Abstract
Background
The prevalence of gestational diabetes mellitus (GDM) is increasing, with approximately 15% of pregnant women affected worldwide, varying by country, ethnicity and diagnostic thresholds. There are associated short‐ and long‐term health risks for women and their babies.
Objectives
We aimed to summarise the evidence from Cochrane systematic reviews on the effects of interventions for preventing GDM.
Methods
We searched the Cochrane Database of Systematic Reviews (6 August 2019) with key words ‘gestational diabetes’ OR ’GDM’ to identify reviews pre‐specifying GDM as an outcome. We included reviews of interventions in women who were pregnant or planning a pregnancy, irrespective of their GDM risk status. Two overview authors independently assessed eligibility, extracted data and assessed quality of evidence using ROBIS and GRADE tools. We assigned interventions to categories with graphic icons to classify the effectiveness of interventions as: clear evidence of benefit or harm (GRADE moderate‐ or high‐quality evidence with a confidence interval (CI) that did not cross the line of no effect); clear evidence of no effect or equivalence (GRADE moderate‐ or high‐quality evidence with a narrow CI crossing the line of no effect); possible benefit or harm (low‐quality evidence with a CI that did not cross the line of no effect or GRADE moderate‐ or high‐quality evidence with a wide CI); or unknown benefit or harm (GRADE low‐quality evidence with a wide CI or very low‐quality evidence).
Main results
We included 11 Cochrane Reviews (71 trials, 23,154 women) with data on GDM. Nine additional reviews pre‐specified GDM as an outcome, but did not identify GDM data in included trials. Ten of the 11 reviews were judged to be at low risk of bias and one review at unclear risk of bias. Interventions assessed included diet, exercise, a combination of diet and exercise, dietary supplements, pharmaceuticals, and management of other health problems in pregnancy. The quality of evidence ranged from high to very low.
Diet
Unknown benefit or harm: there was unknown benefit or harm of dietary advice versus standard care, on the risk of GDM: risk ratio (RR) 0.60, 95% CI 0.35 to 1.04; 5 trials; 1279 women; very low‐quality evidence. There was unknown benefit or harm of a low glycaemic index diet versus a moderate‐high glycaemic index diet on the risk of GDM: RR 0.91, 95% CI 0.63 to 1.31; 4 trials; 912 women; low‐quality evidence.
Exercise
Unknown benefit or harm: there was unknown benefit or harm for exercise interventions versus standard antenatal care on the risk of GDM: RR 1.10, 95% CI 0.66 to 1.84; 3 trials; 826 women; low‐quality evidence.
Diet and exercise combined
Possible benefit: combined diet and exercise interventions during pregnancy versus standard care possibly reduced the risk of GDM: RR 0.85, 95% CI 0.71 to 1.01; 19 trials; 6633 women; moderate‐quality evidence.
Dietary supplements
Clear evidence of no effect: omega‐3 fatty acid supplementation versus none in pregnancy had no effect on the risk of GDM: RR 1.02, 95% CI 0.83 to 1.26; 12 trials; 5235 women; high‐quality evidence.
Possible benefit: myo‐inositol supplementation during pregnancy versus control possibly reduced the risk of GDM: RR 0.43, 95% CI 0.29 to 0.64; 3 trials; 502 women; low‐quality evidence.
Possible benefit: vitamin D supplementation versus placebo or control in pregnancy possibly reduced the risk of GDM: RR 0.51, 95% CI 0.27 to 0.97; 4 trials; 446 women; low‐quality evidence.
Unknown benefit or harm: there was unknown benefit or harm of probiotic with dietary intervention versus placebo with dietary intervention (RR 0.37, 95% CI 0.15 to 0.89; 1 trial; 114 women; very low‐quality evidence), or probiotic with dietary intervention versus control (RR 0.38, 95% CI 0.16 to 0.92; 1 trial; 111 women; very low‐quality evidence) on the risk of GDM. There was unknown benefit or harm of vitamin D + calcium supplementation versus placebo (RR 0.33, 95% CI 0.01 to 7.84; 1 trial; 54 women; very low‐quality evidence) or vitamin D + calcium + other minerals versus calcium + other minerals (RR 0.42, 95% CI 0.10 to 1.73; 1 trial; 1298 women; very low‐quality evidence) on the risk of GDM.
Pharmaceutical
Possible benefit: metformin versus placebo given to obese pregnant women possibly reduced the risk of GDM: RR 0.85, 95% CI 0.61 to 1.19; 3 trials; 892 women; moderate‐quality evidence.
Unknown benefit or harm:eight small trials with low‐ to very low‐quality evidence showed unknown benefit or harm for heparin, aspirin, leukocyte immunisation or IgG given to women with a previous stillbirth on the risk of GDM.
Management of other health issues
Clear evidence of no effect: universal versus risk based screening of pregnant women for thyroid dysfunction had no effect on the risk of GDM: RR 0.93, 95% CI 0.70 to 1.25; 1 trial; 4516 women; moderate‐quality evidence.
Unknown benefit or harm: there was unknown benefit or harm of using fractional exhaled nitrogen oxide versus a clinical algorithm to adjust asthma therapy on the risk of GDM: RR 0.74, 95% CI 0.31 to 1.77; 1 trial; 210 women; low‐quality evidence. There was unknown benefit or harm of pharmacist led multidisciplinary approach to management of maternal asthma versus standard care on the risk of GDM: RR 5.00, 95% CI 0.25 to 99.82; 1 trial; 58 women; low‐quality evidence.
Authors' conclusions
No interventions to prevent GDM in 11 systematic reviews were of clear benefit or harm. A combination of exercise and diet, supplementation with myo‐inositol, supplementation with vitamin D and metformin were of possible benefit in reducing the risk of GDM, but further high‐quality evidence is needed. Omega‐3‐fatty acid supplementation and universal screening for thyroid dysfunction did not alter the risk of GDM. There was insufficient high‐quality evidence to establish the effect on the risk of GDM of diet or exercise alone, probiotics, vitamin D with calcium or other vitamins and minerals, interventions in pregnancy after a previous stillbirth, and different asthma management strategies in pregnancy. There is a lack of trials investigating the effect of interventions prior to or between pregnancies on risk of GDM.
Plain language summary
Interventions to prevent women from developing diabetes during pregnancy: an overview of Cochrane systematic reviews
What is the issue?
Gestational diabetes mellitus (GDM) is defined as high blood glucose levels (hyperglycaemia) first detected during pregnancy. GDM can affect the health of women and their babies.
During pregnancy a woman’s body changes how it processes the nutrients from her food, to ensure that the baby is well nourished. In the first three months the mother has increased sensitivity to insulin. In the second and third trimesters her insulin sensitivity is reduced. Women with GDM have less of an initial increase in sensitivity and their insulin sensitivity is reduced beyond normal later in pregnancy, resulting in the mother developing high blood glucose levels. Her blood levels of fats are also higher than normal, which may contribute to the risk of the baby becoming large for its gestational age.
Why is this important?
Women with GDM are more likely to develop complications in pregnancy including high blood pressure and need labour to be induced. They are at increased risk later of developing type 2 diabetes. Babies born to women with GDM are more likely to be born large, and therefore to experience birth injuries. Once born, the babies are at higher risk of experiencing difficulties in breathing, jaundice and reduced blood sugar levels, and later obesity and diabetes.
There are many risk factors for GDM, making it likely that interventions before/during pregnancy could reduce the risk of women developing GDM. This overview summarises evidence from Cochrane Reviews of randomised controlled trials on interventions that might prevent GDM.
What evidence did we find?
We searched the Cochrane Library (August 2019) and identified 11 Cochrane Reviews that assessed interventions during pregnancy and reported on GDM. The reviews had findings from 71 randomised controlled trials involving 23,154 pregnant women. Interventions included diet, exercise, a combination of diet and exercise, dietary supplements, medications, and management of other health problems. The evidence from the trials ranged from very low to high quality. We identified a further 10 reviews that may provide more information on this topic in the future.
Diet and exercise
Diet and exercise together possibly reduced the risk of a woman developing GDM when compared to standard care (19 trials; 6633 women; moderate‐quality evidence).
Dietary advice alone (5 trials; 1279 women; very low‐quality evidence) and a low glycaemic index diet compared with a moderate to high glycaemic index diet (4 trials; 912 women; low‐quality evidence) had an unclear effect on the risk of GDM. Exercise alone had an unclear effect on the risk of GDM (3 trials; 826 women; low‐quality evidence).
Dietary supplements
Omega‐3 fatty acid supplementation in pregnancy had no effect (12 trials; 5235 women; high‐quality evidence).
Myo‐inositol supplementation during pregnancy possibly reduced the risk of GDM (3 trials with 502 women; low‐quality evidence).
Vitamin D supplementation in pregnancy had a possible benefit in reducing the risk of developing GDM (4 trials with 446 women; low‐quality evidence). These trials were all from Asian countries and the women’s vitamin D levels before supplementation were mostly unknown.
Vitamin D given with calcium supplementation, or with calcium plus other minerals had an unclear effect.
Probiotic with dietary intervention had an unclear effect on the risk of developing GDM.
Medications
The drug metformin had a possible benefit in reducing the risk of developing GDM when given to obese pregnant women (3 trials; 892 women; moderate‐quality evidence).
Low‐ to very low‐quality evidence from eight small trials showed unclear effect on GDM risk for heparin, aspirin, leukocyte immunisation or immunoglobulin (IgG) given to women who had previously experienced a stillbirth.
Management of other health issues
Universal versus risk‐based screening for thyroid problems had no effect on the risk of GDM (1 trial; 4516 women; moderate‐quality evidence). Two different approaches to management of the mothers’ asthma had an unclear effect (low‐quality evidence).
What does this mean?
A combination of exercise and diet, supplementation with myo‐inositol and vitamin D supplementation were of possible benefit in reducing the risk of developing GDM. Further high‐quality evidence from randomised controlled trials is needed to confirm these results, and to look further at the use of metformin. No trials assessed interventions before pregnancy.
Background
Description of the condition
Gestational diabetes mellitus (GDM) is glucose intolerance causing hyperglycaemia with onset during pregnancy. The optimal blood glucose concentration cut‐off to diagnose GDM remains controversial (ACOG 2018; Cheung 2018; Coustan 2010; HAPO 2008; IADPSG 2010; Ministry of Health 2014; NICE 2015). Lower blood glucose concentration thresholds for diagnosis have resulted in more women being diagnosed with GDM (IADPSG 2010; Nankervis 2014; WHO 2013). The prevalence of GDM varies internationally with approximately 15% of pregnant women affected (Bottalico 2007; Egan 2017; Ferrara 2007; Guariguata 2014; McIntyre 2018; Melchior 2017; NICE 2015). Prevalence varies by country, ethnicity and the diagnostic thresholds (Farrar 2016; HAPO 2008; Pu 2015).
Normal pregnancy is characterised by changes in the metabolism of carbohydrate, amino acids and lipids. The result of these combined changes is a switch to maternal use of lipids as a source of energy, sparing glucose and amino acids for the fetus.
In the first trimester of pregnancy there is increased insulin sensitivity (Catalano 1991; Catalano 1992; Catalano 1993) as a result of adaptation of the pancreatic ß‐cells (Van Assche 1978), increased insulin synthesis (Weinhaus 1996) and secretion (Sorenson 1993), leading to improved utilisation and oxidation of glucose. By 14 weeks’ gestation, the first phase of insulin secretion in response to a glucose load has increased by approximately 120% (Bowes 1996; Catalano 1993), resulting in a reduced plasma glucose concentration (Catalano 1992). In the second and third trimesters, insulin sensitivity reduces (Catalano 1991; Catalano 1992; Catalano 1993; Ryan 1985), and hepatic gluconeogenesis increases (Nelson 1994).
In women with GDM, the increase in first phase insulin secretion in response to a glucose load is reduced (Catalano 1993), with inadequate adaptation of ß‐cells leading to impaired glucose homeostasis (Buchanan 2001). In the second and third trimesters the insulin sensitivity is further reduced (Catalano 1998) with resultant maternal hyperglycaemia, elevated glycated haemoglobin (HbA1c) concentrations and increased transport of glucose across the placenta to the developing fetus (Setji 2005).
During normal pregnancy, changes in adipocytes result in more fat stores being laid down with increased synthesis and reduction in clearance of triglycerides (Ginci 1997), resulting in an increase in all plasma lipid components (Butte 2000). Women with GDM have hyperlipidaemia (raised levels of lipids in the blood) beyond that seen in normal pregnancy (Koukkou 1996) due to increased synthesis in the liver and reduced activity of lipoprotein lipase and hepatic lipase (Ginci 1997; Sattar 1997). Higher concentrations of free fatty acids cross the placenta than in normal pregnancy (Larqué 2011), which may contribute to the risk of macrosomia (large baby) (Knopp 1992).
There are multiple risk factors for GDM (Berkowitz 1992;Chu 2007; Khan 2013;Pu 2015; Solomon 1997;Theriault 2014;Xiong 2001). These include advanced maternal age, maternal high or low birthweight, high parity (Petry 2010), polycystic ovarian syndrome (Toulis 2009), a past history of GDM (Kim 2007), family history of first‐degree relatives with GDM or type 2 diabetes (Petry 2010), maternal overweight or obesity (Morisset 2010; Torloni 2009), physical inactivity before or in early pregnancy (Dempsey 2004; Tobias 2011; Zhang 2014), gestational weight gain (Morisset 2010), and a past history of a macrosomic baby or a stillbirth (Petry 2010). Impaired glucose tolerance is common to many of these risk factors, but the exact mechanisms by which each contributes to the development of GDM are uncertain. Some of these risk factors are potentially modifiable through preventive interventions.
Women with GDM have a higher risk of pre‐eclampsia and need for induction of labour (Dodd 2007). Women with a history of GDM have a greater than seven‐fold increased risk of developing type 2 diabetes later, with more than half these women developing type 2 diabetes within 10 years after giving birth (Bellamy 2009; Kim 2002). Infants born to mothers with GDM are at increased risk of being born large‐for‐gestational age (Sacks 2015), and are therefore more likely to experience birth injuries such as nerve palsy, bone fracture and shoulder dystocia. In the neonatal period, they are at higher risk of respiratory distress syndrome, jaundice and hypoglycaemia (reduced levels of blood sugar) (Adams 1998; Crowther 2005; Gonzalez‐Quintero 2007; He 2015; Landon 2009; Langer 2005). Longer‐term health consequences into childhood and adulthood include obesity, diabetes, the metabolic syndrome (Boney 2005; Cho 2000), and adverse neurodevelopmental outcomes (Chatzi 2014; Fraser 2012; Nelson 2000; Torres‐Espinola 2015).
Description of the interventions
Interventions to prevent GDM have been used preconception, during pregnancy and inter‐conception (between pregnancies). Preconception and inter‐conception interventions have been used, particularly in women at high risk of GDM, such as those who are overweight or obese (Yeung 2010), or with a history of GDM (Khan 2013; Shyam 2013). The opportunity exists to intervene with health promotion strategies prior to and between pregnancies for women identified with risk factors for GDM (Hanson 2015; Jack 1990).
Interventions directed at preventing GDM include dietary or exercise interventions, or a combination of these, dietary supplement interventions and pharmaceutical interventions.
The focus of some dietary advice interventions for GDM prevention have been specific, such as increasing fibre intake (Fraser 1983; Fraser 1988) or aiming for a low glycaemic index diet (Kizirian 2017; Markovic 2015). Others have included broader advice regarding “healthy eating” as part of more comprehensive lifestyle interventions (Quinlivan 2011).
Exercise or physical activity interventions for preventing GDM have varied from general advice to specific individualised programs using a range of different activities, such as aerobic activities, stationary cycling or yoga (Barakat 2012; Guelfi 2016; Ong 2009; Rakhshani 2012; Stafne 2012). These have been employed in isolation (Barakat 2012; da Silva 2017; Goodarzi‐Khoigani 2017; Guelfi 2016; Ong 2009; Stafne 2012), or in combination with dietary interventions (Dodd 2014; Harrison 2013; Koivusalo 2016; Luoto 2011; Petrella 2014; Poston 2015; Simmons 2015).
Dietary supplement interventions such as probiotics (Lindsay 2014; Luoto 2010; Wickens 2017), myo‐inositol (D'Anna 2013; Farren 2017; Santamaria 2016), vitamin D (Bao 2017; Soheilykhah 2013), and fish oils have been investigated for GDM prevention (Zhou 2012).
Pharmaceutical therapies, which may have a role in GDM prevention, include sulphonylureas, biguanides, thiazolidinediones, alpha‐glucosidase inhibitors, meglitinides and peptide analogues. Metformin or glibenclamide (also known as glyburide) are the only oral hypoglycaemics recommended in clinical practice guidelines for use in pregnancy (ACOG 2018; NICE 2015). However, there is a paucity of data regarding the safety of many of these in pregnancy (Holt 2014; Kavitha 2013; Slocum 2002). Where safety data are available, this has often been limited to short‐term health outcomes. The Metformin in Gestational diabetes (MiG) trial investigated the use of metformin for women with GDM, and demonstrated metformin was safe in the offspring, at least up to nine years of age (Rowan 2018).
How the intervention might work
Dietary interventions
Different dietary components have direct and indirect effects on glycaemic profile. Interventions that alter these could be utilised to reduce GDM risk (Ley 2011; Rogozinska 2015; Zhang 2006). Insulin sensitivity and secretion are reduced in association with high simple sugar intake (Davis 2005; Reiser 1979). With ongoing high sugar intake, pancreatic exhaustion may ensue with impaired glucose tolerance (Ludwig 2002). Less insulin resistance is seen with a high‐fibre and low‐glycaemic index diet. Fibre slows digestion (Burton‐Freeman 2000; Jenkins 2000; Vahouny 1988) and rate of glucose absorption, thus altering the blood glucose concentration and insulin response (Jenkins 2000; Liese 2005; Mcintosh 2001). Increasing dietary fibre intake may reduce appetite and hence insulin resistance associated with adiposity (Burton‐Freeman 2000). Intake of protein and fats may also reduce appetite (Tannous dit El Khoury 2006) with similar effect (Kantartzis 2009; Kohrt 1993; Pan 1993), and through protection of ß‐cells from oxidative injury (Cai 2012; Lin 2012). A general reduction in calorie intake with resultant weight loss and reduced adiposity improves insulin sensitivity and glycaemic profile (Knopp 1991; Larson‐Meyer 2006). This needs to balanced against potential risks of weight loss during pregnancy such as ketonaemia associated with marked calorie restriction (Churchill 1969;Magee 1990;Metzger 2007; Ornoy 1998;Rizzo 1991). Due to these concerns, international guidelines do not recommend hypocaloric diets during pregnancy (Ireland 2010; NICE 2010; Thompson 2013). There is ongoing debate as to whether calorie restriction might be appropriate in overweight and obese pregnant women (Knopp 1991; Procter 2014). Interventions to aid with weight loss in overweight or obese women preconception or inter‐conception may reduce the risk of GDM in any future pregnancy (Pole 1999).
Exercise interventions
The risk of developing GDM is inversely associated with the amount of regular physical activity before or during pregnancy (Dempsey 2004; Zhang 2014). There is increased energy expenditure and hence glucose consumption during exercise; blood flow through muscle mass and capillary surface area for glucose exchange increases (Rose 2005; Sjøberg 2011). During muscle contraction, there is translocation of the glucose transporter type 4 (GLUT‐4) from within skeletal muscle cells to the surface (Jessen 2005; Kennedy 1999; Rose 2005) with resultant increased glucose uptake. The increase in insulin sensitivity continues beyond the exercise period (Jensen 2012; Perseghin 1996). Muscle mass increases with regular physical activity, and thus glucose tolerance and insulin sensitivity are likely to improve (Yki‐Jarvinen 1983).
Diet and exercise interventions combined
Combined interventions targeting more than one of the multiple risk factors for GDM could be synergistic. Prevention of type 2 diabetes has been demonstrated using combined dietary, exercise and weight loss interventions (Haw 2017; Knowler 2002; Tuomilehto 2001). These might be expected to have a similar effect in prevention of GDM.
Dietary supplement interventions
Probiotics
Probiotic use can change the microbiome of the gut, which may reduce insulin resistance (FAO/WHO 2001; Hill 2014; Kondo 2010), through decreasing inflammatory signalling (Ma 2008), and upregulating genes involved in fat metabolism and insulin sensitivity (Kondo 2010).
Myo‐inositol
Myo‐inositol, a polyol with insulin‐mimetic properties (Croze 2013; Saltiel 1990) involved in insulin transduction signalling (Baillargeon 2010), increases GLUT‐4 translocation to the cell membrane in skeletal muscle (Dang 2010), thus improving insulin sensitivity (Corrado 2011). Supplementary use in polycystic ovarian syndrome results in reduced fasting insulin concentrations (Unfer 2017). Myo‐inositol is present in the diet in some seeds, grains, nuts, beans, vegetables and fruit (Clements 1980).
Vitamin D
Vitamin D deficiency is associated with insulin resistance (Esteghamati 2014) and poor pancreatic β‐cell function (Chiu 2004). Vitamin D may affect insulin secretion by binding to vitamin D receptors in the pancreas and regulating the balance between the extracellular and intracellular calcium pools (Sooy 1999). Vitamin D deficiency may reduce pancreatic insulin secretion (Bourlon 1999; Norman 1980), while supplementation with vitamin D may influence the expression of insulin‐sensitive genes (Alkharfy 2013), thus reducing inflammatory markers and improving glucose uptake (Marcotorchino 2012).
Fish oil
The circulating concentrations of several long‐chain polyunsaturated fatty acids are altered in GDM (Wijendran 1999). Omega‐3 fatty acids have several anti‐inflammatory effects (Calder 2006). The predominant sources of the omega‐3 fatty acids, eicosapentaenoic acid and docosahexaenoic acid, are fish and fish oils (Kris‐Etherton 2000; Kris‐Etherton 2009). The lipid composition of cell membranes is altered with changes to dietary fatty acid composition (Calder 2006; Lardinois 1987), which affects insulin binding and sensitivity. Increased insulin secretion and sensitivity may result from omega‐3 or fish oil supplementation (Baynes 2018). Increased inflammation can result in insulin resistance, while omega‐3 fatty acids inhibit TLR‐2 and TLR‐4 receptors for inflammatory cytokines (Lee 2004).
Pharmaceutical interventions
Metformin, a biguanide, crosses the placenta with similar concentrations found in maternal and fetal circulations (Vanky 2005). Metformin reduces hepatic gluconeogenesis (Stumvoll 1995; Wollen 1988), and enhances peripheral glucose uptake and utilisation (Viollet 2012), improving insulin sensitivity and reducing hyperglycaemia (Jackson 1987). Metformin may enhance insulin sensitivity and preserve pancreatic β‐cell capacity in women with polycystic ovarian syndrome (Ainuddin 2015). Glibenclamide crosses the placenta with fetal blood concentrations approximately 70% of maternal blood concentrations (Hebert 2009). Glibenclamide stimulates insulin secretion, but has been associated with an increased risk of macrosomia, neonatal hypoglycaemia and higher maternal weight gain in comparison to metformin (Liang 2017).
Why it is important to do this overview
A number of risk factors for GDM, such as physical inactivity, being overweight or obese prior to pregnancy, and having a poor diet are potentially modifiable. While different strategies have shown promise in the prevention of GDM, it is currently unclear which strategies are most effective. Primary prevention of GDM rather than treatment would lead to economic (Danyliv 2014) and health benefits.
This overview provides an important resource for all healthcare professionals caring for pregnant women, guideline developers, policy makers, researchers, and pregnant women at risk of developing GDM, and their families.
Use of the overview to identify and target effective preventive interventions may contribute to reducing the increase in rates of GDM seen globally, thus reducing the significant short‐ and long‐term health risks for the mothers and their infants. Further, this overview identifies priority areas requiring further research.
Objectives
We aimed to summarise the evidence from Cochrane systematic reviews on the effects of interventions for preventing gestational diabetes mellitus (GDM).
Methods
Criteria for considering reviews for inclusion
In this overview of systematic reviews, we included only Cochrane systematic reviews, that assessed interventions that may prevent gestational diabetes mellitus (GDM), reporting GDM as a primary or secondary outcome of the review. We identified Cochrane protocols and titles for potential future inclusion in an update of the overview, and classified them as ’ongoing reviews’ (in Appendix 1).
When reviews were identified for inclusion that were more than two years out of date, we contacted the Cochrane Pregnancy and Childbirth Editorial Base to identify whether an update was in progress. We did not contact individual review authors, but noted the publications and search dates of the reviews. When a review was out of date and would not be updated in time to be included in the overview, we included the last published version and acknowledged this as a potential limitation.
Participants
We included women planning a pregnancy, between pregnancies, or pregnant women. We excluded women with pre‐existing type 1 or type 2 diabetes.
Interventions
We included interventions prior to pregnancy (preconception), between pregnancies (inter‐conception), or implemented prior to GDM screening in pregnancy, and for each of these time periods these included:
dietary interventions;
exercise interventions;
dietary and exercise interventions combined;
dietary supplement interventions (e.g. probiotics, myo‐inositol, vitamin D and omega‐3 fatty acids);
pharmaceutical interventions (e.g. oral anti‐diabetic pharmaceutical therapies);
interventions for the management of other health problems during pregnancy.
We included reviews comparing the above interventions with standard care or no intervention (or a placebo), as well as those comparing different interventions.
Outcome
GDM (defined by review authors and trialists).
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews (6 August 2019) using key words ‘gestational diabetes’ OR ’GDM’. We used the search terms to search ’all text’, and did not limit to ’title, abstract, or keywords’. We did not apply any language or date restrictions. The review group was contacted to identify titles for future inclusion and no additional titles were identified.
Data collection and analysis
We based the methodology for data collection and synthesis on Chapter 22, 'Overviews of Reviews' in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Selection of reviews
Two overview authors independently assessed for potential inclusion all Cochrane systematic reviews we identified that evaluated the effects of the aforementioned interventions and reported on the incidence of GDM. We then assessed the methods section of reviews and protocols to ensure those with the appropriate population and pre‐specified outcome were selected for inclusion. We resolved any disagreement through discussion or consultation with a third overview author.
Data extraction and management
Two of the overview authors independently extracted data from each systematic review, using an electronic form which we designed and piloted. We resolved disagreements by consensus or by discussion with a third overview author. When information from the review was missing, we accessed the published papers of the individual study and contacted the systematic review authors for further details. We extracted and tabulated information for the following.
Review characteristics
Review title and authors.
Search date: date of search conducted by review (we considered less than two years ago to be current).
The number of trials in the review, number of women and their infants, and their characteristics.
Risk of bias of the included trials (as reported by the review authors; see ’Risk of bias of included studies within reviews’ below, under Assessment of methodological quality of included reviews).
Interventions and comparisons relevant to this overview.
The prespecified outcome (GDM) relevant to this overview.
Any other characteristics required to assess and report on review quality (see 'Quality of included reviews' under Assessment of methodological quality of included reviews).
Statistical summaries
The summary intervention effects, including the pooled effects (e.g. risk ratios (RRs), odds ratios (ORs), mean differences (MDs), 95% confidence intervals (CIs)), and numbers of studies and participants contributing data to each pooled effect from comparisons and for the outcome relevant to this overview.
Results of any subgroup or sensitivity analyses conducted by the authors for overview outcome.
Information required to assess and report on the quality of the evidence for the intervention effects extracted above (see ’Quality of evidence in included reviews’ under Assessment of methodological quality of included reviews).
All reviews performed meta‐analyses for GDM, the outcome for our overview.
Assessment of methodological quality of included reviews
Quality of included reviews
Two overview authors assessed for relevance, by checking that the population, intervention, comparator and outcomes aligned between the review and overview, and independently assessed the methodological quality of the included systematic reviews using the Risk of Bias in Systematic Reviews (ROBIS) tool (Whiting 2015). We resolved differences through discussion and, when needed, through discussion with a third overview author.
The ROBIS tool assesses risk of bias across four domains.
Study eligibility criteria.
Identification and selection of studies.
Data collection and study appraisal.
Synthesis and findings.
ROBIS uses signalling questions to assess specific concerns about potential biases within the review, and the ratings from these questions are used to judge overall risk of bias. The signalling questions were answered as ’yes’, ’probably yes’, ’probably no’, ’no’ or ’no information’. Each of these four domains were then designated as low, high or unclear risk of bias. If the answers to all signalling questions for a domain were ’yes’ or ’probably yes’, the level of concern could be judged as low. If any signalling question was answered ’no’ or ’probably no’, the potential for concern about bias exists. Finally, a summary judgement based on any concerns, assessment of methods and bias was made of low, high or unclear overall risk of bias for the systematic review.
Quality of evidence in the included reviews
We assessed the quality of the evidence for GDM using GRADE (Grades of Recommendation, Assessment, Development and Evaluation). When available, we used the GRADE assessments from the included Cochrane systematic reviews. When this was not available, we used the GRADE system to review pooled summary statistics and risk of bias of included trials. The GRADE system assesses the following features for the evidence found for selected outcomes.
Risk of bias: internal validity of the evidence.
Inconsistency: heterogeneity or variability in the estimates of effect across studies.
Indirectness: degree of differences between population, intervention and outcome of interest.
Imprecision (random error): extent to which confidence in the effect estimate is adequate to support a particular decision.
Risk of publication bias: degree of selective publication of studies.
The GRADE system rates the quality of the evidence as:
high (further research is very unlikely to change confidence in the estimate of the effect);
moderate (further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate);
low (further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate);
very low (any estimate of effect is very uncertain).
We summarised the evidence in 'Summary of findings' tables which we populated with the summary risk estimates and 95% CIs, number of participants, and the quality of the review for each intervention and whether GDM was a primary or secondary review outcome. We planned to include timing of intervention (preconception, inter‐conception and during pregnancy), but all interventions within our included reviews were during pregnancy.
Risk of bias of included studies within reviews
We did not reassess the risk of bias of included studies within reviews, but instead reported study risk of bias according to the review authors’ assessment. In the case that individual studies were included in two or more Cochrane Reviews, we report this, and any variation regarding review authors’ assessments of study risk of bias. We collected this information during the data extraction process.
Data synthesis
We undertook a narrative description of the included Cochrane systematic reviews. We did not examine indirect comparisons or conduct network meta‐analyses. We summarised the main results of the included reviews by categorising their findings in the following framework, and by intervention focus/topic. We planned to organise by timing of intervention (preconception, inter‐conception and during pregnancy), but no preconception or inter‐conception interventions were identified. We assigned graphic icons to communicate the direction of review effect estimates and our confidence in the available data. This is the framework adopted by Medley and colleagues in their overview on 'Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews' (Medley 2018), and was based on graphics produced by the World Health Organization to describe different types of workers and their roles in maternal and newborn care (http://optimizemnh.org/optimizing-health-worker-roles-maternal-newborn-health/). We used graphic icons to indicate mutually exclusive assessment categories (see Figure 1).
1.

Icon key
Clear evidence of benefit (moderate‐ or high‐quality evidence with CIs not crossing line of no effect).
Clear evidence of harm (moderate‐ or high‐quality evidence with CIs not crossing line of no effect).
Clear evidence of no effect or equivalence (moderate‐ or high‐quality evidence with narrow CIs crossing the line of no effect).
Possible benefit (low‐quality evidence with clear benefit, or moderate‐ or high‐quality evidence with wide CIs crossing the line of no effect).
Possible harm (low‐quality evidence with clear harm, or moderate‐ or high‐quality evidence with wide CIs crossing the line of no effect).
Unknown benefit or harm (low‐quality evidence with wide CIs crossing the line of no effect or very low‐quality evidence).
The choice of category reflected the information synthesised from the included reviews for the overview outcome (GDM). We used separate assessments for different comparisons when required (e.g. where one intervention was compared with both placebo (or no treatment) and with an alternative intervention). This approach to summarising the evidence is based on an earlier overview ‘Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews’ (Shepherd 2017).
We conducted a sensitivity analysis excluding Cochrane systematic reviews with a ROBIS (Risk of Bias in Systematic Reviews) review rating that was of high concern for risk of bias in any domain.
Results
Our search of the Cochrane Database of Systematic Reviews identified 129 results. After excluding six overviews, two protocols which had been withdrawn, and one review which had been superceded, we searched the text of 120 protocols and completed systematic reviews for the outcome, GDM. We assessed the full text of those protocols and reviews which pre‐specified GDM as an outcome. No overview author assessed their own systematic review (several eligible reviews were authored by members of the overview team).
We included 11 reviews in this overview. We excluded 98 reviews that included participants with pre‐existing diabetes or did not pre‐specify GDM as a review outcome and one review (Crowe 2019) for which there was no comparison group (see Figure 2).
2.
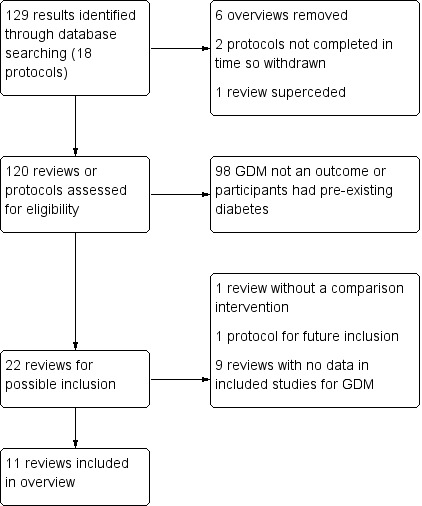
Review flow diagram.
We listed 10 protocols and reviews, that may provide data in future updates of this overview, in the appendices.
Appendix 1, Ongoing reviews, cites one Cochrane protocol that pre‐specifies GDM as a secondary outcome, and will be considered for inclusion in future updates of this overview when it is published as a full review.
Appendix 2, Reviews awaiting further classification, summarises nine Cochrane Reviews that pre‐specified GDM as a primary or secondary outcome, but the included trials had no data reported for this outcome. These reviews will be considered again for inclusion in future updates of this overview.
Description of included reviews
The 11 included systematic reviews all involved pregnant women. They included 71 trials of 23,154 women. There was one review for each of the following:
dietary advice versus standard care or different dietary advice (Tieu 2017);
any type of exercise regimen versus standard care or another type of exercise regimen (Han 2012);
any combined dietary and exercise combination versus standard care or a different dietary and exercise combination (Shepherd 2017);
omega‐3 fatty acid supplementation (supplement or food) versus placebo or no omega‐3 (Middleton 2018);
myo‐inositol versus control or placebo (Crawford 2015);
vitamin D (alone or in combination with other micronutrients) versus other micronutrients or control (Palacios 2019);
probiotics versus "any type" of comparison intervention (Barrett 2014);
metformin (alone or in combination) versus placebo or control in overweight or obese women (Dodd 2018);
any single intervention, combination of interventions or tailored model of care/algorithm/guideline/protocol for improving health outcomes in subsequent pregnancies following stillbirth versus no intervention or standard care (Wojcieszek 2018);
screening for thyroid dysfunction versus no screening or an alternative screening method for improving maternal and infant health (Spencer 2015);
interventions for managing asthma in pregnancy versus alternative interventions, or placebo or control (Bain 2014).
All the included reviews reported interventions during pregnancy. No reviews reported interventions prior to, or between pregnancies. The number of randomised trials that reported data on GDM in the included systematic reviews ranged from one to 19. The number of pregnant women in the included reviews ranged from 217 (Wojcieszek 2018) to 6633 (Spencer 2015). Only one review included trials from low‐income countries (Palacios 2019). Six reviews were published more than two years ago (Bain 2014; Barrett 2014; Crawford 2015; Han 2012; Spencer 2015; Tieu 2017). One review (Shepherd 2017), though published within the last two years had conducted the search more than two years ago. Four reviews (Dodd 2018; Middleton 2018:Palacios 2019; Wojcieszek 2018) had conducted searches in the last two years and were considered up to date. Han 2012 is undergoing an update. There are no known active updates in progress for the remaining reviews.
Further details of the included reviews can be found in Table 1 'Characteristics of included reviews'. Details of the 'Risk of bias' assessments included in each review can be found in Table 2.
1. Characteristics of included reviews.
| Review ID | Date of search; date assessed as up‐to‐date | No. included trials; years of publication and countries | No. participants in included trials | No. participants and trials with data on GDM | Inclusion criteria for “Types of participants” | Relevant comparison interventions (no. trials and participants) | GDM primary or secondary outcome in review |
| Bain 2014 | Search June 2014; Up‐to‐date October 2014 | 8 RCTs published in 1996, 1998, 2004, 2005, 2006, 2011, 2012 (2) in Brazil, USA (2), Egypt, Australia, Germany and multi country | 1181 | 268 (2) | Pregnant women with current asthma (with a health professional's diagnosis), regardless of age, parity, plurality, and severity of asthma | Any intervention to manage asthma (pharmacological/non‐pharmacological) versus placebo/no intervention/alternative intervention | Secondary |
| Barrett 2014 | Search August 2013; Up‐to‐date February 2014 | 1 RCT in 2008 in Finland | 256 | 256 (1) | Pregnant women including those with previous GDM | Probiotic in combination with diet vs diet with placebo or placebo alone | Primary |
| Crawford 2015 | Search November 2015; Up‐to‐date December 2015 | 4 RCTs in 2013 (2), 2014, 2015 in Italy | 567 | 502 (3) | Pregnant women | Any doses of myo‐inositol in pregnancy, alone or in a combination preparation versus no treatment or placebo or another intervention | Primary |
| Dodd 2018 | Search October 2017; Up‐to‐date July 2018 | 3 RCTs in 2015 (2), 2016 in Egypt and UK (2) | 1099 (data for 1034) | 892 (3) | Pregnant women with obesity or who are overweight, defined as women with booking or early pregnancy or pre‐pregnancy body mass index (BMI) ≥ 25.0 kg/m2 | Metformin versus placebo or no metformin | Secondary |
| Han 2012 | Search April 2012; Up‐to‐date July 2012 | 5 RCTs in 2009, 2010 (2), 2011, 2012 in Australia, (2), Norway, Spain, New Zealand | 1115 (data for 922) | 826 (3) | Pregnant women of any age, gestation, parity or plurality | Any types of exercise and lifestyle management versus standard care or different frequencies of same intervention or different interventions |
Primary |
| Middleton 2018 | Search 16 August 2018; up to date 14 November 2018 | 70 RCTs. 1989‐2018. Multiple countries including Bangladesh, Mexico, Venezuela | 19,927 | 5235 (12) | Pregnant women, regardless of their risk for pre‐eclampsia, preterm birth or intrauterine growth restriction. | Omega‐3 fatty acids (usually fish or algal oils) compared with placebo or no omega‐3 fatty acids. Trials that assessed omega‐3 fatty acid co‐interventions (e.g. omega‐3 with another agent) Studies or study arms that compared omega‐3 doses or types of omega‐3 (e.g. DHA versus EPA) directly |
Secondary |
| Palacios 2019 | Search July 2018; Up‐to‐date July 2019 | 30 RCTs in 1980 (1), 1986 (2), 1987 (1), 1988 (1), 1991 (1), 2000 (1), 2002 (1), 2008 (1), 2009 (1), 2010 (1), 2011 (1), 2012 (3), 2013 (6), 2014 (1), 2015 (2), 2016 (4), 2017 (2) in Australia (1), Bangladesh (2), Brazil (1), China (1), France (2), India (5), Iran (12), New Zealand (1), Pakistan (1), Russia (1) and the UK (3) | 7033 | 446 (1) | Pregnant women any gestation or chronological age, parity, number of fetuses | Vitamin D (any dose, duration, time of commencement) during pregnancy (alone or in combination with other micronutrients) versus placebo/no intervention/other vitamins or minerals | Primary |
| Shepherd 2017 | Search November 2016; Up‐to‐date November 2017 | 23 RCTs (2 cluster and 21 individually randomised) in 2002, 2009, 2011 (4), 2012, 2013 (5), 2014 (3), 2015 (3), 2016 (4), 2017 in Australia (2), Brazil, Canada (2), China (2), Denmark, Egypt, Finland (3), Germany, Italy (2), Norway, UK (2), USA (5) | 8918 | 6633 (19) | Pregnant women regardless of age, gestation, parity or plurality | Any type of diet intervention with any type of exercise intervention versus no intervention or a different diet and exercise intervention | Primary |
| Spencer 2015 | Search July 2015; Up‐to‐date September 2015 | 2 RCTs in 2010, 2012 in Italy, UK | 26,408 | 4516 (1) | Women, either pre‐pregnancy or during pregnancy, including both singleton and multiple pregnancies | Any screening method (e.g. tool, program, guideline or protocol) for detecting thyroid dysfunction pre‐pregnancy or during pregnancy versus no screening | Secondary |
| Tieu 2017 | Search January 2016; Up‐to‐date January 2017 | 11 RCTs (1 quasi randomised) in 1983, 1998, 2006, 2008, 2009 (2), 2011 (2), 2012, 2014, 2016 in Australia (4), Brazil, Denmark, Ireland, Finland, UK, USA (2) | 2786 | 2191 (9) | Pregnant women of any age gestation, parity or plurality | Dietary advice before testing for GDM versus no advice or different types of advice | Primary |
| Wojcieszek 2018 | Search June 2018; Up‐to‐date December 2018 | 9 RCTs and 1 quasi randomised in 1964, 1994, 1995, 2002, 2004, 2009, 2012, 2014, 2015, 2016 in Canada (2), Denmark (3), France, Israel, Italy, and Pakistan. One trial was undertaken across both Austria and Germany | 222 | 210 (8) | "Parents" who had experienced a stillbirth of 20 weeks’ gestation or more who were pregnant or considering a subsequent pregnancy. | Any single intervention, combination of interventions or tailored model of care/algorithm/guideline/protocol for improving health outcomes in subsequent pregnancies following stillbirth | Secondary |
Abbreviations:
GDM: gestational diabetes mellitus
RCT: randomised controlled trial
DHA: docosahexaenoic acid
EPA: eicosapentaenoic acid
2. Risk of bias of trials in included reviews.
| Review ID | Summary of trial limitations (risk of bias) |
| Bain 2014 | Sequence generation: 5 low, 3 unclear Allocation concealment: 4 low, 4 unclear Blinding (participants and personnel): 3 low, 3 unclear, 2 high Blinding (outcome assessors): 5 low, 3 unclear Incomplete outcome data: 4 low, 4 unclear Selective reporting: 2 low, 5 unclear, 1 high Other: 3 low, 5 unclear Overall: "Overall we judged two trials to be at a low risk of bias, two trials to be at an unclear risk of bias, and the other four trials to be at a moderate risk of bias." |
| Barrett 2014 | Sequence generation: 1 low Allocation concealment: 1 low Blinding (participants and personnel): 1 low Blinding (outcome assessors): 1 low Incomplete outcome data: 1 low Selective reporting: 1 low Other: 1 low Overall: "The included study was assessed to be at low risk of bias across all domains." |
| Crawford 2015 | Sequence generation: 3 low, 1 unclear Allocation concealment: 2 low, 2 unclear Blinding (participants and personnel): 2 unclear, 2 high Blinding (outcome assessors): 1 low, 3 unclear Incomplete outcome data: 2 low, 1 unclear, 1 high Selective reporting: 2 low, 1 unclear, 1 high Other: 2 low, 1 unclear, 1 high Overall: "Overall, there was unclear risk of bias due to insufficient information provided to enable a judgement of risk, particularly with regard to allocation concealment and blinding of outcome assessment." |
| Palacios 2019 | Sequence generation: 21 low 8 unclear 1 high Allocation concealment: 13 low 16 unclear 1 high Blinding (participants and personnel): 15 low 1 unclear 14 high Blinding (outcome assessors): 10 low 18 unclear 2 high Incomplete outcome data: 16 low 4 unclear 10 high Selective reporting: 29 unclear 1 high Other: 14 low 2 unclear 4 high Overall: "The risk of bias was high for allocation and/or blinding in 14 trials and for attrition in 10 trials." |
| Dodd 2018 | Sequence generation: 2 low, 1 unclear Allocation concealment: 2 low, 1 unclear Blinding (participants and personnel): 2 low, 1 unclear Blinding (outcome assessors): 2 low, 1 unclear Incomplete outcome data: 3 low Selective reporting: 2 low, 1 high Other: 2 low, 1 unclear Overall risk of bias reported: no comment in main text |
| Han 2012 | Sequence generation: 2 low, 3 unclear Allocation concealment: 2 low, 3 unclear Blinding (participants and personnel): 5 high Blinding (outcome assessors): 5 unclear Incomplete outcome data: 2 low, 3 high Selective reporting: 3 low, 2 high Other: 3 low, 2 high Overall: "Overall, the risk of bias was judged to be moderate." |
| Middleton 2018 | Sequence generation: 37 low, 32 unclear, 1 high Allocation concealment: 29 low, 40 unclear, 1 high Blinding (participants and personnel): 52 low, 15 unclear, 3 high Blinding (outcome assessors): 39 low, 41 unclear Incomplete outcome data: 13 low, 30 unclear, 27 high Selective reporting: 13 low, 45 unclear, 12 high Other: 34 low, 34 unclear, 2 high Overall: “Overall study‐level risk of bias was mixed, with selection and performance bias mostly at low risk, but there was high risk of attrition bias in some trials.” |
| Shepherd 2017 | Sequence generation: 17 low, 6 unclear Allocation concealment: 13 low, 10 unclear Blinding (participants and personnel): 23 high Blinding (outcome assessors): 8 low, 15 unclear Incomplete outcome data: 12 low, 7 unclear, 4 high Selective reporting: 3 low, 15 unclear, 5 high Other: 16 low, 6 unclear, 1 high Overall: "Primarily due to lack of reporting, the overall risk of bias was judged to be unclear." |
| Spencer 2015 | Sequence generation: 2 low Allocation concealment: 2 low Blinding (participants and personnel): 1 low, 1 unclear Blinding (outcome assessors): 2 low Incomplete outcome data: 1 low, 1 unclear Selective reporting: 2 unclear Other: 2 low Overall: "Overall, the two trials were judged to be of low risk of bias." |
| Tieu 2017 | Sequence generation: 7 low, 2 unclear, 1 high Allocation concealment: 4 low, 6 unclear, 1 high Blinding (participants and personnel): 11 high Blinding (outcome assessors): 2 low, 9 unclear Incomplete outcome data: 5 low, 5 unclear, 1 high Selective reporting: 2 low, 8 unclear, 1 high Other: 6 low, 5 unclear Overall: "Overall, the risk of bias was judged to be unclear to moderate." |
| Wojcieszek 2018 | Sequence generation: 9 low, 1 high Allocation concealment: 8 low, 1 unclear, 1 high Blinding (participants and personnel): 4 low, 1 unclear, 5 high Blinding (outcome assessors): 10 low Incomplete outcome data: 7 low, 1 unclear, 2 high Selective reporting: 1 low, 9 unclear Other: 7 low, 3 unclear Overall: "We judged the risk of bias in the trials for methodology and reporting to be low to moderate." |
Methodological quality of included reviews
When assessed against the ROBIS domains, 10 reviews (Bain 2014; Crawford 2015; Dodd 2018; Han 2012; Middleton 2018:Palacios 2019; Shepherd 2017; Spencer 2015; Tieu 2017; Wojcieszek 2018) were considered at low risk of bias across all domains, 'Study eligibility criteria', 'Identification and selection of studies', 'Data collection and study appraisal' and 'Synthesis and findings'. One review (Barrett 2014) was assessed as being at unclear risk of bias in the domains 'Data collection and study appraisal' and 'Synthesis and findings'. This review included six papers relating to one trial that was considered to have some design flaws. Only 45% of women, who were considered "high risk" for GDM were tested for GDM. The intervention group (probiotic with dietary intervention) numbers were halved in the Barrett 2014 review analyses as they performed two comparisons: "probiotics versus placebo" and "probiotics versus diet". The review comparison labelled "probiotics vs diet" refers to comparison of probiotic with dietary intervention versus placebo with dietary intervention with both these groups receiving dietary intervention. The review comparison labelled "probiotics versus placebo" refers to probiotics with dietary intervention versus placebo with no dietary intervention. As the GRADE approach was not used in the review to assess the quality of the evidence, we reviewed original trial reports in order assess their risk of bias and quality of evidence using GRADE. The trial included in Barrett 2014 was also in Tieu 2017, but the 'Risk of bias' assessment differed, which could be because different groups in the trial were being compared in these reviews. Barrett 2014 assessed the trial as low risk of bias across all domains. Tieu 2017 assessed risk of bias for the same trial as follows. Sequence generation: low; Allocation concealment: low; Blinding (participants and personnel): high, Blinding (outcome assessors): unclear; Incomplete outcome data: low; Selective reporting: unclear; Other: low.
Details of the ROBIS assessments we made can be found in Table 3.
3. ROBIS assessments for included reviews.
| Review ID | ROBIS domains | Overall risk of bias | |||
| Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | ||
| Bain 2014 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Barrett 2014 | Low risk | Low risk | Unclear risk | Unclear risk | UNCLEAR RISK |
| Crawford 2015 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Palacios 2019 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Dodd 2018 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Han 2012 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Middleton 2018 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Shepherd 2017 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Spencer 2015 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Tieu 2017 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
| Wojcieszek 2018 | Low risk | Low risk | Low risk | Low risk | LOW RISK |
Effect of interventions
We have summarised the main results below. The outcome, GDM is presented for the different intervention types and categorised according to the framework described under 'Data synthesis': clear evidence of benefit; clear evidence of harm; clear evidence of no effect or equivalence; possible benefit; possible harm; or unknown benefit or harm. The first three categories represent GRADE moderate‐ or high‐quality evidence for which we found either clear benefit, clear harm or clear evidence of no effect (i.e. equivalence with a comparator). These categories are identified by a green tick, a red‐cross and a green equal‐sign icon, respectively (see Figure 1). For 'clear' benefit or harm, the confidence interval (CI) associated with the effect size did not cross the line of no effect. For 'clear evidence of no effect or equivalence' we considered a CI approximating the range of risk ratio (RR) 0.75 to 1.25 as sufficiently narrow to indicate a minimal effect relative to the comparator; these are thresholds recommended by GRADE (Guyatt 2011b). Please refer to Figure 3 for a summary of all assessments. Additional details can also be found in the 'Summary of findings' for each intervention type: Table 4; Table 5; Table 6; Table 7; Table 8; Table 9.
3.

Summary of the effect of interventions on the risk of women developing gestational diabetes mellitus
4. Summary of findings: dietary interventions.
| Intervention and comparison | Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with comparator | Corresponding risk with intervention | |||||
| Dietary advice vs standard care in pregnancy Tieu 2017 | GDM | 126 per 1000 82/651 |
76 per 1000 (44 to 131) 54/628 |
RR 0.60 (0.35, 1.04) | 1279 (5) | Very lowa |
| Low GI vs mod‐high GI diet in pregnancy Tieu 2017 | GDM | 110 per 1000 49/444 |
100 per 1000 (70 to 145) 47/468 |
RR 0.91 (0.63, 1.31) | 912 (4) | Lowb |
a Studies contributing data had design limitations; There was considerable variation in the size of the effect in different studies; Wide 95% confidence interval crossing the line of no effect.
b Studies contributing data had design limitations; Wide 95% confidence interval crossing the line of no effect.
5. Summary of findings: exercise interventions.
| Intervention and comparison | Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with comparator | Corresponding risk with intervention | |||||
| Any exercise vs routine care in pregnancy Han 2012 | GDM | 62 per 1000 24/389 |
68 per 1000 (41 to 114) 30/437 |
RR 1.10 (0.66, 1.84) | 826 (3) | Lowa |
a Study design limitations; wide 95% confidence interval crossing the line of no effect.
6. Summary of findings: combined diet and exercise interventions.
| Intervention and comparison | Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with comparator | Corresponding risk with intervention | |||||
| Diet and exercise vs standard care in pregnancy Shepherd 2017 | GDM | 168 per 1000 551/3280 |
143 per 1000 (119 to 170) 525/3353 |
RR 0.85 (0.71, 1.01) | 6633 (19) | Moderatea |
a Study design limitations: 19 RCTs, intervention unable to be blinded (not downgraded for this as outcome is objective); some RCTS with potentially serious design limitations (unclear randomisation, attrition bias); Inconsistency: I² = 42%, possibly largely due to one trial (Dodd 2014), not downgraded))
7. Summary of findings: dietary supplement interventions.
| Intervention and comparison | Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with comparator | Corresponding risk with intervention | |||||
| Omega‐3 fatty acid supplementation in pregnancy vs none Middleton 2018 | GDM | 62 per 1000 158/2569 |
63 per 1000 (51 to 77) 167/2666 |
RR 1.02 (0.83, 1.26) | 5235 (12) | High |
| Myo‐inositol vs control in pregnancy Crawford 2015 | GDM | 283 per 1000 75/265 |
122 per 1000 (82 to 181) 27/237 |
RR 0.43 (0.29, 0.64) | 502 (3) | Lowa |
| Probiotics and diet vs placebo and diet in pregnancy Barrett 2014 | GDM | 333 per 1000 27/76 |
131 per 1000 (53 to 316) 5/38 |
RR 0.37 (0.15, 0.89) | 114 (1) | Very lowb |
| Probiotics and diet vs control in pregnancy Barrett 2014 | GDM | 342 per 1000 25/73 |
130 per 1000 (55 to 315) 5/38 |
RR 0.38 (0.16, 0.92) | 111 (1) | Very lowb |
| Vitamin D vs control/placebo in pregnancy Palacios 2019 | GDM | 127 per 1000 25/197 |
65 per 1000 (34 to 123) 13/249 |
RR 0.51 (0.27, 0.97) | 446 (4) | Lowc |
| Vitamin D+calcium vs control/placebo in pregnancy Palacios 2019 | GDM | 37 per 1000 1/27 |
12 per 1000 (0 to 290) 0/27 |
RR 0.33 (0.01, 7.84) | 54 (1) | Very lowd |
| Vitamin D+calcium+other vitamins or minerals vs calcium+other vitamins or minerals in pregnancy Palacios 2019 | GDM | 12 per 1000 3/259 |
5 per 1000 (1 to 20) 5/1039 |
RR 0.42 (0.10, 1.73) | 1298 (1) | Very lowe |
a Unclear risk of bias for allocation concealment in two of the included trials (one trial did not provide sufficient detail to determine allocation concealment and one trial (reported as a conference abstract) had no details of random sequence generation, allocation concealment or blinding) and for high risk of performance bias for lack of blinding (two trials were open‐label trials with no blinding of participants or researchers, however one trial explicitly described blinding of outcome assessors and was assessed as low risk of detection bias); Studies were conducted in Italy with Caucasian women and generalisability of findings is limited.
b Serious limitations in study design as not all women tested for GDM. Very serious imprecision with one small study and a wide 95% confidence interval crossing the line of no effect.
c Serious limitations in study design due to one study being assessed as high risk of bias for several domains. Serious limitations due to indirectness with studies performed only in Asian countries (this downgrading was added by overview authors).
d Serious limitations in study design due to one study being at high risk of performance and detection bias; Very serious limitations in imprecision due to one small study, with a single event and wide 95% confidence intervals crossing the line of no effect contributing data.
e Very serious limitations in imprecision with only one study, with few events, and wide 95% confidence intervals crossing the line of no effect; Serious indirectness as there were multiple nutrient interventions in addition to vitamin D.
8. Summary of findings: pharmaceutical interventions.
| Intervention and comparison | Outcome | Anticipated absolute effects (95% CI) | Relative effect RR (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with comparator | Corresponding risk with intervention | |||||
| Metformin vs placebo in pregnancy Dodd 2018 | GDM | 143 per 1000 64/448 |
121 per 1000 (87 to 170) 53/444 |
0.85 (0.61, 1.19) | 892 (3) | Moderatea |
| Care prior to and during subsequent pregnancies following stillbirth. Low molecular weight heparin versus no treatment/standard care. Wojcieszek 2018 | GDM | 125 per 1000 5/40 |
160 per 1000 (63 to 406) 8/45 |
1.28 (0.50, 3.25) | 85 (2) | Lowb |
| Care prior to and during subsequent pregnancies following stillbirth. Low‐dose aspirin versus placebo. Wojcieszek 2018 | GDM | 182 per 1000 2/11 |
76 per 1000 (7 to 738) 1/13 |
RR 0.42 (0.04, 4.06) | 24 (1) | Very lowc |
| Care prior to and during subsequent pregnancies following stillbirth. Low‐dose aspirin + low molecular weight heparin versus low‐dose aspirin. Wojcieszek 2018 |
GDM | 77 per 1000 1/13 (aspirin) |
21 per 1000 (1 to 479) 0/16 |
0.27 (0.01, 6.23) | 29 (1) | Very lowc |
| Care prior to and during subsequent pregnancies following stillbirth. Low‐dose aspirin + low molecular weight heparin versus placebo Wojcieszek 2018 |
GDM | 182 per 1000 2/11 |
25 per 1000 (2 to 487) 0/16 |
0.14 (0.01, 2.68) | 27 (1) | Very lowc |
| Care prior to and during subsequent pregnancies following stillbirth. Low molecular weight heparin versus low‐dose aspirin. Wojcieszek 2018 | GDM | 0 per 1000 0/12 (aspirin) |
0 per 1000 (not estimable) 0/10 |
no events | 22 (1) | Very lowd |
| Care prior to and during subsequent pregnancies following stillbirth. Low molecular weight heparin (dose adjusted according to anti‐factor Xa levels) versus low molecular weight heparin (fixed dose). Wojcieszek 2018 | GDM | 143 per 1000 1/7 (fixed dose) |
54 per 1000 (3 to 1000) 0/6 |
0.38 (0.02, 7.93) | 13 (1) | Very lowe |
| Care prior to and during subsequent pregnancies following stillbirth. Leukocyte immunisation versus placebo. Wojcieszek 2018 | GDM | 0 per 1000 0/2 |
0 per 1000 (not estimable) 0/2 |
no events | 4 (1) | Very lowf |
| Care prior to and during subsequent pregnancies following stillbirth. Intravenous IgG versus placebo. Wojcieszek 2018 | GDM | 0 per 1000 0/4 |
0 per 1000 (not estimable) 0/3 |
no events | 7 (2) | Very lowf |
a Study design limitations as one study of the three studies included has unclear risk of bias for random sequence generation, allocation concealment, performance bias, outcome assessor bias and selective reporting bias and reports a much greater effect than the other two studies.
b Limitations in study design; wide 95% confidence intervals crossing the line of no effect.
c Serious limitations in study design; very serious limitations in imprecision with only one study, with few events, and wide 95% confidence intervals crossing the line of no effect.
d Serious limitations in study design; very serious limitations in imprecision with only one study, with few events, and wide 95% confidence intervals crossing the line of no effect.
e Limitations due to indirectness as study population at increased risk of pregnancy loss and GDM not a predefined outcome; Very serious limitations in imprecision with only one study, with few events, and wide 95% confidence intervals crossing the line of no effect.
f Limitations due to indirectness as study population at increased risk of pregnancy loss and GDM not a predefined outcome; Very serious limitations in imprecision with only one study, with few events, and wide 95% confidence intervals crossing the line of no effect.
9. Summary of findings: management of other health issues in pregnancy.
| Intervention and comparison | Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk with comparator | Corresponding risk with intervention | |||||
| Universal screening for thyroid dysfunction vs risk based screening in pregnancy Spencer 2015 | GDM | 40 per 1000 90/2257 |
37 per 1000 (28 to 50) 84/2259 |
RR 0.93 (0.70, 1.25) | 4516 (1) | Moderatea |
| Fractional Exhaled Nitrogen Oxide algorithm vs clinical guideline algorithm to adjust asthma therapy in pregnancy Bain 2014 | GDM | 104 per 1000 11/106 |
77 per 1000 (32 to 184) 8/104 |
RR 0.74 (0.31, 1.77) | 210 (1) | Lowb |
| Pharmacist led multidisciplinary approach to management of maternal asthma vs standard care in pregnancy Bain 2014 | GDM | 69 per 1000 0/29 |
345 per 1000 (17 to 1000) 2/29 |
RR 5.00 (0.25, 99.82) | 58 (1) | Lowb |
a Serious limitation in imprecision with a wide confidence interval crossing the line of no effect
b Very serious limitation in imprecision as a single study with a small sample and a wide 95% confidence interval crossing the line of no effect.
Interventions for prevention of GDM
Dietary interventions
Unknown benefit or harm. Low‐ or very low‐quality evidence with wide CIs
Dietary advice: very low‐quality evidence in Tieu 2017 showed unknown benefit or harm of dietary advice (most focused on giving general guidelines for healthy eating in pregnancy, with a dietician involved in four trials) compared with standard care on the risk of GDM (RR 0.60, 95% CI 0.35 to 1.04; 5 trials; 1279 women). Low‐quality evidence showed unknown benefit or harm on of a low glycaemic index diet versus a moderate‐high glycaemic index diet on the risk of GDM (RR 0.91, 95% CI 0.63 to 1.31; 4 trials; 912 women), Table 4.
Exercise interventions
Unknown benefit or harm. Low‐quality evidence with wide CIs or very low‐quality evidence
Exercise: low‐quality evidence in Han 2012 showed unknown benefit or harm of exercise interventions (individualised plans or a combination of various supervised and unsupervised sessions at least three times a week, starting between 12 and 24 weeks' gestation) on the risk of GDM compared with standard antenatal care (RR 1.10, 95% CI 0.66 to 1.84; 3 trials; 826 women), Table 5.
Combined dietary and exercise interventions
Possible benefit. Low‐quality evidence with clear benefit (CI does not cross the line of no effect), or moderate‐ or high‐quality evidence with wide CI
Diet and exercise combined: moderate‐quality evidence in Shepherd 2017 indicated a possible benefit with reduction in the risk of GDM with combined diet and exercise interventions during pregnancy compared with standard care (RR 0.85, 95% CI 0.71 to 1.01; 19 trials; 6633 women), Table 6. There were no clear subgroup differences for GDM by trial design (Chi2 = 0.22, df = 1 (P = 0.64), I2 = 0.0%), maternal body mass index (BMI) (Chi2 = 1.73, df = 3 (P = 0.63), I2 = 0.0%), or ethnicity (Chi2 = 0.22, df = 3 (P = 0.97), I2 = 0.0%) (Table 10). Sensitivity analysis including only those trials considered at low risk of selection bias (Table 10) did not affect results (RR 0.86; 95% CI 0.68 to 1.09; 11 trials; 5019 women).
10. Summary of findings: subgroup and sensitivity analyses for select comparisons for GDM.
| Intervention and comparison | Outcome | Subgroup or sensitivity analysis | Assumed risk with comparator | Corresponding risk with intervention | Relative effect (95% CI) | Number of participants (trials) | Test for subgroup differences | |
| Combined diet and exercise vs standard care | GDM | Study design | Individually randomised | 546/3222 | 515/3270 | 0.84 (0.70, 1.01) | 6492 (17) | Chi2 = 0.22, df = 1 (P = 0.64), I2 = 0.0% |
| Cluster randomised | 7/58 | 10/83 | 1.05 (0.42, 2.60) | 141 (2) | ||||
| Maternal BMI at or before trial entry | Healthy (BMI < 25 kg/m2) | 8/150 | 8/150 | 0.91 (0.19, 4.24) | 300 (3) | Chi2 = 1.73, df = 3 (P = 0.63), I2 = 0.0% | ||
| Overweight or obese (BMI ≥ 25 kg/m2) | 206/1436 | 210/1465 | 0.77 (0.50, 1.2) | 2901 (8) | ||||
| Obese (BMI ≥ 30 kg/m2) | 204/880 | 191/858 | 0.96 (0.81, 1.13) | 1738 (3) | ||||
| Any | 551/814 | 525/880 | 0.80 (0.63, 1.03) | 1694 (8) | ||||
| Ethnicity | Majority low‐risk ethnicities | 180/1494 | 196/1503 | 0.85 (0.50, 1.43) | 2998 (5) | Chi2 = 0.22, df = 3 (P = 0.97), I2 = 0.0% | ||
| Majority high‐risk ethnicities | 1/29 | 1/27 | 1.07 (0.07, 16.3) | 56 (1) | ||||
| Mixed ethnicities | 242/1046 | 216/1077 | 0.89 (0.76, 1.05) | 2123 | ||||
| Unclear | 128/710 | 112/746 | 0.83 (0.61, 1.12) | 1456 | ||||
| Sensitivity | Low risk of bias | 418/2501 | 409/2518 | 0.86 (0.68, 1.09) | 5019 (11) | Not applicable | ||
| Vit D versus no treatment/placebo | GDM | Gestation at start of supplementation | Less than 20 weeks of pregnancy | 8/70 | 7/70 | 0.88 (0.34, 2.28) | 140 (1) | Chi2 = 2.15, df = 2 (P = 0.34), I2 = 7% |
| 20 weeks of pregnancy or more | 2/84 | 1/135 | 0.43 (0.05, 3.45) | 219 (2) | ||||
| Unknown/unreported/mixed | 15/43 | 5/44 | 0.33 (0.13, 0.82) | 87 (1) | ||||
| Pre‐gestational BMI | BMI 18.5‐24.9 | 9/127 | 8/178 | 0.83 (0.33, 2.05) | 305 (2) | Chi2 = 2.08, df = 1 (P = 0.15), I2 = 52% | ||
| BMI 25 or higher | 16/70 | 5/71 | 0.33 (0.13, 0.79) | 141 (2) | ||||
| Supplementation regimen | Single dose | 1/57 | 1/108 | 0.53 (0.03, 8.28) | 165 (1) | Chi2 = 2.20, df = 2 (P = 0.33), I2 = 9% | ||
| Daily dose | 16/70 | 5/71 | 0.33 (0.13, 0.79) | 141 (2) | ||||
| Weekly/monthly dose | 8/70 | 7/70 | 0.88 (0.34, 2.28) | 140 (1) | ||||
| Season at start of supplementation | Summer | 1/27 | 0/27 | 0.33 (0.01, 7.84) | 54 (1) | Chi2 = 2.09, df = 2 (P = 0.35), I2 = 4% | ||
| Winter | 8/70 | 7/70 | 0.88 (0.34, 2.28) | 140 (1) | ||||
| Mixed/unknown | 16/100 | 6/152 | 0.34 (0.14, 0.82) | 252 (2) | ||||
Dietary supplement interventions
Clear evidence of no effect. Moderate‐ or high‐quality evidence with narrow CI crossing the line of no effect
Omega‐3 fatty acid: high‐quality evidence in Middleton 2018 showed no effect of omega‐3 fatty acid supplementation in pregnancy (doses between 0.22 g and 2.8 g per day, starting between 12 and 24 weeks' gestation) on the risk of GDM (RR 1.02, 95% CI 0.83 to 1.26; 12 trials; 5235 women), Table 7.
Possible benefit. Low‐quality evidence with clear benefit (CI does not cross the line of no effect), or moderate‐ or high‐quality evidence with wide CI
Myo‐inositol: low‐quality evidence in Crawford 2015 showed a possible benefit with a reduction in the risk of GDM with 2 g to 4 g of myo‐inositol per day during pregnancy compared with control (RR 0.43, 95% CI 0.29 to 0.64; 3 trials; 502 women), Table 7.
Vitamin D: low‐quality evidence in Palacios 2019 showed benefit of vitamin D supplementation in pregnancy (varying dose regimens with a weekly total of between 1400 IU and 30,000 IU, starting before 25 weeks' gestation, duration of treatment from a single dose to the remainder of the pregnancy), with a reduction in risk of GDM (RR 0.51, 95% CI 0.27 to 0.97; 4 trials; 446 women), Table 7. These trials were all from Asian countries and the women's vitamin D levels before supplementation were mostly unknown. One study was from India and three from Iran. One study identified whether women in the intervention group were vitamin D deficient prior to supplementation, and gave different vitamin D doses according to serum vitamin D concentrations. There were no subgroup differences for GDM by gestation at start of supplementation (Chi2 = 2.15, df = 2 (P = 0.34), I2 = 7%), maternal pre‐gestational BMI (Chi2 = 2.08, df = 1 (P = 0.15), I2 = 52%), supplementation regimen (Chi2 = 2.20, df = 2 (P = 0.33), I2 = 9%), or season at start of supplementation (Chi2 = 2.09, df = 2 (P = 0.35), I2 = 4%) (Table 10).
Unknown benefit or harm. Low‐quality evidence with wide CIs or very low‐quality evidence
Probiotics: very low‐quality evidence in Barrett 2014 from one trial showed a reduction in the risk of GDM with probiotic and diet intervention combined versus placebo and diet intervention combined (RR 0.37, 95% CI 0.15 to 0.89; 114 women), or probiotic and diet intervention combined versus control (RR 0.38, 95% CI 0.16 to 0.92; 111 women), Table 7.
Vitamin D with calcium: very low‐quality evidence in Palacios 2019 showed unknown benefit or harm of vitamin D with calcium supplementation (RR 0.33, 95% CI 0.01 to 7.84; 1 trial; 54 women) and unknown benefit or harm of vitamin D supplementation + calcium + other vitamins and minerals versus calcium + other vitamins and minerals (RR 0.42, 95% CI 0.10 to 1.73; 1 trial; 1298 women) on the risk of GDM, Table 7.
Pharmaceutical interventions
Possible benefit. Low‐quality evidence with clear benefit (CI does not cross the line of no effect), or moderate‐ or high‐quality evidence with wide CI
Unknown benefit or harm. Low‐quality evidence with wide CIs or very low‐quality evidence
Care prior to and during subsequent pregnancies following stillbirth: evidence in Wojcieszek 2018 showed unknown benefit or harm of several medications on the incidence of GDM: low‐quality evidence for low molecular weight heparin versus no/standard care (RR 1.28, 95% CI 0.50 to 3.25; 2 trials; 85 women); very low‐quality evidence for low‐dose aspirin versus placebo (RR 0.42, 95% CI 0.04 to 4.06; 1 trial; 24 women); very low‐quality evidence for low‐dose aspirin and low molecular weight heparin versus low‐dose aspirin alone (RR 0.27, 95% CI 0.01 to 6.23; 1 trial; 29 women); very low‐quality evidence for low‐dose aspirin and low molecular weight heparin versus placebo (RR 0.14, 95% CI 0.01 to 2.68; 1 trial; 27 women); very low‐quality evidence for low molecular weight heparin versus low‐dose aspirin (no events; one trial; 22 women); very low‐quality evidence for low molecular weight heparin (adjusted dose) versus low molecular weight heparin (fixed dose) (RR 0.38, 95% CI 0.02 to 7.93; 1 trial; 13 women); very low‐quality evidence for leukocyte immunisation versus placebo (no events; one trial; four women); very low‐quality evidence for intravenous IgG versus placebo (no events); two trials; seven women), Table 8.
Management of other health issues
Clear evidence of no effect. Moderate‐ or high‐quality evidence with narrow CI crossing the line of no effect
Universal screening for thyroid dysfunction vs risk based screening: moderate‐quality evidence in Spencer 2015 showed no effect on the risk of GDM with universal screening of pregnant women for thyroid dysfunction compared with risk based screening (RR 0.93, 95% CI 0.70 to 1.25; 1 trial; 4516 women), Table 9.
Unknown benefit or harm. Low‐quality evidence with wide CIs or very low‐quality evidence
Asthma management: low‐quality evidence in Bain 2014 showed unknown benefit or harm of using fractional exhaled nitrogen oxide versus a clinical algorithm to adjust asthma therapy on the incidence of GDM (RR 0.74, 95% CI 0.31 to 1.77; 1 trial; 210 women). Low‐quality evidence in Bain 2014 showed unknown benefit or harm of a pharmacist led multidisciplinary approach to management of maternal asthma versus standard care on the incidence of GDM (RR 5.00, 95% CI 0.25 to 99.82; 1 trial; 58 women), Table 9.
Sensitivity analysis
No sensitivity analysis was required as no reviews were assessed as being of high concern for risk of bias using ROBIS.
Discussion
We have summarised the evidence from relevant Cochrane Reviews on the effectiveness of interventions to prevent gestational diabetes mellitus (GDM), and have assigned interventions to six mutually exclusive categories with graphic icons to provide a quick visual prompt for readers as to the effectiveness or otherwise of these interventions: clear evidence of benefit; clear evidence of harm; clear evidence of no effect or equivalence; possible benefit; possible harm; or unknown benefit or harm.
Summary of main results
This overview, evaluating interventions with GDM as a primary or secondary outcome, included 11 Cochrane Reviews, involving 71 randomised controlled trials and 23,154 women. Five reviews evaluated interventions directed at preventing GDM, and a further six reviews included interventions in pregnant women that may improve maternal or infant health, with GDM as a primary or secondary outcome for the review. All the trials in the 11 systematic reviews included pregnant women; no trials assessed interventions prior to pregnancy or between pregnancies.
Four reviews reported interventions of possible benefit to pregnant women, with a reduction in the risk of GDM: combined diet and exercise (Shepherd 2017), myo‐inositol (Crawford 2015), vitamin D supplementation (Palacios 2019), or metformin (Dodd 2018). Two reviews reported no benefit to pregnant women with universal screening and treatment for thyroid dysfunction (Spencer 2015); nor to pregnant women using omega‐3 fatty acid supplements (Middleton 2018) for reducing the risk of GDM. The evidence was insufficient to assign a level of effectiveness in interventions in seven reviews (Bain 2014; Barrett 2014; Palacios 2019; Dodd 2018; Han 2012; Tieu 2017; Wojcieszek 2018). Importantly, no reviews demonstrated evidence of harm (defined as a clear increase in the risk of GDM).
Overall completeness and applicability of evidence
Our aim was to summarise the evidence relating to strategies that may prevent GDM. However, six reviews were published more than two years ago (Bain 2014; Barrett 2014; Crawford 2015; Han 2012; Spencer 2015; Tieu 2017). There may be trials now published that might alter the results of the reviews, in particular those relating to probiotics and exercise. Exercise interventions used in trials have been widely differing in level of activity, intensity and details of other activity not related to the study, making comparisons between different exercise interventions and pooling results difficult to interpret.
Palacios 2019 reported a possible benefit of vitamin D supplementation in pregnancy with a reduction in the risk of GDM. It is worth noting that this evidence came from four small trials of only 446 women and all trials were conducted in Asian countries. The effect may differ for other ethnicities or geographical locations. Vitamin D concentrations prior to supplementation were determined in two of six trials and it may be that women with vitamin D deficiency are more likely to benefit than those with normal concentrations. The review authors concluded vitamin D supplementation possibly reduces the risk of GDM.
A clear research gap in the published literature is that no randomised trials of interventions in women preconception or inter‐conception for prevention of GDM in a subsequent pregnancy were identified, an important time for intervention. We only included Cochrane systematic reviews in this overview and there are published non‐Cochrane systematic reviews.
Quality of the evidence
We used ROBIS in our evaluation of risks of bias in the included systematic reviews. Cochrane Review methodology includes assessment of risk of bias of included trials. We did not plan to reassess the quality of the included trials in the reviews, though in order to apply GRADE to the outcome pre‐specified in our overview, details of original trial papers were reviewed from Bain 2014, Barrett 2014, Han 2012, Middleton 2018, Spencer 2015 and Wojcieszek 2018. Barrett 2014, suggested a reduction in GDM with the use of probiotics. However, the trial design had a significant limitation with only 45% of women tested for GDM.
Potential biases in the overview process
We were aware that there were risks of introducing bias at all stages of the overview process, and took a number of steps to minimise this. We published a protocol for our overview. At least two overview authors independently assessed reviews as to eligibility for inclusion, carried out data extraction and quality assessment, and assessed the quality of the evidence using the GRADE approach. A potential source of bias relates to authors of this overview being authors for some of the included reviews. As pre‐specified in our protocol, data extraction and quality assessment for such reviews were carried out by two overview authors who were not authors of the individual reviews. We undertook a comprehensive search of the Cochrane Database of Systematic Reviews without language or date restrictions, and identified published reviews, as well as planned and ongoing reviews (protocols).
Agreements and disagreements with other studies or reviews
We found no published overviews of interventions for preventing GDM in women. One review article was identified that included several different types of intervention for preventing GDM: Agha‐Jaffar 2016 reviewed trials investigating, exercise, diet, metformin, myo‐inositol and fish oil as interventions for prevention of GDM. Probiotics and myo‐inositol were possibly effective in reducing the incidence of GDM in women at high risk. Dietary intervention was effective in reducing GDM in obese women. Otherwise, the combination of diet and exercise or exercise alone did not show a clear benefit in reducing the incidence of GDM, and fish oil was of no benefit. Metformin did not reduce the incidence of GDM in women with obesity or polycystic ovarian syndrome. No meta‐analysis was included in this review, but the conclusions were similar to those of this overview. The authors of the review were unable to identify randomised trials with strategies implemented before conception.
Guo 2018 published a systematic review that demonstrated a reduction in the incidence of GDM with dietary intervention (RR 0.75, 95% CI 0.59 to 0.95; 11 trials; 2838 women). This review included five additional trials to Tieu 2017 where data had been published more recently, but also did not include five trials that Tieu 2017 included, likely as result of different search strategies and inclusion criteria. Guo 2018 excluded trials with "severely unbalanced risk factors or dropout rates" between groups. Bennett 2018 systematic review found a reduction in risk of GDM with dietary interventions (RR 0.56, 95% CI 0.36 to 0.86; 9 trials). Song 2016 reported a meta‐analysis of studies in English or Chinese and found no significant effect on risk of GDM with dietary interventions (RR 0.80, CI 0.58 to 1.10; 5 trials; 1279 women).
Song 2016 meta‐analysis did not find a significant reduction in the risk of GDM with exercise interventions (RR 0.77, 95% CI 0.54 to 1.09; 10 trials; 4161 women). However, exercise alone was found to be of benefit in reducing the risk of GDM in several other systematic reviews: Bennett 2018 (RR 0.62, 95% CI 0.50 to 0.78; 10 trials; 2981; women), Yu 2018 (odds ratio (OR) 0.59, 95% CI 0.39 to 0.88; 5 trials; 1370 women), Davenport 2018 (OR 0.62, 95% CI 0.52 to 0.75; 26 trials; 6934 women), Guo 2018 (RR 0.70, 95% CI 0.59 to 0.84; 19 trials; 5883 women), Sanabria‐Martínez 2015 (RR 0.69, 95% CI 0.52 to 0.91; 8 trials; 2501 women) and Zheng 2017 (OR 0.62, 95% CI 0.43 to 0.89; 5 trials; 1872 women). There may be a threshold for frequency and intensity of exercise above which a benefit is seen. In addition, the earlier in pregnancy the intervention was initiated, the more benefit was seen (Guo 2018). A meta‐analysis by Sanabria‐Martínez 2015 indicated a reduction in GDM when an exercise programme was undertaken by pregnant women with previously low levels of activity. All these reviews were published more recently than Han 2012.
A combined diet and exercise intervention did not clearly reduce the risk of GDM in systematic reviews by Bennett 2018 (RR 0.90, 95% CI 0.77 to 1.05; 19 trials; 7274 women) and Guo 2018 (RR 0.86, 95% CI 0.71 to 1.04; 18 trials; 7024 women). The authors of Bennett 2018 suggested the reason for this was that changing both diet and exercise simultaneously was too difficult. Bennett 2018 included search results of Chinese databases and therefore included additional trials not included in the Cochrane systematic reviews. Guo 2018 suggested that the earlier the intervention, the more benefit there was in reduction of GDM risk. The Song 2016 meta‐analysis of interventions included publications in English or Chinese and did not find a significant reduction in risk of GDM with combined exercise and dietary intervention (RR 0.85, 95% CI 0.70 to 1.03; 14 trials; 6047 women).
The Vitagliano 2019 systematic review included an additional trial to Crawford 2015 and also demonstrated a possible reduction in the risk of GDM with myo‐inositol supplementation in pregnancy, though the quality of the evidence was assessed as very low, using GRADE criteria, (OR 0.49, 95% CI 0.24 to1.03; 4 trials; 848 women). Subgroup analysis including the two trials with the higher dose of 4 g my‐inositol per day was advantageous over the lower dose of 1.1 g with OR 0.34, 95% CI 0.21 to 0.53; 3 trials; 608 women.
The Jarde 2018 systematic review, including two studies of 355 women, showed unclear benefit or harm of probiotics in the prevention of GDM (RR 1.25, 95% CI 0.61 to 2.56). The PiP study (Wickens 2017), published since the Jarde review, demonstrated no significant effect on GDM risk when using probiotics compared with placebo, using the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria for diagnosis (RR 0.59, 95% CI 0.32 to 1.08; 373 women), but a reduction was evident when the New Zealand criteria were used for diagnosis (RR 0.32, 95% CI 0.11 to 0.96; 383 women). The recent SPRING trial (Callaway 2019) demonstrated no difference in the rates of GDM in overweight or obese women who received probiotic compared to placebo (OR 1.62, 95% CI 0.91 to 2.89; 411 women).
No other systematic reviews of randomised controlled trials that examined the effect of vitamin D supplementation on risk of GDM were found.
No other systematic reviews of randomised controlled trials that examined the effect of metformin on risk of GDM were found.
Population data from Canada demonstrated no effect of asthma treatment nor asthma severity on the risk of GDM in pregnant asthmatics (Baribeau 2016; Blais 2014).
Authors' conclusions
Our overview included 11 systematic reviews with 71 trials involving 23,154 women. No interventions to prevent GDM in 11 systematic reviews were of clear benefit or harm. Myo‐inositol, metformin, combined exercise and dietary intervention, and vitamin D supplementation in pregnancy, were identified as having a possible benefit in reducing the incidence of GDM. There remains a need for further high‐quality evidence investigating the effect of myo‐inositol, vitamin D, probiotics, metformin, exercise and dietary interventions alone or in combination. There is a lack of trials investigating interventions prior to or between pregnancies for the prevention of GDM.
Implications for practice.
The aim of this overview was to summarise the evidence on the topic of interventions that may prevent GDM, not to provide practice recommendations. Evidence shows a combination of exercise and diet, supplementation with myo‐inositol, vitamin D supplementation in pregnancy and metformin are of possible benefit in reducing the risk of GDM. There is evidence that omega‐3‐fatty acid supplements and universal screening for thyroid dysfunction are of no benefit in pregnancy for preventing GDM, though this does not exclude benefit for other health outcomes. There is insufficient high‐quality evidence to establish the effect on the risk of GDM of diet or exercise alone, probiotics, vitamin D with calcium or other vitamins and minerals, interventions in pregnancy after a previous stillbirth, and different strategies for managing asthma in pregnancy.
Implications for research.
In randomised controlled trials investigating the effect of interventions for preventing GDM, all included participants should be tested for GDM.
Further high‐quality research is needed to determine the possible benefit of a combination of diet and exercise, myo‐inositol, vitamin D supplementation in different ethnic populations and metformin in preventing GDM. The dose of myo‐inositol or metformin and timing of intervention may be important and it would be helpful if future studies were to replicate sound methodology of earlier trials where available, and be of adequate power.
For trials assessing exercise interventions, the intensity, duration, frequency and timing during pregnancy need to be clearly defined and compared as there is heterogeneity in these parameters between trials, making meaningful synthesis of results and meta‐analysis challenging. Dietary interventions are heterogenous in type and timing, which may have an impact on the effect. It is important for trials to record not only allocation of intervention types, but also adherence to the treatment protocol participants are allocated to and other qualities such as pre‐trial diet, activity level or use of additional exercise.
Where possible trials published in using non‐Latin scripts should be included, for example, Chinese.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2020 | Amended | Added plain language summary title. |
History
Protocol first published: Issue 10, 2016 Review first published: Issue 6, 2020
| Date | Event | Description |
|---|---|---|
| 30 April 2019 | Amended | The original protocol published in 2016 (Lawrence 2016) has been amended to reflect a change in scope. |
| 30 April 2019 | New citation required and major changes | The author team have changed to include a new lead author, Rebecca Griffith. Julie Brown and Robyn Lawrence have stepped down from the team. The title has changed from, "Interventions for preventing gestational diabetes mellitus: an overview of Cochrane Reviews", to “Interventions to prevent women developing gestational diabetes: an overview of Cochrane Reviews”. The scope has changed, as outlined below. Description of the condition: expanded to provide a description of the metabolic changes that take place in a normal versus gestational diabetes mellitus (GDM) pregnancy. Description of the interventions: additional recent references added. Outcomes: as the aim of the overview is to summarise the evidence relating to the prevention of GDM, the primary outcome is prevention of GDM. Statistical summaries: subgroup and sensitivity analyses added. Data synthesis: the framework has changed to improve presentation and readability. |
Acknowledgements
The initial version of the protocol for this review (Lawrence 2016) was commented on by an editor, three peer referees and the Group's Statistical Adviser.
As part of the pre‐publication editorial process, this review has been commented on by five peers (an editor and four referees who are external to the editorial team) and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Rachel K Harrison, MD, Jennifer Jury McIntosh, DO, MS, Medical College of Wisconsin, Depatment of OB/GYN; Dr Amanda J Poprzeczny, Women and Children's Hospital, Women's and Babies Division, Robinson Research Institute, The University of Adelaide; Christina Scifres.
We acknowledge the support of the Cochrane Pregnancy and Childbirth Editorial Group, the Australian and New Zealand Satellite of Cochrane Pregnancy and Childbirth, and The Liggins Institute.
This overview was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Robyn Lawrence with colleagues Julie Brown, Philippa Middleton, Emily Shepherd, Stephen Brown and Caroline Crowther in 2016 prepared a protocol for an overview entitled ‘Interventions for preventing gestational diabetes mellitus: an overview of Cochrane Reviews,’ (Lawrence 2016). Robyn Lawrence and Julie Brown then stepped down from the author team. We thank Robyn and Julie for their contribution to the original protocol.
Appendices
Appendix 1. Ongoing reviews
| Protocol citation | Pre‐specified outcome in protocol |
| Bergamaschi DP, Mariath AB, Abbade JF, Grillo LP, DIniz CSG, Hinnig PF. Selenium supplementation during pregnancy for improving maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2012, Issue 3. | Secondary pre‐specified outcomes include GDM. |
Appendix 2. Reviews awaiting further classification
| Review citation | Overview of pre‐specified outcomes in review with no outcome data | Main conclusion(s) of review |
| Showell MG, Mackenzie‐Procter R, Jordan V, Hodgson R, Farquar C. Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2018, Issue 12. | No data in included trials on GDM. Secondary outcomes include GDM. | "Based upon very low‐quality evidence, we are uncertain whether MI improves live birth rate or clinical pregnancy rate for subfertile women with PCOS undergoing IVF pre‐treatment by taking MI versus standard treatment. We are also uncertain whether MI decreases miscarriage rates or multiple pregnancy rates for these same women taking MI compared to standard treatment. No pooled evidence is available for the use of MI compared to placebo, another antioxidant, insulin‐sensitising agents, ovulation induction agents, or another type of inositol for women with PCOS undergoing pre‐treatment for IVF. Also, no pooled evidence is available on the use of MI among women undergoing ovulation induction. We need trialists to further investigate this question using better‐quality placebo‐controlled blinded randomised trials with adequate power to assess the clinically relevant outcomes of live birth, adverse events, and clinical pregnancy and ovulation rates, to determine the efficacy and safety of inositol. We need this research to encompass both women with PCOS who are undergoing pre‐treatment for IVF and women with PCOS who are undergoing ovulation induction. We also need large, good quality randomised controlled trials to compare inositol versus another antioxidant, another type of inositol (D‐chiro‐inositol), insulin‐sensitising agents, and ovulation induction agents." |
| Tieu J, Coat S, Hague W, Middleton P, Shepherd E. Oral anti‐diabetic agents for women with established diabetes/impaired glucose tolerance or previous gestational diabetes planning pregnancy, or pregnant women with pre‐existing diabetes. Cochrane Database of Systematic Reviews 2017, Issue 10. | No data in included trials on GDM. Primary outcomes include GDM. |
"There is currently insufficient evidence to evaluate the use of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes planning a pregnancy or pregnant women with pre‐existing diabetes. Low‐ to very low‐quality evidence suggests possible reductions in pregnancy‐induced hypertension, caesarean section birth and neonatal hypoglycaemia with metformin (an oral anti‐diabetic agent) compared with insulin in pregnant women with type 2 diabetes, and no clear differences in pre‐eclampsia, induction of labour or babies who are large‐for‐gestational age. Therefore, decisions about the use of oral anti‐diabetic agents for these women will probably depend on factors such as women's preferences, available resources, and local or national clinical practice guidelines. Despite limited evidence of the effects of oral anti‐diabetic agents for women included in this review, the potential benefits relating to women's acceptability and adherence with oral anti‐diabetic agents suggest that further evidence is required. Large, high‐quality randomised controlled trials are required to evaluate the effects of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance, or previous gestational diabetes who are planning a pregnancy and pregnant women with pre‐existing diabetes. In particular, trials could compare oral anti‐diabetic agents with insulin or dietary and lifestyle control, and compare different oral anti‐diabetic agents. Trials could attempt to collect and report on the standard outcomes suggested in this review, such as short‐term maternal and infant outcomes including glycaemic control parameters and women's views, long‐term maternal and infant outcomes, and outcomes relating to the use and costs of health services. We have identified three ongoing studies, and four are awaiting classification. We will consider these in the next update of this review." |
| Tieu J, Shepherd E, Middleton P, Crowther CA. Interconception care for women with a history of gestational diabetes for improving maternal and infant outcomes. Cochrane Database of Systematic Reviews 2017, Issue 8. | No trials identified. Primary outcomes include GDM. | The role of inter‐conception care for women with a history of GDM on maternal and infant health outcomes remains unclear. Research should be conducted to investigate the effects of inter‐conception care for women with a history of GDM on health outcomes for mothers and their infants. Although such trials are faced with difficulties in identifying women in this time period between pregnancies, women with a history of GDM do represent a population at risk for potentially reversible poor health outcomes. Trials should consider the role of different forms of intervention including dietary, lifestyle and pharmacological therapies, in addition to the duration of such interventions. Such trials should not only evaluate the effects on maternal and infant health outcomes, but also the acceptability and cost‐effectiveness, to enable translation to clinical practice. Furthermore, future research should focus on long‐term follow‐up, evaluating the effects of such interventions on the long‐term health outcomes associated with GDM for both mothers and their infants." |
| Opray N, Grivell RM, Deussen AR. Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese. Cochrane Database of Systematic Reviews 2015, Issue 7. | No trials identified. Secondary outcomes include GDM. |
"As there is no current evidence from randomised controlled trials to support which, if any, preconception interventions are likely to have a beneficial impact on pregnancy outcomes for overweight and obese women, no conclusions to inform practice can be made at this time. The absence of randomised controlled trials relating to preconception interventions for overweight and obese women to improve pregnancy outcomes reveals an area where further research is required. With increasing numbers of women of reproductive age becoming overweight or obese (Callaway 2006; Vahration 2009), more understanding is required about how to best approach and intervene in the preconception period." |
| Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochrane Database of Systematic Reviews 2015, Issue 6. | No data in included trials on GDM. Secondary maternal outcomes include GDM. | "Little evidence is available from the one included randomised controlled trial (RCT) that contributed data for our prespecified outcomes to evaluate the effect of caffeine on fetal, neonatal and maternal outcomes. However, the included trial did not evaluate all of the prespecified outcomes from our review protocol. There is a need for high‐quality, properly designed RCTs in this field. Proper randomisation, adequate allocation concealment, blinding of outcome assessors, participants and data analysts and clear attrition policies are crucial to ensure appropriate comparisons between caffeinated and decaffeinated groups. Various outcome measurements including fetal and maternal outcomes should be considered in future studies. We recommend a comprehensive RCT to investigate all of the primary and secondary outcomes suggested in our review protocol." |
| Middleton P, Crowther CA. Reminder systems for women with previous gestational diabetes mellitus to increase uptake of testing for type 2 diabetes or impaired glucose tolerance. Cochrane Database of Systematic Reviews 2014, Issue 3. | No data in included trial on GDM. Secondary outcomes include GDM in subsequent pregnancy. |
"While only one trial fulfilled our inclusion criteria and the overall quality of evidence was low, it showed that postal reminders increased the uptake of testing for type 2 diabetes in women with previous gestational diabetes (GDM). Other forms of reminder systems (e.g. email and telephone reminders) could potentially be effective, although our review was not able to compare these approaches due to lack of studies. The number of women diagnosed with GDM is projected to rise due to expected increases in BMI and maternal age, as well as possible changes to diagnostic thresholds, so healthcare systems will require effective postpartum reminder and diabetes screening programmes. The effects of other forms of reminder systems need to be assessed to see whether test uptake is also increased when email and telephone reminders are deployed. We also need a better understanding of why some women fail to take opportunities to be screened postpartum. As the ultimate aim of increasing postpartum testing is to prevent the subsequent development of type 2 diabetes, it is important to determine whether increased test uptake rates also increase women's use of preventive strategies such as lifestyle modifications." |
| Jeffreys AE, Siassakos D, Draycott T, Akande VA, Fox R. Deflation of gastric band balloon in pregnancy for improving outcomes. Cochrane Database of Systematic Reviews 2013, Issue 4. | No trials identified. GDM primary outcome. |
"At present, there is no evidence to favour either elective deflation or maintaining gastric band balloon inflation in pregnancy. This lack of evidence is reflected in the wide variation in management of the gastric band in pregnancy which is seen in observational studies (Carelli 2011; Dixon 2001; Dixon 2005; Lapolla 2010; Sheiner 2009). Management decisions regarding band status in pregnancy should continue to be made according to the clinical context. Maternal weight at the beginning of pregnancy, nausea and vomiting, tolerability of the band, gestational weight gain, fetal growth, maternal wishes, and pregnancy complications should probably all be taken into account, but this review has not produced evidence to guide practice. Issues surrounding management of the gastric band should be discussed with women who should be involved in management decisions. Close surveillance of both mother and fetus should continue throughout pregnancy to identify any complications at an early stage (Guelinckx 2009). Effective care requires communication between all members of the multidisciplinary team including obstetricians, bariatric surgeons, bariatric physicians, dieticians and midwives. As the prevalence of obesity is predicted to rise, so too the incidence of pregnancies following laparoscopic adjustable gastric banding is likely to increase. As the optimal management of women with a gastric band in pregnancy is unknown, there is a need for further research in this area to guide clinicians and pregnant women. This review supports the need for a randomised controlled trial in the area. A systematic review of observational studies could improve our understanding of gastric band management and help to design a randomised controlled trial. A randomised controlled trial would need to have three comparison arms to reflect current practice; elective deflation of the band balloon at the beginning of pregnancy, intention to leave the balloon inflated for the duration of pregnancy, and deflation of the balloon at the beginning of pregnancy with re inflation during the second trimester. Outcome measures should mirror those we intended to assess in this review, as they are both clinically important and meaningful to clinicians and patients (outcomes were selected as important for this review by a Maternity Service User Panel). Such a randomised controlled trial would need to be sufficiently powered to detect differences in primary maternal and perinatal outcomes for different forms of band management. Any bias should be minimised by adequate randomisation using random sequence generation, concealment of allocation to participants, study personnel and outcome assessors. |
| Furber CM, McGowan L, Bower P, Kontopantelis E, Quenby S, Lavender T. Antenatal interventions for reducing weight in obese women for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2013, Issue 1. | No trials identified. Secondary maternal outcomes include GDM. |
"It is interesting to note from observational cohort studies that obese pregnant women may lose weight and have better outcomes than those who gain weight within recommended guidance, especially those who are morbidly obese. However weight loss in morbidly obese pregnant women does not eliminate risks associated with pregnancy (Hinkle 2010; Beyerlein 2011; Blomberg 2011). These observational studies indicate that the impact of weight loss when obese and pregnant are complex, and also variable across obese categories. Although there may be lesser likelihood of pre‐eclampsia, caesarean birth and a large for gestational age fetus at term, the potential for increased risk of small‐for‐gestational age infants indicates that weight loss when pregnant and obese is not without risk. More robust evidence of the outcomes of weight loss when pregnant and obese across obesity categories is required so that we can confidently understand outcomes, especially those that impact on the neonate. As there is no evidence from randomised controlled trials of interventions during pregnancy that weight loss in obese pregnant women is beneficial, recommendations advocating weight reduction in pregnancy when obese cannot be supported. We suggest that until evidence is available, no practice recommendations can be made. The absence of randomised controlled trials related to reducing weight in obese pregnant women may be a reflection of the lack of evidence from observational cohort studies of the safety of weight loss in this group. There is no robust evidence that indicates the benefits, or harm, of losing weight when obese and pregnant. Until evidence is available, it may not be appropriate to conduct a randomised controlled trial designed to promote weight loss in obese women in pregnancy. Furthermore, it is unlikely that an ethics committee would provide favourable opinion to any such study based on current evidence. More understanding is required of the weight trajectory of obese women during pregnancy. Prospective observational cohort studies of obese women during pregnancy will provide more data that explains weight changes for this group, and short and long term outcomes. Further studies are required to explore the efficacy of the latest guidance from the Institute of Medicine (Blomberg 2011; Rasmussen 2009b), especially as this guidance has not stratified recommendations for weight gain across all obese categories (Artal 2010). Qualitative research will provide more insights into the weight management strategies utilised by obese women during pregnancy, especially those who deliberately lose weight." |
| Han S, Crowther CA, Middleton P. Interventions for pregnant women with hyperglycaemia not meeting gestational diabetes and type 2 diabetes diagnostic criteria. Cochrane Database of Systematic Reviews 2012, Issue 1. | No data in included trials on GDM. Secondary outcomes include GDM in subsequent pregnancy. | "This review found interventions for women with pregnancy hyperglycaemia not meeting GDM and T2DM diagnostic criteria helped reduce the number of macrosomic and LGA babies. It is important to note that the results of this review were based on four small randomised trials with moderate to high risk of bias without follow‐up outcomes for women or their babies. Until additional evidence from large well designed randomised trials becomes available, current evidence is insufficient to make conclusive suggestions on management for women with pregnancy hyperglycaemia not meeting GDM and T2DM diagnostic criteria. Further larger trials with sufficient power to assess the effects of lifestyle intervention and metabolic monitoring on maternal and infant health outcomes are needed. Outcomes such as longer term health outcomes for women and their babies after being managed for pregnancy hyperglycaemia during pregnancy and health service cost should be included." |
Contributions of authors
Caroline Crowther and Julie Brown had the original concept for an overview of interventions to prevent women developing GDM. Rebecca Griffith wrote the first draft of this overview. All authors provided review and feedback on the draft versions of the overview and agreed on the final version published.
Sources of support
Internal sources
The Liggins Institute, The University of Auckland, New Zealand
Discipline of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, The University of Adelaide, Australia
External sources
Cochrane Pregnancy and Childbirth Australian and New Zealand Satellite, Australia
Rebecca Griffith receives a Clinical Research Training Fellowship award from the Health Research Council of New Zealand, but the award is not specifically to complete the overview, New Zealand
Declarations of interest
Rebecca Griffith receives a Clinical Research Training Fellowship award from the Health Research Council of New Zealand. The overview will be included in Rebecca’s PhD, but the award is not specifically to complete the overview. There are no other known conflicts of interest.
Abigail E Moore: none known.
Jane Alsweiler is on the steering committee for a randomised controlled trial of tight glycaemic targets for women with gestational diabetes. Jane Alsweiler has been involved as an author or co‐author of Cochrane systematic reviews that are included in this overview review. Overview review authors not involved in those reviews assessed the eligibility for inclusion of these reviews
Emily Shepherd, Philippa Middleton, Jane Alsweiler and Caroline Crowther are authors of reviews that were included in this overview. Overview authors not involved in those particular Cochrane Reviews assessed eligibility, extracted data and GRADE outcomes, where not already undertaken by the review authors.
Stephen Brown: none known.
Edited (no change to conclusions)
References
References to included reviews
Bain 2014
- Bain E, Pierides KL, Clifton VL, Hodyl NA, Stark MJ, Crowther CA, et al. Interventions for managing asthma in pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD010660. [DOI: 10.1002/14651858.CD010660.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrett 2014
- Barrett HL, Dekker NM, Conwell LS, Callaway LK. Probiotics for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD009951. [DOI: 10.1002/14651858.CD009951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2015
- Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD011507. [DOI: 10.1002/14651858.CD011507.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2018
- Dodd JM, Grivell RM, Deussen AR, Hague WM. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD010564. [DOI: 10.1002/14651858.CD010564.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2012
- Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD009021. [DOI: 10.1002/14651858.CD009021.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2018
- Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD003402. [DOI: 10.1002/14651858.CD003402.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacios 2019
- Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD008873. [DOI: 10.1002/14651858.CD008873.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 2017
- Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD010443. [DOI: 10.1002/14651858.CD010443.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2015
- Spencer L, Bubner T, Bain E, Middleton P. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011263. [DOI: 10.1002/14651858.CD011263.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tieu 2017
- Tieu J, Shepherd E, Middleton P, Crowther CA. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD006674. [DOI: 10.1002/14651858.CD006674.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wojcieszek 2018
- Wojcieszek AM, Shepherd E, Middleton P, Lassi ZS, Wilson T, Murphy MM, et al. Care prior to and during subsequent pregnancies following stillbirth for improving outcomes. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD012203. [DOI: 10.1002/14651858.CD012203.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Crowe 2019
- Crowe M, Lee S, Seow CH, Kaplan GG, Metcalfe A, Benchimol EI, et al. The impact of surgical therapies for inflammatory bowel disease on female fertility. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD012711. [DOI: 10.1002/14651858.CD012711.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACOG 2018
- American College of Obstetricians and Gynecologists. Practice bulletin no. 190: Gestational Diabetes Mellitus. Obstetrics & Gynecology 2018;131(2):406-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Adams 1998
- Adams KM, Li H, Nelson RL, Ogburn PL, Danilenko-Dixon DR. Sequelae of unrecognized gestational diabetes. American Journal of Obstetrics and Gynecology 1998;178(6):1321-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agha‐Jaffar 2016
- Agha-Jaffar R, Oliver N, Johnston D, Robinson S. Gestational diabetes mellitus: does an effective prevention strategy exist? Nature Reviews Endocrinology 2016;12(9):533-46. [DOI] [PubMed] [Google Scholar]
Ainuddin 2015
- Ainuddin JA, Kazi S, Aftab S, Kamran A. Metformin for preventing gestational diabetes in women with polycystic ovarian syndrome. Journal of the College of Physicians and Surgeons Pakistan 2015;25(4):237-41. [DOI: ] [PubMed] [Google Scholar]
Alkharfy 2013
- Alkharfy KM, Al-Daghri NM, Yakout SM, Hussain T, Mohammed AK, Krishnaswamy S. Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metabolic Syndrome and Related Disorders 2013;11(4):283-8. [DOI: 10.1089/met.2012.0068] [DOI] [PubMed] [Google Scholar]
Baillargeon 2010
- Baillargeon JP, Iuorno MJ, Apridonidze T, Nestler JE. Uncoupling between insulin and release of a D-chiro-inositol-containing inositolphosphoglycan mediator of insulin action in obese women with polycystic ovary syndrome. Metabolic Syndrome and Related Disorders 2010;8(2):127-36. [DOI: 10.1089/met.2009.0052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bao 2017
- Bao W, Song Y, Bertrand KA, Tobias DK, Olsen SF, Chavarro JE, et al. Pre-pregnancy habitual intake of vitamin D from diet and supplements in relation to risk of gestational diabetes mellitus: a prospective cohort study. Journal of Diabetes 2017;10(5):373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barakat 2012
- Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. British Journal of Sports Medicine 2012;46(9):656-61. [DOI: 10.1136/bjsports-2011-090009] [DOI] [PubMed] [Google Scholar]
Baribeau 2016
- Baribeau V, Beauchesne MF, Rey É, Forget A, Blais L. The use of asthma controller medications during pregnancy and the risk of gestational diabetes. Journal of Allergy and Clinical Immunology 2016;138(6):1732-1733.e6. [DOI] [PubMed] [Google Scholar]
Baynes 2018
- Baynes HW, Mideksa S, Ambachew S. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 2018;0:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellamy 2009
- Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373(9677):1773-9. [DOI] [PubMed] [Google Scholar]
Bennett 2018
- Bennett CJ, Walker RE, Blumfield ML, Gwini S-M, Ma J, Wang F, et al. Interventions designed to reduce excessive gestational weight gain can reduce the incidence of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabetes Research and Clinical Practice 2018;141:69-79. [DOI] [PubMed] [Google Scholar]
Berkowitz 1992
- Berkowitz GS, Lapinski RH, Wein R, Lee D. Race/ethnicity and other risk factors for gestational diabetes. American Journal of Epidemiology 1992;135(9):965-73. [DOI] [PubMed] [Google Scholar]
Blais 2014
- Blais L, Kettani F-Z, Forget A. Associations of maternal asthma severity and control with pregnancy complications [Blais L 1 , Kettani FZ, Forget A.]. Journal of Asthma 2014;51(4):391-8. [DOI] [PubMed] [Google Scholar]
Boney 2005
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115(3):e290-6. [DOI] [PubMed] [Google Scholar]
Bottalico 2007
- Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Seminars in Perinatology 2007;31(3):176-84. [DOI] [PubMed] [Google Scholar]
Bourlon 1999
- Bourlon PM, Billaudel B, Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. Journal of Endocrinology 1999;160(1):87-95. [DOI: 10.1677/joe.0.1600087] [DOI] [PubMed] [Google Scholar]
Bowes 1996
- Bowes SB, Hennessy TR, Umpleby AM, Benn JJ, Jackson NC, Boroujerdi MA, et al. Measurement of glucose metabolism and insulin secretion during normal pregnancy and pregnancy complicated by gestational diabetes. Diabetologia 1996;39(8):976-83. [DOI] [PubMed] [Google Scholar]
Buchanan 2001
- Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. Journal of Clinical Endocrinology & Metabolism 2001;86(3):989-93. [DOI] [PubMed] [Google Scholar]
Burton‐Freeman 2000
- Burton-Freeman B. Dietary fiber and energy regulation. Journal of Nutrition 2000;130(2S):272S-275S. [DOI: 10.1080/10408398.2012.700654] [DOI] [PubMed] [Google Scholar]
Butte 2000
- Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. American Journal of Clinical Nutrition 2000;71(5 Suppl):1256S-61S. [DOI] [PubMed] [Google Scholar]
Cai 2012
- Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation end products (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defences AGE receptor-1 and sirtuin 1. Proceedings of the National Academy of Sciences of the United States of America 2012;109(39):15888-93. [DOI: 10.1073/pnas.1205847109 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Calder 2006
- Calder PC. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition 2006;83(6):1505S-19S. [DOI] [PubMed] [Google Scholar]
Callaway 2019
- Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 2019;42(3):364-71. [DOI] [PubMed] [Google Scholar]
Catalano 1991
- Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. American Journal of Obstetrics and Gynecology 1991;165(6):1667-72. [DOI] [PubMed] [Google Scholar]
Catalano 1992
- Catalano PM, Tyzbir ED, Wolfe RR, Roman NM, B Amini S, Sims EA. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. American Journal of Obstetrics and Gynecology 1992;167(4):913-9. [DOI] [PubMed] [Google Scholar]
Catalano 1993
- Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. American Journal of Physiology - Endocrinology and Metabolism 1993;27:E60-67. [DOI] [PubMed] [Google Scholar]
Catalano 1998
- Catalano PM, Drago NM, Amini SB. Longitudinal changes in pancreatic beta-cell function and metabolic clearance rate of insulin in pregnant women with normal and abnormal glucose tolerance. Diabetes Care 1998;21(3):403-8. [DOI] [PubMed] [Google Scholar]
Chatzi 2014
- Chatzi L, Daraki V, Georgiou V, Koutra K, Kampouri M, A Kyriklaki, et al. Maternal obesity, glucose levels in early pregnancy, and gestational diabetes in association with cognitive and psychomotor development at 4 years of age. Diabetologia 2014;57(1 Suppl 1):S72.
Cheung 2018
- Cheung NW, Moses RG. Gestational diabetes mellitus: is it time to reconsider the diagnostic criteria? Diabetes Care 2018;41(7):1337-8. [DOI] [PubMed] [Google Scholar]
Chiu 2004
- Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. American Journal of Clinical Nutrition 2004;79(5):820-5. [DOI] [PubMed] [Google Scholar]
Cho 2000
- Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. Journal of Pediatrics 2000;136(5):587-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chu 2007
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30(8):2070-6. [DOI: 10.2337/dc06-2559a] [DOI] [PubMed] [Google Scholar]
Churchill 1969
- Churchill JA, Berendes HW, Nemore J. Neuropsychological deficits in children of diabetic mothers. A report from the Collaborative Study of Cerebral Palsy. American Journal of Obstetrics and Gynecology 1969;105(2):257-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clements 1980
- Clements RS Jr, Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. American Journal of Clinical Nutrition 1980;33(9):1954-67. [DOI: 10.1016/j.biochi.2013.05.011] [DOI] [PubMed] [Google Scholar]
Corrado 2011
- Corrado F, D'Anna R, Di Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabetic Medicine 2011;28(8):972-5. [DOI: 10.1111/j.1464-5491.2011.03284.x] [DOI] [PubMed] [Google Scholar]
Coustan 2010
- Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2010;202(6):654.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2005
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian Carbohydrate Intolerance Study in Pregnant Women Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477-86. [DOI] [PubMed] [Google Scholar]
Croze 2013
- Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013;95(10):1811-27. [DOI: 10.1016/j.biochi.2013.05.011] [DOI] [PubMed] [Google Scholar]
D'Anna 2013
- D'Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML, et al. Myo-inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care 2013;36(4):854-7. [DOI: 10.2337/dc12-1371] [DOI] [PMC free article] [PubMed] [Google Scholar]
da Silva 2017
- da Silva SG, Hallal PC, Domingues MR, Bertoldi AD, Silveira MF, Bassani D, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. International Journal of Behavioral Nutrition and Physical Activity 2017;14(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dang 2010
- Dang NT, Mukai R, Yoshida K, Ashida H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Bioscience, Biotechnology, and Biochemistry 2010;74(5):1062-7. [DOI: 10.1271/bbb.90963] [DOI] [PubMed] [Google Scholar]
Danyliv 2014
- Danyliv A, Gillespie P, O'Neill C, Noctor E, O'Dea A, Tierney M, et al. Short and long-term effects of gestational diabetes mellitus on the health care cost: cross-sectional comparative study in the ATLANTIC DIP cohort. Diabetic Medicine 2014;32(4):467-76. [DOI: 10.1111/dme.12678] [DOI] [PubMed] [Google Scholar]
Davenport 2018
- Davenport MH, Ruchat S-M, Poitras VJ, Jaramillo GA, Gray CE, Barrowman N, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. British Journal of Sports Medicine 2018;52(21):1367-75. [DOI] [PubMed] [Google Scholar]
Davis 2005
- Davis JN, Ventura EE, Weigensberg MJ, Ball GD, Cruz ML, Shaibi GQ, et al. The relation of sugar intake to beta cell function in overweight Latino children. American Journal of Clinical Nutrition 2005;82(5):1004-10. [DOI: 10.3945/ajcn.2009.28133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dempsey 2004
- Dempsey JC, Butler CL, Sorensen TK, Lee IM, Thompson, ML, Miller RS, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Research and Clinical Practice 2004;66(2):203-15. [DOI: 10.1016/j.diabres.2004.03.010] [DOI] [PubMed] [Google Scholar]
Dodd 2007
- Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: The effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(4):307-12. [DOI] [PubMed] [Google Scholar]
Dodd 2014
- Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014;348:g1285. [DOI: 10.1136/bmj.g1285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egan 2017
- Egan AM, Vellinga A, Harreiter J, Simmons D, Desoye G, Corcoy R, et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia 2017;60(10):1913-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esteghamati 2014
- Esteghamati A, Aryan Z, Esteghamati AR, Nakhjavani M. Vitamin D deficiency is associated with insulin resistance in nondiabetics and reduced insulin production in type 2 diabetics. Hormone and Metabolic Research 2014;47(04):273-9. [DOI] [PubMed] [Google Scholar]
FAO/WHO 2001
- Food and Agriculture Organization of the United Nations, World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. iqb.es/digestivo/pdfs/probioticos.pdf 2001 (accessed 19 September 2016).
Farrar 2016
- Farrar D, Simmonds M, Griffin S, Duarte A, Lawlor DA, Sculpher M, et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses. Health Technology Assessment 2016;20(86):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farren 2017
- Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The prevention of gestational diabetes mellitus with antenatal oral inositol supplementation: a randomized controlled trial. Diabetes Care 2017;40(6):759-63. [DOI] [PubMed] [Google Scholar]
Ferrara 2007
- Ferrara A. Increasing prevalence of gestational diabetes mellitus. Diabetes Care 2007;30(S2):S141-6. [DOI: 10.2337/dc07-s206] [DOI] [PubMed] [Google Scholar]
Fraser 1983
- Fraser RB, Ford FA, Milner RD. A controlled trial of a high dietary fibre intake in pregnancy—effects on plasma glucose and insulin levels. Diabetologia 1983;25(3):238-41. [DOI] [PubMed] [Google Scholar]
Fraser 1988
- Fraser RB, Ford FA, Lawrence GF. Insulin sensitivity in third trimester pregnancy. A randomized study of dietary effects. British Journal of Obstetrics and Gynaecology 1988;95(3):223-9. [DOI] [PubMed] [Google Scholar]
Fraser 2012
- Fraser A, Nelson Scott M, Macdonald-Wallis C, Lawlor DA. Associations of existing diabetes, gestational diabetes, and glycosuria with offspring IQ and educational attainment: the Avon Longitudinal Study of Parents and Children. Experimental Diabetes Research 2012;2012:963735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginci 1997
- Ginci G, Arezzini L, Terzuoli L, Pizzichini M, Marinello E. Effect of estradiol on serum triglyceride lipoprotein levels and fatty acid composition in castrated rats. Hormone and Metabolic Research 1997;29(10):504-6. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Quintero 2007
- Gonzalez-Quintero VH, Istwan NB, Rhea DJ, Rodriguez LI, Cotter A, Carter J, et al. The impact of glycemic control on neonatal outcome in singleton pregnancies complicated by gestational diabetes. Diabetes Care 2007;30(3):467-70. [DOI: 10.2337/dc06-1875] [DOI] [PubMed] [Google Scholar]
Goodarzi‐Khoigani 2017
- Goodarzi-Khoigani M, Mazloomy Mahmoodabad SS, Baghiani Moghadam MH, Nadjarzadeh A, Mardanian F, Fallahzadeh H, et al. Prevention of insulin resistance by dietary intervention among pregnant mothers: a randomized controlled trial. International Journal of Preventive Medicine 2017;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guariguata 2014
- Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Research and Clinical Practice 2014;103(2):176-85. [DOI] [PubMed] [Google Scholar]
Guelfi 2016
- Guelfi KJ, Ong MJ, Crisp NA, Fournier PA, Wallman KE, Grove JR, et al. Regular exercise to prevent the recurrence of gestational diabetes mellitus. Obstetrics and Gynecology 2016;128(4):819-27. [DOI] [PubMed] [Google Scholar]
Guo 2018
- Guo XY, Shu J, Fu XH, Chen XP, Zhang L, Ji MX, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG: an international journal of obstetrics and gynaecology 2019;126(3):311-20. [DOI] [PubMed] [Google Scholar]
Hanson 2015
- Hanson MA, Bardsley A, De-Regil LM, Moore SE, Oken E, Poston L, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition:“Think Nutrition First”. International Journal of Gynecology & Obstetrics 2015;131(S4):S213-S253. [DOI] [PubMed] [Google Scholar]
HAPO 2008
- Group The HAPO Study Cooperative Research. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358(19):1991-2002. [DOI] [PubMed] [Google Scholar]
Harrison 2013
- Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity 2013;21(5):904-9. [DOI] [PubMed] [Google Scholar]
Haw 2017
- Haw J, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, et al. Long-term sustainability of diabetes prevention approaches. JAMA Internal Medicine 2017;177(12):1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2015
- He XJ, Qin FY, Hu CL, Zhu M, Tian CQ, Li L. Is gestational diabetes mellitus an independent risk factor for macrosomia: a meta-analysis? Archives of Gynecology and Obstetrics 2015;291(4):729-35. [DOI: 10.1007/s00404-014-3545-5] [DOI] [PubMed] [Google Scholar]
Hebert 2009
- Hebert MF, Ma X, Naraharisetti SB, Krudys KM, Umans JG, Hankins GD, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clinical Pharmacology & Therapeutics 2009;85(6):607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2014
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology 2014;11(8):506-14. [DOI: 10.1038/nrgastro.2014.66] [DOI] [PubMed] [Google Scholar]
Holt 2014
- Holt RI, Lambert KD. The use of oral hypoglycaemic agents in pregnancy. Diabetic Medicine 2014;31(3):282-91. [DOI: 10.1007/s13224-012-0312-z] [DOI] [PubMed] [Google Scholar]
IADPSG 2010
- Hadar E, Hod M. Establishing consensus criteria for the diagnosis of diabetes in pregnancy following the HAPO study. Annals of the New York Academy of Sciences 2010;1205(1):88-93. [DOI] [PubMed] [Google Scholar]
Ireland 2010
- Guidelines for the Management of Pre-gestational and Gestational diabetes mellitus from pre conception to the postnatal period. Health Service Executive 2010:84.
Jack 1990
- Jack BW, Culpepper L. Preconception care: risk reduction and health promotion in preparation for pregnancy. JAMA 1990;264(9):1147-9. [DOI: 10.1001/jama.1990.03450090083032] [DOI] [PubMed] [Google Scholar]
Jackson 1987
- Jackson RA, Hawa MI, Jaspan JB, Sim BM, Disilvio L, Featherbe D, et al. Mechanism of metformin action in non-insulin-dependent diabetes. Diabetes 1987;36(5):632-40. [DOI: 10.2337/diabetes.36.5.632] [DOI] [PubMed] [Google Scholar]
Jarde 2018
- Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy and Childbirth 2018;18(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 2000
- Jenkins DJ, Axelsen M, Kendall CW, Augustin LS, Vuksan V, Smith U. Dietary fibre, lente carbohydrates and the insulin-resistant diseases. British Journal of Nutrition 2000;83(S1):S157-63. [DOI: 10.1038/sj.ejcn.1602423] [DOI] [PubMed] [Google Scholar]
Jensen 2012
- Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. Journal of Physiology 2012;590(5):1069-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jessen 2005
- Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. Journal of Applied Physiology 2005;99(1):330-7. [DOI] [PubMed] [Google Scholar]
Kantartzis 2009
- Kantartzis K, Totsikas C, Haring HU, Stefan N. Role of ectopic fat in the pathogenesis of insulin resistance. Future Lipidology 2009;4(4):457-64. [DOI: 10.2217/CLP.09.35] [DOI] [Google Scholar]
Kavitha 2013
- Kavitha N, De Somsubhra, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. Journal of Obstetrics and Gynaecology of India 2013;63(2):82-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 1999
- Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, et al. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999;48(5):1192-7. [DOI: 10.2337/diabetes.48.5.1192] [DOI] [PubMed] [Google Scholar]
Khan 2013
- Khan R, Ali K, Khan Z. Socio-demographic risk factors of gestational diabetes mellitus. Pakistan Journal of Medical Sciences 2012;29(3):843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862-8. [DOI] [PubMed] [Google Scholar]
Kim 2007
Kizirian 2017
- Kizirian NV, Goletzke J, Brodie S, Atkinson FS, Markovic TP, Ross GP, et al. Lower glycemic load meals reduce diurnal glycemic oscillations in women with risk factors for gestational diabetes. BMJ Open Diabetes Research & Care 2017;5(1):e000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knopp 1991
- Knopp RH, Magee MS, Raisys V, Benedetti T. Metabolic effects of hypocaloric diets in management of gestational diabetes. Diabetes 1991;40(S2):165-71. [10.2337/diab.40.2.S165] [DOI] [PubMed] [Google Scholar]
Knopp 1992
- Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 1992;15(11):1605-13. [DOI] [PubMed] [Google Scholar]
Knowler 2002
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346(6):393-403. [DOI: 10.1056/NEJMoa012512] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohrt 1993
- Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes 1993;42(2):273-81. [DOI: 10.2337/diabetes.42.2.273] [DOI] [PubMed] [Google Scholar]
Koivusalo 2016
- Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care 2016;39(1):24-30. [DOI] [PubMed] [Google Scholar]
Kondo 2010
- Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Bioscience, Biotechnology, and Biochemistry 2010;74(8):1656-61. [DOI] [PubMed] [Google Scholar]
Koukkou 1996
- Koukkou E, Watts GF, Lowy C. Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study. Journal of Clinical Pathology 1996;49(8):634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kris‐Etherton 2000
- Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. American Journal of Clinical Nutrition 2000;71(1):179S-188S. [DOI] [PubMed] [Google Scholar]
Kris‐Etherton 2009
- Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins, Leukotrienes and Essential Fatty Acids 2009;81(2):99-104. [DOI: 10.1016/j.plefa.2009.05.011] [DOI] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal Medicine 2009;361(14):1339-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2005
- Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. American Journal of Obstetrics and Gynecology 2005;192(4):989-97. [DOI: 10.1016/j.ajog.2004.11.039] [DOI] [PubMed] [Google Scholar]
Lardinois 1987
- Lardinois CK. The role of omega 3 fatty acids on insulin secretion and insulin sensitivity. Medical Hypotheses 1987;24(3):243-8. [DOI: 10.1016/0306-9877(87)90071-5] [DOI] [PubMed] [Google Scholar]
Larqué 2011
- Larqué E, Demmelmair H, Gil-Sánchez A, Prieto-Sánchez MT, Blanco JE, Pagán A, et al. Placental transfer of fatty acids and fetal implications. American Journal of Clinical Nutrition 2011;94(6 Suppl):1908S-1913S. [DOI] [PubMed] [Google Scholar]
Larson‐Meyer 2006
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006;29(6):1337-44. [DOI: 10.2337/dc05-2565] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2004
- Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. Journal of Biological Chemistry 2004;279(17):16971-9. [DOI] [PubMed] [Google Scholar]
Ley 2011
- Ley SH, Hanley AJ, Retnakaran R, Sermer M, Zinman B, O'Connor DL. Effect of macronutrient intake during the second trimester on glucose metabolism later in pregnancy. American Journal of Clinical Nutrition 2011;94(5):1232-40. [DOI: 10.3945/ajcn.111.018861] [DOI] [PubMed] [Google Scholar]
Liang 2017
- Liang HL, Ma SJ, Xiao YN, Tan HZ. Comparative efficacy and safety of oral antidiabetic drugs and insulin in treating gestational diabetes mellitus. Medicine 2017;96(38):e7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liese 2005
- Liese AD, Schulz M, Fang F, Wolever TM, D'Agostino RB Jr, Sparks KC, et al. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care 2005;28(12):2832-8. [DOI: 10.2337/diacare.28.12.2832] [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin N, Zhang H, Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes & Metabolism 2012;38(3):250-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lindsay 2014
- Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). American Journal of Clinical Nutrition 2014;99(6):1432-9. [DOI: 10.3945/ajcn.113.079723] [DOI] [PubMed] [Google Scholar]
Ludwig 2002
- Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287(18):2414-23. [DOI: 10.1002/mnfr.201400594] [DOI] [PubMed] [Google Scholar]
Luoto 2010
- Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. British Journal of Nutrition 2010;103(12):1792-9. [DOI] [PubMed] [Google Scholar]
Luoto 2011
- Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counselling: a cluster-randomized controlled trial. PLOS Medicine 2011;8(5):e1001036. [DOI: 10.1371/journal.pmed.1001036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2008
- Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. Journal of Hepatology 2008;49(5):821-30. [DOI: 10.1016/j.jhep.2008.05.025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magee 1990
- Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200-kcal diet in obese pregnant women with gestational diabetes. Diabetes 1990;39(2):234-40. [DOI: 10.2337/diab.39.2.234] [DOI] [PubMed] [Google Scholar]
Marcotorchino 2012
- Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, et al. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Molecular Nutrition & Food Research 2012;56(12):1771-82. [DOI: 10.1002/mnfr.201200383] [DOI] [PubMed] [Google Scholar]
Markovic 2015
- Markovic TP, Muirhead R, Overs S, Ross GP, Louie JC, Kizirian N, et al. Randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in women at high risk of gestational diabetes mellitus: The GI Baby 3 Study. Diabetes Care 2015;39(1):31-8. [DOI: 10.2337/dc15-0572] [DOI] [PubMed] [Google Scholar]
Mcintosh 2001
- Mcintosh M, Miller C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutrition Reviews 2001;59(2):52-5. [DOI: 10.1111/j.1753-4887.2001.tb06976.x] [DOI] [PubMed] [Google Scholar]
McIntyre 2018
- McIntyre HD, Jensen DM, Jensen RC, Kyhl HB, Jensen TK, Glintborg D, et al. Gestational diabetes mellitus: does one size fit all? A challenge to uniform worldwide diagnostic thresholds. Diabetes Care 2018;41(7):1339-42. [DOI] [PubMed] [Google Scholar]
Medley 2018
- Medley N, Vogel JP, Care A, Alfirevic Z. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD012505. [DOI: 10.1002/14651858.CD012505.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melchior 2017
- Melchior H, Kurch-Bek D, Mund M. The prevalence of gestational diabetes. Deutsches Arzteblatt international 2017;114(24):412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger 2007
- Metzger BE, Buchanan TA, Coustan DR, De Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care 2007;30(2):S251-60. [DOI: 10.2337/dc07-s225] [DOI] [PubMed] [Google Scholar]
Ministry of Health 2014
- Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A clinical practice guideline. Ministry of Health 2014.
Morisset 2010
- Morisset AS, St-Yves A, Veillette J, Weisnagel SJ, Tchernof A, Robitaille J. Prevention of gestational diabetes mellitus: a review of studies on weight management. Diabetes/metabolism Research and Reviews 2010;26(1):17-25. [DOI] [PubMed] [Google Scholar]
Nankervis 2014
- Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C, et al. ADIPS Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. Australian Government. National Health and Medical Research Council 2014:1-8.
Nelson 1994
- Nelson T, Shulman G, Grainger D, Diamond MP. Progesterone administration induced impairment of insulin suppression of hepatic glucose production. Fertility and Sterility 1994;62(3):491-6. [DOI] [PubMed] [Google Scholar]
Nelson 2000
- Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, DeRegnier R, Georgieff M. Neurocognitive sequelae of infants of diabetic mothers. Behavioral Neuroscience 2000;114(5):950-6. [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence. Weight management before, during and after pregnancy. www.nice.org.uk/guidance/ph27 2010 (accessed 19th September 2016).
NICE 2015
- National Institute for Health and Care Excellence. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. www.nice.org.uk/guidance/ng3 2015 (accessed 2016). [PubMed]
Norman 1980
- Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science (New York, N.Y.) 1980;209(4458):823-5. [DOI: 10.1126/science.6250216] [DOI] [PubMed] [Google Scholar]
Ong 2009
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes & Metabolism 2009;35(5):421. [DOI: 10.1016/j.diabet.2009.04.008] [DOI] [PubMed] [Google Scholar]
Ornoy 1998
- Ornoy A, Ratzon N, Greenbaum C, Peretz E, Soriano D, Dulitzky M. Neurobehaviour of school age children born to diabetic mothers. Archives of Disease in Childhood: Fetal and Neonatal Edition 1998;79(2):F94-9. [DOI: 10.1136/fn.79.2.F94] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 1993
- Pan DA, Storlien LH. Dietary lipid profile is a determinant of tissue phospholipid fatty acid composition and rate of weight gain in rats. Journal of Nutrition 1993;123(3):512-9. [DOI] [PubMed] [Google Scholar]
Perseghin 1996
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, et al. Increased glucose transport–phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. New England Journal of Medicine 1996;335(18):1357-62. [DOI: 10.1056/NEJM199610313351804] [DOI] [PubMed] [Google Scholar]
Petrella 2014
- Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. Journal of Maternal-Fetal & Neonatal Medicine 2014;27(13):1348-52. [DOI] [PubMed] [Google Scholar]
Petry 2010
- Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. British Journal of Nutrition 2010;104(06):775-87. [DOI] [PubMed] [Google Scholar]
Pole 1999
- Pole JD, Dodds LA. Maternal outcomes associated with weight change between pregnancies. Canadian Journal of Public Health 1999;90(4):233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poston 2015
- Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al, UPBEAT Trial Consortium. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinology 2015;3(10):767-77. [DOI] [PubMed] [Google Scholar]
Procter 2014
- Procter SB, Campbell CG. Position of the Academy of Nutrition and Dietetics: nutrition and lifestyle for a healthy pregnancy outcome. Journal of the Academy of Nutrition and Dietetics 2014;114(7):1099-103. [DOI] [PubMed] [Google Scholar]
Pu 2015
- Pu J, Zhao B, Wang EJ, Nimbal V, Osmundson S, Kunz L, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatric and Perinatal Epidemiology 2015;29(5):436-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinlivan 2011
- Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51(2):141-6. [DOI] [PubMed] [Google Scholar]
Rakhshani 2012
- Rakhshani A, Nagarathna R, Mhaskar R, Mhaskar A, Thomas A, Gunasheela S. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: a randomized controlled trial. Preventive Medicine 2012;55(4):333-40. [DOI: 10.1016/j.ypmed.2012.07.020] [DOI] [PubMed] [Google Scholar]
Reiser 1979
- Reiser S, Handler HB, Gardner LB, Hallfrisch JG, Michaelis OE, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. II. Effect on fasting blood insulin, glucose, and glucagon and on insulin and glucose response to a sucrose load. American Journal of Clinical Nutrition 1979;32(11):2206-16. [DOI] [PubMed] [Google Scholar]
Rizzo 1991
- Rizzo T, Metzger BE, Burns WJ, Burns, K. Correlations between antepartum maternal metabolism and intelligence of offspring. New England Journal of Medicine 1991;325(13):911-6. [DOI: 10.1056/NEJM199109263251303] [DOI] [PubMed] [Google Scholar]
Rogozinska 2015
- Rogozinska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLOS One 2015;10(2):e0115526. [DOI: 10.1371/journal.pone.0115526] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2005
- Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology 2005;20(4):260-70. [DOI] [PubMed] [Google Scholar]
Rowan 2018
- Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Research & Care 2018;6(1):e000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 1985
- Ryan EA, O'Sullivan MJ, Skyler JS. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 1985;34(4):380-9. [DOI] [PubMed] [Google Scholar]
Sacks 2015
- Sacks DA, Black MH, Li X, Montoro MN, Lawrence JM. Adverse pregnancy outcomes using the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstetrics and Gynecology 2015;126(1):67-73. [DOI] [PubMed] [Google Scholar]
Saltiel 1990
- Saltiel AR. Second messengers of insulin action. Diabetes Care 1990;13(3):256. [DOI: 10.2337/diacare.13.3.244] [DOI] [PubMed] [Google Scholar]
Sanabria‐Martínez 2015
- Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, Álvarez-Bueno C, Sánchez-López M, Martínez-Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG: an international journal of obstetrics and gynaecology 2015;122(9):1167-74. [DOI] [PubMed] [Google Scholar]
Santamaria 2016
- Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D’Anna R, et al. Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(19):3234-7. [DOI] [PubMed] [Google Scholar]
Sattar 1997
- Sattar N, Greer IA, Louden J, Lindsay G, McConnell M, Shepherd J, et al. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small, dense low density lipoprotein. Journal of Clinical Endocrinology & Metabolism 1997;82(8):2483-91. [DOI] [PubMed] [Google Scholar]
Setji 2005
- Setji TL, Brown AJ, Feinglos MN. Gestational diabetes mellitus. Clinical Diabetes 2005;23(1):17-24. [Google Scholar]
Shyam 2013
- Shyam S, Arshad F, Abdul Ghani R, Wahab NA, Safii NS, Nisak MY, et al. Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: a six months randomized trial. Nutrition Journal 2013;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2015
- Simmons D, Jelsma JG, Galjaard S, Devlieger R, Assche A, Jans G, et al. Results from a European multicenter randomized trial of physical activity and/or healthy eating to reduce the risk of gestational diabetes mellitus: the DALI Lifestyle Pilot. Diabetes Care 2015;38(9):1650–6. [DOI] [PubMed] [Google Scholar]
Sjøberg 2011
- Sjøberg KA, Rattigan S, Hiscock N, Richter EA, Kiens B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. American Journal of Physiology-Heart and Circulatory Physiology 2011;301(2):H450-8. [DOI] [PubMed] [Google Scholar]
Slocum 2002
- Slocum JM, Sosa ME. Use of antidiabetes agents in pregnancy: current practice and controversy. Journal of Perinatal & Neonatal Nursing 2002;2(40):53. [DOI: 10.1016/j.clinthera.2007.12.015] [DOI] [PubMed] [Google Scholar]
Soheilykhah 2013
- Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecological Endocrinology 2013;29(4):396-9. [DOI] [PubMed] [Google Scholar]
Solomon 1997
- Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278(13):1078-83. [DOI: 10.1001/jama.278.13.1078] [DOI] [PubMed] [Google Scholar]
Song 2016
- Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obesity Reviews 2016;17(10):960-9. [DOI] [PubMed] [Google Scholar]
Sooy 1999
- Sooy K, Schermerhorn T, Noda M, Surana M, Rhoten WB, Meyer M, et al. Calbindin-D(28k) controls [Ca(2+)](i) and insulin release. Evidence obtained from calbindin-d(28k) knockout mice and beta cell lines. Journal of Biological Chemistry 1999;274(48):34343-9. [DOI: 10.1074/jbc.274.48.34343] [DOI] [PubMed] [Google Scholar]
Sorenson 1993
- Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 1993;133(5):2227-34. [DOI] [PubMed] [Google Scholar]
Stafne 2012
- Stafne SN, Salvesen KA, Romundstad PR, Eggebo TM, Carlsen SM, Morkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstetrics and Gynecology 2012;119:29-36. [DOI: 10.1097/AOG.0b013e3182393f86] [DOI] [PubMed] [Google Scholar]
Stumvoll 1995
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. New England Journal of Medicine 1995;333(9):550-4. [CENTRAL: 10.1056/NEJM199508313330903] [DOI] [PubMed] [Google Scholar]
Tannous dit El Khoury 2006
- Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Annals of Nutrition & Metabolism 2006;50(3):260-9. [DOI: 10.1159/000091684] [DOI] [PubMed] [Google Scholar]
Theriault 2014
- Theriault S, Forest JC, Masse J, Giguere Y. Validation of early risk-prediction models for gestational diabetes based on clinical characteristics. Diabetes Research & Clinical Practice 2014;103(3):419-25. [DOI: 10.1016/j.diabres.2013.12.009] [DOI] [PubMed] [Google Scholar]
Thompson 2013
- Thompson D, Berger H, Feig D, Gagnon R, Kader T, Keely E, et al. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: diabetes and pregnancy. Canadian Journal of Diabetes 2013;37(S1):S168-83. [DOI: 10.1016/j.jcjd.2013.01.044] [DOI] [Google Scholar]
Tobias 2011
- Tobias DK, Zhang C, Van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care 2011;34(1):223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torloni 2009
Torres‐Espinola 2015
- Torres-Espinola FJ, Berglund SK, Garcia-Valdes LM, Segura MT, Jerez A, Campos D, et al. Maternal obesity, overweight and gestational diabetes affect the offspring neurodevelopment at 6 and 18 months of age - a follow up from the PREOBE cohort. PLOS One 2015;10(7):e0133010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toulis 2009
- Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertility and Sterility 2009;92(2):667-77. [DOI] [PubMed] [Google Scholar]
Tuomilehto 2001
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343-50. [DOI: 10.1056/NEJM200105033441801] [DOI] [PubMed] [Google Scholar]
Unfer 2017
- Unfer V, Facchinetti F, Orrù B, Giordani B, Nestler J. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocrine Connections 2017;6(8):647-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vahouny 1988
- Vahouny GV, Satchithanandam S, Chen I, Tepper SA, Kritchevsky D, Lightfoot FG, et al. Dietary fiber and intestinal adaptation: effects on lipid absorption and lymphatic transport in the rat. American Journal of Clinical Nutrition 1988;47(2):201-6. [DOI: 10.1094/CCHEM-87-3-0190] [DOI] [PubMed] [Google Scholar]
Van Assche 1978
- Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. British Journal of Obstetrics and Gynaecology 1978;85(11):818-20. [DOI] [PubMed] [Google Scholar]
Vanky 2005
- Vanky E, Zahlsen K, Spigset O, Carlsen S. Placental passage of metformin in women with polycystic ovary syndrome. Fertility and Sterility 2005;83(5):1575-8. [DOI] [PubMed] [Google Scholar]
Viollet 2012
- Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical Science 2012;122(6):253-70. [DOI: 10.1042/CS20110386] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitagliano 2019
- Vitagliano A, Saccone G, Cosmi E, Visentin S, Dessole F, Ambrosini G, et al. Inositol for the prevention of gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Archives of Gynecology and Obstetrics 2019;299(1):55-68. [DOI] [PubMed] [Google Scholar]
Weinhaus 1996
- Weinhaus AJ, Stout LE, Sorenson RL. Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology 1996;137(5):1640-9. [DOI] [PubMed] [Google Scholar]
Whiting 2015
- Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology 2015;69:225-34. [DOI: 10.1016/j.jclinepi.2015.06.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO/NMH/MND/13.2 edition. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
Wickens 2017
- Wickens KL, Barthow CA, Murphy R, Abels PR, Maude RM, Stone PR, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. British Journal of Nutrition 2017;117:804-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijendran 1999
- Wijendran V, Bendel RB, Couch SC, Philipson EH, Thomsen K, Zhang X, et al. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. American Journal of Clinical Nutrition 1999;70(1):53-61. [DOI] [PubMed] [Google Scholar]
Wollen 1988
- Wollen N, Bailey CJ. Inhibition of hepatic gluconeogenesis by metformin: synergism with insulin. Biochemical Pharmacology 1988;37(22):4353-8. [DOI] [PubMed] [Google Scholar]
Xiong 2001
- Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. International Journal of Gynecology & Obstetrics 2001;75(3):221-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeung 2010
- Yeung EH, Hu FB, Solomon CG, Chen L, Louis GM, Schisterman E, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia 2010;53(4):668-78. [DOI: 10.1007/s00125-009-1634-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yki‐Jarvinen 1983
- Yki-Jarvinen H, Koivisto VA. Effects of body composition on insulin sensitivity. Diabetes 1983;32(10):965-9. [DOI: 10.2337/diab.32.10.965] [DOI] [PubMed] [Google Scholar]
Yu 2018
- Yu Y, Xie R, Shen C, Shu L. Effect of exercise during pregnancy to prevent gestational diabetes mellitus: a systematic review and meta-analysis. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(12):1632-7. [DOI] [PubMed] [Google Scholar]
Zhang 2006
- Zhang C, Liu S, Solomon CG, Hu FB. Dietary fibre intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006;29(10):2223-30. [DOI: 10.2337/dc06-0266] [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang C, Tobias DK, Chavarro JE, Bao W, Wang D, Ley SH, et al. Adherence to healthy lifestyle before pregnancy and reduced risk of gestational diabetes. BMJ 2014;349:g5450. [DOI: 10.2337/db14-1317-1629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2017
- Zheng J, Wang H, Ren M. Influence of exercise intervention on gestational diabetes mellitus: a systematic review and meta-analysis. Journal of Endocrinological Investigation 2017;40(10):1027-33. [DOI] [PubMed] [Google Scholar]
Zhou 2012
- Zhou SJ, Yelland L, McPhee AJ, Quinlivan J, Gibson RA, Makrides M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. American Journal of Clinical Nutrition 2012;95(6):1378-84. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Griffith 2019
- Griffith RJ, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, Crowther CA. Interventions to prevent women developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012394. [DOI: 10.1002/14651858.CD012394.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrence 2016
- Lawrence RL, Brown J, Middleton P, Shepherd E, Brown S, Crowther CA. Interventions for preventing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD012394. [DOI: 10.1002/14651858.CD012394] [DOI] [PMC free article] [PubMed] [Google Scholar]


