Abstract
Bullous skin diseases are a group of dermatoses characterized by blisters and bullae in the skin and mucous membranes. The etiology and pathogenesis of bullous skin diseases are not completely clear. The most common are pemphigus and bullous pemphigoid (BP). Autoantibodies play critical roles in their pathogenesis. Abnormalities in the adhesion between keratinocytes in patients with pemphigus leads to acantholysis and formation of intra-epidermal blisters. Anti-desmoglein autoantibodies are present both in the circulation and skin lesions of patients with pemphigus. The deficient adhesion of keratinocytes to the basement membrane in BP patients gives rise to subepidermal blisters. Autoantibodies against the components of hemidesmosome can be detected in BP patients. Many novel therapeutics based on knowledge of the pathogenesis have emerged in recent years.
Keywords: Tolerance, Autoimmune skin diseases, Bullous pemphigoid, Autoantibody, Immunosuppression
Highlights
-
•
Bullous skin diseases are characterized by blisters and bullae in the skin and mucosa.
-
•
Autoimmune-mediated response is the main cause of pemphigus and bullous pemphigoid.
-
•
Multiple novel therapeutic approaches have emerged in recent years.
1. Introduction
Bullous skin diseases are a group of common life-threatening dermatoses. The main clinical features are blisters and bullae in the skin and mucous membranes, evolving into erosion in the skin or mucosal surface after rupture. This leads to increased risk of water loss, electrolyte imbalance and infections. Based on the pathogenesis, bullous skin diseases can be divided into autoimmune and non-autoimmune bullous skin diseases. In autoimmune bullous diseases, pathogenic autoantibodies can be detected in both the circulation and skin lesions, while genetic factors play a more significant role in the pathogenesis of non-immune bullous skin diseases. Both these conditions can be further classified into intraepidermal and subepidermal bullous skin diseases, based on the skin layer that is affected (Table 1). Clinically, the most common two types of bullous skin disease are pemphigus and bullous pemphigoid.
Table 1.
Classification of bullous dermatoses.
| Autoimmune bullous dermatoses | Non-autoimmune bullous dermatoses | |
|---|---|---|
| Intraepidermal bullous dermatoses | Pemphigus | Epidermolysis bullosa simplex |
| Familial benign chronic pemphigus (Hailey-Hailey disease) | ||
| Subepidermal bullous dermatoses | Bullous pemphigoid | Epidermolysis bullosa of the border |
| Cicatricial pemphigoid | Epidermolysis bullosa dystrophica | |
| Dermatitis herpetiformis | ||
| Linear IgA bullous dermatosis | ||
| Epidermolysis bullosa acquisita | ||
| Pemphigoid gestationis |
Glucocorticoids and/or immunosuppressive agents are currently the main treatments for autoimmune bullous skin diseases. Due to the long course and recurrent episodes of diseases, the long-term use of glucocorticoids and immunosuppressive agents can lead to frequent and significant adverse reactions, such as osteoporosis, osteonecrosis, hypertension, diabetes, eye problems, gastrointestinal haemorrhage, and myelosuppression. The goals of current research in this field focuses on a better understanding of the pathogenesis of pemphigus and bullous pemphigoid in order to develop safer and more efficient therapeutics for the diseases.
2. Pemphigus
2.1. Clinical manifestations and histology
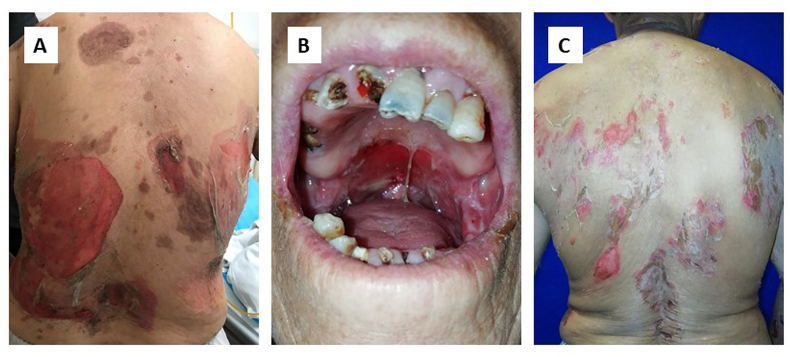
Pemphigus, derived from the Greek pemphix, which means blister or bullae, is a group of chronic, recurrent, and potentially lethal autoimmune bullous dermatoses caused by loss of intercellular adhesions in the epidermis. This disease is more common in people aged 50–60 [1,2]. The incidence of pemphigus varies substantially by race and region, from 0.5 cases per year per million people reported in Germany to 8 cases per year per million people reported in Greece [3,4]. Pemphigus vulgaris (PV) is the most common clinical subtype of pemphigus. Other forms of the disease include pemphigus foliaceus (PF) and erythematous pemphigus. Oral mucosal involvement occurs in almost all PV patients and usually before the appearance of skin lesions. It may be the only presenting sign in the early stages of pemphigus. The buccal and palate mucosa are the most commonly affected sites, followed by the lips, bottom of the mouth, and less invasively, the gums. The lesions present as irregular erosions or ulcerations and gradually spread to the surrounding area. The erosive surface is friable and prone to bleeding and difficult to heal. The patients usually feel burning when eating, chewing and swallowing.
Typical skin lesions are loose blisters or bullae over normal-appearing skin or erythema, followed by erosion. Nikolsky’s sign is positive. Lesions of patients with PF often occur in the skin of the head and face, as well as chest and back, while oral mucosa is rarely involved. The blisters of PF patients are erythematous and easier to rupture than in PV when the blisters are still small, which leads to a smaller erosive surface. Lesions of PF are covered with scales and scabs that are snuff-coloured, oleaginous, and leaf-shaped. The prognosis of PF is better than that of PV (Fig. 1).
Fig. 1.
Clinical features of pemphigus. A. skin lesions of pemphigus vulgaris; B. oral mucosa involvement of pemphigus vulgaris; C. skin lesions of pemphigus foliaceus.
The basic pathological feature in pemphigus is acantholysis. Acantholysis is the destruction of adhesions between cells of the spinous layer, leading to the formation of intraepidermal blisters. Acantholytic cells are found in the blister cavity, characterized by a round-shape, uniformly eosinophilic cytoplasm, large and deeply dyed nuclei surrounded by a light blue halo, and larger than the normal spinous layer cells. The site of acantholysis varies depending on the type of pemphigus. In PF, acantholysis occurs in the upper spinous layer or granular layer, while involvement of deeper layers is characteristic of PV.
Direct immunofluorescence of skin tissue reveals IgG and C3 deposition between the spinous cells, which is distributed in a grid. Fewer patients show IgM and IgA deposition. The immunoglobulin and complement deposition in PV is located below the spinous layer, while in PF, it is located above the spinous cell layer or even in the granular layer. It is difficult to distinguish between PF and PV by immunofluorescence. Indirect immunofluorescence shows that IgG type anti-Dsg autoantibodies exist in the serum of about 80% of pemphigus patients.
2.2. Pathogenesis
2.2.1. Genetic factors
Pemphigus is a polygenic autoimmune disease. Although pemphigus is often sporadic, and there is rarely a case in which more than one patient with pemphigus in a family exists, circulating IgG autoantibodies have been detected more frequently in family members of patients with PV than in the healthy population [5]. Autoimmune diseases such as rheumatoid arthritis (RA) and type 1 diabetes mellitus (T1DM) also have been found more frequently in relatives of patients with PV [6]. This suggests that there exists a genetic predisposition of autoimmunity in general and supports the role of genetic factors in pemphigus. Many researchers have explored the inherited susceptibility to pemphigus. The distribution of related loci varies by region and population. There is evidence that the human leukocyte antigen (HLA) is related to the pathogenesis of pemphigus. DQB1*0503 and DRB1*0402 are HLA alleles that are reported most frequently in PV patients from France, Spain, Slovakia, Italy, Brazil and North America [7].
The largest GWAS in pemphigus was performed by Zhang et al. on 365 PV patients, 104 PF patients and 1105 unaffected controls. They identified specific alleles for PV (HLA-DRB1*04:06 and HLA-DRB1*14:01) and PF (HLA-DQB1*03:02), whereas HLA-DQB1*05:03 was found to be a specific allele both for PV and PF [8]. A meta-analysis performed by Yan et al. on the association between HLA-DRB1 and PV suggests that HLA-DRB1*04, HLA- DRB1*08 and HLA-DRB1*14 are related to a genetic predisposition to develop PV [9]. A study involving 110 Iranian pemphigus patients demonstrated a correlation between Suppression of Tumorigenicity 18 (ST18) polymorphism and pemphigus [10]. ST18, which regulates apoptosis and inflammation, has been shown to be associated with deletion of intracellular adhesion in pemphigus vulgaris [11,12]. It has also been found that ST18 polymorphism plays a significant role in Jewish and Egyptian patients with PV, while it is not linked to German or Chinese susceptibility to PV [13,14]. A risk variant of the ST18 gene may promote cytokine secretion mediated by PV autoantibodies and acantholysis in a p53/p63-dependent manner [15]. Overexpression of ST18 is able to heighten the susceptibility to deletion of keratinocytes adhesion, and enhanced extracellular signal-regulated kinase (ERK) signaling is a possible mechanism [16]. A p.Arg435His variation in IgG3 has been reported to be associated with PV in cohorts including German, Turkish, Egyptian and Iranian patients [17].
2.3. Environmental factors
Environmental factors are known to be critical for the pathogenesis of autoimmune bullous dermatosis. Drugs are the most common trigger for pemphigus. Mercaptan drugs, especially penicillamine [18] and captopril [19], can inhibit the activity of enzymes such as those that facilitate aggregation of keratinocytes (e.g. g-glutamyl transpeptidase or keratinocyte transglutaminase (a calcium-dependent thiol enzyme), and activate enzymes that disaggregate keratinocytes (e.g. proteases, plasminogen activator), leading to disruption of the adhesion ability of keratinocytes. Moreover, some mercaptan drugs may have an impact on the formation of autoantibodies. Phenol drugs may facilitate the releasing of proinflammatory cytokines, such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α, by keratinocytes [20]. The relevance of pemphigus and heroin addiction was first reported by Fellner et al., in 1978 [20]. There are reports of vaccine associated pemphigus as well, with possible associations including influenza [21], rabies [22], hepatitis B [23] and tetanus [24].
Certain allergens may lead to autoimmune responses. Walnut antigens or allergens have been reported to initiate the development of autoantibodies in pemphigus vulgaris via a “hit-and-run” pattern, meaning that pathogenic antigens causing the onset of autoimmune conditions disappear once they have triggered the disease [25]. Kridin et al. conducted a population-based large-scale study, which included 1985 pemphigus patients and 9874 controls, and found a significant association between schizophrenia and pemphigus [26]. The molecular mechanisms of this observation have not yet been determined. Other triggering factors of pemphigus include viral infection (such as herpes simplex virus), nutrients and micronutrients factors, radiotherapy and pregnancy [27].
2.3.1. Autoantigens and autoantibodies
IgG-type desmoglein (Dsg) antibodies, especially anti-Dsg3 and anti-Dsg1, are present in the serum and between the epidermal cells of pemphigus patients. Gene family members of Dsg are distributed on chromosome 18. Dsgs and desmocollins (Dscs) are cadherin-like transmembrane glycoprotein components of desmosomes, which are intracellular junctions that protect cells from the injury of mechanical forces. Intracellular proteins of desmosomes, such as plakophilin 1, plakoglobin and desmoplakin, connect Dscs and Dsgs to keratin intermediate filaments (KIF), which are components of the cytoskeleton.
Passive transfer of IgG-type Dsg antibodies extracted from the serum of pemphigus patients to neonatal mice to generate a passive model of pemphigus demonstrates the pathogenicity of circulating IgG from patients with pemphigus. The IgG 4 subclass has been shown to play a predominant role in PV and PF patients [28,29]. Fc fragments may be non-essential for the occurrence and development of pemphigus on account of the evidence that F(ab’)2 and Fab’ fragment of IgG antibodies from Fogo selvage patients may trigger keratinocyte detachment and epidermal disease in neonatal mice [30].
The distribution and expression levels of Dsg1 and Dsg3 in skin and mucosa are different. Dsg1 is distributed all over the epidermis, and is especially abundant in the surface layer [31], while Dsg3 is mainly expressed in the basal and parabasal cell layers. Dsg1 and Dsg3 are both expressed throughout the epidermis of mucous membranes, but the Dsg3 is much more concentrated than Dsg1. The distribution pattern of Dsg1 and Dsg3 autoantibodies is related to the clinical characteristics of different subclasses of pemphigus. Only anti-Dsg1 antibody can be detected in PF patients, while only anti-Dsg3 antibodies are found in patients with mucosa-dominant pemphigus. Mucocutaneous pemphigus patients have both anti-Dsg1 and anti-Dsg3 autoantibodies.
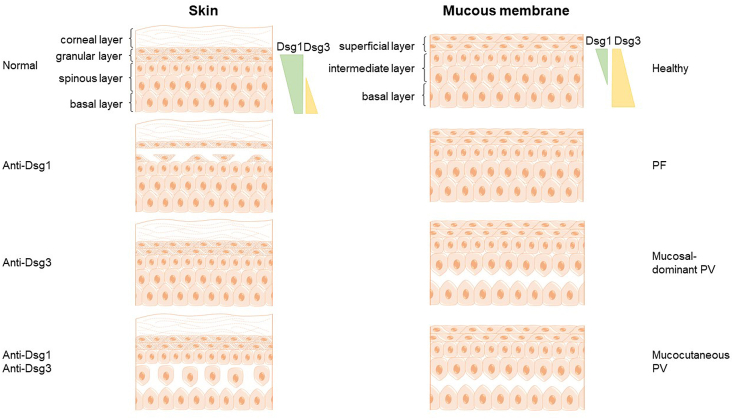
The desmoglein compensation theory clarified the pathogenesis of blister location in the skin and mucosa of pemphigus patients corresponding to the distribution pattern of autoantigens (Fig. 2). Patients with autoantibodies against Dsg3 but without autoantibodies against Dsg1 usually do not suffer from skin lesions, which may result from a compensation effect of Dsg1. For the same reason, patients with pemphigus foliaceus, whose circulation contains only autoantibodies against Dsg1, present with blisters in superficial layers of the skin but not the mucous membranes.
Fig. 2.
Desmoglein compensation in pemphigus. The distribution and expression levels of Dsg1 and Dsg3 vary in the skin and mucous membrane (Dsg1: green pattern; Dsg3: yellow pattern). Desmoglein compensation theory clarifies the association between the site of blisters and the distribution pattern of autoantigens. Patients with pemphigus foliaceus, whose circulation only contain anti-Dsg3 1 antibodies, suffer from superficial blisters in the skin but there is no mucous membrane involvement. It may be due to the compensation effect of Dsg3. Patients with anti-Dsg 3 antibodies but without anti-Dsg 1 antibody usually do not suffer from a skin lesion, presenting with mucosal-dominant PV instead, while mucocutaneous PV patients present with deep-layer blisters in both skin and mucosa. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Autoantibodies have direct pathogenicity independent of complement activation in pemphigus, which differs from other autoimmune-mediated dermatoses such as epidermolysis bullosa and bullous pemphigoid. The IgG4 subclass has been shown to be the predominant isotype of antibodies in PV and PF [29], which does not result in complement activation. Dsg3 knockout mice that have a normal complement system show similar lesions resulted from acantholysis with mucosal-dominant type pemphigus [32]. Transfer of autoantibodies against Dsg3 and Dsg1 into newborn mice can induce a phenotype resembling PV [33,34]. Autoantibody epitope mapping for murine models and pemphigus patients illustrated that pemphigus autoantibodies lead to the formation of a trans-adhesion interface between keratinocytes via binding to the extracellular domain of desmogleins [35]. IgG autoantibodies extracted from sera of PV patients [36,37] or human monoclonal PV antibodies [38] can lead to internalization and degradation of unintegrated Dsg3, interfering with normal desmosome assembly. The resultant incomplete desmosomes leads to reduced intercellular adhesion. Sokol et al. performed ultrastructural examination of pemphigus tissue and found a widened space between keratinocytes layers without obvious acantholysis [39].
Signaling events take place when pathogenic autoantibodies bind to desmogleins on the keratinocytes surface. IgG purified from sera of patients with PF resulted in cultured keratinocyte dissociation without blocking Dsg1 trans-interaction [40]. The MAPK pathway involving P38 mitogen-activated protein kinase (p38MAPK) and the downstream molecule MAPK-activated protein kinase 2 (MK2) has been the most widely studied pathway in pemphigus [[41], [42], [43], [44], [45]]. Keratinocytes lacking plakoglobin are much less sensitive to autoantibodies of pemphigus compared to normal cells, and c-myc may play a role in this process [46,47]. Many other signaling pathways, such as epidermal growth factor receptor, RHO GTPases, caspases and mitochondria, have been implicated in acantholysis. All these factors and pathways may impact the condition and behavior of desmosome and its components, for instance, transfer and internalization. Anti-Dsg1 and Dsg3 autoantibodies may lead to acantholysis by modulating these pathways, which may also become important targets for developing new therapies for pemphigus. However, signaling events are not enough to induce specific histological manifestations of epithelial blisters of pemphigus, indicating the signaling pathways are not indispensable in blisters formation of patients with PV or PF.
2.3.2. B cells and T cells in pemphigus
The critical role of B cells in the production of autoantibodies in autoimmune diseases has been extensively studied, along with the role of T cells and T-B interactions, and the resulting release of proinflammatory cytokines. The role of B cell clones has also been investigated. No Dsg3-specific B cell clonal lineages were detected in pemphigus patients with long-term clinical and serological remission. After complete clinical remission, IgG+ Dsg3 specific B cell clones can exist persistently up to 8.5 years. The same B cell clones may exist for many years in recurrent pemphigus patients [48,49]. The correlation between pathology and B cell clones suggests that therapeutic methods that deplete B cells may be effective for pemphigus. Many novel therapeutic methods targeting B cells have been utilized in the treatment of pemphigus, including anti-CD20 antibodies, desmoglein-specific B cell depleting therapy, anti-CD19 antibodies, and Bruton’s tyrosine kinase inhibitors (BTKI), which targets B cell receptor signaling [50]. By analyzing the Dsg3 specific B cells response in pemphigus patients treated with rituximab, Colliou et al. concluded that rituximab mediates long-term remission by impeding the maturation of B cells and delaying the appearance of memory B cells [51,52].
Therapeutic methods aimed at depleting B cells may lead to deterioration of pemphigus, which may be due to the depletion of regulatory B cells at the same time. The number of regulatory B cells is increased in pemphigus patients compare with healthy controls, but with defective suppressive function on Th1 cells [53]. The level of interleukin-10 producing B cells is decreased in pemphigus patients but not in BP patients [54].
It has been shown that numerous B cells, T cells and plasma cells exist in skin lesions, and there is a larger number of Dsg1-and Dsg3-specific B cells in pemphigus patients compared with normal controls. These results suggest that there is a pathogenic role of locally infiltrating lymphocytes in pemphigus [55].
Dsg3 specific CD4+ T cells can induce a phenotype of interface dermatitis and PV in a mouse model [56]. HLA-II is implicated in the activation of autoreactive T cells in PV. HLA-DRβ1*0402 and/or HLA-DQβ1*0503 is closely related to the recognition of Dsg3 by Th1 and Th2 cells. Autoantibodies against DR and DQ may block the proliferation of autoreactive Th cells [57].
Since autoreactive T cells that mainly produce IL-10 and IFN-γ have also been found in healthy individuals [58], defective regulatory T (Treg) cells may facilitate the onset of pemphigus. A large amount of evidence has proven the pivotal role of Tregs in preventing autoimmune diseases. Langerhans cells present Dsg3 to T cells via the C-type lectin langerin. IL-2 signaling is critical in LC-mediated regulatory T cell expansion [59]. In adoptive transfer mouse models of pemphigus, Yokoyama and colleagues demonstrated that Tregs control the production of anti-Dsg3 autoantibodies and Dsg3 has an impact on the development of Tregs [60]. However, transfer of Dsg3-specific T cells or B cells into Dsg3+/+ Rag2-/- mice did not induce the production of autoantibodies and a pemphigus phenotype, suggesting the interaction of autoreactive T cells and B cells in is required in the pathogenesis of pemphigus [61].
The interaction between autoreactive T cells and B cells are the key events for humoral autoimmunity targeting Dsg3. In normal conditions, autoreactive B cells exist in circulation. The immune system can sense adverse or harmful events such as tissue injury and can shut off the development of antigen-specific B cells in a Fas-mediated manner with the existence of CD4+T cells. In pemphigus vulgaris, this protective mechanism is impaired which results in the expansion of B cells targeting autoantigens [62].
2.4. Diagnosis and therapeutics
2.4.1. Diagnosis
The diagnosis of pemphigus is primarily based on a comprehensive evaluation of clinical manifestations, histopathology and immunopathological features. The disease needs to be differentiated from bullous pemphigoid, linear IgA bullous skin disease and herpetic dermatitis. Pemphigus lesion is often limited to the oral cavity in the early stages of the disease, which renders it difficult to diagnose and distinguish from aphthous mouth ulcers and lichen planus. Cell smears and biopsy at the erosion site can assist in diagnosis.
2.4.2. Novel therapeutics based on pathogenesis
The main treatment of pemphigus are currently glucocorticoids and immunosuppressive agents. Glucocorticoids have significantly reduced the mortality of pemphigus [63]. Glucocorticoids may enhance the synthesis of desmoglein in keratinocytes, which can make up for the internalization and depletion of desmoglein caused by autoantibodies [64]. Immunosuppressants such as mycophenolate mofetil and azathioprine may decrease the production of autoantibodies [65,66]. Treatments that can rapidly decrease levels of circulating autoantibodies, such as plasma exchange and immunosorption or intravenous immunoglobulin injection (IVIg) can be used depending on the patient’s condition.
The main cause of death of pemphigus patients is related to side effects of glucocorticoids and immunosuppressive agents, rather than the disease. Thus, there has been a need to find more innovative effective and safe alternatives for treatment. The ability to develop monoclonal antibodies to target specific mediators of disease has revolutionized the management of many autoimmune diseases. Anti-CD20 antibodies, such as rituximab, can induce the depletion of autoantibodies-secreting B cells. Rituximab combined with short-term systemic corticosteroids is now considered a first-line treatment strategy [67]. Other drugs under investigation which target CD20 for pemphigus include veltuzumab [[68], [69], [70]] and ofatumumab [71]. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, has been proven to be a safe and effective therapeutic option for autoimmune conditions [72]. A phase 3 clinical trial of ofatumumab in the treatment of pemphigus has been completed [73].
Bruton tyrosine kinase (Btk), a critical molecule of the phospholipase Cγ(PCLγ) pathway, plays a key role in BCR signaling. A functional deficiency of Bkt can block the development of B cells at the stage of preB cells. Btk inhibitors (BTKI), such as ibrutinib [74], PRN1008 [75], and PRN473 [76] can suppress B cell activity, survival, proliferation, differentiation and maturation.
Ellebrecht et al. reengineered the chimeric autoantibody receptor-T cells (CAAR-T) that can specifically kill anti-Dsg3 B cells via specific BCR in PV patients without off-target toxicity. CAAR-T is composed of the autoantigen in PV, Dsg3, and CD137-CD3ζ signaling domains [77]. However, there are no long-term studies in preclinical models or human studies regarding the safety and efficacy of CAAR-T.
Other novel therapeutics based on the immune mechanisms for pemphigus include B cell activating factor (BAFF) inhibitors, p38 mitogen-activated protein kinase (p38MAPK) inhibitors, anti-CD154 monoclonal antibody, daclizumab (a monoclonal antibody against CD25), stem cells transplantation, anti-neonatal Fc receptor and polyclonal regulatory T cells (Table 2) [76,78].
Table 2.
Novel therapeutics for pemphigus.
| Therapeutics | Target | Development |
|---|---|---|
| Rituximab | CD20 | First-line treatment combined with short-term systemic corticosteroids |
| Veltuzumab | CD20 | Approved by FDA for the treatment of patients with pemphigus with orphan drug status |
| Ofatumumab | CD20 | A double-blind, randomized, placebo-controlled, phase 3 clinical trial was completed (NCT01920477) |
| PRN1008 | BTK | A randomized, double-blind, placebo-controlled, phase 3 clinical trial is ongoing (NCT03762265) |
| CAAR-T cells | Anti-Dsg3 B cells | Preclinical models |
| VAY736 | BAFF-R | A randomized, placebo-controlled, double-blind, phase 2 clinical trial is ongoing (NCT01930175) |
| SYNT001 | Neonatal Fc receptor | A clinical trial is currently ongoing (NCT03075904) |
| Argx-113 | Neonatal Fc receptor | A clinical trial is currently ongoing (NCT03334058) |
| PolyTregs | A nonrandomized, open-label, phase 1 clinical trial is ongoing (NCT03239470) |
3. Bullous pemphigoid
3.1. Clinical manifestations and histology
Bullous pemphigoid (BP) is an autoimmune skin disease characterized by subepidermal thick-walled tense bullae and is more frequently seen in the elderly population. The mean age of onset in the UK has been reported to be 80 years old. The incidence of BP depends on the population, ranging from 2.5 cases per year per million people reported from Romania [79] to 42.8 cases per year per million people reported from the United Kingdom [80].
The course of the disease usually begins with itching, which lasts from a few days to years, and nonspecific skin lesions in the extremities. Typical lesions are tense blisters developing on normal-appearing skin, or erythema mainly occurring on the chest, abdomen and proximal extremities. The blisters usually have a hemispherical shape with a diameter ranging from less than 1 cm to several centimeters. The blister wall is thick and not easy to break. After rupture, the erosive surface is often covered with scabs, and heals spontaneously. Physical examination shows a negative Nikosky’s sign. Mucosal damage is absent in most patients with BP. A small percentage of patients suffer from superficial blisters and erosions in the mouth. BP often progresses slowly, and the prognosis is better than pemphigus.
The histopathology of BP is characterized by subepidermal blisters, which are single-atrial, with normal skin on the top. The net rack in the blisters consists of fibrin, neutrophils and eosinophils. Infiltrating eosinophils, lymphocytes and neutrophils exist around the dermal papilla. In late stages, subepidermal blisters may become intraepidermal blisters due to the regeneration of basal cells. Direct immunofluorescence performed on the skin shows linear deposition of IgG and complement component C3 in the basement membrane zone in more than 90% of patients. Rarely, IgM and IgA deposition may be observed. Indirect immunofluorescence of serum has shown that circulating IgG autoantibodies against the basement membrane exist in about 75% of BP patients, which is a helpful finding in diagnosing BP.
3.2. Pathogenesis
3.2.1. Genetic factors
There has been significant research that supports a genetic predisposition to BP. Fang H et al. identified susceptibility alleles of major histocompatibility complex class II (MHC II) gene HLA in Chinese patients with BP, including DQA1*01:05, DQA1*05:05, DQA1*05:08, DQB1*03:01, DQB1*05:01 and DRB1*10:01. They also found that DQA1*01:02, DQA1*01:03, DQB1*02: 02 and DRB1*07:01 may play a part in preventing BP. There is a significant increased frequency of DRB1*13, DQA1*05 and DQB1*03 in BP patients compared with normal controls [81].
Research has also shown that HLA-DQB1*0301 is related to BP and mucous membrane pemphigoid (MMP) [[82], [83], [84], [85]]. Dipeptidyl peptidase-4 (DPP-4) inhibitor, a class of drugs commonly used for the treatment of type 2 diabetes, is considered as to be an inducer of BP. HLA-DQB1*0301 is involved in noninflammatory DPP4 induced BP [86,87].
The genetic susceptibility to BP is different among diverse ethnic groups. HLA-DQB1*0301 is the most common marker associated with BP in Chinese [81], Germans [88], Iranians [89] and Americans [90], while HLA- DRB1*1101, DQB1*0302, DRB1*04, DQA1*0301, DQB1*0302, DRB1*1101, DQA1*0505 and DQB1*0302 are more frequently detected in Japanese BP patients [91].
Since differentially expressed cytokines may play a role in the pathogenesis of BP [[92], [93], [94]], the genetic polymorphisms of cytokines may contribute to the susceptibility to BP. Tabatabaei-Panah et al. have shown that the minor allele of IL-8 SNP may protect people from BP in Iran [95]. A study in the Chinese population showed that gene polymorphisms of IL-1β are related to BP in women [96].
Polymorphisms in mitochondrial DNA (mtDNA), especially the mitochondrially encoded ATP synthase 8 gene (MT-ATP8), were significantly associated with BP in German patients [97]. CYP2D6, also known as debrisoquine hydroxylase, is a cytochrome P450 isoenzyme. It is associated with a higher prevalence of BP [98]. These studies suggest that gene polymorphisms apart from HLA may contribute to the genetic predisposition of BP.
3.2.2. Environmental factors
Drug-related BP can be divided into drug-induced BP and drug-triggered BP. Both of these may be initiated by systemic or topical drugs. Resolution occurs after removal of the drug in drug-induced BP but not in drug-triggered BP. The medication history is the most important piece of information required to differentiate drug-induced BP from BP. Age of onset is usually younger in drug-induced BP than BP. Over 50 agents have been reported as a cause of BP, including antibiotics (especially those containing or releasing sulfhydryl), ACE inhibitors, NSAIDs, diuretics (e.g. Captopril, frusemide and spironolactone), anti-diabetics, TNF inhibitors and vaccines [99,100].
Drugs may act as antigenic haptens in the pathogenesis of autoimmune conditions. By combining with molecules in the lamina lucida or basement membrane, hidden epitopes can become exposed and initiate an autoimmune response [101,102]. Molecular mimicry is another potential pathogenic mechanism for drug-related BP. Many drugs may bind to microRNA as well as other transcriptional and translational regulators, causing aberrant immunoregulation and the development of an autoimmune response [100].
There is increasing evidence for the involvement of DPP4is in the pathogenesis of BP. Apart from DPP4is [[103], [104], [105], [106], [107], [108], [109], [110], [111]], other oral diabetic medications have not been shown to be associated with BP [112]. A study of 86 patients with BP and 134 healthy controls demonstrated that loop diuretics were implicated in pathogenesis of BP [113]. Siegel et al. conducted a retrospective study of patients received anti-PD-1/PD-L1 therapy and found that about 1% of patients developed BP [114].
Viral infection, such as H.pylori, Toxoplasma gondii, HBV, HCV, and CMV, have been demonstrated to be a trigger of BP [115,116]. Viruses may elicit BP via molecular mimicry, in which similarities between viral peptides and self-peptides of skin and mucous membrane lead to an immune response against self-antigens [117].
Physical factors, such as radiotherapy [[118], [119], [120], [121]], UV light [122] and photodynamic therapy [123], can also be related to the onset of localized or generalized BP. These factors may change the antigenicity of the basement membrane and stimulate the formation of autoantibodies. An indirect immunomodulatory effect of ionizing irradiation may disturb the balance between helper and regulatory T cells [124]. Other events including surgical procedures, trauma, thermal burns, and insect bites have been identified as triggers of BP [125].
The association between BP and neurologic diseases is widely recognized. Stroke, Parkinson’s disease and dementia are the most common neurologic conditions implicated in BP, although the pathophysiology is still undefined [[126], [127], [128]]. Elevated levels of anti-BP180 and anti-BP230 autoantibodies in the cerebrospinal fluid (CSF) of patients with BP and neurological disorders has been demonstrated [129]. Homologues to BP180 and BP230 exist in the central nervous system and neurologic disease may be associated with the production of antibodies cross-reacting with BP antigens in the skin [130].
3.2.3. Autoantigens and autoantibodies
Two autologous antigens are primarily implicated in the pathogenesis of BP. BP230, also known as BP antigen 1 (BPAG1), is a high molecular weight polypeptide (230kD). BP230 is located in hemidesmosome attachment plaques of basal cells. BP180, also referred to as BPAG2 or collagen XVII, is a transmembrane structural protein with a molecular weight of 180kD. The amino-terminal of BP180 is located in hemidesmosome attachment plaques of basal cells, while the carboxyl terminal is located in the lamina lucida outside of the basal cells. Pathogenic IgGs target the non-collagenous region (NC16A) of BP180, which is just distal to the cell membrane [131,132]. There is a correlation between anti-BP180 NC16A IgG and disease scores of BP which are obtained using the Bullous Pemphigoid Disease Area Index (BPDAI) or the Autoimmune Bullous Skin Disorder Intensity Score (ABSIS). However the presence of anti-BP230 IgG did not show any correlation with such scores [133]. There is a significant correlation between disease activity and the titers of anti-BP180 IgG detected by ELISA [134,135]. These studies support the dominant role of anti-BP180 autoantibodies compared with anti-BP230 autoantibodies in the pathogenesis of BP. There are approximately 8% of BP cases where only anti-BP230 antibodies are found, and this is referred to as BP230-type BP [136].
C3 deposition along the epidermal BMZ can be detected in about 83.1% of BP patients [137], implying a critical role for complement activation in the formation of blisters in BP. The complement deposition is related to the NC16A titer measured by ELISA, which is associated with disease activity [138].
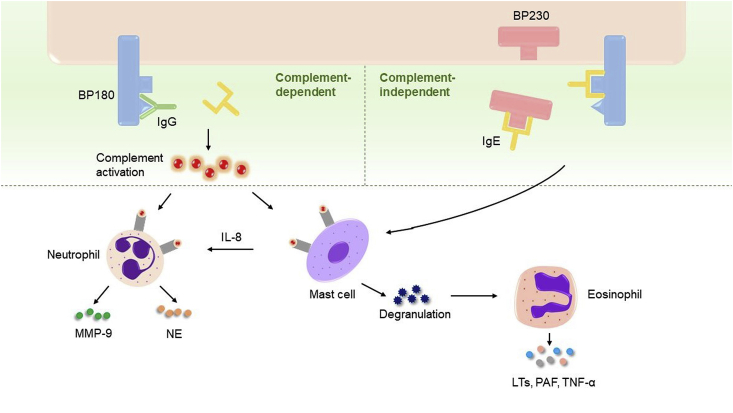
IgG1 and IgG3 autoantibodies can bind to target autoantigens and activate the complement cascade. The formation of activated complement factors, C3a and C5a, induce the chemotaxis of neutrophils and eosinophils, as well as the degranulation of mast cells. Neutrophils at the basement membranes can release proteolytic enzymes, such as neutrophil elastase (NE) and matrix metalloproteinase (MMP)-9, leading to the degradation of the extracellular parts of BP180 and subsequently the deletion of cell-matrix adhesion. Eosinophils can migrate to the basement membranes and release lysosomal enzymes, causing rupture and disappearance of hemidesmosomes and the anchoring filaments of basal cell membranes.
Meanwhile, various mediators, including platelet activating factor (PAF), tumour necrosis factor (TNF-α) and leukotrienes (LTs) are released. Mast cells recruit neutrophils by releasing interleukin (IL)-8. In an experimental BP model, a protease released by mouse mast cells activated MMP-9 and degraded BP180. IL-23 and IL-17 released from mast cells, monocytes, neutrophils as well as lymphocytes lead to an increased release of NE and MMP-9 from neutrophils (Fig. 3) [139]. Low-molecular-weight heparin tinzaparin sodium may be beneficial to BP patients with complement deposits because of their inhibitory effect on complement components [140].
Fig. 3.
Pathogenesis of bullous pemphigoid. The interaction between BP autoantibodies, including IgG and IgE, and their target antigens, BP180 and BP230, lead to pathogenic events in complement-dependent and complement-independent pathways. The release of proteases by neutrophils results in tissue damage, which is enhanced by IL-8 from mast cells. On the other hand, the degranulation of mast cells is implicated in the activation of eosinophils. The production of LTs, PAF and TNF-α is connected with pruritus and urticaria-like lesions. MMP-9: matrix metalloproteinase-9; NE: neutrophil elastase; IL-8: interleukin-8; LTs: leukotrienes; PAF: platelet activating factor; TNF-α: tumor necrosis factor.
Among the autoantibodies against basement membrane, the IgG4 subclass, which lacks the ability to fix complement [141], plays a dominant role in BP [142]. There are approximately 15%–20% of BP patients who do not show complement deposition in their skin biopsy [137,143], suggesting that complement deposition is dispensable and that there may be complement-independent mechanisms involved in the pathogenesis of BP. F(ab’) fragments of anti-Hcol17-IgG and anti-hCol17-IgG4, which lack the ability to activate complement, can induce blister formation in Col17-humanized neonatal mice [144]. In summary, autoantibodies can induce the formation of blisters via direct interaction with their targets [145].
3.2.4. IgE and eosinophils
Elevated total serum IgE levels have been detected in 47%–85% of BP patients [[146], [147], [148]], and positively correlate with anti-BMZ antibody levels as well as disease activity [149,150]. This suggest that not only is IgE important in the pathogenesis of BP, but eosinophils as well. Circulating eosinophils can be detected in about 50%–60% of patients with BP [151,152]. A significant correlation exists between the level of circulating eosinophils and clinical manifestations, such as blisters and erosions [153]. Eosinophils are indispensable for the IgE-mediated formation of blisters in BP mouse models [154].
Zone et al. developed an IgE hybridoma of the LABD97 antigen, a component of the ectodomain of BP180. They transferred the IgE hybridoma into a severe combined immunodeficiency (SCID) mouse, which was engrafted with human skin. About 10 days after injection, the mice presented with erythema and intense scratching. At day 21, histological and immunopathological examination showed infiltration of numerous eosinophils and degranulation of mast cells, leading to blisters in the basement membrane [155]. Another passive transfer mouse model was developed by Fairley and his colleagues, whereby injection of IgE isolated from BP sera led to erythematous and elevated plaques. Infiltration of neutrophils, eosinophils and mast cells were observed in the dermis by histologic and ultrastructural examination [156]. These experimental murine models support the role played by IgE autoantibodies in BP.
Autoantibodies of the IgE isotype mainly target the NC16A domain of BP180 [[157], [158], [159]]. Mast cells coated with IgE exist in perilesional skin of patients with BP. Stimulating the circulating basophils of untreated BP patients with recombinant NC16A leads to release of histamine [160]. The interaction between IgE autoantibodies and the target antigens leads to complement activation, subsequent degranulation of mast cells, accumulation of neutrophils and eosinophils and the release of MMP-9 and NE (Fig. 3). IgE autoantibodies may react with eosinophils and mast cells in the dermis through FcεRI, the high-affinity receptor of IgE [161], and interaction of FcεRI with the extracellular domain of BP180 may induce the activation and degranulation of eosinophils [162].
3.3. Diagnosis and therapeutics
3.3.1. Diagnosis
The diagnosis of BP is confirmed by a comprehensive assessment of the clinical presentation, typical histological characteristics (subepidermal blisters and inflammatory eosinophilic infiltration), and direct immunofluorescence (DIF) examination of the skin, as well as serological tests.
3.3.2. Novel therapeutics based on pathogenesis
Local or systemic application of glucocorticoids is the main treatment for BP. Immunosuppressive agents such as azathioprine can be added as needed. For mild to moderate conditions, tetracycline alone and nicotinamide combined with tetracycline or erythromycin can be alternative therapeutics.
Significant efforts have been devoted to developing novel therapeutics for BP. Omalizumab, a humanized monoclonal antibody of IgE, has been reported to be useful in a pruritic BP patient who was deemed unsuitable for higher doses of glucocorticoids and immunosuppressive agents [[163], [164], [165], [166]], and can be an alternative treatment for BP patients. Bertilimumab, a human monoclonal antibody of eotain-1, may decrease the infiltration of eosinophils in the skin of patients with BP. A phase 2 clinical trial (NCT02226146) of bertilimumab is currently ongoing. Rituximab has been recommended as an effective therapeutic option for severe BP patients. A significant reduction in anti-BP180 and anti-BP230 autoantibodies, disease activity and steroid dosage occurred in BP patients following rituximab therapy [167]. Seventy five percent of BP patients achieved remission following a mean duration of 169 days of rituximab treatment in a retrospective study evaluating the efficacy of rituximab for patients with BP. This study also demonstrated that rituximab can be a relatively safe mode of therapy rituximab for patients with BP [168].
IL-17A has been shown to be a pivotal cytokine in the pathophysiology of BP. An elevated serum level of IL-17 has been found in BP patients [[169], [170], [171]]. A mouse model of BP showed a significant correlation between serum IL-17A levels and disease activity, and IL17A-deficient mouse treated with BP IgG did not demonstrate the phenotype of BP [172]. Ixekizumab, a recombinant humanized monoclonal antibody against IL-17, is in phase 2 clinical trials (NCT03099538) for BP patients [173]. AC-203, a topical ointment formulation which can modulate inflammasome and IL-1β pathways, is also in phase 2 clinical trials [174] and the NRLP3 level positively correlates with disease activity.
The etiology and pathogenesis of autoimmune bullous dermatoses remain to be further explored. Great efforts have been devoted to treatments of patients with pemphigus and BP, and more effective and safer therapeutic methods are being developed for bullous dermatoses.
Conflict of interest
None.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81430074, No. 81602767, No. 81830097, No. 81861138016), National Basic Research Program of China (No. 2014CB541904), the Natural Science Foundation of Hunan Province (2017JJ3453, 2017SK2042, 2018JJ3756) the National Key Research and Development Program of China (2016YFC0903900), and the Natural Key Clinical Specialty Construction Project of National Health and Family Planning Commission of the People’s Republic of China.
References
- 1.Alpsoy E., Akman-Karakas A., Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: pemphigus and bullous pemphigoid. Arch. Dermatol. Res. 2015;307:291–298. doi: 10.1007/s00403-014-1531-1. [DOI] [PubMed] [Google Scholar]
- 2.Shah A.A., Seiffert-Sinha K., Sirois D., Werth V.P., Rengarajan B., Zrnchik W., Attwood K., Sinha A.A. Development of a disease registry for autoimmune bullous diseases: initial analysis of the pemphigus vulgaris subset. Acta Derm. Venereol. 2015;95:86–90. doi: 10.2340/00015555-1854. [DOI] [PubMed] [Google Scholar]
- 3.Bertram F., Brà Cker E., Zillikens D., Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J. der Deutschen Dermatologischen Gesellschaft. 2009;7:434–439. doi: 10.1111/j.1610-0387.2008.06976.x. [DOI] [PubMed] [Google Scholar]
- 4.Michailidou E.Z., Belazi M.A., Markopoulos A.K., Tsatsos M.I., Mourellou O.N., Antoniades D.Z. Epidemiologic survey of pemphigus vulgaris with oral manifestations in northern Greece: retrospective study of 129 patients. Int. J. Dermatol. 2007;46:356–361. doi: 10.1111/j.1365-4632.2006.03044.x. [DOI] [PubMed] [Google Scholar]
- 5.Kricheli D., David M., Frusic-Zlotkin M., Goldsmith D., Rabinov M., Sulkes J., Milner Y. The distribution of pemphigus vulgaris-IgG subclasses and their reactivity with desmoglein 3 and 1 in pemphigus patients and their first-degree relatives. Br. J. Dermatol. 2000;143:337–342. doi: 10.1046/j.1365-2133.2000.03659.x. [DOI] [PubMed] [Google Scholar]
- 6.Firooz A., Mazhar A., Ahmed A.R. Prevalence of autoimmune diseases in the family members of patients with pemphigus vulgaris. J. Am. Acad. Dermatol. 1994;31:434–437. doi: 10.1016/s0190-9622(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 7.Vodo D., Sarig O., Sprecher E. The genetics of pemphigus vulgaris. Front. Med. 2018;5:226. doi: 10.3389/fmed.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S.Y., Zhou X.Y., Zhou X.L., Zhang Y., Deng Y., Liao F., Yang M., Xia X.Y., Zhou Y.H., Yin D.D., Ojaswi P., Hou Q.Q., Wang L., Zhang D.Y., Xia D.M., Deng Y.Q., Ding L., Liu H.J., Yan W., Li M.M., Ma W.T., Ma J.J., Yu Q., Liu B., Yang L., Zhang W., Shu Y., Xu H., Li W. Subtype-specific inherited predisposition to pemphigus in the Chinese population. Br. J. Dermatol. 2019;180:828–835. doi: 10.1111/bjd.17191. [DOI] [PubMed] [Google Scholar]
- 9.Yan L., Wang J.M., Zeng K. Association between HLA-DRB1 polymorphisms and pemphigus vulgaris: a meta-analysis. Br. J. Dermatol. 2012;167:768–777. doi: 10.1111/j.1365-2133.2012.11040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etesami I., Seirafi H., Ghandi N., Salmani H., Arabpour M., Nasrollahzadeh A., Teimourpour A., Daneshpazhooh M., Keramatipour M. The association between ST 18 gene polymorphism and severe pemphigus disease among Iranian population. Exp. Dermatol. 2018;27:1395–1398. doi: 10.1111/exd.13778. [DOI] [PubMed] [Google Scholar]
- 11.Yang J., Siqueira M.F., Behl Y., Alikhani M., Graves D.T. The transcription factor ST18 regulates proapoptotic and proinflammatory gene expression in fibroblasts. FASEB J. 2008;22:3956–3967. doi: 10.1096/fj.08-111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giordano C.N., Sinha A.A. Cytokine networks in Pemphigus vulgaris: an integrated viewpoint. Autoimmunity. 2012;45:427–439. doi: 10.3109/08916934.2012.697593. [DOI] [PubMed] [Google Scholar]
- 13.Sarig O., Bercovici S., Zoller L., Goldberg I., Indelman M., Nahum S., Israeli S., Sagiv N., Martinez De Morentin H., Katz O., Baum S., Barzilai A., Trau H., Murrell D.F., Bergman R., Hertl M., Rosenberg S., Nöthen M.M., Skorecki K., Schmidt E., Zillikens D., Darvasi A., Geiger D., Rosset S., Ibrahim S.M., Sprecher E. Population-specific association between a polymorphic variant in ST18, encoding a pro-apoptotic molecule, and pemphigus vulgaris. J. Investig. Dermatol. 2012;132:1798–1805. doi: 10.1038/jid.2012.46. [DOI] [PubMed] [Google Scholar]
- 14.Yue Z., Fu X., Chen M., Wang Z., Wang C., Yang B., Zhou G., Liu H., Zhang F. Lack of association between the single nucleotide polymorphism of ST18 and pemphigus in Chinese population. J. Dermatol. 2014;41:353–354. doi: 10.1111/1346-8138.12363. [DOI] [PubMed] [Google Scholar]
- 15.Vodo D., Sarig O., Geller S., Ben-Asher E., Olender T., Bochner R., Goldberg I., Nosgorodsky J., Alkelai A., Tatarskyy P., Peled A., Baum S., Barzilai A., Ibrahim S.M., Zillikens D., Lancet D., Sprecher E. Identification of a functional risk variant for pemphigus vulgaris in the ST18 gene. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radeva M.Y., Walter E., Stach R.A., Yazdi A.S., Schlegel N., Sarig O., Sprecher E., Waschke J. ST18 enhances PV-IgG-Induced loss of keratinocyte cohesion in parallel to increased ERK activation. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recke A., Konitzer S., Lemcke S., Freitag M., Sommer N.M., Abdelhady M., Amoli M.M., Benoit S., El-Chennawy F., Eldarouti M., Eming R., Gläser R., Günther C., Hadaschik E., Homey B., Lieb W., Peitsch W.K., Pföhler C., Robati R.M., Saeedi M., Sárdy M., Sticherling M., Uzun S., Worm M., Zillikens D., Ibrahim S., Vidarsson G., Schmidt E. The p.Arg435His variation of IgG3 with high affinity to FcRn is associated with susceptibility for pemphigus vulgaris—analysis of four different ethnic cohorts. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zone J., Ward J., Boyce E., Schupbach C. Penicillamine-induced pemphigus. J. Am. Med. Assoc. 1982;247:2705–2707. [PubMed] [Google Scholar]
- 19.Butt A., Burge S.M. Pemphigus vulgaris induced by captopril. Br. J. Dermatol. 1995;132:315–316. doi: 10.1111/j.1365-2133.1995.tb05038.x. [DOI] [PubMed] [Google Scholar]
- 20.Fellner M.J., Wininger J. Pemphigus erythematosus and heroin addiction. Int. J. Dermatol. 1978;17:308–311. doi: 10.1111/j.1365-4362.1978.tb06083.x. [DOI] [PubMed] [Google Scholar]
- 21.De Simone C., Caldarola G., D’Agostino M., Zampetti A., Amerio P., Feliciani C. Exacerbation of pemphigus after influenza vaccination. Clin. Exp. Dermatol. 2008;33:718–720. doi: 10.1111/j.1365-2230.2008.02835.x. [DOI] [PubMed] [Google Scholar]
- 22.Yalcin B., Alli N. Pemphigus vulgaris following antirabies vaccination. J. Dermatol. 2007;34:734–735. doi: 10.1111/j.1346-8138.2007.00373.x. [DOI] [PubMed] [Google Scholar]
- 23.Berkun Y., Mimouni D., Shoenfeld Y. Pemphigus following hepatitis B vaccination-coincidence or causality? Autoimmunity. 2005;38:117–119. doi: 10.1080/08916930400027078. [DOI] [PubMed] [Google Scholar]
- 24.Korang K., Ghohestain R., Krieg T., Uitto J., Hunzelmann N. Exacerbation of Pemphigus Foliaceus after Tetanus Vaccination Accompanied by Synthesis of Auto-Antibodies against Paraneoplastic Pemphigus Antigens. Acta Derm. Venereol. 2002;82:482–483. doi: 10.1080/000155502762064755. [DOI] [PubMed] [Google Scholar]
- 25.Lin L., Moran T.P., Peng B., Yang J., Culton D.A., Che H., Jiang S., Liu Z., Geng S., Zhang Y., Diaz L.A., Qian Y. Walnut antigens can trigger autoantibody development in patients with pemphigus vulgaris through a “hit-and-run” mechanism. J. Allergy Clin. Immunol. 2019 doi: 10.1016/j.jaci.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kridin K., Zelber-Sagi S., Comaneshter D., Cohen A.D. Association between schizophrenia and an autoimmune bullous skin disease-pemphigus: a population-based large-scale study. Epidemiol. Psychiatr. Sci. 2019;28:191–198. doi: 10.1017/S204579601700052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch. Dermatol. Res. 2018;310:95–106. doi: 10.1007/s00403-017-1790-8. [DOI] [PubMed] [Google Scholar]
- 28.Funakoshi T., Lunardon L., Ellebrecht C.T., Nagler A.R., O’Leary C.E., Payne A.S. Enrichment of total serum IgG4 in patients with pemphigus. Br. J. Dermatol. 2012;167:1245–1253. doi: 10.1111/j.1365-2133.2012.11144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Futei Y., Amagai M., Ishii K., Kuroda-Kinoshita K., Ohya K., Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J. Dermatol. Sci. 2001;26:55–61. doi: 10.1016/s0923-1811(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 30.Rock B., Labib R.S., Diaz L.A. Monovalent Fab’ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J. Clin. Investig. 1990;85:296–299. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naoe Y., Hata T., Tanigawa K., Kimura H., Masunaga T. Bidimensional analysis of desmoglein 1 distribution on the outermost corneocytes provides the structural and functional information of the stratum corneum. J. Dermatol. Sci. 2010;57:192–198. doi: 10.1016/j.jdermsci.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Koch P.J., Mahoney M.G., Ishikawa H., Pulkkinen L., Uitto J., Shultz L., Murphy G.F., Whitaker-Menezes D., Stanley J.R. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J. Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi H., Kouno M., Nagao K., Wada N., Hata T., Nishimoto S., Iwakura Y., Yoshimura A., Yamada T., Kuwana M., Fujii H., Koyasu S., Amagai M. Desmoglein 3-specific CD4+ T cells induce pemphigus vulgaris and interface dermatitis in mice. J. Clin. Investig. 2011;121:3677–3688. doi: 10.1172/JCI57379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anhalt G.J., Labib R.S., Voorhees J.J., Beals T.F., Diaz L.A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N. Engl. J. Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 35.Amagai M., Stanley J.R. Desmoglein as a target in skin disease and beyond. J. Investig. Dermatol. 2012;132:776–784. doi: 10.1038/jid.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato M., Aoyama Y., Kitajima Y. Assembly pathway of desmoglein 3 to desmosomes and its perturbation by pemphigus vulgaris-IgG in cultured keratinocytes, as revealed by time-lapsed labeling immunoelectron microscopy. Lab. Investig. 2000;80:1583–1592. doi: 10.1038/labinvest.3780168. [DOI] [PubMed] [Google Scholar]
- 37.Calkins C.C., Setzer S.V., Jennings J.M., Summers S., Tsunoda K., Amagai M., Kowalczyk A.P. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J. Biol. Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 38.Mao X., Choi E.J., Payne A.S. Disruption of desmosome assembly by monovalent human pemphigus vulgaris monoclonal antibodies. J. Investig. Dermatol. 2009;129:908–918. doi: 10.1038/jid.2008.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokol E., Kramer D., Diercks G., Kuipers J., Jonkman M.F., Pas H.H., Giepmans B. Large-scale electron microscopy maps of patient skin and mucosa provide insight into pathogenesis of blistering diseases. J. Investig. Dermatol. 2015;135:1763–1770. doi: 10.1038/jid.2015.109. [DOI] [PubMed] [Google Scholar]
- 40.Waschke J., Bruggeman P., Baumgartner W., Zillikens D., Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 trans interactions. J. Clin. Investig. 2005;115:3157–3165. doi: 10.1172/JCI23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkowitz P., Hu P., Liu Z., Diaz L.A., Enghild J.J., Chua M.P., Rubenstein D.S. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J. Biol. Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- 42.Chernyavsky A.I., Arredondo J., Kitajima Y., Sato-Nagai M., Grando S.A. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J. Biol. Chem. 2007;282:13804–13812. doi: 10.1074/jbc.M611365200. [DOI] [PubMed] [Google Scholar]
- 43.Berkowitz P., Chua M., Liu Z., Diaz L.A., Rubenstein D.S. Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen-activated protein kinase-dependent signaling in the skin. Am. J. Pathol. 2008;173:1628–1636. doi: 10.2353/ajpath.2008.080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jolly P.S., Berkowitz P., Bektas M., Lee H.E., Chua M., Diaz L.A., Rubenstein D.S. p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. J. Biol. Chem. 2010;285:8936–8941. doi: 10.1074/jbc.M109.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao X., Sano Y., Park J.M., Payne A.S. p38 MAPK activation is downstream of the loss of intercellular adhesion in pemphigus vulgaris. J. Biol. Chem. 2011;286:1283–1291. doi: 10.1074/jbc.M110.172874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldelari R., de Bruin A., Baumann D., Suter M.M., Bierkamp C., Balmer V., Muller E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J. Cell Biol. 2001;153:823–834. doi: 10.1083/jcb.153.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson L., Raess N.A., Caldelari R., Zakher A., de Bruin A., Posthaus H., Bolli R., Hunziker T., Suter M.M., Muller E.J. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 2006;25:3298–3309. doi: 10.1038/sj.emboj.7601224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albers L.N., Liu Y., Bo N., Swerlick R.A., Feldman R.J. Developing biomarkers for predicting clinical relapse in pemphigus patients treated with rituximab. J. Am. Acad. Dermatol. 2017;77:1074–1082. doi: 10.1016/j.jaad.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Hammers C.M., Chen J., Lin C., Kacir S., Siegel D.L., Payne A.S., Stanley J.R. Persistence of anti-desmoglein 3 IgG(+) B-cell clones in pemphigus patients over years. J. Investig. Dermatol. 2015;135:742–749. doi: 10.1038/jid.2014.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musette P., Bouaziz J.D. B cell modulation strategies in autoimmune diseases: new concepts. Front. Immunol. 2018;9:622. doi: 10.3389/fimmu.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed A.R., Kaveri S., Spigelman Z. Long-term remissions in recalcitrant pemphigus vulgaris. N. Engl. J. Med. 2015;373:2693–2694. doi: 10.1056/NEJMc1508234. [DOI] [PubMed] [Google Scholar]
- 52.Colliou N., Picard D., Caillot F., Calbo S., Le Corre S., Lim A., Lemercier B., Le Mauff B., Maho-Vaillant M., Jacquot S., Bedane C., Bernard P., Caux F., Prost C., Delaporte E., Doutre M.S., Dreno B., Franck N., Ingen-Housz-Oro S., Chosidow O., Pauwels C., Picard C., Roujeau J.C., Sigal M., Tancrede-Bohin E., Templier I., Eming R., Hertl M., D’Incan M., Joly P., Musette P. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci. Transl. Med. 2013;5:175ra30. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- 53.Zhu H.Q., Xu R.C., Chen Y.Y., Yuan H.J., Cao H., Zhao X.Q., Zheng J., Wang Y., Pan M. Impaired function of CD19(+) CD24(hi) CD38(hi) regulatory B cells in patients with pemphigus. Br. J. Dermatol. 2015;172:101–110. doi: 10.1111/bjd.13192. [DOI] [PubMed] [Google Scholar]
- 54.Kabuto M., Fujimoto N., Takahashi T., Tanaka T. Decreased level of interleukin-10-producing B cells in patients with pemphigus but not in patients with pemphigoid. Br. J. Dermatol. 2017;176:1204–1212. doi: 10.1111/bjd.15113. [DOI] [PubMed] [Google Scholar]
- 55.Yuan H., Zhou S., Liu Z., Cong W., Fei X., Zeng W., Zhu H., Xu R., Wang Y., Zheng J., Pan M. Pivotal role of lesional and perilesional T/B lymphocytes in pemphigus pathogenesis. J. Investig. Dermatol. 2017;137:2362–2370. doi: 10.1016/j.jid.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi H., Kouno M., Nagao K., Wada N., Hata T., Nishimoto S., Iwakura Y., Yoshimura A., Yamada T., Kuwana M., Fujii H., Koyasu S., Amagai M. Desmoglein 3-specific CD4+ T cells induce pemphigus vulgaris and interface dermatitis in mice. J. Clin. Investig. 2011;121:3677–3688. doi: 10.1172/JCI57379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertl M., Eming R., Veldman C. T cell control in autoimmune bullous skin disorders. J. Clin. Investig. 2006;116:1159–1166. doi: 10.1172/JCI28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hertl M., Amagai M., Sundaram H., Stanley J., Ishii K., Katz S.I. Recognition of desmoglein 3 by autoreactive T cells in pemphigus vulgaris patients and normals. J. Investig. Dermatol. 1998;110:62–66. doi: 10.1046/j.1523-1747.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- 59.Kitashima D.Y., Kobayashi T., Woodring T., Idouchi K., Doebel T., Voisin B., Adachi T., Ouchi T., Takahashi H., Nishifuji K., Kaplan D.H., Clausen B.E., Amagai M., Nagao K. Langerhans cells prevent autoimmunity via expansion of keratinocyte antigen-specific regulatory T cells. EBio Med. 2018;27:293–303. doi: 10.1016/j.ebiom.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yokoyama T., Matsuda S., Takae Y., Wada N., Nishikawa T., Amagai M., Koyasu S. Antigen-independent development of Foxp3+ regulatory T cells suppressing autoantibody production in experimental pemphigus vulgaris. Int. Immunol. 2011;23:365–373. doi: 10.1093/intimm/dxr020. [DOI] [PubMed] [Google Scholar]
- 61.Tsunoda K., Ota T., Suzuki H., Ohyama M., Nagai T., Nishikawa T., Amagai M., Koyasu S. Pathogenic autoantibody production requires loss of tolerance against desmoglein 3 in both T and B cells in experimental pemphigus vulgaris. Eur. J. Immunol. 2002;32:627–633. doi: 10.1002/1521-4141(200203)32:3<627::AID-IMMU627>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 62.Autoreactive B-Cell Elimination by Pathogenic IgG Specific for the Same Antigen: Implications for Peripheral Tolerance. [DOI] [PubMed]
- 63.Kridin K., Sagi S.Z., Bergman R. Mortality and cause of death in patients with pemphigus. Acta Derm. Venereol. 2017;97:607–611. doi: 10.2340/00015555-2611. [DOI] [PubMed] [Google Scholar]
- 64.Jennings J.M., Tucker D.K., Kottke M.D., Saito M., Delva E., Hanakawa Y., Amagai M., Kowalczyk A.P. Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can Be reversed by expression of exogenous Dsg3. J. Investig. Dermatol. 2011;131:706–718. doi: 10.1038/jid.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt E. Rituximab as first-line treatment of pemphigus. Lancet. 2017;389:1956–1958. doi: 10.1016/S0140-6736(17)30787-0. [DOI] [PubMed] [Google Scholar]
- 66.Almugairen N., Hospital V., Bedane C., Duvert-Lehembre S., Picard D., Tronquoy A.F., Houivet E., D’Incan M., Joly P. Assessment of the rate of long-term complete remission off therapy in patients with pemphigus treated with different regimens including medium- and high-dose corticosteroids. J. Am. Acad. Dermatol. 2013;69:583–588. doi: 10.1016/j.jaad.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Joly P., Maho-Vaillant M., Prost-Squarcioni C., Hebert V., Houivet E., Calbo S., Caillot F., Golinski M.L., Labeille B., Picard-Dahan C., Paul C., Richard M., Bouaziz J.D., Duvert-Lehembre S., Bernard P., Caux F., Alexandre M., Ingen-Housz-Oro S., Vabres P., Delaporte E., Quereux G., Dupuy A., Debarbieux S., Avenel-Audran M., D’Incan M., Bedane C., Bénéton N., Jullien D., Dupin N., Misery L., Machet L., Beylot-Barry M., Dereure O., Sassolas B., Vermeulin T., Benichou J., Musette P. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. The Lancet. 2017;389:2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 68.Liebman H.A., Saleh M.N., Bussel J.B., Negrea O.G., Horne H., Wegener W.A., Goldenberg D.M. Low-dose anti-CD20 veltuzumab given intravenously or subcutaneously is active in relapsed immune thrombocytopenia: a phase I study. Br. J. Haematol. 2013;162:693–701. doi: 10.1111/bjh.12448. [DOI] [PubMed] [Google Scholar]
- 69.Du F.H., Mills E.A., Mao-Draayer Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Autoimmunity Highlights. 2017;8 doi: 10.1007/s13317-017-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellebrecht C.T., Choi E.J., Allman D.M., Tsai D.E., Wegener W.A., Goldenberg D.M., Payne A.S. Subcutaneous veltuzumab, a humanized anti-CD20 antibody, in the treatment of refractory pemphigus vulgaris. JAMA Dermatol. 2014;150:1331–1335. doi: 10.1001/jamadermatol.2014.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izumi K., Bieber K., Ludwig R.J. Current clinical trials in pemphigus and pemphigoid. Front. Immunol. 2019;10:978. doi: 10.3389/fimmu.2019.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castillo J., Milani C., Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin. Investig. Drugs. 2009;18:491–500. doi: 10.1517/13543780902832679. [DOI] [PubMed] [Google Scholar]
- 73.Lin T.S. Ofatumumab: a novel monoclonal anti-CD20 antibody. Pharmgenomics Pers Med. 2010;3:51–59. doi: 10.2147/pgpm.s6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee A., Sandhu S., Imlay-Gillespie L., Mulligan S., Shumack S. Successful use of Bruton’s kinase inhibitor, ibrutinib, to control paraneoplastic pemphigus in a patient with paraneoplastic autoimmune multiorgan syndrome and chronic lymphocytic leukaemia. Australas. J. Dermatol. 2017;58:e240–e242. doi: 10.1111/ajd.12615. [DOI] [PubMed] [Google Scholar]
- 75.Smith P.F., Krishnarajah J., Nunn P.A., Hill R.J., Karr D., Tam D., Masjedizadeh M., Funk J.O., Gourlay S.G. A phase I trial of PRN1008, a novel reversible covalent inhibitor of Bruton’s tyrosine kinase, in healthy volunteers. Br. J. Clin. Pharmacol. 2017;83:2367–2376. doi: 10.1111/bcp.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bilgic T.A., Murrell D.F. Pharmacological advances in pemphigus. Curr. Opin. Pharmacol. 2019;46:44–49. doi: 10.1016/j.coph.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., Di Zenzo G., Lanzavecchia A., Seykora J.T., Cotsarelis G., Milone M.C., Payne A.S. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izumi K., Bieber K., Ludwig R.J. Current clinical trials in pemphigus and pemphigoid. Front. Immunol. 2019;10:978. doi: 10.3389/fimmu.2019.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baican A., Baican C., Chiriac G., Chiriac M.T., Macovei V., Zillikens D., Ciuce D., Sitaru C. Pemphigus vulgaris is the most common autoimmune bullous disease in Northwestern Romania. Int. J. Dermatol. 2010;49:768–774. doi: 10.1111/j.1365-4632.2009.04345.x. [DOI] [PubMed] [Google Scholar]
- 80.Langan S.M., Smeeth L., Hubbard R., Fleming K.M., Smith C.J., West J. Bullous pemphigoid and pemphigus vulgaris--incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang H., Shen S., Zheng X., Dang E., Zhang J., Shao S., Qiao P., Li Q., Li C., Wang H., Wang G., Sun L. Association of HLA class I and class II alleles with bullous pemphigoid in Chinese Hans. J. Dermatol. Sci. 2018;89:258–262. doi: 10.1016/j.jdermsci.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y., Liu H., Wang Z., Fu X., Wang C., Mi Z., Sun L., Bao F., Yu G., Zhou G., Zhang F. The HLA-DQB1*03:01 is associated with bullous pemphigoid in the han Chinese population. J. Investig. Dermatol. 2018;138:1874–1877. doi: 10.1016/j.jid.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 83.Chagury A.A., Sennes L.U., Gil J.M., Kalil J., Rodrigues H., Rosales C.B., Miziara I.D. HLA-C*17, DQB1*03:01, DQA1*01:03 and DQA1*05:05 alleles associated to bullous pemphigoid in Brazilian population. Ann. Dermatol. 2018;30:8–12. doi: 10.5021/ad.2018.30.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zakka L.R., Keskin D.B., Reche P., Ahmed A.R. Relationship between target antigens and major histocompatibility complex (MHC) class II genes in producing two pathogenic antibodies simultaneously. Clin. Exp. Immunol. 2010;162:224–236. doi: 10.1111/j.1365-2249.2010.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Setterfield J., Theron J., Vaughan R.W., Welsh K.I., Mallon E., Wojnarowska F., Challacombe S.J., Black M.M. Mucous membrane pemphigoid: HLA-DQB1*0301 is associated with all clinical sites of involvement and may be linked to antibasement membrane IgG production. Br. J. Dermatol. 2001;145:406–414. doi: 10.1046/j.1365-2133.2001.04380.x. [DOI] [PubMed] [Google Scholar]
- 86.Ujiie H., Muramatsu K., Mushiroda T., Ozeki T., Miyoshi H., Iwata H., Nakamura A., Nomoto H., Cho K.Y., Sato N., Nishimura M., Ito T., Izumi K., Nishie W., Shimizu H. HLA-DQB1*03:01 as a biomarker for genetic susceptibility to bullous pemphigoid induced by DPP-4 inhibitors. J. Investig. Dermatol. 2018;138:1201–1204. doi: 10.1016/j.jid.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 87.Takata Y., Higashiyama M., Tanaka A., Suzuki S., Miura H., Fujiwara S. Alogliptin-induced bullous pemphigoid associated with HLA-DQB1*03:01: a case report. Int. J. Dermatol. 2019;58:e132–e133. doi: 10.1111/ijd.14456. [DOI] [PubMed] [Google Scholar]
- 88.Büdinger L., Borradori L., Yee C., Eming R., Ferencik S., Grosse-Wilde H., Merk H.F., Yancey K., Hertl M. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J. Clin. Investig. 1998;102:2082–2089. doi: 10.1172/JCI3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esmaili N., Mortazavi H., Chams-Davatchi C., Daneshpazhooh M., Damavandi M.R., Aryanian Z., Amirzargar A.A. Association between HLA-DQB1*03:01 and Bullous pemphigoid in Iranian patients. Iran J Immunol. 2013;10:1–9. [PubMed] [Google Scholar]
- 90.Delgado J.C., Turbay D., Yunis E.J., Yunis J.J., Morton E.D., Bhol K., Norman R., Alper C.A., Good R.A., Ahmed R. A common major histocompatibility complex class II allele HLA-DQB1* 0301 is present in clinical variants of pemphigoid. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8569–8571. doi: 10.1073/pnas.93.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okazaki A., Miyagawa S., Yamashina Y., Kitamura W., Shirai T. Polymorphisms of HLA-DR and -DQ genes in Japanese patients with bullous pemphigoid. J. Dermatol. 2000;27:149–156. doi: 10.1111/j.1346-8138.2000.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 92.Ameglio F., D’Auria L., Bonifati C., Ferraro C., Mastroianni A., Giacalone B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br. J. Dermatol. 1998;138:611–614. doi: 10.1046/j.1365-2133.1998.02169.x. [DOI] [PubMed] [Google Scholar]
- 93.Inaoki M., Takehara K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J. Dermatol. Sci. 1998;16:152–157. doi: 10.1016/s0923-1811(97)00044-3. [DOI] [PubMed] [Google Scholar]
- 94.Rhodes L.E., Hashim I.A., McLaughlin P.J., Friedmann P.S. Blister fluid cytokines in cutaneous inflammatory bullous disorders. Acta Derm. Venereol. 1999;79:288–290. doi: 10.1080/000155599750010689. [DOI] [PubMed] [Google Scholar]
- 95.Tabatabaei-Panah P.S., Moravvej H., Sadaf Z., Babaei H., Geranmayeh M., Hajmanouchehri S., Karimi A., Sajjadi F., Arghand F., Ludwig R.J., Witte M., Akbarzadeh R. Proinflammatory cytokine gene polymorphisms in bullous pemphigoid. Front. Immunol. 2019;10:636. doi: 10.3389/fimmu.2019.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang Y.T., Liu H.N., Yu C.W., Lin M.W., Huang C.H., Chen C.C., Liu M.T., Lee D.D., Wang W.J., Tsai S.F. Cytokine gene polymorphisms in bullous pemphigoid in a Chinese population. Br. J. Dermatol. 2006;154:79–84. doi: 10.1111/j.1365-2133.2005.06938.x. [DOI] [PubMed] [Google Scholar]
- 97.Hirose M., Schilf P., Benoit S., Eming R., Gläser R., Homey B., Kunz M., Nebel A., Peitsch W.K., Pföhler C., Sárdy M., Schreiber S., Zillikens D., Schmidt E., Ibrahim S.M. Polymorphisms in the mitochondrially encoded ATP synthase 8 gene are associated with susceptibility to bullous pemphigoid in the German population. Exp. Dermatol. 2015;24:715–717. doi: 10.1111/exd.12732. [DOI] [PubMed] [Google Scholar]
- 98.Rychlik-Sych M., Baranska M., Wojtczak A., Skretkowicz J., Zebrowska A., Waszczykowska E. The impact of the CYP2D6 gene polymorphism on the risk of pemphigoid. Int. J. Dermatol. 2015;54:1396–1401. doi: 10.1111/ijd.12967. [DOI] [PubMed] [Google Scholar]
- 99.Heymann W.R. Bullae for you: the increasing importance and implications of drug-induced bullous pemphigoid. J. Am. Acad. Dermatol. 2018;79:1026–1027. doi: 10.1016/j.jaad.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 100.Stavropoulos P.G., Soura E., Antoniou C. Drug-induced pemphigoid: a review of the literature. J. Eur. Acad. Dermatol. Venereol. 2014;28:1133–1140. doi: 10.1111/jdv.12366. [DOI] [PubMed] [Google Scholar]
- 101.Patsatsi A., Vyzantiadis T., Chrysomallis F., Devliotou-Panagiotidou D., Sotiriadis D. Medication history of a series of patients with bullous pemphigoid from northern Greece - observations and discussion. Int. J. Dermatol. 2009;48:132–135. doi: 10.1111/j.1365-4632.2009.03839.x. [DOI] [PubMed] [Google Scholar]
- 102.Ruocco V., Sacerdoti G. Pemphigus and bullous pemphigoid due to drugs. Int. J. Dermatol. 1991;30:307–312. doi: 10.1111/j.1365-4362.1991.tb03867.x. [DOI] [PubMed] [Google Scholar]
- 103.Takama H., Yoshida M., Izumi K., Yanagishita T., Muto J., Ohshima Y., Nishie W., Shimizu H., Akiyama M., Watanabe D. Dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid: recurrence with epitope spreading. Acta Derm. Venereol. 2018;98:983–984. doi: 10.2340/00015555-3010. [DOI] [PubMed] [Google Scholar]
- 104.Béné J., Moulis G., Bennani I., Auffret M., Coupe P., Babai S., Hillaire-Buys D., Micallef J., Gautier S. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case-noncase study in the French Pharmacovigilance Database. Br. J. Dermatol. 2016;175:296–301. doi: 10.1111/bjd.14601. [DOI] [PubMed] [Google Scholar]
- 105.Attaway A., Mersfelder T.L., Vaishnav S., Baker J.K. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors. A case report and review of literature. J. Dermatol. Case Rep. 2014;8 doi: 10.3315/jdcr.2014.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Béné J., Jacobsoone A., Coupe P., Auffret M., Babai S., Hillaire-Buys D., Jean-Pastor M., Vonarx M., Vermersch A., Tronquoy A., Gautier S. Bullous pemphigoid induced by vildagliptin: a report of three cases. Fundam. Clin. Pharmacol. 2015;29:112–114. doi: 10.1111/fcp.12083. [DOI] [PubMed] [Google Scholar]
- 107.Mendonça F.M.I., Martín-Gutierrez F.J., Ríos-Martín J.J., Camacho-Martinez F. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors - one due to linagliptin. Dermatology. 2016;232:249–253. doi: 10.1159/000443330. [DOI] [PubMed] [Google Scholar]
- 108.Mai Y., Nishie W., Sato K., Hotta M., Izumi K., Ito K., Hosokawa K., Shimizu H. Bullous pemphigoid triggered by thermal burn under medication with a dipeptidyl peptidase-IV inhibitor: a case report and review of the literature. Front. Immunol. 2018;9:542. doi: 10.3389/fimmu.2018.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benzaquen M., Borradori L., Berbis P., Cazzaniga S., Valero R., Richard M.A., Feldmeyer L. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J. Am. Acad. Dermatol. 2018;78:1090–1096. doi: 10.1016/j.jaad.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 110.Fania L., Di Zenzo G., Didona B., Pilla M.A., Sobrino L., Panebianco A., Mazzanti C., Abeni D. Increased prevalence of diabetes mellitus in bullous pemphigoid patients during the last decade. J. Eur. Acad. Dermatol. Venereol. 2018;32:e153–e154. doi: 10.1111/jdv.14649. [DOI] [PubMed] [Google Scholar]
- 111.Aouidad I., Fite C., Marinho E., Deschamps L., Crickx B., Descamps V. A case report of bullous pemphigoid induced by dipeptidyl peptidase-4 inhibitors. JAMA Dermatol. 2013;149:243–245. doi: 10.1001/jamadermatol.2013.1073. [DOI] [PubMed] [Google Scholar]
- 112.Varpuluoma O., Försti A., Jokelainen J., Turpeinen M., Timonen M., Tasanen K., Huilaja L. Oral diabetes medications other than dipeptidyl peptidase 4 inhibitors are not associated with bullous pemphigoid: a Finnish nationwide case-control study. J. Am. Acad. Dermatol. 2018;79:1034–1038. doi: 10.1016/j.jaad.2018.05.030. e5. [DOI] [PubMed] [Google Scholar]
- 113.Lloyd-Lavery A., Chi C.C., Wojnarowska F., Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58–62. doi: 10.1001/2013.jamadermatol.376. [DOI] [PubMed] [Google Scholar]
- 114.Siegel J., Totonchy M., Damsky W., Berk-Krauss J., Castiglione F., Sznol M., Petrylak D.P., Fischbach N., Goldberg S.B., Decker R.H., Stamatouli A.M., Hafez N., Glusac E.J., Tomayko M.M., Leventhal J.S. Bullous disorders associated with anti–PD-1 and anti–PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J. Am. Acad. Dermatol. 2018;79:1081–1088. doi: 10.1016/j.jaad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 115.Sagi L., Baum S., Agmon-Levin N., Sherer Y., Katz B.S.P., Barzilai O., Ram M., Bizzaro N., SanMarco M., Trau H., Shoenfeld Y. Autoimmune bullous diseases. Autoimmun. Rev. 2011;10:527–535. doi: 10.1016/j.autrev.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 116.Jang H., Jin Y., Yoon C.H., Kim C., Kim L. Bullous pemphigoid associated with chronic hepatitis C virus infection in a hepatitis B virus endemic area. Medicine. 2018;97 doi: 10.1097/MD.0000000000010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baum H., Butler P., Davies H., Sternberg M.J.E., Burroughs A.K. Autoimmune disease and molecular mimicry: an hypothesis. Trends Biochem. Sci. 1993;18:140–144. doi: 10.1016/0968-0004(93)90022-f. [DOI] [PubMed] [Google Scholar]
- 118.Isohashi F., Konishi K., Umegaki N., Tanei T., Koizumi M., Yoshioka Y. A case of bullous pemphigoid exacerbated by irradiation after breast conservative radiotherapy. Jpn. J. Clin. Oncol. 2011;41:811–813. doi: 10.1093/jjco/hyr049. [DOI] [PubMed] [Google Scholar]
- 119.Olsha O., Lijoretzky G., Grenader T. Bullous pemphigoid following adjuvant radiotherapy for breast cancer. Breast J. 2011;17:204–205. doi: 10.1111/j.1524-4741.2010.01060.x. [DOI] [PubMed] [Google Scholar]
- 120.Kluger N., Mandelin J., Santti K., Jeskanen L., Nuutinen P. Bullous pemphigoid triggered by radiotherapy for breast cancer. Presse Med. 2017;46:128–130. doi: 10.1016/j.lpm.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 121.Shon W., Wada D.A., Kalaaji A.N. Radiation-induced pemphigus or pemphigoid disease in 3 patients with distinct underlying malignancies. Cutis. 2016;97:219–222. [PubMed] [Google Scholar]
- 122.Sacher C., Konig C., Scharffetter-Kochanek K., Krieg T., Hunzelmann N. Bullous pemphigoid in a patient treated with UVA-1 phototherapy for disseminated morphea. Dermatology. 2001;202:54–57. doi: 10.1159/000051588. [DOI] [PubMed] [Google Scholar]
- 123.Rakvit P., Kerr A.C., Ibbotson S.H. Localized bullous pemphigoid induced by photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2011;27:251–253. doi: 10.1111/j.1600-0781.2011.00609.x. [DOI] [PubMed] [Google Scholar]
- 124.Bağcı I.S., Horváth O.N., Ruzicka T., Sárdy M. Bullous pemphigoid. Autoimmun. Rev. 2017;16:445–455. doi: 10.1016/j.autrev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 125.Dănescu S., Chiorean R., Macovei V., Sitaru C., Baican A. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J. Dermatol. 2016;43:134–140. doi: 10.1111/1346-8138.13031. [DOI] [PubMed] [Google Scholar]
- 126.Papakonstantinou E., Limberg M.M., Gehring M., Kotnik N., Kapp A., Gibbs B.F., Raap U. Neurological disorders are associated with bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2019;33:925–929. doi: 10.1111/jdv.15444. [DOI] [PubMed] [Google Scholar]
- 127.Bastuji-Garin S., Joly P., Lemordant P., Sparsa A., Bedane C., Delaporte E., Roujeau J., Bernard P., Guillaume J., Ingen-Housz-Oro S., Maillard H., Pauwels C., Picard-Dahan C., Dutronc Y., Richard M. On behalf of the French study group for bullous diseases, risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J. Investig. Dermatol. 2011;131:637–643. doi: 10.1038/jid.2010.301. [DOI] [PubMed] [Google Scholar]
- 128.Brick K.E., Weaver C.H., Savica R., Lohse C.M., Pittelkow M.R., Boeve B.F., Gibson L.E., Camilleri M.J., Wieland C.N. A population-based study of the association between bullous pemphigoid and neurologic disorders. J. Am. Acad. Dermatol. 2014;71:1191–1197. doi: 10.1016/j.jaad.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meudec L., Amode R., Brunet Possenti F., Mignot S., Descamps V. Presence of anti-BP180 and anti-BP 230 in the CSF of patients with bullous pemphigoid and neurologic disease: is there any intrathecal synthesis? Br. J. Dermatol. 2019 doi: 10.1111/bjd.17882. [DOI] [PubMed] [Google Scholar]
- 130.Messingham K.N., Miller A.D., Narayanan N.S., Connell S.J., Fairley J.A. Demographics and autoantibody profiles of pemphigoid patients with underlying neurologic diseases. J. Investig. Dermatol. 2019;139:1860–1866. doi: 10.1016/j.jid.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daniel B.S., Murrell D.F. Review of autoimmune blistering diseases: the pemphigoid diseases. J. Eur. Acad. Dermatol. Venereol. 2019;33:1685–1694. doi: 10.1111/jdv.15679. [DOI] [PubMed] [Google Scholar]
- 132.Zillikens D., Rose P.A., Balding S.D., Liu Z., Olague-Marchan M., Diaz L.A., Giudice G.J. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J. Investig. Dermatol. 1997;109:573–579. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 133.Daneshpazhooh M., Ghiasi M., Lajevardi V., Nasiri N., Balighi K., Teimourpour A., Khosravi H., Saeidi V., Mahmoudi H., Chams-Davatchi C. BPDAI and ABSIS correlate with serum anti-BP180 NC16A IgG but not with anti-BP230 IgG in patients with bullous pemphigoid. Arch. Dermatol. Res. 2018;310:255–259. doi: 10.1007/s00403-018-1817-9. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y., Li L., Xia Y. BP180 is critical in the autoimmunity of bullous pemphigoid. Front. Immunol. 2017;8:1752. doi: 10.3389/fimmu.2017.01752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zillikens D., Rose P.A., Balding S.D., Liu Z., Olague-Marchan M., Diaz L.A., Giudice G.J. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J. Investig. Dermatol. 1997;109:573–579. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 136.Zheng M., Ujiie H., Iwata H., Muramatsu K., Yoshimoto N., Ito T., Ujiie I., Shimizu S., Sato Matsumura K.C., Shimizu H. Characteristics of IgG subclasses and complement deposition in BP230-type bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2018;33:595–600. doi: 10.1111/jdv.15325. [DOI] [PubMed] [Google Scholar]
- 137.Romeijn T.R., Jonkman M.F., Knoppers C., Pas H.H., Diercks G.F.H. Complement in bullous pemphigoid: results from a large observational study. Br. J. Dermatol. 2017;176:517–519. doi: 10.1111/bjd.14822. [DOI] [PubMed] [Google Scholar]
- 138.Kobayashi M., Amagai M., Kuroda-Kinoshita K., Hashimoto T., Hashimoto K., Shirakata Y., Nishikawa T. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J. Dermatol. Sci. 2002;30:224–232. doi: 10.1016/s0923-1811(02)00109-3. [DOI] [PubMed] [Google Scholar]
- 139.Hammers C.M., Stanley J.R. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu. Rev. Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gutjahr A., Heck F., Emtenani S., Hammers A.K., Hundt J.E., Muck P., Siegel D.L., Schmidt E., Stanley J.R., Zillikens D., Hammers C.M. Bullous pemphigoid autoantibody-mediated complement fixation is abolished by the low-molecular-weight heparin tinzaparin sodium. Br. J. Dermatol. 2019;181:593–594. doi: 10.1111/bjd.18156. [DOI] [PubMed] [Google Scholar]
- 141.Jefferis R., Pound J., Lund J., Goodall M. Effector mechanisms activated by human IgG subclass antibodies: clinical and molecular aspects. Review article. Ann. Biol. Clin. 1994;52:57–65. [PubMed] [Google Scholar]
- 142.Sitaru C., Mihai S., Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch. Dermatol. Res. 2007;299:1–8. doi: 10.1007/s00403-007-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dainichi T., Nishie W., Yamagami Y., Sonobe H., Ujiie H., Kaku Y., Kabashima K. Bullous pemphigoid suggestive of complement-independent blister formation with anti-BP180 IgG4 autoantibodies. Br. J. Dermatol. 2016;175:187–190. doi: 10.1111/bjd.14411. [DOI] [PubMed] [Google Scholar]
- 144.Natsuga K., Nishie W., Shinkuma S., Ujiie H., Nishimura M., Sawamura D., Shimizu H. Antibodies to pathogenic epitopes on type XVII collagen cause skin fragility in a complement-dependent and -independent manner. J. Immunol. 2012;188:5792–5799. doi: 10.4049/jimmunol.1003402. [DOI] [PubMed] [Google Scholar]
- 145.Ujiie H., Sasaoka T., Izumi K., Nishie W., Shinkuma S., Natsuga K., Nakamura H., Shibaki A., Shimizu H. Bullous pemphigoid autoantibodies directly induce blister formation without complement activation. J. Immunol. 2014;193:4415–4428. doi: 10.4049/jimmunol.1400095. [DOI] [PubMed] [Google Scholar]
- 146.van Beek N., Lüttmann N., Huebner F., Recke A., Karl I., Schulze F.S., Zillikens D., Schmidt E. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. 2017;153:30. doi: 10.1001/jamadermatol.2016.3357. [DOI] [PubMed] [Google Scholar]
- 147.Nieboer C. Serum IgE levels in patients with bullous pemphigoid. Acta Derm. Venereol. 1985;65:273–274. [PubMed] [Google Scholar]
- 148.Arbesman C.E., Wypych J.I., Reisman R.E., Beutner E.H. IgE levels in sera of patients with pemphigus or bullous pemphigoid. Arch. Dermatol. 1974;110:378–381. [PubMed] [Google Scholar]
- 149.Saniklidou A.H., Tighe P.J., Fairclough L.C., Todd I. IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: a systematic review. Arch. Dermatol. Res. 2018;310:11–28. doi: 10.1007/s00403-017-1789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Asbrink E., Hovmark A. Serum IgE levels in patients with bullous pemphigoid and its correlation to the activity of the disease and anti-basement membrane zone antibodies. Acta Derm. Venereol. 1984;64:243–246. [PubMed] [Google Scholar]
- 151.Bushkell L.L., Jordon R.E. Bullous pemphigoid: a cause of peripheral blood eosinophilia. J. Am. Acad. Dermatol. 1983;8:648. doi: 10.1016/s0190-9622(83)70073-3. [DOI] [PubMed] [Google Scholar]
- 152.Bernard P., Venot J., Constant F., Bonnetblanc J.M. Blood eosinophilia as a severity marker for bullous pemphigoid. J. Am. Acad. Dermatol. 1987;16:879–881. doi: 10.1016/s0190-9622(87)80227-x. [DOI] [PubMed] [Google Scholar]
- 153.van Beek N., Schulze F.S., Zillikens D., Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev. Clin. Immunol. 2016;12:267–277. doi: 10.1586/1744666X.2016.1123092. [DOI] [PubMed] [Google Scholar]
- 154.Lin L., Hwang B.J., Culton D.A., Li N., Burette S., Koller B.H., Messingham K.A., Fairley J.A., Lee J.J., Hall R.P., An L., Diaz L.A., Liu Z. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J. Investig. Dermatol. 2018;138:1032–1043. doi: 10.1016/j.jid.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zone J.J., Taylor T., Hull C., Schmidt L., Meyer L. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J. Investig. Dermatol. 2007;127:1167–1174. doi: 10.1038/sj.jid.5700681. [DOI] [PubMed] [Google Scholar]
- 156.Fairley J.A., Burnett C.T., Fu C., Larson D.L., Fleming M.G., Giudice G.J. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J. Investig. Dermatol. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- 157.Hofmann S., Thoma-Uszynski S., Hunziker T., Bernard P., Koebnick C., Stauber A., Schuler G., Borradori L., Hertl M. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J. Investig. Dermatol. 2002;119:1065–1073. doi: 10.1046/j.1523-1747.2002.19529.x. [DOI] [PubMed] [Google Scholar]
- 158.Fairley J.A., Fu C.L., Giudice G.J. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J. Investig. Dermatol. 2005;125:467–472. doi: 10.1111/j.0022-202X.2005.23853.x. [DOI] [PubMed] [Google Scholar]
- 159.Dimson O.G., Giudice G.J., Fu C.L., Van den Bergh F., Warren S.J., Janson M.M., Fairley J.A. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J. Investig. Dermatol. 2003;120:784–788. doi: 10.1046/j.1523-1747.2003.12146.x. [DOI] [PubMed] [Google Scholar]
- 160.Dimson O.G., Giudice G.J., Fu C.L., Van den Bergh F., Warren S.J., Janson M.M., Fairley J.A. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J. Investig. Dermatol. 2003;120:784–788. doi: 10.1046/j.1523-1747.2003.12146.x. [DOI] [PubMed] [Google Scholar]
- 161.Messingham K.N., Holahan H.M., Frydman A.S., Fullenkamp C., Srikantha R., Fairley J.A. Human eosinophils express the high affinity IgE receptor, FcepsilonRI, in bullous pemphigoid. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ujiie H. IgE autoantibodies in bullous pemphigoid. Br. J. Dermatol. 2017;177:1481–1482. doi: 10.1111/bjd.16034. [DOI] [PubMed] [Google Scholar]
- 163.Vico Alonso C., Calleja Algarra A., Aragón Miguel R., Sánchez Velázquez A., Velasco Tamariz V., Ortiz Romero P.L., Monsálvez Honrubia V. Omalizumab as an alternative therapeutic tool in the treatment of bullous pemphigoid: a case report. Dermatol. Ther. 2019;32 doi: 10.1111/dth.12829. [DOI] [PubMed] [Google Scholar]
- 164.Balakirski G., Alkhateeb A., Merk H.F., Leverkus M., Megahed M. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: report of two cases and review of literature. J. Eur. Acad. Dermatol. Venereol. 2016;30:1778–1782. doi: 10.1111/jdv.13758. [DOI] [PubMed] [Google Scholar]
- 165.Fairley J.A., Baum C.L., Brandt D.S., Messingham K.A. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J. Allergy Clin. Immunol. 2009;123:704–705. doi: 10.1016/j.jaci.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.James T., Salman S., Stevenson B., Bundell C., Kelly G., Nolan D., John M. IgE blockade in autoimmunity: omalizumab induced remission of bullous pemphigoid. Clin. Immunol. 2019;198:54–56. doi: 10.1016/j.clim.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 167.Hall R.P., Streilein R.D., Hannah D.L., McNair P.D., Fairley J.A., Ronaghy A., Edhegard K.D., Levesque M.C. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J. Investig. Dermatol. 2013;133:2786–2788. doi: 10.1038/jid.2013.236. [DOI] [PubMed] [Google Scholar]
- 168.Polansky M., Eisenstadt R., DeGrazia T., Zhao X., Liu Y., Feldman R. Rituximab therapy in patients with bullous pemphigoid: a retrospective study of 20 patients. J. Am. Acad. Dermatol. 2019;81:179–186. doi: 10.1016/j.jaad.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 169.Plée J., Le Jan S., Giustiniani J., Barbe C., Joly P., Bedane C., Vabres P., Truchetet F., Aubin F., Antonicelli F., Bernard P. Integrating longitudinal serum IL-17 and IL-23 follow-up, along with autoantibodies variation, contributes to predict bullous pemphigoid outcome. Sci. Rep. 2016;5 doi: 10.1038/srep18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kowalski E.H., Kneibner D., Kridin K., Amber K.T. Serum and blister fluid levels of cytokines and chemokines in pemphigus and bullous pemphigoid. Autoimmun. Rev. 2019;18:526–534. doi: 10.1016/j.autrev.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 171.Zebrowska A., Wozniacka A., Juczynska K., Ociepa K., Waszczykowska E., Szymczak I., Pawliczak R. Correlation between IL36alpha and IL17 and activity of the disease in selected autoimmune blistering diseases. Mediat. Inflamm. 2017;2017:8980534. doi: 10.1155/2017/8980534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chakievska L., Holtsche M.M., Künstner A., Goletz S., Petersen B., Thaci D., Ibrahim S.M., Ludwig R.J., Franke A., Sadik C.D., Zillikens D., Hölscher C., Busch H., Schmidt E. IL-17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J. Autoimmun. 2019;96:104–112. doi: 10.1016/j.jaut.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 173.Izumi K., Bieber K., Ludwig R.J. Current clinical trials in pemphigus and pemphigoid. Front. Immunol. 2019;10:978. doi: 10.3389/fimmu.2019.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fang H., Shao S., Cao T., Lei J., Dang E., Zhang J., Wang G. Increased expression of NLRP3 inflammasome components and interleukin-18 in patients with bullous pemphigoid. J. Dermatol. Sci. 2016;83:116–123. doi: 10.1016/j.jdermsci.2016.04.009. [DOI] [PubMed] [Google Scholar]