Abstract
Background
Improved knowledge of different biomarkers is crucial for early diagnosis of rheumatic diseases and to provide important insights for clinical management. In this study, we evaluated the seroreactivity of patients with different connective tissue diseases (CTDs) (rheumatoid arthritis, RA; systemic lupus erythematosus, SLE; systemic sclerosis, SSc; and Sjogren’s syndrome, SSj) to interferon regulatory factor 5 (IRF5) peptide and homologs derived from Epstein-Barr virus (EBV) and Mycobacterium avium subsp. paratuberculosis (MAP). Antigen-induced arthritis (AIA) experiments have been performed in control and IRF5 conditional knockout mice to reinforce the hypothesis that antibodies generated against the three homologous peptides are cross-reactive.
Methods
Reactivity against wild-type (wt) and citrullinated (cit) IRF5 (IRF5424-434), MAP (MAP_402718-32) and EBV (BOLF1305-320) peptides were tested by indirect ELISA in sera from 100 RA patients, 54 patients with other CTDs (14 SLE, 28 SSc and 12 SSj) and 100 healthy subjects (HCs). Antibody responses to the same wt peptides have been tested in AIA mouse sera after immunization with complete Freud’s adjuvant (CFA) and methylated bovine serum albumin (mBSA) to induce arthritis in the knee joint.
Results
BOLF1, MAP_4027 and IRF5 peptides triggered different antibody responses in CTD diseases with a stronger reactivity in RA (p=0.0001). Similar trends were observed in AIA mice with significantly higher reactivity after 7 days from induction of arthritis. We also found statistically significant differences in antibody responses between SSc and HCs for BOLF1 (p=0.003), MAP_4027 (p=0.0076) and IRF5 (p=0.0042). Peripheral reactivity to cit peptides was lower compared to their wt counterparts, except for cit-MAP_402718-32, which induced stronger responses in RA than wt-MAP_402718-32 (46% vs. 26%, p=0.0170).
Conclusion(s): Our results show differential antibody responses to BOLF1, MAP_4027 and IRF5 peptides among CTDs, highlighting their potential as diagnostic biomarkers in these diseases. Experiments performed in IRF5 conditional knockout mice support the hypothesis of cross-reactivity between the investigated homologous antigens.
Keywords: EBV, MAP, IRF5, Citrullination, Rheumatic diseases, Mouse models of rheumatoid arthritis
Highlights
-
•
Serum IgG anti-BOLF1, MAP_4027 and IRF5 Abs responses are significantly higher in RA than in other rheumatic conditions.
-
•
Antibody responses to epitopes of EBV, IRF5 and MAP in AIA mouse model is comparable to results obtained in humans.
-
•
Antigens present in the CFA are homologous to MAP, EBV and IRF5 peptides trigger cross-reactive responses.
-
•
MAP might be a possible triggering factor in the etiology of systemic sclerosis and RA.
1. Introduction
Connective tissue diseases (CTDs) are a group of different autoimmune systemic disorders including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and Sjogren’s syndrome (SSj) that affect the musculoskeletal system and internal organs. The etiology of CTDs is unknown and their pathogenesis is poorly understood. However, the role of immune-mediated responses directed against self-antigens is considered of paramount importance, as highlighted by the shared association with common human leukocyte antigen (HLA) loci and by the expression of a broad range of specific autoantibodies. In RA, specific reactivity of citrullinated peptides is involved in modulation of the autoimmune response. Increased citrullination is observed in the RA synovium, and antibodies against citrullinated peptides (ACPA) are generated during RA-associated autoimmune responses [1,2]. ACPA are demonstrated early in the course of disease and are considered a specific diagnostic and prognostic marker of RA [3,4].
The immune system’s ability to distinguish self from non-self is negatively modulated by genetic factors and environmental triggers including viral and bacterial infections [5].
Amongst genes most frequently reported to be associated to CTDs, IRF5 gene polymorphisms have been linked to the incidence and severity of RA, SLE and SSc, due to regulation of T-cell, B-cell and dendritic cell maturation, as well as production of pro-inflammatory cytokines [[6], [7], [8], [9], [10], [11]].
Among environmental factors, viral and bacterial infections, including those caused by Epstein-Barr virus (EBV) [12], SSJ [13], SLE [14], SSc [15] and Mycobacterium avium subspecies paratuberculosis (MAP), have been associated with different autoimmune diseases and CTDs [[16], [17], [18], [19], [20], [21]].
Different mechanisms have been suggested to cause the onset and/or exacerbate autoimmune diseases. One such mechanism is molecular mimicry, whereby foreign EBV and MAP antigens share sequence or structural similarities with self-antigens. Due to this homology, the immune response against microbial epitopes could also induce undesirable humoral and/or cellular immunity against host proteins. Also, prolonged proinflammatory responses to infections have been associated with the onset and progression of autoimmunity, in a process facilitated by cytokines like type I interferon (IFN), IL-1β, IL-12, IFNγ, IL-17 and TNFα [22]. In RA, it has been established that infections, such as those from Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans and EBV, can ignite innate and secondary immune responses with induction of ACPA production [23,24].
Infectious events may also regulate gene expression to induce auto-reactive responses. For example, EBV infection is able to stimulate the activation of genes that contribute to the development of autoimmune diseases [25]. EBNA2, a protein of the latent EBV cycle, binds to DNA regions associated with high risk of RA, multiple sclerosis (MS), SLE and type 1 diabetes (T1D). In addition, dysregulation of IRF5 synthesis and function could be caused by an antibody (Abs) responses toward IRF5 epitopes following previous exposure to EBV and/or MAP as a likely consequence of the attempt to eliminate the pathogen [16].
Therefore, in the present study we sought to investigate sera reactivity against homologous fragments of IRF5, EBV and MAP in different CTDs in order to analyze the Abs response and a potential association with disease activity and other clinical variables. The Antigen Induced Arthritis (AIA) mouse model has been useful for reinforcing the results obtained in humans.
2. Materials and methods
2.1. Antigens and modifications
Based on our previous reports describing a strong recognition of three homologous protein fragments by Sardinian RA patients [21], the following wild-type (wt) and citrullinated (cit) peptides were employed in this study: BOLF1 (AAVPVLAFDAA-L-LLE and AAVPVLAFDAA-{Cit}-L-{Cit}-LLE), MAP (AVVPVLAYAAA-LLL and AVVPVLAYAAA-{Cit}-LLL) and IRF5 (VVPVAA-LLLE and VVPVAA-{Cit}-LLLE). All peptides were synthesized commercially at >90% purity (LifeTein, South Plainfield, NJ 07080, USA) and kept frozen in single-use aliquots (10 mM) at −80 °C.
2.2. Subjects
Consecutive unselected 100 RA patients (19 males, 65 females; median age 57.65 ± 10.33), 14 SLE patients (no males, 14 females; median age 36.5 ± 11.2), 28 SSc patients (5 males, 23 females; median age 58.9 ± 13.2) and 12 SSj patients (no males, 12 females; median age 59.5 ± 15.4) attending the outpatient clinic of the Rheumatology Unit, Department of Clinical and Experimental Medicine, University Hospital of Sassari, Italy, were enrolled in the study. Only patients satisfying disease specific classification criteria [[26], [27], [28], [29]], were enrolled in the study. Collected data relative to RA patients included: duration of RA; therapy including steroid treatment, disease-modifying anti-rheumatic drugs (DMARDs) and/or anti-tumor necrosis factor-alpha therapy, Tocilizumab, Rituximab and Abatacept; levels of C-reactive protein (CRP), mg/dL; erythrocyte sedimentation rate (ESR) levels, mm/h; rheumatoid factor positivity; anti-cyclic citrullinated peptide positivity (anti-CCP); Disease Activity Score-28 (DAS-28; Wells G, 2009) and Health Assessment Questionnaire (HAQ). The following disease-specific activity scores were also registered: SLEDAI (Systemic lupus erythematosus disease index 2000) for SLE [30]; ESCsG-AI (European Scleroderma Research Group Activity Index, for SSc [31] and ESSDAI (EULAR Sjogren’s syndrome disease activity index, for SSj [32]. 100 healthy controls (HCs; 26 males, 74 females; median age 45.1 ± 11.7) were recruited at the Blood Transfusion Centre of Sassari, Italy.
Demographic and clinical features of all subjects involved in the present study are summarized in Table 1 and Table 2. The study protocols were approved by the ethics committee of Azienda Ospedaliero-Universitaria of Cagliari, Italy (PG/2018/5463) and all participants provided written informed consent.
Table 1.
Demographic and clinical characteristics of groups.
| RA n = 100 | SLE n = 14 | SSc n = 28 | SSj n = 12 | HCs n = 100 | |
|---|---|---|---|---|---|
| Age, years | 57.6 ± 10.3 | 36.5 ± 11.2 | 58.9 ± 13.2 | 59.5 ± 15.4 | 45.1 ± 11.7 |
| Female, n(%) | 80 (80) | 14 (100) | 23 (82) | 12 (100) | 74 (74) |
| DAS28 | 3.45 ± 1.7 | / | / | / | / |
| SLEDAI | / | 3.42 ± 4.7 | / | / | / |
| ESCsG-AI | / | / | 2.23 ± 2.1 | / | / |
| ESSDAI | / | / | / | 2.83 ± 2.16 | / |
RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; SSj, Sjogren’s syndrome; HCs, healthy controls. DAS-28, disease activity score-28 joints; SLEDAI, systemic lupus erythematosus disease index 2000; ESCsG-AI, European Scleroderma Research Group Activity Index; ESSDAI, EULAR Sjogren’s syndrome disease activity index.
Table 2.
Demographic and clinical characteristics of RA patients and HCs.
| RA n = 100 | HCs n = 100 | |
|---|---|---|
| Age, years | 57.6 ± 10.3 | 45.1 ± 11.7 |
| Female, n (%) | 80 (80) | 74 (74) |
| ESR, mm/h | 19.5 ± 25 | / |
| CRP, mg/dL | 1.34 ± 4.8 | / |
| DAS28 score | 3.45 ± 1.7 | / |
| HAQ score | 1.04 ± 0.9 | / |
| ACPA positivity, % | 65 (65) | / |
| RF positivity, % | 73 (73) | / |
| Steroid use, % | 64 | / |
| Steroid dose, mg/day | 1.5 ± 2.3 | / |
| DMARDs use, % | 86 | / |
| TNFi use, % | 27 | / |
| Tocilizumab use, % | 13 | / |
| Abatacept use, % | 4 | / |
| Rituximab use, % | 2 | / |
RA, rheumatoid arthritis; HCs, healthy controls. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS-28, disease activity score-28 joints; HAQ, health assessment questionnaire; ACPA, anti-citrullinated peptide antibodies; RF, rheumatoid factor; DMARDs, disease-modifying anti-rheumatic drugs; TNFi, tumor necrosis factor-alpha inhibitors.
2.3. Antigen-induced arthritis (AIA) in mouse models
In order to better understand the genetic and environmental factors modulating etiopathogenesis of RA, animal models have been extensively employed for studies focused on molecular mechanisms underlying human diseases with the objective to develop new therapeutic strategies [[33], [34], [35]]. A number of rodent models of arthritis have been generated over decades of research in the field and among them mouse models of RA share many features with the relative disease in humans.
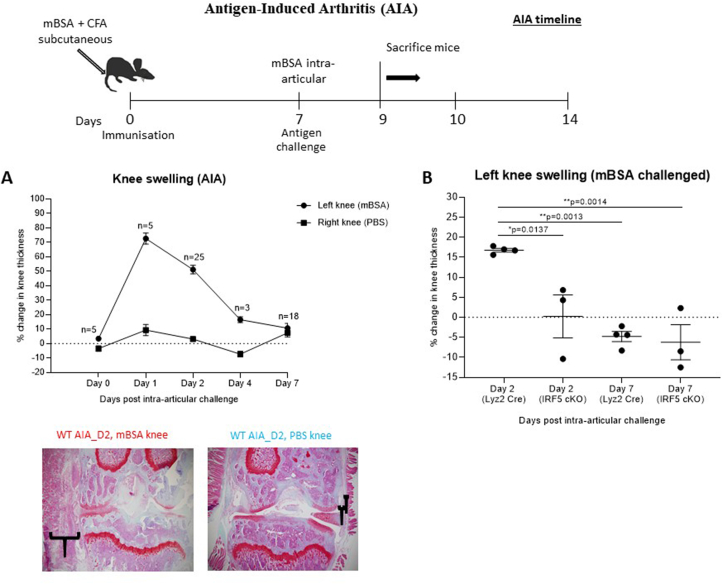
Mice were bred and maintained under SPF conditions in accredited animal facilities at the University of Oxford. All procedures were conducted according to the Operations of Animals in Scientific Procedures Act (ASPA) of 1986 and approved by the Kennedy Institute of Rheumatology Ethics Committee. Animals were housed in individually ventilated cages at a constant temperature with food and water ad libitum. To validate the hypothesis of cross-reactivity, the AIA mouse model was employed in this study for the assessment of immune responses against peptides derived from EBV and MAP, which share sequence homology with IRF5. Wildtype C57BL6/J mice were grouped in the following treatment conditions: n = 6 naïve mice not subjected to immunization, n = 5 immunized mice (Day 0 group) and n = 55 AIA arthritic mice (Day 1–7 groups). AIA was also performed in control Lyz2-Cre mice (B6·129P2-Lyz2tm1(cre)Ifo/J, Jackson Laboratories) and IRF5 conditional knockout mice - generated in the Udalova laboratory by crossing Lyz2-Cre mice with IRF5-LoxP mice (C57BL/6-Irf5tm1Ppr/J, Jackson Laboratories), in order to knockout IRF5 in the Lyz2-expressing myeloid compartment. Arthritis was induced at ~12 wk of age as described elsewhere [6,[36], [37], [38]]. Briefly, after sedation with inhaled isoflurane, mice were immunized by subcutaneous injection at the base of the tail with mBSA (Sigma, 100 μg) emulsified in complete Freud’s adjuvant (Difco, 100 μg). Seven days later (Day 0), mice were challenged by intraarticular injection of 200 μg mBSA into the left knee joint using a sterile 30-gauge microcannula in induce inflammation of the knee joint (Fig. 1). Intraarticular injection of PBS alone was used as a control condition in the right knee joint. Daily caliper measurements were taken to monitor the extent of knee swelling and therefore progression of inflammation in the AIA model. Knee joints and blood were harvested on Days 0–7 post intra-articular challenge from sacrificed mice. Blood was centrifuged at 2000 rpm for 20 min to separate serum for serological analysis.
Fig. 1.
Schematic diagram showing treatment and harvest time points in the antigen-induced arthritis (AIA) model. (A) Percentage change in knee thickness of WT mice immunized with CFA + mBSA, and then challenged by intra-articular injection of either PBS (control, right knee) or mBSA (arthritic, left knee) Safranin O/Fast Green stained histological sections confirmed differences in thickness of the synovial membrane between arthritic and control knees. (B) Knee swelling in wild-type and Lyz2-Cre, IRF5-LoxP conditional knockout mice at Day 2 and Day 7 post mBSA intra-articular challenge. IRF5 conditional knockout mice show reduced knee swelling at Day 2 compared to control Lyz2-Cre mice, and no difference at Day 7 when inflammation has resolved. P-values were determined by 1-way ANOVA with multiple comparisons using Graphpad Prism v. 8.0 software. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.4. Histology
For histological analysis, mice were sacrificed on day 1–7 after onset of arthritis (Fig. 1). Arthritic paws were severed above the ankle and fixed in 10% buffered formalin. Paws were decalcified in 10% EDTA and dehydrated before embedding in paraffin wax. Sagittal and coronal sections were stained with Safranin O/Fast Green with a haemotoxylin and eosin counterstain (Fig. 6, Fig. 7.
2.5. ELISA assays
Indirect ELISA to detect specific antibodies (Abs) against the selected antigens was performed as described previously [16]. The optical density (OD) was read at a wavelength of 405 nm using a SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA 94089, USA). For data normalization, a highly responsive serum with the maximum Abs reactivity fixed at 1.0 arbitrary unit (AU)/ml was included in all experiments involving human sera. Negative control wells were obtained by incubation of immobilized peptides with secondary Abs alone and their mean values subtracted from all samples. Positive control sera were also included in all experiments. OD readings for mouse sera were performed after an overnight incubation with the reaction substrate.
2.6. Statistical analysis
Significant differences between the OD values of RA, SLE, SSc, SSj and HCs groups were determined by ANOVA test. The same test was employed to assess Abs variation between treatment conditions in mice. Significant differences in the proportion of Abs positivity across groups was performed with Mann-Whitney U test and Fisher’s exact test. Differences with p < 0.05 were considered statistically significant. The results were expressed as a mean of three separate experiments and the statistical analyses were performed using Graphpad Prism v. 8.0 software (GraphPad Software Inc., La Jolla, CA 92037, USA).
3. Results
3.1. Abs response in RA and rheumatic diseases
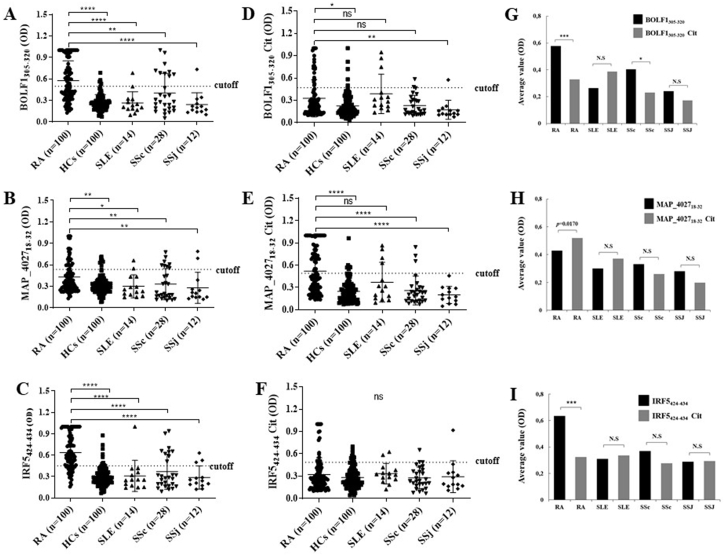
Wt-BOLF1305-320 elicited the highest seroreactivity accounting for 53% (n = 53) among RA patients, 7.14% (n = 1) in SLE, 32.1% (n = 9) in SSc, 7.69% (n = 1) in SSJ and 5% (n = 5) in HCs (p = 0.0001; Fig. 2A), while Abs against cit-BOLF1305-320 were detected in 21% (n = 21) of RA subjects, 21.4% (n = 3) in SLE, 7.14% (n = 2) in SSc and 7.69% (n = 1) in SSj and 5% (n = 5) of HCs, (p = 0.0001; Fig. 2D). Also, we found a statistically significant difference between SSc and HCs (32.1% vs. 5%, respectively, p = 000.3; Fig. 2A) for BOLF1 that highlights the role of EBV in SSc.
Fig. 2.
A-F) ELISA-based analysis of Abs reactivity against human, viral and MAP-derived peptides in RA patients, SLE, SSc, SSj and HCs. The sera were tested against plate-coated BOLF1305-320(A), MAP_402718-32(B) and IRF5424-434(C) peptides. Also, the sera were tested against plate-coated BOLF1305-320 Citrullinated (D), MAP_402718-32 Citrullinated (E) and IRF5424-434 Citrullinated (F) peptides. Bars represent the median ± interquartile range. Thresholds for Abs positivity are indicated by dashed lines. P-values are indicated above the distributions. (G–I) Mean distribution of OD values and Fisher’s exact test.
In RA and SSc, wt-BOLF1305-320 elicited a greater reactivity than its citrullinated counterpart (p = 0.0001 and p = 0.0403, respectively), while no statistically significant difference was attained in SLE and SSj groups (Fig. 2A).
Regarding MAP peptides, wt-MAP_402718-32 elicited the highest seroreactivity among RA patients accounting for 26% (n = 26), 14.29% (n = 2) in SLE, 28.57% (n = 8) in SSc, 15.38% (n = 2) in SSj and 8% (n = 8) in HCs, (p = 0.0001; Fig. 2B), while Abs against cit-MAP_402718-32 were detected in 43% (n = 43) of RA subjects, 28.57% (n = 4) in SLE, 10.71% (n = 3) in SSc, none in SSj and 6% (n = 6) among HCs (p = 0.0001; Fig. 2E). SSc and HCs significantly differed when considering values obtained for MAP_402718-32 (p = 0.0076; Fig. 2B). Of note, a statistical difference was registered in RA patients between the proportion of Abs against wt- and cit-MAP_402718-32 (26 vs. 43%, p = 0.0170; Fig. 2H).
IRF5 peptide elicited a higher seroreactivity reaching 73% (n = 73) among RA patients, 7.14% (n = 1) in SLE, 32.1% (n = 9) in SSc, 23.1% (n = 3) in SSJ and 9% (n = 9) in HCs (p = 0.0001, Fig. 2C), while Abs against cit-IRF5 peptide were detected in 14% (n = 14) of RA subjects, 14.3% (n = 2) in SLE, 10.7% (n = 3) in SSc, 7.69% (n = 1) in SSj and 0% (n = 10) of HCs (p = 0.0001; Fig. 2F). A significant difference was observed for IRF5424-434 between SSc and HCs (p = 0.0042; Fig. 2C).
We then compared the Abs response against wt-IRF5 peptide versus its citrullinated variant in all disease-specific groups. The proportion of anti-wt-IRF5 vs. anti-cit-IRF5 Abs was statistically significant in RA patients only (73% vs. 14%; p = 0.0001; Fig. 2I).
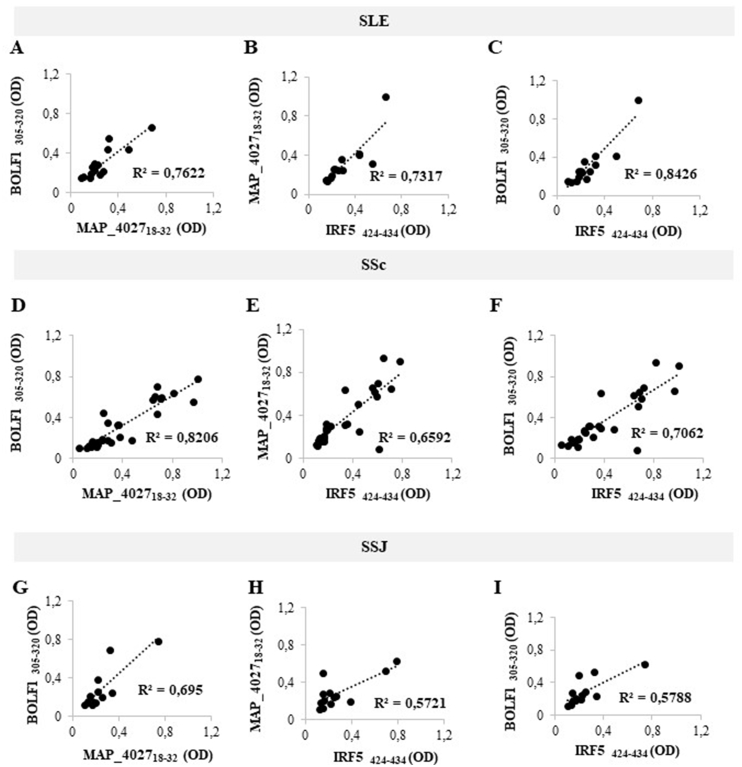
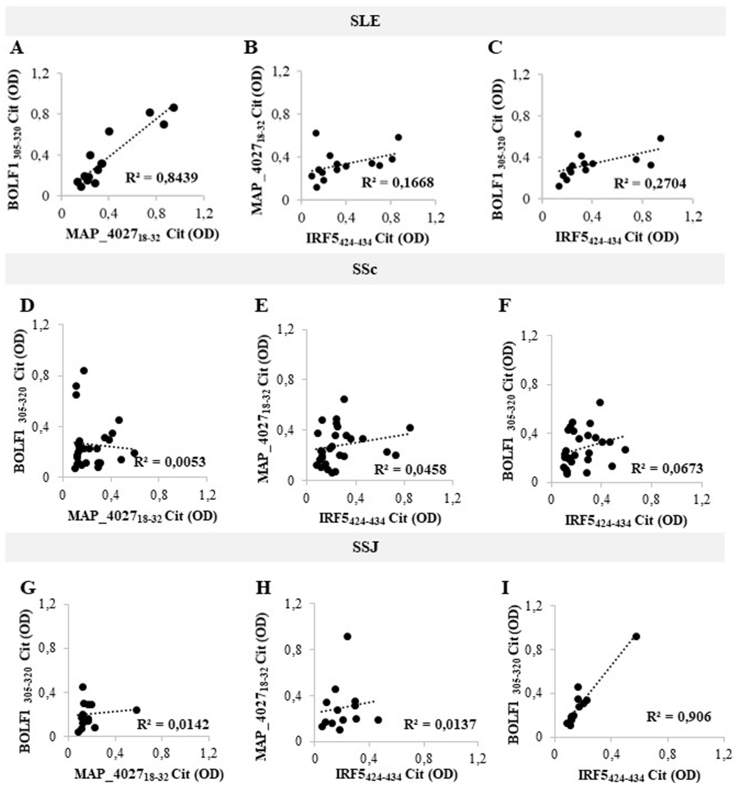
Although there was no statistical significance for the assessed peptides in SLE, SSc and SSJ compared to RA, we performed correlation analyses of Abs positivity values among SLE, SSc and SSJ patients (Fig. 3 and Fig. 4). The highest coefficients were obtained for the homologous epitopes BOLF1305-320, MAP_402718-32 and IRF5424-434 in pairwise plots pointing at cross-reactivity due to shared amino acid sequence (Fig. 3). The lack of correlation was found for all homologous pairs of citrullinated peptides, with the exception of high coefficients observed between cit-BOLF1305-320 and cit-MAP_402718-32 in SLE (R2 = 0.8439; Fig. 4A). Similarly, cit-BOLF1305-320 and cit-IRF5424-434 highly correlated in SSJ (R2 = 0.906; Fig. 4I).
Fig. 3.
A-I) Scatter plot showing correlations between Abs titers recognizing BOLF1305-320 and MAP_402718-32, MAP_402718-32 and IRF5424-434, BOLF1305-320 and IRF5424-434 in SLE (A, B, C), SSc (D, E, F) and SSJ (G, H, I) patients. Pearson’s correlation was calculated through Graphpad Prism v. 8.0 software.
Fig. 4.
A-I) Scatter plot showing correlations between Abs titers recognizing cit-BOLF1305-320 and cit-MAP_402718-32, cit-MAP_402718-32 and cit-IRF5424-434, cit-BOLF1305-320 and cit-IRF5424-434 in SLE (A, B, C), SSc (D, E, F) and SSJ (G, H, I) patients. Pearson’s correlation was calculated through Graphpad Prism v. 8.0 software.
3.2. Abs response against IRF5, MAP, BOLF peptides in mouse models of arthritis
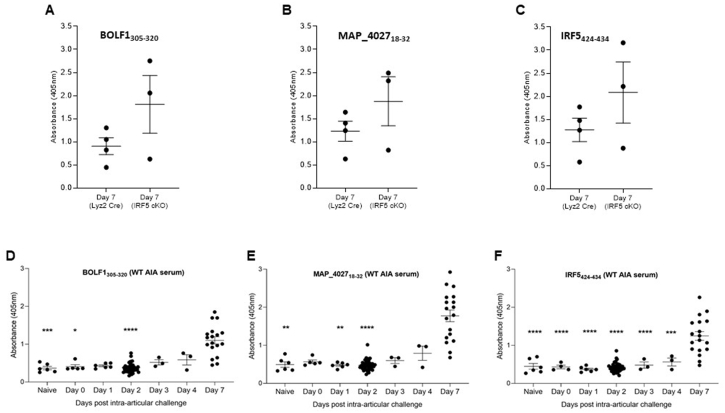
The reactivity to three homologous peptides IRF5, BOLF1 and MAP was tested in serum of three arthritis models (AIA, Collagen-Induced Arthritis (CIA) and Collagen Antibody-Induced Arthritis (CAIA)) and revealed different responses.
The results obtained highlight a statistical difference between Naïve vs. D7, D1 vs. D7 and D2 vs. D7 for BOLF1, between Naïve vs. D7, D1 vs. D7 and D2 vs. D7 for MAP_4027 and between Naïve vs. D7, D1 vs. D7 and D2 vs. D7, D3 vs. D7 and D4 vs. D7 for IRF5 in the AIA model (Fig. 5). No statistical difference for the same peptides was found in the CIA and CAIA models (data not shown). No statistical difference was also observed when IRF5 conditional knockout AIA sera (from mice in which IRF5 was knocked out in Lyz2 expressing myeloid cells) were compared to control sera for each of the 3 peptides (Fig. 5).
Fig. 5.
A-C) ELISA-based analysis of Abs reactivity against EBV, MAP and IRF5 peptides using Lyz2-Cre control and IRF5-LoxP, LysM-Cre conditional knockout serum harvested at Day 7 post-challenge in the AIA (Antigen-Induced arthritis) model. There was no significant difference between the 2 groups for any of the 3 peptides (Mann-Whitney U test performed in Graphpad Prism software); D-F) ELISA-based analysis of Abs reactivity against EBV, MAP and IRF5 peptides using WT serum from an AIA (Antigen-Induced arthritis) timecourse experiment. P-values were determined by ANOVA test with multiple comparisons.
4. Discussion
In this study, we demonstrated that Abs responses to IRF5, EBV and MAP homologous peptides are different across CTDs, with RA sera showing the most significant reactivity against either wild-type or citrullinated peptides. These results confirmed our previous data [16,22] and were reinforced by observations in vivo. Abs formed after immunization with a CFA/mBSA emulsion in the AIA model were able to cross-react with MAP antigens triggering a persistent inflammation towards IRF5 and EBV. To validate this hypothesis, we analyzed the immune responses in IRF5 conditional knockout mice in order to understand whether relative Abs are able to cross-react in a similar way. These mice lack IRF5 expression in Lyz2 expressing cells of the myeloid compartment, in line with previous publications which indicate a role for IRF5 in myeloid cells [39]. Abs responses of the IRF5 conditional knockout mice against the three homologous peptides used (MAP, IRF5 and BOLF) are within the range of the Abs response in control mice. Previous studies confirmed that due to a high homology shared by MAP, BOLF and IRF5 protein fragments, Abs against one of these epitopes are cross-reactive against the remaining two [16]. Moreover, the assessed MAP peptide shares a 100% homology with the antigens of Mycobacterium tuberculosis and Bacillus Calmette–Guérin (BCG) vaccine which is certainly present in the Freund’s adjuvant employed for mice immunization. As IRF5 conditional knockout mice are unable to mount an anti-IRF5 Abs response, observed Abs are mounted against the antigens of Freund’s adjuvant, and therefore against MAP and its EBV/IRF5 homologs. It comes in support of former competition experiments, where the sera of patients positive to all three antigens were preincubated with one peptide, inhibiting the reaction against the other two homologs [16].
Citrullination, a fundamental and ubiquitous post-translational modification with potentially relevant effect on the induction of secondary autoimmune responses, may be triggered by various infective agents, mainly at the level of mucosal surface. Therefore, we supposed that EBV and MAP may also induce citrullination of protein fragments, so we tested the antigenicity of homologous citrullinated peptides derived from IRF5, EBV and MAP. Intriguingly, the response against cit-MAP_402718-32 was significantly higher than that of its wild-type variant, suggesting a role for MAP citrullinated antigens in RA autoimmunity supported by the production of specific ACPA. However, with the exception of anti-cit-MAP, seroreactivity to the other two citrullinated peptides in RA and other CTDs was equal and, in some instances, even significantly lower than responsiveness against their wild-types counterparts.
For the first time in this study, a significant Abs response to homologous peptides of IRF5, MAP and EBV was also shown in SSc patients. It has been demonstrated that exposure to EBV is able to infect human dermal fibroblasts in vitro, inducing pro-fibrotic phenotypic switching, a relevant pathogenetic pathway underlying skin fibrosis in SSc [40]. Moreover, EBV viral transcripts and proteins were demonstrated in fibroblasts and endothelial cells in the skin of SSc patients [40]. EBV chronic replication in SSc primary monocytes has been proved to activate the TLR8 molecular pathway to sustain monocyte-derived inflammation in SSc [15]. In addition, a higher frequency of Abs against EBV has been recently demonstrated in SSc compared to healthy controls [41]. Taken collectively, these data suggest that EBV-specific responses may be an initiating trigger of SSc with persistent viral infection-related tissue injury underlying chronic inflammation and fibrosis. It is probable that defective type I IFN-mediated signaling may blunt anti-viral responses and EBV infection control in patients with SSc, as recently demonstrated in MS [42]. Interestingly, the number of minor rs4728142 alleles of IRF5 has been described as a predictive factor for longer survival in SSc patients [11]. Therefore, it is conceivable that Abs-mediated modulation of IRF5 expression/function in SSc may have an impact on the pathogenesis and severity of disease.
The significant reactivity of SSc sera against MAP peptides demonstrated in our study is intriguing and worthy of further investigation. Although preliminary, our data suggest that SSc and RA patients actuate a similar autoimmune response to MAP-derived antigens, pointing at MAP infection as a common pathogenetic contributor to various CTDs. Weak or insignificant immune responses to the assessed epitopes among patients with SLE and SSj further supports the concept that (auto)immune responses to environmental pathogens are variable across CTDs.
A limitation of this study was the administration of different immunosuppressive therapies to patients at the moment of sample collection, a fact that may have biased the interpretation and significance of humoral responses. In addition, a study of diagnostic performance of Abs against the selected peptides has not been performed due to limited sample size of non-RA CTDs sera. Prospective studies are therefore needed to follow changes in reactivity over time along with disease progression and the effect of therapy. To strengthen our observations, we plan to evaluate the levels of pro-inflammatory cytokines and quantify INF-γ upon stimulation with the analyzed peptides. Also, analysis of immune response against the above peptides in IRF5 conditional knockout mice has been useful in order to understand the cross-reactivity among peptides and to investigate the role of IRF5 in RA, as well as expanding knowledge on possible intervention targets to block Abs production responsible for triggering chronic inflammation. Future comparison of these in vivo results with reactivity against relative citrullinated peptides will provide additional elements on mechanisms involved in RA pathogenesis.
The diagnostic performance of antibodies to BOLF1, MAP and IRF5 homologous peptides in differentiating between healthy condition and CTDs and discriminating between different CTDs, needs to be tested in larger case-control studies including other autoimmune and chronic inflammatory diseases.
Credit author statement
M.B. conceived the study and its experimental design, contributed to sample collection, carried out the sample analysis, analyzed the results, performed research and wrote the paper. M.N. revised the manuscript and contributed to the analysis of results. G.A. helped to collect samples. G.L.E. and G.P. visited patients and diagnosticated the diseases, attended to analyze the results and read the manuscript. E. v.G. and T.E.K helped to collect sera from mice. I.A.U. and H.L.E. provided the AIA mouse model, experimental materials and facility, carried out the mouse serum ELISAs and helped in data analysis. L.A.S. conceived the study and its experimental design, contributed to sample collection, carried out the sample analysis, analyzed the results, and approved the manuscript.
Funding
University of Sassari grant to L.A. Sechi.
Declaration of competing interest
Authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2020.100048.
Contributor Information
Irina A. Udalova, Email: irina.udalova@kennedy.ox.ac.uk.
Leonardo A. Sechi, Email: sechila@uniss.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. 6.
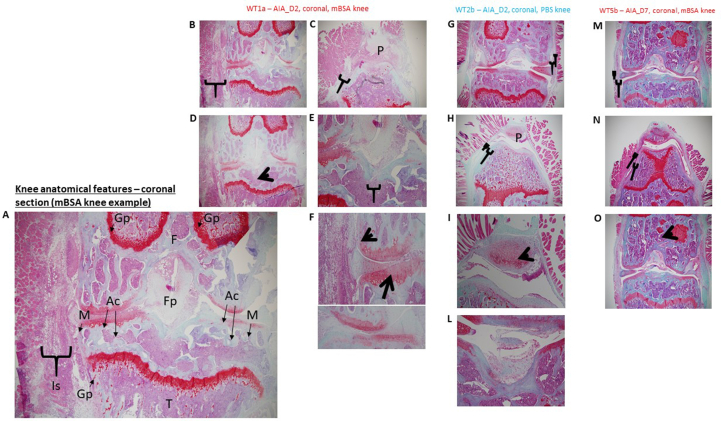
Coronal sectioning Safranin O/Fast Green. Safranin O – stains cartilage in red in proportion to proteoglycan content; Fast green – stains bone blue/green and Haematoxylin counterstain– stains nuclei purple. A) Knee anatomical features- Coronal section (mBSA Knee): Gp (Growth plate), F (Femur), Is (Inflamed synovium/subsynovial lining), M (meniscus, Ac (Articular cartilage), Fp (Fat pad) and T (Tibia). B) Inflammation of synovial and subsynovial lining. C) Inflammation of synovial and subsynovial lining extends to the patella and space between patella and tibia. D) Loss of space in the bone marrow. E) Bone erosion by fat pad, allowing bone marrow to escape. F) Cartilage matrix aging (loss of red stain on surface of cartilage, should be darker stain at surface as the cartilage is younger as shown in PBS knee below) Chondrocyte death (larger pits in which the chondrocytes sit – indicative of cell death). G) Inflammation of synovial and subsynovial lining – none in control knee, space between the two layers of lining. H) Inflammation of synovial and subsynovial lining – none in PBS knee. I) Patella cartilage matrix aging. L) Bone erosion by fat pad, allowing bone marrow to escape. M) Inflammation of synovial and subsynovial lining – reduced at Day 7. N) Inflammation of synovial and subsynovial lining – reduced at Day 7. O) Loss of space in the bone marrow (more white space visible).
Fig. 7.
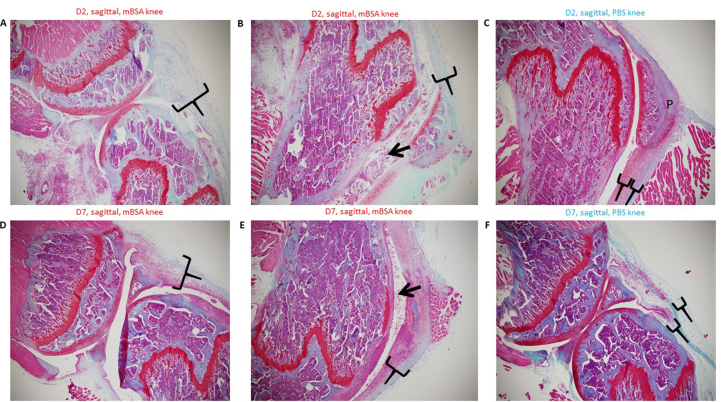
Sagittal sectioning Safranin O/Fast Green. Safranin O – stains cartilage in red in proportion to proteoglycan content; Fast green – stains bone blue/green and Haematoxylin counterstain– stains nuclei purple. A) Inflammation of synovial and subsynovial lining. B) Inflammation of synovial and subsynovial lining. C) Inflammation of synovial and subsynovial lining – none in PBS knee. D) Inflammation of synovial and subsynovial lining – reduced at Day 7. E) Inflammation of synovial and subsynovial lining – reduced at Day 7. F) Inflammation of synovial and subsynovial lining – none in PBS knee.
References
- 1.Yamada R. Citrullinated proteins in rheumatoid arthritis. Front. Biosci. 2005;10:54. doi: 10.2741/1506. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y., Lee J., Jung H., Yi H., Rim Y.A., Jung S.M., Ju J.H. Development of synthetic anti-cyclic citrullinated peptide antibody and its arthritogenic role. Clinical & Translational Immunology. 2015;4:e51. doi: 10.1038/cti.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen L.M., van Schaardenburg D., van der Horst-Bruinsma I., van der Stadt R.J., de Koning M.H., Dijkmans B.A. The predictive value of anti-cyclic citrullinated peptide antibodies in early arthritis. J. Rheumatol. 2003;30(8):1691–1695. [PubMed] [Google Scholar]
- 4.Rantapää-Dahlqvist S., de Jong B.A.W., Berglin E., Hallmans G., Wadell G., Stenlund H., Sundin U., van Venrooij W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 5.Vojdani A. A potential link between environmental triggers and autoimmunity. Autoimmune Dis. 2014:1–18. doi: 10.1155/2014/437231. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss M., Byrne A.J., Blazek K., Saliba D.G., Pease J.E., Perocheau D., Feldmann M., Udalova I.A. IRF5 controls both acute and chronic inflammation. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:11001–11006. doi: 10.1073/pnas.1506254112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffau P., Menn-Josephy H., Cuda C.M., Dominguez S., Aprahamian T.R., Watkins A.A., Yasuda K., Monach P., Lafyatis R., Rice L.M., Kenneth Haines G., Gravallese E.M., Baum R., Richez C., Perlman H., Bonegio R.G., Rifkin I.R. Promotion of inflammatory arthritis by interferon regulatory factor 5 in a mouse model. Arthritis & Rheumatology. 2015;67:3146–3157. doi: 10.1002/art.39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L., Feng D., Bi X., Stone R.C., Barnes B.J. Monocytes from irf5−/− mice have an intrinsic defect in their response to pristane-induced lupus. J. Immunol. 2012;189:3741–3750. doi: 10.4049/jimmunol.1201162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almuttaqi H., Udalova I.A. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J. 2019;286:1624–1637. doi: 10.1111/febs.14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eames H.L., Corbin A.L., Udalova I.A. Interferon regulatory factor 5 in human autoimmunity and murine models of autoimmune disease. Transl. Res. 2016;167:167–182. doi: 10.1016/j.trsl.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Sharif R., Mayes M.D., Tan F.K., Gorlova O.Y., Hummers L.K., Shah A.A., Furst D.E., Khanna D., Martin J., Bossini-Castillo L., Gonzalez E.B., Ying J., Draeger H.T., Agarwal S.K., Reveille J.D., Arnett F.C., Wigley F.M., Assassi S. IRF5 polymorphism predicts prognosis in patients with systemic sclerosis. Ann. Rheum. Dis. 2012;71:1197–1202. doi: 10.1136/annrheumdis-2011-200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erre G.L., Mameli G., Cossu D., Muzzeddu B., Piras C., Paccagnini D., Passiu G., Sechi L.A. Increased epstein-barr virus DNA load and antibodies against EBNA1 and EA in Sardinian patients with rheumatoid arthritis. Viral Immunol. 2015;28:385–390. doi: 10.1089/vim.2015.0035. [DOI] [PubMed] [Google Scholar]
- 13.Sipka S., Zilahi E., Papp G., Chen J.-Q., Nagy A., Hegyi K., Kónya J., Zeher M. Down-regulation of increased TRAF6 expression in the peripheral mononuclear cells of patients with primary Sjögren’s syndrome by an EBV-EBER1-specific synthetic single-stranded complementary DNA molecule. International Journal of Rheumatic Diseases. 2017;20:614–621. doi: 10.1111/1756-185X.13087. [DOI] [PubMed] [Google Scholar]
- 14.Ascherio A., Munger K.L. EBV and autoimmunity. 2015. 365-385. [DOI] [PubMed]
- 15.Farina A., Peruzzi G., Lacconi V., Lenna S., Quarta S., Rosato E., Vestri A.R., York M., Dreyfus D.H., Faggioni A., Morrone S., Trojanowska M., Farina G.A. Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes. Arthritis Res. Ther. 2017;19:39. doi: 10.1186/s13075-017-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bo M., Erre G.L., Niegowska M., Piras M., Taras L., Longu M.G., G Interferon regulatory factor 5 is a potential target of autoimmune response triggered by Epstein-barr virus and Mycobacterium avium subsp. paratuberculosis in rheumatoid arthritis: investigating a mechanism of molecular mimicry. Clin. Exp. Rheumatol. 2018;36:376–381. [PubMed] [Google Scholar]
- 17.Cossu D., Yokoyama K., Tomizawa Y., Momotani E., Hattori N. Altered humoral immunity to mycobacterial antigens in Japanese patients affected by inflammatory demyelinating diseases of the central nervous system. Sci. Rep. 2017;7:3179. doi: 10.1038/s41598-017-03370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slavin Y.N., Bo M., Caggiu E., Sechi G., Arru G., Bach H., Sechi L.A. High levels of antibodies against PtpA and PknG secreted by Mycobacterium avium ssp. paratuberculosis are present in neuromyelitis optica spectrum disorder and multiple sclerosis patients. J. Neuroimmunol. 2018;323:49–52. doi: 10.1016/j.jneuroim.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Niegowska M., Delitala A., Pes G.M., Delitala G., Sechi L.A. Increased seroreactivity to proinsulin and homologous mycobacterial peptides in latent autoimmune diabetes in adults. PloS One. 2017;12 doi: 10.1371/journal.pone.0176584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niegowska M., Rapini N., Piccinini S., Mameli G., Caggiu E., Manca Bitti M.L., Sechi L.A. Type 1 Diabetes at-risk children highly recognize Mycobacterium avium subspecies paratuberculosis epitopes homologous to human Znt8 and Proinsulin. Sci. Rep. 2016;6:22266. doi: 10.1038/srep22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bo M., Niegowska M., Erre G.L., Piras M., Longu M.G., Manchia P., Manca M., Passiu G., Sechi L.A. Rheumatoid arthritis patient antibodies highly recognize IL-2 in the immune response pathway involving IRF5 and EBV antigens. Sci. Rep. 2018;8:1789. doi: 10.1038/s41598-018-19957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Herrath M.G., Fujinami R.S., Whitton J.L. Microorganisms and autoimmunity: making the barren field fertile? Nat. Rev. Microbiol. 2003;1:151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Bañuelos E., Mukherjee A., Darrah E., Andrade F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and aggregatibacter actinomycetemcomitans. J. Clin. Med. 2019;8:1309. doi: 10.3390/jcm8091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratesi F., Tommasi C., Anzilotti C., Chimenti D., Migliorini P. Deiminated Epstein-Barr virus nuclear antigen 1 is a target of anti–citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:733–741. doi: 10.1002/art.21629. [DOI] [PubMed] [Google Scholar]
- 25.Harley J.B., Chen X., Pujato M., Miller D., Maddox A., Forney C., Magnusen A.F., Lynch A., Chetal K., Yukawa M., Barski A., Salomonis N., Kaufman K.M., Kottyan L.C., Weirauch M.T. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat. Genet. 2018;50:699–707. doi: 10.1038/s41588-018-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., Combe B., Costenbader K.H., Dougados M., Emery P., Ferraccioli G., Hazes J.M., Hobbs K., Huizinga T.W., Kavanaugh A., Kay J., Kvien T.K., Laing T., Mease P., Menard H.A., Moreland L.W., Naden R.L., Pincus T., Smolen J.S., Stanislawska-Biernat E., Symmons D., Tak P.P., Upchurch K.S., Vencovsky J., Wolfe F., Hawker G. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. 2010. [DOI] [PubMed] [Google Scholar]
- 27.Petri M., Orbai A.-M., Alarcón G.S., Gordon C., Merrill J.T., Fortin P.R., Bruce I.N., Isenberg D., Wallace D.J., Nived O., Sturfelt G., Ramsey-Goldman R., Bae S.-C., Hanly J.G., Sánchez-Guerrero J., Clarke A., Aranow C., Manzi S., Urowitz M., Gladman D., Kalunian K., Costner M., Werth V.P., Zoma A., Bernatsky S., Ruiz-Irastorza G., Khamashta M.A., Jacobsen S., Buyon J.P., Maddison P., Dooley M.A., van Vollenhoven R.F., Ginzler E., Stoll T., Peschken C., Jorizzo J.L., Callen J.P., Lim S.S., Fessler B.J., Inanc M., Kamen D.L., Rahman A., Steinsson K., Franks A.G., Sigler L., Hameed S., Fang H., Pham N., Brey R., Weisman M.H., McGwin G., Magder L.S. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A., Matucci-Cerinic M., Naden R.P., Medsger T.A., Carreira P.E., Riemekasten G., Clements P.J., Denton C.P., Distler O., Allanore Y., Furst D.E., Gabrielli A., Mayes M.D., van Laar J.M., Seibold J.R., Czirjak L., Steen V.D., Inanc M., Kowal-Bielecka O., Müller-Ladner U., Valentini G., Veale D.J., Vonk M.C., Walker U.A., Chung L., Collier D.H., Ellen Csuka M., Fessler B.J., Guiducci S., Herrick A., Hsu V.M., Jimenez S., Kahaleh B., Merkel P.A., Sierakowski S., Silver R.M., Simms R.W., Varga J., Pope J.E. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 29.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., Rasmussen A., Scofield H., Vitali C., Bowman S.J., Mariette X. American college of rheumatology/European league against rheumatism classification criteria for primary sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis & Rheumatology. 2016;69:35–45. doi: 10.1002/art.39859. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladman Dd I.D.U.M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 31.Valentini G. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 2001;60:592–598. doi: 10.1136/ard.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seror R., Ravaud P., Bowman S.J., Baron G., Tzioufas A., Theander E., Gottenberg J.-E., Bootsma H., Mariette X., Vitali C. EULAR Sjögren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann. Rheum. Dis. 2010;69:1103–1109. doi: 10.1136/ard.2009.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevaart L., Vervoordeldonk M.J., Tak P.P. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum. 2010;62:2192–2205. doi: 10.1002/art.27503. [DOI] [PubMed] [Google Scholar]
- 34.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 35.Kollias G., Papadaki P., Apparailly F., Vervoordeldonk M.J., Holmdahl R., Baumans V., Desaintes C., di Santo J., Distler J., Garside P., Hegen M., Huizinga T.W.J., Jungel A., Klareskog L., McInnes I., Ragoussis I., Schett G., Hart B.t., Tak P.P., Toes R., van den Berg W., Wurst W., Gay S. Animal models for arthritis: innovative tools for prevention and treatment. Ann. Rheum. Dis. 2011;70:1357–1362. doi: 10.1136/ard.2010.148551. [DOI] [PubMed] [Google Scholar]
- 36.Egan P.J., van Nieuwenhuijze A., Campbell I.K., Wicks I.P. Promotion of the local differentiation of murine Th17 cells by synovial macrophages during acute inflammatory arthritis. Arthritis Rheum. 2008;58:3720–3729. doi: 10.1002/art.24075. [DOI] [PubMed] [Google Scholar]
- 37.Midwood K., Sacre S., Piccinini A.M., Inglis J., Trebaul A., Chan E., Drexler S., Sofat N., Kashiwagi M., Orend G., Brennan F., Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 38.Asquith D.L., Miller A.M., McInnes I.B., Liew F.Y. Animal models of rheumatoid arthritis. Eur. J. Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- 39.Khoyratty T.E., Udalova I.A. Diverse mechanisms of IRF5 action in inflammatory responses. Int. J. Biochem. Cell Biol. 2018;99:38–42. doi: 10.1016/j.biocel.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Farina A., Cirone M., York M., Lenna S., Padilla C., Mclaughlin S., Faggioni A., Lafyatis R., Trojanowska M., Farina G.A. Epstein–Barr virus infection induces aberrant TLR activation pathway and fibroblast–myofibroblast conversion in Scleroderma. J. Invest. Dermatol. 2014;134:954–964. doi: 10.1038/jid.2013.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efthymiou G., Liaskos C., Marou E., Dardiotis E., Tsimourtou V., Scheper T., Meyer W., Bogdanos D.P., Hadjigeorgiou G., Sakkas L.I. Scleroderma, Myositis and Related Syndromes. 2018. AB0751The epstein-barr virus infection in systemic sclerosis. pp. 1513.1-1513. [DOI] [Google Scholar]
- 42.Severa M., Rizzo F., Srinivasan S., di Dario M., Giacomini E., Buscarinu M.C., Cruciani M., Etna M.P., Sandini S., Mechelli R., Farina A., Trivedi P., Hertzog P.J., Salvetti M., Farina C., Coccia E.M. A cell type-specific transcriptomic approach to map B cell and monocyte type I interferon-linked pathogenic signatures in Multiple Sclerosis. J. Autoimmun. 2019;101:1–16. doi: 10.1016/j.jaut.2019.04.006. [DOI] [PubMed] [Google Scholar]