Abstract
Introduction
Headache is one of the most frequent neurologic manifestations in COVID-19. We aimed to analyze which symptoms and laboratory abnormalities were associated with the presence of headache and to evaluate if patients with headache had a higher adjusted in-hospital risk of mortality.
Methods
Retrospective cohort study. We included all consecutive patients admitted to the Hospital with confirmed SARS-CoV-2 infection between March 8th and April 11th, 2020. We collected demographic data, clinical variables and laboratory abnormalities. We used multivariate regression analysis.
Results
During the study period, 576 patients were included, aged 67.2 (SD: 14.7), and 250/576 (43.3%) being female. Presence of headache was described by 137 (23.7%) patients. The all-cause in-hospital mortality rate was 127/576 (20.0%). In the multivariate analysis, patients with headache had a lower risk of mortality (OR: 0.39, 95% CI: 0.17–0.88, p = 0.007). After adjusting for multiple comparisons in a multivariate analysis, variables that were independently associated with a higher odds of having headache in COVID-19 patients were anosmia, myalgia, female sex and fever; variables that were associated with a lower odds of having headache were younger age, lower score on modified Rankin scale, and, regarding laboratory variables on admission, increased C-reactive protein, abnormal platelet values, lymphopenia and increased D-dimer.
Conclusion
Headache is a frequent symptom in COVID-19 patients and its presence is an independent predictor of lower risk of mortality in COVID-19 hospitalized patients.
Keywords: COVID-19; Nervous system diseases; Headache disorders, secondary; Mortality, laboratory parameters
Background
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The severity of symptomatic cases varies from mild to critical. In the case of Spain, analyzing data from more than 250,000 infected patients, 38.4% of patients needed hospitalization, 3.9% were admitted to the Intensive Care Unit (ICU) and 8.2% expired [2]. Several factors may affect the prognosis, including male sex, older age, hypertension, diabetes, smoking, cardiovascular disorders, chronic neurological disorders, and pulmonary diseases, among others [3, 4]. Regarding clinical manifestations, fever and dyspnea have been associated with severity and mortality of COVID-19 as well [3].
Headache is one of the most frequent neurologic manifestations in COVID-19. The reported frequency of headache in a recent meta-analysis was 12% of patients [5], ranging from 6.5% [6] to 70.3% [7] depending on the studies, with most of the studies reporting a frequency within 10–30% of patients [8, 9]. Headache might be caused by the release of cytokines, endothelial damage and macrophage activation; or it could be related to the inflammation of lymphatic structures, as the lymphatic organs located in the naso- and oropharynx around the Waldeyer’s ring [10, 11]. The real causes of headache in COVID-19 are still uncertain. Whether the presence of headache reflects a more severe course of COVID-19, or if it is associated with a better prognosis because of a more efficient host response against the virus is also unclear.
In the present study, we aimed to evaluate whether prognosis of hospitalized patients with COVID-19, evaluated by in-hospital all-cause mortality rate, differed in patients with headache compared to those without headache. As a secondary objective, we assessed which variables were associated with the presence of headache in COVID-19 patients. We hypothesized that the presence of headache might be related to a different COVID-19 presentation in terms of severity and inflammatory response.
Methods
We performed an observational analytic study with a retrospective cohort design. The study was done in a tertiary academic public hospital (Clinic University Hospital of Valladolid, Valladolid, Spain) with a reference population of 280,000 patients. The study was performed according to the strengthening the reporting of observational studies in epidemiology (STROBE) statement [12]. The study was designed and data were analyzed by the authors. All co-authors contributed to the collection, analysis and interpretation of data, as well as to the reviewing and approving of the final version of the manuscript.
We included all consecutive patients that were admitted to the Hospital with a confirmed SARS-CoV-2 infection diagnosis between March 8th and April 11th, 2020. The diagnosis was set, according to the World Health Organization (WHO) protocols [13], by real-time reverse transcriptase polymerase -chain reaction (RT-PCR) assay (LightMix Modular SARS-CoV (COVID19) E-gene and LightMix Modular SARS-CoV-2 RdRP, Roche Diagnostics) from oropharyngeal swab, sputum sample, or lower respiratory tract sample; or by the presence of anti-SARS-CoV-2 IgM + IgA antibodies (COVID-19 ELISA IgM + IgA, Vircell, S.L. Granada, Spain) in patients with clinical symptoms. Patients were excluded if the hospitalization was not done from the emergency department (ED) or if the patients’ records were not accessible.
Data sources
Data were collected from electronic medical records. The information was gathered from primary care records, emergency room records, and discharge reports. Data were extracted between April 21st and May 1st, and the minimum follow-up time after admission was 20 days (ranging from 20 to 61 days) and the last patients were contacted on May 25th. Details of the study have been described elsewhere [4]. In those patients in which the presence of headache was not described, a trained neurologist contacted the patient or their relatives by phone and specifically inquired about it. All patients were evaluated after the resolution of all symptoms COVID-19 or after 20 days of COVID-19 symptoms. The detailed description of the headache screening process is available in supplementary materials. We analyzed the presence of any new onset headache developed in temporal relation to the onset of the viral infection, or any headache described as different to the pre-existing headache. During the hospitalization, patients were treated according to the national standard of care [14, 15].
Study objectives
The primary objective was to evaluate if hospitalized COVID-19 patients with headache had a higher risk of all-cause in-hospital mortality compared to those without headache. The secondary objective was to analyze which variables were independently associated with the presence of headache in COVID-19 patients.
Variables
Baseline variables included demographic data, presence of comorbidities, and details about treatment for any chronic diseases. Demographic data included age, sex and baseline performance status according to the modified Rankin scale (mRS) [16]. The evaluated comorbidities included hypertension, diabetes, smoking habits, cardiovascular diseases, pulmonary diseases, cancer, an immunocompromised state, and chronic neurological diseases [4], and we also assessed whether patients had a prior history of headache. We analyzed whether patients were previously treated with angiotensin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers (ARBs), non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or other neuropharmacological therapies.
We evaluated the presence of COVID-19 general symptoms, including anosmia, arthralgia, asthenia, chest pain, cough, cutaneous rash, diarrhea, dyspnea, expectoration, fever, lightheadedness, myalgia, odynophagia, rhinorrhea, syncope, vomiting and weakness. Regarding laboratory parameters, we described both the first determination and the worst value during the hospitalization period, and we also analyzed the frequency of abnormal values. The studied parameters were leukocytes (cell count × 109/L, reference value (RV): 4–10), lymphocytes (count × 109/L, RV: 0.9–5.2), platelets (count × 109/L, RV: 150–400), hemoglobin (g/dL, RV: 12–16), international normalized ratio (INR; RV: 0.8–1.3), D-dimer (ng/dL, RV: < 500), lactate dehydrogenase (LDH; U/L, RV: 135–250), creatine kinase (U/L, RV: 20–170), glomerular filtration rate (GFR) corrected by body area (ml/min/1.73m2, RV > 90), C-reactive protein (CRP; mg/L, RV: 1–5), procalcitonin (PCT; ng/mL, RV < 5), interleukin-6 (IL-6; pg/mL, RV < 5.9), and ferritin (ng/mL, RV: 15–150). We described whether chest imaging was abnormal by using either X-ray or computerized tomography scan. We analyzed the all-cause in-hospital mortality.
Ethics
The study protocol was approved by the Institutional Review Board of the East Valladolid health area (PI-20-1751). Because of the risk of contagion, the retrospective nature of the study and the urgent need to obtain data, written informed consent was waived. However, all patients were informed about the nature of the study and were invited to participate when contacted by phone. In those patients that were personally interviewed, written informed consent was obtained. Personal information about patients was codified before entry into the database, and the identities of patients were anonymous. The study was done in accordance with the principles of the Declaration of Helsinki [17].
Statistical analysis
We describe qualitative and ordinal variables as frequencies and percentages, respectively, and quantitative continuous variables as means and standard deviations (SD) if the distribution was normal or medians and interquartile ranges (IQR) otherwise. We assessed normality by using the Kolmogorov-Smirnov test. We used two-tailed chi-square tests or Fisher’s exact tests for hypothesis testing of qualitative variables, and an independent samples Student’s t-test or a median test for hypothesis testing of quantitative, continuous variables if the distribution was normal or not normal, respectively. The level of significance was set to 0.05, adjusting for multiple comparisons by using the Bonferroni method. We managed missing data by using complete case analysis. For the primary objective, we employed a univariate logistic regression analysis to determine which variables were associated with higher odds of mortality, including the presence of headache. Those variables with p-values less than or equal to 0.2 were included in a subsequent multivariate logistic regression analysis. We present the odds ratio (OR) and the 95% confidence interval (CI). To analyze which variables were associated with the presence of headache, we employed a univariate regression analysis. We were more stringent due to the high number of studied variables, so we further studied only those variables that had a p-value less than or equal to 0.1 in the univariate regression model. The multivariate logistic regression analysis was performed following the Wald procedure and a backwards strategy. We adjusted for multiple comparisons by using the False Discovery Rate (FDR) with Benjamini-Hochberg procedure [18]. Statistical significance was achieved if the p-value was < 0.05 after correcting for multiple comparisons. We analyzed multicollinearity by using the variance inflation factor (VIF). We considered multicollinearity as critical when VIF was > 5. We did not estimate the sample size a priori and the analysis proceeded on the available data. The analysis of the study was preplanned. Statistical analysis was performed with SPSS v.26 (IBM Corp. Armonk, NY).
Results
During the study period, 580 consecutive patients fulfilled inclusion criteria. Three patients were excluded because they were not admitted from the ED and one because there was no available information. Diagnosis was confirmed by RT-PCR in 546/576 (94.8%) of cases and/or serology in 175/576 (30.4%). The mean age was 67.2 (SD: 14.7), and 250/576 (43.3%) were female.
Presence of headache was described by 137 (23.7%) patients. The headache was already present at the moment of the ED consultation in 124/137 (90.5%) patients. The headache was the first symptom in 27/104 (26.0%) patients and was present within 24 h in 40/104 (38.5%) patients, within 48 h in 65/104 (62.5%) patients and within 72 h in 77/104 (74.0%) patients. Female sex and prior history of headache were more frequent in patients with headache, while mean Rankin scale, and frequency of hypertension, smoking habits, cardiological disorders and chronic neurological disorders were more frequent in patients without headache after adjusting for multiple comparisons (p = 0.0029). Demographic data, comorbidities, and data about the chronic treatment of the full sample and the group of patients with and without headache are available in Table 1. Prior episodes of headache associated with systemic viral infections were described by 50/106 (47.2%) of patients with headache, and the present episode was described to be similar to the former in 23/50 (46.0%) of the cases. During the hospitalization, 55 (40.1%) patients with headache received systemic steroids compared to 245 (55.9%) patients without headache (p = 0.002). All-cause in-hospital mortality rate was 127/576 (20.0%) in the whole sample and 8/137 (5.8%) in headache patients.
Table 1.
Frequency of baseline variables the entire sample and comparison between patients with and without headache
| Variable | All patients (n = 576) |
Headache (n = 137) |
No headache (n = 439) |
Adjusted p-value |
|---|---|---|---|---|
| Female sex | 250 (43.3%) | 80 (58.4%) | 170 (38.7%) | < 0.001 |
| Age (years) | 67.2 (SD: 14.7) | 59.25 (SD: 12.4) | 69.6 (SD: 14.5) | < 0.001 |
| Modified Rankin scale (mean) | 0.61 (SD: 1.12) | 0.15 (SD: 0.5) | 0.75 (SD: 1.2) | < 0.001 |
| Hypertension | 300 (52.1%) | 52 (38%) | 248 (56.5%) | < 0.001 |
| Diabetes | 113 (19.6%) | 20 (14.6%) | 93 (21.2%) | 0.090 |
| Smoking | 118 (20.5%) | 20 (14.6%) | 98 (22.3%) | 0.050 |
| Cardiological disorders | 154 (26.7%) | 19 (13.9%) | 135 (30.8%) | < 0.001 |
| Pulmonary disorders | 145 (25.2%) | 33 (24.1%) | 112 (25.5%) | 0.737 |
| Cancer | 94 (16.3%) | 18 (13.1%) | 76 (17.3%) | 0.249 |
| Immuno-suppression | 24 (4.2%) | 4 (2.9%) | 20 (4.6%) | 0.409 |
| Chronic neurological disorders | 105 (18.3%) | 13 (9.5%) | 92 (2%) | 0.002 |
| Prior history of headache | 32 (5.6%) | 15 (10.9%) | 17 (3.9%) | 0.002 |
| ACEis /ARBs | 215 (37.3%) | 42 (30.7%) | 173 (39.4%) | 0.064 |
| NSAIDs | 75 (13%) | 12 (8.8%) | 66 (14.4%) | 0.090 |
| Corticosteroids | 35 (6.1%) | 7 (5.1%) | 28 (6.4%) | 0.587 |
| Neuropharmacological treatment | 203 (35.2%) | 42 (30.7%) | 161 (36.7%) | 0.198 |
| Time symptoms-ED (days) | 7.34 (SD: 6.16) | 8.51 (SD: 5.90) | 6.98 (SD: 6.21) | 0.011 |
ACEis angiotensin-converting enzyme inhibitors, ARBs angiotensin II receptor blockers, NSAIDs non-steroidal anti-inflammatory drugs, ED Emergency department. In bold, variables that were statistically significant after adjusting for multiple comparisons (threshold p < 0.0029)
Primary objective: mortality
In the univariate regression analysis, baseline disability, age, hypertension, diabetes, smoking habit, cardiological disorders, cancer, and chronic neurological disorders were associated with higher odds of death, while presence of headache and time between onset of symptoms and ED visit were associated with a lower odds of death. In the multivariate regression analysis, baseline disability (OR: 4.10, 95% CI: 2.09–8.05; p-value: < 0.001, FDR-corrected p-value: < 0.001), age (OR: 1.06, 95% CI 1.03–1.08; p-value: < 0.001, FDR-corrected p-value: < 0.001) and headache (OR: 0.39, 95% CI: 0.17–0.88; p-value: 0.028, FDR-corrected p-value: 0.007) remained statistically significant. Full results of the regression analysis are presented in Table 2.
Table 2.
Predictors of mortality: univariate and multivariate logistic regression analysis
| Type of analysis | OR | 95% CI | p-value | |
|---|---|---|---|---|
| mRS > 2 | Univariate | 11.371 | 6.376–20.278 | < 0.001 |
| Multivariate | 3.714 | 1.880–7.338 | < 0.001 | |
| Age | Univariate | 1.090 | 1.069–1.112 | < 0.001 |
| Multivariate | 1.058 | 1.055–1.031 | < 0.001 | |
| Female sex | Univariate | 0.682 | 0.454–1.024 | 0.065 |
| Multivariate | 0.703 | 0.413–1.196 | 0.194 | |
| Hypertension | Univariate | 3.534 | 2.272–5.495 | < 0.001 |
| Multivariate | 1.379 | 0.802–2.370 | 0.245 | |
| Diabetes | Univariate | 2.129 | 1.353–3.351 | 0.001 |
| Multivariate | 1281 | 0.739–2.221 | 0.377 | |
| Smoking | Univariate | 1.589 | 1.004–2.514 | 0.048 |
| Multivariate | 1.907 | 0.997–3.275 | 0.051 | |
| Cardiological disorders | Univariate | 2.955 | 1.950–4.478 | < 0.001 |
| Multivariate | 1.263 | 0.760–2.100 | 0.368 | |
| Pulmonary disorders | Univariate | 1.434 | 0.928–2.217 | 0.105 |
| Multivariate | 0.898 | 0.517–1.559 | 0.702 | |
| Cancer | Univariate | 1.641 | 1.001–2.690 | 0.049 |
| Multivariate | 1.254 | 0.694–2.266 | 0.453 | |
| Chronic neurological disorders | Univariate | 3.961 | 2.516–6.234 | < 0.001 |
| Multivariate | 1.659 | 0.947–2.906 | 0.077 | |
| Immunosuppression | Univariate | 1.295 | 0.405–4.138 | 0.663 |
| Headache | Univariate | 0.167 | 0.079–0.351 | < 0.001 |
| Multivariate | 0.399 | 0.175–0.906 | 0.028 | |
| Time between onset of symptoms and ED visit | Univariate | 0.908 | 0.870–0.947 | < 0.001 |
| Multivariate | 0.962 | 0.925–1.000 | 0.050 |
OR Odds Ratio, CI Confidence-interval, mRS Modified Rankin scale, ED Emergency department
Associated symptoms and laboratory abnormalities
Anosmia, arthralgia, cough, lightheadedness, and myalgia were statistically more frequent in patients with headache after adjusting for multiple comparisons (p = 0.0029). The frequencies of associated symptoms in the full sample and comparisons between patients with and without headache are presented in Table 3. When comparing patients with and without headache, we observed significant differences in median values of laboratory parameters on admission for CRP, PCT, and D-dimer (p < 0.0025 for all after adjusting for multiple comparisons). Of the worst experienced laboratory values during hospitalization, median values were significantly different for leukocytes, lymphocytes, LDH, CRP, PCT, ferritin, IL-6, D-dimer and INR for those with and without headache. Table 4 shows the median values of laboratory parameters.
Table 3.
Frequency of associated symptoms in the entire sample and the comparison between patients with and without headache
| Variable | All patients (n = 576) | Headache (n = 137) | No Headache (n = 439) | Adjusted p-value |
|---|---|---|---|---|
| Anosmia | 146 (25.3%) | 64 (46.7%) | 82 (18.7%) | < 0.001 |
| Arthralgia | 35 (6.1%) | 18 (13.1%) | 17 (3.9%) | < 0.001 |
| Asthenia | 242 (42.1%) | 70 (51.1%) | 172 (39.2%) | 0.014 |
| Chest pain | 99 (17.2%) | 33 (24.1%) | 66 (15.1%) | 0.015 |
| Cough | 403 (70.2%) | 113 (82.5%) | 290 (66.2%) | < 0.001 |
| Cutaneous rash | 11 (1.9%) | 4 (2.9%) | 7 (1.6%) | 0.322 |
| Diarrhea | 192 (33.4%) | 54 (39.4%) | 138 (31.4%) | 0.084 |
| Dyspnea | 292 (50.8%) | 73 (53.3%) | 219 (49.9%) | 0.487 |
| Expectoration | 90 (15.7%) | 24 (17.5%) | 66 (15.0%) | 0.484 |
| Fever | 462 (80.3%) | 121 (88.3%) | 341 (77.7%) | 0.006 |
| Lightheadedness | 60 (10.4%) | 19 (13.9%) | 41 (9.3%) | 0.001 |
| Myalgia | 139 (24.1%) | 55 (40.1%) | 85 (19.1%) | < 0.001 |
| Odynophagia | 60 (10.4%) | 22 (16.1%) | 38 (9.7%) | 0.892 |
| Rhinorrhea | 12 (2.1%) | 2 (1.5%) | 10 (2.3%) | 0.558 |
| Syncope | 43 (7.5%) | 9 (6.6%) | 34 (7.7%) | 0.648 |
| Vomiting | 47 (8.2%) | 11 (8%) | 136 (8.2%) | 0.949 |
| Weakness | 90 (15.7%) | 26 (19.0%) | 64 (14.6%) | 0.216 |
In bold, variables that were statistically significant after adjusting for multiple comparisons (threshold p < 0.0029)
Table 4.
Median values of laboratory parameters in the whole sample and the comparison between patients with and without headache
| Variable | All patients (n = 576) |
Headache (n = 137) |
No headache (n = 439) |
p-value |
|---|---|---|---|---|
| Leukocytes at admission | 6710 (4945–8885.00) | 6290.00 (4682.50–8257.50) | 6785.00 (5137.50–9302.50) | 0.202 |
| Worst leukocyte count | 9425.00 (4442.50–14,182.50) | 6245.00 (3875.00–11,422.50) | 9975.00 (4722.50–14,625.00) | < 0.001 |
| Lymphocytes at admission | 990.00 (710.00–1387.50) | 1117.00 (850.00–1495.00) | 940.00 (680.00–1350.00) | 0.003 |
| Worst lymphocyte count | 690.00 (430.00–1050.00) | 910.00 (575.00–1220.00) | 650.00 (410.00–990.00) | 0.001 |
| Platelets at admission | 195,000.00 (156,000.00–257,000.00) | 197,500.00 (170,500.00–240,750.00) | 194,000.00 (149,000.00–264,000.00) | 0.677 |
| Worst platelet count | 192,500.00 (137,000.00–341,250.00) | 206,000.00 (163,000.00–384,500.00) | 188,000.00 (134,000.00–333,500.00) | 0.111 |
| LDH at admission | 285.00 (225.00–372.00) | 263.00 (215.00–341.00) | 292.50 (227.00–381.50) | 0.019 |
| Worst LDH value | 285.00 (225.00–372.00) | 301.50 (237.00–411.75) | 365.00 (268.50–487.00) | < 0.001 |
| CK at admission | 83.00 (49.00–372.00) | 72.00 (49.5–110) | 86.00 (47.00–166.00) | 0.605 |
| Worst CK value | 89.00 (53.25–179.75) | 79.00 (49.00–136.00) | 94.00 (55.50–192.00) | 0.026 |
| CRP at admission | 65.90 (23.37–122.15) | 40.55 (14.15–102.26) | 73.40 (28.55–130.05) | 0.002 |
| Worst CRP value | 105.00 (51.25–198.02) | 67.90 (21.70–164.40) | 113.40 (58.45–208.62) | < 0.001 |
| PCT at admission | 0.10 (0.06–0.26) | 0.07 (0.04–0.14) | 0.11 (0.07–0.30) | 0.001 |
| Worst PCT value | 0.13 (0.06–0.43) | 0.07 (0.04–0.18) | 0.15 (0.08–0.54) | < 0.001 |
| Worst ferritin value | 979.00 (444.40–1819.00) | 615.00 (306.80–1449.70) | 1094.80 (534.75–1945.47) | < 0.001 |
| Worst IL-6 value | 27.80 (12.40–61.87) | 17.95 (9.76–44.15) | 33.50 (13.32–67.30) | 0.001 |
| D-Dimer at admission | 761.50 (462.75–1369.00) | 550.00 (380–864) | 855.50 (515–50-1570.00) | 0.001 |
| Worst D-Dimer value | 1300.00 (674.75–3231.75) | 810.00 (504.50 (1458.50) | 1534.00 (795.00–3580.00) | < 0.001 |
| INR at admission | 1.18 (1.10–1.29) | 1.15 (1.08–1.23) | 1.19 (1.11–1.32) | 0.003 |
| Worst INR value | 1.25 (1.15–1.40) | 1.19 (1.11–1.30) | 1.26 (1.17–1.45) | < 0.001 |
LDH Lactate dehydrogenase, CK Creatine Kinase, CRP C-reactive protein, PCT Procalcitonin, IL-6 interleukin 6, INR International normalized ratio. In bold, variables that were statistically significant after adjusting for multiple comparisons (threshold p < 0.0025)
Variables associated with the presence of headache
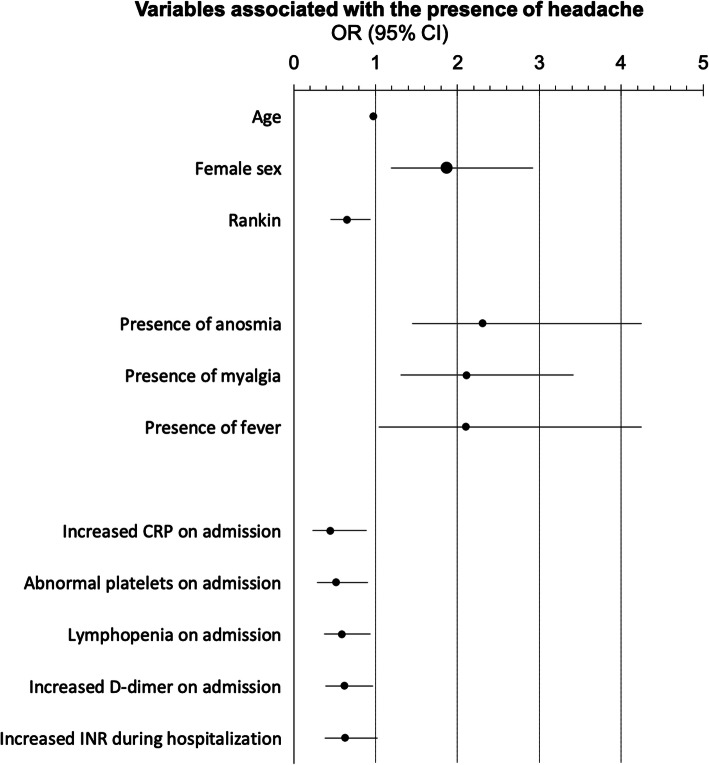
In the univariate regression analysis, 56 different variables were included in the model, of which 39 had a p-value < 0.1 (supplementary Table 1). We included those 39 variables in a multivariate regression analysis, and 11 variables were statistically significant. After FDR adjustments, 10 variables remained statistically significant. The variables that were independently associated with a higher odds of headache in hospitalized COVID-19 patients were anosmia, myalgia, female sex, and fever; while the variables that were independently associated with a lower odds of having headache were age, modified Rankin scale, increased CRP on admission, abnormal platelet value on admission, lymphopenia on admission, and increased D-dimer on admission. The VIF was less than or equal to 1.131 in all cases. Full results of the multivariate regression analysis are shown in Table 5 and Fig. 1.
Table 5.
Multivariate regression analysis of variables that were associated with the presence of headache
| Variable | OR | 95% CI | p-value | Corrected p-value (Benjamini-Hochberg) |
|---|---|---|---|---|
| Presence of anosmia | 2.306 | 1.449–4.247 | < 0.001 | 0.001 |
| Presence of myalgia | 2.114 | 1.308–3.418 | 0.002 | 0.011 |
| Age | 0.976 | 0.959–0.993 | 0.005 | 0.018 |
| Female sex | 1.861 | 1.186–2.921 | 0.007 | 0.019 |
| Rankin | 0.654 | 0.455–0.940 | 0.022 | 0.048 |
| Increased CRP on admission | 0.451 | 0.227–0.897 | 0.023 | 0.042 |
| Abnormal platelets on admission | 0.516 | 0.291–0.912 | 0.023 | 0.036 |
| Lymphopenia on admission | 0.59 | 0.368–0.945 | 0.028 | 0.031 |
| Presence of fever | 2.105 | 1.043–4.247 | 0.038 | 0.046 |
| Increased D-dimer on admission | 0.616 | 0.388–0.978 | 0.04 | 0.044 |
| Increased INR during hospitalization | 0.627 | 0.384–1.024 | 0.062 | 0.062 |
In bold, variables that were statistically significant after adjusting for multiple comparisons (Benjamini-Hochberg procedure)
Fig. 1.
Odds ratio and 95% confidence interval of variables associated with the presence of headache
Discussion
In this study we analyzed which variables were associated with the presence of headache in COVID-19 patients and whether patients with headache had a worse prognosis, assessed by the risk of all-cause, in-hospital mortality. For this, we studied a large cohort of hospitalized patients with confirmed COVID-19, and we systematically interrogated them about the presence of headache.
SARS-CoV-2 causes respiratory symptoms in most patients. However, neurological symptoms are among the most frequent extrapulmonary symptoms [8, 9]. After an incubation period of 2–14 days [1], most patients present general symptoms for 1–3 weeks [19]. Patients with comorbidities are particularly vulnerable to the virus [4, 20], and there are several risk factors that have been associated with a worse prognosis [3, 21, 22]. The prompt diagnosis, isolation and treatment of patients is crucial [23]. Every clinician should be prepared for facing the virus, as the pandemic has extended worldwide causing hundreds of thousands of deaths and millions of cases [2].
Headache is a frequent symptom of COVID-19. It was present in almost a quarter of our sample. This frequency was higher than in other previously reported series, which described a 6–15% prevalence [5, 6, 8, 9]. On the one hand, this finding must be interpreted with caution given that we only included hospitalized patients. On the other hand, this could be explained because we interviewed every patient or relative about the presence of headache, reaching them by telephone if necessary. We also extensively reviewed primary care records, screening for the presence of headache. Further studies will be necessary to clarify the real prevalence of headache in COVID-19 patients, including outpatient series.
The main finding of our study was that headache was associated with a lower probability of death. In our sample, headache patients had a different clinical picture, as the frequency of symptoms such as anosmia, cough, myalgia and arthralgia were higher. The higher frequency of symptoms could suggest that those patients visited the ED earlier; however, in our sample, patients with headache presented to the ED later than those without headache. This could reflect that patients without headache suffer from a more severe case of COVID-19 and therefore they seek medical attention earlier [24].
Many laboratory results were also different in patients with headache compared with the rest of the sample, although these results should be interpreted cautiously [25]. Headache patients were younger, more frequently female, less disabled, and had lower frequencies of hypertension, smoking habits, cardiac disorders, and chronic neurological disorders. All of those could influence the crude results [3, 4], and therefore we did a multivariate regression analysis to assess which variables were independently associated with the presence of headache.
The baseline factors that independently increased the odds of having headache were the female sex, younger age, and lower disability level at the baseline. These variables have been associated with a lower risk of death from COVID-19 [4, 26]. However, in our study, headache was independently associated with a lower risk of death in the multivariate model.
Some symptoms were associated with higher odds of headache, such as fever, anosmia and myalgia [27]. Fever, myalgia and headache are common in other systemic viral infections [28–30]. These symptoms can be related to lymphocyte and macrophage activation, interferon secretion and cytokine release [31, 32]. An efficient innate immunity response is associated with a better COVID-19 prognosis. However, in some patients, the immune response persists and causes endothelial dysfunction, thrombosis, persistent macrophage activation, fibrinoid organizing pneumonia, acute respiratory distress syndrome, multi-organ failure and death [33].
Laboratory biomarkers reportedly associated with COVID-19 pathophysiology include lymphocytes, CRP, D-dimer [34], PCT, troponins, ferritin, and IL-6, among others [22, 35]. In our sample, most of them differed between patients with and without headache. In the multivariate analysis, patients with headache had lower odds of having increased CRP, abnormal platelet values, lymphopenia and increased D-dimer at the ED visit, and these are factors that are typically associated with the cytokine storm described in COVID-19 patients [32, 36]. In our opinion, this could partially explain the lower risk of death experienced by COVID-19 patients with headache [36]. However, it was also an unexpected finding, as we hypothesized that the presence of headache could be partially explained by those factors. We could not explain the lower levels of CRP or IL-6 due to the use of NSAIDs or steroids, as patients with headache received them less frequently than the rest of the sample. The specific causes of headache might be further studied via imaging and systematic cerebrospinal fluid evaluation.
Our findings should be interpreted with caution. In a previous prospective study that included 179 hospitalized COVID-19 patients with pneumonia, mortality was higher in patients with headache (5/21 (23.8%)) than those without headache (12/158 (7.6%)); however, it did not remain statistically significant in the multivariate regression analysis [37]. In a meta-analysis that included 19 studies (2874 patients) published between January 1, 2020 and February 21, 2020, presence of headache was not associated with a worse prognosis [38]. We propose that the impact of headache in COVID-19 prognosis should be studied in deeper detail. Compared with other symptoms, headache might have different phenotypes and each presentation might have a different meaning [39, 40]. We hypothesize that the impact of headache on prognosis might be linked to the causes of headache [41]. If the presence of headache represents a more effective immune response, then it could be related to a lower mortality. In those cases in which the headache is associated with systemic complications, the prognosis might be worse. However, in our study, most of the patients reported the headache early in the course of the disease, and therefore the precise significance of later or different headache presentations should also be analyzed.
This study has notable weaknesses. It was a single-center study, so the results may not be applicable to other centers or countries. In addition, the sample included only hospitalized patients, which could limit the generalizability of the results. Further multicentric studies with larger sample sizes and inclusion of non-hospitalized patients will be necessary to evaluate our hypothesis and to clarify whether the prevalence of headache differs between admitted and non-admitted patients, particularly in patients with prior headache history. Finally, our study was retrospective and the collected data may not be complete; despite the extensive search, the frequency of headache could be underreported due to the lack of prospective and iterative evaluation, the unavailability of some patients, and the fact that in some cases we contacted the relatives instead of the patients. Some variables may be associated with the lower age and higher frequency of female sex seen in patients with headache, so direct comparisons should be interpreted cautiously. We did not assess the impact on prognosis of the different possible headache phenotypes. We encourage other researchers to pool the data and create a larger series that could better clarify the role of prior history of headache disorders in the prognosis and phenotyping of headache in COVID-19.
Conclusion
Headache is a frequent symptom in patients with COVID-19. The presence of headache is an independent predictor of lower risk of mortality in COVID-19 hospitalized patients. Anosmia, myalgia, fever and female sex were independent predictors of headache in hospitalized COVID-19 patients, while lower age and lower baseline disability, CRP, platelet value, lymphocyte count and D-dimer values on admission were independently associated with the presence of headache.
Supplementary information
Additional file 1: Summary of the screening proccess.
Acknowledgements
We thank the patients for their collaboration and all the people who are facing COVID-19. We thank the Institute of Health Carlos III (ISCIII) and the University of Valladolid.
Abbreviations
- ACEis
Angiotensin-converting enzyme inhibitors
- ARBs
Angiotensin II receptor blockers
- NSAIDs
Non-steroidal anti-inflammatory drugs
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- ED
Emergency department
- GRR
Glomerular filtration rate
- ICU
Intensive Care Unit
- IL-6
Interleukin-6
- INR
International normalized ratio
- LDH
Lactate dehydrogenase
- mRS
Modified Rankin scale
- NSAIDs
Non-steroidal anti-inflammatory drugs
- RV
Reference value
- RT-PCR
Real-time reverse-transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- time symptoms-ED
Time since the beginning of the symptoms to the arrival to emergency department
- STROBE
Strengthening the reporting of observational studies in epidemiology
- SD
Standard deviation
- IQR
Inter-Quartile range
- OR
Odds ratio
- FDR
False Discovery Rate
- VIF
Variance Inflation Factor
- WHO
World Health Organization
Authors’ contributions
JT and DGA were the major contributors in writing the manuscript. APG, EMP, BT, IHP, GVP, PSC, MDL, ACM, CLS, MGS, EMV, MP, AS, BGV collected and analyzed the data. JFA and ALG designed and conceptualized the study, analysed the data and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Clinical Research Ethics Committee of East Valladolid Area approved the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s10194-020-01165-8.
References
- 1.Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020;2:1–10. doi: 10.1007/s11684-019-0733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Epidemiologic Surveillance Network . Covid-19 report number 33. 2020. [Google Scholar]
- 3.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;23:S0163–4453(20)30234-6. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Azorin D, Martínez-Pías D, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, Talavera B et al (2020) Neurological comorbidity is a predictor of death in Covid-19 disease: a cohort study on 576 patients. Front Neurol. 10.3389/fneur.2020.00781 [DOI] [PMC free article] [PubMed]
- 5.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, Von Groote TC, Jayarajah U, Weerasekara I, et al. Novel Coronavirus Infection (COVID-19) in humans: a scoping review and meta-analysis. J Clin Med. 2020;9:941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q et al (2020) Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Int Med. 10.1111/joim.13089 [DOI] [PMC free article] [PubMed]
- 8.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, LIang Y et al (2020) COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol:1–7. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed]
- 10.Masieri S, Trabattoni D, Incorvaia C, De Luca MC, Dell'Allbani I, Leo G, et al. A role for Waldeyer's ring in immunological response to alergens. Curr Med Res Opin. 2014;30(2):203–5. 10.1185/03007995.2013.855185. [DOI] [PubMed]
- 11.El-Anwar MW, Elzayat S, Fouad YA. ENT manifestation in COVID-19 patients. Auris Nasus Larynx. 2020;15:S0385-8146(20):30146–2. 10.1016/j.anl.2020.06.003. [DOI] [PMC free article] [PubMed]
- 12.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance). Accessed 19 May 2020.
- 14.Ministry of Health . Technical documents. Hospital management of COVID-19. 2020. [Google Scholar]
- 15.World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019- nCoV) infection is suspected: interim guidance. 2020. [Google Scholar]
- 16.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin scale: a systematic review. Stroke. 2009;40:3393–3395. doi: 10.1161/STROKEAHA.109.557256. [DOI] [PubMed] [Google Scholar]
- 17.International Conference on Harmonisation . Proceedings of the International conference on harmonisation of technical requirements for registrations of pharmaceuticals for human use. Geneva: International Conference on Harmonisation; 1996. Good clinical practice: consolidate guideline. [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–230. [Google Scholar]
- 19.Kaur N, Gupta I, Singh H, Karia R, Ashraf A, Habib A et al (2020) Epidemiological and clinical characteristics of 6635 COVID-19 patients: a pooled analysis. SN Compr Clin Med:1–5. 10.1007/s42399-020-00393-y PMC7343407 [DOI] [PMC free article] [PubMed]
- 20.Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P et al (2020) Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med:1–8. 10.1007/s42399-020-00363-4 PMCID: PMC7314621 [DOI] [PMC free article] [PubMed]
- 21.Bwire GM (2020) Coronavirus: Why men are more vulnerable to Covid-19 than women? SN Compr Clin Med:1–3. 10.1007/s42399-020-00341-w PMCID: PMC7271824 [DOI] [PMC free article] [PubMed]
- 22.Pennica A, Conforti G, Falangone F, Martocchia A, Tafaro L, Sentimentale A, Marini V et al (2020) Clinical management of adult coronavirus infection disease 2019 (COVID-19) positive in the setting of low and medium intensity of care: a short practical review. SN Compr Clin Med:1–6. 10.1007/s42399-020-00333-w PMCID: PMC7258606 [DOI] [PMC free article] [PubMed]
- 23.Shen B, Chen L, Zhang L, Zhang M, Li J, Wu J et al (2020) Wuchang Fangcang Shelter Hospital: Practices, experiences, and lessons learned in controlling COVID-19. SN Compr Clin Med:1–6. 10.1007/s42399-020-00382-1 PMCID: PMC7334130 [DOI] [PMC free article] [PubMed]
- 24.O’Reilly GM, Mitchell RD, Rajiv P, Wu J, Brennecke H, Brichko L, Noonan MP et al (2020) Epidemiology and clinical features of emergency department patients with suspected COVID-19: initial results from the COVID 19 emergency department quality improvement project (COVED-1). Emerg Med Australas. 10.1111/1742-6723.13540 [DOI] [PubMed]
- 25.Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58:e00512–e00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vacchiano V, Riguzzi P, Volpi L, Tappatà M, Avoni P, Rizzo G et al (2020) Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci:1–3. 10.1007/S10072-020-04525-z [DOI] [PMC free article] [PubMed]
- 28.Eccles R, Loose I, Jawad M, Nyman L. Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med. 2003;4:118–124. doi: 10.1046/j.1526-4637.2003.03019.x. [DOI] [PubMed] [Google Scholar]
- 29.Ng S, Cowling BJ, Fang VJ, Chang KH, Ip DKM, Cheng CKY, et al. Effects of Oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;50:707–714. doi: 10.1086/650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii T, Sasaki Y, Maeda T, Komatsu F, Suzuki T, Urita Y. Clinical differentiation of infectious mononucleosis that is caused by Epstein-Barr virus or cytomegalovirus: a single-center case-control study in Japan. J Infect Chemother. 2019;25:431–436. doi: 10.1016/j.jiac.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;ciaa310. 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed]
- 32.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `cytokine Storm' in COVID-19. J Inf Secur. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Med Clin. 2020;S0025–7753(20):30344–30344. doi: 10.1016/jmedcli.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”. Inflamm Res. 2020;11:1–15. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong-Hui D, Li-Rong L, Cheng-Qing Y, Wen W, Tan-Ze C, Ming L. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana P, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belvis R (2020) Headaches during COVID-19: my clinical case and review of the literature. Headache. 10.1111/head.13841 [DOI] [PMC free article] [PubMed]
- 40.Porta-Etessam J, Matías-Guiu JA, Gonzalez-García N, Gomez Iglesias P, Santos-Bueso E, Arriola-Villalobos P et al (2020) Spectrum of headaches associated with SARS-CoV-2 infection: study of healthcare professionals. Headache. 10.1111/head.13902 [DOI] [PMC free article] [PubMed]
- 41.García-Azorín D, Trigo J, Talavera B, Martínez-Pías E, Sierra A, Porta-Etessam J et al (2020) Frequency and type of red flags in patients with Covid-19 and headache: a series of 104 hospitalized patients. Headache. 10.1111/head.13927 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Summary of the screening proccess.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.