Abstract
The aim of the present study was to determine the analgesic effects of ropivacaine combined with different doses of dexmedetomidine for ultrasound-guided transversus abdominis plane (TAP) block immediately following laparotomy in patients with gynecologic malignancies. A further aim was to determine the appropriate clinical dose of dexmedetomidine as an adjuvant for ropivacaine. Patients with gynecologic malignancies scheduled for laparotomy were randomly assigned to group R (TAP block with 0.3% ropivacaine), group RD1 (TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine), group RD2 (TAP block with ropivacaine and 1 µg/kg dexmedetomidine) and group RD3 (TAP block with ropivacaine and 2 µg/kg dexmedetomidine). TAP blocks were performed post-operatively. The four groups all received patient-controlled intravenous analgesia (PCIA) after the operation. The numerical rating scale (NRS) as well as the Ramsay sedation scale (RSS) scores, the first request time for PCIA bolus, oxycodone hydrochloride consumption, the plasma concentration of ropivacaine, the incidence of post-operative complications and adverse events, and patient satisfaction were recorded. Post-operative NRS scores at rest exhibited significant differences between the R group and all the RD groups at 24 h after surgery (P<0.05). Compared with the other groups, the NRS score in the RD3 group was decreased (P<0.05). The RSS scores were higher in all of the RD groups compared with those in the R group at 2 h (P<0.05) and were highest in the RD3 group compared with those in all other groups at 4 h (P<0.05). The first request time for PCIA was significantly longer in the RD3 group compared with that in the RD2, RD1 and R groups (510.47±102.67, 595.47±100.11, 682.43±104.46 and 776.42±143.91 min, respectively; P<0.05). Cumulative opioid consumption based on the number of PCIA bolus requested at 24 and 48 h post-operatively indicated that the total number of PCIA boluses was significantly lower in the RD groups compared with those in the R group at 24 and 48 h (P<0.05). The ropivacaine concentration did not differ among the four groups. There was no significant difference between groups with respect to post-operative nausea and vomiting, bradycardia and hypotension; however, all RD groups had a higher patient satisfaction than group R (P<0.05). Compared with that in the other groups, the duration of post-anesthesia care unit stay in group RD3 was relatively longer due to excessive sedation (P<0.05). In conclusion, TAP blockade using 0.5-2 µg/kg dexmedetomidine combined with 0.3% ropivacaine is a safe and effective treatment for analgesia in laparotomy procedures for gynecologic malignancies. The study was registered in the Chinese Clinical Trial Registry (CHICTR; www.chictr.org.cn) on January 15th, 2019 (registration no. ChiCTR1900020995).
Keywords: dexmedetomidine, transversus abdominis plane, ropivacaine, adjuvant
Introduction
Common gynecological malignancies, including cervical, ovarian and endometrial cancer, are potentially life-threatening. Early surgical intervention is the first-line treatment for early-stage forms of these cancer types. For patients with early-stage cervical cancer, open abdominal radical hysterectomy has higher rates of disease-free survival and overall survival than minimally invasive radical hysterectomy (1). However, patients who undergo major open abdominal surgery under general anesthesia frequently experience severe post-operative pain. Traditional use of systemic opioids may provide effective pain relief but undesired side effects are frequent (2).
Transversus abdominis plane (TAP) block is an integral part of multimodal analgesia and traditionally involves the delivery of local anesthetics into the abdominal wall to enhance analgesia following abdominal surgery (3,4). Ropivacaine, the most commonly used long-acting local anesthetic, on its own may provide analgesia for 9-14 h. However, pain after open surgery may last for 24-72 h (5). Identifying an adjuvant to the local anesthetic that may prolong the duration of the TAP block has garnered significant interest.
Dexmedetomidine is a highly selective α2-adrenoceptor agonist that possesses sedative, analgesic and anti-inflammatory properties. Dexmedetomidine has been suggested to have a longer duration of analgesia than other adjuvants without inducing neurotoxic effects (6). Furthermore, it has previously been demonstrated to prolong the duration of analgesia when combined with local anesthetics in various regional blocks. Numerous clinical studies have highlighted the benefits of dexmedetomidine when combined with ropivacaine for TAP block (7-9). However, the optimal dosing of dexmedetomidine for adjunctive use in TAP blocks has remained to be determined.
The objective of the present study was to observe the safety and efficacy of varying doses of adjunctive dexmedetomidine combined with ropivacaine for TAP block following major open abdominal surgery in patients with gynecological malignancies.
Materials and methods
Patients
This prospective, randomized, double-blinded, controlled clinical trial was approved by the ethics committee of Hunan Cancer Hospital (Changsha, China) and registered on January 15th, 2019 in the Chinese Clinical Trial Registry (CHICTR; www.chictr.org.cn; registration no. ChiCTR1900020995). All patients provided written informed consent.
A total of 105 patients (age, 18-60 years) with American Society of Anesthesiologists physical status of I-II and a body mass index (BMI) of 18.5-23.9 kg/m2, who were scheduled for elective laparotomy for gynecological malignant tumor under general anesthesia between January 1st, 2019 and April 31st, 2019, were enrolled in the present study. The exclusion criteria were as follows: Local infection at the site of injection; coagulopathy; severe cardiovascular or respiratory disease; hepatorenal insufficiency; hypersensitivity or allergy to dexmedetomidine or ropivacaine; chronic use of analgesics; mental disorders; or an inability to understand and express the pain score on the numerical rating scale (NRS). During the pre-operative visit, patients were taught how to operate the analgesic pump and were further educated on the standardized pain scales that would be utilized in this study.
Study design and randomization
The randomization was performed by an anesthesia nurse who was not involved in the anesthetic management of the patients or the collection of data. The randomization scheme was generated using a random sampling number table.
All patients were randomly allocated to one of the following groups: i) Ropivacaine group (group R) and ii) dexmedetomidine combined with ropivacaine group (group RD). Group RD was further divided into three subgroups according to the dose of dexmedetomidine: i) 0.5 µg/kg dexmedetomidine combined with ropivacaine (group RD1), ii) 1 µg/kg dexmedetomidine combined with ropivacaine (group RD2) and iii) 2 µg/kg dexmedetomidine combined with ropivacaine (group RD3). The dose of dexmedetomidine was determined according to each patient's ideal body weight. Following surgical site closure, patients in group R received TAP block with 50 ml of 0.3% ropivacaine, while patients in group RD received TAP block with the various doses of dexmedetomidine, combined with 50 ml of 0.3% ropivacaine.
Anesthesia and TAP block procedure
The anesthetic technique was standardized for all patients. General anesthesia was induced with midazolam (0.05 mg/kg), sufentanyl (4 µg/kg), propofol (1 mg/kg) and rocuronium (0.6 mg/kg). Propofol, remifentanil and sevoflurane were used for anesthetic maintenance. The bispectral index (BIS) was maintained at 40-60 and intra-operative blood pressure and heart rate fluctuations within 20% of the baseline. Cisatracurium was administered as required during the surgery. Remifentanil was discontinued 10 min prior to the end of the surgery and 10 µg of sufentanil and 5 mg of tropisetron were injected. After wound closure, ultrasound-guided bilateral dual TAP blocks were performed by experienced anesthesiologists according to Lee et al (10). All blocks were performed using the same real-time ultrasound (Sonosite) and linear probe (5-12 Hz). For the subcostal approach, the probe, covered by a protective plastic sheath, was placed over the anterior abdominal wall immediately inferior and parallel to the costal margin. The external oblique, internal oblique and transversus abdominis muscles (the three lateral abdominal wall muscles) were identified. After visualization of these layers, the probe was moved toward the midline until the rectus abdominis muscle was identified. A 21 G needle (Pajunk GmbH) was inserted through the skin at the end of the ultrasound probe and inserted in-plane to lie between the posterior rectus abdominis and transversus abdominis muscle or posterior rectus sheath. For the posterior TAP block, the probe was placed in a transverse orientation over the anterolateral aspect of the abdominal wall, between the iliac crest and costal margin. When the three lateral abdominal wall muscles were noted, the same needle was inserted through the skin and in-plane to lie between the internal oblique and transversus abdominis muscle. The injection was performed at approximately the mid-axillary line. The needle tip was observed using ultrasound-guided visualization in the determined plane and 1 ml of saline was injected to ensure accurate drug diffusion in the appropriate location. The volumes of the study drug used at each side were 15 ml for the posterior approach and 10 ml for the subcostal approach, respectively.
Post-operative analgesia management
After the TAP blocks, all patients were transported to the post-anesthesia care unit (PACU) for resuscitation and treated with a wireless intravenous analgesic pump using the following analgesic formula: Oxycodone hydrochloride (50 mg) and tropisetron (5 mg), dissolved in normal saline and diluted to 100 ml. The analgesia pump parameters were set as follows: No initial dose; no basal infusion; bolus, 3 ml (1.5 mg); lockout time, 5 min; maximum rate, 20 ml/h. Patients with persistent pain received an intramuscular injection of 30 mg ketorolac butanediol as rescue analgesia.
Measurements
Patient characteristics (age, weight, height, BMI, blood pressure, heart rate and any complications), surgical information (duration of surgery, incision range, incision length and blood loss) were recorded. The primary outcomes in this present study were the NRS and Ramsay sedation scale (RSS) scores at 2, 4, 12, 24 and 48 h after surgery while at rest; the number of patient-controlled intravenous analgesia (PCIA) boluses requested and oxycodone hydrochloride consumption during the first two days after surgery; and the time to first PCIA bolus requested following the TAP blocks. The incidence of post-operative nausea and vomiting (PONV), respiratory depression, hypotension (mean arterial pressure decrease by >20% from pre-induction values) and bradycardia (HR <50 beats/min) as well as duration of PACU stay, any complication with the TAP blocks, analgesia satisfaction, block failure rate (defined as cases with an NRS pain score ≥4 at rest within 2 h of surgery) and rescue analgesia requirements were recorded.
Radial artery blood was collected at 10, 25, 60, 120, 240 and 360 min after ropivacaine administration. Post-operative visits were performed by an anesthesiologist who was not involved in the patient's anesthesia. Data collection from the wireless analgesic pump system was performed by a trained staff member.
The standard NRS was used to evaluate post-operative pain: Score 0, no pain or discomfort; score <3, slight pain; score 4-6, moderate pain that affects sleep but is tolerable; score 7-10, severe pain that is intolerable. The RSS was used to evaluate the depth of sedation: 1 point, irritability or anxiety; 2 points, good directive force and cooperation ability; 3 points, drowsiness, but ability to respond to instructions; 4 points, able to respond to strong sound stimuli or eyebrow reaction; 5 points, slow response to strong sound stimuli or eyebrow reaction; and 6 points, no response to strong sound stimuli or light eyebrow reaction Overall patient satisfaction with pain control was assessed using the Likert's scale (11) from patients at 48 h post operation. The scale was defined as follows: 1, very dissatisfied; 2, dissatisfied; 3, unsure; 4, satisfied; and 5, very satisfied. The ratio of patients with scores ≥4, to the total number of patients in each group was used to evaluate patient satisfaction with the post-operative analgesia.
The ropivacaine standard (purity, 99.9%) was purchased from The China Institute of Drug Control. Acetonitrile and methanol MS grade were obtained from Dikma Technologies, Inc. Samples were centrifuged for 10 min at -1006.2 x g at 4˚C within 30 min of collection. Plasma samples were stored at -80˚C until further use. Determination of the ropicacaine concentration was performed using Acquity equipment (Shimadzu Corp.) and ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry (Shanghai AB SCIEX Analytical Instrument Trading Co.). Chromatographic separation was performed on a Phenomenex Kinetex 1.7 µm C18 100A 100x2.1 mm. The mobile phase was 0.1% formic acid in water and acetonitrile (1:1, v/v), the flow rate was 0.3 ml/min and the elution time of 3 min. To measure the plasma concentration of ropivacaine, 100 µl acetonitrile was added to 100 µl plasma samples in a 1.5-ml tube. Samples were vortexed for 1 min and centrifuged for 10 min at 15984 x g, at 4˚C. The supernatant was then filtered through a 0.45-µm filter. Finally, 100 µl of supernatant was injected into the UPLC system. A standard solution of ropivacaine hydrochloride was prepared by diluting the stock solution with patients' plasma to a concentration of 0.1, 0.2, 0.4, 0.8, 2 or 4 µg/ml and stored at 4˚C prior to further use.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp). Normally distributed data (age, weight, height, BMI, operation time, length of incision, blood loss, cumulative opioid consumption, the first request time for PCIA, curation of PACU stay and time for TAP block) are expressed as the mean ± standard deviation and differences between groups were evaluated by one-way analysis of variance and least-significant differences tests. Non-normally distributed interval and ordinal data (NRS and RSS scores) are expressed as the median (range or interquartile range) and were compared among groups using the Kruskal-Wallis H-test. Post-hoc analysis was performed using Bonferroni corrections for multiple comparisons (four groups, P=0.05/4=0.0125). Categorical variables were analyzed using Fisher's exact test or Pearson's χ2 test. P<0.05 was considered to indicate statistical significance.
Results
Patient characteristics
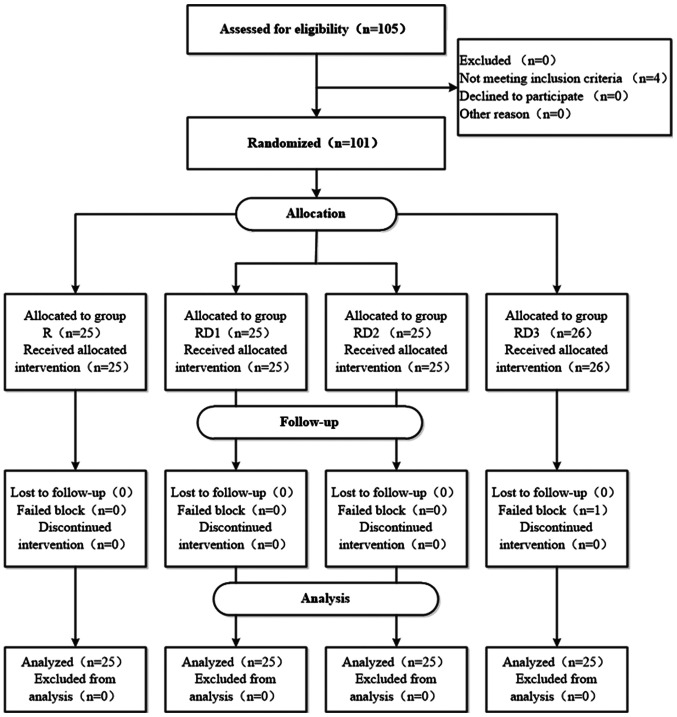
Among the 105 patients who were eligible for the study, 4 patients did not satisfy the inclusion criteria. A total of 101 patients met the eligibility criteria and were randomized into one of the four groups. Only 1 patient did not complete the experiment due to failed regional blockade (Fig. 1). There were no significant differences in patient characteristics and surgical profiles between the groups (Table I).
Figure 1.
Flow diagram demonstrating the number of patients at each phase of the study. R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine.
Table I.
Comparison of patient characteristics and surgical profiles between groups.
| Item | Group R (n=25) | Group RD1 (n=25) | Group RD2 (n=25) | Group RD3 (n=26) |
|---|---|---|---|---|
| Age (years) | 49.28±8.23 | 50.16±6.16 | 48.64±7.17 | 48.73±9.10 |
| BMI (kg/m2) | 21.04±1.74 | 20.92±1.81 | 20.91±1.63 | 20.99±1.65 |
| ASA class I/II status | 11/14 | 13/12 | 14/11 | 12/14 |
| Type of surgery | ||||
| Cervical cancer | 16 | 17 | 15 | 14 |
| Ovarian cancer | 9 | 8 | 10 | 12 |
| Operation time (min) | 181.92±33.02 | 180.56±34.23 | 175.40±34.70 | 185.08±32.57 |
| Length of incision (cm) | 21.14±2.05 | 21.80±1.80 | 21.36±1.98 | 22.08±1.68 |
| Blood loss (ml) | 317.00±107.94 | 340.20±79.81 | 338.20±83.24 | 326.80±85.58 |
All values are presented as mean ± SD or number of patients. There were no significant difference between groups. R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine; BMI, body mass index; ASA, American Society of Anesthesiologists; TAP, transversus abdominis plane.
Post-operative NRS scores
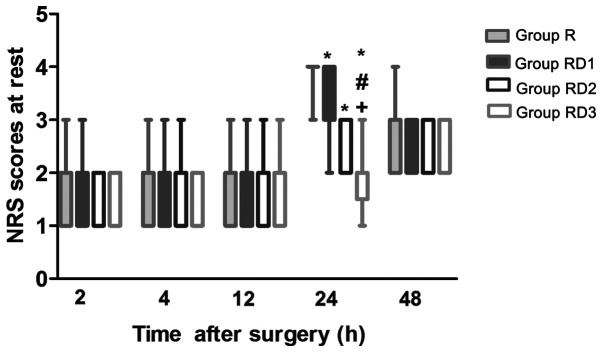
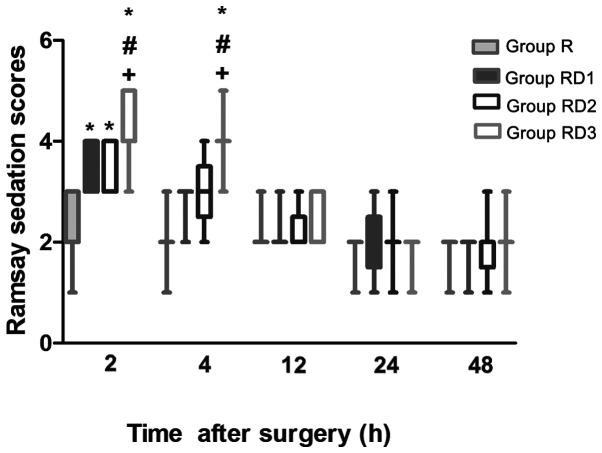
Post-operative NRS scores at rest are presented in Fig. 2. There were no significant differences between group R and any of the RD groups during the first 12 h after surgery (P>0.05). Compared with those in group R, the NRS scores were significantly lower in all of the RD groups, with the scores in group RD3 being significantly lower than those in groups RD1 and RD2 at 24 h (P<0.05). The NRS scores were comparable among all of the groups at 48 h after surgery (P>0.05). The RSS scores were higher in all of the RD groups compared with those in group R at 2 h (P<0.05) and were still higher in group RD3 compared with those in the other three groups at 4 h (P<0.05). There were no significant differences in the RSS scores among the four groups at 12, 24 and 48 h (Fig. 3).
Figure 2.
Box plots of post-operative NRS scores at different time points between the four groups. Data are presented as median (interquartile range) and extreme vales. *P<0.05 vs. group R. #P<0.05 vs. group RD1. +P<0.05 vs. group RD2. R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine.
Figure 3.
Box plots of post-operative Ramsay sedation scores at different time points between the four groups. Data are presented as median (interquartile range) and extreme values. *P<0.05 vs. group R. #P<0.05 vs. group RD1. +P<0.05 vs. group RD2. R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine.
PCIA
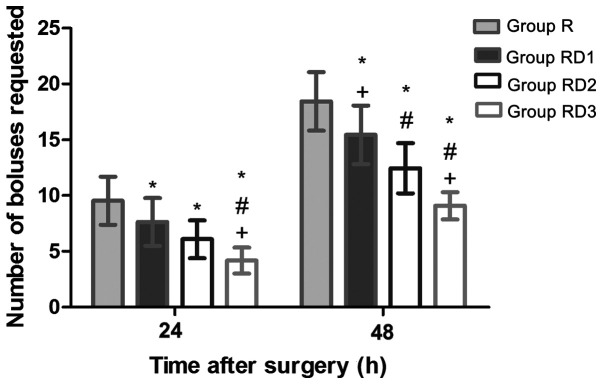
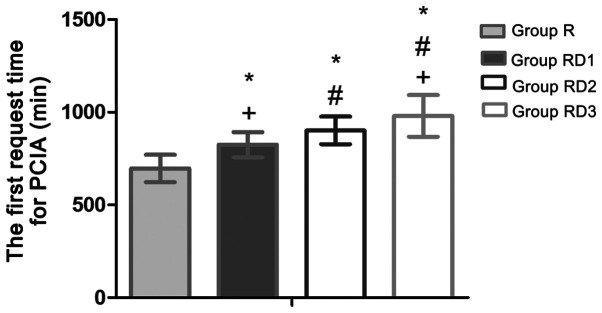
Cumulative opioid consumption based on the number of PCIA boluses requested at 24 and 48 h post-operatively revealed that the total number of PCIA boluses was significantly lower in the RD groups compared with that in group R (P<0.05; Fig. 4). As indicated in Fig. 5, the first request time for PCIA was significantly later in group RD3 compared with that in other groups (510.47 min in group R, 595.47 min in group RD1, 682.43 min in group RD2, and 776.42 min in group RD3; P<0.05). Thus, Compared with group R, group RD3, RD2 and RD1 extended 265.95, 171.96 and 85.00 min of analgesia time respectively (P<0.05).
Figure 4.
Cumulative opioid consumption based on the number of patient-controlled intravenous analgesia bolus requested at 24 and 48 h post-operatively. Data are presented as mean ± SD. *P<0.05. vs. group R. #P<0.05 vs. group RD1. +P<0.05 vs. group RD2. R R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine.
Figure 5.
The first request time for PCIA in four groups. Data are presented as mean ± SD. Notes: *P<0.05 vs. group R. #P<0.05 vs. group RD1. +P<0.05 vs. group RD2. PCIA, patient-controlled intravenous analgesia; R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine.
Post-operatively, 3 patients in group R (3/25) required analgesia with 30 mg IM of ketorolac, compared to 1 patient in group RD1 (1/25). However, the differences were not statistically significant among all the groups (P=0.189).
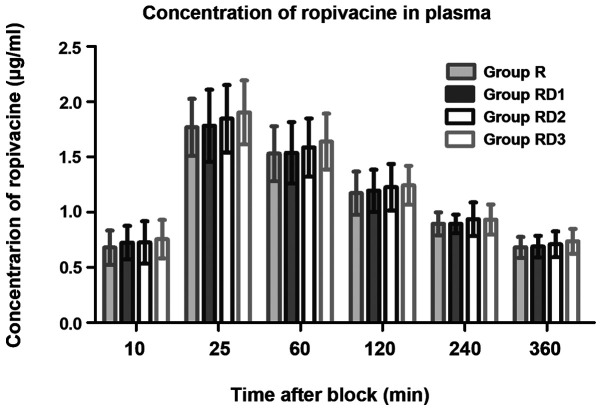
Plasma ropivacaine
There were no significant differences in ropivacaine plasma levels among all groups at any of the time-points (P>0.05; Fig. 6).
Figure 6.
Ropivacaine plasma concentration at any time point in four groups. Data are presented as mean ± SD. R, ropivacaine; RD, ropivacaine with dexmedetomidine.
Post-operative complications and patient satisfaction
Table II outlines the results of the assessment of the incidence of post-operative complications and patient satisfaction on post-operative day 2 between groups. There was no significant difference between all groups with respect to PONV, bradycardia and hypotension (P>0.05). No differences were observed in the incidence of respiratory depression and TAP block complication rates among the four groups (P>0.05). Compared with that in the other groups, the duration of PACU stay in group RD3 was relatively longer due to excessive sedation (P<0.05). Each RD group demonstrated higher patient satisfaction scores than group R (P<0.05).
Table II.
Comparison of post-operative parameters among all groups.
| Item | Group R (n=25) | Group RD1 (n=25) | Group RD2 (n=25) | Group RD3 (n=25) | P-value |
|---|---|---|---|---|---|
| Nausea | 4 | 3 | 2 | 2 | 0.897 |
| Vomiting | 3 | 2 | 2 | 1 | 0.956 |
| Respiratory depression | 0 | 0 | 0 | 0 | - |
| Bradycardia | 0 | 1 | 3 | 3 | 0.303 |
| Hypotension | 0 | 0 | 3 | 4 | 0.079 |
| Duration of PACU stay (min) | 51.32±10.47 | 53.24±14.76 | 52.20±9.22 | 62.68±15.40 | 0.007 |
| Complications of TAP block | 0 | 0 | 0 | 0 | - |
| Patient satisfaction | 15 | 19 | 22 | 23 | 0.025a |
All values are presented as mean ± SD or number of patients.
aP<0.05 vs. group R. R, TAP block with 0.3% ropivacaine; RD1, TAP block with ropivacaine and 0.5 µg/kg dexmedetomidine; RD2, TAP block with ropivacaine and 1 µg/kg dexmedetomidine; RD3, TAP block with ropivacaine and 2 µg/kg dexmedetomidine; PACU, post-anesthesia care unit; TAP, transversus abdominis plane.
Discussion
The results of the present prospective study suggested that the addition of dexmedetomidine to ropivacaine improves the efficacy of TAP block in a dose-dependent fashion, prolonging the duration of analgesia, reducing the requirement of analgesics and reducing post-operative pain in patients with gynecological malignancies undergoing open abdominal surgery. In 2011, Brummett et al (12) observed a prolonged duration of analgesia following perineurally administered ropivacaine with dexmedetomidine adjuvant for sciatic nerve block in rats. Subsequently, numerous studies have indicated that the addition of dexmedetomidine to local anesthetics may prolong the action time of the latter in brachial plexus nerve block (13), sciatic nerve block (12), femoral nerve block (14) and TAP block (7-9), without increasing associated complications. The results of the present study suggest that dexmedetomidine prolongs the duration of the action of ropivacaine and enhances its analgesic effects, which is consistent with previous studies (7-9).
Despite previous studies providing promising results for dexmedetomidine as an adjunct for TAP blockade, its optimal dosing has remained to be determined. Xu et al (8) reported that ropivacaine combined with 0.5 µg/kg dexmedetomidine for TAP block and rectus sheath block were more effective than ropivacaine on its own in prolonging the duration of analgesia and reducing post-operative pain in elderly high-risk patients undergoing emergency abdominal surgery. Luan et al (9) also indicated that 0.5 µg/kg dexmedetomidine is able to potentiate the analgesic properties of ropivacaine in TAP block after abdominal hysterectomy surgery. A separate study suggested that dexmedetomidine used at 50, 100 or 150 µg, mixed with 3 ml of 0.75% ropivacaine, for ulnar nerve blockade, increases the duration of sensory blockade in a dose-dependent fashion (15). Jung et al (16) added three different doses of dexmedetomidine (1, 1.5 and 2 µg/kg) to ropivacaine for interscalene brachial plexus blockade after arthroscopic shoulder surgery and obtained no significant difference in the duration of analgesia between the groups treated with 1 and 1.5 µg/kg dexmedetomidine. Only patients treated with dexmedetomidine at 2 µg/kg had significantly prolonged analgesia; however, this also increased the risk of hypotension. Based on the above, the present study included three doses of dexmedetomidine (0.5, 1 and 2 µg/kg) in order to observe the effect of dexmedetomidine in adjunct to TAP blocks and to determine the optimal dose while minimizing the incidence of adverse events. In the present study, no significant differences were observed in the post-operative NRS scores among all four groups during the first 12 h after surgery. This suggests that the duration of action of ropivacaine was sufficient to provide analgesia within 12 h after TAP blockade. By contrast, at 24 h post-operation, the pain score in the R group was significantly higher than that in the RD groups. The analgesic effect of the TAP blocks appeared to be in parallel with the increase in the dose of adjunctive dexmedetomidine. The first request time for PCIA was significantly longer in the RD3 group. Although the RSS scores in the RD3 group were higher at 4 h after surgery, the clinical observations of the current study have indicated that there was no difference in the RSS scores of each group at 6 h; therefore, a short period of excessive sedation should not reduce the demand for PCIA after surgery. The results of the present study suggested that the addition of dexmedetomidine (0.5-2 µg/kg) to ropivacaine for TAP blockade may improve the duration and quality of analgesia, as well as reduce oxycodone hydrochloride consumption after surgery, in a dose-dependent fashion.
The common adverse events to dexmedetomidine are hypotension and bradycardia. However, There were no statistically significant differences in the incidence of bradycardia and hypotension among the four groups. These results differ from those of Jung et al (16) and may be attributable to the fact that the TAP blocks in this present study were performed immediately after surgery. Furthermore, the patients were emerging from anesthesia, during which the patients' enhanced sympathetic activity may have potentially offset the hypotension and decreased the heart rate typically caused by dexmedetomidine. These differences may become apparent, as the number of cases increased, particularly in the high-dose dexmeditomidine group. In addition, 4 patients treated with 2 µg/kg dexmedetomidine had delayed tracheal extubation, attributed to excessive sedation. Therefore, it appears that a dose of 1 µg/kg dexmedetomidine presented a good balance between prolongation of the analgesic effect and the intensity of sedation.
In 2017, Ding et al (17) proposed that adding dexmedetomidine (1 µg/kg) to ropivacaine does not significantly improve the quality or duration of TAP block. The possible reasons may be as follows: In the study by Ding et al (17), the type of operation performed was open gastrectomy and the block location was near the costal margin to the midline, which was different from the block location in the present study. The total capacity of blocking drugs was also different. A total of 15 ml was given on each side, compared with 25 ml on each side (15 ml in the posterior approach and 10 ml in the subcostal approach) in the present study. There are 3 types of TAP block. Subcostal TAP block is effective for upper abdominal surgery where the surgical incision extends from T6 to T9 dermatomes. Lateral TAP blocks and posterior TAP blocks are effective in providing analgesia after lower abdominal surgery where the incision extends from T10 to L1 dermatomes. Niraj et al (18) compared the four-quadrant TAP block (bilateral subcostal and lateral TAP block) with epidural analgesia in patients undergoing laparoscopic colorectal surgery and determined that the two methods have a similar analgesic efficacy. The four-quadrant TAP block was utilized in the present study, as the laparotomy incision for gynecological malignant tumors frequently extends from the upper abdomen to the lower abdomen.
There were two purposes of measuring the plasma concentration of ropivacaine in the present study. One purpose was to ensure that the patients did not develop local anesthetic poisoning. The other purpose was to determine whether dexmedetomidine would affect the plasma concentration of ropivacaine. In order to achieve adequate analgesia, TAP blocks require injection of a large dose of local anesthetic (2.5-3 mg/kg of ropivacaine) in the form of a single high-volume bolus into a relatively vascular plane. However, Griffiths et al (19) tested plasma concentrations of ropivacaine after administering 3 mg/kg of ropivacaine in TAP block to adult healthy females and the mean maximum plasma ropivacaine concentration reached 2.54 µg/ml without symptoms of local anesthetic toxicity at 30 min post-block, which exceeded the potentially toxic threshold of 2.2 µg/ml. Other studies have reported similar results, even in patients without cardiac or renal failure (20,21). Another study observed that in a patient with cardiac and renal failure, 1.8 mg/kg of ropivacaine caused local anesthetic systemic toxicity (22). In the present study, all subjects had gynecological malignancies. Certain participants had undergone chemotherapy prior to surgery, which may have potentially caused a certain degree of organ dysfunction. In the present study, the average blood concentration of ropivacaine was 1.9 µg/ml, with the highest reaching 2.35 µg/ml without any symptoms of local anesthetic toxicity. The potential intoxication threshold was exceeded. Most of the patients had received chemotherapy pre-operatively, suggesting a requirement to reduce the local anesthetic dose for TAP block in those patients.
The mechanism of action by which dexmedetomidine mediates peripheral nerve block has not been fully elucidated. In the present study, there were no significant differences in the plasma concentration of ropivacaine among all of the groups, which is consistent with previous studies (17,23). These results indicated that the prolonged block duration conferred by the adjuvant dexmedetomidine was neither achieved by changing the plasma concentration of ropivacaine nor by delaying the peak plasma concentration of ropivacaine. The underlying mechanism of action is thought to involve the blocking of the hyperpolarization-activated cation current, enhancing the membrane hyperpolarization and inhibiting the subsequent action potential (12). Dexmedetomidine may also cause local vasoconstriction, thus prolonging the time for the local anesthetic to enter the blood vessels and be removed from the site of injection (24). Further studies are required to clarify the mechanism through which adjuvant dexmedetomidine prolongs the duration of TAP block.
There were several limitations to the present study. First, the study participants were diagnosed with different gynecologic malignancies. As a result, various surgical approaches were employed, which may lead to a variation in the procedures. Furthermore, TAP block was completed after wound closure surgery. As patients were not fully recovered, it was not possible to analyze whether dexmedetomidine may affect the onset time of TAP block. Finally, clinical signs or symptoms of neurotoxicity were not assessed.
In conclusion, in patients undergoing open surgery for gynecological malignant tumors, adjuvant dexmedetomidine (0.5-2 µg/kg) with ropivacaine may prolong the duration of TAP blockade and confer a greater analgesic effect than ropivacaine alone, in a dose-dependent fashion. This result has the potential to significantly reduce post-operative opioid consumption. The prolonged TAP block duration seen with adjuvant dexmedetomidine is not achieved by altering ropivacaine plasma concentrations.
Furthermore, the dose of ropivacaine utilized for TAP blockade should be decreased in patients who receive pre-operative chemotherapy to avoid toxic local anesthetic plasma concentrations secondary to chemotherapy-induced organ dysfunction and altered drug metabolism.
The results of the present study demonstrated that TAP blocks with adjuvant dexmedetomidine (0.5-2 µg/kg) combined with 0.3% ropivacaine are effective in patients with gynecological malignancies undergoing open abdominal surgery. However, patients treated with 2 µg/kg dexmedetomidine had an increased risk of delayed tracheal extubation due to excessive sedation. It is therefore recommended that 1µg/kg dexmedetomidine is used, combined with 0.3% ropivacaine to optimize the safety and efficacy of TAP blocks for open surgery for gynecologic malignancies.
Acknowledgements
Not applicable.
Funding
The current work was funded by the Foundation of Hunan Natural Resources Fund in China (grant no. 2019JJ40180).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YZ performed the experiments. YW collected and analyzed data. JY designed the study. HS conceived and designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of Hunan Cancer Hospital in Changsha, China and registered on January 15th, 2019 in the Chinese Clinical Trial Registry (CHICTR; www.chictr.org.cn; registration no. ChiCTR1900020995). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, Seagle BL, Alexander A, Barber EL, Rice LW, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivard C, Dickson EL, Vogel RI, Argenta PA, Teoh D. The effect of anesthesia choice on post-operative outcomes in women undergoing exploratory laparotomy for a suspected gynecologic malignancy. Gynecol Oncol. 2014;133:278–282. doi: 10.1016/j.ygyno.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S, Kalra S, Sharma B, Sahai C, Sood J. Evaluation of ultrasound-guided transversus abdominis plane block for postoperative analgesia in patients undergoing intraperitoneal onlay mesh repair. Anesth Essays Res. 2019;13:126–131. doi: 10.4103/aer.AER_176_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang H, Rimel BJ, Li AJ, Cass I, Karlan BY, Walsh C. Ultrasound guided transversus abdominis plane (TAP) block utilization in multimodal pain management after open gynecologic surgery. Gynecol Oncol Rep. 2018;26:75–77. doi: 10.1016/j.gore.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suresh S, Ecoffey C, Bosenberg A, Lonnqvist PA, de Oliveira GS Jr, de Leon Casasola O, de Andrés J, Ivani G. The European society of regional anaesthesia and pain therapy/American society of regional anesthesia and pain medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43:211–216. doi: 10.1097/AAP.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 6.Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49:104–116. doi: 10.1097/AIA.0b013e31820e4a49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarvesh B, Shivaramu BT, Sharma K. Addition of dexmedetomidine to ropivacaine in subcostal transversus abdominis plane block potentiates postoperative analgesia among laparoscopic cholecystectomy patients: A prospective randomized controlled trial. Anesth Essays Res. 2018;12:809–813. doi: 10.4103/aer.AER_141_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Hu Z, Shen J, McQuillan PM. Efficacy of US-guided transversus abdominis plane block and rectus sheath block with ropivacaine and dexmedetomidine in elderly high-risk patients. Minerva Anestesiol. 2018;84:18–24. doi: 10.23736/S0375-9393.17.11538-5. [DOI] [PubMed] [Google Scholar]
- 9.Luan H, Zhang X, Feng J, Zhu P, Li J, Zhao Z. Effect of dexmedetomidine added to ropivacaine on ultrasound-guided transversus abdominis plane block for postoperative analgesia after abdominal hysterectomy surgery: A prospective randomized controlled trial. Minerva Anestesiol. 2016;82:981–988. [PubMed] [Google Scholar]
- 10.Lee TH, Barrington MJ, Tran TM, Wong D, Hebbard PD. Comparison of extent of sensory block following posterior and subcostal approaches to ultrasound-guided transversus abdominis plane block. Anaesth Intensive Care. 2010;38:452–460. doi: 10.1177/0310057X1003800307. [DOI] [PubMed] [Google Scholar]
- 11.Lobo Prabhu K, Cleghorn MC, Elnahas A, Tse A, Maeda A, Quereshy FA, Okrainec A, Jackson TD. Is quality important to our patients? The relationship between surgical outcomes and patient satisfaction. BMJ Qual Saf. 2017;27:48–52. doi: 10.1136/bmjqs-2017-007071. [DOI] [PubMed] [Google Scholar]
- 12.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–843. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Boghdadly K, Brull R, Sehmbi H, Abdallah FW. Perineural dexmedetomidine is more effective than clonidine when added to local anesthetic for supraclavicular brachial plexus block: A systematic review and meta-analysis. Anesth Analg. 2017;124:2008–2020. doi: 10.1213/ANE.0000000000002014. [DOI] [PubMed] [Google Scholar]
- 14.Abdulatif M, Fawzy M, Nassar H, Hasanin A, Ollaek M, Mohamed H. The effects of perineural dexmedetomidine on the pharmacodynamic profile of femoral nerve block: A dose-finding randomised, controlled, double-blind study. Anaesthesia. 2016;71:1177–1185. doi: 10.1111/anae.13603. [DOI] [PubMed] [Google Scholar]
- 15.Keplinger M, Marhofer P, Kettner SC, Marhofer D, Kimberger O, Zeitlinger M. A pharmacodynamic evaluation of dexmedetomidine as an additive drug to ropivacaine for peripheral nerve blockade: A randomised, triple-blind, controlled study in volunteers. Eur J Anaesthesiol. 2015;32:790–796. doi: 10.1097/EJA.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 16.Jung HS, Seo KH, Kang JH, Jeong JY, Kim YS, Han NR. Optimal dose of perineural dexmedetomidine for interscalene brachial plexus block to control postoperative pain in patients undergoing arthroscopic shoulder surgery. Medicine. 2018;97(e0440) doi: 10.1097/MD.0000000000010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding W, Li W, Zeng X, Li J, Jiang J, Guo C, Li W. Effect of Adding dexmedetomidine to ropivacaine on ultrasound-guided dual transversus abdominis pane block after Gastrectomy. J Gastrointest Surg. 2017;21:936–946. doi: 10.1007/s11605-017-3402-5. [DOI] [PubMed] [Google Scholar]
- 18.Niraj G, Kelkar A, Hart E, Horst C, Malik D, Yeow C, Singh B, Chaudhri S. Comparison of analgesic efficacy of four-quadrant transversus abdominis plane (TAP) block and continuous posterior TAP analgesia with epidural analgesia in patients undergoing laparoscopic colorectal surgery: An open-label, randomised, non-inferiority trial. Anaesthesia. 2014;69:348–355. doi: 10.1111/anae.12546. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105:853–856. doi: 10.1093/bja/aeq255. [DOI] [PubMed] [Google Scholar]
- 20.Weiss E, Jolly C, Dumoulin JL, Meftah RB, Blanié P, Laloë PA, Tabary N, Fischler M, Le Guen M. Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean analgesia. Reg Anesth Pain Med. 2014;39:248–251. doi: 10.1097/AAP.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths JD, Le NV Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110:996–1000. doi: 10.1093/bja/aet015. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Tanaka S, Sakamoto A, Hirabayashi T, Kawamata M. Plasma ropivacaine concentration after TAP block in a patient with cardiac and renal failure. Local Reg Anesth. 2018;11:57–60. doi: 10.2147/LRA.S173877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, Gerner P, Brummett CM. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective 1houlder surgery when compared with ropivacaine slone. Reg Anesth Pain Med. 2014;39:37–47. doi: 10.1097/AAP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 24.Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25:403–409. doi: 10.1017/S0265021507003079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.