Abstract
Disruption of epithelial barriers is a key pathogenic event of mucosal inflammation: It ignites the exaggerated immune response and accelerates tissue damage. Loss of barrier function is attributed to the abnormal structure and permeability of epithelial adherens junctions and tight junctions, driven by inflammatory stimuli through a variety of cellular mechanisms. This review focuses on roles of the actin cytoskeleton in mediating disruption of epithelial junctions and creation of leaky barriers in inflamed tissues. We summarize recent advances in understanding the role of cytoskeletal remodeling driven by actin filament turnover and myosin II-dependent contractility in the homeostatic regulation of epithelial barriers and barrier disruption during mucosal inflammation. We also discuss how the altered biochemical and physical environment of the inflamed tissues could affect the dynamics of the junction-associated actomyosin cytoskeleton, leading to the disruption of epithelial barriers.
Keywords: adherens junctions, actin turnover, non-muscle myosin II, tight junctions, permeability, cytoskeletal forces
Introduction
Epithelial layers form protective barriers that separate internal organs from the external environment and demarcate boundaries between different tissue compartments. Integrity of these barriers is regulated by elaborate intercellular contacts, most notably, adherens junctions (AJs) and tight junctions (TJs). Epithelial junctions are composed of adhesive transmembrane and cytoplasmic scaffolding proteins that readily self-associate to form dynamic multiprotein platforms at the plasma membrane [1,2]. Self-organization, stability and remodeling of epithelial junctions are regulated by their coupling to the underlying cortical actin cytoskeleton. Indeed, a junction-associated cortical actin belt represents one of the most recognizable cytoskeletal structures in epithelial and endothelial cells, and roles of the actin cytoskeleton in AJ/TJ assembly and functions have been demonstrated by a variety of pharmacological and genetic approaches [3–5]. Disruption of epithelial barriers is a hallmark of tissue inflammation. An excessive and prolonged increase in epithelial permeability has profound detrimental effects on tissue homeostasis by increasing body exposure to harmful environmental agents and igniting uncontrollable inflammation [6–8]. Several mechanisms are known to mediate the disruption of epithelial barriers in inflamed tissues [9]. Among them, remodeling of the junction-associated actin cytoskeleton could be particularly important. In this review we summarize recent evidence regarding the roles of actin filaments, actin motors and actin-binding proteins in regulating disruption of epithelial barriers during mucosal inflammation. Based on the available data, we focus on gastrointestinal and pulmonary inflammation. Actin-dependent regulation of endothelial barriers in inflamed tissues has been recently reviewed elsewhere [10,11].
Actin-dependent regulation of intercellular junctions: a battle of the cytoskeletal motors
Actin is a highly abundant eukaryotic ATPase that readily polymerizes into a dynamic network of filaments [12,13]. This network undergoes a constant remodeling driven by actin filament turnover and interactions with myosin motors. Actin filament turnover is a polarized process, at which the polymerization step occurs at a so-called ‘barbed’ end, while depolymerization events happen at the opposite, pointed filament end [12,13]. This cyclic process is controlled by specific sets of actin-polymerizing and depolymerizing proteins. Thus, the actin-related proteins (Arp) 2/3 complex and formins are specialized in assembling branched and linear actin filaments, respectively [12–14]. Members of the actin-depolymerizing factor (ADF)/cofilin protein family play essential roles in actin filament severing and depolymerization [15]. Arrangement and motility of actin filaments in epithelial cells are further regulated by non-muscle myosin II (NM II) motors that either slide parallel actin filaments, or compress a loose filament meshwork into tightly packed actomyosin bundles [16,17]. One of the key consequences of the actin filament dynamics is creation of directional mechanical forces that act upon all actomyosin-coupled organelles and subcellular structures. A protrusive (pushing) force is generated by the orchestrated polymerization of actin filaments, whereas a contractile (pulling) force is driven by the activity of bipolar NM II fibrils and actin filament sliding [3,17,18]. Both AJs and TJs are physically coupled to the underlying actomyosin cytoskeleton via several actin-binding molecules, such as α-catenin, vinculin, afadin and zonula occludens (ZO) proteins [19,20]. Therefore, these junctions constantly experience cytoskeletal pushing and pulling forces that dramatically affect their assembly and permeability of epithelial barriers [3,4,16–18]. Net functional effects of the cytoskeletal forces on epithelial junctions depend on the force directionality, which is influenced by the spatial arrangement of the perijunctional actomyosin network [17,18].
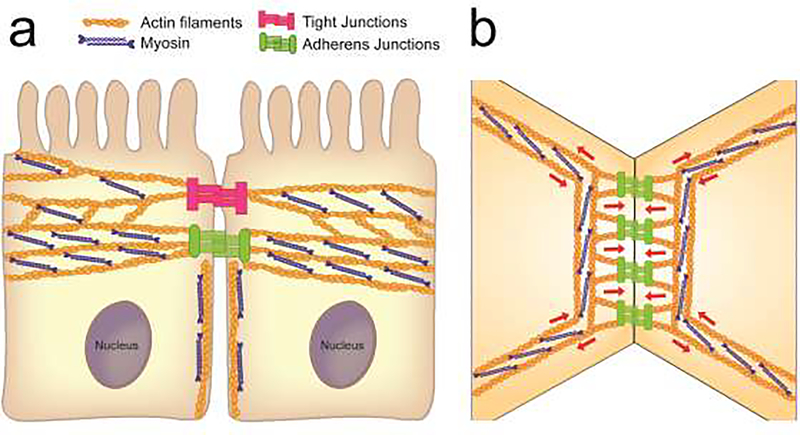
Intercellular adhesions of columnar epithelial cells are regulated by different cortical actin cytoskeletal structures (Fig. 1A). The most recognizable structure, is a circumferential actin belt associated with mature AJs and, probably, with the basal aspect of TJs [21,22]. This belt is flanked by the apical TJ-associated actin network [23] and lateral cortical actin filaments coupled with non-AJ cadherin-catenin complexes [24]. The circumferential belt is composed of thick actin filament bundles that run in parallel to the cell membrane and intercellular contact areas (Fig. 1B) [21,23]. They are enriched in bipolar NM II filaments, which in some epithelia are organized in periodic sarcomeric-type structures resembling the skeletal muscle myofibrils [21]. It is unclear if the circumferential actomyosin bundles directly associate with E-cadherin-catenin complexes at the plasma membrane. At endothelial, and possible epithelial AJs, junctional proteins are physically linked to the network of orthogonal actin filaments [23,25]. The orthogonal filaments originate from the circumferential actomyosin bundles and extend toward the plasma membrane (Fig. 1B) as either a branched network in endothelial cells [25], or linear microspikes in the columnar epithelium [26]. These actin filaments undergo a constant turnover driven by the Arp 2/3-dependent actin polymerization. [25,26]. The described organization of the actomyosin cytoskeleton enables generation of different mechanical forces acting upon epithelial junctions (Fig. 1B). Protrusive forces generated by polymerizing orthogonal actin network push plasma membranes of contacting cells toward each other, thereby promoting E-cadherin trans-interactions and strengthening AJs (Fig. 1B). The contractive/pulling forces generated by parallel actomyosin bundles (Fig. 1B) could either stabilize/repair intercellular adhesions [25,27,28], or trigger their disassembly [26].
Figure 1.
Organization of the actin cytoskeleton and cytoskeleton-generated forces at epithelial junctions.
a. A diagram depicts different fractions of the actin cytoskeleton associated with the apical junctions and lateral contacts of adjacent columnar epithelial cells.
b. A putative spatial organization of the actomyosin cytoskeleton at epithelial apicaljunctions. Red arrows indicate directionality of forces generated by the perijunctional cytoskeleton.
In contrast to the well-defined structure of the circumferential actomyosin belt, little is known about the composition of apical-TJ-associated actin filaments. They appear to be enriched in a specific actin isoform, γ-cytoplasmic actin, whereas β-cytoplasmic actin is more abundant at AJ-associated/lateral actin filaments [29]. Yet, TJ- and AJ- associated cytoskeletal structures cross-talk and compete for the available pool of monomeric actin. Thus, loss TJ-associated actin filaments in ZO protein-depleted epithelium dramatically enhances the assembly and contractility of the circumferential actomyosin belt [30,31]. Actin filaments associated with the lateral plasma membranes also contribute to intercellular adhesions and epithelial barrier development [24,32]; however, their ultrastructure remains poorly understood. These filaments generate both protrusive and contractile forces and may regulate the cortical flow along the lateral membrane that delivers AJ and TJ proteins to apical junctions.
Barrier-protective roles of actin filament turnover during mucosal inflammation
Actin filament turnover is known to be essential for AJ and TJ assembly in cultured epithelial cell monolayers, in which both actin filament-polymerizing and depolymerizing proteins have been implicated in the formation of epithelial barriers [9,33]. Surprisingly, some of these actin regulators could be dispensable for the homeostatic functions of epithelial barriers in vivo. For example, mice with intestinal epithelial specific knockout of a key component of the Arp2/3 complex, ArpC3, did not show noticeable defects in the organization of epithelial junctions and apical actin filaments [34]. Likewise, intestinal epithelial-specific loss of essential Arp2/3 activator, a neural Wiskott - Aldrich syndrome protein, increased normal gut permeability, but caused no global alterations in the architecture of TJ and the perijunctional actomyosin cytoskeleton [35]. By contrast, mice deficient in another regulator of actin polymerization, cortactin, showed abnormal molecular composition of intestinal epithelial TJs, along with the deformation of colonic crypts [36]. However, the defects in intestinal mucosal organization caused by cortactin depletion most likely reflect diverse functional roles of this protein in the intestinal epithelium that involve not only control of the actin filament turnover, but also regulation of RhoA signaling [36].
Recent studies suggest that balanced actin filament turnover protects epithelial barriers and attenuates tissue injury during mucosal inflammation in vivo. For example, cortactin-deficient mice developed more severe dextran sodium sulfate (DSS)-induced colitis as compared to wild type controls [36]. Likewise, ADF-null mice while not displaying vivid intestinal epithelium abnormalities under homeostatic conditions, demonstrated exaggerated barrier disruption, colonic mucosal injury and inflammation during DSS colitis [37]. Interestingly, loss of ADF functions was not compensated by its close homolog, cofilin-1, which is highly expressed in the intestinal epithelium. Disruption of the airway’s epithelial barrier during bacterial endotoxin-induced acute lung injury was associated with the perturbed balance of key actin monomer-binding proteins, profilin-1 and β-thymosin [38]. Normalizing epithelial profilin-1 and β-thymosin expression attenuated barrier disruption in the airway and neutrophil influx into the lungs. These examples demonstrate that an elaborate cross talk between different regulators of actin filament turnover creates a critical protective mechanism that attenuates epithelial injury and/or promotes mucosal repair during inflammation.
NM II-dependent regulation of epithelial barriers: detrimental effects of strong and weak cytoskeletal forces
Activation of junction-associated NM II is considered a key mechanism driving epithelial barrier disruption during mucosal inflammation [6,7]. Such activation occurs via phosphorylation of regulatory myosin light chains (RMLC), creating excessive contractile forces that tear apart intercellular junctions [6]. While the increased RLMC phosphorylation in inflamed epithelia has been well-documented, neither remodeling of the perijunctional actin cytoskeleton, nor contractile/tension forces applied to epithelial junctions during inflammation have been properly examined. RMLC phosphorylation is mediated by several kinases, most notably myosin light chain kinase (MLCK) and Rho-associated kinases (ROCK) [5,6]. These kinases are activated by a variety of proinflammatory stimuli, including bacterial pathogens, cytokines and extracellular proteinases [39,40]. A recent study revealed an unusual cellular mechanism of epithelial actomyosin activation by tumor necrosis factor (TNF)-α. This activation is driven by MLCK recruitment to the apical/perijunctional actomyosin cytoskeleton [41]. A small molecular compound, divertin, preventing apical MLCK translocation, displayed potent barrier-protective effects in different types of experimental inflammation [41]. However, other evidence suggests that NM II-dependent tension/contractility serves as an important enhancer of epithelial barrier integrity that strengthens adhesive interactions between different AJ and TJ proteins [3,4,16] and activates a RhoA-dependent protective response to repair junctional breaks [27,28]. Either pharmacological or genetic inhibition of NM II was shown to attenuate both AJ/TJ assembly and disassembly in vitro [42,43]. Furthermore, intestinal epithelial specific knockout of NM IIA in mice triggered leaky gut barrier along with spontaneous intestinal inflammation, and exaggerated mucosal injury during DSS colitis [44]. These studies suggest that homeostatic integrity of epithelial barriers is regulated by balanced tensile/contractile NM II forces. Loss of tension due to decreased NM II activity, or increased tension/contractility caused by NM II activation, could lead to the barrier breakdown and exaggerated mucosal injury.
Recent studies highlight the roles of so-called ‘unconventional’ myosins in the regulation of epithelial barriers. For example, an unconventional myosin IXb is a unique cytoskeletal motor that also modulates RhoA activity via its Rho GTPase activating domain. Loss of this motor in the intestinal epithelium promoted epithelial cell apoptosis resulting in the development of spontaneous colitis [45]. The epithelial injury in myosin IXb null animals was associated with RhoA activation and stimulation of NM II contractility. Another example is an unconventional myosin 1D that localizes at the lateral membrane of intestinal epithelial cells and couples cortical actin filaments to the membrane phospholipids [46]. Mutations that decrease myosin 1D expression in different murine tissues increased animal sensitivity to DSS colitis, via mechanisms which remain to be determined [46].
Mechanisms that mediate altered organization of the epithelial cytoskeleton during mucosal inflammation
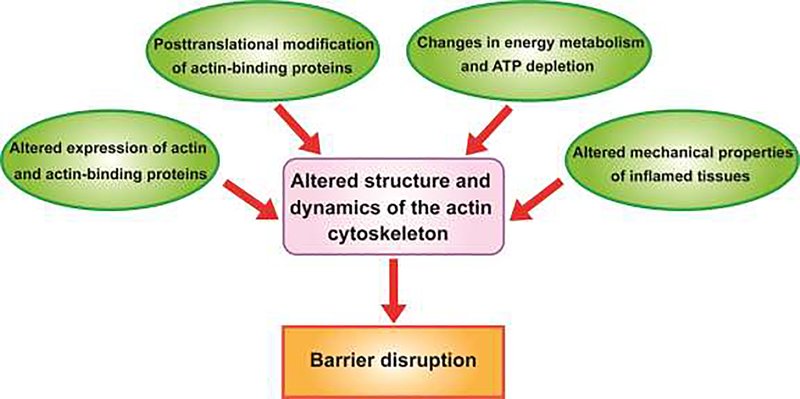
Mucosal inflammation results in profound changes in the biochemical composition and mechanical properties of surrounding tissues, which contribute to the disruption of epithelial barriers. Many of these environmental changes could be sensed and transduced to epithelial junctions by the actin cytoskeleton. While altered organization of the apical/junctional actomyosin bundles has been reported in clinical and experimental inflammation [33], the mechanisms and functional impact of such alterations remain poorly understood. Below we discuss the most likely mechanisms by which inflammatory environment affects structure and dynamics of the epithelial actomyosin cytoskeleton (Fig. 2).
Figure 2.
Multiple mechanisms affecting the organization and dynamics of the epithelial actin cytoskeleton during mucosal inflammation.
The diagram presents most likely mechanisms by which inflammatory environment can alter the structure and remodeling of the perijunctional actomyosin cytoskeleton in epithelial cells, resulting in breakdown of epithelial barriers.
Altered expression of actin and actin-binding proteins
Accumulation of different cytokines, free radicals and active lipids in inflamed tissues triggers robust transcriptional reprogramming of epithelial cells that involves marked alterations in AJ and TJ protein expression [7,9]. It is likely that inflammatory mediators also affect expression of different cytoskeletal proteins, although this possibility has been investigated in only few studies. For example, a comparative proteomic analysis of tissue samples from IBD patients and non-IBD subjects documented altered expression of different actin isoforms along with several actin-binding proteins in the inflamed IBD intestinal mucosa [47,48].
Posttranslational modification of actin-binding proteins
Inflammatory mediators stimulate a variety of signaling cascades in epithelial cells resulting in phosphorylation of different cytoskeletal proteins. A classic example is RMLC phosphorylation triggered by TNFα and other cytokines that enhances actomyosin contractility and drives junctional disassembly [6,7]. Phosphorylation events could also modulate the perijunctional actin filament turnover in inflamed epithelia. For example, cortactin phosphorylation disrupts its interaction with AJ proteins and underlying actin filaments, thereby uncoupling epithelial junctions from the cortical cytoskeleton [49]. Increased cortactin phosphorylation was associated with disruption of the airway’s epithelial barrier caused by respiratory syncytial virus infection [50]. Altered Rho/ROCK signaling in inflamed mucosa is known to affect actin filament turnover via inhibitory phosphorylation of ADF/cofilin proteins [15,51]. For example, Shigela infection destabilized intestinal epithelial barrier by causing activatory de-phosphorylation of ADF/cofilin [52]. In contrast, increased phosphorylation and inhibition of ADF/cofilin was observed in the intestinal mucosa of patients with irritable bowel syndrome [53].
Altered energy metabolism and ATP depletion
Both actin filament turnover and NM II-dependent contractility are driven by the chemical energy of ATP hydrolysis [13,17]. Therefore, organization and dynamics of the actomyosin cytoskeleton could be affected by metabolic alterations and fluctuations in the cellular ATP level. Chemical ATP depletion readily disrupts the integrity of model epithelial barriers in parallel with disassembly of perijunctional actin filaments [54,55]. Importantly, inflammation frequently decreases cellular ATP level, due to tissue ischemia and defects in mitochondrial respiration [56,57]. However, causal links between altered energy metabolism/ATP depletion, disassembly of the actomyosin cytoskeleton, and disruption of the epithelial barriers during mucosal inflammation in vivo await future investigations.
Altered mechanical properties of inflamed tissues
Since assembly and dynamics of the actomyosin filaments depend on the applied external forces, the cytoskeleton is able to sense the mechanical properties of tissues and transduce this information to epithelial junctions. Indeed, AJs and TJs are known to be sensitive to external mechanical forces in vitro, including shear stress and cyclic stretch [58–60]. Because of such mechanosensitivity, permeability of epithelial barriers could be affected by mechanical stresses associated with physiological processes in vivo. Examples include stretching of airway and intestinal epithelia due to lung breeding and gut peristalsis, as well as compression of the epithelial segments during intestinal crypt invagination. Inflammatory processes markedly affect biomechanical properties of different tissues, which could alter mechanical forces applied to epithelial barriers [61]. Several changes could be envisioned. They include altered magnitude and frequency of the cyclic stretch due to abnormal lung ventilation and gut peristalsis, increased osmotic pressure by tissue edema and increased stiffness of the underlining connective tissues in fibrotic organs.
Conclusions
The actin cytoskeleton is a master regulator of the assembly and remodeling of epithelial junctions and establishment of tissue barriers. Epithelial junctions are subjected to constant protrusive, tensile and contractile forces generated by two cytoskeletal motors: actin filament polymerization and myosin II-dependent contractility. Activity of these motors is controlled by biochemical and mechanical properties of the environment. Surprisingly, little is known about alterations in cytoskeletal forces that could affect structure and permeability of epithelial barriers in inflamed tissues. Our understanding of the junction-associated actomyosin cytoskeleton has been derived from studying epithelial cell monolayers existing under excessive mechanical stress due to their attachment to stiff substrates in vitro. These conditions do not reflect physical features of the environment surrounding epithelial barriers in vivo and cannot model altered mechanical properties of tissues in different diseases. Introduction of novel models, such as ex vivo primary epithelial cell organoids and animals with epithelial specific knockouts of different actin regulators should provide critical novel insights to our understanding the roles and mechanisms of cytoskeletal regulation of epithelial barriers during mucosal inflammation.
Acknowledgments
Financial support: This work was supported by the NIH-NIDDK grant RO1 DK108278 to A.I.I.
Footnotes
Conflict of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubsam M, Broussard JA, Wickstrom SA, Nekrasova O, Green KJ, Niessen CM: Adherens Junctions and Desmosomes Coordinate Mechanics and Signaling to Orchestrate Tissue Morphogenesis and Function: An Evolutionary Perspective. Cold Spring Harb Perspect Biol 2018, 10:a02907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Itallie CM, Anderson JM: Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 2014, 36:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charras G, Yap AS: Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr Biol 2018, 28:R445–R457. [DOI] [PubMed] [Google Scholar]
- 4.Citi S: The mechanobiology of tight junctions. Biophys Rev 2019, 11:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varadarajan S, Stephenson RE, Miller AL: Multiscale dynamics of tight junction remodeling. J Cell Sci 2019, 132:jcs229286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley A, Turner JR: Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb Perspect Biol 2018, 10:a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruder B, Atreya R, Becker C: Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int J Mol Sci 2019, 20:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugita K, Kabashima K: Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J Leukoc Biol 2020, 107:749–762. [DOI] [PubMed] [Google Scholar]
- 9.Lechuga S, Ivanov AI: Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim Biophys Acta Mol Cell Res 2017, 1864:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alon R, van Buul JD: Leukocyte Breaching of Endothelial Barriers: The Actin Link. Trends Immunol 2017, 38:606–615. [DOI] [PubMed] [Google Scholar]
- 11.Belvitch P, Htwe YM, Brown ME, Dudek S: Cortical Actin Dynamics in Endothelial Permeability. Curr Top Membr 2018, 82:141–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merino F, Pospich S, Raunser S: Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin Cell Dev Biol 2020, 102:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard TD: Actin and Actin-Binding Proteins. Cold Spring Harb Perspect Biol 2016, 8:a018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann D, Kovar DR: Feeling the force: formin’s role in mechanotransduction. Curr Opin Cell Biol 2019, 56:130–140. [DOI] [PubMed] [Google Scholar]
- 15.Coumans JVF, Davey RJ, Moens PDJ: Cofilin and profilin: partners in cancer aggressiveness. Biophys Rev 2018, 10:1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal P, Zaidel-Bar R: Principles of Actomyosin Regulation In Vivo. Trends Cell Biol 2019, 29:150–163. [DOI] [PubMed] [Google Scholar]
- 17.Svitkina TM: Ultrastructure of the actin cytoskeleton. Curr Opin Cell Biol 2018, 54:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang VW: Cell-cell adhesion interface: orthogonal and parallel forces from contraction, protrusion, and retraction. F1000Res 2018, 7:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spadaro D, Le S, Laroche T, Mean I, Jond L, Yan J, Citi S: Tension-Dependent Stretching Activates ZO-1 to Control the Junctional Localization of Its Interactors. Curr Biol 2017, 27:3783–3795 e3788. [DOI] [PubMed] [Google Scholar]
- 20.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M: alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010, 12:533–542. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahim S, Fujita T, Millis BA, Kozin E, Ma X, Kawamoto S, Baird MA, Davidson M, Yonemura S, Hisa Y, et al. : NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol 2013, 23:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonemura S, Itoh M, Nagafuchi A, Tsukita S: Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 1995, 108:127–142. [DOI] [PubMed] [Google Scholar]
- 23.Hirokawa N, Tilney LG: Interactions between actin filaments and between actin filaments and membranes in quick-frozen and deeply etched hair cells of the chick ear. J Cell Biol 1982, 95:249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kannan N, Tang VW: Myosin-1c promotes E-cadherin tension and force-dependent recruitment of alpha-actinin to the epithelial cell junction. J Cell Sci 2018, 131:jcs211334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efimova N, Svitkina TM: Branched actin networks push against each other at adherens junctions to maintain cell-cell adhesion. J Cell Biol 2018, 217:1827–1845.** This study uses a platinum replica electron microscopy to visualize a high resolution organization of the actomyosin cytoskeleton associated with endothelial AJs. It demonstrates a direct coupling of AJ proteins with an orthogonal actin filament network that is assembled via Arp2/3 dependent actin polymerization.
- 26.Li JXH, Tang VW, Brieher WM: Actin protrusions push at apical junctions to maintain E-cadherin adhesion. Proc Natl Acad Sci U S A 2020, 117:432–438.* Similar to the finding reported in Ref. 25, this study signifies the roles of Arp2/3 driven actin polymerization in creating membrane protrusions that strengthen mature epithelial apical junctions. An interesting finding is that forces created by the membrane protrusions (spikes) are antagonized by contractile forces generated by the circumferential perijunctional actomyosin bundles.
- 27.Acharya BR, Nestor-Bergmann A, Liang X, Gupta S, Duszyc K, Gauquelin E, Gomez GA, Budnar S, Marcq P, Jensen OE, et al. : A Mechanosensitive RhoA Pathway that Protects Epithelia against Acute Tensile Stress. Dev Cell 2018, 47:439–452. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson RE, Higashi T, Erofeev IS, Arnold TR, Leda M, Goryachev AB, Miller AL: Rho Flares Repair Local Tight Junction Leaks. Dev Cell 2019, 48:445–459.** This elegant study uses live cell imaging to examine homeostatic remodeling of TJs and associated actomyosin cytoskeleton. It reveals the existence of periodic flares of active RhoA at TJs that triggers actin filament polymerization and actomyosin contractility to repair spontaneous TJ breaks.
- 29.Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, Ivanov AI: Nonredundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Mol Biol Cell 2012, 23:3542–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartagena-Rivera AX, Van Itallie CM, Anderson JM, Chadwick RS: Apical surface supracellular mechanical properties in polarized epithelium using noninvasive acoustic force spectroscopy. Nat Commun 2017, 8:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odenwald MA, Choi W, Kuo WT, Singh G, Sailer A, Wang Y, Shen L, Fanning AS, Turner JR: The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J Biol Chem 2018, 293:17317–17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luong P, Hedl M, Yan J, Zuo T, Fu TM, Jiang X, Thiagarajah JR, Hansen SH, Lesser CF, Wu H, et al. : INAVA-ARNO complexes bridge mucosal barrier function with inflammatory signaling. Elife 2018, 7:e38539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov AI, Parkos CA, Nusrat A: Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 2010, 177:512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou K, Sumigray KD, Lechler T: The Arp2/3 complex has essential roles in vesicle trafficking and transcytosis in the mammalian small intestine. Mol Biol Cell 2015, 26:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garber JJ, Mallick EM, Scanlon KM, Turner JR, Donnenberg MS, Leong JM, Snapper SB: Attaching-and-Effacing Pathogens Exploit Junction Regulatory Activities of N-WASP and SNX9 to Disrupt the Intestinal Barrier. Cell Mol Gastroenterol Hepatol 2018, 5:273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Citalan-Madrid AF, Vargas-Robles H, Garcia-Ponce A, Shibayama M, Betanzos A, Nava P, Salinas-Lara C, Rottner K, Mennigen R, Schnoor M: Cortactin deficiency causes increased RhoA/ROCK1-dependent actomyosin contractility, intestinal epithelial barrier dysfunction, and disproportionately severe DSS-induced colitis. Mucosal Immunol 2017, 10:1237–1247.** A rare and important investigation of the effects of actin-polymerizing proteins on the severity of mucosal inflammation. The study utilizes cortactin null mice and shows their increased sensitivity to DSS colitis. Furthermore, it describes a dual mechanism of cortactin-dependent regulation of the intestinal epithelial barrier that involves modulation of the actin filament turnover and RhoA signaling.
- 37.Wang D, Naydenov NG, Feygin A, Baranwal S, Kuemmerle JF, Ivanov AI: Actin-Depolymerizing Factor and Cofilin-1 Have Unique and Overlapping Functions in Regulating Intestinal Epithelial Junctions and Mucosal Inflammation. Am J Pathol 2016, 186:844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen B, Yang Z, Yang C, Qin W, Gu J, Hu C, Chen A, Ning J, Yi B, Lu K: A self-organized actomyosin drives multiple intercellular junction disruption and directly promotes neutrophil recruitment in lipopolysaccharide-induced acute lung injury. FASEB J 2018:fj201701506RR.** This is the first report that implicates a perturbed balance of two major actin monomer binding proteins, proflin-1 and β4-thymosin, in the disruption of the airway’s epithelial barrier during lipopolysaccharide-induced acute lung injury.
- 39.Al-Sadi R, Youssef M, Rawat M, Guo S, Dokladny K, Haque M, Watterson MD, Ma TY: MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am J Physiol Gastrointest Liver Physiol 2019, 316:G278–G290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nighot M, Rawat M, Al-Sadi R, Castillo EF, Nighot P, Ma TY: Lipopolysaccharide-Induced Increase in Intestinal Permeability Is Mediated by TAK-1 Activation of IKK and MLCK/MYLK Gene. Am J Pathol 2019, 189:797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham WV, He W, Marchiando AM, Zha J, Singh G, Li HS, Biswas A, Ong M,Jiang ZH, Choi W, et al. : Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat Med 2019, 25:690–700.* The authors describe a non-canonical mechanism that mediates leakiness of the intestinal epithelial barrier during experimental inflammation. This mechanism involves recruitment of a myosin light chain kinase to the perijunctional actomyosin, resulting in the stimulation of contractility and junctional disassembly.
- 42.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA: A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2007, 2:e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA: Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 2005, 16:2636–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naydenov NG, Feygin A, Wang D, Kuemmerle JF, Harris G, Conti MA, Adelstein RS, Ivanov AI: Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci Rep 2016, 6:24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegan PS, Chandhoke SK, Barone C, Egan M, Bahler M, Mooseker MS: Mice lacking myosin IXb, an inflammatory bowel disease susceptibility gene, have impaired intestinal barrier function and superficial ulceration in the ileum. Cytoskeleton 2016, 73:163–179. [DOI] [PubMed] [Google Scholar]
- 46.McAlpine W, Wang KW, Choi JH, San Miguel M, McAlpine SG, Russell J, Ludwig S, Li X, Tang M, Zhan X, et al. : The class I myosin MYO1D binds to lipid and protects against colitis. Dis Model Mech 2018, 11:dmm035923.* A random chemical mutagenesis screen in mice identified mutations of an unconventional myosin 1d as a cause of the exaggerated intestinal mucosal injury during DSS colitis. The observed colitis-promoting effects of myosin 1d deficiency are linked to non-hematopoietic tissue compartments.
- 47.Poulsen NA, Andersen V, Moller JC, Moller HS, Jessen F, Purup S, Larsen LB: Comparative analysis of inflamed and non-inflamed colon biopsies reveals strong proteomic inflammation profile in patients with ulcerative colitis. BMC Gastroenterol 2012, 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shkoda A, Werner T, Daniel H, Gunckel M, Rogler G, Haller D: Differential protein expression profile in the intestinal epithelium from patients with inflammatory bowel disease. J Proteome Res 2007, 6:1114–1125. [DOI] [PubMed] [Google Scholar]
- 49.Sroka R, Van Lint J, Katz SF, Schneider MR, Kleger A, Paschke S, Seufferlein T, Eiseler T: Cortactin is a scaffolding platform for the E-cadherin adhesion complex and is regulated by protein kinase D1 phosphorylation. J Cell Sci 2016, 129:2416–2429. [DOI] [PubMed] [Google Scholar]
- 50.Rezaee F, DeSando SA, Ivanov AI, Chapman TJ, Knowlden SA, Beck LA, Georas SN: Sustained protein kinase D activation mediates respiratory syncytial virus-induced airway barrier disruption. J Virol 2013, 87:11088–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee MH, Kundu JK, Chae JI, Shim JH: Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch Pharm Res 2019, 42:481–491. [DOI] [PubMed] [Google Scholar]
- 52.Maldonado-Contreras A, Birtley JR, Boll E, Zhao Y, Mumy KL, Toscano J, Ayehunie S, Reinecker HC, Stern LJ, McCormick BA: Shigella depends on SepA to destabilize the intestinal epithelial integrity via cofilin activation. Gut Microbes 2017, 8:544–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodino-Janeiro BK, Martinez C, Fortea M, Lobo B, Pigrau M, Nieto A, Gonzalez-Castro AM, Salvo-Romero E, Guagnozzi D, Pardo-Camacho C, et al. : Decreased TESK1-mediated cofilin 1 phosphorylation in the jejunum of IBS-D patients may explain increased female predisposition to epithelial dysfunction. Sci Rep 2018, 8:2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.JanssenDuijghuijsen LM, Grefte S, de Boer VCJ, Zeper L, van Dartel DAM, van der Stelt I, Bekkenkamp-Grovenstein M, van Norren K, Wichers HJ, Keijer J: Mitochondrial ATP Depletion Disrupts Caco-2 Monolayer Integrity and Internalizes Claudin 7. Front Physiol 2017, 8:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JS, Wang RX, Alexeev EE, Lanis JM, Battista KD, Glover LE, Colgan SP: Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function. J Biol Chem 2018, 293:6039–6051.** This study provides an elegant quantitative analysis of the relationship between energy supply, cytoskeletal assembly, and barrier function in model intestinal epithelial cell monolayers. One of the most valuable aspects of this paper is that it determines the percentage of the total energy expenditure that is directed to the actin polymerization in epithelial cells.
- 56.Campbell EL, Colgan SP: Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2019, 16:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang HS, Noh MR, Kim J, Padanilam BJ: Defective Mitochondrial Fatty Acid Oxidation and Lipotoxicity in Kidney Diseases. Front Med 2020, 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavanaugh KJ Jr., Oswari J, Margulies SS: Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 2001, 25:584–591. [DOI] [PubMed] [Google Scholar]
- 59.Gao X, Acharya BR, Engl WCO, De Mets R, Thiery JP, Yap AS, Viasnoff V: Probing compression versus stretch activated recruitment of cortical actin and apical junction proteins using mechanical stimulations of suspended doublets. APL Bioeng 2018, 2:026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samak G, Gangwar R, Crosby LM, Desai LP, Wilhelm K, Waters CM, Rao R: Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2014, 306:G947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Discher D, Dong C, Fredberg JJ, Guilak F, Ingber D, Janmey P, Kamm RD, Schmid-Schonbein GW, Weinbaum S: Biomechanics: cell research and applications for the next decade. Ann Biomed Eng 2009, 37:847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]