Abstract
Fine particulate matter, a major air pollutant across the world, causes a series of pulmonary diseases. Vitamin D is a typical vitamin with emerging roles in inflammation and fibrosis. Different situations and diseases need different doses and modes of vitamin D administration, which challenges the existing vitamin D supplementary rules. Thus, studies of vitamin D applications and their mechanisms in various diseases are important for its future therapeutic applications. In this study, the therapeutic application of vitamin D3 in chronic particle-exposure-associated lung fibrosis and tissue remodeling was investigated. In vivo studies showed that vitamin D3 significantly attenuated fibrosis effects by decreasing α-smooth muscle actin-regulated extracellular matrix deposition and restoring expressions of E-cadherin and N-cadherin. With the importance of activated macrophage in the regulation of local epithelium and fibroblast in the process of tissue fibrosis, two separate in vitro systems of co-culture of macrophages with lung epithelium or fibroblast were built. The results confirmed that vitamin D3 promoted the proliferation of lung epithelium and depressed the fibrosis effects of fibroblasts as well. In addition, our results indicated that the therapeutic effects of vitamin D3 were through Nrf2 signals. Our work provides convincing experimental evidence for vitamin D therapeutic application to promote tissue repair and improve particle-associated lung fibrosis.
Keywords: Nrf2 signals, fine particulate matter, vitamin D, α-SMA, lung fibrosis
Introduction
Fine particulate matter is one of the major air pollutants worldwide, which has an aerodynamic diameter less than 2.5 μm (PM2.5) [1–4]. Epidemiological studies reported that chronic PM2.5 exposure is associated with pulmonary function reduction, chronic respiratory disease exacerbation, and cancer’s incidence and mortality [5–7]. Experimental studies indicated that PM2.5 particle-instillation causes tissue oxidative stress, vascular hyper-permeability, and alveolar epithelial dysfunction, a comprehensive syndrome along with progressive inflammatory and fibrotic responses [8, 9]. Current therapies mainly focus on symptomatic treatment of inflammatory response, without effective blocking progressive lung injury and fibrosis [10, 11]. Thus, development of novel therapeutic strategies for prevention or reversal of respiratory system injury become a priority.
SiO2 containing fine particulate matter is a major one that caused lung injury [12]. By phagocytosing the inhaled particles, macrophages secreted a series of pro-inflammatory cytokines, which stimulate the neighboring epithelial cells, macrophages, and even myofibroblasts. The inhaled particles induced oxidative stress and caused the lung inflammation [13]. Then the dysregulated tissue repair (activation of myofibroblasts, proliferation of fibroblast, and accumulation of ECM) causes the lung fibrosis [13, 14]. Vitamin D is a highly versatile molecule with emerging roles in inflammation and fibrosis-related diseases [15]. Recently, it has been unveiled that vitamin D deficiency is associated with the prevalence and risks of several chronic diseases and toxin-induced tissue inflammation and fibrosis [16–18]. Vitamin D3 is a major form of vitamin D, which can be activated in the kidney to its active form (1,25(OH)2D3), also known as calcitriol [19]. Nrf2 is a potential therapeutic protein in the process of several diseases by regulating a series of genes with the effects of anti-oxidation and anti-inflammation by binding with antioxidant response elements in their promoters to inhibit free radicals and remove environmental toxins [20]. TGF (Transforming growth factor) β1 is the major isoform of TGFβ superfamily in respiratory diseases, which served as the pro-fibrotic switch to activate myofibroblasts and inhibit lung epithelial cells’ proliferation [21, 22]. It also regulates the expression of α-SMA and its targets, such as components of the extracellular matrix and EMT-related transcription factors [23].
Our previous study has reported that vitamin D3 opposed particle-induced lung oxidative stress, inflammation, and cell apoptosis through Nrf2 signals [24]. However, long-term chronic inflammation leads to lung fibrotic tissue repair, which are responsible for lung destruction and abnormal physiology. Therefore, the purpose of this study was to clarify the potential mechanisms of vitamin D3 in fine particle-associated lung fibrosis, which provides convincing experimental evidence of vitamin D3’s therapeutic application on pulmonary injury.
Materials and Methods
Chemicals, antibodies, and cell culture
Vitamin D3 was purchased from Shanghai General Pharmaceutical Company (Shanghai, China). Calcitriol (1,25-dihydroxy vitamin D3, D1530) was observed from Sigma-Aldrich (St. Louis, MO). Primary antibodies against Nrf2 (sc-13032), NQO1 (sc-32793), Ki67 (sc-23900), collagen IV (sc-398655), fibronectin (FN) (sc-18827), α-SMA (sc-53142), cleave-caspase 3 (CC3: sc-7272), E-cadherin (E-cad: sc-8426), N-cadherin (N-cad: sc-59987), and GAPDH (sc-32233) were purchased from Santa Cruz. 8-oxo-dG (134-MC-050) antibody was purchased from Trevigen (Gaithersburg, MD). VDR (12550S) antibody was observed from Cell signaling Technology (Danvers, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Immunoway (Plano, TX: RS0001 (Mouse), RS0002 (Rabbit)). Human acute monocytic leukemia cell line (THP-1) and human bronchial epithelial cell line (BEAS-2B) were observed from ATCC (Manassas, VA). Human primary fibroblast cells (HFLIII) were purchased from JCRB (National Institute for Biomedical Innovation, Japan). THP-1 cells were cultured in RPMI1640 medium containing 10% FBS (Hyclone, Logan, UT), 0.1% gentamycin (Invitrogen, Carlsbad, CA), and 5 ng/ml phorbol-12-myristate-13-acetate (PMA) (Sigma) were treated for cell differentiation. BEAS-2B cells were cultured in BEBM with additives from Lonza/Clonetics Corporation (CC-3170). HFLIII cells were cultured in F12 medium supplemented with 15% FBS, 0.1% gentamycin. The cells were kept in a humidified incubator at 37 °C with 5% CO2.
Small interfering RNA transfections
Cells were transfected with either a non-target small interfering RNA (siRNA) (Ctrl siRNA, 1027281; Qiagen, Valencia, CA), an Nrf2-specific siRNA (Nrf2 siRNA, SI00657937), or a VDR-specific siRNA (VDR siRNA, SI03649163) with HiperFect Transfection Reagent (Qiagen, 301702) based on a previous study [24]. Briefly, differentiated THP-1 cells and BEAS-2B or HFLIII cells were plated in the six wells-transwell plate (THP-1 on the upper chamber, BEAS-2B or HFLIII on the bottom). Followed by the transfection of Ctrl siRNA, Nrf2 siRNA, or VDR siRNA, BEAS-2B or HFLIII cells were passaged on the bottom of the plates and the THP-1 cells on the upper chamber were exposed with the indicated treatments for another 24 h; the cells were then harvested for the following assays.
Cell viability assay
The cell viability was detected by functional impairment of the mitochondria using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, M5655) based on the protocol used in the previous study. MTT 40 μg was used for each well. Absorbance at 570 nm was captured by a Synergy 2 Multi-Mode Microplate Reader (Biotek, Seattle, USA).
Immunoblot analysis
After lysed with the Laemmli buffer (62.5 mM Tris-Hcl (pH = 6.9), 3% SDS, 10% glycerol, 5% Beta-mercaptoethanol, and 0.1% bromophenol blue), cells were sonicated on ice and boiled. Then, lysates were electrophoresed in the SDS-polyacrylamide gel, and the expression of indicated protein was detected by immunoblot analysis. The bands were quantified by the Syngene gel document system along with GeneTools software from Syngene (Frederick, MD).
Indirect immunofluorescence staining
Cells were passaged on glass-coverslips (Fisher Scientific). Followed by fixation with pre-chilled methanol, coverslips were incubated with primary and secondary antibody. After mounted onto glass slides with anti-fade mounting medium (Invitrogen), coverslips were detected with LEICA DM 2500 fluorescence microscope and quantified by GeneTools software from Syngene (Frederick, MD).
In vitro scratch assay
The scratch assay was performed as described in our previous study [13]. Briefly, UV-sterilized polydimethylsiloxane (PDMS) blocks (1 mm × 2 cm) were located transversally on the bottom of the plates. Cells were passaged in the plates. After the indicated treatments accordingly, the slab was removed to allow the cell migration. The images were captured with the microscope and quantified by GeneTools software from Syngene (Frederick, MD).
Animals and treatments
Seven-weeks-old male C57BL/6 mice were purchased from SLAC laboratory Animal Co. Ltd. (Shanghai, China). With standard food and water consumption ad libitum, mice were raised in 12 h/12 h light/dark cycle, climate-controlled pathogen-free rooms. Mice handling in our study was according to the Guide of Care and Use of Laboratory Animals, and protocols were approved by Soochow University Institutional Animal Care and Use Committee. After adaptively fed for 1 week, mice were randomly separated into four groups (10 mice/group): ctrl (corn oil); particles; vitamin D3 (40 000 U/kg, dissolved in corn oil); combination (Vitamin D3 + Particles). Particles (3 mg/50 μl sterile saline) were intratracheally instilled. Vitamin D3 was administrated once a week (1 week before particles instillation) until the end of the experiment. The body weight was recorded every week and was sacrificed at day 56 after particle instillation.
Particle preparation
The particles of quartz DQ 12 were observed from Doerentrup Quarz GmbH (Germany). The content of the free dust was SiO2 (more than 99%). 90% particles were less than 2.3 μm, 50% less than 1.1 μm, and 10% less than 0.6 μm. The particles were grinded in saline for 3 h and boiled in 1 N HCl. With resuspension in sterile saline, particles were subjected to sonication for 10 min before use.
Hematoxylin and eosin and immunohistochemical staining
Lung tissues were paraffin embedding and cut (4 μm). Tissue sections were deparaffinized for hematoxylin and eosin (H&E) or immunohistochemical (IHC) staining. Pathology analysis was detected by H&E staining. And IHC staining was performed using the EnVision+System-HRP kit (Dako) based on the manufacturer’s instructions. The slides were boiled with citric acid monohydrate (2.1 g/l in H2O, pH = 6.0) for antigen retrieval. After quench endogenous peroxidase activity with 0.3% peroxidase and blocked with normal goat serum, tissue sections were incubated with various primary antibodies. For 8-oxo-dG staining, the sections were incubated with proteinase K (800 U/ml, in PBS) and RNase A (10 mg/ml in buffer containing 150 mM NaCl and 15 mM sodium citrate). Followed by denatured DNA with 2 N HCl for 5 min, the sections were incubated with 8-oxo-dG antibody dissolved with 5% normal goat serum for 2 h.
Statistical analysis
Three independent experiments were performed in triplicate, and data are shown as mean ± SD. Statistical analysis was performed with SPSS 19.0 (SPSS Inc., Chicago, IL). Unpaired Student’s t test was employed to compare the difference of two groups. One-way ANOVA with Bonferroni’s correction was used to compare the means of more than three groups. And P < 0.05 was considered to be significant.
Results
Vitamin D3 activates Nrf2 signals and promotes particle exposure-caused lung tissue damage
Lung tissues from the mice exposed with particles have increased oxidative stress. Nrf2+/+ (n = 10) and Nrf2−/− (n = 10) C57BL/6 mice were exposed with particles (Supplementary Fig. S1A). The levels of 8-oxo-dG were increased in the mice with particles exposure. As expected, higher 8-oxo-dG and more inflammation were observed in the lung tissues of Nrf2−/− mice with particle exposure. The results indicate that Nrf2−/− mice suffered a stronger lung damage due to lack of Nrf2-mediated compensatory protection. Therefore, it was proposed that therapeutic induction of Nrf2 protects against particle-caused lung damage. Vitamin D is an essential natural vitamin in the human daily life and considered a highly multifunctional molecule with roles in toxin-induced inflammation and fibrosis, immunity, infectious diseases, and even cancer [25]. In addition, our previous study has demonstrated that the active form of vitamin D3 is an Nrf2 inducer [24]. To investigate the protective effects and detailed mechanisms of vitamin D3 in the process of lung fibrosis associated with long-term particles exposure, mice were intratracheally instilled with particles on day 0. Vitamin D3 or control vehicle was injected 1 week before particle instillation and once a week until the end of the experiment. No significant differences in the body weight and lung weight among each group were observed (Fig. 1B and Supplementary Fig. S1A). Since vitamin D is a typical vitamin, which plays a critical role in calcium and phosphorus homeostasis, the level of serum calcium was determined. The results showed that vitamin D3 supplementation did not change the level of serum calcium in the mice (Supplementary Fig. S1C). Furthermore, induction of Nrf2 signals and VDR (vitamin D receptor) in the lung tissues was confirmed according to our previous study (Fig. 1C).
Figure 1.
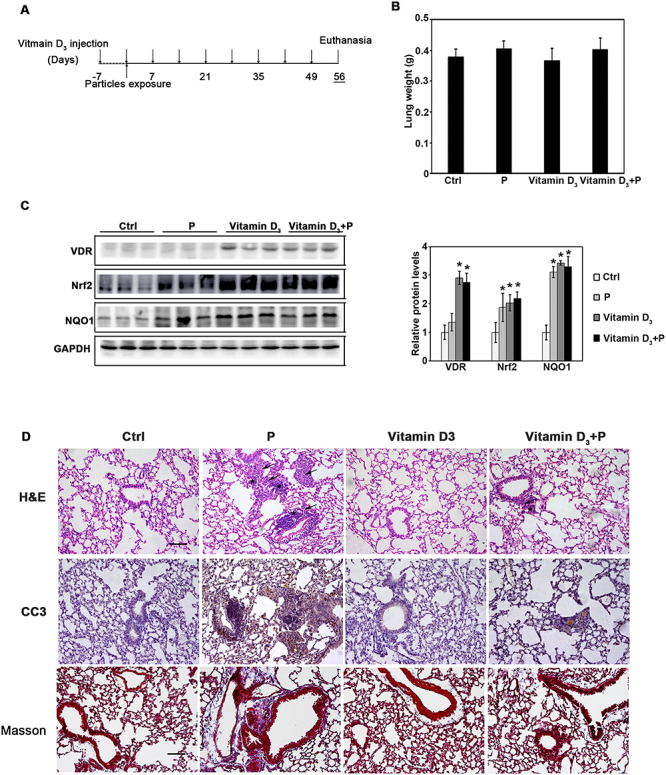
Vitamin D3 activates Nrf2 signals and promotes the particle exposure-caused lung tissue damage. (A) The animal study was performed as follows: 8-week old C57BL/6 mice were randomly allocated into four experimental groups (10 mice/group): Ctrl (control), P (particles), vitamin D3 (40 000U/kg/week), and vitamin D3 + P (vitamin D3 + particles). Each group of mice received i.p. injection of carrier control (corn oil) or vitamin D3 1 week before the particle instillation and administrated every week until the end. Particles were intratracheally instilled at day 0. The mice were harvested at day 56. (B) Lung weight of mice from the indicated groups. (C) Lung tissue lysates from each group were subjected to immunoblot analysis. The intensity of the indicated antibody’s bands from three independent experiments were quantified (*P < 0.05, Ctrl vs. treatment groups). (D) H&E, IHC staining of CC3, and Masson staining of lung tissue section from the indicated groups were performed (n = 10, and a representative image from each group is shown (scale bar = 100 μm, the black arrows indicate the infiltration of inflammatory cells; asterisks indicate the cell nodules).
Next, the prevention of vitamin D3 on lung injury and fibrotic effects were investigated. Increased inflammatory cells’ infiltration and thickened alveolar septal were observed with particle exposure by H&E staining. Vitamin D3 alone did not affect the lung morphology. However, it dramatically attenuated the pulmonary pathological changes caused by particle instillation (Fig. 1D). Measurement of apoptotic cell death with CC3 expression showed that particle exposure dramatically upregulated the cell apoptosis, which was suppressed by vitamin D3. Besides that, Masson staining also showed that the induced fibrotic effects were attenuated by vitamin D3 administration. Taken together, these results demonstrated that vitamin D3 protected against particle-induced lung injury by decreasing inflammation and cell apoptosis, as well as fibrosis effects.
Vitamin D3 modulates the expression of α-SMA and FN caused by particle instillation
Given that vitamin D3 diminished lung fibrosis associated with particles instillation, the molecular mechanisms were explored. α-SMA (α-smooth muscle actin) is a protein marker of myofibroblast transdifferentiation [26], and FN is a critical ECM deposition-related protein. Particle instillation significantly upregulated protein levels of α-SMA and FN, which were significantly abrogated by vitamin D3 administration (Fig. 2A and B). Taken together, these results indicated that vitamin D3 treatment inhibits the lung tissue fibrosis by inhibiting the levels of α-SMA and FN.
Figure 2.
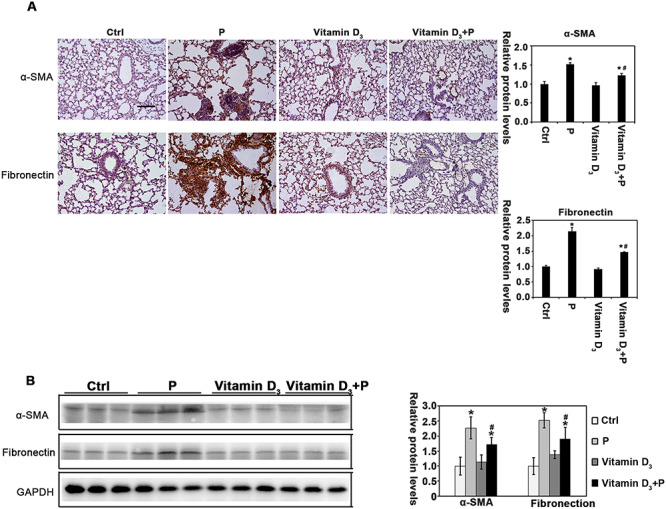
Vitamin D3 modulates the expression of α-SMA and FN caused by particle instillation. (A) IHC staining of α-SMA, and FN were performed [n = 10, and a representative image of lung tissue from each group is shown (scale bar: 100 μm)]. The quantification of the IHC images is shown. Results are expressed as mean ± SD (*P < 0.05, Ctrl vs. treatment groups; #P < 0.05, P vs. vitamin D3 + P). (B) Lung tissue lysates from each group were subjected to immunoblot analysis with α-SMA and FN antibodies. The intensity of the indicated antibody’s bands from three independent experiments was quantified.
Vitamin D3 restores the expression of E-cadherin and N-cadherin in the particle-exposure lung tissue
The above data suggest that vitamin D3 opposed the fibrosis effects associated with particle instillation by inhibition of α-SMA and FN expression. In addition to its effects on myofibroblasts transdifferentiation and ECM deposition, another process termed epithelial-mesenchymal transformation (EMT) has got public attention recently due to its potential role in lung fibrosis. E-cadherin and N-cadherin are the important proteins related to EMT. With particle instillation, the expression of E-cadherin was increased and the N-cadherin was decreased accordingly, which was restored by vitamin D3 administration (Fig. 3). These results indicated that vitamin D3 attenuated the EMT-like transform of epithelial cells.
Figure 3.
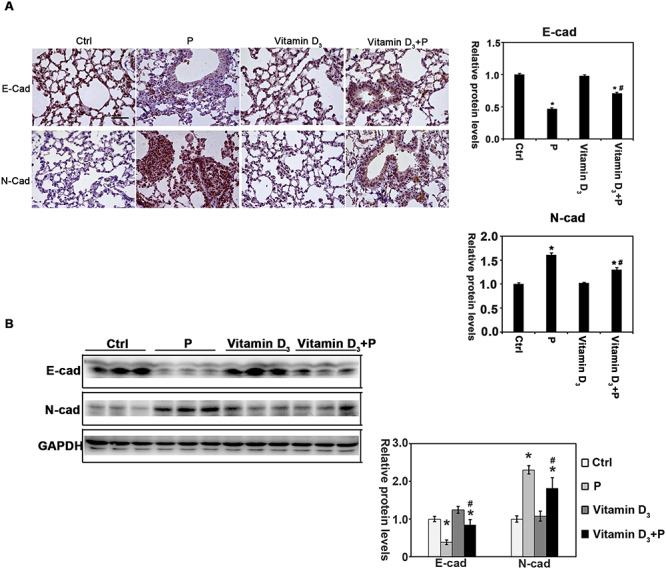
Vitamin D3 regulates the levels of E-cadherin and N-cadherin in the particle-exposure lung tissue. (A) IHC staining of E-cadherin, and N-cadherin was performed [n = 10, and a representative image of the lung tissue from each group is shown (scale bar: 100 μm)]. The quantification of the above IHC images is shown. Results are expressed as mean ± SD (*P < 0.05, Ctrl vs. treatment groups; #P < 0.05, P vs. Vitamin D3 + P). (B) Lung tissue lysates from each group were subjected to immunoblot analysis with E-cadherin and N-cadherin antibodies. The intensity of the indicated antibody’s bands from three independent experiments was quantified.
Calcitriol activates Nrf2 and VDR in both human epithelial cells and primary lung fibroblast cells
According to previous studies, lung epithelial cells and fibroblast cells are two major cell types related to the process of lung tissue repair and fibrosis. To understand the detailed molecular mechanisms of vitamin D3 abrogation, the fibrosis effects associated with particle instillation, lung epithelial cells BEAS-2B and cultured primary fibroblast cells HFLIII were used. Cell viability assay showed that there was no significant decrease with calcitriol treatment (0–200 nM) in BEAS-2B cells and HFLIII cells (Fig. 4A and B). Furthermore, the results from western blot analysis indicated that VDR and Nrf2 signaling pathways were upregulated by calcitriol treatment in a dose-dependent manner in both cell lines (Fig. 4E and F). As expected, immunofluorescence images showed that calcitriol upregulated VDR and Nrf2, which were mostly located in the nucleus (Fig. 4C and D). These results indicate that calcitriol activates Nrf2 and VDR signals in both epithelial cells and primary lung fibroblast cells.
Figure 4.
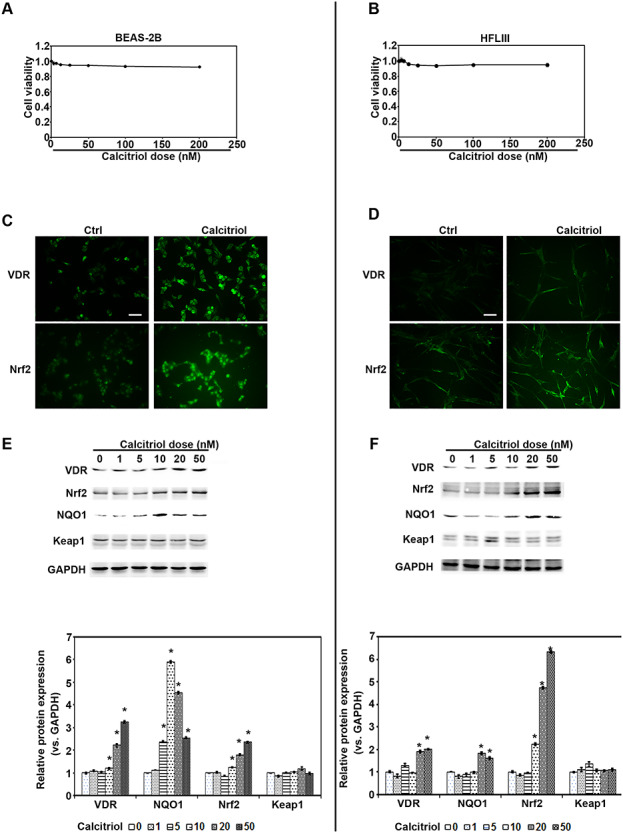
Calcitriol activates Nrf2 and VDR in both human epithelial cells and primary lung fibroblast cells. (A) BEAS-2B and (B) HFLIII cells were treated with the indicated doses of calcitriol for 48 h. Cell viability was measured. (C) BEAS-2B and (D) HFLIII cells were left untreated or treated with calcitriol 20 nM for 24 h. VDR and Nrf2 immunofluorescence images were captured (scale bar: 100 μm). (E) BEAS-2B and (F) HFLIII cells were treated with the indicated doses of calcitriol for 24 h. Cell lysates were subjected to immunoblot analyses with VDR, Nrf2, NQO1, Keap1, and GAPDH antibodies. The intensity of the blots from three independent experiments were quantified (lower panel). Results are expressed as mean ± SD (*P < 0.05, Ctrl vs. treatment groups; #P < 0.05, P vs. calcitriol + P).
Nrf2 activated by calcitriol is functionally in the effects of fibroblast cell viability and proliferation, myofibroblast transdifferentiation, and matrix expression
Our above results have confirmed that vitamin D3 injection opposed the fibrosis effects and attenuated the EMT-like tissue repair. And based on the previous study, macrophage plays an important role in regulating tissue inflammation and fibrosis [13]. Two separate in vitro systems with co-culture of macrophages & lung epithelial cells and macrophages & fibroblast cells were set up. Firstly, human primary fibroblast cells (HFLIII) were co-cultured with differentiated THP1 cells in the transwell-plate. With Ctrl siRNA, Nrf2 siRNA, or VDR siRNA 24 h-transfection, HFLIII cells were passaged on the bottom of plate, and in the upper chamber differentiated THP1 cells were separately treated with Ctrl, calcitriol, particles, or combination (calcitriol + particles) and co-cultured for another 48 h. HFLIII cells were subjected to MTT assay for cell viability. The results revealed that calcitriol only did not affect the cell viabilities. However, calcitriol pre-treated blunted the increase of cell viability caused by particles exposure in Ctrl siRNA group (Fig. 5A), while calcitriol treatment opposed the cell viabilities by particle exposure in Nrf2 siRNA and VDR siRNA groups. To confirm these results, Ki67 was detected by immunofluorescence. Accordingly, there was higher Ki67 expression in cells with particle exposure, which was depressed by calcitriol. However, Ki67 was not blunted by calcitriol in Nrf2 siRNA and VDR siRNA transfected cells (Fig. 5C). In addition, the expression of FN, collagen IV, and α-SMA was determined. After similar treatment with differentiated THP1 cells, the cell lysis was harvested and subjected with immunoblot analysis with the indicated antibodies. The results showed particle exposure increased the ECM accumulation (FN, Collagen IV), and myofibroblast transdifferentation (α-SMA), which were attenuated by calcitriol in a VDR-Nrf2-dependent manner (Fig. 5D).
Figure 5.
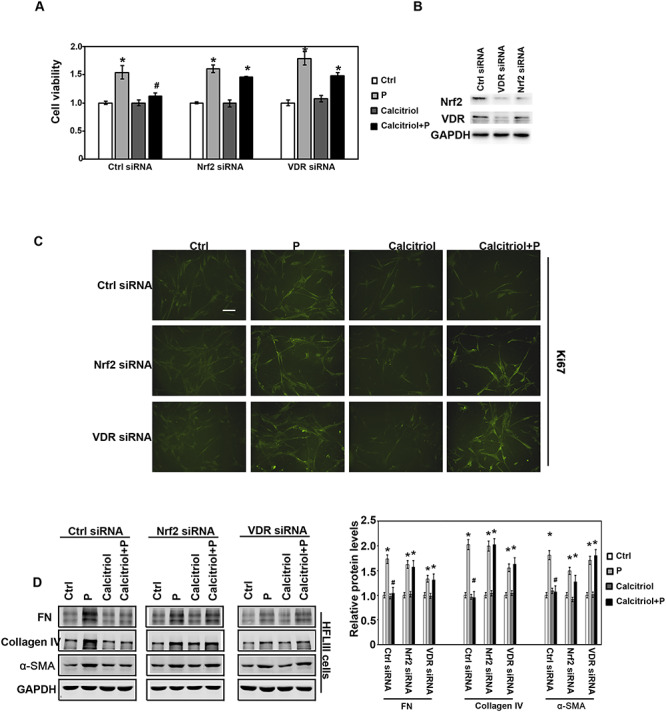
Calcitriol decreased the fibroblast cell viability, proliferation, myofibroblast transdifferentation, and matrix expression through Nrf2 signaling pathway. (A) HFLIII and THP-1 cells were pre-transfected with Ctrl siRNA, Nrf2 siRNA, or VDR siRNA for 24 h and then co-cultured with the indicated treatment for another 48 h. HFLIII cells were harvested, and cell viability was measured by MTT assay. (B) The effects of Nrf2 siRNA and VDR siRNA were determined by immunoblot analysis. (C) HFLIII cells were harvested after 24 h with the same treatment above. Ki67 immunofluorescence images were captured (scale bar: 100 μm). (D) The same batch of the HFLIII cells was harvested, and the lysates were subjected to immunoblot analysis with FN, collagen IV, α-SMA, and GAPDH antibodies. The intensity of the blots from three independent experiments were quantified (right panel). Results are expressed as mean ± SD (*P < 0.05, Ctrl vs. treatment groups; #P < 0.05, P vs. calcitriol + P).
Calcitriol limits fibroblast cells’ migration through Nrf2 signals
To explore if calcitriol could suppress lung fibroblast cells’ migration, and attenuated lung fibrosis, an in vitro scratch assay was performed. Differentiated THP-1 cells (upper chamber) and HFLIII cells (bottom) were co-cultured in the transwell-plates. Followed by transfection with Ctrl siRNA, Nrf2 siRNA, or VDR siRNA, THP-1 cells were treated with Ctrl, calcitriol, particles, or combination for 24 h. An in vitro scratch assay was performed in HFLIII cells up to 48 h. The results indicated that particle exposure significantly increased the HFLIII cells’ migration, which shortened scratch area closure time; while calcitriol and particle co-treatment significantly slowed down open area closure compared with cells in particles exposure group, calcitriol could not suppress the upregulated migration caused by particles exposure in the cells with Nrf2 or VDR siRNA pre-transfection (Fig. 6 and Supplementary Fig. S2).
Figure 6.
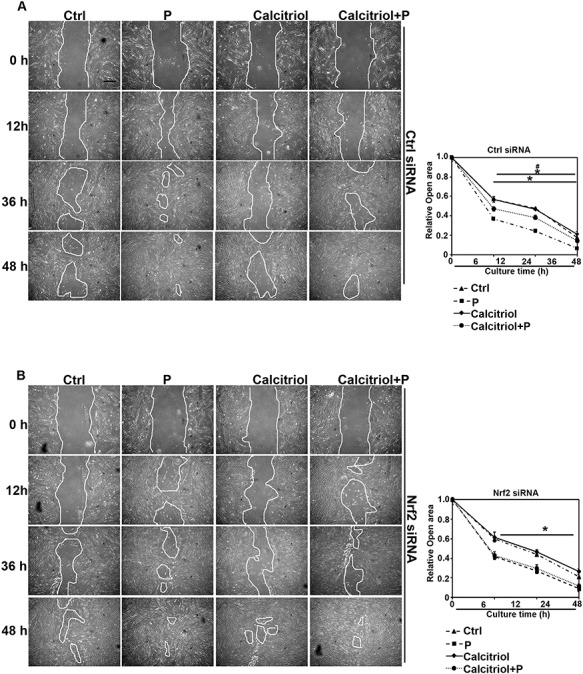
Calcitriol limits fibroblast cell migration through Nrf2 signals. (A, B) In vitro scratch assay of lung primary fibroblast cells (HFLIII). HFLIII cells were transfected with either Ctrl or Nrf2 siRNA for 24 h, followed by removal of PDMS slab above and co-cultured with differentiated THP-1 cells with the indicated treatments for another 24 h. Representative cell images from each treatment group in the indicated time points after removal of PDMS slab are shown (scale bar: 50 μm). Quantification of open areas is shown. Results are expressed as mean ± SD (*P < 0.05, Ctrl vs. treatment groups; #P < 0.05, P vs. calcitriol + P).
Particle-induced effects of EMT is antagonized by calcitriol through Nrf2 signals
Except for the fibroblast cells, lung epithelial cells also played an important role in the lung fibrosis. BEAS-2B cells were employed to further unveil the detailed mechanism in lung fibrosis. First, a similar scratch assay was performed in BEAS-2B cells. The results indicated that particles inhibited the migration of BEAS-2B cells, while calcitriol co-treatment significantly accelerated closure compared with cells of particle exposure. No difference was detected between cells’ migration when Nrf2 was silenced in the cells (Fig. 7A), which was consistent with the results of Ki67 IHC staining in Fig. 2D. Next, the EMT-related protein levels were determined in the cells with the indicated treatment. BEAS-2B cells (bottom) and differentiated THP-1 cells (upper chamber) were seeded in the transwell-plate. Followed by transfection with Ctrl siRNA, Nrf2 siRNA, or VDR siRNA, BEAS-2B cells were co-cultured with THP-1 cells with the indicated treatments for another 24 h. Cells were harvested and subjected to western blot analysis. As expected, VDR expression was upregulated with calcitriol treatment except for VDR siRNA transfection group. Nrf2 levels were increased with particles, calcitriol, and combination treatment in Ctrl siRNA transfected cells, which indicated that particle exposure caused the cell oxidative stress. Additionally, particle exposure caused the epithelial cell EMT-related protein dysregulation. The protein levels of α-SMA and its downstream gene N-cadherin were increased, while the expression of E-cadherin and β-catenin (epithelium related proteins) decreased. Calcitriol co-treatment restore these proteins’ expression, whereas in the Nrf2 or VDR siRNA cells, calcitriol did not function as it in Ctrl siRNA group (Fig. 7B). Then the growth rate of BEAS-2B cells were assessed with the role of epithelial cell’s proliferation in the re-epithelialization. By co-culturing the THP-1 and BEAS-2B cells in transwell-plate, cells were silenced with either Ctrl siRNA or Nrf2 siRNA. After THP-1 cells were treated with Ctrl, calcitriol, particles, or combination for 24 h, the expression of Ki67 in BEAS-2B cells was detected by immunofluorescence. Obviously, particle exposure decreased Ki67 expression, and calcitriol reversed its expression, whereas in Nrf2-silenced cells, Ki67 was further decreased (Fig. 7C). These results indicated that Nrf2 induction by calcitriol positively modulates epithelial cell’s proliferation.
Figure 7.
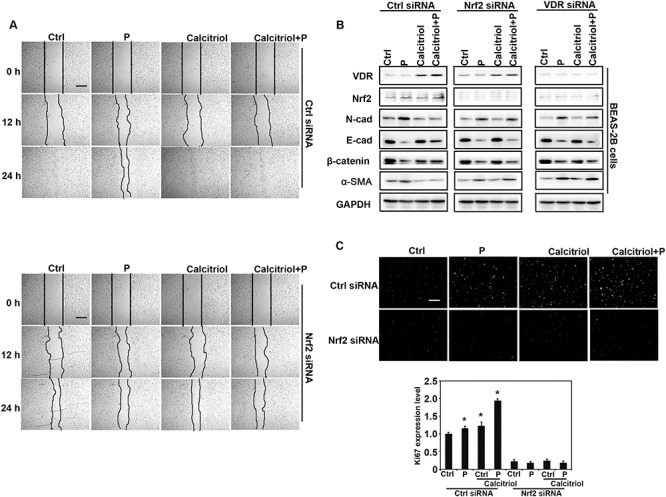
Particle-induced EMT-like effects is antagonized by calcitriol in an Nrf2-dependent manner. (A) In vitro scratch assay of lung epithelial cells. BEAS-2B cells were transfected with either Ctrl or Nrf2 siRNA for 24 h, followed by removal of PDMS slab above and co-cultured with differentiated THP-1 cells with the indicated treatments for another 24 h. Representative cell images from each treatment group in the indicated time points after removal of PDMS slab are shown (scale bar: 50 μm). (B) BEAS-2B cells were transfected with Ctrl, Nrf2, or VDR siRNA for 24 h and then co-cultured with differentiated THP-1 cells with the indicated treatments for another 24 h. Cell lysates were harvested and subjected to immunoblot analysis with the indicated antibodies. (C) Calcitriol promotes the proliferation of BEAS-2B cells through Nrf2 signals. Ki67 immunofluorescence image (upper) and quantification of fluorescence intensity (bottom). Similarly treated and siRNA-transfected BEAS-2B cells were subjected to immunofluorescence analysis with Ki67 antibody (scale bar: 100 μm).
Discussion
Vitamin D is a typical vitamin regarding with a multi-function with roles in inflammation- and fibrosis-related diseases. In addition, it modulates bone development by regulating calcium and phosphorus homeostasis [27]. In this study, the therapeutic application and detailed roles of vitamin D3 (a highly versatile molecule) in chronic particle exposure-induced lung inflammation, repair, fibrosis, and remodeling was investigated. First, animal study confirmed that vitamin D3 supplied the protective effects on the development of particle-induced lung injury. Particle exposure induced the expression of Nrf2, and NQO1 as well, consistent with the role of Nrf2 as a stress rescuer. Particle exposure caused a series of inflammatory response, including inflammatory cell infiltration and alveolar septal thickening and cell apoptosis (Fig. 1). Vitamin D3 improved the particle-induced inflammation and apoptosis, as well as increased the epithelial cell proliferation. To explore the detailed effects of vitamin D3 against the particle-induced injury, in vitro experiments with macrophages, lung epithelium, and primary lung fibroblast were performed. Here, we employed the calcitriol in our cell study, which is the active form of vitamin D3. The results further demonstrated that pharmacological Nrf2 induction with calcitriol treatment contributes to important events of lung tissue repair and fibrosis progression caused by particle inhalation, including attenuating oxidative stress, depressing inflammation, decreasing cell apoptosis, promoting epithelial cells’ proliferation, and migration, inhibiting fibrosis effects as well. Furthermore, these data also demonstrated that calcitriol-mediated protection is through the negative regulation of α-SMA and FN through Nrf2 signals. Obviously, in this study, we used different forms of vitamin D3 for the in vivo and in vitro studies. The reason for this is mice have a metabolic system that can transform vitamin D3 to 1,25(OH)2D3 in their kidney, while cells could not. In order to assess the protective mechanism of vitamin D3 in cell-based study, the active form of vitamin D3 was required.
Since vitamin D is a typical vitamin, which plays a critical role in calcium and phosphorus homeostasis, the level of serum calcium was determined. The results showed that vitamin D3 supplementation did not change the level of serum calcium in the mice (Supplementary Fig. S1C). Particle inhalation causes oxidative stress and lung inflammation. Increased oxidative stress damages the cyto-proteins, lipids, and DNA, which further leads to cell apoptosis, tissue dysfunction, and injury [28]. Our previous study and this research identified that particle exposure induces oxidative stress (8-oxo-dG) and increased apoptosis (CC3) [24]. Besides that, the induced level of oxidative stress caused an abnormal tissue repair as detection of fibroblast proliferation and lung epithelial cells migration. Thus, Nrf2 signals were accordingly activated to contend the injury and regulate the tissue repair; however, toxins-caused injury has already occurred. Oppositely, pharmacological activation of Nrf2 signals could protect the tissue directly against particles-caused oxidative stress and inflammation, with increased antioxidative ability available. Taken together, therapeutically pre-induction of Nrf2 signaling pathway supplies the cells with the prevention against oxidative-caused damage, and apoptosis. Furthermore, here we also found that it could regulate the expression of other proteins important for tissue repair and remodeling (α-SMA, FN, Collogen IV and migration & proliferation-related genes) through direct and indirect mechanisms with vitamin D3 treatment, which still need to be clarified in the next research.
Currently, most of the studies focused on the macrophage in the lung tissue injury with particle exposure, and few studies work on the mechanism and preventive role of VD-Nrf2 signals in lung epithelial and fibroblast cell tissue repair and remodeling. However, these studies have been reported in other types of tissue. Studies showed that activation of Nrf2 signaling pathway improves the diabetic wound healing by upregulation of keratinocyte cells’ proliferation and migration [29, 30]. Paradoxically, some studies reported that no significant differences were observed in the course of cell proliferation and migration of Nrf2−/− mice tissues compared to wild type mice expect for serious inflammation caused by toxins [31]. The conflicts may be because of the different pattern of Nrf2 activation. In addition, particle inhalation caused oxidative stress is progressive activity, which leads to more ROS-induced Nrf2 expression to provide antioxidant roles and increase DNA oxidative damage and apoptosis at the same time. Taken together, the beneficial effect of vitamin D3 treatment in reducing oxidative stress, improving lung epithelial cell’s proliferation and migration, and opposing fibrosis effects, as well as lung tissue repair was figured out in our research via Nrf2 signals, which is consistent with the previous reports [32, 33].
Totally, there are three major cell types involved in the process of lung inflammation and fibrosis. Epithelial and fibroblast cells play an essential role in tissue repair and remodeling [13, 34]. But most of the current studies focused on the effects of alveolar macrophage during the course of lung injury [9, 35]. Particle exposure leads to ROS generation, DNA damage, and cell apoptosis. Thus, in this study, we established the in vitro models by co-cultured macrophage and epithelial cells or fibroblast cells together to figure out the regulation effects on local cells by macrophage. By phagocytosing particles, macrophages were activated and secreted cytokines, including IL6 and TGFβ1. TGFβ1 regulates the pro-fibrosis switch, activates myofibroblasts, and inhibits epithelial cell’s proliferation [14, 36]. It also increases the expression of α-SMA, which induces the accumulation of extracellular matrix components and activates EMT-associated transcription factors [37]. Our results indicated that the α-SMA level was increased after being exposed to the particles, which was partially restored by vitamin D3. These results indicated that activation of Nrf2 signals by vitamin D3 improved epithelial cells’ proliferation and migration not only through attenuating the generation of ROS, but also by inhibiting the α-SMA level, which could facilitate the epithelial cell migration [13].
Conclusions
Our research provides convincing experimental evidence that vitamin D3 therapeutically improves tissue repair and inhibits the fibrosis effects induced by particle exposure, which contributes to the pulmonary injury improvement.
Conflicts of Interest. The authors declare no conflicts of interest.
Supplementary Material
Acknowledgements
The grants that supported our study are as follows: National Natural Science Foundation of China (Grant ref: 81703205); Youth Foundation of Jiangsu Province (Grant ref: BK20160333); Postdoctoral Research Foundation of China (Grant ref: 2016M600440, 2017T100402); Postdoctoral Science Foundation of Jiangsu Province (Grant ref: 1601079C); a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1. Chan TC, Zhang Z, Lin BC et al. Long-term exposure to ambient fine particulate matter and chronic kidney disease: a cohort study. Environ Health Perspect 2018;126:107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jaganathan S, Jaacks LM, Magsumbol M et al. Association of long-term exposure to fine particulate matter and cardio-metabolic diseases in low- and middle-income countries: A systematic review. Int J Environ Res Public Health 2019;16 pii: E2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lao XQ, Guo C, Chang LY et al. Long-term exposure to ambient fine particulate matter (PM2.5) and incident type 2 diabetes: a longitudinal cohort study. Diabetologia 2019;62:759–69. [DOI] [PubMed] [Google Scholar]
- 4. Zhen AX, Piao MJ, Hyun YJ et al. Diphlorethohydroxycarmalol attenuates fine particulate matter-induced subcellular skin dysfunction. Mar Drugs 2019;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun Q, Liu C, Chen R et al. Association of fine particulate matter on acute exacerbation of chronic obstructive pulmonary disease in Yancheng, China. Sci Total Environ 2019;650:1665–70. [DOI] [PubMed] [Google Scholar]
- 6. Weber SA, Insaf TZ, Hall ES et al. Assessing the impact of fine particulate matter (PM2.5) on respiratory-cardiovascular chronic diseases in the New York City metropolitan area using hierarchical Bayesian model estimates. Environ Res 2016;151:399–409. [DOI] [PubMed] [Google Scholar]
- 7. Wang C, Cai J, Chen R et al. Personal exposure to fine particulate matter, lung function and serum club cell secretory protein (Clara). Environ Pollut 2017;225:450–55. [DOI] [PubMed] [Google Scholar]
- 8. Deng X, Zhang F, Wang L et al. Airborne fine particulate matter induces multiple cell death pathways in human lung epithelial cells. Apoptosis: Int J Program Cell Death 2014;19:1099–112. [DOI] [PubMed] [Google Scholar]
- 9. Xue L, Zhang H, Zhang J et al. Bixin protects against particle-induced long-term lung injury in an NRF2-dependent manner. Toxicol Res (Camb) 2018;7:258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Familari M, Naav A, Erlandsson L et al. Exposure of trophoblast cells to fine particulate matter air pollution leads to growth inhibition, inflammation and ER stress. PLoS One 2019;14:e0218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong Z, Guo Z, Zhang R et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med 2016;239:117–25. [DOI] [PubMed] [Google Scholar]
- 12. Guo C, Xia Y, Niu P et al. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-kappaB signaling. Int J Nanomedicine 2015;10:1463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Xue L, Li B et al. Therapeutic potential of bixin in PM2.5 particles-induced lung injury in an Nrf2-dependent manner. Free Radic Biol Med 2018;126:166–76. [DOI] [PubMed] [Google Scholar]
- 14. Zheng R, Tao L, Jian H et al. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol Environ Saf 2018;163:612–9. [DOI] [PubMed] [Google Scholar]
- 15. Berridge MJ. Vitamin D: a custodian of cell signalling stability in health and disease. Biochem Soc Trans 2015;43:349–58. [DOI] [PubMed] [Google Scholar]
- 16. Herr C, Greulich T, Koczulla RA et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res 2011;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slominski AT, Li W, Bhattacharya SK et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol 2011;131:1167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slominski A, Janjetovic Z, Tuckey RC et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab 2013;98:E298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014;21:319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vega MR, Dodson M, Gross C et al. Role of Nrf2 and autophagy in acute lung injury. Current Pharmacol Rep 2016;2:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 2004;85:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang H, Gao L, Mao J et al. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-kappaB and TGF-beta1/Smad-2/-3 pathways. Cell Stress Chaperones 2016;21:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo Y, Wu XQ, Zhang C et al. Effect of indole-3-carbinol on ethanol-induced liver injury and acetaldehyde-stimulated hepatic stellate cells activation using precision-cut rat liver slices. Clin Exp Pharmacol Physiol 2010;37:1107–13. [DOI] [PubMed] [Google Scholar]
- 24. Tao S, Zhang H, Xue L et al. Vitamin D protects against particles-caused lung injury through induction of autophagy in an Nrf2-dependent manner. Environ Toxicol 2019;34:594–609. [DOI] [PubMed] [Google Scholar]
- 25. Stocklin E, Eggersdorfer M. Vitamin D, an essential nutrient with versatile functions in nearly all organs. Int J Vit Nutr Res Internationale Zeitschrift fur Vit- und Ernahrungsforschung J Int de vitaminologie et de Nutr 2013;83:92–100. [DOI] [PubMed] [Google Scholar]
- 26. Shen H, Wang J, Min J et al. Activation of TGF-beta1/alpha-SMA/col I profibrotic pathway in fibroblasts by Galectin-3 contributes to atrial fibrosis in experimental models and patients. Cell Physiol Biochem 2018;47:851–63. [DOI] [PubMed] [Google Scholar]
- 27. Dusso AS. Update on the biologic role of the vitamin D endocrine system. Curr Vasc Pharmacol 2014;12:272–7.23713878 [Google Scholar]
- 28. Liu H, Fang S, Wang W et al. Macrophage-derived MCPIP1 mediates silica-induced pulmonary fibrosis via autophagy. Part Fibre Toxicol 2016;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashi R, Himori N, Taguchi K et al. The role of the Nrf2-mediated defense system in corneal epithelial wound healing. Free Radic Biol Med 2013;61:333–42. [DOI] [PubMed] [Google Scholar]
- 30. Long M, Rojo de la Vega M, Wen Q et al. An essential role of NRF2 in diabetic wound healing. Diabetes 2016;65:780–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braun S, Hanselmann C, Gassmann MG et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol 2002;22:5492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaiprasongsuk A, Janjetovic Z, Kim TK et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol 2019;24:101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janjetovic Z, Tuckey RC, Nguyen MN et al. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol 2010;223:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lachapelle P, Li M, Douglass J, et al. Safer approaches to therapeutic modulation of TGF-beta signaling for respiratory disease. Pharmacol Ther 2018;187:98–113. [DOI] [PubMed] [Google Scholar]
- 35. Han XB, Li HX, Jiang YQ et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis 2017;8:e2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ren DQ, Liu M, Guo YZ et al. Liver X receptor agonist T0901317 inhibits TGF-beta1-induced alpha-SMA expression in normal human lung fibroblasts. Nan fang yi ke da xue xue bao = J Southern Med Univ 2011;31:744–8. [PubMed] [Google Scholar]
- 37. Chung Y, Fu E, Chin YT et al. Role of Shh and TGF in cyclosporine-enhanced expression of collagen and alpha-SMA by gingival fibroblast. J Clin Periodontol 2015;42:29–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


