Abstract
Background
Conversion and dissociative disorders are conditions where people experience unusual neurological symptoms or changes in awareness or identity. However, symptoms and clinical signs cannot be explained by a neurological disease or other medical condition. Instead, a psychological stressor or trauma is often present. The symptoms are real and can cause significant distress or problems with functioning in everyday life for the people experiencing them.
Objectives
To assess the beneficial and harmful effects of psychosocial interventions of conversion and dissociative disorders in adults.
Search methods
We conducted database searches between 16 July and 16 August 2019. We searched Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and eight other databases, together with reference checking, citation searching and contact with study authors to identify additional studies.
Selection criteria
We included all randomised controlled trials that compared psychosocial interventions for conversion and dissociative disorders with standard care, wait list or other interventions (pharmaceutical, somatic or psychosocial).
Data collection and analysis
We selected, quality assessed and extracted data from the identified studies. Two review authors independently performed all tasks. We used standard Cochrane methodology. For continuous data, we calculated mean differences (MD) and standardised mean differences (SMD) with 95% confidence interval (CI). For dichotomous outcomes, we calculated risk ratio (RR) with 95% CI. We assessed and downgraded the evidence according to the GRADE system for risk of bias, imprecision, indirectness, inconsistency and publication bias.
Main results
We included 17 studies (16 with parallel‐group designs and one with a cross‐over design), with 894 participants aged 18 to 80 years (female:male ratio 3:1).
The data were separated into 12 comparisons based on the different interventions and comparators. Studies were pooled into the same comparison when identical interventions and comparisons were evaluated. The certainty of the evidence was downgraded as a consequence of potential risk of bias, as many of the studies had unclear or inadequate allocation concealment. Further downgrading was performed due to imprecision, few participants and inconsistency.
There were 12 comparisons for the primary outcome of reduction in physical signs.
Inpatient paradoxical intention therapy compared with outpatient diazepam: inpatient paradoxical intention therapy did not reduce conversive symptoms compared with outpatient diazepam at the end of treatment (RR 1.44, 95% CI 0.91 to 2.28; 1 study, 30 participants; P = 0.12; very low‐quality evidence).
Inpatient treatment programme plus hypnosis compared with inpatient treatment programme: inpatient treatment programme plus hypnosis did not reduce severity of impairment compared with inpatient treatment programme at the end of treatment (MD –0.49 (negative value better), 95% CI –1.28 to 0.30; 1 study, 45 participants; P = 0.23; very low‐quality evidence).
Outpatient hypnosis compared with wait list: outpatient hypnosis might reduce severity of impairment compared with wait list at the end of treatment (MD 2.10 (higher value better), 95% CI 1.34 to 2.86; 1 study, 49 participants; P < 0.00001; low‐quality evidence).
Behavioural therapy plus routine clinical care compared with routine clinical care: behavioural therapy plus routine clinical care might reduce the number of weekly seizures compared with routine clinical care alone at the end of treatment (MD –21.40 (negative value better), 95% CI –27.88 to –14.92; 1 study, 18 participants; P < 0.00001; very low‐quality evidence).
Cognitive behavioural therapy (CBT) compared with standard medical care: CBT did not reduce monthly seizure frequency compared to standard medical care at end of treatment (RR 1.56, 95% CI 0.39 to 6.19; 1 study, 16 participants; P = 0.53; very low‐quality evidence). CBT did not reduce physical signs compared to standard medical care at the end of treatment (MD –4.75 (negative value better), 95% CI –18.73 to 9.23; 1 study, 61 participants; P = 0.51; low‐quality evidence). CBT did not reduce seizure freedom compared to standard medical care at end of treatment (RR 2.33, 95% CI 0.30 to 17.88; 1 trial, 16 participants; P = 0.41; very low‐quality evidence).
Psychoeducational follow‐up programmes compared with treatment as usual (TAU): no study measured reduction in physical signs at end of treatment.
Specialised CBT‐based physiotherapy inpatient programme compared with wait list: no study measured reduction in physical signs at end of treatment.
Specialised CBT‐based physiotherapy outpatient intervention compared with TAU: no study measured reduction in physical signs at end of treatment.
Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) compared with standard care: brief psychotherapeutic interventions did not reduce conversion symptoms compared to standard care at end of treatment (RR 0.12, 95% CI 0.01 to 2.00; 1 study, 19 participants; P = 0.14; very low‐quality evidence).
CBT plus adjunctive physical activity (APA) compared with CBT alone: CBT plus APA did not reduce overall physical impacts compared to CBT alone at end of treatment (MD 5.60 (negative value better), 95% CI –15.48 to 26.68; 1 study, 21 participants; P = 0.60; very low‐quality evidence).
Hypnosis compared to diazepam: hypnosis did not reduce symptoms compared to diazepam at end of treatment (RR 0.69, 95% CI 0.39 to 1.24; 1 study, 40 participants; P = 0.22; very low‐quality evidence).
Outpatient motivational interviewing (MI) and mindfulness‐based psychotherapy compared with psychotherapy alone: psychotherapy preceded by MI might decrease seizure frequency compared with psychotherapy alone at end of treatment (MD 41.40 (negative value better), 95% CI 4.92 to 77.88; 1 study, 54 participants; P = 0.03; very low‐quality evidence).
The effect on the secondary outcomes was reported in 16/17 studies. None of the studies reported results on adverse effects. In the studies reporting on level of functioning and quality of life at end of treatment the effects ranged from small to no effect.
Authors' conclusions
The results of the meta‐analysis and reporting of single studies suggest there is lack of evidence regarding the effects of any psychosocial intervention on conversion and dissociative disorders in adults. It is not possible to draw any conclusions about potential benefits or harms from the included studies.
Plain language summary
Therapeutic and social interventions for conversion and dissociative disorders
The aim of this review is to provide a better understanding of what is an effective and useful intervention (treatment) for people with conversion disorders and dissociative disorders. The interventions we look at are non‐medical. Instead they concern therapy or social interventions. Background
Conversion and dissociative disorders are conditions where people experience unusual neurological symptoms (relating to the nerves and nervous system) or changes in awareness or identity. Neurological disease or other medical conditions cannot explain these clinical signs; often a psychological (affecting or arising in the mind) stressor or trauma is present. The symptoms are real and can cause distress or problems with functioning in everyday life for the people experiencing them.
This review seeks to help these patients, as well as the clinicians, policy makers and healthcare services working with these disorders.
Review question
What is the evidence for psychosocial (relating to social factors and individual thought and behaviour) intervention of conversion and dissociative disorders?
Search date
We searched medical databases between 16 July and 16 August 2019.
Results of search
We read 3048 summaries of articles, resulting in 17 studies that met our criteria for the conditions, the groups of people, the interventions and the types of studies that are the focus of this review.
The 17 studies had 894 participants, and each study was relatively small.
More studies are under way, and we will include them in updates of this review.
Study characteristics
The studies took place in nine different countries worldwide, with adults aged 18 to 80 years, who had a diagnosis of conversion or dissociative disorder for any length of time. Some studies were conducted in either psychiatric or neurological settings. Some included people already in hospital, some included people attending outpatient clinics.
The interventions were all psychosocial, meaning that they focused on psychological or social interventions such as therapy, hypnosis or simply teaching people about their illness. The number of sessions varied.
The included studies all compared the intervention to a control group to see if the interventions made any difference. The control groups received a different psychosocial intervention, medication or the care that people would normally get if they had the same condition but were not part of a research study.
The primary outcome we looked for was a reduction in physical signs.
Key results
We investigated the effect of different types of psychosocial interventions, ranging from hypnosis to behavioural therapy. None of the studies were conducted to a high enough standard to be able to say anything conclusive about the evidence of the results.
There was a reduction in physical signs at the end of treatment for three interventions.
Hypnosis reduced the severity of impairment compared to people on a wait list for treatment; behavioural therapy, given on top of routine care to inpatients, reduced the number of weekly seizures (fits) and symptom severity compared with people receiving routine care alone; and psychotherapy preceded by motivational interviewing (a talking therapy that attempts to move an person away from a state of indecision or uncertainty to positivity) compared with psychotherapy alone reduced seizure frequency.
Quality of the evidence
Most of the included studies had methodological flaws and the quality of evidence used to assess the effectiveness of the different treatments was judged as low or very low. Due to this low‐quality evidence, we cannot say how reliable the results are.
Conclusion
The results of the meta‐analysis and reporting of single studies suggest that there is lack of evidence regarding the effects of any psychosocial intervention of conversion and dissociative disorders in adults. Therefore, it is not possible to draw any conclusions about potential benefits or harms from the included studies.
However, the review shows that research in this area is possible.
Summary of findings
Background
Conversion and dissociative disorders have been described in psychology and medicine in a scientific context since Freud, Charcot and Janet (Charcot 1887; Freud 1896; Janet 1907). These early pioneers proposed some of the first theories of both cause and effective treatment.
In the following, we have described the current definitions of these conditions, given a brief overview of the range of different psychosocial interventions that are now used, as well as linked these to the different theories regarding how an intervention might work.
Description of the condition
In this review, we include both dissociative disorders and conversion disorders as defined in the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM‐5) (APA 2013), and the International Classification of Diseases, Tenth Revision (ICD‐10) (WHO 1993). In ICD‐10, conversion disorders are included in the category of dissociative disorders (WHO 1993), whereas DSM‐5 defines the conditions separately (APA 2013).
Dissociation is a collapse of usually integrated functions such as awareness, memory, orientation, or sensory and motor function in response to, or by reactivating, a severe emotional stress or trauma, whereas examples of conversion disorders (in the DSM‐5 definition) are psychogenic non‐epileptic seizures (PNES), paralysis, gait disturbances, anaesthesia, tremor, dystonia (involuntarily muscle contractions, causing repetitive or twisting movements), functional blindness or aphonia (loss of voice due to disturbance of the vocal organs).
DSM‐5 (APA 2013) defines conversion disorder as:
one or more symptoms of altered voluntary motor or sensory function;
clinical findings that show evidence of incompatibility between the symptoms and recognised neurological or medical conditions;
symptoms or deficit that are not better explained by another medical or mental disorder;
symptoms or deficit that cause clinically significant distress or impairment in social, occupational or other important areas of functioning or warrants medical evaluation.
In DSM‐5, the dissociative disorders are placed independently and separated from the conversion section and next to, but not as part of, the trauma‐ and stressor‐related disorders, reflecting the close relationship between these diagnostic classes. Dissociative disorders in DSM‐5 include dissociative identity disorder (disruption of identity characterised by two or more distinct personality states), depersonalisation/derealisation disorder (experiences of unreality or detachment from one's mind or self or detachment from one's surroundings) and dissociative amnesia (inability to recall autobiographical information). It may sometimes involve travel or confused wandering away from one's life (dissociative fugue).
Common for these conditions is that a psychological stressor or trauma is often present, but is not a requirement to establish the diagnosis.
The epidemiology of conversion disorder is complicated by case definition, case ascertainment and identifying a suitable study population, but community surveys suggest a minimum prevalence of 50 per 100,000. Deveci 2007 found the prevalence in a city population to be 5.6% and Stone 2009 found conversion symptoms for 18%, among people referred to neurological outpatient clinics in the UK. There is no good evidence to suggest that conversion disorder is becoming less common or that it is more often found in low‐income countries. Conversion symptoms can occur in both men and women although all case series show a predominance of women (Akagi 2002). Several studies have pointed out that childhood trauma, specifically physical or sexual abuse, emotional or physical neglect, and a greater number of stressful life events and traumatic episodes characterise conversion disorders (Kranick 2011; Roelofs 2002; Sar 2009). Scevola 2013 found trauma history in 49% of people with PNES and sexual abuse is particularly prevalent in this group, present in 30% (Stone 2004) to 45% (Selkirk 2008) of people with PNES.
With regard to dissociative disorders, Johnson surveyed a population of 658 people in New York and found a one‐year prevalence of dissociative disorders of 9.1% (conversion disorders not included) (Johnson 2006). A different study likewise found a prevalence of dissociative disorders of 10% (Ross 1991).
In some clinical terminology, the concept of dissociation may be used synonymously with psychogenic dissociation (e.g. amnesia, fugue, stupor, trance or dissociative personality disorder), whereas the concept of conversion may be described clinically as somatoform dissociation. We included both somatoform dissociation (conversion) and psychoform dissociation in this review.
The prognosis for people with conversion disorder is poorly studied, but results from several studies suggest the prospects for immediate recovery are good but a significant number of people will relapse. Factors associated with quick recovery are an acute onset and prompt treatment (Ron 2001). Prognosis may also be influenced by symptom pattern and some studies suggest that non‐epileptic attacks and people presenting with tremor or amnesia have a poorer prognostic outcome than those with hysterical blindness, aphonia and motor disorders (Toone 1990). Trauma in childhood seems to have an impact on the seriousness of the development and progress of conversion disorder (Selkirk 2008; Stone 2004).
Description of the intervention
Psychosocial interventions include all psychological interventions specified in the UK Department of Health review of psychological therapies (Department of Health 2001), social interventions such as social skills training and befriending and packages of interventions that have a psychosocial focus.
Many psychosocial interventions have been developed for conversion and dissociative disorders. Most have been developed over decades based on observations and theory formation in the early years of psychiatry and psychology by pioneers such as Charcot, Freud and Janet (Charcot 1887; Freud 1896; Janet 1907), and later as part of behavioural or cognitive approaches.
Some of the most common psychosocial interventions include the following.
Behavioural therapy consists of graded exposure to the body sensations or to situations perceived as threatening in order to reduce the patient’s apprehensive reaction towards them. If trauma is a part of the condition, it may be practised as 'prolonged exposure' (Myers 2017), or be combined with other forms of therapy.
Cognitive behavioural therapy (CBT) is a structured, present‐oriented psychotherapy directed towards solving current problems and teaching the patients skills to modify unhelpful thinking and behaviour. One important part of CBT is helping clients change their unhelpful thinking and behaviour, which leads to enduring improvement in their mood and functioning.
Psychoeducation is connected to CBT and concerns teaching patients about their condition, thereby empowering them to take an active part in the management of the condition and the recovery from it (Colom 2011; Zhao 2015).
Hypnosis is a therapeutic technique in which clinicians make suggestions to people who have undergone a procedure designed to relax them and focus their minds. Hypnosis strengthens the ability to handle emotional stress and it often forms part of a treatment package, for example, together with CBT or eye movement desensitisation reprocessing (EMDR) (Fine 2012; Fine 2001).
Psychodynamic andpsychoanalytic therapies consist of a set of psychological therapies, where psychological symptoms can be seen as manifestations of intrapsychic or unconscious conflicts. These therapies use different therapeutic strategies to reveal, interpret and resolve such conflicts. In the field of dissociation or conversion, early life events and trauma experience may be an important part of the therapy.
Specialised physiotherapy contains elements of CBT and aims to retrain motor or sensory function by redirecting attention and addressing unhelpful illness beliefs and behaviours. The patient's problems are considered in a broad biopsychosocial framework where symptom‐predisposing, precipitating and perpetuating factors can be addressed within a multidisciplinary environment (Nielsen 2016).
Paradoxical intention therapy is where the therapist encourages the patient to engage in the unwanted behaviour, promoting the worsening of the symptoms rather than their removal.
Models of dissociation and trauma treatment. In phase‐oriented treatments, patients are working from establishing safety and stability, for example, symptom reduction, emphasised emotion regulation and impulse control (Stage 1), to focus on maintaining stability while exploring trauma narratives and resolving trauma‐related emotions (Stage 2) and finally to emphasise integration and living without reliance on dissociation (Stage 3) (Dorahy 2014; van der Hart 2012).
Eye movement desensitisation reprocessing (EMDR) is a structured therapy that encourages the patient to briefly focus on the trauma memory while simultaneously experiencing bilateral stimulation (eye movements), which is associated with a reduction in the vividness and emotion associated with the trauma memories. It is a treatment divided into phases where desensitisation in relation to a trauma is essential.
Psychosocial interventions can be delivered individually or in group formats, sometimes following a very structured manual, at others adapting the intervention to the individual or group in question.
How the intervention might work
Because most theories about the origin of conversion and dissociative disorders are concerned with underlying psychological stressors or trauma, the common hypothesis is that offering treatments that work with and address this in patients' lives might produce a positive treatment effect and a reduction in symptoms.
The basis for how the different methods and procedures in different treatment approaches to conversion and dissociative disorder are usually associated with a specific theoretical grounding, which often present divergent views on aetiology as well as on what they regard as active mediators in treatment. In general, however, for most models, the mechanism of action of therapy is the integration of dissociated parts of the personality or therapeutic work with maladaptive thoughts towards more adaptive and appropriate thoughts. For example, in therapy, one can work on integration of dissociated elements of the self (psychodynamic) or to weaken maladaptive schemas and construct new, more adaptive schemas (in cognitive therapy a schema is an organised pattern of thought and behaviour or a mental structure of preconceived ideas representing some aspect of the world).
It must be pointed out that not just the method but also the relationship and the therapeutic alliance between the patient and the therapist are main factors for a satisfactory outcome of a therapy course (Horvath 1991; Martin 2000).
Cognitive behavioural approaches
These are based on Aaron Beck's personality model, which suggests that the personality is composed of different modes that are collections of schemas responsible for coding cognitive, affective, behavioural and physiological information and for generating response. A mode with associated schemas is activated only when a particular schema for orientation, related to a particular situation or feeling, triggers it. Based on this theory, Kennedy 2013 has developed a model for dissociation.
At level 1, scary stimuli can result in dissociation of the schema for orientation, which results in incorrect integration of incoming information. The level involves 'detachment', that is, a change of consciousness that is a result of the fight/flight/freeze response, which results in depersonalisation or derealisation or 'compartmentalisation' (i.e. where trauma‐related information is partially stored without integration into the normal memory system and thus is inaccessible). However, when situations occur that are similar to the trauma, trauma‐related information can be activated with severe anxiety and discomfort, as in flashbacks in post‐traumatic stress disorder (PTSD). The therapy works by breaking this 'compartmentalisation' and establishing reintegration.
Level 2 describes dissociative compartmentalisation that can occur within the single mode between the various schemas for coding cognitive, affective, behavioural and physiological information. A mode associated with a traumatic experience or an inter/intrapsychic issue can activate a dissociative process between the individual schemas. An effective schema that is unacceptable to consciousness (e.g. one unbearable feeling related to a traumatic event) can be isolated and kept out of consciousness, or a schema of behaviour can be dissociated so that the person experiences losing a physical skill in connection with a trauma or retrauma (PNES, sensory loss, paralysis or gait disturbance), which also falls within the concept of compartmentalisation.
Level 3 describes dissociation between different modes in Beck's personality model. Generally, integrated modes representing the well‐integrated personality may be subject to dissociation upon severe stress or trauma. An example is the dissociative identity disorder or the disintegration occurring in borderline personality disorder. Based on this model, an adapted traditional cognitive treatment plan can be prepared, taking into account the dissociation level, in order to establish reintegration within schemas and modes.
Brown 2013 has introduced The Integrative Cognitive Model, which is based on the individual's way of storing information in memory. During a dissociative process, the patient activates a primary attentional system and the most active hypothesis for an event (e.g. a misinterpretation of a symptom) combined with the corresponding sensory data creates a counterproductive working model or primary representation. This 'rogue' representation is the starting point in the case formulation that is the basis of the treatment plan. In the course of the treatment, a 'socialisation' takes place, which refers to some form of emotional neutralisation in relation to the patient's notions of the condition. Brown emphasises that not all dissociative reactions are the result of traumatic painful memories, but when it is the case, he recommends follow‐up treatment with trauma‐focused cognitive (Ehlers 2000) or metacognitive therapy (Wells 2004).
The structural dissociation and trauma approach
The two Dutch psychologists, Ellert Nijenhuis and Onno van der Hart, are the most prominent in the theory formation and research on dissociation and trauma (Nijenhuis 2009; Nijenhuis 2010; Steele 2009). Based on Janet 1907, they define structural dissociation as a lack of personality integration, manifested by the existence of two or more inadequately integrated and dissociated parts of the personality. Traumatic dissociation leads to fragmentation in the individual's personality (i.e. of the entire dynamic, biopsychosocial system), which constitutes the mental and behavioural conditions of the person. They emphasise that this is fundamentally different from that mentioned in the cognitive model above regarding changes in perception and memory.
Regarding symptom formation, Nijenhuis and van der Hart distinguish between psychoform and somatoform consciousness and identity (e.g. amnesia, fugue, flashbacks and dissociative identity disorder), while they consider somatoform dissociation as the proper term for conversion, more specifically neurological conditions and other conditions involving the body.
The model is based on Janet's phase‐divided treatment. In phase 1, one strives to provide reassurance and the ability to experience bodily reactions; in phase 2, one looks at the integration of the different parts of the personality by working with phobias for traumatic memories and for anxious attachment; and in phase 3, one works with personality reintegration, phobias for ordinary life, change and intimacy (van der Hart 2012).
The psychodynamic approach
There exist only a few guidelines and general recommendations on conducting psychoanalytic and psychodynamic psychotherapy for dissociative and conversion disorders. Some authors describe a broader perspective (Kalogjera‐Sackellares 2004; Kaplan 2014), while Matthews 1997 propose a psychodynamic psychotherapy for people who have experienced severe childhood trauma. Howell 2011 discusses the identification and diagnosis of dissociative identity disorder and outlines a phase‐oriented treatment plan, which includes facilitating a therapeutic relationship, emphasising the multiplicity of transferences (the patient redirects feelings or desires for another person to an entirely different person, frequently the therapist), countertransferences (redirection of a therapist's feelings towards a patient) and the potential enactments of this transference and countertransference.
The comprehensive guidelines for treating dissociative identity disorder in adults proposes a phase‐oriented treatment (Chu 2011). In phase 1, safety, stabilisation and symptom reduction are established; phase 2 involves confronting, working through and integrating traumatic memories; and in phase 3 the objectives are identity integration and rehabilitation. The guideline is referring to individual psychodynamic‐oriented psychotherapy as the most common, often incorporating other techniques as CBT techniques, hypnosis or EMDR.
Significant contributions from contemporary psychoanalysis to the theory and treatment of dissociative disorders concerns attachment and relational theory (Bradfield 2011; Bromberg 2009; Howell 2005).
Dynamic interpersonal therapy for functional somatic disorders is a mentalisation‐based approach (Bateman 2013), which entails a focus on restoring the capacity for stress regulation by fostering the use of more adaptive attachment strategies in response to stress, and recovery of the capacity for (embodied) mentalising (Luyten 2013). Mentalisation is the ability to understand the mental state, of oneself or others, that underlies overt behaviour. Dynamic interpersonal therapy consists of three phases. The first phase focuses on the treatment alliance and the collaborative formulation of a treatment (i.e. formulation of a shared and acceptable illness theory that recognises the complexity of the disorder through consensus). The second phase consists of the working through of interpersonal affective focus (how the patient perceives others in relation to a self‐perception, and the affect that links these two experiences) and consolidation of treatment gains. This is used as a guide to explore the typical interpersonal patterns with the aim to foster patients' capacities to reflect on the bodily self, others and the self‐in‐relation‐to‐others. The final phase focuses on the aims to transfer what one has 'learned' during treatment to the everyday context of the patient to prevent relapses and to foster autonomy and resilience long‐term.
The contextual approach
This treatment model, described by Gold 2009, is not based on a specific theory but involves elements from both cognitive and psychodynamic methods, based on practical clinical experience and empirical evidence. Attachment theory, though, with a specific focus on disorganised attachment and dissociation, has particular theoretical and practical significance in this model (Barach 1991; Liotti 2006; Williams 2019), and the overall approach of the therapy is based on the observation of the absence of reliable attachment resources for these patients. Disorganised attachment is an insecure attachment style, hypothesised to be an outcome of childhood abuse and trauma, and closely related to the development of dissociation. Focus on therapeutic relationship and treatment alliance are also considered essential. The primary objective of the treatment is to improve adaptive functioning, to focus on problems that find expression in the present and teaching the patient skills that can be applied to difficulties as they arise. The treatment strategy is based on the fact that these patients may have difficulty with affect management, and identifying the presence, intensity and type of affect resulting in an associated inappropriate and incomprehensible behaviour.
Affective arousal is so intense that cognitive processing does not sufficiently contribute to behavioural regulation and a result is that the patient experiences the dissociative episode as 'not me' and may feel shame. The intervention concerns fostering capacity for emotional de‐escalation and increasing the cognitive capacities for recognising the presence of affect. Another aspect is to reduce the propensity of chronic high arousal. Gold 2013 recommends training with a log sheet, relaxation techniques and a 'grounding' programme. He states that trauma exposure is inappropriate for these patients.
The neurobiological approach
Neuroimaging techniques have contributed to the understanding of the basic mechanisms of conversion and dissociative disorders and on some points, pathophysiological equivalents exist for the psychological theories mentioned above and have reinforced their treatment strategies (Aybek 2014; Perez 2015; Perez 2017).
Psychoeducation
Psychoeducation concerns teaching patients about their condition. It is connected to CBT in that it is focused on the present and works with empowering patients to engage with their illness in more informed and helpful ways, the theory being that the more informed patients are, the better equipped they will be in dealing with their condition and with working on recovering from it.
Psychoeducation can be performed in many ways, both formally and informally, and can last from one session to more elaborate programmes over longer periods (Colom 2011; Zhao 2015).
Why it is important to do this review
It is apparent that conversion and dissociative disorders cause clinically significant suffering and that developing effective interventions is important. The main purpose of this review is to update the knowledge about treating conversion and dissociative disorders. This is important for people with conversion and dissociative disorder to understand the evidence supporting the treatments offered to them and to help clinicians prescribe effective treatments.
Previous reviews on this theme
The review is an update of the Cochrane Review "Psychosocial interventions for conversion disorder", published in 2005 (Ruddy 2005). This study included conversion disorder motor and sensory symptoms or impairment, that cannot be explained by a neurological cause (conversion disorder in Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM‐IV)), and all dissociative states in DSM‐IV and ICD‐10. The review included all randomised controlled trials (RCT) that compared psychosocial interventions for conversion disorder with standard care (SC) or other interventions (biological or psychosocial). Only three studies (119 participants) met the inclusion criteria. One study was concerned with paradoxical injunction therapy and the other two studied the value of hypnosis. All studies were of poor methodological quality and it was, therefore, difficult to place much value on the results. The authors concluded that the use of psychosocial interventions for conversion disorder required more research and it was not possible to draw any conclusions about their potential benefits or harms from the included studies.
The latest Cochrane Review that deals with conditions broadly within the field of conversion disorders is "Psychological and behavioural treatments for adults with non‐epileptic attack disorder" (Martlew 2007; Martlew 2014). In the 2014 update, 12 studies met the inclusion criteria (four RCTs and eight non‐controlled studies). Overall, three examined CBT, two investigated hypnosis, one assessed paradoxical intention and one had a mixed intervention design. They classified two included studies at low risk of bias, one at unclear and nine at high risk of bias. For quality of the evidence (GRADE), six studies were of very low, two were of low and three were of moderate quality. However, most included studies reported improved outcomes for the intervention under investigation. The authors concluded that there is little reliable evidence to support the use of any treatment, including CBT, in the treatment of non‐epileptic seizures.
Since the last Cochrane Review in 2014 (Martlew 2014), one meta‐analysis on PNES has been published. Carlson 2017 synthesised data from 13 studies, of which 2/13 studies used an RCT design. Studies were included if they evaluated the effectiveness of at least one psychological intervention undertaken to lessen the frequency of PNES and using seizure frequency as an outcome measure. They found a moderate to high level of heterogeneity (I2 = 58% to 78%) and in general serious bias regarding incomplete data, primary and secondary outcome measures, intervention application and duration. The findings highlight the potential for psychological interventions as a favourable alternative to the lack of treatment options offered to people with PNES, but brought no new evidence to the field.
Another meta‐analysis from 2014 dealt with the effectiveness of psychotherapy for severe somatoform disorder (Koelen 2014). The authors included prospective studies. The patients had a diagnosis of somatoform disorder, primarily severe conversion and somatisation (diagnosis of hypochondriasis and body dysmorphic disorder was excluded) and received psychotherapy in secondary or tertiary care. The review included 10 randomised and six non‐randomised studies. Study quality was moderately poor (ranging from very poor to very good), measured using the Psychotherapy Quality Rating Scale (Kocsis 2010), and heterogeneity was high. The effect size for physical symptoms was large, for psychological symptoms moderate to large and for functional impairment moderate, but effect sizes were generally lower than those typically found for other mental disorders. Post‐hoc analysis indicated that psychodynamic interventions were more effective in improving functioning than cognitive interventions, although not in improving symptoms and there was no difference in the effectiveness of group versus individual therapy was found.
With regard to physiotherapy for conversion disorder, one systematic review by Nielsen 2013 identified 29 studies evaluating the effect of physical treatment of adults. No RCTs and only one controlled study was described. The Nielsen review does not define one primary outcome, it only defines that they look at the same range of outcomes as used in the individual studies, and that these showed encouraging results with improvement in 60% to 70% of patients. Combining motor relearning with behavioural therapy was the most common approach. They concluded that the evidence to guide physiotherapy treatment for conversion disorder was of low quality. Likewise, there was limited and poor‐quality evidence for the efficacy of physiotherapy management of child and adolescents with conversion disorder (FitzGerald 2015).
Finally, Brand 2009 reviewed 20 empirical reports of treatment for dissociative disorders, mainly dissociative identity disorder.
Collectively, these reports suggest that treatment for dissociative disorders is associated with decreased symptoms of dissociation, depression, PTSD, distress and suicidality. Effect sizes, based on pre/post measures, were in the medium to large range across studies. However, there were significant methodological limitations illustrating a serious lack of well‐designed studies in the treatment of dissociative disorders. The authors proposed good arguments for using case studies instead of RCTs when treating people with severe psychopathology, high comorbidity and the need for long‐term treatment.
Conclusion
To conclude, several reviews and meta‐analyses have been published since the 2005 version of this Cochrane Review. However, none have consistently examined the same diagnostic spectrum (i.e. both conversion and dissociative disorders). In addition, we are following the revised and expanded Cochrane Handbook for Systematic Reviews of Interventions and are updating the methods from the first review (Higgins 2019).
Objectives
To assess the beneficial and harmful effects of psychosocial interventions of conversion and dissociative disorders in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs that met our inclusion criteria. We included studies irrespective of language, publication type and publication status.
Types of participants
Participants were adults aged 18 to 80 years, of any gender and nationality. Participants fulfilled the criteria for having a conversion or dissociative disorder according to the DSM‐IV (APA 1994), DSM‐5 (APA 2013), or ICD‐10 (WHO 1993) criteria. As some studies may have been conducted prior to DSM‐IV or ICD‐10, we included all studies where a large majority (80% or greater) of participants fulfilled current diagnostic criteria or any earlier diagnostic equivalent. We consider the use of conversion and dissociative disorders to cover the diagnostic criteria equivalent to ICD‐10 F.44 codes (F44.0 Dissociative amnesia, F44.1 Dissociative fugue, F44.2 Dissociative stupor, F44.3 Trance and possession disorders, F44.4 Dissociative motor disorders, F44.5 Dissociative convulsions, F44.6 Dissociative anaesthesia and sensory loss, F44.7 Mixed dissociative [conversion] disorders, F44.8 Other dissociative [conversion] disorders, F44.9 Dissociative [conversion] disorder, unspecified) and in the DSM system the DSM‐IV codes 300.11 Conversion disorder, 300.12 Dissociative amnesia, 300.13 Dissociative fugue, 300.14 Dissociative identity disorder, 300.6 Depersonalisation disorder and 300.15 Dissociative disorder not otherwise specified. Studies with participants with any length of illness were included, as well as studies with participants being treated in any intervention setting, as long as the diagnostic criteria were fulfilled.
If the search identified studies where comorbidity occurred, we planned to comment on this and the participants included if they had conversion or dissociative disorder according to the DSM‐IV (APA 1994), DSM‐5 (APA 2013), or ICD‐10 (WHO 1993) criteria.
Types of interventions
We included RCTs that compared a psychosocial (including psychotherapeutic) intervention for conversion or dissociative disorder with another intervention (pharmacological or psychosocial, or mixed) or with SC or wait list controls.
Experimental interventions
Psychosocial interventions
Our understanding of psychosocial interventions follows the traditional use of this term in medical health, to cover interventions of either a psychological nature, a social nature, or both. Often what makes an intervention psychosocial is the theoretical framework it places itself within, giving a particular approach to how that intervention is regarded. Psychosocial interventions are described in much more detail in the Description of the intervention section, and include the following.
Behavioural therapy
Cognitive behavioural therapy (CBT)
Psychoeducation
Hypnosis
Psychodynamic andpsychoanalytic therapies
Specialised physiotherapybased on cognitive behavioural therapy (CBT)
Paradoxical intention therapy
Models of dissociation and trauma treatment
Eye movement desensitisation reprocessing (EMDR)
Controls
Controls could be SC, wait list controls, pharmaceutical interventions or another psychosocial intervention.
Pharmacological interventions: any pharmaceutical medication officially recognised by US or European law.
SC: standard medical care (SMC) or treatment as usual (TAU) is the care that a person would normally receive had they not been included in the research study. What this contains varies greatly in different settings and we describe this with the studies.
Wait list controls: wait list controls are those patients referred to the same intervention, but who have not yet received it due to the allocation in the study. They are usually free to pursue other interventions outside of the study, but will not receive any controlled intervention while waiting. They will receive the same intervention as the active group, only delayed in time.
Comparisons
If possible, we compared the effects of any similar interventions with controls on both primary and secondary outcomes. By 'similar', we mean interventions that broadly speaking fall under the same category of psychosocial interventions listed above, namely the same forms of psychotherapy, interventions consisting of psychoeducation or comparable other psychosocial training. As the field of psychosocial interventions contains a variety of different approaches, the actual decision of what to group into comparisons is based on clinical judgement.
Types of outcome measures
If a study had more than one measure on the same outcome, only data from one measure for each outcome were included in the analysis. When several measures on the same outcome appeared in a study, we discussed this within the review author team and choose one measure based on which measure was most widely used and which we deemed most clinically relevant.
Time points
Studies were included that measured the effects at end of treatment as the main time point, and if available, follow‐up effects, which were then divided into shorter term (up to and including five months after end of treatment) and longer term (from six months after end of treatment).
Primary outcomes
Reduction in physical signs.
This would be expected to be seen in the improvement of physical functioning or in reduction of conversion experiences such as seizures or other discrete conversion episodes.
There are no golden standards in scales for this outcome, but possible ways could be using scales measuring physical functioning such as the physical function dimension of 36‐item Short‐Form (SF‐36) (Jenkinson 1996), or by the Somatoform Dissociation Questionnaire (SDQ‐5 and SDQ‐20) (Nijenhuis 1996). It could also be by counting number of seizures daily or weekly, or by using binary outcomes such as improvement or no improvement.
Secondary outcomes
Level of functioning: this is a new addition to this updated version of the review and has been added as a secondary outcome because level of functioning is often one of the main ways of assessing a patient's ability to participate in life despite any illness or incapacity. This could be measured using the Global Assessment of Functioning (GAF) scale (APA 1994), the Work and Social Adjustment Scale (WSAS) (Mundt 2002), or similar.
Quality of life: this was part of the secondary outcomes in the original review, and is still very relevant in current research. This can be measured using the full version of the SF‐36 (Jenkinson 1996), Quality of Life in Epilepsy‐31 (QOLIE31) (Cramer 1998), or similar.
Mental state: this secondary outcome was also used in the original review, though, in this update, we decided to divide it into anxiety and depression where possible, to get a more accurate picture of patients' experiences, resulting in three secondary outcomes: mental state, anxiety and depression. The general component of mental state can be measured using instruments such as the Symptom Check List 90 (SCL‐90) (Derogatis 1977), or the mental component of the 12‐‐item Short Form (SF‐12)/SF‐36 (Jenkinson 1996). Anxiety could be measured using instruments such as Becks Anxiety Index (BAI) (Beck 1988), or the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983). Depression could be measured using instruments such as Becks Depression Index version II (BDI‐II) (Beck 1996), Hamilton Depression Rating Scale (HDRS) (Hamilton 1960), or by the depression subscale from the HADS (Zigmond 1983).
Dropout rate/leaving the study early: this would often be measured by numbers or percentage.
Use of health service resources: often measured in how many subsequent visits a patient has to hospital/accident and emergency department or local general practitioner/clinician.
Adverse effects: in psychotherapy, the general adverse effects are described by Barlow 2010 and Crawford 2016. Examples could be the risk of psychotic decompensation or self‐injury through psychological intervention of psychologically fragile patients or that participants experience more positive attention from staff or family by being in a study (secondary gains arise), which could be maintaining the symptoms.
Search methods for identification of studies
For this update, the main review author (CG) in co‐operation with an Information Specialist of the Cochrane Common Mental Disorder Group and an Academic Research Librarian of the Research Unit in Region Zealand, revised the search strategies in line with current Cochrane Common Mental Disorder Group practices (Higgins 2019).
Electronic searches
We searched the electronic resources listed below, using the strategy detailed in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL) (2019, Issue 7 in the Cochrane Library). Searched on 16 July 2019.
MEDLINE via Ovid (from 1946). Searched on 16 July 2019.
CINAHL via EBSCO. Searched on 16 July 2019.
Embase via Ovid from 1974. Searched on 16 July 2019.
PsycINFO via Ovid from 1806. Searched on 16 July 2019.
ERIC via EBSCO. Searched on 16 August 2019.
Web of Science Core Collection (Thomson Reuters). Searched on 16 July 2019.
We searched online clinical trial registers for ongoing or recently completed studies on 16 July 2019, including the ISRCTN Registry (www.isrctn.com/), US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), the World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/trialsearch/), and the EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
There were no limitations on languages.
Searching other resources
We checked references, screened reference lists, citation searching and contacted relevant study authors to identify additional studies.
Data collection and analysis
We conducted the review according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). In the following section, we report only the methods that we were able to use in this update.
Selection of studies
Following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), the main review author (CG) plus one of the other members of the author team did the initial screening of abstracts using Covidence. This was divided so the main review author (CG) read all abstracts, and three other authors (OJS, RR, US) read a third of the abstracts each. When disagreements arose about inclusion of abstracts, this was discussed between the two voting review authors. In case of no consensus, we consulted a third review author. A record of all papers rejected on the basis of their abstracts were documented in Covidence but are not included in the review.
We retrieved the full papers of all remaining abstracts and other potentially relevant articles identified by the various search strategies (reference checking, personal communications, etc.). All papers in languages other than English were (as far as possible) translated or reviewed by someone who spoke the language. Two review authors independently reviewed all articles, following the same format as for abstracts, with the main review author (CG) reading all full texts, and three other review authors (OJS, RR, US) reading a third each. The process of full‐text screening was done using Covidence, which was also used for documenting the results of each reviewer's judgement.
When disagreements arose about inclusion of a full text, the two voting review authors discussed them, and, if no consensus was reached, a third author was consulted. If there was a lack of clarity about the suitability of inclusion of a study, then we contacted the authors of the article for more information.
Any ongoing studies were followed up by contacting the principal investigators to enquire whether any data were available that could be included in this review.
Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references
We documented the reasons for exclusion of the full papers in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (CG, HC) completed the extraction of data using Covidence.
They independently extracted data onto a data collection form. We extracted information on title, authors, year of publication, source, setting, country, participant characteristics, diagnosis, comorbidity, study design and methods, interventions, outcomes and relevant information for 'Risk of bias' assessments. We exported data to Review Manager 5 and one review author (OJS) performed data analysis (Review Manager 2014). We resolved differences by discussion. In cases of insufficient data, or where data in the published study reports were unclear, we contacted the study authors requesting them to clarify the missing information (see Dealing with missing data).
We completed a Characteristics of included studies table.
Assessment of risk of bias in included studies
In the original version of this review, methodological quality of included studies were assessed by criteria sent out in the Cochrane Handbook for Systematic Reviews of Interventions current at the time; however, following the publication of the revised and expanded Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we updated our methods accordingly.
Working independently, two review authors (CG and HC) assessed the risk of bias of included studies using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following items.
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated intervention adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed?
Selective outcome reporting: were reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias?
We included quotations from the text of included studies and comments on how we assessed the risk of bias in the Characteristics of included studies table. We assigned studies to one of three categories (low risk of bias, uncertain risk of bias and high risk of bias), according to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. We subsequently included the risk of bias assessment in the GRADE evaluation that was used to collectively assess the certainty of the estimates based on the risk of bias, imprecision, indirection and publication bias.
If disputes arose we achieved resolution after consulting with the third review author (OJS).
Measures of treatment effect
Outcomes were assessed using continuous (e.g. changes on physical function scales); categorical measures (e.g. one of three categories on a quality of life scale, such as 'better', 'worse' or 'no change') or dichotomous measures (e.g. either returned to employment or did not return to employment).
Continuous data
We compared the mean score between the two groups to give a mean difference (MD) and presented this with 95% confidence intervals (CI). We wanted to use the overall MD, where possible, to compare the outcome measures from studies. However, because some of the included studies used different rating scales for measuring the same outcome, we needed to use the standardised mean difference (SMD) in some analyses. We considered a statistical significant SMD effect size of: 0.15 or less to have no clinically meaningful effect; 0.15 to 0.40 to have a clinical meaningful but small effect; 0.40 to 0.75 to have a moderate effect; and greater than 0.75 to have a large treatment effect (Cohen 1988; Thalheimer 2002).
Many rating scales are available to measure outcomes in psychosocial studies. These scales vary in the quality of their validation and reliability. Therefore, if a rating scale's validation had not been published in a peer‐reviewed journal, the data were included but we noted that it was not validated. In addition, it is preferable for the rating scale to be either self‐report or completed by an independent observer or relative. We included studies that used the same rating scales to evaluate the effect on a specific outcome in the same analysis for direct comparison.
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the problems of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: 1. standard deviations and means had to be reported in the paper or be obtainable from the authors, 2. when a scale started from a finite number (such as zero), the standard deviation, when multiplied by two must have been less than the mean (otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution) (Altman 1996). If data were non‐parametric, then we reported this in the text but did not used them in the meta‐analysis.
Dichotomous data
For dichotomous outcomes, we calculated the risk ratio (RR) with its associated 95% CI was estimated.
Unit of analysis issues
We planned to include data from randomised cross‐over studies up to the point of first cross‐over (first period only) (Curtin 2002). We planned to include cluster randomised studies; however, we found none. If we find cluster‐randomised studies for updates of this review, they will be eligible for inclusion. We will anticipate that investigators have presented their results for cluster RCTs after appropriately checking for clustering effects (robust standard errors or hierarchical linear models). If it is unclear whether a cluster‐randomised trial has used appropriate checks for clustering, we will contact the investigators for further information. Where appropriate checks are not used, we will request and re‐analyse individual participant data using multilevel models that check for clustering. Following this, we will analyse effect sizes and standard errors in Review Manager 5 (Review Manager 2014), using the generic inverse method (Higgins 2019).
Dealing with missing data
We conducted analyses 'as reported' for continuous outcomes. We attempted to retrieve any missing data from the study authors. We contacted the authors of three studies with unclear or missing data and requested the necessary data. If data remained unavailable, we tried to estimate the missing data using the available information (e.g. if the standard deviation (SD) was missing, we estimated it from the standard error, if reported). When we were unable to obtain missing data, we conducted analyses using available (incomplete) data.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining variability in the participants, interventions and outcomes described in each study. We assessed methodological heterogeneity by inspecting variability in the design of the studies and statistical heterogeneity by assessing the difference in the studies' intervention effects. We assessed heterogeneity between studies by visual inspection of the forest plot for overlapping CIs, using the Chi2 test for homogeneity with a significance level of α (alpha) = 0.10, and the I2 statistic for quantifying inconsistency (estimating the percentage of variation in effect estimates due to heterogeneity rather than sampling error). We judged I2 values of 0% to 40% to indicate little heterogeneity; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; and 75% to 100%, considerable heterogeneity. For values including overlapping judgements, the degree of heterogeneity was considered as an interval (e.g. with a heterogeneity of 55% being considered as moderate to substantial heterogeneity) (Higgins 2019).
Assessment of reporting biases
We handled different forms of reporting bias, especially publication bias and outcome reporting bias, according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019; Section 10.1). If more than 10 studies had been included in a given meta‐analysis, we had planned to draw funnel plots to give a visual assessment on whether effects were associated with the size of the study (Egger 1997). Due to the small number of eligible trials (i.e. fewer than 10 studies for each comparisons), this was not possible.
Data synthesis
We included and analysed studies undertaken in any configuration or setting; for instance, in groups, hospitals, people's homes and clinics. We summarised data in a meta‐analysis when they were available. If clinical heterogeneity was not excessive (e.g. there was similarity in participants' characteristics), we performed statistical analysis in Review Manager 5 (Review Manager 2014), according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019; Section 9.4.1). We synthesised data using final values and the inverse variance method in the meta‐analyses. We generally used the fixed‐effect model because most of the analyses included only one study. When more than one study was included, we used the random‐effects model. A random‐effects model has the assumption that apparent differences between study effects are random, but the estimated difference follows a normal distribution. This method gives more weight to small studies, whereas the fixed‐effect model gives more weight to large studies (Higgins 2019; Section 9.5.4).
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses for:
different symptoms groups;
group versus individual therapy;
manual‐driven versus non‐manual‐driven therapies.
We planned to conduct these specific analyses to investigate if treatment effects vary between symptoms groups. We also wished to determine if treatment effects are influenced by how the therapy is provided (group versus individual therapy) or if therapies are based on a manual or not, or both.
Sensitivity analysis
We planned to conduct sensitivity analyses for:
attrition rate: more than 50% attrition compared with less than 50% attrition;
use of intention to treat analyses: the studies using intention to treat analyses compared with the studies not using intention‐to‐treat analyses;
differences between cluster and non‐cluster randomised studies;
differences between self‐reported and observer‐reported outcomes.
Summary of findings and assessment of the certainty of the evidence
We constructed 12 'Summary of findings' tables for the comparisons. As the field of psychosocial interventions contains a variety of different approaches we were only able to predefine that, when possible, we would group studies together based on clinical judgement as to which interventions were most alike and create the actual comparisons after identification of the included studies. For the studies identified in this version of the review, the team agreed on the following comparisons:
inpatient paradoxical intention therapy versus outpatient diazepam;
inpatient treatment programme plus hypnosis versus inpatient treatment programme;
outpatient hypnosis versus wait list;
behavioural therapy plus routine clinical care versus routine clinical care;
CBT versus SMC;
psychoeducational follow‐up programmes versus TAU;
specialised CBT‐based physiotherapy inpatient programme versus wait list;
specialised CBT‐based physiotherapy outpatient intervention versus TAU;
brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus SC;
CBT plus APA versus CBT alone;
hypnosis versus diazepam;
psychotherapy preceded by motivational interviewing compared with psychotherapy alone.
We included the primary outcome reduction in physical signs and the secondary outcomes level of functioning and quality of life assessed at the relevant time points.
We used the GRADE approach to assess the certainty of the evidence associated with each of these outcomes (Guyatt 2008). The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Considerations are due to: within‐study risk of bias, directness of evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Andrews 2013a; Andrews 2013b; Balshem 2011; Brunetti 2013; Guyatt 2011; Mustafa 2013).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables for detailed information on the individual studies and Figure 1 for an overview of the search process.
1.
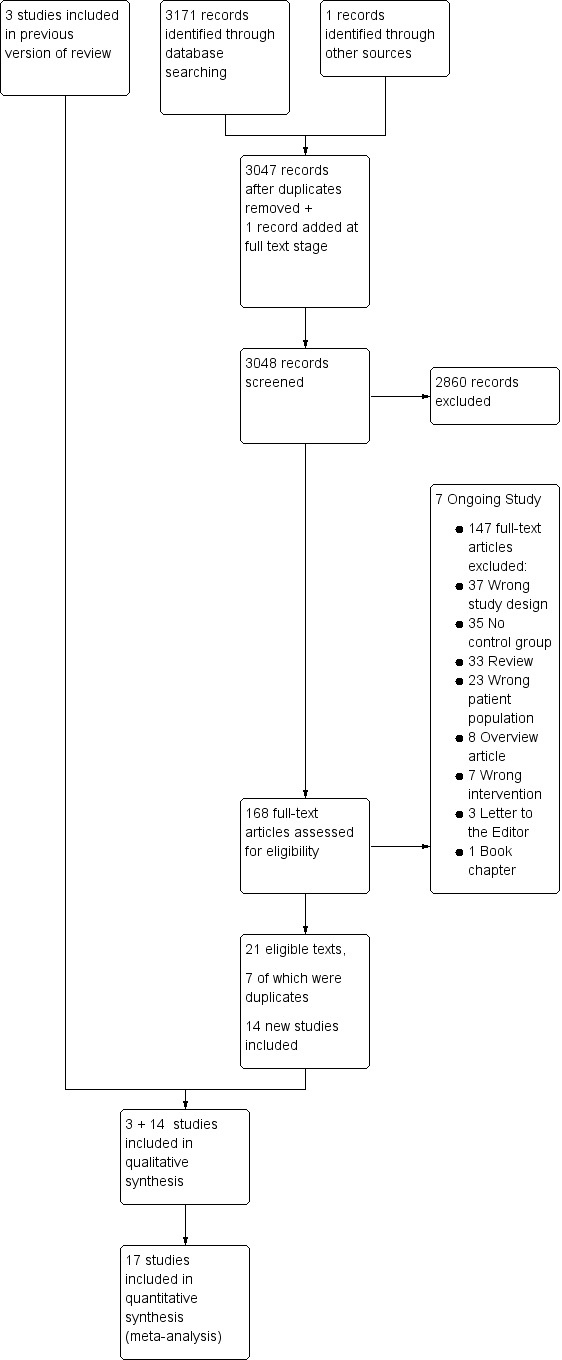
PRISMA flow diagram.
Results of the search
The electronic searches resulted in 3171 references, some of which were duplicates and thus removed. This left 3048 texts to be screened in Covidence, of which 2860 were judged irrelevant on the basis of their abstracts. In the process of obtaining full texts on the relevant studies, we identified one more relevant study that was not found by the electronic search and added it to the full texts in Covidence, giving a total of 168 full‐text papers that were then read and assessed for eligibility.
For planned and ongoing studies, we contacted the relevant authors and requested possible results and timeframes (see Characteristics of ongoing studies table for details). None of these had any results ready to share and we designated seven studies as ongoing at this stage.
In a few otherwise relevant studies it was unclear whether the data could be extracted, in which case, we contacted the authors, all of whom responded and some supplied us with missing data and missing information about methodology (Chen 2018; Thompson 2018; Vermeulen 2018). For the Pleizier 2017 study, our question was with regard to the patient population, which the authors (Vermeulen 2018) confirmed fulfilled the criteria to be included in the review. For two other studies, our queries were with regard to the outcome data. This was not available for the Thompson 2013 study, but Chen 2014 did present data on secondary outcomes. All three studies were then included.
We excluded 147 papers after reading the full texts and contacting the authors (see Characteristics of excluded studies table).
This resulted in 21 full texts being included, seven of which were duplicates of the same study, thus the total number of studies included in the analysis was 17 (14 new, and three studies from the original review; see Characteristics of included studies table for details).
A flowchart diagram of this process can be found in Figure 1.
Included studies
Seventeen studies met the inclusion criteria: Aamir 2012; Ataoglu 2003; Chen 2014; Dallocchio 2016; Drane 2016; Goldstein 2010; Hubschmid 2015; Jordbru 2014; Khattak 2006; LaFrance 2014; Moene 2002; Moene 2003; Mousavi 2008; Nielsen 2017; Pleizier 2017; Thompson 2013; Tolchin 2019. Each study is described in detail in the Characteristics of included studies table.
One cross‐over study was included in the review (Jordbru 2014). We used the data from the first period of this study as recommended by Curtin 2002.
Design
All the 17 studies were RCTs, 16 with parallel‐group designs, and one with a cross‐over design (Jordbru 2014).
Sample sizes
The 17 studies included 894 participants. The studies varied in sizes between 16 participants and 195 participants.
Setting
The studies took place in a variety of settings. Four were conducted in psychiatric inpatient settings (Aamir 2012; Ataoglu 2003; Khattak 2006; Moene 2002); three in psychiatric outpatient settings (Goldstein 2010; Hubschmid 2015; Moene 2003); two in neurology departments, one in day‐patient settings (Nielsen 2017) and one in a general outpatient setting (Pleizier 2017); one was in a veteran medical centre with outpatients (Chen 2014); one in an emergency unit (Mousavi 2008); one was in a hospital rehabilitation clinic with inpatients (Jordbru 2014); one with outpatients in three different academic hospital settings (LaFrance 2014); three originated in an epilepsy unit, two of those mainly taking place at the hospital (Thompson 2013) or in local clinics (Tolchin 2019), and one mainly by telephone (Drane 2016); and one study gave no information on the setting (Dallocchio 2016).
Country
The studies took place in nine countries, with a single study in each of Iran, Italy, Norway, Switzerland and Turkey; two studies from each of Pakistan and the UK, three studies taking place in The Netherlands and four in the USA. Thus, the locations spanned a wide range of countries given the small number of studies included.
Participants
Participants were all adults aged 18–80 years with a confirmed diagnosis of conversion disorder or dissociative disorder. In one study, there were doubts about whether this was the case (Pleizier 2017), but by contacting the author, it was confirmed that the majority of participants did have conversion disorder (Vermeulen 2018). The duration of symptoms varied from a few hours in one study (Mousavi 2008) to several months or years in most other studies. The total number of participants was 894 with 673 women (75%) and 221 men (25%), giving a female:male ratio of 3:1.
Interventions
The interventions were varied and included CBT (Goldstein 2010; LaFrance 2014), hypnosis (Moene 2002; Moene 2003; Mousavi 2008), a psychoeducative focus (Chen 2014; Drane 2016; Pleizier 2017; Thompson 2013), physical rehabilitation within a cognitive behavioural framework (Dallocchio 2016; Jordbru 2014; Nielsen 2017); psychodynamic therapy (Hubschmid 2015); paradoxical intervention (Ataoglu 2003); behavioural therapy (Aamir 2012; Khattak 2006); and motivational interviewing given prior to the therapy (Tolchin 2019).
Controls
Elleven studies received TAU or SMC as controls (Aamir 2012; Chen 2014; Goldstein 2010; Hubschmid 2015; Khattak 2006; LaFrance 2014; Moene 2002; Nielsen 2017; Pleizier 2017; Thompson 2013; Tolchin 2019), two studies had wait list controls (Jordbru 2014; Moene 2003), while two studies had psychopharmaceutical interventions as controls (Ataoglu 2003; Mousavi 2008).
One study had four interventions and no control, only one of which was clearly a psychosocial intervention and one clearly a pharmaceutical intervention, thus for the purpose of using the data in this review, we chose the psychosocial as active intervention and the pharmaceutical as control (Mousavi 2008).
In one study the reported control group was not part of the original randomisation for the study and data for that group thus not eligible for inclusion here (Dallocchio 2016). As that study had two intervention arms where the participants were randomised correctly, we decided to extract data from those instead, using the least invasive intervention as control.
One study had a design where similar interventions were given to both the active and control groups, but with the active group having additional components compared with the controls (Drane 2016).
None of the studies had any restrictions on, or documentation of, whether their control groups received other interventions outside of the study.
Duration of study
The duration of studies varied between a few hours (Mousavi 2008) and more than four months (Goldstein 2010; LaFrance 2014). Interventions for inpatients or day patients tended to be relatively short, lasting five‐days to one week in hospital, some with additional follow‐up afterwards. Interventions given to outpatients of psychoeducative nature tended to have few actual contacts spread out over some months, while therapeutic interventions delivered for outpatients tended to require regular weekly or bi‐weekly visits to an outpatient clinic over 12 weeks.
Outcomes
The studies were primarily clinical, with one feasibility study (Nielsen 2017), which had relevant outcome measures for use of this review.
Rating scales and measurements
Studies used the following scales and measures.
Primary outcomes
For the primary outcome of a reduction in physical signs, several studies used patients seizure diaries as the main measurement, where patients, relatives or staff noted the number of daily or weekly seizures (Aamir 2012; Drane 2016; Goldstein 2010; LaFrance 2014; Mousavi 2008; Tolchin 2019). This would then be used to judge change either as a change in numbers, or percentage when. One study devised its own scale (Drane 2016).
One study measured the primary outcome by looking at the number of participants without conversive attacks in the last two weeks (Ataoglu 2003).
Two studies used a video rating scale developed by the authors, to judge the severity of symptoms (Moene 2002; Moene 2003).
Some studies used different standardised scales to measure reduction in physical signs (Dallocchio 2016; Hubschmid 2015; Khattak 2006; Nielsen 2017; Pleizier 2017). In those studies, the following scales was used:
physical component of the SF‐36 (Jenkinson 1996);
SDQ‐20 (Nijenhuis 1996);
Psychogenic Movement Disorder Scale (PMDRS) total score (Hinson 2005);
Clinical Global Impression (CGI) (Guy 1976).
Finally, three studies did not report on our primary outcome (Chen 2014; Jordbru 2014; Thompson 2013).
Secondary outcomes
Studies used the following scales.
Level of functioning:
GAF (APA 1994; Jones 1995);
Functional Independence Measure (FIM) (McDowell 1996);
WSAS (Mundt 2002).
Quality of life:
full version SF‐36 (Jenkinson 1996);
QOLIE31 (Cramer 1998);
Quality of Life in Epilepsy 10 (QOLIE10‐P) (Cramer 1996).
General mental state:
SCL‐90 (Derogatis 1977);
SF‐36 or SF‐12 Mental Component) (Jenkinson 1996).
Depression:
HADS – Depression Component (Zigmond 1983);
HRSD (Hamilton 1960);
BDI‐II (Beck 1996).
Anxiety:
BAI (Beck 1988);
Hamilton Rating Scale for Anxiety (HRSA) (Hamilton 1959);
Hospital Anxiety and Depression Scale – Anxiety Component (Zigmond 1983).
Use of health service resources:
Client Service Receipt Inventory (adjusted);
number of general practitioner consultations (Beecham 2019).
Excluded studies
We excluded 147 full‐text articles for the following reasons: 37 studies had a wrong study design, 35 studies had no control group, 23 studies had a wrong population and seven studies had a wrong intervention. Eight texts were overview articles, 33 were reviews, three were letters to the Editor and one was a book chapter.
We provided references for all the excluded full texts together with the reasons in the Characteristics of excluded studies table with excluded studies.
Studies awaiting assessment
There are no studies awaiting assessment.
Ongoing studies
We found seven ongoing studies. Some had very clear and updated information on the study in the ClinicalTrials Register, in which case we have given that information below, while other studies gave less clear information and were explored via personal communication with the principal investigators, in order to obtain all available data. The following projects are all RCTs, either in preliminary phases, still performing data collection, have not yet made their data available or have not yet published their results.
Professor Laura Goldstein 2018 is heading a large research project in the UK (CODES), which has been described in various articles (Goldstein 2016; Goldstein 2017; Robinson 2017). Their data and publication is not yet ready (Goldstein 2018), but is likely to be relevant for future updates of this review.
Modum Bad 2018, a treatment institution in Norway specialising in trauma, is also undertaking a research project in this area (NCT02450617b), with data collection hopefully being completed in 2019 according to the principal investigator Harald Baekkelund (Modum Bad 2018).
Dr Fobian 2018 is principal investigator on a project "Treatment outcomes of CBT for PNES" (NCT02801136), which is due to publish results soon according to the author (Fobian 2018).
Dr Meinlschmidt has conducted a study on "Treatment of globus sensations with psychotherapy (NCT01590992), but according to the author no results are available yet (Meinlschmidt 2018).
Another study, "The role of the temporo‐parietal junction in functional neurological disorders. A study with mindfulness‐based stress reduction therapy" had received ethical approval and was due to start late 2018 according to the principal investigator Professor Aybek 2018 (Aybek 2018; DRKS00012997).
Dr Kim D Bullock is leading a study on "Embodied virtual reality therapy for functional neurological symptom/conversion disorder', which is currently recruiting participants and expects to be complete by January 2022 (NCT02764476).
Dr Anna Philine Senf‐Beckenbach is heading a study named "Evaluation of the effect of a psychotherapy program with body movement focus for patients with dissociative seizures." (DRKS00014251).
Risk of bias in included studies
We assessed the risk of bias of each included study using the Cochrane 'Risk of bias' tool (Higgins 2011). A summary of our assessment is displayed in Figure 2 and Figure 3 and the details of the assessment of each study can be found in the Characteristics of included studies table. We assessed most studies to be studies with an overall high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered random sequence generation at low risk of bias in 13 studies (Aamir 2012; Ataoglu 2003; Chen 2014; Dallocchio 2016; Drane 2016; Goldstein 2010; Hubschmid 2015; Jordbru 2014; LaFrance 2014; Nielsen 2017; Pleizier 2017; Thompson 2013; Tolchin 2019), at high risk in one study (Mousavi 2008), and at unclear risk of bias in three studies (Khattak 2006; Moene 2002; Moene 2003).
Allocation concealment
We considered allocation concealment at low risk of bias in five studies (Goldstein 2010; Hubschmid 2015; Jordbru 2014; Moene 2002; Tolchin 2019), and at unclear risk of bias in 12 studies (Aamir 2012; Ataoglu 2003; Chen 2014; Dallocchio 2016; Drane 2016; Khattak 2006; LaFrance 2014; Moene 2003; Mousavi 2008; Nielsen 2017; Pleizier 2017; Thompson 2013).
Blinding
We considered the method of blinding of participants and personal unclear in nine studies (Aamir 2012; Chen 2014; Drane 2016; Hubschmid 2015; Jordbru 2014; Moene 2002; Mousavi 2008; Pleizier 2017; Thompson 2013), and at high risk of bias in eight studies (Ataoglu 2003; Dallocchio 2016; Goldstein 2010; Khattak 2006; LaFrance 2014; Moene 2003; Nielsen 2017; Tolchin 2019).
We considered the method of blinding of outcome assessment at low risk of bias in five studies (Ataoglu 2003; Dallocchio 2016; LaFrance 2014; Moene 2002; Tolchin 2019), and unclear in eight studies (Aamir 2012; Chen 2014; Drane 2016; Goldstein 2010; Jordbru 2014; Moene 2003; Pleizier 2017; Thompson 2013). In four studies there was a high risk of bias regarding blinding of outcome assessment (Hubschmid 2015; Khattak 2006; Mousavi 2008; Nielsen 2017).
Incomplete outcome data
Eight studies displayed low risk of bias in regards to incomplete data (Ataoglu 2003; Chen 2014; Dallocchio 2016; Goldstein 2010; Hubschmid 2015; Jordbru 2014; Khattak 2006; Pleizier 2017), and six studies displayed high risk of bias (Drane 2016; Moene 2003; Mousavi 2008; Nielsen 2017; Thompson 2013; Tolchin 2019). In three studies, there was lack of information and we could not assess whether the method used to handle missing data was likely to bias the effect estimate (Aamir 2012; LaFrance 2014; Moene 2002).
Selective reporting
Five studies displayed low risk of bias with regard to selective reporting (Goldstein 2010; Hubschmid 2015; LaFrance 2014; Nielsen 2017; Tolchin 2019), and five studies displayed high risk of bias (Chen 2014; Khattak 2006; Moene 2002; Mousavi 2008; Pleizier 2017). In seven studies, it was unclear whether authors reported all predefined outcomes (Aamir 2012; Ataoglu 2003; Dallocchio 2016; Drane 2016; Jordbru 2014; Moene 2003; Thompson 2013).
Other potential sources of bias
In 15 studies there were no other sources of bias (Aamir 2012; Ataoglu 2003; Chen 2014; Dallocchio 2016; Goldstein 2010; Hubschmid 2015; Jordbru 2014; Khattak 2006; LaFrance 2014; Moene 2002; Moene 2003; Mousavi 2008; Nielsen 2017; Pleizier 2017; Thompson 2013). One study measured the primary outcome using a self‐developed scale that was not properly validated (Drane 2016) and this was assessed to likely bias the effect estimate. In one study, it was unclear whether there was any other sources of bias (Tolchin 2019).
Subgroup analysis
We did not perform any subgroup or sensitivity analyses due to lack of data
Sensitivity analysis
We did not perform any sensitivity analyses due to lack of data
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12
Summary of findings 1. Paradoxical intention therapy compared with diazepam.
| Paradoxical intention therapy compared with diazepam for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatient and inpatient Intervention: paradoxical intention therapy Comparison: diazepam over 45 days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Paradoxical intention therapy | |||||
|
Reduction in physical signs (number of patients without any conversive attacks in last 2 weeks) End of treatment |
Study population | RR 1.44 (0.91 to 2.28) | 30 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Paradoxical intention therapy may have no effect on physical signs at end of treatment. | |
| 600 per 1000 | 864 per 1000 (54 less to 768 more) | |||||
| Level of functioning | — | — | — | — | — | No studies assessed this outcome. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded two levels due to imprecision (wide confidence intervals; based on one study with few patients). bDowngraded one level due to high risk of bias.
Summary of findings 2. Hypnosis plus treatment as usual compared with treatment as usual.
| Hypnosis + treatment as usual compared with treatment as usual for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: inpatient Intervention: hypnosis + TAU Comparison: TAU | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Hypnosis + TAU | |||||
|
Reduction in physical signs (Severity of impairment) Measured by the VRMC scale (higher is better) Range: 1–7 End of treatment |
The mean reduction in physical signs in the control group was 5.9 |
MD 0.49 lower (1.28 lower to 0.30 higher) |
— | 45 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Hypnosis + TAU may have no effect on physical signs at end of treatment |
| Level of functioning | — | — | — | — | — | No studies assessed this outcome |
| Quality of life | — | — | — | — | — | No studies assessed this outcome |
| Adverse events | — | — | — | — | — | No studies assessed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; TAU: treatment as usual; VRMC: The Video Rating Scale for Motor Conversion Symptoms. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two levels due to imprecision (wide confidence intervals; based on 1 study with few patients).
Summary of findings 3. Hypnosis compared with wait list.
| Hypnosis compared with wait list for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatient Intervention: hypnosis Comparison: wait list | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Wait list | Hypnosis | |||||
|
Reduction in physical signs (Severity of impairment) Measured by the VRMC scale (higher is better) Range: 1–7 End of treatment |
The mean reduction in physical signs in the control group was 3.8 |
MD 2.10 higher (1.34 higher to 2.86 higher) |
— | 49 (1 study) | ⊕⊕⊝⊝ Lowa,b | Hypnosis may have little effect on reduction in physical signs at end of treatment |
| Level of functioning | — | — | — | — | — | No studies assessed this outcome |
| Quality of life | — | — | — | — | — | No studies assessed this outcome |
| Adverse events | — | — | — | — | — | No studies assessed this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; VRMC: Video Rating Scale for Motor Conversion Symptoms. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded one level due to imprecision (based on 1 study with few patients).
Summary of findings 4. Behavioural therapy plus treatment as usual compared with treatment as usual.
| Behavioural therapy + TAU compared with TAU for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: inpatient Intervention: behavioural therapy + TAU Comparison: TAU | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Behavioural therapy + TAU | |||||
|
Reduction in physical signs Number of weekly seizures, as assessed by daily self‐reported treatment diary End of treatment |
The mean reduction in physical signs in the control group was 27.8 |
MD 21.40 lower (27.88 lower to 14.92 lower) |
— | 18 (1 study) |
Very lowa,b ⊕⊝⊝⊝ |
Behavioural therapy + TAU may have little effect on reduction in physical signs at end of treatment. |
|
Reduction in physical signs (symptom severity) Measured by Clinical Global Impression scale (lower is better) Range: 1–7 End of treatment |
The mean reduction in physical signs in the control group was 4.48 |
MD 2.90 lower (3.41 lower to 2.39 lower) |
— | 90 (1 study) |
Very lowa,b ⊕⊝⊝⊝ |
Behavioural therapy + TAU may have little effect on reduction in physical signs at end of treatment. |
| Level of functioning | — | — | — | — | — | No studies assessed this outcome. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded two levels due to high risk of bias. bDowngraded one level due to imprecision (data based on 1 study with few participants).
Summary of findings 5. Cognitive behavioural therapy as compared with standard medical care.
| Cognitive behavioural therapy compared with standard medical care for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatient Intervention: cognitive behavioural therapy Comparison: standard medical care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard medical care | Cognitive behavioural therapy | |||||
|
Reduction in physical signs Reduction in monthly seizure frequency as assessed by a daily self‐reported seizure diary End of treatment |
Study population | RR 1.56 (0.39 to 6.19) | 16 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Cognitive behavioural therapy may have little or no effect on reducing physical signs at end of treatment. | |
| 286 per 1000 | 446 per 1000 (174 less to 1484 more) | |||||
|
Reduction in physical signs Monthly seizure frequency as assessed by a daily self‐reported seizure diary (lower is better) End of treatment |
The mean reduction in physical signs in the control group was 6.75 |
MD –4.75 lower (18.73 lower to 9.23 higher) |
— | 61 (1 study) | ⊕⊕⊝⊝ Lowb | Cognitive behavioural therapy may have little or no effect on reducing physical signs at end of treatment. |
|
Reduction in physical sign Seizure freedom as assessed by a daily self‐reported seizure diary End of treatment |
Study population | RR 2.33 (0.30 to 17.88) | 16 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Cognitive behavioural therapy may have little or no effect on reducing physical signs at end of treatment. | |
| 143 per 1000 | 333 per 1000 (100 less to 2414 more) | |||||
|
Level of functioning As measured by the GAF (range 0–100) scale and the WSAS scale (0–40) (lower is better) End of treatment |
— |
SMD 0.44 higher (1.69 lower to 2.57 higher) I2 = 91% |
— | 74 (2 studies) | ⊕⊝⊝⊝ Very lowa,c,d | Cognitive behavioural therapy may have little or no effect on level of functioning at end of treatment. |
|
Quality of life As assessed by QOLIE31 (higher is better) Range: 15–97 End of treatment |
The mean quality of life in the control group was 9.7 |
MD 11.20 higher (7.98 lower to 30.38 higher) |
— | 16 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Cognitive behavioural therapy may have little or no effect on quality of life at end of treatment. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; GAF: global assessment of functioning; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; QOLIE31: quality of life in epilepsy inventory; RR: Risk Ratio; SMD: standardised mean difference; WSAS: work and social adjustment scale. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two levels due to imprecision (wide confidence intervals; 1 study with few participants). cDowngraded one level due to imprecision (wide confidence intervals). dDowngraded one level due to inconsistency (I2 = 91%).
Summary of findings 6. Psychoeducational follow‐up programme compared with treatment as usual.
| Systematic follow‐up programme compared with TAU for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatient Intervention: systematic follow‐up programme Comparison: TAU | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Systematic follow‐up programme | |||||
|
Reduction in physical signs End of treatment |
— | — | — | — | — | No studies assessed this outcome at end of treatment. |
|
Level of functioning As assessed by WSAS scale (lower is better) Range: 0–40 End of treatment |
The mean level of functioning in the control group was 25.52 |
MD 7.12 lower (12.47 lower to 1.77 lower) |
— | 43 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Psychoeducational follow‐up programme may have little effect on level of functioning at end of treatment. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; TAU: treatment as usual; WSAS: Work and Social Adjustment Scale. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded two levels due to high risk of bias. bDowngraded one level due to imprecision (wide confidence interval and based on one study with few participants).
Summary of findings 7. Specialised cognitive behavioural therapy‐based physiotherapy compared with waitlist.
| Specialised CBT‐based physiotherapy programme compared with wait list for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: inpatient Intervention: specialised CBT‐based physiotherapy programme Comparison: wait list | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Wait list | Specialised CBT‐based physiotherapy | |||||
|
Reduction in physical signs End of treatment |
— | — | — | — | — | No studies assessed this outcome. |
|
Level of functioning As measured by FIM (higher is better) Range: 18–126 End of treatment |
The mean level of functioning in the control group was 80.9 |
MD 9.20 higher (6.06 higher to 12.34 higher) |
— | 118 (1 study) | ⊕⊕⊝⊝ Lowa,b | Specialised CBT‐based physiotherapy may slightly improve level of functioning at end of treatment. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBT: cognitive behavioural therapy; CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; FIM: Functional Independence Measure Motor; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded one level due to imprecision (based on one study with few patients).
Summary of findings 8. Specialised cognitive behavioural therapy‐based physiotherapy intervention compared with treatment as usual.
| Specialised CBT‐based physiotherapy‐led intervention compared with TAU for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatients at day hospital Intervention: specialised CBT‐based physiotherapy‐led intervention Comparison: TAU | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Specialised CBT‐based physiotherapy‐led intervention | |||||
|
Reduction in physical signs End of treatment |
— | — | — | — | — | No studies assessed this outcome at end of treatment. |
|
Level of functioning As assessed by WSAS scale (lower is better) Range: 0–40 End of treatment |
The mean level of functioning in the control group was 26.9 |
MD 7.10 lower (11.40 lower to 2.80 lower) |
— | 54 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Specialised CBT‐based physiotherapy intervention may slightly improve level of functioning at end of treatment. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CBT: cognitive behavioural therapy; CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; TAU: treatment as usual; WSAS: Work and Social Adjustment Scale. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two levels due to imprecision (wide confidence interval and based on one study with few patients).
Summary of findings 9. Brief psychotherapeutic intervention compared with standard care.
| Brief psychotherapeutic intervention compared with standard care for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatients Intervention: brief psychotherapeutic intervention Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Brief psychotherapeutic intervention | |||||
|
Reduction in physical signs As assessed by SDQ‐20 (lower is better) Range: 20–100 End of treatment |
Study population | RR 0.12 (0.01 to 2.00) | 19 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Brief psychotherapeutic intervention may have no effect on physical signs at end of treatment. | |
| 400 per 1000 | 48 per 1000 (396 less to 400 more) | |||||
| Level of functioning | — | — | — | — | — | No studies assessed this outcome. |
|
Quality of life As assessed by SF‐36 (lower is better) Range: 0–100 End of treatment |
The mean quality of life in the control group was 50.56 |
MD 6.99 lower (28.09 lower to 14.11 higher) |
16 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Brief psychotherapeutic intervention may have little effect on quality of life after end of treatment. | |
| Adverse events | No studies assessed this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; RR: Risk Ratio; SDQ‐20: somatoform dissociation questionnaire; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two levels due to imprecision (Wide confidence intervals and based on one study with few patients).
Summary of findings 10. Cognitive behavioural therapy plus adjunctive physical activity compared with cognitive behavioural therapy alone.
| CBT + APA compared with CBT alone for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatients Intervention: CBT + APA Comparison: CBT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| CBT | CBT + APA | |||||
|
Reduction in physical signs (overall physical impact) As measured by PMDRS, total score (lower is better) Range: 0–128 End of treatment |
The mean reduction in physical signs in the control group was 33.2 |
MD 5.60 higher (15.48 lower to 26.68 higher) |
— | 21 (1 study) | ⊕⊝⊝⊝ Very lowa,b | CBT + APA may have no effect on physical signs at end of treatment. |
| Level of functioning | — | — | — | — | — | No studies assessed this outcome. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). APA: adjunctive physical activity; CBT: cognitive behavioural therapy; CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; PMDRS: Psychogenic Movement Disorders Rating Scale. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two level due to imprecision (wide confidence interval, based on one study with few participants).
Summary of findings 11. Hypnosis compared with diazepam.
| Hypnosis compared with diazepam for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: emergency unit Intervention: hypnosis Comparison: diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Hypnosis | |||||
|
Reduction in physical signs Number with symptom freedom End of treatment |
Study population | RR 0.69 (0.39 to 1.24) | 40 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Hypnosis may have no effect on physical signs at end of treatment. | |
| 650 per 1000 | 448 per 1000 (397 less to 156 more) | |||||
| Level of functioning | — | — | — | — | — | No studies assessed this outcome. |
| Quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two levels due to imprecision (Wide confidence interval and based on one study with few patients).
Summary of findings 12. Psychotherapy preceded by motivational interviewing compared with psychotherapy alone.
| Psychotherapy preceded by motivational interviewing compared with psychotherapy alone for conversion disorder | ||||||
|
Patient or population: people with conversion disorder according to DSM‐IV or ICD‐10 criteria Settings: outpatient Intervention: psychotherapy preceded by motivational interviewing Comparison: psychotherapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Psychotherapy | Psychotherapy preceded by motivational interviewing | |||||
|
Reduction in physical signs Decrease in seizure frequency End of treatment |
The mean reduction in physical signs in the control group was 34.8 |
MD 41.40 higher (4.92 higher to 77.88 higher) |
— | 54 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Psychotherapy preceded by motivational interviewing may have little effect on physical signs at end of treatment. |
| Level of functioning | — | — | — | — | — | No studies assessed this outcome. |
|
Quality of life As measured by QOLIE10 (lower is better) Range: 10–50 End of treatment |
The mean quality of life in the control group was 1.8 |
MD 5.40 higher (0.26 higher to 10.54 higher) |
— | 47 (1 study) | ⊕⊝⊝⊝ Very lowa,b | Psychotherapy preceded by motivational interviewing may have little effect on quality of life at end of treatment. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders 4th Edition; ICD‐10:International Classification of Diseases, Tenth Revision; MD: mean difference; QOLIE10: quality of life in epilepsy. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to high risk of bias. bDowngraded two level due to imprecision (wide confidence interval, based on one study with few participants).
We present the results for each of the primary and secondary outcomes connected to the 12 comparisons below. Most are presented as MDs because there was only one study; where there is more than one study and the outcomes were reported on different scales, we present the standardised mean difference (SMD). We considered a statistical significant SMD effect size of: 0.15 or less to have no clinically meaningful effect; 0.15 to 0.40 to have a clinical meaningful but small effect; 0.40 to 0.75 to have a moderate effect; and greater than 0.75 to have a large treatment effect (Cohen 1988; Thalheimer 2002).
We contacted the authors of three studies with unclear or missing data and requested the necessary information. All three replied, which led to one study being included but with no data available (Thompson 2013), one study included in the final analysis on basis of secondary outcomes as no data were available on primary outcomes (Chen 2014), and one study included in the final analysis as it was clarified that the population consisted of people with conversion and dissociative disorders (Pleizier 2017).
We considered most studies at high risk of bias due to systematic errors. However, we used all eligible studies in the meta‐analyses, as it is recommend in the Cochrane Handbook for Systematic Reviews of Interventions to do so when all the studies are assigned the same risk of bias. We took into account our 'Risk of bias' assessment when considering the quality of the evidence using the GRADE approach, to ensure that judgements about risk of bias and other factors affecting the certainty of the evidence are taken into account when interpreting the results of the review (Higgins 2019; Section 8.8.3.1).
Comparison 1: inpatient paradoxical intention therapy compared with outpatient diazepam
One study compared inpatient paradoxical intention therapy with outpatient diazepam (Ataoglu 2003).
1.1 Reduction in physical signs
Inpatient paradoxical intention therapy did not reduce conversive symptoms, measured by number of participants without conversive attacks in the last week, compared with outpatient diazepam et end of treatment (RR 1.44, 95% CI 0.91 to 2.28; 1 study, 30 participants; P = 0.12) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Inpatient paradoxical intention therapy versus outpatient diazepam, Outcome 1: Reduction in physical signs: no conversion symptoms in last 2 weeks
1.2 Level of functioning
The study did not measure level of functioning.
1.3 Quality of life
The study did not measure quality of life.
1.4 Mental state
1.4.1 Anxiety
Inpatient paradoxical intention therapy might reduce anxiety symptoms at end of treatment, measured by HADS, compared with outpatient diazepam at end of treatment (MD –3.73, 95% CI –6.96 to –0.50; 1 study, 30 participants; P = 0.02) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Inpatient paradoxical intention therapy versus outpatient diazepam, Outcome 2: Mental state – anxiety (Hamilton)
1.5 Dropout rate
In Ataoglu 2003, no participants left the study before the end from either group.
1.6 Use of health service resources
The study did not measure use of health service resources.
1.7 Adverse effects
The study did not measure adverse effects.
Comparison 2: inpatient treatment programme plus hypnosis compared with inpatient treatment programme
One study compared inpatient treatment programme plus hypnosis with inpatient treatment programme (Moene 2002).
2.1 Reduction in physical signs
Inpatient treatment programme plus hypnosis did not reduce severity of impairment, measured using a video rating scale, compared with inpatient treatment programme at the end of treatment (MD –0.49, 95% CI –1.28 to 0.30; 1 study, 45 participants; P = 0.23) or at follow‐up (MD 0.13, 95% CI –0.55 to 0.81; 1 study, 45 participants; P = 0.71) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Inpatient treatment programme plus hypnosis versus inpatient treatment programme, Outcome 1: Reduction in physical signs: severity of impairment (VRMC)
2.2 Level of functioning
The study did not measure level of functioning.
2.3 Quality of life
The study did not measure quality of life.
2.4 Mental state
Inpatient treatment programme plus hypnosis did not reduce symptoms of mental state, measured using SCL‐90, compared with inpatient treatment programme at the end of treatment (MD 1.42 95% CI –36.02 to 38.86; 1 study, 45 participants; P = 0.94) or at follow‐up (MD –5.97, 95% CI –44.22 to 32.28; 1 study, 45 participants; P = 0.76) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Inpatient treatment programme plus hypnosis versus inpatient treatment programme, Outcome 2: Mental state (SCL‐90)
2.5 Dropout rate
In Moene 2002, 2/26 participants left the treatment programme and hypnosis group compared with 2/23 of the participants in the treatment programme and individual sessions group before the end of treatment (RR 0.88, 95% CI 0.14 to 5.79; 1 study, 49 participants; P = 0.90) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Inpatient treatment programme plus hypnosis versus inpatient treatment programme, Outcome 3: Dropout rate
2.6 Use of health service resources
The study did not measure use of health service resources.
2.7 Adverse effects
The study did not measure adverse effects.
Comparison 3: outpatient hypnosis compared with wait list
One study compared outpatient hypnosis with wait list (Moene 2003).
3.1 Reduction in physical signs
3.1.1 Severity of impairment
Outpatient hypnosis might reduce severity of impairment, measured using a video rating scale, compared with wait list at the end of treatment (MD 2.10, 95% CI 1.34 to 2.86; 1 study, 49 participants; P < 0.00001) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Outpatient hypnosis versus wait list, Outcome 1: Reduction in physical signs: severity of impairment (VRMC)
3.2 Level of functioning
The study did not measure level of functioning.
3.3 Quality of life
The study did not measure quality of life.
3.4 Mental state
The study did not measure mental state.
3.5 Dropout rate
Outpatient hypnosis did not reduce dropout compared with wait list at end of treatment (RR 4.17, 95% CI 0.50 to 34.66; 1 study, 49 participants; P = 0.19) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Outpatient hypnosis versus wait list, Outcome 2: Dropout rate
3.6 Use of health service resources
The study did not measure use of health service resources.
3.7 Adverse effects
No study measured adverse effects.
Comparison 4: behavioural therapy plus routine clinical care compared with routine clinical care
Two studies compared behavioural therapy plus routine clinical care with routine clinical care (Aamir 2012; Khattak 2006).
4.1 Reduction in physical signs
4.1.1 Number of weekly seizures
Behavioural therapy plus routine clinical care might reduce the number of weekly seizures at the end of treatment, measured using participant's seizure diaries, compared with routine clinical care alone at end of treatment (MD –21.40, 95% CI –27.88 to –14.92; 1 study, 18 participants; P < 0.00001) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Behavioural therapy plus routine clinical care versus routine clinical care, Outcome 1: Reduction in physical signs: number of weekly fits
4.1.2 Symptom severity
Behavioural therapy plus routine clinical care might reduce symptom severity at the end of treatment, measured using the CGI scale, compared with routine clinical care alone at end of treatment (MD –2.90 95% CI –3.41 to –2.39; 1 study, 90 participants; P < 0.00001) (Analysis 4.2).
4.2. Analysis.

Comparison 4: Behavioural therapy plus routine clinical care versus routine clinical care, Outcome 2: Reduction in physical signs: symptom severity (Clinical Global Impression CGI)
4.2 Level of functioning
Neither study measured level of functioning.
4.3 Quality of life
Neither study measured quality of life.
4.4 Mental state
4.4.1 Anxiety
Behavioural therapy plus routine clinical care might reduce symptoms of anxiety at the end of treatment, measured using Hamilton Anxiety Scale, compared with routine clinical care alone at end of treatment (MD –5.47, 95% CI –7.08 to –3.86; 2 studies, 108 participants; P < 0.00001) (Analysis 4.3).
4.3. Analysis.

Comparison 4: Behavioural therapy plus routine clinical care versus routine clinical care, Outcome 3: Mental state – anxiety (HADS)
4.4.2 Depression
Behavioural therapy plus routine clinical might reduce symptoms of depression at follow‐up, measured using Hamilton Depression Scale, compared with routine clinical care alone at follow‐up (MD –4.99, 95% CI –6.36 to –3.62; 2 studies, 108 participants; P < 0.00001) (Analysis 4.4).
4.4. Analysis.

Comparison 4: Behavioural therapy plus routine clinical care versus routine clinical care, Outcome 4: Mental state – depression (HADS)
4.5 Dropout rate
Behavioural therapy plus routine clinical care was associated with fewer dropouts compared with routine clinical care alone at end of treatment (RR 0.24, 95% CI 0.06 to 0.90; 2 studies, 118 participants; P = 0.04) (Analysis 4.5).
4.5. Analysis.

Comparison 4: Behavioural therapy plus routine clinical care versus routine clinical care, Outcome 5: Dropout rate
4.6 Use of health service resources
Neither study measure used of health service resources.
4.7 Adverse effects
Neither study measured adverse effects.
Comparison 5: cognitive behavioural therapy compared with standard medical care
Two studies compared CBT with SMC (Goldstein 2010; LaFrance 2014).
5.1 Reduction in physical signs
5.1.1 Monthly seizure frequency (reduction in %)
CBT did not reduce monthly seizure frequency, measured using participants' seizure diaries, compared with SMC at end of treatment (RR 1.56, 95% CI 0.39 to 6.19; 1 study, 16 participants; P = 0.53) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 1: Reduction in physical signs: monthly seizure frequency (reduction in %)
5.1.2 Monthly seizure frequency (median interquartile range)
CBT did not reduce monthly seizure frequency, measured using participants' seizure diaries, compared with SMC at end of treatment (MD –4.75, 95% CI –18.73 to 9.23; 1 study, 61 participants; P = 0.51) or at follow‐up (MD –3.50, 95% CI –12.69 to 5.69; 1 study, 59 participants; P = 0.46) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 2: Reduction in physical signs: monthly seizure frequency
5.1.3 Seizure freedom
CBT did not reduce seizure freedom, measured using participants' seizure diaries, compared with SMC at end of treatment (RR 2.33, 95% CI 0.30 to 17.88; 1 study, 16 participants; P = 0.41) (Analysis 5.3).
5.3. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 3: Reduction in physical sign: seizure freedom
5.2 Level of functioning
CBT did not increase level of functioning compared with SMC at end of treatment (SMD 0.44, 95% CI –1.69 to 2.57; 2 studies, 74 participants; P = 0.69) or at follow‐up (SMD –0.63, 95% CI –1.18 to –0.08; 1 study, 53 participants; P = 0.03) (Analysis 5.4).
5.4. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 4: Level of functioning
5.3 Quality of life
CBT did not increase quality of life, measured using QOLIE31, compared with SMC at end of treatment (MD 11.20, 95% CI –7.98 to 30.38; 1 study, 16 participants; P = 0.25) (Analysis 5.5).
5.5. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 5: Quality of life (QOLIE31)
5.4 Mental state
5.4.1 Anxiety
CBT did not reduce symptoms of anxiety compared with SMC at end of treatment (SMD –0.31, 95% CI –0.78 to 0.15; 2 studies, 74 participants; P = 0.18) or at follow‐up (SMD –0.31, 95% CI –0.85 to 0.23; 1 study, 53 participants; P = 0.25) (Analysis 5.6).
5.6. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 6: Mental state – anxiety
5.4.2 Depression
CBT did not reduce symptoms of depression compared with SMC at end of treatment (SMD –0.25, 95% CI –0.71 to 0.21; 2 studies, 74 participants; P = 0.28) or at follow‐up (SMD –0.32, 95% CI –0.86 to 0.23; 1 study, 53 participants; P = 0.25) (Analysis 5.7).
5.7. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 7: Mental state – depression
5.4.3 Symptoms of general mental state
CBT might reduce negative mental state symptoms, measured using SCL‐90, compared with SMC at end of treatment (MD –70.60, 95% CI –121.59 to –19.61; 1 study, 16 participants; P = 0.007) (Analysis 5.8).
5.8. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 8: Mental state (SCL‐90)
5.5 Dropout rate
CBT did not decrease dropout compared with SMC at end of treatment (RR 0.37, 95% CI 0.02 to 8.01; 2 studies, 83 participants; P = 0.52) or at follow‐up (RR 1.41, 95% 0.25 to 7.87; 1 study, 64 participants; P = 0.70) (Analysis 5.9).
5.9. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 9: Dropout rate
5.6 Use of health service resources
CBT did not decrease primary health service use, measured using numbers of general practitioner consultations, compared with SMC at follow‐up (MD –1.00, 95% CI –3.32 to 1.32; 1 study, 46 participants; P = 0.40) (Analysis 5.10).
5.10. Analysis.

Comparison 5: Cognitive behavioural therapy versus standard medical care, Outcome 10: Use of health services (number of general practitioner consultations)
5.7 Adverse effects
Neither study measured adverse effects.
Comparison 6: psychoeducational follow‐up programmes compared with treatment as usual
Two studies compared psychoeducational follow‐up programmes with TAU (Drane 2016; Pleizier 2017).
6.1 Reduction in physical signs
6.1.1 Seizure frequency
Psychoeducational follow‐up programmes might reduce seizure frequency, measured using a self‐developed scale, compared with TAU at follow‐up (MD –0.80, 95% CI –1.44 to –0.16; 1 study, 27 participants; P = 0.01) (Analysis 6.1).
6.1. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 1: Reduction in physical signs: seizure frequency (self‐made scale)
6.1.2 Physical symptoms load
Psychoeducational follow‐up programmes did not reduce physical symptoms load, measured using SSF‐36 Physical Component scale, compared with TAU at follow‐up (MD –0.26, 95% CI –3.76 to 3.24; 1 study, 186 participants; P = 0.88) (Analysis 6.2).
6.2. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 2: Reduction in physical signs: physical symptom load (SF‐36 – physical)
6.2 Level of functioning
Psychoeducational follow‐up programmes might improve level of functioning, measured using WSAS, compared with TAU at end of treatment (MD –7.12, 95% CI –12.47 to –1.77; 1 study, 43 participants; P = 0.009), and at follow‐up (MD –6.11, 95% CI –11.67 to –0.55; 1 study, 43 participants; P = 0.03) (Analysis 6.3).
6.3. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 3: Level of functioning (WSAS)
6.3 Quality of life
Psychoeducational follow‐up programmes might improve quality of life, measured using QOLIE10‐P, compared with TAU at follow‐up (MD –9.30, 95% CI –14.06 to –4.54; 1 study, 27 participants; P = 0.0001) (Analysis 6.4).
6.4. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 4: Quality of life (QOLIE10‐P)
6.4 Mental state
6.4.1 Anxiety
Psychoeducational follow‐up programmes did not reduce anxiety symptoms, measured using HADS – Anxiety, compared with TAU at follow‐up (MD –0.47, 95% CI –1.67 to 0.73; 1 study, 192 participants; P = 0.44) (Analysis 6.5)
6.5. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 5: Mental state – anxiety (HADS)
6.4.2 Depression
Psychoeducational follow‐up programmes did not reduce depression symptoms compared with TAU at follow‐up (SMD –0.30, 95% CI –1.08 to 0.48; 2 studies, 219 participants; P = 0.45) (Analysis 6.6).
6.6. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 6: Mental state – depression
6.5 Dropout rate
Psychoeducational follow‐up programmes did not decrease dropout compared with TAU at end of treatment (RR 1.76, 95% CI 0.82 to 3.78; 1 study, 64 participants; P = 0.14) or at follow‐up (RR 0.54, 95% CI 0.00 to 70.37; 2 studies, 259 participants; P = 0.81) (Analysis 6.7).
6.7. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 7: Dropout rate
6.6 Use of health service resources
Psychoeducational follow‐up programmes did not decrease number of hospital visits compared with TAU at end of treatment (RR 0.18, 95% CI 0.02 to 1.43; 1 study, 64 participants; P = 0.10) (Analysis 6.8).
6.8. Analysis.

Comparison 6: Psychoeducational follow‐up programmes versus treatment as usual, Outcome 8: Use of health services (number hospital visits)
6.7 Adverse effects
Neither study measured adverse effects.
Comparison 7: specialised cognitive behavioural therapy‐based physiotherapy inpatient programme compared with wait list
One study compared CBT with wait list (Jordbru 2014).
7.1 Reduction in physical signs
The study did not measure reduction in physical signs.
7.2 Level of functioning
Specialised CBT‐based physiotherapy inpatient programme might improve level of functioning, measured using FIM, compared with wait list at end of treatment (MD 9.20, 95% CI 6.06 to 12.34; 1 study, 118 participants; P < 0.00001 (Analysis 7.1).
7.1. Analysis.

Comparison 7: Specialised cognitive behavioural therapy‐based physiotherapy inpatient programme versus wait list, Outcome 1: Level of functioning (Functional Independence Measure Motor (FIM))
7.3 Quality of life
The study did not measure quality of life.
7.4 Mental state
Specialised CBT‐based physiotherapy inpatient programme might improve mental state, measured using SF‐12, compared with wait list at end of treatment (MD 9.10, 95% CI 4.96 to 13.24; 1 study, 118 participants; P < 0.0001) (Analysis 7.2).
7.2. Analysis.

Comparison 7: Specialised cognitive behavioural therapy‐based physiotherapy inpatient programme versus wait list, Outcome 2: Mental state (SF‐12)
7.5 Dropout rate
Specialised CBT‐based physiotherapy inpatient programme did not reduce dropout compared with wait list at end of treatment (RR 0.23, 95% CI 0.03 to 1.97; 1 study, 60 participants; P = 0.18) (Analysis 7.3).
7.3. Analysis.

Comparison 7: Specialised cognitive behavioural therapy‐based physiotherapy inpatient programme versus wait list, Outcome 3: Dropout rate
7.6 Use of health service resources
The study did not measure use of health service resources.
7.7 Adverse effects
The study did not measure adverse effects.
Comparison 8: specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared with treatment as usual
One study compared specialised CBT‐based physiotherapy outpatient intervention with TAU (Nielsen 2017).
8.1 Reduction in physical signs
8.1.1 Physical symptom load
Specialised CBT‐based physiotherapy outpatient intervention might reduce physical symptom load, measured using SF‐36 – Physical Component), compared with TAU at follow‐up (MD 9.20, 95% CI 4.00 to 14.40; 1 study, 57 participants; P = 0.0005) (Analysis 8.1).
8.1. Analysis.

Comparison 8: Specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared to treatment as usual (TAU), Outcome 1: Reduction in physical signs: physical symptom load (SF‐36 – Physical Component)
8.2 Level of functioning
Specialised CBT‐based physiotherapy outpatient intervention might improve level of functioning, measured using WSAS, compared with TAU at end of treatment (MD –7.10, 95% CI –11.40 to –2.80; 1 study, 54 participants; P = 0.001) or at follow‐up (MD –6.70, 95% CI –12.07 to –1.33; 1 study, 57 participants; P = 0.01) (Analysis 8.2).
8.2. Analysis.

Comparison 8: Specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared to treatment as usual (TAU), Outcome 2: Level of functioning (WSAS)
8.3 Quality of life
The study did not measure quality of life.
8.4 Mental state
8.4.1 Anxiety
Specialised CBT‐based physiotherapy outpatient intervention did not reduce symptoms of anxiety, measured using HADS, compared with TAU at end of treatment (MD –0.60, 95% CI –3.05 to 1.85; 1 study, 54 participants; P = 0.63) or at follow‐up (MD –1.00, 95% CI –3.71 to 1.71; 1 study, 57 participants; P = 0.47) (Analysis 8.3).
8.3. Analysis.

Comparison 8: Specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared to treatment as usual (TAU), Outcome 3: Mental state – anxiety (HADS)
8.4.2 Depression
Specialised CBT‐based physiotherapy outpatient intervention might reduce symptoms of depression, measured using HADS, compared with TAU at end of treatment (MD –3.60, 95% CI –7.13 to –0.07; 1 study, 54 participants; P =0.05) and at follow‐up (MD –3.20, 95% CI –5.33 to –0.87; 1 study, 57 participants; P = 0.007) (Analysis 8.4).
8.4. Analysis.

Comparison 8: Specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared to treatment as usual (TAU), Outcome 4: Mental state – depression (HADS)
8.5 Dropout rate
Specialised CBT‐based physiotherapy outpatient intervention did not improve dropout compared with TAU at end of treatment (RR 0.20, 95% CI 0.02 to 1.61; 1 study, 60 participants; P = 0.13) or at follow‐up (RR 0.50, 95% CI 0.05 to 5.22; 1 study, 60 participants; P = 0.56) (Analysis 8.5).
8.5. Analysis.

Comparison 8: Specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared to treatment as usual (TAU), Outcome 5: Dropout rate
8.6 Use of health service resources
The study did not measure use of health service resources.
8.7 Adverse effects
The study did not measure adverse effects.
Comparison 9: brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) compared with standard care
One study compared brief psychotherapeutic interventions with SC (Hubschmid 2015).
9.1 Reduction in physical signs
9.1.1 Conversion symptoms
Brief psychotherapeutic interventions did not reduce conversion symptoms, measured using SDQ‐20, compared with SC at end of treatment (RR 0.12, 95% CI 0.01 to 2.00; 1 study, 19 participants; P = 0.14), at four months' follow‐up (RR 1.13, 95% CI 0.08 to 15.19; 1 study, 17 participants; P = 0.93) or at 10 months' follow‐up (RR 0.16, 95% 0.01 to 2.66; 1 study, 15 participants; P = 0.20) (Analysis 9.1).
9.1. Analysis.

Comparison 9: Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus standard care, Outcome 1: Reduction in physical signs: conversions symptoms (SDQ20)
9.2 Level of functioning
The study did not measure level of functioning.
9.3 Quality of life
Brief psychotherapeutic interventions did not improve quality of life, measured using SF‐36 – Physical Component, compared with SC at end of treatment (MD –6.99, 95% CI –28.09 to 14.11; 1 study, 16, participants; P = 0.52), at four months' follow‐up (MD –19.53, 95% CI –43.91 to 4.85; 1 study, 16 participants; P = 0.12) or at 10 months' follow‐up (MD –11.43, 95% CI –36.16 to 13.30; 1 study, 14 participants; P = 0.37) (Analysis 9.2).
9.2. Analysis.

Comparison 9: Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus standard care, Outcome 2: Quality of life (SF‐36)
9.4 Mental state
9.4.1 Depression
Brief psychotherapeutic intervention did not reduce depression symptoms, measured using BDI‐II, compared with SC at end of treatment (RR 1.29, 95% CI 0.10 to 17.14; 1 study, 16 participants; P = 0.85), at four months' follow‐up (RR 3.86, 95% CI 0.50 to 29.55; 1 study, 16 participants; P = 0.19) or at 10 months' follow‐up (RR 1.00, 95% CI 0.08 to 13.02; 1 study, 14 participants; P = 1.00) (Analysis 9.3).
9.3. Analysis.

Comparison 9: Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus standard care, Outcome 3: Mental state – depression (BDI‐II)
9.5 Dropout rate
Brief psychotherapeutic intervention did not reduce dropout compared with SC at end of treatment (RR 1.09, 95% CI 0.18 to 6.48; 1 study, 23 participants; P = 0.92), at four months' follow‐up (RR 1.09, 95% CI 0.28 to 4.32; 1 study, 23 participants; P = 0.90) or at 10 months' follow‐up (RR 0.82, 95% CI 0.23 to 2.87; 1 study, 23 participants; P = 0.75) (Analysis 9.4).
9.4. Analysis.

Comparison 9: Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus standard care, Outcome 4: Dropout rate
9.6 Use of health service resources
Brief psychotherapeutic intervention did not reduce use of health service compared with SC at end of treatment (MD –0.16 95% CI –1.25 to 0.93; 1 study, 19 participants; P = 0.77) (Analysis 9.5).
9.5. Analysis.

Comparison 9: Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus standard care, Outcome 5: Use of health services (emergency department visits)
9.7 Adverse effects
The study did not measure adverse effects.
Comparison 10: cognitive behavioural therapy plus adjunctive physical activity compared with cognitive behavioural therapy alone
One study compared CBT plus APA with CBT alone (Dallocchio 2016).
10.1 Reduction in physical signs
10.1.1 Overall physical impacts
CBT plus APA did not reduce overall physical impacts, measured using PMDRS total score, compared with CBT alone at end of treatment (MD 5.60, 95% CI –15.48 to 26.68; 1 study, 21 participants; P = 0.60) (Analysis 10.1).
10.1. Analysis.

Comparison 10: Cognitive behavioural therapy plus adjunctive physical activity versus cognitive behavioural therapy alone, Outcome 1: Reduction in physical signs: overall physical impact (Psychogenic Movement Disorder Scale (PMDRS)
10.2 Level of functioning
The study did not measure level of functioning.
10.3 Quality of life
The study did not measure quality of life.
10.4 Mental state
10.4.1 Anxiety
CBT plus APA did not reduce symptoms of anxiety, measured using BAI, compared with CBT alone at end of treatment (MD –3.40, 95% CI –8.01 to 1.21; 1 study, 21 participants; P = 0.15) (Analysis 10.2).
10.2. Analysis.

Comparison 10: Cognitive behavioural therapy plus adjunctive physical activity versus cognitive behavioural therapy alone, Outcome 2: Mental state – anxiety (BAI)
10.4.2 Depression
CBT plus APA did not reduce symptoms of depression, measured using Hamilton Depression Scale, compared with CBT alone at end of treatment (MD –0.50, 95% CI –3.32 to 2.32; 1 study, 21 participants; P = 0.73) (Analysis 10.3).
10.3. Analysis.

Comparison 10: Cognitive behavioural therapy plus adjunctive physical activity versus cognitive behavioural therapy alone, Outcome 3: Mental state – depression (Hamilton)
10.5 Dropout rate
CBT plus APA did not reduce dropout compared with CBT alone at end of treatment (MD 1.87, 95% CI 0.40 to 8.65; 1 study, 29 participants; P = 0.42) (Analysis 10.4).
10.4. Analysis.

Comparison 10: Cognitive behavioural therapy plus adjunctive physical activity versus cognitive behavioural therapy alone, Outcome 4: Dropout rate
10.6 Use of health service resources
The study did not measure use of health service resources.
10.7 Adverse effects
The study did not measure adverse effects.
Comparison 11: hypnosis compared with diazepam
One study compared hypnosis with diazepam (Mousavi 2008).
11.1 Reduction in physical signs
11.1.1 Symptom freedom
Hypnosis did not increase symptom freedom, measured using PMDRS Total Score, compared with diazepam at end of treatment (RR 0.69, 95% CI 0.39 to 1.24; 1 study, 40 participants; P = 0.22) (Analysis 11.1).
11.1. Analysis.

Comparison 11: Hypnosis versus diazepam, Outcome 1: Reduction in physical signs: symptom freedom
11.2 Level of functioning
The study did not measure level of functioning.
11.3 Quality of life
The study did not measure quality of life.
11.4 Mental state
The study did not measure mental state.
11.5 Dropout rate
The study did not measure dropouts.
11.6 Use of health service resources
The study did not measure use of health service resources.
11.7 Adverse effects
The study did not measure adverse effects.
Comparison 12: outpatient motivational interviewing and mindfulness‐based psychotherapy compared with psychotherapy alone
One study compared psychotherapy preceded by MI with psychotherapy alone (Tolchin 2019).
12.1 Reduction in physical signs
12.1.1 Decrease in seizure frequency
Psychotherapy preceded by MI might have a slight effect on physical signs, measured using participants' seizure diaries, compared with psychotherapy alone at end of treatment (MD 41.40, 95% CI 4.92 to 77.88; 1 study, 54 participants; P = 0.03) (Analysis 12.1).
12.1. Analysis.

Comparison 12: Outpatient motivational interviewing and mindfulness‐based psychotherapy compared with psychotherapy alone, Outcome 1: Reduction in physical signs: decrease in seizure frequency
12.2 Level of functioning
The study did not measure level of functioning.
12.3 Quality of life
Psychotherapy preceded by MI might have a slight effect on quality of life compared with psychotherapy alone at end of treatment (MD 5.40, 95% CI 0.26 to 10.54; 1 study, 47 participants; P = 0.04) (Analysis 12.3).
12.3. Analysis.

Comparison 12: Outpatient motivational interviewing and mindfulness‐based psychotherapy compared with psychotherapy alone, Outcome 3: Quality of life
12.4 Mental state
The study did not measure mental state.
12.5 Dropout rate
Psychotherapy preceded by MI did not change dropouts compared with psychotherapy alone at end of treatment (RR 1.60, 95% CI 0.29 to 8.92; 1 study, 60 participants; P = 0.59) (Analysis 12.4).
12.4. Analysis.

Comparison 12: Outpatient motivational interviewing and mindfulness‐based psychotherapy compared with psychotherapy alone, Outcome 4: Dropout rate
12.6 Use of health service resources
12.6.1 Change in monthly visits
Psychotherapy preceded by MI did not change use of health service resources compared with psychotherapy alone at end of treatment (MD –0.21, 95% CI –0.55 to 0.13; 1 study, 54 participants; P = 0.23) (Analysis 12.2).
12.2. Analysis.

Comparison 12: Outpatient motivational interviewing and mindfulness‐based psychotherapy compared with psychotherapy alone, Outcome 2: Changes in monthly visits
12.7 Adverse effects
The study did not measure adverse effects.
Discussion
We conducted this systematic review to examine the effects of psychosocial interventions of conversion and dissociative disorders in adults. We considered 168 full‐text reports from which we included 17 studies published in 21 articles in this review. We used all studies with eligible data in the meta‐analyses, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Summary of main results
In the following, we give a brief summary and present a short discussion on the results found for the primary and secondary outcomes of the review. As the overall GRADE analysis deemed all studies to be of low to very low quality, none of the following is to be taken as recommendations, but as indications of what might have effect, knowing very well that this would need to be replicated by studies of higher quality.
Primary outcomes
Reduction in physical signs
For the primary outcome, only five comparisons showed statistically significant positive effects on reduction in physical signs. These were:
hypnosis treatment for outpatients (Comparison 3);
behavioural therapy plus routine clinical care for inpatients (Comparison 4);
psychoeducational follow‐up programmes for outpatients (Comparison 6);
specialised CBT‐based physiotherapy‐led intervention (Comparison 8);
psychotherapy preceded by MI (Comparison 12).
Comparison 3 consisted of one study only, which showed that hypnosis compared to wait list reduced the severity of physical impairment at the end of treatment (Moene 2003). Interestingly, hypnosis for inpatients did not have the same effect (Moene 2002), perhaps because for inpatients the hypnosis treatment was an addition to an already full treatment programme, whereas for the outpatients it was the sole treatment. It could be argued that any treatment is better than none, but we cannot know this is the case without considering adverse effects and these were not reported.
Both studies in Comparison 4 had positive results on primary outcomes (Aamir 2012; Khattak 2006). Aamir 2012 showed a statistically significant reduction in the number of weekly seizures at end of treatment, while Khattak 2006 produced a statistically significant reduced symptom severity at end of treatment. It is noticeable that these two studies (of which Aamir 2012 was modelled on the Khattak 2006) are the studies most alike of all our comparisons, and also the only comparison group that had a positive effect on the primary outcome in more than one study. It would be of interest to see if similar results were obtained by new RCTs of a similar design, but with a much larger number of participants and lower risk of bias.
In Comparison 6, the studies showed different results. Drane 2016 showed a statistically significant reduction in seizure frequency compared to TAU at follow‐up, while Pleizier 2017 showed that psychoeducational follow‐up programmes did not reduce physical symptom load compared to TAU at follow‐up. The studies used different measures (a self‐developed scale based on number of seizures versus the SF‐36 Physical Component), but it would still be expected that similar effects would show on both measures for this outcome.
Comparison 8 only had one study, which was a five‐day outpatient specialised physiotherapy‐led intervention compared to TAU (Nielsen 2017). This was conducted as a feasibility study, inspired by the Jordbru 2014 study (which was inpatient cognitive behavioural‐based physiotherapy), but as one was inpatients and the other outpatients, we decided to have the two studies in separate comparisons.
In Comparison 7, Jordbru 2014 found no statistically significant effects on this outcome, while Comparison 8 found a reduction in physical signs at four months' follow‐up. It would be interesting if the authors of Nielsen 2017 decided to conduct a new and more comprehensive RCT, and see if the indication of effect found here could be affirmed.
Comparison 12 consisted of only one study, which showed that intervention led to a decrease in seizure frequency at end of treatment (Tolchin 2019). This study was conducted to investigate whether adherence to treatment would be higher if psychotherapy was preceded by MI immediately after diagnosis. Both groups thus received psychotherapy, but as the active group attended more sessions (i.e. adherence was better), it could be argued as to what might actually be the active mediator in the intervention. Perhaps a new study that compared the effect of the same number of actually received psychotherapy sessions in each arm might give some information on the extent MI or psychotherapy is the active mediator.
Secondary outcomes
The overall findings of the analysis do point towards statistically significant treatment effects of psychosocial interventions on some of the secondary outcomes.
Level of functioning
Three different comparisons showed an effect on level of functioning. Comparison 5 showed that CBT increased level of functioning compared to SMC at follow‐up (Goldstein 2010), while both Comparison 6 (psychoeducational follow‐up programmes versus TAU) (Chen 2014), and Comparison 8 (specialised CBT‐based physiotherapy led intervention compared to TAU) (Nielsen 2017), showed improved level of functioning both at end of treatment and follow‐up.
Quality of life
Comparison 6 found effects on improved quality of life at follow‐up (Drane 2016), and comparison 12 found improved quality of life at end of treatment (Tolchin 2019).
Mental state, anxiety and depression
Two comparisons showed effects on general mental state. Comparison 5 found that CBT compared to SMC reduced symptoms of general mental state at end of treatment (LaFrance 2014), while Comparison 7 showed that specialised CBT‐based physiotherapy in an inpatient programme improved mental state compared to wait list at end of treatment (Jordbru 2014).
Two different comparisons showed effects on anxiety. Comparison 1 showed that inpatient paradoxical intention therapy reduced anxiety symptoms at end of treatment compared to outpatient diazepam (Ataoglu 2003), while Comparison 4 (behavioural therapy plus routine clinical care compared to routine clinical care alone) likewise found effects in reduction in symptoms of anxiety at end of treatment (Aamir 2012; Khattak 2006). Comparison 4 also found effects on reduction in symptoms of depression at follow‐up.
Dropout rate
Only Comparison 4 showed statistical significant reduction in dropout at end of treatment (Aamir 2012; Khattak 2006).
Use of health service resources
We found no statistically significant results for use of health services resources.
Adverse effects
We found no statistically significant results for adverse effects.
Overall completeness and applicability of evidence
The review contains a mixture of strengths and issues in terms of overall completeness and applicability of evidence.
Issues concerning the overall completeness and applicability of the evidence
The review highlights some major issues concerning the overall completeness and applicability of the evidence of the benefits and harms of psychosocial interventions on people diagnosed with conversion or dissociative disorders compared with SC; wait‐list controls; or with another intervention, either pharmaceutical/biological or a different psychosocial intervention.
The impact of the applicability of findings related to the duration of studies, choice of interventions and comparators, diagnostic criteria, issues related to the ratings scales and lack of data of adverse effects.
Duration of studies
The duration of studies was very short, ranging from a few hours to four months. The mean duration of studies was 9.5 weeks (range two hours to 20 weeks). Due to lack of data it was not possible to investigate the long‐term benefits of psychological interventions for conversion and dissociative disorders. Overall, the evidence on long‐term effects on psychosocial interventions for people with conversion and dissociative disorders is lacking, and it is possible that the small, possibly beneficial effects found in this review might be diminished over the time.
Interventions and comparators
The studies in this review compared one type of psychosocial intervention with either another intervention (either pharmacological or psychosocial, or mixed) or with SC/SMC or wait list controls.
We had hoped to have combined those comparators in meta‐analyses; however, this was not possible due to variations in the interventions.
Lack of validated scales
The field of research on conversion disorder, dissociative disorder and related conditions has no clear guidance about the best way to measure the efficacy of treatments. There seem to be a variety of views on this, from researchers developing their own instruments (e.g. Moene 2002 has created VMCR), some studies using scales that are not widely used (e.g. Nielsen 2017, which used PMDRS), while others prefer to simply ask their participants to note the number of seizures in a day, week or month (e.g. Goldstein 2010). Some authors also mentioned that the nature of these disorders makes it difficult to decide on which aspect to focus, be it physical ability or quality of life.
This lack of common measures and lack of commonly used validated scales means it becomes almost impossible to compare the effects of different treatments. The findings of this review highlight the wide variety of different scales used to measure the primary outcome across the studies.
Lack of adverse effects
The studies did not describe adverse effects. As it is unusual for any treatment, psychosocial, medical or otherwise, to have no adverse effects, we would expect some of the studies in the review to have observed adverse effects, even if these are not reported.
Lack of participant involvement
None of the studies included report on whether they involved participants in deciding which outcomes to focus on. This would be a major improvement for future studies, to investigate which outcomes are most important to patients and include those. For this update of the review, we amended the secondary outcomes from the original review to be more in line with the interest of patients, but direct patient involvement could also be improved further in future updates.
Strengths in the overall completeness and applicability of the evidence
Countries and settings
One strength of the review was that the studies were from a wide range of countries and settings, which included low‐, medium‐ and high‐income economies, showing how widespread this condition is, as well as the interest in the psychosocial treatment of it.
Diagnostic criteria
A different strength of the review was that it concerned a diagnostic area usually very consistent in identifying the conditions in it, with only 4% of patients being misdiagnosed (Stone 2005). Furthermore, in a follow‐up study of Stone 2009, only 0.4% had acquired an organic disease diagnosis that was unexpected at initial assessment. These percentages are very low compared with other conditions, both psychiatric and somatic ones.
This means clinicians and policy makers can rest assured that whatever the findings of the review (or future versions of it), it will be applicable to conditions diagnosed in this way.
Quality of the evidence
As assessed by GRADE, the overall quality of the evidence ranged from low to very low. We assessed most of studies to have high risk of bias, which may cause systematic errors, that is overestimation of benefits and underestimation of harms (Higgins 2019). The certainty of the evidence was downgraded as a consequence of potential risk of bias, as many of the studies had unclear or inadequate allocation concealment, making them prone to selection bias. Due to the nature of psychological interventions, maintaining an adequate level of blinding of both personal and participants is often difficult. Despite this, it should be noted that most studies included in this review still managed a considerable level of blinding.
Our results were based on 17 studies with a limited number of participants (894). Due to the heterogeneous reporting of outcomes, it was not possible to combine results into a collective analysis to investigate for common effects across studies. Therefore, the results are based on individual studies with only few participants included. This led to imprecision and inconsistency of the estimates, ultimately resulting in further downgrading of the evidence. We were unable to perform funnel plot or any other analyses to assess whether there was risk of publication bias.
These important methodological limitations have reduced the reliability and robustness of the results in this review. Currently, there is insufficient evidence to draw any conclusions on the effect of any form of psychosocial intervention of conversion and dissociative disorders in adults. Further research may change the estimates of the treatment effect, but such studies ought to be conducted without risk of systematic errors (bias), random errors (play of chance) and design errors (Keus 2010). Overall, we found consistency between conclusions in the previous (Ruddy 2005) and the current review.
To advance this field, there is a need for high‐quality RCTs with large numbers of participants, that include similar reporting of core outcomes related to this disorder.
Potential biases in the review process
We conducted extensive searches of relevant databases. Two review authors independently selected studies for inclusion and extracted data. Disagreements were resolved by discussion with team members. We assessed risk of bias in all studies according to the recommendations provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data collection as risk of bias
To our knowledge we did not introduce any bias in the data collection process, as we used a comprehensive search strategy and made all available attempts to retrieve any missing data.
We do not know whether there could be any publication bias, in that it is possible that studies have been conducted but not been published, which would have been of interest to this review.
Deciding on rating scales
As many studies used several scales measuring our predefined outcomes, we made a choice for each study about which one to include. This was based on what the review author team deemed most relevant and of best quality, with reference to those scales highlighted in our Methods section. As the selection process was subjective and not a guideline‐based decision process, this could have introduced bias. We would assume that the various instruments assessing similar aspects would provide similar results if the instruments had been of similar quality. However, this is not necessarily the case as many self‐created and unvalidated rating scales were used.
Most of the 12 comparators had data on our primary outcome: 'Reduction in physical signs', although this was measured in many different ways. There were also data on many of our predefined secondary outcomes, most of them measuring important outcomes for these people. We had six secondary outcomes, which in fact was eight because we decided to divide the mental health outcome into anxiety and depression where possible. This high number of outcomes was chosen due to the clinical importance of these outcomes. However, we are aware that this might be problematic as it increases the likelihood of finding a statistical significant result just by chance (Jakobsen 2014).
Subjective judgements as risk of bias
It is inevitable that there will be certain aspects in the many steps of the process which may have or will have had subjective judgements involved.
For this review, there are a few areas we would like to address.
First, the risk of bias assessment itself will have had some subjective discussion behind the final decisions. Whether to be very strict on a judgement may or may not be clouded by the review authors' backgrounds and daily routines. In the case of this review, the main author is also a clinician, thus has a knowledge of what is possible and desirable in everyday clinical practice. The other review author working on risk of bias has a different background, one of extensive research, and we deliberately chose this combination to minimise the effects of any particular perspective. We followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), and discussed any discrepancies and found consensus on all items. Hence, we do not judge that our processes would have introduced bias, but want to point out that this could be perceived if one is not aware of the knowledge and experience each review author brings to the process.
Second, in choosing which studies to group to create the possibility for comparisons, a risk of bias is also possible. As there are no guidelines on which comparisons to make, the choice presented in this review was based on our clinical judgement, discussed at length among the review author team to provide some coherence in comparing studies, while also ensuring fair and relevant groupings of studies. This should not have affected judgements about overall risk of bias, as the studies were chosen independently of any results, and the comparisons are made on the primary and secondary outcomes as predefined by this review, rather than by the individual studies. However, it is worth noting that such a decision will most likely depend on the clinical experience of the review authors, when studies are as diverse as the ones included here.
In conclusion, we judge that the review author team and the processes followed will not have introduced bias in the production of this review, but wanted to mention these areas for future updates and other teams to be aware of.
Agreements and disagreements with other studies or reviews
To our knowledge, this update of the previous Cochrane Review from Ruddy 2005 is the most extensive and comprehensive systematic review of RCTs of psychosocial interventions for conversion and dissociative disorders. The 2005 review identified 260 references and only three RCTs (119 participants) met the inclusion criteria. In the current review, we identified 3045 relevant research papers and included 17 RCTs (894 participants).
The review authors' conclusions in the 2005 study was that "If psychosocial interventions are available for people with conversion disorder then at present they should be viewed as experimental with slight evidence in favour of help rather than harm" and "It is unclear what effects these interventions and other psychosocial interventions have on social functioning, interpersonal relationships, quality of life or satisfaction with care."
It was reasonable to presume with more studies since 2005 and that there may be a better evidence base for drawing conclusions about treatment of these conditions. Unfortunately, despite there being some tentative conclusions about effectiveness in the current review, the overall findings are inconclusive because of the poor quality of the studies and because there was little opportunity to combine results from studies given a wide variety of different psychosocial interventions.
The results were assessed to be of very low to low quality of evidence measured by GRADE and most studies had a high risk of bias. Therefore, we likewise found no evidence of any conclusive benefits of any psychosocial intervention for conversion and dissociative disorders in adults. However, comparing the two reviews, we can see some improvement in the method and quality of studies in this area and find it encouraging to know that potentially high‐quality studies will appear soon (see 'Ongoing studies' and Characteristics of ongoing studies table).
Several other reviews and meta‐analyses have been published since the previous version of our review. However, none of them have consistently examined the same diagnostic spectrum (i.e. both conversion and dissociation). Our results confirm the findings of Martlew 2014 who concluded that there is little reliable evidence to support the use of any specific treatment for non‐epileptic seizures. They used the same methods as us, but found only four RCTs, which we included in our review.
In a review of Carlson 2017 the results of the analyses indicated that psychological interventions for non‐epileptic seizures may lead to greater rates of seizure reduction and seizure freedom compared to those who do not receive psychotherapy. When compared with the existing evidence, the results of the meta‐analyses indicated that psychological interventions may yield greater rates of seizure reduction (82%) and seizure freedom (47%) compared with those who do not receive psychotherapy (14% to 23%). However, they found there was a high risk of bias and a there was no proper analysis of the quality of evidence. They only found two RCTs, both are included in our review.
Some non‐randomised studies found a medium‐ to large‐effect size of psychotherapy and physical treatment for a range of psychological symptoms, physical symptoms and functional impairment (Brand 2009; Koelen 2014; Nielsen 2013). However, these studies had methodological limitations.
A more detailed description of these studies can be found in the 'Previous reviews on this theme' in the Why it is important to do this review section.
Authors' conclusions
Implications for practice.
The interventions of this review represent a broad spectrum of psychosocial interventions, covering behavioural therapy, cognitive behavioural therapy, hypnosis, psychodynamic therapy, specialised physiotherapy, paradoxical intervention and psychoeducation.
However, there was no high‐quality, unequivocal evidence to support one psychosocial intervention over others or other comparisons for conversion or dissociative disorders in adults. Most studies had a high risk of bias and the results were of very low‐ to low‐certainty evidence due to imprecision, few participants and inconsistency.
In relation to implications for practice, we must await ongoing and new studies that may bring forward valid evidence of treatment effect for these conditions, which also includes both patient and clinician involvements.
Until then, evidence‐based practice must be based on critically appraised topics on available literature or national guidelines (external evidence), background information or expert opinions (internal evidence), and patient preferences and values.
Implications for research.
This review highlights the need for long‐term, high‐quality studies with low risk of bias and with sufficient numbers of participants investigating the benefits and harms of psychological interventions for people with conversation disorders and dissociative disorders. If the CONSORT recommendations were followed in reporting future studies, including prepublished protocols to combat the problem of publication bias, we would be more aware of the effects of psychosocial interventions for conversion disorder (Moher 2001). Important data from several of the included studies were lost due to poor reporting.
Researchers may wish to investigate further the interventions included in this review in the ways suggested or to explore other psychosocial interventions for these disorders. Whatever intervention is studied it is important to have an appropriate control group that is receiving a comparable intervention. Some way of compensating for the additional time spent with people by allocation to a psychosocial intervention, particularly an inpatient one, may be desirable.
Further studies of psychosocial interventions for conversion and dissociative disorders should include clinically meaningful outcomes such as: clinically significant changes in physical functioning, mental state, relapse, admission to hospital, engagement with services, quality of life, leaving the study early, satisfaction with care, social functioning, adverse effects and economic outcomes (cost‐effectiveness and cost‐benefit). It will also be important to create studies that explicitly include greater patient involvement from early design to final research in order to integrate end‐user perspectives more.
Some studies are based on people with severe and complicated conditions with high comorbidity, severe psychopathology and need for long‐term treatment. Here, in particular for ethical reasons, it may be difficult to operate with control groups. A recommendation for these participants is to prepare a high standard non‐randomised design that allows optimal reviews and evaluation with ROBINS‐I in assessing the risk of bias (Sterne 2016).
What's new
| Date | Event | Description |
|---|---|---|
| 13 July 2020 | New search has been performed | We updated the searches and included 14 new studies. |
| 13 July 2020 | New citation required and conclusions have changed | Review updated. New studies added. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 5 November 2008 | Amended | Converted to new review format. |
| 23 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Notes
None.
Acknowledgements
We would like to thank the Psychiatric Research Unit, Region Zealand Denmark, and the Department of Specialized Treatment, Psychiatry Region Zealand Denmark, for their willingness to support this project.
We would like to thank Cochrane Mental Health Group for help with formulating both the search and the aims of the update. In addition, Information Specialist Trine Kaestel, Psychiatric Research Unit, for her assistance on executing the search and various other helpful assistance.
We would like to thank Rachel Ruddy and Alan House for writing the original version of this review back in 2005, and for their encouragement for a new team to take up this work.
Finally, we would like to thank our co‐author Ulf Sogaard for instigating this project and to Dr Kjartan Bergqvist for doing some of the initial research to determine if it was feasible with an update.
Appendices
Appendix 1. Search Strategies
Cochrane CENTRAL Issue 7 of 12, 2019
Date Run: 16/07/2019 09:40:13
ID Search
#1 MeSH descriptor: [Conversion Disorder] explode all trees
#2 (conversion NEXT (disorder* or reaction* or hysteria)):ti,ab,kw (Word variations have been searched)
#3 "functional neurological disorder" or "functional neurological disorders"
#4 MeSH descriptor: [Dissociative Disorders] explode all trees
#5 dissociative NEXT (possession* or disorder* or amnesia or stupor or convulsion* or symptom*)
#6 trance NEXT disorder*
#7 fugue*
#8 hysteri*:ti,ab,kw (Word variations have been searched)
#9 ("multiple personality disorder"):ti,ab,kw (Word variations have been searched)
#10 non‐epileptic or nonepileptic or pseudoseizure* or pseudo‐seizure*
#11 ("Ganser"):ti,ab,kw (Word variations have been searched)
#12 MeSH descriptor: [Factitious Disorders] explode all trees
#13 PNES
#14 psychogenic NEXT (nonepileptic or non‐epileptic or non epileptic) NEXT seizure*
#15 dissociative NEXT (possession* or disorder* amnesia or stupor or convulsion* or symptom* or identit*)
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to July 15, 2019>
1 Conversion Disorder/ (2772)
2 (conversion disorder* or conversion reaction* or conversion hysteria or functional neurological disorder*).ab,kw,ot,ti. (2190)
3 exp Dissociative Disorders/ (4158)
4 (dissociative possession* or dissociative disorder* or possession disorder* or trance disorder* or fugue* or dissociative amnesia or dissociative stupor or dissociative convulsion* or dissociative symptom* or dissociative identit*).ti,ab,kw,ot. (2056)
5 hysteri*.ab,kw,ot,ti. (4611)
6 multiple personality disorder*.ab,kw,ot,ti. (367)
7 (non‐epileptic or nonepileptic or (pseudo‐seizure* or pseudoseizure*) or psychogenic non‐epileptic seizure* or psychogenic nonepileptic seizure* or psychogenic non epileptic seizure* or PNES).ab,kw,ot,ti. (3605)
8 ganser.ab,kw,ot,ti. (91)
9 or/1‐8 (15429)
10 randomized controlled trial.pt. (485438)
11 controlled clinical trial.pt. (93159)
12 randomized.ab. (448955)
13 placebo*.ab. (199936)
14 Clinical Trials as Topic/ (187646)
15 randomly.ab. (314577)
16 trial.ti. (201751)
17 (TAU or "treatment as usual" or waitlist or waiting list).ab,ti. (51572)
18 or/10‐17 (1271558)
19 exp Animals/ not Humans/ (4598525)
20 18 not 19 (1164432)
21 9 and 20 (415)
Ovid Embase <1974 to 2019 July 15>
1 conversion disorder/ (2283)
2 (conversion disorder* or conversion reaction* or conversion hysteria or functional neurological disorder*).ab,kw,ot,ti. (2745)
3 exp dissociative disorder/ (8138)
4 (dissociative possession* or dissociative disorder* or possession disorder* or trance disorder* or fugue* or dissociative amnesia or dissociative stupor or dissociative convulsion* or dissociative symptom* or dissociative identit*).ab,kw,ot,ti. (2880)
5 hysteri*.ab,kw,ot,ti. (4766)
6 multiple personality disorder*.ab,kw,ot,ti. (464)
7 (non‐epileptic or nonepileptic or pseudo‐seizure* or pseudoseizure* or psychogenic non‐epileptic seizure* or psychogenic nonepileptic seizure* or psychogenic non epileptic seizure* or PNES).ab,kw,ot,ti. (5389)
8 ganser.ab,kw,ot,ti. (121)
9 or/1‐8 (21699)
10 randomized controlled trial/ (558691)
11 controlled clinical trial/ (464028)
12 random*.ab. (1394024)
13 trial.ti. (274930)
14 (TAU or treatment as usual or waitlist or waiting list).ti. (14896)
15 or/10‐14 (1773148)
16 9 and 15 (865)
CINAHL via EBSCO Date 16.07.2019
# Query
S1 (MH "Somatoform Disorders+")
S2 TX “conversion disorder*” or “conversion reaction*” or “conversion hysteria” or “functional neurological disorder*”
S3 (MH "Dissociative Disorders+")
S4 TX (“dissociative possession*” or “dissociative disorder*” or “possession disorder*” or “trance disorder*” or fugue* or “dissociative amnesia” or “dissociative stupor” or “dissociative convulsion*” or “dissociative symptom*” or “dissociative identit*”)
S5 TX hysteri*
S6 TX multiple personality disorder*
S7 TX ((non‐epileptic or nonepileptic) or (pseudo‐seizure* or pseudoseizure*) or ("psychogenic non‐epileptic seizure*" or "psychogenic nonepileptic seizure*" or "psychogenic non epileptic seizure*" or “psychogenic non‐epileptic seizure*”) or ("PNES"))
S8 TX ganser*
S9 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8)
S10 (MH "Randomized Controlled Trials")
S11 AB randomi?ed
S12 AB placebo*
S13 AB randomly
S14 TI trial
S15 TX TAU or "treatment as usual" or waitlist or "waiting list"
S16 (S10 OR S11 OR S12 OR S13 OR S14 OR S15)
S17 (S9 AND S16 )
Ovid PsycINFO <1806 to July Week 2 2019>
1 exp Conversion Disorder/ (1360)
2 (conversion disorder* or conversion reaction* or conversion hysteria* or functional neurological disorder*).ab,ot,ti,tw. (1534)
3 exp Dissociative Disorders/ (5161)
4 (dissociative possession* or dissociative disorder* or possession disorder* or trance disorder* or fugue* or dissociative amnesia or dissociative stupor or dissociative convulsion* or dissociative symptom* or dissociative identit*).ab,ot,ti,tw. (4176)
5 hysteri*.ab,ot,ti,tw. (7671)
6 multiple personality disorder*.ab,ot,ti,tw. (1138)
7 (non‐epileptic or nonepileptic or (pseudo‐seizure* or pseudoseizure*) or psychogenic non‐epileptic seizure* or psychogenic nonepileptic seizure* or psychogenic non epileptic seizure* or PNES).ab,ot,ti,tw. (1870)
8 ganser.ab,ot,ti,tw. (100)
9 exp Treatment Effectiveness Evaluation/ (23608)
10 exp Treatment Outcomes/ (118065)
11 exp PLACEBO/ (5281)
12 exp Followup Studies/ (12368)
13 (placebo* or random* or comparative stud* or (clinical adj3 trial*) or (research adj3 design) or (evaluat* adj3 stud*) or (prospectiv* adj3 stud*) or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ab,ot,ti,tw. (25466)
14 (TAU or "treatment as usual" or waitlist or waiting list).ab,ot,ti,tw. (15322)
15 or/1‐8 (17481)
16 or/9‐14 (184443)
17 15 and 16 (589)
Web of Science Core Collection, Thomson Reuters, 16.07.2019 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI Timespan=All years
#11 #9 AND #10
#10 TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#9 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#8 TOPIC: ("multiple personality disorder*")
#7 TOPIC: ("pseudoseizure*")
#6 TOPIC: ("PNES")
#5 TOPIC: ("psychogenic nonepileptic seizure*")
#4 TOPIC: ("psychogenic non‐epileptic seizure*")
#3 TOPIC: ("dissociative disorder*")
#2 TOPIC: ("functional neurological disorder*")
#1 TOPIC: ("conversion disorder*")
ERIC via EBSCO (original search date April 2018, updated July 2019, later problems were discovered with this search, so we corrected and updated the search again on 23 Jan 2020)
S1 TX conversion disorder* or conversion reaction* or conversion hysteria or functional neurological disorder*
S2 TX dissociative possession* or dissociative disorder* or possession disorder* or trance disorder* or fugue* or dissociative amnesia or dissociative stupor or dissociative convulsion* or dissociative symptom* or dissociative identit*
S3 TX hysteri*
S4 TX multiple personality disorder*
S5 TX non‐epileptic or nonepileptic or pseudoseizure* or pseudo‐seizure* or "psychogenic non‐epileptic seizure" or "psychogenic nonepileptic seizure" or "psychogenic non epileptic seizure" or PNES
S6 TX ganser
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S8 DE "Randomized Controlled Trials"
S9 AB randomly
S10 TI trial
S11 TX TAU or treatment as usual or waitlist or waiting list
S12 TX random* or control* or comparat* or stud* or blind* or mask*
S13 S8 OR S9 OR S10 OR S11 OR S12
S14 S7 AND S13
Following searches were also executed on the 16 July 2019:
ClinicalTrials.gov Search: Conversion Disorder, Interventional studies
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/)
"Conversion Disorder"
ISRCTN (http://www.isrctn.com/)
"conversion disorder"
WHO ICTRP
Search title: dissociative or "conversion disorder" or "functional neurological" or "psychogenic non‐epileptic seizure" or PNES or "multiple personality disorder"
Data and analyses
Comparison 1. Inpatient paradoxical intention therapy versus outpatient diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Reduction in physical signs: no conversion symptoms in last 2 weeks | 1 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.44 [0.91, 2.28] |
| 1.1.1 End of treatment | 1 | 30 | Risk Ratio (IV, Fixed, 95% CI) | 1.44 [0.91, 2.28] |
| 1.2 Mental state – anxiety (Hamilton) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.96, ‐0.50] |
| 1.2.1 End of treatment | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.96, ‐0.50] |
| 1.3 Dropout rate | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.3. Analysis.

Comparison 1: Inpatient paradoxical intention therapy versus outpatient diazepam, Outcome 3: Dropout rate
Comparison 2. Inpatient treatment programme plus hypnosis versus inpatient treatment programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Reduction in physical signs: severity of impairment (VRMC) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 End of treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐1.28, 0.30] |
| 2.1.2 Follow‐up | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.55, 0.81] |
| 2.2 Mental state (SCL‐90) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 End of treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [‐36.02, 38.86] |
| 2.2.2 Follow‐up | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐5.97 [‐44.22, 32.28] |
| 2.3 Dropout rate | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.14, 5.79] |
Comparison 3. Outpatient hypnosis versus wait list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Reduction in physical signs: severity of impairment (VRMC) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.34, 2.86] |
| 3.1.1 End of treatment | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.34, 2.86] |
| 3.2 Dropout rate | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 End of treatment | 1 | 49 | Risk Ratio (IV, Fixed, 95% CI) | 4.17 [0.50, 34.66] |
Comparison 4. Behavioural therapy plus routine clinical care versus routine clinical care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Reduction in physical signs: number of weekly fits | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐21.40 [‐27.88, ‐14.92] |
| 4.1.1 End of treatment | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐21.40 [‐27.88, ‐14.92] |
| 4.2 Reduction in physical signs: symptom severity (Clinical Global Impression CGI) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐3.41, ‐2.39] |
| 4.2.1 End of treatment | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐3.41, ‐2.39] |
| 4.3 Mental state – anxiety (HADS) | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐5.47 [‐7.08, ‐3.86] |
| 4.3.1 End of treatment | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐5.47 [‐7.08, ‐3.86] |
| 4.4 Mental state – depression (HADS) | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐6.44, ‐3.53] |
| 4.4.1 Follow‐up | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐6.44, ‐3.53] |
| 4.5 Dropout rate | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.5.1 End of treatment | 2 | 118 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.06, 0.90] |
Comparison 5. Cognitive behavioural therapy versus standard medical care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Reduction in physical signs: monthly seizure frequency (reduction in %) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 End of treatment | 1 | 16 | Risk Ratio (IV, Fixed, 95% CI) | 1.56 [0.39, 6.19] |
| 5.2 Reduction in physical signs: monthly seizure frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 End of treatment | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐4.75 [‐18.73, 9.23] |
| 5.2.2 Follow‐up | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐12.69, 5.69] |
| 5.3 Reduction in physical sign: seizure freedom | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 End of treatment | 1 | 16 | Risk Ratio (IV, Fixed, 95% CI) | 2.33 [0.30, 17.88] |
| 5.4 Level of functioning | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4.1 End of treatment | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.69, 2.57] |
| 5.4.2 Follow‐up | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.18, ‐0.08] |
| 5.5 Quality of life (QOLIE31) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.20 [‐7.98, 30.38] |
| 5.5.1 End of treatment | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.20 [‐7.98, 30.38] |
| 5.6 Mental state – anxiety | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.6.1 End of treatment | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.78, 0.15] |
| 5.6.2 Follow‐up | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.85, 0.23] |
| 5.7 Mental state – depression | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.7.1 End of treatment | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.71, 0.21] |
| 5.7.2 Follow‐up | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.86, 0.23] |
| 5.8 Mental state (SCL‐90) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐70.60 [‐121.59, ‐19.61] |
| 5.8.1 End of treatment | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐70.60 [‐121.59, ‐19.61] |
| 5.9 Dropout rate | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.9.1 End of treatment | 2 | 83 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.02, 8.01] |
| 5.9.2 Follow‐up | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.25, 7.87] |
| 5.10 Use of health services (number of general practitioner consultations) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.32, 1.32] |
| 5.10.1 Follow‐up | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.32, 1.32] |
Comparison 6. Psychoeducational follow‐up programmes versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Reduction in physical signs: seizure frequency (self‐made scale) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.44, ‐0.16] |
| 6.1.1 Follow‐up | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.44, ‐0.16] |
| 6.2 Reduction in physical signs: physical symptom load (SF‐36 – physical) | 1 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐3.76, 3.24] |
| 6.2.1 Follow‐up | 1 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐3.76, 3.24] |
| 6.3 Level of functioning (WSAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 End of treatment | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐7.12 [‐12.47, ‐1.77] |
| 6.3.2 Follow‐up | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐6.11 [‐11.67, ‐0.55] |
| 6.4 Quality of life (QOLIE10‐P) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐9.30 [‐14.06, ‐4.54] |
| 6.4.1 Follow‐up | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐9.30 [‐14.06, ‐4.54] |
| 6.5 Mental state – anxiety (HADS) | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.67, 0.73] |
| 6.5.1 Follow‐up | 1 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.67, 0.73] |
| 6.6 Mental state – depression | 2 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.08, 0.48] |
| 6.6.1 Follow‐up | 2 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.08, 0.48] |
| 6.7 Dropout rate | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.7.1 End of treatment | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 1.76 [0.82, 3.78] |
| 6.7.2 Follow‐up | 2 | 259 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.00, 70.37] |
| 6.8 Use of health services (number hospital visits) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 6.8.1 End of treatment | 1 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 0.18 [0.02, 1.43] |
Comparison 7. Specialised cognitive behavioural therapy‐based physiotherapy inpatient programme versus wait list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Level of functioning (Functional Independence Measure Motor (FIM)) | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 9.20 [6.06, 12.34] |
| 7.1.1 End of treatment | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 9.20 [6.06, 12.34] |
| 7.2 Mental state (SF‐12) | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 9.10 [4.96, 13.24] |
| 7.2.1 End of treatment | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 9.10 [4.96, 13.24] |
| 7.3 Dropout rate | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 End of treatment | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.03, 1.97] |
Comparison 8. Specialised cognitive behavioural therapy‐based physiotherapy outpatient intervention compared to treatment as usual (TAU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Reduction in physical signs: physical symptom load (SF‐36 – Physical Component) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 9.20 [4.00, 14.40] |
| 8.1.1 Follow‐up | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 9.20 [4.00, 14.40] |
| 8.2 Level of functioning (WSAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.2.1 End of treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐11.40, ‐2.80] |
| 8.2.2 Follow‐up | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐12.07, ‐1.33] |
| 8.3 Mental state – anxiety (HADS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.3.1 End of treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.05, 1.85] |
| 8.3.2 Follow‐up | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.71, 1.71] |
| 8.4 Mental state – depression (HADS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.4.1 End of treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐7.13, ‐0.07] |
| 8.4.2 Follow‐up | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐5.53, ‐0.87] |
| 8.5 Dropout rate | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 8.5.1 End of treatment | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 0.20 [0.02, 1.61] |
| 8.5.2 Follow‐up | 1 | 60 | Risk Ratio (IV, Fixed, 95% CI) | 0.50 [0.05, 5.22] |
Comparison 9. Brief psychotherapeutic intervention (psychodynamic interpersonal treatment approach) versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Reduction in physical signs: conversions symptoms (SDQ20) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1.1 End of treatment | 1 | 19 | Risk Ratio (IV, Fixed, 95% CI) | 0.12 [0.01, 2.00] |
| 9.1.2 Follow‐up | 1 | 17 | Risk Ratio (IV, Fixed, 95% CI) | 1.12 [0.08, 15.19] |
| 9.1.3 Follow‐up 10 months | 1 | 15 | Risk Ratio (IV, Fixed, 95% CI) | 0.16 [0.01, 2.66] |
| 9.2 Quality of life (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.2.1 End of treatment | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐6.99 [‐28.09, 14.11] |
| 9.2.2 Follow‐up 4 months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐19.53 [‐43.91, 4.85] |
| 9.2.3 Follow‐up 10 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐11.43 [‐36.16, 13.30] |
| 9.3 Mental state – depression (BDI‐II) | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.3.1 End of treatment | 1 | 16 | Risk Ratio (IV, Fixed, 95% CI) | 1.29 [0.10, 17.14] |
| 9.3.2 Follow‐up 4 months | 1 | 16 | Risk Ratio (IV, Fixed, 95% CI) | 3.86 [0.50, 29.55] |
| 9.3.3 Follow‐up 10 months | 1 | 14 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.08, 13.02] |
| 9.4 Dropout rate | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 9.4.1 End of treatment | 1 | 23 | Risk Ratio (IV, Fixed, 95% CI) | 1.09 [0.18, 6.48] |
| 9.4.2 Follow‐up 4 months | 1 | 23 | Risk Ratio (IV, Fixed, 95% CI) | 1.09 [0.28, 4.32] |
| 9.4.3 Follow‐up 10 months | 1 | 23 | Risk Ratio (IV, Fixed, 95% CI) | 0.82 [0.23, 2.87] |
| 9.5 Use of health services (emergency department visits) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.25, 0.93] |
| 9.5.1 End of treatment | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.25, 0.93] |
Comparison 10. Cognitive behavioural therapy plus adjunctive physical activity versus cognitive behavioural therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Reduction in physical signs: overall physical impact (Psychogenic Movement Disorder Scale (PMDRS) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐15.48, 26.68] |
| 10.1.1 End of treatment | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐15.48, 26.68] |
| 10.2 Mental state – anxiety (BAI) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐8.01, 1.21] |
| 10.2.1 End of treatment | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐8.01, 1.21] |
| 10.3 Mental state – depression (Hamilton) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐3.32, 2.32] |
| 10.3.1 End of treatment | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐3.32, 2.32] |
| 10.4 Dropout rate | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.4.1 End of treatment | 1 | 29 | Risk Ratio (IV, Fixed, 95% CI) | 1.87 [0.40, 8.65] |
Comparison 11. Hypnosis versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Reduction in physical signs: symptom freedom | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1.1 End of treatment | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | 0.69 [0.39, 1.24] |
Comparison 12. Outpatient motivational interviewing and mindfulness‐based psychotherapy compared with psychotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Reduction in physical signs: decrease in seizure frequency | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 41.40 [4.92, 77.88] |
| 12.1.1 End of treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 41.40 [4.92, 77.88] |
| 12.2 Changes in monthly visits | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.55, 0.13] |
| 12.2.1 End of treatment | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.55, 0.13] |
| 12.3 Quality of life | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [0.26, 10.54] |
| 12.3.1 End of treatment | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [0.26, 10.54] |
| 12.4 Dropout rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.29, 8.92] |
| 12.4.1 End of treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.29, 8.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aamir 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Duration of study: 9 weeks Randomisation method: lottery method Allocation concealment method: no information Blinding of outcome assessors: no information Adequate power (evidence of power calculation): no information Check of blinding: no information |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: people diagnosed with conversion disorder (having pseudo seizures only) as per ICD‐10 criteria thoroughly investigated having no comorbid psychiatric or physical illness and whose total duration of illness was not > 6 months, of both sexes, aged 18 to 50 years. Exclusion criteria: all dissociative (conversion) disorders other than fits (pseudo seizures), aged < 18 years and > 50 years and those who did not consent. Pretreatment: no statistically significant differences at baseline between groups |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Number of weekly seizures
Mental state – anxiety (HADS)
Mental state – depression (HADS)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were than randomly assigned to the behavior therapy (n=9) and control group (n=9) by lottery method." |
| Allocation concealment (selection bias) | Unclear risk | Nothing information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The control was commented on. It was unclear with the intervention group, and the personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data were reported, including dropout rates. The distribution of dropout was similar across groups (1 vs 3), but the reasons for dropout were not specified, neither was the time point for dropout. |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided. |
| Other bias | Low risk | No other apparent sources of bias. |
Ataoglu 2003.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): none mentioned Allocation concealment method: no information Blinding of outcome assessors: participants were assessed by a psychiatrist who was unaware of the treatment group Check of blinding: none mentioned Duration of study: 6 weeks Randomisation method: computer |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: people admitted to the emergency unit with pseudoseizure. The diagnoses were based on DSM‐IV criteria for conversion disorder. Exclusion criteria: abnormal EEG, organic disease, axis I or II disorder, previous psychiatric treatment Pretreatment: no statistically significant differences at baseline between groups |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Dropout
No conversion symptoms in last 2 weeks
Mental state – anxiety (HDRS)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Finally, thirty patients (29 women and 1 man), diagnosed as conversion disorder were randomly divided into two groups by means of a computer." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients treated with diazepam were offered appointments at the days 10‐20‐30‐45 of treatment to review their progress, to reinforce the use of diazepam, and to regulate the dosage of diazepam." Quote: "The patients in the PI group were informed about the nature of the treatment, what was expected of them, and approximately how long the treatment would last. The relationship between." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patients were assessed by a psychiatrist who was undisclosed to the subjects' group throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No apparent sources of bias. |
Chen 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): none mentioned Allocation concealment method: none mentioned Blinding of outcome assessors: none mentioned Check of blinding: none mentioned Duration of study: June 2011 to October 2012 Randomisation method: computerised/even‐odd numbers |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: VEEG‐confirmed non‐epileptic events of interest, which were interpreted to be of psychogenic origin based on combined features of ictal semiology, psychosocial history and results from psychological screening instruments. Exclusion criteria: main place of dwelling beyond commutable distance (patients referred from outside VA medical centres); suspected mixed disorder of PNES and epilepsy (people with prior EEG documentation of electrographic seizures or interictal epileptiform abnormalities); and Mini‐Mental Status Examination score of 25, when assessed during the EMU admission. Pretreatment: no statistically significant differences at baseline between groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Level of functioning (WSAS)
Dropout
Healthcare use – hospital visits
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were each independently designated a computer generated." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 withdrew in the intervention group and 9 withdrew in the control group. Withdrawal was accounted for. |
| Selective reporting (reporting bias) | High risk | The background data that are used for describing the PNES frequency were not available even though they were used in the article. The primary outcome was not sufficiently reported. No protocol available. |
| Other bias | Low risk | No other apparent sources of bias. |
Dallocchio 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): none mentioned Allocation concealment method: none mentioned Blinding of outcome assessors: a single rater blinded to the aims of the study and the time evaluation (PMDRS) Check of blinding: none Duration of study: no information Randomisation method: block randomisation (size = 4 with balance combinations) |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: people with functional movement disorder (conversion disorder, in accordance to DSM‐IV) Exclusion criteria: none mentioned Pretreatment: not assessed. No statistically significant differences apparent at baseline between groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Overall physical impact (PMDRS total score) (SD)
Mental state – anxiety (BAI)
Mental state – depression (HDSR)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block procedure (block size = 4 with balanced combinations). |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Mentioned as single‐blind, but only the rater was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A single rater blinded to the aims of the study and time valuation completed the PMDRS in a randomised order. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No apparent sources of bias. |
Drane 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): not adequate in power. Power analysis suggested a sample of ≥ 23 participants per group in order to detect a statistically significant difference in event frequency at 8 weeks. While we were unable to achieve this enrolment goal, our findings nevertheless achieved statistical significance. Allocation concealment method: none mentioned Blinding of outcome assessors: none mentioned Check of blinding: none mentioned Duration of study: July 2011 to May 2012 Randomisation method: simple randomisation (preset randomisation chart that was based on computer generation of random numbers). |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: diagnosis of PNES based on recognised criteria. Exclusion criteria: epileptiform activity during an episode and semiology characterised by: 1. a definitive motor component (e.g. shaking or writhing of the torso or limbs, convulsive or rocking movements, head shaking) or 2. a discrete episode of unresponsiveness and 3. the clinical impression that the event could not be explained by another physiological cause (e.g. syncope, sleep disturbance). Severe cognitive impairment or active homicidal or suicidal ideation. Pretreatment: age was the only baseline variable to significantly differ between groups (standard practice 45.3 (SD 11.5) years; structured inpatient feedback 37.7 (SD 10.5) years, structured ongoing feedback 34.1 (SD 9.5) years). |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Mental state – depression (BDI)
Quality of life (QOLIE10‐P)
Seizure frequency (self‐made scale)
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a preset randomisation chart that was based on computer generation of random numbers (simple randomisation)." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The distribution of dropouts among groups was not specified. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Primary outcome was measured on a self‐made scale. |
Goldstein 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): sample size calculation. Based on previous experience, we assumed a true mean change in monthly seizure frequency of 7.3 in the CBT group, no change in the SMC group, and a common SD of 9.5 seizures. Therefore, 28 per group were required to detect a difference with this effect size (Cohen 0.768) with 80% power at P < 0.05. Allowing for a 20% dropout rate, the study authors sought to recruit 35 participants per group; resource limitations resulted in recruitment of 33 per group. Allocation concealment method: developed from a table of random numbers, using unstratified permuted blocks of 4, and concealed in sealed envelopes. The envelopes were then numbered consecutively and given to an independent clinician who allocated them in order as patients gave written informed consent. Blinding of outcome assessors: no information provided Check of blinding: no information provided Duration of study: June 2001 to April 2007 Randomisation method: independently prepared sequence of consecutive, randomised treatment assignments. |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: aged 18–70 years; clinical diagnosis of PNES primarily confirmed by VEEG telemetry, and only if this was not feasible by ictal EEG, or where the referrer and consultant neuropsychiatrists involved in the study agreed that there was no doubt about the diagnosis and that further investigation was unjustified ('clinical consensus'). Exclusion criteria: coexistent diagnosis (past occurrence) of epilepsy; 2 seizures per month; current drug or alcohol misuse; benzodiazepine use exceeding the equivalent of diazepam 10 mg/day; IQ 70 Pretreatment: similar demographic characteristics and seizure histories at enrolment. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Monthly seizure frequency
Mental state – anxiety (HADS)
Mental state – depression (HADS)
Level of functioning (WSAS)
Primary health service use (number of GP consultations)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to CBT or SMC using an independently prepared sequence of consecutive, randomised treatment assignments. This was developed from a table of random numbers, using unstratified permuted blocks of 4, and concealed in sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "This was developed from a table of random numbers, using unstratified permuted blocks of 4, and concealed in sealed envelopes. The envelopes were then numbered consecutively and given to an independent clinician who allocated them in order as patients gave written informed consent." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Therapy sessions were audio‐recorded. Two independent raters evaluated the content of sessions 4 and 9. Overall ratings of therapeutic alliance and CBT were calculated." Comment: no blinding due to the nature of the study, but independent rating of therapy to assure consistency. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts accounted for. |
| Selective reporting (reporting bias) | Low risk | Clinical trials register NCT00688727. The study followed this. |
| Other bias | Low risk | No other apparent sources of bias. |
Hubschmid 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): no information provided Allocation concealment method: randomisation to IPI or SC was done through 24 identical, non‐transparent, sealed envelopes, half containing a paper stipulating 'treatment' and the other half 'standard'. Blinding of outcome assessors: no, to minimise dropout of participants Check of blinding: no blinding Duration of study: November 2010 to January 2013 Randomisation method: randomisation to IPI or SC using 24 identical, non‐transparent, sealed envelopes, half containing a paper stipulating 'treatment' and the other half 'standard.' Envelopes were independently prepared and sealed, and given to a third person unaware of the content to mix. They were numbered consecutively and given in chronological order to patients as written informed consent was signed. |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: aged 16–65 years; newly diagnosed conversion disorder according to the (DSM‐IV‐TR) (within 12 months) with motor or NEA symptoms assessed by experienced neurologists. Exclusion criteria: lack of verbal fluency in French; neurological comorbidity with motor or gait symptoms, or concomitant epilepsy diagnosed by an experienced epileptologist; psychiatric comorbidity of psychosis, acute suicidality or current substance abuse; or current psychotherapy at the time of inclusion. Pretreatment: no statistically significant differences at baseline between groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Conversions symptoms (SDQ‐20)
Quality of life (SF‐36)
Use of health service emergency department
Mental state – depression (BDI‐II),
Dropout
|
|
| Notes | The use of health services at 4 and 10 months' follow‐up were not included in this review, as data did not allow for inclusion in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to IPI or SC was done through 24 identical, nontransparent, sealed envelopes, half containing a paper stipulating "treatment" and the other half “standard." The envelopes were independently prepared by H.M. and sealed, and then given to a third person unaware of their content to mix. They were numbered consecutively and given in chronological order to patients as written informed consent was signed." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization to IPI or SC was done through 24 identical, nontransparent, sealed envelopes, half containing a paper stipulating "treatment” and the other half "standard." The envelopes were independently prepared by H.M. and sealed, and then given to a third person unaware of their content to mix. They were numbered consecutively and given in chronological order to patients as written informed consent was signed." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of personnel delivering the intervention to keep participants throughout the study. So the authors had actively made a decision. Nothing mentioned for participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This trial was not blinded, as part of the measures was rated by the therapists themselves, to try and limit the dropout rate. Other measures were self‐administered." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts accounted for. |
| Selective reporting (reporting bias) | Low risk | No changes to trial outcomes were made after trial started. The following outcome measures were analysed as per protocol. |
| Other bias | Low risk | No other apparent sources of bias. |
Jordbru 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over Adequate power (evidence of power calculation): no specific power calculation mentioned. Allocation concealment method: the first author was blinded to information about intervention or control group, which was kept in sealed envelopes. Blinding of outcome assessors: first author (assessing FIM) was blinded to randomisation. Check of blinding: no information provided. Duration of study: May 2007 to October 2010. Randomisation method: randomisation procedure was performed at a statistical office at a site remote from where the study was conducted. Participants were randomised consecutively and equally (with a 1:1 ratio) to immediate 3 weeks of treatment or 4 weeks on a wait list. Those on the wait list received treatment after the waiting period. |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: disabling walking disturbance resembling psychogenic gait with no organic explanation after neurological examination; aged 18–69 years; duration < 5 years, and willingness to participate in the study. Exclusion criteria: people who needed inpatient psychiatric treatment, people with coexistent somatic disorders (multiple sclerosis, cerebral palsy, etc.) or people who did not want to take part in active rehabilitation. Pretreatment: no statistically significant differences at baseline between groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Level of functioning (FIM)
Mental state (SF‐12)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation procedure was performed at a statistical office at a site remote from where the study was conducted. the first author was blinded to information about intervention or control group, which was kept in sealed envelopes. the envelopes were allocated to patients consecutively in the same order as patients had given written informed consent." |
| Allocation concealment (selection bias) | Low risk | Participants randomly assigned by blocks of 4, balanced for sex, to intervention or control groups. The randomisation procedure was performed at a statistical office at a site remote from where the study was conducted. The first author was blinded to information about intervention or control group, which was kept in sealed envelopes. The envelopes were allocated to participants consecutively in the same order as participants had given written informed consent. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the patients from the intervention group, as well as the control group, were consecutively admitted to the ward, and the team did not know to which group the patients were allocated." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The first author handled all data collection and was not involved in the treatment." Comment: the first author was blinded to information about randomisation. Some outcomes were self‐reported and participants were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout was accounted for well up to the latest time point. |
| Selective reporting (reporting bias) | Unclear risk | No protocol. |
| Other bias | Low risk | No apparent other sources of bias. |
Khattak 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): no information provided Allocation concealment method: no information provided Blinding of outcome assessors: no information provided Check of blinding: no information provided Duration of study: April 2004 to September 2004 Randomisation method: method not mentioned |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: symptoms according to ICD‐10 (WHO classification of psychiatric diseases) criteria for dissociative disorder were included in the study. People presenting with convulsions only were included in this study. Exclusion criteria: co‐existing physical illness or another psychiatric disorder except anxiety and depressive disorder Pretreatment: no statistically significant differences at baseline between groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Symptom severity (CGI)
Mental state – anxiety (HADS)
Mental state – depression (HADS)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After entry into the study, patients were allocated randomly, either to the intervention or control group on 1:1 ratio." Comment: method not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel were not blinded, unclear if patient groups were kept separately. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "absence of a blind rater," |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Did not seem so. Dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Baseline characteristics were not provided, which hindered an assessment on whether sufficient randomisation was obtained. |
| Other bias | Low risk | No other apparent sources of bias. |
LaFrance 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): quote: "not being powered for differences between groups," but no data provided. Allocation concealment method: no information provided Blinding of outcome assessors: treatment‐blinded trained raters assessed clinician‐scored outcomes Check of blinding: no information provided Duration of study: September 2008 to February 2012 Randomisation method: computer‐generated block randomisation |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: aged 18–65 years with a VEEG‐confirmed diagnosis of lone PNES and ≥ 1 event in the month prior. Criteria for the diagnosis of events consisted of stereotypic motor manifestations with or without change in level of consciousness. Exclusion criteria: concurrent mixed epilepsy and PNES or equivocal VEEG findings in discerning between epileptic seizures and PNES; use of monoamine oxidase inhibitor or pimozide within 30 days prior to study entry; current use of sumatriptan succinate or other serotonin‐1 receptor agonist; allergy or sensitivity to sertraline; current enrolment in CBT for PNES; current or past‐year self‐mutilation; frank psychosis; current suicidality with intent to self‐harm; serious illness; active substance or alcohol use or dependence that could interfere with participation; pending litigation and current application for long‐term disability. Pretreatment: no statistically significant differences at baseline between groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
The study included two other interventions, Sertraline hydrochloride (25‐200 mg/d), and CBT+ Sertraline hydrochloride (25‐200 mg/d) which were not relevant to this review. |
|
| Outcomes |
Monthly seizure frequency (reduction in %)
Seizure freedom
Level of functioning (GAF)
Mental state (SCL‐90)
Mental state – depression (BDI)
Mental state – anxiety (BAI)
Quality of life (QOLIE31)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised 1:1:1:1 into 1 of 4 treatment arms using a computer‐generated blocked randomisation." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "blocked schedule." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Given the nature of interventions delivery, clinicians in the study were not blinded to the intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment‐blinded trained raters assessed clinician‐scored outcomes after reliability was established. Interrater reliability was established by having raters score a sample of the same patients and having the results reviewed." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data. |
| Selective reporting (reporting bias) | Low risk | Matches study protocol. |
| Other bias | Low risk | No other apparent sources of bias. |
Moene 2002.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): Quote: "A power of 80% and an alpha (two‐sided) of 5% showed that 25 patients would have been sufficient for each group in order to detect differences between groups with an effect size of d=0.8" Allocation concealment method: none described Blinding of outcome assessors: assessors were blinded Check of blinding: no information provided Duration of study: 1991–1996 Randomisation method: block randomisation using blocks with the following sized: 3 × 4, 2 × 6, 2 × 8, 2 × 4 and 2 × 2. |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: positive diagnosis of somatisation disorder with conversion symptoms of the motor type according to DSM‐III‐R criteria; duration of ≥ 1 month; aged 18–65 years; no problem speaking the Dutch language. Patients had to be available for full course of treatment and assessment sessions, and agree to received no other treatment during project and not to change their medication except when indicated and temporarily (e.g. temazepam 1 mg to sleep or oxazepam 20 mg during the day). Exclusion criteria: evidence of a neurological disorder explaining the conversion symptom; major affective disorder of the melancholy type or other severe psychiatric diagnosis requiring immediate treatment. Pretreatment: no statistically significant differences at baseline between groups. Diagnosis consistent with conversion disorder as specified in ICD‐10 or DSM‐IV |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Severity of impairment (VRMC)
Mental state (SCL‐90)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for randomisation was not provided. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from the therapist and assessor. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible, due to the interventions believer. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Allocation was concealed from the therapist and assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear dropout reporting in terms of total number of participants in each group at each stage. |
| Selective reporting (reporting bias) | High risk | Baseline demographic factors not stratified between groups. |
| Other bias | Low risk | No other apparent sources of bias. |
Moene 2003.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): No. Quote: "Another limitation is the lack of power of the study because of the small sample size". Allocation concealment method: participants were told they would receive same treatment, only at different starting points. Blinding of outcome assessors: 3 trained and independent raters blind to group membership and study rational rated the performance of motor tasks. Check of blinding: no information provided Duration of study: 1991–1996 Randomisation method: block randomisation |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: positive diagnosis of conversion disorder, motor type (such as paresis or paralysis, gait disturbances, co‐ordinations problems, aphonia, seizures, or pseudo‐epileptic seizures with motor activity) or a diagnosis of somatisation disorder with conversion symptoms, motor type according to DSM‐III‐R criteria; duration of symptoms ≥ 1 month; aged 18–65 years; no problem speaking the Dutch language; available for full course of treatment and assessment sessions; no other psychological treatment during the project; no imminent change in medication. Exclusion criteria: evidence of a neurological disorder explaining the conversion symptom; major affective disorder or other severe psychiatric diagnosis requiring immediate treatment. Pretreatment: no significant differences between groups with respect to demographic variable, psychiatric history or dependent values at baseline. All Chi2 P values were > 0.15. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Severity of impairment (VRMC)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used for randomisation was not provided. |
| Allocation concealment (selection bias) | Unclear risk | All participants knew they would get the same treatment at different times. No evidence of this being a problem. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants knew they would get the same treatment as informed of cross‐over study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 2 trained and independent raters viewed the pretreatment video followed randomly by the posttreatment or follow‐up video. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data as data were not reported for the 2 groups (control and intervention) separately. Dropouts accounted for. But no baseline characteristics reported. |
| Selective reporting (reporting bias) | Unclear risk | Results only reported for end of treatment, as follow‐up data were not comparable, as the control/wait list group had started treatment. |
| Other bias | Low risk | None described. |
Mousavi 2008.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): no information provided Allocation concealment method: no information provided Blinding of outcome assessors: not blinded to assist treatment alliance Check of blinding: no information provided Duration of study: 2005–2006 Randomisation method: no information provided |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: outpatient referrals with conversion disorder except epileptiform subtype; aged 10–50 years; onset of illness in the past 24 hours; no history of conversion disorder in the year before study Exclusion criteria: non‐consenting patients or careers; non‐compliance after initial agreement; failing to enter in trance state; no accessibility for 1‐month follow‐up after treatment. Pretreatment: uncertain. No baseline demographics given per groups. |
|
| Interventions |
Intervention characteristics Intervention
Control
The study included two other interventions, relaxation and suggestion, which were not relevant to the review. |
|
| Outcomes |
Symptom freedom
Relapse
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We managed to include 20 patients in each group randomly." Comment: no information on how this was done. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the psychiatrist who carried out the assessment and the therapeutic works was not blind to the intervention groups. This was to improve the rapport and therapeutic relationship, which is necessary for treatment of conversion disorder." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear what the outcomes were and how the outcomes were measured. There was a lack of untreated controls. |
| Selective reporting (reporting bias) | High risk | No baseline characteristics, or individual group data |
| Other bias | Low risk | No apparent sources of bias. |
Nielsen 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): a power calculation was not performed as the primary aim of this study was to assess feasibility. Allocation concealment method: none. Both participants and clinicians were unmasked to treatment allocation. Blinding of outcome assessors: participants were immediately informed of their treatment allocation. Both participants and clinicians were unmasked to treatment allocation. Check of blinding: none. Both participants and clinicians were unmasked to treatment allocation. Duration of study: 8 September 2014 to 4 June 2015 Randomisation method: randomly allocated (1:1) to the intervention or control group using a secure online randomisation application (Sealed Envelope, London, UK) |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: clinically established diagnosis of FMS according to Fahn‐Williams criteria; aged ≥ 18 years; completed diagnostic investigations; acceptance of the diagnosis on the balance of probability (i.e. quote: "we did not exclude patients who continued to express some doubt over the diagnosis"); FMS duration ≥ 6 months; symptoms severe enough to cause distress or impairment in social or occupational functioning. Exclusion criteria: unable to understand English; pain or fatigue that was the primary cause of the patient’s disability; prominent dissociative seizures for which the patient required assistance to manage; clinically evident anxiety or depression that required assessment before starting physiotherapy treatment; high level of disability that prevented participation in an outpatient/day hospital environment; unable to attend 5 consecutive days of treatment. Pretreatment: inspection of baseline data suggested that the control group had generally worse scores than the intervention group, which were accounted for in the analysis. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Physical symptom load (SF‐36 – Physical Component)
Mental state – anxiety (HADS)
Mental state – depression (HADS)
Level of functioning (WSAS)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible consenting participants were randomly allocated (1:1) to the intervention or control group using a secure online randomisation application (Sealed Envelope, London, UK)." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants were immediately informed of their treatment allocation. Both participants and clinicians were unmasked to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Participants were immediately informed of their treatment allocation. Both participants and clinicians were unmasked to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were missing from end of treatment on the parameter. The study authors considered the SF‐36 – Physical Component the best measurement. |
| Selective reporting (reporting bias) | Low risk | Matches study protocol. |
| Other bias | Low risk | Baseline group differences, but they were adjusted for. |
Pleizier 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): with a sample size of 200 participants (100 per treatment arm), there was 90% power to detect an effect size of d = 0.50, using a 2‐group Student’s t‐test with a 0.05 2‐sided significance level. Allocation concealment method: none. Weakness described that 90% randomised by 1 neurologist. Randomisation in a 1:1 ratio was stratified for type of functional symptoms (pain, 'pseudo' neurological symptoms or 'positive' sensory symptoms) with permuted blocks within the strata. Blinding of outcome assessors: no information provided Check of blinding: no information provided Duration of study: August 2009 to November 2013 Randomisation method: the randomisation procedure was web based (using a validated TENALEA Clinical Trial Data Management System). Randomisation in a 1:1 ratio was stratified for type of functional symptoms (pain, 'pseudo' neurological symptoms or 'positive' sensory symptoms) with permuted blocks within the strata. |
|
| Participants |
Baseline characteristics Intervention
Control
Overall
Inclusion criteria: pain: tension‐type headache (headache without alarming symptoms and not consistent with 1 of the headache syndromes such as migraine, analgesic abuse and cluster headache) and ≥ 1 other FNS; back or neck pain (pain not caused by spinal pathology such as fractures, spondylitis and metastases; myelopathy; radiculopathy; plexopathy or neuropathy) and ≥ 1 other functional symptom; 'pseudo' neurological symptoms: functional movement disorders (movement disorders not consistent with known 'organic' movement disorders); motor impairment other than in movement disorders (motor impairment that cannot be explained by central or peripheral nervous system disorders) or sensory impairment (loss of sensory perception that can neither be explained by central nor by peripheral nervous system disorders), or both; dissociative attacks or PNESs (seizures without evidence for epilepsy on EEGs); 'positive' sensory symptoms: hypersensory perception that can neither be explained by central nor by peripheral nervous system disorders. Exclusion criteria: aged ≤ 18 years; if the duration of the functional symptoms since the first consultation at the GP surgery was > 1 year; known to have psychiatric disorders other than somatoform, depressive or anxiety disorders; primary diagnosis of a severe mood, generalised anxiety or psychotic disorder requiring psychiatric treatment; treated with psychotherapy; known to simulate the symptoms; in dispute about financial or social benefit; experiencing a major somatic disease; and insufficient understanding of the Dutch language. Pretreatment: groups were generally well matched. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Physical symptom load (SF‐36 – Physical Component)
Mental state – anxiety (HADS)
Mental state – depression (HADS)
Dropout
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation procedure was web based (using a validated TENALEA Clinical Trial Data Management System). Randomisation in a 1:1 ratio was stratified for type of functional symptoms (pain, ‘pseudo’neurological symptoms or ‘positive’ sensory symptoms) with permuted blocks within the strata." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The GPs were not informed about the randomisation." Participants were not blinded. Personnel unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information (a nurse prompted the participants, but it did not state whether she also scored the replies). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data available for end of treatment, but as the protocol specified outcome data after 12 months, this was not incomplete. |
| Selective reporting (reporting bias) | High risk | Protocol was changed shortly before the first submission of the article and outcomes etc were changed. The reported outcomes here match the updated protocol. |
| Other bias | Low risk | No other apparent sources of bias. |
Thompson 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): the sample size of 19 participants was small, but the effect size of the primary outcome (follow‐up with therapist) was large for this pilot study. Based on the preliminary study where 100% followed up with a mental health professional with the intervention compared with 10% before, the sample size (nQuery Advisor 6.01, 1995–2005) would have 84% power to detect similar results (using a conservative estimate of 80% vs 10% following up with a therapist, or 90% vs 20% following up). Allocation concealment method: no information provided Blinding of outcome assessors: at 5‐week postdischarge, the principal investigator sent a reminder letter about the upcoming telephone interview and the QOLIE31, with instructions to complete prior to the telephone interview between weeks 6 and 8. The research assistant conducted telephone interviews with the 19 participants. Check of blinding: no information provided Duration of study: 6 weeks Randomisation method: table of random numbers |
|
| Participants |
Baseline characteristics Overall
Inclusion criteria: able to provide written informed consent; diagnosis of PNES established by a neurologist using history, examination and VEEG capturing ≥ 1 of their typical events; and not have a comorbid neurological disease or confirmed medical condition causing the seizures. Exclusion criteria: people with legal guardians; concurrent epilepsy; history of psychiatric disorders that included psychotic features (hallucinations or delusions, or both). Pretreatment: no information provided. |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes | ||
| Notes | There were no data available for the primary or secondary outcomes specified for the review, as it was not possible for the author to provide these (Thompson 2018). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 19 subjects were randomly assigned into either the control or treatment group by using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[The] attending neurologist identified appropriate candidates while they were in the EMU before the final diagnosis was provided." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The personnel were not blinded when delivering the intervention and control. Quote: "The study was explained and informed consent was obtained by the PI [principal investigator] to subjects in the treatment group. She used a consent script (...) The study was explained and informed consent obtained by an advanced practice registered nurse to subjects in the control group." Quote: "The principal investigator (PI), who was also the interventionist, had no contact with subjects who were randomly assigned to the control group. She did not have contact with treatment group subjects prior to meeting them for the intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether the outcome assessor was blinded. Quote: "At the 5‐week postdischarge time, the PI [principal investigator] sent a reminder letter about the upcoming telephone interview and the QOLIE‐31, with instructions to complete prior to the telephone interview between weeks 6 and 8. The research assistant (RA) conducted telephone interviews with the 19 subjects." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only data for the 19 participants were included, even if the text described 25 participants to consent to participate. Quote: "Twenty‐five subjects consented to be in the study. We lost six subjects. One subject was not eligible because she had been diagnosed with psychosis. Two subjects were not eligible as they were also diagnosed with epilepsy. We were unable to find three subjects at the time of the second interview." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | No apparent sources of bias. |
Tolchin 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Adequate power (evidence of power calculation): no power calculation Allocation concealment method: sequentially numbered opaque envelopes were used to conceal allocation until after baseline assessments were completed. Blinding of outcome assessors: yes. Telephone interviewers blinded to study arm assessed participants for adherence to psychotherapy, weekly PNES frequency, 4‐week seizure freedom, and monthly emergency department visits, and administered the QOLIE10 instrument. Participants were instructed not to reveal their group assignment to the interviewer and to avoid use of treatment language or terminology. Events where the blind was broken were tracked. Check of blinding: yes Duration of study: 16 weeks Randomisation method: 1:1 ratio using the Stata function RUNIFORM |
|
| Participants |
Baseline Characteristics Intervention
Control
Overall
Inclusion criteria: aged ≥ 18 years; diagnosed with documented PNES by board‐certified epileptologists at BWH via VEEG review of all types of habitual seizure‐like events, without epileptiform or electrocardiographic abnormalities immediately before, during or following the events and with semiologies (clinical signs and symptoms) that were consistent with PNES. Exclusion criteria: active alcohol or drug‐use disorders; pregnancy; severe medical illness expected to prevent regular participation in psychotherapy; clinically judged significant cognitive impairment; lack of fluent spoken English (given concerns that MI may be less effective in the setting of significant cognitive impairment or when delivered via interpreter). |
|
| Interventions |
Intervention characteristics Intervention
Control
|
|
| Outcomes |
Dropout
Quality of life
Change in monthly emergency department visits
Decrease in seizure frequency
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization assignments to one of two study arms (psychotherapy alone vs psychotherapy preceded by a 30‐minute session of MI) were generated with a 1:1 ratio using the Stata function RUNIFORM. Randomization assignments were created before the study began." |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially numbered opaque envelopes were used to conceal allocation until after baseline assessments were completed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Immediately following informational sessions, participants randomized to MI plus psychotherapy received 30 minutes of MI in the seizure clinic. MI was conducted by a board‐ certified neurologist (B.T.)." Quote: "Participants were instructed not to reveal their group assignment to the interviewer and to avoid use of treatment language or terminology." Comment: the study's principal investigator performed the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At 16‐ week follow‐up, telephone interviewers blinded to study arm assessed participants for adherence to psychotherapy, weekly PNES frequency, 4‐week seizure freedom, and monthly ED visits, and administered the QOLIE‐10 instrument." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: intervention had adherence of 65.4%, while control only had 31.0%. This did not seem comparable. |
| Selective reporting (reporting bias) | Low risk | Comment: matched study protocol. |
| Other bias | Unclear risk | Comment: the results were not presented for actual active participants (17 vs 9), but for the whole groups of both intervention and controls. This did not give a correct view of the effect. |
AED: antiepileptic drug; APA: adjunctive physical activity; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; CBT: cognitive behavioural therapy; CGI: Clinical Global Impression; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders 4th Edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders 4th Edition, Text Revision; EEG: electroencephalogram; EMU: emergency medical unit; FIM: Functional Independence Measure Motor; FMS: functional motor symptom; FNS: functional neurological symptom; GAF: Global Assessment of Functioning; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; HRSA: Hamilton Rating Scale for Anxiety; ICD‐10: International Classification of Diseases; ICIDH; International Classification of Impairments, Disabilities and Handicaps; IQ: intelligence quotient; IPI: interdisciplinary psychotherapeutic intervention; max: maximum; MI: motivational interviewing; n: number of participants; NEA: non‐epileptic attack; PI; paradoxical intention therapy; PMDRS: Psychogenic Movement Disorder Scale; PNES: psychogenic non‐epileptic seizures; QOLIE: Quality of Life in Epilepsy Inventory; RCC: routine clinical care; SC: standard care; SCL‐90; Symptom Checklist; SD: standard deviation; SDQ‐20: 20‐item Somatoform Dissociation Questionnaire; SF‐36: 36‐item Short Form; SHCS; Stanford Hypnotic Clinical Scale for Adults; SMC: standard medical care; SRSS; National Institute of Mental Health Self‐ Rating Symptom Scale; TAU: treatment as usual; VA: Veterans Affairs; VEEG: video‐electroencephalogram; VRMC; Video Rating Scale for Motor Conversion Symptoms; WHO: World Health Organization; WSAS: Work and Social Adjustment Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aamland 2012 | Review. |
| ACTRN12615001176550 | Wrong study design. |
| ACTRN12618000181202 | Wrong study design. |
| Allen 2006 | Wrong patient population. |
| Aybek 2013 | Wrong study design. |
| Baker 2007 | Review. |
| Baslet 2012 | Review. |
| Behr 1996 | No control group. |
| Bellamy 1989 | No control group. |
| Berkwitz 1952 | No control group. |
| Berney 2012 | Wrong study design. |
| Bhattacharyya 1971 | No control group. |
| Binzer 1997 | No control group. |
| Bouchal 1991 | Wrong study design. |
| Brand 2009 | Review. |
| Brand 2012a | Review. |
| Brand 2012b | Review. |
| Brooks 2007a | Review. |
| Brooks 2007b | Review. |
| Cardenas 1986 | No control group. |
| Carlson 2017 | Review. |
| Carson 2012 | Review. |
| Carter 1949 | No control group. |
| Casacchia 1989 | Wrong intervention. |
| Conwill 2014 | Wrong study design. |
| Dahlhauser 2017 | Wrong study design. |
| Deeley 2016 | Wrong study design. |
| Delargy 1986 | No control group. |
| Demartini 2014 | Wrong study design. |
| Dickes 1974 | No control group. |
| Diseth 2005 | Review. |
| DRKS00007139 | Wrong study design. |
| Dworetzky 2014 | Review. |
| Egan 2015 | Overview article. |
| Ellason 1997 | No control group. |
| Escobar 2007 | Wrong patient population. |
| EUCTR2008 004167‐19‐NL | Wrong study design. |
| Fackler 1997 | No control group. |
| Feinstein 2011 | Wrong study design. |
| Fritzsche 2004 | Wrong patient population. |
| Garcin 2018 | Overview article. |
| Gaynor 2009 | Review. |
| Geetha 1980 | Wrong study design. |
| Ghaffar 2017 | Review. |
| Ghosh 2018 | Wrong intervention. |
| Goldstein 2004 | Wrong study design. |
| Gooch 1997 | No control group. |
| Grattan‐Smith 1988 | No control group. |
| Guida 1954 | No control group. |
| Guimaraes 1979 | Wrong study design. |
| Gyllensten 2003 | Wrong patient population. |
| Gyllensten 2009 | Wrong patient population. |
| Hafeiz 1980 | No control group. |
| Halpern 1944 | No control group. |
| Hilmarsdottir 2016 | Review. |
| Hoedeman 2010 | Review. |
| Hoogduin 1993 | No control group. |
| Ilic 2005 | Wrong study design. |
| ISRCTN51225587 | Wrong intervention. |
| Kelley 2011 | Wrong study design. |
| Koelen 2014 | Review. |
| Kolk 2004 | Wrong patient population. |
| Kompoliti 2015 | Overview article. |
| Koufman 1982 | No control group. |
| Kroenke 2007 | Review. |
| Kroenke 2009 | Review. |
| Krull 1990 | No control group. |
| Kupper 1947 | No control group. |
| Lampe 2008 | Wrong patient population. |
| Lehmkuhl 1989 | No control group. |
| Leslie 1988 | No control group. |
| Lipsitt 2006 | Overview article. |
| Margalit 2008 | Wrong patient population. |
| Martlew 2009 | Review. |
| Martlew 2014 | Review. |
| Masiwal 2015 | Wrong study design. |
| Mayor 2010 | Wrong study design. |
| McCormack 2014 | Wrong study design. |
| McKenzie 2010 | Wrong study design. |
| Minnen 2016 | Wrong patient population. |
| Moene 1998 | Wrong study design. |
| Morriss 2006 | Wrong patient population. |
| Myrick 2012 | Wrong study design. |
| Myrick 2017 | Wrong study design. |
| NCT00159965 | Wrong intervention. |
| NCT00630981 | Wrong study design. |
| NCT01422278 | Wrong study design. |
| NCT01643161 | Trial terminated. |
| NCT01778517 | Trial terminated. |
| Nidich 2016 | Wrong patient population. |
| Nielsen 2013 | Review. |
| Nielsen 2016 | Overview article. |
| NTR4496 | Wrong study design. |
| O'Kearney 2001 | Wrong patient population. |
| Payne 2010 | Wrong patient population. |
| Perez 2016 | Review. |
| Pollak 2014 | Review. |
| Poole 2010 | Review. |
| Powell 1998 | Letter to the editor. |
| Pringsheim 2017 | Review. |
| Pu 1986 | No control group. |
| Puhakka 1988 | Wrong patient population. |
| Ramani 1982 | No control group. |
| Rampello 1996 | Wrong intervention. |
| Rangaswami 1985 | No control group. |
| Resick 2012 | Wrong patient population. |
| Reuber 2007 | Wrong study design. |
| Rosebush 2011 | Overview article. |
| Rosendal 2007 | Wrong patient population. |
| Ross 1998 | Letter to the editor. |
| Rudegeair 2013 | Review. |
| Russell 1950 | No control group. |
| Saxe 2002 | Wrong study design. |
| Scallet 1976 | Wrong intervention. |
| Schade 2011 | Wrong patient population. |
| Schilte 2001 | Wrong study design. |
| Schonenberg 2015 | Wrong intervention. |
| Schweden 2016 | Wrong patient population. |
| Shapiro 1997 | No control group. |
| Shapiro 2004a | No control group. |
| Shapiro 2004b | Wrong study design. |
| Sharpe 2011 | Wrong patient population. |
| Shokrolahi 2017 | Wrong study design. |
| Silberg 1996 | Book chapter. |
| Speed 1996 | Wrong study design. |
| Stone 2010 | Wrong study design. |
| Stone 2014a | Review. |
| Stone 2014b | Letter to the editor. |
| Stone 2015 | Review. |
| Stone 2016 | Review. |
| Suzuki 1979 | No control group. |
| Sveinsson 2009 | Review. |
| Tazaki 2006 | Review. |
| Terhune 2017 | Wrong study design. |
| Thenganatt 2015 | Overview article. |
| Tsui 2017 | Overview article. |
| Turgay 1990a | No control group. |
| Turgay 1990b | Wrong study design. |
| Urbanek 2014 | Wrong study design. |
| van Bokhoven 2009 | Wrong intervention. |
| Watanabe 1998 | No control group. |
| Wetzelaer 2014 | Wrong patient population. |
| White 1988 | No control group. |
| Williams 1979 | No control group. |
| Wiseman 2015 | Review. |
| Wolf 2016 | Wrong patient population. |
| Yaskin 1936 | No control group. |
| Zlotnick 1997 | Wrong patient population. |
| Zonneveld 2009 | Wrong patient population. |
| Zonneveld 2012 | Wrong study design. |
Characteristics of ongoing studies [ordered by study ID]
DRKS00012997.
| Study name | The role of the temporo‐parietal junction in functional neurological disorders. A study with mindfulness‐based stress reduction therapy |
| Methods | Randomised controlled trial |
| Participants | Adults with dissociative [conversion] disorders |
| Interventions | 8 weeks of "Mindfulness‐based stress reduction therapy", as developed by Jon Kabat‐Zinn + treatment as usual in people with FND compared with treatment as usual in FND. |
| Outcomes | Primary outcomes
|
| Starting date | 18 March 2020 |
| Contact information | Professor Selma Aybek, Department of Neurology, Bern University Hospital Inselspital C. L. Lora Haus, Freiburgstrasse 41G, 3010 Bern, Switzerland |
| Notes |
DRKS00014251.
| Study name | Evaluation of the effect of a psychotherapy program with body movement focus for patients with dissociative seizures |
| Methods | Randomised controlled trial |
| Participants | Adults with diagnosis of DS |
| Interventions | Short‐term group psychotherapy programme with body movement focus compared with a support group therapy in people with psychogenic non‐epileptic seizures. The programme will run for 10 weeks with a 90‐minute session per week |
| Outcomes |
|
| Starting date | Date of first enrollment: 5 May 2018 |
| Contact information | Dr Philine Senf‐Beckenbach, Charitéplatz 1, 10967 Berlin, Germany; telephone: 004930450553602 E‐mail: philine.senf at charite.de |
| Notes |
Goldstein 2015.
| Study name | Cognitive behavioural therapy vs standardised medical care for adults with dissociative non‐epileptic seizures (CODES): a multicentre randomised controlled trial protocol |
| Methods | Randomised controlled trial |
| Participants | Adults with DS |
| Interventions | CBT |
| Outcomes | Primary outcome
|
| Starting date | 2015 |
| Contact information | Laura.goldstein@kcl.ac.uk |
| Notes |
Goldstein 2016.
| Study name | Cognitive behavioural therapy vs standardised medical care for adults with dissociative non‐epileptic seizures (CODES): a multicentre randomised controlled trial protocol |
| Methods | Randomised controlled trial |
| Participants | Adults with dissociative non‐epileptic seizures |
| Interventions | CBT |
| Outcomes | Primary outcome
|
| Starting date | 2015 |
| Contact information | Laura.goldstein@kcl.ac.uk |
| Notes |
Goldstein 2017.
| Study name | Cognitive behavioural therapy vs standardised medical care for adults with dissociative non‐epileptic seizures (CODES): a multicentre randomised controlled trial protocol |
| Methods | Randomised controlled trial |
| Participants | Adults with dissociative non‐epileptic seizures |
| Interventions | CBT |
| Outcomes | Primary outcome
|
| Starting date | 2015 |
| Contact information | Laura.goldstein@kcl.ac.uk |
| Notes |
ISRCTN05681227.
| Study name | Cognitive behavioural therapy vs standardised medical care for adults with dissociative non‐epileptic seizures (CODES): a multicentre randomised controlled trial protocol |
| Methods | Randomised controlled trial |
| Participants | Adults with dissociative non‐epileptic seizures |
| Interventions | CBT |
| Outcomes | Primary outcome
|
| Starting date | 2015 |
| Contact information | Laura.goldstein@kcl.ac.uk |
| Notes |
NCT01590992.
| Study name | Psychotherapy and psychobiology of somatoform disorders (globus sensations): a randomized controlled trial |
| Methods | |
| Participants | 175 |
| Interventions | Exposure‐based psychotherapy for somatic symptoms and relaxation therapy |
| Outcomes |
|
| Starting date | May 2012 |
| Contact information | Gunther Meinlschmidt, PhD, University of Basel, Ruhr‐University Bochum |
| Notes | We contacted the PI on this study, who informed us that the study did not really get off the ground and was terminated without any results. |
NCT02325544.
| Study name | Cognitive behavioural therapy vs standardised medical care for adults with dissociative non‐epileptic seizures (CODES): a multicentre randomised controlled trial protocol |
| Methods | Randomised controlled trial |
| Participants | Adults with dissociative non‐epileptic seizures |
| Interventions | CBT |
| Outcomes | Primary outcome
|
| Starting date | 2015 |
| Contact information | Laura.goldstein@kcl.ac.uk |
| Notes |
NCT02450617.
| Study name | Stabilizing group treatment of complex trauma: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants | People with complex trauma |
| Interventions | Stabilising group treatment |
| Outcomes |
|
| Starting date | 2015 |
| Contact information | Modum Bad |
| Notes |
NCT02764476.
| Study name | Embodied virtual reality therapy for functional neurological symptom/conversion disorder |
| Methods | Single‐blind, randomised controlled trial |
| Participants | People with conversion disorder, psychogenic movement disorder, functional movement disorder, FND or non‐epileptic seizures |
| Interventions | Embodied virtual reality therapy |
| Outcomes |
|
| Starting date | 2016 |
| Contact information | kbullock@stanford.edu |
| Notes |
NCT02801136.
| Study name | Treatment outcomes of CBT for PNES |
| Methods | Randomised control trial |
| Participants | People with non‐epileptic convulsions |
| Interventions | Cognitive‐behavioural therapy for psychogenic non‐epileptic seizures |
| Outcomes |
|
| Starting date | 2016 |
| Contact information | afobian@uabmc.edu |
| Notes |
Robinson 2017.
| Study name | Cognitive behavioural therapy versus standardised medical care for adults with dissociative non‐epileptic seizures (CODES) |
| Methods | Randomised control trial |
| Participants | Adult outpatients with DS |
| Interventions | CBT + SMC compared with SMC alone for adult outpatients with DS |
| Outcomes | Primary outcome
|
| Starting date | No information |
| Contact information | Emily J Robinson. Department of Biostatistics & Health Informatics, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, UK. emily.robinson@kcl.ac.uk. |
| Notes |
CBT: cognitive behavioural therapy; DS: dissociative seizures; FND: functional neurological disorder; fMRI: functional magnetic resonance imaging; SMC: standard medical care; TPJ: temporo‐parietal junction.
Differences between protocol and review
The original review did not publish its protocol, so this update has taken the 2005 review itself to be protocol.
This update follows the aims and primary outcome of the original review, but there are certain differences between the original review and this update.
Title: we changed the title, from "Psychosocial interventions for conversion disorders" to "Psychosocial interventions for conversion and dissociative disorders for adults". This is in order to reflect the scope of what is being investigated, both here and in the original review.
Secondary outcomes: we added 'level of functioning' as a secondary outcome as this is often reported by patients as being important to them in their everyday lives. In addition, we divided the secondary outcome of mental state, so it allows focus on depression and anxiety separately, as well as mental state as a whole.
As this added up to a total of eight secondary outcomes, we decided not to include certain secondary outcomes from the original review, namely relapse, social adjustment, and patient and carer satisfaction.
Time points: to create consistency in the data, the authors decided to change the time points, so 'end of treatment' is now the primary time point, with short‐ and long‐term follow‐up (short term being up five months and less and long term being six months or more).
Participants: to ensure better quality data that more accurately reflect the population of interest, we have tightened the inclusion criteria for the number of participants with a relevant disorder. Where the previous review used over 50%, we now only considered the study for inclusion where over 80% of participants fulfilled current diagnostic criteria or any earlier diagnostic equivalent.
The main author of the original review (RR) has been part of the author team for this update and confirmed the differences.
Contributions of authors
CAG has been principal investigator and thus is overall responsible for the review update. Part of this has been co‐ordinating the team, screening all abstracts and full texts, performing data extraction and risk of bias, and cowriting the final review.
OJS has participated in screening of abstracts and full texts, is responsible for all the data analysis and results, has cowritten the final review, and has contributed with knowledge and advice on Cochrane procedures and gold standards for good practice.
HEC has part‐taken in data extraction and risk of bias assessment, assisted with the data analysis, contributed invaluable knowledge on the process of working with evidence‐based research, and prepared the 'Summary of findings' tables and GRADE assessment.
RR has contributed with knowledge as the author of the original review, has partaken in screening of abstracts and full texts, and has been part of quality assurance of the final text.
US has contributed with the original idea to update the review, has partaken in screening of abstracts and full texts, has contributed with specialised knowledge on the topic, written the background sections on the disorder, and participated in the preparation of the discussion and conclusion.
Dr Rachel Ruddy and Professor Allan House conceived and wrote the original review published in 2005.
Sources of support
Internal sources
-
Clinic of Liaison Psychiatry and Department of Specialized Treatment, Region Zealand , Denmark
These clinics have funded part of Christina A Ganslevs' participation as principal investigator, have also funded part of Ulf Søgaards' time, as well as provided invaluable support and clinical knowledge on the topic.
-
Department of Psychiatric Research, Region Zealand Healthcare Service, Denmark , Denmark
The research department assisting and supporting the production of this update. This department has also part funded the participation of Christina A Ganslev, Ole Jakob Storebø and Ulf Søgaard.
External sources
-
No external sources of support, UK
This review had no external sources of support
Declarations of interest
CAG: none.
OJS: none.
HEC: none.
RR: none.
US: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aamir 2012 {published data only}
- Aamir S, Hamayon S, Sultan S. Behavior therapy in dissociative convulsion disorder. Journal of Depression & Anxiety 2012;1(1):100-3. [DOI: 10.4172/2167-1044.1000103] [DOI] [Google Scholar]
Ataoglu 2003 {published data only}
- Ataoglu A, Ozcetin A, Icmeli C, Ozbulut O. Paradoxical therapy in conversion reaction. Journal of Korean Medical Science 2003;18(4):581-4. [DOI: 10.3346/jkms.2003.18.4.581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen DK, Maheshwari A, Franks R, Trolley GC, Robinson JS, Hrachovy RA. Brief group psychoeducation for psychogenic nonepileptic seizures: a neurologist-initiated program in an epilepsy center. Epilepsia 2014;55(1):156-6. [DOI: 10.1111/epi.12481] [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Trolley G, Franks R, Chen DK. An abbreviated support group therapy for psychogenic nonepileptic events: a neurologist-initiated program in an epilepsy center. Epilepsy Currents 2013;1:133-4. [DOI: 10.5698/1535-7511-13.s1.1] [DOI] [PubMed] [Google Scholar]
Dallocchio 2016 {published data only}
- Dallocchio C, Tinazzi M, Bombieri F, Arno N, Erro R. Cognitive behavioural therapy and adjunctive physical activity for functional movement disorders (conversion disorder): a pilot, single-blinded, randomized study. Psychotherapy and Psychosomatics 2016;85(6):381-3. [DOI: 10.1159/000446660] [DOI] [PubMed] [Google Scholar]
- Erro R, Tinazzi M, Dallocchio C. Cognitive behavioural therapy and adjunctive physical therapy for functional movement disorders. 20th International Congress of Parkinson's Disease and Movement Disorders; 2019 Jun 19-23; Berlin, Germany . [DOI: 10.1002/mds.26688] [DOI]
Drane 2016 {published data only}
- Drane Dl, LaRoche SM, Ganesh GA, Teagarden D, Loring DW. A standardized diagnostic approach and ongoing feedback improves outcome in psychogenic nonepileptic seizures. Epilepsy & Behavior 2016;54:34-9. [DOI: 10.1016/j.yebeh.2015.10.026] [DOI] [PubMed] [Google Scholar]
- Ganesh G, Drane D, Loring D, Teagarden D, Kress K, Laroche SM. Treatment strategies for psychogenic nonepileptic seizures: a pilot study. Epilepsy Currents 2013;13:135-6. [DOI: 10.5698/1535-7511-13.s1.1] [DOI] [Google Scholar]
Goldstein 2010 {published data only}
- Goldstein LH, Chalder T, Chigwedere C, Khondoker MR, Moriarty J, Toone BK, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology 2010;74(24):1986-94. [DOI: 10.1212/WNL.0b013e3181e39658] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LH, Chalder T, Chigwedere C, Moriarty J, Toone BK, Mellers JD. A randomised controlled trial of CBT and standard medical care in patients with dissociative (non-epileptic) seizures. Epilepsia 2009;11:221-2. [Google Scholar]
- NCT00688727. Cognitive behavioural therapy in dissociative seizures. clinicaltrials.gov/ct2/show/NCT00688727 (first received 3 June 2008).
- NCT02325544. Comparing different treatments in reducing dissociative seizure occurrence (CODES). clinicaltrials.gov/ct2/show/NCT02325544 (first received 25 December 2014).
Hubschmid 2015 {published data only}
- Hubschmid M, Aybek S, Maccaferri GE, Chocron O, Gholamrezaee MM, Rossetti AO, et al. Efficacy of brief interdisciplinary psychotherapeutic intervention for motor conversion disorder and nonepileptic attacks. General Hospital Psychiatry 2015;37(5):448-55. [DOI: 10.1016/j.genhosppsych.2015.05.007] [DOI] [PubMed] [Google Scholar]
Jordbru 2014 {published data only}
- Jordbru AA, Smedstad LM, Klungsøyr O, Martinsen EW. Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one-year follow-up. Journal of Rehabilitation Medicine 2014;46(2):181-7. [DOI: 10.2340/16501977-1246] [DOI] [PubMed] [Google Scholar]
Khattak 2006 {published data only}
- Khattak T, Farooq S, Jan B. Behavior therapy in dissociative convulsions disorder. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP 2006;16(5):359-63. [PMID: PMID: 16756783] [PubMed] [Google Scholar]
LaFrance 2014 {published data only}
- Baird Gl, Harlow Ll, Machan JT, LaFrance WC. Cluster reduction in patients in a pilot treatment trial for psychogenic nonepileptic seizures. Epilepsy & Behavior 2017;73:273-9. [DOI: 10.1016/j.yebeh.2017.04.015] [DOI] [PubMed] [Google Scholar]
- LaFrance WC Jr, Frank Webb A, Blum AS, Keitner GI, Barry J, Szaflarski J. Multi-center treatment trial pilot for psychogenic nonepileptic seizures. Epilepsy Currents 2013;1:99. [DOI: 10.5698/1535-7511-13.s1.1] [DOI] [Google Scholar]
- LaFrance WC, Baird Gl, Barry JJ, Blum AS, Frank WA, Keitner GI, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014;71(9):997-1005. [DOI: 10.1001/jamapsychiatry.2014.817] [DOI] [PubMed] [Google Scholar]
- NCT00835627. Treatment trial for psychogenic nonepileptic seizures (NEST-T_1). clinicaltrials.gov/ct2/show/NCT00835627 (first received 3 February 2009).
Moene 2002 {published data only}
- Moene FC, Spinhoven P, Hoogduin KA, Dyck R. A randomised controlled clinical trial on the additional effect of hypnosis in a comprehensive treatment programme for in-patients with conversion disorder of the motor type. Psychotherapy and Psychosomatics 2002;71(2):66-76. [DOI: 10.1159/000049348] [DOI] [PubMed] [Google Scholar]
Moene 2003 {published data only}
- Moene FC, Spinhoven P, Hoogduin KA, Dyck R. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. International Journal of Clinical and Experimental Hypnosis 2003;51(1):29-50. [DOI: 10.1076/iceh.51.1.29.14067] [DOI] [PubMed] [Google Scholar]
Mousavi 2008 {published data only}
- Mousavi SG, Rahimi J, Afshar H. Comparison of four different treatment options in the management of acute conversion disorder. Iranian Journal of Psychiatry and Behavioral Sciences 2008;2(1):21-5. [Google Scholar]
Nielsen 2017 {published data only}
- NCT02275000. Feasibility study of physiotherapy for functional motor symptoms. clinicaltrials.gov/ct2/show/NCT02275000 (first received 27 October 2014).
- Nielsen G, Buszewicz M, Stevenson F, Hunter R, Holt K, Dudziec M, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. Journal of Neurology, Neurosurgery, and Psychiatry 2017;88(6):484-90. [DOI: 10.1136/jnnp-2016-314408] [DOI] [PubMed] [Google Scholar]
Pleizier 2017 {published data only}
- NTR2570. Treatment of functional neurological symptoms. www.trialregister.nl/trial/2454 (first received 19 June 2009).
- Pleizier M, De Haan RJ, Vermeulen M. Management of patients with functional neurological symptoms: a single-centre randomised controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 2017;88(5):430-6. [DOI: 10.1136/jnnp-2015-312889] [DOI] [PubMed] [Google Scholar]
Thompson 2013 {published data only}
- Thompson N, Connelly L, Peltzer J, Nowack WJ, Hamera E, Hunter EE. Psychogenic nonepileptic seizures: a pilot study of a brief educational intervention. Perspectives in Psychiatric Care 2013;49(2):78-83. [DOI: 10.1111/j.1744-6163.2012.00353.x] [DOI] [PubMed] [Google Scholar]
Tolchin 2019 {published data only}
- NCT02598076. Motivational interviewing to enhance adherence of patients with psychogenic non-epileptic seizures. clinicaltrials.gov/ct2/show/NCT02598076 (first received 5 November 2015).
- Tolchin B, Baslet G, Suzuki J, Martino S, Blumenfeld H, Hirsch LJ, et al. Randomized controlled trial of motivational interviewing for psychogenic nonepileptic seizures. Epilepsia 2019;60(5):986-95. [DOI: 10.1111/epi.14728] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aamland 2012 {published data only}
- Aamland A, Malterud K, Werner EL. Phenomena associated with sick leave among primary care patients with medically unexplained physical symptoms: a systematic review. Scandinavian Journal of Primary Health Care 2012;30(3):147-55. [DOI: 10.3109/02813432.2012.704812] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12615001176550 {published data only}
- ACTRN12615001176550. In patients with conversion disorder and healthy volunteers, does a short term stress reduction technique followed by psychotherapy lead to improved processing of stress? www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369315 (first received 2 November 2015).
ACTRN12618000181202 {published data only}
- ACTRN12618000181202. Brief psychodynamic therapy for functional neurological symptoms. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373698 (first received 5 February 2018).
Allen 2006 {published data only}
- Allen LA, Woolfolk RL, Escobar JI, Gara, MA, Hamer RM. Cognitive-behavioral therapy for somatization disorder: a randomized controlled trial. Archives of Internal Medicine 2006;166(14):1512-8. [DOI] [PubMed] [Google Scholar]
Aybek 2013 {published data only}
- Aybek S, Hubschmid M, Mossinger C, Berney A, Vingerhoets F. Early intervention for conversion disorder: neurologists and psychiatrists working together. Acta Neuropsychiatrica 2013;25(1):52-6. [DOI: 10.1111/j.1601-5215.2012.00668.x] [DOI] [PubMed] [Google Scholar]
Baker 2007 {published data only}
- Baker GA, Brooks JL, Goodfellow L, Bodde N, Aldenkamp A. Treatments for non-epileptic attack disorder. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006370] [DOI] [PubMed] [Google Scholar]
Baslet 2012 {published data only}
- Baslet G. Psychogenic nonepileptic seizures: a treatment review. What have we learned since the beginning of the millennium? Neuropsychiatric Disease and Treatment 2012;8:585-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Behr 1996 {published data only}
- Behr J. The role of physiotherapy in the recovery of patients with conversion disorder. Physiotherapy Canada 1996;48(3):197-202. [Google Scholar]
Bellamy 1989 {published data only}
- Bellamy RF, McCaughey BG, Fragala MR. Slight hysteria. Military Medicine 1989;154(2):94-7. [PubMed] [Google Scholar]
Berkwitz 1952 {published data only}
- Berkwitz NJ. Outpatient treatment with faradic (non-convulsive electric) stimulation. Confinia Neurologica 1952;12:362-3. [DOI] [PubMed] [Google Scholar]
Berney 2012 {published data only}
- Berney A, Aybek S, Hubschmid M, Vingerhoets F. Short term structured joint neurological and psychiatric intervention improves long term outcome of conversion disorder patients. Journal of Psychosomatic Research 2012;72(6):472. [Google Scholar]
Bhattacharyya 1971 {published data only}
- Bhattacharyya DD, Singh R. Behavior therapy of hysterical fits. American Journal of Psychiatry 1971;128(5):602-6. [DOI] [PubMed] [Google Scholar]
Binzer 1997 {published data only}
- Binzer M, Andesen PM, Kullgren G. Clinical characteristics of patients with motor disability due to conversion disorder: a prospective control group study. Journal of Neurology, Neurosurgery, and Psychiatry 1997;63:83-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouchal 1991 {published data only}
- Bouchal M, Skoda C. Catamnesis of neuroses after 11 years of treatment with 3 different therapeutic programs. Ceskoslovenska Psychiatrie 1991;87(5-6):230-41. [PubMed] [Google Scholar]
Brand 2009 {published data only}
- Brand BL, Classen CC, McNary SW, Zaveri P. A review of dissociative disorders treatment studies. Journal of Nervous & Mental Disease 2009;197(9):646-54. [DOI: ] [DOI] [PubMed] [Google Scholar]
Brand 2012a {published data only}
- Brand BL. What we know and what we need to learn about the treatment of dissociative disorders. Journal of Trauma & Dissociation 2012;13(4):387-96. [DOI: ] [DOI] [PubMed] [Google Scholar]
Brand 2012b {published data only}
- Brand BL, Lanius R, Vermetten E, Loewenstein RJ, Spiegel D. Where are we going? An update on assessment, treatment, and neurobiological research in dissociative disorders as we move toward the DSM-5. Journal of Trauma & Dissociation 2012;13(1):9-31. [DOI: 10.1080/15299732.2011.620687] [DOI] [PubMed] [Google Scholar]
Brooks 2007a {published data only}
- Brooks JL, Goodfellow L, Bodde NM, Aldenkamp A, Baker GA. Nondrug treatments for psychogenic nonepileptic seizures: what's the evidence? Epilepsy and Behavior 2007;11(3):367-77. [DOI] [PubMed] [Google Scholar]
Brooks 2007b {published data only}
- Brooks JL, Baker GA, Goodfellow L, Bodde N, Aldenkamp A. Behavioural treatments for non-epileptic attack disorder. Cochrane Database of Systematic Reviews 2007, Issue CD006370. [DOI: 10.1002/14651858.CD006370] [DOI] [PubMed] [Google Scholar]
Cardenas 1986 {published data only}
- Cardenas DD, Larson J, Egan KJ. Hysterical paralysis in the upper extremity of chronic pain patients. Archives of Physical Medicine & Rehabilitation 1986;67(3):190-3. [DOI] [PubMed] [Google Scholar]
Carlson 2017 {published data only}
- Carlson P, Nicholson PK. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure 2017;45:142-50. [DOI: ] [DOI] [PubMed] [Google Scholar]
Carson 2012 {published data only}
- Carson AJ, Brown R, David AS, Duncan R, Edwards MJ, Goldstein LH, et al. Functional (conversion) neurological symptoms: research since the millennium. Journal of Neurology Neurosurgery and Psychiatry 2012;83(8):842-50. [DOI: 10.1136/jnnp-2011-301860] [DOI] [PubMed] [Google Scholar]
Carter 1949 {published data only}
- Carter AB. The prognosis of certain hysterical symptoms. British Medical Journal 1949;1:1076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casacchia 1989 {published data only}
- Casacchia M, Farolfi A, Priore P, Magni G, Stratta P, Cesana B, et al. A double-blind, placebo-controlled study of alpidem, a novel anxiolytic of imidazopyridine structure, in chronically anxious patients. Acta Psychiatrica Scandinavica 1989;80(2):137-41. [DOI] [PubMed] [Google Scholar]
Conwill 2014 {published data only}
- Conwill M, Oakley L, Evans K, Cavanna AE. CBT-based group therapy intervention for nonepileptic attacks and other functional neurological symptoms: a pilot study. Epilepsy & Behavior 2014;34:68-72. [DOI: 10.1016/j.yebeh.2014.03.012] [DOI] [PubMed] [Google Scholar]
Dahlhauser 2017 {published data only}
- Dahlhauser SE, Theuer A, Hollman J. Satisfaction and occupational performance in patients with functional movement disorder. Open Journal of Occupational Therapy (OJOT) 2017;5(2):1-7. [DOI: 10.15453/2168-6408.1287] [DOI] [Google Scholar]
Deeley 2016 {published data only}
- Deeley Q. Hypnosis as therapy for functional neurologic disorders. Handbook of Clinical Neurology 2016;139:585-95. [DOI] [PubMed] [Google Scholar]
Delargy 1986 {published data only}
- Delargy MA, Peatfield RC, Burt AA. Successful rehabilitation in conversion paralysis. BMJ 1986;292:1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demartini 2014 {published data only}
- Demartini B, Batla A, Petrochilos P, Fisher L, Edwards MJ, Joyce, E. Multidisciplinary treatment for functional neurological symptoms: a prospective study. Journal of Neurology 2014;261(12):2370-7. [DOI: 10.1007/s00415-014-7495-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickes 1974 {published data only}
- Dickes RA. Brief therapy of conversion reactions: an in-hospital technique. American Journal of Psychiatry 1974;131:584-6. [PubMed] [Google Scholar]
Diseth 2005 {published data only}
- Diseth TH, Christie HJ. Trauma-related dissociative (conversion) disorders in children and adolescents – an overview of assessment tools and treatment principles. Nordic Journal of Psychiatry 2005;59(4):278-92. [DOI: 10.1080/08039480500213683] [DOI] [PubMed] [Google Scholar]
DRKS00007139 {published data only}
- DRKS00007139. The significance of adverse experience, emotional processing and body perception for functional (pseudo-) neurological symptoms: a longitudinal study (pre-post-comparison). www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00007139 (first received 19 August 2015).
Dworetzky 2014 {published data only}
- Dworetzky BA. Neglected patients, few treatments, and minimal evidence: the updated Cochrane review on psychological and behavioral treatments for nonepileptic seizures. Epilepsy Currents 2014;14(6):329-31. [DOI: 10.5698/1535-7597-14.6.329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egan 2015 {published data only}
- Egan RA, LaFrance WC. Functional vision disorder. Seminars in Neurology 2015;35(5):557-63. [DOI: 10.1055/s-0035-1563580] [DOI] [PubMed] [Google Scholar]
Ellason 1997 {published data only}
- Ellason JW, Ross CA. Two-year follow-up of inpatients with dissociative disorder. American Journal of Psychiatry 1997;154:832-9. [DOI] [PubMed] [Google Scholar]
Escobar 2007 {published data only}
- Escobar JI, Gara MA, Diaz-Martinez AM, Interian A, Warman M, Allen LA, et al. Effectiveness of a time-limited cognitive behavior therapy type intervention among primary care patients with medically unexplained symptoms. Annals of Family Medicine 2007;5(4):328-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2008 004167‐19‐NL {published data only}
- EUCTR2008-004167-19-NL. The effectiveness of antidepressants and psychological intervention in treating conversion disorder, motor type: a randomized placebo controlled clinical trial. www.clinicaltrialsregister.eu/ctr-search/trial/2008-004167-19/NL (first received 29 September 2008).
Fackler 1997 {published data only}
- Fackler SM, Anfinson TJ, Rand JA. Serial sodium amytal interviews in the clinical setting. Psychosomatics 1997;38:558-64. [DOI] [PubMed] [Google Scholar]
Feinstein 2011 {published data only}
- Feinstein A. Conversion disorder: advances in our understanding. Canadian Medical Association Journal 2011;183(8):915-20. [DOI: 10.1503/cmaj.110490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fritzsche 2004 {published data only}
- Fritzsche K, Larisch A, Cierpka M, Wirsching M. Improving the biopsychosocial competence of German primary care physicians in diagnosing and treating somatoform disorders. Families, Systems & Health 2004;22(3):352-64. [Google Scholar]
Garcin 2018 {published data only}
- Garcin B. Motor functional neurological disorders: an update. Revue Neurologique 2018;174(4):203-11. [DOI: 10.1016/j.neurol.2017.11.003] [DOI] [PubMed] [Google Scholar]
Gaynor 2009 {published data only}
- Gaynor D, Cock H, Agrawal N. Psychological treatments for functional non-epileptic attacks: a systematic review. Acta Neuropsychiatrica 2009;21(4):158-68. [DOI] [PubMed] [Google Scholar]
Geetha 1980 {published data only}
- Geetha PR, Channabasavanna SM, Bhatti RS. The study of efficacy of family ward treatment in hysteria in comparison with the open ward and the outpatient treatment. Indian Journal of Psychiatry 1980;22(4):317-21. [PMC free article] [PubMed] [Google Scholar]
Ghaffar 2017 {published data only}
- Ghaffar O, Modirrousta M, LaFrance C, Williams N, Shura R, Ducharme S, et al. Treatment of functional neurological disorders: towards expert consensus led guidelines for the American neuropsychiatric association committee for research. Movement Disorders 2017;32(Suppl 2):874. [Google Scholar]
Ghosh 2018 {published data only}
- Ghosh A, Mehta VS, Munda SK. Efficacy of adjunctive cathodal TDCS in psychogenic non-epileptic seizure in children and adolescents: a randomized double blinded sham controlled study. Indian Journal of Psychiatry 2018;60(5):53. [Google Scholar]
Goldstein 2004 {published data only}
- Goldstein LH, Deale AC, Mitchell-O'Malley SJ, Toone BK, Mellers JC, Aboukasm A. An evaluation of cognitive behavioral therapy as a treatment for dissociative seizures: a pilot study. Cognitive and Behavioral Neurology 2004;17(1):41-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gooch 1997 {published data only}
- Gooch JL, Wolcott R, Speed J. Behavioral management of conversion disorder in children. Archives of Physical Medicine & Rehabilitation 1997;78(3):264-8. [DOI] [PubMed] [Google Scholar]
Grattan‐Smith 1988 {published data only}
- Grattan-Smith P, Fairley M, Procopis P. Clinical features of conversion disorder. Archives of Disease in Childhood 1988;63(4):408-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guida 1954 {published data only}
- Guida A. Observations on scopochloralose. Neurone 1954;2:133-61. [Google Scholar]
Guimaraes 1979 {published data only}
- Guimaraes AR. Topical and systemic analysis and treatment of conversion disorders: a radical behaviorist analysis. Dissertation Abstracts International 1979;40(1-b):450-1. [Google Scholar]
Gyllensten 2003 {published data only}
- Gyllensten AL, Hansson L, Ekdahl C. Outcome of basic body awareness therapy. A randomized controlled study of patients in psychiatric outpatient care. Advances in Physiotherapy 2003;5(4):179-90. [Google Scholar]
Gyllensten 2009 {published data only}
- Gyllensten AL, Ekdahl C, Hansson L. Long-term effectiveness of basic body awareness therapy in psychiatric outpatient care. A randomized controlled study. Advances in Physiotherapy 2009;11(1):2-12. [Google Scholar]
Hafeiz 1980 {published data only}
- Hafeiz HB. Hysterical conversion: a prognostic study. British Journal of Psychiatry 1980;136:548-51. [DOI] [PubMed] [Google Scholar]
Halpern 1944 {published data only}
- Halpern HJ. Hysterical amblyopia. Bulletin. United States. Army Medical Dept 1944;72:84-7. [Google Scholar]
Hilmarsdottir 2016 {published data only}
- Hilmarsdottir D, Birk S, Rask CU. Psychotherapeutic treatment of psychogenic non-epileptic seizures. Ugeskrift for Laeger 2016;178(48):28. [PubMed] [Google Scholar]
Hoedeman 2010 {published data only}
- Hoedeman R, Blankenstein AH, Feltz-Cornelis CM, Krol B, Stewart R, Groothoff JW. Consultation letters for medically unexplained physical symptoms in primary care. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD006524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoogduin 1993 {published data only}
- Hoogduin K, Akkermans M, Oudshoorn D, Reinders M. Hypnotherapy and contractures of the hand. American Journal of Clinical Hypnosis 1993;36(2):106-12. [DOI] [PubMed] [Google Scholar]
Ilic 2005 {published data only}
- Ilic A, Ristic AJ, Vojvodic N, Petrovic I, Jankovic S, Sokic D. Placebo induction benefits and clinical features assessment in patients with psychogenic nonepileptic seizures. Epilepsia 2005;46:344. [Google Scholar]
ISRCTN51225587 {published data only}
- ISRCTN51225587. A feasibility study of magnetic stimulation (using TMS – transcranial magnetic stimulation) of the brain to improve limb weakness in motor conversion (functional neurological) disorder. www.isrctn.com/ISRCTN51225587 (first received 18 September 2017).
Kelley 2011 {published data only}
- Kelley SD, Bozorg A. Outcomes of trauma-induced psychogenic nonepileptic attacks treated with eye movement densensitization and reprocessing. Epilepsy Currents 2011;11(Suppl 1):1. [Google Scholar]
Koelen 2014 {published data only}
- Koelen JA, Houtveen JH, Abbass A, Luyten P, Eurelings-Bontekoe EH, Van Broeckhuysen-Kloth SA, et al. Effectiveness of psychotherapy for severe somatoform disorder: meta-analysis. British Journal of Psychiatry 2014;204(1):12-9. [DOI] [PubMed] [Google Scholar]
Kolk 2004 {published data only}
- Kolk A, Schagen S, Hanewald Gj. Multiple medically unexplained physical symptoms and health care utilization: outcome of psychological intervention and patient-related predictors of change. Journal of Psychosomatic Research 2004;57(4):379-89. [DOI: 10.1016/j.jpsychores.2004.02.012] [DOI] [PubMed] [Google Scholar]
Kompoliti 2015 {published data only}
- Kompoliti K. Cognitive therapies for psychogenic movement disorders. Journal of the Neurological Sciences 2015;1:e479. [Google Scholar]
Koufman 1982 {published data only}
- Koufman JA, Blalock PD. Classification and approach to patients with functional voice disorders. Annals of Otology, Rhinology, and Laryngology 1982;91:372-7. [DOI] [PubMed] [Google Scholar]
Kroenke 2007 {published data only}
- Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosomatic Medicine 2007;69(9):881-8. [DOI] [PubMed] [Google Scholar]
Kroenke 2009 {published data only}
- Kroenke K. Efficacy of treatment for somatoform disorders: A review of randomized controlled trials. In: Somatic Presentations of Mental Disorders: Refining the Research Agenda for DSM-V. Washington (DC): American Psychiatric Publishing, 2009:143-63. [Google Scholar]
Krull 1990 {published data only}
- Krull F, Schifferdecker M. Inpatient treatment of conversion disorder: a clinical investigation of outcome. Psychotherapy and Psychosomatics 1990;53:161-5. [DOI] [PubMed] [Google Scholar]
Kupper 1947 {published data only}
- Kupper WH. Observations on the use of a phonograph record of battle sounds employed in conjunction with pentothal in the treatment of 14 cases of severe conversion hysteria caused by combat. Journal of Nervous and Mental Disease 1947;105:56-60. [DOI] [PubMed] [Google Scholar]
Lampe 2008 {published data only}
- Lampe A, Mitmansgruber H, Gast U, Schüssler G, Reddemann L. Treatment outcome of psychodynamic trauma therapy in an inpatient setting. Neuropsychiatrie : Klinik, Diagnostik, Therapie und Rehabilitation 2008;22(3):189-97. [PubMed] [Google Scholar]
Lehmkuhl 1989 {published data only}
- Lehmkuhl G, Blanz B, Lehmkuhl U, Braun-Scharm H. Conversion disorder (DSM-III 300.11): symptomatology and course in childhood and adolescence. European Archives of Psychiatry and Neurological Sciences 1989;238:155-60. [DOI] [PubMed] [Google Scholar]
Leslie 1988 {published data only}
- Leslie SA. Diagnosis and treatment of hysterical conversion reactions. Archives of Disease in Childhood 1988;63:506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipsitt 2006 {published data only}
- Lipsitt DR, Starcevic V. Psychotherapy and pharmacotherapy in the treatment of somatoform disorders. Psychiatric Annals 2006;36(5):341-8. [Google Scholar]
Margalit 2008 {published data only}
- Margalit AP, El-Ad A. Costly patients with unexplained medical symptoms: a high-risk population. Patient Education & Counseling 2008;70(2):173-8. [DOI] [PubMed] [Google Scholar]
Martlew 2009 {published data only}
- Martlew J, Baker GA, Goodfellow L, Bodde N, Aldenkamp A. Behavioural treatments for non-epileptic attack disorder. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006370] [DOI] [PubMed] [Google Scholar]
Martlew 2014 {published data only}
- Martlew J, Pulman J, Marson AG. Psychological and behavioural treatments for adults with non-epileptic attack disorder. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD006370.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masiwal 2015 {published data only}
- Masiwal D, Tripathi M, Sagar R. To evaluate quality-of-life (QOL), before and after administration of cognitive behavioural therapy (CBT) as a treatment in patients diagnosed with psychogenic non-epileptic seizures (PNES) and assess the same on WHOQOL-BREF (field trial version) and M.I.N.I scale. Epilepsia 2015;1:20. [Google Scholar]
Mayor 2010 {published data only}
- Mayor R, Howlett S, Grunewald R, Reuber M. Long-term outcome of brief augmented psychodynamic interpersonal therapy for psychogenic nonepileptic seizures: seizure control and health care utilization. Epilepsia 2010;51(7):1169-76. [DOI] [PubMed] [Google Scholar]
McCormack 2014 {published data only}
- McCormack R, Moriarty J, Mellers JD, Shotbolt P, Pastena R, Landes N, et al. Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. Journal of Neurology Neurosurgery, and Psychiatry 2014;85(8):893-8. [DOI: 10.1136/jnnp-2013-305716] [DOI] [PubMed] [Google Scholar]
McKenzie 2010 {published data only}
- McKenzie P, Oto M, Russell A, Pelosi A, Duncan R. Early outcomes and predictors in 260 patients with psychogenic nonepileptic attacks. Neurology 2010;74(1):64-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Minnen 2016 {published data only}
- Minnen A, Vleugel BM, Berg DP, Bont PA, Roos C, Gaag M, et al. Effectiveness of trauma-focused treatment for patients with psychosis with and without the dissociative subtype of post-traumatic stress disorder. British Journal of Psychiatry 2016;209(4):347-8. [DOI: 10.1192/bjp.bp.116.185579] [DOI] [PubMed] [Google Scholar]
Moene 1998 {published data only}
- Moene FC, Hoogduin KA, Van Dyck R. The inpatient treatment of patients suffering from (motor) conversion symptoms: a description of eight cases. International Journal of Clinical and Experimental Hypnosis 1998;46(2):171-90. [DOI: ] [DOI] [PubMed] [Google Scholar]
Morriss 2006 {published data only}
- Morriss R, Dowrick C, Salmon P, Peters S, Rogers A, Dunn G, et al. Turning theory into practice: rationale, feasibility and external validity of an exploratory randomized controlled trial of training family practitioners in reattribution to manage patients with medically unexplained symptoms (the MUST). General Hospital Psychiatry 2006;28(4):343-51. [DOI] [PubMed] [Google Scholar]
Myrick 2012 {published data only}
- Myrick AC, Brand BL, McNary SW, Classen CC, Lanius R, Loewenstein RJ, et al. An exploration of young adults' progress in treatment for dissociative disorder. Journal of Trauma & Dissociation 2012;13(5):582-95. [DOI: ] [DOI] [PubMed] [Google Scholar]
Myrick 2017 {published data only}
- Myrick AC, Webermann AR, Loewenstein RJ, Lanius R, Putnam FW, Brand BL. Six-year follow-up of the treatment of patients with dissociative disorders study. European Journal of Psychotraumatology 2017;8(1):1344080. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00159965 {published data only}
- NCT00159965. Treatments for psychogenic nonepileptic seizures (NES). clinicaltrials.gov/ct2/show/NCT00159965 (first received 12 September 2005).
NCT00630981 {published data only}
- NCT00630981. Psychotherapy and pharmacotherapy in dissociative disorders. clinicaltrials.gov/ct2/show/NCT00630981 (first received 7 March 2008).
NCT01422278 {published data only}
- NCT01422278. Rehabilitation of conversion gait disorder. clinicaltrials.gov/ct2/show/NCT01422278 (first received 23 August 2011).
NCT01643161 {published data only}
- NCT01643161. Guided self-help for functional neurological symptoms. clinicaltrials.gov/ct2/show/NCT01643161 (first received 18 July 2012).
NCT01778517 {published data only}
- NCT01778517. Treatment of functional movement disorders with psychotherapy. clinicaltrials.gov/ct2/show/NCT01778517 (first received 29 January 2013).
Nidich 2016 {published data only}
- Nidich S, O'Connor T, Rutledge T, Duncan J, Compton B, Seng A, et al. Reduced trauma symptoms and perceived stress in male prison inmates through the transcendental meditation program: a randomized controlled trial. Permanente Journal 2016;20(4):43-7. [DOI: 10.7812/TPP/16-007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2013 {published data only}
- Nielsen G, Stone J, Edwards MJ. Physiotherapy for functional (psychogenic) motor symptoms: a systematic review. Journal of Psychosomatic Research 2013;75(2):93-102. [DOI] [PubMed] [Google Scholar]
Nielsen 2016 {published data only}
- Nielsen G. Chapter 45 – physical treatment of functional neurologic disorders. In: Hallett M, Stone J, Carson A, editors(s). Handbook of Clinical Neurology. Vol. 139. London: Elsevier, 2016:555-69. [DOI] [PubMed] [Google Scholar]
NTR4496 {published data only}
- NTR4496. Innovation in the treatment of dissociative identity disorder: the application of schema therapy. www.trialregister.nl/trial/4356 (first received 1 June 2014).
O'Kearney 2001 {published data only}
- O'Kearney R. Cognitive behavioural therapy reduced distress and doctor visits in patients with medically unexplained symptoms. Evidence-Based Mental Health 2001;4:22. [Google Scholar]
Payne 2010 {published data only}
- Payne H, Stott D. Change in the moving bodymind: quantitative results from a pilot study on the use of the BodyMind approach (BMA) to psychotherapeutic group work with patients with medically unexplained symptoms (MUSs). Counselling & Psychotherapy Research 2010;10(4):295-306. [DOI: 10.1080/14733140903551645] [DOI] [Google Scholar]
Perez 2016 {published data only}
- Perez DL, LaFrance WC. Nonepileptic seizures: an updated review. CNS Spectrums 2016;21(3):239-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollak 2014 {published data only}
- Pollak TA, Nicholson TR, Edwards MJ, David AS. A systematic review of transcranial magnetic stimulation in the treatment of functional (conversion) neurological symptoms. Journal of Neurology, Neurosurgery, and Psychiatry 2014;85(2):191-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Poole 2010 {published data only}
- Poole NA, Wuerz A, Agrawal N. Abreaction for conversion disorder: systematic review with meta-analysis. British Journal of Psychiatry 2010;197(2):91-5. [DOI: 10.1192/bjp.bp.109.066894] [DOI] [PubMed] [Google Scholar]
Powell 1998 {published data only}
- Powell RA, Howell AJ. Treatment outcome for dissociative identity disorder. American Journal of Psychiatry 1998;155(9):1304. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pringsheim 2017 {published data only}
- Pringsheim T, Edwards M. Functional movement disorders: five new things. Neurology. Clinical Practice 2017;7(2):141-7. [DOI: 10.1212/cpj.0000000000000350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pu 1986 {published data only}
- Pu T, Mohamed E, Iman K, El-Roey A. One hundred cases of hysteria in Eastern Libya: a socio-demographic study. British Journal of Psychiatry 1986;148:606-9. [DOI] [PubMed] [Google Scholar]
Puhakka 1988 {published data only}
- Puhakka HJ, Kirveskari P. Globus hystericus: globus syndrome? Journal of Laryngology and Otology 1988;102(3):231-4. [DOI] [PubMed] [Google Scholar]
Ramani 1982 {published data only}
- Ramani V, Gumnit RJ. Management of hysterical seizures in epileptic patients. Archives of Neurology 1982;39:78-81. [DOI] [PubMed] [Google Scholar]
Rampello 1996 {published data only}
- Rampello L, Raffaele R, Nicoletti G, Le Pira F, Malaguarnera M, Drago F. Hysterical neurosis of the conversion type: therapeutic activity of neuroleptics with different hyperprolactinemic potency. Neuropsychobiology 1996;33:186-8. [DOI] [PubMed] [Google Scholar]
Rangaswami 1985 {published data only}
- Rangaswami K. Treatment of hysterical conditions by avoidance conditioning. Dayalbagh Educational Institute Research Journal of Education 1985;3:53-6. [Google Scholar]
Resick 2012 {published data only}
- Resick PA, Suvak MK, Johnides BD, Mitchell KS, Iverson KM. The impact of dissociation on PTSD treatment with cognitive processing therapy. Depression and Anxiety 2012;29(8):718-30. [DOI] [PubMed] [Google Scholar]
Reuber 2007 {published data only}
- Reuber M, Burness C, Howlett S, Brazier J, Grunewald R. Tailored psychotherapy for patients with functional neurological symptoms: a pilot study. Journal of Psychosomatic Research 2007;63(6):625-32. [DOI] [PubMed] [Google Scholar]
Rosebush 2011 {published data only}
- Rosebush PI, Mazurek MF. Treatment of conversion disorder in the 21st century: have we moved beyond the couch? Current Treatment Options in Neurology 2011;13(3):255-66. [DOI: 10.1007/s11940-011-0124-y] [DOI] [PubMed] [Google Scholar]
Rosendal 2007 {published data only}
- Rosendal M, Olesen F, Fink P, Toft T, Sokolowski I, Bro F. A randomized controlled trial of brief training in the assessment and treatment of somatization in primary care: effects on patient outcome. General Hospital Psychiatry 2007;29(4):364-73. [DOI] [PubMed] [Google Scholar]
Ross 1998 {published data only}
- Ross CA, Ellason J. "Treatment of dissociative identity disorder": Dr. Ross and Ms. Ellason reply. American Journal of Psychiatry 1998;155(10):1462-3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rudegeair 2013 {published data only}
- Rudegeair T. Review of understanding and treating dissociative identity disorder: a relational approach. Journal of Trauma & Dissociation 2013;14(3):365-6. [DOI: ] [Google Scholar]
Russell 1950 {published data only}
- Russell RJ. Symptomatic treatment of hysteria with intensified electroconvulsant therapy. Lancet 1950;258:135-6. [Google Scholar]
Saxe 2002 {published data only}
- Saxe GN, Chawla N, Kolk B. Self-destructive behavior in people with dissociative disorders. Suicide & Life-threatening Behavior 2002;32(3):313-20. [DOI] [PubMed] [Google Scholar]
Scallet 1976 {published data only}
- Scallet A, Cloninger CR, Othmer E. The management of chronic hysteria: a review and double-blind trial of electrosleep and other relaxation methods. Diseases of the Nervous System 1976;37:347-53. [PubMed] [Google Scholar]
Schade 2011 {published data only}
- Schade N, Torres P, Beyebach M. Cost-efficiency of a brief family intervention for somatoform patients in primary care. Families, Systems & Health 2011;29(3):197-205. [DOI] [PubMed] [Google Scholar]
Schilte 2001 {published data only}
- Schilte AF, Portegijs PJ, Blankenstein AH, Horst HE, Latour MB, Eijk JT, et al. Randomised controlled trial of disclosure of emotionally important events in somatisation in primary care. BMJ 2001;323(7304):86-9. [DOI: 10.1136/bmj.323.7304.86] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schonenberg 2015 {published data only}
- Schonenberg M, Jusyte A, Hohnle N, Mayer SV, Weber Y, Hautzinger M, et al. Theory of mind abilities in patients with psychogenic nonepileptic seizures. Epilepsy & Behavior 2015;53:20-4. [DOI: 10.1016/j.yebeh.2015.09.036] [DOI] [PubMed] [Google Scholar]
Schweden 2016 {published data only}
- Schweden TL, Pittig A, Brauer D, Klumbies E, Kirschbaum C, Hoyer J. Reduction of depersonalization during social stress through cognitive therapy for social anxiety disorder: a randomized controlled trial. Journal of Anxiety Disorders 2016;43:99-105. [DOI] [PubMed] [Google Scholar]
Shapiro 1997 {published data only}
- Shapiro AP, Teasell RW. Strategic-behavioural intervention in the inpatient rehabilitation of non-organic (factitious/conversion) motor disorders. Neurorehabilitation 1997;8:183-92. [DOI] [PubMed] [Google Scholar]
Shapiro 2004a {published data only}
- Shapiro AP, Teasell W. Behavioural interventions in the rehabilitation of acute v chronic non-organic (conversion/ factitious) motor disorders. British Journal of Psychiatry 2004;185:140-6. [DOI] [PubMed] [Google Scholar]
Shapiro 2004b {published data only}
- Shapiro AP, Teasell RW. Behavioural interventions in the rehabilitation of acute v. chronic non-organic (conversion/factitious) motor disorders. British Journal of Psychiatry 2004;185:140-6. [DOI: 10.1192/bjp.185.2.140] [DOI] [PubMed] [Google Scholar]
Sharpe 2011 {published data only}
- Sharpe M, Walker J, Williams C, Stone J, Cavanagh J, Murray G, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011;77(6):564-72. [DOI: 10.1212/WNL.0b013e318228c0c7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokrolahi 2017 {published data only}
- Shokrolahi M, Tavakoli M, Esmaeili M, Barekatain M. The effect of emotion regulation intervention on quality of life, cognitive emotion regulation, and seizures in patients with nonepileptic seizures. Journal of Isfahan Medical School 2017;35(434):677-85. [Google Scholar]
Silberg 1996 {published data only}
- Silberg JL, Waters FS. Factors associated with positive therapeutic outcome. In: The Dissociative Child: Diagnosis, Treatment, and Management. 2nd edition. Derwood (MD): The Sidran Press, 1996:103-12. [Google Scholar]
Speed 1996 {published data only}
- Speed J. Behavioral management of conversion disorder: retrospective study. Archives of Physical Medicine and Rehabilitation 1996;77(2):147-54. [DOI: 10.1016/s0003-9993(96)90159-8] [DOI] [PubMed] [Google Scholar]
Stone 2010 {published data only}
- Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain 2010;133(Pt 5):1537-51. [DOI: 10.1093/brain/awq068] [DOI] [PubMed] [Google Scholar]
Stone 2014a {published data only}
- Stone J. Functional neurological disorders: the neurological assessment as treatment. Neurophysiologie Clinique [Clinical Neurophysiology] 2014;44(4):363-73. [DOI: 10.1016/j.neucli.2014.01.002] [DOI] [PubMed] [Google Scholar]
Stone 2014b {published data only}
- Stone J. Psychotherapy for severe somatoform disorder: problems with missing studies. British Journal of Psychiatry 2014;204(3):243-4. [DOI: 10.1192/bjp.204.3.243] [DOI] [PubMed] [Google Scholar]
Stone 2015 {published data only}
- Stone J, Carson A. Functional neurologic disorders. Continuum 2015;21(3):818-37. [DOI] [PubMed] [Google Scholar]
Stone 2016 {published data only}
- Stone J, Carson A, Hallett M. Explanation as treatment for functional neurologic disorders. Handbook of Clinical Neurology 2016;139:543-53. [DOI: 10.1016/b978-0-12-801772-2.00044-8] [DOI] [PubMed] [Google Scholar]
Suzuki 1979 {published data only}
- Suzuki J, Yamauchi Y, Yamamoto H, Komuro U. Fasting therapy for psychosomatic disorders in Japan. Psychotherapy and Psychosomatics 1979;31(1-4):307-14. [DOI] [PubMed] [Google Scholar]
Sveinsson 2009 {published data only}
- Sveinsson OA, Stefansson SB, Hjaltason H. Conversion disorder – review. Laeknabladid 2009;95(4):269-76. [PubMed] [Google Scholar]
Tazaki 2006 {published data only}
- Tazaki M, Landlaw K. Behavioural mechanisms and cognitive-behavioural interventions of somatoform disorders. International Review of Psychiatry 2006;18(1):67-73. [DOI] [PubMed] [Google Scholar]
Terhune 2017 {published data only}
- Terhune DB, Cleeremans A, Raz A, Lynn SJ. Hypnosis and top-down regulation of consciousness. Neuroscience and Biobehavioral Reviews 2017;81:59-74. [DOI: 10.1016/j.neubiorev.2017.02.002] [DOI] [PubMed] [Google Scholar]
Thenganatt 2015 {published data only}
- Thenganatt MA, Jankovic J. Psychogenic Movement Disorders. Neurologic Clinics 2015;33(1):205. [DOI: 10.1016/j.ncl.2014.09.013] [DOI] [PubMed] [Google Scholar]
Tsui 2017 {published data only}
- Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Current Pain and Headache Reports 2017;21(6):10. [DOI: 10.1007/s11916-017-0627-7] [DOI] [PubMed] [Google Scholar]
Turgay 1990a {published data only}
- Turgay A. Treatment outcome for children and adolescents with conversion disorder. Canadian Journal of Psychiatry 1990;35:585-9. [DOI] [PubMed] [Google Scholar]
Turgay 1990b {published data only}
- Turgay A. Treatment outcome for children and adolescents with conversion disorder. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 1990;35(7):585-9. [DOI] [PubMed] [Google Scholar]
Urbanek 2014 {published data only}
- Urbanek M, Harvey M, McGowan J, Agrawal N. Regulation of emotions in psychogenic nonepileptic seizures. Epilepsy & Behavior 2014;37:110-5. [DOI: 10.1016/j.yebeh.2014.06.004] [DOI] [PubMed] [Google Scholar]
van Bokhoven 2009 {published data only}
- Bokhoven MA, Koch H, Weijden T, Grol RP, Kester AD, Rinkens PE, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints. Annals of Family Medicine 2009;7(2):112-20. [DOI: 10.1370/afm.958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 1998 {published data only}
- Watanabe TK, O'Dell MW, Togliatti TJ. Diagnosis and rehabilitation strategies for patients with hysterical hemiparesis: a report of four cases. Archives of Physical Medicine & Rehabilitation 1998;79:709-14. [DOI] [PubMed] [Google Scholar]
Wetzelaer 2014 {published data only}
- Wetzelaer P, Farrell J, Evers SM, Jacob GA, Lee CW, Brand O, et al. Design of an international multicentre RCT on group schema therapy for borderline personality disorder. BMC Psychiatry 2014;14:319. [DOI: 10.1186/s12888-014-0319-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1988 {published data only}
- White A, Corbin D, Coope B. The use of thiopentone in the treatment of non-organic locomotor disorders. Journal of Psychosomatic Research 1988;32:249-53. [DOI] [PubMed] [Google Scholar]
Williams 1979 {published data only}
- Williams DT, Gold AP, Shrout P, Shaffer D, Adams D. The impact of psychiatric intervention on patients with uncontrolled seizures. Journal of Nervous and Mental Disease 1979;167:626-31. [DOI] [PubMed] [Google Scholar]
Wiseman 2015 {published data only}
- Wiseman H, Reuber M. New insights into psychogenic nonepileptic seizures 2011–2014. Seizure 2015;29:69-80. [DOI] [PubMed] [Google Scholar]
Wolf 2016 {published data only}
- Wolf EJ, Lunney CA, Schnurr PP. The influence of the dissociative subtype of posttraumatic stress disorder on treatment efficacy in female veterans and active duty service members. Journal of Consulting and Clinical Psychology 2016;84(1):95-100. [DOI: 10.1037/ccp0000036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaskin 1936 {published data only}
- Yaskin JC. The psychoneuroses and neuroses. A review of 100 cases with special reference to treatment and end results. American Journal of Psychiatry 1936;93:107-25. [Google Scholar]
Zlotnick 1997 {published data only}
- Zlotnick C, Shea TM, Rosen K, Simpson E, Mulrenin K, Begin A, et al. An affect-management group for women with posttraumatic stress disorder and histories of childhood sexual abuse. Journal of Traumatic Stress 1997;10(3):425-36. [DOI] [PubMed] [Google Scholar]
Zonneveld 2009 {published data only}
- Zonneveld LN, Spijker A, Passchier J, Busschbach JJ, Duivenvoorden HJ. The effectiveness of a training for patients with unexplained physical symptoms: protocol of a cognitive behavioral group training and randomized controlled trial. BMC Public Health 2009;9:251. [DOI: 10.1186/1471-2458-9-251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zonneveld 2012 {published data only}
- Zonneveld LN, Rood YR, Kooiman CG, Timman RT, Spijker A, Busschbach JJ. Predicting the outcome of a cognitive-behavioral group training for patients with unexplained physical symptoms: a one-year follow-up study. BMC Public Health 2012;12:848. [DOI: 10.1186/1471-2458-12-848] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
DRKS00012997 {published data only}
- DRKS00012997. The role of the temporo-parietal junction in functional neurological disorders. a study with mindfulness-based stress reduction therapy. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00012997 (first received 8 September 2017).
DRKS00014251 {published data only}
- DRKS00014251. Evaluation of the effect of a psychotherapy program with body movement focus for patients with dissociative seizures. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00014251 (first received 6 April 2018).
Goldstein 2015 {published data only}
- Goldstein LH, Mellers JDC, Landau S, Stone J, Carson A, Medford N, et al. Cognitive behavioural therapy vs standardised medical care for adults with dissociative non-epileptic seizures (CODES): a multicentre randomised controlled trial protocol. BMC Neurology 2015;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 2016 {published data only}
- Goldstein LH, Chalder T, Carson AJ, Landau S, McCrone P, Medford N, et al. Cognitive behavioural therapy vs. standardised medical care for adults with dissociative non-epileptic seizures (codes): progress in a randomised controlled trial. Epilepsia 2016;57(Suppl 2):28-9. [DOI: [DOI: 10.1111/epi.13609]] [Google Scholar]
Goldstein 2017 {published data only}
- Goldstein LH, Chalder T, Carson AJ, Landau S, McCrone P, Medford N, Murray J, et al. Cognitive behavioural therapy vs standardised medical care for adults with dissociative non-epileptic seizures (codes): update on a pragmatic randomised controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 2017;88(8):e26. [DOI: 10.1136/jnnp-2017-BNPA.56] [DOI] [Google Scholar]
ISRCTN05681227 {published data only}
- ISRCTN05681227. Cognitive behavioural therapy vs standardised medical care for dissociative non-epileptic seizures. www.isrctn.com/ISRCTN05681227 (first received 2 March 2014).
NCT01590992 {published data only}
- NCT01590992. Treatment of globus sensations with psychotherapy. clinicaltrials.gov/ct2/show/NCT01590992 (first received 3 May 2012).
NCT02325544 {published data only}
- NCT02325544. Comparing different treatments in reducing dissociative seizure occurrence (CODES). clinicaltrials.gov/ct2/show/NCT02325544 (first received 25 December 2014).
NCT02450617 {published data only}
- NCT02450617. Stabilizing group treatment of complex trauma: a randomized controlled trial (STAB). clinicaltrials.gov/ct2/show/NCT02450617 (first received 21 May 2015).
NCT02764476 {published data only}
- NCT02764476. Embodied virtual reality therapy for functional neurological symptom/conversion disorder. clinicaltrials.gov/ct2/show/NCT02764476 (first received 6 May 2016).
NCT02801136 {published data only}
- NCT02801136. Treatment outcomes of CBT for PNES. clinicaltrials.gov/ct2/show/NCT02801136 (first received 15 June 2016).
Robinson 2017 {published data only}
- Robinson EJ, Goldstein LH, McCrone P, Perdue I, Chalder T, Mellers JD, et al. Cognitive behavioural therapy versus standardised medical care for adults with dissociative non-epileptic seizures (CODES): statistical and economic analysis plan for a randomised controlled trial. Trials 2017;18(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Akagi 2002
- Akagi H, House AO. The epidemiology of hysteria: vanishingly rare or just vanishing? Psychological Medicine 2002;32:191-4. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2013a
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719-25. [DOI: 10.1016/j.jclinepi.2012.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Andrews 2013b
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation – determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726-35. [DOI: 10.1016/j.jclinepi.2013.02.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
APA 2013
- American Psychiatric Association. DSM 5. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Aybek 2014
- Aybek S, Nicholson TR, Zelaya F, O'Daly OG, Craig TJ, David AS, et al. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 2014;71(1):52-60. [DOI] [PubMed] [Google Scholar]
Aybek 2018
- Aybek S [pers comm]. Question about research trial – results. Email to: Ganslev CA 14 August 2018.
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI: 10.1016/j.jclinepi.2010.07.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barach 1991
- Barach PM. Multiple personality disorder as an attachment disorder. Dissociation: Progress in the Dissociative Disorders 1991;4(3):117-23. [Google Scholar]
Barlow 2010
- Barlow DH. Negative effects from psychological treatments: a perspective. American Psychologist 2010;65(1):13-20. [DOI] [PubMed] [Google Scholar]
Bateman 2013
- Bateman A, Fonagy P. Mentalization-based treatment. Psychoanalytic Inquiry 2013;33(6):595-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893-7. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. Journal of Personality Assessment 1996;67(3):588-97. [DOI] [PubMed] [Google Scholar]
Beecham 2019
- Beecham J, Knapp M. Costing psychiatric interventions. In: Measuring Mental Health Needs. Bideford (UK): Gaskell, 2001:200-24. [Google Scholar]
Bradfield 2011
- Bradfield B. The dissociation of lived experience: a relational psychoanalytic analysis of the intergenerational transmission of trauma. International Journal of Psychoanalytic Self Psychology 2011;6:531-50.
Bromberg 2009
- Bromberg PM. Multiple self-states, the relational mind and dissociation: a psychoanalytic perspective. In: Dissociation and the Dissociative Disorders: DSM-V and Beyond. Abingdon (UK): Routledge/Taylor & Francis Group, 2009:637-52. [Google Scholar]
Brown 2013
- Brown RJ. Dissociation and somatoform disorders. In: Kennedy F, Kennerley H, Pearson D, editors(s). Cognitive Behavioural Approaches to the Understanding and Treatment of Dissociation. New York (NY): Routledge, 2013. [Google Scholar]
Brunetti 2013
- Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology 2013;66(2):140-50. [DOI: 10.1016/j.jclinepi.2012.04.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Charcot 1887
- Charcot JM, Babiński J, Bernard J. Oeuvres Complètes de J. M. Charcot: Leçons sur les Maladies du Système Nerveux. Paris: Bureaux du Progrès Médical, 1887. [Google Scholar]
Chen 2018
- Chen DK [pers comm]. Question about data for Cochrane article. Email to: Ganslev CA 17 October 2018.
Chu 2011
- Chu JA, Dell PF, Van der Hart O, Cardeña E, Barach PM, Somer E, et al. International society for the study of trauma and dissociation. Guidelines for treating dissociative identity disorder in adults, third revision. Journal of Trauma and Dissociation 2011;12:115-87. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Publishers, 1988. [Google Scholar]
Colom 2011
- Colom F. Keeping therapies simple: psychoeducation in the prevention of relapse in affective disorders. British Journal of Psychiatry 2011;198(5):338-40. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cramer 1996
- Cramer JA, Perrine K, Devinsky O, Meador K. A brief questionnaire to screen for quality of life in epilepsy: the QOLIE-10. Epilepsia 1996;37(6):577-82. [DOI] [PubMed] [Google Scholar]
Cramer 1998
- Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 1998;39(1):81-8. [DOI] [PubMed] [Google Scholar]
Crawford 2016
- Crawford MJ, Thana L, Farquharson L, Palmer L, Hancock E, Bassett P, et al. Patient experience of negative effects of psychological treatment: results of a national survey dagger. British Journal of Psychiatry 2016;208(3):260-5. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III: the issue of carry-over. Statistics in Medicine 2002;21(15):2161-73. [DOI] [PubMed] [Google Scholar]
Department of Health 2001
- Department of Health. Treatment Choice in Psychological Therapies and Counselling. Evidence based clinical practice guidelines. London (UK): Department of Health, 2001. [Google Scholar]
Derogatis 1977
- Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the SCL-90: a study in construct validation. Journal of Clinical Psychology 1977;33(4):981-9. [Google Scholar]
Deveci 2007
- Deveci A, Taskin O, Dinc G, Yilmaz H, Demet MM, Erbay-Dundar P, et al. Prevalence of pseudoneurologic conversion disorder in an urban community in Manisa, Turkey. Social Psychiatry and Psychiatric Epidemiology 2007;42(11):857-64. [DOI] [PubMed] [Google Scholar]
Dorahy 2014
- Dorahy MJ, Brand BL, Sar V, Kruger C, Stavropoulos P, Martinez-Taboas A, et al. Dissociative identity disorder: an empirical overview. Australian and New Zealand Journal of Psychiatry 2014;48(5):402-17. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehlers 2000
- Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy 2000;38(4):319-45. [DOI] [PubMed] [Google Scholar]
Fine 2001
- Fine CG, Berkowitz AS. The wreathing protocol: the imbrication of hypnosis and EMDR in the treatment of dissociative identity disorder and other dissociative responses. Eye Movement Desensitization Reprocessing. American Journal of Clinical Hypnotherapy 2001;43(3-4):275-90. [DOI] [PubMed] [Google Scholar]
Fine 2012
- Fine CG. Cognitive behavioral hypnotherapy for dissociative disorders. American Journal of Clinical Hypnotherapy 2012;54(4):331-52. [DOI] [PubMed] [Google Scholar]
FitzGerald 2015
- FitzGerald TL, Southby AK, Haines TP, Hough JP, Skinner EH. Is physiotherapy effective in the management of child and adolescent conversion disorder? A systematic review. Journal of Paediatrics and Child Health 2015;51(2):159-67. [DOI] [PubMed] [Google Scholar]
Fobian 2018
- Fobian A [pers comm]. Question about research trial. Email to: Ganslev CA 16 August 2018.
Freud 1896
- Freud S. The aetiology of hysteria, Standard Edition Vol. 3. Freud, S. (1920), Beyond the pleasure principle, Standard Edition 1896;18:7-64. [Google Scholar]
Gold 2009
- Gold SN, Seibel SL. Treating dissociation: a contextual approach. In: Dissociation and the Dissociative Disorders: DSM-V and Beyond. Abingdon (UK): Routledge/Taylor & Francis Group, 2009:625-636. [Google Scholar]
Gold 2013
- Gold S. Not Trauma Alone: Therapy for Child Abuse Survivors in Family and Social Context. Abingdon (UK): Routledge, 2013. [Google Scholar]
Goldstein 2018
- Goldstein [pers comm]. Question about research on dissociative seizures. Email to: Ganslev CA 8 August 2018.
Guy 1976
- Guy W, National Institute of Mental Health (US), Psychopharmacology Research Branch, Early Clinical Drug Evaluation Program. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380-2. [DOI: 10.1016/j.jclinepi.2010.09.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton MC. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50-5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hinson 2005
- Hinson VK, Cubo E, Comella CL, Goetz CG, Leurgans S. Rating scale for psychogenic movement disorders: scale development and clinimetric testing. Movement Disorders 2005;20(12):1592-7. [DOI] [PubMed] [Google Scholar]
Horvath 1991
- Horvath AO, Symonds DB. Relation between working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology 1991;38(2):139-49. [Google Scholar]
Howell 2005
- Howell EF. The Dissociative Mind. London (UK): The Analytic Press, 2005. [Google Scholar]
Howell 2011
- Howell E. Understanding and Treating Dissociative Identity Disorder. New York (NK): Routledge, 2011. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janet 1907
- Janet P. The Major Symptoms of Hysteria. New York (NY): Macmillan. Reprint of 1920 edition (1965), 1907. [Google Scholar]
Jenkinson 1996
- Jenkinson C, Layte R, Wright L, Coulter A. The UK SF-36: an Analysis and Interpretation Manual. Oxford (UK): Health Services Research Unit, 1996. [Google Scholar]
Johnson 2006
- Johnson JG, Cohen P, Kasen S, Brook JS. Dissociative disorders among adults in the community, impaired functioning, and axis I and II comorbidity. Journal of Psychiatric Research 2006;40(2):131-40. [DOI] [PubMed] [Google Scholar]
Jones 1995
- Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale: reliability and validity of the Global Assessment of Functioning (GAF). British Journal of Psychiatry 1995;166(5):654-9. [DOI] [PubMed] [Google Scholar]
Kalogjera‐Sackellares 2004
- Kalogjera-Sackellares D. Psychodynamics and Psychotherapy of Pseudoseizures. Norwalk (CT): Crown House Publishing Limited, 2004. [Google Scholar]
Kaplan 2014
- Kaplan MJ. A psychodynamic perspective on treatment of patients with conversion and other somatoform disorders. Psychodynamic Psychiatry 2014;42(4):593-615. [DOI] [PubMed] [Google Scholar]
Kennedy 2013
- Kennedy F, Kennerley H, Pearson D, editor(s). Cognitive Behavioural Approaches to the Understanding and Treatment of Dissociation. New York (NY): Routledge, 2013. [Google Scholar]
Keus 2010
- Keus F, Wetterslev J, Gluud C, Laarhoven CJ. Evidence at a glance: error matrix approach for overviewing available evidence. BMC Medical Research Methodology 2010;10:90. [DOI: 10.1186/1471-2288-10-90] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocsis 2010
- Kocsis JH, Gerber AJ, Milrod B, Roose SP, Barber J, Thase ME, et al. A new scale for assessing the quality of randomized clinical trials of psychotherapy. Comprehensive Psychiatry 2010;51(3):319-24. [DOI] [PubMed] [Google Scholar]
Kranick 2011
- Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Movement Disorders 2011;26(10):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liotti 2006
Luyten 2013
- Luyten P, Van Houdenhove B, Lemma A, Target M, Fonagy P. Vulnerability for functional somatic disorders: a contemporary psychodynamic approach. Journal of Psychotherapy Integration 2013;23(3):250. [Google Scholar]
Martin 2000
- Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. Journal of Consult Clinical Psychology 2000;68(3):438-50. [PubMed] [Google Scholar]
Martlew 2007
- Martlew J, Baker GA, Goodfellow L, Bodde N, Aldenkamp A. Behavioural treatments for non-epileptic attack disorder. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006370] [DOI] [PubMed] [Google Scholar]
Matthews 1997
- Matthews JA, Chu JA. Psychodynamic therapy for patients with early childhood trauma. In: Trauma and Memory: Clinical and Legal Controversies. New York (NY): Oxford University Press, 1997:316-343. [Google Scholar]
McDowell 1996
- McDowell I, Newell C. Measuring Health, a Guide to Rating Scales and Questionnaires. New York (NY): Oxford University Press, 1996. [Google Scholar]
Meinlschmidt 2018
- Meinlschmidt [pers comm]. Question about research trial. Email to: CA Ganslev 4 September 2018.
Modum Bad 2018
- Bækkelund [pers comm]. Spørgsmål om forskning – stabilizing group treatment of complex trauma. Email to: Ganslev CA 5 September 2018.
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987-91. [DOI] [PubMed] [Google Scholar]
Mundt 2002
- Mundt JC. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. British Journal of Psychiatry 2002;180(5):461-4. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42. [DOI: 10.1016/j.jclinepi.2013.02.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Myers 2017
- Myers L, Vaidya-Mathur U, Lancman M. Prolonged exposure therapy for the treatment of patients diagnosed with psychogenic non-epileptic seizures (PNES) and post-traumatic stress disorder (PTSD). Epilepsy Behaviour 2017;66:86-92. [DOI] [PubMed] [Google Scholar]
Nijenhuis 1996
- Nijenhuis ER, Spinhoven P, Dyck R, Hart Van O, Vanderlinden J. The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ-20). Journal of Nervous and Mental Disease 1996;184(11):688-94. [DOI] [PubMed] [Google Scholar]
Nijenhuis 2009
- Nijenhuis Ellert RS, den Boer Johan A. Psychobiology of Traumatization and Trauma-related Structural Dissociation of the Personality. I: Dell PF, O'Neil JA [red]. Dissociation and the Dissociative Disorders: DSM-V and Beyond. New York (NY): Routledge/Taylor & Francis Group, 2009. [Google Scholar]
Nijenhuis 2010
- Nijenhuis E, Hart O, Steele K. Trauma-related structural dissociation of the personality. Activitas Nervosa Superior 2010;52(1):2010. [Google Scholar]
Perez 2015
- Perez DL, Dworetzky BA, Dickerson BC, Leung L, Cohn R, Baslet G, et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clinical EEG and Neuroscience 2015;46(1):4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez 2017
- Perez DL, Matin N, Barsky A, Costumero-Ramos V, Makaretz SJ, Young SS, et al. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. Journal of Neurology and Neurosurgical Psychiatry 2017;88(6):491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Center, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014.
Roelofs 2002
- Roelofs K, Keijsers GP, Hoogduin KA, Näring GW, Moene FC. Childhood abuse in patients with conversion disorder. American Journal of Psychiatry 2002;159(11):1908-13. [DOI] [PubMed] [Google Scholar]
Ron 2001
- Ron M. The prognosis of hysteria/somatization disorder. In: Contemporary Approaches to the Study of Hysteria. Oxford (UK): Oxford University Press, 2001. [Google Scholar]
Ross 1991
- Ross CA. Epidemiology of multiple personality disorder and dissociation. Psychiatric Clinics of North America 1991;14:503-17. [PubMed] [Google Scholar]
Ruddy 2005
- Ruddy R, House A. Psychosocial interventions for conversion disorder. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005331] [DOI] [PubMed] [Google Scholar]
Sar 2009
- Sar V, Islam S, Ozturk E. Childhood emotional abuse and dissociation in patients with conversion symptoms. Psychiatry and Clinical Neurosciences 2009;63(5):670-7. [DOI] [PubMed] [Google Scholar]
Scevola 2013
- Scevola L, Teitelbaum J, Oddo S, Centurion E, Loidl CF, Kochen S, et al. Psychiatric disorders in patients with psychogenic nonepileptic seizures and drug-resistant epilepsy: a study of an Argentine population. Epilepsy and Behavior 2013;29(1):155-60. [DOI] [PubMed] [Google Scholar]
Selkirk 2008
- Selkirk M, Duncan R, Oto M, Pelosi A. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia 2008;49(8):1446-50. [DOI] [PubMed] [Google Scholar]
Steele 2009
- Steele K, Hart O, Nijenhuis E. The theory of trauma-related structural dissociation of the personality. In: Dissociation and the Dissociative Disorders: DSM-V and Beyond. Abingdon (UK): Routledge/Taylor & Francis Group, 2009:239-58. [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2004
- Stone J, Sharpe M, Binzer M. Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics 2004;45(6):492-9. [DOI] [PubMed] [Google Scholar]
Stone 2005
- Stone J, Smyth R, Carson A, Lewis S, Prescott R, Warlow C, et al. Systematic review of misdiagnosis of conversion symptoms and "hysteria". BMJ 2005;331(7523):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2009
- Stone J, Carson A, Duncan R, Coleman R, Roberts R, Warlow C, et al. Symptoms 'unexplained by organic disease' in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain 2009;132(Pt 10):2878-88. [DOI] [PubMed] [Google Scholar]
Thalheimer 2002
- Thalheimer W, Cook S. How to calculate effect sizes from published research articles: a simplified methodology. www.worklearning.com/2016/08/11/using-effect-sizes-and-considering-meta-analyses/ (accessed 16 July 2020).
Thompson 2018
- Thompson N [pers comm]. Question about research data on PNES study. Email to: Ganslev CA 19 October 2018.
Toone 1990
- Toone BK. Disorders of hysterical conversion. In: Bass C, editors(s). Somatization: Physical Symptoms and Psychological Illness. Oxford (UK): Blackwell Scientific Publications, 1990. [Google Scholar]
van der Hart 2012
- Hart O. The use of imagery in phase 1 treatment of clients with complex dissociative disorders. European Journal of Psychotraumatology 2012;3:ArtID 8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 2018
- Vermeulen M [pers comm]. Question about research data for Cochrane review. Email to: Ganslev CA 25 October 2018.
Wells 2004
- Wells A, Sembi S. Metacognitive therapy for PTSD: a core treatment manual. Cognitive and Behavioral Practice 2004;11(4):365-77. [Google Scholar]
WHO 1993
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization, 1993. [Google Scholar]
Williams 2019
- Williams B, Ospina JP, Jalilianhasanpour R, Fricchione GL, Perez DL. Fearful attachment linked to childhood abuse, alexithymia, and depression in motor functional neurological disorders. Journal of Neuropsychiatry and Clinical Neurosciences 2019;31(1):65-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2015
- Zhao S, Sampson S, Xia J, Jayaram MB. Psychoeducation (brief) for people with serious mental illness. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD010823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]


