Abstract
Idiopathic inflammatory myopathies (IIM) are a heterogeneous group of connective tissue diseases, collectively known as myositis. Diagnosis of IIM is challenging while timely recognition of an IIM is of utter importance considering treatment options and otherwise irreversible (severe) long-term clinical complications. With the EULAR/ACR classification criteria (2017) considerable advancement has been made in the diagnostic workup of IIM. While these criteria take into account clinical parameters as well as presence of one autoantibody, anti-Jo-1, several autoantibodies are associated with IIM and are currently evaluated to be incorporated into classification criteria. As individual antibodies occur at low frequency, the development of line blots allowing multiplex antibody analysis has improved laboratory diagnostics for IIM. The Euroline myositis line-blot assay (Euroimmun) allows screening and semi-quantitative measurement for 15 autoantibodies, i.e. myositis specific antibodies (MSA) to SRP, EJ, OJ, Mi-2α, Mi-2β, TIF1-γ, MDA5, NXP2, SAE1, PL-12, PL-7, Jo-1 and myositis associated antibodies (MAA) to Ku, PM/Scl-75 and PM/Scl-100. To evaluate the clinical significance of detection and levels of these autoantibodies in the Netherlands, a retrospective analysis of all Dutch requests for extended myositis screening within a 1 year period was performed. A total of 187 IIM patients and 632 non-IIM patients were included. We conclude that frequencies of MSA and MAA observed in IIM patients in a routine diagnostic setting are comparable to cohort-based studies. Weak positive antibody levels show less diagnostic accuracy compared to positive antibody levels, except for anti-NXP2. Known associations between antibodies and skin involvement (anti-MDA5, anti-TIF1-γ), lung involvement (anti-Jo-1), and malignancy (anti-TIF1-γ) were confirmed in our IIM study population. The availability of multiplex antibody analyses will facilitate inclusion of additional autoantibodies in clinical myositis guidelines and help to accelerate diagnosing IMM with rare but specific antibodies.
Keywords: Idiopathic inflammatory myopathies, Myositis, Diagnostic parameters, Multiplex assay, Validation
Highlights
-
•
Similar frequency of MAA and MSA in diagnostic setting compared to cohort studies.
-
•
Positive levels of MSA/MAA show better diagnostic accuracy compared to weak positive levels.
-
•
Anti-NXP2 differentiates already at weak positive level between IIM and non-IIM patients.
-
•
Anti-TIF1-γ is associated with malignancy and anti-MDA5 and TIF1-γ with skin disease.
-
•
Anti-Jo-1 is associated with lung disease.
Abbreviations
- sCK
serum creatine kinase
- DM
dermatomyositis
- IBM
inclusion body myositis
- IIM
idiopathic inflammatory myopathies
- IMNM
immune mediated necrotizing myopathy
- JDM
juvenile dermatomyositis
- MAA
myositis associated antibodies
- MSA
myositis specific antibodies
- PM
polymyositis
1. Introduction
Idiopathic inflammatory myopathies (IIM), collectively known as myositis, are a heterogeneous group of diseases characterized by muscle weakness and inflammation and include anti-synthetase-syndrome (ASS), dermatomyositis (DM), non-specific (‘overlap’) myositis, immune mediated necrotizing myopathy (IMNM), inclusion body myositis (IBM), and, in children, juvenile dermatomyositis (JDM). Other organs are frequently affected in IIM’s including joints, heart, lungs, gastrointestinal tract and skin. Extra-muscular symptoms, such as skin manifestations, arthritis or interstitial lung disease, might be the presenting or predominant feature when muscle symptoms are mild or absent in IIM [[1], [2], [3]]. The EULAR/ACR classification criteria defined in 2017 [2] aim to differentiate IIM from non-IIM. These criteria are based on clinical parameters as well as laboratory parameters. Until now only presence of Jo-1 auto-antibodies is included in the classification criteria but it is increasingly recognized that presence of other myositis specific antibodies (MSA) can contribute to identify subgroups of IIM [4].
Multiple auto-antibodies are currently known to associate with myositis. These can be classified into MSA that are primarily found in IIM patients i.e. antibodies to SRP, Mi-2α, Mi-2β, TIF1-γ, MDA5, NXP2, SAE1, EJ, OJ, PL-12, PL-7, Jo-1, HMGCR, and cN1A and myositis associated antibodies (MAA) that also occur in patients with other rheumatic disorders i.e. antibodies to Ku, PM/Scl-75 and PM/Scl-100 [5]. Like in other systemic autoimmune diseases, distinct clinical phenotypes or organ involvement are associated with specific autoantibody targets in patients [6,7]. For example, anti-TIF1-γ, anti-MDA5 and anti-NXP2 are associated with DM, of which MDA5 is more frequently seen in the so called ‘dermato-pulmonary syndrome’ while anti-TIF1-γ is associated with an increased risk of malignancy [8].
The vast majority of MSA and MAA have been discovered using immunoprecipitation (IP) and IP is therefore considered the gold standard assay [9]. However, this assay is time consuming and subjective to interpretive errors and consequently not suited for use in large scale routine diagnostics. Multiplex assays such as immunodot or line immunoassays, on the other hand, are very suitable for high throughput analysis in routine diagnostic laboratories, but might not be the optimal detection method for all antibodies. The conventional technologies, employing more often native antigens, generally allow better detection of antibodies directed to conformational epitopes as well as to epitopes depending on post-translational modifications. Adequate validation of both the IP as well as the line immunoassays are hampered by the low prevalence of the distinct MSA and MAA, and also by the heterogeneity of the clinical picture of myositis. One solution to overcome this problem is to use clinically defined disease- and control cohorts. Although this approach is often considered as the gold standard, it may show a selection bias as preferentially patients are included that fulfill all clinical classification criteria. In addition, retrospective cohorts often do not include sufficient patients with low frequent autoantibodies and therefore results for associations between autoantibodies and clinical subgroups differ dependent on the cohort studied [5]. Large multi-center initiatives, like EuroMyositis [10], could overcome these problems due to the large cohort size; currently more than 4000 IIM patients are enlisted in this registry.
While validation of multiplex techniques like the Euroline myositis line-blot assay of Euroimmun, are still ongoing, the assay is already widely used in routine diagnostics. So far, only clinically defined retrospective cohort studies or single center results have been published [5,11]. In the Netherlands, extended MSA/MAA analysis is performed in 5 centers. All centers use the Euroline myositis line-blot assay of Euroimmun. This allows an alternative validation approach by performing a nation-wide validation study with a high number of consecutive patients with a request for extended MSA/MAA analysis. The aim of our study is to compare frequencies and clinical associations of MSA/MAA obtained with the Euroline myositis line-blot assay in routine diagnostics to previously reported results of cohort studies.
2. Material and methods
2.1. Patients and sera
In the Netherlands, extended myositis antibody determination is performed in only 5 centers: St. Antonius Hospital, Sanquin Diagnostic Services, Catharina Hospital Eindhoven, Amsterdam UMC location AMC and Erasmus MC. All use the Euroline myositis line-blot assay of Euroimmun. Patients from other hospitals in the Netherlands are referred to one of the 5 centers for extended myositis antibody testing. In this retrospective study, all requests of extended myositis related antibody determination in the Netherlands between 1-7–2016 and 30-6-2017 were examined. Redundant requests were excluded by including only the first sample if more than one sample of a given patient in that period was present. In total, 819 patients from 22 general hospitals and all 8 university hospitals were included. Determination of anti-Jo-1 is part of regular extractable nuclear antibody (ENA) testing. Patients found positive for anti-Jo-1 as part of ENA testing will generally not be referred to extended myositis antibody testing and are therefore underrepresented in our study population. Since patients from 30 hospitals are included structural selection bias is unlikely.
Results of the Euroline myositis line blot were queried from the locally used laboratory information system (LIMS) and for each hospital an overview was prepared including patient identification number and results of antibody determination. Retrospectively, clinical data were provided by the local hospital by entering the following information to the overview: Diagnosis; IIM Yes/No; organ involvement (muscles, skin, joints, lung, heart; in which blank values were scored as negative); diagnosed with cancer at time of diagnosis ±3 years; serum creatinine kinase (sCK) activity results closest to time of blood draw within ±6 month. Patients were subdivided into IIM patients (n = 187) and non-IIM patients (n = 632) based on judgment of the treating physician. Completed overviews containing results for antibody determination and clinical data were anonymized by the local hospital and provided to the investigators. Occurrence of MSA/MAA in IIM patients were compared to non-IIM patients, which form the control group of our study. Patients with multi-specificities were not excluded from analyses.
The study was approved by the ethics committee of the Erasmus University Medical Centre under protocol number MEC-2016-606.
2.2. Determination of MSA/MAA
The Euroline myositis line-blot assay of Euroimmun (Lübeck, Germany) was performed according to the instructions of the manufacturer. This blot allows for detection of multiple MSA, namely antibodies against SRP, EJ, OJ, Mi-2α, Mi-2β, TIF1-γ, MDA5, NXP2, SAE1, PL-12, PL-7 and Jo-1, and MAA, specifically antibodies against Ku, PM/Scl-75 and PM/Scl-100. Anti-Ro52 was excluded from the analysis because anti-Ro52 is not specific for myositis or connective tissue diseases [12]. HGMCoAR antibodies and cN1A antibodies have not been systematically tested in this cohort of patients, since they are not part of the above-mentioned assay.
All immunoblot strips were analyzed with the EUROLineScan (Euroimmun) according to the manufacturers recommendations, and scored negative, weakly positive (+) and positive (++ or +++). This corresponds to intensity levels 0–10, 11–25 and > 25, respectively. The strong positive intensity level (>50) was not reported by all centers, therefore all intensity levels >25 were included in the positive category.
2.3. Statistics
Statistical analysis was carried out using SPSS (IBM SPSS statistics version 24). Difference in sCK levels between IIM and non-IIM or differences in age and the occurrence of malignancy in anti-TIF1-γ positive IIM patients was tested with a two-sided unpaired student T test assuming unequal variances. Differences in frequencies of dichotomous variables were analyzed by chi-squared test or Fisher’s exact test. A logistic regression model was used for testing antibody level and associations with IIM. Generated odds ratios are shown and P-values <0.05 were considered to be statistically significant. In the case of multiple hypothesis testing (organ involvement), post-hoc Bonferroni adjustment was carried out and P-values <0.01 were considered statistically significant.
Diagnostic accuracy for IIM was measured by sensitivity, specificity, likelihood ratio, positive- and negative-predictive values for any antibody specificity investigated. Sensitivity (%) for IIM = (number of MSA/MAA-positive patients with IIM/total patients with IIM) × 100. Specificity (%) for IIM (with respect to disease controls) = (number of MSA/MAA-negative non-IIM patients/total non-IIM patients) × 100. Likelihood ratios were calculated using sensitivity/(1-specificity). LRs greater than 10 generally argue for the presence of disease [13,14]. Positive predictive values (PPV) was calculated using (number of true positives)/(number of true positives + number of false positives) and subsequently the negative predictive value (NPV) by (number of true negatives)/(number of true negatives + number of false negatives).
3. Results
3.1. Frequencies of MSA and MAA and associations with IIM in Dutch samples with a request for extended myositis antibody testing
A total of 840 samples of individual Dutch patients with results from the Euroline myositis line-blot assay were included. Of these, clinical data of 819 patients were available of which 187 patients (22.8%) were diagnosed with IIM; the other 632 patients were classified as non-IIM and considered as disease controls.
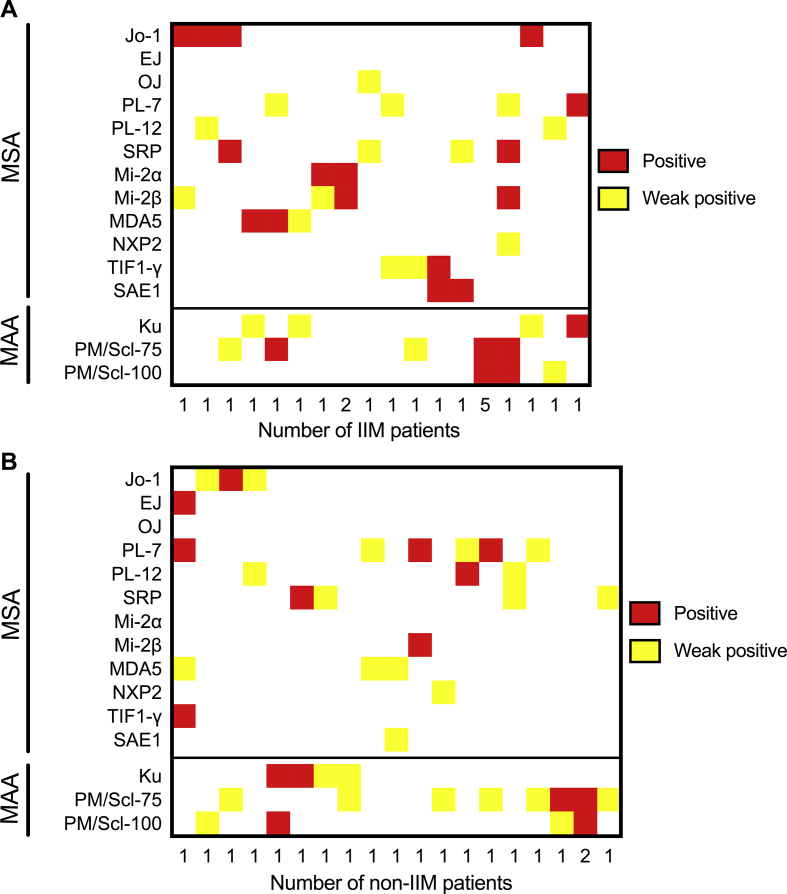
In the vast majority of antibody-positive IIM patients (n = 119 in total) and antibody-positive non-IIM patients (n = 160 in total) only one MSA/MAA was detected. Twenty-three out of 119 antibody-positive IIM patients presented with 2 (n = 20), 3 (n = 2), or with 6 (n = 1) distinct antibodies. Twenty out of 160 antibody-positive non-IIM patients showed 2 (n = 19) or 4 (n = 1) antibodies (Figure Supplementary Fig. 1). Of the 23 IIM patients with multi-specificities, 5 patients showed only a combination of anti-PM/Scl-75 and anti-PM/Scl-100, and 3 a combination of only anti-Mi-2α and anti-Mi-2β. Of the 20 non-IIM patients with multi-specificities, 3 showed only a combination of anti-PM/Scl-75 and anti-PM/Scl-100. Of all 43 sera with multiple antibodies, 18 sera showed a combination of two or more antibodies at the positive intensity level. All other sera showed a combination of antibodies at the weak positive and the positive level (n = 10) or only at the weak positive level (n = 15).
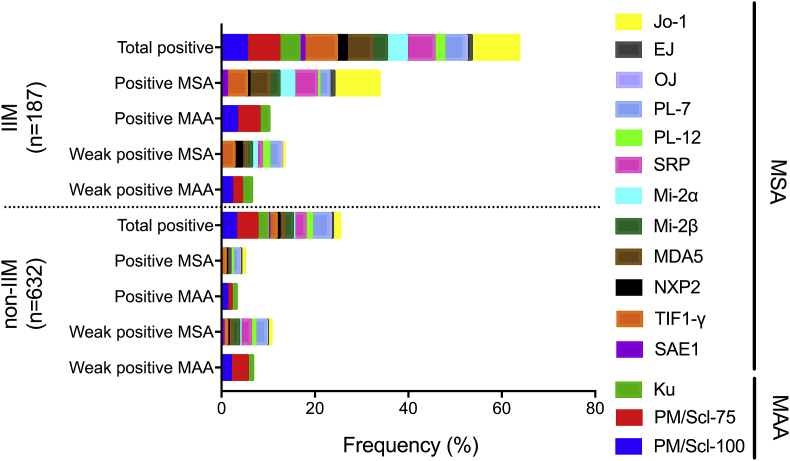
Median age and female to male ratio of IIM and non-IIM patients were comparable (Table 1). Date of birth was available from 182 IIM patients including 7 patients younger than 18 years and from 558 non-IIM patients including 26 patients younger than 18 years. Due to the low number of patients below 18 years included no sub-analyses for children and adults were performed. Median sCK levels were higher in IIM patients compared to non-IIM patients but the difference did not reach statistical significance (p = 0.111). Frequencies of MSA and MAA were different between the two groups (Fig. 1). Antibody frequencies differed, e.g. anti-Jo-1 showed the highest frequency (10.16%), followed by anti-PM/Scl-75, anti-SRP, anti-MDA5 and anti-PM/Scl-100, while the lowest frequency was found for anti-OJ (0.53%) (Table 2). These results are in agreement with earlier studies [4,9,13,[15], [16], [17]].
Table 1.
Study population characteristics.
| IIM | non-IIM | Pf | |
|---|---|---|---|
| Number | 187 | 632 | |
| Female: male | 61 : 39 | 50:50 | |
| Median age (range) | 62b (6–93) | 62c (5–95) | |
| Median sCKa level (range) | 702d (25–35.000) U/L | 106e (10–100.000) U/L | 0.111 |
Serum creatinine kinase.
Data available from 182 patients including 7 patients <18 years of age.
Data available from 558 patients including 26 patients <18 years of age.
Data available from 177 patients.
Data available from 365 patients.
Two-sided unpaired T-test assuming unequal variances.
Fig. 1.
Individual frequency per intensity level for IIM and non-IIM for different antibodies as measured with the Euroline myositis line-blot assay (Euroimmun). IIM; idiopathic inflammatory myopathy, MSA; myositis specific antibodies, MAA; myositis associated antibodies. Data is not corrected for multiple antibodies per patient.
Table 2.
Frequency of MSA and MAA in IIM patients and non-IIM patients.
| Antibody | IIM (n = 187)a |
non-IIM (n = 632)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # Posa | % Pos | % Lit.b | # Posa | % Pos | PPVc | NPVd | LRe | 95% CIf | Pg | |
| Jo-1 | 19 | 10.2 | 15–30 | 10 | 1.6 | 65.5 | 78.7 | 6.4 | 3.0–13.6 | <0.001 |
| EJ | 2 | 1.1 | <2 | 3 | 0.5 | 40.0 | 77.3 | 2.3 | 0.4–13.4 | 0.321 |
| OJ | 1 | 0.5 | <2 | 2 | 0.3 | 33.3 | 77.2 | 1.7 | 0.2–18.5 | 0.541 |
| PL-7 | 8 | 4.3 | 3–4 | 23 | 3.6 | 25.8 | 77.3 | 1.2 | 0.5–2.6 | 0.665 |
| PL-12 | 4 | 2.1 | 3–4 | 9 | 1.4 | 30.8 | 77.3 | 1.5 | 0.5–4.8 | 0.507 |
| SRP | 11 | 5.9 | 5 | 15 | 2.5 | 42.3 | 77.8 | 2.5 | 1.2–5.3 | 0.029 |
| Mi-2α | 8 | 4.3 | 5–10 | 2 | 0.3 | 80.0 | 77.9 | 13.5 | 2.9–63.1 | <0.001 |
| Mi-2β | 6 | 3.2 | 5–10 | 12 | 1.9 | 33.3 | 77.4 | 1.7 | 0.6–4.4 | 0.267 |
| MDA5 | 10 | 5.4 | 0–13h | 6 | 0.9 | 62.5 | 78.0 | 5.6 | 2.1–15.3 | 0.001 |
| NXP2 | 4 | 2.1 | 2–20 | 4 | 0.6 | 50.0 | 77.4 | 3.4 | 0.9–13.4 | 0.085 |
| TIF1-γ | 13 | 7.0 | 20–40 | 10 | 1.6 | 56.5 | 78.1 | 4.4 | 2.0–9.9 | <0.001 |
| SAE1 |
2 |
1.1 |
2–8 |
2 |
0.3 |
50.0 |
77.3 |
3.4 |
0.5–23.8 |
0.225 |
| All MSAi |
88 |
47.1 |
98 |
18.4 |
47.3 |
84.4 |
3.0 |
2.4–3.9 |
<0.001 |
|
| Ku | 8 | 4.3 | 20–30 | 14 | 2.2 | 36.4 | 77.5 | 1.9 | 0.8–4.5 | 0.128 |
| PM/Scl-75 | 13 | 7.0 | 10–15 | 29 | 4.6 | 31.0 | 77.6 | 1.5 | 0.8–2.9 | 0.192 |
| PM/Scl-100 |
10 |
5.4 |
10–15 |
19 |
3.0 |
34.5 |
77.6 |
1.8 |
0.8–3.8 |
0.173 |
| All MAAi | 31 | 16.6 | 62 | 10.9 | 33.3 | 78.5 | 1.7 | 1.1–2.5 | 0.010 | |
Data is not corrected for multiple antibodies in one patient.
PPV; positive predictive value.
NPV; negative predictive value.
LR; likelihood ratio of presence of an IIM for a given antibody.
95% Confidence Interval of Fisher’s exact test.
Fisher’s exact test two sided IIM vs non-IIM with and without antibody.
In Caucasians.
Sum of all patients with at least one positive antibody.
A significant association was found between the occurrence of all MSA or MAA together with IIM, with likelihood ratios (LR) of 3.0 and 1.7, although the positive predictive value (PPV) was rather low, being 47% and 33%, respectively (Table 2). LR > 10 were found only for anti-Mi-2α, with a PPV of 80%. Other antibodies displaying a LR > 4 in combination with a significant association with IIM were anti-Jo-1, anti-TIF1-γ and anti-MDA5 (Table 2). The PPV calculated for these antibodies ranged from 65% to 56%. LR were <2 and PPV values were <40% for MSA occurring at low prevalence, e.g. antibodies to PL-7, PL-12, OJ, Mi-2β, and all MAA (Table 2).
3.2. Auto-antibody level and association with IIM
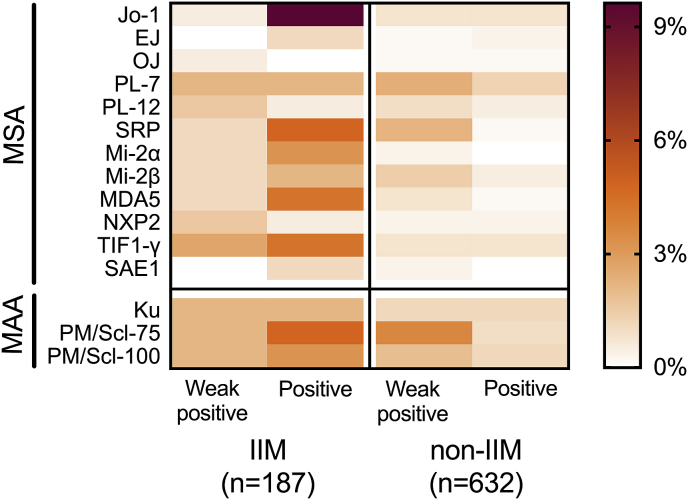
In general, MSA and MAA intensity levels varied largely between patients. Antibodies such as anti-Jo-1, anti-SRP, anti-MDA5 and anti-TIF1-γ frequently revealed positive intensity levels in samples from IIM patients, while other antibodies, such as anti-PL-12 and anti-NXP2, primarily revealed weak positive intensity levels (Fig. 2). At the positive intensity level, antibodies to Jo-1, SRP, Mi-2α, MDA5, TIF1-γ, SAE, and PM/Scl-75 were significantly associated with myositis (Table 3). Odds ratios were more than 3 times higher for anti-Jo-1, anti-SRP, anti-Mi-2β, anti-PM/Scl-75 and anti-MDA5 when the positive intensity level was compared to the weak positive intensity level. In case of anti-PL-12, anti-PL-7, anti-TIF1-γ, anti-Ku and anti-PM/Scl-100 odds ratios were similar for both intensity levels and for anti-EJ, anti-OJ, anti-Mi-2α and anti-SAE1 statistical analysis could not be performed as no events were available in either category. In contrast, anti-NXP2 showed higher odds ratios at the weak positive compared to the positive intensity level.
Fig. 2.
Heatmap of individual frequency per intensity level for IIM and non-IIM for different antibodies as measured with the Euroline myositis line-blot assay (Euroimmun). IIM; idiopathic inflammatory myopathy, MSA; myositis specific antibodies, MAA; myositis associated antibodies. Data is not corrected for multiple antibodies per patient.
Table 3.
Associations of MSA/MAA with IIM at different antibody intensity levels.
| Antibody | IIM (n = 187) |
non-IIM (n = 632) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Number Neg |
Number Weak posa | Number Posa |
Number Neg | Number Weak posa | Number Posa | OR pb OR wpb | 95% CIc | Pd | |
| Jo-1 | 179 | 1 | 18 | 622 | 5 | 5 | 13.3 | 4.87–36.42 | <0.001 |
| 0.7 | 0.08–6.38 | ||||||||
| EJ | 176 | 0 | 2 | 629 | 1 | 2 | 3.4 | 0.47–24.30 | 0.372 |
| ∞ | ∞ | ||||||||
| OJ | 185 | 1 | 0 | 630 | 1 | 1 | ∞ | ∞ | 0.567 |
| 3.4 | 0.21–54.41 | ||||||||
| PL-7 | 186 | 4 | 4 | 609 | 15 | 8 | 1.7 | 0.50–5.71 | 0.673 |
| 0.9 | 0.29–2.76 | ||||||||
| PL-12 | 179 | 3 | 1 | 623 | 6 | 3 | 1.1 | 0.11–10.97 | 0.748 |
| 1.7 | 0.42–6.87 | ||||||||
| SRP | 181 | 2 | 9 | 617 | 14 | 1 | 31.56 | 3.97–250.73 | <0.001 |
| 0.5 | 0.11–2.24 | ||||||||
| Mi-2α | 174 | 2 | 6 | 630 | 2 | 0 | ∞ | ∞ | <0.001 |
| 3.5 | 0.49–25.16 | ||||||||
| Mi-2β | 177 | 2 | 4 | 620 | 9 | 3 | 4.6 | 1.01–20.59 | 0.089 |
| 0.86 | 0.16–3.55 | ||||||||
| MDA5 | 183 | 2 | 8 | 626 | 5 | 1 | 28.3 | 3.51–227.73 | <0.001 |
| 1.4 | 0.27–7.35 | ||||||||
| NXP2 | 185 | 3 | 1 | 628 | 2 | 2 | 1.7 | 0.15–19.03 | 0.126 |
| 5.1 | 0.85–31.04 | ||||||||
| TIF1-γ | 183 | 5 | 8 | 622 | 5 | 5 | 5.7 | 1.84–17.70 | <0.001 |
| 3.6 | 1.02–12.48 | ||||||||
| SAE1 | 179 | 0 | 2 | 630 | 2 | 0 | ∞ | ∞ | 0.025 |
| ∞ | ∞ | ||||||||
| Ku | 168 | 4 | 4 | 618 | 7 | 7 | 2.0 | 0.57–6.81 | 0.309 |
| 2.0 | 0.57–6.81 | ||||||||
| PM/Scl-75 | 174 | 4 | 9 | 603 | 23 | 6 | 5.2 | 1.82–14.80 | 0.002 |
| 0.6 | 0.20–1.77 | ||||||||
| PM/Scl-100 | 177 | 4 | 6 | 613 | 12 | 7 | 3.0 | 0.98–8.94 | 0.126 |
| 1.2 | 0.36–3.62 | ||||||||
Data is not corrected for multiple antibodies in one patient.
OR; odds ratio at weak positive level (wp), odds ratio at positive level (p) calculated using logistic regression analysis.
95% confidence interval of odds ratio’s.
Logistic regression analysis of IIM vs non-IIM with positive, weak positive and negative antibody.
3.3. Associations of MSA/MAA with organ involvement and malignancy
When specific organ involvement was evaluated (skin, heart, lungs, muscles or joints), known associations were confirmed (Table 4), e.g. anti-TIF1-γ (LR indefinite) and anti-MDA5 were significantly associated with skin involvement (LR 10.0) and joint involvement (LR 10.2) within IIM patients (sup Table 1). Of note, although it did not reach statistical significance, of the 10 patients with anti-MDA5 antibodies, three had interstitial lung disease. Within IIM patients, anti-Jo-1 was significantly associated with lung involvement (Table 4), with a LR of 4.7 (sup Table 1). No significant association between total sum of MAA and MSA with specific organ involvement was found (data not shown). Only anti-TIF1-γ antibody positivity was significantly associated with malignancy (LR 5.5) within IIM patients (sup Table 2). Additionally, there was a highly significant association between the occurrence of malignancy and age in anti-TIF1-γ positive IIM patients (p < 0.001) (data not shown). There was no overlap in age between the two groups, with anti-TIF1-γ positive IIM patients with malignancy being all older than 65 years and anti-TIF1-γ positive IIM patients without malignancy being all younger than 57 years.
Table 4.
Association of MSA/MAA with organ involvement in IIM patients.
| Antibody | # Muscle |
# No Muscle |
# Skin |
# No skin |
# Joints |
# No joints |
# Lungs |
# No lungs |
# Heart |
# No Heart |
# Muscle |
# No Muscle |
# Skin |
# No skin |
# Joints |
# No joints |
# Lungs |
# No lungs |
# Heart |
# No Heart |
P muscleb | P skinc | P jointsd | P lungse | P heartf | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive for respective antibodya | Negative for respective antibodya | |||||||||||||||||||||||||
| Jo-1 | 16 | 3 | 8 | 11 | 8 | 11 | 12 | 7 | 0 | 19 | 138 | 21 | 75 | 81 | 42 | 116 | 35 | 122 | 13 | 143 | 0.725 | 0.808 | 0.180 | <0.001 | 0.366 | |
| EJ | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 2 | 153 | 23 | 81 | 92 | 49 | 126 | 45 | 129 | 13 | 160 | 0.252 | 0.224 | 0.486 | 0.070 | 1.000 | |
| OJ | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 153 | 24 | 82 | 92 | 50 | 126 | 47 | 128 | 13 | 161 | 1.000 | 0.474 | 1.000 | 1.000 | 1.000 | |
| PL-7 | 4 | 4 | 3 | 5 | 4 | 4 | 3 | 5 | 2 | 6 | 150 | 20 | 80 | 87 | 46 | 123 | 44 | 124 | 11 | 156 | 0.013 | 0.723 | 0.224 | 0.442 | 0.111 | |
| PL-12 | 3 | 1 | 1 | 3 | 0 | 4 | 1 | 3 | 0 | 4 | 151 | 23 | 82 | 89 | 50 | 123 | 46 | 126 | 13 | 158 | 0.443 | 0.623 | 0.578 | 1.000 | 1.000 | |
| SRP | 11 | 0 | 5 | 6 | 2 | 9 | 2 | 9 | 2 | 9 | 143 | 24 | 78 | 86 | 48 | 118 | 45 | 120 | 11 | 153 | 0.365 | 1.000 | 0.730 | 0.730 | 0.191 | |
| Mi-2α | 7 | 1 | 6 | 1 | 1 | 7 | 2 | 6 | 0 | 8 | 147 | 23 | 77 | 91 | 49 | 120 | 45 | 123 | 13 | 154 | 1.000 | 0.054 | 0.444 | 1.000 | 1.000 | |
| Mi-2β | 6 | 0 | 4 | 2 | 3 | 3 | 1 | 5 | 1 | 5 | 148 | 24 | 79 | 90 | 47 | 124 | 46 | 124 | 12 | 157 | 1.000 | 0.425 | 0.353 | 1.000 | 0.375 | |
| MDA5 | 7 | 3 | 9 | 1 | 8 | 2 | 4 | 5 | 0 | 9 | 147 | 21 | 74 | 91 | 42 | 125 | 43 | 124 | 13 | 153 | 0.137 | 0.007 | 0.001 | 0.250 | 1.000 | |
| NXP2 | 4 | 0 | 2 | 2 | 2 | 2 | 0 | 4 | 2 | 2 | 150 | 24 | 81 | 90 | 48 | 125 | 47 | 125 | 11 | 160 | 1.000 | 1.000 | 0.317 | 0.575 | 0.028 | |
| TIF1-γ | 10 | 3 | 13 | 0 | 1 | 12 | 1 | 12 | 1 | 12 | 144 | 21 | 70 | 92 | 49 | 115 | 46 | 117 | 12 | 150 | 0.389 | <0.001 | 0.114 | 0.189 | 1.000 | |
| SAE1 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 152 | 24 | 81 | 92 | 50 | 125 | 47 | 127 | 13 | 160 | 1.000 | 0.224 | 1.000 | 1.000 | 1.000 | |
| Ku | 6 | 2 | 3 | 4 | 5 | 2 | 3 | 4 | 1 | 6 | 148 | 22 | 80 | 88 | 45 | 125 | 44 | 125 | 12 | 156 | 0.294 | 1.000 | 0.020 | 0.386 | 0.423 | |
| PM/Scl-75 | 11 | 2 | 7 | 6 | 6 | 7 | 4 | 9 | 2 | 11 | 143 | 22 | 76 | 86 | 44 | 120 | 43 | 120 | 11 | 151 | 0.689 | 0.775 | 0.197 | 0.749 | 0.249 | |
| PM/Scl-100 | 9 | 1 | 5 | 5 | 3 | 7 | 2 | 8 | 2 | 8 | 145 | 23 | 78 | 87 | 47 | 120 | 45 | 121 | 11 | 154 | 1.000 | 1.000 | 1.000 | 1.000 | 0.163g | |
# = number IIM patients with or without given organ involvement.
Data is not corrected for multiple antibodies or organ involvement in one patient. The number of missing values in the data base is 9; 12; 10; 11; 12 for muscles, skin, joints, lung and heart respectively.
Fisher’s exact test two sided within IIM antibody positive vs antibody negative vs muscle vs no muscle.
Fisher’s exact test two sided within IIM antibody positive vs antibody negative vs skin vs no skin.
Fisher’s exact test two sided within IIM antibody positive vs antibody negative vs joints vs no joints.
Fisher’s exact test two sided within IIM antibody positive vs antibody negative vs lungs vs no lungs.
Fisher’s exact test two sided within IIM antibody positive vs antibody negative vs heart vs no heart.
95% Confidence Interval of Fisher’s exact test values are listed in supplementary table 2.
4. Discussion
We performed a nation-wide one-year evaluation of detection of MSA/MAA with the Euroimmun Euroline myositis line blot in a routine diagnostic setting. Inclusion was based on the first request for MSA/MAA detection. Selection bias was prevented because consecutive patients were included that eventually were diagnosed as having IIM or not (non-IIM). Furthermore, samples were primarily obtained at the time of diagnostic work-up preventing possible interference with immunomodulating therapies. Frequencies of MSA/MAA in our large retrospective Dutch routine diagnostic population, including 187 IIM and 632 non-IIM patients, confirm previously published frequencies in clinically defined cohort studies with patients of North-American/European ancestry [9,13,15,[18], [19], [20], [21], [22], [23]]. The frequency of anti-Jo1 was lower than reported in literature, probably because anti-Jo-1 is part of regular ENA testing and anti-Jo-1 positive patients are not further tested for presence of other MSA/MAA. Our data show that association of MSA and MAA with IIM is much higher at the positive antibody level when compared with the weak positive level. The only exception was anti-NXP2, which showed a higher odds ratio at weak positive intensity level compared to positive intensity level. Known associations between MSA or MAA with organ involvement or malignancy were confirmed in our study. Our data substantially extend previous reports that were based on a low numbers of patients [19,20]. Re-defining cut-off levels for individual antibody specificities detected with the Euroimmun Euroline myositis line blot can lead to better discriminative performance of the assay but larger multi-center studies are required to reach that goal.
In general, associations of autoantibodies with IIM in our study are similar to the literature, but differences were also found compared to what has been published earlier using the same line-blot of Euroimmun [12,13,[16], [17], [18], [19], [20], [21],23]. Differences could be explained by study set-up, cohort based versus retrospective analysis of consecutive patients referred to university and general hospitals. Cohort studies include patients who fulfill stringent predefined classification criteria while in our study classification in IIM and non-IIM is based on the evaluation of the treating physician reflecting the daily clinical practice. In addition, although we performed a one-year survey of all Dutch patients for whom extended myositis antibody analysis was requested, the number of patients classified as IIM is still limited (n = 187). Vulsteke and colleagues [13] found that antibodies to Jo-1, Mi-2α, Mi-2β, MDA-5, TIF1-γ and SAE1 were significantly associated with IIM when comparing a cohort of IIM patients and selected disease controls. In our study we found a similar picture but surprisingly anti-Mi-2β was not significantly associated with IIM. Furthermore, we found anti-SRP but not anti-SAE1 to be significantly associated with the presence of IIM. Anti-SAE1 is frequently reported in dermatomyositis as shown in various assays such as line-blot of Euroimmun [13] or Alphadia dot immunoassay [22], but this association was not significant in our study population. Other known associations were confirmed, such as anti-MDA-5 and anti-TIF1-γ with skin involvement [[24], [25], [26]] and anti-Jo-1 with lung involvement [22,27].
In studies focusing on dermatomyositis patients, anti-MDA5 has been reported to be significantly associated with interstitial lung disease [28]. Although 3 out of 10 anti-MDA5 positive IIM patients showed lung involvement in our study, this association was not significant. Also significance was not reached for this association in the study by Vulsteke et al. [13]. Again, these differences emphasize the influence of patient selection on study outcome. We confirmed a previously reported association of anti-MDA5 with occurrence of symmetric polyarthropathy when using immunoprecipitation or dot immunoassays of Alphadia respectively [26,29]. As expected, anti-TIF1-γ was significantly associated with malignancy confirming the observation in a Chinese study in which the line-blot of Euroimmun was used [21], while this was not the case in the Belgian study using the same read out [13]. Recently, an association between occurrence of cancer and age in TIF1-γ positive dermatomyositis patients was described [30]. In line with this observation, we found that all IIM patients with TIF1-γ antibodies and malignancy were older (all >65 years of age) than IIM patients with TIF1-γ antibodies without malignancy (all <57 years of age).
In our study, diagnostic accuracy of MSA was generally lower than reported in studies using clinically defined cohorts. In the Netherlands, assessment of MSA and MAA has become daily practice in the work-up of patients with any clinical suspicion of myositis. Since IIM is a rare disease, pre-test probability in our situation is relatively low, which explains the lower PPV and NPV values. The strength of our study is that the use of consecutive patient samples included without knowing the diagnosis at time of testing reflects the prevalence of MSA/MAA in the total patient population for which the test is ordered. Hence it reflects the current diagnostic setting better than cohort-based studies. Furthermore, the relation in time between occurrence of antibodies and clinical symptoms of IIM still remains to be established. It is known that autoantibodies can be present years before onset of clinical symptoms of systemic rheumatic diseases [31]. Potentially, patients were classified as non-IIM because they have not yet been diagnosed with myositis (or another connective tissue disease) but they may develop the disease over time. On the other hand, negative laboratory results do not exclude the diagnosis of an IIM. These reasons probably contribute to the low PPV and NPV with this study setup. Of note, most antibodies detected in non-IIM patients were classified as weakly positive while antibodies in the IIM group generally occurred at positive level, confirming results previously reported in a French study, in which the same line-blot of Euroimmun was used [20].
Since our aim was to study myositis related antibodies in a routine diagnostic setting, our data is not corrected for multiple antibodies per patient. In our study, 23 of 119 antibody positive sera of IIM patients showed multi-reactivity (19.3%) and 20 of 160 antibody positive sera in non-IIM patients (12.5%). Montagnese et al. [11] recently reported a frequency of multi-reactive IIM sera of 8.1% and Vulsteke et al. found a frequency of 45% (personal communication). Both studies used the same line-blot of Euroimmun. We observed that the majority of multi-reactive sera showed a combination of anti-PM/Scl-75 and anti-PM/Scl-100, a combination of Mi-2α and Mi-2β in various intensity levels. Additionally, of all multi-reactive sera, 23% showed a combination of weak positive and positive antibody levels. The latter observations, together with our finding that weak antibodies have a lower diagnostic accuracy, emphasizes that antibody-specific cut-offs need to be established [19,20]. This is a major challenge that can only be overcome by meta-analysis of primary laboratory line-blot results.
We conclude that the Euroline myositis line-blot assay is suited for routine diagnostic use in patients suspected of IIM since known frequencies and clinical associations published in other cohort studies were confirmed. Presence of MSA and MAA antibodies at a weak positive level have a low diagnostic accuracy. The method can be improved by establishing antibody-specific cut-off values. However, since line blot might not be the optimal method for detection of all MAA/MSA, development of new detection methods that combine the diagnostic accuracy of IP with ease of use in routine setting of line blots is needed. This will pave the way for autoantibody detection in clinical guidelines [32,33] in which improved diagnosis, treatment and prognosis of IIM is facilitated.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributors
DH, BW and AP designed the study, JL and AK advised on collection of clinical data, BW prepared the data files for each participating hospital, AP, JL, JB, HB, JC, JD, MH, GK ML, EL LM, CS, HV, AK, EL, MV, MS and BM provided clinical data and edited the manuscript, AP analyzed the data, AP, BM and DH drafted the manuscript.
Competing interests
The authors declare no conflicting interests.
Patient consent
Retrospective study using routine diagnostic samples.
Ethics committee
The study was approved by the ethics committee of the Erasmus University Medical Centre under protocol number MEC-2016-606.
Acknowledgements
We thank all clinicians and laboratory specialists from contributing centers; Liesbeth Bakker-Jonges from Reinier de Graaf Group, Martine Deckers from OLVG location West, Michael Fouraux from Albert Schweitzer Hospital, Arend Jan van Houte from Diakonessen Hospital, Carin Koelman from Meander Hospital, Leroy Lard from LangeLand Hospital, Helen Leavis from University Medical Center Utrecht, Annemarie Meenhuis from Tergooi Hospital, Walentina Slieker from Northwest Clinics and all technicians and patients from centers that contributed to this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jtauto.2019.100013.
Contributor Information
Anouk C.M. Platteel, Email: a.platteel@antoniusziekenhuis.nl.
Brigitte A. Wevers, Email: bwevers@atalmedial.nl.
Johan Lim, Email: J.Lim@hagaziekenhuis.nl.
Jaap A. Bakker, Email: J.A.Bakker@lumc.nl.
Hetty J. Bontkes, Email: HJ.Bontkes@vumc.nl.
Joyce Curvers, Email: joyce.curvers@catharinaziekenhuis.nl.
Jan Damoiseaux, Email: jan.damoiseaux@mumc.nl.
Michiel Heron, Email: m.heron@etz.nl.
Gijs de Kort, Email: G.deKort@labwest.nl.
Maarten Limper, Email: M.Limper-2@umcutrecht.nl.
Ellen G. van Lochem, Email: EvanLochem@rijnstate.nl.
A.H. Leontine Mulder, Email: L.Mulder@medlon.nl.
Christiaan G.J. Saris, Email: C.Saris@radboudumc.nl.
Hester van der Valk, Email: h.van.der.valk@umcg.nl.
Anneke J. van der Kooi, Email: a.j.kooi@amc.uva.nl.
Ester M.M. van Leeuwen, Email: e.m.vanleeuwen@amc.uva.nl.
Marcel Veltkamp, Email: m.veltkamp@antoniusziekenhuis.nl.
Marco W.J. Schreurs, Email: m.schreurs@erasmusmc.nl.
Bob Meek, Email: b.meek@antoniusziekenhuis.nl.
Dörte Hamann, Email: D.Wenzlau@umcutrecht.nl.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Fig. 1.
Heatmap of multi-reactive sera within IIM (A) and non-IIM (B) patients. Shown are all sera with two or more specificities. MSA; myositis specific antibodies, MAA; myositis associated antibodies.
References
- 1.Mariampillai K., Granger B., Amelin D., Guiguet M., Hachulla E., Maurier F., Meyer A., Tohme A., Charuel J.L., Musset L., Allenbach Y., Benveniste O. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol. 2018;75:1528–1537. doi: 10.1001/jamaneurol.2018.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg I.E., Tjarnlund A., Bottai M., Werth V.P., Pilkington C., de Visser M., Alfredsson L., Amato A.A., Barohn R.J., Liang M.H., Singh J.A., Aggarwal R., Arnardottir S., Chinoy H., Cooper R.G., Danko K., Dimachkie M.M., Feldman B.M., Garcia-De La Torre I., Gordon P., Hayashi T., Katz J.D., Kohsaka H., Lachenbruch P.A., Lang B.A., Li Y., Oddis C.V., Olesinska M., Reed A.M., Rutkowska-Sak L., Sanner H., Selva-O’Callaghan A., Song Y.W., Vencovsky J., Ytterberg S.R., Miller F.W., Rider L.G., International Myositis Classification criteria Project Consortium, the Euromyositis register, and the juvenile dermatomyositis cohort Biomarker study and Repository (UK and Ireland) 2017 European League against rheumatism/American college of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheum. 2017;69:2271–2282. doi: 10.1002/art.40320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rietveld A., Lim J., de Visser M., van Engelen B., Pruijn G., Benveniste O., van der Kooi A., Saris C. Autoantibody testing in idiopathic inflammatory myopathies. Pract. Neurol. 2019;4:284–294. doi: 10.1136/practneurol-2017-001742. [DOI] [PubMed] [Google Scholar]
- 4.Betteridge Z., Tansley S., Shaddick G., Chinoy H., Cooper R.G., New R.P., Lilleker J.B., Vencovsky J., Chazarain L., Danko K., Nagy-Vincze M., Bodoki L., Dastmalchi M., Ekholm L., Lundberg I.E., McHugh N., contributors U.K.M. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J. Autoimmun. 2019;101:48–55. doi: 10.1016/j.jaut.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damoiseaux J., Vulsteke J.B., Tseng C.W., Platteel A.C.M., Piette Y., Shovman O., Bonroy C., Hamann D., De Langhe E., Musset L., Chen Y.H., Shoenfeld Y., Allenbach Y., Bossuyt X. Autoantibodies in idiopathic inflammatory myopathies: clinical associations and laboratory evaluation by mono- and multispecific immunoassays. Autoimmun. Rev. 2019;18:293–305. doi: 10.1016/j.autrev.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Casciola-Rosen L., Mammen A.L. Myositis autoantibodies. Curr. Opin. Rheumatol. 2012;24:602–608. doi: 10.1097/BOR.0b013e328358bd85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredi M., Cavazzana I., Franceschini F. The clinico-serological spectrum of overlap myositis. Curr. Opin. Rheumatol. 2018;30:637–643. doi: 10.1097/BOR.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg I.E., de Visser M., Werth V.P. Classification of myositis. Nat. Rev. Rheumatol. 2018;14:269–278. doi: 10.1038/nrrheum.2018.41. [DOI] [PubMed] [Google Scholar]
- 9.Betteridge Z., McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J. Intern. Med. 2016;280:8–23. doi: 10.1111/joim.12451. [DOI] [PubMed] [Google Scholar]
- 10.Lilleker J.B., Vencovsky J., Wang G., Wedderburn L.R., Diederichsen L.P., Schmidt J., Oakley P., Benveniste O., Danieli M.G., Danko K., Thuy N.T.P., Vazquez-Del Mercado M., Andersson H., De Paepe B., deBleecker J.L., Maurer B., McCann L.J., Pipitone N., McHugh N., Betteridge Z.E., New P., Cooper R.G., Ollier W.E., Lamb J.A., Krogh N.S., Lundberg I.E., Chinoy H., all EuroMyositis contributors The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann. Rheum. Dis. 2018;77:30–39. doi: 10.1136/annrheumdis-2017-211868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montagnese F., Babacic H., Eichhorn P., Schoser B. Evaluating the diagnostic utility of new line immunoassays for myositis antibodies in clinical practice: a retrospective study. J. Neurol. 2019;266:1358–1366. doi: 10.1007/s00415-019-09266-4. [DOI] [PubMed] [Google Scholar]
- 12.Ghirardello A., Rampudda M., Ekholm L., Bassi N., Tarricone E., Zampieri S., Zen M., Vattemi G.A., Lundberg I.E., Doria A. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology. 2010;49:2370–2374. doi: 10.1093/rheumatology/keq281. [DOI] [PubMed] [Google Scholar]
- 13.Vulsteke J.B., De Langhe E., Claeys K.G., Dillaerts D., Poesen K., Lenaerts J., Westhovens R., Van Damme P., Blockmans D., De Haes P., Bossuyt X. Detection of myositis-specific antibodies. Ann. Rheum. Dis. 2018;78:e7. doi: 10.1136/annrheumdis-2017-212915. [DOI] [PubMed] [Google Scholar]
- 14.Bossuyt X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun. Rev. 2009;8:543–548. doi: 10.1016/j.autrev.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-De La Torre I. Clinical Usefulness of autoantibodies in idiopathic inflammatory myositis. Front. Immunol. 2015;6:331. doi: 10.3389/fimmu.2015.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronnelid J., Barbasso Helmers S., Storfors H., Grip K., Ronnblom L., Franck-Larsson K., Nordmark G., Lundberg I.E. Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun. Rev. 2009;9:58–61. doi: 10.1016/j.autrev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Cruellas M.G., Viana Vdos S., Levy-Neto M., Souza F.H., Shinjo S.K. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics. 2013;68:909–914. doi: 10.6061/clinics/2013(07)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan T.C., Wienholt L., Adelstein S. TEST performance of a myositis panel in a clinical immunology laboratory in New South Wales, Australia. Int. J. Rheum. Dis. 2016;19:996–1001. doi: 10.1111/1756-185X.12792. [DOI] [PubMed] [Google Scholar]
- 19.Bundell C., Rojana-Udomsart A., Mastaglia F., Hollingsworth P., McLean-Tooke A. Diagnostic performance of a commercial immunoblot assay for myositis antibody testing. Pathology. 2016;48:363–366. doi: 10.1016/j.pathol.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Lecouffe-Desprets M., Hemont C., Neel A., Toquet C., Masseau A., Hamidou M., Josien R., Martin J.C. Clinical contribution of myositis-related antibodies detected by immunoblot to idiopathic inflammatory myositis: a one-year retrospective study. Autoimmunity. 2018;51:89–95. doi: 10.1080/08916934.2018.1441830. [DOI] [PubMed] [Google Scholar]
- 21.Yang H., Peng Q., Yin L., Li S., Shi J., Zhang Y., Lu X., Shu X., Zhang S., Wang G. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res. Ther. 2017;19:259. doi: 10.1186/s13075-017-1469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tampoia M., Notarnicola A., Abbracciavento L., Fontana A., Giannini M., Louis Humbel R., Iannone F. A new immunodot assay for multiplex detection of autoantibodies in a cohort of Italian patients with idiopathic inflammatory myopathies. J. Clin. Lab. Anal. 2016;30:859–866. doi: 10.1002/jcla.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavazzana I., Fredi M., Ceribelli A., Mordenti C., Ferrari F., Carabellese N., Tincani A., Satoh M., Franceschini F. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods. 2016;433:1–5. doi: 10.1016/j.jim.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Scholtissek B., Ferring-Schmitt S., Maier J., Wenzel J. Expression of the autoantigen TRIM33/TIF1gamma in skin and muscle of patients with dermatomyositis is upregulated, together with markers of cellular stress. Clin. Exp. Dermatol. 2017;42:659–662. doi: 10.1111/ced.13180. [DOI] [PubMed] [Google Scholar]
- 25.Ceribelli A., Fredi M., Taraborelli M., Cavazzana I., Tincani A., Selmi C., Chan J.Y., Chan E.K., Satoh M., Franceschini F. Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin. Exp. Rheumatol. 2014;32:891–897. [PubMed] [Google Scholar]
- 26.Hall J.C., Casciola-Rosen L., Samedy L.A., Werner J., Owoyemi K., Danoff S.K., Christopher-Stine L. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. 2013;65:1307–1315. doi: 10.1002/acr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Sadeleer L.J., De Langhe E., Bodart N., Vigneron A., Bossuyt X., Wuyts W.A. Prevalence of myositis-specific antibodies in idiopathic interstitial pneumonias. Lung. 2018;196:329–333. doi: 10.1007/s00408-018-0108-8. [DOI] [PubMed] [Google Scholar]
- 28.Moghadam-Kia S., Oddis C.V., Aggarwal R. Anti-MDA5 antibody spectrum in western world. Curr. Rheumatol. Rep. 2018;20:78. doi: 10.1007/s11926-018-0798-1. [DOI] [PubMed] [Google Scholar]
- 29.Best M., Jachiet M., Molinari N., Manna F., Girard C., Pallure V., Cosnes A., Lipsker D., Hubiche T., Schmutz J.L., Le Corre Y., Cordel N., Dandurand M., Dereure O., Guillot B., Du-Thanh A., Bulai Livideanu C., Chasset F., Bouaziz J.D., Frances C., Bengoufa D., Vincent T., Bessis D., Study Group of Systemic Diseases in Dermatology (EMSED: Etude des Maladies Systemiques en Dermatologie Distinctive cutaneous and systemic features associated with specific antimyositis antibodies in adults with dermatomyositis: a prospective multicentric study of 117 patients. J. Eur. Acad. Dermatol. Venereol. 2018;32:1164–1172. doi: 10.1111/jdv.14759. [DOI] [PubMed] [Google Scholar]
- 30.Oldroyd A., Sergeant J.C., New P., McHugh N.J., Betteridge Z., Lamb J.A., Ollier W.E., Cooper R.G., Chinoy H., UKMyoNet The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatology. 2019;58:650–655. doi: 10.1093/rheumatology/key357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W.T., Chang C., Gershwin M.E., Lian Z.X. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: a comprehensive review. J. Autoimmun. 2017;83:95–112. doi: 10.1016/j.jaut.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Malaviya A.N. 2017 EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: little emphasis on autoantibodies, why? Ann. Rheum. Dis. 2017;77:e77. doi: 10.1136/annrheumdis-2017-212701. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg I.E., Tjarnlund A. Response to: ‘2017 EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: little emphasis on autoantibodies, why?’ by Malaviya. Ann. Rheum. Dis. 2017;77:e78. doi: 10.1136/annrheumdis-2017-212709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.