Abstract
Cathelicidin LL-37 is an antimicrobial peptide that is synthesized by epithelial cells, neutrophils, or lymphocytes and act as an essential defense mechanism against bacterial, viral, or fungi infection of eukaryotic organisms. However, in recent years, this cathelicidin has gained the interest of the scientific community because, besides its antimicrobial properties, LL-37 is an immunomodulator that can contribute to the development of autoimmune diseases. The other non-antimicrobial function of this cathelicidin is its ability to form complexes with the DNA, stimulating plasmacytoid dendritic cells (pDCs) to produce type I IFN, deciding the course of autoimmune diseases, including systemic lupus erythematosus (SLE). The chronic activation of pDCs by surrounding complexes is a crucial factor for the early development of autoimmunity in SLE patients. This stimulation is given by the complexes (LL-37-DNA/anti-DNA) recognized by the receptor FcγRII on pDCs, allowing its endocytosis and its recognition via TLR9, leading to the activation of pDCs and enhanced type I IFN production. In this article, we reviewed the structure, function, and importance of LL-37 in innate immunity, as well as its biological plausibility in the pathophysiology of autoimmune diseases such as SLE. In this narrative review, we included primary journal articles describing the function, structure, prevalence, and importance of LL-37 in various manifestations of SLE, as well as LL-37 and anti-LL37 antibodies in patients with SLE or other autoimmune diseases. In conclusion, LL-37 is an essential molecule in the pathophysiology of SLE, mainly by its role in increasing the production of IFN by pDCs, which postulates it as a crucial molecule in the pathophysiology of SLE and, given plausibility biology, could serve as a biomarker of the disease.
Keywords: LL-37, Cathelicidin, Antibodies, Antigen, NETosis, Lupus
Highlights
-
•
LL-37 is an essential molecule in inflammatory processes.
-
•
The complexes LL-37/DNA stimulates the production of type I interferon in plasmacytoid dendritic cells.
-
•
Based on its pathophysiology, LL-37 is a useful biomarker of SLE activity.
1. Introduction
The innate immune response provides a defense against a variety of pathogens and is often the initiator of a cascade of inflammatory responses. Antimicrobial peptides (AMP) act as an important defense mechanism against bacterial, viral or fungi infection of eukaryotic organisms. These innate immune molecules are synthesized by epithelial cells or lymphocytes [1]. Though there several AMPs, the Cathelicidin LL-37 (henceforth referred to as LL-37) peptide is the only member of the family of human cathelicidins. In recent years, this peptide has gained the interest of the scientific community because, besides its antimicrobial properties, LL-37 is an immunomodulator that can contribute to the development of autoimmune diseases. This review will focus on the structure, function, and importance of the LL-37 in innate immunity, as well as the biologic plausibility of its role in the pathophysiology of certain autoimmune diseases mainly systemic lupus erythematosus (SLE).
2. Structure of LL-37
LL-37 is a cationic protein of 37 amino acids encoded as an inactive predecessor by cAMP on chromosome 3. This gene encodes the human cationic antimicrobial peptide 18 (hCAP 18), which has an atomic weight of 18 kDa [1,2]. Under physiologic conditions, LL-37 assumes a secondary alpha helix structure and acquires amphipathic properties that allow its interaction with bacterial membranes or other anionic components [3,4]. The hydrophobic portion is mainly composed of positively charged residues that interact with negatively charged molecules such as lipopolysaccharide (LPS), genetic material, and bacterial cell wall [5]. Its cationic amphipathic alpha helix structure has three domains—an N-terminal alpha helix adjacent to a C terminal alpha helix and a C-terminal tail—each with a unique function [[5], [6], [7]]. The N-terminal alpha helix is involved in chemotaxis of innate immune cells, formation of peptide oligomers, proteolytic protection of the cell, and has hemolytic activity in humans. The C terminal alpha helix consists of the antimicrobial peptide core and, therefore, is responsible of antimicrobial, antineoplastic and antiviral activity of LL-37. The C-terminal tail is essential for the formation of peptide tetramers, interacting primarily with negatively charged molecules, such as anionic phosphatidylglycerols, LPS of gram-negative bacteria, and teichoic acid of gram-positive bacteria. This domain provides target specificity against bacterial anionic membranes, while protecting eukaryotic cationic membranes, as the latter are composed of cholesterol and phospholipids [3,8].
2.1. Induction and synthesis of LL-37
LL-37 was initially thought to be a peptide only present constitutively in the secondary granules of neutrophils [2]. This molecule is now known to be synthesized in multiple cells, such as Natural Killer lymphocytes (NK), macrophages, and epithelial cells of the intestine, airway, genitalia, eye surface, skin, and some endocrine glandules, among others [5,9]. The constitutive expression of LL-37 in multiple epithelial cells confers on it a crucial role in the defense against pathogen-induced diseases. It is known that LL-37 concentration rises in response to wounds, UV radiation, direct damage to the epithelial barrier, certain components of the bacterial cell wall, and endoplasmic reticulum stress, among many others [6,10]. LL-37 is stored as a precursor molecule in granules within neutrophils, NK cells, and mastocytes, from where it released in response to Toll-like receptor (TLR) or cytokine signaling in response to infections or tissue damage [6]. First, the inactive precursor hCAP 18 is released to the extracellular space, where it is cleaved in its C-terminal domain by serine proteases of the kallikrein family in keratinocytes [2,9] and by proteinase 3 in neutrophils [11]. The neutrophils, by virtue of the high concentrations of LL-37 they release at sites of inflammation, play an important role as they amplify the immune response to the point of eradicating the infection [10].
Among the signaling pathways responsible for LL-37 production, two play an important role—vitamin D-induced LL-37 expression naturally under non-inflammatory conditions, and nuclear factor K–B (NF-KB)-induced expression that is activated under during inflammation and endoplasmic reticulum stress [[12], [13], [14]]. The former pathway is inhibited by the NF-KB, which plays an important role in the regulation of CAMP. This stimulates not only protein expression, but also its secretion from cells and its activation through proteolytic processes [13]. Ultimately, the objective of LL-37 is to stimulate the repair of epithelial functions after an immune assault.
2.2. LL-37 and its functions in innate immunity
In recent years, LL-37 has been shown to be immensely important due to its multi-functional role in the primary defense against invasion by bacteria, fungi, and viruses. As an antimicrobial agent, high concentrations of LL-37 at inflammatory sites confer protection against both gram-positive and gram-negative bacteria [15,16]. LL-37 also functions as a chemokine [17], by modulating or stimulating immune cells [18]. As well, LL-37 participates in wound healing, reepithelization, and scarring mediated by transactivation of the epidermal growth factor receptor, inducing the migration of keratinocytes [19].
Perhaps its most important role is as an antimicrobial peptide; LL-37 has the ability to form pores in bacterial cell wall [5,6]. Upon reaching the external layer of the bacterial cell wall, LL-37 forms complexes with diverse molecules attaining a concentration threshold that permits translocation of monomers to the periplasmic space and to the internal surface of the internal layer. This allows the formation of pores and leak of cytoplasmic material to the extracellular space [5]. Besides this, LL-37 can perturb the integrity of the bacterial cell wall through interactions with electron transport proteins, thereby altering membrane homeostasis.
Another interesting feature of LL-37 is its ability to deliver double-stranded ribonucleic acid (dsRNA) of viruses and bacterial plasmids to endosomal TLRs that are located in the proximity of the nucleus. In the absence of LL-37, plasmids are degraded by nucleases, but in its presence it protects the DNA forming a complex LL-37-Plasmid DNA. This complex allows and increase the internalization capacity of the plasmid DNA into the mammal cells. Once inside the cell, the plasmid DNA stimulates TLR-7 and TLR-9 to produce type I IFN. In the matter of viral infection, the inflammation and damaged of tissues causes the release of dsRNA from viruses and necrotic cells. In vitro, using human bronchial epithelial and human embryonic kidney cells transiently transfected with TLR3, it was found that dsRNA forms a complex with LL-37 to activate TLR-3. The TLR3 agonist polyinosinic:polycytidylic acid (poly(I:C)) enhances this activation, causing the translocation of the transcription factor NF-kB and interferon regulatory factor 3 (IRF3) to the nucleus increasing the production of type I IFN [[20], [21], [22]].
By lowering the permeability of epithelial cells, LL-37 confers cell wall the robustness required to protect cells against bacterial infection, proteins of the viral capsid, and against fusion of viral envelope membrane [23]. LL-37 also exhibits antimycotic properties by interacting with the membrane of certain fungi and directly inducing reactive oxygen species (ROS), aiding in the separation and rapid disintegration of the membrane in vesicles [24]. Table 1 summarizes the functions of LL-37 in the innate immunity.
Table 1.
LL-37 functions in innate immunity.
| Antimicrobial agent | Protection against gram-positive and negative bacteria. | [15,16] |
| Protection to infection | LL-37 lowers the permeability of epithelial cells inhibiting the entry of microorganisms to them. | [5] |
| Chemokine function | LL-37 modulates and stimulates the function of monocytes, lymphocytes, neutrophils and mastocytes. | [54] |
| Wound healing | Reepithelization and scarring by transactivation of epidermal growth factor receptor, inducing keratinocytes migration. | [19] |
| Improves coestimulation | High concentrations of LL-37 induce a higher expression of membrane CD86. | [52] |
| Enhance type I IFN response | LL-37 delivers dsRNA to TLR3 with their agonist poly(I:C) which produces the transcription of NF-kB and IRF3 and enhances the type I IFN response. | [22] |
| Prevention of sepsis | LL-37 binds to LPS to prevent the activation of TLR4. | [31] |
| Delivers plasmids to the nuclei | It prevents the degradation of plasmids from nucleases and form complexes with the DNA to deliver them to the nuclei by lipid rafts to stimulate TLR7 and TLR9. | [55] |
| Enhances the function of dendritic cells | Dendritic cells derived from stimuli of LL-37 show a better capacity of endocytic capacity, high concentrations of phagocytic receptors, high concentrations of coestimulation molecules and high levels of type I IFN secretion which stimulates a better Th1 response against viruses and bacteria. | [53,56] |
Abbreviations: Double-stranded ribonucleic acid (dsRNA), Toll-like receptor (TLR), lipopolysaccharide (LPS), nuclear factor K–B (NF-kB), Polyinosinic: polycytidylic acid (poly(I:C)), interferon regulatory factor 3 (IRF3).
2.3. Inflammatory and anti-inflammatory properties of LL-37
Besides its antimicrobial functions, LL-37 acts as an immunomodulator by exerting both pro- and anti-inflammatory effects depending on the microenvironment where it is secreted [10]. Several in vitro studies highlight these characteristics. During differentiation of monocytes into macrophages, the presence of 10 μg/mL of LL-37 creates a pro-inflammatory mileu, resulting in downregulation of IL-10 and increase in IL-12p40 that favors M1 macrophages population over the M2 type [25]. These findings show the strong influence of LL-37 in the development and production of pro-inflammatory cytokines in macrophages. Additionally, the LL-37 also plays chemotactic roles among different types of immune cells. LL-37 can modulate the formyl-peptide receptor (FPR 2 receptor) to induce migration of eosinophils and neutrophils [17]. In experimental models, LL-37 is capable of inducing the transcription of the chemokine, CXCL8 [26] and the secretion of MCP-1/CCL-2, a recruitment factor of monocytes, regardless of the dosage [27]. In the same way, some studies showed that LL37 enables keratinocytes and macrophages to recognize self-non-coding U1 RNA l by facilitating binding to cell surface scavenger receptors allowing the production of inflammatory cytokines such as IL-6 [28].Furthermore, the cytokine TGF-β released from intestinal epithelial cells after being exposed to LL-37, exerts important chemotactic properties by inducing the migration of epithelial cells that aid in wound healing [29]. Therefore, LL-37 released at the site of infections or injuries is capable of promoting an inflammatory response and initiating wound repair.
The anti-inflammatory properties of LL-37 were identified through its antagonistic actions against IFN-γ, TNF-α, IL-4, and IL-12 responses in several types of cells [30,31]. LL-37 downregulates LPS-mediated TLR-4 signaling [32], and interrupts the function of TLR-4 receptor in dendritic cells and macrophages [33,34] in the presence of LPS, resulting in lower levels of pro-inflammatory cytokine production.
3. LL-37 and its role in SLE pathogenesis
SLE is an autoimmune disease characterized by the loss of tolerance to nuclear antigens, the deposition of immune complexes on tissues, and multi-organic compromise [35]. Commonly, the cells implied in SLE pathogenesis are primarily from the adaptive immune system, with B-lymphocytes being the most relevant. Nevertheless, its pathophysiology is not totally understood. However, in recent years, the key participation of other cells in SLE development has been demonstrated. Particularly, the chronic activation of pDCs by surrounding immune complexes is a key factor for the early development of autoimmunity in SLE patients [36]. The activation of pDCs leads to production of type I IFNs, which is a hallmark feature of most SLE patients, which is corroborated by the finding that genomic studies have identified 95% of children and 70% of adults with SLE have a high IFN-inducible gene expression profile [37,38]. The mechanisms leading to chronic activation of pDCs have also been elucidated and in the recent years, the role of neutrophils has taken center stage in SLE pathogenesis.
Neutrophils are one of the main cells in circulation and are usually the first to arrive at sites of inflammation. Once there, activated neutrophils are capable of phagocytosing or releasing AMP, proteolytic enzymes, and ROS; however, in addition, another antimicrobial effector mechanism has been identified. Termed, NETosis, it consists of a process of condensation and release of the intracellular content of neutrophils to create an extracellular net (NETs) that allows the destruction of the microorganisms, but which also leads to death of neutrophils [39,40]. It is plausible that in SLE patients, the alteration in apoptotic bodies clearance, via the NETs, by DNAses inhibitors or by anti-NET autoantibodies will lead to an affected function of NET [38,[40], [41], [42]].
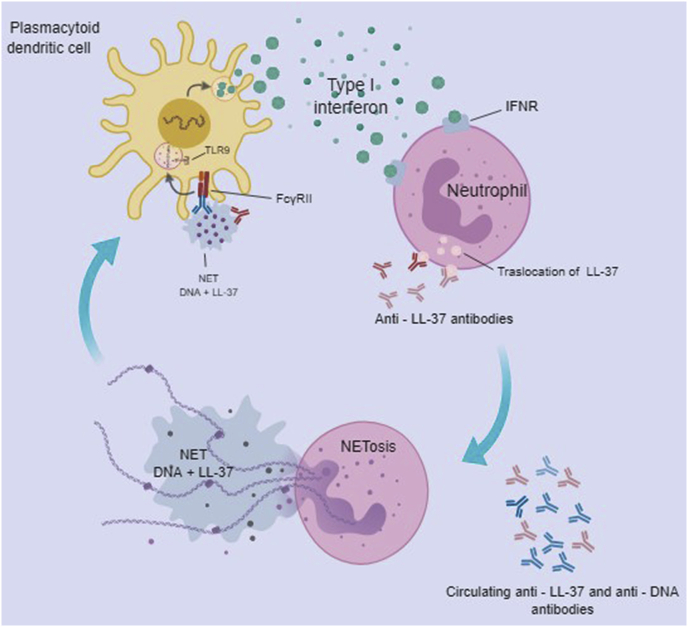
It was demonstrated that SLE patients have higher serum levels of LL-37 than in healthy individuals [41], and this molecule along with human neutrophils peptides (HNP), were essential for the immunogenicity of immune complexes containing DNA, that are resistant to DNAse-mediated degradation. It was also demonstrated that anti-LL-37 and anti-HNP antibodies were present in these patients. The complexes (LL-37-DNA/anti-DNA) are recognized by the receptor FcγRII on pDC, allowing its endocytosis and its recognition via TLR9, leading to the activation of pDC and enhanced type I IFN production [41]. Indeed, LL-37 has been demonstrated as a key molecule in the activation of pDC in psoriasis patients [43]. Since the immune complexes activated neutrophils to generate NETs, it was concluded that peptide-antimicrobial/DNA complexes were essential for formation of NETs. Others have demonstrated the role of AMPs in NETs, activation of pDC and the production of type I IFN [44,45]. The positive correlation between anti-LL-37 and anti-HNP antibodies with the anti-DNA antibodies in SLE patients suggests that neutrophil-derived AMPs along with DNA may act as B cell autoantigens [38] (See Fig. 1).
Fig. 1.
Activation of Plasmacytoid Dendritic Cells by cathelicidin LL-37. Anti-LL37 antibodies and circulating anti-DNA antibodies activate neutrophils to form NETs, leading to the release of DNA + LL-37 immune complexes which are recognized by the FcγRII receptor on pDCs, via TLR 9, enhancing the activation and production of type I IFN in pDCs. The IFN, thus released, is recognized by IFN receptor (IFNR) on the neutrophil, leading to cellular activation to form the NETs, allowing the translocation of LL-37 to the cell membrane, where it is recognized by the anti-LL37 antibody.
Given that type I IFN signature is characteristic of SLE patients and reflects disease perpetuation and severity, it seems biologically plausible to use LL-37 as a biomarker of lupus activity. Although, the in vitro role of LL-37 in SLE pathogenesis has been demonstrated; in vivo LL-37 serum measures by EIA are not conclusive. Some studies show a lack of association between LL-37 and disease activity or organic compromise [46,47], without this association being altered by other variables such as age, sex or duration of the disease; though higher levels of LL-37 have been associated with presence of chronic cutaneous lupus erythematosus [48,49]. However, the inconsistency in findings warrants additional studies to investigate whether LL-37, which may have a biological role in SLE pathogenesis, could serve as a marker of lupus activity.
4. LL-37 in other autoimmune/inflammatory diseases
LL-37 has caught the attention of researchers in the field of autoimmune/inflammatory diseases because immune complexes participate in the pathophysiology and disease perpetuation. An example of this is psoriasis, an inflammatory disease where the impact of LL-37 in disease perpetuation has been extensively studied [50]. Psoriasis is characterized by skin inflammation, keratinocyte proliferation, enhanced type I IFN signature, and increased LL-37 levels [43]. In other way, the LL-37/DNA complex can promote the loss of tolerance and stimulate TLR9 on pDCs, leading to an increase in type I IFN and the activation of additional inflammatory pathways to perpetuate the disease [43]. Moreover, whether the increase in LL-37 in rheumatoid arthritis (RA) patients [51] was a component of the disease pathogenesis was investigated, and it was demonstrated that LL-37 can induce osteoblast apoptosis, and participate in periarticular osteopenia, seen in RA patients [52]. Furthermore, LL-37 is known to undergo citrullination, which is associated with RA pathogenesis. Citrullination potentiates LL-37’s chemotaxis capacity, thereby contributing to RA pathophysiology [53]. The findings in various studies of LL-37 strongly indicate that LL-37 participates as an important mediator perpetuating inflammation in RA patients. Nevertheless, additional studies are needed to corroborate this association.
5. Conclusion
In recent years, LL-37 has caught the attention of the scientific community because of its demonstrated overexpression and its capacity to form DNA-immune complexes, leading to amplification of type I IFN signaling in autoimmune pathologies such as SLE. Given its biological plausibility, it is possible to postulate LL-37 as a useful biomarker of disease activity; however, more studies are needed to support this premise.
Funding
No funding received.
Declaration of competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
Not apply for this article.
References
- 1.Wang Z., Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32:590D–592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larrick J.W., Hirata M., Balint R.F. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucki R., Janmey P.A. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob. Agents Chemother. 2006;50:2932–2940. doi: 10.1128/AAC.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oren Z., Lerman J.C., Gudmundsson G.H. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999;341(Pt 3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 5.Vandamme D., Landuyt B., Luyten W., Schoofs L. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell. Immunol. 2012;280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Dürr U.H.N., Sudheendra U.S., Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008;283:32637–32643. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- 8.Turner J., Cho Y., Dinh N.N. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamasaki K., Schauber J., Coda A. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 10.Kahlenberg J.M., Kaplan M.J. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013;191:4895–4901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sørensen O.E., Johnsen A.H., Calafat J. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 12.Liu P.T., Stenger S., Tang D.H., Modlin R.L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179 doi: 10.4049/jimmunol.179.4.2060. 2060–3. [DOI] [PubMed] [Google Scholar]
- 13.Park K., Elias P.M., Oda Y. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J. Biol. Chem. 2011;286:34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauber J., Gallo R.L. The vitamin D pathway: a new target for control of the skins immune response? Exp. Dermatol. 2008;17:633–639. doi: 10.1111/j.1600-0625.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iimura M., Gallo R.L., Hase K. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 2005;174:4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 16.Majewski K., Kozłowska E., Żelechowska P., Brzezińska-Błaszczyk E. Serum concentrations of antimicrobial peptide cathelicidin LL-37 in patients with bacterial lung infections. Cent. Eur. J. Immunol. 2018;43:453–457. doi: 10.5114/ceji.2018.81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tjabringa G.S., Ninaber D.K., Drijfhout J.W. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int. Arch. Allergy Immunol. 2006;140:103–112. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 18.van Harten R., van Woudenbergh E., van Dijk A., Haagsman H. Cathelicidins: immunomodulatory antimicrobials. Vaccines. 2018;6:63. doi: 10.3390/vaccines6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokumaru S., Sayama K., Shirakata Y. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Oglęcka K., Sandgren S. Dual functions of the human antimicrobial peptide LL-37—target membrane perturbation and host cell cargo delivery. Biochim. Biophys. Acta Biomembr. 2010;1798:2201–2208. doi: 10.1016/j.bbamem.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Chamilos G., Gregorio J., Meller S. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood. 2012;120:3699–3707. doi: 10.1182/blood-2012-01-401364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai Y., Adhikarakunnathu S., Bhardwaj K. LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasin B., Pang M., Turner J.S. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000;19:187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 24.den HERTOG A.L., van MARLE J., van VEEN H.A. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005;388:689–695. doi: 10.1042/BJ20042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Does A.M., Beekhuizen H., Ravensbergen B. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 2010;185:1442–1449. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Takai T., Xie Y. Human antimicrobial peptide LL-37 modulates proinflammatory responses induced by cytokine milieus and double-stranded RNA in human keratinocytes. Biochem. Biophys. Res. Commun. 2013;433:532–537. doi: 10.1016/j.bbrc.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Scott M.G., Davidson D.J., Gold M.R. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T., Kulkarni N.N., Lee E.Y. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-22409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otte J.-M., Zdebik A.-E., Brand S. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul. Pept. 2009;156:104–117. doi: 10.1016/j.regpep.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Yu J., Mookherjee N., Wee K. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J. Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 31.Barlow P.G., Li Y., Wilkinson T.S. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenfeld Y., Papo N., Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. J. Biol. Chem. 2006;281:1636–1643. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]
- 33.Brown K.L., Poon G.F.T., Birkenhead D. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J. Immunol. 2011;186:5497–5505. doi: 10.4049/jimmunol.1002508. [DOI] [PubMed] [Google Scholar]
- 34.Di Nardo A., Braff M.H., Taylor K.R. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 35.Tsokos G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365:2110–2121. doi: 10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- 36.Panda S.K., Kolbeck R., Sanjuan M.A. Plasmacytoid dendritic cells in autoimmunity. Curr. Opin. Immunol. 2017;44:20–25. doi: 10.1016/j.coi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Obermoser G., Pascual V. The interferon-α signature of systemic lupus erythematosus. 2010. [DOI] [PMC free article] [PubMed]
- 38.Bosch X., Ph D. Clinical implications of basic research Systemic Lupus Erythematosus and the Neutrophil. N. Engl. J. Med. 2011;365:758–760. doi: 10.1056/NEJMcibr1107085. [DOI] [PubMed] [Google Scholar]
- 39.Zawrotniak M., Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta Biochim. Pol. 2013;60:277–284. [PubMed] [Google Scholar]
- 40.Yu Y., Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J. Clin. Cell. Immunol. 2013 doi: 10.4172/2155-9899.1000139. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chamilos G., Lande R., Fukuhara S. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001180. 73ra19-73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leffler J., Martin M., Gullstrand B. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 43.Lande R., Gregorio J., Facchinetti V. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 44.Villanueva E., Yalavarthi S., Berthier C.C. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Romo G.S., Caielli S., Vega B. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001201. 73ra20-73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kienhöfer D., Hahn J., Schubert I. No evidence of pathogenic involvement of cathelicidins in patient cohorts and mouse models of lupus and arthritis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roshandel G., Sahebari M., Abdolahi N. Cathelicidin (LL-37) and its correlation with pro-oxidant, antioxidant balance and disease activity in systemic lupus erythematosus: a cross-sectional human study. Lupus. 2017;26:975–982. doi: 10.1177/0961203317691368. [DOI] [PubMed] [Google Scholar]
- 48.Kreuter A., Jaouhar M., Skrygan M. Expression of antimicrobial peptides in different subtypes of cutaneous lupus erythematosus. J. Am. Acad. Dermatol. 2011;65:125–133. doi: 10.1016/j.jaad.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Sun C.-L., Zhang F.-Z., Li P., Bi L.-Q. LL-37 expression in the skin in systemic lupus erythematosus. Lupus. 2011;20:904–911. doi: 10.1177/0961203311398515. [DOI] [PubMed] [Google Scholar]
- 50.Reinholz M., Ruzicka T., Schauber J. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012;24:126. doi: 10.5021/ad.2012.24.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen F., Pufe T., Conradi L. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J. Pathol. 2002;198:369–377. doi: 10.1002/path.1224. [DOI] [PubMed] [Google Scholar]
- 52.Säll J., Carlsson M., Gidlöf O. The antimicrobial peptide LL-37 alters human osteoblast Ca 2+ handling and induces Ca 2+ -independent apoptosis. J Innate Immun. 2013;5:290–300. doi: 10.1159/000346587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilsgård O., Andersson P., Malmsten M. Peptidylarginine deiminases present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am. J. Respir. Cell Mol. Biol. 2012;46:240–248. doi: 10.1165/rcmb.2010-0500OC. [DOI] [PubMed] [Google Scholar]
- 54.Davidson D.J., Currie A.J., Reid G.S.D. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004;172 doi: 10.4049/jimmunol.172.4.2704-a. 2704.2-2704. [DOI] [PubMed] [Google Scholar]
- 55.Sandgren S., Wittrup A., Cheng F. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J. Biol. Chem. 2004;279:17951–17956. doi: 10.1074/jbc.M311440200. [DOI] [PubMed] [Google Scholar]
- 56.Ganguly D., Chamilos G., Lande R. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]