Abstract
Systemic lupus erythematosus (SLE) is the prototypical autoimmune disease that can affect any organ of the body. Multiple mechanisms may contribute to the pathophysiology of systemic lupus, including failure to remove apoptotic bodies, hyperactivity of self-reactive B and T lymphocytes, abnormal exposure to autoantigens, and increased levels of B-cell stimulatory cytokines. The involvement of the kidney, called lupus nephritis (LN), during the course of the disease affects between 30% and 60% of adult SLE patients, and up to 70% of children. LN is an immune-mediated glomerulonephritis that is a common and serious finding in patients with SLE. Nowadays, renal biopsy is considered the gold standard for classifying LN, besides its degree of activity or chronicity. Nevertheless, renal biopsy lacks the ability to predict which patients will respond to immunosuppressive therapy and is a costly and risky procedure that is not practical in the monitoring of LN because serial repetitions would be necessary. Consequently, many serum and urinary biomarkers have been studied in SLE patients for the complementary study of LN, existing conventional biomarkers like proteinuria, protein/creatinine ratio in spot urine, 24 h urine proteinuria, creatinine clearance, among others and non-conventional biomarkers, like Monocyte chemoattractant protein-1 (MCP-1), have been correlated with the histological findings of the different types of LN. In this article, we review the advances in lupus nephritis urinary biomarkers. Such markers ideally should be capable of predicting early sub-clinical flares and could be used to follow response to therapy. In addition, some of these markers have been found to be involved in the pathogenesis of lupus nephritis.
Keywords: Lupus nephritis, Urinary biomarkers, Systemic lupus erythematosus, Lupus biomarkers, Non-invasive biomarkers, Renal injury, Urine analyte, Proteomics, Chemokine, Cytokines, Adhesion molecules, Protein biomarkers
Highlights
-
•
Cytokines, chemokines, complement proteins, adhesion molecules have been the most studied urinary biomarkers for LN.
-
•
Ideal biomarker should be capable of predicting early sub-clinical flares and could be used to follow response to therapy.
-
•
TWEAK and MCP1 are molecules that have the best evidence to use as urinary biomarkers.
-
•
Ceruloplasmin, STAT-1, HMGB-1 and Osteoprotegerin need more studies to determinate its role like a good urinary biomarker.
-
•
A single biomarker is probable not strong enough for clinical follow-up.
1. Systemic lupus erythematosus and lupus nephritis
Systemic lupus erythematosus (SLE) is the prototypical autoimmune disease that can affect any organ of the body. The involvement of the kidney, called lupus nephritis (LN), during the course of the disease affects between 30% and 60% of adult SLE patients, and up to 70% of children. Hence, the kidney is one of the main organs affected by SLE and the cause of the greater burden of morbidity and lower survival in these patients [1].
Despite management guidelines and established immunosuppressive treatments, approximately 10%–20% of SLE patients will progress to end-stage renal disease within 5 years after diagnosis, whereas 40% of patients with LN III-V will develop some degree of chronic kidney disease (CKD)[2].
Renal biopsy is considered the gold standard for classifying and determine the degree of activity or chronicity of LN. It can serve as a guide for immunosuppressive therapy selection. However, the current LN classification system that is based on renal biopsy is difficult to reproduce, resulting in doubts about its clinical application [3]. Furthermore, the current classification of LN does not include certain histological findings—thrombotic microangiopathy, vasculitic lesions, and lupus vasculopathy—that can modify the management decision and be associated with a worse renal prognosis. In addition to this limitation, renal biopsy lacks the ability to predict which patients will respond to immunosuppressive therapy and is a costly and risky procedure that is not practical in the monitoring of LN because serial repetitions would be necessary [4].
Consequently, many serum and urinary biomarkers have been studied in SLE patients for the complementary study of LN. Thus, conventional biomarkers for the determination of LN—proteinuria, protein/creatinine ratio in spot urine, 24 h urine proteinuria, creatinine clearance, double-chain anti-DNA levels, and serum complement, among others—have been extensively researched in LN. Nevertheless, these biomarkers may not be useful enough to appropriately predict relapse, accurately monitor response to treatment, or identify the degree of disease activity and chronic damage. Also, non-conventional biomarkers have been correlated with the histological findings of the different types of LN. For this reason, the investigation of new potential biomarkers that overcome these obstacles are clearly necessary [5].
2. Biomarker definition
A biomarker refers to a biological, genetic or chemical, characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathological processes, or pharmacological responses to a therapeutic intervention [6]. Biomarkers can be: 1) prognostic, identifying individuals at risk of developing the disease or those likely to experience an outbreak; 2) diagnostic, confirming the presence or subtype of a disease; 3) predictive of the response to treatment; 4) pharmacodynamic, determining the optimal therapeutic doses; and 5) surrogate endpoint, which is defined as a biomarker intended to substitute for a clinical endpoint, the latter being a characteristic or variable that reflects how a patient feels, functions, or survives [7].
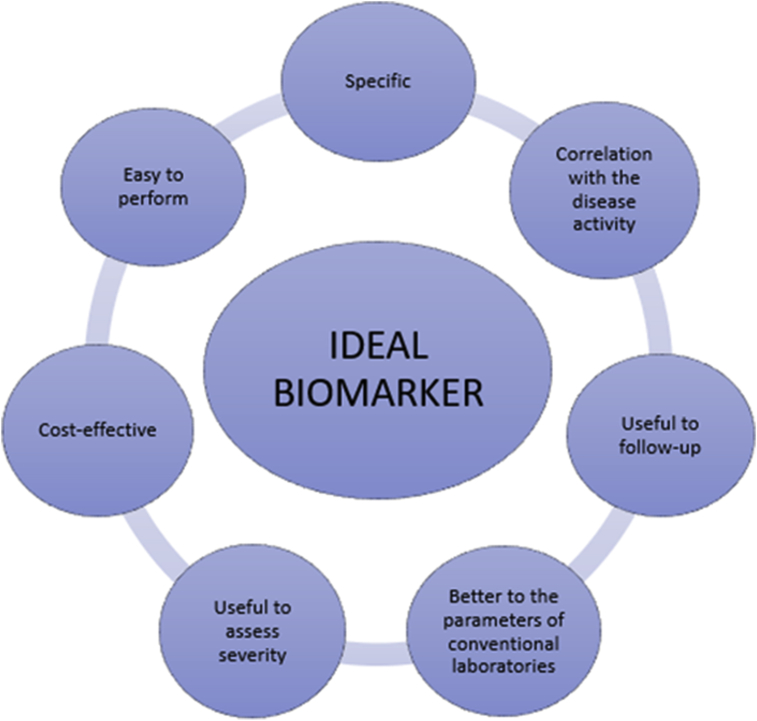
The ideal biomarker in SLE patients with suspicion or confirmation of LN should have the following properties: 1) be specific for renal involvement, 2) have a good correlation with kidney activity or damage, 3) be useful for serial monitoring, 4) be superior to conventional clinical or laboratory parameters, 5) possess the ability to assess the severity of renal involvement, 6) be cost-effective, and 7) easy to perform and available in most clinical laboratories [8]. Fig. 1 summarizes these characteristics.
Fig. 1.
Characteristics of an ideal biomarker.
3. Urinary biomarkers
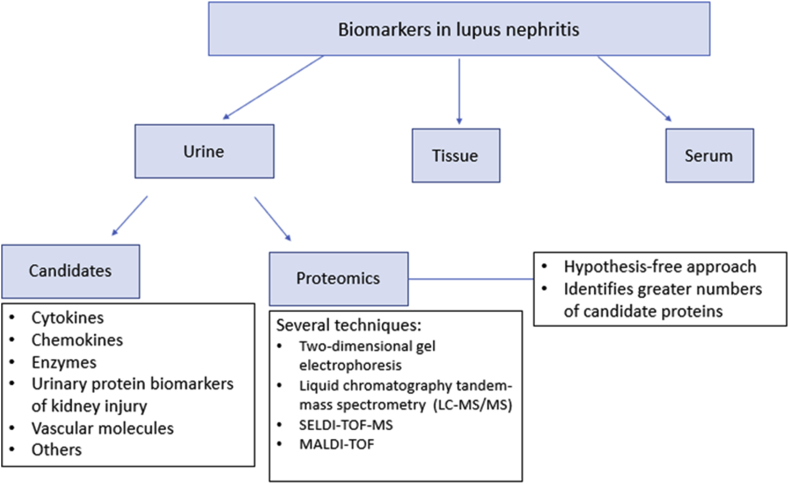
Unlike other sample sources (serum or tissue), obtaining urine is not an invasive procedure, and it can be easily collected, allowing for sequential sampling. In the case of LN, the urine is physically close to the site of activity of the disease, being an interesting sample for monitoring of patients with LN [9]. Therefore, proteins, such as cytokines, chemokines, complement proteins, adhesion molecules, and autoantibodies, have been identified as possible biomarkers of disease activity in cross-sectional studies. In most of the studies, this disease activity is measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), which is an index that allows identify the level of systemic activity the patient [classifying the patient as follows: no activity (SLEDAI = 0), mild activity (SLEDAI = 1–5), moderate activity (SLEDAI = 6–10), high activity (SLEDAI = 11–19), very high activity (SLEDAI = 20)], and thus correlate it with the urinary biomarker studied [10]. However, few candidate biomarkers have been validated in longitudinal cohorts, and none have been used successfully even in the context of a clinical trial (see Fig. 2). Table 1, Table 2 summarize the most important biomarkers and the types of clinical study designs in which they have been described.
Fig. 2.
Approach for the assessment of urinary biomarkers in patients with lupus nephritis.
Table 1.
Urinary biomarkers in Lupus Nephritis.
| Biomarker | Specificity for renal involvement in SLE patients | Correlation with renal disease activity | Correlation with renal damage | Predictor of LN flares | Decrease after treatment | Main Detection method | Correlation with renal histology |
|---|---|---|---|---|---|---|---|
| Cytokines | |||||||
| TWEAK | + | + | ns | + | + | EIA | ns |
| IL-17 | + | + | + | ns | ns | EIA | + (class III/IV nephritis) |
| IL-6 | + | + | ns | ns | + | EIA | + (class IV nephritis) |
| Adiponectin | + | + | ns | + | ns | EIA | ns |
| TGF-β | + | + | – | ns | + | PCR/mRNA | + (class IV nephritis) |
| Osteoprotegerin | + | + | ns | ns | ns | EIA | ns |
| TNFR1 | + | + | ns | ns | ns | EIA | ns |
| Chemokines | |||||||
| MCP-1 | + | + | ns | + | + | EIA | + (class III/IV nephritis) |
| CCL5/RANTES | + | + | – | + | ns | PCR/mRNA | – |
| CXCL10/IP10 | + | + | ns | ns | ns | PCR/mRNA | + (class IV nephritis) |
| CXCL16 | + | + | – | ns | – | EIA | + (class IV nephritis) |
| IL-8 | + | + | ns | ns | + | EIA | ns |
| Urinary enzymes | |||||||
| NGAL | + | + | ns | + | + | EIA | ns |
| NAG | + | + | ns | ns | + | EIA | ns |
| Injury proteins | |||||||
| KIM-1 | + | + | + | ns | ns | EIA | ns |
| HMGB1 | + | + | ns | ns | ns | EIA | ns |
| Vascular molecules | |||||||
| Angiostatin | + | + | + | ns | ns | EIA | ns |
| VCAM – ICAM | + | + | + | – | ns | EIA | + (class III/IV nephritis) |
| Endothelin | + | + | ns | ns | + | EIA | ns |
| VEGF | + | + | ns | ns | ns | EIA | ns |
| Others biomarkers | |||||||
| FOXP 3 | + | + | ns | + | + | PCR/mRNA | ns |
| Ceruloplasmin | + | + | ns | ns | ns | EIA | ns |
| Transferrin | + | + | ns | + | + | EIA | ns |
| STAT-1 | + (biopsies) | + | ns | ns | ns | IHC | ns |
| Th1/Th2 Factors | + (Th1) | + (Th1) | ns | ns | ns | PCR/EIA | ns |
| LTh1 CD4 + CXCR3 + | + | + | ns | ns | ns | FCT | ns |
TWEAK: TNF-like weak inducer of apoptosis; TGF-β: transforming growth factor beta; TNFR1: Tumor necrosis factor receptor 1; MCP-1: monocyte chemoattractant protein-1; RANTES: Regulated upon Activation, Normal T cell Expressed, and Secreted; IP-10: Interferon gamma-induced protein 10; NGAL: neutrophil gelatinase-associated lipocallin; NAG: N-Acetyl-β-D Glucosaminidase; KIM-1: Urinary kidney injury molecule-1; HMGB1: High mobility group box 1; VCAM: vascular cell adhesion molecule 1; ICAM: Intercellular Adhesion Molecule 1; VEGF: Vascular Endothelial Growth Factor; FOXP3: forkhead box protein P3; STAT-1: Signal transducer and activator of transcription 1; Th1: T helper 1; Th2: T helper 2; EIA: Enzyme immunoassay; PCR: Polymerase chain reaction; mRNA: Messenger ribonucleic acid; IHC: Immunohistochemistry; CT: Flow cytometry. (+) indicates a positive and statistically significant relationship documented in at least one study; (−) indicate that there is no statistically significant relationship; “ns” denotes “not studied”. It must be taken into account that, for several molecules, the data are only available in 1–2 studies, and these findings should be updated as more validation tests are carried out.
Table 2.
Different urinary biomarkers in relation to the activity of lupus nephritis.
| Biomarkers that correlate with activity in longitudinal studies | Biomarkers that correlate with activity in cross-sectional studies | Biomarkers that correlate with prognosis |
|---|---|---|
| MCP-1 | Urine Osteoprotegerin | MCP-1 |
| NGAL | Urine CXCR3⁺-CD4⁺ T cells | VEGF |
| TWEAK | Urine FoxP3 mRNA expression | STAT-1 |
| Urine endothelin-1 | IP-10 | |
| VCAM-1 | CXCR3 | |
| TGF-β | TGF-β |
AbbreviationsMCP-1: monocyte chemoattractant protein-1; NGAL: neutrophil gelatinase-associated lipocallin; TWEAK: TNF-like weak inducer of apoptosis; FOXP3: forkhead box protein P3; VCAM: vascular cell adhesion molecule 1; TGF-β: transforming growth factor beta; VEGF: Vascular Endothelial Growth Factor;; STAT-1: Signal transducer and activator of transcription; IP-10: Interferon gamma-induced protein 10.
We will present an update of the main urinary biomarkers, grouped by their biological characteristics or functions, which until now have demonstrated a potential role in LN. Additionally, we will describe the techniques used to identify them and their validation in clinical practice. It should be emphasized that none of the biomarkers described below have an approval for commercial use in clinical practice.
3.1. Urinary cytokines
Several cytokines that are involved in the adaptive and innate immune responses, and potentially play a role in the pathophysiology of LN, have been studied. We will summarize the cytokines most described in the literature.
3.1.1. Urinary TNF-like weak inducer of apoptosis (TWEAK)
This cytokine is a part of the tumor necrosis factor (TNF) superfamily [11]. Its mRNA is expressed in a large number of tissues, primarily the kidneys, lungs, and placenta, and also in active monocytes and macrophages, which are the cells most closely related to its production [12]. One of the most relevant characteristics of TWEAK is the pro-apoptotic function in several tumor lines, such as KYM-1 (rhabdomyosarcoma), HSC3 (squamous cell carcinoma), HT-29 (colon cancer)[13,14], and proinflammatory properties in fibroblasts and synoviocytes [15]. In relation to SLE and LN, the main receptor of TWEAK, Fibroblast growth factor-inducible 14 (Fn14), has been observed as expressed in mesangial cells, podocytes, and tubular cells. Moreover, TWEAK stimulates the expression of a large number of proinflammatory mediators, such as Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES), Monocyte chemoattractant protein-1 (MCP-1), Interferon gamma-induced protein-10 (IP-10), Matrix metalloproteinase-1 (MMP-1), MMP-9, vascular cell adhesion molecule-1 (VCAM-1), Intercellular Adhesion Molecule-1 (ICAM-1), and macrophage inflammatory protein-1 alpha (MIP-1a), conferring it as having an important role in the pathogenesis of the disease [16].
For the above mentioned, the measurement of this cytokine in the urine is interesting as a potential biomarker of LN. SLE patients with active kidney disease have significantly higher urinary TWEAK levels than SLE patients without active renal disease. These levels correlate very significantly with Systemic Lupus Erythematosus Disease Activity Index renal domain (SLEDAI-R) > 4[17]. Interestingly, urinary levels of TWEAK are specifically characteristic of LN, showing substantially elevated levels in nephritis compared with other systemic manifestations. More strikingly, the levels of this cytokine rise at the beginning of the renal flare, obtain its peak at the time of diagnosis, and progressively decrease after the resolution of the flare [18], suggesting that the monitoring of TWEAK levels may be useful for predicting flares or the response to these [19]. Besides, it has been described that TWEAK is also significantly related to anti-dsDNA levels and has a high specificity for the proteinuria presence, notably together with the MCP-1 levels (explained below)[20].
3.1.2. Urinary interleukin (IL-17)
Interleukin (IL)-17 is a transmembrane protein of 17 kDa. It has a remarkable proinflammatory capacity, and through its effects on the recruitment of monocytes and neutrophils, it increases the local production of chemokines, such as MCP-1, IL-8, among others [21]. Although it is mostly produced by CD3⁺ Th17 lymphocytes, IL-17 can be produced by a large number of cells, such as CD4⁺ T cells, CD8⁺ T cells, γδ T cells, NK cells, and neutrophils [22]. This IL plays an important role in the immune response against bacteria, such as Klebsiella pneumoniae, and fungi, such as Candida albicans [23]. Further, IL-17 has also been described in several autoimmune diseases, such as rheumatoid arthritis [24], systemic sclerosis [25], inflammatory bowel disease [26], and SLE, particularly in cutaneous involvement and serositis [27].
Regarding LN, it is known that IL-17 is associated with renal injury and plays a role in the pathogenesis of SLE [28]. The levels of this cytokine are characteristically elevated in the urine, to a greater extent in patients with severe renal damage. This correlates with the histological findings of class III/IV LN. Likewise, its diagnostic capacity is adequate, with a high area under the curve (AUC 0.7)[29]. Another study found elevated expression of IL-17 in the urinary sediment of patients with LN. These levels correlated with an active SLEDAI [30]. On the other hand, a more recent study reported high urinary levels of IL-17 in patients with LN, as well as a correlation with lymphocyte count, proteinuria, erythrocyte sedimentation rate, and blood urine nitrogen. The diagnostic capacity of IL-17 in urine and Th-17 in serum were evaluated and found to be highly sensitive and very specific, respectively [31]. Despite the evidence of the role of this protein in LN, more studies are still needed.
3.1.3. Urinary Interleukin-6
IL-6 is a pleiotropic cytokine produced by monocytes, T and B lymphocytes, fibroblasts, endothelial cells, and mesangial cells [32]. This signaling molecule is related to multiple functions, such as the production of plasma cells, differentiation and growth of T cells, megakaryocyte maturation, osteoclast development, synthesis of acute phase proteins in hepatocytes, and growth factor in myeloma/plasmacytoma [33]. Additionally, it is associated with other types of neoplasms, for example, colorectal cancer [34].
The IL-6 production in mesangial cells, tubuloepithelial cells, endothelial cells, and podocytes, have linked this cytokine to CKD, acute kidney disease (AKD), IgA nephropathy, diabetic nephropathy (DN), and LN [35]. Regarding LN, the involvement of IL-6 in the pathogenesis of this disease and the production of anti-dsDNA has been demonstrated in murine models [36]. One study reported the correlation between high urinary levels of IL-6 with histological class IV and its decrease in the urine levels after treatment [37]. Tsai et al. found significantly elevated levels of IL-6 in the urinary sediment in patients with active LN and also described a decrease of this cytokine in conjunction with IL-8 after the 6-month therapy with cyclophosphamide, raising the possible utility of the measurement of this molecule to monitor response to treatment [38].
However, a research that was conducted in 2006 and included 143 SLE patients did not find a significant difference in the urinary levels of IL-6 in patients with LN compared with patients without LN. This study has the largest number of patients compared with previous publications, causing some controversy about the clinical usefulness of this cytokine.
3.1.4. Urinary Adiponectin
Adiponectin is produced almost entirely by adipocytes and performs various homeostatic functions, mostly in immunomodulation [39,40]. It is known that adiponectin participates in insulin sensitivity, and the serum levels of this molecule are diminished in pathologies, such as type II diabetes mellitus and obesity [41]. Additionally, it has been linked as a protective factor in osteoarthritis and atherosclerosis [42]. In spite of the above, adiponectin has proinflammatory activity [43].
The Ohio SLE Study identified urinary levels of this cytokine in SLE patients with renal flare compared with SLE patients without renal involvement, describing that adiponectin levels began to increase 2 months before renal manifestations [44]. Moreover, in the same study, a correlation was found between proteinuria and an increase in serum creatinine. A more recent paper measured the adiponectin ability, along with other molecules, to correlate with activity index in kidney biopsies and finding positive results [45]. They also described that adiponectin has a high AUC to determine the activity of the LN. Furthermore, pediatric studies have reported a correlation between adiponectin and albuminuria [46].
3.1.5. Transforming growth factor-beta
Transforming growth factor-beta (TGF-β) is a dimeric peptide with a wide spectrum of physiological activities, such as morphogenic functions, modulation of the immune system, regulation of tissue remodeling, and wound repair [47]. It is a key factor in the inflammatory response, participating both in the early response through the recruitment of monocytes and in the repair phase, favoring the production of angiogenic factors and fibrosis [48,49]. As mentioned, TGF-β is expressed in repair processes and has been observed to induce the production of collagen types I, III, IV, and V in cell cultures. It also increases the transcription of collagen genes types I and IV [50,51]. For this reason, TGF-β has been related to several pathological processes, performing important roles in the pathogenesis of hepatic fibrosis [52], remodeling in respiratory pathologies, such as Chronic Obstructive Pulmonary Disease and asthma [53], and its participation in renal fibrosis [54], regulating the accumulation of proteins and other components in the extracellular matrix [49,54].
TGF-β is expressed in the glomerulus [55] and in the renal interstitium during inflammation, where it decreases the production of MCP-1 in proximal tubular epithelial cells [56] and promotes the production of Vascular Endothelial Growth Factor (VEGF) and IL-8, causing greater recruitment of proinflammatory cells and proteinuria due to increased vascular permeability [57]. The three isoforms of this cytokine (TBRI, II, and III) have been associated with renal pathologies because of their expression in the glomerulus and interstitium of patients with IgA nephropathy, focal and segmental glomerulosclerosis, membranoproliferative glomerulonephritis, ANCA glomerulonephritis, and LN [58].
Studies have been conducted on the expression of TGF-β in patients with LN and its association with the disease activity. Avihingsanon et al. demonstrated that the measurement of mRNA levels of TGF-β in urine by real-time PCR is associated with a high level of specificity to class IV LN [59]. In addition, they reported that TGF-β levels were reduced after treatment but remained high in patients who did not respond to immunosuppressive therapy [59]. An Indian series described a correlation between TGF-β urine levels with the disease activity and the biopsy activity indexes (BAIs), but no correlation with the chronicity indexes [60]. In pediatric patients, it has been observed that urinary TGF-β levels are significantly higher in those with active LN and are positively associated with anti-dsDNA titers [61]. Interestingly, the plasma levels of TGF-β in these pediatric patients were decreased compared with the levels in the urine. Finally, recent research described a relationship between high levels of TGF-β and severe LN. In the same study, the diagnostic capacity of this cytokine was measured together with neutrophil gelatinase-associated lipocalin (NGAL), IL-17, and MCP-1, and it was found that TGF-β was the weakest in the group. However, AUC was greater than 50% [62].
3.1.6. Osteoprotegerin
Osteoprotegerin (OPG) belongs to the superfamily of TNF receptors whose main function is the regulation of bone resorption, by decreasing osteoclastogenesis [63]. This protein is expressed in various body tissues including the lung, kidney, liver, cardiovascular system, and in immune tissues, such as the spleen, bone marrow, thymus, and to a large extent B lymphocytes [63,64]. For several years it has been known that the OPG regulation is linked to different cytokines, such as IL-1α, IL-1β, IL-6, IL-11, IL-17, TNF-α, hormones such as PTH, and other substances such as PGE2[65].
Elevated levels of OPG have been associated with CDK, polycystic kidney disease, IgA nephropathy, and increased cardiovascular risk because of its probable relationship with arteriosclerosis [66]. The kidney plays an important role in the excretion of this protein; thus, one hypothesis proposes that OPG concentration can be increased in active LN because of the rise in production and excretion of this molecule by the endothelial cells of the renal microvasculature [67]. In several reports, elevated urinary OPG levels correlated significantly with active nephritis, specifically in the presence of hematuria, elevated proteinuria/creatinuria index, and anti-dsDNA [67]. One study found an association between elevated urinary levels of OPG and rSLEDAI. Also, the diagnostic capacity of OPG markedly increased with the measured in conjunction with TWEAK, MCP-1, and IL-8[68]. In addition, it was found that urinary measurement of this cytokine had a better AUC to detect renal active disease compared with conventional serum markers, such as C3, C4, and anti-dsDNA [69].
3.1.7. Urinary Tumor necrosis factor receptor 1
Tumor necrosis factor receptor (TNFR) is part of the superfamily of TNF receptors which regulate the signaling of survival, proliferation, differentiation, and action of the immune and non-immune system cells [70]. This molecule is a pleiotropic receptor with the main function of activating the signaling pathways in apoptotic processes through the caspase pathway and programmed cell necrosis [71]. This protein is expressed in the peritubular capillaries in the endothelium of the kidney, and its elevated serum levels have been linked to the progressive deterioration of renal function [72]. Additionally, TNFR-1 is expressed in the glomerulus and distal and collecting tubules. High urinary levels correlate significantly in patients with active LN (according to elevated proteinuria/creatinuria index and SLEDAI)[73]. Although the usefulness of TNFR-1 as a urinary biomarker for LN has been confirmed, it has only been described in one study, which is why more evidence is required to support this finding.
3.2. Urinary chemokines
The main functions of chemokines are the regulation of cellular traffic, as well as selectively recruiting monocytes, neutrophils, and lymphocytes that are involved in the inflammatory response [74]. Because of their relevant role in the immune response, chemokines have been evaluated as potential biomarkers of LN.
3.2.1. Monocyte chemoattractant protein-1 (MCP-1)
MCP-1 has been one of the most investigated urinary biomarkers for LN. MCP-1 belongs to the CC-chemokine family, is encoded on chromosome 17, and is composed of 76 amino acids [75]. In the kidney, it is produced by mesangial, tubular, epithelial cells, and in smooth muscle [76]. It is mainly expressed by monocytes, activated macrophages, T cells, and NK cells [74].
MCP-1 levels are significantly higher in patients with LN compared with healthy controls [77]. In experimental murine models, MCP-1 participates in the pathogenesis of tubulointerstitial damage, recruiting monocytes, and producing fibrosis in the interstitium [78]. This elevation in urinary concentration has also been related to the decrease in renal function [79]. Moreover, this cytokine has been associated with disease activity and evaluated with tools, such as rSLEDAI, suggesting that high levels of MCP-1 indicate active LN, chiefly in classes III and IV nephritis [80]. In fact, MCP-1 levels dramatically increase in renal exacerbation and decrease in conjunction with the resolution of this condition [81]. Furthermore, urinary levels may increase between 2 and 4 months before renal relapse and may be an interesting predictive factor [81].
Despite the well-known correlation between MCP-1 function and LN, high urinary levels of this protein are not exclusive for LN. For instance, it has been observed that MCP-1 is an important promoter of inflammation, renal injury, and fibrosis in DN [82]. It has also been associated with IgA nephropathy, membranous glomerulonephritis, focal and segmental glomerulosclerosis, polycystic kidney disease, and renal allograft rejection [83]. All the same, recent studies have shown that urinary measurement of MCP-1, in combination with TWEAK (described above), correlated more precisely with BAI and the rSLEDAI, being even more accurate than the measurement of C3 and C4 in serum [84].
Currently, therapeutic alternatives using MCP-1 antagonist molecules are being explored in murine models, observing a decrease in the incidence and progression of renal lesions in LN [85].
3.2.2. Chemokine ligand 5/RANTES
RANTES is a beta-chemokine that promotes the attraction and transit of T lymphocytes and plays an important role in the recruitment of leukocytes to the area of inflammation [86]. This molecule has been observed as expressed in the kidney of murine models [87], in relation to kidney injury processes, such as ischemia, and rejection of kidney transplantation [88]. Additionally, elevated serum levels of circulating RANTES have been described in SLE patients[89], and it has been found that the expression of RANTES mRNA in the urinary sediment is elevated in patients with active LN.
One study reported that RANTES correlates with proteinuria and SLEDAI levels, but it is not a good predictor of renal flare and has no correlation with the activity index, chronicity index, or with any histopathological characteristics [90]. In contrast to a subsequent study including a larger number of patients who were followed for approximately 24 months, it was found that persistently high levels of RANTES and macrophage colony stimulating factor (M-CSF) measured by ELISA, were predictors of renal flare [91]. Lastly, a more recent research using ELISA measured urinary CCL2, CXCL10, RANTES, CCL2, CCL4, and IL-10 and concluded that the first three had a good ability to detect disease activity; however, CCL2 and CXCL10 were better than RANTES [92].
3.2.3. Interferon gamma-induced protein 10 or C-x-C motif chemokine 10
Interferon gamma-induced protein 10 (IP-10) or C-X-C motif chemokine 10 (CXCL10) is part of the CXC chemokine family. The chemokines of this family have proinflammatory properties and act as modulators of angiogenesis in conditions of scarring, ischemia, and neoplasia. For this reason, they share specific receptors on leukocytes and endothelial cells [93]. Therefore, CXC chemokines play an essential role in the control of homeostatic and inflammatory basal movement of leukocytes and have strong antiangiogenic activity [94]. IP-10 is inducible by IFN-γ, TNF-α, viruses, and microbial components directly or indirectly by the activation of the NF-kB pathway [93]. It has been proposed that this chemokine is also involved in the recruitment and enhancement of Th1 cells and is also involved in the pathogenesis of different types of diseases, such as certain infectious, inflammatory, and autoimmune diseases including multiple sclerosis, Grave’s disease, autoimmune thyroiditis, type I diabetes mellitus, and SLE93 95.
Serum levels of CXCL10 in SLE patients are significantly elevated and correlate with the SLEDAI. Additionally, the expression of CXCL10 mRNA and its receptor CXCR3 in urine is related to patients with LN and is undetectable in control patients [96]. Moreover, in a cross-sectional study by Marie et al., a statistically significant positive correlation of CXCL10 was found with the level of proteins excreted in the urine in 24 h, SLEDAI, activity index, and the LN class in renal biopsy [95]. The aforementioned refers to the fact that the CXCL10 elevated levels in urine are related to LN, in SLE patients with high SLEDAI in general and particularly with renal involvement. Also, this chemokine has a remarkable sensitivity (100%) and specificity (98%) when levels are positive (≥93 pg/dL)[95].
3.2.4. C-X-C motif chemokine 16
CXCL16 is a cytokine expressed in dendritic cells and macrophages, which participates in the recruitment of inflammatory cells, such as NK cells and T lymphocytes, and the intracellular adhesion is mediated by interactions with CXC 6 receptor (CXCR6) [97,98]. Despite its role as an inflammatory chemokine, CXCL16 is described in the development of atherosclerosis, phagocytosis of gram-positive and gram-negative bacteria, and as an inducer of NF-kB activation [[99], [100], [101]]. CXCL16 has been postulated as a chemokine implicated in the pathophysiology of LN since several studies have reported its major expression in the urine of patients with LN compared with SLE patients without LN [102].
One of the first groups to postulate this chemokine as a biomarker of renal involvement in SLE was Teramoto et al. They used microarray techniques to analyze the mRNA and found that in murine kidneys with LN, the expression of several chemokines was increased—including CXCL16 and its receptor CXCR6—involving them as recruiters of T lymphocytes polarized to the Th1 profile [103]. Taking this into account, in recent years, the role of CXCL16 as a urinary biomarker has been studied. It has been found that its expression is associated with higher rates of disease activity (elevated SLEDAI, high values of 24 h proteinuria [104], and high anti-DNA titers [105]). Also, CXCL16 is histologically correlated with class IV LN and with higher activity indexes, whereas it does not correlate with the chronicity indexes [106].
3.2.5. Interleukin-8
IL-8 belongs to the family of proinflammatory chemokines. Its synthesis is carried out in fibroblasts, endothelial cells, monocytes, macrophages, and dendritic cells. It is a potent neutrophil chemotactic factor, regulates the production of adhesion proteins, and amplifies the local inflammatory response [107].
Wada et al. were the first to study the role of this cytokine as a urinary biomarker for LN. They measured IL-8 levels in patients with polycausal glomerular disease and found that 11/15 patients with LN had increased IL-8 levels in the urine [108]. To date, few studies have evaluated the effectiveness of IL-8 as a urinary biomarker. However, polymorphisms of this cytokine have been studied as a risk factor for the development of severe LN because the polymorphism of IL-8-845C predisposes African–American patients to a higher risk of severe LN [109]. Similarly, it is known that the levels of IL-8 in urine are higher in patients with active LN compared with inactive LN, and these values decrease after the administration of pharmacological therapy, such as cyclophosphamide [38]. Although this chemokine has evidence as a biomarker of LN activity, more studies are still required to postulate IL-8 as a useful biomarker in clinical practice.
3.3. Urinary enzymes
3.3.1. Neutrophil gelatinase-associated lipocalin (NGAL)
NGAL is a 25 kDa molecule that belongs to the lipocalins superfamily. It is highly expressed in activated leukocytes and tubular epithelial cells [110]. Increased levels of this molecule have been reported in various renal diseases, such as AKD, after cardiovascular surgery in the pediatric population, renal failure after transplantation, hemolytic uremic syndrome, contrast-induced nephropathy [111], cisplatin-induced nephrotoxicity [112], and CKD [113]. With regards to LN, urinary levels of NGAL are significantly high in SLE patients with active renal disease (rSLEDAI > 4), unlike in patients with SLE without renal manifestations [114]. A significant increase in the uNGAL1/creatinuria ratio (up to 104%) can be detected up to 3 months before the LN exacerbation.
Interestingly, some studies have reported that urinary levels of NGAL increase very early during renal flare, raising the possibility of its use as an early biomarker of LN [77]. It has been seen that urinary levels of NGAL in patients with LN decrease after the administration of immunosuppressants, such as cyclosporine and mycophenolate mofetil. During follow-up at 6 months in these patients, NGAL level decreases significantly with a complete response, thereby showing even greater utility than conventional markers, such as proteinuria [115]. Additionally, high urinary levels of NGAL in conjunction with kidney injury molecule–1 (KIM-1) (described below) have been considered as risk factors for poor renal prognosis [77].
3.3.2. N-acetyl-b-D-glucosaminidase (NAG)
NAG is a hydrolase-class enzyme, found abundantly in the lysosomes of cells located in the proximal tubules [116]. Normally it is not filtered through the glomerulus and is only excreted in the urine when there is dysfunction in the tubular epithelial cells [117]. The urinary measurement of NAG has been useful to detect tubular damage in several cases. It has been observed that high urinary levels of this enzyme are predictors of micro and macroalbuminuria in individuals with type 1 diabetes mellitus [118]. These elevated levels can also be observed in the urine of patients with glomerulonephritis of any cause but mainly secondary to primary glomerular disease [119]. It has also been linked to proteinuria and CKD (particularly stage 3) [116].
Elevated urinary levels of NAG have been found in patients with LN compared with healthy individuals [120]. Additionally, high levels of this enzyme correlate with proteinuria but not with SLEDAI [121]. In addition, after 7 days of oral prednisolone treatment, NAG levels decreased after 30 days [122]. However, this evidence of improvement occurs long after the proteinuria decreases and renal function improves; therefore, it is not considered a useful tool in the monitoring of optimal response to treatment. Albeit, more studies should be conducted.
3.4. Urinary protein biomarkers of kidney injury
3.4.1. Kidney injury molecule–1(KIM-1)
KIM-1 is a transmembrane protein with an immunoglobulin-like domain, as well as a mucin domain in the extracellular portion. KIM-1 is expressed at high levels in the proximal tubular epithelial cells in regeneration and is responsible for repairing and regenerating the damaged regions of the nephron after kidney injury [123], specifically in post-ischemic states and in response to nephrotoxicity. The usefulness of this protein has been reported as a biomarker of kidney damage considering that is not expressed in healthy kidneys [124]. Additionally, it has been related to acute tubular necrosis and renal cell carcinoma [125,126].
Regarding LN, KIM-1 levels are significantly higher in SLE patients with active kidney disease, compared with patients without renal activity. Elevated levels of this protein are significantly associated with tubular impairment and proteinuria. It is also very sensitive at detecting cellular glomerular crescents and is highly specific for detecting interstitial infiltration [127]. In addition, other researchers have described a significant correlation between high urinary levels of KIM-1 with AKD, nephrotic syndrome, and hematuria in SLE patients [77]. As previously mentioned, the combination of different molecules can increase their clinical utility. In this case, the combined measurement of KIM-1, NGAL, and MCP-1 has an optimal diagnostic capacity (AUC 0.796) to predict active or chronic tubulointerstitial lesions in SLE patients [77].
3.4.2. High-mobility group box 1 proteins (HMGB1)
HMGB1 are members of the High-Mobility Group proteins located in the nucleus of eukaryotic cells [128,129]. These proteins bind to several types of DNA—double-stranded DNA (dsDNA), single-stranded DNA, distorted DNA, and nucleosomes—and participate in their transcriptional regulation [130]. HMGB1 is encoded in human chromosome 13q12-13 and is composed of three distinct domains: box A, box B, and an acid C-terminal tail. The latter contains a stretch of approximately 30 continuous residues of glutamic and aspartic acid [131]. One of the best-characterized functions of this protein is its role as damage-associated molecular patterns that are released passively by cells during necrosis or immunogenic apoptosis, being recognized by Toll-like receptors (TLRs) 2 and 4, and activating the inflammatory response [132,133].
As an LN biomarker, HMGB1 serum levels are high in SLE patients with renal involvement compared with SLE patients without renal manifestations [134,135]. Certainly, the cytoplasmic and extracellular presence of HMGB1 has been found in kidney biopsies of patients with LN [136,137]. HMGB1 can be detected in the urine of patients with LN compared with that of patients without active LN. Additionally, it is positively correlated with serum levels of HMGB1, proteinuria, and SLEDAI. It is inversely correlated with C3 complement levels [138]. Thus, it is possible that HMGB1 can be used as a biomarker of SLE activity and severity, particularly in renal compromise. However, more studies are needed to support this association.
3.5. Vascular molecules
3.5.1. Angiostatin
Angiostatin is an internal proteolytic fragment plasminogen of 38 kDa with antiangiogenic and antitumor properties. It inhibits the proliferation of endothelial cells by regulating the expression of cyclin-dependent kinase 5 (CDK5) [139]. Recently, this molecule has been linked to the immune system. It has been demonstrated that it inhibits the activation and migration of neutrophils [140]. It has been described, even in proteomics studies, that angiostatin levels are elevated in SLE patients, but the molecular mechanism is not yet known. One study reported that high levels of angiostatin are significantly correlated with the SLEDAI, rSLEDAI, renal SLICC (Systemic Lupus International Collaborating Clinical damage index for systemic Lupus erythematous), and the chronicity and activity indexes used in kidney biopsies. The most remarkable finding is that angiostatin levels are higher in SLE patients in remission with a previous LN history, in comparison with SLE patients in remission without prior LN [106]. Mok et al. evaluated the urinary levels of angiostatin, VCAM, and CXCL4 and found that angiostatin has the best AUC of the three markers to discriminate SLE with disease activity and renal involvement vs. SLE patients with active disease but no renal compromise, although there was no correlation with the chronicity indexes in the kidney biopsies [141]. To conclude, angiostatin may be useful in discriminating SLE patients with kidney injury; nevertheless, it is necessary to conduct further studies to determine its utility as a possible biomarker of progression and chronicity of the disease.
3.5.2. Adhesion molecules (VCAM and ICAM)
VCAM-1 or CD106 is an adhesion molecule expressed by endothelial cells that is involved in the migration and recruitment of inflammatory cells through its interaction with integrin very late antigen 4 (VLA-4), which is present in infiltrating leukocytes [142]. Leukocyte infiltration in the kidney is distinctive of severe kidney disease and is one of the morphological features that contribute to LN. Consequently, the production and VCAM-1 urine concentration are elevated in patients with SLE and LN. Singh et al. conducted a cross-sectional study, including 74 patients with LN, 13 controls, and other individuals with other renal diseases. They identified that urinary VCAM-1 was significantly elevated in patients with kidney disease compared with control patients [143]. It is probable that VCAM-1 levels fall in patients with LN when activity decreases and chronicity is established. This would be beneficial for the long-term monitoring of LN; however, the findings indicate that VCAM-1 is not specific for SLE and that it is a more general marker of kidney injury caused by other types of inflammatory nephritis [142]. This suggests that VCAM-1 is potentially useful in LN associated with another biomarker.
3.5.3. Endothelin-1
Endothelin-1 (ET-1) is a peptide of 21 amino acids produced by endothelial cells and renal cells [144]. It is considered as the most powerful endogenous vasoconstrictor [145]. Nonetheless, other functions at the renal and vascular levels—cell proliferation [146], inflammation [147], and fibrosis—have been associated with ET-1[148]. Also, ET-1 is implicated in the development and progression of CKD [144].
There are few studies that have evaluated ET-1 as a urinary biomarker. Scotland et al. evaluated the levels of renal excretion of ET-1 in patients with renal impairment secondary to different etiology and found that active renal damage secondary to SLE presented the highest values of ET-1, thereby postulating it as an activity biomarker. In addition, they described a reduction in ET-1 levels after immunomodulatory therapy in patients with LN [149]. Certainly, more studies are needed to substantiate the role of ET-1 as a useful LN activity biomarker.
3.5.4. Angiogenic cytokine vascular endothelial growth factor(VEGF)
Endothelial cell injury is a pathophysiological mechanism of LN [150,151], characterized by an increase in the apoptosis of these cells [152], endothelial dysfunction [153], and a persistent endothelial inflammatory state [154].
VEGF is an angiogenic cytokine produced by endothelial cells, monocytes, macrophages, activated T lymphocytes, and other cell types [155]. This molecule achieves functions that promote the survival of endothelial cells, as well as the maintenance of vascular homeostasis. Its production increases in response to inflammatory stimuli [156]. Hence, increased VEGF production has been associated with some pathologies like SLE [157] and is considered a serum biomarker of lupus activity [158].
Regarding LN, it has been seen that VEGF is elevated in the urine of patients with active LN, compared with patients with inactive LN or quiescent lupus [159]. Notwithstanding, the understanding of this cytokine as an activity biomarker is not entirely clear because it has also been found that higher levels of VEGF in kidney biopsies is associated with a lower activity of LN. Its low levels could act as a glomerular damage progression marker [160]. Therefore, in order to strengthen its role as a biomarker, more studies are needed to understand the participation of VEGF in the pathogenesis of SLE.
3.6. Other urinary biomarkers
3.6.1. Forkhead box P3
Forkhead box P3 (FoxP3) is a crucial transcription factor in the regulation of the development and function of Treg [161]. This molecule is very important in the homeostasis of the immune system. Mutation of the FoXP3 gene causes a complex multi-organ autoimmune disease called immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX syndrome)[162]. The participation of Foxp3 in the pathogenesis of SLE has been reported, and the expression of this transcription factor in CD4⁺ T cells has been correlated with the disease activity [163].
Increased levels of FoXP3 mRNA in the urine have been described in patients with proliferative LN, associated with high rSLEDAI and high titers of anti-dsDNA. Additionally, FoXP3 has been correlated with the histological activity index, and its levels decreased significantly in SLE patients who received treatment with a complete posterior response [164]. In fact, it is reported that FoXP3 starts to elevate approximately 8 weeks before the renal flare [165]. According to the results found in the reviewed studies, the measurement of this transcription factor could be useful for determining the activity of the disease, predicting relapses, and monitoring the response to treatment.
3.6.2. Transferrin
Transferrin weighs an average of 76–81 kDa and participates in the homeostasis of the organism since it binds to two ferric iron molecules and is responsible for transporting this metal to supply most of the body’s needs [166]. Recently, transferrin was isolated in the urine of pediatric patients with active LN, using proteomics studies [167]. It has been found that transferrin levels are elevated in patients with active LN, correlate with SLEDAI, and have an excellent diagnostic value for LN [168]. One research reported that elevated levels of transferrin are associated with capillary and mesangial proliferation and the formation of cellular glomerular crescent [169].
An additional study reported that the urinary measurement of transferrin, combined with the measurement of MCP-1 and liver-type fatty acid binding protein, had a good prognostic utility for deterioration in renal function defined as a decrease in the GFR of at least 20% or 25% increase in creatinine compared with baseline [79]. It has been documented that the combined measurement of transferrin with α1-acid-glycoprotein (AGP) and lipocalin-like prostaglandin D synthase (L-PGDS) may have predictive utility for remission of LN, with persistently elevated levels of these after treatment being a risk factor for therapeutic failure [46].
3.6.3. Ceruloplasmin
Ceruloplasmin (CP) is a 122 kDa protein that contains the majority of circulating copper. It has the function of iron oxidase and is associated with transferrin because CP oxidizes Fe2+ (ferrous) to Fe3+ (ferric) so that it can later bind to transferrin [170]. This is important since it has been seen that molecules associated with iron metabolism (such as CP and ferritin) are increased in SLE patients because their production can be induced by proinflammatory cytokines such as IL-6 and IL-1[171].
So far there are few studies that have evaluated the role of urinary CP as a biomarker for LN, although it has been reported that CP levels are elevated in SLE patients with LN in comparison with SLE patients without renal involvement [167]. Similarly, urinary CP levels are higher in patients with active LN and are related to a higher activity index. On the contrary, no association was found between urinary CP levels with a high SLEDAI value or with a particular LN class [168].
3.6.4. Th1/Th2 transcription factors
SLE is a disease physiologically characterized by aberrant activation of the T lymphocytes. This leads to an increased production of proinflammatory cytokines [172]. The populations of Th1 and Th2 lymphocytes play an important role in abnormal cytokines production since it has been seen that the imbalance between the cytokines produced by lymphocytes Th1 (LTh1) and lymphocytes Th2 (LTh2) predisposes to an inflammatory state characteristic of SLE [173]. To date, the reasons that lead to this imbalance are unclear; however, it has been identified that transcription factors T-bet and GATA-3 are essential for the differentiation to the Th1 and Th2 profiles, respectively [[173], [174], [175]]. For this reason, the alteration in the expression of T-bet and GATA-3 leads to an imbalance between Th1 and Th2, which has been implicated in multiple immunological diseases [176,177].
For the above mentioned, the urinary levels of these two transcription factors have been studied in patients with active LN, compared with patients with inactive LN, as well as the mRNA of each of them. It was found that T-bet levels are raised in patients with active LN and are associated with both higher SLEDAI and histological activity index. In contrast, GATA-3 mRNA and protein levels are lower in the urine of SLE patients with active LN in comparison with patients with remission or quiescent LN. In conclusion, it appears that in patients with LN there is a greater differentiation toward the Th1 profile because of the greater expression of T-bet. The measurement of the mRNA of these transcription factors could possibly be used as a urinary biomarker of disease activity [178]. Nevertheless, more studies are required to support the clinical utility of Th1/Th2 transcription factors.
3.6.5. CXCR3⁺ CD4⁺ T cells
Classically, LN has been associated with the deposition of immune complexes and complement activation, which causes renal damage and further progression of the disease [179]. However, some studies of renal biopsies of patients with LN have revealed a leukocytic infiltrate (mainly CD4⁺ T cells) that could contribute to kidney injury. Some relate the extension of the infiltrate with kidney injury and a worse prognosis of LN [[180], [181], [182]]. In murine SLE models with altered B-lymphocyte function (incapable of producing antibodies), it has been observed that they also develop LN. The presence of leukocyte infiltrate in the kidney biopsies of these mice has also been described [183].
Understanding how CD4⁺ T cells are recruited to the renal tissue is important because this may allow for the proposal of new therapeutic targets or markers for renal injury. The CXCR3 receptor is a chemokine receptor that is located chiefly in CD4⁺ T cells (producer of IFN-ɣ) and has been identified as one of the key receptors for general leukocytic infiltrate in LN. P. Enghard et al. evaluated, using immunohistochemistry and immunofluorescence, the leukocytic infiltrate of 18 patients with LN. In parallel, they studied by flow cytometry, the cell types (CD3⁺ T cells, CXCR3⁺ cells, and CXCL10⁺ cells) in peripheral blood and urine and found in renal biopsies that 63% of the cells of the infiltrate were CXCR3⁺ CD4⁺ T cells. In urine, the most frequent cell type was CXCR3⁺, which was associated with greater activity of the disease (according to SLEDAI). Therefore, they concluded that CXCR3⁺ CD4⁺ T cells represent an interesting marker of LN activity and could be postulated as a possible therapeutic target [184]. More studies are needed to further understand the role of these cells as biomarkers of activity.
3.6.6. Signal transducer and activator of transcription(STAT)-1
STAT-1 is the main transcription factor that mediates the biological effects of IFN-g [185,186]. The JAK-STAT molecular pathway is activated by IFN-gamma in target cells, causing activated STAT-1 to bind to ɣ-activated sequence (GAS) that is contained within the promoters of inflammatory immune genes inducible by IFN-ɣ[187,188]. On the other hand, STAT1 regulates the expression of proteins, such as Fas and Fas ligand, that can trigger apoptosis and are known to be deregulated in SLE [189].
L Martinez-Lostao et al. studied the biopsies of 15 patients with diffuse proliferative LN and found that all patients expressed STAT-1, but those with higher levels of this transcription factor had a higher disease activity (measured by the SLEDAI) and higher creatinuria rates. This suggested that one of the main mechanisms that IFN uses to mediate renal injury in SLE patients is the STAT-1 activation; thus, its measurement could be postulated with a marker of disease activity [190].
3.6.7. Renal Activity Index for Lupus (RAIL)
Each of the previously described biomarkers represents, by itself, a useful tool that in the future may help the clinician to assess the activity and severity of LN in a noninvasive manner. However, it is most likely that a single biomarker is not enough to determine the activity and severity of LN. Therefore, Brunner et al. performed an assessment of 16 urinary biomarkers to determine the performance of these and postulated an index to predict the activity of LN. Of the 16 biomarkers evaluated, 6 (NGAL, MCP-1, CP, adiponectin, hemopexin, and KIM-1) were considered to construct the renal Activity Index for Lupus (RAIL). The activity statuses of LN were calculated according to the National Institutes of Health activity index (NIH-AI) and the tubulointerstitial activity index (TIAI). These urinary biomarkers predicted the LN activity state (NIH-AI) with over 92% accuracy and the LN activity state (TIAI) with up 80% accuracy; therefore, RAIL represents an interesting tool that will allow the clinician to determine, in a noninvasive way, the LN activity [191].
4. MicroRNA
MicroRNA are molecules of non-coding RNA, between 18 and 25 nucleotides and are part of one of the most abundant classes of regulatory molecules in the genome. They intervene in gene expression at the post-transcriptional level [192] and affect the expression of a large proportion of the genome. This is reflected in several regulatory functions, such as cell proliferation, apoptosis, and proliferation [193]. Recently, it has been documented that the expression of specific microRNAs in renal tissue is altered in several kidney diseases, including LN [193].
It has been reported that urinary levels of miR-146a and miR-155 are elevated in patients with SLE. Additionally, miR-146a and miR-155 correlated with eGFR, proteinuria and SLEDAI, respectively. Also, the levels of these two decreased after the administration of calcitriol [194]. In one study, urinary levels of miR-221, miR-222, miR-339–3P, and miR-339–5P were measured since they have a key role in the regulation of ICAM-1. It was found that urinary sediment levels of miR-339–3P are significantly associated with proteinuria, and that miR-221 and miR-222 are inversely related to serum anti-dsDNA. Also, miR-221 specifically correlated with serum C3 levels [195]. On the other hand, one study evaluated the urinary levels of miR-200a, miR-200c, miR-141, miR-429, and miR-192. It described that these were significantly decreased in the sediment of SLE patients and do not correlate with any clinical parameter or serum levels. Regarding serum levels (miR-200a, miR-200c, miR-141, miR-429, and miR-192), an association was found with some clinical characteristics, raising the hypothesis that these could have a role in the pathophysiology of LN that is not reflected in urinary sediment [196].
Studies of murine models have found that treatment with hydroxychloroquine and prednisone decreases urinary levels of miR-let-7a and miR-21[197]. Likewise, the identification of microRNA in urinary exosomes has also been conducted. One study described the presence of miR-29c in exosomes of patients with LN showing a high predictive power for a high chronicity index in kidney biopsies, acquiring a potential function as an early predictor of progression to fibrosis in patients with LN [198]. Moreover, a recent study identified that the expression of miR-3135b, miR-654–5p, and miR-146a-5p in the exosomes correlated with class IV LN with good diagnostic efficacy [199].
5. Urinary proteomic profiles
As mentioned in this review, the traditional approach for the determination of urinary biomarkers has been directed toward the detection of specific molecules or proteins that are considered to be involved in the pathogenesis of LN. Alternatively, another interesting approach for the determination of urinary biomarkers is using proteomics techniques, where multiple proteins can be detected simultaneously, allowing the establishment of a profile according to the patient’s clinical condition. Although separation and analysis of proteins in biological samples is a technically complex task, especially with two-dimensional gel electrophoresis [19], there are alternatives, such as liquid chromatography tandem-mass spectrometry (LC-MS/MS). In LC-MS/MS, proteins are initially digested by a protease, then the peptides are separated using LC, and subsequently—using mass spectrometry—the previously fragmented proteins are identified, simplifying the entire process and improving the specificity [200]. Finally, the use of surface-enhanced laser desorption/ionization-time-of-flight-mass spectrometry (SELDI-TOF-MS) has demonstrated the ability to analyze larger numbers of samples in less time and also makes it possible to identify low molecular weight proteins (<20,000 Da) [19].
With the use of SELDI-TOF-MS, one study detected that two protein ions (3340 and 3980) were useful for distinguishing active from inactive LN, with a specificity superior to traditional urinary biomarkers, as well as being useful for predicting relapses or response to immunosuppressive treatment [201]. Additionally, using electrospray ionization quadrupole time-of-flight mass spectrometry (ESI-Q-TOF MS/MS), two potential candidate proteins were identified: Zinc-alpha 2-glycoprotein (ZA2G) and Prostaglandin H2D-isomerase (PGDS). ZA2G was elevated in both LN and other glomerular diseases, whereas PGDS was specifically elevated in active LN, thereby the latter being interesting for further investigation [202]. Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS/MS), three urinary proteins have been identified: alpha-1 antichemotrypsin (ACT), haptoglobin (Hp), and retinol binding protein (RBP), which were subsequently validated by ELISA. These proteins (ACT, Hp, RBP) showed a correlation with SLEDAI and rSLEDAI, with a considerable capacity to detect disease activity, especially with ACT. Furthermore, patients were followed for 12 months after treatment, and the levels of these proteins were found to decrease after the administration of immunosuppressive drugs. This could be used as a marker of response to therapy [203].
Other investigations have been conducted in the pediatric population, and eight potential markers have been determined as useful to effectively discriminate active LN from inactive LN [204]. Subsequently, MALDI-TOF-MS/MS was used to identify specific proteins such as AGP, L-PGDS, transferrin, and PC. The first three were found to be useful regarding predicting flares in pediatric patients with LN [205]. Table 3 summarizes some possible biomarkers obtained by proteomics based on the study of Rovin and collaborators [206].
Table 3.
Proteomics studies (Adaptation of the results obtained by Rovin and collaborators [206]).
| Urinary samples | |
|---|---|
| Proteomics | Metabolomics |
| Prostaglandin D synthetase (PGDS) | Citrate |
| Lipocalin-type Prostaglandin D synthetase (L-PGDS) | Taurine |
| Serum amyloid-P (SAP) | Discriminate between class III/IV LN vs. Class V LN |
| Superoxide dismutase | |
| Transferrin | |
| Ceruloplasmin | |
| Albumin | |
| Hepcidin | |
| A-1 antitrypsin | |
6. Limitations to the use of urine as a sample
Despite the ease of urine collection for the potential determination of biomarkers, several aspects must be considered at the time of interpretation. For example, changes in urinary pH, due to physiological or pathological conditions, can modify the results. Also, daytime concentrations of urine may vary. Besides, the presence of bacteriuria or urinary infections can alter the results. All these aspects must be considered at the time of assessment.
7. Conclusions
Notwithstanding that there are many urinary biomarkers evaluated to predict and assess the activity of LN, until now there is not enough evidence in large-scale longitudinal studies in populations with different ethnic groups to validate the usefulness of these biomarkers and their possible use in clinical practice. On the other hand, the use of a single biomarker is not strong enough for clinical follow-up. Instead, several biomarkers are required to develop a useful index for the monitoring of LN patients. Finally, although urinary biomarkers are interesting tools in patients with LN, it should be remembered that similar serum and tissue biomarkers exist and have equal weight in the study of these patients; therefore, the development of an activity score will require the use of both.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Davidson A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 2016;12:143–153. doi: 10.1038/nrrheum.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houssiau F.A., Lauwerys B.R. Current management of lupus nephritis. Best Pract. Res. Clin. Rheumatol. 2013;27:319–328. doi: 10.1016/j.berh.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Restrepo-Escobar M., Granda-Carvajal P.A., Jaimes F. Systematic review of the literature on reproducibility of the interpretation of renal biopsy in lupus nephritis. Lupus. 2017;26:1502–1512. doi: 10.1177/0961203317706556. [DOI] [PubMed] [Google Scholar]
- 4.Giannico G., Fogo A.B. Lupus nephritis: is the kidney biopsy currently necessary in the management of lupus nephritis? Clin. J. Am. Soc. Nephrol. 2013;8:138–145. doi: 10.2215/CJN.03400412. [DOI] [PubMed] [Google Scholar]
- 5.Berthier C.C., Kretzler M., Davidson A. From the large scale expression analysis of lupus nephritis to targeted molecular medicine. J. Data Min. Genom. Proteonomics. 2012;3:3. doi: 10.4172/2153-0602.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartung E.A. Biomarkers and surrogate endpoints in kidney disease. Pediatr. Nephrol. 2016;31:381–391. doi: 10.1007/s00467-015-3104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arriens C., Wren J.D., Munroe M.E. Systemic lupus erythematosus biomarkers: the challenging quest. Rheumatology. 2017;56:i32–i45. doi: 10.1093/rheumatology/kew407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birmingham D.J., Merchant M., Waikar S.S. Biomarkers of lupus nephritis histology and flare: deciphering the relevant amidst the noise. Nephrol. Dial. Transplant. 2017;32:i71–i79. doi: 10.1093/ndt/gfw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harpole M., Davis J., Espina V. Current state of the art for enhancing urine biomarker discovery. Expert Rev. Proteomics. 2016;13:609–626. doi: 10.1080/14789450.2016.1190651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths B., Mosca M., Gordon C. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract. Res. Clin. Rheumatol. 2005;19:685–708. doi: 10.1016/j.berh.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Chicheportiche Y., Bourdon P.R., Xu H. TWEAK , a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis *. J. Biol. Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 12.Wiley S.R., Winkles J.A. TWEAK , a member of the TNF superfamily , is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 14. 2003;14:241–249. doi: 10.1016/s1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M., Ishidoh K., Kayagaki N. Multiple pathways of TWEAK-induced cell death. J. Immunol. 2002;168:734–774. doi: 10.4049/jimmunol.168.2.734. [DOI] [PubMed] [Google Scholar]
- 14.Schneider P., Schwenzer R., Haas E. TWEAK can induce cell death via endogenous TNF and TNF receptor 1. Eur. J. Immunol. 1999:1785–1792. doi: 10.1002/(SICI)1521-4141(199906)29:06<1785::AID-IMMU1785>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Chicheportiche Y., Chicheportiche R., Sizing I. Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes : blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res. 2002;4:126–133. doi: 10.1186/ar388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell S., Michaelson J., Burkly L., Putterman C. The role of tweak/fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front. Biosci. 2004;9:2273–2284. doi: 10.2741/1395. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz N., Su L., Burkly L.C. Urinary TWEAK and the activity of lupus nephritis. J. Autoimmun. 2006;27:242–250. doi: 10.1016/j.jaut.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz N., Rubinstein T., Burkly L.C. Research article Urinary TWEAK as a biomarker of lupus nephritis : a multicenter cohort study. Arthritis Res. Ther. 2011;11:1–10. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes-thomas J., Blanco I., Putterman C. Urinary biomarkers in lupus nephritis. Clin. Rev. Allergy Immunol. 2012;40:138–150. doi: 10.1007/s12016-010-8197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong X., Zheng Z., Luo X. Combined utilization of untimed single urine of MCP-1 and TWEAK as a potential indicator for proteinuria in lupus nephritis. Medicine (Baltim.) 2018;16:1–7. doi: 10.1097/MD.0000000000010343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nalbandian A., Crispín J.C. Interleukin- 17 and systemic lupus erythematosus : current concepts. Br Soc Immunol Clin Exp Immunol. 2009:209–215. doi: 10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn T., Oukka M., Kuchroo B. Th17 cells: effector T cells with inflammatory properties. Semin. Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W., Na L., Fidel P.L. Requirement of interleukin-17a for systemic anti – Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 24.Miossec P. Interleukin-17 in rheumatoid arthritis if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 25.Kurasawa K., Hirose K., Sano H. Increased INTERLEUKIN-17 production IN patients with systemic sclerosis. Arthritis Rheum. 2000;43:2455–2463. doi: 10.1002/1529-0131(200011)43:11<2455::AID-ANR12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Shih D.Q., Targan S.R., McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr. Gastroenterol. Rep. 2009;10:568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mok M.Y., Wu H.J., Lo Y., Lau C.S. The relation of interleukin 17 ( IL-17 ) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J. Rheumatol. 2010;37:2046–2052. doi: 10.3899/jrheum.100293. [DOI] [PubMed] [Google Scholar]
- 28.Crispín J.C., Oukka M., Bayliss G. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the. J. Immunol. 2018;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susianti H., Iriane V.M., Dharmanata S. Analysis of urinary TGF-  1 , MCP-1 , NGAL , and IL-17 as biomarkers for lupus nephritis. Pathophysiology. 2015;22:65–71. doi: 10.1016/j.pathophys.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Kwan B.C., Tam L., Lai K. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology. 2018;48:1491–1497. doi: 10.1093/rheumatology/kep255. [DOI] [PubMed] [Google Scholar]
- 31.Saber N.Z., Maroof S.H., Soliman D.A. The Egyptian Rheumatologist Expression of T helper 17 cells and interleukin 17 in lupus nephritis patients. Egypt Rheumatol J. 2017;39:151–157. [Google Scholar]
- 32.Kishimoto T., Akira S., Narazaki M. Interleukin-6 family of cytokines and gp130. J Am Soc Hematol. 2018;86:1243–1254. [PubMed] [Google Scholar]
- 33.Hirano T. Interleukin 6 and its Receptor : TenYears later. Inrem Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 34.Brozek W., Bises G., Fabjani G. Clone-specific expression, transcriptional regulation, and action of interleukin-6 in human colon carcinoma cells. BMC Canc. 2008;9:1–9. doi: 10.1186/1471-2407-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H., Lei C., Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front. Immunol. 2017;8:1–10. doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards B.H.B., Satoh M., Shaw M. Interleukin 6 dependence of anti-DNA antibody Production : evidence for two pathways of autoantibody formation in pristane-induced lupus. J. Exp. Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwano M., Dohi K., Hirata E. Urinary levels of IL-6 in patients with active lupus nephritis. Clin. Nephrol. 1993;40:16–21. [PubMed] [Google Scholar]
- 38.Tsai C., Wu T., Yu C. Increased excretions of ß 2 -microglobulin , IL-6 , and IL-8 and decreased excretion of tamm-horsfall glycoprotein in urine of patients. Nephron. 2000;85:207–214. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- 39.Scherer P.E., Williams S., Fogliano M. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26750. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z.V., Scherer P.E. Adiponectin , the past two decades. J. Mol. Cell Biol. 2016;8:93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cnop M., Havel P.J., Utzschneider K.M. Relationship of adiponectin to body fat distribution , insulin sensitivity and plasma lipoproteins : evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 42.Chen T., Chen L., Hsieh M. Evidence for a protective role for adiponectin in osteoarthritis. Biochim. Biophys. Acta. 2006;1762:711–718. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Hutcheson J., Ye Y., Han J. Resistin as a potential marker of renal disease in lupus nephritis. Br Soc Immunol Clin Exp Immunol. 2014;179:435–443. doi: 10.1111/cei.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rovin B.H., Song H., Hebert L.E. Plasma , urine , and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–1833. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 45.Landolt-marticorena C., Prokopec S.D., Morrison S. A discrete cluster of urinary biomarkers discriminates between active systemic lupus erythematosus patients with and without glomerulonephritis. Arthritis Res. Ther. 2016:1–12. doi: 10.1186/s13075-016-1120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunner H.I., Bennett M.R., Gulati G. Urine biomarkers to predict response to lupus nephritis therapy in children and young adults. J. Rheumatol. 2017;44:1239–1248. doi: 10.3899/jrheum.161128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart A.G., Thomas B., Koff M.J. TGF- β : master regulator of inflammation and fibrosis. Respirology. 2018;23:1096–1097. doi: 10.1111/resp.13415. [DOI] [PubMed] [Google Scholar]
- 48.Wahl S.M., Mccartney-frands N., Mergenhagen S.E. Inflammatory and immunomodulatory roles of TGF-B. Immunol. Today. 1989;10:258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- 49.Border Wayne A., Noble Nancy A. Fibrosis linked to TGF-B in yet another disease. J. Clin. Invest. 1995;96:2565–2566. doi: 10.1172/JCI118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grande J.P. Mechanisms of progression of renal damage in lupus nephritis:Pathogenesis of renal scarring. Lupus. 1998;7:604–610. doi: 10.1191/096120398678920721. [DOI] [PubMed] [Google Scholar]
- 51.Mackay K., Striker L.J., Stauffer J.W. Transforming growth factor-B. J. Clin. Invest. 1989;83:1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabregat I, Caballero-díaz D. Transforming growth factor- β -induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front Oncol; 8. Epub ahead of print 2018. DOI: 10.3389/fonc.2018.00357. [DOI] [PMC free article] [PubMed]
- 53.Aghasafari P., George U., Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019;68:59–74. doi: 10.1007/s00011-018-1191-2. [DOI] [PubMed] [Google Scholar]
- 54.Isaka Y. Targeting TGF- β signaling in kidney fibrosis. Int J Mol Sci Rev. 2018;19:1–13. doi: 10.3390/ijms19092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackay K., Kondaiah P., Danielpour D. Expression of transforming growth factor-B1 and B2 in rat glomeruli. Kidney Int. 1990;38:1095–1100. doi: 10.1038/ki.1990.318. [DOI] [PubMed] [Google Scholar]
- 56.Gerritsma J.S.J., Kooten C.V., Gerritsen A.F. Transforming growth factor- B1 regulates chemokine and complement production by human proximal tubular epithelial cells. Kidney Int. 1998;53:609–616. doi: 10.1046/j.1523-1755.1998.00799.x. [DOI] [PubMed] [Google Scholar]
- 57.Kitamura S., Maeshima Y., Sugaya T. Transforming growth factor- ß 1 induces vascular endothelial growth factor expression in murine proximal tubular epithelial cells. Nephron Exp. Nephrol. 2003;95:e79–86. doi: 10.1159/000073675. [DOI] [PubMed] [Google Scholar]
- 58.Tatsuo Y., Takuya W., Ikegaya N. Expression of types I, II, and III TGF-beta receptors in human glomerulonephritis. J. Am. Soc. Nephrol. 1998;9:2253–2261. doi: 10.1681/ASN.V9122253. [DOI] [PubMed] [Google Scholar]
- 59.Avihingsanon Y., Phumesin P., Benjachat T. Measurement of urinary chemokine and growth factor messenger RNAs : a noninvasive monitoring in lupus nephritis. Kidney Int. 2006;69:747–753. doi: 10.1038/sj.ki.5000132. [DOI] [PubMed] [Google Scholar]
- 60.Torabinejad S., Mardani R., Habibagahi Z. Urinary monocyte chemotactic protein-1 and transforming growth factor- b in systemic lupus erythematosus. Indian J. Nephrol. 2012;22:5–12. doi: 10.4103/0971-4065.91179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammad A.M., Youssef H.M., El-arman M.M. Transforming growth factor beta 1 in children with systemic lupus erythematosus : a possible relation. Lupus. 2006;15:608–612. doi: 10.1177/0961203306071873. [DOI] [PubMed] [Google Scholar]
- 62.Susianti H., Iriane V.M., Dharmanata S. Analysis of urinary TGF-β1, MCP-1, NGAL, and IL-17 as biomarkers for lupus nephritis. Pathophysiology. 2015;22:65–71. doi: 10.1016/j.pathophys.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Simonet W.S., Lacey D.L., Dunstan C.R. Osteoprotegerin : a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 64.Yun T.J., Chaudhary P.M., Shu G.L. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J. Immunol. 1998;161:6113–6121. [PubMed] [Google Scholar]
- 65.Hofbauer L.C., Khosla S., Dunstan C.R. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 66.Montañez-Barragán A., Gómez-Barrera I., Sanchez-Niño M.D. Osteoprotegerin and kidney disease. J. Nephrol. 2014;27:607–617. doi: 10.1007/s40620-014-0092-x. [DOI] [PubMed] [Google Scholar]
- 67.Kiani A.N., Johnson K., Chen C. Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J. Rheumatol. 2009;36:2224–2230. doi: 10.3899/jrheum.081112. [DOI] [PubMed] [Google Scholar]
- 68.El-shehaby A., Darweesh H. Correlations of urinary biomarkers , TNF-like weak inducer of apoptosis ( TWEAK ), osteoprotegerin ( OPG ), monocyte chemoattractant protein-1 ( MCP-1 ), and IL-8 with lupus nephritis. J Clin mmunol. 2011;1:848–856. doi: 10.1007/s10875-011-9555-1. [DOI] [PubMed] [Google Scholar]
- 69.Gupta R., Aggarwal A., Sinha S. Urinary osteoprotegerin : a potential biomarker of lupus nephritis disease activity. Lupus. 2016;1–7 doi: 10.1177/0961203316636470. [DOI] [PubMed] [Google Scholar]
- 70.Dostert C., Grusdat M., Letellier E. The TNF family of ligands and receptors: system and beyond. Physiol. Rev. 2018;99:115–160. doi: 10.1152/physrev.00045.2017. [DOI] [PubMed] [Google Scholar]
- 71.Li J., Yin Q., Wu H. first ed. Elsevier Inc.; 2013. Structural Basis of Signal Transduction in the TNF Receptor Superfamily. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhatraju P.K., Zelnick L.R., Kestenbaum B. Association of soluble TNFR-1 concentrations with long-term decline in kidney Function : the multi-ethnic study of atherosclerosis. J. Am. Soc. Nephrol. 2018;29:1–9. doi: 10.1681/ASN.2018070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu T., Xie C., Wang H.W. Murine lupus strains and human lupus nephritis 1. J. Immunol. 2007;179:7166–7175. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- 74.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1(MCP-1): an overview. J. Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Coillie E., Van Damme J., Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 76.Ghobrial E.E., Hamshary AA El, Mohamed A.G. Urinary monocyte chemoattractant protein-1 as a biomarker of lupus nephritis activity in children. Saudi J Kidney Dis Transplant. 2015;26:507–515. doi: 10.4103/1319-2442.157350. [DOI] [PubMed] [Google Scholar]
- 77.Ding Y., Nie L., Pang Y. Composite urinary biomarkers to predict pathological tubulointerstitial lesions in lupus nephritis. Lupus. 2018;1:1–12. doi: 10.1177/0961203318788167. [DOI] [PubMed] [Google Scholar]
- 78.Barbado J., Vega L., Bermejo-martin F. MCP-1 en orina como biomarcador de lupus renal en ausencia de citoquinas, interferon gamma y factores de crecimiento. Reumatol. Clínica. 2010;6:296–298. doi: 10.1016/j.reuma.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 79.Abulaban K.M., Song H., Zhang X. Predicting decline of kidney function in lupus nephritis using urine biomarkers. Lupus. 2017;25:1012–1018. doi: 10.1177/0961203316631629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosa R.F., Takei K., Araújo N.C. Monocyte chemoattractant-1 as a urinary biomarker for the diagnosis of activity of lupus nephritis in Brazilian patients. J. Rheumatol. 2012;39:1948–1954. doi: 10.3899/jrheum.110201. [DOI] [PubMed] [Google Scholar]
- 81.Rovin B.H., Song H., Birmingham D.J. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. 2005;16:467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 82.Tesch G.H. MCP-1/CCL2 : a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 83.Kim M.J., Tam F.W.K. Urinary monocyte chemoattractant protein-1 in renal disease. Clin. Chim. Acta. 2011;412:2022–2030. doi: 10.1016/j.cca.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 84.Dong X.W., Zheng Z.H., Ding J. Combined detection of uMCP-1 and uTWEAK for rapid discrimination of severe lupus nephritis. Lupus. 2018;27:971–981. doi: 10.1177/0961203318758507. [DOI] [PubMed] [Google Scholar]
- 85.Hasegawa H., Kohno M., Sasaki M. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum. 2003;48:2555–2566. doi: 10.1002/art.11231. [DOI] [PubMed] [Google Scholar]
- 86.Taub D.D., Oppenheim J.J. Chemokines, inflammation and the immune system. Ther. Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- 87.Pérez de Lema G., Maier H., Nieto E. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 2001;12:1369–1382. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 88.Segerer S., Nelson P.J., Ondorff D.S. Chemokines , chemokine receptors , and renal Disease : from basic science to pathophysiologic and therapeutic studies. J. Am. Soc. Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- 89.Kaneko H., Ogasawara H., Naito T. Circulating levels of beta-chemokines in systemic lupus erythematosus. J. Rheumatol. 1999;26:568–573. [PubMed] [Google Scholar]
- 90.Chan R.W., Lai F.M., Li E.K. Messenger RNA expression of RANTES in the urinary sediment of patients with lupus nephritis. SUMMARY : Nephrology. 2006;11:219–225. doi: 10.1111/j.1440-1797.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 91.Tian S., Li J., Wang L. Urinary levels of RANTES and M-CSF are predictors of lupus nephritis fl are. Inflamm. Res. 2007;56:304–310. doi: 10.1007/s00011-007-6147-x. [DOI] [PubMed] [Google Scholar]
- 92.Jakiela B., Kosa J., Plutecka H. Urinary cytokines and mRNA expression as biomarkers of disease activity in lupus nephritis. Lupus. 2018;27:1259–1270. doi: 10.1177/0961203318770006. [DOI] [PubMed] [Google Scholar]
- 93.Gotsch F., Romero R., Friel L. CXCL10/IP-10 : a missing link between inflammation and anti-angiogenesis in preeclampsia ? 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenkilde M.M., Schwartz T.W. The chemokine system - a major regulator of angiogenesis in health and disease. Apmis. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 95.Marie M.A., Abu Khalil R.E., Habib H.M. Urinary CXCL10: a marker of nephritis in lupus patients. Reumatismo. 2013;73:292–297. doi: 10.4081/reumatismo.2013.719. [DOI] [PubMed] [Google Scholar]
- 96.Avihingsanon Y., Phumesin P., Benjachat T. Measurement of urinary chemokine and growth factor messenger RNAs: a noninvasive monitoring in lupus nephritis. Kidney Int. 2006;69:747–753. doi: 10.1038/sj.ki.5000132. [DOI] [PubMed] [Google Scholar]
- 97.Gough P.J., Garton K.J., Wille P.T. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J. Immunol. 2004;172:3678–3685. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- 98.Shimaoka T., Nakayama T., Fukumoto N. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J. Leukoc. Biol. 2004;75:267–274. doi: 10.1189/jlb.1003465. [DOI] [PubMed] [Google Scholar]
- 99.Minami M., Kume N., Shimaoka T. Expression of scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX) in human atheroma. Ann. N. Y. Acad. Sci. 2001;947:373–376. doi: 10.1111/j.1749-6632.2001.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 100.Chandrasekar B., Bysani S., Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J. Biol. Chem. 2004;279:3188–3196. doi: 10.1074/jbc.M311660200. [DOI] [PubMed] [Google Scholar]
- 101.Shimaoka T., Nakayama T., Kume N. Cutting edge: SR-PSOX/CXC chemokine ligand 16 mediates bacterial phagocytosis by APCs through its chemokine domain. J. Immunol. 2003;171:1647–1651. doi: 10.4049/jimmunol.171.4.1647. [DOI] [PubMed] [Google Scholar]
- 102.Wen S., He F., Zhu X. IFN-γ, CXCL16, uPAR: potential biomarkers for systemic lupus erythematosus. Clin. Exp. Rheumatol. 2018;36:36–43. [PubMed] [Google Scholar]
- 103.Teramoto K., Negoro N., Kitamoto K. Microarray analysis of glomerular gene expression in murine lupus nephritis. J. Pharmacol. Sci. 2008;106:56–67. doi: 10.1254/jphs.fp0071337. [DOI] [PubMed] [Google Scholar]
- 104.El-Gamasy M.A., El-Naghy W. Urinary neutrophil gelatinase-associated lipocalin and urinary soluble CXCL16 as biomarkers of activity in pediatric lupus nephritis. Indian J. Nephrol. 2018;28:427–432. doi: 10.4103/ijn.IJN_265_17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Hassan A.M., Farghal N.M.A., Hegab D.S. Serum-soluble CXCL16 in juvenile systemic lupus erythematosus: a promising predictor of disease severity and lupus nephritis. Clin. Rheumatol. 2018;37:3025–3032. doi: 10.1007/s10067-018-4203-2. [DOI] [PubMed] [Google Scholar]
- 106.Wu T., Du Y., Han J. Urinary angiostatin - a novel putative marker of renal pathology chronicity in lupus nephritis. Mol. Cell. Proteomics. 2013;12:1170–1179. doi: 10.1074/mcp.M112.021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gonzalez-Aparicio M., Alfaro C. Influence of interleukin-8 and neutrophil extracellular trap (NET) formation in the tumor microenvironment: is there a pathogenic role? J Immunol Res. 2019;2019:6252138. doi: 10.1155/2019/6252138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsai C.Y., Wu T.H., Yu C.L. Increased excretions of β2-microglobulin, IL-6, and IL-8 and decreased excretion of tamm-horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85:207–214. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- 109.Rovin B.H., Lu L., Zhang X. A novel interleukin-8 polymorphism is associated with severe systemic lupus erythematosus nephritis. Kidney Int. 2002;62:261–265. doi: 10.1046/j.1523-1755.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 110.Bolignano D., Donato V., Coppolino G. Neutrophil Gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 111.Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand. J. Clin. Lab. Invest. Suppl. 2008:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mishra J., Ma Q., Lipocalin Barasch J. A novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004;3039:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 113.Malyszko J., Malyszko J.S., Poniatowski B. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant. Proc. 2009;41:158–161. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 114.Rubinstein T., Pitashny M., Levine B. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology. 2010;49:960–971. doi: 10.1093/rheumatology/kep468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Satirapoj B., Kitiyakara C., Leelahavanichkul A. Urine neutrophil gelatinase-associated lipocalin to predict renal response after induction therapy in active lupus nephritis. BMC Nephrol. 2017;18:263. doi: 10.1186/s12882-017-0678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Genctoy G., Arikan S. Urinary N-Acetyl-Beta-D glucosaminidase activity is associated with inflammation and proteinuria in diabetic and non-diabetic patients with different stages of chronic kidney disease. Turkish J Nephrol. 2015;24:166–173. [Google Scholar]
- 117.Bazzi C., Petrini C., Rizza V. Urinary N -acetyl- b -glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol. Dial. Transplant. 2002;17:1890–1896. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- 118.Kern E.O., Erhard P., Sun W. Early urinary markers of diabetic kidney disease: a nested case-control study from the diabetes control and complications trial (DCCT) Am J Kidney. 2010;55:824–834. doi: 10.1053/j.ajkd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holdt-lehmann B., Lehmann A., Korten G. Diagnostic value of urinary alanine aminopeptidase and N -acetyl- b - D -glucosaminidase in comparison to a 1 -microglobulin as a marker in evaluating tubular dysfunction in glomerulonephritis patients. Clin. Chim. Acta. 2000;297:93–102. doi: 10.1016/s0009-8981(00)00237-0. [DOI] [PubMed] [Google Scholar]
- 120.Marks S.D., Shah V., Pilkington C. Renal tubular dysfunction in children with systemic lupus erythematosus. Pediatr. Nephrol. 2005;20:141–148. doi: 10.1007/s00467-004-1707-6. [DOI] [PubMed] [Google Scholar]
- 121.Erdener D., Aksu K., Biçer I. Urinary N-acetyl- b - D -glucosaminidase ( NAG ) in lupus nephritis and rheumatoid arthritis. J. Clin. Lab. Anal. 2005;176:172–176. doi: 10.1002/jcla.20073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gluhovschi C., Velciov S., Kaycsa A. The dynamics of urinary N -acetyl- β -d-glucosaminidase ( NAG ), a marker of renal tubular dysfunction , in patients with lupus nephritis undergoing oral prednisone therapy. Immunopharmacol. Immunotoxicol. 2012;34:163–169. doi: 10.3109/08923973.2011.585343. [DOI] [PubMed] [Google Scholar]
- 123.Ichimura T., Bonventre J.V., Wei H. Kidney injury molecule-1 ( KIM-1 ), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain , is up-regulated in renal cells after injury. J. Biol. Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 124.Ichimura T., Hung C.C., Yang S.A. Kidney injury molecule-1 : a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 2018;286:552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 125.Han W.K., Bailly V., Abichandani R. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 126.Han W.K., Alinani A., Wu C. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J. Am. Soc. Nephrol. 2005;16:1126–1134. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nozaki Y., Kinoshita K., Yano T. Estimation of kidney injury molecule-1 ( Kim-1 ) in patients with lupus nephritis. Lupus. 2014;23:769–777. doi: 10.1177/0961203314526292. [DOI] [PubMed] [Google Scholar]
- 128.Reeves R. Nuclear functions of the HMG proteins. Biochim Biophys Acta - Gene Regul Mech. 2010;1799:3–14. doi: 10.1016/j.bbagrm.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goodwin G.H., Sanders C., Johns E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 130.Bianchi M.E., Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 131.Landsman D., Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993;15:539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- 132.Rojo-León V., Aguilar-Cázares D., Prado-García H. Participation of damage-associated molecular patterns in conventional treatment of cancer | Participación de los patrones moleculares asociados al daño en el tratamiento convencional del cáncer. Rev. Investig. Clin. 2012;64:284–293. [PubMed] [Google Scholar]
- 133.Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 134.Li J., Xie H., Wen T. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J. Rheumatol. 2010;37:766–775. doi: 10.3899/jrheum.090663. [DOI] [PubMed] [Google Scholar]
- 135.Abdulahad D.A., Westra J., Bijzet J. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res. Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zickert A., Palmblad K., Sundelin B. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res. Ther. 2012;14:R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abdulahad D.A., Westra J., Bijzet J. Urine levels of HMGB1 in Systemic Lupus Erythematosus patients with and without renal manifestations. Arthritis Res. Ther. 2012;14:1–11. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burbano C., Gómez-Puerta J.A., Muñoz-Vahos C. HMGB1 + microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur. J. Immunol. 2019;49:323–335. doi: 10.1002/eji.201847747. [DOI] [PubMed] [Google Scholar]
- 139.Sharma M.R., Tuszynski G.P., Sharma M.C. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J Celullar Biochem. 2004;91:398–409. doi: 10.1002/jcb.10762. [DOI] [PubMed] [Google Scholar]
- 140.Aulakh G.K., Balachandran Y., Liu L. Angiostatin inhibits activation and migration of neutrophils. Cell Tissue Res. 2014;355:375–396. doi: 10.1007/s00441-013-1753-0. [DOI] [PubMed] [Google Scholar]
- 141.Mok C.C., Soliman S., Ho L.Y. Urinary angiostatin , CXCL4 and VCAM-1 as biomarkers of lupus nephritis. Arthritis Res. Ther. 2018;20:6. doi: 10.1186/s13075-017-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singh S., Wu T., Vanarsa K. Urine VCAM1 is a good indicator of renal pathology activity index in lupus nephritis. Arthritis Res. Ther. 2012:1–11. doi: 10.1186/ar3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spronk P.E., Bootsma H., Huitema M.G. Levels of soluble VCAM-1, soluble ICAM-1, and soluble E-selectin during disease exacerbations in patients with systemic lupus erythematosus (SLE); a long term. Clin. Exp. Immunol. 1994:439–444. doi: 10.1111/j.1365-2249.1994.tb06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dhaun N., Goddard J., Webb D.J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- 145.Yanagisawa M., Kurihara H., Kimura S. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 146.Simonson M.S., Wann S., Mené P. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J. Clin. Invest. 1989;83:708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sasser J.M., Sullivan J.C., Hobbs J.L. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J. Am. Soc. Nephrol. 2007;18:143–154. doi: 10.1681/ASN.2006030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hocher B., Thöne-Reineke C., Rohmeiss P. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dhaun N., Lilitkarntakul P., Macintyre I.M. Urinary endothelin-1 in chronic kidney disease and as a marker of disease activity in lupus nephritis. Am. J. Physiol. Ren. Physiol. 2009;296:F1477–F1483. doi: 10.1152/ajprenal.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Appel G.B., Pirani C.L., D’Agati V. Renal vascular complications of systemic lupus erythematosus. J. Am. Soc. Nephrol. 1994;4:1499–1515. doi: 10.1681/ASN.V481499. [DOI] [PubMed] [Google Scholar]
- 151.Banfi G., Bertani T., Boeri V. Renal vascular lesions as a marker of poor prognosis in patients with lupus nephritis. Gruppo Italiano per lo Studio della Nefrite Lupica (GISNEL) Am. J. Kidney Dis. 1991;18:240–248. doi: 10.1016/s0272-6386(12)80885-7. [DOI] [PubMed] [Google Scholar]
- 152.Rajagopalan S., Somers E.C., Brook R.D. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 153.El-Magadmi M., Bodill H., Ahmad Y. Systemic lupus erythematosus. Circulation. 2004;110:399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 154.Kahlenberg J.M., Kaplan M.J. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus. Arthritis Res. Ther. 2010;13:203. doi: 10.1186/ar3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mor F., Quintana F.J., Cohen I.R. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J. Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 156.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 157.Robak E., Sysa-Jedrzejewska A., Robak T. Vascular endothelial growth factor and its soluble receptors VEGFR-1 and VEGFR-2 in the serum of patients with systemic lupus erythematosus. Mediat. Inflamm. 2003;12:293–298. doi: 10.1080/09629350310001619726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Edelbauer M., Kshirsagar S., Riedl M. Soluble VEGF receptor 1 promotes endothelial injury in children and adolescents with lupus nephritis. Pediatr. Nephrol. 2012;27:793–800. doi: 10.1007/s00467-011-2062-z. [DOI] [PubMed] [Google Scholar]
- 159.Adhya Z., El Anbari M., Anwar S. Soluble TNF-R1, VEGF and other cytokines as markers of disease activity in systemic lupus erythematosus and lupus nephritis. Lupus. 2019;28:713–721. doi: 10.1177/0961203319845487. [DOI] [PubMed] [Google Scholar]
- 160.Avihingsanon Y., Benjachat T., Tassanarong A. Decreased renal expression of vascular endothelial growth factor in lupus nephritis is associated with worse prognosis. Kidney Int. 2009;75:1340–1348. doi: 10.1038/ki.2009.75. [DOI] [PubMed] [Google Scholar]
- 161.Sakaguchi S., Yamaguchi T., Nomura T. Review regulatory T cells and immune tolerance. Cell. 2008:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 162.Copland A., Bending D. Foxp3 molecular dynamics in Treg in juvenile idiopathic arthritis. Front. Immunol. 2018;9:1–7. doi: 10.3389/fimmu.2018.02273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bonelli M., Dalwigk K Von, Savitskaya A. Foxp3 expression in CD4 + T cells of patients with systemic lupus erythematosus : a comparative phenotypic analysis. Ann. Rheum. Dis. 2008:664–671. doi: 10.1136/ard.2007.074690. [DOI] [PubMed] [Google Scholar]
- 164.Wang G., Lai F.M., Tam L. Urinary FOXP3 mRNA in patients with lupus nephritis — relation with disease activity and treatment response. Rheumatology. 2009;48:755–760. doi: 10.1093/rheumatology/kep074. [DOI] [PubMed] [Google Scholar]
- 165.Szeto C. Urinary mRNA and lupus disease flare. Nephrology. 2017;4:27–30. doi: 10.1111/nep.13151. [DOI] [PubMed] [Google Scholar]
- 166.Winter W.E., Bazydlo L.A.L., Harris N.S. Review. Lab Med Spring. 2014;45:92–102. doi: 10.1309/lmf28s2gimxnwhmm. [DOI] [PubMed] [Google Scholar]
- 167.Suzuki M., Wiers K., Brooks E.B. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr. Res. 2009;65:530–536. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Urrego T., Ortiz-Reyes B., Vanegas-García A.L. Utility of urinary transferrin and ceruloplasmin in patients with systemic lupus erythematosus for differentiating patients with lupus nephritis. Reumatol. Clínica. 2018 Mar 9;(18) doi: 10.1016/j.reuma.2018.02.002. pii: S1699-258X 30039-1. [DOI] [PubMed] [Google Scholar]
- 169.Bennett M.R. Development of a novel renal activity index of lupus nephritis in children and young adults. Arthritis Care Res. 2017;68:1003–1011. doi: 10.1002/acr.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.White K.N., Conesa C., Sánchez L. The transfer of iron between ceruloplasmin and transferrins. Biochim. Biophys. Acta Gen. Subj. 2012;1820:411–416. doi: 10.1016/j.bbagen.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 171.Vanarsa K., Ye Y., Han J. Inflammation associated anemia and ferritin as disease markers in SLE. Arthritis Res. Ther. 2012;14:R182. doi: 10.1186/ar4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Horwitz D.A., Jacob C.O. The cytokine network in the pathogenesis of systemic lupus erythematosus and possible therapeutic implications. Springer Semin. Immunopathol. 1994;16:181–200. doi: 10.1007/BF00197516. [DOI] [PubMed] [Google Scholar]
- 173.Foster M.H., Kelley V.R. Lupus nephritis: update on pathogenesis and disease mechanisms. Semin. Nephrol. 1999;19:173–181. [PubMed] [Google Scholar]
- 174.Farrar J.D., Asnagli H., Murphy K.M. T helper subset development: roles of instruction, selection, and transcription. J. Clin. Invest. 2002;109:431–435. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Noble A. Molecular signals and genetic reprogramming in peripheral T-cell differentiation. Immunology. 2000;101:289–299. doi: 10.1046/j.1365-2567.2000.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ray A., Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Invest. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Neurath M.F., Weigmann B., Finotto S. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chan R.W.Y., Lai F.M.M., Li E.K.M. Imbalance of Th1/Th2 transcription factors in patients with lupus nephritis. Rheumatology. 2006;45:951–957. doi: 10.1093/rheumatology/kel029. [DOI] [PubMed] [Google Scholar]
- 179.Tsokos G.C. Systemic Lupus Erythematosus. J Chem Inf Model. 2011;53:160. [Google Scholar]
- 180.Boucher A., Droz D., Adafer E.N.L. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. - PubMed - NCBI. Kidney Int. 1986;29:1043–1049. doi: 10.1038/ki.1986.105. [DOI] [PubMed] [Google Scholar]
- 181.Caligaris-Cappio F., Bergui L., Tesio L. HLA-Dr+ T cells of the Leu 3 (helper) type infiltrate the kidneys of patients with systemic lupus erythematosus. Clin. Exp. Immunol. 1985;59:185–189. [PMC free article] [PubMed] [Google Scholar]
- 182.Alexopoulos E., Seron D., Hartley R.B. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37:100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- 183.Chan O.T.M., Hannum L.G., Haberman A.M. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Enghard P., Humrich J.Y., Rudolph B. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 2009;60:199–206. doi: 10.1002/art.24136. [DOI] [PubMed] [Google Scholar]
- 185.Meraz M.A., White J.M., Sheehan K.C. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 186.Durbin J.E., Hackenmiller R., Simon M.C. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 187.Horvath C.M., Darnell J.E. The state of the STATs: recent developments in the study of signal transduction to the nucleus. Curr. Opin. Cell Biol. 1997;9:233–239. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 188.Darnell J.E., Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 189.Battle T.E., Frank D.A. The role of STATs in apoptosis. Curr. Mol. Med. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 190.Martinez-Lostao L., Ordi-Ros J., Balada E. Activation of the signal transducer and activator of transcription-1 in diffuse proliferative lupus nephritis. Lupus. 2007;16:483–488. doi: 10.1177/0961203307079618. [DOI] [PubMed] [Google Scholar]
- 191.Brunner H.I., Bennett M.R., Abulaban K. Development of a novel renal activity index of lupus nephritis in children and young adults. Arthritis Care Res. 2016;68:1003–1011. doi: 10.1002/acr.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bartel D.P., Lee R., Feinbaum R. MicroRNAs : genomics , biogenesis , mechanism , and function Genomics : the miRNA genes. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 193.Szeto C. Urine miRNA in nephrotic syndrome. Clin. Chim. Acta. 2014;436:308–313. doi: 10.1016/j.cca.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 194.Wang G., Tam L., Kwan B.C. Expression of miR-146a and miR-155 in the urinary sediment of systemic lupus erythematosus. Clin. Rheumatol. 2012;31:435–440. doi: 10.1007/s10067-011-1857-4. [DOI] [PubMed] [Google Scholar]
- 195.Guan J., Wang G., Tam L. Urinary sediment ICAM-1 level in lupus nephritis. Lupus. 2012;21:1190–1195. doi: 10.1177/0961203312451334. [DOI] [PubMed] [Google Scholar]
- 196.Wang G. Serum and urinary free microRNA level in patients with systemic lupus erythematosus. Lupus. 2011;20:493–500. doi: 10.1177/0961203310389841. [DOI] [PubMed] [Google Scholar]
- 197.Cha C.B., Regna N.L., Hammond S.E. Cellular and urinary microRNA alterations in NZB/W mice with hydroxychloroquine or prednisone treatment. Int. Immunopharm. 2013;17:894–906. doi: 10.1016/j.intimp.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Solé C., Cortés-hernández J., Felip M.L. miR-29c in urinary exosomes as predictor of early renal fi brosis in lupus nephritis. Nephrol. Dial. Transplant. 2015;30:1488–1496. doi: 10.1093/ndt/gfv128. [DOI] [PubMed] [Google Scholar]
- 199.Li Y., Xu X., Tang X. MicroRNA expression profile of urinary exosomes in Type IV lupus nephritis complicated by cellular crescent. J. Biol. Res. 2018;25:1–13. doi: 10.1186/s40709-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin D., Tabb D.L., Yates J.R. Large-scale protein identification using mass spectrometry. Biochim. Biophys. Acta. 2003;1646:1–10. doi: 10.1016/s1570-9639(02)00546-0. [DOI] [PubMed] [Google Scholar]
- 201.Mosley K., Tam F.W.K., Edwards R.J. Urinary proteomic profiles distinguish between active and inactive lupus nephritis. Rheumatology. 2006;45:1497–1504. doi: 10.1093/rheumatology/kel351. [DOI] [PubMed] [Google Scholar]
- 202.Somparn P., Hirankarn N., Leelahavanichkul A. Urinary proteomics revealed prostaglandin H 2 D-isomerase , not Zn- α 2-glycoprotein , as a biomarker for active lupus nephritis. J Proteomics. 2012;75:3240–3247. doi: 10.1016/j.jprot.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 203.Aggarwal A., Gupta R., Negi V.S. Urinary haptoglobin , alpha-1 anti-chymotrypsin and retinol binding protein identified by proteomics as potential biomarkers for lupus nephritis. J Transl Immmunology. 2017;188:254–262. doi: 10.1111/cei.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Suzuki M., Ross G.F., Wiers K. Identification of a urinary proteomic signature for lupus nephritis in children. Pediatr. Nephrol. 2007;22:2047–2057. doi: 10.1007/s00467-007-0608-x. [DOI] [PubMed] [Google Scholar]
- 205.Suzuki M., Wiers K., Brooks E.B. Nitial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr. Res. 2010;65:530–536. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu C.Y., Ph D, Hebert L.A. Biomarker discovery in human SLE nephritis. 2007;65:187–193. [PubMed] [Google Scholar]