Abstract
Background
With increasing survival estimates for individuals with cystic fibrosis, long‐term management has become an important focus. Psychological interventions are largely concerned with adherence to treatment, emotional and social adaptation and health‐related quality of life. We are unaware of any relevant systematic reviews.
Objectives
To determine whether psychological interventions for people with cystic fibrosis provide significant psychosocial and physical benefits in addition to standard medical care.
Search methods
Studies were identified from two Cochrane trials registers (Cystic Fibrosis and Genetic Disorders Group; Depression, Anxiety and Neurosis Group), Ovid MEDLINE and PsychINFO; unpublished trials were located through professional networks and Listserves. Most recent search of the Cystic Fibrosis and Genetic Disorders Group's register: 19 December 2013.
Most recent search of the Depression, Anxiety and Neurosis Group's register: 12 November 2013.
Selection criteria
Randomised controlled studies of a broad range of psychological interventions evaluating subjective and objective health outcomes, such as quality of life or pulmonary function, in individuals of all ages with cystic fibrosis and their immediate family. We were interested in psychological interventions, including psychological methods within the scope of psychotherapeutic or psychosomatic mechanism of action (e.g. cognitive behavioural, cognitive, family systems or systemic, psycho‐dynamic, or other, e.g. supportive, relaxation, or biofeedback), which were aimed at improving psychological and psychosocial outcomes (e.g. quality of life, levels of stress or distress, psychopathology, etc.), adaptation to disease management and physiological outcomes.
Data collection and analysis
Three authors were involved in selecting the eligible studies and two of these authors assessed their risk of bias.
Main results
The review includes 16 studies (eight new studies included in this update) representing data from 556 participants. Studies are diverse in their design and their methods. They cover interventions with generic approaches, as well as interventions developed specifically to target disease‐specific symptoms and problems in people with cystic fibrosis. These include cognitive behavioural interventions to improve adherence to nutrition or psychosocial adjustment, cognitive interventions to improve adherence or those associated with decision making in lung transplantation, a community‐based support intervention and other interventions, such as self‐hypnosis, respiratory muscle biofeedback, music therapy, dance and movement therapy, and a tele‐medicine intervention to support patients awaiting transplantation.
A substantial proportion of outcomes relate to adherence, changes in physical status or other specific treatment concerns during the chronic phase of the disease.
There is some evidence that behavioural interventions targeting nutrition and growth in children (4 to 12 years) with cystic fibrosis are effective in the short term. Evidence was found that providing a structured decision‐making tool for patients considering lung transplantation improves patients' knowledge of and expectations about the transplant, and reduces decisional conflict in the short term. One study about training in biofeedback‐assisted breathing demonstrated some evidence that it improved some lung function measurements. Currently there is insufficient evidence for interventions aimed at other aspects of the disease process.
Authors' conclusions
Currently, insufficient evidence exists on psychological interventions or approaches to support people with cystic fibrosis and their caregivers, although some of the studies were promising. Due to the heterogeneity between studies, more of each type of intervention are needed to support preliminary evidence. Multicentre studies, with consequent funding implications, are needed to increase the sample size of these studies and enhance the statistical power and precision to detect important findings. In addition, multicentre studies could improve the generalisation of results by minimizing centre or therapist effects. Psychological interventions should be targeted to illness‐specific symptoms or behaviours to demonstrate efficacy.
Plain language summary
Psychological treatments to help individuals with cystic fibrosis and their caregivers manage the disease
Cystic fibrosis is a genetic disorder that damages many of the body's organs and can shorten a person's life span. The disease is progressive, stressful to manage, and needs complex and time‐consuming treatments, leaving patients and caregivers stressed due to the challenges of the treatment. Thus, individuals with cystic fibrosis and their family members often need help to cope better and to deal with their thoughts and feelings. They also need assistance in managing the demands of the prescribed treatment schedules. In addition, infection control guidelines recommend the isolation of people with cystic fibrosis from others with the same disease, leading to a lack of peer support and potential social isolation.
We looked for studies of psychological treatments in individuals of all ages with cystic fibrosis and their families which aimed to reduce anxiety and depression, to improve adjustment, quality of life, and even medical outcomes, as well as knowledge, skills, and decisions regarding care. The review includes 16 studies with a total of 556 participants. Even though there many different psychological interventions, only a few have been evaluated for individuals with CF and their families. Due to the lack of high quality studies, it is not possible to currently show which psychological treatments are most helpful to those with cystic fibrosis and their caregivers. Five out of the 16 studies we found evaluated behavioural interventions to improve dietary intake. We found that in children aged 4 to 12 years receiving a nutritional intervention plus behavioural management training, consumed about 276 calories per day more than children just receiving the nutritional intervention. We also found that a structured decision‐making tool for adults considering lung transplantation improved their knowledge, assisted in setting realistic expectations, and reduced indecision.
In summary, there is some evidence that behavioural interventions targeting specific illness‐related symptoms and behaviours can work. More studies on psychological interventions with more people are urgently needed. There are several ongoing randomised controlled studies aimed at improving adherence to prescribed treatments, but final results are not yet available. We recommend multicentre studies to provide evidence for which interventions are most effective for the key issues faced by people with cystic fibrosis and their caregivers.
Summary of findings
Summary of findings 1. Cognitive behavioral interventions to improve adherence.
| Nutrional intervention plus behavioural management training (BEH) compared with Nutritional intervention alone (NUT)for children with CF (4‐12 years) | ||||
|
Patient or population: Children from 4‐12 years with a diagnosis of CF by sweat test, pancreatic insufficiency; and weight for age and height < 40th percentile Settings: group setting Intervention: Nutrional intervention plus behavioural management training Comparison: Nutritional intervention alone | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Comments | |
| Assumed risk | Corresponding risk | |||
| Nutritional intervention alone | Nutrional intervention plus behavioural management training | |||
|
Change in weight pre‐ and post‐treatment in (kg) |
The mean change in weight ranged across control groups from 0.92 to 1.75kg | The mean change in weight in the intervention groups was
0.11kghigher (0.84 lower to 1.07 higher) |
75 (2) | Primary outcome |
|
BMIz change range: ‐1.00 to 1.00 2‐year follow up |
The mean BMIz change in the control group was ‐0.22 | The mean BMIz change in the intervention group was
0.35 higher (0 to 0.7 higher) |
67 (1) | Primary outcome |
|
Total calories per day post‐intervention |
The mean total calories per day ranged across control groups from 1316 to 2315 calories | The mean total calories per day in the intervention groups was 275.8 calories higher (66.65 to 485.05 higher) | 83 (3) | Secondary outcome |
|
Change in calorie intake pre‐ and post‐treatment |
The mean change in calorie intake ranged across control groups from 303.9 to 489 calories | The mean change in calorie intake in the intervention groups was 364.06 calories higher (191.99 to 536.13 higher) | 82 (3) | Secondary outcome |
|
Estimated energy requirements (%EER) post‐intervention |
The mean %EER in the control group was 127% | The mean %EER in the intervention group was 21% higher (7.76 to 34.24 higher) | 67 (1) | Secondary outcome |
|
Change in estimated energy requirements (%EER) pre‐ and post‐treatment |
The mean of change in %EER in the control group was 27% | The mean of change in %EER in the intervention groups was
21% higher (9.22 to 32.78 higher) |
67 (1) | Secondary outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||
Summary of findings 2. Cognitive interventions associated with decision making in lung transplantation.
| Decision aid for patients considering lung transplantation compared with usual carefor patients with advanced CF considering referral for lung transplantation | ||||
|
Patient or population: patients with advanced CF considering referral for lung transplantation Settings: individual Intervention: decision aid for patients considering lung transplantation Comparison: usual care | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Comments | |
| Assumed risk | Corresponding risk | |||
| Usual care | Decision aid for patients considering lung transplantation | |||
|
Participants' knowledge 4‐item knowledge questionnaire (range 0 to 4) 3 week follow‐up |
The mean score of participants' knowledge in the control group was 1.974 | The mean score of participants' knowledge in the intervention group was 0.98 higher (0.66 to 1.31 higher) | 149 (1) | Secondary outcome |
|
Participants' knowledge ‐ change in knowledge 4‐item knowledge questionnaire (range 0 to 4) week 3 ‐ baseline |
The mean of change in participants' knowledge in the control group was 0.3 |
The mean of change in participants' knowledge in the intervention group was 0.94 higher (0.53 to 1.35 higher) | 149 (1) | Secondary outcome |
|
Patient expectations 2‐item questionnaire (range 0 to 2) 3 week follow‐up |
The mean of the expectation score in the control group was 0.58 | The mean of the expectation score in the intervention group was 0.73 higher (0.51 to 0.95 higher) | 149 (1) | Secondary outcome |
|
Patient expectations ‐ change in expectation score 2‐item questionnaire (range 0 to 2) week 3 ‐ baseline |
The mean change in the expectation score in the control group was 0.05 | The mean change in the expectation score in the intervention group was 0.66 higher (0.37 to 0.95 higher) | 149 (1) | Secondary outcome |
|
Decisional conflict ‐ Total score range 0 to 100 (low decisional conflict to high decisional conflict) 3 week follow‐up |
The mean of the total score in the control group was 20.4 |
The mean of the total score in the intervention group was 8.8 lower (13.7 to 3.9 lower) | 149 (1) | Secondary outcome |
|
Decisional conflict ‐ Change in total score range 0 to 100 (low decisional conflict to high decisional conflict) week 3 ‐ baseline |
The mean of changes in the total score of the decisional conflict questionnaire in the control group was 13.1 |
The mean of changes in the total score of the decisional conflict questionnaire in the intervention group was 3.3 higher (2.28 lower to 8.88 higher) |
149 (1) | Secondary outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||
Summary of findings 3. Other interventions ‐ Biofeedback.
| Biofeedback assisted breathing re‐training (BRT) compared with biofeedback assisted relaxation training (RLXT)for individuals with CF (10 ‐ 41 years) | ||||
|
Patient or population: individuals with CF (10 ‐ 41 years) Settings: individual Intervention: biofeedback assisted breathing re‐training (BRT) Comparison: biofeedback assisted relaxation training (RLXT) | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Comments | |
| Assumed risk | Corresponding risk | |||
| Biofeedback assisted relaxation training (RLXT) | Biofeedback assisted breathing re‐training (BRT) | |||
| Pulmonary function ‐ Forced expiratory volume (FEV1) in litres per second | The mean FEV1 in the control group was 0.78 | The mean FEV1 in the intervention group was 0.54 higher (0.15 to 0.93 higher) | 24 (1) | Primary outcome |
| Pulmonary function ‐ Forced vital capacity (FVC) in litres | The mean FVC in the control group was 2.31 | The mean FVC in the intervention group was
0.87 higher (0.09 lower to 1.83 higher) |
26 (1) | Primary outcome |
| Pulmonary function ‐ FEF25-75% in litres per second | The mean FEF25-75% in the control group was 1.39 | The mean FEF25-75% in the intervention group was
0.67 higher (0.1 to 1.24 higher) |
26 (1) | Primary outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||
Summary of findings 4. Other interventions ‐ Massage therapy.
| Massage therapycompared with bedtime reading controlfor children and adolescents with CF (5 ‐ 18 years) | ||||
|
Patient or population: children and adolescents with CF (5 ‐ 18 years) Settings: individual Intervention: massage therapy Comparison: bedtime reading control | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Comments | |
| Assumed risk | Corresponding risk | |||
| Bedtime reading control | Massage therapy | |||
|
Pulmonary function ‐ Peak Air Flow (PEFR) Follow‐up day 30 |
The mean Peak Air Flow in the control group was 244 | The mean Peak Air Flow in the intervention group was 53.9 higher (43.27 lower to 151.07 higher) | 20 (1) | Primary outcome |
|
Parent Anxiety State Trait Anxiety Inventory (STAI; range from 0 to 80 with a higher score reflecting more anxiety) |
The mean parent anxiety score in the control group was 40 | The mean parent anxiety score in the intervention group was 9.1 lower (17.84 to 0.36 lower) | 20 (1) | Primary outcome |
|
Child Anxiety ‐ State Trait Anxiety Inventory for Children (STAIC; range from 0 to 80 with a higher score reflecting more anxiety) |
The mean child anxiety score in the control group was 32.9 | The mean child anxiety score in the intervention group was 8.2 lower (12.36 to 4.04 lower) | 20 (1) | Primary outcome |
|
Child Mood Profile of Mood States (POMS)‐ depression subscale modified (19 items 5‐point‐scale 0 to 4, score range 0 to 76) |
The mean score of the depression subscale in the control group was 7.2 | The mean score of the depression subscale in the intervention group was 5.5 lower (8.81 to 2.19 lower) | 20 (1) | Primary outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||
Background
Cystic fibrosis (CF) is the most common life‐limiting, hereditary disease among those of European descent; between four and five per cent of these people carry the recessive gene and 1 per 2500 live births are affected worldwide (Bobadilla 2002). The incidence of CF is rare in native African, Asian and Oriental people (Tsui 1990). The disease is manifested by generalised dysfunction of the exocrine glands, which produce excessively viscous mucus secretions. The pancreas and lungs are the main organs affected and prognosis is largely determined by the extent of the pulmonary damage. The treatment regimen for CF is complex and time‐consuming, and is primarily aimed at slowing or preventing the secondary effects of the disease. Primary components of the current daily regimen include the following.
Airway clearance or chest physiotherapy and postural drainage using a variety of devices (acapella, flutter, chest wall oscillation)
Nebulised medications ‐ bronchodilators, antibiotics, mucous thinning agents
Antibiotics – oral, nebulised, or intravenous
Replacement of pancreatic enzymes
Increased calorie intake through foods or nutritional supplementation ‐ orally, nasogastrically or through a gastrostomy tube
Gene correctors for specific mutations of the CF gene
Significant increases in life span have been achieved due mainly to the availability of specialist multidisciplinary CF centres, early diagnosis via newborn screening, and treatment breakthroughs. In the USA in 1990, the mean and median ages at diagnosis were 2.9 years and 7 months, respectively (FitzSimmons 1993). The median survival age in the USA doubled between 1969 and 1990, from 14 to 28 years (FitzSimmons 1993) and those children born in the UK in 2000 have a predicted median age of survival of greater than 50 years (Dodge 2007). Data from the CF Foundation Patient Registry show that in 2010, more than 47.5 per cent of all people with CF in the USA are 18 years or older. The median predicted age of survival in 2010 was 38.3 years, and the median age at diagnosis was five months (US Patient Registry Annual Data Report 2010).
Thus, CF is no longer solely a childhood disease, and as a consequence, long‐term management issues have become more relevant, with increasing emphasis placed upon adherence to the medical regimen and the transition to adulthood while living with a chronic, life‐threatening condition. In addition to the profound, emotional impact of a disease that is life‐shortening, a major challenge for families is to balance the demands of daily CF care with the developmental needs of the affected individual and other family members (Quittner 1992a; Quittner 1992b; Quittner 1998). The ultimate goal is to attend to the health needs of the individual without sacrificing overall quality of life of that person or their family. Thus, the major areas for concern from a psychosocial perspective include emotional and social adjustment, adherence to treatment, and health‐related quality of life. These challenges must be placed within the context of the individual's age, from infancy through adolescence to adulthood, and within the context of the disease process, from initial diagnosis to death.
Although there have been descriptive overviews of psychosocial research in the area (Stark 1995; Miller 1999; Bluebond‐Langner 2001) there have been no systematic reviews of intervention studies for those affected by CF until the first version of the current review was published in 2003 (Glasscoe 2003). A meta‐analysis of behavioural and emotional problems and self‐concept in children aged 3 to 19 years with a variety of chronic medical conditions showed that these children had an increased risk of overall adjustment problems (Lavigne 1992). Those with CF fell into a group of moderate impact, with a pooled effect size of ∂ = 0.5 in studies comparing those with CF to normative data (n = 9) and ∂ = 0.44 in studies making comparisons to controls (n = 7) (Cohen 1992). The most prevalent psychiatric diagnoses reported by Thompson in a 7 to 14 year age group with CF were anxiety (37%) and oppositional disorder (23%) (Thompson 1990), while Pearson reported anxiety (22%) and depression (42%) as most prevalent in a 16 to 40 year age group with CF (Pearson 1991). Targeted interventions aimed at addressing these symptoms of distress are a major focus for psychological interventions, utilizing a diverse range of theoretical perspectives for example with the paediatric population (Beale 2006). Although these older, cross‐sectional studies do not document the development of psychological dysfunction over time, they generally demonstrate that a majority of those affected with CF adapt well, which is partially in line with more current data. Depression is one of the major mental health problems worldwide with prevalence rates estimated between 6.9% in Europe (Wittchen 2011) and 8.6% in the USA (Kessler 2012). In the context of chronic conditions depression is of high relevance, because it is known as a risk factor for poor adherence to treatment (DiMatteo 2000). Quittner reviewed studies examining rates of depression in patients with CF and reported higher rates of depression compared to healthy population (Quittner 2008). Within a large epidemiological study (TIDES study) data from a representative sample of German patients with CF (N = 670, age range 12 to 64 years) showed that about one in 10 patients reported high levels of depressive symptoms, but elevated depression was not more common than in community samples, except for those with severe disease (Goldbeck 2011b). In summary, epidemiological studies examining symptoms of depression in CF were not conclusive in the question whether they occur more frequently in individuals with CF compared to the general population. Nevertheless, the Goldbeck study revealed that elevated anxiety scores were documented in about one in five patients and that they were more prevalent in adult German patients with CF compared to a community sample (Goldbeck 2011b).
Regarding parent caregivers, a multicentre study within the TIDES study showed that anxious and depressive symptoms are more common in German parents of minors with CF (N = 650) than in a community sample of adults matched by age and gender (Besier 2011a). More than one‐third (37.2%) of parents showed elevated levels of anxious symptoms, compared to 18.9% of a population sample and significantly more parents reported elevated levels of depressive symptoms (28% versus 21%). Only a minority of affected caregivers (13.6%) received any form of specialist treatment for their mental health problems (Besier 2011b). A study conducted in the USA also found elevated levels of symptoms of depression in children (29%), in mothers (35%), and in fathers (23%), whereupon adherence to airway clearance was lowest for children with symptoms of depression and a less secure child‐parent relationship (Smith 2010).
Adherence to the prescribed treatment regimen is one of the most challenging aspects of managing CF and parents often feel critically responsible for ensuring that these treatments are performed on a daily basis. Studies identifying rates of non‐adherence in chronic paediatric conditions report that it affects about 50% to 75% of the children and adolescents (Rapoff 2010) and about 50% of adult patients (WHO 2003). For CF, reported rates vary widely, depending on different types of treatment and attempts to measure adherence. For example rates of approximately 45% (Quittner 2000) and 64% to 74% (Modi 2006) for adherence to airway clearance in children with CF were reported. Moreover, rates for enzyme medication were reported as ranging from 27% to 46% (Modi 2006). Abbott described amounts of adherence reported in the CF literature and pointed out, that it is higher for simple treatment‐related behaviours than for behaviours of more complexity (Abbott 2009). Adherence to the major pulmonary medications, including inhaled mucolytics, inhaled antibiotics, and azithromycin, is for instance generally 50% or less (Modi 2010; Eakin 2011). Dietary management is also necessary to prevent pulmonary decline. Although nutritional status improves, for example data from the 2011 German CF Registry showed that about 26% the of children and adolescents still have a BMI scoring below the 15th percentile and approximately 23.5% of adults have a BMI lower than 19 kg/m2 (Stern 2012). Hence, a substantial proportion of patients need to improve their nutritional status. Overall, there is evidence that poor adherence reduces the effectiveness of treatment, might be responsible for disease progression and ultimately might reduce survival in chronic conditions in general (DiMatteo 2002).
As psychological interventions have to be adapted to the developmental level of the patients, the broad age range of individuals with CF (from infancy to later adulthood) and the corresponding developmental tasks, a variety of intervention methods and aims are required in order to cope with the disease. Furthermore, psychological interventions should be designed according to the stage of the disease, ranging from the normal health status of infants diagnosed by newborn screening to the severe disease of patients awaiting a lung transplantation and those in the terminal stage of CF needing palliative interventions. Interventions aimed at reducing stress and improving coping may improve both psychological outcomes and disease management, including adherence to prescribed treatments and thus hopefully improving medical outcomes (Bartholomew 1991; Smith 2010). Thus, the potential of psychological interventions goes beyond a mere improvement of mental health or psychosocial functioning, once the interactions of health‐related behaviour and medical outcomes are considered. Therefore, a range of psychological interventions may be needed according to the broad scope of CF‐related challenges. The scope of this review is therefore not limited to a certain type of problem, intervention, or category of outcome. Rather, the literature research is oriented towards a broad range of options for psychological interventions, with a focus on sound methodological studies.
Objectives
To describe the extent and quality of existing research on the efficacy of psychological interventions for individuals with CF and their families.
To establish whether the effects of psychological interventions, in addition to standard multidisciplinary care, provide significant benefits for the psychological and physical well‐being of individuals with CF and their families.
To compare the effectiveness of different psychological interventions within major target areas, such as newborn screening, adherence to nutrition, airway clearance, and other medications, decision making in lung transplant, coping and adapting to treatment regimen, transitioning toward independence and adult care, and maintaining or improving health‐related quality of life.
If psychological interventions were shown to be effective in these areas, then we planned to explore whether these effects varied depending on demographic characteristics of the family, socioeconomic status, age of the individual, or stage of the disease.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled studies, published and unpublished.
We also considered quasi‐randomised controlled studies, such as those using alternation and controlled studies, if a reasonable assumption could be made that the baseline was similar in both groups.
Types of participants
Children, adolescents and adults diagnosed with CF ‐ individuals of all ages, from any ethnic group, and during all stages of the disease process will be included.
Parents or caregivers of a child or an adult with CF.
Siblings of a child or an adult with CF.
Nuclear family of a child or an adult with CF in which these individuals were identified collectively as a family unit.
Types of interventions
A precise definition of psychological interventions is often missing in reviews on psychological interventions for patients with chronic illnesses (e.g. cancer (Hodges 2011)). The following definition was used to identify possible interventions for the current review (modelled after the definition used by Pai for paediatric oncology (Pai 2006).
An intervention was defined as a psychological intervention and eligible for the review if it fulfilled the following:
included psychological methods within the scope of psychotherapeutic or psychosomatic mechanism of action (e.g. cognitive behavioural, cognitive, family systems or systemic, psycho‐dynamic, or other like supportive, relaxation, biofeedback) and were provided in structured interactions between a participant and a facilitator;
was facilitated by psychologists, psychotherapists, and therapists in training or other trained professionals supervised by a clinical psychologist or therapist;
targeted areas for treatment set out in the aims and objectives for this review. The main targets for psychological interventions are: genetic screening for CF, adherence to treatments (e.g. nutrition, airway clearance, or other medication), coping or adapting to prescribed treatments, decision making in lung transplantation, and transitions towards independence and adult care;
was aimed at improving psychological or psychosocial outcomes (e.g. quality of life, levels of stress or distress, psychopathology etc.), adaptation to disease management or physiological outcomes (or both);
was compared to either no psychological intervention (apart from standard multidisciplinary care providing psychological support) or to an alternative psychological intervention;
was conducted in an individually‐oriented, family‐oriented or group setting.
The following broad categories were used to classify the different types of interventions.
1. Cognitive behavioral
2. Cognitive
3. Family systems or systemic
4. Psychodynamic
5. Other interventions
Within the five categories, subcategories will be used to classify the included studies. Those subcategories arise from the predetermined target areas for psychological interventions highlighted in the objectives. The main targets with implications for psychological interventions are: genetic screening for CF; adherence to treatment; nutrition; airway clearance; decision making in lung transplantation; and transition toward independence and adult care.
A review on interventions aimed exclusively at supporting adherence to treatment for CF is currently underway. Therefore, non‐psychological interventions to promote adherence are not included in this review. Interventions only containing educational methods or educational approaches aimed at improving self‐management were excluded in the current update because a separate Cochrane Review on ‘Self‐management education for cystic fibrosis’ was published in 2011 (Savage 2011).
Types of outcome measures
The following outcome measures were of interest for the review. Due to the range of interventions and potential outcomes we classified them into broad categories according to their clinical relevance. We accepted any measure with adequate psychometric properties demonstrating reliability and validity in relation to psychological, social outcomes, or quality of life outcomes or a combination of these. All outcome data that were reported at a single time point post‐intervention (or follow up) or were calculated as change scores were included.
Primary Outcomes
-
Well‐being and functioning
Measures assessing health‐related quality of life or psychopathology or distress and focusing on the affected child, adolescent or adult or their family or both.
Assessment of psychopathology or distress (any standardized interview that identifies a psychiatric disorder with 'The Diagnostic and Statistical Manual of Mental Disorders' (DSM) or an 'International Classification of Diseases' (ICD) category in an affected child or adult with CF or any self‐report measure of emotional difficulties, such as anxiety or depression, or behavioural difficulties).
-
Pulmonary function
Objective measures of lung function (in infants younger than six years of age: chest radiograph scores and in older children, adolescents and adults: forced vital capacity (FVC), forced expiratory volume at one second (FEV1), residual volume/total lung capacity (RV/TLC), 25% to 75% of forced expiratory flow (FEF25-75%) (as % predicted)).
Weight and height or body mass index (BMI)
Secondary Outcomes
-
Adherence to treatment
Measures of adherence (child, adolescent, adult or parent) to the treatment regimen including: pill counts; electronic monitors; calorie and dietary intake; self‐report forms; semi‐structured interviews; and diary reports.
-
Social functioning
Measures of how well the child, adolescent or adult is able to participate in developmentally appropriate social activities, to access social support systems, and to develop appropriate peer relationships and social skills.
Measures of academic performance deficits or achievements or employment in a child, adolescent or adult with CF.
-
Cognitive psychological outcomes
Measures of the coping strategies.
Measures of decision‐making strategies or conflicts.
Measures of self‐esteem, social competence, perceptions of their own appearance or body image, locus of control, self‐efficacy or sense of coherence.
-
Relational or family adjustment
Measures of the relationship between the parents of the affected child, adolescent or adult or with their partner, of family functioning or communication and interaction patterns.
-
Utilization and costs of health‐care services
Measures estimating the financial cost of the intervention in relation to its efficacy for the individual with CF or their family (or both) or costs for the health care services and utilization.
Number of hospital admissions, excluding the visits in CF centres for routine assessment.
Number of days hospitalised, excluding the visits in CF centres for routine assessment.
Search methods for identification of studies
Electronic searches
Search strategies developed by the Cochrane Depression, Anxiety and Neurosis Group and the Cochrane Cystic Fibrosis and Genetic Disorders Group were undertaken. Relevant studies were identified from the Cystic Fibrosis Trials Register using the terms: motivation OR behaviour OR mental health in CF OR genetic counselling.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (updated each new issue), quarterly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journal ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work was identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis andGenetic Disorders Group Module.
For the current update of the review, searches were updated in June 2012 (Appendix 1) and were carried out for all years in the Cochrane Central Register of Controlled Trials (CENTRAL), OVID MEDLINE, OVID Embase, and OVID PsycINFO.
Date of the most recent search of the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register: 19 December 2013. Date of the most recent search of the Depression, Anxiety and Neurosis Group: 12 November 2013.
In addition, the Clinical Trial Registry (www.clinicaltrials.gov) was used to identify possible ongoing studies using the following term: 'cystic fibrosis'.
Searching other resources
Efforts were made to locate unpublished findings through personal contacts with researchers active in this field and professional networks including listserves.
Data collection and analysis
Selection of studies
Three authors (LG, AF, MH), working independently in groups of two, selected the studies relevant for inclusion in the current update of the review. The authors LG and AF checked one third of the studies, AF and MH checked a further third and LG and MH checked the remaining studies. The authors used a study eligibility form devised for the selection process. If two authors could not reach a consensus, then they asked one of the other authors to decide. In the case of studies published by one of the authors, a co‐author assessed this for eligibility.
For previous versions of the review, two authors (CG and AQ) independently selected the studies that were relevant for inclusion in the review using a study eligibility form devised for this purpose.
Data extraction and management
Two authors (AF and MH) independently extracted data using a data extraction form. If they could not reach a consensus, then they asked one of the remaining authors to decide.
For previous versions of the review two authors (CG and AQ) independently extracted the data to be analysed using a data extraction form.
Measures of treatment effect
For binary outcomes with sufficient data, the authors calculated a pooled estimate of the treatment effect for each outcome. A comparison was made between the odds of an outcome occurring in the group receiving the treatment of interest versus the control group (odds ratio (OR) and 95% confidence interval (CI)).
For continuous outcomes, the authors calculated a pooled estimate of the treatment effect with a mean difference (MD) with 95% CIs.
Unit of analysis issues
In the event that groups are cluster‐randomised, the authors will use the inverse variance method and report results as a fixed MD (Deeks 2011). Furthermore, the authors will explore whether the study reported appropriate analyses, taking the cluster design into account. If this is not the case they will perform correct analyses, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
In the event that a study compared more than one intervention, the authors will compare the primary active treatment to usual care (or at least biased control condition).
Deeks recommends for cross‐over studies to first of all prove whether the cross‐over design is appropriate for the intervention and outcomes, which need to be reversible (Deeks 2011). Secondly, to take into consideration the possibility of substantial carry‐over or period effects. Different options are available for the incorporation of cross‐over study data into meta‐analyses different options are available ranging from conservative methods to just analysing data from the first period. The selection of an appropriate method will be based on the characteristics of the study and the reported data.
Assessment of heterogeneity
When appropriate, the authors pooled results and they used the I2 statistic to judge whether the results of different studies were inconsistent. The authors assumed the following overlapping percentage intervals of the I2 statistic for quantifying heterogeneity (Deeks 2011): 0% to 40% (might not be important); 30% to 60% (may represent moderate heterogeneity); 50% to 90% (may represent substantial heterogeneity); and 75% to 100% (considerable heterogeneity). Additionally, the authors conducted a visual inspection of the forest plot and chi2 test.
Data synthesis
We have analysed data using a random‐effects model.
The authors investigated the pooling of results when the interventions were:
of the same type of interventions defined in the methods section 'Types of interventions';
were comparable in the psychological methods used, the setting, the addressed outcomes and participants;
being compared to an equal control condition.
The authors extracted data at post‐treatment and when available, including short‐ and long‐term follow‐up data.
Subgroup analysis and investigation of heterogeneity
If the authors identify heterogeneity of pooled results, they will use subgroup analyses to explore the possible sources of heterogeneity.
They plan to group studies into conceptually similar interventions and, if sufficient data are available, they will perform subgroup analyses with respect to the following list.
Socioeconomic level of the individual and family (education level, income level, occupation)
Age of the affected individual with CF (infants, toddlers, and preschool children under five years of age, school‐aged children ages 5 to 12 years, adolescents ages 13 to 18 years, adults 19 years+)
Stage of the disease process (pre‐diagnosis stage, diagnosis phase through the the first year post‐diagnosis, chronic phase ‐ intervening years between the diagnosis and terminal phase, terminal phase ‐ when the affected child or adult's condition was such that active treatment had ceased and only palliative measures were being offered)
Sensitivity analysis
In future, if sufficient data are available, the authors will undertake a sensitivity analysis comparing the findings of studies with a high risk of bias to results when these studies are excluded. Due to the small number of studies currently included and the different types, interventions and outcome measures, a sensitivity analysis was not feasible.
Results
Description of studies
Results of the search of previous versions of the review
Originally, a total of 70 RCTs (99 reports) were reviewed; 28 studies (50 reports) were relevant. In a previous version of the review, 13 RCTs (25 reports) were included (Delk 1994; Stark 1996; Cheuvront 1998; Trapp 1998; Hernandez‐Reif 1999; Grasso 2000; Powers 2003; Stapleton 2001; Chernoff 2002; Davis 2002; Stark 2003; Powers 2005; Downs 2006) and five studies were classified as ongoing (Kalnins 1996; Quittner 2000; Quinn 2004; Watson 2004; Watson 2006). Two of the ongoing studies were concerned with adherence (Quittner 2000; Quinn 2004). Ten studies were classified as 'awaiting information from authors' (Williams 1987; Klig 1989; Davis 1990; Petzel 1991; Mischler 1998; Stark 1998; Cannon 1999; Christian 2006; Bryon 2000; Powers 2003a). A total of 42 studies (49 reports) were not eligible for inclusion in the review.
Results of the new search for the current update
For the current update of the review a total of 1705 titles and abstracts (1365 without duplicates) were identified. A total of 33 new studies (55 reports) were identified for reviewing as potentially relevant for inclusion. Three of these are still ongoing (Quittner 2011; Quittner 2012; Riekert 2012). We are awaiting further information from authors for 12 studies (14 reports) (Wainwright 2009; Huang 2010; Jessup 2010; Patel 2010; Cummings 2011; Hatziagorou 2011;Ruddy 2011; Irons 2012; Hass 2012; Goldbeck 2013; Powers 2013; Widman 2013). Eleven studies were not eligible for inclusion (Dodd 2001; Stark 2002; Rodrigue 2005; Ralston 2008; Bingham 2010; Pop Jordanova 2010; Castellani 2011; Georgiopoulos 2011; Goldbeck 2011a; Rault 2012; Rozenfeld 2012). Seven newly identified studies (15 reports) are included in the current update of the review (Belsky 1994; Taylor 2003; Goodill 2005; Christian 2006; Wilkinson 2008; Stark 2009; Vandemheen 2009). Details of the number of reports yielded by the new search are shown in Figure 1. Where necessary, we contacted authors of the included studies for additional data to allow quantitative analysis. We have not been able to locate contact details on any of the authors concerning one study (Belsky 1994). For three studies only narrative reporting of results were possible (Belsky 1994; Goodill 2005;Wilkinson 2008).
1.
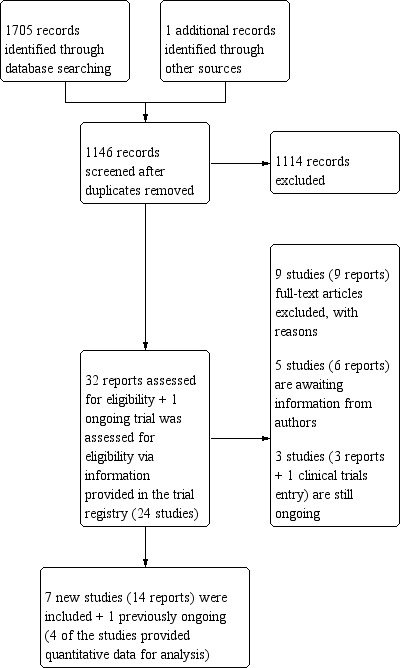
Number of reports yielded by the new search for the current update (2014)
Four of the 12 newly identified studies awaiting classification were published as abstracts (Wainwright 2009; Jessup 2010; Patel 2010; Hatziagorou 2011) and one journal article did not include any quantitative data (Cummings 2011). The remaining seven studies were identified in the latest search in December 2013 and are still awaiting classification for inclusion (Huang 2010; Ruddy 2011; Hass 2012; Irons 2012; Goldbeck 2013; Powers 2013; Widman 2013). Information available to date on the studies is presented in the 'Characteristics of studies awaiting classification' tables.
One of the previous studies assessed as ongoing is now awaiting information from authors (Kalnins 1996) and one is now included in the review (Quinn 2004). Additionally, two previously ongoing studies were not eligible for the review due to an educational approach of the intervention (Watson 2004; Watson 2006).
Report characteristics of all previous and new relevant studies are listed in an additional table (Table 5).
1. Report characteristics of relevant studies.
| Study ID | Country of Origin | Language of Report | Type of Report | Contact Author | Reply | Assessment |
| Belsky 1994 | USA | English | Journal article | Yes | No | awaiting |
| Bingham 2010 | USA | English | Journal article | No | No | exclude |
| Bryon 2000 | UK | English | Abstract | Yes | No | awaiting |
| Cannon 1999 | USA | English | Abstract | Yes | No | awaiting |
| Castellani 2011 | Italy | English | Journal article | No | No | exclude |
| Chernoff 2002 | USA | English | Journal article | No | NA | include |
| Christian 1999 | USA | English | Abstract | Yes | No | awaiting |
| Christian 2006 | USA | English | Journal article | No | No | include |
| Cummings 2011 | Australia | English | Journal article | No | No | awaiting |
| Davis 1990 | USA | English | Abstract | Yes | No | awaiting |
| Delk 1994 | USA | English | Journal article | Yes | No | include |
| Dodd 2001 | Ireland | English | Abstract | Yes | Yes | exclude |
| Georgiopoulus 2011 | USA | English | Journal article | No | No | exclude |
| Goodill 2005 | USA | English | Journal article | Yes | No | awaiting |
| Goldbeck 2011 | Germany | English | Journal article | No | No | exclude |
| Grasso 2000 | Australia | English | Journal article | Yes | Yes | include |
| Hatziagorou 2010 | Greece | English | Abstract | Yes | Yes | awaiting |
| Hernandez‐Reif 1999 | USA | English | Journal article | Yes | Yes | include |
| Jessup 2010 | Australia | English | Abstract | Yes | Yes | awaiting |
| Jessup 2011 | Australia | English | Abstract | Yes | Yes | awaiting |
| Kalnins 1996 | USA | English | Abstract | Yes | No | awaiting |
| Klig 1989 | USA | English | Abstract | Yes | Yes | awaiting |
| Marciel 2010 | USA | English | Journal Article / Summary of clinical trial registration |
Yes | Yes | ongoing |
| Mischler 1998 | USA | English | Journal article | No | No | awaiting |
| Patel 2010 | UK | English | Abstract | Yes | Yes | exclude |
| Petzel 1991 | USA | English | Abstract | Yes | No | awaiting |
| Pop Jordanova 2010 | Macedonia | English | Journal Article | No | No | exclude |
| Powers 2003 | USA | English | Journal article | Yes | Yes | include |
| Powers 2005 | USA | English | Journal article | No | NA | include |
| Quinn 2004 | UK | English | Abstract | Yes | Yes | include |
| Quittner 2000 | USA | English | Book chapter | Yes | Yes | awaiting |
| Quittner 2002 | USA | English | Personal correspondence | Yes | Yes | awaiting |
| Quittner 2011 | USA | English | Abstract | Yes | Yes | ongoing |
| Ralston 2008 | USA | English | Journal article | Yes | No | exclude |
| Ranström 2000 | France | English | Journal article | Yes | No | exclude |
| Riekert 2012 | USA | English | Summary of clinical trials registration | No | No | ongoing |
| Rodrigue 2005 | USA | English | Journal Article | No | No | exclude |
| Stark 1996 | USA | English | Journal article | Yes | Yes | include |
| Stark 1998 | USA | English | Abstract | Yes | Yes | awaiting |
| Stark 2001 | USA | English | Unpublished report | Yes | Yes | include |
| Stark 2002 | USA | English | Journal Article | No | No | exclude |
| Stark 2003 | USA | English | Journal article | No | NA | include |
| Stark 2009 | USA | English | Journal article | Yes | Yes | include |
| Taylor 2003 | USA | English | Journal Article | Yes | Yes | include |
| Vandemheen 2009 | Australia / Canada | English | Journal article | Yes | Yes | awaiting |
| Wainwright 2009 | Australia | English | Abstract | Yes | Yes | awaiting |
| Wilkinson 2008 | UK | English | Journal article | Yes | No | awaiting |
| Williams 1997 | UK | English | Abstract | Yes | Yes | awaiting |
| Wolter 1997 | Australia | English | Journal article | Yes | Yes | exclude |
Included studies
Sixteen RCTs and one CCT (a total of 33 reports), representing data from 556 participants, had sufficient data for inclusion in the analyses of this review (Belsky 1994; Delk 1994; Stark 1996; Hernandez‐Reif 1999; Grasso 2000; Chernoff 2002; Powers 2003; Stark 2003; Taylor 2003; Quinn 2004; Goodill 2005; Powers 2005; Christian 2006; Wilkinson 2008; Stark 2009; Vandemheen 2009).
Twelve of the included studies were conducted in the USA, one in Australia (Grasso 2000), one in Australia and Canada (Vandemheen 2009), and two in the UK (Quinn 2004; Wilkinson 2008). One study was ongoing at the time of the original review and has not yet been published in full (Quinn 2004). Summary details of these studies are given in the 'Characteristics of included studies' section.
Participants were children in one study (Christian 2006), children and their caregivers in seven of the studies (Stark 1996; Hernandez‐Reif 1999; Grasso 2000; Powers 2003; Stark 2003; Powers 2005; Stark 2009), children and adolescents in one study (Belsky 1994), adolescents and their families in one study (Chernoff 2002) and only adults in two of the included studies (Quinn 2004; Vandemheen 2009). In four further studies, patients with a broad age range were enrolled (Delk 1994; Taylor 2003; Goodill 2005; Wilkinson 2008).
Due to the wide scope of this review, included studies were diverse in their design and methods. Those that were relevant for inclusion sometimes involved differing combinations of interventions for various aspects of the psychological management of CF. On occasion individual studies compared two or more types of intervention with each other.
All the included studies can be considered within the different methods the interventions contained as stated in the section 'Types of interventions'. Further distinction within these groupings arises from the predetermined target areas for psychological interventions highlighted in the 'Objectives' section (newborn screening, adherence to treatment, transplantation, adapting to treatment regimen, and maintaining or improving health‐related quality of life, and transitioning toward independence and adult care).
1. Cognitive behavioral
1.1 Cognitive behavioral interventions to improve adherence
There are five studies in this group (Stark 1996; Powers 2003; Stark 2003; Powers 2005; Stark 2009). These involved educational or cognitive behavioural interventions, or both, with a stronger cognitive component in the three Stark studies (Stark 1996; Stark 2003; Stark 2009). The outcomes measured were mostly physical, i.e. dietary input specifically linked to nutrition status, and oriented towards adherence.
The Stark study compared the effectiveness of a group behavioural intervention in primary school age children and their parents in improving dietary input with a wait list control (Stark 1996). The earlier study by Powers examined the effectiveness of two nutritional interventions with children younger than three years of age and their parents (Powers 2003). Behavioural management training and nutrition education for parents were compared to nutrition education alone. The primary outcome indicator was number of calories consumed per day. The more recent Powers study is more focused and systematic in its design and assessment of individualised nutritional counselling compared to behavioural parent training, with children under five years of age (Powers 2005). Another study by Stark compared the effectiveness of two group approaches to promoting dietary input in school‐age children and their parents (Stark 2003). One intervention involved nutrition education and the other involved behavioural management training for parents. The primary outcome for all of these studies was the number of calories consumed per day. The latest study by Stark also compared the effectiveness of two approaches (nutrition education versus behavioural plus nutrition education) for children ages from four to twelve years and their parents, with the aim of improving caloric intake and weight (Stark 2009).
1.2 Cognitive behavioral interventions to improve psychosocial adjustment
This subcategory includes one study that assessed the effectiveness of an intervention consisting of an individually tailored intervention during a home visit and a structured group session (Christian 2006). The intervention was based on teaching problem solving and training social skills. The children aged eight to twelve years were educated and supported with the following specific problems: finding out about the CF‐diagnosis, explaining CF‐related differences, dealing with teasing about CF, and keeping up with peers during physical activity. The control group received treatment as usual. Outcomes were assessed to measure differences between groups on psychosocial adjustment, functional health and physiological health status.
2. Cognitive
2.1 Cognitive interventions to improve adherence
Both studies in this group utilized a cognitive approach to improve adherence or health care utilization (Taylor 2003; Quinn 2004). One study provided a written self‐disclosure intervention compared to standard care and was designed to improve health‐care utilization in adolescents or adults with CF (Taylor 2003). Participants were asked to write three times over a 20‐minute period (once in the CF clinic, twice at home) without being concerned with spelling or syntax. The first topic related to the most distressing experience of their life with possible connections between the topic to their relationships with others (e.g. family, friends etc.) and to their past, present, or future. For the following two writing sessions, participants could change from one topic to another. The second study assessed the effectiveness of motivational interviewing provided via telephone over a three‐month period (Quinn 2004). It is a non‐confrontational, patient‐centred counselling style for enhancing behaviour change by helping patients explore and resolve ambivalence. Comparisons of the intervention to usual treatment were made on the primary outcome of adherence to aerosolised antibiotics; lung function was also measured pre‐ and post‐intervention.
2.2 Cognitive interventions associated with decision making in lung transplantation
There is one study in this category (Vandemheen 2009). In addition to usual counselling, patients with advanced CF were provided with an evidence‐based decision aid as they considered referral for lung transplantation. Comparisons were made with usual care. Patients received a paper version of the decision aid and could also access it online using the provided password. The aim was to improve patient knowledge about options when considering lung transplantation and facilitate more realistic expectations about transplantation to decrease decisional conflict.
3. Family systems or systemic
The study included in this category assessed its effectiveness as a psychosocial intervention for parenting a child with a chronic illness (Chernoff 2002). It was designed to mobilise community‐based support linking new parent or child dyads (or both) with experienced parent or professional dyads. Each experienced dyad consisted of a trained network mother and a 'child life specialist'. This experienced pair initiated home visits, telephone calls and special family events aimed at enhancing adaptation and reducing stress. The generic program was targeted to children with diabetes, sickle cell, CF or moderate to severe asthma. However, the emotional outcomes reported by caregivers were reported separately, enabling the specific effect for CF to be estimated.
4. Psychodynamic
No studies were eligible for this category.
5. Other interventions
Six studies are included within this category (Belsky 1994; Delk 1994; Hernandez‐Reif 1999; Grasso 2000; Goodill 2005; Wilkinson 2008).The CCT by Belsky assessed the effects of self‐hypnosis on psychological and physiological functioning in children aged from 7 to 18 years with CF (Belsky 1994). Children were taught a hypnotic technique within a two‐week period. Outcomes were physiological and psychological indicators. The Delk study was an RCT to assess the effectiveness of a respiratory muscle biofeedback technique used with adolescent and adult participants (Delk 1994). It was specifically targeted at enhancing the efficacy of breathing during physiotherapy using electromyographic trapezius muscle feedback in comparison to promoting relaxation, not specifically associated with physiotherapy. The primary outcome was pulmonary function. The Hernandez‐Reif RCT that assessed the effectiveness of massage therapy compared to bedtime reading, as an adjunct to physiotherapy when delivered to primary school‐aged children by parents (Hernandez‐Reif 1999). The primary outcome was pulmonary function. The Grasso RCT assessed the effectiveness of music therapy in enhancing the experience of physiotherapy in mothers and infants under two years of age, diagnosed with CF (Grasso 2000). The primary outcomes were ratings of emotional (child and parent enjoyment) and cognitive (parents' perception of the time it took to perform physiotherapy) variables. The Goodill RCT assessed the effectiveness of dance and movement therapy in adult hospitalised patients compared to controls (Goodill 2005). Mood state, adherence, self‐care expectations and body image were targeted for improvement. The remaining RCT by Wilkinson evaluated the feasibility of a video link to support patients on the transplantation list and their families (Wilkinson 2008). On a weekly basis tele‐medicine sessions were used to discuss relevant issues between the consultant and the patient. Outcomes were quality of life, symptoms of anxiety and depression, and healthcare utilisation.
A summary of the categories of outcome measures reported in studies included in this review can be viewed in 'Additional tables' (Table 6).
2. Types of outcome measure used in included studies.
| Study ID | Primary Outcomes | Secondary Outcomes |
| Belsky 1994 | Physiological: pulmonary function ‐ Average PEFR
Height
Weight Well‐being: child behavior, anxiety |
Impact on family Ratings of the parents' assessment of the child's illness Locus of control and health locus of control Self‐concept |
| Chernoff 2002 | Well being: depression, anxiety | |
| Christian 2006 | Pulmonary function Height Weight |
Perceived Illness Experience Scale
Children's Loneliness Scale Social Support Scale for Children Self‐Perception Profile for Children Functional Health Status |
| Delk 1994 | Pulmonary function | |
| Goodill 2005 | Well‐being: mood state | Body image Adherence |
| Grasso 2000 | Enjoyment & perception of time | |
| Hernandez‐Reif 1999 | Well‐being: anxiety/depression Pulmonary function |
|
| Powers 2003 | Weight | Calorie intake Relational |
| Powers 2005 | Weight Height | Calorie consumption |
| Quinn 2004 | Well‐being: anxiety, depression, quality of life Pulmonary function | Adherence behaviour to aerosolised antibiotics |
| Stark 1996 | Pulmonary function Weight and height | Activity level |
| Stark 2003 | Weight | Daily calorie intake Quality of parent/child interaction at mealtime CF coping skills Adherence to CF regimen |
| Stark 2009 | Change in weight BMI Pulmonary function |
Caloric intake, energy (%EER) |
| Taylor 2003 | Pulmonary function BMI | Health care utilization |
| Vandemheen 2009 | Participants’ knowledge and realistic expectations Decisional conflict | |
| Wilkinson 2008 | Well‐being: quality of life, anxiety, depression | Coping of carers Health care utilization |
Some parts are empty for the following reason: We summarized all reported outcomes of the 16 included studies in this table and categorized them according to our determined primary and secondary outcomes.
BMI: body mass index EER: estimated energy requirements PEFR: peak expiratory flow rate
Subgroups
1. Age group of the individual with CF
Three studies involved infants (Grasso 2000; Powers 2003; Powers 2005). Seven studies involved school‐aged children (Belsky 1994, Stark 1996; Hernandez‐Reif 1999; Chernoff 2002; Stark 2003, Christian 2006; Stark 2009); two study involved adolescents and adults (Delk 1994; Taylor 2003); and the remaining four studies involved adults only (Quinn 2004, Goodill 2005; Wilkinson 2008, Vandemheen 2009).
2. Socioeconomic status (SES)
Eight of the included studies conducted an assessment of SES for the purposes of establishing equivalent group characteristics at baseline (Stark 1996; Hernandez‐Reif 1999; Chernoff 2002; Powers 2003; Stark 2003; Powers 2005; Stark 2009; Vandemheen 2009). No attempt was made to stratify samples on the basis of SES groupings.
3. Stage of CF condition
Three studies were conducted during or soon after the diagnosis of CF (Grasso 2000; Powers 2003; Powers 2005). Two studies were conducted within a period when patients were considered for lung transplantation (Wilkinson 2008; Vandemheen 2009), but no study was conducted explicitly with those individuals affected with CF during the terminal stage of their disease state (palliative care). In the remaining 14 studies, stage of disease severity was not specified, although most occurred during the chronic management period.
Details of the subgroup membership of studies in this review can be viewed in 'Additional tables' (Table 7). No subgroup analyses were conducted due to insufficient data.
3. Subgroup membership of included studies.
| Study ID | Socioeconomic Level | Age | Stage of Disease |
| Belsky 1994 | 7 ‐ 18 years | ||
| Chernoff 2002 | Mothers' educational level | 7 ‐ 11 years | chronic? |
| Christian 2006 | 8 – 12 years | chronic | |
| Delk 1994 | 10 ‐ 41 years | ||
| Goodill 2005 | > 17 years | ||
| Grasso 2000 | < 2 years | post diagnosis | |
| Hernandez‐Reif 1999 | Hollingshead | 5 ‐ 15 years | chronic? |
| Powers 2003 | Hollingshead | < 3 years | post diagnosis |
| Powers 2005 | 18 ‐ 48 months | post diagnosis | |
| Quittner 2000 | 9.5 to 16.5 years |
||
| Quinn 2004 | Employment status | ||
| Stark 1996 | Holligshead, Control group ‐ II, Intervention group III | 3 ‐10 years control, 5 ‐ 10 years intervention | chronic? |
| Stark 2003 | Hollingshead & family income | 4 ‐ 12 years | |
| Stark 2009 | Mother’s and father’s education, income before taxes | 4 – 12 years | |
| Taylor 2003 | > 15 years | ||
| Vandemheen 2009 | highest level of education | > 18 years | considering transplantation |
| Wilkinson 2008 | 21 ‐ 41 years | on transplantation list |
Not all characteristics were reported for every study. That is the reason why some parts are left empty.
Excluded studies
Fifty‐nine studies with 66 reports were excluded. The reasons for exclusion for each individual study are listed in the 'Characteristics of excluded studies' section.
Five previously included studies on educational interventions (Cheuvront 1998; Trapp 1998; Stapleton 2001; Davis 2002; Downs 2006) have now been excluded following the publication of a separate review in 2011 (Savage 2011).
Studies awaiting assessment
A total of 22 studies are now awaiting information from authors (Williams 1987; Klig 1989; Davis 1990; Petzel 1991; Kalnins 1996; Mischler 1998; Stark 1998; Cannon 1999; Bryon 2000; Powers 2003a; Wainwright 2009; Huang 2010; Jessup 2010; Patel 2010; Cummings 2011; Hatziagorou 2011; Ruddy 2011; Hass 2012; Irons 2012; Goldbeck 2013; Powers 2013; Widman 2013).
Ongoing studies
To date, four studies are listed as ongoing (Quittner 2000; Quittner 2011; Quittner 2012; Riekert 2012).
Risk of bias in included studies
For judging the risk of bias for the studies being included in this update the evaluation criteria proposed by Higgins in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1) (Higgins 2011b) were used.
Details for every study and reasons for judgement are described within the risk of bias tables in the section ‘Characteristics of included studies’. Only one of the included studies was judged as adequately meeting all criteria (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
It should be mentioned that most of the included studies were small in their sample size, which might have influenced the ability of randomisation to deliver equivalence at baseline.
Allocation (selection bias)
Sequence generation was judged to be low risk in eight of the studies (Taylor 2003; Stark 2003; Quinn 2004; Powers 2005; Christian 2006; Wilkinson 2008; Stark 2009; Vandemheen 2009), was unclear for seven studies (Delk 1994; Stark 1996; Hernandez‐Reif 1999; Grasso 2000; Chernoff 2002; Powers 2003; Goodill 2005) and was judged as high for one study (Belsky 1994).
Allocation concealment was judged to be low in six of the studies (Stark 2003; Powers 2005; Christian 2006; Wilkinson 2008; Stark 2009; Vandemheen 2009), to be high in one study (Belsky 1994) and unclear in the other nine studies (Delk 1994; Stark 1996; Hernandez‐Reif 1999; Grasso 2000; Chernoff 2002; Powers 2003; Taylor 2003; Quinn 2004; Goodill 2005).
Blinding (performance bias and detection bias)
Blinding participants and providers of psychological interventions is quite difficult. To keep them unaware of the received or provided treatment is almost impossible. Details on blinding especially regarding the blinding of outcome assessors were judged as high risk in five studies (Stark 1996; Hernandez‐Reif 1999; Powers 2003; Stark 2003; Powers 2005), low risk in six studies (Chernoff 2002; Taylor 2003; Quinn 2004; Goodill 2005; Christian 2006; Vandemheen 2009) and unclear in five studies (Belsky 1994; Delk 1994; Grasso 2000; Wilkinson 2008;Stark 2009).
Incomplete outcome data (attrition bias)
Judgments of possible biases due to the nature, amount or handling incomplete outcome data were assessed as the following: low risk for nine studies (Delk 1994; Stark 1996; Hernandez‐Reif 1999; Powers 2003; Quinn 2004; Powers 2005; Christian 2006; Stark 2009; Vandemheen 2009), high risk for three studies (Chernoff 2002; Goodill 2005; Wilkinson 2008) and unclear risk for four studies (Belsky 1994; Chernoff 2002; Delk 1994; Powers 2003; Stark 2003; Taylor 2003).
Selective reporting (reporting bias)
Assessment of selective outcome reporting revealed the following results for the studies: low risk for four studies (Stark 2003; Taylor 2003; Stark 2009; Vandemheen 2009), high risk for four studies (Belsky 1994; Chernoff 2002; Goodill 2005; Wilkinson 2008) and unclear risk for the other eight studies (Delk 1994; Stark 1996; Hernandez‐Reif 1999; Grasso 2000; Powers 2003; Quinn 2004, Powers 2005; Christian 2006).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The studies included in this review were so diverse in their methodologies that the objective of pooling results became impossible. A large number of different outcome measures were used and are described below for clarity and readability. Of the outcomes defined in 'Types of outcome measures', only those reported in one or more of the included primary studies are described below. If an outcome was not described, it is not reported in any of the target groups. Within psychological interventions adverse events or harms are not usually an issue and none of the included studies reported any such information. There were insufficient data to conduct subgroup analyses with respect to age, stage of condition or SES within any data comparisons.
Please note: the comparison groups and outcome groups refer to the graphs located in Data collection and analysis. We report only those outcomes which have relevant data included in the primary reports.
A brief overview of all main findings can be seen in the summary of findings tables. The findings were classified in accordance to the types of interventions. Main findings for cognitive behavioural interventions can be found in the Table 1, for cognitive interventions in the Table 2, and for other interventions in Table 3 and Table 4.
1. Cognitive behavioral
1.1 Cognitive behavioral interventions to improve adherence
Comparision 1 ‐ Behavioural group treatment versus wait list control
One study with eight participants is included (Stark 1996).
PRIMARY OUTCOMES
Analysis 1.1: Pulmonary function Subcategory 1.1.1: FEV1% predicted (absolute values) Subcategory 1.1.2: FVC% predicted (absolute values) Subcategory 1.1.3: FEF25-75% predicted (absolute values)This behavioural intervention group did not show any demonstrable effect on pulmonary function as measured by three independent indicators: FEV1 % predicted, MD ‐11.70 (95% CI ‐40.35 to 16.95); FVC % predicted, MD ‐6.00 (95% CI ‐31.16 to 19.16); and FEF25-75% predicted, MD ‐32.90 (95% CI ‐98.93 to 33.13).
1.1. Analysis.

Comparison 1: Behavioural group treatment versus wait list control, Outcome 1: Pulmonary function
Analysis 1.2: Anthropometric measures Subcategory 1.2.1: Weight (z score) Subcategory 1.2.2: Height (z score) A significant effect demonstrated that a group behavioural intervention increased children's weight when scores were converted to z scores, MD 1.02 (95% CI 0.04 to 2.00) (P = 0.04). However, no significant group differences were observed in height z scores, MD 0.00 (95% CI ‐1.46 to 1.46).
1.2. Analysis.

Comparison 1: Behavioural group treatment versus wait list control, Outcome 2: Anthropometric measures
SECONDARY OUTCOMES
Analysis 1.3: Adherence Subcategory 1.3.1: Activity level ‐ resting energy expenditure (% predicted) Subcategory 1.3.2: Activity level ‐ resting energy expenditure (Kcal/24 hours) We were unable to find any evidence that a behavioural group intervention had any effect on resting energy expenditure either in terms of % predicted, MD 9.87 (95% CI ‐11.58 to 31.32) or Kcal/24 hours, MD 148.00 (95% CI ‐182.61 to 478.61).
1.3. Analysis.

Comparison 1: Behavioural group treatment versus wait list control, Outcome 3: Adherence
Subcategory 1.3.3: Activity level ‐ activity points There was no significant effect on the activity points, MD ‐1.00 (95% CI ‐9.75 to 7.75).
Subcategory 1.3.4: Change in Kcalorie intake The data for this outcome were reported in the source paper as calories and were converted to Kcalories in order to reduce the scale and thus contain the confidence intervals on the graph. For change in Kcalorie intake there was a significant effect in favour of the group behavioural intervention, MD 0.93 (95% CI 0.31 to 1.55) (P = 0.003).
Analysis 1.4: Nutrition status (additional outcome) Subcategory 1.4.1: % Body fat No significant group differences were observed in % body fat, MD ‐0.65 (95% CI ‐4.41 to 3.11).
1.4. Analysis.

Comparison 1: Behavioural group treatment versus wait list control, Outcome 4: Nutrition status
Comparision 2 ‐ Nutritional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT)
Three studies are included: one with eight participants (Powers 2003); one with seven participants (Stark 2003); and one with 67 participants (Stark 2009). The interventions involved educational and cognitive behavioural components. The three studies utilised the same design and intervention, allowing the data to be pooled.
PRIMARY OUTCOMES
Analysis 2.1: Anthropometric change scores
2.1. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 1: Anthropometric change scores
Subcategory 2.1.1: Change in weight (kg) When data from two studies were combined, no significant differences were found for change in weight, MD 0.11 (95% CI ‐0.84 to 1.07) (Powers 2003; Stark 2009). The result should be interpreted carefully due to the fact that the two included studies reported converse effects and the I2 statistic coefficient for pooling the two studies is relatively high (I2 = 73%). Subcategory 2.1.2: Change in height (cm) Data reported by Powers showed no significant differences for change in height, MD ‐2.03 (95% CI ‐4.87 to 0.81) between the two groups post‐intervention (Powers 2003).
Subcategory 2.1.3: Change in weight (kg) two‐year follow‐up and 2.1.4: Change in height (cm) two‐year follow up Data reported by Stark showed no significant differences at two‐year follow‐up for change in weight, MD 0.52 (95% CI ‐1.34 to 2.38) and change in height, MD ‐0.20 (95% ‐1.45 to 1.05) (Stark 2009). Subcategories 2.1.5: BMIz change post‐intervention and 2.1.6: BMIz change two‐year follow up Data reported by Stark on BMIz change scores did not show a significant difference between the two compared groups, MD 0.20 (95% CI ‐0.02 to 0.42), but did show a difference with borderline statistical significance (P = 0.05) at two‐year follow up, MD 0.35 (95% CI 0.00 to 0.70) in favour with the behavioural management intervention (Stark 2009). Subcategory 2.1.7: HAZ change two‐year follow up Again, this outcome was only reported by Stark, the HAZ change scores (height for age z‐scores) at two‐year follow up did not reveal a significant difference between the groups, MD ‐0.01 (95% CI ‐0.17 to 0.15) (Stark 2009).
Analysis 2.2% Anthropometric
2.2. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 2: % Anthropometric
Subcategories 2.2.1 and 2.2.2: % Ideal body weight and 2.2.3 and 2.2.4: Weight % for age The final two anthropometric measures, only reported by Powers, showed a significant effect size in favour of the intervention (Powers 2003). Although these data showed that the behavioural intervention succeeded in achieving substantial increases at follow up in % ideal body weight, MD 49.49 (95% CI 23.39 to 75.59) (P = 0.00002) and weight % for age, MD 24.70 (95% CI 12.77 to 36.63) (P < 0.0001), the values at baseline for both these measures were also significantly different in the same direction, MD 50.40 (95% CI 5.28 to 95.52) (P = 0.03); MD 25.30 (95% CI 2.70 to 47.90) (P = 0.03)).
Analysis 2.3: Anthropometric
2.3. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 3: Antropometric
Subcategory 2.3.1: Weight (kg) post intervention Subcategory 2.3.2: Weight (kg) two‐year follow up Subcategory 2.3.3: Height (cm) two‐year follow up Subcategory 2.3.4: HAZ two‐year follow up Subcategory 2.3.5: BMIz post intervention Subcategory 2.3.6: BMIz two‐year follow up One study reported on this outcome and found no differences between the two groups in either of the assessed measures at post intervention or at two‐year follow up: weight post intervention, MD ‐0.28 (95% CI ‐3.84 to 3.28); weight two‐year follow up, MD ‐1.00 (95% CI ‐6.26 to 4.26); height two‐year follow up, MD ‐2.80 (95% CI ‐10.88 to 5.28); HAZ two‐year follow up, MD ‐0.15 (95% CI ‐0.54 to 0.24); BMIz post intervention, MD ‐0.08 (95% CI ‐0.54 to 0.38); BMIz two‐year follow up, MD 0.15 (95% CI ‐0.26 to 0.56) (Stark 2009).
Analysis 2.4: Pulmonary function
2.4. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 4: Pulmonary function
Subcategories 2.4.1: FEV1 two‐year follow up and 2.4.2: FEV1 change two‐year follow up One study measured pulmonary function at two‐year follow up via FEV1, no significant differences could be observed in the FEV1 score, MD 0.00 (95% CI ‐13.03 to 13.03) or in the FEV1 change score, MD 5.16 (95% CI ‐8.49 to 18.81) (Stark 2009).
SECONDARY OUTCOMES
Analysis 2.5: Adherence (nutrition)
2.5. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 5: Adherence (nutrition)
Subcategory 2.5.1: % RDA (Kcal/day) One study showed no significant difference between the comparison groups for % RDA (Kcal/day), MD 8.52 (95% CI ‐18.95 to 35.99) (Powers 2003).
Subcategory 2.5.2: Total calories consumed per day Three studies reported on this outcome (Powers 2003; Stark 2003; Stark 2009). They showed a significant difference (P = 0.010) in the total number of calories consumed per day, MD 275.85 (95% CI 66.65 to 485.05) favouring behavioural management training as compared to nutrition education alone.
Subcategory 2.5.3: Change in calorie intake Data from the three studies showed a significant difference between the comparison groups for change in calorie intake, MD 364.06 (95% CI 191.99 to 536.13) (P < 0.0001) (Powers 2003; Stark 2003; Stark 2009).
Subcategory 2.5.4: Two‐year follow up caloric intake One study showed no significant differences at two‐year follow up in caloric intake, MD 112.00 (95% CI ‐216.56 to 440.56) (Stark 2009). Subcategory 2.5.5: Two‐year follow up change in caloric intake Similiarly, Stark reported no significant differences between the groups could be shown in change in caloric intake scores after the two‐year follow up period, MD 188.00 (95% CI ‐75.57 to 451.57) (Stark 2009). Subcategory 2.5.6: Estimated energy requirements (% EER) Stark showed a significant difference favouring the behavioural intervention in % EER, MD 21.00 (95% CI 7.76 to 34.24) (P = 0.002) (Stark 2009). Subcategory 2.5.7: Change in % EER Stark showed a significant difference favouring the behavioural intervention was also found in change in % EER, MD 21.00 (95% CI 9.22 to 32.78) (P = 0.0005) (Stark 2009). Subcategory 2.5.8: Two‐year follow up % EER In % EER at two‐year follow up, Stark showed no significant difference between the two groups, MD 9.00 (95% CI ‐5.09 to 23.09) (Stark 2009). Subcategory 2.5.9: two‐year follow‐up change in % EER Stark showed no significant difference between the groups in change in %EER at two‐year follow up, MD 9.00 (95% CI ‐4.73 to 22.73) (Stark 2009).
Analysis 2.6: Relational ‐ Parent‐child interaction Subcategory 2.1.1: Nursing Satellite Assessment Training Feeding Scale (NCAST) ‐ carer or infant total score (Sumner 1994) This is an observational measure administered during a family meal. It has 76‐items that assess a set of empirically derived and objectively observed behaviours that characterise carer‐child communication and interaction during feeding. This measure is a reliable and valid observation tool with internal consistency (Chronbach alpha) ranging from 0.73 to 0.86. From the only study reporting this outcome, we were unable to find evidence that behavioural management training improves parent‐child communication and interaction during feeding as assessed by the NCAST total score, MD 4.60 (95% CI ‐0.40 to 9.60) (Powers 2003).
2.6. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 6: Relational
Analysis 2.7: Nutritional status
2.7. Analysis.

Comparison 2: Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT), Outcome 7: Nutritional status
Subcategory 2.7.1: % Body fat Powers showed no statistical difference was evidenced between the groups in % body fat, MD 0.20 (95% CI ‐9.43 to 9.83) (Powers 2003).
Comparision 3 ‐ Behavioral and nutritional intervention (BEH) versus standard care control (CTL)
One study with 10 participants was included (Powers 2005).
SECONDARY OUTCOMES
Analysis 3.1: Total energy intake per day (kcal) There was a substantially larger intake of calories per day in the children in the intervention group post‐treatment, MD 979.10 (95% CI 266.17 to 1692.03) (P = 0.007) compared with the control group (Powers 2005). The design of the study did not permit this comparison to be repeated at follow up, although calorie intake remained stable from post‐treatment to three‐ and 12‐month follow up. This represents a clinically significant finding that should be replicated with a larger study.
3.1. Analysis.

Comparison 3: Behavioral and nutritional intervention (BEH) versus standard care control (CTL), Outcome 1: total energy intake per day (kcal)
Analysis 3.2: % Fat intake The finding for fat intake was in the predicted direction although not significant statistically, MD 10.40 (95% CI ‐0.51 to 21.31) (Powers 2005).
3.2. Analysis.

Comparison 3: Behavioral and nutritional intervention (BEH) versus standard care control (CTL), Outcome 2: % fat intake
1.2 Cognitive behavioral interventions to improve psychosocial adjustment
Comparision 4 ‐ Educational problem‐solving and social skills interventions versus usual care
One study with 116 participants was included for this comparison (Christian 2006).
PRIMARY OUTCOMES
Analysis 4.1: Pulmonary function (FEV1)
4.1. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 1: Pulmonary function (FEV1)
Subcategories 4.1.1: Three months; 4.1.2: six months; and 4.1.3: Nine months There were no statistically significant differences in pulmonary function (FEV1) between the two groups at any time point: three months, MD 2.02 (95% CI ‐4.78 to 8.82); six months, MD 3.35 (95% CI ‐3.28 to 9.98); nine months, MD ‐2.44 (95% CI ‐9.04 to 4.16) (Christian 2006).
Analysis 4.2:Height in cm
4.2. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 2: Height (cm)
Subcategories 4.2.1: Three months; 4.2.2: Six months; and 4.2.3: Nine months No statistically significant differences in height in cm between the intervention and usual care could be found at: three months, MD 1.00 (95% CI ‐2.23 to 4.23); at six months, MD 1.83 (95% CI ‐3.03 to 6.69); and at nine months, MD ‐0.54 (95% CI ‐3.92 to 2.84) (Christian 2006).
Analysis 4.3: Weight in kg
4.3. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 3: Weight in (kg)
Subcategories 4.3.1: Three months; 4.3.2: Six months; and 4.3.3: Nine months The outcome weight in kg did not statistically significant differ at any of the three points in time between the two groups: three months, MD 0.17 (95% CI ‐1.98 to 2.32); six months, MD 0.27 (95% CI ‐2.09 to 2.63); nine months, MD ‐0.53 (95% CI ‐3.03 to 1.97) (Christian 2006).
SECONDARY OUTCOMES
Analysis 4.4: Perceived illness experience
4.4. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 4: Perceived illness experience
Subcategories 4.4.1: Three months; 4.4.2: Six months; and 4.4.3: Nine months This outcome was measured with 'The perceived Illness Experience Scale' (Eiser 1995). Regarding the perceived illness experience (impact of chronic illness) by the participating children, no statistically significant differences between the intervention and usual care group were found at: three months, MD ‐4.31 (95% CI ‐12.69 to 4.07); six months, MD ‐1.09 (95% CI ‐9.03 to 6.85); nine months, MD ‐ 6.60 (95% CI ‐13.98 to 0.78)) (Christian 2006).
Analysis 4.5: Children's loneliness
4.5. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 5: Children's loneliness
Subcategories 4.5.1: Three months; 4.5.2: Six months; and 4.5.3: Nine months Children's loneliness was measured with the 'Children's Loneliness Scale' (Asher 1984). No statistically significant differences between the groups at any point in time were observed at: three months, MD ‐0.76 (95% CI ‐4.26 to 2.74); six months, MD 0.39 (95% CI ‐2.78 to 3.56); nine months, MD ‐2.17 (95% CI ‐5.73 to 1.39) (Christian 2006).
Analysis 4.6: Social support peers
4.6. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 6: Social support ‐ peers
Subcategory 4.6.1: three months, sub‐category 4.6.2: six months, and sub‐category 4.6.3: nine months This outcome is measured with the subscale 'Peers' of the 'Social Support Scale for Children' (Harter 1986). No statistically significant differences were found between the two groups at: three months MD 0.75 (95% CI ‐0.59 to 2.09); at six months MD ‐0.05 (95% CI ‐1.13 to 1.03); and at nine months, MD ‐0.09 (95% CI ‐1.13 to 0.95) (Christian 2006).
Analysis 4.7: Social support classmates
4.7. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 7: Social support ‐ classmates
Subcategories 4.7.1: Three months; 4.7.2: Six months; and 4.7.3: Nine months This outcome is measured with the subscale 'Classmates' of the 'Social Support Scale for Children' (Harter 1986). No statistically significant differences were found between the two groups at: three months, MD 0.06 (95% CI ‐1.59 to 1.71); at six months, MD 0.35 (95% CI ‐1.11 to 1.81); and at nine months, MD 1.33 (95% CI ‐0.20 to 2.86) (Christian 2006).
Analysis 4.8: Self‐competence –subscale 'Global self‐worth'
4.8. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 8: Self competence ‐ subscale 'global self‐worth'
Subcategory 4.8.1: three months, sub‐category 4.8.2: six months, and sub‐category 4.8.3: nine months This outcome is measured with the subscale 'Global self‐worth' of the 'Self‐perception Profile for Children' (Harter 1985). No statistically significant differences were observed at any point in time: three months, MD 0.36 (95% CI ‐0.84 to 1.56); six months, MD 0.31 (95% CI ‐0.81 to 1.43); nine months, MD 0.63 (95% CI ‐0.54 to 1.80) (Christian 2006).
Analysis 4.9: Self‐competence ‐ subscale 'scholastic competence'
4.9. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 9: Self‐competence ‐ subscale 'scholastic competence'
Subcategories 4.9.1: Three months; 4.9.2: Six months; and 4.9.3: Nine months This outcome is measured with the subscale 'Scholastic competence' of the 'Self‐perception Profile for Children' (Harter 1985). This measure did not show any statistically significant effect between the groups at any point in time: three months, MD 0.28 (95% CI ‐1.38 to 1.94); six months, MD 0.55 (95% CI ‐0.93 to 2.03); nine months, MD 0.76 (95% CI ‐0.65 to 2.17) (Christian 2006).
Analysis 4.10: Self‐competence – subscale 'Social acceptance'
4.10. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 10: Self‐competence ‐ subscale 'social acceptance'
Subcategories 4.10.1: Three months; 8.10.2: Six months; and 8.10.3: Nine months This outcome is measured with the subscale 'Social acceptance' of the 'Self‐perception Profile for Children' (Harter 1985). No statistically significant difference was found at: three months, MD ‐0.38 (95% CI ‐1.85 to 1.09); six months, MD 0.24 (95% CI ‐1.09 to 1.57); or nine months, MD 0.52 (95% CI ‐0.84 to 1.88) between the intervention and usual care group (Christian 2006).
Analysis 4.11: Self‐competence – subscale 'Athletic competence'
4.11. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 11: Self‐competence ‐ subscale 'athletic competence'
Subcategories 4.11.1: Three months, 4.11.2: Six months; and 4.11.3: Nine months This outcome is measured with the subscale 'Athletic competence' of the 'Self‐perception Profile for Children' (Harter 1985). No statistically significant differences between the groups at any point in time were observed: three months, MD 0.59 (95% CI ‐0.90 to 2.08); six months, MD 0.33 (95% CI ‐1.09 to 1.75); nine months, MD 0.12 (95% CI ‐1.38 to 1.62) (Christian 2006).
Analysis 4.12: Self‐competence – subscale 'physical appearance'
4.12. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 12: Self‐competence ‐ subscale 'physical appearance'
Subcategories 4.12.1: Three months; 4.12.2: Six months; and 4.12.3: Nine months This outcome is measured with the subscale 'Physical appearance' of the 'Self‐perception Profile for Children' (Harter 1985). No statistically significant differences were found between the two groups at: three months, MD 0.38 (95% CI ‐0.94 to 1.70); at six months, MD 0.45 (95% CI ‐0.82 to 1.72); and at nine months, MD 0.75 (95% CI ‐0.49 to 1.99) (Christian 2006).
Analysis 4.13: Self‐competence – subscale ‘behavioural conduct’
4.13. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 13: Self‐competence ‐ subscale 'behavioral conduct'
Subcategories 4.13.1: Three months; 4.13.2: Six months; and 4.13.3: Nine months This outcome is measured with the subscale 'Behavioural conduct' of the 'Self‐perception Profile for Children' (Harter 1985). No statistically significant differences between the groups at any point in time were observed: three months, MD 0.62 (95% CI ‐0.89 to 2.13); six months, MD 0.14 (95% CI ‐1.35 to 1.63); nine months, MD 0.16 (95% CI ‐1.24 to 1.56) (Christian 2006).
Analysis 4.14: Functional health status
4.14. Analysis.

Comparison 4: Educational problem‐solving and social skills intervention versus usual care, Outcome 14: Functional health status
Subcategory 4.14.1: three months; sub‐category 4.14.2: six months, and sub‐category 4.14.3: nine months This outcome was measured with 'The Functional Disability Inventory' (Walker 1991). Functional health status was not significantly different at all three points in time between the intervention and usual care group: three months, MD ‐0.05 (95% CI ‐1.86 to 1.76); six months, MD 0.17 (95% CI ‐1.43 to 1.77); nine months, MD 0.73 (95% CI ‐0.80 to 2.26) (Christian 2006).
2. Cognitive
2.1 Cognitive interventions to improve adherence
Comparision 5 ‐ Written self‐disclosure intervention versus standard care
One study with 39 participants was included (Taylor 2003).
PRIMARY OUTCOMES
Analysis 5.1: Subjective health status‐ PHQ
5.1. Analysis.

Comparison 5: Written self‐disclosure intervention versus standard care, Outcome 1: Subjective health status ‐ PHQ
Subcategory 5.1.1: Depression scale three‐month follow up Subcategory 5.1.2: Anxiety scale three‐month follow up Subcategory 5.1.3: Somatic complaints three‐month follow up Subcategory 5.1.4: Stressful life events three‐month follow up Subjective health status was measured with the ‘Patient Health Questionnaire (PHQ; Spitzer 1994). On the subscales 'Anxiety', MD ‐0.80 (95% CI ‐2.28 to 0.68), 'Depression', MD ‐1.50 (95% CI ‐4.11 to 1.11), and 'Stressful life events', MD 0.50 (95% CI ‐2.95 to 3.95), the two groups did not differ significantly at three‐months follow up. The subscale 'Somatic complaints' showed a statistically significant difference at the three‐month follow up (P = 0.0007) in favour of the written self‐disclosure intervention, MD ‐3.10 (95% CI ‐5.36 to ‐0.84) (Taylor 2003).
Analysis 5.2: Subjective health status –SF‐12
5.2. Analysis.

Comparison 5: Written self‐disclosure intervention versus standard care, Outcome 2: Subjective health status ‐ SF‐12
Subcategory 5.5.1: Physical health status three‐month follow up Subcategory 5.5.2: Mental health status three‐month follow up The subjective health status of the participating adolescents and adults with CF measured with the SF‐12 (Ware 1993). At the three‐month follow up, the two groups did not differ statistically on the 'Physical health' subscale, MD 0.50 (95% CI ‐5.90 to 6.90) or on the 'Mental health' subscale, MD 4.20 (95% CI ‐1.01 to 9.41) (Taylor 2003).
Analysis 5.3: FEV1 three‐months follow up FEV1 as a physiological disease severity measure did not show a statistically significant difference between the intervention and standard medical care group, MD ‐1.90 (95% CI ‐14.93 to 11.13) (Taylor 2003).
5.3. Analysis.

Comparison 5: Written self‐disclosure intervention versus standard care, Outcome 3: FEV1 3‐month follow up
Analysis 5.4: BMI three months follow up At the three‐month follow up, BMI as a physiological disease severity measure did not differ between the two groups, MD 0.30 (95% CI ‐1.35 to 1.95) (Taylor 2003).
5.4. Analysis.

Comparison 5: Written self‐disclosure intervention versus standard care, Outcome 4: BMI 3‐month follow up
SECONDARY OUTCOMES
Analysis 5.5: Health care utilization
5.5. Analysis.

Comparison 5: Written self‐disclosure intervention versus standard care, Outcome 5: Health care utilization
Subcategory 5.5.1: Outpatient utilization three‐month follow up Subcategory 5.5.2: Inpatient utilization three‐month follow up The data for health care utilization was retrieved from patient chart review. The number of outpatient visits and inpatient hospitalisation days were determined for the three months prior to and after enrolment in the study. There were no significantly different differences regarding outpatient visits, MD ‐0.80 (95% CI ‐1.93 to 0.33) and the inpatient hospitalisation days, MD ‐2.80 (95% CI ‐8.03 to 2.43) at three‐month follow up, although the authors reported a significant reduction of the number of days patients spent in hospital due to the intervention (Taylor 2003). This indicated the importance of reporting change scores.
Comparision 6 ‐ Motivational Interviewing via telephone (three‐month period) versus treatment as usual
One study with 35 participants was included (Quinn 2004).
PRIMARY OUTCOMES
Analysis 6.1: Quality of Life
6.1. Analysis.

Comparison 6: Motivational Interviewing via telephone (3‐month period) versus treatment as usual, Outcome 1: Quality of life
Subcategory 6.1.1: Physical functioning Subcategory 6.1.2: Social functioning Subcategory 6.1.3: Treatment issues Subcategory 6.1.4: Chest symptoms Subcategory 6.1.5: Emotional functioning Subcategory 6.1.6: Concerns for the future Subcategory 6.1.7: Interpersonal relationships Subcategory 6.1.8: Body image Subcategory 6.1.9: Career concerns This group of outcomes includes different domains of CF‐specific quality of life. These data were collected using the CFQOL[A1] (Gee 2000). We could only find a significant effect (P = 0.03) of the 'Motivational Interviewing' intervention on the domain ‘Body image’, MD 15.17 (95% CI 1.85 to 28.49). On the domain 'Emotional functioning', no significant effect (P = 0.07) of the intervention was observed, MD 7.88 (95% CI ‐0.53 to 16.29). Further, no significant group differences were found in: 'Physical functioning', MD 1.75 (95% CI ‐6.64 to 10.14); 'Social functioning', MD 6.78 (95% CI ‐3.55 to 17.11); 'Treatment issues', MD ‐3.09 (95% CI ‐20.10 to 13.92); 'Chest symptoms', MD 4.39 (95% CI ‐5.91 to 14.69); 'Concerns for the future', MD ‐1.30 (95% CI ‐18.06 to 15.46); 'Interpersonal relationships', MD 1.92 (95% CI ‐10.76 to 14.60); or 'Career concerns', MD 4.63 (95% CI ‐12.81 to 22.07) (Quinn 2004).
Analysis 6.2: Psychological distress
6.2. Analysis.

Comparison 6: Motivational Interviewing via telephone (3‐month period) versus treatment as usual, Outcome 2: Psychological distress
Subcategory 6.2.1: STAI‐Short Form Subcategory 6.2.2: HADS anxiety Subcategory 6.2.3: HADS depression Post intervention, there were no significant effects of the 'Motivational Interviewing' intervention on symptoms of anxiety measured with STAI ‐Short Form, MD ‐5.50 (95% CI ‐12.05 to 1.05), symptoms of anxiety measured with HADS, MD ‐0.65 (95% CI ‐2.76 to 1.46), or symptoms of depression measured with HADS, MD 0.07 (95% CI ‐1.64 to 1.78) (Quinn 2004).
Analysis 6.3:Lung function
6.3. Analysis.

Comparison 6: Motivational Interviewing via telephone (3‐month period) versus treatment as usual, Outcome 3: Lung function
Subcategory 6.3.1: FEV1 Subcategory 6.3.2: FVC We were unable to find any evidence that a 'Motivational Interviewing' intervention via telephone had any effect on the lung function post‐intervention in either: FEV1, MD 1.88 (95% CI ‐11.44 to 15.20); or in FVC, MD ‐2.07 (95% CI ‐14.49 to 10.35) (Quinn 2004).
SECONDARY OUTCOME
Analysis 6.4:% Adherence behaviour
6.4. Analysis.

Comparison 6: Motivational Interviewing via telephone (3‐month period) versus treatment as usual, Outcome 4: % adherence behavior post intervention
There were no significant group differences observed in rate of adherence post‐intervention, MD 4.32 (95% CI ‐26.77 to 35.41) (Quinn 2004).
2.2 Cognitive interventions associated with transplantation
Comparision 7 ‐ Decision aid for patients considering lung transplantation versus usual care
One study with 149 participants was included (Vandemheen 2009).
SECONDARY OUTCOMES
Analysis 7.1: Participants’ knowledge
7.1. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 1: Participants' knowledge
Subcategory 7.1.1: Three‐week follow up Subcategory 7.1.2: Change in knowledge (week three to baseline) We were able to find evidence that a decision aid for patients considering lung transplantation improves patients’ knowledge: three‐week follow‐up score, MD 0.98 (95% CI 0.66 to 1.31 (P < 0.00001); and change in knowledge, MD 0.94 (95% CI 0.53 to 1.35) (P < 0.0001) (Vandemheen 2009).
Analysis 7.2: Patients' expectations
7.2. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 2: Patient expectations
Subcategory 7.2.1: Three‐week follow up Subcategory 7.2.2: Change in expectation score (week three to baseline) Realistic expectations scores were used to measure patients’ perceptions of the level of risk for surgery and probabilities of survival. Significant differences between the two groups were found for scores at the three‐week follow up, MD 0.73 (95% CI 0.51 to 0.95) (P < 0.00001) and change scores, MD 0.66 (95% CI 0.37 to 0.95) (P < 0.0001) in favour of the decision aid intervention (Vandemheen 2009).
Analysis 7.3: Decisional conflict
7.3. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 3: Decisional conflict
Subcategory 7.3.1: Total score three‐week follow up Subcategory 7.3.2: Subscale ‐ certainty three‐week follow up Subcategory 7.3.3: Subscale ‐ informed three‐week follow up Subcategory 7.3.4: Subscale ‐ values three‐week follow up Subcategory 7.3.5: Subscale ‐ support three‐week follow up Subcategory 7.3.6: Subscale ‐ satisfaction with decision three‐week follow up Subcategory 7.3.7: Change in total score (week three to baseline) This outcome was measured with the 'Decisional Conflict Scale' (O'Connor 1995). The scale includes five subscales and one total score. Scores range from 0 (low decisional conflict) to 100 (high decisional conflict). A minimal clinically important difference was established: patients scoring of 25 or less tend to make decisions.
Significant differences between both groups in favour of the decision aid intervention were found for the total score at three‐week follow up, MD ‐8.80 (95% CI ‐13.70 to ‐3.90) (P = 0.0004); the subscale 'certainty', MD ‐10.00 (95% CI ‐18.63 to ‐1.37) (P = 0.02); the subscale 'informed', MD ‐12.70 (95% CI ‐17.77 to ‐7.63) (P < 0.00001); the subscale 'values', MD ‐6.90 (95% CI ‐13.12 to ‐0.68) (P = 0.03); the subscale 'support', MD ‐7.60 (95% CI ‐12.15 to ‐2.75) (P = 0.002) and the subscale 'satisfaction with decision', MD ‐7.50 (95% CI ‐13.42 to ‐1.58) (P = 0.01). In contrast, no significant differences were observed in the change in the total score from baseline to three‐week follow up, MD 3.30 (95% CI ‐2.28 to 8.88) (P = 0.25). All means across these scales, at the three‐week follow up, fell under the MID cut‐off of 25 points indicating absence of relevant decisional conflict, except the subscale 'certainty' (Decision aid group (mean (SD)): 26.4 (25.9); usual care group: 36.4 (27.8)) (Vandemheen 2009).
Analysis 7.4: Patient's stated choice three‐week follow up
7.4. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 4: Patient's stated choice at 3‐week follow‐up
Subcategory 7.4.1: Referral for transplantation Subcategory 7.4.2: Declined referral for transplantation Subcategory 7.4.3: Unsure For all subcategories no significant differences in the rate of patients were found: for 'Referral for transplantation', OR 0.85 (95% CI 0.43 to 1.72); for 'Declined referral for transplantation', OR 1.69 (95% CI 0.61 to 4.71); and for 'Unsure', OR 0.88 (95% CI 0.39 to 2.00) (Vandemheen 2009).
Analysis 7.5: Durability of patient decision (12‐month follow up)
7.5. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 5: Durability of patient decisions (12‐month follow up)
The outcome also considered whether patients chose the same option as reported at three‐week follow up or not. No significant difference between groups was found on durability of the patients’ decisions observed at 12‐month follow up, OR 2.47 (95% CI 0.94 to 6.52) (P = 0.06) (Vandemheen 2009).
Analysis 7.6: Preparation for decision‐making three‐week follow up
7.6. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 6: Preparation for decision‐making (3‐week follow up)
This outcome was measured with a questionnaire asking whether patients felt prepared to make a decision by using the provided information. The two groups differed significantly at the three‐week follow up, providing evidence that the participants in the decision aid group felt better prepared to make a decision, MD 11.30 (95% CI 2.95 to 19.65) (P = 0.009) (Vandemheen 2009).
Analysis 7.8:Values congruence
7.8. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 8: Acceptability
The choices of patients were consistent with their values across both groups (decision aid versus usual care) (Vandemheen 2009, p.765). Patients choosing a referral for lung transplantation rated living longer as very important, OR 1.80 (CI 95% 1.02 to 3.18) (P = 0.04); avoiding hassle, stress, and worry of lung transplantation less important, OR 0.77 (95% CI 0.60 to 0.99) (P = 0.047), in comparison to those declining lung transplantation (Vandemheen 2009).
Analysis 7.8: Acceptability
Subcategory 7.8.1: Received the right amount of information Subcategory 7.8.2: Materials were helpful in helping to arrive at a decision Subcategory 7.8.3: Definitely or probably recommend the materials Both groups received material with information on lung transplantation. Regarding acceptability of this information, a significant higher number of participants reported that they received the right amount of information, OR 4.47 (95% CI 2.22 to 9.02) (P = 0.008) and would definitely or probably recommend the materials they received, OR 4.93 (95% CI 1.04 to 23.32) (P = 0.04) in the decision aid than comparison group. In contrast to the authors, we did not find a significant difference between groups in whether the material was perceived as helpful in arriving at a decision, OR 1.72 (95% CI 0.89 to 3.33) between the two groups (Vandemheen 2009).
3. Family systems or Systemic
Comparision 8 ‐ Community‐based support program versus contact telephone number
One study with 13 participants was included (Chernoff 2002).
PRIMARY OUTCOMES
Analysis 8.1: Psychiatric Symptom Index ‐ Anxiety subscale ‐ mothers
8.1. Analysis.

Comparison 8: Community‐based support program versus contact telephone number, Outcome 1: Psychiatric Symptom Index ‐ Anxiety subscale ‐ mothers
Subcategory 8.1.1: Mother's anxiety ‐ 12 months post‐baseline This is an 11‐item anxiety subscale which returns a standardised score with a possible range of 0 to 100 (Ilfeld 1976). Although the reported effects for the whole group of carers of children with a chronic illness in the source article showed reduced anxiety following the intervention, when comparisons were made for the subgroup of carers with a child with CF, no significant difference was found between groups, MD ‐3.60 (95% CI ‐18.14 to 10.94) at 12‐month post‐baseline. This subgroup was small and unlikely to demonstrate a clear effect, however it served to illustrate the pattern of the data within the group (Chernoff 2002).
4. Psychodynamic
No comparisons to report.
5. Other interventions
Comparision 9 ‐ Biofeedback assisted breathing re‐training (BRT) versus biofeedback assisted relaxation training (RLXT)
One study with 26 participants was included (Delk 1994).
PRIMARY OUTCOME
Analysis 9.1: Pulmonary function (the study author's primary outcome indicator) Subcategory 9.1.1: FEV1 (litres per second) (absolute values) Subcategory 9.1.2: FVC (litres) (absolute values) Subcategory 9.1.3: FEF25-75% (litres per second) (absolute values) Two of three measures of lung function were better in the intervention than control group post‐study (at four weeks). These were FEV1, MD 0.54 (95% CI 0.15 to 0.93) (P = 0.007) and FEF25-75%, MD 0.67 (95% CI 0.10 to 1.24) (P = 0.02). The FVC measure was not significantly different between the groups at this time point, MD 0.87 (95% CI ‐0.09 to 1.83). This set of findings shows that biofeedback‐assisted breathing re‐training is associated with improvement in some measures of spirometric lung function in adolescents with CF. These findings have clinical significance for the combined effects of physiotherapy and biofeedback (Chernoff 2002).
9.1. Analysis.

Comparison 9: Biofeedback assisted breathing re‐training (BRT) versus biofeedback assisted relaxation training (RLXT), Outcome 1: Pulmonary function
Comparision 10 ‐ Massage therapy versus bedtime reading control
One study with 20 participants was included (Hernandez‐Reif 1999).
PRIMARY OUTCOMES
Analysis 10.1: Well‐being Three measures were utilised; one was aimed at the parents' emotional state (subcategory 10.1.1) and the remaining two assessed the child's emotional state (sub‐categories 10.1.2 and 10.1.3).
10.1. Analysis.

Comparison 10: Massage therapy versus bedtime reading control, Outcome 1: Well‐being
Subcategory 10.1.1: State Trait Anxiety Inventory for parent (STAI) (Spielberger 1970) This inventory measures anxiety of adults as an emotional state, i.e. the anxiety that a person experiences under certain conditions, and personality trait, i.e. a relatively enduring personality characteristic that reflects the individual's propensity to respond with anxiety to a broad range of conditions. The measure has 20 items and has acceptable concurrent validity and internal consistency. This emotional outcome measure showed a significant effect 30 days after completing the intervention in favour of massage for the parent, MD ‐9.10 (95% CI ‐17.84 to ‐0.36) (P = 0.04) in reducing anxiety (Hernandez‐Reif 1999).
Subcategory 10.1.2: State Trait Anxiety Inventory for children (STAIC) (Spielberger 1973) This is an adaptation of the adult STAI that has already been described above (Outcome 3.1.1) for children of school age and adolescents who are below average reading level. The scale has 20 items with acceptable internal validity. This emotional outcome measure showed a significant effect 30 days after completing the intervention in favour of massage for the child, MD ‐8.20 (95% CI ‐12.36 to ‐4.04) (P = 0.0001) in reducing anxiety (Hernandez‐Reif 1999). Subcategory 10.1.3: Profile of Mood States (POMS) (McNair 1971) (child only) This measure is a modification for children of the original POMS designed for adults; items that focused on depressed symptoms were simplified based on the POMS manual. Only the depression subscale was utilised, which comprised 19 adjectives describing how the child felt right now, rated on a 5‐point rating scale. The measure has good face validity for measuring depressed mood in children. Significant improvements in this outcome measure were found at post‐day 30 in favour of massage for overall mood state, MD ‐5.50 (95% CI ‐8.81 to ‐2.19) (P = 0.001) (Hernandez‐Reif 1999).
In summary, these three emotional outcome measures showed significant effects in favour of: massage for the child and the parent with respect to anxiety and mood state in children, suggesting that massage reduced anxiety levels in both the parent and the child and reduced the child's depressive symptoms. Although the sample was small, the consistency of these results indicate a clinically important result, with implications for managing affective states.
Analysis 10.2: Pulmonary function
10.2. Analysis.

Comparison 10: Massage therapy versus bedtime reading control, Outcome 2: Pulmonary function
Subcategory 10.2.1: Peak air flow (PEFR) at day 1 Subcategory 10.2.2: Peak air flow (PEFR) at day 30. At day 1 the two groups did not differ statistically on their pulmonary function, MD 16.50 (95% CI ‐64.04 to 97.04). We did not find that massage therapy affected pulmonary function at day 30, MD 53.90 (95% CI ‐43.27 to 151.07) (Hernandez‐Reif 1999).
Comparision 11 ‐ Music therapy versus familiar music and no audiotape control
One study with 20 participants was included (Grasso 2000).
PRIMARY OUTCOMES
Analysis 11.1: Enjoyment (the study author's primary outcome indicator) Subcategories 11.1.1 and 11.1.2: Enjoyment ‐ parent & child This outcome utilised an ad hoc seven point bi‐polar rating scale measuring enjoyment of the routine. These outcome measures are highly subjective and their validity has not been tested. Parents ranked their own enjoyment and their perception of their child's enjoyment of chest physiotherapy (CPT) in the comparison groups (music therapy versus familiar music tape and no audiotape) on these scales. Significant effects were found in favour of music therapy for enjoyment during CPT by both child, MD 2.05 (95% CI 0.51 to 3.59) (P = 0.009) and parent, MD 1.20 (95% CI 0.22 to 2.18) (Grasso 2000).
11.1. Analysis.

Comparison 11: Music therapy versus familiar music and no audiotape control, Outcome 1: Enjoyment
Analysis 11.2: Cognitive (the study author's primary outcome indicator) Subcategory 11.2.1: Parent perception of time This measured the parents' perception of how long they felt it took to complete the CPT in minutes. The score was compared to the actual time the parent recorded on a diary chart. The MD had wide CIs, so we did not find any evidence of an effect here, MD ‐4.70 (95% CI ‐16.93 to 7.53) (Grasso 2000).
11.2. Analysis.

Comparison 11: Music therapy versus familiar music and no audiotape control, Outcome 2: Cognitive
Comparision 12 ‐ Self‐hypnosis versus control
One study with twelve participants was included (Belsky 1994).
We were not able to perform quantitative analysis based on the data the authors provided in their report and were not able to contact the authors to provide detailed data. Therefore we report the results the authors described.
PRIMARY OUTCOMES
Analysis 12.1: Anxiety
12.1. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 1: Anxiety: State Trait Anxiety Inventory for Children (STAIC)
| Anxiety: State Trait Anxiety Inventory for Children (STAIC) | |
| Study | Narrative reported results |
| Belsky 1994 | No group differences were observed (U=15, p>.05) for State Anxiety. A significant difference between experimental and control group was found for Trait Anxiety (U=1.5, p<.004) with change scores reflecting decreases in Trait Anxiety for the experimental group and increases for the control group. |
The authors reported the following results: No group differences were observed (U = 15, P > 0.05) for 'State Anxiety'. A significant difference between experimental and control group was found for 'Trait Anxiety' (U = 1.5, P < 0.004) with change scores reflecting decreases in 'Trait Anxiety' for the experimental group and increases for the control group (Belsky 1994).
Analysis 12.2: Impact on 'Family Scale'
12.2. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 2: Impact on Family Scale
| Impact on Family Scale | |
| Study | Narrative reported results |
| Belsky 1994 | No group differences were observed at post‐intervention. |
The authors reported that there were no group differences observed (Belsky 1994).
Analysis 12.3: 'Child Behavior Checklist'
12.3. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 3: Child Behavior Checklist
| Child Behavior Checklist | |
| Study | Narrative reported results |
| Belsky 1994 | No group differences were observed at post‐intervention. |
The authors reported that there were no group differences observed (Belsky 1994).
Analysis 12.4: Average peak flow expiratory flow rate
12.4. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 4: Average peak expiratory flow rate (PEFR)
| Average peak expiratory flow rate (PEFR) | |
| Study | Narrative reported results |
| Belsky 1994 | The mean PEFR score showed an increase of approximately 76 points from pre‐ to post‐test in the experimental group, whereas it dropped approximately 12 points in the control group. This difference proved to be significant (U=0, p<.001). The difference between the two groups was also significant at the 4‐month follow up. |
The authors reported the following result: The mean PEFR score showed an increase of approximately 76 points from pre‐ to post‐test in the experimental group, whereas it dropped approximately 12 points in the control group. This difference proved to be significant (U = 0, P < 0.001). The difference between the two groups was also significant at the four‐month follow up (Belsky 1994).
Analysis 12.5: Height and weight
12.5. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 5: Height and weight
| Height and weight | |
| Study | Narrative reported results |
| Belsky 1994 | No group differences were observed (U=125, p>.05) at 4 month follow up. |
No group differences were observed by the authors (U = 125, P > 0.05) at four month follow up (Belsky 1994).
SECONDARY OUTCOMES
Analysis 12.6: Rating of the parents' assessment of the child's illness
12.6. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 6: Rating of the parents' assessment of the child's illness
| Rating of the parents' assessment of the child's illness | |
| Study | Narrative reported results |
| Belsky 1994 | No group differences were observed (U=15, p>.05) at post‐intervention. |
No group differences were observed (U = 15, P > 0.05) (Belsky 1994).
Analysis 12.7: 'Locus of Control'
12.7. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 7: Locus of Control (LOC): Nowicki Strickland (1973) LOC for Children
| Locus of Control (LOC): Nowicki Strickland (1973) LOC for Children | |
| Study | Narrative reported results |
| Belsky 1994 | Significant differences were found in change of Locus of Control (U=0, p=.001) between experimental and control group: The scores of the experimental group decreased, which indicates more internality. |
Significant differences were found by the authors in change of 'Locus of Control' (U = 0, P = 0.001) between the experimental and the control groups: scores of the experimental group decreased, which indicates more internality (Belsky 1994).
Analysis 12.8: 'Health Locus of Control'
12.8. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 8: Health Locus of Control: Children's Health Locus of Control (HLOC)
| Health Locus of Control: Children's Health Locus of Control (HLOC) | |
| Study | Narrative reported results |
| Belsky 1994 | Significant differences were found in change of Children’s Health Locus of Control (U=0, p=.001) between experimental and control group: The scores of the experimental group increased, which means that children’s internal expectancies related to health improved more in the experimental group. |
Significant differences were found in change of Children’s Health Locus of Control (U = 0, P = 0.001) between the experimental and control groups. The scores of the experimental group increased, which means that children’s internal expectancies related to health improved more in the experimental group (Belsky 1994).
Analysis 12.9: Self‐concept
12.9. Analysis.
Comparison 12: Self‐hypnosis versus control, Outcome 9: Self‐concept: Piers Harris Children's Self‐Concept Scale
| Self‐concept: Piers Harris Children's Self‐Concept Scale | |
| Study | Narrative reported results |
| Belsky 1994 | Self‐concept scores improved significantly more in the experimental group than in the control group (U=1.5, p=.003) at post‐intervention. |
Self‐concept scores improved significantly more in the experimental group than control groups (U = 1.5, P = 0.003) (Belsky 1994).
Comparision 13 ‐ Home telemedicine for patients awaiting transplantation versus usual care
One study with seven participants was included here (Wilkinson 2008).
We were not able to perform quantitative analysis based on the data the authors provided in their report and did not receive a response on our query for detailed data. However, we describe the provided results of the report.
PRIMARY OUTCOMES
Analysis 13.1: Quality of life
13.1. Analysis.
Comparison 13: Home telemedicine for patients awaiting transplantation versus usual care, Outcome 1: Quality of Life: Cystic Fibrosis Quality of Life Questionnaire (Gee 2000)
| Quality of Life: Cystic Fibrosis Quality of Life Questionnaire (Gee 2000) | |
| Study | Narrative reported results |
| Wilkinson 2008 | Telemedicine group a significant improvement in the subjects’ perception of body image (P=.002). |
The quality of life was measured with the 'Cystic Fibrosis Quality of Life Questionnaire' (Gee 2000). The authors reported that the intervention group showed a significant improvement in the subjects’ perception of body image (P = 0.002) (Wilkinson 2008).
Analysis 13.2: Anxiety
13.2. Analysis.
Comparison 13: Home telemedicine for patients awaiting transplantation versus usual care, Outcome 2: Anxiety: Beck Anxiety Inventory (Beck 1988) + Borg anxiety scale (Borg 1982)
| Anxiety: Beck Anxiety Inventory (Beck 1988) + Borg anxiety scale (Borg 1982) | |
| Study | Narrative reported results |
| Wilkinson 2008 | "There was no significant change in anxiety before and after link‐up for the telemedicine group or before and after clinic attendance for either group. There was no difference in the change of anxiety from pre to post link‐up for the telemedicine group or for the control group in pre and post clinic attendance." (Wilkinson 2008, p.184) |
The authors reported: "There was no significant change in anxiety before and after link‐up for the telemedicine group or before and after clinic attendance for either group. There was no difference in the change of anxiety from pre to post link‐up for the telemedicine group or for the control group in pre and post clinic attendance" (Wilkinson 2008, p.184).
Analysis 13.3: Depression
13.3. Analysis.
Comparison 13: Home telemedicine for patients awaiting transplantation versus usual care, Outcome 3: Depression: Beck Depression Inventory (Beck 1987)
| Depression: Beck Depression Inventory (Beck 1987) | |
| Study | Narrative reported results |
| Wilkinson 2008 | "At the end of the study there was a higher reporting of depression, which was associated with a higher reporting of anxiety, in both groups." (Wilkinson 2008, p.184) |
The authors reported: "At the end of the study there was a higher reporting of depression, which was associated with a higher reporting of anxiety, in both groups" (Wilkinson 2008, p.184).
SECONDARY OUTCOME
Analysis 13.4: Coping of carers
13.4. Analysis.
Comparison 13: Home telemedicine for patients awaiting transplantation versus usual care, Outcome 4: Coping of carers: COPE questionnaire (Carver 1997)
| Coping of carers: COPE questionnaire (Carver 1997) | |
| Study | Narrative reported results |
| Wilkinson 2008 | No significant differences between the two groups were found. |
Regarding the coping of the participating carers no significant differences between the two groups were found by the authors (Wilkinson 2008).
Analysis 13.5: health care utilization
13.5. Analysis.
Comparison 13: Home telemedicine for patients awaiting transplantation versus usual care, Outcome 5: Health care utilization: seven‐point questionnaire (number of visits, general practitioner, courses of IV antibiotics, length of hospital inpatient stay, visits either to hospital or GP)
| Health care utilization: seven‐point questionnaire (number of visits, general practitioner, courses of IV antibiotics, length of hospital inpatient stay, visits either to hospital or GP) | |
| Study | Narrative reported results |
| Wilkinson 2008 | No significant differences between the two groups were observed. |
The authors reported that the two groups did not show any significant differences on the following outcomes: number of visits; general practitioner; courses of IV antibiotics; length of hospital inpatient stay; or visits either to hospital or GP (Wilkinson 2008).
Comparision 14 ‐ Dance/Movement therapy for adults versus control
One study with 14 participants was included here (Goodill 2005).
We were not able to perform quantitative analysis based on the data the authors provided in their report and we did not receive detailed data from the authors. Therefore, we report the results the author described in the published paper (Goodill 2005).
PRIMARY OUTCOMES
Analysis 14.1: Mood state
14.1. Analysis.
Comparison 14: Dance/movement therapy for adults versus control, Outcome 1: Mood state: ’The Profile of Mood States’ (POMS; Mc Nair 1992)
| Mood state: ’The Profile of Mood States’ (POMS; Mc Nair 1992) | |
| Study | Narrative reported results |
| Goodill 2005 | Scores were analysed performing a repeated measures ANOVA: Found within‐subjects interaction of Time, group, and Gender on Levels of Confusion (F (2,20) =5.99, P=.007, eta2=.231). Men in the treatment group showed the strongest effect. |
The mood state was measured with 'The Profile of Mood States' (POMS) (McNair 1992).The author described that the repeated measures ANOVA revealed a statistically significant effect of time group, and gender on the subscale 'confusion‐bewilderment' (F (2,20) = 5.99, P = 0.007, eta2 = 0.231). Men participating in the treatment group showed the strongest effect regarding changes in the mood state of participants (Goodill 2005).
SECONDARY OUTCOMES
Analysis 14.2: Adherence to exercise and nutrition regimen
14.2. Analysis.
Comparison 14: Dance/movement therapy for adults versus control, Outcome 2: Adherence to exercise and nutrition regimen
| Adherence to exercise and nutrition regimen | |
| Study | Narrative reported results |
| Goodill 2005 | No significant differences on exercise at post‐intervention or follow‐up (1 month later) and not for nutrition at post‐intervention. But significant differences at follow‐up between groups on adherence to nutrition regimes (Chi2 (1, N=23) = 4.485, P=.03) with lower levels in the control group. |
The author reported the following results: No significant differences on exercise or nutrition post‐intervention or one‐month follow up (one month later). However, significant differences were found at follow up between groups on adherence to nutrition regimens (Chi2 (1, N = 23) = 4.485, P = 0.03), with lower levels of adherence in the control group (Goodill 2005).
Analysis 14.3: Body image
14.3. Analysis.
Comparison 14: Dance/movement therapy for adults versus control, Outcome 3: Body Image: Human figure drawing task
| Body Image: Human figure drawing task | |
| Study | Narrative reported results |
| Goodill 2005 | No group differences were observed. |
The author stated that no group differences were observed (Goodill 2005).
Discussion
Summary of main results
Psychological interventions for patients with CF are heterogenous in many respects, including their target populations (caregivers, young children, adults), focus of the intervention (e.g., adherence, nutrition, depression and anxiety, improved health‐related quality of life), age and stage of disease, and outcome measures. This makes it extremely difficult to compare and evaluate the efficacy of these interventions using meta‐analytic techniques. In addition, the range of psychosocial challenges faced by this population, which include high treatment burden, poor adherence, risk for depression and anxiety, transition to adult care, and ultimately, declining health, increases the difficulty of accumulating intervention data in a particular area. A recent consensus conference, organized by the European Cystic Fibrosis Society, published a prioritised list of the key issues that should be addressed in research in the next decade (Bradley 2012), along with reviews and recommendations of the best outcome measures in allied health professionals and nursing research. This included next to adherence, physical activity, nutritional interventions as well as interventions for newborns. Developing and seeking funding for psychological intervention studies in high priority areas would be one way to build the evidence base that is needed.
In terms of the complex psychological issues in coping with CF and its treatment regimen, only limited data on interventions is available. The retrieved primary studies used interventions with generic approaches utilized in the context of patients with CF, e.g. providing motivational interviewing to adult patients via telephone, behavioral group interventions for children and caregivers with nutritional problems, or interventions providing a decision aid for patients considering lung transplantation.
Given the heterogeneity of the interventions and outcome measure, it is not currently possible to identify an overall effect of psychological interventions for patients with CF and their caregivers. For approximately half of the retrieved studies, some positive evidence fort he intervention was found. Most of the successful interventions were disease‐specific.
Interventions with the most evidence were those that combined educational and behavioral techniques to improve dietary intake and nutritional status. The combined intervention was compared to nutrition education alone. By pooling the results of three studies, it was shown that these behavioral interventions were effective in increasing calorie intake in underweight children with CF in the short‐term (Stark 2003; Powers 2005; Stark 2009). Additionally, one of the three studies showed that this intervention was also effective in increasing the proportion of estimated energy requirements of these children over a short period of time. Longer studies are needed to see if these effects are maintained over time or if 'booster sessions' are needed.
Promising interventions were reported in single studies. A combination of an educational session plus a decision‐making aid (Vandemheen 2009) improved patients' knowledge of and expectations about the transplant, with decisional conflict reduce in the short‐term
Furthermore, on the basis of three single studies, we found some evidence to suggest that biofeedback, massage and music therapy might be a helpful adjunct during physical therapy. Biofeedback‐assisted breathing re‐training (Delk 1994) compared to biofeedback assisted relaxation training over the course of eight sessions was superior in enhancing lung function, especially FEV1. Massage therapy compared to bedtime reading (Hernandez‐Reif 1999) was superior to reducing symptoms of anxiety in parents and children, and symptoms of depression in children.
Overall completeness and applicability of evidence
In relation to the broad area of psychosocial challenges in CF, only a few studies with rigorous research designs were identified for this review. A number of clinically relevant psychological issues have not yet been studied. Of note, few interventions have been developed or evaluated to address parental responses to newborn screening, transitions to adult care, or palliative care. In addition, areas of high clinical relevance across stages of the disease, such as adherence, depression and anxiety, and health‐related quality of life are underrepresented or currently absent from the literature.
For example, despite published national data indicating that caregivers and other members of the family are at high risk for psychological symptoms due to the illness (Besier 2011a; Besier 2011b), no studies addressing these issues were available and eligible for inclusion. Moreover, no studies investigating the application of evidence‐based psychotherapy approaches to prevent or reduce comorbid psychological distress were found. In sum, although recent evidence has demonstrated that there are significant psychological needs to be met for both caregivers and patients, the evidence on psychological interventions applied to this population is highly incomplete.
Interventions were delivered in a variety of modes, including individual, group, face‐to‐face, and telephone or Internet‐based communication. Given recent infection control guidelines (Kerem 2005; Miroballi 2012), group‐based psychological interventions for patients are not recommended. More research is needed on the efficacy and effectiveness of psychological interventions delivered via the Internet using Skype or other video‐conferencing software.
Importantly, patients and family members face a number of barriers in attempting to access psychological interventions, including:
distance to the centre;
insurance coverage and cost;
availability of a psychologist or trained mental health provider on the CF team or in hospital; and
availability of a trained provider in the patient’s local community.
Applicability of group interventions into the individual outpatient treatment setting requires availability of a number of eligible families at the same time.
Another critical issue is the availability of trained mental health providers on CF specialty teams. Data from the iCARE study suggest that CF team members need additional training in counselling, but can quickly be trained to deliver a structured, behavioral intervention with brief phone supervision. If we are hoping to integrate highly structured, manualised psychological interventions into CF care, we will have to address the need for mental health training and expertise within CF teams. Currently, there are only a few comprehensive, patient‐centred CF Centers that routinely collect and display data on the psychosocial functioning of its CF cohort (e.g. Sophia Children’s Hospital, ErasmusMC, Netherlands; CF Center, Medical University Innsbruck, Austria). Implementation of evidence‐based psychological treatments will require carefully trained, specialized providers to implement disease‐specific, evidence‐based treatments. It is possible that these interventions, aimed at improving adherence for example, may provide costs savings in the long‐run by reducing pulmonary exacerbations and therefore, inpatient hospitalisation costs.
Furthermore, the improvements of therapy and care in CF lead to the assumption that there is a contemporary era of care effect in the included studies. Hence, the validity of newer studies is estimated to be higher for today’s individuals with CF.
Footnote: It is important to note that this review did not include data on primarily educational interventions because they were included in a separate review (Savage 2011). However, since many psychosocial interventions combine education with other strategies (e.g., problem‐solving, MI) it is somewhat artificial to draw the line between purely educational versus psychological interventions.
Quality of the evidence
The evidence presented in this review, which is based on data from only a few very heterogenous studies (16), involving a total of 556 participants, is limited by the quality of the available research. One major challenge in conducting high quality research in CF, which is a rare disease, is having large enough samples. In this review, more than half of the included studies had small sample sizes given their aims and only a few studies conducted a formal power calculation to ensure that they had the necessary sample to detect statistically significant differences. Due to the small number of studies and limited number of participants we were not able to explore whether the effect varied by type of intervention, socioeconomic status, age of the individual, or stage of the disease. Given that CF is a rare disease, larger samples with more diverse patients will require implementation and analysis of multicentre studies. This increases the complexity of obtaining funding and maintaining standardization of procedures and methods, but is likely a critical step in evaluating the efficacy of psychological interventions. Several multi‐site studies are currently in process (e.g. Quittner 2011; Quittner 2012) and will likely report their results in the next two years.
The assessment of risk of bias indicated that the quality of many of the earlier intervention studies, in terms of study design and methods, fell far short of typical requirements. Several important studies were excluded from previous versions of the review, because participants were not randomised (e.g. Bartholomew 1997; Hains 1997; Johnson 2001).
We would also like to acknowledge that the quality of RCTs is improving and that most of the newly included studies paid more rigorous attention to the design, methods and reporting standards for RCTs than those in the past. Missing information on important details of the study design and procedures was associated with unclear risk of bias. Although most authors reported some information about the randomisation procedure, few provided enough detailed information to determine whether allocation concealment occurred. Although it is generally not feasible to blind therapists to the type of psychological intervention being delivered or participants from whether they are in the treatment or control arm of the study, it is always possible to blind assessors; however, this aspect of blinding was only reported in a few studies. It was also more difficult to detect significant differences between groups because data on changes in the outcome variables were not provided. Additionally, three studies of interest did not provide enough quantitative data to perform analysis. Another weakness in reporting is that some of the studies did not describe whether they pre‐specified a primary outcome. Psychological interventions often have several endpoints, making it critical to specify which outcome variable is the primary and which are considered secondary. Another common limitation is inadequate correction for multiple tests in the analytic phase. Most of the retrieved studies, especially the newer ones, provided sufficient information about the intervention (duration, qualification of therapists etc.) or the treatment manuals were available online (e.g. Stark 2009; Vandemheen 2009). However, just four of the studies reported some actions or methods to ensure treatment fidelity (Grasso 2000; Chernoff 2002; Quinn 2004; Stark 2009).
Quality indicators should have been grouped for the purposes of a sensitivity analysis. However, we could not distinguish between levels of quality (e.g., good, fair, poor) due to the fact that most studies were single studies Replications by independent groups are missing for the majority of positive findings. Currently, the body of evidence is of insufficient quality and quantity to draw any firm conclusions.
Potential biases in the review process
We are confident to have identified all relevant studies guided by the objectives of this review. The selection of studies, the assessment of risk of bias, and data extraction were conducted independently by two authors. Potentially disagreements were discussed within the working group. A potential conflict of interest should be mentioned. The authors LG and AQ are investigators of some of the considered interventions studies for this review. Unfortunately, for three of the included studies, we were only able to describe the results because of insufficient quantitative data. However, most of the authors we contacted were extremely helpful and provided a great deal of data and information. There are several ongoing studies that should be ready to publish their results fairly soon (Quittner 2000; Quittner 2011; Quittner 2012; Riekert 2012).
Agreements and disagreements with other studies or reviews
The findings and respectively the drawn evidence for psychological interventions for patients with CF and their families are in line with other reviews focusing research on psychological interventions for chronically ill children, adolescents, and adults. In common with other Cochrane Reviews, e.g. on psychological interventions for children or adults with asthma (Yorke 2005; Yorke 2006) the evidence base is still lacking in quantity and quality.
In terms of other chronic illnesses, such as cancer in children and adolescents, more sufficient data of studies on psychological interventions are available to draw conclusions on the evidence in this particular illness. Two reviews highlight the support for the evidence of psychological interventions for caregivers and siblings in some of the relevant outcomes (Pai 2006; Prchal 2009). In a more recent systematic review (Sansom‐Daly 2012), the most positive results were found for skills‐based, multiple‐session interventions for young adults with chronic illnesses; this is comparable to the results we found for the behavioral interventions for young children with feeding problems.
In general, most authors of reviews, regardless systematically or not, state that there is a need for greater rigor in designing, performing and reporting intervention studies for patients with a chronic illness and their families more rigorously (e.g. Plante 2001; Beale 2006; Seitz 2009; Sansom‐Daly 2012).
Authors' conclusions
Implications for practice.
The studies of CF‐specific interventions developed especially for patients with CF, such as interventions for those considering lung transplantation or behavioral interventions for children with feeding problems are supported by the evidence to date. Providing an additional decision aid to patients considering lung transplantation was associated with improvements patient's knowledge and realistic expectations and enabled the patient to reduce decisional conflict. The structured decision aid used in this trial is available via the Internet (in English and French language). After failure of nutrition counselling alone to improve calorie intake, behavioral interventions aiming at improving intake should be considered, in the short‐term, as an alternative to tube‐feeding with its medical and psychosocial side effects (e.g. Oliver 2004; Van Biervliet 2004; Hazel 2006). Multi‐component, behavioural interventions have shown the greatest promise in improving adherence, one of the greatest challenges faced by both patients and care teams. Current RCTs testing these strategies in CF are currently underway and should report results within the next year or two. Other interventions, such as biofeedback combined with physiotherapy are promising, although there are too few studies at this point to provide clear evidence. Therefore, we cannot currently recommend these interventions for implementation in clinical practice. Importantly, to utilize these interventions, psychologists or other members of the multidisciplinary team must be trained in their implementation. Health care providers with these skills are often not available in CF centres and thus, it may also be necessary to increase the behavioural expertise of providers or develop clinical pathways to referrals outside the CF centre.
Additionally, practitioners and clinical researchers should also be aware of other systematic reviews that have demonstrated that psychological interventions are beneficial. These include behavioural strategies to cope with procedural pain and distress (e.g., anxiety about needles, pic line insertions). These studies are not focused exclusively on patients with CF and thus, were not the focus of the current review (Uman 2013).
Implications for research.
To summarise our findings: high quality efficacy and effectiveness studies of psychological interventions for CF are becoming more common, but there is an urgent need for studies with larger samples, more diverse characteristics (e.g., inclusion of minorities), replication of promising results, and better reporting of the methodological aspects of these studies. We are encouraged to report that some of the studies in this review utilized this approach and more are in the pipeline. We must also acknowledge that conducting large‐scale, multicentre studies requires large funding sources, complex coordination of procedures and methods across sites, and complex multi‐level modelling of both predictors and primary and secondary outcomes. To move this effort forward may require leadership from the various national CF associations in Europe and the US, and collaborative efforts by clinical researchers testing psychological interventions. The International TIDES study on comorbid depression and anxiety in patients with CF and their caregivers (seehttp://www.tides-cf.org/) with multiple funding in nine different countries, coordinated by one international study centre, involving multiple researchers and clinicians across the nine countries and finally in a joint effort collecting data of more than 6000 patients (Quittner 2012b) could serve as a model for effective international research collaboration and could be applied in international intervention studies with a need of larger samples.
A number of technological advances may also be helpful in overcoming the challenges of this rare disease, for example having online access for a decision aid for patients considering lung transplantation (Vandemheen 2009). The development of patient registries by individual countries (UK, Germany, US), as well as ECFS, represents an enormous technological advance that improves our ability to collect demographic and health outcome data on a large scale. The implementation of electronic medical records (EMR), which may be accessed with patient permission, may also facilitate data collection in key areas, such as adherence. In addition, development of electronic monitoring devices that measure ingestion of medications and pulmonary function, may also improve the accuracy of data collection and enable data collection on a much larger scale.
Our review also highlighted some important and common pitfalls of intervention research. Selection of a primary outcome is critical and if several outcomes are tested, attention should be paid to statistical adjustments for multiple tests to reduce the frequency of Type I errors. It is also important in the design phase to select reliable, well‐validated measures that are consistent with the targeted outcome (i.e. adherence behavior, decisional conflict etc.) and are thus, able to demonstrate the efficacy of the psychological intervention.It should also be noted that the likelihood of finding evidence is higher for studies which target specific aspects of the treatment regimen (e.g. behavioural nutrition interventions) and pre‐specified outcomes, rather than conducting non‐specific interventions using more generic approaches and general outcomes.
We identified areas of need that have not been targeted to date in clinical research of psychological interventions. Key among these are: interventions addressing at improving adherence, for patients at the end stage of the disease, interventions aimed at the psychological consequences of newborn screening/diagnosis, supportive interventions for caregivers, families, and siblings of patients with CF, and psychotherapy interventions for co‐morbid mental disorders. To deliver interventions to patients/caregivers more conveniently, researchers and clinicians should take advantage of new technologies, such as the Internet, in designing their studies. If effective, Internet‐based interventions could improve access to a larger, more diverse group of patients and potentially reduce common barriers (e.g., distance to centre, need to miss school or work). Thus, psychological interventions could become more accessible, potentially less costly, and more convenient for patients.
In terms of reporting, there is room for improvement in implementing the standards in reporting methods that are set out in the CONSORT statement (Moher 2001) and it's extension for non‐pharmacological interventions (Boutron 2008), including the reporting of change scores.
What's new
| Date | Event | Description |
|---|---|---|
| 21 May 2020 | Amended | An small error has been corrected in the 'Plain language summary'. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 11 June 2014 | New citation required but conclusions have not changed | Leading author changed (new: Lutz Goldbeck) and new co‐authors joined the team (Astrid Fidika and Marion Herle). The title has been changed from 'Psychological interventions from people with cystic fibrosis and their families'. The definition of psychological intervention was changed to be more precise and the classification of types of interventions, as well as specification of types of outcomes has been changed. |
| 11 June 2014 | New search has been performed | Searches carried out identified 28 new relevant reports. Ten reports related to seven newly identified studies (Belsky 1994; Taylor 2003; Goodill 2005; Christian 2006; Wilkinson 2008; Stark 2009; Vandemheen 2009) were included in this review. One previous classified as ongoing is now included (Quinn 2004). Nine of the new relevant reports were not eligible for inclusion. Three studies (Quittner 2011; Quittner 2012, Riekert 2012) were recorded as ongoing and six studies are awaiting classification/information from authors. Due to a new Cochrane Review on 'Self‐management education for cystic fibrosis' (Savage 2011) all five previously included studies with an purely educational approach were excluded (Cheuvront 1998; Trapp 1998; Stapleton 2001; Davis 2002; Downs 2006). |
| 12 August 2009 | Amended | Contact details updated. |
| 29 April 2008 | Amended | Converted to new review format. |
| 29 April 2008 | New search has been performed | Twenty new reports were found. Eleven reports related to five new studies (Chernoff 2002; Davis 2002; Downs 2006; Powers 2005; Stapleton 2001) which were included in this review and three reports related to studies already included. A single report of one study was excluded and four reports related to three studies which were recorded as ongoing. It was unclear whether one further report represented a new study or simply an additional report of an existing study and so awaits classification. |
| 29 April 2008 | New citation required but conclusions have not changed | The title has been changed from 'Psychological interventions for cystic fibrosis'. |
| 21 March 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We appreciate the support and guidance of the Cochrane Cystic Fibrosis and Gentic Disorders Group. We would like to thank the Neurosis, Anxiety and Depression Group from the Cochrane Collaboration for their help with the search strategy for psychological interventions.
Appendices
Appendix 1. New Search for the updated review
Psychological Therapies for Cystic Fibrosis: Search strategies June 2012
1. Cochrane Central Register of Controlled Trials (CENTRAL)
| Intervention: Psychotherapy | |
| #1 | MeSH descriptor PSYCHOTHERAPY explode all trees |
| #2 | (CBT or coping skills or counsel* or hypno* or mindfulness or psychoanal* or psychoeducat* or psychodynamic or psychotherapy* or rehabilitat*):ti,ab,kw |
| #3 | (psychologic* or behavior or behaviour or cognitive):ti,ab,kw |
| #4 | (Abreaction or (Acting NEXT Out) or Adlerian or (Adolescent NEXT Psych*) or (Anger NEXT Control) or (Anger NEXT Management) or (Art NEXT Therap*) or (Assertive* NEXT Training) or (Autogenic NEXT Training) or Autosuggestion or (Aversion NEXT Therap*) or (Balint NEXT Group) or Bibliotherap* or Biofeedback or (Caregiver NEXT Support) or (Child NEXT Psych*) or (Client NEXT Cent*) or (Color NEXT Therap*) or (Colour NEXT Therap*) or (Mind NEXT Training) or (Conjoint NEXT Therap*) or (Contingency NEXT Management) or (Conversion NEXT Therap*) or (Conversational NEXT Therap*) or Countertransference or (Couples NEXT Therap*) or (Covert NEXT Sensitization) or (Crisis NEXT Intervention)):ti,ab,kw |
| #5 | ((Dance NEXT Therap*) or Dialectic* or (Dream* NEXT Analys*) or Eclectic or (Emotion* NEXT Focus*) or (Emotional NEXT Freedom NEXT Technique) or (Encounter NEXT Group) or (Existential NEXT Therap*) or (Exposure NEXT Therap*) or (Eye NEXT Movement NEXT Desensiti*) or (Family NEXT Therap*) or (Free NEXT Association) or (Gestalt NEXT Therap*) or Griefwork or (Group NEXT Therap*) or (Guided NEXT Image*) or Imagery or (Implosive NEXT Therap*) or (Insight NEXT Therap*) or Integrative or Interpersonal):ti,ab,kw |
| #6 | (Logotherap* or (Marital NEXT Therap*) or Meditation or (Mental NEXT Healing) or Metacognitive or (Milieu NEXT Therap*) or (Morita NEXT Therap*) or (Music NEXT Therap*) or (Narrative NEXT Therap*) or (Nondirective NEXT Therap*) or (Personal NEXT Construct) or (Person NEXT Cent*) or Persuasion or (Pet NEXT Therap*) or (Play NEXT Therap*) or (Primal NEXT Therap*) or (Problem NEXT Solv*) or Psychodrama or Psychotherapeutic):ti,ab,kw |
| #7 | ((Rational NEXT Emotive) or (Reality NEXT Therap*) or (Reciprocal NEXT Inhibition) or (Relationship NEXT Therap*) or Relaxation or (Stress NEXT Manage*) or Reminiscence or (Role NEXT Play*) or (Self NEXT Analys*) or (Self NEXT Esteem) or (Sensitivity NEXT Training) or (Sex NEXT Therap*) or (Sleep NEXT Phase NEXT Chronotherap*) or (Socioenvironmental NEXT Therap*) or Sociotherap* or (Solution NEXT Focus*) or (Stress Manage*) or (Support NEXT Group*) or (Systematic NEXT Desensiti*) or (Therapeutic NEXT Communit*) or (Transactional NEXT Analysis) or (Validation NEXT Therap*)):ti,ab,kw |
| #8 | (Support NEAR/3 Psycho*):ti,ab,kw |
| #9 | MeSH descriptor ASSOCIATION explode tree 1 |
| #10 | MeSH descriptor HYPNOSIS explode tree 1 |
| #11 | MeSH descriptor CYSTIC FIBROSIS explode all trees with qualifiers: PX,RH,TH |
| #12 | MeSH descriptor NUTRITION THERAPY explode all trees |
| #13 | ((nutrition* or diet*) NEAR/7 (behavi* or educat* or intervention or support* or therap*)):ti,ab,kw |
| #14 | MeSH descriptor DIET explode all trees with qualifiers: PX,TH |
| #15 | MeSH descriptor SELF CARE explode all trees |
| #16 | MeSH descriptor SELF EFFICACY, this term only |
| #17 | (self NEAR/3 (care* or educat* or efficacy or help or manage*)):ti,ab,kw |
| #18 | MeSH descriptor HEALTH EDUCATION explode all trees |
| #19 | MeSH descriptor COUNSELING explode all trees |
| #20 | MeSH descriptor GENETIC COUNSELING explode all trees |
| #21 | (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20) |
| Condition: Cystic Fibrosis | |
| #22 | MeSH descriptor CYSTIC FIBROSIS, this term only |
| #23 | (cystic NEXT fibrosis):ti,ab,kw |
| #24 | Mucoviscidos*:ti,ab,kw |
| #25 | ((cysti* or cisti*) NEAR/10 fibros*):ti,ab,kw |
| #26 | (fibrocystic* NEAR pancrea*):ti,ab,kw |
| #27 | (#22 or #23 or #24 or #25 or #26) |
| #28 | (#21 and #27) |
2. OVID MEDLINE
| Intervention: Psychotherapy | |
| 1 | exp Psychotherapy/ |
| 2 | (CBT or coping skills or counsel?ing or hypno$ or mindfulness or psychoanal$ or psychoeducat$ or psychodynamic or psychotherap$ or rehabilitat$).tw. |
| 3 | (psychologic$ or behavio?r or cognitive).tw. |
| 4 | (Abreaction or Acting Out or Adlerian or Adolescent Psych$ or Analytical Psychotherap$ or Anger Control or Anger Management or Art Therap$ or Assertive$ Training or Autogenic Training or Autosuggestion or Aversion Therap$ or Balint Group or Behavio?r Contracting or Behavio?r Modification or Behavio?r Therap$ or Bibliotherap$ or Biofeedback or Body Psychotherap$ or Brief Psychotherap$ or Caregiver Support or Child Psych$ or Client Cent$ or Cognitive Behavio?r* or Cognitive$ Rehabilit$ or Cognitive Therap$ or Colo?r Therap$ or Compassionate Mind Training or Conjoint Therap$ or Contingency Management or Conversion Therap$ or Conversational Therap$ or Countertransference or Couples Therap$ or Covert Sensitization or Crisis Intervention).mp. |
| 5 | (Dance Therap$ or Dialectic$ or (Dream$ adj3 Analys$) or Eclectic or Emotion$ Focus$ or Emotional Freedom Technique or Encounter Group Therap$ or Existential Therap$ or Experiential Psychotherap$ or Exposure Therap$ or Expressive Psychotherap$ or Eye Movement Desensiti#ation or Family Therap$ or Feminist Therap$ or Free Association or Geriatric Psychotherap$ or Gestalt Therap$ or Griefwork or Group Psychotherap$ or Group Therap$ or Guided Image$ or Holistic Psychotherap$ or Humanistic Psychotherap$ or Hypnosis or Hypnotherap$ or Hypnoti#zability or Imagery or Implosive Therap$ or Individual Psychotherap$ or Insight Therap$ or Integrative Psychotherap$ or Integrative Therap$ or Interpersonal).mp. |
| 6 | (Logotherap$ or Marathon Group Therap$ or Marital Therap$ or Meditation or Mental Healing or Metacognitive Therap$ or Milieu Therap$ or Morita Therap$ or Music Therap$ or Narrative Therap$ or Nondirective Therap$ or Personal Construct Therap$ or Person Cent$ Therap$ or Persuasion Therap$ or Pet Therap$ or Play Therap$ or Primal Therap$ or Problem Solving or Psychoanaly$ or Psychodrama or Psychodynamic or Psychotherapeutic Counsel$ or Psychotherapeutic Processes or Psychotherapeutic Training).mp. |
| 7 | (Rational Emotive or Reality Therap$ or Reciprocal Inhibition or Relationship Therap$ or Relaxation or Stress Management or Reminiscence Therap$ or Role Play$ or Self Analys$ or Self Esteem or Sensitivity Training or Sex Therap$ or Sleep Phase Chronotherap$ or Socioenvironmental Therap$ or Sociotherap$ or Solution Focused or Stress Management or Support Group$ or (Support adj3 Psycho$) or Systematic Desensiti#ation or Therapeutic Communit$ or Transactional Analysis or Validation Therap$).mp. |
| 8 | ASSOCIATION/ or ASSOCIATION LEARNING/ |
| 9 | exp HYPNOSIS/ |
| 10 | CYSTIC FIBROSIS / px, rh, th [Psychology, Rehabilitation,Therapy] |
| 11 | exp NUTRITION THERAPY/ (to include diet therapy/ and nutritional support/) |
| 12 | ((nutrition* or diet*) adj7 (behavi* or educat* or intervention or support* or therap*)).tw. |
| 13 | DIET/px [Psychology] |
| 14 | SELF CARE/ |
| 15 | SELF EFFICACY/ |
| 16 | ((self) adj3 (care* or educat* or efficacy or help or manage*)).tw. |
| 17 | exp HEALTH EDUCATION/ |
| 18 | exp COUNSELING/ |
| 19 | exp GENETIC COUNSELING/ |
| 20 | or/1‐19 |
| Condition: Cystic Fibrosis | |
| 21 | Cystic Fibrosis/ |
| 22 | cystic fibrosis.tw. |
| 23 | mucoviscidos$.tw. |
| 24 | ((cysti$ or cisti$) adj10 fibros$).tw, ot. |
| 25 | (fibrocystic* and pancreas*).tw. |
| 26 | or/15‐19 |
| Filter: RCTs | |
| 27 | randomized controlled trial.pt. |
| 28 | controlled clinical trial.pt. |
| 29 | randomi#ed.ti,ab. |
| 30 | randomly.ab. |
| 31 | placebo.ab. |
| 32 | trial.ab. |
| 33 | groups.ab. |
| 34 | (control$ adj3 (trial or study)).ab,ti. |
| 35 | ((singl$ or doubl$ or tripl$ or trebl$) adj3 (blind$ or mask$ or dummy)).mp. |
| 36 | (animals not (humans and animals)).sh. |
| 37 | or/27‐35 |
| 38 | 37 not 36 |
| 39 | (20 and 26 and 38) |
3. OVID EMBASE
| Intervention: Psychotherapy | |
| 1 | exp Psychotherapy/ |
| 2 | (CBT or coping skills or counsel?ing or hypno$ or mindfulness or psychoanal$ or psychoeducat$ or psychodynamic or psychotherap$ or rehabilitat$).tw. |
| 3 | (psychologic$ or behavio?r or cognitive).tw. |
| 4 | (Abreaction or Acting Out or Adlerian or Adolescent Psych$ or Analytical Psychotherap$ or Anger Control or Anger Management or Art Therap$ or Assertive$ Training or Autogenic Training or Autosuggestion or Aversion Therap$ or Balint Group or Behavio?r Contracting or Behavio?r Modification or Behavio?r Therap$ or Bibliotherap$ or Biofeedback or Body Psychotherap$ or Brief Psychotherap$ or Caregiver Support or Child Psych$ or Client Cent$ or Cognitive Behavio?r* or Cognitive$ Rehabilit$ or Cognitive Therap$ or Colo?r Therap$ or Compassionate Mind Training or Conjoint Therap$ or Contingency Management or Conversion Therap$ or Conversational Therap$ or Countertransference or Couples Therap$ or Covert Sensitization or Crisis Intervention).mp. |
| 5 | (Dance Therap$ or Dialectic$ or (Dream$ adj3 Analys$) or Eclectic or Emotion$ Focus$ or Emotional Freedom Technique or Encounter Group Therap$ or Existential Therap$ or Experiential Psychotherap$ or Exposure Therap$ or Expressive Psychotherap$ or Eye Movement Desensiti#ation or Family Therap$ or Feminist Therap$ or Free Association or Geriatric Psychotherap$ or Gestalt Therap$ or Griefwork or Group Psychotherap$ or Group Therap$ or Guided Image$ or Holistic Psychotherap$ or Humanistic Psychotherap$ or Hypnosis or Hypnotherap$ or Hypnoti#zability or Imagery or Implosive Therap$ or Individual Psychotherap$ or Insight Therap$ or Integrative Psychotherap$ or Integrative Therap$ or Interpersonal).mp. |
| 6 | (Logotherap$ or Marathon Group Therap$ or Marital Therap$ or Meditation or Mental Healing or Metacognitive Therap$ or Milieu Therap$ or Morita Therap$ or Music Therap$ or Narrative Therap$ or Nondirective Therap$ or Personal Construct Therap$ or Person Cent$ Therap$ or Persuasion Therap$ or Pet Therap$ or Play Therap$ or Primal Therap$ or Problem Solving or Psychoanaly$ or Psychodrama or Psychodynamic or Psychotherapeutic Counsel$ or Psychotherapeutic Processes or Psychotherapeutic Training).mp. |
| 7 | (Rational Emotive or Reality Therap$ or Reciprocal Inhibition or Relationship Therap$ or Relaxation or Stress Management or Reminiscence Therap$ or Role Play$ or Self Analys$ or Self Esteem or Sensitivity Training or Sex Therap$ or Sleep Phase Chronotherap$ or Socioenvironmental Therap$ or Sociotherap$ or Solution Focused or Stress Management or Support Group$ or (Support adj3 Psycho$) or Systematic Desensiti#ation or Therapeutic Communit$ or Transactional Analysis or Validation Therap$).mp. |
| 8 | Association/ |
| 9 | Suggestion/ or Transference/ |
| 10 | CYSTIC FIBROSIS / rh, th [Rehabilitation,Therapy] |
| 11 | NUTRITION EDUCATION/ |
| 12 | ((nutrition* or diet*) adj7 (behavi* or educat* or intervention or support* or therap*)).tw. |
| 13 | exp DIET THERAPY/ |
| 14 | DIET/th |
| 15 | exp SELF CARE/ |
| 16 | exp SELF CONCEPT/ |
| 17 | ((self) adj3 (care* or educat* or efficacy or help or manage*)).tw. |
| 18 | exp HEALTH EDUCATION/ |
| 19 | exp COUNSELING/ |
| 20 | or/1‐19 |
| Condition: Cystic Fibrosis | |
| 21 | Cystic Fibrosis/ |
| 22 | cystic fibrosis.tw. |
| 23 | mucoviscidos$.tw. |
| 24 | ((cysti$ or cisti$) adj10 fibros$).tw, ot. |
| 25 | (fibrocystic* and pancreas*).tw. |
| 26 | or/21‐25 |
| Filter: RCTs | |
| 27 | randomized controlled trial.de. |
| 28 | randomization.de. |
| 29 | placebo.de. |
| 30 | placebo.ab. |
| 31 | randomi#ed.ti,ab. |
| 32 | randomly.ab. |
| 33 | ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. |
| 34 | factorial$.ti,ab. |
| 35 | (allocat$ or assign$).ti,ab. |
| 36 | crossover procedure.de. |
| 37 | (crossover$ or cross over$).ti,ab. |
| 38 | (quasi adj (experimental or random$)).mp. |
| 39 | (control$ adj3 (trial$ or study or studies or group$)).ti,ab. |
| 40 | ((animal or nonhuman) not (human and (animal or nonhuman))).de. |
| 41 | or/27‐39 |
| 42 | 41 not 40 |
| 43 | (20 and 26 and 42) |
4. OVID PsycINFO(Condition + RCT filter only)
| Condition: Cystic Fibrosis | |
| 1 | Cystic Fibrosis/ |
| 2 | cystic fibrosis.tw. |
| 3 | mucoviscidos$.tw. |
| 4 | ((cysti$ or cisti$) adj10 fibros$).tw, ot. |
| 5 | (fibrocystic* and pancreas*).tw. |
| 6 | or/21‐25 |
| Filter: RCTs | |
| 7 | treatment effectiveness evaluation.sh. |
| 8 | clinical trials.sh. |
| 9 | mental health program evaluation.sh. |
| 10 | randomly.ab. |
| 11 | randomi#ed.ti,ab. |
| 12 | (placebo or (attention control)).ab. |
| 13 | (waitlist$ or (wait$ adj2 list$)).ab. |
| 14 | trial.ti,ab. |
| 15 | ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. |
| 16 | (control$ adj3 (trial$ or study or studies or group$)).ti,ab. |
| 17 | factorial$.ti,ab. |
| 18 | (allocat$ or assign$).ti,ab. |
| 19 | (crossover$ or cross over$).ti,ab. |
| 20 | (quasi adj (experimental or random$)).mp. |
| 21 | "2000".md. |
| 22 | or/7‐21 |
| 23 | (6 and 22) |
Data and analyses
Comparison 1. Behavioural group treatment versus wait list control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pulmonary function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.3 FEF25-75% (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Anthropometric measures | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Weight (z score) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 Height (z score) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Adherence | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.1 Activity level ‐ Resting energy expenditure (% predicted) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.2 Activity level ‐Resting energy expenditure (Kcal/24 hours) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.3 Activity level ‐Activity points | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.4 Change in Kcalorie intake | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Nutrition status | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4.1 % Body Fat | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Nutrional intervention plus behavioural management training (BEH) versus nutritional intervention alone (NUT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Anthropometric change scores | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Change in weight (kg) | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.84, 1.07] |
| 2.1.2 Change in height (cm) | 1 | 7 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐4.87, 0.81] |
| 2.1.3 Change in weight (kg) 2‐year follow up | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐1.34, 2.38] |
| 2.1.4 Change in height (cm) 2‐year follow up | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.45, 1.05] |
| 2.1.5 BMIz change post intervention | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.02, 0.42] |
| 2.1.6 BMIz change 2‐year follow up | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.00, 0.70] |
| 2.1.7 HAZ change 2‐year follow up | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.17, 0.15] |
| 2.2 % Anthropometric | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.1 % ideal body weight ‐ baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.2 %Ideal body weight ‐ follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.3 Weight % for age ‐baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2.4 Weight % for age ‐ follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Antropometric | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 weight (kg) post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.2 weight (kg) 2‐year follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.3 height (cm) 2‐year follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.4 HAZ 2‐year follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.5 BMIZ post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.6 BMIZ 2‐year follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Pulmonary function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 FEV1 2‐year follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.2 FEV1 change 2‐year follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Adherence (nutrition) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 % RDA Kcal/day | 1 | 8 | Mean Difference (IV, Random, 95% CI) | 8.52 [‐18.95, 35.99] |
| 2.5.2 Total calories per day | 3 | 82 | Mean Difference (IV, Random, 95% CI) | 275.85 [66.65, 485.05] |
| 2.5.3 Change in calorie intake | 3 | 82 | Mean Difference (IV, Random, 95% CI) | 364.06 [191.99, 536.13] |
| 2.5.4 2‐year follow up caloric intake | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 112.00 [‐216.56, 440.56] |
| 2.5.5 2‐year follow up change in caloric intake | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 188.00 [‐75.57, 451.57] |
| 2.5.6 Estimated energy requirements (%EER) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 21.00 [7.76, 34.24] |
| 2.5.7 Change in %EER | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 21.00 [9.22, 32.78] |
| 2.5.8 2‐year follow up %EER | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 9.00 [‐5.09, 23.09] |
| 2.5.9 2‐year follow up change in %EER | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 9.00 [‐4.73, 22.73] |
| 2.6 Relational | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6.1 Nursing Child Assessment Satellite Training Feeding Scale (NCAST) ‐ Caregiver / Infant total score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.7 Nutritional status | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 % Body fat | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Behavioral and nutritional intervention (BEH) versus standard care control (CTL).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 total energy intake per day (kcal) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.1 post‐treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2 % fat intake | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2.1 post‐treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Educational problem‐solving and social skills intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pulmonary function (FEV1) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Height (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Weight in (kg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4 Perceived illness experience | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5 Children's loneliness | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.6 Social support ‐ peers | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.6.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.6.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.6.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.7 Social support ‐ classmates | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.7.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.7.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.7.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8 Self competence ‐ subscale 'global self‐worth' | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9 Self‐competence ‐ subscale 'scholastic competence' | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.10 Self‐competence ‐ subscale 'social acceptance' | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.10.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.10.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.10.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.11 Self‐competence ‐ subscale 'athletic competence' | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.11.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.11.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.11.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.12 Self‐competence ‐ subscale 'physical appearance' | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.12.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.12.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.12.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.13 Self‐competence ‐ subscale 'behavioral conduct' | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.13.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.13.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.13.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14 Functional health status | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Written self‐disclosure intervention versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Subjective health status ‐ PHQ | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.1 PHQ‐ Depression scale 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.2 PHQ‐ Anxiety scale 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.3 PHQ‐ Somatic complaints 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.4 PHQ‐ Stressfull life events 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Subjective health status ‐ SF‐12 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.1 Physical health status 3 month follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.2 Mental health status 3 month follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 FEV1 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4 BMI 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5 Health care utilization | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5.1 Outpatient utilization 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5.2 Inpatient utilization 3‐month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Motivational Interviewing via telephone (3‐month period) versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.1 Physical functioning | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.2 Social functioning | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.3 Treatment issues | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.4 Chest symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.5 Emotional functioning | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.6 Concerns for the future | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.7 Interpersonal relationships | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.8 Body image | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.9 Career concerns | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.2 Psychological distress | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.2.1 STAI post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.2.2 HADS anxiety post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.2.3 HADS depression post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3 Lung function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3.1 FEV1 post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.3.2 FVC post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.4 % adherence behavior post intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Decision aid for patients considering lung transplantation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Participants' knowledge | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1.1 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1.2 change in knowledge (week 3 ‐ baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2 Patient expectations | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2.1 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2.2 change in expectation score (week 3 ‐ baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3 Decisional conflict | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.1 Total score 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.2 Subscale ‐ certainty 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.3 Subscale ‐ informed 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.4 Subscale ‐ values 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.5 Subscale ‐ support 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.6 Subscale ‐ satisfaction with decision 3 week follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3.7 Change in total score (week 3 ‐ baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.4 Patient's stated choice at 3‐week follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4.1 Referral for transplantation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4.2 Declined referral for transplantation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4.3 Unsure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.5 Durability of patient decisions (12‐month follow up) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.6 Preparation for decision‐making (3‐week follow up) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7 Values congruence | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.7.1 Importance of living longer | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.7.2 Importance of avoiding hassle, stress, and worry of lung transplantation | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7.8 Acceptability | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.1 Received the right amount of information. | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.2 Materials were helpful in helping to arrive at a decision. | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.3 Definitely or probably recommend the materials. | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
7.7. Analysis.

Comparison 7: Decision aid for patients considering lung transplantation versus usual care, Outcome 7: Values congruence
Comparison 8. Community‐based support program versus contact telephone number.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Psychiatric Symptom Index ‐ Anxiety subscale ‐ mothers | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1.1 Mothers' anxiety ‐ baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1.2 Mothers' anxiety ‐ 12 months post‐baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Biofeedback assisted breathing re‐training (BRT) versus biofeedback assisted relaxation training (RLXT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Pulmonary function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.1 Forced expiratory volume (FEV1) in litres per second | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.2 Forced vital capacity (FVC) in litres | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.3 FEF25-75% in litres per second | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 10. Massage therapy versus bedtime reading control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Well‐being | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1.1 Parent Anxiety ‐ State Trait Anxiety Inventory (STAI) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1.2 Child Anxiety ‐ State Trait Anxiety Inventory for Children (STAIC) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1.3 Child Mood ‐ Profile of Mood States (POMS) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.2 Pulmonary function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.2.1 Peak Air Flow (PEFR) ‐ Day 1 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.2.2 Peak Air Flow (PEFR) ‐ Day 30 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 11. Music therapy versus familiar music and no audiotape control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Enjoyment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1.1 Parent enjoyment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1.2 Child enjoyment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.2 Cognitive | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.2.1 Parent perception of time | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 12. Self‐hypnosis versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Anxiety: State Trait Anxiety Inventory for Children (STAIC) | 1 | Other data | No numeric data | |
| 12.2 Impact on Family Scale | 1 | Other data | No numeric data | |
| 12.3 Child Behavior Checklist | 1 | Other data | No numeric data | |
| 12.4 Average peak expiratory flow rate (PEFR) | 1 | Other data | No numeric data | |
| 12.5 Height and weight | 1 | Other data | No numeric data | |
| 12.6 Rating of the parents' assessment of the child's illness | 1 | Other data | No numeric data | |
| 12.7 Locus of Control (LOC): Nowicki Strickland (1973) LOC for Children | 1 | Other data | No numeric data | |
| 12.8 Health Locus of Control: Children's Health Locus of Control (HLOC) | 1 | Other data | No numeric data | |
| 12.9 Self‐concept: Piers Harris Children's Self‐Concept Scale | 1 | Other data | No numeric data |
Comparison 13. Home telemedicine for patients awaiting transplantation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Quality of Life: Cystic Fibrosis Quality of Life Questionnaire (Gee 2000) | 1 | Other data | No numeric data | |
| 13.2 Anxiety: Beck Anxiety Inventory (Beck 1988) + Borg anxiety scale (Borg 1982) | 1 | Other data | No numeric data | |
| 13.3 Depression: Beck Depression Inventory (Beck 1987) | 1 | Other data | No numeric data | |
| 13.4 Coping of carers: COPE questionnaire (Carver 1997) | 1 | Other data | No numeric data | |
| 13.5 Health care utilization: seven‐point questionnaire (number of visits, general practitioner, courses of IV antibiotics, length of hospital inpatient stay, visits either to hospital or GP) | 1 | Other data | No numeric data |
Comparison 14. Dance/movement therapy for adults versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Mood state: ’The Profile of Mood States’ (POMS; Mc Nair 1992) | 1 | Other data | No numeric data | |
| 14.2 Adherence to exercise and nutrition regimen | 1 | Other data | No numeric data | |
| 14.3 Body Image: Human figure drawing task | 1 | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belsky 1994.
| Study characteristics | ||
| Methods | CCT. Pilot study that assessed the effects of self‐hypnosis on psychological and physiological functioning of children ages 7 to 18 years with CF. Hypothesis: hypnosis is associated with enhanced performance on clinical rating criteria and with improved scores in self‐concept and other psychological tests. The study used a pre‐ and post‐test design with control group and repeated measures. |
|
| Participants | 12 children with CF ages 7 ‐ 18 years attending a local CF clinic. Population of interest: 30 (met eligibility). Number included: 12. Control group: 5 girls and 2 boys. |
|
| Interventions | The parents and children in the experimental group had three appointments within a 2‐week period during which time the children were taught a hypnotic technique. The hypnotic induction is described in detail in the article. |
|
| Outcomes |
CF illness ratings: Shwachman‐ Kulzcycki system standard form has been completed by the physician at baseline, which is based on 100 maximum points measuring four categories: general activity, physical examination, nutrition, and radiographic findings. Other illness variables included each patient's average PEFR, height, weight, and number of hospital admissions during 2 years prior to the study. Parents' measures: 'Impact on Family Scale' (measures family functioning); 'Child Behavior Checklist' (parents' perception of behavioral problems and competencies); rating of the parents' assessment of the child's illness; parents also provided a record of school days missed by their child for 3 years before the pretest. Children's measures: LOC was assessed with the Nowicki Strickland (1973) LOC for Children and with the Children's HLOC (Parcel & Mayer, 1978) which is a 20‐item area specific measure of expectancies regarding locus of control and prediction of health‐related behavior. Self‐concept was assessed with the Piers Harris Children's Self‐Concept Scale (1964). Anxiety was assessed with the STAIC (Spielberger 1973). |
|
| Notes | None of the authors could be contacted for questions on provided data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The 2 groups were not randomised. The authors stated that "2 groups were formed on the basis of comparability of age and clinical ratings" (Belsky 1994, p. 284). |
| Allocation concealment (selection bias) | High risk | Because groups were not randomised, participants and/or investigators enrolling participants could probably foresee assignment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of participants and personnel providing the intervention was not possible. The authors provided no information on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on dropouts were provided by the authors. |
| Selective reporting (reporting bias) | High risk | The number of participants for post‐intervention analysis were not mentioned. Outcomes of interest in the review are reported incompletely so that they cannot be entered in a an analysis (N, means and standard deviations for some of the outcomes). |
Chernoff 2002.
| Study characteristics | ||
| Methods | Parallel RCT. Comparing the effect for carers of children with a chronic illness of a comprehensive community‐based support programme with having a contact telephone number for an experienced mother of a child with a similar illness. For children it was hypothesised that those in the experimental group would have better mental health at the end of the intervention than controls. For the mothers it was hypothesised that those in the intervention group would report fewer psychological symptoms than control mothers. | |
| Participants | Population of interest N = 193. Sample agreeing to participate N = 161 children with a chronic illness and their mothers. Children with diabetes, sickle cell, CF or moderate to severe asthma were included. For CF subgroup: n = 7 assigned to the intervention group and n = 6 controls. Included children aged 7 ‐ 11 years living within 80 km radius of Baltimore, with no learning disability, with a mother as a primary carer, on the telephone, speaking English, and diagnosed > 6 months ago. | |
| Interventions | 1. Community‐based support programme over 15 months ‐ trained network mother + child life specialist pairing. a. 7 home visits b. twice‐weekly telephone calls c. 3 special family events 2. Control group are given a contact number for an experienced mother. | |
| Outcomes | Children a. personal adjustment and role skills b. depression c. anxiety d. self‐perception Mothers a. anxiety b. depression c. stressful life events | |
| Notes | POI: anxiety subscale ‐ 11 items self‐report from 'Psychiatric Symptom Index'.
The groups were equivalent at baseline although these data not reported for individual disease subgroups.
Network mothers were trained and supervised and a manual was produced. Treatment fidelity: the authors reported that weekly meetings took place to ensure that intervention was provided as planned (Chernoff 2002, p.534/35). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not describe the randomisation process in detail. The stated that of the 161 families being eligible and who agreed to participate were 'randomly assigned to the experimental group and [...] to the control group (Chernoff 2002; p. 773). |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Due to the nature of the intervention blinding of participants is not possible adequately, but the authors provided the information that 'interviews were completed by paid interviewers who had undergone extensive training and were blind to group assignment' (Chernoff 2002; p. 534). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors reported that about 15.5% of the randomised participants were not available for follow‐up analysis. 'of the 161 families randomised, 86 were randomised to the experimental group and 75 to the control group. Overall, 25 families, 14 and 11 from the experimental and the control group, respectively, were lost to follow up [...] (Chernoff 2002; p. 536). |
| Selective reporting (reporting bias) | High risk | The presentation of the results was selective. The subgroup analysis reported on p. 537 and figure 2 e.g. was not pre‐specified. |
Christian 2006.
| Study characteristics | ||
| Methods | RCT. 2‐group, experimental, repeated‐measures design (baseline, 3, 6, and 9 months intervals) with randomly assigned participants to intervention and usual care group. Hypothesis: children with CF who receive the intervention will improve significantly on 3 parameters (psychosocial adjustment, functional health status, physiologic health) over the time compared to children who received usual care. |
|
| Participants | Population of interest: N = 128. Number of randomised participants: N = 116. Control group 'usual care': N = 58. Intervention group 'educational problem‐solving and social skills intervention': N = 58. Included children with CF aged 8 ‐ 12 years receiving care from one of four CF centres in North Carolina (USA). |
|
| Interventions | Educational problem‐solving and social skills intervention (N = 58) versus usual care (N = 58). Children in the problem‐solving group received individual, tailored intervention during one home visit and a structured group session (conducted approximately 2 weeks after individual home visit). Intervention was designed to support children (8 ‐ 12years) with the following specific problems: 1. finding out about the CF diagnosis; 2. explaining CF‐related differences; 3. dealing with teasing about CF; 4. keeping up with peers during physical activity. The intervention contains 4 modules. In every module there was a focus on one of the 4 mentioned areas of problems (1‐4). The intervention team received detailed information about the intervention protocol and was trained and supervised conducting the intervention. |
|
| Outcomes | 5 questionnaires which are developed for children were read aloud by research assistant to the children. The following questionnaires were used to the assess different constructs: Psychosocial adjustment was assessed by: ‐ Perceived Illness Experience Scale (35 items); ‐ Children's Loneliness Scale (16 items); ‐ Social Support Scale for Children (16 items); ‐ Self‐Perception Profile for Children (30 items). Functional Health Status was assessed by: ‐ Functional Disability Inventory (15 items). Physiolgical Health Status: ‐ Pulmonary function (FEV1). ‐ Height and weight. ‐ BMI. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors describe that the children were randomly assigned to the intervention and control group "using a computer‐generated randomization plan". (Christian 2006, p. 302) |
| Allocation concealment (selection bias) | Low risk | Assignment could not been foreseen by participants and investigators enrolling participants due to a computer‐generated randomisation plan. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The authors state that "group assignments were unknown to the CF clinics and research assessment team". (Christian 2006; p. 302) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors stated that "all children completed the baseline and 3 follow‐up assessments" (Christian 2006, p.301). That means that there was no attrition or loss of any participant during the study and follow‐up period. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the published records are reported. It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. |
Delk 1994.
| Study characteristics | ||
| Methods | Parallel RCT. Comparing the effects of therapy that it is targeted with aim of facilitating physiotherapy and when it is aimed at increasing relaxation. | |
| Participants | N = 26. Aged 10 ‐ 41 years. Groups matched by age and severity of disease using Shwachman‐Kulczycki scoring system prior to randomisation. | |
| Interventions | 1. Biofeedback assisted breathing re‐training (N = 13). 2. Biofeedback assisted relaxation training (N = 13). 8 sessions over 4 weeks. Delivered by psychology and biofeedback students under supervision. | |
| Outcomes | Lung function: 1. FVC; 2. FEV1; 3. FEF 25-75%. Evaluation at: 1. 3 pre‐study assessments, 18 months prior to study commencement; 2. baseline; 3. post‐study; 4. follow up. | |
| Notes | POI: Pulmonary function. The groups were equivalent at baseline on age, severity of disease, height, weight, and pre‐treatment lung functions. Both groups believed they were being given an intervention so effective blinding of participants ‐ no information about blinding of trainers and pulmonary function technicians. Not clear how sample was selected or from what population they were drawn. Analysed as ITT apart from one missing matched pair in FEV1 outcome measure. Limited follow‐up data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random generation process were not provided by the authors. They just reported that 'subjects were screened medically, matched by age and severity disease, and randomly assigned to either the experimental group receiving the biofeedback‐assisted BRT or to the control groups receiving peripheral temperature biofeedback‐assisted relaxation training (RLXT)' (Delk 1994, p. 23). |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Due to the nature of the intervention blinding of participants is not possible adequately. Insuffiecent information about blinding of outcome assessment available, |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were analysed as intention‐to‐treat, but it has to be mentioned that for FEV1 results of one participant is missing (n = 25). Reasons were not explained by the authors. |
| Selective reporting (reporting bias) | Unclear risk | Information is insufficient to judge whether 'low' or 'high' risk. |
Goodill 2005.
| Study characteristics | ||
| Methods | RCT. Compared brief dance/movement therapy for adult CF patients during an exacerbation of CF symptomatology to standard care. In the study it was explored whether treatment group participants would show more positive mood state, better adherence with self‐care expectations, and a healthier and/or more positive body image. |
|
| Participants | Participants were 17 years or older and hospitalised for CF treatment. All were Caucasian except one, who was African‐American. Pre‐test and post‐test data were collected for 42 participants, follow‐up data for 24 participants (14 in the treatment group and 10 in the control group). |
|
| Interventions | The intervention consisted of 3 individual or small group DMT sessions over a 7‐ to l0‐day time period. DMT, a creative arts psychotherapy, utilizes guided movement activity with creative and non‐verbal communication processes for increasing self‐awareness, enhancing expressive competence, and integrating cognitive, physical, social, and emotional functioning. | |
| Outcomes | Mood state was measured with 'The Profile of Mood States' (POMS): scores for 'Total Mood Disturbance' and 6 subscales: 'Tension'; 'Depression'; 'Fatigue'; 'Confusion'; 'Anger'; and 'Vigor'. Body image was assessed with human figure drawings created by the participants and rated by trained raters (levels of acceptable agreement r = .85). Adherence: an adherence questionnaire developed for this study posed two simple questions regarding adherence with physician instructions for exercise and nutrition regimens: "How much did you do of what was instructed for exercise? for nutrition?" Response options were, "l did almost everything" (or "about half" or "little or nothing") of "what I was expected to do.” |
|
| Notes | Contacted the leading author for detailed data on the outcome measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The author only provided the information that "randomization formed groups similar in age, gender, and disease severity" (Goodill 2005, p. 76). |
| Allocation concealment (selection bias) | Unclear risk | The author did not describe details of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel providing the intervention were not able to be blinded due to the nature of the intervention. Research assistants, who conducted pre‐ and post‐testing were blinded to group assignments (Goodill 2005, p.76). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The author reported a high rate of dropouts at follow up. The percentage of dropouts in each group is unclear. A complete data set was only for 14 participants available, although 42 participants have been randomised. |
| Selective reporting (reporting bias) | High risk | Means and SDs for all outcome parameters for intervention and control group were not reported in the published article. Of the reported measure it was not prespecified which is the primary outcome. |
Grasso 2000.
| Study characteristics | ||
| Methods | Parallel RCT. Comparing the effects of music therapy in enhancing the experience of physiotherapy with no effective intervention. Random allocation to 2 groups. Groups matched for spread of ages, frequency of CPT and number of rural families. Control group experienced 2 consecutive conditions ‐ control and placebo control with repeated measures. | |
| Participants | Population of interest N = 26. Five refused. Recruitment rate 87.5%. Sample comprised N = 21. N = 1 withdrew from intervention group. Caregivers of one or more children with CF ‐ parent most responsible for CPT. Children aged 6 to 24 months with diagnosis at least two months prior to start of study. Exclusions ‐ children admitted to hospital with a chest infection. | |
| Interventions | Intervention group (N = 10): 1. Music Therapy Tape (TT) with 5 minute explanation and instruction to use as often as they liked plus diary chart (N = 10). Control group (N = 10): 2. No audiotape (NT control). 3. Familiar music tape (FT placebo control) plus diary chart. The study lasted for 12 weeks with the control group experiencing 2 conditions each for a period of 6 weeks in the order of 2. then 3. | |
| Outcomes | 1. Enjoyment ‐ child & parent. 2. Perception of time. Evaluation at baseline, 6 weeks and 12 weeks. Ad hoc test measure. | |
| Notes | POI: enjoyment in child and parent and perception of time in parent.
Equivalence at baseline for:
1. age;
2. sex;
3. stage of condition.
Analysed as ITT apart from one dropout.
Participants not informed about whether they were in treatment group. Treatment fidelity/compliance: The authors reported that the 'participants were asked to complete a diary chart to monitor frequency of tape use' (Grasso 2000, p.373). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not describe details of random generation process. They just stated that 'the participants were randomly allocated into control and treatment groups.' (Grasso 2000, p. 372) |
| Allocation concealment (selection bias) | Unclear risk | The authors did not describe the method of concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The authors reported that the participants were not informed about the group assignment, but blinding for control condition (no audiotape and later familiar music tape) is doubtful. All outcomes were assessed via self‐reports by the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors report that 21 families were assigned to one of the groups and that 'one family was withdrawn when the child was hospitalised for a chest infection, leaving a total of 10 in each group' (Grasso 2000, p.373). That means that the attrition during the study was less than 15%. |
| Selective reporting (reporting bias) | Unclear risk | The available information is insufficient to judge whether 'low' or 'high' risk. |
Hernandez‐Reif 1999.
| Study characteristics | ||
| Methods | Parallel RCT. Control group offered intervention on completion of study. Comparing the effect of massage in enhancing pulmonary function. | |
| Participants | Population of interest N = 24, aged 5 ‐ 18 years. 1 refused and 3 dropped out. Total sample n = 20 parent/child dyads. Children aged 5 ‐ 12 years. Intervention group: 9 mothers, 1 father & 10 children. Control group: 10 mothers & 10 children. Mean baseline scores on NIH clinical score: intervention group 82.2; control group 82.0. | |
| Interventions | 1. Massage therapy (N = 10). 2. Bedtime reading control (N = 10). Every evening for 30 days. | |
| Outcomes | Parents: anxiety (STAI). Children: anxiety (STAIC); mood (POMS); peak flow (PEFR). Assessment at baseline (Day 1) & Day 30. Assessment: pre‐ and post‐Day 1; pre‐ and post‐Day 30. | |
| Notes | POI: peak flow. Fewer than 15% refused or dropped out. Equivalence at baseline for: 1. sex of parent; 2. age of child; 3. SES; 4. severity. No blinding of participants or therapists and blinding of assessors unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not describe details of random generation process. They just stated that the participants were 'randomly assigned to a massage therapy or a reading control group' (Hernandez‐Reif 1999, p. 177). |
| Allocation concealment (selection bias) | Unclear risk | The authors did not present information about the allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Due to the nature of the interventions the participants and the interventionists could not be blinded. The authors did not report whether the research assistants, who were calling the participants and checking for compliance, were blinded (as well as outcome assessment). Due to the lack of blinding the performance might have been influenced. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported that out of 23 participants, 20 were available for post analyses. Those three families 'were later dropped from the study due to noncompliance' and 'had failed to keep their 30‐day appointment' (Hernandez‐Reif 1999, p. 177). Two were assigned to the control condition and one family to the massage therapy condition. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes, which have been assessed, were reported. |
Powers 2003.
| Study characteristics | ||
| Methods | Parallel RCT. Examine the effectiveness of 2 nutritional interventions. Hypotheses: 1. children participating in both groups will increase their calorie intake; 2. children participating in both groups will show significant increases in weight. Sequence of randomisation ‐ number drawn from a box. | |
| Participants | N = 12. Less than 3 years old. Confirmed diagnosis of CF with pancreatic insufficiency. No other disease or condition known to affect growth. | |
| Interventions | 1. Nutrition intervention with strategies for enhancing calorie intake ‐ behavioral management training for parents designed to encourage children to eat food consistent with CF dietary recommendations (N = 7). 2. Nutrition intervention only (N = 5). Both groups received 8 sessions (45 to 60 minutes) over 1 year: Sessions 1 to 4 (3 months) intensive education; Sessions 5 to 8 (9 months) review of education material. | |
| Outcomes | 1. Average number of calories consumed per day. 2. Weight (kg). Assessments at baseline and follow up 1 year later. | |
| Notes | POI: daily calorie intake. Attrition: one family failed to complete follow‐up assessment at 1 year. 3 familles withdrew after the third treatment session. No significant difference between those that completed study and those that dropped out. Groups were comparable with regard to age, parent's marital status and SES of family. Equivalence at baseline doubtful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors recorded the drop‐outs (33%) and presented the reasons. However, reasons for drop‐outs are not reported separately for both conditions. They additionally reported that a comparison of children who withdrew from the study and those who completed the study protocol yielded no significant differences on demographic and anthropometric data' (Powers 2003 p. 304). |
Powers 2005.
| Study characteristics | ||
| Methods | Parallel RCT. Comparing behavioral and nutrition treatment (BEH) with usual standard care (CTL). Cross‐over from CTL to BEH offered after stage 1 of study finished with 3‐ and 12‐month follow up. Hypotheses: 1. BEH would improve child's energy intake relative to CTL; 2. improvements would be maintained for 12 months. Computer‐generated block randomisation code with independent blind allocation. | |
| Participants | Population of interest N = 21 with n = 14 meeting criteria. Four withdrew or lost to follow up. Thus, n = 10 participants included aged 18 ‐ 48 months with confirmed diagnosis of CF with pancreatic insufficiency; diagnosis for at least 3 months; and, on unrestricted fat diet. Exclusions for those with developmental delay; receiving supplemental enteral nutrition; an additional diagnosis or medication for condition known to affect growth; baseline diet record of 120% RDA or greater. | |
| Interventions | 1. BEH (N = 4) nutritional counselling ‐ targeted at 1 meal each week. 3 focus areas: a. increasing calorie and fat intake; b. dosage and timing of pancreatic enzymes; c. teaching parent management skills. Treatment over 8‐week period with baseline at week 1 and 6; intervention sessions during weeks 3 ‐ 6. 2. CTL (n = 6) scheduled clinic visits every 3 months with CF and dietitian consulted whenever diet and growth issues were identified. | |
| Outcomes | Change in average energy intake % fat intake measured with 7‐day diet diaries 1 week before intervention and 1 week after intervention (8 weeks apart). | |
| Notes | POI: change in average energy intake per day over a 7‐day period from pre‐ to post‐treatment. 71% recruitment rate. No significant difference between the groups at baseline for age, weight, height, sex, nutritional status ethnicity or marital status of caregiver. Attrition: 2 families did not attend regularly and 2 declined to participate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors described the randomisation process in detail. 'The computer‐generated randomisation code was developed independently by the study biostatistician.' (Powers 2005, p. 1443) |
| Allocation concealment (selection bias) | Low risk | The method of concealment was described in detail by the authors. They reported that a decentralised blinded research staff member then was given a copy of the list. Once a family was enrolled [...], another research staff member called the decentralised research staff member and obtained the random assignment.' (Powers 2005, p. 1443) The authors noted additionally that all relevant study team members were unaware of the specific allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and personnel providing intervention could not be blinded to intervention as usual for psychological interventions. The primary outcome 'daily calorie intake' was assessed via self‐reported daily diet dairies provided by the parents. The measure, which is not objective, might be influenced by the knowledge of allocation to one of the conditions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported that ITT analysis has been conducted for the three‐month follow up. Two families failed to complete the dietary record at this time point. Scores for the two participants were imputed 'by calculating the mean change in: (1) energy intake per day; (2) percentage of RDA; and (3) percentage of fat intake from post‐treatment to 3‐month follow up for the 7 completers. These mean changes then were used to calculate estimated 3‐month follow up data for the 2 noncompleters' (Powers 2005, p. 1446). Furthermore, they reported that two other approaches for imputation revealed similar results. |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s pre‐specified outcomes have been reported. It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. |
Quinn 2004.
| Study characteristics | ||
| Methods | RCT. The effectiveness of brief telephone 'Motivational Interviewing' intervention in improving adherence to nebulised antibiotics in adults with CF has been evaluated (compared to treatment as usual). |
|
| Participants | Population of interest, N = 102 (patients prescribed nebulised colistimethate). Patients who met eligibility, n = 47 (patients invited to the study). Number of invited patients who declined, n = 8. Prior to randomisation all participants were given a Prodose nebuliser and used it for a 3‐month period to become accustomed to it and to generate baseline data on adherence. Number of dropouts, n = 4. Number of patients who received intended treatment, n = 35. Inclusion criteria: ‐ participants were diagnosed with CF prior to the study (by 2 positive sweat tests or recognised CF genotypes); ‐ age > 18 years; ‐ attending regional CF clinic; ‐ prescribed Colistimethate sodium delivered via a ProdoseTM Adaptive Aerosol Delivery system nebuliser. Exclusion criteria: ‐ lung function FVC < 30%; ‐ being involved in other trials; ‐ being pregnant. |
|
| Interventions | 1. Treatment as usual plus brief 'Motivational Interviewing' intervention (n = 16). Delivered via telephone by the principal investigator of the study. Was delivered over a 3‐month period with up to 6 MI sessions (fortnightly). Audiotapes of intervention were supervised and checked to ensure treatment fidelity. 2. Treatment as usual alone (n = 19). |
|
| Outcomes |
Primary outcome Difference in adherence (to aerosolised antibiotics) pre‐ and post‐intervention measured electronically monitored using Prodose. (Calculated for the previous 3 months in terms of the number of times the devise was used over the number of times its use was prescribed; 0‐100+%). Lung function measured as FEV1 and FVC. Secondary outcomes Were measured with questionnaires for anxiety (STAI‐Short Form; HADS‐A), depression (HADS‐D), and quality of life (CFQOL). Physiotherapists who administered questionnaires pre‐randomisation and immediately post‐intervention were blind to study allocation. |
|
| Notes | Tratement fidelity: the authors reported that audiotapes of the consultations were used in supervision, and were checked by GL to ensure treatment fidelity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors provided the information that the participants were randomised using a computer generated pseudo‐random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised using a computer generated pseudo‐random number generator. But the authors did not provide information about adequate concealment of allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel providing the MI telephone intervention could not be blinded to intervention as usual for psychological interventions. Physiotherapists who administered questionnaires were blind to study allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors provided the information that of the 39 participants randomised 4 dropped out during the study for the following reasons: possible side effects of the medication, preferring the previous nebuliser, receiving a lung transplant. Four participants were repeatedly unavailable, but were included in the intention to treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | The information is insufficient to judge whether 'low' or 'high' risk. |
Stark 1996.
| Study characteristics | ||
| Methods | Parallel RCT with half participants receiving intervention first then other half 3 months later ‐ not reported. 4 families changed group after randomisation ‐ due to conflicting vacation scheduling. Thus not truly randomised. | |
| Participants | N = 10. 1 withdrew from control group after randomisation. Total sample n = 9. Age range: 5.3 years to 10.1 years. Mean age: 7.3 years (SD = 1.7). | |
| Interventions | 1. Group behavioural intervention (n = 5). 7 weekly sessions ‐ baseline assessment plus snack, breakfast, relaxation skills training, lunch, dinner and maintenance strategies targeted over following 7 sessions. 2. Wait list control (n = 4). Parent meeting and 7‐day food diaries at times corresponding to baseline and last week of intervention. | |
| Outcomes | 1. Calorie intake. 2. Anthropometric measures ‐ weight, height and skinfold. 3. PFT. 4. REE. 5. Physical activity ‐ Caltrac electronic accelerator. Assessments at baseline, then 3 months and 6 months post‐treatment. | |
| Notes | Unclear what population the sample was drawn from. POI: Daily calorie intake. ITT ‐ unclear. Blinding ‐ unclear. No significant difference on z scores for weight or age before treatment. Control group average SES group II ‐ technical workers. Intervention group average SES III ‐ skilled labour. Equaivalence at baseline doubtful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not describe details of random generation process. It is just stated that 'the nine subjects were randomly assigned to either a behavioral intervention or a wait list control group' (Stark 1996). |
| Allocation concealment (selection bias) | Unclear risk | The authors did not provide information about adequate concealment of allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and personnel providing the intervention were not able to be blinded due to the nature of the intervention and the study design (wait‐list‐control design). The main outcome 'calorie intake' is assessed via self‐reported daily diet dairies. Due to that the measure is not objective and might be influenced by the knowledge of allocation to one of the conditions. All other objective measures (e.g. weight) are not likely to be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported that there was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | The authors reported alls pre‐specified outcomes. It is unclear if additional outcomes were pre‐specified in the study protocol but not reported. |
Stark 2003.
| Study characteristics | ||
| Methods | Parallel RCT. Comparing calorie intake and weight gain in 2 group interventions. Hypothesised that those receiving a behavioral intervention would eat more calories and gain more weight. | |
| Participants | Population of interest, N = 10. 3 dropped out. Total sample, n = 7. Children aged 4 to 12 years with CF and their parents. Children had chronic pulmonary disease, pancreatic insufficiency and weight below the 40th percentile. Mild disease, chronic phase. | |
| Interventions | Group educational & behavioural interventions: 1. nutritional education (n = 4); 2. behavioural intervention (n = 3). 7 sessions: baseline, 5 sessions (90 min each) plus 1 follow up session. | |
| Outcomes | 1. Calorie intake. 2. Weight. 3. Quality of parent/child interaction at mealtime. 4. CF coping skills. 5. Adherence to CF regimen. Assessments at baseline, post‐treatment and 6, 12 & 24 months follow up. | |
| Notes | POI: daily calorie intake. Unclear what the population of interest was and how sample was selected. Attrition ‐ 3 dropped out. One spoke only Spanish. 1 had 3 children with CF, 1 of whom was hospitalised. 1 had conflicting time commitments. Equivalence at baseline unclear but unlikely. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors described that families were assigned based on family availability to time slots, which were in a second step then randomly assigned to one of the conditions 'via a coin flip' (Stark 2003, p. 244). |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and personnel providing the intervention were not able to be blinded due to the nature of the intervention. One primary outcome 'daily calorie intake' is assessed via self‐reported daily diet dairies. Due to that the measure is not objective and might be influenced by the knowledge of allocation to one of the conditions. The other objective measure used as primary outcome ('weight') is not likely to be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors described in detail the attrition and the reasons for families who dropped out, but it is relatively unclear what the population of interest was and how many families were randomly assigned. Out of originally 15 families, who agreed to participate, seven families completed treatment and follow up (Stark 2003, p. 240). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcome variables (primary and secondary) are reported. |
Stark 2009.
| Study characteristics | ||
| Methods | RCT. Compared behavioral intervention plus nutrition education to nutrition education alone to improve caloric intake and weight in children with CF. Hypothesis: Children receiving the behavioral plus nutrition education intervention would have a significantly greater increase from pre‐treatment to post‐treatment and through 24‐month follow up on primary and secondary outcomes. |
|
| Participants | Population of interest, N = 177 (met eligibility). Number randomised, n = 79. Number of participants received the intervention = 67. group 'nutrition education', n = 34. group 'behavioral intervention plus nutrition education', n = 33. There were 6 drop outs in both arms prior to treatment, leaving 67 participants for analysis. Recruited from 5 CF centres located in the Eastern, Midwestern, and Southern USA. Children from 4‐12 years with a diagnosis of CF by sweat test, pancreatic insufficiency; and weight for age and height ≤ 40th percentile. |
|
| Interventions | Behavioral intervention and nutrition education in group setting. 1. Nutrition education (n = 34), 2. Behavioral intervention for change around nutrition an energy (Be‐In‐CHARGE!; n = 33) (available online at www.oup.com/us/pediatricpsych). 7 sessions (each 90 minutes): pre‐treatment (session 1), 2 weeks later 5 weekly groups sessions (sessions 2 to 6), 2 weeks later post‐treatment (session 7; follow up). |
|
| Outcomes |
Primary Outcomes Change in caloric intake and weight pre‐ to post‐treatment. Secondary outcomes %EER (percentage of the estimated energy requirements; calculated by subtracting EER for an active child of same age and gender from individual subject's calorie intake X 100), BMIZ (BMI z scores), weight, %EER, BMIZ (assessed at baseline and post‐treatment) and additionally height, HAZ, and FEV1 were examined at 24 months following treatment. Caloric intake was assessed at baseline, post‐treatment, 3, 6, 12, 18 and 24, months follow up. |
|
| Notes | In one report effects of the intervention on 'Family Interactions at Mealtime' were reported, but data was not sufficient. For detailed information on this specific outcome the leading author has been contacted and we are waiting information. Treatment fidelity: the authors reported that 'treatment fidelity was assessed by raters coding 4 videotapes from each of the 7 sessions for each intervention.' (Stark 2009, p.917). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were 'randomised to the treatment arms by coin flip by research assistant and postdoctoral fellow together' (Stark 2009, p.916). |
| Allocation concealment (selection bias) | Low risk | Assignment could not been foreseen by participants and investigators enrolling participants because of coin flipping by research assistant and postdoctoral fellow together. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The authors of the study state that 'families were never explicitly told which treatment they had been assigned' (Stark et al 2009, p.916). But, 'as with any behavioral intervention, it is not possible to keep subjects unaware of the treatment they are receiving or therapists the treatment they are providing' (Stark 2009, p.921). No details are provided about blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 79 enrolled children 40 were assigned to the nutrition education group (NE) and 39 to the behaviour plus nutrition education group. There have been 6 drop outs in both arms prior to treatment. Data of 67 children was available for analysis post‐treatment (NE n = 33 and behavioural plus nutrition education intervention n = 34). 24 month follow‐up data of 28 children in the behaviour plus nutrition education intervention group and of 31 children in the NE group was available for analysis. The authors provided a flow diagram of participants randomised to both study arms and assessed at each point in time from baseline to 24‐month follow up (seeStark 2009, p.916 Figure 1). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified outcomes have been reported. |
Taylor 2003.
| Study characteristics | ||
| Methods | RCT. Compared written self‐disclosure intervention to standard care (wait‐list control condition). Hypothesis: patients who completed the written self‐disclosure procedure would evidence improvement in health care utilization, disease severity and subjective health status 3 month following the intervention. |
|
| Participants | Population of interest: N = 81 (met eligibility). Number randomised: 70. Number of participants received the intervention: 70. Dropouts: 14. Excluded from data analysis because of unusual health care utilization patterns: 17. Final sample size: 39, intervention group (WSD) n = 18, control group (SMC) n = 21. Recruited from 2 CF centres located at 2 children’s hospitals in the southeastern part of the USA. Initial eligibility criteria included: (1) a diagnosis of CF; (2) age at least 15 years; (3) enrolled in the CF clinic for at least 9 months; (4) physical and mental, ability to complete the research protocol as judged by, the project staff and CF physician or nurse; (5) willingness, to write for 20 min on 3 separate occasions, over a 5‐day period and completion of self‐report, measures on two occasions over the next 3 months; and (6) access to a telephone on the days that the participants were to write at home. |
|
| Interventions | Written self‐disclosure intervention: 3‐sessions written self‐disclosure intervention, adapted from Pennebaker's laboratory‐based protocol First session in a private room within the CF clinic, two home‐writing sessions prompted by a telephone call (each 20 minutes). Instructions were in accord with those of Pennebaker (1989, 1993, 1997) and required that participants write about their "deepest thoughts and feelings about the most distressing experience of their entire life for a period of 20 minutes" (Pennebaker 1997). |
|
| Outcomes | Health care utilization: number of (1) outpatient visits; and (2) inpatient hospitalizations days. Physiological disease severity: (1) FEV1; (2) BMI. Subjective health status: (1) 'The Patient Health Questionnaire' (PHQ; Spitzer Taylor, Wallander, Anderson, Beasley, and Brown et al 1994; Spitzer, Kronke, Williams et al 1999): symptoms of (a) depression; (b) anxiety; (c) somatic complaints; and (d) psychological distress. (2) 'The Stressful Life Events Scale': 10 psychosocial complaints common among health care seeking populations (e.g., difficulties with family support, problems with significant others, and financial concerns); (3) SF‐12: severity and frequency of 12 physical and mental health problems, as well as of their impact on the patients' overall perceived health status. Feasibility and acceptability: modified version of the 'Visit Specific Satisfaction Questionnaire' (VSQ; Ware & Hays 1988). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors provide insufficient information about the sequence generation. After contacting the authors they provided the information that they followed a published random numbers sequence and assigned the patient to one of the two treatments based on the next number in the sequence being odd or even. |
| Allocation concealment (selection bias) | Unclear risk | The authors provide insufficient information on the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The physician and the clinic staff were not informed as to whether individual patients were in the WSD or the SMC condition. Patients and providers of the intervention knew who was in the WSD or the SMC condition (Taylor 2003). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of drop‐outs in each group is not reported, demographic comparison data between those excluded and included in the final sample, using T‐tests and chi‐square analyses, revealed no significant differences between the two groups. |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified outcomes have been reported. |
Vandemheen 2009.
| Study characteristics | ||
| Methods | Single blind RCT with 1‐year follow up. Compared an evidence‐based decision aid for patients with advanced CF considering referral for lung transplantation to usual care. Primary objectives: to assess whether the decision aid increased knowledge about patient options, improved realistic expectations, and decreased decisional conflict. |
|
| Participants | Population of interest: N = 155 (assessed for eligibility). Number randomised: 149. Number of participants received the intervention: 148 (intervention group n = 70; control group n = 79). Final sample size after 1‐year follow up: N = 125 (intervention group n = 60; control group n = 65). Recruited from nine Canadian and five Australian outpatient CF centres. Eligibility criteria: 1. > 18 years of age; 2. FEV1 ≦ 40% predicted; 3. Able to read English or French. Exclusion criteria: 1. patients who previously received a lung transplant; 2. patients on lung transplantation waiting list. |
|
| Interventions | All study patients received an educational session on the process of referral and the risks and benefits of lung transplantation from their CF physician and/or CF team. All patients received a copy of a pamphlet entitled 'Cystic Fibrosis and Lung Transplantation' and were provided addresses to access two generic websites on lung transplantation. After randomisation: Intervention group: Received a sealed package containing a paper version of the decision aid and a website address with a user name and password, where they could access the decision aid online (http://decisionaid.ohri.ca/decaids.html). Control group: received a package contained blank pages and a letter explaining why blank pages were included. |
|
| Outcomes | The following primary outcomes were assessed at baseline and 3 weeks after randomisation: participants’ knowledge and realistic expectations with designed questionnaire (structured format as per the International Patient Decision Aids Standards Guidelines); decisional conflict scores (Decisional Conflict Scale, a validated 16‐item scale). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors reported that randomisation was performed via a "a randomization schedule prepared through a computer‐generated random listing of the two treatment allocations blocked in variable blocks of two or four and stratified by site and by infection status with Burkholderia cepacia" (Vandemheen 2009, p.762). |
| Allocation concealment (selection bias) | Low risk | The authors reported that a central allocation was conducted (Vandemheen 2009, p. 762). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The authors provided the following information regarding blinding: "Neither research staff nor medical staff as aware of the treatment assignment before and after randomization" (Vandemheen 2009, p.762). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of drop‐outs for 3‐week follow up and 12‐month follow up were reported for each group. |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were pre‐specified as primary and secondary outcomes. |
Wilkinson 2008.
| Study characteristics | ||
| Methods | RCT. Prospective pilot study to investigate the feasibility of a video link to support patients on the transplantation waiting list and their families. |
|
| Participants | Patients on the transplantation list, at least 16 years of age, with a confirmed diagnosis of CF and willing to have an ISDN line installed in their home. Number randomised: 16, median age 27 years (range 21 – 41). Number of participants completed the intervention: 7 (4 on telemedicine and 3 in the control arm). Of those who did not complete the study four patients died, three patients received a transplant, one withdrew following randomisation and one was too unwell to continue. |
|
| Interventions | Telemedicine additional to standard care: the participants were provided with an ISDN line to their home and a videoconferencing unit was connected to their home television set. Participants were also given a micro‐spirometer, pulse‐oximeter and a supply of single use clinical thermometers. Contact was made, on a weekly basis, at a time agreed by the patient and assessor (senior physiotherapist or nurse consultant). The topics which were discussed included: non‐invasive ventilation; haemoptysis; physiotherapy and amount of sputum; mobility; difficulties with any clinical procedures; appetite and weight; and any other problems as appropriate. Control group: standard medical care. |
|
| Outcomes | 1. 'Cystic Fibrosis Quality of Life Questionnaire'. 2. 'Beck Anxiety Inventory'. 3. 'Beck Depression Inventory'. 4. Seven‐point questionnaire which included the number of visits to the cystic fibrosis clinic, general practitioner (GP), courses of intravenous antibiotics, length of hospital inpatient stay and how many visits were made either to a hospital or to the GP. 5. 'Borg Anxiety Scale', which was completed before and after each clinic attendance and after telemedicine link‐up. 6. Coping of carers: COPE questionnaire (Carver 1997). Patients and their caregivers were asked to complete a telemedicine satisfaction questionnaire on completion of the study. |
|
| Notes | Authors were contacted for detailed quantitative data on outcome measures, but we did not receive a response within the time updating the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a physiotherapist distributing a pre‐prepared sealed envelope, which was made by a third party not involved with recruitment (Wilkinson 2008, p. 183). |
| Allocation concealment (selection bias) | Low risk | Participants and investigator could not foresee assignments because the authors reported that they used sequentially numbered sealed envelopes (Wilkinson 2008, p.183). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Due to the nature of the intervention the participants and teams providing the intervention could been blinded, but he authors provided no information on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors reported a high number of dropouts. The number of dropouts for each group is unclear. Reasons for dropouts were reported: 4 patients died, 3 patients received transplant, 1 withdrew, and 1 was too unwell to continue (Wilkinson 2008, p. 183). |
| Selective reporting (reporting bias) | High risk | Means and SDs for all outcome parameters for intervention and control group were not reported in the published article. |
ACT: airway clearance techniques BEH: nutritional and behavioural intervention BMI: body mass index CCT: controlled clinical trial CF: cystic fibrosis CFK: CF knowledge CPT: chest physiotherapy CTL: control condition FEV1: forced expiratory volume at one second FVC: forced vital capacity HLOC: health locus of control KG: kilogram ITT: intention‐to‐treat LOC: locus of control Mean FEF25-75%: forced expiratory flow during the middle of the maneuver NIH: National Institute of Health PEFR: peak expiratory flow rate PFT: pulmonary functioning POI: primary outcome indicator POMS: profile of mood states QOL: quality of life RCT: randomised controlled trial RDA: recommended daily allowance REE: resting energy expenditure RISCS: role play inventory of situations and coping skills SD: standard deviation SES: socioeconomic status STAI: state trait anxiety inventory STAIC: state trait anxiety inventory (children)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barbero 1996 | This is a lecture with a broad remit and does not describe an intervention study. |
| Bartholomew 1997 | This was pre‐post design ‐ participants were not randomised to the treatment and comparison groups. |
| Bingham 2010 | This is a study on breath biofeedback ‐ the study had no comparision group (no RCT design). |
| Brady 1991 | The intervention is neither psychological nor does it have a psychosocial focus. |
| Brandt 1996 | This is not an intervention study ‐ it is a cross‐sectional survey. |
| Brown 1994 | This is not an intervention study ‐ it is a case‐control study without a psychosocial focus. |
| Castellani 2011 | This is a study on an interactive computer‐based program for education in cystic fibrosis carrier tests. The participants were not a population of interest (participants did not have CF or were family members of patients with CF). |
| Cheuvront 1998 | This is a parallel RCT to evaulate two approaches to CF gene pre‐test education and carrier testing. Genetic clinic education and testing versus Home‐based education and testing. The approaches were both educational which are by defintion not eligible for this update of the review. |
| Clayton 1995 | This a study of population screening. The participants do not have CF nor related to anyone with CF ‐ they were also not CF carriers and had no higher than the average risk of being a carrier. |
| Davis 2002 | This is a parallel RCT comparing an educational intervention using a CD‐ROM with a wait list control. The approach was educational which is by defintion not eligible for this update of the review. |
| Dodd 1997 | This is not an intervention study ‐ it is a study validating a psychological measure on a CF population. |
| Dodd 2001 | This is a study on motivational interviewing for exercise performance in patients with cystic fibrosis. Data has not been published in a journal article and is not available via the author anymore. |
| Donaldson 1995 | This study is a survey of attitudes and a comparison of 2 approaches to population screening. The participants do not have CF nor are they related to anyone with CF ‐ there is no higher risk of their being a carrier than average. |
| Downs 2006 | This is a multi‐centre parallel RCT investigating the effectiveness of a self‐management education programme called ’Airways’. The approach was educational which is by defintion not eligible for this update of the review. |
| Eng 1997 | This was a study of attitudes towards screening for CF in a population of Askenazi Jews referred for TSD testing. There was no comparison group and no higher risk of being a CF carrier than average. |
| Falkman 1977 | This is not an intervention study ‐ it is a descriptive study of a random sample of children with CF. |
| Gee 2000 | This is not an intervention study ‐ it is a report of a part of the development of a psychological measure for CF. |
| Georgiopoulos 2011 | This is a study on ADHD treatment. It is a chart review. |
| Goa 1997 | This is not an intervention study it is a review of the literature. The intervention reviewed is not psychological although the reported outcomes are psychosocial. |
| Goldbeck 2001 | This study has a pre‐post design ‐ there is no comparison group. |
| Goldbeck 2011a | This is a study on an inpatient rehabilitation program (including psychological intervention), but there was no control group for comparing outcomes of the program. |
| Gonciartz 1988 | This study is not concerned with CF although some of the participants may have had CF. The intervention is not psychological nor does it have a psychosocial focus. |
| Green 1995 | This is a report of a survey of obstetricians views and not an intervention study. The participants do not have CF nor are they related to anyone with CF although they may have a professional relationship with someone with CF. |
| Gremse 1998 | This is a descriptive case‐control study and not an intervention study. |
| Grody 1997 | This is a study of population screening ‐ the participants have no history of CF and no higher than the average risk of being a carrier for CF. |
| Gulmans 1999 | There was no comparison group in this study. |
| Haddow 1999 | This was not an intervention study ‐ it was a summary of the views of professionals who were attending a conference regarding screening for CF. |
| Hains 2001 | This intervention study has no comparison group. |
| Hains 1997 | This intervention study has no comparison group. |
| Hodson 1997 | This is a descriptive case‐control study ‐ it is not an intervention study. |
| Jelalian 1998 | This is not an intervention study ‐ it is a review of the literature and meta‐analysis of interventions for CF. Only 1 of the studies described is a RCT and that is reported separately here (Stark 1996). |
| Johnson 2001 | This intervention study has no comparison group. |
| Kollberg 1982 | This is a descriptive account ‐ it is not an intervention study. |
| Krauth 1999 | The intervention in this study was not psychological nor did it have a psychosocial focus. |
| Le Heuzey 1997 | This is not an intervention study ‐ it is a descriptive single group assessment psychological adjustment. |
| Leonard 1995 | This is not an RCT it used a quasi experimental design to compare two approaches to education information about carrier testing for CF. The participants do not have CF nor do they have any higher risk of being a carrier than average. |
| Lindström 1994 | This is not an intervention study ‐ it is a descriptive single group study and it is not wholly concerned with CF although some of the participants had CF. |
| Loader 1996 | This was a study of attitudes and responses to population screening ‐ the participants did not have CF nor were they and higher risk of being a carrier. Of the group identified as carriers there was no comparison group and no psychological intervention was offered. |
| Magnussen 1992 | This study was not wholly concerned with CF ‐ it was one of several lung diseases investigated. The intervention was not psychological nor did it have a psychosocial focus. |
| Phillipson 2000 | This study had no comparison group and the intervention was not psychological nor did it have a psychosocial focus ‐ no psychosocial outcomes were included. |
| Pollitt 1997 | This is not an intervention study it is a systematic review of the literature with respect to the cost‐effectiveness of population screening. There is no review of psychological interventions nor is there a psychosocial focus. |
| Pop Jordanova 2010 | This is a study on 'Biofeedback', but there was no control group which did not receive the intervention. |
| Ralston 2008 | This is a study on of smoking cessation counselling for parents, but CF parents were not a major subgroup of the study sample. No information about the number of participating CF parents is provided. |
| Ramström 2000 | Although this study did not have a psychological intervention there was a psychosocial focus. It was however considered to be more concerned with service delivery. |
| Rault 2012 | This is a study investigating a CF quality improvement program. It is a pilot study testing the applicability and effectiveness of Clinical Microsystems Approach to improve CF care in France. No control condition. |
| Rodrigue 2005 | This study is a study on quality‐of‐life therapy for patients awaiting lung transplantation. The study included mostly patients with COPD (only 2 patients with CF). |
| Rozenfeld 2012 | This is a study investigating Snoezeln in infants ages 0‐3 years with CF in a single centre without a comparator. |
| Stapleton 2001 | This is a parallel RCT comparing the effectiveness of an educational programme with standard care for knowledge about nutrition. The approach was educational which is by defintion not eligible for this update of the review. |
| Stark 2002 | Altough the article describes outcomes of a RCT study, only data for intervention group was collected and is provided for the outcome 'Calcium intake'. |
| Thompson 1990 | This is not an intervention study ‐ it is a cross‐sectional, controlled, descriptive study of psychological adjustment in children with CF. |
| Thornton 1995 | This is a study comparing three methods for conveying information about population screening. The participants do not have CF nor do they have any higher risk of being a carrier than average. |
| Trapp 1998 | This ia parallel CCT of 2 interventions with a convenience control group sample to investigate whether self‐administration of drugs leads to greater knowledge about medications. The approach was educational which is by defintion not eligible for this update of the review. |
| Tullis 1995 | This is not an intervention study ‐ it is an argument for a CF specific psychological measure. |
| Villari 1994 | This is not an intervention study and it is not concerned with CF although some of the participants may have had CF. There is no psychosocial focus to this cross‐sectional case‐control study. |
| Ward 1999 | This is not an intervention study ‐ it is a descriptive study comparing three groups with respect to energy expenditure. |
| Watson 2004 | The previous ongoing study is now published as a randomized controlled trial of a new behavioral home‐based nutrition education program, "Eat Well with CF," in adults with cystic fibrosis. The approach was educational which is by defintion not eligible for this update of the review. |
| Watson 2006 | The previous ongoing study is now published as a randomized controlled trial of a new behavioral home‐based nutrition education program, "Eat Well with CF," in adults with cystic fibrosis. The approach was educational which is by defintion not eligible for this update of the review. |
| Welkenhuysen 1996 | This is not an intervention study ‐ it is a survey of attitudes towards population screening in adolescents. The participants do not have CF nor are they related to anyone with CF. They have no higher risk of being a carrier for CF then average. |
| Williams 1997 | This intervention study does not have a comparison group. |
| Wolter 1997 | This is not a psychological intervention albeit with a psychosocial focus. It was considered to be more concerned with service delivery. |
CF: cystic fibrosis TSD: Tay Sachs Disease
Characteristics of studies awaiting classification [ordered by study ID]
Bryon 2000.
| Methods | Assessed a new developed home visit program to improve health status and psychosocial functioning, randomised allocation to intervention group vs waiting list control group. |
| Participants | Children with CF, are from families with significant psychosocial stresses (consequences: sub‐optimal treatment, irregular clinic attendance, erratic health status). |
| Interventions | Program included at least four home visits from both nurse and psychologist (in addition to routine nurse specialist input) over a six‐month period. |
| Outcomes | 'General Health Questionnaire', 'Parenting Stress Index', 'Harter self‐esteem' (for children aged over 8 years), pulmonary function (FVC, FEV1), Take‐up of clinic appointments, drug prescription and planned admissions. |
| Notes | Published as an abstract. |
Cannon 1999.
| Methods | Explored the feasibility and benefit of in‐home videoconferencing using inexpensive equipment interfacing to a patient’s standard telephone and television set, 2 groups (study vs control). |
| Participants | 10 patients aged 6 ‐ 13 years. |
| Interventions | Study group received reinforcement of the education message from each clinic visit with weekly in‐home videoconferencing contacts by nurse, TR dietitian, or social worker, plus routine clinic education; control group received only routine clinic education. |
| Outcomes | PFT, weight triceps skin fold thickness, mid‐arm muscle circumference, prealbumin, a simple test of basic CF knowledge, the Hopelessness Scale for Children, the Family Empowerment Scale, Client Satisfaction Survey. |
| Notes | Published as abstract. |
Cummings 2011.
| Methods | Pilot study that aimed to assess a mentor‐based behavioural modification intervention to enhance self‐efficacy for self‐management skills and QoL, single‐blind RCT, 3 groups (mentor vs mentor plus mobile phone vs control). |
| Participants | 17 adolescents and adults aged 14 ‐ 48 years. |
| Interventions | Mentor‐based behavioural modification intervention with mentorship and self‐monitoring via a modified mobile phone with immediate visual feedback on clinical status. Duration: 6 months. |
| Outcomes |
Primary outcome
Self‐efficacy. Secondary outcomes CF‐related and generic QoL, participant feedback on their experience via semi‐structured interviews, lung function and acute healthcare utilization. |
| Notes | Published as abstract. |
Davis 1990.
| Methods | Examined the psychosocial effects, safety and efficacy of HIVAT comparing to conventional hospital therapy over short term (one exacerbation). |
| Participants | 78 CF patients with acute exacerbation (1 dropout). |
| Interventions | Home IV antibiotic therapy as part of a multicentre randomised study, all patient were treated with IV tobramycin and ceftazidime every 8 hours (one exception: tobramycin and piperacillin). |
| Outcomes | Self‐concept (< 18: PH; Adults: TSC), Anxiety (8th grade and under: STAIC, all other: STAI), CHIP Weight, resting respiratory rate, NIH score, FEV1, FVC. |
| Notes | Published as abstract. |
Goldbeck 2013.
| Methods | |
| Participants | 151 CF patients aged 6 years or older recruited in 3 CF outpatient clinics. 300 patients from other CF centres receiving usual care and matched for age, sex and lung function were recruited as controls. |
| Interventions | Follow‐up period: 2 years. Patients in intervention group: program based on 'Individual Treatment Agreements' (ITA) between patient or caregiver and CF physician. ITA's may focus on medical, nutritional and psychotherapeutic measures. |
| Outcomes |
Primary outcome Quality of care as assessed by the frequency of BMI and lung function measurements per year. Secondary outcomes Range of medical (mortality, lung function, BMI) and psychosocial (QoL, adherence, caregiver distress). Use of economic resources also assessed |
| Notes | Commenced clinically in May 2012. Co‐funded by the German Ministry of Health and the German CF‐association Mukoviszidose e.V. |
Hass 2012.
| Methods | Randomised, open‐label, parallel study on the effects of massage therapy on QoL in youth/young adults with CF. |
| Participants | Diagnosis of CF. Ages: 8 ‐ 21 years. Estimated enrolment: 24. |
| Interventions | Active Comparator: massage therapy. Treatment group receives pre‐determined massage therapy protocol x 5 over 10 ‐ 12 weeks. massage therapy protocol includes a blend of Swedish strokes and myofascial trigger point therapy. Initially, dosing will be more frequent. Treatments will be spaced out to determine the ability of the body to maintain a more efficient musculoskeletal system, especially related to respiratory and postural efforts. Each session will end with resting hands and relaxation strokes to signal the end of the session. This protocol invites increased mobility in the musculoskeletal system. The ultimate goal is to return connective tissue (including muscles and fascia) to a more relaxed and neutral state, thus allowing expansion and ease of movement of the areas of the musculoskeletal system being worked. Control group: no intervention. |
| Outcomes |
Primary outcome measures QoL Secondary outcome measures Pulmonary function Other outcome measures Pain |
| Notes | Study start date: July 2013; estimated completion date: December 2014. Country: USA. |
Hatziagorou 2011.
| Methods | Compares behavioral and nutritional intervention (BEH) with a usual care control condition (CTL) for CF children with pancreatic insufficiency. |
| Participants | Twenty‐four children with CF (aged 8 months to 18 years) were assigned to the BEH group. |
| Interventions | BEH included nutrition counselling to increase energy intake and child behavioral management training. |
| Outcomes | Energy intake (calories per day), weight gain, BMI z scores. |
| Notes | Published as abstract. Contact to leading author. Detailed data is not published. |
Huang 2010.
| Methods | Randomised open‐label, parallel study investigating the use of texting to promote chronic disease management. |
| Participants | Enrolment: 81. Ages: 14 to 22 years. Have CF, type 1 diabetes, or inflammatory bowel disease for at least 6 months. |
| Interventions | Experimental: SMS and Internet. The SMS and Internet group will receive information, tips, strategies, and questions related to the self management of chronic disease (cystic fibrosis, inflammatory bowel disease, or type 1 diabetes) on a web‐based program and via SMS messages. No intervention: control. The control group will receive monthly tip sheets on various health topics for adolescents and young adults. |
| Outcomes |
Primary outcome measures 1. Health related self‐efficacy Secondary outcome measures 1. Health knowledge 2. Disease health knowledge 3. Health literacy 4. QoL |
| Notes | Start date: October 2009; study completion: March 2012. Country: USA. |
Irons 2012.
| Methods | RCT. |
| Participants | 51 hospitalized children (mean age¼ 11.6 years, 35% male). |
| Interventions | Participants undertook 8 singing or 8 recreational sessions. |
| Outcomes | Respiratory muscle strength using maximal inspiratory and expiratory pressure (MIP and MEP, respectively), spirometry, and QoL were assessed at baseline, postintervention, and follow up. |
| Notes | Brisbane, Australia. |
Jessup 2010.
| Methods | RCT. Pilot study that aimed to assist patients to achieve increased levels of self‐efficacy through interactions with volunteer mentors coupled with technology supported self‐monitoring. |
| Participants | Adolescents and adults with CF. |
| Interventions | Volunteer mentors coupled with technology supported self‐monitoring. |
| Outcomes | QoL. Clinical outcomes measures. Feedback on all aspects of the intervention: the process, including the mentor relationship with the help of semi‐structured interviews |
| Notes | Published as abstract. Contact to the leading author MJ ‐ data is not published, yet. |
Kalnins 1996.
| Methods | |
| Participants | N = 13. Over ten years of age, less than 90% ideal weight for height or 5% reduction in weight over a three month period. Exclusion criteria: routine use of supplements, liver disease, insulin dependent diabetes and oxygen dependence. |
| Interventions | 1. Dietary counselling. 2. Nutritional supplement ‐ Boost Plus over a three month period. |
| Outcomes | 1. Pulmonary function FEV1. 2. Nutritional status: a. weight; b. height; c. weight for height; d. calorie intake; e. calorie intake; as per cent of predicted energy needs. |
| Notes |
Klig 1989.
| Methods | Effectiveness and psychosocial advantage of a self‐administered method of pulmonary therapy. FET was compared to CPT in a 2‐year prospective study. |
| Participants | 50 patients with mild to percussion lung disease, age from 8 ‐ 22 years |
| Interventions | FET included postural drainage, diaphragmatic breathing and forced expiration. CPT entailed postural drainage, percussion and vibration. |
| Outcomes | FET/CPT compliant, noncompliant PET`s, clincal scores, weight, x‐ray scores. |
| Notes |
Mischler 1998.
| Methods | Comprehensive RCT to study both benefits and risks of newborn screening and to determine if early diagnosis would improve the prognosis of children with CF. Compares the nutritional status of patients with CF identified by neonatal screening or by standard diagnostic methods. |
| Participants | 650 340 infants, 135 families of children diagnosed as having CF, 206 false‐positive families. |
| Interventions | |
| Outcomes | Height percentiles, weight percentiles, head‐circumference percentile, anthropometric indexes. Assessments of reproductive knowledge, attitudes and behaviours. |
| Notes |
Patel 2010.
| Methods | Parallel randomised controlled pilot study, single centre study. Research question: Effect of incorporating the CLCF‐Q into the annual review process. To assess the impact of a PROM on the Self Efficacy (SE) of parents and carers of children with CF. |
| Participants | Parents/carers of children with CF. |
| Interventions | Participants in the intervention group complete the CLCF‐Q. They receive feedback at their next clinic appointment from the team in the form of colour coded tables illustrating both the positive and negative issues raised in the CLCF‐Q. They are invited to talk to members of the CF team and others (consultants, CF nurses, dietitians, physiotherapists, psychologists, pharmacists, family doctors and the school) about the issues raised in the CLCF‐Q. |
| Outcomes | SE‐Questionnaire (SE‐Q) as an outcome measure. Participants complete the SE‐Q at 2 time points. Between the two time points the intervention group received the intervention. |
| Notes | Information is retrieved from an abstract. Information from the author: Data is not published, yet. |
Petzel 1991.
| Methods | The study use several objective adherence indices to evaluate adherence to medical/behavioral interventions across a broad‐age range of nonhospitalized CF patients, identify psychosocial attributes of patients and families associated opportunities for adherence‐promoting interventions. |
| Participants | Parents of 45 children with CF |
| Interventions | Health education intervention. 3 interventions: ‐ problem‐solving lesson in computerized form; ‐ problem‐solving lesson in written form. |
| Outcomes | Sociodemographic data, psychological test results, measures of the child health status (% predicted wt., x‐ray scores), compliance. |
| Notes |
Powers 2003a.
| Methods | Clinical study that compares a behavioral intervention to standard of care over a 2‐month period |
| Participants | Children ages 18 months to 4 years. |
| Interventions | Behavioral interventions to standard of care, and separate multiple baseline studies across meals (RDA guidelines). |
| Outcomes | Change in calorie intake per day. |
| Notes | Information is retrieved from Symposium Session Summary. |
Powers 2013.
| Methods | RCT. Children from 7 CF centres. |
| Participants | Children (aged 2 ‐ 6 years) with CF and pancreatic insufficiency. |
| Interventions | Behavioural and nutritional treatment versus an attention control group (CTL). Treatments occurred weekly for 8 weeks and then monthly for 4 months. Participants returned to standard care and a 12‐month follow up was conducted. CTL provided education and served as a behavioural placebo. |
| Outcomes | Daily energy intake (7‐day, weighed diet diaries) and weight for age z score change from baseline to post‐treatment (6 months) and height for age z score change from baseline to follow up (18 months). |
| Notes | ClincialTrials.gov: #NCT00241969. USA. |
Ruddy 2011.
| Methods | Randomised, open‐label, parallel study. |
| Participants | Estimated enrolment: 20. Ages: 12 ‐ 25 years. |
| Interventions | Active comparator: yoga (immediate start) group. This arm will start yoga sessions immediately after screening and will continue sessions for 8 weeks. This group will then continue with home yoga for an additional 8 weeks. Active comparator: Yoga (waitlist) group. This arm will continue regular CF therapies for 8 weeks and will start yoga sessions at week 9. |
| Outcomes |
Primary outcome measures 1. Number of participants with adverse events as a measure of safety 2. Pain scores on the Visual Analog Scale as a measure of tolerability Participants will be given a tolerance questionnaire after each yoga session which will assess their degree of musculoskeletal and chest pain on a visual analog scale. Vital signs will also be measured before and after each yoga session. Secondary outcome measures 1. QoL 2. Respiratory symptoms 3. Treatment adherence 4. Pulmonary function 5. Ease of breathing 6. BMI |
| Notes | Start date: February 2011, estimated end date: October 2011. Country: USA. |
Stark 1998.
| Methods | Multi‐site, clinical study examining two innovative approaches to diet education. |
| Participants | 33 children between ages 4 and 12 years have completed treatment and 26 of these have completed the 6‐month follow up. |
| Interventions | Nutrition Education (NE) addresses the issue of lack of dietary knowledge by providing families an intensive 7‐session program on nutritional suggestions for meeting the CF RDA for energy on a meal by meal basis. Behavioral Education (BE), combines the nutritional education component, as described, above plus training on behavioral child management skills such as contingent attention to appropriate eating, ignoring behaviours incompatible with eating, and use of contingent privileges for meeting calorie goals. |
| Outcomes | At post treatment the children in the BE groups demonstrated a significantly greater increase in caloric intake than the children in the NE group t(30) = 2.24, P = The BE group also showed a significantly greater weight gain compared to the NE. At the 6 month follow up both groups demonstrated a similar rate of weight gain from post treatment. Differences on weight gain post treatment to 6 months and pretreatment to 6 months were not significant between the two groups. |
| Notes | Information is retrieved from Symposium Session Summary. |
Wainwright 2009.
| Methods | RCT. Pilot study of an education and behavioural adaptation program (delivered via a mentorship system, telephone and IT tools) designed to enhance self‐management in adolescents. |
| Participants | 46 adolescents aged 12‐19 with CF. |
| Interventions | 3 groups: standard care (controls; N = 15); standard care + phone mentoring (N = 16); standard care, phone mentoring + IT‐tool (N = 15). Treatment lasted for 6 months with a further 6 months follow up, outcomes were re‐assessed at 3, 6, and 12 months. |
| Outcomes |
Primary outcomes Stanford Self‐Efficacy Scale
QoL (measured with CFQ‐R) Secondary outcomes Spirometry Height and weight z scores Qualitative data from 10 intervention participants and mentors |
| Notes | Contacted the leading author who provided the information that they are going to publish, but the data is not published, yet. |
Widman 2013.
| Methods | Randomised open‐label cross‐over study ‐ a pilot study of behavioural intervention for nutrition in CF. |
| Participants | Anticipate 30 into 2 groups. |
| Interventions | This is a feasibility study to try out a multi‐component behavioural intervention. The intervention is designed to help people with CF use their nutritional support to gain weight. The first group receive the behavioural intervention immediately. The second group receive the intervention after 3 months. All participants will be followed up for 6 months. Data will be collected every 6 weeks during clinic reviews. Experimental: Behaviour change intervention. Intensive behaviour change intervention for 3 months, followed by a maintenance phase for 3 months. Wait‐list ControlUsual care for 3 months, followed by intensive behaviour change intervention for 3 months. Note: Behavioral: Behaviour change intervention: the behaviour change intervention consists of feedback via a web‐based food diary 'coaching' with problem‐solving and implementation plansThe intensive intervention phase consists of 2 home visits, 8 telephone calls and 6 reminder emails.The maintenance phase consists of 3 telephone calls and 4 reminder emails. |
| Outcomes |
Primary outcome measures
1. Proportion of patients who accepted invitation to participate as a marker of feasibility
2. Proportion of people with CF who are randomised into the study after accepting the invitation to participate, as a marker of feasibility Secondary outcome measures 1. Participants' opinion about the behavioural intervention 2. Participant attrition rate and phases of study whereby the attrition occurs 3. Participants' opinion about the study processes 4. Participants' suggestions for further improvement of the intervention and study processes 5. Proportion of adults who fulfils the BMI criteria for recruitment with IT facilities 6. Resources needed by the investigators to deliver the intervention 7. Proportion of days with missing nutritional data, as a marker for feasibility Participants are requested to fill in their nutritional intake daily on a web‐based food diary. The investigators are interested to find out what the proportion of such data will be missing to determine the feasibility of collecting data with the web‐based food diary. Other outcome measures 1. BMI 2. Weight 3. BMQ score 4. The mean SRBAI score |
| Notes | Start date: Feb 2014. Anticipated end date: March 2015. Country: UK. |
Williams 1987.
| Methods | Explored the effects of intensive dietary counselling in adolescents with CF upon nutritional status. The participants were randomly divided into three groups (control vs counselling and normal food vs counselling and enteric coated pancreatic enzyme supplements). The groups were matched for age sex and Schwachman score. |
| Participants | 28 adolescents with CF aged 9 ‐ 18 years. |
| Interventions | The participants in the first group were the controls. Then the second group received a counselling to improve dietary intake to > 130% of RDI calculated form expected weight for height), using normal foods and simple supplements; the final group received counselling and enteric coated pancreatic enzyme supplements instead of conventional supplements. Duration: 1 year. |
| Outcomes | Change in weight; dietary intake; improvements in MAC. |
| Notes | Published as an abstract. |
BMQ: Beliefs about Medicines Questionnaire CF: cystic fibrosis CPT: chest physical therapy FET: forced expiration technique HIAT: home intravenous antibiotic therapy MAC: mid‐arm circumference PFT: pulmonary function tests QoL: quality of life RDI: recommended dietary intake SRBAI: Self‐Report Behavioural Automaticity Index
Characteristics of ongoing studies [ordered by study ID]
Quittner 2000.
| Study name | |
| Methods | RCT. The aim of the study was to compare the effects of BFST, an empirically supported treatment, to both the Family Learning Program (FLP) and standard care (SC). |
| Participants | 117 adolescents with CF ages 10 to 17 years.
Inclusion criteria ‐ Age 9.5 to 16.5 years ‐ Diagnosed with CF for a minimum of one year ‐ FEV1 greater than 35% predicted ‐ no other chronic disease (other than CF‐specific diabetes) ‐ no history of psychiatric treatment within the past years ‐ No evidence of intellectual impairment Stratified by age, sex, illness severity and family conflict |
| Interventions | Standard care versus 2 manualised and structured interventions. 11 family sessions with a registered nurse or licensed clinical psychologist 1. Standard care (n = 36) Teens received their usual care at the CF centre. 2. Family Learning Program (n = 40) 10 90‐minute psycheducational sessions over 6 months aimed at increasing knowledge about CF and its management. 3. 'Behavioral Family Systems Therapy' (n = 41) 10 treatment sessions (90 minutes) over 6 months plus 1 booster session 3 months later. Family problem‐solving, communication skills training, and cognitive restructuring were included in the intervention. |
| Outcomes | Evaluation post‐treatment and at 6, 12, & 18 months subsequently.
1. Treatment adherence:
a. electronic monitors;
b. self‐report;
c. daily‐phone diary.
2. Family functioning (family conflict;coping skills; communication):
a. self‐report;
b. audiotaped role‐play vignettes;
c. videotaped family discussions/interactions.
3. Quality of life:
a. CF questionnaire. Additionally: long‐term health outcomes and cost‐effectiveness. |
| Starting date | |
| Contact information | Prof. Alexandra Quittner, University of Miami, Department of Psychology. |
| Notes |
Quittner 2011.
| Study name | RCT of a behavioral adherence intervention for adolescents with CF: I change adherence and raise expectations (iCARE) |
| Methods | Study design: clustered‐randomised controlled study. The study is evaluating two adherence promotion interventions for adolescents, implemented by CF care teams. The centres are randomised to one of the types of interventions. Half of the centres will receive the Comprehensive Adherence Program (CAP) for 2 years. The other half will receive the Adherence Dashboard only in year 1 of the study and CAP in year 2 of the study. |
| Participants | Enrolled: 632 adolescents. Population of interest: adolescents diagnosed with CF who are prescribed chronic use of a pulmonary medication. Inclusion criteria: 1. male or female patients age 11‐120 years old;. 2. patients with a diagnosis of CF; 3. patients attend the accredited care centre for regularly scheduled clinic visits; 4. patient must be prescribed at least one of the following medications for at least 6 months prior to signing the informed consent (azithromycin; hypertonic saline; Pulmozyme®; TOBI®; inhaled compounded tobramycin); 5. Patient has consented to provide data to the CF Foundation Registry prior to conversion to PORTCFv. |
| Interventions | Comprehensive Adherence Program (CAP) consists of: 1. Adherence Dashboard ‐ a web‐based resource offering automated/regularly updated patients adherence estimates (based on pharmacy refill patterns and health information retrieved from registry); 2. Training in the CF My Way program ‐ a validated problem‐solving adherence promotion intervention. |
| Outcomes |
Primary outcome Medication adherence will be assessed via MPR derived from pharmacy refill records. (Time frame 24 months.) Secondary outcomes (Time frame 24 months.) CF knowledge Skills associated with CF treatments Health‐related quality of life (CFQ‐R) Lung function (FEV1) Pulmonary exacerbation CF hospitalizations |
| Starting date | October 2009. |
| Contact information | Prof. Alexandra Quittner, University of Miami, Department of Psychology. |
| Notes | This study is ongoing and is currently recruiting participants (31st July 2012). http://www.clinicaltrials.gov/ct2/show/record/NCT01232478?term=icare+adherence&rank=1) |
Quittner 2012.
| Study name | CFfone: A cell phone support program for adolescents with CF. |
| Methods | The investigators propose to evaluate the efficacy of a cell phone support program for adolescents and young adults (CFfone) in improving CF knowledge, treatment adherence, and quality of life compared to a CF‐related educational website intervention (control group). Study type: interventional. Study design: efficacy study, randomised allocation. |
| Participants | Inclusion criteria 1. Have a diagnosis of CF. 2. Be within the target age range of 11‐20 years old at enrolment. 3. Have regular access to an internet‐connected computer that does not prevent access to websites (i.e. firewall). Exclusion criteria 1. Have a developmental disorder that would affect ability to respond to survey questions. Estimated enrolment: n = 146. |
| Interventions | Cell phone support program for adolescents and young adults (CFfone): Is a behavioral intervention which contains a password‐protected, secure website, which can be accessed via web capable cell phone. The website provides age appropriate medical and behavioral information, disease management tools and social networking features for adolescents. CF‐related educational website intervention (control group): The interventions contains a CF‐related educational website which has some areas of CF information and services relevant to adolescents. |
| Outcomes |
Primary outcome The participants' CF‐related knowledge will be assessed via the CFK. It will be administered at baseline, month 3, month 6 and month 9. Secondary outcomes The participants' quality of life will be assessed via the CFQ‐R. The adolescent/adult version will be used for participants aged 15‐20 years and the CFQ‐R Older Child version will be used for participants ages 11‐14 years. Quality of life will be administered at baseline, month 3, month 6 and month 9. To measure treatment adherence a print out of CF related medications and pharmacy refill data will be collected. To obtain participants' prescription information in order to have adherence calculations for three month periods, the attending physician will fill out a prescribed treatment plan at every clinic visit during the study. Pharmacy refill data will be collected at baseline for the 12 months prior to enrolment; and again after the 9 month clinic visit for the 9 months of the study. The prescribed treatment plan will be collected at baseline, month 3, month 6 and month 9. Exploratory end point: social support. |
| Starting date | September 2010. |
| Contact information | Prof. Alexandra Quittner, University of Miami, Department of Psychology. |
| Notes | Information provided by the investigator Prof. Quittner: "This study is ongoing, an already completed enrolment with a sample size of 100 adolescents ages 11 to 20. The study is going on at 6 CF Centers. We anticipate that all measures will be completed in April of 2013." |
Riekert 2012.
| Study name | Building adherence to live with and navigate my CF experience (BALANCE). |
| Methods | The investigators propose to evaluate the relative efficacy of a Motivational Interviewing‐focused intervention (MI) in improving adherence and reducing CF‐related morbidity compared to a CF education intervention (CFE; attention control group). The investigators hypothesize that MI will result in improved regimen adherence and reduced CF morbidity compared to the CFE control group. Study type: Interventional. Study design: efficacy study; randomised allocation. |
| Participants | Inclusion criteria
Exclusion criteria
Estimated enrolment: n = 153. |
| Interventions |
CF Education This intervention is designed to increase knowledge and enhance the skills needed to optimise CF‐management. The strategies used to achieve improved adherence include providing didactic education and skills training, and proscriptively using behavioral modification strategies, such as positive reinforcement for desired behaviours, and problem‐solving training to overcome barriers. Motivational Interviewing The overarching goal for the intervention is to motivate and assist the participant to improve his/her adherence to the CF pulmonary medications. The intervention will begin by providing the patient personal feedback on their adherence(using pharmacy refill data) and health outcomes (e.g., trajectory of lung function values, frequency of exacerbations) as well as clinic‐level figures showing the association between adherence and health outcomes. |
| Outcomes |
Primary outcome A MPR will be calculated for each prescribed drug that is being monitored for adherence (time frame: 12 months). Secondary outcome Change in FEV1 % predicted (time frame: 12 months). |
| Starting date | July 2009. |
| Contact information | Kristin A. Riekert, PhD, The Johns Hopkins Adherence Research Center. |
| Notes | This study is ongoing, but not recruiting participants at the moment (20th July 2012). Information from http://www.clinicaltrials.gov/ct2/show/NCT01013896?term=adherence+cystic+fibrosis&rank=1). |
BFST: Behavioral‐Family Systems Therapy CF: cystic fibrosis CFK: Cystic Fibrosis Knowledge Questionnaire CFQ‐R: Cystic Fibrosis Questionnaire‐Revised FEV1: forced expiratory volume litres per second MPR: medication possession ratio QoL: quality of life RCT: randomised controlled trial SRBAI: Self‐Report Behavioural Automaticity Index
Differences between protocol and review
The Definition of psychological intervention was been changed to a more precisely definition of psychological intervention and classification types of intervention has been changed.
Due to a new Cochrane Review on 'Self‐management education for cystic fibrosis', studies with a purely educational approach are not longer included for this review (Savage 2011).
Contributions of authors
Lutz Goldbeck (LG) is taking principal responsibility for updating the review. He screened titles, abstracts, and reviewed papers against inclusion criteria. He drafted the updated review. He was appraising quality of papers and interpreted results of data analysis.
Astrid Fidika (AF) is co‐author for the review. She was screening titles, abstracts and, retrieved papers against the inclusion criteria. She extracted data from included papers, appraised quality of papers, took the leading role in contacting authors of papers for additional information. She investigated data analysis and interpretation, data management for the review, and entered data into RevMan. She provided advice on conducting the update of the systematic review and was involved in drafting the update.
Marion Herle (MH) is co‐author for the review. She was screening titles, abstracts and, retrieved papers against the inclusion criteria. She extracted data from included papers and appraised quality of papers. She also cross‐checked data entry of results for each outcome to Revman.
Alexandra L. Quittner (AQ) provided general advice on the review and interpretation of results for the current update. She has contributed a broad overview to the process of updating this review. She has supported CG for the original review and previous updates, and fine‐tuned the strategy for reviewing intervention studies within this vast and complex area. She also initiated the debate surrounding evidence‐based interventions with the international psychosocial interest group, which enhanced the discussion of the findings.
Claire A. Glasscoe (CG) was guarantor for the previous updates and the original review. She drafted the review and drew on the expertise of others to refine it.
Sources of support
Internal sources
Royal Liverpool Children's NHS Trust, UK
National Institutes of Health, USA
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Belsky 1994 {published data only}
- Belsky J, Khanna P. The effects of self-hypnosis for children with cystic fibrosis: a pilot study. American Journal of Clinical Hypnosis 1994;36(4):282-92. [DOI] [PubMed] [Google Scholar]
Chernoff 2002 {published data only}
- Chernoff RG, Ireys HT, DeVet KA, Kim Y. A randomized, controlled trial of a community-based support program for families of children with chronic illness: Pediatric outcomes. Archives of Pediatrics & Adolescent Medicine 2002;156(6):533-539. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ireys HT, Chernoff R, DeVet KA, Kim Y. Maternal outcomes of a randomized controlled trial of a community-based support program for families of children with chronic illnesses. Archives of Pediatrics & Adolescent Medicine 2001;155(7):771-777. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Christian 2006 {published data only}
- Christian B, D'Auria J, Belyea M. Loneliness in school age children with cystic fibrosis [abstract]. Pediatric Pulmonology 2003;Suppl 25:366. 5500100000002391 [Google Scholar]
- Christian B. Functional disability and quality of life of school-age children with cystic fibrosis [abstract]. Pediatric Pulmonology 2004;38 Suppl 27:356. 5500100000002744 [Google Scholar]
- Christian B. The impact of chronic illness on quality of life in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2006;41 Suppl 29:398. 5500100000003069 [Google Scholar]
- Christian B. The interface between physical activity, growth, and pulmonary function with the psychosocial impact of cystic fibrosis in children [abstract]. Pediatric Pulmonology 2005;40 Suppl 28:370. 5500100000002985 [Google Scholar]
- Christian BJ & D'Auria JP. Building life skills for children with cystic fibrosis: Effectiveness of an intervention. Nursing Research 2006;55(5):300-7. [DOI] [PubMed] [Google Scholar]
- Christian BJ, D'Auria JP, Belyea MJ, Retsch-Bogart GZ. Psychosocial status of school-age children with cystic fibrosis [abstract]. Pediatric Pulmonology 1999;Suppl 19:329. [Google Scholar]
Delk 1994 {published data only}
- Delk KK, Gevirtz R, Hicks DA, Carden F, Rucker R. The effects of biofeedback assisted breathing retraining on lung functions in patients with cystic fibrosis. Chest 1994;105(1):23-8. [DOI] [PubMed] [Google Scholar]
Goodill 2005 {published data only}
- Goodill SW. Dance/movement therapy for adults with cystic fibrosis: pilot data on mood and adherence. Alternative Therapies in Health & Medicine 2005;11(1):76-7. [PubMed] [Google Scholar]
Grasso 2000 {published data only}
- Grasso MC, Button BM, Allison DJ, Sawyer SM. Benefits of music therapy as an adjunct to chest physiotherapy in infants and toddles with cystic fibrosis. Pediatric Pulmonology 2000;29(5):371-81. [DOI] [PubMed] [Google Scholar]
- Grasso MC, Button BM, Sawyer SM, Allison DJ. Music: Meeting the challenge of adherence to chest physiotherapy for infants and toddlers with cystic fibrosis [abstract]. Pediatric Pulmonology 1998;Suppl 17:397. [DOI] [PubMed] [Google Scholar]
Hernandez‐Reif 1999 {published data only}
- Hernandez-Reif M, Field T, Krasnegor J, Martinez E, Schwartzman M, Mavunda K. Children with cystic fibrosis benefit from massage therapy. Journal of Pediatric Psychology 1999;24(2):175-81. [DOI] [PubMed] [Google Scholar]
Powers 2003 {published and unpublished data}
- Powers SW, Byars KC, Mitchell MJ, Schindler T, Patton SR, Zeller MH. A randomized pilot study of behavioral treatment to increase calorie intake in toddlers with cystic fibrosis. Child Health Care 2003;32:297-311. [Google Scholar]
- Powers SW, Byars KC, Mitchell MJ, Schindler T, Patton SR, Zeller MH. Nutritional benefits of two early intervention programs for toddlers with cystic fibrosis: A randomized pilot study. personal communication 2001.
- Powers SW, Schindler T, Schwarber L, Deeks CM, Byars KC, Arthur S, et al. Behavioral treatment to improve nutrition in toddlers with cystic fibrosis [abstract]. Pediatric Pulmonology 1999;Suppl 19:329. [Google Scholar]
Powers 2005 {published data only}
- Powers SW, Jones JS, Ferguson KS, Heidemann M, Henry R, Piazza-Waggoner C, et al. Behavioural treatment for toddlers and pre-schoolers with cystic fibrosis produces recommended energy intake and normal rates of growth [abstract]. Pediatric Pulmonology 2004;38(27):361. [Google Scholar]
- Powers SW, Jones JS, Ferguson KS, Henry R, Heidemann M, Patton SR, et al. Overcoming barriers to evidence-based nutrition treatment for preschoolers such as distance from cystic fibrosis center and multiple food allergies [abstract]. Pediatric Pulmonology 2004;38(27):341. [Google Scholar]
- Powers SW, Jones JS, Ferguson KS, Piazza-Waggoner C, Daines C, Acton JD. Randomized clinical trial of behavioral and nutrition treatment to improve energy intake and growth in toddlers and preschoolers with cystic fibrosis. Pediatrics 2005;116(6):1442-50. [DOI] [PubMed] [Google Scholar]
- Powers SW, Piazza-Waggoner C, Jones J, Daines C, Acton J. Impact of behavioral and nutrition treatment for toddlers and preschoolers with CF on energy intake and growth maintains for 12 and 18 months [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):372. [Google Scholar]
- Powers SW. Nutrition in early childhood: focus on parent interaction [abstract]. Pediatric Pulmonology 2003;Suppl 25:138. [Google Scholar]
Quinn 2004 {published and unpublished data}
- Quinn J, Latchford G, Duff A, Conner M, Pollard K, Morrison L, Conway S, Marsden R. Measuring, predicting and improving adherence to inhalation therapy in patients with CF: Randomised controlled study of motivational interviewing [abstract]. Pediatric Pulmonology 2004;38(S27):360. [MEDLINE: ]
Stark 1996 {published data only}
- Stark LJ, Mulvihill MM, Powers SW, Jelalian E, Keating K, Creveling S, et al. Behavioral intervention to improve calorie intake of children with cystic fibrosis: Treatment versus wait list control. Journal of Pediatric Gastroenterology and Nutrition 1996;22(3):240-53. [DOI] [PubMed] [Google Scholar]
Stark 2003 {published and unpublished data}
- Stark LJ, Opipari LC, Spieth LE, Jelalian E, Quittner AL, Higgins L, et al. Contribution of behavior therapy to dietary treatment in cystic fibrosis: A randomized controlled study within 2-year follow-up. Behavior Therapy 2003;34(2):237-258. [MEDLINE: ] [Google Scholar]
- Stark LJ. Contribution of behavior therapy to nutrition adherence in cystic fibrosis: A two-year randomized controlled study. Personal communication 2001.
Stark 2009 {published data only}
- Janicke DM, Mitchell MJ, Quittner AL, Piazza-Waggoner C, Stark LJ. The impact of behavioral intervention on family interactions at mealtime in pediatric cystic fibrosis. Children's Health Care 2008;37(1):49-66. [Google Scholar]
- Stark LJ, Opipari-Arrigan L, Quittner AL, Bean J, Powers SW. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatric Pulmonology 2011;46(1):31-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LJ, Quittner AL, Powers SW, Opipari Arrigan L, Bean JA, Duggan C, et al. A randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Archives of Pediatrics & Adolescent Medicine 2009;163(10):915-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor LA, Wallander JL, Anderson D, Beasley P, Brown RT. Improving health care utilization, improving chronic disease utilization, health status, and adjustment in adolescents and young adults with cystic fibrosis: A preliminary report. Journal of Clinical Psychology in Medical Settings 2003;10(1):9-16. [Google Scholar]
Vandemheen 2009 {published data only}
- Vandemheen K, Stacey D, Hennessey R, Gooyers T, Salgado J, Freitag A, et al. Translating research into practice: implementing a decision aid for adult cystic fibrosis patients [abstract]. Pediatric Pulmonology 2011;46 (Suppl 34):427. 5500100000011188 [Google Scholar]
- Vandemheen KL, O'Connor A, Bell SC, Freitag A, Bye P, Jeanneret A, et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation. American Journal of Respiratory and Critical Care Medicine 2009;180(8):761-8. [DOI] [PubMed] [Google Scholar]
Wilkinson 2008 {published data only}
- Wilkinson OM, Duncan-Skingle F, Pryor JA, Hodson ME. A feasibility study of home telemedicine for patients with cystic fibrosis awaiting transplantation. Journal of Telemedicine and Telecare 2008;14(4):182-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barbero 1996 {published data only}
- Barbero GJ. The science and humanity of cystic fibrosis. Current Problems in Pediatrics 1996;26(10):345-54. [DOI] [PubMed] [Google Scholar]
Bartholomew 1997 {published data only}
- Bartholomew LK, Czyzewski DI, Parcel GS, Swank PR, Sockrider MM, Mariotto MJ, et al. Self-management of cystic fibrosis: Short-term outcomes of the Cystic Fibrosis Family Education Program. Health Education and Behavior 1997;24(5):652-666. [DOI] [PubMed] [Google Scholar]
Bingham 2010 {published data only}
- Bingham PM, Bates JH, Thompson Figueroa J, Lahiri T. A breath biofeedback computer game for children with cystic fibrosis. Clinical Pediatrics 2010;49(4):337-42. [DOI] [PubMed] [Google Scholar]
Brady 1991 {published data only}
- Brady MS, Rickard K, Yu PL, Eigen H. Effectiveness and safety of small versus large doses of enteric coated pancreatic enzymes in reducing steatorrhea in children with cystic fibrosis. Pediatric Pulmonology 1991;10(2):79-85. [DOI] [PubMed] [Google Scholar]
Brandt 1996 {published data only}
- Brandt NJ, Schwartz M, Skovby F, Clausen H. A follow-up study of carriers of cystic fibrosis. Ugeskrift for laeger 1996;158(33):4623-7. [PubMed] [Google Scholar]
Brown 1994 {published data only}
- Brown RK, Kelly FJ. Evidence of increased oxidative damage in patients with cystic fibrosis. Pediatric Research 1994;36(4):487-93. [DOI] [PubMed] [Google Scholar]
Castellani 2011 {published data only}
- Castellani C, Perobelli S, Bianchi V, Seia M, Melotti P, Zanolla L, et al. An interactive computer program can effectively educate potential users of cystic fibrosis carrier tests. American Journal of Medical Genetics. Part A 2011;155A(4):778-85. [DOI] [PubMed] [Google Scholar]
Cheuvront 1998 {published data only}
- Cheuvront B, Sorenson JR, Callanan NP, Stearns SC, DeVellis BM. Psychosocial and educational outcomes associated with home- and clinic-based pretest education and cystic fibrosis carrier testing among a population of at-risk relatives. American Journal of Medical Genetics 1998;75(5):461-8. [PubMed] [Google Scholar]
- Sorenson JR, Cheuvront B, Bruning A, Talton S, DeVellis BM, Kock G, et al. Proband and parent assistance in identifying relatives for cystic fibrosis carrier testing. American Journal of Medical Genetics 1996;63(3):419-25. [DOI] [PubMed] [Google Scholar]
- Sorenson JR, Cheuvront B, DeVellis BM, Callanan N, Silverman L, Kock G, et al. Acceptance of home and clinic-based cystic fibrosis carrier education and testing by first, second, and third degree relatives of cystic fibrosis patients. American Journal of Medical Genetics 1997;70(2):121-9. [PubMed] [Google Scholar]
Clayton 1995 {published data only}
- Clayton EW, Hannig VL, Pfotenhauer JP, Parker RA, Campbell PW, Phillips JA. Teaching about cystic fibrosis carrier screening by using written and video information. American Journal of Human Genetics 1995;57(1):171-81. [PMC free article] [PubMed] [Google Scholar]
Davis 2002 {published data only}
- Davis MA, Quittner AL, Stack CM, Yang MC. Controlled evaluation of the STARBRIGHT CD-ROM program for children and adolescents with Cystic Fibrosis. Journal of Pediatric Psychology 2004;29(4):259-67. 5500100000002613 [DOI] [PubMed] [Google Scholar]
- Davis MA, Quittner AL, Stack CM. Controlled evaluation of the STARBRIGHT Explorer Series CD-Rom program for children and adolescents with cystic fibrosis [abstract]. Pediatric Pulmonology 2002;Suppl 24:351. 5500100000002209 [DOI] [PubMed] [Google Scholar]
- Quittner AL, Drotar D. Controlled trial of family interventions for cystic fibrosis. NIH Research Grant, Oct 1997 to Aug 2003 1997.
- Wade SL. Commentary: Computer-based interventions in pediatric psychology. Journal of Pediatric Psychology 2004;29(4):269-272. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dodd 1997 {published data only}
- Dodd ME, Abbott J, Haworth CS, Moorcroft AJ, Webb AK. Validity of a visual numerical general quality of life scale and chest scale in adults with cystic fibrosis [abstract]. Thorax 1997;52 Suppl 6:A45. [Google Scholar]
Dodd 2001 {published data only}
- Dodd J, Barry S, Moran A, Gallagher C. The effect of verbal motivation on exercise performance in patients with cystic fibrosis [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163 (5 Suppl):A624. [Google Scholar]
Donaldson 1995 {published data only}
- Donaldson C, Shackley P, Abdalla M, Miedzybrodzka Z. Willingness to pay for antenatal carrier screening for cystic fibrosis. Health Economics 1995;4(6):439-52. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Michie S, Miedzybrodzka ZH, Allanson A. Incorrect recall of residual risk three years after carrier screening for cystic fibrosis: A comparison of two-step and couple screening. American Journal of Obstetrics & Gynecology 1999;181(12):165-9. [DOI] [PubMed] [Google Scholar]
- Miedzybrodzka ZH, Haites N, Hall M, Templeton A, Marteau T, Dean J, et al. Two approaches to ante-natal carrier screening for cystic fibrosis [abstract]. In: Proceedings of the 11th International Cystic Fibrosis Congress. 1992:TP20.
- Miedzybrodzka ZH, Hall MH, Mollison J, Templeton A, Russell IT, Dean JC, et al. Antenatal screening for carriers of cystic fibrosis: randomised trial of stepwise versus couple screening. British Medical Journal 1995;310(6976):353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedzybrodzka ZH, Marteau TM. Recall of residual risk three years after carrier screening for CF: a comparison of two-step and couple screening [abstract]. Journal of Medical Genetics 1999;36 Suppl 1:570. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Brock DJH, Haddow EJ, Doherty RA. Antenatal screening for cystic fibrosis [letter]. British Medical Journal 1995;310(6988):1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Downs 2006 {published data only}
- Downs JA, Roberts CM, Blackmore AM, Le Souëf PN, Jenkins SC. Benefits of an education programme on the self-management of aerosol and airway treatments for children with cystic fibrosis. Chronic Respiratory Disease 2006;3:19-27. [DOI] [PubMed] [Google Scholar]
Eng 1997 {published data only}
- Eng CM, Schechter C, Robinowitz J, Fulop G, Burgert T, Levy B, et al. Prenatal genetic carrier testing using triple disease screening. Journal of the American Medical Association 1997;278(15):1268-72. [PubMed] [Google Scholar]
Falkman 1977 {published data only}
- Falkman C. Cystic fibrosis - A psychological study of 52 children and their families. Acta Paediatrica Scandinavia 1977;Suppl 269:7-93. [PubMed] [Google Scholar]
Gee 2000 {published data only}
- Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Georgiopoulos 2011 {published data only}
- Georgiopoulos AM, Hua LL. The diagnosis and treatment of attention deficit-hyperactivity disorder in children and adolescents with cystic fibrosis: A retrospective study. Psychosomatics: Journal of Consultation Liaison Psychiatry 2011;52(2):160-166. [DOI] [PubMed] [Google Scholar]
Goa 1997 {published data only}
- Goa KL, Lamb H. Dornase alfa. A review of pharmacoeconomic and quality-of-life aspects of its use in cystic fibrosis. Pharmacoeconomics 1997;12(3):409-22. [DOI] [PubMed] [Google Scholar]
Goldbeck 2001 {published data only}
- Goldbeck L, Babka C. Development and evaluation of a multi-family psychoeducational program for cystic fibrosis. Patient Education and Counselling 2001;44(2):187-92. [DOI] [PubMed] [Google Scholar]
Goldbeck 2011a {published data only}
- Goldbeck L, Holling I, Schlack R, West C, Besier T. The impact of an inpatient family-oriented rehabilitation program on parent-reported psychological symptoms of chronically ill children. Klinische Padiatrie 2011;223(2):79-84. [DOI] [PubMed] [Google Scholar]
Gonciartz 1988 {published data only}
- Gonciartz Z, Besser P, Lelek E, Gundermann KJ, Johannes KJ. Randomised placebo-controlled double blind trial on 'essential' phospholipids in the treatment of fatty liver associated with diabetes. Medicine & Chirurgie Digestives 1988;17:61-5. [Google Scholar]
Green 1995 {published data only}
- Green JM. Obstetricians' views on prenatal diagnosis and termination of pregnancy: 1980 compared with 1993. British Journal of Obstetrics and Gynaecology 1995;102(3):228-32. [DOI] [PubMed] [Google Scholar]
Gremse 1998 {published data only}
- Gremse DA, Lytle JM, Sacks AI, Balistreri WF. Characterization of failure to imbibe in infants. Clinical Pediatrics 1998;37(5):305-9. [DOI] [PubMed] [Google Scholar]
Grody 1997 {published data only}
- Grody WW, Dunkel-Schetter C, Tatsugawa ZH, Fox MA, Fang CY, Cantor RM, et al. PCR based screening for cystic fibrosis carrier mutations in an ethnically diverse pregnant population. American Journal of Human Genetics 1997;60(4):935-47. [PMC free article] [PubMed] [Google Scholar]
Gulmans 1999 {published data only}
- Gulmans VA, Meer K, Brackel HJL, Faber JAJ, Berger R, Helders PJM. Outpatient Exercise training in Children with Cystic Fibrosis: Physiological Effects, Perceived Competence, and Acceptability. Pediatric Pulmonology 1999;28(1):39-46. [DOI] [PubMed] [Google Scholar]
Haddow 1999 {published data only}
- Haddow JE, Bradley LA, Palomaki GE, Doherty RA. Issues for implementing prenatal screening for cystic fibrosis: results of a working conference. Journal of Medical Screening 1999;6(2):60-6. [DOI] [PubMed] [Google Scholar]
Hains 1997 {published data only}
- Hains AA, Davies WH, Behrens D, Biller JA. Cognitive behavioral interventions for adolescents with cystic fibrosis. Journal of Pediatric Psychology 1997;22(5):669-87. [DOI] [PubMed] [Google Scholar]
Hains 2001 {published data only}
- Hains AA, Davies WH, Behrens D, Freeman ME, Biller JA. Effectiveness of a cognitive behavioral intervention for adults with cystic fibrosis. Journal of Clinical Psychology in Medical Settings 2001.
Hodson 1997 {published data only}
- Hodson ME. Psychosocial aspects for the management of adults with cystic fibrosis. Pediatric Pulmonology 1997;Suppl 16:113-4. [DOI] [PubMed] [Google Scholar]
Jelalian 1998 {published data only}
- Jelalian E, Stark LJ, Reynolds L, Seifer R. Nutrition intervention for weight gain in cystic fibrosis: A meta-analysis. Journal of Pediatrics 1998;132(3 Pt 1):486-92. [DOI] [PubMed] [Google Scholar]
Johnson 2001 {published data only}
- Johnson KB, Ravert RD, Everton A. Hopkins Teen Central: Assessment of an internet-based support system for children with cystic fibrosis. Pediatrics 2001;107(2):E24. [DOI] [PubMed] [Google Scholar]
Kollberg 1982 {published data only}
- Kollberg H. Sociomedical conditions for Swedish patients with cystic fibrosis. A review of the past twelve years. International Journal of Rehabilitation Research 1982;5(3):345-61. [DOI] [PubMed] [Google Scholar]
Krauth 1999 {published data only}
- Krauth C, Busse R, Smaczny C, Ullrich G, Wagner TO, Weber J, et al. Cost comparison of hospital and ambulatory I.V. therapy in adult cystic fibrosis patients: Results of a controlled prospective study. Medizinische Klinik 1999;94(10):541-8. [DOI] [PubMed] [Google Scholar]
Le Heuzey 1997 {published data only}
- Le Heuzey MF, Mouren-Simeoni MC, Navarro J. Psychological adjustment of children and adolescents with cystic fibrosis [abstract]. Pediatric Pulmonology 1997;Suppl 16:259. [DOI] [PubMed] [Google Scholar]
Leonard 1995 {published data only}
- Leonard KP, Bartholomew LK, Swank PR, Parcel GS. A comparison of two approaches to education about carrier testing for cystic fibrosis. Journal of Genetic Counselling 1995;4(2):97-113. [DOI] [PubMed] [Google Scholar]
Lindström 1994 {published data only}
- Lindström B. The essence of existence. On the quality of life of children in the Nordic countries. Thesis - NHV - Report, The Nordic School of Public Health, Göteborg, Sweden 1994.
Loader 1996 {published data only}
- Loader S, Caldwell P, Kozyra A, Levenkron JC, Boehm CD, Kazazian HH, et al. Cystic fibrosis carrier population screening in the primary care setting. American Journal of Human Genetics 1996;59(1):234-47. [PMC free article] [PubMed] [Google Scholar]
Magnussen 1992 {published data only}
- Magnussen H, Scheidt Mackes M. The effect of flupertine on respiratory drive in healthy probands and patients with various lung diseases. Pneumologie 1992;46:580-6. [PubMed] [Google Scholar]
Phillipson 2000 {published data only}
- Phillipson GT, Petrucco OM, Matthews CD. Congenital bilateral absence of the vas deferens, cystic fibrosis mutation analysis and intracytoplasmic sperm injection. Human Reproduction 2000;15(2):431-5. [DOI] [PubMed] [Google Scholar]
Pollitt 1997 {published data only}
- Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al. Neonatal screening for inborn errors of metabolism: cost, yield and outcome. Health Technology Assessment 1997;1(7:i-iv):1-202. [PubMed]
Pop Jordanova 2010 {published data only}
- Pop Jordanova N, Gucev Z. Game-based peripheral biofeedback for stress assessment in children. Pediatrics International 2010;52(3):428-31. [DOI] [PubMed] [Google Scholar]
Ralston 2008 {published data only}
- Ralston S, Roohi M. A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatric Pulmonology 2008;43(6):561-66. [DOI] [PubMed] [Google Scholar]
Ramström 2000 {published data only}
- Ramström H, Erwander I, Mared L, Kornfält R, Seiving B. Pharmaceutical intervention in the care of cystic fibrosis patients. Journal of Clinical Pharmaceutical Therapy 2000;25(6):427-34. [DOI] [PubMed] [Google Scholar]
Rault 2012 {published data only}
- David V, Rault G, Pougheon-Bertrand D, Dorenlot E. Therapeutic patient education and quality improvement program: What synergy? Journal of Cystic Fibrosis 2013;12 (Suppl 1):S136. [Google Scholar]
- Rault G, Pougheon-Bertrand D, David V, Minguet G, Lombrail P. CF quality improvement program: A pilot phase to implement the us QIP approach in France [abstract]. Pediatric Pulmonology 2012;47 Suppl 35:401-2. [Google Scholar]
Rodrigue 2005 {published data only}
- Rodrigue JR, Baz MA, Widows MR, Ehlers SL. A randomized evaluation of quality-of-life therapy with patients awaiting lung transplantation. American Journal of Transplantation 2005;5(10):2425-32. [DOI] [PubMed] [Google Scholar]
Rozenfeld 2012 {published data only}
- Rozenfeld S, Mantin H, Mussaffi H, Taizi T, Landau E, Kadosh D, et al. Effect of Snoezlen on pain perception and anxiety in CF infants during sputum suction [abstract]. Pediatric Pulmonology 2012;47 (Suppl 35):441. 5500125000000042 [Google Scholar]
Stapleton 2001 {published data only}
- Stapleton D, Tunnicliffe L, McGuiness D, Sheriff J, Sly P. Development of a nutrition and behaviour intervention program: Go and Grow with CF. Australian Journal of Nutrition and Diet 1998;55:130-7. [Google Scholar]
- Stapleton DR, Gurrin LC, Zubrick SR, Silburn SR, Sherriff JL, Sly PD. What do children with cystic fibrosis and their parents know about nutrition and pancreatic enzymes? Journal of the American Dietetic Association 2000;100(12):1494-500. [DOI] [PubMed] [Google Scholar]
- Stapleton DR, Gurrin LC, Zubrick SR, Silburn SR, Sly PD. The effect of 'Go and Grow with CF' on nutrition and pancreatic enzyme knowledge of children with cystic fibrosis. Australian Journal of Nutrition and Dietetics 2001;58(3):164-168. [MEDLINE: ] [Google Scholar]
Stark 2002 {published data only}
- Stark LJ, Mackner LM, Kessler JH, Opipari LiC, Quittner AL. Preliminary findings for calcium intake in children with cystic fibrosis following behavioral intervention for caloric intake. Children's Health Care 2002;31(2):107-18. [Google Scholar]
Thompson 1990 {published data only}
- Thompson RJ, Hodges K, Hamlett KW. A matched comparison of adjustment in children with cystic fibrosis and psychiatrically referred and nonreferred children. Journal of Pediatric Psychology 1990;15(6):745-59. [DOI] [PubMed] [Google Scholar]
Thornton 1995 {published data only}
- Thornton JG, Hewison J, Lilford RJ, Vail A. A randomised trial of three methods of giving information about prenatal testing. British Medical Journal 1995;311(7013):1127-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trapp 1998 {published and unpublished data}
- Trapp M, Barton S, Morgan H, Lockyer L. Self-administration of drugs for cystic fibrosis. Professional Nurse 1998;14(3):199-203. [PubMed] [Google Scholar]
Tullis 1995 {published data only}
- Tullis DE, Guyatt GH. Quality of life in cystic fibrosis. Pharmacoeconomics 1995;8(1):23-33. [DOI] [PubMed] [Google Scholar]
Villari 1994 {published data only}
- Villari B, Hess OM, Piscione F, Vassalli G, Weber KT, Chiariello M. Heart function in chronic pressure overload caused by aortic stenosis: The role of collagen tissue. Cardiologia 1994;39(6):411-20. [PubMed] [Google Scholar]
Ward 1999 {published data only}
- Ward SA, Tomezsko JL, Holsclaw DS, Paolone AM. Energy expenditure and substrate utilization in adults with cystic fibrosis and diabetes mellitus. American Journal of Clinical Nutrition 1999;69(5):913-9. [DOI] [PubMed] [Google Scholar]
Watson 2004 {published data only}
- Watson H, Truby H, Haworth CS, Bilton D. Pilot study to evaluate a home-based behavioural nutrition programme for adults. Journal of Cystic Fibrosis 2004;3 Suppl 1:S75. [MEDLINE: ]
Watson 2006 {published data only}
- Watson H, Bilton D, Truby H. A randomised controlled trial of a behavioural nutrition education programme "Eat Well with CF" for adults with CF. Journal of Cystic Fibrosis 2006;5 Suppl 1:S73. [DOI] [PubMed] [Google Scholar]
Welkenhuysen 1996 {published data only}
- Welkenhuysen M, Evers-Kiebooms G, Decruyenaere M, Van-den-Berghe H, Bande-Knops J, Van-Gerven V. Adolescents' attitudes towards carrier testing for cystic fibrosis and its relative stability over time. European Journal of Human Genetics 1996;4(1):52-62. [DOI] [PubMed] [Google Scholar]
Williams 1997 {published data only}
- Williams PD, Hanson S, Karlin R, Liebergen A, Olson J, Barnard MU, et al. Outcomes of a nursing intervention for siblings of chronically ill children: A pilot study. Journal of Social Pediatric Nursing 1997;2(3):127-37. [DOI] [PubMed] [Google Scholar]
Wolter 1997 {published data only}
- Wolter JM, Bowler SD, McCormack JG, Nolan PJ. Home versus hospital therapy including intravenous antibiotics in cystic fibrosis [abstract]. In: Proceedings of the Annual Scientific Meeting of the 11th Thoracic Society of Australia and New Zealand (TSANZ). 1994:148.
- Wolter JM, Bowler SD, Nolan PJ, McCormack JG. Home intravenous therapy in cystic fibrosis: a prospective randomized trial examining clinical, quality of life and cost aspects. European Respiratory Journal 1997;10(4):896-900. [PubMed] [Google Scholar]
- Wolter JM, Bowler SD, Nolan PJ, McCormack JG. Intravenous home therapy - a randomised trial in adult cystic fibrosis patients [abstract]. Australian and New Zealand Journal of Medicine 1995;25:567. [Google Scholar]
References to studies awaiting assessment
Bryon 2000 {published data only}
- Bryon M, Burton J, Tostevin M, Madge S. A home visit programme to improve health status and psychosocial functioning of families with a child with cystic fibrosis [abstract]. Pediatric Pulmonology 2000;Suppl 20:336. [Google Scholar]
Cannon 1999 {published data only}
- Cannon C, Benitez J, Scarbary J, Taylor K, Guill M. In-home videoconferencing for cystic fibrosis patient education [abstract]. Pediatric Pulmonology 1999;Suppl 19:333. [Google Scholar]
Cummings 2011 {published data only}
- Cummings E, Hauser J, Cameron-Tucker H, Fitzpatrick P, Jessup M, Walters EH, et al. Enhancing self-efficacy for self-management in people with cystic fibrosis. Studies in Health Technology and Informatics 2011;169:33-7. [PubMed] [Google Scholar]
- Jessup MM, Hauser J, Cameron-Tucker H, Cummings E, Turner P, Blizzard L, Reid D. Facilitating self-management in adolescents and adults with cystic fibrosis: a pilot study [abstract]. Pediatric Pulmonology 2011;46 Suppl 34:405. [Google Scholar]
Davis 1990 {published data only}
- Davis P, Davis S, Mather F, Waring W. A randomized trial of home intravenous antibiotic therapy (HIVAT) in cystic fibrosis (CF): Short-term psychological effects [abstract]. Pediatric Pulmonology 1990;Suppl 5:281-2. [Google Scholar]
- Davis S, Mather F, Tankersly P, Waring W. A randomized trial of home intravenous antibiotic therapy (HIVAT) in cystic fibrosis (CF): Short-term safety and efficacy [abstract]. Pediatric Pulmonology 1990;Suppl 5:245. [Google Scholar]
Goldbeck 2013 {published data only}
- Goldbeck L, Hebestreit H, Junge S, Sens B, Smaczny C, Frank M, et al. Vemse-CF - A prospective controlled care-research study investigating the effects of a comprehensive psychosocial intervention [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:430. 5500125000000138 [Google Scholar]
Hass 2012 {published data only}
- NCT01729585. The effects of massage therapy on QOL in youth/young adults with cystic fibrosis. http://clinicaltrials.gov/show/NCT01729585NCT01729585 (accessed 01 Apil 2014).
Hatziagorou 2011 {published data only}
- Hatziagorou E, Chourdakis M, Chrisochoou E, Avramido V, Tsanakas J. Nutritional education improves energy intake and weight gain in children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2011;10 Suppl1:S73.
Huang 2010 {published data only}
- NCT01253733. TAHLC - texting to promote adolescent health liaisons and chronic disease management.. http://clinicaltrials.gov/show/NCT01253733 (accessed 01 April 2014). 5500100000011528
Irons 2012 {published data only}
- Irons JY, Kenny DT, McElrea M, Chang AB. Singing therapy for young people with cystic fibrosis: a randomized controlled pilot study. Music and Medicine 2012;4(3):136-45. 5500100000007892 [Google Scholar]
Jessup 2010 {published data only}
- Jessup MM, Cameron-Tucker H, Cummings E, Hauser J, Joseph L, Saddington H, et al. 'Someone to talk to': Adolescent and adult CF patients' feedback on their experience of a mentoring and IT intervention [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S107. [Google Scholar]
Kalnins 1996 {published data only}
- Kalnins D, Durie P. Oral supplements versus normal food intake in children and adults [abstract]. Israel Journal of Medical Sciences 1996;32 Suppl:SD120-1. 5500100000001421 [Google Scholar]
- Kalnins D, Durie PR, Corey M, Ellis L, Pencharz P, Tullis E. Are oral dietary supplements effective in the nutritional management of adolescents and adults with CF? [abstract]. Pediatric Pulmonology 1996;381(Suppl 13):314-5. 5500100000001420 [Google Scholar]
Klig 1989 {published data only}
- Klig S, Denning C, Jacoby J, Xia F, Gaerlan P, Bisberg D, et al. Biopsychosocial examination of two methods of pulmonary therapy [abstract]. Pediatric Pulmonology 1989;Suppl 4:145. [Google Scholar]
Mischler 1998 {published data only}
- Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, et al. Nutritional benefits of neonatal screening for cystic fibrosis. New England Journal of Medicine 1997;337(14):963-9. [DOI] [PubMed] [Google Scholar]
- Farrell PM, Mischler EH. Newborn screening for cystic fibrosis. Advances in Pediatrics 1992;39:36-69. [PubMed] [Google Scholar]
- Fost N, Farrell PM. A prospective randomized trial of early diagnosis and treatment of cystic fibrosis: A unique ethical dilemma. Clinical Research 1989;37:495-500. [PubMed] [Google Scholar]
- Mischler EH, Wilford BS, Fost N, Laxona A, Reiser C, Sauer CM, et al. Cystic fibrosis newborn screening: Impact on reproductive behavior and implications for genetic counseling. Pediatrics 1998;102(1 Pt 1):44-52. [DOI] [PubMed]
Patel 2010 {published data only}
- Patel L, Glasscoe C, Dixon C, Dyer K, Southern KW. Does administering a parent reported outcome measure during the annual review process improve the self efficacy of the carer of a child with CF? [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:441. [Google Scholar]
Petzel 1991 {published data only}
- Petzel SV, Finkelstein J, Budd J, Ellis LBM. Adherence in cystic fibrosis [abstract]. Pediatric Pulmonology 1991;Suppl 6:308. [Google Scholar]
Powers 2003a {published data only}
- Powers SW. Nutrition in early childhood: Focus on parent interaction. Pediatric Pulmonology 2003;Suppl 25:138-9. [MEDLINE: ]
Powers 2013 {published data only}
- Chamberlin LA, Sullivan S, Stark L, Powers SW. Adjusting recruitment strategies and intervention format to meet enrollment goals in a multicenter trial of a behavioural treatment [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:438. 5500125000000140 [Google Scholar]
- Powers SW, Stark L, Chamberlin L, Sullivan S, Filigno S, Rausch J. A multi-site, randomized, controlled clinical trial of behavioral and nutrition treatment for preschoolers with cystic fibrosis [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:402. 5500125000000143 [Google Scholar]
Ruddy 2011 {published data only}
- NCT01325766. Study of yoga as a therapy for cystic fibrosis (CF) patients. http://clinicaltrials.gov/show/NCT01325766 (accessed 01 April 2014).
Stark 1998 {published data only}
- Stark LJ, Quittner AL, Opipari LC, Jones E, Powers SW, Higgins L, et al. The contribution of behavior therapy to enhancing adherence in school-age children with CF: The example of diet [abstract]. Pediatric Pulmonology 1998;Suppl 17:110-1. [Google Scholar]
Wainwright 2009 {published data only}
- Wainwright C, Saddington H, Busch J, Blizzard L, Cameron-Tucker H, Cheney J, et al. Improving self efficacy in adolescents and young adults with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2009;8 Suppl 2:S93. [Google Scholar]
Widman 2013 {published data only}
- NCT01957072. Pilot study of behavioural intervention for nutrition in cystic fibrosis. http://clinicaltrials.gov/show/NCT01957072 (accessed 01 April 2014).
Williams 1987 {published data only}
- Williams J, Handy DA, Booth IW, Weller PH. Intensive dietary counselling with adolescents with cystic fibrosis: What are the effects? [abstract]. In: Proceedings of the 10th International Cystic Fibrosis Congress; 1998 March 5-10; Sydney. 1988:165.
- Williams J, Handy DA, Weller PH, Booth IW. Intensive dietary counselling In adolescents with cystic fibrosis. Gut 1987;28:A1352. [Google Scholar]
References to ongoing studies
Quittner 2000 {published data only}
- DeLambo KE, Ievers-Landis CE, Drotar D, Quittner AL. Association of observed family relationship quality and problem-solving skills with treatment adherence in older children and adolescents with cystic fibrosis. Journal of Pediatric Psychology 2004;29(5):343-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Quittner AL, Drotar D, Ievers-Landis C, Slocum N, Seidner D, Jacobsen J. Adherence to medical treatments in adolescents with cystic fibrosis: The development and evaluation of family-based interventions. In: Drotar D, editors(s). Promoting adherence to medical treatment in childhood chronic illness: Concepts, methods, and interventions. First edition. Mahjah NJ: Lawrence Earlbaum Associates, 2000:383-407. [Google Scholar]
- Quittner AL, Drotar D, Ievers-Landis C. Intervention to increase adolescent adherence to treatment: preliminary comparisons of family therapy and cystic fibrosis family education [abstract]. Pediatric Pulmonology 1998;Suppl 17:113-4. [Google Scholar]
- Quittner AL, Drotar D, Ivers-Landis C, Slocum N, Buu A. Measuring adherence to treatment in adolescents with cystic fibrosis: Pros and cons of electronic monitoring [abstract]. In: Conference proceedings of the 8th Florida Conference on Child Health Psychology; 2001 April; Florida. 2001.
- Quittner AL, Johnson SB, Modi A. Controlled evaluation of a clinic-based intervention to promote adherence in children with cystic fibrosis. Personal communication 2002.
Quittner 2011 {published and unpublished data}
- Alpern AN, McLean KA, Marciel KK, Zhang J, Riekert KA, Quittner AL. Effects of clinical supervision on treatment fidelity in icare [abstract]. Pediatric Pulmonology 2012;47(S35):433-4. [Google Scholar]
- Barker D, Quittner AL, Riekert KA. Relating patterns of social support from friends and family to health-related quality of life [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:433. 5500125000000139 [Google Scholar]
- Blackwell LS, Romero SL, Marciel KK, Romero CV, Quittner AL. Pain in adolescents and young adults with CF: location, severity and predictors [abstract]. Pediatric Pulmonology 2012;47 Suppl 35:430. 5500125000000041 [Google Scholar]
- McLean KA, Quittner AL, Alpern AN, Marciel KK, Riekert KA. Healthcare provider acceptability of problem-solving in the I change adherence and raise expectations (iCARE) study [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:437. 5500125000000126 [Google Scholar]
- Quittner A, Kimberg C, Marciel K, Zhang J, Riekert K. Randomized, Controlled trial of a behavioral adherence intervention for adolescents with cystic fibrosis: I change adherence and raise expectations (iCARE) [abstract]. Chest 2011;140(4 Suppl):908a. [Google Scholar]
- Quittner AL, Alpern AN, McLean KA, Marciel KK, Zhang J, Riekert KA. Clinical supervision improves treatment fidelity to an adherence intervention [abstract]. Journal of Cystic Fibrosis 2012;11(Suppl 1):S9. [Google Scholar]
- Quittner AL, Riekert KA. I Change adherence and raise expectations: the iCARE study. Pediatric Pulmonology 2013;48 Suppl 36:136. 5500125000000125 [Google Scholar]
Quittner 2012 {published and unpublished data}
- Blackwell LS, Romero SL, Romero CV, McLean KA, Dawkins K, Hoag J, et al. CFfone: a social networking site for adolescents and young adults with CF [abstract]. Pediatric Pulmonology 2012;47 Suppl 35:430. 5500100000011184 [Google Scholar]
- Marciel KK, Saiman L, Quittell LM, Dawkins K, Quittner AL. Cell Phone Intervention to Improve Adherence: CysticFibrosis Care Team, Patient, and Parent Perspectives. Pediatric Pulmonology 2010;45(2):157-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, Marciel KK, Romero CV, Dawkins K, et al. Effect of CFfone on knowledge of disease management, psychological well-being, and health-related quality of life in adolescents and young adults with CF [abstract]. Journal of Cystic Fibrosis 2012;11 Suppl 1:S137. [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, McLean KA, Monzon AD, Dawkins K. Efficacy of an online social networking site: CFFONE results. Pediatric Pulmonology 2013;48 Suppl 36:135. 5500125000000144 [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, Romero CV, Marciel KK, Dawkins K, et al. Preliminary results on the efficacy of an online social network for adolescents with CF: age and disease severity group comparisons [abstract]. Pediatric Pulmonology 2012;47 Suppl 35:388. 5500100000011541 [Google Scholar]
Riekert 2012 {unpublished data only}
- Riekert KA, Borrelli B, Bilderback AV, green A, Eakin MN. Building adherence to live and navigate my CF experience: the balance study. Pediatric Pulmonology 2013;48 (Suppl 36):133. 5500125000000142 [Google Scholar]
Additional references
Abbott 2009
- Abbott J, Havermans T, Harta A. Adherence to the medical regimen: clinical implications of new findings. Current Opinion in Pulmonary Medicine 2009;15(9):597-603. [DOI] [PubMed] [Google Scholar]
Asher 1984
- Asher SR, Hymel S, Renshaw PD. Loneliness in children. Child Development 1984;55(4):1456-46. [Google Scholar]
Bartholomew 1991
- Bartholomew LK, Parcel GS, Seilheimer DK, Czyzewski D, Spinelli SH, Congdon B. Development of a health education program to promote the self-management of cystic fibrosis.. Health Education Quarterly 1991;18(4):429-443. [DOI] [PubMed] [Google Scholar]
Beale 2006
- Beale IL. Scholarly literature review: Efficacy of psychological interventions for pediatric chronic illnesses. Journal of Pediatric Psychology 2006;31(5):437-451. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Besier 2011a
- Besier T, Born A, Henrich G, Hinz A, Quittner AL, Goldbeck L, et al. Anxiety, depression, and life satisfaction in parents caring for children with cystic fibrosis. Pediatric Pulmonology 2011;46(7):672-86. [DOI] [PubMed] [Google Scholar]
Besier 2011b
- Besier T, Goldbeck L. Anxiety and depression in adolescents with CF and their caregivers. Journal of Cystic Fibrosis 2011;10(6):435-42. [DOI] [PubMed] [Google Scholar]
Bluebond‐Langner 2001
- Bluebond-Langner M, Lask B, Angst DB. Psychosocial Aspects of Cystic Fibrosis. London: Arnold, 2001. [Google Scholar]
Bobadilla 2002
- Bobadilla JL, Macek Jr M, Fine JP, Farrell PM. Cystic Fibrosis: A worldwide analysis of CFTR mutations - correlation with incidence data and application to screening. Human Mutation 2002;19:575-606. [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, the CONSORT Group. Extending the CONSORT Statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295-309. [DOI] [PubMed] [Google Scholar]
Bradley 2012
- Bradley JM, Madge S, Morton AM, Quittner AL, Elborn JS, Allied Health and Nursing Professions Working Group, European Cystic Fibrosis Society. Cystic fibrosis research in allied health and nursing professions. Journal of Cystic Fibrosis 2012;11(5):387-92. [DOI] [PubMed] [Google Scholar]
Cohen 1992
- Cohen J. Quantitative Methods in Psychology: A Power Primer. Psychological Bulletin 1992;112(1):155-159. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta-analysis. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The CochraneCollaboration, 2011. Available from www.cochrane-handbook.org, 2011. [Google Scholar]
DiMatteo 2000
- DiMatteo M, Lepper H, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160(14):2101-2107. [DOI] [PubMed] [Google Scholar]
DiMatteo 2002
- DiMatteo M, Giordani P, Lepper H, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Medical Care 2002;40(9):794-811. [DOI] [PubMed] [Google Scholar]
Dodge 2007
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947-2003. European Respiratory Journal 2007;29:522-526. [DOI] [PubMed] [Google Scholar]
Eakin 2011
- Eakin MN, Bilderback A, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. Journal of Cystic Fibrosis 2011;10:258-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eiser 1995
- Eiser C, Havermans T, Craft A, Kernahan J. Development of a measure to assess the perceived illness experience after treatment for cancer. Archives of Disease in Childhood 1995;72(4):302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
FitzSimmons 1993
- FitzSimmons SC. The changing epidemiology of cystic fibrosis. Journal of Pediatrics 1993;122(1):1-9. [DOI] [PubMed] [Google Scholar]
Goldbeck 2011b
- Goldbeck L, Besier T, Hinz A, Singer S, Quittner AL, TIDES Group. Prevalence of symptoms of anxiety and depression in German patients with cystic fibrosis. Chest 2010;138(4):929-36. [DOI] [PubMed] [Google Scholar]
Harter 1985
- Harter S. Manual: Self-perception profile for children. Denver: CO: University of Denver, 1985. [Google Scholar]
Harter 1986
- Harter S. Manual: Social support scale for children. Denver: CO: University of Denver, 1986. [Google Scholar]
Hazel 2006
- Hazel R. The psychosocial impact on parents of tube feeding their child. Paediatric Nursing 2006;18(4):19-22. [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The CochraneCollaboration, 2011. Available from www.cochrane-handbook.org., 2011. [Google Scholar]
Hodges 2011
- Hodges LJ, Walker J, Kleiboer AM, Ramirez AJ, Richardson A, Velikova G, et al. What is a psychological intervention? A metareview and practical proposal. Psychooncology 2011;20(5):470-8. [DOI] [PubMed] [Google Scholar]
Ilfeld 1976
- Ilfeld F. Further validation of a psychiatric symptom index in a normal population. Psychological Reports 1976;39:1215-8. [Google Scholar]
Kerem 2005
- Kerem E, Conway S, Elborn S, Heijerman H. Standards of care for patients with cystic fibrosis: a European consensus. Journal of Cystic Fibrosis 2005;41:7-26. [DOI] [PubMed] [Google Scholar]
Kessler 2012
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research 2012;21(3):169-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavigne 1992
- Lavigne JV, Faier-Routman J. Psychological adjustment to pediatric physical disorders: A meta-analytic review. Journal of Pediatric Psychology 1992;17(2):133-58. [DOI] [PubMed] [Google Scholar]
McNair 1971
- McNair DM, Lorr M, Droppleman LF. POMS Profile of Mood States. San Diego: Educational and Industrial Testing Service, 1971. [Google Scholar]
McNair 1992
- McNair D M, Lorr M, Droppleman LF. Revised manual for the Profile of Mood States. San Diego: CA: Educational and Industrial Testing Services, 1992. [Google Scholar]
Miller 1999
- Miller DL, Jelalian E, Stark LJ. Cystic Fibrosis. In: Goreczny AJ, Hersen M, editors(s). Handbook of Pediatric and Adolescent Health Psychology. First edition. Boston: Allyn and Bacon, 1999. [Google Scholar]
Miroballi 2012
- Miroballi Y, Garber E, Jia H, Zhou JJ, Alba L, Quittell LM, et al. Infection control knowledge, attitudes, and practices among cystic fibrosis patients and their families. Pediatric Pulmonology 2012;47(2):144-52. [DOI] [PubMed] [Google Scholar]
Modi 2006
- Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. Journal of Cystic Fibrosis 2006;5:177-85. [DOI] [PubMed] [Google Scholar]
Modi 2010
- Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. Journal of Pediatric Psychology 2010;35:1028-37. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. [PubMed] [Google Scholar]
O'Connor 1995
- O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making 1995;15(1):25-30. [DOI] [PubMed] [Google Scholar]
Oliver 2004
- Oliver MR, Heine RG, Ng CH, Volders E, Olinsky A. Factors affecting clinical outcome in gastrostomy-fed children with cystic fibrosis. Pediatric Pulmonology 2004;37:324-29. [DOI] [PubMed] [Google Scholar]
Pai 2006
- Pai AL, Drotar D, Zebracki K, Moore M, Youngstrom E. A meta-analysis of the effects of psychological interventions in pediatric oncology on outcomes of psychological distress and adjustment. Journal of Pediatric Psychology 2006;31(9):978-88. [DOI] [PubMed] [Google Scholar]
Pearson 1991
- Pearson DA, Pumariega AJ, Seilheimer DK. The development of psychiatric symptomatology in patients with cystic fibrosis. Journal of the American Academy of Child & Adolescent Psychiatry 1991;30(2):290-7. [DOI] [PubMed] [Google Scholar]
Plante 2001
- Plante WA, Lobato D, Engel R. Review of group interventions for pediatric chronic conditions. Journal of Pediatric Psychology 2001;26(7):435-53. [DOI] [PubMed] [Google Scholar]
Prchal 2009
- Prchal A, Landolt MA. Psychological interventions with siblings of pediatric cancer patients: a systematic review. Psychooncology 2009;18(12):1241-51. [DOI] [PubMed] [Google Scholar]
Quittner 1992a
- Quittner AL, DiGirolamo AM, Michel M, Eigen H. Parental Response to Cystic Fibrosis: A Contextual Analysis of the Diagnosis Phase. Journal of Pediatric Psychology 1992;17(6):683-704. [DOI] [PubMed] [Google Scholar]
Quittner 1992b
- Quittner AL, Opipari LC, Regoli MJ, Jacobsen J, Eigen H. The impact of caregiving and role strain on family life: Comparisons between mothers of children with cystic fibrosis and matched controls. Rehabilitation Psychology 1992;37(4):275-90. [Google Scholar]
Quittner 1998
- Quittner AL, Espelage DL, Opipari LC, Carter B, Eid N, Eigen H. Role strain in couples with and without a child with a chronic illness: Associations with marital satisfaction, intimacy, and daily mood. Health Psychology 1998;17(2):112-24. [DOI] [PubMed] [Google Scholar]
Quittner 2008
- Quittner AL, Barker DH, Snell C, Grimley ME, Marciel K, Cruz I. Prevalence and impact of depression in cystic fibrosis. Current Opinion in Pulmonary Medicine 2008;14:582-8. [DOI] [PubMed] [Google Scholar]
Quittner 2012b
- Quittner AL. Setting Up a Multi-National Trial: Lessons Learned in the TIDES study. Paper presented at the European Cystic Fibrosis Society Conference, Dublin, Irl. http://www.ecfs.eu/files/webfm/webfiles/File/AHP_Nursing%20Research/Dublin%20Presentations/TIDES%20Lessons%20Learned%206-7-12.pdf (accessed 05 December 2012).
Rapoff 2010
- Rapoff M. Adherence to Pediatric Medical Regimens. 2nd ed. Issues in Clinical Child Psychology edition. New York: Springer Science+Business Media, 2010. [Google Scholar]
Sansom‐Daly 2012
- Sansom-Daly UM, Peate M, Wakefield CE, Bryant RA, Cohn RJ. A systematic review of psychological interventions for adolescents and young adults living with chronic illness. Health Psychology 2012;31(3):380-93. [DOI] [PubMed] [Google Scholar]
Savage 2011
- Savage E, Beirne PV, Ni Chroinin M, Duff A, Fitzgerald T, Farrell D. Self-management education for cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007641.pub2] [DOI] [PubMed] [Google Scholar]
Seitz 2009
- Seitz DC, Besier T, Goldbeck L. Psychosocial interventions for adolescent cancer patients: a systematic review of the literature. Psychooncology 2009;18(7):683-90. [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith BA, Modi AC, Quittner AL, Wood BL. Depressive symptoms in children with cystic fibrosis and parents and its effects on adherence to airway clearance. Pediatric Pulmonology 2010;45:756-63. [DOI] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RC, Lushene RE. The State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychological Press, 1970. [Google Scholar]
Spielberger 1973
- Spielberger CD. State Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychological Press, 1973. [Google Scholar]
Spitzer 1994
- Spitzer R, Williams J, Kroenke D, Linzer M, deGruy F, Hahn S, et al. Utility of a new procedure for diagnosing mental disorders in primary care: The PRIME-MD 1000 study. JAMA 1994;272(22):1749-55. [PubMed] [Google Scholar]
Stark 1995
- Stark LJ, Jelalian E, Miller DL. Cystic Fibrosis. In: Roberts MC, editors(s). Handbook of Pediatric Psychology. 2nd edition. New York: Guildford Press, 1995. [Google Scholar]
Stern 2012
- Sens B, Stern M. Qualitaetssicherung Mukoviszidose - Ueberblick ueber den Gesundheitszustand der Patienten 2011. Bad Honnef: Hippocampus Verlag, 2012. [Google Scholar]
Sumner 1994
- Sumner G, Spietz A. Caregiver/parent-child interaction teaching manual. Seattle, WA: NCAST Publications, 1994. [Google Scholar]
Tsui 1990
- Tsui LC. Population Analysis of the Major Mutation in Cystic Fibrosis. Human Genetics 1990;85(4):391-445. [DOI] [PubMed] [Google Scholar]
Uman 2013
- Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ, Kisely SR. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD005179.pub3] [DOI] [PubMed] [Google Scholar]
US Patient Registry Annual Data Report 2010
- US Patient Registry Anual Data Report 2010. http://www.cff.org/UploadedFiles/LivingWithCF/CareCenterNetwork/PatientRegistry/2010-Patient-Registry-Report.pdf (accessed 06 August 2012).
Van Biervliet 2004
- Van Biervliet S, De Waele K, Van Winckel M, Robberecht E. Percutaneous endoscopic gastrostomy in cystic fibrosis: patient acceptance and effect of overnight tube feeding on nutritional status. Acta Gastroenterol Belg 2004;67(3):241-4. [PubMed] [Google Scholar]
Walker 1991
- Walker LS, Greene JW. The functional disability inventory: Measuring a neglected dimension of child health status. Journal of Pediatric Psychology 1991;16(1):39-58. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware J, Snow K, Kosinski M, Gandek B. SF-36 Health Survey: Manual and interpretation guide. The Health Institute, New England Medical Center 1993.
WHO 2003
- World Health Organisation. Adherence to long term therapies: evidence for action. Geneva: WHO, 2003. [Google Scholar]
Wittchen 2011
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Joensson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology 2011;21(9):655-79. [DOI] [PubMed] [Google Scholar]
Yorke 2005
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for children with asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003272.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yorke 2006
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD002982.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Glasscoe 2003
- Glasscoe CA, Quittner AL. Psychological interventions for cystic fibrosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003148.pub2] [DOI] [PubMed] [Google Scholar]


