Abstract
Down syndrome (DS) is a multisystem disorder affecting one in 800 births worldwide. Advancing technology, medical treatment, and social intervention have dramatically increased life expectancy, yet there are many etiologies of this disorder that are in need of further research. The advent of the ability to capture extracellular vesicles (EVs) in blood from specific cell types allows for the investigation of novel intracellular processes. Exosomes are one type of EVs that have demonstrated great potential in uncovering new biomarkers of neurodegeneration and disease, and also that appear to be intricately involved in the transsynaptic spread of pathogenic factors underlying Alzheimer’s disease (AD) and other neurological diseases. Exosomes are nanosized vesicles, generated in endosomal multivesicular bodies (MVBs) and secreted by most cells in the body. Since exosomes are important mediators of intercellular communication and genetic exchange, they have emerged as a major research focus and have revealed novel biological sequelae involved in conditions afflicting the DS population. This review summarizes current knowledge on exosome biology in individuals with DS, both early in life and in aging individuals. Collectively these studies have demonstrated that complex multicellular processes underlying DS etiologies may include abnormal formation and secretion of extracellular vesicles such as exosomes.
Keywords: Down syndrome, Alzheimer’s disease, Neurodegeneration, Extracellular vesicles, Biomarkers
1. Introduction
Down syndrome (DS) is the most common survivable genetic developmental condition, caused by a triplication of Chromosome 21 (Hsa21); (Hartley et al., 2015) and with an incidence of about 1 in 800 live births worldwide. The phenotype of DS includes intellectual disabilities, congenital heart disease, hypotonia, muscle weakness and other developmental abnormalities, as well as early onset Alzheimer’s disease (AD) (see e.g. Gibson, 1973). In addition, using imaging studies, there is evidence for smaller total brain volume, total gray matter and white matter volumes affecting cortical lobar, hippocampal, and cerebellar volumes (Hamner et al., 2018). Into adolescence, frontal gray matter volume continues to be significantly smaller in those with DS (Hamner et al., 2018), providing a potential neuroanatomical basis for alterations in attention and working memory deficits in individuals with DS (Kirk et al., 2017; Naerland et al., 2017). In adults with DS, data suggest an early cortical degenerative process prior to the onset of an AD-like phenotype, suggesting that cortical regions are highly vulnerable in DS (Fonseca et al., 2016). The amyloid precursor protein gene (APP) is located on Chromosome 21 (Cooper et al., 2016), and individuals with DS exhibit accumulation of toxic amyloid peptides early in life (Prasher et al., 2010), leading to aggregation of amyloid plaques in their third or fourth decade (Neale et al., 2018; Rafii et al., 2017). Recent studies have demonstrated that overexpression of the APP gene is necessary, albeit not the only culprit, to develop AD in those with DS (Millan Sanchez et al., 2012). It is also well known that those with DS accumulate intracellular neurofibrillary tangles (NFTs) early in life (Head et al., 2003; Rafii, 2018) as well as progressive loss of certain neuronal populations including basal forebrain cholinergic neurons (BFCNs) and locus coeruleus noradrenergic (LC-NE) neurons (German et al., 1992; Sendera et al., 2000). Because these are behavioral and pathological hallmarks of Alzheimer’s disease (AD), a majority of individuals with DS are diagnosed with AD in their fourth to sixth decade (Davidson et al., 2018; Firth et al., 2018; Head et al., 2016). These findings are replicated in mouse models of DS. For example, Ts65Dn mice, the most commonly used mouse model for DS (Costa et al., 2010; Reeves et al., 1995), exhibit significant hyperactivity as well as working and reference memory deficits along with neuronal cell loss, oxidative stress, and inflammation when they are middle-aged (Bimonte-Nelson et al., 2003; Demas et al., 1996; Escorihuela et al., 1998; Granholm et al., 2000; Hamlett et al., 2016a). However, the pathogenetic mechanisms responsible for various DS phenotypes including alterations in brain development and aging have not yet been revealed.
Several other candidate genes on the triplicated segment of Hsa21 are thought to contribute to both developmental and age-related brain alterations. One component of the triplicated genes that appears to be important, both for development and adult brain function, is the gene encoding for the dual specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A), which plays a role both in controlling the differentiation of prenatal neurons as well as in synaptic plasticity later in life (Arbones et al., 2018). In fact, overexpression of DYRK1A results in altered cognitive abilities, defective cortical microarchitecture and imbalance of cortical excitation/inhibition, as evidenced by decreased excitability and deficits in gamma frequency in the prefrontal cortex (Ruiz-Mejias et al., 2016). DYRK1A also causes phosphorylation of APP, which leads to its cleavage in Aβ40 and Aβ42, playing a critical role in the early onset of AD pathologies in DS (Pathak et al., 2018). This effect of DYRK1A may be especially detrimental in people with DS, because the APP gene is located on Hsa21 (see above and Glasson et al., 2014), leading to an overexpression of APP, and the formation of toxic amyloid peptides early in life (Schoeppe et al., 2018). In addition, overexpression of DYRK1A contributes to the hyperphosphorylation of the microtubule-associated protein Tau, resulting in the development of NFTs in adults with DS (Ryoo et al., 2007). Tau hyperphosphorylation by DYRK1A also inhibits the ability of Tau to promote microtubule assembly, leading to dysfunction of neuronal axonal transport. Interestingly, our previous studies indicated that Tau hyperphosphorylation is already found in exosomal cargo in young children with DS, suggesting that this is an early neurodegenerative event in people with this disorder (Hamlett et al., 2016b).
Functionally, extracellular vesicles (EVs) have emerged as intercellular agents of transfer and communication (Armstrong and Wildman, 2018; Gamez-Valero et al., 2018). A sub-type of EVs, called exosomes, are released by most cell types and are present in eukaryotic fluids, including blood, saliva and urine (Armstrong and Wildman, 2018; Properzi et al., 2013). Because exosomes of different cellular origins can be isolated from either serum or plasma, it is relatively easy to examine their cargo and obtain information regarding neuron- or glia-related pathology from a blood draw. The current review will include information regarding exosome development, origin, release, and cargo both in typically developing individuals and those with DS. Exosome biology reveals not only biomarkers of disease, but also mechanisms of disease spreading throughout the neuro-axis and periphery. In addition, it may be possible to harness exosomes to function as delivery vessels for novel treatment paradigms that could prevent adult-onset pathologies in those with DS.
2. Formation of extracellular vesicles
EVs include a heterogeneous family of lipid-enclosed vesicles that consist of apoptotic bodies, microvesicles, and exosomes. EVs were first described in 1967 as products of platelets that appeared as small “dust” under electron microscopic examination (Wolf, 1967). Today, EVs are recognized as important intracellular communication mechanisms in normal and pathological processes. Exosome biogenesis starts with invagination of the plasma membrane, transporting and enclosing materials into an endosome (Figure 1). Subsequently, the endosome will mature into a multivesicular body (MVB). The MVB undergoes inward budding, generating, and accumulating intraluminal vesicles that can either be directed to the lysosome for degradation or fused with the plasma membrane for subsequent release. Exosomes are vesicles sized 30–150 nm that are released to the extracellular environment after fusion of the MVBs with the plasma membrane (Kowal et al., 2014). Once released, these intraluminal vesicles, called exosomes (Johnstone et al., 1987), transit for short and long distances between cells. Different molecules promote the intracellular formation of exosomes and their subsequent secretion suggesting the existence of different sub-types of exosomes, even within the same cell type. There is growing evidence that predominant extracellular vesicle (EV) subtypes including exosomes from the endosomal origin can be stratified into distinct subtypes (Greening and Simpson, 2018). In addition, there is also emerging evidence supporting the notion that endogenous exosomes released by neurons or glial cells in the brain affect cell survival and proliferation, thus regulating neuronal protection and repair (Xiong et al., 2017).
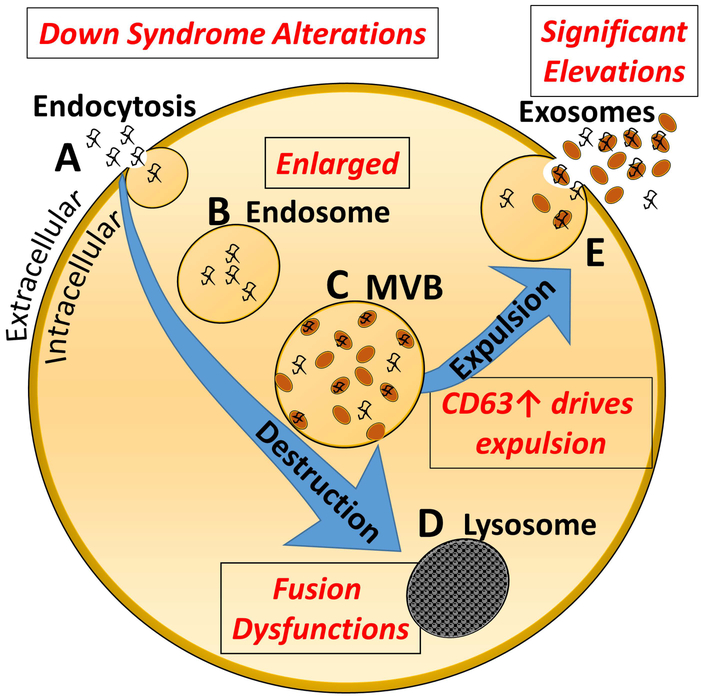
Figure 1. Exosome biogenesis and secretion.
A. Invagination of the plasma membrane into signaling endosomes. B. Transport of the vesicle into a larger endosome. C. The endosome will mature into a multivesicular body (MVB). D. The MVB undergoes inward budding, generating, and accumulating intraluminal vesicles that can either be directed to the lysosome for degradation (E) or fused to the plasma membrane for subsequent release (F). Alterations noted in the DS population, or in DS mouse models, are shown in Red in the figure.
3. Exosome release mechanisms
3.1. Exosome release mechanisms in normal conditions
Exosome secretion occurs in a constitutive manner although cellular stress or activation signals can modulate their secretion. Exosomes contain tiny amounts of the cytoplasm derived from their cells of origin. Thus, exosomal cargo essentially represents a sample of the intracellular environment and can be isolated from cerebrospinal fluid (CSF), blood, saliva, urine, and milk (Armstrong and Wildman, 2018; Vaswani et al., 2017). While initially observed in immune cells, they have now been observed in almost all cell types. Exosomes, which contain lipids, proteins and RNAs, represent an efficient way to transfer biologically active cargo from one cell to another. It has now been revealed that exosomes play a critical role in complex and coordinated communication among neurons, astrocytes and microglia in the brain, both in healthy conditions and in neurodegenerative diseases by propagating pathologic factors between brain regions (Lai and Breakefield, 2012). The inside-out budding of EVs carries not only membrane-associated material but also a substantial portion of cytosol. The outward budding of exosomal membranes at the cell membrane, which results in its release, is regulated by the endosomal sorting complex required for transport (ESCRT) (Juan and Furthauer, 2018), or by an ESCRT-independent manner via sphingomyelinase (Menck et al., 2017). The ESCRT machinery is a cytosolic protein complex, which together with accessory proteins enables a unique mode of membrane remodeling that results in budding (Babst, 2011).
Several cellular activities can activate or reduce the release of exosomes from central nervous system (CNS) cells (Chivet et al., 2012). For example, potassium-induced depolarization in neurons can increase exosome release (Faure et al., 2006). Intracellular calcium changes in cultured cortical neurons (Faure et al., 2006) and oligodendrocytes (Kramer-Albers et al., 2007) induce exosome release. Furthermore, in cultured cortical neurons, exosomal release is modulated by glutaminergic activity (Lachenal et al., 2011). In addition, exercise increases exosome release, at least from endothelial progenitor cells (Ma et al., 2018). Moreover, it has been suggested that beneficial effects of exercise on heart and vascular health could be related to an exercise-induced increase in miR-126 in the exosome cargo (Ma et al., 2018), which is known to promote vascular repair and angiogenesis.
Even though the data presented above strongly indicate that exosomes play a crucial role for brain function, we are only just beginning to explore mechanisms involved in the release, transport, and target uptake of EVs in the CNS. Nonetheless, two major biological needs of the cell are satisfied by the release of exosomes - expelling unneeded cellular material for disposal and transferring signaling biomolecules between cells.
3.2. Exosome release mechanisms in AD and DS
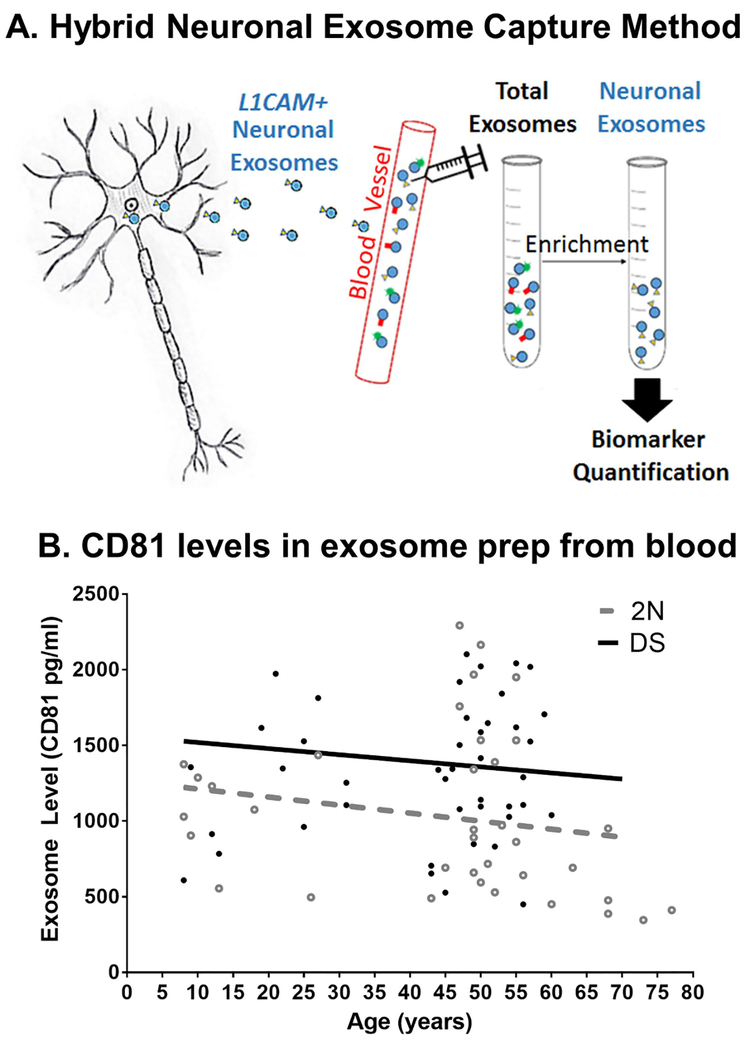
Enhanced exosome secretion is thought to be a compensatory mechanism in response to cellular stressors. Recent molecular studies suggest that specific molecular checkpoints, such as ATG5, ATG7 and CD63, interface with the control of exosomal release (Abdulrahman et al., 2018; Uddin et al., 2018). Tetraspanins, a family of transmembrane proteins (e.g., CD63 and CD81) are among the most abundant surface proteins on exosomes (Booth et al., 2006; Raposo and Stoorvogel, 2013) and are often used to quantify total exosome secretion. Exosome release has been demonstrated to increase by induced hypoxia (King et al., 2012), DNA damage (Lehmann et al., 2008; Xiao et al., 2014), and by oxidative stress (Atienzar-Aroca et al., 2016). However, not all pathological conditions may induce exosomal secretion. In a recent study, investigators found that there were no differences in the total number or size distribution of neuronal exosomes isolated from the blood of subjects with a clinical diagnosis of mild cognitive impairment (MCI) a prodromal stage of AD compared to control participants (Winston et al., 2016). As described earlier, individuals with AD and DS have dysfunctions in endosomal transport and endosomal enlargement (Cataldo et al., 2004; Ginsberg et al., 2010), a cellular pathology which may be compensated by exosomal secretion. In a recent study, Levy and colleagues support this notion with evidence that post mortem brain tissue isolated from individuals with DS expelled nearly 40% more exosomes that age-matched non-DS controls (Gauthier et al., 2017). They showed that this enhanced exosome secretion phenomenon also occurred in the TS2 mouse model of DS, which also exhibits age-related endosome abnormalities (Levine et al., 2009). In support of this finding, we quantified levels of CD81 expression from two cohorts that included a population of participants with DS (8–62 years old) and a population of age-matched control participants (8–77 years old)(Hamlett et al., 2016b). These populations were age- and gender-matched but a near 40% increase in neuron-derived exosomes (NDEs) was observed in blood samples obtained from individuals with DS compared to non-DS controls (Hamlett et al., 2018). CD81 displayed a trend for elevated levels over broad cross-sections of ages as early as 8 years of age in the DS population (Figure 2B) that remained high into the sixth decade of life, compared to non-DS controls. Collectively, the results support that there is enhanced exosome secretion in the DS brain, which can also be observed in NDE abundance in the blood. This phenomenon could either be due to alterations in the lysosomal pathway, as discussed above (Cataldo et al., 2004; Nixon, 2017), or to other alterations associated with the Hsa21 trisomy.
Figure 2. Exosome purification method.
A. Plasma or serum samples are obtained and the Exoquick polymer employed to obtain total exosomes from the fluid. Thereafter, an L1CAM antibody capture system is utilized to purify all exomes of neuronal origin from the sample. B. CD81 levels in DS and control NDEs. Multiple blood samples at each age from DS and a typically developing population were obtained, exosomes purified and CD81 levels measured by ELISA. As can be seen here, most DS samples contained elevated amounts of CD81, indicating an increase in exosome release in this population at all ages analyzed. [Original data Hamlett, Ledreux and Granholm et al]
Further studies by Levy’s group using DS fibroblasts revealed increased levels of the tetraspanin CD63, and rab35 (Gauthier et al., 2017), both important regulators of exosome biogenesis. They found that DS fibroblasts secreted more exosomes into the cell culture media than non-DS (2N) fibroblasts. Interestingly, CD63 knockdown in the DS fibroblasts significantly reduced exosome release and significantly increased the number of intracellular endosomes (Gauthier et al., 2017). Together these data suggest that increased CD63 expression in DS is a compensatory cellular mechanism that enhances exosome release to relieve endosomal abnormalities (see also Figure 1). The CD63 compensatory enhancement seems to be associated with DS aneuploidy rather than with pathological Aβ alone. Tg2576 mice also overexpress APP but do not secrete more exosomes than their littermate controls at an age after which amyloid pathology has fully developed (Perez-Gonzalez et al., 2012). Thus enhanced exosome secretion may be a distinct cellular phenotype that could be observed across all cell types in various tissues and organs of individuals with DS. Interestingly, the apolipoprotein Apoɛ4 variant allele, whether homozygous or heterozygous with an ɛ3 allele, gives rise to secretion of lower numbers of exosomes in the brain extracellular space (Peng et al., 2018). These investigators showed that expression of the Apoɛ4 allele gave rise to reduced expression levels of the exosome pathway regulators tumor susceptibility gene 101 (TSG101) and Ras-related protein Rab35, and the investigators therefore argued that having the ɛ4 allele leads to a downregulation of exosome biosynthesis and release (Peng et al., 2018). Reduced exosome production is likely to have adverse effects, including diminished ability to eliminate damaging materials via the endosomal-lysosomal system. Peng and collaborators postulated that the reduction in brain exosome levels in 12-month-old ApoE ɛ4 mice occurred earlier than endosomal pathway dysfunction, arguing that an ɛ4-driven failure in exosome production plays a primary role in endosomal and lysosomal deficits in these mice and potentially also in humans with one or two copies of the ɛ4 allele (Peng et al., 2018).
For individuals with DS, defects in endocytosis and autophagy/lysosomal recycling have emerged as key drivers of accumulation of cellular waste products, progressive neuronal dysfunction, and neurodegeneration (Colacurcio et al., 2018).
4. Interaction between exosomes and autophagy
The autophagosomal pathway mediates the specific degradation of damaged or old proteins as well as organelles. This pathway is important for cellular protection, homeostasis, as well as life span in many organisms. Autophagy malfunction is often linked to a variety of human diseases, such as cancer, neurodegeneration, and microbial infection. A review of the relationship between exosomes and autophagy and their involvement in brain health and disease can be found in (Baixauli et al., 2014). As indicated in Figure 1, there are at least two alternative pathways – the lysosomal and the exosomal pathways – that coordinate the removal of harmful or unwanted materials from the cell to preserve cellular integrity. Autophagy occurs constitutively but can also be triggered or enhanced by different stimuli such as nutrients, growth factors, energy and oxidative stress or inflammation (Papandreou and Tavernarakis, 2017). Several lines of evidence suggest that there is close coordination between autophagy and biogenesis and release of exosomes from cells (Xu et al., 2018). For example, in conditions that stimulate autophagy, the multivesicular bodies (MVBs) are directed towards the autophagic/lysosomal pathway, leading to a reduction in exosome release (Fader et al., 2008). On the other hand, studies have shown that where there is a neuronal lysosomal dysfunction, secretion of unique exosomes is increased. These are enriched for undigested lysosomal substrates, including amyloid precursor protein C-terminal fragments (APP-CTFs) and sphingolipids which normally reside in endolysosomes (Miranda et al., 2018). The mechanistic consequence of increased exosomal release will be discussed further below in the context of AD.
Once released into extracellular space, exosomes can travel vast distances both within in the brain parenchyma and also across the blood-brain barrier (BBB) and other tissue barriers (Das et al., 2018; Osorio-Querejeta et al., 2018). Exosomes can, for example, propagate inflammation across the BBB (Holm et al., 2018) but can also deliver drugs and other therapies across the BBB as a novel therapeutic delivery system (Tian et al., 2018).
5. Exosome and cargo detection methods
Studies of NDEs have been greatly enabled by unique neuron-specific surface markers that are abundant in brain tissue but to a lesser extent in peripheral tissues. Specifically, a neural cell adhesion molecule, L1-cell adhesion molecule (L1CAM, or CD171), is a highly expressed surface protein in the brain, and to a lesser extent in other organs (Figure 3) (Uhlen et al., 2015). While L1CAM is observed in other human tissues, brain expression predominates significantly, as seen in Figure 3, thus targeted capture of L1CAM-positive exosomes from circulating biological fluids allows for relatively stringent enrichment of neuronal exosomes (Fiandaca et al., 2015; Goetzl et al., 2018; Goetzl et al., 2015; Goetzl et al., 2016; Hamlett et al., 2016b).
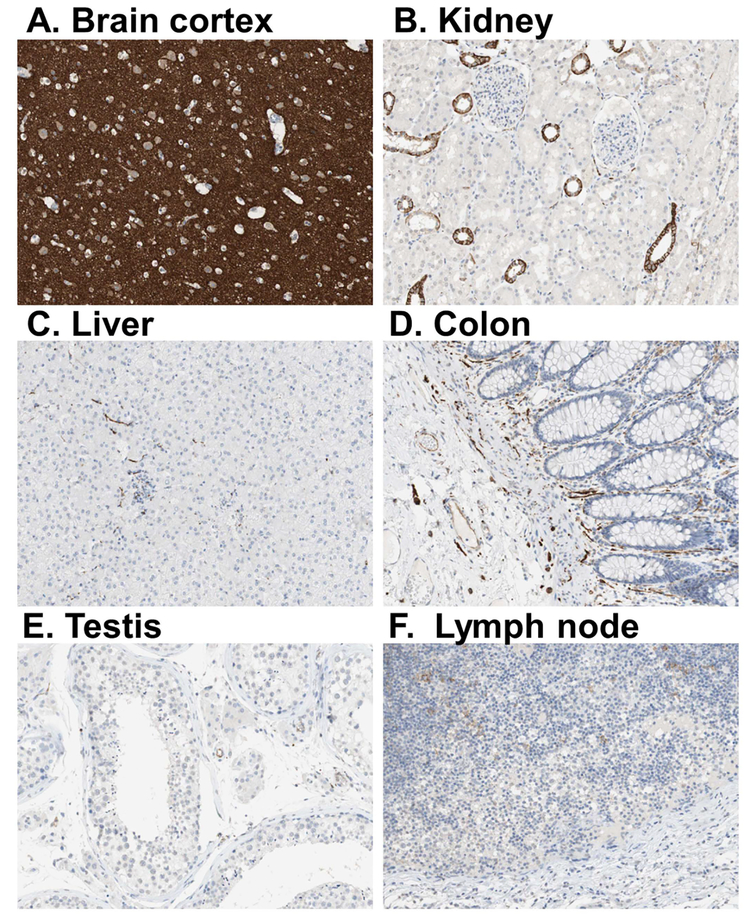
Figure 3. L1CAM immunostaining in various organs in human post mortem tissues.
L1CAM immunostaining is enriched in (A) cortical tissues, and is much less abundant to near absent in (B-F) other organs. Thus, this antibody represents an excellent tool for immunoprecipitation of brain-derived exosomes, as used in our protocols. Photos were obtained from the Human Protein Atlas which is licensed under the Creative Commons Attribution-ShareAlike 3.0 International License (thus not copyrighted). From Uhlen et al., 2015.
Our laboratory uses the ExoQuick polymer solution (System Biosciences) to isolate total exosomes from serum or plasma (Hamlett et al., 2016b). Thereafter, samples are incubated with mouse biotinylated anti-human CD171 monoclonal antibody (see Figure 2A). Then, streptavidin-Plus Ultralink resin (Thermo Scientific, Inc.) plus BSA is added to the suspension and incubated at room temperature. Immunoabsorbed samples are centrifuged followed by removal of supernatants and each resin pellet suspended in 0.1 M glycine-HCl (pH 3.0), incubated and centrifuged according to standard protocols (Goetzl et al., 2018; Goetzl et al., 2019; Goetzl et al., 2016; Hamlett et al., 2016b). Supernatants containing the CNS-derived exosomes are neutralized with 1 M Tris-HCl and then lysed with M-PER lysis buffer. The final sample can be examined for purity and size of exosomes using different methods. For example, electron microscopy will allow determination of particle size and can therefore be used to distinguish between exosomes and other vesicles (Baglio et al., 2015). Nanosight technology uses optical microscopy to quantify small particles like exosomes and can be used to assess the size, distribution and number of exosomes (see e.g. Street et al., 2017; www.nanosight.com). Finally, ELISA kits (e.g., ExoELISA, Franquesa et al., 2014) allow exosome particle quantification. Unfortunately, there is not yet a consensus for a gold standard of exosome isolation methods from blood, nor is there a consensus for quantification of exosome size and quantity. It is therefore difficult to determine the best combination of methods for the ideal approach.
6. Exosomal Cargo
6.1. Exosomal cargo in control conditions
Exosomes contain proteins, messenger RNA (mRNA) and microRNA (miRNA) that reflect their cellular origin and play a key role in cellular signaling, removal of unwanted proteins, and transfer of cellular pathogens to other cells (Coleman and Hill, 2015). Proteomic studies of EVs released by primary cell cultures, cell lines, tissue cultures or isolated from biofluids have yielded extensive catalogs of the protein abundance in different types of EVs. Several recent investigations have reported that exosomes contain proteins associated with neurodegenerative diseases, including Aβ, α-synuclein, and phospho-Tau (Emmanouilidou et al., 2010; Goetzl et al., 2018; Hamlett et al., 2016b; Winston et al., 2016). Because exosomes have found some success as diagnostic biomarkers in cancer (Gao and Jiang, 2018; Properzi et al., 2013; Rofi et al., 2018), it was thought that they might also be utilized to measure biomarkers of neurodegenerative disease. Public on-line databases are available that catalog EV-associated components including Vesiclepedia, EVpedia (Kim et al., 2015) and ExoCarta (Mathivanan et al., 2012). Exosomes contain a multitude of protein profiles that differ by cellular origin, small differences in physical properties and composition (Zaborowski et al., 2015), and cell topography (basolateral vs. apical) (Tauro et al., 2013). Therefore, the same cell type may secrete different subgroups of vesicles dependent on the sum of these factors as well as dependent on cellular responses to stress or pathological burdens over time. Because of these heterogeneous interactions and the vast number of cellular subtypes, over 40,000 different proteins have been identified as cargo inside exosomes (Kim et al., 2015).
6.2. Exosome cargo in AD and DS-AD
About 10 years ago, it was discovered that amyloid might be transported out of the cell via the endosome/exosome pathway (Rajendran et al., 2006; Vella et al., 2008). Previous studies revealed that endocytic perturbations including endosome enlargement, which occurred even before Aβ levels increase during the early stages of AD (Cataldo et al., 2004; Ginsberg et al., 2010). The same is true for individuals with DS, who also exhibit early dysfunction of the endosomal machinery in the CNS (Jiang et al., 2010). Aβ accumulates in abnormal neuronal endosomes suggesting that endosomal trafficking contributes to the early elevation of Aβ (Cataldo et al., 2004), and this AD pathology is found in exosomes prior to the onset of AD symptoms (Yuyama and Igarashi, 2017; Yuyama et al., 2015). Aβ also accumulates in MVBs, which are precursors of exosomes (Babst, 2011). It has been reported that Aβ is released in association with exosomes from APP-transfected cells to the culture medium (Rajendran et al., 2006). In addition, Alix, an exosomal marker involved in exosome secretion (Chiasserini et al., 2014), is enriched around amyloid plaques in post mortem human tissues, suggesting that Aβ associated with exosomes may contribute to plaque formation. APP and APP C-terminal fragments (CTFs) are also present in exosomes and released to the extracellular space (Vingtdeux et al., 2007).
The process by which Aβ binds to exosomes is complex. Glycosphingolipids (GSLs), which are involved in exosome release mechanisms, bind to cytosolic Aβ and are present both in the cell membrane and in exosomes (Dinkins et al., 2017). Degradation of GSL-glycans with endoglycoceramidase (EGCase) prevent the association between Aβ and exosomes (Yuyama and Igarashi, 2017; Yuyama et al., 2015). It was also shown that significantly more GSLs in exosomes from neurons than in those from glial cells (Yuyama and Igarashi, 2017). Since the autophagosome and other degradation pathways are disrupted in both AD and DS (Colacurcio et al., 2018; Nixon, 2017; Tramutola et al., 2016; Uddin et al., 2018), Aβ may accumulate and propagate by disrupting the normal degradation of amyloid peptides. Collective evidence strongly suggests that this is an early event, prior to the accumulation of other pathological hallmarks (Nixon, 2017).
Interestingly, Yuyama and collaborators (Yuyama et al., 2015) found that infusion of NDEs purified from neuronal cell cultures intracranially into APP transgenic mice decreased amyloid levels, plaque load, and attenuated synaptic densities in the hippocampus, suggesting that NDEs were involved in amyloid clearance. These investigators also demonstrated that intracranial infusion of NDEs led to an almost exclusive uptake of injected exosomes into microglia vs. other cell types, suggesting that exosomes may participate in Aβ clearance via microglial mechanisms. These findings are interesting, and perhaps also controversial based on the findings from others suggesting that exosomes may spread pathologic proteins from one brain region to another. Studies found that depolarization of neurons promoted the release of Tau-containing exosomes in culture, suggesting that neuronal activity contributes to the spread of pathology and exosomes were also found to mediate the trans-neuronal transfer of Tau depending on synaptic connectivity in multi-fluidic culture chambers (Wang et al., 2017). These findings suggested that Tau propagation by direct transmission of exosomes between neurons and may be a diametrically different function of exosomes compared to the Aβ clearance mediated by exosomes described above. As this field emerges, more specific biological mechanisms for the role of exosomes in both clearance and spreading of AD pathology will be revealed in more detail.
The findings regarding Tau seeding and spreading via exosomes were corroborated by Winston and collaborators (Winston et al., 2016) who injected purified NDEs from humans with AD into wild type mice. Several weeks after infusion, they found large numbers of p-Tau staining of neurons within the hippocampus, suggesting the spread of toxic hyper-phosphorylated Tau from individual to individual via exosomes. We have recently undertaken pilot experiments with injections of NDEs purified from blood of individuals with DS-AD and discovered a significant amount of p-Tau immunostaining in neurons of the pyramidal cell layer and the dentate gyrus in the hippocampus 6 weeks following the injection (Ledreux et al., unpublished results). P-Tau immunostaining (P-Tau S396, Invitrogen) was not observed in the hippocampus of mice who received NDEs from non-DS control participants. These findings suggest that toxic Tau species can spread from individual to individual via exosome transfer. Studies continue in our laboratory focusing on which Tau species that are present in NDEs from those with DS-AD as well as how early in development these aggregating Tau species can be detected in NDE samples obtained from blood.
Neuropathological biomarkers of AD, including Aβ40 and Aβ42, have been detected in brain tissue and cerebrospinal fluid (CSF) decades before the onset of dementia in general (Blennow and Zetterberg, 2018) and DS population (Dekker et al., 2017). The progressive accumulation of Alzheimer’s disease (AD) pathology in the DS population suggests that there is a preclinical phase to DS-AD since there are at least two decades between the onset of AD pathology and dementia diagnosis in those with DS (Head et al., 2016; Prasher et al., 2010; Schupf et al., 2015; Wiseman et al., 2015). Although CSF is a more reliable source of biomarkers for AD than blood, performing lumbar punctures in those with DS to acquire CSF can be challenging. Thus, exosomal biomarkers of dementia from blood samples would allow a minimally invasive method once further validated.
Evidence has been mounting suggesting that exosomes, isolated from either blood (Gauthier et al., 2017; Hamlett et al., 2016b) or CSF (Gomes DeAndrade et al., 2018; Otake et al., 2019), might be useful in capturing relevant neuropathological processes within CNS neurons. Although only one manuscript could be found where investigators compared cargo of exosomes derived from blood vs CSF (Chen et al., 2019), this is indeed a promising new direction. The investigators did find differences in exosome cargo between these two sources; specifically, alterations in miRNAs as a result of pathology were different between the two. However, as it is more difficult to obtain LPs from those with DS, CSF exosome investigations may not be a mainstream focus in this field. In relation to AD neuropathology, NDEs have been shown to be involved in the processing of APP by self-contained proteases (Rajendran et al., 2006; Yuyama et al., 2015). As described above, exosomes receive APP from early endosomes which is cleaved into Aβ peptides and then secreted from the cells in exosome clearance strategies (Yuyama and Igarashi, 2017). NDEs contain Aβ peptide products with neuropathogenic potential (Coleman and Hill, 2015), suggesting that NDEs extracted from either plasma or CSF could be used to assess neuropathological processes within CNS neurons (Chiasserini et al., 2014; Coleman and Hill, 2015; Fiandaca et al., 2015; Goetzl et al., 2018).
The first research group to explore the use of NDEs isolated from blood as biomarkers for AD was the Kapiogannis/Goetzl research group at the National Institutes of Health (NIH, see Fiandaca et al., 2015; Goetzl et al., 2018; Goetzl et al., 2015; Goetzl et al., 2016; Kapogiannis et al., 2015). These investigators found significantly higher levels of Aβ42, p-Tau-T181, and p-Tau-S396 in NDEs purified from the blood of patients with AD. They further found that the levels of each protein predicted AD with an accuracy of more than 90%. When combined, the predictive ability of these three AD biomarkers in exosomes approached 100 percent (Fiandaca et al., 2015). They also discovered that the same three proteins were elevated in NDEs obtained from patients 1–10 years prior to the onset of AD, suggesting a strong predictive capability of AD biomarkers in NDEs. Based on these findings, we hypothesized that NDEs obtained from blood of individuals with DS might portray a novel window to better define the preclinical AD phase of DS-AD. Analysis of NDEs obtained from blood samples of participants with DS (8–64 years of age) showed elevated levels of Aβ1–42 peptides and p-Tau already early in life (Hamlett et al., 2016b). It was not surprising that neuronal exosome levels of Aβ42 were elevated in DS since the gene encoding for APP is located on Hsa21 (Cooper et al., 2016; Head et al., 2016), and previous studies have shown elevations of toxic amyloid peptides in the plasma of young adults with DS (Fortea et al., 2018). Positron emission tomography (PET) imaging studies established that accumulation of Aβ occurs almost without exceptions in the DS brain in the 3rd to 5th decade (Annus et al., 2016; Cole et al., 2017; Handen et al., 2012; Hartley et al., 2015; Hartley et al., 2017; Lao et al., 2017; Neale et al., 2018; Rafii et al., 2017; Wilcock et al., 2016). Increased levels of Aβ42 have been reported in the CSF of adults with DS without dementia (Dekker et al., 2017; Fortea et al., 2018). Interestingly, findings from the Dominantly Inherited Alzheimer Network (DIAN), an international registry of individuals at risk for developing autosomal dominant AD, suggest that early high levels of Aβ in the CSF, followed by reduced CSF Aβ levels at more progressive disease stages successfully mark the onset of AD (Moulder et al., 2013).
Diffuse amyloid plaques have been described as early as age fifteen in post mortem brain samples from people with DS (Lemere et al., 1996), and elevated Aβ levels are observed in plasma already in young adults with DS (Mehta et al., 1998; Prasher et al., 2010). In a similar vein, we observed a marked increase in Aβ42 levels in NDEs of individuals with DS as early as eight years of age (Hamlett et al., 2016b) suggesting that intracellular pathology is present long before silver staining or PET imaging can identify lesions. In addition to the pronounced Aβ deposition seen in subjects with DS, abundant NFTs and increased phospho-Tau levels also occur (Head et al., 2003; Hof et al., 1995; Kasai et al., 2017; Rafii, 2018). NFTs are first seen in the entorhinal cortex in the mid‐thirties, hippocampus in mid‐forties, and neocortex in the mid‐fifties, but variability exists between individuals with DS (Davidson et al., 2018; Dekker et al., 2017; Hartley et al., 2015). In a neuropathological study of people with DS and of different ages, NFT pathology was observed in 4 cases below 36 years of age and in all 20 cases above that age, again suggesting individual variability in terms of onset (Wegiel et al., 1996). However, although frank NFTs may not appear before the age of 20 in those with DS, a recent report demonstrated abnormal phosphorylation of Tau already in the brain of fetuses with DS, suggesting that alterations in Tau conformation or phosphorylation state is an early event in DS and may contribute to later development of AD pathology (Milenkovic et al., 2018). Tau phosphorylation at the carboxyl terminus has been shown to be one of the earliest pathological events in DS-AD (Mondragon-Rodriguez et al., 2014).
Similar to the findings described above from the Milenkovic lab (Milenkovic et al., 2018), in our study we found that levels of phosphorylated Tau residues (p-(S396) and p-(T181)-Tau) were also significantly increased as early as eight years of age in NDEs obtained from blood samples of children with DS (Hamlett et al., 2016b). The biological mechanisms driving elevated p-Tau levels early in those with DS remain unknown but recent studies show that exosomes are capable of transferring Tau pathology via seeding in the rodent brain. Exosomes isolated from Tau transgenic mouse brains (Polanco et al., 2018) or from humans with AD (Winston et al., 2018) propagate seeding of Tau pathology when injected into wild-type mice. In addition, as discussed above in the Introduction, several other gene targets on Hsa21 have been shown to affect Tau phosphorylation, both in vivo and in vitro. These include RCAN1 (Jung et al., 2011), APP (Hartley et al., 2015) and DYRK1A (Jung et al., 2011; Pathak et al., 2018; Ryoo et al., 2007), which are all overexpressed in DS mouse models and humans with DS. Further studies are warranted to examine the effects of these overexpressed genes, gene interactions between them, as well as other gene targets, on Hsa21, and their role for exosome formation or secretion as well as spreading of toxic agents between brain regions and between cell types or individuals.
6.3. Exosome RNA cargo
Exosomes contain not only surface markers and proteins, but they have also been shown to contain different forms of RNA including mRNA, microRNA (miRNA) and long non-coding RNA (Gamez-Valero et al., 2018; Ren, 2018). Hill and collaborators (Cheng et al., 2015) have identified 14 upregulated and 3 downregulated miRNAs in patients with AD or mild cognitive impairment (MCI) compared to controls. The altered miRNAs that were most predictive of AD or MCI were associated with APP processing, apoptosis, or cellular stress, indicating that these miRNAs may be involved in the disease process. These data suggest that exosomal RNA biomarker panels may become a suitable peripheral screening tool for AD. Independent of these exosome RNA studies, others have shown that specific miRNAs are involved in age-associated changes in the brain as well as cognitive impairment. Candidate miRNAs have been linked to age-associated alterations in cortical thickness, cortical glucose metabolism, and cognitive performance (Maldonado-Lasuncion et al., 2018). These include for example miR29-b, miR-125b, and miR-146a – miRNAs which have all been found in exosomal cargo (Kim et al., 2015; Mathivanan et al., 2012). It has been suggested that some miRNAs and tRNAs are overrepresented in exosomes compared to the cell of origin, for example following an inflammatory or other stress events (Baglio et al., 2015) or in neurodegenerative diseases (Bellingham et al., 2012a). This is an interesting field that deserves more attention, and that may lead to novel diagnostic or predictive tools for AD and DS-related AD.
7. Exosome transportation and signaling
Once released, exosomes may have both short- and long-range destinations (Bellingham et al., 2012b). The mechanisms underlying exosome transport into biological fluids is likely driven by endothelial intracellular transport, which seems to be governed, in part, by exosomal surface markers. In a recent study, investigators discovered that removal of exosome surface proteins with proteinase K decreased endothelial transport rates of exosomes by 45% while inhibition of endocytosis, with cytochalasin D, caused a 50% decrease in transport (Kusuma et al., 2016). It seems that regardless of tissue or cellular origin, exosomes are actively transported from interstitial space through endothelial cells into the blood which makes them an extremely mobile vehicle in vivo. Thus exosomes are proposed to serve as signaling vectors and/or off-loading mechanisms to neighboring recipient cells, and likely can impart a long- and short-range “bystander effect” in vivo (Xu et al., 2015). Because of their small size, secreted exosomes diffuse into biological fluids (blood, CSF and urine) and circulate in the interstitial space, both in brain and periphery, and can cross the BBB in both directions (Salido-Guadarrama et al., 2014). The ability to capture exosomes directly from fluids makes them an extremely attractive tool to probe various etiologies associated with neurological conditions.
In the context of enhanced exosome secretion, for example, in neurodegenerative diseases, it is important to consider that long-range exosome transmittance could affect any other cell in the entire body. For example, Aβ aggregates, generally thought to be specific to brain tissue, have also been found in the skin, skeletal muscle and gut tissue of AD patients (Joachim et al., 1989). Additionally, it has been discovered that Aβ aggregates are present in the myocardium of patients with AD (Troncone et al., 2016). The occurrence of Aβ deposits in multiple tissues suggests that the protein may be either produced locally in numerous organs or derived from a common circulating precursor (Jensen and Willis, 2016). These findings indicate that the bystander effect imparted by exosomes from a specific cell type could be ubiquitously modulating other cellular recipients across several organs. Exosome kinetics in the brain and peripheral organs have yet to be reported but are currently under investigation. Systemic studies of whole-body kinetics in mice revealed that labeled exosomes, when injected into the blood, are transferred to liver and lungs to a much higher degree than spleen, kidney, and heart (Lee et al., 2018). Altogether, in studies of exosome influence upon a specific cell or tissue, one must consider the cellular exosome source, especially during pathological situations. Recent elegant work using a microfluidics culture system showed that extracellular exosomes could be internalized by a nearby neuron and transferred to another neuron (Polanco et al., 2018) by utilizing secretory endosomes. Together, these findings suggest that the endosomal/exosomal machinery can spread pathogenic agents across neuronal connectomes and increase the radius of action of pathogenic cargoes. We are currently investigating the travel patterns of labeled human NDEs when injected either into the bloodstream or directly into brain tissue of intact mice, to examine their movement patterns across long distances and across the blood-brain barrier (BBB).
8. Challenges in the Field
As mentioned above, the exosome biomarker field is yet in its infancy and much remains to be investigated. There are challenges which are summarized in this section. First, we do not yet know whether exosome cargo remains the same within a person with DS during longitudinal sampling. However, this experiment has been performed in patients with prodromal AD, where exosomal cargo was examined in the prodromal phase and then 1–10 years later (Goetzl et al., 2015). The investigators demonstrated that exosomal cargo predicted conversion to AD which occurred later in the same patient and thus we expect a similar progression also in patients with DS and prodromal AD.
Secondly, it will be interesting to discuss utilizing exosomes obtained from saliva or urine instead of blood in future studies. There are experiments performed purifying exosomes from both urine and saliva in the cancer field (Armstrong and Wildman, 2018; Properzi et al., 2013) but these have not yet been performed in DS or AD. As discussed above, secreted exosomes diffuse into blood, CSF and can cross the BBB in both directions (Salido-Guadarrama et al., 2014). Because, at least theoretically, NDEs can be isolated from all these body fluids, their cargo should be similar whether obtained from blood, saliva or urine based on the neuronal surface markers used in the precipitation. However, this has not been examined in DS yet, and remains an unanswered question. Cargo found within these NDEs must be considered of brain rather than peripheral origin. Purifying NDEs from brain tissue vs. blood samples from the same individual to compare cargo is a focus of a recently funded grant in the Ledreux laboratory.
Another challenge with new biomarkers is replicability across several cohorts. A recent manuscript from our group has specifically addressed this challenge by demonstrating identical findings when NDEs were purified from blood samples obtained from 4 different sources (one in Europe and three in the US, see Hamlett et al., 2016B). Because the NDE cargo is normalized against exosomal markers, similar results were obtained whether exosomes were purified from serum or plasma from the same individual (Hamlett et al., 2016B).
Finally, as with every novel biomarker method, a challenge would be the rate of throughput for translating the new method into the clinic. This is, indeed, a challenge with exosome biomarkers. Although purification of NDEs from blood samples is relatively straightforward, obtaining their cargo is technically difficult and is only mastered by a few laboratories to date. As with other novel techniques, methodological development will no doubt rapidly translate the method into a clinical rate technique.
9. Conclusions
The NDE-based studies described above suggest that alterations in AD biomarkers may already be occurring in children with DS. While neuritic plaques and NFTs occur earlier in the DS brain than in typically developing individuals, alterations in synapse number, cortical neuronal population and dendritic structure are seen even earlier in DS, already during fetal development (Wisniewski, 1990). Furthermore, early impairment in neuronal differentiation leads to reduced gray matter volume especially in the frontal cortex as discussed above (Hamner et al., 2018). Thus a lifelong complex interplay between developmental alterations and age-related deterioration can be studied in the DS population from the aging paradigm but also from a developmental perspective in this unique population.
The exosome biomarker approach offers several benefits for the interrogation of DS etiologies. Once validated, exosomal screening could allow insight into the profile of proteins secreted from neurons throughout the lifespan with minimally invasive techniques. Another significant benefit is that outcome measures can be reliably obtained either from serum or plasma samples from a consortium of biobanks since data are normalized against the same exosomal markers. The exosome approach may also prove useful in evaluating potential drug targets by enabling reliable measures of therapeutic efficacy. Future studies will include comparison studies between whole plasma and NDE biomarker levels, as well as expanded search for other putative biomarkers in neuronal and glial exosomes that may predict dementia and/or other conditions in adults with DS.
Despite the usefulness of exosome biomarkers, there remain several challenges to their optimal use. In particular, methods for large-scale isolation must be developed and are currently too costly to be utilized in a routine clinical environment. Other caveats include methods for isolation and storage which should be carefully optimized since other extracellular vesicles or other cellular components may share major biophysical and biochemical characteristics, reducing precision in exosome isolation (Tkach et al., 2018). Additionally, further studies of therapeutic delivery of exosomes are needed to maximize the potential of this exciting vesicle-based technology. This novel approach has already been extensively tested in the cancer field (Gao and Jiang, 2018), but to our knowledge not for neurodegenerative conditions.
Studies of individuals with DS and mouse models of DS show new interactions between brain pathology, inflammatory pathways, autoimmune disorders and the endosomal/exosomal machinery which may inform future diagnostics, preventions, and therapies in the DS population. We postulate that recent advances in exosome technology may enable improvements in precision medicine and diagnostics and promote healthy development and aging. Such benefits could prevent the onset of aging-related diseases like AD in those with DS.
Acknowledgments
This work was made possible by grants from the National Institutes on Aging to ACG (R21AG056974) and EJM (RO1AG14449) and a grant from the Alzheimer’s Association to AL (AARG-18–565696). This work was also supported by research grants from the Carlos III Institute of Health, Spain (PI14/01126 and PI17/01019), “Marató TV3” (20141210), PERIS SLT006/17/125 Fundació Bancaria La Caixa and NIH (1 R01 AG056850–01A1) grants to JF. The authors would like to thank Ms. Laura Columbo and Mr. Hammam Belgasem for expert technical assistance.
Footnotes
Declaration of Interest: The authors have no conflict of interest with companies or other organizations that can bias this review.
Data Availability Statement: The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Abdulrahman BA, Abdelaziz DH, Schatzl HM, 2018. Autophagy regulates exosomal release of prions in neuronal cells. J Biol Chem 293, 8956–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annus T, Wilson LR, Hong YT, Acosta-Cabronero J, Fryer TD, Cardenas-Blanco A, Smith R, Boros I, Coles JP, Aigbirhio FI, Menon DK, Zaman SH, Nestor PJ, Holland AJ, 2016. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dement 12, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbones ML, Thomazeau A, Nakano-Kobayashi A, Hagiwara M, Delabar JM, 2018. DYRK1A and cognition: A lifelong relationship. Pharmacol Ther. 2019 February;194:199–221. doi: 10.1016/j.pharmthera.2018.09.010. Epub 2018 Sep 28. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Wildman DE, 2018. Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J Pathol Transl Med 52, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S, Perez-Cremades D, Garcia-Verdugo JM, Diaz-Llopis M, Romero FJ, Sancho-Pelluz J, 2016. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med 20, 1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, 2011. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol 23, 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM, 2015. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, Lopez-Otin C, Mittelbrunn M, 2014. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 5, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Coleman BM, Hill AF, 2012a. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res 40, 10937–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Guo BB, Coleman BM, Hill AF, 2012b. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 3, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC, 2003. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res 139, 47–57. [DOI] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H, 2018. The Past and the Future of Alzheimer’s Disease Fluid Biomarkers. J Alzheimers Dis 62, 1125–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ, 2006. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol 172, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA, 2004. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging 25, 1263–1272. [DOI] [PubMed] [Google Scholar]
- Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, Martins RN, Rowe CC, Macaulay SL, Masters CL, Hill AF, Australian Imaging B, Lifestyle Research G, 2015. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry 20, 1188–1196. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhou Y, Wang ZY, Yan BX, Zhou WF, Wang TT, Zheng M, Man XY. Exosomal microRNA profiles from serum and cerebrospinal fluid in neurosyphilis. Sex Transm Infect. 2019. March 29 pii: sextrans-2018–053813. doi: 10.1136/sextrans-2018-053813. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chiasserini D, van Weering JR, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE, de Wit H, Jimenez CR, 2014. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics 106, 191–204. [DOI] [PubMed] [Google Scholar]
- Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R, 2012. Emerging role of neuronal exosomes in the central nervous system. Front Physiol 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colacurcio DJ, Pensalfini A, Jiang Y, Nixon RA, 2018. Dysfunction of autophagy and endosomal-lysosomal pathways: Roles in pathogenesis of Down syndrome and Alzheimer’s Disease. Free Radic Biol Med 114, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Annus T, Wilson LR, Remtulla R, Hong YT, Fryer TD, Acosta-Cabronero J, Cardenas-Blanco A, Smith R, Menon DK, Zaman SH, Nestor PJ, Holland AJ, 2017. Brain-predicted age in Down syndrome is associated with beta amyloid deposition and cognitive decline. Neurobiol Aging 56, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BM, Hill AF, 2015. Extracellular vesicles--Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol 40, 89–96. [DOI] [PubMed] [Google Scholar]
- Cooper SA, Ademola T, Caslake M, Douglas E, Evans J, Greenlaw N, Haig C, Hassiotis A, Jahoda A, McConnachie A, Morrison J, Ring H, Starr J, Stiles C, Sirisena C, Sullivan F, 2016. Towards onset prevention of cognition decline in adults with Down syndrome (The TOP-COG study): A pilot randomised controlled trial. Trials 17, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Schmidt C, Davisson MT, 2010. Behavioral validation of the Ts65Dn mouse model for Down syndrome of a genetic background free of the retinal degeneration mutation Pde6b(rd1). Behav Brain Res 206, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, Mandal M, 2018. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol Pharm. [DOI] [PubMed] [Google Scholar]
- Davidson YS, Robinson A, Prasher VP, Mann DMA, 2018. The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer’s disease in individuals with Down syndrome. Acta Neuropathol Commun 6, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker AD, Fortea J, Blesa R, De Deyn PP, 2017. Cerebrospinal fluid biomarkers for Alzheimer’s disease in Down syndrome. Alzheimers Dement (Amst) 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ, 1996. Spatial memory deficits in segmental trisomic Ts65Dn mice. Behav Brain Res 82, 85–92. [DOI] [PubMed] [Google Scholar]
- Dinkins MB, Wang G, Bieberich E, 2017. Sphingolipid-Enriched Extracellular Vesicles and Alzheimer’s Disease: A Decade of Research. J Alzheimers Dis 60, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K, 2010. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30, 6838–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorihuela RM, Vallina IF, Martinez-Cue C, Baamonde C, Dierssen M, Tobena A, Florez J, Fernandez-Teruel A, 1998. Impaired short- and long-term memory in Ts65Dn mice, a model for Down syndrome. Neurosci Lett 247, 171–174. [DOI] [PubMed] [Google Scholar]
- Fader CM, Sanchez D, Furlan M, Colombo MI, 2008. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic 9, 230–250. [DOI] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R, 2006. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31, 642–648. [DOI] [PubMed] [Google Scholar]
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ, 2015. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11, 600–607 e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth NC, Startin CM, Hithersay R, Hamburg S, Wijeratne PA, Mok KY, Hardy J, Alexander DC, LonDown SC, Strydom A, 2018. Aging related cognitive changes associated with Alzheimer’s disease in Down syndrome. Ann Clin Transl Neurol 5, 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca LM, Yokomizo JE, Bottino CM, Fuentes D, 2016. Frontal Lobe Degeneration in Adults with Down Syndrome and Alzheimer’s Disease: A Review. Dement Geriatr Cogn Disord 41, 123–136. [DOI] [PubMed] [Google Scholar]
- Fortea J, Carmona-Iragui M, Benejam B, Fernandez S, Videla L, Barroeta I, Alcolea D, Pegueroles J, Munoz L, Belbin O, de Leon MJ, Maceski AM, Hirtz C, Clarimon J, Videla S, Delaby C, Lehmann S, Blesa R, Lleo A, 2018. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol 17, 860–869. [DOI] [PubMed] [Google Scholar]
- Franquesa M, Hoogduijn MJ, Ripoll E, Luk F, Salih M, Betjes MG, Torras J, Baan CC, Grinyo JM, Merino AM, 2014. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Front Immunol 5, 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamez-Valero A, Beyer K, Borras FE, 2018. Extracellular vesicles, new actors in the search for biomarkers of dementias. Neurobiol Aging 74, 15–20. [DOI] [PubMed] [Google Scholar]
- Gao D, Jiang L, 2018. Exosomes in cancer therapy: a novel experimental strategy. Am J Cancer Res 8, 2165–2175. [PMC free article] [PubMed] [Google Scholar]
- Gauthier SA, Perez-Gonzalez R, Sharma A, Huang FK, Alldred MJ, Pawlik M, Kaur G, Ginsberg SD, Neubert TA, Levy E, 2017. Enhanced exosome secretion in Down syndrome brain - a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol Commun 5, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM, 1992. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 32, 667–676. [DOI] [PubMed] [Google Scholar]
- Gibson D, 1973. Karyotype variation and behavior in Down’s syndrome: methodological review. Am J Ment Defic 78, 128–133. [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, Che S, 2010. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol Psychiatry 68, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson EJ, Dye DE, Bittles AH, 2014. The triple challenges associated with age-related comorbidities in Down syndrome. J Intellect Disabil Res 58, 393–398. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB, 2018. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J 32, 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Kapogiannis D, 2015. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Elahi FM, Mustapic M, Kapogiannis D, Pryhoda M, Gilmore A, Gorgens KA, Davidson B, Granholm AC, Ledreux A, 2019. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J, fj201802319R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL, 2016. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 30, 3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes de Andrade G, Reck Cechinel L, Bertoldi K, Galvão F, Valdeci Worm P, Rodrigues Siqueira I. The Aging Process Alters IL-1β and CD63 Levels Differently in Extracellular Vesicles Obtained from the Plasma and Cerebrospinal Fluid. Neuroimmunomodulation. 2018;25(1):18–22. doi: 10.1159/000488943. Epub 2018 Jul 18. . [DOI] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA, Crnic LS, 2000. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol 161, 647–663. [DOI] [PubMed] [Google Scholar]
- Greening DW, Simpson RJ, 2018. Understanding extracellular vesicle diversity - current status. Expert Rev Proteomics 15, 887–910. [DOI] [PubMed] [Google Scholar]
- Hamlett ED, Boger HA, Ledreux A, Kelley CM, Mufson EJ, Falangola MF, Guilfoyle DN, Nixon RA, Patterson D, Duval N, Granholm AC, 2016a. Cognitive Impairment, Neuroimaging, and Alzheimer Neuropathology in Mouse Models of Down Syndrome. Curr Alzheimer Res 13, 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett ED, Goetzl EJ, Ledreux A, Vasilevko V, Boger HA, LaRosa A, Clark D, Carroll SL, Carmona-Iragui M, Fortea J, Mufson EJ, Sabbagh M, Mohammed AH, Hartley D, Doran E, Lott IT, Granholm AC, 2016b. Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimers Dement. 2017 May;13(5):541–549. doi: 10.1016/j.jalz.2016.08.012. Epub 2016 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett ED, Ledreux A, Potter H, Chial HJ, Patterson D, Espinosa JM, Bettcher BM, Granholm AC, 2018. Exosomal biomarkers in Down syndrome and Alzheimer’s disease. Free Radic Biol Med 114, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner T, Udhnani MD, Osipowicz KZ, Lee NR, 2018. Pediatric Brain Development in Down Syndrome: A Field in Its Infancy. J Int Neuropsychol Soc 24, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL, Cohen AD, Channamalappa U, Bulova P, Cannon SA, Cohen WI, Mathis CA, Price JC, Klunk WE, 2012. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimers Dement 8, 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, Granholm AC, Iqbal K, Krams M, Lemere C, Lott I, Mobley W, Ness S, Nixon R, Potter H, Reeves R, Sabbagh M, Silverman W, Tycko B, Whitten M, Wisniewski T, 2015. Down syndrome and Alzheimer’s disease: Common pathways, common goals. Alzheimers Dement 11, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Handen BL, Devenny D, Mihaila I, Hardison R, Lao PJ, Klunk WE, Bulova P, Johnson SC, Christian BT, 2017. Cognitive decline and brain amyloid-beta accumulation across 3 years in adults with Down syndrome. Neurobiol Aging 58, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Lott IT, Hof PR, Bouras C, Su JH, Kim R, Haier R, Cotman CW, 2003. Parallel compensatory and pathological events associated with tau pathology in middle aged individuals with Down syndrome. J Neuropathol Exp Neurol 62, 917–926. [DOI] [PubMed] [Google Scholar]
- Head E, Lott IT, Wilcock DM, Lemere CA, 2016. Aging in Down Syndrome and the Development of Alzheimer’s Disease Neuropathology. Curr Alzheimer Res 13, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH, 1995. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down’s syndrome. Quantitative regional analysis and comparison with Alzheimer’s disease. Arch Neurol 52, 379–391. [DOI] [PubMed] [Google Scholar]
- Holm MM, Kaiser J, Schwab ME, 2018. Extracellular Vesicles: Multimodal Envoys in Neural Maintenance and Repair. Trends Neurosci 41, 360–372. [DOI] [PubMed] [Google Scholar]
- Jensen BC, Willis MS, 2016. The Head and the Heart: The Alzheimer’s Connection. J Am Coll Cardiol 68, 2408–2411. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA, 2010. Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A 107, 1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim CL, Mori H, Selkoe DJ, 1989. Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature 341, 226–230. [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C, 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262, 9412–9420. [PubMed] [Google Scholar]
- Juan T, Furthauer M, 2018. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 74, 66–77. [DOI] [PubMed] [Google Scholar]
- Jung MS, Park JH, Ryu YS, Choi SH, Yoon SH, Kwen MY, Oh JY, Song WJ, Chung SH, 2011. Regulation of RCAN1 protein activity by Dyrk1A protein-mediated phosphorylation. J Biol Chem 286, 40401–40412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ, 2015. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Tatebe H, Kondo M, Ishii R, Ohmichi T, Yeung WTE, Morimoto M, Chiyonobu T, Terada N, Allsop D, Nakagawa M, Mizuno T, Tokuda T, 2017. Increased levels of plasma total tau in adult Down syndrome. PLoS One 12, e0188802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, Kim SH, Park KS, Kim OY, Park HT, Seo JH, Aikawa E, Baj-Krzyworzeka M, van Balkom BW, Belting M, Blanc L, Bond V, Bongiovanni A, Borras FE, Buee L, Buzas EI, Cheng L, Clayton A, Cocucci E, Dela Cruz CS, Desiderio DM, Di Vizio D, Ekstrom K, Falcon-Perez JM, Gardiner C, Giebel B, Greening DW, Gross JC, Gupta D, Hendrix A, Hill AF, Hill MM, Nolte-’t Hoen E, Hwang DW, Inal J, Jagannadham MV, Jayachandran M, Jee YK, Jorgensen M, Kim KP, Kim YK, Kislinger T, Lasser C, Lee DS, Lee H, van Leeuwen J, Lener T, Liu ML, Lotvall J, Marcilla A, Mathivanan S, Moller A, Morhayim J, Mullier F, Nazarenko I, Nieuwland R, Nunes DN, Pang K, Park J, Patel T, Pocsfalvi G, Del Portillo H, Putz U, Ramirez MI, Rodrigues ML, Roh TY, Royo F, Sahoo S, Schiffelers R, Sharma S, Siljander P, Simpson RJ, Soekmadji C, Stahl P, Stensballe A, Stepien E, Tahara H, Trummer A, Valadi H, Vella LJ, Wai SN, Witwer K, Yanez-Mo M, Youn H, Zeidler R, Gho YS, 2015. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HW, Michael MZ, Gleadle JM, 2012. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk HE, Gray K, Riby DM, Taffe J, Cornish KM, 2017. Visual attention and academic performance in children with developmental disabilities and behavioural attention deficits. Dev Sci. 2017 November; 20(6). doi: 10.1111/desc.12468. Epub 2016 Sep 21. [DOI] [PubMed] [Google Scholar]
- Kowal J, Tkach M, Thery C, 2014. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29, 116–125. [DOI] [PubMed] [Google Scholar]
- Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J, 2007. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl 1, 1446–1461. [DOI] [PubMed] [Google Scholar]
- Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J, 2016. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am J Physiol Cell Physiol 310, C800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R, 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46, 409–418. [DOI] [PubMed] [Google Scholar]
- Lai CP, Breakefield XO, 2012. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol 3, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao PJ, Handen BL, Betthauser TJ, Mihaila I, Hartley SL, Cohen AD, Tudorascu DL, Bulova PD, Lopresti BJ, Tumuluru RV, Murali D, Mathis CA, Barnhart TE, Stone CK, Price JC, Devenny DA, Mailick MR, Klunk WE, Johnson SC, Christian BT, 2017. Longitudinal changes in amyloid positron emission tomography and volumetric magnetic resonance imaging in the nondemented Down syndrome population. Alzheimers Dement (Amst) 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Kim JH, Choi ES, Cho JH, Kim E, 2018. Effect of young exosomes injected in aged mice. Int J Nanomedicine 13, 5335–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM, 2008. Senescence-associated exosome release from human prostate cancer cells. Cancer Res 68, 7864–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ, 1996. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis 3, 16–32. [DOI] [PubMed] [Google Scholar]
- Levine S, Saltzman A, Levy E, Ginsberg SD, 2009. Systemic pathology in aged mouse models of Down’s syndrome and Alzheimer’s disease. Exp Mol Pathol 86, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wang J, Liu H, Chen Y, Ma X, Chen S, Chen Y, Bihl JI, Yang YI, 2018. Moderate Exercise Enhances Endothelial Progenitor Cell Exosomes Release and Function. Med Sci Sports Exerc 50, 2024–2032. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lasuncion I, Atienza M, Sanchez-Espinosa MP, Cantero JL, 2018. Aging-Related Changes in Cognition and Cortical Integrity are Associated With Serum Expression of Candidate MicroRNAs for Alzheimer Disease. Cereb Cortex. 2018 December 22. doi: 10.1093/cercor/bhy323. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ, 2012. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40, D1241–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PD, Dalton AJ, Mehta SP, Kim KS, Sersen EA, Wisniewski HM, 1998. Increased plasma amyloid beta protein 1–42 levels in Down syndrome. Neurosci Lett 241, 13–16. [DOI] [PubMed] [Google Scholar]
- Menck K, Sonmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, Erdmann G, Kling S, Boutros M, Binder C, Gross JC, 2017. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles 6, 1378056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic I, Jarc J, Dassler E, Aronica E, Iyer A, Adle-Biassette H, Scharrer A, Reischer T, Hainfellner JA, Kovacs GG, 2018. The physiological phosphorylation of tau is critically changed in fetal brains of individuals with Down syndrome. Neuropathol Appl Neurobiol 44, 314–327. [DOI] [PubMed] [Google Scholar]
- Millan Sanchez M, Heyn SN, Das D, Moghadam S, Martin KJ, Salehi A, 2012. Neurobiological elements of cognitive dysfunction in down syndrome: exploring the role of APP. Biol Psychiatry 71, 403–409. [DOI] [PubMed] [Google Scholar]
- Miranda AM, Lasiecka ZM, Xu Y, Neufeld J, Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA, Di Paolo G, 2018. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat Commun 9, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon-Rodriguez S, Perry G, Luna-Munoz J, Acevedo-Aquino MC, Williams S, 2014. Phosphorylation of tau protein at sites Ser(396–404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol Appl Neurobiol 40, 121–135. [DOI] [PubMed] [Google Scholar]
- Moulder KL, Snider BJ, Mills SL, Buckles VD, Santacruz AM, Bateman RJ, Morris JC, 2013. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimers Res Ther 5, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naerland T, Bakke KA, Storvik S, Warner G, Howlin P, 2017. Age and gender-related differences in emotional and behavioural problems and autistic features in children and adolescents with Down syndrome: a survey-based study of 674 individuals. J Intellect Disabil Res 61, 594–603. [DOI] [PubMed] [Google Scholar]
- Neale N, Padilla C, Fonseca LM, Holland T, Zaman S, 2018. Neuroimaging and other modalities to assess Alzheimer’s disease in Down syndrome. Neuroimage Clin 17, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, 2017. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J 31, 2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Querejeta I, Alberro A, Munoz-Culla M, Mager I, Otaegui D, 2018. Therapeutic Potential of Extracellular Vesicles for Demyelinating Diseases; Challenges and Opportunities. Front Mol Neurosci 11, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake K, Kamiguchi H, Hirozane Y. Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid. BMC Med Genomics. 2019. January 10;12(1):7. doi: 10.1186/s12920-019-0473-z. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou ME, Tavernarakis N, 2017. Autophagy and the endo/exosomal pathways in health and disease. Biotechnol J. 2017 January; 12(1). doi: 10.1002/biot.201600175. Epub 2016 Dec 15. [DOI] [PubMed] [Google Scholar]
- Pathak A, Rohilla A, Gupta T, Akhtar MJ, Haider MR, Sharma K, Haider K, Yar MS, 2018. DYRK1A kinase inhibition with emphasis on neurodegeneration: A comprehensive evolution story-cum-perspective. Eur J Med Chem 158, 559–592. [DOI] [PubMed] [Google Scholar]
- Peng KY, Perez-Gonzalez R, Alldred MJ, Goulbourne CN, Morales-Corraliza J, Saito M, Saito M, Ginsberg SD, Mathews PM, Levy E, 2018. Apolipoprotein E4 genotype compromises brain exosome production. Brain. 2019 January 1;142(1):163–175. doi: 10.1093/brain/awy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E, 2012. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287, 43108–43115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco JC, Li C, Durisic N, Sullivan R, Gotz J, 2018. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol Commun. 2018 February 15; 6(1):10. doi: 10.1186/s40478-018-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher VP, Sajith SG, Mehta P, Zigman WB, Schupf N, 2010. Plasma beta-amyloid and duration of Alzheimer’s disease in adults with Down syndrome. Int J Geriatr Psychiatry 25, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Properzi F, Logozzi M, Fais S, 2013. Exosomes: the future of biomarkers in medicine. Biomark Med 7, 769–778. [DOI] [PubMed] [Google Scholar]
- Rafii MS, 2018. Tau PET Imaging for Staging of Alzheimer’s Disease in Down Syndrome. Dev Neurobiol. 2018 December 8 doi: 10.1002/dneu.22658. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- Rafii MS, Lukic AS, Andrews RD, Brewer J, Rissman RA, Strother SC, Wernick MN, Pennington C, Mobley WC, Ness S, Matthews DC, Down Syndrome Biomarker I, the Alzheimer’s Disease Neuroimaging, I., 2017. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimers Dis 60, 439–450. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K, 2006. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103, 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W, 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT, 1995. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet 11, 177–184. [DOI] [PubMed] [Google Scholar]
- Ren K, 2018. Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology. 2018 October 15. doi: 10.1007/s10266-018-0395-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofi E, Vivaldi C, Del Re M, Arrigoni E, Crucitta S, Funel N, Fogli S, Vasile E, Musettini G, Fornaro L, Falcone A, Danesi R, 2018. The emerging role of liquid biopsy in diagnosis, prognosis and treatment monitoring of pancreatic cancer. Pharmacogenomics. 2019 January;20(1):49–68. doi: 10.2217/pgs-2018-0149. Epub 2018 Dec 6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Mejias M, Martinez de Lagran M, Mattia M, Castano-Prat P, Perez-Mendez L, Ciria-Suarez L, Gener T, Sancristobal B, Garcia-Ojalvo J, Gruart A, Delgado-Garcia JM, Sanchez-Vives MV, Dierssen M, 2016. Overexpression of Dyrk1A, a Down Syndrome Candidate, Decreases Excitability and Impairs Gamma Oscillations in the Prefrontal Cortex. J Neurosci 36, 3648–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo SR, Jeong HK, Radnaabazar C, Yoo JJ, Cho HJ, Lee HW, Kim IS, Cheon YH, Ahn YS, Chung SH, Song WJ, 2007. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease. J Biol Chem 282, 34850–34857. [DOI] [PubMed] [Google Scholar]
- Salido-Guadarrama I, Romero-Cordoba S, Peralta-Zaragoza O, Hidalgo-Miranda A, Rodriguez-Dorantes M, 2014. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther 7, 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeppe F, Rossi A, Levin J, Reiser M, Stoecklein S, Ertl-Wagner B, 2018. Increased cerebral microbleeds and cortical superficial siderosis in pediatric patients with Down syndrome. Eur J Paediatr Neurol. 2019 January;23(1):158–164. doi: 10.1016/j.ejpn.2018.09.004. Epub 2018 Sep 12. [DOI] [PubMed] [Google Scholar]
- Schupf N, Lee A, Park N, Dang LH, Pang D, Yale A, Oh DK, Krinsky-McHale SJ, Jenkins EC, Luchsinger JA, Zigman WB, Silverman W, Tycko B, Kisselev S, Clark L, Lee JH, 2015. Candidate genes for Alzheimer’s disease are associated with individual differences in plasma levels of beta amyloid peptides in adults with Down syndrome. Neurobiol Aging 36, 2907 e2901–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendera TJ, Ma SY, Jaffar S, Kozlowski PB, Kordower JH, Mawal Y, Saragovi HU, Mufson EJ, 2000. Reduction in TrkA-immunoreactive neurons is not associated with an overexpression of galaninergic fibers within the nucleus basalis in Down’s syndrome. J Neurochem 74, 1185–1196. [DOI] [PubMed] [Google Scholar]
- Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS, 2017. Urine Exosomes: An Emerging Trove of Biomarkers. Adv Clin Chem 78, 103–122. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA, Coleman BM, Hill AF, Kusebauch U, Hallows JL, Shteynberg D, Moritz RL, Zhu HJ, Simpson RJ, 2013. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics 12, 2148–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J, 2018. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150, 137–149. [DOI] [PubMed] [Google Scholar]
- Tkach M, Kowal J, Thery C, 2018. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci. 2018 January 5;373(1737). pii: 20160479. doi: 10.1098/rstb.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramutola A, Lanzillotta C, Arena A, Barone E, Perluigi M, Di Domenico F, 2016. Increased Mammalian Target of Rapamycin Signaling Contributes to the Accumulation of Protein Oxidative Damage in a Mouse Model of Down’s Syndrome. Neurodegener Dis 16, 62–68. [DOI] [PubMed] [Google Scholar]
- Troncone L, Luciani M, Coggins M, Wilker EH, Ho CY, Codispoti KE, Frosch MP, Kayed R, Del Monte F, 2016. Abeta Amyloid Pathology Affects the Hearts of Patients With Alzheimer’s Disease: Mind the Heart. J Am Coll Cardiol 68, 2395–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM, Stankiewicz AM, 2018. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front Aging Neurosci 10, 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, 2015. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- Vaswani K, Koh YQ, Almughlliq FB, Peiris HN, Mitchell MD, 2017. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod Biol 17, 341–348. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF, 2008. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J 37, 323–332. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Hamdane M, Loyens A, Gele P, Drobeck H, Begard S, Galas MC, Delacourte A, Beauvillain JC, Buee L, Sergeant N, 2007. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem 282, 18197–18205. [DOI] [PubMed] [Google Scholar]
- Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, Mandelkow EM, 2017. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Wisniewski HM, Dziewiatkowski J, Popovitch ER, Tarnawski M, 1996. Differential susceptibility to neurofibrillary pathology among patients with Down syndrome. Dementia 7, 135–141. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Schmitt FA, Head E, 2016. Cerebrovascular contributions to aging and Alzheimer’s disease in Down syndrome. Biochim Biophys Acta 1862, 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Aulston B, Rockenstein EM, Adame A, Prikhodko O, Dave KN, Mishra P, Rissman RA, Yuan SH, 2018. Neuronal Exosome-Derived Human Tau is Toxic to Recipient Mouse Neurons in vivo. J Alzheimers Dis. 2019;67(2):541–553. doi: 10.3233/JAD-180776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA, 2016. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VL, Fisher EM, Strydom A, 2015. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci 16, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, 1990. Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. Am J Med Genet Suppl 7, 274–281. [DOI] [PubMed] [Google Scholar]
- Wolf P, 1967. The nature and significance of platelet products in human plasma. Br J Haematol 13, 269–288. [DOI] [PubMed] [Google Scholar]
- Xiao H, Lasser C, Shelke GV, Wang J, Radinger M, Lunavat TR, Malmhall C, Lin LH, Li J, Li L, Lotvall J, 2014. Mast cell exosomes promote lung adenocarcinoma cell proliferation - role of KIT-stem cell factor signaling. Cell Commun Signal 12, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2017. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res 12, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Camfield R, Gorski SM, 2018. The interplay between exosomes and autophagy - partners in crime. J Cell Sci 131. [DOI] [PubMed] [Google Scholar]
- Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, Hua J, Wei W, Zhu Q, 2015. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol 12, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Igarashi Y, 2017. Exosomes as Carriers of Alzheimer’s Amyloid-ss. Front Neurosci 11, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Kimura N, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y, 2015. A potential function for neuronal exosomes: sequestering intracerebral amyloid-beta peptide. FEBS Lett 589, 84–88. [DOI] [PubMed] [Google Scholar]
- Zaborowski MP, Balaj L, Breakefield XO, Lai CP, 2015. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 65, 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]