Abstract
Childhood-onset systemic lupus erythematosus (cSLE) is a rare, chronic and systemic autoimmune disease generally with a more severe clinical phenotype than the adult-onset SLE. In both conditions, it is known that females are predominantly affected; therefore, the possible overlap of SLE and sex chromosomal abnormalities has attracted attention. Our case report describe the clinical manifestations and immunological profile of a Brazilian female with cSLE and trisomy X. The 22 year-old patient, diagnosed with cSLE at age of 11, present some features related to 47, XXX, such as difficulties at school and communication, although this was not enough to investigate for chromosome abnormalities. Cytoscan HD array screening allowed the comprehensive diagnosis for this patient. We also characterized her ancestral composition, showing that she has 6.2% higher African component than the mean from health subjects from the same geographical area. This report reinforces the role of the X chromosome dose effect for sex bias in SLE, as well as the importance of African ancestry composition in cLES. It also throws lights upon the application of high-throughput molecular analysis in a large scale cohort can be useful to detect the impact of the genomic findings for more accurate epidemiological data.
Keywords: Triple X syndrome, Childhood-onset systemic lupus erythematosus, Autoimmune disease, Cytoscan HD array
Highlights
-
•
This report reinforces the role of the X chromosome dose effect for sex bias in SLE.
-
•
We highlight the importance of African ancestry composition in cSLE.
-
•
The application of high-throughput molecular analysis in a large scale cohort can be useful to detect the impact of the genomic findings for more accurate epidemiological data.
1. Introduction
Childhood-onset systemic lupus erythematosus (cSLE) is a chronic systemic autoimmune disease with onset before the age 18 [1]. Increased prevalence has been observed in women with SLE, independently of age at disease-onset [2]. Several factors have been described to account for the sex difference observed in autoimmune disease, including hormonal and genetic factors. With peak incidence during the reproductive age, sexual hormones have been studied for decades as possible causes and triggers [[3], [4], [5]]. An imbalance between increased estrogens and prolactine levels and a reduction of androgen levels are frequently observed in both men and women with SLE [6].
Genetic factors include an excessive chromosome X that can be observed in women (47, XXX) or in men (46, XXY) [7,8].
Herein we describe clinical and immunological features of a female patient with cSLE with trisomy X and review the literature for similar cases.
2. Material and methods
The patient was followed at the pediatric rheumatology with diagnosis for cSLE [1,9]. The patient was enrolled in a genetic study to analyze copy number variations in SLE and signed the consent form approved by the Research Ethics Committee of our University (920/2007). The medical chart was reviewed for cumulative clinical and immunological features.
DNA was extracted from the blood sample using QIAamp® DNA Blood Maxi Kit (QIAGEN, Hilden, Germany). The genome-wide human Cytoscan HD array (Thermo Fisher Scientific, MA, USA) was used to performed cytogenetic research analysis according to the manufacturer’s protocol. Scanned data files were generated using GeneChip Command Console software v. 1.2. Quality control of the chip was carried out using Chromosome Analysis Suite (ChAS) software v. 3.1. Single nucleotide polymorphisms (SNPs) were mapped according to the human genome reference assembly based on National Centre for Biotechnology Information build 38 (UCSC version hg18).
A panel of 345 ancestry informative markers (AIMs) based on SNP data from Cytoscan HD array was used to infer the proportion of European, African and Amerindian ancestries of the case [10]. The individual ancestral composition based on the SNP-AIMs set was estimated with the Admixture v. 1.23 software [11], a Bayesian model-based algorithm used for clustering the genetic data. For the ancestral inference analysis, genotype frequencies of European and African parental populations were obtained from the HapMap project data (CEU − Utah residents exhibiting Northern and Western European ancestry and YRI − Yoruba in Ibadan, Nigeria). Brazilian Amerindian reference population was based on the Karitiana and Surui populations from the HGDP-CEPH Human Genome Diversity Cell Line Panel.
3. Case description
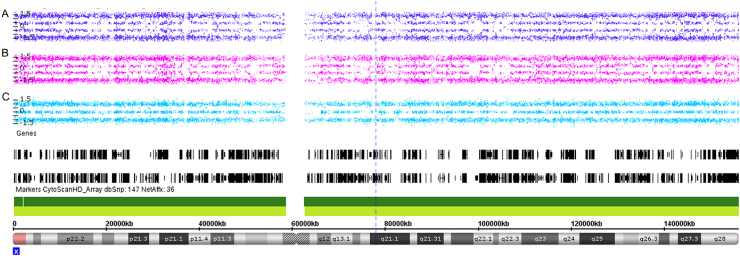
After performing Cytoscan HD array analysis in 90 female cSLE patients, we detect an extra X chromosome in 1 (1.1%) patient. A technical duplication was performed in the same patient to confirm the triple X syndrome (Fig. 1).
Fig. 1.
Allele diference of polymorphic markers from X chromosome for the female cSLE patient confirming 47, XXX diagnosed. The data obtained by Cytoscan HD array displays technical duplications from the case (A, B) compared with a female 46, XX karyotype (C).
The patient is a 22 year-old women diagnosed with SLE at age of 11. Her presenting symptoms were arthritis, hemolytic anemia, nephritis (nephrotic syndrome) and cutaneous vasculitis. Laboratory investigation revealed a positive antinuclear antibodies with titers 1/1280, positive double-stranded DNA (dsDNA), low complement (C3 and C4) and positive lupus anticoagulant. Her previous history, including birth and neuropsychological development was unremarkable. The patient was treated with prednisone 1 mg/kg and, sodium mycophenolate for nephritis induction and hydroxychloroquine. Prednisone was steadily reduced and she was maintained with azathioprine and hydroxychloroquine over the years. The patient presented menarche at the age of 12 and had normal menstrual cycles. Over the years, difficulties in school performance was noted, however she finished high school in time. She had several episodes of flares over the years (arthritis, alopecia, leucopenia) treated with additional prednisone. Since 2015 the patient is in remission with no medication.
Ancestral composition analysis using Admixure software revealed that the main component of the patient was European (73.4%), followed by African (16.0%) and Amerindian (10.6%) (Fig. 2).
Fig. 2.
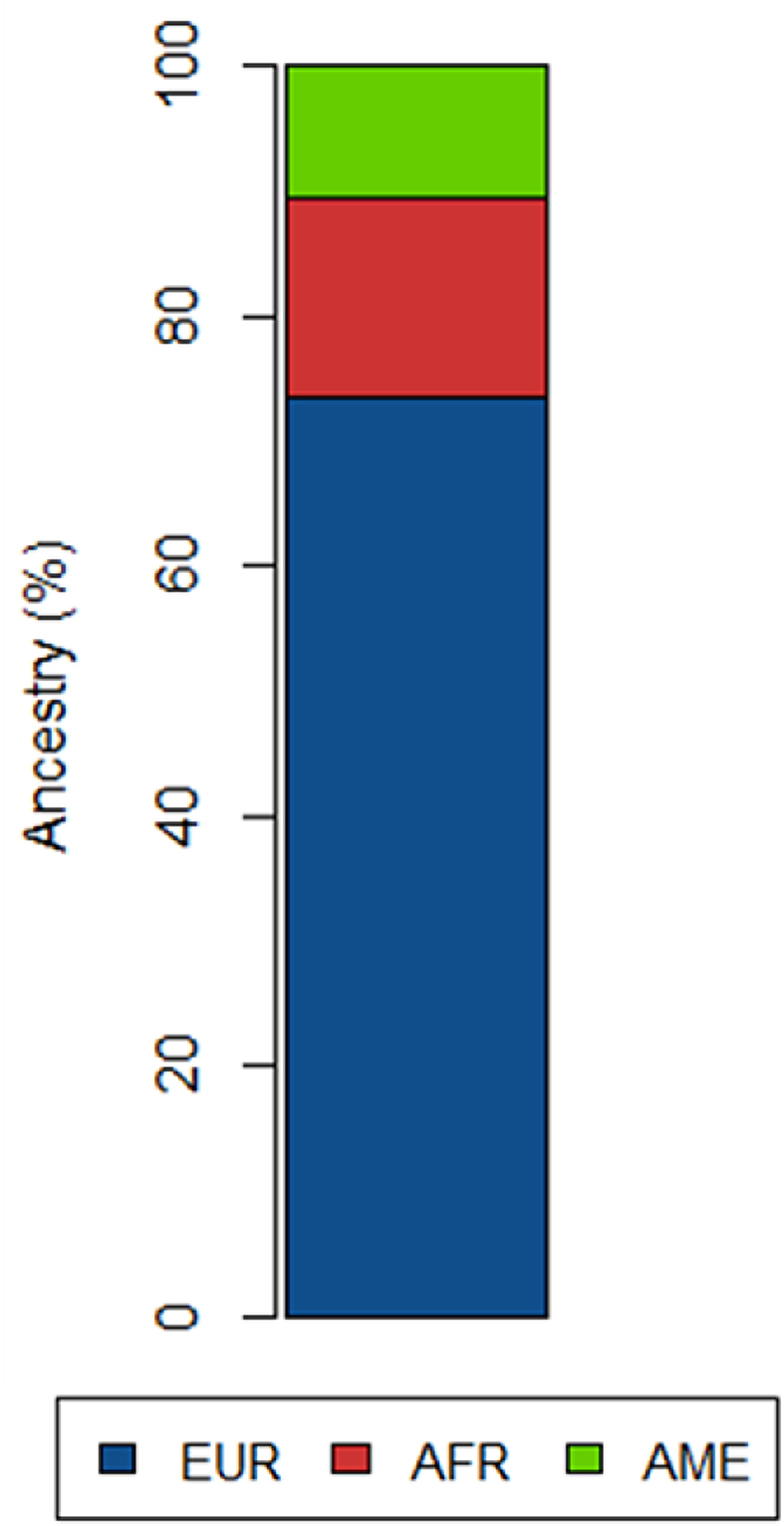
Ancestral composition in female patient with cSLE and Triple X syndrome showing European (EUR), African (AFR) and Amerindian (AME) proportions.
4. Discussion
We observed 1/90 of cSLE women with 47, XXX. There are lack of widespread epidemiological studies describing incidence of 47, XXX and SLE in Brazil. SLE and 47, XXX both occur in 1 in 1000 women as reported in international literature [12,13]. If the conditions were independent, then only 1 in 1 million would have both SLE and 47, XXX. Therefore we observed an increased incidence of 47, XXX in our cSLE cohort. In a large study including 2826 patients with SLE, 1/404 47, XXX were identified, corresponding to an estimate prevalence of SLE and 47, XXX of 2.5 times higher than women with 46, XX and 25 times higher than in men with normal karyotype [12].
In the literature, additional 5 47, XXX SLE patients are reported with detailed clinical and laboratory features [[14], [15], [16], [17]]. Hemolytic anemia was observed in 4/5 case reports, followed by mesangial glomerulonephritis and arthritis 2/5 reports. Four out of 5 patients had positive dsDNA and low complement. These were all features also observed in our patient, however they are not sufficient to characterize a subgroup of SLE patients.
The increased prevalence of SLE in women and the peak incidence of SLE during reproductive age, has raised the role of sex hormones in the pathogenesis of SLE. Increased estrogen and prolactin levels and decreased progesterone, testosterone and DHEA/DHEAS levels have been observed in women with SLE. In males, however, increased levels of prolactin has been observed, while estradiol and testosterone are within normal values and inconclusive data are available for progesterone and DHEA/DHEAS [6]. Estrogen and prolactin affect maturation and selection of autoreactive B cells and autoantibody production [18].
On the other hand, an extra X chromosome can predispose to SLE even in the absence of abnormal sex hormones. Each additional X chromosome, which is normally inactivated by methylation, could exert an effect if methylation is reversed or if additional X chromosomes affect methylation-related regulation of autosomal genes [19]. In a study including 2500 patients, 3 of them had a triple mosaic consisting of 45, X/46, XX/47, XXX, suggesting more attention in genes within partial trisomy of the distal p arm of the X chromosome as the mediators of the X chromosome dose effect for sex bias in SLE [19]. Reports of SLE in mice demonstrated that the disease is associated with the number of X chromosomes, despite the phenotypic sex [20,21].
In men, the presence of an additional X chromosome (46, XX or 47, XXY) is associated with an increased risk of SLE [[22], [23], [24]]. This risk is similar to women 46, XX, however no difference in disease phenotype is observed [7]. Taking together, these finding supports that the gene dosage of the X chromosome is a major determining factor in the female predominance of SLE, highlighting the extreme importance of detecting chromosomal abnormalities in patients to improve epidemiological research in this subject.
Most cases of 47, XXX are undiagnosed, since no significant facial dysmorphology are observed in the majority of the affected women [25]. Minor physical findings described in women 47, XXX were the presence of epicanthal folds, hypertelorism, upslanting palpebral fissures, clinodactyly, overlapping, digits, pes planus, pectus excavatum, hypotonia and joint hyperextensibility. In children 47, XXX under the age of 6, average weight at birth was reported 400–500 g lower than 46, XX women. In addition, head circumference is generally below 50th% and height above 50th%. Delayed motor development, as well as delayed receptive and expressive language development has been reported in 50% of affected girls [26,27]. In older children, increased growth velocity between 4 and 8 years of age is observed. Educational problems are frequently observed. Coordination problems, sensory–motor–visual integration problems, lower IQ, difficulties in language and behavioral problems have been described [26,28,29]. Our patient presented some of these features, such as difficulties at school and communication, however, these are not striking features to investigate 47, XXX chromosome abnormalities.
Screening of cSLE patients using Cytoscan HD array approach allowed the precise diagnosis the triple X condition. Using SNP data from this methodology, women with 47, XXX are recognized by the pattern of allele frequency plot showing four lines in X chromosome (AAA, AAB, ABB, BBB), whereas women with normal karyotype (46, XX) were identified by the three lines (AA, AB, BB) as we shown in Fig. 1.
From the SNP data from the array, ancestry composition analysis revealed that the case report has major European contribution (73.4%), followed by African and Amerindian (Fig. 2). Comparing this ancestral profile with the mean for healthy subjects from the same geographical area, we observed that the African component of the patient (16%) is 6.2% higher than the mean of non-SLE individuals (9.8%) [10]. This corroborates with international findings that incidence SLE rates were higher in African American female subjects compared to Caucasian female subjects, including those with childhood-onset [30].
In conclusion, we observed an increased frequency of 47, XXX in our cSLE cohort. No specific phenotypic features or SLE manifestations were observed that would lead to investigate for chromosomal abnormalities in the patient described here. Since trisomy X is underdiagnosed because the lack of a specific phenotype, but the understand of X chromosome gene dosage is crucial to elucidate the SLE pathogenesis, this report showed that use of high-density molecular approach in a large scale cohort can be applied for more accurate epidemiological data.
Funding information
Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP, grant number (2008/02917–0, 2019/06632–5), Coordination of Superior Level Staff Improvement (CAPES), Brazil 001, Brazilian National Council for Scientific and Technological Development (CNPq), Brazil (306,723/2019–0, 401,477/2016–9, 305,985/2017–5).
Potential competing interests
The authors declare that they have no competing interests.
Specific author contributions
FBB: design the study, data acquisition, data analysis, manuscript writing and reviewing.
NAS: design the study, data acquisition, data analysis, manuscript reviewing.
PRJ: data acquisition, data analysis, manuscript reviewing.
ACL: data acquisition, data analysis, manuscript reviewing.
RM: data acquisition, data analysis, manuscript reviewing.
VLGSL: design the study, data acquisition, data analysis, manuscript writing and reviewing.
SA: design the study, data acquisition, data analysis, manuscript writing and reviewing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: None.
References
- 1.Silva C.A., Avcin T., Brunner H.I. Taxonomy for systemic lupus erythematosus with onset before adulthood. Arthritis Care Res. 2012;64:1787–1793. doi: 10.1002/acr.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mina R., Brunner H.I. Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Res. Ther. 2013;15:218. doi: 10.1186/ar4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutolo M., Capellino S., Sulli A., Serioli B., Secchi M.E., Villaggio B. Estrogens and autoimmune diseases. Ann. N. Y. Acad. Sci. 2006;1089:538–547. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 4.Lleo A., Battezzati P.M., Selmi C., Gershwin M.E., Podda M. Is autoimmunity a matter of sex? Autoimmun. Rev. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson D.L., Gange S.J., Rose N.R., Graham N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 6.McMurray R.W., May W. Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum.: Official Journal of the American College of Rheumatology. 2003;48:2100–2110. doi: 10.1002/art.11105. [DOI] [PubMed] [Google Scholar]
- 7.Margery-Muir A.A., Bundell C., Nelson D., Groth D.M., Wetherall J.D. Gender balance in patients with systemic lupus erythematosus. Autoimmun. Rev. 2017;16:258–268. doi: 10.1016/j.autrev.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Dillon S.P., Kurien B.T., Li S., Bruner G.R., Kaufman K.M., Harley J.B. Sex chromosome aneuploidies among men with systemic lupus erythematosus. J. Autoimmun. 2012;38:J129–J134. doi: 10.1016/j.jaut.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatology. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa F.B., Cagnin N.F., Simioni M., Farias A.A., Torres F.R., Molck M.C. Ancestry informative marker panel to estimate population stratification using genome-wide human array. Ann. Hum. Genet. 2017;81:225–233. doi: 10.1111/ahg.12208. [DOI] [PubMed] [Google Scholar]
- 11.Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K., Kurien B.T., Zimmerman S.L., Kaufman K.M., Taft D.H., Kottyan L.C. X chromosome dose and sex bias in autoimmune diseases: increased prevalence of 47, XXX in systemic lupus erythematosus and sjögren’s syndrome. Arthritis & Rheumatology. 2016;68:1290–1300. doi: 10.1002/art.39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartaglia N.R., Howell S., Sutherland A., Wilson R., Wilson L. A review of trisomy X (47, XXX) Orphanet J. Rare Dis. 2010;5:8. doi: 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosawa S., Kimura O., Sagawa A. Systemic lupus erythematosus in a patient with the 47, XXX karyotype. Arthritis Rheum.: Official Journal of the American College of Rheumatology. 1991;34:371–372. doi: 10.1002/art.1780340317. [DOI] [PubMed] [Google Scholar]
- 15.Slae M., Heshin-Bekenstein M., Simckes A., Heimer G., Engelhard D., Eisenstein E.M. Seminars in Arthritis and Rheumatism. Elsevier; 2014. Female polysomy-X and systemic lupus erythematosus; pp. 508–512. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto T., Fujimoto M., Ikeda K., Saku A., Makita S., Furuta S. Manifestations of systemic lupus erythematosus in female patients with polysomy X: possible roles of chromosome X. Mod. Rheumatol. 2019;29:192–194. doi: 10.1080/14397595.2016.1205800. [DOI] [PubMed] [Google Scholar]
- 17.Alvaro-Gracia J., Humbría A., García-Vicuña R., Ariza A., García-Vadillo A., Laffón A. Systemic lupus erythematosus and tetrasomy-X. J. Rheumatol. 1989;16:1486–1488. [PubMed] [Google Scholar]
- 18.Desai M.K., Brinton R.D. Autoimmune disease in women: endocrine transition and risk across the lifespan. Front. Endocrinol. 2019;10:265. doi: 10.3389/fendo.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma R., Harris V.M., Cavett J., Kurien B.T., Liu K., Koelsch K.A. Brief report: rare X chromosome abnormalities in systemic lupus erythematosus and sjögren’s syndrome. Arthritis & Rheumatology. 2017;69:2187–2192. doi: 10.1002/art.40207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasidhar M.V., Itoh N., Gold S.M., Lawson G.W., Voskuhl R.R. The XX sex chromosome complement in mice is associated with increased spontaneous lupus compared with XY. Annals of the rheumatic diseases. 2012;71:1418–1422. doi: 10.1136/annrheumdis-2011-201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith-Bouvier D.L., Divekar A.A., Sasidhar M., Du S., Tiwari-Woodruff S.K., King J.K. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen A., Sevier S., Kelly J.A., Glenn S.B., Aberle T., Cooney C.M. The lupus family registry and repository. Rheumatology. 2011;50:47–59. doi: 10.1093/rheumatology/keq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scofield R.H., Bruner G.R., Namjou B., Kimberly R.P., Ramsey-Goldman R., Petri M. Klinefelter’s syndrome (47, XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon S., Aggarwal R., Harding J.W., Li L.J., Weissman M.H., Li S. Klinefelter’s syndrome (47, XXY) among men with systemic lupus erythematosus. Acta Paediatr. 2011;100:819–823. doi: 10.1111/j.1651-2227.2011.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustavson K. Triple X syndrome deviation with mild symptoms. The majority goes undiagnosed. Lakartidningen. 1999;96:5646. [PubMed] [Google Scholar]
- 26.Robinson A., Ha L. Summary of clinical findings: profiles of children with 47. XXY. 1979:47. XXX and 47, XYY karyotypes. [PubMed] [Google Scholar]
- 27.Robinson A., Lubs H.A., Bergsma D. Sex chromosome aneuploidy: prospective studies on children. Birth Defects Orig. Artic. Ser. 1979:15. [Google Scholar]
- 28.Ratcliffe S., Butler G., James M. Wiley-Liss for the National Foundation-March of Dimes; New York: 1990. Children and Young Adults with Sex Chromosome Aneuploidy. Edinburgh Study of Growth and Development of Children with Sex Chromosome Abnormalities; pp. 59–115. [Google Scholar]
- 29.Ratcliffe S.G., Paul N. 1986. Prospective Studies on Children with Sex Chromosome Aneuploidy. [PubMed] [Google Scholar]
- 30.Somers E.C., Marder W., Cagnoli P., Lewis E.E., DeGuire P., Gordon C. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis & rheumatology. 2014;66:369–378. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]