Abstract
Behçet’s disease (BD) is a relapsing, multisystem and inflammatory condition characterized by systemic vasculitis of small and large vessels. Although the etiopathogenesis of BD remains unknown, immune-mediated mechanisms play a major role in the development of the disease. BD patients present leukocyte infiltration in the mucocutaneous lesions as well as neutrophil hyperactivation. In contrast to neutrophils, whose involvement in the pathogenesis of BD has been extensively studied, the biology of monocytes during BD is less well known. In this study, we analyzed the phenotype and function of circulating monocytes of 38 BD patients from Hospital of Braga. In addition, we evaluated the impact of inflammatory and metabolomic plasma environment on monocyte biology. We observed a worsening of mitochondrial function, with lower mitochondrial mass and increased ROS production, on circulating monocytes of BD patients. Incubation of monocytes from healthy donors with the plasma of BD patients mimicked the observed phenotype, strongly suggesting the involvement of serum mediators. BD patients, regardless of their symptoms, had higher serum pro-inflammatory TNF-α and IP-10 levels and IL-1β/IL-1RA ratio. Untargeted metabolomic analysis identified a dysregulation of glycerophospholipid metabolism on BD patients, where a significant reduction of phospholipids was observed concomitantly with an increase of lysophospholipids and fatty acids. These observations converged to an enhanced phospholipase A2 (PLA2) activation. Indeed, inhibition of PLA2 with dexamethasone or the downstream cyclooxygenase (COX) enzyme with ibuprofen was able to significantly revert the mitochondrial dysfunction observed on monocytes of BD patients. Our results show that the plasma inflammatory environment coupled with a dysregulation of glycerophospholipid metabolism in BD patients contribute to a dysfunction of circulating monocytes.
Keywords: Behçet’s disease, Monocytes, Phospholipid metabolism, Inflammation
Highlights
-
•
BD patients present high serum inflammatory mediators regardless to symptomatology.
-
•
IL-1β/IL-1RA ratio as a marker of BD inactive phase.
-
•
Monocytes of BD patients have increased ROS production and lower mitochondrial mass.
-
•
Dysregulation of glycerophospholipid metabolism leads to dysfunction of monocytes.
-
•
Phospholipase A2 and Cyclooxygenase enzyme are key players of BD pathology.
1. Introduction
Behçet’s disease (BD) is a chronic relapsing inflammatory condition, characterized by systemic vasculitis of small and large vessels [[1], [2], [3]]. Recurrent oral and genital ulcers and uveitis are the main symptoms observed in active patients [2]. In more serious cases or in late stages of the disease, patients can present involvement of other organs accompanied by gastrointestinal, neurological, and articular manifestations [1,2]. Despite a worldwide BD distribution due to migration, higher prevalence has been found in the ancient silk route [[1], [2], [3], [4]].
Although the precise etiology is still unknown, genetic predisposition coupled with environmental factors have been implicated in the immunological deviation observed during the disease, where both innate and adaptive immunity were shown to play a role [5,6]. Mucocutaneous lesions in BD are characterized by leukocyte infiltration, primarily by neutrophils, monocytes and T cells [7]. Neutrophil hyperactivation described in the form of increased production of reactive oxygen species (ROS), endothelial adhesion, chemotaxis, and phagocytosis has been correlated with tissue injury observed in lesions of BD [8]. These patients also display monocyte hyperactivity contributing to increased neutrophil adhesion and immune tissue infiltration with the development of a pro-inflammatory phenotype [9,10]. This immune hyperactivation is not only responsible for the acute attacks of the disease but also for generating an inflammatory loop that perpetuates high levels of inflammatory cytokines, leading to tissue damage and vasculitis. As such, current treatment is focused on immunomodulatory and immunosuppressive agents aiming to suppress the inflammatory attacks of the disease. Granulocyte and monocyte adsorption apheresis without additional anti-symptomatic therapy has been proved to induce a significant recovery of BD lesions [11], pinpointing the crucial role for innate immunity in triggering initial inflammation. While the involvement of neutrophils in the pathogenesis of BD has been extensively studied, less is known about the dysregulated activity of mononuclear cells during BD. Moreover, monocytes are divided into three subsets, namely classical, intermediate and non-classical. Accumulating evidence showed that monocyte activation, particularly the intermediate and non-classical subsets, is associated with the disease progression in distinct autoimmune and autoinflammatory diseases [12]. However, it remains to be determined how monocytes contribute to the BD process and which subset is involved.
Emerging evidences indicate that immune cells tightly coordinate their metabolic programs to support immunological functions [13]. Moreover, the environmental metabolome is a crucial factor influencing the activity of the immune system [13]. Alterations of these delicate signalling networks could lead to the onset of chronic inflammation. In this context, clinical and experimental data have suggested that the pathogenesis of immune-mediated disorders, such as BD, might involve circulating mediators bridging metabolism and immunity [14].
Herein, we aimed to understand how plasma microenvironment impacts monocyte metabolism and function in BD patients. Our results show that the chronic systemic inflammatory environment combined with a dysregulation of glycerophospholipid metabolism are responsible for a worsening of mitochondrial function, with lower mitochondrial mass and increased ROS production, on circulating monocytes of BD patients.
2. Material and methods
2.1. Ethics statement
Study approval was obtained from the Ethics Subcommittee for Life and Health Sciences of the University of Minho, Portugal (SECVS, 059/2014) and the Ethics Committee for Health of the Hospital of Braga, Portugal (CESHB) in July 22nd, 2014 with an upgrade for the metabolomic studies in July 4th, 2016 by the SECVS.
2.2. Study population
A total of 38 patients with either definitive or probable diagnostic of BD (according to the International Study Group of Behçet (ISGB) criteria) at the Department of Internal Medicine of Hospital of Braga, Braga (Portugal) between 2015 and 2017, were recruited to the study. The clinical characteristics of the patients are detailed in Table 1. Samples from age- and sex-matched healthy individuals were used as controls. Patients with other systemic inflammatory conditions and those who developed infection complications during the study were excluded. Disease activity was assessed by Behçet’s Disease Clinical Activity Form (BDCAF) [15]. The presence of oral ulcers, genital ulcers, any skin lesions (erythema nodosum or papulopustular lesions), eye involvement, joint tenderness and/or swelling, gastrointestinal, neurological, and vascular involvement were all evaluated by thorough clinical evaluation and specific investigations whenever needed. Active patients were defined as those with BDCAF equal to or higher than 2 [16]. Patients were divided into four treatment groups: without treatment (only 2 patients in inactive stage), treated with glucocorticoids (11 active patients and 22 inactive patients), with administration of anti-TNFα (only 1 patient in inactive stage) and with disease-modifying antirheumatic drugs (DMARDs) (8 active patients and 7 inactive patients).
Table 1.
Clinical characteristics at baseline.
| Parameter | Active (n = 13) | Inactive (n = 25) |
|---|---|---|
| Clinical Manifestations, n (%) | ||
| Oral ulcers | 13 (100) | 25 (100) |
| Pathergy reaction | 10 (77) | 23 (92) |
| Skin lesions | 10 (77) | 20 (80) |
| Genital ulcers | 9 (69) | 17 (68) |
| Articular involvement | 6 (46) | 10 (40) |
| Ocular lesions | 5 (38) | 7 (28) |
| Vascular involvement | 0 (0) | 4 (16) |
| Gastrointestinal involvement | 0 (0) | 1 (4) |
| Neurological involvement | 0 (0) | 1 (4) |
| Treatment, n (%) | ||
| Without treatment | 0 (0) | 2 (8) |
| Glucocorticoid | 11 (85) | 22 (88) |
| Anti-TNFα | 0 (0) | 1 (4) |
| Other DMARD | 8 (62) | 7 (28) |
Legend: DMARD: Disease-Modifying Antirheumatic Drugs.
2.3. Monocyte isolation and culture with plasma samples
Peripheral blood mononuclear cells (PBMC) were isolated using equal volumes of peripheral blood and Histopaque 1077 (MilliporeSigma, St Louis, Missouri, USA). After centrifugation, plasma samples were collected and stored (−80 °C). Monocytes were then separated by positive selection using magnetically labelled CD14 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) on an OctoMACS separator. Isolated monocytes were resuspended in complete RPMI medium (RPMI supplemented with 10%FBS, 1%PenStrep and 1%HEPES), seeded at a concentration of 1 × 106 monocytes per mL in a 96-well plate (Corning, Corning, NY) and incubated for 24h with 50% of control or BD patient plasma and 50% complete RPMI medium. In some cases, 1 μM Dexamethasone (Sigma) and 100 μM Ibuprofen (Sigma) was added to the monocyte culture incubated with plasma from BD patients.
2.4. Phenotypic analysis of peripheral blood leukocytes
Blood samples were collected using K3EDTA tubes (Greiner Bio-One). Red blood cells were lysed with the ACK Lysis Buffer (150 mM NH₄Cl, 10 mM KHCO3, 0.01 mM EDTA). Surface staining was performed with the following antibodies: BV711-CD3 (clone OKT3), BV786-CD19 (clone HIB19), BV605-CD56 (clone HCD56), FITC-CD66b (clone G10F5), APC-CY7-CD16 (clone 3G8), BV650-CD14 (clone M5E2), BV510-CD86 (clone IT2.2), PE-Cy7-CD163 (clone GH1/61), APC-CD206 (clone 15-2) and PB-HLA-DR (clone L243). The anti-human antibodies used to perform this study were purchased from BioLegend (CA, USA). PBMC-derived monocytes and cultured monocytes were stained for the quantification of superoxide [Dihydroethidium (DHE)] and mitochondrial mass [nonylacridine orange (NAO)]. Staining was performed using DHE (45 μM in FACS buffer (2% FBS in PBS, both from Thermo Fisher Scientific, Waltham, Massachusetts, USA)) for 10 min at 37 °C and NAO (1.25 μM in complete RPMI) for 30 min at 37 °C (Sigma, St. Louis, Missouri, EUA). After incubation, DHE stained cells were acquired directly, while NAO staining was washed with FACS buffer before acquisition. Samples were acquired on LSRII flow cytometer (BD Biosciences) using the DIVA Software and data was analyzed using FlowJo software. The gating strategy is depicted on Supplementary Fig. 1 [17].
2.5. Cytokine quantification
Cytokine quantification of plasma samples was performed with Human Macrophage/Microglia Panel Legendplex kit (ref. 740,502, BioLegend), according to manufacturer’s instructions.
2.6. Metabolomics
2.6.1. Reagents and solvents
Methanol (MEOH) MS grade and methyl tert-butyl ether (MTBE) were obtained from Sigma–Aldrich (Steinheim, Germany). Acetonitrile (ACN) MS grade (Fluka Chromassol, Spain), pyridine (Carlo Erba Reagents SAS, France), isopropanol (IPA) (Fischer, Austria), ammonium hydroxide 28% (VWR Collection Chemicals, USA) and formic acid 98% obtained from Sigma-Aldrich (St Louis, USA). Heptane MS grade, sodium hydroxide (NaOH) and C18: 0, N, O-Bis (trimethylsilyl) trifluoroacetamide with 1% trimethylchlorosilane (TMCS) obtained from Sigma Aldrich, Spain. Ultra-pure water Milli-Q-Plus 185 (Millipore, USA). A mixture of standards of alkanes (Supelco, United States), methionine sulfone, O-methoxamine, a mixture of methyl acids and fatty acids (FAME C8–C22) and tricosane were obtained from Sigma Aldrich, Spain. 30 k/Daltons Centrifree protein cutoff cut filters were obtained from Millipore, United States.
2.6.2. LC-ESI-qTOF/MS
50 μl of plasma samples were aliquoted and added 350 μl of a mixture of MTBE/Ethanol (50:50 v/v), followed by vortex for approximately 30s and centrifugation at 15 min 4000 g, at 15 °C [18]. The supernatant was collected and transferred to the sterile vials to LC-MS analysis. Analysis was performed by HPLC 1200 Agilent system accoupled to mass spectrometry, with a RP C8 column Agilent Poroshell (150 mm × 2.1 mm, 2.7 μm) as previously described [19]. Mobile Phases were composed by 5 mmol L−1 NH4HCO2 in ultra-pure water (phase A) and 5 mmol L−1 NH4HCO2 in MEOH (85%) and IPA (15%) in phase B in positive mode; formic acid 0.1% in ultra-pure water (phase A) and formic acid 0.1% in MEOH (85%) and IPA (15%) for the negative mode, pumped 0.5 mL/min. Quadrupole-time of flight (qTOF) mass spectrometry (6520, Agilent Technologies) was performed in both polarities ESI (positive and negative) in mode full scan from 50 to 1200 m/z. The capillary voltage (V) was 3500 kV in positive mode and 4500 kV in negative mode. Reference masses were applied in all analyses, which were 121.0509 m/z and 922.0098 m/z for the positive mode and 112.9856 m/z and 1033.9881 m/z for the negative mode to performe the mass correction. Data files were collected in centroide mode, scan rate of 1.02 scans/s.
2.6.3. CE-ESI-Tof MS
100 μL of plasma samples were vortexed for 2min and added 100 μL of 0.2 mol L−1 formic acid with ACN 5% and 0.4 mmol L−1 methionine sulfone (internal standard). Samples were vortexed (1 min) and then transferred to Milipore filter (30 kDa protein cutoff), followed by centrifugation (70 min, 2000 rpm at 4 °C). The supernatant was transferred to a vial for analysis. Electrophoresis capillary (6200, Agilent Technologies) system accoupled to (TOF MS) mass spectrometry (6500, Agilent Technologies) was used to sample analysis following a previously published method [20]. The separation was performed by silica capillary (50 μm i.d., 96 cm length) and background electrolyte (BGE) composed by MEOH 10.8% and formic acid 3.78% v/v. All sample analysis was performed in positive mode with ESI source. In source fragmentation was made from 200 V to obtain the spectra profile of compounds.
2.6.4. GC-EI-q-MS
GC-MS analysis was performed following a protocol developed in CEMBIO [21]. Briefly, 40 μL of plasma sample was homogenized for 2 min and added 120 μL of cold ACN, mixed for 2 min and let stand the samples for 5 min on ice for deproteinization. Samples were methoximated and silylated with BSTFA Gas chromatography coupled to single quadrupole mass spectrometry (7693, Agilent Technologies), electron ionization (EI) source, Split/splitless injector, ccoupled to a Mass Selective Detector. Column (DB-5 ms, 30 m × 250 μm x 0.25 μm, 95% dimethyl/5% phenyl polysiloxane film (Agillent Technologies).
2.6.5. Blank and quality control (QC) sample preparation
Blank and QCs were prepared to each platform from of ultra-pure water volume and a pooling equal volume of each of the plasma samples, respectively. The same protocol of treatment was applied to blank as well as QC samples [22].
2.6.6. Data treatment
All data were controlled and acquired using Mass Hunter Qualitative Analysis B.07.00 (Agilent Technologies). Data obtained from LC-MS and CE-MS were cleaned of background noise, unrelated ions, peak detection, deconvolution and alignment were performed by the recursive feature extraction (RFE) using Profinder Software B.08.00 (Agilent Technologies). Data obtained from GC-MS were processed by Unknowns Analysis Software B.09.00 (Agilent Technologies) to perform peak extraction and deconvolution. Alignment and integration were performed by Mass Profiler Professional 14.9 and MS Quantitative Analysis (Quant-My-Way) B.09.00. Blank subtraction and filtering by frequency of at least 50% of the QC and 70% of each group and relative standard deviation (RSD) less than 20% (LC-MS) and 30% (CE-MS and GC-MS) in QC were performed, to keep only the relevant features. Feature with higher RSD was removed from the data set. Missing values were substituted by KNN algorithm using MetaboAnalyst 4.0 Software [23]. Normalization by QC was applied to all data matrix and I.S applied only to GC-MS data. Beyond this, all data were log-transformed to get a normal distribution and used pareto scaling.
2.6.7. Metabolites annotation and pathway analysis
Features obtained by LC-MS and CE-MS representing significant differences between the class were annotated using CEU Mass Mediator Online Platform as the database source [24]. Accurate masses, retention time, protonation, deprotonation, adducts formation, neutral loss, isotopic distribution and formula calculation tool Qualitative Analysis B.07.00 (Agilent Technologies), were used to annotate the metabolites. Additionally, CE-MS features were annotated using an in-house library developed at CEMBIO (Madrid, Spain) to match the fragmentation profile and the relative migration time (RMT). Features annotation by GC-MS was performed by using the Fienh 15 [25] Library using retention time information, the in house CEMBIO library and the NIST 17 mass spectra library using retention index determination obtained from n-alkanes (C8–C28). Biochemical pathway was created from the metabolites annotated using Pathway Analysis of MetaboAnalyst 4.0 with the following parameters: Hypergeometric Test and Relative-betweenness Centrality Algorithms and Homo sapiens (KEGG) pathway library [23].
2.7. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 6 software. Regarding the small sample size and the non-normality observed in our variables, Kruskal–Wallis test was used to identify statistical differences. For variables that reached global significance, pairwise comparisons were performed by the Mann–Whitney U test. Correlations were calculated using Spearman’s correlation: Spearman coefficient and p value were reported. For metabolomics, Multivariate Analysis as Principal Components Analysis (PCA) and Partial Least Square Discriminant Analysis (PLS-DA) and Variables Importance Projection (VIP) Score with Leave-one-out cross-validation method (LOOCV) were performed in order to exploratory and discrimination of the classes. Univariate analysis was performed by t-tests with false discover rate (FDR) adjusted P-value. Combination of the VIP Score >1.0 and p < 0.05 were used as features statistically significant. Multivariate and univariate analyses were performed by MetaboAnalyst 4.0 Software online [23]. Statistically significant values are as follows: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0,0001.
3. Results
3.1. Characterization of BD patients and control group
Our study enrolled 77 subjects including 39 healthy age-matched controls (17 males and 22 females, mean age ± SD, 32.95 ± 1.30 years), 25 inactive BD patients (10 males and 15 females; mean age ± SD, 41.38 ± 2.64 years) and 13 active BD patients (2 males and 11 females; mean age ± SD, 29.76 ± 2.03 years) from the Hospital of Braga. The mean disease duration was 12.16 ± 8.24 years in inactive patients and 9.08 ± 8.04 years in active patients. The mean age of diagnose was 34.68 ± 10.23 years in inactive patients and 38.08 ± 10.34 years in active patients. The clinical manifestations of the disease are presented in Table 1, being oral ulcers, pathergy reaction and skin lesions the most common in both inactive and active BD patients.
3.2. Cytokine profile of BD patients
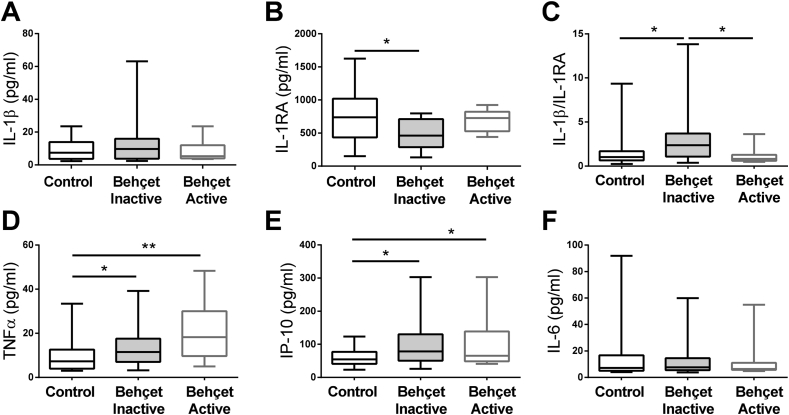
Plasma samples from BD patients and healthy controls were used to quantify the levels of circulating cytokines. Inflammatory (IL-1β, TNFα, IL-6, IL-12p70, IFN-gamma, IL-23, and IP-10) and anti-inflammatory (arginase, IL-4, IL-10 and IL1-RA) mediators were evaluated. While no significant differences among groups were observed on the IL-1β levels (Fig. 1A), IL-1RA levels were significantly decreased in BD patients without active disease (p = 0.0107, Fig. 1B). As a result, the IL-1β/IL-1RA ratio was found significantly higher in inactive BD patients in comparison to active BD patients and healthy controls (p = 0.0147 and p = 0,0368, respectively, Fig. 1C). Among the inflammatory mediators, both TNFα and IP-10 levels were significantly increased in both inactive and active patients in comparison to healthy individuals (p = 0.0391 and p = 0.0037 for TNFα and p = 0.0117 and p = 0.0355 for IP-10, respectively, Fig. 1D–E). No significant differences were found between groups for IL-6, IL-12p70, IFN-gamma and IL-23 (Fig. 1F and Supplementary Figs. 2A–C). Of note, the other tested anti-inflammatory mediators were also similar between all groups (Supplementary Figs. 2D–F). Our data demonstrates increased levels of inflammatory mediators on the plasma of BD patients, being the higher IL-1β/IL-1RA ratio exclusively of patients without active disease.
Fig. 1.
Plasma cytokine profile in BD patients. The levels of (A) IL-1β, (B) IL-1RA, (C) IL-1β/IL-1RA ratio, (D) TNF-α, (E) IP-10, (F) IL-6 were quantified on the plasma of healthy controls (n = 39) and inactive (n = 25) and active (n = 13) BD patients. Data are shown in a box and whisker plot format; ∗p < 0.05; ∗∗p < 0.01.
3.3. BD patients monocytes display loss of mitochondrial mass
We extended our analysis to blood immune cell populations. While no major changes were observed in the percentage of neutrophil and lymphocyte populations, an increased neutrophil-to-lymphocyte (NLR) ratio was detected in active patients, corroborating previous publications (Supplementary Fig. 3A) [26,27]. Similarly, the percentage of circulating monocytes was similar between all groups (Fig. 2A). Monocytes of BD patients, particularly from those with active disease, displayed higher surface levels of the antigen-presenting molecule human leukocyte antigen-DR (HLA-DR) and the co-stimulatory molecule CD86, both suggestive of monocyte activation (Fig. 2B). In contrast, no significant variations were observed on the surface levels of the anti-inflammatory markers CD163 and CD206 (Fig. 2B). A deeper analysis was performed on the three sub-populations of monocytes based on the surface expression of CD14 and CD16. The percentages of classic (CD14++ CD16−), intermediate (CD14++, CD16++) and non-classic (CD14+ CD16++) were similar between groups, which corroborate the data on total monocytes (Supplementary Fig. 3B). Interestingly, the significant increase of the surface HLA-DR and CD86 levels on the monocyte population was due to a specific increase on the non-classical (p = 0.0262 and p = 0.0319 for CD86 and p = 0.0393 and p = 0.0134 for HLA-DR for inactive and active, respectively, compared to control) and, partially the intermediate (p = 0.0483 for active vs control and p = 0.0410 for active vs inactive for CD86), monocyte populations (Supplementary Fig. 4). As observed in total monocytes, no significant differences were found on the surface levels of CD163 and CD206 for each sub-population (Supplementary Fig. 5). A functional characterization of circulating monocytes was performed through the evaluation of superoxide anion levels and mitochondrial mass. Corroborating previous studies, we observed a significantly increase of superoxide anion levels, both of mitochondrial and non-mitochondrial origin (data not shown), in BD patients, with a tendency to be higher in active patients (p = 0.0214 and p = 0.0030, respectively, Fig. 3A) [28,29]. Interestingly, a significant decreased mitochondrial mass was found in both inactive and active BD patients (p = 0.0203 and p = 0.0242, respectively), being more drastic in the latter (Fig. 3B), when compared to healthy controls. This mitochondrial mass phenotype was observed in each of the three subsets of monocytes (Supplementary Fig. S3C). Interestingly, we observed a negative correlation between the DHE and NAO levels only on BD patients (p = 0.0287; r = 0.4377) (Fig. 3C). Furthermore, the mitochondrial mass was inversely correlated with the surface expression of the HLA-DR and CD86 activation markers (Fig. 3D). While the phenotype was already observed in control samples (p = 0.0320; r = −0.4930 for HLA-DR and p = 0.1608; r = −0.3024 for CD86), it was significantly enhanced on monocytes of BD patients (p = 0.0068; r = −0.5592 for HLA-DR and p = 0.0018; r = −0.5551 for CD86). Collectively, our data suggests that the higher monocyte activation of BD patients is associated with a worse mitochondrial function characterized by lower mitochondrial mass.
Fig. 2.
Circulating monocytes of BD patients display a pro-inflammatory profile (A) Percentages of the monocyte population based on gating strategy on Fig S1 (B) The mean fluorescence intensity of M1-like prototype markers HLA-DR and CD86 and M2-like prototype markers CD163 and CD206. Data are shown in a box and whisker plot format; ∗p < 0.05 MFI - Mean Fluorescent Intensity.
Fig. 3.
BD patients display a worsening of monocyte mitochondrial function with lower mitochondrial mass and increased ROS production. (A) The superoxide production (DHE) and (B) mitochondrial mass (NAO) was quantified on the gated monocyte population. (C) Correlation between the monocyte mitochondrial mass (NAO) and superoxide production (DHE) in control (n = 17) or BD patients (n = 25). (D) Correlation between the monocyte mitochondrial mass (NAO) and HLA-DR or CD86 in control (n = 23) or BD patients (n = 22). Individual values are shown. ∗p < 0.05; ∗∗p < 0.01. MFI - Mean Fluorescent Intensity.
3.4. Untargeted metabolomic analysis of plasma samples from BD patients
Recent reports have suggested the involvement of several inflammatory mediators in the general hyperactivity of the immune response observed in BD [5,30,31]. Yet, it is still unclear how the metabolic environment in the blood stream will impact immune cell phenotype and effector functions contributing to BD symptomatology and response to therapy. To address this issue, we performed an untargeted metabolomic analysis of plasma samples from BD patients and compared it against healthy samples. The principal components analysis (PCA; Supplementary Fig. 6) models show the clustering of the quality control samples (QC, light blue spheres) of each technique, evidencing a satisfactory stability and performance of the analytical techniques. Further, PCA models for LC-MS both ionization modes and CE-MS (Supplementary Fig. 6A) showed two separate clusters representing BD and control group. Yet, no differences were found between BD active and inactive patients (Supplementary Fig. 6A). For this reason, the PLS-DA (Fig. 4A, a-d) supervised models were built only with two classes (Behçet and control). Cross validation (CV) of PLS-DA model was measured from determination (R2) and prediction (Q2) coefficients that were considerate to be good quality models with high R2 and Q2. CV of each platform was: LC-MS ESI+ (R2: 0.90, Q2: 0.88), LC-MS ESI- (R2: 0.96, Q2: 0.95), CE-MS (R2: 0.70, Q2: 0.63) and GC-MS (R2: 0.39, Q2: 0.10). Statistical analysis showed a total of 130 significantly different features, from which 45 metabolites were annotated. Features of each technique are summarized in Tables S1–S3. Univariate analysis performed on the metabolites allow to discriminate 12 and 24 metabolites that were increased and decreased, respectively, on BD patient’s plasma in comparison to healthy samples (Fig. 4B). Among the most significant metabolites, we found glycerophospholipids and fatty acids (Fig. 4C). A pathway analysis overview pinpointed glycerophospholipid metabolism, serine, glycine and threonine metabolism, linoleic acid metabolism and amino acyl-tRNA biosynthesis as the most significantly modulated (Fig. 4D and Supplementary Fig. 6B), being the biochemical pathway related to lipid metabolism the more relevant. Moreover, the level of phospholipids was found to be significantly decreased concomitantly with a trend of increased lysophospholipid levels in BD patients in comparison to the control group (Fig. 4E–F). Of note, no significant differences were found in the plasma levels of phospholipid and lysophospholipid between active and inactive patient (Supplementary Fig. S7). Overall, an untargeted metabolomic analysis identified the glycerophospholipids metabolism as the most significantly modulated pathway on BD patients.
Fig. 4.
Metabolic profiling of BD patients’ plasma (A) Principal Components Analysis Score Plot of untargeted metabolomics of BD patients and control group by multiplatform analysis. HPLC-MS positive (a) and negative (b) modes analysis, CE-MS (c) and GC-MS (d) techniques. (B) Volcano plot highlighting significant metabolites annotated between Behçet and control group. (C) Heat map of the lipids annotated with significantly different abundance between Behçet and control group. (D) Graphical representation of the major pathways modified in the plasma of BD patients. Quantification of (E) phospholipids and (F) lysophospholipids in the plasma of BD patients and healthy controls. Data are shown in a box and whisker plot format; ∗p < 0.05.
3.5. Plasma from BD patients decreases mitochondrial mass in healthy monocytes through a phospholipid-mediated inflammatory response
Our results revealed the activation status and functional mitochondrial changes in monocytes from BD patients, along with a significant modulation of inflammatory status of the environment and lipid metabolism. As such, we hypothesized that the inflammatory soluble mediators on the BD patient’s plasma contribute to the dysregulation of lipid metabolism that in turn would be responsible for the observed monocyte phenotype. Incubation of monocytes recovered from healthy donors with BD patient’s plasma, but not plasma from control donors, induced a significantly decrease on the mitochondrial mass (p < 0.0001; Fig. 5A). Considering the dysregulation of glycerophospholipid metabolism observed in the metabolomic analysis, the role of PLA2 in this mitochondrial mass reduction was evaluated. Inhibition of PLA2 by dexamethasone or the downstream cyclooxygenase (COX) enzyme with ibuprofen significantly reverted the loss of mitochondrial mass (p = 0.0358 and p = 0.0165, respectively) (Fig. 5A–B). Taken together, our data show that the dysregulated metabolism of phospholipids found in the metabolomic analysis are directly linked to monocyte’s phenotype, once the inhibition of arachidonic acid metabolism prevented a pronounced decrease of mitochondrial mass in monocytes.
Fig. 5.
Inhibition of PLA2with dexamethasone and cyclooxygenase with ibuprofen was able to partially revert the mitochondrial dysfunction observed on monocytes of BD patients. (A) Monocytes isolated from healthy individuals were incubated with a 50% dilution of plasma recovered from healthy individuals (Control) and BD patients in the absence (BD) or presence of dexamethasone (Dexa) or Ibuprofen (IB) for 24 h. Mitochondrial mass was quantified by flow cytometry. Data are shown in a box and whisker plot format; ∗p < 0.05; ∗∗∗∗p < 0,0001. (B) The mean fluorescent intensity (MFI) of nonylacridine orange (NAO), as an indicator of mitochondrial mass, of each individual condition is shown for each individual plasma tested. Data are shown as mean ± SD, n = 9 plasma/group. ∗p < 0.05.
4. Discussion
Neutrophils and lymphocytes have already been pointed out as important players of the chronic inflammatory response developed during BD [[32], [33], [34], [35]]. Although increased monocyte activation has been reported in BD patients [9], the exact mechanism behind it is still unclear. Our results show that a chronic inflammatory environment that results in dysregulated phospholipid metabolism contributes to mitochondrial dysfunction and to the pro-inflammatory phenotype of circulating monocytes of BD patients.
While most studies have demonstrated increased levels of inflammatory cytokines [30,[36], [37], [38], [39]], other have found no differences [40]. Our cohort presented similar plasma levels of IL-1β, IL-6, IL-12p70 and IFN-gamma between BD patients and control group, highlighting the heterogeneity of the cohorts under study in terms of the severity of disease or undergoing treatment. Of note, 87% of patients enrolled in our study were treated with glucocorticoids, which may explain the low levels in most of the pro-inflammatory cytokines tested. However, IP-10 and TNFα levels were found significantly increased in BD patients, when compared to heathy controls, proving that, despite the treatment, a pro-inflammatory environment is still present. Increased levels of IP-10 were previously reported in BD as well as in Kawasaki disease, an acute febrile vasculitis, suggesting an important role of this chemokine in vasculitis development [41,42]. IL-1RA levels, an antagonist of the IL-1β receptor used as therapeutic agent in inflammatory diseases as Still’s Disease and BD [43,44], were highly decreased in inactive BD patients. This lead to a significantly increase of IL-1β/IL-1RA ratio in these patients, a phenomena similarly observed in other inflammatory diseases such as osteoarthritis [45]. Our data show a significant increase of TNFα and IP-10 levels regardless of disease stage. This was further confirmed by the metabolic signature of BD that is also independent of disease activity. Interestingly, IL-1RA/IL-1β ratio is increased exclusively in inactive BD patients, which suggests a low-grade but constant systemic inflammatory phenotype in BD patients even in the absence of overt disease.
In contrast to several other studies that have shown that circulating CD16+ monocytes (intermediates and non-classical) are found in large numbers in patients with inflammation processes [46] and infectious diseases [47,48], we did not observed any imbalance on the monocytes sub-populations in our cohort of BD patients. Yet, a consensus exists on the higher activation status of circulating monocytes in BD patients. Previous studies demonstrated a higher production of inflammatory cytokines and increased surface expression levels of HLA-DR and CD86 on monocytes of BD patients when compared to healthy controls [34,[49], [50], [51]]. We found a similar phenotype in our cohort. Moreover, here we show that the increased activation status was observed only on the intermediate and non-classical monocyte populations, whereas no modifications were observed on classical monocytes. This is a phenotype that was already observed in other inflammatory conditions, namely systemic lupus erythematous, sepsis and atherosclerosis [17,52,53]. In opposition to the classical subtype, intermediate and non-classical monocytes display a more pro-inflammatory phenotype more prone to become activated and capable of secreting inflammatory cytokines and antimicrobial molecules [17,54]. Therefore, the activation, without expansion, of the two minority monocyte populations in BD comes in line with the cytokine signature, pinpointing a low-level but constant systemic inflammatory activation.
Monocytes from BD patients displayed a lower mitochondrial mass with higher superoxide anion levels when compared to the control group. Increased levels of superoxide production have already been reported in neutrophils and macrophages of BD patients [[55], [56], [57], [58]]. High levels of superoxide induce mitochondrial damage and the consequent increased permeability leads to the accumulation of reactive oxygen species (ROS), generating a mitochondrial damage loop, that culminates in mitochondrial dysfunction [59,60]. We observed a negative correlation between superoxide anion levels and mitochondrial mass in BD patients. Also, an inverse correlation between mitochondrial mass and activation markers (HLA-DR and CD86) was observed, which was more evident in BD patients than in the control group. Thus, our data strongly suggest that the pro-inflammatory environment in BD patients (mainly due to increased levels of pro-inflammatory cytokines) leads to activation of monocytes towards a pro-inflammatory phenotype increasing the production of inflammatory products, namely ROS, that ultimately contribute to the mitochondrial dysfunction through loss of mitochondrial mass (Fig. 6).
Fig. 6.
Proposed model for mitochondrial dysfunction in monocytes from BD patients. Circulating pro-inflammatory cytokines (TNFα, IL-1β and IL-6) enhance Phospholipase A 2 (PLA2) cleavage of phospholipids, producing lysophospholipids and fatty acid, and releasing arachidonic acid from monocyte membrane. Resulting lipids can induce PLA2 activity, leading to an inflammatory loop. Arachidonic acid is converted in prostaglandins by Cyclooxygenase complex (COX-1/COX-2). Inflammatory cytokines can also induce production of reactive oxygen species (ROS). In consequence, monocytes lose mitochondrial mass and become dysfunctional. Dexamethasone and ibuprofen are selective inhibitors of PLA2 and COX-1/COX-2, respectively.
Glycerophospholipid metabolism was the major significantly modified pathway in the plasma of BD patients. The activation of this pathway produces bioactive lipids as omega 3 and 6 fatty acids, polyunsaturated fatty acids (PUFAs) and arachidonic acid [61]. Some of these bioactive lipids can be metabolized to mediators that participate in the pro-inflammatory signalling [61]. Inflammatory cytokines induce the transport of the phospholipase A2 (PLA2) enzymes to the cellular membrane that contains glycerophospholipid in the composition. Some glycerophospholipids are activated and converted to PUFAs, producing oxylipins that will promote an inflammatory loop [[61], [62], [63]]. In BD patients, decreased levels of glycerophospholipids [phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS)] may be related to their metabolization to produce oxylipins that were also found to be increased in BD patients. In addition, lysophospholipid, in particular lysophosphatidylcholines (LPC), and fatty acids levels were increased in BD patients suggesting an activation of PLA2, which comes in line with the observed ongoing inflammatory processes. Our data suggest an interplay between lipid metabolism and inflammation at multiple levels that may exacerbate monocyte dysfunction in the development of BD.
In order to explore the mechanism underlying the mitochondrial dysfunction observed, plasma from BD patients was incubated with healthy monocytes, significantly leading to a reduction of the mitochondrial mass of these cells. Phenotypic induction through plasma mediators has already been described in BD [64]. Considering the changes observed in glycerophospholipid metabolism with the untargeted metabolome analysis, we hypothesized that phospholipid metabolism could be enrolled in this BD-phenotype induction. Expression of PLA2 can be induced through pro-inflammatory cytokines, such as IL-1 or TNFα [36,65]. PLA2 cleaves membrane phospholipids into fatty acids and lysophospholipids, and, upon stimulation, promotes the release of arachidonic acid (AA) that unleashes the production of prostaglandins by cyclooxygenase-2 (COX-2) [65]. The resulting lipids can activate PLA2 through specific lipid-receptors, enhancing this inflammatory loop [65]. Thus, dexamethasone and ibuprofen were used to suppress cytokine-induced expression of PLA2 and inhibit COX-1/COX-2, respectively [65,66]. Dexamethasone and ibuprofen treatment significantly reverted the loss of mitochondrial mass experienced by monocytes in the presence of plasma from BD patients. Prostaglandins have been associated with mitochondrial dysfunction and to significantly potentiate cytokine-induced inflammatory responses in rheumatoid arthritis and other inflammatory disorders [[67], [68], [69]]. Our results suggest a direct or indirect association between the production of prostaglandins and the loss of mitochondrial mass (Fig. 6). Although future studies are necessary to define the precise mechanism, the loss of mitochondrial mass can occur through increased mitophagy or decreased biogenesis [70].
In conclusion, we demonstrate the existence of a low-grade continuous inflammatory environment that associates with an increased glycerophospholipid metabolism in the plasma of BD patients. Interestingly, some mechanisms such as the increased IL1-RA/IL-1β ratio are specific for inactive disease while others as inflammatory cytokines, monocyte activation and metabolite signature, are specific of BD, irrespectively to the disease stage. Consequently, circulating intermediate and non-classical monocytes display an activated phenotype that correlates with worsening of mitochondrial function, which may contribute to the inflammatory loop. Together with the already known inflammatory players, namely neutrophils and lymphocytes, monocytes promote the inflammatory environment present in BD patients. Finally, inhibition of PLA2 with dexamethasone or the downstream cyclooxygenase (COX) with ibuprofen was able to significantly revert the mitochondrial dysfunction observed on monocytes of BD patients. Our results bridge metabolism and immunity to unravel the mechanisms of BD.
Funding
This work was supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) (NORTE-01-0145-FEDER-000013) and the Fundação para a Ciência e Tecnologia (FCT) (contracts UMINHO/BD/57/2018 to AMF and IF/00021/2014 to RS). D. Z. S.F. received funding from Airbus Defense and Space through the CLX-2 program developed with Comando da Aeronáutica (COMAER) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. This work was supported by The Clinical Academic Center (2CA-Braga) through the grant 2017 2CA 004.
Author Contributions
Ana Mendes-Frias: Investigation, Validation, Formal Analysis, Data Curation, Writing - Original Draft, Visualization Bruno Santos-Lima: Investigation, Methodology, Formal Analysis, Data Curation Danielle Zildeana Sousa Furtado: Investigation, Formal Analysis, Data Curation Francisco J Ruperez: Investigation, Methodology, Validation, Data Curation, Supervision Nilson Antonio Assunção: Resources Maria João Matias: Investigation, Methodology, Formal Analysis, Data Curation Vânia Gomes: Investigation, Methodology, Formal Analysis, Data Curation Joana Gaifem: Investigation, Methodology Coral Barbas: Methodology, Validation, Data Curation, Supervision, Writing - Review & Editing António Gil Castro Conceptualization, Methodology, Writing - Review & Editing Carlos Capela Conceptualization, Methodology, Resources, Supervision, Writing - Review & Editing, Project Management, Ricardo Silvestre Conceptualization, Methodology, Supervision, Writing - Original Draft, Writing - Review & Editing, Project Management, Funding Acquisition
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper..
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2020.100056.
Contributor Information
Carlos Capela, Email: carloscapela@med.uminho.pt.
Ricardo Silvestre, Email: ricardosilvestre@med.uminho.pt.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Islam S.M.S., Sohn S. HSV-induced systemic inflammation as an animal model for Behçet’s disease and therapeutic applications. Viruses. 2018;10 doi: 10.3390/v10090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mat C., Yurdakul S., Sevim A., Özyazgan Y., Tüzün Y. Behçet’s syndrome: facts and controversies. Clin. Dermatol. 2013;31:352–361. doi: 10.1016/j.clindermatol.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kapsimali V.D., Kanakis M.A., Vaiopoulos G.A., Kaklamanis P.G. Etiopathogenesis of Behçet’s disease with emphasis on the role of immunological aberrations. Clin. Rheumatol. 2010;29:1211–1216. doi: 10.1007/s10067-010-1491-6. [DOI] [PubMed] [Google Scholar]
- 4.Davatchi F. Behçet’s disease. Int. J. Rheum. Dis. 2018;21:2057–2058. doi: 10.1111/1756-185X.13465. [DOI] [PubMed] [Google Scholar]
- 5.Mesquida M., Molins B., Llorenç V., Sainz de la Maza M., Hernandez M.V., Espinosa G., Adán A. Proinflammatory cytokines and c-reactive protein in uveitis associated with Behçet’s disease. Mediat. Inflamm. 2014:2014. doi: 10.1155/2014/396204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu J., Kaneko F., Suzuki N. Skewed helper T-cell responses to IL-12 family cytokines produced by antigen-presenting cells and the genetic background in Behcet’s disease. Genet. Res. Int. 2013 doi: 10.1155/2013/363859. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller W., Lehner T. Quantitative electron microscopical analysis of leukocyte infiltration in oral ulcers of Behcet’s syndrome. Br. J. Dermatol. 1982;106:535–544. doi: 10.1111/j.1365-2133.1982.tb04556.x. [DOI] [PubMed] [Google Scholar]
- 8.Eksioglu-Demiralp E., Direskeneli H., Kibaroglu A., Yavuz S., Ergun T., Akoglu T. Neutrophil activation in Behcet’s disease. Clin. Exp. Rheumatol. 2001;19:19–24. [PubMed] [Google Scholar]
- 9.Şahin Ş., Lawrence R., Direskeneli H., Hamuryudan V., Yazici H., Akoǧlu T. Monocyte activity in Behçet’s disease. Br. J. Reumatology. 1996;35:424–429. doi: 10.1093/rheumatology/35.5.424. [DOI] [PubMed] [Google Scholar]
- 10.de Chambrun M.P., Wechsler B., Geri G., Cacoub P., Saadoun D. New insights into the pathogenesis of Behçet’s disease. Autoimmun. Rev. 2012;11:687–698. doi: 10.1016/j.autrev.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Kanekura T., Gushi A., Iwata M., Fukumaru S., Sakamoto R., Kawahara K., Maruyama I., Kanzaki T. Treatment of Behçet’s disease with granulocyte and monocyte adsorption apheresis. J. Am. Acad. Dermatol. 2004;51:83–87. doi: 10.1016/j.jaad.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Hirose S., Lin Q., Ohtsuji M., Nishimura H., Verbeek J.S. Monocyte subsets involved in the development of systemic lupus erythematosus and rheumatoid arthritis. Int. Immunol. 2019;31:687–696. doi: 10.1093/intimm/dxz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill L.A.J., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolowczuk I., Verwaerde C., Viltart O., Delanoye A., Delacre M., Pot B., Grangette C. Feeding our immune system: impact on metabolism. Clin. Dev. Immunol. 2008:2008. doi: 10.1155/2008/639803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhakta B.B., Brennan P., James T.E., Chamberlain M.A., Noble B.A., Silman A.J. Behçet’s disease: evaluation of a new instrument to measure clinical activity. Rheumatology. 1999;38:728–733. doi: 10.1093/rheumatology/38.8.728. [DOI] [PubMed] [Google Scholar]
- 16.Neves F. de S., Caldas C.A.M., de Medeiros D.M., de Moraes J.C.B., Gonçalves C.R. Cross-cultural adaptation of simplified version (s) of Behçet’s Disease Current Activity Form (BDCAF) and comparison between two different instruments with Brazilian versions for evaluating Behçet’s Disease Activity: BR-BDCAF and BR-BDCAF(s) Rev. Bras. Reumatol. 2009;49:20–31. doi: 10.1590/s0482-50042009000100003. [DOI] [Google Scholar]
- 17.Mukherjee R., Barman P.K., Thatoi P.K., Tripathy R., Das B.K., Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci. Rep. 2015;5 doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villaseñor A., Garcia-Perez I., Garcia A., Posma J.M., Fernández-López M., Nicholas A.J., Modi N., Holmes E., Barbas C. Breast milk metabolome characterization in a single-phase extraction, multiplatform analytical approach. Anal. Chem. 2014;86:8245–8252. doi: 10.1021/ac501853d. [DOI] [PubMed] [Google Scholar]
- 19.Godzien J., Ciborowski M., Armitage E.G., Jorge I., Camafeita E., Burillo E., Martín-Ventura J.L., Rupérez F.J., Vázquez J., Barbas C. A single in-vial dual extraction strategy for the simultaneous lipidomics and proteomics analysis of HDL and LDL fractions. J. Proteome Res. 2016;15:1762–1775. doi: 10.1021/acs.jproteome.5b00898. [DOI] [PubMed] [Google Scholar]
- 20.Naz S., Garcia A., Rusak M., Barbas C. Method development and validation for rat serum fingerprinting with CE-MS: application to ventilator-induced-lung-injury study. Anal. Bioanal. Chem. 2013;405:4849–4858. doi: 10.1007/s00216-013-6882-5. [DOI] [PubMed] [Google Scholar]
- 21.Mastrangelo A., Ferrarini A., Rey-Stolle F., García A., Barbas C. From sample treatment to biomarker discovery: a tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta. 2015;900:21–35. doi: 10.1016/j.aca.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Dudzik D., Barbas-Bernardos C., García A., Barbas C. Quality assurance procedures for mass spectrometry untargeted metabolomics. a review. J. Pharmaceut. Biomed. Anal. 2018;147:149–173. doi: 10.1016/j.jpba.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 23.Chong J., Wishart D.S., Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinf. 2019;68:1–128. doi: 10.1002/cpbi.86. [DOI] [PubMed] [Google Scholar]
- 24.Gil-De-La-Fuente A., Godzien J., Saugar S., Garcia-Carmona R., Badran H., Wishart D.S., Barbas C., Otero A. CEU mass mediator 3.0: a metabolite annotation tool. J. Proteome Res. 2019;18:797–802. doi: 10.1021/acs.jproteome.8b00720. [DOI] [PubMed] [Google Scholar]
- 25.Kind T., Wohlgemuth G., Lee D.Y., Lu Y., Palazoglu M., Shahbaz S., Fiehn O. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009;81:10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammad M., Shehata O.Z., Abdel-Latif S.M., El-Din A.M.M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in Behçet’s disease: which and when to use? Clin. Rheumatol. 2018;37:2811–2817. doi: 10.1007/s10067-018-4194-z. [DOI] [PubMed] [Google Scholar]
- 27.Ozturk C., Balta S., Balta I., Demirkol S., Celik T., Turker T., Iyisoy A., Eksioglu M. Neutrophil-lymphocyte ratio and carotid-intima media thickness in patients with Behçet disease without cardiovascular involvement. Angiology. 2015;66:291–296. doi: 10.1177/0003319714527638. [DOI] [PubMed] [Google Scholar]
- 28.Becatti M., Emmi G., Silvestri E., Bruschi G., Ciucciarelli L., Squatrito D., Vaglio A., Taddei N., Abbate R., Emmi L., Goldoni M., Fiorillo C., Prisco D. Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation. 2016;133:302–311. doi: 10.1161/CIRCULATIONAHA.115.017738. [DOI] [PubMed] [Google Scholar]
- 29.Liang L., Tan X., Zhou Q., Zhu Y., Tian Y., Yu H., Kijlstra A., Yang P. IL-1β triggered by peptidoglycan and lipopolysaccharide through TLR2/4 and ROS-NLRP3 inflammasome- dependent pathways is Involved in ocular Behçet’s disease. Invest. Ophthalmol. Vis. Sci. 2013;54:402–414. doi: 10.1167/iovs.12-11047. [DOI] [PubMed] [Google Scholar]
- 30.Cantarini L., Pucino V., Vitale A., Talarico R., Lucherini O.M., Magnotti F., De Rosa V., Galgani M., Alviggi C., Marone G., Galeazzi M., Matarese G. Immunometabolic biomarkers of inflammation in Behçet’s disease: relationship with epidemiological profile, disease activity and therapeutic regimens. Clin. Exp. Immunol. 2016;184:197–207. doi: 10.1111/cei.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopalco G., Lucherini O.M., Vitale A., Talarico R., Lopalco A., Galeazzi M., Lapadula G., Cantarini L., Iannone F. Putative role of serum Amyloid-A and proinflammatory cytokines as biomarkers for Behcet’s disease. Medicine (Baltim.) 2015:94. doi: 10.1097/MD.0000000000001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köse O. Development of immunopathogenesis strategies to treat Behçet’s disease. Pathol. Res. Int. 2012 doi: 10.1155/2012/261989. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frassanito M.A., Dammacco R., Cafforio P., Dammacco F. Th1 polarization of the immune response in Behçet’s disease. A putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999;42:1967–1974. doi: 10.1002/1529-0131(199909)42:9<1967::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 34.Pay S., Şimşek I., Erdem H., Dinç A. Immunopathogenesis of Behçet’s disease with special emphasize on the possible role of antigen presenting cells. Rheumatol. Int. 2007;27:417–424. doi: 10.1007/s00296-006-0281-6. [DOI] [PubMed] [Google Scholar]
- 35.Neves F.S., Spiller F. Possible mechanisms of neutrophil activation in Behçet’s disease. Int. Immunopharm. 2013;17:1206–1210. doi: 10.1016/j.intimp.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Hamzaoui K., Hamzaoui A., Guemira F., Bessioud M., Hamza M., Ayed K. Cytokine profile in Behçet’s disease patients: relationship with disease activity. Scand. J. Rheumatol. 2002;31:205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 37.Perazzio S.F., Soeiro-Pereira P.V., de Souza A.W.S., Condino-Neto A., Andrade L.E.C. Behçet’s disease heterogeneity: cytokine production and oxidative burst of phagocytes are altered in patients with severe manifestations. Clin. Exp. Rheumatol. 2015;33:85–95. [PubMed] [Google Scholar]
- 38.Sugi-Ikai N., Nakazawa M., Nakamura S., Ohno S., Minami M. Increased frequencies of interleukin-2- and interferon-γ-producing T cells in patients with active Behçet’s disease. Invest. Ophthalmol. Vis. Sci. 1998;39:996–1004. [PubMed] [Google Scholar]
- 39.Yosipovitch G., Shohat B., Bshara J., Wysenbeek A., Weinberger A. Elevated serum interleukin 1 receptors and interleukin 1B in patients with Behçet’s disease: correlations with disease activity and severity. Isr. J. Med. Sci. 1995;31:345–348. [PubMed] [Google Scholar]
- 40.Akkurt Z.M., Bozkurt M., Uçmak D., Yüksel H., Uçak H., Sula B., Özkurt Z.G., Yildiz M., Akdeniz D., Arica M. Serum cytokine levels in Behçet’s disease. J. Clin. Lab. Anal. 2015;29:317–320. doi: 10.1002/jcla.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambrose N., Khan E., Ravindran R., Lightstone L., Abraham S., Botto M., Johns M., Haskard D.O. The exaggerated inflammatory response in Behçet’s syndrome: identification of dysfunctional post-transcriptional regulation of the IFN-γ/CXCL10 IP-10 pathway. Clin. Exp. Immunol. 2015;181:427–433. doi: 10.1111/cei.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko T.-M., Kuo H.-C., Chang J.-S., Chen S.-P., Liu Y.-M., Chen H.-W., Tsai F.-J., Lee Y.-C., Chen C.-H., Wu J.-Y., Chen Y.-T. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ. Res. 2015;116:876–883. doi: 10.1161/CIRCRESAHA.116.305834. [DOI] [PubMed] [Google Scholar]
- 43.Vitale A., Rigante D., Lopalco G., Selmi C., Galeazzi M., Iannone F., Cantarini L. Interleukin-1 inhibition in Behçet’s disease. Isr. Med. Assoc. J. 2016;18:171–176. [PubMed] [Google Scholar]
- 44.Dayer J.-M., Oliviero F., Punzi L. A brief history of IL-1 and IL-1 Ra in Rheumatology. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richette P., François M., Vicaut E., Fitting C., Bardin T., Corvol M., Savouret J.F., Rannou F. A high interleukin 1 receptor antagonist/IL-1β ratio occurs naturally in knee osteoarthritis. J. Rheumatol. 2008;35:1650–1654. [PubMed] [Google Scholar]
- 46.Mizuno K., Toma T., Tsukiji H., Okamoto H., Yamazaki H., Ohta K., Ohta K., Kasahara Y., Koizumi S., Yachie A. Selective expansion of CD16highCCR2- subpopulation of circulating monocytes with preferential production of haem oxygenase (HO)-1 in response to acute inflammation. Clin. Exp. Immunol. 2005;142:461–470. doi: 10.1111/j.1365-2249.2005.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fingerle-Rowson G., Auers J., Kreuzer E., Fraunberger P., Blumenstein M., Ziegler-Heitbrock L.H.W. Expansion of CD14+CD16+ monocytes in critically ill cardiac surgery patients. Inflammation. 1998;22:367–379. doi: 10.1023/A:1022316815196. [DOI] [PubMed] [Google Scholar]
- 48.Horelt A., Belge K., Steppich B., Prinz J., Ziegler-Heitbrock L. The CD14+CD16+ monocytes in erysipelas are expanded and show reduced cytokine production. Eur. J. Immunol. 2002;32:1319–1327. doi: 10.1002/1521-4141(200205)32:5<1319::AID-IMMU1319>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 49.Kose O., Stewart J., Waseem A., Lalli A., Fortune F. Expression of cytokeratins, adhesion and activation molecules in oral ulcers of Behçet’s disease. Clin. Exp. Dermatol. 2008;33:62–69. doi: 10.1111/j.1365-2230.2007.02558.x. [DOI] [PubMed] [Google Scholar]
- 50.Islam S.M.S., Kim H.A., Choi B., Jung J.Y., Lee S.M., Suh C.H., Sohn S. Differences in expression of human leukocyte antigen class II subtypes and T cell subsets in Behçet’s disease with Arthritis. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gül A., Esin S., Dilsen N., Koniçe M., Wigzell H., Biberfeld P. Immunohistology of skin pathergy reaction in Behçet’s disease. Br. J. Dermatol. 1995;132:901–907. doi: 10.1111/j.1365-2133.1995.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 52.Naranjo-Gómez J.S., Castillo J.A., Rojas M., Restrepo B.N., Diaz F.J., Velilla P.A., Castaño D. Different phenotypes of non-classical monocytes associated with systemic inflammation, endothelial alteration and hepatic compromise in patients with dengue. Immunology. 2019;156:147–163. doi: 10.1111/imm.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel V.K., Williams H., Stephen C.H.L., Fletcher J.P., Medbury H.J. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis. 2017;263:15–23. doi: 10.1016/j.atherosclerosis.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 54.Sanmarco L.M., Eberhardt N., Ponce N.E., Cano R.C., Bonacci G., Aoki M.P. New insights into the immunobiology of mononuclear phagocytic cells and their relevance to the pathogenesis of cardiovascular diseases. Front. Immunol. 2018;8 doi: 10.3389/fimmu.2017.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeno M., Kariyone A., Yamashita N., Takiguchi M., Mizushima Y., Kaneoka H., Sakane T. Excessive function of peripheral blood neutrophils from patients with behcet’s disease and from HLA-B51 transgenic mice. Arthritis Rheum. 1995;38:426–433. doi: 10.1002/art.1780380321. [DOI] [PubMed] [Google Scholar]
- 56.Freitas J.P., Filipe P., Yousefi A., Emerit I., Rodrigo F.G. Oxidative stress in adamantiades-behçet’s disease. Dermatology. 1998;197:343–348. doi: 10.1159/000018029. [DOI] [PubMed] [Google Scholar]
- 57.Mege J., Dilsen N., Sanguedolce V., Gul A., Bongrand P., Roux H., Ocal L., Inanç M., Capo C. Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behçet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J. Reumatology. 1993;20:1544–1549. [PubMed] [Google Scholar]
- 58.Zeidan M.J., Saadoun D., Garrido M., Klatzmann D., Six A., Cacoub P. Behçet’s disease physiopathology: a contemporary review. Autoimmunity Highlights. 2016;7 doi: 10.1007/s13317-016-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter C., Park J.W., Ames B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Batandier C., Leverve X., Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J. Biol. Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 61.Stephenson D.J., Hoeferlin L.A., Chalfant C.E. Lipidomics in translational research and the clinical significance of lipid-based biomarkers. Transl. Res. 2017;189:13–29. doi: 10.1016/j.trsl.2017.06.006.Lipidomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirano Y., Gao Y.G., Stephenson D.J., Vu N.T., Malinina L., Simanshu D.K., Chalfant C.E., Patel D.J., Brown R.E. Structural basis of phosphatidylcholine recognition by the c2–domain of cytosolic phospholipase A2α. ELife. 2019;8 doi: 10.7554/eLife.44760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soubhye J., van Antwerpen P., Dufrasne F. Targeting cytosolic Phospholipase A2a for novel anti-inflammatory agents. Curr. Med. Chem. 2018;25 doi: 10.2174/0929867325666180117103919. [DOI] [PubMed] [Google Scholar]
- 64.Alpsoy E., Kodelja V., Goerdt S., Orfanos C.E., Zouboulis C.C. Serum of patients with Behçet’s disease induces classical (pro-inflammatory) activation of human macrophages in vitro. Dermatology. 2003;206:225–232. doi: 10.1159/000068888. [DOI] [PubMed] [Google Scholar]
- 65.Murakami M., Nakatani Y., Atsumi G., Inoue K., Kudoa I. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 2017;37:127–195. doi: 10.1016/j.bbalip.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Rainsford K.D. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- 67.Valcárcel-Ares M.N., Vaamonde-García C., Riveiro-Naveira R.R., Lema B., Blanco F.J., López-Armada M.J. A novel role for mitochondrial dysfunction in the inflammatory response of rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:A56. [Google Scholar]
- 68.Figueiredo-Pereira M.E., Rockwell P., Schmidt-Glenewinkel T., Serrano P. Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front. Mol. Neurosci. 2015;7 doi: 10.3389/fnmol.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kar R., Mishra N., Singha P.K., Venkatachalam M.A., Saikumar P. Mitochondrial remodeling following fission inhibition by 15d-PGJ2 involves molecular changes in mitochondrial fusion protein OPA1. Biochem. Biophys. Res. Commun. 2010;399:548–554. doi: 10.1016/j.bbrc.2010.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melser S., Lavie J., Bénard G. Mitochondrial degradation and energy metabolism. Biochim. Biophys. Acta. 2015;1853:2812–2821. doi: 10.1016/j.bbamcr.2015.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.