Abstract
Regulatory B cells (Breg) are crucial immunoregulators that maintain peripheral tolerance and suppress inflammatory autoimmune responses. In recent years, our understanding on the nature and mechanism of action of Bregs has revealed the important role of cytokines in promoting the regulatory properties of this unique B cell subset, both in animal and human models. In this review, we compiled the cytokines that have been reported by multiple studies to induce the expansion of Breg. The Breg-inducing cytokines which are currently known include IL-21, IL-6, IL1β, IFNα, IL-33, IL-35, BAFF and APRIL. As cytokines are also known to play a pivotal role in the pathogenesis of autoimmune diseases, in parallel we reviewed the pattern of expression of the Breg-inducing cytokines in Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), Inflammatory Bowel Diseases (IBD) and Multiple Sclerosis (MS). We show here that Breg-inducing cytokines are commonly implicated in these inflammatory diseases where they typically have a higher expression than in healthy individuals, suggesting their paradoxical nature. Interestingly, despite the general overexpression of Breg-inducing cytokines, it is known that Breg cells are often numerically or functionally impaired in various autoimmune conditions. Considering these alterations, we explored the possible parameters that may influence the function of Breg-inducing cytokines in exhibiting either their regulatory or pro-inflammatory properties in the context of autoimmune conditions.
Keywords: Autoimmune diseases, Systemic lupus erythematosus, Rheumatoid arthritis, Inflammatory bowel diseases, Multiple sclerosis, Regulatory B cells, Cytokines, Tolerance, Inflammation
Highlights
-
•
Cytokines such as IL-21, IL-6, IL1β.
-
•
Breg-inducing cytokines are typically associated in various autoimmune diseases, suggesting their paradoxical nature.
-
•
In autoimmune disease patients, despite the upregulation of Breg-inducing cytokines, Breg impairments have been identified.
-
•
Many factors may influence the dual role of Breg-inducing cytokines such as dose and environmental milieu.
-
•
Understanding the paradoxical role of Breg-inducing cytokines is essential for developing therapeutic strategies.
1. Introduction
Traditionally, B cells have always been known to directly serve as regulators of humoral immunity through antibody production against specific antigens. They also participate in cellular immunity, such as facilitating antigen presentation, providing essential co-stimulations to T cells, producing cytokines and defending against microbial invasion. This paradigm changed with the discovery of regulatory B cells (Breg) in 1974, led by James Turk [1]. His group found that transfer of splenocytes depleted of B cells failed to inhibit delayed-type hypersensitivity (DTH) reaction on skin. This observation suggested that B cells could exhibit a novel regulatory property contributing to the suppression of immune responses [1]. It was around 20 years later that the regulatory properties of B cells were further described by Janeway’s group for multiple sclerosis (MS), in an experimental autoimmune encephalomyelitis (EAE) model [2]. In that case, EAE mice that had their B cells genetically removed developed a more severe pathological disease upon immunization with the myelin basic protein (MBP) peptide. Owing to its potent immune suppressive ability, Mizoguchi et al. coined this subset as regulatory B cells (Bregs) [3]. Subsequently, extensive characterization and functional implication of Bregs has been studied in various animal models of inflammation and autoimmune diseases [4]. Compelling evidence demonstrated that the adoptive transfer of Bregs in animal models effectively induces immune tolerance, delays the onset and reduces the incidence of autoimmune disease progression. Characterization of human subsets of Bregs has also been made. For example, Breg cells with a phenotype of CD19+CD24hiCD38hi were identified in healthy individuals to possess suppressive capacity [5].
Dynamic changes in the Breg population have been well implicated in the development of human and murine autoimmune. It is currently well accepted that Breg numbers and functional activity often are inversely correlated with the severity and progression of autoimmunity. In summary, large amount of preclinical and clinical data point towards a strong relationship between an impaired function and/or reduced number of Breg cells with the development of different types of autoimmune pathologies. These findings have been extensively reviewed by others [[4], [5], [6]]. Given these premises, manipulating Bregs represents a promising therapeutic tool to modulate immune responses and avert uncontrolled inflammation.
Since their first discovery, our understanding of the nature and role of Breg as well as the signals that can promote their differentiation has improved considerably. One of the important cues involved in Breg induction are cytokines; a group of small peptides that can facilitate cell signaling and interaction. To date, various cytokines have been described to expand Breg cells both in vitro and in vivo. Nevertheless, they have simultaneously been implicated in the pathogenesis of autoimmune diseases. This article reviews the current knowledge on Breg-inducing cytokines, highlighting their potential paradoxical role, both as a disease-driving factors and as regulators of immune responses through Breg generation. A special focus will be put on the major autoimmune diseases, namely Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), Inflammatory Bowel Diseases (IBD) and Multiple Sclerosis (MS).
2. Breg-inducing cytokines
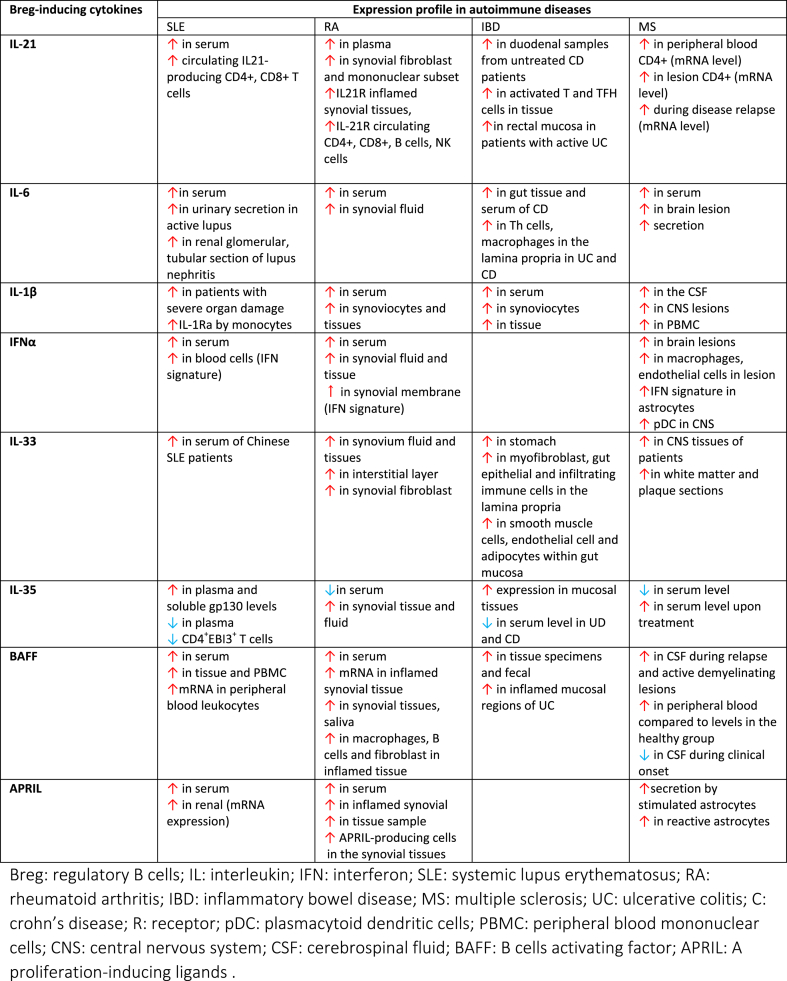
Here, we review the cytokines that play an integral role in Breg induction, and their expression profile in different autoimmune disorders (Table 1). Reviewing the literature, we obtained a list of cytokines involved in both processes: IL-21, IL-6, IL-1β, IFN-α, IL-33, IL-35, BAFF and APRIL. Based on such knowledge, we will explore the concept of cytokines duality and its implication in the context of Breg impairment in autoimmune diseases.
Table 1.
Expression profile of Breg-inducing cytokines in SLE, RA, IBD and MS.
2.1. Interleukin-21 (IL-21)
IL-21 was first described by Yoshizaki et al. as a potent factor in shifting naïve B cells towards immunosuppressive phenotypes [7]. The first observation was that the IL-10 producing B (B10) cells responsible for EAE disease suppression highly expressed the IL-21 receptor (IL-21R), MHC-II and CD40, which suggested a role for IL-21 in driving B10 expansion [7]. It was confirmed when murine splenic B cells primed with IL-21 prior to restimulation with LPS, PMA and Ionomycin resulted in over 4-fold increase of IL-10 secretion. Furthermore, the group demonstrated that IL-21-induced IL-10 expression in B cells was similar to the level achieved by LPS-stimulated B cells. IL-21-induced B10 cells were found to be a subset of CD1dhiCD5+ progenitor cells and played essential roles during the initial phases of autoimmune disease. Subsequent analysis showed that in vitro culture of pre-activated purified splenic B cells in the presence of IL-21, CD40 ligand (CD40L) and B lymphocyte stimulator (BLyS)- expressing cells remarkably induced B10 cells. IL-21 was shown to play a pivotal role in the maturation of progenitor B10 towards a fully functional Breg upon CD40 engagement. Several studies further confirmed the potent role of IL-21 in murine Breg differentiation [[8], [9], [10], [11]]. Furthermore, a recent study demonstrated that the combination of IL-21 with ligands of CD40 and mucin domain 1 (TIM1) significantly increased IL-10 expression in splenic B cells. Treatment of palatal tissue with this stimulation cocktail enhanced in vivo Breg differentiation and function leading to amelioration of ligature-induced gum inflammation in mice [11].
In human, IL-21 has been shown to induce a phenotypically unique subset of granzyme-B expressing Breg (GraB) [9]. Stimulated B cells in the presence of IL-21 differentiated into an enzymatically active Breg subset with the ability to inhibit T cell proliferation, via granzyme-B-dependent degradation of TCR. Phenotypic analysis showed that GraB expressed a combination of surface markers such as CD38, CD1d, CD25, IgM and CD147. Additionally, this subset also displayed high expression of immune suppressive molecules such as IL-10 and indoleamine-2,3-dioxygenase (IDO).
2.2. The expression of IL-21 in autoimmune diseases
IL-21 has emerged as one of the important factors in modulating autoimmunity. Elevated IL-21 expression in animal models and patients of SLE, RA, IBD and MS has been identified by different groups [[12], [13], [14], [15], [16], [17], [18]]. IL-21 intracellular expression was upregulated in CD4+ T cells of patients with active SLE, resulting in altered B cell function [14]. Serum IL-21 was also significantly higher in SLE patients [19]. In agreement, Terrier et al. reported that SLE patients displayed a greater number of circulating IL-21-producing CD4+ T cells and this increase correlated well with disease severity [20].
Plasma IL-21 level was also significantly higher in RA patients in comparison to healthy controls [21,22]. Furthermore, IL-21/IL-21 receptor (IL-21R) expression were markedly upregulated in macrophages and synovial fibroblast in this group of patients [23]. Li et al. demonstrated that inflamed synovial tissues exhibit a higher expression of IL-21R [24]. Additional assessment revealed that circulating CD4+ and CD8+ T cells, B cells, NK cells and synoviocytes mononuclear subset of RA patients highly expressed IL-21R [24,25].
In line with SLE and RA data, overexpression of IL-21/IL-21R in different subgroups of IBD patients have been reported. Monteleone et al. observed that IL-21 is significantly elevated in the gut of both patients with Crohn’s disease (CD) and Ulcerative Colitis (UC) [26]. Moreover, enhanced transcriptional and protein expression of IL-21 was detected in activated T and T follicular helper (TFh) cells of duodenal samples of IBD tissue [27,28]. IL-21 mRNA has been found significantly elevated both during the IBD active phase and throughout IBD disease relapse [29], and patients with active UC displayed a profound IL-21 expression in rectal mucosa [30].
IL-21 upregulation has also been consistently reported in MS patients, with elevated mRNA and protein expression of IL-21/IL-21R in circulating CD4+ T cells of progressive MS patients [31]. Follow-up studies subsequently demonstrated that CD4+ T cells infiltrating white matter lesions expressed higher IL-21 mRNA levels in acute, chronic and relapse MS patients [15,32]. Collectively, the available data from the discussed studies evidences the excessive production of IL-21/IL-21R in patients with active SLE, RA, IBD and MS.
2.3. Interleukin-6 (IL-6) and interleukin 1 β (IL-1β)
The unique capacity of IL-6 and IL-1β to expand IL-10 expressing Breg cells was described by Rosser and colleagues [33]. In response to inflammation, gut microbiota drove the production of IL-6 and IL-1β by macrophages and dendritic cells in gut-associated lymphoid tissues (GALT) and in periphery, which resulted in direct expansion of Breg in mice. Alteration of gut microbiota by treatment with different antibiotics or changes in housing environment prevented Breg induction due to a decrease of IL-6 and IL-1β production. Furthermore, in vivo decrease of IL-6 and IL-1β affected other B cell compartments, including a diminution in transitional T2 precursor B cells.
Supporting the initial hypothesis, mice with IL-6R- and IL-1R-deficient B cells, housed in non-sterile condition, developed more severe arthritis due to failure in Breg development [33]. Additional in vitro experiments demonstrated that IL-6 and IL-1β, in concert with CD40 ligation not only effectively induced Bregs, but also enhanced its function to reciprocally generate Treg and suppress pro-inflammatory T cells. Inhibition of disease progression was observed upon treatment with the combination of the two cytokines and anti-CD40L antibody through an increase of IL-10-producing B cells.
2.3.1. The expression of IL-6 in autoimmune diseases
IL-6 has been widely implicated in autoimmune inflammatory diseases. The strong association is based on numerous studies reporting excessive levels of IL-6 in both patients and experimental models. For instance, elevated levels of IL-6 has been detected in different samples of SLE patients, including serum [34], urine [35] and cerebrospinal fluid [36]. Furthermore, patients with active disease and lupus nephritis have higher concentration of serum IL-6 [37,38]. In-situ analysis also demonstrated high expression of IL-6 in renal glomerular and tubular sections of lupus nephritis patients [39].
IL-6 has also been suggested to play a pivotal role in the enhancement of joint and systemic inflammation of RA. Several studies reported on increased level of IL-6 in serum and synovial fluid of RA patients [40,41]. Further analysis demonstrated the association of IL-6 level with RA disease development and severity, concomitant with reduced serum IL-6 upon successful treatment [42].
The involvement of IL-6 signaling in IBD was demonstrated by increased levels of IL-6 in both serum and gut tissue of CD patients [43]. Consistently, IL-6R and IL-6 common receptor subunit, glycoprotein (gp) 130 were also expressed at excessive levels in peripheral lymphocytes [44]. Additionally, T helper cells and macrophages in the lamina propria were found to abundantly secrete IL-6 in both UC and CD patients [45].
In MS, on top of higher plasma concentration, IL-6 is also overproduced by infiltrating immune cells, microglia and astrocytes in the brain lesion [46]. Moreover, B cells of MS patients exhibit greater capacity to secrete IL-6, subsequently leading to a greater ratio of IL-6/IL-10–producing B-cells [47].
It is therefore currently accepted that IL-6 is excessively produced in the aforementioned autoimmune diseases and this increase is paralleled with its known role in promoting local and systemic inflammation.
2.3.2. The expression of IL-1β in autoimmune diseases
The expression profile of IL-1β in SLE patients is controversial. Some studies observed a significant association between the upregulation of IL-1β in serum and the patients’ disease severity [48,49]. On the contrary, other groups failed to observe similar results. Notably, IL-1β plays a more significant role in RA, IBD and MS.
IL-1β is one of the key regulators of RA pathogenesis, where it plays a vital role in establishing inflammation in the joint, by triggering the expression of chemokines and adhesion molecules [50]. Elevated levels of IL-1β was detected in serum of RA patients, and a consistent correlation with disease severity has been reported [51,52]. Furthermore, the concentration of IL-1β in the joint and synovial fluid was markedly increased compared to healthy controls and patients with osteoarthritis [53]. In vitro culture of synovial cells derived from RA patients displayed a significant increase in IL-1β production [54], which led to an enhanced recruitment of effector cells to the local inflammation. This accumulation of IL-1β in the joint induced synoviocytes and chondrocytes to release MMPs and other proteinases that promote cartilage degradation, osteoclast differentiation and expression of pro-inflammatory genes [55]. These observations eventually prompted the development of an agent to block IL-1β (anakinra) as an effective treatment of RA [56].
The same is also true in IBD, where a progressive increase of IL-1β with ongoing mucosal inflammation has been reported [57]. Patients with active CD exhibit a greater secretion of IL-1β by monocytes in the colon lamina propria [58]. Furthermore, greater levels of IL-1β were observed in many animal models of colitis [59].
These observations can be extended to early studies of MS patients, in which IL-1β protein and mRNA were detected in the cerebrospinal fluid (CSF) and central nervous system (CN)S lesion [60]. A more recent study subsequently reported that as compared to the healthy population, PBMC derived from relapsing-remitting MS patients displayed elevated transcript levels of IL-1β along with inflammasome components [61]. Further analysis found a correlation between the amount of IL-1β and the severity of brain cortical demyelinating lesions in the CSF [62].
Collectively, although less clear in SLE pathogenesis, these results indicate that IL-1β expression is highly upregulated in RA, IBD and MS, highlighting the association of IL-1β with autoimmune diseases.
2.4. Interferon-α (IFNα)
The role of IFNα in inducing a suppressive capacity in B cells was first described by Menon and colleagues [63]. They demonstrated that upon co-culturing with TLR9-stimulated pDCs, B cells purified from healthy individuals differentiated into a suppressive subset, with CD24+CD38hi phenotype. Further phenotypic analysis revealed that pDC-induced Breg expressed higher levels of IgD and IgM with negative expression of CD27, suggestive to be an immature B cell population. IFNα-induced Breg were capable of exclusively express and secrete IL-10 but no other key pro-inflammatory cytokines. Additional analysis confirmed the inhibitory effect of CD24+CD38hi Breg on the differentiation of naïve T cells towards effector inflammatory T cells [63]. The group also identified immature B cells as a direct precursor for pDC-expanded Breg cells. A detailed mechanistic investigation established IFN-α as the important cytokine responsible for this observation. CpG-stimulated pDCs secreted high amounts of IFNα and the addition of IFNα/IFNR neutralizing antibody abruptly inhibited the Breg differentiation. Additionally, pDC-induced Breg exhibited an upregulation in CD40L, and its blocking with monoclonal antibody partially suppressed Breg induction, suggesting CD40 engagement as one of the involved mechanisms. Furthermore, addition of exogenous IFNα into the culture of activated B cells led to a significant expansion of Breg with the same phenotypes confirming the in vitro regulatory capacity of this cytokine.
2.4.1. The expression of IFNα in autoimmune diseases
Elevated levels of IFNα have been observed in patients and animal models of autoimmune diseases, particularly in SLE, in large datasets from numerous studies [64]. Specifically, an increased concentration of circulating IFNα and expression of IFN-I-signature genes have been recorded in patients with SLE, accompanied by a higher IFNα activity as compared to healthy control and patient cohorts with other autoimmune diseases such as RA [65,66]. Similar observations have also been demonstrated in pediatric patients upon genetic profiling, while around 60–80% of adult patients display high IFNα-gene signature in various cell types [67]. The increase is most prominent in patients with exacerbate disease activity and IFNα is considered as an essential biomarker for SLE disease development. The excessive level of IFNα was later discovered to be mainly secreted by pDCs [68].
On the other side, a small number of studies have also reported increased IFNα levels in RA and MS. Increased level of IFNα has been detected both in the circulation and within the synovial fluids [69], concomitant with elevated of IFN-I-signature expression in the peripheral blood of RA patients. Moreover, synovial membrane of RA patients expressed an abundant level of IFNα-gene signature [70].
Correspondingly, IFNα protein expression has been detected in chronic brain lesions of MS patients, although with modest levels as compared to IFNγ. IFNα was predominantly expressed by macrophages and to a lesser extent by the endothelial cells of MS patients [71]. Furthermore, upregulation of the specific IFN-signature gene Myxovirus resistance gene (MxA) was observed in astrocytes, infiltrating immune cells and in the serum of MS patients [72]. These data suggested an increased local expression of IFNα. IFNα producers, pDCs were also found abundantly present in MS lesions and in cerebrospinal fluid [73].
Taken together, IFNα and IFN-I-signatures are predominantly increased in SLE with established disease driving roles, and is expressed at modest level in IBD, RA and MS.
2.5. Interleukin-33 (IL-33)
The regulatory role of IL-33 was discovered recently, when it was reported to induce IL-33 membrane-bound receptor (ST2)-expressing Treg and the alternatively-activated M2 macrophages with suppressive ability [74,75]. Subsequently, the role of IL-33 in B cell biology was studied by Sattler et al. who showed that the intraperitoneal treatment of IL-33 exacerbated gut inflammation in IL-10-KO but not in IL-10-sufficient wild-type mice [76]. The observed protective role of IL-33 in IL-10-sufficient mice was identified to be mediated by a unique subset of Breg. This subset was coined as BregIL−33 and phenotypically characterized as positive for CD25, CD1d and IgM but negative for CD23 and TIM1. Additionally, adoptive transfer of BregIL−33 successfully inhibited the development of spontaneous colitis in IL-10-KO mice, indicating the potent suppressive effect of this Breg subset. These findings were confirmed by Zhu et al. demonstrating an increase of IL-10-expressing Breg responses in the mesenteric lymph node upon IL-33 treatment in dextran sulfate sodium (DSS)-induced colitis models [77].
2.5.1. The expression of IL-33 in autoimmune diseases
Early studies characterizing the serum profile of SLE patients reported increased serum IL-33R, the IL-33 soluble decoy receptor sST2 protein [78]. This was confirmed by a study in active disease in a cohort of Hong Kong SLE patients, describing a marked increase of sST2 in the circulation as compared to healthy individuals and patients with inactive disease [79]. sST2 level was also found to positively correlate with disease severity and anti-dsDNA antibody. On the other side, Chinese SLE patients also displayed a significant elevation of serum IL-33, albeit lower than in RA patients [80].
In addition, a large body of research reported on the increased expression of IL-33 in RA patients. Earlier studies demonstrated the elevated expression of IL-33 in synovium tissue, synovial fluid and interstitial layer in patients with RA [[81], [82], [83]]. A marked increase of serum IL-33 and ST2 has also been observed by different laboratories, particularly in RA patients with severe disease activity [84,85]. Furthermore, the expression level of IL-33 in the resting synovial fibroblast of RA patients was dramatically enhanced upon stimulation with pro-inflammatory cytokines [82].
Likewise, several studies have also supported the notion that IL-33 is upregulated and mediate MS development. This was prompted by the high expression of IL-33/ST2 detected in spinal cord of EAE mice, and later confirmed in central nervous system (CNS) tissues of MS patients, particularly in the brain and spinal cord region [[86], [87], [88]]. White matter and plaque tissue sections of MS patients dramatically displayed high IL-33 level as compared to healthy controls [88]. In line with this data, patients with relapsing-remitting MS had a significant increase of serum IL-33 accompanied by abundant IL-33 mRNA level in the stimulated lymphocytes and macrophages [88].
In parallel, there is increasing evidence showing an elevated expression of IL-33 in IBD, with abundant IL-33 in the stomach, and able to cause detrimental changes in the GI track and lamina propria [89,90]. IL-33 is highly expressed by myofibroblasts [91], gut epithelia and infiltrating immune cells in the lamina propria during intestinal inflammation [92,93]. Further studies also demonstrated the expression of IL-33 in smooth muscle cells, endothelial cells and adipocytes within gut mucosa [90,94]. In UC, but not in CD, IL-33 is highly detected in tissue samples, although the correlation to disease severity has not been well established [95]. However, several studies with conflicting results on IL-33 expression in IBD has also been reported [96]. For instance, IL-33 was found to be downregulated in the serum of patients with IBD as compared to healthy control and was suggested to promote tissue repair and attenuation of colitis through M2 switching [97].
Despite some discrepancies, a general consensus is that IL-33 is strongly upregulated in SLE, RA, MS and IBD.
2.6. Interleukin-35 (IL-35)
While initially described as an immune regulatory cytokine exclusively secreted by Treg, IL-35 has been recently demonstrated as a new mediator of Breg differentiation and function. In 2014 it was first described the capability of IL-35 to induce IL-10 and IL-23 in B cells, using genetically engineered murine heterodimeric IL-35 (rIL-35) [98]. rIL-35 effectively expanded both a CD5+CD19+B220lo Breg population and a unique subset of IL-35-producing Breg (IL-35+Breg). IL-35 potently inhibited autoimmune uveitis development and such effect was attributed to the expansion of Breg. Subsequently, the adoptive transfer of IL-35+Breg also suppressed an established autoimmune uveitis evidenced by reduced ocular inflammation. The regulatory potential of IL-35 as Breg inducer was further complemented by a number of other studies. In 2017, Dambuza and colleagues revealed that the compelling role of IL-35 in converting Breg and Treg cells was largely attributed by its IL-12p35 subunit, which is shared with IL-12 [99]. IL12p35 alone could exhibit regulatory functions which include inhibition of lymphocyte proliferation and their differentiation to effector T cells lineages as well as augmenting IL-10 and IL-35 expression in B and T cells.
2.6.1. The expression of IL-35 in autoimmune diseases
The characterization of the immune regulatory profile in SLE patients done by Qiu et al. revealed a significant increased level of IL-35, and treatment with anti-inflammatory prednisone suppressed IL-35 concentrations [100]. This was in line with reports by Cai et al. which described higher serum IL-35 levels in patients with active SLE disease as compared to patients with inactive disease and healthy controls [101]. In contrast, significant reduction of IL-35 serum level in active SLE patients in patients with active disease and lupus nephritis, in comparison with patients with inactive disease has also been reported [102].
In patients with RA, IL-35 and its subunits were found to be significantly increased in the synovial fluid of patients with naïve early disease, as compared to cohorts with established RA and control patients with osteoarthritis [103]. Li et al. reported that IL-35 serum level was greatly increased in comparison with healthy control [104]. Furthermore, the increase was associated with RA clinical parameters which include level of rheumatoid factor and anticyclic citrullinate peptide antibodies. However, contradictory findings on lower concentration of IL-35 in sera of patients with RA has also been reported [105]. At the same time, diminished IL-35 serum levels was reported in patients with UC and CD as compared to healthy controls and linked to a decrease count in IL-35-producing Treg [106], a notable increase of IL-35 in the mucosal tissues has also been described [106]. On the other hand, studies of IL-35 expression in MS is less extensive. One report described a lower serum concentration of IL-35 in MS patients, and its upregulation as a mediator of beneficial treatment response [107].
As a new addition to the IL-12 cytokine family, the apparent involvement of IL-35 in mediating inflammation and autoimmunity has sparked the community enthusiasm, albeit more investigation is needed to further elucidate its precise role. Interestingly, there seems to be an increasing number of conflicting studies documenting both increased and decreased levels of IL-35 depending on the different biological compartments and disease models studied.
2.7. B-cell activating factor (BAFF)
The novel role BAFF in inducing IL-10 expression in B cells was reported by Yang et al. [108]. Evaluation on murine splenic B cells cultured in the presence of BAFF led to a dose-dependent increased in the frequency of IL-10-producing B cells. In addition, blockade of BAFF receptor by TACI:Fc protein abrogated BAFF-mediated Breg induction. Subsequently, surface marker analysis showed that BAFF-induced Breg exhibit similar phenotype as B10 cells in regard of its high CD5 and CD1d expression. In vivo analysis in murine revealed a selective expansion of Breg within the marginal zone region upon exogenous administration of BAFF.
In a different study, BAFF has also been shown to promote IL-10 expression and secretion in B cells of animal model and patients of chronic lymphocytic leukemia (CLL) [109]. BAFF concentration were significantly associated with IL-10 level in both animal and patients sample of CLL. Based on this observation, the dual nature of BAFF in promoting IL-10 production and B cells survival was suggested.
More recently, Zhang et al. demonstrated the potent regulatory role of BAFF in inducing IL-35-producing-Breg [110]. Exogenous addition of BAFF with various concentrations to lupus-prone MRL-Faslpr/lpr mice-derived splenic culture resulted in an increase of IL-35 expression in B cells. BAFF-induced-IL-35+ Breg was identified to be of the marginal zone B cell population and greatly expressed BAFF-receptor, transmembrane activator and calcium-modulator and cytophilin ligand interactor (TACI). Furthermore, the subset exhibit CD5+CD1dhiFcγRIIbhi phenotype. In addition, functional study revealed the potent regulatory role of BAFF-induced IL-35-producing-Breg in inhibiting IFN-γ expression in CD4+CD25− T cells. Interestingly, BAFF-induced-IL-35 producing Breg also promoted the expansion of CD4+CD25+ Tregs and enhanced IL-35 secretion in T cells. BAFF-driven IL-35 expression in B cells was shown to be induced via TACI-BAFF interaction and the classical NF-κB1 pathway. Moreover, intravenous treatment of Faslpr/lpr mice with anti-BAFF led to a reduction of p35+EBI3+B cells, confirming the in vivo Breg-inducing role of BAFF.
2.7.1. The expression of BAFF in autoimmune diseases
The involvement of BAFF as a key pathogenic factor and its overexpression in SLE have been supported by extensive evidences. In the investigation of both serum and plasma of SLE patients, it was demonstrated that the level of BAFF were higher than those healthy controls [111,112]. Elevated concentration of BAFF was also strongly correlated with different classes of anti-double-stranded DNA antibody, including of the IgA, IgG and IgM [111]. Another study measuring BAFF in patients with numerous systemic immune-based rheumatic diseases found a significant increase of serum BAFF, of which this observation was also associated with autoantibody titer [113]. Additionally, serum BAFF levels has been shown to significantly correlate with disease activity and SLEDAI score [114,115]. Moreover, aside from elevated BAFF expression in the peripheral leukocytes, PBMC from SLE patients often exhibit high expression of BAFF-receptor [116]. Additionally, patients with active lupus displayed greater BAFF mRNA expression as compared to patients with more stable disease [116].
The same is true for RA patients, of which BAFF and BAFF-R expression were shown to be highly expressed in synovial tissues and serum. In inflamed synovium, BAFF was shown to be highly produced by tissue macrophages, B cells and fibroblasts [117]. BAFF level has also been associated with the production of rheumatoid factors and anti-dsDNA autoantibodies [118,119]. Furthermore, RA patients with higher circulating BAFF had more advanced disease activity [120]. Additionally, BAFF-R expression was greatly expressed in RA fibroblast-like synoviocytes. Dendritic cells in the collagen induced arthritis (CIA) animal model displayed a marked increase of BAFF protein production which resulted in enhanced B cell proliferation [117].
Studies related to the role and expression level of BAFF in IBD and MS are less extensive. Investigation in IBD mouse models reported on the overexpression of BAFF albeit with less clear role [121]. More recent papers showed that serum, tissue specimens and fecal levels of BAFF were elevated along with positive correlation with disease activity [122]. Fu et al. demonstrated the strong association of fecal BAFF with endoscopic inflammatory IBD score [123]. Furthermore, inflamed mucosal regions of UC patients displayed a marked upregulation of BAFF expression in the lamina propria [122].
BAFF expression was shown to be markedly upregulated in brain lesions of MS patients [[124], [125]]. Moreover, high level of BAFF-R was detected in CNS tissue and stimulated astrocytes of these patients could highly secrete active BAFF [126]. Additionally, gene expression of BAFF in monocytes, and its receptor in B and T cells were shown to be higher in MS patients than in normal controls [127].
Taken together, these clinical evidences highlight the notion that BAFF is substantially upregulated and involved in the pathogenesis of autoimmune diseases, especially in SLE and RA. The presence of high BAFF level in serum of SLE and RA patients is now very well-accepted. How it modulates the immune response in such pathological condition remains elusive. But it may serve as a secondary response to counteract the overwhelming pro-inflammatory activation in these clinical scenarios.
2.8. A proliferation-inducing ligand (APRIL)
The novel function of APRIL in augmenting IL-10 mRNA and protein expression in B cells was explored by Hua et al. [128]. CpG-stimulated PBMC from healthy donors cultured in the presence of APRIL was assessed for the presence of IL-10-expressing cells. Subsequently, a dose dependent increase of IL-10 expression was observed in B cells but not in other cell subsets such as monocytes and T cells. Further analysis revealed that APRIL-induced-B10 cells mediate their suppressive effect through STAT3 signaling and displayed high expression of TACI. Furthermore, stimulation of B cells with APRIL successfully inhibit the upregulation of proinflammatory cytokine such as IFNγ and TNFα.
In line with these findings, APRIL upregulates Breg cells which featured shared human Breg markers, CD24hiCD38hi, and contributes to the immunosuppressive environment in the bone marrow [129].
Earlier study had also demonstrated the capability of APRIL in expanding peritoneal B-1 B cell with regulatory property in mice. Transgenic mice overexpressing APRIL develop hyperplasia with accumulation of IL-10-producing B1 cells [130].
Adding to the regulatory properties of APRIL, a more recent study found that APRIL enhanced the induction of naïve human B cells to IgA-positive IL-10-expressing B cells [131]. Different subsets of B cells, including naïve, IgM-, IgG- and IgA-expressing were stimulated by APRIL, IL-21 and CD40L-expressing fibroblasts. Subsequently, IgA+ B cells was identified to preferentially express IL-10. Assessment of other Breg markers, co-stimulatory and inhibitory molecules demonstrated that APRIL-induced IgA+ Breg exhibited higher expression of FasL, PD-L1 and T cell immunoreceptor with Ig and ITIM domains (TIGIT), suggesting distinct phenotypes from other reported Breg markers. Furthermore, addition of blocking antibodies to TACI diminished the initial observation suggesting the role of TACI signaling in the induction of APRIL-driven IgA+ Breg cells. Functional experiments confirmed the regulatory properties of APRIL-driven IgA+ Breg cells in inhibiting T cells proliferation, TNF production in macrophages as well as expanding FOXP3+ Treg. Interestingly, APRIL transgenic mice were protected from CIA induction of which APRIL-driven IgA+ Breg cells was shown to be one of the mechanisms for the observed disease inhibition.
2.8.1. The expression of APRIL in autoimmune diseases
Similar to BAFF, there have been extensive clinical evidences on the involvement of APRIL in autoimmune diseases. Multiple investigations on serum samples of SLE patients found a significant elevation of APRIL [132,133]. Additionally, this increase has also been correlated to SLE worst prognostic parameters such as glomerulonephritis, the British Isles Lupus Assessment Group (BILAG) index and hemolytic anemia [116,134]. Additionally, a correlation between serum APRIL and renal expression and severity of renal diseases in SLE has been observed. However, correlations between APRIL levels and autoantibodies production in SLE remains controversial with some studies noted either inverse or weak correlations between these two parameters [132,133].
Further to its involvement in SLE, high expression of APRIL has also been detected in serum of RA patients. Moura et al. showed an abundant expression of serum APRIL in patients with very early RA (VERA) [135]. Furthermore, increasing data has reported the accumulation of APRIL in inflamed synovial compartment and tissues samples of patients [136]. Inflamed synovial tissues displayed a significant elevation of APRIL mRNA expression [136]. Accumulation of APRIL-secreting cells has also been observed in synovial tissues of RA patients. Moreover, in comparison to healthy individuals of which APRIL expression are only detected in nonclassical monocytes, myeloid cells and all monocytes in RA patients exhibited upregulation of surface APRIL expression with an association to disease severity [137]. Stimulation of synoviocytes from the patients also resulted to higher production of pro-inflammatory cytokines and upregulation of receptor activator of nuclear factor kappa-B ligand (RANKL) expression. Increased expression of APRIL receptor in synoviocytes of RA patients has also been noted [138] with an association between serum APRIL and synovitis [117].
In agreement with the observation made in SLE and RA patients, MS patients displayed raised APRIL protein expression in reactive astrocytes of the CNS [139]. This was confirmed by another study showing how infiltrating macrophages contributed to the increase level of APRIL in CNS of patients with MS [125].
However, APRIL expression profile in IBD patients hasn’t been well-examined and most likely it plays a less important role as B cells are not the primary mediator of IBD.
Summarizing, APRIL is excessively expressed in many autoimmune diseases, and its exact role remains to be defined for it may also be a secondary response to aberrant immune activation.
3. Breg-inducing cytokines: double-edged sword and outstanding questions
Here we compiled the available data on Breg-inducing cytokines and their expression profile in common autoimmune diseases; highlighting their general overexpression. If we take a closer look, cytokines identified to induce the expression of IL-10 or other regulatory molecules in B cells are mostly categorized as pro-inflammatory, perhaps with the exception of IL-35. IL-35 possesses a potent immune suppressive role and is predominantly produced by Treg [140]. However, it should be noted that IL-35 is not constitutively expressed in tissues, but its expression is upregulated in response to underlying inflammation [141].
As summarized in Table 2, the ability of the listed cytokines to induce Breg were reported either in animal models, clinical cohorts or both. For instance, it is yet unclear whether IL-33, IL-6 and IL-1 could have the same effect on human cells in regards of their ability to induce Breg expansion, as they have only been studied in the murine model. For this reason, it is critical to validate whether the observed functions are species-restricted, or they can be extended to humans.
Table 2.
Breg-inducing cytokines.
| Cytokines | Breg phenotype | Stimulus | In vitro/vivo | Species | Reference |
|---|---|---|---|---|---|
| IL-21 | CD1dhiCD5+ B10 cells | cells expressing CD40L and BLyS | In vitro | mouse | [7] |
| IL10+ | anti-Tim1+CD40L | In vitro | mouse | [11] | |
| CD5+CD86+CD43+CD147+ | IL-21+CD40L−Th cells | In vitro | human | [147] | |
| GrB+CD38+CD1d+IgM + CD147+ IDO+CD25+ | Anti-BCR | In vitro | human | [9] | |
| IL-6 | IL-10+ T2-MZPs, IL-10+CD5+ cells, IL-10+Tim-1+ | CD40L | In vitro | mouse | [33] |
| IL10+ | CD40L | In vivo | mouse | [33] | |
| IL-1β | IL-10+ T2-MZPs, IL-10+CD5+ cells, IL-10+Tim-1+ | CD40L | In vitro | mouse | [33] |
| IL-10+ | CD40L | In vivo | mouse | [33] | |
| IFNα | IL-10+CD24+CD38hi | CpGC-stimulated pDCs | In vitro | human | [63] |
| IL-10+CD24+CD38hi | TLR9-activated | In vitro | human | [63] | |
| IL-10+CD24+CD38hi | R848 (TLR7/8 agonist) | In vitro | human | [63] | |
| IL-33 | CD25+CD1dhiIgMhiCD5−CD23−Tim-1- | In vivo | mouse | [76] | |
| IL-35 | p35+Ebi3+ (IL-35+) CD19+IL10+ |
LPS PMA |
In vitro In vitro |
Mouse human | [98] [98] |
| CD5+B220lo | In vivo | mouse | [98] | ||
| BAFF | CD1dhiCD5+ B10 |
In vitro In vivo |
mouse | [148] | |
| CD5+CD1dhiFcγRIIbhiIL-35+ |
In vitro In vivo |
mouse | [110] | ||
| APRIL | IL-10+ | CpG | In vitro | human | [149] |
| IgA+FasL+PDL1+TIGIT+IL10+ | CD40L-expressing fibroblast, IL21 |
In vitro, In vivo |
mouse, human |
[131] |
Breg: regulatory B cells; IL: interleukin; BAFF: B cells activating factor; APRIL: A proliferation-inducing ligands; CD40L: CD40 ligand; BLys: B lymphocyte stimulator; TIM1: T-cell immunoglobulin and mucin domain 1; IDO: indoleamine 2,3-dioxygenase; GrB: granzyme B; Th: T-helper cells; BCR: B cell receptor; BAFF: B-cell activating factor; APRIL: A proliferation-inducing ligand; TLR: toll-like receptor; MZP: marginal-zone precursor; T2-MZP: transitional 2 marginal-zone precursor; FcγR: FC gamma receptor.
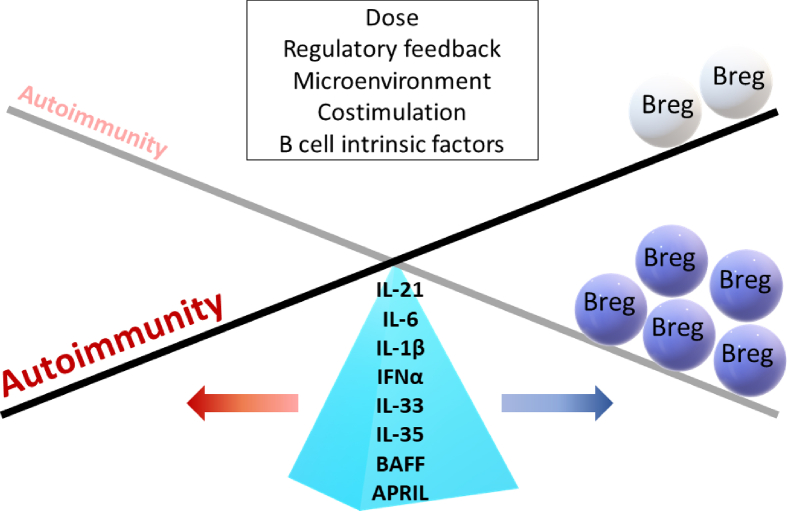
The paradoxical effect attributed to inflammatory cytokines, that is, expanding Bregs while also sustaining inflammatory disease progression (Fig. 1), may not be surprising, since it has been previously proposed that the differentiation and activation of Bregs occurs under the same set of signals that initiate inflammatory responses [6].
Fig. 1.
The paradoxical roles of Breg-inducing cytokines. IL-21, IL-6, IL-1β, IFNα, IL-33, IL-35, BAFF and APRIL promote regulatory responses through Breg induction. On the other hand, these cytokines are generally overexpressed and associated in most autoimmune diseases. Multiple factors may influence the behavior of these cytokines in the context of autoimmunity of which Breg cells are often impaired in function or number. The proposed factors include dose of cytokines, alteration of regulatory feedback, microenvironment, costimulation and B cells intrinsic factors.
The notion that cytokines can constitute a double-edged sword in mediating and tuning the immune responses is fascinating, yet a crucial aspect to consider when defining the outcome of inflammation, whether it derives into resolution or autoimmunity. One of the critical questions posed by the above-mentioned observations relates to the mechanism of aberrant Breg number and functions in autoimmune conditions. In other words, if the general consensus pointed towards upregulation of Breg-inducing cytokines in most of autoimmune diseases, what factors could account for the failure of Breg proper expansion and function? Here we address some of the potential means:
Dose of cytokine-several lines of evidence support the idea that a precise cytokine concentration seems to be essential for Breg induction. Menon et al. [63] demonstrated that high-dose IFNα failed to promote Breg development in human, and instead enhanced plasma cells differentiation. In a similar manner for its thymic counterpart, low-dose IL-2 selectively promotes the expansion and activation of Treg cells, but not high-dose [142]. Therefore, while cytokines play a crucial role in driving regulatory responses, excessive production during inflammation may paradoxically cause harmful and pathogenic effects.
Defective regulatory feedback- Other cells with immunomodulatory properties have been shown to modulate Breg expansion. Park et al. [143] reported that myeloid-derived suppressor cells (MDSCs) promote Breg, while suppressing the formation of germinal center and plasma B cells in mice. In the context of autoimmunity, such cell interaction could possibly be altered. For example, the failure of crosstalk between pDC and B cells in SLE patients could be leading to dysfunctional Breg expansion and thus disease progression [63].
Environmental- The alteration of the microenvironment may interfere with the in vivo generation of Bregs. To date, the majority of studies and concepts developed related to Breg are based on in vitro studies. Furthermore, the in vitro expansion and suppression assays were mostly performed under specific culture conditions, often with the addition of selective reagents, growth and stimulatory factors. In the context of autoimmune diseases, the unique proinflammatory environment such as cytokine storms, cell death, free radicals/metabolites and the presence of other cell types including effector and stromal cells may impair the progression of Breg and their functional behavior.
Cytokine networks- It cannot be overruled that the presence of multiple cytokines in physiological conditions may affect cytokine-induced Breg expansion. One of the limitations of the presented studies is using isolated cytokines that may not represent the true environmental milieu, especially in the context of autoimmune and inflammatory conditions. As cytokines are pleiotropic, redundant and share common receptors, this may lead to a wide range of responses. The complex cytokine networks may contribute to synergism, regulatory and inhibitory effects between different cytokines. For instance, the interplay between IL-21 and BAFF leading to a synergistic effect in driving memory B cells into IgG-secreting plasma cell has been documented [25], and IL-6 and IL-33 have been demonstrated to synergistically amplify IgE-mediated mast cell priming [144], to cite few examples. However, the role of these cytokines interactions and synergies on Breg function and regulation remains unknown.
Altered confounding co-stimulation or co-inhibition– Aside from cytokines, other stimuli have also been shown to significantly influence Breg function and expansion. These factors include signal transduction through B cell receptor, Toll like receptors and costimulatory molecules. Recent works have presented many evidences relating dysregulated B cell signaling with the promotion of autoimmunity [145]. Dysregulated B cell signaling not only can lead to defective BCR repertoire and autoantibody-producing B cells but may skew the naïve B cells to become resistant to Breg induction.
Intensity of co-stimulation- The level of activation and duration of co-stimulation may have a substantial impact during Breg generation. For example, CD40 signaling has been nicely demonstrated to play a pivotal role during Breg activation, while it is also known that aberrant CD40−CD40L axis is widely implicated in a variety of autoimmune diseases [146]. Therefore, whether the strength of co-stimulatory signals may alter Breg generation remains an open question.
B-cells intrinsic defect– It is widely appreciated that most autoimmune conditions are associated with abnormal genetic variants resulting in altered B cell function and BCR repertoires. This raised the possibility that B cell intrinsic defects might impede Breg function.
As it is known that Breg impairment contributes to the development of autoimmunity, it is noteworthy to mention that decreased Breg numbers and/or function might also be a consequence of the established ongoing inflammatory responses.
As our knowledge of the biology and immunomodulatory role of Breg increases, modulating Bregs might be a useful tool to restore immunological tolerance, especially in the context of autoimmune inflammation. One of the promising therapeutic approach is to efficiently and reproducibly expand the Breg population as well as to maintain its functional integrity. This could be done by using a combination of different cytokines and co-stimulations as detailed above. In order to fully achieve this goal, it is meaningful to define the optimum signal which acts as Breg drivers, including the paradoxical role of Breg-inducing cytokines.
4. Concluding remarks
As highlighted in this review, many cytokines involved in the pathogenesis of autoimmune diseases are at the same time capable of inducing Breg in mice and humans. This suggests the complex and paradoxical responses exhibited by immune molecules to propagate both effector and regulatory responses. Yet, despite the rise of the mentioned cytokines in autoimmunity, functional impairment of Bregs has been identified as an autoimmune disease-driving factor. Therefore, the current challenge lies on understanding the balance of action of these cytokines and to efficiently modulate their effect. This will not only push forward our knowledge on the fundamental mechanism of Breg dysregulation in autoimmunity but would also enhance the design of more effective diagnostics and therapies.
Conflicts of interest
None of the authors have declared any financial or commercial conflicts of interest.
Acknowledgement
We thank Dr. M. Monguio for technical editing and reviewing the manuscript.
This work was supported by grants from Instituto de Salud Carlos III, Spain (PI17/00335), SGR program of Generalitat de Catalunya, Spain (2017-SGR-301 REMAR Group) and ISCIII-REDinREN (RD16/0009 Feder Funds). FEB is a researcher from Fundació Institut de Recerca en Ciències de la Salut Germans Trias i Pujol, supported by the Health Department of the Catalan Government (Direcció General de Recerca i Innovació, Dept. Salut, Generalitat de Catalunya); Fatin N. Mohd Jaya is funded by postgraduate scholarship, The University of Hong Kong; and Marcella Franquesa is funded by the Catalan Health Department (Generalitat de Catalunya) contract PERIS (SLT002/16/00069).
References
- 1.Parker Katz, D., Turk J.L. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974 doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- 2.Wolf S.D., Dittel B.N., Hardardottir F., Janeway C.A. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 1996;184(6):2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizoguchi A., Mizoguchi E., Takedatsu H., Blumberg R.S., Bhan A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002 doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 4.Miyagaki T., Fujimoto M., Sato S. Regulatory B cells in human inflammatory and autoimmune diseases: from mouse models to clinical research. Int. Immunol. 2015 doi: 10.1093/intimm/dxv026. [DOI] [PubMed] [Google Scholar]
- 5.Blair P.A., Noreña L.Y., Flores-Borja F., Rawlings D.J., Isenberg D.A., Ehrenstein M.R., Mauri C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010 doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Mauri C., Menon M. Human regulatory B cells in health and disease: therapeutic potential. J. Clin. Investig. 2017 doi: 10.1172/JCI85113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshizaki A., Miyagaki T., Dilillo D.J., Matsushita T., Horikawa M., Kountikov E.I., Spolski R., Poe J.C., Leonard W.J., Tedder T.F. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012 doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagn M., Sontheimer K., Dahlke K., Brueggemann S., Kaltenmeier C., Beyer T., Hofmann S., Lunov O., Barth T.F., Fabricius D., Tron K., Nienhaus G.U., Simmet T., Schrezenmeier H., Jahrsdörfer B. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol. Cell Biol. 2012;90:457–467. doi: 10.1038/icb.2011.64. [DOI] [PubMed] [Google Scholar]
- 9.Lindner S., Dahlke K., Sontheimer K., Hagn M., Kaltenmeier C., Barth T.F.E., Beyer T., Reister F., Fabricius D., Lotfi R., Lunov O., Nienhaus G.U., Simmet T., Kreienberg R., Moller P., Schrezenmeier H., Jahrsdorfer B. Interleukin 21-induced granzyme b-expressing b cells infiltrate tumors and regulate t cells. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 10.Bankó Z., Pozsgay J., Szili D., Tóth M., Gáti T., Nagy G., Rojkovich B., Sármay G. Induction and differentiation of IL-10–producing regulatory B cells from healthy blood donors and rheumatoid arthritis patients. J. Immunol. 2017 doi: 10.4049/jimmunol.1600218. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Yu P., Yu X., Hu X., Kawai T., Han X. IL-21/anti-Tim1/CD40 ligand promotes B10 activity in vitro and alleviates bone loss in experimental periodontitis in vivo. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2017;1863:2149–2157. doi: 10.1016/j.bbadis.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troncone E., Marafini I., Pallone F., Monteleone G. Th17 cytokines in inflammatory bowel diseases: discerning the good from the bad. Int. Rev. Immunol. 2013 doi: 10.3109/08830185.2013.823421. [DOI] [PubMed] [Google Scholar]
- 13.Ohl K., Tenbrock K. Inflammatory cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2011;2011:1–14. doi: 10.1155/2011/432595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakou M., Papadimitraki E.D., Fanouriakis A., Bertsias G.K., Choulaki C., Goulidaki N., Sidiropoulos P., Boumpas D.T. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin. Exp. Rheumatol. 2013;31(2):172–179. [PubMed] [Google Scholar]
- 15.Tzartos J.S., Craner M.J., Friese M.A., Jakobsen K.B., Newcombe J., Esiri M.M., Fugger L. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am. J. Pathol. 2011 doi: 10.1016/j.ajpath.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok S.K., La Cho M., Park M.K., Oh H.J., Park J.S., Her Y.M., Lee S.Y., Youn J., Ju J.H., Park K.S., Il Kim S., Kim H.Y., Park S.H. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.33390. [DOI] [PubMed] [Google Scholar]
- 17.Hovhannisyan Z., Treatman J., Littman D.R., Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011 doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Nitto D., Sarra M., Pallone F., Monteleone G. Interleukin-21 triggers effector cell responses in the Gut. World J. Gastroenterol. 2010 doi: 10.3748/wjg.v16.i29.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb R., Merrill J.T., Kelly J.A., Sestak A., Kaufman K.M., Langefeld C.D., Ziegler J., Kimberly R.P., Edberg J.C., Ramsey-Goldman R., Petri M., Reveille J.D., Alarcón G.S., Vilá L.M., Alarcón-Riquelme M.E., James J.A., Gilkeson G.S., Jacob C.O., Moser K.L., Gaffney P.M., Vyse T.J., Nath S.K., Lipsky P., Harley J.B., Sawalha A.H. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009 doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrier B., Costedoat-Chalumeau N., Garrido M., Geri G., Rosenzwajg M., Musset L., Klatzmann D., Saadoun D., Cacoub P. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J. Rheumatol. 2012 doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- 21.Ma J., Zhu C., Ma B., Tian J., Baidoo S.E., Mao C., Wu W., Chen J., Tong J., Yang M., Jiao Z., Xu H., Lu L., Wang S. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012 doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen T.K., Andersen T., Hvid M., Hetland M.L., Hørslev-Petersen K., Stengaard-Pedersen K., Holm C.K., Deleuran B. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J. Rheumatol. 2010 doi: 10.3899/jrheum.100259. [DOI] [PubMed] [Google Scholar]
- 23.Andersson A.K., Feldmann M., Brennan F.M. Neutralizing IL-21 and IL-15 inhibits pro-inflammatory cytokine production in rheumatoid arthritis. Scand. J. Immunol. 2008 doi: 10.1111/j.1365-3083.2008.02118.x. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Shen W., Kong K., Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand. J. Immunol. 2006 doi: 10.1111/j.1365-3083.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger R., Sims G.P., Robbins R., Withers D., Fischer R.T., Grammer A.C., Kuchen S., Lipsky P.E. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J. Immunol. 2007 doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 26.Monteleone G., Monteleone I., Fina D., Vavassori P., Del Vecchio Blanco G., Caruso R., Tersigni R., Alessandroni L., Biancone L., Naccari G.C., Macdonald T.T., Pallone F. Interleukin-21 enhances T-helper cell type I signaling and interferon-γ production in Crohn’s disease. Gastroenterology. 2005 doi: 10.1053/j.gastro.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 27.Sarra M., Monteleone I., Stolfi C., Fantini M.C., Sileri P., Sica G., Tersigni R., Macdonald T.T., Pallone F., Monteleone G. Interferon-gamma-expressing cells are a major source of interleukin-21 in inflammatory bowel diseases. Inflamm. Bowel Dis. 2010 doi: 10.1002/ibd.21238. [DOI] [PubMed] [Google Scholar]
- 28.Fina D., Sarra M., Caruso R., Del Vecchio Blanco G., Pallone F., MacDonald T.T., Monteleone G. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut. 2008 doi: 10.1136/gut.2007.129882. [DOI] [PubMed] [Google Scholar]
- 29.Ghalamfarsa G., Mahmoudi M., Mohammadnia-Afrouzi M., Yazdani Y., Anvari E., Hadinia A., Ghanbari A., Setayesh M., Yousefi M., Jadidi-Niaragh F. IL-21 and IL-21 receptor in the immunopathogenesis of multiple sclerosis. J. Immunotoxicol. 2016 doi: 10.3109/1547691X.2015.1089343. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto-Furusho J.K., Miranda-Pérez E., Fonseca-Camarillo G., Sánchez-Muñoz F., Barreto-Zuñiga R., Dominguez-Lopez A. Interleukin 21 expression is increased in rectal biopsies from patients with ulcerative colitis. Inflamm. Bowel Dis. 2010 doi: 10.1002/ibd.21135. [DOI] [PubMed] [Google Scholar]
- 31.Christensen J.R., Börnsen L., Ratzer R., Piehl F., Khademi M., Olsson T., Sørensen P.S., Sellebjerg F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013 doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tegla C.A., Cudrici C.D., Azimzadeh P., Singh A.K., Trippe R., Khan A., Chen H., Andrian-Albescu M., Royal W., Bever C., Rus V., Rus H. Dual role of Response gene to complement-32 in multiple sclerosis. Exp. Mol. Pathol. 2013 doi: 10.1016/j.yexmp.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Rosser E.C., Oleinika K., Tonon S., Doyle R., Bosma A., Carter N.A., Harris K.A., Jones S.A., Klein N., Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med. 2014 doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- 34.Grondal G., Gunnarsson I., Ronnelid J., Rogberg S., Klareskog L., Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2000;18(5):565–570. [PubMed] [Google Scholar]
- 35.Reyes-Thomas J., Blanco I., Putterman C. Urinary biomarkers in lupus nephritis. Clin. Rev. Allergy Immunol. 2011 doi: 10.1007/s12016-010-8197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirohata S., Miyamoto T. Elevated levels of interleukin-6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system invol vement. Arthritis Rheum. 1990 doi: 10.1002/art.1780330506. [DOI] [PubMed] [Google Scholar]
- 37.Linker-Israeli M., Deans R.J., Wallace D.J., Prehn J., Ozeri-Chen T., Klinenberg J.R. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J. Immunol. 1991;147(1):117–123. [PubMed] [Google Scholar]
- 38.Tackey E., Lipsky P.E., Illei G.G. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004 doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukatsu A., Matsuo S., Tamai H., Sakamoto N., Matsuda T., Hirano T. Distribution of interleukin-6 in normal and diseased human kidney. Lab. Investig. 1991 [PubMed] [Google Scholar]
- 40.Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimi M., Maid R., Feldmann M., Kishimoto T. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur. J. Immunol. 1988 doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- 41.Srirangan S., Choy E.H. The role of Interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010 doi: 10.1177/1759720X10378372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madhok R., Crilly A., Watson J., Capell H.A. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann. Rheum. Dis. 1993 doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross V., Andus T., Caesar I., Roth M., Schölmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology. 1992 doi: 10.1016/0016-5085(92)90098-J. [DOI] [PubMed] [Google Scholar]
- 44.Mitsuyama K., Toyonaga A., Sasaki E., Ikeda H., Tateishi H., Nishiyama T., Tanikawa K., Elisa S. Soluble interleukin-6 receptors in inflammatory bowel disease : relation to circulating interleukin-6. Gut. 1995;36(1):45–49. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atreya R., Mudter J., Finotto S., Müllberg J., Jostock T., Wirtz S., Schütz M., Bartsch B., Holtmann M., Becker C., Strand D., Czaja J., Schlaak J.F., Lehr H.A., Autschbach F., Schürmann G., Nishimoto N., Yoshizaki K., Ito H., Kishimoto T., Galle P.R., Rose-John S., Neurath M.F. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 2000 doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 46.Maimone D., Guazzi G.C., Annunziata P. IL-6 detection in multiple sclerosis brain. J. Neurol. Sci. 1997 doi: 10.1016/S0022-510X(96)00283-3. [DOI] [PubMed] [Google Scholar]
- 47.Guerrier T., Labalette M., Launay D., Lee-Chang C., Outteryck O., Lefèvre G., Vermersch P., Dubucquoi S., Zéphir H. Proinflammatory B-cell profile in the early phases of MS predicts an active disease. Neurol. Neuroimmunol. NeuroInflammation. 2018 doi: 10.1212/NXI.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umare V., Pradhan V., Nadkar M., Rajadhyaksha A., Patwardhan M., Ghosh K.K., Nadkarni A.H. Effect of proinflammatory cytokines (IL-6, TNF, and IL-1 β) on clinical manifestations in indian SLE patients. Mediat. Inflamm. 2014 doi: 10.1155/2014/385297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brugos B., Vincze Z., Sipka S., Szegedi G., Zeher M. Serum and urinary cytokine levels of SLE patients. Die Pharmazie. 2012 doi: 10.1691/ph.2012.1694. [DOI] [PubMed] [Google Scholar]
- 50.Dayer J.M., Oliviero F., Punzi L. A brief history of IL-1 and IL-1 Ra in rheumatology. Front. Pharmacol. 2017 doi: 10.3389/fphar.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eastgate J.A., Wood N.C., Di Giovine F.S., Symons J.A., Grinlinton F.M., Duff G.W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 doi: 10.1016/S0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 52.Shoda H., Nagafuchi Y., Tsuchida Y., Sakurai K., Sumitomo S., Fujio K., Yamamoto K. Increased serum concentrations of IL-1 beta, IL-21 and Th17 cells in overweight patients with rheumatoid arthritis. Arthritis Res. Ther. 2017 doi: 10.1186/s13075-017-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahle P., Saal J.G., Schaudt K., Zacher J., Fritz P., Pawelec G. Determination of cytokines in synovial fluids: correlation with diagnosis and histomorphological characteristics of synovial tissue. Ann. Rheum. Dis. 1992 doi: 10.1136/ard.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chomarat P., Vannier E., Dechanet J., Rissoan M.C., Banchereau J., Dinarello C.A., Miossec P. Balance of IL-1 receptor antagonist/IL-1 beta in rheumatoid synovium and its regulation by IL-4 and IL-10. J. Immunol. 1995;154(3):1432–1439. [PubMed] [Google Scholar]
- 55.Jung S.M., Kim K.W., Yang C.W., Park S.H., Ju J.H., Mamura M. Cytokine-mediated bone destruction in rheumatoid arthritis. J. Immunol. Res. 2014 doi: 10.1155/2014/263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sokolov A.V., Shmidt A.A., Lomakin Y.A. B cell regulation in autoimmune diseases. Acta Nat. 2018 doi: 10.32607/20758251-2018-10-3-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balzola F., Cullen G., Ho G.T., Russell R.K., Wehkamp J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+Th17 cells, Inflamm. Bowel Dis. Monit. 2012 doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahida Y.R., Wu K., Jewell D.P. Enhanced production of interleukin 1-β by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn’s disease. Gut. 1989 doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCall R.D., Haskill S., Zimmermann E.M., Lund P.K., Thompson R.C., Sartor R.B. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rats. Gastroenterology. 1994 doi: 10.1016/0016-5085(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 60.Lin C.-C., Edelson B.T. New insights into the role of IL-1β in EAE and MS. Can. J. Physiol. Pharmacol. 2017;198:4553–4560. doi: 10.4049/jimmunol.1700263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossi S., Studer V., Motta C., Germani G., Macchiarulo G., Buttari F., Mancino R., Castelli M., De Chiara V., Weiss S., Martino G., Furlan R., Centonze D. Cerebrospinal fluid detection of interleukin-1β in phase of remission predicts disease progression in multiple sclerosis. J. Neuroinflammation. 2014 doi: 10.1186/1742-2094-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seppi D., Puthenparampil M., Federle L., Ruggero S., Toffanin E., Rinaldi F., Perini P., Gallo P. Cerebrospinal fluid IL-1β correlates with cortical pathology load in multiple sclerosis at clinical onset. J. Neuroimmunol. 2014 doi: 10.1016/j.jneuroim.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Menon M., Blair P.A., Isenberg D.A., Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity. 2016 doi: 10.1016/j.immuni.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crow M.K. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014 doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes K.M., Baechler E.C., Batliwalla F.M., Ortmann W.A., Karypis G., Gaffney P.M., Espe K.J., Shark K.B., Grande W.J., Gregersen P.K., Behrens T.W., Kapur V. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. 2003 doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooks J.J., Moutsopoulos H.M., Geis S.A., Stahl N.I., Decker J.L., Notkins A.L. Immune interferon in the circulation of patients with autoimmune disease. N. Engl. J. Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 67.Becker A.M., Dao K.H., Han B.K., Kornu R., Lakhanpal S., Mobley A.B., Li Q.Z., Lian Y., Wu T., Reimold A.M., Olsen N.J., Karp D.R., Chowdhury F.Z., Farrar J.D., Satterthwaite A.B., Mohan C., Lipsky P.E., Wakeland E.K., Davis L.S. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS One. 2013 doi: 10.1371/journal.pone.0067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rönnblom L., Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008 doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez-Carrio J., De Paz B., López P., Prado C., Alperi-López M., Javier Ballina-García F., Suárez A. IFNα serum levels are associated with endothelial progenitor cells imbalance and disease features in rheumatoid arthritis patients. PLoS One. 2014 doi: 10.1371/journal.pone.0086069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hopkins S.J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin. Exp. Immunol. 1988 doi: 10.1002/aic.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Traugott U., Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann. Neurol. 1988 doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- 72.Al-Masri A.N., Heidenreich F., Walter G.F. Interferon-induced Mx proteins in brain tissue of multiple sclerosis patients. Eur. J. Neurol. 2009 doi: 10.1111/j.1468-1331.2009.02573.x. [DOI] [PubMed] [Google Scholar]
- 73.Longhini A.L.F., von Glehn F., Brandão C.O., de Paula R.F.O., Pradella F., Moraes A.S., Farias A.S., Oliveira E.C., Quispe-Cabanillas J.G., Abreu C.H., Damasceno A., Damasceno B.P., Balashov K.E., Santos L.M.B. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J. Neuroinflammation. 2011 doi: 10.1186/1742-2094-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joshi A.D., Oak S.R., Hartigan A.J., Finn W.G., Kunkel S.L., Duffy K.E., Das A., Hogaboam C.M. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010 doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schiering C., Krausgruber T., Chomka A., Fröhlich A., Adelmann K., Wohlfert E.A., Pott J., Griseri T., Bollrath J., Hegazy A.N., Harrison O.J., Owens B.M.J., Löhning M., Belkaid Y., Fallon P.G., Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014 doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sattler S., Ling G.S., Xu D., Hussaarts L., Romaine A., Zhao H., Fossati-Jimack L., Malik T., Cook H.T., Botto M., Lau Y.L., Smits H.H., Liew F.Y., Huang F.P. IL-10-producing regulatory B cells induced by IL-33 (BregIL-33) effectively attenuate mucosal inflammatory responses in the gut. J. Autoimmun. 2014 doi: 10.1016/j.jaut.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu J., Xu Y., Zhao J., Li X., Meng X., Wang T., Zou B., Zhao P., Liu Q., Lu C., Zheng F., Liu H. IL-33 protects mice against DSS-induced chronic colitis by increasing both regulatory B cell and regulatory T cell responses as well as decreasing Th17 cell response. J. Immunol. Res. 2018 doi: 10.1155/2018/1827901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuroiwa K., Arai T., Okazaki H., Minota S., ichi Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem. Biophys. Res. Commun. 2001 doi: 10.1006/bbrc.2001.5090. [DOI] [PubMed] [Google Scholar]
- 79.Mok M.Y., Huang F.P., Ip W.K., Lo Y., Wong F.Y., Chan E.Y.T., Lam K.F., Xu D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology. 2009 doi: 10.1093/rheumatology/kep402. [DOI] [PubMed] [Google Scholar]
- 80.Yang Z., Liang Y., Xi W., Li C., Zhong R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin. Exp. Med. 2011 doi: 10.1007/s10238-010-0115-4. [DOI] [PubMed] [Google Scholar]
- 81.Roussel L., Carriere V., Lacorre D.-A., Ortega N., Americh L., Girard J.-P., Bouche G., Aguilar L. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. 2006 doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mu R., Jiang H.-R., Pitman N., Xu D., McInnes I.B., Liew F.Y., Li Y., Kurowska-Stolarska M., Fraser A.R., McKenzie A.N.J., Kewin P. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. 2008 doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiangyang Z., Lutian Y., Lin Z., Liping X., Hui S., Jing L. Increased levels of interleukin-33 associated with bone erosion and interstitial lung diseases in patients with rheumatoid arthritis. Cytokine. 2012 doi: 10.1016/j.cyto.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 84.Matsuyama Y., Okazaki H., Tamemoto H., Kimura H., Kamata Y., Nagatani K., Nagashima T., Hayakawa M., Iwamoto M., Yoshio T., Tominaga S.I., Minota S. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J. Rheumatol. 2010 doi: 10.3899/jrheum.090492. [DOI] [PubMed] [Google Scholar]
- 85.Kunisch E., Chakilam S., Gandesiri M., Kinne R.W. IL-33 regulates TNF-α dependent effects in synovial fibroblasts. Int. J. Mol. Med. 2012 doi: 10.3892/ijmm.2012.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang H.R., Milovanović M., Allan D., Niedbala W., Besnard A.G., Fukada S.Y., Alves-Filho J.C., Togbe D., Goodyear C.S., Linington C., Xu D., Lukic M.L., Liew F.Y. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur. J. Immunol. 2012 doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 87.Li M., Li Y., Liu X., Gao X., Wang Y. IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J. Neuroimmunol. 2012 doi: 10.1016/j.jneuroim.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Christophi G.P., Gruber R.C., Panos M., Christophi R.L., Jubelt B., Massa P.T. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin. Immunol. 2012 doi: 10.1016/j.clim.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liew Y., Grencis Neil E Humphreys R.K., Xu D., Hepworth M.R., Humphreys N.E., Liew F.Y., Grencis R.K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. Ref. J. Immunol. 2016 doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 90.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D.M., Bazan J.F., Kastelein R.A. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005 doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Kobori A., Yagi Y., Imaeda H., Ban H., Bamba S., Tsujikawa T., Saito Y., Fujiyama Y., Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 2010 doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 92.Balzola F., Bernstein C., Ho G.T., Lees C. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental TH1/TH2 driven enteritis: commentary. Inflamm. Bowel Dis. Monit. 2010;107(17):8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seidelin J.B., Bjerrum J.T., Coskun M., Widjaya B., Vainer B., Nielsen O.H. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol. Lett. 2010 doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Miller A.M., Xu D., Asquith D.L., Denby L., Li Y., Sattar N., Baker A.H., McInnes I.B., Liew F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008 doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sponheim J., Pollheimer J., Olsen T., Balogh J., Hammarström C., Loos T., Kasprzycka M., Sørensen D.R., Nilsen H.R., Küchler A.M., Vatn M.H., Haraldsen G. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am. J. Pathol. 2010 doi: 10.2353/ajpath.2010.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beltrán C.J., Núñez L.E., Díaz-Jiménez D., Farfan N., Candia E., Heine C., López F., González M.J., Quera R., Hermoso M.A. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010 doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- 97.Seo D.H., Che X., Kwak M.S., Kim S., Kim J.H., Ma H.W., Kim D.H., Il Kim T., Kim W.H., Kim S.W., Cheon J.H. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci. Rep. 2017 doi: 10.1038/s41598-017-00840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang R.X., Yu C.R., Dambuza I.M., Mahdi R.M., Dolinska M.B., Sergeev Y.V., Wingfield P.T., Kim S.H., Egwuagu C.E. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 2014 doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dambuza I.M., He C., Choi J.K., Yu C.R., Wang R., Mattapallil M.J., Wingfield P.T., Caspi R.R., Egwuagu C.E. IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat. Commun. 2017 doi: 10.1038/s41467-017-00838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qiu F., Song L., Yang N., Li X. Glucocorticoid downregulates expression of IL-12 family cytokines in systemic lupus erythematosus patients. Lupus. 2013 doi: 10.1177/0961203313498799. [DOI] [PubMed] [Google Scholar]
- 101.Cai Z., Wong C.K., Dong J., Chu M., Jiao D., Kam N.W., Lam C.W.K., Tam L.S. Remission of systemic lupus erythematosus disease activity with regulatory cytokine interleukin (IL)-35 in Murphy Roths Large (MRL)/lpr mice. Clin. Exp. Immunol. 2015;181:253–266. doi: 10.1111/cei.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He D., Liu M., Liu B. Interleukin-35 as a new biomarker of renal involvement in lupus nephritis patients. Tohoku J. Exp. Med. 2018 doi: 10.1620/tjem.244.263. [DOI] [PubMed] [Google Scholar]
- 103.Šenolt L., Šumová B., Jandová R., Hulejová H., Mann H., Pavelka K., Vencovský J., Filková M. Interleukin 35 synovial fluid levels are associated with disease activity of rheumatoid arthritis. PLoS One. 2015 doi: 10.1371/journal.pone.0132674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Yao L., Liu S., Wu J., Xia L., Shen H., Lu J. Elevated serum IL-35 levels in rheumatoid arthritis are associated with disease activity. J. Investig. Med. 2019 doi: 10.1136/jim-2018-000814. [DOI] [PubMed] [Google Scholar]
- 105.Ning X., Jian Z., Wang W. Low serum levels of interleukin 35 in patients with rheumatoid arthritis. Tohoku J. Exp. Med. 2015 doi: 10.1620/tjem.237.77. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y., Wang Y., Wang Y., Li Y., Li Y., Lu X., Zuo X. The possible role of the novel cytokines IL-35 and IL-37 in inflammatory bowel disease. Mediat. Inflamm. 2014;2014:1–10. doi: 10.1155/2014/136329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jafarzadeh A., Jamali M., Mahdavi R., Ebrahimi H.A., Hajghani H., Khosravimashizi A., Nemati M., Najafipour H., Sheikhi A., Mohammadi M.M., Daneshvar H. Circulating levels of interleukin-35 in patients with multiple sclerosis: evaluation of the influences of FOXP3 gene polymorphism and treatment program. J. Mol. Neurosci. 2015 doi: 10.1007/s12031-014-0443-z. [DOI] [PubMed] [Google Scholar]
- 108.Ko K.-H., Lu L., Zheng B.-J., Cao X., Yang M., Xu H., Wang S., Sun L. Cutting edge: novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J. Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 109.Saulep-Easton D., Vincent F.B., Quah P.S., Wei A., Ting S.B., Croce C.M., Tam C., MacKay F. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia. 2016 doi: 10.1038/leu.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y., Li J., Zhou N., Zhang Y., Wu M., Xu J., Shen C., An X., Shen G., Yang M., Zhang C., Tao J. The unknown aspect of BAFF: inducing IL-35 production by a CD5 + CD1d hi FcγRIIb hi regulatory B-cell subset in lupus. J. Investig. Dermatol. 2017 doi: 10.1016/j.jid.2017.07.843. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J., Roschke V., Baker K.P., Wang Z., Alarcón G.S., Fessler B.J., Bastian H., Kimberly R.P., Zhou T. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 2001 doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 112.McCarthy E.M., Lee R.Z., Gabhann J.N., Smith S.n., Cunnane G., Doran M.F., Howard D., O’Connell P., Kearns G., Jefferies C.A. Rheumatol; United Kingdom: 2013. Elevated B Lymphocyte Stimulator Levels Are Associated with Increased Damage in an Irish Systemic Lupus Erythematosus Cohort. [DOI] [PubMed] [Google Scholar]
- 113.Cheema G.S., Roschke V., Hilbert D.M., Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44(6):1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 114.Samy E., Wax S., Huard B., Hess H., Schneider P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 2017 doi: 10.1080/08830185.2016.1276903. [DOI] [PubMed] [Google Scholar]
- 115.Marín-Rosales M., Cruz A., Salazar-Camarena D.C., Santillán-López E., Espinoza-García N., Muñoz-Valle J.F., Ramírez-Dueñas M.G., Oregón-Romero E., Orozco-Barocio G., Palafox-Sánchez C.A. High BAFF expression associated with active disease in systemic lupus erythematosus and relationship with rs9514828C>T polymorphism in TNFSF13B gene. Clin. Exp. Med. 2019 doi: 10.1007/s10238-019-00549-8. [DOI] [PubMed] [Google Scholar]
- 116.Zhao L.D., Li Y., Smith M.F., Wang J.S., Zhang W., Tang F.L., Tian X.P., Wang H.Y., Zhang F.C., Ba D.N., He W., Zhang X. Expressions of BAFF/BAFF receptors and their correlation with disease activity in Chinese SLE patients. Lupus. 2010 doi: 10.1177/0961203310375268. [DOI] [PubMed] [Google Scholar]
- 117.Bosello S., Youinou P., Daridon C., Tolusso B., Bendaoud B., Pietrapertosa D., Morelli A., Ferraccioli G.F. Concentrations of BAFF correlate with autoantibody levels, clinical disease activity, and response to treatment in early rheumatoid arthritis. J. Rheumatol. 2008;1256(1264) [PubMed] [Google Scholar]
- 118.Thorn M., Lewis R.H., Mumbey-Wafula A., Kantrowitz S., Spatz L.A. BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion. Cell. Immunol. 2010 doi: 10.1016/j.cellimm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pers J.O., Daridon C., Devauchelle V., Jousse S., Saraux A., Jamin C., Youinou P. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann. N. Y. Acad. Sci. 2005 doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 120.Geng Y., li Zhang Z. Comparative study on the level of B lymphocyte stimulator (BlyS) and frequency of lymphocytes between sero-negative and sero-positive rheumatoid arthritis patients. Int. J. Rheum. Dis. 2012 doi: 10.1111/j.1756-185X.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- 121.Browning J.L., Tschopp J., Mackay F., Schneider P., Lawton P., Ambrose C., Woodcock S.A., Baetscher M. Mice transgenic for baff develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 2002;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang P., Liu X., Guo A., Xiong J., Fu Y., Zou K. B cell-activating factor as a new potential marker in inflammatory bowel disease. Dig. Dis. Sci. 2016;61:2608–2618. doi: 10.1007/s10620-016-4136-z. [DOI] [PubMed] [Google Scholar]
- 123.Fu Y., Wang L., Xie C., Zou K., Tu L., Yan W., Hou X. Comparison of non-invasive biomarkers faecal BAFF, calprotectin and FOBT in discriminating IBS from IBD and evaluation of intestinal inflammation. Sci. Rep. 2017 doi: 10.1038/s41598-017-02835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ponath G., Park C., Pitt D. The role of astrocytes in multiple sclerosis. Front. Immunol. 2018 doi: 10.3389/fimmu.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baert L., Benkhoucha M., Popa N., Ahmed M.C., Manfroi B., Boutonnat J., Sturm N., Raguenez G., Tessier M., Casez O., Marignier R., Ahmadi M., Broisat A., Ghezzi C., Rivat C., Sonrier C., Hahne M., Baeten D., Vives R.R., Lortat-Jacob H., Marche P.N., Schneider P., Lassmann H.P., Boucraut J., Lalive P.H., Huard B. A proliferation-inducing ligand–mediated anti-inflammatory response of astrocytes in multiple sclerosis. Ann. Neurol. 2019 doi: 10.1002/ana.25415. [DOI] [PubMed] [Google Scholar]
- 126.Theil D., Rosenwald A., Hohlfeld R., Wekerle H., Schrader F., Monoranu C.-M., Krumbholz M., Aloisi F., Derfuss T., Meinl E., Serafini B., Hess D.M., Kalled S.L. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thangarajh M., Gomes A., Masterman T., Hillert J., Hjelmström P. Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. J. Neuroimmunol. 2004 doi: 10.1016/j.jneuroim.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 128.Hua C., Audo R., Yeremenko N., Baeten D., Hahne M., Combe B., Morel J., Daïen C. A proliferation inducing ligand (APRIL) promotes IL-10 production and regulatory functions of human B cells. J. Autoimmun. 2016;73:64–72. doi: 10.1016/j.jaut.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 129.Tai Y.-T., Lin L., Xing L., Xing L., Wen K., Munshi N., Richardson P., Anderson K. Abstract 3832: a proliferation-inducing ligand (APRIL) directly impacts immune regulatory subsets to regulate suppressive multiple myeloma bone marrow microenvironment. Cancer Res. 2018 doi: 10.1158/1538-7445.am2018-3832. [DOI] [Google Scholar]
- 130.Planelles L., Carvalho-Pinto C.E., Hardenberg G., Smaniotto S., Savino W., Gómez-Caro R., Alvarez-Mon M., De Jong J., Eldering E., Martínez-A C., Medema J.P., Hahne M. APRIL promotes B-1 cell-associated neoplasm. Cancer Cell. 2004 doi: 10.1016/j.ccr.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 131.Fehres C.M., van Uden N.O., Yeremenko N.G., Fernandez L., Franco Salinas G., van Duivenvoorde L.M., Huard B., Morel J., Spits H., Hahne M., Baeten D.L.P. APRIL induces a novel subset of IgA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koyama T., Tsukamoto H., Miyagi Y., Himeji D., Otsuka J., Miyagawa H., Harada M., Horiuchi T. Raised serum APRIL levels in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2005 doi: 10.1136/ard.2004.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morel J., Roubille C., Planelles L., Rocha C., Fernandez L., Lukas C., Hahne M., Combe B. Serum levels of tumour necrosis factor family members a proliferation-inducing ligand (APRIL) and B lymphocyte stimulator (BLyS) are inversely correlated in systemic lupus erythematosus. Ann. Rheum. Dis. 2009 doi: 10.1136/ard.2008.090928. [DOI] [PubMed] [Google Scholar]
- 134.Salazar-Camarena D.C., Ortiz-Lazareno P.C., Cruz A., Oregon-Romero E., Machado-Contreras J.R., Muñoz-Valle J.F., Orozco-López M., Marín-Rosales M., Palafox-Sánchez C.A. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus. 2016 doi: 10.1177/0961203315608254. [DOI] [PubMed] [Google Scholar]
- 135.Moura R.A., Cascão R., Perpétuo I., Canhão H., Vieira-sousa E., Mourão A.F., Rodrigues A.M., Polido-Pereira J., Queiroz M.V., Rosário H.S., Souto-Carneiro M.M., Graca L., Fonseca J.E. Cytokine pattern in very early rheumatoid arthritis favours B-cell activation and survival. Rheumatology. 2011 doi: 10.1093/rheumatology/keq338. [DOI] [PubMed] [Google Scholar]
- 136.Gabay C., Krenn V., Bosshard C., Seemayer C.A., Chizzolini C., Huard B. Synovial tissues concentrate secreted APRIL. Arthritis Res. Ther. 2009 doi: 10.1186/ar2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weldon A.J., Moldovan I., Cabling M.G., Hernandez E.A., Hsu S., Gonzalez J., Parra A., Benitez A., Daoud N., Colburn K., Payne K.J. Surface April is elevated on myeloid cells and is associated with disease activity in patients with rheumatoid arthritis. J. Rheumatol. 2015 doi: 10.3899/jrheum.140630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nagatani K., Itoh K., Nakajima K., Kuroki H., Katsuragawa Y., Mochizuki M., Aotsuka S., Mimori A. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by APRIL. Arthritis Rheum. 2007 doi: 10.1002/art.22929. [DOI] [PubMed] [Google Scholar]
- 139.Thangarajh M., Masterman T., Hillert J., Moerk S., Jonsson R. A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand. J. Immunol. 2007 doi: 10.1111/j.1365-3083.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 140.Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 141.Li X., Mai J., Virtue A., Yin Y., Gong R., Sha X., Gutchigian S., Frisch A., Hodge I., Jiang X., Wang H., Yang X.F. IL-35 is a novel responsive anti-inflammatory cytokine - a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012 doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ye C., Brand D., Zheng S.G. Targeting IL-2: an unexpected effect in treating immunological diseases. Signal Transduct. Target. Ther. 2018 doi: 10.1038/s41392-017-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park M.J., Lee S.H., Kim E.K., Lee E.J., Park S.H., Kwok S.K., La Cho M. Myeloid-derived suppressor cells induce the expansion of regulatory B cells and ameliorate autoimmunity in the sanroque mouse model of systemic lupus erythematosus. Arthritis Rheum. 2016 doi: 10.1002/art.39767. [DOI] [PubMed] [Google Scholar]
- 144.Cop N., Ebo D.G., Bridts C.H., Elst J., Hagendorens M.M., Mertens C., Faber M.A., De Clerck L.S., Sabato V. Influence of IL-6, IL-33, and TNF-α on human mast cell activation: lessons from single cell analysis by flow cytometry. Cytom. B Clin. Cytom. 2018 doi: 10.1002/cyto.b.21547. [DOI] [PubMed] [Google Scholar]
- 145.Rawlings D.J., Metzler G., Wray-Dutra M., Jackson S.W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017 doi: 10.1038/nri.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karnell J.L., Rieder S.A., Ettinger R., Kolbeck R. Targeting the CD40-CD40L pathway in autoimmune diseases: humoral immunity and beyond. Adv. Drug Deliv. Rev. 2019 doi: 10.1016/j.addr.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 147.Kaltenmeier C., Gawanbacht A., Beyer T., Lindner S., Trzaska T., van der Merwe J.A., Härter G., Grüner B., Fabricius D., Lotfi R., Schwarz K., Schütz C., Hönig M., Schulz A., Kern P., Bommer M., Schrezenmeier H., Kirchhoff F., Jahrsdörfer B. CD4+ T cell-derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J. Immunol. 2015;194:3768–3777. doi: 10.4049/jimmunol.1402568. [DOI] [PubMed] [Google Scholar]
- 148.Yang M., Sun L., Wang S., Ko K.-H., Xu H., Zheng B.-J., Cao X., Lu L. Cutting edge: novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J. Immunol. 2010 doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 149.Hua C., Audo R., Yeremenko N., Baeten D., Hahne M., Combe B., Morel J., Daïen C. A proliferation inducing ligand (APRIL) promotes IL-10 production and regulatory functions of human B cells. J. Autoimmun. 2016 doi: 10.1016/j.jaut.2016.06.002. [DOI] [PubMed] [Google Scholar]