Abstract
Osteoarthritis (OA) is a joint disease caused by a variety of factors, including aging, obesity and trauma. MicroRNAs (miRNAs) have been reported to be crucial regulators during OA progression. The present study aimed to investigate the role of miR-17-5p and miR-19b-3p during OA development. Interleukin (IL)-1β-treated chondrocytes were used to mimic OA in vitro. The expression levels of miR-17-5p and enhancer of zeste homolog 2 (EZH2) were measured in cartilage tissues and chondrocytes using reverse transcription-quantitative PCR or western blotting. Apoptosis was assessed by flow cytometry. The protein expression levels of extracellular matrix (ECM)-associated genes were detected by western blotting. The binding sites between miR-17-5p or miR-19b-3p and EZH2 were predicted using the MicroT-CDS online database and verified using dual-luciferase reporter and RIP assays. miR-17-5p expression was downregulated, whereas EZH2 expression was upregulated in OA cartilage tissues and IL-1β-induced chondrocytes compared with that in the control tissues and cells. miR-17-5p mimics inhibited IL-1β-induced apoptosis and ECM degradation in chondrocytes. EZH2 was the target of miR-17-5p and miR-19b-3p in chondrocytes, and enhanced apoptosis and ECM degradation in IL-1β-stimulated chondrocytes. Rescue experiments revealed that miR-17-5p or miR-19b-3p mimic-induced inhibition of OA progression was reversed by EZH2 overexpression. In conclusion, miR-17-5p and miR-19b-3p inhibited OA progression by targeting EZH2, which may serve as a potential therapeutic target for OA.
Keywords: microRNA-17-5p, microRNA-19b-3p, enhancer of zeste homolog 2, osteoarthritis, progression
Introduction
Osteoarthritis (OA) is a common degenerative joint disease that usually occurs in elderly individuals (1). The pathogenesis of OA, which is caused by the imbalance of joint tissue repair and destruction, is complex (2). Chondrocytes are the cells of the cartilage tissue that participate in the production of cartilage extracellular matrix (ECM) (3), which serves an important role in the maintenance of cartilage structure and function (4). With an increased global prevalence and limited treatment options, the therapeutic strategies for OA are still unsatisfactory (5,6); therefore, identifying novel biomarkers for OA treatment is important.
MicroRNAs (miRNAs) are highly conserved short non-coding RNAs 18-25 nucleotides long (7). Increasing evidence has indicated that miRNAs serve vital roles during cartilage formation and remodeling (8). Furthermore, a number of miRNAs have been identified as regulators of OA progression, including apoptosis and ECM degradation processes (9). For example, miRNA (miR)-103 restrained chondrocyte proliferation by targeting Sox6 to trigger OA progression (10). Additionally, miR-9-5p overexpression hindered chondrocyte apoptosis by inhibiting tenascin C expression in a mouse model of OA (11). In addition, miR-21-5p upregulated collagen type II-α1 chain expression to facilitate cartilage formation in interleukin (IL)-1β-induced chondrocytes (12). Therefore, miRNAs may serve as novel therapeutic targets for OA. Of note, miR-17-5p has been demonstrated to reverse HOX transcript antisense RNA and fucosyltransferase 2-induced chondrocyte apoptosis (13); however, the molecular mechanism underlying miR-17-5p in OA is not completely understood.
Enhancer of zeste homolog 2 (EZH2), a histone methyltransferase, participates in the pathogenesis of different types of cancer, including breast cancer, colon cancer and prostate cancer (14). Emerging evidence has demonstrated that EZH2 is often dysregulated at the transcriptional and post-transcriptional level in a number of diseases, such as prostate cancer and leukemia (15). EZH2 dysregulation is a hallmark of disease progression in a number of different types of cancer. For example, in gastric cancer tissues, EZH2 expression was abnormally enhanced and EZH2 accelerated cell proliferation by targeting p21(16). Lui et al (17) reported that EZH2 was a regulator of chondrocyte proliferation and hypertrophy; however, the relationship between miR-17-5p and EZH2 has not been reported.
Therefore, the present study investigated the effect of miR-17-5p on chondrocyte apoptosis and ECM degradation in IL-1β-treated chondrocytes, as well as the molecular mechanisms underlying miR-17-5p and miR-19b-3p activity in OA.
Materials and methods
Specimen collection
A total of 35 OA cartilage specimens from patients with OA (age, 61.77±4.66 years; 23 female, 12 male) who underwent joint replacement and 35 normal cartilage tissues from patients (age, 41.51±4.01 years; 19 female, 16 male) with traumatic emergency amputation without a history of OA or rheumatoid arthritis were acquired from the People's Hospital of Rizhao between July 2016 and August 2018. The present study was approved by the Ethics Committee of the People's Hospital of Rizhao. All participants provided written informed consent.
Cell culture
Cartilage samples were cut into small slices (<1 mm3) and digested with 0.2% trypsin for 30 min at 37˚C, followed by 0.2% collagenase type II for 8 h at 37˚C. After filtration and centrifugation at 1000 x g for 10 min, chondrocytes were incubated in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5% CO2.
Cell transfection and IL-1β treatment
Vectors and oligonucleotides were synthesized by Guangzhou RiboBio Co., Ltd. The following vectors and oligonucleotides were used for transfection: miR-17-5p mimic (miR-17-5p, 5'-CAAAGUGCUUACAGUGCAGGUAG-3'; 50 nM), mimic negative control (miR-NC, 5'-UCGCUUGGUGCAGGUCGGGAA-3'; 50 nM), miR-17-5p inhibitor (in-miR-17-5p, 5'-CUACCUGCACUGUAAGCACUUUG-3'; 100 nM), inhibitor control (in-miR-NC, 5'-CAGUACUUUUGUGUAGUACAA-3'; 100 nM), EZH2 overexpression vector (EZH2; 50 nM), empty overexpression vector (pcDNA; 50 nM), small interfering RNA (si-RNA) targeting EZH2 (si-EZH2, 5'-GAGGGAAAGUGUAUGAUAATT-3'; 100 nM), siRNA control (si-NC, 5'-GGGAAAGAGUAUAUAGUGATT-3'; 100 nM), miR-19b-3p mimic (miR-19b-3p, 5'-UGUGCAAAUCCAUGCAAAACUGA-3'; 50 nM) and miR-19b-3p inhibitor (in-miR-19b-3p, 5'-UCAGUUUUGCAUGGAUUUGCACA-3'; 100 nM). At 70% confluency, cells were transfected using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. After transfection for 48 h at 37˚C, chondrocytes were treated with 10 ng/ml IL-1β (Beijing Solarbio Science and Technology Co., Ltd.) for 24 h at 37˚C.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cartilage tissues or chondrocytes using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Subsequently, RNA was reverse transcribed into cDNA using the FastQuant RT kit (Tiangen Biotech Co., Ltd.) or miScript Reverse Transcription kit (Qiagen GmbH), according to the manufacturer's protocol. qPCR was performed using the SYBR Green PCR Master Mix (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The reactions were incubated at 95˚C for 30 sec, followed by 40 cycles of 95˚C for 5 sec, 60˚C for 10 sec, 95˚C for 5 sec and 60˚C for 10 sec. The following primer pairs were purchased from (Guangzhou RiboBio Co., Ltd.) and used for qPCR: miR-17-5p forward, 5'-CGGCGGCAAAGTGCTTACAG-3' and reverse, 5'-GTGCAGGGTCCGAGGT-3'; miR-19b-3p forward, 5'-ACACTCCAGCTGGGTGTGCAAATCCATGCAA-3' and reverse, 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAGTTT-3'; EZH2 forward, 5'-AATCAGAGTACATGCGACTGAGA-3' and reverse, 5'-AATCAGAGTACATGCGACTGAGA-3'; GAPDH forward, 5'-GCTGAGTATGTCGTGGAGTC-3' and reverse, 5'-AGTTGGTGGTGCAGGATGC-3'; and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'. mRNA and miRNA levels were normalized to the internal reference genes GAPDH and U6, respectively. Expression levels were quantified via the 2−ΔΔCq method (18).
Western blot analysis
Total protein was extracted using RIPA buffer (Sigma-Aldrich; Merck KGaA). Protein samples were quantified using a BCA Protein Assay Kit (cat. no. ab102536; Abcam). Equal amounts of protein samples (20 µg) were separated by 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). Subsequently, the membrane was blocked with 5% skimmed milk for 2 h at room temperature. The membrane was incubated with primary antibodies overnight at 4˚C against matrix metalloproteinase-13 (MMP13; cat. no. ab39012; 60 KDa; dilution, 1:4,000; Abcam), Collagen II (cat. no. ab34712; 142 KDa; dilution, 1:2,000; Abcam), Aggrecan (cat. no. ab36861; 110 KDa; dilution, 1:2,000; Abcam), EZH2 (cat. no. ab186006; 85 KDa; dilution, 1:1,000; Abcam) and β-actin (cat. no. ab8227; 42 KDa; dilution, 1:2,000; Abcam). Following primary antibody incubation, the membranes were incubated for 2 h at room temperature with a secondary anti-rabbit antibody marked by horseradish peroxidase (cat. no. ab7090; dilution, 1:20,000; Abcam). Protein bands were visualized using ECL reagents (EMD Millipore) and quantified by densitometry analysis using ImageJ software (version 1.6.0; National Institutes of Health, Inc.). β-actin was used as the loading control.
Flow cytometry
Chondrocytes (1x105 cells/well) were seeded into 6-well plates and washed twice with cold PBS. Apoptotic cells were detected using the Annexin V-FITC/propidium iodide Apoptosis Detection kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Early apoptotic cells were analyzed using an Attune NxT flow cytometer (Thermo Fisher Scientific, Inc.) and calculated using Cell Quest acquisition software (version 2.9; BD Biosciences, Inc.).
Dual-luciferase reporter assay
The binding sequences between miR-17-5p or miR-19b-3p and EZH2 were predicted using MicroT-CDS software (diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index). The EZH2 3'-untranslated region (3'-UTR) containing wild-type (WT) or mutant (MUT) binding sites for miR-17-5p or miR-19b-3p (Guangzhou RiboBio Co., Ltd.) were inserted into a pGL3 vector (Promega Corporation). Subsequently, the luciferase reporter and miR-17-5p, miR-19b-3p, miR-NC, in-miR-17-5p, in-miR-19b-3p or in-miR-NC were co-transfected into chondrocytes (5x104 cells/well) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Following 48 h incubation at 37˚C, luciferase activity was detected using a Dual-Lucy assay kit (Beijing Solarbio Science and Technology Co., Ltd.), according to the manufacturer's protocol. Renilla luciferase activity was used for normalization.
Radioimmunoprecipitation (RIP) assay
The Magna RIP kit (EMD Millipore) was used to perform the RIP assay, according to the manufacturer's protocol. Briefly, chondrocytes were transfected with miR-17-5p, miR-19b-3p or miR-NC. Subsequently, chondrocytes were harvested and lysed using RIP lysis buffer. The cell lysates were collected and incubated with RIP-argonaute RISC catalytic component 2 (Ago2) antibody (EMD Millipore) or RIP-immunoglobulin G (IgG) antibody (EMD Millipore) overnight at 4˚C. EZH2 expression was detected by RT-qPCR.
Statistical analysis
Data are presented as the mean ± standard deviation. GraphPad Prism 7.0 software (GraphPad Software, Inc.) was used to perform statistical analyses. The correlation between miR-17-5p and EZH2 levels in OA cartilage tissues was analyzed using Spearman's correlation coefficient. Data were analyzed using the Student's t-test or one-way ANOVA followed by Tukey's post hoc test. All experiments were performed at least three times. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-17-5p is downregulated and EZH2 is upregulated in OA cartilage tissues
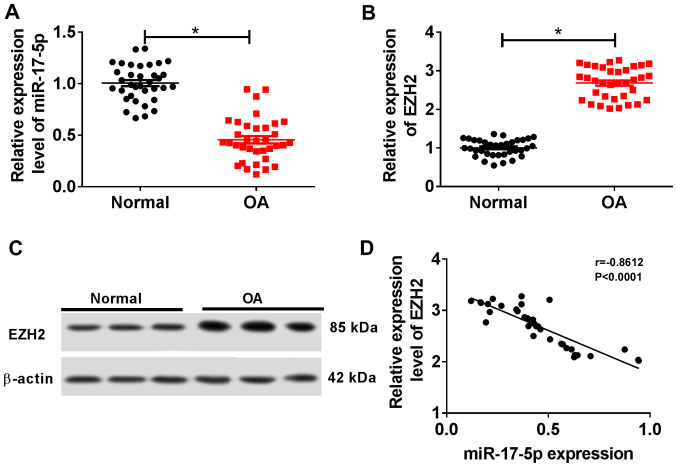
The RT-qPCR results suggested that miR-17-5p expression was significantly decreased in OA cartilage tissues compared with normal cartilage tissues (Fig. 1A). By contrast, EZH2 expression was significantly increased in OA cartilage tissues compared with normal cartilage tissues (Fig. 1B and C). Additionally, miR-17-5p expression was negatively correlated with EZH2 expression in OA cartilage tissues (Fig. 1D). These results suggested that miR-17-5p may serve a role in OA progression.
Figure 1.
miR-17-5p and EZH2 expression in cartilage tissues of patients with OA. (A and B) The expression levels of (A) miR-17-5p and (B) EZH2 were detected in cartilage tissues of normal patients (n=35) and patients with OA (n=35) by reverse transcription-quantitative PCR. (C) The expression of EZH2 protein in cartilage tissues was measured using western blotting. (D) The correlation between miR-17-5p and EZH2 expression in OA cartilage tissues was verified using Spearman's correlation coefficient analysis. *P<0.05. miR, microRNA; EZH2, enhancer of zeste homolog 2; OA, osteoarthritis.
miR-17-5p inhibits IL-1β-induced chondrocyte apoptosis and ECM degradation
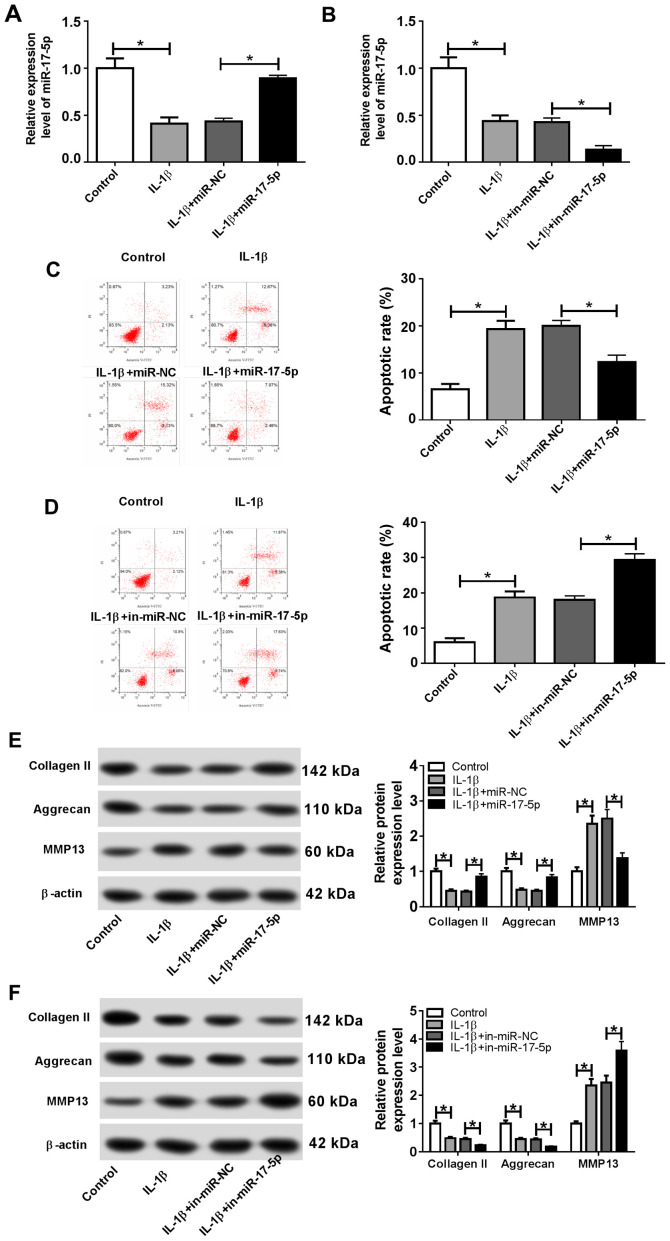
To investigate the role of miR-17-5p during the pathogenesis of OA, chondrocytes were transfected with miR-17-5p, miR-NC, in-miR-17-5p or in-miR-NC and subsequently treated with 10 ng/ml IL-1β for 24 h. Transfection efficiency of the miR-17-5p mimics and inhibitor was determined by RT-qPCR (Fig. S1A). The expression of miR-17-5p was significantly decreased in the IL-1β group compared with that in the control group, and the miR-17-5p mimic significantly reversed the IL-1β-induced effect (Fig. 2A). miR-17-5p expression was also significantly reduced in the IL-1β + in-miR-17-5p group compared with the IL-1β + in-miR-NC group (Fig. 2B). Furthermore, flow cytometry demonstrated that the proportion of apoptotic cells was significantly increased in the IL-1β group compared with that in the control group. In addition, the miR-17-5p mimics inhibited the IL-1β-induced apoptosis, and miR-17-5p inhibition significantly increased the IL-1β-induced apoptosis (Fig. 2C and D). IL-1β treatment also resulted in a significant decrease in the protein expression levels of the cartilage formation proteins Collagen II and Aggrecan, and a significant increase in the protein expression level of the cartilage-degrading enzyme MMP13 compared with the control group. By contrast, the miR-17-5p mimics counteracted the IL-1β-induced effects, and miR-17-5p inhibition enhanced the IL-1β-induced effects on cartilage-related protein expression (Fig. 2E and F). These results indicated that miR-17-5p modulated apoptosis and ECM degradation in IL-1β-induced chondrocytes.
Figure 2.
miR-17-5p decreases IL-1β-induced cell apoptosis and ECM degradation in chondrocytes. Chondrocytes were transfected with miR-17-5p mimic, miR-NC, in-miR-17-5p or in-miR-NC prior to IL-1β treatment. (A and B) The expression of miR-17-5p was measured by reverse transcription-quantitative PCR in chondrocytes transfected with (A) the miR-17-5p mimics and (B) in-miR-17-5p. (C and D) The proportion of apoptotic chondrocytes was determined by flow cytometry following transfection with (C) the miR-17-5p mimics and (D) in-miR-17-5p. (E and F) The protein expression levels of ECM-associated proteins Collagen II, Aggrecan and MMP13 were determined by western blotting in chondrocytes transfected with (E) the miR-17-5p mimics and (D) in-miR-17-5p. *P<0.05. miR, microRNA; IL-1β, interleukin-1β; ECM, extracellular matrix; NC, negative control; in, inhibitor; MMP13, matrix metalloproteinase 13; PI, propidium iodide.
EZH2 is a target of miR-17-5p in chondrocytes
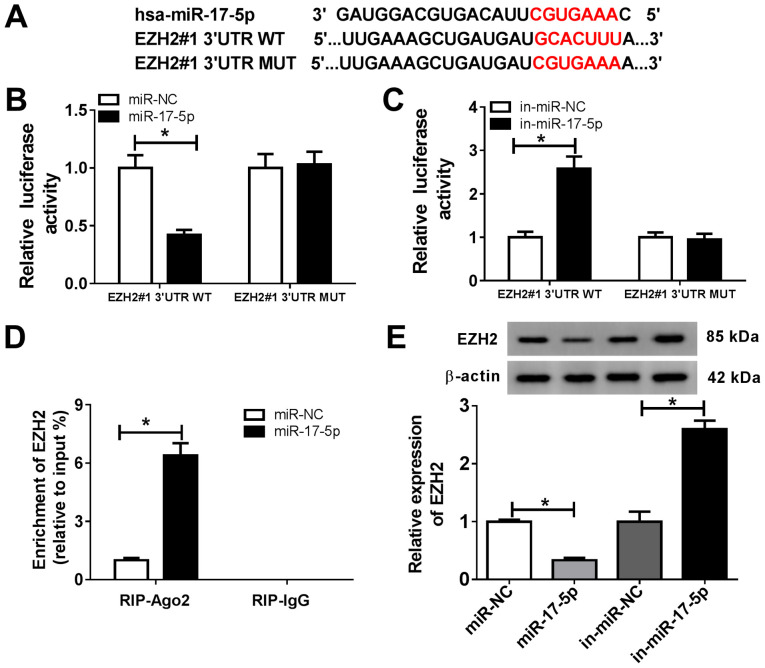
To explore the mechanism underlying miR-17-5p activity during OA progression, the MicroT-CDS online database was used, which predicted that miR-17-5p and EZH2 3'UTR exhibited putative binding sites (Fig. 3A). The dual-luciferase reporter assay revealed that the miR-17-5p mimics significantly reduced the luciferase activity of EZH2#1 3'UTR-WT, but displayed no effect on the luciferase activity of EZH2#1 3'UTR-MUT compared with the control group (Fig. 3B). In addition, the luciferase activity of EZH2#1 3'UTR-WT was significantly enhanced by the miR-17-5p inhibitor, but was not altered for EZH2#1 3'UTR-MUT compared with the control group (Fig. 3C). The RIP assay was performed to further investigate the relationship between miR-17-5p and EZH2. The results demonstrated that EZH2 was significantly enriched in the miR-17-5p group coated with the Ago2 antibody compared with the control group (Fig. 3D). Furthermore, the protein expression levels of EZH2 were measured in chondrocytes transfected with miR-NC, miR-17-5p, in-miR-NC or in-miR-17-5p. The results suggested that the miR-17-5p mimics significantly decreased the expression of EZH2, and miR-17-5p inhibition significantly increased the expression level of EZH2 in chondrocytes (Fig. 3E). These results suggested that EZH2 was a direct target of miR-17-5p in chondrocytes.
Figure 3.
EZH2 is a target of miR-17-5p in chondrocytes. (A) The predicted binding sites of miR-17-5p and EZH2 3'UTR. (B and C) The luciferase activity in chondrocytes co-transfected with EZH2#1 3'UTR-WT or EZH2#1 3'UTR-MUT and (B) miR-NC or the miR-17-5p mimic, or (C) in-miR-NC or in-miR-17-5p was determined using a dual-luciferase reporter assay. (D) The RIP assay was used to validate the relationship between miR-17-5p and EZH2. (E) EZH2 protein expression was measured in chondrocytes transfected with miR-NC, miR-17-5p mimic, in-miR-NC or in-miR-17-5p. *P<0.05 vs. NC. EZH2, enhancer of zeste homolog 2; miR, microRNA; 3'UTR, 3'-untranslated region; WT, wild-type; MUT, mutant; NC, negative control; in, inhibitor; Ago2, argonaute RISC catalytic component 2; IgG, immunoglobulin G.
EZH2 facilitates IL-1β-induced apoptosis and ECM degradation in chondrocytes
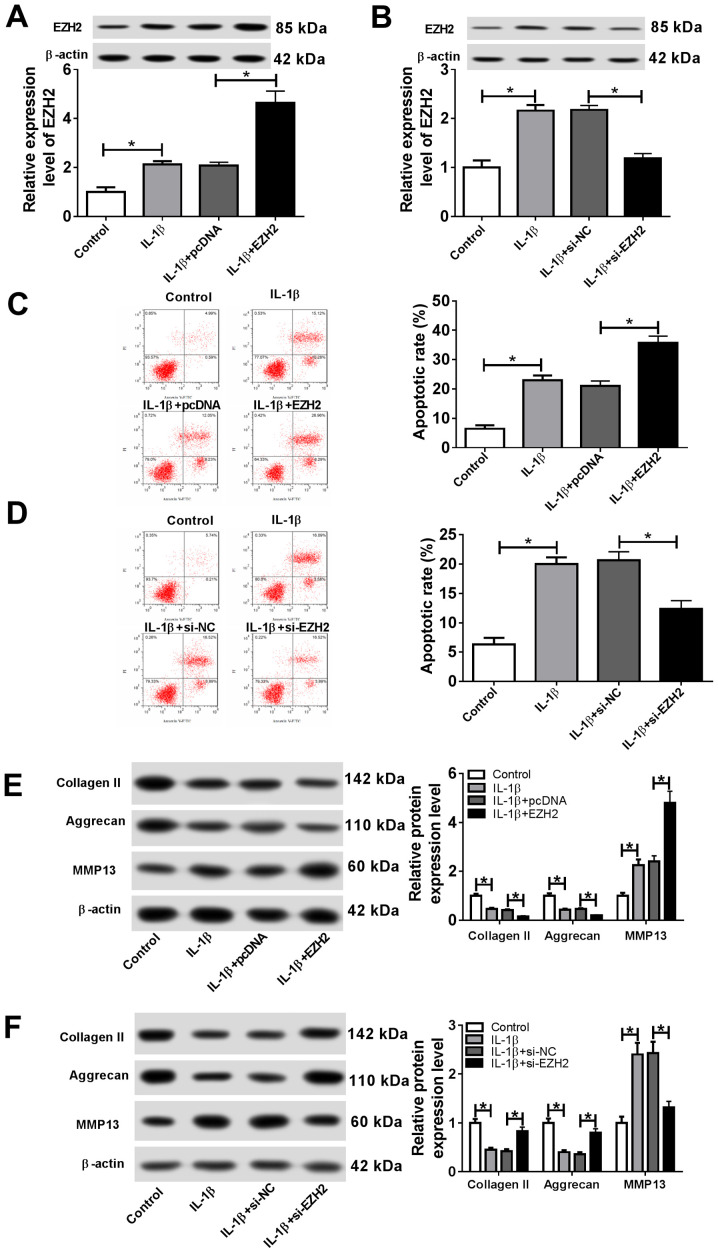
To investigate the effects of EZH2 on OA progression, chondrocytes were transfected with EZH2, pcDNA, si-EZH2 or si-NC. Subsequently, transfected cells were stimulated with IL-1β. The transfection efficiency of EZH2 overexpression and knockdown were detected by RT-qPCR (Fig. S1C). The results suggested that IL-1β stimulation significantly increased EZH2 expression, whereas EZH2 overexpression significantly enhanced EZH2 expression, and EZH2 knockdown significantly decreased EZH2 expression in IL-1β-stimulated chondrocytes (Fig. 4A and 4B). Additionally, the proportion of apoptotic cells was significantly increased following EZH2 overexpression, whereas si-EZH2 significantly reduced the proportion of apoptotic IL-1β-treated chondrocytes (Fig. 4C and 4D). EZH2 overexpression significantly decreased the expression level of the cartilage formation proteins Collagen II and Aggrecan, and significantly increased the expression level of the cartilage-degrading enzyme MMP13; however, EZH2 knockdown displayed the opposite effect (Fig. 4E and 4F). The results suggested that EZH2 regulated cell apoptosis and ECM degradation in IL-1β-induced chondrocytes.
Figure 4.
EZH2 facilitates IL-1β-induced chondrocyte apoptosis and ECM degradation. Chondrocytes were transfected with EZH2 overexpression vector, pcDNA, si-EZH2 or si-NC prior to IL-1β stimulation. (A and B) The protein expression of EZH2 was measured by western blotting in chondrocytes transfected with (A) the EZH2 overexpression vector and (B) si-EZH2. (C and D) The proportion of apoptotic cells was assessed by flow cytometry in chondrocytes transfected with (C) the EZH2 overexpression vector and (D) si-EZH2. (E and F) The protein expression levels of extracellular matrix-associated proteins Collagen II, Aggrecan and MMP13 were detected by western blotting in chondrocytes transfected with (E) the EZH2 overexpression vector and (F) si-EZH2. *P<0.05. EZH2, enhancer of zeste homolog 2; IL-1β, interleukin-1β; si, small interfering RNA; NC, negative control; MMP13, matrix metalloproteinase 13; PI, propidium iodide.
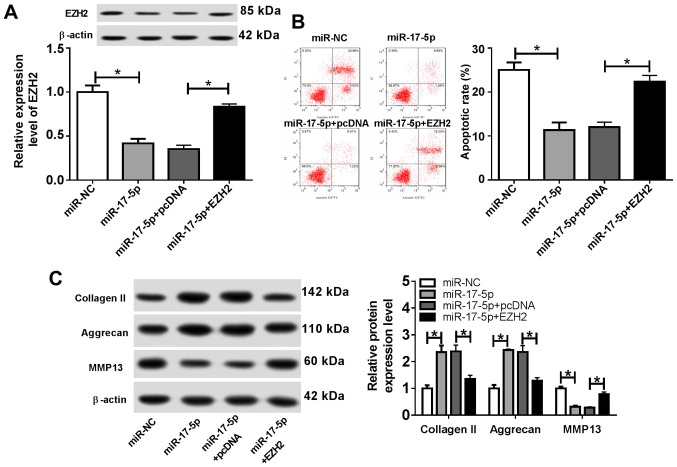
EZH2 overexpression reverses the effect of miR-17-5p overexpression on the progression of OA
To further investigate the role of miR-17-5p and EZH2 during OA progression, chondrocytes were transfected with miR-NC, miR-17-5p, miR-17-5p + pcDNA or miR-17-5p + EZH2, and subsequently stimulated with IL-1β for 24 h. The results indicated that EZH2 expression was significantly decreased by miR-17-5p overexpression, which was reversed by EZH2 overexpression (Fig. 5A). Furthermore, the miR-17-5p mimics significantly reduced the proportion of apoptotic chondrocytes, and EZH2 overexpression reversed the miR-17-5p-induced effects on chondrocyte apoptosis (Fig. 5B). In addition, transfection with the miR-17-5p mimics resulted in a significant increase in the expression levels of Collagen II and Aggrecan and a significant decrease in MMP13 expression compared with those in the control group. EZH2 overexpression reversed the miR-17-5p mimic-induced effects on cartilage-associated protein expression (Fig. 5C). These results suggested that EZH2 overexpression counteracted the effects of the miR-17-5p mimics on OA progression.
Figure 5.
EZH2 overexpression reverses the effects of the miR-17-5p mimics on osteoarthritis progression. Chondrocytes were transfected with miR-NC, miR-17-5p mimic, miR-17-5p mimic + pcDNA or miR-17-5p + EZH2 overexpression vector, and treated with IL-1β for 24 h. (A) EZH2 protein expression was determined using western blotting. (B) The proportion of apoptotic cells was determined by flow cytometry. (C) The protein expression levels of extracellular matrix-associated proteins were detected by western blotting. *P<0.05, as indicated. EZH2, enhancer of zeste homolog 2; miR, microRNA; NC, negative control; IL-1β, interleukin-1β; PI, propidium iodide; MMP13, matrix metalloproteinase 13.
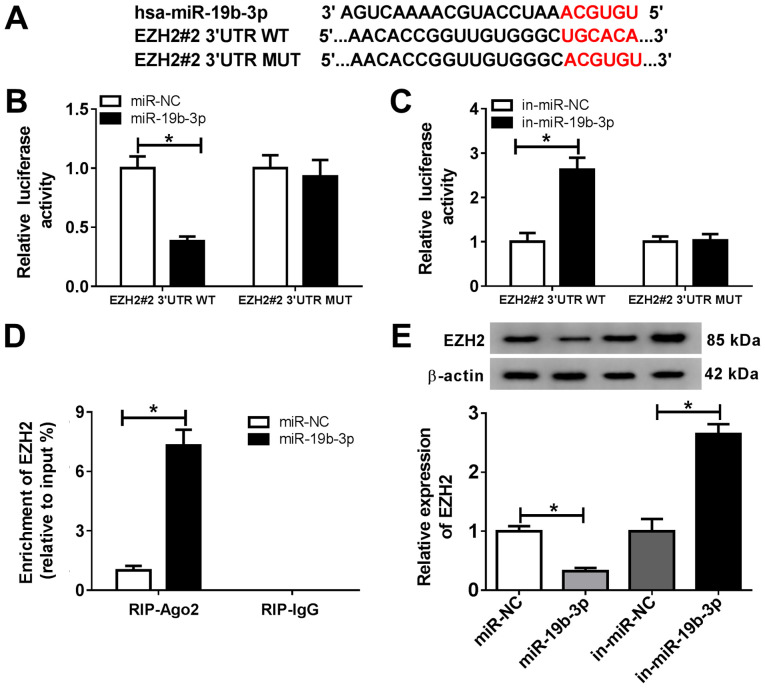
miR-19b-3p directly targets EZH2 in chondrocytes. The MicroT-CDS online database also predicted that miR-19b-3p and EZH2 3'UTR displayed putative binding sites (Fig. 6A). The dual-luciferase reporter assay suggested that the miR-19b-3p mimics significantly decreased the luciferase activity of EZH2#2 3'UTR-WT, and the miR-19b-3p inhibitor significantly enhanced the luciferase activity of EZH2#2 3'UTR-WT (Fig. 6B and C). The RIP assay results indicated that EZH2 was significantly enriched in the miR-19b-3p group coated with the Ago2 antibody compared with the control group (Fig. 6D). The transfection efficiency of miR-19b-3p was verified by RT-qPCR (Fig. S1B). In addition, the protein expression of EZH2 was significantly decreased in chondrocytes transfected with the miR-19b-3p mimic compared with that in the miR-NC group. EZH2 expression was significantly increased in chondrocytes transfected with in-miR-19b-3p compared with that in the in-miR-NC group (Fig. 6E). These results suggested that miR-19b-3p directly targeted EZH2 and negatively regulated EZH2 expression in chondrocytes.
Figure 6.
miR-19b-3p directly targets EZH2. (A) The putative binding sites between miR-19b-3p and EZH2 3'UTR. (B and C) EZH2#2 3'UTR-WT or EZH2#2 3'UTR-MUT and (B) miR-NC or the miR-19b-3p mimics, or (C) in-miR-NC or in-miR-19b-3p were co-transfected into chondrocytes, and a dual luciferase reporter assay was performed to evaluate the luciferase activity. (D) The RIP assay was performed to verify the relationship between miR-19b-3p and EZH2. (E) EZH2 expression was detected in chondrocytes transfected with miR-NC, the miR-19b-3p mimics, in-miR-NC or in-miR-19b-3p. *P<0.05 vs. control. miR, microRNA; EZH2, enhancer of zeste homolog 2; 3'UTR, 3'-untranslated region; WT, wild-type; MUT, mutant; NC, negative control; in, inhibitor; Ago, argonaute RISC catalytic component 2; IgG, immunoglobulin G.
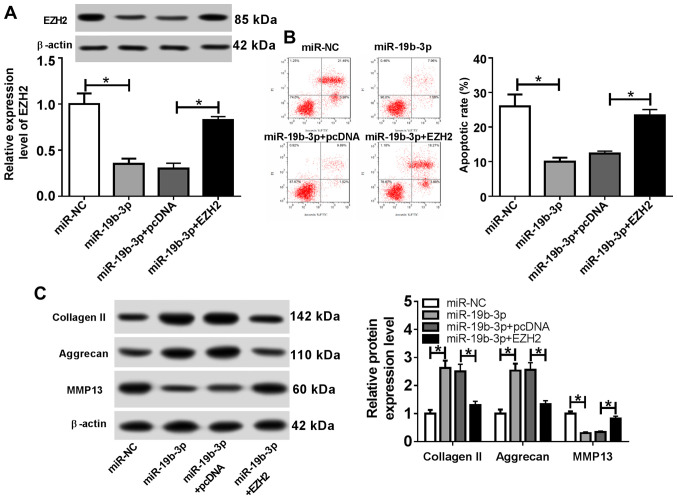
EZH2 overexpression reverses the effects of the miR-19b-3p mimics on OA progression
To further investigate the role of miR-19b-3p during the development of OA, transfected chondrocytes were treated with IL-1β for 24 h. The western blotting results indicated that EZH2 expression was significantly decreased in the miR-19b-3p mimics group compared with that in the miR-NC group, whereas EZH2 overexpression reversed the miR-19b-3p-induced effects on EZH2 expression (Fig. 7A). The proportion of apoptotic cells was significantly reduced in the miR-19b-3p mimics group, and EZH2 overexpression reversed the effect of the miR-19b-3p mimics on chondrocyte apoptosis (Fig. 7B). Furthermore, the levels of Collagen II and Aggrecan expression were significantly increased and MMP13 expression was significantly decreased in the miR-19b-3p mimics group compared with the miR-NC group. Similarly, the miR-19b-3p mimic-induced effects were reversed by EZH2 overexpression (Fig. 7C). These results suggested that EZH2 overexpression reversed the effects of miR-19b-3p overexpression on OA progression.
Figure 7.
EZH2 overexpression reverses the effects of the miR-19b-3p mimics on osteoarthritis progression. Following transfection with miR-NC, miR-19b-3p mimic, miR-19b-3p mimic + pcDNA or miR-19b-3p mimic + EZH2 overexpression vector, chondrocytes were stimulated with IL-1β for 24 h. (A) EZH2 protein expression was measured by western blotting. (B) The proportion of apoptotic cells was assessed using flow cytometry. (C) The protein expression levels of extracellular matrix-related proteins were examined by western blotting. *P<0.05, as indicated. EZH2, enhancer of zeste homolog 2; miR, microRNA; NC, negative control; IL-1β, interleukin-1β; PI, propidium iodide; MMP13, matrix metalloproteinase 13.
Discussion
OA is characterized by cartilage destruction, and the pathogenesis of the disease involves chondrocyte apoptosis and ECM degradation (19). Musumeci et al (20) reported that the proportion of apoptotic chondrocytes was significantly increased after injury. In a rat model of OA, adenylate cyclase-activating polypeptide 1 prevented IL-1β-induced chondrocyte apoptosis in vitro (21). Molecular markers that regulate chondrocyte apoptosis are important during the development of OA (22). According to the literature, ECM degradation participates in the pathogenesis of OA, leading to the loss of cartilage tissue (23). To reduce the incidence of degenerative joint diseases, a number of OA treatment strategies have been developed in recent years, including the use of fibrates or collagen cell carrier scaffolds (24,25). Increasing evidence has suggested that a number of miRNAs are associated with OA progression (26); therefore, the role of miRNAs in chondrocyte apoptosis and ECM degradation was investigated in the present study.
Previous studies have reported that miRNA-17-5p modulates cell autophagy and apoptosis to affect cellular senescence, aging and cancer (27). For example, aberrant expression of miNA-17-5p regulates the osteoblastic differentiation of mesenchymal stem cells by targeting SMAD7(28). Moreover, increasing evidence has indicated that miRNA-17-5p may serve as a tumor inhibitor in breast cancer and an oncogene in pancreatic cancer (29,30). miRNA-19b-3p blocks the progression of breast cancer by mediating the PI3K/Akt signaling pathway (31). A previous study reported that miRNA-17-5p was significantly downregulated in OA chondrocytes and facilitated autophagy by decreasing p62 expression (32). Additionally, miRNA-19b-3p decreases IL-1β-induced ECM degradation and inflammatory injury in chondrocytes by regulating G protein-coupled receptor kinase 6 expression (33). Similar to previous studies, the results of the present study indicated that miR-17-5p expression was decreased in OA chondrocytes compared with that in the control cells. The results also suggested that miR-17-5p modulated OA progression by inhibiting chondrocyte apoptosis and ECM degradation.
Histone methyltransferase EZH2 inhibits osteoblast maturation and bone development (34). A number of studies have reported that EZH2 upregulation in different tumors is associated with adverse outcomes (35,36). Previous studies have also demonstrated that the level of EZH2 in OA chondrocytes was significantly elevated; therefore, the use of EZH2 inhibitors may serve as a therapeutic strategy for OA (37,38). Consistent with previous studies, EZH2 expression was significantly increased in OA chondrocytes compared with control cells in the present study. In addition, it has been reported that miRNAs serve a vital role in a number of diseases, including prostate cancer, osteoporosis and osteoarthritis (39,40), by targeting the 3'UTR of target genes to downregulate protein expression (40). In the present study, the MicroT-CDS online database predicted that EZH2 and miR-17-5p or miR-19b-3p displayed putative binding sites, which was confirmed by the dual-luciferase reporter and RIP assays. In addition, the rescue experiments indicated that miR-17-5p and miR-19b-3p modulated OA progression by targeting EZH2.
In conclusion, the results of the present study suggested that miR-17-5p and miR-19b-3p inhibited chondrocyte apoptosis and ECM degradation by targeting EZH2, which indicated that these miRNAs may serve as promising preventative and therapeutic biomarkers for OA. However, animal experiments are required to verify the results of the present study before the miRNAs can be used in the clinic.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
FY and YS conceived the study and performed the experiments. YS, XG and YL analyzed and interpreted the data. YL, FY and YS drafted and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethical Review Committee of The People's Hospital of Rizhao (Rizhao, China). Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–508. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, Malaise M, de Seny D. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Şahin Ş, Tuncel SA, Salimi K, Bilgiç E, Korkusuz P, Korkusuz F. Advanced injectable alternatives for osteoarthritis. Adv Exp Med Biol. 2018;1077:183–96. doi: 10.1007/978-981-13-0947-2_11. [DOI] [PubMed] [Google Scholar]
- 5.Ghouri A, Conaghan PG. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis. 2019;11(1759720X19864492) doi: 10.1177/1759720X19864492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinatier C, Merceron C, Guicheux J. Osteoarthritis: From pathogenic mechanisms and recent clinical developments to novel prospective therapeutic options. Drug Discov Today. 2016;21:1932–1937. doi: 10.1016/j.drudis.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Yao Q, Chen Y, Zhou X. The roles of microRNAs in epigenetic regulation. Curr Opin Chem Biol. 2019;51:11–17. doi: 10.1016/j.cbpa.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Tian B, Qu X, Liu F, Tang T, Qin A, Zhu Z, Dai K. MicroRNAs play a role in chondrogenesis and osteoarthritis (review) Int J Mol Med. 2014;34:13–23. doi: 10.3892/ijmm.2014.1743. [DOI] [PubMed] [Google Scholar]
- 9.Malemud CJ. MicroRNAs and osteoarthritis. Cells 7: pii. 2018;(E92) doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Wu X. MicroRNA-103 contributes to osteoarthritis development by targeting Sox6. Biomed Pharmacother. 2019;118(109186) doi: 10.1016/j.biopha.2019.109186. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Yang J, Tan Z. Upregulation of microRNA-9-5p inhibits apoptosis of chondrocytes through downregulating Tnc in mice with osteoarthritis following tibial plateau fracture. J Cell Physiol. 2019;234:23326–23336. doi: 10.1002/jcp.28900. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Yan X, Zhang M, Ji F, Wang S. miR-21-5p protects IL-1β-induced human chondrocytes from degradation. J Orthop Surg Res. 2019;14(118) doi: 10.1186/s13018-019-1160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Wang Z, Shan Y, Pan Y, Ma J, Jia L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis. 2018;9(711) doi: 10.1038/s41419-018-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan KS, Lin CY, Liao TW, Peng CM, Lee SC, Liu YJ, Chan WP, Chou RH. EZH2 in cancer progression and potential application in cancer therapy: A friend or foe? Int J Mol Sci. 2017;18: pii(E1172) doi: 10.3390/ijms18061172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamagishi M, Uchimaru K. Targeting EZH2 in cancer therapy. Curr Opin Oncol. 2017;29:375–381. doi: 10.1097/CCO.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Wang Z, Lu W, Jiang H, Lu J, Qiu J, Ye G. EZH2 promotes gastric cancer cells proliferation by repressing p21 expression. Pathol Res Pract. 2019;215(152374) doi: 10.1016/j.prp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Lui JC, Garrison P, Nguyen Q, Ad M, Keembiyehetty C, Chen W, Jee YH, Landman E, Nilsson O, Barnes KM, Baron J. EZH1 and EZH2 promote skeletal growth by repressing inhibitors of chondrocyte proliferation and hypertrophy. Nat Commun. 2016;7(13685) doi: 10.1038/ncomms13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Zhang FJ, Luo W, Lei GH. Role of HIF-1α and HIF-2α in osteoarthritis. Joint Bone Spine. 2015;82:144–147. doi: 10.1016/j.jbspin.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Musumeci G, Castrogiovanni P, Loreto C, Castorina S, Pichler K, Weinberg AM. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: A morphological study. Int J Mol Sci. 2013;14:15767–15784. doi: 10.3390/ijms140815767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giunta S, Castorina A, Marzagalli R, Szychlinska MA, Pichler K, Mobasheri A, Musumeci G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci. 2015;16:5922–5944. doi: 10.3390/ijms16035922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musumeci G, Castrogiovanni P, Trovato FM, Weinberg AM, Al-Wasiyah MK, Alqahtani MH, Mobasheri A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16:20560–20575. doi: 10.3390/ijms160920560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Hu X, Cheng J, Zhang X, Zhao F, Shi W, Ren B, Yu H, Yang P, Li Z, et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat Commun. 2019;10(1914) doi: 10.1038/s41467-019-09839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szychlinska MA, Ravalli S, Musumeci G. Pleiotropic effect of fibrates on senescence and autophagy in osteoarthritis. EBioMedicine. 2019;45:11–12. doi: 10.1016/j.ebiom.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szychlinska MA, Castrogiovanni P, Nsir H, Di Rosa M, Guglielmino C, Parenti R, Calabrese G, Pricoco E, Salvatorelli L, Magro G, et al. Engineered cartilage regeneration from adipose tissue derived-mesenchymal stem cells: A morphomolecular study on osteoblast, chondrocyte and apoptosis evaluation. Exp Cell Res. 2017;357:222–235. doi: 10.1016/j.yexcr.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Trachana V, Ntoumou E, Anastasopoulou L, Tsezou A. Studying microRNAs in osteoarthritis: Critical overview of different analytical approaches. Mech Ageing Dev. 2018;171:15–23. doi: 10.1016/j.mad.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Dellago H, Bobbili MR, Grillari J. MicroRNA-17-5p: At the crossroads of cancer and aging-a mini-review. Gerontology. 2017;63:20–28. doi: 10.1159/000447773. [DOI] [PubMed] [Google Scholar]
- 28.Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu X, Feng Y, Dai Z. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46(e107) doi: 10.1038/emm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Lai Y, Ma J, Liu Y, Bi J, Zhang L, Chen L, Yao C, Lv W, Chang G, et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer. 2017;17(745) doi: 10.1186/s12885-017-3674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, Gu J, Li Y, Peng C, Shi M, Wang X, Wei G, Ge O, Wang D, Zhang B, et al. MiR-17-5p enhances pancreatic cancer proliferation by altering cell cycle profiles via disruption of RBL2/E2F4-repressing complexes. Cancer Lett. 2018;412:59–68. doi: 10.1016/j.canlet.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Jin J, Sun Z, Yang F, Tang L, Chen W, Guan X. miR-19b-3p inhibits breast cancer cell proliferation and reverses saracatinib-resistance by regulating PI3K/Akt pathway. Arch Biochem Biophys. 2018;645:54–60. doi: 10.1016/j.abb.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Miao D, Zhu Q, Huang J, Lu G, Xu W. MicroRNA-17-5p contributes to osteoarthritis progression by binding p62/SQSTM1. Exp Ther Med. 2018;15:1789–1794. doi: 10.3892/etm.2017.5622. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Duan L, Duan D, Wei W, Sun Z, Xu H, Guo L, Wu X. MiR-19b-3p attenuates IL-1β induced extracellular matrix degradation and inflammatory injury in chondrocytes by targeting GRK6. Mol Cell Biochem. 2019;459:205–214. doi: 10.1007/s11010-019-03563-2. [DOI] [PubMed] [Google Scholar]
- 34.Camilleri ET, Dudakovic A, Riester SM, Galeano-Garces C, Paradise CR, Bradley EW, McGee-Lawrence ME, Im HJ, Karperien M, Krych AJ, et al. Loss of histone methyltransferase Ezh2 stimulates an osteogenic transcriptional program in chondrocytes but does not affect cartilage development. J Biol Chem. 2018;293:19001–19011. doi: 10.1074/jbc.RA118.003909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stazi G, Zwergel C, Mai A, Valente S. EZH2 inhibitors: A patent review (2014-2016) Expert Opin Ther Pat. 2017;27:797–813. doi: 10.1080/13543776.2017.1316976. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Hu B, Shen H, Zhou H, Xue X, Chen Y, Chen S, Han Y, Yuan B, Zhao H, et al. Clinical and prognostic relevance of EZH2 in breast cancer: A meta-analysis. Biomed Pharmacother. 2015;75:218–225. doi: 10.1016/j.biopha.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Wu Y, Wu Y, Wang Y, Sun L, Li F. The inhibition of EZH2 ameliorates osteoarthritis development through the Wnt/β-catenin pathway. Sci Rep. 2016;6(29176) doi: 10.1038/srep29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trenkmann M, Brock M, Gay RE, Kolling C, Speich R, Michel BA, Gay S, Huber LC. Expression and function of EZH2 in synovial fibroblasts: epigenetic repression of the Wnt inhibitor SFRP1 in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1482–1488. doi: 10.1136/ard.2010.143040. [DOI] [PubMed] [Google Scholar]
- 39.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore BT, Xiao P. MiRNAs in bone diseases. Microrna. 2013;2:20–31. doi: 10.2174/2211536611302010004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.