Abstract
Introduction:
The use of screening can prevent death from colorectal cancer, yet people without regular healthcare visits may not realize the benefits of this preventive intervention. The objective of this study was to determine the effectiveness of a mailed screening invitation or mailed fecal immunochemical test in increasing colorectal cancer screening uptake in eterans without recent primary care encounters.
Study design:
Three-arm pragmatic randomized trial.
Setting/participants:
Participants were screening-eligible veterans aged 50–75 years, without a recent primary care visit who accessed medical services at the Corporal Michael J. Crescenz Veteran Affairs Medical Center between January 1, 2017, and July 31, 2017. All data were analyzed from March 1, 2018, to July 31, 2018.
Intervention:
Participants were randomized to (1) usual opportunistic screening during a healthcare visit (n=260), (2) mailed invitation to screen and reminder phone calls (n=261), or (3) mailed fecal immunochemical test outreach plus reminder calls (n=61).
Main outcome measures:
The main outcome under investigation was the completion of colorectal cancer screening within 6 months after randomization.
Results:
Of 782 participants in the trial, 53.9% were aged 60–75 years and 59.7% were African American. The screening rate was higher in the mailed fecal immunochemical test group (26.1%) compared with usual care (5.8%) (rate difference=20.3%, 95% CI=14.3%, 26.3%; RR=4.52, 95% CI=2.7, 7.7) or screening invitation (7.7%) (rate difference=18.4%, 95% CI=12.2%, 24.6%; RR=3.4, 95% CI=2.1, 5.4). Screening completion rates were similar between invitation and usual care (rate difference=1.9%, 95% CI= −2.4%, 6.2%; RR=1.3, 95% CI=0.7, 2.5).
Conclusions:
Mailed fecal immunochemical test screening promotes colorectal cancer screening participation among veterans without a recent primary care encounter. Despite the addition of reminder calls, an invitation letter was no more effective in screening participation than screening during outpatient appointments.
Trial registration:
This study is registered at clinicaltrials.gov NCT02584998.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of death from cancer, with approximately 140,250 new cases and 50,630 deaths in the U.S. in 2018.1 CRC incidence and mortality rates have been decreasing steadily in recent decades, attributed largely to improvements in screening uptake.2,3 Failure to screen using tests recommended by national groups, including the U.S. Preventive Services Task Force,4,5 increases the risk of CRC death.6
The delivery of CRC screening within the Veterans Affairs (VA) health system generally relies on outpatient office visits and therefore is sensitive to factors that hinder access to healthcare services or competing medical needs.7–9 Veterans who remain unscreened despite ongoing efforts to promote, primarily visit-based, screening may be marginalized and may not regularly keep primary care appointments. They may face barriers to receiving CRC screening such as lacking veteran status–related coverage, living long distances from VA facilities, difficulty with access to reliable transportation, and living with mental health conditions.10,11
About 3% of all CRC cases in the U.S., including about 6% of cases among adult men, are among veterans.12,13 A study found that 80% of veterans who attended primary care visits at a VA health facility participated in CRC screening.14 That study, however, excluded screening-eligible people who did not have recent visits at a VA. Studies show that an interaction with a primary provider is strongly correlated with undergoing screening.15–18 Few interventions specifically target improving participation in cancer screening interventions among veterans with limited attendance at primary care. The goal of this study was to determine whether sending a screening invitation or directly mailing a fecal immunochemical test (FIT) to eligible veterans without a recent office visit is effective at increasing participation in CRC screening.
METHODS
This study was a 3-arm pragmatic RCT comparing the effectiveness of mailed screening invitation or mailed FIT relative to usual care and to each other (Figure 1). Eligible participants were randomized in a 1:1:1 ratio to 1 of 3 groups: (1) usual care (group A); (2) a mailed screening invitation for clinic-based screening plus reminders (group B); or (3) mailed home FIT with prenotification letter and reminders (group C).
Figure 1.
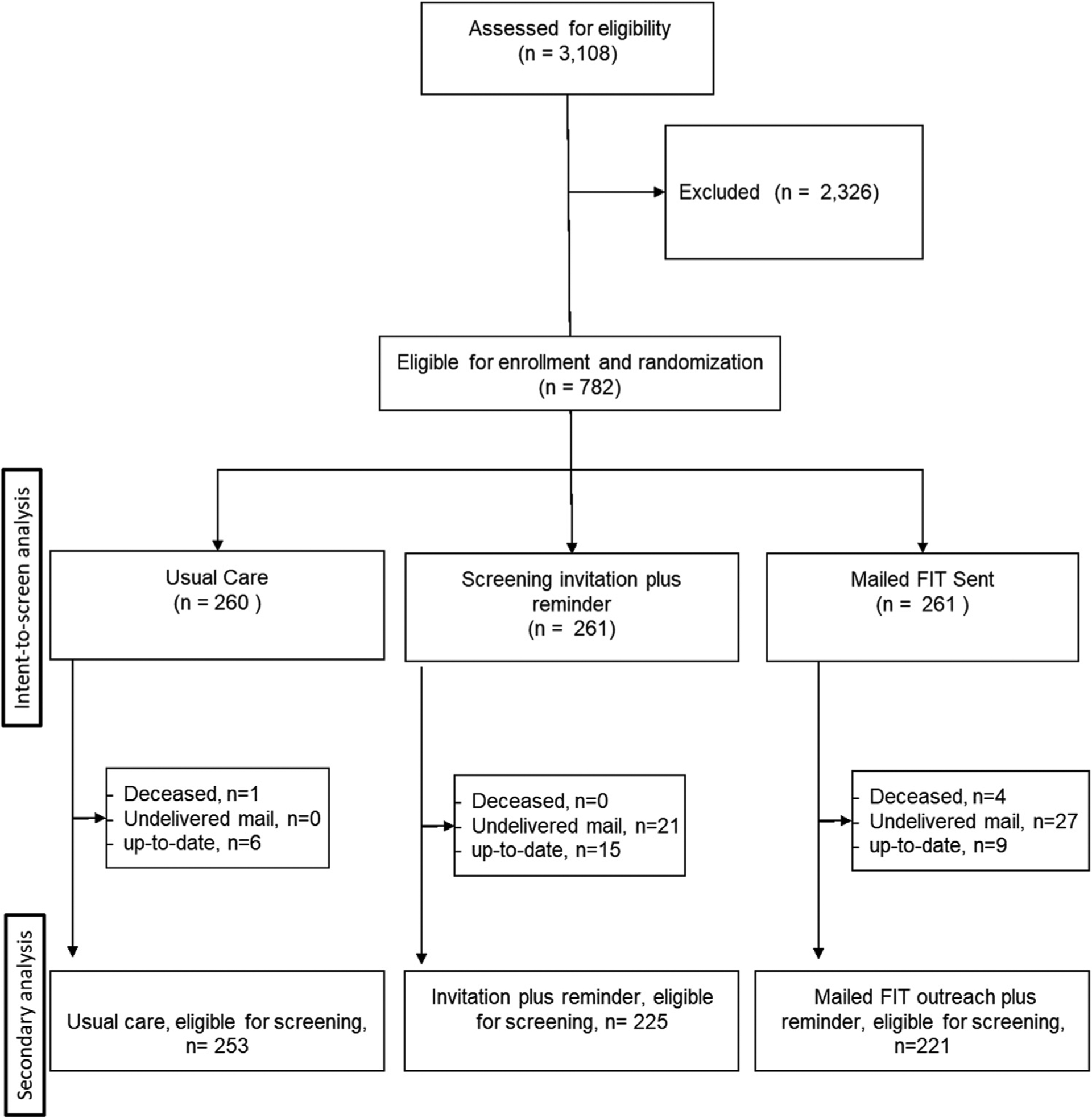
Flow diagram of participant inclusion (CONSORT).
The IRB of the Corporal Michael J. Crescenz VA Medical Center (CMC-VAMC) in Philadelphia, PA, approved the study. The study received a waiver of the requirement for individual informed consent because doing so would be impracticable and result in selection bias.19,20 The protocol was registered at clinicaltrials.gov (NCT02584998). The protocol and statistical analysis plan can be found in the Appendix (available online).
Study Population
The study was conducted between January 1, 2017, and July 31, 2017, at the CMC-VAMC with follow-up through December 31, 2017. The CMC-VAMC serves almost 100,000 veterans in Southeastern Pennsylvania and Southern New Jersey. This region is one of the largest metropolitan areas in the U.S., with notably high levels of poverty.
Participants were aged 50–75 years; received care through the CMC-VAMC in the 18–48 months before enrollment; on standardized chart review, had no documented primary care visit within 18 months of study enrollment; and had an active CRC screening reminder in the VA electronic health record (EHR), the Computerized Patient Record System. The record for each potentially eligible patient was individually abstracted by a study investigator (ECP).
Patients meeting 1 or more of the following criteria based on the EHR were excluded: (1) symptoms of rectal bleeding, unexplained weight loss, or change in bowel habits; (2) 1 or more first degree relative with CRC; (3) personal history of inflammatory bowel disease; (4) history of colon or rectal resection; (5) up-to-date on CRC screening; and (6) documentation of primary care service access outside of VAMC. The up-to-date screening was based on Computerized Patient Record System–documented colonoscopy within 10 years, sigmoidoscopy or barium enema within 5 years, or fecal occult blood testing (FOBT) within 1 year of study enrollment. Patients with documented overwhelming comorbidity or expected mortality within 6 months were excluded at the discretion of the reviewing investigator. For the secondary analysis, a participant was considered up-to-date on screening post-randomization if the mailed screening was returned or up-to-date screening status was indicated in reminder calls.
Measures
Patients were blocked randomized in groups of 87. Randomization was performed by a data analyst not involved in the identification of participants or the intervention or follow-up. Using SAS, version 9.4, the 87 participants in a block were assigned a random seed value between 0 and 1. The participants were then ranked and assigned to 1 of 3 study groups based on the numeric seed.
Participants randomized to usual care arm, received the current practice of CRC screening at CMC-VAMC, whereby providers, most during primary care visits, offer screening. Participants randomized to the mailed clinic-based screening invitation received usual care plus a letter inviting them to make an appointment with their doctor for screening. The letter provided lay-audience descriptions of screening tests such as FIT and colonoscopy, as well as symptoms such as a change in bowel habits or rectal bleeding that may warrant further diagnostic workup. In addition, a prepaid postage card was included to encourage the veteran to update their contact information or screening history as needed. Research team information was provided in the mailed documentation to facilitate contact with study staff if the veteran believed they were not eligible for CRC screening at the time the letter was received. The letter informed participants that they would receive a follow-up telephone call 4, 5, and 6 weeks from receipt of the invitation letter if screening remained incomplete.
Participants randomized to mailed home FIT received usual care, the clinic-based screening invitation packet (as described for Group B), and a FIT prenotification letter detailing the home stool screening modality. The letter informed participants that they would receive a follow-up telephone call 4, 5, and 6 weeks from receipt of the invitation letter if screening remained incomplete. One week after mailing the prenotification letter, a home kit containing a1-sample FIT was mailed along with instructions on how to return the screening test. For patients in the mailed FIT arm who returned the FIT test, the study staff sent the patient a letter with the results and education about future testing. Participants who had a positive FIT result (≥20 μg hemoglobin/g) were sent a letter and also received a live phone call from trained research staff to explain the findings and assist in scheduling a colonoscopy.
The primary outcome was documented completion of CRC screening within 6 months of study initiation in the EHR. The secondary outcome was the EHR-documented rate of FIT return within 6 months of study initiation among participants randomized to mailed home FIT. Age, race, and sex were abstracted from patient EHR.
Statistical Analysis
Power and sample size calculations were based on an expected 15% screening rate in the usual care group, 25% in the invitation reminder group, and 40% in the mailed FIT group. Thus, sample sizes of 250 participants per group for the usual care versus screening invitation comparison, and 152 per group in the screening invitation versus mailed home FIT comparison would provide 80% power with a 2-sided α=0.05. Accounting for an assumed 20% participant ineligibility post-randomization, we enrolled 783 participants in the trial.
All data were analyzed from March 1, 2018 to July 31, 2018. The Pearson chi-squared test of independence was used to evaluate the null hypothesis that screening completion rates did not differ by intervention group. RRs and rate difference (RD) were calculated with 95% CIs for completion of CRC screening using contingency tables in intention-to-treat analyses. Subgroup analyses were performed to evaluate differences in screening completion by age and race. A secondary analysis was performed, excluding patients who were ineligible to screen post-randomization. Values of p<0.05 were considered statistically significant. All statistical analyses were performed using Stata, version 14.1.
RESULTS
A total of 3,108 patients aged 50–75 years were identified for the study through an automated EHR search. After chart review, 782 eligible participants were randomized to usual care (n=260), mailed clinic-based screening invitation (n=261), and mailed FIT outreach (n=261). One participant was assigned to both the usual care and screening invitation groups, and so was included in group B because a mailed screening invitation was sent. As a result, the total sample size of the usual care group was 260 (Figure 1); 46.1% of the sample was aged 50–59 years, 59.7% were African American, and 97.2% were men (Table 1). The age, race/ethnicity, and sex distributions were balanced across intervention groups.
Table 1.
Participant Demographic Characteristic by Intervention Group
| Characteristics | Total(n=782) | Usual care(n=260) | lnvitation(n=26) | Mailed FIT(n=261) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Age, years | ||||
| 50–59 | 360 (46.1) | 125 (48.1) | 126 (48.3) | 109 (41.8) |
| 60–69 | 328 (41.9) | 102 (39.2) | 105 (40.2) | 120 (46.0) |
| 70–75 | 94 (12.0) | 33 (12.7) | 30 (11.5) | 32 (12.2) |
| Race/ethnicity | ||||
| African American | 467 (59.7) | 160 (61.6) | 149 (57.1) | 158 (60.5) |
| White/Caucasian | 252 (32.3) | 76 (29.2) | 92 (35.2) | 84 (32.2) |
| Hispanic | 30 (3.8) | 12 (4.6) | 9 (3.5) | 9 (3.5) |
| Unknown | 33 (4.2) | 12 (4.6) | 11 (4.2) | 10 (3.8) |
| Sex | ||||
| Male | 760 (97.2) | 252 (96.9) | 254 (97.3) | 254 (97.3) |
| Female | 22 (2.8) | 8 (3.1) | 7 (2.7) | 7 (2.7) |
Notes: All comparisons were nonsignificant with p>0.05.
FIT, fecal immunochemical test.
Fifteen (5.8%) participants randomized to usual care, 20 (7.7%) to mailed clinic-based screening invitation, and 68 (26.1%) to mailed home FIT completed CRC screening within 6 months of the intervention (Figure 2). The CRC screening completion rate was significantly higher in the mailed FIT group compared with the usual care group (RD=20.3%, 95% CI=14.3%, 26.3%; RR=4.5, 95% CI=2.7, 7.7, p<0.001). The CRC screening completion rate was also significantly higher for participants randomized to mailed FIT outreach than for those randomized to mailed screening invitation plus reminders (RD=18.4%, 95% CI=12.2%, 24.6%; RR=3.4, 95% CI=2.1, 5.4, p<0.001) (Figure 2). There was no statistically significant difference in screening completion between the usual care and the invitation reminder groups (RD=1.9%, 95% CI= − 2.4%, 6.2%; RR=1.3, 95% CI=0.7, 2.5, p=0.39) (Figure 2).
Figure 2.
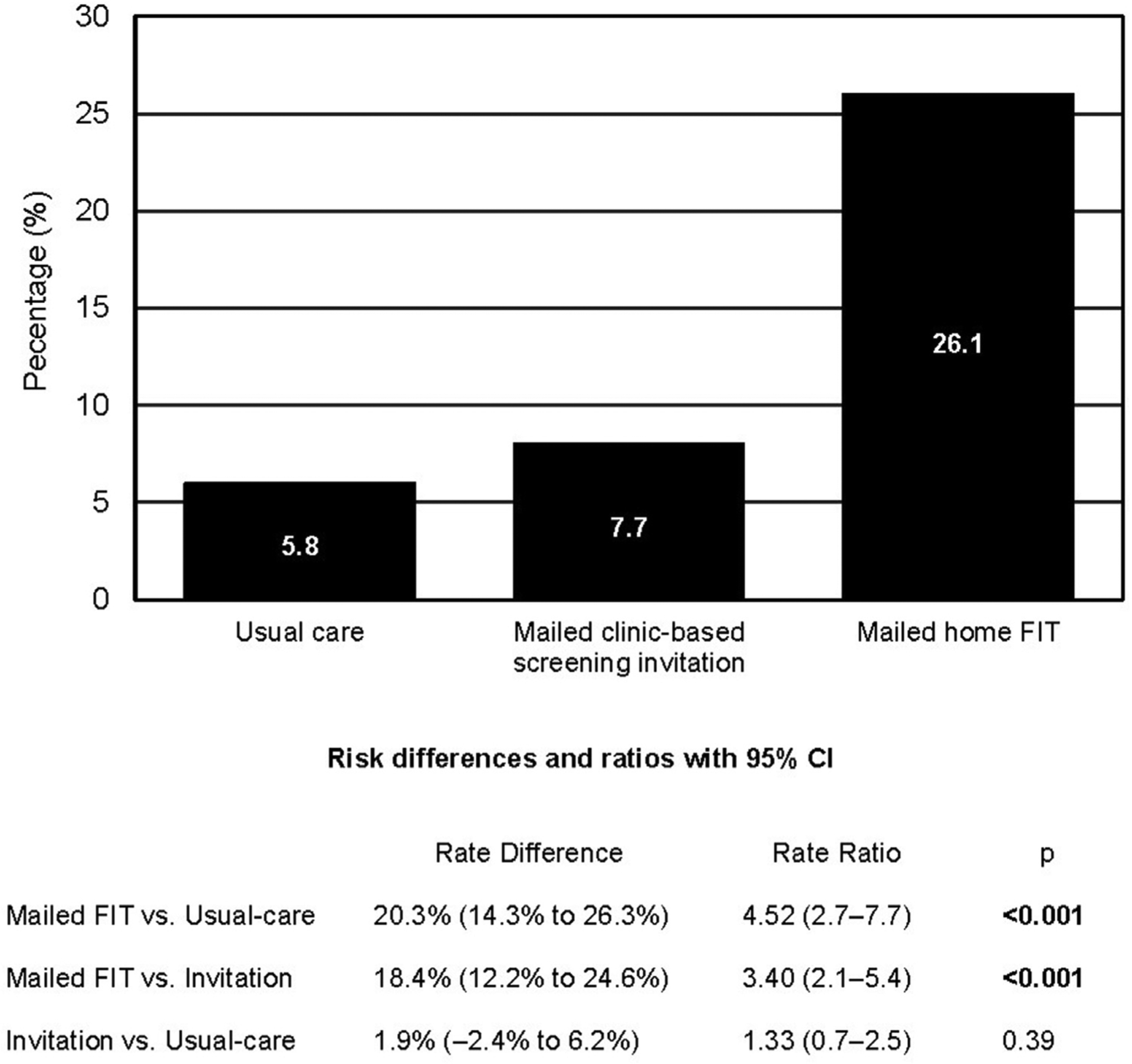
Rate of CRC screening completion by study intervention group.
Note: Boldface indicates statistical significance (p<0.05).
CRC, colorectal cancer.
Fifteen (5.8%) participants randomized to usual care, 20 (7.7%) participants randomized to screening invitation plus reminder, and 68 (26.1%) randomized to mailed home FIT completed CRC screening within 6 months of the intervention. Of those randomized to usual care, 11 (73.3%) participants had FOBT through the VA, and 4 (26.7%) participants had a colonoscopy. Of those randomized to screening invitation plus reminder, 10 (50.0%) had FOBT through the VA, 6 (30.0%) had a colonoscopy, 1 (5.0%) had a flexible sigmoidoscopy, and 3 (15.0%) reported screening on a follow-up call without more detail about modality. Of those randomized to mailed FIT, 62 (91.2%) had negative FIT testing, 4 (5.9%) had FOBT through the VA, and 2 (2.9%) reported screening on a follow-up call without more detail about modality.
Six participants had a positive FIT screening test. Of those with a positive screen, 2 contacted by phone reported undergoing colonoscopy within the 12 months before the FIT test. One patient was contacted and received follow-up colonoscopy at a non-VA hospital. One patient died within 4 months of a positive FIT screen. Two patients were lost to follow-up despite multiple efforts by the study team to contact them and help schedule a colonoscopy.
There was no statistically significant difference in screening completion by either age or race/ethnicity (Table 2). Sixty-two participants randomized to the mailed FIT returned it (Appendix Table 1, available online). Age, race/ethnicity, and sex were not significantly different between those who did and those who did not complete screening.
Table 2.
Screening Completion Rate by Intervention Group and Patient Demographic Characteristics.
| Characteristics | Usual care (n=260) | Invitation (n=261) | Mailed FIT (n=261) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age, years | |||
| 50–59 | 10 (8.0) | 9 (7.1) | 24 (22.0) |
| 60–69 | 4 (3.9) | 6 (5.7) | 34 (28.3) |
| 70–75 | 1 (3.0) | 5 (16.7) | 10 (31.3) |
| Race/ethnicity | |||
| Black/African American | 11 (6.9) | 10 (6.7) | 39 (24.7) |
| White/Caucasian | 3 (3.9) | 7 (7.6) | 22 (26.2) |
| Hispanic | 1 (8.3) | 2 (22.2) | 4 (44.4) |
| Unknown | 0 (0.0) | 1 (9.1) | 3 (30.0) |
| Sex | |||
| Male | 15 (5.9) | 20 (7.9) | 66 (26.0) |
| Female | 0 (0.0) | 0 (0.0) | 2 (28.6) |
Notes: All comparisons were nonsignificant with p>0.05.
FIT, fecal immunochemical test.
A secondary analysis was performed excluding patients with undeliverable letters (group B, n=21 [8.1%]; group C, n=27 [10.3%]), or who were up-to-date on screening at randomization (group A, n=6 [2.4%]; group B, n=15[5.8%]; group C, n=9 [3.5%]), or were deceased at randomization or had died during the 6-month study (n=5) (Figure 1). After these exclusions, 6-month CRC screening completion rates were 5.5% (14 of 253) for group A, 8.9% (20 of 225) for group B, and 28.4% (63 of 222) for group C. The association between mailed FIT and usual care (RD=22.8%, 95% CI=16.3%, 29.4%; RR=5.13, 95% CI=3.0, 8.9, p<0.001) and between mailed FIT and clinic-based screening invitation (RD=19.5%, 95% CI=12.5%, 26.5%; RR=3.19, 95% CI=2.00, 5.10, p<0.001) remained statistically significant. Clinic-based screening invitation and reminders was not statistically different from usual care (RD=3.4%, 95% CI= −1.3%, 8.0%; RR=1.6, 95% CI=0.8, 3.1, p=0.15). There were no statistically significant differences in FIT return rate by either age or race/ethnicity in this sub analysis.
DISCUSSION
This study demonstrates that a strategy of mailing in-home FIT kits accompanied by a prenotification letter and reminders promotes CRC screening for veterans without a recent primary care visit in the VA health system. This study shows that veterans who receive mailed home FIT are more likely to complete CRC screening in 6 months than those receiving the usual anticipatory guidance regarding screening during primary care visits or a proactive screening invitation plus live telephone reminders. The latter strategy is often used in delivery systems to reach patients who are not up-to-date on preventive services. This study shows that for veterans without a recent primary care visit within the VA, communication through an invitation by mail and a follow-up reminder call was no more effective than waiting for them to present for an appointment.
The results are consistent with research on the effectiveness of home FIT for population-based CRC screening, including a systematic review of 15 prior studies that were published in 2018.21,22 Notably, included studies varied in methodology and patient setting with resultant heterogeneity in the magnitude of the effect. In almost all studies, mailed fecal-based screening increased participation rates when compared with usual care.21 A clinical trial by co-authors on this paper demonstrated a mailed FIT completion rate of 29.1%, similar to the 26.1% completion rate found here.23 That study, however, only included patients who had at least 2 primary care visits in the 2 years before study enrollment. Another randomized trial performed in a rural VA setting demonstrated a mailed FIT completion rate of 21% compared with 6% in usual care.24 Again, included participants were “regular users of VA primary care services” who had at least 2 primary care visits in the 13 months before study enrollment. The effectiveness of mailed FIT outreach in these diverse settings may be due to the ability of mailed screening to overcome structural barriers and the convenience of completing screening in the privacy of home but may also derive from the endowment effect of having a relatively simple screening kit in hand.25,26
This research represents the first pragmatic RCT of mailed FIT outreach screening among veterans who have not recently (18 months) used primary care services offered by the VA. In this work, there were large relative, and absolute differences in CRC screening participation rate between veterans offered home FIT screening and those who received usual care (RR=4.52, RD=20.2%) or a mailed invitation plus reminders (RR=3.40, RD=18.4%). These results are encouraging given that patients who do not make regular appointments may be hard to reach and may not have opportunities to get screened without a face-to-face encounter.
Another strength is the study setting. The CMC-VAMC is located in urban Philadelphia, PA, and serves almost 100,000 veterans in Southeastern Pennsylvania and Southern New Jersey. In this environment, the underuse of healthcare services may be due to socioeconomic factors. Thus, the efficacy of mailed FIT in this setting highlights the strategy’s potential for high impact in populations that are historically medically disadvantaged with low screening rates, high disease burden, and who live in proximity to follow-up services.27
Limitations
This study was not powered to identify differences in screening completion or FIT return by patient demographic characteristics. Although not statistically significant, older veterans had a higher response rate to both mailed clinic-based screening invitations and mailed home FIT than their younger counterparts (Table 2 and Appendix Table 1). Further research is necessary to clarify whether veteran demographic characteristics are associated with home FIT screening completion to better define populations for targeted screening efforts. The sample used for this research was randomized from predominantly male veterans cared for at a single northeastern U.S. VAMC; thus, there is limited generalizability to other populations. Although there is limited external validity, the consistency of these results with similar trials performed in different settings, it is fair to assume that mailed FIT is likely a useful CRC screening strategy across differing populations. Importantly, follow-up and subsequent evaluation of FIT-positive participants is important to the success of a mailed FIT intervention. Of the 3 FIT-positive participants who should have received follow-up evaluation (2 were already up-to-date but still returned the mailed FIT, and 1 patient died), only 1 underwent colonoscopy. The challenge of FIT to colonoscopy is a study limitation among participants who already do not use care regularly at the CMC-VAMC.
CONCLUSIONS
There are important implications of this study. By focusing on a population that was not regular users of healthcare visits, this research provides strong evidence for the use of a directly mailed in-home screening test to overcome structural barriers related to screening provided in face-to-face office visits. This work supports the potential for a mailed FIT to address racial/ethnic disparities in CRC screening related to differences in the use of healthcare use.8,17 Only 8%–10% of mails were undeliverable, which supports the feasibility of this strategy.
The use of mailed in-home FIT represents a feasible and effective approach to deliver CRC screening among veterans in an urban VAMC without a recent primary care visit. Despite providing up to three reminders calls, a mailed clinic-based invitation to screening approach was no more effective than the usual practice of in-clinic screening. The results show that relying solely on office visits may result in missed opportunities to offer CRC screening. Further research is needed to evaluate the effectiveness of mailed FIT in promoting ongoing screening after initiation in diverse underserved populations.
Supplementary Material
ACKNOWLEDGMENTS
The funders had no role in the study design; collection, analysis, or interpretation of the data; or in the writing of the report. This trial was funded by an award from the Veterans Affairs Health Services Research and Development Service VA (Principal Investigators: ECP and CAD). CAD was supported by grant number R01CA213645, and SJM was supported by grant number K08CA234326 from the National Cancer Institute of the National Institutes of Health.
CAD is a member of the U.S. Preventive Services Task Force. CAD also authors topics on UpToDate. This article does not represent the views and policies of the U.S. Preventive Services Task Force or UpToDate.
Footnotes
No financial disclosures were reported by the authors of this paper.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2020.02.014.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 3.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann Epidemiol. 2015;25(3):208–213.e1. 10.1016/j.annepidem.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575. 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 5.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 6.Doubeni CA, Fedewa SA, Levin TR, et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. 2019;156(1):63–74.e6. 10.1053/j.gastro.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaén CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38(2):166–171. [PubMed] [Google Scholar]
- 8.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2170–2175. 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarnall KS, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28(19):3176–3181. 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May FP, Yano EM, Provenzale D, Neil Steers W, Washington DL. The association between primary source of healthcare coverage and colorectal cancer screening among U.S. Veterans. Dig Dis Sci. 2017;62(8):1923–1932. 10.1007/s10620-017-4607-x. [DOI] [PubMed] [Google Scholar]
- 12.Zullig LL, Smith VA, Jackson GL, et al. Colorectal cancer statistics from the Veterans Affairs Central Cancer Registry. Clin Colorectal Cancer. 2016;15(4):e199–e204. 10.1016/j.clcc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693–701. 10.7205/milmed-d-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long MD, Lance T, Robertson D, Kahwati L, Kinsinger L, Fisher DA. Colorectal cancer testing in the national Veterans Health Administration. Dig Dis Sci. 2012;57(2):288–293. 10.1007/s10620-011-1895-4. [DOI] [PubMed] [Google Scholar]
- 15.Halm EA, Beaber EF, McLerran D, et al. Association between primary care visits and colorectal cancer screening outcomes in the era of population health outreach. J Gen Intern Med. 2016;31(10):1190–1197. 10.1007/s11606-016-3760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laiyemo AO, Adebogun AO, Doubeni CA, et al. Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among U.S. adults. Prev Med. 2014;67:1–5. 10.1016/j.ypmed.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doubeni CA, Laiyemo AO, Young AC, et al. Primary care, economic barriers to health care, and use of colorectal cancer screening tests among Medicare enrollees over time. Ann Fam Med. 2010;8(4):299–307. 10.1370/afm.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41(1):23–29. 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Asch DA, Ziolek TA, Mehta SJ. Misdirections in informed consent-impediments to health care innovation. N Engl J Med. 2017;377(15):1412–1414. 10.1056/NEJMp1707991. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(12):1645–1658. 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MM, Freeman M, Shannon J, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States-How, what and when? BMC Cancer. 2018;18(1):40 10.1186/s12885-017-3813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta SJ, Khan T, Guerra C, et al. A randomized controlled trial of opt-in versus opt-out colorectal cancer screening outreach. Am J Gastroenterol. 2018;113(12):1848–1854. 10.1038/s41395-018-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlton ME, Mengeling MA, Halfdanarson TR, et al. Evaluation of a home-based colorectal cancer screening intervention in a rural state. J Rural Health. 2014;30(3):322–332. 10.1111/jrh.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahneman D, Knetsch JL, Thaler RH. Experimental tests of the endowment effect and the Coase theorem. J Polit Econ. 1990;98(6):1325–1348. 10.1086/261737. [DOI] [Google Scholar]
- 26.CDC. Multicomponent interventions recommended to increase cancer screening; 2016. The community guide. www.thecommunityguide.org/content/multicomponent-interventions-recommended-increase-cancer-screening. Published 2016. Accessed February 26, 2020.
- 27.Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev. 2015;24(8):1151–1156. 10.1158/1055-9965.EPI-15-0082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


