Abstract
Autoimmune diseases evolve from complex interactions between the immune system and self-antigens and involve several genetic attributes, environmental triggers and diverse cell types. Research using experimental mouse models has contributed key knowledge on the mechanisms that underlie these diseases in humans, but differences between the mouse and human immune systems can and, at times, do undermine the translational significance of these findings. The use of human immune system (HIS) mice enables the utility of mouse models with greater relevance for human diseases. As the name conveys, these mice are reconstituted with mature human immune cells transferred directly from peripheral blood or via transplantation of human hematopoietic stem cells that nucleate the generation of a complex human immune system. The function of the human immune system in HIS mice has improved over the years with the stepwise development of better models. HIS mice exhibit key benefits of the murine animal model, such as small size, robust and rapid reproduction and ease of experimental manipulation. Importantly, HIS mice also provide an applicable in vivo setting that permit the investigation of the physiological and pathological functions of the human immune system and its response to novel treatments. With the gaining popularity of HIS mice in the last decade, the potential of this model has been exploited for research in basic science, infectious diseases, cancer, and autoimmunity. In this review we focus on the use of HIS mice in autoimmune studies to stimulate further development of these valuable models.
Keywords: Humanized mice, Human immune system mice, Autoimmunity, Tolerance, SCID mice
Highlights
-
•
Human immune system (HIS) mice bear components of the human immune system.
-
•
HIS mice engraft with human blood or hematopoietic stem cells, and sometimes thymus.
-
•
HIS mice are used to investigate development and function of the human immune system.
-
•
Immunological tolerance and autoimmune responses can be studied in HIS mice.
-
•
HIS models of autoimmunity vary in complexity and in ability to represent disease.
1. Introduction
The immune system has evolved to ensure the development and maturation of functional lymphocytes able to recognize and quickly respond to foreign antigens while maintaining tolerance to self. Despite the presence of multiple tolerance checkpoints throughout lymphocyte development, a small fraction of autoreactive B and T cells escape and survive these mechanisms. These autoreactive lymphocytes are generally short-lived and anergic, remaining incapable of attacking self. However, under certain environmental and genetic settings, these clones are able to proliferate and respond to self-antigens, the degree and target of these responses determine the severity and tissue distribution of the clinical manifestations. These autoimmune responses can evolve into full-fledged chronic diseases for which there are no cures. Autoimmunity affects up to 5% of the human population, women predominantly, causing a variety of debilitating illnesses and significantly shortening the lifespan. While some autoimmune diseases have effective treatments, the administration of insulin in type 1 diabetes (T1D) is an example, other diseases – e.g., systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) – rely mainly on broad anti-inflammatory and immunosuppressive drugs with mixed responses and a variety of side effects. In addition to the spontaneous development of autoimmunity caused by the synergy of genetic and environmental predisposing factors, recently we have been faced by an additional challenge: the rise of autoimmune complications that follow the use of novel immunotherapy protocols in cancer patients.
Autoimmune diseases have a complex origin and development. Although our understanding of these diseases has vastly improved, their complexity has hampered a concrete elucidation of their causes. The biological mechanisms driving the onset of autoimmunity remain largely undefined, also because they are multifaceted and diverse for different patients. Moreover, the variability in the response of patients to a given treatment is often unexplained. This fosters a great and unmet need to better understand disease pathogenesis to find new ways to treat successfully a higher number of patients, and also to develop protocols that may altogether prevent disease onset in people at risk. Over time, the escalation in scientific and technological exploration of human biology has been justifiably accompanied by a rise of ethical considerations and regulations. For instance, testing whether a new vaccine works as Jenner did at the end of the 18th century (i.e., inoculating an experimental vaccine into a child who is then challenged with a deadly virus) is unthinkable nowadays. Small mammals, including rabbits, rats and mice but also larger non-human primates, have become accepted systems to model human diseases, understand mechanisms, and test new treatments. Mice have been particularly useful in this regard: they can live in small spaces, are easy to handle, reproduce robustly and, most importantly, their immune system and responses to infections and vaccines, as well as the development of autoimmune responses, are remarkably similar to those of humans. Mouse models exist that spontaneously develop autoimmune diseases, such as the non-obese diabetic (NOD) with T1D, and the MRL and the NZBXNZW that develop lupus. These strains are still heavily used as models for basic and translational research in autoimmunity. However, can we always be sure that mice faithfully represent human biology? This is definitively a problem when dealing with pathogens that do not infect mice, but it is also an issue when considering developing and testing new treatments that target human cells.
The desire to improve the modeling of human diseases and probing their molecular mechanisms have led investigators to play with the idea of “humanizing” mice. This is a loose term with different meanings, but usually refers to the introduction of a human component, an individual gene, a locus, a cell type, or even a tissue into the mouse, to generate what we call humanized mice (hu-mice). Some of the alleles of the highly polymorphic Human Leukocyte Antigen (HLA) locus, which encodes the human MHC proteins, display the strongest association with autoimmunity [1]. Their involvement in autoimmunity is based on the fact that MHC proteins present host and foreign peptides to T cells, thus playing a crucial role in T cell development and adaptive immune responses. For these reasons, HLA transgenic mice were among the first “humanized” animal models of autoimmune diseases [[2], [3], [4], [5]]. These models have become even more sophisticated by introducing additional genetic mutations, such as a recent study that created C57BL/6 mice lacking mouse MHC II and instead expressing human HLA-DR4, a high risk allele for T1D development, and human CD80, for additional co-stimulation of T cells [6]. Upon immunization with murine proinsulin-2, these mice developed a disease similar to human T1D [6], with myeloid and lymphoid murine cell infiltration into the pancreatic islets and the production of insulin-reactive autoantibodies [6,7]. These and other studies have considerably advanced our knowledge of autoimmunity development and progression. Nevertheless, significant discoveries in mice on the mechanisms and treatment of diseases do not often replicate in human patients [8,9].
Despite the many similarities, there are also clearly significant differences between the mouse and human immune systems, differences that have not yet been all resolved. Moreover, laboratory mice belong to inbred strains with limited genetic variation. Thus, discoveries made in the mouse must be first verified and further investigated in humans [[10], [11], [12], [13]], particularly when considering using new drugs in patients based on knowledge acquired in mice. Since testing mechanisms and drugs in humans is hampered by ethical, practical, and safety reasons, the pressure of improving the “humanization” of mice has steadily increased, leading to the idea of transplanting these animals with cellular components of the human immune system. In this review we focus on hu-mice that carry a human immune system (HIS) either via transplantation of human blood cells or of hematopoietic stem cells.
2. Development and improvement of human immune system mice
Mouse models of murine autoimmunity remain important tools for investigating the immune system at the mechanistic level. On the other hand, HIS hu-mice offer the additional potential to test whether these mechanisms truly operate in the human immune system, to add genetic variation to the modeling of immune responses, and for testing the effect of novel therapies on human cells [14]. HIS hu-mice are generated by transplanting human hematopoietic cells, such as peripheral blood monocytes (PBMCs), T cells, or hematopoietic stem cells (HSCs), into immunodeficient mice. After cell transplantation and the establishment of cell chimerism, these animals allow for experimentation on human immune cells in vivo, within a small animal model setting. PBMCs are typically injected intravenous (i.v.) in adult mice in the absence or after a small irradiation dose. Transplant of HSCs, which are CD34+ cells generally isolated from umbilical cord blood after live births or from fetal liver after voluntary termination of pregnancy, requires sublethal irradiation of the recipient animals. Chemical myeloablation (e.g., with busulfan) has been sometimes used in place of radiation. HSC transplant is performed by injecting cells either into newborn mice, i.v. into the facial vein or directly into the liver, or into adult mice, typically by i.v. tail vein injection. To distinguish HIS hu-mice made with different sources of cells we use the term hu-PBL for mice generated by the transfer of human PBMCs and hu-HSC for mice generated by the transfer of human HSCs.
Improvement of the HIS hu-mouse model over the years followed the introduction of genetic modifications in recipient mice that eventually culminated in the development of more stringent immunodeficient host animals. The first of these genetic modifications came from the discovery of a natural mutation in the protein kinase DNA-activated catalytic polypeptide (Prkdc) found in Severe Combined Immunodeficiency (SCID) mice on the CB17 genetic background. Given the essential role of Prkdc in non-homologous end joining DNA repair during V(D)J recombination, the PrkdcSCID mutation leads to a severe deficiency in B and T lymphocytes, allowing for the engraftment of human cells in a mouse host without the issue of rejection by the adaptive immune system [[15], [16], [17]]. In one of the first autoimmune studies using SCID mice, injection of human PBMCs from autoimmune patients was performed to determine whether this led to the development of autoantibodies and disease symptoms similar to those of patients [18,19]. Indeed, autoantibodies were occasionally observed. However, disease manifestations did not develop, possibly because many of the human effector cells transferred into the mice did not survive long enough to generate a functional immune system. Furthermore, these studies were generally hampered by the development of graft versus host disease (GVHD) that arises in the context of MHC mismatch between donor and recipient cells. As it turns out, GVHD per se can cause the production of autoantibodies, confounding interpretations [20,21]. Other limitations observed in this model were the high numbers of mouse NK cells, which can directly limit human cell engraftment. Moreover, the PrkdcSCID mutation also affects the ability of myeloid cells to repair DNA damage, a concern when exposing mice to the ionizing radiations required for the engraftment of human HSCs [22,23]. Finally, while most of the SCID mice lack lymphocytes, as they age some accumulate functional (mouse) T and B cells due to a ‘leaky’ phenotype whereby alternate DNA repair mechanisms are able to rescue defective V(D)J gene recombination [24,25]. These issues significantly affected the ability to use SCID mice as recipients of a transplanted human immune system.
Not long after the discovery of the PrkdcSCID mutation two different groups used the recently developed technique of homologous gene recombination to generate knock-out mice for the recombination activating genes Rag1 and Rag2. These RAG-deficient animals completely lacked mature B and T cells due to an absolute inability to perform B cell receptor (BCR) and T cell receptor (TCR) V(D)J gene rearrangements, events that are absolutely necessary for antigen receptor expression and subsequent signaling [26,27]. Moreover, genetic deletion of Rag1 or Rag2 genes had a permanent and specific impact on lymphocyte development but not elsewhere, meaning it could overcome both the radio sensitivity and the “leakiness” issues typical of SCID mice [26,27]. Nevertheless, RAG knockout mice did not significantly improve the engraftment and maintenance of human cells because of the presence of mouse NK cells, the number of which expand to fill the void left by the absence of mature B and T cells [26,27].
In the meantime, to address the low human cell engraftment observed in CB17-SCID hu-mice, the PrkdcSCID mutation was backcrossed onto different genetic backgrounds including the NOD mouse strain. Human cell engraftment was greatly improved in NOD-SCID mice, both in percentage and in kinetics [28]. In addition to developing diabetes, NOD mice are appreciated to display poor NK cell activity, which likely contributed to the improved human chimerism [[29], [30], [31]]. Nevertheless, even in NOD-SCID hu-mice the establishment of a human immune system maintained significant problems that restricted a wider use of this model for human immunological studies. For instance, the NK cell population in NOD-SCID hu-mice was only diminished but not abolished, still causing some tissue rejection. Moreover, these mice displayed spontaneous development of thymic lymphomas with increased mortality after 5 months of age [30]. In the mid 1990s, the realization that mutations in the interleukin-2 (IL-2) receptor γ-chain locus (IL2Rγ or CD132) lead to severe immunodeficiency [[32], [33], [34]] was finally instrumental for improving HIS hu-mice. IL2rγ is an essential component for the intracellular signaling of IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 cytokine receptors. Mutations in Il2rγ result in X-linked severe combined immunodeficiency (XSCID) in humans and mice [[32], [33], [34], [35], [36], [37]]. This is characterized by a profound defect in T cell development but also an arrest of NK cell development due to, at least in part, a decreased ability to respond to IL-7 and IL-15, respectively [[37], [38], [39]]. IL-7 is also important for B cell development in mice [40], further reducing the host lymphocyte population and increasing the chances of human cell repopulation. The targeted mutations of the Il2rγ gene were introduced into the NOD-SCID background creating the NOG strain first [41,42] and the NSG strain later [43]. They were also introduced into the BALB/c-Rag2null background creating the BRG strain [44], and the NOD-RAG−/− background, creating the NRG strain [45]. The inactivation of IL2Rγ led to a crucial improvement of human chimerism in HIS hu-mice, with superior human cell engraftment and the development of a functional adaptive immune system [[42], [43], [44], [45]]. With the creation of NSG, BRG and NRG immunodeficient mice, the use of HIS hu-mouse models became more widespread and was paralleled by the range of areas investigated, including infections, transplantation, tumor responses, autoimmunity, but also basic immunological studies [[46], [47], [48]].
The quest for a “better” humanized mouse model led to the simultaneous development of immunodeficient strains in different genetic backgrounds. Among the mouse strains historically used to study immune responses, it was found that the BALB/c genetic background, in contrast to C57BL/6, allowed the establishment of human hematopoietic chimerism [44,49]. Nevertheless, even when sharing the same genetic modifications, NSG or NRG HIS mice still exhibited higher human cell engraftment relative to BRG HIS mice. The basis for this difference was subsequently demonstrated by an elegant study from Danska and colleagues showing that the NOD, but not the BALB/c (or C57BL/6) variant of the Signal Regulatory Protein-α (SIRP-α), a protein expressed on the surface of mouse macrophages, is able to bind human CD47 on human cells. This binding, which represents a “don’t eat me” signal, inhibits phagocytosis, leading to higher human chimerism in NOD immunodeficient strains [50]. This discovery guided the development of BRG mice harboring either the NOD or human SIRP-α variants (strains named BRGS) and resulting in levels of human hematopoietic engraftment similar to those of NOD strains [51,52]. This line of study also led to the development of a BL/6-Rag2nullIL2rγnull strain that upon the deletion of the CD47 locus allows the development of human hematopoietic cells [53]. Despite these genetic manipulations, however, myeloablative irradiation still remains a common limiting requirement for the development of HIS mice. Such conditioning is necessary for opening niches in bone marrow microenvironments in which human HSCs can engraft, but it can also lead to tissue damage and other defects, particularly in SCID strains. The need for irradiation was relieved when mutations in the Kit gene (encoding c-Kit or CD117) were backcrossed into the BRG and NSG strains [54,55]. C-Kit, the receptor for stem cell factor, is expressed on hematopoietic cells and is required for hematopoiesis. Defects in murine HSCs caused by the KitW41 mutation allows engraftment of multilineage human hematopoietic cells in intact NSGW41 mice at levels similar to those achieved in irradiated NSG mice [54,55].
The response of conventional T cells relies on the ability of their TCRs to recognize (and bind) a specific peptide in the context of an MHC molecule that was expressed in the thymus during their initial development. Recognition of these selective peptides occurs mostly on thymic epithelial cells, although thymic dendritic cells and B cells also contribute to some extent to the selection of the emerging T cell repertoire [56]. In HIS mice generated by transplanting human HSCs, human T cells develop in the mouse thymus where they simultaneously undergo maturation and selection via recognition of mouse MHC molecules expressed by mouse epithelial and dendritic cells. HIS mouse models in which the T cells are educated, and therefore restricted, to mouse MHC are not ideal for studies that require these cells to coordinate their response with human antigen presenting cells (APCs), which express human MHC (i.e., HLA) receptors. From the early implementation of hu-mice, investigators interested in studying human T cell development and function collected from the same human fetus fragments of thymus and liver tissues that were then implanted beneath the kidney capsule of SCID mice. This procedure led transplanted SCID mice to generate a transient wave of human CD4 and CD8 T cells that had undergone selection within the transplanted thymic fragments, on human HLA [16,57]. The human T cells developing in these animals, however, were not functional due to the lack of human APCs. To achieve a HIS hu-mouse model in which T cells are HLA educated and able to coordinate a functional immune response with human APCs, Lan and colleagues in 2006 transplanted human CD34+ cells isolated from human fetal liver into NOD-SCID mice that were implanted with liver and thymus from the same fetus, a model later named bone marrow-liver-thymus (BLT) [58]. In BLT hu-mice, the human T cells develop within the human thymus (implanted under the mouse kidney capsule), and are, therefore, HLA-restricted, while human APCs mature within the human liver or in the mouse bone marrow. Together, these cells can mount strong and coordinated immune responses [58,59]. Since then the BLT hu-mouse has become the model of choice for certain studies, such as HIV infection, both because of the robust production and more physiological MHC education of T cells, but also due to the enhanced presence of T cells and other human cells in mucosal tissue [60].
A shortcoming of early BLT models was that they were prone to developing fatal GVHD [61], a major obstacle for studying long-term immune responses such as autoimmunity. Methods that reduce number and/or function of the human T cells that are endogenous to the donor thymus, such as freezing and thawing the fetal thymus tissue before implant and treating the thymus-implanted mice with anti-hCD2 antibodies, are some of the strategies shown to attenuate or altogether eliminate GVHD [62]. Deleting MHC class I and II in the recipient mice has also been shown to greatly reduce GVHD, at least in hu-PBL animals [[62], [63], [64]]. The generation of BLT hu-mice includes additional issues. One is about the availability of fetal thymus, which is not often easy to procure. Other concerns are of ethical and legal nature. In some countries, voluntary abortion and/or the use of related fetal tissue for research are not legal. Even when legalized, some investigators do not approve the use of this tissue for personal ethical reasons. In the USA, federal government agencies have recently imposed additional oversight for approval of grants proposing fetal tissue research, and federal researchers are currently not allowed to work with this tissue altogether. An alternative to fetal thymus is neonatal thymus. This tissue can be acquired from pediatric cardiology units as it is often discarded during infant heart surgery, and one recent study suggests it can function similar to fetal thymus when implanted into mice, supporting the HLA education of developing human thymocytes [65]. However, although neonatal thymus does not possess the ethical problems associated with the use of fetal tissue, it does bear the critical drawback of lacking a source of fully HLA-matched hematopoietic stem cells, reducing the functionality of the immune system it supports. In these animals, in fact, HLA screening of human cord blood or bone marrow HSCs must be performed to select those with partial allele matches to available thymi.
In spite of the many improvements implemented over the years, HIS hu-mice in NSG, NRG and BRGS strains retain important limitations. This is because cytokines required for the development and maintenance of leukocytes are often produced by non-hematopoietic cells, and some of the mouse cytokines are not as functional on human cells as their human orthologs due to sequence divergence in ligands and/or receptors [14,66]. To improve the generation and function of human leukocytes, different labs have generated recipient strains with transgenes encoding relevant human cytokines. For instance, replacing the mouse Il-6 gene with human Il-6 in BRGS mice, has led to enhanced human thymopoiesis and peripheral T cell numbers, while also increasing the number of class-switched memory B cells and IgG production [67]. Lymphocytes aside, the human myeloid populations are even more significantly impacted in HIS hu-mice. This is because, besides chemokine and receptor divergence, human myeloid cells have to compete during their development with the mouse host myeloid populations. The generation of NSG strains expressing human IL-3, GM-CSF and stem cell (or Steel) factor (strains named NSG-SGM3 or NSGS) has led to great improvement in the numbers of human neutrophils and monocytes, positively impacting also the number of CD4 T cells and mature B cells [[68], [69], [70]]. Related genetic modifications (the introduction of human M-CSF, IL-3, GM-CSF, and thrombopoietin) have been implemented in BRG/S mice to generate strains named MSTRG and MISTRG, which perform similar to NSGS mice upon transplantation with human HSCs [71]. Although these strains greatly improve human monocyte development, they also have some drawbacks such as the mobilization of bone marrow HSCs that reduces their regeneration potential, and the development of anemia and the consequent shortened lifespan [72,73]. An additional drawback is that these animals develop a dendritic cell population that is greatly skewed in favor of mouse instead of human cells. Deleting the mouse receptor tyrosine kinase Flk2/Flt3 gene has led to the development of a BRGS strain (named BRGSF) that, with the administration of human Flt3L, significantly improves the numbers of human DCs and their function [74], leading also to a better reconstitution and function of human NK cells and innate lymphoid cells (ILCs) in lymphoid and mucosal tissues [75]. Other studies have shown that transgenic expression of human IL-15, or injection of human IL-15 coupled to IL-15 receptor alpha, substantially improves NK cell development and function in HIS mice generated in different genetic backgrounds [[76], [77], [78], [79]]. Finally, while the mutation of the Il2rγ gene has tremendously improved the generation of a human immune system in mice, the deficiency in IL2Rγ/CD132 signaling has the unfortunate drawback of preventing the formation of secondary lymphoid tissue due to defective IL-7 responses and the consequent lack of lymphoid tissue inducer cells [80]. A BRGS strain (named BRGST) with a transgene-mediated overexpression of murine thymic stromal cell derived lymphopoietin (TSLP) was recently generated to obviate this defect [81]. In addition to developing lymph nodes in larger numbers and size, BRGST HIS mice exhibit significantly higher numbers of T follicular helper cells, a more organized distribution of B and T cells in follicles, and improved IgG antibody responses.
3. Using HIS mice to study tolerance and autoimmunity
HIS mice represent an experimental in vivo model to study the complex dynamics of the human immune system in a controlled setting, a model in which extensive technical manipulations and analyses can be performed in ways that are not tenable in human studies. Not only can this animal model help elucidate human biological processes, it can also provide an ideal setting for testing new therapies. The HIS mouse model has been used to explore many diverse properties and functions of the human immune system [14,[82], [83], [84], [85]]. Here we discuss studies in which this model was used to investigate properties related to immunological tolerance and autoimmunity.
3.1. Tolerance in HIS mice
Human T cells develop relatively well in HIS mice, but studies on T cell function must rely on a model in which the T cell repertoire is not only diverse but also tolerant to the host. Studies in mice have demonstrated that the development and selection of thymocytes relies on a regulated migration through distinct thymic microenvironments and the local interaction with different cell types [56]. Moreover, T cell development has been well characterized to progress through several phenotypically distinct stages defined by the expression of the co-receptors CD4 and CD8 and the rearrangement of their T cell receptor (TCR) genes [86,87]. Within the thymic cortex, cells at the CD4/CD8 double negative (DN) stage undergo V(D)J recombination of the β chain of the TCR genes leading to the expression of the pre-TCR complex, an event followed by the expression of both co-receptors CD4 and CD8. These double positive (DP) cells, while still in the thymic cortex, undergo VJ recombination at the TCR α chain genes leading to the expression of a mature TCR and the first repertoire selection event, known as the positive selection [[87], [88], [89], [90], [91]]. DP thymocytes that are able to bind weakly to the MHC-peptide complex present on the surface of cortical thymic epithelial cells (cTEC) receive survival stimuli and are positively selected to continue their development. After this event, DP thymocytes downregulate the expression of the co-receptor that was not engaged in binding the MHC-peptide complex, thus becoming (CD4 or CD8) single positive (SP) thymocytes. SP thymocytes migrate to the medullary region where they are tested for autoreactivity during the negative selection process. Negative selection is promoted mostly by medullary thymic epithelial cells (mTEC), but also by thymic conventional and plasmacytoid dendritic cells and by B cells. This process leads to the deletion of self-reactive T cells, i.e., T cells that bind too strongly to the MHC-peptide complex [56,92,93]. As we described above, when human T cells develop in HIS mice transplanted only with human HSCs, they undergo maturation in a mouse thymus and, thus, they are mostly restricted by mouse MHC. In recipients that express transgenic human HLA alleles, or that have been implanted with fragments of a human thymus, the T cells are selected also, or entirely, on human HLA, depending on whether mouse MHC molecules and/or the mouse thymus are still present. Despite these differences, in all HIS models the thymic maturation of human conventional T cells has been found to be normal, resulting in the development of a peripheral T cell population with a diverse repertoire [[94], [95], [96]]. On the other hand, the development of regulatory T cells (Tregs) is greatest when a human thymic organoid is present [96,97]. An important question is whether the development of thymocytes in HIS mouse models includes the negative selection of autoreactive T cells and the generation of a peripheral T cell population tolerant to the host. In their seminal study, Traggiai and colleagues used a mixed lymphocyte reaction to show that the peripheral T cell population of hu-HSC HIS mice is tolerant to both mouse and human MHC, indicating that in this model, the human T cells developing in the mouse thymus undergo selection on MHC antigens of both species [44]. Thus, their findings suggest that human APCs seeding the mouse thymus present antigens to human thymocytes and are partly responsible for the development of a tolerant T cell population. A recent study specifically investigated the ability of human thymic APCs to promote negative selection of CD8 T cells. To do so, BLT hu-mice were transplanted with a mix of human CD34+ HSCs: some were transduced with vectors encoding either a MART1 or a control antigen, while others were transduced with a TCR specific for a MART1 peptide in the context of HLA-A2 [98]. In these animals, T cells expressing the MART1-specific TCR developed among other T cells and in the presence of APCs that either did or did not express the MART1 peptide. The mice with APCs expressing the MART1 peptide displayed profound clonal deletion of their developing MART1-reactive CD8 T cells, indicating an ability of human APCs to populate the mouse thymus and participate in the process of negative selection [98]. Another recent study utilized high-throughput sequencing of TCRβ Complementarity-Determining Region 3 (CDR3) and single-cell TCR sequencing to demonstrate that NSG BLT hu-mice generate a very diverse T cell repertoire that develops via positive selection mediated by weak interaction with self-peptides [99]. Overall, these data indicate that whether in the mouse thymus or in a human thymic organoid, human thymocytes developing in HIS mice are properly selected by both thymic epithelial cells and thymic APCs.
In mammals after birth, B cells develop within the bone marrow tissue through a stepwise differentiation process. This process is regulated mostly in a cell-intrinsic manner by antigen receptors containing immunoglobulin (Ig) heavy and light chain proteins expressed following productive Ig V(D)J gene rearrangement events [100,101]. Similar to the rearrangements that produce the TCR in T cells, these Ig gene rearrangements are random events, producing Ig genes encoding BCRs that can potentially bind foreign antigens but also those that bind self-antigens [102,103]. The mechanisms of tolerance that purge the B cell population of autoreactive clones have been carefully investigated in mice by employing Ig transgenic and knock-in animals with Igs of defined antigen specificities. These studies have shown that newly generated autoreactive B cells with medium to high avidity for self-antigen are eliminated within the bone marrow by two mechanisms of central tolerance: receptor editing, a process using secondary Ig light chain gene rearrangements to edit the antigen receptor, or clonal deletion, which leads to cell death [[104], [105], [106], [107]]. In contrast, autoreactive B cells with low avidity for self-antigens are able to leave the bone marrow, but for the most part, they are anergic and short-lived, which promotes tolerance in the peripheral B cell population [108]. Our group has spent significant effort investigating the development and function of human B cells in HIS hu-mice. Using the model generated by transplanting human HSCs from umbilical cord blood into BRG and BRGS neonates, we have shown that human B cells are robustly produced in these animals and that their bone marrow development is largely normal [109,110]. Once exported outside the bone marrow, however, newly generated human B cells are impaired from developing into fully mature cells, and we have found that their maturation is aided by the accumulation of T cells [109] but not by the addition of human BAFF [110].
We were curious to understand whether human autoreactive B cells are properly selected in HIS mice and, if so, to use this model to elucidate the mechanisms of human B cell tolerance. Peripheral B cell tolerance is implemented by both B cell-intrinsic and extrinsic mechanisms [104,111], but extrinsic restraint (or lack thereof) may be less than optimal in HIS hu-mice because of the suboptimal coordination of B and T cell responses. Given that central B cell tolerance is, to large extent, a B cell-intrinsic process, we setup a HIS model to study the bone marrow selection of human autoreactive immature B cells. To achieve this goal we tailored a system established by Nemazee and colleagues to study central tolerance of wild-type mouse B cells [112]. In this system, transgenic mice ubiquitously express a synthetic membrane antigen that serves as a ‘self’ antigen by incorporating antibody variable region genes specific for Ig constant regions. Thus, a recipient BRG strain expressing a synthetic self-antigen specific for the constant region of human Igκ would be expected to censor all Igκ+, but not Igλ+, human B cells while they develop in the bone marrow. Indeed, using this system we demonstrated that central B cell tolerance is intact in HIS mice [113]. In this model, developing human autoreactive (κ+) B cells undergo central tolerance by both receptor editing and clonal deletion. This was shown by augmented expression of RAG1/2 genes and increased extrusion of Igλ excision DNA circles in bone marrow κ+ B cells, accompanied by a slightly reduced number of B cells entering the spleen [113]. In these animals, the peripheral B cell population is almost exclusively composed of λ+ B cells, demonstrating that, similar to mouse B cell development, central tolerance for human B cells is extremely efficient. In agreement with our findings, repertoire studies have shown that newly generated B cells in the spleen of NSG HIS mice comprise only rare clones reacting with common self-antigens such as DNA and those expressed by Hep2 cells. This further suggests autoreactive human B cells developing in HIS mice are generally eliminated in the bone marrow [114]. B cells in the spleen of HIS hu-mice also display reduced frequency of autoreactive VH4-34+ clones relative to their bone marrow counterpart [115]. These overall findings demonstrate that central B cell tolerance is intact in HIS hu-mice. Therefore, this model could also be used to elucidate the molecular mechanisms that either drive or impair the negative selection of autoreactive B cells. In such a study, NSG mice were transplanted with genetically altered human HSCs to demonstrate that central B cell tolerance is either reinforced or diminished by the respective expression of the AID gene or the PTPN22 autoimmune-associated variant, consistent with observations in patients with relevant genetics [114,116]. Overall these studies show that HIS hu-mice are useful for investigating mechanisms of central lymphoid tolerance and repertoire generation.
But can this model system be used to also study full-fledged autoimmunity? Autoimmunity is a complex process involving multiple cell types, genetic loci, and environmental drivers. Due to its complexity, achieving the development of disease models that faithfully represent the overall disease process requires the establishment of a sophisticated human immune system that can also interact with non-hematopoietic cell types and the environment. Though HIS hu-mice develop a human immune system, the function of this system is imperfect due to many differences in the biological environments of mice and humans. Thus, while some aspects of autoimmunity can be recapitulated and studied in this model, others are not yet attainable. Nevertheless, significant progress has been made in understanding the use and limitations of HIS mice for studying autoimmune diseases (Fig. 1, Table 1).
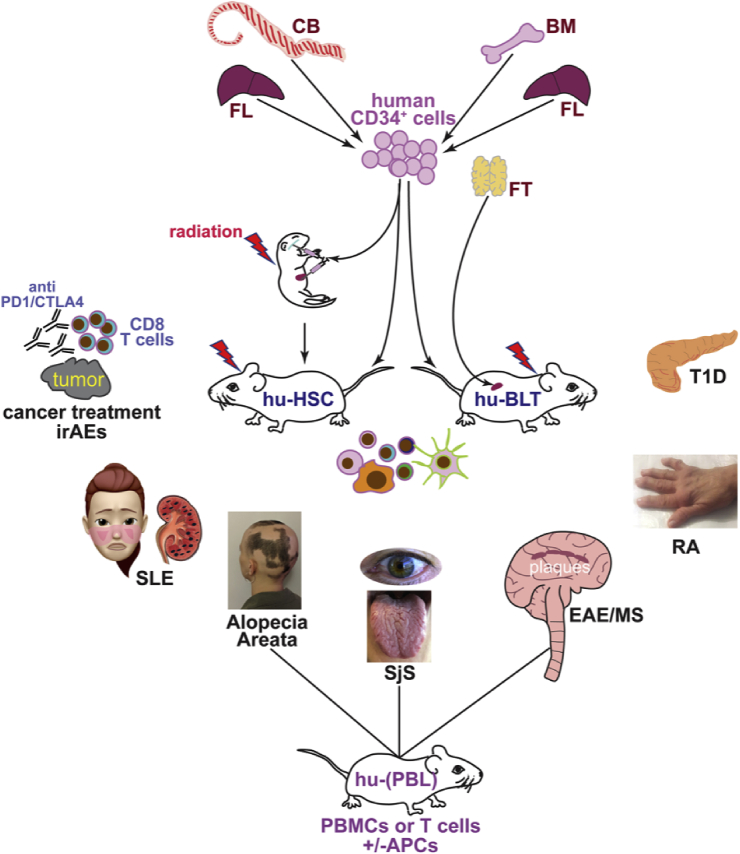
Fig. 1.
Human immune system mice development and use for autoimmune studies. Human hematopoietic cells used as source for the generation of HIS mice vary according to the model. In the hu-HSC model, human hematopoietic stem cells (HSCs) usually in the form of CD34+ cells, are enriched from either umbilical cord blood (CB) obtained after live births or from fetal livers (FT) that are collected subsequent elective and voluntary abortion procedures. These HSCs are then transplanted into either newborn or adult immunodeficient mice that have been preconditioned with a sub-lethal irradiation dose. HSCs are generally injected intra-venously, but they can also be injected intra-hepatically in newborn mice. To setup the hu-BLT model, adult immunodeficient mice are transplanted with thymus fragments obtained either from human fetal tissue or from infants undergoing heart surgery. The thymic fragments are engrafted under the kidney capsule of recipient mice. In addition, these mice are sublethally irradiated and injected with human HSCs isolated from the liver of the same thymus donor fetal tissue (to achieve complete HLA match), or from CB or adult bone marrow (BM) samples that have partial HLA match to the fetal thymus. Injection of the HSCs can occur at the same time of the thymus engraftment or few days after this surgery. In addition to the hu-HSC and hu-BLT models, HIS mice can be generated in a less sophisticated way by injecting immunodeficient mice with total human PBMCs (hu-PBL mice), or with T cells isolated from the blood or other tissues (e.g., the skin). These T cells can be injected as naïve or after culture with APCs (e.g., from the related blood) and specific antigens to enrich for antigen-reactive T cell clones. The figure depicts autoimmune diseases that have been investigated so far using HIS mice: type 1 diabetes (T1D) which manifests in the pancreas, rheumatoid arthritis (RA) which exhibits joint inflammation, experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS) which manifests in the central nervous system, Sjogren’s syndrome (SjS) which displays dryness of eyes, mouth and tongue, alopecia areata which leads to partial or complete hair loss, and systemic lupus erythematosus (SLE) which has multiple manifestations, skin rash and kidney disease being some of these. Alopecia, SjS, and EAE have only been investigated in HIS mice transplanted with human PBMCs or T cells. In addition to the studies of these autoimmune diseases, HIS mice are also starting to be utilized to study the autoimmune manifestations, or immune-related adverse events (irAEs), that arise following cancer immunotherapies with anti-PD1 and anti-CTLA4 antibodies.
Table 1.
Autoimmune studies with HIS hu-mice.
| Strain | Additional genetic manipulation | Model and human cell/tissue source | Autoimmune studies |
|---|---|---|---|
| CB17Scid | None | Hu-(skin T cells) – skin T cells reactive with hair follicles + hairless scalp biopsies from alopecia patients | Alopecia areata: hairless scalp biopsies regrew hair in few weeks after engrafting into SCID mice. Intra-dermal injection of in vitro activated skin T cells from patients reduced hair growth (132) |
| NODScid | None | Hu-(blood T cells&APCs) – islet-reactive T cell clones + HLA-matched peptide-pulsed APCs from healthy PBMCs | T1D: streptozotocin treatment before T cell adoptive transfer; islet-reactive T cell clones homed to pancreas tissue in the presence of peptide-loaded APCs (121) |
| None | Hu-(synovium) – inflamed synovium from RA patients | RA: Blocking BLyS and APRIL modulated B and T cell function in the engrafted synovium. Stimulatory effects were observed when synovium contained germinal center structures. (127) | |
| NODScid Il2rγ−/- (NSG) | None | Hu-PBL – PBMCs from SjS patients | SjS: local inflammation in salivary and lacrimal glands with increased production of inflammatory cytokines in mice transferred with patients PBMCs (130) |
| None | Hu-PBL – healthy PBMCs | EAE/MS: adoptive transfer of PBMCs and myelin primed human DCs followed by immunization with myelin peptides in CFA; observed subclinical CNS inflammation (131) | |
| HLA-DR4 transgene | Hu-(blood T cells) – CD4 T cells from either T1D or control patients with T1D associated HLA-DR4 | T1D: CD4 T cell stimulated with pancreas peptides; healthy and T1D T cells homed to pancreas; reduced insulin production in pancreas of hu-mice that received T1D T cells (123) | |
| None | Hu-HSCs (FL) | SLE: pristane injection; observed lupus-like disease with ANA production and lupus nephritis (118) | |
| MHC II KO; HLA-DR1 transgene | Hu-HSCs (BM) – HSCs from IPEX patient | IPEX: spontaneous multi-organ systemic and fatal autoimmunity (119) | |
| None | Hu-HSCs (CB) | RA: CFA injection into joints; observed clinical and histological RA with inflammatory infiltrate, swelling and bone erosion (129) | |
| None | Hu-HSCs (FL) | Anti-CTLA4 immunotherapy: observed autoimmune hepatitis, liver lymphocytic infiltration, ANA production, T cell activation, weight loss (144) | |
| None | Hu-HSCs (FL) | Anti-CTLA4 or PD1 immunotherapy: observed massive lymphocytic infiltration in many tissues and weight loss but only in mice treated with anti-CTLA4 (143) | |
| MHC II KO; HLA-DQ8 transgene | Hu-HSCs (CB) – HLA-DQ8+ HSCs | T1D vaccine study: infusion of insulin mimetopes induces functional Foxp3+ Tregs that suppress insulin-reactive effector T cells (125) | |
| None | Hu-BLT – BM HSCs from T1D patients and T1D-relevant HLA-matched FT | T1D: observed increased proportion of activated and memory T cells with T1D-derived bone marrow HSCs (62) | |
| MHC II KO; HLA-DQ8 transgene | Hu-BLT –HLA-DQ8+ FL HSCs and FT | T1D: streptozotocin treatment before transfer of insulin-reactive CD4 T cells and insulin peptide immunization in CFA; observed insulitis and diabetes (124) | |
| None or pig cytokine transgenes (IL-3, GM-CSF, SCF) | Hu-BLT – BM HSCs from T1D or RA patients and disease-relevant HLA-matched FT | T1D and RA: observed increased frequency of autoreactive and polyreactive B cells (122) | |
| NOD/Shi-scid/IL-2Rγnull (NOG) | None | Hu-HSCs (CB) | RA: low dose of EBV infection; observed erosive arthritis closely related to RA with pannus formation and inflammatory infiltrate in the synovium (128) |
| None or with human cytokine transgenes (IL-3, GM-CSF) | Hu-BLT – FL HSCs and FT | Anti-PD1 immunotherapy: observed pneumonitis, hepatitis, nephritis, dermatitis, adrenalitis, T cell activation (145) |
BM = bone marrow; CB = cord blood; FL = fetal liver; FT = fetal thymus; HSCs = hematopoietic stem cells; ANA = anti-nuclear antibodies.
3.2. Systemic lupus erythematosus and IPEX in HIS mice
Systemic lupus erythematosus is one of the most studied autoimmune diseases. This disease is highly complex and diverse but appears to be generally dominated by pathogenic B cells and autoantibodies. While there are mouse strains that due to their genetic makeup spontaneously develop a lupus-like disease, it is also possible to trigger a similar dysfunction in healthy mice by treating these animals with a single injection of the long chain hydrocarbon pristane [117]. When pristane was used in HIS hu-mice generated by transplanting fetal HSCs into NSG animals, it led to the development of key SLE features similar to those that develop in intact wild-type mice upon treatment. These features manifested with an increased production of anti-nuclear autoantibodies and pro-inflammatory cytokines, and also with the development of lupus nephritis and pulmonary serositis [118].
While SLE is a multigenic disease where a diverse number of genetic loci contribute to a lesser or larger extent, other autoimmune diseases are caused by rare monogenic alterations. An example is Immunodysregulation Polyendocrinopathy Enteropathy X-linked (IPEX) syndrome, a multi-organ and fatal autoimmune disease that is caused by mutations in the FOXP3 gene and, thus, a defective generation of Tregs. A study by Goettel et al. showed that this monogenic autoimmune syndrome can be modeled in hu-HSC HIS mice by transplanting HSCs from a patient with IPEX into an NSG strain deficient for MHC-II and transgenic for HLA-DR1 [119]. This model can also be used to test genetic therapies, such as the transplantation of HSCs transduced with a lentivirus encoding a normal FOXP3 gene [120].
3.3. Type 1 diabetes in HIS mice
With respect to type 1 diabetes and other autoimmune diseases, the immune system produced by HIS and BLT hu-mice might not be sufficiently functional to fully recapitulate all aspects of a disease, although it may still mimic some key features. A study that focused on the tissue recruitment of pancreas-specific human T cells showed that islet-reactive human T cell clones that were primed in vitro by autologous APCs pulsed with relevant peptides, home to the pancreas of NOD-SCID mice that were pre-treated with streptozotocin to stress pancreatic β-cells. This tissue recruitment was contingent on proper in vivo antigen stimulation (i.e., the coinjection of antigen-pulsed APCs) and the pre-treatment of the animals with streptozotocin [121]. These mice, however, did not develop diabetes, suggesting they lacked critical components of disease development.
As mentioned earlier, the BLT model allows endogenous human T cells to be educated by human HLA antigens. This model is most often setup using thymic fragments and CD34+ HSCs isolated from the same fetus, which results in a complete match between the thymic “education” of human T cells and the HLAs expressed by human APCs in the peripheral tissue (although not to the MHC of mouse APCs and the mouse tissue itself). The BLT model can also be generated using fetal thymus expressing HLA alleles that partially match those expressed by HSCs isolated from the bone marrow of pediatric or adult individuals. This enables the development of BLT mice bearing immune systems from patients with distinct genetic characteristics, including those responsible for the onset of autoimmunity. In a pioneering study from the group of Megan Sykes, NSG animals received bone marrow HSCs from either T1D or healthy donors and were also implanted with fetal thymus fragments expressing the T1D-associated HLA*A201 and DRB*0302 and/or DQB*0301 HLA alleles to match those expressed by the transplanted HSCs. In these mice, while HSCs from T1D and control donors generated similar numbers of Treg cells, the peripheral T cells of T1D hu-mice showed increased proportion of activated and memory T cells compared to controls, suggesting a decreased propensity for immune regulation [62]. In addition to differences in T cell function, this group also showed that BLT hu-mice generated with HSCs from T1D or RA patients display B-cell abnormalities when compared to mice transplanted with HSCs from healthy controls [122]. This was demonstrated by an increased frequency of autoreactive and polyreactive clones in the peripheral B cell population, indicating HIS mice can be used to study the contribution of genetic polymorphisms to autoimmune-related B cell defects [122]. These animals, however, did not display the tissue damage that is distinctive of T1D (e.g., insulitis) and RA (joint inflammation). Thus, this model does not appear to reflect critical aspects of disease pathogenesis.
A potential explanation for the lack of disease in the above model is that their T cells do not react with peptides presented by mouse tissue cells and mouse APCs because they are only educated on human HLAs. In fact, insulitis was observed in a model in which CD4 T cells isolated from DRB1*0401 (DR4) T1D patients or healthy controls were pulsed with pancreatic peptides and then injected into NSG-DR4Tg mice in which mouse cells express DR4 [123]. However, only the pancreas of NSG-DR4Tg mice that received patient T cells displayed reduced insulin production despite the fact that both T1D and control T cells infiltrated the organ to a similar extent [123]. Moreover, the degree of lymphocytic infiltration of the islets varied significantly depending on the donor and the peptide used to stimulate the T cells, suggesting the contribution of additional genetic factors and/or individual variations in T cell repertoire. Nevertheless, these mice did not develop overt hyperglycemia, indicating they were lacking cell types (e.g., CD8 T cells) that are important for β-cell destruction.
The establishment of full-fledged insulitis and T1D, in fact, appears to require additional antigen-specific T cell interactions. This was demonstrated in a recent study in which NSG-DQ8Tg recipients were transplanted with HLA-DQ8+ human fetal thymus and HSCs to develop both CD4 and CD8 T cells and other human leukocytes. These mice were injected with streptozotocin to stress the pancreatic β-cells, adoptively transferred with autologous human CD4 T cells transduced to express a TCR reactive with the insulin B:9–23 peptide in the context of HLA-DQ8, and finally immunized with the B:9–23 peptide in Freund’s complete adjuvant [124]. While this represents a large amount of sophisticated experimental manipulations to model the full pathologic state, it can instead be viewed as an opportunity for learning what is required to recapitulate whole human immune responses. In fact, it is because of what is lacking in hu-mice that we can sometimes learn what are the essential components needed to be added in order to establish physiologic and pathologic immunity. This humanized T1D preclinical model can now be used to elucidate the immunopathogenesis of T1D and furthermore to test novel therapies [124]. For instance, a recent translational study utilized NSG-DQ8Tg HIS mice generated by transplanting HLA-DQ8+ HSCs, to test the efficacy of T cell vaccines in promoting the expansion of functional Tregs. A two-week infusion with sub-immunogenic amounts of insulin mimetope peptides induced long lasting and stable Foxp3+ Tregs that were efficient in suppressing the response of insulin-reactive effector T cells [125].
3.4. Rheumatoid arthritis in HIS mice
Rheumatoid arthritis research groups have also taken advantage of HIS hu-mice to investigate disease pathogenesis, environmental cues and pre-clinical treatments [126]. Beside the study with BLT hu-mice described above in the T1D section [122], most studies have used either PBMCs or synovial tissue from RA patients as source of human cells. As an example, Seyler and colleagues [127] transplanted human inflamed synovium from RA patients into NOD-SCID mice to study how the B cell growth factors B Lymphocyte Stimulator (BLyS) and A Proliferation-Inducing Ligand (APRIL) contribute to B cell and T cell functions in this disease. By blocking these cytokines with a decoy receptor (TACI:Fc), they found that BLyS and APRIL regulate both B and T cell function but their effects varied depending on the presence or absence of germinal center structures, with a strong immunostimulatory effect on both types of lymphocytes only in germinal center-positive synovium [127]. Similar studies providing important contributions for disease treatment, have been described in a recent review of humanized mouse models of RA [126].
Environmental factors play a crucial role in the development of autoimmune diseases, chief among these are infections. An investigation on the role of Epstein-Barr virus (EBV) in the development of RA, found that NOG HIS hu-mice (hu-HSCs) develop erosive arthritis with pannus formation and inflammatory cell infiltration when infected with EBV at a dose not promoting lymphomagenesis [128]. In a different study, injection of Freund’s complete adjuvant into the ankle or knee joints was sufficient to trigger acute inflammatory arthritis in NSG HIS hu-mice [129]. The development of clinical and histological arthritis in these animals manifested with human immune cell infiltration, swelling, and bone erosion, symptoms that were decreased upon treatment with a TNF-α inhibitor [129].
3.5. Other autoimmune diseases in HIS mice
While the development of sophisticated HIS mouse models for T1D, RA and SLE has significantly progressed, those for other autoimmune diseases are lagging behind. For instance, Sjogren’s syndrome (SjS) and multiple sclerosis (MS) have only been modeled in hu-PBL mice, and alopecia areata has only been modeled by transplanting skin and T cells.
In a study using NSG mice injected with PBMCs from either SjS patients or healthy donors, recipients of SjS cells produced higher amounts of inflammatory cytokines relative to mice with control cells [130]. Moreover, these mice displayed inflammation and lymphocyte infiltration specifically in salivary and lacrimal glands, the target organs of SjS [130].
Hu-PBL mice were also used to induce experimental autoimmune encephalomyelitis (EAE), a model of MS. In this study an NSG hu-PBL mouse model was developed by injecting a mix of healthy PBMCs and donor-matched dendritic cells (DCs) that were pulsed in vitro with myelin antigens [131]. Animals were then boosted a week later by subcutaneous injection of immature DCs and myelin peptides emulsified in Freund’s complete adjuvant. These treatments led to the development of subclinical inflammation in the central nervous system, as seen by the presence of infiltrating CD4 and CD8 T cells expressing IFN-γ and GM-CSF [131]. In this model, the adoptive transfer of antigen-primed DCs was necessary for this outcome. However, the mice did not develop disease symptoms characteristic of EAE and MS, such as tail and leg paralysis, indicating an absence of important immunopathogenic mediators.
Alopecia areata, an autoimmune condition directed to hair follicles and leading to the partial or even total loss of body hair, has been investigated in a humanized animal model in which hairless scalp biopsies from patients are engrafted in the skin of SCID mice. In the absence of human or mouse lymphocytes the grafts were able to regrow some of the hair within several weeks. However, this hair growth was hampered if the grafts were injected with patient T cells isolated from affected skin patches and activated first by culture with hair follicle homogenates and peripheral blood APCs [132]. These studies show that effector T cells reactive with hair follicles are present in the patients’ skin.
Although the SjS, EAE, and alopecia studies represent important contributions for understanding how human immune cells respond to self-antigens and cause tissue damage in vivo, there are significant limitations due to the models used. Hu-PBL mice feature a poorly diverse immune system, mainly composed of clones of mature and xenoreactive T cells that expand to occupy the peripheral niches. These cells are by nature not tolerant to the host antigens, causing the development of GVHD that kills the mice around 4 weeks after transfer [133]. In the alopecia studies in which only hair follicle-reactive T cells were transferred, this model lacks potential regulatory cells that can reduce T cells effector functions [134]. When human CD34+ HSCs are transplanted instead of PBMCs or isolated cell fractions they lead to a more robust reconstitution of an immune system that is more complex and sophisticated, long-lived, self-replicating, functional, and also generally tolerant to the host.
3.6. Autoimmune responses as a consequence of cancer immunotherapy
In addition to the many autoimmune diseases that are driven by genetics and environment, newly developed immunotherapy protocols that are used nowadays in many cancer patients have as side-effects the development of autoimmune responses [135,136]. These therapies use antibodies that block the inhibitory receptors PD-1 (or PD-L1) and CTLA-4, increasing (and at times unleashing) the response of T cells to tumors, but also to other tissues. HIS hu-mice are becoming a model of choice for preclinical investigation of tumor-specific immunotherapies in combination or not with chemotherapies. These studies demonstrate that HIS hu-mice develop an immune system capable of rejecting some but not all tumors, and that this response can be enhanced by the use of immune checkpoint inhibitors, and modulated by some chemotherapies [[137], [138], [139], [140], [141], [142], [143]]. Importantly, these animals appear to replicate many of the immune-related adverse events (irAEs) that have been observed in cancer patients treated with immune checkpoint inhibitor drugs, such as hepatitis, nephritis, dermatitis, adrenalitis, and development of anti-nuclear auto-antibodies [[143], [144], [145]]. In addition, and similar to patients, these irAEs are more severe in mice treated with anti-CTLA-4 than anti-PD-1 antibodies. Thus, the ability of immunotherapy to trigger aberrant autoimmune responses could be investigated through the use of HIS hu-mice. These animals could also be useful for testing ways to prevent irAEs following cancer therapy.
4. Conclusions
While many models of HIS hu-mice exist, that use diverse recipient strains and human cell sources, none display a human immune system that completely recapitulates all aspects of the “normal” human immune system. Nonetheless, the human immune system that establishes in HIS hu-mice can often replicate complex responses that mimic those observed in human physiology and pathology. Several studies demonstrate that HIS hu-mice can be used as models to study autoimmune diseases, in their totality or in some of their components. The growing use of HIS mice in immunological research in the last 10 years has demonstrated that with empirical methods, this model can be successfully adapted to virtually any study. The development of proper HIS hu-mouse models of autoimmunity is important for closing the gap of knowledge on the initiation and progression of these diseases, a gap caused by the problems associated with accessing the disease target tissues in patients and in establishing the mechanisms of disease. Moreover, human disease heterogeneity caused by genetic polymorphisms and contrasting environmental exposure renders treatment inadequate for many patients. This issue might find some answers in personalized medicine approach based on the use of HIS hu-mice.
Conflict of interest
None.
Acknowledgements
This work was supported by National Institutes of Health [grant numbers R01-AI124474 to R.P. and R01-AI136534 to R.M.T.]. We thank Dr. Sophina Taitano for some initial editing.
References
- 1.Bodis G., Toth V., Schwarting A. Role of human leukocyte antigens (HLA) in autoimmune diseases. Rheumatol. Ther. 2018;5:5–20. doi: 10.1007/s40744-018-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer R.E., Maika S.D., Richardson J.A., Tang J.P., Taurog J.D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 3.Taneja V., David C.S. HLA transgenic mice as humanized mouse models of disease and immunity. J. clin. investig. 1998;101:921–926. doi: 10.1172/JCI2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotturi M.F., Assarsson E., Peters B., Grey H., Oseroff C., Pasquetto V. Of mice and humans: how good are HLA transgenic mice as a model of human immune responses? Immunome Res. 2009;5:3. doi: 10.1186/1745-7580-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luckey D., Bastakoty D., Mangalam A.K. Role of HLA class II genes in susceptibility and resistance to multiple sclerosis: studies using HLA transgenic mice. J. Autoimmun. 2011;37:122–128. doi: 10.1016/j.jaut.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhagen J., Smith E.L., Whettlock E.M., Macintyre B., Peakman M. Proinsulin-mediated induction of type 1 diabetes in HLA-DR4-transgenic mice. Sci. Rep. 2018;8:14106. doi: 10.1038/s41598-018-32546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In’t Veld P. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin. Immunopathol. 2014;36:569–579. doi: 10.1007/s00281-014-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roep B.O. Are insights gained from NOD mice sufficient to guide clinical translation? Another inconvenient truth. Ann. N. Y. Acad. Sci. 2007;1103:1–10. doi: 10.1196/annals.1394.018. [DOI] [PubMed] [Google Scholar]
- 9.von Herrath M., Nepom G.T. Remodeling rodent models to mimic human type 1 diabetes. Eur. J. Immunol. 2009;39:2049–2054. doi: 10.1002/eji.200939429. [DOI] [PubMed] [Google Scholar]
- 10.Roep B.O., Atkinson M., von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat. Rev. Immunol. 2004;4:989–997. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- 11.Hegen M., Keith J.C., Jr., Collins M., Nickerson-Nutter C.L. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann. Rheum. Dis. 2008;67:1505–1515. doi: 10.1136/ard.2007.076430. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Nun A., Kaushansky N., Kawakami N., Krishnamoorthy G., Berer K., Liblau R. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J. Autoimmun. 2014;54:33–50. doi: 10.1016/j.jaut.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Richard M.L., Gilkeson G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med. 2018;5 doi: 10.1136/lupus-2016-000199. e000199-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rongvaux A., Takizawa H., Strowig T., Willinger T., Eynon E.E., Flavell R.A. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu. Rev. Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosma G.C., Custer R.P., Bosma M.J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 16.McCune J.M., Namikawa R., Kaneshima H., Shultz L.D., Lieberman M., Weissman I.L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 17.Mosier D.E., Gulizia R.J., Baird S.M., Wilson D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 18.Duchosal M.A., McConahey P.J., Robinson C.A., Dixon F.J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J. Exp. Med. 1990;172:985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashany D., Hines J., Gharavi A., Mouradian J., Elkon K.B. Analysis of autoantibody production in SCID-systemic lupus erythematosus (SLE) chimeras. Clin. Exp. Immunol. 1992;88:84–90. doi: 10.1111/j.1365-2249.1992.tb03043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T., Young J.S., Johnston H., Ni X., Deng R., Racine J. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J. Immunol. 2013;191:488–499. doi: 10.4049/jimmunol.1300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Gao Q., Feng Y., Zhang X. Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Br. J. Haematol. 2019;184:323–336. doi: 10.1111/bjh.15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulop G.M., Phillips R.A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 23.Tomita Y., Sachs D.H., Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939. [PubMed] [Google Scholar]
- 24.Kotloff D.B., Bosma M.J., Ruetsch N.R. V(D)J recombination in peritoneal B cells of leaky scid mice. J. Exp. Med. 1993;178:1981–1994. doi: 10.1084/jem.178.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greiner D.L., Hesselton R.A., Shultz L.D. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 26.Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 27.Shinkai Y., Rathbun G., Lam K.-P., Oltz E.M., Stewart V., Mendelsohn M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 28.Hesselton R.M., Greiner D.L., Mordes J.P., Rajan T.V., Sullivan J.L., Shultz L.D. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J. Infect. Dis. 1995;172:974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka S., Satoh J., Fujiya H., Toyota T., Suzuki R., Itoh K. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983;32:247–253. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- 30.Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., Tennent B. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 31.Christianson S.W., Greiner D.L., Schweitzer I.B., Gott B., Beamer G.L., Schweitzer P.A. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell. Immunol. 1996;171:186–199. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 32.DiSanto J.P., Rieux-Laucat F., Dautry-Varsat A., Fischer A., de Saint Basile G. Defective human interleukin 2 receptor gamma chain in an atypical X chromosome-linked severe combined immunodeficiency with peripheral T cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9466–9470. doi: 10.1073/pnas.91.20.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi M., Yi H., Rosenblatt H.M., Filipovich A.H., Adelstein S., Modi W.S. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 34.Sugamura K., Asao H., Kondo M., Tanaka N., Ishii N., Ohbo K. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 35.Ohbo K., Suda T., Hashiyama M., Mantani A., Ikebe M., Miyakawa K. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 36.Tangye S.G. Advances in IL-21 biology - enhancing our understanding of human disease. Curr. Opin. Immunol. 2015;34:107–115. doi: 10.1016/j.coi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 37.DiSanto J.P., Muller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. U. S. A. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore T.A., von Freeden-Jeffry U., Murray R., Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 -/- mice. J. Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 39.Liu C.C., Perussia B., Young J.D. The emerging role of IL-15 in NK-cell development. Immunol. Today. 2000;21:113–116. doi: 10.1016/s0167-5699(99)01581-9. [DOI] [PubMed] [Google Scholar]
- 40.Grabstein K.H., Waldschmidt T.J., Finkelman F.D., Hess B.W., Alpert A.R., Boiani N.E. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J. Exp. Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 44.Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J.C., Lanzavecchia A. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 45.Pearson T., Shultz L.D., Miller D., King M., Laning J., Fodor W. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin. Exp. Immunol. 2008;154:270–284. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shultz L.D., Ishikawa F., Greiner D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 47.Macchiarini F., Manz M.G., Palucka A.K., Shultz L.D. Humanized mice: are we there yet? J. Exp. Med. 2005;202:1307–1311. doi: 10.1084/jem.20051547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito M., Kobayashi K., Nakahata T. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 2008;324:53–76. doi: 10.1007/978-3-540-75647-7_3. [DOI] [PubMed] [Google Scholar]
- 49.Kwant-Mitchell A., Pek E.A., Rosenthal K.L., Ashkar A.A. Development of functional human NK cells in an immunodeficient mouse model with the ability to provide protection against tumor challenge. PLoS One. 2009;4:e8379. doi: 10.1371/journal.pone.0008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takenaka K., Prasolava T.K., Wang J.C., Mortin-Toth S.M., Khalouei S., Gan O.I. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 51.Strowig T., Rongvaux A., Rathinam C., Takizawa H., Borsotti C., Philbrick W. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Legrand N., Huntington N.D., Nagasawa M., Bakker A.Q., Schotte R., Strick-Marchand H. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13224–13229. doi: 10.1073/pnas.1101398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavender K.J., Pang W.W., Messer R.J., Duley A.K., Race B., Phillips K. BLT-humanized C57BL/6 Rag2-/-gammac-/-CD47-/- mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013;122:4013–4020. doi: 10.1182/blood-2013-06-506949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosgun K.N., Rahmig S., Mende N., Reinke S., Hauber I., Schafer C. Kit regulates HSC engraftment across the human-mouse species barrier. Cell. St.Cell. 2014;15:227–238. doi: 10.1016/j.stem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 55.McIntosh B.E., Brown M.E., Duffin B.M., Maufort J.P., Vereide D.T., Slukvin I.I. Nonirradiated NOD,B6.SCID Il2rgamma-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. St. Cell. Rep. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breed E.R., Lee S.T., Hogquist K.A. Directing T cell fate: how thymic antigen presenting cells coordinate thymocyte selection. Semin. Cell Dev. Biol. 2018;84:2–10. doi: 10.1016/j.semcdb.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCune J.M., Peault B., Streeter P.R., Rabin L. Preclinical evaluation of human hematolymphoid function in the SCID-hu mouse. Immunol. Rev. 1991;124:45–62. doi: 10.1111/j.1600-065x.1991.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 58.Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.-G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 59.Melkus M.W., Estes J.D., Padgett-Thomas A., Gatlin J., Denton P.W., Othieno F.A. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006;12(11):1316–1322. doi: 10.1038/nm1431. 12b1316-22. [DOI] [PubMed] [Google Scholar]
- 60.Denton P.W., Nochi T., Lim A., Krisko J.F., Martinez-Torres F., Choudhary S.K. IL-2 receptor gamma-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol. 2012;5:555–566. doi: 10.1038/mi.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenblatt M.B., Vrbanac V., Tivey T., Tsang K., Tager A.M., Aliprantis A.O. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS One. 2012:7be44664. doi: 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalscheuer H., Danzl N., Onoe T., Faust T., Winchester R., Goland R. A model for personalized in vivo analysis of human immune responsiveness. Sci. Transl. Med. 2012;4:125ra30. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King M.A., Covassin L., Brehm M.A., Racki W., Pearson T., Leif J. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin. Exp. Immunol. 2009;157(1):104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brehm M.A., Kenney L.L., Wiles M.V., Low B.E., Tisch R.M., Burzenski L. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019;33:3137–3151. doi: 10.1096/fj.201800636R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown M.E., Zhou Y., McIntosh B.E., Norman I.G., Lou H.E., Biermann M. A humanized mouse model generated using surplus neonatal tissue. Stem. cell. reports. 2018;10:1175–1183. doi: 10.1016/j.stemcr.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Q., Khoury M., Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu H., Borsotti C., Schickel J.N., Zhu S., Strowig T., Eynon E.E. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood. 2017;129:959–969. doi: 10.1182/blood-2016-04-709584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller P.H., Cheung A.M., Beer P.A., Knapp D.J., Dhillon K., Rabu G. Enhanced normal short-term human myelopoiesis in mice engineered to express human-specific myeloid growth factors. Blood. 2013;121 doi: 10.1182/blood-2012-09-456566. e1-4. [DOI] [PubMed] [Google Scholar]
- 69.Coughlan A.M., Harmon C., Whelan S., O’Brien E.C., O’Reilly V.P., Crotty P. Myeloid engraftment in humanized mice: impact of granulocyte-colony stimulating factor treatment and transgenic mouse strain. Stem Cells Dev. 2016;25:530–541. doi: 10.1089/scd.2015.0289. [DOI] [PubMed] [Google Scholar]
- 70.Jangalwe S., Shultz L.D., Mathew A., Brehm M.A. Improved B cell development in humanized NOD-scid IL2Rgamma(null) mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 2016;4:427–440. doi: 10.1002/iid3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saito Y., Ellegast J.M., Rafiei A., Song Y., Kull D., Heikenwalder M. Peripheral blood CD34(+) cells efficiently engraft human cytokine knock-in mice. Blood. 2016;128:1829–1833. doi: 10.1182/blood-2015-10-676452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicolini F.E., Cashman J.D., Hogge D.E., Humphries R.K., Eaves C.J. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia. 2004;18:341–347. doi: 10.1038/sj.leu.2403222. [DOI] [PubMed] [Google Scholar]
- 73.Rongvaux A., Willinger T., Martinek J., Strowig T., Gearty S.V., Teichmann L.L. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y., Mention J.J., Court N., Masse-Ranson G., Toubert A., Spits H. A novel Flt3-deficient HIS mouse model with selective enhancement of human DC development. Eur. J. Immunol. 2016;46:1291–1299. doi: 10.1002/eji.201546132. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Lastra S., Masse-Ranson G., Fiquet O., Darche S., Serafini N., Li Y. A functional DC cross talk promotes human ILC homeostasis in humanized mice. Blood. Adv. 2017;1:601–614. doi: 10.1182/bloodadvances.2017004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huntington N.D., Legrand N., Alves N.L., Jaron B., Weijer K., Plet A. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herndler-Brandstetter D., Shan L., Yao Y., Stecher C., Plajer V., Lietzenmayer M. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E9626–E9634. doi: 10.1073/pnas.1705301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katano I., Nishime C., Ito R., Kamisako T., Mizusawa T., Ka Y. Long-term maintenance of peripheral blood derived human NK cells in a novel human IL-15- transgenic NOG mouse. Sci. Rep. 2017;7:17230. doi: 10.1038/s41598-017-17442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brehm M.A., Aryee K.-E., Bruzenksi L., Greiner D.L., Shultz L.D., Keck J. Transgenic expression of human IL15 in NOD-<em>scid IL2rg</em><em>null</em> (NSG) mice enhances the development and survival of functional human NK cells. J. Immunol. 2018;200 103.20-.20. [Google Scholar]
- 80.van de Pavert S.A., Mebius R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 81.Li Y., Masse-Ranson G., Garcia Z., Bruel T., Kok A., Strick-Marchand H. A human immune system mouse model with robust lymph node development. Nat. Methods. 2018;15:623–630. doi: 10.1038/s41592-018-0071-6. [DOI] [PubMed] [Google Scholar]
- 82.Shultz L.D., Brehm M.A., Garcia-Martinez J.V., Greiner D.L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ito R., Takahashi T., Katano I., Ito M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walsh N.C., Kenney L.L., Jangalwe S., Aryee K.E., Greiner D.L., Brehm M.A. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Allen T.M., Brehm M.A., Bridges S., Ferguson S., Kumar P., Mirochnitchenko O. Humanized immune system mouse models: progress, challenges and opportunities. Nat. Immunol. 2019;20:770–774. doi: 10.1038/s41590-019-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blackburn C.C., Manley N.R. Developing a new paradigm for thymus organogenesis. Nat. Rev. Immunol. 2004;4:278–289. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 87.Germain R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 88.Dudley E.C., Petrie H.T., Shah L.M., Owen M.J., Hayday A.C. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 89.Capone M., Hockett R.D., Jr., Zlotnik A. Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12522–12527. doi: 10.1073/pnas.95.21.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Starr T.K., Jameson S.C., Hogquist K.A. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 91.Gameiro J., Nagib P., Verinaud L. The thymus microenvironment in regulating thymocyte differentiation. Cell. Adhes. Migrat. 2010;4:382–390. doi: 10.4161/cam.4.3.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stein J.V., Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Forster R., Davalos-Misslitz A.C., Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 94.Huntington N.D., Alves N.L., Legrand N., Lim A., Strick-Marchand H., Plet A. Autonomous and extrinsic regulation of thymopoiesis in human immune system (HIS) mice. Eur. J. Immunol. 2011;41:2883–2893. doi: 10.1002/eji.201141586. [DOI] [PubMed] [Google Scholar]
- 95.Brugman M.H., Wiekmeijer A.S., van Eggermond M., Wolvers-Tettero I., Langerak A.W., de Haas E.F. Development of a diverse human T-cell repertoire despite stringent restriction of hematopoietic clonality in the thymus. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6020–E6027. doi: 10.1073/pnas.1519118112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halkias J., Yen B., Taylor K.T., Reinhartz O., Winoto A., Robey E.A. Conserved and divergent aspects of human T-cell development and migration in humanized mice. Immunol. Cell Biol. 2015;93:716–726. doi: 10.1038/icb.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Onoe T., Kalscheuer H., Danzl N., Chittenden M., Zhao G., Yang Y.G. Human natural regulatory T cell development, suppressive function, and post thymic maturation in a humanized mouse model. J. Immunol. 2011;187:3895–3903. doi: 10.4049/jimmunol.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y., Teteloshvili N., Tan S., Rao S., Han A., Yang Y.G. Humanized mice reveal new insights into the thymic selection of human autoreactive CD8(+) T cells. Front. Immunol. 2019;10:63. doi: 10.3389/fimmu.2019.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khosravi-Maharlooei M., Obradovic A., Misra A., Motwani K., Holzl M., Seay H.R. Crossreactive public TCR sequences undergo positive selection in the human thymic repertoire. J. clin. investig. 2019;130:2446–2462. doi: 10.1172/JCI124358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajewsky K. Early and late B-cell development in the mouse. Curr. Opin. Immunol. 1992;4:171–176. doi: 10.1016/0952-7915(92)90008-3. [DOI] [PubMed] [Google Scholar]
- 101.Melchers F., Rolink A., Grawunder U., Winkler T.H., Karasuyama H., Ghia P. Positive and negative selection events during B lymphopoiesis. Curr. Opin. Immunol. 1995;7:214–227. doi: 10.1016/0952-7915(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 102.Grandien A., Fucs R., Nobrega A., Andersson J., Coutinho A. Negative selection of multireactive B cell clones in normal adult mice. Eur. J. Immunol. 1994;24:1345–1352. doi: 10.1002/eji.1830240616. [DOI] [PubMed] [Google Scholar]
- 103.Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 104.Goodnow C.C., Sprent J., Fazekas de St Groth B., Vinuesa C.G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 105.Pelanda R., Torres R.M. Receptor editing for better or for worse. Curr. Opin. Immunol. 2006;18:184–190. doi: 10.1016/j.coi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 106.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat. Rev. Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 107.Shlomchik M.J. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 108.Cambier J.C., Gauld S.B., Merrell K.T., Vilen B.J. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lang J., Kelly M., Freed B.M., McCarter M.D., Kedl R.M., Torres R.M. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J. Immunol. 2013;190:2090–2101. doi: 10.4049/jimmunol.1202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lang J., Zhang B., Kelly M., Peterson J.N., Barbee J., Freed B.M. Replacing mouse BAFF with human BAFF does not improve B-cell maturation in hematopoietic humanized mice. Blood. Adv. 2017;1:2729–2741. doi: 10.1182/bloodadvances.2017010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goodnow C.C. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 112.Ait-Azzouzene D., Verkoczy L., Peters J., Gavin A., Skog P., Vela J.L. An immunoglobulin C kappa-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J. Exp. Med. 2005;201:817–828. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lang J., Ota T., Kelly M., Strauch P., Freed B.M., Torres R.M. Receptor editing and genetic variability in human autoreactive B cells. J. Exp. Med. 2016;213:93–108. doi: 10.1084/jem.20151039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cantaert T., Schickel J.N., Bannock J.M., Ng Y.S., Massad C., Oe T. Activation-induced cytidine deaminase expression in human B cell precursors is essential for central B cell tolerance. Immunity. 2015;43:884–895. doi: 10.1016/j.immuni.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ippolito G.C., Hoi K.H., Reddy S.T., Carroll S.M., Ge X., Rogosch T. Antibody repertoires in humanized NOD-scid-IL2Rgamma(null) mice and human B cells reveals human-like diversification and tolerance checkpoints in the mouse. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schickel J.N., Kuhny M., Baldo A., Bannock J.M., Massad C., Wang H. PTPN22 inhibition resets defective human central B cell tolerance. Sci. Immunol. 2016;1 doi: 10.1126/sciimmunol.aaf7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reeves W.H., Lee P.Y., Weinstein J.S., Satoh M., Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gunawan M., Her Z., Liu M., Tan S.Y., Chan X.Y., Tan W.W.S. A novel human systemic lupus erythematosus model in humanised mice. Sci. Rep. 2017;7:16642. doi: 10.1038/s41598-017-16999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goettel J.A., Biswas S., Lexmond W.S., Yeste A., Passerini L., Patel B. Fatal autoimmunity in mice reconstituted with human hematopoietic stem cells encoding defective FOXP3. Blood. 2015;125:3886–3895. doi: 10.1182/blood-2014-12-618363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Masiuk K.E., Laborada J., Roncarolo M.G., Hollis R.P., Kohn D.B. Lentiviral gene therapy in HSCs restores lineage-specific Foxp3 expression and suppresses autoimmunity in a mouse model of IPEX syndrome. Cell. St.Cell. 2019;24:309–317 e7. doi: 10.1016/j.stem.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 121.van Halteren A.G.S., Kardol M.J., Mulder A., Roep B.O. Homing of human autoreactive T cells into pancreatic tissue of NOD-scid mice. Diabetologia. 2005;48:75–82. doi: 10.1007/s00125-004-1613-2. [DOI] [PubMed] [Google Scholar]
- 122.Borsotti C., Danzl N.M., Nauman G., Holzl M.A., French C., Chavez E. HSC extrinsic sex-related and intrinsic autoimmune disease-related human B-cell variation is recapitulated in humanized mice. Blood. Adv. 2017;1:2007–2018. doi: 10.1182/bloodadvances.2017006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Viehmann Milam A.A., Maher S.E., Gibson J.A., Lebastchi J., Wen L., Ruddle N.H. A humanized mouse model of autoimmune insulitis. Diabetes. 2014;63:1712–1724. doi: 10.2337/db13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tan S., Li Y., Xia J., Jin C.H., Hu Z., Duinkerken G. Type 1 diabetes induction in humanized mice. Proc. Natl. Acad. Sci. U. S. A. 2017;114:10954–10959. doi: 10.1073/pnas.1710415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serr I., Furst R.W., Achenbach P., Scherm M.G., Gokmen F., Haupt F. Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nat. Commun. 2016;7:10991. doi: 10.1038/ncomms10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schinnerling K., Rosas C., Soto L., Thomas R., Aguillon J.C. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front. Immunol. 2019;10:203. doi: 10.3389/fimmu.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seyler T.M., Park Y.W., Takemura S., Bram R.J., Kurtin P.J., Goronzy J.J. BLyS and APRIL in rheumatoid arthritis. J. clin. investig. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuwana Y., Takei M., Yajima M., Imadome K., Inomata H., Shiozaki M. Epstein-Barr virus induces erosive arthritis in humanized mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Misharin A.V., Haines G.K., 3rd, Rose S., Gierut A.K., Hotchkiss R.S., Perlman H. Development of a new humanized mouse model to study acute inflammatory arthritis. J. Transl. Med. 2012;10:190. doi: 10.1186/1479-5876-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Young N.A., Wu L.C., Bruss M., Kaffenberger B.H., Hampton J., Bolon B. A chimeric human-mouse model of Sjogren’s syndrome. Clin. Immunol. 2015:8. doi: 10.1016/j.clim.2014.10.004. 156b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zayoud M., El Malki K., Frauenknecht K., Trinschek B., Kloos L., Karram K. Subclinical CNS inflammation as response to a myelin antigen in humanized mice. J. Neuroimmune. Pharmacol. : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:1037–1047. doi: 10.1007/s11481-013-9466-4. [DOI] [PubMed] [Google Scholar]
- 132.Gilhar A., Ullmann Y., Berkutzki T., Assy B., Kalish R.S. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J. clin. investig. 1998;101:62–67. doi: 10.1172/JCI551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Rijn R.S., Simonetti E.R., Hagenbeek A., Hogenes M.C., de Weger R.A., Canninga-van Dijk M.R. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2-/- gammac-/- double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 134.Ghraieb A., Keren A., Ginzburg A., Ullmann Y., Schrum A.G., Paus R. iNKT cells ameliorate human autoimmunity: lessons from alopecia areata. J. Autoimmun. 2018;91:61–72. doi: 10.1016/j.jaut.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 135.Fecher L.A., Agarwala S.S., Hodi F.S., Weber J.S. Ipilimumab and its toxicities: a multidisciplinary approach. The Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.June C.H., Warshauer J.T., Bluestone J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017;23:540–547. doi: 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 137.Morton J.J., Bird G., Keysar S.B., Astling D.P., Lyons T.R., Anderson R.T. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35:290–300. doi: 10.1038/onc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gammelgaard O.L., Terp M.G., Preiss B., Ditzel H.J. Human cancer evolution in the context of a human immune system in mice. Mol.Oncol. 2018;12:1797–1810. doi: 10.1002/1878-0261.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosato R.R., Davila-Gonzalez D., Choi D.S., Qian W., Chen W., Kozielski A.J. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20:108. doi: 10.1186/s13058-018-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang M., Yao L.C., Cheng M., Cai D., Martinek J., Pan C.X. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018;32:1537–1549. doi: 10.1096/fj.201700740R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Capasso A., Lang J., Pitts T.M., Jordan K.R., Lieu C.H., Davis S.L. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J. immunother. cancer. 2019;7:37. doi: 10.1186/s40425-019-0518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao L.C., Aryee K.E., Cheng M., Kaur P., Keck J.G., Brehm M.A. Creation of PDX-bearing humanized mice to study immuno-oncology. Methods Mol. Biol. 2019;1953:241–252. doi: 10.1007/978-1-4939-9145-7_15. [DOI] [PubMed] [Google Scholar]
- 143.Zhao Y., Shuen T.W.H., Toh T.B., Chan X.Y., Liu M., Tan S.Y. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut. 2018;67:1845–1854. doi: 10.1136/gutjnl-2017-315201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vudattu N.K., Waldron-Lynch F., Truman L.A., Deng S., Preston-Hurlburt P., Torres R. Humanized mice as a model for aberrant responses in human T cell immunotherapy. J. Immunol. 2014;193:587–596. doi: 10.4049/jimmunol.1302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weaver J.L., Zadrozny L.M., Gabrielson K., Semple K.M., Shea K.I., Howard K.E. BLT-immune humanized mice as a model for nivolumab-induced immune-mediated adverse events: comparison of the NOG and NOG-EXL strains. Toxicol. Sci. :Off. J. Soc. Toxicol. 2019;169:194–208. doi: 10.1093/toxsci/kfz045. [DOI] [PubMed] [Google Scholar]