Abstract
Background:
Inertial sensors are increasingly useful to clinicians and researchers to detect gait deficits. Reference values are necessary for comparison to children with gait abnormalities.
Objective:
To present a normative database of spatiotemporal gait and turning parameters in 162 typically developing children and young adults ages 5–30 utilizing the APDM Mobility Lab® system.
Methods:
Participants completed the i-WALK test at both self-selected (SS) and fast as possible (FAP) walking speeds. Spatiotemporal gait and turning parameters included stride length, stride length variability, gait speed, cadence, stance, swing, and double support times, and initial contact, toe-off, and toe-out angles, turn duration, peak turn velocity and number of steps to turn.
Results:
Absolute stride length and gait speed increased with age. Normalized gait speed, absolute and normalized cadence, and stride length variability decreased with age. Normalized stride length and all parameters of gait cycle phase and foot position remained unaffected by age except for greater FSA in children 7–8. Foot position parameters in children 5–6 were excluded due to aberrant values and high standard deviations. Turns were faster in children ages 5–13 and 7–13 in the SS and FAP conditions, respectively. There were no differences in number of steps to turn. Similar trends were observed in the FAP condition except: normalized gait speed did not demonstrate a relationship with age and children ages 5–8 demonstrated increased stance and double support times and decreased swing time compared to children 11–13 and young adults (ages 5–6 only). Females ages 5–6 demonstrated increased stride length variability in the SS condition; males ages 7–8 and 14–30 had increased absolute stride length in the FAP condition. Similarities and differences were found between our values and previous literature.
Significance:
This normative database can be used by clinicians and researchers to compare abnormal gait patterns and responses to interventions.
Keywords: gait, normative, APDM MobilityLab®, inertial sensors, children
1. Introduction
The analysis of gait is critical in evaluating the effectiveness of interventions for children with motor impairments. Gait requires complex neuromuscular coordination [1, 2] and walking forms a key part of development by allowing the child to explore and interact with the environment. Reference values from typically developing children and young adults are therefore necessary to inform clinicians and researchers on typical gait maturation patterns, guide clinical practice and compare motor outcomes in children with gait abnormalities.
Gait analysis tools such as visual observation, questionnaires, and timed tests including the Timed Up and Go (TUG) and two-minute walk tests are commonly used by clinicians and researchers but may be limited in their clinical usefulness because some are qualitative measures and are not sensitive enough to detect subtle but important changes in movement patterns [1, 3, 4]. In contrast, more quantitative gait analysis tools such as the GAITRite® are limited by the relatively short length of the walkway and inability to measure trunk motion, turning, and upper extremity parameters frequently impaired in clinical populations with neurological impairments. Other methods including 3D motion capture are prohibitive in their clinical utility due to cost, lack of portability or difficulty of use [1, 3].
Inertial sensors provide a useful alternative and are relatively low cost, easy to transport, and more objective than traditional clinical assessments [1, 4–6]. Increasingly popular to investigate gait, balance and functional mobility impairments in neurological populations including Parkinson’s Disease [7–9], cerebellar ataxia [5, 10], multiple sclerosis [11–13], and traumatic brain injury [14–16], inertial sensors have added benefits in both pediatric and neurological populations. They are easy to apply, do not limit gait analysis to a given space or walkway, and do not restrict natural movement patterns [6]. One such system, the commercially available APDM Mobility Lab® Opal system, utilizes accelerometers, gyroscopes, and magnetometers to measure spatiotemporal parameters of gait and turning while participants perform standard gait and functional mobility assessments such as the TUG and two-minute walk tests [17, 18]. The Mobility Lab® system has been shown to be valid and repeatable in healthy young adults [4], and was partially validated in typically developing children (ages 3.0–8.3) against 3D motion analysis [6], but test-retest repeatability in children is still lacking.
Previous studies have presented normative data for spatiotemporal gait parameters in children utilizing 3D motion capture [19] and GAITRite® [20–23]. A partial normative database utilizing the APDM Mobility Lab® system has been established in adults [1], but has yet to be established in children. Such a database will enable the more than 1000 users of this system to reliably compare gait measures obtained in pediatric populations with gait impairments [24]. Therefore, the purpose of this study was to 1) present a normative database of spatiotemporal gait parameters in healthy children and young adults 5–30 years and 2) analyze age specific changes in spatiotemporal gait and turning parameters using the APDM Mobility Lab® system.
2. Methods
2.1. Participants
A total of 175 typically developing children and young adults was recruited from the local community through school systems, park districts, and health fairs in the Chicago metropolitan area and through students, friends or family members of employees from Rush University Medical Center (RUMC) or the NIH Clinical Research Center, Bethesda, MD. Inclusion criteria were typically developing children and young adults of both sexes ages 5–30 years and ability to follow verbal directions. Exclusion criteria included any known neurological, developmental, neuromuscular, and/or orthopedic impairments, inability to follow directions, requiring an assistive device for ambulation and current or past medical diagnoses affecting balance. Approval was obtained from both the RUMC and NIH IRBs. All participants, or their parents/legal guardians for those under 18 years, provided signed written consent.
2.2. Data Collection
Demographic measures were: age, sex, height (cm), weight (kg), BMI (kg/m2) and ethnicity. Participants were instructed to wear comfortable clothing and standard walking or gym shoes. Tests took place in school gyms or hallways with minimal distractions. Each participant was given standardized instructions prior to each session and before each condition.
Gait analysis was performed using the APDM Mobility Lab® six Opal inertial sensor system (APDM Mobility Lab 2®; Oregon, Version 2.0.0.201903301). Participants wore six sensors over their clothing: on the dorsal aspect of each foot and wrist, on the lumbar trunk at approximately L5, and approximately two centimeters below the sternal notch. A laptop wirelessly collected data from the sensors which was processed by the Mobility Lab 2® software containing the instrumented walk test (i-WALK) plug-in from which spatiotemporal parameters of gait and turns were quantified using algorithms developed by the manufacturer. The i-WALK was performed along a 25 meter walkway under two conditions: 1) walking at a self-selected pace (SS); and 2) walking as fast as possible (FAP) without running. The FAP condition was included because numerous studies investigate fast paced gait as measures of both endurance and locomotor skills [25–27]. Both conditions were performed on a flat, straight, non-carpeted surface and both ends of the walkway were designated by two cones and bright masking tape. The test administrator demonstrated the task and instructed the participant to remain still for approximately 5 seconds before and after test completion. A three second countdown started each test. The participant walked from one end of the walkway towards the opposite end at a self-selected pace, pivot turned 180 degrees and returned to the starting line, repeating the procedure until two minutes elapsed. The participant was given two minutes to rest if needed before repeating the procedure in the FAP condition.
Data was stratified into six age groups: 5–6, 7–8, 9–10, 11–13, 14–21 and 22–30 years old. These groups were chosen based on precedence in the literature examining gait differences between similar age groups [19–23, 28, 29]. Prior research also suggested that gait may not be mature until age 12 or 13 [20, 28], leading to the selection of 14–21 years olds as one category. The 13 metrics selected for analysis were chosen a priori from 74 gait and turn metrics provided by the APDM Mobility Lab V2.0 system as these metrics are most typically reported in the literature examining gait and turn abnormalities in children and commonly investigated in normative gait studies [1, 19–23, 28, 29]. The parameters selected for this study included: stride length, stride length variability (Coefficient of variation (CoV) = SD/mean), gait speed, cadence, stance, swing, and double support times, foot strike angle (FSA), toe-off angle (TOA), toe-out angle, turn duration, peak turn velocity and number of steps to turn. A turn is defined by a sustained heading change greater than 45 degrees from the line of progression. Illustrations with definitions of foot angles are included in Supplementary Figure 1. It should be noted that the algorithm in Mobility Lab V2.0 for stride length calculation was changed from V1.0; it uses zero velocity updates and does not require any type of correction or calibration that relates to the stature or anatomy of the subjects. However, due to precedence for the use of normalization procedures in the pediatric gait literature [19, 21–23, 28, 29], we additionally expressed stride length, gait speed and cadence as a % of total height. Normalization to height, as has been done in previous studies [21–23], was chosen over leg length for increased efficiency for clinicians and researchers utilizing the APDM Mobility Lab system.
2.3. Data Analysis
Descriptive statistics were applied to all parameters (means and SD), and data was analyzed using SPSS 19.0 and SAS 9.4 software. Data distributed more than three SD from the mean were excluded as outliers (SS: n=13; FAP: n=9). Normal distribution of variables was checked using the Kolmogorov-Smirnov and Shapiro-Wilk normality tests. Intraclass correlation coefficients (ICCs) were calculated for bilateral gait variables between the right and left side. ICCs were high for FSA (0.98) and TOA (0.95), swing and stance phase times (>0.99) and stride length (0.95); therefore, these bilateral values were averaged for statistical analysis as in the literature [19–21, 23, 25, 26]. Toe-out angle ICC was 0.29, also reported as low or fair in prior studies [21, 22]. Stride length, velocity and cadence were normalized to height (% stature) and subjects were categorized as normal weight (18.5kg/m2≤BMI≤28 kg/m2) or obese (BMI>28 kg/m2). The effects of age, sex and the interaction between age and sex on gait performance was explored using a two-way ANOVA. A one-way ANOVA with Tukey’s HSD post hoc comparisons was used to assess differences between age groups to control for multiple comparisons. Two-sample t tests were used to compare differences between sexes. Significance was set to p<0.05.
3. Results
3.1. Participant Demographics
Demographics of the 162 participants included in the analyses are presented in Table 1. Five subjects were in the obese category: four in the 14–21 age group and one in the 9–10 age group. No significant differences were found between obese and non-obese subjects. 62% percent of participants were Caucasian, 14% were African-American, 12% were Hispanic, 7% were Asian, and 5% were mixed-race.
Table 1.
Participant demographics.
| Age (years) | |||||||
|---|---|---|---|---|---|---|---|
| 5–6 | 7–8 | 9–10 | 11–13 | 14–21 | 22–30 | ||
| n | |||||||
| Female | 11 (50 %) | 15 (54 %) | 10 (48 %) | 15 (50 %) | 15 (60 %) | 17 (47 %) | |
| Male | 11 (50 %) | 13 (46 %) | 11 (52 %) | 15 (50 %) | 10 (40 %) | 19 (53 %) | |
| Total | 22 | 28 | 21 | 30 | 25 | 36 | |
| Age | |||||||
| Female | 5.40 ± 0.52 | 7.36 ± 0.50 | 9.50 ± 0.53 | 11.95 ± 0.77 | 16.40 ± 2.16 | 24.59 ± 2.06 | |
| Male | 5.56 ± 0.53 | 7.46 ± 0.52 | 9.54 ± 0.52 | 12.23 ± 0.89 | 16.20 ± 2.20 | 24.26 ± 0.99 | |
| Total | 5.47 ± 0.51 | 7.41 ± 0.50 | 9.50 ± 0.51 | 12.09 ± 0.83 | 16.32 ± 2.14 | 24.41 ± 1.57 | |
| Height (cm) | |||||||
| Female | 113.50 ± 6.52 | 127.29 ± 0.10 | 140.10 ± 4.95 | 158.53 ± 8.80 | 162.87 ± 10.88 | 165.71 ± 8.33 | |
| Male | 115.89 ± 8.60 | 131.15 ± 0.08 | 144.00 ± 6.20 | 158.71 ± 12.30 | 174.70 ± 7.00 | 182.32 ± 4.76 | |
| Total | 114.63 ± 7.31 | 129.15 ± 8.88 | 142.00 ± 5.97 | 158.62 ± 10.44 | 167.60 ± 11.06 | 174.47 ± 10.68 | |
| Weight (kg) | |||||||
| Female | 20.10 ± 1.97 | 26.14 ± 7.21 | 38.70 ± 11.15 | 48.73 ± 12.78 | 70.40 ± 27.55 | 62.06 ± 8.91 | |
| Male | 21.89 ± 4.46 | 30.92 ± 8.96 | 41.18 ± 10.20 | 47.86 ± 12.33 | 74.50 ± 12.96 | 77.42 ± 7.69 | |
| Total | 20.95 ± 3.41 | 28.44 ± 8.30 | 40.00 ± 10.46 | 48.31 ± 12.35 | 72.04 ± 22.59 | 70.17 ± 11.28 | |
| BMI (kg/m2) | |||||||
| Female | 15.72 ± 2.30 | 15.89 ± 2.64 | 19.65 ± 5.26 | 19.13 ± 3.74 | 26.43 ± 9.27 | 22.57 ± 2.42 | |
| Male | 16.15 ± 1.49 | 17.77 ± 3.49 | 19.84 ± 4.79 | 18.75 ± 2.91 | 24.45 ± 4.31 | 23.25 ± 1.61 | |
| Total | 15.93 ± 1.92 | 16.80 ± 3.16 | 19.75 ± 4.89 | 18.95 ± 3.31 | 25.64 ± 7.62 | 22.93 ± 2.04 | |
3.2. Self-selected (SS) Walking Pace
Means and SD for all parameters in the SS condition are presented in Table 2. When male and female values were found to be significantly different they are reported separately. Age impacted all gait parameters except double support time and the number of steps to turn, whereas sex only affected cadence and normalized gait speed (p=0.0005 and p=0.007, respectively), where both were significantly higher in females (Table 4). The interaction of age and sex was significant in stride length variability (p=0.006), with post-hoc comparisons demonstrating greater variability in females ages 5–6 (p<0.0001).
Table 2.
Normative reference gait and turn data for the self-selected pace condition.
| i-WALK domain parameters | 5–6 (n=22) | 7–8 (n=29) | 9–10 (n=22) | 11–13 (n=_0) | 14–21 (n=25) | 22–30 (n=35) |
|---|---|---|---|---|---|---|
| Gait speed | ||||||
| Stride length (m) | 0.99 ± 0.11 | 1.10 ± 0.13 | 1.20 ± 0.13 | 1.3 2 ± 0.13 | 1.34 ± 0.16 | 1.42 ± 0.12 |
| Stride length (% stature) | 85.46 ± 9.38 | 85.37 ± 7.79 | 84.70 ± 8.73 | 83.53 ± 7.07 | 79.69 ± 7.97 | 81.56 ± 6.16 |
| Gait speed (m/s) | 1.11 ± 0.12 | 1.19 ± 0.16 | 1.25 ± 0.17 | 1.34 ± 0.14 | 1.28 ± 0.15 | 1.36 ± 0.17 |
| Gait speed (% stature/s) | 96.41 ± 10.13 | 92.70 ± 12.00 | 87.8 7 ± 11.68 | 85.23 ± 11.69 | 76.57 ± 9.62 | 78.34 ± 10.22 |
| Gait variability | ||||||
| Stride length CoV | 0.14 ± 0.12 | 0.09 ± 0.05 | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.01 |
| Female | 0.18 ± 0.15**** | 0.08 ± 0.04 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.02 |
| Male | 0.09 ± 0.05**** | 0.09 ± 0.07 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.01 |
| Gait rhythm | ||||||
| Cadence (steps/min) | 138.95 ± 9.06 | 131.09 ± 11.79 | 124.20 ± 9.02 | 122.03 ± 10.94 | 115.35 ± 8.18 | 114.84 ± 9.78 |
| Normalized cadence | 120.65 ± 11.87 | 102.24 ± 14.23 | 87.54 ± 7.53 | 77.78 ± 11.91 | 69.29 ± 8.44 | 66.18 ± 8.40 |
| Gait cycle phase | ||||||
| Swing (%) | 42.09 ± 1.82 | 41.32 ± 1.99 | 41.38 ± 1.59 | 42.19 ± 1.22 | 41.07 ± 1.64 | 41.21 ± 1.07 |
| Stance (%) | 57.9 1 ± 1.82 | 58.68 ± 1.99 | 58.62 ± 1.59 | 57.81 ± 1.22 | 58.93 ± 1.64 | 58.79 ± 1.07 |
| Double support (%) | 16.29 ± 3.61 | 17.57 ± 3.79 | 17.28 ± 3.11 | 15.67 ± 2.37 | 17.85 ± 3.29 | 17.58 ± 2.12 |
| Movement transition | ||||||
| Peak turn velocity (degrees/s) | 275.86 ± 55.05 | 318.01 ± 77.25 | 308.33 ± 75.17 | 288.79 ± 47.94 | 235.68 ± 45.47 | 220.93 ± 44.76 |
| Turn duration (s) | 1.50 ± 0.37 | 1.50 ± 0.33 | 1.61 ± 0.25 | 1.61 ± 0.27 | 1.82 ± 0.33 | 1.93 ± 0.31 |
| Number steps to Turn | 3.07 ± 0.82 | 2.93 ± 0.97 | 3.01 ± 0.57 | 2.99 ± 0.52 | 3.18 ± 0.64 | 3.38 ± 0.52 |
| Foot position | ||||||
| FSA (degrees) | ---- | 35.88 ± 19.06 | 28.07 ± 4.61 | 26.82 ± 3.78 | 25.76 ± 4.83 | 25.96 ± 3.90 |
| TOA (degrees) | ---- | 33.43 ± 14.06 | 38.77 ± 2.86 | 38.92 ± 2.00 | 39.37 ±3.24 | 39.43 ± 3.53 |
| Toe out angle | ---- | 3.21 ± 8.73 | 4.60 ± 6.27 | 4.71 ± 4.52 | 5.82 ± 7.37 | 6.24 ± 5.28 |
Data presented as female and male only when statistically significant differences were observe d between the sexes. Key: FSA, Foot strike angle; TOA, Toe off angle
p<0.0001
Table 4.
The influence of age group, sex, and age group x sex interactions on gait parameters in the self-selected and fast as possible conditions.
| SS | Age group (p) | Sex (p) | Age group * sex (p) | FAP | Age Group (p) | Sex (p) | Age group * sex (p) | |
|---|---|---|---|---|---|---|---|---|
| Stride length (m) | <0.0001 | 0.12 | <0.0001 | 0.002 | 0.001 | |||
| Stride length (% stature) | 0.04 | 0.19 | 0.73 | 0.14 | ||||
| Gait speed (m/s) | <0.0001 | 0.46 | <0.0001 | 0.0008 | ||||
| Gait speed (% stature) | <0.0001 | 0.007 | <0.0001 | 0.46 | ||||
| Stride length CoV | <0.0001 | 0.09 | 0.006 | <0.0001 | 0.547 | |||
| Cadence (steps/min) | <0.0001 | 0.0005 | <0.0001 | 0.57 | ||||
| Swing | 0.04 | 0.73 | 0.01 | 0.01 | ||||
| Stance | 0.04 | 0.73 | 0.01 | 0.01 | ||||
| Double Support | 0.06 | 0.72 | 0.001 | 0.02 | ||||
| Peak turn velocity | <0.0001 | 0.49 | <0.0001 | 0.38 | ||||
| Turn duration (s) | <0.0001 | 0.95 | <0.0001 | 0.19 | ||||
| Number steps to turn | 0.11 | 0.45 | 0.22 | 0.37 | ||||
| FSA (degrees) | <0.0001 | 0.68 | <0.0001 | 0.55 | ||||
| TOA (degrees) | <0.0001 | 0.25 | <0.0001 | 0.53 | ||||
| Toe out angle (degrees) | <0.0001 | 0.18 | <0.0001 | 0.43 | ||||
Key: SS, self-selected pace; FAP, fast as possible pace; FSA, Foot strike angle; TOA, Toe off angle. Significant values are bolded.
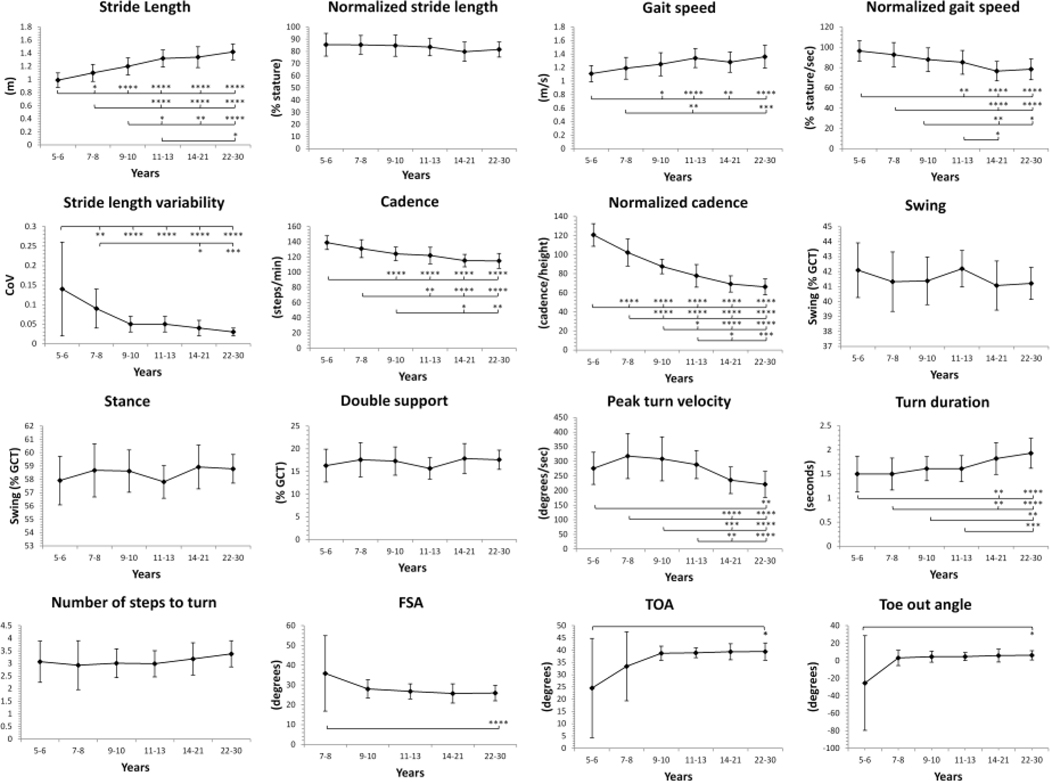
Significant differences between children and adults were observed across all gait domains except gait phase cycle (Figure 1; p-values are presented in Supplemental Table 1). Absolute stride length progressively increased with age, where children ages 5–13 had shorter stride length than young adults. After normalization to height, no differences in stride length were observed between age groups. Absolute gait speed was slowest in children 5–8, while normalized gait speed was fastest in children ages 5–10 and progressively decreased with age. Stride length variability decreased with age and was greatest in children ages 5–6 compared to all older age groups and in children 7–8 compared to the two oldest age groups. Absolute cadence decreased with age, with children ages 5–10 walking more steps per minute than older age groups at least two respective age tiers above. Similarly, normalized cadence decreased progressively with age and was higher in children 5–13 years compared to all older age groups. Time spent in swing, stance, and double support phase did not differ between age groups.
Figure 1 Legend.
Spatiotemporal gait and turn metrics in children and young adults (ages 5 to 30) in the self-selected pace condition. All data reported as Mean ± SD. Key: GCT, gait cycle time; CoV, Coefficient of variation = SD/mean; FSA, foot strike angle; TOA, toe off angle. Significant differences between age groups are noted as *p<0.05, **p<0.01, ***p<0.001 ****p<0.0001
Toe-out angle was aberrant in children ages 5–6, with negative values and a large SD (25.52 ± 54.12 degrees). Similarly, all measures of foot position in children 5–6 years also had large SDs; therefore, these values were excluded from the reference table. Children 7–8 years showed greater FSA than adults, but these values may also be invalid for the same reason (SD=19.06). No other differences were observed for foot position. Children ages 5–13 had faster turns than the two eldest age groups with higher peak velocity and shorter turn duration. No differences were observed in number of steps to turn.
3.3. Fast as Possible (FAP) Walking Pace
Means and SD for all parameters in the FAP condition are presented in Table 3. Age impacted all parameters except normalized stride length and number of steps to turn, whereas sex affected absolute gait speed, stride length and all three gait cycle phases (Table 4). Absolute gait speed, stride length and time spent in swing were greater in males (p=0.0008, p=0.002, and p=0.01, respectively), and time spent in stance and double support were greater in females (p=0.01 and p=0.02, respectively). The interaction of age and sex was significant in absolute stride length (p=0.001), with post-hoc comparisons demonstrating greater absolute stride length in males in the 7–8, 14–21, and 22–30 year age groups (p=0.03, p=0.0009, and p<0.0001, respectively).
Table 3.
Normative reference gait and turn data for the fast pace condition.
| i-WALK domain parameters | 5–6 (n=21) | 7–8 (n=26) | 9–10(n=22) | 11–13 (n-29) | 14–21 (n=24) | 22–30 (n=37) |
|---|---|---|---|---|---|---|
| Gait speed | ||||||
| Stride length (m) | 1.04 ± 0.09 | 1.17 ± 0.16 | 1.31 ± 0.13 | 1.45 ± 0.16 | 1.50 ± 0.18 | 1.61 ± 0.19 |
| Female | 1.08 ± 0.07 | 1.11 ± 0.16* | 1.29 ± 0.15 | 1.45 ± 0.13 | 1.42 ± 0.14*** | 1.5 ± 0.18**** |
| Male | 0.97 ± 0.06 | 1.23 ± 0.15* | 1.33 ± 0.11 | 1.45 ± 0.18 | 1.62 ± 0.16*** | 1.72 ± 0.12**** |
| Stride length (% stature) | 89.08 ± 10.45 | 90.88 ± 9.50 | 92.15 ± 8.88 | 91.20 ± 8.36 | 89.31 ± 8.37 | 92.58 ± 7.95 |
| Gait speed (m/s) | 1.26 ± 0.17 | 1.46 ± 0.14 | 1.59 ± 0.19 | 1.70 ± 0.16 | 1.65 ± 0.17 | 1.81 ± 0.22 |
| Gait speed (% stature/s) | 107.87 ± 14.17 | 114.30 ± 8.55 | 111.95 ± 13.08 | 106.85 ± 13.02 | 98.58 ± 10.35 | 103.84 ± 10.88 |
| Gait variability | ||||||
| Stride length CoV | 0.19 ± 0.21 | 0.10 ± 0.08 | 0.07 ± 0.05 | 0.06 ± 0.05 | 0.04 ± 0.03 | 0.03 ± 0.01 |
| Gait rhythm | ||||||
| Cadence (steps/min) | 147.62 ± 17.80 | 150.92 ± 9.97 | 148.04 ± 11.03 | 140.80 ± 13.23 | 132.73 ± 8.84 | 134.32 ± 9.34 |
| Normalized cadence | 126.52 ± 16.08 | 118.41 ± 13.22 | 104.17 ± 9.11 | 88.83 ± 13.73 | 79.49 ± 9.53 | 77.63 ± 8.61 |
| Gait cycle phase | ||||||
| Swing (%) | 41.76 ± 3.58 | 42.63 ± 2.42 | 43.61 ± 1.48 | 44.48 ± 1.32 | 43.09 ± 1.84 | 43.59 ± 1.35 |
| Stance (%) | 58.24 ± 3.58 | 57.37 ± 2.42 | 56.39 ± 1.48 | 55.52 ± 1.32 | 56.91 ± 1.84 | 56.41 ± 1.35 |
| Double support (%) | 15.74 ± 5.47 | 15.34 ± 4.48 | 13.56 ± 2.16 | 11.45 ± 3.03 | 14.14 ± 3.31 | 13.27 ± 2.22 |
| Movement transition | ||||||
| Peak turn velocity | 300.21 ± 58.07 | 360.45 ± 69.84 | 357.33 ± 72.57 | 341.14 ± 50.18 | 281.75 ± 45.34 | 277.63 ± 54.84 |
| Turn duration (s) | 1.55 ± 0.36 | 1.40 ± 0.20 | 1.39 ± 0.27 | 1.47 ± 0.23 | 1.65 ± 0.27 | 1.67 ± 0.25 |
| Number steps to turn | 3.07 ± 0.98 | 3.11 ± 0.60 | 3.03 ± 0.84 | 3.16 ± 0.60 | 3.40 ± 0.74 | 3.41 ± 0.64 |
| Foot position | ||||||
| FSA (degrees) | ---- | 36.78 ± 19.94 | 28.91 ± 4.35 | 29.22 ± 2.98 | 28.89 ± 4.85 | 28.74 ± 3.83 |
| TOA (degrees) | ---- | 35.28 ± 15.18 | 40.67 ± 3.17 | 40.57 ± 1.85 | 41.71 ± 2.90 | 41.17 ± 3.94 |
| Toe out angle (degrees) | ---- | 6.21 ± 13.12 | 4.18 ± 6.73 | 4.22 ± 4.84 | 5.39 ± 6.66 | 4.49 ± 4.92 |
Data presented as female and male only when statistically significant differences were observed between the sexes. Key: FSA, Foot strike angle; TOA, Toe off angle
p<0.05
p<0.001
p<0.0001
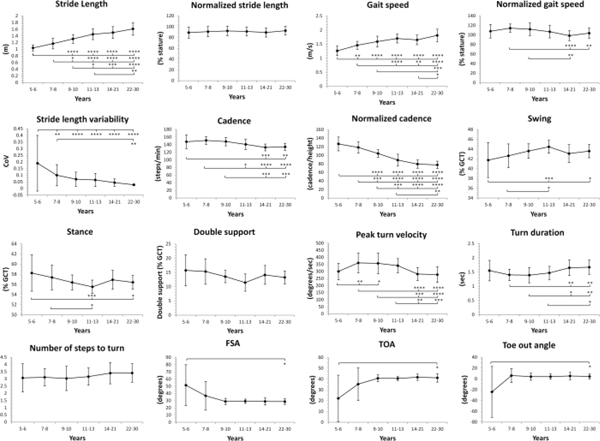
Age-related differences in the FAP condition are shown in Figure 2 with p-values presented in Supplemental Table 1. Absolute stride length progressively increased with age, and was longer in adults compared to children ages 5–13. After normalization, no differences in stride length were observed. Absolute gait speed increased with age, with children ages 5–10 and adolescents 14–21 walking slower than young adults. Normalized gait speed was faster in children ages 7–8 compared to 14–21 year olds. Stride length variability was greatest in children ages 5–6 compared to all older age groups and in children 7–8 compared to adults. Absolute cadence was greatest in children ages 5–10, reaching adult-like patterns by ages 11–13, whereas normalized cadence did not reach adult-like patterns until 14–21 years. Children ages 5–6 spent more time in stance phase and less time in swing phase than adults and children 11–13 years.
Figure 2 Legend.
Spatiotemporal gait and turn metrics in children and young adults (ages 5 to 30) in the fast as possible pace condition. All data reported as Mean ± SD. Key: GCT, gait cycle time; CoV, Coefficient of variation = SD/mean; FSA, foot strike angle; TOA, toe off angle. Significant differences between age groups are noted as *p<0.05, **p<0.01, ***p<0.001 ****p<0.0001
Similar to the SS condition, toe-out angle was aberrant in children ages 5–6; therefore, this data was also excluded from the reference table. There were no other differences in foot position. Peak turn velocity and turn duration in children ages 7–13 were both faster than adults. There were no differences in number of steps to turn.
4. Discussion
This is the first study to present normative spatiotemporal gait and turn parameters derived from the commercially available APDM Mobility Lab ® Opal inertial sensor system in typically developing children and young adults ages 5–30. As a system that is increasingly used in clinical practice and research [1, 5, 7, 10, 12, 14–16, 30], clinicians and investigators can now use this reference data to compare to pediatric populations with gait impairments.
Our findings of increasing absolute stride length and gait speed and decreasing cadence with advancing developmental age in both speed conditions supports previous findings [19–23, 28, 29] and is likely to be related to increasing stature. This was supported by our finding that stride length normalized to height did not vary between age groups in either condition, also similar to prior findings in which normalization was applied [19, 21, 28, 29]. In contrast to prior studies in which normalized gait speed and cadence remained stable in children ages 6–10 [19] and between children 5–13 and young adults 18–27 years [28] we found that normalized gait speed and cadence decreased with age, reaching adult-like patterns by ages 14–21 in the SS condition. Discrepancies between our findings and those of prior studies may be due to the exact normalization method. Some used leg length [19, 28, 29] and others used height [21–23], which may underlie the differences obtained depending upon the developing anthropometrics of the entire body versus the lower extremity alone.
Our values for all gait cycle parameters in the SS condition were similar to prior findings [21, 23] with no differences between age groups. These results are somewhat in contrast to another report where young adults demonstrated increased stance and double support time compared to children [28]; however their values in young adults for double support time (18.6%) were more similar to our values across all age groups (~16–17%) than those they obtained in children ages 5–13 (13.5%). Walking speed influenced gait cycle parameters, such that children ages 5–6 spent more time in stance phase and conversely less in swing time than adults and children 11–13 in the FAP condition. Younger children may still be developing gait stability requiring more time in stance to enhance stability at faster walking speeds.
Previous normative studies have been limited by the inability to capture trunk motion and ankle position utilizing GAITRite® [20–23, 28, 29], or by the area available for motion capture [19]; therefore this is the first study to present normative data on turns, FSA and TOA in children. Children ages 5–13 turned faster in both speed conditions while number of steps to turn remained constant. Higher FSA in children ages 7–8 suggests that ankle stability may still be developing in young children. However, it is worth mentioning that all measures of foot position in children ages 7–8 demonstrated high standard deviations, possibly reflecting the maturation of the neural control mechanisms for gait stability. Our adult values for FSA were very similar to those of a previous normative study using the APDM system in adults [1], however, this was not the case for TOA, for which our values were much lower. Additionally, our average TOA is higher than the motion capture literature (~20 degrees plantarflexion) [19, 31], likely due to differences in how Mobility Lab 2® defines these angles relative to the horizontal surface versus the actual ankle angle. We posit that the aberrant values obtained for toe angles in children ages 5–6 were abnormal because the algorithms used in the Mobility Lab 2® system were derived based on an adult model reference which would likely create errors when calculating these metrics in shorter children.
Similar to prior findings [19–21, 23, 28], there was no significant interaction of age and sex for all parameters in the SS condition, except for increased stride length variability in females 5–6 years old. Stride length variability was highest in this age group and achieved adultlike levels by age 9 in both conditions. Increased variability in younger children could be related to either the maturation of the neural control mechanisms for gait stability or increased distractibility in younger children, a hypothesis posited by Dusing & Thorpe [21]. Sex differences became apparent with fast walking in ages 14–30, where males demonstrated longer absolute stride length, likely due to increased height in adolescence; this finding supports prior hypotheses that sex differences may not become apparent until adolescence [23].
Sutherland et al. [32] suggested that most gait patterns achieve adult-like levels by age 4. Our results are in contrast to this hypothesis with the majority of our gait parameters in the SS condition achieving adult-like patterns by ages 9–10 (absolute gait speed), 11–13 (absolute cadence) or ages 14–21 (absolute stride length, normalized gait speed and cadence). In fact, greater stride length variability in children ages 5–6 as well as large standard deviations for all measures of foot position in children 7–8 suggest that these components of gait may still be maturing in younger children. These results align with prior hypotheses that gait may not be mature until age 12 [20] or 13 [28].
One limitation of this study is that our values of FSA and TOA were not consistent with prior studies [1, 19, 31]. Thus, further validation of these measures in both children and adults is recommended. Another limitation is that data collection occurred in large gymnasiums or hallways. All attempts were made to avoid distractions from activities in the surrounding building, however some children may have become distracted during testing. In addition, some of the younger children appeared to want to walk fast in the SS condition while others did not. When this occurred we would restart the trial and repeat the directions to walk at a normal self-selected pace. We believe that our results are valid and that there are indeed inherent natural differences in self-selected and fast walking speeds in both children and adults without gait abnormalities that would also be present in a clinical setting. However, the gait and turn metrics obtained in the present study would be most applicable to a very similar walking track and duration versus aggregation of shorter discrete trials. Prior studies have shown that walking speed and gait variability measures are higher during discrete trials, compared to continuous trials, perhaps due to disruptions in gait rhythm with greater number of turns [33, 34]. Finally, test retest reliability studies using the APDM® inertial sensor system are lacking in children and are recommended in future work.
5. Conclusion
This study provides meaningful and clinically relevant normative spatiotemporal gait and turning values using the commercially available APDM® inertial sensor system in children and young adults ages 5–30. As this system is increasingly utilized for gait analysis by clinicians and researchers, other groups can now compare their results in children with atypical gait and/or children in research studies investigating response to interventions to a relatively large reference of typically developing children and young adults. This work also contributes significantly to the body of literature regarding the maturation of gait.
Supplementary Material
Highlights.
Normative gait metrics using the APDM® system in 5–30 year olds are provided.
Database allows comparisons to children and young adults with abnormal gait.
This work contributes to the literature regarding gait maturation.
Acknowledgements
The authors would like to thank the volunteers who participated in this study, as well as Dr. Anne Hoffman, Nicollette Purcell, Caitlin Bailey, Chris D’Arco, Colleen Huml, Nicole Gamboa, Keith Kravis and Lauren Baker for data collection and study recruitment support.
Funding
This work was supported by: NIH (K01 HD088762) (JAO) The Hope for Haley and Samantha’s Search for the Cure foundations
Elizabeth Berry-Kravis (EBK) has received funding from Seaside Therapeutics, Novartis, Roche, Alcobra, Neuren, Cydan, Fulcrum, Neurotrope, BioMarin, GW, Marinus, Zynerba, Ovid, Yamo, AMO, Acadia, Ionis, GeneTx, Vtesse/Sucampo/Mallinckrodt, Neurogene, and Orphazyme Pharmaceuticals to consult on trial design or development strategies and/or conduct clinical trials in genetic neurodevelopmental or neurodegenerative diseases, and from Asuragen Inc to develop testing standards and resources for FMR1 testing, and has research support from NICHD, NINDS, NIMH, CDC, NCATS, FRAXA Research Foundation and the John Merck Fund. She reports no conflicts of interests related to this manuscript.
Joan A. O’Keefe receives research support from the NIH (K01 HD088762) and has received consulting fees from Avexis, Inc. She reports no conflicts of interests related to this manuscript.
Footnotes
Conflict of interest statement
None of the authors has or has had any financial connection to the manufacturers of the APDM Mobility Lab® Opal inertial sensor system (APDM, Inc., Oregon) and have not received any form of compensation for the conduct of the present study. None of the authors have conflicts of interests related to this manuscript.
Stephanie Voss reports no disclosures or conflicts of interests related to this manuscript.
Jessica Joyce reports no disclosures or conflicts of interests related to this manuscript.
Alexandras Biskis reports no disclosures or conflicts of interests related to this manuscript.
Medha Parulekar reports no disclosures or conflicts of interests related to this manuscript.
Nicholas Armijo reports no disclosures or conflicts of interests related to this manuscript.
Cris Zampieri reports no disclosures or conflicts of interests related to this manuscript.
Rachel Tracy reports no disclosures or conflicts of interests related to this manuscript.
Sasha Palmer reports no disclosures or conflicts of interests related to this manuscript.
Marie Fefferman reports no disclosures or conflicts of interests related to this manuscript.
Bichun Ouyang no disclosures or conflicts of interests related to this manuscript.
Yuanqing Liu reports no disclosures or conflicts of interests related to this manuscript
Declaration of Competing Interest
None.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fang X, Liu C, Jiang Z, Reference values of gait using APDM movement monitoring inertial sensor system, R Soc Open Sci 5(1) (2018) 170818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kraan CM, Tan AHJ, Cornish KM, The developmental dynamics of gait maturation with a focus on spatiotemporal measures, Gait Posture 51 (2017) 208–217. [DOI] [PubMed] [Google Scholar]
- [3].Muro-de-la-Herran A, Garcia-Zapirain B, Mendez-Zorrilla A, Gait analysis methods: an overview of wearable and non-wearable systems, highlighting clinical applications, Sensors (Basel) 14(2) (2014) 3362–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C, Validity and repeatability of inertial measurement units for measuring gait parameters, Gait Posture 55 (2017) 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O’Keefe JA, Robertson-Dick EE, Hall DA, Berry-Kravis E, Gait and Functional Mobility Deficits in Fragile X-Associated Tremor/Ataxia Syndrome, Cerebellum 15(4) (2016) 475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lanovaz JL, Oates AR, Treen TT, Unger J, Musselman KE, Validation of a commercial inertial sensor system for spatiotemporal gait measurements in children, Gait Posture 51 (2017) 14–19. [DOI] [PubMed] [Google Scholar]
- [7].Dewey DC, Miocinovic S, Bernstein I, Khemani P, Dewey RB 3rd, Querry R, et al. , Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease, J Neurol Sci 345(1–2) (2014) 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Horak FB, Mancini M, Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors, Mov Disord 28(11) (2013) 1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stack E, Agarwal V, King R, Burnett M, Tahavori F, Janko B, et al. , Identifying balance impairments in people with Parkinson’s disease using video and wearable sensors, Gait Posture 62 (2018) 321–326. [DOI] [PubMed] [Google Scholar]
- [10].Schmitz-Hubsch T, Brandt AU, Pfueller C, Zange L, Seidel A, Kuhn AA, et al. , Accuracy and repeatability of two methods of gait analysis - GaitRite und Mobility Lab - in subjects with cerebellar ataxia, Gait Posture 48 (2016) 194–201. [DOI] [PubMed] [Google Scholar]
- [11].Pau M, Caggiari S, Mura A, Corona F, Leban B, Coghe G, et al. , Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure, Mult Scler Relat Disord 10 (2016) 187–191. [DOI] [PubMed] [Google Scholar]
- [12].Storm FA, Nair KPS, Clarke AJ, Van der Meulen JM, Mazza C, Free-living and laboratory gait characteristics in patients with multiple sclerosis, PLoS One 13(5) (2018) e0196463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Witchel HJ, Oberndorfer C, Needham R, Healy A, Westling CEI, Guppy JH, et al. , Thigh-Derived Inertial Sensor Metrics to Assess the Sit-to-Stand and Stand-to-Sit Transitions in the Timed Up and Go (TUG) Task for Quantifying Mobility Impairment in Multiple Sclerosis, Front Neurol 9 (2018) 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fino PC, Parrington L, Walls M, Sippel E, Hullar TE, Chesnutt JC, et al. , Abnormal Turning and Its Association with Self-Reported Symptoms in Chronic Mild Traumatic Brain Injury, J Neurotrauma 35(10) (2018) 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fino PC, Wilhelm J, Parrington L, Stuart S, Chesnutt JC, King LA, Inertial Sensors Reveal Subtle Motor Deficits When Walking With Horizontal Head Turns After Concussion, J Head Trauma Rehabil 34(2) (2019) E74–E81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Newman MA, Hirsch MA, Peindl RD, Habet NA, Tsai TJ, Runyon MS, et al. , Reliability of the sub-components of the instrumented timed up and go test in ambulatory children with traumatic brain injury and typically developed controls, Gait Posture 63 (2018) 248–253. [DOI] [PubMed] [Google Scholar]
- [17].Podsiadlo D, Richardson S, The timed “Up & Go”: a test of basic functional mobility for frail elderly persons, J Am Geriatr Soc 39(2) (1991) 142–8. [DOI] [PubMed] [Google Scholar]
- [18].Bohannon RW, Wang YC, Bubela D, Gershon RC, Normative Two-Minute Walk Test Distances for Boys and Girls 3 to 17 Years of Age, Phys Occup Ther Pediatr 38(1) (2018) 39–45. [DOI] [PubMed] [Google Scholar]
- [19].Smith Y, Louw Q, Brink Y, The three-dimensional kinematics and spatiotemporal parameters of gait in 6–10 year old typically developed children in the Cape Metropole of South Africa - a pilot study, BMC Pediatr 16(1) (2016) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thevenon A, Gabrielli F, Lepvrier J, Faupin A, Allart E, Tiffreau V, et al. , Collection of normative data for spatial and temporal gait parameters in a sample of French children aged between 6 and 12, Ann Phys Rehabil Med 58(3) (2015) 139–44. [DOI] [PubMed] [Google Scholar]
- [21].Dusing SC, Thorpe DE, A normative sample of temporal and spatial gait parameters in children using the GAITRite electronic walkway, Gait Posture 25(1) (2007) 135–9. [DOI] [PubMed] [Google Scholar]
- [22].Holm I, Tveter AT, Fredriksen PM, Vollestad N, A normative sample of gait and hopping on one leg parameters in children 7–12 years of age, Gait Posture 29(2) (2009) 317–21. [DOI] [PubMed] [Google Scholar]
- [23].Moreno-Hernandez A, Rodriguez-Reyes G, Quinones-Uriostegui I, Nunez-Carrera L, Perez-Sanpablo AI, Temporal and spatial gait parameters analysis in non-pathological Mexican children, Gait Posture 32(1) (2010) 78–81. [DOI] [PubMed] [Google Scholar]
- [24].APDM, Inc. https://www.apdm.com/about-apdm/. (accessed Sept. 17 2019).
- [25].Martakis K, Stark C, Alberg E, Bossier C, Semler O, Schonau E, et al. , Motor Function Improvement in Children with Ataxia Receiving Interval Rehabilitation, Including Vibration-Assisted Hometraining: A Retrospective Study, Klin Padiatr 231(6) (2019) 304–312. [DOI] [PubMed] [Google Scholar]
- [26].Flachenecker F, Gassner H, Hannik J, Lee DH, Flachenecker P, Winkler J, et al. , Objective sensor-based gait measures reflect motor impairment in multiple sclerosis patients: Reliability and clinical validation of a wearable sensor device, Mult Scler Relat Disord 39 (2019) 101903. [DOI] [PubMed] [Google Scholar]
- [27].Carvalho C, Sunnerhagen KS, Willen C, Walking speed and distance in different environments of subjects in the later stage post-stroke, Physiother Theory Pract 26(8) (2010) 519–27. [DOI] [PubMed] [Google Scholar]
- [28].Lythgo N, Wilson C, Galea M, Basic gait and symmetry measures for primary school-aged children and young adults whilst walking barefoot and with shoes, Gait Posture 30(4) (2009) 502–6. [DOI] [PubMed] [Google Scholar]
- [29].Lythgo N, Wilson C, Galea M, Basic gait and symmetry measures for primary school-aged children and young adults. II: walking at slow, free and fast speed, Gait Posture 33(1) (2011) 29–35. [DOI] [PubMed] [Google Scholar]
- [30].Berry-Kravis E, Chin J, Hoffmann A, Winston A, Stoner R, LaGorio L, et al. , Long-Term Treatment of Niemann-Pick Type C1 Disease With Intrathecal 2-Hydroxypropyl-beta-Cyclodextrin, Pediatr Neurol 80 (2018) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Loudon JK, Swift M, Bell SL, The clinical orthopedic assessment guide, Human Kinetics, Kansas, 2008, pp. 395–408. [Google Scholar]
- [32].Sutherland DH, Olshen R, Cooper L, Woo SL, The development of mature gait, J Bone Joint Surg Am 62(3) (1980) 336–53. [PubMed] [Google Scholar]
- [33].Brown MJ, Hutchinson LA, Rainbow MJ, Deluzio KJ, De Asha AR, A Comparison of Self-Selected Walking Speeds and Walking Speed Variability When Data Are Collected During Repeated Discrete Trials and During Continuous Walking, J Appl Biomech 33(5) (2017) 384–387. [DOI] [PubMed] [Google Scholar]
- [34].Paterson KL, Lythgo ND, Hill KD, Gait variability in younger and older adult women is altered by overground walking protocol, Age Ageing 38(6) (2009) 745–8. https://www.ncbi.nlm.nih.gov/pubmed/19726433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.