Abstract
Background
While there are several existing eHealth technologies for drug-drug interactions and stand-alone drug adverse effects, it appears that considerably less attention is focussed on that of complementary and alternative medicine (CAM). Despite poor knowledge of their potential interactions and side effects, many patients use CAM. This justifies the need to identify what eHealth technologies are assisting in identifying potential 1) adverse drug interactions with CAM, 2) adverse CAM-CAM interactions or 3) standalone CAM adverse events or side effects.
Methods
A scoping review was conducted to identify eHealth technologies assisting in identifying potential adverse interactions with CAM or standalone CAM adverse events or side effects, following Arksey and O’Malley’s five-stage scoping review framework. MEDLINE, EMBASE, and AMED databases and the Canadian Agency for Drugs and Technologies in Health website were systematically searched. Eligible articles had to have assessed or referenced an eHealth technology assisting in identifying potential one or more of the three aforementioned items. We placed no eligibility restrictions on type of eHealth technology.
Results
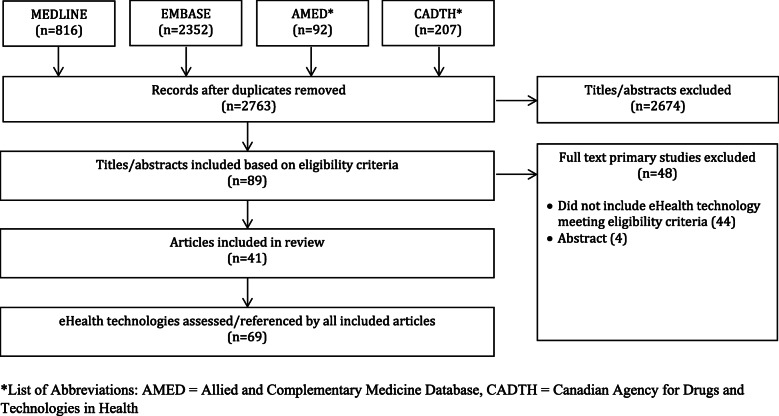
Searches identified 3467 items, of which 2763 were unique, and 2674 titles and abstracts were eliminated, leaving 89 full-text articles to be considered. Of those, 48 were not eligible, leaving a total of 41 articles eligible for review. From these 41 articles, 69 unique eHealth technologies meeting our eligibility criteria were identified. Themes which emerged from our analysis included the following: the lack of recent reviews of CAM-related healthcare information; a large number of databases; and the presence of government adverse drug/event surveillance.
Conclusions
The present scoping review is the first, to our knowledge, to provide a descriptive map of the literature and eHealth technologies relating to our research question. We highlight that while an ample number of resources are available to healthcare providers, researchers, and patients, we caution that the quality and update frequency for many of these resources vary widely, and until formally assessed, remain unknown. We identify that a need exists to conduct an updated and systematically-searched review of CAM-related healthcare or research resources, as well as develop guidance documents associated with the development and evaluation of CAM-related eHealth technologies.
Keywords: Adverse events, Complementary and alternative medicine, eHealth, eHealth technologies, Herb-drug interactions, mHealth, Scoping review, Side effects
Background
Considerable research has established that the concurrent use of pharmaceutical drugs and complementary and alternative medicines (CAMs) can lead to unwanted interactions; furthermore, certain CAMs have been shown to cause standalone adverse reactions [1]. It is currently estimated that more than 70% of North Americans have tried at least one form of CAM, which includes traditional medicines [2–4], and collectively spend billions of dollars on these therapies each year [5, 6]. An integral part of CAMs include natural health products (NHPs) in Canada, which are sold directly to Canadians and do not require a prescription nor the oversight of a healthcare professional, despite being regulated by Health Canada [7]. A 2010 Ipsos-Reid poll showed that 73% of Canadians took NHPs regularly [8], yet only 19% of Canadians surveyed by Health Canada considered themselves well-informed when purchasing NHPs. Furthermore, 12% of Canadians who used NHPs report that they experienced adverse reactions, and only 41% of Canadians who experienced adverse effects from NHPs reported them [8].
eHealth is used widely in managing patients’ medications, however, it is currently unknown what types of eHealth technologies are available to detect potential drug interactions with CAM or standalone CAM adverse effects. With regard to pharmaceutical technologies, there are some useful medication databases available that can be integrated into hospitals’ electronic data and used as a tool for computerized decision support systems (CDSS). Studies on programs such as the Swedish Finnish Interaction X-referencing or SFINX database have demonstrated a correlation between medication alert systems and a decrease in potentially adverse drug interactions [9]. In some hospitals, medication-related CDSSs are used which evaluate drug dosage, patients’ age, and comorbidities and send alarms when possible drug interactions and side effects are detected [10].
Additionally, there are several mobile apps that provide beneficial information regarding appropriate dosing, potential drug-drug interactions, and side effects. WebMD, Medscape, Epocrates, The Blue Book, and Micromedex are some of the most commonly used mobile drug information apps [11]. These types of mobile health (mHealth) apps are used by both health professionals and patients, and many are publicly available in the Google Play or the Apple App Store [12]. Some mobile apps have been developed to assist patients taking high-risk medications to manage their symptoms. For example, an app was developed by a team of collaborators in Oxford for patients undergoing treatment for colorectal cancer. This type of mHealth system shares the data recorded by the patient in symptom diaries with their health professional and will generate an alarm if a serious side effect occurs [13].
Moreover, computer-assisted history-taking systems (CAHTS) are other eHealth tools that have the potential to improve the monitoring of drug interactions and side-effects. CAHTS allow patients to enter their medication history prior to consultations, resulting in a more comprehensive record of medication information [14].
While there are several existing eHealth technologies for drug-drug interactions and stand-alone drug adverse effects, it appears that considerably less attention is focussed on CAM-drug interactions, CAM-CAM interactions, and standalone CAM adverse effects. Despite poor knowledge of their potential interactions and side effects, many patients use CAM. This justifies the need to identify what eHealth technologies exist for such CAM-related interactions and adverse effects.
Methods
Approach
A scoping review was conducted to identify eHealth technologies assisting in identifying potential adverse interactions with CAM or standalone CAM adverse events or side effects, following Arksey and O’Malley’s [15] five-stage scoping review framework, and supplemented by Levac, Colquhoun, & O’Brien [16] and Daudt, van Mossel, & Scott [17] which build upon Arksey and O’Malley’s work. The five steps are as follows: (1) identifying the research question, (2) identifying relevant studies, (3) selecting the studies, (4) charting the data, and (5) collating, summarizing, and reporting the results. This method was chosen in order to fulfill the prerequisites of a scoping review, which involve searching for and assessing the available literature on a given topic in order to identify the characteristics of eligible articles, summarize their contents and highlight knowledge gaps. We did not register a protocol.
Step 1: identifying the research question
The research question for this scoping review was as follows: “What eHealth technologies are assisting in identifying potential 1) adverse drug interactions with CAM, 2) adverse CAM-CAM interactions or 3) standalone CAM adverse events or side effects?”. While a multitude of definitions for “eHealth” exists [18], for the purpose of this scoping review, in order to define parameters of eHealth, we considered 3 domains and their subcategories based on a study by Shaw et al. [19]. The framework for defining eHealth technologies are summarized in Table 2 in Appendix 3 of their paper. CAM has also been defined in a multitude of ways [20], however, the National Center for Complementary and Integrative Health (NCCIH) defines a non-mainstream practice used together with conventional medicine as “complementary”, a non-mainstream practice used in place of conventional medicine as “alternative”, and the coordinated delivery or use of conventional and complementary approaches as “integrative” [21]. For the purpose of this scoping review, in order to define parameters of eHealth, we considered Wieland et al.’s [22] bibliometric and content analysis of the Cochrane Complementary Medicine Field specialized register of controlled trials, where the authors collected the number of CAM field specialized register citations classified by type of CAM therapies. What was included as CAM are shown in Table 4 in Appendix 3 of their paper. Finally, we define the term “adverse event” as “any untoward medical occurrence that may present during treatment with a medicine but which does not necessarily have a causal relationship with this treatment”, and “side effect” as “any unintended effect of a pharmaceutical product [or CAM] occurring at doses normally used in man, which is related to the pharmacological proprieties of the drug [or CAM]”. Our definition of the terms “adverse event” and “side effect” correspond with that of the World Health Organization [23].
Step 2: finding relevant studies
Following a preliminary scan of the literature, an experienced academic librarian was consulted to assist in devising a comprehensive, systematic search strategy on MEDLINE, EMBASE, and AMED academic databases. The search included literature published from 1995 up until November 6, 2019, as eHealth was only popularized in the late 1990s with the term itself was coined in 1999 [24]. The search strategy included Medical Subject Headings and keywords that reflect terms commonly used in the literature to refer to both eHealth and CAM. Following preliminary searches, it was decided not to also include search terms relating to adverse events or side effects, as many eligible articles were found to not be indexed using them and thus this would have excluded them. Additionally, the Canadian Agency for Drugs and Technologies in Health (CADTH) website (https://www.cadth.ca/) was also searched to account for any grey literature; terms searched included “eHealth”, “mHealth”, “complementary and alternative medicine” and “herbal”. A search strategy we used including Medical Subject Headings and keywords that reflect terms commonly used in the literature to refer to CAM and eHealth can be found in Appendix 1.
Step 3: selecting the studies
Preliminary searches indicated that the academic literature on this subject area exists as eligible articles could be found. We included primary research articles and research protocols; any relevant reviews were used to source additional eligible primary research articles or research protocols. In order to be included, the article had to have included an eHealth technology (either the authors’ own or referenced) of any kind that was assisting in identifying potential 1) adverse drug interactions with CAM, 2) adverse CAM-CAM interactions, or 3) standalone CAM adverse events or side effects, otherwise they were excluded. At this stage, articles were excluded if they did not make reference to our research question. Publications in the form of conference abstracts were not eligible. We also restricted eligibility to articles published in the English language and that were either publicly available or could be ordered through our library system. If there was any uncertainty, the article’s full-text was reviewed to determine eligibility. We placed no eligibility restrictions on type of eHealth technology; even if they were only accessible in a non-English language, we included them as long as English literature was written about them. All three authors (JYN, MM, and VM) pilot-screened a subset of all titles and abstracts independently and met to verify their agreement in applying the inclusion criteria prior to screening all items, including the full-texts of potentially eligible articles, independently in triplicate. Disagreement was solved by discussion, and in the case that consensus could not be reached, a majority vote was used to determine eligibility.
Step 4: charting the data
Articles meeting the inclusion criteria were critically reviewed using Arksey and O’Malley’s [15] descriptive-analytical narrative method. For each eligible article, the following data were extracted and charted: article title; author(s); year of publication; study country; study design; whether the article was original research or a review of resources; study aim; and name of eHealth technology(s) assessed or referenced in the eligible article that we assessed in this scoping review. For each included eHealth technology, the following data were extracted and charted: name; URL (if available), type (i.e. adverse drug reporting system, database, factsheets, etc.), format (i.e. website, mobile app, etc.), year established; if the eHealth technology still exists; whether it has been used in any context outside of the authors’ study; whether it is free and/or available to anyone; developer (and category); purpose; and intended user(s). All three authors (JYN, MM, and VM) participated in a pilot data extraction of a subset of eligible articles/eHealth technologies, and MM and VM independently extracted data from all eligible articles as well as from all eHealth technologies (i.e. the authors’ own or referenced). All three authors then met to discuss and resolve discrepancies. We did not conduct a critical appraisal of included sources of evidence nor did we collect included articles’ sources of funding, as no prior scoping review had been conducted on this topic before, thus we only aimed to provide a descriptive map of the literature and highlight a number of key themes that emerged from our analysis.
Step 5: collating, summarizing, and reporting the results
Charted data was summarized in the format of tables, and the descriptive data were analysed using content analysis. All three authors (JYN, MM, and VM) reviewed the descriptive data, and JYN identified codes relative to the findings, organized codes into thematic groups, and presented a narrative relating to the research question as well as highlighted knowledge gaps in the currently existing literature. All three authors then met to discuss and resolve discrepancies.
Results
Search results
Searches identified a total of 3467 items, of which 2763 were unique, and 2674 titles and abstracts were eliminated, leaving 89 full-text articles to be considered. Of those, 48 were not eligible, because they did not include eHealth technology meeting eligibility criteria (n=44) or were an abstract (n=4), leaving a total of 41 articles that were included in this scoping review [25–65]. A PRISMA diagram can be found in Fig. 1 of Appendix 2.
Eligible article characteristics
Eligible articles were published from 1997 to 2019 and originated from the United States (n=16), China (n=8), Germany (n=3), Republic of Korea (n=3), Singapore (n=3), Canada (n=1), Denmark (n=1), Greece (n=1), Italy (n=1), Sweden (n=1), and United Kingdom (n=1). Additionally, one study involved researchers from Australia, China, and Germany (n=1), and another from China and the United Kingdom (n=1). Of the 41 articles included, 27 were primary research articles with the following study designs: development (n=12) or evaluation (n=7) of an eHealth technology, analysis of data collected by an eHealth technology (n=7), and a usability study (n=1); the remaining 14 articles were reviews of one or more medical information resource(s) including at least one containing an eHealth technology addressing our research question. Here, we define a “review” as an article that reviewed an aspect of the entirety of an eligible eHealth technology, and not necessarily a systematic or scoping review. The details associated with all eligible article characteristics, including study aims, can be found in Table 1 of Appendix 3.
eHealth technology characteristics
Of the 69 included eHealth technologies, we characterized them as follows: databases (n=34), factsheets/healthcare information (n=13), adverse drug/event alerting, reporting and/or signal detection systems (n=11), search engines (n=4), interaction checkers (standalone) (n=1), bulletin (n=1), continuing education module (n=1), electronic pharmacovigilance system (n=1), model (n=1), and serious game (n=1). Additionally, one eHealth technology is both an adverse drug reaction detection/spontaneous reporting system and a database. These eHealth technologies were offered in the following formats: websites/web-based only (n=38), website and mobile app (n=10), mobile app only (n=6), software (n=2), artificial intelligence (n=1). The format was unclear for the remaining twelve. In many cases, it was difficult to ascertain the year the eHealth technology was created (n=29), however, 15 were identified to have been created from at least the mid-1970s to 2000, another 15 from 2001 to 2010, and 10 from 2011 to 2019. Forty-eight eHealth technologies were identified to currently exist, while 2 did not, and 1 was replaced by one of the 48, and for the remaining 18, their current existence was unclear. We found that 60 eHealth technologies had been used in any context outside of the authors’ study; for example, the technology or its data were cited in another research article not conducted by the developing authors. Thirty-two eHealth technologies were found to be available and entirely free to use by anyone, 12 are only accessible with a subscription, 1 was partially available without a subscription, required a partial-subscription, 3 were confirmed to be not available to the general public, and the status of the remaining 21 was unclear. eHealth technologies were developed by the following: companies (n=24), researchers or research groups (n=22) (11 of which by the authors of one or more of the eligible articles), government agencies/departments (n=17), botanical council (n=1), not-for-profit (n=1), practitioners (n=1), unclear (n=1). Additionally, 2 were developed by organizations that involved both researchers and government. Intended users of the eHealth technologies were not always clear, and we used our discretion in cases where this information was not provided explicitly. Our assessment was as follows: for healthcare providers (n=54), for researchers (n=36), for patients/public (n=32). These numbers reflect a large amount of overlap among these user types across many of the eHealth technologies. The details associated with all included eHealth technologies can be found in Table 2 of Appendix 3.
Findings from thematic analysis
In total, three main themes emerged from our analysis and are described below.
Obsolete reviews of CAM-related healthcare information
Upon accounting for all eligible articles, one immediate and striking finding was the fact that while a number of reviews have been published providing overviews or summaries of CAM-related healthcare or research resources, they have all been published in excess of one decade ago, with the exception of Xie et al. [41]‘s review, however it is only specific to natural product databases. The most recent reviews providing information on CAM, in general, were published approximately 15–20 years ago [25, 28, 32, 33, 48, 50, 56, 65]. As a result, the information contained within these articles is all, to varying extents, obsolete, including resources that are no longer available or updated. Furthermore, as these articles were published years before the methodologies of systematic or scoping reviews were published, many resources included in these reviews were found unsystematically or based on the authors’ knowledge.
Large number of databases
It was found that a large number of databases exist, primarily offered in website and/or mobile app formats, making up the largest category of types of eHealth technology in this scoping review (35 of 69). New databases have been created (and maintained) since the 1970s up to the present day, and their developers and content vary fairly widely. Perhaps unsurprisingly, the vast majority of databases developed by companies focus on the provision of resources for healthcare providers. These include a number of well-recognized, large companies in the healthcare industry, providing items such as monographs, clinical decision suites, dosing calculators and adverse event/interaction checkers in their respective databases [66–71]. In contrast, databases developed by government organizations or agencies collect information at the population level regarding pharmacovigilance and adverse events [49, 72, 73], though databases such as the Drug Product Database available on the Health Canada website also provides a search tool for drugs and certain natural health products available nationally with associated monographs. Interestingly, most databases developed by researchers or research groups emerge from Asia (notably China and the Republic of Korea). These databases serve a different purpose than the aforementioned company- and government-developed ones, instead housing information on traditional medicine (i.e. traditional Chinese medicine) ingredients such as interactions, mechanisms of action, compound structures, and their relationship to genes and diseases [30, 31, 39–41, 47, 55].
We did not formally assess the usability of these databases or the quality of the information contained within them, as this exceeded the scope of this scoping review. Despite this, it should be noted that initial impressions obtained simply by completing the data extraction step for this review indicated that these databases likely vary largely in quality, and the authors intend on assessing this in a future research study. The frequency of updates for each database is presented in Table 3 in Appendix 3; some of the most recent databases (updated and/or created within the last 3 years) are presented in Table 4 in Appendix 3.
Government adverse drug/event surveillance
We identified that the majority of adverse drug/event surveillance eHealth technologies including CAM identified were government initiated, and found across the following countries: China, India, the Republic of Korea, and the United States. These eHealth technologies are equipped with detection [34] and reporting systems [27, 31, 34, 74] and safety alerts [74, 75]. The countries with such government initiatives outside of the United States are unsurprising, given that a very large proportion of traditional and indigenous medical systems originate from these parts of the world, notably traditional Chinese and Korean medicine and Ayurvedic medicine [76]. Though a not-for-profit, as opposed to a government agency, our review also captured the Institute for Safe Medication Practices in Canada which runs a medication error reporting program and publishes newsletters, reports and safety alerts based on information received [77]. These government initiatives highlight the growing need to collect information surrounding CAM-related adverse events and side effects given they are widely used across the globe [78].
Discussion
The purpose of the present scoping review was to identify eHealth technologies that assist in identifying potential 1) adverse drug interactions with CAM, 2) adverse CAM-CAM interactions, or 3) standalone CAM adverse events or side effects. The amount of available literature on this topic as well as the number of eHealth technologies, while not overly voluminous, presents a broad range of different eHealth technologies that have emerged since the popularization of (and even before) the term “eHealth”. Given that, to our knowledge, this is the first study to present such eHealth technologies using a systematic search of the peer-reviewed and grey literature, it is hoped that these findings will provide both healthcare providers and researchers with an awareness of what research has taken place over the past few decades at the intersection of CAM and eHealth.
Resources for practitioners, researchers and patients: ample, but of unclear quality
This review also provides readers with a list of currently existing resources that they may not have been aware of to date, which may aid in their clinical practice or research. While these resources have been developed, evaluated, studied or assessed, at least to some degree, by academic researchers, this review was only designed to scope out the number of eHealth technologies and their key characteristics. Unfortunately, we did not find that any authors of included articles expressed an intention to create new upcoming eHealth technologies in this topic area, beyond improving their own existing resource. As expected, our scoping review captured some well-known and authoritative resources, such as Natural Medicines [69], MicroMedex [67] and the NCCIH’s Herbs at a Glance [79], however, some others may be less known and their quality may be questionable. Therefore, we encourage users of any of these resources to exercise caution and use their professional judgement when utilizing any of these resources, especially those which may be unfamiliar to them.
Areas identified for future research
We have identified a couple of areas for future research based on our findings.
Today there exists more information on the Internet about CAM and CAM-related adverse events and side effects than ever before, yet the quality of much of it is arguably questionable [80]. Clinicians, researchers, governments and policymakers, and patients alike all need resources that provide them with reliable, trustworthy, and current information. Combined with our finding that the vast majority of reviews on CAM-related healthcare or research resources are now decades old, this undoubtedly justifies a need for an updated review of CAM-related healthcare information given that many changes have occurred in the way in which both guidelines inform clinical practice and research methodologies have evolved.
While we did not formally assess the quality of the eHealth technologies (nor the information they contained), it was evident at face-value that they varied across eHealth technologies across all types, developers and content areas. We hypothesize that this may relate to the fact that little guidance exists in developing or evaluating CAM-related eHealth technologies, as the growth of research conducted in this area has been slow and the information limited. While there have been discussions surrounding the creation of comprehensive information resources about CAM, it appears that the most recent ones took place nearly two decades ago [81, 82], based on preliminary searches we conducted prior to finalizing our systematic search strategy. The development of CAM-related eHealth technologies should be viewed as a research method in itself similar to how guidelines for different study types exist [83]. As a result, additional and updated guidance documents are needed in creating eHealth technologies, let alone CAM-related ones, as to date only one appears to exist [84].
Strengths and limitations
Notable strengths of this study included the use of a comprehensive systematic search strategy to identify eligible articles, devised with the assistance of an experienced academic librarian. Interpretation of these findings was strengthened by the fact that all three authors independently screened, data extracted, and summarized the findings.
Limitations include the fact that this scoping review did not include non-English language articles, which perhaps would have been of importance given our finding that there is an emergence of new CAM-related eHealth technologies in Asia, among other countries. Additionally, it should be noted that many apps exist that likely also detect CAM-related adverse events or side effects which were not captured by this search. Though this is a limitation, it can be inferenced that higher-quality apps (or at least those that have been exposed to peer-review) would be those that would be captured in the published literature, which would be of greater use to healthcare providers and researchers as the primary audience of this review. Furthermore, a very large number of poor-quality apps exist on platforms such as the Google Play Store and the Apple App Store, and it would not be practical nor feasible to review them all.
Conclusion
The present scoping review involved a systematic search of the literature to identify eHealth technologies assisting in identifying potential 1) adverse drug interactions with CAM, 2) adverse CAM-CAM interactions, or 3) standalone CAM adverse events or side effects. Having identified 69 unique eHealth technologies that fall into this category, we provide a descriptive map of the literature on this area and highlight a number of key themes that emerged from our analysis. Additionally, we highlight that while an ample number of resources are available to healthcare providers, researchers, and patients, we caution that the quality and update frequency for many of these resources vary widely, and until formally assessed, remain unknown. Lastly, we identify that a need exists to conduct an updated and systematically-searched review of CAM-related healthcare or research resources, as well as develop guidance documents associated with the development and evaluation of CAM-related eHealth technologies.
Acknowledgements
We gratefully acknowledge librarian Laura Banfield for providing guidance in constructing the search strategy. JYN was awarded a Research Scholarship and an Entrance Scholarship from the Department of Health Research Methods, Evidence and Impact, Faculty of Health Sciences at McMaster University.
Abbreviations
- CADTH
Canadian Agency for Drugs and Technologies in Health
- CAM
Complementary and alternative medicine
- CAHTS
Computer-assisted history-taking systems
- CDSS
Computerized decision support systems
- mHealth
Mobile health
- NCCIH
National Center for Complementary and Integrative Health
- NHP
Natural health product
Appendix 1
Sample Search Strategy
MEDLINE Search Strategy for Scoping Review of eHealth Technologies Assisting in Identifying Potential 1) Adverse Drug Interactions with CAM, 1) Adverse CAM-CAM Interactions or 3) Standalone CAM Adverse Events or Side Effects Executed November 6, 2019
|
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions(R) <1946 to November 05, 2019> Search Strategy: | |
|
1 ((Alternative or Traditional or Complementary or Integrat*) adj2 (Therap* or Medicine*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (103649) 2 Complementary Therapies/ or Integrative Medicine/ or exp Medicine, East Asian Traditional/ or exp Medicine, Chinese Traditional/ or exp Herbal Medicine/ or exp Plants, Medicinal/ or exp Phytotherapy/ or exp Drugs, Chinese Herbal/ or exp Medicine, Ayurvedic/ (151984) 3 (CAM or TCM or Traditional Chinese Medicine or Ayurved* or Medicinal Plant* or Herbalism).mp. (69971) 4 (Herb* adj1 (Medic* or Therap* or Supplement*)).mp. (23276) 5 or/1-4 (248865) 6 (Telemedicine or Telehealth or eHealth or e-Health or mHealth or m-Health or Mobile Health or Health Records, Personal or Mobile Application* or E-Prescription* or Electronic Prescri* or Electronic Health Record* or Electronic Medical Record* or Medical Records System* or Health Informatics or Medical Informatics or Computeri?ed Decision Support or Data Mining or Decision Support System* or Wearable Electronic Device* or Wearable Technolog* or Smartphone or Iphone or I-phone or Android or Handheld Computer or Personal* Digital or Deep Learning or Artificial Intelligence).mp. (166979) 7 Telemedicine/ or Health Records, Personal/ or Mobile Applications/ or Electronic Prescribing/ or Medical Records Systems, Computerized/ or Medical Informatics/ or Drug Therapy, Computer-Assisted/ or Drug Information Services/ or Hospital Information System/ or Computing Methodologies/ or Wearable Electronic Devices/ or Artificial Intelligence/ or "Neural Networks (Computer)"/ (112744) 8 or/6-7 (199016) 9 5 and 8 (1157) 10 limit 9 to (english language and yr="1995 -Current") (815) *************************** |
Appendix 2
Fig. 1.
PRISMA Diagram. *List of Abbreviations: AMED = Allied and Complementary Medicine Database, CADTH = Canadian Agency for Drugs and Technologies in Health
Appendix 3
Data Extraction Tables
Table 1
Table 1.
Eligible article characteristics (n = 41)
| First Author and Year | Article title | Study Country | Study Design | Article type | Study aim |
|---|---|---|---|---|---|
| ORIGINAL RESEARCH ARTICLES (n = 27) | |||||
| Archer et al. 2014 [35] | Development of an alert system to detect drug interactions with herbal supplements using medical record data | USA | Development of alert system prototype | Original Research | To develop an automated herb-drug interaction alert system prototype designed to alert physicians and patients of potential herb-drug interactions |
| Boehmer et al. 2011 [53] | Evaluating the value of a web-based natural medicine clinical decision tool at an academic medical center | USA | Evaluation of web-based clinical decision tool | Original Research | To evaluate the impact that a natural medicine clinical decision tool has on faculty attitudes, practice experiences, and needs with respect to herbal and natural products |
| Brink et al. 2004 [60] | Cancer CAM(TM): Web-based continuing education for health professionals | USA | Evaluation of online continuing education prototype module | Original Research | To describe “a formative evaluation of a web-based continuing education program for nurses and patient health educators on CAM for cancer patients” (p. 44) |
| Chen et al. 2002 [29] | Computer automated prediction of potential therapeutic and toxicity protein targets of bioactive compounds from Chinese medicinal plants | Singapore | Evaluation of software | Original Research | To determine the therapeutic and toxicity protein targets of Chinese medicinal plant compounds |
| Chen et al. 2006 [30] | Database of traditional Chinese medicine and its application to studies of mechanism and to prescription validation | China | Development of database and artificial intelligence systems | Original Research | To collect quantitative information about traditional Chinese medicine (TCM) prescriptions, constituent herbs and herbal ingredients to create/test a database to assist with the studying and exploring of TCM |
| Ee et al. 2018 [64] | Herbopolis - A mobile serious game to educate players on herbal medicines | Singapore | Usability study for a mobile game prototype | Original Research | To develop a mobile game to motivate users to learn more about herbal medicine |
| Faubert et al. 2010 [54] | A pilot study to compare natural health product-drug interactions in two databases in Canada | Canada | Evaluation of databases | Original Research | To evaluate and compare two natural health product databases for the purpose of integrating them into a pharmacy information system in Canada |
| Fischer et al. 2005 [44] | Complementary and alternative medical reference software for personal digital assistants: evidence of clinical applicability | USA | Evaluation of databases | Original Research | To evaluate the value and clinical applicability of new complementary and alternative medicine software products |
| Fucik et al. 2002 [49] | Building a computerized herbal substance register for implementation and use in the World Health Organisation International Drug Monitoring Programme | Sweden | Development of database | Original Research | To build a computerized herbal substance register for implementation and use in the World Health Organisation International Drug Monitoring Programme |
| Gao et al. 2015 [59] | Pharmacovigilance in China: Issues of concern identified through an analysis of the Chinese Adverse Drug Reaction Information Bulletin 2001 to 2014 | China | Analysis of Adverse Drug Reaction Information Bulletin (ADRIB) reports | Original Research | To analyse the reports in the ADRIB since its first publication in 2001 to give international readers a better appreciation of the pharmacovigilance issues addressed |
| Gregory et al. 2016 [42] | Characterization of complementary and alternative medicine-related consultations in an academic drug information service | USA | Analysis of complementary and alternative medicine drug information consultations | Original Research | “To evaluate and characterize consultation requests received through our academic drug information consultation service related to complementary and alternative medicines” (p. 540) |
| Hamre et al. 2017 [61] | Use and safety of anthroposophic medicinal products: An analysis of 44,662 patients from the EvaMed Pharmacovigilance Network | Germany | Analysis of EvaMed Pharmacovigilance Network diagnoses and prescriptions data | Original Research | To determine the frequency of adverse drug reactions to anthroposophic medicinal products (AMPs), relative to the number of AMP prescriptions. |
| Kim et al. 2019 [45] | Drug repositioning of herbal compounds via a machine-learning approach | Republic of Korea | Development of algorithm | Original Research | “To predict new indications for existing drugs and additional herbal compounds based on a machine-learning approach” (p. 34) |
| Lee et al. 2015 [40] | PharmDB-K: Integrated bio-pharmacological network database for traditional Korean medicine | Republic of Korea | Development of database | Original Research | To construct PharmDB-K, which offers comprehensive information relating to Traditional Korean Medicine-associated drugs (compound), disease indication, and protein relationships |
| Li et al. 2009 [26] | A web-based quantitative signal detection system on adverse drug reaction in China | China | Development of signal detection system | Original Research | “To establish a web-based quantitative signal detection system for adverse drug reactions based on spontaneous reporting to the Guangdong province drug monitoring database in China” (p. 729) |
| Ogultarhan et al. 2016 [36] | KATIS: An eHealth system for complementary medicine | Germany | Development of database and mobile app | Original Research | To aggregate knowledge on complementary and alternative medicine (CAM) into one database to allow for the search of CAM therapies, indications and interactions |
| Olesen et al. 2013 [43] | Absence of ‘over-the-counter’ medicinal products in on-line prescription records: A risk factor of overlooking interactions in the elderly | Denmark | Analysis of online prescription record data | Original Research | “To assess possible origins of harmful interactions in elderly patients arising from the current absence of information on over-the counter (OTC) medicines in the Danish ‘on-line prescription record’” (p. 145) |
| Spanakis et al. 2019 [46] | PharmActa: Empowering patients to avoid clinical significant drug-herb interactions | Greece | Evaluation of mobile app | Original Research | To discuss the use of personal health services and mobile health applications for patients and healthcare providers to avoid and manage drug-herb interactions, and to discuss a recently developed personalized pharmaceutical mobile health application called PharmActa |
| Sun et al. 2019 [55] | Development of quantitative structure-activity relationship models to predict potential nephrotoxic ingredients in traditional Chinese medicines | China | Development and testing of model | Original Research | To develop a quantitative structure-activity relationship models to predict potential nephrotoxic ingredients in traditional Chinese medicines |
| Tabali et al. 2012 [62] | Adverse drug reactions for CAM and conventional drugs detected in a network of physicians certified to prescribe CAM drugs | Germany | Analysis of adverse drug reactions in database | Original Research | “To describe and quantify the volume and severity of adverse drug reactions for complementary and alternative medicine (CAM) and conventional drugs in a proprietary database created from prescriptions and patient data of primary care CAM physicians who participate in the EvaMed Network” (p. 427) |
| Walker 2002 [51] | Evaluation of the ability of seven herbal resources to answer questions about herbal products asked in drug information centers | USA | Evaluation of databases | Original Research | “To evaluate the ability of seven widely known herbal references and electronic databases to answer questions about herbal products asked at drug information centers” (p. 1611) |
| Woo et al. 2019 [31] | Safety of herbal medicine for elderly patients with chronic disease in the Republic of Korea | Republic of Korea | Analysis of spontaneous adverse event reports | Original Research | To investigate the detection of drug safety signals associated with herbal medicine by analyzing spontaneous adverse event reports in elderly patients with chronic diseases to generate new safety information |
| Xu et al. 2019 [47] | ETCM: An encyclopaedia of traditional Chinese medicine | China | Development of web-based encyclopedia | Original Research | To develop an online encyclopedia to provide users information on traditional Chinese medicine herbs and formulas |
| Yao et al. 2019 [63] | An ontology-based artificial intelligence model for medicine side-effect prediction: taking traditional Chinese medicine as an example | Australia, China, Germany | Development of artificial intelligence | Original Research | To develop an ontology-based model for artificial intelligence-assisted medicine side-effect prediction, and validate a proposed model consisting of three main components, including the drug model, the treatment model, and the artificial intelligence-assisted prediction model |
| Yap et al. 2012 [39] | Utilizing mobile networks for the detection of clinically relevant interactions between chemotherapy regimens and complementary and alternative medicines | Singapore | Development of an iPhone app | Original Research | “To develop a novel database application for the Mobile Internet called OncoRx-MI, the purpose of which is to detect drug-complementary and alternative medicine interactions of both single-agent and multiple-agent chemotherapy regimen prescriptions” (p. 166) |
| Ye et al. 2009 [34] | A computerized system for signal detection in spontaneous reporting system of Shanghai China | China | Development of signal detection system and reporting system | Original Research | To develop a computerized signal detection system to detect adverse drug reactions |
| Zhang 2018 [27] | Pharmacovigilance of herbal and traditional medicines | China | Analysis of pharmacovigilance of herbal and traditional medicines | Original Research | To differentiate the concepts of traditional/complementary medicine and their products, and introduce the supervision and management systems of the China Food and Drug Administration and the differences between conventional medicine and traditional/complementary medicine products, taking drugs used in traditional Chinese medicine as an example |
| REVIEWS (n = 14) | |||||
| Allais et al. 2000 [65] | Access to databases in complementary medicine | Italy | Review of medical information resource(s) | Review | To review and categorize biomedical databases for complementary and alternative medicine |
| Boddy et al. 2008 [52] | Review of reliable information sources related to integrative oncology | UK | Review of medical information resource(s) | Review | “To provide an overview of reliable integrative oncology information from various resources.” (p. 620) |
| Clauson et al. 2008 [37] | Clinical decision support tools: Personal digital assistant versus online dietary supplement databases | USA | Review and evaluation of databases and personal digital assistants | Review | To “assess and compare the content of PDA dietary supplement databases and their online counterparts used as clinical support decision tools” p. 1593 |
| Fitzpatrick 2010 [38] | Natural standard database | USA | Review of medical information resource(s) | Review | To “provide an overview of Natural Standard and its content and scope, as well as provide some basics for searching this resource”(p. 154) |
| Jackson 2001 [50] | An overview of information resources for herbal medicinals and dietary supplements | USA | Review of medical information resource(s) | Review | To “provide a comprehensive, annotated listing of reliable resources of information on herbs and dietary supplements divided into the following categories: journals, databases and websites, and books and compendia” (p. 36) |
| Jackson et al. 2001 [25] | Resources for information on herbal medicinals and dietary supplements | USA | Review of medical information resource(s) | Review | To assess information on herbal medicinals and dietary supplements on AltMedDex |
| Kiefer et al. 2001 [32] | Finding information on herbal therapy: a guide to useful sources for clinicians | USA | Review of medical information resource(s) | Review | To provide primary care clinicians with a list of general, clinically oriented, evidence-based, English language references for Western herbal therapeutics that may be of practical use in the clinical setting |
| Meyer et al. 2004 [33] | Evaluation of herbal-drug interaction data in tertiary resources | USA | Review of medical information resource(s) | Review | To objectively evaluate various tertiary resources using a set of predetermined criteria to assess which provide the most complete, current, and accurate herbal-drug interaction information |
| Molassiotis & Zu 2004 [58] | Quality and safety issues of web-based information about herbal medicines in the treatment of cancer | China, UK | Review of medical information resource(s) | Review | To assess the quality and safety of the information presented on the internet about medicinal herbs specifically in the field of cancer |
| Motl et al. 2004 [28] | Health information websites by therapeutic category for healthcare professionals | USA | Review of medical information resource(s) | Review | “To compile and evaluate health information websites to aid healthcare professionals in locating information on select therapeutic categories” (p. 106) |
| Sweet et al. 2003 [56] | Usefulness of herbal and dietary supplement references | USA | Review of medical information resource(s) | Review | “To describe the usefulness of some of the most common tertiary references that healthcare professionals employ to answer requests about herbal and dietary supplements” (p. 494) |
| Tomasulo 2003 [57] | Natural Standard--New integrative medicine database | USA | Review of medical information resource(s) | Review | To provide an overview of the Natural Medicines (Natural Standards) database |
| Wootton 1997 [48] | Directory of databases for research into alternative and complementary medicine: An update | USA | Review of medical information resource(s) | Review | To provide a directory of databases for research into complementary and alternative medicine |
| Xie et al. 2015 [41] | Review of natural product databases | China | Review of medical information resource(s) | Review | To provide an overview and analysis of current natural product databases and discuss trends of future database development |
Table 2
Table 2.
eHealth technology characteristics (n = 69)
| Name of eHealth Technology | Source Eligible Article(s) Included in Review | URL of eHealth Technology? | Type of eHealth Technology | Format | Year Established | Still in Existence? | Used Outside of the Authors’ Study? | Available for Anyone to Use? | Developer of eHealth Technology | Type of Developer | Purpose of eHealth Technology | Intended User(s) of eHealth Technology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Drug/Event Alerting, Reporting and/or Signal Detection Systems (n = 11) | ||||||||||||
| Center for Food Safety and Applied Nutrition | Jackson et al. 2001 [25] | https://www.fda.gov/about-fda/office-foods-and-veterinary-medicine/center-food-safety-and-applied-nutrition-cfsan | Adverse event reporting tool and safety alerts | Website | 1984 | Yes | Yes | Yes, entirely free | U.S. Food and Drug Administration | Government | Website containing safety alert publications, guide documents and a reporting tool for dietary supplement adverse events | Healthcare providers, researchers, patients/public |
| Guangdong Quantitative Signal Detection System (GDQSDS) | Li et al. 2009 [26]; Zhang 2018 [27] | No | Adverse drug reaction signal detection and spontaneous reporting system | Web-based | Unclear | Unclear | Yes | Unclear | Authors | Researchers (authors) | “A web-based system comprising three software modules that prepare data, detect associations, and generate reports, was developed based on the Guangdong ADR monitoring platform” p. 730 | Researchers |
| Institute for Safe Medication Practices (ISMP) | Motl et al. 2004 [28] | https://www.ismp.org | Adverse event reporting system | Website | 1995 (website) | Yes | Yes | Yes, entirely free | The Institute for Safe Medication Practices (ISMP) | Not-for-Profit | Contains a medication error reporting program, and publishes newsletters, reports and safety alerts based on information received | Healthcare providers, patients/public |
| INVDOCK | Chen et al. 2002 [29]; Chen et al. 2006 [30] | Unclear | Adverse drug reaction signal detection | Software | 2001 | Yes | Yes | Unclear | Chen & Zhi (2001). See: 10.1002/1097-0134(20010501)43:2%3C217::AID-PROT1032%3E3.0.CO;2-G | Researchers | Software that allows for “computer-aided identification of potential protein targets of a small molecule” (Chen & Zhi 2001, p. 225 [85]) | Researchers |
| Korea Institute of Drug Safety and Risk Management Korea Adverse Event Reporting System database (KIDS-KD) | Woo et al. 2019 [31] | Unclear | Adverse event reporting system and database | Unclear | 2012 | Unclear | Yes | Unclear | Korean Institute of Drug Safety & Risk Management (KIDS) | Researchers/Government | An adverse drug reaction reporting system | Healthcare providers |
| MedWatch | Jackson et al. 2001 [25]; Kiefer et al. 2001 [32]; Meyer et al. 2004 [33]; Motl et al. 2004 [28] | https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/medical-product-safety-information | Adverse event reporting tool and safety reports | Website | Unclear | Yes | Yes | Yes, entirely free | U.S. Food and Drug Administration | Government | Safety alerts on drugs, natural products and other medical products | Healthcare providers, researchers, patients/public |
| National Coordination Centre, Pharmacovigilance Programme of India (NCC-PvPI) Database | Zhang 2018 [27] | No | Adverse drug reaction reporting system | Unclear | 2010 | Yes | Yes | Unclear | Government of India | Government | To conduct traditional medicines surveillance though a drug adverse event reporting system which reports to Vigibase | Healthcare providers, researchers, health organizations, patients/public |
| Shanghai Adverse Drug Reaction Spontaneous Reporting System (ADR-SRS) | Ye et al. 2009 [34] | Unclear | Adverse drug reaction detection/spontaneous reporting system and database | Unclear | 2001 | Yes | Yes | Unclear | National Adverse Drug Reaction Monitoring Center of China | Government | Web-based ADR detection system and reporting tool for TCM and chemical medicine | Healthcare providers, pharmaceutical manufacturers, researchers |
| Shanghai Drug Monitoring and Evaluative System (SDMES) | Ye et al. 2009 [34]; Zhang 2018 [27] | Unclear | Adverse drug reaction surveillance system | Unclear | 2001 | Unclear | Yes | Unclear | Shanghai Center for Adverse Drug Reaction Monitoring | Government | To locally monitor marketed drugs (including herbs) by working in partnership with ten hospitals in Shanghai that permit direct access to patient adverse drug reaction information | Researchers |
| Special Nutritionals Adverse Event Monitoring System | Jackson et al. 2001 [25] | Unclear | Adverse event reporting system | Web-based | Unclear | Unclear | Yes | Yes, entirely free | U.S. Food and Drug Administration | Government | Collects adverse event reports on dietary supplements, infant formulas, and medical foods from a variety of sources | Healthcare providers, researchers, patients/public |
| Unnamed | Archer et al. 2014 [35] | No | Adverse drug reaction alerting system | Unclear | Unclear | Unclear | Unclear | Unclear | Authors | Researchers (authors) | To automatically detect herb-drug interactions and classify their severity | Healthcare providers, researchers |
| Yellow Card Scheme | Ye et al. 2009 [34] | https://yellowcard.mhra.gov.uk/ | Adverse event reporting system | Website | Unclear | Yes | Yes | Yes, entirely free | Medicines and Healthcare Products Regulatory Agency | Researchers/Government | A reporting tool that monitors the safety of healthcare products, including herbal products, in the UK | Healthcare providers (including pharmacists), patients/public |
| Databases (n = 34) | ||||||||||||
| ABDAMED | Ogultarhan et al. 2016 [36] | https://abdata.de/datenangebot/abdamed/ | Database | Website | Unclear | Yes | Yes | Unclear | ABDATA Pharma Data Service | Company | “ABDAMED is a commercial pharmaceutical database, which contains approved drug-related data, such as active ingredients, excipients, risks, indications, contraindications and adverse effects” (Ogultarhan et al. 2016, p. 169 [36]) | Healthcare providers, researchers, patients |
| Alticopeia Herbal Medicine Database | Clauson et al. 2008 [37]; Fischer et al. 2005 [44] | http://www.ddhsoftware.com/gallery.html?show=number&record=527 | Database | Mobile app | 2001 | Yes | Yes | Yes, entirely free | DDH Software | Company | “To facilitate understanding of herbal medicine by physicians and other ‘traditional’ health care workers.” (See: http://www.ddhsoftware.com/gallery.html?show=number&record=527) | Healthcare providers |
| ALTMEDA | Ogultarhan et al. 2016 [36] | Unclear | Database | Unclear | 2016 | Unclear | Unclear | Unclear | Authors | Researchers (authors) | A database that includes complementary and drug-related data (Ogultarhan et al. 2016, p. 167 [36]) | Healthcare providers, researchers |
| American Botanical Council | Fitzpatrick 2010 [38]; Jackson et al. 2001 [25]; Kiefer et al. 2001 [32]; Motl et al. 2004 [28] | http://abc.herbalgram.org/site/PageServer | Database | Website | 1988 | Yes | Yes | Yes, partially without subscription | American Botanical Council | Council | A nonprofit, research and education organization, providing access to several databases (i.e. HerbMedPro, the Complete German Commission E Monographs) among other herbal resources (See: www.herbalgram.org) | Healthcare providers, researchers, patients/public |
| Caremark Drug Interactions | Yap et al. 2012 [39] | http://cpref.goldstandard.com/inter.asp?r=8084 | Database | Website | Unclear | Yes | Yes | Yes, entirely free | Gold standard Inc. | Company | A tool for checking drug, herb and vitamin interactions | Patients/public |
| China Natural Products Database (CNPD) | Lee et al. 2015 [40] | Unclear | Database | Unclear | Unclear | Unclear | Unclear | Unclear | No information | Unclear | No information available | Researchers |
| Chinese Ethnic Minority Traditional Drug Database (CEMTDD) | Xie et al. 2015 [41] | http://www.cemtdd.com/ (inaccessible) | Database | Website | 2015 | Unclear | Yes | Unclear | Jinhui Wang | Researchers | A database containing Chinese minority herbs, built on data retrieved from various resources (mainly from Kazakh and Uygur traditional drugs) containing a variety of modules p. 399 | Healthcare providers, researchers |
| Clinical Pharmacology (also known as Gold Standard) | Gregory et al. 2016 [42] | https://www.clinicalpharmacology.com/ | Database | Website | Unclear | Yes | Yes | Yes, but only with a subscription | Elsevier | Company | Monographs, reports, guides, resources and drug information of prescription drugs and herbal/nutritional products | Healthcare providers, researchers |
| Danish National Drug Interaction Database | Olesen et al. 2013 [43] | http://www.interaktionsdatabasen.dk/ | Database | Website | 2003 | Yes | Yes | Yes, entirely free | Danish Medicines Agency | Government | “The Danish Drug Interaction database is an electronic search tool for searching and learning about the effects of and adverse reactions from taking two or more different kinds of medication” (See: https://www.danishhealthdata.com/find-health-data/Interaktionsdatabasen) | Healthcare providers |
| DoubleCheckMD | Yap et al. 2012 [39] | Unclear | Database | Unclear | Unclear | No | Yes | Unclear | Enhanced Medical Decisions Inc. | Company | Tool for users to check for drug interactions and side effects | Healthcare providers, patients/public |
| DrDrugs | Clauson et al. 2008 [37]; Fischer et al. 2005 [44] | https://www.skyscape.com/product/drdrugs-drug-guide-for-physicians | Database | Website and mobile app | Unclear | Yes | Yes | Yes, but only with a subscription | Skyscape | Company | Drug information, dosing calculators, monographs, safety alerts | Healthcare providers |
| Drug Information (formerly DrugDigest) | Motl et al. 2004 [28] | https://www.express-scripts.com/medco/consumer/ehealth/druginfo/dlmain.jsp?WC=N | Database | Website | Unclear | Yes | Yes | Yes, entirely free | Express Scripts Inc | Company | To provide consumers with information on drugs and CAM supplements, including adverse effects | Patients/public |
| Drug Product Database | Motl et al. 2004 [28] | https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html | Database | Website | Unclear | Yes | Yes | Yes, entirely free | Health Canada | Government | Search tool for drugs and certain natural health products in Canada containing monographs | Healthcare providers, patients/public |
| Drugbank | Kim et al. 2019 [45]; Lee et al. 2015 [40]; Spanakis et al. 2019 [46] | https://www.drugbank.ca/ | Database | Website | 2006 | Yes | Yes | Yes, entirely free | Wishart Research Group | Researchers | A bioinformatics and cheminformatics resource that combines detailed drug data with comprehensive drug target information | Healthcare providers, researchers, patients/public |
| Encyclopedia of Traditional Chinese Medicine (ETCM) | Xu et al. 2019 [47] | http://www.nrc.ac.cn:9090/ETCM/ | Database | Website | Unclear | Yes | Yes | Yes, entirely free | Authors | Researchers (authors) | Contains information and data on traditional Chinese medicine herbal drugs, formulas, predicted drug targets and diseases | Healthcare providers, researchers |
| Epocrates Rx (plus or pro) | Clauson et al. 2008 [37]; Fischer et al. 2005 [44]; Yap et al. 2012 [39] | https://online.epocrates.com/ | Database | Website and mobile app | Unclear | Yes | Yes | Yes, but only with a subscription | Athenahealth | Company | Provides monographs, a drug interaction checker, pill identifier, dosing calculators, and formularies | Healthcare providers |
| Guide to Popular Natural Products | Clauson et al. 2008 [37]; Fischer et al. 2005 [44] | http://www.skyscape.com/EStore/ProductDetail.aspx?ProductID=951 (inaccessible) | Database | Mobile app | Unclear | No | Yes | Unclear | Skyscape | Company | No information available | Unclear |
| IBIS: Integrative BodyMind Information System | Allais et al. 2000 [65]; Kiefer et al. 2001 [32]; Wootton 1997 [48] | http://www.ouribis.com/ | Database | Software | 1992 | Yes | Yes | Yes, but only with a subscription | Medicineworks | Company | Provides clinical reference database with drug-herb and drug-nutrient interactions, among other information about nutritional supplements and botanical preparations | Healthcare providers, researchers |
| International Drug Information System (INTDIS) | Fucik et al. 2002 [49] | No | Database | Unclear | 1978 | No (has been replaced by Vigibase) | Yes | No | World Health Organization International Drug Monitoring Program | Government | To collect and store information on herbal medicines, allopathic drugs and adverse drug reactions from various countries | National centers (i.e. World Health Organization), researchers, pharmaceutical companies |
| Lexi-Natural (includes LexiComp) | Clauson et al. 2008 [37]; Gregory et al. 2016 [42]; Fischer et al. 2005 [44]; Motl et al. 2004 [28]; Yap et al. 2012 [39] | http://webstore.lexi.com/Store/Individual-Databases/Lexi-Natural-Products | Database | Website and mobile app | Unclear | Yes | Yes | Yes, but only with a subscription | Wolters Kluwer | Company | Provides information on natural products, including interactions, adverse reactions, toxicology, etc. and includes monographs | Healthcare providers (including pharmacists, physicians, nurses, dentists), researchers |
| MedicinesComplete (includes Herbal Medicines; formerly known as the British National Formulary) | Jackson 2001 [50]; Jackson et al. 2001 [25]; Yap et al. 2012 [39] | https://about.medicinescomplete.com/publication/herbal-medicines/ | Database | Website | Unclear | Yes | Yes | Yes, but only with a subscription | Pharmaceutical Press Editorial, The Royal Pharmaceutical Society | Company | Resources on herbal medicines including “uses, dosage, evidence of efficacy, adverse effects, contraindications, use in pregnancy and lactation, drug interactions” (See: https://www.pharmpress.com/product/MC_HERB/herbal-medicines) | Healthcare providers (including pharmacists) |
| Micromedex (includes DrugDex, Drug-Reax, AltMedDex) | Clauson et al. 2008 [37]; Fischer et al. 2005 [44]; Jackson 2001 [50]; Jackson et al. 2001 [25]; Kiefer 2001 [32]; Meyer et al. 2004 [33]; Walker 2002 [51]; Yap et al. 2012 [39] | https://www.micromedexsolutions.com/home/dispatch/ssl/true | Database | Website and mobile app | mid-1970s | Yes | Yes | Yes, but only with a subscription | IBM | Company | Contains evidence-based documents on prescription and non-prescription drugs; contains information such as dosing, adverse effects, interactions, warnings and efficacy | Healthcare providers, researchers |
| Natural Medicines (formerly Natural Medicine Comprehensive Database (NMCD) and Natural Standard Database (NSD)) | Archer et al. 2014 [35]; Boddy et al. 2011 [52]; Boehmer et al. 2011 [53]; Clauson et al. 2008 [37]; Faubert et al. 2010 [54]; Fischer et al. 2005 [44]; Fitzpatrick 2010 [38]; Gregory et al. 2016 [42]; Jackson et al. 2001 [25]; Kiefer et al. 2001 [32]; Motl et al. 2004 [28]; Sun et al. 2019 [55]; Sweet al al. 2003 [56]; Tomasulo 2003 [57]; Walker 2002 [51]; Yap et al. 2012 [39] | https://naturalmedicines.therapeuticresearch.com/ | Database | Website and mobile app | early 2000s | Yes | Yes | Yes, but only with a subscription | Therapeutic Research Center | Company | Contains information about herbs and dietary supplements, including 15 categories of information which address the most common questions faced by practitioners; also provides interactive tools for safety, effectiveness and interactions | Healthcare providers, researchers, patients |
| OncoRx Database (called Onco-Rx as a website, and OncoRx-MI as a mobile app) | Yap et al. 2012 [39] | http://www.onco-informatics.com/oncorx/ | Database | Website and mobile app | 2007 (website), unclear (mobile app) | Yes | Yes | Yes, but only with a subscription | Dr. Kevin Yap Research Group | Researchers | Detects drug-CAM interactions (DCIs) and provides information on “interaction effects, severities, mechanisms of interactions, substantiating evidences and references, as well as overall management plans for the chemotherapy regimens” (Yap et al. 2012, p. 167 [39]) | Healthcare providers, researchers, patients/public |
| PEPID Drug Information Database | Clauson et al. 2008 [37]; Fischer et al. 2005 [44] | https://www.pepid.com/ | Database | Website and mobile app | Unclear | Yes | Yes | Yes, but only with a subscription | PEPID | Company | Clinical decision suites for a variety of healthcare providers, providing information on drugs, herbal medicines and supplements, and an Interaction checker and toxicology resources | Healthcare providers |
| PharmDB-K | Lee et al. 2015 [40] | http://pharmdb-k.org/ | Database | Website | Unclear | Yes | Yes | Yes, entirely free | Authors, Information Center for Bio-pharmacological Network (i-Pharm) | Researchers (authors) | Provides comprehensive traditional Korean medicine-associated compound, drug, disease indication, and protein relationship information | Researchers |
| PharmGKB | Yap et al. 2012 [39] | https://www.pharmgkb.org/ | Database | Website | 2001 | Yes | Yes | Yes, entirely free | Shriram Center for Bioengineering and Chemical Engineering | Researchers | A pharmacogenomics knowledge resource providing clinical information including clinical guidelines and drug labels, potentially clinically actionable gene-drug associations and genotype-phenotype relationships | Healthcare providers (including pharmacists), researchers |
| Tarascon Pocket Pharmacopoeia | Clauson et al. 2008 [37]; Fischer et al. 2005 [44] | https://www.tarascon.com/products/mobile/ | Database | Mobile app | Unclear | Yes | Yes | Yes, but only with a subscription | Tarascon Publishing | Company | Contains drug information to help clinicians make better decisions at the point of care | Healthcare providers |
| TCM Assistant | Yap et al. 2012 [39] | http://www.tcmassistant.com/ (inaccessible) | Database | Website | 2004 | Unclear | Yes | Unclear | e-MS Inc | Company | Provides resources on traditional Chinese medicine herbs, including herbs name, their formulas, their usage in curing disease and patent description | Healthcare providers, researchers |
| TCM Database@Taiwan | Lee et al. 2015 [40]; Sun et al. 2019 [55]; Xie et al. 2015 [41] | http://tcm.cmu.edu.tw/ (inaccessible) | Database | Website | 2011 | Unclear | Yes | Unclear | Calvin Yu-Chian Chen | Researchers | A database for Chinese traditional medicinal compounds, providing freely downloadable 3D compound structures of ingredients used in traditional Chinese medicine | Researchers |
| TCMGeneDIT | Xu et al. 2019 [47] |
http://tcm.lifescience.ntu.edu.tw/ (inaccessible) |
Database | Website | Unclear | Unclear | Yes | Unclear | Fang et al. 2018 See: 10.1186/1472-6882-8-58 | Researchers | A traditional Chinese medicine database that provides information about genes, diseases, effects and ingredients | Researchers |
| TCM-MESH | Xu et al. 2019 [47] | http://mesh.tcm.microbioinformatics.org/ | Database | Website | Unclear | Yes | Yes | Yes, entirely free | Zhang et al. 2017 See: 10.1038/s41598-017-03039-7 | Researchers | Database that contains TCM-related information on herbs, compounds, diseases and genes. Includes side-effects and has a search tool | Healthcare providers, researchers |
| Traditional Chinese Medicine Information Database (TCM-ID) | Chen et al. 2006 [30]; Lee et al. 2015 [40]; Xu et al. 2019 [47] | http://bidd.nus.edu.sg/group/TCMsite/ | Database | Unclear | 2005 | Yes | Yes | Yes, entirely free | Authors | Researchers (authors) | Provides information about traditional Chinese medicines including prescriptions, constituent herbs, herbal ingredients, molecular structure and functional properties of active ingredients, therapeutic and side effects, and clinical indications | Healthcare providers, researchers |
| Vigibase (includes Vigilyze) | Ye et al. 2009 [34]; Zhang 2018 [27] | https://www.who-umc.org/vigibase/vigibase/ | Database | Website | 1971 | Yes | Yes | Yes, but only with a subscription | WHO Uppsala Monitoring Center, World Health Organization | Government | A global pharmacovigilance database of individual case safety reports, with over 20 million reports of suspected adverse effects of medicines | Healthcare providers (including physicians, dentists, nurses, and pharmacists) |
| Factsheets/Healthcare Information (n = 13) | ||||||||||||
| About Herbs | Boddy et al. 2008 [52]; Molassiotis et al. 2004 [58]; Yap et al. 2012 [39] | https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine | Factsheets/healthcare information | Website and mobile app | Unclear | Yes | Yes | Yes, entirely free | Memorial Sloan-KetteringCancer Center | Researchers | Resources on herbs, botanicals and other CAM drugs. Includes information on CAM interactions | Healthcare providers, researchers, patients |
| ALMEKO | Ogultarhan et al. 2016 [36] | https://play.google.com/store/apps/details?id=de.icancode.almeko&hl=de | Factsheets/healthcare information | Mobile app | 2016 | Yes | Unclear | Unclear | Authors | Researchers (authors) | ALMEKO bundles information on different areas of complementary and alternative medicine, providing an indication check, and overview of potential therapies for the alleviation of symptoms (See: https://apkcombo.com/almeko/de.icancode.almeko/) | Healthcare providers, public |
| AlternativeDr.com | Motl et al. 2004 [28] | http://www.alternativedr.com | Factsheets/healthcare information | Unclear | Unclear | Unclear | Yes | Yes, entirely free | Advisory panel of MDs and experts in natural medicine | Practitioners | “Lists herbal interactions with several prescription and over the-counter medications” (Motl et al. 2004, p. 109 [28]) | Healthcare providers, public |
| Complementary and Alternative Medicine | Boddy et al. 2008 [52] | https://www.cancer.gov/about-cancer/treatment/cam | Factsheets/healthcare information | Website | Unclear | Yes | Yes | Yes, entirely free | National Cancer Institute, National Institutes of Health | Government | Provides summaries on commonly used complementary and alternative medicines for patients and healthcare providers | Healthcare providers, patients/public |
| Dietary Supplement Fact Sheets | Motl et al. 2004 [28] | https://ods.od.nih.gov/factsheets/list-all/ | Factsheets/healthcare information | Website | 1998 | Yes | Yes | Yes, entirely free | Office of Dietary Supplements, National Institute of Health | Government | Contains factsheets on supplements, vitamins and minerals | Healthcare providers, patients/public |
| Herb-Drug Interactions, NCCIH Clinical Digest | Allais et al. 2000 [65]; Boddy et al. 2008 [52]; Gregory et al. 2016 [42]; Jackson et al. 2001 [25]; Motl et al. 2004 [28] | https://nccih.nih.gov/health/providers/digest/herb-drug | Factsheets/healthcare information | Website | 2015 | Yes | Yes | Yes, entirely free | National Center for Complementary and Integrative Health (NCCIH) (formerly the National Center for Complementary and Alternative Medicine (NCCAM)) | Government | Provides healthcare providers with information about several herbs and their potential interactions with other agents | Healthcare providers |
| Herbs at a Glance | Boddy et al. 2008 [52]; Jackson et al. 2001 [25]; Gregory et al. 2016 [42]; Motl et al. 2004 [28] | https://nccih.nih.gov/health/herbsataglance.htm | Factsheets/healthcare information | Website | Unclear | Yes | Yes | Yes, entirely free | National Center for Complementary and Integrative Health (NCCIH) (formerly the National Center for Complementary and Alternative Medicine (NCCAM)) | Government | Provides basic information about specific herbs/botanicals, including common names, scientific evidence, potential side effects and cautions, and resources for more information | Healthcare providers, researchers, patients |
| KATIS (based off ALTMEDA) | Ogultarhan et al. 2016 [36] | http://www.komplementaeremedizin.de | Factsheets/healthcare information | Website | 2016 | Unclear | Unclear | Unclear | Authors | Researchers (authors) | A web-based information system for complementary medicine, designed to assist patients and health care professionals search for alternative therapies efficiently | Healthcare providers, patients |
| Medline Plus | Motl et al. 2004 [28] | https://medlineplus.gov/druginformation.html | Factsheets/healthcare information | Website | 1998 | Yes | Yes | Yes, entirely free | U.S. National Library of Medicine, National Institutes of Health | Government | Information resource on health, drugs and herbal supplements | Patients/public |
| Medscape | Motl et al. 2004 [28]; Spanakis et al. 2019 [46] | https://www.medscape.com/ | Factsheets/healthcare information | Website and mobile app | 1995 | Yes | Yes | Yes, entirely free | WebMD (formerly SCP Communications Inc.) | Company | Provides clinical resources, news, and drug/disease information, as well as a drug interaction checker tool | Healthcare providers |
| PharmActa | Spanakis et al. 2019 [46] | No | Factsheets/healthcare information | Mobile app | Unclear | Unclear | Unclear | No | Authors | Researchers (authors) | An “app for personalized pharmaceutical care with information regarding drug-herbal medicinal products interactions” (Spanakis et al. 2019, p. 1 [46]) | Patients |
| RxList (owned by WebMD) | Motl et al. 2004 [28]; Spanakis et al. 2019 [46] | https://www.rxlist.com/script/main/hp.asp | Factsheets/healthcare information | Website | 1995 | Yes | Yes | Yes, entirely free | WebMD (formerly a team of pharmacists) | Company | An online medical resource providing pharmaceutical information on brand and generic drugs and CAM, including an interaction checker and factsheets | Healthcare providers, patients/public |
| RxMed | Motl et al. 2004 [28] | https://www.rxmed.com | Factsheets/healthcare information | Website | 1994 | Yes | Yes | Yes, entirely free | RxMed Medical Advisory | Company | Provides illness and medication information, including CAM factsheets, as well as access to various medical products and services | Healthcare providers, patients |
| Search Engines (n = 4) | ||||||||||||
| Drugs.com | Motl et al. 2004 [28]; Spanakis et al. 2019 [46]; Yap et al. 2012 [39] | https://www.drugs.com/ | Search engine | Website and mobile app | 2001 | Yes | Yes | Yes, entirely free | Cerner Multum and Thomson Micromedex (and Wolters Kluwer) | Company | Contains information about prescriptions and CAM therapies, as well as an interaction checker tool | Healthcare providers, patients/public |
| Electronic Medicines Compendium (EMC) | Motl et al. 2004 [28] | https://www.medicines.org.uk/emc/ | Search engine | Website | 1999 | Yes | Yes | Yes, entirely free | Datapharm Ltd. | Company | Provides information, including leaflets, on drugs (including CAM) in the UK, as well as an adverse effect reporting tool | Healthcare providers (specifically pharmacists), patients/public |
| European Medicines Agency (EMA) | Spanakis et al. 2019 [46] | https://www.ema.europa.eu/en | Search engine | Website | Unclear | Yes | Yes | Yes, entirely free | European Medicines Agency | Government | Search tool to find information on herbal drugs and provides drug profiles | Healthcare providers, researchers, patients/public |
| Merck Manual | Motl et al. 2004 [28] | https://www.merckmanuals.com/en-ca/ | Search engine | Website | 1999 | Yes | Yes | Yes, entirely free | Merck & Co. Inc. | Company | A search tool that allows users to find information on drugs and supplements (including side effects and interactions) | Healthcare providers, patients/public |
| Other (n = 6) | ||||||||||||
| Adverse Drug Reaction Information Bulletin (ADRIB) | Gao et al. 2015 [59]; Zhang 2018 [27] | http://www.sda.gov.cn/WS01/CL0078/ (inaccessible) | Bulletin | Website | 2001 | Unclear | Yes | Unclear | China Food and Drug Admininstration | Government | Technical bulletin that provides information on ADRs and potential hazardous drugs to highlight current pharmacovigilance concerns in China | Public |
| Cancer CAM™ Web-based continuing education program | Brink et al. 2004 [60] | http://www.nurse-ceus-stat.com/ (inaccessible) | Continuing education module | Web-based | 2004 | Unclear | Unclear | Unclear | HealthMark Multimedia | Company | To provide continuing education to nurses, allied health and health educators on the use of CAM for prostate cancer patients | Healthcare providers (including nurses and allied health professions), health/patient educators |
| Evaluation of Anthroposophical Medicine (EvaMed) Pharmacovigilance Network | Hamre et al. 2017 [61]; Tabali et al. 2012 [62] | No | Electronic Pharmacovigilance System | Unclear | 2004 | Unclear | Yes | No | Havelhoehe Research Institute | Researchers | Online adverse drug reaction reporting tool that connects with the hospital EMR system and integrates into the daily clinical practice of physicians | Healthcare providers, researchers |
| HIV Drug Interactions | Motl et al. 2004 [28] | https://www.hiv-druginteractions.org/checker | Interaction checker | Website | 1999 | Yes | Yes | Yes, entirely free | Liverpool HIV pharmacology group from the University of Liverpool | Researchers | Tool to check for interaction of HIV drugs and other drugs (includes complementary and alternative medicines) | Healthcare providers, researchers, patients |
| Ontology-based Artificial Neural Network Model for Drug Side-Effects | Yao et al. 2019 [63] | No | Model | Artificial Intelligence | 2019 | Unclear | Unclear | Unclear | Authors | Researchers (authors) | An ontology-based model for artificial intelligence-assisted medicine side-effect prediction | Healthcare providers, researchers |
| Herbopolis | Ee et al. 2018 [64] | https://play.google.com/store/apps/details?id=com.herbopolisgame.alpha&hl=en | Serious game | Mobile app | 2017 | Yes | Unclear | Yes, entirely free | Authors | Researchers (authors) | To motivate players to learn more about herbal medicine | Public |
Table 3
Table 3.
Frequency of updates by database identified via eligible articles
| Database name | URL | Frequency of updates |
|---|---|---|
| ABDAMED | https://abdata.de/datenangebot/abdamed/ | The content is updated daily. |
| Alticopeia Herbal Medicine Database | http://www.ddhsoftware.com/gallery.html?show=number&record=527 | The website states that this database was last updated on January 11, 2009. The frequency of updates is not available. |
| ALTMEDA | Unclear | This database cannot be located outside the author’s original publication. The frequency of updates is not available. |
| American Botanical Council | http://abc.herbalgram.org/site/PageServer | Dates of updates to ABC’s product-specific and botanical ingredient monographs range from 2009 to 2019. Information on the frequency of updates made to ABC Clinical Guide to Herbs database is not available. |
| Caremark Drug Interactions | http://cpref.goldstandard.com/inter.asp?r=8084 | Interaction reports list dates of revisions that range from 2016 to 2019. The frequency of updates is not available. |
| China Natural Products Database (CNPD) | Unclear | While this database has been mentioned in other papers, the database cannot be located, and the frequency of updates is not available. |
| Chinese Ethnic Minority Traditional Drug Database (CEMTDD) | http://www.cemtdd.com/ | The database is still publicly available and maintained but no updates are apparent since the database was created 2014. |
| Clinical Pharmacology | https://www.clinicalpharmacology.com/ | The content is updated daily. |
| Danish National Drug Interaction Database | http://www.interaktionsdatabasen.dk/ | The website states that this database is updated “constantly”. The precise frequency of updates is not explicitly listed. |
| DoubleCheckMD | Unclear | While this database has been mentioned in other papers, the database cannot be located, and the frequency of updates is not available. |
| DrDrugs | https://www.skyscape.com/product/drdrugs-drug-guide-for-physicians | The exact frequency of updates will vary based on the publisher’s availability, but drug databases are updated on a monthly or quarterly basis. |
| Drug Information- formerly named DrugDigest | https://www.express-scripts.com/medco/consumer/ehealth/druginfo/dlmain.jsp?WC=N | The website states that the site homepage was last updated on 04/20/2020. However, dates are not listed for the drug profiles in the database, therefore the frequency of updates is not available. |
| Drug Product Database | https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html | The website states that monographs are updated after a marketing drug has been authorized, but update frequency is not explicitly stated. Database appears to be updated up until 2020. |
| DrugBank | https://www.drugbank.ca/ | The content is updated daily. |
| Encyclopedia of Traditional Chinese Medicine (ETCM) | http://www.nrc.ac.cn:9090/ETCM/ | In their paper, Xu et al. 2019 [47] advised that the database would continue to be updated. The database is maintained but the frequency of updates is not explicitly listed. |
| Epocrates Rx (plus or pro) | https://online.epocrates.com/ | The content is updated daily. |
| Guide to Popular Natural Products | http://www.skyscape.com/EStore/ProductDetail.aspx?ProductID=951 (inaccessible) | This database no longer exists. The frequency of updates when the database was in existence is not available. |
| IBIS: Integrative BodyMind Information System | http://www.ouribis.com/ | Update frequency is not mentioned clearly. The database was not working for a period of time, and became available again in 2010, The last update appears to be in 2013. |
| International Drug Information System (INTDIS): | N/A | The INTDIS no longer exists, as it has been replaced by Vigibase. |
| Lexi-Natural (includes LexiComp) | http://webstore.lexi.com/Store/Individual-Databases/Lexi-Natural-Products |
The content of LexiComp is updated daily. It is stated that the online version has been updated 1–5 time(s) a year, but the last update is recorded as June 2018. |
| MedicinesComplete (includes Herbal Medicines; formerly known as the British National Formulary) | https://about.medicinescomplete.com/publication/herbal-medicines/ | MedicinesComplete is updated 3–4 times a year, usually in January, April, July, and October. |
| Micromedex (includes DrugDex, Drug-Reax, AltMedDex) | https://www.micromedexsolutions.com/home/dispatch/ssl/true | The content is updated daily. |
| Natural Medicines (formerly Natural Medicine Comprehensive Database (NMCD) and Natural Standard Database (NSD)) | https://naturalmedicines.therapeuticresearch.com/ | The content is updated daily. |
| OncoRx Database (called Onco-Rx as a website, and OncoRx-MI as a mobile app) | http://www.onco-informatics.com/oncorx/ | The update frequency is not clear, though likely at least annually. The last update was reported in 2019. |
| PEPID Drug Information Database | https://www.pepid.com/ | Update frequency is not mentioned clearly. It was mentioned that the content is updated “continuously” and “regularly”. |
| PharmDB-K | There is no information available. Neither links provided lead to an accessible website. | |
| PharmGKB | https://www.pharmgkb.org | Literature annotations and variant annotations are added in real time as a curator annotates the paper. Very important pharmacogene (VIP) summaries, curated pathways and clinical annotations are reviewed periodically. New clinical guideline annotations and drug label annotations are added as they become evident. Gene, drug and disease data that is automatically retrieved is updated in response to scheduled updates at the external resource. |
| Tarascon Pocket Pharmacopoeia | https://www.tarascon.com/products/mobile/ | The frequency of updates is not clear, but the last update was in 2020. |
| TCM Assistant | http://www.tcmassistant.com/ | There is no information available regarding update frequency, and the website is inaccessible. |
| TCM Database@Taiwan | http://tcm.cmu.edu.tw/ | There is no information available regarding update frequency, and the website is inaccessible. |
| TCMGeneDIT | http://tcm.lifescience.ntu.edu.tw/ | There is no information available regarding update frequency, and the website is inaccessible. |
| TCM-MESH | http://mesh.tcm.microbioinformatics.org/ | There is no information available regarding update frequency, and the website is inaccessible. |
| Traditional Chinese Medicine Information Database (TCM-ID) |
http://bidd.nus.edu.sg/group/TCMsite/ New URL: http://119.3.41.228:8000/tcmid/ 2017 Version: Huang L, Xie D, Yu Y, Liu H, Shi Y, Shi T, Wen C. TCMID 2.0: a comprehensive resource for TCM. Nucleic acids research. 2018 Jan 4;46(D1):D1117–20. |
The first version of TCM-ID was released in 2012. Since this time, however, a second version was released in 2017 (see new URL with accompanying citation). The frequency of updates since 2017 is unclear. |
| Vigibase (includes Vigilyze) | https://www.who-umc.org/vigibase/vigibase/ | VigiBase is continuously updated with reports, sometimes daily, as they are submitted by member countries of the World Health Organization Programme for International Drug Monitoring. Most national centres report quarterly or even more frequently. The date of occurrence is noted when supplied, and the date of entry into VigiBase is recorded. |
Table 4
Table 4.
Databases created and/or updated within the last 3 years identified via eligible articles
| Databases | URL | Summary |
|---|---|---|
| Publicly Available | ||
| American Botanical Council | http://abc.herbalgram.org/site/PageServer | The American Botanical Council provides a searchable database titled “ABC Clinical Guide to Herbs” that includes monographs, clinical overviews, and patient information sheets for various herbs. While access to this database requires a paid subscription, the resource also offers free databases with information on herbal and plant-based ingredients, herb profiles and select herbal monographs. |
| Caremark Drug Interactions | http://cpref.goldstandard.com/inter.asp?r=8084 | Copyrighted through Gold Standard, the Caremark Drug Interactions database includes a drug-interaction search tool. Reports are provided for interactions between drugs, CAM, food, alcohol, and other substances. |
| Danish National Drug Interaction Database | http://www.interaktionsdatabasen.dk/ | The Danish Medicines Agency provides a searchable interactive database for prescription drugs, herbal remedies, vitamins, minerals, and grapefruit juice. |
| Drug Information- formerly named DrugDigest | https://www.express-scripts.com/medco/consumer/ehealth/druginfo/dlmain.jsp?WC=N | The Drug Information database includes a search tool to find monographs and information on medications, herbal drugs, and supplements. While several drugs are indexed in the database, the majority of drugs do not have a monograph available. |
| Drug Product Database | https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html | The Drug Product Database is maintained by Health Canada through the Government of Canada and contains a searchable database for drugs authorized for sale in Canada. While several drugs are indexed in the database, many do not have product monographs available. |
| DrugBank | https://www.drugbank.ca/ | The Drugbank database provides information on drugs and drug/chemical target information. |
| Encyclopedia of Traditional Chinese Medicine (ETCM) | http://www.nrc.ac.cn:9090/ETCM/ | ETCM is an online searchable database that provides data on the ingredients, herbs, and formulas of Traditional Chinese Medicine. |
| PharmGKB | https://www.pharmgkb.org | PharmGKB is a searchable pharmacogenomic database targeted for researchers that provides data on the relationship between genetic variations and drug response. Molecules, genes, or variants can be searched within the database. |
| Traditional Chinese Medicine Information Database (TCM-ID) | http://119.3.41.228:8000/tcmid/ | In its second revision, the TCM-ID database provides information on the relationships between the herbs and formulas and the pharmacology or biochemistry of Traditional Chinese Medicine. |
| Requiring Paid Subscription | ||
| Clinical Pharmacology | https://www.clinicalpharmacology.com/ | Clinical Pharmacology is a drug information resource that is powered by Clinical Key and owned by Gold Standard. The database contains monographs for prescription drugs as well as CAM products. |
| DrDrugs | https://www.skyscape.com/product/drdrugs-drug-guide-for-physicians | DrDrugs provides a mobile and web-based database of monographs for drugs and herbal products. |
| Epocrates Rx (plus or pro) | https://online.epocrates.com/ | Epocrates offers a web-based and mobile version of their database of drug monographs and interaction reports. |
| Lexi-Natural (includes LexiComp) | http://webstore.lexi.com/Store/Individual-Databases/Lexi-Natural-Products | Lexi-Natural is part of the Lexicomp database and includes drug monographs and patient handouts for both prescription drugs and CAM products. |
| MedicinesComplete (includes Herbal Medicines; formerly known as the British National Formulary) | https://about.medicinescomplete.com/publication/herbal-medicines/ | Developed by the Royal Pharmaceutical Society, MedicinesComplete provides a database of drug and CAM monographs and drug interaction reports. |
| Micromedex (includes DrugDex, Drug-Reax, AltMedDex) | https://www.micromedexsolutions.com/home/dispatch/ssl/true | IBM Micromedex provides a searchable database of drug and CAM monographs as well as disease and toxicology |
| Natural Medicines (formerly Natural Medicine Comprehensive Database (NMCD) and Natural Standard Database (NSD)) | https://naturalmedicines.therapeuticresearch.com/ | Natural Medicines is a database with several sub-databases on databases on food, herbs and supplements, herbal combinations, drug manufacturers, sports medicine, health and wellness and a comparative effectiveness database. The database also features tools including an interaction checker, effectiveness checker, nutrient depletion, pregnancy and lactation checker and adverse event checker. |
| OncoRx Database (called Onco-Rx as a website, and OncoRx-MI as a mobile app) | http://www.onco-informatics.com/oncorx/ | Onco Rx is a database of interaction information for oncology drugs, chemotherapy regimens and CAM products. |
| PEPID Drug Information Database | https://www.pepid.com/ | PEPID Knowledgebase is a web-accessible database hosted through PEPID Connect. Features of PEPID Connect include: Drug interaction checker; drug-allergy checker; pill identification; IV Compatibility tool; lab manuals; clinical calculators; drug database; differential diagnosis and symptom checker; news and alerts; PEPID PGx pharmacogenomic tool. |
| Tarascon Pocket Pharmacopoeia | https://www.tarascon.com/products/mobile/ | The Tarascon Pocket Pharmacopoeia database contains drug guides and information on drug interactions. The database comes in a PDA format and also includes information on herbal supplements. |
Authors’ contributions
JYN: conceptualized and designed the study, collected the data, interpreted and analysed the data, drafted the manuscript, and gave final approval of the version to be submitted. MM: collected the data, interpreted and analysed the data, provided contributions and critically revised the manuscript, and gave final approval of the version to be submitted. VM: collected the data, interpreted and analysed the data, provided contributions and critically revised the manuscript, and gave final approval of the version to be submitted. All authors have read and approved the manuscript.
Funding
This study was not funded.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Ethics approval and consent to participate
This study involved a systematic search of the literature only; it did not require ethics approval or consent to participate.
Consent for publication
All authors consent to this manuscript’s publication in the order in which the authors are listed in the cover sheet.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeremy Y. Ng, Email: ngjy2@mcmaster.ca, https://orcid.org/0000-0003-0031-5873;
Maryam Mooghali, Email: mooghalm@mcmaster.ca, https://orcid.org/0000-0001-9347-494X;.
Vanessa Munford, Email: munforvh@mcmaster.ca.
References
- 1.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355(9198):134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Semin Integr Med. 2004;2(2):54–71. [PubMed] [Google Scholar]
- 3.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompav M, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 4.Public Health Agency of Canada . Canada.ca: Government of Canada. 2019. [Google Scholar]
- 5.Esmail N. Complementary and alternative medicine in Canada: trends in use and public attitudes, 1997-2006. Public Policy Sources. 2007;87:1–53. [Google Scholar]
- 6.Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Rep. 2009;(18):1–14. [PubMed]
- 7.Health Canada. Summary report on the consultation on a framework for consumer health products: what we heard [Internet]. Canada.ca: Government of Canada; 2016 [updated 2016 Apr 01; cited 2019 Dec 14]. Available from http://www.hc-sc.gc.ca/dhp-mps/consultation/natur/sum_chpf-som_cpsc-eng.php.
- 8.Ipsos Reid . Natural health product tracking survey - 2010 final report. Ottawa: Health Canada; 2011. [Google Scholar]
- 9.Andersson ML, Böttiger Y, Lindh JD, Wettermark B, Eiermann B. Impact of the drug-drug interaction database SFINX on prevalence of potentially serious drug-drug interactions in primary health care. Eur J Clin Pharmacol. 2013;69(3):565–571. doi: 10.1007/s00228-012-1338-y. [DOI] [PubMed] [Google Scholar]
- 10.Hammar T, Lidström B, Petersson G, Gustafson Y, Eiermann B. Potential drug-related problems detected by electronic expert support system: physicians’ views on clinical relevance. Int J Clin Pharm. 2015;37(5):941–948. doi: 10.1007/s11096-015-0146-8. [DOI] [PubMed] [Google Scholar]
- 11.Apidi NA, Murugiah MK, Muthuveloo R, Soh YC, Caruso V, Patel R, et al. Mobile medical applications for dosage recommendation, drug adverse reaction, and drug interaction: review and comparison. Ther Innov Regul Sci. 2017;51(4):480–485. doi: 10.1177/2168479017696266. [DOI] [PubMed] [Google Scholar]
- 12.Kim BY, Sharafoddini A, Tran N, Wen EY, Lee J. Consumer mobile apps for potential drug-drug interaction check: systematic review and content analysis using the mobile app rating scale (MARS) JMIR Mhealth and Uhealth. 2018;6(3):e74. doi: 10.2196/mhealth.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen M, Rowntree J, Young A, Pearson S, Smith J, Gibson O, et al. Chemotherapy side-effect management using mobile phones. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5152–5155. doi: 10.1109/IEMBS.2008.4650374. [DOI] [PubMed] [Google Scholar]
- 14.Car J, Tan WS, Huang Z, Sloot P, Franklin BD. eHealth in the future of medications management: personalisation, monitoring and adherence. eHealth in the future of medications management: personalisation, monitoring and adherence. BMC Med. 2017;15(1):73. doi: 10.1186/s12916-017-0838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 16.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2016;13(1):48. doi: 10.1186/1471-2288-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh H, Rizo C, Enkin M, Jadad A. What is eHealth (3): a systematic review of published definitions. J Med Internet Res. 2005;7(1):e1. doi: 10.2196/jmir.7.1.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw T, Mcgregor D, Brunner M, Keep M, Janssen A, Barnet S. What is eHealth (6)? Development of a conceptual model for eHealth: qualitative study with key informants. J Med Internet Res. 2017;19(10):e324. doi: 10.2196/jmir.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng JY, Boon HS, Thompson AK, Whitehead CR. Making sense of “alternative”, “complementary”, “unconventional” and “integrative” medicine: exploring the terms and meanings through a textual analysis. BMC Complement Altern Med. 2016;16(1):134. doi: 10.1186/s12906-016-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Complementary and Integrative Health (NCCIH). Complementary, alternative, or integrative health: what's in a name? [Internet] U.S. Department of Health and Human Services; 2018 [updated 2018 Jul; cited 2019 Dec 14]. Available from: https://nccih.nih.gov/health/integrative-health.
- 22.Wieland LS, Manheimer E, Sampson M, Barnabas JP, Bouter LM, Cho K, et al. Bibliometric and content analysis of the Cochrane complementary medicine field specialized register of controlled trials. Syst Rev. 2013;2(1):51. doi: 10.1186/2046-4053-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) Safety monitoring of medicinal products: guidelines for setting up and running a pharmacovigilance centre [Internet] Geneva: World Health Organization and the Uppsala Monitoring Centre; 2000. [Google Scholar]
- 24.Mitchell JG. From telehealth to e-health: the unstoppable rise of e-health. Canberra, A.C.T.: Commonwealth Department of Communications, Information Technology and the Arts; 1999. [Google Scholar]
- 25.Jackson EA, Kanmaz T. An overview of information resources for herbal medicinals and dietary supplements. J Herb Pharmacother. 2001;1(1):35–61. [PubMed] [Google Scholar]
- 26.Li C, Xia J, Deng J, Chen W, Wang S, Jiang J, et al. A web-based quantitative signal detection system on adverse drug reaction in China. Eur J Clin Pharmacol. 2009;65(7):729–741. doi: 10.1007/s00228-009-0638-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L. Pharmacovigilance of herbal and traditional medicines. Methods in pharmacology and toxicology evidence-based pharmacovigilance. New York: Humana Press; 2018. pp. 37–65. [Google Scholar]
- 28.Motl SE, Timpe EM, Robinson M, Corsberg C, Phillips K. Health information web sites by therapeutic category for healthcare professionals. J Pharm Technol. 2004;20(2):106–118. [Google Scholar]
- 29.Chen YZ, Ung CY. Computer automated prediction of potential therapeutic and toxicity protein targets of bioactive compounds from Chinese medicinal plants. Am J Chin Med. 2002;30(1):139–154. doi: 10.1142/S0192415X02000156. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Zhou H, Liu YB, Wang JF, Li H, Ung CY, et al. Database of traditional Chinese medicine and its application to studies of mechanism and to prescription validation. Br J Pharmacol. 2006;149(8):1092–1103. doi: 10.1038/sj.bjp.0706945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo Y, Hyun MK. Safety of herbal medicine for elderly patients with chronic disease in the Republic of Korea. Eur J Integr Med. 2019;30:100934. [Google Scholar]
- 32.Kiefer D, Shah S, Gardiner P, Wechkin H. Finding information on herbal therapy: a guide to useful sources for clinicians. Altern Ther Health Med. 2001;7(6):74–78. [PubMed] [Google Scholar]
- 33.Meyer JR, Generali JA, Karpinski JL. Evaluation of herbal–drug interaction data in tertiary resources. Hosp Pharm. 2004;39(2):149–160. [Google Scholar]
- 34.Ye X, Fu Z, Wang H, Du W, Wang R, Sun Y, et al. A computerized system for signal detection in spontaneous reporting system of Shanghai China. Pharmacoepidemiol Drug Saf. 2009;18(2):154–158. doi: 10.1002/pds.1695. [DOI] [PubMed] [Google Scholar]
- 35.Archer M, Proulx J, Shane-McWhorter L, Bray BE, Zeng-Treitler Q. Development of an alert system to detect drug interactions with herbal supplements using medical record data. AMIA Annu Symp Proc. 2014;2014:249–255. [PMC free article] [PubMed] [Google Scholar]
- 36.Ogultarhan V, Shoshi A, Magnucki R, Kormeier B, Hofestädt R. KATIS: an eHealth system for complementary medicine. IOS Press Ebooks. 2016;223:167–173. [PubMed] [Google Scholar]
- 37.Clauson KA, Polen HH, Peak AS, Marsh WA, DiScala SL. Clinical decision support tools: personal digital assistant versus online dietary supplement databases. Ann Pharmacother. 2008;42(11):1592–1599. doi: 10.1345/aph.1L297. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick RB. Natural Standard Database. Med Ref Serv Q. 2010;29(2):154–165. doi: 10.1080/02763861003723341. [DOI] [PubMed] [Google Scholar]
- 39.Yap KY, See CS, Kuo EY, Chui WK, Chan A. Utilizing mobile networks for the detection of clinically relevant interactions between chemotherapy regimens and complementary and alternative medicines. J Altern Complement Med. 2012;18(2):165–174. doi: 10.1089/acm.2010.0846. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Park KM, Han DJ, Bang NY, Kim DH, Na H, et al. PharmDB-K: integrated bio-pharmacological network database for traditional Korean medicine. PLoS One. 2015;10(11):e0142624. doi: 10.1371/journal.pone.0142624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie T, Song S, Li S, Ouyang L, Xia L, Huang J. Review of natural product databases. Cell Prolif. 2015;48(4):398–404. doi: 10.1111/cpr.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregory PJ, Jalloh MA, Abe AM, Hu J, Hein DJ. Characterization of complementary and alternative medicine-related consultations in an academic drug information service. J Pharm Pract. 2016;29(6):539–542. doi: 10.1177/0897190015579450. [DOI] [PubMed] [Google Scholar]
- 43.Olesen C, Harbig P, Barat I, Damsgaard EM. Absence of ‘over-the-counter’ medicinal products in on-line prescription records: a risk factor of overlooking interactions in the elderly. Pharmacoepidemiol Drug Saf. 2013;22(2):145–150. doi: 10.1002/pds.3362. [DOI] [PubMed] [Google Scholar]
- 44.Fischer JE, Crowell K, Curtis P. Complementary and alternative medical reference software for personal digital assistants: evidence of clinical applicability. Complement Health Pract Rev. 2005;10(1):57–72. [Google Scholar]
- 45.Kim E, Choi AS, Nam H. Drug repositioning of herbal compounds via a machine-learning approach. BMC Bioinformatics. 2019;20(S10):247. doi: 10.1186/s12859-019-2811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spanakis M, Sfakianakis S, Sakkalis V, Spanakis E. PharmActa: empowering patients to avoid clinical significant drug–herb interactions. Medicines. 2019;6(1):26. doi: 10.3390/medicines6010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu HY, Zhang YQ, Liu ZM, Chen T, Lv CY, Tang SH, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(D1):D976–D982. doi: 10.1093/nar/gky987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wootton JC. Directory of databases for research into alternative and complementary medicine. J Altern Complement Med. 1997;3(2):179–190. doi: 10.1089/acm.1997.3.179. [DOI] [PubMed] [Google Scholar]
- 49.Fucik H, Backlund A, Farah M. Building a computerized herbal substance register for implementation and use in the World Health Organisation international drug monitoring Programme. Drug Inform J. 2002;36(4):839–854. [Google Scholar]
- 50.Jackson EA. Resources for information on herbal medicinals and dietary supplements. J Herb Pharmacother. 2001;1(2):89–98. [PubMed] [Google Scholar]
- 51.Walker JB. Evaluation of the ability of seven herbal resources to answer questions about herbal products asked in drug information centers. Pharmacotherapy. 2002;22(12):1611–1615. doi: 10.1592/phco.22.17.1611.34126. [DOI] [PubMed] [Google Scholar]
- 52.Boddy K, Ernst E. Review of reliable information sources related to integrative oncology. Hematol Oncol Clin North Am. 2008;22(4):619–630. doi: 10.1016/j.hoc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Boehmer S, Karpa K. Evaluating the value of a web-based natural medicine clinical decision tool at an academic medical center. BMC Health Serv Res. 2011;11(1):279. doi: 10.1186/1472-6963-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faubert G, Lebe D, Bussières JF. A pilot study to compare natural health product–drug interactions in two databases in Canada. Pharm World Sci. 2010;32(2):179–186. doi: 10.1007/s11096-010-9364-2. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y, Shi S, Li Y, Wang Q. Development of quantitative structure-activity relationship models to predict potential nephrotoxic ingredients in traditional Chinese medicines. Food Chem Toxicol. 2019;128:163–170. doi: 10.1016/j.fct.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 56.Sweet BV, Gay WE, Leady MA, Stumpf JL. Usefulness of herbal and dietary supplement references. Ann Pharmacother. 2003;37(4):494–499. doi: 10.1345/aph.1C046. [DOI] [PubMed] [Google Scholar]
- 57.Tomasulo P. Natural standard–new integrative medicine database. Med Ref Serv Q. 2003;22(3):33–38. doi: 10.1300/J115v22n03_04. [DOI] [PubMed] [Google Scholar]
- 58.Molassiotis A, Xu M. Quality and safety issues of web-based information about herbal medicines in the treatment of cancer. Complement Ther Med. 2004;12(4):217–227. doi: 10.1016/j.ctim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Gao P, Zhang CL, Zeng FD, Pu R, Zhang J, Huang R, et al. Pharmacovigilance in China: issues of concern identified through an analysis of the Chinese adverse drug reaction information bulletin 2001 to 2014. J Clin Pharm Ther. 2015;40(5):594–598. doi: 10.1111/jcpt.12317. [DOI] [PubMed] [Google Scholar]
- 60.Brink SG, Mcfarren AE, Lincoln JM, Birney AJ. Cancer CAM™: web-based continuing education for health professionals. Complement Health Pract Rev. 2004;9(1):43–60. [Google Scholar]
- 61.Hamre HJ, Glockmann A, Heckenbach K, Matthes H. Use and safety of anthroposophic medicinal products: an analysis of 44,662 patients from the EvaMed Pharmacovigilance network. Drugs Real World Outcomes. 2017;4(4):199–213. doi: 10.1007/s40801-017-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tabali M, Ostermann T, Jeschke E, Witt CM, Matthes H. Adverse drug reactions for CAM and conventional drugs detected in a network of physicians certified to prescribe CAM drugs. J Manag Care Pharm. 2012;18(6):427–438. doi: 10.18553/jmcp.2012.18.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao Y, Wang Z, Li L, Lu K, Liu R, Liu Z, et al. An ontology-based artificial intelligence model for medicine side-effect prediction: taking traditional Chinese medicine as an example. Comput Math Methods Med. 2019;2019:1–7. doi: 10.1155/2019/8617503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ee RW, Yap KZ, Yap KY. Herbopolis – a mobile serious game to educate players on herbal medicines. Complement Ther Med. 2018;39:68–79. doi: 10.1016/j.ctim.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Allais G, Voghera D, Lorenzo CD, Mana O, Benedetto C. Access to databases in complementary medicine. J Altern Complement Med. 2000;6(3):265–274. doi: 10.1089/acm.2000.6.265. [DOI] [PubMed] [Google Scholar]
- 66.Clinical Pharmacology. Clinical pharmacology [Internet]. Elsevier Gold Standard [cited 2019 Dec 14]. Available from: https://www.clinicalpharmacology.com/.
- 67.IBM Watson Health Products . Micromedex web applications access [Internet] Armonk: IBM Corporation; 2019. [Google Scholar]
- 68.Lexicomp . Lexi-natural products. Alphen aan den Rijn: Wolters Kluwer Clinical Drug Information, Inc; 2019. [Google Scholar]
- 69.Therapeutic Research Center . Natural medicines. Stockton: Therapeutic Research Center; 2019. [Google Scholar]
- 70.MedicinesComplete . Herbal medicines. London: The Royal Pharmaceutical Society; 2019. [Google Scholar]
- 71.PEPID . Clinical decision support. Phoenix: PEPID; 2019. [Google Scholar]
- 72.Interaktionsdatabasen Danish Medicines Agency. Interaktionsdatabasen.dk [Internet]. [cited 2019 Dec 14]. Available from: http://www.interaktionsdatabasen.dk/.
- 73.Uppsala Monitoring Centre . Vigibase. Swedan: WHO Collaborating Centre for International Drug Monitoring; 2019. [Google Scholar]
- 74.U.S. Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition (CFSAN) [Internet] Silver Spring: FDA; 2018. [Google Scholar]
- 75.U.S. Food and Drug Administration (FDA) Medical product safety information [Internet] Silver Spring: FDA; 2019. [Google Scholar]
- 76.World Health Organization (WHO) WHO global report on traditional and complementary medicine 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 77.Institute For Safe Medication Practices . About us [Internet]. Horsham: Institute for Safe Medication Practices; 2019. [Google Scholar]
- 78.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.National Center for Complementary and Integrative Health . Herbs at a glance [Internet] Bethesda: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 80.Scarton LA, Del Fiol G, Treitler-Zeng Q. Completeness, accuracy, and presentation of information on interactions between prescription drugs and alternative medicines: an internet review. Stud Health Technol Inform. 2013;192:841–845. [PMC free article] [PubMed] [Google Scholar]
- 81.Kronenberg F, Molholt P, Zeng ML, Eskinazi D. A comprehensive information resource on traditional, complementary, and alternative medicine: toward an international collaboration. J Altern Complement Med. 2001;7(6):723–729. doi: 10.1089/10755530152755289. [DOI] [PubMed] [Google Scholar]
- 82.Richardson J. Building CAM databases: the challenges ahead. J Altern Complement Med. 2002;8(1):7–8. doi: 10.1089/107555302753507122. [DOI] [PubMed] [Google Scholar]
- 83.The EQUATOR Network . Your one-stop-shop for writing and publishing high-impact health research [Internet] Headington: UK EQUATOR Centre; 2019. [Google Scholar]
- 84.Baker TB, Gustafson DH, Shaw B, Hawkins R, Pingree S, Roberts L, et al. Relevance of CONSORT reporting criteria for research on eHealth interventions. Patient Educ Couns. 2010;81:S77–S86. doi: 10.1016/j.pec.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen YZ, Zhi DG. Ligand–protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins Struct Funct Genet. 2001;43:217–226. doi: 10.1002/1097-0134(20010501)43:2<217::aid-prot1032>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].