Abstract
The protein corona has served as a central dogma and a nuisance to the applications of nanomedicine and nanobiotechnology for well over a decade. Here we introduce the emerging field of amyloidosis inhibition, which aims to understand and harness the interfacial phenomena associated with a nanoparticle interacting with pathogenic amyloid proteins. Much of this interaction correlates with our understanding of the protein corona, and yet much differs, as elaborated for the first time in this Perspective. Specifically, we examine the in vitro, in silico and in vivo features of the new class of “amyloid protein corona”, and discuss how the interactions with nanoparticles may halt the self-assembly of amyloid proteins. As amyloidosis is driven off pathway by the nanoparticles, the oligomeric and protofibrillar populations are suppressed to ameliorate their cytotoxicity. Furthermore, as amyloid proteins spread via the transport of bodily fluids or cross seeding, amyloidosis is inherently associated with dynamic proteins and ligands to evoke the immune system. Accordingly, we ponder the structural and medical implications of the amyloid protein corona in the presence of their stimulated cytokines. Understanding and exploiting the amyloid protein corona may facilitate the development of new theranostics against a range of debilitating amyloid diseases.
Keywords: amyloidosis, nanoparticle inhibitor, protein corona, cytokine, amyloid protein corona
Graphical Abstract
1. A brief introduction to the protein corona
One of the biggest challenges in the field of nanomedicine is that, when nanomaterials enter a biological fluid, they spontaneously take on a new biological identity through fouling [1–3]. Here the nanomaterials encounter a smorgasbord of possible binding partners in the complex and concentrated mix of biomolecules, including proteins, lipids, and polysaccharides, of which the proteins are the most abundant and hence the generic name of the protein “corona” is assigned to represent the nanoparticle-biomolecular complexes [4, 5]. This inevitable formation of the nano-bio interface regulates the overall physicochemical, pharmacological and toxicological profiles of the nanomaterials in an unpredictable manner, including rapid clearance from the bloodstream, loss of targeting capacity, and activation of the immune response (Fig. 1) [6–16]. Therefore, understanding the nano-bio interface is necessary for advancing nanoscale science and engineering and for facilitating the clinical translation of nanomedicines [17–22]. More importantly, exploiting new strategies to attenuate the adverse effects of the protein corona offers new opportunities for developing rational nano-platforms for theranostics [6, 23]. In general, the manipulation and utilization of proteins mainly aims at: (1) attenuating the formation of the protein corona, (2) controlling the surface properties of nanomaterials, and (3) pre-coating nanomaterials with specific/functional proteins or peptides. For a comprehensive introduction to the history, foundation, characterization, implication as well as application of the protein corona, interested readers may refer to the reviews by Walkey and Chan [2], Cai and Chen [6], Doctor et al. [24], del Pino et al. [10] and Ke et al. [25].
Figure 1. The “conventional” protein corona and the amyloid protein corona.

The amyloid protein corona owes its name to the protein corona, but entails unique structural characteristics and pathological implications due to the propensity of amyloid proteins for aggregation.
Amyloids may be regarded as native nanomaterials that form in the pathological fluids, via a process called amyloidosis which underlies a range of devastating human diseases. In recent years, a new strategy against amyloidosis has involved the use of engineered nanomaterials to target amyloid proteins, where a nano-bio interface arises [26–28]. However, the design of these nanoparticle-based strategies has largely ignored the role of the protein corona that is inevitably formed in vivo.
Here we present the “amyloid protein corona” [29] that is at the front and center of amyloidosis inhibition but has yet to gain much attention within the field. It is only logical to contend that the collective entity of the amyloids and their protein corona mediates their pathological outcomes and therapeutic effects, in terms of (1) impacting the targeting capacity of anti-amyloid agents, (2) boosting the immune response by the structural changes of coronal proteins, and (3) accelerating amyloid protein fibrillization and their downstream physiopathological manifestations (Fig. 1) [30]. Understanding the foundation and implications of the amyloid protein corona is, therefore, essential for guiding the development of clinically viable anti-amyloid strategies, a viewpoint to convey by this Perspective.
2. Exploiting the amyloid protein corona
In the following, we focus on a special class of the protein corona comprised of amyloid proteins and peptides (abbreviated as “amyloid proteins” hereafter for simplicity) associated with nanoparticles. We discuss the inner workings of such corona from the perspective of amyloidosis, i.e., the spontaneous conversion of monomeric proteins/peptides into toxic intermediates and fibrils, and its modulation by the physicochemical properties of their antagonist – the nanoparticle inhibitors. We highlight our recent progress in delineating this new class of protein corona in vitro, in silico and in vivo, motivated by the need of harnessing their theranostic potential for combating neurological disorders, systemic amyloidosis, as well as metabolic disorders.
2a. Amyloid protein sequestration in vitro and in vivo
Alzheimer’s and Parkinson’s disease (AD, PD) as well as type 2 diabetes (T2D) are epidemics impacting an estimated global population of 400 million. Despite their different origin and capacity for cross-talk, these diseases share a pathological hallmark of extracellular deposits of fibrils and plaques and intracellular tangles derived from the amyloidosis of peptides and proteins [31]. Kinetically, amyloidosis is characterized by the three consecutive phases of nucleation, elongation and saturation of protein fibrillization, often indicated by the fluorescence fluctuations of thioflavin T (ThT) or Congo red which possesses a high affinity for the cross-β backbone that is ubiquitous to all amyloid fibrils. Primary nucleation involves the assembly of monomeric proteins and peptides into the oligomers that can grow into amyloid fibrils, while secondary nucleation utilizes the surface of newly-formed fibrils and their fragments to seed peptide assembly [32, 33]. Contributions of both mechanisms give rise to the polymorphism of amyloid fibrils on the nanoscale [34]. Although biophysical in nature, the term of amyloidosis entails strong biological, functional and pathological connotations central to the wellbeing of microorganisms, animals and humans.
Historically, our understanding of amyloidosis has not been straightforward. While the amyloid hypothesis [35] initially attributed neurodegeneration in AD to amyloid β (Aβ) fibrils, a growing body of literature in the past two decades has implicated oligomers as the most toxic species of amyloid proteins, regardless of their origin and sequence [36]. Accordingly, small molecules (including polyphenols such as epigallocatechin gallate), natural and synthetic peptides as well as monoclonal antibodies have been designed to mitigate amyloidosis [31, 37–42]. Overall, these efforts have shown great promise in vitro, moderate successes in vivo with mouse models, and abysmal outcomes in human clinical trials.
Within the past decade, a slew of nanomaterials have been designed to utilize their physicochemical properties to engage the structural characteristics of – primarily – Aβ, alpha-synuclein (αS) and human islet amyloid polypeptide (IAPP), the amyloid proteins associated with AD, PD and T2D pathologies, respectively [43–58]. While the choices of the nanomaterial inhibitors appeared sporadic in the early going [47, 59, 60], it has emerged from these efforts that a key to amyloidosis inhibition is to disrupt protein-protein association by promoting nanoparticle-protein interaction [61]. It is worth noting that both protein self-assembly and nanoparticle-protein interaction are mediated primarily by hydrophobic interaction, π-stacking and hydrogen bonding. In any case, amyloidosis inhibition with nanoparticles renders a corona of sequestrated proteins/peptides on the nanoparticle surface, captured in the forms of disordered and alpha-helical monomers, alpha-helical and β-sheet rich oligomers, or β sheet-rich (proto)fibrils and fragments. As the hydrophobicity of amyloid proteins increases evolving from monomers to oligomers and protofibrils, and decreases as protofilaments assemble into amyloid fibrils, the toxicity of these aggregation species rises and falls accordingly [62, 63]. Such polymorphic structures of the amyloid proteins, coupled with the rich physicochemical properties (shape, charge, size and surface chemistry) of the nanoparticle core and the presence of even richer environmental factors such as cell membranes [64], organelles, ligands, metals [65] and chaperones [66], entail numerous forms of the amyloid protein corona as well as their complex biological, toxicological and pathological outcomes. The response of the amyloid protein corona to dynamic physiological conditions, such as the low pH and high Zn2+ concentration of the pancreatic islets, may be harnessed for monitoring pathogenic developments in vivo [67].
From a materials’ viewpoint, a nanoparticle inhibitor against amyloidosis can be rather simple (e.g., graphene oxide, graphene quantum dots, ceria nanocrystals, OH-terminated dendrimers, hydroxylated fullerene, star polymers, silica nanoribbons, gold, ZnO and iron oxide nanoparticles) or elaborate in composition and function (e.g., pH sensitive carboxylate-terminated gold-carbon nanoparticles, transition-metal dichalcogenide nanosheets, metallohelices and multifunctional peptide-polymer nanosweepers) [43, 60, 61, 68–72]. Regardless, structural sophistication appears to have no direct bearing on the potency of a nanoparticle inhibitor but affords additional functionalities such as targeting and sensing. Biophysically, a nanoparticle inhibitor binds an amyloid protein of interest through interactions no different from those governing the formation of a nanoparticle-protein corona, with two distinctions that amyloid proteins a) do tend to aggregate into cross-beta architectures driven by energetics and b) are pathogenic rather than functional, like cellular or plasma proteins.
While amyloidosis inhibition often leads to suppressed peptide toxicity, the amyloidosis of IAPP was accelerated by star polymers through the association of the IAPP amyloidogenic segments with the relatively hydrophobic and rigid star polymer branches. As a result, the IAPP monomer and oligomer populations were rapidly exhausted, giving rise to a peculiar form of the protein corona where amyloid fibrils emanated from a star polymer core as well as subdued IAPP toxicity [73]. Similarly, accelerated IAPP aggregation coupled with reduced toxicity was observed with right-handed silica nanoribbons, which possessed more binding and nucleation sites per surface area than their left-handed counterpart to sequester IAPP aggregates in an embryonic zebrafish model [74].
Among the simple forms of nanoparticle inhibitors, graphene quantum dots (GQDs) have emerged as one of the most promising candidates due to their small size, ease of functionalization, minimal toxicity and strong capacity in translocating across the blood-brain barrier. Indeed, GQDs bound IAPP to effectively drive the peptide aggregation off-pathway and eliminate the toxic intermediates [75]. Using a PD mouse model, Kim et al. demonstrated that GQDs were capable of inhibiting and remodelling αS fibrillization, mitigating neurodegeneration, synaptic loss and Lewy body formation, and preventing the neuronal transmission of αS pathology [76]. In the case of Aβ, nano-graphene oxide extracted the peptide in its monomeric form from their mature fibrils by π-stacking between the aromatic residues of the peptide and the nanostructure [77]. In a separate study, graphene oxide destabilized the β-sheet architecture of Aβ fibrils and invited an immune response to clear the Aβ plaques [78].
Within the category of composite nanoparticle inhibitors, we have recently devised two new schemes against amyloidosis through the construction of functional-pathogenic double protein coronae. Specifically, a carbon nanotube was first deposited with functional amyloid fragments of whey protein beta lactoglobulin (bLg CNTs) [79], and a gold nanoparticle was stabilized by chaperone protein β casein (βcas AuNPs) [80], respectively (Fig. 2). These nanoparticle-functional protein constructs then sequestered toxic IAPP and Aβ in embryonic, larval or adult zebrafish models (to simulate the different stages of ageing), and were effective in recovering the hatching, behavior and cognitive function of the organism. For bLg CNTs, the cross-β architecture of the bLg amyloid fragments served as a template for the deposition of β-sheet rich IAPP oligomers and protofibrils to give rise to double protein coronae [79]. For βcas AuNPs, the chaperone-like capacity of β casein, a disordered and relatively hydrophobic protein, facilitated the association of disordered and hydrophobic Aβ1–42 through the latter’s C-terminus to establish the double protein coronae (Fig. 2) [80]. PEGylated (MW: 2,000) co-polymer nanoparticles (poly-alkyl cyanoacrylate and poly-lactic acid) captured the Aβ monomers and soluble oligomers with a great efficiency. This was accomplished by a combination of hydrophilic and hydrophobic interactions between the PEG shell and Aβ that induced the adsorption of the peptide on the nanoparticle surface, even in the presence of serum proteins [81]. Similarly, Luo et al. constructed a nano-sweeper by conjugating the PEGylated chitosan with a GKLVFF peptide. The GKLVFF peptide aided the nanoparticles to target, capture and co-assemble with Aβ and promoted immunological clearance of Aβ [45]. Polymeric nanoparticles have also been designed for both imaging and drug delivery against amyloidosis. For example, PEGylated poly-lactic acid nanoparticles were conjugated with Aβ-targeting tri-peptides TGN and QSH, and were further loaded with a DiY dye [82]. The nanoparticles reached Aβ plaques in the mouse brain, enabled in vivo fluorescence imaging of Aβ deposits and simultaneous delivery of curcumin as a drug cargo.
Figure 2. Amyloidosis sensing and mitigation strategies exploiting the protein corona concept.

(A) AuNPs ligated with β-casein (βCas) sequestered toxic Aβ species. When βCas AuNPs and Aβ were co-administered to the heart and brain of zebrafish, respectively, βCas AuNPs minimized the cerebral deposition of Aβ plaques and mitigated its cognitive pathology [80]. Functional beta lactoglobulin amyloid fragments (bLg Am) capped on gold nanoparticles (AuNPs) intercalated with IAPP via β-sheet stacking. This strategy was exploited for X-ray induced destruction of IAPP amyloids and for imaging amyloid-immune cell interaction [83]. Citrate- and polyethylene glycol (PEG)-coated AuNPs, upon interacting with IAPP monomers (IAPPm), oligomers (IAPPo) or fibrils (IAPPa), elicited different surface plasmon resonances and induced the release of immune cytokines. This response was driven by the length of the AuNP surface ligands and the spatial separation between the AuNPs and the IAPP species [84]. (B) IONPs conjugated with a two-photon dye NaP-labelled polymer yielded a fluorescent probe NaP-NH2 upon acid hydrolysis in pancreatic β-cells. This strategy was used to inhibit IAPP amyloidosis and image β-cell mass in vitro and ex vivo [67]. (C) bLg Am adsorbed on the surface of multi-walled carbon nanotubes (CNTs) sequestered IAPP to render functional-pathogenic bLg Am-IAPP double protein coronae, and rescued zebrafish embryos from the toxicity of IAPP [79]. Panels A-C were reproduced from refs. 67, 79, 80, 83 and 84. Copyrights 2020 American Chemical Society, 2018 American Chemical Society, 2019 Springer Nature, 2017 The American Chemical Society, 2019 The American Chemical Society.
While a protein corona can readily form on the surface of a nanoparticle, it can also be constructed by cellular and plasma proteins as well as ligands on the surface of an amyloid fibril, mediated by hydrogen bonding, electrostatic and hydrophobic interactions [85]. Due to the specific morphology of amyloid fibrils, the proteins which show high affinity for the fibrils are typically linear, or possess multi-domains of high flexibility to maximize their association with the amyloid (Fig. 3) [29]. As a result of such corona formation, recognition of the fibril by its structural antibody was markedly hindered [30], suggesting a potential pitfall for morphology-based therapeutic strategies that have dominated human clinical trials against AD.
Figure 3. The amyloid fibrillar protein corona.

Amyloid fibrils spontaneously collect a corona of linear or multi-domain proteins in a biological milieu, which may consequently alter their pathogenic fingerprints and targeting by morphology-based therapeutic strategies.
In contrast to pathogenic amyloids, functional amyloids such as the extracellular curli and FapC amyloid networks associated with the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa are much less studied [86, 87]. While functional amyloids share structural characteristics with pathogenic amyloids, they also provide support for polysaccharides deposition in biofilm formation and facilitate cell communication, adhesion and quorum sensing. We have recently synthesized silver nanoparticles and nanoclusters (AgNPs and AgNCs, of 10 and 3 nm in diameter, respectively) functionalized with cationic branched polyethylenimine (bPEI). When applied to Pseudomonas aeruginosa, AgNCs were bactericidal through their disruption of cell membranes as well as elevated production of reactive oxygen species (ROS), while AgNPs at biocompatible concentrations sequestered FapC to render a functional protein corona and inhibited the bacterial biofilm formation [88]. This anti-amyloidosis nanotechnology may prove valuable against microbial multidrug resistance (MDR) that is a paramount challenge to the pharmaceutical and healthcare industries.
2b. Theoretical development in amyloidosis inhibition
Accurate and efficient modelling of amyloidosis and inhibition at the nanoscale is crucial for designing de novo anti-amyloid nanomedicines. We have applied discrete molecular dynamics (DMD) [89], a rapid and predictive molecular dynamics (MD) algorithm capable of simulating large systems and reaching long timescales, to characterize in silico amyloidosis and inhibition at the molecular level. The physics-based force field used to describe interatomic interactions in DMD is transferable [90], allowing us to model various types of biomolecules and nanoparticles. Using multiscale DMD simulations, the formation of a single AgNP-ubiquitin corona was observed in silico for the first time [91]. Taking advantage of the high predictive power and computational efficiency, DMD simulations have been utilized to characterize the aggregation process of multiple amyloid peptides - including amyloidogenic fragments of IAPP, Aβ, αS and FapC [92–94] - from isolated monomers to fibril-like cross-β structures. Using a large number of independent DMD simulations, the aggregation free-energy landscape (i.e., the potential of mean force) as a function of the oligomer size (defined as the number of composite peptides) and β-content has been obtained. As expected, the initial monomers and final cross-β aggregates correspond to two deep free-energy basins. Starting from monomers, amyloid peptides first form coil and helix-rich oligomers. Only in oligomers larger than the critical aggregation nucleus, the β-rich structures stabilized by inter-peptide backbone hydrogen bonds can be nucleated and start to grow into cross-β aggregates. The coil and helix-rich oligomers and the β-rich oligomers are separated by free energy barriers. Although the β-sheet content is not the sole determinant of oligomer toxicity [95], toxic oligomers usually possess high β-sheet contents. Reducing the population of β-sheet rich oligomers, therefore, is crucial for designing anti-amyloidosis inhibitors.
In silico studies have demonstrated that the β-rich oligomers often adopt distinct and well-defined 3D structures, such as β-barrels [93]. Nanoparticles engineered with certain physicochemical properties can modulate the aggregation free energy landscape by selectively accumulating a corona of peptide monomers, coil or helix-rich oligomers and fibrils instead of the β-rich oligomers (Fig. 4). The first strategy is to stabilize monomers with soft polymeric nanoparticles or nanoparticles functionalized by soft molecules capable of binding and encapsulating peptide monomers via dynamic conformation changes. Combining DMD simulations with biophysical characterizations we examined the inhibition potentials of OH-terminated PAMAM dendrimer [69] and βcas AuNPs [80] against IAPP and Aβ aggregation. Specifically, the dendrimer encapsulated IAPP monomers within its dynamic porous interior [69] to render a stable and conformationally unique dendrimer-IAPP “corona”, while an Aβ monomer became more helical and an Aβ hexamer started to lose β-sheet structure upon binding the intrinsically disordered βcas with high conformational flexibility [80]. Moreover, the Aβ- and AuNP-binding sites of βcas did not overlap, suggesting that chaperone-like βcas in the AuNP corona could trap Aβ monomers in the aggregation-incompetent forms while remaining AuNP surface-adsorbed and mobile.
Figure 4. Examples of DMD simulations of amyloidosis inhibition with nanoparticles.
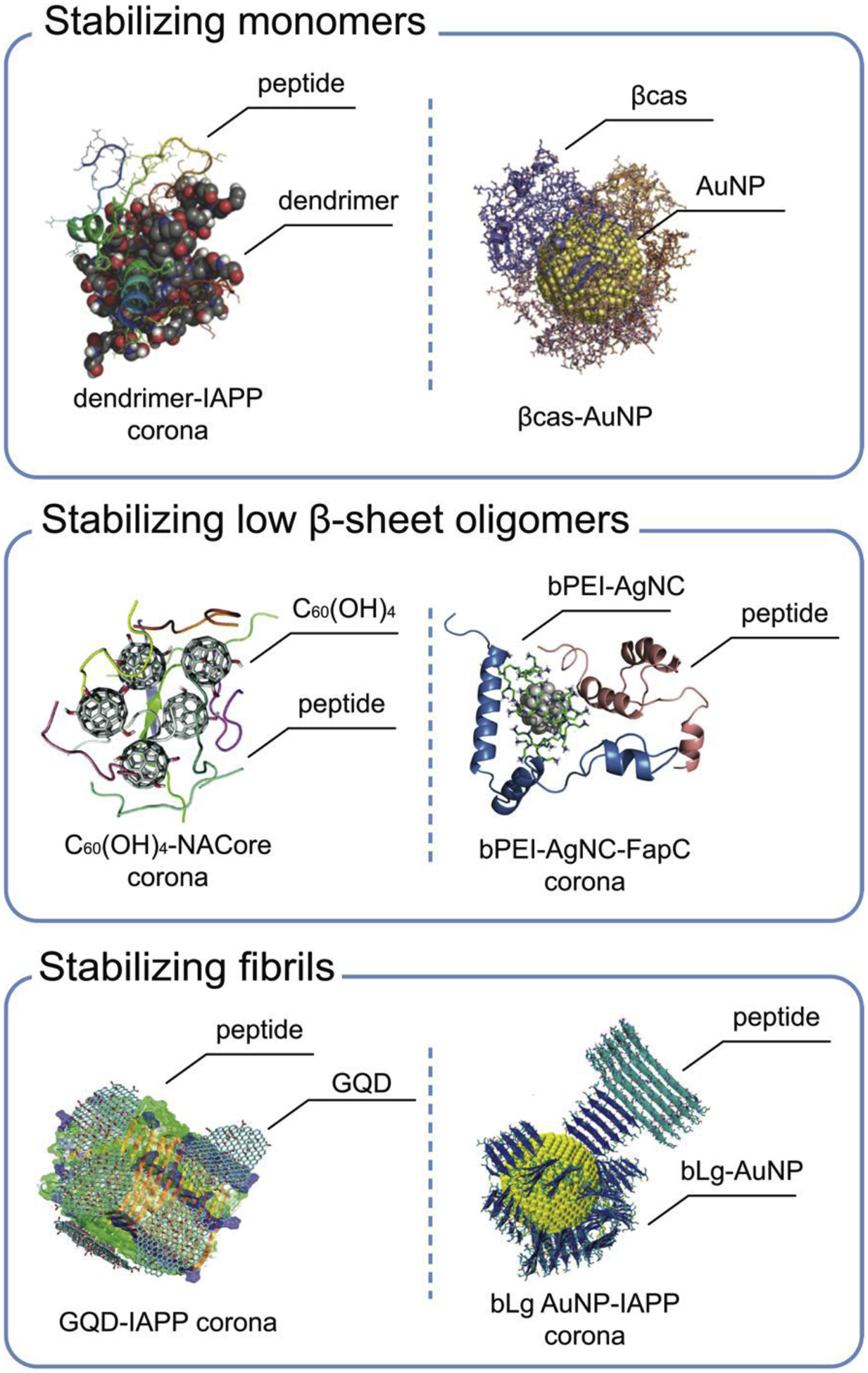
Top row: OH-terminated PAMAM dendrimer binding with IAPP [69] (left) and βcas AuNP (right) sequestering Aβ (not shown) [80]. Middle row: C60(OH)4 nanoparticles complexing with the NACore (the amyloidogenic core region of the non-amyloid-β component in αS) [96] (left) and bPEI-coated silver nanocluster (bPEI-AgNC) binding with bacterial functional amyloid protein FapC (here represented by its fragment L1R2) [88] (right). Lower row: graphene quantum dots (GQDs) interacting with an IAPP fibril [97] (left) and beta lactoglobulin amyloid fragments seeding IAPP fibrillization [83] (right). Panels reproduced from refs. 69, 80, 83, 88, 96 and 97. Copyrights 2016 John Weily and Sons, 2019 Springer Nature, 2017 The American Chemical Society, 2020 John Wiley and Sons, 2019 Royal Society of Chemistry, 2019 Springer Nature.
With DMD simulations, GQDs (Fig. 5) [75], fullerenols C60(OH)24 [96], polyphenol nanoclusters [98] and bPEI-coated nano silvers [88] were found to inhibit amyloidosis by forming coronae of coil and helix-rich oligomers. Common to all these nanostructures, their capability in mediating hydrogen bonds with the peptide backbones competed with the formation of inter-peptide hydrogen bonds. Most importantly, structural mismatches between the random distributions of hydrophobic/hydrophilic mosaics on these amphiphilic nanoparticle surfaces and the ordered β-sheet peptide structures discourage the nucleation of β-rich oligomers in the corona. Hence, the peptide coronae of the nanoparticles remained stable in the coil and helix-rich conformations without evolving further into β-rich oligomers and fibrils.
Figure 5. An example illustrating the molecular insights offered by DMD simulation of IAPP amyloidosis inhibition with GQDs [75].

Average secondary structure contents (a) and secondary structure propensities per residue (b) were computed for an IAPP dimer in the absence and presence of a GQD. (c) Intra- and inter-chain contact frequency maps of the IAPP dimer. Changes of IAPP contact frequencies upon binding of GQD are presented. (d) Potential mean force (PMF) of the IAPP dimer (top left) or IAPP dimer + GQD (bottom left) is shown as a function of the total number of hydrogen bonds (Num. Hbond) and radius of gyration (Rg, nm). Three snapshots of the IAPP dimer (top right) or IAPP dimer + GQD (bottom right) near the corresponding free energy basins (the coordinates labelled in the PMF on the left are given in the parentheses on the right). Figure reproduced from ref. 75. Copyright 2018 Royal Society of Chemistry.
With star polymers as mentioned in section 2a, DMD simulations revealed that their poly(2-hydroxyethyl acrylate) arms were rigid with a large persistent length and served as a rodlike scaffold for IAPP binding, subsequently accelerating IAPP aggregation by increasing local peptide concentration [73]. Using DMD-aided docking simulations, we revealed that IAPP fibrils and bLg amyloid fragments, both rich in the cross-β architecture, bound each other with a strong propensity owning to their structural compatibilities. IAPP fibrillization was subsequently initiated by the bLg amyloid fragment corona of the AuNPs and the “cross-seeding” significantly reduced the aggregation free-energy barrier to accelerate IAPP fibrillization with AuNPs embedded in the growing fibrils (Fig. 4).
In addition to atomistic DMD simulations, coarse-grained (CG) simulations can be used to capture the mesoscale properties of amyloid aggregation. Recently, we developed an 11-bead peptide model which could form left-handed fibrils as commonly observed for most amyloid fibrils. Due to complementary morphologies, CG peptides adsorbed onto a left-handed SiO2 nanoribbon formed fibrils parallel to the ribbon axis. In contrast, CG peptides on the right-handed SiO2 nanoribbon surface could only form fibrils perpendicular to the ribbon axis. With excess IAPP peptides, the right-handed nanoribbon possessed more available surfaces for peptide binding and nucleation, while the left-handed nanoribbon was rapidly covered by fibrils to resist further peptide binding and fibril nucleation [74].
Together, these simulation studies provided crucial molecular insights into the structure and dynamics of the amyloid protein corona in close connection with the physicochemical properties of nanoparticle inhibitors. With structural, dynamic and morphological complementarity, nanoparticles can be engineered to form corona selectively with amyloid aggregation species other than the structurally distinct β-rich oligomers and reduce the overall population of toxic oligomers, thereby mitigating amyloid cytotoxicity.
2c. New immunological identity of the amyloid protein corona
It is well documented that misfolded proteins and protein aggregates, such as those rendered by amyloidosis, can trigger diverse immune responses via different signalling pathways, exerting a major influence on immune homeostasis and disease progression. Meanwhile, the interactions of nanoparticles – here intended amyloidosis inhibitors or probes – with their physiological environments can result in alterations to the secondary and tertiary structures of their surface adsorbed proteins. These biomolecular coatings may define the cellular uptake pathway of the nanoparticle-protein corona entity via altering the recognition between predominant coronal proteins and specific receptors on the cell membrane and ultimately dictate their biological fate. Understanding the amyloid protein corona, especially from the perspective of their interplay with the immune system in complex biological environments (Fig. 6), may therefore provide crucial new insights into the physiopathology and facilitate the treatment of amyloid diseases.
Figure 6. Amyloid deposition-induced proinflammatory cytokine release.

Secretion of the cytokines from immune cells may be triggered by the influence of nanoparticle inhibitors, plasma proteins and extracellular matrix components. NP: nanoparticle.
Amyloidosis can be divided into two groups, namely localized amyloidosis and systemic amyloidosis, according to the accumulation sites of protein plaque deposition. Localized amyloidosis primarily occurs in one specific tissue or organ, such as IAPP amyloids in the extracellular space of pancreatic beta cells, or Aβ plaques and tau tangles in the extracellular and intracellular spaces of neuronal cells, although these peptides and their aggregates do spread to other bodily organs through cross-seeding or other mechanisms [99, 100], which, while unclear, most certainly involves the complication of the protein corona. In contrast, systemic amyloidosis refers to the deposition of amyloids and plaques, usually produced in the bone marrow, in the heart, kidneys, liver and other organs, in the forms of amyloid light chain amyloidosis (AL), A amyloidosis (AA), transthyretin amyloidosis (ATTR) and beta2-microglobulin amyloidosis (Aβ2M). Together, for all these amyloid diseases, inflammation reactions have been commonly found over most of the states in disease progression. For example, the Aβ aggregates can activate microglia, one type of resident immune cells in the brain, through antigen-receptor recognition, thereby triggering innate and adaptive immune responses and inflammatory cytokine (TNF and IL-1β) secretion. The Aβ oligomers can induce mitochondrial ROS via activated pyrin domain containing 3 (NLRP3) and NADPH oxidase-mediated ROS through active caspase-1 and promote transformation of pro-IL-1β into mature IL-β microglia, yielding synaptic plasticity damage and long-term potentiation. Overexpression of IL-β induces an aberrant activation of p38-MAPK and glycogen synthase kinase 3 (GSK3), accelerating tau phosphorylation and tangle formation [101]. Abnormal activation of microglia and overproduced inflammatory mediator for a long period can induce a loss of the phagocytic capacity of microglia and further accelerate the amyloid deposition and neuroinflammation (Fig. 7a) [102]. Similarly, AA usually co-occurs with chronic inflammation diseases, where secretory inflammatory cytokines IL-6 upregulate the hyper-expression of serum amyloid A (SAA) to facilitate amyloid deposition to reinforce systematic inflammatory responses and rapid tissue damage [103].
Figure 7. Aβ amyloidosis and inflammation in the brain, cytokine detection and the role of the protein corona on Aβ toxicity.

A. Feedback loop between microglia and neurons in healthy and diseased brain [102]. B. Commonly used methods for biomarker analysis. C. Cell viability and morphology when exposed to Aβ oligomers (Aβo) or fibrils (Aβf) with or without plasma proteins [104]. LSPR: localized surface plasmon resonance. AuNRs: gold nanorods. Panels A and C were reproduced from refs. 102 and 104. Copyrights 2019 John Wiley and Sons and 2019 The American Chemical Society.
Increased ROS generation during amyloidosis can cause oxidative stress and contribute to elevated release of proinflammatory cytokines such as TNF, IL-6, IFN-γ and IL-18 from immune cells via mitogen-activated protein kinase (MAPK), activator protein-1 (AP-1), and nuclear factor kappa light chain enhancer of activated B cells (NF-κB) pathways [105, 106]. Neuron-derived proinflammatory cytokines are generated at the earliest stages of the AD neuropathological cascade even before plaques deposition and cell degeneration [107]. While the exact mechanism underlying the toxicity of amyloid oligomers is still under debate, a recent study has attributed that to their greater diffusivity and ability to interact with cell membranes and other cellular components. Surface hydrophobicity, which often entails a propensity for cell membranes, is also a key factor in determining the toxicity of amyloid aggregates [63]. Together, these results have implicated an inherent connection between amyloidosis and their induced immune toxicity, thereby highlighting cytokines as potential diagnostic and treatment markers for amyloid diseases [108].
The current “gold standard” methods, such as enzyme-linked immunosorbent assay (ELISA) and flow cytometry, allow in vitro quantifications of multiple cytokines simultaneously. Fluorescent immunohistochemistry, on the other hand, provides information on the distribution and localization of target cytokine biomarkers in tissues via staining. More recently, a microfluidic-based localized surface plasmon resonance (LSPR) biosensor has been developed to detect multiple cytokines in real-time (Fig. 7b), which has enabled a dynamic study of interactions between immune cells, amyloid aggregates and the amyloid protein corona in a biologically relevant microenvironment. It is known that steric repulsion from plasma protein-coated particles could reduce the access of Aβ to the nanoparticle surface and prevent Aβ amyloidosis [59]. However, such inhibition effect should be assessed along with the induced immune responses at different stages of Aβ amyloidosis (Fig. 7c). By employing the LSPR biosensor, we performed a dynamic analysis of inflammatory cytokines (IL-6 and TNF) secretion from immune cells when exposing to Aβ oligomers (Aβo) and Aβ fibrils (Aβf) with and without the presence of plasma proteins [104]. Binding with plasma proteins prevented further aggregation of Aβo and maintained their small size and surface hydrophobicity, which further enhanced the association of the peptide with immune cell membranes to promote toxicity and inflammatory cytokine secretion. Aβf, in comparison, exhibited lower structural plasticity and less exposed hydrophobic residues, and induced less toxicity after binding with plasma proteins through charged and polar groups. In addition, the Aβ aggregation-induced immune responses were cell type-dependent. The plasma protein corona partially shielded the exposed Aβo activation motifs, blocking the signal pathways for specific cytokine secretion. For example, TNF secretion from THP-1 cells induced by Aβo was suppressed in the presence of plasma proteins, likely due to the blockage of receptor-specific signalling pathways via the Mac-1 receptor. In a separate study, we adopted a similar approach to investigate the immunotoxicity of IAPP aggregation in the presence of a gold nanoparticle inhibitor. The oligomeric IAPP-incited immune response was suppressed by the polyethylene glycol or citrate corona of the gold nanoparticle inhibitor [84]. A most recent study has shown that the recognition and internalization of protein corona by macrophage is modulated by CD35 (a complement receptor type 1) or CD63 (a scavenger receptor), which further pinpoints on the essential role of surface chemistry on the regulation of protein adsorption on nanoparticle inhibitors in the blood plasma and the subsequent effects of receptor-mediated endocytosis of the protein corona [109]. Hence, a comprehensive analysis of protein composition, intrinsic physicochemical properties of nanoparticles as well as the exposed surface chemistry of the protein corona entity when interacting with the immune system is necessary for the design of nanoparticle inhibitors and their in vivo applications.
While in vitro studies usually examine the aggregation of amyloid proteins in a simple medium – a buffer or water – amyloidosis in vivo is a far more complex and far slower process as the amyloid proteins interact with, for example, chaperones and extracellular matrix components such as serum amyloid P (SAP), heparan sulfate proteoglycan (HSPG), laminin, collagen IV, entactin and apolipoprotein E. These extracellular constitutions not only become a part of the amyloid fibrils in situ, but also play an important role in amyloid deposition and toxicity. For example, SAP-enriched amyloid fibrils have been shown to contribute to amyloid deposit stabilization [110]. HSPGs have been found in amyloid-associated organs displaying highly sulfated and altered proportion of disaccharides from healthy individuals. HSPGs can bind to the secondary structure of amyloid proteins and accelerate amyloid plague deposition. Heparan sulfate (HS), as a member of HSPGs, has been shown to facilitate cellular uptake and mediate the neurotoxicity and immune responses induced by amyloid proteins [111]. Deficiency in neuronal HS moderated the activation of plaque-associated microglia and reduced Aβ aggregation, leading to a decreased neuroinflammation and secretion of proinflammatory cytokines [112]. Therefore, future research into these largely uncharted areas, i.e., interactions between extracellular matrix components and amyloid aggregates as well as the interactions between cytokines and the amyloid protein corona, could offer new insights into understanding and controlling the immunological identity of the nanoparticle inhibitors towards their translation into amyloidosis nanomedicines.
3. Outlook
Amyloidosis involves myriad types of peptides and proteins and entails the pathologies of AD, PD, T2D, prions, and many more. Triggered by ageing, familial and environmental factors, amyloid proteins misfold and can spread to different compartments of the body via the bloodstream, cerebrospinal fluid (CSF) and the gut-brain axis, promoted by compromised gut-brain barrier, blood-brain barrier and immune response [113, 114]. The pervasive presence of amyloid proteins, therefore, offers countless scenarios of amyloid proteins interfacing with cellular and plasma proteins, chaperones, cytokines and metabolites to give rise to amyloid coronal structures which have rarely been examined so far. The use of nanoparticle inhibitors or probes, furthermore, offers vast new opportunities of constructing the protein corona through nano-bio self-assembly and molecular crowding, another little explored area that is central to the advancement of amyloid nanomedicines. While the modes of interaction between amyloid proteins and nanoparticles are now understood to be mediated primarily by hydrophobic and electrostatic forces as well as H-bonding, the design of a nanoparticle inhibitor which can effectively “freeze”/trap amyloid proteins in their nontoxic heterogeneous forms is nontrivial and is executed on a case-by-case basis through trial and error. This is partly due to the rich structural feature and aggregation landscape of amyloid proteins, coupled with the complexity of their in vivo conditions as well as the vast parameter space of nanomaterials synthesis [115]. Furthermore, while nanomaterials have emerged in recent years as a promising alternative to peptidomimetics and monoclonal antibodies against AD, PD and T2D, to our knowledge no studies have so far validated the potential of nanomedicines against systemic amyloidosis. Fundamentally, these efforts must prioritize the design of smart nanostructures which preferentially sequester toxic amyloid proteins over functional cellular and plasma proteins without evoking inflammation and toxicity. In this regard, the construction of functional-pathogenic double protein coronae and the use of state-of-the-art cytokine detection techniques, as demonstrated in this Perspective, appear to be a viable starting point. In addition to experimental efforts, synergic computational methodologies as exemplified in this Perspective are crucially needed for delineating the structure, function and dynamics of the amyloid protein corona and for guiding the design of nanomedicines against amyloidosis. While much progress has been made in the past decades, especially with the advancement of mass spectroscopy, NMR, cryo-electron microscopy and single-molecule techniques, how to quantify and correlate the miniscule changes of the analytes of amyloid proteins, cytokines and bacterial metabolites in the blood, CSF and other organs and how to characterize the dynamic amyloid protein corona [25, 116] in vivo offers numerous opportunities for scientific discovery and poses tremendous challenges moving forward.
Highlights.
The “amyloid protein corona” refers to amyloidogenic peptides and proteins sequestered by a nanoparticle inhibitor, or a nanomedicine.
The structural characteristics of the amyloid protein corona partially resemble that of a protein corona, but also significantly differ from the latter owing to the remarkable aggregation capacity of the pathogenic peptides and proteins.
The amyloid protein corona evokes cytokine release from the immune system, with intriguing structural and medical implications that are not yet understood.
Harnessing the amyloid protein corona is a new arena for the development of preventative and precision nanomedicines against debilitating amyloid diseases that are now global epidemics.
Funding
This work was supported by the Australian Research Council Project No. CE140100036 (Davis), NSF CAREER CBET-1553945 (Ding), NIH MIRA R35GM119691 (Ding), NIH MIRA R35GM133795 (P. Chen), the National Basic Research Plan of China (MOST Program 2016YFA0201600, C. Chen) and the Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000, C. Chen), CAMS Innovation Fund for Medical Sciences (CIFMS 2019-I2M-5–018, C. Chen).
Biographies

Pengyu Chen is an Assistant Professor in Materials Engineering, Department of Mechanical Engineering at Auburn University, USA. He obtained his Ph.D. in nanomaterials and biophysics from Clemson University in 2012 and worked as a research fellow (2013–2016) in Mechanical Engineering at the University of Michigan. He was a recipient of Maximizing Investigators’ Research Award (US National Institute of Health, 2019) and CAREER Award (US National Science Foundation, 2020). His research interests include nanomaterials, protein-nanoparticle interaction, plasmonic biosensors, immune biosensing and immunotherapy.

Feng Ding is an Associate Professor of Physics at Clemson University, USA. He obtained his PhD from Boston University (2004) and worked as a postdoctoral fellow (2004–2006), research associate (2006–2008), and Research Assistant Professor (2008–2012) at the University of North Carolina at Chapel Hill before being hired as an Assistant Professor at Clemson University (2012–2017). His research focuses on understanding the structure, dynamics, and function interrelationship of biomolecules and molecular complexes. He was recipient of a Postdoctoral Award for Research Excellence (UNC, 2005), CAREER Award (US National Science Foundation, 2016) and Outstanding Young Researcher Award (Clemson, 2017).

Rong Cai is an Associate Professor at the National Center for Nanoscience and Technology (NCNST), Chinese Academy of Sciences (CAS). She obtained her PhD in Materials Science and Engineering at the University of Tsukuba and the National Center for Materials Science (NIMS), Japan (2012–2015) and worked as Assistant Professor at NCNST (2015–2018). Her research focuses on the nano-bio interface and interactions, evaluation of the biomedical effects of nanomaterials and nanosafety.

Ibrahim Javed is a Postdoctoral Fellow at the Australian Institute for Bioengineering and Nanotechnology (AIBN) and the University of Queensland node of the ARC Centre of Excellence in Convergent Bio-Nano Science and Technology (CBNS). He obtained his PhD (2019) with Profs. Thomas Davis and Pu Chun Ke from Monash Institute of Pharmaceutical Sciences at Monash University, and was a recipient of the Shanghai Belt and Road fellowship (2018) and CBNS most significant publication award (2019). His research concerns the in vivo disease modelling of amyloidogenesis, the pharmaco-efficacy and bio-nano interaction of nanomedicines, and the gut-brain axis on neurological disorders.

Wen Yang is currently pursuing her PhD degree in Material Engineering under the supervision of Prof. Pengyu Chen at Auburn University. She received her BSc in 2011 from the Department of Material Engineering at Huaqiao University. Her research focuses on plasmonic materials for the development of biosensors and understanding the immune responses induced by nanoparticle-protein interactions and amyloidosis.

Zhenzhen Zhang is a PhD student advised by Prof. Feng Ding in the Department of Physics and Astronomy at Clemson Unviersity, USA. She received her BSc degree from the Department of Physics at Nankai University in 2017. Her current research focuses on studying the effects of nanoparticles on protein aggregation, RNA folding, as well as RNA sequence design.

Yuhuan Li is a Postdoctoral Fellow with Profs. Thomas Davis and Pu Chun Ke at CBNS, Monash Institute of Pharmaceutical Sciences, Monash University in Australia. She obtained her BSc (Pharmaceutics, 2011) and PhD degrees (Microbiology and Biochemical Pharmacy, 2016) from Jilin University, China. Her research is focused on understanding cellular responses to amyloidogenic peptides and nanoparticles, polymer chemistry, and the delivery of therapeutics and diagnostic nanomedicines against cancer.

Thomas P. Davis is the Director of CBNS and a Fellow of the Australian Academy of Science. He is a Professor at Monash University and Chair Professor of Precision Medicine at the University of Queensland. Prior to his appointment at the University of Queensland he spent 21 years as a senior academic at the University of New South Wales in Sydney. Thomas’s research focuses on the application of polymer science and nanotechnology to therapeutic applications.

Pu Chun Ke is a Professor at Zhongshan Hospital of Fudan University and a Signature Project Leader at CBNS, Monash University. He was a Distinguished Visiting Scientist at CSIRO, Australia (2014) and Assistant and then tenured Associate Professor at Clemson University, USA (2003–2013). He was recipient of CBNS Inaugural Supervision Excellence Award (2019), Faculty Achievement in the Sciences Award (2012), US National Science Foundation CAREER Award (2008) and LJIS Postdoctoral Fellowship in Biophysics at the University of California, San Diego (2001–2003). His research concerns the mitigation of brain and metabolic diseases, the protein corona and environmental science.

Chunying Chen is a Principal Investigator at CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety in the National Center for Nanoscience and Technology of China. Prof. Chen received her Bachelor’s degree in Chemistry in 1991 and her Ph.D. degree in Biomedical Engineering from Huazhong University of Science and Technology of China in 1996. Prof. Chen provides input to WHO, ISO, and OECD working groups investigating the health effects of nanomaterials. Her research interests mainly include nanotoxicology, nanomedicine, the nano–bio interfaces, and the transformation and fate of nanomaterials in biological systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None.
References
- [1].Monopoli MP, Åberg C, Salvati A, Dawson KA, Nature Nanotechnology, 7 (2012) 779–786. [DOI] [PubMed] [Google Scholar]
- [2].Walkey CD, Chan WCW, Chemical Society Reviews, 41 (2012) 2780–2799. [DOI] [PubMed] [Google Scholar]
- [3].Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ, ACS Nano, 9 (2015) 6996–7008. [DOI] [PubMed] [Google Scholar]
- [4].Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S, Proceedings of the National Academy of Sciences, 104 (2007) 2050–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lynch I, Dawson KA, Nano Today, 3 (2008) 40–47. [Google Scholar]
- [6].Cai R, Chen C, Advanced Materials, 31 (2019) 1805740. [DOI] [PubMed] [Google Scholar]
- [7].Lu X, Zhu Y, Bai R, Wu Z, Qian W, Yang L, Cai R, Yan H, Li T, Pandey V, Liu Y, Lobie PE, Chen C, Zhu T, Nature Nanotechnology, 14 (2019) 719–727. [DOI] [PubMed] [Google Scholar]
- [8].Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z, Chen C, Proceedings of the National Academy of Sciences, 108 (2011) 16968–16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang L, Li J, Pan J, Jiang X, Ji Y, Li Y, Qu Y, Zhao Y, Wu X, Chen C, Journal of the American Chemical Society, 135 (2013) 17359–17368. [DOI] [PubMed] [Google Scholar]
- [10].del Pino P, Pelaz B, Zhang Q, Maffre P, Nienhaus GU, Parak WJ, Materials Horizons, 1 (2014) 301–313. [Google Scholar]
- [11].Treuel L, Malissek M, Gebauer JS, Zellner R, ChemPhysChem, 11 (2010) 3093–3099. [DOI] [PubMed] [Google Scholar]
- [12].Treuel L, Eslahian KA, Docter D, Lang T, Zellner R, Nienhaus K, Nienhaus GU, Stauber RH, Maskos M, Physical Chemistry Chemical Physics, 16 (2014) 15053–15067. [DOI] [PubMed] [Google Scholar]
- [13].Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J, Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C, Sirirattanapan J, Mann W, Treuel L, Zellner R, Maskos M, Schild H, Stauber RH, ACS Nano, 5 (2011) 7155–7167. [DOI] [PubMed] [Google Scholar]
- [14].Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes VF, Small, 7 (2011) 3479–3486. [DOI] [PubMed] [Google Scholar]
- [15].Cai R, Chen C, Science Bulletin, 62 (2017) 976. [DOI] [PubMed] [Google Scholar]
- [16].Baimanov D, Wu J, Chu R, Cai R, Wang B, Cao M, Tao Y, Liu J, Guo M, Wang J, Yuan X, Ji C, Zhao Y, Feng W, Wang L, Chen C, ACS Nano, 14 (2020) 5529–5542. [DOI] [PubMed] [Google Scholar]
- [17].Peng F, Setyawati MI, Tee JK, Ding X, Wang J, Nga ME, Ho HK, Leong DT, Nature Nanotechnology, 14 (2019) 279–286. [DOI] [PubMed] [Google Scholar]
- [18].Corbo C, Molinaro R, Tabatabaei M, Farokhzad OC, Mahmoudi M, Biomaterials Science, 5 (2017) 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cui J, Richardson JJ, Björnmalm M, Faria M, Caruso F, Accounts of Chemical Research, 49 (2016) 1139–1148. [DOI] [PubMed] [Google Scholar]
- [20].Liu J, Wang L, Shen X, Gao X, Chen Y, Liu H, Liu Y, Yin D, Liu Y, Xu W, Cai R, You M, Guo M, Wang Y, Li J, Li Y, Chen C, Nano Today, 34 (2020) 100907. [Google Scholar]
- [21].Jin J, Ovais M, Chen C, Nano Today, 22 (2018) 83–99. [Google Scholar]
- [22].Ren J, Cai R, Wang J, Daniyal M, Baimanov D, Liu Y, Yin D, Liu Y, Miao Q, Zhao Y, Chen C, Nano Letters, 19 (2019) 4692–4701. [DOI] [PubMed] [Google Scholar]
- [23].Fang G, Li W, Shen X, Perez-Aguilar JM, Chong Y, Gao X, Chai Z, Chen C, Ge C, Zhou R, Nature Communications, 9 (2018) 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer SK, Stauber RH, Chemical Society Reviews, 44 (2015) 6094–6121. [DOI] [PubMed] [Google Scholar]
- [25].Ke PC, Lin S, Parak WJ, Davis TP, Caruso F, ACS Nano, 11 (2017) 11773–11776. [DOI] [PubMed] [Google Scholar]
- [26].Linse S, Cabaleiro-Lago C, Xue W-F, Lynch I, Lindman S, Thulin E, Radford SE, Dawson KA, Proceedings of the National Academy of Sciences, 104 (2007) 8691–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kogan MJ, Bastus NG, Amigo R, Grillo-Bosch D, Araya E, Turiel A, Labarta A, Giralt E, Puntes VF, Nano Letters, 6 (2006) 110–115. [DOI] [PubMed] [Google Scholar]
- [28].Li J, Pylypchuk I, Johansson DP, Kessler VG, Seisenbaeva GA, Langton M, Scientific Reports, 9 (2019) 8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pilkington EH, Gustafsson OJR, Xing Y, Hernandez-Fernaud J, Zampronio C, Kakinen A, Faridi A, Ding F, Wilson P, Ke PC, Davis TP, ACS Nano, 12 (2018) 6066–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nandakumar A, Xing Y, Aranha RR, Faridi A, Kakinen A, Javed I, Koppel K, Pilkington EH, Purcell AW, Davis TP, Faridi P, Ding F, Ke PC, Biomacromolecules, 21 (2020) 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ke PC, Sani MA, Ding F, Kakinen A, Javed I, Separovic F, Davis TP, Mezzenga R, Chem Soc Rev, 46 (2017) 6492–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP, Proc Natl Acad Sci U S A, 110 (2013) 9758–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peduzzo A, Linse S, Buell AK, ACS Chemical Neuroscience, 11 (2020) 909–918. [DOI] [PubMed] [Google Scholar]
- [34].Kakinen A, Sun Y, Javed I, Faridi A, Pilkington EH, Faridi P, Purcell AW, Zhou R, Ding F, Lin S, Ke PC, Davis TP, Science Bulletin, 64 (2019) 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hardy JA, Higgins GA, Science, 256 (1992) 184–185. [DOI] [PubMed] [Google Scholar]
- [36].Lansbury PT, Lashuel HA, Nature, 443 (2006) 774–779. [DOI] [PubMed] [Google Scholar]
- [37].McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MHL, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HTJ, Przybylski M, St George-Hyslop P, Nature Medicine, 8 (2002) 1263–1269. [DOI] [PubMed] [Google Scholar]
- [38].Bieschke J, Herbst M, Wiglenda T, Friedrich RP, Boeddrich A, Schiele F, Kleckers D, Lopez del Amo JM, Grüning BA, Wang Q, Schmidt MR, Lurz R, Anwyl R, Schnoegl S, Fändrich M, Frank RF, Reif B, Günther S, Walsh DM, Wanker EE, Nature Chemical Biology, 8 (2012) 93–101. [DOI] [PubMed] [Google Scholar]
- [39].Krysmann MJ, Castelletto V, Kelarakis A, Hamley IW, Hule RA, Pochan DJ, Biochemistry, 47 (2008) 4597–4605. [DOI] [PubMed] [Google Scholar]
- [40].Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE, Proceedings of the National Academy of Sciences, 107 (2010) 7710–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen J, Armstrong AH, Koehler AN, Hecht MH, Journal of the American Chemical Society, 132 (2010) 17015–17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Palhano FL, Lee J, Grimster NP, Kelly JW, Journal of the American Chemical Society, 135 (2013) 7503–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ke PC, Pilkington EH, Sun Y, Javed I, Kakinen A, Peng G, Ding F, Davis TP, Adv Mater, 32 (2019) 1901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li M, Zhao A, Dong K, Li W, Ren J, Qu X, Nano Research, 8 (2015) 3216–3227. [Google Scholar]
- [45].Luo Q, Lin Y-X, Yang P-P, Wang Y, Qi G-B, Qiao Z-Y, Li B-N, Zhang K, Zhang J-P, Wang L, Wang H, Nature Communications, 9 (2018) 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang J, Liu L, Ge D, Zhang H, Feng Y, Zhang Y, Chen M, Dong M, Chemistry – A European Journal, 24 (2018) 3397–3402. [DOI] [PubMed] [Google Scholar]
- [47].Cabaleiro-Lago C, Lynch I, Dawson KA, Linse S, Langmuir, 26 (2010) 3453–3461. [DOI] [PubMed] [Google Scholar]
- [48].Huang F, Wang J, Qu A, Shen L, Liu J, Liu J, Zhang Z, An Y, Shi L, Angewandte Chemie International Edition, 53 (2014) 8985–8990. [DOI] [PubMed] [Google Scholar]
- [49].Debnath K, Shekhar S, Kumar V, Jana NR, Jana NR, ACS Applied Materials & Interfaces, 8 (2016) 20309–20318. [DOI] [PubMed] [Google Scholar]
- [50].Nedumpully-Govindan P, Gurzov EN, Chen P, Pilkington EH, Stanley WJ, Litwak SA, Davis TP, Ke PC, Ding F, Physical Chemistry Chemical Physics, 18 (2016) 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Song Y, Moore EG, Guo Y, Moore JS, Journal of the American Chemical Society, 139 (2017) 4298–4301. [DOI] [PubMed] [Google Scholar]
- [52].Yousaf M, Huang H, Li P, Wang C, Yang Y, ACS Chemical Neuroscience, 8 (2017) 1368–1377. [DOI] [PubMed] [Google Scholar]
- [53].Liu Y, Xu L-P, Wang Q, Yang B, Zhang X, ACS Chemical Neuroscience, 9 (2018) 817–823. [DOI] [PubMed] [Google Scholar]
- [54].Shin J, Lee S, Cha M, Medicinal Chemistry Communications, 8 (2017) 625–632.30108779 [Google Scholar]
- [55].Mo Y, Brahmachari S, Lei J, Gilead S, Tang Y, Gazit E, Wei G, ACS Chemical Neuroscience, 9 (2018) 2741–2752. [DOI] [PubMed] [Google Scholar]
- [56].Liu F, Wang W, Sang J, Jia L, Lu F, ACS Chemical Neuroscience, 10 (2019) 588–598. [DOI] [PubMed] [Google Scholar]
- [57].Jung H, Chung YJ, Wilton R, Lee CH, Lee BI, Lim J, Lee H, Choi J-H, Kang H, Lee B, Rozhkova EA, Park CB, Lee J, Advanced Functional Materials, 30 (2020) 2070094. [Google Scholar]
- [58].Sahoo BR, Genjo T, Nakayama TW, Stoddard AK, Ando T, Yasuhara K, Fierke CA and Ramamoorthy A, Chem. Sci, 10 (2019), 3976–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Lindman S, Minogue AM, Thulin E, Walsh DM, Dawson KA, Linse S, J Am Chem Soc, 130 (2008) 15437–15443. [DOI] [PubMed] [Google Scholar]
- [60].Mahmoudi M, Akhavan O, Ghavami M, Rezaee F, Ghiasi SM, Nanoscale, 4 (2012) 7322–7325. [DOI] [PubMed] [Google Scholar]
- [61].Gladytz A, Abel B, Risselada HJ, Angew Chem Int Ed, 55 (2016) 11242–11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Campioni S, Mannini B, Zampagni M, Pensalfini A, Parrini C, Evangelisti E, Relini A, Stefani M, Dobson CM, Cecchi C, Chiti F, Nat Chem Biol, 6 (2010) 140–147. [DOI] [PubMed] [Google Scholar]
- [63].Mannini B, Mulvihill E, Sgromo C, Cascella R, Khodarahmi R, Ramazzotti M, Dobson CM, Cecchi C, Chiti F, ACS Chemical Biology, 9 (2014) 2309–2317. [DOI] [PubMed] [Google Scholar]
- [64].Sahoo BR, Genjo T, Cox SJ, Stoddard AK, Anantharamaiah GM, Fierke C, Ramamoorthy A, Journal of Molecular Biology, 430 (2018) 4230–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gao N, Sun H, Dong K, Ren J, Duan T, Xu C, Qu X, Nature Communications, 5 (2014) 3422. [DOI] [PubMed] [Google Scholar]
- [66].Ahmed R, Huang J, Weber DK, Gopinath T, Veglia G, Akimoto M, Khondker A, Rheinstädter MC, Huynh V, Wylie RG, Bozelli JC, Epand RM, Melacini G, Journal of the American Chemical Society, (2020). [DOI] [PubMed] [Google Scholar]
- [67].Xin F, Li Y, Fu C, Javed I, Huang X, Schaschkow A, Garcia Ribeiro RS, Gurzov EN, Davis TP, Zhang X, Ke PC, Qiao R, Chemistry of Materials, 32 (2020) 1080–1088. [Google Scholar]
- [68].Chen Q, Du Y, Zhang K, Liang Z, Li J, Yu H, Ren R, Feng J, Jin Z, Li F, Sun J, Zhou M, He Q, Sun X, Zhang H, Tian M, Ling D, ACS Nano, 12 (2018) 1321–1338. [DOI] [PubMed] [Google Scholar]
- [69].Gurzov EN, Wang B, Pilkington EH, Chen P, Kakinen A, Stanley WJ, Litwak SA, Hanssen EG, Davis TP, Ding F, Ke PC, Small, 12 (2016) 1615–1626. [DOI] [PubMed] [Google Scholar]
- [70].Zhao Y, Cai J, Liu Z, Li Y, Zheng C, Zheng Y, Chen Q, Chen H, Ma F, An Y, Xiao L, Jiang C, Shi L, Kang C, Liu Y, Nano Letters, 19 (2019) 674–683. [DOI] [PubMed] [Google Scholar]
- [71].Kakinen A, Xing Y, Hegoda Arachchi N, Javed I, Feng L, Faridi A, Douek AM, Sun Y, Kaslin J, Davis TP, Higgins MJ, Ding F, Ke PC, Nano Letters, 19 (2019) 6535–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Guan Y, Du Z, Gao N, Cao Y, Wang X, Scott P, Song H, Ren J, Qu X, Science Advances, 4 (2018) eaao6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pilkington EH, Lai M, Ge X, Stanley WJ, Wang B, Wang M, Kakinen A, Sani MA, Whittaker MR, Gurzov EN, Ding F, Quinn JF, Davis TP, Ke PC, Biomacromolecules, 18 (2017) 4249–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Faridi A, Sun Y, Okazaki Y, Peng G, Gao J, Kakinen A, Faridi P, Zhao M, Javed I, Purcell AW, Davis TP, Lin S, Oda R, Ding F, Ke PC, Small, 14 (2018) 1802825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang M, Sun Y, Cao X, Peng G, Javed I, Kakinen A, Davis TP, Lin S, Liu J, Ding F, Ke PC, Nanoscale, 10 (2018) 19995–20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, Park MJ, Lee M, Choi S, Kwon SH, Lee S, Kwon SH, Kim S, Park YJ, Kinoshita M, Lee YH, Shin S, Paik SR, Lee SJ, Lee S, Hong BH, Ko HS, Nat Nanotechnol, 13 (2018) 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yang Z, Ge C, Liu J, Chong Y, Gu Z, Jimenez-Cruz CA, Chai Z, Zhou R, Nanoscale, 7 (2015) 18725–18737. [DOI] [PubMed] [Google Scholar]
- [78].Li X, Li K, Chu F, Huang J, Yang Z, Chemico-Biological Interactions, 325 (2020) 109126. [DOI] [PubMed] [Google Scholar]
- [79].Javed I, Yu T, Peng G, Sánchez-Ferrer A, Faridi A, Kakinen A, Zhao M, Mezzenga R, Davis TP, Lin S, Ke PC, Nano letters, 18 (2018) 5797–5804. [DOI] [PubMed] [Google Scholar]
- [80].Javed I, Peng G, Xing Y, Yu T, Zhao M, Kakinen A, Faridi A, Parish CL, Ding F, Davis TP, Ke PC, Lin S, Nat Commun, 10 (2019) 3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Brambilla D, Verpillot R, Le Droumaguet B, Nicolas J, Taverna M, Kóňa J, Lettiero B, Hashemi SH, De Kimpe L, Canovi M, Gobbi M, Nicolas V, Scheper W, Moghimi SM, Tvaroška I, Couvreur P, Andrieux K, ACS Nano, 6 (2012) 5897–5908. [DOI] [PubMed] [Google Scholar]
- [82].Zhang C, Wan X, Zheng X, Shao X, Liu Q, Zhang Q, Qian Y, Biomaterials, 35 (2014) 456–465. [DOI] [PubMed] [Google Scholar]
- [83].Javed I, Sun Y, Adamcik J, Wang B, Kakinen A, Pilkington EH, Ding F, Mezzenga R, Davis TP, Ke PC, Biomacromolecules, 18 (2017) 4316–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Javed I, He J, Kakinen A, Faridi A, Yang W, Davis TP, Ke PC, Chen P, ACS Applied Materials & Interfaces, 11 (2019) 10462–10471. [DOI] [PubMed] [Google Scholar]
- [85].Pilkington EH, Xing Y, Wang B, Kakinen A, Wang M, Davis TP, Ding F, Ke PC, Sci Rep, 7 (2017) 2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ, Science, 295 (2002) 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR, Trends Microbiol, 20 (2012) 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Huma Z.-e., Javed I, Zhang Z, Bilal H, Sun Y, Hussain SZ, Davis TP, Otzen DE, Landersdorfer CB, Ding F, Hussain I, Ke PC, Small, n/a (2020) 1906674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rapaport DC, The Art of Molecular Dynamics Simulation, Cambridge University Press, New York, 2004. [Google Scholar]
- [90].Ding F, Tsao D, Nie H, Dokholyan NV, Structure, 16 (2008) 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ding F, Radic S, Chen R, Chen P, Geitner NK, Brown JM, Ke PC, Nanoscale, 5 (2013) 9162–9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun Y, Wang B, Ge X, Ding F, Phys Chem Chem Phys, 19 (2017) 28414–28423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sun Y, Ge X, Xing Y, Wang B, Ding F, Scientific Reports, 8 (2018) 10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sun Y, Kakinen A, Xing Y, Faridi P, Nandakumar A, Purcell AW, Davis TP, Ke PC, Ding F, Small, 15 (2019) 1805166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vivoli Vega M, Cascella R, Chen SW, Fusco G, De Simone A, Dobson CM, Cecchi C, Chiti F, ACS Chemical Biology, 14 (2019) 1593–1600. [DOI] [PubMed] [Google Scholar]
- [96].Sun Y, Kakinen A, Zhang C, Yang Y, Faridi A, Davis TP, Cao W, Ke PC, Ding F, Nanoscale, 11 (2019) 11933–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Faridi A, Sun Y, Mortimer M, Aranha RR, Nandakumar A, Li Y, Javed I, Kakinen A, Fan Q, Purcell AW, Davis TP, Ding F, Faridi P, Ke PC, Nano Research, 12 (2019) 2827–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Nedumpully-Govindan P, Kakinen A, Pilkington EH, Davis TP, Ke PC, Ding F, Sci Rep, 6 (2016) 19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L, Proc Natl Acad Sci U S A, 98 (2001) 12245–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS, Neuron, 103 (2019) 627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Parajuli B, Sonobe Y, Horiuchi H, Takeuchi H, Mizuno T, Suzumura A, Cell Death & Disease, 4 (2013) e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Webers A, Heneka MT, Gleeson PA, Immunology & Cell Biology, 98 (2020) 28–41. [DOI] [PubMed] [Google Scholar]
- [103].Tuppo EE, Arias HR, Int J Biochem Cell Biol, 37 (2005) 289–305. [DOI] [PubMed] [Google Scholar]
- [104].Faridi A, Yang W, Kelly HG, Wang C, Faridi P, Purcell AW, Davis TP, Chen P, Kent SJ, Ke PC, Biomacromolecules, 20 (2019) 4208–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wang WY, Tan MS, Yu JT, Tan L, Ann Transl Med, 3 (2015) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM, Annals of the New York Academy of Sciences, 1281 (2013) 16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Welikovitch LA, Do Carmo S, Magloczky Z, Malcolm JC, Loke J, Klein WL, Freund T, Cuello AC, Proc Natl Acad Sci U S A, 117 (2020) 6844–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT, Nat Med, (2020). [Google Scholar]
- [109].Cai R, Ren J, Ji Y, Wang Y, Liu Y, Chen Z, Farhadi Sabet Z, Wu X, Lynch I, Chen C, ACS Applied Materials & Interfaces, 12 (2020) 1997–2008. [DOI] [PubMed] [Google Scholar]
- [110].Botto M, Hawkins PN, Bickerstaff MC, Herbert J, Bygrave AE, McBride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB, Nat Med, 3 (1997) 855–859. [DOI] [PubMed] [Google Scholar]
- [111].Wall JS, Richey T, Stuckey A, Donnell R, Macy S, Martin EB, Williams A, Higuchi K, Kennel SJ, Proc Natl Acad Sci U S A, 108 (2011) E586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Liu CC, Zhao N, Yamaguchi Y, Cirrito JR, Kanekiyo T, Holtzman DM, Bu G, Sci Transl Med, 8 (2016) 332ra344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, Tsaava T, Addorisio ME, Putzel GG, Zhou L, Bessman NJ, Yang R, Moriyama S, Parkhurst CN, Li A, Meyer HC, Teng F, Chavan SS, Tracey KJ, Regev A, Schroeder FC, Lee FS, Liston C, Artis D, Nature, 574 (2019) 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kim JD, Yoon NA, Jin S, Diano S, Cell Metab, 30 (2019) 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Andrikopoulos N, Li Y, Cecchetto L, Nandakumar A, Da Ros T, Davis TP, Velonia K, Ke PC, Nanoscale, (2020) DOI: 10.1039/D0NR04273K. [DOI] [PubMed] [Google Scholar]
- [116].Carril M, Padro D, del Pino P, Carrillo-Carrion C, Gallego M, Parak WJ, Nature Communications, 8 (2017) 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]


