Abstract
The inflammatory response has been implicated in various cardiac and systemic diseases. Epigallocatechin-3-gallate (EGCG), the major polyphenol extracted from green tea, has various biological and pharmacological properties, such as anti-inflammation, anti-oxidative and anti-tumorigenesis. To some extent, the mechanism of EGCG in the inflammatory response that characterizes myocardial dysfunction is not fully understood. The present study aimed to investigate the inhibiting effect of EGCG on lipopolysaccharide (LPS)-induced inflammation in vitro. Treatment with LPS affected rat H9c2 cardiomyocytes and induced an inflammatory response. However, the LPS-induced effects were attenuated after treatment with EGCG. The present results demonstrated that EGCG treatment repressed several inflammatory mediators, such as vascular endothelial growth factor, chemokine ligand 5, chemokine ligand 2, intercellular adhesion molecule-1, matrix metalloproteinase-2, tumor necrosis factor-α and nitric oxide (induced by LPS), and the repressing effect of EGCG on inflammatory response was dose-dependent in the range of 6.25-100 µM. EGCG inhibited these marked inflammatory key signaling molecules by reducing the expression of phospho-nuclear factor-κB p65, -Akt, -ERK and -MAPK p38 while the total protein level of these signal proteins were not affected. In conclusion, the present findings suggested that EGCG possesses cardiomyocyte-protective action in reducing the LPS-induced inflammatory response due to the inhibition of the phosphorylation of Akt and ERK signaling molecules.
Keywords: EGCG, LPS, inflammation, H9c2, inflammatory mediators
Introduction
The inflammatory response is the key pathogenesis of the most common forms of heart disease and its processes underlie various conditions related with injury of the cardiac muscle, such as cardiomyopathy, myocardial infarction, sepsis and heart failure (1,2). Lipopolysaccharide (LPS) is a major component of the bacterial outer membrane, and plays a crucial role in the initiation of several diseases (3). Numerous previous studies demonstrated that LPS contributes to inflammation and apoptosis; for example, LPS-induced acute respiratory distress syndrome, systemic inflammation induced by a low dose of LPS in mice and LPS-stimulated acute kidney injury (4,5). LPS can also induce inflammation and apoptosis in cardiomyocytes (6).
LPS, as a stimulus, contributes to pro-inflammatory responses, in addition to the increased expression of numerous inflammatory cytokines, including tumor necrosis factor-α (TNF-α), monocyte chemo-attractant protein (MCP)-1 and intercellular adhesion molecule (ICAM)-1 in the heart (7). A previous study has demonstrated that TNF-α shows direct negative inotropic effects and several features of heart failure (HF). In HF, inflammation might be associated with dysregulation of the TNF-α feedback system (8). A previous study identified that cardiac inflammation is accompanied by overexpression of ICAM-1 and vascular cell adhesion molecule (VCAM)-1(9). During the development of inflammation, cellular adhesion molecules (CAMs) mediate the transendothelial migration of immune-cells into the cardiac tissue. Those infiltrated cells, as well as cardiomyocytes, produce pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1β and IL-18. These cytokines stimulate the expression of CAMs in a positive feedback system. Furthermore, they have direct and indirect detrimental effects on the heart (10).
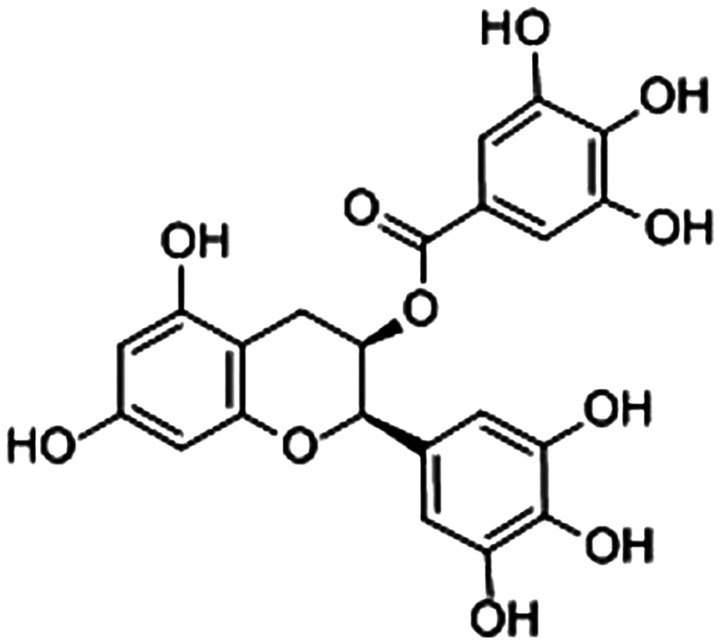
Epigallocatechin-3-gallate [EGCG; Fig. 1; (11)] is the major polyphenol extracted from green tea, which is the most abundant and well-studied catechin (12). Previous studies demonstrated the beneficial effects of EGCG, including potential anti-oxidative, anti-inflammatory and anti-tumorigenesis properties in the treatment and prevention of several chronic diseases, including heart diseases, cancer, obesity and endocrine disorders (13,14). Among these effects, the anti-inflammatory activity of EGCG plays a vital role against these diseases.
Figure 1.
Structure of (-)-epigallocatechin-gallate (11).
EGCG was demonstrated to decrease expression of inflammatory genes, such as TNF-α, IL-1β, IL-6 and IL-8, when EGCG (10 µg/ml) was applied to inflamed human corneal epithelial cells (15). Similarly, there was a significant downregulation of the expression of some kidney injury markers and pro-inflammatory mediators in unilateral ureteral obstruction (16). Previous studies demonstrated that cardiac injuries were associated with the activation of p38, PI3K-Akt and ERK 1/2(17). The PI3K-Akt pathway plays a vital role in many biological reactions, including inflammatory responses, chemotaxis, cellular activation and apoptosis, which also regulates the expressions of inflammatory genes (18). Previous studies have demonstrated that inflammation stimulates the activation of PI3K/Akt signaling pathway in cardiomyocytes (19,20). A previous study demonstrated that EGCG repressed the PI3K/Akt system in fibroblast cells and phosphorylation of ERK and Akt kinases in epidermal growth factor stimulated cells (21). However, to the best of the authors' knowledge, few studies have investigated the effect of EGCG on LPS-induced inflammation in H9c2 cells.
Therefore, the aim of the present study was to investigate the regulation of EGCG on inflammatory mediators, and examine whether EGCG treatment could ameliorate inflammatory responses induced by LPS in H9c2 and the underlying mechanisms in vitro.
Materials and methods
Reagents
EGCG (purity, 98%) was purchased from Sigma-Aldrich (Merck KGaA). LPS was obtained from Sigma-Aldrich (Merck KGaA). The Cell Titer 96 Aqueous cell viability assay kit was procured from Promega Corporation. ELISA kits for vascular endothelial growth factor (VEGF; cat. no. DY493), Rantes (cat. no. DY478), MCP-1 (cat. no. SMJE00B), ICAM-1 (cat. no. MIC100), matrix metalloproteinase 2 (MMP-2; cat. no. SMMP200) and TNF-α (cat. no. SMTA00B), as well as nitric oxide (NO; cat. no. SKGE001) assay kits were purchased from R&D Systems, Inc. Anti-Akt (cat. no. 4685), anti-nuclear factor-κB (NF-κB) p65 (cat. no. 3034), anti-p38 (cat. no. 9212), anti-Erk (cat. no. 4695), anti-phosphorylated (p)-Akt (cat. no. 4060), anti-p-NF-κB p65 (cat. no. 3033) and anti-p-ERK (cat. no. 4376) were all obtained from Cell Signaling Technology, Inc. Mouse anti-β-actin (cat. no. sc-58673) was obtained from Santa Cruz Biotechnology, Inc.
Cell culture
The rat embryonic-heart derived cell line H9c2 was obtained from The American Type Culture Collection. Cells were cultured in DMEM (Sigma-Aldrich; Merck KGaA) supplemented with 10% heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.), 25 mM D-glucose, 100 U/ml penicillin and 100 U/ml streptomycin, and maintained in a humidified atmosphere of 5% CO2 at 37˚C. The medium was changed every 2 days.
Viability evaluation
Cell viability was determined by an MTS assay (Promega Corporation). Cultured H9c2 cells (1x104 cells/well in 96-well plate) were treated with EGCG (0, 1.5, 3.0, 6.25, 12.5, 25, 50, 100 and 200 µmol/l) for 48 h, and 20 µl MTS solution was added to each well. The cells were incubated for 1 h at 37˚C in 5% CO2 and the absorbance was measured at 490 nm. The cell survival rate was determined using the optical density (OD) as follows:
Cell survival rate (%)=(ODtreated-ODblank)/(ODcontrol-ODblank) x100.
ELISA analysis
Cultured H9c2 cells were treated with LPS (250 ng/ml) or LPS (250 ng/ml) + EGCG (0, 1.5, 3.0, 6.25, 12.5, 25, 50 and 100 µmol/l) for 24 h. Cultured supernatants were collected and analyzed for the release of cyto- and chemokines using commercial ELISA test systems. Levels of VEGF, Rantes, MCP-1, ICAM-1, MMP-2 and TNF-α were determined using ELISA kits (R&D Systems, Inc.). Absorbance was measured at 450 nm, with the correction wavelength set at 540 or 570 nm.
NO assay
Cultured H9c2 cells were treated with LPS (250 ng/ml) or LPS (250 ng/ml) + EGCG (0, 1.5, 3.0, 6.25, 12.5, 25, 50 and 100 µmol/l) for 24 h. Nitrite determination was detected using Griess reagent. The absorbance was measured at 540 nm using a flow-through spectrophotometer. The sensitivity of the NO assay was <0.78 µmol/l.
Western blot analysis
Cultured H9c2 cells (5x106/10 cm dish) were treated with LPS (250 ng/ml) or LPS (250 ng/ml) + EGCG (0, 1.5, 3.0, 6.25, 12.5, 25, 50 and 100 µmol/l) for 24 h. After treatment, cells were washed twice with cold PBS and lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology) for 30 min at 4˚C. Extracted protein in each cell lysate was determined using a BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts (20 µg) of protein were separated by SDS-PAGE on 10% gels. Proteins were transferred to a PVDF membrane and blocked with 5% non-fat dry milk in PBS with 0.02% v/v Tween-20 for 1 h at room temperature. The membrane was incubated for 16 h with primary antibodies (all 1:1,000) in PBS-Tween at 4˚C. The membrane was washed and incubated for 1 h at room temperature with a peroxidase-labeled secondary antibody in PBS-Tween (anti-mouse, cat. no. P0447; anti-rabbit, cat. no. P0448; Dako; Agilent Technologies, Inc.). After further washing, the mumembrane was detected with ECL chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.). ImageQuantTL software (LAS 4000; GE Healthcare) was used for densitometry.
Statistical analysis
All experiments were performed in triplicate. The data are presented as the mean ± SEM. One-way ANOVA and Tukey's test were used to determine the statistical significance of differences among the experimental groups and the control group. SPSS 20.0 (IBM Corp) was used for statistical analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
EGCG does not markedly affect cell viability in H9c2 cells
The effects of EGCG on cell viability in H9c2 were examined by the MTS assay. Cultured H9c2 cells (1x104 cells/well in a 96-well plate) were incubated without or with EGCG (1.5, 3.0, 6.25, 12.5, 25, 50, 100 and 200 µmol/l) for 48 h. Cell viability did not change significantly with respect to the control up to 100 µM. However, 200 µM EGCG significantly reduced cell viability and caused cytotoxicity in H9c2 cells (Fig. 2). Therefore, concentrations <200 µM of EGCG were selected for use in the subsequent experiments.
Figure 2.
Effects of EGCG on the viability and toxicity of H9c2 cells. Data are presented as the mean ± SEM. n=3 in each group. **P<0.01 vs. control group. EGCG, epigallocatechin-3-gallate.
EGCG attenuates LPS-induced inflammation in H9c2 cardiac cells
To investigate the effects of EGCG on the inflammatory response in H9c2 cells, inflammatory cytokine expressions were determined using ELISA. LPS (250 ng/ml) significantly increased the TNF-α (Fig. 3A; P<0.001), ICAM-1 (Fig. 3B; P<0.001) and MMP-2 (Fig. 3C; P<0.001) protein levels in the medium supernatant compared with the control, according to ELISA, whereas EGCG suppressed the release of these cytokines in LPS-treated cells in a dose-dependent manner (Table I). The levels of TNF-α and MMP-2 were significantly alleviated after EGCG intervention (≥12.5 µmol/l). Meanwhile, treatment with EGCG (≥6.25 µmol/l) significantly reduced the expression of ICAM-1 induced by LPS.
Figure 3.
EGCG attenuates LPS-induced cytokine release in H9c2 cells. (A) TNF-α content in the culture medium of cultured H9c2 cells. (B) ICAM-1 content in the culture medium of cultured H9c2 cells. (C) MMP-2 content in the culture medium of cultured H9c2 cells. Effects of LPS and EGCG in H9c2 cells and the supernatant levels of (D) MCP-1 and (E) Rantes of H9c2 incubated with different concentrations of EGCG for 24 h were determined by ELISA. (F) EGCG inhibited LPS-induced upregulation of VEGF. Data are presented as the mean ± SEM. n=3 in each group. *P<0.05, **P<0.01, ***P<0.001 vs. control group; #P<0.05, ##P<0.01, ###P<0.001 vs. LPS group. EGCG, epigallocatechin-3-gallate; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1; MMP-2, matrix metalloproteinase 2; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein 1; VEGF, vascular endothelial growth factor.
Table I.
Effects of LPS and EGCG in H9c2 cells and the supernatant levels of inflammatory mediators of H9c2 incubated with different concentrations of EGCG for 24 h were determined by ELISA.
| Groups Inflammatory mediators | Control | 250 ng/ml LPS | 250 ng/ml LPS + 6.25 µmol/l EGCG | 250 ng/ml LPS + 12.5 µmol/l EGCG | 250 ng/ml LPS + 25 µmol/l EGCG | 250 ng/ml LPS + 50 µmol/l EGCG | 250 ng/ml LPS + 100 µmol/l EGCG |
|---|---|---|---|---|---|---|---|
| TNF-α, pg/ml | 25.67±4.16 | 131.33±6.66c | 117±10.54c | 99.33±13.58c,d | 68.67±12.66b,e | 43.67±15.95f | 38±12.53f |
| ICAM-1, ng/ml | 2.04±0.30 | 17.45±0.89c | 11.81±1.13c,e | 5.68±0.83b,f | 3.96±0.36b,f | 3.31±0.25b,f | 2.81±0.41f |
| MMP-2, ng/ml | 0.81±0.10 | 13.17±0.87c | 11.33±1.28c | 5.78±0.39c,f | 3.71±0.39c,f | 1.68±0.12c,f | 1.18±0.19a,f |
| MCP-1, pg/ml | 123.67±16.56 | 859.33±76.20c | 876±51.03c | 770.67±31.26c | 576.33±55.10c,e | 345.33±30.24a,f | 233.33±26.08b,f |
| Rantes, ng/ml | 40.67±14.74 | 498.67±35.16c | 424.33±33.01c | 359.67±22.89c,e | 238.33±34.65c,f | 162.67±12.10c,f | 134.67±6.51c,f |
| VEGF, pg/ml | 39.33±9.07 | 293±49.11c | 242.67±16.5c | 194.67±15.82c,d | 133.67±15.57c,e | 87±15.13b,e | 63.67±13.58e |
Data are presented as the mean ± SD. n=3 in each group.
aP<0.05,
bP<0.01,
cP<0.001 vs. control group;
dP<0.05,
eP<0.01,
fP<0.001 vs. LPS group. EGCG, epigallocatechin-3-gallate; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1; MMP-2, matrix metalloproteinase 2; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein 1; VEGF, vascular endothelial growth factor.
EGCG inhibits LPS-induced chemokine expression related to inflammation
To further analyze the cardioprotective role of EGCG, the concentrations of chemokines MCP-1 and Rantes in the H9c2 cell medium supernatant were analyzed after LPS stimulation. The present data demonstrated that LPS induced a significant upregulation of the expression of MCP-1 (Fig. 3D; P<0.001) and Rantes (Fig. 3E; P<0.001) compared with the control group. EGCG treatment significantly attenuated the LPS-induced increased MCP-1 and Rantes in a dose-dependent manner when the concentrations of EGCG were ≥25 or ≥6.25 µmol/l, respectively (Fig. 3D and E; Table I).
Inhibitory effect of EGCG on LPS-induced upregulation of VEGF in H9c2 cells
To evaluate the inhibitory effect of EGCG on the LPS-induced upregulation of VEGF, the levels of VEGF in the supernatant of H9c2 cells were determined by ELISA. Cultured H9c2 cells were treated without or with LPS (250 ng/ml), or LPS (250 ng/ml) + EGCG (6.25, 12.5, 25, 50 and 100 µmol/l) for 24 h. EGCG (≥12.5 µmol/l) significantly diminished the LPS-induced upregulation of VEGF (Fig. 3F).
EGCG suppresses NO expression in LPS-induced inflammation of H9c2 cardiomyocytes
The effect of EGCG on the NO content in the culture medium was additionally determined. LPS significantly increased the NO content in the culture medium (Fig. 4; Table II; P<0.001). However, EGCG (≥25 µmol/l) significantly counteracted the induction of NO release into the culture medium (Fig. 4).
Figure 4.
EGCG suppresses LPS-induced upregulation of NO release. Data are presented as the mean ± SEM. n=3 in each group. **P<0.01, ***P<0.001 vs. control group; ##P<0.01, ###P<0.001 vs. LPS group. EGCG, epigallocatechin-3-gallate; LPS, lipopolysaccharide; NO, nitric oxide.
Table II.
EGCG suppresses LPS-induced upregulation of NO release.
| Group | NO, µmol/l |
|---|---|
| Control | 3.52±0.45 |
| 250 ng/ml LPS | 43.76±6.08a |
| 250 ng/ml LPS + 6.25 µmol/l EGCG | 42.11±3.62a |
| 250 ng/ml LPS + 12.5 µmol/l EGCG | 33.73±1.65a |
| 250 ng/ml LPS + 25 µmol/l EGCG | 23.70±2.29a,b |
| 250 ng/ml LPS + 50 µmol/l EGCG | 15.42±1.53a,b |
| 250 ng/ml LPS + 100 µmol/l EGCG | 8.42±1.13a,c |
Data are presented as the mean ± SD. n=3 in each group.
aP<0.001 vs. control group;
bP<0.01,
cP<0.001 vs. LPS group. EGCG, epigallocatechin-3-gallate; LPS, lipopolysaccharide; NO, nitric oxide.
Potential mechanisms of EGCG inhibition of LPS-induced inflammatory responses in H9c2 cells
To further elucidate the mechanism underlying the anti-inflammatory effects of EGCG on LPS-induced H9c2 cells, western blot analysis was used to detect the potential pathways. LPS (250 ng/ml) was identified to significantly increase p-Akt, p-ERK, p-NF-κB p65 and p-p38 compared with the control (Fig. 5). Conversely, treatment with EGCG (≥12.5 µmol/l) significantly suppressed the LPS-induced inflammatory protein activation as demonstrated by the downregulated phosphorylation of Akt (Fig. 5B), NF-κB p65 (Fig. 5D) and p38 (Fig. 5E) compared with the LPS group. The induction of p-ERK expression by LPS was significantly reduced by ≥50 µmol/l EGCG (Fig. 5C).
Figure 5.
EGCG suppresses LPS-induced marked inflammatory protein activation. Cultured H9c2 cells were treated with control or LPS (250 ng/ml) or LPS + EGCG (0, 6.25, 12.5, 25, 50 and 100 µmol/l) for 24 h. After immunoblotting, the phosphorylation or the total levels of Akt, ERK, p38, nuclear factor-κB p65 were identified through their phosphor-specific or non-phosphor-specific antibodies. (A) The protein expressions of phosphorylated/total Akt, ERK, p38, nuclear factor-κB and p65 are presented. The expressions of (B) p-Akt/Akt, (C) p-ERK/ERK, (D) p-p65/p65 and (E) of p-p38/p38 are demonstrated. Data are presented as the mean ± SEM. n=3 in each group. *P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. LPS group. EGCG, epigallocatechin-3-gallate; LPS, lipopolysaccharide; p, phosphorylated.
Discussion
The present study provided evidence that H9c2 cells with LPS-induced inflammation, exhibited enhanced inflammatory mediators, such as VEGF, Rantes, MCP-1, ICAM-1, MMP-2, TNF-α and NO. However, the upregulation of the inflammatory mediators were attenuated by EGCG treatment. Additionally, EGCG suppressed the inflammatory signal pathway by downregulating p-NF-κB p65, p-Akt, p-ERK and p-p38.
EGCG is the main and most significantly bioactive polyphenol found in solid green tea extract, accounting for ~65% of the catechin content; 250 mg EGCG is present in a brewed cup of green tea (22). Several previous studies showed that EGCG has important anti-atherogenic and anti-inflammatory properties (23,24). The anti-inflammatory effects of EGCG have been demonstrated in numerous previous studies related to the pathological conditions where inflammation is a core-driving factor (23,24). For example, previous studies identified that EGCG was effective in preventing IL-8 production in airway epithelial cells through stimulation of IL-1β, restraining the development of respiratory inflammation (25,26). Moreover, EGCG treatment ameliorated cigarette smoke (CS)-induced airway inflammation and mucus secretion in a CS-exposed rat model (27). EGCG was also demonstrated to reduce cardiac apoptosis by decreasing the inflammatory response (28) and attenuating the serum level of cardiac function biomarker enzymes in rats (29). However, EGCG possesses several limitations, such as poor stability and low bioavailability, which are associated with the concentration of EGCG. High doses of EGCG achieve a positive result of antioxidant and pro-oxidative properties. However, a series of toxic side effects are induced by high doses of EGCG (30). Previous clinical studies have identified that the side effects of EGCG include nausea, insomnia and hepatotoxicity (31,32). When the present study was conducted, to the best of the authors' knowledge there were no previous studies in the literature to check the optimal EGCG concentration. Specific concentrations ranging from 50-400 µM were adopted according to the cell viability experiment in different literatures (33,34). Therefore, in the present study the safest maximum concentration (100 µM) was used in the present experiments. Recent studies on the optimum concentrations have since been conducted, the cell viability and Actin-Tracker Green technique in these recent studies demonstrated that the optimal range of concentration for the protective effects of EGCG was 50-100 µM (35,36).
H9c2 is a traditional cell line used to study myocardial disease, which was preserved in the laboratory of Hangzhou Red Cross Hospital/Hospital of Integrated Traditional Chinese and Western Medicine. Moreover, animal cells are also one of the most important ways to establish disease models, so that research is not limited to using human cell lines. Many previous studies on the effects of LPS on cardiomyocytes also used the H9c2 cell line (37-39). Additionally, subsequent validation experiments were performed in rats in addition to H9c2 cell lines. Therefore, the present study selected the H9c2 cell line for the present investigations.
NO is generated from all cell types composing the myocardium and regulates cardiac function, including coronary vessel tone, proliferative and inflammatory properties (40). Under severe inflammatory conditions, excessive NO production causes decreased vascular tonus and consequently hypotension, which is a characteristic of sepsis (19).
LPS, released from the surface of the cell membrane of Gram-negative bacteria, contributes to an inflammatory response. The cellular response to LPS includes the production of reactive oxygen species and other mediators, such as NO and pro-inflammatory cytokines (41). Oxidative stress, a condition caused by excessive production of free radicals, plays an important role in the progression of an inflammatory condition (42). Previous clinical studies demonstrated that the levels of TNF-α and serum concentration of soluble tumor necrosis factor receptors and IL are increased in patients with chronic HF (43-45). Indeed, concentrations of TNF-α are associated with the stimulation of NO synthase, contributing to reduced cardiac contractility in HF (46). As for NO, three NO synthases support various involvements of NO in cardiac physiology. Induced excessive NO from inflammatory cells and LPS-stimulated cardiomyocytes themselves, may lead to profound cellular disturbances resulting in HF (47). In the present study, with LPS-induced inflammation, H9c2 exhibited upregulated expression of inflammatory factors and oxidative stress molecules, such as Rantes, MCP-1, ICAM-1, MMP-2, TNF-α and NO. Moreover, the effects of LPS (250 µg/ml) on cell viability were examined and it was identified that LPS (250 µg/ml) had no effect on the cell viability rate or cytotoxicity in H9c2 cells compared with the control group (101.52±9.0% vs. 100%; P>0.05; data not shown) and the chosen concentration of LPS (250 µg/ml) was lower the concentration used in literature (48,49). The pro-inflammatory cytokines play an important role in the inhibition of cardiac function and the progression from cardiac injury to failure (50). Treatment with EGCG after LPS-stimulated inflammation significantly decreased the levels of pro-inflammatory cytokines and adhesion molecules, suggesting its anti-inflammatory potential.
To demonstrate how EGCG plays a role in the inflammation process, the effects on the inflammatory response of H9c2 cells stimulated by LPS were investigated. H9c2 cells stimulated by LPS demonstrated an increased activation of p-Akt. However, EGCG treatment inhibited the expression of p-Akt. PI3K/Akt signaling is an important pathway involved in controlling cardiomyocyte function and survival (51). One of the downstream effectors of Akt in the PI3K/Akt pathway is endothelial NO synthase, which after phosphorylation, leads to the production of NO (52). Excessive NO delivery from inflammatory cells and cardiomyocytes may influence cellular abnormal and cardiac contractility (40). The present results demonstrated that EGCG significantly counteracted the induction of NO release into the culture medium. Furthermore, the activation of p-p38, p-NF-κB p65 and p-ERK was increased by stimulation of LPS in H9c2 cells. EGCG suppressed the activation of these phosphorylated proteins. NF-κB, p38 and ERK are the most important factors playing a vital role in mediating inflammatory responses to a variety of signals, including inflammatory cytokines (53). Therefore, these associated pathways may comprise important molecular mechanisms responsible for cardiomyocyte inflammation induced by LPS, and EGCG ameliorates LPS-induced inflammation in H9c2 cells through the PI3K/Akt and p38 signaling pathways. A potential mechanism is that EGCG affects the kinase upstream, and then p38 and ERK regulate the downstream target NF-κB. Furthermore, future studies should broaden the scope to determine the crosstalk between the ERK and p38 pathways in H9c2 cells after EGCG treatment by inhibiting ERK and p38.
In the present study, H9c2 cells were incubated without or with EGCG at different concentrations for 48 h. The MTS assay showed no effect on the cell viability with EGCG at concentrations <200 µM. Although, further investigations will be necessary to further study the effects of EGCG on cell viability in H9c2 cells at multiple time points, such as 24, 48 and 96 h. The present results should also be verified in human cardiomyocytes in future studies.
The present findings provided evidence for the inhibiting effects of EGCG on LPS-stimulated inflammation in H9c2 cells. These results suggested the therapeutic potential of EGCG in cardiac inflammation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science Foundation for Youth of China (grant no. 30800533) and the Zhejiang Provincial Natural Science Foundation of China (gran no. LY14H070002).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZL, ZS, HY, ST, XL and FW designed the present study, analyzed the data and wrote and revised the manuscript. Moreover, FW gave final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Coggins M, Rosenzweig A. The fire within: Cardiac inflammatory signaling in health and disease. Circ Res. 2012;110:116–25. doi: 10.1161/CIRCRESAHA.111.243196. [DOI] [PubMed] [Google Scholar]
- 2.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res. 2012;110:126–144. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 3.Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557–581. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan QQ, Wu D, Ye QF. The expression profiles of circRNAs in lung tissues from rats with lipopolysaccharide-induced acute respiratory distress syndrome: A microarray study. Biochem Biophys Res Commun. 2017;493:684–689. doi: 10.1016/j.dib.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Yang S, Chen F, Li H, Chen B. Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by activating SIRT1 via inhibiting the NF-κB signaling pathway. Cell Biosci. 2017;7(44) doi: 10.1186/s13578-017-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazier WJ, Xue J, Luce WA, Liu Y. MAPK signaling drives inflammation in LPS-stimulated cardiomyocytes: The route of crosstalk to G-protein-coupled receptors. PLoS One. 2012;7(e50071) doi: 10.1371/journal.pone.0050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 8.Cocco G, Jerie P, Amiet P, Pandolfi S. Inflammation in heart failure: Known knowns and unknown unknowns. Expert Opin Pharmacother. 2017;18:1225–1233. doi: 10.1080/14656566.2017.1351948. [DOI] [PubMed] [Google Scholar]
- 9.Tschöpe C, Walther T, Escher F, Spillmann F, Du J, Altmann C, Schimke I, Bader M, Sanchez-Ferrer CF, Schultheiss HP, Noutsias M. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J. 2005;19:2057–2059. doi: 10.1096/fj.05-4095fje. [DOI] [PubMed] [Google Scholar]
- 10.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 11.Sun TL, Liu Z, Qi ZJ, Huang YP, Gao XQ, Zhang YY. (-)-Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem Toxicol. 2016;93:102–110. doi: 10.1016/j.fct.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 14.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 15.Tseng CL, Hung YJ, Chen ZY, Fang HW, Chen KH. Synergistic effect of artificial tears containing epigallocatechin gallate and hyaluronic acid for the treatment of rabbits with dry eye syndrome. PLoS One. 2016;11(e0157982) doi: 10.1371/journal.pone.0157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammad FT, Lubbad L. The effect of epigallocatechin-3-gallate on the renal dysfunction in the obstructed kidney in the rat. Int J Physiol Pathophysiol Pharmacol. 2017;9:119–126. [PMC free article] [PubMed] [Google Scholar]
- 17.Bliksoen M, Kaljusto ML, Vaage J, Stensløkken KO. Effects of hydrogen sulphide on ischaemia-reperfusion injury and ischaemic preconditioning in the isolated, perfused rat heart. Eur J Cardiothorac Surg. 2008;34:344–349. doi: 10.1016/j.ejcts.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Suo M, Yu G, Zhang M. Antinociceptive and anti-inflammatory effects of cryptotanshinone through PI3K/Akt signaling pathway in a rat model of neuropathic pain. Chem Biol Interact. 2019;305:127–133. doi: 10.1016/j.cbi.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Franceschelli S, Pesce M, Ferrone A, Gatta DM, Patruno A, Lutiis MA, Quiles JL, Grilli A, Felaco M, Speranza L. Biological effect of licochalcone C on the regulation of PI3K/Akt/eNOS and NF-kappaB/iNOS/NO signaling pathways in H9c2 cells in response to LPS stimulation. Int J Mol Sci. 2017;18: pii(E690) doi: 10.3390/ijms18040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Jiang L, Lin S, He Y, Shen G, Cai Z, Ling M, Ni J, Zhang H, Zhang M. Inhibition of mTORC1 renders cardiac protection against lipopolysaccharide. Int J Clin Exp Pathol. 2014;7:8432–8442. [PMC free article] [PubMed] [Google Scholar]
- 21.Sah JF, Balasubramanian S, Eckert RL, Rorke EA. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J Biol Chem. 2004;279:12755–12762. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 22.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou HC, Song TY, Yeh YC, Huang CY, Yang SF, Chiu TH, Tsai KL, Chen KL, Wu YJ, Tsai CS, et al. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol (1985) 2010;108:1745–1756. doi: 10.1152/japplphysiol.00879.2009. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Lui WT, Chu CY, Ng PS, Wang CC, Rogers MS. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. 2009;24:608–618. doi: 10.1093/humrep/den417. [DOI] [PubMed] [Google Scholar]
- 25.Kim IB, Kim DY, Lee SJ, Sun MJ, Lee MS, Li H, Cho JJ, Park CS. Inhibition of IL-8 production by green tea polyphenols in human nasal fibroblasts and A549 epithelial cells. Biol Pharm Bull. 2006;29:1120–1125. doi: 10.1248/bpb.29.1120. [DOI] [PubMed] [Google Scholar]
- 26.Ge M, Xiao Y, Chen H, Luo F, Du G, Zeng F. Multiple antiviral approaches of (-)-epigallocatechin-3-gallate (EGCG) against porcine reproductive and respiratory syndrome virus infection in vitro. Antiviral Res. 2018;158:52–62. doi: 10.1016/j.antiviral.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Liu KWK, Yeung SC, Li X, Ip MSM, Mak JCW. (-)-Epigallocatechin-3-gallate reduces cigarette smoke-induced airway neutrophilic inflammation and mucin hypersecretion in rats. Front Pharmacol. 2017;8(618) doi: 10.3389/fphar.2017.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeed NM, El-Naga RN, El-Bakly WM, Abdel-Rahman HM, Salah ElDin RA, El-Demerdash E. Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: A mechanistic study. Biochem Pharmacol. 2015;95:145–155. doi: 10.1016/j.bcp.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Othman AI, Elkomy MM, El-Missiry MA, Dardor M. Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction. Eur J Pharmacol. 2017;794:27–36. doi: 10.1016/j.ejphar.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Peter B, Bosze S, Horvath R. Biophysical characteristics of proteins and living cells exposed to the green tea polyphenol epigallocatechin-3-gallate (EGCg): Review of recent advances from molecular mechanisms to nanomedicine and clinical trials. Eur Biophys J. 2017;46:1–24. doi: 10.1007/s00249-016-1141-2. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Li P, Qu Z, Xiong W, Liu A, Zhang S. Advances in the antagonism of Epigallocatechin-3-gallate in the treatment of digestive tract tumors. Molecules. 2019;24: pii(E1726) doi: 10.3390/molecules24091726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Mei H, Jia L, Zhao H, Li X, Meng X, Zhao X, Xing L, Yu J. doi: 10.1007/s10637-019-00871-8. Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: A prospective, non-randomised, phase 1 trial. Invest New Drugs: Nov 7, 2019 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 33.Seok JK, Lee JW, Kim YM, Boo YC. Punicalagin and (-)-Epigallocatechin-3-Gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin Pharmacol Physiol. 2018;31:134–143. doi: 10.1159/000487400. [DOI] [PubMed] [Google Scholar]
- 34.Holczer M, Besze B, Zámbó V, Csala M, Bánhegyi G, Kapuy O. Epigallocatechin-3-gallate (EGCG) promotes autophagy-dependent survival via influencing the balance of mTOR-AMPK pathways upon endoplasmic reticulum stress. Oxid Med Cell Longev. 2018;2018(6721530) doi: 10.1155/2018/6721530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Hu Y, Wu J, Wen J, Li S, Zhang L, Zhang J, Li Y, Li J. Epigallocatechin-3-gallate inhibits angiotensin II-induced cardiomyocyte hypertrophy via regulating Hippo signaling pathway in H9c2 rat cardiomyocytes. Acta Biochim Biophys Sin (Shanghai) 2019;51:422–430. doi: 10.1093/abbs/gmz018. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Liao P, Liang R, Zheng X, Jian J. Epigallocatechin gallate prevents mitochondrial impairment and cell apoptosis by regulating miR-30a/p53 axis. Phytomedicine. 2019;61(152845) doi: 10.1016/j.phymed.2019.152845. [DOI] [PubMed] [Google Scholar]
- 37.Magi S, Nasti AA, Gratteri S, Castaldo P, Bompadre S, Amoroso S, Lariccia V. Gram-negative endotoxin lipopolysaccharide induces cardiac hypertrophy: Detrimental role of Na(+)-Ca(2+) exchanger. Eur J Pharmacol. 2015;746:31–40. doi: 10.1016/j.ejphar.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Liu X, Du A, Zhu X, Yu B. miR-203 accelerates apoptosis and inflammation induced by LPS via targeting NFIL3 in cardiomyocytes. J Cell Biochem. 2019;120:6605–6613. doi: 10.1002/jcb.27955. [DOI] [PubMed] [Google Scholar]
- 39.Su Q, Yao J, Sheng C. Geniposide Attenuates LPS-induced injury via Up-regulation of miR-145 in H9c2 cells. Inflammation. 2018;41:1229–1237. doi: 10.1007/s10753-018-0769-8. [DOI] [PubMed] [Google Scholar]
- 40.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: Ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 41.Franceschelli S, Pesce M, Ferrone A, Patruno A, Pasqualone L, Carlucci G, Ferrone V, Carlucci M, de Lutiis MA, Grilli A, et al. A Novel biological role of α-mangostin in modulating inflammatory response through the activation of sirt-1 signaling pathway. J Cell Physiol. 2016;231:2439–2451. doi: 10.1002/jcp.25348. [DOI] [PubMed] [Google Scholar]
- 42.Franceschelli S, Pesce M, Ferrone A, De Lutiis MA, Patruno A, Grilli A, Felaco M, Speranza L. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2-production. PLoS One. 2014;9(e88359) doi: 10.1371/journal.pone.0088359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hori M, Yamaguchi O. Is tumor necrosis factor-alpha friend or foe for chronic heart failure? Circ Res. 2013;113:492–494. doi: 10.1161/CIRCRESAHA.113.302024. [DOI] [PubMed] [Google Scholar]
- 44.Khodadadi I, Vahedi MS, Abdi M, Daneshkhah N, Rahbari R, Menbari S, Ahmadi D, Ahmadi A, Lahoorpour F, Hakhamaneshi MS, et al. Evaluation of adenosine deaminase (ADA) isoenzymes activity and tumor necrosis factor-α (TNFα) concentration in chronic heart failure. EXCLI J. 2014;13:58–66. [PMC free article] [PubMed] [Google Scholar]
- 45.Eskandari V, Amirzargar AA, Mahmoudi MJ, Rahnemoon Z, Rahmani F, Sadati S, Rahmati Z, Gorzin F, Hedayat M, Rezaei N. Gene expression and levels of IL-6 and TNFα in PBMCs correlate with severity and functional class in patients with chronic heart failure. Ir J Med Sci. 2018;187:359–368. doi: 10.1007/s11845-017-1680-2. [DOI] [PubMed] [Google Scholar]
- 46.Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother. 2008;42:1002–1016. doi: 10.1345/aph.1K272. [DOI] [PubMed] [Google Scholar]
- 47.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-Inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Li H, Ge A, Guo E, Liu S, Zhang L. Long non-coding RNA TUG1 inhibits apoptosis and inflammatory response in LPS-treated H9c2 cells by down-regulation of miR-29b. Biomed Pharmacother. 2018;101:663–669. doi: 10.1016/j.biopha.2018.02.129. [DOI] [PubMed] [Google Scholar]
- 49.Hao R, Su G, Sun X, Kong X, Zhu C, Su G. Adiponectin attenuates lipopolysaccharide-induced cell injury of H9c2 cells by regulating AMPK pathway. Acta Biochim Biophys Sin (Shanghai) 2019;51:168–177. doi: 10.1093/abbs/gmy162. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez A, Ravassa S, Beaumont J, López B, Díez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–1843. doi: 10.1016/j.jacc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y, Xia ZY, Meng QT, Zhu J, Lei S, Xu J, Dou J. Shen-Fu injection preconditioning inhibits myocardial ischemia-reperfusion injury in diabetic rats: Activation of eNOS via the PI3K/Akt pathway. J Biomed Biotechnol. 2011;2011(384627) doi: 10.1155/2011/384627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Shen L, Wang Z, Jiang HP, Liu LX. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2016;84:106–114. doi: 10.1016/j.biopha.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 53.Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60:1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.