Abstract
Introduction
Autoimmune thyroid disease (AITD) is the most common autoimmune disorder worldwide. Remarkably, it is commonly accompanied by other autoimmune diseases, such as rheumatoid arthritis (RA). The immunopathogenic mechanisms behind the coexistence of these disorders are still not completely understood. Immunogenetics influences the physiopathology of these diseases since ethnicity plays an essential role in the inheritance of susceptibility markers.
Methods
High-resolution HLA class II typing was performed using a sequence-based method.
Results
The allele frequency of HLA-DRB1∗04:04 and -DRB1∗03:01 were significantly increased in patients with AITD and RA compared to healthy individuals, pC = 0.021, OR = 2.4, 95%CI = 1.19–4.75 and pC = 0.009, OR = 3.4, 95%CI = 1.42–7.93, respectively. Remarkably, these patients have a combined risk given by susceptibility HLA-DRB1 alleles that contain the shared epitope, pC = 0.03, OR = 1.7, IC95% = 1.07–2.76, and a lack of protective alleles carrying aspartic acid70, pC = 0.009, OR = 0.5, IC95% = 0.32–0.84.
Discussion
The results suggest that patients with AITD and RA have an immunogenetic mechanism that combines the susceptibility alleles associated with both diseases. Importantly, it seems to be linked mainly to the lack of protective alleles with aspartic acid in the position 70, along with the presence of susceptibility alleles that have the sequences QRRAA, QKRAA, and RRRAA at positions 70–74.
Conclusion
Patients with AITD and RA have a characteristic immunogenetic signature, which could be useful for determining multiple autoimmunities and assessing their relatives’ risk of developing it.
Keywords: Multiple autoimmunities, Rheumatoid arthritis, Autoimmune thyroid disease, Graves’ disease, Hashimoto’s disease, Shared epitope, HLA-DRB1
Highlights
-
•
The HLA-DRB1∗04:04 and -DRB1∗03:01 are the susceptibility alleles in RA and AITD.
-
•
The susceptibility in RA and AITD involves HLA-DRB1 shared epitope alleles.
-
•
The Mexican mestizo admixture patterns explain the variety of SE alleles in RA and AITD.
-
•
The immunopathogenic mechanism involves lack of DRB1 alleles with aspartic acid at 70 position.
1. Introduction
Autoimmune diseases are relatively frequent worldwide, particularly in Mexican Mestizos. Among all the autoimmune diseases, Autoimmune Thyroid Disease (AITD) is by far the most prevalent, affecting up to 5% of the general population, although subclinical disease might be even more frequent [1]. The incidence in people with European ancestry is 27–448 per 100,000 individuals, and 100 per 100,000 individuals in Mexicans [2].
Regarding musculoskeletal autoimmune diseases, rheumatoid arthritis (RA) is one of the most common around the world, with an incidence of 0.5–1% in industrialized countries [1]. Notably, in the Mexican population, RA has a prevalence of 1.6% [3]. It is a chronic, progressive, autoimmune systemic disease that leads to joint destruction and organ impairment [1].
The coexistence of AITD and RA might be a result of their high frequency; however, a common physiopathogenic mechanism has been proposed. Several studies have highlighted the importance of shared genetic susceptibility for the development of RA and AITD, mainly Human Leukocyte Antigen (HLA) polymorphisms [4,5]. The most analyzed region in the genome for RA development is the Major Histocompatibility Complex (MHC), which includes the HLA class I and class II genes [4,6,7]. Association studies have consistently shown the presence of HLA-DRB1∗04 alleles in different ethnicities [[8], [9], [10], [11], [12], [13]]. Additionally, the variants associated with increased susceptibility share the epitope QRRAA, QKRAA, and RRRAA at positions 70–74. The most common are HLA-DRB1∗01:01, -DRB1∗10:01, -DRB1∗14:02, DRB1∗14:06 [14,15]. These studies have been replicated in the Mexican population. Additionally, DRB1∗04 alleles have been associated with high levels of rheumatoid factor [16].
Moreover, early studies showed the association of HLA-DRB1∗03 with Graves’ disease in Caucasians, while others have explored the relation of HLA-DRB1∗03 with AITD [5,[17], [18], [19]]. However, the association of HLA-DRB1∗03 with Hashimoto’s disease is not as strong as with Graves’ disease. The prevalence of AITD is estimated to be 5%; however, the prevalence of antithyroid antibodies may be even higher [20].
However, there is a fraction of patients who develop multiple autoimmunities. The most common in Mexico is the combination of RA and AITD. It is well known that patients with autoimmune rheumatological disorders are at higher risk of developing AITD [21]. Such overlap has been reported in up to 27% of patients [1,22]. A meta-analysis documented that RA patients have a frequency of antithyroid antibodies 2.3 to 3.1 times higher compared to healthy individuals [22].
The Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran (INCMNSZ) is a reference health care center for patients with rheumatological and endocrine autoimmune diseases. Its rheumatology outpatient clinic attends nearly 7500 patients, of which approximately 3000 have been diagnosed with RA, most of which show signs and symptoms of AITD.
Until today, there have been no studies on the prevalence of both diseases in the Mexican population or the shared immunogenetic mechanisms that predispose to them. Thus, this work aims to define genetic susceptibility to the development of AITD in Mexican patients with RA.
2. Materials and methods
2.1. Patient samples
Eligible patients included those born in Mexico whose parents and grandparents were also born in Mexico, referred to as Mexican Mestizos [5,16]. Forty-five patients who were diagnosed with AITD and RA were included. The RA diagnosis was based on clinical evaluation and fulfillment of the classification criteria for RA established by ACR/EULAR 2010 or ACR 1987 [23,24]. All patients were recruited from the outpatient rheumatology clinic at the INCMNSZ. AITD comprises either Graves’ disease or Hashimoto’s disease. The AITD diagnosis was based on clinical manifestations and laboratory results per the established criteria. The evaluation included thyroid hormone levels, and antithyroid antibodies, such as anti-thyroglobulin, anti-antithyroid peroxidase, and anti-thyrotropin receptor. Thyroid ultrasound was also employed to asses increased size [4].
As the control group, 234 unrelated Mexican admixed individuals were included [25]. In both groups, allelic and haplotypic gene frequencies of HLA Class I; HLA-A, HLA -B, HLA-C, and HLA Class II; HLA-DR and HLA-DQ were reported. The ethnicity of Mexican Mestizos was evaluated by a questionnaire, and an admixture estimate was verified with HLA alleles and STRs assays and calculations [25].
Clinical and sociodemographic information was registered for each patient. The information included age, gender, birthplace, and residence. Diagnosis of other autoimmune diseases, family members with autoimmune disorders, time of RA evolution, and interval time between the diagnosis of RA and AITD were included. Additionally, in the study group, the analysis included the percentage of patients who required more than 2 Disease-Modifying Anti-Rheumatic Drugs (DMARDs) for the control of extra-joint manifestations such as rheumatoid nodules.
2.2. Ethics statement
The Ethics Committee of the INCMNSZ reviewed and approved the protocol for this genetic study. All subjects were of adult age, provided a signed informed consent, authorizing the collection and processing of their blood sample and storage of their DNA samples at the INCMNSZ repositories for this and future studies.
2.3. Statistical analysis
Differences in the frequencies of HLA class II alleles were analyzed using X2, and p-values less than 0.05 were considered statistically significant. If appropriate, the p-values were corrected using the Bonferroni method (for allele frequencies, multiplying the original p-value by the number of alleles). Odds ratios (OR) and 95% confidence intervals (95%CI) were calculated to measure association strength with the program Epi Info™ 7.2 version. Clinical and demographic variables were analyzed with the IBM SPSS Statistics 25 program.
2.4. Sanger sequencing-based HLA typing
Genomic DNA was obtained from whole blood using the QIAamp DNA mini kit (Qiagen, Valencia, CA, USA). The DNA purity was evaluated with Qubit Fluorometric Quantitation (Invitrogen), and DNA integrity was assessed through gel electrophoresis. High-resolution HLA classI and II typing was performed using a sequence-based method (SBM) as described previously [26]. Briefly, as example we amplified exon 2 from HLA-DRB1. Polymerase chain reactions (PCRs) utilized 1.5 mm KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 200 mM dNTPs, 10 pM of each primer, 30 ng of DNA and 0.5 U of Taq DNA polymerase in a final volume of 25 μl. Amplifications were performed on a PE9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) under the following cycling conditions: 95 °C for 30 s, 65 °C for 30 s, 72 °C for 1 min, preceded by 5 min at 95 °C and followed by a final elongation step at 72 °C for 5 min. The amplified products were sequenced independently in both directions using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems™) on the ABI PRISM® 3730xl Genetic Analyzer (Applied Biosystems). Then, the BigDye XTerminator® Purification Kit (Applied Biosystems™) is used as a simple purification method for DNA sequencing reactions that remove unincorporated BigDye® terminator and salts. Data were analyzed with matching allele assignment software (Applied Biosystems) using the IMGT/HLA sequence database alignment tool http://www.ebi.ac.uk/imgt/hla/align.html [27]. Ambiguities were solved using group-specific sequencing primers (GSSPs) that had been previously reported and validated [26].
3. Results
3.1. Clinical manifestations
Table 1 shows the main clinical manifestations and demographic characteristics of Mexican AITD and RA patients included in this study. Ninety-five percent of patients were women (40 patients). Eighty-three percent of patients showed a positive reaction to the rheumatoid factor, and 47.6% had a positive reaction to anti-cyclic citrullinated peptides (anti-CCPs). Additionally, 7.1% of patients presented rheumatoid nodules, and 47.6% used more than 2 DMARDs to control their disease at the time of inclusion in the study. The mean age was 52.4 years, with a range of 26–75 years. Thirteen patients had diagnostics of Graves’ disease and 32 of Hashimoto’s thyroiditis. The mean interval of time between diagnoses was 9.4 years.
Table 1.
Clinical characteristics of Mexican patients with Autoimmune Thyroid Disease and Rheumatoid Arthritis.
| Characteristics | Patients |
|---|---|
| (N = 45) | |
| Female sex (%) | 95 |
| Mean age in years | 52.4 |
| Mean years since RA onset | 13.1 |
| Time between diagnoses in years(range) | 9.4 (0–31) |
| Rheumatoid factor + (%) | 83 |
| Anti-CCP + (%) | 47.6 |
| Rheumatoid nodules (%) | 7.1 |
| >2 DMARDs (%) | 47.6 |
| Graves’ disease n(%) | 13 (29%) |
| Hashimoto’s disease n(%) | 32 (71%) |
Abbreviations: disease-modifying anti-rheumatic drugs (DMARDs), anti-citrullinated protein antibody (Anti-CCP), rheumatoid arthritis (RA).
First degree relatives were reported to have autoimmune diseases in 69% of the cases. These diseases included systemic lupus erythematosus, rheumatoid arthritis, vitiligo, and autoimmune thyroid disease. Remarkably, 16% of patients have symptoms related to cardiovascular risk or ischemic heart disease.
3.2. HLA alleles in patients with rheumatoid arthritis and autoimmune thyroid disease
The allele frequency of HLA-DRB1∗04:04 was increased in the group of patients with RA and AITD when compared with healthy individuals, showing a statistically significant difference with a pC = 0.021, OR = 2.4, IC95% = 1.19–4.75. Furthermore, the frequency of HLA-DRB1∗03:01 was also increased when compared to healthy individuals, pC = 0.009, OR = 3.4, IC95% = 1.42–7.93.(Table 2)
Table 2.
Gene frequencies of HLA-DRB1 alleles in patients with Autoimmune Thyroid Disease and Rheumatoid Arthritis.
| HLA-DRB1 | AITD and RA |
Healthy Individuals |
pC | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| N = 90 |
N = 468 |
|||||||
| n | gf | n | gf | |||||
| DRB1∗04:04 | 13 | 0.144 | 31 | 0.066 | 0.021 | 2.4 | 1.19 | 4.75 |
| DRB1∗08:02 | 11 | 0.122 | 91 | 0.194 | 0.140 | 0.6 | 0.29 | 1.13 |
| DRB1∗03:01 | 9 | 0.100 | 15 | 0.032 | 0.009 | 3.4 | 1.42 | 7.93 |
| DRB1∗04:07 | 9 | 0.100 | 55 | 0.118 | 0.766 | 0.8 | 0.40 | 1.76 |
| DRB1∗14:06 | 8 | 0.089 | 47 | 0.100 | 0.886 | 0.9 | 0.40 | 1.92 |
| DRB1∗11:01 | 4 | 0.044 | 6 | 0.013 | 0.102 | 3.6 | 0.99 | 12.96 |
| DRB1∗04:01 | 3 | 0.033 | 3 | 0.006 | 0.090 | 5.3 | 1.06 | 26.92 |
| DRB1∗11:04 | 3 | 0.033 | 8 | 0.017 | 0.548 | 2.0 | 0.52 | 7.62 |
| DRB1∗14:02 | 3 | 0.033 | 11 | 0.024 | 0.859 | 1.4 | 0.39 | 5.24 |
| DRB1∗15:01 | 3 | 0.033 | 17 | 0.036 | 1.000 | 0.9 | 0.26 | 3.19 |
| DRB1∗01:01 | 2 | 0.022 | 9 | 0.019 | 1.000 | 1.2 | 0.25 | 5.46 |
| DRB1∗01:02 | 2 | 0.022 | 11 | 0.024 | 1.000 | 0.9 | 0.21 | 4.33 |
| DRB1∗07:01 | 2 | 0.022 | 33 | 0.071 | 0.135 | 0.3 | 0.07 | 1.27 |
| DRB1∗10:01 | 2 | 0.022 | 6 | 0.013 | 0.839 | 1.8 | 0.35 | 8.81 |
| DRB1∗13:03 | 2 | 0.022 | 3 | 0.006 | 0.397 | 3.5 | 0.58 | 21.39 |
| DRB1∗15:02 | 2 | 0.022 | 5 | 0.011 | 0.700 | 2.1 | 0.40 | 11.02 |
| DRB1∗16:02 | 2 | 0.022 | 30 | 0.064 | 0.188 | 0.3 | 0.08 | 1.41 |
| Other Alleles | 10 | 0.111 | 381 | 0.186 | ||||
Abbreviations: Autoimmune Thyroid Disease (AITD), confidence interval (CI), gene frequency (gf), odds ratio (OR), corrected probability value (pC), Rheumatoid Arthritis (RA).
Moreover, it was found that the frequencies of some HLA-DQB1 alleles were increased in the patients group, showing statistically significant differences compared to healthy individuals. These alleles were HLA-DQB1∗02:01 (pC = 1E-09, OR = 26.2, 95%IC = 7.29–93.97), HLA-DQB1∗03:02(pC = 1E-09, OR = 37.6, 95%IC = 13.95–101.46), and HLA-DQB1∗03:01 (pC = 1E-09, OR = 11.6, 95%IC = 5.45–24.56). These results obey the strong linkage disequilibrium between HLA-DRB1∗03:01-DQB1∗02:01. Meanwhile, HLA-DRB1∗14:06 and -DRB1∗14:02 show linkage with DQB1∗03:01. This data is shown in Table 1 in the supplementary materials.
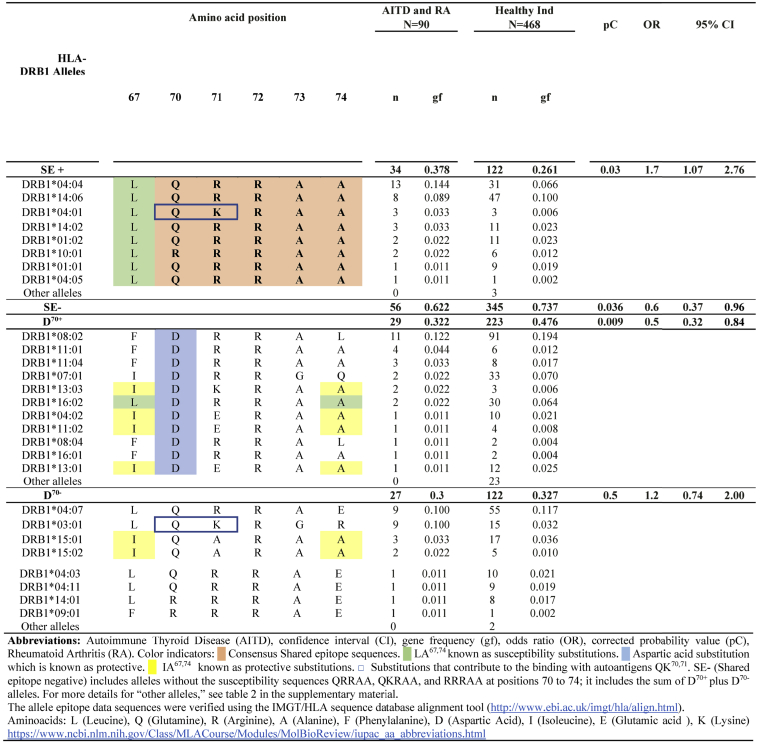
3.3. Both shared epitope alleles and D70+ alleles influence the susceptibility to multiple autoimmunities
Early studies in 1976 about HLA-DRB1 in RA patients showed that the presence of HLA-DRB1∗04 strongly predisposes to RA [28]. In 1986, the shared epitope (SE) hypothesis was postulated [15]. This hypothesis explains that polymorphisms in HLA-DRB1 are associated with RA because of the presence of the sequences QRRAA, QKRAA, and RRRAA, in amino acid positions 70–74. These alleles are collectively called HLA-SE alleles. It was hypothesized that this sequence directly influences peptide presentation or T-cell recognition [[29], [30], [31]]. The association of SE alleles with RA in Mexican Mestizos has been confirmed in several studies [16].
However, this mechanism has not been described in patients with multiple autoimmunities, like RA and AITD patients. The association of HLA-DRB1 SE alleles with AITD and RA was studied in this work. It was found that the proportion of patients carrying at least one HLA-DRB1 allele encoding the SE was 57%, contrasting with just 12% of the healthy individuals. In the patients group 18% were SE homozygotes, but only 2.6% of healthy individuals.(Table 5)
Table 5.
Genotypes absolute frequencies of HLA-DRB1 shared epitope, D70+ and D70- in patients with Autoimmune Thyroid Disease and Rheumatoid Arthritis.
| Group Genotype | RA & AITD N = 45 |
Healthy Individuals N = 234 |
pC | OR | IC95% | |||
|---|---|---|---|---|---|---|---|---|
| N | F | N | f | |||||
| SE/SE | 8 | 0.178 | 6 | 0.026 | 0.00009 | 8.2 | 2.7 | 25.03 |
| SE/D70- | 12 | 0.267 | 17 | 0.073 | 0.0003 | 4.6 | 2.03 | 10.59 |
| SE/D70- | 6 | 0.133 | 6 | 0.026 | 0.004 | 5.8 | 1.79 | 19.05 |
| D70-/D70- | 2 | 0.044 | 5 | 0.021 | 0.699 | 2.1 | 0.4 | 11.34 |
| D70-/D70+ | 11 | 0.244 | 7 | 0.030 | 5E-07 | 10.5 | 3.81 | 28.92 |
| D70+/D70+ | 6 | 0.133 | 16 | 0.068 | 0.239 | 2.1 | 0.77 | 5.69 |
Abbreviations: Autoimmune Thyroid Disease (AITD), confidence interval (CI), frequency (f), odds ratio (OR), corrected probability value (pC), Rheumatoid Arthritis (RA). Alleles with aspartic acid at position 70 (D70+), which confers a protective role to the carrier allele. Alleles with a different amino acid from aspartic acid at position 70 (D70-). For detailed information of alleles included in each group see Table 2 in supplementary materials.
In agreement with this observation, the frequency of HLA-DRB1 SE alleles was increased in RA and AITD patients, pC = 0.03, OR = 1.7, 95% CI = 1.07–2.76. The most frequent HLA-DRB1 allele encoding the SE in AITD and RA cases was HLA-DRB1∗04:04 (0.144 vs. 0.066) and HLA-DRB1∗04:01 (0.033 versus 0.006). However, lack of protective alleles seemed to be as important as the presence of SE risk alleles, since the proportion of D70+ alleles was significantly lower in patients with AITD and RA when compared with healthy individuals, pC = 0.009, OR = 0.5, 95%IC = 0.32–0.82. Unsurprisingly, neutral alleles (which do not confer susceptibility with SE and do not confer protection with D70+), showed no statistically significant differences between patients and healthy individuals (Table 3).
Table 3.
Gene frequencies of HLA-DRB1 shared epitope alleles vs. non-shared epitope alleles in patients with Autoimmune Thyroid Disease and Rheumatoid Arthritis.
Another factor that seemed to influence the susceptibility of patients was the presence of the epitope LA67,74. This epitope is important for citrullinated autoantigens binding. Notably, all SE alleles carry this substitution (Table 3). In contrast, the IA67,74 epitope shows a protective function [32]. IA67,74 was mainly found in D70+ alleles, which were found to be decreased in the patients group.
Finally, another epitope, QK70, 71, is relevant due to its capacity to form hydrogen bonds with citrullinated antigens; Hence, probably adding susceptibility. It is found in HLA-DRB1∗04:01 and -DRB1∗03:01—both significant susceptibility alleles in RA and AITD, respectively.
3.4. Distribution of HLA-B/-DRB1 blocks in patients and healthy individuals
Regarding haplotypes, it was found that HLA-DRB1∗04:04 was in linkage disequilibrium with HLA-B∗39:06 and HLA-B∗35:12. While the HLA-DRB1∗03:01 was associated with the HLA-B∗18:01 and HLA-B∗08:01. When performing a haplotype analysis, a significant difference was found for these two HLA haplotypes, B∗39:06/-DRB1∗04:04, pC = 0.0031, OR = 11.7, 95%IC = 2.10–64.66, and B∗08:01/-DRB1∗03:01, pC = 0.0003, OR = 14.8, 95%IC = 2.81–77.33. (Table 4)
Table 4.
Gene frequencies of HLA-B/-DRB1 haplotypes in patients with AITD and RA.
| HLA-B/-DRB1 Haplotypes | AITD and RA |
Healthy individuals |
pC | OR | 95% CI | |
|---|---|---|---|---|---|---|
| N = 90 |
N = 468 |
|||||
| N | n | |||||
| B∗08:01 - DRB1∗03:01 | 5 | 2 | 0.0003 | 14.8 | 2.81 | 77.33 |
| B∗39:06 - DRB1∗04:04 | 4 | 2 | 0.0031 | 11.7 | 2.10 | 64.66 |
| B∗18:01 - DRB1∗03:01 | 3 | 3 | 0.0697 | 5.7 | 1.14 | 28.94 |
| B∗35:12 - DRB1∗08:02 | 3 | 7 | 0.3847 | 2.4 | 0.62 | 9.63 |
| B∗35:17 - DRB1∗08:02 | 3 | 14 | 1.0000 | 1.2 | 0.34 | 4.27 |
| B∗39:06 - DRB1∗14:06 | 2 | 16 | 0.8732 | 0.7 | 0.16 | 3.05 |
| B∗14:02 - DRB1∗01:02 | 2 | 5 | 0.6452 | 2.3 | 0.43 | 11.84 |
| B∗13:02 - DRB1∗07:01 | 2 | 4 | 0.5023 | 2.8 | 0.51 | 15.70 |
| B∗48:01 - DRB1∗04:04 | 2 | 3 | 0.3552 | 3.8 | 0.62 | 22.98 |
| B∗39:02 - DRB1∗08:02 | 2 | 2 | 0.2130 | 5.7 | 0.79 | 40.91 |
| B∗40:02 - DRB1∗14:02 | 2 | 2 | 0.2130 | 5.7 | 0.79 | 40.91 |
| B∗40:02 - DRB1∗04:07 | 1 | 4 | 1.0000 | 1.4 | 0.15 | 12.66 |
| B∗40:02 - DRB1∗04:04 | 1 | 3 | 1.0000 | 1.9 | 0.19 | 18.17 |
| B∗35:01 - DRB1∗04:07 | 1 | 3 | 1.0000 | 1.9 | 0.19 | 18.17 |
| B∗35:01 - DRB1∗08:02 | 1 | 3 | 1.0000 | 1.9 | 0.19 | 18.17 |
| B∗52:01 - DRB1∗14:06 | 1 | 3 | 1.0000 | 1.9 | 0.19 | 18.17 |
| B∗40:02 - DRB1∗14:06 | 1 | 2 | 0.9440 | 2.8 | 0.25 | 31.31 |
| B∗35:12 - DRB1∗04:04 | 1 | 2 | 0.9440 | 2.8 | 0.25 | 31.31 |
| B∗35:12 - DRB1∗04:07 | 1 | 2 | 0.9440 | 2.8 | 0.25 | 31.31 |
| Other Haplotypes | 52 | 386 | ||||
Abbreviations: Autoimmune Thyroid Disease (AITD), confidence interval (CI), gene frequency (gf), odds ratio (OR), corrected probability value (pC), Rheumatoid Arthritis (RA).
4. Discussion
In summary, the results suggest that patients with RA and AITD have an immunogenetic mechanism that combines the susceptibility alleles of both diseases. At the same time, results show a decreased frequency of protective alleles. Therefore, the analysis of both the increase in susceptibility factors and the diminishment of protection factors is furtherly dissected. Both mechanisms depend on the HLA-DRB1 chain sequence and alleles.
The first factor: Known susceptibility alleles. The HLA-DRB1 susceptibility alleles for each disease, separately, were concurrently carried by the AITD and RA patients. The alleles found were HLA-DRB1∗04:04 and HLA-DRB1∗03:01, which are the most frequently occurring alleles in RA and Graves’ disease in Mexican patients, respectively [5,16,17].
The second factor: Shared epitope susceptibility alleles. The HLA-DRB1 SE alleles found were HLA-DRB1∗04:04, -DRB1∗14:06, -DRB1∗04:01, -DRB1∗14:02, -DRB1∗01:02, -DRB1∗10:01, -DRB1∗01:01 and -DRB1∗04:05. These alleles encode the SE sequences QRRAA, QKRAA, and RRRAA at positions 70–74 in the binding-peptide groove [[16], [35]]. Most of these alleles did not show a statistically significant difference in the AITD and RA group compared to healthy individuals. Regardless of the discrepancy in the frequency of individual alleles, analyzing SE alleles conjointly showed a significant increase in the study group compared to the control group. The presence and dose of HLA-DRB1 alleles encoding the SE have been associated with more severe disease, extra-articular manifestations, higher degree and extent of joint damage, and a higher likelihood of positive rheumatoid factor [33,34]. This finding suggests that the SE hypothesis also encompasses multiple autoimmunities, in the form of RA with concurrent AITD.
The third factor: Other susceptibility epitopes in the HLA-DRB1 chain. The SE hypothesis is widely accepted; nevertheless, the reason why the SE alleles confer susceptibility has not yet been clearly explained. Nonetheless, the epitope LA67,74 has been strongly associated with a higher risk of developing RA. The mechanism by which LA67,74 confers susceptibility might be due to the creation of the appropriate pocket in which citrullinated antigens can bind to HLA-DRB1 molecules through interaction with these residues [32]. Importantly, all SE alleles also contain this residue, which could represent an added factor of susceptibility or could suggest an extended epitope, as previous studies have [15]. Remarkably, this model proposes an additional participation of QK70,71, which could form hydrogen bonds with the self-peptide in RA patients, thus playing a critical role in peptide binding [32]. Concurrently, HLA-DRB1∗03:01, the most common susceptibility allele for AITD and the strongest risk allele for Graves’ disease, also carries QK70,71. This could suggest a common mechanism for peptide binding in both RA and AITD, favoring, at least in part, the development of multiple autoimmunities (Table 3).
The fourth factor: Diminished protection alleles in the patients group. Another crucial element in the group of patients was the lack of protective alleles carrying D70. The presence of this residue has been identified as the strongest protective factor against RA development [35]. The mechanism appears to be related to the inability to bind citrullinated antigens when position 70 is occupied by an aspartic acid [32]. Furthermore, the residue IA67,74 has also been identified as protective and is concurrently diminished in the patients group.
The fifth factor: amino acid in position 74. A common fact for AITD and RA is the importance of amino acid at position 74 of the DRB1 chain for the binding of self-antigens. For Graves’ disease, R74 substitution favors the binding of thyroid-stimulating hormone receptor and thyroglobulin peptides [17]. The HLA-DRB1∗03:01 allele meets this feature. For RA, A74 is part of an indispensable epitope for the binding of citrullinated antigens, and the SE alleles meet this feature perfectly.
Having analyzed the aforementioned immune genetic factors that increase the risk of developing RA and AITD, it is also crucial to consider the origin of all these factors. A possible candidate is the interaction between autochthonous and imported alleles, which is a result of the thorough admixture process that has taken place in Mexico ever since the Spanish conquest. For instance, the HLA-DRB1∗04:04 allele, which carries the SE and was one of the susceptibility alleles in the study group, is also one of the most frequent HLA-DRB1 alleles in autochthonous groups, such as Nahuas and Mazatecans, and Mexican Mestizos [36]. Contrastingly, HLA-DRB1∗03:01 is an imported allele that is more frequent in Caucasian populations, and has also been consistently associated with autoimmunity, such as Graves’ disease [17,19]. The presence of multiple susceptibility alleles from different ethnic origins explains, in part, the high incidence of autoimmunity among Mexican Mestizos.
The distribution of HLA SE risk alleles varies geographically, according to ancestry and ethnicity. Therefore, ethnicity is recognized as an important risk factor in RA. Namely, in North America and Northern Europe the HLA-DRB1∗01:01, -DRB1∗04:01, -DRB1∗04:04, and -DRB1∗04:05 SE alleles predominate; with the latter being one of the main SE alleles in Chinese, Japanese, Korean, and Asian-Indian patients [[8], [9], [10], [11], [12], [13]]. Del Rincon et al. performed an HLA study in a group of Mexican Americans with RA. They reported a significant increase of the HLA-DRB1∗14:02 allele in the patients group compared to healthy controls, along with a significantly higher frequency of homozygotes among RA patients [37]. All of these SE risk alleles were present in the study group, although only certain alleles reached statistical significance individually. Such finding provides further evidence of the importance of admixture in Mexicans. This phenomenon is not replicated in AITD, with the susceptibility allele HLA-DRB1∗03:01, which explains the HLA-haplotype susceptibility due to the linkage disequilibrium -B∗08 –C∗07 -DQB1∗02 [38].
According to the susceptibility conferred by the HLA haplotypes, previous studies have shown that the interaction of variants in DR-DQ alleles may generate complex haplotypes patterns that are associated with genetic susceptibility for the development of autoimmune diseases [6]. In the present study, haplotypes B∗39:06/-DRB1∗04:04 and B∗08:01/-DRB1∗03:01 were associated with an increased predisposition for the development of both autoimmune diseases. Interestingly, DRB1∗03:01 does not belong to the shared epitope.
From a clinical point of view, identifying Mexican individuals at high risk to develop RA due to anti-CCP serology or positivity for an HLA SE allele could be an opportunity to decrease the risk of developing RA. In this sense, there is evidence that elevated levels of omega-3 fatty acids bound to erythrocytes could reduce the risk for the development of RA [39,40]. Prospective studies or clinical trials have not corroborated these findings.
It is incredibly relevant for rheumatologists to be aware of this association as a lower threshold to suspect multiple autoimmunities would lead to prompt diagnosis and treatment of AITD, as a delay can increase the risk for developing diabetes and cardiovascular disease [21]. Although routine screening of thyroid autoantibodies is not recommended in the general population, it could be justified in RA patients.
As for the clinical implications, Sakr and colleagues reported the occurrence of multiple autoimmunities in a series of RA patients from Egypt. When compared to a group of healthy individuals, RA patients had a significantly increased occurrence of high titers of TGO and Tg autoantibodies. Additionally, patients with RA plus AITD were more commonly female, younger, had higher erythrocyte sedimentation rate, and higher Disease Activity Score 28 (DAS28) [41].
This course of ability can change the approach and management given to patients during their care. However, this association does not appear to be restricted to alleles included in the shared epitope.
In conclusion, the analysis of the MHC haplotypes in the co-occurrence of AITD and RA patients could identify the risk for multiple autoimmunities, both in the patient and in first degree relatives.
Author contributions
Valdés-Corona LF: Methodology, and Writing - original draft. Hernández-Doño S: Formal analysis, Investigation, Writing - original draft, Writing - review & editing, and Visualization. Rodríguez-Reyna TS: Formal analysis, Writing - review & editing, and Visualization. García-Silva R: Writing - original draft, and Writing - review & editing. Jakez J: Investigation. Escamilla-Tilch M: Investigation. Lima G: Investigation. Llorente L: Formal analysis, Writing - review & editing, and Visualization. Pineda C: Conceptualization. Yunis E: Conceptualization. Granados J: Conceptualization, Project administration, Funding acquisition, Writing - original draft, and Writing - review & editing
Declaration of competing interest
The author(s) declared no potential conflicts of interest concerning the research, authorship, and publication of this article.
Acknowledgments
The authors thank all individuals who have participated in this genetic study. This work was supported by grant 000 000 000273175 from the CONACYT (Consejo Nacional de Ciencia y Tecnología de México).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2020.100057.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lazurova I., Jochmanova I., Benhatchi K., Sotak S. Autoimmune thyroid disease and rheumatoid arthritis: relationship and the role of genetics. Immunol. Res. 2014;60:193–200. doi: 10.1007/s12026-014-8598-9. [DOI] [PubMed] [Google Scholar]
- 2.McLeod D.S., Cooper D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252–265. doi: 10.1007/s12020-012-9703-2. [DOI] [PubMed] [Google Scholar]
- 3.Pelaez-Ballestas I., Sanin L.H., Moreno-Montoya J., Alvarez-Nemegyei J., Burgos-Vargas R., Garza-Elizondo M. Epidemiology of the rheumatic diseases in Mexico. A study of 5 regions based on the COPCORD methodology. J. Rheumatol. Suppl. 2011;86:3–8. doi: 10.3899/jrheum.100951. [DOI] [PubMed] [Google Scholar]
- 4.Fallahi P., Ferrari S.M., Ruffilli I., Elia G., Biricotti M., Vita R. The association of other autoimmune diseases in patients with autoimmune thyroiditis: review of the literature and report of a large series of patients. Autoimmun. Rev. 2016;15:1125–1128. doi: 10.1016/j.autrev.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Trevino O., Yamamoto-Furusho J.K., Cutino-Moguel T., Hernandez-Martinez B., Rodriguez-Reyna T.S., Ruiz-Morales J.A. HLA study on two Mexican Mestizo families with autoimmune thyroid disease. Autoimmunity. 2002;35:265–269. doi: 10.1080/0891693021000010712. [DOI] [PubMed] [Google Scholar]
- 6.Miyadera H., Tokunaga K. Associations of human leukocyte antigens with autoimmune diseases: challenges in identifying the mechanism. J. Hum. Genet. 2015;60:697–702. doi: 10.1038/jhg.2015.100. [DOI] [PubMed] [Google Scholar]
- 7.Cho J.H., Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat. Med. 2015;21:730–738. doi: 10.1038/nm.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapitány A., Zilahi E., Szántó S., Szücs G., Szabó Z., Végvári A. Association of rheumatoid arthritis with HLA-DR1 and HLA-DR4 in Hungary. Ann. N. Y. Acad. Sci. 2005;1051:263–270. doi: 10.1196/annals.1361.067. [DOI] [PubMed] [Google Scholar]
- 9.Jaraquemada D., Ollier W., Awad J., Young A., Silman A., Roitt I.M. HLA and rheumatoid arthritis: a combined analysis of 440 British patients. 1986;45:627–636. doi: 10.1136/ard.45.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepúlveda-Delgado J., Rizo-Pinto A., Granados-Arriola J., Mena-Vela B.A., Cetina-Díaz J.H., García-Silva R. Role of HLA-DRB1∗04 in the susceptibility and HLA-DRB1∗08 in the protection for development of rheumatoid arthritis in a population of Southern Mexico: brief report. Clin. Rheumatol. 2020 doi: 10.1007/s10067-020-05060-0. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi F., Nakano K., Matsuta K., Nabeta H., Bannai M., Tanimoto K. Positive and negative association of HLA-DR genotypes with Japanese rheumatoid arthritis. Clin. Exp. Rheumatol. 1996;14:17–22. [PubMed] [Google Scholar]
- 12.Hong G.H., Park M.H., Takeuchi F., Oh M.D., Song Y.W., Nabeta H. Association of specific amino acid sequence of HLA-DR with rheumatoid arthritis in Koreans and its diagnostic value. J. Rheumatol. 1996;23:1699–1703. [PubMed] [Google Scholar]
- 13.Toussirot E., Auge B., Tiberghien P., Chabod J., Cedoz J.P., Wendling D. HLA-DRB1 alleles and shared amino acid sequences in disease susceptibility and severity in patients from eastern France with rheumatoid arthritis. J. Rheumatol. 1999;26:1446–1451. [PubMed] [Google Scholar]
- 14.Kim K., Bang S.Y., Lee H.S., Bae S.C. Update on the genetic architecture of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:13–24. doi: 10.1038/nrrheum.2016.176. [DOI] [PubMed] [Google Scholar]
- 15.Gregersen P.K., Silver J., Winchester R.J. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Morales J.A., Vargas-Alarcon G., Flores-Villanueva P.O., Villarreal-Garza C., Hernandez-Pacheco G., Yamamoto-Furusho J.K. HLA-DRB1 alleles encoding the "shared epitope" are associated with susceptibility to developing rheumatoid arthritis whereas HLA-DRB1 alleles encoding an aspartic acid at position 70 of the beta-chain are protective in Mexican Mestizos. Hum. Immunol. 2004;65:262–269. doi: 10.1016/j.humimm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson E.M., Huber A., Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J. Autoimmun. 2008;30:58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Criswell L.A., Pfeiffer K.A., Lum R.F., Gonzales B., Novitzke J., Kern M. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am. J. Hum. Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt P.J., Marshall S.E., Weetman A.P., Bunce M., Bell J.I., Wass J.A. Histocompatibility leucocyte antigens and closely linked immunomodulatory genes in autoimmune thyroid disease. Clin. Endocrinol. 2001;55:491–499. doi: 10.1046/j.1365-2265.2001.01356.x. [DOI] [PubMed] [Google Scholar]
- 20.Antonelli A., Ferrari S.M., Corrado A., Di Domenicantonio A., Fallahi P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015;14:174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Cardenas Roldan J., Amaya-Amaya J., Castellanos-de la Hoz J., Giraldo-Villamil J., Montoya-Ortiz G., Cruz-Tapias P. vol. 2012. Arthritis; 2012. p. 864907. (Autoimmune Thyroid Disease in Rheumatoid Arthritis: a Global Perspective). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X.F., Gu J.Q., Shan Z.Y. Increased risk of thyroid autoimmunity in rheumatoid arthritis: a systematic review and meta-analysis. Endocrine. 2015;50:79–86. doi: 10.1007/s12020-015-0533-x. [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. 2010. [DOI] [PubMed] [Google Scholar]
- 24.Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Zuniga J., Yu N., Barquera R., Alosco S., Ohashi M., Lebedeva T. HLA class I and class II conserved extended haplotypes and their fragments or blocks in Mexicans: implications for the study of genetic diversity in admixed populations. PloS One. 2013;8 doi: 10.1371/journal.pone.0074442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebedeva T.V., Mastromarino S.A., Lee E., Ohashi M., Alosco S.M., Yu N. Resolution of HLA class I sequence-based typing ambiguities by group-specific sequencing primers. Tissue Antigens. 2011;77:247–250. doi: 10.1111/j.1399-0039.2010.01616.x. [DOI] [PubMed] [Google Scholar]
- 27.Robinson J. IMGT/HLA Database--a sequence database for the human major histocompatibility complex. Nucleic Acids Res. 2001;29:210–213. doi: 10.1093/nar/29.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stastny P. Mixed lymphocyte cultures in rheumatoid arthritis. J. Clin. Invest. 1976;57:1148–1157. doi: 10.1172/JCI108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winchester R., Gregersen P. Springer Semin Immunopathol; 1988. The Molecular Basis of Susceptibility to Rheumatoid Arthritis: the Conformational Equivalence Hypothesis; pp. 119–139. [DOI] [PubMed] [Google Scholar]
- 30.van Heemst J., van der Woude D., Huizinga T.W., Toes R.E. HLA and rheumatoid arthritis: how do they connect? Ann. Med. 2014;46:304–310. doi: 10.3109/07853890.2014.907097. [DOI] [PubMed] [Google Scholar]
- 31.Morgan A.W., Haroon-Rashid L., Martin S.G., Gooi H.-C., Worthington J., Thomson W. The shared epitope hypothesis in rheumatoid arthritis: evaluation of alternative classification criteria in a large UK Caucasian cohort. Arthritis Rheum. 2008;58:1275–1283. doi: 10.1002/art.23432. [DOI] [PubMed] [Google Scholar]
- 32.Freed B.M., Schuyler R.P., Aubrey M.T. Association of the HLA-DRB1 epitope LA67, 74 with rheumatoid arthritis and citrullinated vimentin binding. Arthritis Rheum. 2011;63:3733–3739. doi: 10.1002/art.30636. [DOI] [PubMed] [Google Scholar]
- 33.Perdriger A., Chalès G., Semana G., Guggenbuhl P., Meyer O., Quillivic F. Role of HLA-DR-DR and DR-DQ associations in the expression of extraarticular manifestations and rheumatoid factor in rheumatoid arthritis. J. Rheumatol. 1997;24:1272–1276. [PubMed] [Google Scholar]
- 34.Koh W.H., Chan S.H., Lin Y.N., Boey M.L. Association of HLA-DRB1∗0405 with extraarticular manifestations and erosions in Singaporean Chinese with rheumatoid arthritis. J. Rheumatol. 1997;24:629–632. [PubMed] [Google Scholar]
- 35.Mattey D.L., Dawes P.T., Gonzalez-Gay M.A., Garcia-Porrua C., Thomson W., Hajeer A.H. HLA-DRB1 alleles encoding an aspartic acid at position 70 protect against development of rheumatoid arthritis. J. Rheumatol. 2001;28:232–239. [PubMed] [Google Scholar]
- 36.Vargas-Alarcón G., Gamboa R., Zuñiga J., Hernández-Pacheco G., Ramos-Kuri M., Castillo E. HLA-DR4 allele frequencies on Indian and Mestizo population from Mexico. Hum. Immunol. 2000;61:341–344. doi: 10.1016/s0198-8859(99)00180-9. [DOI] [PubMed] [Google Scholar]
- 37.Del Rincón I., Escalante A.N. HLA-DRB1 alleles associated with susceptibility or resistance to rheumatoid arthritis, articular deformities, and disability in Mexican Americans. Arthritis Rheum. 1999;42:1329–1338. doi: 10.1002/1529-0131(199907)42:7<1329::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Stenszky V., Kozma L., Balazś C., Bear J.C., Farid N.R. The role of HLA antigens in the manifestation and course of Graves’ disease. Mol. Biol. Med. 1986;3:53–62. [PubMed] [Google Scholar]
- 39.Sparks J.A., Costenbader K.H. Rheumatoid arthritis in 2017: protective dietary and hormonal factors brought to light. Nat. Rev. Rheumatol. 2018;14:71–72. doi: 10.1038/nrrheum.2017.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gan R.W., Bemis E.A., Demoruelle M.K., Striebich C.C., Brake S., Feser M.L. The association between omega-3 fatty acid biomarkers and inflammatory arthritis in an anti-citrullinated protein antibody positive population. Rheumatology. 2017;56:2229–2236. doi: 10.1093/rheumatology/kex360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakr B.R., Elfishawi M.M., ElArousy M.H., Hatw A.K., AbdulKarim A.N., Tammam A.B. Rheumatoid arthritis: a single-center Egyptian experience. Immunol. Invest. 2018;47:293–302. doi: 10.1080/08820139.2018.1425700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.