐91; discussion 489‐91. [DOI] [PubMed] [Google Scholar]
Coselli 2000
- Coselli JS, LeMaire SA, Miller CC, 3rd, Schmittling ZC, Koksoy C, Pagan J, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Annals of Thoracic Surgery 2000;69(2):409‐14. [DOI] [PubMed] [Google Scholar]
Crawford 1986
- Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. Journal of Vascular Surgery 1986;3(4):578‐82. [DOI] [PubMed] [Google Scholar]
Dake 1994
- Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent‐grafts for the treatment of descending thoracic aortic aneurysms. New England Journal of Medicine 1994;331(26):1729‐34. [DOI] [PubMed] [Google Scholar]
Deeb 1995
- Deeb GM, Jenkins E, Bolling SF, Brunsting LA, Williams DM, Quint LE, et al. Retrograde cerebral perfusion during hypothermic circulatory arrest reduces neurologic morbidity. Journal of Thoracic and Cardiovascular Surgery 1995;109(2):259‐68. [DOI] [PubMed] [Google Scholar]
Demers 2004
- Demers P, Miller DC, Mitchell RS, Kee ST, Sze D, Razavi MK, et al. Midterm results of endovascular repair of descending thoracic aortic aneurysms with first‐generation stent grafts. Journal of Thoracic and Cardiovascular Surgery 2004;127(3):664‐73. [DOI] [PubMed] [Google Scholar]
Ehrlich 1998
- Ehrlich M, Grabenwoeger M, Cartes‐Zumelzu F, Grimm M, Petzl D, Lammer J, et al. Endovascular stent graft repair for aneurysms on the descending thoracic aorta. Annals of Thoracic Surgery 1998;66(1):19‐24; discussion 24‐5. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- Available from www.gradepro.org. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Greenberg 2000
- Greenberg R, Resch T, Nyman U, Lindh M, Brunkwall J, Brunkwall P, et al. Endovascular repair of descending thoracic aortic aneurysms: an early experience with intermediate‐term follow‐up. Journal of Vascular Surgery 2000;31(1 Pt 1):147‐56. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2004
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30‐day operative mortality results: randomised controlled trial. Lancet 2004;364(9437):843‐8. [DOI] [PubMed] [Google Scholar]
Griepp 1999
- Griepp RB, Ergin MA, Galla JD, Lansman SL, McCullough JN, Nguyen KH, et al. Natural history of descending thoracic and thoracoabdominal aneurysms. Annals of Thoracic Surgery 1999;67(6):1927‐30; discussion 1953‐8. [DOI] [PubMed] [Google Scholar]
Hamerlijnck 1989
- Hamerlijnck RP, Rutsaert RR, Geest R, Brutel de la Riviere A, Defauw JJ, Vermeulen FE. Surgical correction of descending thoracic aortic aneurysms under simple aortic cross‐clamping. Journal of Vascular Surgery 1989;9(4):568‐73. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirose 1993
- Hirose Y, Hamada S, Takamiya M, Imakita S, Naito H. Growth rates of aortic aneurysms as a risk factor in rupture: an evaluation with CT. Nippon Igaku Hoshasen Gakkai Zasshi 1993;53(6):635‐40. [PubMed] [Google Scholar]
Huynh 2002
- Huynh TT, Miller CC 3rd, Estrera AL, Porat EE, Safi HJ. Thoracoabdominal and descending thoracic aortic aneurysm surgery in patients aged 79 years or older. Journal of Vascular Surgery 2002;36(3):469‐75. [DOI] [PubMed] [Google Scholar]
Johansson 1995
- Johansson G, Markstrom U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. Journal of Vascular Surgery 1995;21(6):985‐8. [DOI] [PubMed] [Google Scholar]
Joyce 1964
- Joyce JW, Fairbairn JF II, Kincaid OW, Juergen JL. Aneurysms of the thoracic aorta. A clinical study with special reference to prognosis. Circulation 1964;29(2):176‐81. [PubMed] [Google Scholar]
Juvonen 1997
- Juvonen T, Ergin MA, Galla JD, Lansman SL, Nguyen KH, McCullough JN, et al. Prospective study of the natural history of thoracic aortic aneurysms. Annals of Thoracic Surgery 1997;63(6):1533‐45. [DOI] [PubMed] [Google Scholar]
LeMaire 2003
- LeMaire SA, Miller CC 3rd, Conklin LD, Schmittling ZC, Coselli JS. Estimating group mortality and paraplegia rates after thoracoabdominal aortic aneurysm repair. Annals of Thoracic Surgery 2003;75(2):508‐13. [DOI] [PubMed] [Google Scholar]
Lobato 1998
- Lobato AC, Puech‐Leao P. Predictive factors for rupture of thoracoabdominal aortic aneurysm. Journal of Vascular Surgery 1998;27(3):446‐53. [DOI] [PubMed] [Google Scholar]
Marcheix 2006
- Marcheix B, Dambrin C, Bolduc J‐P, Arnaud C, Cron C, Hollington L, et al. Midterm results of endovascular treatment of atherosclerotic aneurysms of the descending thoracic aorta. Journal of Thoracic and Cardiovascular Surgery 2006;132(5):1030‐6. [DOI] [PubMed] [Google Scholar]
Neuhauser 2005
- Neuhauser B, Czermak BV, Fish J, Perkmann R, Jaschke W, Chemelli A, et al. Type A dissection following endovascular thoracic aortic stent‐graft repair. Journal of Endovascular Therapy 2005;12(1):74‐81. [DOI] [PubMed] [Google Scholar]
Parodi 1991
- Parodi JC, Palmaz JC, Barone HD, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Annals of Vascular Surgery 1991;5(6):491‐9. [DOI] [PubMed] [Google Scholar]
Perko 1995
- Perko MJ, Norgaard M, Herzog TM, Olsen PS, Schroeder TV, Pettersson G. Unoperated aortic aneurysm: a survey of 170 patients. Annals of Thoracic Surgery 1995;59(5):1204‐9. [DOI] [PubMed] [Google Scholar]
Prinssen 2004
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. New England Journal of Medicine 2004;351(16):1607‐18. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health technology assessment (Winchester, England) 2012;16(35):1‐82. [PUBMED: 22989478] [DOI] [PubMed] [Google Scholar]
Svensson 2008
- Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent‐grafts. Annals of Thoracic Surgery 2008;85 Suppl 1:1‐41. [DOI] [PubMed] [Google Scholar]
Thurnher 2002
- Thurnher SA, Grabenwoger M. Endovascular treatment of thoracic aortic aneurysms: a review. European Radiology 2002;12(6):1370‐87. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. British Medical Journal (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Abraha 2007
- Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006796] [DOI] [Google Scholar]
Abraha 2009
- Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006796.pub2] [DOI] [PubMed] [Google Scholar]
Abraha 2013
- Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD006796.pub3] [DOI] [PubMed] [Google Scholar]


